false
0001280776
0001280776
2024-05-08
2024-05-08
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
May 8, 2024
IMMUNIC, INC.
(Exact name of registrant as specified in its
charter)
| Delaware |
001-36201 |
56-2358443 |
(State or other jurisdiction
of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
1200 Avenue of the Americas, Suite 200
New York, NY 10036
USA
(Address of principal executive offices)
Registrant’s telephone number, including
area code: (332) 255-9818
Check the appropriate box below if the Form 8-K filing is intended
to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of exchange on which registered |
| Common Stock, par value $0.0001 |
IMUX |
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth
company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange
Act of 1934 (§ 240.12b2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant
has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant
to Section 13(a) of the Exchange Act. Yes ☐ No ☐
Item 2.02. Results of Operations and Financial
Condition
On May 8, 2024, Immunic, Inc. (the “Company”)
issued a press release announcing its financial results for the quarter ended March 31, 2024 and providing a corporate update (the “Press
Release”).
The information contained in Item 2.02 of this Current
Report on Form 8-K, including the Press Release, shall not be deemed “filed” for the purposes of Section 18 of the Securities
Exchange Act of 1934, as amended, or otherwise subject to the liability of that section or Sections 11 and 12(a)(2) of the Securities
Act of 1933, as amended. In addition, this information shall not be deemed incorporated by reference into any of the Company’s filings
with the Securities and Exchange Commission, except as shall be expressly set forth by specific reference in any such filing.
Item 8.01. Other Events
On May 8, 2024, the Company posted an updated presentation
(the “Presentation”) on its website. A copy of the Presentation is filed herewith as Exhibit 99.2 and is incorporated herein
by reference.
Cautionary Note Regarding Forward-Looking Statements
Certain statements in this Current Report on Form 8-K, the Press Release
and the Presentation are “forward-looking statements” that involve substantial risks and uncertainties for purposes of the
safe harbor provided by the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts,
included in this Current Report on Form 8-K, the Press Release and the Presentation regarding strategy, future operations, future financial
position, future revenue, projected expenses, sufficiency of cash and cash runway, expected timing, development and results of clinical
trials, prospects, plans and objectives of management are forward-looking statements. Examples of such statements include, but are not
limited to, statements relating to Immunic's development programs and the targeted diseases; the potential for Immunic's development programs
to safely and effectively target diseases; preclinical and clinical data for Immunic's development programs; the timing of current and
future clinical trials and anticipated clinical milestones; the nature, strategy and focus of the Company and further updates with respect
thereto; the development and commercial potential of any product candidates of the Company; and the Company's expected cash runway. Immunic
may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in the forward-looking
statements and you should not place undue reliance on these forward-looking statements. Such statements are based on management's current
expectations and involve substantial risks and uncertainties. Actual results and performance could differ materially from those projected
in the forward-looking statements as a result of many factors, including, without limitation, the COVID-19 pandemic, increasing inflation,
impacts of the Ukraine – Russia conflict and the conflict in the Middle East on planned and ongoing clinical trials, risks and uncertainties
associated with the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations,
the availability of sufficient financial and other resources to meet business objectives and operational requirements including the ability
to satisfy the conditions required to receive funding in tranche 2 and 3 of the January 2024 private placement, the fact that the results
of earlier preclinical studies and clinical trials may not be predictive of future clinical trial results, the protection and market exclusivity
provided by Immunic's intellectual property, risks related to the drug development and the regulatory approval process and the impact
of competitive products and technological changes. A further list and descriptions of these risks, uncertainties and other factors can
be found in the section captioned “Risk Factors,” in the Company’s Annual Report on Form 10-K for the fiscal year ended
December 31, 2023, filed with the SEC on February 22, 2024, and in the Company’s subsequent filings with the SEC. Copies of these
filings are available online at www.sec.gov or ir.imux.com/sec-filings.
The statements made in this Current Report on Form 8-K, the Press Release
and the Presentation speak only as of the date stated herein, and subsequent events and developments may cause the Company’s expectations
and beliefs to change. While the Company may elect to update these forward-looking statements publicly at some point in the future, the
Company specifically disclaims any obligation to do so, whether as a result of new information, future events or otherwise, except as
required by law. These forward-looking statements should not be relied upon as representing the Company’s views as of any date after
the date stated herein.
Item 9.01. Financial Statements and Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934,
as amended, the Registrant has duly caused this report to be signed on its behalf by the undersigned, hereunto duly authorized.
| Dated: May 8, 2024 |
Immunic, Inc. |
| |
|
|
| |
By: |
/s/ Daniel Vitt |
| |
|
Daniel Vitt |
| |
|
Chief Executive Officer |

Immunic, Inc. Reports First
Quarter 2024 Financial Results
and Provides Corporate Update
–
Substantially Bolstered Balance Sheet Through a Three-Tranche Private Placement Totaling Up to $240 Million, Extending Cash Runway Into
the Third Quarter of 2025, Based on Initial $80 Million Tranche –
–
Received Fourth U.S. Patent Directed to Use of Vidofludimus Calcium in Multiple Sclerosis; Multilayered Intellectual Property Strategy
Provides Protection Into 2041 in the United States –
–
Twin Phase 3 ENSURE Trials in Relapsing Multiple Sclerosis and Phase 2 CALLIPER Trial in Progressive Multiple Sclerosis Remain Underway
–
–
Webcast to be Held Today, May 8, 2024, at 8:00 am ET –
NEW YORK,
May 8, 2024 – Immunic, Inc. (Nasdaq:
IMUX), a biotechnology company developing a clinical pipeline of orally administered, small molecule therapies for chronic
inflammatory and autoimmune diseases, today announced financial results for the first quarter ended March 31, 2024, and provided a corporate
update.
“During the first quarter and subsequent
period, we have continued to advance both the phase 2 CALLIPER trial in patients with progressive multiple sclerosis (PMS) and the twin
phase 3 ENSURE trials in relapsing multiple sclerosis (RMS), for our potentially groundbreaking, orally available lead asset, nuclear
receptor related 1 (Nurr1) activator, vidofludimus calcium (IMU-838),” stated Daniel Vitt, Ph.D., Chief Executive Officer and President
of Immunic. “Just last month, we hosted a Multiple Sclerosis (MS) R&D Day, highlighting the latest developments in the MS landscape
as well as our highly encouraging preclinical and clinical data supporting the neuroprotective potential and reduced disability-worsening
associated with vidofludimus calcium, which represent important distinctions compared to currently available MS therapies. We also shared
our strong belief that vidofludimus calcium could elevate today’s standard of care by providing a holistic solution for the full
spectrum of MS patients, given that it is designed to selectively manage all three components of smoldering MS with its neuroprotective,
anti-inflammatory and antiviral effects. Importantly, the clear separation from placebo in serum neurofilament light chain (NfL) levels
in patients with PMS and non-relapsing secondary progressive multiple sclerosis (SPMS), which was observed in the recent interim analysis
from our phase 2 CALLIPER trial, is significant, and, if this separation is also evident in the top-line CALLIPER data expected in April
of next year, we may also be able to position vidofludimus calcium as the first oral treatment option for non-relapsing SPMS. Additionally,
as it relates to our phase 3 ENSURE program, we expect to read-out the first of the ENSURE trials in the second quarter of 2026 and anticipate
reading out the second ENSURE trial in the second half of 2026.”
“We are well capitalized to execute on our
upcoming MS milestones, following our successful January 2024, three-tranche private placement of up to $240 million, with a group of
top-tier new and existing investors, which we believe reflects the enormous potential of our clinical programs. In addition to strengthening
our capital position, we also continued to build on the several layers of patents, currently protecting vidofludimus calcium into 2041
in the United States, with the United States Patent and Trademark Office's (USPTO) Notice of Allowance last month for our fourth patent
application covering a key composition-of-matter of a specific polymorph of vidofludimus calcium and related production of the material.”

Dr. Vitt concluded, “Our second program,
IMU-856, an orally available, systemically acting small molecule modulator that targets Sirtuin
6 (“SIRT6”), a protein which serves as a transcriptional regulator of intestinal barrier function and physiological regeneration
of bowel epithelium, also shows great promise. As we have noted previously, based on initial clinical proof-of-concept data, we
believe that IMU-856 could be an entirely new therapeutic approach to treating gastrointestinal
disorders by restoring a healthy gut through renewal of the bowel wall. In particular, the data from our phase 1b clinical trial in celiac
disease patients during periods of gluten-free diet and gluten challenge demonstrated positive effects for IMU-856 over placebo in four
key dimensions of celiac disease pathophysiology: protection of the gut architecture, improvement of patients’ symptoms, biomarker
response, and enhancement of nutrient absorption. We are currently preparing clinical phase 2 testing of IMU-856 in patients with ongoing
active celiac disease (OACD) despite gluten-free diet, while also exploring the possibility of additional clinical uses in other gastrointestinal
conditions.”
First Quarter 2024 and Subsequent Highlights
| · | April 2024: Hosted an MS R&D Day, during which management discussed
the latest developments in the MS landscape, along with recent preclinical and clinical data supporting the neuroprotective potential
of vidofludimus calcium. |
| · | March 2024: Received Notice of Allowance from the USPTO for patent
application 16/981,122 entitled, “Calcium salt polymorphs as anti-inflammatory, immunomodulatory and anti-proliferative agents,”
covering the composition-of-matter of a specific polymorph of vidofludimus calcium and a related method of production of the material. |
| · | February 2024: Presented data from the company's phase 2 CALLIPER
and CALVID-1 clinical trials of vidofludimus calcium, in two poster presentations at the Americas Committee for Treatment and Research
in Multiple Sclerosis (ACTRIMS) Forum 2024. |
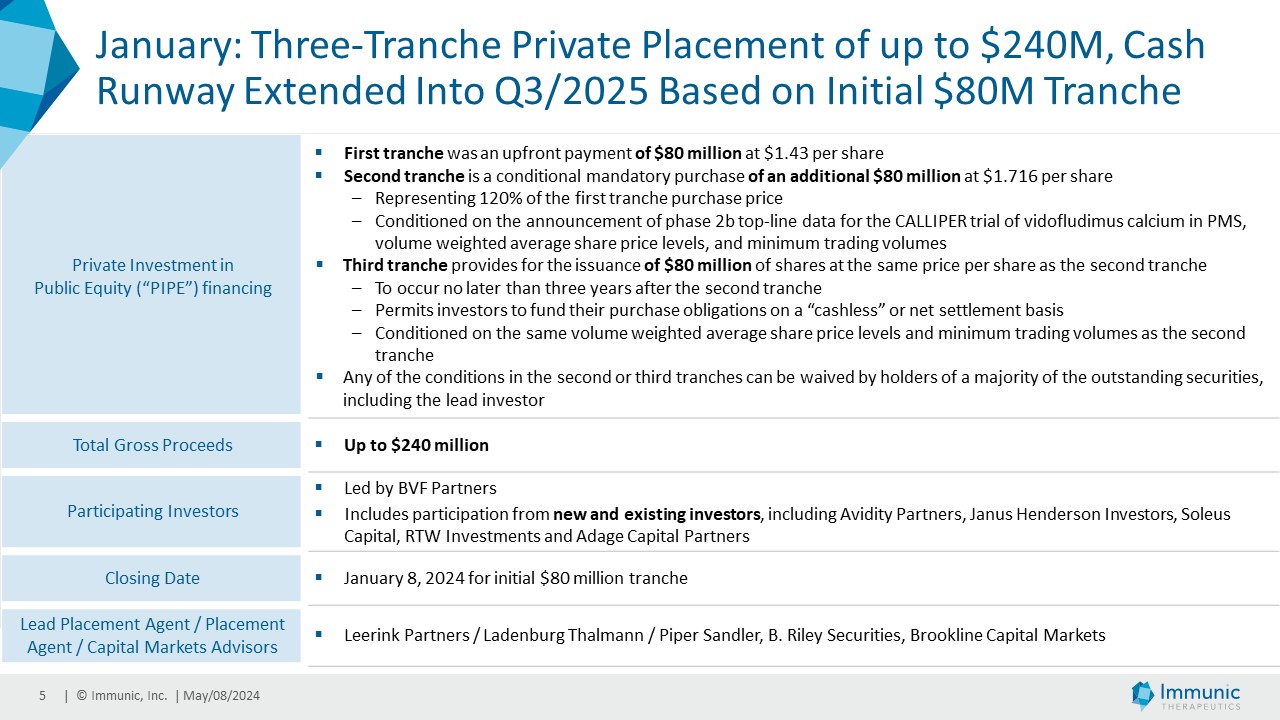
| · | January 2024: Announced a three-tranche private placement totaling
up to $240 million, with participation from select new and existing investors. These included lead investor BVF Partners, alongside Avidity
Partners, Janus Henderson Investors, Soleus Capital, RTW Investments and Adage Capital Partners. The initial tranche successfully closed
on January 8, 2024, with Immunic securing $80 million in gross proceeds. |
Clinical Development Programs
| · | Vidofludimus calcium in MS: Top-line data from the phase 2 CALLIPER trial of vidofludimus calcium
in PMS is expected in April 2025. An interim futility analysis of the ENSURE program is expected in late 2024. The read-out of the first
of the ENSURE trials is currently anticipated in the second quarter of 2026; and the second ENSURE trial in the second half of 2026. |
| · | IMU-856 in celiac disease: Based on the positive data from the phase 1b clinical trial, the company
is preparing for clinical phase 2 testing of IMU-856 in OACD patients despite gluten-free diet. |
Financial and Operating Results
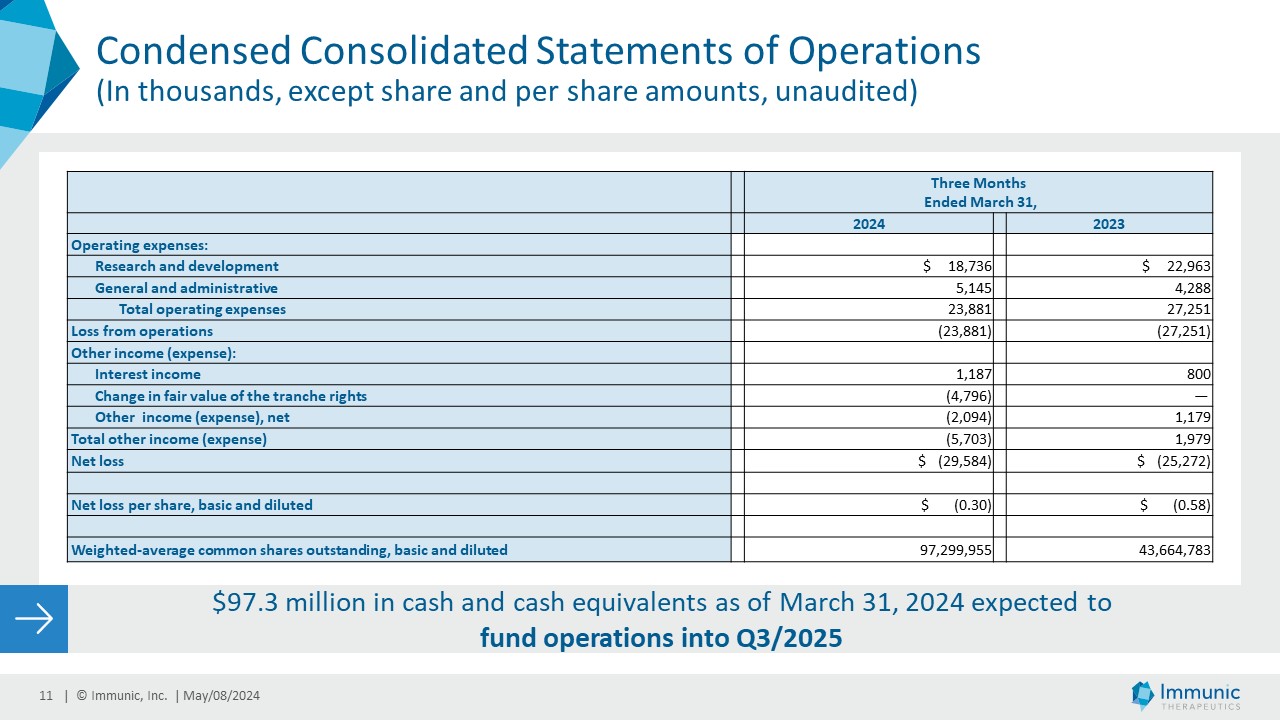
| · | Research and Development (R&D) Expenses were $18.7 million for the three months ended March
31, 2024, as compared to $22.9 million for the three months ended March 31, 2023. The $4.2 million decrease reflects (i) a decrease of
$2.4 million from deprioritizing the izumerogant program in psoriasis and castration-resistant prostate cancer and (ii) a $2.5 million
decrease in external development costs related to the vidofludimus calcium and IMU-856 programs. The decreases were partially offset by
a $0.7 million increase in personnel costs, $0.3 million of which is related to non-cash stock compensation and the remainder of which
is due to an increase in headcount. |

| · | General and Administrative (G&A) Expenses were $5.1 million for the three months ended March
31, 2024, as compared to $4.2 million for the same period ended March 31, 2023. The $0.9 million increase was primarily due to (i) a $0.8
million increase in personnel expense in general and administrative, $0.5 million of which is related to non-cash stock compensation expense
and the remainder of which is related to an increase in headcount and (ii) $0.1 million in legal and consultancy expenses. |
| · | Interest Income was $1.2 million for the three months ended March 31, 2024, as compared to $0.8
million for the same period ended March 31, 2023. The $0.4 million increase was due to higher interest rates. |
| · | The Change in Fair Value of the Tranche Rights of $4.8 million for the three months ended March
31, 2024 was a non-cash charge related to the change in value of the tranche rights associated with the future tranches 2 and 3 of the
January 2024 private placement. |
| · | Other Income (Expense) was ($2.1 million) for the three months ended March 31, 2024, as compared
to $1.2 million for the same period ended March 31, 2023. The $3.3 million decrease was primarily attributable to (i) a $1.7 million expense
related to the portion of deal costs from the January 2024 private placement related to the tranche rights that were established at the
time of the closing of tranche 1, (ii) the German Federal Ministry of Finance grant of $1.1 million being recognized in the fourth quarter
of 2023 which was one quarter earlier than in the prior year when the grant was recognized in the first quarter of 2023 and (iii) a $0.5
million decrease in research and development tax incentives for clinical trials in Australia as a result of decreased spending on clinical
trials in Australia. |
| · | Net Loss for the three months ended March 31, 2024, was approximately $29.6 million, or $0.30 per
basic and diluted share, based on 97,299,955 weighted average common shares outstanding, compared to a net loss of approximately $25.3
million, or $0.58 per basic and diluted share, based on 43,664,783 weighted average common shares outstanding for the same period ended
March 31, 2023. |
| · | Cash and Cash Equivalents as of March 31, 2024 were $97.3 million. With these funds, Immunic expects
to be able to fund its operations into the third quarter of 2025. |
Webcast Information
Immunic will host a webcast today at 8:00 am ET.
To participate in the webcast, please register in advance at: https://imux.zoom.us/webinar/register/WN_K6jgjaMURFiJjgA5i9-M8g
or on the “Events and Presentations” section of Immunic’s website at: ir.imux.com/events-and-presentations.
Registrants will receive a confirmation email containing a link for online participation or a telephone number for dial in access.

An archived replay of the webcast will be available
approximately one hour after completion on Immunic’s website at: ir.imux.com/events-and-presentations.
About Immunic,
Inc.
Immunic, Inc. (Nasdaq:
IMUX) is a biotechnology company developing a clinical pipeline of orally administered, small molecule therapies for chronic inflammatory
and autoimmune diseases. The company's lead development program, vidofludimus calcium (IMU-838), is currently in phase 3 and phase 2 clinical
trials for the treatment of relapsing and progressive multiple sclerosis, respectively, and has shown therapeutic activity in phase 2
clinical trials in patients suffering from relapsing-remitting multiple sclerosis, progressive multiple sclerosis and moderate-to-severe
ulcerative colitis. Vidofludimus calcium combines neuroprotective effects, through its mechanism as a first-in-class nuclear receptor
related 1 (Nurr1) activator, with additional anti-inflammatory and anti-viral effects, by selectively inhibiting the enzyme dihydroorotate
dehydrogenase (DHODH). IMU-856, which targets the protein Sirtuin 6 (SIRT6), is intended to restore intestinal barrier function and regenerate
bowel epithelium, which could potentially be applicable in numerous gastrointestinal diseases, such as celiac disease, for which it is
currently in preparations for a phase 2 clinical trial. IMU-381, which currently is in preclinical testing, is a next generation molecule
being developed to specifically address the needs of gastrointestinal diseases. For further information, please visit: www.imux.com.
Cautionary
Statement Regarding Forward-Looking Statements
This press release contains "forward-looking
statements" that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation
Reform Act of 1995. All statements, other than statements of historical facts, included in this press release regarding strategy, future
operations, future financial position, future revenue, projected expenses, sufficiency of cash and cash runway, expected timing, development
and results of clinical trials, prospects, plans and objectives of management are forward-looking statements. Examples of such statements
include, but are not limited to, statements relating to Immunic's development programs and the targeted diseases; the potential for Immunic's
development programs to safely and effectively target diseases; preclinical and clinical data for Immunic's development programs; the
timing of current and future clinical trials and anticipated clinical milestones; the nature, strategy and focus of the company and further
updates with respect thereto; the development and commercial potential of any product candidates of the company; and the company's expected
cash runway. Immunic may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in
the forward-looking statements and you should not place undue reliance on these forward-looking statements. Such statements are based
on management's current expectations and involve substantial risks and uncertainties. Actual results and performance could differ materially
from those projected in the forward-looking statements as a result of many factors, including, without limitation, the COVID-19 pandemic,
increasing inflation, impacts of the Ukraine – Russia conflict and the conflict in the Middle East on planned and ongoing clinical
trials, risks and uncertainties associated with the ability to project future cash utilization and reserves needed for contingent future
liabilities and business operations, the availability of sufficient financial and other resources to meet business objectives and operational
requirements including the ability to satisfy the conditions required to receive funding in tranche 2 and 3 of the January 2024 private
placement, the fact that the results of earlier preclinical studies and clinical trials may not be predictive of future clinical trial
results, the protection and market exclusivity provided by Immunic's intellectual property, risks related to the drug development and
the regulatory approval process and the impact of competitive products and technological changes. A further list and descriptions of these
risks, uncertainties and other factors can be found in the section captioned "Risk Factors," in the company's Annual Report
on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC on February 22, 2024, and in the company's subsequent filings
with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov or ir.imux.com/sec-filings. Any
forward-looking statement made in this release speaks only as of the date of this release. Immunic disclaims any intent or obligation
to update these forward-looking statements to reflect events or circumstances that exist after the date on which they were made. Immunic
expressly disclaims all liability in respect to actions taken or not taken based on any or all the contents of this press release.

Contact Information
Immunic, Inc.
Jessica Breu
Vice President Investor Relations and Communications
+49 89 2080 477 09
jessica.breu@imux.com
US IR Contact
Rx Communications Group
Paula Schwartz
+1 917 633 7790
immunic@rxir.com
US Media Contact
KOGS Communication
Edna Kaplan
+1 617 974 8659
kaplan@kogspr.com

Financials
Immunic, Inc.
Condensed Consolidated Statements of Operations
(In thousands, except share and per share amounts)
(Unaudited)
| | |
Three
Months Ended
March 31, |
| | |
2024 | |
2023 |
| Operating expenses: | |
| | | |
| | |
| Research and development | |
$ | 18,736 | | |
$ | 22,963 | |
| General and administrative | |
| 5,145 | | |
| 4,288 | |
| Total operating expenses | |
| 23,881 | | |
| 27,251 | |
| Loss from operations | |
| (23,881 | ) | |
| (27,251 | ) |
| Other income (expense): | |
| | | |
| | |
| Interest income | |
| 1,187 | | |
| 800 | |
| Change in fair value of the tranche rights | |
| (4,796 | ) | |
| — | |
| Other income (expense), net | |
| (2,094 | ) | |
| 1,179 | |
| Total other income (expense) | |
| (5,703 | ) | |
| 1,979 | |
| Net loss | |
$ | (29,584 | ) | |
$ | (25,272 | ) |
| | |
| | | |
| | |
| Net loss per share, basic and diluted | |
$ | (0.30 | ) | |
$ | (0.58 | ) |
| | |
| | | |
| | |
| Weighted-average common shares outstanding, basic and diluted | |
| 97,299,955 | | |
| 43,664,783 | |

Immunic, Inc.
Condensed Consolidated Balance Sheets
(In thousands, except share and per share amounts)
(Unaudited)
| | |
March 31,
2024 | |
December 31,
2023 |
| | |
(Unaudited) | |
|
| Assets | |
| | | |
| | |
| Current assets: | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 97,312 | | |
$ | 46,674 | |
| Other current assets and prepaid expenses | |
| 5,303 | | |
| 5,860 | |
| Total current assets | |
| 102,615 | | |
| 52,534 | |
| Property and equipment, net | |
| 442 | | |
| 466 | |
| Right-of-use assets, net | |
| 1,098 | | |
| 1,299 | |
| Total assets | |
$ | 104,155 | | |
$ | 54,299 | |
| Liabilities and Stockholders’ Equity | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Accounts payable | |
$ | 6,767 | | |
$ | 5,099 | |
| Accrued expenses | |
| 12,419 | | |
| 18,664 | |
| Other current liabilities | |
| 960 | | |
| 966 | |
| Total current liabilities | |
| 20,146 | | |
| 24,729 | |
| Long-term liabilities | |
| | | |
| | |
| Operating lease liabilities | |
| 433 | | |
| 639 | |
| Total long-term liabilities | |
| 433 | | |
| 639 | |
| Total liabilities | |
| 20,579 | | |
| 25,368 | |
| Commitments and contingencies | |
| | | |
| | |
| Stockholders’ equity: | |
| | | |
| | |
| Preferred stock, $0.0001 par value; 20,000,000 authorized and no shares issued or outstanding as of March 31, 2024 and December 31, 2023 | |
| — | | |
| — | |
| Common stock, $0.0001 par value; 500,000,000 and 130,000,000 shares authorized as of March 31, 2024 and December 31, 2023, respectively, and 90,079,016 and 45,177,730 shares issued and outstanding as of March 31, 2024 and December 31, 2023, respectively | |
| 8 | | |
| 4 | |
| Additional paid-in capital | |
| 519,757 | | |
| 436,060 | |
| Accumulated other comprehensive income | |
| 4,287 | | |
| 3,759 | |
| Accumulated deficit | |
| (440,476 | ) | |
| (410,892 | ) |
| Total stockholders’ equity | |
| 83,576 | | |
| 28,931 | |
| Total liabilities and stockholders’ equity | |
$ | 104,155 | | |
$ | 54,299 | |













v3.24.1.u1
Cover
|
May 08, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
May 08, 2024
|
| Entity File Number |
001-36201
|
| Entity Registrant Name |
IMMUNIC, INC.
|
| Entity Central Index Key |
0001280776
|
| Entity Tax Identification Number |
56-2358443
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
1200 Avenue of the Americas
|
| Entity Address, Address Line Two |
Suite 200
|
| Entity Address, City or Town |
New York
|
| Entity Address, State or Province |
NY
|
| Entity Address, Country |
US
|
| Entity Address, Postal Zip Code |
10036
|
| City Area Code |
(332)
|
| Local Phone Number |
255-9818
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, par value $0.0001
|
| Trading Symbol |
IMUX
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionISO 3166-1 alpha-2 country code.
| Name: |
dei_EntityAddressCountry |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:countryCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Grafico Azioni Immunic (NASDAQ:IMUX)
Storico
Da Feb 2025 a Mar 2025

Grafico Azioni Immunic (NASDAQ:IMUX)
Storico
Da Mar 2024 a Mar 2025
