Hyloris Announces Launch of Maxigesic® IV, a Novel Non-Opioid Pain Treatment, in Key European Markets
08 Luglio 2021 - 7:00AM

Marks first European launches of Maxigesic IV, a
well-tolerated and effective non-opioid pain treatment
Liège, Belgium
– 8 July 2021
– Hyloris Pharmaceuticals
SA (Euronext Brussels: HYL), a specialty biopharma
company committed to addressing unmet medical needs through
reinventing existing medications, today announces that Maxigesic IV
is now available in Germany, the largest European pharmaceutical
market, and Austria.
Maxigesic IV is a novel, patented, non-opioid
treatment for post-operative pain and is a unique combination of
1000mg paracetamol and 300mg ibuprofen solution for infusion.
Hyloris’ partner AFT Pharmaceuticals works together with
distribution partners with strong local presence to commercialise
the product globally.
Maxigesic IV is currently licensed in more than
100 countries across the globe, and it has been registered in 24
countries. Following the launch in Germany and Austria, the product
is now available in 5 countries: Australia, New Zealand, The United
Arab Emirates, Germany, and Austria.
Stijn Van Rompay, Chief Executive
Officer of Hyloris, commented: “We are pleased that AFT
and its partner Ever Pharma have now launched Maxigesic IV in
Germany and Austria. We are convinced that Ever Pharma is the ideal
partner to make this valuable new non-addictive pain treatment
available to patients in Germany and Austria given their strong
footprint in key European markets, and their expertise with complex
injectables in multiple therapeutic areas, including anaesthesia.
We look forward to continuing to update the market as we, and our
partner AFT, make further progress in the regulatory activities,
launches and further roll-out of Maxigesic IV across the
globe.”
Annually, over 5.2 million surgical procedures
are performed in Germany, and the market for postoperative pain in
Germany is expected to grow to $166.5 million by 2028 at a CAGR of
11.58% from 2017-2028.1
About
Maxigesic®
IV
Maxigesic IV has been developed under the
development collaboration agreement signed in 2012 between Hyloris
and AFT Pharmaceuticals. Maxigesic IV is a unique combination of
1000mg paracetamol and 300mg ibuprofen solution for infusion for
use post-operatively. Results from a randomised, double-blind,
placebo-controlled Phase 3 trial in 276 patients following bunion
surgery demonstrated that Maxigesic IV was well-tolerated and had a
faster onset of action and offered higher pain relief compared to
ibuprofen IV or paracetamol IV alone in the same doses. Moreover,
the superior analgesic effect of Maxigesic IV was supported by a
range of secondary endpoints, including reduced opioid consumption
compared to the paracetamol IV and ibuprofen IV treatment groups
(P<0.005).2 In addition, the safety and tolerability of repeated
doses of Maxigesic IV over an extended period was assessed in an
open-label, multi-centre, single arm study in 232 patients
undergoing orthopaedic or plastic surgery. This extension study
demonstrated that Maxigesic IV, administered 6-hourly as a
15-minute infusion between 48 hours to 5 days was safe and
well-tolerated, and was perceived positively by study participants,
supporting a favourable risk benefit profile.3 Under the terms of
the collaboration agreement with AFT, Hyloris is eligible to a high
minority share of Maxigesic IV related income generated by AFT,
excluding income generated in Australia and New Zealand.
About Hyloris
Pharmaceuticals
Hyloris is a specialty biopharma company focused
on innovating, reinventing, and optimising existing medications to
address important healthcare needs and deliver relevant
improvements for patients, healthcare professionals and payors.
Hyloris has built a broad, patented portfolio of 13 reformulated
and repurposed value-added medicines that have the potential to
offer significant advantages over available alternatives. Two
products are currently commercialised with partners: Sotalol IV for
the treatment of atrial fibrillation, and Maxigesic® IV, a
non-opioid post-operative pain treatment. The Company’s development
strategy primarily focuses on the FDA’s 505(b)2 regulatory pathway,
which is specifically designed for pharmaceuticals for which safety
and efficacy of the molecule have already been established. This
pathway can reduce the clinical burden required to bring a product
to market, and significantly shorten the development timelines and
reduce costs and risks. Hyloris is based in Liège, Belgium. For
more information, visit www.hyloris.com and follow-us on
LinkedIn.
For more information, please
contact Hyloris
Pharmaceuticals:
Marieke VermeerschVP Investor Relations and
Corporate CommunicationsM: +32 (0)479 490
603marieke.vermeersch@hyloris.com
Disclaimer and forward-looking
statements
Hyloris means “high yield, lower risk”, which
relates to the 505(b)(2) regulatory pathway for product approval on
which the Issuer focuses, but in no way relates or applies to an
investment in the Shares.Certain statements in this press release
are “forward-looking statements.” These forward-looking statements
can be identified using forward-looking terminology, including the
words "believes", "estimates," "anticipates", "expects", "intends",
"may", "will", "plans", "continue", "ongoing", "potential",
"predict", "project", "target", "seek" or "should", and include
statements the Company makes concerning the intended results of its
strategy. These statements relate to future events or the Company’s
future financial performance and involve known and unknown risks,
uncertainties, and other factors, many of which are beyond the
Company’s control, that may cause the actual results, levels of
activity, performance or achievements of the Company or its
industry to be materially different from those expressed or implied
by any forward-looking statements. The Company undertakes no
obligation to publicly update or revise forward-looking statements,
except as may be required by law.
1 Postoperative Pain Market Insights, Epidemiology and Market
Forecast – 2028. DELVEINSIGHT2 Daniels et al, 2019, Clinical
Therapeutics3 Gottlieb et al, 2021, Biomedicine &
Pharmacotherapy
- 210708 Hyloris Press Release Maxigesic EU launch_ENG
Grafico Azioni Hyloris Pharmaceuticals (EU:HYL)
Storico
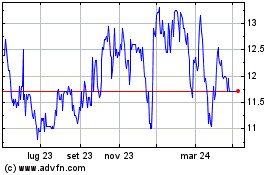
Da Mar 2024 a Apr 2024
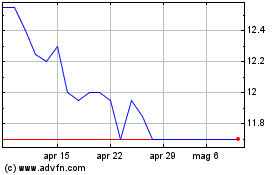
Grafico Azioni Hyloris Pharmaceuticals (EU:HYL)
Storico
Da Apr 2023 a Apr 2024