false 0001894562 0001894562 2024-01-08 2024-01-08
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
January 8, 2024
Date of Report (Date of earliest event reported)
Prime Medicine, Inc.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
001-41536 |
|
84-3097762 |
| (State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(I.R.S. Employer Identification No.) |
|
|
|
| 21 Erie Street Cambridge, MA |
|
02139 |
| (Address of principal executive offices) |
|
(Zip Code) |
(617) 564-0013
(Registrant’s telephone number, including area code)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common stock, par value $.00001 per share |
|
PRME |
|
The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule12b-2 of the Securities Exchange Act of 1934 (§250.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
Prime Medicine, Inc. (the “Company”) will be conducting meetings with participants attending the 42nd Annual J.P. Morgan Healthcare Conference (the “Conference”) during the week of January 8, 2024. A copy of the slides to be presented by the Company at the Conference is furnished as Exhibit 99.1 to this Current Report on Form 8-K, which is incorporated herein by reference.
The information in this Item 7.01, including Exhibit 99.1 attached hereto, is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
Date: January 8, 2024
|
|
|
| Prime Medicine, Inc. |
|
|
| By: |
|
/s/ Keith Gottesdiener |
| Name: |
|
Keith Gottesdiener, M.D. |
| Title: |
|
President and Chief Executive Officer |

Exhibit 99.1 Delivering on the promise of Prime Editing JP Morgan
Healthcare Conference January 2024 1

Forward Looking Statements This presentation contains forward-looking
statements of Prime Medicine, Inc. ( Prime , we or our ) within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. These forward-looking statements contain information about our current and future prospects and our
operations, which are based on currently available information. All statements other than statements of historical facts contained in this presentation, including statements regarding our strategy, projects and plans are forward-looking statements.
In some cases, you can identify forward-looking statements by terminology such as “aim,” “anticipate,” “assume,” “believe,” “contemplate,” “continue” “could,”
“design,” “due,” “estimate,” “expect,” “goal,” “hope,” “intend,” “may,” “might,” “objective,” “opportunity,”
“plan,” “predict,” “positioned,” “possible,” “potential,” “project,” “seek,” “should,” “strategy,” “target,” “will,”
“would” and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. These forward-looking statements include, but are not limited to,
express or implied statements about Prime’s beliefs and expectations regarding: the initiation, timing, progress and results of our research and development programs, preclinical studies and future clinical trials, and the release of data
related thereto; our ability to demonstrate, and the timing of, preclinical proof-of-concept in vivo for multiple programs; our ability to pursue our four strategic indication categories: immediate target indications, differentiation target
indications, “blue sky” indications and “march up the chromosome” approaches; our ability to quickly leverage programs within our initial target indications and to progress additional programs to further develop our pipeline;
the potential of Prime Editors to reproducibly correct disease-causing genetic mutations across different tissues, organs and cell types, and the capacity of our PASSIGE technology to edit CAR-T cells for the treatment of certain cancers and immune
diseases; the timing of our regulatory filings, including our anticipated initial IND submission for CGD as early as 2024 with additional filings anticipated in 2025; our ability to demonstrate superior off-target profiles for Prime Editing
programs; the further advancement of Prime Editors to maximize their versatility, precision and efficiency; the continued development and optimization of various non-viral and viral delivery systems, including our universal liver-targeted LNP
delivery approach; the expansion of Prime Editing’s therapeutic potential to extend the reach and impact of Prime Editing to areas beyond our current areas of focus; the potential of Prime Editing to offer curative genetic therapies for a wide
spectrum of diseases; the scope of protection we are able to establish and maintain for intellectual property rights covering our Prime Editing technology; developments related to our competitors and our industry; our ability to leverage the
clinical, regulatory, and manufacturing advancements made by gene therapy and gene editing programs to accelerate our clinical trials and approval of product candidates; the research collaboration with Cimeio to combine our and Cimeio’s
respective technologies, including our Prime Editing platform and Cimeio’s SCIP platform, and the goals of such collaboration, the potential benefits of such collaboration and technology thereunder, including the ability to cure various
diseases and replace existing treatments such as transplantation; the implementation of our strategic plans for our business, programs and technology, including our ability to identify and enter into future license agreements and collaborations;
regulatory developments in the United States and foreign countries; our ability to attract and retain key scientific and management personnel; and our estimates of our expenses, capital requirements, and needs for additional financing as well as our
cash runway into 2024. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make due to a number of risks and uncertainties. These and other risks, uncertainties
and important factors are described in the section entitled Risk Factors in our most recent Annual Report on Form 10-K, as well as any subsequent filings with the Securities and Exchange Commission. Any forward- looking statements represent our
views only as of the date of this presentation and we undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, the occurrence of certain events or otherwise subject to any obligations under
applicable law. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. No representations or warranties (expressed or
implied) are made about the accuracy of any such forward-looking statements. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and our own
internal estimates and research. While we believe these third-party studies, publications, surveys and other data to be reliable as of the date of this presentation, we have not independently verified, and make no representation as to the adequacy,
fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent source has evaluated the reasonableness or accuracy of our internal estimates or research and no reliance should be made on any
information or statements made in this presentation relating to or based on such internal estimates and research. 2

“The age of human therapeutic gene editing isn’t just
coming. It’s already here.”* 3 * David Liu, Ph.D., Co-Founder of Prime Medicine

“The age of human therapeutic gene editing isn’t just
coming. It’s already here.”* 4 * David Liu, Ph.D., Co-Founder of Prime Medicine

“The age of human therapeutic gene editing isn’t just
coming. It’s already here.”* 5 * David Liu, Ph.D., Co-Founder of Prime Medicine

“The age of human therapeutic gene editing isn’t just
coming. It’s already here.”* 6 * David Liu, Ph.D., Co-Founder of Prime Medicine

“The age of human therapeutic gene editing isn’t just
coming. It’s already here.”* 7 * David Liu, Ph.D., Co-Founder of Prime Medicine

Now is our moment: Prime Medicine brings together the right people and
the right technology at the right time we are building on decades of progress to deliver the promise of one-time, curative genetic therapies to address the widest spectrum of diseases 8
OPERATIONAL EXECUTION BROAD OPPORTUNITY TO ADDRESS LARGE MARKETS
Delivering on the promise of Prime Editing Now is our moment: DIFFERENTIATED SAFETY PROFILE Prime Medicine brings together the PLATFORM MODULARITY right people and the right technology at the right time ENTERING THE CLINIC we are building on decades
of progress to deliver the promise of one-time, curative genetic therapies to address the widest spectrum of diseases STRATEGIC PIPELINE ALIGNED TO FOUR CORE PILLARS 9
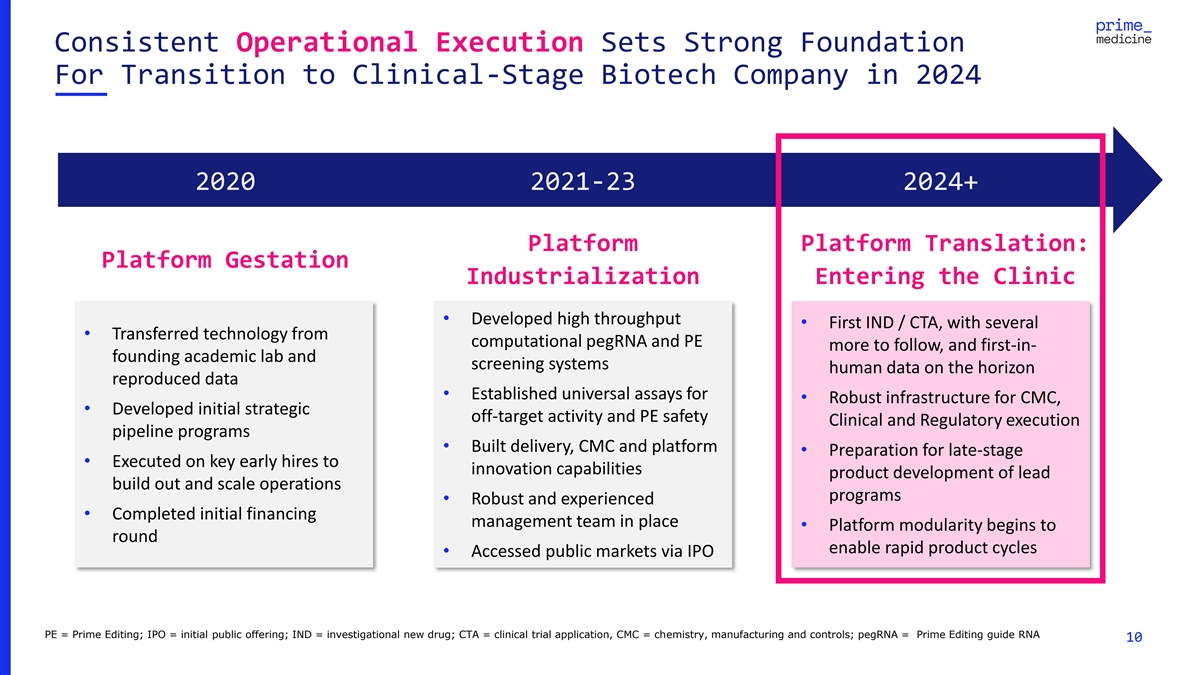
Consistent Operational Execution Sets Strong Foundation For Transition
to Clinical-Stage Biotech Company in 2024 2020 2021-23 2024+ Platform Platform Translation: Platform Gestation Industrialization Entering the Clinic • Developed high throughput • First IND / CTA, with several • Transferred
technology from computational pegRNA and PE more to follow, and first-in- founding academic lab and screening systems human data on the horizon reproduced data • Established universal assays for • Robust infrastructure for CMC, •
Developed initial strategic off-target activity and PE safety Clinical and Regulatory execution pipeline programs • Built delivery, CMC and platform • Preparation for late-stage • Executed on key early hires to innovation
capabilities product development of lead build out and scale operations programs • Robust and experienced • Completed initial financing management team in place • Platform modularity begins to round enable rapid product cycles
• Accessed public markets via IPO PE = Prime Editing; IPO = initial public offering; IND = investigational new drug; CTA = clinical trial application, CMC = chemistry, manufacturing and controls; pegRNA = Prime Editing guide RNA 10
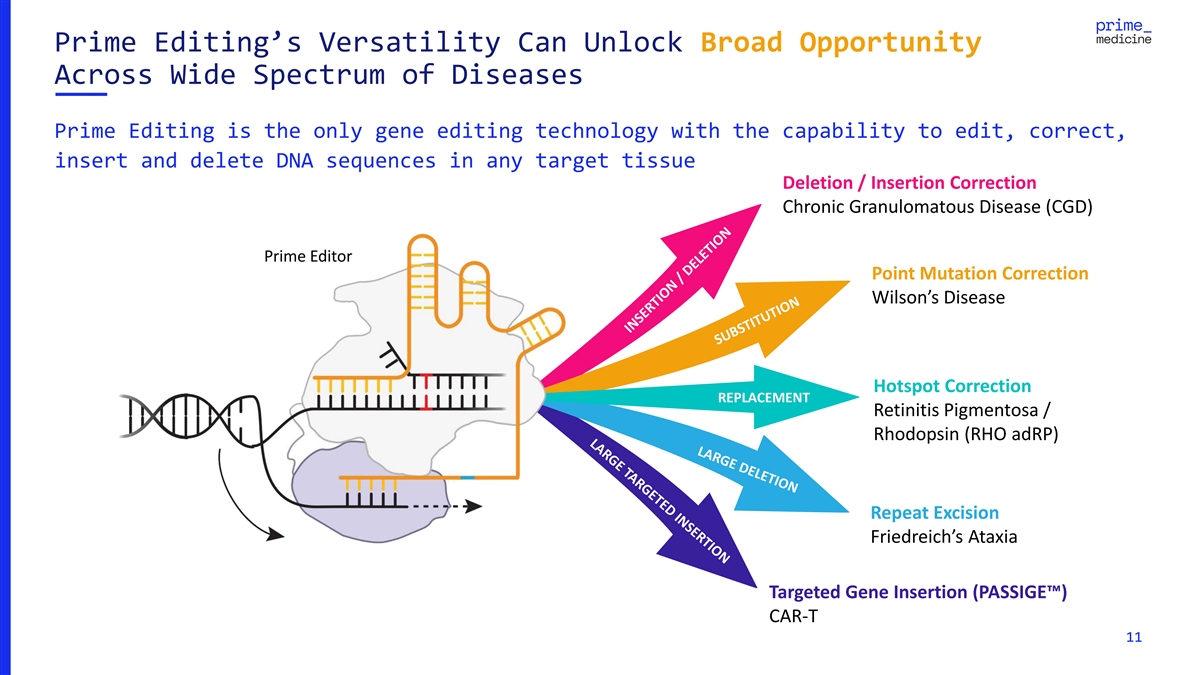
Prime Editing’s Versatility Can Unlock Broad Opportunity Across
Wide Spectrum of Diseases Prime Editing is the only gene editing technology with the capability to edit, correct, insert and delete DNA sequences in any target tissue Deletion / Insertion Correction Chronic Granulomatous Disease (CGD) Prime Editor
Point Mutation Correction Wilson’s Disease Hotspot Correction REPLACEMENT Retinitis Pigmentosa / Rhodopsin (RHO adRP) Repeat Excision Friedreich’s Ataxia Targeted Gene Insertion (PASSIGE™) CAR-T 11
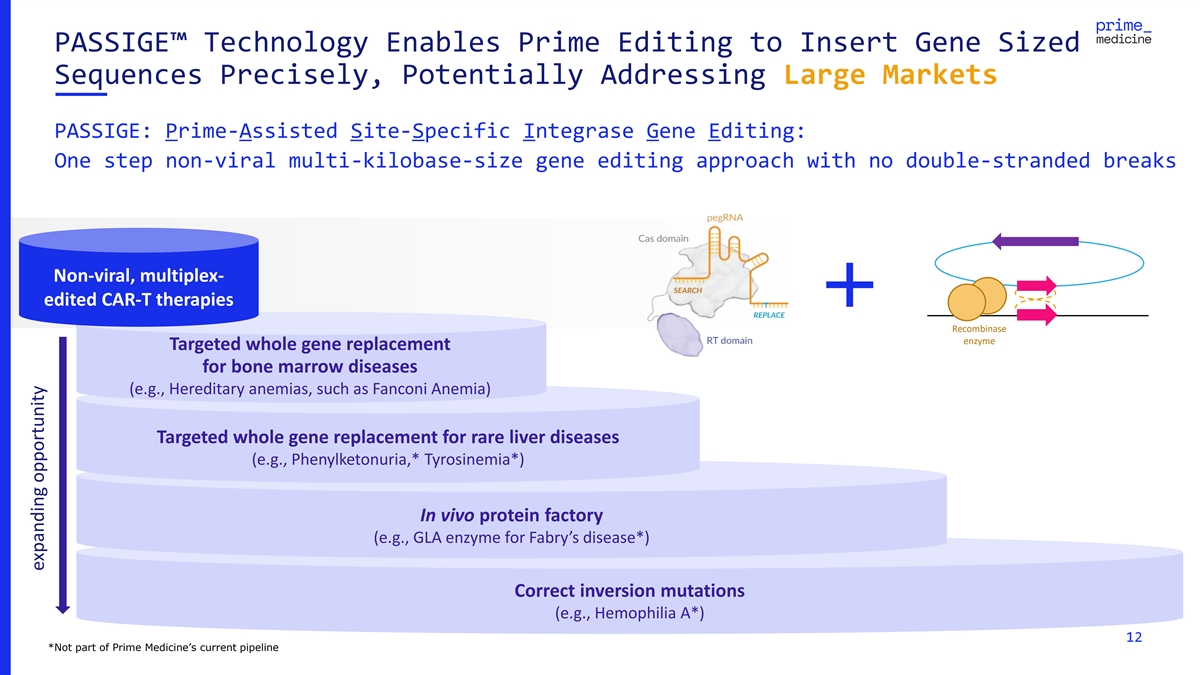
PASSIGE™ Technology Enables Prime Editing to Insert Gene Sized
Sequences Precisely, Potentially Addressing Large Markets PASSIGE: Prime-Assisted Site-Specific Integrase Gene Editing: One step non-viral multi-kilobase-size gene editing approach with no double-stranded breaks Non-viral, multiplex- edited CAR-T
therapies Recombinase enzyme Targeted whole gene replacement for bone marrow diseases (e.g., Hereditary anemias, such as Fanconi Anemia) Targeted whole gene replacement for rare liver diseases (e.g., Phenylketonuria,* Tyrosinemia*) In vivo protein
factory (e.g., GLA enzyme for Fabry’s disease*) Correct inversion mutations (e.g., Hemophilia A*) 12 *Not part of Prime Medicine’s current pipeline expanding opportunity
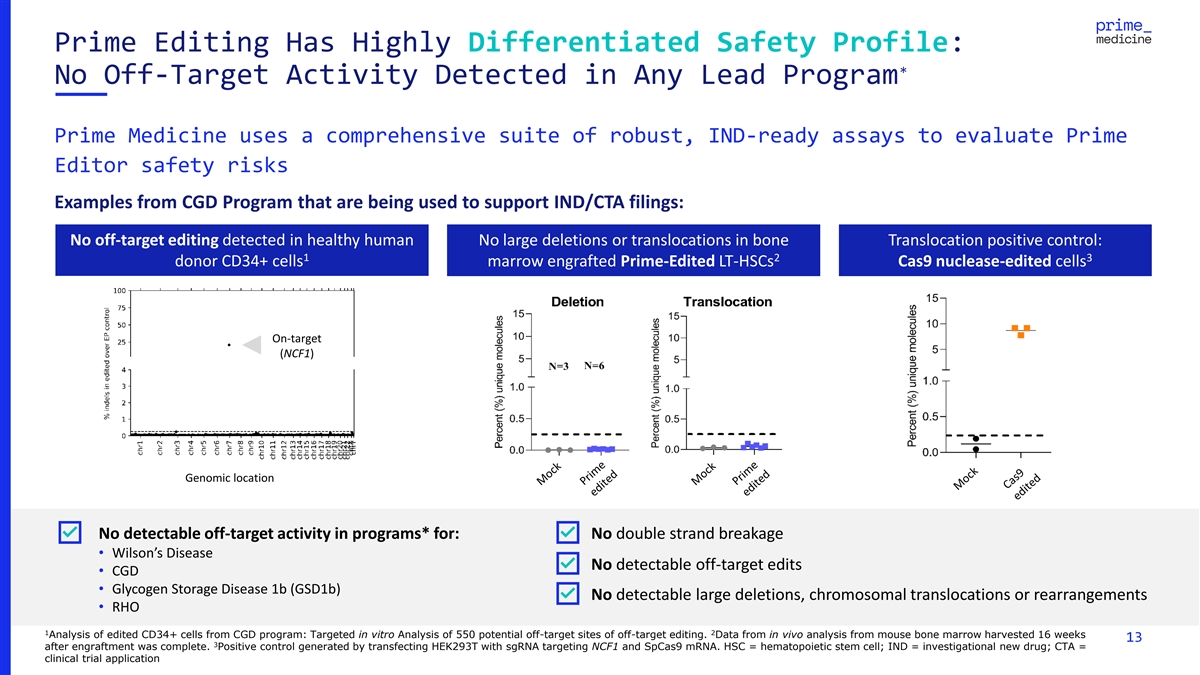
Prime Editing Has Highly Differentiated Safety Profile: * No Off-Target
Activity Detected in Any Lead Program Prime Medicine uses a comprehensive suite of robust, IND-ready assays to evaluate Prime Editor safety risks Examples from CGD Program that are being used to support IND/CTA filings: No off-target editing
detected in healthy human No large deletions or translocations in bone Translocation positive control: 1 2 3 donor CD34+ cells marrow engrafted Prime-Edited LT-HSCs Cas9 nuclease-edited cells On-target (NCF1) Genomic location No detectable
off-target activity in programs* for: No double strand breakage • Wilson’s Disease No detectable off-target edits • CGD • Glycogen Storage Disease 1b (GSD1b) No detectable large deletions, chromosomal translocations or
rearrangements • RHO 1 2 Analysis of edited CD34+ cells from CGD program: Targeted in vitro Analysis of 550 potential off-target sites of off-target editing. Data from in vivo analysis from mouse bone marrow harvested 16 weeks 13 3 after
engraftment was complete. Positive control generated by transfecting HEK293T with sgRNA targeting NCF1 and SpCas9 mRNA. HSC = hematopoietic stem cell; IND = investigational new drug; CTA = clinical trial application
Platform Modularity Accelerates and De-Risks Ongoing Efforts and
Enables Rapid Generation of New Product Candidates Core components can be readily leveraged to drive pipeline acceleration, efficiency and execution Clinical Regulatory Manufacturing Delivery Off-Target Assays Prime Editors 14
Prime Medicine is Entering the Clinic at the Right Time: Evolving
Landscape Favors Innovation in Cell and Gene Therapy Positive regulatory interactions in U.S. and globally set stage for near-term clinic entry In 2023, FDA: In 2023, Prime Medicine: Established Office of Therapeutic Products under Dr. Nicole
Engaged in multiple formal and informal interactions with Verdun global regulatory agencies on PM359 program and Prime Introduced novel initiatives for expediting development of Editing platform genetic medicines – INTERACT and pre-IND
meetings with the FDA – Platform designation: allows companies to leverage data across – Highly positive interactions with one ex-U.S. agency to-date; two programs using modular components additional pending for early 2024 – START
program: increased regulatory feedback for therapies Prime Medicine has aligned with FDA recommendations targeting rare diseases with morbidity in first decade of life regarding: Allowed first clinical trials of base editing- and in vivo –
Preclinical data CRISPR-based therapies to proceed – Toxicology – CMC Approved first BLA of CRISPR-based therapy in Vertex’s exa-cel – Off-target – Clinical development plans On-track to file first IND in 1H 2024 15 BLA
= biologics license application; IND = investigational new drug
Prime Medicine is Focused Internally on Four Pillars, Each with
Demonstrated High Efficiency, Precise in Vivo Editing Business development can extend reach and impact, bolstering our financial resources and maximizing the potential of Prime Editing Hematology & Business Liver Ocular Neuromuscular Immunology
Development Strong company foundation Expert at designing guide RNAs, Clinical and Regulatory CMC expertise mRNA and vector genome sequence know-how 16
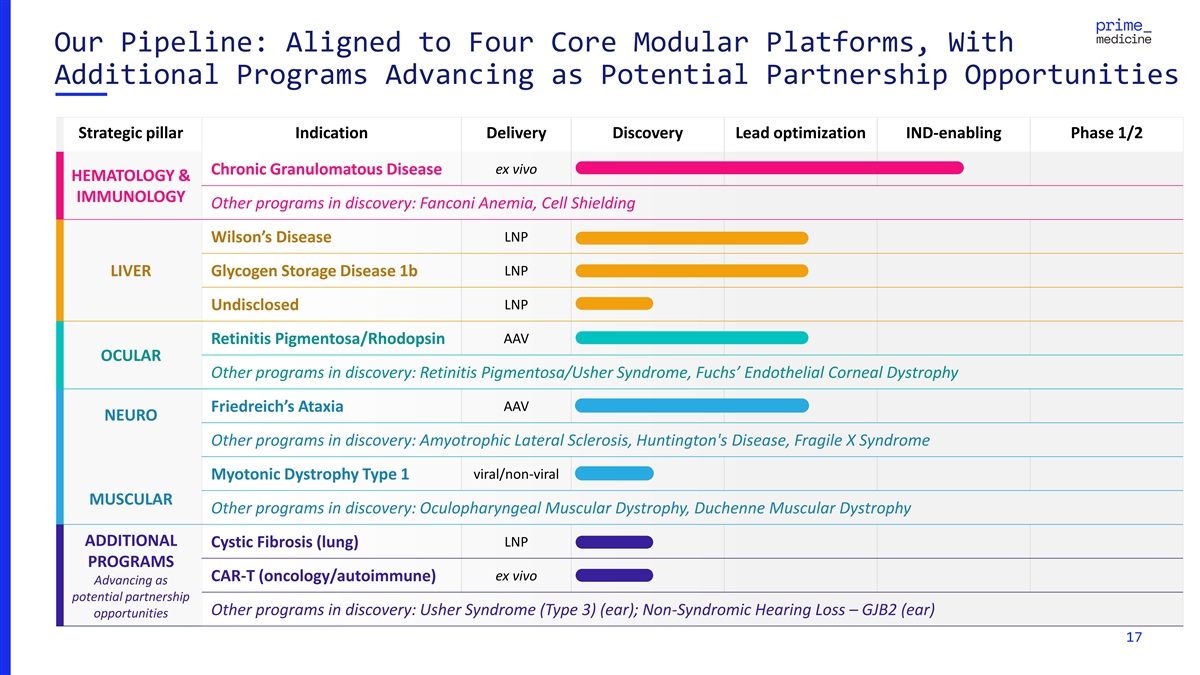
Our Pipeline: Aligned to Four Core Modular Platforms, With Additional
Programs Advancing as Potential Partnership Opportunities Strategic pillar Indication Delivery Discovery Lead optimization IND-enabling Phase 1/2 ex vivo Chronic Granulomatous Disease HEMATOLOGY & IMMUNOLOGY Other programs in discovery: Fanconi
Anemia, Cell Shielding LNP Wilson’s Disease LIVER Glycogen Storage Disease 1b LNP Undisclosed LNP Retinitis Pigmentosa/Rhodopsin AAV OCULAR Other programs in discovery: Retinitis Pigmentosa/Usher Syndrome, Fuchs’ Endothelial Corneal
Dystrophy AAV Friedreich’s Ataxia NEURO Other programs in discovery: Amyotrophic Lateral Sclerosis, Huntington's Disease, Fragile X Syndrome viral/non-viral Myotonic Dystrophy Type 1 MUSCULAR Other programs in discovery: Oculopharyngeal
Muscular Dystrophy, Duchenne Muscular Dystrophy ADDITIONAL LNP Cystic Fibrosis (lung) PROGRAMS CAR-T (oncology/autoimmune) ex vivo Advancing as potential partnership Other programs in discovery: Usher Syndrome (Type 3) (ear); Non-Syndromic Hearing
Loss – GJB2 (ear) opportunities 17
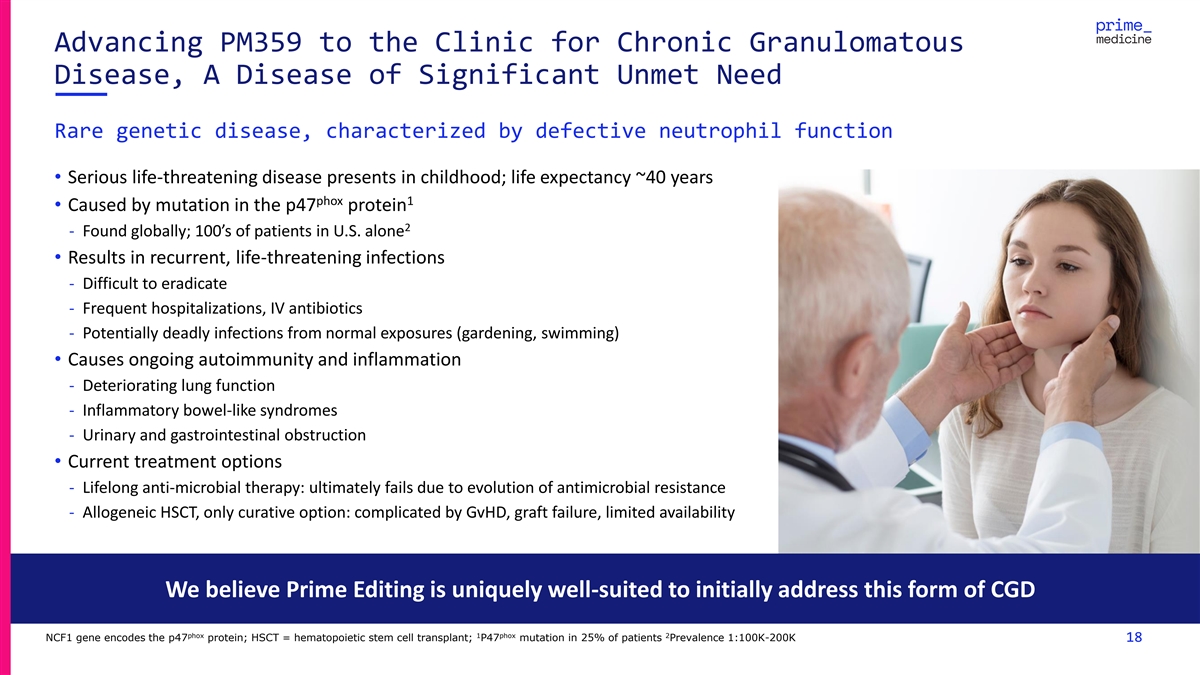
Advancing PM359 to the Clinic for Chronic Granulomatous Disease, A
Disease of Significant Unmet Need Rare genetic disease, characterized by defective neutrophil function • Serious life-threatening disease presents in childhood; life expectancy ~40 years phox 1 • Caused by mutation in the p47 protein 2 -
Found globally; 100’s of patients in U.S. alone • Results in recurrent, life-threatening infections - Difficult to eradicate - Frequent hospitalizations, IV antibiotics - Potentially deadly infections from normal exposures (gardening,
swimming) • Causes ongoing autoimmunity and inflammation - Deteriorating lung function - Inflammatory bowel-like syndromes - Urinary and gastrointestinal obstruction • Current treatment options - Lifelong anti-microbial therapy:
ultimately fails due to evolution of antimicrobial resistance - Allogeneic HSCT, only curative option: complicated by GvHD, graft failure, limited availability We believe Prime Editing is uniquely well-suited to initially address this form of CGD
phox 1 phox 2 NCF1 gene encodes the p47 protein; HSCT = hematopoietic stem cell transplant; P47 mutation in 25% of patients Prevalence 1:100K-200K 18
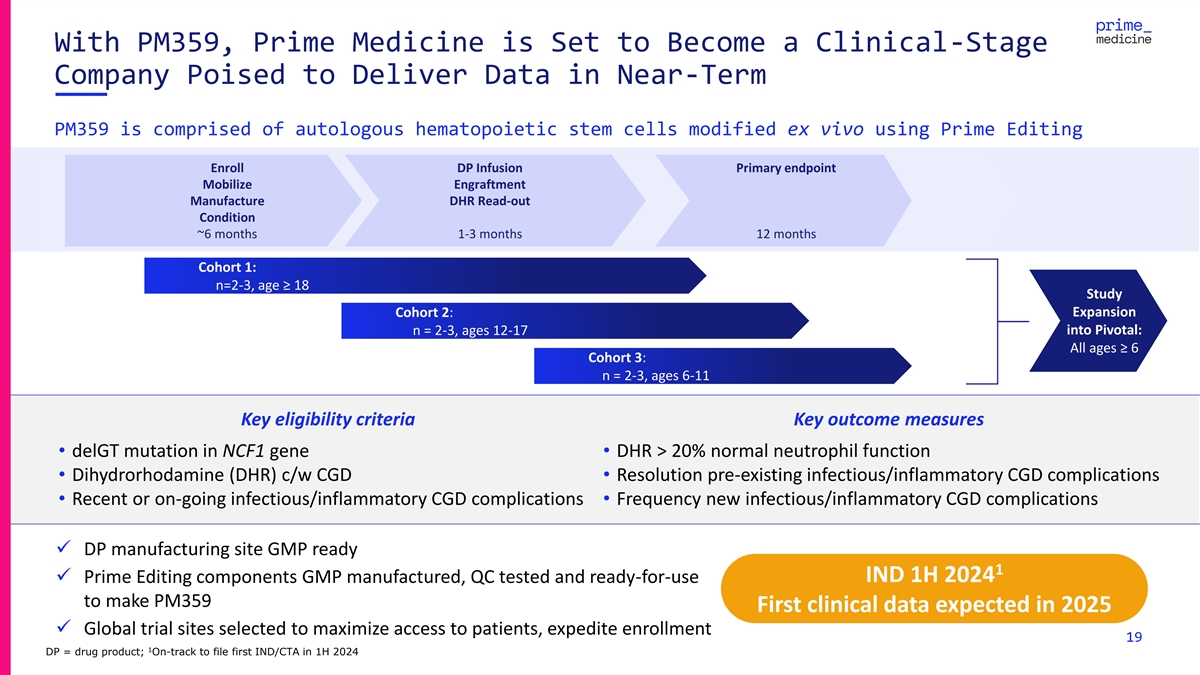
With PM359, Prime Medicine is Set to Become a Clinical-Stage Company
Poised to Deliver Data in Near-Term PM359 is comprised of autologous hematopoietic stem cells modified ex vivo using Prime Editing Enroll DP Infusion Primary endpoint Mobilize Engraftment Manufacture DHR Read-out Condition ~6 months 1-3 months 12
months Cohort 1: n=2-3, age ≥ 18 Study Expansion Cohort 2: n = 2-3, ages 12-17 into Pivotal: All ages ≥ 6 Cohort 3: n = 2-3, ages 6-11 Key eligibility criteria Key outcome measures • delGT mutation in NCF1 gene• DHR > 20%
normal neutrophil function • Dihydrorhodamine (DHR) c/w CGD• Resolution pre-existing infectious/inflammatory CGD complications • Recent or on-going infectious/inflammatory CGD complications• Frequency new
infectious/inflammatory CGD complications ✓ DP manufacturing site GMP ready 1 IND 1H 2024 ✓ Prime Editing components GMP manufactured, QC tested and ready-for-use to make PM359 First clinical data expected in 2025 ✓ Global trial
sites selected to maximize access to patients, expedite enrollment 19 1 DP = drug product; On-track to file first IND/CTA in 1H 2024
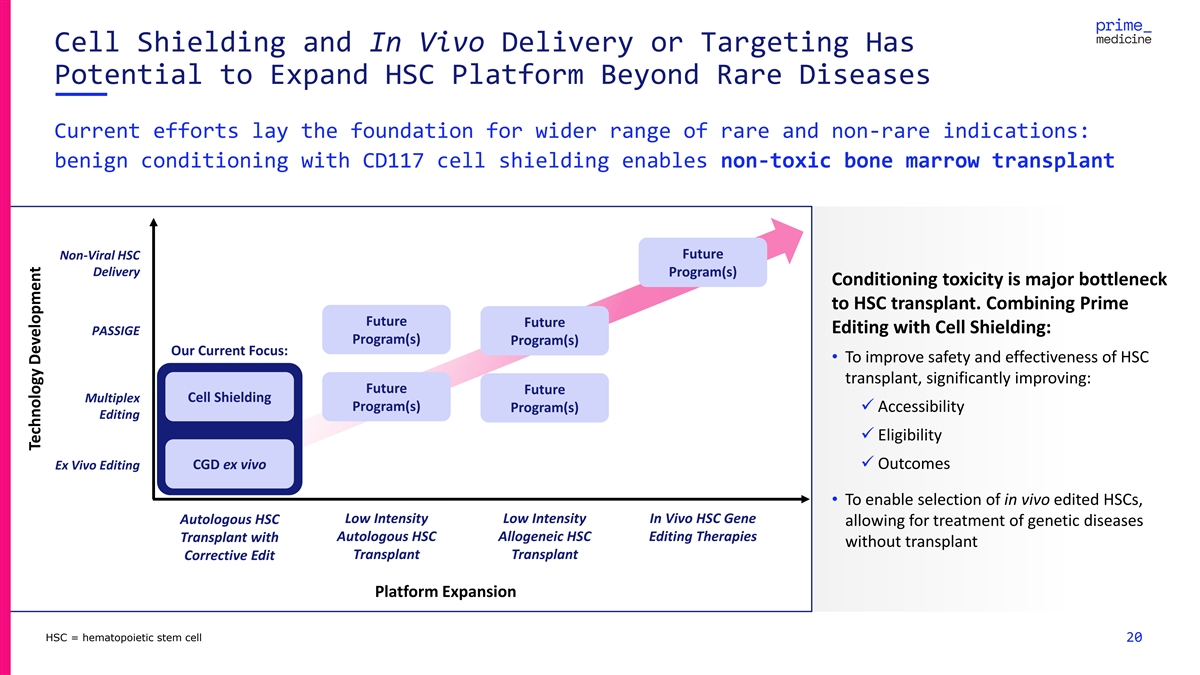
Cell Shielding and In Vivo Delivery or Targeting Has Potential to
Expand HSC Platform Beyond Rare Diseases Current efforts lay the foundation for wider range of rare and non-rare indications: benign conditioning with CD117 cell shielding enables non-toxic bone marrow transplant Future Non-Viral HSC Delivery
Program(s) Conditioning toxicity is major bottleneck to HSC transplant. Combining Prime Future Future Editing with Cell Shielding: PASSIGE Program(s) Program(s) Our Current Focus: • To improve safety and effectiveness of HSC transplant,
significantly improving: Future Future Multiplex Cell Shielding Program(s) Program(s)✓ Accessibility Editing ✓ Eligibility CGD ex vivo✓ Outcomes Ex Vivo Editing • To enable selection of in vivo edited HSCs, Autologous HSC
Low Intensity Low Intensity In Vivo HSC Gene allowing for treatment of genetic diseases Autologous HSC Allogeneic HSC Editing Therapies Transplant with without transplant Transplant Transplant Corrective Edit Platform Expansion HSC = hematopoietic
stem cell 20 Technology Development
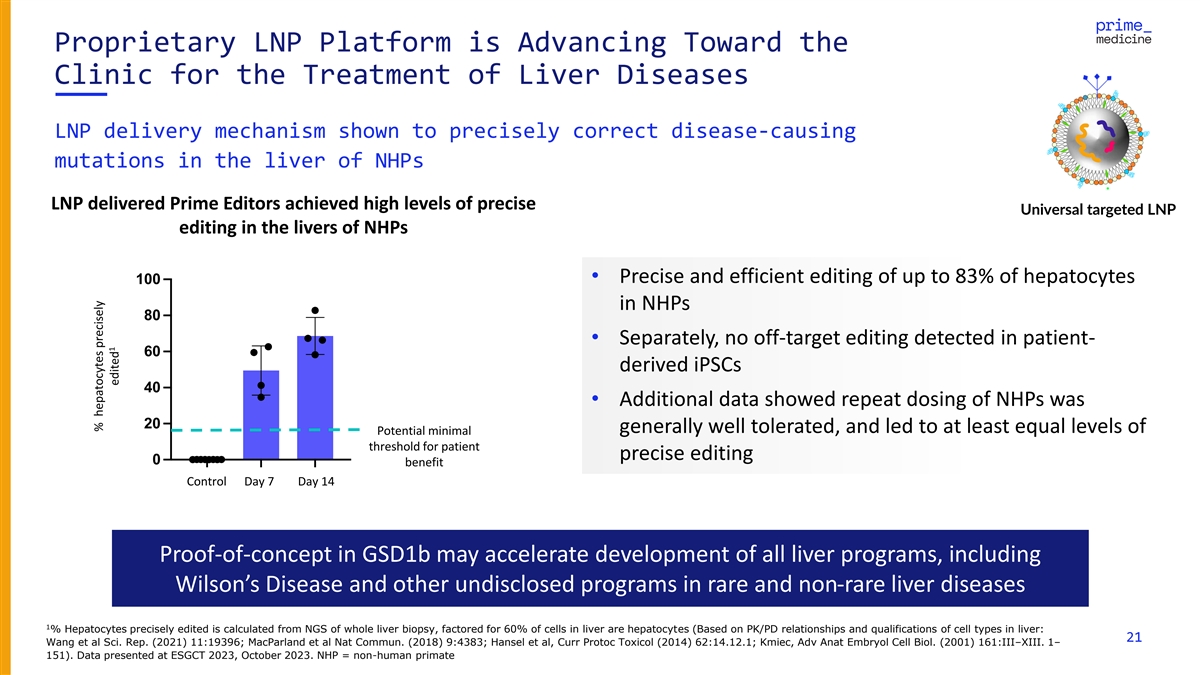
Proprietary LNP Platform is Advancing Toward the Clinic for the
Treatment of Liver Diseases LNP delivery mechanism shown to precisely correct disease-causing mutations in the liver of NHPs LNP delivered Prime Editors achieved high levels of precise Universal targeted LNP editing in the livers of NHPs 100•
Precise and efficient editing of up to 83% of hepatocytes in NHPs 80 • Separately, no off-target editing detected in patient- 60 derived iPSCs 40 • Additional data showed repeat dosing of NHPs was 20 generally well tolerated, and led to
at least equal levels of Potential minimal threshold for patient precise editing 0 benefit control Day 7 Day 14 Control Day 7 Day 14 Proof-of-concept in GSD1b may accelerate development of all liver programs, including Wilson’s Disease and
other undisclosed programs in rare and non-rare liver diseases 1 % Hepatocytes precisely edited is calculated from NGS of whole liver biopsy, factored for 60% of cells in liver are hepatocytes (Based on PK/PD relationships and qualifications of cell
types in liver: 21 Wang et al Sci. Rep. (2021) 11:19396; MacParland et al Nat Commun. (2018) 9:4383; Hansel et al, Curr Protoc Toxicol (2014) 62:14.12.1; Kmiec, Adv Anat Embryol Cell Biol. (2001) 161:III–XIII. 1– 151). Data presented at
ESGCT 2023, October 2023. NHP = non-human primate % hepatocytes precisely % hepatocytes 1 edited precisely edited
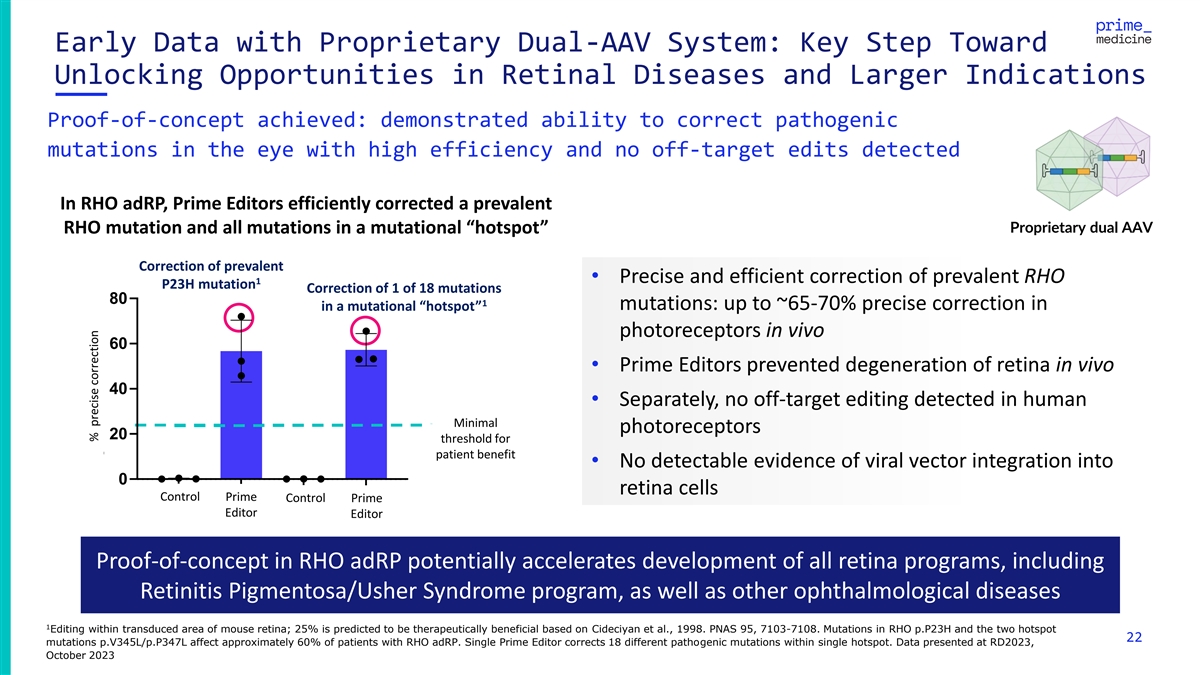
Early Data with Proprietary Dual-AAV System: Key Step Toward Unlocking
Opportunities in Retinal Diseases and Larger Indications Proof-of-concept achieved: demonstrated ability to correct pathogenic mutations in the eye with high efficiency and no off-target edits detected In RHO adRP, Prime Editors efficiently
corrected a prevalent Proprietary dual AAV RHO mutation and all mutations in a mutational “hotspot” Correction of prevalent • Precise and efficient correction of prevalent RHO 1 P23H mutation Correction of 1 of 18 mutations 80 1
mutations: up to ~65-70% precise correction in in a mutational “hotspot” photoreceptors in vivo 60 • Prime Editors prevented degeneration of retina in vivo 40 • Separately, no off-target editing detected in human Minimal
photoreceptors 20 threshold for patient benefit • No detectable evidence of viral vector integration into 0 retina cells Control Prime control Prime cCo on nt tr ro oll Pr Prim ime e Editor Editor Editor Editor Proof-of-concept in RHO adRP
potentially accelerates development of all retina programs, including Retinitis Pigmentosa/Usher Syndrome program, as well as other ophthalmological diseases 1 Editing within transduced area of mouse retina; 25% is predicted to be therapeutically
beneficial based on Cideciyan et al., 1998. PNAS 95, 7103-7108. Mutations in RHO p.P23H and the two hotspot 22 mutations p.V345L/p.P347L affect approximately 60% of patients with RHO adRP. Single Prime Editor corrects 18 different pathogenic
mutations within single hotspot. Data presented at RD2023, October 2023 % precise correction % precise correction
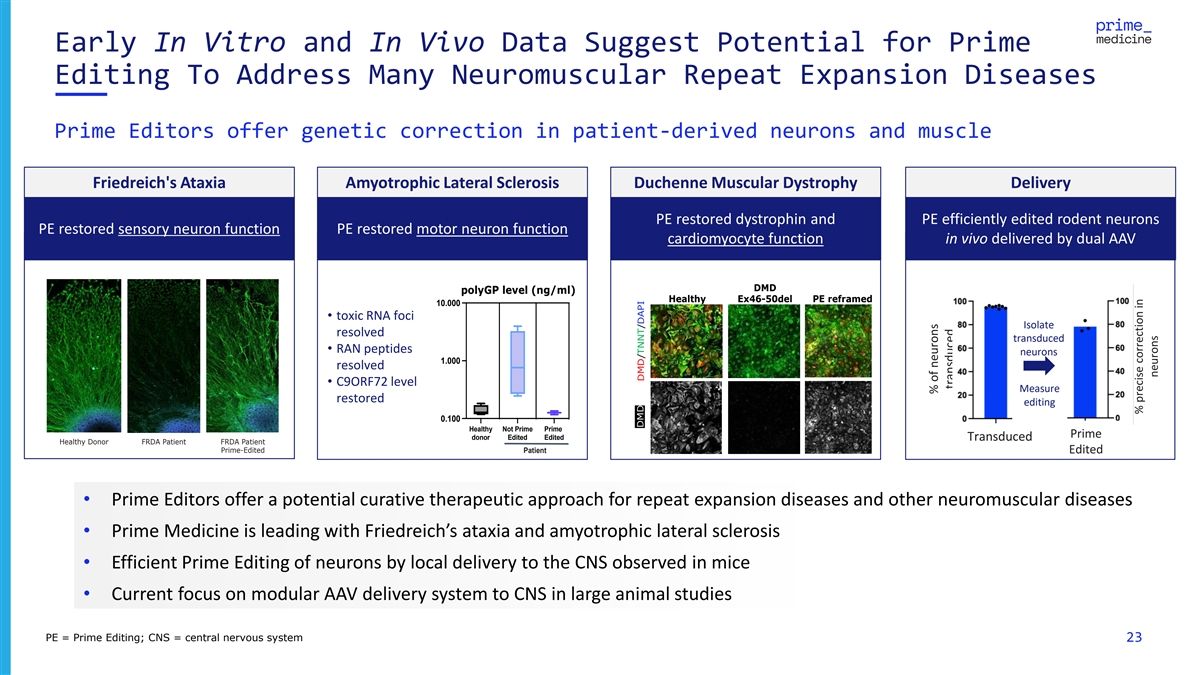
Early In Vitro and In Vivo Data Suggest Potential for Prime Editing To
Address Many Neuromuscular Repeat Expansion Diseases Prime Editors offer genetic correction in patient-derived neurons and muscle Friedreich's Ataxia Amyotrophic Lateral Sclerosis Duchenne Muscular Dystrophy Delivery PE restored dystrophin and PE
efficiently edited rodent neurons PE restored sensory neuron function PE restored motor neuron function cardiomyocyte function in vivo delivered by dual AAV DMD polyGP level (ng/ml) Healthy Ex46-50del PE reframed 10.000 • toxic RNA foci
Isolate resolved transduced • RAN peptides neurons 1.000 resolved • C9ORF72 level Measure restored editing 0.100 Healthy Not Prime Prime Prime donor Edited Edited Transduced Healthy Donor FRDA Patient FRDA Patient Prime-Edited Patient
Edited • Prime Editors offer a potential curative therapeutic approach for repeat expansion diseases and other neuromuscular diseases • Prime Medicine is leading with Friedreich’s ataxia and amyotrophic lateral sclerosis •
Efficient Prime Editing of neurons by local delivery to the CNS observed in mice • Current focus on modular AAV delivery system to CNS in large animal studies PE = Prime Editing; CNS = central nervous system 23 DMD DMD/TNNT/DAPI % of neurons
transduced % precise correction in neurons
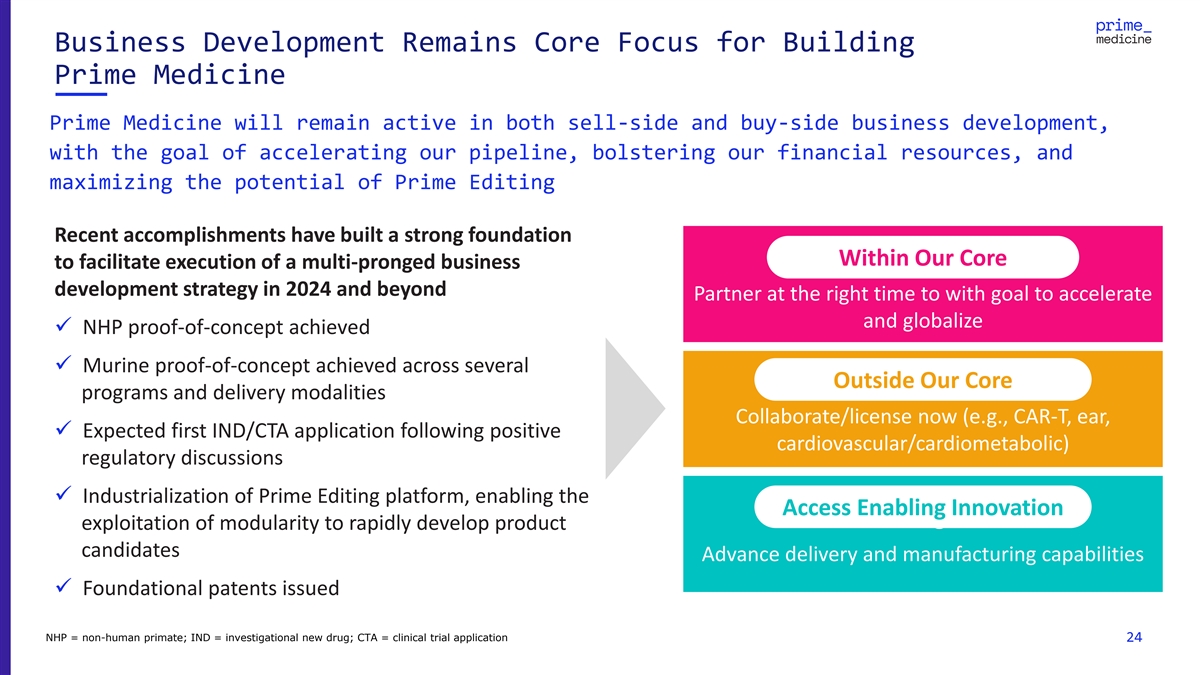
Business Development Remains Core Focus for Building Prime Medicine
Prime Medicine will remain active in both sell-side and buy-side business development, with the goal of accelerating our pipeline, bolstering our financial resources, and maximizing the potential of Prime Editing Recent accomplishments have built a
strong foundation Within the Core Within Our Core to facilitate execution of a multi-pronged business development strategy in 2024 and beyond Partner at the right time to with goal to accelerate and globalize ✓ NHP proof-of-concept achieved
✓ Murine proof-of-concept achieved across several Outside Our Core Outside Our Core programs and delivery modalities Collaborate/license now (e.g., CAR-T, ear, ✓ Expected first IND/CTA application following positive
cardiovascular/cardiometabolic) regulatory discussions ✓ Industrialization of Prime Editing platform, enabling the Access Enabling Innovation Access Enabling Innovation exploitation of modularity to rapidly develop product candidates Advance
delivery and manufacturing capabilities ✓ Foundational patents issued NHP = non-human primate; IND = investigational new drug; CTA = clinical trial application 24
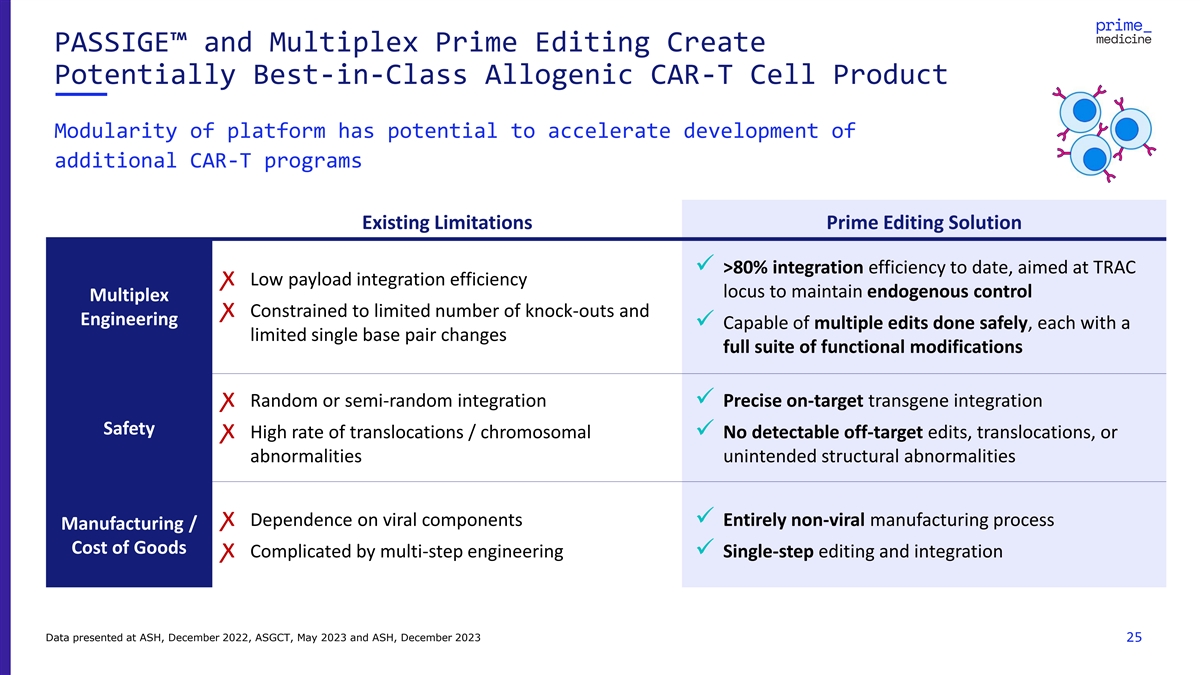
PASSIGE™ and Multiplex Prime Editing Create Potentially
Best-in-Class Allogenic CAR-T Cell Product Modularity of platform has potential to accelerate development of additional CAR-T programs Existing Limitations Prime Editing Solution ✓ >80% integration efficiency to date, aimed at TRAC
ꭗ Low payload integration efficiency locus to maintain endogenous control Multiplex ꭗ Constrained to limited number of knock-outs and Engineering ✓ Capable of multiple edits done safely, each with a limited single base pair
changes full suite of functional modifications ꭗ Random or semi-random integration✓ Precise on-target transgene integration Safety ꭗ High rate of translocations / chromosomal ✓ No detectable off-target edits,
translocations, or abnormalities unintended structural abnormalities ꭗ Dependence on viral components✓ Entirely non-viral manufacturing process Manufacturing / Cost of Goods ꭗ Complicated by multi-step engineering✓
Single-step editing and integration Data presented at ASH, December 2022, ASGCT, May 2023 and ASH, December 2023 25
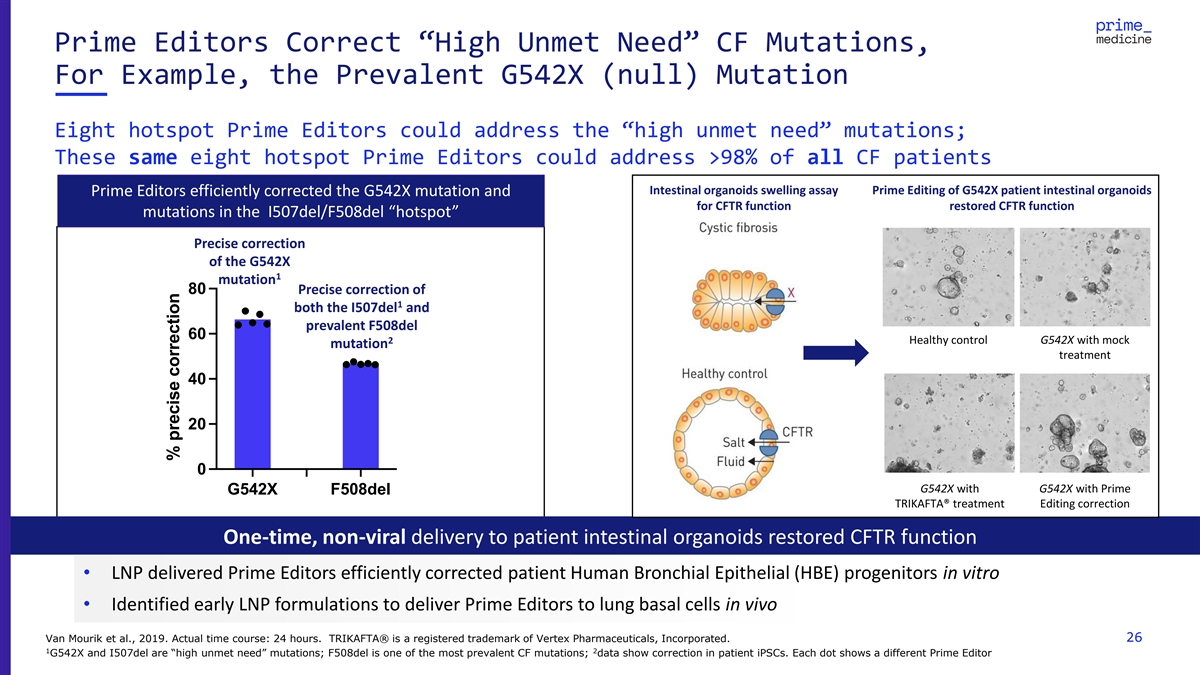
Prime Editors Correct “High Unmet Need” CF Mutations, For
Example, the Prevalent G542X (null) Mutation Eight hotspot Prime Editors could address the “high unmet need” mutations; These same eight hotspot Prime Editors could address >98% of all CF patients Intestinal organoids swelling assay
Prime Editing of G542X patient intestinal organoids Prime Editors efficiently corrected the G542X mutation and for CFTR function restored CFTR function mutations in the I507del/F508del “hotspot” Precise correction of the G542X 1 mutation
80 Precise correction of 1 both the I507del and prevalent F508del 60 2 Healthy control G542X with mock mutation treatment 40 20 0 G542X with G542X with Prime G542X F508del TRIKAFTA® treatment Editing correction One-time, non-viral delivery to
patient intestinal organoids restored CFTR function • LNP delivered Prime Editors efficiently corrected patient Human Bronchial Epithelial (HBE) progenitors in vitro • Identified early LNP formulations to deliver Prime Editors to lung
basal cells in vivo Van Mourik et al., 2019. Actual time course: 24 hours. TRIKAFTA® is a registered trademark of Vertex Pharmaceuticals, Incorporated. 26 1 2 G542X and I507del are “high unmet need” mutations; F508del is one of the
most prevalent CF mutations; data show correction in patient iPSCs. Each dot shows a different Prime Editor % precise correction

Prime Medicine Holds Extensive Intellectual Property for Prime Editing
Technologies Prime Medicine’s IP includes: • Multiple configurations of RNA-templated gene editing - Prime Editor protein configurations: fusion, separate and split configurations - pegRNA configurations: fusion, split, separate and
engineered configurations - Dual flap and dual guide RNA editing systems • Broad diversity of RNA-templated gene editing systems Seminal Prime Dual Flap Prime Editing PASSIGE System - Large variety of nucleic acid programmable DNA binding
proteins Editing Publication - Extensive range of RNA-dependent DNA polymerases (reverse transcriptases) • PASSIGE™: System using Prime Editing and recombinase to insert gene- sized DNA at chosen target location in genome - PASSIGE
systems include various gene editing configurations and recombinases • Additional gene editing technology including DNA-dependent DNA PE4, PE5, and PEmax Engineered pegRNAs pegRNA Enhancements polymerase editing • Program-specific patent
filings for all pipeline programs Prime Medicine has 3 issued US patents and 1 allowed US application - Numerous pending applications worldwide with broad coverage Novel PE Proteins Improved recombinases - Aggressive filing strategy covering
technological advances 27

Key Upcoming Events will Drive Prime Medicine Forward, Support Our
Maturation into a Clinical-Stage Company Summary of key ongoing activities and planned next steps for Prime Medicine in 2024-2025 Hematology & Immunology - Open IND and/or CTA for Phase 1/2 study in Chronic Granulomatous disease in 1H 2024, with
anticipated initial clinical data in 2025 - Advance Shielded HSC and Immunotherapy Pairs (SCIP) technology, establish proof-of-concept in HSC and immunotherapy, and identify first clinical program(s) with this approach in 2024 - Advance Prime
Medicine’s differentiated CAR-T program (using PASSIGE™) into lead optimization Liver Pipeline - Continue to advance preclinical studies for our 3 liver programs, and initiate IND-enabling activities for at least one in 2024, leading to
an IND/CTA in 2H 2025/1H 2026 Ocular - Nominate development candidate for Retinitis Pigmentosa / Rhodopsin (RHO) in 2024 and initiate IND-enabling activities in 2024 Neuromuscular - Continue to advance Friedreich’s Ataxia, and advance one
other program into lead optimization in 2024 Delivery - Nominate first development candidate using Prime Medicine’s liver-targeted universal LNP platform in 2024 - In large animal studies, establish AAV delivery platform and a route of
administration for neuromuscular programs in 2024 Platform Regulatory - Advance discussions with Regulatory agencies on platform strategy for streamlined development As of September 30, 2023, Prime Medicine had cash, cash equivalents, and
investments of $165.3 million, excluding restricted cash, or $178.8 million, including restricted cash IND = investigational new drug; CTA = clinical trial application; HSC = hematopoietic stem cell 28

Delivering on the promise of Prime Editing primemedicine.com
29
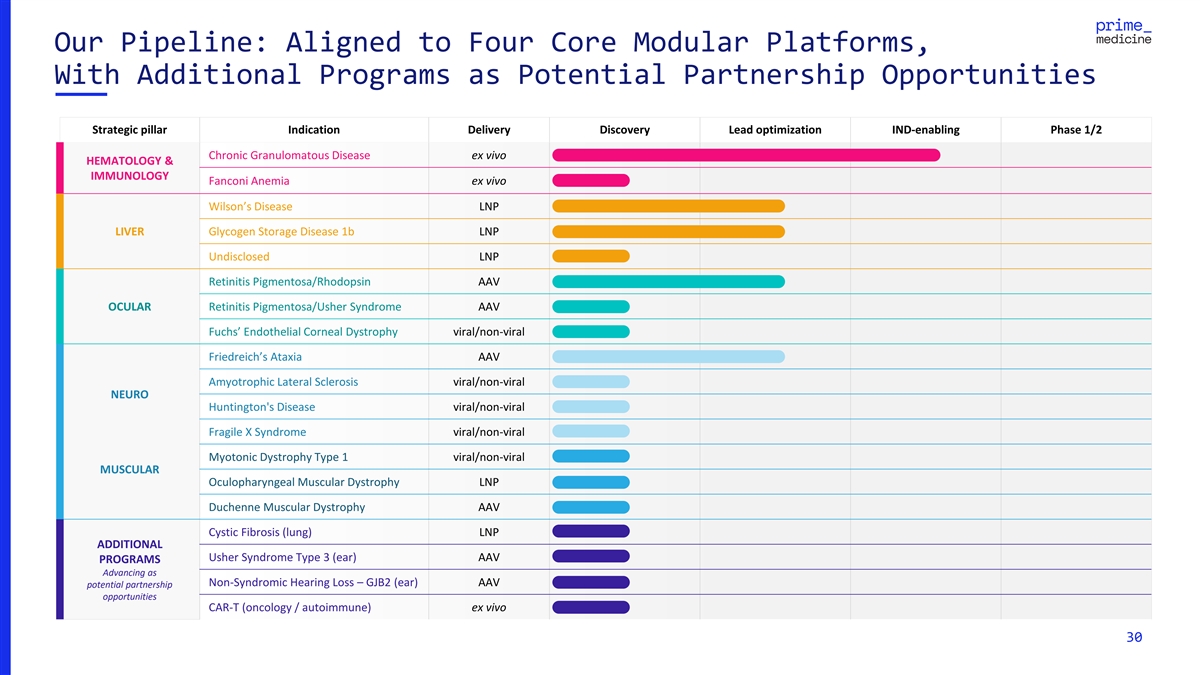
Our Pipeline: Aligned to Four Core Modular Platforms, With Additional
Programs as Potential Partnership Opportunities Strategic pillar Indication Delivery Discovery Lead optimization IND-enabling Phase 1/2 Chronic Granulomatous Disease ex vivo HEMATOLOGY & IMMUNOLOGY Fanconi Anemia ex vivo Wilson’s Disease
LNP LIVER Glycogen Storage Disease 1b LNP Undisclosed LNP Retinitis Pigmentosa/Rhodopsin AAV OCULAR Retinitis Pigmentosa/Usher Syndrome AAV Fuchs’ Endothelial Corneal Dystrophy viral/non-viral Friedreich’s Ataxia AAV Amyotrophic Lateral
Sclerosis viral/non-viral NEURO Huntington's Disease viral/non-viral Fragile X Syndrome viral/non-viral Myotonic Dystrophy Type 1 viral/non-viral MUSCULAR Oculopharyngeal Muscular Dystrophy LNP Duchenne Muscular Dystrophy AAV Cystic Fibrosis (lung)
LNP ADDITIONAL Usher Syndrome Type 3 (ear) AAV PROGRAMS Advancing as Non-Syndromic Hearing Loss – GJB2 (ear) AAV potential partnership opportunities CAR-T (oncology / autoimmune) ex vivo 30
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Grafico Azioni Prime Medicine (NASDAQ:PRME)
Storico
Da Feb 2025 a Mar 2025

Grafico Azioni Prime Medicine (NASDAQ:PRME)
Storico
Da Mar 2024 a Mar 2025
