British American Tobacco's Covid-19 Vaccine Approved for Human Trials
16 Dicembre 2020 - 5:22PM
Dow Jones News
By Joe Hoppe
British American Tobacco PLC said Wednesday that its
Investigational New Drug application for a coronavirus vaccine has
been approved by the U.S. Food and Drug Administration, allowig it
to proceed to Phase 1 clinical trials in adult volunteers.
BAT--which produces cigarettes, electronic and vape products and
other tobacco products--said its vaccine candidate will shortly
enroll 180 healthy volunteers for a trial, with results expected in
mid-2021. If positive, the results would allow for a Phase 2 study,
subject to regulatory approval.
The candidate vaccine, developed by BAT's U.S. bio-technology
arm Kentucky BioProcessing, uses fast-growing plant-based
technology. The approach brings a number of possible advantages,
including the rapid production of vaccine active ingredients in six
weeks rather than several months, and has the potential to be
stable at room temperature.
"Moving into human trials with both our Covid-19 and seasonal
flu vaccine candidates is a significant milestone and reflects our
considerable efforts to accelerate the development of our emerging
biologicals portfolio," BAT's Director of Scientific Research David
O'Reilly said.
Shares at 1551 GMT were up 6.5 pence, or 0.2%, at 2844.5
pence.
Write to Joe Hoppe at joseph.hoppe@wsj.com
(END) Dow Jones Newswires
December 16, 2020 11:07 ET (16:07 GMT)
Copyright (c) 2020 Dow Jones & Company, Inc.
Grafico Azioni British American Tobacco (LSE:BATS)
Storico
Da Mar 2024 a Apr 2024
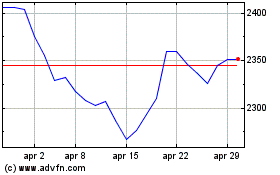
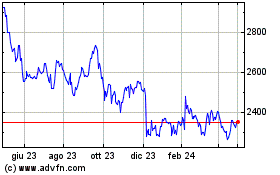
Grafico Azioni British American Tobacco (LSE:BATS)
Storico
Da Apr 2023 a Apr 2024