Johnson & Johnson Profit Is Boosted by Pharmaceuticals Business
26 Gennaio 2021 - 2:35PM
Dow Jones News
By Matt Grossman
Strong sales growth from pharmaceuticals lifted Johnson &
Johnson's revenue in the latest quarter, even as the coronavirus
pandemic weighed on other parts of the company's business.
As the health-products company nears the release of pivotal data
from a final-stage study of its Covid-19 vaccine, Johnson &
Johnson said the pandemic was continuing to drag down sales of its
medical devices as people postponed elective procedures. But modest
growth in consumer-health products also contributed to the sales
boost in the latest quarter.
Alex Gorsky, the company's chairman and chief executive, said
that Johnson & Johnson would soon share data from its
final-stage study of the coronavirus vaccine it is developing.
Emergency approval from the Food & Drug Administration could
follow, and would bolster the U.S. supply of shots aimed at ending
the pandemic amid a rocky vaccine rollout.
Johnson & Johnson's fourth-quarter revenue was $22.48
billion, compared with $20.75 billion in the year-before quarter.
Analysts surveyed by FactSet were expecting revenue of $21.66
billion.
Drugs such as Stelara, for inflammatory diseases, and Darzalex,
for multiple myeloma, helped drive sales growth of 16% for the New
Brunswick, N.J., company's pharmaceutical division, to $12.27
billion. Revenue from the consumer-health division, ticked up by
1.4% to $3.62 billion.
Sales from medical devices slipped 0.7% year over year to $6.59
billion as the pandemic cut demand for products used in surgery,
orthopedics and vision care.
The company posted net earnings of $1.74 billion, or 65 cents a
share, compared with $4.01 billion, or $1.50 a share, in the same
three-month period a year earlier.
Johnson & Johnson said it expects sales growth in 2021 to
boost its full-year revenue to between $90.5 billion and $91.7
billion. Analysts had been forecasting full-year sales of $88.59
billion.
The company's Covid-19 vaccine, if approved, would be especially
helpful to the nation's supply because it is designed to work with
just one dose. The two vaccines that have received emergency U.S.
approval, one produced by Moderna Inc. and the other by Pfizer Inc.
and BioNTech SE, require two doses to reach their full protective
strength.
The rollout of vaccines across the country has been slowed by
technical challenges and supply constraints as manufacturers and
health officials race to make and distribute the shots.
Merck & Co. is discontinuing development of its Covid-19
vaccine after results from a clinical trial showed disappointing
results, the company said Monday.
(END) Dow Jones Newswires
January 26, 2021 08:20 ET (13:20 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
Grafico Azioni Johnson and Johnson (NYSE:JNJ)
Storico
Da Mar 2024 a Apr 2024
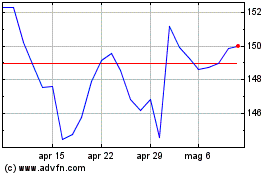
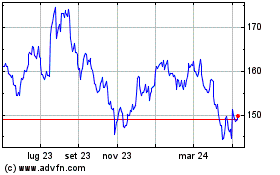
Grafico Azioni Johnson and Johnson (NYSE:JNJ)
Storico
Da Apr 2023 a Apr 2024