As filed with the Securities and Exchange Commission
on March 25, 2025.
Registration
No. 333-285862
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
Amendment No. 1
to
FORM
S-1
REGISTRATION
STATEMENT
UNDER
THE SECURITIES ACT OF 1933
VIRPAX
PHARMACEUTICALS, INC.
(Exact
name of registrant as specified in its charter)
| Delaware |
|
2834 |
|
82-1510982 |
(State or other jurisdiction
of
incorporation or organization) |
|
(Primary Standard Industrial
Classification Code Number) |
|
(I.R.S. Employer
Identification No.) |
1055
Westlakes Drive, Suite 300
Berwyn,
PA 19312
(610)
727-4597
(Address,
including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Jatinder Dhaliwal
Chief Executive Officer
Virpax Pharmaceuticals, Inc.
1055 Westlakes Drive, Suite 300
Berwyn, PA 19312
(610) 727-4597
(Name,
address, including zip code, and telephone number, including area code, of agent for service)
with
copies to:
Ross D. Carmel,
Esq.
Benjamin E. Sklar, Esq.
Sichenzia Ross Ference Carmel LLP
1185 Avenue of the Americas, 31st Floor
New York, NY 10036
(212) 930-9700 |
|
Joseph M.
Lucosky, Esq.
Scott E. Linsky, Esq.
Lucosky Brookman LLP
101 Wood Avenue South, 5th Floor
Woodbridge, NJ 08830
(732) 395-4400 |
Approximate
date of commencement of proposed sale to public: As soon as practicable after this registration statement is declared effective.
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the
Securities Act check the following box. ☒
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the
following box and list the Securities Act registration statement number of the earlier effective registration statement for the same
offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the
Securities Act registration number of the earlier effective registration statement for the same offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the
Securities Act registration number of the earlier effective registration statement for the same offering. ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting
company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,”
“smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer |
☐ |
Accelerated
filer |
☐ |
| Non-accelerated filer |
☒ |
Smaller reporting company |
☒ |
| |
|
Emerging growth company |
☒ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The
registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the
registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective
in accordance with section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date
as the Commission, acting pursuant to section 8(a), may determine.
The information in this
preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with
the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities, and we are
not soliciting an offer to buy these securities, in any jurisdiction where the offer or sale is not permitted.
| PROSPECTUS |
SUBJECT
TO COMPLETION |
DATED
MARCH 25, 2025 |

Virpax Pharmaceuticals, Inc.
Up to 2,415,459 Shares
of Common Stock
Up 2,415,459 Pre-Funded
Warrants to Purchase 2,415,459 Shares of Common Stock
Up to 2,415,459 Shares of Common Stock Issuable Upon Exercise of such Pre-Funded Warrants
We are offering on a
best efforts basis up to 2,415,459 shares of our common stock, par value $0.00001 per share (the “Common Stock”).
We are also offering to each
purchaser, if any, whose purchase of shares of Common Stock in this offering would otherwise result in the purchaser, together with its
affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of such purchaser, 9.99%) of our outstanding
Common Stock immediately following the consummation of this offering, the opportunity to purchase, if the purchaser so chooses, pre-funded
warrants (the “Pre-Funded Warrants”), in lieu of shares of Common Stock that would otherwise result in the purchaser’s
beneficial ownership exceeding 4.99% (or, at the election of such purchaser, 9.99%) of our outstanding shares of Common Stock. There
can be no assurance that we will sell any of the Pre-Funded Warrants being offered. Each Pre-Funded Warrant will be immediately
exercisable for one share of Common Stock and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full.
The purchase price of each Pre-Funded Warrant will equal the price per share at which one share of Common Stock is being sold to the public
in this offering, minus $0.00001, and the exercise price of each Pre-Funded Warrant will be $0.00001 per share.
For each Pre-Funded Warrant
we sell, the number of shares of Common Stock we are offering will be decreased on a one-for-one basis. This offering also relates to
the shares of Common Stock issuable upon exercise of any Pre-Funded Warrants sold in this offering. We refer to the shares of Common Stock
and Pre-Funded Warrants to be sold in this offering collectively as the “Securities.”
Our Common Stock is listed
on the Nasdaq Capital Market under the symbol “VRPX”. The last reported sale price of our Common Stock on Nasdaq on March
21, 2025 was $2.07 per share. We have assumed a public offering price of $2.07 per share of Common Stock, which was the last reported
sale price on Nasdaq of our shares of Common Stock on March 21, 2025. The actual offering price per share of Common Stock and Pre-Funded
Warrant will be negotiated between us and the investors, in consultation with the placement agent based on, among other things, the trading
price of our Common Stock prior to the offering and may be at a discount to the current market price. Therefore, the assumed public offering
price used throughout this prospectus may not be indicative of the final offering price. In addition, there is no established public
trading market for the Pre-Funded Warrants, and we do not expect a market to develop. We do not intend to apply to have the Pre-Funded
Warrants listed on any national securities exchange or any other nationally recognized trading system.
We have engaged Spartan Capital
Securities, LLC to act as our exclusive placement agent (the “Placement Agent”) in connection with this offering. The Placement
Agent has agreed to use its reasonable best efforts to arrange for the sale of the Securities offered by this prospectus. The Placement
Agent is not purchasing or selling any of the Securities we are offering, and the Placement Agent is not required to arrange the purchase
or sale of any specific number of Securities or dollar amount. We have agreed to pay to the Placement Agent the fees set forth in the
table below, which assumes that we sell all of the Securities offered by this prospectus. See “Plan of Distribution”.
The Securities are expected
to be issued in a single closing and the public offering price per share of Common Stock and Pre-Funded Warrant will be fixed for the
duration of this offering. We will deliver all Securities to be issued in connection with this offering delivery versus payment (“DVP”)/receipt
versus payment (“RVP”) upon receipt of investor funds received by us. There is no minimum offering requirement as a condition
of closing of this offering. Because there is no minimum offering amount required as a condition to closing this offering, we may sell
fewer than all of the Securities offered hereby, which may significantly reduce the amount of proceeds received by us, and investors in
this offering will not receive a refund in the event that we do not sell an amount of Securities sufficient to pursue our business goals
described in this prospectus. Further, any proceeds from the sale of Securities offered by us will be available for our immediate use,
despite uncertainty about whether we would be able to use such funds to effectively implement our business plan. See “Risk Factors”
on page 13 of this prospectus for more information.
This offering will terminate
on April 15, 2025, unless the offering is fully subscribed before that date, or we decide to terminate the offering (which we may do at
any time in our discretion) prior to that date. We will bear all costs associated with the offering. No escrow agent has been appointed
or will be involved in this offering.
We are an “emerging
growth company” and a “smaller reporting company” as defined under the federal securities laws and, as such, we may
elect to comply with certain reduced public company reporting requirements for this prospectus and future filings. See “Prospectus
Summary – Implications of Being an Emerging Growth Company” and “Prospectus Summary – Implications of Being a
Smaller Reporting Company”.
You
should read this prospectus, together with additional information described under the heading “Where You Can Find More Information”
carefully before you invest in any of our Securities.
Investing
in our securities involves substantial risks. Please read carefully the section entitled “Risk Factors” beginning on page
13 of this prospectus, as well as the other information included or incorporated by reference in this prospectus, before buying any of
our securities.
Neither the Securities
and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the accuracy
or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
| |
|
Per Share |
|
Per Pre-
Funded
Warrant |
|
Total |
| Public offering price |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
| Placement Agent fees(1) |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
| Proceeds to us, before expenses(2) |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
| (1) |
Represents a cash fee equal to 2.0% of the gross proceeds of the offering. We have also agreed to reimburse the Placement Agent for certain offering-related legal and other expenses and to pay to the Placement Agent at the closing of the offering a non-accountable expense allowance equal to 1.0% of the gross proceeds of the offering. See “Plan of Distribution”. |
| (2) |
The proceeds to us, before expenses does not give effect to any exercise of any Pre-Funded Warrants. |
We expect to deliver the Securities to investors
on or about __________, 2025.
Sole
Placement Agent
Spartan
Capital Securities, LLC
The
date of this prospectus is _______________, 2025
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
We have not authorized anyone to provide any information
to you or to make any representations other than those contained, or incorporated by reference, in this prospectus, any amendment or supplement
to this prospectus, or in any free writing prospectuses prepared by or on behalf of us or to which we have referred you. We take no responsibility
for, and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus is an offer
to sell only the securities offered hereby, and only under circumstances and in jurisdictions where offers and sales are permitted. You
should not assume that the information contained in this prospectus or any applicable prospectus supplement is accurate on any date subsequent
to the date set forth on the front of the document or that any information we have incorporated by reference is correct on any date subsequent
to the date of the document incorporated by reference, even though this prospectus or any applicable prospectus supplement is delivered,
or securities are sold, on a later date. Our business, financial condition, results of operations and prospects may have changed since
the date on the front cover of this prospectus.
We may also file a prospectus
supplement or post-effective amendment to the registration statement of which this prospectus forms a part that may contain
material information relating to this offering. The prospectus supplement or post-effective amendment may also add, update or
change information contained in this prospectus. If there is any inconsistency between the information in this prospectus and the applicable
prospectus supplement or post-effective amendment, you should rely on the prospectus supplement or post-effective amendment,
as applicable. Before purchasing any securities, you should carefully read this prospectus, any post-effective amendment, and
any applicable prospectus supplement, together with the additional information described under the heading “Where You Can Find More
Information” and “Incorporation of Certain Information by Reference.”
This prospectus contains summaries of certain provisions
contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the
summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed,
will be filed or will be incorporated by reference as exhibits to the registration statement of which this prospectus is a part, and you
may obtain copies of those documents as described below under the section entitled “Where You Can Find More Information.”
Information contained in,
and that can be accessed through our website, www.virpaxpharma.com, shall not be deemed to be part of this prospectus
or incorporated herein by reference and should not be relied upon by any prospective investors for the purposes of determining whether
to purchase the Common Stock offered hereunder.
Unless the context otherwise
requires, the terms “we,” “us,” “our,” “the Company,” “Virpax” and “our
business” refer to Virpax Pharmaceuticals, Inc. and “this offering” refers to the offering contemplated in this prospectus.
INDUSTRY AND MARKET DATA
Unless otherwise indicated, information in this prospectus
concerning economic conditions, our industry, our markets and our competitive position is based on a variety of sources, including information
from third-party industry analysts and publications and our own estimates and research. Some of the industry and market data contained
in this prospectus are based on third-party industry publications. This information involves a number of assumptions, estimates and limitations.
The industry publications,
surveys and forecasts and other public information generally indicate or suggest that their information has been obtained from sources
believed to be reliable. We believe this information is reliable as of the applicable date of its publication, however, we have not independently
verified the accuracy or completeness of the information included in or assumptions relied on in these third-party publications.
In addition, the market and industry data and forecasts that may be included in this prospectus, any post-effective amendment
or any prospectus supplement may involve estimates, assumptions and other risks and uncertainties and are subject to change based on various
factors, including those discussed under the heading “Risk Factors” contained in this prospectus, any post-effective amendment,
any prospectus supplement and under similar headings in the other documents that are incorporated by reference into this prospectus. Accordingly,
investors should not place undue reliance on this information.
TRADEMARKS, SERVICE MARKS
AND TRADE NAMES
We own or have rights to
use a number of registered and common law trademarks, service marks and/or trade names in connection with our business in the United States
and/or in certain foreign jurisdictions.
Solely for convenience, the
trademarks, service marks, logos and trade names referred to in this prospectus are without the ® and ™
symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable
law, our rights or the rights of the applicable licensors to these trademarks, service marks and trade names. This prospectus contains
additional trademarks, service marks and trade names of others, which are the property of their respective owners. All trademarks, service
marks and trade names appearing in this prospectus are, to our knowledge, the property of their respective owners. We do not intend our
use or display of other companies’ trademarks, service marks, copyrights or trade names to imply a relationship with, or endorsement
or sponsorship of us by, any other companies.
Virpax® is a registered
tradename for Virpax Pharmaceuticals, Inc. It was registered under the United States Patent and Trademark Office under serial number 87897821
on December 11, 2018. Our logo is a registered tradename for Virpax Pharmaceuticals, Inc. It was registered under the United States Patent
and Trademark Office (the “USPTO”) under serial number 87897809 on January 1, 2019. In this prospectus, Virpax® is referred
to as Virpax. Additionally, “we”, “our”, “the company” will be synonymous with Virpax. We have obtained
a notice of allowance for our trademark AnQlar™. We have filed for trademark protection with the USPTO for Probudur™, Epoladerm™,
NobrXiol™, and Envelta™.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Certain statements in this prospectus may contain
“forward-looking statements” within the meaning of the federal securities laws. Our forward-looking statements include, but
are not limited to, statements about us and our industry, as well as statements regarding our or our management team’s expectations,
hopes, beliefs, intentions or strategies regarding the future. Additionally, any statements that refer to projections, forecasts or other
characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. We intend the
forward-looking statements to be covered by the safe harbor provisions of the federal securities laws. Words such as “may,”
“should,” “could,” “would,” “predicts,” “potential,” “continue,”
“expects,” “anticipates,” “future,” “intends,” “plans,” “believes,”
“estimates,” and similar expressions, as well as statements in future tense, may identify forward-looking statements, but
the absence of these words does not mean that a statement is not forward-looking.
Forward-looking statements should not be read as a
guarantee of future performance or results and may not be accurate indications of when such performance or results will be achieved. Forward-looking
statements are based on information we have when those statements are made or management’s good faith belief as of that time with
respect to future events, and are subject to significant risks and uncertainties that could cause actual performance or results to differ
materially from those expressed in or suggested by the forward-looking statements. Important factors that could cause such differences
include, but are not limited to:
| |
● |
Our expected use of proceeds from this offering; |
| |
● |
Our limited operating history makes it difficult for us to evaluate our future business prospects; |
| |
● |
Our ability to continue as a going concern; |
| |
● |
The expectation that we will incur significant operating losses for the foreseeable future and will need significant additional capital; |
| |
● |
Our current and future capital requirements to support our development and commercialization efforts for our product candidates and our ability to satisfy our capital needs; |
| |
● |
Risks relating to ownership of our Common Stock, including high volatility and dilution; |
| |
● |
Our lack of operating history; |
| |
● |
The outcome of certain current litigation in which we and our former Chief Executive Officer are named as defendants; |
| |
● |
Our ability to raise additional capital; |
| |
● |
Our dependence on our product candidates, which are still in preclinical or early stages of clinical development; |
| |
● |
Our, or that of our third-party manufacturers, ability to manufacture current good manufacturing practice (“cGMP”) quantities of our product candidates as required for preclinical and clinical trials and, subsequently, our ability to manufacture commercial quantities of our product candidates; |
| |
● |
Our ability to complete required clinical trials for our product candidates and obtain approval from the US Food and Drug Administration (“FDA”) or other regulatory agencies in different jurisdictions; |
| |
● |
Our lack of a sales and marketing organization and our ability to commercialize our product candidates if we obtain regulatory approval; |
| |
● |
Our dependence on third parties to manufacture our product candidates; |
| |
● |
Our reliance on third-party contract research organizations (“CROs”) to conduct our clinical trials; |
| |
● |
Our ability to maintain or protect the validity of our intellectual property; |
| |
● |
Our ability to internally develop new inventions and intellectual property; |
| |
● |
Interpretations of current laws and the passages of future laws; |
| |
● |
Acceptance of our business model by investors; |
| |
● |
The accuracy of our estimates regarding expenses and capital requirements; |
| |
|
|
| |
● |
Our ability to maintain retention of key directors, officers and employees due to the recent cuts in salaries, insurance coverage and resources, which could result in significant disruptions to our business; |
| |
● |
Our ability to maintain our Nasdaq listing; and |
| |
● |
Our ability to adequately support organizational and business growth. |
The risks and uncertainties
included here are not exhaustive or necessarily in order of importance. Other sections of this prospectus, including “Risk Factors”
beginning on page 13, our Annual Report, as updated with the Risk Factors set forth in our Annual Report for year ended December
31, 2024, and other reports that we file with the SEC include additional factors that could affect our businesses and financial performance.
Moreover, we operate in a rapidly changing and competitive environment. New risk factors emerge from time to time, and it is not possible
for management to predict all such risk factors.
Further, it is not possible
to assess the effect of all risk factors on our businesses or the extent to which any factor, or combination of factors, may cause actual
results to differ materially from those contained in any forward-looking statements. Given these risks and uncertainties, investors should
not place undue reliance on forward-looking statements as a prediction of actual results. In addition, we disclaim any obligation to correct
or update any forward-looking statements to reflect events or circumstances that occur after the date of this prospectus.
PROSPECTUS SUMMARY
The following summary highlights information
contained elsewhere in this prospectus or incorporated by reference herein and does not contain all the information that may be important
to purchasers of our Securities. Prospective purchasers of our securities should carefully read the entire prospectus, including the
risks of investing in our securities discussed under in the section entitled “Risk Factors” commencing on Page 13 of
this prospectus and under similar headings in the other documents that are incorporated by reference into this prospectus. Prospective
purchasers of our securities should also carefully read the information incorporated by reference into this prospectus, including our
financial statements, and the exhibits to the registration statement of which this prospectus is a part.
Our Company
We are a preclinical-stage
pharmaceutical company focused on developing novel and proprietary drug delivery systems across various pain indications in order to enhance
compliance and optimize each product candidate in our pipeline. Our drug-delivery systems and drug-releasing technologies being developed
are focused on advancing non-opioid and non-addictive pain management treatments and treatments for central nervous system (“CNS”)
disorders to enhance patients’ quality of life.
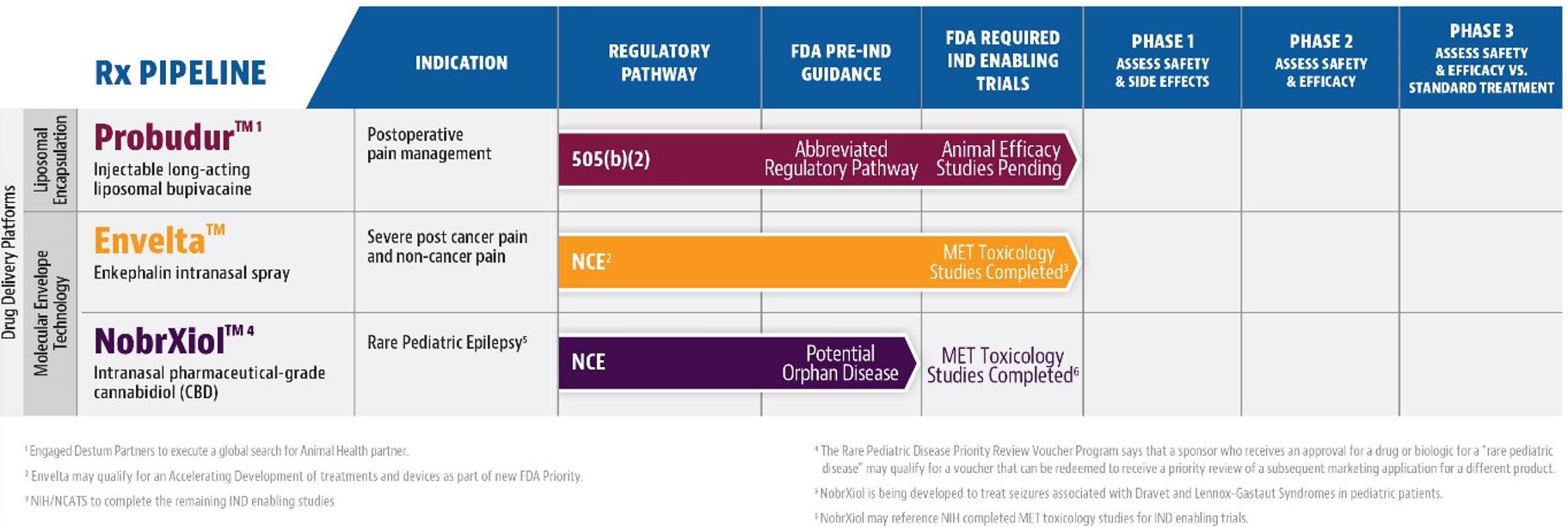
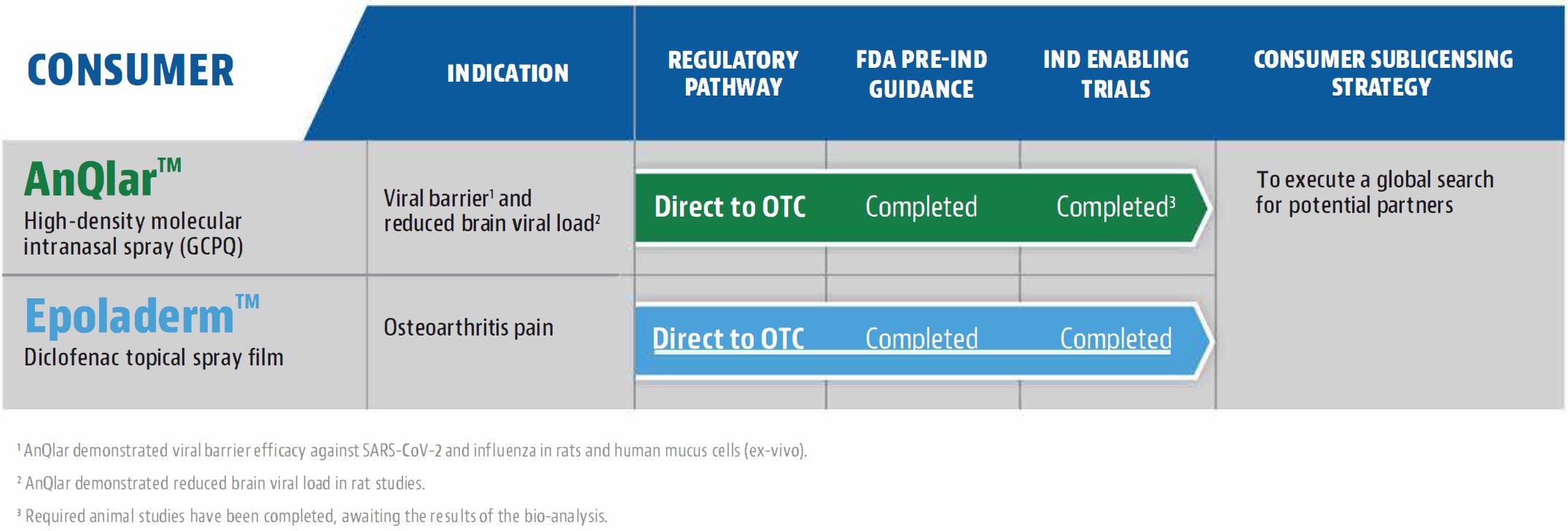
We have exclusive global
rights to the following proprietary patented technologies: (i) Molecular Envelope Technology (“MET”) that uses an intranasal
device to deliver enkephalin for the management of severe pain, including post cancer pain (Envelta™) and post-traumatic stress
disorder (“PTSD”), (ii) Injectable “local anesthetic” Liposomal Technology for postoperative pain management (Probudur™),
and (iii) Investigational formulation delivered via the nasal route to enhance pharmaceutical-grade cannabidiol (“CBD”) transport
to the brain (“NobrXiol™”, formerly VRP324) to potentially treat epileptic seizures associated with Lennox-Gastaut syndrome
(LGS) and Dravet syndrome (DS) in pediatric patients two years of age and older. We are also exploring value creative opportunities for
our two nonprescription product candidates including seeking regulatory approval for commercialization of such products: AnQlar™,
which is being developed as a 24-hour prophylactic viral barrier to inhibit viral infection by influenza or SARS-CoV-2, and Epoladerm™,
which is a topical diclofenac epolamine metered dosed spray film formulation being developed to manage pain associated with osteoarthritis.
Our portfolio currently consists of multiple preclinical
stage product candidates: Epoladerm, Probudur, Envelta, AnQlar and NobrXiol. We anticipate commencing clinical trials for Probudur in
2025 and Envelta in 2026 but there can be no assurances that the trials will commence on the anticipated timeline presented, or at all.
This estimate assumes no disruptions to the ongoing operations and the product development process. We have recently implemented significant
cost-cutting measures and eliminated our directors and officers insurance coverage, as described further in the risk factors. There is
a risk that the Company will not be able to retain or replace key directors, officers, and employees, who may seek alternative employment
opportunities, which could result in significant disruptions to our business and cause delay in the above mentioned clinical trial timeline.
Recent Developments
On March 5, 2025, the Board
of Directors (the “Board”) approved a 1-for-25 reverse stock split (the “Reverse Split”) of the Company’s
outstanding shares of common stock. The Company filed a certificate of amendment to its Amended and Restated Certificate of Incorporation
on March 12, 2025, to effect the Reverse Split, which will become effective at 12:01 a.m. Eastern Time on March 21, 2025. All share and
per share amounts in this prospectus have been retroactively adjusted for all periods presented to give effect to the Reverse Split.
Corporate Governance
On February 5, 2025, the
Board appointed Mr. Charn Deol as an independent director of the Company, effective immediately. Mr. Deol was appointed to fill the vacancy
created by the resignation of Jaydriane (Jay) Panis, who resigned from the Board on February 3, 2025. In connection with his appointment,
Mr. Deol has also been designated as the Chair of the Audit Committee, and as a member of the Compensation Committee and the Nominating
and Corporate Governance Committee.
On January 17, 2025, the
Board, by written consent, approved several key governance appointments. Mr. Jatinder Dhaliwal, a current director and Chief Executive
Officer, was appointed Chairman of the Board, and Judy Su was named Lead Independent Director. Esha Randhawa was appointed Chair of the
Science and Technology Committee, while Jaydriane Panis was appointed Chair of the Audit Committee and joined the Compensation and Nominating
and Corporate Governance Committees. Additionally, Ms. Randhawa and Mr. Panis were appointed as Class III Directors.
Bylaws
On November 27, 2024, we amended our Amended and Restated
Bylaws to modify the quorum requirement for shareholder meetings. The amendment reduced the quorum threshold from a majority of the voting
power of the Company’s outstanding shares entitled to vote at a meeting to 34% of such voting power.
Directors
Mr. Jaydriane (Jay) Panis
On December 17, 2024, we appointed Mr. Jaydriane (Jay)
Panis to our Board to fill the vacancy created by the resignation of Mr. Gary Herman on December 6, 2024. Mr. Panis is a Certified Financial
Planner (CFP) with more than 20 years of experience in the financial services industry. He is the founder of his own financial planning
practice and serves as a director at a prominent Canadian financial planning firm based in British Columbia. Mr. Panis holds diplomas
in Financial Management and Business Management and has a strong track record of helping clients achieve their financial goals through
effective planning and leadership.
On February 3, 2025, we accepted the resignation of
Mr. Jay Panis as a member of the Board and from all committees on which he served, effective immediately.
Mr. Gary Herman
On December 6, 2024, we accepted the resignation of
Mr. Gary Herman as a member of the Board and from all committees on which he served, effective immediately.
Mr. Herman’s resignation was not due to any
disagreement with the Company, its management, the Board, or any matter relating to our operations, policies, or practices. We expressed
our gratitude to Mr. Herman for his service and contributions to the Board.
Mr. Eric Floyd
On September 20, 2024, Eric Floyd, Chairman of the
Board, notified the Company of his intention to resign from the Board, effective immediately.
Ms. Esha Randhawa
On November 19, 2024, we appointed Ms. Esha Randhawa
as an independent director and member of the Audit Committee. Ms. Randhawa’s appointment remedied the deficiencies resulting from
the resignation of Dr. Eric Floyd on September 20, 2024, which had caused us to fall out of compliance with Nasdaq Listing
Rules 5605(b)(1) and 5605(c)(2).
Randhawa serves as a member of the following committees
of the Board: (1) the Audit Committee, alongside Ms. Judy Su and Mr. Deol, (2) the Compensation Committee, where she serves as Chair alongside
Ms. Su and Mr. Deol, (3) the Nominating and Corporate Governance Committee, where she serves as Chair alongside Ms. Su and Mr. Deol, and
(4) the Science and Technology Committee, where she serves alongside Mr. Dhaliwal, Ms. Su, and Ms. Katharyn Field.
Ms. Randhawa is an experienced pharmacist and entrepreneur
with a strong background in pharmaceutical sciences. She holds a Bachelor of Pharmacy degree, with a focus in Pharmaceutical Sciences,
and a Bachelor of Science in Biology, both completed at The University of British Columbia in Vancouver. Ms. Randhawa is the Co-Founder
of Green Light Cannabis, where she applies her extens1ive knowledge of the healthcare and retail industries to develop innovative solutions
and strategic initiatives. Her expertise in pharmaceutical sciences and business operations brings a unique perspective to the Board and
supports the Company’s commitment to advancing its mission.
Mr. Charn Deol
On February 5, 2025, the Board appointed Mr. Charn
Deol as an independent director of the Company, effective immediately. Mr. Deol was appointed to fill the vacancy created by the resignation
of Jaydriane (Jay) Panis, who resigned from the Board on February 3, 2025. In connection with his appointment, Mr. Deol has also been
designated as the Chair of the Audit Committee, and as a member of the Compensation Committee and the Nominating and Corporate Governance
Committee. Mr. Deol will receive a monthly compensation of $3,000 for his service as a director. There are no arrangements or understandings
between Mr. Deol and any other persons pursuant to which he was appointed as a director.
Mr. Deol is a multi-industry executive with over 35
years of public company management experience. His recent experience includes serving as a director or in management roles for numerous
Canadian private and public companies, including his current positions as a director of Bayridge Resources Inc., Trilogy AI Corp, and
Green Battery Minerals Inc., as well as Chief Financial Officer at Akanda Corp. He has extensive experience in the public markets, including
guiding private Canadian companies through the regulatory process of going public.
Chief Executive Officer
On November 19, 2024, we entered into an Independent
Contractor Agreement (the “Agreement”) with Jatinder Dhaliwal, the Company’s Chief Executive Officer (the “CEO”)
and a member of the Board, through his personal corporation, Jat Consulting Corp., a company incorporated under the laws of the Province
of British Columbia, Canada.
On December 30, 2024, we entered into an amendment
(the “Amendment”) to the Agreement to clarify certain provisions. The Amendment confirms that the term “Contractor”
in the Agreement refers exclusively to Jat Consulting Corp., through which Mr. Dhaliwal provides his services as CEO. The Amendment also
clarifies that all payments under the Agreement are made directly to Jat Consulting Corp., which is responsible for compensating its personnel,
including Mr. Dhaliwal. Additionally, the Amendment addresses tax responsibilities, indemnification provisions, and other terms to ensure
compliance with applicable laws and regulations.
Mr. Dhaliwal was appointed as the Company’s
CEO on October 6, 2024. Under the Agreement, Mr. Dhaliwal will provide strategic leadership, oversight, and advisory services, including
advancing the Company’s drug development programs and regulatory applications, achieving key regulatory milestones, and performing
other responsibilities consistent with his position as CEO. Mr. Dhaliwal brings extensive leadership experience in the pharmaceutical
and biotechnology industries. His expertise positions him to lead the Company in achieving its strategic objectives. There are no family
relationships between Mr. Dhaliwal and any director, executive officer, or other significant person at the Company. Additionally, Mr.
Dhaliwal has not been involved in any legal proceedings required to be disclosed under Item 401(e) of Regulation S-K in the past ten years.
Under the terms of the Agreement, the Company will
pay Jat Consulting Corp. an annual fee of $200,000, payable in equal monthly installments, and milestone-based incentive payments tied
to the achievement of key regulatory and clinical development objectives, including Investigational New Drug (IND) status, commencement
of clinical trials, and FDA approval. The Agreement also includes confidentiality obligations that survive termination and provides for
a severance payment equivalent to $200,000 in the event of termination without cause or resignation for good reason.
Notice of Special Meeting
The 2025 Special Meeting
of Stockholders (the “2025 Special Meeting”) of the Company was held on January 15, 2025. At the meeting, the Company’s
stockholders approved an amendment to the Company’s Amended and Restated Certificate of Incorporation to authorize a reverse stock
split of the Company’s issued and outstanding common stock, par value $0.00001 per share, including stock held by the Company as
treasury shares. The reverse stock split will be implemented at a ratio within a range of 1-for-2 to 1-for-240, with the exact ratio to
be determined by the Board of Directors and announced publicly. Stockholders also elected Jatinder Dhaliwal, Katharyn Field, Jaydriane
Panis, Judy Su, and Esha Randhawa to serve as directors until the 2025 Annual Meeting of Shareholders or until their respective successors
are duly elected and qualified. Additionally, stockholders approved a proposal to adjourn the 2025 Special Meeting, if necessary, to solicit
additional votes in favor of the reverse stock split proposal or the election of directors. Only stockholders of record at the close of
business on November 20, 2024, were entitled to receive notice of and vote at the 2025 Special Meeting or any postponement or adjournment
thereof.
Chief Financial Officer
On November 18, 2024, we appointed Mr. Usama Chaudhry
as Chief Financial Officer of the Company under an Independent Contractor Agreement with Chaudhry U Consulting Inc., a Canadian corporation
represented by Mr. Chaudhry. In this role, Mr. Chaudhry will oversee the Company’s financial reporting, budgeting, and compliance
functions, as well as develop and implement financial strategies.
On December 30, 2024, we entered into an amendment
(the “Amendment”) to the Agreement to clarify certain provisions. The Amendment confirms that the term “Contractor”
in the Agreement refers exclusively to Chaudhry U Consulting Inc., through which Mr. Chaudhry provides his services as CFO. The Amendment
also clarifies that all payments under the Agreement are made directly to Chaudhry U Consulting Inc., which is responsible for compensating
its personnel, including Mr. Chaudhry. Additionally, the Amendment addresses tax responsibilities, indemnification provisions, and other
terms to ensure compliance with applicable laws and regulations.
Mr. Chaudhry is a seasoned executive with extensive
expertise in corporate development, investor relations, financial reporting, and corporate governance. He currently serves on several
public company boards and has a track record of aligning strategic objectives with cost-control measures to enhance organizational performance.
Mr. Chaudhry earned a Bachelor of Commerce degree, majoring in accounting, from the University of Northern British Columbia.
There are no family relationships between Mr. Chaudhry
and any director, executive officer, or other significant person at the Company. Additionally, Mr. Chaudhry has not been involved in any
legal proceedings required to be disclosed under Item 401(e) of Regulation S-K in the past ten years.
Under the terms of the Independent Contractor Agreement,
we will pay Chaudhry U Consulting Inc. an annual fee of $180,000, payable in monthly installments, and reimburse reasonable, pre-approved
expenses incurred in the performance of services.
Investor Relations Agreement
On November 12, 2024, we
entered into an investor relations agreement (the “IR Agreement”) with IR Agency LLC (the “Consultant”). Under
the IR Agreement, the Consultant will provide marketing and advertising services to promote the Company to the financial community. In
consideration for these services, we paid the Consultant a fee, for an initial term of one month, after which the agreement may be extended
by mutual agreement, of two million U.S. Dollars ($2,000,000), payable in cash. Either party may terminate the IR Agreement at any time
by providing written notice. A copy of the IR Agreement is filed as Exhibit 10.48 to this Form S-1.
New Management
On October 5, 2024, Gerald
Bruce, the former Chief Executive Officer of the Company, and Vinay Shah, the former Chief Financial Officer of the Company, notified
the Company of their resignations from their respective positions, effective immediately.
On October 6, 2024, the Board
appointed Jatinder Dhaliwal, a current member of the Board, as Chief Executive Officer of the Company, effective immediately. Mr. Dhaliwal
will continue to serve as a member of the Board, and Katharyn Field will replace him on the Board’s Audit Committee. Mr. Dhaliwal
will also serve as the Principal Financial Officer of the Company.
Litigation
On February 29, 2024, Sorrento Therapeutics, Inc.
(“Sorrento”), and Scilex Pharmaceuticals Inc. (“Scilex” and together with Sorrento, the “Plaintiffs”)
and the Company entered into a Settlement Agreement and Mutual Release (the “Settlement Agreement”) to fully resolve all claims
by the Plaintiffs against the Company related to the litigation in the Chancery Court of the State of Delaware (the “Chancery
Court”) captioned Sorrento Therapeutics, Inc. and Scilex Pharmaceuticals Inc. v. Anthony Mack and Virpax Pharmaceuticals,
Inc., Case No. 2021-0210-PAF, (the “Action”), subject to the entry by the United States Bankruptcy Court for the Southern
District of Texas, which is handling the Sorrento bankruptcy filing (the “Bankruptcy Court”), of an order approving the Settlement
Agreement (the “Settlement Order”). On March 1, 2024, the Plaintiffs filed a motion with the Bankruptcy Court to approve the
Settlement Agreement and grant the related relief. On March 14, 2024, the Bankruptcy Court entered an order approving the Settlement Agreement
and on March 20, 2024 the Plaintiffs filed a Stipulation of Dismissal with the Chancery Court dismissing the Action. The Chancery Court
has not yet acted upon the filing. See “Part II—Item 1—Legal Proceedings” in our Quarterly Report on Form 10-Q
for the three and nine months ended September 30, 2024 incorporated herein by reference for additional information regarding the litigation
with the Plaintiffs.
Pursuant to the Settlement
Agreement, we agreed to pay Sorrento and Scilex a total cash payment of $6 million, of which $3.5 million was paid two business days after
the date that the Settlement Order was entered by the Bankruptcy Court (the “Effective Date”), which payment was made on March
18, 2024 and the remaining $2.5 million was paid on July 8, 2024. Additionally, we agreed to pay to Plaintiffs royalties of 6% of annual
net sales of products developed from drug candidates Epoladerm, Probudur and Envelta until the earlier of the expiration of the last-to-expire
valid patent claim of such product and the expiration of any period of regulatory exclusivity for such product.
Pursuant to the Settlement
Agreement, each of the Plaintiffs and we provided mutual releases of all claims as of the Effective Date, whether known or unknown, arising
from any allegations set forth in the Action. Plaintiffs’ release relates to claims against us only. Plaintiffs’ release as
to us was effective upon our initial payment of $3.5 million, and our release of the Plaintiffs was effective on the Effective Date.
The Plaintiffs can still
pursue claims against Mr. Mack. Our bylaws require us to “indemnify any person who was or is a party or is threatened to be made
a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other
than an action by or in the right of the Corporation) by reason of the fact that such person is or was a director or officer of the Corporation,
or, while a director or officer of the Corporation.” Such indemnification, however, is limited to circumstances where the covered
person “acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the
Corporation.” Mr. Mack may attempt to claim he is entitled to indemnification, should the Chancery Court find him liable for damages
in the Action. Given the findings in the Memorandum Opinion issued in the Action, we believe we have a strong position that Mr. Mack would
not be entitled to indemnification. There is a risk, however, that a court could find he is entitled to such indemnification. Additionally,
per Section 7.6 of the bylaws, we had been advancing Mr. Mack’s attorneys’ fees and costs for the Action. It is likely Mr.
Mack will contend he is still entitled to advancement of any fees and/or costs for the Action going forward and may seek judicial intervention.
However, as per the bylaws, Mr. Mack is only entitled to advancement of expenses for indemnifiable actions. As noted above, given the
Memorandum Opinion in the Action, we believe that we have a strong position that Mr. Mack is not entitled to indemnification, and therefore,
not entitled to advancement of expenses. However, there is a risk that a court could find that Mr. Mack is entitled to such advancement.
Further, Mr. Mack may attempt to seek damages from us based on the Chancery Court’s final judgment on damages under the theory of
joint and several liability and/or seek contribution from us for any monetary judgment.
Given the Settlement Agreement
does not release Mr. Mack from liability related to the Action, the Chancery Court has requested supplemental briefing as to whether the
Chancery Court can dismiss us from the lawsuit, as well as any claims Mr. Mack has against us arising from the Action. While we believe
that any damages assessed may be awarded against Mr. Mack alone, Plaintiffs cannot seek additional damages from us. However, there is
a risk that Mr. Mack will still seek contribution from us for any damages claim arising from the Action and, there is a risk that the
Chancery Court will rule in Mr. Mack’s favor. Any such amounts for indemnification, contribution or other amounts awarded by the
Chancery Court in Mr. Mack’s favor could be significant.
No further reimbursements are permitted from our insurance
policy with respect to the litigation. Accordingly, if Mr. Mack were to successfully seek indemnification from us, we would have to pay
such amounts in cash which would further reduce our cash position.
Employment Dispute
On September 3, 2024, we
received a letter from Kagen, Caspersen & Bogart PLLC, legal counsel to Gerald Bruce, our former Chief Executive Officer, and Vinay
Shah, our former Chief Financial Officer, regarding their employment agreements with the Company. In the letter, they alleged that the
Company had violated their employment agreements by reducing their contractual base compensation by 50%, and claim that, as a result,
the executives may resign for good reason after the 30-day cure period and be entitled to severance compensation. The Company disagrees
with their interpretation of the employment agreements and considers its actions to be consistent with the terms of the agreements. Accordingly,
the Company denies the allegations and intends to vigorously defend the matter. On October 5, 2024, Gerald Bruce, our former Chief Executive
Officer and member of the Board, and Vinay Shah, our former Chief Financial Officer, notified the Company of their resignation from their
respective positions, effective immediately.
Nasdaq Compliance
Stockholders’ Equity. On April 2, 2024,
we received a letter from the Listing Qualifications Staff of the Nasdaq Stock Market LLC (“Nasdaq”) notifying us that our
stockholders’ equity as reported in our Annual Report on Form 10-K for the period ended December 31, 2023 (the “Annual Report”),
did not meet the minimum stockholders’ equity requirement for continued listing on the Nasdaq Capital Market. Nasdaq Listing Rule
5550(b)(1) requires companies listed on the Nasdaq Capital Market to maintain stockholders’ equity of at least $2,500,000 (the “Minimum
Stockholders’ Equity Rule”). In the Annual Report, we reported stockholders’ equity of $1,934,321, which is below the
minimum stockholders’ equity required for continued listing pursuant to Nasdaq Listing Rule 5550(b)(1). Additionally, as of the
date of this prospectus, we do not meet the alternative Nasdaq continued listing standards under Nasdaq Listing Rules. In our Report on
Form 10-K for the year ended December 31, 2024, we reported stockholders’ deficit of $0.9 million.
Pursuant to Nasdaq’s Listing Rules, we were
given 45 calendar days (or until May 17, 2024), to submit a plan to evidence compliance with the Minimum Stockholders’ Equity Rule
(a “Compliance Plan”). We submitted a Compliance Plan within the required time period, which included raising equity in public
offerings. On July 29, 2024, we received notice from the Listing Qualifications Staff (the “Staff”)
of Nasdaq that we were granted an extension through
September 30, 2024 to regain compliance with
Nasdaq Listing Rule 5550(b)(1). On October 3, 2024, we received a delist determination letter from the Staff advising the Company
that the Staff had determined that the Company did not meet the terms of the extension. As a result, we requested an appeal of the
Staff’s determination. We submitted a hearing request to the Nasdaq Hearings Panel (the “Panel”), which request is expected
to stay any delisting action by the Staff at least until the hearing process concludes and any extension granted by the Panel expires.
On January 6, 2025, we received a decision letter
from the Panel, granting the Company’s request to continue its listing on Nasdaq, subject to certain conditions. The Panel’s
decision provides the Company with an exception until April 1, 2025, to demonstrate compliance with Minimum Stockholders’ Equity
Rule.
The Panel reviewed the Company’s compliance
plan, which included recent and planned fundraising activities, cost-cutting measures, and strategies for achieving compliance with the
Minimum Stockholders’ Equity Rule. As part of the conditions outlined in the Panel’s decision, the Company is required to
file public disclosures by April 1, 2025, describing the transactions and financial measures undertaken to regain compliance, along with
additional income projections and evidence of compliance with all applicable Nasdaq listing criteria.
We may not be able to comply with the Minimum Stockholders’
Equity Rule even after this offering due to our cash burn rate, operating expenses, and payment obligations. As a result, we may be required
to raise additional funds after this offering in order to achieve compliance.
Minimum Bid Price. On June 28, 2024, we received
written notice from the Listing Qualifications Department of Nasdaq notifying us that for the preceding 30 consecutive business days (May
15, 2024 through June 27, 2024), the Company’s Common Stock did not maintain a minimum closing bid price of $1.00 (“Minimum
Bid Price Rule”) per share as required by Nasdaq Listing Rule 5550(a)(2).
In accordance
with Nasdaq Listing Rule 5810(c)(3)(A), we were given a compliance period of 180 calendar days, or until December 26, 2024, to regain
compliance with Nasdaq Listing Rule 5550(a)(2).
On July 22,
2024, the Company received a notice from the Listing Qualifications Department of Nasdaq notifying the Company that the staff has determined
that for 10 consecutive business days, from July 8, 2024 to July 19, 2024, the closing bid price of the Company’s shares of Common
Stock had been at $1.00 per share or greater. Accordingly, the staff had determined that the Company had regained compliance with Listing
Rule 5550(a)(2).
On October 4,
2024, the Company received a deficiency letter from the Staff of Nasdaq notifying the Company that, for the last 30 consecutive business
days, the closing bid price for the Company’s common stock had been below the minimum $1.00 per share required for continued listing
on Nasdaq pursuant to Nasdaq Listing Rule 5550(a)(2). The Nasdaq deficiency letter has no immediate effect on the listing of the Company’s
common stock, and our common stock will continue to trade on The Nasdaq Capital Market under the symbol “VRPX”.
In accordance
with Nasdaq Listing Rule 5810(c)(3)(A), the Company has been given 180 calendar days, or until April 2, 2025, to regain compliance with
the Minimum Bid Price Rule. If at any time before April 2, 2025, the bid price of the Company’s common stock closes at $1.00 per
share or more for a minimum of 10 consecutive business days, the Staff will provide written confirmation that the Company has achieved
compliance.
If the Company
does not regain compliance with the Minimum Bid Price Rule by April 2, 2025, the Company may be afforded a second 180 calendar days period
to regain. The Company would be required to notify Nasdaq of its intent to cure the deficiency during the second compliance period. If
the Company does not regain compliance with the Minimum Bid Price Rule by the end of the compliance period (or the second compliance period,
if applicable), the Company’s common stock will become subject to delisting. In the event that the Company receives notice that
its common stock is being delisted, the Nasdaq listing rules permit the Company to appeal a delisting determination by the Staff to a
hearings panel.
The Company
intends to monitor the closing bid price of its common stock and may, if appropriate, consider available options to regain compliance
with the Minimum Bid Price Rule, including initiating a reverse stock split. However, there can be no assurance that the Company will
be able to regain compliance with the Minimum Bid Price Rule or will otherwise be in compliance with other Nasdaq Listing Rules.
Independent Director
and Audit Committee Requirements. On November 19, 2024, we received a letter from Nasdaq notifying the Company that, as a result
of the resignation of Dr. Eric Floyd from the Board and Audit Committee on September 20, 2024, we were no longer in compliance with Nasdaq’s
independent director and Audit Committee requirements under Listing Rules 5605(b)(1) and 5605(c)(2).
On November 25, 2024, we received a letter from
Nasdaq notifying us that we had regained compliance with the independent director and audit committee requirements for continued listing
on the Nasdaq Capital Market, as set forth in Listing Rules 5605(b)(1) and 5605(c)(2). Nasdaq has confirmed that, as of November
25, 2024, we regained compliance with the rules, and the matter is now closed.
Financing
On January 27, 2025, the Company entered into a placement
agency agreement with Spartan Capital Securities, LLC, and a securities purchase agreement with investors pursuant to which the Company
agreed to issue and sell, in a “reasonable best efforts” public offering (the “Offering”) (i) 138,000 shares (the
“Shares”) of the Company’s common stock, par value $0.00001 per share (the “Common Stock”), and (ii) pre-funded
warrants to purchase up to 1,062,000 shares of Common Stock (the “Pre-Funded Warrants”) at an offering price of $5.00 per
Share, less $0.00001 per Pre-Funded Warrant, for aggregate gross proceeds of $6,000,000, assuming the full exercise of the Pre-Funded
Warrants, and before deducting placement agent fees and other offering expenses. As part of its compensation for acting as Placement Agent
for the Offering, the Company paid the Placement Agent a cash fee of 2.5% of the aggregate gross proceeds, in addition to 1.0% of the
gross proceeds for non-accountable expenses, and reimbursed the Placement Agent for other expenses incurred by the Placement Agent, including
legal fees. The Company intends to use the net proceeds of the Offering to fund development activities for commencing a clinical trial
for Probudur, the Company’s formulation injected at a wound site to provide pain relief, $2,000,000 for marketing and
advertising services, as well as other general corporate purposes.
On January 29, 2025, the Company entered into an investor
relations agreement (the “IR Agreement”) with IR Agency LLC (the “Consultant”). Under the IR Agreement, the Consultant
will provide marketing and advertising services to promote the Company to the financial community. In consideration for these services,
the Company will pay the Consultant a fee, for an initial term of one month, after which it may be extended by mutual agreement, of one
million seven hundred thousand U.S. Dollars ($1,700,000), payable in cash. Either party may terminate the IR Agreement at any time by
providing written notice.
On January 30, 2025, the Company entered into an investor
relations agreement (the “Marketing Agreement”) with Sideways Frequency LLC (“Sideways”). Under the Marketing
Agreement, Sideways will provide marketing and advertising services to promote the Company to the financial community. In consideration
for these services, the Company will pay Sideways a fee, for an initial term of one month, after which it may be extended by mutual agreement,
of three hundred thousand U.S. Dollars ($300,000), payable in cash. Either party may terminate the Marketing Agreement at any time by
providing written notice.
The Offering closed on January 29, 2025. The securities
sold in the Offering were offered and sold pursuant to a registration statement on Form S-1 (File No. 333-284089), which was initially
filed with the Securities and Exchange Commission (the “Commission”) on December 30, 2024, and amended on January 15, 2025
and January 17, 2025, and declared effective by the Commission on January 27, 2025.
Positive Results
On July 10, 2024, we issued
a press release announcing positive results for a Swine Model pilot study for Probudur, our long-acting liposomal bupivacaine formulation.
The pharmacokinetics (“PK”) and safety study of Probudur in the Swine Model was designed to determine the PK profile of Probudur
as well as to ascertain any adverse effects on the pigs. Probudur was subcutaneously injected into four juvenile domestic pigs at a dose
of 30 mg/kg and was well-tolerated by all of the pigs and demonstrated a long-term, slow-release profile. Histopathology was also conducted
at the injection site and Probudur was well-tolerated by all pigs in this study.
Elimination of Directors
and Officers Insurance
As part of recent cost-cutting measures, we have eliminated
our directors and officers (“D&O”) insurance coverage. This decision was made in an effort to preserve capital and manage
our financial resources more effectively. Without D&O insurance, our directors and officers will not have insurance protection in
the event of legal claims made against them in their capacities as executives of the Company. In such circumstances, the Company may be
required to indemnify its directors and officers under applicable law and agreements, which could result in a significant financial burden.
The absence of D&O insurance may also impact our ability to retain and attract qualified individuals to serve as directors and officers
of the Company.
Discontinuation of Out-Licensing Agreements
In July 2024, we submitted a plan to Nasdaq that included
the pursuit of out-licensing agreements for certain of our product candidates. However, as part of our efforts to reduce expenditures,
we have elected to discontinue our pursuit of any out-licensing agreements at this time.
Corporate Information
We were incorporated under
the laws of the State of Delaware on May 12, 2017. Our principal executive offices are located at 1055 Westlakes Drive, Suite 300, Berwyn,
Pennsylvania 19312. Our telephone number is (610) 727-4597.
Our website address is www.virpaxpharma.com.
The information contained in, or accessible through, our website does not constitute a part of this prospectus. You should not rely on
any such information in making your decision whether to purchase our Common Stock.
Implications of Being an Emerging Growth Company
We qualify as an “emerging growth company”
as defined under the Securities Act of 1933, as amended (the “Securities Act”). As a result, we are permitted to, and intend
to, rely on exemptions from certain disclosure requirements that are otherwise applicable to public companies. These provisions include,
but are not limited to:
| |
● |
being permitted to present only two years of audited financial statements and only two years of related “Management’s Discussion and Analysis of Financial Condition and Results of Operations”; |
| |
● |
not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended (or the Sarbanes-Oxley Act); |
| |
● |
reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements; and |
| |
● |
exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
In addition, an emerging growth company can take advantage
of an extended transition period for complying with new or revised accounting standards. This provision allows an emerging growth company
to delay the adoption of some accounting standards until those standards would otherwise apply to private companies. We have elected to
avail ourselves of this extended transition period. We will remain an emerging growth company until the earliest to occur of: (i) our
reporting $1.235 billion or more in annual gross revenues; (ii) the end of fiscal year 2026; (iii) our issuance, in a three year period,
of more than $1 billion in non-convertible debt; and (iv) the last day of the fiscal year in which we are deemed to be a large accelerated
filer, which generally means that we have been public for at least 12 months, have filed at least one annual report, and the market value
of our Common Stock that is held by non-affiliates exceeds $700 million as of the last day of our then-most recently completed second
fiscal quarter.
We have elected to take advantage of certain of the
reduced disclosure obligations and may elect to take advantage of other reduced reporting requirements in future filings. As a result,
the information that we provide to our stockholders may be different than the information you might receive from other public reporting
companies in which you hold equity interests.
Implications of Being a Smaller Reporting Company
We also qualify as a “smaller reporting company,”
as such term is defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and to the
extent we continue to qualify as a “smaller reporting company,” after we cease to qualify as an “emerging growth company,”
certain of the exemptions available to us as an “emerging growth company” may continue to be available to us as a smaller
reporting company, including: (1) not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley
Act; (2) scaled executive compensation disclosures; and (3) the ability to provide only two years of audited financial statements, instead
of three years.
The Offering
| Shares being offered |
|
Up to 2,415,459 shares of Common Stock at an assumed public offering price of $2.07 per share (the last reported sale price of our Common Stock on the Nasdaq Capital Market on March 21, 2025). |
| |
|
|
| Pre-Funded Warrants being offered |
|
We are also offering up to 2,415,459 Pre-Funded Warrants to purchase up to 2,415,459 shares of Common Stock in lieu of shares of Common Stock to any purchaser whose purchase of shares of Common Stock in this offering would otherwise result in such purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the purchaser’s election, 9.99%) of our outstanding shares of Common Stock immediately following the consummation of this offering. Each Pre-Funded Warrant will be exercisable for one share of Common Stock at an exercise price of $0.00001 per share, will be immediately exercisable, and will not expire prior to exercise. For each Pre-Funded Warrant that we sell, the number of shares of Common Stock that we are selling will be decreased on a one-for-one basis. This prospectus also relates to the offering of the shares of Common Stock issuable upon exercise of the Pre-Funded Warrants. |
| |
|
|
| Number of shares of Common Stock outstanding immediately before this offering |
|
1,278,504 shares. |
| |
|
|
| Number of shares of Common Stock to be outstanding after this offering (1) |
|
3,693,963 shares (assuming all of the shares of Common Stock we are offering under this prospectus are sold and assuming no sale of Pre-Funded Warrants, which, if sold, would reduce the number of shares of Common Stock that we are offering on a one-for-one basis). |
| |
|
|
| Reasonable best efforts |
|
We have agreed to offer and sell the securities offered hereby to the purchasers through the Placement Agent. The Placement Agent is not required to buy or sell any Securities offered hereby but rather has agreed to use its reasonable best efforts to solicit offers to purchase the Securities offered by this prospectus. See “Plan of Distribution”. |
| |
|
|
| Use of proceeds |
|
Assuming 2,415,459 shares of Common Stock are sold in this offering at an assumed public offering price of $2.07 per share of Common Stock, which represents the closing price of shares of our Common Stock on Nasdaq on March 21, 2025, and assuming no issuance of Pre-Funded Warrants in this offering, we estimate that our net proceeds from the this offering will be approximately $4.7 million, after deducting Placement Agent fees, inclusive of financial advisor fees, and estimated offering expenses payable by us. However, this is a best efforts offering with no minimum number of Securities or amount of proceeds as a condition to closing, and we may not sell all or any of these Securities offered pursuant to this prospectus; as a result, we may receive significantly less in net proceeds. |
| |
|
We intend to use the net proceeds
from this offering to fund our ongoing drug development activities, as well as for working capital and other general corporate purposes.
In addition, we may use $1.7 million for marketing and advertising services to communicate information about the Company to the financial
community. In addition, if Mr. Mack were to seek indemnification and/or damages from us and if he were successful in his claim, we
may determine to use a portion of the proceeds from this offering to make such payments. See “Litigation” under “Recent
Developments” in the Prospectus Summary, above and see “Use of Proceeds” below. |
| |
|
|
| Lock-up Agreements |
|
The Company and our directors, executive
officers, and holders of 5% or more of the issued and outstanding shares of our Common Stock have agreed with the Placement Agent,
subject to certain exceptions, not to sell, transfer or dispose of, directly or indirectly, any of our Common Stock or securities
convertible into or exercisable or exchange for Common Stock for 60 days, after the closing of this offering. See “Plan of
Distribution” for more information. |
| |
|
|
| Nasdaq Capital Market symbol |
|
Shares of our Common Stock are listed on
the Nasdaq Capital Market under the symbol “VRPX”. We do not intend to apply to have the Pre-Funded Warrants listed on
any national securities exchange or other nationally recognized trading system. See “Risk Factors— There is no public
market for the Pre-Funded Warrants being offered in this offering”. |
| |
|
|
| Risk factors |
|
Investing in our securities involves a high
degree of risk. See “Risk Factors” beginning on page 13 of this prospectus and other information included, or incorporated
by reference, in this prospectus for a discussion of factors you should consider carefully before deciding to invest in our securities. |
| |
(1) |
The number of shares of our Common Stock to be outstanding immediately after this offering is based on shares of our Common Stock outstanding as of March 12, 2025, which excludes: |
| |
● |
4,603 shares of Common Stock issuable upon exercise of stock options outstanding as of March 12, 2025, at a weighted-average exercise price of $655.09 per share; |
| |
● |
568,420 shares of Common Stock issuable upon exercise of warrants outstanding as of March 12, 2025, at a weighted-average exercise price of $3.43 per share; and |
| |
● |
48,521 shares of our Common Stock that are available for future issuance under our Virpax Pharmaceuticals, Inc. 2022 Equity Incentive Plan (the “2022 Plan”) or shares that will become available under our 2022 Plan. |
Unless otherwise indicated,
this prospectus reflects and assumes the following:
| |
● |
No exercise of outstanding options or warrants described above; and |
| |
● |
No sale of the Pre-Funded Warrants in this offering. |
RISK FACTORS
Investing in our Securities involves a high degree
of risk. You should consider carefully the risks described below, together with all of the other information included or incorporated
by reference in this prospectus, including the risks and uncertainties discussed under “Risk Factors” in the Annual Report
on Form 10-K for the year ended December 31, 2024, which has been filed with the SEC and is incorporated by reference in this prospectus,
as well as any updates thereto contained in subsequent filings with the SEC or any free writing prospectus, before deciding whether to
purchase our Securities in this offering. All of these risk factors are incorporated herein in their entirety. The risks described below
and incorporated by reference are material risks currently known, expected or reasonably foreseeable by us. However, the risks described
below and incorporated by reference are not the only ones that we face. Additional risks not presently known to us or that we currently
deem immaterial may also affect our business, operating results, prospects or financial condition. If any of these risks actually materialize,
our business, prospects, financial condition, and results of operations could be seriously harmed. This could cause the trading price
of our Common Stock to decline, resulting in a loss of all or part of your investment.
This prospectus also contains forward-looking statements
that involve risks and uncertainties. Our actual results could differ materially from those anticipated in the forward-looking statements
as a result of a number of factors, including the risks described below or incorporated by reference. See the section titled “Cautionary
Note Regarding Forward-Looking Statements.”
Risks Related to Our Financial Position
We require substantial additional capital to
fund our operations, and if we fail to obtain necessary financing, we will not be able to complete the development and commercialization
of our drugs.
Our operations have consumed
substantial amounts of cash since inception. As of December 31, 2024, our cash position totaled approximately $1.5 million and as of
March 21, 2025, our cash position totaled approximately $3.4 million. Our current cash position is not sufficient to enable us to fund
our operations through the second quarter of 2025. Even if we are able to raise the maximum offering amount being offered in this offering,
we will require additional capital again after this offering in the near future and we will be prohibited from using our equity to raise
capital for thirty (30) days after the closing of this offering. There can be no assurance that we will be able to raise capital when
needed. Our failure to raise such additional capital or sufficient capital in this offering could result in us being forced to liquidate
assets or initiate bankruptcy proceedings.
Recent litigation has also
negatively impacted our cash position. As a result of the $6.0 million payment that has been made to the Plaintiffs pursuant to the Settlement
Agreement, our cash position has been significantly decreased. Moreover, the payment of the royalties to the Plaintiffs pursuant to the
terms of the Settlement Agreement, will significantly impact our future revenue and may make it more difficult for us to engage in collaborations,
licenses or the acquisition of certain product candidates, and may result in us ceasing to develop certain product candidates or all of
our product candidates if we determine that it will not be financially profitable to do so. In addition, litigation-related indemnification
and/or contribution payments, if any, that we make to our former Chief Executive Officer, and which may be significant, will further reduce
our cash position.
We will need to spend
substantial amounts to advance the clinical development of and launch and commercialize our product candidates.
The amount we will spend for our clinical development
of and launch and commercialization of our product candidates is difficult to estimate. For example, we estimate that we will require
at least a total of approximately $7.5 million for the completion of planned development for commencing a clinical trial for Probudur
in 2025 and other expenditures that we will need to incur in order to develop our other product candidates and our ongoing operations
during that period, potential cash separation payments to our former Chief Executive Officer and potential marketing and advertising services
expenditure to communicate information about the Company to the financial community. Our estimate of our clinical trial timing may change
and we may need substantially more funds to commence our planned clinical trial for Probudur. The estimate assumes no disruptions to the
ongoing operations and the product development process. We have recently implemented significant cost-cutting measures and eliminated
our D&O insurance coverage,
as described in the risks below. There is a risk that
the Company will not be able to retain or replace key directors, officers, and employees who may seek alternative employment opportunities,
which could result in significant disruptions to our business and cause delay in the above-mentioned clinical trial timeline. The Company
will require additional funding if there is a delay in commencing a clinical trial. Even if we sell the maximum number of Securities in
this offering, we will need to raise additional capital in order to commence our clinical trial. If we are unable to raise capital when
needed or on attractive terms, we could be forced to delay, reduce or eliminate our research and development programs or any future commercialization
efforts. In addition, our strategy for AnQlar and Epoladerm is to license out or partner these assets as we continue to focus our efforts
on our prescription drug pipeline. If we are unsuccessful in our partnering activities and/or financing activities, we may be unable to
develop AnQlar and Epoladerm.
We may face challenges in retaining key personnel
and maintaining business continuity as a result of recent cost-cutting measures, including salary reductions, which may also affect our
product development and timelines.
We recently implemented significant cost-cutting measures.
While these actions were deemed necessary to conserve capital, they may negatively affect employee morale and retention. There is a risk
that key directors, officers, and employees may seek alternative employment opportunities, which could result in significant disruptions
to our business. If we are unable to retain or replace qualified personnel, it could adversely affect our ability to execute our business
strategy, maintain continuity in our operations, and meet key development milestones.
In addition, these measures may affect our ability
to meet projected timelines for product development and clinical trials. Any delays in the development and commercialization of our products
or services could materially impact our business prospects, financial condition, and results of operations. As a result of these cost-cutting
measures, sublicensing work on AnQlar and Epoladerm have been suspended, which will further delay our development timelines.
The elimination of our directors and officers
insurance may affect retention, expose our directors and officers to personal liability, and impact our financial condition.
We have recently eliminated our D&O insurance
coverage as part of cost-saving measures. This decision may negatively impact our ability to retain and attract key directors and officers,
as they may be more likely to leave or hesitate to join the Company due to the increased personal liability exposure. If key personnel
depart, it could disrupt our business operations, affect continuity, and hinder our ability to achieve our strategic objectives.
Without D&O insurance, the Company will not have
coverage in the event of lawsuits filed against our directors and officers, which may require us to indemnify them under applicable law
and contractual obligations. If the Company faces significant legal claims, we may not have sufficient financial resources to fully indemnify
our directors and officers. In such a situation, directors and officers may be personally liable, which could further increase the risk
of departures and negatively affect our business continuity and financial condition.
The cancellation of our cybersecurity insurance
may expose us to increased financial and operational risks.
We have recently cancelled our cybersecurity insurance
coverage, which may increase our vulnerability to the financial and operational impacts of cyberattacks. Without this coverage, we may
face significant costs if a cybersecurity breach occurs, including expenses related to data recovery, legal claims, regulatory penalties,
and reputational damage. These incidents could disrupt our operations and delay the development of our drug-delivery systems and therapies.
The lack of insurance coverage could have a material adverse effect on our financial condition and business operations.
The recent reduction in salaries and the elimination
of directors and officers insurance may negatively impact our ability to retain key personnel and disrupt our business operations.
We have recently implemented significant cost-cutting
measures, and have eliminated our directors and officers insurance coverage. These actions could result in key directors, officers, and
employees seeking alternative employment opportunities. For example, on September 3, 2024, we received a letter from Kagen, Caspersen
& Bogart PLLC, legal counsel to Gerald Bruce, our former Chief Executive Officer, and Vinay Shah, our former Chief Financial Officer,
regarding their employment agreements with the Company. In said letter, they made certain allegations that the Company violated their
employment agreements as a result of the 50% reduction of the executives’ contractual base compensation and, as a result, the executive
may resign after the 30-day cure period for good reason and be entitled to payment of severance compensation. On October 5, 2024, Gerald
Bruce, our former Chief Executive Officer and member of the Board, and Vinay Shah, our former Chief Financial Officer, notified the Company
of their resignation from their respective positions, effective immediately. The further departure of key personnel could cause disruptions
to our business operations and negatively impact our ability to execute our business strategy. If we are unable to retain or replace key
personnel, our business, financial condition, and results of operations could be materially and adversely affected.
Our arrangements relating to independent contractors
may be questioned by the relevant authorities and if such authorities determine that such arrangements constitute employment, we may be
liable to make certain back payments and payments of interest and penalties to such authorities.
We believe that we have a reasonable basis to treat
our CEO and CFO as independent contractors and not as employees pursuant to the independent contractor agreements we have entered with
each of them. However, if the Internal Revenue Service (IRS) or any relevant state tax authority nonetheless deem that such arrangements
constitute an employment relationship between us and our CEO and CFO, we may be liable to pay such amounts in income and payroll taxes
as we would have paid had such persons been treated as employees, the employer portion of any applicable payroll taxes, interest on such
payments from the date such payments would have been due to the payment date and any applicable statutory or other penalties assessed.
There can be no assurance that the arrangements will
be deemed compliant with the relevant tax legislation or that if they are found not to be, that the amounts that may be ultimately assessed
against us would have not a material adverse effect on our financial condition and results of operations.
Risks Related to this Offering and Our Common
Stock
We will need additional
future financing which may not be available on acceptable terms, if at all and will result in the issuance of additional securities being
issued which will cause investors to experience further dilution.
We expect to require substantial
additional capital until our operations generate sufficient revenue to cover our expenses. We have not generated any revenue since inception
and may never generate revenues unless any of our products are approved by the FDA and other regulatory authorities, which may never happen.
Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. There are currently no
other commitments by any person for future financing. Our securities may be offered to other investors in other offerings at a price lower
than the price per share offered in this offering, or upon terms which may be deemed more favorable than those offered to investors in
this offering. In addition, the issuance of securities in any future financing may dilute an investor’s equity ownership and have
the effect of depressing the market price for our securities. Moreover, we may issue securities convertible or exchangeable into Common
Stock, in future transactions. The issuance of any such derivative securities, which is at the discretion of our Board of Directors, may
further dilute the equity ownership of our stockholders.
Our management has
broad discretion in using the net proceeds from this offering.
Our management will have
broad discretion with respect to the use of proceeds from this offering. See “Use of Proceeds.” We cannot, with any assurance,
be more specific at this time. We will have broad discretion in the timing of the expenditures and application of proceeds received in
this offering. If we fail to apply the net proceeds effectively, we may not be successful in bringing our proposed products to market.
You will not have the opportunity to evaluate all of the economic, financial or other information upon which we may base our decisions
to use the net proceeds from this offering. We may use the proceeds of this offering in ways that do not increase our operating results
or enhance the value of our Common Stock.
We may not be able to maintain a listing of
our common stock on Nasdaq.
We are subject meet certain requirements to maintain
the listing of shares of our common stock on Nasdaq If we fail to meet any of Nasdaq’s continued listing standards, shares of our
common stock may be delisted. In addition, our board of directors may determine that the cost of maintaining our listing on a national
securities exchange outweighs the benefits of such listing. A delisting from Nasdaq may materially impair the ability of stockholders
to buy and sell shares of our common stock and could have an adverse effect on the market price of, and the efficiency of the trading
market for, shares of our common stock. The delisting of shares of our common stock could significantly impair our ability to raise capital
and the value of your investment.
If shares of our common stock become subject
to the penny stock rules, it would become more difficult to trade our shares.
The SEC has adopted rules that regulate broker-dealer
practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00,
other than securities registered on certain national securities exchanges or authorized for quotation on certain automated quotation systems,
provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system.
If we do not retain a listing on Nasdaq or another national securities exchange and if the price of our shares of common stock is less
than $5.00 per share, our common stock could be deemed a penny stock. The penny stock rules require a broker-dealer, before a transaction
in a penny stock not otherwise exempt from those rules, to deliver a standardized risk disclosure document containing specified information.
In addition, the penny stock rules require that before effecting any transaction in a penny stock not otherwise exempt from those rules,
a broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive
(i) the purchaser’s written acknowledgment of the receipt of a risk disclosure statement; (ii) a written agreement to transactions
involving penny stocks; and (iii) a signed and dated copy of a written suitability statement. These disclosure requirements may have the
effect of reducing the trading activity in the secondary market for our common stock, and therefore stockholders may have difficulty selling
their shares.
Our failure to meet
the continued listing requirements of the Nasdaq Capital Market could result in a delisting of our Common Stock.
Our shares of Common Stock
are listed for trading on the Nasdaq Capital Market under the symbol “VRPX”. If we fail to satisfy the continued listing requirements
of the Nasdaq Capital Market, such as the corporate governance requirements, the Minimum Stockholders’ Equity Rule or the Minimum
Bid Price Rule, the Nasdaq Capital Market may take steps to delist our Common Stock.
On April 2, 2024, we received
a notification letter from the Listing Qualifications Staff of the Nasdaq notifying us that our stockholders’ equity as reported
in our Annual Report, did not meet the minimum stockholders’ equity requirement for continued listing on the Nasdaq Capital Market.
Nasdaq Listing Rule 5550(b)(1) requires companies listed on the Nasdaq Capital Market to maintain stockholders’ equity of at least
$2,500,000 (the “Minimum Stockholders’ Equity Rule”). In the Annual Report, we reported stockholders’ equity of
$1,934,321, which is below the minimum stockholders’ equity required for continued listing pursuant to Nasdaq Listing Rule 5550(b)(1).
Additionally, as of the date of this prospectus, we do not meet the alternative Nasdaq continued listing standards under Nasdaq Listing
Rules. In our Report on Form 10-K for the year ended December 31, 2024, we reported stockholders’ deficit of $0.9 million.
Pursuant to Nasdaq’s Listing Rules, we were
given 45 calendar days (or until May 17, 2024), to submit a plan to evidence compliance with the Minimum Stockholders’ Equity Rule
(a “Compliance Plan”). We submitted a Compliance Plan within the required time period, which included raising funds from equity
offerings. On July 29, 2024, we received notice from the Listing Qualifications Staff of The Nasdaq Stock Market LLC that we were granted
an extension through September 30, 2024 to regain compliance with Nasdaq Listing Rule 5550(b)(1). On October 3, 2024, we received
a delist determination letter from the Staff advising the Company that the Staff had determined that the Company did not meet the
terms of the extension. As a result, we requested an appeal of the Staff’s determination. We submitted a hearing request to the
Nasdaq Hearings Panel, which request is expected to stay any delisting action by the Staff at least until the hearing process concludes
and any extension granted by the Panel expires. Notwithstanding the foregoing, there can be no assurance that the Panel will grant the
Company’s request or an additional extension period, or that the Company will ultimately regain compliance with all applicable requirements
for continued listing on The Nasdaq Capital Market. We will not be able to comply with the Minimum Stockholders’ Equity Rule as
a result of this offering due to our cash burn rate, operating expenses, and payment obligations. As a result, we will be required to
raise additional funds after this offering in order to achieve compliance.
In addition, on June 28,
2024, we received written notice from the Listing Qualifications Department of the Nasdaq notifying us that for the preceding 30 consecutive
business days (May 15, 2024 through June 27, 2024), the Common Stock did not maintain a minimum closing bid price of $1.00 (“Minimum
Bid Price Rule”) per share as required by Nasdaq Listing Rule 5550(a)(2).
On July 22,
2024, the Company received a notice from the Listing Qualifications Department of Nasdaq notifying the Company that the staff has determined
that for 10 consecutive business days, from July 8, 2024 to July 19, 2024, the closing bid price of the Company’s Common Stock had
been at $1.00 per share or greater. Accordingly, the staff had determined that the Company had regained compliance with Listing Rule 5550(a)(2).
On October 4,
2024, the Company received a deficiency letter from the Staff of Nasdaq notifying the Company that, for the last 30 consecutive business
days, the closing bid price for the Company’s common stock had been below the minimum $1.00 per share required for continued listing
on Nasdaq pursuant to Nasdaq Listing Rule 5550(a)(2). The Nasdaq deficiency letter has no immediate effect on the listing of the Company’s
common stock, and our common stock will continue to trade on The Nasdaq Capital Market under the symbol “VRPX”.
In accordance
with Nasdaq Listing Rule 5810(c)(3)(A), the Company has been given 180 calendar days, or until April 2, 2025, to regain compliance with
the Minimum Bid Price Rule. If at any time before April 2, 2025, the bid price of the Company’s common stock closes at $1.00 per
share or more for a minimum of 10 consecutive business days, the Staff will provide written confirmation that the Company has achieved
compliance.
If the Company
does not regain compliance with the Minimum Bid Price Rule by April 2, 2025, the Company may be afforded a second 180 calendar days period
to regain. The Company would be required to notify Nasdaq of its intent to cure the deficiency during the second compliance period. If
the Company does not regain compliance with the Minimum Bid Price Rule by the end of the compliance period (or the second compliance period,
if applicable), the Company’s common stock will become subject to delisting. In the event that the Company receives notice that
its common stock is being delisted, the Nasdaq listing rules permit the Company to appeal a delisting determination by the Staff to a
hearings panel.
The Company
intends to monitor the closing bid price of its common stock and may, if appropriate, consider available options to regain compliance
with the Minimum Bid Price Rule, including initiating a reverse stock split. However, there can be no assurance that the Company will
be able to regain compliance with the Minimum Bid Price Rule or will otherwise be in compliance with other Nasdaq Listing Rules.
Any perception that we may not regain compliance or
a delisting of our Common Stock by Nasdaq could adversely affect our ability to attract new investors, decrease the liquidity of the outstanding
shares of our Common Stock, reduce the price at which such shares trade and increase the transaction costs inherent in trading such shares
with overall negative effects for our stockholder. In addition, delisting of our Common Stock from Nasdaq could deter broker-dealers from
making a market in or otherwise seeking or generating interest in our Common Stock and might deter certain institutions and persons from
investing in our Common Stock.
If our shares are delisted from Nasdaq, we may
become subject to the securities laws of various states where we may offer securities in the future which may make it more difficult for
us to raise finance.
The National Securities Markets
Improvement Act of 1996, which is a federal statute, prevents or preempts the states from regulating the sale of certain securities, which
are referred to as “covered securities.” Because our Common Stock is listed on the Nasdaq Capital Market, shares of our Common
Stock are covered securities. If we were to be delisted from Nasdaq, our shares would cease to be covered securities and we would be subject
to regulation in each state in which we offer our securities Should this occur, we would be required to register or qualify offerings
of securities with the various states where we offer our securities, which may make it more difficult to raise finance via public offerings
of our securities.
This is a reasonable
best efforts offering, with no minimum amount of Securities required to be sold, and we may sell fewer than all of the Securities offered
hereby.
The Placement Agent has agreed
to use its reasonable best efforts to solicit offers to purchase the Securities in this offering. The Placement Agent has no obligation
to buy any of the Securities from us or to arrange for the purchase or sale of any specific number or dollar amount of the Securities.
There is no required minimum number of Securities that must be sold as a condition to completion of this offering, and there can be no
assurance that the offering contemplated hereby will ultimately be consummated. Even if we sell Securities offered hereby, because there
is no minimum offering amount required as a condition to closing of this offering, the actual offering amount is not presently determinable
and may be substantially less than the maximum amount set forth on the cover page of this prospectus. We may sell fewer than all
of the Securities offered hereby, which may significantly reduce the amount of proceeds received by us. Thus, we may not raise the amount
of capital we believe is required for our operations in the short-term and may need to raise additional funds, which may not be available
or available on terms acceptable to us.
Because there is no
minimum required for the offering to close, investors in this offering will not receive a refund in the event that we do not sell an amount
of securities sufficient to pursue the business goals outlined in this prospectus.
We have not specified a minimum
offering amount. Because there is no minimum offering amount, investors could be in a position where they have invested in our company,
but we are unable to fulfill our objectives due to a lack of interest in this offering. Further, because there is no minimum investment
amount, any proceeds from the sale of Securities offered by us will be available for our immediate use, despite uncertainty about whether
we would be able to use such funds to effectively implement our business plan. Investor funds will not be returned under any circumstances
whether during or after the offering.
If you purchase shares
of our Common Stock sold in this offering, you will experience immediate and substantial dilution in the net tangible book value of your
shares. In addition, we may issue additional equity or convertible debt securities in the future, which may result in additional dilution
to investors.
The price per share of our
Common Stock being offered may be higher than the net tangible book value per share of our outstanding Common Stock prior to this offering,
which may result in new investors in this offering incurring immediate dilution. To the extent outstanding stock options are exercised,
there will be further dilution to new investors. For a more detailed discussion of the foregoing, see the section entitled “Dilution”
below. To the extent additional stock options or warrants are issued, there will be further dilution to new investors.
This offering may cause
the trading price of our Common Stock to decrease.
The price per share, together
with the number of shares of Common Stock we issue if this offering is completed, may result in an immediate decrease in the market price
of our Common Stock. This decrease may continue after the completion of this offering.
Because we will not
declare cash dividends on our Common Stock in the foreseeable future, stockholders must rely on appreciation of the value of our Common
Stock for any return on their investment.
We have never declared or
paid cash dividends on our Common Stock. We currently anticipate that we will retain future earnings for the development, operation and
expansion of our business and will not declare or pay any cash dividends in the foreseeable future. As a result, only appreciation of
the price of our Common Stock, if any, will provide a return to investors in this offering.
There is no public market for the Pre-Funded Warrants being offered
in this offering.
There is no established public
trading market for the Pre-Funded Warrants being offered in this offering, and we do not expect a market to develop. In addition, we do
not intend to apply to have the Pre-Funded Warrants listed on Nasdaq or any national securities exchange or other nationally recognized
trading system. Without an active market, the liquidity of the Pre-Funded Warrants will be limited.
Holders of the Pre-Funded
Warrants offered hereby will have no rights as Common Stockholders with respect to the shares our Common Stock issuable upon exercise
of the Pre-Funded Warrants until such holders exercise their Pre-Funded Warrants and acquire our Common Stock, except as otherwise provided
in the Pre-Funded Warrants.
Until holders of the Pre-Funded
Warrants acquire shares of our Common Stock upon exercise thereof, such holders will have no rights with respect to the shares of our
Common Stock issuable upon exercise of such Pre-Funded Warrants, except to the extent that holders of such Pre-Funded Warrants will have
certain rights to participate in distributions or dividends paid on our Common Stock as set forth in the Pre-Funded Warrants. Upon exercise
of the Pre-Funded Warrants, the holders will be entitled to exercise the rights of a Common Stockholder only as to matters for which the
record date occurs after the exercise date.
Purchasers who purchase
our Securities in this offering pursuant to a securities purchase agreement may have rights not available to purchasers that purchase
without the benefit of a securities purchase agreement.
In addition to rights and
remedies available to all purchasers in this offering under federal securities and state law, the purchasers that enter into a securities
purchase agreement will also be able to bring claims of breach of contract against us. The ability to pursue a claim for breach of contract
provides those investors with the means to enforce the covenants uniquely available to them under the securities purchase agreement, including:
a covenant to not enter into any equity financings for thirty (30) days from closing of the offering, subject to certain exceptions.
USE OF PROCEEDS
We estimate that we will
receive net proceeds from this offering of approximately $4.7 million (assuming the sale of the maximum number of Securities offered
hereby), based upon an assumed public offering price of $2.07 per share (which is the last reported sale price of shares of our Common
Stock on Nasdaq on March 21, 2025), after deducting the estimated Placement Agent fees and estimated offering expenses payable by us
and assuming no issuance of any Pre-Funded Warrants. However, because this is a reasonable best efforts offering with no minimum number
of securities or amount of proceeds as a condition to closing, the actual offering amount, Placement Agent fees, and net proceeds to
us are not presently determinable and may be substantially less than the maximum amounts set forth on the cover page of this prospectus,
and we may not sell all or any of the Securities we are offering. As a result, we may receive significantly less in net proceeds. Based
on the assumed offering price set forth above, we estimate that our net proceeds from the sale of 75%, 50%, and 25% of the Securities
offered in this offering would be approximately $3.4 million, $2.2 million, and $1.0 million, respectively, after deducting the estimated
Placement Agent fees, inclusive of financial advisor fees, and estimated offering expenses payable by us, and assuming no issuance of
any Pre-Funded Warrants.
We
intend to use the net proceeds from this offering to fund our ongoing development activities
for commencing clinical trial for Probudur, as well
as for working capital and other general corporate purposes. In addition, we may use
$1.7 million for marketing and advertising services to communicate information about the
Company to the financial community. Pending our use of the net proceeds from this offering,
we intend to invest the net proceeds in a variety of capital preservation investments, including
short-term, investment-grade, interest-bearing instruments and U.S. government securities.
This expected use of net
proceeds from this offering represents our intentions based upon our current plans and business conditions, which could change in the
future as our plans, business conditions and cash position evolve. Our management will have significant flexibility and discretion in
the timing and application of the net proceeds of the offering. In addition, if our former Chief Executive Officer, Mr. Mack, were to
seek indemnification and/or damages from us and if he were successful in his claim, we may determine to use a portion of the proceeds
from this offering to make such payments. See “Litigation” under “Recent Developments” in the Prospectus Summary,
above. Unforeseen events or changed business conditions may result in application of the proceeds of the offering in a manner other than
as described in this prospectus. Our stockholders may not agree with the manner in which our management chooses to allocate and spend
the net proceeds. See “Risk Factors”.
CAPITALIZATION
The following table sets
forth our cash and our capitalization as of December 31, 2024:
| |
● |
on a pro forma basis, giving effect to (i) the issuance on January 30,
2025 of 138,000 shares of common stock and 1,062,000 pre-funded warrants at an offering price of $5.00 per share, less $0.00001 per pre-funded
warrant, for net proceeds of $5.6 million; (ii) the issuance of 545,000 shares of Common Stock upon exercise of the pre-funded warrants;
and |
| |
● |
on a pro forma as adjusted basis, giving effect to the pro forma
adjustments set forth above and the sale of 2,415,459 shares of Common Stock based on an assumed public offering price of $2.07 per
share (the last reported sale price of our Common Stock on the Nasdaq Capital Market on March 21, 2025), after deducting estimated
Placement Agent fees and estimated offering expenses payable by us and assuming no sale of Pre-Funded Warrants. |
The “pro forma as adjusted” information
set forth in the table below is illustrative only and will be adjusted based on the actual public offering price and other terms of this
offering as determined at pricing. You should read the information in this table together with our condensed consolidated financial statements
and related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in the
Company’s Annual Report on Form 10-K for the year ended December 31, 2024, incorporated by reference in this prospectus.
| |
|
As of
December 31,
2024 |
|
Pro Forma |
|
Pro Forma
As Adjusted |
| |
|
Actual |
|
|
|
|
| Cash: |
|
$ |
1,513,057 |
|
|
$ |
7,126,843 |
(1) |
|
$ |
11,782,827 |
|
| Stockholders’ (deficit) equity: |
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock, $0.00001 par value; 100,000,000
shares authorized, and 595,504 shares issued and outstanding, actual; 1,278,504 shares issued and outstanding pro forma and 3,693,963 shares
issued and outstanding pro forma as adjusted |
|
$ |
6 |
|
|
$ |
13 |
|
|
$ |
37 |
|
| Additional paid-in capital |
|
|
70,697,594 |
|
|
|
76,311,373 |
|
|
|
80,967,333 |
|
| Accumulated deficit |
|
|
(71,611,360 |
) |
|
|
(71,611,360 |
) |
|
|
(71,611,360) |
|
| Total stockholders’ (deficit) equity |
|
$ |
(913,760) |
) |
|
$ |
4,700,026 |
|
|
$ |
9,356,010 |
|
| Total capitalization |
|
$ |
(913,760 |
) |
|
$ |
4,700,026 |
|
|
$ |
9,356,010 |
|
| (1) |
In January 2025, we entered into the IR Agreement with IR Agency LLC. Under the IR Agreement, the Consultant will provide marketing and advertising services to promote the Company to the financial community. In consideration for these services, we paid the Consultant a fee of $1.7 million. In January 2025, we entered into an investor relations agreement with Sideways Frequency LLC to provide marketing and advertising services to promote the Company to the financial community. In consideration for these services, we paid Sideways Frequency LLC a fee of $300,000. These cash payments are not reflected in the pro-forma capitalization table. |
DILUTION
If you invest in shares of
our Common Stock in this offering, your ownership interest will be diluted immediately to the extent of the difference between the public
offering price per share of our Common Stock and the as adjusted net tangible book value per share of our Common Stock immediately after
this offering.
Dilution results from the
fact that the public offering price per share is substantially in excess of the book value per share attributable to the existing stockholders
for the presently outstanding shares of Common Stock. We calculate net tangible book value per share by dividing the net tangible book
value (total tangible assets less total liabilities) by the number of outstanding shares of Common Stock.
Our historical net tangible
book value deficit as of December 31, 2024 was approximately $(0.9) million, or ($1.53) per share. Our historical net tangible book value
deficit is the amount of our total tangible assets less our total liabilities. Historical net tangible book value deficit per share represents
our historical net tangible book value deficit divided by the 595,504 shares of our Common Stock outstanding as of December 31, 2024.
Our pro forma net tangible
book value as of December 31, 2024 was approximately $4.7 million, or $3.68 per share. Pro forma net tangible book value represents the
amount of our tangible book value as adjusted to take into account (i) the issuance of 138,000 shares of common stock and 1,062,000 pre-funded
warrants at an offering price of $5.00 per Share, less $0.00001 per pre-funded warrant, for net proceeds of $5.6 million; (ii) the issuance
of 545,000 shares of Common Stock upon exercise of the pre-funded warrants.
After giving effect to the pro forma adjustments
set forth above and the receipt of the estimated maximum net proceeds from our sale of Securities in this offering, based on an assumed
public offering price of $2.07 per share (the last reported sale price of our Common Stock on the Nasdaq Capital Market on March 21,
2025), after deducting estimated Placement Agent fees, inclusive of financial advisor fees, and estimated offering expenses payable by
us and assuming no sale of Pre-Funded Warrants, our pro forma as adjusted net tangible book value at December 31, 2024 would have been
approximately $9.4 million, or $2.54 per share. This represents an immediate decrease in net tangible book value per share of $(1.14)
to existing stockholders and an immediate dilution per share of $(0.47) to you. The following table illustrates this dilution on a per
share basis to new investors:
| Assumed public offering price per
share |
|
|
|
|
|
$ |
2.07 |
|
| Pro Forma net tangible book value per share as
of December 31, 2024 |
|
$ |
3.68 |
|
|
|
|
|
| Decrease in pro forma net tangible book value
per share attributable to this offering |
|
$ |
(1.14) |
|
|
|
|
|
| Pro forma as adjusted net tangible book value
per share after this offering |
|
|
|
|
|
$ |
2.53 |
|
| Dilution per share to new investors purchasing
Common Stock in this offering |
|
|
|
|
|
$ |
(0.47) |
|
The dilution information
discussed above is illustrative only and will change based on the actual public offering price and other terms of this offering determined
at pricing.
Each $0.10 increase (decrease) in the assumed
public offering price of $2.07 per share of Common Stock (the last reported sale price of our Common Stock on the Nasdaq Capital Market
on March 21, 2025) would increase (decrease) our pro forma as adjusted net tangible book value per share after this offering by approximately
$0.2 million or $0.06 per share and would change dilution per share to new investors purchasing Securities in this offering by approximately
$0.04, assuming that the number of Securities offered by us, as set forth on the cover page of this prospectus, remains the same and
after deducting the estimated Placement Agent fees, inclusive of financial advisor fees, and estimated offering expenses payable by us
and assuming no sale of Pre-Funded Warrants.
Each increase (decrease)
in the number of shares of Common Stock offered by 200,000 shares of Common Stock would increase (decrease) our pro forma as adjusted
net tangible book value as of March 21, 2025 after this offering by approximately $0.4 million or $(0.03) per share, and would change
the dilution to investors in this offering by approximately $0.03 per share, assuming that the assumed offering price per share, as set
forth on the cover page of this prospectus, remains the same, after deducting Placement Agent fees, inclusive of financial advisor fees,
and estimated offering expenses payable by us and assuming no sale of Pre-Funded Warrants.
Each increase in the number of shares of Common
Stock offered by 500,000 shares of Common Stock would increase our pro forma as adjusted net tangible book value as of December 31, 2024
after this offering by approximately $1.0 million, or $(0.06) per share, and would change the dilution to investors in this offering
by approximately $0.06 per share, assuming that the assumed offering price per share, as set forth on the cover page of this prospectus,
remains the same, after deducting Placement Agent fees, inclusive of financial advisor fees, and estimated offering expenses payable
by us and assuming no sale of Pre-Funded Warrants.
The table and discussion above do not include:
| |
● |
5,561 shares of Common Stock issuable upon exercise of stock options outstanding as of December 31, 2024, at a weighted-average exercise price of $632.25 per share; |
| |
● |
17,777 shares of our Common Stock that are available for future issuance under our 2022 Plan or shares that will become available under our 2022 Plan; and |
| |
● |
51,420 shares of Common Stock issuable upon exercise of warrants outstanding as of December 31, 2024, at a weighted-average exercise price of $38.00 per share. |
Unless otherwise indicated,
this prospectus reflects and assumes the following:
| |
● |
No exercise of outstanding options or warrants described above; and |
| |
● |
No sale of the Pre-Funded Warrants in this offering. |
To the extent any outstanding
options or other equity awards are exercised or become vested or any additional options or other equity awards are granted and exercised
or become vested or other issuances of our Common Stock are made, there may be further economic dilution to new investors.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS
AND
MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets
forth information with respect to the beneficial ownership of our Common Stock, as of March 21, 2025 :
| |
● |
each person or group of affiliated persons known by us to beneficially own more than 5% of our Common Stock; |
| |
● |
each of our executive officers; |
| |
● |
each of our directors; and |
| |
● |
all of our current executive officers and directors as a group. |
The number of shares beneficially owned by each
stockholder is determined under rules issued by the SEC. Under these rules, beneficial ownership includes any shares as to which the
individual or entity has sole or shared voting power or investment power. We have based our calculation of the percentage of beneficial
ownership of our Common Stock before this offering based on 1,278,504 shares of Common Stock outstanding as of March 21, 2025. We have
based our calculation of the percentage of beneficial ownership of our Common Stock after this offering of 2,415,459 shares of our Common
Stock, which gives effect to the issuance of 3,693,963 shares of Common Stock in this offering. In computing the number of shares beneficially
owned by an individual or entity and the percentage ownership of that person, shares of Common Stock subject to the exercise of options,
warrants or other rights held by such person that are currently exercisable or will become exercisable within 60 days of March 21, 2025
are counted as outstanding. Unless noted otherwise, the address of all listed stockholders is 1055 Westlakes Drive, Suite 300, Berwyn,
Pennsylvania 19312. Each stockholder listed has sole voting and investment power with respect to the shares beneficially owned by the
stockholder unless noted otherwise, subject to community property laws where applicable.
| | |
Shares Beneficially Owned Prior to
this Offering | |
Shares Beneficially Owned After this
Offering |
| Name of Beneficial Owner | |
Shares of Common Stock | |
Percentage of Common Stock | |
Shares of Common Stock | |
Percentage of Common Stock |
| Named Executive Officers and Directors | |
| | | |
| | | |
| | | |
| | |
| Gerald Bruce(1) | |
| 17 | | |
| * | | |
| 17 | | |
| * | |
| Vinay Shah(2) | |
| — | | |
| — | | |
| — | | |
| — | |
| Jatinder Dhaliwal | |
| — | | |
| — | | |
| — | | |
| — | |
| Sheila Mathias, PhD, J.D., MBA(3) | |
| 791 | | |
| * | | |
| 791 | | |
| * | |
| Katharyn Field | |
| — | | |
| — | | |
| — | | |
| — | |
| Esha Randhawa | |
| — | | |
| — | | |
| — | | |
| — | |
| Jaydriane Panis (4) | |
| — | | |
| — | | |
| — | | |
| — | |
| Charn Doel | |
| — | | |
| — | | |
| — | | |
| — | |
| Usama Chaudhry | |
| — | | |
| — | | |
| — | | |
| — | |
| Judy Su | |
| — | | |
| — | | |
| — | | |
| — | |
| All current executive officers and directors as a group (7 persons) | |
| 791 | | |
| * | | |
| 791 | | |
| * | |
| 5% or Greater Stockholders | |
| | | |
| | | |
| | | |
| | |
| * |
Less than 1%. |
| |
|
| (1) |
Gerald Bruce, resigned as our Chief Executive Officer effective
October 5, 2024. |
| |
|
| (2) |
Vinay Shah resigned as our Chief Financial Officer, effective October
5, 2024. |
| |
|
| (3) |
Includes 791 shares of common stock issuable upon exercise
of stock options that are exercisable within 60 days of March 21, 2025. |
| |
|
| (4) |
Jaydriane Panis resigned as a director, effective February 3, 2025. |
DESCRIPTION OF SECURITIES
WE ARE OFFERING
We are offering up to
2,415,459 shares of our Common Stock.
We are offering to certain purchasers whose purchase
of shares in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially
owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of Common Stock immediately following
the consummation of this offering, the opportunity to purchase, if any such purchaser so chooses, Pre-Funded Warrants, in lieu of shares
of Common Stock that otherwise would result in such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the
purchaser, 9.99%) of our outstanding shares of Common Stock, to purchase up to 2,415,459 shares of Common Stock.
For each Pre-Funded Warrant we sell, the number of
shares of Common Stock we are offering will be decreased on a one-for-one basis.
Common Stock
The material terms and provisions
of our Common Stock are described under the caption “Description of Our Capital Stock” in this prospectus.
Pre-Funded Warrants
The following summary
of certain terms and provisions of the Pre-Funded Warrants that are being offered hereby is not complete and is subject to, and qualified
in its entirety by, the provisions of the form of the Pre-Funded Warrant, which is filed as an exhibit to the registration statement of
which this prospectus forms a part. Prospective investors should carefully review the terms and provisions set forth in the form of Pre-Funded
Warrants.
Duration and Exercise
Price
Each Pre-Funded Warrant offered
hereby will have an initial exercise price equal to $0.00001 per share of Common Stock. The Pre-Funded Warrants will be immediately exercisable
and may be exercised at any time until the Pre-Funded Warrants are exercised in full. The exercise price and number of shares issuable
upon exercise is subject to appropriate proportional adjustment in the event of share dividends, share splits, reclassification or similar
events affecting our Common Stock. The Pre-Funded Warrants will be issued in certificated form only.
Exercisability
The Pre-Funded Warrants will
be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice. A holder may not
exercise any portion of the Pre-Funded Warrant to the extent that the holder, together with its affiliates and any other persons acting
as a group together with any such persons, would own more than 4.99% (or, at the election of the purchaser, 9.99%) of the number of shares
of Common Stock outstanding immediately after exercise (the “Beneficial Ownership Limitation”); provided that a holder with
a Beneficial Ownership Limitation of 4.99%, upon notice to us and effective sixty-one (61) days after the date such notice is delivered
to us, may increase the Beneficial Ownership Limitation so long as it in no event exceeds 9.99% of the number of shares of Common Stock
outstanding immediately after exercise.
Cashless Exercise
In lieu of making the cash
payment otherwise contemplated to be made to us upon exercise of the Pre-Funded Warrants in payment of the aggregate exercise price, the
holder may exercise its Pre-Funded Warrants (either in whole or in part), at such time by means of a cashless exercise in which the holder
shall be entitled to receive upon such exercise the net number of shares of Common Stock determined according to a formula set forth in
the Pre-Funded Warrants, which generally provides for a number of shares equal to (A) (1) the volume weighted average price on the trading
day preceding the notice of exercise, if the notice of exercise is executed and delivered (x) on a day that is not a trading day or (y)
prior to the opening of “regular trading hours” on a trading day or (2) the VWAP on the trading day immediately preceding
the date of the notice of exercise, if the notice of exercise is executed and delivered during “regular trading hours” on
a trading day, or (3) the bid price on the day of the notice of exercise, if the notice of exercise is executed after the close of “regular
trading hours” on a trading day, less (B) the exercise price, multiplied by (C) the number of shares of Common Stock the Pre-Funded
Warrant was exercisable into, with such product then divided by the number determined under clause (A) in this sentence.
Fractional Shares
No fractional shares of Common Stock will be issued
upon the exercise of the Pre-Funded Warrants. Rather, we will, at our election, and in lieu of the issuance of such fractional share,
either (i) pay cash in an amount equal to such fraction multiplied by the exercise price or (ii) round up to the next whole share issuable
upon exercise of the Pre-Funded Warrant.
Transferability
Subject to applicable laws,
a Pre-Funded Warrant may be transferred at the option of the holder upon surrender of the Pre-Funded Warrant to us together with the appropriate
instruments of transfer and funds sufficient to pay any transfer taxes payable upon such transfer.
Trading Market
There is no trading market
available for the Pre-Funded Warrants on any securities exchange or nationally recognized trading system. We do not intend to list the
Pre-Funded Warrants on any securities exchange or nationally recognized trading system.
Rights as a Stockholder
Except as otherwise provided
in the Pre-Funded Warrants or by virtue of such holder’s ownership of shares of Common Stock, the holders of the Pre-Funded Warrants
do not have the rights or privileges of holders of our Common Stock, including any voting rights, until they exercise their Pre-Funded
Warrants. The Pre-Funded Warrants will provide that holders have the right to participate in distributions or dividends paid on Common
Stock.
Fundamental Transaction
In the event of a fundamental
transaction, as described in the Pre-Funded Warrants and generally including any reorganization, recapitalization or reclassification
of our Common Stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation
or merger with or into another person, the consummation of a business combination with another person or group of persons whereby such
other person or group acquires greater than 50% of the voting power of the outstanding Common Stock and preferred stock, the holders of
the Pre-Funded Warrants will be entitled to receive upon exercise of the Pre-Funded Warrants the kind and amount of securities, cash or
other property that the holders would have received had they exercised the Pre-Funded Warrants immediately prior to such fundamental transaction.
MATERIAL UNITED STATES
FEDERAL INCOME TAX CONSIDERATIONS
The following discussion
describes the material U.S. federal income tax consequences of the acquisition, ownership and disposition of the Common Stock acquired
in this offering. This discussion is based on the current provisions of the Internal Revenue Code of 1986, as amended, referred to as
the Code, existing and proposed U.S. Treasury regulations promulgated thereunder, and administrative rulings and court decisions in effect
as of the date hereof, all of which are subject to change at any time, possibly with retroactive effect. No ruling has been or will be
sought from the Internal Revenue Service, or IRS, with respect to the matters discussed below, and there can be no assurance the IRS will
not take a contrary position regarding the tax consequences of the acquisition, ownership or disposition of the Common Stock and Pre-Funded
Warrants or that any such contrary position would not be sustained by a court.
We assume in this discussion
that the shares of Common Stock and Pre-Funded Warrants will be held as capital assets (generally, property held for investment). This
discussion does not address all aspects of U.S. federal income taxes, does not discuss the potential application of the Medicare contribution
tax or the alternative minimum tax and does not address state or local taxes or U.S. federal gift and estate tax laws, except as specifically
provided below with respect to non-U.S. holders, or any non-U.S. tax consequences that may be relevant to holders in light of their particular
circumstances. This discussion also does not address the special tax rules applicable to particular holders, such as:
| |
● |
persons who acquired our Common Stock and Pre-Funded Warrants as compensation for services; |
| |
● |
traders in securities that elect to use a mark-to-market method of accounting for their securities holdings; |
| |
● |
persons that own, or are deemed to own, more than 5% of our Common Stock (except to the extent specifically set forth below); |
| |
● |
persons required for U.S. federal income tax purposes to conform the timing of income accruals to their financial statements under Section 451(b) of the Code (except to the extent specifically set forth below); |
| |
● |
persons for whom our Common Stock constitutes “qualified small business stock” within the meaning of Section 1202 of the Code or “Section 1244 stock” for purposes of Section 1244 of the Code; |
| |
● |
persons deemed to sell our Common Stock and Pre-Funded under the constructive sale provisions of the Code; |
| |
● |
banks or other financial institutions; |
| |
● |
brokers or dealers in securities or currencies; |
| |
● |
tax-exempt organizations or tax-qualified retirement plans; |
| |
● |
regulated investment companies or real estate investment trusts; |
| |
● |
persons that hold the Common Stock and Pre-Funded Warrants as part of a straddle, hedge, conversion transaction, synthetic security or other integrated investment; |
| |
● |
controlled foreign corporations, passive foreign investment companies, or corporations that accumulate earnings to avoid U.S. federal income tax; and |
| |
● |
certain U.S. expatriates, former citizens, or long-term residents of the United States. |
In addition, this discussion
does not address the tax treatment of partnerships (including any entity or arrangement classified as a partnership for U.S. federal income
tax purposes) or other pass-through entities or persons who hold shares of Common Stock through such partnerships or other entities which
are pass-through entities for U.S. federal income tax purposes. If such a partnership or other pass-through entity holds shares of Common
Stock and Pre-Funded Warrants, the treatment of a partner in such partnership or investor in such other pass-through entity generally
will depend on the status of the partner or investor and upon the activities of the partnership or other pass-through entity. A partner
in such a partnership and an investor in such other pass-through entity that will hold shares of Common Stock and Pre-Funded Warrants
should consult his, her or its own tax advisor regarding the tax consequences of the ownership and disposition of shares of Common Stock
and Pre-Funded through such partnership or other pass-through entity, as applicable.
This discussion of U.S.
federal income tax considerations is for general information purposes only and is not tax advice. Prospective investors should consult
their own tax advisors regarding the U.S. federal, state, local and non-U.S. income and other tax considerations of acquiring, holding
and disposing of our Common Stock and Pre-Funded Warrants.
For the purposes of this
discussion, a “U.S. Holder” means a beneficial owner of shares of Common Stock and Pre-Funded Warrants that is for U.S. federal
income tax purposes (a) an individual citizen or resident of the United States, (b) a corporation (or other entity taxable as
a corporation for U.S. federal income tax purposes), created or organized in or under the laws of the United States, any state thereof
or the District of Columbia, (c) an estate the income of which is subject to U.S. federal income taxation regardless of its source,
or (d) a trust if it (1) is subject to the primary supervision of a court within the United States and one or more U.S. persons
(within the meaning of Section 7701(a)(30) of the Code) has the authority to control all substantial decisions of the trust or (2) has
a valid election in effect under applicable U.S. Treasury regulations to be treated as a domestic trust. A “Non-U.S. Holder”
is, for U.S. federal income tax purposes, a beneficial owner of shares of Common Stock and Pre-Funded Warrants that is not a U.S. Holder
or a partnership for U.S. federal income tax purposes.
Treatment of Pre-Funded
Warrants
Although it is not entirely
free from doubt, a pre-funded warrant should be treated as a share of Common Stock for U.S. federal income tax purposes and a holder of
Pre-Funded Warrants should generally be taxed in the same manner as a holder of Common Stock, as described below. Accordingly, no gain
or loss should be recognized upon the exercise of a Pre-Funded Warrant and, upon exercise, the holding period of a Pre-Funded Warrant
should carry over to the share of Common Stock received. Similarly, the tax basis of the Pre-Funded Warrant should carry over to the share
of Common Stock received upon exercise, increased by the exercise price of $0.00001 per share. Each holder should consult his, her or
its own tax advisor regarding the risks associated with the acquisition of Pre-Funded Warrants pursuant to this offering (including potential
alternative characterizations). The balance of this discussion generally assumes that the characterization described above is respected
for U.S. federal income tax purposes.
Tax Considerations Applicable
to U.S. Holders
Distributions
As discussed above, we currently
anticipate that we will retain future earnings, if any, to finance the growth and development of our business and do not intend to pay
cash dividends in respect of shares of Common Stock in the foreseeable future. In the event that we do make distributions on our Common
Stock to a U.S. Holder, those distributions generally will constitute dividends for U.S. tax purposes to the extent paid out of our current
or accumulated earnings and profits (as determined under U.S. federal income tax principles). Distributions in excess of our current and
accumulated earnings and profits will constitute a return of capital that is applied against and reduces, but not below zero, a U.S. Holder’s
adjusted tax basis in our Common Stock. Any remaining excess will be treated as gain realized on the sale or exchange of shares of Common
Stock as described below under the section titled “Disposition of Common Stock and Pre-Funded Warrants.”
Certain Adjustments
to Pre-Funded Warrants
The number of shares of Common
Stock issued upon the exercise of the Pre-Funded Warrants and the exercise price of Pre-Funded Warrants are subject to adjustment in certain
circumstances. Adjustments (or failure to make adjustments) that have the effect of increasing a U.S. Holder’s proportionate interest
in our assets or earnings and profits may, in some circumstances, result in a constructive distribution to the U.S. Holder. Adjustments
to the conversion rate made pursuant to a bona fide reasonable adjustment formula which has the effect of preventing the dilution of the
interest of the holders of Pre-Funded Warrants generally should not be deemed to result in a constructive distribution. If an adjustment
is made that does not qualify as being made pursuant to a bona fide reasonable adjustment formula, a U.S. Holder of Pre-Funded Warrants
may be deemed to have received a constructive distribution from us, even though such U.S. Holder has not received any cash or property
as a result of such adjustment. The tax consequences of the receipt of a distribution from us are described above under “Distributions.”
Disposition of Common
Stock and Pre-Funded Warrants
Upon a sale or other taxable
disposition (other than a redemption treated as a distribution, which will be taxed as described above under “Distributions”)
of shares of Common Stock and, Pre-Funded Warrants, a U.S. Holder generally will recognize capital gain or loss in an amount equal to
the difference between the amount realized and the U.S. Holder’s adjusted tax basis in the Common Stock and, Pre-Funded Warrants
sold. Capital gain or loss will constitute long-term capital gain or loss if the U.S. Holder’s holding period for the Common Stock
and Pre-Funded Warrants exceeds one year. The deductibility of capital losses is subject to certain limitations. U.S. Holders who recognize
losses with respect to a disposition of shares of Common Stock and Pre-Funded Warrants should consult their own tax advisors regarding
the tax treatment of such losses.
Information Reporting
and Backup Reporting
Information reporting requirements
generally will apply to payments of distributions (including constructive distributions) on the Common Stock and Pre-Funded Warrants and
to the proceeds of a sale or other disposition of Common Stock and Pre-Funded Warrants paid by us to a U.S. Holder unless such U.S. Holder
is an exempt recipient, such as a corporation. Backup withholding will apply to those payments if the U.S. Holder fails to provide the
holder’s taxpayer identification number, or certification of exempt status, or if the holder otherwise fails to comply with applicable
requirements to establish an exemption.
Backup withholding is not
an additional tax. Rather, any amounts withheld under the backup withholding rules will be allowed as a refund or a credit against
the U.S. Holder’s U.S. federal income tax liability provided the required information is timely furnished to the IRS. U.S. Holders
should consult their own tax advisors regarding their qualification for exemption from information reporting and backup withholding and
the procedure for obtaining such exemption.
Tax Considerations Applicable
to Non-U.S. Holders
Certain Adjustments
to Warrants
As described under “—U.S.
Holders—Certain Adjustments to Pre-Funded Warrants,” an adjustment to the Pre-Funded Warrants could result in a constructive
distribution to a Non-U.S. Holder, which would be treated as described under “Distributions” below. Any resulting withholding
tax attributable to deemed dividends would be collected from other amounts payable or distributable to the Non-U.S. Holder. Non-U.S. Holders
should consult their tax advisors regarding the proper treatment of any adjustments to the Pre-Funded Warrants.
In addition, regulations
governing “dividend equivalents” under Section 871(m) of the Code may apply to the Pre-Funded Warrants. Under those
regulations, an implicit or explicit payment under Pre-Funded Warrants that references a dividend distribution on our Common Stock would
possibly be taxable to a Non-U.S. Holder as described under “Distributions” below. Such dividend equivalent amount would be
taxable and subject to withholding whether or not there is actual payment of cash or other property, and the Company may satisfy any withholding
obligations it has in respect of the Pre-Funded Warrants by withholding from other amounts due to the Non-U.S. Holder. Non-U.S. Holders
are encouraged to consult their own tax advisors regarding the application of Section 871(m) of the Code to the Pre-Funded Warrants.
Distributions
As discussed above, we currently
anticipate that we will retain future earnings, if any, to finance the growth and development of our business and do not intend to pay
cash dividends in respect of our Common Stock in the foreseeable future. In the event that we do make distributions on our Common Stock
to a Non-U.S. Holder, those distributions generally will constitute dividends for U.S. federal income tax purposes as described in “—U.S.
Holders—Distributions.” To the extent those distributions do not constitute dividends for U.S. federal income tax purposes
(i.e., the amount of such distributions exceeds both our current and our accumulated earnings and profits), they will constitute a return
of capital and will first reduce a Non-U.S. Holder’s basis in our Common Stock (determined separately with respect to each share
of Common Stock), but not below zero, and then will be treated as gain from the sale of that share Common Stock as described below under
the section titled “—Disposition of Common Stock and Pre-Funded Warrants .”
Any distribution (including
constructive distributions) on shares of Common Stock that is treated as a dividend paid to a Non-U.S. Holder that is not effectively
connected with the holder’s conduct of a trade or business in the United States will generally be subject to withholding tax at
a 30% rate or such lower rate as may be specified by an applicable income tax treaty between the United States and the Non-U.S. Holder’s
country of residence. To obtain a reduced rate of withholding under a treaty, a Non-U.S. Holder generally will be required to provide
the applicable withholding agent with a properly executed IRS Form W-8BEN, IRS Form W-8BEN-E or other appropriate form,
certifying the Non-U.S. Holder’s entitlement to benefits under that treaty. Such form must be provided prior to the payment of dividends
and must be updated periodically. If a Non-U.S. Holder holds stock through a financial institution or other agent acting on the holder’s
behalf, the holder will be required to provide appropriate documentation to such agent. The holder’s agent may then be required
to provide certification to the applicable withholding agent, either directly or through other intermediaries. If you are eligible for
a reduced rate holding tax under an income tax treaty, you should consult with your own tax advisor to determine if you are able to obtain
a refund or credit of any excess amounts withheld by timely filing an appropriate claim for a refund with the IRS.
We generally are not required
to withhold tax on dividends paid (or constructive dividends deemed paid) to a Non-U.S. Holder that are effectively connected with the
holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, are attributable
to a permanent establishment or fixed base that the holder maintains in the United States) if a properly executed IRS Form W-8ECI,
stating that the dividends are so connected, is furnished to us (or, if stock is held through a financial institution or other agent,
to the applicable withholding agent). In general, such effectively connected dividends will be subject to U.S. federal income tax on a
net income basis at the regular tax rates applicable to U.S. persons. A corporate Non-U.S. Holder receiving effectively connected dividends
may also be subject to an additional “branch profits tax,” which is imposed, under certain circumstances, at a rate of 30%
(or such lower rate as may be specified by an applicable treaty) on the corporate Non-U.S. Holder’s effectively connected earnings
and profits, subject to certain adjustments.
See also the sections below
titled “Backup Withholding and Information Reporting” and “Foreign Accounts” for additional withholding rules that
may apply to dividends paid to certain foreign financial institutions or non-financial foreign entities.
Disposition of Common
Stock and Pre-Funded Warrants
Subject to the discussions
below under the sections titled “Backup Withholding and Information Reporting” and “Foreign Accounts,” a Non-U.S.
Holder generally will not be subject to U.S. federal income or withholding tax with respect to gain recognized on a sale or other disposition
(other than a redemption treated as a distribution, which will be taxable as described above under “Distributions”) of shares
of Common Stock and , Pre-Funded Warrants unless:
| |
● |
the gain is effectively connected with the Non-U.S. Holder’s conduct of a trade or business in the United States, and if an applicable income tax treaty so provides, the gain is attributable to a permanent establishment or fixed base maintained by the Non-U.S. Holder in the United States; in these cases, the Non-U.S. Holder will be taxed on a net income basis at the regular tax rates and in the manner applicable to U.S. persons, and if the Non-U.S. Holder is a corporation, an additional branch profits tax at a rate of 30%, or a lower rate as may be specified by an applicable income tax treaty, may also apply; |
| |
● |
the Non-U.S. Holder is a nonresident alien present in the United States for 183 days or more in the taxable year of the disposition and certain other requirements are met, in which case the Non-U.S. Holder will be subject to a 30% tax (or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder’s country of residence) on the net gain derived from the disposition, which may be offset by certain U.S.-source capital losses of the Non-U.S. Holder, if any; or |
| |
● |
the Common Stock constitutes a U.S. real property interest because we are, or have been at any time during the five-year period preceding such disposition (or the Non-U.S. Holder’s holding period of the Common Stock and Pre-Funded Warrants , if shorter), a “U.S. real property holding corporation,” unless the Common Stock is regularly traded on an established securities market, as defined by applicable Treasury Regulations, and the Non-U.S. Holder held no more than 5% of our outstanding Common Stock, directly or indirectly, during the shorter of the five-year period ending on the date of the disposition or the period that the Non-U.S. Holder held the Common Stock. Special rules may apply to the determination of the 5% threshold in the case of a holder of Pre-Funded Warrants. Non-U.S. Holders are urged to consult their own tax advisors regarding the effect of holding Pre-Funded Warrants on the calculation of such 5% threshold. Generally, a corporation is a “U.S. real property holding corporation” if the fair market value of its “U.S. real property interests” (as defined in the Code and applicable regulations) equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus its other assets used or held for use in a trade or business. Although there can be no assurance, we believe that we are not currently, and we do not anticipate becoming, a “U.S. real property holding corporation” for U.S. federal income tax purposes. No assurance can be provided that the Common Stock will be regularly traded on an established securities market for purposes of the rules described above. Non-U.S. Holders are urged to consult their own tax advisors regarding the U.S. federal income tax considerations that could result if we are, or become a “U.S. real property holding corporation.” |
See the sections titled “Backup
Withholding and Information Reporting” and “Foreign Accounts” for additional information regarding withholding rules that
may apply to proceeds of a disposition of the Common Stock and Pre-Funded Warrants paid to foreign financial institutions or non-financial
foreign entities.
Backup Withholding
and Information Reporting
We must report annually to
the IRS and to each Non-U.S. Holder the gross amount of the distributions (including constructive distributions) on the Common Stock and
Pre-Funded Warrants paid to such holder and the tax withheld, if any, with respect to such distributions. Non-U.S. Holders may have to
comply with specific certification procedures to establish that the holder is not a U.S. person (as defined in the Code) in order to avoid
backup withholding at the applicable rate, currently 24%, with respect to dividends (or constructive dividends) on the Common Stock and
Pre-Funded Warrants. Generally, a holder will comply with such procedures if it provides a properly executed IRS Form W-8BEN (or
other applicable Form W-8) or otherwise meets documentary evidence requirements for establishing that it is a Non-U.S. Holder, or
otherwise establishes an exemption. Dividends paid to Non-U.S. Holders subject to withholding of U.S. federal income tax, as described
above under the heading “Distributions,” will generally be exempt from U.S. backup withholding.
Information reporting and
backup withholding generally will apply to the proceeds of a disposition of the Common Stock and Pre-Funded Warrants by a Non-U.S. Holder
effected by or through the U.S. office of any broker, U.S. or foreign, unless the holder certifies its status as a Non-U.S. Holder and
satisfies certain other requirements, or otherwise establishes an exemption. Generally, information reporting and backup withholding will
not apply to a payment of disposition proceeds to a Non-U.S. Holder where the transaction is effected outside the United States through
a non-U.S. office of a broker. However, for information reporting purposes, dispositions effected through a non-U.S. office of a broker
with substantial U.S. ownership or operations generally will be treated in a manner similar to dispositions effected through a U.S. office
of a broker. Non-U.S. Holders should consult their own tax advisors regarding the application of the information reporting and backup
withholding rules to them.
Copies of information returns
may be made available to the tax authorities of the country in which the Non-U.S. Holder resides or is incorporated under the provisions
of a specific treaty or agreement.
Backup withholding is not
an additional tax. Any amounts withheld under the backup withholding rules from a payment to a Non-U.S. Holder can be refunded or
credited against the Non-U.S. Holder’s U.S. federal income tax liability, if any, provided that an appropriate claim is timely filed
with the IRS.
Foreign Accounts
The Foreign Account Tax Compliance
Act, or FATCA, generally imposes a 30% withholding tax on dividends (including constructive dividends) on the Common Stock and Pre-Funded
Warrants if paid to a non-U.S. entity unless (i) if the non-U.S. entity is a “foreign financial institution,” the non-U.S.
entity undertakes certain due diligence, reporting, withholding, and certification obligations, (ii) if the non-U.S. entity is not
a “foreign financial institution,” the non-U.S. entity identifies certain of its U.S. investors, if any, or (iii) the
non-U.S. entity is otherwise exempt under FATCA.
Withholding under FATCA generally
will apply to payments of dividends (including constructive dividends) on our Common Stock and Pre-Funded Warrants. While withholding
under FATCA would have also applied to payments of gross proceeds from a sale or other disposition of the Common Stock and, Pre-Funded
Warrants, under proposed U.S. Treasury Regulations withholding on payments of gross proceeds is not required. Although such regulations
are not final, applicable withholding agents may rely on the proposed regulations until final regulations are issued.
An intergovernmental agreement
between the United States and an applicable foreign country may modify the requirements described in this section. Under certain circumstances,
a holder may be eligible for refunds or credits of the tax. Holders should consult their own tax advisors regarding the possible implications
of FATCA on their investment in the Common Stock and Pre-Funded Warrants.
The preceding discussion
of material U.S. federal tax considerations is for information only. It is not tax advice. Prospective investors should consult their
own tax advisors regarding the particular U.S. federal, state, local and non-U.S. tax consequences of purchasing, holding and disposing
of the Common Stock and Pre-Funded Warrants including the consequences of any proposed changes in applicable laws.
PLAN OF DISTRIBUTION
We engaged Spartan Capital
Securities, LLC to act as our exclusive Placement Agent to solicit offers to purchase the Securities offered by this prospectus on a reasonable
best-efforts basis, subject to the terms and conditions of a placement agency agreement between the Company and the Placement Agent. The
Placement Agent is not purchasing or selling any of the securities offered by this prospectus, nor is it required to arrange the purchase
or sale of any specific number or dollar amount of securities, but has agreed to use its reasonable best efforts to arrange for the sale
of the Securities offered hereby. Therefore, we may not sell the entire amount of securities offered pursuant to this prospectus. The
Placement Agent may engage one or more sub-placement agents or selected dealers to assist with the offering. We will enter into a securities
purchase agreement directly with certain investors, at the investor’s option, who purchase our securities in this offering. Investors
who do not enter into a securities purchase agreement shall rely solely on this prospectus and the documents incorporated by reference
herein in connection with the purchase of our securities in this offering.
We will deliver the securities
being issued to the investors upon receipt of such investor’s funds for the purchase of the securities offered pursuant to this
prospectus. We expect this offering to be completed not later than one (1) business day following the commencement of this offering. We
will deliver all Securities to be issued in connection with this offering delivery versus payment (“DVP”)/receipt versus payment
(“RVP”) upon receipt of investor funds received by us.
We have agreed to indemnify
the Placement Agent and specified other persons against specified liabilities, including liabilities under the Securities Act, and to
contribute to payments the Placement Agent may be required to make in respect thereof.
Fees and Expenses
The
following table shows the per share, the per Pre-Funded Warrant and total of the public offering price, Placement Agent fees and proceeds
to us before expenses. These amounts are shown assuming the sale of all of the Securities we are offering.
| |
|
Per Share |
|
Per Pre- Funded Warrant |
|
Total |
| Public offering price |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
| Placement Agent fees (1) |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
| Proceeds to us, before expenses (2) |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
| (1) |
Represents a cash fee equal to 2.0% of the
gross proceeds of the offering. We have also agreed to reimburse the Placement Agent for certain offering-related legal and other
expenses and to pay to the Placement Agent at the closing of the offering a non-accountable expense allowance equal to 1.0% of the
gross proceeds of the offering. |
| (2) |
Does not include proceeds from the exercise of the Pre-Funded Warrants
in cash, if any. |
We have agreed to pay all
of the expenses relating to the offering, including but not limited to the following: (a) all filing
fees and communication expenses relating to the registration of the securities to be sold in this offering with the SEC; (b) all filing
fees and communication expenses associated with the review of the offering by FINRA; (c) all fees, expenses and disbursements relating
to the registration or qualification of the securities to be sold in this offering under the” securities or “blue sky”
laws of such states and other jurisdictions as the Placement Agent may reasonably designate (including, without limitation, all filing
and registration fees, and the reasonable fees and disbursements of “blue sky” counsel); (d) the costs of preparing,
printing, mailing and delivering the Registration Statement, the preliminary and final prospectus contained therein and amendments thereto,
post-effective amendments and supplements thereto, any other offering materials, the placement agent agreement and related documents (all
in such quantities as the Placement Agent may reasonably require); (e) preparing and printing stock certificates and warrant certificates;
the costs of any “due diligence” meetings; (f) expenses incurred by the Placement Agent
for any roadshow for the offering; (g) the costs associated with the use of a third-party electronic road show service (such as Net Roadshow);
(h) the fees, expenses and disbursements relating to background checks of the our executive officers and directors; (i) the preparation
of leather bound volumes and Lucite cube mementos in such quantities as the Placement Agent may reasonably request; (j) any applicable
transfer taxes; (k) the fees and expenses of our transfer agent and registrar and warrant agent relating to the offering; (l) the
fees and expenses of our accountants; (m) the fees and expenses of our legal counsel and other agents and representatives; (n) the fees
and expenses of the counsel to the Placement Agent; provided that reimbursement of such accountable expenses to the Placement Agent or
the payment of such accountable expenses on behalf of the Placement Agent shall not exceed $65,000. We have also agreed to pay closing
costs incurred by the Placement Agent, including the out-of-pocket cost of any escrow agent or clearing agent in an amount not to exceed
$12,500.
We have also agreed to pay
the Placement Agent the closing of the offering a non-accountable expense allowance equal to 1% of the gross proceeds of the offering.
We estimate the total
expenses payable by us for this offering, excluding the Placement Agent fees and expenses, will be approximately $194,016.
Lock-Up Agreement
Pursuant to “lock-up”
agreements, we, our executive officers and directors, and holders of 5% or more of the issued and outstanding shares of our Common Stock
have agreed for a period of sixty (60) days after the closing of the offering, subject to customary exceptions, without the prior written
consent of the Placement Agent, not to, directly or indirectly, offer pledge, sell, contract to sell, sell any option or contract to purchase,
purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of, directly
or indirectly, any shares of our capital stock or any securities convertible into or exercisable or exchangeable for shares of our capital
stock; (ii) file or caused to be filed any registration statement with the SEC relating to the offering of any shares of our capital stock
or any securities convertible into or exercisable or exchangeable for shares of our capital stock; (iii) in the case of the Company, complete
any offering of debt securities, other than entering into a line of credit with a traditional bank or (iv) enter into any swap or other
arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of shares of our capital stock,
whether any such transaction described in clause (i), (ii), (iii) or (iv) above is to be settled by delivery of shares of our capital
stock or such other securities, in cash or otherwise.
Electronic Distribution
A prospectus in electronic
format may be made available on a website maintained by the Placement Agent. In connection with the offering, the Placement Agent or
selected dealers may distribute prospectuses electronically. No forms of electronic prospectus other than prospectuses that are printable
as Adobe® PDF will be used in connection with this offering.
Other than the prospectus
in electronic format, the information on the Placement Agent’s website and any information contained in any other website maintained
by the Placement Agent is not part of the prospectus or the registration statement of which this prospectus forms a part, has not been
approved and/or endorsed by us or the Placement Agent in its capacity as placement agent and should not be relied upon by investors.
Listing
Shares of our Common
Stock are listed on Nasdaq under the symbol “VRPX”. On March 21, 2025, the last reported sale price of shares of our Common
Stock on Nasdaq was $2.07 per share. We do not plan to list the Pre-Funded Warrants on Nasdaq or any other securities exchange or trading
market.
Regulation M
The Placement Agent may be
deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any commissions received by it and any profit
realized on the resale of the Securities sold by it while acting as principal might be deemed to be underwriting discounts or commissions
under the Securities Act. As an underwriter, the Placement Agent would be required to comply with the requirements of the Securities Act
and the Exchange Act, including, without limitation, Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations may
limit the timing of purchases and sales of our Securities by the placement agent acting as principal. Under these rules and regulations,
the Placement Agent (i) may not engage in any stabilization activity in connection with our Securities and (ii) may not bid for or purchase
any of our Securities or attempt to induce any person to purchase any of our securities, other than as permitted under the Exchange Act,
until it has completed its participation in the distribution.
Other Relationships
From time to time, the Placement
Agent and/or its affiliates may have provided, and may in the future provide, various investment banking and other financial services
for us for which they may receive customary fees. In the course of its business, the Placement Agent and its affiliates may actively trade
our securities or loans for its own account or for the accounts of customers, and, accordingly, the Placement Agent and its respective
affiliates may at any time hold long or short positions in such securities or loans.
Selling
Restrictions
Other than in the United
States of America, no action has been taken by us or the Placement Agent that would permit a public offering of the Securities offered
by this prospectus in any jurisdiction where action for that purpose is required. The Securities offered by this prospectus may not be
offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the
offer and sale of any such Securities be distributed or published in any jurisdiction, except under circumstances that will result in
compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised
to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus
does not constitute an offer to sell or a solicitation of an offer to buy any Securities offered by this prospectus in any jurisdiction
in which such an offer or a solicitation is unlawful.
European Economic Area
In relation to each Member
State of the European Economic Area (each, a Member State), no Securities have been offered or will be offered pursuant to this offering
to the public in that Member State prior to the publication of a prospectus in relation to our Securities which has been approved by the
competent authority in that Member State or, where appropriate, approved in another Member State and notified to the competent authority
in that Member State, all in accordance with the Prospectus Regulation, except that offers of securities may be made to the public in
that Member State at any time under the following exemptions under the Prospectus Regulation:
| |
(a) |
to any legal entity which is a qualified investor as defined in the Prospectus Regulation; |
| |
(b) |
by the placement agent to fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Regulation), subject to obtaining the prior written consent of the representatives for any such offer; or |
| |
(c) |
in any other circumstances falling within Article 1(4) of the Prospectus Regulation, provided that no such offer of our Securities shall result in a requirement for us or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Regulation or supplement a prospectus pursuant to Article 23 of the Prospectus Regulation. |
Each person in a Member State
who initially acquires any of our Securities or to whom any offer is made will be deemed to have represented, acknowledged, and agreed
with us and the representatives that it is a qualified investor within the meaning of the Prospectus Regulation.
In the case of any of our
Securities are being offered to a financial intermediary as that term is used in Article 5(1) of the Prospectus Regulation, each such
financial intermediary will be deemed to have represented, acknowledged and agreed that the Securities acquired by it in the offer have
not been acquired on a non-discretionary basis on behalf of, nor have they been acquired with a view to their offer or resale to, persons
in circumstances which may give rise to an offer to the public other than their offer or resale in a Member State to qualified investors,
in circumstances in which the prior written consent of the representatives has been obtained to each such proposed offer or resale.
We, the placement agent,
and their affiliates will rely upon the truth and accuracy of the foregoing representations, acknowledgments, and agreements.
For the purposes of this
provision, the expression an “offer to the public” in relation to any of our Securities in any Member State means the communication
in any form and by any means of sufficient information on the terms of the offer and any of our Securities to be offered so as to enable
an investor to decide to purchase or subscribe for our Securities, and the expression “Prospectus Regulation” means Regulation
(EU) 2017/1129.
United Kingdom
No securities have been offered
or will be offered pursuant to this offering to the public in the United Kingdom prior to the publication of a prospectus in relation
to the securities which has been approved by the Financial Conduct Authority, except that the securities may be offered to the public
in the United Kingdom at any time:
| |
(a) |
to any legal entity which is a qualified investor as defined under Article 2 of the UK Prospectus Regulation; |
| |
(b) |
to fewer than 150 natural or legal persons (other than qualified investors as defined under Article 2 of the UK Prospectus Regulation), subject to obtaining the prior consent of the representatives for any such offer; or |
| |
(c) |
in any other circumstances falling within Section 86 of the Financial Services and Markets Act 2000, or FSMA; |
provided that no such offer
of the securities shall require the us or any placement agent to publish a prospectus pursuant to Section 85 of the FSMA or supplement
a prospectus pursuant to Article 23 of the UK Prospectus Regulation. For the purposes of this provision, the expression an “offer
to the public” in relation to the securities in the United Kingdom means the communication in any form and by any means of sufficient
information on the terms of the offer and any shares to be offered so as to enable an investor to decide to purchase or subscribe for
any securities and the expression “UK Prospectus Regulation” means Regulation (EU) 2017/1129 as it forms part of domestic
law by virtue of the European Union (Withdrawal) Act 2018.
Switzerland
Our Securities may not be
publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange, or the SIX, or on any other stock exchange or regulated
trading facility in Switzerland. This document does not constitute a prospectus within the meaning of, and has been prepared without regard
to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure
standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated
trading facility in Switzerland. Neither this document nor any other offering or marketing material relating to our Securities or this
offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor
any other offering or marketing material relating to this offering, our company or our Securities have been or will be filed with or approved
by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of our Securities will not be supervised
by, the Swiss Financial Market Supervisory Authority and the offer of our Securities have not been and will not be authorized under the
Swiss Federal Act on Collective Investment Schemes, or the CISA. The investor protection afforded to acquirers of interests in collective
investment schemes under the CISA does not extend to acquirers of our Securities.
Canada
The Securities may be sold
in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National
Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in
National Instrument 31 103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the Securities must
be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities
laws.
Securities legislation in
certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus supplement
(including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by
the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser
should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars
of these rights or consult with a legal advisor.
Pursuant to section 3A.3
of National Instrument 33 105 Underwriting Conflicts (NI 33 105), the placement agent are not required to comply with the disclosure requirements
of NI 33-105 regarding placement agent conflicts of interest in connection with this offering.
LEGAL MATTERS
Sichenzia Ross Ference Carmel
LLP, New York, New York, is acting as counsel to the Company in connection with this offering and will pass upon the validity of the shares
of Common Stock offered by this prospectus and certain other legal matters. Lucosky Brookman LLP is acting as counsel to the Placement
Agent.
EXPERTS
The consolidated balance
sheets of Virpax Pharmaceuticals, Inc. as of December 31, 2024, and 2023, and the related consolidated statements of operations, changes
in stockholders’ equity, and cash flows for each of the years then ended, have been audited by Bush & Associates CPA LLC, independent
registered public accounting firm, as stated in their report, which is incorporated herein by reference. This report includes an explanatory
paragraph expressing substantial doubt about the Company’s ability to continue as a going concern. These financial statements have
been incorporated by reference in reliance on the report of Bush & Associates CPA LLC, given upon their authority as experts in accounting
and auditing.
WHERE YOU CAN FIND MORE
INFORMATION
We have filed with the Securities
and Exchange Commission (the “SEC”) a registration statement on Form S-1 under the Securities Act with respect to the
Securities offered hereby. This prospectus, which constitutes a part of the registration statement, does not contain all of the information
set forth in the registration statement or the exhibits and schedules filed therewith. For further information about us and the Securities
offered hereby, we refer you to the registration statement and the exhibits and schedules filed thereto. Statements contained in this
prospectus regarding the contents of any contract or any other document that is filed as an exhibit to the registration statement are
not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other
document filed as an exhibit to the registration statement. The SEC also maintains an Internet website that contains reports, proxy statements
and other information about registrants, like us, that file electronically with the SEC. The address of that site is www.sec.gov.
We are required to file periodic reports, proxy statements,
and other information with the SEC pursuant to the Exchange Act. These reports, proxy statements, and other information will be available
on the website of the SEC referred to above.
We also maintain a website at www.virpaxpharma.com,
through which you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with,
or furnished to, the SEC. Information contained on or accessed through our website is not a part of this prospectus and the inclusion
of our website address in this prospectus is an inactive textual reference only.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference”
information from other documents that we file with it, which means that we can disclose important information to you by referring you
to those documents. The information incorporated by reference is considered to be part of this prospectus. Information in this prospectus
supersedes information incorporated by reference that we filed with the SEC prior to the date of this prospectus.
We incorporate by reference into this prospectus and
the registration statement of which this prospectus is a part the information or documents listed below that we have filed with the SEC
(Commission File No. 001-40064):
| |
● |
Our Annual Report on Form 10-K for the fiscal December 31, 2024 (the “Annual Report”) with the SEC on March 3, 2025; and |
| |
|
|
| |
● |
The description of our Common Stock, as set forth in our registration statement
on Form 8-A filed with the SEC on filed on February 11, 2021, and as subsequently updated by the description of our Common Stock filed
as Exhibit 4.4 to our Annual Report on Form 10-K for the year ended December 31, 2023, filed with the SEC on March 26, 2024, including
any amendments or reports filed for the purpose of updating such description. |
We also incorporate by reference any future filings
(other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that are related to such
items unless such Form 8-K expressly provides to the contrary) made with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the
Exchange Act, including those made (i) on or after the date of the initial filing of the registration statement of which this prospectus
forms a part and prior to effectiveness of such registration statement, and (ii) on or after the date of this prospectus but prior to
the termination of the offering (i.e., until the earlier of the date on which all of the securities registered hereunder have been sold
or the registration statement of which this prospectus forms a part has been withdrawn). Information in such future filings updates and
supplements the information provided in this prospectus. Any statements in any such future filings will automatically be deemed to modify
and supersede any information in any document we previously filed with the SEC that is incorporated or deemed to be incorporated herein
by reference to the extent that statements in the later filed document modify or replace such earlier statements.
We will furnish without charge to each person, including
any beneficial owner, to whom a prospectus is delivered, upon written or oral request, a copy of any or all of the documents incorporated
by reference into this prospectus but not delivered with the prospectus, including exhibits that are specifically incorporated by reference
into such documents. You should direct any requests for documents to:
Virpax Pharmaceuticals, Inc.
1055 Westlakes Drive, Suite 300
Berwyn, Pennsylvania 19312
Telephone (610) 727-4597
Attention: Corporate Secretary
You may also access these documents, free of charge,
on the SEC’s website at www.sec.gov or on our website at https://virpaxpharma.com/investors/sec-filings.
The information contained in, or that can be accessed through, our website is not incorporated by reference in, and is not part of, this
prospectus or any accompanying prospectus supplement.
In accordance with Rule 412 of the Securities Act,
any statement contained in a document incorporated by reference herein shall be deemed modified or superseded to the extent that a statement
contained herein or in any other subsequently filed document which also is or is deemed to be incorporated by reference herein or deemed
to be a part of the registration statement or prospectus that is part of the registration statement modifies or supersedes such statement.
Up to 2,415,459 Shares
of Common Stock
Up to 2,415,459 Pre-Funded Warrants to Purchase 2,415,459 Shares of Common Stock
Up to 2,415,459 Shares of Common Stock Issuable Upon Exercise of such Pre-Funded Warrants

VIRPAX PHARMACEUTICALS, INC.
PROSPECTUS
Sole Placement Agent
Spartan Capital Securities, LLC
, 2025
PART II — INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the expenses in connection
with this registration statement. All of such expenses are estimates, other than the SEC registration fee and the FINRA filing fee.
| | |
Amount to
be paid |
| SEC registration fee | |
$ | 766 | |
| FINRA filing fee | |
$ | 1,250 | |
| Accounting fees and expenses | |
$ | 2,000 | |
| Legal fees and expenses | |
$ | 150,000 | |
| Miscellaneous expenses | |
$ | 40,000 | |
| Total | |
$ | 194,016 | |
Item 14. Indemnification of Directors and Officers
Section 145 of the Delaware
General Corporation Law (the “DGCL”) empowers a corporation to indemnify its directors and officers and to purchase insurance
with respect to liability arising out of their capacity or status as directors and officers, provided that the person acted in good faith
and in a manner the person reasonably believed to be in our best interests, and, with respect to any criminal action, had no reasonable
cause to believe the person’s actions were unlawful. The DGCL further provides that the indemnification permitted thereunder shall
not be deemed exclusive of any other rights to which the directors and officers may be entitled under the corporation’s bylaws,
any agreement, a vote of stockholders or otherwise.
Section 102(b)(7) of the
Delaware General Corporation Law permits a corporation to provide in its certificate of incorporation that a director or officer of the
corporation shall not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duty as
a director or officer, except (i) for any breach of the director’s or officer’s duty of loyalty to the corporation or
its stockholders; (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of
law; (iii) a director for payments of unlawful dividends or unlawful stock repurchases or redemptions; (iv) for any transaction
from which the director or officer derived an improper personal benefit; or (v) an officer in any action by or in the right of the corporation.
Our amended and restated bylaws provides that we will
indemnify our directors and executive officers to the fullest extent permitted by law, and may indemnify other officers, employees and
other agents. Our amended and restated bylaws also provide that we are obligated to advance expenses incurred by a director or executive
officer in advance of the final disposition of any action or proceeding. In addition, as permitted by Delaware law, our amended and restated
certificate of incorporation includes provisions that eliminate the personal liability of our directors and officers for monetary damages
resulting from breaches of certain fiduciary duties as a director or officer, as applicable, except to the extent such an exemption from
liability thereof is not permitted under the DGCL.
We have entered into indemnification agreements with
each of our directors and executive officers. These agreements will require us to indemnify these individuals to the fullest extent permitted
under Delaware law against liabilities that may arise by reason of their service to us and to advance expenses incurred as a result of
any proceeding against them as to which they could be indemnified.
The Registrant has an insurance policy in place that
covers its officers and directors with respect to certain liabilities, including liabilities arising under the Securities Act or otherwise.
Item 15. Recent Sales of Unregistered Securities
Except as set forth below, the Company has not issued
unregistered securities to any person within the last three years.
On July 5, 2024, the Company
entered into a Securities Purchase Agreement with an institutional investor pursuant to which, on July 5, 2024, the Company issued to
the Investor a senior secured promissory note in the principal amount of $2.5 million for $2.5 million. The issuance was exempt from registration
pursuant to Section 4(a)(2) of the Securities Act of 1933, as amended, and/or Regulation D promulgated thereunder.
On September 30, 2024, we entered into an extension
agreement with the Investor pursuant to the terms of the Purchase Agreement.
Pursuant to the terms of the Extension Agreement,
the Investor agreed to amend certain provisions of the Purchase Agreement related to a potential financing arrangement of not less than
$5 million. Under the Extension Agreement, the Investor retains the exclusive right to negotiate the terms of and consummate any Subsequent
Financing until November 30, 2024. Additionally, the Investor holds a right of first refusal for any Subsequent Financing that may occur
on or before the Outside Date. If a financing arrangement of not less than $5 million is not provided by the Investor (or its affiliate(s)
and/or third-party designee(s)) by the Outside Date, the Investor’s nominated members on the Company’s Board of Directors
will resign, effective immediately.
Item 16. Exhibits
EXHIBIT INDEX
The exhibits listed in the accompanying Exhibit Index
are filed or incorporated by reference as part of this registration statement.
| Exhibit No. |
|
Description of Document |
| 1.1* |
|
Form of Placement Agency Agreement |
| 3.1 |
|
Amended and Restated Certificate of Incorporation of Virpax Pharmaceuticals, Inc. (incorporated by reference to Exhibit 3.1 to the Company’s Annual Report on Form 10-K (File No. 001-40064) filed on March 31, 2021). |
| 3.2 |
|
Amended and Restated Bylaws of Virpax Pharmaceuticals, Inc. (incorporated by reference to Exhibit 3.2 to the Company’s Annual Report on Form 10-K (File No. 001-40064) filed with the SEC on March 31, 2021). |
| 3.3 |
|
Amendment to By-Laws dated June 5, 2023 (incorporated by reference to Exhibit 3.1 to Company’s Current Report on Form 8-K (File No. 001-40064) filed with the SEC on June 7, 2023). |
| 3.4 |
|
Certificate of Amendment to the Certificate of Incorporation (incorporated by reference to Exhibit 3.1 of the Company’s Current Report on Form 8-K (File No. 001-40064) filed with the SEC on March 1, 2024) |
| 3.5 |
|
Amended and Restated Bylaws of Virpax Pharmaceuticals, Inc. (incorporated by reference to Exhibit 3.1 of the Company’s Current Report on Form 8-K (File No. 001-40064) filed with the SEC on November 29, 2024). |
| 4.1 |
|
Specimen Certificate representing shares of Common Stock of Virpax Pharmaceuticals, Inc. (incorporated by reference to Exhibit 4.1 of the Company’s Registration Statement on Form S-1 (333-249417) filed with the SEC on October 9, 2020). |
| 4.2 |
|
Form of Consultant Warrant (incorporated by reference to Exhibit 4.3 of the Company’s Registration Statement on Form S-1 (333-249417) filed with the SEC on October 9, 2020). |
| 4.3 |
|
Form of Underwriter’s Warrant (incorporated by reference to Exhibit 4.2 of the Company’s Registration Statement on Form S-1/A (333-249417) filed with the SEC on February 2, 2021). |
| 4.4 |
|
Form of Series A-1 Warrants (incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K (File No. 001-40064) filed with the SEC on May 17, 2024) |
| 4.5 |
|
Form of Series A-2 Warrant (incorporated by reference to Exhibit 4.2 of the Company’s Current Report on Form 8-K (File No. 001-40064) filed with the SEC on May 17, 2024) |
| 4.6 |
|
Form of Secured Note (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K (File No. 001-40064) filed with the SEC on July 8, 2024) |
| 4.7 |
|
Form of Pre-Funded Warrant (incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K (File No. 001-40064) filed with the SEC on January 30, 2025) |
| 4.8* |
|
Form of Pre-Funded Warrant for this Offering |
| 5.1** |
|
Opinion of Sichenzia Ross Ference Carmel LLP |
| 10.1† |
|
Virpax Pharmaceuticals, Inc. 2017 Equity Incentive Plan (incorporated by reference to Exhibit 10.2 of the Company’s Registration Statement on Form S-1 (333-249417) filed with the SEC on October 9, 2020) † |
| 10.2† |
|
Form of Nonqualified Stock Option Award under 2017 Equity Incentive Plan (incorporated by reference to Exhibit 10.3 of the Company’s Registration Statement on Form S-1 (333-249417) filed with the SEC on October 9, 2020).† |
| 10.3† |
|
Form of Incentive Stock Option Award under 2017 Equity Incentive Plan (incorporated by reference to Exhibit 10.4 of the Company’s Registration Statement on Form S-1 (333-249417) filed with the SEC on October 9, 2020) † |
| 10.4† |
|
Employment Agreement by and between Virpax Pharmaceuticals, Inc. and Anthony Mack, dated as of September 18, 2018 (incorporated by reference to Exhibit 10.5 of the Company’s Registration Statement on Form S-1 (333-249417) filed with the SEC on October 9, 2020) † |
| 10.5† |
|
Consulting Agreement by and between Virpax Pharmaceuticals, Inc. and Gerald Bruce, dated as of March 11, 2020 (incorporated by reference to Exhibit 10.6 of the Company’s Registration Statement on Form S-1 (333-249417) filed with the SEC on October 9, 2020).† |
| 10.6 |
|
Form of Indemnification Agreement entered into by Virpax Pharmaceuticals, Inc. with its Officers and Directors (incorporated by reference to Exhibit 10.1 of the Company’s Registration Statement on Form S-1/A (333-249417) filed with the SEC on November 20, 2020). |
| 10.7# |
|
License Agreement by and between MedPharm Limited and Virpax Pharmaceuticals, Inc., dated as of June 6, 2017 (incorporated by reference to Exhibit 10.7 of the Company’s Registration Statement on Form S-1/A (333-249417) filed with the SEC on November 20, 2020) |
| 10.8# |
|
First Amendment to the License Agreement by and between MedPharm Limited and Virpax Pharmaceuticals, Inc., dated as of September 2, 2017 (incorporated by reference to Exhibit 10.8 of the Company’s Registration Statement on Form S-1/A (333-249417) filed with the SEC on November 20, 2020) |
| 10.9# |
|
Second Amendment to the License Agreement by and between MedPharm Limited and Virpax Pharmaceuticals, Inc., dated as of October 31, 2017 (incorporated by reference to Exhibit 10.9 of the Company’s Registration Statement on Form S-1/A (333-249417) filed with the SEC on November 20, 2020) |
| 10.10# |
|
Research and Option Agreement by and between MedPharm Limited and Virpax Pharmaceuticals, Inc., dated as of April 11, 2017 (incorporated by reference to Exhibit 10.10 of the Company’s Registration Statement on Form S-1/A (333-249417) filed with the SEC on November 20, 2020). |
| 10.11# |
|
First Amendment to the Research and Option Agreement by and between MedPharm Limited and Virpax Pharmaceuticals, Inc., dated as of May 30, 2018 (incorporated by reference to Exhibit 10.11 of the Company’s Registration Statement on Form S-1/A (333-249417) filed with the SEC on November 20, 2020). |
| 10.12# |
|
License and Sublicense Agreement by and between LipoCureRx, Ltd. and Virpax Pharmaceuticals, Inc., dated as of March 19, 2018 (incorporated by reference to Exhibit 10.12 of the Company’s Registration Statement on Form S-1/A (333-249417) filed with the SEC on November 20, 2020). |
| 10.13# |
|
Collaboration and License Agreement by and between Nanomerics Ltd. and Virpax Pharmaceuticals, Inc., dated as of April 11, 2019 (incorporated by reference to Exhibit 10.13 of the Company’s Registration Statement on Form S-1/A (333-249417) filed with the SEC on November 20, 2020). |
| 10.14# |
|
Amendment to the Collaboration and License Agreement by and between Nanomerics Ltd. and Virpax Pharmaceuticals, Inc., dated as of December 30, 2019 (incorporated by reference to Exhibit 10.14 of the Company’s Registration Statement on Form S-1/A (333-249417) filed with the SEC on November 20, 2020). |
| 10.15# |
|
Collaboration and License Agreement between Nanomerics Ltd. and Virpax Pharmaceuticals, Inc., dated August 7, 2020 (incorporated by reference to Exhibit 10.17 of the Company’s Registration Statement on Form S-1/A (333-249417) filed with the SEC on February 2, 2021). |
| 10.16 |
|
Paycheck Protection Program Term Note, dated May 4, 2020, between Virpax Pharmaceuticals, Inc. and PNC Bank, National Association. (incorporated by reference to Exhibit 10.26 of the Company’s Registration Statement on Form S-1 (333-249417) filed with the SEC on February 2, 2021). |
| 10.17 |
|
Cooperative Research and Development Agreement, dated August 25, 2020, between the U.S. Department of Health and Human Services, as represented by National Center for Advancing Translational Sciences an Institute or Center of the National Institutes of Health and Virpax Pharmaceuticals, Inc. (incorporated by reference to Exhibit 10.27 of the Company’s Registration Statement on Form S-1 (333-249417) filed with the SEC on February 2, 2021). |
| 10.18 |
|
Amendment No. 1 to the Collaboration and License Agreement between Nanomerics Ltd. and Virpax Pharmaceuticals, Inc., dated as of December 31, 2020 (incorporated by reference to Exhibit 10.31 of the Company’s Registration Statement on Form S-1/A (333-249417) filed with the SEC on February 2, 2021). |
| 10.19† |
|
Employment Agreement, dated as of April 7, 2021, by and between Christopher M. Chipman and Virpax Pharmaceuticals, Inc. (incorporated by reference to Exhibit 10.1 of the Company’s current report on Form 8-K (File No. 001-40064) filed with the SEC on April 13, 2021). |
| 10.20† |
|
Employment Agreement, dated as of April 15, 2021, by and between Jeffrey Gudin, MD and Virpax Pharmaceuticals, Inc. (incorporated by reference to Exhibit 10.1 of the Company’s current report on Form 8-K (File No. 001-40064) filed with the SEC on April 19, 2021). |
| 10.21 |
|
Amendment to the Collaboration and License Agreement dated April 11, 2019, as amended, between Nanomerics Ltd. and Virpax Pharmaceuticals, Inc., dated April 6, 2021 (incorporated by reference to Exhibit 10.3 of the Company’s quarterly report on Form 10-Q (File No. 001-40064) filed with the SEC on August 10, 2021). |
| 10.22 |
|
Amendment to the Collaboration and License Agreement dated April 11, 2019, as amended, between Nanomerics Ltd. and Virpax Pharmaceuticals Inc., dated May 5, 2021 (incorporated by reference to Exhibit 10.4 of the Company’s quarterly report on Form 10-Q (File No. 001-40064) filed with the SEC on August 10, 2021). |
| 10.23† |
|
Amendment No. 1 to the Amended and Restated Virpax Pharmaceuticals, Inc. 2017 Equity Incentive Plan (incorporated by reference to Exhibit 10.5 of the Company’s Quarterly Report on Form 10-Q (File No. 001-40064) filed with the SEC on August 10, 2021). |
| 10.24 |
|
Agreement for Rendering of Research Services between LipoCureRx, Ltd. and Virpax Pharmaceuticals, Inc., dated June 29, 2021 (incorporated by reference to Exhibit 10.16 of the Company’s Registration Statement on Form S-1 (File No. 333-259421) filed with the SEC on September 9, 2021). |
| 10.25† |
|
Virpax Pharmaceuticals, Inc. 2022 Equity Incentive Plan (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K (File No. 001-40064) filed with the SEC on July 25, 2022). |
| 10.26† |
|
Virpax Pharmaceuticals, Inc. Form of Nonqualified Stock Option Grant Agreement (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K (File No. 001-40064) filed with the SEC on July 25, 2022). |
| 10.27† |
|
Virpax Pharmaceuticals, Inc. Form of Incentive Stock Option Grant Agreement (incorporated by reference to Exhibit 10.3 to the Current Report on Form 8-K (File No. 001-40064) filed with the SEC on July 25, 2022). |
| 10.28† |
|
Virpax Pharmaceuticals, Inc. Form of Restricted Stock Award Agreement (incorporated by reference to Exhibit 10.4 to the Current Report on Form 8-K (File No. 001-40064) filed with the SEC on July 25, 2022). |
| 10.29† |
|
Virpax Pharmaceuticals, Inc. Form of Restricted Stock Unit Award Agreement (incorporated by reference to Exhibit 10.5 to the Current Report on Form 8-K (File No. 001-40064) filed with the SEC on July 25, 2022). |
| 10.30# |
|
Amended and Restated Collaboration and License Agreement between Nanomerics Ltd. and Virpax Pharmaceuticals, Inc., dated as of March 9, 2022 (incorporated by reference to Exhibit 10.26 of the Annual Report on Form 10-K (File No. 001-40064) filed with the SEC on March 22, 2023). |
| 10.31† |
|
Amendment No. 1, dated March 29, 2022, to the Employment Agreement by and between Virpax Pharmaceuticals, Inc. and Anthony Mack, dated September 18, 2017 (incorporated by reference to Exhibit 10.7 of the Company’s Annual Report on Form 10-K (File No. 001-40064) filed with the SEC on March 22, 2023) |
| 10.32† |
|
Amendment No. 1, dated March 29, 2022, to the Employment Agreement by and between Virpax Pharmaceuticals, Inc. and Jeffrey Gudin, MD, dated April 15, 2021 (incorporated by reference to Exhibit 10.11 of the Company’s annual report on Form 10-K (File No. 001-40064) filed with the SEC on March 22, 2023) |
| 10.33† |
|
Employment Agreement, dated June 20, 2023, by and between Virpax Pharmaceuticals, Inc. and Vinay Shah (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K (File No. 001-40064) filed with the SEC on June 21, 2023). |
| 10.34† |
|
Separation Agreement, dated June 18, 2023, by and between Virpax Pharmaceuticals, Inc. and Christopher Chipman incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K (File No. 001-40064) filed with the SEC on June 21, 2023). |
| 10.35† |
|
Amendment No. 2 to Employment Agreement, dated August 15, 2023, by and between Virpax Pharmaceuticals, Inc. and Anthony Mack (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K (File No. 001-40064) filed with the SEC on August 16, 2023). |
| 10.36† |
|
Employment Agreement, dated December 6, 2023, by and between Virpax Pharmaceuticals, Inc. and Gerald Bruce (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K (File No. 001-40064) filed with the SEC on December 7, 2023). |
| 10.37 |
|
Settlement Agreement and Mutual Release between Virpax Pharmaceuticals, Inc. and Sorrento Therapeutics, Inc. and Scilex Pharmaceuticals Inc. (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K (File No. 001-40064) filed with the SEC on March 1, 2024) |
| 10.38† |
|
Employment Agreement, dated April 7, 2021, by and between Virpax Pharmaceuticals, Inc. and Sheila Mathias (incorporated by reference to Exhibit 10.38 of the Company’s Annual Report on Form 10-K (File No. 001-40064) filed with the SEC on March 26, 2024) |
| 10.39 |
|
Indemnification Agreement, dated March 25, 2024, by and between Virpax Pharmaceuticals, Inc. and Vinay Shah (incorporated by reference to Exhibit 10.39 of the Company’s Annual Report on Form 10-K (File No. 001-40064) filed with the SEC on March 26, 2024) |
| 10.40* |
|
Form of Securities Purchase Agreement to be entered into in this Offering |
| 10.41 |
|
Securities Purchase Agreement, dated July 5, 2024 by and between Virpax Pharmaceuticals, Inc. and Corbo Capital Inc. (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K (File No. 001-40064) filed with the SEC on July 8, 2024) |
| 10.42 |
|
Security Agreement, dated July 5, 2024 by and between Virpax Pharmaceuticals, Inc. and Corbo Capital Inc. (incorporated by reference to Exhibit 10.2 of the Company’s Current Report on Form 8-K (File No. 001-40064) filed with the SEC on July 8, 2024) |
| 10.43† |
|
Amendment to the Virpax Pharmaceuticals 2022 Equity Incentive Plan (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K (File No. 001-40064) filed with the SEC on July 30, 2024) |
| 10.44† |
|
Amendment to the Virpax Pharmaceuticals 2022 Equity Incentive Plan (incorporated by reference to Exhibit 10.2 of the Company’s Current Report on Form 8-K (File No. 001-40064) filed with the SEC on July 30, 2024) |
| 10.45 |
|
Extension Agreement, dated September 30, 2024, by and between Virpax Pharmaceuticals, Inc. and Corbo Capital Inc. (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K (File No. 001-40064) filed with the SEC on October 3, 2024) |
| 10.46 |
|
Form of Independent Contractor Agreement, dated November 18, 2024, by and between Virpax Pharmaceuticals, Inc. and Chaudhry U Consulting Inc. (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K (File No. 001-40064) filed with the SEC on November 20, 2024) |
| 10.47 |
|
Form of Independent Contractor Agreement, dated November 19, 2024, by and between Virpax Pharmaceuticals, Inc. and Jat Consulting Corp., the personal corporation of Mr. Jatinder Dhaliwal (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K (File No. 001-40064) filed with the SEC on November 22, 2024) |
| 10.48 |
|
Form of Investor Relations Agreement, dated November 12, 2024, between the Company and IR Agency LLC (incorporated by reference to Exhibit 10.3 of the Company’s Current Report on Form 8-K (File No. 001-40064) filed with the SEC on November 15, 2024) |
| 10.49 |
|
Form of Investor Relations Agreement, by and between the Company and IR Agency LLC (incorporated by reference to Exhibit 10.3 of the Company’s Current Report on Form 8-K (File No. 001-40064) filed with the SEC on January 30, 2025) |
| 10.50 |
|
Form of Investor Relations Agreement, by and between the Company and Sideways Frequency LLC (incorporated by reference to Exhibit 10.4 of the Company’s Current Report on Form 8-K (File No. 001-40064) filed with the SEC on January 30, 2025) |
| 10.51 |
|
First Amendment to Independent Contractor Agreement with Jat Consulting Corp. (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K (File No. 001-40064) filed with the SEC on December 30, 2024) |
| 10.52 |
|
First Amendment to Independent Contractor Agreement with Chaudhry U Consulting Inc. (incorporated by reference to Exhibit 10.2 of the Company’s Current Report on Form 8-K (File No. 001-40064) filed with the SEC on December 30, 2024) |
| 10.53 |
|
Form of Placement Agency Agreement (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K (File No. 001-40064) filed with the SEC on January 30, 2025) |
| 10.54 |
|
Form of Securities Purchase Agreement (incorporated by reference to Exhibit 10.2 of the Company’s Current Report on Form 8-K (File No. 001-40064) filed with the SEC on January 30, 2025) |
| 21.1 |
|
List of Subsidiaries (incorporated by reference to Exhibit 21.1 of the Company’s Annual Report on Form 10-K (File No. 001-40064) filed with the SEC on March 26, 2024). |
| 23.1** |
|
Consent of Bush & Associates CPA LLC |
| 23.2** |
|
Consent of Sichenzia Ross Ference Carmel LLP (contained in Exhibit 5.1) |
| 24.1** |
|
Power of Attorney (reference is made to the signature page hereto) |
| 107* |
|
Filing Fee Table |
| * |
Previously filed |
| ** |
Filed herewith |
| † |
Denotes management compensation plan or contract. |
| # |
Certain portions of this exhibit have been
omitted because the omitted information is (i) not material and (ii) would likely cause competitive harm to the Company
if publicly disclosed. |
Item 17. Undertakings.
The undersigned registrant hereby undertakes:
| (1) |
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| |
i. |
to include any prospectus required by Section 10(a)(3) of the Securities Act; |
| |
ii. |
to reflect in the prospectus any acts or events arising after the effective date of this registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in this registration statement (notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of a prospectus filed with the Commission pursuant to Rule 424(b) under the Securities Act if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement); and |
| |
iii. |
to include any material information with respect to the plan of distribution not previously disclosed in this registration statement or any material change to such information in this registration statement; |
| |
|
|
| |
|
provided, however, that subparagraphs (i), (ii) and (iii) do not apply if the information required to be included in a post-effective amendment by those subparagraphs is contained in periodic reports filed with or furnished to the Commission by the Registrant pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934, that are incorporated by reference in this registration statement, or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration statement. |
| (2) |
That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (3) |
To remove from registration, by means of a post-effective amendment, any of the securities being registered which remain unsold at the termination of the offering. |
| (4) |
That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
| (5) |
That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: |
| |
|
| |
The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| |
i. |
Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 (§ 230.424 of this chapter); |
| |
ii. |
Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| |
iii. |
The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| |
iv. |
Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
| (6) |
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by a Registrant of expenses incurred or paid by a director, officer or controlling person of a Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, that Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue. |
| (7) |
For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
| |
|
| (8) |
For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (9) |
For purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
SIGNATURES
Pursuant to the requirements of the Securities
Act, the registrant has duly caused this amendment no. 1 to registration statement on Form S-1 to be signed on its behalf by the undersigned,
thereunto duly authorized, in Berwyn, Pennsylvania, on March 25, 2025.
| |
VIRPAX PHARMACEUTICALS, INC. |
| |
|
| |
By: |
/s/ Jatinder Dhaliwal |
| |
|
Name: |
Jatinder Dhaliwal |
| |
|
Title: |
Chief Executive Officer |
Pursuant to the requirements of the Securities
Act, this amendment no. 1 to registration statement on Form S-1 has been signed below by the following persons in the capacities and
on the dates indicated.
| Signature |
|
Title |
|
Date |
| |
|
|
|
|
| /s/ Jatinder Dhaliwal |
|
Chief Executive Officer (Principal Executive |
|
March 25, 2025 |
| Jatinder Dhaliwal |
|
Officer) and Director |
|
|
| |
|
|
|
|
| /s/ Usama Chaudhry |
|
Chief Financial Officer (Principal Financial |
|
March 25, 2025 |
| Usama Chaudhry |
|
Officer) |
|
|
| |
|
|
|
|
| /s/ Judy Su |
|
Director |
|
March 25, 2025 |
| Judy Su |
|
|
|
|
| |
|
|
|
|
| /s/ Katharyn Field |
|
Director and Vice President |
|
March 25, 2025 |
| Katharyn Field |
|
|
|
|
| |
|
|
|
|
| /s/ Esha Randhawa |
|
Director |
|
March 25, 2025 |
| Esha Randhawa |
|
|
|
|
| |
|
|
|
|
| /s/ Charn Deol |
|
Director |
|
March 25, 2025 |
| Charn Deol |
|
|
|
|
II-9
Exhibit 5.1

March 25, 2025
Virpax Pharmaceuticals, Inc.
1055 Westlakes Drive, Suite 300
Berwyn, PA 19312
Ladies and Gentlemen:
We have acted as counsel for Virpax Pharmaceuticals,
Inc., a Nevada corporation (the “Company”), in connection with the preparation and filing of a Registration Statement on Form
S-1 (the “Registration Statement”), including a related prospectus filed with the Registration Statement (the “Prospectus”),
with the Securities and Exchange Commission (the “Commission”) pursuant to the Securities Act of 1933, as amended (the “Securities
Act”), covering the offer and sale of up to 2,415,459 shares (the “Shares”) of common stock, par value $0.00001 per
share (the “Common Stock”), and pre-funded warrants (the “Pre-Funded Warrants”), in lieu of shares of Common Stock
that would otherwise result in the purchaser’s beneficial ownership exceeding 4.99% (or, at the election of such purchaser, 9.99%)
of the outstanding shares of Common Stock. Each Pre-Funded Warrant will be immediately exercisable for one share of Common Stock and may
be exercised at any time until all of the Pre-Funded Warrants are exercised in full. The purchase price of each Pre-Funded Warrant will
equal the price per share at which one share of Common Stock is being sold to the public in this offering, minus $0.00001, and the exercise
price of each Pre-Funded Warrant will be $0.00001 per share. This opinion is being rendered in connection with the filing of the Registration
Statement with the Commission.
In connection with this opinion, we have examined
originals or copies (certified or otherwise identified to our satisfaction) of (i) the Company’s Articles of Incorporation, as currently
in effect, (ii) the Company’s Bylaws as currently in effect, (iii) the Registration Statement and related Prospectus and (vi) such
corporate records, agreements, documents and other instruments, and such certificates or comparable documents of public officials or of
officers and representatives of the Company, as we have deemed relevant and necessary as a basis for the opinion hereinafter set forth.
In such examination, we have assumed the genuineness
of all signatures, the legal capacity of all natural persons, the authenticity of all documents submitted to us as originals, the conformity
to original documents of all documents submitted to us as certified, conformed or photostatic copies, and the authenticity of the originals
of such latter documents. As to certain questions of fact material to this opinion, we have relied upon certificates or comparable documents
of officers and representatives of the Company and have not sought to independently verify such facts.
Based on the foregoing, and in reliance thereon, and
subject to the qualifications, limitations, exceptions and assumptions set forth herein, we are of the opinion that, (i) the Shares and
the Pre-Funded Warrants have been duly authorized and when issued as described in the Registration Statement will be duly and validly
issued, fully paid and non-assessable and (ii) the shares of Common Stock issuable upon the exercise of the Pre-Funded Warrants have been
duly authorized and, upon the exercise of the Pre-Funded Warrants in accordance with the terms thereof, such Shares will be duly and validly
issued, fully paid and non-assessable shares of common stock of the Company. The Pre-Funded Warrants, when issued and delivered in accordance
with the terms described in the Registration Statement, will constitute valid and binding obligations of the Company, enforceable against
the Company in accordance with their terms under the laws of the State of New York, subject to the effects of bankruptcy, insolvency,
reorganization, moratorium, or other similar laws affecting creditors’ rights generally and to general principles of equity.
We express no opinion herein as to the laws of any
state or jurisdiction other than the federal laws of the United States of America, the Delaware General Corporation Law (the “DGCL”),
and, with respect to our opinion relating to the enforceability of the Pre-Funded Warrants, the laws of the State of New York.
This opinion speaks only as of the date hereof and
we assume no obligation to update or supplement this opinion if any applicable laws change after the date of this opinion or if we become
aware after the date of this opinion of any facts, whether existing before or arising after the date hereof, that might change the opinions
expressed above.
This opinion is furnished in connection with the filing
of the Registration Statement and may not be relied upon for any other purpose without our prior written consent in each instance.
We assume no obligation to update or supplement any
of our opinions to reflect any changes of law or fact that may occur. We hereby consent to the filing of this letter as an exhibit to
the Registration Statement and to the reference to our firm under the caption “Legal Matters” in the Prospectus which is a
part of the Registration Statement. In giving such consents, we do not thereby admit that we are in the category of persons whose consent
is required under Section 7 of the Securities Act or the rules and regulations of the Commission promulgated thereunder.
Very truly yours,
| /s/ Sichenzia Ross Ference Carmel LLP |
|
| Sichenzia Ross Ference Carmel LLP |
|
EXHIBIT 23.1

To Whom It May Concern:
We hereby consent
to the use in the Registration Statement of Virpax Pharmaceuticals, Inc.
on Form S-1 of our Report of Independent Registered Public Accounting Firm, dated
February 28, 2025, on the balance sheet of Virpax Pharmaceuticals,
Inc. as of December 31, 2024,
and the related statements of operations, changes in stockholder’s equity and cash flows for
the year then ended.
We also
consent to the referenc’es
to us under the headings “Experts” in
such Registration Statement.
| Very truly yours |
|
| |
|
 |
|
| Bush & Associates CPA LLC (PCAOB 6797)
|
|
| Henderson, Nevada |
|
| March 25, 2025 |
|
179 N. Gibson Rd.,
Henderson, NV 89014 • 702.703.5979
• www.bushandassociatescpas .com
Grafico Azioni Virpax Pharmaceuticals (NASDAQ:VRPX)
Storico
Da Mar 2025 a Apr 2025

Grafico Azioni Virpax Pharmaceuticals (NASDAQ:VRPX)
Storico
Da Apr 2024 a Apr 2025
