Hyloris Provides Additional Information on U.S. FDA Review of NDA Application for the Registration of Maxigesic IV®
01 Luglio 2022 - 8:00PM

- FDA did not report any issues with clinical data
- Hyloris is asked to submit a report describing potential
extractable and leachable compounds expected to be present in the
drug product based on the drug product packaging
- Hyloris is confident that it can deliver the requested
information to FDA
- Hyloris is committed bringing Maxigesic IV to patients in the
U.S. as soon as possible and continues to work with the FDA to
support the application review
Liège, Belgium - 01 July 2022 – 8.00 PM
CET - Regulated information - Inside Information - Hyloris
Pharmaceuticals SA (Euronext Brussels: HYL) a specialty
biopharma company committed to addressing unmet medical needs
through reinventing existing medications, today provided additional
details about the United States (U.S.) Food and Drug Administration
(FDA)’s review of the New Drug Application (NDA) for the
registration of Maxigesic IV®.
The FDA has informed Hyloris’ development
partner, AFT Pharmaceuticals, via a Complete Response Letter, that
it was unable to complete its review of the NDA for Maxigesic IV®
and has provided recommendations needed to address the
application’s deficiency. Importantly, the agency did not report
any issues related to data generated during Maxigesic IV®’s
clinical development program, and the deficiency is confined solely
to the Quality section of the application dossier, and more
specifically to revise a risk assessment of the required leachable
compound study.
“We are corresponding with the FDA to address
the recommendations highlighted in the letter as soon as possible,”
said Stijn Van Rompay, Chief Executive Officer of
Hyloris. “Hyloris initially submitted a toxicological risk
assessment for all leachable compounds, however, we will need to
re-submit this information based on confirmed compounds quantified
using validated methods. As previously stated, this request
required by the FDA falls well within the parameters of our normal
operational budget and can be completed expeditiously, requiring no
additional clinical data to be generated. Hyloris remains committed
to Maxigesic IV®, and ensuring the product fulfills its commercial
potential in the U.S. We will be updating the market on revisions
to the U.S. commercialization timeline for this product candidate
as we gain more clarity about the FDA’s request.”
About Maxigesic IV®Maxigesic IV has been
developed under the development collaboration agreement signed in
2012 between Hyloris and AFT Pharmaceuticals, and is to date,
licensed in more than 100 countries, approved in 39 countries
and marketed in seven countries. Maxigesic IV® is a unique
combination of 1000mg paracetamol with 300mg ibuprofen solution for
infusion for use post-operatively. The superior analgesic effect of
Maxigesic IV demonstrated in a phase 3 trial was supported by a
range of secondary endpoints, including reduced opioid consumption
compared to the paracetamol IV and ibuprofen IV treatment groups
(P<0.005). An additional exposure study demonstrated Maxigesic
IV’s efficacy and safety in an expanded population group over a
longer treatment period. Maxigesic IV is protected by several
granted and pending patent applications.
About Hyloris Pharmaceuticals Hyloris is a
specialty biopharma company focused on innovating, reinventing, and
optimizing existing medications to address important healthcare
needs and deliver relevant improvements for patients, healthcare
professionals and payors. Hyloris has built a broad, patented
portfolio of 14 reformulated and repurposed value-added medicines
that have the potential to offer significant advantages over
available alternatives. Outside of its core strategic focus, the
Company also has 4 high barrier generic products in development.
Two products are currently in initial phases of commercialization
with partners: Sotalol IV for the treatment of atrial fibrillation,
and Maxigesic® IV, a non-opioid post-operative pain treatment. The
Company’s development strategy primarily focuses on the FDA’s
505(b)2 regulatory pathway, which is specifically designed for
pharmaceuticals for which safety and efficacy of the molecule have
already been established. This pathway can reduce the clinical
burden required to bring a product to market, and significantly
shorten the development timelines and reduce costs and risks.
Hyloris is based in Liège, Belgium. For more information,
visit www.hyloris.com and follow-us on LinkedIn.
For more information contact:
Hyloris Pharmaceuticals, Investors and
Mediainvestorrelations@hyloris.com Disclaimer and
forward-looking statements Hyloris means “high yield,
lower risk”, which relates to the 505(b)(2) regulatory pathway for
product approval on which the Issuer focuses, but in no way relates
or applies to an investment in the Shares. Certain statements in
this press release are “forward-looking statements.” These
forward-looking statements can be identified using forward-looking
terminology, including the words "believes", "estimates,"
"anticipates", "expects", "intends", "may", "will", "plans",
"continue", "ongoing", "potential", "predict", "project", "target",
"seek" or "should", and include statements the Company makes
concerning the intended results of its strategy. These statements
relate to future events or the Company’s future financial
performance and involve known and unknown risks, uncertainties, and
other factors, many of which are beyond the Company’s control, that
may cause the actual results, levels of activity, performance or
achievements of the Company or its industry to be materially
different from those expressed or implied by any forward-looking
statements. The Company undertakes no obligation to publicly update
or revise forward-looking statements, except as may be required by
law.
- 220701_Hyloris_Press_Release_CRL_Updated_final
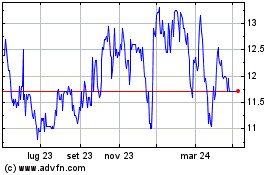
Grafico Azioni Hyloris Pharmaceuticals (EU:HYL)
Storico
Da Mar 2024 a Apr 2024
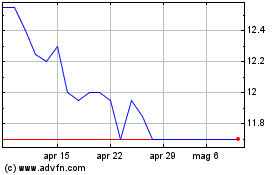
Grafico Azioni Hyloris Pharmaceuticals (EU:HYL)
Storico
Da Apr 2023 a Apr 2024