- Five proprietary platforms fueling multiple clinical
programs
- Targeting large indications including epilepsy, bipolar
disorder, depression, obsessive-compulsive disorder, migraine,
pain, obesity, Alzheimer's disease, Parkinson's disease, multiple
sclerosis, rheumatoid arthritis, and cancer. Plus,
rare autoimmune and inflammatory diseases, including
myasthenia gravis, cardiomyopathy, spinal muscular atrophy and IgA
nephropathy
NEW
HAVEN, Conn. and SAN
FRANCISCO, Jan. 8, 2024 /PRNewswire/ -- Biohaven Ltd.
(NYSE: BHVN) (Biohaven or the Company), a clinical-stage
biopharmaceutical company focused on the discovery, development and
commercialization of life-changing therapies to treat a broad range
of rare and common diseases, today highlighted substantial progress
and outlined 2024 milestones related to its broad portfolio at the
42nd Annual J.P. Morgan Healthcare Conference in San Francisco. A copy of the slide
presentation is available on the Events and Presentations section
of the Biohaven website.
Vlad Coric, M.D., Chairman and
Chief Executive Officer of Biohaven, said, "Building upon
our groundbreaking legacy of success in migraine, we have
re-emerged a year after the spinoff from the Pfizer transaction
with one of the most innovative portfolios in biotech with multiple
clinical programs primarily focused on neuroscience, immunology,
and oncology. We are very excited about each of these programs with
particular emphasis on those with near-term potential, including
our selective Kv7 activator platform. We have shown CNS target
engagement and differentiated tolerability without the typical CNS
side effects of other non-selective agents in this class. Given
this remarkable profile, we are advancing BHV-7000 in an array of
clinical studies for focal epilepsy, generalized epilepsy, bipolar
disorder, and major depressive disorder. BHV-7000 has the potential
to change the treatment paradigm in mood and epilepsy.
Further, our innovative extracellular protein degrader platform
has generated multiple new investigational agents that are rapidly
advancing into the clinic, initially targeting IgG, IgA, and β1-AR
autoantibodies to treat both common and rare autoimmune diseases.
This highlights the uniqueness of the platform to efficiently
deliver highly differentiated assets that are finely tuned to
specific clinical targets in relatively short development periods.
For example, our newly disclosed β1-AR degrader went from concept
to lead drug candidate in approximately one year. Our lead degrader
candidate for β1-AR autoantibodies, BHV-1600, offers to re-route
pathogenic antibodies to the liver where they can be degraded and
help alleviate β1-AR+ heart failure. In addition, we have quickly
advanced our next generation IgG degrader, BHV-1310, and shown that
it can achieve ultra-rapid 90% IgG depletion after a single dose in
preclinical models. This is particularly well suited to address
indications such as acute myasthenia gravis, transplant rejection
and other indications that require rapid clearance of
disease-causing antibodies. In addition to completing enrollment in
a pivotal spinal muscular atrophy clinical study, we have been
opportunistic in exploring our myostatin inhibitor, taldefgrobep
alfa, as a potential treatment approach for obesity demonstrating
that it reduces adipose tissue and improves lean mass. Addressing
the growing public health crisis of obesity by reducing fat while
preserving and improving muscle mass would be a tremendous
advancement in the field, especially as the field evaluates the
potential long-term impact of reducing muscle mass that has been
observed with GLP-1 medications. Finally, we look forward to an
important milestone in our glutamate modulating program with the
first quarter database lock for our interim efficacy analysis of
our Phase 3 study of troriluzole in OCD. There have been no novel
treatments in OCD in over 20 years and if troriluzole proves to be
efficacious this will be an important breakthrough for the millions
of patients suffering from this disorder."
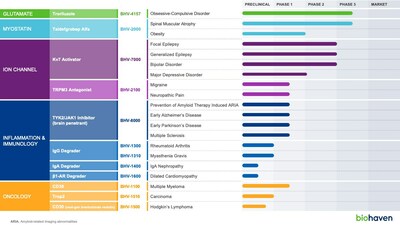
Anticipated 2024 Clinical Milestones
Biohaven is
positioned to achieve significant, value-creating milestones in
2024 across numerous programs:
Selective Kv7 Activator: BHV-7000 is a selective
activator of Kv7.2/7.3 potassium channels, a breakthrough target in
neurology and neuropsychiatry with blockbuster potential. Kv7
activation is a clinically validated target for treating epilepsy.
More recently, clinical proof-of-concept studies have also
demonstrated that Kv7 activators have robust antidepressant effects
and rapid onset of action, providing strong clinical support for
the transformative potential of BHV-7000 as a novel treatment for
major depressive disorder.
- Initiate BHV-7000 Phase 2/3 program in focal epilepsy in 1Q
2024
- Initiate BHV-7000 Phase 2/3 study in bipolar disorder in 1Q
2024
- Initiate BHV-7000 Phase 2 study in major depressive disorder in
1Q 2024
- Initiate BHV-7000 Phase 2/3 study in generalized epilepsy in 2Q
2024
Troriluzole: Troriluzole is a novel glutamate modulator
currently in Phase 3 development for obsessive-compulsive disorder
(OCD). It is being evaluated as an adjunctive therapy in patients
with an inadequate response to existing standard of care treatment.
The troriluzole Phase 2 trial in OCD demonstrated consistent
numerical benefits vs. placebo on the Yale-Brown Obsessive
Compulsive Scale (primary endpoint) at all timepoints and informed
the Phase 3 study design.
- Database lock in 1Q 2024 and report troriluzole Phase 3 interim
efficacy analysis topline results in OCD in 2Q 2024
Taldefgrobep alfa: Taldefgrobep is a novel myostatin
inhibitor that is optimized to block both myostatin and activin A
signaling, two key regulators of muscle growth, in a balanced
manner. Biohaven is studying taldefgrobep in a global Phase 3 study
in Spinal Muscular Atrophy (SMA), as an adjunctive therapy to
enhance muscle mass and function in patients treated with
standard-of-care treatments. Further, taldefgrobep also has
significant promise as a potential treatment for obesity. In
preclinical models, taldefgrobep demonstrated meaningful reductions
in fat mass, the primary pathogenic tissue in obesity, while
increasing lean mass. This is a paradigm-shift from obesity
therapies that cause significant losses in muscle mass.
- Initiate taldefgrobep Phase 2 study in obesity in 2Q 2024
- Report taldefgrobep Phase 3 topline results in SMA in 2H
2024
First-in-class TRPM3 Antagonist: BHV-2100 is a
first-in-class, oral, selective TRPM3 antagonist that offers a
novel, non-addictive treatment for migraine and neuropathic pain.
The preliminary pharmacokinetic and safety data from the ongoing
Phase 1 study in healthy volunteers supports evaluation of BHV-2100
in acute migraine. It is rapidly absorbed and achieves 90%
inhibitory concentrations within 1 hour, and it is well tolerated
at projected therapeutic concentrations.
- Initiate BHV-2100 Phase 2 study in acute migraine in 2H
2024
- Conduct BHV-2100 POC study for neuropathic pain in 2H 2024
TYK2/JAK1 Inhibitor: BHV-8000 is a
first-in-class, oral, brain-penetrant, selective TYK2/JAK1
inhibitor with broad potential for neuroinflammatory
disorders. In the ongoing Phase 1 study in healthy volunteers,
Biohaven has successfully dosed three single ascending dose cohorts
and one multiple ascending dose cohort. Projected therapeutic
concentrations were achieved, and BHV-8000 was well tolerated.
- Initiate BHV-8000 Phase 2 study in Multiple Sclerosis in 2Q
2024
- Initiate BHV-8000 Phase 2a study in prevention of amyloid
therapy induced ARIA in 2H 2024
- Initiate BHV-8000 Phase 2/3 study in early Parkinson's disease
in 2H 2024
- Initiate BHV-8000 Phase 2/3 study in early Alzheimer's disease
in 2H 2024
Extracellular protein degradation platform: Four agents
quickly advancing
From Biohaven's targeted
extracellular protein degradation platform, the Company is planning
four INDs across a number of indications. The lead program,
BHV-1300, offers a mechanism of action that is differentiated from
FcRn targeting agents with the potential for a faster onset of
action, deeper reductions in IgG, no mechanistic effects on albumin
or cholesterol, self-administered subcutaneous dosing, and ability
to dose in conjunction with Fc-containing biologic therapeutic
agents. In a preclinical model, BHV-1300 demonstrated that it can
be co-administered with Fc-containing biologics, such as Humira®,
supporting Biohaven's strategy of advancing BHV-1300 in
combination with standard of care treatments for rheumatoid
arthritis (unlike the FcRn class which have limitations on
co-administration with Fc containing biologics).
- BHV-1300 clinical data regarding first-in-human of IgG lowering
expected in 1Q 2024
- A total of 4 INDs are expected for the degrader program in
2024
Next Generation ADC Platform: Biohaven's ADC technology
is focused on novel conjugation chemistry with the potential to be
superior to the current industry standard maleimide and lipophilic
click chemistry. The goal of its technology is to provide more
stable and consistent drug antibody ratio (DAR) for use in
oncology.
- Initiate Phase 1 trial of BHV-1510 (Trop2) in 2Q 2024
- File IND for BHV-1500 (next gen brentuximab ADC) in 2H
2024
Dr. Coric concluded, "2024 will be a groundbreaking year for
Biohaven. We have the foundation and momentum to drive success
across numerous clinical and preclinical programs, where our
ability to execute with speed and efficiency is well proven. In
only one short year, we have built a new company and portfolio that
is well positioned to drive patient and shareholder value led by an
experienced leadership team with a strong financial foundation to
succeed."

About Biohaven
Biohaven is a biopharmaceutical company focused on the
discovery, development and commercialization of life-changing
treatments in key therapeutic areas including neuroscience,
immunology and oncology. The company is advancing one of the
industry's most innovative therapeutic portfolios, leveraging its
proven drug development experience and multiple, proprietary drug
development platforms. Biohaven's extensive clinical and
preclinical programs include Kv7 ion channel modulation for
epilepsy and mood disorders; extracellular protein degradation for
immunological diseases; TRPM3 activation for migraine and
neuropathic pain; TYK2/JAK1 inhibition for neuroinflammatory
disorders; glutamate modulation for obsessive-compulsive disorder;
myostatin inhibition for neuromuscular and metabolic diseases,
including obesity; and antibody recruiting, bispecific molecules
and antibody drug conjugates for cancer. For more information,
visit www.biohaven.com.
Forward-looking Statements
This presentation includes
forward-looking statements within the meaning of the Private
Securities Litigation Reform Act of 1995, including statements
about Biohaven Ltd. (the "Company") and our planned and ongoing
clinical trials, the timing of and the availability of data from
those trials, the timing and our decisions to proceed with our
planned regulatory filings, the timing of and our ability to obtain
regulatory approvals for our product candidates, the clinical
potential utility of our product candidates, alone and as compared
to other existing potential treatment options, and the potential
advancement of our early phase programs. The use of certain words,
including "continue", "plan", "will", "believe", "may", "expect",
"anticipate" and similar expressions, is intended to identify
forward-looking statements. Investors are cautioned that any
forward-looking statements, including statements regarding the
future development, timing and potential marketing approval and
commercialization of our development candidates, are not guarantees
of future performance or results and involve substantial risks and
uncertainties. Actual results, developments and events may differ
materially from those in the forward-looking statements as a result
of various factors including: the expected timing, commencement and
outcomes of Biohaven's planned and ongoing clinical trials; the
timing of planned interactions and filings with the FDA; the timing
and outcome of expected regulatory filings; complying with
applicable U.S. regulatory requirements; the potential
commercialization of Biohaven's product candidates; the potential
for Biohaven's product candidates to be first-in-class and
best-in-class therapies; the anticipated consummation of the Trop2
transaction, and the effectiveness and safety
of Biohaven's product candidates. You should, therefore,
not rely on these forward-looking statements as representing our
views as of any date subsequent to the date of this presentation.
Additional important factors to be considered in connection with
forward-looking statements are described in the Company's filings
with the Securities and Exchange Commission, including within the
sections titled "Risk Factors" and "Management's Discussion and
Analysis of Financial Condition and Results of Operations". The
forward-looking statements are made as of the date of this
presentation, and Biohaven does not undertake any
obligation to update any forward-looking statements, whether as a
result of new information, future events or otherwise, except as
required by law. This presentation also contains market data
and other information based on industry publications, reports by
market research firms or published independent sources. Some market
data and information is also based on the Company's good faith
estimates, which are derived from management's knowledge of its
industry and such independent sources referred to above.
Humira® is a registered trademark of AbbVie Inc.
Investor Contact:
Jennifer
Porcelli
Vice President, Investor Relations
jennifer.porcelli@biohavenpharma.com
+1 (201) 248-0741
Media Contact:
Mike
Beyer
Sam Brown Inc.
mikebeyer@sambrown.com
+1 (312) 961-2502
 View original content to download
multimedia:https://www.prnewswire.com/news-releases/biohaven-highlights-progress-across-innovative-portfolio-and-outlines-2024-anticipated-milestones-at-the-42nd-annual-jp-morgan-healthcare-conference-established-extensive-portfolio-across-20-therapeutic-indications-in-neuroscie-302027933.html
View original content to download
multimedia:https://www.prnewswire.com/news-releases/biohaven-highlights-progress-across-innovative-portfolio-and-outlines-2024-anticipated-milestones-at-the-42nd-annual-jp-morgan-healthcare-conference-established-extensive-portfolio-across-20-therapeutic-indications-in-neuroscie-302027933.html
SOURCE Biohaven Ltd.