Pfizer, Lilly See Positive Top-Line Results From Phase 3 Trial of Tanezumab
18 Luglio 2018 - 1:43PM
Dow Jones News
By Chris Wack
Pfizer Inc. (PFE) and Eli Lilly and Co. (LLY) said Wednesday
that a 16-week Phase 3 study in patients with osteoarthritis pain
evaluating subcutaneous administration of tanezumab, an
investigational humanized monoclonal antibody, met all three
co-primary endpoints.
The study demonstrated that patients who received two doses of
tanezumab separated by eight weeks experienced a statistically
significant improvement in pain, physical function and the
patients' overall assessment of their osteoarthritis, compared to
those receiving a placebo, the drug companies in a release.
"We are encouraged by these results, which speak to the
potential of tanezumab as a non-opioid treatment option for pain
reduction and improvement in physical function in people living
with osteoarthritis pain," Pfizer's Ken Verburg said.
Preliminary safety data showed that tanezumab was generally well
tolerated, with about 1% of patients discontinuing treatment due to
adverse events.
Pfizer and Lilly received U.S. Food and Drug Administration Fast
Track designation for tanezumab for the treatment of osteoarthritis
pain and chronic lower back pain in June 2017.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
July 18, 2018 07:28 ET (11:28 GMT)
Copyright (c) 2018 Dow Jones & Company, Inc.
Grafico Azioni Pfizer (NYSE:PFE)
Storico
Da Mar 2024 a Apr 2024
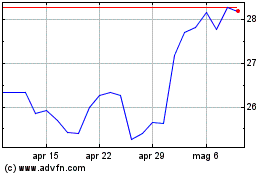
Grafico Azioni Pfizer (NYSE:PFE)
Storico
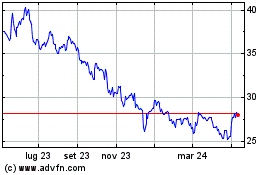
Da Apr 2023 a Apr 2024