GlaxoSmithKline's Belantamab Mafodotin Gets Recommendation From FDA Panel
15 Luglio 2020 - 8:47AM
Dow Jones News
By Adria Calatayud
GlaxoSmithKline PLC said Wednesday that a U.S. Food and Drug
Administration committee has voted in favor of its belantamab
mafodotin antibody drug as therapy for the treatment of relapsed or
refractory multiple myeloma.
The FDA's oncologic drugs advisory committee voted in favor of a
positive benefit-risk profile for the drug to help patients with
relapsed or refractory multiple myeloma who have received at least
four prior therapies, the British pharmaceutical company said. The
FDA will consider the recommendation of the committee but isn't
obligated to follow it, GSK said.
Belantamab mafodotin isn't currently approved for use anywhere
in the world, the company said.
Write to Adria Calatayud at adria.calatayud@dowjones.com
(END) Dow Jones Newswires
July 15, 2020 02:32 ET (06:32 GMT)
Copyright (c) 2020 Dow Jones & Company, Inc.
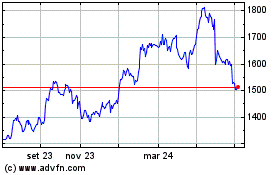
Grafico Azioni Gsk (LSE:GSK)
Storico
Da Mar 2024 a Apr 2024
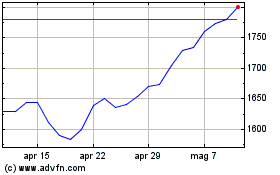
Grafico Azioni Gsk (LSE:GSK)
Storico
Da Apr 2023 a Apr 2024