Cara Therapeutics to Host Notalgia Paresthetica (NP) Virtual KOL Event on Wednesday, March 27, 2024
07 Marzo 2024 - 1:00PM

Cara Therapeutics, Inc. (Nasdaq: CARA), a development-stage
biopharmaceutical company leading a new treatment paradigm to
improve the lives of patients suffering from pruritus, today
announced the Company will host a virtual key opinion leader (KOL)
event titled “Meet the NP Experts” on Wednesday, March 27, 2024, at
10:00 a.m. EDT.
The event will feature a panel of leaders in the
field of neuropathic pruritus who will discuss the epidemiology,
diagnosis, treatment landscape, and significant unmet medical need
in notalgia paresthetica (NP). The live panel discussion will be
moderated by Joana Goncalves, MD, Chief Medical Officer of Cara
Therapeutics. The event will also highlight oral difelikefalin in
NP and its potential in this underserved neuropathy. A live
question and answer session will follow the formal
presentation.
Participants will include:
- Christopher Posner, President and Chief Executive Officer, Cara
Therapeutics
- Joana Goncalves, MD, Chief Medical Officer, Cara
Therapeutics
- Brian Kim, MD, MTR, FAAD, Vice Chair of Research, Department of
Dermatology at the Icahn School of Medicine at Mount Sinai
- Gil Yosipovitch, MD, FAAD, Professor of Dermatology at the
Miller School of Medicine at University of Miami
- Melinda Gooderham, MSc, MD, FRCPC, Medical Director at the SKiN
Centre for Dermatology in Ontario, Canada
To register for the event, please click here.
A live webcast of the event will be accessible under “Events
& Presentations” in the Investors section of the Company’s
website at www.CaraTherapeutics.com. A replay of the webcast will
be archived on the Company’s website following the event.
About Cara Therapeutics
Cara Therapeutics is a development-stage
biopharmaceutical company leading a new treatment paradigm to
improve the lives of patients suffering from pruritus. The Company
is developing an oral formulation of difelikefalin, a selective,
peripherally acting, non-scheduled kappa opioid receptor agonist,
for the treatment of chronic pruritus associated with notalgia
paresthetica (NP), a common, underdiagnosed neuropathy affecting
the upper back for which there are no FDA-approved therapies. The
Company is conducting a Phase 2/3 clinical program in NP with
topline results of the dose-finding portion expected in the third
quarter of 2024. Cara Therapeutics also developed an IV formulation
of difelikefalin, which is approved in the United States, EU, and
multiple other countries for the treatment of moderate-to-severe
pruritus associated with advanced chronic kidney disease in adults
undergoing hemodialysis. The IV formulation is out-licensed
worldwide. For more information, visit www.CaraTherapeutics.com and
follow the company on X (Twitter), LinkedIn and Instagram.
MEDIA CONTACT:Annie Spinetta6
Degrees973-768-2170aspinetta@6degreespr.com
INVESTOR CONTACT:Iris Francesconi, Ph.D.Cara
Therapeutics203-406-3700investor@caratherapeutics.com
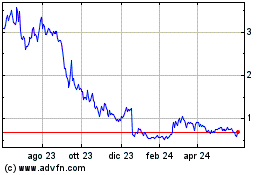
Grafico Azioni Cara Therapeutics (NASDAQ:CARA)
Storico
Da Ott 2024 a Nov 2024
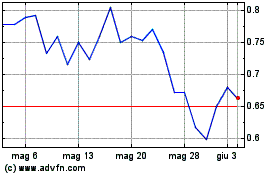
Grafico Azioni Cara Therapeutics (NASDAQ:CARA)
Storico
Da Nov 2023 a Nov 2024