Aurinia Announces First Participant Dosed in AUR200 Single Ascending Dose Trial
05 Settembre 2024 - 12:00PM
Business Wire
Aurinia Pharmaceuticals Inc. (NASDAQ: AUPH) (Aurinia or the
Company) announced today that the first participant has been dosed
in a Phase 1a single ascending dose (SAD) study of AUR200, a
differentiated, potential best-in-class therapy for autoimmune
diseases that targets both BAFF (B-cell Activating Factor) and
APRIL (A Proliferation-Inducing Ligand).
The SAD study will assess the safety, tolerability,
pharmacokinetics, and changes in biomarkers for AUR200 in healthy
volunteers, with data expected in the first half of 2025.
“The start of the single ascending dose study is an important
milestone for developing AUR200, which has the potential to serve
as a best-in-class treatment in autoimmune diseases with high unmet
need. We believe AUR200 is a more potent compound with a higher
binding affinity compared to the two other TACI-Fc molecules
designed to bind both BAFF and APRIL that were tested in our
preclinical research. We expect the data from this early-stage
study to provide key points of differentiation that will inform
further clinical development efforts,” said Dr. Greg Keenan, Chief
Medical Officer of Aurinia.
The Company intends to develop AUR200 in disease states where
there are currently few market entrants, including one larger
indication and one fast-to-market smaller indication that meets the
FDA criteria for orphan and rare diseases. The Company will
communicate specific indications and development plans pending
progress and outcomes of early-stage clinical trials and based on
ongoing assessment of the competitive landscape in relevant
indications. The Company anticipates funding this development
program with available cash flow, which is not anticipated to
impact previously announced post-restructuring operating expense
targets.
About AUR200
AUR200, a highly potent and specific immune modulator, is an
IgG4 Fc-fusion protein with no appreciable effector function.
AUR200 contains a unique, structurally engineered B-cell maturation
antigen (BCMA) domain for enhanced binding to both BAFF and APRIL,
two cytokines that play important roles in regulating B-cell
survival and differentiation. Dual inhibition of BAFF and APRIL is
a clinically validated mechanism that has demonstrated great
therapeutic potential for a wide range of autoimmune diseases.
In animal data presented at the annual American College of
Rheumatology Convergence 2022, AUR200 dosed therapeutically reduced
several markers of disease activity and improved overall survival
in a mouse model of lupus. AUR200 was also well-tolerated in both
mice and cynomolgus monkeys, with no adverse effects. These
findings highlight the potential value of AUR200 in the treatment
of autoimmune diseases.i
About Aurinia
Aurinia Pharmaceuticals is a fully integrated biopharmaceutical
company focused on delivering therapies to people living with
autoimmune diseases with high unmet medical needs. In January 2021,
the Company introduced LUPKYNIS® (voclosporin), the first
FDA-approved oral therapy dedicated to the treatment of adult
patients with active lupus nephritis. The Company’s head office is
in Edmonton, Alberta, with its U.S. commercial office in Rockville,
Maryland. The Company focuses its development efforts globally.
Forward-Looking Statements
Certain statements made in this press release may constitute
forward-looking information within the meaning of applicable
Canadian securities law and forward-looking statements within the
meaning of applicable United States securities law. These
forward-looking statements or information include but are not
limited to statements or information with respect to: Aurinia’s
belief that AUR200 is a potential best-in-class therapy for
autoimmune diseases that targets both BAFF and APRIL; Aurinia’s
expectation that data from the SAD study will be available in the
first half of 2025; Aurinia’s belief that AUR200 has the potential
to serve as a best-in-class treatment in autoimmune diseases with
high unmet need; Aurinia’s belief that AUR200 is a more potent
compound with a higher binding affinity compared to other TACI-Fc
molecules designed to bind both BAFF and APRIL; Aurinia’s
expectations that the SAD study will provide key points of
differentiation that will inform further clinical development
efforts; Aurinia’s intention to develop AUR200 in diseases states
where there are currently few market entrants; and Aurinia’s
anticipation to fund the AUR200 development program with available
cash flow. It is possible that such results or conclusions may
change. Words such as “anticipate”, “will”, “believe”, “estimate”,
“expect”, “intend”, “target”, “plan”, “goals”, “objectives”, “may”
and other similar words and expressions, identify forward-looking
statements. We have made numerous assumptions about the
forward-looking statements and information contained herein,
including among other things, assumptions about: the burn rate of
Aurinia’s cash for operations; that pre-clinical data for AUR200
will be reproduceable in clinical studies; and that Aurinia’s third
party service providers will comply with their contractual
obligations. Even though the management of Aurinia believes that
the assumptions made, and the expectations represented by such
statements or information are reasonable, there can be no assurance
that the forward-looking information will prove to be accurate.
Forward-looking information by their nature are based on
assumptions and involve known and unknown risks, uncertainties and
other factors which may cause the actual results, performance, or
achievements of Aurinia to be materially different from any future
results, performance or achievements expressed or implied by such
forward-looking information. Should one or more of these risks and
uncertainties materialize, or should underlying assumptions prove
incorrect, actual results may vary materially from those described
in forward-looking statements or information. Such risks,
uncertainties and other factors include, among others, the
following: Aurinia’s actual future financial and operational
results may differ from its expectations; Aurinia may have to pay
unanticipated expenses; the future prospects for AUR200 may not be
as Aurinia has anticipated, or Aurinia may not be able to fully
capitalize on the opportunities presented by AUR200; and Aurinia’s
third party service providers may not, or may not be able to,
comply with their obligations under their agreements with Aurinia.
Although Aurinia has attempted to identify factors that would cause
actual actions, events, or results to differ materially from those
described in forward-looking statements and information, there may
be other factors that cause actual results, performances,
achievements, or events to not be as anticipated, estimated or
intended. Also, many of the factors are beyond Aurinia’s control.
There can be no assurance that forward-looking statements or
information will prove to be accurate, as actual results and future
events could differ materially from those anticipated in such
statements. Accordingly, you should not place undue reliance on
forward-looking statements or information.
All forward-looking information contained in this press release
is qualified by this cautionary statement. Additional information
related to Aurinia, including a detailed list of the risks and
uncertainties affecting Aurinia and its business, can be found in
Aurinia’s most recent Annual Report on Form 10-K available by
accessing the Canadian Securities Administrators’ System for
Electronic Document Analysis and Retrieval (SEDAR) website at
www.sedarplus.ca or the U.S. Securities and Exchange Commission’s
Electronic Document Gathering and Retrieval System (EDGAR) website
at www.sec.gov/edgar, and on Aurinia’s website at
www.auriniapharma.com.
___________________________________ i Morales S, Cross J,
Huizinga R. AUR200: An Improved BAFF/APRIL Inhibitor with Increased
Potency and Safety for the Treatment of B Cell-Mediated Diseases
[abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9).
https://acrabstracts.org/abstract/aur200-an-improved-baff-april-inhibitor-with-increased-potency-and-safety-for-the-treatment-of-b-cell-mediated-diseases/.
Accessed December 6, 2023.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20240905970274/en/
Media & Investor Inquiries: Andrea Christopher,
Corporate Communications & Investor Relations, Aurinia
achristopher@auriniapharma.com
General Investor Inquiries: ir@auriniapharma.com
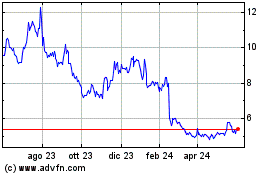
Grafico Azioni Aurinia Pharmaceuticals (NASDAQ:AUPH)
Storico
Da Gen 2025 a Feb 2025
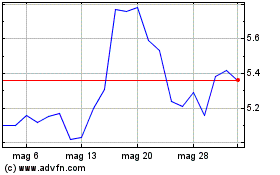
Grafico Azioni Aurinia Pharmaceuticals (NASDAQ:AUPH)
Storico
Da Feb 2024 a Feb 2025