GlaxoSmithKline Receives European Authorization for Self-Injectable Benlysta
13 Novembre 2017 - 12:47PM
Dow Jones News
By Carlo Martuscelli
GlaxoSmithKline PLC (GSK.LN) reported on Monday that it had
received European marketing authorization for the self-injectable
formulation of Benlysta--a drug for the treatment of systemic lupus
erythematosus.
The pharmaceutical giant said that the European Commission had
approved the new formulation as an add-on therapy for patients
afflicted by a high degree of disease activity despite standard
therapy.
The approval is for a single-dose pre-filled syringe and a
single-dose pre-filled pen--as a once-weekly injection of 200
milligrams, the company said.
The newly approved version of the drug adds to the existing
intravenous formulation, which was licensed for use in Europe in
2011.
Write to Carlo Martuscelli at carlo.martuscelli@dowjones.com
(END) Dow Jones Newswires
November 13, 2017 06:32 ET (11:32 GMT)
Copyright (c) 2017 Dow Jones & Company, Inc.
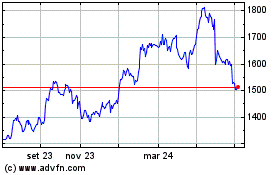
Grafico Azioni Gsk (LSE:GSK)
Storico
Da Mar 2024 a Apr 2024
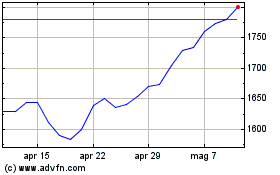
Grafico Azioni Gsk (LSE:GSK)
Storico
Da Apr 2023 a Apr 2024