false000167158400016715842025-03-202025-03-20
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): March 20, 2025 |
APTEVO THERAPEUTICS INC.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-37746 |
81-1567056 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
2401 4th Avenue Suite 1050 |
|
Seattle, Washington |
|
98121 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: (206) 838-0500 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, $0.001 par value |
|
APVO |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On March 20, 2025, Aptevo Therapeutics Inc. ("Aptevo" or the "Company") made available its investor presentation which details the Company's current drug pipeline, including clinical product candidates mipletamig and ALG.APV-527 and preclinical candidates APVO711, APVO603 and APVO442.
A copy of the presentation is attached hereto as Exhibit 99.1 and is incorporated by reference herein.
Item 8.01 Other Events.
On March 20, 2025, the Company issued a press release announcing two additional patients achieved remission within 30 days in Cohort 2 of the RAINIER frontline acute myeloid leukemia (AML) Phase 1b trial, building on previously reported outcomes from RAINIER's Cohort 1 in which 100% of patients achieved remission.
A copy of the press release is attached hereto as Exhibit 99.2 and is incorporated by reference herein.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
APTEVO THERAPEUTICS INC. |
|
|
|
|
Date: |
March 20, 2025 |
By: |
/s/ Marvin L. White |
|
|
|
President and Chief Executive Officer |

20, March 2025 | Nasdaq: APVO

Forward-Looking Statements This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical fact, including, without limitation, Aptevo's expectations about the activity, efficacy and safety of its therapeutic candidates and potential use of any such candidates as therapeutics for treatment of disease, statements related to the progress of Aptevo’s clinical programs, including statements related to the Phase 1b/2 trial initiation for mipletamig, whether the Phase 1b/2 protocol will be successful, whether further study of mipletamig in Phase 1b/2 trial focusing on a targeted patient population will continue to show clinical benefit, whether Aptevo’s strategy will translate into an improved overall survival rate in acute myeloid leukemia (AML), statements related to the durability of mipletamig and whether its duration of remission results will be indicative of later stage clinical trials, whether the mipletamig data in combination therapy and monotherapy will be indicative of later stage clinical trials, mipletamig’s potential for multiple indications, and the timing for its expected data readouts, ALG.APV-527’s potential for multiple indications, and the timing for its expected preliminary data, the possibility of meaningful data readouts for ALG.APV-527, whether the diversified pipeline candidates will demonstrate the ability to fight a range of solid malignancies, expectations regarding the effectiveness of its ADAPTIR and ADAPTIR-FLEX platforms, whether Aptevo will continue to have momentum in its business in the future, statements related to Aptevo’s ability to generate stockholder value, and any other statements containing the words "may," "believes," "expects," "anticipates," "hopes," "intends," "optimism," "potential," "designed," "engineered," "innovative," "innovation," "promising," "plans," "forecasts," “estimates,” “will” and similar expressions. Investors are, therefore, cautioned not to place undue reliance on any forward-looking statement. These forward-looking statements are based on Aptevo's current intentions, beliefs, and expectations regarding future events. Aptevo cannot guarantee that any forward-looking statement will be accurate. Investors should realize that if underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could differ materially from Aptevo’s expectations. There are several important factors that could cause Aptevo's actual results to differ materially from those indicated by such forward-looking statements, including a deterioration in Aptevo's business or prospects; further assessment of preliminary data or different results from later clinical trials, adverse events and unanticipated problems, adverse developments in clinical development, including unexpected safety issues observed during a clinical trial; the market potential of Aptevo’s therapeutic candidates; and changes in regulatory, social, macroeconomics and political conditions. For instance, actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including the uncertainties inherent in the results of preliminary data and pre-clinical studies being predictive of the results of later-stage clinical trials, initiation, enrollment and maintenance of patients, and completion of clinical trials, availability and timing of data from ongoing clinical trials, the trial design includes combination therapies that may make it difficult to accurately ascertain the benefits of a product candidate, expectations for the timing and steps required in the regulatory review process, expectations for regulatory approvals, the impact of competitive products, our ability to enter into agreements with strategic partners or raise funds on acceptable terms or at all, and other matters that could affect the availability or commercial potential of the Company's product candidates or business, economic disruptions due to catastrophes or other events, including natural disasters or public health crises such as the coronavirus (referred to as COVID- 19), and geopolitical risks, including the current war between Russian and Ukraine as well as the war between Israel and Hamas and macroeconomic conditions such as economic uncertainty, rising inflation and interest rates, conditions in the banking system and financial markets, including the failure of banks and financial institutions, increased market volatility and decreased consumer confidence. These risks are not exhaustive. Aptevo faces known and unknown risks. Additional risks and factors that may affect results are set forth in Aptevo's filings with the Securities and Exchange Commission, including its Annual Report on Form 10-K for the fiscal year ended December 31, 2023, and its subsequent quarterly reports on Form 10-Q and current reports on Form 8-K. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from Aptevo's expectations in any forward-looking statement. Any forward-looking statement speaks only as of the date of this presentation, and, except as required by law, Aptevo does not assume any obligation to update any forward-looking statement to reflect new information, events, or circumstances. December 2024 | Nasdaq: APVO
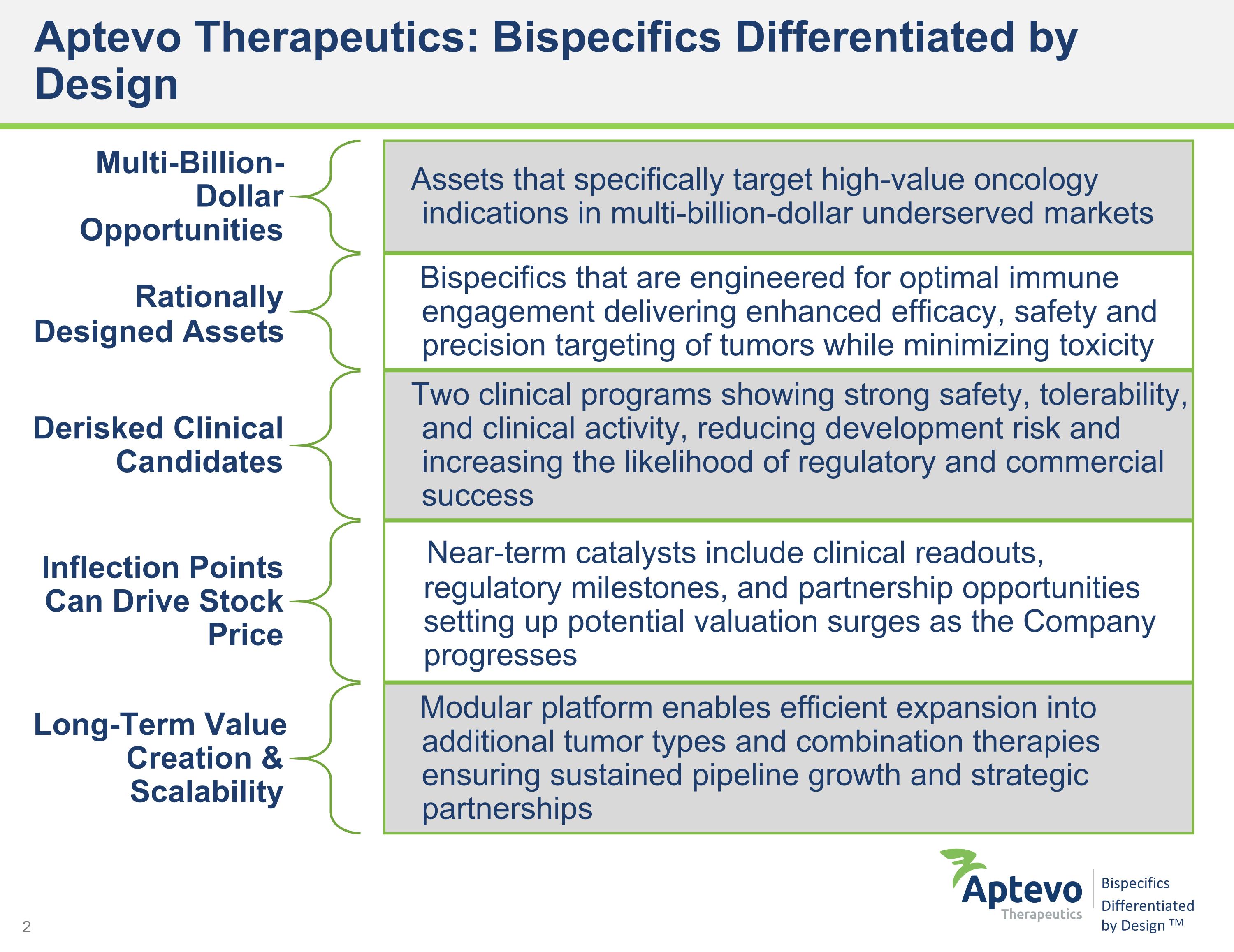
Aptevo Therapeutics: Bispecifics Differentiated by Design Multi-Billion-Dollar Opportunities Assets that specifically target high-value oncology indications in multi-billion-dollar underserved markets Rationally Designed Assets Bispecifics that are engineered for optimal immune engagement delivering enhanced efficacy, safety and precision targeting of tumors while minimizing toxicity Derisked Clinical Candidates Two clinical programs showing strong safety, tolerability, and clinical activity, reducing development risk and increasing the likelihood of regulatory and commercial success Inflection Points Can Drive Stock Price Near-term catalysts include clinical readouts, regulatory milestones, and partnership opportunities setting up potential valuation surges as the Company progresses Long-Term Value Creation & Scalability Modular platform enables efficient expansion into additional tumor types and combination therapies ensuring sustained pipeline growth and strategic partnerships

Combinable with standard of care or other experimental therapies (i.e. radioisotopes, antibody drug conjugates (ADC’s), adaptive T cells, checkpoint molecules, other bispecifics) CHO production cell lines used with antibody-like purification Aptevo’s Differentiated & Diversified Bispecifics Built to Overcome Common Safety Challenges Unique CD3 designed to reduce cytokine release syndrome (CRS) as seen in prior mipletamig clinical results Stimulatory binding domains only function when crosslinked, eliminating systemic off target immune activation, potentially reducing or eliminating toxicities Enhance Tumor-Specific Functions Binding domains used are known to be expressed on tumor or within the tumor microenvironment that localize the bispecific to tumors Reduced affinity CD3 with potential to increase tumor localization (APVO442) Checkpoint inhibitor to block the PD-1/PD-L1 pathways (APVO711) Costimulation via 4-1BB, OX40 or CD40 augment the antitumor response (APVO603, ALG.APV-527, APVO711)
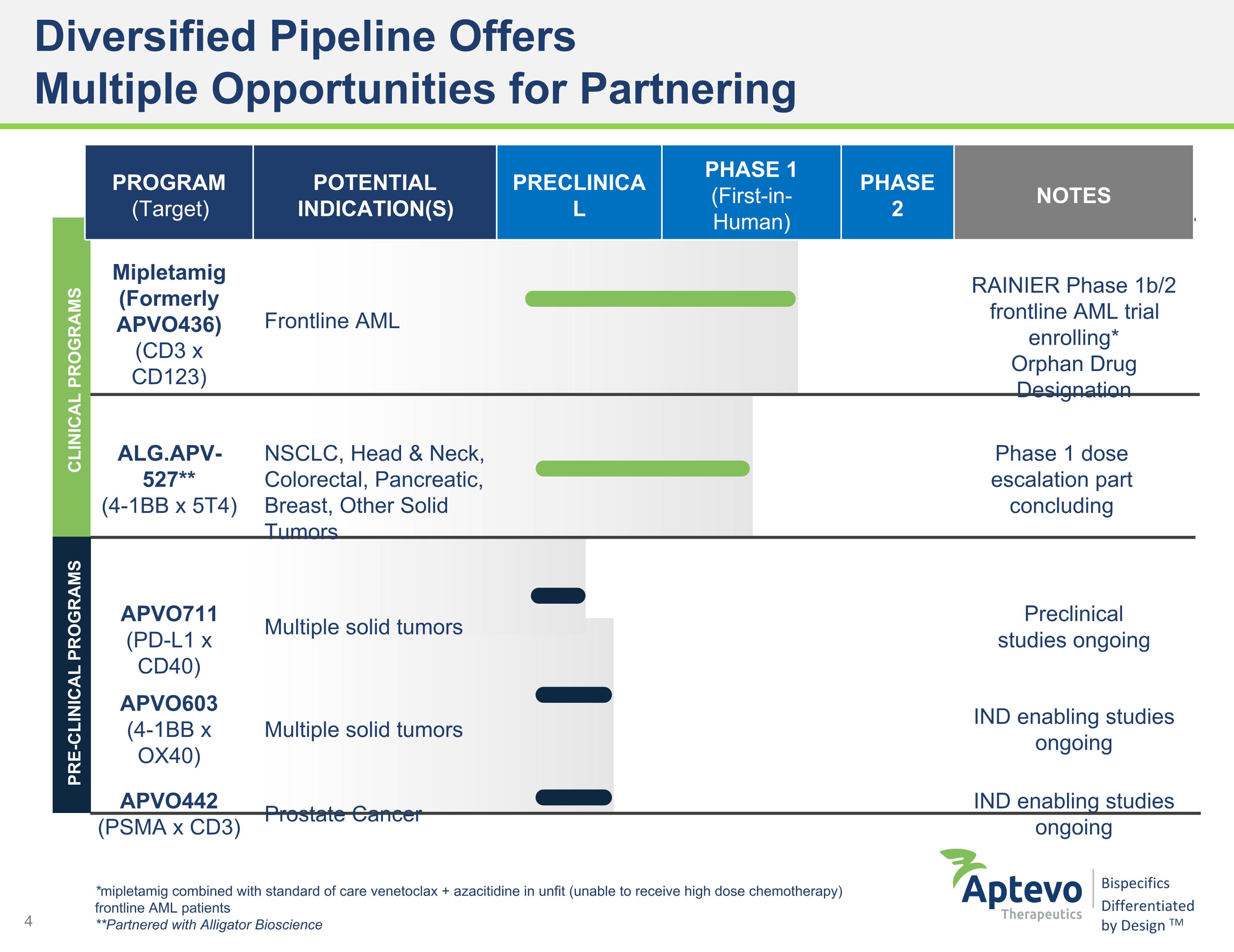
Diversified Pipeline Offers �Multiple Opportunities for Partnering *mipletamig combined with standard of care venetoclax + azacitidine in unfit (unable to receive high dose chemotherapy) frontline AML patients **Partnered with Alligator Bioscience CLINICAL PROGRAMS PRE-CLINICAL PROGRAMS PROGRAM (Target) POTENTIAL INDICATION(S) PRECLINICAL PHASE 1 (First-in-Human) PHASE 2 NOTES Mipletamig (Formerly APVO436) (CD3 x CD123) Frontline AML RAINIER Phase 1b/2 frontline AML trial enrolling* Orphan Drug Designation ALG.APV-527** (4-1BB x 5T4) NSCLC, Head & Neck, Colorectal, Pancreatic, �Breast, Other Solid Tumors Phase 1 dose escalation part concluding APVO711 (PD-L1 x CD40) Multiple solid tumors Preclinical �studies ongoing APVO603 (4-1BB x OX40) Multiple solid tumors IND enabling studies ongoing APVO442 (PSMA x CD3) Prostate Cancer IND enabling studies ongoing

Aptevo is developing multiple molecules capable of targeting hematologic and solid tumor malignancies with large market potential Market Opportunity Select Global Market Opportunities* Size AML/MDS/Other leukemias $6.3B Breast Cancer $32.1B Non-Small Cell Lung Cancer $24.2B Colorectal Cancer $15.8B Head and Neck Cancer $2.8B Pancreatic Cancer $2.7B Cervical Cancer $2.2B * Source: Global Data 2022

Mipletamig (CD3 X CD123 Bispecific) �AML, MDS and Other Leukemias “The mipletamig results are promising and show that it is well-suited to combine with the venetoclax + azacitidine standard of care regimen. We see a very manageable safety profile and promising efficacy, including duration of remission results.” Justin Watts, MD, Associate Professor of Medicine, Division of Hematology, Chief, Leukemia Section, University of Miami/Sylvester Comprehensive Cancer Center
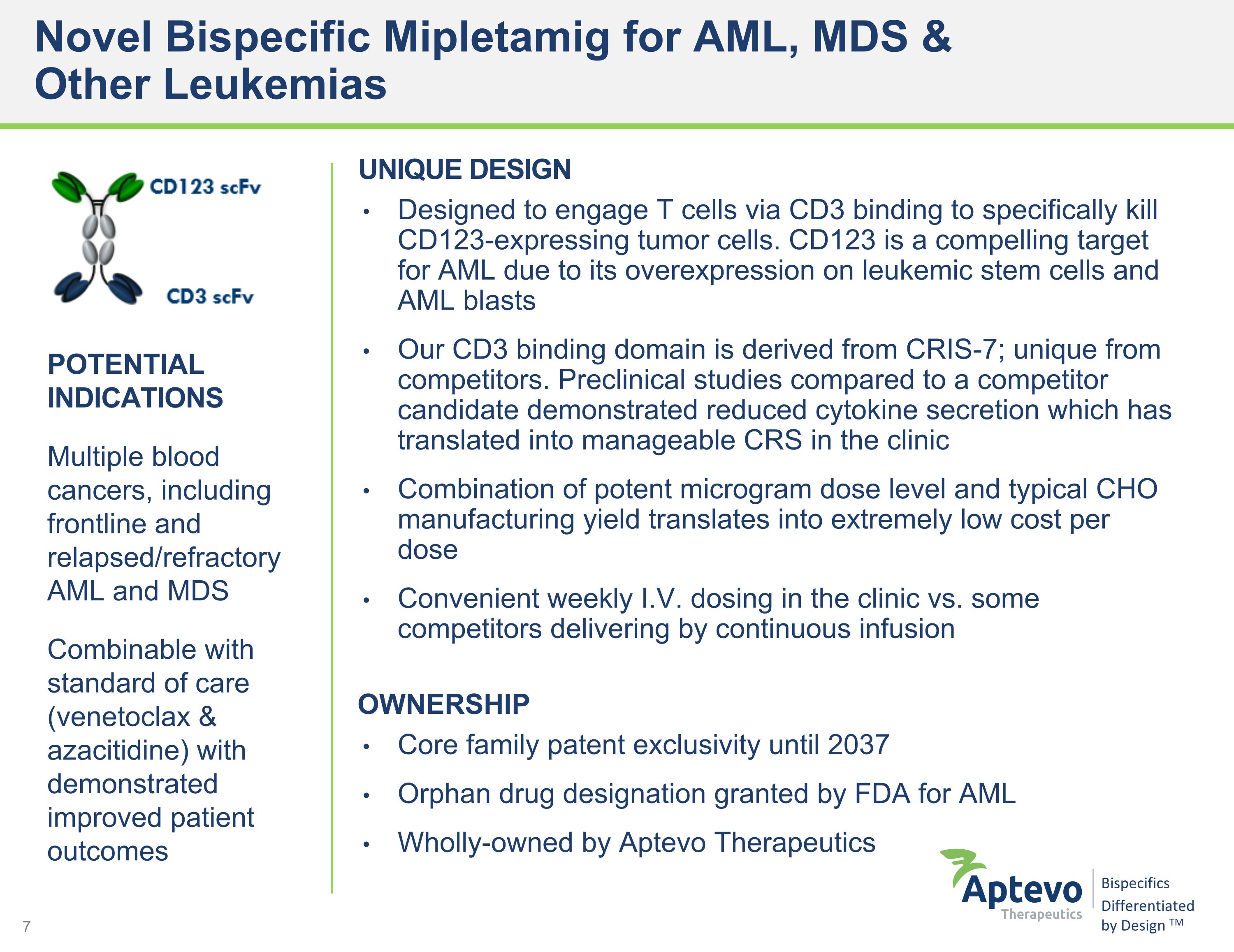
Novel Bispecific Mipletamig for AML, MDS & �Other Leukemias POTENTIAL INDICATIONS Multiple blood cancers, including frontline and relapsed/refractory AML and MDS Combinable with standard of care (venetoclax & azacitidine) with demonstrated improved patient outcomes UNIQUE DESIGN Designed to engage T cells via CD3 binding to specifically kill CD123-expressing tumor cells. CD123 is a compelling target for AML due to its overexpression on leukemic stem cells and AML blasts Our CD3 binding domain is derived from CRIS-7; unique from competitors. Preclinical studies compared to a competitor candidate demonstrated reduced cytokine secretion which has translated into manageable CRS in the clinic Combination of potent microgram dose level and typical CHO manufacturing yield translates into extremely low cost per dose Convenient weekly I.V. dosing in the clinic vs. some competitors delivering by continuous infusion OWNERSHIP Core family patent exclusivity until 2037 Orphan drug designation granted by FDA for AML Wholly-owned by Aptevo Therapeutics
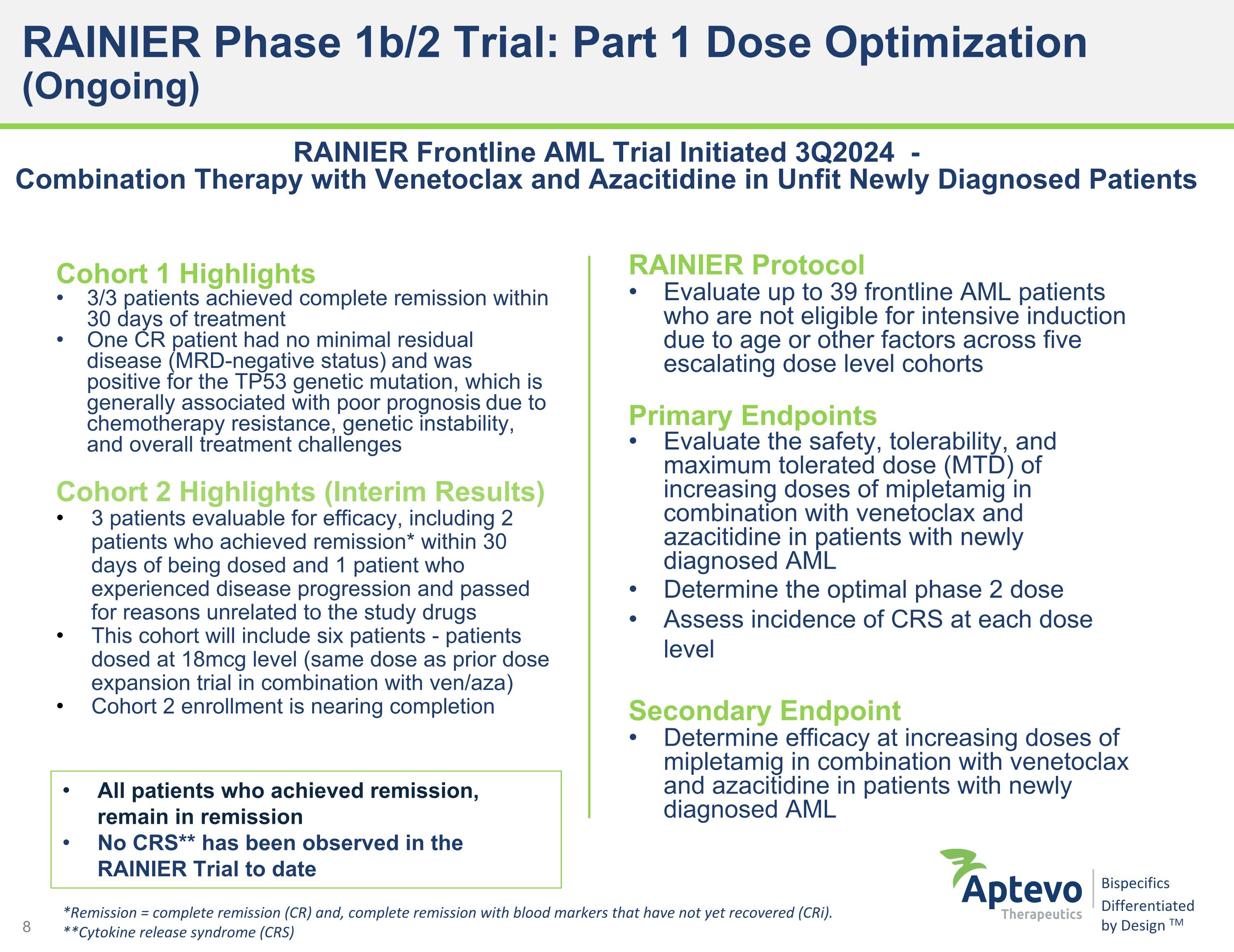
RAINIER Phase 1b/2 Trial: Part 1 Dose Optimization (Ongoing) Cohort 1 Highlights 3/3 patients achieved complete remission within 30 days of treatment One CR patient had no minimal residual disease (MRD-negative status) and was positive for the TP53 genetic mutation, which is generally associated with poor prognosis due to chemotherapy resistance, genetic instability, and overall treatment challenges Cohort 2 Highlights (Interim Results) 3 patients evaluable for efficacy, including 2 patients who achieved remission* within 30 days of being dosed and 1 patient who experienced disease progression and passed for reasons unrelated to the study drugs This cohort will include six patients - patients dosed at 18mcg level (same dose as prior dose expansion trial in combination with ven/aza) Cohort 2 enrollment is nearing completion RAINIER Frontline AML Trial Initiated 3Q2024 - Combination Therapy with Venetoclax and Azacitidine in Unfit Newly Diagnosed Patients *Remission = complete remission (CR) and, complete remission with blood markers that have not yet recovered (CRi). **Cytokine release syndrome (CRS) RAINIER Protocol Evaluate up to 39 frontline AML patients who are not eligible for intensive induction due to age or other factors across five escalating dose level cohorts Primary Endpoints Evaluate the safety, tolerability, and maximum tolerated dose (MTD) of increasing doses of mipletamig in combination with venetoclax and azacitidine in patients with newly diagnosed AML Determine the optimal phase 2 dose Assess incidence of CRS at each dose level Secondary Endpoint Determine efficacy at increasing doses of mipletamig in combination with venetoclax and azacitidine in patients with newly diagnosed AML All patients who achieved remission, remain in remission No CRS** has been observed in the RAINIER Trial to date
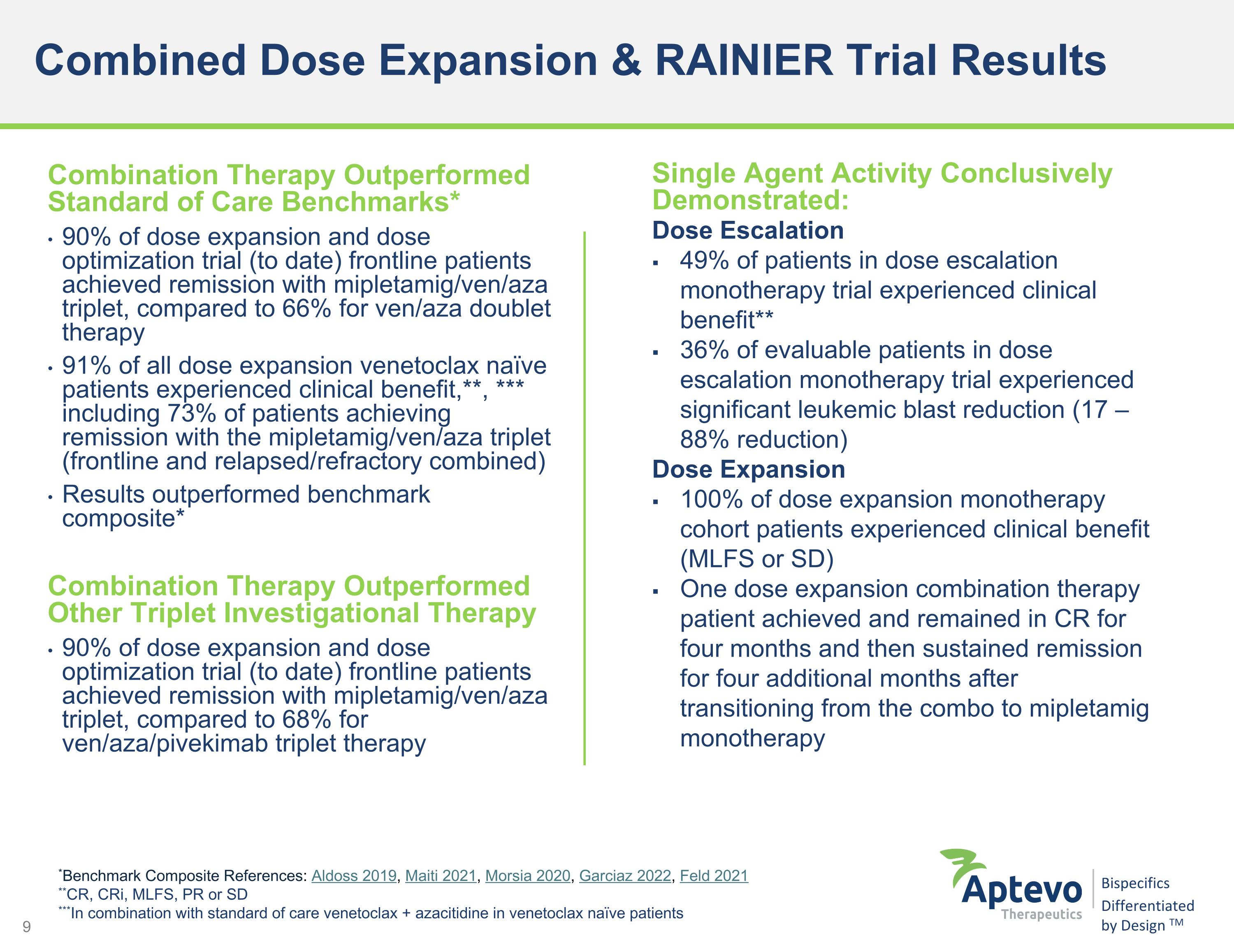
Combined Dose Expansion & RAINIER Trial Results *Benchmark Composite References: Aldoss 2019, Maiti 2021, Morsia 2020, Garciaz 2022, Feld 2021 **CR, CRi, MLFS, PR or SD ***In combination with standard of care venetoclax + azacitidine in venetoclax naïve patients Combination Therapy Outperformed Standard of Care Benchmarks* 90% of dose expansion and dose optimization trial (to date) frontline patients achieved remission with mipletamig/ven/aza triplet, compared to 66% for ven/aza doublet therapy 91% of all dose expansion venetoclax naïve patients experienced clinical benefit,**, *** including 73% of patients achieving remission with the mipletamig/ven/aza triplet (frontline and relapsed/refractory combined) Results outperformed benchmark composite* Combination Therapy Outperformed Other Triplet Investigational Therapy 90% of dose expansion and dose optimization trial (to date) frontline patients achieved remission with mipletamig/ven/aza triplet, compared to 68% for ven/aza/pivekimab triplet therapy Single Agent Activity Conclusively Demonstrated: Dose Escalation 49% of patients in dose escalation monotherapy trial experienced clinical benefit** 36% of evaluable patients in dose escalation monotherapy trial experienced significant leukemic blast reduction (17 – 88% reduction) Dose Expansion 100% of dose expansion monotherapy cohort patients experienced clinical benefit (MLFS or SD) One dose expansion combination therapy patient achieved and remained in CR for four months and then sustained remission for four additional months after transitioning from the combo to mipletamig monotherapy
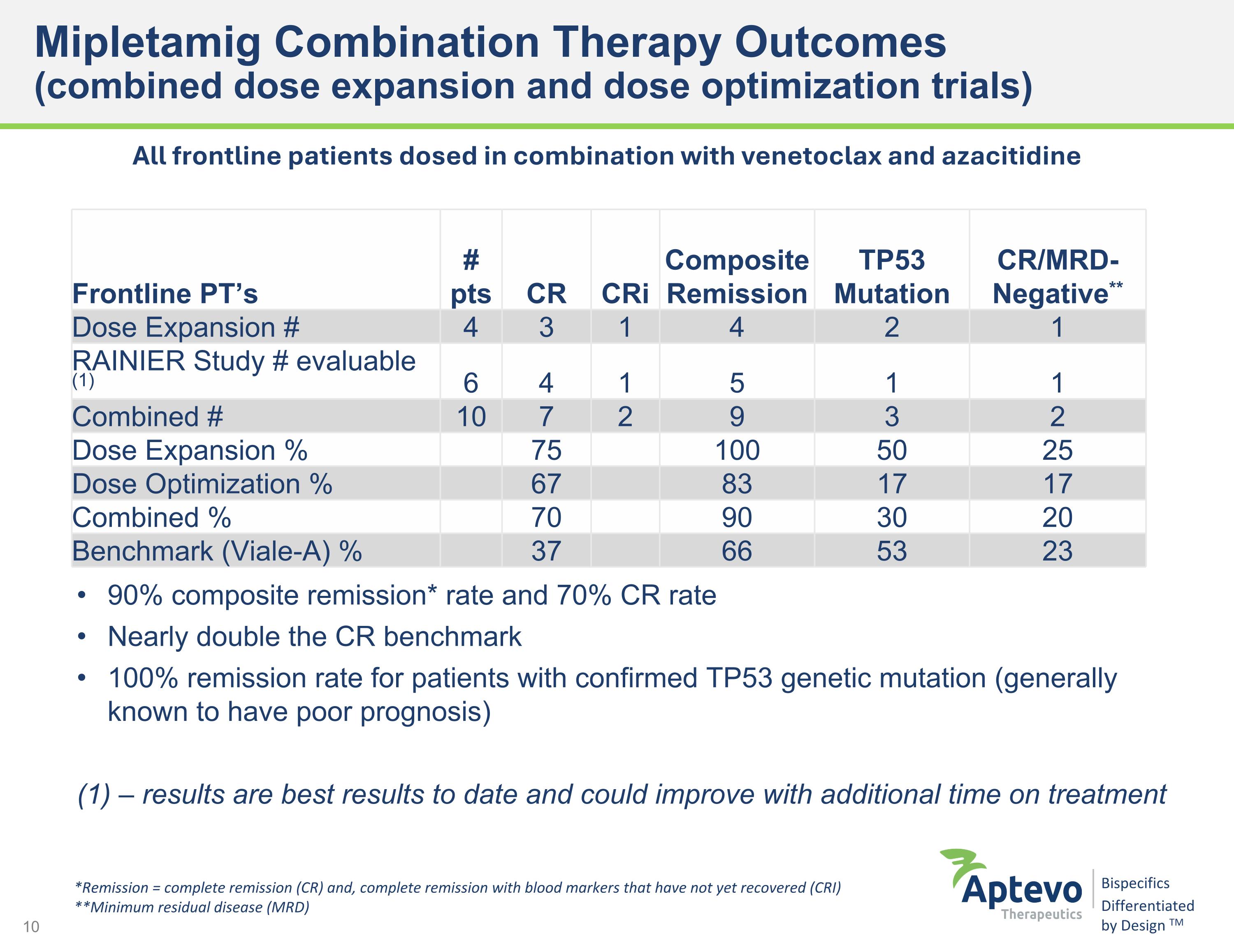
Mipletamig Combination Therapy Outcomes (combined dose expansion and dose optimization trials) 90% composite remission* rate and 70% CR rate Nearly double the CR benchmark 100% remission rate for patients with confirmed TP53 genetic mutation (generally known to have poor prognosis) (1) – results are best results to date and could improve with additional time on treatment Frontline PT’s # pts CR CRi Composite Remission TP53 Mutation CR/MRD- Negative** Dose Expansion # 4 3 1 4 2 1 RAINIER Study # evaluable (1) 6 4 1 5 1 1 Combined # 10 7 2 9 3 2 Dose Expansion % 75 100 50 25 Dose Optimization % 67 83 17 17 Combined % 70 90 30 20 Benchmark (Viale-A) % 37 66 53 23 *Remission = complete remission (CR) and, complete remission with blood markers that have not yet recovered (CRI) **Minimum residual disease (MRD) All frontline patients dosed in combination with venetoclax and azacitidine
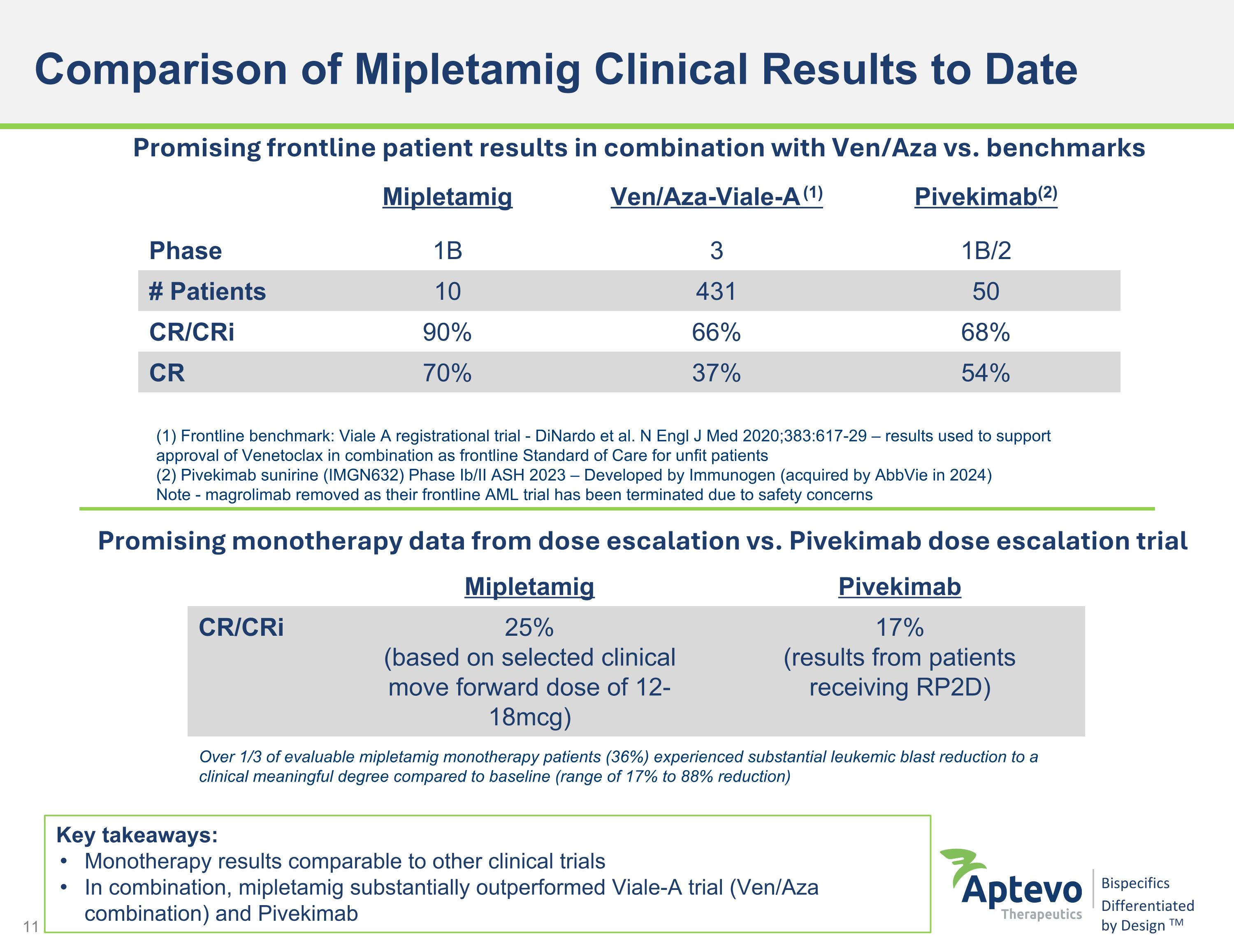
Comparison of Mipletamig Clinical Results to Date Promising frontline patient results in combination with Ven/Aza vs. benchmarks Mipletamig Ven/Aza-Viale-A (1) Pivekimab(2) Phase 1B 3 1B/2 # Patients 10 431 50 CR/CRi 90% 66% 68% CR 70% 37% 54% (1) Frontline benchmark: Viale A registrational trial - DiNardo et al. N Engl J Med 2020;383:617-29 – results used to support approval of Venetoclax in combination as frontline Standard of Care for unfit patients (2) Pivekimab sunirine (IMGN632) Phase Ib/II ASH 2023 – Developed by Immunogen (acquired by AbbVie in 2024) Note - magrolimab removed as their frontline AML trial has been terminated due to safety concerns Promising monotherapy data from dose escalation vs. Pivekimab dose escalation trial Over 1/3 of evaluable mipletamig monotherapy patients (36%) experienced substantial leukemic blast reduction to a clinical meaningful degree compared to baseline (range of 17% to 88% reduction) Mipletamig Pivekimab CR/CRi 25% (based on selected clinical move forward dose of 12-18mcg) 17% (results from patients receiving RP2D) Key takeaways: Monotherapy results comparable to other clinical trials In combination, mipletamig substantially outperformed Viale-A trial (Ven/Aza combination) and Pivekimab
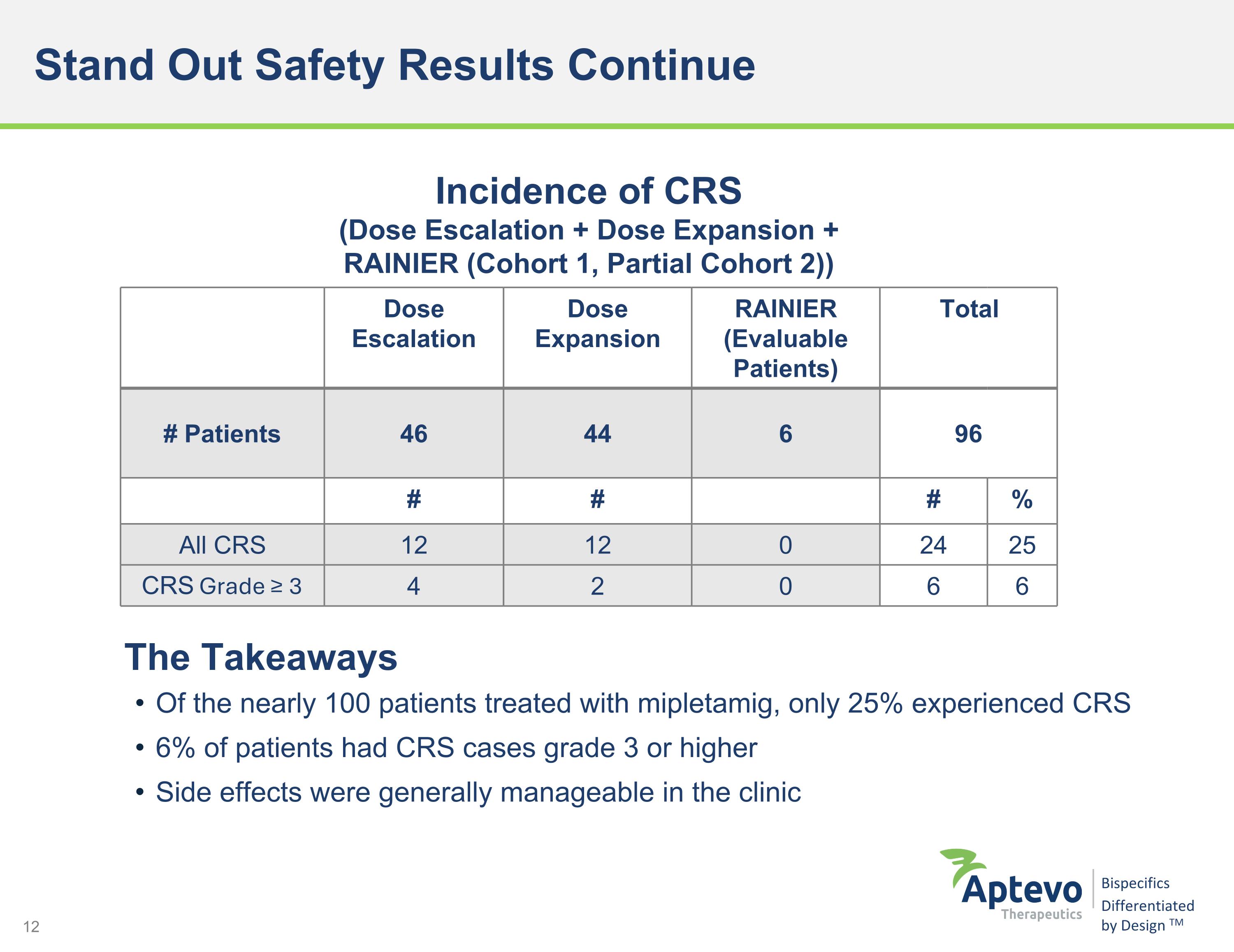
Stand Out Safety Results Continue Of the nearly 100 patients treated with mipletamig, only 25% experienced CRS 6% of patients had CRS cases grade 3 or higher Side effects were generally manageable in the clinic Incidence of CRS (Dose Escalation + Dose Expansion + RAINIER (Cohort 1, Partial Cohort 2)) Dose Escalation Dose Expansion RAINIER (Evaluable Patients) Total # Patients 46 44 6 96 # # # % All CRS 12 12 0 24 25 CRS Grade ≥ 3 4 2 0 6 6 The Takeaways
Monotherapy Trial Laid the Foundation: Evidence of Single Agent Activity in Dose Escalation and Expansion Studies Mipletamig was explored in the dose escalation study as a single agent. Given the evidence of AML blast cell reduction as well as clinical benefit in a heterogenous and difficult to treat patient population along with a favorable safety profile, the 18 mcg dose was chosen for further study in the expansion part of the study and extensively explored in combination with standard of care. Evidence of single agent activity in escalation phase: Approximately 1/2 of evaluable monotherapy patients (49%) experienced clinical benefit (CR, CRi, MLFS, PR or SD) 19 of 39 evaluable patients Over 1/3 of evaluable monotherapy patients (36%) experienced substantial leukemic blast reduction to a clinical meaningful degree compared to baseline (range of 17% to 88% reduction), proving evidence of the pharmacodynamic effect of the drug 2 complete remissions were observed at the 12 mcg and 18 mcg dose levels, which aligned with the two largest percentage reductions in AML blast counts These results show the clinical activity of mipletamig as a single agent in a heterogenous AML patient population Evidence of single agent activity in expansion phase: Of 4 evaluable Cohort 3 patients who had AML and were treated with single agent, 1 patient achieved MLFS and 3 achieved SD as their best overall response indicating antileukemic activity
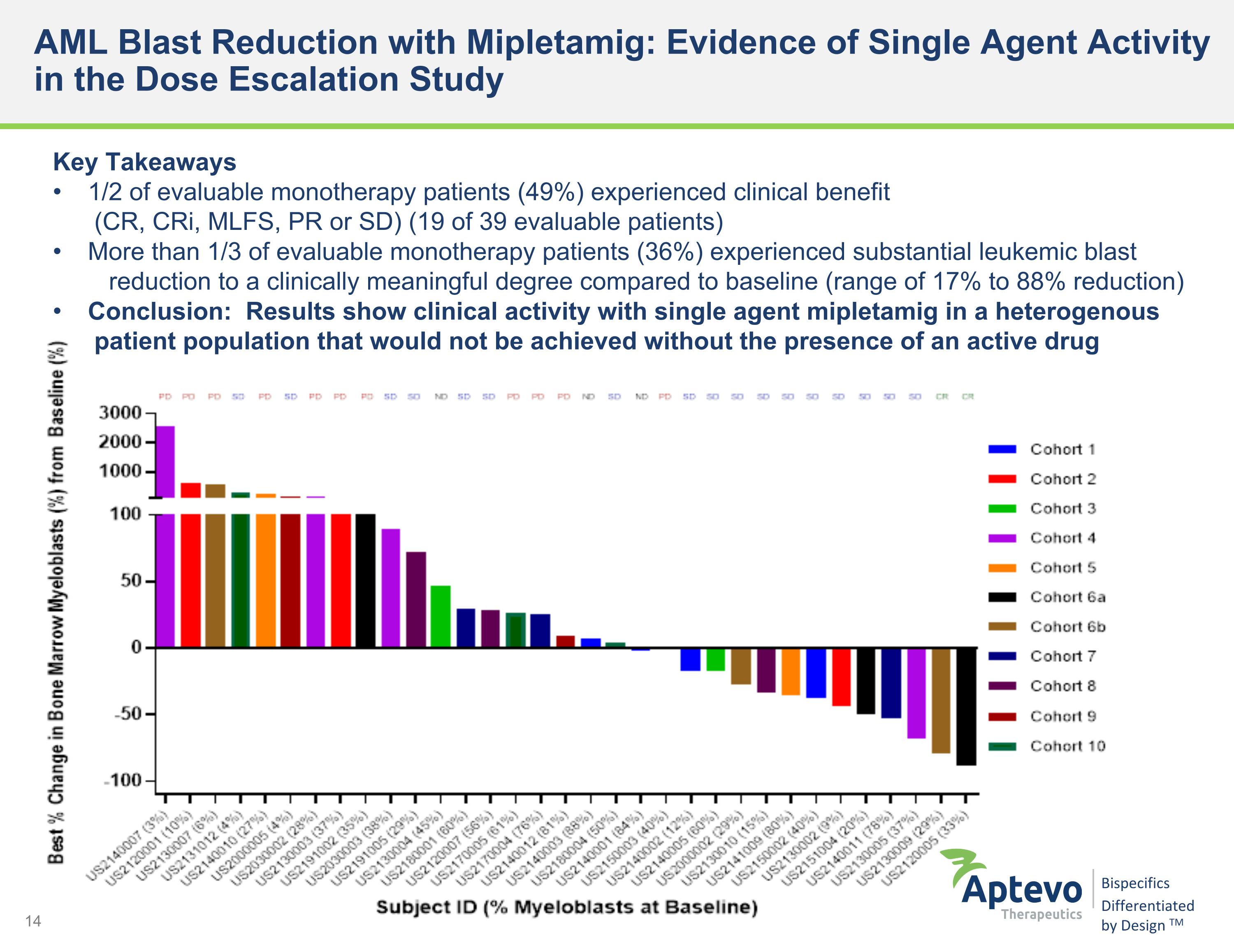
AML Blast Reduction with Mipletamig: Evidence of Single Agent Activity in the Dose Escalation Study Key Takeaways 1/2 of evaluable monotherapy patients (49%) experienced clinical benefit (CR, CRi, MLFS, PR or SD) (19 of 39 evaluable patients) More than 1/3 of evaluable monotherapy patients (36%) experienced substantial leukemic blast reduction to a clinically meaningful degree compared to baseline (range of 17% to 88% reduction) Conclusion: Results show clinical activity with single agent mipletamig in a heterogenous patient population that would not be achieved without the presence of an active drug
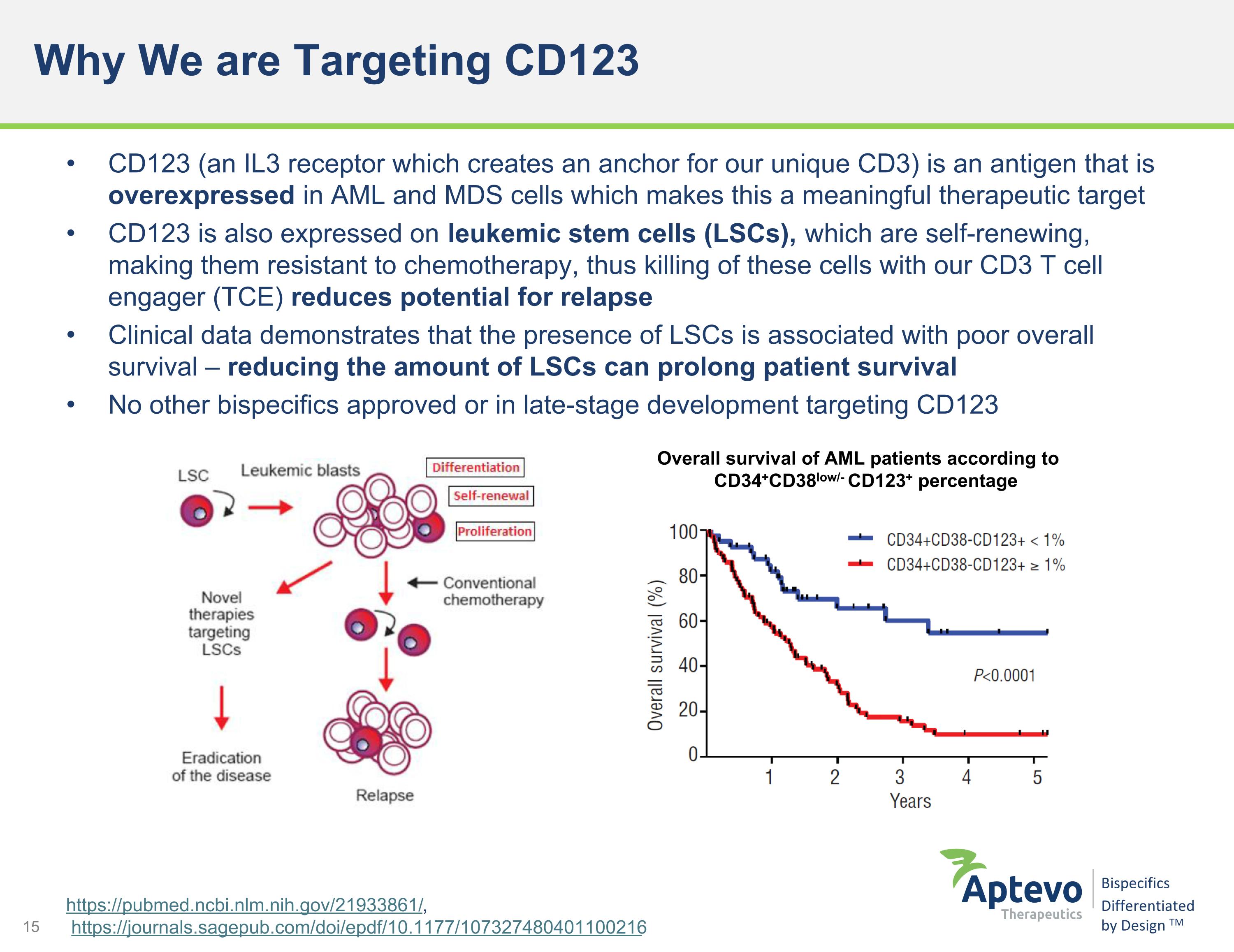
Why We are Targeting CD123 CD123 (an IL3 receptor which creates an anchor for our unique CD3) is an antigen that is overexpressed in AML and MDS cells which makes this a meaningful therapeutic target CD123 is also expressed on leukemic stem cells (LSCs), which are self-renewing, making them resistant to chemotherapy, thus killing of these cells with our CD3 T cell engager (TCE) reduces potential for relapse Clinical data demonstrates that the presence of LSCs is associated with poor overall survival – reducing the amount of LSCs can prolong patient survival No other bispecifics approved or in late-stage development targeting CD123 https://pubmed.ncbi.nlm.nih.gov/21933861/, https://journals.sagepub.com/doi/epdf/10.1177/107327480401100216 Overall survival of AML patients according to CD34+CD38low/- CD123+ percentage

ALG.APV-527�Multiple Solid Tumor Type Expressing 5T4 ALG.APV-527 is differentiated from other solid tumor-targeting bispecifics because it combines precise tumor specificity (via 5T4 targeting) with controlled immune activation through 4-1BB stimuli. The drug candidate incorporates the innovative design advantages of Aptevo’s proprietary ADAPTIR platform. These features are designed to improve safety and efficacy and broaden its potential impact across multiple solid tumor types.
Novel Bispecific ALG.APV-527 for Multiple Solid Tumors POTENTIAL INDICATIONS Multiple solid tumor cancers, including lung, breast, head & neck, colorectal, pancreatic, and other solid tumors with significant markets Unique Design Unique mechanism of action allows for targeting of both 4-1BB (co-stimulatory receptor) and 5T4 (tumor antigen) Designed to overcome safety issues of others’ first-generation 4-1BB agonists by designing ALG.AVP-527 to require 5T4-dependent immune activation Promotes the activity of antigen-primed CD8 T cells by increasing survival and enhancing their ability to kill tumor Designed for combinability with other drugs Ownership Joint 50/50 ownership and co-development agreement with Alligator Bioscience Patent exclusivity until 2038 (+ up to 5 years patent term extension)

ALG.APV-527 Dose Escalation Trial Yields Positive Data Promising data from the ALG.APV-527 multi-center Phase 1 dose escalation trial, were reported as follows: Clinical activity | Signs of clinical activity were observed, 10 of 17 efficacy evaluable patients (59%) have a best overall response to date of stable disease (SD) Safety and tolerability | Treatment was overall well-tolerated, and a maximum tolerated dose has not yet been determined No severe liver toxicity observed, a side effect associated with dose limiting toxicity Pharmacokinetics | ALG.APV-527 could be measured in all patients with serum concentration of ALG.APV-527 consistent with the administered dose and predicted exposure Pharmacodynamics | Biomarker analyses indicate the expression of the targets (4-1BB and 5T4) in tumor biopsies, treatment resulted in increased soluble 4-1BB in blood and an overall increase in number of CD8 T cells in the tumor, confirming biological activity of ALG.APV-527 The longest SD duration was in a breast cancer patient who entered the study with progressive disease, achieved stable disease and remained on study for >11 months. This patient successfully transitioned to a higher dose level twice One colon cancer patient remained on study and in SD for six months One prostate cancer patient in stable disease remained on study and in SD for more than four months Data support continued clinical evaluation for the treatment of multiple solid tumors
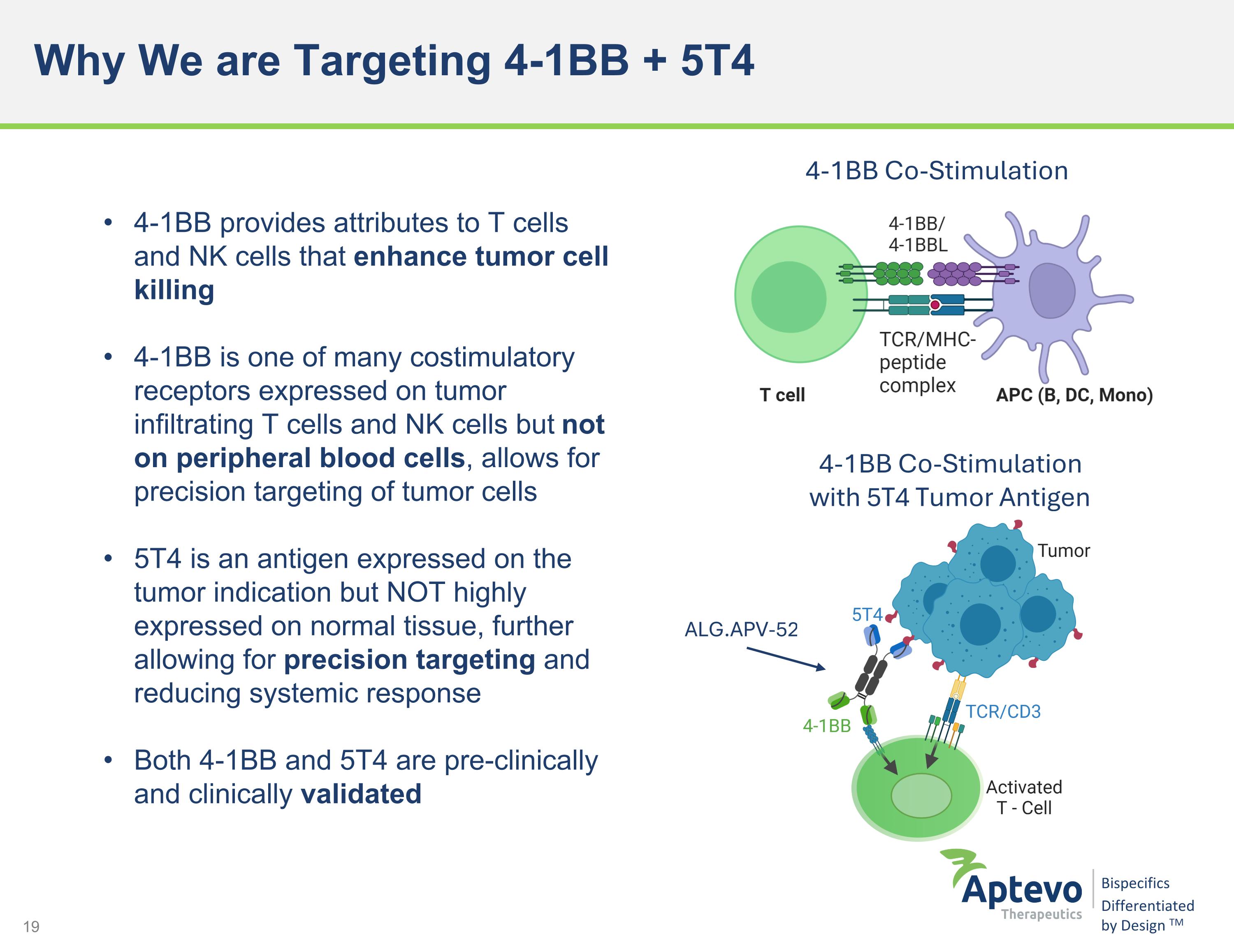
Why We are Targeting 4-1BB + 5T4 4-1BB provides attributes to T cells and NK cells that enhance tumor cell killing 4-1BB is one of many costimulatory receptors expressed on tumor infiltrating T cells and NK cells but not on peripheral blood cells, allows for precision targeting of tumor cells 5T4 is an antigen expressed on the tumor indication but NOT highly expressed on normal tissue, further allowing for precision targeting and reducing systemic response Both 4-1BB and 5T4 are pre-clinically and clinically validated 4-1BB Co-Stimulation 4-1BB Co-Stimulation with 5T4 Tumor Antigen Dependency ALG.APV-527

Preclinical Pipeline & �Platforms
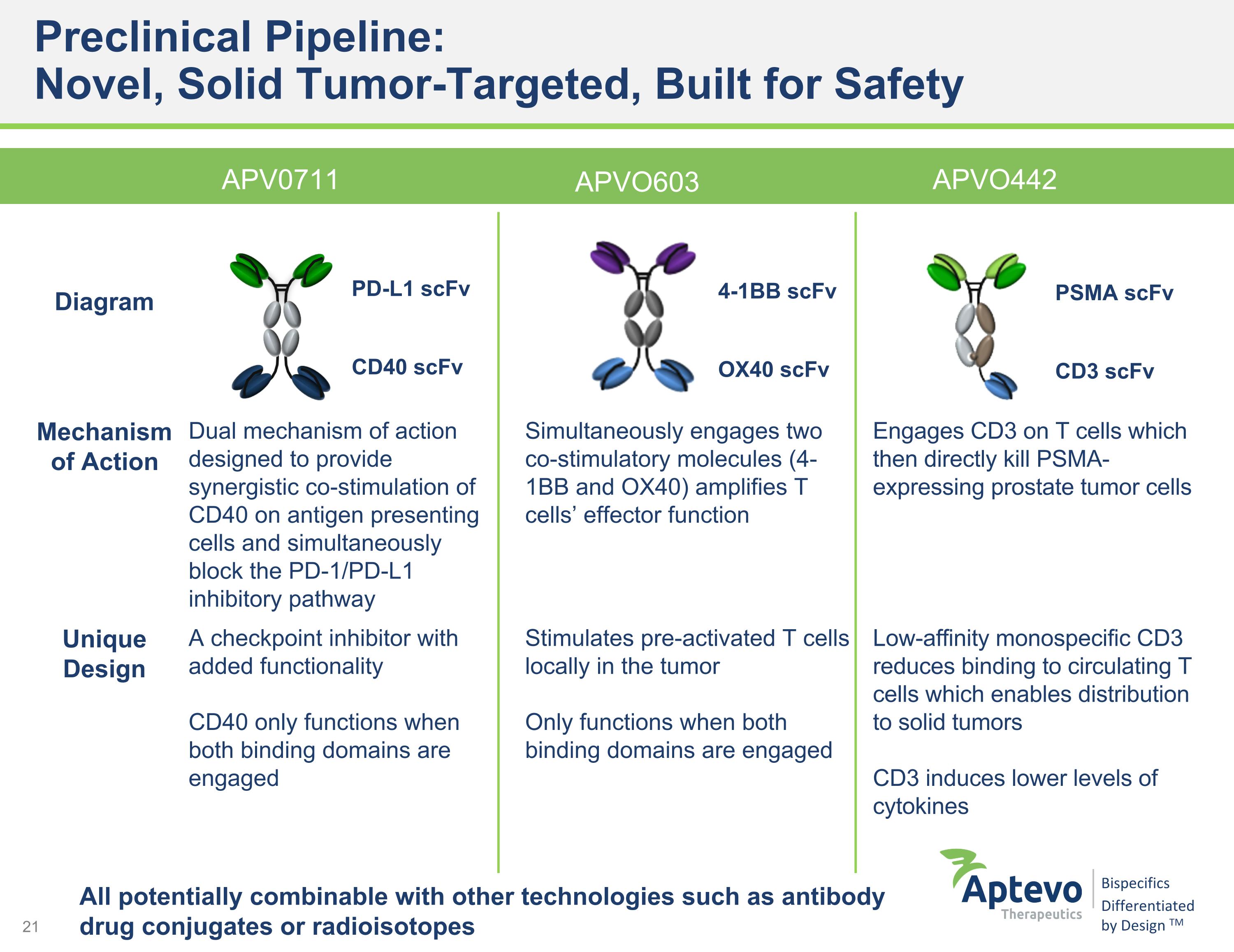
Preclinical Pipeline: �Novel, Solid Tumor-Targeted, Built for Safety APV0711 APVO603 Diagram Mechanism of Action Dual mechanism of action designed to provide synergistic co-stimulation of CD40 on antigen presenting cells and simultaneously block the PD-1/PD-L1 inhibitory pathway Simultaneously engages two co-stimulatory molecules (4-1BB and OX40) amplifies T cells’ effector function Engages CD3 on T cells which then directly kill PSMA-expressing prostate tumor cells Unique Design A checkpoint inhibitor with added functionality CD40 only functions when both binding domains are engaged Stimulates pre-activated T cells locally in the tumor Only functions when both binding domains are engaged Low-affinity monospecific CD3 reduces binding to circulating T cells which enables distribution to solid tumors CD3 induces lower levels of cytokines APVO442 4-1BB scFv OX40 scFv PSMA scFv CD3 scFv All potentially combinable with other technologies such as antibody drug conjugates or radioisotopes PD-L1 scFv CD40 scFv
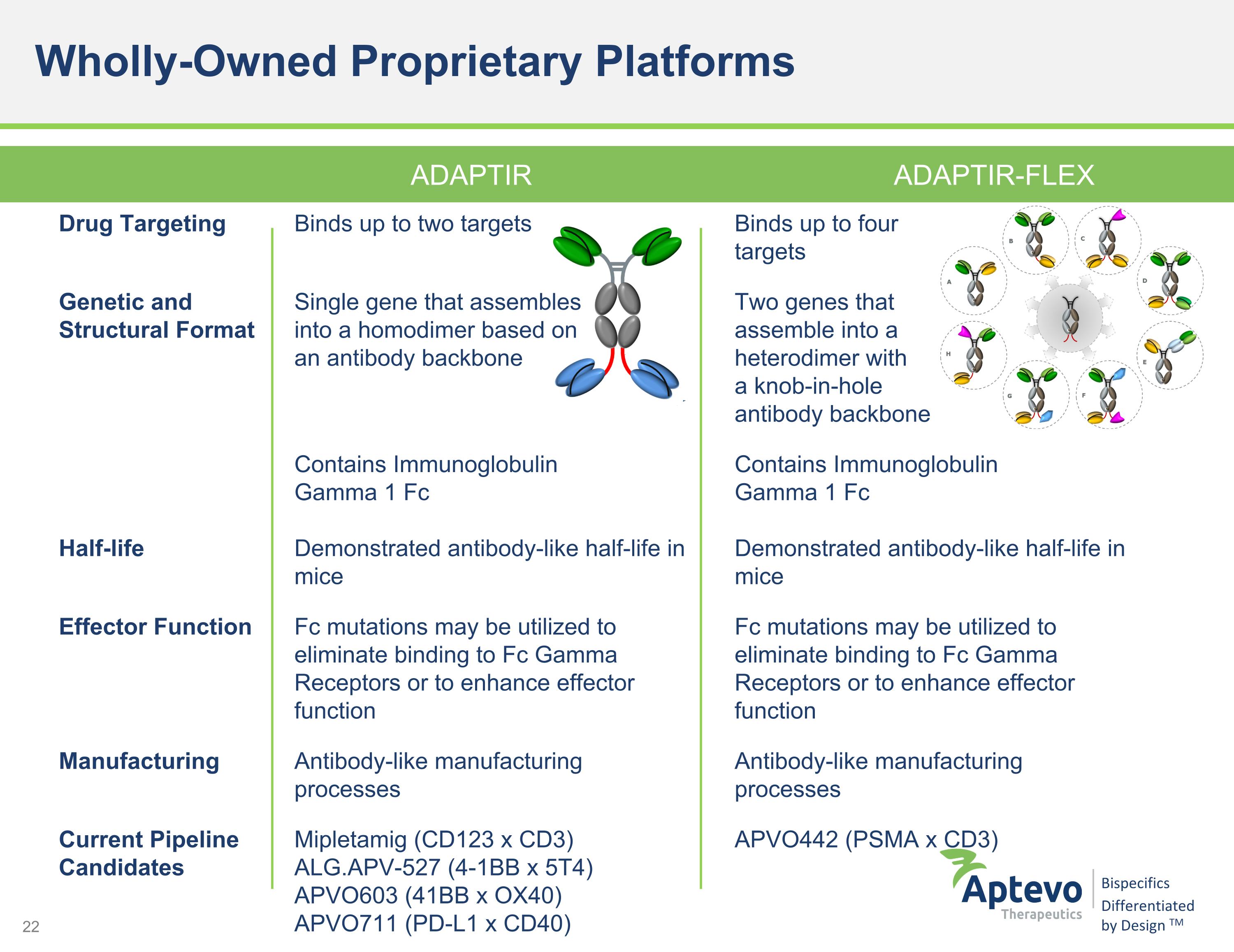
ADAPTIR ADAPTIR-FLEX Drug Targeting Binds up to two targets Binds up to four targets Genetic and Structural Format Single gene that assembles into a homodimer based on an antibody backbone Two genes that assemble into a heterodimer with a knob-in-hole antibody backbone Half-life Contains Immunoglobulin Gamma 1 Fc Demonstrated antibody-like half-life in mice Contains Immunoglobulin Gamma 1 Fc Demonstrated antibody-like half-life in mice Effector Function Fc mutations may be utilized to eliminate binding to Fc Gamma Receptors or to enhance effector function Fc mutations may be utilized to eliminate binding to Fc Gamma Receptors or to enhance effector function Manufacturing Antibody-like manufacturing processes Antibody-like manufacturing processes Current Pipeline Candidates Mipletamig (CD123 x CD3) ALG.APV-527 (4-1BB x 5T4) APVO603 (41BB x OX40) APVO711 (PD-L1 x CD40) APVO442 (PSMA x CD3) Wholly-Owned Proprietary Platforms ADAPTIR ADAPTIR-FLEX

The Company

Experienced & Expert Leadership Extensive R&D, manufacturing, clinical and financial background Senior Management Marvin White, President & CEO Jeff Lamothe, Chief Operating Officer Daphne Taylor, Chief Financial Officer SoYoung Kwon, General Counsel, Business Development & Corporate Affairs Dirk Huebner, MD, Chief Medical Officer Board of Directors John Niederhuber, MD, Chairman Zsolt Harsanyi, Ph.D., Director Barbara Lopez Kunz, Director Daniel Abdun-Nabi, Director Grady Grant, III, Director Marvin White, Director
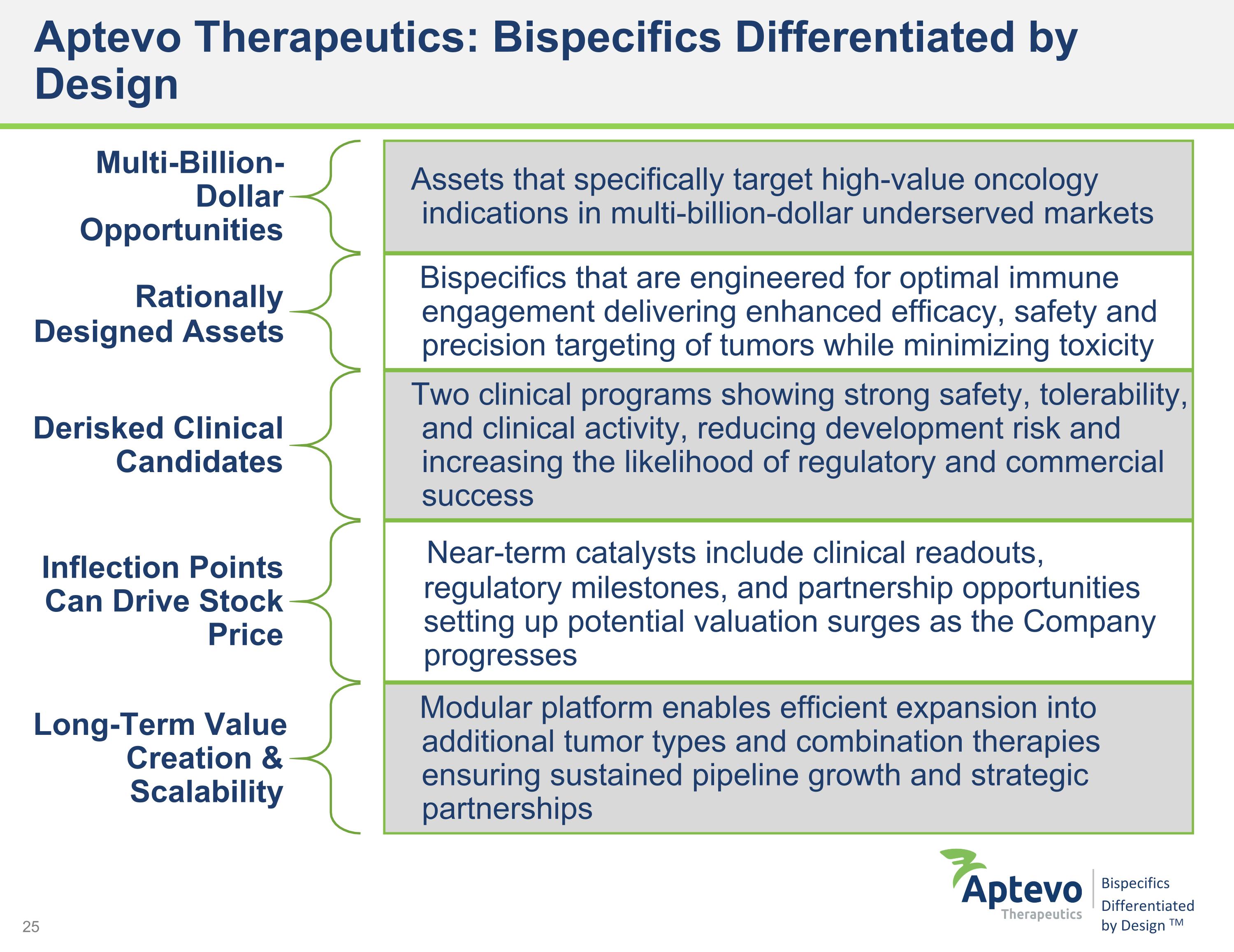
Aptevo Therapeutics: Bispecifics Differentiated by Design Multi-Billion-Dollar Opportunities Assets that specifically target high-value oncology indications in multi-billion-dollar underserved markets Rationally Designed Assets Bispecifics that are engineered for optimal immune engagement delivering enhanced efficacy, safety and precision targeting of tumors while minimizing toxicity Derisked Clinical Candidates Two clinical programs showing strong safety, tolerability, and clinical activity, reducing development risk and increasing the likelihood of regulatory and commercial success Inflection Points Can Drive Stock Price Near-term catalysts include clinical readouts, regulatory milestones, and partnership opportunities setting up potential valuation surges as the Company progresses Long-Term Value Creation & Scalability Modular platform enables efficient expansion into additional tumor types and combination therapies ensuring sustained pipeline growth and strategic partnerships

Thank You Aptevo Therapeutics Inc. 2401 4th Avenue, Suite 1050 Seattle, WA 98121 General Inquiries Tel: 206-838-0500 Fax: 206-838-0503 Investor Relations & Communications Miriam Weber Miller Tel: 206-859-6628 Email: ir@apvo.com or Millerm@apvo.com

Exhibit 99.2
RAINIER Trial Data Update: Two Additional AML Patients Achieve Remission Within 30 Days of Treatment
Across two trials, 9 of 10 frontline AML patients achieved remission when treated with mipletamig in combination with the standard of care
Triplet Combination with mipletamig continues to outperform doublet combination benchmark
No Cytokine Release Syndrome (CRS) has been observed in the RAINIER trial to date
Cohort 2 enrollment nears completion
Seattle, Washington, March 20, 2025 – Aptevo Therapeutics (“Aptevo”) (Nasdaq: APVO), a clinical-stage biotechnology company developing novel bispecific immuno-oncology therapeutics based on its proprietary ADAPTIR® and ADAPTIR-FLEX® platform technologies, today announced two additional frontline AML patients have achieved remission* within 30 days of treatment in the Company’s RAINIER dose optimization trial evaluating mipletamig in combination with standard of care for patients unfit for intensive chemotherapy. In total, 9 of 10 frontline patients across two trials achieved remission* when receiving the triplet combination of mipletamig + venetoclax + azacitidine (ven/aza). Notably, no CRS has been reported in the RAINIER trial to date.
The data builds on previously reported favorable outcomes from RAINIER’s Cohort 1 and the completed dose expansion trial where 100% of frontline patients achieved remission. Together with the addition of these interim Cohort 2 results, mipletamig has achieved a compelling overall remission rate of 90% among frontline patients. This outperforms the doublet remission* rate from a venetoclax + azacitidine only study, of 66%. Additionally, the frontline patient triplet therapy CR rate of 70% outperforms the CR rate from a venetoclax + azacitidine only study of 36% (Viale-A Pivotal trial). Thus far, all RAINIER patients who achieved remission remain in remission.
Cohort 2 will include six patients, dosed at the 18mcg level, the same dose used in combination with ven/aza in the completed expansion trial.
Three patients evaluable for efficacy achieved the following outcomes:
•Two patients achieved remission withing 30 days of being dosed
•One patient progressed after the first cycle and passed away for reasons unrelated to study drugs
•Cohort 2 enrollment is nearing completion
“We’re now past the halfway mark in Cohort 2 of the RAINIER trial and are thrilled by the continued, highly favorable remission results,” said Dirk Huebner, MD, Chief Medical Officer of Aptevo. “ This emerging pattern further supports mipletamig’s impact on treatment outcomes in frontline AML patients who are not fit for intensive chemotherapy and who would otherwise
receive ven/aza as the standard of care. One of our primary goals with the RAINIER trial is to demonstrate the contribution of mipletamig’s unique mechanism of action when used in combination with venetoclax and azacitidine. By targeting AML this way, our approach has the potential to improve outcomes, particularly for elderly patients who have limited treatment options.”
Mipletamig, a differentiated by design CD3 x CD123 bispecific antibody built on Aptevo’s ADAPTIR platform and driven by a unique CRIS-7 derived binding domain, is being investigated as frontline therapy in combination with venetoclax and azacitidine, the current standard of care for AML patients who are unfit for intensive chemotherapy. These latest results further reinforce mipletamig's potential as a transformative treatment, supported by impressive efficacy, safety, and tolerability data from two prior clinical trials involving almost 100 patients.
About RAINIER
RAINIER, a frontline AML study, is a Phase 1b/2 dose optimization, multi-center, multi-cohort, open label study of up to 39 patients who are being treated across five dose levels ranging from 9 mcg – 140 mcg in combination with venetoclax and azacitidine (ven/aza). Subjects will be adults aged 18 or older, newly diagnosed with AML who are not eligible for intensive induction chemotherapy. Phase 1b consists of 28-day cycles of treatment in five sequential cohorts. Aptevo has partnered with Prometrika (https://www.prometrika.com/), a premier contract research organization for the trial. RAINIER will be conducted in two parts. First, a Phase 1b dose optimization study in frontline AML patients followed by a Phase 2 study.
Cohort 1 included 3 patients, dosed at the 9mcg level.
•All patients achieved remission* within 30 days
•Thus far, all patients who achieved remission remain in remission.
•One CR patient had no minimal residual disease (MRD-negative status) and was positive for the TP53 genetic mutation, which is generally associated with poor prognosis due to chemotherapy resistance, genetic instability, and overall treatment challenges
*Remission = complete remission (CR) and, complete remission with blood markers that have not yet recovered (CRi).
About Mipletamig
Aptevo's wholly owned lead proprietary drug candidate, mipletamig, targeting AML, MDS and other leukemias, is differentiated by design to redirect the immune system of the patient to destroy leukemic cells and leukemic stem cells expressing the target antigen CD123, which is a compelling target for AML due to its overexpression on leukemic stem cells and AML blasts. This antibody-like recombinant protein therapeutic is designed to engage both leukemic cells and T cells of the immune system and bring them closely together to trigger the destruction of leukemic cells. Mipletamig is purposefully designed to reduce the likelihood and severity of CRS by use of a unique CD3 derived from CRIS-7 vs. the CD3 used by other competitors. Mipletamig has received orphan drug designation ("orphan status") for AML according to the Orphan Drug Act.
Mipletamig has been evaluated in almost 100 patients over three trials to date. RAINIER, Aptevo’s Phase 1b/2 frontline AML program, was initiated in 3Q24.
About Aptevo Therapeutics
Aptevo Therapeutics Inc. (Nasdaq: APVO) is a clinical-stage biotechnology company focused on developing novel bispecific immunotherapies for the treatment of cancer. The company has two clinical candidates. Mipletamig is currently being evaluated in RAINIER, a Phase 1b/2 trial for the treatment of frontline acute myeloid leukemia in combination with standard of care venetoclax + azacitidine. Mipletamig has orphan status for AML according to the Orphan Drug Act. ALG.APV-527, a bispecific conditional 4-1BB agonist that is only active upon simultaneous binding to 4-1BB and 5T4, is being co-developed with Alligator Bioscience and is being evaluated in a Phase 1 clinical trial for the treatment of multiple solid tumor types likely to express 5T4. Aptevo has three pre-clinical candidates with different mechanisms of action designed to target a range of solid tumors. All pipeline candidates were created from two proprietary platforms, ADAPTIR and ADAPTIR-FLEX. The Aptevo mission is to improve treatment outcomes and transform the lives of cancer patients. For more information, please visit www.aptevotherapeutics.com.
Safe Harbor Statement
This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical fact, including, without limitation, Aptevo’s expectations about the activity, efficacy, safety, tolerability and durability of its therapeutic candidates and potential use of any such candidates, including in combination with other drugs, as therapeutics for treatment of disease, its expectations regarding the effectiveness of its ADAPTIR and ADAPTIR-FLEX platforms, statements related to the progress of Aptevo’s clinical programs, including statements related to anticipated clinical and regulatory milestones, whether further study of mipletamig in a Phase 1b dose optimization trial focusing on multiple doses of mipletamig in combination with venetoclax + azacitidine on a targeted patient population will continue to show remissions, whether Aptevo’s final remission data or trial results will vary from its earlier assessment, whether Aptevo’s strategy will translate into an improved overall survival in AML, especially among patient subgroups with poor prognosis, whether further study of ALG.APV-527 across multiple tumor types will continue to show clinical benefit, the possibility and timing of future preliminary or interim data readouts for ALG.APV-527, statements related to the progress of and enthusiasm for Aptevo's clinical programs, statements related to Aptevo’s ability to generate stockholder value, whether Aptevo will continue to have momentum in its business in the future, and any other statements containing the words “may,” “continue to,” “believes,” “knows,” “expects,” “optimism,” “potential,” “designed,” “promising,” “plans,” “will” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based on Aptevo’s current intentions, beliefs, and expectations regarding future events. Aptevo cannot guarantee that any forward-looking statement will be accurate. Investors should realize that if underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could differ materially from Aptevo’s expectations. Investors are, therefore, cautioned not to place undue reliance on any forward-looking statement.
There are several important factors that could cause Aptevo’s actual results to differ materially from those indicated by such forward-looking statements, including a deterioration in Aptevo’s business or prospects; further assessment of preliminary or interim data or different results from later clinical trials; adverse events and unanticipated problems, adverse developments in clinical development, including unexpected safety issues observed during a clinical trial; and changes in regulatory, social, macroeconomic and political conditions. For instance, actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including the uncertainties inherent in the results of preliminary or interim data and preclinical studies being predictive of the results of later-stage clinical trials, initiation, enrollment and maintenance of patients, and the completion of clinical trials, the availability and timing of data from ongoing clinical trials, the trial design includes combination therapies that may make it difficult to accurately ascertain the benefits of mipletamig, expectations for the timing and steps required in the regulatory review process, expectations for regulatory approvals, the impact of competitive products, our ability to enter into agreements with strategic partners or raise funds on acceptable terms or at all and other matters that could affect the availability or commercial potential of Aptevo’s product candidates, business or economic disruptions due to catastrophes or other events, including natural disasters or public health crises such as the coronavirus (referred to as COVID-19), geopolitical risks, including the current war between Russia and Ukraine, war between Israel and Hamas, and macroeconomic conditions such as economic uncertainty, rising inflation and interest rates, continued market volatility and decreased consumer confidence. These risks are not exhaustive, Aptevo faces known and unknown risks. Additional risks and factors that may affect results are set forth in Aptevo’s filings with the Securities and Exchange Commission, including its Annual Report on Form 10-K for the fiscal year ended December 31, 2024, and its subsequent reports on Form 10-Q and current reports on Form 8-K. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from Aptevo’s expectations in any forward-looking statement. Any forward-looking statement speaks only as of the date of this press release, and, except as required by law, Aptevo does not assume any obligation to update any forward-looking statement to reflect new information, events, or circumstances.
Aptevo Therapeutics
Miriam Weber Miller
Aptevo Therapeutics
IR@apvo.com or millerm@apvo.com
+1 (206) 859 6629
v3.25.1
Document And Entity Information
|
Mar. 20, 2025 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Mar. 20, 2025
|
| Entity Registrant Name |
APTEVO THERAPEUTICS INC.
|
| Entity Central Index Key |
0001671584
|
| Entity Emerging Growth Company |
false
|
| Entity File Number |
001-37746
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Tax Identification Number |
81-1567056
|
| Entity Address, Address Line One |
2401 4th Avenue
|
| Entity Address, Address Line Two |
Suite 1050
|
| Entity Address, City or Town |
Seattle
|
| Entity Address, State or Province |
WA
|
| Entity Address, Postal Zip Code |
98121
|
| City Area Code |
(206)
|
| Local Phone Number |
838-0500
|
| Entity Information, Former Legal or Registered Name |
Not Applicable
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, $0.001 par value
|
| Trading Symbol |
APVO
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Grafico Azioni Aptevo Therapeutics (NASDAQ:APVO)
Storico
Da Mar 2025 a Apr 2025

Grafico Azioni Aptevo Therapeutics (NASDAQ:APVO)
Storico
Da Apr 2024 a Apr 2025
