UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of the Securities
Exchange Act of 1934 (Amendment No.)
 |
Filed by the Registrant |
 |
Filed by a Party other than the Registrant |
| Check the appropriate
box: |
 |
Preliminary
Proxy Statement |
 |
Confidential,
for use of the Commission only (as permitted by Rule 14a-6(e)(2)) |
 |
Definitive
Proxy Statement |
 |
Definitive
Additional Materials |
 |
Soliciting
Material Pursuant to §240.14a-12 |
ULTRAGENYX PHARMACEUTICAL INC.

(Name of Registrant as Specified In
Its Charter)
(Name of Person(s) Filing Proxy Statement,
if other than the Registrant)
| Payment of Filing Fee
(Check all boxes that apply): |
 |
No
fee required |
 |
Fee
paid previously with preliminary materials |
 |
Fee
computed on table in exhibit required by Item 25(b) per Exchange Act Rules 14a-6(i)(1) and 0-11 |


60 Leveroni Court
Novato, California 94949
Notice of Annual Meeting of Stockholders
|
To Be Held on
May 15, 2025 at
9:00 a.m. Pacific Time
|
|
Dear Stockholder:
You are cordially invited to attend the Annual
Meeting of Stockholders of Ultragenyx Pharmaceutical Inc., a Delaware corporation (we, us, Ultragenyx or the Company), which will
be held on May 15, 2025, at 9:00 a.m. Pacific Time virtually via the Internet at www.virtualshareholdermeeting.com/RARE2025
(Annual Meeting). Instructions on how to participate in the Annual Meeting and demonstrate proof of stock ownership are included
in this proxy statement (Proxy Statement). The webcast of the Annual Meeting will be archived for one year after the date of the
Annual Meeting at www.virtualshareholdermeeting.com/RARE2025. Only stockholders who held stock at the close of business
on the record date, March 24, 2025, may vote at the Annual Meeting or any adjournment or postponement thereof.
In the event of a technical malfunction or
other situation that the meeting chair determines may affect the ability of the Annual Meeting to satisfy the requirements for
a meeting of stockholders to be held by means of remote communication under the Delaware General Corporation Law, or that otherwise
makes it advisable to adjourn the Annual Meeting, the chair or secretary of the Annual Meeting will convene the meeting at 12:00
p.m. Pacific Time on the date specified above and at the Company’s address specified above solely for the purpose of adjourning
the meeting to reconvene at a date, time and physical or virtual location announced by the meeting chair. Under either of the foregoing
circumstances, we will post information regarding the announcement on the Investors page of the Company’s website at https://ir.ultragenyx.com.
At the Annual Meeting, you will be asked
to consider and vote upon: (1) the election of the two Class III director nominees named in the Proxy Statement; (2) approval of
our Second Amended and Restated 2023 Incentive Plan (Second A&R 2023 Plan), (3) the ratification of the selection of Ernst
& Young LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2025; (4) an advisory
(non-binding) resolution to approve the compensation of our named executive officers; and (5) any other business that may properly
come before the Annual Meeting or any adjournment or postponement thereof. No other items of business are expected to be considered,
and no other director nominees will be entertained, at the Annual Meeting.
The accompanying Proxy Statement more fully
describes the details of the business to be conducted at the Annual Meeting. Proposal No. 1 relates solely to the election of the
two directors nominated by the Board of Directors. After careful consideration, our Board of Directors has unanimously approved
the proposals and recommends that you vote FOR each of the director nominees, and FOR each of the other proposals
described in the Proxy Statement.
|
We are pleased to make use of the U.S. Securities
and Exchange Commission (SEC) rules that allow companies to furnish proxy materials to their stockholders via the Internet. We
believe the ability to deliver proxy materials electronically allows us to provide our stockholders with the information they need,
while lowering the costs of delivery and reducing the environmental impact from the distribution of our Annual Meeting materials.
We look forward to speaking with you at the Annual Meeting.
Sincerely,
Emil D. Kakkis, M.D., Ph.D.
President and Chief Executive Officer
March 28, 2025
WHETHER OR NOT YOU EXPECT TO ATTEND THE
ANNUAL MEETING, PLEASE VOTE VIA THE INTERNET AS INSTRUCTED IN THE NOTICE OF INTERNET AVAILABILITY OR, IF YOU REQUESTED AND RECEIVED
A PRINTED COPY OF THE PROXY MATERIALS, COMPLETE, DATE, SIGN, AND RETURN THE ENCLOSED PROXY CARD USING THE ENCLOSED RETURN ENVELOPE
OR VOTING INSTRUCTION FORM PROVIDED WITH THE PRINTED PROXY MATERIALS, AS PROMPTLY AS POSSIBLE SO THAT YOUR SHARES MAY BE REPRESENTED
AT THE ANNUAL MEETING. YOU MAY ALSO VOTE AT THE VIRTUAL ANNUAL MEETING.
IMPORTANT NOTICE REGARDING THE AVAILABILITY
OF PROXY MATERIALS FOR THE ANNUAL MEETING OF STOCKHOLDERS TO BE HELD ON MAY 15, 2025:
The Proxy Statement and Annual Report on
Form 10-K for the fiscal year ended December 31, 2024 are available at www.proxyvote.com.
ULTRAGENYX PHARMACEUTICAL INC. •
2025 Proxy Statement 3
Proxy Statement Overview
This overview highlights certain information
contained elsewhere in this Proxy Statement and does not contain all of the information that you should consider. You should read
the entire Proxy Statement carefully before voting. For more complete information regarding our business and 2024 performance,
please review our 2024 Annual Report on Form 10-K as filed with the U.S. Securities and Exchange Commission (SEC) in February 2025.
Meeting and Voting Information
| |
|
|
|
|
 |
|
 |
|
 |
| Meeting Date and Meeting Time |
|
Record Date |
|
Location |
May 15, 2025
9:00 a.m. Pacific Time |
|
March 24, 2025 |
|
Live via audio webcast at
www.virtualshareholdermeeting.com/RARE2025 |
| |
|
|
|
|
We intend to mail the Notice Regarding the
Availability of Proxy Materials containing instructions on how to access this Proxy Statement and our 2024 Annual Report on Form
10-K beginning on or about March 28, 2025 to all stockholders entitled to vote at the Annual Meeting.
| Proposals |
|
Board Vote
Recommendation |
For More
Information
See Page |
| Proposal 1 |
Election of Class III Directors |
FOR each nominee |
8 |
| Proposal 2 |
Approval of the Second A&R 2023 Plan |
FOR |
13 |
| Proposal 3 |
Ratification of the selection of Ernst & Young LLP as our independent auditor |
FOR |
22 |
| Proposal 4 |
Say on Pay |
FOR |
25 |
Business Highlights
2024 was a pivotal year for the Company as
we expanded access and grew revenue from our four global commercial products while also advancing our six late-stage programs in
serious genetic conditions. See page 37 for a summary of our achievements in 2024.
|
Raised
and Beat
Full
Year Financial
Guidance
|
|
78%
growth in
Crysvita® sales
in Latin America
and Turkey
|
|
Impact Report Highlights
At Ultragenyx, we are committed to bringing
novel products to patients for the treatment of rare and ultrarare diseases, with a focus on serious, debilitating genetic diseases.
Our purpose is to lead the future of rare disease medicine as we seek to treat individuals afflicted by diseases with limited or
no treatment options. We recognize that their lives and well-being are dependent upon our efforts to develop new therapies. For
this reason, we are passionate about developing these therapies with the utmost urgency and care. For more information about our
efforts and initiatives related to patient access, innovation and responsible business practices, please see our Impact Report
for fiscal 2024 (2024 Impact Report), which will be available on our website at www.ultragenyx.com under “Ultra-Committed”.
Website references throughout this document are provided for convenience only, and the content on the referenced websites is not
incorporated by reference into this document.
See page 31 for more information about our
Impact Report.
|
| |
|
|
|
ULTRAGENYX PHARMACEUTICAL INC. •
2025 Proxy Statement 4
Board of Directors
The following table provides summary information
about each nominee for director at the Annual Meeting and our continuing directors, as of the date of this Proxy Statement.
| Nominee/Director Name |
|
Age |
|
Principal Occupation |
|
Director
Since |
|
Year Current
Term Expires |
|
Current
Director Class |
|
Independent |
| Director Nominees |
|
|
|
|
|
|
|
|
|
|
|
|
| Matthew K. Fust |
|
60 |
|
Board member and advisor to various life science companies |
|
2014 |
|
2025 |
|
III |
|
 |
| Amrit Ray, M.D. |
|
52 |
|
Board member and advisor to various life science companies |
|
2022 |
|
2025 |
|
III |
|
 |
| Continuing Directors |
|
|
|
|
|
|
|
|
|
|
|
|
| Emil D. Kakkis, M.D., Ph.D. |
|
64 |
|
Founder, President and CEO, Ultragenyx |
|
2010 |
|
2026 |
|
I |
|
 |
| Shehnaaz Suliman, M.D. |
|
53 |
|
Chief Executive Officer of ReCode Therapeutics |
|
2019 |
|
2026 |
|
I |
|
 |
| Daniel G. Welch |
|
67 |
|
Board member and advisor to various life science companies |
|
2015 |
|
2026 |
|
I |
|
 |
| Deborah Dunsire, M.D. |
|
62 |
|
Board member and advisor to various life science companies |
|
2017 |
|
2027 |
|
II |
|
 |
| Michael Narachi |
|
65 |
|
Board member and advisor to various life science companies |
|
2015 |
|
2027 |
|
II |
|
 |
| Corsee D. Sanders, Ph.D. |
|
68 |
|
Board member and advisor to various life science companies |
|
2021 |
|
2027 |
|
II |
|
 |
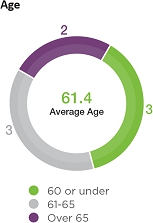
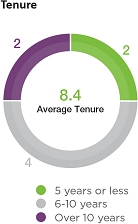
| Board
Composition Highlights |
 |
|
|
 |
ULTRAGENYX PHARMACEUTICAL INC. •
2025 Proxy Statement 5
Building the Right Board
Our Nominating and Corporate Governance Committee
is responsible for reviewing with the entire Board from time to time the appropriate skills and characteristics required of directors
in the context of the current Board composition and the anticipated needs of the Board and the Company. Our Nominating and Corporate
Governance Committee actively considers whether potential candidates would assist in achieving a mix of Board members that represents
a broad range of knowledge, skills, and experience, including whether the nominee has specific strengths that would augment existing
skills and experiences of the Board and in a manner that is aligned with the Company’s strategic direction. The following
matrix highlights each director’s primary skills or knowledge in these areas as identified by the Nominating and Corporate
Governance Committee. The matrix does not encompass all of the knowledge, skills, experiences or attributes of our directors, and
the fact that a particular knowledge, skill, experience or attribute is not listed does not mean that a director does not possess
it. In addition, the absence of a particular knowledge, skill, experience or attribute with respect to any of our directors does
not mean the director in question is unable to contribute to the decision-making process in that area. The type and degree of knowledge,
skill and experience listed below may vary among the members of the Board.
| |
|
Welch |
|
Kakkis |
|
Dunsire |
|
Fust |
|
Narachi |
|
Ray |
|
Sanders |
|
Suliman |
| Skills and Experience |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Core Board Capabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Biopharma C-level leadership |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
| Scientific and research leadership |
|
|
|
 |
|
|
|
|
|
|
|
 |
|
 |
|
 |
| Clinical development leadership |
|
|
|
 |
|
 |
|
|
|
 |
|
 |
|
 |
|
 |
| Regulatory leadership |
|
|
|
 |
|
|
|
|
|
 |
|
 |
|
 |
|
|
| Rare disease commercial experience |
|
 |
|
 |
|
 |
|
|
|
|
|
|
|
|
|
|
| Biopharma commercial leadership |
|
 |
|
|
|
 |
|
|
|
 |
|
 |
|
|
|
|
| Global access, pricing and reimbursement |
|
 |
|
|
|
 |
|
|
|
 |
|
 |
|
|
|
|
| Global business operations |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
| Finance and capital markets |
|
 |
|
|
|
 |
|
 |
|
 |
|
|
|
|
|
 |
| Corporate strategy and/or business development leadership |
|
 |
|
 |
|
 |
|
|
|
 |
|
 |
|
|
|
 |
| Corporate governance and board experience |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Relevant / Advisory Capabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Compliance (GXP, commercial) |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
|
| Corporate legal and IP |
|
 |
|
|
|
 |
|
|
|
 |
|
|
|
|
|
 |
| Government affairs and policy |
|
 |
|
 |
|
 |
|
|
|
 |
|
 |
|
|
|
|
| Human resources and organizational development |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
| Manufacturing/supply chain |
|
 |
|
 |
|
 |
|
|
|
 |
|
|
|
|
|
|
| Background |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Gender |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Male |
|
 |
|
 |
|
|
|
 |
|
 |
|
 |
|
|
|
|
| Female |
|
|
|
|
|
 |
|
|
|
|
|
|
|
 |
|
 |
| Race/Ethnicity |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Asian |
|
|
|
|
|
|
|
|
|
|
|
 |
|
 |
|
 |
| White |
|
 |
|
 |
|
 |
|
 |
|
 |
|
|
|
|
|
|
| LGBTQ+ |
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
|
|
ULTRAGENYX PHARMACEUTICAL INC. •
2025 Proxy Statement 6
Corporate Governance Overview
We are committed to maintaining good corporate
governance practices and we periodically review our practices. We believe that good corporate governance, including the practices
listed below, promotes the long term interests of our stockholders.
| • |
All of our directors are independent, other than our President and Chief Executive Officer |
| • |
Director Resignation Policy that applies when a director fails to receive majority support in an uncontested election |
| • |
Completely independent Audit Committee, Nominating and Corporate Governance Committee and Compensation Committee |
| • |
Effective and active independent Chairman |
| • |
Annual Board and committee self-evaluations |
| • |
Active stockholder engagement program |
| • |
All our current directors attended 100% of the Board and committee meetings of which the director was a member in 2024 |
| • |
Minimum stock ownership requirements for named executive officers and directors |
| • |
Director Overboarding Policy limiting the total number of public company boards that a director may serve to five total public company boards and public company CEO directors to three total public company boards |
| • |
Board and committees are authorized to engage outside advisors independently of management |
| • |
Clawback Policy that complies with Rule 10D-1 under the Securities Exchange Act of 1934, as amended (Exchange Act) and also includes a discretionary recoupment provision that permits recovery of all incentive compensation (including time-based and performance-based equity awards) in the event of fraud or intentional misconduct (see section entitled “Clawback Policy” of this Proxy Statement for additional details) |
| • |
Prohibition against hedging and pledging transactions by our directors and employees, including our executive officers |
| • |
Mixture of short, medium and long-tenured directors |
| • |
Corporate Governance Guidelines and robust Global Code of Conduct |
ULTRAGENYX PHARMACEUTICAL INC. •
2025 Proxy Statement 7
PROPOSAL 1
Election of Class III Directors
Our Amended and Restated Certificate of Incorporation
provides that the Board is to be divided into three classes as nearly equal in number as reasonably possible, with directors in
each class generally serving three-year terms. The total Board size is currently fixed at eight directors. The Class III directors
(whose terms expire at the Annual Meeting) are Matthew K. Fust and Amrit Ray, M.D. Mr. Fust and Dr. Ray were most recently elected
by stockholders at the 2022 Annual Meeting. The Class I directors (whose terms expire at the 2026 Annual Meeting) are Emil D. Kakkis,
M.D., Ph.D., Shehnaaz Suliman, M.D., and Daniel G. Welch. The Class II directors (whose terms expire at the 2027 Annual Meeting)
are Deborah Dunsire, M.D., Michael Narachi and Corsee D. Sanders, Ph.D. The Class III directors elected at the Annual Meeting will
hold office until the 2028 Annual Meeting or until their successors are elected and qualified, unless they resign or their seats
become vacant due to death, removal, or other cause in accordance with our Amended and Restated Bylaws (bylaws).
As described below, the Board, upon the recommendation
of the Nominating and Corporate Governance Committee, has nominated the director nominees listed above for election as directors
at the Annual Meeting. They have indicated their willingness and ability to serve if elected. Should any of them become unable
or, for good cause, unwilling to serve, the persons named on the enclosed proxy card as proxy holders may vote all proxies given
in response to this solicitation for the election of a substitute nominee(s) chosen by the Board or the Board may reduce the size
of the Board.
Nomination of Directors
The Nominating and Corporate Governance Committee
reviews and recommends to the Board potential nominees for election to the Board. In reviewing potential nominees, the Nominating
and Corporate Governance Committee considers the qualifications of each potential nominee in light of the Board’s existing
and desired mix of experience and expertise. Specifically, the Nominating and Corporate Governance Committee considers each potential
nominee’s personal and professional ethics, integrity, values, experience, interest in the Company, and commitment to the
representation of the long-term interests of the stockholders. As described more fully above under “Proxy Statement Overview
– Building the Right Board”, the Nominating and Corporate Governance Committee also considers each potential nominee’s
contribution to the Board’s composition as whole with in achieving a mix of knowledge, skills, experience, and background.
The Board has adopted a policy in the Company’s Corporate Governance Guidelines, which provides that as part of the search
process for each new director, the Nominating and Corporate Governance Committee will add diverse candidates into the pool of candidates
(and instructs any search firm the Nominating and Corporate Governance Committee engages to do so). Directors ultimately are selected
based on the skills, experiences and qualifications that best support the Company in the context of the Board as a whole.
Additionally, the Nominating and Corporate
Governance Committee considers whether a nominee will be able to dedicate sufficient time to, and focus on, his or her duties as
a member of the Board. The Board membership criteria are set forth in our Corporate Governance Guidelines, a copy of which is available
on our website at www.ultragenyx.com in the “Corporate Governance” subsection of the “Investors”
tab. The Nominating and Corporate Governance Committee assesses its effectiveness in balancing these considerations in connection
with its annual evaluation of the composition of the Board.
After reviewing the qualifications of potential
Board candidates, the Nominating and Corporate Governance Committee presents its recommendations to the Board, which selects the
final director nominees. We did not pay any fees to any third party to identify or assist in identifying or evaluating nominees
for the Annual Meeting.
The Nominating and Corporate Governance Committee
considers stockholder-recommended director nominees using the same criteria set forth above and in the same manner as director
candidates recommended by other sources. Stockholders who wish to recommend a potential nominee to the Nominating and Corporate
Governance Committee for consideration for election at a future annual meeting of stockholders must submit such recommendation
to the Nominating and Corporate Governance Committee as described in the section entitled “Stockholder Communications”
and provide the Nominating and Corporate Governance Committee with the same information that would be required to nominate a director
candidate in accordance with the process and within the deadline for nominating director candidates set forth below under the question
“When are other proposals and director nominations for next year’s annual meeting due?” in the section
entitled “Additional Information”.
ULTRAGENYX PHARMACEUTICAL INC. •
2025 Proxy Statement 8
Nominees and Incumbent Directors
Information regarding our director nominees
and our current directors, including their respective age as of the date of this Proxy Statement and their principal occupation,
is set forth below.
Class III Directors Nominated for Election
| Matthew K. Fust |
| Board member and advisor to various life science companies |
Age: 60
Director since: 2014
INDEPENDENT
Committees: 2
Other Public Directorships: 3
|
|
Mr. Fust is a board member and advisor to life science
companies. Mr. Fust currently serves on the board of directors of Atara Biotherapeutics, Inc., Crinetics
Pharmaceuticals, Inc. and Neumora Therapeutics Inc., all of which are publicly traded biopharmaceutical
companies. Mr. Fust also previously served on the board of directors of Dermira, Inc., a biopharmaceutical
company, from August 2014 to February 2020 and the board of MacroGenics, Inc., a biotechnology company,
from March 2014 to May 2020. He retired as Executive Vice President of Onyx Pharmaceuticals, Inc.,
a biopharmaceutical company, where he served from January 2009 to January 2014. From May 2003 to December
2008, Mr. Fust served as Chief Financial Officer at Jazz Pharmaceuticals, Inc., a specialty pharmaceutical
company. From 2002 to 2003, Mr. Fust served as Chief Financial Officer at Perlegen Sciences, Inc.,
a biopharmaceutical company. Previously, he was Senior Vice President and Chief Financial Officer at
ALZA Corporation, a pharmaceutical company, where he was an executive from 1996 to 2002. From 1991
to 1996, Mr. Fust was a manager in the healthcare strategy practice at Andersen Consulting, a consulting
company. Mr. Fust holds a B.A. in Accounting from the University of Minnesota and an M.B.A. from the
Stanford Graduate School of Business.
Skills and Qualifications specific to Ultragenyx:
We believe that Mr. Fust is qualified to serve on our Board due to his extensive
experience in the life sciences industry, his financial experience and ability to be our “audit committee financial
expert,” and his service as a director of other public biopharmaceutical companies.
|
| Amrit Ray, M.D., M.B.A. |
| Board member and advisor to various life science companies |
Age: 52
Director since: 2022
INDEPENDENT
Committees: 1
Other Public Directorships: 1
|
|
Dr. Ray is a board member and advisor
to life science companies. Dr. Ray currently serves on the board of directors of Fortrea Holdings Inc., a publicly traded,
global clinical research organization, and on the board of directors of several privately owned life science companies.
Previously, Dr. Ray served as Chief Patient Officer at Biohaven Ltd. a public biopharmaceutical company, from March 2022
to December 2022 when acquired by Pfizer. Prior to his role at Biohaven, he served as Senior Adviser to Bain Capital Life
Sciences, an investment company, from February 2021 to March 2022. Prior to Bain Capital, Dr. Ray served as Global President,
Head of R&D and Medical, for Pfizer Essential Health and subsequently the Pfizer Upjohn division at Pfizer, Inc.,
a public pharmaceutical company, from 2017 to January 2021. Prior to Pfizer, he held positions of increasing responsibility
at Johnson & Johnson, a public pharmaceutical company, including serving as Senior Vice President, External Affairs
(Science and Medicine) in 2017, Senior Vice President, Chief Medical Officer of Janssen from 2012 to 2017 and Senior Vice
President, Chief Safety Officer from 2009 to 2012. Dr. Ray currently serves as a Trustee at the Board of the Hastings
Center for Bioethics, and as a Visiting Professor of Practice, Faculty of Medical Sciences at Newcastle University in
the United Kingdom. Dr. Ray holds a B.S., with Honours, in Immunology and a M.D. (M.B., Ch.B.) from the University of
Edinburgh. He also holds an M.B.A. from the Tuck School of Business at Dartmouth College.
Skills
and Qualifications specific to Ultragenyx:
We believe that Dr. Ray is qualified
to serve on our Board due to his extensive experience in the life sciences industry, and particularly his research and
development expertise.
|
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 9
Class I Directors Continuing in Office until 2026
| Emil D. Kakkis, M.D., Ph.D.
|
| Founder, President and CEO |
Age: 64
Director since: 2010
NOT INDEPENDENT
Committees: 1
Other Public Directorships: 0
|
|
Dr. Kakkis is our founder and has
served as our President and Chief Executive Officer and as a member of our Board since our inception in April 2010. Dr.
Kakkis served as our interim principal financial officer following the departure of the Company’s Chief Financial
Officer in November 2022 until the appointment of Mr. Horn as the Company’s Chief Financial Officer in October 2023.
Prior to Ultragenyx, from September 1998 to February 2009, Dr. Kakkis served in various executive capacities, and ultimately
as Chief Medical Officer, at BioMarin Pharmaceutical Inc., a biopharmaceutical company. Dr. Kakkis then served as a development
consultant to BioMarin from 2009 to 2010. Dr. Kakkis is also Founder of EveryLife Foundation for Rare Diseases, a non-profit
organization he started in 2009 to accelerate biotechnology innovation for rare diseases. Dr. Kakkis received the Termeer
Visionary Leadership Award in 2019 and the Leadership Award from the California Life Sciences Association in 2021. Dr.
Kakkis is board certified in Medical Genetics and was board certified in Pediatrics. He holds a B.A. in Biology from Pomona
College and combined M.D. and Ph.D. degrees from the UCLA School of Medicine’s Medical Scientist Training Program
where he received the Bogen prize for his research.
Skills
and Qualifications specific to Ultragenyx:
We believe that Dr. Kakkis possesses
specific expert knowledge of genetics and rare diseases and operational experience in the life sciences sector that qualify
him to serve on our Board.
|
| Shehnaaz Suliman, M.D., M.Phil.,
M.B.A |
| Chief Executive Officer of ReCode Therapeutics |
Age: 53
Director since: 2019
INDEPENDENT
Committees: 2
Other Public Directorships: 1
|
|
Dr. Suliman has served as Chief
Executive Officer of ReCode Therapeutics, a privately-held, integrated genetic medicines company, since January 2022.
Prior to joining ReCode Therapeutics, Dr. Suliman served as President and Chief Operating Officer of Alector, Inc., a
clinical stage biotechnology company, from December 2019 to December 2021 and previously served as Senior Vice President,
Corporate Development and Strategy of Theravance Biopharma, Inc., a biopharmaceutical company, from July 2017 to March
2019. Prior to her position at Theravance, Dr. Suliman worked for Genentech, Inc., a biopharmaceutical company, as Group
Leader and Project Team Leader in the R&D Portfolio Management and Operations Group from September 2010 to May 2015
and then as Vice President and Global Therapeutic Head, Roche Partnering from June 2015 to July 2017. Prior to Genentech,
Dr. Suliman held various management roles of increasing responsibility at Gilead Sciences, Inc., a biopharmaceutical company,
from January 2005 and September 2010. Prior to Gilead, Dr. Suliman was an investment banker with Lehman Brothers and Petkevich &
Partners, advising public and private companies on buy- and sell-side transactions. She is a member of the board of directors
of 10X Genomics, Inc., a publicly traded life science technology company. Dr. Suliman received her M.D. at the University
of Cape Town Medical School, South Africa, and holds an M.B.A, with distinction, and M.Phil. in Development Studies from
Oxford University, where she was a Rhodes Scholar.
Skills
and Qualifications specific to Ultragenyx:
We believe that Dr. Suliman is
qualified to serve on our Board due to her extensive operational experience with global biopharmaceutical companies, and
particularly her expertise in business development, corporate strategy and clinical drug development.
|
| Daniel G. Welch |
| Board member and advisor to various life science companies |
Age: 67
Director since: 2015
INDEPENDENT CHAIRMAN
Committees: 2
Other Public Directorships: 2
|
|
Mr. Welch is a board member and
advisor to life science companies. Mr. Welch currently serves as chairman on the boards of Structure Therapeutics, Inc.
and Prothena Corporation plc, each a publicly traded biotechnology company. Between January 2015 and January 2018, he
was an Executive Partner at Sofinnova Ventures, a venture capital firm. Prior to Sofinnova, Mr. Welch served as Chairman,
Chief Executive Officer, and President of InterMune, Inc., a biotechnology company, from May 2008 to October 2014 and
served as President and Chief Executive Officer of InterMune and a member of its board of directors from September 2003
to May 2008. From August 2002 to January 2003, Mr. Welch served as Chairman and Chief Executive Officer of Triangle Pharmaceuticals,
Inc., a pharmaceutical company. From October 2000 to June 2002, Mr. Welch served as President of the pharmaceutical division
of Elan Corporation, plc. Mr. Welch previously served as chairman of the board of directors of Nuvation Bio from July
2020 to September 2024 and as a director on the boards of directors of Intercept Pharmaceuticals, Inc from November 2015
to June 2021 and Seagan Inc. from July 2007 to December 2023. Mr. Welch holds a B.S. from the University of Miami and
an M.B.A. from the University of North Carolina.
Skills
and Qualifications specific to Ultragenyx:
We believe that Mr. Welch is a strong
operating executive with operational and strategic expertise in the global pharmaceutical market, whose experience contributes
valuable insight to the Board.
|
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 10
Class II Directors Continuing in Office until 2027
| Deborah Dunsire, M.D.
|
| Board member and advisor to various life science companies |
Age: 62
Director since: 2017
INDEPENDENT
Committees: 2
Other Public Directorships: 1
|
|
Dr. Dunsire is a board member and
advisor to life science companies. Dr. Dunsire served as President and Chief Executive Officer of H. Lundbeck A/S, a pharmaceutical
company, from September 2018 until August 2023. She previously served as President and Chief Executive Officer and as
a director of Xtuit Pharmaceuticals, Inc., a private biopharmaceutical company, from January 2017 to March 2018. Prior
to her position at Xtuit, she served as President and Chief Executive Officer and a director of FORUM Pharmaceuticals
Inc., a private pharmaceutical company, from July 2013 to May 2016. Prior to FORUM, Dr. Dunsire worked for Takeda Pharmaceutical
Company Limited, a publicly traded pharmaceutical company, as a corporate officer from June 2010 to June 2011 and a director
from June 2011 to June 2013. She served as President and Chief Executive Officer and as a director of Millennium Pharmaceuticals,
Inc., a publicly traded biopharmaceutical company, between 2005 and 2008, when it was acquired by Takeda, and then as
President and Chief Executive Officer of Millennium: The Takeda Oncology Company after the acquisition between 2008 and
2013. Prior to Millennium, Dr. Dunsire held various roles of increasing responsibility at Novartis Pharma AG between 1988
and 2005. Dr. Dunsire currently serves on the board of McKesson Corporation, a publicly traded healthcare services company.
She previously served on the board of directors of Syros Pharmaceuticals, a publicly traded biotech company, from September
2021 to November 2024 and of Alexion Pharmaceuticals Inc., a publicly traded biotech company, from January 2018 until
July 2021 when it was acquired by AstraZeneca. She obtained an MBBCh from the University of the Witwatersrand.
Skills
and Qualifications specific to Ultragenyx:
We believe that Dr. Dunsire is
qualified to serve on our Board due to her extensive experience in the biotechnology and pharmaceutical sectors, including
service as the chief executive officer of various pharmaceutical companies, which gives her the skills to provide us with
operational and strategic insights.
|
| Michael Narachi |
| Board member and advisor to various life science companies |
Age: 65
Director since: 2015
INDEPENDENT
Committees: 2
Other Public Directorships: 0
|
|
Mr. Narachi previously served as
President and Chief Executive Officer of CODA Biotherapeutics, Inc., a private biotherapeutics company, from August 2018
through October 2022 and served as a director of CODA Biotherapeutics until the company’s sale in March 2023. Between
March 2009 and July 2018, Mr. Narachi served as President, Chief Executive Officer and director of Orexigen Therapeutics,
Inc., a biotechnology company. Orexigen filed for reorganization under Chapter 11 of the U.S. Bankruptcy Code in March
2018. Previously, Mr. Narachi served as Chairman, Chief Executive Officer, and President of Ren Pharmaceuticals, Inc.,
a private biotechnology company, from November 2006 to March 2009. In 2004, Mr. Narachi retired as Vice President of Amgen
Inc., a leading therapeutics company, where he served as General Manager of Amgen’s Anemia Business from 1999 to
2003, until his retirement in 2004. Mr. Narachi joined Amgen in 1984 and held various senior positions throughout the
organization over a 20-year career including global development leader for Neupogen/Neulasta, Vice President of development
and representative director for Amgen Japan; head of corporate strategic planning; Chief Operations Officer of Amgen Colorado;
and vice president, licensing and business development. Mr. Narachi previously served on the board of directors of BIO,
the Biotechnology Innovation Organization, as a member of the board of directors of PhRMA, the Pharmaceutical Research
and Manufacturers of America, and as the chairman of the board of directors of Celladon Corporation, a publicly traded
gene therapy company, from October 2013 to March 2016, and as a director of AMAG Pharmaceuticals, Inc., a publicly traded
specialty pharmaceutical company, from November 2006 to April 2014. Mr. Narachi holds a B. S. in Biology and an M. A.
in Biology and Genetics from the University of California, Davis. He also holds an M.B.A. from the Anderson Graduate School
of Management at the University of California, Los Angeles.
Skills and Qualifications
specific to Ultragenyx:
We believe that Mr. Narachi is
qualified to serve on our Board due to his extensive experience in the life sciences industry, his service as the chief
executive officer of various biotechnology companies, and his membership on various boards of directors in the biotechnology
and pharmaceutical sectors, all of which give him the skills to provide us with operational and strategic insights.
|
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 11
| Corsee D. Sanders, Ph.D.
|
| Board member and adviser to various life science companies |
Age: 68
Director since: 2021
INDEPENDENT
Committees: 2
Other Public Directorships: 3
|
|
Dr. Sanders is a board member and
advisor to life science companies. Dr. Sanders currently serves as a member of the board of directors of Beigene Ltd., Molecular Templates Inc. and Legend Biotech Corporation, each a publicly traded biotechnology company. She currently
serves as a co-chair of the Board of Advisors, and Chair of the Science and Technology Advisory Committee for the Fred
Hutchinson Cancer Center. She most recently served as Executive Vice President at Juno Therapeutics, Inc., a biopharmaceutical
company, from 2017 to 2018 and strategic advisor to the Chief Medical Officer of Celgene Corporation, a biopharmaceutical
company, from 2018 to 2019 following Juno’s acquisition by Celgene. Following the acquisition of Celgene by Bristol
Myers Squibb, Dr. Sanders served as Transition Advisor to the Clinical Development team at BMS from 2019 to 2020. Prior
to her role at BMS, she held positions of increasing responsibility at Genentech/Roche, a biotechnology company, from
1994 to 2017, including serving as Senior Vice President, Global Head of Clinical Operations and Industry Collaboration,
from 2012 to 2017. Dr. Sanders holds a B.S. and M.S. in Statistics, magna cum laude, from the University of the
Philippines. She also holds an M.A. and Ph.D. in Statistics from the Wharton Doctoral Program at the University of Pennsylvania.
Skills and Qualifications
specific to Ultragenyx:
We believe that Dr. Sanders is
qualified to serve on our Board due to her established and extensive experience in global clinical development and her
role as an advisor and director to various companies in the biotechnology and pharmaceutical sectors, all of which give
her the skills to provide us with operational and strategic insights.
|
Vote Required
The two nominees who receive the greatest
number of affirmative votes will be elected as Class III directors. Shares as to which a stockholder withholds voting authority
and broker non-votes, if any, are not considered votes cast and therefore will have no effect on the vote outcome.
Director Resignation Policy
We have a Director Resignation Policy, which
is set forth in our Corporate Governance Guidelines, a copy of which is available on our website in the “Corporate Governance”
subsection of the “Investors” tab. The policy establishes that any director nominee who receives more “withhold”
votes than “for” votes in an uncontested election of directors is expected to tender his or her resignation promptly
following the certification of the election results. Broker non-votes, if any, are not counted as either a “withhold”
or “for” vote.
The Nominating and Corporate Governance Committee will promptly consider the tendered resignation and make a recommendation
to the Board. The Board will act on the recommendation of the Nominating and Corporate Governance Committee no later than
90 days following the certification of the election results. The Board will promptly and publicly disclose its decision and,
if applicable, the reasons for rejecting the tendered resignation.
 |
THE BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR”
EACH OF THE DIRECTOR NOMINEES IDENTIFIED ABOVE. |
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 12
PROPOSAL 2
Approval of the Ultragenyx Pharmaceutical Inc. Second Amended and Restated 2023 Incentive Plan
On March 24, 2025, the Board approved
a second amendment and restatement of the Ultragenyx Pharmaceutical Inc. 2023 Incentive Plan (the 2023 Plan and as amended
and restated, the Second A&R 2023 Plan), subject to stockholder approval at the Annual Meeting. The Second A&R 2023
Plan provides for an additional 3.0 million shares of our common stock to be available for issuance thereunder and extends
the term of the plan through May 15, 2035. We believe that our equity compensation policies enable us to attract and retain a
highly-skilled team of executives and aligns our executives’ interests with those of our stockholders by rewarding
short-term and long-term performance and tying compensation to increases in stockholder value. If the Second A&R 2023
Plan is approved by our stockholders, we intend to file a Form S-8 with the SEC following the Annual Meeting during the
second or third quarter that covers the additional shares reserved for issuance under the Second A&R 2023 Plan.
Share Reserve Under the Second A&R 2023 Plan
The maximum number of shares of our common
stock authorized for issuance under the Second A&R 2023 Plan is (i) 11.5 million shares plus (ii) the number of shares subject
to any award outstanding under the 2014 Incentive Plan (2014 Plan) or the 2011 Incentive Plan (2011 Plan and together with the
2014 Plan, the Prior Plans) as of June 7, 2023 that after June 7, 2023 are not issued because such award is forfeited, canceled,
terminates, expires or otherwise lapses without being exercised, or is settled in cash. Based on the recent range of our stock
price, our current compensation practices, our anticipated future awards, as well as our three-year burn rate, we believe the
Second A&R 2023 Plan share reserve will be sufficient for us to grant equity awards for approximately one year based on our
stock price and compensation philosophy and policies. If stockholders do not approve this Proposal No. 2, we anticipate that the
shares that remain available for grant would be insufficient for us to continue to provide equity incentives at a competitive
market level, limiting our ability to attract and retain talented employees and other service providers, and requiring a greater
cash allocation to support our incentive programs.
As part of the Compensation Committee’s recommendation to the Board to approve the Second A&R 2023 Plan and the
additional shares available for issuance under the Second A&R 2023 Plan, the Compensation Committee considered advice
from Aon, its independent compensation consultant. The Compensation Committee also carefully analyzed our historical burn
rate, anticipated future equity award needs to help drive our long-term strategic plan, and the dilutive impact of the Second
A&R 2023 Plan’s share reserve.
Reasons for Seeking Stockholder Approval
We believe the following are important
considerations for stockholders in determining whether to approve the Second A&R 2023 Plan:
| • |
Equity Awards are Essential to Talent Acquisition and Retention: Equity awards, similar to
those typically offered by our competitors are, and we believe will continue to be, an integral component of our overall
compensation program, enabling us to attract qualified and skilled employees and directors, retain our existing
employees, including our experienced management team, and provide incentives for our employees to exert maximum efforts for
our success, ultimately contributing to the creation of stockholder value. If the Second A&R 2023 Plan is not approved by
our stockholders at the Annual Meeting, we will not have adequate shares to continue to grant equity awards to all our
employees and directors in 2025. Historically, we have granted equity awards deeply in our organization, believing that a
culture of ownership is important to our ability to achieve our short- and long-term business objectives and that our success
is dependent on our employees feeling invested in our future. In fiscal 2024, we granted equity awards to 1,376
employees.
|
| • |
We are Committed to Executing on our Strategic Priorities: The use
of equity awards assists us and will continue to assist us in ensuring that our executives and employees are focused on long
term value creation for our stockholders and in enabling us to attract and retain the talent needed to execute on our
strategic priorities while managing our cash flow. We believe that the approval of the Second A&R 2023 Plan as described
in this proposal is instrumental to our ongoing success and our ability to provide increased value to our
stockholders.
|
| • |
We are Managing our Annual Burn Rate: We carefully and thoughtfully manage our equity
award use, balancing attraction, retention and incentivization of our employees against dilution and burn rate
considerations. We have relied more heavily on the grant of restricted stock units (RSUs) and for our executives, performance
stock units (PSUs) as opposed to options in an effort to manage our burn rate. Further, we regularly review and consider our
burn rate against those of our peer companies in the biotech industry to provide incentives at a competitive market level as
part of our human capital management strategy.
|
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 13
| • |
Our Equity Program is Performance-Based: The
Compensation Committee believes that PSUs align the objectives of management with those of our stockholders with respect to
long-term performance and success of the Company. For 2024, the equity grants to our executive officers, other than to our
Chief Executive Officer, reflected an equal value split among option, RSUs and PSUs. To further align the compensation of
our executive officers with stockholder interests and to increase the portion of compensation tied to Company performance
measures, the Compensation Committee made the following changes: |
| |
• |
In 2024, the Compensation Committee changed
the equity award value split for our Chief Executive Officer from an equal value split among options, RSUs and PSUs to 20%
options, 20% RSUs and 60% PSUs; |
| |
• |
In 2025, the Compensation Committee increased the portion of PSUs for our other executive
officers from 25% to 50% resulting in an awards split of 25% RSUs, 25% options and 50% PSUs. |
| • |
Forecasted Usage. If stockholders
approve the Second A&R 2023 Plan, the Compensation Committee currently anticipates that we will likely need
to again request additional shares at our 2026 Annual Meeting of Stockholders, based on our current share price, recent
burn rate history, and historical new hire and annual grant practices. In making this determination, the Compensation
Committee took into account the potential impact of any expected future changes in employee population on projected
share use, in particular given our ongoing efforts to execute on our strategic priorities. We cannot predict our
future equity grant practices, the future price of our shares or future hiring activity with any degree of certainty
at this time, and the share reserve under the Second A&R 2023 Plan could last for a shorter or longer time. |
Key Features and Governance Best Practices
The Second A&R 2023 Plan reflects a number of provisions
that protect stockholders and reflect corporate governance best practices, including the following:
| • |
No Repricing
and No Reload Options: Without stockholder approval, the Second A&R 2023 Plan prohibits the repricing of stock options
and SARs (defined below), the exchange or substitution of one award for another award that has the effect of reducing the
exercise or purchase price, and the cancellation or exchange of underwater awards for cash, another award or other property,
except in the event of a change in our capitalization or a covered transaction (described below). Reload options are not permitted
under the Second A&R 2023 Plan. |
| • |
No Dividends on Unvested Awards: The
Second A&R 2023 Plan provides that dividends and dividend equivalent rights may never be paid on any unvested award. |
| • |
No Liberal Share Recycling: The Second
A&R 2023 Plan prohibits liberal share recycling. |
| • |
Limit on Non-Employee Director Compensation: The
Second A&R 2023 Plan contains an annual limit on cash and equity-based compensation that may be paid or granted, whether
under the Second A&R 2023 Plan or otherwise, to our non-employee directors of $900,000 (or $1,500,000 in the calendar
year that the non-employee director first joins the Board or if the non-employee director is serving as chairman or lead director
of the Board). |
| • |
Clawback Provision:
Awards under the Second A&R 2023 Plan are subject to our clawback policy. In addition, the Plan Administrator (as
defined below) may cancel or limit awards under the Second A&R 2023 Plan if a participant is not in compliance with the
applicable award agreement or if a participant breaches any confidentiality agreement with the Company. |
| • |
No Automatic Single Trigger Acceleration: In
the event of a covered transaction, the Second A&R 2023 Plan does not provide for automatic single trigger acceleration.
|
| • |
Term and Exercise Price Limits on Options and
SARs: Options and SARs granted under the Second A&R 2023 Plan are subject to a maximum term of 10 years and may
not be granted at a discount to the fair market value of our common stock on the grant date. |
| • |
No Tax Gross-Ups. The Second A&R
2023 Plan does not provide for any tax gross-ups. |
| • |
No Evergreen: There is no automatic share
reload or “evergreen” provision in the Second A&R 2023 Plan. |
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 14
Shares Remaining Under the Prior Plans
As of March 7, 2025, a total
of 93,892,528 shares of our common stock were outstanding and the fair market value of our common stock was $38.62 based on the
closing sale price of our common stock on the Nasdaq Global Select Market as of that date. The following table sets forth information
regarding outstanding equity awards and shares available for future equity awards under the 2023 Plan (without giving effect to
approval of the Second A&R 2023 Plan), the Prior Plans, our Employment Inducement Plan, as amended (the “Inducement
Plan), the Dimension Therapeutics, Inc. 2015 Stock Option and Incentive Plan (the “DT 2015 Plan), the Dimension Therapeutics,
Inc. 2013 Stock Plan (the “DT 2013 Plan”), and the shares available for future equity awards under our Amended and
Restated 2014 Employee Stock Purchase Plan (ESPP) as of March 7, 2025.
| |
|
2023 Plan |
|
2014 Plan |
|
2011 Plan |
DT 2015
Plan |
DT 2013
Plan |
|
Inducement
Plan |
|
Aggregate
Under All
Plans |
|
| Total shares underlying outstanding stock options |
|
2,399,511 |
|
8,459,896 |
|
— |
|
21,403 |
|
|
221 |
|
|
268,183 |
|
11,149,214 |
|
| Weighted average exercise price of outstanding stock options |
|
48.43 |
|
68.50 |
|
— |
|
49.33 |
|
|
31.78 |
|
|
46.88 |
|
63.62 |
|
| Weighted average remaining life of outstanding stock options |
|
9.4 |
|
4.8 |
|
— |
|
1.1 |
|
|
0.2 |
|
|
7.7 |
|
5.9 |
|
| Total shares underlying outstanding RSUs |
|
4,301,616 |
|
1,265,530 |
|
— |
|
— |
|
|
— |
|
|
587,676 |
|
6,154,822 |
|
| Total shares underlying outstanding PSUs (assuming target performance) |
|
619,640 |
|
115,318 |
|
— |
|
— |
|
|
— |
|
|
— |
|
734,958 |
|
| Total shares available for issuance (assuming outstanding PSUs vest at maximum performance) |
|
2,493,498 |
|
— |
|
— |
|
— |
|
|
— |
|
|
200,413 |
|
2,693,911 |
|
Dilution
Dilution is commonly measured
by “overhang,” which generally refers to the total number of equity awards outstanding plus the total number of shares
available for grant under our equity plans, divided by the sum of the total common stock outstanding, the number of equity awards
outstanding and the total number of shares available for grant under our equity plans. If the Second A&R 2023 Plan is approved,
our overhang will be approximately 21% as of March 7, 2025; however, many of the stock options attributing to our overhang are
underwater. As shown above, we had approximately 11,149,214 stock options outstanding, with a weighted average exercise price
of $63.62 as of March 7, 2025. That included 7,727,822 options currently exercisable, with a weighted average exercise price of
$70.07, and 3,421,392 options not yet vested, with a weighted average exercise price of $49.05. That compares to a closing stock
price on March 7, 2025, of $38.62. The 7,638,237 stock options currently underwater account for 6% of our overhang if the Second
A&R 2023 Plan is approved.
Historical Burn Rate
Our equity plan share usage over 2022, 2023 and 2024 represented
a three-year average burn rate of 5.75%, as described in the table below.
| Year |
|
Weighted
Average
Common Stock
Outstanding |
|
Time-based
Stock Options
Granted |
|
Performance-
based Stock
Options
Granted |
|
|
Performance-
based Stock
Options Earned |
|
RSUs
Granted |
|
PSUs Granted |
|
|
PSUs Earned |
|
|
Annualized
Burn Rate(1) |
| 2022 |
|
69,914,225 |
|
2,293,950 |
|
1,827,449 |
|
|
0 |
|
1,347,125 |
|
166,730 |
|
|
28,990 |
|
|
5.25% |
| 2023 |
|
73,543,862 |
|
2,123,256 |
|
0 |
|
|
152,593 |
|
2,447,170 |
|
362,470 |
|
|
27,581 |
|
|
6.46% |
| 2024 |
|
90,538,118 |
|
1,445,364 |
|
0 |
|
|
342,622 |
|
3,169,688 |
|
274,484 |
|
|
47,464 |
|
|
5.53% |
| Three-Year Average |
5.75% |
| (1) |
Annualized burn rate defined as: time-based stock options granted, performance-based
stock options earned, RSUs granted and PSUs earned as a percentage of weighted average common shares outstanding. |
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 15
Summary of the Second A&R 2023 Plan
The following summary describes the material
terms of the Second A&R 2023 Plan. This summary of the Second A&R 2023 Plan is not a complete description of all provisions
of the Second A&R 2023 Plan and is qualified in its entirety by reference to the Second A&R 2023 Plan, which is attached
hereto as Appendix A. Stockholders are encouraged to read the Second A&R 2023 Plan in its entirety.
Purpose
The purpose of the Second A&R 2023 Plan
is to advance the Company’s interests by providing for the grant to participants of equity and other incentive awards.
Plan Administration
The Compensation Committee serves as the
primary administrator of the Second A&R 2023 Plan, except that the Compensation Committee may, subject to applicable law,
delegate authority to one or more members of the Board or one or more of our officers or employees (the Compensation Committee
or such delegee, the Plan Administrator).
The Plan Administrator
has the authority to, among other things, (i) interpret and construe the Second A&R 2023 Plan, any rules and regulations under
the Second A&R 2023 Plan and the terms and conditions of any award granted under the Second A&R 2023 Plan, (ii) determine
eligibility for and grant awards under the Second A&R 2023 Plan, (iii) determine, modify or waive the terms and conditions
of awards under the Second A&R 2023 Plan, (iv) prescribe forms, rules and procedures relating to the Second A&R 2023 Plan,
(v) establish and verify the extent of satisfaction of any performance goals or other conditions applicable to the grant, issuance,
retention, vesting, exercisability or settlement of any award under the Second A&R 2023 Plan, and (vi) otherwise do all things
necessary or appropriate to carry out the purposes of the Second A&R 2023 Plan. The Plan Administrator’s determinations
under the Second A&R 2023 Plan are conclusive and binding.
Authorized Shares
Subject to adjustment as described below,
the maximum number of shares of our common stock that may be delivered in satisfaction of awards under the Second A&R 2023
Plan is (i) 11.5 million, plus (ii) the number of shares of common stock subject to any award under a Prior Plan as of June 7,
2023 that become available as a result of the termination, cancellation or forfeiture of such awards under such Prior Plan after
June 7, 2023.
Shares of our common stock to be issued
under the Second A&R 2023 Plan may be authorized but unissued shares of our common stock or previously issued shares acquired
by us. If any shares of our common stock underlying awards that are settled in cash, canceled, forfeited or otherwise expire or
lapse without being exercised (to the extent applicable), the shares of common stock allocable to the terminated portion of such
award will again be available for issuance under the Second A&R 2023 Plan.
However, notwithstanding
the foregoing, the following shares of common stock will not be available for issuance under the Plan: (a) shares withheld from
an award under the Second A&R 2023 Plan or a Prior Plan to satisfy the tax withholding obligations with respect to such award,
(b) shares withheld from an award under the Second A&R 2023 Plan or a Prior Plan in payment of the exercise price of an award
requiring exercise, (c) shares repurchased on the open market by us using proceeds from the exercise price paid with respect to
awards under the Second A&R 2023 Plan or a Prior Plan, or (d) gross shares subject to an SAR granted under the Second A&R
2023 Plan or a Prior Plan that are not issued in connection with the stock-settlement of such SAR.
Limits on Non-Employee Director Compensation
The aggregate dollar value of
equity-based (based on the grant date fair value of equity-based awards determined for financial reporting purposes) and cash
compensation granted under the Second A&R 2023 Plan or otherwise to any one non-employee director during any fiscal year
will not exceed $900,000, with up to $1,500,000 to be permitted for a non-employee director in the fiscal year he or she
first joins our Board or is first designated as Chairman of our Board or Lead Director.
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 16
Eligibility
The Plan Administrator will select
participants from among our, and our affiliates’, executives, employees in good performance standing, directors,
consultants and advisors who are in a position to contribute significantly to our success and the success of our affiliates.
Eligibility for options intended to be incentive stock options, or ISOs, is limited to our employees or the employees of
certain of our affiliates. As of March 7, 2025, there were approximately 1,279 employees (including eight executive officers)
and seven non-employee directors who would be eligible to participate in the Second A&R 2023 Plan. As of March 7, 2025,
no consultants or advisors would be eligible to participate in the Second A&R 2023 Plan.
Awards Under the Second A&R 2023 Plan
The Second A&R 2023
Plan provides for grants of stock options, SARs, restricted and unrestricted stock, stock units (including RSUs), performance
awards, cash awards and other awards convertible into or otherwise based on shares of our common stock. Dividend equivalents may
also be provided in connection with an award under the Second A&R 2023 Plan (other than stock options and SARs), provided
that such dividend equivalents will be subject to the same limits or restrictions as the awards to which they relate, and will
not be payable until such awards vest.
Stock Options
A stock option is an award that
entitles the participant to receive, upon exercise, shares of our common stock upon payment of the exercise price. The
exercise price of an option may not be less than the fair market value (or, in the case of an ISO granted to a ten percent
shareholder, 110% of the fair market value) of a share of our common stock on the date of grant. The Plan Administrator will
determine the time or times at which stock options become exercisable and the terms on which they remain exercisable. The
maximum term of a stock option is 10 years (or, in the case of an ISO granted to a ten percent shareholder, five years).
Reload stock options are prohibited under the Second A&R 2023 Plan.
SARs
SARs entitle the participant to
receive, upon exercise, shares of our common stock or cash equal to the excess of the value of the shares subject to the SAR
over the exercise price. The exercise price of an SAR may not be less than the fair market value of a share of our common
stock on the date of grant. The Plan Administrator will determine the time or times at which SARs become exercisable and the
terms on which they remain exercisable. The maximum term of an SAR is 10 years.
Restricted and Unrestricted Stock
A restricted stock award
is an award of our common stock subject to restrictions requiring that it be redelivered or offered for sale to the Company if
specified conditions are not satisfied, while an unrestricted stock award is an award of our common stock that is not subject
to any restrictions. The Plan Administrator will determine the terms of awards of restricted and unrestricted stock.
Stock Units
A stock unit award is denominated in shares
of our common stock and entitles the participant to receive stock or cash measured by the value of the shares in the future. An
RSU is a stock unit award that is subject to the satisfaction of performance conditions or other vesting conditions. The Plan
Administrator will determine the terms of awards of stock units, including RSUs.
Performance Awards
A performance award is an award under the
Second A&R 2023 Plan where the vesting, settlement or exercisability is subject to specified performance criteria.
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 17
Cash Awards
Cash awards are awards under the Second A&R 2023 Plan that
are denominated in cash.
Termination of Employment
The Plan Administrator will
determine the effect of termination of employment or service on an award under the Second A&R 2023 Plan. Unless otherwise
expressly provided by the Plan Administrator, upon a termination of a participant’s employment all unvested options then
held by the participant and other awards requiring exercise will terminate and all other unvested awards will be forfeited and
all vested stock options and SARs then held by the participant will remain outstanding for three months, or one year in the case
of death, or, in each case, until the applicable expiration date, if earlier. All stock options and SARs held by a participant
immediately prior to the participant’s termination of employment will immediately terminate upon termination of employment
if the termination is for cause or occurs in circumstances that, in the determination of the Plan Administrator, would have constituted
grounds for the participant’s employment to be terminated for cause.
Transferability
Awards under the Second A&R 2023 Plan
may not be transferred except by will or by the laws of descent and distribution. For awards other than ISOs, the Plan Administrator
may permit the transfer not for value to any transferee eligible to be covered by the provisions of Form S-8.
Clawback; Recovery of Compensation
Awards granted under the Second A&R
2023 Plan are subject to forfeiture, termination and rescission, and a participant will be obligated to return to us the value
received with respect to awards, to the extent provided by the Plan Administrator in an award agreement, pursuant to Company policy
relating to the recovery of erroneously-paid incentive compensation (including our Clawback Policy), or as otherwise required
by law or applicable stock exchange listing standards.
Covered Transaction
In the event of a covered
transaction (as defined in the Second A&R 2023 Plan), the Plan Administrator may, among other things, provide for (i) continuation
or assumption of outstanding awards, (ii) new grants in substitution of outstanding awards, (iii) the accelerated vesting or delivery
of shares under awards or for a cash-out of outstanding awards, in each case on such terms and with such restrictions as it deems
appropriate. However, if the award is not continued or assumed or a new grant is not substituted for the award, then stock options
and SARs will become fully exercisable and all other awards shall become vested (with any performance based on target or actual
performance as determined by the Plan Administrator).
Prohibition Against Repricing
We will not, without stockholder approval
(except in the case of a change in our capitalization, as described below), (i) reduce the exercise price of a stock option or
SAR, (ii) other than in the case of covered transaction, at any time when the exercise price of a stock option or SAR is above
the fair market value of a share of stock, cancel and re-grant or exchange such stock option or SAR for cash or a new award having
a lower (or no) exercise price, or (iii) take any other action with respect to an award that would be treated as a repricing under
generally accepted accounting principles.
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 18
Adjustment
In the event of a stock dividend, stock
split or combination of shares including a reverse stock split, recapitalization or other change in our capital structure
that constitutes an equity restructuring within the meaning of the Financial Accounting Standards Board, Accounting Standards
Codification Topic 718, Compensation — Stock Compensation, the Plan Administrator will make
appropriate adjustments to the maximum number of shares that may be delivered under the Second A&R 2023 Plan, and will
also make appropriate adjustments to the number and kind of shares of stock or securities subject to awards, the exercise
prices of such awards or any other terms of awards affected by such change. The Plan Administrator will also make the types
of adjustments described above to take into account distributions and other events other than those listed above if
it determines that such adjustments are appropriate to avoid distortion and preserve the value of awards.
Amendment and Termination
The Plan Administrator will be able to amend
the Second A&R 2023 Plan or outstanding awards for any purpose which may at the time be permitted by law, or terminate the
Second A&R 2023 Plan as to future grants of awards, except that the Plan Administrator will not be able to alter the terms
of an award if it would affect materially and adversely a participant’s rights under the award without the participant’s
consent (unless expressly provided in the Second A&R 2023 Plan or the right to alter the terms of an award was expressly reserved
by the Plan Administrator at the time the award was granted). Stockholder approval will be required for any amendment to the Second
A&R 2023 Plan to the extent such approval is required by law, including the Internal Revenue Code of 1986, as amended (the
“Code”), or applicable stock exchange requirements. No awards may be made under the Second A&R 2023 Plan after
10 years from June 7, 2023.
New Plan Benefits
As described above, the selection of participants
who will receive awards under the Second A&R 2023 Plan and the size and types of awards will be determined by the Plan Administrator
in its discretion. Therefore, the amount of any future awards under the Second A&R 2023 Plan is not yet determinable and it
is not possible to predict the benefits or amounts that will be received by, or allocated to, particular individuals or groups
of employees.
Awards Granted Under the 2023 Plan
No awards granted under the 2023 Plan prior
to the date of the Annual Meeting were subject to stockholder approval of the Second A&R 2023 Plan. Pursuant to the
SEC rules, the following table sets forth information with respect to awards that have been granted under the 2023 Plan to
the groups named below as of March 7, 2025, with PSUs based on achievement of target performance. No associate of any
director, executive officer or director nominee has received awards under the 2023 Plan, and no person has received more than
5% of all awards under the 2023 Plan.
| Name and Position |
|
Stock Options
Granted |
|
RSUs and PSUs
Granted |
| Emil D. Kakkis, M.D., Ph.D., President and Chief Executive Officer |
|
148,542 |
|
326,783 |
| Howard Horn, Chief Financial Officer and Executive Vice President, Corporate Strategy |
|
62,360 |
|
85,760 |
| Erik Harris, Chief Commercial Officer and Executive Vice President |
|
64,446 |
|
89,240 |
| Eric Crombez, M.D., Chief Medical Officer and Executive Vice President |
|
64,446 |
|
89,240 |
| John R. Pinion II, Chief Quality Officer and Executive Vice President, Translational Sciences |
|
57,474 |
|
79,082 |
| All current executive officers as a group (8) |
|
543,616 |
|
863,869 |
| All current directors who are not executive officers as a group (7) |
|
69,300 |
|
37,415 |
| Matthew K. Fust |
|
9,900 |
|
5,345 |
| Amrit Ray, M.D. |
|
9,900 |
|
5,345 |
| All current employees, including all current officers who are not executive officers, as a group (1,221) |
|
1,899,221 |
|
4,893,071 |
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 19
U.S. Federal Income Tax Consequences
The following is a summary of the U.S. federal
income tax treatment applicable to us and the participants who receive awards under the Second A&R 2023 Plan based on the federal
income tax laws in effect on the date of this Proxy Statement. This summary is not intended to be exhaustive and does not address
all matters relevant to a particular participant based on their specific circumstances. The summary expressly does not discuss
the income tax laws of any state, municipality, or non-U.S. taxing jurisdiction, or the gift, estate, excise (including the rules
applicable to deferred compensation under Section 409A of the Code), or other tax laws other than U.S. federal income tax law.
Because individual circumstances may vary, we recommend that all participants consult their own tax advisor concerning the tax
implications of awards granted under the Second A&R 2023 Plan.
Stock Option Grants
Stock options granted under the Second A&R
2023 Plan may be either ISOs, which satisfy the requirements of Section 422 of the Code, or non-statutory stock options (NSOs),
which are not intended to meet such requirements. The U.S. federal income tax treatment for the two types of options differs
as follows:
Incentive Stock Options
No taxable income is recognized by the participant
at the time of the grant of an ISO, and no taxable income is recognized for ordinary income tax purposes at the time the ISO is
exercised, although taxable income may arise at that time for alternative minimum tax purposes. Unless there is a disqualifying
disposition, as described below, the participant will recognize long-term capital gain in an amount equal to the excess of (i)
the amount realized upon the sale or other disposition of the purchased shares over (ii) the exercise price paid for the shares.
A disqualifying disposition occurs if the disposition
is less than two years after the date of grant or less than one year after the exercise date. If there is a disqualifying disposition
of the shares, then the excess of (i) the fair market value of those shares on the exercise date or (if less) the amount realized
upon such sale or disposition over (ii) the exercise price paid for the shares will be taxable as ordinary income to the participant.
Any additional gain or loss recognized upon the disposition will be a capital gain or loss.
If the participant makes a disqualifying disposition
of the purchased shares, then we will be entitled to an income tax deduction, for the taxable year in which such disposition occurs,
equal to the amount of ordinary income recognized by the participant as a result of the disposition. We will not be entitled to
any income tax deduction if the participant makes a qualifying disposition of the shares.
Non-Statutory Stock Options
No taxable income is recognized by a participant
upon the grant of an NSO. The participant in general will recognize ordinary income, in the year in which the NSO is exercised,
equal to the excess of the fair market value of the purchased shares on the exercise date over the exercise price paid for the
shares, and the participant will be required to satisfy the tax withholding requirements applicable to such income. We will be
entitled to an income tax deduction equal to the amount of ordinary income recognized by the participant with respect to the exercised
NSO.
SARs
No taxable income is recognized upon receipt
of an SAR. The holder will recognize ordinary income in the year in which the SAR is exercised, in an amount equal to the excess
of (i) the fair market value of the underlying shares of common stock on the exercise date over (ii) the base price in effect for
the exercised right, and the holder will be required to satisfy the tax withholding requirements applicable to such income. We
will be entitled to an income tax deduction equal to the amount of ordinary income recognized by the holder in connection with
the exercise of the SAR.
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 20
Stock Awards
Participants will recognize ordinary income at
the time unrestricted stock awards are granted in an amount equal to the excess of (i) the fair market value of the shares on the
grant date over (ii) the cash consideration (if any) paid for the shares. The holder will be required to satisfy the tax withholding
requirements applicable to the income.
No taxable income is recognized at the time restricted
stock awards are issued, but the participant will have to report as ordinary income, as and when those shares subsequently vest,
an amount equal to the excess of (i) the fair market value of the shares on the vesting date over (ii) the cash consideration (if
any) paid for the shares. The participant may, however, elect under Section 83(b) of the Code to include as ordinary income in
the year the unvested shares are issued an amount equal to the excess of (a) the fair market value of those shares on the issue
date over (b) the cash consideration (if any) paid for such shares. If the Section 83(b) election is made, the participant will
not recognize any additional income as and when the shares subsequently vest.
We will be entitled to an income tax deduction
equal to the amount of ordinary income recognized by the participant at the time such ordinary income is recognized by the participant.
Other Awards
Generally, no taxable income is recognized upon
receipt of stock units (including RSUs), performance awards or cash awards. The holder will recognize ordinary income in the year
in which the shares subject to the award are actually issued or in the year in which the award is settled in cash. The amount of
that income will be equal to the fair market value of the shares on the date of issuance or the amount of the cash paid in settlement
of the award, and the holder will be required to satisfy the tax withholding requirements applicable to the income.
We will be entitled to an income tax deduction
equal to the amount of ordinary income recognized by the holder at the time the shares are issued or the cash amount is paid.
Deductibility of Executive Compensation
Section 162(m) of the Code limits the deductibility
for federal income tax purposes of certain compensation paid to any “covered employee” in excess of $1 million. For
purposes of Section 162(m), the term “covered employee” includes any individual who serves as chief executive officer,
chief financial officer or one of the other three most highly compensated executive officers for 2017 or any subsequent calendar
year (and beginning in 2027, also including the next five highest-compensated employees for the applicable year). It is expected
that compensation deductions for any covered employee with respect to awards under the Second A&R 2023 Plan will be subject
to the $1 million annual deduction limitation.
Vote Required
Approval of the Second A&R 2023 Plan requires
the affirmative vote of a majority of the votes cast on this proposal. Because abstentions and broker non-votes, if any, are not
counted as votes cast for or against this resolution, they will have no effect on the outcome of the vote.
 |
THE BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR” PROPOSAL NO. 2. |
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 21
PROPOSAL 3
Ratification of the Selection of Independent Registered Public Accounting Firm
Our Audit Committee has selected Ernst &
Young LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2025, and has further directed
that we submit the selection of Ernst & Young LLP for ratification by our stockholders at the Annual Meeting.
We are not required to submit the selection of
our independent registered public accounting firm for stockholder approval, but are submitting our selection of Ernst &
Young LLP for stockholder ratification as a matter of good corporate governance. If the stockholders do not ratify this selection,
the Audit Committee will reconsider its selection of Ernst & Young LLP. Even if the selection is ratified, our Audit Committee
may direct the appointment of a different independent registered public accounting firm at any time during the year if the Audit
Committee determines that the change would be in our best interests.
The Audit Committee reviews and pre-approves
all audit and non-audit services performed by our independent registered public accounting firm. The Audit Committee may delegate
its pre-approval authority to one or more of its members and has delegated such authority to the Chairman of the Audit Committee;
any pre-approval decisions made by the Chairman are reported to the Audit Committee at the next scheduled committee meeting. The
Audit Committee may pre-approve specified audit-related services (assurance and related services that are reasonably related to
the performance of the audit or review of our financial statements and that are traditionally performed by the independent auditor)
as well as specified tax services that the Audit Committee believes would not impair the independence of the independent auditor,
and that are consistent with rules on auditor independence established by the SEC and the Public Company Accounting Oversight Board
(PCAOB). The Audit Committee may also pre-approve those permissible non-audit services classified as “all other services”
that it believes are routine and recurring services and would not impair the independence of the independent auditor and are consistent
with SEC and PCAOB rules on auditor independence. All requests or applications for services to be provided by the independent auditor
will be submitted to the Chief Financial Officer and must include a detailed description of the services to be rendered. The Chief
Financial Officer or the Principal Accounting Officer, as the case may be, will authorize those services that have been pre-approved
by the Audit Committee. If there is any question as to whether a proposed service fits within the pre-approved categories of services,
the Chairman of the Audit Committee is to be consulted for a determination. For services that have not been pre-approved by the
Audit Committee, requests or applications to provide services will be submitted to the Audit Committee by both the independent
auditor and the Chief Financial Officer, and must include a joint oral or written statement as to whether, in their view, the request
or application is consistent with the SEC’s and PCAOB’s rules on auditor independence.
All services rendered by Ernst & Young
LLP in fiscal 2024 were approved in accordance with these policies. In its review of non-audit services, the Audit
Committee considers, among other things, the possible impact of the performance of such services on the independent
registered public accounting firm’s independence. The Audit Committee has determined that the non-audit services
performed by Ernst & Young LLP in the fiscal year ended December 31, 2024 were compatible with maintaining the
independent registered public accounting firm’s independence. Additional information concerning the Audit Committee and
its activities can be found in the following sections of this Proxy Statement: “Board of Directors
and Committees—Board Committees” and “Report of the Audit Committee.”
Ernst & Young LLP
has audited our financial statements since our inception. Representatives of Ernst & Young LLP are expected to be
present at the Annual Meeting, will have the opportunity to make a statement if they desire to do so, and are expected to be
available to respond to appropriate stockholder questions.
Fees for Independent Registered Public Accounting
Firm
The following is a summary of the aggregate audit
fees billed or expected to be billed by Ernst & Young LLP for the indicated fiscal years and the fees billed by Ernst &
Young LLP for all other services rendered during the indicated fiscal years.
| | |
2024 | | |
2023 | |
| Audit fees(1) | |
$ | 2,820,000 | | |
$ | 2,240,000 | |
| Audit-related fees | |
| — | | |
| — | |
| Tax fees(2) | |
| 93,000 | | |
| 77,000 | |
| All other fees | |
| — | | |
| — | |
| TOTAL | |
$ | 2,913,000 | | |
$ | 2,317,000 | |
| (1) |
Audit fees consist of the aggregate fees billed for professional services rendered for the
audit of our annual financial statements included in our annual reports on Form 10-K; the review of our interim financial
statements included in our quarterly reports on Form 10-Q; consultation on technical accounting matters; assistance with registration
statements filed with the SEC; and the issuance of comfort letters and consents. |
| (2) |
Tax fees principally include fees for tax compliance and tax advice. |
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 22
Vote Required
Ratification of the selection of the independent
registered public accounting firm requires the affirmative vote of a majority of the votes cast. Because abstentions and broker
non-votes, if any, are not counted as votes cast for or against this proposal, they will have no effect on the outcome of the vote.
 |
THE BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR” PROPOSAL
NO. 3. |
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 23
Report of the Audit Committee
The Audit Committee evaluates auditor performance,
manages relations with our independent registered public accounting firm, and evaluates policies and procedures relating to internal
control systems. The Audit Committee operates under a written Audit Committee Charter that has been adopted by the Board, a copy of
which is available on our website at www.ultragenyx.com. All members of the Audit Committee currently meet the
independence and qualification standards for Audit Committee membership set forth in the listing standards and rules of Nasdaq and
the SEC.
No member of the Audit Committee is a professional
accountant or auditor. The members’ functions are not intended to duplicate or to certify the activities of management and
the independent registered public accounting firm. The Audit Committee serves a board-level oversight role in which it provides
advice, counsel, and direction to management and the auditors on the basis of the information it receives, discussions with management
and the auditors, and the experience of the Audit Committee’s members in business, financial, and accounting matters.
The Audit Committee oversees our financial reporting
process on behalf of the Board. Our management has the primary responsibility for the financial statements and reporting process,
including our system of internal controls over financial reporting. In fulfilling its oversight responsibilities, the Audit Committee
reviewed and discussed with management the audited financial statements included in the Annual Report on Form 10-K for the fiscal
year ended December 31, 2024. This review included a discussion of the quality and the acceptability of our financial reporting,
including the nature and extent of disclosures in the financial statements and the accompanying notes.
The Audit Committee discussed with our independent
registered public accounting firm, which is responsible for expressing an opinion on the conformity of the audited financial
statements with generally accepted accounting principles in the U.S., their judgments as to the quality and the acceptability of our
financial reporting and such other matters as are required to be discussed with the Committee pursuant to applicable rules of the
PCAOB and the SEC. The Audit Committee has received the written disclosures and the letter from the independent registered public
accounting firm required by the applicable rules of the PCAOB regarding the independent accountant’s communications with the
Audit Committee concerning independence. The Audit Committee discussed with the independent registered public accounting firm their
independence.
In addition to the matters specified above, the
Audit Committee discussed with our independent registered public accounting firm the overall scope, plans, and estimated costs
of their audit. The Audit Committee met with the independent registered public accounting firm periodically, with and without management
present, to discuss the results of the independent registered public accounting firm’s examinations, the overall quality
of our financial reporting, and the independent registered public accounting firm’s reviews of the quarterly financial statements
and drafts of the quarterly and annual reports.
Based on the reviews and discussions referred
to above, the Audit Committee recommended to the Board that our audited financial statements should be included in our Annual Report
on Form 10-K for the fiscal year ended December 31, 2024.
Submitted by the Audit Committee of the Board of
Directors
Matthew K. Fust, Chairperson
Michael Narachi
Corsee D. Sanders, Ph.D.
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 24
PROPOSAL 4
Advisory (Non-Binding) Vote to Approve the Compensation of our Named Executive Officers
Background
Section 14A of the Exchange Act, as adopted pursuant
to the Dodd-Frank Wall Street Reform and Consumer Protection Act (Dodd-Frank Act) requires that stockholders have the opportunity
to cast an advisory (non-binding) vote to approve the compensation of our named executive officers (say-on-pay vote).
The say-on-pay vote is a non-binding vote on
the compensation of our “named executive officers,” as described in the Compensation Discussion and Analysis section,
the tabular disclosure regarding such compensation, and the accompanying narrative disclosure, set forth in this Proxy Statement.
The say-on-pay vote is not a vote on our general compensation policies, compensation of our Board, our compensation policies as
they relate to risk management, or our pay ratio.
Our philosophy in setting compensation policies
for executive officers has two fundamental objectives: (1) to attract and retain a highly-skilled team of talented executives and
(2) to align our executives’ interests with those of our stockholders by rewarding short-term and long-term performance and
tying compensation to increases in stockholder value. The Compensation Committee believes that executive compensation should be
directly linked to performance, including continuous improvements in corporate performance and accomplishments of our strategic
plan that are expected to increase stockholder value. The Compensation Discussion and Analysis section starting on page 37 provides
a more detailed discussion of the executive compensation program and compensation philosophy.
The vote under this Proposal No. 4
is advisory and therefore not binding on us, the Board, or our Compensation Committee. However, our Board, including our
Compensation Committee, values the opinions of our stockholders and, to the extent there is any significant vote against this
proposal, we will consider our stockholders’ concerns and evaluate what actions may be appropriate to address those
concerns. The Dodd-Frank Act requires us to hold the say-on-pay vote at least once every three years, and we have determined
to hold a say-on-pay vote every year. Unless the Board modifies its policy on the frequency of holding say-on-pay advisory
votes, the next say-on-pay vote will occur in 2026.
Stockholders will be asked at the Annual Meeting
to approve the following resolution pursuant to this Proposal No. 4:
RESOLVED, that the stockholders of Ultragenyx
Pharmaceutical Inc. approve, on an advisory basis, the compensation of the Company’s “named executive officers”
(as defined in the Company’s definitive proxy statement for the 2025 Annual Meeting of Stockholders (the “Proxy Statement”)),
as such compensation is described in the Compensation Discussion and Analysis section, the tabular disclosure regarding such compensation,
and the accompanying narrative disclosure, set forth in the Company’s Proxy Statement.
Vote Required
Approval of this resolution requires the affirmative vote of a majority of the votes cast on this proposal. Because abstentions
and broker non-votes, if any, are not counted as votes cast for or against this resolution, they will have no effect on the
outcome of the vote.
 |
THE BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR” PROPOSAL NO. 4. |
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 25
Corporate Governance
Director Independence
Our Board currently consists of eight members.
Our Board has determined that Dr. Dunsire, Mr. Fust, Mr. Narachi, Dr. Ray, Dr. Sanders, Dr. Suliman, and Mr. Welch qualify as “independent”
directors in accordance with Nasdaq listing requirements and rules. Dr. Kakkis is not considered independent because he is an employee
of the Company. Under Nasdaq rules, the Board’s determination of a director’s independence considers objective tests,
such as whether the director is, or has been within the last three years, an employee of the Company and whether the director or
any of his or her family members has engaged in certain types of business dealings with us. Under Nasdaq rules, our Board also
evaluates whether any relationships exist that, in the opinion of our Board, would interfere with the exercise of independent judgment
in carrying out the responsibilities of a director. In making these independence determinations, our Board reviewed and discussed
information provided by the directors and us with regard to each director’s business and personal activities and relationships
as they may relate to us and our management. There are no family relationships among any of our directors or executive officers.
Director Overboarding Policy
Our Board believes that all members of the Board
must have sufficient time to focus on his or her Board duties. Our Corporate Governance Guidelines limit the total number of public
company boards that a director may serve as follows:
| • |
Director who is not a public company Chief Executive Officer: five total public company boards |
| • |
Director who serves as a Chief Executive Officer of a public company: three total public company boards |
The Nominating and Corporate Governance Committee
regularly assesses compliance with this policy as part of the director nomination process. All of our directors are currently in
compliance with our policy.
Global Code of Conduct
We have adopted a Global Code of Conduct that
applies to all of our employees, officers, and directors, including our principal executive officer, principal financial officer,
principal accounting officer or controller, or persons performing similar functions. Our Global Code of Conduct is available on
our website, www.ultragenyx.com, under the “Corporate Governance” subsection of the “Investors”
tab. We intend to promptly disclose on our website any future changes or amendments to, or waivers of, to the Global Code of Conduct
that we are required to disclose under applicable rules. Our Board is responsible for applying and interpreting the Global Code
of Conduct in situations where questions are presented to it.
Insider Trading, Anti-Hedging and Anti-Pledging
Policy
We have adopted insider trading policies and
procedures governing the purchase, sale and other transactions in Ultragenyx securities by our directors, officers and employees,
and other covered persons, or the Company itself, that we believe are reasonably designed to promote compliance with insider trading
laws, rules and regulations, and Nasdaq listing rules, as applicable.
As part of these policies and procedures, we
prohibit our directors, officers, employees and other covered persons from engaging in (a) short-term trading; (b) short sales;
(c) transactions involving publicly traded options or other derivatives, such as trading in puts or calls with respect to Company
securities; (d) hedging transactions; and (e) pledging Ultragenyx securities as collateral for a loan.
Stockholder Communications
Generally, stockholders who have questions or
concerns regarding the Company should contact our Investor Relations department at (844) 280-7681. However, any stockholders who
wish to address questions regarding our business or affairs directly with the Board, or any individual director, should direct
his or her questions in writing to the Chairman of the Board, c/o Ultragenyx Pharmaceutical Inc., 60 Leveroni Court, Novato, California
94949. At the request of the Chairman of the Board, the Corporate Secretary reviews all correspondence addressed to the Chairman,
organizes the correspondence, and provides it to the Chairman or to individual directors, as appropriate. Our independent directors
have requested that certain items that are inappropriate or unrelated to the Board’s duties, such as spam, junk mail, mass
mailings, solicitations, resumes, and job inquiries not be provided to directors.
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 26
Board of Directors and Committees
During fiscal 2024, our Board met four times.
Each of our current directors attended 100% of the aggregate meetings of the Board and meetings of the committees of which the
director was a member in fiscal 2024 held during the period when the director served on the Board or the committees, as applicable.
The Board has a standing Audit Committee, Compensation
Committee, Nominating and Corporate Governance Committee, and Research and Development Committee. All members of the Audit, Compensation,
and Nominating and Corporate Governance Committees are non-employee directors whom the Board has determined are independent under
applicable independence standards (including the heightened independence standards that apply to Audit Committee and Compensation
Committee members).
Five of the directors serving at the time of
the 2024 Annual Meeting attended such annual meeting. Each director who is up for election at an annual meeting of stockholders
or who has a term that continues after such annual meeting is encouraged to attend the annual meeting of stockholders.
Board Leadership Structure
We currently separate the positions of Chairman
of the Board and Chief Executive Officer, which allows our Chief Executive Officer, Dr. Kakkis, to focus on our day-to-day business,
while allowing the Chairman of the Board, Mr. Welch, to lead the Board in its fundamental role of providing advice to and independent
oversight of management. Independent oversight of management is an important goal of the Board, which is why our Corporate Governance
Guidelines provide that a lead independent director will be appointed by the Board if the Chairman is not independent. Additionally,
our Board recognizes the time, effort, and energy that the Chief Executive Officer is required to devote to his position in the
current business environment, as well as the commitment required to serve as our Chairman. Our Board also believes that the separation
of the Chairman and Chief Executive Officer positions fosters a greater role for the independent directors in the oversight of
our Company and active participation of the independent directors in setting agendas and establishing priorities and procedures
for the work of our Board. The benefits of the separated Chairman and Chief Executive Officer positions are augmented by the independence
of seven of our eight current directors, including our Chairman, and our independent Board committees that provide appropriate
oversight in the areas described below. At executive sessions of independent directors, these directors can speak candidly on any
matter of interest. The independent directors of the Board regularly meet in executive sessions, and met four times in 2024, and
the Chairman presides at these sessions. We believe this structure provides effective oversight of our management and the Company.
The Board believes that its programs for risk
oversight, as described under “Role of the Board in Risk Oversight” below, would be effective under a variety of leadership
frameworks. Accordingly, the Board’s risk oversight function did not significantly impact its selection of the current leadership
structure.
Role of the Board in Risk Oversight
The Board has overall responsibility for the
oversight of our risk management process, which is designed to support the achievement of organizational objectives, including
strategic objectives, to improve long-term organizational performance, and enhance stockholder value. Risk management includes
not only understanding company-specific risks and the steps management implements to manage those risks, but also assessing what
level of risk is acceptable and appropriate for us, taking into account the immediacy of any such risks, and evaluating risks and
circumstances across various timeframes, including the short, medium and long term. Management is responsible for establishing
our business strategy, identifying and assessing the related risks, and implementing appropriate risk management practices. The
Board periodically reviews our business strategy and management’s assessment of the related risk and discusses with management
the appropriate level of risk for us. The Board also periodically evaluates and discusses potential emerging risks with members
of senior management as well as third-party advisors and experts. In 2024, the Board and the committees, as appropriate, reviewed
with management the various risks and mitigation strategies related to cost containment strategies, prioritization of the Company’s
pipeline programs, cybersecurity risks and the Company’s initiatives related to our Impact Report. The Board also delegates
to Board committees oversight of selected elements of risk as set forth below.
Board Committees
Our Board currently has a standing Audit Committee,
Compensation Committee, Nominating and Governance Committee and Research and Development Committee. Each of these committees operates
under a written charter which sets forth the functions and responsibilities of the committee, a copy of which is available on our
website at www.ultragenyx.com under the “Corporate Governance” subsection of the “Investors” tab.
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 27
| Audit Committee |
|
Key Responsibilities |
Members:
Matthew K. Fust (Chairperson)
Michael Narachi
Corsee D. Sanders, Ph.D.
All members of the Audit Committee satisfy the
current independence and financial literacy standards promulgated by Nasdaq and the SEC, and the Board
has determined that Mr. Fust qualifies as an “audit committee financial expert,” as the SEC
has defined that term in Item 407 of Regulation S-K
Meetings held during 2024: Six |
|
The Audit Committee has been delegated the task of overseeing
significant financial risks facing us and steps management has undertaken to mitigate these risks. The Audit Committee
reports back to the Board regarding these risks. In 2024, the Audit Committee reviewed with management, in particular,
the risks and mitigation strategies related to cybersecurity and the security programs related to our information technology
systems. The Audit Committee is also responsible for the following:
•
Appoints, approves the compensation of, reviews the performance
of, and assesses the independence of our independent registered public accounting firm
•
Approves audit and permissible non-audit services, and the
terms of such services, to be provided by our independent registered public accounting firm
•
Reviews the audit plan with the independent registered public
accounting firm and members of management responsible for preparing our financial statements
•
Reviews and discusses with management and the independent
registered public accounting firm our annual and quarterly financial statements and related disclosures and critical accounting
policies
•
Discusses with management, and as applicable, the independent
auditor, disclosure controls and procedures over environmental and sustainability reporting data and disclosures
•
Reviews the adequacy of our internal control over financial
reporting; establishes policies and procedures for the receipt and retention of accounting-related complaints and concerns
•
Recommends whether our audited financial statements shall
be included in our annual reports on Form 10-K
•
Prepares the audit committee report to be included in our
annual proxy statements
•
Reviews all related-person transactions
•
Reviews policies related to financial risk assessment and
management
•
Establishes, maintains, and oversees our Global Code of Conduct
•
Assists the Nominating and Corporate Governance Committee
by overseeing our compliance program with respect to legal and regulatory requirements impacting areas of financial risk
•
Annually reviews and reassesses the adequacy of the Audit
Committee charter and performs other duties, as specified in the charter |
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 28
| Compensation Committee |
|
Key Responsibilities |
Members:
Michael Narachi (Chairperson)
Deborah Dunsire, M.D.
Daniel G. Welch
All members of the Compensation Committee satisfy
the current Nasdaq and SEC independence standards
Meetings held during 2024: Five |
|
The Compensation Committee has been delegated the task of
overseeing risks related to our compensation policies and programs. In 2024, the Compensation Committee reviewed with
management in particular the risks and mitigation strategies related to human capital management, succession planning
for senior management positions, and strategies related to the Company’s management of outstanding equity awards.
The Compensation Committee is also responsible for the following:
•
Annually reviews and approves corporate goals and objectives
relevant to the compensation of our executive officers
•
Evaluates the performance of our executive officers in light
of such goals and objectives, and determines the compensation of our executive officers
•
Appoints, compensates, and oversees the work of any compensation
consultant, legal counsel, or other advisor retained by the Compensation Committee
•
Conducts the independence assessment outlined in Nasdaq rules
with respect to any compensation consultant, legal counsel, or other advisor retained by the Compensation Committee
•
Oversees, and has the authority to administer, our compensation
and benefit plans
•
Oversees administration of our clawback policy
•
Reviews and approves our policies and procedures for the
grant of equity-based awards
•
Reviews and makes recommendations to the Board with respect
to director compensation
•
Reviews and discusses with management the compensation discussion
and analysis, if any, to be included in our annual proxy statements or annual reports on Form 10-K
•
Oversees the maintenance and presentation to the Board of
management’s plans for succession to senior management positions
•
Annually reviews and reassesses the adequacy of the Compensation
Committee charter and performs other duties, as specified in the charter |
Nominating and Corporate
Governance Committee |
|
Key Responsibilities |
Members:
Shehnaaz Suliman, M.D.
(Chairperson)
Matthew K. Fust
Daniel G. Welch
All members of the Nominating and Corporate Governance
Committee satisfy the current Nasdaq and SEC independence standards
Meetings held during 2024: Three |
|
The Nominating and Corporate Governance Committee has been
delegated the task of overseeing all risks facing us, other than those overseen by the Audit Committee and by the Compensation
Committee, and reporting back to the Board regarding the same. In 2024, the Nominating and Corporate Governance Committee
reviewed with management, in particular, CEO succession planning, the risks and mitigation strategies related to matters
covered in our Impact Report, our director search process and the Company’s governance structure to assess the continued
appropriateness of the classified board and other structural elements for the Company. The Nominating and Corporate Governance
Committee is also responsible for the following:
•
Develops and recommends to the Board criteria for Board and
committee membership
•
Establishes procedures for identifying and evaluating Board
candidates, including nominees recommended by stockholders; identifies individuals qualified to become members of the
Board
•
Recommends to the Board the persons to be nominated for election
as directors and to each of the Board’s committees
•
Develops and recommends to the Board a set of corporate governance
guidelines
•
Oversees the maintenance and presentation to the Board of
plans for succession to the position of Chief Executive Officer
•
Assists the Compensation Committee in its oversight of succession
planning for other senior management positions
•
Oversees our compliance program
•
Recommends to the Board and reviews on regular basis with
Board our corporate responsibility and sustainability matters relevant to our business
•
Annually reviews and reassesses the adequacy of the Nominating
and Corporate Governance Committee charter and performs other duties, as specified in the charter |
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 29
Research and Development
Committee |
|
Key Responsibilities |
Members:
Amrit Ray, M.D. (Chairperson)
Emil D. Kakkis, M.D., Ph.D.
Deborah Dunsire, M.D.
Corsee D. Sanders, Ph.D.
Shehnaaz Suliman, M.D.
Meetings held during 2024: Two |
|
The Research and Development Committee assists the Board
in its oversight of the strategic direction for our pipeline and investment in research and development. In 2024, the
Research and Development Committee reviewed with management in particular the risks and mitigation strategies related
to the Company’s prioritization of its clinical and pre-clinical programs. The Research and Development Committee
is also responsible for the following:
•
Evaluates and advises on our key R&D activities and early
pipeline development goals and strategy
•
Evaluates and provides input with respect to the quality
of the science being conducted and overall program execution
•
Assesses the overall quality of the R&D programs and
prospects for progression to monitor our pipeline to maintain product flow
•
Evaluate and advise on our clinical-stage pipeline and the
development strategies
•
Performs other duties as specified in the Research and Development
Committee Charter |
Compensation Committee Interlocks and Insider
Participation
During fiscal 2024, the Compensation Committee
consisted of Dr. Dunsire and Messrs. Narachi and Welch. None of the members of our Compensation Committee has at any time during
the prior three years been one of our officers or employees. None of our executive officers currently serves, or in the past fiscal
year has served, as a member of the board of directors or compensation committee of any entity that has one or more executive officers
serving on our Board or Compensation Committee.
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 30
Impact Report
At Ultragenyx, our purpose is to lead the future
of rare disease medicine as we seek to treat individuals afflicted by diseases with limited or no treatment options, and we recognize
that their lives and well-being are dependent upon our collective efforts to develop new therapies. For this reason, we are passionate
about developing these therapies with the utmost urgency and care. Our strong commitment to ethics, integrity and responsible business
practices is centered around partnering with the rare disease community, improving access to treatments, maintaining a people-first
culture, and investing in innovation.
Our Impact Report for fiscal 2024 (2024 Impact
Report) will be available in digital format on our website at www.ultragenyx.com under “Ultra-Committed – Corporate
Responsibility”. Website references throughout this document are provided for convenience only, and the content on the referenced
websites is not incorporated by reference into this document. Our 2024 Impact Report is based on our strategic framework, which
centers on six pillars: Innovation, Patients, People, Communities, Planet and Governance. The contents of our Impact Report are
not deemed to be part of this Proxy Statement or incorporated by reference herein.
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 31
Executive Officers
Our current executive officers, their respective
ages as of the date of this Proxy Statement, and positions are set forth in the following table. Biographical information regarding
each executive officer (other than Dr. Kakkis) is set forth following the table. Biographical information for Dr. Kakkis is set
forth above under Proposal No. 1 (Election of Class III Directors).
| Name |
|
Age |
|
Position |
| Emil D. Kakkis, M.D.,
Ph.D. |
|
64 |
|
President and Chief Executive Officer,
Director |
| Eric Crombez, M.D. |
|
52 |
|
Chief Medical Officer and Executive Vice
President |
| Erik Harris |
|
55 |
|
Chief Commercial Officer and Executive
Vice President |
| Howard Horn |
|
47 |
|
Chief Financial Officer, Corporate Strategy
and Executive Vice President |
| Dennis
Huang |
|
60 |
|
Chief Technical Operations Officer and
Gene Therapy Operations and Executive Vice President |
| Thomas Kassberg |
|
64 |
|
Chief Business Officer and Executive Vice
President |
| Karah Parschauer |
|
47 |
|
Chief Legal Officer & Corporate Affairs
and Executive Vice President |
| John
R. Pinion II |
|
58 |
|
Chief Quality
Officer and Executive Vice President of Translational Sciences |
Dr. Eric Crombez has served as our Chief Medical
Officer, Executive Vice President since March 2023. Dr. Crombez previously served as our Chief Medical Officer for gene therapy
and inborn errors of metabolism, overseeing global clinical development and execution for the Company’s gene therapy programs,
after he joined the Company following the acquisition of Dimension Therapeutics, a biotechnology company, in November 2017. At
Dimension Therapeutics, Dr. Crombez served as Chief Medical Officer from December 2014 to November 2017 where he led the clinical
development efforts for clinical gene therapy programs in hemophilia B, hemophilia A, ornithine transcarbamylase (OTC) deficiency
and glycogen storage disease type Ia (GSDIa). Previously, he worked at Shire plc, a biopharmaceutical company, in its Human Genetics
Therapy business unit. Dr. Crombez is an appointed industry representative on the FDA Cellular, Tissue and Gene Therapies Advisory
Committee. He previously served as assistant professor, Department of Pediatrics, Division of Medical Genetics at the David Geffen
School of Medicine at the University of California, Los Angeles (UCLA). Dr. Crombez currently serves on the board of directors
of Abeona Therapeutics Inc., a publicly traded biopharmaceutical company. Dr. Crombez is a board-certified clinical geneticist
and completed residencies in pediatrics and medical genetics and a fellowship in clinical biochemical genetics at the UCLA School
of Medicine. Dr. Crombez obtained his B.S. in biology from the University of Michigan, Ann Arbor, and his M.D. from Wayne State
University School of Medicine, Detroit.
Erik Harris has served as our Chief Commercial Officer
and Executive Vice President since June 2019. Prior to his appointment as our Chief Commercial Officer, Erik served as our Senior
Vice President and Head of North American Commercial Operations from July 2017 to June 2019. Prior to Ultragenyx, Mr. Harris spent
six years at Crescendo Bioscience, Inc., a molecular diagnostic company, most recently as Vice President of Commercial. Earlier
in his career, Mr. Harris served as Vice President of Marketing at InterMune, Inc., a biotechnology company, and also held positions
in the commercial organizations at Elan Pharmaceuticals, Inc., Genentech, Inc., and Bristol-Myers Squibb Company. At the start
of his professional career, Mr. Harris served as a Lieutenant Commander in Naval Aviation and Congressional Fellow for the United
States Navy. Mr. Harris currently serves on the board of directors of Denali Therapeutics Inc. and Inozyme Pharma, Inc., each
a publicly traded biopharmaceutical company. Mr. Harris holds a B.S. from the United States Naval Academy and an M.B.A. from the
Wharton School of Business.
Howard Horn has served as
our Chief Financial Officer, Corporate Strategy and Executive Vice President since October 2023. He previously served as
Chief Financial Officer and as a member of the founding management team at Vir Biotechnology, a public biotech company from
March 2017 to April 2023, where he led the accounting, finance, investor relations and facilities functions. Prior to Vir
Biotechnology, Mr. Horn was at Biogen, a biotechnology company, where he served first as Vice President, Strategic Corporate
Finance and led Biogen’s corporate capital allocation processes, and then as Vice President, Business Planning and led
Biogen’s resource allocation processes across all functions and regions. Previously, he held positions of increasing
responsibility as a consultant in the Pharmaceutical and Medical Products Practice at McKinsey & Company, and as an
equity research analyst in the Life Sciences group at UBS Group AG. Mr. Horn received his B.A. in Economics from
Princeton University and his M.B.A. from the Wharton School of the University of Pennsylvania.
Dennis Huang has served as our Executive Vice President
since January 2016, our Chief Technical Operations Officer since May 2015 and as our Chief Technical Operations Officer and Gene
Therapy Operations since December 2021. From May 2015 to January 2016, he served as our Senior Vice President. Prior to Ultragenyx,
Mr. Huang served as Senior Vice President of Manufacturing and Supply Chain at InterMune, Inc., a biotechnology company, from
August 2013 to March 2015. Prior to InterMune, Mr. Huang served as Vice President of Biologic Manufacturing and Development at
Allergan, Inc., a global pharmaceutical company, from May 2006 to August 2013. Mr. Huang currently serves on the board of directors
of CytoDel, Inc., a private biopharmaceutical company. Mr. Huang holds a B.A. in Chemistry from Knox College in Galesburg, Illinois.
Thomas Kassberg has served
as our Executive Vice President since January 2016 and our Chief Business Officer since November 2011. From November 2011 to
January 2016, he served as our Senior Vice President. Prior to Ultragenyx, Mr. Kassberg worked as Vice President of Business
Development at Corium International, Inc., a biotechnology company, from July 2010 to October 2011. Prior to his work at
Corium
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 32
International, Inc., Mr. Kassberg
worked as an independent consultant in corporate development and business strategy and consulted with a number of companies
from March 2009 to June 2010, including Corium International, Inc., a biopharmaceutical company, and Rib-X Pharmaceuticals,
Inc., a pharmaceutical company focused on the development of novel antibiotics. Before becoming a consultant, Mr. Kassberg
worked at Proteolix, Inc., a biotechnology company subsequently acquired by Onyx Pharmaceuticals, from January 2008 to
February 2009, where he served as Senior Vice President of Corporate Development. Mr. Kassberg currently serves on the board
of directors of Passage Bio, Inc., a publicly traded biotechnology company. Mr. Kassberg holds a B.A. in Business
Administration from Gustavus Adolphus College and an M.B.A. from Northwestern University.
Karah Parschauer has
served as our Chief Legal Officer & Corporate Affairs, Executive Vice President since February 2023, as our Chief
Legal Officer and Executive Vice President since December 2021 and as our General Counsel and Executive Vice President since
June 2016. Prior to Ultragenyx, Ms. Parschauer served in various executive capacities, and most recently as Vice President,
Associate General Counsel, at Allergan plc, a pharmaceutical company, from June 2005 to June 2016. Prior to Allergan, Ms.
Parschauer was an attorney at Latham & Watkins LLP, where she practiced in the areas of mergers and acquisitions,
securities offerings and corporate governance. Ms. Parschauer currently serves on the board of directors of Evolus, Inc., a
publicly traded performance beauty company, and the board of directors of Tenaya Therapeutics, a publicly traded
biotechnology company. Ms. Parschauer holds a B.A. in Biology from Miami University and a J.D. from Harvard Law School.
John R. Pinion II has served as our
Chief Quality Officer and Executive Vice President of Translational Sciences since September 2017. From January 2016 to
September 2017, he served as our Executive Vice President of Analytical Sciences and Research, and from July 2015 to
September 2017, as our Chief Quality Operations Officer. From July 2015 to January 2016, he served as our Senior Vice
President of Analytical Sciences and Research. Prior to Ultragenyx, Mr. Pinion served in various roles with increasing
responsibilities at Genentech, a pharmaceutical company, from 2005 to June 2015, including his most recent position as the
Senior Vice President and Global Head of Quality and Compliance for Roche/Genentech Pharma Technical Operations from October
2009 to July 2015. Mr. Pinion currently serves on the board of directors of Aroa Biosurgery, a soft tissue regeneration
company listed on the Australian Securities Exchange. Mr. Pinion holds a B.S. in Mechanical Engineering from the University
of West Virginia.
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 33
Certain Relationships and Related-Person
Transactions
Related-Person Transactions
Since January 1, 2024, we have not become,
and are not currently proposed to be, a participant in any transactions required to be disclosed in the Proxy Statement under
SEC rules with any “related persons,” which are generally considered to be our directors and executive officers, nominees
for director, holders of more than 5% of our outstanding common stock, and members of their immediate families.
Procedures for Related-Person Transactions
We have adopted a written
related-person transactions policy that governs the review, approval, and/or ratification of transactions with a related
person where the amount involved exceeds $100,000 and in which any related person has or will have a direct or indirect
interest. Under the policy, a “related person” is defined as any person described in Item 404(a) of Regulation
S-K and includes any director, nominee for director, or executive officer of the Company; a beneficial owner of more than
five percent of any class of our voting securities; and a person who is an immediate family member of any such director,
nominee for director, executive officer, or more-than-five percent beneficial owner (the term “immediate family
member” includes any child, stepchild, parent, stepparent, spouse, sibling, mother-in-law, father-in-law, son-in-law,
daughter-in-law, brother-in-law, or sister-in-law and any person (other than a tenant or employee) sharing the household of
any such director, nominee for director, executive officer, or more-than-five percent beneficial owner).
Pursuant to this policy, prior to
entering into a transaction with a related person, our Chief Financial Officer (or Chief Legal Officer, in the case where the
Chief Financial Officer has a direct or indirect interest in the transaction) will review the proposed transaction to
determine if such transaction qualifies as a related-person transaction. If the Chief Financial Officer (or Chief Legal
Officer, if applicable) determines that the proposed transaction is a related-person transaction, then the proposed
transaction will be submitted to the Audit Committee for consideration at the next Audit Committee meeting; provided,
however, that if the Chief Financial Officer (or Chief Legal Officer, if applicable),in consultation with the
Chief Executive Officer,determines that it is not practicable or desirable to wait until the next meeting of the
Audit Committee, then the Chief Financial Officer (or Chief Legal Officer, if applicable) shall submit the proposed
transaction to the chairperson of the Audit Committee (who possesses delegated authority to act between meetings of the Audit
Committee to pre-approve or ratify, as applicable, any related-person transaction in which the aggregate amount involved is
expected to be less than $1 million).
In the event that our Chief Executive Officer
or Chief Financial Officer (or Chief Legal Officer, if applicable) becomes aware of a related-person transaction that has not
been previously approved or previously ratified under our related-person transaction policy, the transaction, if pending or ongoing,
will be promptly submitted to the Audit Committee or the chairperson of the Audit Committee for consideration. If the transaction
is already completed, the Audit Committee or the chairperson of the Audit Committee shall evaluate the transaction to determine
if rescission of the transaction and/or any disciplinary action is appropriate.
In evaluating these transactions, the Audit
Committee or the chairperson of the Audit Committee, as applicable, will consider all of the relevant facts and circumstances
available, including (if applicable) but not limited to: the benefits to us; the impact on a director’s independence in
the event the related person is a director, an immediate family member of a director, or an entity in which a director has a position
or relationship; the availability of other sources for comparable products or services; the terms of the transaction; and the
terms available to unrelated third parties or to employees generally. The Audit Committee or the Chairperson of the Audit Committee,
as applicable, will only approve related-person transactions that are in, or are not inconsistent with, the best interests of
the Company and its stockholders, as the Audit Committee or the Chairperson of the Audit Committee determines in good faith.
No member of the Audit Committee shall participate
in any review, consideration or approval of any related-person transaction with respect to which such member or any of his or
her immediate family members is the related person.
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 34
Security Ownership of Certain Beneficial
Owners and Management
The following table sets forth information
relating to the beneficial ownership of our common stock as of March 7, 2025 (unless otherwise indicated in the footnotes), by:
| • | each
person, or group of affiliated persons, known by us to beneficially own more than 5%
of our outstanding shares of common stock; |
| • | each
of our directors and nominees; |
| • | each
of our named executive officers; and |
| • | all
current directors and executive officers as a group. |
The number of shares beneficially owned
by each entity, person, director or executive officer is determined in accordance with the rules of the SEC, and the information
is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any
shares over which the person has sole or shared voting power or investment power as well as any shares that the person has the
right to acquire within 60 days through the exercise of any stock options, warrants or other rights. We believe, based on the
information furnished to us, that except as otherwise indicated, and subject to applicable community property laws, the persons
named in the table below have sole voting and investment power with respect to all shares of common stock held by that person.
The percentage of shares beneficially owned
is computed on the basis of 93,892,528 shares of our common stock outstanding as of March 7, 2025. Shares of our common stock
that a person has the right to acquire within 60 days are deemed outstanding for purposes of computing the percentage ownership
of the person holding such rights, but are not deemed outstanding for purposes of computing the percentage ownership of any other
person, except with respect to the percentage ownership of all directors and executive officers as a group. Unless otherwise indicated
below, the address for each beneficial owner listed is c/o Ultragenyx Pharmaceutical Inc., 60 Leveroni Court, Novato, California
94949.
| |
|
Beneficial Ownership |
| Name and Address of Beneficial Owner |
|
Number of Shares |
|
% of Total
|
| Stockholders Owning Greater than 5%: |
|
|
|
|
| The Vanguard Group(1) |
|
8,673,432 |
|
9.2% |
| BlackRock, Inc.(2) |
|
5,238,400 |
|
5.6% |
| Directors and Named Executive Officers: |
|
|
|
|
| Deborah Dunsire, M.D.(3) |
|
70,210 |
|
* |
| Matthew K. Fust(4) |
|
61,590 |
|
* |
| Michael Narachi(5) |
|
68,335 |
|
* |
| Amrit Ray, M.D.(6) |
|
35,160 |
|
* |
| Corsee D. Sanders, Ph.D.(7) |
|
27,274 |
|
* |
| Shehnaaz Suliman, M.D.(8) |
|
61,585 |
|
* |
| Daniel G. Welch(9) |
|
68,335 |
|
* |
| Emil D. Kakkis, M.D., Ph.D.(10) |
|
3,293,909 |
|
3.5% |
| Eric Crombez, M.D.(11) |
|
98,113 |
|
* |
| Erik Harris(12) |
|
184,922 |
|
* |
| Howard Horn(13) |
|
78,344 |
|
* |
| John R. Pinion II(14) |
|
336,097 |
|
* |
| All current executive officers and directors
as a group(15) (15 persons) |
|
5,261,347 |
|
5.5% |
| * | Indicates
beneficial ownership of less than 1% of the total outstanding common stock. |
| (1) | Based
on information set forth in a Schedule 13G/A filed with the SEC on February 13, 2024
by The Vanguard Group. The Schedule 13G/A reported that, as of December 29, 2023, The
Vanguard Group has shared voting power with respect to 33,125 shares, sole dispositive
power with respect to 8,556,929 shares, and shared dispositive power with respect to
116,503 shares. The principal business address for The Vanguard Group is listed in such
filing as 100 Vanguard Blvd., Malvern, PA 19355. |
| (2) | Based
on information set forth in a Schedule 13G/A filed with the SEC on January 29, 2024 by
BlackRock, Inc. The Schedule 13G/A reported that, as of December 31, 2023, BlackRock,
Inc. has sole voting power with respect to 4,967,673 shares and sole dispositive power
with respect to 5,238,400 shares. The principal business address for BlackRock Inc. is
listed in such filing as 50 Hudson Yards, New York, NY 10001. |
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 35
| (3) |
Consists of (a) 19,730 shares
of common stock, (b) 50,480 shares of common stock issuable pursuant to stock options exercisable within 60 days of the date
of this table. |
| (4) |
Consists of (a) 14,860 shares of common stock
and (b) 46,730 shares of common stock issuable pursuant to stock options exercisable within 60 days of the date of this table.
|
| (5) |
Consists of (a) 21,605 shares of common stock
and (b) 46,730 shares of common stock issuable pursuant to stock options exercisable within 60 days of the date of this table.
|
| (6) |
Consists of (a) 10,573 shares of common stock
and (b) 22,880 shares of common stock issuable pursuant to stock options exercisable within 60 days of the date of this table,
and (c) 1,707 shares of common stock issuable pursuant to the vesting of RSUs within 60 days of the date of this table. |
| (7) |
Consists of (a) 6,664 shares of common stock
and (b) 20,610 shares of common stock issuable pursuant to stock options exercisable within 60 days of the date of this table.
|
| (8) |
Consists of (a) 14,855 shares of common stock
and (b) 46,730 shares of common stock issuable pursuant to stock options exercisable within 60 days of the date of this table.
|
| (9) |
Consists of (a) 21,605 shares of common stock
and (b) 46,730 shares of common stock issuable pursuant to stock options exercisable within 60 days of the date of this table.
|
| (10) |
Consists of (a) 2,158,985 shares of common
stock held by the Emil Kakkis and Jenny Soriano Living Trust, dated June 18, 2009, (b) 533,532 shares of common stock held
by Dr. Kakkis and (c) 601,392 shares of common stock issuable pursuant to stock options exercisable within 60 days of the
date of this table 4. Dr. Kakkis shares voting and dispositive power over the 2,158,985 shares of common stock held by the
Emil Kakkis and Jenny Soriano Living Trust, dated June 18, 2009; each of Dr. Kakkis and Dr. Soriano is a trustee of such trust.
Dr. Kakkis has sole voting and dispositive power over the 533,532 shares of common stock held by him and the 2,158,985 shares
of common stock issuable pursuant to stock options held by Dr. Kakkis. |
| (11) |
Consists of (a) 32,081 shares of common stock
and (b) 64,367 shares of common stock issuable pursuant to stock options exercisable within 60 days of the date of this table,
and (c) 1,665 shares of common stock issuable pursuant to the vesting of RSUs within 60 days of the date of this table. |
| (12) |
Consists of (a) 41,992 shares of common stock
and (b) 142,930 shares of common stock issuable pursuant to stock options exercisable within 60 days of the date of this table.
|
| (13) |
Consists of (a) 15,692 shares of common stock
and (b) 62,652 shares of common stock issuable pursuant to stock options exercisable within 60 days of the date of this table.
|
| (14) |
Consists of (a) 66,084 shares of common stock
and (b) 270,013 shares of common stock issuable pursuant to stock options exercisable within 60 days of the date of this table.
|
| (15) |
Consists of (a) 3,300,328 shares of common
stock, (b) 1,957,647 shares of common stock issuable pursuant to stock options that are exercisable within 60 days of the
date of this table, and (c) 3,372 shares of common stock issuable pursuant to the vesting of RSUs within 60 days of the date
of this table. |
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 36
Executive Compensation
Compensation Discussion and Analysis
The following compensation discussion and analysis describes
the material elements of compensation earned in fiscal 2024 by each of the executive officers identified below, who are referred
to collectively as our “named executive officers.” Our named executive officers with respect to the fiscal year that
ended on December 31, 2024 are:
| |
|
 |
|
|
|
 |
|
|
|
 |
|
|
|
 |
|
|
|
 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
EMIL
D. KAKKIS, M.D., PH.D.
President and Chief Executive Officer |
|
HOWARD
HORN
Chief Financial Officer, Corporate Strategy and Executive Vice President |
|
ERIK
HARRIS
Chief Commercial Officer and Executive Vice President |
|
ERIC
CROMBEZ, M.D.
Chief Medical Officer and Executive Vice President |
|
JOHN
R. PINION II
Chief Quality Officer
and Executive
Vice President
of Translational
Sciences |
|
These persons constitute our principal executive officer, our
principal financial offer and our three other most highly paid executive officers serving during fiscal 2024.
Business Highlights
2024 was a pivotal year for the
Company as we expanded access and grew revenue from our four global commercial products while also advancing our six
late-stage programs in serious genetic conditions. The following company performance highlights from the year directly
contributed to achievement under our 2024 annual incentive program.
Financial and Commercial
Highlights in 2024
| • | Raised
our total revenue guidance in mid-2024 to the range of $530 million to $550 million
and exceeded the upper end of the revised guidance range with total revenue of $560 million
for the year. Total revenue in 2024 represented 29% growth compared to 2023. |
| • | Generated
2024 Crysvita® product sales from Latin America and Turkey, where we lead
commercialization activities of $135 million, representing 78% growth compared to 2023.
|
| • | Continued
commercial expansion outside of the United States with Evkeeza® launches
in Canada, Europe and Japan. |
Clinical Program Highlights
in 2024
| • | UX143
for the treatment of Osteogenesis Imperfect (OI). |
| ■ | Announced
positive 14-month results from the Phase 2 portion of the ongoing Phase 2/3 Orbit
study demonstrating that, as of the data cut-off date, treatment with setrusumab
continued to show statistically significant reductions in the incidence of fractures
in patients with OI compared to the pre-treatment period and also resulted in ongoing
and meaningful improvements in lumbar spine bone mineral density at month 12 without
evidence of plateau. |
| ■ | Received
Breakthrough Designation from the FDA as a treatment to reduce the risk of fracture associated
with OI Type I, III, or IV in patients 2 years of age and older. |
| • | GTX-102
for the treatment of Angelman syndrome |
| ■ | Announced
positive interim data from the Phase 1/2 study reflecting that as of the data cut-off
date, patients in the Expansion Cohorts A & B treated with GTX-102 showed rapid and
clinically meaningful improvement across multiple domains consistent with or exceeding
Dose Escalation Cohorts 4-7 data at Day 170. As of the data cut-off date, treatment of
the Dose Escalation Cohorts 4-7 showed long-term increasing and sustained clinical benefit
far exceeding natural history data at Day 758. |
| ■ | Began
enrollment in our global Phase 3 Aspire study. |
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 37
| • | UX111
for the treatment of Sanfilippo syndrome type A (MPS IIIA) |
| ■ | Jointly
sponsored a workshop with the Regan-Udall Foundation for the FDA on qualifying biomarkers
to support accelerated approval in rare disease drug development – an example of
the way we are leading and driving changes in the field of rare disease. |
| ■ | Submitted
our BLA to the FDA, which was subsequently accepted and granted priority review by the FDA, positioning UX111 to becoming
our first commercialized gene therapy product, if approved. |
| • | DTX401
for the treatment of Glycogen Storage Disease Type Ia, or GSDIa |
| ■ | Announced
positive topline results in May 2024 from our Phase 3 GlucoGene study for the
treatment of patients aged eight years and older with the study achieving its primary
endpoint, demonstrating that treatment with DTX401 resulted in a statistically significant
and clinically meaningful reduction in daily cornstarch intake compared with placebo
at Week 48. In November 2024, we announced positive updated, longer-term Phase 3 data
from the study. |
| • | UX701
for the treatment of Wilson disease |
| ■ | Announced
positive data from the dose-finding stage of the Cyprus2+ study of UX701 that
demonstrated clinical activity as well as improvements in copper metabolism for patients
treated in Stage 1 of the study and our plans to initiate a fourth dose-finding cohort
to enhance the efficiency and efficacy of the gene therapy. |
| • | DTX301
for the treatment of Ornithine Transcarbamylase, or OTC, deficiency |
| ■ | Continued
enrollment of our Phase 3 study of DTX301. |
Stockholder Outreach
Stockholder Advisory Vote on Executive Compensation
Each year, our stockholders are provided
the opportunity to cast an advisory vote on the compensation of our named executive officers, or the “say-on-pay”
vote, and the Compensation Committee considers the outcome of the prior year’s say-on-pay vote when making decisions relating
to the compensation of our named executive officers and our executive compensation programs. We received 74% support for our say-on-pay
proposal at our 2024 Annual Meeting. While this result reflects broad support of our compensation philosophy and pay practices,
we were nevertheless disappointed by the outcome. In light of this result, we are continuing to engage with our stockholders as
we have in prior years, reflecting our commitment to engagement, communication and transparency.
Stockholder Engagement
We regularly engage with our stockholders
to solicit their feedback on a variety of topics, including our equity plan, executive compensation, corporate governance, corporate
responsibility and other topics in order to gain a better understanding of their perspective on these issues. Stockholder feedback
is important, and the information we glean from these engagements is highly valued. Stockholders also regularly meet with members
of our senior management team to discuss our strategy and review our business, goals and performance. During our recent engagement
season, we reached out to stockholders representing approximately 70% of our outstanding shares as of September 30, 2024, including
almost all of our top 25 largest stockholders and met with those who responded to our engagement request, representing approximately
25% of our outstanding shares as of September 30, 2024. Participants at these meetings included our Chief Legal Officer, our Chief
Human Resources Officer and Michael Narachi, an independent director and Chairperson of our Compensation Committee. Topics discussed
during the meetings included our equity plan, executive compensation, corporate governance and sustainability matters. Our Board
and management team reviewed and considered feedback received throughout our engagement activities and adopted certain changes
in response to such feedback as described below.
Outcomes from Engagement
| Feedback
Received |
|
Action
Taken |
| More of executive’s compensation
should be based on performance and “at risk” |
 |
We have increased the percentage of PSUs from 33% to
50% for our executive officers (excluding our Chief Executive Officer) for the 2025 equity awards. For our Chief Executive
Officer, we maintained the percentage of PSUs of 60% for 2024 and 2025 annual grants.
We have also incorporated a “cap” on the
portion of the PSUs subject to relative TSR performance for 2025 such that no more than the target number of PSUs may
be earned if our absolute TSR is negative, regardless of our relative TSR performance.
|
| Greater transparency related to corporate
goals and goals achievement |
 |
We have included a more detailed summary regarding our
bonus program and the impact of corporate goals and individual goals achievements on the bonus payout.
We have also included additional detailed disclosure
related to achievement of our corporate goals, including the achievement of each goal category. See the section below
entitled “Annual Bonuses”.
|
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 38
Compensation Highlights
As described below under “Compensation
Philosophy and Objectives”, our Compensation Committee believes that executive compensation should be directly linked to
short-term and long-term performance and should result in long-term value creation for stockholders. A few of the key decisions
made by the Compensation Committee aligned with such philosophy are as follows:
| • | Modest
(or no) base salary adjustments in 2024; no base salary increase for CEO in 2025: Base
salary increases in 2024 were 4% for Dr. Kakkis, our Chief Executive Officer, and 3%
for each of Mr. Harris and Mr. Pinion. Mr. Horn who joined our company in October 2023
did not receive a base salary increase in 2024. Dr. Crombez received a base salary increase of 15% to align closer
to the median for similar roles at peer companies. Salary increases were generally based
on competitive market positioning and individual performance and were aligned to increases
provided to all employees in good performance standing. In 2025, Dr. Kakkis opted not to receive
a base salary increase. |
| • | Increasing
emphasis on performance-based awards: We maintained our equity mix at one-third
of each PSUs, RSUs and stock options for named executive officers other than our Chief
Executive Officer for 2024 annual grants but we increased the percentage of PSUs to 50%
for the 2025 annual grant for our named executive officers (other than our Chief Executive
Officer) without increasing the size of the pay package. For our Chief Executive Officer,
PSUs were 60% of his annual grants in 2024 and 2025, consistent with the allocation implemented
in 2023. |
| • | Lengthening
the time horizon for strategic performance portion of the PSUs: We lengthened
the performance period for the strategic performance portion of the PSUs from two years
to three years and, in doing so, we will require sustained performance over a longer-term
timeframe. |
| • | Increasing
emphasis on relative TSR portion and the strategic performance portion of PSU awards:
We shifted the PSU award mix to 1/3 for each of the revenue portion (decrease),
strategic performance portion (increase), and relative TSR portion (increase) to add
rigor and complexity to our PSU program. |
| • | Further
limit PSU upside: Beginning in 2025, the relative TSR portion of the PSUs is
subject to a “cap” such that no more than the target number of PSUs may be
earned if our absolute TSR is negative, regardless of our relative TSR performance. |
| • | PSU
payouts linked to performance: Our revenue portion of the 2023 PSUs paid out
below target, at 74%. |
| • | Pay
mix is highly “at risk”: The percentage of pay that is “at
risk” for our CEO and named executive officers is 94% and 85%, respectively, helping
us align pay with performance. |
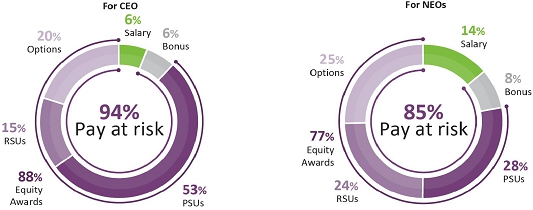
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 39
CEO Realizable Compensation
To ensure ongoing alignment to the compensation
philosophy, in 2024 our Compensation Committee evaluated the relationship between the target and realizable value of the compensation
granted to our CEO from 2022 through 2024.
The average realizable pay of our CEO
over 2022 through 2024 is lower than the average target compensation for such three-year period, primarily as a result of the
decrease in value of in-flight equity awards resulting from our stock performance as of the end of 2024 and the payouts
from PSUs issued in 2022 and 2023 that paid out below target, as reflected in the following chart and described below.
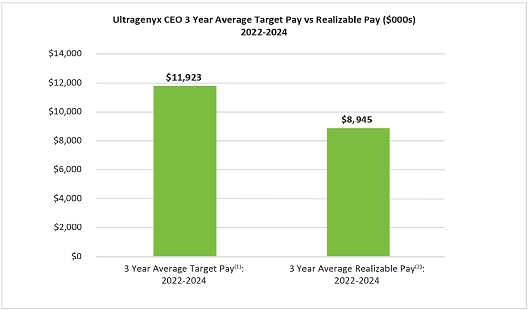
| (1) |
Target value represents the base salary rate in effect
in each year, target annual bonus, and the grant date fair value of long-term incentive awards (PSUs and RSUs). |
| (2) |
Realizable value is calculated as
the sum of the compensation deliverable in each year, including actual salary paid, annual bonus earned, and the value of
long-term incentive plan components (PSUs, RSUs, and stock options) as valued on December 31, 2024 using our year-end stock
price of $42.07 per share. The realizable value for PSUs granted in 2022 was adjusted to reflect the recent payout at 25%
of target. The realizable value for PSUs granted in 2023 was adjusted to reflect recent payouts based on Revenue and Strategic
performance, and the Relative TSR payout was assumed to be at 100% of target. The realizable value for PSUs granted in 2024
(for which the performance period has not ended) assumes payout at 100%. |
Compensation Philosophy and Objectives
Our philosophy in setting compensation policies
for executive officers has two fundamental objectives: (1) to attract and retain a highly-skilled team of executives and (2) to
align our executives’ interests with those of our stockholders by rewarding short-term and long-term performance and tying
compensation to increases in stockholder value. The Compensation Committee believes that executive compensation should be directly
linked to both continuous improvements in corporate performance (pay for performance) and accomplishments that are expected to
increase stockholder value. In furtherance of this goal, the Compensation Committee has adhered to the following guidelines as
a foundation for decisions that affect the levels of compensation:
| • | provide
a competitive total compensation package that enables us to attract and retain highly
qualified executives with the skills and experience required for the achievement of business
goals; |
| • | align
compensation elements with our annual goals and long-term business strategies and objectives; |
| • | promote
the achievement of key strategic and financial performance measures by linking short-term
and long-term cash and equity incentives to the achievement of measurable corporate and
individual performance goals and objectives; and |
| • | align
executives’ incentives with the creation of stockholder value. |
The Compensation Committee has historically
compensated executive officers with three primary compensation components: a base salary, an annual bonus opportunity, and equity-based
compensation. The Compensation Committee believes that cash compensation in the form of base salary and an annual bonus opportunity
provides our executive officers with short-term rewards for success in achieving annual goals and objectives, and that long-term
compensation through the award of stock options, RSUs and PSUs aligns the objectives of management with those of our stockholders
with respect to long-term performance and success of the Company.
In setting compensation levels for our executive
officers, the Compensation Committee does not formulaically benchmark against any one specific reference point. Instead, it considers
a variety of factors, including peer group survey data, tenure, role, responsibilities, performance, and competitive market practices.
Compensation paid to our named executive officers is delivered primarily through at-risk pay, based on both short-term and long-term
incentives, including the achievement of corporate and individual goals and objectives.
In addition to our compensation elements,
the following compensation program features are designed to align our executive team’s interests with stockholder interests
and market best practices.
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 40
| What
We Do |
|
What
We Don’t Do |
| |
|
|
|
|
 |
Our Compensation Committee is comprised solely of independent directors. |
|
 |
We do not offer any tax gross-up payments to our executive team for any
change-of-control payments. |
 |
Our Compensation Committee engages its own independent, external compensation
consultant to assist the committee in its review of executive and director compensation practices. |
|
 |
As discussed above under “—Corporate Governance”, we
prohibit our employees, including our executive officers, from hedging our securities. |
 |
We proactively engage with our stockholders throughout the year. |
|
 |
We prohibit our employees, including our executive team, from pledging
our securities. |
 |
We have a clawback policy that permits recovery of all incentive compensation
(including time-based and performance-based equity awards) in the event of fraud or intentional misconduct by our current
or former executive officers; see “—Clawback Policy”. |
|
 |
We do not offer our executive team any substantially enhanced benefits
or perquisites when compared with our overall employee population. |
 |
We require our executive officers and directors to hold shares of our common
stock in order to align their long-term interests with those of our stockholders; see “—Minimum Stock Ownership
Requirements”. |
|
 |
We prohibit the repricing of outstanding stock options without stockholder
approval. |
 |
We have double-trigger vesting of outstanding equity awards following covered
transactions under our employment arrangements with our executive officers. See “Summary Compensation Table—Narrative
Disclosure to Summary Compensation Table—Covered Transaction”. |
|
 |
The Second A&R 2023 Plan does not include an “evergreen”
feature. |
 |
We have established a long-term incentive program applicable to all current
employees, including our executive officers, to further tie compensation to performance and focus employee efforts on corporate
goals and objectives; see “—Equity Compensation—Annual Equity Grants in Fiscal 2024” and “—Fiscal
2025 Compensation – Equity Grants.” |
|
|
|
 |
We hold an annual say-on-pay vote for stockholders. |
|
|
|
Roles in Determining Compensation
Compensation Committee
The Board has delegated to the Compensation
Committee the responsibility to ensure that total compensation paid to our executive officers, including our named executive officers,
is consistent with our compensation policy and objectives. The Compensation Committee oversees and approves all compensation arrangements
and actions for our executive officers, including our named executive officers. While the Compensation Committee draws on a number
of resources, including input from the Board, the Chief Executive Officer, and its independent compensation consultants, to make
decisions regarding our executive compensation program, ultimate decision-making authority rests with the Compensation Committee.
The Compensation Committee retains discretion over base salaries, annual bonuses, and equity compensation for executive officers.
The Compensation Committee relies upon the judgment of its members in making compensation decisions, after reviewing our corporate
performance and carefully evaluating an executive’s performance during the year against established goals, operational performance,
and business responsibilities. In addition, the Compensation Committee utilizes discretion in the assessment process to respond
to and adjust to a dynamic business environment. The Compensation Committee may delegate its authority to one or more subcommittees.
The Compensation Committee may also delegate authority to review and approve the compensation of our employees to certain of our
executive officers, including the grant of equity awards under the 2023 Plan (as amended and restated).
Compensation Consultant
The Compensation Committee retains the services
of an independent, external compensation consultant, Aon’s Human Capital Solutions Practice, a division of Aon plc (Aon).
The mandate of the consultant is to assist the Compensation Committee in its review of executive and director compensation practices,
including the competitiveness of pay levels, executive compensation design, benchmarking with our peers in the industry, and other
technical considerations, including tax- and accounting-related matters. The Compensation Committee annually evaluates Aon’s
performance and determines whether to engage Aon or another compensation consultant and has the final authority to engage and
terminate Aon’s services. In 2024, the cost of Aon’s consulting services related to executive compensation, director
compensation and equity plan design and considerations provided to the Compensation Committee was approximately $208,597. In
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 41
addition, in 2024, management also engaged
Aon to provide survey data relating to broad-based compensation matters. The aggregate cost of the survey data provided by Aon
in 2024 was approximately $33,205. No other consulting services were provided by Aon or its affiliates to the Company in 2024.
Our Compensation Committee has assessed
the independence of Aon consistent with Nasdaq listing standards and has concluded that the engagement of Aon does not raise any
conflict of interest.
Chief Executive Officer
The Chief Executive Officer, and other executive
officers, attends Compensation Committee meetings and works with the Compensation Committee Chairman and Aon to develop compensation
recommendations for the executive officers (excluding the Chief Executive Officer), based upon individual experience and breadth
of knowledge, internal considerations, individual performance during the fiscal year, competitive market considerations, and other
factors deemed relevant by the Compensation Committee. The recommendations are then submitted to the Compensation Committee for
review and consideration. The Compensation Committee works directly with Aon and the other non-employee directors of the Board
to evaluate the performance of the Chief Executive Officer and determine compensation actions for the Chief Executive Officer,
and the Chief Executive Officer does not participate in the portions of meetings in which his compensation is discussed and determined.
Defining and
Comparing Compensation to Peer Group Benchmarks
While we do not establish compensation levels
based solely on benchmarking, pay practices at other companies are an important factor that the Compensation Committee considers
in assessing the reasonableness of compensation and ensuring that our compensation practices are competitive in the marketplace.
Market data is one element considered by the Compensation Committee when making executive compensation decisions, but the Compensation
Committee does not set compensation levels based solely on market data. Rather, the Compensation Committee reviews the 25th,
50th and 75th percentiles of relevant market data as one frame of reference in making its executive compensation
decisions. Final executive compensation decisions reflect a variety of factors, including each executive’s experience, performance
rating, the relative importance of the executive’s role within the organization, as well as where each executive’s
pay level falls relative to the market data.
In order to evaluate the level of compensation
for our named executive officers for 2024, our Compensation Committee, using information provided by Aon, established a peer group
of publicly traded, national, and regional companies in the biopharmaceutical and biotechnology industries based on a balance
of the following criteria:
| • | companies
with emphasis on orphan pharmaceutical products; |
| • | companies
with comparable market capitalizations (i.e., in the range of $2 billion to $16 billion); |
| • | companies
with revenue of between $200 million and $1.4 billion; and |
| • | companies
with headcounts between 300 to 4,000 employees. |
Our 2024 peer
group is comprised of the following 20 companies in the pharmaceutical and biotechnology industries, reflecting the removal of
Alnylam Pharmaceuticals Inc., Biohaven Pharmaceutical Holding Company, Ltd., Global Blood Therapeutics, Inc. and United Therapeutics
Corporation from our peer group used to evaluate compensation for our named executive officers in 2023 in order to add BridgeBio
Pharma, Inc. Intra-Cellular Therapies Inc. and Sage Therapeutics,
Inc. who our Compensation Committee determined better fit the balanced criteria described above.
| ACADIA Pharmaceuticals Inc. |
|
BridgeBio Pharma, Inc. |
|
Ionis Pharmaceuticals, Inc. |
| Alkermes plc |
|
Corcept Therapeutics Inc. |
|
Jazz Pharmaceuticals plc |
| Amicus Therapeutics |
|
Exelixis Inc. |
|
Neurocrine Biosciences, Inc. |
| Apellis Pharmaceuticals, Inc. |
|
FibroGen, Inc. |
|
PTC Therapeutics, Inc. |
| BioCryst Pharmaceuticals, Inc. |
|
Halozyme Therapeutics |
|
Sage Therapeutics, Inc. |
| BioMarin Pharmaceutical Inc. |
|
Insmed Incorporated |
|
Sarepta Therapeutics, Inc. |
| Blueprint
Medicines Corporation |
|
Intra-Cellular
Therapies Inc. |
|
|
We believe that the
compensation practices of our 2024 peer group provided us with appropriate benchmarks for evaluating the compensation of our
named executive officers for 2024 because of the developmental, market and organizational characteristics we shared with our
peer group. At the time that we selected our 2024 peer group we were at approximately the 26th percentile of the
peer group in terms of market capitalization, the 81st percentile in terms of employee headcount and the 44th percentile
in terms of revenue.
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 42
Annual Performance Reviews
Our Compensation Committee conducts an annual performance review of our named executive officers and approves their compensation. By the end of the first quarter of each year, base salaries and equity awards for the fiscal year are approved and, for purposes of determining potential payments under our corporate bonus plan (the bonus plan), target bonuses, annual corporate goals and individual performance objectives are established and set forth in writing. After the end of each year, our Compensation Committee determines the amounts that will be paid to our executive officers under our bonus plan after carefully (1) reviewing overall corporate performance; (2) evaluating each named executive officer’s annual performance against established corporate goals; and (3) in the case of executive officers other than our Chief Executive Officer, reviewing the achievement of individual performance objectives.
At its first regularly scheduled meeting
each year, our Compensation Committee, with input from the Board, evaluates our Chief Executive Officer’s individual performance,
determines whether to adjust his base salary, and determines the amount of equity awards and his bonus, if any, under our bonus
plan.
Our Compensation Committee may also review
and adjust the compensation of our executive officers throughout the course of the year.
Base Salary
Overview
The Compensation Committee believes it is
important to provide adequate fixed compensation to our executive officers working in a highly volatile and competitive industry.
The Compensation Committee’s choice of actual pay levels versus our competitive market reflects consideration of our stockholders’
interests in paying what is necessary to achieve our corporate goals, while setting competitive levels which are essential to retain
these key executives. In determining appropriate base salary levels for a given executive officer, the Compensation Committee considers
the following factors:
| • |
individual performance of, and overall management of the function by, the executive, as well as our overall corporate performance, during the prior year; |
| • |
level of responsibility, including breadth, scope, and complexity of the position; |
| • |
level of experience and expertise of the executive; |
| • |
internal review of the executive’s compensation relative to other executives to ensure internal equity; |
| • |
executive officer compensation levels at other similar companies to ensure competitiveness; and |
| • |
recruiting and retention market dynamics. |
The effective date of annual merit increases to base salary is March 1.
2024 Base Salaries
The Compensation Committee engaged Aon to
conduct a competitive review and analysis of our current executive compensation program relative to our 2024 peer group. Aon prepared
an Executive Compensation Assessment report in February 2024 that provided a competitive assessment of our executive compensation
program as compared to the 2024 peer group data for base salaries, target total cash compensation, and equity compensation.
For 2024, base salary increases for our
Chief Executive Officer was 4% annualized and approximately 3% annualized for Mr. Harris and Mr. Pinion. Mr. Horn who joined
our company in October 2023 did not receive a base salary increase for 2024. Dr. Crombez received a base salary increase of
15% following an independent compensation market analysis, which identified that his prior base salary was below the median
for similar roles at peer companies. This adjustment brought his salary into line with market norms for the position while
ensuring competitiveness in attracting and retaining talent. The overall 2024 merit budget was based on an Aon trend report
regarding projected market merit spends for 2024. Individual increases in base salaries were also based on achievement of
2023 individual goals.
The following table shows the increases in
base salaries for our named executive officers between fiscal 2023 and fiscal 2024. Mr. Horn joined the Company in October 2023
and as such, did not receive an increase in his base salary for fiscal 2024.
| Name | |
Title | |
Fiscal 2023
Base Salary (as of
December 31, 2023) | | |
Fiscal 2024
Base Salary (as of
December 31, 2024) | | |
Percentage
Increase
(%) |
| Emil D. Kakkis, M.D., Ph.D. | |
President and Chief Executive Officer | |
$ | 828,000 | | |
$ | 861,000 | | |
4.0 |
| Howard Horn | |
Chief Financial Officer, Corporate Strategy and Executive Vice President | |
| 590,000 | | |
| 590,000 | | |
— |
| Erik Harris | |
Chief Commercial Officer and Executive Vice President | |
| 589,000 | | |
| 607,000 | | |
3.1 |
| Eric Crombez, M.D. | |
Chief Medical Officer and Executive Vice President | |
| 525,000 | | |
| 604,000 | | |
15.0 |
| John R. Pinion II | |
Chief Quality Officer and Executive Vice President, Translational Sciences | |
| 555,000 | | |
| 572,000 | | |
3.1 |
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 43
Annual Bonus
Overview
Our annual incentive program provides the
opportunity for cash bonus awards based on the achievement of annual performance goals. For executive officers, other than the
Chief Executive Officer, bonus awards are based on both corporate and individual performance, with corporate performance serving
as the primary driver.
| • |
Corporate performance goals focus on business, financial, and operational measures or objectives. |
| • |
Individual performance goals reflect an executive’s contributions toward achieving corporate goals and leadership within their respective function. |
| • |
The Chief Executive Officer’s bonus is entirely based on corporate performance. |
At the beginning of each performance year,
the Chief Executive Officer sets and communicates individual goals to each executive officer. At the end of the year, the Compensation
Committee evaluates corporate performance and each executive officer’s contributions (excluding the Chief Executive Officer)
to determine whether a bonus will be awarded and, if so, the amount. The annual bonus is not guaranteed and is entirely at risk.
The Chief Executive Officer’s annual
bonus award is based 100% on corporate performance achievement.
For executive officers other than the Chief
Executive Officer, the annual bonus is calculated as follows:

While individual performance is a key factor,
corporate performance is the primary driver in the formula. If corporate goals are not met, an executive may not receive an annual
bonus award, even if the executive performed well individually. Similarly, if corporate goals are met, an executive with low individual
performance may receive a reduced award or no bonus award.
The corporate performance achievement percentage
is capped at 150% and the individual performance achievement percentage is capped at 133%, resulting in a maximum bonus of 150%
of target for the Chief Executive Officer and 200% of target for the other executive officers. The corporate performance score
determines the overall bonus pool funding. If corporate performance achievement is at or below 50%, the corporate component of
the bonus is not funded and no bonuses will be awarded, regardless of individual performance.
Actual payouts are at risk and contingent
on both corporate and individual performance. Corporate performance payouts are based on the achievement of predefined goals, while
individual performance is assessed across three key areas: attainment of individual goals, execution of key role deliverables,
and demonstration of the Company’s values and behaviors.
Subject to the rights contained in any agreement
between the Company and the executive officer, an executive officer must be employed by the Company on the bonus payment date to
be eligible to receive a bonus payment.
Fiscal 2024 Bonuses
Annual corporate goals for fiscal 2024 were
proposed by our executive officers and approved by our Compensation Committee in early 2024. Individual objectives for our executive
officers, other than the Chief Executive Officer, for 2024 were proposed by each executive officer, with review, input and confirmation
from our Chief Executive Officer.
Each executive officer’s annual target
bonus is based on their role, responsibilities, accountability, potential impact on corporate performance and peer group benchmarks.
The target bonuses, as a percentage of base salary, for the named executive officers for fiscal 2024 are set forth in the following
table.
| Name |
|
Target Bonus
for Fiscal 2024
(% of Base Salary) |
| Emil D. Kakkis, M.D., Ph.D. |
|
80 |
| Howard Horn |
|
50 |
| Erik Harris |
|
50 |
| Eric Crombez, M.D. |
|
50 |
| John R. Pinion II |
|
50 |
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 44
In February 2025, the Compensation Committee
assessed our overall 2024 performance against the achievement of the corporate goals to determine a total percentage of achievement
between 0% and 150%. The Compensation Committee considered the following performance goals, as well as the relative weighting of
these goals, in assessing overall performance for the 2024 fiscal year:
| Goal (Weighting) |
|
Key Results |
|
Achievement
Against Goal |
|
Weighted
Achievement |
Commercial (30%)
Revenue goals for commercial assets |
|
Exceeded. Achieved revenue targets between base and outperform |
|
123% |
|
37% |
Development (60%)
Clinical program-based goals focused on clinical development and enrollment-targets and milestone, including enrollment of the phase 3 portion of our study for UX143 and the BLA filing for UX111 by specified timelines. Highest weight was placed on goals related to GTX-102, UX143, UX701 and DTX401. |
|
Met. Overall achievement of base target for development goals |
|
100% |
|
60% |
| People and Governance (10%) |
|
Met most. As summarized below, met or exceeded goals related to culture and governance. |
|
70% |
|
7.0% |
|
• Budget:
Maintaining operating cash usage within 5% of budget (5%)
|
|
• Did not meet. Minimum target goal was not achieved primarily due to expenses from acceleration of select manufacturing and development activities as well as timing of certain cash flows (0% achievement)
|
|
• Culture: Maintain high retention of employees in good performance standing (1.5%)
|
|
• Exceeded. Retention rate exceeded outperform target (150% achievement)
|
• Culture: Goals related to maintaining and improving employee engagement, inclusion and well being (each 0.5%, together
1.5%) |
|
• Employee engagement: Met base target for goal (100% achievement)
• Inclusion: Exceeded outperform goal (150% achievement)
• Well-being: Met base target for goal (100% achievement)
|
|
• Governance: Goals related to continuous compliant and ethical practices (2%) |
|
• Exceeded. Achieved outperform target goals (150% achievement) |
In establishing these goals, the Compensation
Committee selected performance goals that it considered aggressive, meaning that they are goals that were considered achievable,
but only with a high degree of diligence and success in execution.
In assessing performance against these goals,
the Compensation Committee reviewed each goal and determined whether or not it was achieved. The Compensation Committee then referred
to the relative importance of the goals, based on the previously established weightings of each goal. The Compensation Committee
also considered additional key corporate achievements that were not represented in the 2024 corporate goals, including completion
of a successful financing transaction and significant work to gain alignment with the FDA to support the submission of the BLA
for UX111 for accelerated approval. For all goals combined, including additional achievements by the Company not previously defined,
the Compensation Committee determined an overall 110% achievement for fiscal year 2024.
In February 2025, in addition to assessing
the foregoing corporate goals, the Compensation Committee (after consultation with our Chief Executive Officer) assessed the individual
accomplishments of our named executive officers for purposes of determining the individual component of their annual bonus, other
than our Chief Executive Officer whose annual bonus is based solely on the corporate goals described above. Key individual achievements
for these named executive officers are summarized below.
| Named Executive Officer |
|
Key 2024 Achievements |
Howard Horn
Chief Financial Officer and EVP, Corporate Strategy |
|
• Successful
execution of follow on equity offering for gross proceeds of $403 million
• Informed
decision making and drove execution of business development transactions, 2024 strategy and budget and long-range plan
• Execution
of defined operational excellence projects in collaboration with business teams
|
Erik Harris
Chief Commercial Officer and EVP |
|
• Achieved
continued growth in product sales to support achievement of global revenue targets, including exceeding top range of revenue guidance
• Executed
commercial planning for launch readiness for late-stage programs
• Retention
of key contributors for commercial leadership team
|
Eric Crombez, M.D.
Chief Medical Officer and EVP |
|
• Led
development team in achievement of high priority clinical milestones
• Effective
resource planning and right-sizing of development teams to achieve milestones
• Continued
close collaboration with cross-functional executive team to elevate development team performance and drive the business
|
John R. Pinion II
Chief Quality Officer and Executive Vice President, Translational Sciences |
|
• Led
and sponsored non-clinical activities to enable development and advancement of translational science portfolio
• Successfully
led start-up and advancement of Amlogenyx operations and other specified pre-clinical development, per plan
• Led
reliable quality supply across commercial and clinical products to support patient needs, revenue growth and clinical program success
|
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 45
Dr. Kakkis evaluated the performance of Mr.
Horn, Mr. Harris, Dr. Crombez and Mr. Pinion after considering the above achievements and provided a proposed bonus amount for
each such officer to the Compensation Committee in light of such officer’s achievements during 2024.
Achievement of Goals and Relationship
to Compensation Awarded
The overall 110% achievement score for the
2024 corporate goals, combined with Dr. Kakkis’ assessment of the individual performance and achievement of Mr. Horn, Mr. Harris,
Dr. Crombez and Mr. Pinion during fiscal 2024 resulted in the Compensation Committee approving bonus awards for performance in
2024 as set forth in the following table. The bonuses awarded under our 2024 annual incentive program were paid in March 2025.
| Name | |
Title | |
Corporate
Component Score (%)
(100% for CEO) | |
Individual
Component
Score (%) | |
Total Fiscal 2024
Bonus | | Bonus
Achieved
(as %
of Base
Salary) |
| Emil D. Kakkis, M.D., Ph.D. | |
President and Chief Executive Officer | |
110 | |
— | |
$ | 757,768 | | |
88% |
| Howard Horn | |
Chief Financial Officer and Executive Vice President, Corporate Strategy | |
110 | |
106 | |
$ | 343,970 | | |
58% |
| Erik Harris | |
Chief Commercial Officer and Executive Vice President | |
110 | |
110 | |
$ | 367,235 | | |
61% |
| Eric Crombez, M.D. | |
Chief Medical Officer and Executive Vice President | |
110 | |
115 | |
$ | 382,030 | | |
63% |
| John R. Pinion II | |
Chief Quality Officer and Executive Vice President, Translational Sciences | |
110 | |
108 | |
$ | 339,768 | | |
59% |
Equity Compensation
Overview
Stock Options, Restricted Stock Units
and Performance Stock Units. Executive officers are eligible to
receive equity compensation in the form of stock options, RSUs and/or PSUs. The Compensation Committee grants stock options,
RSUs and/or PSUs annually to executive officers to recognize their contributions to the achievement of corporate objectives,
to align their interests with those of our stockholders by creating value tied to the performance of our stock price, and for
retention purposes. In determining the form and value of an annual grant, the Compensation Committee considers the
contributions and responsibilities of each executive officer, appropriate incentives for the achievement of our long-term
growth, the size and value of grants made to other executives at peer companies holding comparable positions, individual
achievement of designated performance goals, and our overall performance relative to corporate objectives. The Compensation
Committee also grants stock options and RSUs to new executive officer hires.
Under the terms of our 2023 Plan, pursuant
to which all equity grants are currently made, the exercise price of any stock options awarded must be equal to at least 100% of
the fair market value of our common stock (the closing sales price on The Nasdaq Global Select Market) on the date of grant.
Authority to make equity grants to executive
officers rests with the Compensation Committee. Recommendations for equity grants are made by Aon based on grant values for similarly
situated executive positions in our peer group companies and accounting for dilution constraints and management of our burn rate.
Our CEO recommends grants for individual executives within those guidelines. The Compensation Committee then reviews and considers
our CEO’s recommendation and approves the final grant amounts. The Compensation Committee also determines and approves the
final grant amounts to our CEO.
We believe that annual equity awards serve as a useful performance recognition mechanism, encouraging the retention of executive officers by maintaining their focus on our long-term performance, as well as on the achievement of specific performance goals. Our typical option awards to executive officers (including our named executive officers) have a term of 10 years and vest and become exercisable over a period of four years, with 25% of the underlying shares vesting on the first anniversary of the grant date and the remainder monthly over the next three years. Our typical RSU awards to executive officers (including our named executive officers) vest and become exercisable over a period of four years, with 25% of the underlying shares vesting on each anniversary of the grant date.
In addition to the new hire and annual equity
awards described above, the Compensation Committee considers grants of other equity awards, as needed, to align business strategy
with our compensation practices.
Annual Equity Grants in
Fiscal 2024
Grants of Ultragenyx Awards
In March 2024, the Compensation Committee
approved equity grants that reflected an equal value split among options, RSUs and PSUs for our named executive officers other
than our Chief Executive Officer, who instead received 60% of his grant in the form of PSUs,
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 46
with the remaining portion split equally
among options and RSUs. The increased portion of our Chief Executive Officer’s annual equity grant that is performance-based
is intended to enhance the alignment with stockholder interests.
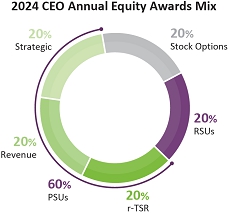
The PSU awards granted to our executive officers in March 2024 (2024 PSUs) consist of a revenue portion (1/3 of the 2024 PSUs, which reflects a decrease from 50% of the 2023 PSUs) with a two-year performance period, a relative TSR portion (1/3 of the 2024 PSUs, which reflects an increase compared to 25% of the 2023 PSUs) with a three-year performance period and a strategic performance portion (1/3 of the 2024 PSUs, which reflects an increase compared to 25% of the 2023 PSUs) with a three-year performance period, which is longer than the two-year performance period for the strategic portion of the 2023 PSUs. The increased emphasis on relative TSR portion and the strategic performance portion of the PSU awards reduces the overall portion of our incentive compensation program that is focused on revenue and creates a more balanced mix of performance goals that are key to creation of long-term success. The increased performance period for the strategic performance portion of the 2024 PSUs results in additional program rigor due to a sustained performance requirement over a longer timeframe.
The revenue-based 2024 PSUs will vest based
upon achievement of revenue-based targets during the period beginning January 1, 2024 and ending December 31, 2025, with all of
the earned revenue-based PSUs vesting on the later of (i) the date in which the Compensation Committee certifies such achievement
and (ii) March 1, 2026. The revenue-based PSUs will be earned at 50% of target for threshold performance and 200% of target for
maximum performance, with no PSUs being earned for below threshold performance.
The relative TSR-based 2024 PSUs will vest
based upon our TSR performance relative to the TSR of the companies in the Nasdaq Biotechnology Index during the period beginning
January 1, 2024 and ending December 31, 2026, with all of the earned relative TSR-based PSUs vesting on the later of (i) the date
in which the Compensation Committee certifies such achievement and (ii) March 1, 2027. The relative TSR-based PSUs will be earned
as follows based on our percentile ranking among the Nasdaq Biotechnology Index during the performance period:
| Level of Performance |
|
TSR Percentile |
|
Earned TSR PSUs |
| Threshold |
|
25th |
|
25% |
| Target |
|
50th |
|
100% |
| Stretch |
|
75th |
|
150% |
| Maximum |
|
90th |
|
200% |
The strategic performance based 2024 PSUs
will vest upon achievement of specified strategic performance objectives during the period between January 1, 2024 and ending December
31, 2026, with all of the earned strategic performance based PSUs vesting on the later of (i) the date in which the Compensation
Committee certifies such achievement and (ii) March 1, 2027. The strategic performance based PSUs will be earned as follows based
on the number of strategic goals achieved during the performance period. No PSUs are earned under the strategic performance based
2024 PSUs for below threshold performance.
| Level of Performance |
|
Number of Strategic Goals Achieved |
|
Earned Strategic PSUs |
| Threshold |
|
2 out of 5 |
|
50% |
| Target |
|
3 out of 5 |
|
100% |
| Stretch |
|
4 out of 5 |
|
150% |
| Maximum |
|
5 out of 5 |
|
200% |
The tables below set forth all equity awards
granted in fiscal 2024 to our named executive officers.
| Name |
|
Date of Grant |
|
Number of Options |
|
Number of RSUs |
|
Target Number of PSUs |
| Emil D. Kakkis, M.D., Ph.D. |
|
3/1/2024 |
|
70,094 |
|
38,428 |
|
115,284 |
| Howard Horn |
|
3/1/2024 |
|
34,200 |
|
19,400 |
|
19,400 |
| Erik Harris |
|
3/1/2024 |
|
34,200 |
|
19,400 |
|
19,400 |
| Eric Crombez, M.D. |
|
3/1/2024 |
|
34,200 |
|
19,400 |
|
19,400 |
| John R. Pinion II |
|
3/1/2024 |
|
31,400 |
|
17,800 |
|
17,800 |
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 47
Grants of Performance Stock Options (PSOs)
in Amlogenyx Inc. (Amlogenyx)
In addition to grants of Ultragenyx options,
RSUs and PSUs in fiscal 2024 as described above, in recognition of their contributions to Amlogenyx, Dr. Kakkis, Mr. Horn and Mr.
Pinion also received grants of PSOs from Amlogenyx, a privately-held subsidiary of Ultragenyx in which Ultragenyx currently holds
a majority interest, during fiscal 2024. The Amlogenyx PSOs will vest upon achievement of specified performance criteria related
to clinical milestones, with each criteria weighted 25%. If the performance criteria are not achieved by the end of the 18 month
performance period on April 21, 2026, the unvested portion of the Amlogenyx PSOs will be forfeited.
| Name |
|
Date of Grant |
|
Number of Amlogenyx PSOs |
| Emil D. Kakkis, M.D., Ph.D. |
|
10/21/2024 |
|
350,000 |
| Howard Horn |
|
10/21/2024 |
|
22,500 |
| John R. Pinion II |
|
10/21/2024 |
|
100,000 |
Performance of 2023 PSUs
The PSU awards granted to our executive officers
in March 2023 (2023 PSUs) consisted of a revenue portion with a two-year performance period (50% of the PSUs), a relative TSR portion
(25% of the PSUs) with a three-year performance period and a strategic performance portion (25% of the PSUs) with a two-year performance
period. The performance periods of the revenue portion and strategic performance portion of the 2023 PSUs ended on December 31,
2024 and the revenue portion and strategic performance portion of the 2023 PSUs vested on March 1, 2025, as described below. The
performance period for the relative TSR-based 2023 PSUs extends through December 31, 2025, and if earned, such portion will vest
in early 2026.
Achievement of Revenue Portion of 2023
PSUs
The revenue-based 2023 PSUs vested based upon achievement of the following revenue-based targets (and application of linear interpolation) during the period beginning January 1, 2023 and ending December 31, 2024, with all of the earned revenue-based PSUs vesting on the later of (i) the date in which the Compensation Committee certifies such achievement and (ii) March 1, 2025.
| Level of Performance |
Aggregate Revenue |
|
Earned Revenue-Based PSU Percentage |
| Threshold |
$ |
945M |
|
50% |
| Actual |
$ |
995M |
|
74% |
| Target |
$ |
1,050M |
|
100% |
| Maximum |
$ |
1,260M |
|
200% |
In February 2025, based on the Company’s
2023 and 2024 revenue performance measured in accordance with U.S. GAAP (GAAP), the Compensation Committee certified that the revenue
based 2023 PSUs were earned at 74% of target, and such earned 2023 PSUs vested on March 1, 2025.
Achievement of Strategic Performance Portion
of 2023 PSUs
The strategic performance based 2023 PSUs vested upon achievement of specified strategic performance objectives during the period between January 1, 2023 and ending December 31, 2024, with all of the earned strategic performance based PSUs vesting on the later of (i) the date in which the Compensation Committee certifies such achievement and (ii) March 1, 2025.
| Level of Performance |
|
Number of Strategic Goals Achieved |
|
Earned Strategic PSUs |
| Threshold |
|
2 out of 5 |
|
50% |
| Target |
|
3 out of 5 |
|
100% |
| Actual |
|
3.5 out of 5 |
|
125% |
| Stretch |
|
4 out of 5 |
|
150% |
| Maximum |
|
5 out of 5 |
|
200% |
In February 2025, based on the Company’s
achievement of 3.5 out of 5 strategic performance objectives, the Compensation Committee certified that the strategic performance
portion of 2023 PSUs were earned at 125% of target, and such earned 2023 PSUs vested on March 1, 2025.
Performance of 2022 PSUs
The PSU awards granted to our executive officers
in March 2022 (2022 PSUs) consisted of a revenue portion with a two-year performance period (80% of the PSUs) and a relative TSR
portion with a three-year performance period (20% of the PSUs). In February 2024, based on the Company’s 2022 and 2023
revenue performance measured in accordance with GAAP, the Compensation Committee certified that the threshold level of revenue-based
2022 PSUs had not been achieved and as a result, the revenue-based portion of the 2022 PSUs were forfeited.
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 48
The relative TSR-based 2022 PSUs vested based
upon our TSR performance relative to the TSR of the companies in the Nasdaq Biotechnology Index during the period beginning January
1, 2022 and ending December 31, 2024, with all of the earned relative TSR-based PSUs vesting on the later of (i) the date in which
the Compensation Committee certifies such achievement and (ii) March 1, 2025. The relative TSR-based PSUs was earned as follows
based on our percentile ranking among the Nasdaq Biotechnology Index during the performance period:
| Level of Performance |
|
TSR Percentile |
|
Earned TSR PSUs |
| Threshold |
|
25th |
|
25% |
| Target |
|
50th |
|
100% |
| Actual |
|
62nd |
|
124% |
| Stretch |
|
75th |
|
150% |
| Maximum |
|
90th |
|
200% |
Based on the Company’s ranking relative
to the TSR of the companies in the Nasdaq Biotechnology Index of the 62nd percentile during the performance period,
in February 2025, the Compensation Committee certified that the relative TSR-based 2022 PSUs were earned at 124% of target, and
such earned 2022 PSUs vested on March 1, 2025.
Fiscal 2025 Compensation
Peer Group
The Compensation Committee reviews our list
of peer companies annually to determine if revisions are needed to reflect changes in our development status, market capitalization,
changes in individual peer companies, and other factors. The Compensation Committee engaged Aon to assist in reviewing our peer
group and in suggesting revisions, as appropriate.
Based on Aon’s assessment and recommendations,
the Compensation Committee selected 20 publicly traded companies in the pharmaceutical and biotechnology industries to serve as
our new list of peer companies for 2025, referred to as our 2025 peer group, by balancing the following criteria:
| • |
companies with emphasis on orphan pharmaceutical products; |
| • |
companies with comparable market capitalizations (i.e., in the range of $2 billion to $14 billion); |
| • |
companies with revenue of between $250 million and $1.6 billion; and |
| • |
companies with headcounts between 300 to 4,000 employees. |
Our 2025 peer group is comprised of the following 19 companies in the pharmaceutical and biotechnology industries, reflecting the addition of Axsome Therapeutics, Inc., Denali Therapeutics Inc. and Vericel Corporation and the removal of BioCryst Biopharmaceuticals, Inc., FibroGen, Inc. and Sage Therapeutics, Inc. from our 2024 peer group due to misalignment with our market capitalization and the removal of BioMarin Biopharmaceutical Inc. who exceeded our market capitalization and revenue ranges.
| ACADIA Pharmaceuticals Inc. |
|
Corcept Therapeutics Inc. |
|
Jazz Pharmaceuticals plc |
| Alkermes plc |
|
Denali Therapeutics Inc. |
|
Neurocrine Biosciences, Inc. |
| Amicus Therapeutics |
|
Exelixis Inc. |
|
PTC Therapeutics, Inc. |
| Apellis Pharmaceuticals, Inc. |
|
Halozyme Therapeutics |
|
Sarepta Therapeutics, Inc. |
| Axsome Therapeutics, Inc. |
|
Insmed Incorporated |
|
Vericel Corporation |
| Blueprint Medicines Corporation |
|
Intra-Cellular Therapies Inc. |
|
|
| BridgeBio Pharma, Inc. |
|
Ionis Pharmaceuticals, Inc. |
|
|
We believe that the compensation practices
of our 2025 peer group provided us with appropriate compensation benchmarks for evaluating the compensation of our named executive
officers for 2025.
Base Salaries
For 2025, increases in base salaries for
our named executive officers, were 5.1% annualized for Dr. Crombez to align to the external market peer group, 2.9% annualized
for Mr. Horn, 2.5% annualized for Mr. Harris and 2.8% annualized for Mr. Pinion. Dr. Kakkis opted not to receive an increase to
his base salary for 2025. The overall 2025 merit budget was based on an Aon trend report regarding projected market merit budgets
and spend for 2025.
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 49
Annual Bonuses
For 2025, the Compensation Committee maintained
the target annual bonus of 80% for our Chief Executive Officer and maintained the target bonus amount of 50% for all other named
executive officers. No significant changes were made to the bonus plan for 2025.
Equity Compensation
For 2025, the Compensation Committee determined
to increase the PSU component of the annual equity grants to our executive officers, other than our Chief Executive Officer, from
33% to 50%, with the remaining portion of the grant equally split at 25% options and 25% RSUs. For our Chief Executive Officer,
the Compensation Committee maintained the equity split of 60% PSUs, 20% options and 20% RSUs.
The PSU awards granted to our executive officers in 2025 (2025 PSUs) consist of a revenue portion with a two-year performance period (1/3 of the 2025 PSUs), a relative TSR portion with a three-year performance period (1/3 of PSUs) and a strategic performance portion (1/3 of the PSUs) with a three-year performance period. The revenue-based 2025 PSUs will vest based upon achievement of revenue-based targets during the period beginning January 1, 2025 and ending December 31, 2026, with all of the earned revenue-based PSUs vesting on the later of (i) the date in which the Compensation Committee certifies such achievement and (ii) March 1, 2027. The relative TSR-based 2025 PSU awards will vest based upon our TSR performance relative to the TSR of the companies in the Nasdaq Biotechnology Index during the period beginning January 1, 2025 and ending December 31, 2027, with a maximum payout of 100% of target in the event our absolute TSR is negative, with all of the earned relative TSR-based PSUs vesting on the later of (i) the date in which the Compensation Committee certifies such achievement and (ii) March 1, 2028. The strategic-based 2025 PSU awards will vest based on achievement of specified strategic goals during the period beginning January 1, 2025 and ending December 31, 2027, with all of the earned strategic based PSUs vesting on the later of (i) the date in which the Compensation Committee certifies such achievement and (ii) March 1, 2028. The PSUs will be earned at 50% of target for threshold performance (or 25% of target for the relative TSR-based PSUs) and 200% of target for maximum performance, with no PSUs becoming earning for below threshold performance.
Employee Benefits Program
Executive officers are eligible to participate
in all of our employee benefit plans, including medical, dental, vision, group life, disability, and accidental death and dismemberment
insurance. In each case, participation is on the same basis as other employees, subject to applicable law. We also provide vacation
and other paid holidays to all employees, including executive officers, all of which we believe to be comparable to those provided
at peer companies. These benefit programs are designed to enable us to attract and retain our workforce in a competitive marketplace.
Reliable and competitive health, welfare and vacation benefits ensure that we have a productive and focused workforce.
In addition, our named executive officers
are eligible to participate in a retirement savings plan (401(k) Plan), which is a tax-qualified defined contribution plan pursuant
that allows participants to contribute certain amounts of their annual compensation, subject to limits prescribed by the Internal
Revenue Service. All of our employees are eligible to participate in the 401(k) Plan on the same terms as the named executive officers.
Our named executive officers, along with
our other highly-compensated U.S. based employees and non-employee directors of our Board, are also eligible to participate in
our nonqualified deferred compensation plan. Please see ” -Nonqualified Deferred Compensation Plan” below for a summary
of the plan.
Clawback Policy
Our Board has adopted a Clawback Policy intended
to comply with the listing standards from Nasdaq implementing Exchange Act Rule 10D-1. In the event the Company is required to
prepare an accounting restatement of the Company’s financial statements due to material non-compliance with any financial
reporting requirement under the federal securities laws, the Company will recover, on a reasonably prompt basis, the excess incentive-based
compensation received by any covered executive officer, including the named executive officers, during the prior three fiscal years
that exceeds the amount that the named executive officer otherwise would have received had the incentive-based compensation been
determined based on the restated financial statements. The Clawback Policy also permits recoupment of all incentive compensation,
including time-based and performance-based equity awards and bonuses, in the event of fraud or intentional misconduct by a current
or former executive officer, including the named executive officers.
Minimum Stock Ownership
Requirements
We maintain stock ownership guidelines in
order to align the long-term interests of our executive officers and directors with those of our stockholders. The guidelines require
holding shares of our common stock with value equivalent to 3x the annual retainer for Board members, 3x base salary for our Chief
Executive Officer and 1x base salary for the other named executive officers. Shares that count toward satisfaction of these guidelines
include shares owned outright by the individual (including RSUs that have vested), shares
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 50
retained after an option exercise or issuance
under another type of equity award granted under the Company’s equity incentive plans, shares retained after purchase under
the ESPP and shares held in trust for the benefit of the individual. Unexercised options and unvested and unearned RSUs and PSUs do
not count towards satisfaction of these guidelines. These guidelines were required to be achieved by the end of 2022 for our named
executive officers and Board members who have been named executive officers and Board members as of the date the policy was
implemented and within five years of appointment for newly appointed named executive officers and Board members. As of December 31,
2024, each of our Board members and named executive officers who were required to be in compliance with the guidelines by the end of
2024 had met the ownership guidelines.
Equity Grant Timing
Generally, the Compensation Committee grants annual equity awards, including stock options, on March 1 of each year following the filing of our Annual Report on Form 10-K and grants new hire equity awards, including stock options, on the 16th of each month following the individual’s start date. The Compensation Committee may also grant equity awards at other times during the year, such as in connection with special retention awards, performance recognition, or in the event of a significant change in job responsibilities. Under the 2014 Employee Stock Purchase Plan, eligible employees may purchase shares at a discount, with purchase dates generally on April 30 and October 31 of each year, using payroll deductions accumulated during the prior six-month period. The Compensation Committee did not take material nonpublic information into account when determining the timing and terms of equity awards, including stock options, during 2024, and we did not time the disclosure of material nonpublic information for the purpose of affecting the value of executive compensation. During 2024, no stock options were granted to the named executive officers in the period beginning four business days before and ending one business day after the filing or furnishing of any Form 10-Q, Form 10-K or Form 8-K that disclosed material nonpublic information.
Risk Management and Mitigation
In reviewing the compensation structure in
fiscal 2024, the Compensation Committee also considered how our compensation policies may affect our risk profile and how compensation
policies may be used to mitigate risks facing us. More specifically, the Compensation Committee considered the general design philosophy
of our policies for employees whose conduct would be most affected by incentives established by compensation policies. In considering
these issues, the Compensation Committee concluded that the use of performance-based bonuses and long-term equity awards did not
appear to create undue risks for us or encourage excessive risk-taking behavior on the part of employees.
With respect to bonus awards, the amount
of an individual’s award depends principally (exclusively, in the case of our Chief Executive Officer) on our overall performance,
which reduces the ability and incentive for an individual to take undue risks in an effort to increase the amount of his or her
bonus award for a particular year. For fiscal 2024, our corporate goals were reviewed and approved by the Board in early 2024,
upon the recommendation of the Compensation Committee, and are considered to be generally of the nature that would not encourage
or reward excessive risk taking. Additionally, the Compensation Committee monitors our performance throughout the year and has
the ability to intervene in instances where our actions vis-à-vis our performance goal attainment would be considered unduly
risky, so that the Compensation Committee may act to prevent or penalize such actions.
With respect to equity awards, these awards
typically vest over several years, meaning that long-term value creation, contrasted with short-term gain, presents the best opportunity
for employees to benefit from these awards.
Compensation Committee Report
The Compensation Committee has reviewed and
discussed with management the Compensation Discussion and Analysis required by Item 402(b) of Regulation S-K. Based on this review
and discussion, the Compensation Committee recommended to the Board of Directors that the foregoing Compensation Discussion and
Analysis be included in this Proxy Statement and incorporated by reference in our Annual Report on Form 10-K for the fiscal year
ended December 31, 2024.
Submitted by the Compensation Committee of
the Board of Directors
Michael Narachi, Chairman
Deborah Dunsire, M.D.
Daniel G. Welch
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 51
Summary Compensation Table
The following table sets forth the compensation earned during
the years ended December 31, 2024, 2023, and 2022 by our Chief Executive Officer, our Chief Financial Officer and our next three
highest-paid executives. We refer to these officers as our named executive officers.
| Name and Principal Position |
|
Year |
|
|
Salary |
|
|
Bonus |
|
|
Stock
Award(1) |
|
|
Option
Award(2) |
|
|
Non-Equity
Incentive Plan
Compensation(3) |
|
|
All Other
Compensation(4) |
|
|
Total |
|
Emil D. Kakkis, M.D., Ph.D.
President and Chief Executive Officer
|
|
|
2024 |
|
|
$ |
856,008 |
|
|
$ |
— |
|
|
$ |
9,374,511 |
|
|
$ |
2,715,600 |
|
|
$ |
757,768 |
|
|
$ |
13,800 |
|
|
$ |
13,717,687 |
|
| |
|
2023 |
|
|
|
823,692 |
|
|
|
— |
|
|
|
9,456,663 |
|
|
|
2,161,782 |
|
|
|
622,656 |
|
|
|
13,200 |
|
|
|
13,077,993 |
|
| |
|
2022 |
|
|
|
796,154 |
|
|
|
— |
|
|
|
7,463,295 |
|
|
|
3,310,681 |
|
|
|
558,000 |
|
|
|
12,200 |
|
|
|
12,140,330 |
|
Howard Horn
Chief Financial Officer, Corporate Strategy and Executive Vice President
|
|
|
2024 |
|
|
|
590,000 |
|
|
|
— |
|
|
|
2,271,944 |
|
|
|
1,076,335 |
|
|
|
343,970 |
|
|
|
13,800 |
|
|
|
4,296,049 |
|
| |
|
2023 |
|
|
|
124,808 |
|
|
|
60,000 |
|
|
|
2,867,459 |
|
|
|
2,888,773 |
|
|
|
— |
|
|
|
— |
|
|
|
5,941,040 |
|
Erik Harris
Chief Commercial Officer and Executive Vice President
|
|
|
2024 |
|
|
|
604,231 |
|
|
|
— |
|
|
|
2,271,944 |
|
|
|
1,038,603 |
|
|
|
367,235 |
|
|
|
13,800 |
|
|
|
4,295,813 |
|
| |
|
2023 |
|
|
|
585,969 |
|
|
|
— |
|
|
|
2,169,750 |
|
|
|
1,020,050 |
|
|
|
279,598 |
|
|
|
13,200 |
|
|
|
4,068,567 |
|
| |
|
2022 |
|
|
|
566,331 |
|
|
|
— |
|
|
|
2,007,768 |
|
|
|
892,550 |
|
|
|
269,706 |
|
|
|
12,200 |
|
|
|
3,748,555 |
|
Eric Crombez, M.D.
Chief Medical Officer and Executive Vice President
|
|
|
2024 |
|
|
|
591,846 |
|
|
|
— |
|
|
|
2,271,944 |
|
|
|
1,038,603 |
|
|
|
382,030 |
|
|
|
13,800 |
|
|
|
4,298,223 |
|
John R. Pinion II
Chief Quality Officer and Executive Vice President, Translational
Sciences
|
|
|
2024 |
|
|
|
570,366 |
|
|
|
— |
|
|
|
2,084,548 |
|
|
|
1,121,271 |
|
|
|
339,768 |
|
|
|
13,800 |
|
|
|
4,129,753 |
|
| |
|
2023 |
|
|
|
552,092 |
|
|
|
— |
|
|
|
2,169,750 |
|
|
|
1,020,050 |
|
|
|
294,761 |
|
|
|
13,200 |
|
|
|
4,049,853 |
|
| |
|
2022 |
|
|
|
533,315 |
|
|
|
— |
|
|
|
2,007,768 |
|
|
|
892,550 |
|
|
|
260,678 |
|
|
|
12,200 |
|
|
|
3,706,511 |
|
| (1) |
The amounts reported in this column for
a fiscal year represent the grant date fair value of the RSUs and PSUs granted to our named executive officers during the
fiscal year, as computed in accordance with ASC Topic 718, not including any estimates of forfeitures, and, with respect to
the PSUs, assuming the most probable outcome of the performance conditions as of the grant date. The assumptions used in calculating
the grant date fair value of the RSUs and PSUs reported in this column are set forth in the notes to our financial statements
included in our Annual Report. The amounts reported in this column reflect the accounting cost for these RSUs and PSUs and
do not correspond to the actual economic value that may be realized by the named executive officers from the RSUs and the
PSUs. The value of the PSUs reported in this column for 2024, assuming achievement of the maximum performance level, was as
follows: $14,622,623 for Dr. Kakkis, $2,460,715 for each of Mr. Horn, Dr. Crombez and Mr. Harris and $2,257,733 for Mr. Pinion. |
| (2) |
The amounts reported in this column for a fiscal year
represent the grant date fair value of the stock options granted to our named executive officers during the fiscal year, as
computed in accordance with ASC Topic 718, not including any estimates of forfeitures. Amounts included in this column for
Dr. Kakkis, Mr. Horn and Mr. Pinion also include the grant date fair value of the PSOs granted from Amlogenyx, a privately-held
subsidiary of Ultragenyx in which Ultragenyx currently holds a majority interest, as described above under “ —Annual
Equity Grants in Fiscal 2024”. The grant date fair value of the Amlogenyx PSOs is as follows: $586,950 for Dr. Kakkis,
$37,733 for Mr. Horn and $167,700 for Mr. Pinion. The assumptions used in calculating the grant date fair value of the Amlogenyx
PSOs reported in this column were as follows: expected term of 5.75 years, risk free rate of 3.98%, volatility of 85% and
exercise price of $2.31. The amounts reported in this column reflect the accounting cost for these stock options, and do not
correspond to the actual economic value that may be received by the named executive officers from the options. |
| (3) |
Amounts for a fiscal year represent annual cash bonuses
earned in that fiscal year and paid in the subsequent fiscal year based on achievement of performance goals and other factors
deemed relevant by our Compensation Committee under our annual incentive program. |
| (4) |
Amounts reported in this column for the 2024 fiscal
year reflects 401(k) matching contributions. |
Narrative Disclosure to Summary Compensation
Table
Employment Arrangements with Our Named Executive Officers
Dr. Kakkis, our Chief Executive Officer,
is party to an employment agreement with us that provides for base salary and participation in our employee benefit plans, subject
to the terms of those plans. Pursuant to the terms of the employment agreement, the employment of Dr. Kakkis is at will; we may
terminate his employment at any time, without advance notice, for any reason or for no reason at all, and Dr. Kakkis may terminate
his employment at any time, upon four weeks’ prior written notice, for any reason or for no reason at all.
Each
of our other named executive officers is party to an offer letter with us that provides for base salary, an annual bonus opportunity,
and an initial grant of equity. They are eligible to participate in our employee benefit plans, subject to the terms of those plans.
Pursuant to the terms of the offer letters, their employment is at will and may be
terminated either by us or by them, with or without advance notice, for any reason or for no reason at all. Pursuant to Mr. Horn’s
offer letter, he received a $60,000 sign-on bonus in 2023, which was subject to repayment in the event his employment is terminated
for “cause” (as defined below) or as a result of his resignation that is not a “constructive termination”
(as defined below) within 12 months of his start date, which obligation lapsed in 2024.
Each of these employment arrangements and
offer letters, as amended, also contain provisions that provide for certain payments and benefits in the event of an involuntary
termination of employment. In addition, the named executive officers may be entitled to accelerated vesting of their outstanding
and unvested awards in certain circumstances. The information below describes certain compensation that may become due and payable
as a result of certain events as reflected in the amended arrangements.
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 52
Involuntary Termination of Employment
Pursuant to their employment arrangements
or offer letters, each named executive officer is eligible to receive certain payments and benefits in the event of certain qualifying
terminations, including termination of his or her employment by us without “cause” (as defined below) or resignation
of his or her employment with “good reason” or because of a “constructive termination” (each, as defined
below). Certain benefits are enhanced in the event the qualifying termination occurs within a specified period of time (12 months
for Dr. Kakkis, and 18 months for Mr. Horn, Mr. Harris, Dr. Crombez and Mr. Pinion) after the consummation of a “covered
transaction” (as defined below), which we refer to as the covered transaction protection period. Upon the timely execution
of a general release of claims, each named executive officer is eligible to receive the following payments and benefits:
| • |
if Dr. Kakkis is terminated by us other
than for cause (and not as a result of death or disability) or he resigns for good reason, he will be entitled to receive
(i) the sum of 24 months of his base salary and his target bonus for the year in which the termination occurs (or, if such
termination occurs within the covered transaction protection period, the sum of 24 months of his base salary and 2x his target
bonus), (ii) reimbursement for monthly COBRA premiums for the 24-month period following his termination, (iii) if such termination
occurs within the covered transaction protection period, accelerated vesting of any unvested equity-based compensation; and |
| • |
if Mr. Horn, Mr. Harris, Dr. Crombez or Mr. Pinion is
terminated by us without cause (and not as a result of death or disability) or upon a resignation due to a constructive termination,
the executive will be entitled to the following severance benefits: (i) extension of the exercise period applicable to any
options to purchase our stock held by the executive at the time of termination until 12 months from the date of such termination
(or, if earlier, until the expiration of the term of the option set forth in the applicable option award agreement), (ii)
the sum of 12 months of the executive’s base salary and the executive’s target bonus for the year in which the
termination occurs (or, if such termination occurs within the covered transaction period, the sum of 18 months of the executive’s
base salary and 1.5x the executive’s target bonus), (iii) reimbursement for monthly COBRA premiums for the 12-month
period following the executive’s termination (or, if such termination occurs within the covered transaction protection
period, for the 18-month period following the executive’s termination), (iv) if such termination occurs within the covered
transaction period, accelerated vesting of any unvested equity-based compensation, and (v) if such termination occurs within
the covered transaction period, extension of the exercise period applicable to any outstanding options held by the executive
until 12 months from the date of such termination (or, if earlier, until the expiration of the term of the option
set forth in the applicable option award agreement). |
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 53
Definitions
For purposes of Dr.Kakkis’ employment agreement, “cause”
means his:
| • |
commission of a felony or any crime involving dishonesty,
breach of trust, or physical harm to any person; |
| • |
willful engagement in conduct that is in bad faith and materially injurious
to us, including but not limited to misappropriation of trade secrets, fraud, or embezzlement; |
| • |
material breach of his employment agreement that is not cured within
10 days after written notice to him from us; or |
| • |
willful refusal to implement or follow a lawful policy or directive of
ours, which breach is not cured within 10 days after written notice to him from us. |
For purposes of each of the offer letters
with Mr. Horn, Mr. Harris, Dr.Crombez and Mr.Pinion “cause” means the named executive officer’s:
| • |
willful engagement in conduct that is materially injurious
to the Company (for Mr. Harris) or gross negligence in carrying out, or material failure to carry out, his or her duties for
us (including, without limitation, failure to cooperate in any Company investigation), after notice from the Board and a reasonable
opportunity to cure (if deemed curable); |
| • |
breach of his or her fiduciary duties to us, after notice from the Board
and a reasonable opportunity to cure (if deemed curable); |
| • |
conviction of, or plea of guilty or no contest to, any felony; |
| • |
act of fraud or embezzlement with respect to his or her obligations
to us or otherwise relating to our business; |
| • |
willful refusal to implement or follow a material policy or directive
(for Mr. Horn, Dr. Crombez and Mr. Harris) or a material violation of any of our policies (for Mr. Pinion), in each case
other than for Mr. Pinion and Dr. Crombez after notice from the Board and a reasonable opportunity to cure (if deemed
curable); |
| • |
material breach of any agreement entered into with us (subject to notice
from the Board and a reasonable opportunity to cure (if deemed curable) for Mr. Horn); or |
| • |
unauthorized use or disclosure of confidential information or trade
secrets of ours or of our affiliates (subject to notice from the Board and a reasonable opportunity to cure (if deemed curable)
for Mr. Horn). |
For purposes of Dr. Kakkis’ employment
agreement, “good reason” means any of the following events without his consent, subject to standard notice and cure
requirements:
| • |
a change in his position with us that materially reduces
his level of responsibility; |
| • |
a material reduction in his base salary, except for reductions that are
comparable to reductions generally applicable to similarly situated executives of ours; or |
| • |
a relocation of his principal place of employment by more than 50 miles. |
For purposes of each of the offer letters
with Mr. Horn, Mr. Harris, Dr. Crombez and Mr. Pinion, “constructive termination” means the occurrence of any of the
following events without the named executive officer’s consent, subject to standard notice and cure requirements:
| • |
a material reduction or change in the executive’s job
duties, responsibilities and requirements from the executive’s job duties, responsibilities and requirements immediately
prior to such reduction or change, taking into account the differences in job title and duties that are normally occasioned
by reason of an acquisition of one company by another; |
| • |
a material reduction of the executive’s base salary (other than
an equal, across-the-board reduction in the compensation of all similarly-situated employees of ours or the surviving entity
that is approved by the Board); or |
| • |
a requirement that the executive relocate to a principal office that
increases his or her one-way commute by more than 50 miles relative to the executive’s immediately preceding principal
office. |
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 54
Grants of Plan-Based Awards
The following table sets forth certain information
regarding grants of plan-based awards to the named executive officers during fiscal 2024.
| |
|
|
|
|
Estimated Future Payouts Under
Non-Equity Incentive Plan Awards(1) |
|
Estimated Future Payouts Under
Equity Incentive Plan Awards(2) |
|
Stock
Awards:
Number
of Shares
of Stock |
|
Option
Awards:
Number of
Securities
Underlying |
|
Exercise
Price of |
|
Grant Date
Fair Value
of Stock |
| Name |
|
Grant Date |
|
|
Threshold
($) |
|
Target
($) |
|
Maximum
($) |
|
Threshold
(#) |
|
Target
($) |
|
Maximum
($) |
|
or Units
Granted
(#)(3) |
|
Options
Granted
(#) |
|
Option
Awards
($/Share) |
|
and Option
Awards
($)(4) |
| Emil D. Kakkis, |
|
|
|
|
344,400 |
|
688,800 |
|
1,033,200 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
| M.D., Ph.D. |
|
3/1/2024 |
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
70,094 |
|
53.69 |
|
2,128,650 |
| |
|
3/1/2024 |
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
38,428 |
|
— |
|
— |
|
2,063,199 |
| |
|
3/1/2024 |
|
|
— |
|
— |
|
— |
|
48,045 |
|
115,284 |
|
230,568 |
|
— |
|
— |
|
— |
|
7,311,311 |
| |
|
10/21/2024 |
(5) |
|
— |
|
— |
|
— |
|
— |
|
350,000 |
|
— |
|
— |
|
— |
|
2.31 |
|
586,950 |
| Howard Horn |
|
|
|
|
— |
|
295,000 |
|
588,525 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
| |
|
3/1/2024 |
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
34,200 |
|
53.69 |
|
1,038,603 |
| |
|
3/1/2024 |
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
19,400 |
|
— |
|
— |
|
1,041,586 |
| |
|
3/1/2024 |
|
|
— |
|
— |
|
— |
|
8,085 |
|
19,400 |
|
38,800 |
|
— |
|
— |
|
— |
|
1,230,358 |
| |
|
10/21/2024 |
(5) |
|
— |
|
— |
|
— |
|
— |
|
22,500 |
|
— |
|
— |
|
— |
|
2.31 |
|
37,733 |
| Erik Harris |
|
|
|
|
— |
|
303,500 |
|
605,483 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
| |
|
3/1/2024 |
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
34,200 |
|
53.69 |
|
1,038,603 |
| |
|
3/1/2024 |
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
19,400 |
|
— |
|
— |
|
1,041,586 |
| |
|
3/1/2024 |
|
|
— |
|
— |
|
— |
|
8,085 |
|
19,400 |
|
38,800 |
|
— |
|
— |
|
— |
|
1,230,358 |
| Eric Crombez., |
|
|
|
|
— |
|
302,000 |
|
602,490 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
| M.D. |
|
3/1/2024 |
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
34,200 |
|
53.69 |
|
1,038,603 |
| |
|
3/1/2024 |
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
19,400 |
|
— |
|
— |
|
1,041,586 |
| |
|
3/1/2024 |
|
|
— |
|
— |
|
— |
|
8,085 |
|
19,400 |
|
38,800 |
|
— |
|
— |
|
— |
|
1,230,358 |
| John R. Pinion II |
|
|
|
|
— |
|
286,000 |
|
570,570 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
| |
|
3/1/2024 |
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
31,400 |
|
53.69 |
|
953,571 |
| |
|
3/1/2024 |
|
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
17,800 |
|
— |
|
— |
|
955,682 |
| |
|
3/1/2024 |
|
|
— |
|
— |
|
— |
|
7,418 |
|
17,800 |
|
35,600 |
|
— |
|
— |
|
— |
|
1,128,866 |
| |
|
10/21/2024 |
(5) |
|
— |
|
— |
|
— |
|
— |
|
100,000 |
|
— |
|
— |
|
— |
|
2.31 |
|
167,700 |
| (1) |
The amounts in these columns represent the threshold (for Dr.
Kakkis only), target and maximum amount of each named executive officer’s cash payments under our 2024 annual incentive
program as established by the Board and described in “Compensation Discussion and Analysis” above. Actual payments
made for fiscal 2024 are provided in the Summary Compensation Table. |
| (2) |
The amounts in these columns represent the threshold, target and maximum
level of achievement for the 2024 PSUs granted under our 2023 Incentive Plan. The PSU awards consist of a revenue portion
(33.4% of the PSUs) with a two-year performance period, a relative total stockholder return (TSR) portion (33.3% of the PSUs)
with a three-year performance period and a strategic performance portion (33.3% of the PSUs) with a three-year performance
period. See “—Annual Equity Grants in Fiscal 2024” above for a description of the 2024 PSUs. |
| (3) |
The amounts in this column represents the RSUs granted under our 2023
Plan during 2024. |
| (4) |
This column reflects the aggregate grant date fair value of equity awards
granted in 2024 as computed in accordance with ASC Topic 718, not including any estimates of forfeitures. The assumptions
used in calculating the grant date fair value of the stock and option awards reported in this column are set forth in Note
13 to our financial statements included in our Annual Report for fiscal 2024. |
| (5) |
Reflects PSOs granted by Amlogenyx, a privately-held subsidiary of Ultragenyx
in which Ultragenyx currently holds a majority interest, as described above under “—Annual Equity Grants in
Fiscal 2024”. The Amlogenyx PSOs will vest upon achievement of specified clinical performance criteria, with each
criteria weighted 25%. If the performance criteria are not achieved during the 18-month performance period ending April 21,
2026, any unvested portion of the Amlogenyx PSOs will be forfeited. |
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 55
Outstanding Equity Awards at December 31, 2024
The following table sets forth information
concerning the outstanding equity awards held by each of the named executive officers as of December 31, 2024.
| |
|
|
|
Option Awards(1) |
|
Stock Awards |
| Name |
|
Grant Date |
|
Number of
Securities
Underlying
Unexercised
Options (#)
Exercisable |
|
Number of
Securities
Underlying
Unexercised
Options (#)
Unexercisable |
|
Equity Incentive
Plan Awards:
Number of
Securities
Underlying
Unexercised
Unearned
Options (#)(2) |
|
Option
Exercise
Price
($) |
|
Option
Expiration
Date |
|
Number of
Shares or
Units of
Stock
That Have
Not Vested
(#)(3) |
|
Market
Value of
Shares or
Units of
Stock That
Have Not
Vested
($) |
|
Equity Incentive
Plan Awards:
Number of
Unearned
Shares,
Units
or Other Rights
That Have Not
Vested (#)(4) |
|
Equity Incentive Plan
Awards: Market
or Payout Value of
Unearned Shares,
Units or Other Rights
That Have Not
Vested
($) |
Emil D. Kakkis,
M.D., Ph.D. |
|
10/21/2024 |
|
— |
|
— |
|
350,000 |
|
2.31 |
|
10/21/2034 |
|
— |
|
— |
|
— |
|
— |
| |
3/1/2024 |
|
— |
|
70,094 |
|
— |
|
53.69 |
|
3/1/2034 |
|
38,428 |
|
1,616,666 |
|
115,284 |
|
4,849,998 |
| |
3/1/2023 |
|
36,531 |
|
46,969 |
|
— |
|
45.65 |
|
3/1/2033 |
|
129,036 |
|
5,428,545 |
|
44,314 |
|
1,864,290 |
| |
3/1/2022 |
|
63,498 |
|
28,862 |
|
— |
|
67.37 |
|
3/1/2032 |
|
38,926 |
|
1,637,617 |
|
— |
|
— |
| |
3/1/2021 |
|
33,750 |
|
2,250 |
|
— |
|
142.47 |
|
3/1/2031 |
|
4,500 |
|
189,315 |
|
— |
|
— |
| |
3/1/2020 |
|
56,100 |
|
— |
|
— |
|
56.08 |
|
3/1/2030 |
|
— |
|
— |
|
— |
|
— |
| |
3/1/2019 |
|
66,000 |
|
— |
|
— |
|
67.55 |
|
3/1/2029 |
|
— |
|
— |
|
— |
|
— |
| |
3/1/2018 |
|
94,500 |
|
— |
|
— |
|
48.43 |
|
3/1/2028 |
|
— |
|
— |
|
— |
|
— |
| |
3/1/2017 |
|
78,000 |
|
— |
|
— |
|
88.80 |
|
3/1/2027 |
|
— |
|
— |
|
— |
|
— |
| |
6/1/2016 |
|
63,700 |
|
— |
|
— |
|
70.57 |
|
6/1/2026 |
|
— |
|
— |
|
— |
|
— |
| |
5/21/2015 |
|
68,300 |
|
— |
|
— |
|
84.89 |
|
5/21/2025 |
|
— |
|
— |
|
— |
|
— |
| Howard Horn |
|
10/21/2024 |
|
— |
|
— |
|
22,500 |
|
2.31 |
|
10/21/2034 |
|
— |
|
— |
|
— |
|
— |
| |
3/1/2024 |
|
— |
|
34,200 |
|
— |
|
53.69 |
|
3/1/2034 |
|
19,400 |
|
816,158 |
|
19,400 |
|
816,158 |
| |
10/9/2023 |
|
40,971 |
|
99,498 |
|
— |
|
35.68 |
|
10/9/2033 |
|
60,274 |
|
2,535,727 |
|
— |
|
— |
| Erik Harris |
|
3/1/2024 |
|
— |
|
34,200 |
|
— |
|
53.69 |
|
3/1/2034 |
|
19,400 |
|
816,158 |
|
19,400 |
|
816,158 |
| |
3/1/2023 |
|
17,238 |
|
22,162 |
|
— |
|
45.65 |
|
3/1/2033 |
|
31,471 |
|
1,323,985 |
|
7,029 |
|
295,710 |
| |
3/1/2022 |
|
17,119 |
|
7,781 |
|
— |
|
67.37 |
|
3/1/2032 |
|
10,472 |
|
440,557 |
|
— |
|
— |
| |
3/1/2021 |
|
10,313 |
|
687 |
|
— |
|
142.47 |
|
3/1/2031 |
|
1,375 |
|
57,846 |
|
— |
|
— |
| |
3/1/2020 |
|
22,000 |
|
— |
|
— |
|
56.08 |
|
3/1/2030 |
|
— |
|
— |
|
— |
|
— |
| |
6/19/2019 |
|
12,000 |
|
— |
|
— |
|
63.27 |
|
6/19/2029 |
|
— |
|
— |
|
— |
|
— |
| |
3/1/2019 |
|
13,000 |
|
— |
|
— |
|
67.55 |
|
3/1/2029 |
|
— |
|
— |
|
— |
|
— |
| |
3/1/2018 |
|
3,900 |
|
— |
|
— |
|
48.43 |
|
3/1/2028 |
|
— |
|
— |
|
— |
|
— |
| |
7/6/2017 |
|
30,000 |
|
— |
|
— |
|
63.28 |
|
7/6/2027 |
|
— |
|
— |
|
— |
|
— |
Eric Crombez,
M.D. |
|
3/1/2024 |
|
— |
|
34,200 |
|
— |
|
53.69 |
|
3/1/2034 |
|
19,400 |
|
816,158 |
|
19,400 |
|
816,158 |
| |
5/1/2023 |
|
3,310 |
|
5,052 |
|
— |
|
44.03 |
|
5/1/2033 |
|
18,464 |
|
776,780 |
|
7,029 |
|
295,710 |
| |
3/1/2023 |
|
4,308 |
|
5,537 |
|
— |
|
45.65 |
|
3/1/2033 |
|
5,538 |
|
232,984 |
|
— |
|
— |
| |
3/1/2022 |
|
7,491 |
|
8,249 |
|
— |
|
67.37 |
|
3/1/2032 |
|
1,587 |
|
66,765 |
|
— |
|
— |
| |
4/16/2021 |
|
— |
|
— |
|
— |
|
— |
|
|
|
500 |
|
21,035 |
|
— |
|
— |
| |
3/1/2021 |
|
4,041 |
|
269 |
|
— |
|
142.47 |
|
3/1/2031 |
|
360 |
|
15,145 |
|
— |
|
— |
| |
3/1/2020 |
|
13,000 |
|
— |
|
— |
|
56.08 |
|
3/1/2030 |
|
— |
|
— |
|
— |
|
— |
| |
3/1/2019 |
|
8,000 |
|
— |
|
— |
|
67.55 |
|
3/1/2029 |
|
— |
|
— |
|
— |
|
— |
| |
11/17/2017 |
|
7,500 |
|
— |
|
|
|
48.11 |
|
11/17/2027 |
|
— |
|
— |
|
— |
|
— |
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 56
| |
|
|
|
Option Awards(1) |
|
Stock Awards |
| Name |
|
Grant Date |
|
Number
of
Securities
Underlying
Unexercised
Options (#)
Exercisable |
|
Number of
Securities
Underlying
Unexercised
Options (#)
Unexercisable |
|
Equity Incentive
Plan Awards:
Number of
Securities
Underlying
Unexercised
Unearned
Options (#)(2) |
|
Option
Exercise
Price
($) |
|
Option
Expiration
Date |
|
Number of
Shares or
Units of
Stock
That Have
Not Vested
(#)(3) |
|
Market
Value of
Shares or
Units of
Stock That
Have Not
Vested
($) |
|
Equity Incentive
Plan Awards:
Number of
Unearned
Shares, Units
or Other Rights
That Have Not
Vested (#)(4) |
|
Equity Incentive Plan
Awards: Market
or Payout Value of
Unearned Shares,
Units or Other Rights
That Have Not
Vested
($) |
| John R. Pinion II |
|
10/21/2024 |
|
— |
|
— |
|
100,000 |
|
2.31 |
|
10/21/2034 |
|
— |
|
— |
|
— |
|
— |
| |
3/1/2024 |
|
— |
|
31,400 |
|
— |
|
53.69 |
|
3/1/2034 |
|
17,800 |
|
748,846 |
|
17,800 |
|
748,846 |
| |
|
3/1/2023 |
|
17,238 |
|
22,162 |
|
— |
|
45.65 |
|
3/1/2033 |
|
31,471 |
|
1,323,985 |
|
7,029 |
|
295,710 |
| |
|
3/1/2022 |
|
17,119 |
|
7,781 |
|
— |
|
67.37 |
|
3/1/2032 |
|
10,472 |
|
440,557 |
|
— |
|
— |
| |
|
3/1/2021 |
|
10,313 |
|
687 |
|
— |
|
142.47 |
|
3/1/2031 |
|
1,375 |
|
57,846 |
|
— |
|
— |
| |
|
3/1/2020 |
|
22,000 |
|
— |
|
— |
|
56.08 |
|
3/1/2030 |
|
— |
|
— |
|
— |
|
— |
| |
|
3/1/2019 |
|
23,000 |
|
— |
|
— |
|
67.55 |
|
3/1/2029 |
|
— |
|
— |
|
— |
|
— |
| |
|
3/1/2018 |
|
27,000 |
|
— |
|
— |
|
48.43 |
|
3/1/2028 |
|
— |
|
— |
|
— |
|
— |
| |
|
3/1/2017 |
|
18,000 |
|
— |
|
— |
|
88.80 |
|
3/1/2027 |
|
— |
|
— |
|
— |
|
— |
| |
|
6/3/2016 |
|
11,000 |
|
— |
|
— |
|
69.53 |
|
6/3/2026 |
|
— |
|
— |
|
— |
|
— |
| |
|
6/1/2016 |
|
17,800 |
|
— |
|
— |
|
70.57 |
|
6/1/2026 |
|
— |
|
— |
|
— |
|
— |
| |
|
7/16/2015 |
|
90,000 |
|
— |
|
— |
|
124.87 |
|
7/16/2025 |
|
— |
|
— |
|
— |
|
— |
| (1) |
The options vest with respect to 1/4th of the shares underlying
the option on the one-year anniversary of the applicable grant date, and with respect to 1/48th of the shares underlying the
option, on each monthly anniversary thereafter, subject to the holder’s continued service to us through each such vesting
date. Please see the section entitled “—Narrative Disclosure to Summary Compensation Table—Covered Transaction”
for accelerated vesting provisions that apply on certain terminations of employment. |
| (2) |
Reflects PSOs from Amlogenyx, a privately-held subsidiary of Ultragenyx
in which Ultragenyx currently holds a majority interest. The Amlogenyx PSOs will vest upon achievement of specified clinical
performance criteria, with each criteria weighted 25%. If the performance criteria are not achieved during the 18-month performance
period ending April 21, 2026, any unvested portion of the Amlogenyx PSOs will be forfeited. |
| (3) |
Amounts in this column reflects unvested RSUs as well as earned but unvested
PSUs. The RSUs vest with respect to 1/4th of the underlying shares on each anniversary of the grant date over a four-year
period. For (i) Dr. Kakkis, 12,906 of the total earned PSUs listed in the March 1, 2022 row and 94,386 of the total earned
PSUs listed in the March 1, 2023 row vested on March 1, 2025 and for (ii) each of Mr. Harris and Mr. Pinion, 3,472 of the
total earned PSUs listed in the March 1, 2022 row, for Mr. Harris and Mr. Pinion, 14,971 of the total earned PSUs listed in
the March 1, 2023 row vested on March 1, 2025 and for Dr. Crombez, 14,971 of the of the total earned PSUs listed in the
May 1, 2023 row vested on March 1, 2025. Please see the section entitled “—Narrative Disclosure to Summary Compensation
Table—Covered Transaction” for accelerated vesting provisions that apply on certain terminations of employment. |
| (4) |
The PSUs set forth in the table are reported at target achievement. The
PSUs vest as described above under “Compensation Discussion and Analysis—Equity Compensation”. Please see
the section entitled “—Narrative Disclosure to Summary Compensation Table—Covered Transaction” for
accelerated vesting provisions that apply on certain terminations of employment. |
Option Exercises and Stock Vested
The following table sets forth certain information concerning
the stock awards vested for our named executive officers during fiscal 2024. No named executive officers exercised any option awards
during fiscal 2024.
| |
Stock Awards |
| Name |
Number of
Shares Acquired
on Vesting
(#) |
|
Value
Realized on
Vesting
($)(1) |
| Emil D. Kakkis, M.D., Ph.D. |
36,442 |
|
1,956,571 |
| Howard Horn |
20,092 |
|
1,072,511 |
| Erik Harris |
13,282 |
|
713,111 |
| Eric Crombez, M.D. |
5,781 |
|
292,588 |
| John R. Pinion II |
11,620 |
|
623,878 |
| (1) |
Value realized is equal to the closing price of our
common stock on The Nasdaq Global Select Market on each vesting date multiplied by the number of shares of stock that vested
on such date. |
Pension Benefits
We do not have a defined benefit plan. Our
named executive officers did not participate in, or otherwise receive any special benefits under, any pension or defined benefit
retirement plan sponsored by us during fiscal 2024.
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 57
Nonqualified Deferred Compensation
In June 2021, we adopted a non-qualified
deferred compensation plan (Deferred Compensation Plan). Our Deferred Compensation Plan permits highly-compensated U.S. based employees,
including our named executive officers, as well as non-employee members of the Board to defer up to 75% of their base salary and
up to 100% of director compensation and other types of compensation, including annual cash bonuses, RSUs and PSUs awarded.
Generally, a deferral election must be made
no later than December 31 of the previous year and is irrevocable. Deferrals with respect to salary are deducted from the participant’s
salary in equal installments for the period of January 1 to December 31 of each year. These deferral elections are for the salary
earned by the participant for the particular salary pay period during that year, which would otherwise be payable to the participant
in such pay period. The election to defer salary under the Deferred Compensation Plan is in addition to any deferral election made
by the participant under our 401(k) Plan. Deferrals for performance-based annual bonuses are for those bonuses earned during the
year for which the election applies, which are payable the following year. The Deferred Compensation Plan is intended to provide
participants with a tax deferral opportunity for compensation paid by us. The deferred amounts are not subject to income tax or
income tax withholding when earned and deferred, but are fully taxable (and withheld appropriately) when distributed.
The
Deferred Compensation Plan authorizes the Company to make matching contributions at our sole discretion. The participant is 100%
vested at all times in his or her deferred cash account (including any company
matching contributions), and deferrals of any compensation subject to vesting (such as RSUs and PSUs) shall vest in accordance
with the provisions of the underlying award. No matching contributions were made by the Company in 2024.
The Deferred Compensation Plan provides
for distribution of deferred compensation and earnings thereon upon a participant’s separation from service with us,
his or her retirement, a date specified by the participant in his or her compensation deferral agreement, the death of a
participant (in such a case, to the designated beneficiary) or a “change in control.” Payment distributions can
be made in a lum psum, annual installments of up to five years at the participant’s election or for “specified
date accounts only”, installments of up to 15 years, at the participant’s election.
The Deferred Compensation Plan credits gains
and losses to deferred amounts based upon “deemed” investments in mutual funds investing in equity instruments or debt
securities chosen by each participant (which the participant may change at any time) from a “menu” of fund options
provided by us. The investment returns credited to participants’ accounts in the Deferred Compensation Plan correspond to
actual returns of the chosen funds. The performance of the mutual funds fluctuates with the conditions of the capital markets and
the economy generally, and is affected by prevailing interest rates and credit risks. The investment options under the Deferred
Compensation Plan include:
| Fund |
2024 Rate of Return (%) |
| PIMCO VIT Short-Term Admin |
6.1 |
| Vanguard VIF Total Bond Market Index |
1.2 |
| Western Asset Core Plus Vit I |
(0.4) |
| DFA VIT Inflation-Protected Secs Instl. |
1.9 |
| Empower Multi-Sector Bond Investor (MXLMX) |
5.1 |
| American Funds IS®
Capital World Bond 2 |
(3.0) |
| Vanguard VIF Equity Income |
15.1 |
| Fidelity®
VIP Index 500 Initial |
24.9 |
| Invesco VI Equally Wtd S&P 500 I |
12.7 |
| T. Rowe Price Blue Chip Growth Port |
35.5 |
| American Century Vp Mid Cap Value I (AVIPX) |
8.7 |
| Empower S&P Mid
Cap 400® Index Inv (MXMDX) |
13.3 |
| MFS® VIT Mid Cap Growth Init |
14.7 |
| Dimensional VA US Targeted Value |
8.1 |
| Empower S&P Smallcap 600® Index Inv
(MXISX) |
7.9 |
| Clearbridge Variable Small Cap Growth I (QLMSIX) |
4.5 |
| Vanguard VIF Total Intl Stock Market Index |
5.1 |
| Vanguard VIF International |
9.0 |
| American Funds IS® New World 2 |
6.6 |
| MFS®
VIT III Global Real Estate Initial |
(2.7) |
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 58
For fiscal year 2024, other than Mr. Pinion,
none of our named executive officers, participated in the Deferred Compensation Plan.
| Name |
|
Executive
Contributions
in Last FY
($) |
|
Company
Contributions
in Last FY
($) |
|
Aggregate
Earnings
in Last FY
($) |
|
Aggregate
Withdrawals/
Distributions
($) |
|
Aggregate
Balance
at Last FYE
($) |
| Emil D. Kakkis, M.D., Ph.D., President and Chief Executive Officer |
|
— |
|
— |
|
— |
|
— |
|
— |
| Howard Horn Chief Financial Officer, Corporate Strategy and Executive Vice President |
|
— |
|
— |
|
— |
|
— |
|
— |
| Erik Harris, Chief Commercial Officer and Executive Vice President |
|
— |
|
— |
|
— |
|
— |
|
— |
| Eric Crombez, M.D., Chief Medical Officer and Executive Vice President |
|
— |
|
— |
|
— |
|
— |
|
— |
| John R. Pinion II, Chief Quality Officer and Executive Vice President, Translational Sciences |
|
86,011(1) |
|
— |
|
29,798 |
|
— |
|
333,174 |
| (1) |
Represents the value of 1,602 RSUs as of the applicable vesting
date that were deferred into the Deferred Compensation Plan upon the partial vesting of such RSUs. |
Potential Payments Upon Termination or Change of Control
The amount of compensation and benefits
payable to each named executive officer in various termination and change in control situations, as described above under
“—Narrative Disclosure to Summary Compensation Table—Involuntary Termination of Employment” and
“—Narrative Disclosure to Summary Compensation Table—Covered Transaction, has been estimated in the tables
below. The tables below do not include the values of any amounts that a named executive officer may receive under the
Deferred Plan as a result of a termination of employment or a change in control, as all amounts under the Deferred Plan are
fully vested benefits.
The value of the option, RSU, and PSU
vesting acceleration was calculated for each of the tables below based on the assumption that the change in control and
executive’s employment termination occurred on December 31, 2024. The closing price of our common stock on The Nasdaq
Global Select Market as of December 31, 2024 was $42.07, which was used as the value of our common stock for purposes of the
following tables. The value of the option vesting acceleration was calculated by multiplying the number of unvested option
shares subject to vesting acceleration as of December 31, 2024 by the difference between the closing price of our common
stock as of December 31, 2024 and the exercise price for such unvested option shares. No value is attributed to unvested
options subject to acceleration which have exercise prices above the closing market price of our common stock as of December
31, 2024. The value of the RSU and PSU vesting acceleration was calculated by multiplying the number of unvested RSUs, earned
but unvested PSUs and unearned PSUs (based on an assumed target level of performance) subject to vesting acceleration as of
December 31, 2024 by the closing price of our common stock as of the last trading day of 2023. The value of COBRA
reimbursements was calculated for each of the tables below based on the elections for each named executive officer in effect
in December 2024 and the applicable COBRA premiums for December 2024.
Dr. Emil Kakkis
The following table describes the potential
payments upon employment termination for Emil Kakkis, our President and Chief Executive Officer, as if his employment terminated
as of December 31, 2024, the last business day of the fiscal year. Qualifying termination includes a termination by the Company
without cause or a resignation by Dr. Kakkis for good reason.
Potential Payments Upon Termination
or Change of Control | |
Qualifying
Termination | | |
Qualifying Termination
following a Covered
Transaction | |
| Base Salary | |
$ | 1,722,000 | | |
$ | 1,722,000 | |
| Bonus | |
| 688,800 | | |
| 1,377,600 | |
| Acceleration of equity awards | |
| — | | |
| 15,586,430 | |
| COBRA reimbursements | |
| 40,086 | | |
| 40,086 | |
| TOTAL | |
$ | 2,450,886 | | |
$ | 18,726,116 | |
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 59
Howard Horn
The following table describes the potential
payments upon employment termination for Howard Horn, our Chief Financial Officer, Corporate Strategy and Executive Vice President,
as if his employment terminated as of December 31, 2024, the last business day of the fiscal year. Qualifying termination includes
a termination by the Company without cause or a resignation by Mr. Horn due to a constructive termination.
Potential Payments Upon Termination
or Change of Control | |
Qualifying
Termination | | |
Qualifying Termination
following a Covered
Transaction |
| Base Salary | |
$ | 590,000 | | |
$ | 885,000 |
| Bonus | |
| 295,000 | | |
| 442,500 |
| Acceleration of equity awards | |
| — | | |
| 4,803,835 |
| COBRA reimbursements | |
| 26,378 | | |
| 39,567 |
| TOTAL | |
$ | 911,378 | | |
$ | 6,170,902 |
Erik Harris
The following table describes the potential
payments upon employment termination for Erik Harris, our Chief Commercial Officer and Executive Vice President as if his employment
terminated as of December 31, 2024, the last business day of the fiscal year. Qualifying termination includes a termination by
the Company without cause or a resignation by Mr. Harris due to a constructive termination.
Potential Payments Upon Termination
or Change of Control | |
Qualifying
Termination | | |
Qualifying Termination
following a Covered
Transaction |
| Base Salary | |
$ | 607,000 | | |
$ | 910,500 |
| Bonus | |
| 303,500 | | |
| 455,250 |
| Acceleration of equity awards | |
| — | | |
| 3,750,414 |
| COBRA reimbursements | |
| 8,935 | | |
| 13,402 |
| TOTAL | |
$ | 919,435 | | |
$ | 5,129,566 |
Eric Crombez, M.D.
The following table describes the potential
payments upon employment termination for Eric Crombez, M.D., our Chief Medical Officer and Executive Vice President, as if his
employment terminated as of December 31, 2024, the last business day of the fiscal year. Qualifying termination includes a termination
by the Company without cause or a resignation by Mr. Pinion due to a constructive termination.
Potential Payments Upon Termination
or Change of Control | |
Qualifying
Termination | | |
Qualifying Termination
following a Covered
Transaction |
| Base Salary | |
$ | 604,000 | | |
$ | 906,000 |
| Bonus | |
| 302,000 | | |
| 453,000 |
| Acceleration of equity awards | |
| — | | |
| 3,040,735 |
| COBRA reimbursements | |
| 20,043 | | |
| 30,064 |
| TOTAL | |
$ | 926,043 | | |
$ | 4,429,799 |
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 60
John R. Pinion II
The following table describes the potential
payments upon employment termination for John Pinion, our Chief Quality Officer and Executive Vice President, Translational Sciences
as if his employment terminated as of December 31, 2024, the last business day of the fiscal year. Qualifying termination includes
a termination by the Company without cause or a resignation by Mr. Pinion due to a constructive termination.
Potential Payments Upon Termination
or Change of Control | |
Qualifying
Termination | | |
Qualifying Termination
following a Covered
Transaction |
| Base Salary | |
$ | 572,000 | | |
$ | 858,000 |
| Bonus | |
| 286,000 | | |
| 429,000 |
| Acceleration of equity awards | |
| — | | |
| 3,615,790 |
| COBRA reimbursements | |
| 39,974 | | |
| 59,961 |
| TOTAL | |
$ | 897,974 | | |
$ | 4,962,751 |
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 61
Equity Compensation Plan Information
The table below discloses information as
of December 31, 2024 with respect to our equity compensation plans that have been approved by stockholders and equity compensation
plans that have not been approved by stockholders.
| Plan Category |
|
Number of Securities
to be Issued
upon Exercise of
Outstanding Options,
Warrants and Rights(a)(1) |
|
Weighted-Average
Exercise Price of
Outstanding Options,
Warrants and Rights |
|
Number of Securities
Remaining Available
for Future Issuance
under Equity
Compensation Plans
(Excluding Securities
Reflected in Column(a)) |
| Equity compensation plans approved by security holders: |
|
|
|
|
|
|
| 2014 Incentive Plan |
|
11,190,128 |
|
$68.34 |
|
— |
| 2014 Employee Stock Purchase Plan |
|
— |
|
— |
|
6,409,256 |
| 2023 Equity Incentive Plan |
|
4,327,714 |
|
$52.87 |
|
6,139,766 |
| Equity compensation plans not approved by security holders |
|
|
|
|
|
|
| Dimension Therapeutics, Inc. 2015 Stock Option and Incentive Plan(2) |
|
21,531 |
|
$49.12 |
|
— |
| Dimension Therapeutics, Inc. 2013 Stock Plan(2) |
|
940 |
|
$28.66 |
|
— |
| Employment Inducement Plan |
|
854,219 |
|
$47.66 |
|
211,628 |
| TOTAL |
|
16,394,532 |
|
$65.75 |
|
12,760,650 |
| (1) |
Amounts in this column include outstanding stock options, RSUs and PSUs (assuming
target performance for any unearned PSUs. |
| (2) |
In connection with our acquisition of Dimension Therapeutics, Inc. on November 7, 2017, we
assumed these plans and outstanding option awards thereunder (whether or not then vested or exercisable). The assumed awards
continue to have, and are subject to, the same terms and conditions as were applicable prior to the acquisition as set forth
in the applicable plan (including any applicable award agreement, other agreement or other document evidencing such awards),
except that the awards are exercisable for shares of our common stock with exercise prices adjusted to reflect the terms of
the acquisition, all as set forth in the merger agreement. No new awards can be made under these plans. |
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 62
Director Compensation
Our Board has adopted a non-employee director
compensation policy that is designed to provide a total compensation package that enables us to attract and retain, on a long-term
basis, high caliber non-employee directors. A Board compensation review prepared by Aon in February 2024 provided a competitive
assessment of our compensation practices for non-employee directors in connection with the Compensation Committee’s evaluation
of the level of compensation for our non-employee directors for 2024.
A summary of the non-employee director cash compensation arrangements
for fiscal 2024 is set forth below:
| | |
Annual Retainer |
| Board of Directors: | |
| |
| Chairman | |
$ | 85,000 |
| Non-Chairman members | |
$ | 50,000 |
| Audit Committee: | |
| |
| Chairman | |
$ | 25,000 |
| Non-Chairman members | |
$ | 12,500 |
| Compensation Committee: | |
| |
| Chairman | |
$ | 20,000 |
| Non-Chairman members | |
$ | 10,000 |
| Nominating and Corporate Governance Committee: | |
| |
| Chairman | |
$ | 12,000 |
| Non-Chairman members | |
$ | 6,000 |
| Research and Development Committee: | |
| |
| Chairman | |
$ | 15,000 |
| Non-Chairman members | |
$ | 7,500 |
In 2025, upon recommendation of the Compensation
Committee, the Board maintained the annual cash compensation levels under the non-employee director compensation policy.
Under the non-employee director compensation policy for fiscal 2024, each non-employee director who was
initially appointed or elected to the Board received an equity award with a target value of $600,000, comprised 50% of options
to purchase shares of our common stock and 50% of RSUs on the date he or she first becomes a non-employee director. The options
vest monthly over a three-year period and the RSUs vest annually in equal amounts over a three-year period, in each case subject
to the director’s continued service to us through each such vesting date. In addition, for fiscal year 2024, on the date
of the annual meeting of stockholders, each continuing non-employee director received an annual equity award at a target value
of $400,000, comprised of 50% options to purchase shares of our common stock and 50% RSUs, each of which would vest in full upon
the earlier of (1) our subsequent annual meeting of stockholders and (2) the first anniversary of the date of grant, subject to
the director’s continued service to us through such vesting date. The exercise price of all of the foregoing options was
equal to the fair market value of a share of our common stock on the date of grant.
In 2025, upon recommendation of the Compensation
Committee, the Board maintained the target values of annual awards for non-employee directors and the target annual awards for
newly appointed directors.
Dr. Kakkis, our President and Chief Executive
Officer, receives no compensation for his service as a director.
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 63
The following table shows the compensation earned in fiscal 2024
by our non-employee directors.
| Name | |
Fees Earned or
Paid in Cash in
Fiscal 2024 | | |
Stock
Awards(1) | | |
Option
Awards(2) | | |
Total | |
| Daniel G. Welch | |
$ | 100,750 | | |
$ | 200,010 | | |
$ | 199,374 | | |
$ | 500,134 | |
| Deborah Dunsire, M.D. | |
| 67,375 | | |
| 200,010 | | |
| 199,374 | | |
| 466,759 | |
| Matthew K. Fust | |
| 80,750 | | |
| 200,010 | | |
| 199,374 | | |
| 480,134 | |
| Michael Narachi | |
| 82,500 | | |
| 200,010 | | |
| 199,374 | | |
| 481,884 | |
| Amrit Ray, M.D. | |
| 64,750 | | |
| 200,010 | | |
| 199,374 | | |
| 464,134 | |
| Corsee D. Sanders, Ph.D. | |
| 69,875 | | |
| 200,010 | | |
| 199,374 | | |
| 469,259 | |
| Shehnaaz Suliman, M.D. | |
| 68,875 | | |
| 200,010 | | |
| 199,374 | | |
| 468,259 | |
| (1) |
The amounts reported in this column for a fiscal year represent the grant date
fair value of the RSUs granted to our non-employee directors during fiscal 2024, as computed in accordance with ASC Topic
718, not including any estimates of forfeitures. The assumptions used in calculating the grant date fair value of the RSUs
reported in this column are set forth in the notes to our financial statements included in our Annual Report. The amounts
reported in this column reflect the accounting cost for these RSUs, and do not correspond to the actual economic value that
may be received by the non-employee directors from the RSUs. As of December 31, 2024, each of our non-employee directors had
5,345 outstanding RSUs. |
| (2) |
The amounts reported in this column represent the grant date fair value of the stock options
granted to our non-employee directors during fiscal 2024, as computed in accordance with ASC Topic 718, not including any
estimates of forfeitures. The assumptions used in calculating the grant date fair value of the stock options reported in this
column are set forth in the notes to our financial statements included in our Annual Report. The amounts reported in this
column reflect the accounting cost for these stock options, and do not correspond to the actual economic value that may be
received by the non-employee directors from the options. As of December 31, 2024, our then non-employee directors had the
following outstanding options: Mr. Welch – 74,130; Dr. Dunsire – 60,380; Mr. Fust – 56,630; Mr. Narachi
– 74,130; Dr. Ray – 32,780; Dr. Sanders – 30,510; and Dr. Suliman – 56,630. |
CEO Pay Ratio
We are required by SEC rules adopted under
the Dodd-Frank Act to disclose the ratio of our median employee’s annual total compensation to the annual total compensation
of our principal executive officer. This disclosure provides a measure of the equitability of pay within our company. We believe
our compensation philosophy and process yield an equitable result for all of our employees. For 2024, the annual total compensation
for Dr. Emil Kakkis, our Chief Executive Officer and President, was $13,717,687 and for our median employee was $325,295, resulting
in a pay ratio of 42:1.
In accordance with Item 402(u) of Regulation
S-K, we identified the median employee by (i) aggregating for each applicable employee (A) base salary for 2024 on the calculation
date, (B) the target bonus for 2024, and (C) the accounting value of any equity awards granted during 2024, and (ii) ranking this
annual compensation measure for our employees from lowest to highest. This calculation encompasses individuals, excluding our CEO,
employed by us on October 1, 2024, whether employed on a full-time, part-time, or seasonal basis. For any permanent employees who
were only employed for part of the 2024 fiscal year, we annualized their compensation to present a more accurate representation
of their comparative annual compensation. On October 1, 2024, we had 1,291 employees. The total compensation of our identified
median employee using the same methodology we use for our named executive officers as set forth in the Summary Compensation Table.
The SEC’s rules for identifying the
median employee and calculating the pay ratio based on that employee’s annual total compensation allow companies to adopt
a variety of methodologies, to apply certain exclusions, and to make reasonable estimates and assumptions that reflect their employee
populations and compensation practices. As a result, the pay ratio reported by other companies may not be comparable to the pay
ratio reported above, as other companies have different employee populations and compensation practices and may utilize different
methodologies, exclusions, estimates and assumptions in calculating their own pay ratios.
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 64
Pay versus Performance
As required by Section 953(a) of the Dodd-Frank
Wall Street Reform and Consumer Protection Act, and Item 402(v) of Regulation S-K, we are providing the following information about
the relationship between Compensation Actually Paid (CAP) and certain financial performance of the Company. For further information
concerning the Company’s pay for performance philosophy and how the Company’s aligns executive compensation with the
Company’s performance, refer to “Executive Compensation – Compensation Discussion and Analysis.”
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | |
| | |
| | |
Average | | |
| | |
Value of Initial Fixed $100 Investment Based On: | | |
| | |
| |
| Year | |
Summary
Compensation Table
Total for PEO(1) | | |
Compensation
Actually Paid to
PEO(2) | | |
Summary
Compensation
Table Total for
Non-PEO NEOs(3) | | |
Average
Compensation
Actually Paid to
Non-PEO NEOs(4) | | |
Total
Shareholder
Return(5) | | |
Peer Group
Total
Shareholder
Return(6) | | |
Net Income
(Loss)
(in thousands)(7) | | |
Revenue
(in thousands)(8) | |
| 2024 | |
$ | 13,717,687 | | |
$ | 8,186,261 | | |
$ | 4,254,959 | | |
$ | 2,790,240 | | |
$ | 99 | | |
$ | 114 | | |
$ | (569,183) | | |
$ | 560,230 | |
| 2023 | |
$ | 13,077,993 | | |
$ | 11,293,489 | | |
$ | 4,524,001 | | |
$ | 4,829,669 | | |
$ | 112 | | |
$ | 115 | | |
$ | (606,639) | | |
$ | 434,249 | |
| 2022 | |
$ | 12,140,330 | | |
$ | 3,311,432 | | |
$ | 3,911,773 | | |
$ | (175,590) | | |
$ | 108 | | |
$ | 111 | | |
$ | (707,421) | | |
$ | 363,329 | |
| 2021 | |
$ | 9,522,738 | | |
$ | (2,152,804) | | |
$ | 3,314,256 | | |
$ | (1,211,638) | | |
$ | 197 | | |
$ | 125 | | |
$ | (454,025) | | |
$ | 351,406 | |
| 2020 | |
$ | 5,144,220 | | |
$ | 22,726,743 | | |
$ | 2,706,102 | | |
$ | 8,015,000 | | |
$ | 324 | | |
$ | 126 | | |
$ | (186,566) | | |
$ | 271,030 | |
| (1) |
The dollar amounts reported are the amounts in the “Total” column
of the Summary Compensation Table in each applicable year for Dr. Kakkis, who served as our Chief Executive Officer during
2020, 2021, 2022, 2023 and 2024. |
| (2) |
The dollar amounts reported represent the amount of CAP, as computed in accordance with SEC
rules. The dollar amounts do not reflect the actual amount of compensation earned by or paid during the applicable year. In
accordance with SEC rules, the following adjustments were made to total compensation to determine the CAP: |
| |
Compensation Actually Paid to PEO | |
2024 | | |
2023 | | |
2022 | | |
2021 | | |
2020 | |
| |
Summary Compensation Table Total | |
$ | 13,717,687 | | |
$ | 13,077,993 | | |
$ | 12,140,330 | | |
$ | 9,522,738 | | |
$ | 5,144,220 | |
| |
Less, value of “Stock Awards” and “Option Awards”
reported in Summary Compensation Table | |
| (12,090,111) | | |
| (11,618,444) | | |
| (10,773,976) | | |
| (8,246,092) | | |
| (3,709,589) | |
| |
Plus, year end fair value of outstanding and unvested equity awards
granted during the year | |
| 8,562,675 | | |
| 11,288,072 | | |
| (6,264,762) | | |
| 4,152,033 | | |
| 10,163,438 | |
| |
Plus (less), change in fair value from prior year end to current year
end of outstanding and unvested equity awards granted in prior years | |
| (2,033,745) | | |
| (1,167,400) | | |
| (2,934,552) | | |
| (5,753,632) | | |
| 9,346,926 | |
| |
Plus (less), change in fair value from prior year end to vesting date
of equity awards granted in prior years that vested in the year | |
| 29,755 | | |
| (286,732) | | |
| (1,385,132) | | |
| (1,827,851) | | |
| 1,781,748 | |
| |
Less, prior year-end fair value of any equity awards that failed to
meet vesting conditions in the year | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| |
Compensation Actually Paid to
Dr. Kakkis | |
$ | 8,186,261 | | |
$ | 11,293,489 | | |
$ | 3,311,432 | | |
$ | (2,152,804) | | |
$ | 22,726,743 | |
| (3) |
The dollar amounts reported represent the average of the amounts reported for
the Company’s named executive officers (NEOs) as a group (excluding our CEO) in the “Total” column of the
Summary Compensation Table in each applicable year. The names of each of the NEOs (excluding our CEO) included for purposes
of calculating the average amounts in each applicable year are as follows: (i) for 2024, Mr. Horn, Erik Harris, Dr. Crombez
and Mr. Pinion, (ii) for 2023, Mr. Horn, Mr. Harris, Mr. Pinion and Ms. Parschauer, (iii) for 2022 and 2021, Dr. Camille Bedrosian,
Mr. Harris, Mr. Pinion and Mardi Dier; and (iv) for 2020, Dr. Bedrosian, Ms. Dier, Mr. Harris, Mr. Pinion, and Shalini Sharp. |
| (4) |
The dollar amounts reported represent the average amount of CAP to the NEOs as a group (excluding
our CEO), as computed in accordance with SEC rules. The dollar amounts do not reflect the actual average amount of compensation
earned by or paid to the NEOs as a group (excluding our CEO) during the applicable year. In accordance with the SEC rules,
the following adjustments were made to average total compensation for the NEOs as a group (excluding our CEO) for each year
to determine the compensation actually paid, using the same methodology described above in Note 2: |
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 65
| |
Compensation Actually Paid to Non-PEO NEOs | |
2024 | | |
2023 | | |
2022 | | |
2021 | | |
2020 | |
| |
Summary Compensation Table Total | |
$ | 4,254,959 | | |
$ | 4,524,001 | | |
$ | 3,911,773 | | |
$ | 3,314,256 | | |
$ | 2,706,102 | |
| |
Less, value of “Stock Awards” and “Option Awards” reported in
Summary Compensation Table | |
| (3,293,798) | | |
| (3,831,408) | | |
| (3,130,468) | | |
| (2,519,639) | | |
| (2,027,088) | |
| |
Plus, year end fair value of outstanding and unvested equity awards granted during the
year | |
| 2,412,702 | | |
| 4,476,201 | | |
| 1,264,910 | | |
| 1,268,695 | | |
| 4,150,071 | |
| |
Plus (less), change in fair value from prior year end to current year end of outstanding
and unvested equity awards granted in prior years | |
| (607,133) | | |
| (237,537) | | |
| (784,234) | | |
| (2,359,539) | | |
| 2,748,610 | |
| |
Plus (less), change in fair value from prior year end to vesting date of equity awards
granted in prior years that vested in the year | |
| 23,510 | | |
| (101,588) | | |
| (573,020) | | |
| (915,411) | | |
| 437,305 | |
| |
Less, prior year-end fair value of any equity awards that failed to meet vesting conditions
in the year | |
| — | | |
| — | | |
| (864,551) | | |
| — | | |
| — | |
| |
Compensation Actually Paid to Non-PEO NEOs | |
$ | 2,790,240 | | |
$ | 4,829,669 | | |
$ | (175,590) | | |
$ | (1,211,638) | | |
$ | 8,015,000 | |
| (5) |
Cumulative TSR is calculated by dividing (a) the sum of (i) the
cumulative amount of dividends for the measurement period, assuming dividend reinvestment, and (ii) the difference between
the Company’s share price at the end and the beginning of the measurement period by (b) the Company’s share price
at the beginning of the measurement period. The beginning of the measurement period for each year in the table is December
31, 2019. |
| (6) |
Represents the weighted peer group TSR, weighted according to the respective
companies’ stock market capitalization at the beginning of each period for which a return is indicated. The peer group
used for this purpose is the following published industry index: Nasdaq Biotechnology Index. |
| (7) |
The dollar amounts reported represent the amount of net income calculated
in accordance with GAAP reflected in the Company’s audited financial statements for the applicable year. |
| (8) |
The dollar amounts reported represent the amount of revenue calculated in
accordance with GAAP reflected in the Company’s audited financial statements for the applicable year. |
Performance Measures
As described in greater detail in
“Executive Compensation – Compensation Discussion and Analysis,” the Company’s executive compensation
program reflects a variable pay-for-performance philosophy. The metrics that the Company uses for both our long-term and
short-term incentive awards are selected based on an objective of incentivizing our NEOs to increase the value of our
enterprise for our stockholders. The most important performance measures used by the Company to link executive compensation
actually paid to the Company’s NEOs, for the most recently completed fiscal year, to the Company’s performance
are as follows:
| • |
Total Revenue |
| • |
Relative Total Stockholder Return |
| • |
Net Cash Used in Operations |
| • |
Clinical Development of Product Candidates |
Description of the Relationship between Pay and Performance
As described in more detail in the section
“Executive Compensation – Compensation Discussion and Analysis,” the Company’s executive compensation program
reflects a variable pay-for-performance philosophy. While the Company utilizes several performance measures to align executive
compensation with Company performance, all of those Company measures are not presented in the Pay versus Performance table. Moreover,
the Company generally seeks to incentivize long-term performance, and therefore does not specifically align the Company’s
performance measures with compensation that is actually paid (as computed in accordance with SEC rules) for a particular year.
In accordance with SEC rules, the Company is providing the following descriptions of the relationships between information presented
in the Pay versus Performance table.
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 66
The following graphs provide visual representations of the relationship
between both the CAP of our PEO and the average CAP of our non-PEO NEOs and our (i) TSR, (ii) net income, and (iii) Revenue.
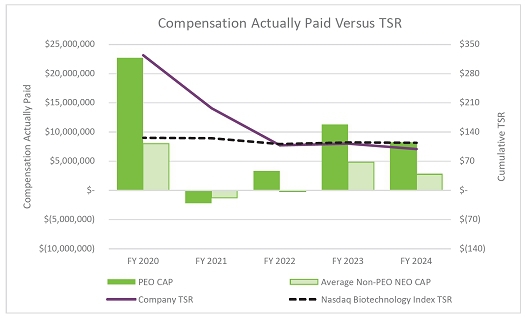
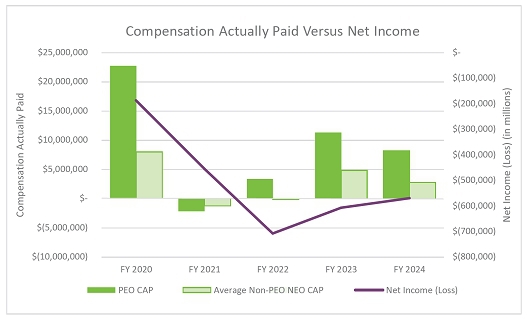
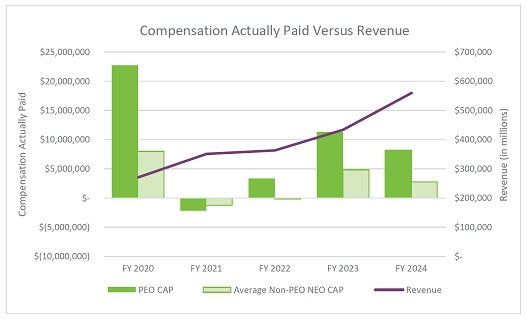
ULTRAGENYX PHARMACEUTICAL INC. • 2025 Proxy Statement 67
Additional Information
Questions and Answers About these Proxy Materials and Voting
Why did I receive a one-page notice
in the mail regarding the Internet availability of proxy materials instead of a full set of proxy materials?
Pursuant to rules adopted by the SEC, we
have elected to provide access to our proxy materials over the Internet. Accordingly, we are sending an Important Notice Regarding
the Availability of Proxy Materials (the Notice of Internet Availability) to our stockholders of record. All stockholders will
have the ability to access the proxy materials on the website referred to in the Notice of Internet Availability free of charge
or request to receive a printed set of the proxy materials for the Annual Meeting. Instructions on how to access the proxy materials
over the Internet or to request a printed copy may be found in the Notice of Internet Availability.
We intend to mail the Notice of Internet
Availability beginning on or about March 28, 2025 to all stockholders of record entitled to vote at the Annual Meeting.
What if I received more
than one Notice of Internet Availability?
If you receive more than one Notice of Internet
Availability, your shares may be registered in more than one name or are registered in different accounts. Please follow the voting
instructions on each Notice of Internet Availability and cast your vote with respect to each set of proxy materials to ensure
that all of your shares are voted.
When and where will
the Annual Meeting be held?
The Annual Meeting will be held on May 15,
2025 at 9:00 a.m. Pacific Time virtually via the Internet at www.virtualshareholdermeeting.com/RARE2025. We conduct the
Annual Meeting virtually via the Internet to facilitate stockholder attendance and participation and have done so every year since
our initial public offering. We believe the virtual format for the Annual Meeting enhances stockholder access by allowing our
stockholders to participate fully, and equally, from any location around the world at no cost. Taking advantage of this virtual
approach reduces our expenses and eliminates the time we would otherwise spend managing the various aspects of holding a physical
meeting. We believe the virtual format is the right choice for us, not only because it brings cost savings to us and our stockholders,
but because it increases our ability to engage with all stockholders, regardless of size, resources, or physical location.
We are aware of concerns that virtual meetings
may diminish stockholder voice or reduce accountability and have taken steps to address these concerns. For example, we believe
that our virtual meeting format enhances, rather than constrains, stockholder access, participation, and communication because
the online format allows stockholders to communicate with us during the Annual Meeting. Stockholders can ask questions of our
Board, management, and a representative from our independent registered public accounting firm during the meeting. During the
live Q&A session, we will answer questions as they come in, as time permits, and in accordance with the meeting rules of conduct
that will be available at the virtual meeting website. Following the Annual Meeting, we intend to publish and answer any questions
received that comply with the meeting of conduct and that are not answered at the meeting. Although the live webcast is available
only to stockholders as of the Record Date (defined below) at the time of the Annual Meeting, the webcast of the Annual Meeting
will be archived for the public for one year after the date of the Annual Meeting at www.virtualshareholdermeeting.com/RARE2025.
Our annual meetings are
only one aspect of our stockholder outreach program, which is a year-long effort by our management to engage with our stockholders
in a continuous and meaningful way. Our stockholders can raise questions or concerns regarding the Company at any time by calling
our Investor Relations department at (844) 280-7681 or contacting our Board by following the process described under “Corporate
Governance—Stockholder Communications”.
To participate in the Annual Meeting, you
must access the meeting website above, enter your 16-digit control number found on your Notice of Internet Availability, proxy
card or voting instruction form, and follow the instructions on the website. If your shares are held in street name and your Notice
of Internet Availability or voting instruction form indicates that you may vote those shares through www.proxyvote.com, then
you may access, participate in and vote at the Annual Meeting with the 16-digit access code indicated on that Notice of Internet
Availability or voting instruction form. Otherwise, stockholders who hold their shares in street name should contact their bank,
broker or other nominee (preferably at least five days before the Annual Meeting) and obtain a “legal proxy” in order
to be able to attend, participate in or vote at the Annual Meeting.
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 68
What am I voting on?
At the Annual Meeting, you will be asked to consider and vote
upon:
| 1. |
The election of the two directors named in the Proxy Statement as Class III directors; |
| 2. |
The approval of the Second A&R 2023 Plan; |
| 3. |
The ratification of the selection of Ernst & Young LLP as our independent registered public accounting firm for the
fiscal year ending December 31, 2025; and |
| 4. |
An advisory (non-binding) resolution to approve the compensation of our named executive officers. |
What if another matter is properly brought
before the Annual Meeting?
The Board of Directors is not aware of any
other matter that will be presented for consideration at the Annual Meeting. If any other matters are properly brought before
the Annual Meeting, the persons named in the accompanying proxy will vote on those matters in accordance with their best judgment.
What is the Board of Directors’ voting
recommendation?
The Board of Directors recommends that you
vote your shares:
| • |
“FOR” the election of each of the Class III director nominees; |
| • |
“FOR” the approval of the Second A&R 2023 Plan; |
| • |
“FOR” the ratification of the selection of Ernst & Young LLP as our independent registered public
accounting firm for the fiscal year ending December 31, 2025; and |
| • |
“FOR” the approval, on an advisory basis, of the compensation of our named executive officers |
How many votes do I have?
Each share of common stock is entitled to
one vote on all matters to be voted upon at the Annual Meeting. Holders of common stock do not have the right to cumulate votes
in the election of directors.
When is the record date for the Annual Meeting?
The Board of Directors has fixed the close
of business on March 24, 2025 as the record date for the Annual Meeting (Record Date).
How many shares must be represented in order
to hold the Annual Meeting?
A quorum of stockholders is necessary to
hold a valid stockholder meeting. A quorum will be present if stockholders holding at least a majority of the outstanding shares
entitled to vote are present or represented by proxy at the Annual Meeting. On the Record Date, there were 93,899,667 shares outstanding
and entitled to vote.
Your shares will be counted towards the
quorum if you submit a valid proxy by mail, over the phone or via the Internet (or one is submitted on your behalf by your broker,
bank or other nominee) or if you attend the Annual Meeting and vote. In addition, abstentions and broker non-votes will be counted
towards the quorum requirement. If there is no quorum, the chair of the Annual Meeting may adjourn the meeting to another date.
How do I vote?
With regard to Proposal No. 1, the election of
directors, you may vote “For” all the nominees to the Board or you may “Withhold” your vote for all the
nominees or any individual nominee you specify. With regard to Proposals No. 2, 3 and 4 you may vote “For” or “Against”
or abstain from voting.
The procedures for voting depend on whether your shares are registered
in your name or are held by a bank, broker or other nominee:
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 69
Registered Holders: Shares Registered in Your Name
If you are a stockholder
of record, you may vote at the Annual Meeting, which will be held virtually via the Internet, vote by proxy over the telephone,
vote by proxy via the Internet, or vote by proxy using a proxy card that you may request or that we may elect to deliver at a
later time. Whether or not you plan to attend the Annual Meeting, we urge you to vote by proxy to ensure your vote is counted.
You may still attend the Annual Meeting and vote online even if you have already voted by proxy.
| • |
To vote via the Internet, vote at www.proxyvote.com prior to 11:59 p.m.
Eastern Time the day before the Annual Meeting. |
| • |
If you have received a paper copy of the proxy materials, vote
over the telephone by calling 1-800-690-6903 prior to 11:59 p.m. Eastern Time the day before the Annual Meeting or by returning
an executed proxy card (that we must receive before the Annual Meeting). |
| • |
Registered holders who attend the Annual Meeting may also vote during the Annual Meeting by
going to www.virtualshareholdermeeting.com/RARE2024 and following the instructions regarding voting. |
Beneficial Holders: Shares Registered in Name of Broker,
Bank or Other Nominee
Persons who hold shares of Ultragenyx common
stock indirectly on the Record Date through a brokerage firm, bank or other financial institution (beneficial holders) may vote
before the Annual Meeting as follows:
| • |
In accordance with the voting instructions provided by the institution
that holds their shares, which may provide for voting over the telephone or via the Internet, or |
| • |
By returning a voting instruction form provided to them by the institution
that holds their shares, in order to have their shares voted on their behalf. |
| • |
Beneficial holders who attend the Annual Meeting may also vote during the
Annual Meeting by going to www.virtualshareholdermeeting.com/RARE2025 and following the instructions regarding voting. |
What if I return a proxy card or otherwise
vote but do not make specific choices?
If you are a stockholder of record and return
a signed and dated proxy card or otherwise vote without marking voting selections, your shares will be voted in accordance with
the Board’s recommendations as describe under “What is the Board of Directors’ voting recommendation?”.
If any other matter is properly presented at the Annual Meeting, your proxy holder (one of the individuals named on your proxy
card) will vote your shares in his or her discretion.
Are my shares voted if I do not provide a
proxy or voting instructions?
If you are a stockholder
of record and do not provide a proxy, you must attend the Annual Meeting in order to vote. If you hold shares through an account
with a bank or broker and do not provide voting instructions, the bank or broker is permitted to vote your shares only on certain
proposals considered “routine” matters. Whether a proposal is considered “routine” or “non-routine”
is subject to stock exchange rules and final determination by the stock exchange. Even with respect to routine matters, some brokers
are choosing not to exercise discretionary voting authority. Uninstructed shares that banks and brokers do not vote are counted
as “broker non-votes.” As a result, we urge you to direct your broker, fiduciary or custodian how to vote your shares
to ensure that your interests are represented at the Annual Meeting.
What vote is required to approve each proposal
and how are votes counted?
Proposal No. 1 — Election of directors
Directors are elected by a plurality of
the votes cast, with the two nominees obtaining the greatest number of affirmative votes being elected as directors. Shares as
to which a stockholder withholds voting authority and broker non-votes, if any, are not considered votes cast and therefore will
have no effect on the vote outcome. As described above under “Proposal No. 1 – Election of Class III Directors,”
any nominee for director who receives a greater number of “withhold” votes for his or her election than votes “for”
his or her election is expected to promptly tender his or her resignation to the Board following certification of the election
results.
Each of the Other Proposals
Each of the other proposals must be approved
by a majority of the votes cast on the proposal (meaning the number of shares voted “for” this proposal must exceed
the number of shares voted “against” such proposal). As a result, abstentions and broker non-votes, if any, will have
no effect on the vote outcome.
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 70
Can I change my vote after submitting my
proxy?
Yes. If you are the registered holder of
your shares, you may change or revoke a delivered proxy by:
| • |
Executing and returning a new, later-dated proxy card by mail,
or submitting a new vote via telephone or through the Internet, as instructed above in advance of the applicable deadline;
|
| • |
Delivering a written revocation to the Corporate Secretary before the Annual
Meeting; or |
| • |
Voting at the Annual Meeting. Simply attending the Annual Meeting will not,
by itself, revoke or change your proxy. |
If you hold your shares beneficially through
a brokerage firm, bank or other financial institution, you should contact your brokerage firm, bank or other financial institution
for information on how to change or revoke your proxy.
Who is paying for this proxy solicitation?
We are making these proxy
materials available to you in connection with the solicitation of proxies by the Board of Directors of the Company. We will pay
all of the costs of soliciting proxies. We will provide copies of our proxy materials to brokerage firms, fiduciaries, and custodians
for forwarding to beneficial owners who request printed copies of these materials and will reimburse these persons for their costs
of forwarding these materials. We have retained Innisfree, a proxy solicitation firm, for assistance in connection with the Annual
Meeting at an estimated cost of up to approximately $25,000 plus reasonable out-of-pocket expense. Our directors, officers, and
employees may also solicit proxies by telephone, facsimile, or personal solicitation; however, we will not pay these individuals
additional compensation for any of these services.
When are stockholder proposals for inclusion
in our proxy statement for next year’s annual meeting due?
Stockholders wishing to
present proposals for inclusion in our proxy statement for the 2026 Annual Meeting of Stockholder (2026 Annual Meeting) pursuant
to Rule 14a-8 of the Exchange Act must submit their proposals so that they are received by us at our principal executive offices
no later than the close of business (5:00 p.m. Pacific Time) on November 28, 2025. However, if our 2026 Annual Meeting is advanced
or delayed by more than 30 days before or after the anniversary date of the 2024 annual meeting, then the deadline will be a reasonable
time prior to the time that we begin to print and mail our proxy materials.
Proposals for inclusion
in our proxy statement for the 2026 Annual Meeting should be sent to the Company’s Corporate Secretary at 60 Leveroni Court,
Novato, California 94949 and must satisfy the requirements of Rule 14a-8 of the Exchange Act. We reserve the right to exclude
from our proxy statement any proposals not meeting such requirement.
When are other proposals and director nominations
for next year’s annual meeting due?
With respect to director nominations and
proposals of other business (other than those to be included in our proxy statement pursuant to and in compliance with Rule 14a-8),
our bylaws provide that stockholders who intend to present a stockholder proposal or director nomination at the 2026 Annual Meeting
must deliver written notice of the proposal or nomination to our Corporate Secretary between 90 and 120 days prior to the one-year
anniversary date of the Annual Meeting (that is, between January 15, 2026 and the close of business (5:00 p.m. Pacific Time) on
February 14, 2026). If the 2026 Annual Meeting date is advanced by more than 30 days before or delayed by more than 60 days after
the anniversary date of the Annual Meeting, then such notice must be received on or before the close of business (5:00 p.m. Pacific
Time) on the later of 90th day before the date of the 2026 Annual Meeting or the 10th day after the day on which the date of the
2026 Annual Meeting is first disclosed in a public announcement. Notice of any such stockholder proposals and director nominations
must satisfy the requirements set forth in our bylaws (which include timing and information required under Rule 14a-19 of the
Exchange Act).
If a stockholder fails to meet these deadlines
and fails to satisfy the requirements of Rule 14a-4 under the Exchange Act, we may exercise discretionary voting authority under
proxies we solicit to vote on any such proposal as we determine appropriate. Proposals and nominations not meeting the requirements
set forth in our bylaws will not be entertained at the Annual Meeting. All notices of proposals or nominations, as applicable,
must be addressed to our Corporate Secretary at 60 Leveroni Court, Novato, California 94949. We reserve the right to reject, rule
out of order, or take other appropriate action with respect to any nomination or proposal that does not comply with these and
other applicable requirements.
How can I find out the result of the voting
at the Annual Meeting?
Preliminary voting results will be announced at the Annual Meeting.
In addition, final voting results will be published in a current report on Form 8-K that we expect to file with the SEC within
four business days after the Annual Meeting.
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 71
Other Business
We know of no other matters
to be submitted to a vote of stockholders at the Annual Meeting. In order for any stockholder to nominate a candidate or to submit
a proposal for other business to be acted upon at a given annual meeting, he or she must provide timely written notice to our
Corporate Secretary in the form prescribed by our bylaws, as described above.
Forward-Looking Statements
Certain of the statements made in this Proxy
Statement are “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended,
Section 21E of the Exchange Act and the Private Securities Litigation Reform Act of 1995, including, among others, statements
related to our expectations and projections regarding our future operating results and financial performance, anticipated cost
or expense reductions, the timing, progress and plans for our clinical programs and clinical studies, future regulatory interactions,
goals and other statements regarding our activities and initiatives covered in our Impact Report, anticipated effects of our executive
compensation structure and programs, and the components and timing of regulatory submissions. Such forward-looking statements
involve substantial risks and uncertainties that could cause our clinical development programs, collaboration with third parties,
future results, performance or achievements to differ significantly from those expressed or implied by the forward-looking statements.
Such risks and uncertainties include, among others, business and operating results, risks related to reliance on third party partners
to conduct certain activities on the Company’s behalf, uncertainty and potential delays related
to clinical drug development, smaller than anticipated market opportunities for the Company’s products and product candidates,
manufacturing risks, competition from other therapies or products, and other matters that could affect sufficiency of existing
cash, cash equivalents and short-term investments to fund operations, the Company’s future operating results and financial
performance, the timing of clinical trial activities and reporting results from same, and the availability or commercial potential
of Ultragenyx’s products and drug candidates. The Company expressly disclaims any obligation to update or revise any forward-looking
statements. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed
in these forward-looking statements, as well as risks relating to the business of Ultragenyx in general, see our Annual Report
on Form 10-K for the fiscal year ended December 31, 2024 filed with the SEC in February 2025, and our subsequent annual and periodic
reports filed with the SEC.
Delivery Of Proxy Materials
Our annual report to
stockholders for the fiscal year ended December 31, 2024, including audited financial statements, accompanies this Proxy Statement.
We will provide copies of our Annual Report without charge upon written request of a stockholder to our investor relations department
at 60 Leveroni Court, Novato, California 94949. Copies of these materials are also available online through the SEC at www.sec.gov.
We may satisfy SEC rules regarding delivery of proxy materials, including the Proxy Statement, Annual Report and Notice of
Internet Availability by delivering a single copy of the proxy materials to stockholders who have the same address and do not
participate in electronic delivery of proxy materials, a procedure adopted by the SEC called “householding.” This
delivery method can result in meaningful cost savings for us and conservation of natural resources. Under this procedure, only
one copy of the proxy materials will be delivered to multiple stockholders who share an address, unless contrary instructions
are received from one or more stockholders at that address. We undertake to deliver promptly upon written or oral request a separate
copy of the Proxy statement, Annual Report or Notice of Internet Availability to a stockholder at a shared address to which a
single copy of these materials was delivered. If you hold stock as a registered holder and prefer to receive separate copies of
these materials either now or in the future, please contact our investor relations department at 60 Leveroni Court, Novato, California
94949 or by telephone at (415) 483-8800. Similarly, if you share an address with another stockholder and have received multiple
copies of the proxy materials, you may write or call us at the address and phone number above to request delivery of a single
copy of these materials in the future. If your stock is held through a brokerage firm, bank or other financial institution and
you received a single copy of the proxy materials and prefer to receive separate copies of the proxy materials, either now or
in the future, or if you received multiple copies of the proxy materials and prefer to receive a single copy in the future, please
contact your brokerage firm, bank or other financial institution.
EACH STOCKHOLDER IS URGED
TO VOTE VIA THE INTERNET AS INSTRUCTED IN THE NOTICE OF INTERNET AVAILABILITY OR, IF YOU REQUESTED AND RECEIVED A PRINTED COPY
OF THE PROXY MATERIALS, BY COMPLETING, DATING, SIGNING AND RETURNING THE ENCLOSED PROXY CARD USING THE ENCLOSED RETURN ENVELOPE
OR VOTING INSTRUCTION FORM PROVIDED WITH THE PRINTED PROXY MATERIALS, AS PROMPTLY AS POSSIBLE SO THAT YOUR SHARES MAY BE REPRESENTED
AT THE ANNUAL MEETING.
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 72
APPENDIX A
Ultragenyx Pharmaceutical Inc. Second Amended and Restated 2023 Incentive Plan
1. Defined Terms
Exhibit A, which is incorporated by reference,
defines the terms used in the Plan and sets forth certain operational rules related to those terms.
2. Purpose
The Plan has been established to advance
the interests of the Company by providing for the grant to Participants of Stock-based and other incentive Awards. The Plan was
originally approved by the Board on April 20, 2023 effective on the Original Effective Date and was amended and restated effective
as June 18, 2024 and on May 15, 2025 (the “Effective Date”).
3. Administration
The Administrator has discretionary
authority, subject only to the express provisions of the Plan, to (a) interpret and construe the Plan, any rules and regulations
under the Plan and the terms and conditions of any Award; (b) determine eligibility for and grant Awards; (c) determine, modify
or waive the terms and conditions of any Award; (d) prescribe forms, rules and procedures relating to the Plan; (e) establish
and verify the extent of satisfaction of any Performance Criteria or other conditions applicable to the grant, issuance, retention,
vesting, exercisability or settlement of any Award; (f) determine the extent to which adjustments are required pursuant to Section
7; (g) approve corrections in the documentation or administration of any Award; and otherwise do all things necessary or appropriate
to carry out the purposes of the Plan. Determinations of the Administrator made under the Plan will be conclusive and will bind
all parties.
4. Limits on Awards Under the Plan
| |
(i) |
The maximum number of shares of Stock
that may be delivered in satisfaction of Awards under the Plan is (A) 11,500,000, plus (B) the number of shares of
Stock subject to any Award outstanding under a Prior Plan as of the Original Effective Date that after the Original Effective
Date are not issued because such Award is forfeited, canceled, terminates, expires or otherwise lapses without being exercised
(to the extent applicable), or is settled in cash. Up to 8,500,000 shares of Stock may be issued in satisfaction of ISOs,
but nothing in this Section 4(a) will be construed as requiring that any, or any fixed number of, ISOs be awarded under the
Plan. The limits set forth in this Section 4(a) shall be construed to comply with Section 422. |
| |
(ii) |
For purposes of this Section 4(a), if an
outstanding Award is forfeited, canceled, terminates, expires or otherwise lapses without being exercised (to the extent
applicable), or is settled in cash, the shares of Stock allocable to the terminated portion of such Award or such forfeited shares
of Stock shall again be available for issuance under the Plan. Notwithstanding the foregoing, the following shares shall not be
available for issuance under the Plan: (A) shares withheld from an Award under the Plan or a Prior Plan to satisfy the tax
withholding obligations with respect to such Award, (B) shares withheld from an Award under the Plan or a Prior Plan in payment of
the exercise price of an Award requiring exercise, (C) shares repurchased on the open market by the Company using proceeds from the
exercise price paid with respect to Awards under the Plan or a Prior Plan, or (D) gross shares subject to an SAR granted under the
Plan or a Prior Plan that are not issued in connection with the Stock-settlement of such SAR.
|
| (b) |
Type of Shares. Stock delivered by the Company under the Plan
may be authorized but unissued Stock or previously issued Stock acquired by the Company. No fractional shares of Stock will
be delivered under the Plan. |
| (c) |
Limit on Non-Employee Director Compensation.
The aggregate dollar value of equity-based (based on the grant date fair value of equity-based Awards determined for financial
reporting purposes) and cash compensation granted under the Plan or otherwise to any non-employee director shall not exceed
$900,000 during any calendar year for services provided as a non-employee director; provided, however, that in the calendar
year in which a non-employee director first joins the Board or during any calendar year in which a non-employee director is
designated as Chairman of the Board or Lead Director, the maximum aggregate dollar value of equity-based and cash compensation
granted to the non-employee director may be up to $1,500,000. |
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 73
5. Eligibility and Participation
The Administrator will select Participants
from among key Employees and directors of, and consultants and advisors to, the Company and its Affiliates. Eligibility for ISOs
is limited to individuals described in the first sentence of this Section 5 who are employees of the Company or of a “parent
corporation” or “subsidiary corporation” of the Company as those terms are defined in Section 424 of the Code.
Eligibility for Stock Options other than ISOs is limited to individuals described in the first sentence of this Section 5 who
are providing direct services on the date of grant of the Stock Option to the Company or to a subsidiary of the Company that would
be described in the first sentence of Section 1.409A-1(b)(5)(iii)(E) of the Treasury Regulations.
6. Rules Applicable to Awards
| |
(i) |
Award Provisions. The Administrator
will determine the terms of all Awards, subject to the limitations provided herein. By accepting (or, under such rules as
the Administrator may prescribe, being deemed to have accepted) an Award, the Participant will be deemed to have agreed to
the terms of the Award and the Plan. Notwithstanding any provision of this Plan to the contrary, awards of an acquired company
that are converted, replaced or adjusted in connection with the acquisition may contain terms and conditions that are inconsistent
with the terms and conditions specified herein, as determined by the Administrator. |
| |
(ii) |
Term of Plan. No Awards may be made after 10
years from the Effective Date, but previously granted Awards may continue beyond that date in accordance with their terms.
Notwithstanding the foregoing, no ISOs may be granted after 10 years from Date of Adoption.
|
| |
(iii) |
Transferability. Neither ISOs nor, except as
the Administrator otherwise expressly provides in accordance with the second sentence of this Section 6(a)(iii), other Awards
may be transferred other than by will or by the laws of descent and distribution. During a Participant’s lifetime, ISOs
(and, except as the Administrator otherwise expressly provides in accordance with the second sentence of this Section 6(a)(iii),
SARs and NSOs) may be exercised only by the Participant. The Administrator may permit the gratuitous transfer (i.e., transfer
not for value) of Awards other than ISOs to any transferee eligible to be covered by the provisions of Form S-8 (under the
Securities Act), subject to such limitations as the Administrator may impose. |
| |
(iv) |
Vesting, etc. The Administrator will determine the time or times at which
an Award will vest or become exercisable and the terms on which a Stock Option or SAR will remain exercisable. Without limiting
the foregoing, the Administrator may at any time accelerate the vesting or exercisability of an Award, regardless of any adverse
or potentially adverse tax or other consequences resulting from such acceleration. Unless the Administrator expressly provides
otherwise, however, the following rules will apply if a Participant’s Employment ceases: |
| |
(A) |
Immediately upon the cessation of the Participant’s Employment and
except as provided in (B) and (C) below, each Stock Option and SAR that is then held by the Participant or by the Participant’s
permitted transferees, if any, will cease to be exercisable and will terminate and all other Awards that are then held by
the Participant or by the Participant’s permitted transferees, if any, to the extent not already vested will be forfeited. |
| |
(B) |
Subject to (C) and (D) below, all Stock Options and SARs held by the Participant or the Participant’s
permitted transferees, if any, immediately prior to the cessation of the Participant’s Employment, to the extent then
exercisable, will remain exercisable for the lesser of (i) a period of three months or (ii) the period ending on the latest
date on which such Stock Option or SAR could have been exercised without regard to this Section 6(a)(iv), and will thereupon
immediately terminate. |
| |
(C) |
All Stock Options and SARs held by a Participant or the Participant’s permitted transferees,
if any, immediately prior to the Participant’s death, to the extent then exercisable, will remain exercisable for the
lesser of (i) the one year period ending with the first anniversary of the Participant’s death or (ii) the period ending
on the latest date on which such Stock Option or SAR could have been exercised without regard to this Section 6(a)(iv), and
will thereupon immediately terminate. |
| |
(D) |
All Stock Options and SARs (whether or not exercisable) held by a Participant or the Participant’s
permitted transferees, if any, immediately prior to the cessation of the Participant’s Employment will immediately terminate
upon such notice of cessation of Employment if the termination is for Cause or occurs in circumstances that in the sole determination
of the Administrator would have constituted grounds for the Participant’s Employment to be terminated for Cause. |
| |
(v) |
Additional Restrictions. The Administrator may cancel, rescind, withhold or otherwise limit or restrict
any Award at any time if the Participant is not in compliance with all applicable provisions of the Award agreement and the
Plan, or if the Participant breaches any agreement with the Company or its Affiliates with respect to confidentiality. Without
limiting the generality of the foregoing, the Administrator may recover Awards made under the Plan and payments under or gain
in respect of any Award to the extent required to comply with Company policy, including the Company’s Clawback Policy. |
| |
(vi) |
Taxes. The delivery, vesting and retention of Stock, cash or other property under an Award are conditioned upon |
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 74
| |
|
full satisfaction by the Participant of all tax withholding
requirements with respect to the Award. The Administrator will prescribe such rules for the withholding of taxes as it deems
necessary. The Administrator may, but need not, hold back shares of Stock from an Award or permit a Participant to tender
previously owned shares of Stock in satisfaction of tax withholding requirements (but not in excess of the maximum statutory
withholding rate). |
| |
(vii) |
Dividend Equivalents, Etc. The Administrator may provide for the payment
of amounts (on terms and subject to conditions established by the Administrator, except to the extent provided otherwise
in this Section 6(a)(vii)) in lieu of cash dividends or other cash distributions with respect to Stock subject to an
Award whether or not the holder of such Award is otherwise entitled to share in the actual dividend or distribution in
respect of such Award. Any entitlement to dividend equivalents or similar entitlements will be established and
administered either consistent with an exemption from, or in compliance with, the requirements of Section 409A. Dividends
or dividend equivalent amounts payable in respect of Awards shall be subject to the same limits or restrictions as the
Awards to which they relate and shall not be payable until such Awards vest. Notwithstanding the foregoing, no Stock
Options or SARs shall provide for payment or accrual of dividends or dividend equivalents. |
| |
(viii) |
Rights Limited. Nothing in the Plan will be construed as giving any person the right to continued
employment or service with the Company or its Affiliates, or any rights as a stockholder except as to shares of Stock actually
issued under the Plan. The loss of existing or potential profit in Awards will not constitute an element of damages in the
event of termination of Employment for any reason, even if the termination is in violation of an obligation of the Company
or any Affiliate to the Participant. |
| |
(ix) |
Coordination with Other Plans. Awards under the Plan may be granted
in tandem with, or in satisfaction of or substitution for, other Awards under the Plan or awards made under other compensatory
plans or programs of the Company or its Affiliates. For example, but without limiting the generality of the foregoing, awards
under other compensatory plans or programs of the Company or its Affiliates may be settled in Stock (including, without limitation,
Unrestricted Stock) if the Administrator so determines, in which case the shares delivered will be treated as awarded under
the Plan (and will reduce the number of shares thereafter available under the Plan in accordance with the rules set forth
in Section 4). |
| |
(x) |
Section 409A. Each Award will contain such terms as the Administrator determines, and will
be construed and administered, such that the Award either qualifies for an exemption from the requirements of Section 409A
or satisfies such requirements. |
| (b) |
Stock Options and SARs. |
| |
(i) |
Time and Manner of Exercise. Unless the Administrator expressly provides otherwise, no Stock Option
or SAR will be deemed to have been exercised until the Administrator receives a notice of exercise (in form acceptable to
the Administrator), which may be an electronic notice, signed (including electronic signature in form acceptable to the Administrator)
by the appropriate person and accompanied by any payment required under the Award. A Stock Option or SAR exercised by any
person other than the Participant will not be deemed to have been exercised until the Administrator has received such evidence
as it may require that the person exercising the Award has the right to do so. |
| |
(ii) |
Exercise Price. The exercise price (or the base value from which appreciation is to be measured) of each Award requiring
exercise will be no less than 100% (or in the case of an ISO granted to a ten-percent shareholder within the meaning of Section
422, 110%) of the Fair Market Value of the Stock subject to the Award, determined as of the date of grant, or such higher
amount as the Administrator may determine in connection with the grant. |
| |
(iii) |
Payment of Exercise Price. Where the exercise of an Award is to be accompanied by payment,
payment of the exercise price will be by cash or check acceptable to the Administrator or by such other legally permissible
means, if any, as may be acceptable to the Administrator. |
| |
(iv) |
Maximum Term. Stock Options and SARs will have a maximum term not
to exceed 10 years from the date of grant (or five years from the date of grant in the case of an ISO granted to a ten-percent
shareholder described in Section 6(b)(ii) above); provided, however, that, if a Participant still holding an outstanding but
unexercised NSO or SAR 10 years from the date of grant (or, in the case of an NSO or SAR with a maximum term of less than
10 years, such maximum term) is prohibited by applicable law from exercising such Stock Options or SARs, and if at such time
the Stock is publicly traded (as determined by the Administrator), the maximum term of such Award will instead be deemed to
expire on the 30th day following the date the Participant is no longer prohibited from engaging in such open market
sales. |
| |
(v) |
No Repricing without Stockholder Approval. The Company shall not, without stockholder approval (except in the case of
a change in the Company’s capitalization (as described in Section 7(b)), (A) reduce the exercise price of a Stock Option
or SAR, (B) other than in the case of Covered Transaction, at any time when the exercise price of a Stock Option or SAR is
above the Fair Market Value of a share of Stock, cancel and re-grant or exchange such Stock Option or SAR for cash or a new
Award having a lower (or no) exercise price, or (c) take any other action with respect to an Award that would be treated as
a repricing under generally accepted accounting principles. |
| |
(vi) |
No Reload Grants. Stock Options shall not be granted under the Plan in consideration for, and shall not be conditioned
upon the delivery of, shares of Stock to the Company in payment of the exercise price and/or tax withholding obligation under
any other employee stock option. |
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 75
7. Effect of Certain Transactions
| (a) |
Mergers, etc. Except as otherwise provided in an Award agreement, the following provisions will apply
in the event of a Covered Transaction: |
| |
(i) |
Assumption or Substitution. If the Covered Transaction
is one in which there is an acquiring or surviving entity, the Administrator may (but, for the avoidance of doubt, need not)
provide (i) for the assumption or continuation of some or all outstanding Awards or any portion thereof or (ii) for the grant
of new awards in substitution therefor by the acquiror or survivor or an affiliate of the acquiror or survivor. Notwithstanding
any provision of this Section 7, in the event of a Covered Transaction in which the acquiring or surviving entity does not
assume or continue outstanding Awards or provide for the grant of new awards in substitution therefor, all Awards that are
not assumed, continued or substituted for shall be treated as follows effective immediately prior to the Covered Transaction:
(A) in the case of a Stock Option or SAR, the Participant shall have the ability to exercise such Stock Option or SAR, including
any portion of the Stock Option or SAR not previously exercisable, (B) in the case of any Award the vesting of which is in
whole or in part subject to Performance Criteria, all conditions to the grant, issuance, retention, vesting or transferability
of, or any other restrictions applicable to, such Award shall immediately lapse and the Participant shall have the right to
receive a payment based on target level achievement or actual performance through a date determined by the Committee, as determined
by the Committee, and (C) in the case of any other Award all conditions to the grant, issuance, retention, vesting or transferability
of, or any other restrictions applicable to, such Award shall immediately lapse. In no event shall any action be taken pursuant
to this Section 7(a)(i) that would change the payment or settlement date of an Award in a manner that would result in the
imposition of any additional taxes or penalties pursuant to Section 409A of the Code. |
| |
(ii) |
Cash-Out of Awards. Subject to Section 7(a)(v) below the
Administrator may (but, for the avoidance of doubt, need not) provide for payment (a “cash- out”), with
respect to some or all Awards or any portion thereof, equal in the case of each affected Award or portion thereof to the
excess, if any, of (A) the fair market value of one share of Stock (as determined by the Administrator in its reasonable
discretion) times the number of shares of Stock subject to the Award or such portion, over (B) the aggregate exercise or
purchase price, if any, under the Award or such portion (in the case of an SAR, the aggregate base value above which
appreciation is measured), in each case on such payment terms (which need not be the same as the terms of payment to
holders of Stock) and other terms, and subject to such conditions, as the Administrator determines; provided, however,
that if the fair market value of one share of Stock (as determined by the Administrator in its reasonable discretion)
does not exceed the exercise price of such Award, then the Award shall be cancelled without any payment of consideration
therefor. |
| |
(iii) |
Acceleration of Certain Awards. Subject to Section 7(a)(v) below,
the Administrator may (but, for the avoidance of doubt, need not) provide that any Award requiring exercise will become exercisable,
in full or in part and/or that the delivery of any shares of Stock remaining deliverable under any outstanding Award of Stock
Units (including Restricted Stock Units and Performance Awards to the extent consisting of Stock Units) will be accelerated
in full or in part, in each case on a basis that gives the holder of the Award a reasonable opportunity, as determined by
the Administrator, following exercise of the Award or the delivery of the shares, as the case may be, to participate as a
stockholder in the Covered Transaction. |
| |
(iv) |
Termination of Awards Upon Consummation of Covered Transaction.
Except as the Administrator may otherwise determine in any case, each Award will automatically terminate (and in the case
of outstanding shares of Restricted Stock, will automatically be forfeited) upon consummation of the Covered Transaction,
other than Awards assumed or continued pursuant to Section 7(a)(i) above. |
| |
(v) |
Additional Limitations. Any share of Stock and any cash or other property delivered pursuant
to Section 7(a)(ii) or Section 7(a)(iii) above with respect to an Award may, in the discretion of the Administrator, contain
such restrictions, if any, as the Administrator deems appropriate to reflect any Performance Criteria or other vesting conditions
to which the Award was subject and that did not lapse (and were not satisfied) in connection with the Covered Transaction.
For purposes of the immediately preceding sentence, a cash-out under Section 7(a)(ii) above or acceleration under Section
7(a)(iii) above will not, in and of itself, be treated as the lapsing (or satisfaction) of a Performance Criteria or other
vesting condition. In the case of Restricted Stock that does not vest and is not forfeited in connection with the Covered
Transaction, the Administrator may require that any amounts delivered, exchanged or otherwise paid in respect of such Stock
in connection with the Covered Transaction be placed in escrow or otherwise made subject to such restrictions as the Administrator
deems appropriate to carry out the intent of the Plan. |
| (b) |
Changes in and Distributions With Respect to Stock. |
| |
(i) |
Basic Adjustment Provisions. In the event of a stock
dividend, stock split or combination of shares (including a reverse stock split), recapitalization or other change in the
Company’s capital structure that constitutes an equity restructuring within the meaning of FASB ASC 718, the Administrator
will make appropriate adjustments to the maximum number of shares specified in Section 4(a) that may be delivered under the
Plan and will also make appropriate adjustments to the number and kind of shares of stock or securities subject to Awards
then outstanding or subsequently granted, any exercise prices relating to Awards and any other provision of Awards affected
by such change. |
| |
(ii) |
Certain Other Adjustments. The Administrator may also make adjustments
of the type described in Section 7(b)(i) above to take into account distributions to stockholders other than |
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 76
| |
|
those provided for in Section 7(a) and 7(b)(i), or any other event, if the Administrator
determines that adjustments are appropriate to avoid distortion in the operation of the Plan, having due regard for the qualification
of ISOs under Section 422 and the requirements of Section 409A, where applicable. |
| |
(iii) |
Continuing Application of Plan Terms. References in the Plan to shares of Stock will be construed
to include any stock or securities resulting from an adjustment pursuant to this Section 7. |
8. Legal Conditions on Delivery of Stock
The Company will not be
obligated to deliver any shares of Stock pursuant to the Plan or to remove any restriction from shares of Stock previously delivered
under the Plan until: (a) the Company is satisfied that all legal matters in connection with the issuance and delivery of such
shares have been addressed and resolved; (b) if the outstanding Stock is at the time of delivery listed on any stock exchange
or national market system, the shares to be delivered have been listed or authorized to be listed on such exchange or system upon
official notice of issuance; and (c) all conditions of the Award have been satisfied or waived. The Company may require, as a
condition to exercise of the Award, such representations or agreements as counsel for the Company may consider appropriate to
avoid violation of the Securities Act or any applicable state or non-U.S. securities law. Any Stock required to be issued to Participants
under the Plan will be evidenced in such manner as the Administrator may deem appropriate, including book-entry registration or
delivery of stock certificates. In the event that the Administrator determines that Stock certificates will be issued to Participants
under the Plan, the Administrator may require that certificates evidencing Stock issued under the Plan bear an appropriate legend
reflecting any restriction on transfer applicable to such Stock, and the Company may hold the certificates pending lapse of the
applicable restrictions.
9. Amendment and Termination
The Administrator may at any time or times
amend the Plan or any outstanding Award for any purpose which may at the time be permitted by law, and may at any time terminate
the Plan as to any future grants of Awards; provided, that except as otherwise expressly provided in the Plan the Administrator
may not, without the Participant’s consent, alter the terms of an Award so as to affect materially and adversely the Participant’s
rights under the Award, unless the Administrator expressly reserved the right to do so at the time the Award was granted. Any
amendments to the Plan will be conditioned upon stockholder approval only to the extent, if any, such approval is required by
law (including the Code and applicable stock exchange requirements), as determined by the Administrator.
10. Other Compensation Arrangements
The existence of the Plan or the grant of any Award will not
in any way affect the Company’s right to Award a person bonuses or other compensation in addition to Awards under the Plan.
11. Miscellaneous
| (a) |
Waiver of Jury Trial. By accepting an Award under
the Plan and to the extent permitted under applicable law, each Participant waives any right to a trial by jury in any action,
proceeding or counterclaim concerning any rights under the Plan and any Award, or under any amendment, waiver, consent, instrument,
document or other agreement delivered or which in the future may be delivered in connection therewith, and agrees that any
such action, proceedings or counterclaim will be tried before a court and not before a jury. By accepting an Award under the
Plan, each Participant certifies that no officer, representative, or attorney of the Company has represented, expressly or
otherwise, that the Company would not, in the event of any action, proceeding or counterclaim, seek to enforce the foregoing
waivers. Notwithstanding anything to the contrary in the Plan, nothing herein is to be construed as limiting the ability of
the Company and a Participant to agree to submit disputes arising under the terms of the Plan or any Award made hereunder
to binding arbitration or as limiting the ability of the Company to require any eligible individual to agree to submit such
disputes to binding arbitration as a condition of receiving an Award hereunder. |
| (b) |
Limitation of Liability. Notwithstanding anything to the contrary in the Plan, neither the
Company, nor any Affiliate, nor the Administrator, nor any person acting on behalf of the Company, any Affiliate, or the Administrator,
will be liable to any Participant or to the estate or beneficiary of any Participant or to any other holder of an Award by
reason of any acceleration of income, or any additional tax (including any interest and penalties), asserted by reason of
the failure of an Award to satisfy the requirements of Section 422 or Section 409A or by reason of Section 4999 of the Code,
or otherwise asserted with respect to the Award. |
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 77
12. Establishment of Sub-Plans
The Administrator may from
time to time establish one or more sub-plans under the Plan for purposes of satisfying applicable blue sky, securities or tax
laws of various jurisdictions. The Administrator will establish such sub-plans by adopting supplements to the Plan setting forth
(a) such limitations on the Administrator’s discretion under the Plan as it deems necessary or desirable and (b) such additional
terms and conditions not otherwise inconsistent with the Plan as it deems necessary or desirable. All supplements so established
will be deemed to be part of the Plan, but each supplement will apply only to Participants within the affected jurisdiction (as
determined by the Administrator).
13. Governing Law
| (a) |
Certain Requirements of Corporate Law. Awards will be granted and administered
consistent with the requirements of applicable Delaware law relating to the issuance of stock and the consideration to be
received therefor, and with the applicable requirements of the stock exchanges or other trading systems on which the Stock
is listed or entered for trading, in each case as determined by the Administrator. |
| (b) |
Other Matters. Except as otherwise provided by the express terms
of an Award agreement, under a sub-plan described in Section 12 or as provided in Section 13(a) above, the provisions of the
Plan and of Awards under the Plan and all claims or disputes arising out of or based upon the Plan or any Award under the
Plan or relating to the subject matter hereof or thereof will be governed by and construed in accordance with the domestic
substantive laws of the State of Delaware without giving effect to any choice or conflict of laws provision or rule that would
cause the application of the domestic substantive laws of any other jurisdiction. |
| (c) |
Jurisdiction. By accepting an Award, each Participant will be deemed to (a) have submitted
irrevocably and unconditionally to the jurisdiction of the federal and state courts located within the geographic boundaries
of the United States District Court for the Northern District of California for the purpose of any suit, action or other
proceeding arising out of or based upon the Plan or any Award; (b) agree not to commence any suit, action or other
proceeding arising out of or based upon the Plan or an Award, except in the federal and state courts located within the
geographic boundaries of the United States District Court for the Northern District of California; and (c) waive, and
agree not to assert, by way of motion as a defense or otherwise, in any such suit, action or proceeding, any claim that
it is not subject personally to the jurisdiction of the above-named courts that its property is exempt or immune from
attachment or execution, that the suit, action or proceeding is brought in an inconvenient forum, that the venue of the
suit, action or proceeding is improper or that the Plan or an Award or the subject matter thereof may not be enforced in
or by such court. |
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 78
EXHIBIT
A
Definition of Terms
The following terms, when
used in the Plan, will have the meanings and be subject to the provisions set forth below:
“Administrator”:
The Compensation Committee, except that the Compensation Committee may, subject to applicable law, delegate (i) to one or
more of its members (or one or more other members of the Board (including the full Board)) such of its duties, powers and responsibilities
as it may determine; (ii) to one or more officers or Employees of the Company the power to grant Awards to the extent permitted
by Section 157(c) of the Delaware General Corporation Law; and (iii) to such Employees or other persons as it determines such
ministerial tasks as it deems appropriate. In the event of any delegation described in the preceding sentence, the term “Administrator”
will include the person or persons so delegated to the extent of such delegation.
“Affiliate”: Any corporation
or other entity that stands in a relationship to the Company that would result in the Company and such corporation or other entity
being treated as one employer under Section 414(b) and Section 414(c) of the Code.
“Award”: Any or a combination
of the following:
| |
(i) |
Stock Options. |
| |
(ii) |
SARs. |
| |
(iii) |
Restricted Stock. |
| |
(iv) |
Unrestricted Stock. |
| |
(v) |
Stock Units, including Restricted Stock Units. |
| |
(vi) |
Performance Awards. |
| |
(vii) |
Cash Awards. |
| |
(viii) |
Awards (other than Awards described in (i) through (vii) above) that are convertible into
or otherwise based on Stock. |
“Board”: The Board of Directors of the Company.
“Cash Award”: An Award denominated in cash.
“Cause”:
In the case of any Participant who is party to an employment or severance-benefit agreement that contains a definition of
“Cause,” the definition set forth in such agreement will apply with respect to such Participant under the Plan. In
the case of any other Participant, “Cause” will mean, as determined by the Administrator in its reasonable judgment,
(i) a substantial failure of the Participant to perform the Participant’s duties and responsibilities to the Company or
subsidiaries or substantial negligence in the performance of such duties and responsibilities; (ii) the commission by the Participant
of a felony or a crime involving moral turpitude; (iii) the commission by the Participant of theft, fraud, embezzlement, material
breach of trust or any material act of dishonesty involving the Company or any of its subsidiaries; (iv) a significant violation
by the Participant of the code of conduct of the Company or its subsidiaries of any material policy of the Company or its subsidiaries,
or of any statutory or common law duty of loyalty to the Company or its subsidiaries; (v) material breach of any of the terms
of the Plan or any Award made under the Plan, or of the terms of any other agreement between the Company or subsidiaries and the
Participant; or (vi) other conduct by the Participant that could be expected to be harmful to the business, interests or reputation
of the Company.
“Code”: The U.S. Internal
Revenue Code of 1986 as from time to time amended and in effect, or any successor statute as from time to time in effect.
“Company”: Ultragenyx
Pharmaceutical Inc., and except as utilized in the definition of Covered Transaction, any successor corporation.
“Compensation Committee”:
The Compensation Committee of the Board.
“Covered Transaction”: The occurrence of any
one of the following events:
| |
(i) |
a consolidation, merger, or similar transaction or
series of related transactions in which the Company is not the surviving corporation or which results in the acquisition of
all or substantially all of the Company’s then outstanding Stock by a single person or entity or by a group of persons
and/or entities acting in concert (a “Transaction”), excluding a Transaction which would result in the
holders of the voting securities of the Company outstanding immediately prior to such merger or consolidation continuing to
represent (either by remaining outstanding or by being converted into voting securities of the surviving entity or any parent
thereof) at least 50% of the combined voting power of the securities of the Company or such surviving entity or any parent
thereof outstanding immediately after such merger or consolidation; |
| |
(ii) |
a sale or transfer of all or substantially all the Company’s assets, excluding a sale
or transfer to an entity, at least 50% of the combined voting power of the voting securities of which is owned by stockholders
of the Company in substantially the same proportions as their ownership of the Company immediately prior to such sale; or
|
| |
(iii) |
a dissolution or liquidation of the Company. |
Where a Covered Transaction involves
a tender offer that is reasonably expected to be followed by a merger described in clause (i) (as determined by
the Administrator), the Covered Transaction will be deemed to have occurred upon consummation of the tender
offer. Notwithstanding the foregoing, for each Award that constitutes deferred compensation under
Section 409A, and to the extent required to avoid accelerated taxation and/or tax penalties under Section 409A, a Covered
Transaction shall be deemed to have occurred under the Plan with respect to such Award only if a change in the ownership or
effective control of the Company or a change in ownership of a substantial portion of the assets of the Company shall also be
deemed to have occurred under Section 409A.
“Date of Adoption”: March 24, 2025
“Employee”: Any person who is employed by
the Company or an Affiliate.
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 79
“Employment”:
A Participant’s employment or other service relationship with the Company and its Affiliates, which may include service
as a director, consultant or independent contractor. Employment will be deemed to continue, unless the Administrator expressly
provides otherwise, so long as the Participant is employed by, or otherwise is providing services in a capacity described in Section
5 to the Company or an Affiliate. If a Participant’s employment or other service relationship is with an Affiliate and that
entity ceases to be an Affiliate, the Participant’s Employment will be deemed to have terminated when the entity ceases
to be an Affiliate unless the Participant transfers Employment to the Company or its remaining Affiliates. Notwithstanding the
foregoing and the definition of “Affiliate” above, in construing the provisions of any Award relating to the payment
of “nonqualified deferred compensation” (subject to Section 409A) upon a termination or cessation of Employment, references
to termination or cessation of employment, separation from service, retirement or similar or correlative terms will be construed
to require a “separation from service” (as that term is defined in Section 1.409A-1(h) of the Treasury Regulations)
from the Company and from all other corporations and trades or businesses, if any, that would be treated as a single “service
recipient” with the Company under Section 1.409A-1(h)(3) of the Treasury Regulations. The Company may, but need not, elect
in writing, subject to the applicable limitations under Section 409A, any of the special elective rules prescribed in Section
1.409A-1(h) of the Treasury Regulations for purposes of determining whether a “separation from service” has occurred.
Any such written election will be deemed a part of the Plan.
“Fair Market Value”:
| (a) |
If the Stock is readily traded on an established
national exchange or trading system (including the Nasdaq Global Market), the closing price of the Stock as reported by the
principal exchange on which such Stock is traded; provided, however, that if such day is not a trading day, Fair Market Value
will mean the reported closing price of the Stock for the immediately preceding day that is a trading day. |
| (b) |
If the Stock is not traded on an established national exchange or trading system, the average
of the bid and ask prices for such Stock where the bid and ask prices are quoted. |
| (c) |
If the Stock cannot be valued pursuant to clauses (a) or (b), the value as determined in good
faith by the Board in its sole discretion consistent with the rules of Section 422 and Section 409A to the extent applicable. |
“ISO”: A Stock Option
intended to be an “incentive stock option” within the meaning of Section 422. Each Stock Option granted pursuant to
the Plan will be treated as providing by its terms that it is to be an NSO unless, as of the date of grant, it is expressly designated
as an ISO.
“NSO”: A Stock Option
that is not intended to be an “incentive stock option” within the meaning of Section 422.
“Participant”:
A person who is granted an Award under the Plan.
“Original Effective Date”:
June 7, 2023.
“Performance Award”: An
Award subject to Performance Criteria.
“Performance Criteria”: Specified
criteria, other than the mere continuation of Employment or the mere passage of time, the satisfaction of which is a condition
for the grant, exercisability, vesting or full enjoyment of an Award. Performance Criteria will include but not be limited to
any objectively determinable measure of performance relating to any, or any combination, of the following (measured either absolutely
or by reference to an index or indices and determined either on a consolidated basis or, as the context permits, on a divisional,
subsidiary, line of business, project or geographical basis or in combinations thereof): sales; revenues; assets; expenses; earnings
from operations; earnings before or after deduction for all or any portion of interest, taxes, depreciation, amortization, incentives,
service fees or extraordinary or special items, whether or not on a continuing operations or an aggregate or per share basis;
net income or net income per common share (basic or diluted); return on equity, investment, capital or assets; one or more operating
ratios; borrowing levels, leverage ratios or credit rating; market share; capital expenditures; cash flow, free cash flow, cash
flow return on investment, or net cash provided by operations; stock price, dividends or total stockholder return; development
of new technologies or products; sales of particular products or services; economic value created or added; operating margin or
profit margin; customer acquisition or retention; raising or refinancing of capital; successful hiring of key individuals; resolution
of significant litigation; acquisitions and divestitures (in whole or in part); joint ventures and strategic alliances; spin-offs,
split-ups and the like; reorganizations; recapitalizations, restructurings, financings (issuance of debt or equity) or refinancings;
or strategic business criteria, consisting of one or more objectives based on the following goals: meeting specified market penetration
or value added, product development or introduction (including, without limitation, any clinical trial accomplishments, regulatory
or other filings or approvals, or other product development milestones), geographic business expansion, cost targets, cost reductions
or savings, customer satisfaction, operating efficiency, acquisition or retention, employee satisfaction, information technology,
corporate development (including, without limitation, licenses, innovation, research or establishment of third party collaborations),
manufacturing or process development, legal compliance or risk reduction, patent application or issuance goals, or goals relating
to acquisitions or divestitures (in whole or in part), joint ventures or strategic alliances. Performance Criteria and any targets
with respect thereto determined by the Administrator need not be based upon an increase, a positive or improved result or avoidance
of loss.
“Plan”: The Ultragenyx
Pharmaceutical Inc. 2023 Incentive Plan as amended and restated effective as of the Effective Date, and as from time to time amended
and in effect.
“Prior Plans”: The Ultragenyx
Pharmaceutical Inc. 2011 Incentive Plan and the Ultragenyx Pharmaceutical Inc. 2014 Incentive Plan, in each case as amended from
time to time.
“Restricted Stock”: Stock
subject to restrictions requiring that it be redelivered or offered for sale to the Company if specified conditions are not satisfied.
“Restricted Stock Unit”:
A Stock Unit that is, or as to which the delivery of Stock or cash in lieu of Stock is, subject to the satisfaction of specified
performance or other vesting conditions.
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 80
“SAR”:
A right entitling the holder upon exercise to receive an amount (payable in cash or in shares of Stock of equivalent value)
equal to the excess of the Fair Market Value of the shares of Stock subject to the right over the base value from which appreciation
under the SAR is to be measured.
“Section 409A”: Section
409A of the Code.
“Section 422”: Section 422 of the Code.
“Securities Act”: The U.S. Securities
Act of 1933, as amended.
“Stock”: Common stock
of the Company, par value $0.001 per share.
“Stock Option”: An option
entitling the holder to acquire shares of Stock upon payment of the exercise price.
“Stock Unit”: An unfunded
and unsecured promise, denominated in shares of Stock, to deliver Stock or cash measured by the value of Stock in the future.
“Unrestricted Stock”: Stock
not subject to any restrictions under the terms of the Award.
ULTRAGENYX
PHARMACEUTICAL INC. • 2025 Proxy Statement 81