false000178222300017822232025-03-182025-03-18
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): March 18, 2025 |
Pyxis Oncology, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-40881 |
83-1160910 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
321 Harrison Avenue |
|
Boston, Massachusetts |
|
02118 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: (617) 453-3596 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, par value $0.001 per share |
|
PYXS |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Item 2.02 Results of Operations and Financial Condition.
On March 18, 2025, Pyxis Oncology, Inc. (“the Company”) issued a press release announcing its financial results for the full year ended December 31, 2024 and provided a corporate update. A copy of the press release is furnished as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
Item 5.02. Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers.
On March 18, 2025, the Company announced that Ken Kobayashi, M.D., F.A.C.P, is stepping down from his position as the Company's Chief Medical Officer. His resignation will be effective as of March 18, 2025 and is not associated with, or attributable to, any disagreement regarding any matter related to the Company's operations, policies or practices. Lara S. Sullivan, M.D., President and Chief Executive Officer will assume the role of Chief Medical Officer along with her current role as President and Chief Executive Officer.
Item 7.01 Regulation FD Disclosure.
On March 18, 2025, the Company made available an updated corporate presentation on the Company’s website. A copy of the corporate presentation is furnished as Exhibit 99.2 to this Current Report on Form 8-K and is incorporated herein by reference.
The information contained in Items 2.02 and 7.01 of this Current Report, including Exhibits 99.1 and 99.2, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference into any other filing with the U.S. Securities and Exchange Commission made by the Company, regardless of any general incorporation language in such filings.
Item 9.01 Financial Statements and Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
|
Pyxis Oncology, Inc. |
|
|
|
|
Date: |
March 18, 2025 |
By: |
/s/ Pamela Connealy |
|
|
|
Pamela Connealy
Chief Financial Officer and Chief Operating Officer
|
Exhibit 99.1
Pyxis Oncology Reports Fourth Quarter and Full Year 2024 Financial Results and Provides Business Update
-Recently reported positive preliminary data from Phase 1 dose escalation trial of micvotabart pelidotin (“MICVO,” formerly PYX-201), including a confirmed 50% objective response rate by RECIST 1.1 in recurrent and metastatic head and neck squamous cell carcinoma (R/M HNSCC)
-Received Fast Track Designation from the U.S. Food and Drug Administration for MICVO for the treatment of adult patients with R/M HNSCC whose disease has progressed following treatment with platinum-based chemotherapy and an anti-PD-(L)1 therapy
-Initiated monotherapy expansion cohorts of MICVO for 2L and 3L R/M HNSCC patients who have received prior platinum-based chemotherapy and prior PD-(L)1 inhibitor therapy with preliminary data expected in 2H25 and 2/3L R/M HNSCC patients who have received prior EGFRi and PD-1 inhibitor therapy with preliminary data expected 1H26
-Initiated MICVO in combination with Merck’s anti-PD-1 therapy, KEYTRUDA® (pembrolizumab), in 1/2L+ R/M HNSCC patients as part of a recently announced Clinical Trial Collaboration Agreement with Merck (known as MSD outside of the US and Canada) with preliminary data expected in 2H25
-Streamlined organization and implemented operational initiatives to focus resources on the execution of the MICVO clinical program, including workforce reduction of approximately 20%
-Expected cash runway into 2H26
BOSTON, March 18, 2025 (GLOBE NEWSWIRE) — Pyxis Oncology, Inc. (Nasdaq: PYXS), a clinical-stage company developing next-generation therapeutics for difficult-to-treat cancers, today reported financial results for the year and quarter ended December 31, 2024, and provided a business update.
“We are committed to the development of a novel therapy for patients with recurrent or metastatic head and neck squamous cell carcinoma who will progress following platinum-based therapies and prior PD-(L)1 therapy, and those that progress after current and emerging EGFRi therapies,” said Lara S. Sullivan, M.D., President and Chief Executive Officer. “We look forward to expanding upon the encouraging safety and efficacy results observed from our Phase 1 trial evaluating micvotabart pelidotin, and we believe targeting Extradomain-B Fibronectin (EDB+FN) will offer a novel approach to addressing the limitations of existing therapies.”
“Given the positive micvotabart pelidotin data, it is critical that we ensure the flawless execution of our clinical programs on the fastest possible timeline,” said Dr. Sullivan. “To support this goal, we have streamlined our organization to allocate resources in a way that gives us the greatest opportunity to deliver on our mission and bring meaningful therapies to patients who need them most. I am confident that our focused approach will drive value for both patients and shareholders,” concluded Dr. Sullivan.
Pipeline Updates
In 2024 the Company established that its lead therapeutic candidate, micvotabart pelidotin (MICVO, formerly referred to as PYX-201), has profound monotherapy effect on multiple tumor types with significant tumor regression demonstrated during the Phase 1 dose escalation study. MICVO is a first-in-concept antibody-drug conjugate antibody-drug conjugate (ADC) that targets EDB+FN, a non-cellular structural component of the tumor extra-cellular matrix.
•Recently reported positive preliminary data from the ongoing Phase 1 dose-escalation trial of micvotabart pelidotin evaluating its safety and efficacy in multiple solid tumor types. In six heavily pretreated HPV-positive and HPV-negative efficacy evaluable patients who had received a median of four prior lines of therapy with R/M HNSCC, micvotabart pelidotin achieved a confirmed 50% objective response rate (ORR) based on RECIST 1.1 criteria, including one complete response and a disease control rate (DCR) of 100%.
•Initiated Part 2 monotherapy expansion cohorts of the ongoing Phase 1 clinical trial to evaluate micvotabart pelidotin in 2L and 3L R/M HNSCC patients who have received prior platinum and PD-1 inhibitor therapy, and 2L and 3L R/M HNSCC patients who have received prior EGFRi and PD-1 inhibitor therapy. Preliminary data from patients who have received prior platinum and PD-1 inhibitor therapy are expected in the second half of 2025 and preliminary data from patients who have received prior EGFRi and PD-1 inhibitor therapy are expected in the first half of 2026. R/M HNSCC continues to be an area of high medical need despite improvements in treatment options.
•Initiated Phase 1/2 combination study of micvotabart pelidotin and Merck’s anti-PD-1 therapy, KEYTRUDA® (pembrolizumab), in patients with R/M HNSCC and other advanced solid tumors. We aim to select a dose of micvotabart pelidotin in combination with pembrolizumab by mid-year 2025 and share preliminary data from the trial in the second half of 2025.
KEYTRUDA® is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
•Received Fast Track Designation from the U.S. Food and Drug Administration (FDA) for micvotabart pelidotin for the treatment of adult patients with R/M HNSCC whose disease has progressed following treatment with platinum-based chemotherapy and an anti-PD-(L)1 therapy.
•In December 2024, suspended further development of PYX-106 — a fully human IgG1 monoclonal antibody targeting Siglec-15 to allocate resources toward advancing micvotabart pelidotin.
Business Updates
•Pyxis Oncology recently announced a portfolio prioritization, focusing resources on advancing its lead clinical program, micvotabart pelidotin. In connection with the portfolio prioritization, the Company today announced it has reduced its workforce by approximately 20%, with a majority of the headcount reductions from the Company’s G&A and preclinical group. In addition, Ken Kobayashi, M.D., F.A.C.P, is stepping down as Chief Medical Officer and Lara S. Sullivan, M.D., President and Chief Executive Officer will assume the role of Chief Medical Officer along with her current role as President and Chief Executive Officer.
Full Year 2024 Financial Results
•As of December 31, 2024, Pyxis Oncology had cash and cash equivalents, including restricted cash, and short-term investments, of $128.4 million. The Company believes that its current cash, cash equivalents, and short-term investments will be sufficient to fund its operations into the second half of 2026.
•Research and development expenses were $58.7 million for the year ended December 31, 2024, compared to $49.6 million for the year ended December 31, 2023. The increase was primarily due to increased clinical trial-related expenses, including manufacturing of drug product and drug substance for Phase 1 clinical trials of micvotabart pelidotin and the recently attrited PYX-106 asset.
•General and administrative expenses were $25.4 million for the year ended December 31, 2024, compared to $32.6 million for the year ended December 31, 2023. The decrease was primarily due to lower employee costs including stock-based compensation and decrease in legal, professional and consulting fees.
•During the fourth quarter of 2024, Pyxis Oncology recorded a non-cash impairment loss of $21.0 million for in-process research and development (IPR&D) intangible asset related to PYX-107, which was acquired by the Company in August 2023 as part of the acquisition of Apexigen. The impairment loss was mainly due to de-prioritization of clinical development of PYX-107. Despite the impairment loss, acquisition of Apexigen remains a net accretive transaction for the Company wherein we received $9.5 million of cash since acquisition from the sale of royalty rights and royalty payments.
•Net loss was $77.3 million, or ($1.32) per common share, for the year ended December 31, 2024, compared to $73.8 million, or ($1.85) per common share, for the year ended December 31, 2023. Excluding non-cash stock-based compensation expense and impairment loss, the net loss for the year ended December 31, 2024, was $43.4 million, compared to net loss of $56.8 million for the year ended December 31, 2023.
•As of March 17, 2025, the outstanding number of shares of Common Stock of Pyxis Oncology was 61,590,415.
About Pyxis Oncology, Inc.
Pyxis Oncology, Inc. is a clinical stage company focused on defeating difficult-to-treat cancers. The company is efficiently building next generation therapeutics that hold the potential for monotherapy and combination indications. The lead product candidate, micvotobart pelidotin (“MICVO” formerly PYX-201), is an antibody-drug conjugate (ADC) that uniquely targets Extradomain-B Fibronectin (EDB+FN), a non-cellular structural component of the tumor extra-cellular matrix. MICVO has been evaluated in ongoing Phase 1 clinical studies in multiple types of solid tumors with a go-forward development focus on treating patients with recurrent and metastatic head and neck squamous cell carcinoma (R/M HNSCC) based on the strength of the HNSCC signal that emerged. MICVO is designed to generate a multi-pronged attack on difficult-to-treat cancers by directly killing cancer cells, reducing extra-cellular matrix (ECM) density, inhibiting tumor angiogenesis and mobilizing an anti-tumor immune response.
To learn more, visit www.pyxisoncology.com or follow us on X (formerly known as Twitter) and LinkedIn.
Forward Looking Statements
This press release contains forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. These statements are often identified by the use of words such as “anticipate,” “believe,” “can,” “continue,” “could,” “estimate,” “expect,” “intend,” “likely,” “may,” “might,” “objective,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “to be,” “will,” “would,” or the negative or plural of these words, or similar expressions or variations, although not all forward-looking statements contain these words. We cannot assure you that the events and circumstances reflected in the forward-looking statements will be achieved or occur and actual results could differ materially from those expressed or implied by these forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those identified herein, and those discussed in the section titled “Risk Factors” set forth in Part II, Item 1A. of the Company’s Annual Report on Form 10-K filed with SEC on March 18, 2025, and our other filings, each of which is on file with the Securities and Exchange Commission. These risks are not exhaustive. New risk factors emerge from time to time, and it is not possible for our management to predict all risk factors, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date hereof and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain, and investors are cautioned not to unduly rely upon these statements. Except as required by law, we undertake no obligation to update any forward-looking statements to reflect events or circumstances after the date of such statements.
Pyxis Oncology Contact
Pamela Connealy
CFO and COO
ir@pyxisoncology.com
PYXIS ONCOLOGY, INC.
Consolidated Statements of Operations and Comprehensive Loss
(In thousands, except share and per share amounts)
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|
|
|
2024 |
|
|
2023 |
|
Revenues |
|
|
|
|
|
|
Royalty revenues |
|
$ |
8,146 |
|
|
$ |
— |
|
Sale of royalty rights |
|
|
8,000 |
|
|
|
— |
|
Total revenues |
|
|
16,146 |
|
|
|
— |
|
Costs and operating expenses |
|
|
|
|
|
|
Cost of revenues |
|
|
475 |
|
|
|
— |
|
Research and development |
|
|
58,747 |
|
|
|
49,586 |
|
General and administrative |
|
|
25,420 |
|
|
|
32,610 |
|
Impairment of in-process research and development intangible asset |
|
|
20,964 |
|
|
|
— |
|
Total costs and operating expenses |
|
|
105,606 |
|
|
|
82,196 |
|
Loss from operations |
|
|
(89,460 |
) |
|
|
(82,196 |
) |
Other income, net |
|
|
|
|
|
|
Interest and investment income |
|
|
7,039 |
|
|
|
6,630 |
|
Sublease income |
|
|
2,926 |
|
|
|
1,776 |
|
Total other income, net |
|
|
9,965 |
|
|
|
8,406 |
|
Loss before income taxes |
|
|
(79,495 |
) |
|
|
(73,790 |
) |
Income tax benefit |
|
|
(2,164 |
) |
|
|
— |
|
Net loss |
|
$ |
(77,331 |
) |
|
$ |
(73,790 |
) |
Net loss per common share - basic and diluted |
|
$ |
(1.32 |
) |
|
$ |
(1.85 |
) |
Weighted average shares of common stock outstanding - basic and diluted |
|
|
58,445,765 |
|
|
|
39,904,603 |
|
PYXIS ONCOLOGY, INC.
Consolidated Balance Sheets
(In thousands, except share and per share amounts)
|
|
|
|
|
|
|
|
|
|
|
December 31, 2024 |
|
|
December 31, 2023 |
|
Assets |
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
19,473 |
|
|
$ |
9,664 |
|
Marketable debt securities, short-term |
|
|
107,458 |
|
|
|
109,634 |
|
Restricted cash |
|
|
1,472 |
|
|
|
1,472 |
|
Prepaid expenses and other current assets |
|
|
4,037 |
|
|
|
3,834 |
|
Total current assets |
|
|
132,440 |
|
|
|
124,604 |
|
Property and equipment, net |
|
|
9,899 |
|
|
|
11,872 |
|
Intangible assets, net |
|
|
2,600 |
|
|
|
24,308 |
|
Operating lease right-of-use asset |
|
|
12,242 |
|
|
|
12,942 |
|
Total assets |
|
$ |
157,181 |
|
|
$ |
173,726 |
|
Liabilities and Stockholders’ Equity |
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
Accounts payable |
|
$ |
4,859 |
|
|
$ |
3,896 |
|
Accrued expenses and other current liabilities |
|
|
11,371 |
|
|
|
12,971 |
|
Operating lease liabilities, current portion |
|
|
1,450 |
|
|
|
1,232 |
|
Deferred revenues |
|
|
— |
|
|
|
7,660 |
|
Total current liabilities |
|
|
17,680 |
|
|
|
25,759 |
|
Operating lease liabilities, net of current portion |
|
|
18,650 |
|
|
|
20,099 |
|
Financing lease liabilities, net of current portion |
|
|
100 |
|
|
|
— |
|
Deferred tax liability, net |
|
|
— |
|
|
|
2,164 |
|
Total liabilities |
|
|
36,430 |
|
|
|
48,022 |
|
Commitments and contingencies |
|
|
|
|
|
|
Stockholders’ equity: |
|
|
|
|
|
|
Preferred stock |
|
|
— |
|
|
|
— |
|
Common stock |
|
|
60 |
|
|
|
45 |
|
Additional paid-in capital |
|
|
484,077 |
|
|
|
411,821 |
|
Accumulated other comprehensive income |
|
|
170 |
|
|
|
63 |
|
Accumulated deficit |
|
|
(363,556 |
) |
|
|
(286,225 |
) |
Total stockholders’ equity |
|
|
120,751 |
|
|
|
125,704 |
|
Total liabilities and stockholders’ equity |
|
$ |
157,181 |
|
|
$ |
173,726 |
|

Building a Differentiated ADC Company Nasdaq: PYXS March 18, 2025 Exhibit 99.2

Forward Looking Statement This presentation contains forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. All statements other than statements of historical facts contained in this presentation and press release, including without limitation statements regarding the Company's plans to develop, manufacture and commercialize its product candidates, including micvotabart pelidotin, formerly referred to as PYX-201; initial results, timing and progress of the Company's ongoing clinical trials; the expected results of the Company's clinical trials including those of its lead product candidate, micvotabart pelidotin; the expected benefits of the pipeline prioritization; the ability of initial and topline clinical data to de-risk micvotabart pelidotin and be confirmed with clinical trial progression, including the safety, tolerability, and potential efficacy of micvotabart pelidotin; the potential differentiation, advantage or effectiveness of micvotabart pelidotin compared to other approved products or products in development; the dosage and treatment potential of micvotabart pelidotin; the size and future of the market; the plans and objectives of management, and the future results of operations and financial position of the Company, are forward-looking statements. These statements are neither promises nor guarantees, but are statements that involve known and unknown risks, uncertainties and other important factors that are in some cases beyond the Company's control that may cause actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: the risks inherent in drug research and development, the Company's projected cash runway and potential needs for additional funding; the lengthy, expensive, and uncertain process of clinical drug development, including potential delays in or failure to obtain regulatory approvals; the Company's reliance on third parties and collaborators to conduct clinical trials, manufacture their product candidates, and develop and commercialize their product candidates; and the Company's ability compete successfully against other drug candidates. Accordingly, investors should not rely upon forward-looking statements as predictions of future events. Except as required by applicable law, the Company undertakes no obligation to update publicly or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. Factors that could cause or contribute to differences include, but are not limited to, those identified herein, and those discussed in the section titled “Risk Factors” set forth in Part II, Item 1A. of the Company’s Annual Report on Form 10-K filed with SEC on March 18, 2025, and our other filings, each of which is on file with the Securities and Exchange Commission. These risks are not exhaustive. New risk factors emerge from time to time, and it is not possible for our management to predict all risk factors, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date hereof and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain, and investors are cautioned not to unduly rely upon these statements. Except as required by law, we undertake no obligation to update any forward-looking statements to reflect events or circumstances after the date of such statements.
Positioned to be a Differentiated ADC Company *Balance sheet as of December 31, 2024 First-in-Concept Extra-cellular ADC Technology Deeply Experienced �Team of Oncology Drug Developers Multiple Clinical Data Catalysts �in 2025 Strong Balance �Sheet* with $128M Cash Runway �into 2H 2026
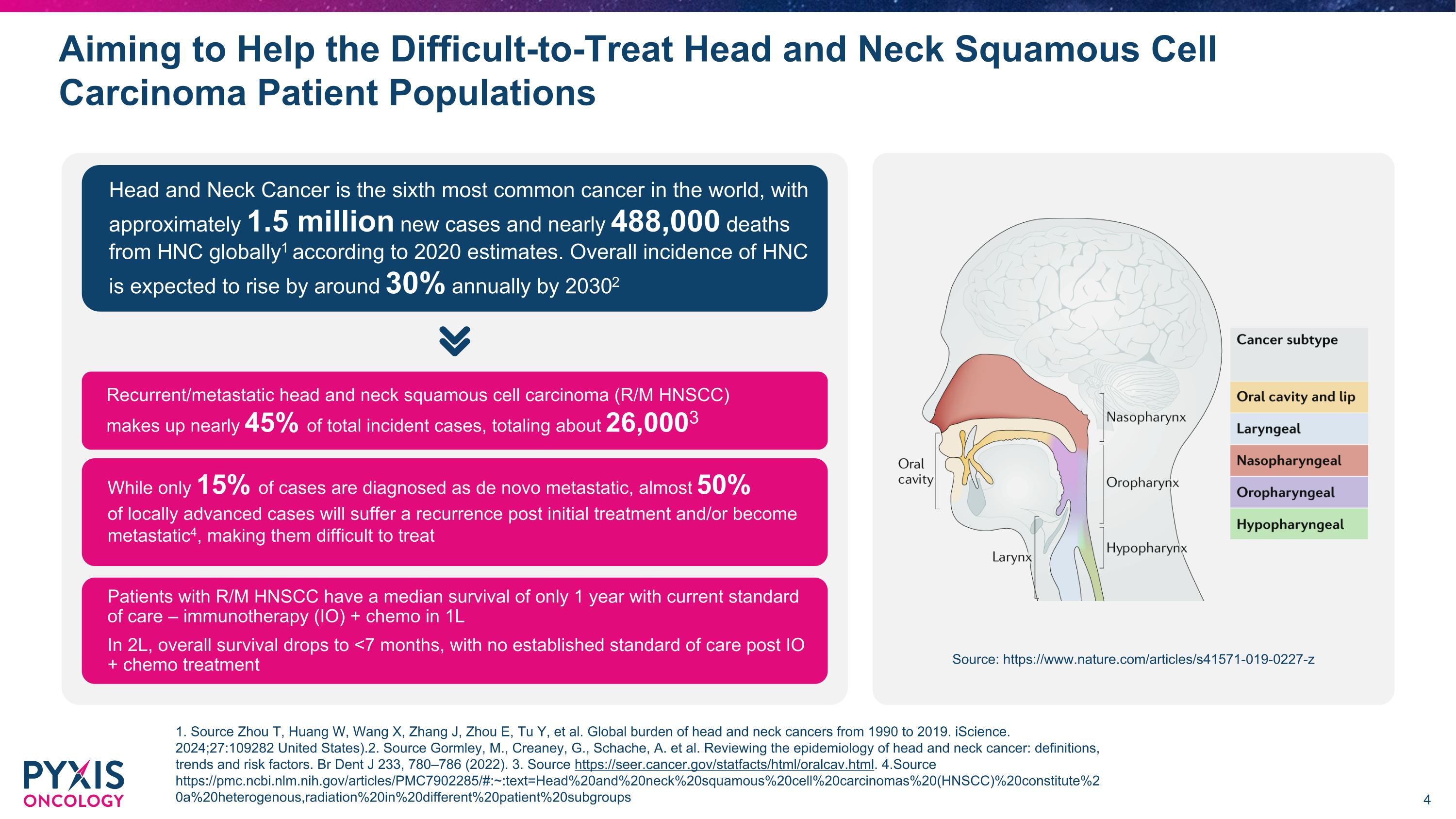
Aiming to Help the Difficult-to-Treat Head and Neck Squamous Cell Carcinoma Patient Populations Head and Neck Cancer is the sixth most common cancer in the world, with approximately 1.5 million new cases and nearly 488,000 deaths from HNC globally1 according to 2020 estimates. Overall incidence of HNC is expected to rise by around 30% annually by 20302 Recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) �makes up nearly 45% of total incident cases, totaling about 26,0003 While only 15% of cases are diagnosed as de novo metastatic, almost 50% �of locally advanced cases will suffer a recurrence post initial treatment and/or become metastatic4, making them difficult to treat Patients with R/M HNSCC have a median survival of only 1 year with current standard �of care – immunotherapy (IO) + chemo in 1L In 2L, overall survival drops to <7 months, with no established standard of care post IO + chemo treatment Source: https://www.nature.com/articles/s41571-019-0227-z 1. Source Zhou T, Huang W, Wang X, Zhang J, Zhou E, Tu Y, et al. Global burden of head and neck cancers from 1990 to 2019. iScience. 2024;27:109282 United States).2. Source Gormley, M., Creaney, G., Schache, A. et al. Reviewing the epidemiology of head and neck cancer: definitions, trends and risk factors. Br Dent J 233, 780–786 (2022). 3. Source https://seer.cancer.gov/statfacts/html/oralcav.html. 4.Source https://pmc.ncbi.nlm.nih.gov/articles/PMC7902285/#:~:text=Head%20and%20neck%20squamous%20cell%20carcinomas%20(HNSCC)%20constitute%20a%20heterogenous,radiation%20in%20different%20patient%20subgroups
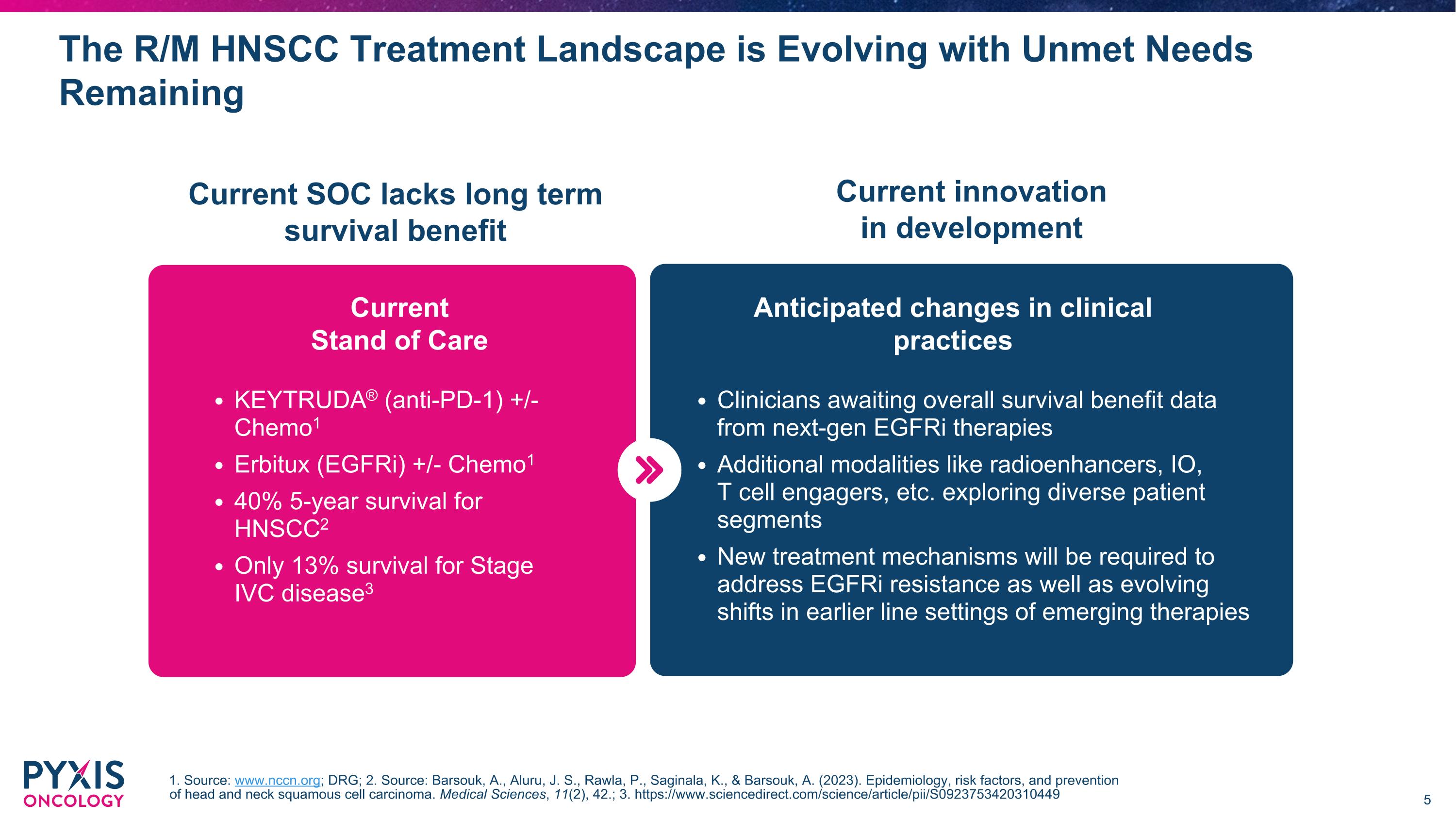
The R/M HNSCC Treatment Landscape is Evolving with Unmet Needs Remaining Current SOC lacks long term survival benefit Current innovation in development Current�Stand of Care Anticipated changes in clinical practices KEYTRUDA® (anti-PD-1) +/- Chemo1 Erbitux (EGFRi) +/- Chemo1 40% 5-year survival for HNSCC2 Only 13% survival for Stage IVC disease3 Clinicians awaiting overall survival benefit data from next-gen EGFRi therapies Additional modalities like radioenhancers, IO, �T cell engagers, etc. exploring diverse patient segments New treatment mechanisms will be required to address EGFRi resistance as well as evolving shifts in earlier line settings of emerging therapies 1. Source: www.nccn.org; DRG; 2. Source: Barsouk, A., Aluru, J. S., Rawla, P., Saginala, K., & Barsouk, A. (2023). Epidemiology, risk factors, and prevention of head and neck squamous cell carcinoma. Medical Sciences, 11(2), 42.; 3. https://www.sciencedirect.com/science/article/pii/S0923753420310449
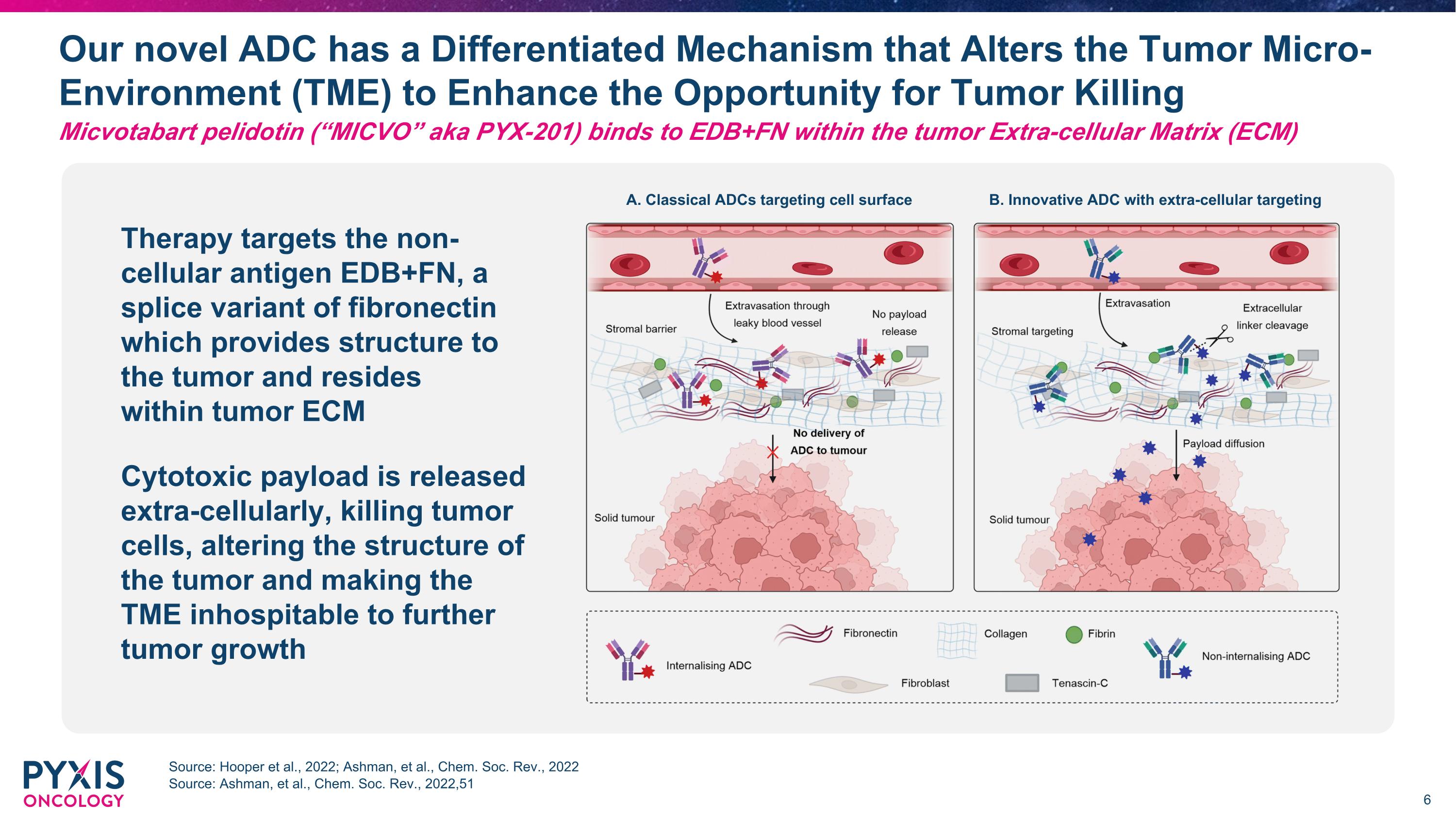
Our novel ADC has a Differentiated Mechanism that Alters the Tumor Micro-Environment (TME) to Enhance the Opportunity for Tumor Killing Source: Hooper et al., 2022; Ashman, et al., Chem. Soc. Rev., 2022 Source: Ashman, et al., Chem. Soc. Rev., 2022,51 Micvotabart pelidotin (“MICVO” aka PYX-201) binds to EDB+FN within the tumor Extra-cellular Matrix (ECM) Therapy targets the non-cellular antigen EDB+FN, a splice variant of fibronectin which provides structure to the tumor and resides within tumor ECM Cytotoxic payload is released extra-cellularly, killing tumor cells, altering the structure of the tumor and making the TME inhospitable to further tumor growth A. Classical ADCs targeting cell surface B. Innovative ADC with extra-cellular targeting Low amount of ADC reaches tumor cells
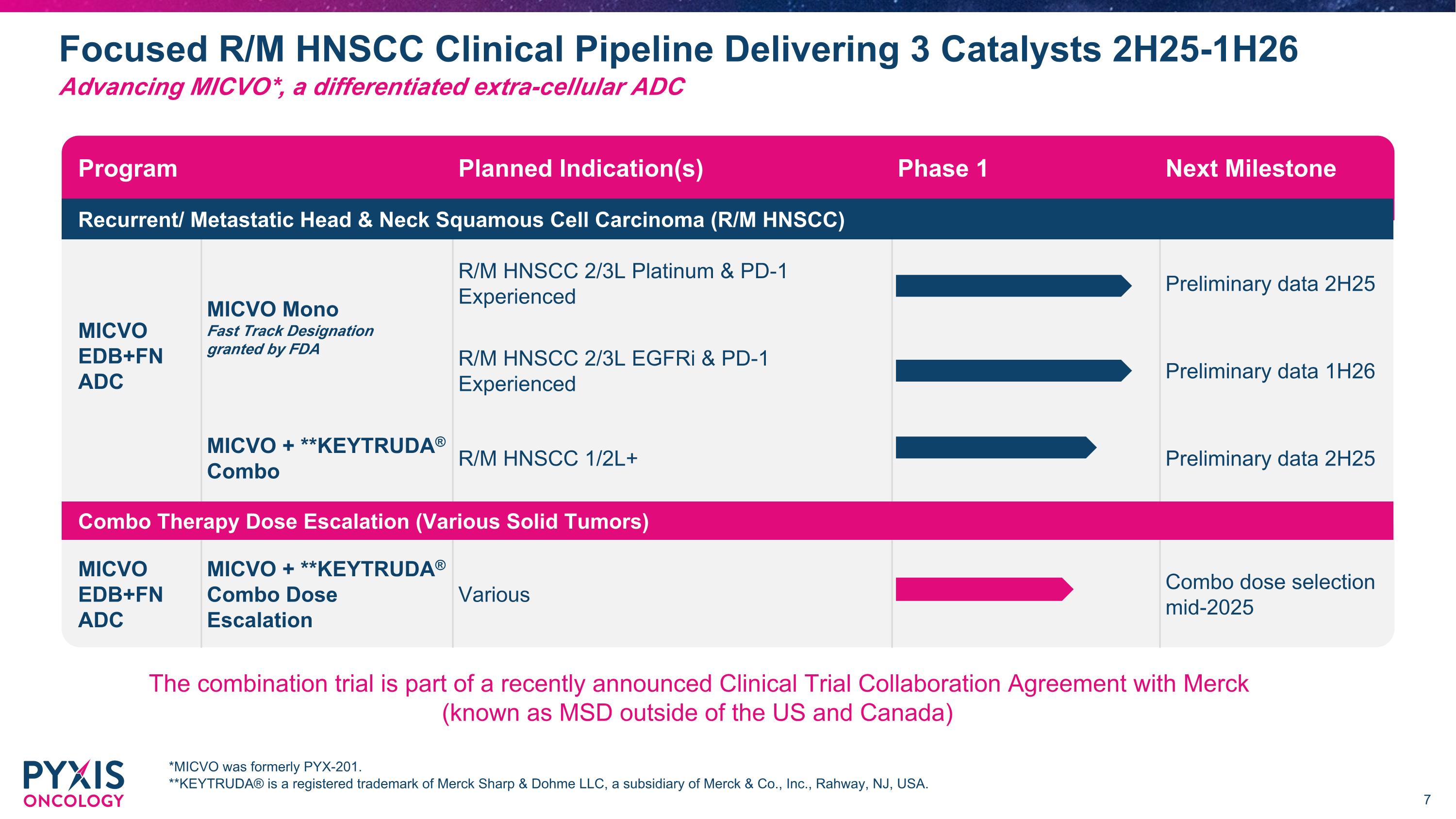
Focused R/M HNSCC Clinical Pipeline Delivering 3 Catalysts 2H25-1H26 *MICVO was formerly PYX-201. **KEYTRUDA® is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Advancing MICVO*, a differentiated extra-cellular ADC Program Planned Indication(s) Phase 1 Next Milestone Recurrent/ Metastatic Head & Neck Squamous Cell Carcinoma (R/M HNSCC) MICVO Mono Fast Track Designation granted by FDA R/M HNSCC 2/3L Platinum & PD-1 Experienced Preliminary data 2H25 MICVO EDB+FN ADC PYX-201 EDB+FN ADC R/M HNSCC 2/3L EGFRi & PD-1 Experienced Preliminary data 1H26 MICVO + **KEYTRUDA® Combo R/M HNSCC 1/2L+ Preliminary data 2H25 Combo Therapy Dose Escalation (Various Solid Tumors) MICVO EDB+FN ADC MICVO + **KEYTRUDA® Combo Dose Escalation Various Combo dose selection mid-2025 The combination trial is part of a recently announced Clinical Trial Collaboration Agreement with Merck (known as MSD outside of the US and Canada)

Leadership Lara Sullivan, MD President and Chief Executive Officer Balu Balasubramanian, PhD Interim Chief Technology Officer Thuy Craveiro, PharmD Head of Clinical Pharmacology Marsha Crochiere, PhD Head of Translational Medicine Shui He, PhD Head Of Biometrics Pam Connealy, MBA Chief Financial Officer and Chief Operating Officer Stephane Marzabal, PhD Interim Global Program Lead Supriya Roth, PhD Head Commercial Development Sondra Smyrnios Head of Global Clinical Operations Jitu Wadhane, CA, CPA Chief Accounting Officer Hongwei Wang, MD, PhD Head of ADC Development & Clinical Strategy Stephen Worsley Chief Business Officer Michael Bui, MD Head Of Global Regulatory Jennifer D’Auteuil Executive Office Administration Board of Directors John Flavin Chairman Lara S. Sullivan, MD President and Chief Executive Officer Thomas Civik Director Darren Cline Director Jakob Dupont, MD Director Rachel Humphrey, MD Director Freda Lewis-Hall, MD Director Michael A. Metzger Director Santhosh Palani, PhD, CFA Director PYXS Team Members Have Collectively Contributed to >60 Oncology Drug Approvals
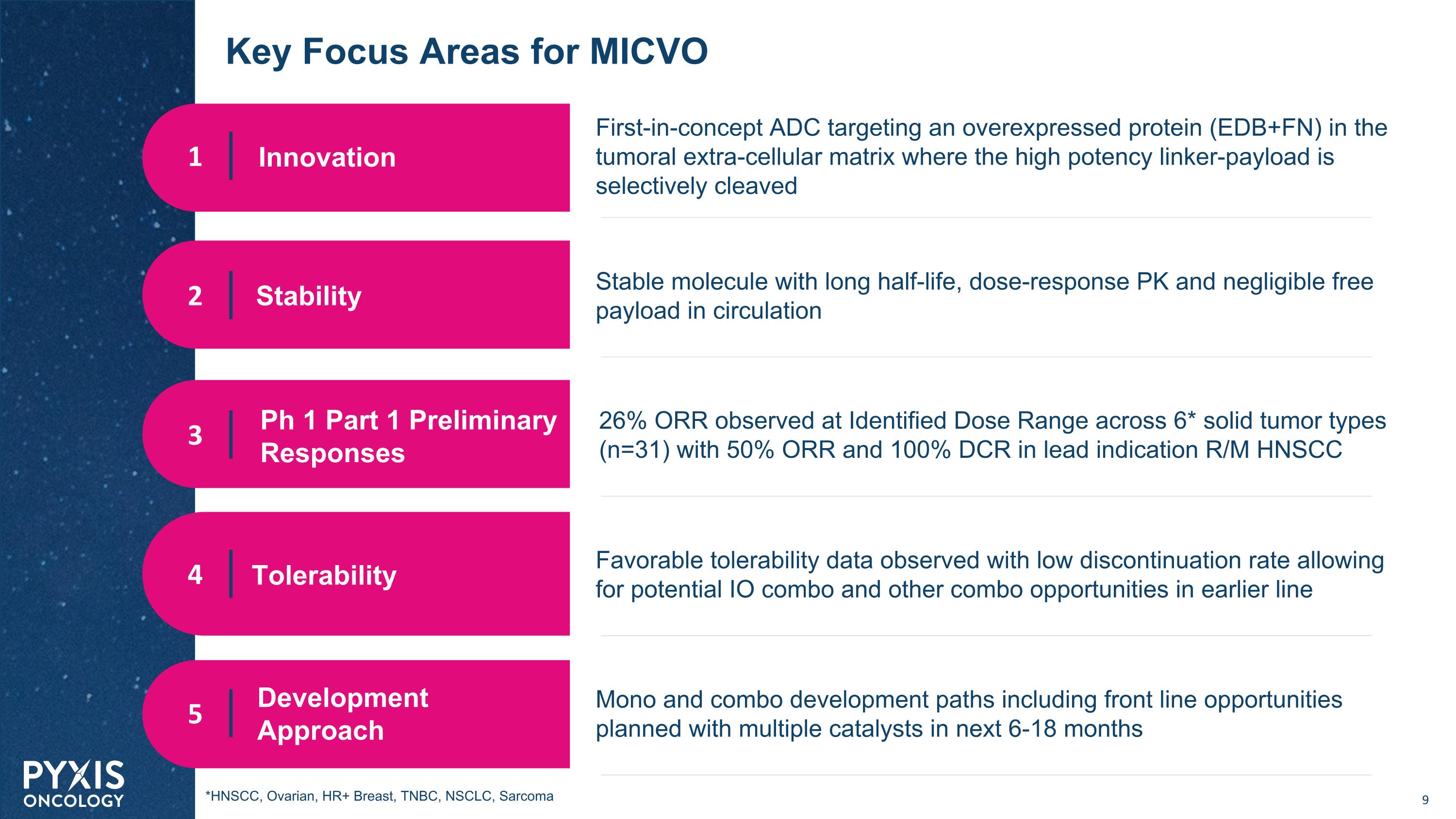
Key Focus Areas for MICVO Innovation Stability Tolerability Ph 1 Part 1 Preliminary Responses Development Approach 1 2 3 4 5 First-in-concept ADC targeting an overexpressed protein (EDB+FN) in the tumoral extra-cellular matrix where the high potency linker-payload is selectively cleaved Stable molecule with long half-life, dose-response PK and negligible free payload in circulation Favorable tolerability data observed with low discontinuation rate allowing for potential IO combo and other combo opportunities in earlier line Mono and combo development paths including front line opportunities planned with multiple catalysts in next 6-18 months *HNSCC, Ovarian, HR+ Breast, TNBC, NSCLC, Sarcoma 26% ORR observed at Identified Dose Range across 6* solid tumor types (n=31) with 50% ORR and 100% DCR in lead indication R/M HNSCC
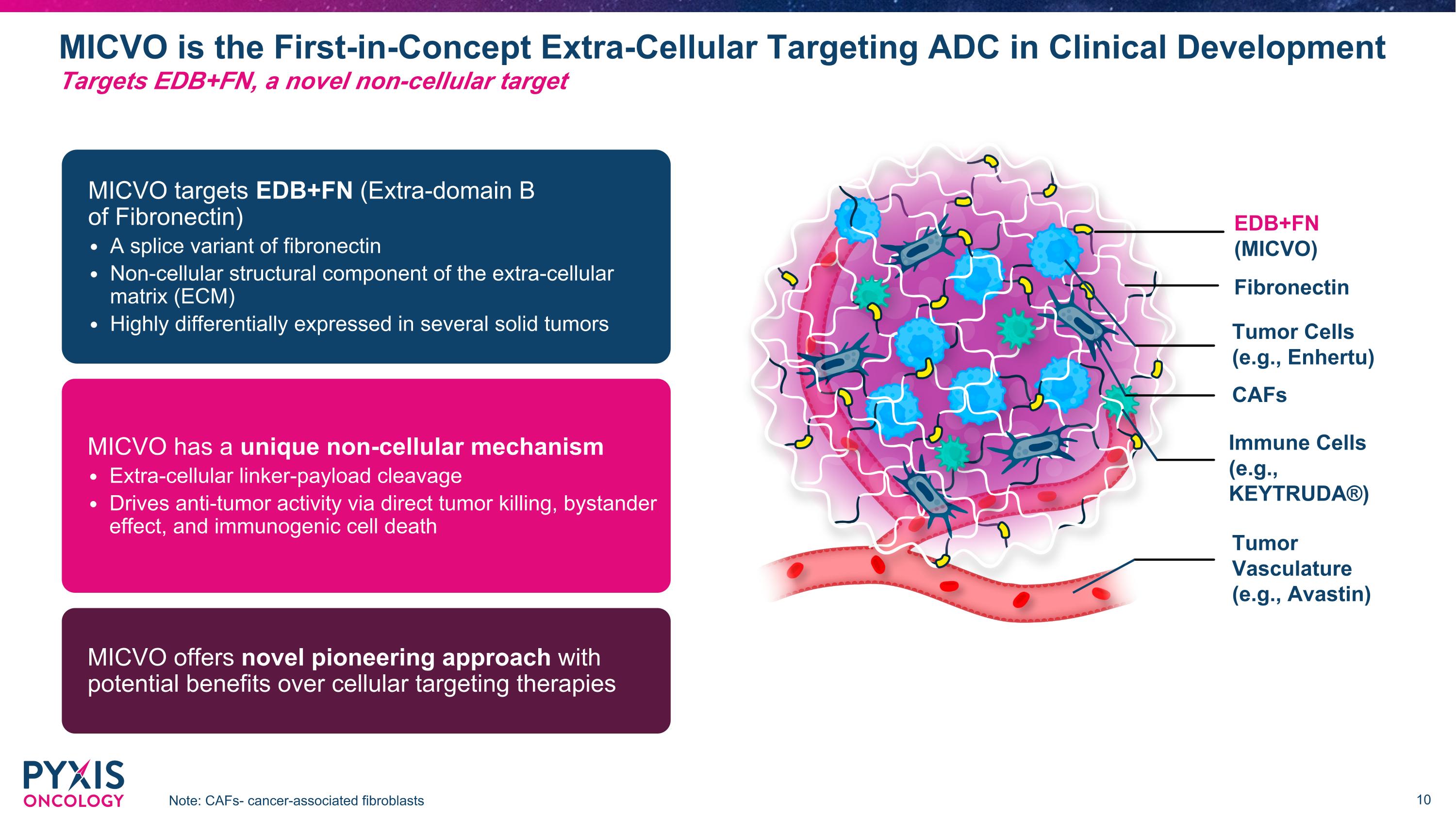
MICVO is the First-in-Concept Extra-Cellular Targeting ADC in Clinical Development Note: CAFs- cancer-associated fibroblasts Targets EDB+FN, a novel non-cellular target Tumor Vasculature (e.g., Avastin) Fibronectin CAFs EDB+FN (MICVO) Immune Cells (e.g., KEYTRUDA®) Tumor Cells (e.g., Enhertu) MICVO targets EDB+FN (Extra-domain B �of Fibronectin) A splice variant of fibronectin Non-cellular structural component of the extra-cellular matrix (ECM) Highly differentially expressed in several solid tumors MICVO has a unique non-cellular mechanism Extra-cellular linker-payload cleavage Drives anti-tumor activity via direct tumor killing, bystander effect, and immunogenic cell death MICVO offers novel pioneering approach with potential benefits over cellular targeting therapies
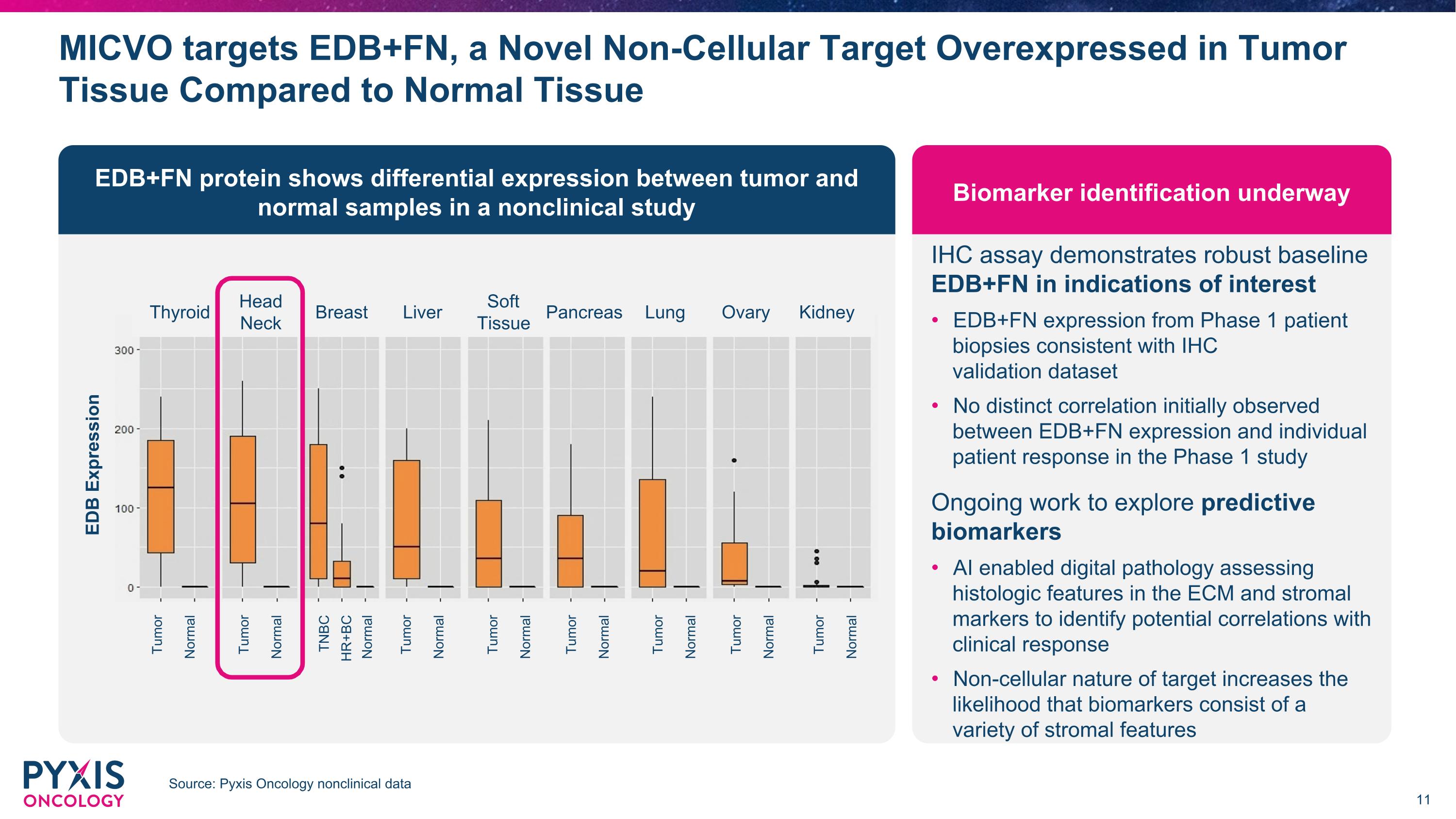
MICVO targets EDB+FN, a Novel Non-Cellular Target Overexpressed in Tumor Tissue Compared to Normal Tissue Source: Pyxis Oncology nonclinical data IHC assay demonstrates robust baseline �EDB+FN in indications of interest EDB+FN expression from Phase 1 patient biopsies consistent with IHC �validation dataset No distinct correlation initially observed between EDB+FN expression and individual patient response in the Phase 1 study Ongoing work to explore predictive biomarkers AI enabled digital pathology assessing histologic features in the ECM and stromal markers to identify potential correlations with clinical response Non-cellular nature of target increases the likelihood that biomarkers consist of a variety of stromal features EDB+FN protein shows differential expression between tumor and normal samples in a nonclinical study Biomarker identification underway EDB Expression Tumor Normal Tumor Normal TNBC Normal HR+BC Tumor Normal Tumor Normal Tumor Normal Tumor Normal Tumor Normal Tumor Normal Thyroid Head Neck Breast Liver Soft Tissue Lung Ovary Kidney Pancreas
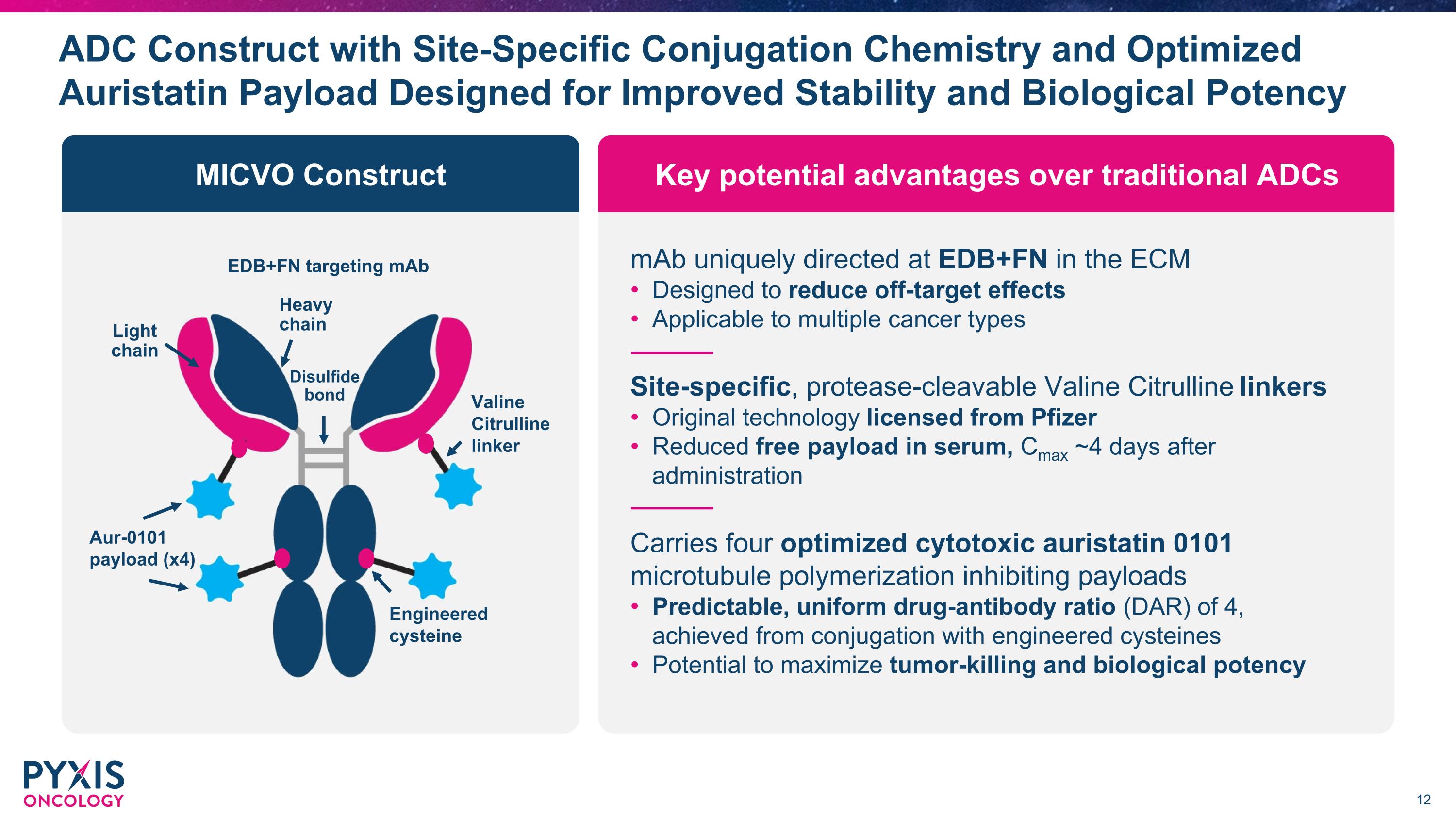
Key potential advantages over traditional ADCs MICVO Construct ADC Construct with Site-Specific Conjugation Chemistry and Optimized Auristatin Payload Designed for Improved Stability and Biological Potency mAb uniquely directed at EDB+FN in the ECM Designed to reduce off-target effects Applicable to multiple cancer types Site-specific, protease-cleavable Valine Citrulline linkers Original technology licensed from Pfizer Reduced free payload in serum, Cmax ~4 days after administration Carries four optimized cytotoxic auristatin 0101 microtubule polymerization inhibiting payloads Predictable, uniform drug-antibody ratio (DAR) of 4, achieved from conjugation with engineered cysteines Potential to maximize tumor-killing and biological potency EDB+FN targeting mAb Aur-0101 payload (x4) Valine Citrulline linker Light chain Heavy chain Engineered cysteine Disulfide bond
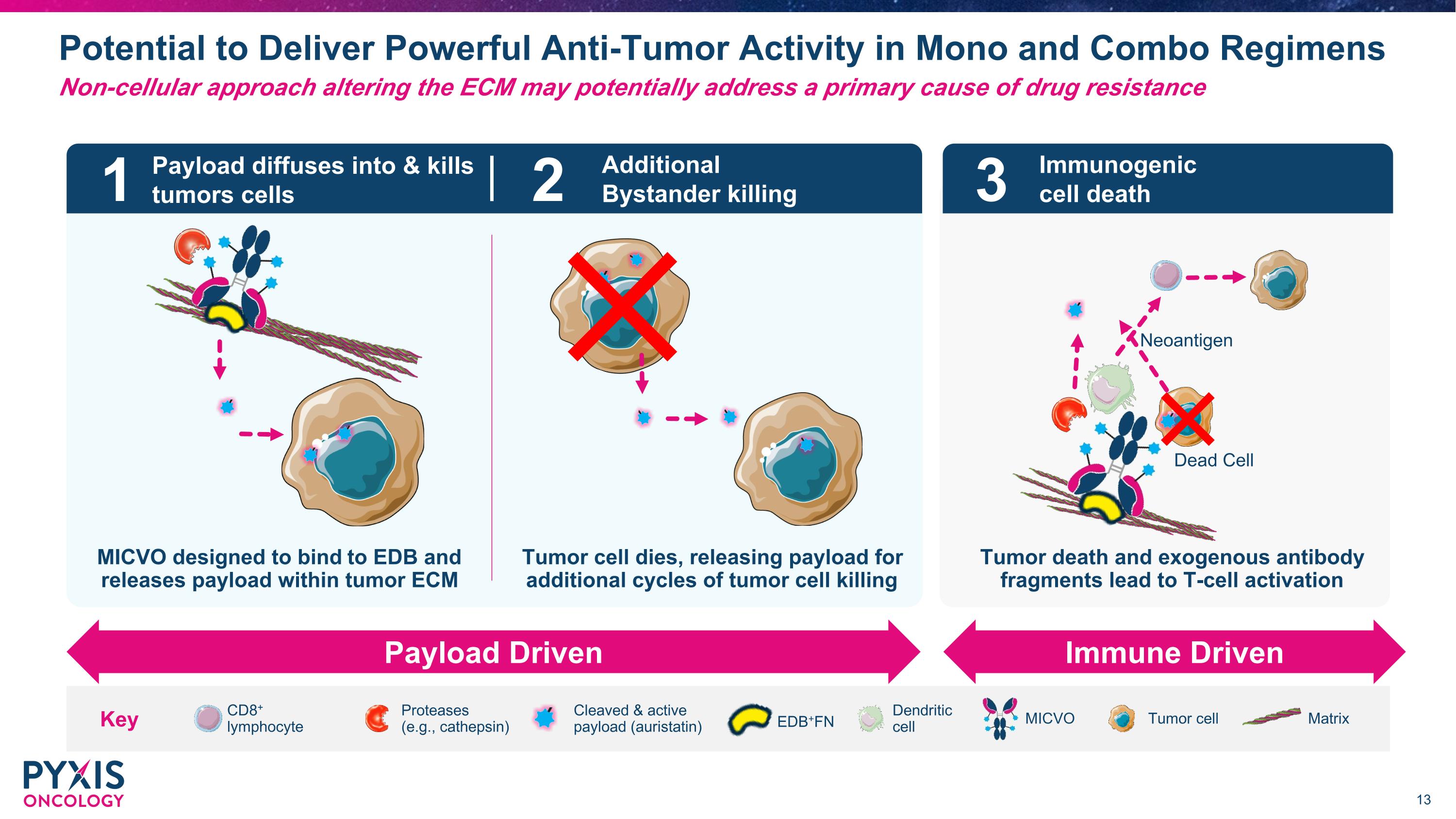
Potential to Deliver Powerful Anti-Tumor Activity in Mono and Combo Regimens Non-cellular approach altering the ECM may potentially address a primary cause of drug resistance MICVO designed to bind to EDB and releases payload within tumor ECM CD8+ lymphocyte Dendritic �cell MICVO Cleaved & active payload (auristatin) Tumor cell Matrix Proteases (e.g., cathepsin) Key 1 2 3 Payload diffuses into & kills tumors cells Additional Bystander killing Immunogenic cell death Payload Driven Immune Driven Dead Cell Neoantigen Tumor cell dies, releasing payload for additional cycles of tumor cell killing Tumor death and exogenous antibody fragments lead to T-cell activation EDB+FN
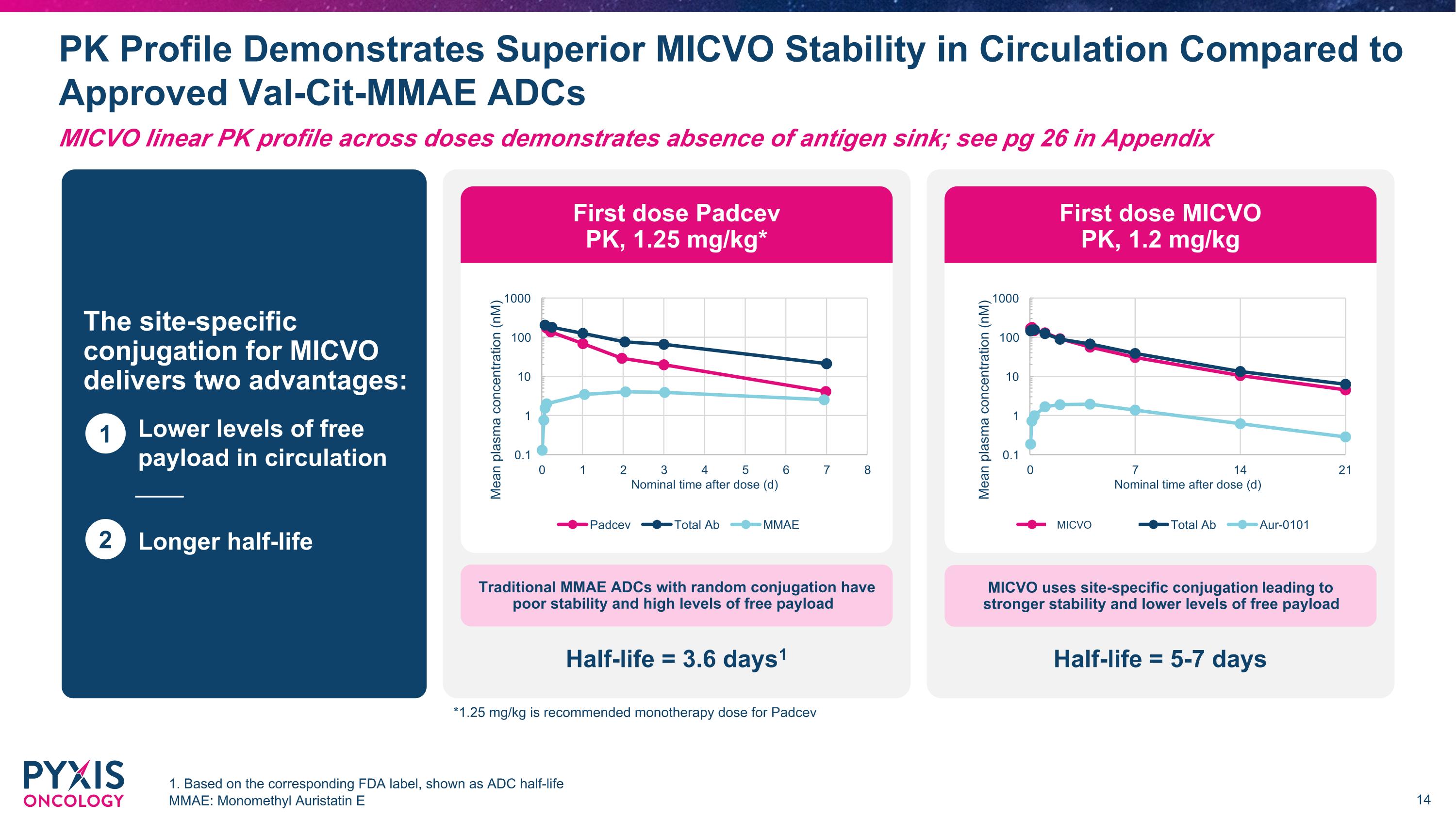
PK Profile Demonstrates Superior MICVO Stability in Circulation Compared to Approved Val-Cit-MMAE ADCs 1. Based on the corresponding FDA label, shown as ADC half-life MMAE: Monomethyl Auristatin E The site-specific conjugation for MICVO delivers two advantages: Lower levels of free payload in circulation Longer half-life 1 2 Traditional MMAE ADCs with random conjugation have poor stability and high levels of free payload MICVO uses site-specific conjugation leading to �stronger stability and lower levels of free payload Half-life = 3.6 days1 Half-life = 5-7 days First dose Padcev �PK, 1.25 mg/kg* First dose MICVO �PK, 1.2 mg/kg MICVO *1.25 mg/kg is recommended monotherapy dose for Padcev MICVO linear PK profile across doses demonstrates absence of antigen sink; see pg 26 in Appendix
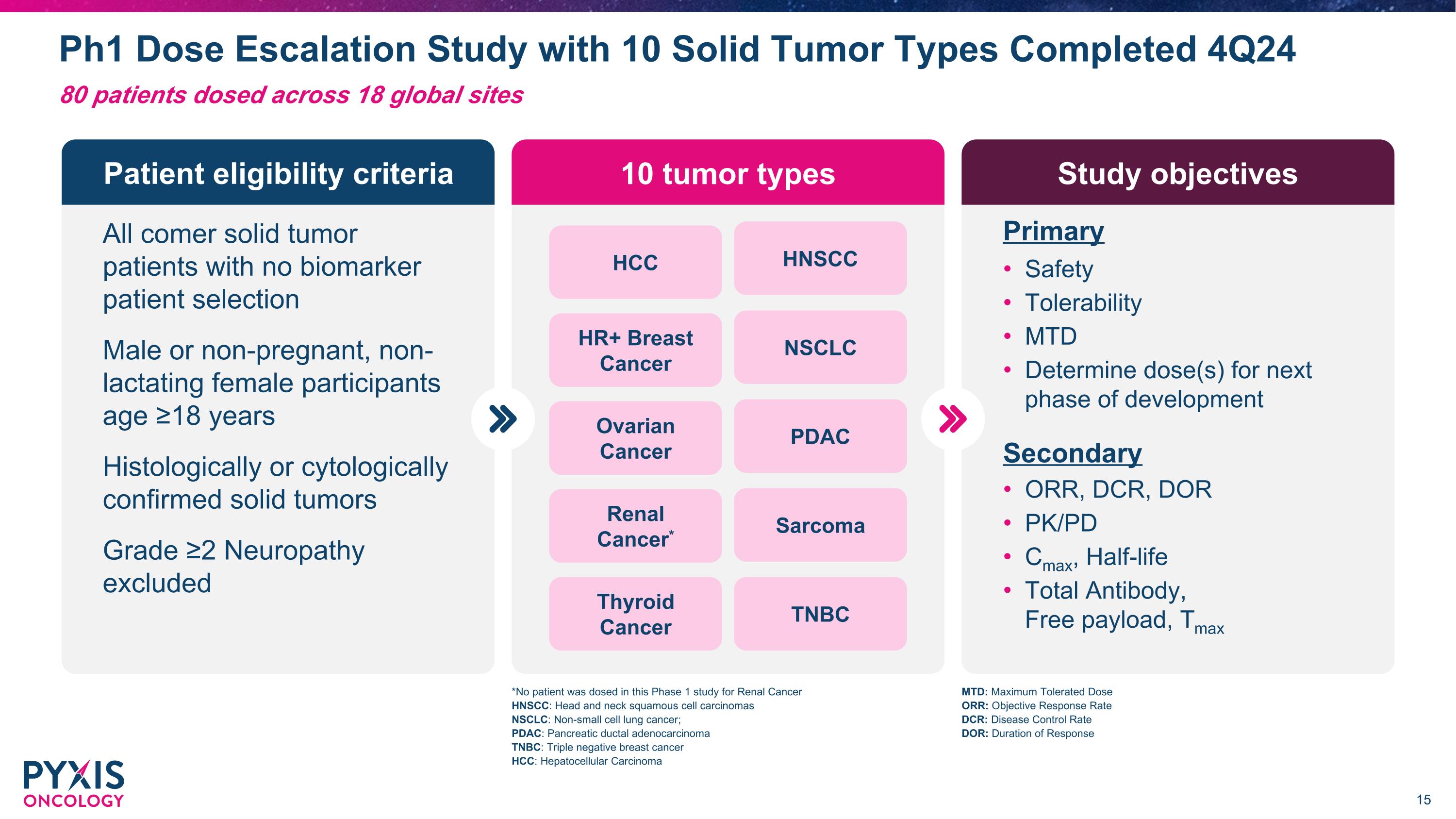
Ph1 Dose Escalation Study with 10 Solid Tumor Types Completed 4Q24 Patient eligibility criteria 10 tumor types Study objectives All comer solid tumor patients with no biomarker patient selection Male or non-pregnant, non-lactating female participants age ≥18 years Histologically or cytologically confirmed solid tumors Grade ≥2 Neuropathy excluded HCC HNSCC HR+ Breast Cancer NSCLC Ovarian �Cancer PDAC Renal �Cancer* Sarcoma Thyroid Cancer TNBC Safety Tolerability MTD Determine dose(s) for next phase of development Primary ORR, DCR, DOR PK/PD Cmax, Half-life Total Antibody, �Free payload, Tmax Secondary MTD: Maximum Tolerated Dose ORR: Objective Response Rate DCR: Disease Control Rate DOR: Duration of Response *No patient was dosed in this Phase 1 study for Renal Cancer HNSCC: Head and neck squamous cell carcinomas NSCLC: Non-small cell lung cancer; PDAC: Pancreatic ductal adenocarcinoma TNBC: Triple negative breast cancer HCC: Hepatocellular Carcinoma 80 patients dosed across 18 global sites
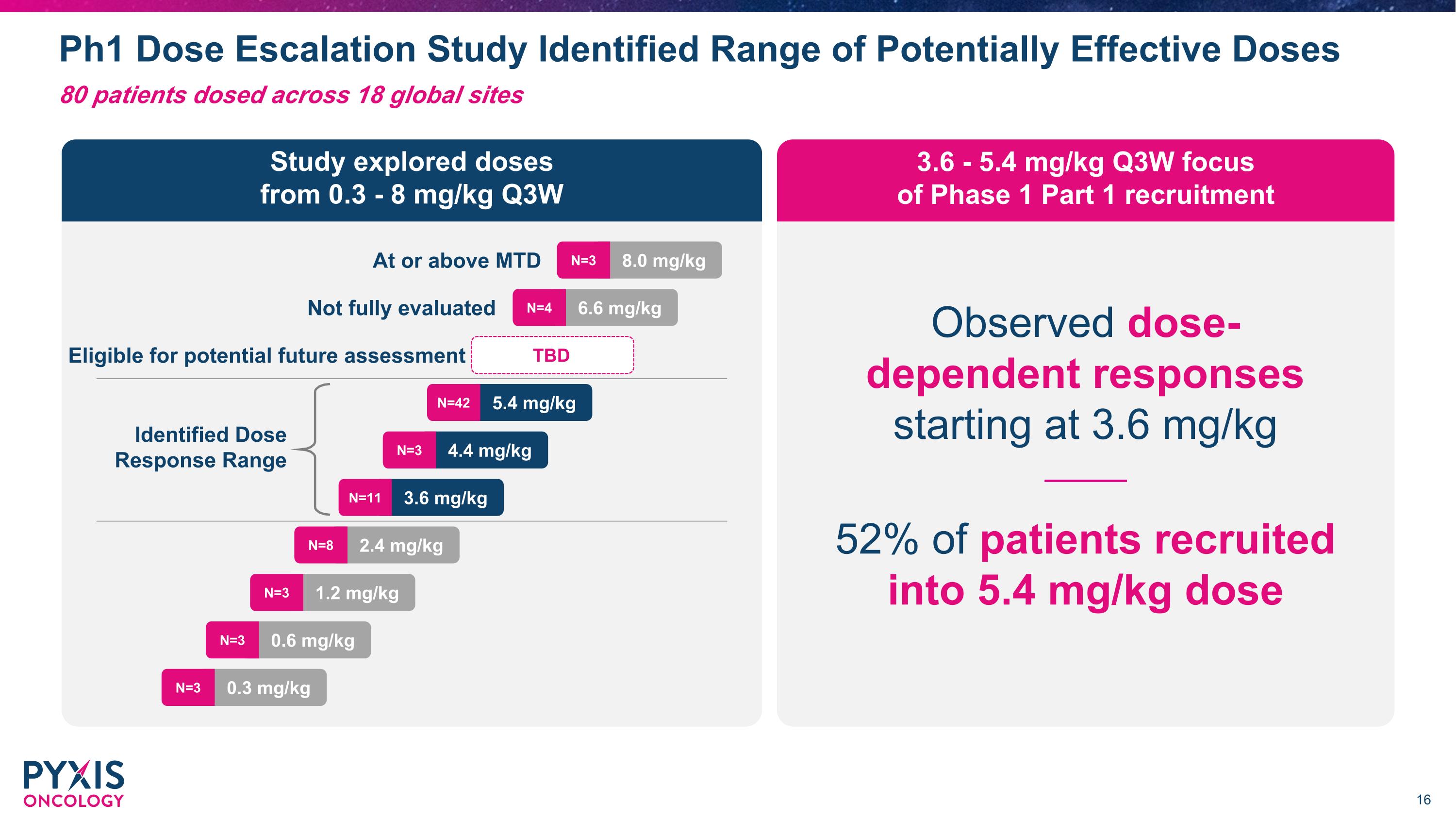
Ph1 Dose Escalation Study Identified Range of Potentially Effective Doses 80 patients dosed across 18 global sites 3.6 - 5.4 mg/kg Q3W focus �of Phase 1 Part 1 recruitment Study explored doses �from 0.3 - 8 mg/kg Q3W Observed dose-�dependent responses starting at 3.6 mg/kg 52% of patients recruited �into 5.4 mg/kg dose 8.0 mg/kg N=3 TBD Identified Dose Response Range Eligible for potential future assessment Not fully evaluated At or above MTD 6.6 mg/kg N=4 5.4 mg/kg N=42 4.4 mg/kg N=3 3.6 mg/kg N=11 2.4 mg/kg N=8 1.2 mg/kg N=3 0.6 mg/kg N=3 0.3 mg/kg N=3
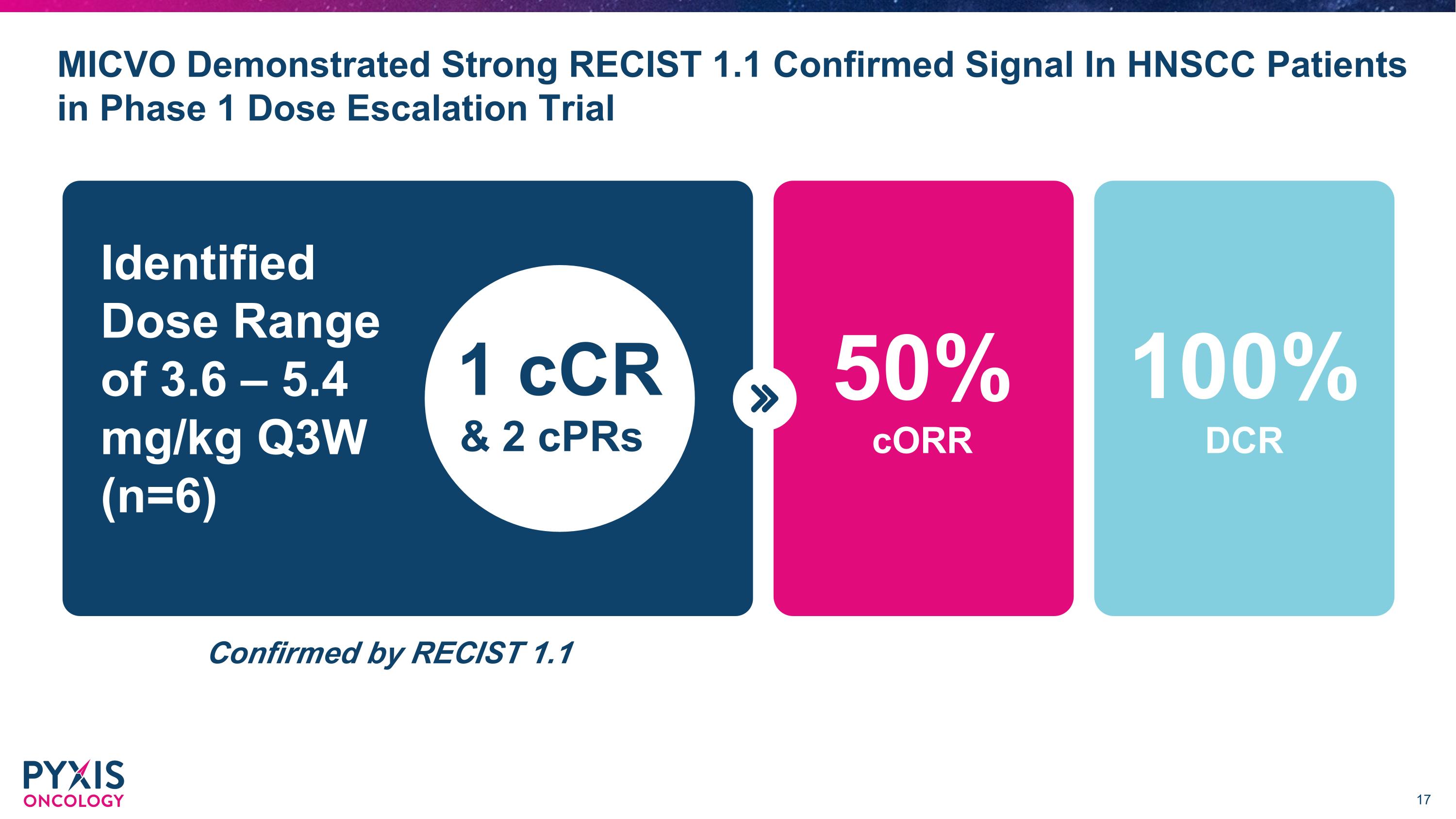
MICVO Demonstrated Strong RECIST 1.1 Confirmed Signal In HNSCC Patients in Phase 1 Dose Escalation Trial Identified Dose Range �of 3.6 – 5.4 mg/kg Q3W (n=6) & 2 cPRs 1 cCR Confirmed by RECIST 1.1 100% DCR cORR 50%
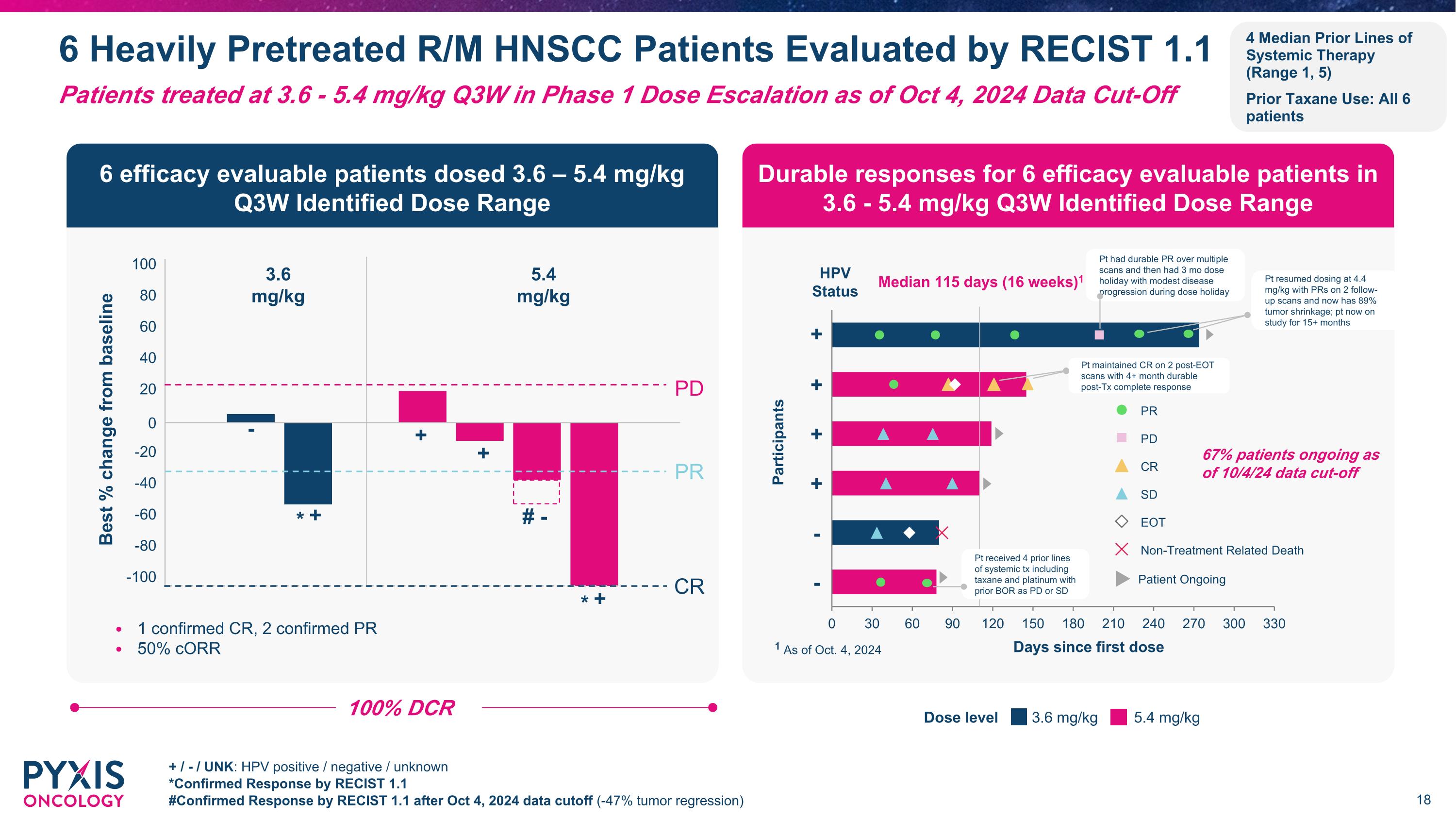
6 Heavily Pretreated R/M HNSCC Patients Evaluated by RECIST 1.1 + / - / UNK: HPV positive / negative / unknown *Confirmed Response by RECIST 1.1 #Confirmed Response by RECIST 1.1 after Oct 4, 2024 data cutoff (-47% tumor regression) Patients treated at 3.6 - 5.4 mg/kg Q3W in Phase 1 Dose Escalation as of Oct 4, 2024 Data Cut-Off 100% DCR 6 efficacy evaluable patients dosed 3.6 – 5.4 mg/kg �Q3W Identified Dose Range Durable responses for 6 efficacy evaluable patients in�3.6 - 5.4 mg/kg Q3W Identified Dose Range -100 -80 -60 -40 -20 0 20 40 60 80 100 Best % change from baseline # * + + + - - CR * + 3.6 mg/kg 5.4 mg/kg Participants Days since first dose Median 115 days (16 weeks)1 1 As of Oct. 4, 2024 - + HPV �Status + - + + 3.6 mg/kg 5.4 mg/kg Dose level 67% patients ongoing as of 10/4/24 data cut-off PR PD CR SD EOT Non-Treatment Related Death Patient Ongoing Pt had durable PR over multiple scans and then had 3 mo dose holiday with modest disease progression during dose holiday PR PD 1 confirmed CR, 2 confirmed PR 50% cORR 4 Median Prior Lines of Systemic Therapy (Range 1, 5) Prior Taxane Use: All 6 patients Pt maintained CR on 2 post-EOT scans with 4+ month durable post-Tx complete response Pt resumed dosing at 4.4 mg/kg with PRs on 2 follow-up scans and now has 89% tumor shrinkage; pt now on study for 15+ months Pt received 4 prior lines of systemic tx including taxane and platinum with prior BOR as PD or SD
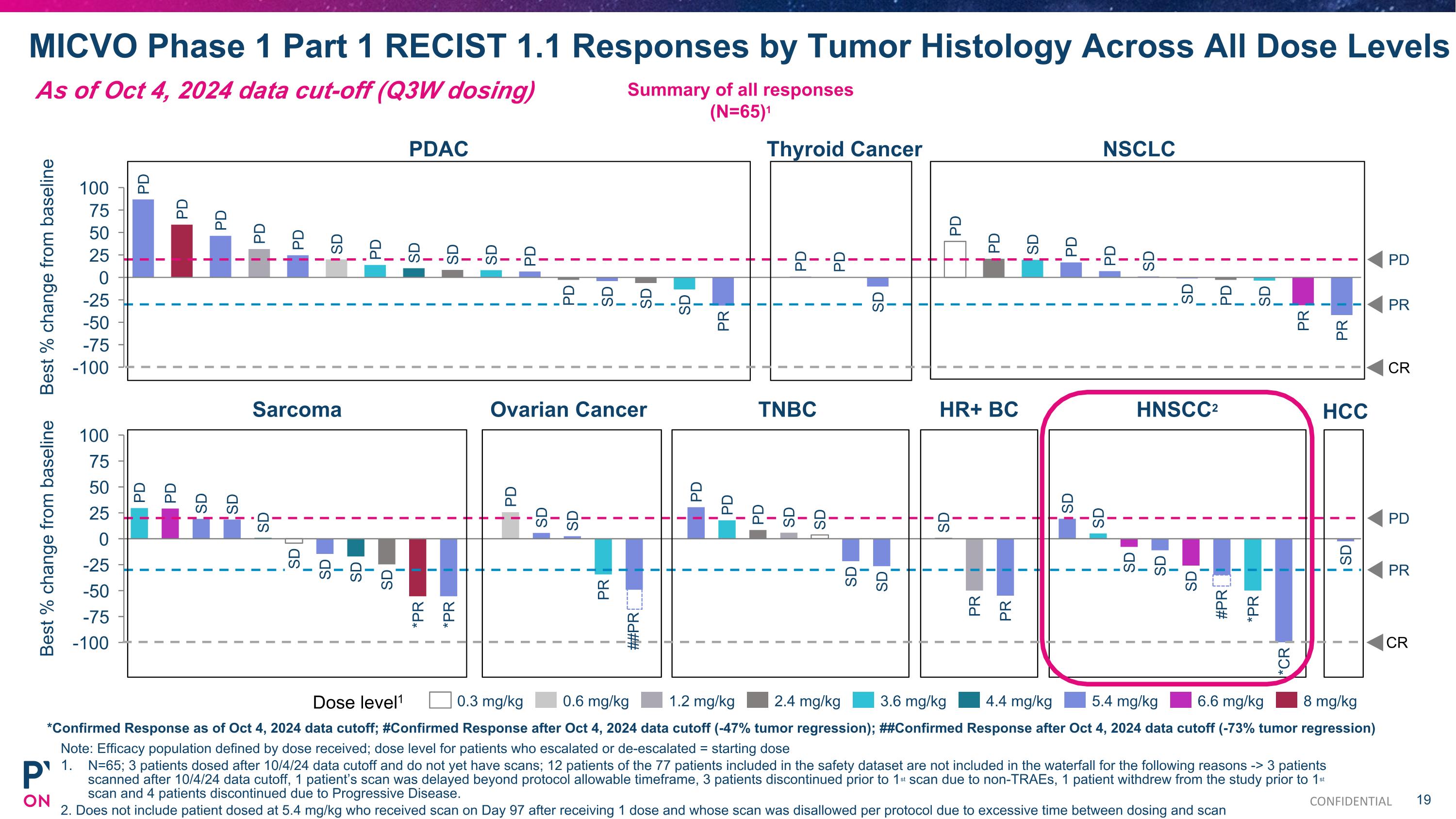
MICVO Phase 1 Part 1 RECIST 1.1 Responses by Tumor Histology Across All Dose Levels Note: Efficacy population defined by dose received; dose level for patients who escalated or de-escalated = starting dose N=65; 3 patients dosed after 10/4/24 data cutoff and do not yet have scans; 12 patients of the 77 patients included in the safety dataset are not included in the waterfall for the following reasons -> 3 patients scanned after 10/4/24 data cutoff, 1 patient’s scan was delayed beyond protocol allowable timeframe, 3 patients discontinued prior to 1st scan due to non-TRAEs, 1 patient withdrew from the study prior to 1st scan and 4 patients discontinued due to Progressive Disease. 2. Does not include patient dosed at 5.4 mg/kg who received scan on Day 97 after receiving 1 dose and whose scan was disallowed per protocol due to excessive time between dosing and scan Summary of all responses (N=65)1 Best % change from baseline PD PR PD PD PD PD PD SD PD SD SD SD PD PD SD SD SD PR PD PD SD PD PD SD PD PD SD SD PD SD PR PR Dose level1 0.3 mg/kg 0.6 mg/kg 1.2 mg/kg 2.4 mg/kg 3.6 mg/kg 4.4 mg/kg 5.4 mg/kg 6.6 mg/kg 8 mg/kg PDAC Thyroid Cancer NSCLC CR *Confirmed Response as of Oct 4, 2024 data cutoff; #Confirmed Response after Oct 4, 2024 data cutoff (-47% tumor regression); ##Confirmed Response after Oct 4, 2024 data cutoff (-73% tumor regression) PR Best % change from baseline PD PD SD SD SD SD SD SD SD *PR *PR PD SD SD PR PD PD PD SD SD PD SD SD PR PR SD SD SD SD SD *PR *CR SD SD Sarcoma Ovarian Cancer TNBC HR+ BC HNSCC2 ##PR #PR CR HCC As of Oct 4, 2024 data cut-off (Q3W dosing) CONFIDENTIAL
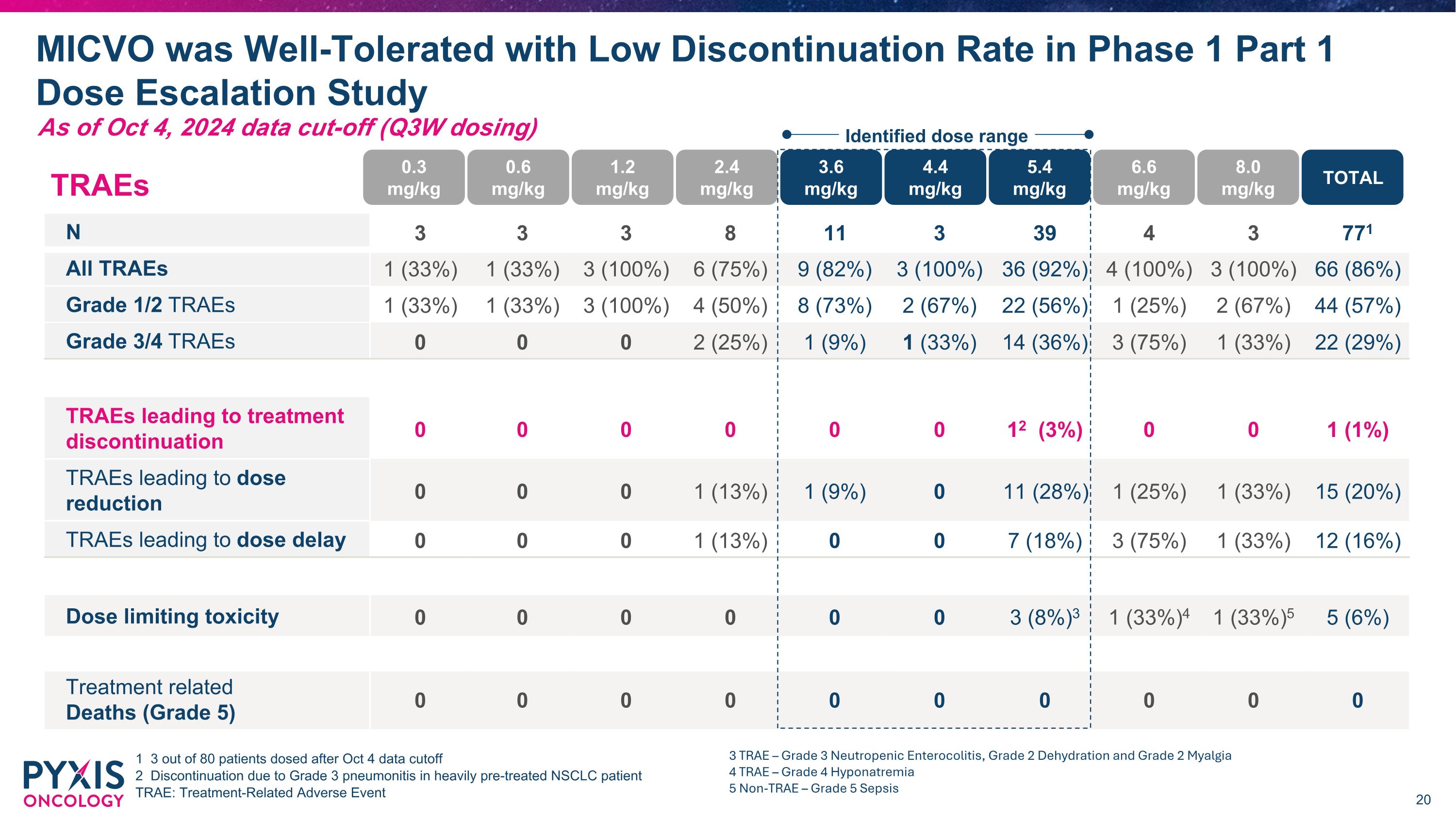
TRAEs N 3 3 3 8 11 3 39 4 3 771 All TRAEs 1 (33%) 1 (33%) 3 (100%) 6 (75%) 9 (82%) 3 (100%) 36 (92%) 4 (100%) 3 (100%) 66 (86%) Grade 1/2 TRAEs 1 (33%) 1 (33%) 3 (100%) 4 (50%) 8 (73%) 2 (67%) 22 (56%) 1 (25%) 2 (67%) 44 (57%) Grade 3/4 TRAEs 0 0 0 2 (25%) 1 (9%) 1 (33%) 14 (36%) 3 (75%) 1 (33%) 22 (29%) TRAEs leading to treatment discontinuation 0 0 0 0 0 0 12 (3%) 0 0 1 (1%) TRAEs leading to dose reduction 0 0 0 1 (13%) 1 (9%) 0 11 (28%) 1 (25%) 1 (33%) 15 (20%) TRAEs leading to dose delay 0 0 0 1 (13%) 0 0 7 (18%) 3 (75%) 1 (33%) 12 (16%) Dose limiting toxicity 0 0 0 0 0 0 3 (8%)3 1 (33%)4 1 (33%)5 5 (6%) Treatment related�Deaths (Grade 5) 0 0 0 0 0 0 0 0 0 0 MICVO was Well-Tolerated with Low Discontinuation Rate in Phase 1 Part 1 Dose Escalation Study 1 3 out of 80 patients dosed after Oct 4 data cutoff 2 Discontinuation due to Grade 3 pneumonitis in heavily pre-treated NSCLC patient TRAE: Treatment-Related Adverse Event 0.3�mg/kg 0.6�mg/kg 1.2�mg/kg 2.4�mg/kg 3.6�mg/kg 4.4�mg/kg 5.4�mg/kg 6.6�mg/kg 8.0�mg/kg TOTAL Identified dose range 3 TRAE – Grade 3 Neutropenic Enterocolitis, Grade 2 Dehydration and Grade 2 Myalgia 4 TRAE – Grade 4 Hyponatremia 5 Non-TRAE – Grade 5 Sepsis As of Oct 4, 2024 data cut-off (Q3W dosing)
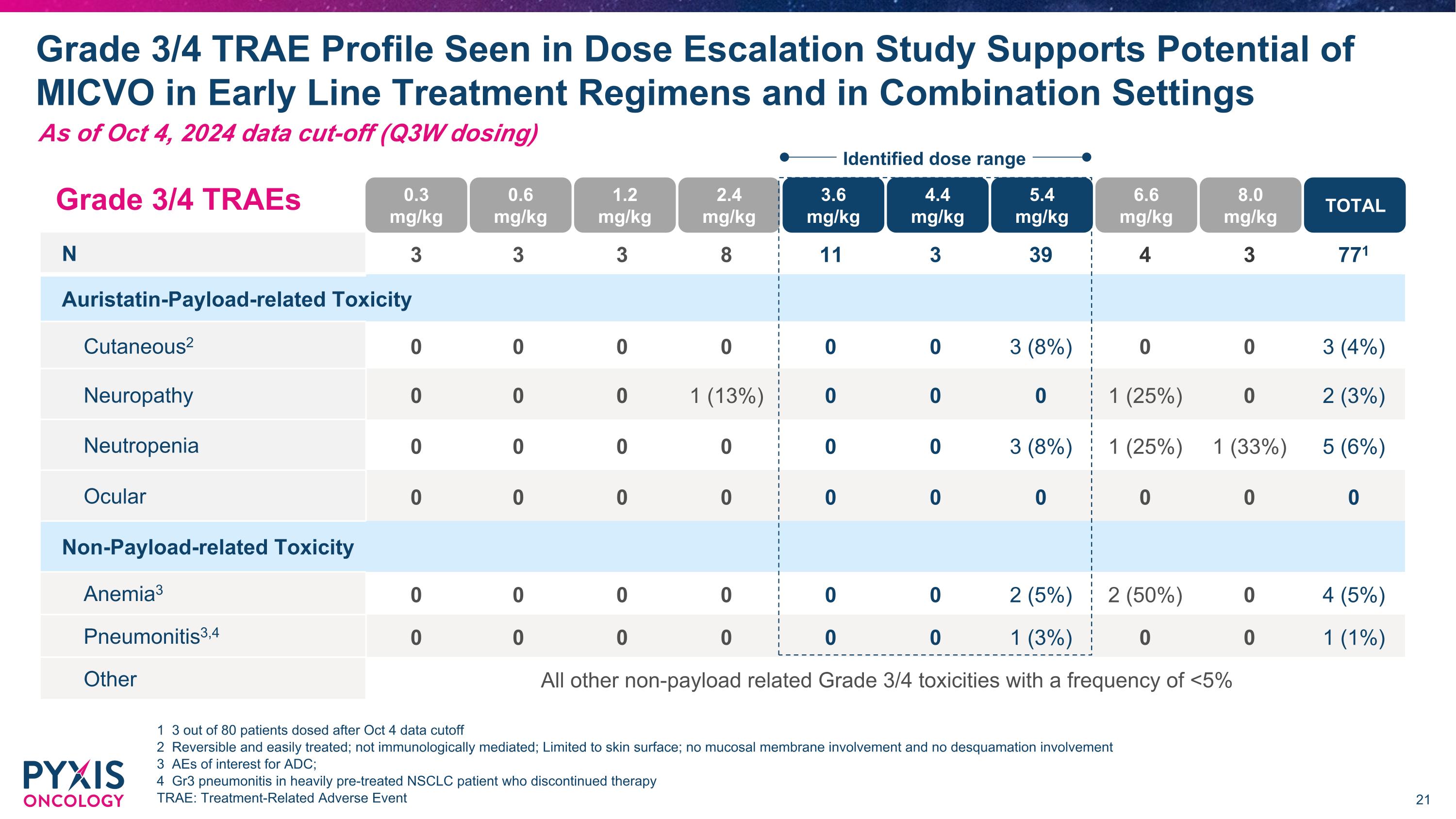
N 3 3 3 8 11 3 39 4 3 771 Auristatin-Payload-related Toxicity Cutaneous2 0 0 0 0 0 0 3 (8%) 0 0 3 (4%) Neuropathy 0 0 0 1 (13%) 0 0 0 1 (25%) 0 2 (3%) Neutropenia 0 0 0 0 0 0 3 (8%) 1 (25%) 1 (33%) 5 (6%) Ocular 0 0 0 0 0 0 0 0 0 0 Non-Payload-related Toxicity Anemia3 0 0 0 0 0 0 2 (5%) 2 (50%) 0 4 (5%) Pneumonitis3,4 0 0 0 0 0 0 1 (3%) 0 0 1 (1%) Other All other non-payload related Grade 3/4 toxicities with a frequency of <5% Grade 3/4 TRAE Profile Seen in Dose Escalation Study Supports Potential of MICVO in Early Line Treatment Regimens and in Combination Settings 1 3 out of 80 patients dosed after Oct 4 data cutoff 2 Reversible and easily treated; not immunologically mediated; Limited to skin surface; no mucosal membrane involvement and no desquamation involvement 3 AEs of interest for ADC; 4 Gr3 pneumonitis in heavily pre-treated NSCLC patient who discontinued therapy TRAE: Treatment-Related Adverse Event Grade 3/4 TRAEs 0.3�mg/kg 0.6�mg/kg 1.2�mg/kg 2.4�mg/kg 3.6�mg/kg 4.4�mg/kg 5.4�mg/kg 6.6�mg/kg 8.0�mg/kg TOTAL Identified dose range As of Oct 4, 2024 data cut-off (Q3W dosing)
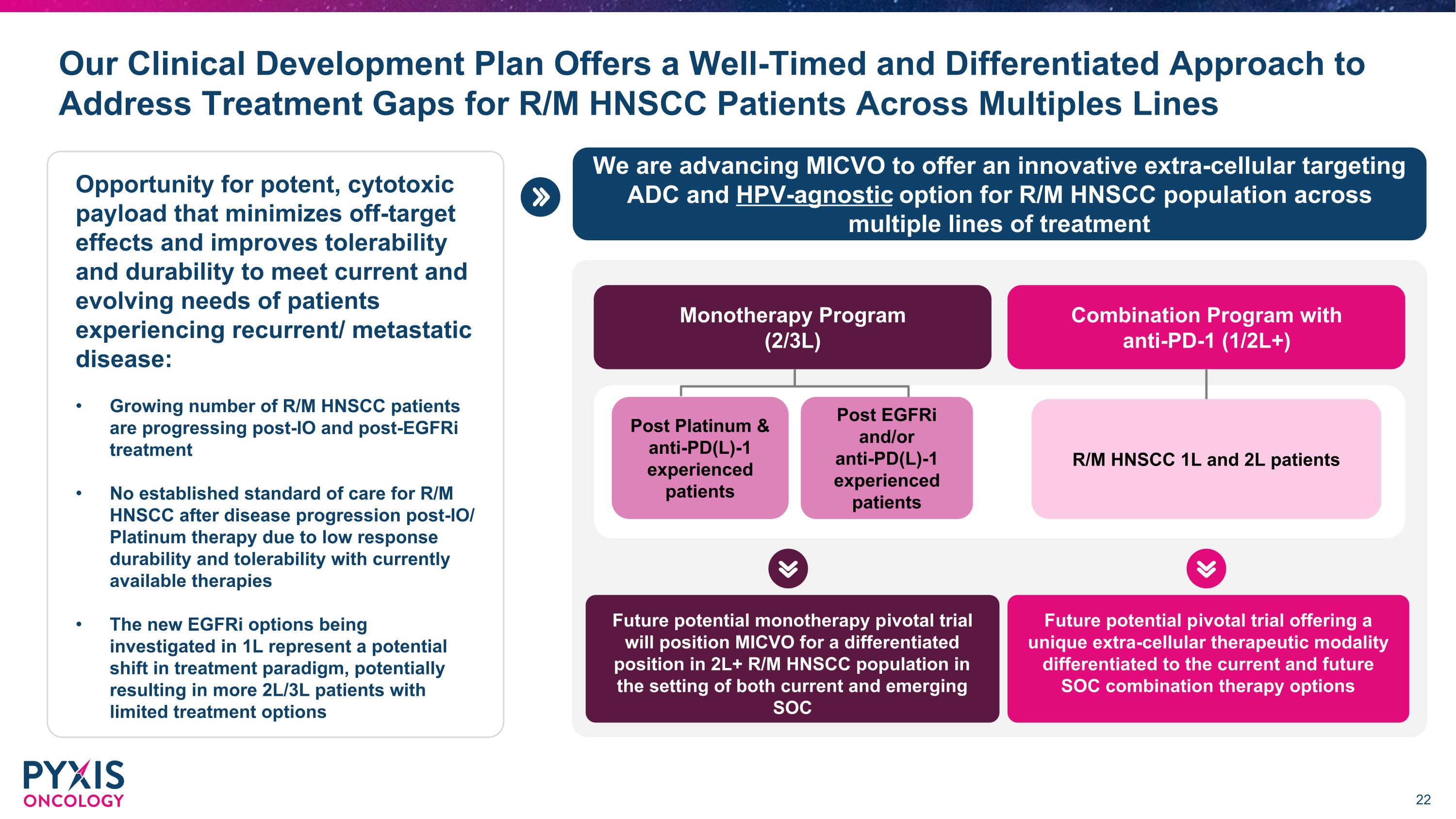
Our Clinical Development Plan Offers a Well-Timed and Differentiated Approach to Address Treatment Gaps for R/M HNSCC Patients Across Multiples Lines Monotherapy Program (2/3L) Combination Program with anti-PD-1 (1/2L+) Future potential monotherapy pivotal trial will position MICVO for a differentiated position in 2L+ R/M HNSCC population in the setting of both current and emerging SOC Future potential pivotal trial offering a unique extra-cellular therapeutic modality differentiated to the current and future SOC combination therapy options R/M HNSCC 1L and 2L patients Post Platinum & anti-PD(L)-1 experienced patients Post EGFRi and/or anti-PD(L)-1 experienced patients We are advancing MICVO to offer an innovative extra-cellular targeting ADC and HPV-agnostic option for R/M HNSCC population across multiple lines of treatment Opportunity for potent, cytotoxic payload that minimizes off-target effects and improves tolerability and durability to meet current and evolving needs of patients experiencing recurrent/ metastatic disease: Growing number of R/M HNSCC patients are progressing post-IO and post-EGFRi treatment No established standard of care for R/M HNSCC after disease progression post-IO/ Platinum therapy due to low response durability and tolerability with currently available therapies The new EGFRi options being investigated in 1L represent a potential shift in treatment paradigm, potentially resulting in more 2L/3L patients with limited treatment options
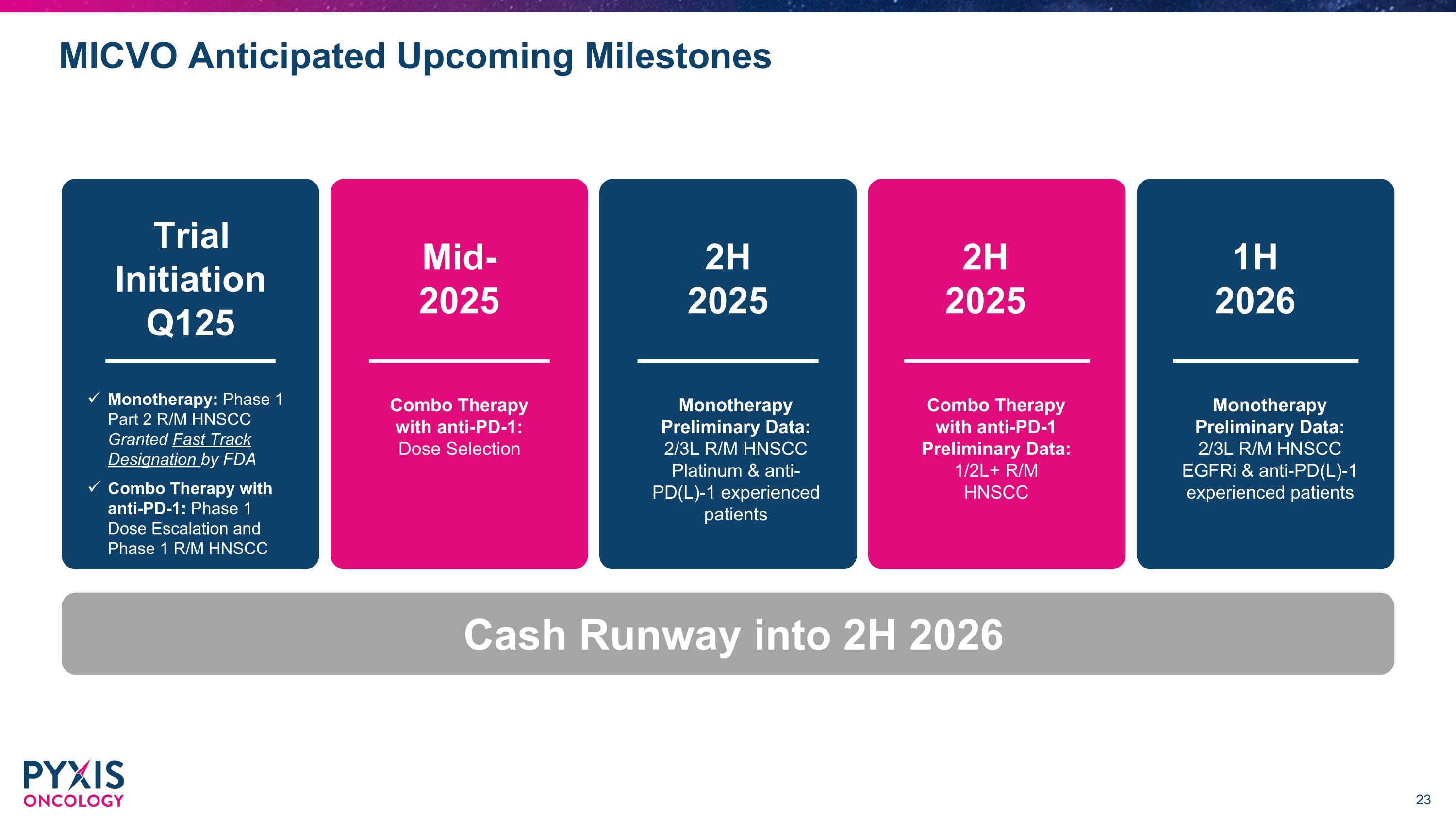
MICVO Anticipated Upcoming Milestones Trial Initiation Q125 Monotherapy: Phase 1 Part 2 R/M HNSCC �Granted Fast Track�Designation by FDA Combo Therapy with anti-PD-1: Phase 1 Dose Escalation and Phase 1 R/M HNSCC Mid-2025 Combo Therapy with anti-PD-1: Dose Selection 2H �2025 Monotherapy Preliminary Data: 2/3L R/M HNSCC Platinum & anti-PD(L)-1 experienced patients 2H �2025 Combo Therapy with anti-PD-1 Preliminary Data: 1/2L+ R/M HNSCC 1H �2026 Monotherapy Preliminary Data: 2/3L R/M HNSCC EGFRi & anti-PD(L)-1 experienced patients Cash Runway into 2H 2026
Positioned to be a Differentiated ADC Company *Balance sheet as of December 31, 2024 First-in-Concept Extra-cellular ADC Technology Deeply Experienced �Team of Oncology Drug Developers Multiple Clinical Data Catalysts �in 2025 Strong Balance �Sheet* with $128M Cash Runway �into 2H 2026

Appendix
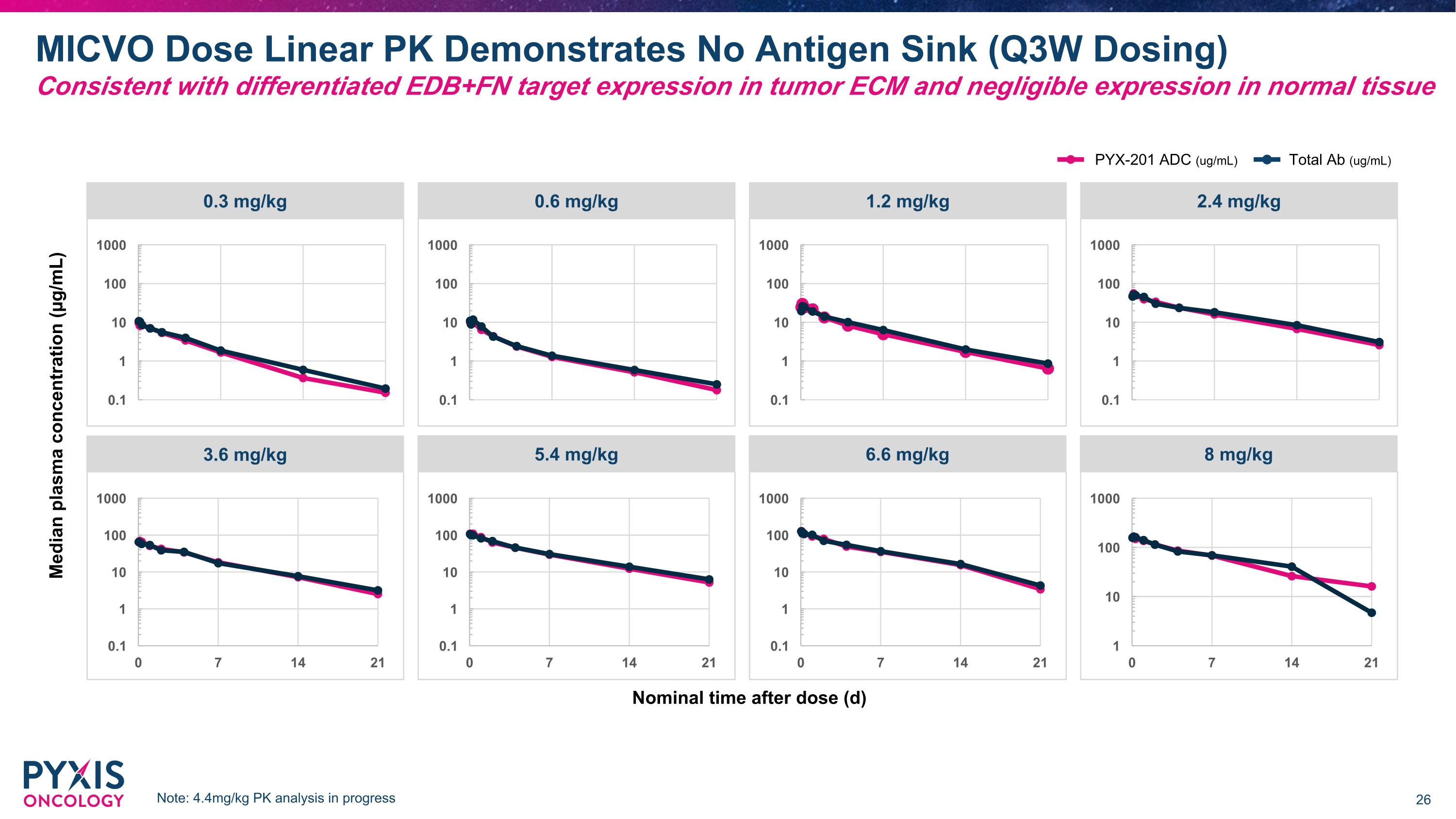
MICVO Dose Linear PK Demonstrates No Antigen Sink (Q3W Dosing)�Consistent with differentiated EDB+FN target expression in tumor ECM and negligible expression in normal tissue Nominal time after dose (d) Median plasma concentration (µg/mL) 0.3 mg/kg 0.6 mg/kg 1.2 mg/kg 2.4 mg/kg 3.6 mg/kg 5.4 mg/kg 6.6 mg/kg 8 mg/kg PYX-201 ADC (ug/mL) Total Ab (ug/mL) Note: 4.4mg/kg PK analysis in progress
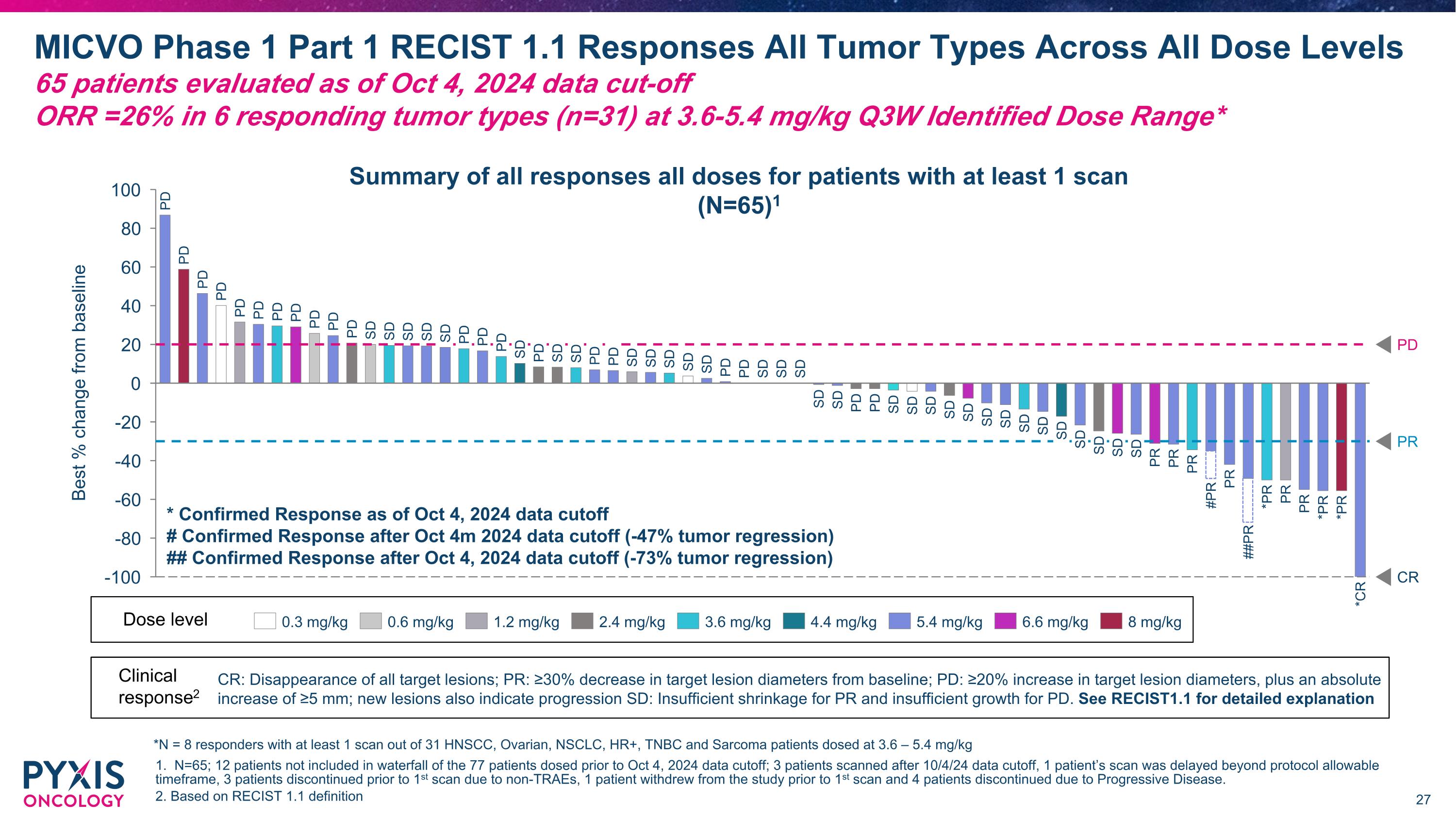
Best % change from baseline PR CR PD PD PD PD PD PD PD PD PD PD PD SD SD SD SD SD PD PD PD SD PD SD SD PD PD SD SD SD SD SD PD PD SD SD SD SD SD PD PD SD SD SD SD PD SD SD SD SD SD SD SD SD SD PR SD PR #PR PR ##PR *PR PR PR *PR *PR *CR PR MICVO Phase 1 Part 1 RECIST 1.1 Responses All Tumor Types Across All Dose Levels�65 patients evaluated as of Oct 4, 2024 data cut-off�ORR =26% in 6 responding tumor types (n=31) at 3.6-5.4 mg/kg Q3W Identified Dose Range* 1. N=65; 12 patients not included in waterfall of the 77 patients dosed prior to Oct 4, 2024 data cutoff; 3 patients scanned after 10/4/24 data cutoff, 1 patient’s scan was delayed beyond protocol allowable timeframe, 3 patients discontinued prior to 1st scan due to non-TRAEs, 1 patient withdrew from the study prior to 1st scan and 4 patients discontinued due to Progressive Disease. 2. Based on RECIST 1.1 definition 0.3 mg/kg 0.6 mg/kg 1.2 mg/kg 2.4 mg/kg 3.6 mg/kg 4.4 mg/kg 5.4 mg/kg 6.6 mg/kg 8 mg/kg Dose level Summary of all responses all doses for patients with at least 1 scan (N=65)1 Clinical response2 CR: Disappearance of all target lesions; PR: ≥30% decrease in target lesion diameters from baseline; PD: ≥20% increase in target lesion diameters, plus an absolute increase of ≥5 mm; new lesions also indicate progression SD: Insufficient shrinkage for PR and insufficient growth for PD. See RECIST1.1 for detailed explanation * Confirmed Response as of Oct 4, 2024 data cutoff # Confirmed Response after Oct 4m 2024 data cutoff (-47% tumor regression) ## Confirmed Response after Oct 4, 2024 data cutoff (-73% tumor regression) *N = 8 responders with at least 1 scan out of 31 HNSCC, Ovarian, NSCLC, HR+, TNBC and Sarcoma patients dosed at 3.6 – 5.4 mg/kg
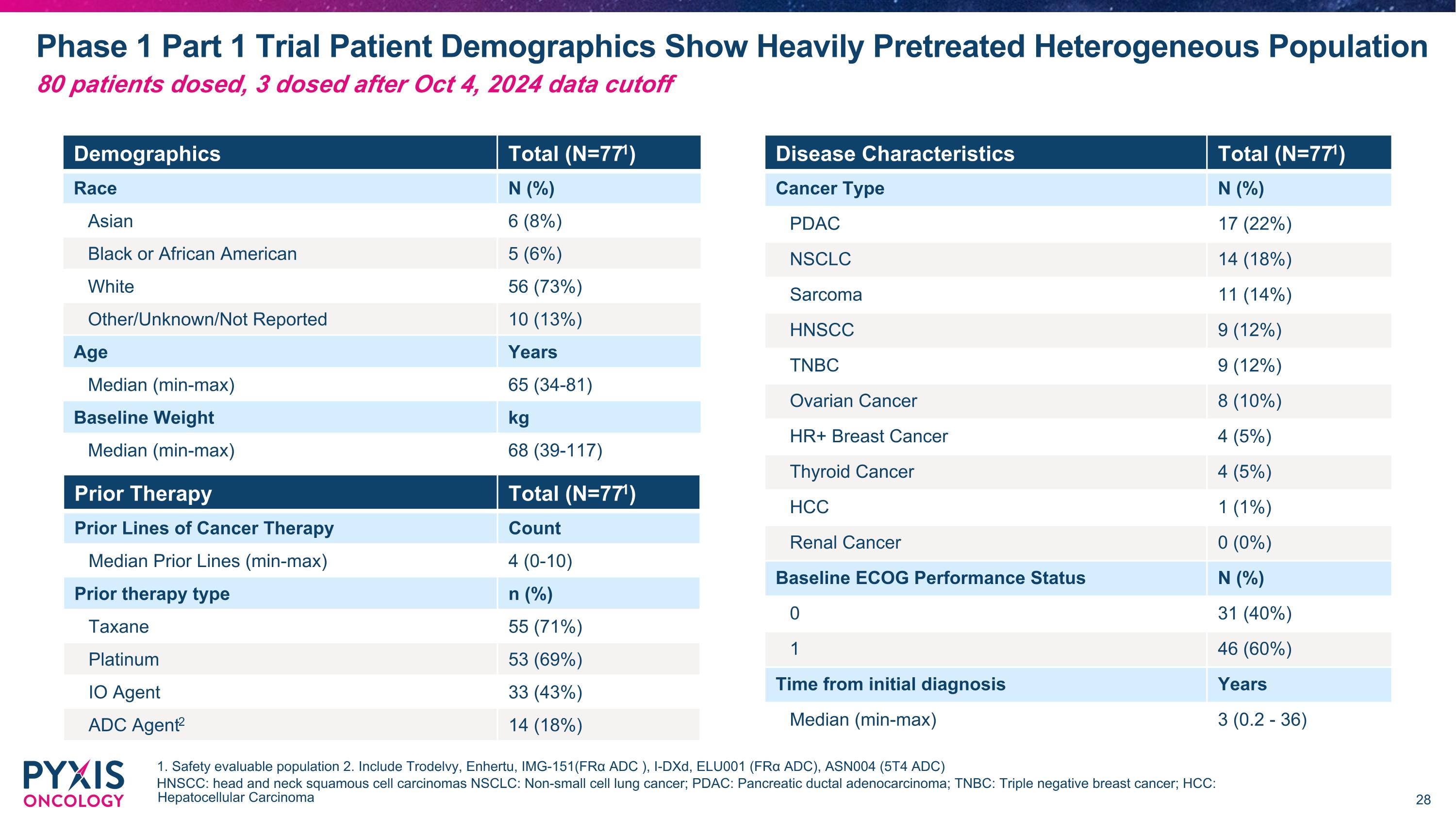
Phase 1 Part 1 Trial Patient Demographics Show Heavily Pretreated Heterogeneous Population 1. Safety evaluable population 2. Include Trodelvy, Enhertu, IMG-151(FRα ADC ), I-DXd, ELU001 (FRα ADC), ASN004 (5T4 ADC) HNSCC: head and neck squamous cell carcinomas NSCLC: Non-small cell lung cancer; PDAC: Pancreatic ductal adenocarcinoma; TNBC: Triple negative breast cancer; HCC: Hepatocellular Carcinoma Demographics Total (N=771) Race N (%) Asian 6 (8%) Black or African American 5 (6%) White 56 (73%) Other/Unknown/Not Reported 10 (13%) Age Years Median (min-max) 65 (34-81) Baseline Weight kg Median (min-max) 68 (39-117) Prior Therapy Total (N=771) Prior Lines of Cancer Therapy Count Median Prior Lines (min-max) 4 (0-10) Prior therapy type n (%) Taxane 55 (71%) Platinum 53 (69%) IO Agent 33 (43%) ADC Agent2 14 (18%) Disease Characteristics Total (N=771) Cancer Type N (%) PDAC 17 (22%) NSCLC 14 (18%) Sarcoma 11 (14%) HNSCC 9 (12%) TNBC 9 (12%) Ovarian Cancer 8 (10%) HR+ Breast Cancer 4 (5%) Thyroid Cancer 4 (5%) HCC 1 (1%) Renal Cancer 0 (0%) Baseline ECOG Performance Status N (%) 0 31 (40%) 1 46 (60%) Time from initial diagnosis Years Median (min-max) 3 (0.2 - 36) 80 patients dosed, 3 dosed after Oct 4, 2024 data cutoff

Building a Differentiated ADC Company Nasdaq: PYXS Thank you
v3.25.1
Document And Entity Information
|
Mar. 18, 2025 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Mar. 18, 2025
|
| Entity Registrant Name |
Pyxis Oncology, Inc.
|
| Entity Central Index Key |
0001782223
|
| Entity Emerging Growth Company |
true
|
| Entity File Number |
001-40881
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Tax Identification Number |
83-1160910
|
| Entity Address, Address Line One |
321 Harrison Avenue
|
| Entity Address, City or Town |
Boston
|
| Entity Address, State or Province |
MA
|
| Entity Address, Postal Zip Code |
02118
|
| City Area Code |
(617)
|
| Local Phone Number |
453-3596
|
| Entity Information, Former Legal or Registered Name |
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Ex Transition Period |
true
|
| Title of 12(b) Security |
Common Stock, par value $0.001 per share
|
| Trading Symbol |
PYXS
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Grafico Azioni Pyxis Oncology (NASDAQ:PYXS)
Storico
Da Mar 2025 a Mar 2025

Grafico Azioni Pyxis Oncology (NASDAQ:PYXS)
Storico
Da Mar 2024 a Mar 2025
