Saint-Herblain (France), April 16,
2025 – Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a
specialty vaccine company, today announced its presence at the
forthcoming 25th World Vaccine Congress, which will take place from
April 21-24, 2025 at the Walter E. convention center in Washington,
D.C. Under the leadership of the Company´s Chief Executive Officer,
Thomas Lingelbach, a team of Valneva´s senior managers will
participate in multiple events, including an important presentation
on the Company´s vaccine IXCHIQ®, the world’s first licensed
chikungunya vaccine.
The Company will have a display in the exhibit
area of the congress at booth #424.
On April 22, from 11:40am to 12:20pm EST,
Valneva’s Chief Science Officer, Dr. Hanneke Schuitemaker will host
an interactive work group session focused on “Prophylactic Enteric
Disease Vaccines and Antimicrobial Resistance”.
In the evening of April 22, Valneva will attend
the Vaccine Industry Excellence Awards ceremony, where it is a
finalist for the Best Biotech Award and Best Prophylactic Vaccine
Award for VLA15, the most advanced Lyme disease vaccine candidate
worldwide, currently in Phase 3, partnered with Pfizer.
The following day on April 23 at 9:40am EST,
Susanne Eder-Lingelbach, Vice President Clinical Development at
Valneva, will present “Valneva’s chikungunya vaccine: key data in
support of licensure and plans to confirm effectiveness".
On April 24, at 9:00am EST, Valneva’s Chief
Medical Officer, Dr. Juan Carlos Jaramillo, will take part in the
“The Urgent Need for Vaccines Against Mosquito-Borne Diseases”
panel discussion alongside Gabrielle Breugelmans, Director of
Epidemiology and Data Science, at the Coalition for Epidemic
Preparedness Innovations, Trevor Wellington, Director, Clinical
Trials Center, Walter Reed Army Institute of Research, and
moderated by Sushant Sahastrabuddhe, Deputy Director General,
International Vaccine Institute.
About ChikungunyaChikungunya
virus (CHIKV) is a mosquito-borne viral disease spread by the bites
of infected Aedes mosquitoes which causes fever, severe joint and
muscle pain, headache, nausea, fatigue and rash. Joint pain is
often debilitating and can persist for weeks to years1. In 2004,
the disease began to spread quickly, causing large-scale outbreaks
around the world. Since the re-emergence of the virus, CHIKV has
now been identified in over 110 countries in Asia, Africa, Europe
and the Americas2. Between 2013 and 2023, more than 3.7 million
cases were reported in the Americas3 and the economic impact is
considered to be significant. The medical and economic burden is
expected to grow with climate change as the mosquito vectors that
transmit the disease continue to spread geographically. As such,
the World Health Organization (WHO) has highlighted chikungunya as
a major public health problem.4
About IXCHIQ®
IXCHIQ® is the world’s first licensed chikungunya vaccine available
to address this significant unmet medical need. The single-shot
vaccine is approved for the prevention of disease caused by the
chikungunya virus in people aged 12 years and older in the European
Union, and in people aged 18 years and older in the United States
(U.S.)5, Canada6, United Kingdom (U.K.)7 and Brazil8. Label
extension applications to adolescents were submitted in the U.S.,
Canada and the U.K.Please refer to the full prescribing information
approved by the relevant governmental authority, as applicable.
About Valneva SEWe are a
specialty vaccine company that develops, manufactures, and
commercializes prophylactic vaccines for infectious diseases
addressing unmet medical needs. We take a highly specialized and
targeted approach, applying our deep expertise across multiple
vaccine modalities, focused on providing either first-, best- or
only-in-class vaccine solutions.We have a strong track record,
having advanced multiple vaccines from early R&D to approvals,
and currently market three proprietary travel vaccines, including
the world’s first chikungunya vaccine, as well as certain
third-party vaccines.Revenues from our growing commercial business
help fuel the continued advancement of our vaccine pipeline. This
includes the only Lyme disease vaccine candidate in advanced
clinical development, which is partnered with Pfizer, the world’s
most clinically advanced Shigella vaccine candidate, as well as
vaccine candidates against the Zika virus and other global public
health threats. More information is available at
www.valneva.com.
About CEPICEPI was launched in
2017 as an innovative partnership between public, private,
philanthropic and civil organizations. Its mission is to accelerate
the development of vaccines and other biologic countermeasures
against epidemic and pandemic threats so they can be accessible to
all people in need. CEPI has supported the development of more than
50 vaccine candidates or platform technologies against multiple
known high-risk pathogens or a future Disease X. Central to CEPI’s
pandemic-beating five-year plan for 2022-2026 is the ‘100 Days
Mission’ to compress the time taken to develop safe, effective,
globally accessible vaccines against new threats to just 100
days.
Learn more at CEPI.net. Follow us on X
(@CEPIvaccines), LinkedIn and Facebook.
About Horizon EuropeHorizon
Europe — #HorizonEU — is the European Union's flagship
Research and Innovation programme, part of the EU-long-term
Multiannual Financial Framework (MFF) with a budget of €95,5
billion to spend over a seven-year period
(2021-2027). Under Horizon Europe, health research will
be supported with the aim to find new ways to keep people healthy,
prevent diseases, develop better diagnostics and more effective
therapies, use personalised medicine approaches to improve
healthcare and wellbeing, and take up innovative health
technologies, such as digital ones.
Media and Investor Relations
Contacts
|
Valneva Laetitia Bachelot-FontaineVP Global
Communications & European Investor RelationsM +33 (0)6 4516
7099laetitia.bachelot-fontaine@valneva.com
|
Joshua Drumm,
Ph.D.VP Global Investor RelationsM +001 917 815
4520joshua.drumm@valneva.com |
CEPIpress@cepi.net +44 7387 055214
Forward-Looking Statements
This press release contains certain
forward-looking statements relating to the business of Valneva,
including with respect to business partnerships and the progress,
timing, results and completion of technology transfer and
regulatory approvals in additional markets. In addition, even if
the actual results or development of Valneva are consistent with
the forward-looking statements contained in this press release,
those results or developments of Valneva may not be sustained in
the future. In some cases, you can identify forward-looking
statements by words such as “could,” “should,” “may,” “expects,”
“anticipates,” “believes,” “intends,” “estimates,” “aims,”
“targets,” or similar words. These forward-looking statements are
based largely on the current expectations of Valneva as of the date
of this press release and are subject to a number of known and
unknown risks and uncertainties and other factors that may cause
actual results, performance or achievements to be materially
different from any future results, performance or achievement
expressed or implied by these forward-looking statements. In
particular, the expectations of Valneva could be affected by, among
other things, uncertainties and delays involved in the development
and manufacture of vaccines, unexpected clinical trial results,
unexpected regulatory actions or delays, competition in general,
currency fluctuations, the impact of the global and European credit
crisis, and the ability to obtain or maintain patent or other
proprietary intellectual property protection. Success in
preclinical studies or earlier clinical trials may not be
indicative of results in future clinical trials. In light of these
risks and uncertainties, there can be no assurance that the
forward-looking statements made in this press release will in fact
be realized. Valneva is providing this information as of the date
of this press release and disclaims any intention or obligation to
publicly update or revise any forward-looking statements, whether
as a result of new information, future events, or otherwise.
1 https://jvi.asm.org/content/jvi/88/20/11644.full.pdf 2
https://cmr.asm.org/content/31/1/e00104-163 PAHO/WHO data: Number
of reported cases of chikungunya fever in the Americas (Cumulative
Cases 2018-2023 and Cases per year 2013-2017).
https://www.paho.org/data/index.php/en/mnu-topics/chikv-en/550-chikv-weekly-en.html.
Last accessed 01 Aug 2023.4 Geographical expansion of cases of
dengue and chikungunya beyond the historical areas of transmission
in the Region of the Americas (who.int)5 Valneva Announces U.S. FDA
Approval of World’s First Chikungunya Vaccine, IXCHIQ® - Valneva6
Valneva Announces Health Canada Approval of the World’s First
Chikungunya Vaccine, IXCHIQ® - Valneva7 Valneva Receives Marketing
Authorization in the UK for the World’s First Chikungunya Vaccine,
IXCHIQ® - Valneva8 Valneva Receives First Marketing Authorization
for IXCHIQ® in a Chikungunya Endemic Country - Valneva
- 2025_04_16_WVC2025_PR_EN_Final
Grafico Azioni Valneva (EU:VLA)
Storico
Da Mar 2025 a Apr 2025
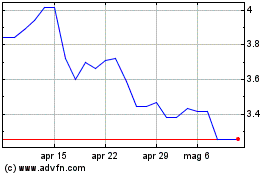
Grafico Azioni Valneva (EU:VLA)
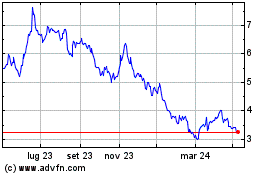
Storico
Da Apr 2024 a Apr 2025