Ionis: FDA to Review Eplontersen in ATTRv-PN
08 Marzo 2023 - 8:05AM
Dow Jones News
By Colin Kellaher
Ionis Pharmaceuticals Inc. on Tuesday said the U.S. Food and
Drug Administration will review its application seeking approval of
its eplontersen drug candidate for the treatment of the rare
multisystemic disease hereditary transthyretin-mediated amyloid
polyneuropathy, or ATTRv-PN.
The Carlsbad, Calif., pharmaceutical company said the FDA set a
target action date of Dec. 22 for the application, adding that the
agency indicated that it isn't planning to hold an advisory
committee meeting.
ATTRv-PN is a debilitating disease that leads to peripheral
nerve damage with motor disability within five years of diagnosis
and is generally fatal within a decade without treatment.
Ionis and Anglo-Swedish drugmaker AstraZeneca PLC in late 2021
inked an agreement to jointly develop and commercialize eplontersen
in the U.S., with AstraZeneca developing and commercializing the
drug in the rest of the world with the exception of Latin
America.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
March 07, 2023 07:47 ET (12:47 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
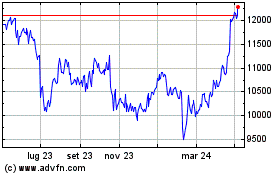
Grafico Azioni Astrazeneca (LSE:AZN)
Storico
Da Mar 2024 a Apr 2024
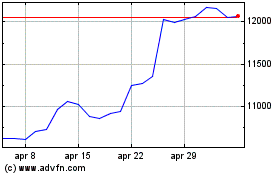
Grafico Azioni Astrazeneca (LSE:AZN)
Storico
Da Apr 2023 a Apr 2024