UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Proxy
Statement Pursuant to Section 14(a) of the
Securities
Exchange Act of 1934
Filed by
the Registrant ☒
Filed by
a Party other than the Registrant ☐
Check the
appropriate box:
☐
Preliminary Proxy Statement
☐ Confidential, For Use of the Commission Only (As Permitted by Rule 14a-6(e)(2))
☒ Definitive Proxy Statement
☐ Definitive Additional Materials
☐ Soliciting Material under Rule 14a-12
RENOVARO
BIOSCIENCES INC.
(Name of Registrant as Specified In Its Charter)
(Name of Person(s) Filing Proxy Statement,
if
other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
☐
No fee required
☒
Fee paid previously with preliminary materials.
☐
Fee computed on table in exhibit required by Item 25(b) per Exchange Act
Rules 14a-6(i)(1) and 0-11.
RENOVARO BIOSCIENCES INC.
2080 Century Park East, Suite 906
Los Angeles, CA 90067
NOTICE OF SPECIAL MEETING OF STOCKHOLDERS
TO BE HELD ON JANUARY 25, 2024
Dear Stockholder:
You are invited to attend a special meeting of the stockholders of Renovaro
Biosciences Inc., a Delaware corporation (“Renovaro”), which will be held on Thursday, January 25, 2024, at 12:00 p.m., Eastern
Standard Time (the “Special Meeting”), at K&L Gates LLP, 200 S Biscayne Blvd., Suite 3900, Miami, FL 33131, unless postponed
or adjourned to a later date. The Special Meeting is an important meeting that will impact your investment in Renovaro.
On September 28, 2023, Renovaro entered into a Stock Purchase Agreement, dated September 28, 2023, as amended
on December 20, 2023 (the “Stock Purchase Agreement”), with GEDi Cube Intl Ltd., a private company formed under the
laws of England and Wales (“GEDi Cube”), the other sellers party thereto (together with the other shareholders of GEDi
Cube who deliver to Renovaro a joinder to the Stock Purchase Agreement, the “Sellers” and each, a “Seller”),
and Yalla Yalla Ltd., a private limited liability company registered and incorporated under the laws of Malta, in its capacity
as the representative of the Sellers (the “Sellers’ Representative”), pursuant to which Renovaro will acquire
all of the issued and outstanding shares and other equity interests of GEDi Cube from the Sellers after which GEDi Cube will become
a wholly-owned subsidiary of Renovaro (the “Transaction”).
Under the terms of the Stock Purchase Agreement, at the effective time of the
Transaction (the “Effective Time”), the Sellers will sell and transfer to Renovaro all of the equity interests of GEDi Cube
(each, a “GEDi Cube Share”) owned by Sellers that are issued and outstanding as of immediately prior to the Effective Time
in exchange for (i) an aggregate number of shares of common stock, par value $0.0001 per share, of Renovaro (“Common Stock”)
equal to a specified percentage of the shares of Common Stock issued and outstanding as of the Effective Time (minus (a) 1 million shares
of Common Stock issued to a consultant assisting with the Transaction and (b) 1 million shares of Common Stock issued to Avram Miller
prior to the Closing pursuant to his Advisory Agreement, dated October 11, 2023, by and between Mr. Miller and Renovaro (the “Miller
Consulting Agreement”)), which percentage shall be a ratio of the aggregate number of GEDi Cube Shares owned by the Sellers divided
by the aggregate number of GEDi Cube Shares issued and outstanding, in each case, as of the Effective Time (the “Closing Consideration”),
and (ii) the right to receive earn-out shares of Common Stock to be issued pro rata to the Sellers upon the exercise or conversion of
any of Renovaro’s derivative securities (subject to certain exceptions) which are outstanding at the Effective Time (the “Earnout
Shares”). For a complete description of how the Closing Consideration and Earnout Shares will be determined, see “The Stock
Purchase Agreement—Exchange Consideration” in the accompanying proxy statement. Based on the number of shares of Common
Stock outstanding as of December 29, 2023 and the terms of the Stock Purchase Agreement governing the determination of the Closing Consideration,
Renovaro estimates that, immediately following the closing of the Transaction, the Sellers will own approximately 49% of the issued and
outstanding shares of Common Stock (before giving effect to the issuance of the Earnout Shares). In addition, following the closing of
the Transaction, Renovaro will change its name to “Renovaro AI,” or such other name as mutually agreed by Renovaro and GEDi Cube.
At the Effective Time of
the Transaction, the Board of Directors of Renovaro (the “Renovaro Board”) is expected to include Mark R. Dybul, as
Chief Executive Officer of Renovaro, four of the other existing directors of Renovaro, three of whom must qualify as independent
directors under the rules of The Nasdaq Stock Market (“Nasdaq”), and four persons designated by GEDi Cube, two of whom
shall qualify as independent directors under the Nasdaq rules.
The Common Stock is currently
listed on Nasdaq under the symbol “RENB.” After the closing of the Transaction, Renovaro expects that its Common Stock
will continue trading on Nasdaq under the symbol “RENB.”
At the Special Meeting,
the stockholders of Renovaro will be asked to consider and vote on the following matters, as more fully described in the accompanying
proxy statement:
| ● | To approve, for purposes of Nasdaq Listing Rule 5635, the issuance of shares of Common Stock
pursuant to the Stock Purchase Agreement (the “Share Issuance Proposal”), which approval is necessary to consummate
the transactions contemplated by the Stock Purchase Agreement; |
| ● | To approve an amendment to Renovaro’s Certificate of Incorporation,
as amended (the “Certificate of Incorporation”), to increase the total number of authorized shares of capital stock of Renovaro
from one hundred ten million (110,000,000) to three hundred sixty million (360,000,000) and to increase the total number of authorized
shares of Common Stock from one hundred million (100,000,000) to three hundred fifty million (350,000,000) (the “Authorized Share
Proposal”), which approval is necessary to consummate the transactions contemplated by the Stock Purchase Agreement; |
| ● | To approve a decrease in the exercise price of the outstanding stock options held by Renovaro’s
employees and consultants granted pursuant to the Enochian BioSciences Inc. 2019 Equity Incentive Plan (the “2019 Plan”)
and the Renovaro Biosciences Inc. 2023 Equity Incentive Plan (the “2023 Plan” and, together with the 2019 Plan, the
“Plans”) with current exercise prices above the closing price of the Common Stock as reported on Nasdaq on the date
of the closing of the Transaction to such reported closing price, as required by Nasdaq Listing Rule 5635 and the terms of the
Plans (the “Repricing Proposal”); |
| ● | To approve an amendment to the 2023 Plan to increase the number of shares
of Common Stock available for awards under the 2023 Plan by five million (5,000,000) shares (the “2023 Plan Amendment Proposal”);and |
| ● | To adjourn the Special Meeting, if necessary, for the purpose of soliciting additional proxies
to approve any one or more of the matters identified above for consideration at the Special Meeting (the “Adjournment Proposal”). |
After careful consideration, the Renovaro Board unanimously approved the Stock Purchase Agreement, the Transaction
and each of the proposals referred to above, and has determined that they are advisable, fair and in the best interests of Renovaro’s
stockholders. Accordingly, the Renovaro Board unanimously recommends that Renovaro’s stockholders vote “FOR”
the Share Issuance Proposal, “FOR” the Authorized Share Proposal, “FOR” the Repricing Proposal, “FOR”
the 2023 Plan Amendment Proposal and “FOR” the Adjournment Proposal.
More information about Renovaro,
GEDi Cube, the Stock Purchase Agreement, the Transaction and the proposals referred to above is contained in the accompanying proxy
statement. Renovaro urges you to read the accompanying proxy statement carefully and in its entirety. IN PARTICULAR, YOU SHOULD
CAREFULLY CONSIDER THE MATTERS DISCUSSED UNDER “RISK FACTORS” BEGINNING ON PAGE 22.
The Renovaro Board fixed December 29, 2023, as the record date (the “Record
Date”) for the Special Meeting. Only holders of record of shares of Common Stock and shares of Series A Convertible Preferred Stock,
par value $0.0001 per share, of Renovaro (“Series A Preferred Stock”) at the close of business on the Record Date are entitled
to notice of, and to vote at, the Special Meeting. At the close of business on the Record Date, Renovaro had 67,224,089 shares of Common
Stock outstanding and entitled to vote at the Special Meeting and 561,010 shares of Series A Preferred Stock outstanding and entitled
to vote at the Special Meeting. Each holder of record of shares of Common Stock on the Record Date will be entitled to one vote for each
share held on all matters to be voted upon at the Special Meeting. Each holder of record of shares of Series A Preferred Stock on the
Record Date will be entitled to ten (10) votes for each share held on all matters to be voted upon at the Special Meeting.
Whether or not you plan
to attend the Special Meeting, Renovaro encourages you to vote as soon as possible to ensure that your shares are represented at
the Special Meeting. The accompanying proxy statement explains more about proxy voting, so please read it carefully. Please complete,
date, sign and return the accompanying proxy card in the enclosed envelope or vote online or by telephone using the instructions
included on the proxy card, to ensure the presence of a quorum at the Special Meeting. Even if you have voted by proxy, and you
attend the Special Meeting, you may, if you prefer, revoke your proxy and vote your shares in person. Please note, however, that
if your shares are held of record by a broker, bank or other nominee and you wish to vote at the Special Meeting, you will not
be permitted to vote in person at the Special Meeting unless you first obtain a legal proxy issued in your name from the record
holder.
The proxy statement is dated January 3, 2024 and is expected to be first mailed
to stockholders of Renovaro on or about January 4, 2024. The proxy statement is available at http://www.viewproxy.com/Renovaro/2024.
Renovaro is excited about
the opportunities that the Transaction brings to Renovaro’s stockholders and thanks you for your consideration and continued
support.
| Sincerely, |
| |
| /s/ Mark R. Dybul |
|
| Mark R. Dybul |
| Chief Executive Officer and Director |
January 3, 2024
TABLE
OF CONTENTS
RENOVARO BIOSCIENCES INC.
PROXY STATEMENT FOR THE
SPECIAL MEETING OF STOCKHOLDERS
TO BE HELD ON JANUARY 25, 2024
Renovaro Biosciences Inc. (“we,” “us,” “our,”
“Renovaro,” or the “Company”) is providing these proxy materials in connection with the special meeting of Renovaro
stockholders to be held on Thursday, January 25, 2024, at 12:00 p.m., Eastern Standard Time (the “Special Meeting”),
at K&L Gates LLP, 200 S Biscayne Blvd., Suite 3900, Miami, FL 33131, unless postponed or adjourned to a later date. This proxy statement
contains important information for you to consider when deciding how to vote on the matters to be brought before the Special Meeting.
The following section provides answers to frequently asked questions about the Special Meeting and the Transaction (as defined below).
This section, however, only provides summary information. For a more complete response to these questions and for additional information,
please refer to the cross-referenced sections. Renovaro urges its stockholders to read this document in its entirety prior to making any decision.
QUESTIONS AND ANSWERS ABOUT THE SPECIAL
MEETING AND THE TRANSACTION
| Q: |
What is the Transaction? |
| |
|
| A: |
On September 28, 2023, Renovaro entered into
a Stock Purchase Agreement, dated September 28, 2023 (the “Stock Purchase Agreement”), with GEDi Cube Intl Ltd., a
private company formed under the laws of England and Wales (“GEDi Cube”), the other sellers party thereto (together
with the other shareholders of GEDi Cube who deliver to Renovaro a joinder to the Stock Purchase Agreement, the “Sellers”
and each, a “Seller”), and Yalla Yalla Ltd., a private limited liability company registered and incorporated under
the laws of Malta, in its capacity as the representative of the Sellers (the “Sellers’ Representative”), pursuant
to which Renovaro will acquire all of the issued and outstanding shares and other equity interests of GEDi Cube from the Sellers
after which GEDi Cube will become a wholly-owned subsidiary of Renovaro (the “Transaction”).
Under the terms of the Stock Purchase Agreement, at the effective time of the
Transaction (the “Effective Time”), the Sellers will sell and transfer to Renovaro all of the equity interests of GEDi Cube
(each, a “GEDi Cube Share”) owned by Sellers that are issued and outstanding as of immediately prior to the Effective Time
in exchange for (i) an aggregate number of shares of common stock, par value $0.0001 per share, of Renovaro (“Common Stock”)
equal to a specified percentage of the shares of Common Stock issued and outstanding as of the Effective Time (minus (a) 1 million shares
of Common Stock issued to a consultant assisting with the Transaction and (b) 1 million shares of Common Stock issued to Avram Miller
prior to the Closing pursuant to his Advisory Agreement, dated October 11, 2023, by and between Mr. Miller and Renovaro (the “Miller
Consulting Agreement”)), which percentage shall be a ratio of the aggregate number of GEDi Cube Shares owned by the Sellers divided
by the aggregate number of GEDi Cube Shares issued and outstanding, in each case, as of the Effective Time (the “Closing Consideration”),
and (ii) the right to receive earn-out shares of Common Stock to be issued pro rata to the Sellers upon the exercise or conversion of
any of Renovaro’s derivative securities (subject to certain exceptions) which are outstanding at the Effective Time (the “Earnout
Shares” and, together with the Closing Consideration, the “Exchange Consideration”). For a complete description of how
the Closing Consideration and Earnout Shares will be determined, see “The Stock Purchase Agreement—Exchange Consideration”
in the accompanying proxy statement. Based on the number of shares of Common Stock outstanding as of December 29, 2023 and the terms of
the Stock Purchase Agreement governing the determination of the Closing Consideration, Renovaro estimates that, immediately following
the closing of the Transaction (the “Closing”), the Sellers will own approximately 49% of the issued and outstanding shares
of Common Stock (before giving effect to the issuance of the Earnout Shares).
Following the consummation of the Transaction, Renovaro will change its name to “Renovaro
AI,” or such other name as mutually agreed by Renovaro and GEDi Cube. Renovaro expects that, following consummation of the Transaction,
the Common Stock will continue trading on The Nasdaq Stock Market (“Nasdaq”) under the symbol “RENB.”
References to the “combined company”
in this proxy statement are references to Renovaro following the Closing. For a more complete description of the Transaction and
the Exchange Consideration, please see the section titled “The Stock Purchase Agreement” in this proxy statement. |
| Q: |
Why is Renovaro proposing to acquire GEDi Cube? |
| |
|
| A: |
GEDi Cube is an artificial intelligence (“AI”) medical technology company.
If completed, the Transaction will result in a combined company that will offer advanced early diagnosis and early identification
of recurring cancer as well as potential therapies for several critical diseases such as pancreatic cancer and other solid tumors
with poor life expectancy. It is expected that the combined company will have a unique advantage: Renovaro’s pre-clinical
and clinical trial data could be utilized to accelerate GEDi Cube’s AI capabilities that, in turn, could potentially help
to accelerate Renovaro’s development of potential new therapies. AI will be used to advance the fields of diagnosis and treatment
with the aim of redefining the future of medicine.
For a more complete description of the reasons
for the Transaction, please see the sections titled “The Transaction—Reasons for the Transaction” beginning
on page 57 of this proxy statement. |
| Q: |
What is required to consummate the Transaction? |
| |
|
| A: |
The consummation of the Transaction is subject
to a number of closing conditions, including the conditions that the Renovaro stockholders approve the issuance of shares of Common
Stock in the Transaction under Nasdaq Listing Rule 5635 and the amendment of the Certificate of Incorporation of Renovaro, as
amended (the “Certificate of Incorporation”), to increase the number of authorized shares of Common Stock to allow
for the issuance of shares of Common Stock under the Stock Purchase Agreement.
For a more complete description of the closing
conditions under the Stock Purchase Agreement, please see the section titled “The Stock Purchase Agreement—Conditions
to Completion of the Transaction” beginning on page 71 of the proxy statement. |
| Q: |
Are there any federal or state regulatory requirements that must be complied with or federal or state regulatory approvals or clearances that must be obtained in connection with the Transaction? |
| |
|
| A: |
Neither Renovaro nor GEDi Cube is required to make any filings or to obtain any approvals or clearances from any antitrust regulatory authorities in the United States or other countries to consummate the Transaction. In the United States, Renovaro must comply with applicable federal and state securities laws and the Nasdaq rules in connection with the issuance of shares of Common Stock in the Transaction, including the filing with the U.S. Securities and Exchange Commission (the “SEC”) of this proxy statement and the required Renovaro stockholder approval for the issuance of shares of Common Stock under the Stock Purchase Agreement under Nasdaq Listing Rule 5635 and the repricing of outstanding stock options under the Enochian BioSciences Inc. 2019 Equity Incentive Plan (the “2019 Plan”) and the Renovaro Biosciences Inc. 2023 Equity Incentive Plan (the “2023 Plan” and, together with the 2019 Plan, the “Plans”) under Nasdaq Listing Rule 5635. |
| Q: |
Why am I receiving these proxy materials? |
| |
|
| A: |
You are receiving this proxy statement because you have been identified as
a stockholder of Renovaro as of December 29, 2023, the record date for the Special Meeting, and you are entitled to vote to approve the
matters set forth herein. The Board of Directors of Renovaro (the “Renovaro Board”) is soliciting your proxy to vote at the
Special Meeting, which is to be held on Thursday, January 25, 2024, at 12:00 p.m., Eastern Standard Time, at K&L Gates LLP, 200
S Biscayne Blvd., Suite 3900, Miami, FL 33131 or at any adjournments or postponements thereof, for the purposes set forth in this proxy
statement. You are invited to attend the Special Meeting to vote on the proposals described in this proxy statement. However, you do not
need to attend the Special Meeting to vote your shares. This proxy statement includes information that we are required to provide to you
under SEC rules and is designed to assist you in voting your shares.
In addition, you are receiving copies of our
Annual Report on Form 10-K for the fiscal year ended June 30, 2023, as filed with the SEC on October 2, 2023 (the “Renovaro
2023 Form 10-K”), and our Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2023, as filed with
the SEC on November 14, 2023 (the “Renovaro First Quarter 2024 Form 10-Q”), along with this proxy statement. Such reports
include Renovaro’s audited consolidated financial statements for the fiscal years ended June 30, 2023 and June 30, 2022 and
Renovaro’s unaudited condensed consolidated financial statements for the quarter ended September 30, 2023 and certain other
financial information, which are incorporated by reference herein. See “Information Incorporated by Reference”
for further information.
If you are a stockholder of Renovaro as of
the record date and have received a printed copy of these materials by mail, you may complete, sign and return the enclosed proxy
card or follow the instructions below to submit your proxy over the telephone or on the Internet. If you did not receive a printed
copy of these materials by mail and are accessing them on the Internet, you may submit your proxy on the Internet or over the telephone,
as described below. |
| Q: |
What is a proxy? |
| |
|
| A: |
The Renovaro Board is asking for your proxy. This means you authorize persons selected by us to vote your shares at the Special Meeting in the way that you instruct. All shares represented by valid proxies received before the Special Meeting will be voted in accordance with the stockholder’s specific voting instructions. |
| Q: |
What proposals will be voted on at the Special Meeting? |
| |
|
| A: |
Pursuant to the terms of the Stock Purchase
Agreement, the following proposals must be approved by the Renovaro stockholders at the Special Meeting to consummate the Transaction:
● Proposal
1: To approve, for purposes of Nasdaq Listing Rule 5635, the issuance of shares of Common Stock pursuant to the Stock Purchase
Agreement (the “Share Issuance Proposal”); and
● Proposal
2: To approve an amendment to the Certificate of Incorporation to increase the total number of authorized shares
of capital stock of Renovaro from one hundred ten million (110,000,000) to three hundred sixty million (360,000,000) and to increase
the total number of authorized shares of Common Stock from one hundred million (100,000,000) to three hundred fifty million (350,000,000)
(the “Authorized Share Proposal”).
The Transaction and the issuance of the shares
of Common Stock in the Transaction will not be consummated unless the Share Issuance Proposal and the Authorized Share Proposal
are approved by the Renovaro stockholders.
In addition to the proposals listed above,
the Renovaro stockholders will be asked to approve the following proposals at the Special Meeting:
● Proposal
3: To approve a decrease in the exercise price of the outstanding stock options
held by Renovaro’s employees and consultants granted pursuant to the Plans with current exercise prices above the closing price
of the Common Stock as reported on Nasdaq on the date of the Closing to such reported closing price, as required by Nasdaq Listing Rule
5635 and the terms of the Plans (the “Repricing Proposal”);
● Proposal
4: To approve an amendment to the 2023 Plan to increase the number of shares of Common Stock available for awards under the 2023 Plan
by five million (5,000,000) shares (the “2023 Plan Amendment Proposal”); and
● Proposal
5: To adjourn the Special Meeting, if necessary, for the purpose of soliciting additional proxies to approve any one or more of
the matters identified above for consideration at the Special Meeting (the “Adjournment Proposal”).
The Renovaro Board is not aware of any other
matters to be brought before the Special Meeting. If other matters are properly raised at the meeting, the proxy holders may vote
any shares represented by proxy in their discretion. |
| Q: |
When and where will the Special Meeting take place? |
| |
|
| A: |
The Special Meeting will be held on Thursday, January 25, 2024, at 12:00 p.m,
Eastern Standard Time. The Special Meeting will be held at K&L Gates LLP, 200 S Biscayne Blvd., Suite 3900, Miami, FL 33131. Subject
to space availability, all Renovaro stockholders as of the Record Date (as defined below), or their duly appointed proxies, may attend
the Special Meeting. |
| Q: |
Who is entitled to vote at the Special Meeting? |
| |
|
| A: |
Renovaro’s Board of Directors fixed December 29, 2023 as the record date (the “Record
Date”) for the Special Meeting. Only holders of record of shares of Common Stock or shares of Series A Convertible Preferred Stock,
par value $0.0001 per share, of Renovaro (“Series A Preferred Stock”) at the close of business on the Record Date are entitled
to notice of, and to vote at, the Special Meeting. The notice of the Special Meeting, proxy statement and form of proxy are first expected
to be mailed to stockholders on or about January 4, 2024.
A stockholder of record as of the Record Date
will be able to attend the Special Meeting and vote during the Special Meeting in person. Even if you plan to attend the Special
Meeting in person, Renovaro requests that you sign and return the enclosed proxy to ensure that your shares will be represented
at the Special Meeting if you are unable to attend.
Beneficial owners of Common Stock or Series
A Preferred Stock who are not stockholders of record are also invited to attend the Special Meeting. However, beneficial owners
may not vote during the Special Meeting unless they have obtained a legal proxy from the bank, broker or other nominee that holds
their shares, giving them the right to vote at the Special Meeting.
At the close of business on the Record Date, Renovaro had 67,224,089 shares
of Common Stock outstanding and entitled to vote at the Special Meeting and 561,010 shares of Series A Preferred Stock outstanding and
entitled to vote at the Special Meeting. Each holder of record of shares of Common Stock on the Record Date will be entitled to one vote
for each share held on all matters to be voted upon at the Special Meeting. Each holder of record of shares of Series A Preferred Stock
on the Record Date will be entitled to ten (10) votes for each share held on all matters to be voted upon at the Special Meeting. |
| Q: |
What is a stockholder of record? |
| |
|
| A: |
A stockholder of record is a stockholder whose ownership of our Common Stock or Series A Preferred Stock is reflected directly on the books and records of our transfer agent, Securities Transfer Corporation. If you hold stock through an account with a bank, broker or similar organization, you are considered the beneficial owner of shares held in “street name” and are not a stockholder of record. For shares held in street name, the stockholder of record is your bank, broker or similar organization. We only have access to stock ownership information for stockholders of record. As described below, if you are not a stockholder of record, you will not be able to vote your shares unless you have a proxy from the stockholder of record authorizing you to vote your shares. |
| Q: |
What are the quorum requirements for the Special Meeting? |
| |
|
| A: |
The presence in person or by proxy of at least one-third (1/3) of the issued and outstanding shares of Common Stock and Series A Preferred Stock entitled to vote at the Special Meeting constitutes a quorum. Your shares will be counted as present at the Special Meeting for purposes of determining whether there is a quorum if a proxy card has been properly submitted by you or on your behalf, or you vote at the Special Meeting. Abstaining votes and broker non-votes are counted for purposes of establishing a quorum. |
| |
|
|
| Q: |
How can I find out the results of the voting at the Special Meeting? |
| |
|
| A: |
Preliminary voting results will be announced at the Special Meeting. In addition, final voting results will be published in a current report on Form 8-K that we expect to file within four (4) business days after the Special Meeting. If final voting results are not available to us in time to file a Form 8-K within four (4) business days after the Special Meeting, we intend to file a Form 8-K to publish preliminary results and, within four (4) business days after the final results are known to us, file an additional Form 8-K to publish the final results. |
| Q: |
What vote is required to approve the proposals to be presented at the Special Meeting? |
| |
|
| A: |
Provided a quorum is present at the Special
Meeting:
● each
of Proposal 1 and Proposal 3 must be approved by the affirmative vote of a majority of the votes cast in person or by proxy at
the Special Meeting, as required by Nasdaq Listing Rule 5635;
● each of Proposal 2 and Proposal 4 must be approved by the affirmative vote of a majority of the votes cast
in person or by proxy at the Special Meeting, as required by the General Corporation Law of the State of Delaware; and
● Proposal 5 must be approved by the affirmative vote of a majority of the votes cast in person or by proxy
at the Special Meeting, as required by the By-laws of Renovaro, as amended (the “By-laws”). |
The approval of Proposals 1 and 2 is a condition
to the consummation of the Transaction pursuant to the Stock Purchase Agreement.
Pursuant to the terms of the Series A Preferred
Stock, the holders of Series A Preferred Stock will vote together with the holders of Common Stock, as a single class, on all matters
to be presented to the Renovaro stockholders at the Special Meeting.
For each of the Proposals 1, 2, 3, 4 and 5, broker non-votes, if any, and a properly marked “ABSTAIN”
with respect to any such matter will not be voted “for” or “against,” and will not be counted as a vote
cast for purposes of determining the number of votes cast with respect to such proposals. Accordingly, broker non-votes and abstentions
will not be considered as a vote cast with respect to such matters.
| Q: |
What are the Renovaro Board’s voting recommendations? |
| |
|
| A: |
The Renovaro Board recommends that you vote your shares: |
| |
● |
“FOR” the Share Issuance Proposal; |
| |
|
|
| |
● |
“FOR” the Authorized Share Proposal; |
| |
|
|
| |
● |
“FOR” the Repricing Proposal; |
| |
● |
“FOR” the 2023 Plan Amendment Proposal; and |
| |
|
|
| |
● |
“FOR” the Adjournment Proposal. |
| Q: |
How do I vote? |
| |
|
| A: |
You may vote by any of the following methods: |
If you are a registered holder:
| |
● |
By Internet or telephone. You may vote over the Internet at www.AALvote.com/RENBSM or vote by telephone at 1-866-804-9616. Please see your proxy card for voting instructions. |
| |
|
|
| |
● |
By mail. If you elected to receive printed proxy materials by mail, you may vote by signing and returning the proxy card provided. Please allow sufficient time for mailing if you decide to vote by mail. |
| |
|
|
| |
● |
During the Special Meeting. You may vote by attending the Special Meeting and voting in person. |
| |
|
|
| If you are a beneficial owner of shares held in “street name”: |
| |
|
|
| |
● |
You may vote by following the voting instructions provided to you by your bank or broker. |
| Q: |
How can I change or revoke my vote? |
| ● |
Stockholders of record. You may change or revoke your vote as follows: |
| |
● |
by submitting a written notice of revocation to our Secretary at our principal executive office, Attention: Luisa Puche, Chief Financial Officer and Corporate Secretary; |
| |
|
|
| |
● |
by timely delivering a properly executed, later-dated proxy or submitting a proxy with new voting instructions using the telephone or Internet voting system; or |
| |
|
|
| |
● |
by attending the Special Meeting and voting
in person.
For all methods of voting, the
last vote cast will supersede all previous votes. |
| ● |
Beneficial owners of shares held in “street name.” You may change or revoke your voting instructions by following the specific directions provided to you by your bank, broker or other nominee. |
| Q: |
What if I do not specify a choice for a matter when returning a proxy? |
| A: |
Your proxy will be treated as follows: |
| |
|
| |
Stockholders of record. If you are a stockholder of record and you sign and return a proxy card without giving specific voting instructions, then the proxy holders will vote your shares in the manner recommended by the Renovaro Board on all matters presented in this proxy statement and as the proxy holders may determine in their discretion for any other matters properly presented for a vote at the Special Meeting. |
| |
Beneficial owners of shares held in street name. If you are a beneficial owner of shares held in street name and do not provide the broker or other nominee that holds your shares with specific voting instructions, the nominee that holds your shares may generally vote on “routine” matters but cannot vote on “non-routine” matters under applicable stock exchange rules. If the nominee that holds your shares does not receive instructions from you on how to vote your shares on a non-routine matter, the organization that holds your shares will inform the inspector of election that it does not have the authority to vote on this matter with respect to your shares. This is referred to as a “broker non-vote.” |
| Q: |
Which ballot measures are considered “routine” or “non-routine”? |
| A: |
All proposals to be presented at the Special Meeting are considered to be non-routine matters under applicable stock exchange rules. A broker or other nominee cannot vote without instructions on non-routine matters, and therefore there may be broker non-votes on each of the proposals to be presented at the Special Meeting. |
| Q: |
How are proxies solicited and what is the cost? |
| |
|
| A: |
We will bear all expenses incurred in connection with the solicitation of proxies for the Special Meeting. In addition to solicitation by mail, our directors, officers and employees may solicit proxies from stockholders by telephone, letter, email or in person without compensation. We will reimburse banks, brokerage firms and other custodians, nominees and fiduciaries for their reasonable out-of-pocket expenses for sending proxy materials to stockholders and obtaining their votes. |
| |
|
| Q: |
What should I do if I have questions regarding the Special Meeting? |
| |
|
| A: |
If you have any questions about the Special Meeting or would like additional copies of any of the documents
referred to in this proxy statement, you should email our Investor Relations department at ir@renovarobio.com. |
| Q: |
How are votes counted? |
| A: |
Votes will be counted by the inspector of election for the Special Meeting, who will separately count votes “For”, “Withhold”, “Against”, abstentions, and if applicable, broker non-votes applicable for each proposal. |
SUMMARY
This summary highlights selected information
from this proxy statement and may not contain all of the information that is important to you. To better understand the Transaction
and the other proposals being considered at the Special Meeting, you should read this entire proxy statement carefully, including
the materials attached as annexes, as well as other documents referred to or incorporated by reference herein. This proxy statement
also incorporates by reference certain information from the Renovaro 2023 Form 10-K and the Renovaro First Quarter 2024 Form 10-Q,
copies of which have been delivered together with this proxy statement. You can obtain additional copies of these report and the
other documents incorporated by reference into this proxy statement without charge by following the instructions under the section
of this proxy statement titled “Where You Can Find More Information”.
The Companies
Renovaro Biosciences Inc.
2080 Century Park East, Suite 906
Los Angeles, CA 90067
(305) 918-1980
Renovaro is a biotechnology company committed
to developing advanced allogeneic cell and gene therapies to promote stronger immune system responses potentially for long-term
or life-long cancer remission in some of the deadliest cancers, and potentially to treat or cure serious infectious diseases such
as Human Immunodeficiency Virus (HIV) and Hepatitis B virus (HBV) infection. Renovaro has developed advanced cell, gene and immunotherapy
platforms designed to renew the body’s natural tumor-fighting capabilities against cancer and infectious diseases. Renovaro
expects to begin human Phase 1/2 clinical trials of its leading candidate for pancreatic cancer and other solid tumors with poor
life expectancy by the second half of 2024.
GEDi Cube, Intl Ltd.
71-75 Shelton Street
Covent Garden
London
WC2H 9JQ
GEDi Cube is pioneering a multi-modal approach
for the early detection of cancer and its recurrence involving blood biopsies, imaging and multi-omic analysis. GEDi Cube has a
strategic partnership with Nvidia Corporation (NASDAQ: NVDA), the leader in semiconductors for AI. GEDi Cube has been developing
its innovative technologies over the last decade and has already validated early diagnosis of lung cancer in humans at a leading
university hospital. GEDi Cube has also validated technology to target 12 additional cancers, including pancreatic and breast cancer.
The Transaction (see page 54)
Under the Stock Purchase Agreement, Renovaro will acquire all of the issued and
outstanding shares and other equity interests of GEDi Cube from the Sellers after which GEDi Cube will become a wholly-owned subsidiary
of Renovaro. Subject to the terms and conditions of the Stock Purchase Agreement, at the Effective Time, the Sellers will sell and transfer
to Renovaro all of GEDi Cube Shares owned by Sellers that are issued and outstanding as of immediately prior to the Effective Time in
exchange for (i) the Closing Consideration and (ii) the right to receive Earnout Shares. Following the Closing, Renovaro will change its
name to “Renovaro AI,” or such other name as mutually agreed by Renovaro and GEDi Cube. Based on the number of shares of Common
Stock outstanding as of December 29, 2023 and the terms of the Stock Purchase Agreement governing the determination of the Closing Consideration,
Renovaro estimates that, immediately following the Closing, the Sellers will own approximately 49% of the issued and outstanding shares
of Common Stock (before giving effect to the issuance of the Earnout Shares). References to the “combined company” in this
proxy statement are references to Renovaro following the consummation of the Transaction.
Renovaro expects the Transaction to close during
the first half of calendar year 2024, subject to satisfying certain closing conditions.
Reasons for the Transaction (see page 57)
Renovaro anticipates that the Transaction will
result in a combined company that will offer advanced early diagnosis and early identification of recurring cancer as well as potential
therapies for several critical diseases such as pancreatic cancer and other solid tumors with poor life expectancy. The combined
company is also expected to have a unique advantage of the potential utilization of Renovaro’s pre-clinical and clinical
trial data to accelerate GEDi Cube’s AI capabilities that, in turn, could potentially help to accelerate Renovaro’s
development of potential new therapies. AI will be used by the combined company to advance the fields of diagnosis and treatment
with the aim of redefining the future of medicine.
For a more complete description of the factors
on which the Renovaro Board based its decision to approve the Stock Purchase Agreement and the transactions contemplated by the
Stock Purchase Agreement, please see the section titled “The Transaction -- Reasons for the Transaction”
in this proxy statement.
The Stock Purchase Agreement (see page 64)
Exchange Consideration
If the Transaction is consummated, Renovaro will
acquire from the Sellers all of the issued and outstanding GEDi Cube Shares. In exchange therefor and subject to the terms and
conditions of the Stock Purchase Agreement, at the Effective Time, the Sellers shall become entitled to receive from Renovaro (i)
an aggregate number of shares of Common Stock equal to a specified percentage of the shares of Common Stock issued and outstanding
as of the Effective Time (minus (a) 1 million shares of Common Stock issued to a consultant assisting with the Transaction and
(b) 1 million shares of Common Stock issued to Avram Miller prior to the Closing pursuant to the Miller Consulting Agreement), which
percentage shall be a ratio of the aggregate number of GEDi Cube Shares owned by the Sellers divided by the aggregate number of
GEDi Cube Shares issued and outstanding, in each case, as of the Effective Time (which is referred to herein as the “Closing
Consideration”), and (ii) earn-out shares of Common Stock to be issued pro rata to the Sellers upon the exercise or conversion
of any of Renovaro’s derivative securities (subject to certain exceptions) which are outstanding at the Effective Time (which
is referred to herein as the “Earnout Shares” and, together with the Closing Consideration, the “Exchange Consideration”).
The aggregate number of Earnout Shares to be issued to the Sellers will be equal to a percentage of the shares of Common Stock
issued upon exercise or conversion of Renovaro’s derivative securities, which percentage shall be a ratio of the aggregate
number of GEDi Cube Shares owned by the Sellers divided by the aggregate number of GEDi Cube Shares issued and outstanding, in
each case, as of the Effective Time.
Each Seller will receive its pro rata portion
of the Exchange Consideration based on such Seller’s pro rata ownership of the GEDi Cube Shares transferred to Renovaro.
No fractional shares of Common Stock will be issued in connection with the Transaction, and no cash will be paid for fractional
shares. Any fractional shares of Common Stock that a Seller would otherwise be entitled to receive will be rounded down to the
nearest whole share.
Immediately following the Closing, the Sellers are
expected to own approximately 49% of the issued and outstanding shares of Common Stock (before giving effect to the issuance of the
Earnout Shares).
Conditions to Completion of the Transaction
The obligation of Renovaro, on the one hand,
and the Sellers and GEDi Cube, on the other hand, to consummate the Transaction is subject to certain customary conditions, unless
otherwise waived, including the following:
| ● | approval of Proposals 1 and 2 by the Renovaro stockholders; |
| ● | amendment of the Certificate of Incorporation to reflect the amendment contemplated by Proposal 2; |
| ● | accuracy of the respective representations and warranties of Renovaro, on the one hand, and GEDi Cube and the Sellers, on the
other hand, as of the Closing, except that, in the case of representations and warranties other than those deemed to be fundamental
representations, except where such inaccuracy would not have a material adverse effect on the business of Renovaro or GEDi Cube,
as applicable, or the ability of Renovaro, or the Sellers or GEDi Cube, as applicable, to consummate the Transaction or perform
their respective obligations under the Stock Purchase Agreement; |
| ● | the Renovaro Board being composed of those individuals designated by the Stock Purchase Agreement and otherwise specified by
Renovaro or GEDi Cube, where required, as of the Effective Time; |
| ● | all of the holders of GEDi Cube Shares immediately prior to the Closing having delivered a joinder to become a party to the
Stock Purchase Agreement as a Seller; and |
| ● | compliance in all material respects by Renovaro, on the one hand, and the Sellers and GEDi Cube, on the other hand, with their
respective covenants and obligations in the Stock Purchase Agreement. |
The conditions that must be satisfied or waived
prior to the consummation of the Transaction are set forth in the section titled “The Stock Purchase Agreement—Conditions
to Completion of the Transaction.”
Exclusivity
Each of Renovaro, on the one hand, and GEDi Cube
and the Sellers, on the other hand, agreed to not, and to cause their respective representatives to not, subject to certain exceptions
under the Stock Purchase Agreement, from the date of the Stock Purchase Agreement until the earlier of the consummation of the
Transaction or the termination of the Stock Purchase Agreement in accordance with its terms:
| ● | solicit, assist, initiate or facilitate the making, submission or announcement of, or intentionally encourage, any Acquisition
Proposal (as defined under “The Stock Purchase Agreement—Exclusivity”); |
| ● | furnish any non-public information regarding such party or its affiliates or their respective businesses, operations, assets,
liabilities, financial condition, prospects or employees to any person or group of persons (other than the other parties to the
Stock Purchase Agreement or their respective representatives) in connection with or in response to an Acquisition Proposal, other
than as required by applicable law; |
| ● | engage or participate in discussions or negotiations with any person or group of persons with respect to, or that could reasonably
be expected to lead to, an Acquisition Proposal; |
| ● | approve, endorse or recommend, or publicly propose to approve, endorse or recommend, any Acquisition Proposal; |
| ● | negotiate or enter into any letter of intent, agreement in principle, acquisition agreement or other similar agreement related
to any Acquisition Proposal; or |
| ● | release any third person from, or waive any provision of, any confidentiality agreement to which such party is a party. |
Termination
The Stock Purchase Agreement may be terminated
by Renovaro or by GEDi Cube or the Sellers’ Representative under certain circumstances, including, among others (and as further
described under “The Stock Purchase Agreement—Termination”):
| ● | by mutual written consent of Renovaro and GEDi Cube; |
| ● | by Renovaro or the Sellers’ Representative if any of the conditions to the Closing under the Stock Purchase Agreement
have not been satisfied or waived by February 28, 2024, so long as no breach or violation by the terminating party or any of its
representations, warranties, covenants or obligations under the Stock Purchase Agreement was the cause of, or otherwise resulted
in, the failure of the Closing to occur before such date; |
| ● | by Renovaro or GEDi Cube if a governmental entity of competent jurisdiction has issued an order or taken any other action that
permanently restricts, restrains, enjoins or otherwise prohibits the Transaction and such order or other action has become final
and non-appealable, so long as no failure of the terminating party or any of its affiliates to comply with any provision of the
Stock Purchase Agreement has been a substantial cause of, or substantially resulted in, such action by such governmental entity; |
| ● | by Renovaro or GEDi Cube, as applicable, upon a breach of any representation, warranty, covenant or agreement on the part of
GEDi Cube or any Seller, or on the part of Renovaro, respectively, set forth in the Stock Purchase Agreement, or if any representation
or warranty of such other parties or party shall have become inaccurate, in either case, such that the closing conditions concerning
accuracy of such other parties’ or party’s representations and warranties and compliance with covenants would not be
satisfied as of the later of the date of the Stock Purchase Agreement or the time of such breach or inaccuracy, if such breach
or inaccuracy is incapable of being cured or is not cured within the earlier of 20 days after prior written notice of such breach
or inaccuracy provided by the terminating party or February 28, 2024; |
| ● | by Renovaro upon a material adverse effect occurring with respect to the business of GEDi Cube or the ability of the Sellers
or GEDi Cube to consummate the Transaction or perform their respective obligations under the Stock Purchase Agreement; |
| ● | by Renovaro or GEDi Cube, if the Special Meeting is held and has concluded, and the Renovaro stockholders have voted on Proposals
1 and 2 and any of such proposals have not been approved or adopted at the Special Meeting; |
| ● | by Renovaro, if there has been a material adverse change, in Renovaro’s reasonable determination, to GEDi Cub’s
AI technologies, intellectual property rights or capital structure, except as contemplated by the Stock Purchase Agreement on or
prior to the date that is five business days following the date of the delivery of GEDi Cube’s disclosure schedules to the
Stock Purchase Agreement; provided that Renovaro is not in material uncured breach of the Stock Purchase Agreement; or |
| ● | by the Sellers’ Representative, if there has been a material adverse change, in the Sellers’ Representative’s
reasonable determination, to Renovaro’s intellectual property rights or capital structure, except as contemplated by the
Stock Purchase Agreement on or prior to the date that is five business days following the date of the delivery of Renovaro’s
disclosure schedules to the Stock Purchase Agreement; provided that none of GEDi Cube or the Sellers are in material uncured breach
of the Stock Purchase Agreement. |
In the event of termination, the Stock Purchase
Agreement (other than certain provisions as set forth in the Stock Purchase Agreement) will become void and of no effect with no
liability or obligation on the part of any party to the Stock Purchase Agreement or any of its officers or directors, except that
no party will be relieved of any liability or damages arising from fraud or willful breach by such party of its representations,
warranties or covenants in the Stock Purchase Agreement.
Registration Rights Agreement (see page 76)
Upon the consummation of the Transaction, Renovaro
and each Seller will enter into a Registration Rights Agreement, pursuant to which Renovaro will undertake certain resale shelf
registration obligations and each Seller will be granted customary demand and piggyback registration rights with respect to its
shares of Common Stock acquired in the Transaction.
Management of the Combined Company Following the Closing (see
page 61)
Following the Closing, the combined company’s
executive officers are expected to remain the same as Renovaro’s current executive officers, including the following individuals:
| Name |
Position with the Combined Company |
Current Position |
| Mark R. Dybul, M.D. |
Chief Executive Officer |
Chief Executive Officer |
| Luisa Puche |
Chief Financial Officer & Corporate Secretary |
Chief Financial Officer & Corporate Secretary |
| François Binette, PhD |
Chief Operating Officer & Executive Vice President for Research & Development |
Chief Operating Officer & Executive Vice President for Research & Development |
Directors of the Combined Company Following the Closing (see
page 62)
The Stock Purchase Agreement obligates the parties
to take all necessary action so that, effective as of the consummation of the Transaction, the combined company will have a board
of directors consisting of nine individuals, including the Chief Executive Officer of Renovaro, four persons designated by Renovaro
(at least three of whom must qualify as an “independent director” under Nasdaq Listing Rules), and four person designated
by GEDi Cube (at least two of whom must qualify as an “independent director” under Nasdaq Listing Rules), subject to
diversity and other requirements under applicable law and Nasdaq Listing Rules.
Interests of the Renovaro Directors and Executive Officers
in the Transaction (see page 60)
In considering the recommendation of Renovaro’s
board of directors to approve the Share Issuance Proposal and the Authorized Share Proposal, Renovaro’s stockholders should
be aware that members of the Renovaro Board and executive officers of Renovaro have interests in the Transaction that may be different
from, or in addition to, your interests.
Each of Renovaro’s executive officers is
expected to continue, immediately following the consummation of the Transaction, as officers of the combined company. In addition,
certain current directors of Renovaro are expected to continue after the consummation of the Transaction as directors of the combined
company.
As of December 29, 2023, all directors and executive
officers of Renovaro, together with their affiliates, beneficially owned approximately 20.18% of the outstanding shares of Common Stock
(which excludes any shares of Common Stock issuable upon exercise or settlement of outstanding stock options or restricted stock units
under the Plans and outstanding warrants held by such individuals).
See “Proposal 3: Approval of the Repricing
of Outstanding Stock Options Granted under the Enochian BioSciences Inc. 2019 Equity Incentive Plan and the Renovaro Biosciences
Inc. 2023 Equity Incentive Plan—Eligible Option Holders” for additional information regarding the Eligible Options
(as defined below) held by Renovaro’s executive officers that may be subject to the Option Repricing (as defined below).
Federal Securities Law Consequences (see page 62)
The issuance of Common Stock in the Transaction to
Renovaro stockholders will be effected by means of a private placement, which is exempt from registration under the U.S. Securities
Act of 1933, as amended (the “Securities Act”), in reliance on Section 4(a)(2) of the Securities Act and Rule 506 of
Regulation D or Regulation S promulgated thereunder and such shares will be “restricted securities.” The shares issued
in connection with the Transaction will not be registered under the Securities Act upon issuance and will not be freely
transferable. Holders of such shares may not sell their respective shares unless the shares are registered under the Securities Act
or an exemption is available under the Securities Act. Upon the consummation of the Transaction, Renovaro will enter into a
registration rights agreement with each Seller granting to each such Seller certain customary registration rights with respect to
such Seller’s shares of Common Stock acquired in the Transaction, as further described under “Agreements Related to
the Transaction—Registration Rights Agreement”.
Material U.S. Federal Income Tax Consequences of the Transaction
(see page 63)
The Transaction has been structured to qualify
as a reorganization within the meaning of Section 368(a) of the U.S. Internal Revenue Code of 1986, as amended (the “Code”).
Renovaro stockholders will not sell, exchange or dispose of any shares of Common Stock as a result of the Transaction. Thus, there
will be no material U.S. federal income tax consequences to Renovaro or its stockholders as a result of the Transaction.
For a more complete description of the material
U.S. federal income tax consequences of the Transaction, see “The Transaction— Material U.S. Federal Income Tax
Consequences of the Transaction” beginning on page 63 of this proxy statement.
Regulatory Approvals (see page 63)
Neither Renovaro nor GEDi Cube is required to
make any filings or to obtain approvals or clearances from any antitrust regulatory authorities in the United States or other countries
to consummate the Transaction. In the United States, Renovaro must comply with applicable federal and state securities laws and
the Nasdaq rules in connection with the issuance of shares of Common Stock in the Transaction, including the filing with the SEC
of this proxy statement.
Anticipated Accounting Treatment (see page 63)
In accordance with accounting principles generally
accepted in the United States (“U.S. GAAP”), Renovaro intends to account for the Transaction using the acquisition
method of accounting for business combinations. See “The Transaction—Anticipated Accounting Treatment”
elsewhere in this proxy statement for additional information.
Appraisal Rights (see page 63)
Renovaro stockholders are not entitled to appraisal
rights in connection with the Transaction.
Risk Factors (see page 22)
Both Renovaro and GEDi Cube are subject to various
risks associated with their businesses and industries. In addition, the Transaction, and the possibility that the Transaction may
not be completed, poses a number of risks to each Renovaro and GEDi Cube and its respective securityholders, including those risks
discussed under the section titled “Risk Factors” in this proxy statement. Renovaro encourages you to read and
consider all of these risks carefully.
MARKET PRICE AND DIVIDEND INFORMATION
The Common Stock trades on Nasdaq under the symbol
“RENB”. GEDi Cube is a private company and its GEDi Cube Shares are not publicly traded.
As of September 27, 2023, the last full trading
day immediately preceding the public announcement of the signing of the Stock Purchase Agreement, the closing price of the Common
Stock as reported on Nasdaq was $3.80 per share. Because the market price of the Common Stock is subject to fluctuation, the market
value of the shares of the Common Stock that the Sellers will be entitled to receive in the Transaction may increase or decrease
after the Effective Time.
As of December 29, 2023, the Record Date for the Special
Meeting, there were approximately 189 holders of record of the Common Stock. The number of record holders was determined from the records
of our transfer agent and does not include beneficial owners of our Common Stock whose shares are held in the names of various banks,
brokers or other nominees.
Assuming approval of the Share Issuance
Proposal, the Authorized Share Proposal and the Repricing Proposal and following the consummation of the Transaction, the
Common Stock is expected to continue trading on Nasdaq under the symbol “RENB.”
Dividends
Renovaro has not declared or paid any cash dividends
on the Common Stock and does not intend to declare or pay any cash dividends in the foreseeable future. The payment of dividends,
if any, is within the discretion of the Renovaro Board and will depend on Renovaro’s earnings, if any, its capital requirements
and financial condition and such other factors as the Renovaro Board may consider.
RISK FACTORS
In addition to the other information
contained in this proxy statement, you should carefully consider the material risks described below before deciding how to
vote your shares of Common Stock. You should also read and consider the risks associated with the business of Renovaro
because these risks may also affect the combined company—these risks can be found in the Renovaro 2023 Form 10-K and
the Renovaro First Quarter 2024 Form 10-Q, copies of which were delivered with this proxy statement, and are incorporated by
reference herein. You should also read and consider the other information in this proxy statement and the other documents
incorporated by reference into this proxy statement. Please see the section titled “Where You Can Find More
Information” on page 178 of this proxy statement.
Summary of Risk Factors
Risks Related to the Transaction
| ● | If the proposed Transaction with GEDi Cube is not consummated, Renovaro’s business could suffer materially and the price
of the Common Stock could decline. |
| ● | The Transaction may be completed even though material adverse changes may occur prior to the Closing. |
| ● | Renovaro’s stockholders will have a reduced ownership and voting interest in, and will exercise less influence over the
management of, Renovaro following the Closing and may not realize a benefit from the Transaction commensurate with the ownership
dilution resulting from the Transaction. |
| ● | Certain provisions of the Stock Purchase Agreement may discourage third parties from submitting competing proposals, and, during
the pendency of the Transaction, Renovaro may not be able to pursue alternatives to the Transaction or enter into a business combination
or acquisition transaction with another party on more favorable terms because of restrictions in the Stock Purchase Agreement. |
| ● | Due to the lack of a public market for the GEDi Cube Shares, the Sellers may receive consideration in the Transaction that
is greater than or less than the fair market value of the GEDi Cube Shares. |
| ● | Renovaro and GEDi Cube may become involved in securities litigation or stockholder derivative litigation in connection with
the Transaction. |
| ● | If the conditions to the Transaction are not met or waived, the Transaction will not occur. |
| ● | The unaudited pro forma financial information included in this proxy statement may not be representative of the combined company’s
results following the Closing. |
| ● | Following the Closing, the combined company may be unable to integrate successfully the businesses of Renovaro and GEDi Cube
and realize the anticipated benefits of the Transaction. |
Risks Related to Renovaro
Please refer to the section titled “Item
1A. Risk Factors” set forth in the Renovaro 2023 Form 10-K and the section titled “Item 1A. Risk Factors” set
forth in the Renovaro First Quarter 2024 Form 10-Q.
Risks Related to GEDi Cube
| ● | GEDi Cube operates in a rapidly evolving field and has a limited operating history, which makes
it difficult to evaluate its current business and to predict its future performance. |
| ● | GEDi Cube has a history of net losses and has never generated revenue
related to its AI platform, and anticipates that it may continue to incur net losses for the foreseeable future. |
| ● | If GEDi Cube fails to obtain the substantial financing needed for its current and future operations,
it may be unable to successfully commercialize its AI platform or commercialize or develop additional products. |
| ● | GEDi Cube’s AI platform and future products may not perform as expected. |
| ● | Following any future commercialization of GEDi Cube’s AI platform and other products, the
commercial success of such platform and products is uncertain and market acceptance may be less than anticipated. |
| ● | GEDi Cube’s success is heavily dependent on the successful development, regulatory approval,
insurance coverage and commercialization of its AI platform and future products. |
| ● | Any harm or injury to patients from GEDi Cube’s products or competitors’ products,
whether directly or indirectly, may subject GEDi Cube to significant reputation and liability risks. |
| ● | GEDi Cube’s AI platform and products will face significant competition. |
| ● | GEDi Cube will rely on third parties to conduct certain portions of its business operations, and
such third parties may not successfully carry out their contractual duties or meet expected deadlines. |
| ● | GEDi Cube’s business is subject to risks associated with operating internationally. |
| ● | GEDi Cube may not be able to attract and keep key personnel and members of management. |
| ● | GEDi Cube may fail to scale its operations, including the establishment of sales and marketing
capabilities, or adequately manage the growth of its business. |
| ● | Changes in laws and regulations, or their application, including new developments related to AI
technology, may adversely affect GEDi Cube’s business, financial condition and results of operations. |
| ● | GEDi Cube may be subject to financial and reputational risks due to product and professional liability
claims, and could face substantial liabilities resulting from defending such claims that exceed its resources. |
| ● | GEDi Cube conducts business in a heavily regulated industry, and may expend significant resources
in complying with the numerous statutes and regulations pertaining to its business. |
| ● | GEDi Cube relies significantly on information technology, including machine learning and cloud-based
computing technology, and any failures or breaches of GEDi Cube’s information technology systems or those of its third-party
service providers may result in liability to GEDi Cube or harm to its reputation, or cause delays in the development and commercialization
of its products. |
| ● | GEDi Cube primarily relies on trade secret protection and confidentiality agreements to protect
proprietary information, which may not be effective. |
| ● | GEDi Cube’s trademarks and trade names are not adequately protected, leaving them open to
challenge, infringement or other impairment (including third-party infringement claims). |
| ● | Litigation or other proceedings or third-party claims of intellectual property infringement or
misappropriation could require GEDi Cube to spend significant time and money. |
Risks Related to the Transaction
If the proposed Transaction with GEDi Cube is not consummated,
Renovaro’s business could suffer materially and the price of the Common Stock could decline.
The consummation
of the Transaction with GEDi Cube is subject to the satisfaction of a number of closing conditions, including the receipt of Renovaro
stockholder approval on Proposals 1 and 2; the occurrence of the Special Meeting; all of the holders of GEDi Cube Shares
becoming a party to the Stock Purchase Agreement; and other closing conditions. For a more detailed
discussion of these and other closing conditions, see the section titled “The Stock Purchase Agreement—Conditions
to Completion of the Transaction.” Renovaro expects the Transaction to close during the first half of 2024, subject
to satisfying certain closing conditions.
If the Transaction
is not consummated, Renovaro may be subject to a number of material risks, and its business and stock price could be adversely
affected, as follows:
| ● | Renovaro has incurred and expects to continue to incur significant expenses related to the Transaction with GEDi Cube, even
if the Transaction is not consummated. |
| ● | The Stock Purchase Agreement contains covenants restricting Renovaro’s
solicitation of competing acquisition proposals and the conduct of Renovaro’s business between the date of signing the Stock
Purchase Agreement and the Closing. As a result, significant business decisions and transactions before the Closing require the
consent of GEDi Cube and the Sellers. Accordingly, Renovaro may be unable to pursue business opportunities that would otherwise
be in its best interest as a standalone company. |
| ● | Renovaro’s collaborators and other business partners and investors in general may view the failure to consummate the
Transaction as a poor reflection on its business or prospects. |
| ● | As a result of the proposed Transaction, current and prospective employees could experience uncertainty about their future
roles within the combined company. This uncertainty may adversely affect Renovaro’s ability to retain its key employees,
who may seek other employment opportunities. |
| ● | Renovaro’s management team may be distracted from day-to-day operations as a result of the proposed Transaction. |
| ● | The market price of the Common Stock may decline to the extent that the
current market price reflects a market assumption that the proposed Transaction will be completed. |
The market price of the Common Stock may decline as a result
of the Transaction.
The market price of the Common Stock may decline
as a result of the Transaction for a number of reasons including if:
| ● | the combined company does not achieve the perceived benefits of the Transaction as rapidly or to the extent anticipated by
financial or industry analysts; |
| ● | the effect of the Transaction on the combined company’s business and prospects is not consistent with the expectations
of financial or industry analysts; or |
| ● | investors react negatively to the effect on the combined company’s business and prospects from the Transaction. |
The Transaction may be completed even though material adverse
changes may occur prior to the Closing.
In general, either party can refuse to complete
the Transaction if there is a material adverse change affecting the other party between the date of the Stock Purchase Agreement
and the Closing. However, some types of changes do not permit either party to refuse to complete the Transaction, even if such
changes would have a material adverse effect on Renovaro or GEDi Cube, to the extent they resulted from the following:
| ● | changes generally affecting the economy, financial or securities markets, or political conditions; |
| ● | the execution and delivery of the Stock Purchase Agreement or consummation of the Transaction, except with respect to any representation
or warranty intended to address the consequences of the execution and delivery or consummation of the Stock Purchase Agreement); |
| ● | any change in applicable law, U.S. GAAP or other applicable accounting standards; |
| ● | acts of war or terrorism or the escalation thereof; |
| |
● | natural disasters, epidemics, pandemics, or disease outbreaks (including the COVID-19 virus),
public health emergencies, or other force majeure events; |
| ● | general conditions in Renovaro’s or GEDi Cube’s industry, as applicable; |
| |
● | the failure of Renovaro or GEDi Cube to meet internal or published projections, forecasts,
estimates, or predictions in respect of revenues, earnings, or other financial or operating metrics for any period; or |
| ● | the taking of any action required or specifically permitted to be taken by the Stock Purchase Agreement or any actions or omissions
taken with the other party’s consent, except in each case with respect to the first, third, fourth, fifth and sixth items
listed above, to the extent disproportionately affecting either company and its subsidiaries, taken as a whole, relative to other
participants in the industries in which either company conducts its business, as applicable. |
If adverse changes occur but Renovaro and GEDi
Cube must still complete the Transaction, the combined company’s stock price may suffer.
Renovaro’s stockholders will have a reduced ownership
and voting interest in, and will exercise less influence over the management of, Renovaro following the Closing as compared to
their current ownership and voting interest in Renovaro.
After the completion of the Transaction, the
stockholders of Renovaro before the Effective Time of the Transaction will own a smaller percentage of the combined company than
their ownership in Renovaro prior to the Transaction. Consequently, the stockholders of Renovaro will not be able to exercise the
same degree of influence over the management and policies of the combined company following the Closing.
Renovaro’s stockholders may not realize a benefit from
the Transaction commensurate with the ownership dilution they will experience in connection with the Transaction.
If the combined company is unable to realize
the strategic and financial benefits currently anticipated from the Transaction, Renovaro’s stockholders will have experienced
substantial dilution of their ownership interest without receiving any commensurate benefit. Significant management attention and
resources will be required to integrate the two companies. Delays in this process could adversely affect the combined company’s
business, financial results, financial condition and stock price following the Closing. Even if the combined company is able to
integrate the business operations successfully, there can be no assurance that this integration will result in the realization
of the full benefits of synergies, innovation and operational efficiencies that may be possible from this integration and that
these benefits will be achieved within a reasonable period of time.
Certain provisions of the Stock Purchase Agreement may discourage
third parties from submitting competing proposals, including proposals that may be superior to the transactions contemplated by
the Stock Purchase Agreement. During the pendency of the Transaction, Renovaro may not be able to pursue alternatives to the Transaction
or enter into a business combination or acquisition transaction with another party on more favorable terms because of restrictions
in the Stock Purchase Agreement, which could adversely affect its business prospects.
The terms of the Stock Purchase Agreement
generally prohibit each of Renovaro and GEDi Cube from soliciting competing acquisition proposals or discussing or
negotiating with, or furnishing information to, persons making unsolicited acquisition proposals, except in limited
circumstances. Covenants in the Stock Purchase Agreement impede the ability of each of Renovaro and GEDi Cube to engage in
certain transactions involving the acquisition of each company’s business during the pendency of the Transaction,
subject to specified exceptions. As a result, if the Transaction is not completed, the parties may be at a disadvantage to
their competitors during that period. In addition, while the Stock Purchase Agreement is in effect, each party is generally
prohibited from soliciting, assisting, initiating, or facilitating the making, submission or announcement of, or
intentionally encouraging any acquisition proposal or inquiry or engaging or participating in discussions or negotiations
with respect to or that could reasonably be expected to lead to any acquisition proposal or inquiry, including a merger, sale
of assets or other business combination, subject to specified exceptions. Any such transactions could be favorable to such
party’s securityholders, but the parties may be unable to pursue them. For more information, see the section titled
“The Stock Purchase Agreement—Exclusivity” beginning on page 70 of this proxy statement.
Because the lack of a public market for the GEDi Cube Shares
makes it difficult to evaluate the fairness of the Transaction, the Sellers may receive consideration in the Transaction that is
greater than or less than the fair market value of the GEDi Cube Shares.
The outstanding shares and other equity interests
in GEDi Cube are privately held and are not traded in any public market. The lack of a public market makes it extremely difficult
to determine the fair market value of GEDi Cube. Since the percentage of Renovaro’s Common Stock to be issued to the Sellers
was determined based on negotiations between the parties, it is possible that the value of the Common Stock to be issued in connection
with the Transaction will be greater than the fair market value of GEDi Cube. Alternatively, it is possible that the value of the
shares of Common Stock to be issued in connection with the Transaction will be less than the fair market value of GEDi Cube.
The combined company will incur significant transaction
costs as a result of the Transaction, including investment banking, legal and accounting fees. In addition, the combined company
will incur significant consolidation and integration expenses which cannot be accurately estimated at this time. Actual transaction
costs may substantially exceed estimates and may have an adverse effect on the combined company’s financial condition and
operating results.
Renovaro and GEDi Cube may become involved in securities litigation
or stockholder derivative litigation in connection with the Transaction in the future, and this could divert the attention of Renovaro
and GEDi Cube management and harm the combined company’s business, and insurance coverage may not be sufficient to cover
all related costs and damages.
Securities litigation or stockholder derivative
litigation frequently follows the announcement of certain significant business transactions, such as the sale of a business division
or the announcement of a business combination transaction. Renovaro and GEDi Cube may become involved in this type of litigation
in connection with the Transaction, and the combined company may become involved in this type of litigation in the future. Litigation
often is expensive and diverts management’s attention and resources, which could adversely affect the business of Renovaro,
GEDi Cube and the combined company, and insurance coverage may not be sufficient to cover all related costs and damages.
If the conditions of the Transaction are not met or waived,
the Transaction will not occur.
Even if the Share Issuance Proposal and the Authorized
Share Proposal, as described in this proxy statement, are approved by the Renovaro stockholders, specified conditions set forth
in the Stock Purchase Agreement must be satisfied or waived to complete the Transaction. These conditions are described in the
section titled “The Stock Purchase Agreement—Conditions to Completion of the Transaction” beginning on
page 71 of this proxy statement. Renovaro cannot assure you that all of the conditions to the consummation of the Transaction
will be satisfied or waived. If the conditions are not satisfied or waived, the Transaction may not occur or the Closing may be
delayed, and Renovaro and GEDi Cube each may lose some or all of the intended benefits of the Transaction.
The unaudited pro forma financial information included in
this proxy statement may not be representative of the combined company’s results following the Closing.
The unaudited pro forma financial information
included in this proxy statement has been presented for informational purposes only and is not necessarily indicative of the financial
position or results of operations that actually would have occurred had the Transaction been completed as of the date indicated,
nor is it indicative of the combined company’s future operating results or financial position. The pro forma financial statements
have been derived from the historical financial statements of Renovaro and GEDi Cube and adjustments and assumptions have been
made regarding the combined company after giving effect to the Transaction. The information upon which these adjustments and assumptions
have been made is preliminary, and these kinds of adjustments and assumptions are difficult to make with accuracy. Moreover, the
pro forma financial statements do not reflect all costs that are expected to be incurred by the combined company in connection
with the Transaction. As a result, the actual financial condition of the combined company following the Closing may not be consistent
with, or evident from, these pro forma financial statements. The assumptions used in preparing the pro forma financial information
may not prove to be accurate, and other factors may affect the combined company’s financial condition following the Closing.
Following the Closing, the combined company may be unable
to integrate successfully the businesses of Renovaro and GEDi Cube and realize the anticipated benefits of the Transaction.
The Transaction involves the combination of two
companies which currently operate as independent companies. Following the Closing, the combined company will be required to devote
significant management attention and resources to integrating its business practices and operations. The combined company may fail
to realize some or all of the anticipated benefits of the Transaction if the integration process takes longer than expected or
is more costly than expected. Some of the potential difficulties the combined company may encounter in the integration process
include the following:
| ● | the inability to successfully combine the businesses of Renovaro and GEDi Cube in a manner that permits the combined company
to achieve the anticipated benefits from the Transaction, which would result in those benefits of the Transaction not being realized
partly or wholly in the time frame currently anticipated or at all; |
| ● | creation of uniform standards, controls, procedures, policies and information systems; and |
| ● | potential unknown liabilities and unforeseen increased expenses, delays or regulatory conditions associated with the Transaction. |
In addition, Renovaro and GEDi Cube have operated and, until the completion of the Transaction will continue
to operate, independently. It is possible that the integration process also could result in the diversion of each company’s
management’s attention, the disruption or interruption of, or the loss of momentum in, each company’s ongoing businesses
or inconsistencies in standards, controls, procedures and policies, any of which could adversely affect the combined company’s
ability to maintain its business relationships or the ability to achieve the anticipated benefits of the Transaction, or could
otherwise adversely affect the business and financial results of the combined company.
Risks Related to Renovaro
For risks related to
the business of Renovaro, please refer to the section titled “Item 1A. Risk Factors” set forth in the Renovaro 2023
Form 10-K and the section titled “Item 1A. Risk Factors” set forth in the Renovaro First Quarter 2024 Form 10-Q, each
of which is incorporated by reference herein.
Risks Related to GEDi Cube
Risks
Related to GEDi Cube’s Limited Operating History, Financial Position and Capital Requirements
GEDi
Cube is an artificial intelligence (“AI”)-driven healthcare technology company operating in a rapidly evolving field
and has a limited operating history, which makes it difficult to evaluate GEDi Cube’s current business and predict GEDi
Cube’s future performance.
GEDi
Cube is an AI-driven healthcare technology company operating in a rapidly
evolving field and, having commenced operations in 2013, has a limited operating history. GEDi Cube shifted its business from the
financial technology (or FinTech) industry to cancer diagnostics only in 2018. GEDi Cube does not currently have a commercial product
for sale. GEDi Cube has never generated any revenue relating to its cancer diagnostics AI platform. GEDi Cube’s short operating
history as a company makes any assessment of its current business or future success and viability subject to significant uncertainty.
GEDi Cube expects to encounter risks and difficulties, including those frequently experienced by early-stage companies in rapidly
evolving fields. If GEDi Cube does not address these risks and difficulties successfully, its business will suffer.
GEDi Cube has
a history of net losses and anticipates that it may continue to incur net losses for the foreseeable future.
Grace Systems (GEDi Cube’s predecessor) has primarily incurred net losses since its inception in 2013
and has never generated any revenue relating to its cancer diagnostics AI platform. GEDi Cube anticipates that it may continue
to incur primarily net losses in the foreseeable future. GEDi Cube has invested significant financial resources in research and
development activities, including to develop its technology and investigational products and plan for commercial launch of its
AI platform. The amount of GEDi Cube’s future net losses will depend, in part, on the level of GEDi Cube’s future expenditures
and its ability to generate revenue following the commercialization of its AI platform. Moreover, GEDi Cube’s net losses
may fluctuate significantly from quarter to quarter and year to year, such that a period-to-period comparison of GEDi
Cube’s results of operations may not be a good indication of GEDi Cube’s future performance.
GEDi
Cube expects to continue to incur significant expenses and operating losses for the foreseeable future if, and as, it:
| |
● |
attracts, hires, and retains qualified personnel; |
| |
● |
continues its research and development activities; |
| |
● |
initiates and conducts additional clinical validation to support the development and commercialization of its products; |
| |
● |
expands its technological and operating capabilities and introduces laboratory capacity as GEDi Cube prepares for commercial scale; |
| |
● |
seeks regulatory approvals and any other marketing authorizations or clearances that may be necessary or desired for its products; |
| |
● |
establishes sales, marketing and distribution infrastructure to commercialize its products; |
| |
● |
acquires or in-licenses additional intellectual property and technologies; |
| |
● |
makes milestone, royalty, or other payments due under any license or collaboration agreements; |
| |
● |
obtains, maintains, protects and enforces its intellectual property portfolio, including intellectual property obtained through license agreements; |
| |
● |
provides additional infrastructure to support its continued research and development operations and any planned commercialization efforts in the future; |
| |
● |
as part of the combined company following the Closing, meets the requirements and demands of being a public company; and |
| |
● |
defends against any product liability claims or other lawsuits related to its products. |
GEDi Cube has
never generated revenue from its cancer diagnostics AI platform, and does not expect any near-term revenue to offset
GEDi Cube’s ongoing operating expenses, and may never be able to maintain profitability.
GEDi
Cube’s ability to generate revenue from product sales and maintain profitability in the future depends on its ability to
commercialize its products. While GEDi Cube plans to commercially launch its AI platform in the European Union and United Kingdom
in 2024, GEDi Cube cannot assure you that it will successfully be able to do so as planned, if at all, and GEDi Cube’s failure
to do so would prevent GEDi Cube from generating revenue. Furthermore, even if GEDi Cube is able to launch its AI platform or other
products in a timely manner, GEDi Cube may not be able to generate sufficient revenue to offset its costs and maintain profitability.
GEDi Cube’s ability to generate future revenue from product sales depends heavily on its success in:
| |
● |
completing clinical development and additional validation of GEDi Cube’s products and continuing to improve product performance and expand product features over time; |
| |
● |
seeking, obtaining and maintaining marketing approvals, clearances, licenses, or exemptions that may be necessary or desired for any future products that GEDi Cube develops; |
| |
● |
establishing a sales force, marketing, medical affairs and distribution infrastructure or, alternatively, collaborating with a commercialization partner sufficient to launch and commercialize its products; |
| |
● |
obtaining market acceptance by consumers, including self-insured employers, integrated health systems, healthcare providers, patients and third-party payors; |
| |
● |
establishing and maintaining supply and manufacturing relationships with third parties that can timely and consistently provide adequate, in both amount and quality, products and services to support clinical development and the market demand for GEDi Cube’s future products; |
| |
● |
achieving adequate coverage or reimbursement recognition from governments, health insurance organizations and other third-party payors for products that GEDi Cube launches; |
| |
● |
addressing any technological and market developments, including competing products; |
| |
● |
negotiating favorable terms in any collaboration, licensing, or other arrangements into which GEDi Cube may enter, and maintaining such existing or future arrangements; |
| |
● |
achieving general adoption and acceptance of GEDi Cube’s products by the medical community; |
| |
● |
maintaining, protecting and expanding GEDi Cube’s portfolio of intellectual property rights, including patents, trade secrets, know-how and trademarks; |
| |
● |
defending against third-party interference or infringement claims, if any; and |
| |
● |
attracting, hiring and retaining qualified personnel. |
GEDi
Cube anticipates incurring substantial costs to commercialize GEDi Cube’s products. GEDi Cube’s expenses could increase
beyond expectations if it is required by the U.S. Food and Drug Administration (the “FDA”), the European Medicines
Agency (the “EMA”), the Medicines and Healthcare products Regulatory Agency (“MHRA”) or other regulatory
agencies to delay its launch, narrow or change its intended use or product claims, modify or expand its clinical validation, perform
future additional clinical validation, either pre- or post-approval, or conduct clinical trials. Even if GEDi Cube
is able to generate revenue from the sale of any products, GEDi Cube may not become profitable and may need to obtain additional
funding to continue its operations.
GEDi Cube will
require additional financing in order to start its commercialization efforts and develop additional products.
To
date, GEDi Cube has financed its operations primarily through the sale of its equity securities and cash flow from consulting services
previously provided by GEDi Cube. GEDi Cube’s product development and clinical validation activities are expensive, and GEDi
Cube expects to start to spend substantial amounts as it prepares for the launch and commercialization of its AI platform, continues
to enhance its AI platform, broadens the applications of its AI platform and develops new products. In addition, obtaining any
necessary or desirable regulatory approvals and clearances from the FDA, the MHRA, or the EMA for GEDi Cube’s products will
require substantial additional funding.
GEDi
Cube will require additional capital for the development of its AI platform and future products. GEDi Cube’s future capital
requirements depend on many additional factors, including:
| |
● |
the cost of development and commercialization activities for GEDi Cube’s products, including marketing, sales and distribution costs; |
| |
● |
the timing of, and the costs involved in, obtaining any required or desired regulatory approvals and clearances for GEDi Cube’s products; |
| |
● |
the timing, scope, progress, results and costs of developing additional products, and of conducting additional clinical validation or future clinical studies, if required; |
| |
● |
the costs involved in preparing, filing, prosecuting, maintaining, expanding, defending and enforcing patent and other intellectual property rights and claims, including litigation costs and the outcome of such litigation; |
| |
● |
the timing and amount of sales of GEDi Cube’s products, if any, and collection of related receivables; |
| |
● |
the extent to which GEDi Cube’s products are eligible for coverage and/or reimbursement from third-party payors; |
| |
● |
the emergence of new technologies or any competing tests, products, or services and other adverse market developments; and |
| |
● |
other potential adverse developments. |
Risks Related
to GEDi Cube’s Business Operations, Products and Industry
GEDi Cube’s
products may not perform as expected, which could materially and adversely affect GEDi Cube’s business, financial condition,
results of operations and growth prospects.
GEDi
Cube does not currently have a commercial product. GEDi Cube’s success depends on the market’s confidence that it can
provide reliable, high-quality products. Assuming successful launch, GEDi Cube’s products may not perform as expected.
If this were to occur, GEDi Cube’s business, financial condition, results of operations and growth prospects would suffer.
GEDi
Cube’s AI platform requires a number of complex and sophisticated biochemical and bioinformatics processes, many of which
are highly sensitive to external factors. An operational or technological failure in one of these complex processes or fluctuations
in external variables may result in sensitivity and specificity rates that are lower than GEDi Cube anticipates or that vary between
test runs or in a higher than anticipated number of tests that fail to produce consistent results. In addition, GEDi Cube regularly
evaluates and refines its algorithms and other processes under development. These refinements may inadvertently result in unanticipated
issues that may reduce GEDi Cube’s sensitivity and specificity rates or otherwise adversely affect the performance of the
tests supported by GEDi Cube’s AI platform and the results of such tests.
Further,
GEDi Cube plans to iterate and improve, enhancing product performance, offerings, scalability and/or cost of goods. However, GEDi
Cube may not be successful in transitioning its AI platform to a new or enhanced version or iteration. Product development involves
a lengthy and complex process and GEDi Cube may be unable to commercialize, validate, or improve performance of any of its AI platform
or products on a timely basis, or at all. GEDi Cube’s failure to successfully develop new and/or improved products (including
new versions of its existing platform and other products) on a timely basis could have a material adverse effect on GEDi Cube’s
results of operations and business.
Even if GEDi
Cube commercially launches its AI platform and other products, they may fail to achieve the degree of market acceptance necessary
for commercial success.
The
commercial success of GEDi Cube’s AI platform and other future products will depend upon the degree of market acceptance
by consumers, including self-insured employers, integrated health systems, healthcare providers, patients and, over the long-term, third-party payors.
The degree of market acceptance of GEDi Cube’s products will depend on a number of factors, including:
| |
● |
the performance and clinical utility of its products as demonstrated in clinical validation and published in peer-reviewed journals; |
| |
● |
GEDi Cube’s ability to demonstrate the clinical utility of its products and their potential advantages to the medical community; |
| |
● |
the ability of GEDi Cube’s products to demonstrate the same performance in real-world intended use populations as in clinical validation; |
| |
● |
the willingness of consumers, including self-insured employers, integrated health systems, healthcare providers, patients and others in the medical community to utilize GEDi Cube’s products; |
| |
● |
the willingness of commercial third-party payors and government payors to cover and reimburse for GEDi Cube’s products, the scope and amount of which will likely affect an individual’s willingness or ability to pay for GEDi Cube’s products and likely heavily influence healthcare providers’ decisions to recommend GEDi Cube’s products; |
| |
● |
with respect to products under development that GEDi Cube intends to launch for use in a broad asymptomatic population, the concern that such products could lead to over-diagnosis or a high false-positive rate and unnecessary medical procedures and costs; |
| |
● |
the introduction of competing products, including the expansion of the capabilities of existing products; |
| |
● |
the market acceptance of existing competitive products, including tests that are currently reimbursed; |
| |
● |
publicity concerning GEDi Cube’s products or competing products; and |
| |
● |
the strength of GEDi Cube’s marketing and distribution support. |
The
failure of GEDi Cube’s AI platform, once introduced, to be listed in physician guidelines or of any future clinical validation
to produce favorable results or to be published in peer-reviewed journals could limit the adoption of its AI platform.
In addition, healthcare providers and third-party payors, including Medicare, may rely on physician guidelines issued
by industry groups, medical societies and other key organizations, such as the U.S. Preventive Services Task Force, before utilizing
or reimbursing the cost of any diagnostic or screening test. Although GEDi Cube has conducted prior clinical validation of its
AI platform, this platform is not yet, and may never be, listed in any such guidelines.
Further,
if GEDi Cube’s products and the technology underlying them do not receive sufficient favorable exposure in peer-reviewed publications,
the rate of physician and market acceptance of GEDi Cube’s products and positive reimbursement or coverage decisions for
GEDi Cube’s products could be negatively affected. The publication of clinical data in peer-reviewed journals is
a crucial step in commercializing and obtaining reimbursement or coverage for GEDi Cube’s products, and GEDi Cube’s
inability to control when, if ever, results are published may delay or limit GEDi Cube’s ability to derive sufficient revenues
from any of its products that is developed using data from a clinical study.
Failure
to achieve broad market acceptance of GEDi Cube’s products, once launched, would materially harm GEDi Cube’s business,
financial condition and results of operations.
GEDi Cube may
be unable to develop and commercialize new products.
GEDi
Cube continues to expand its research and development efforts to use its proprietary AI platform to develop new products, including
in disease areas beyond cancer. The commercialization of any new products will require the completion of certain clinical development
activities, regulatory activities and the expenditure of additional cash resources. GEDi Cube cannot assure you that it can successfully
complete the clinical development of any such products.
GEDi
Cube also cannot assure you that it will be able to reduce its expenditures sufficiently, generate sufficient revenue from products
that it successfully commercializes or otherwise mitigate the risks associated with its business to raise enough capital to develop
and commercialize new products. In addition, once GEDi Cube’s development efforts for a product are completed, commercialization
efforts, including allocation of resources necessary to comply with applicable laws and regulations, will require significant expenditures.
Any failure by GEDi Cube to develop and commercialize new products could have a material adverse effect on GEDi Cube’s ability
to implement its strategy and grow its business.
One of the key
elements of GEDi Cube’s strategy is to expand access to its tests by pursuing reimbursement and/or coverage from third-party payors.
If GEDi Cube’s products do not receive adequate coverage or reimbursement from third-party payors, its ability
to expand access to its tests beyond its initial sales channels and its overall commercial success will be limited.
GEDi
Cube anticipates that it will not have broad-based coverage or reimbursement at the initial commercial launch. However,
a key element to GEDi Cube’s strategy is to expand access to its tests by pursuing coverage and/or reimbursement by third-party payors,
including government payors. Coverage and reimbursement by third-party payors, including managed care organizations,
private health insurers and government healthcare programs, such as Medicare and Medicaid in the United States and similar programs
in other countries, for the types of early detection and post-diagnosis service tests that GEDi Cube provides can be limited and
uncertain. Healthcare providers may not order GEDi Cube’s products unless third-party payors cover or provide adequate
reimbursement for a substantial portion of the price of GEDi Cube’s products. If GEDi Cube is not able to obtain adequate
coverage or an acceptable level of reimbursement for its products from third-party payors, there could be a greater co-insurance or co-payment obligation
for any individual for whom a test is ordered. The individual may be forced to pay the entire cost of a test out-of-pocket, which
could dissuade physicians from ordering GEDi Cube’s products and, if ordered, could result in delay in, or decreased likelihood
of, GEDi Cube’s collection of payment. GEDi Cube believes its revenue and revenue growth will depend on its success in achieving
broad coverage and adequate reimbursement for its products from third-party payors.
Coverage
and reimbursement by a third-party payor may depend on a number of factors, including a payor’s determination that
a product is appropriate, medically necessary and cost-effective. Each payor will make its own decision as to whether
to establish a policy or enter into a contract to cover GEDi Cube’s products and the amount it will reimburse for such products.
Any determination by a payor to cover and the amount it will reimburse for GEDi Cube’s products would likely be made on an indication-by-indication basis.
For example, because GEDi Cube intends to cover a broad asymptomatic population with its future products which could potentially
generate a significant number of false-positive results on an absolute basis, GEDi Cube may face additional scrutiny
in obtaining reimbursement from third-party payors given the additional costs of further diagnostic workup. As a result,
obtaining approvals from third-party payors to cover GEDi Cube’s products and establishing adequate coding recognition
and reimbursement levels is an unpredictable, challenging, time-consuming and costly process and GEDi Cube may never
be successful. If third-party payors do not provide adequate coverage or reimbursement for GEDi Cube’s products,
GEDi Cube’s ability to succeed commercially will be limited.
Even
if GEDi Cube establishes relationships with payors to provide its products at negotiated rates, such agreements would not obligate
any healthcare providers to order its products or guarantee that it would receive reimbursement for its products from these or
any other payors at adequate levels. Thus, these payor relationships, or any similar relationships, may not result in acceptable
levels of coverage or reimbursement for GEDi Cube’s products or meaningful increases in the number of billable tests it sells
to healthcare providers. GEDi Cube believes it may take several years to achieve coverage or adequate reimbursement with a majority
of third-party payors, including with those payors offering negotiated rates. In addition, GEDi Cube cannot predict whether,
under what circumstances, or at what payment levels payors will cover or reimburse for its products. If GEDi Cube fails to establish
and maintain broad-based coverage or reimbursement for its products, its ability to expand access to its products, generate
increased revenue and grow its test volume and customer base will be limited and its overall commercial success will be limited.
If GEDi Cube’s
products, or the products of its competitors, directly or indirectly result in harm or injury to patients, GEDi Cube could be subject
to significant reputational and liability risks, and its operating results, reputation and business could suffer.
GEDi
Cube’s success will depend on the market’s confidence that its developed products can provide reliable, high-quality results,
once such products are launched. GEDi Cube believes that patients, physicians and regulators are likely to be particularly sensitive
to errors in the use of its products or failure of its products to perform as described, and there can be no guarantee that its
products will meet their expectations. GEDi Cube’s initial product is intended to be used to detect a cancer signal in patients,
but its results are not diagnostic. If a cancer signal is detected, the product would be used to localize the origin of the cancer
signal. A “cancer signal detected” test result would need to be followed up by appropriate diagnostic methods. Because
this product cannot detect all cancer signals, and may not detect signals for all cancer types, a negative test would not rule
out the presence of cancer. Additionally, a patient undergoing unnecessary diagnostic tests on the basis of a false-positive result
or an erroneous location of cancer signal result could expose GEDi Cube to significant liability and reputational risks notwithstanding
the emotional and mental health effects to which the patient may be exposed. Similarly, a patient who receives a cancer diagnosis
shortly following a “no cancer signal detected” test result may create negative publicity about GEDi Cube’s product,
which would discourage adoption. Performance failures could establish a negative perception of GEDi Cube’s products among
physicians, patients and regulators, jeopardize GEDi Cube’s ability to successfully commercialize its products, impair GEDi
Cube’s ability to obtain regulatory approvals or secure favorable coverage or reimbursement, or otherwise result in reputational
harm. In addition, GEDi Cube may be subject to legal claims arising from any errors in the use, manufacture, design, labelling
or performance of its products, including any false-positive or false-negative results.
In
addition, other companies are developing competing cancer detection tests and technologies focused on improving cancer care with
cancer detection tests and post-diagnostic products. If any tests marketed or being developed by GEDi Cube’s competitors
that are similar to its products do not perform in accordance with expectations or cause harm or injury to patients, such failure
to perform, harm or injury may result in lower confidence in early disease detection and post-diagnosis tests in general,
which could potentially adversely affect confidence in GEDi Cube’s products and result in an adverse impact on its operating
results and reputation.
If GEDi Cube’s
facilities or those of its third-party collaborators become inoperable, GEDi Cube’s ability to provide its products
will be significantly impaired and its business will be harmed.
GEDi
Cube relies on its third-party collaborators, consultants, contractors, vendors, suppliers and service providers. The
facilities of these partners could be subject to earthquakes, power shortages, telecommunications failures, water shortages, floods,
tornadoes, hurricanes, fires, extreme weather conditions, medical epidemics, pandemics, global conflict, war and other natural
or man-made disasters or business interruptions. In addition, they may be affected by government shutdowns, changes to
applicable laws, regulations and policies, or withdrawn funding. The occurrence of any of these business disruptions could seriously
harm their ability to complete their contracted services to GEDi Cube, which may adversely impact its operations and financial
condition.
GEDi Cube’s
business and results of operations will suffer if it fails to compete effectively.
The
testing and diagnostic products industry is intensely competitive. GEDi Cube has competitors both in Europe and abroad, including
Grail, Inc., Exact Sciences Corporation, Freenome, Inc. and Thrive Earlier Detection Corp., that have stated that they are developing
tests designed to detect cancer. GEDi Cube’s competitors have, or may have, substantially greater financial, technical and
other resources, such as larger research and development staff and well-established marketing and sales forces, and they
may operate in jurisdictions where lower standards of evidence are required to bring products to market. GEDi Cube’s competitors
may succeed in developing, acquiring, or licensing, on an exclusive basis or otherwise, tests or services that are more effective
or less costly than GEDi Cube’s products. In addition, established medical technology, biotechnology, or pharmaceutical companies
may invest heavily to accelerate the discovery and development of tests that could make GEDi Cube’s products less competitive
than GEDi Cube anticipates.
GEDi
Cube’s ability to compete successfully will depend largely on its ability to:
| |
● |
successfully
commercialize its products; |
| |
● |
demonstrate
compelling advantages in the performance and convenience of its products, including on a cost-competitive basis; |
| |
● |
achieve
market acceptance of its products by consumers, including self-insured employers, integrated health systems, healthcare
providers and patients; |
| |
● |
achieve
adequate coverage or reimbursement by third-party payors for its products; |
| |
● |
differentiate
its product from the other tests and products of current and potential competitors; |
| |
● |
attract
qualified scientific, data science, clinical development, product development and commercial personnel; |
| |
● |
obtain,
maintain, defend and enforce patents and other intellectual property rights and claims as necessary for its products; |
| |
● |
obtain
and maintain any necessary or desirable clearance or approval from regulators in Europe, the United Kingdom, the United States
and other jurisdictions; |
| |
● |
successfully
collaborate with institutions in the discovery, development and commercialization of its products; and |
| |
● |
successfully
expand its operations and implement a successful sales and marketing strategy to support commercialization. |
GEDi
Cube may not be able to compete effectively if GEDi Cube is unable to accomplish one or more of these or similar objectives.
If GEDi Cube
cannot enter new collaborations in a timely manner and on acceptable terms, its efforts to develop and commercialize its products
could be delayed or adversely affected.
From
time to time, GEDi Cube expects to engage in discussions with potential development and/or commercial collaborators that may or
may not lead to collaborations. However, GEDi Cube cannot guarantee that any discussions will result in development or commercial
collaborations. Further, once news of discussions regarding possible collaborations are known in the general public, regardless
of whether the news is accurate, failure to announce a collaboration agreement, or the entity’s announcement of a collaboration
with an entity other than GEDi Cube, could result in adverse speculation about GEDi Cube, its products or its technology, resulting
in harm to its reputation and its business. In addition, establishing collaborations is difficult and time-consuming and
may require GEDi Cube’s significant financial investment. Potential collaborators may elect not to work with GEDi Cube based
on their assessment of its financial, regulatory, or intellectual property position. Even if GEDi Cube establishes new collaborations,
they may not result in the successful development or commercialization of its products or technology.
If GEDi Cube
is unable to establish sales and marketing capabilities, it may not be successful in commercializing GEDi Cube’s products.
GEDi
Cube has only limited sales and marketing infrastructures and no experience as a company in the sale, marketing and distribution
of screening or diagnostic tests. In preparation of a commercial launch, GEDi Cube is rapidly hiring additional personnel in GEDi
Cube’s sales and marketing organization.
Factors that may inhibit
GEDi Cube’s efforts to commercialize any of its products include:
| |
● |
its inability to recruit and retain adequate numbers of effective sales, marketing, reimbursement, customer service, medical affairs and other support personnel; |
| |
● |
the inability of sales personnel to persuade adequate numbers of customers, including healthcare systems and healthcare providers, to use its products; |
| |
● |
the inability to price its products at a sufficient price point to ensure an adequate and attractive level of profitability; |
| |
● |
its inability to effectively market to, collaborate with, and secure coverage or reimbursement from third-party payors; |
| |
● |
its failure to comply with applicable regulatory requirements governing the sale, marketing, reimbursement and commercialization of its products; and |
| |
● |
unforeseen costs and expenses associated with creating an independent commercialization organization. |
GEDi Cube’s
business is subject to economic, political, regulatory and other risks associated with international operations.
GEDi
Cube’s business is subject to risks associated with conducting business internationally. For example, some of GEDi Cube’s
suppliers and parties with whom it has collaborative relationships are located outside of the Netherlands, including in the United
Kingdom and Israel. Accordingly, GEDi Cube’s future results could be harmed by a variety of factors, including:
| |
● |
economic weakness, including inflation, or political instability in particular foreign economies and markets; |
| |
● |
challenges enforcing its contractual and intellectual property rights, especially in those foreign jurisdictions that do not respect and protect intellectual property rights to the same extent as the Netherlands; |
| |
● |
changes in foreign laws, regulations and customs, tariffs and trade barriers; |
| |
● |
changes in foreign currency exchanges rates; |
| |
● |
changes in a specific country’s or region’s political or economic environment; |
| |
● |
negative consequences from changes in tax laws; |
| |
● |
trade protection measures, import or export licensing requirements or other restrictive actions by the Netherlands or foreign governments; |
| |
● |
compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; |
| |
● |
workforce uncertainty in countries where labor unrest is more common than in the Netherlands; |
| |
● |
difficulties associated with staffing and managing international operations, including differing labor relations; and |
| |
● |
business interruptions resulting from geo-political actions, including war and terrorism, pandemics, or natural disasters, including earthquakes, typhoons, floods and fires. |
Risks Related
to GEDi Cube’s Employee Matters and Managing Growth
GEDi Cube is
highly dependent on its key personnel. If GEDi Cube is not successful in attracting, motivating and retaining highly qualified
personnel, it may not be able to successfully implement its business strategy.
GEDi
Cube’s ability to compete in the highly competitive biotechnology industry depends upon its ability to attract, motivate
and retain highly qualified personnel. GEDi Cube is highly dependent on its executive management team and its scientific, medical,
technological and engineering personnel, all of whom have been working together as a group for only a limited period of time. The
loss of the services provided by any of GEDi Cube’s executive officers, other key employees and other scientific and medical
advisors, and GEDi Cube’s inability to find suitable replacements as needed, could result in delays in commercialization
of its products and harm its business. GEDi Cube does not maintain “key person” insurance policies on the lives of
these individuals or the lives of any of its other employees.
GEDi
Cube is headquartered in Amsterdam, the Netherlands, a region in which many other healthcare companies, technology companies and
academic and research institutions are headquartered. Competition for personnel is intense and the turnover rate can be high, which
may limit GEDi Cube’s ability to hire and retain highly qualified personnel on acceptable terms or at all. GEDi Cube expects
that it may need to recruit talent from outside of its region, and doing so may be costly and difficult. If GEDi Cube is unable
to attract and retain highly qualified personnel, its ability to develop and commercialize its products may be limited.
If GEDi Cube
is unable to scale its operations successfully to support demand for its products following the launch of its platform, its business
could suffer.
As
and to the extent demand increases beyond GEDi Cube’s expectations following the launch of GEDi Cube’s platform, GEDi
Cube will likely need to start to ramp up operating capacity. GEDi Cube will need to implement new infrastructure, data processing
capabilities, customer service, billing and systems processes, and expand GEDi Cube’s internal quality assurance program
and technology to support operations on a larger scale. GEDi Cube will also need collaboration arrangements with third-party laboratories
to process its physical tests or, if processing of such tests is not fully outsourced to support demand, will need to obtain equipment
and certified and licensed laboratory personnel to process these physical tests internally. GEDi Cube may face difficulties increasing
the scale of its operations, including implementing changes in infrastructure or programs or acquiring additional equipment or
personnel. As GEDi Cube refines its products and develops additional products, GEDi Cube may need to introduce new equipment, implement
new systems, technology, controls and procedures, and hire personnel with different qualifications, licenses or certifications.
The
value of GEDi Cube’s products will depend, in part, on GEDi Cube’s ability to perform tests, whether through a licensed
provider or internally, and return results to providers on a timely basis and at an appropriate quality standard, and on GEDi Cube’s
reputation for such timeliness and quality. Failure to establish necessary arrangements with licensed providers, to implement necessary
procedures, to transition to new equipment or processes, or to hire the appropriately qualified personnel could result in higher
costs of processing, longer turnaround times or an inability to meet market demand. There can be no assurance that GEDi Cube or
any such licensed provider will be able to perform tests on a timely basis at a level consistent with demand, that GEDi Cube will
be able to maintain the quality of its test results as GEDi Cube scales its commercial operations, or that GEDi Cube will be successful
in responding to the potential growing complexity of its operations, including the related data analysis requirements.
In
addition, GEDi Cube’s growth may place a significant strain on its management, operating and financial systems, research
and development, and its sales, marketing, and administrative resources. As a result of GEDi Cube’s growth, its operating
costs may escalate even faster than planned, and some of its internal systems may need to be enhanced or replaced. If GEDi Cube
cannot effectively manage its expanding operations and its costs, GEDi Cube may not be able to grow successfully or it may grow
at a slower pace, and its business could be adversely affected.
GEDi Cube will
need to grow the size and capabilities of its organization, and it may experience difficulties in managing this growth.
As
of December 20, 2023, GEDi Cube had 11 full-time employees and four independent contractors, including
two full-time. As GEDi Cube’s growth plan and strategies develop, it must add a significant number of additional managerial,
operational, financial and other personnel. Future growth will impose significant added responsibilities on members of management, including:
| |
● |
identifying, recruiting, integrating, retaining and motivating additional employees; |
| |
● |
managing its internal development efforts effectively, including creating compliant programs and processes, and managing the regulatory requirements for its products, while complying with its contractual obligations to contractors and other third parties; |
| |
● |
expanding its operational, financial and management controls, reporting systems and procedures; and |
| |
● |
managing the increasing complexity associated with a larger organization and expanded operations. |
GEDi
Cube’s future financial performance and its ability to commercialize its products will depend, in part,
on its ability to effectively manage any future growth. GEDi Cube’s management may also have to divert a disproportionate
amount of its attention away from day-to-day activities in order to manage these growth activities. GEDi Cube’s
ability to successfully manage its expected growth is uncertain given the fact that GEDi Cube has been in full operation as an
independent company focusing on cancer diagnostics AI only since 2018. GEDi Cube’s executive management team’s lack
of long-term experience working together may adversely impact their ability to effectively manage its business and growth.
If
GEDi Cube is not able to effectively expand its organization by hiring new employees, it may not be able to successfully implement
the tasks necessary to commercialize its products, which would have a negative impact on GEDi Cube’s business and result
of operations.
Risks Related
to GEDi Cube’s Legal Compliance and Litigation
Changes in
funding or disruptions at the EMA, the MRHA, and other government agencies caused by funding shortages or global health concerns
could hinder GEDi Cube’s ability to hire and retain key leadership and other personnel, or otherwise prevent new or modified
products from being developed, approved or commercialized in a timely manner or at all, or otherwise prevent those agencies from
performing normal business functions on which the operation of GEDi Cube’s business may rely, which could negatively impact
its business.
The
ability of the EMA, the MRHA, and, in the future, the FDA and other government agencies to review and clear or approve new products
or changes to existing products can be affected by a variety of factors, including government budget and funding levels, statutory,
regulatory and policy changes, the agency’s ability to hire and retain key personnel and accept the payment of user fees,
government shutdowns and other events that may otherwise affect the agency’s ability to perform routine functions. For example,
average review times at the FDA have fluctuated in recent years as a result of such factors. In addition, government funding of
other government agencies that fund research and development activities is subject to the political process, which is inherently
fluid and unpredictable. Disruptions at the EMA, the MRHA, and, in the future, the FDA and other agencies may also slow the time
necessary for new medical devices or modifications to cleared or approved medical devices to be reviewed and/or approved by necessary
government agencies, which would adversely affect GEDi Cube’s business.
If GEDi Cube
is sued for product or professional liability, it could face substantial liabilities that exceed its resources.
GEDi
Cube’s business depends upon its ability to obtain reliable and accurate test results that incorporate rapidly evolving understanding
of how to interpret minute signals detected by GEDi Cube’s assays as indications of potential presence of disease. Actual
or perceived errors resulting from laboratory or reporting errors, false positive or false negative test results, or the manufacture,
design, or labelling of GEDi Cube’s products, could subject GEDi Cube to product liability or professional liability claims.
A product liability or professional liability claim against GEDi Cube could result in substantial damages and be costly and time-consuming to
defend. Any liability claim brought against GEDi Cube, with or without merit, could increase its insurance rates or prevent it
from securing insurance coverage in the future. Additionally, any liability lawsuit could damage GEDi Cube’s reputation or
force it to delay or suspend sales of its products. The occurrence of any of these events could have a material adverse effect
on GEDi Cube’s business, results of operations, financial condition and prospects.
GEDi Cube’s
employees, independent contractors, consultants, commercial partners and vendors may engage in misconduct or other improper activities,
including noncompliance with regulatory standards and requirements.
GEDi
Cube is exposed to the risk of fraud, misconduct, or other illegal activity by its employees, independent contractors, consultants,
commercial partners and vendors. Misconduct by these parties could include intentional, reckless and negligent conduct that fails
to comply with the rules and regulations of the Centers for Medicare & Medicaid Services (the “CMS”), the FDA,
the EMA, the MRHA and other comparable regulatory authorities; provide true, complete and accurate information to such regulatory
authorities; comply with manufacturing and clinical laboratory standards; comply with healthcare fraud and abuse laws in the United
Kingdom, Europe and, in the future, the United States and similar fraudulent misconduct laws; or report financial information or
data accurately or to disclose unauthorized activities to GEDi Cube. When GEDi Cube begins commercializing its products in the
United Kingdom and Europe and, in the future,
the United States, its potential exposure under such laws will increase significantly,
and its costs associated with compliance with such laws are also likely to increase. In particular, research, sales, marketing,
education and other business arrangements in the healthcare industry are subject to extensive laws designed to prevent fraud, kickbacks, self-dealing and
other abusive practices, as well as off-label product promotion. These laws and regulations may restrict or prohibit
a wide range of pricing, discounting, educating, marketing and promotion, sales and commission, certain customer incentive programs
and other business arrangements generally. Activities subject to these laws also involve the improper use of information obtained
in the course of clinical validation, which could result in regulatory sanctions and cause serious harm to GEDi Cube’s reputation.
GEDi Cube has adopted a code of business conduct and ethics, but it is not always possible to identify and deter misconduct by
employees and third parties, and the precautions GEDi Cube takes to detect and prevent this activity may not be effective in controlling
unknown or unmanaged risks or losses or in protecting GEDi Cube from governmental investigations or other actions or lawsuits stemming
from a failure to be in compliance with such laws. If any such actions are instituted against GEDi Cube, and GEDi Cube is not successful
in defending itself or asserting its rights, those actions could have a significant impact on GEDi Cube’s business, including
the imposition of significant fines or other sanctions. Even if it is later determined after an action is instituted against GEDi
Cube that GEDi Cube was not in violation of these laws, GEDi Cube may be faced with negative publicity, incur significant expenses
defending its actions and have to divert significant management resources from other matters.
GEDi Cube’s products, if used
for the diagnosis of disease, could be subject to government regulation, and the regulatory approval and maintenance process for
such products may be expensive, time-consuming, and uncertain both in timing and in outcome.
GEDi Cube’s products
are not subject to FDA or other government regulatory clearance or approval if they are not intended to be used for the diagnosis,
treatment or prevention of disease. However, as GEDi Cube expands its product line to encompass products that are intended to be
used for the diagnosis of disease, certain of its products will become subject to regulation by the FDA, or comparable international
agencies, including requirements for regulatory clearance or approval of such products before they can be marketed. Such regulatory
approval processes or clearances may be expensive, time-consuming, and uncertain, and GEDi Cube’s failure to obtain or comply
with such approvals and clearances could have an adverse effect on its business, financial condition, or operating results. In
addition, changes to the current regulatory framework, including the imposition of additional or new regulations, could arise at
any time during the development or marketing of GEDi Cube’s future products, which may negatively affect its ability to obtain
or maintain FDA or comparable regulatory approval of its products, if required.
Diagnostic products
are regulated as medical devices by the FDA and comparable international agencies and may require either clearance from the FDA
or such other comparable agencies following the 510(k) pre-market notification process or pre-market approval from the FDA, in
each case prior to marketing. Obtaining the requisite regulatory approvals can be expensive and may involve considerable delay.
If GEDi Cube fails to obtain, or experiences significant delays in obtaining, regulatory approvals for diagnostic products that
it develops in the future, GEDi Cube may not be able to launch or successfully commercialize such products in a timely manner,
or at all.
In addition, if GEDi
Cube’s products labelled as “For Research Use Only. Not for use in diagnostic procedures,” or RUO, are used,
or could be used, for the diagnosis of disease, the regulatory requirements related to marketing, selling, and supporting such
products could change or be uncertain, even if such use by GEDi Cube’s customers is without its consent. If the FDA or other
regulatory agencies assert that any of GEDi Cube’s RUO products are subject to regulatory clearance or approval, GEDi Cube’s
business, financial condition, or results of operations could be adversely affected.
Regulatory and legislative developments
on the use of AI and machine learning could adversely affect GEDi Cube’s use of such technologies in its platform and other
products.
As the regulatory framework
for machine learning technology and AI evolves, GEDi Cube’s business, financial condition, and results of operations may
be adversely affected. The regulatory framework for machine learning technology, AI and automated decision-making is evolving.
It is possible that new laws and regulations will be adopted in the United Kingdom, the European Union, the United States and/or
other foreign jurisdictions, or that existing laws and regulations may be interpreted in ways that would affect the operation of
GEDi Cube’s AI platform and data analytics and the way in which GEDi Cube uses AI and machine learning technology. Further,
the cost to comply with such laws or regulations could be significant and would increase GEDi Cube’s operating expenses,
which could adversely affect its business, financial condition and results of operations.
For example, in Europe,
on April 21, 2021, the European Commission proposed a regulation seeking to establish a comprehensive, risk-based governance framework
for AI in the European Union market. The proposed legislation is intended to apply to companies that develop, use and/ or provide
AI in the European Union and includes requirements around transparency, conformity assessments and monitoring, risk assessments,
human oversight, security and accuracy, and proposes fines for breach of up to 6% of worldwide annual turnover. In addition, on
September 28, 2022, the European Commission proposed the AI Liability Directive and the revised Product Liability Directive seeking
to establish a harmonized civil liability regime for AI in the European Union in order to facilitate civil claims in respect of
harm caused by AI and to include AI-enabled products within the scope of the European Union’s existing product liability
regime. If enacted, this regulatory framework is expected to have a material impact on the way AI is regulated in the European
Union, and together with developing guidance and/or decisions in this area, may affect GEDi Cube’s use of AI and its ability
to provide and to improve its services, require additional compliance measures and changes to its operations and processes, result
in increased compliance costs and potential increases in civil claims against GEDi Cube, and could adversely affect its business,
operations and financial condition.
Risks Related
to GEDi Cube’s Technology and Intellectual Property
Issues in the development and use of AI, including machine
learning and computer vision, in GEDi Cube’s AI platform may result in reputational harm or liability.
AI
is integrated into GEDi Cube’s platform and is a significant element of its business offerings going forward. As with many
developing technologies, AI presents risks, challenges and unintended consequences that could affect its further development, adoption,
and use, and therefore GEDi Cube’s business. AI algorithms and training methodologies may be flawed. Data sets may be insufficient,
of poor quality, or contain biased information. Inappropriate or controversial data practices by data scientists, engineers, and
end-users of GEDi Cube’s systems could impair the acceptance of AI solutions. If the analyses that AI applications assist
in producing are deficient or inaccurate, GEDi Cube could be subjected to competitive harm, potential legal liability, and brand
or reputational harm. Some uses of AI present ethical issues, and GEDi Cube’s judgment as to the ethical concerns may not
be perceived as accurate. While GEDi Cube aims to develop and use AI responsibly and attempts to identify and mitigate ethical
and legal issues presented by its use, GEDi Cube may be unsuccessful in identifying or resolving issues before they arise. If GEDi
Cube uses AI as part of its platform in a manner that is controversial or perceived as unethical, this may lead to adverse results
for GEDi Cube’s financial condition and operations or the financial condition and operations of its collaborators or vendors,
which may further lead to GEDi Cube experiencing competitive harm, legal liability and brand or reputational harm. In addition,
AI-related issues, deficiencies and/or failures could give rise to legal and/or regulatory action, including with respect
to proposed legislation regulating AI in jurisdictions such as the European Union and others, and as a result of new applications
of existing data protection, privacy, intellectual property, and other laws.
Failure of,
or defects in, GEDi Cube’s machine learning and cloud-based computing infrastructure, or increased regulation in
the machine learning space, could impair GEDi Cube’s ability to process its data, develop products, or provide test results,
and harm its business and results of operations.
The
design, development, maintenance and operation of GEDi Cube’s technology over time is expensive and complex, and may involve
unforeseen difficulties including material performance problems, undetected defects or errors. Overcoming technical obstacles
and correcting defects or errors could prove to be impossible or impracticable, and the costs incurred may be substantial and adversely
affect GEDi Cube’s results of operations. Additionally, regulation in the machine learning space is constantly evolving and
may make it difficult for GEDi Cube to continue using its machine learning approach. If GEDi Cube’s technology does not function
reliably, fails to meet expectations in terms of performance, or cannot be fully utilized due to increasing regulation, GEDi Cube
may be unable to provide, or its customers may stop using, its products.
GEDi
Cube currently hosts all of its data on, and conducts its data analysis through, local hosting facilities. Any technical problems
or outages that may arise in connection with these hosting facilities could result in the loss of GEDi Cube’s data or delayed
or ineffective data processing. A variety of factors, including infrastructure changes, human or software errors, viruses, malware,
security attacks, fraud, spikes in customer usage, or denial of service issues could cause interruptions in GEDi Cube’s service.
Such service interruptions may reduce or inhibit GEDi Cube’s ability to provide its products, delay any further clinical
validation and any future clinical studies, and damage its relationships with its customers. GEDi Cube could also be exposed to
potential lawsuits, liability claims or regulatory actions, if, for example, GEDi Cube’s local servers experienced a data
privacy breach. If GEDi Cube was required to transfer its data to an alternative hosting provider, the transfer and acclimation
to the new provider could result in significant business delays and require additional resources.
Real or perceived errors, failures, or bugs in GEDi Cube’s
platform and future products could adversely affect its business, results of operations, financial condition, and growth prospects.
GEDi Cube’s platform is, and its future
products will be, complex, and therefore, undetected errors, failures, bugs, or defects may be present in such platform or products
or occur in the future in its platform or products, its technology or software or the technology or software GEDi Cube licenses from third parties, including open source software, especially when updates or new products are released. Such software and
technology is used in information technology (“IT”) environments with different operating systems, system management
software, devices, databases, servers, storage, middleware, custom and third-party applications, and equipment and networking configurations,
which may cause errors, failures, bugs, or defects in the IT environment into which such software and technology is deployed. This
diversity increases the likelihood of errors, failures, bugs, or defects in those IT environments. Some of the features in GEDi
Cube’s platform are powered by machine learning and AI, which depend on datasets and algorithms that could be flawed,
including through inaccurate, insufficient, outdated, or biased data. Despite testing by GEDi Cube, real or perceived errors, failures,
bugs, or defects may not be found until GEDi Cube’s customers use its products. Real or perceived errors, failures, bugs,
or defects in GEDi Cube’s products could result in negative publicity, loss of or delay in market acceptance of its platform
or future products and harm to its brand, loss of investor confidence, weakening of its competitive position, claims by customers
for losses sustained by them, or failure to meet the stated service level commitments in its customer agreements. In such an event,
GEDi Cube may be required, or may choose, for customer relations or other reasons, to expend significant additional resources in
order to help correct the problem. Any real or perceived errors, failures, bugs, or defects in GEDi Cube’s products could
also impair its ability to attract new customers, retain existing customers, or expand their use of its products, which would adversely
affect GEDi Cube’s business, results of operations and financial condition.
GEDi Cube may also be subject to liability claims for damages related to real
or perceived errors, failures, bugs, or defects in its platform or future products. A material liability claim or other occurrence that
harms GEDi Cube’s reputation or decreases market acceptance of its platform or future products may harm its business and results
of operations. Finally, since some of GEDi Cube’s customers use its products for compliance reasons, any errors, failures, bugs,
defects, disruptions in service or other performance problems with GEDi Cube’s products may damage its customers’ businesses
and could hurt its reputation.
GEDi Cube’s
internal computer systems, or those expected to be used by its third-party research institution collaborators or other contractors
or consultants, may fail or suffer security breaches.
Despite
the implementation of security and back-up measures, GEDi Cube’s internal computer, server and other information
technology systems as well as those of its third-party collaborators, consultants, contractors, suppliers and service
providers, may be vulnerable to damage from physical or electronic break-ins, computer viruses, malware, ransomware,
denial of service and other cyber-attacks or disruptive incidents that could result in unauthorized access to, use or
disclosure of, corruption of, or loss of sensitive and/or proprietary data, including personal and health information, and could
subject GEDi Cube to significant liabilities, regulatory and enforcement actions and reputational damage. For example, the loss
of clinical study data from future clinical studies could result in delays in any regulatory clearance or approval efforts and
significantly increase GEDi Cube’s costs to recover or reproduce the data, and subsequently commercialize its future products.
If GEDi Cube or its third-party collaborators, consultants,
contractors, suppliers or service providers were to suffer
an attack or breach, for example, that resulted in the unauthorized access to or use or disclosure of personal or health information,
GEDi Cube may have to notify physicians, patients, partners, collaborators, government authorities and the media, and may be subject
to investigations, civil penalties, administrative and enforcement actions and litigation, any of which could harm GEDi Cube’s
business and reputation. Likewise, GEDi Cube relies on its third-party research institution collaborators and other third
parties to conduct clinical validation, and similar events relating to their computer systems could also have a material adverse
effect on GEDi Cube’s business. To the extent that any disruption or security breach were to result in a loss of, or damage
to, GEDi Cube’s data or systems, or inappropriate or unauthorized access to or disclosure or use of confidential, proprietary,
or other sensitive, personal, or health information, GEDi Cube could incur liability and suffer reputational harm, and the development
and commercialization of its products could be delayed.
GEDi
Cube’s insurance policies may not be adequate to compensate it for the potential losses arising from such disruptions, failure,
or security breach. In addition, such insurance may not be available to GEDi Cube in the future on economically reasonable terms,
or at all. Further, GEDi Cube’s insurance may not cover all claims made against it and defending a suit, regardless of its
merit, could be costly, divert management attention and harm GEDi Cube’s reputation.
If GEDi Cube
is unable to protect the confidentiality of its trade secrets, GEDi Cube’s business and competitive position would be harmed.
GEDi
Cube relies on trade secrets and confidentiality agreements to protect its know-how, technology, data and other proprietary
information and to maintain its competitive position. Trade secrets and know-how can be difficult to protect. GEDi Cube
expects its trade secrets and know-how to, over time, be disseminated within the industry through independent development,
the publication of journal articles describing the methodology and the movement of personnel from academic to industry scientific
positions.
GEDi
Cube seeks to protect these trade secrets and other proprietary technology, in part, by entering into non-disclosure and
confidentiality agreements with parties who have access to them, such as GEDi Cube’s employees, directors, corporate collaborators,
outside scientific collaborators, contract research organizations (“CROs”), contract manufacturers, suppliers, service
providers, consultants, advisors and other third parties. GEDi Cube also enters into confidentiality and invention or patent assignment
agreements with its employees and consultants, and reminds departing employees when they leave their employment of their continuing
confidentiality obligations. GEDi Cube cannot guarantee that it has entered into such agreements with each party that may have,
or have had, access to GEDi Cube’s trade secrets or proprietary technology and processes. Despite GEDi Cube’s efforts,
any of these parties may breach the agreements and disclose GEDi Cube’s proprietary information, including GEDi Cube’s
trade secrets, and GEDi Cube may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally
disclosed or misappropriated a trade secret is difficult, expensive, and time-consuming, and the outcome is unpredictable.
Some courts outside The Netherlands are less willing or unwilling to protect trade secrets. For example, in China, claims regarding
infringement or misappropriation of trade secrets are difficult to prove, and consequently plaintiffs are rarely successful in
bringing these claims. If any of GEDi Cube’s trade secrets were to be lawfully obtained or independently developed by a competitor
or other third party, GEDi Cube would have no right to prevent them from using that technology or information to compete with GEDi
Cube. If any of GEDi Cube’s trade secrets were to be misappropriated by, disclosed to, or independently developed by a competitor
or other third party, GEDi Cube’s competitive position could be materially and adversely harmed.
GEDi
Cube has and may enter into collaboration, license, contract research and/or manufacturing relationships with contract organizations
that operate in certain countries that are at heightened risk of theft of technology, data
and intellectual property through direct intrusion by private parties or foreign actors, including those affiliated with or controlled
by state actors. Accordingly, GEDi Cube’s efforts to protect and enforce GEDi Cube’s intellectual property rights around
the world may be inadequate to obtain a significant commercial advantage from the intellectual property that GEDi Cube develops
or licenses, and GEDi Cube may be at heightened risk of losing its proprietary intellectual property rights around the world, including
outside of such countries, to the extent such theft or intrusion destroys the proprietary nature of its intellectual property.
GEDi Cube depends
on its information technology and telecommunications systems and those of third parties, the failure or disruption of which could
harm its business.
GEDi
Cube depends on information technology and telecommunications systems, including those provided by third parties and their vendors,
for significant elements of its operations, such as its information management systems, research and development, scientific and
medical data analysis and general administrative activities. In addition, GEDi Cube’s third-party service providers
depend upon technology and telecommunications systems provided by outside vendors. Following the Closing, GEDi Cube expects that
the combined company will expand and strengthen a number of enterprise software systems that affect a broad range of business processes
and functions, including, for example, systems handling human resources, financial controls and reporting, customer relationship
management, regulatory compliance, security controls and other infrastructure operations. These expansions may prove more difficult
than GEDi Cube expects and could cause disruptions in its operations or additional expense.
Information
technology and telecommunications systems are vulnerable to damage from a variety of sources, including telecommunications or network
failures, malicious human acts and natural disasters. Moreover, despite network security and back-up measures, some of
GEDi Cube’s servers are potentially vulnerable to physical or electronic break-ins, computer viruses and similar
disruptive events. Despite the precautionary measures GEDi Cube has taken to detect and prevent or solve problems that could affect
its information technology and telecommunications systems, failures or significant downtime of these systems or those used by its third-party service
providers and their vendors could prevent GEDi Cube from conducting tests, preparing and providing reports to future customers,
billing payors, conducting research and development activities, maintaining its financial controls and other reporting functions,
and managing the administrative aspects of its business. Any disruption or loss of information technology or telecommunications
systems on which critical aspects of GEDi Cube’s operations depend could have an adverse effect on its business.
If GEDi Cube’s
trademarks and trade names are not adequately protected, GEDi Cube may not be able to build name recognition in its markets
of interest and its business may be adversely affected.
GEDi
Cube uses certain unregistered trademarks and trade names in its business. These trademarks or trade names may be challenged, infringed
or declared generic or determined to be infringing on other marks. GEDi Cube may not be able to protect its rights to these trademarks
and trade names in the future, which GEDi Cube views as valuable to building name recognition among potential partners and customers
in its markets of interest. At times, competitors or other third parties have adopted or may adopt trade names or trademarks similar
to GEDi Cube’s trade names and/or trademarks, thereby impeding its ability to build brand identity and possibly leading to
market confusion and/or litigation. In addition, there could be potential trade name or trademark infringement claims brought by
owners of registered trademarks or trademarks that incorporate variations of GEDi Cube’s unregistered trademarks or trade
names. Over the long term, if GEDi Cube is unable to establish name recognition based on its trademarks and trade names, then GEDi
Cube may not be able to compete effectively and GEDi Cube’s business may be adversely affected. GEDi Cube’s efforts
to enforce, protect or defend its proprietary rights related to trademarks and trade names may be ineffective and could result
in substantial costs and diversion of resources and could adversely affect GEDi Cube’s business, financial condition, results
of operations and prospects.
Intellectual
property litigation may lead to unfavorable publicity that harms GEDi Cube’s reputation.
During
the course of any intellectual property litigation, there could be public announcements of the initiation of the litigation as
well as results of hearings, rulings on motions and other interim proceedings in the litigation. Such announcements could harm
GEDi Cube’s reputation, the perceived value of GEDi Cube’s intellectual property or the potential market for its products,
which could have a material adverse effect on its business.
GEDi Cube’s
success depends on its ability to develop and commercialize its technology without infringing, misappropriating, or otherwise violating
the intellectual property of third parties. Third parties may initiate legal proceedings alleging that GEDi Cube is infringing
their intellectual property rights, and if they prevail, could block sales of GEDi Cube’s products and force GEDi Cube to
make large damages and/or royalty payments, which could have a material adverse effect on the success of its business.
GEDi
Cube’s commercial success in part depends upon its ability, and the ability of its collaborators, to market, sell and distribute
GEDi Cube’s products and use GEDi Cube’s proprietary technologies without infringing, misappropriating or otherwise
violating the proprietary rights of third parties. There is considerable intellectual property litigation in the medical technology,
biotechnology, diagnostic and pharmaceutical industries. GEDi Cube may become party to, or threatened with, future adversarial
proceedings or litigation regarding intellectual property rights with respect to its products, including interference proceedings
before the United Kingdom Intellectual Property Office, the European Patent Office,
the United States Patent and Trademark Office and similar bodies in other jurisdictions. Third parties may assert infringement
claims against GEDi Cube based on existing patents or patents that may be issued in the future.
If
GEDi Cube is found to infringe, misappropriate, or otherwise violate a third party’s intellectual property rights, it could
be required to obtain a license from such third party to continue developing, marketing, selling and distributing GEDi Cube’s
products, or to cease using the infringing technology. However, GEDi Cube may not be able to obtain any required license on commercially
reasonable terms or at all. Even if GEDi Cube were able to obtain a license, it could be non-exclusive, thereby giving
GEDi Cube’s competitors access to the same technologies licensed to GEDi Cube. In addition, GEDi Cube could be found liable
for monetary damages, including treble damages if it is found to have willfully infringed a patent and attorneys’ fees if
the court finds the case to be exceptional. A finding of infringement, misappropriation, or other violation could prevent GEDi
Cube from commercializing its products or force GEDi Cube to cease some of its operations, which could materially harm its business.
Claims that GEDi Cube has misappropriated the confidential information or trade secrets of third parties could have a similar negative
impact on GEDi Cube’s business.
Even
if resolved in GEDi Cube’s favor, litigation or other legal proceedings relating to intellectual property claims may cause
GEDi Cube to incur significant expenses and could distract GEDi Cube’s personnel from their normal responsibilities. Such
litigation or proceedings could substantially increase GEDi Cube’s operating losses and reduce the resources available for
development activities or any future sales, marketing, or distribution activities. GEDi Cube may not have sufficient financial
or other resources to conduct such litigation or proceedings adequately. Some of GEDi Cube’s competitors may be able to sustain
the costs of such litigation or proceedings more effectively than GEDi Cube can because of their greater financial resources and
more mature and developed intellectual property portfolios. Uncertainties resulting from the initiation and continuation of patent
litigation or other proceedings could have a material adverse effect on GEDi Cube’s ability to compete in the market place.
GEDi Cube’s
use of open-source software could subject GEDi Cube’s proprietary technology to unwanted open-source license
conditions that could negatively impact its business.
A
portion of GEDi Cube’s technology capabilities incorporates open-source software, and GEDi Cube may incorporate open-source software
into other offerings or products in the future. If an author or other third party that distributed such open-source software
to GEDi Cube were to allege that GEDi Cube had not complied with the conditions of one or more of these licenses, GEDi Cube could
be required to incur significant legal expenses defending against such allegations. Further, the outcome of such litigation may
be particularly uncertain in some cases, because there is little legal precedent governing the interpretation of certain terms
of common open source licenses. In addition, if GEDi Cube combines its proprietary software with open-source software
in a certain manner and makes it available to others, under some open-source licenses, it could be required to license
or make available the source code of its proprietary software, which could substantially help its competitors develop products
that are similar to or better than GEDi Cube’s and harm its business.
The success
and growth of GEDi Cube’s business depends upon its ability to continuously innovate and develop new products and technologies.
GEDi
Cube’s solution is a technology-driven platform that relies on innovation
to remain competitive. The process of developing new technologies and products is complex, and GEDi Cube has built and seeks to further
develop its own technology using the latest in AI and machine learning, cloud-based technologies and other tools to differentiate
GEDi Cube’s products and technologies. In addition, GEDi Cube’s dedication to incorporating technological advancements into
its AI platform requires significant financial and personnel resources and talent. GEDi Cube’s development efforts with respect
to these initiatives could distract management from current operations and could divert capital and other resources from other growth
initiatives important to GEDi Cube’s business. GEDi Cube operates in an industry experiencing rapid technological change and frequent
product introductions. GEDi Cube may not be able to make technological improvements as quickly as demanded by its customers, or GEDi Cube
may not be able to accurately predict the demand or growth of its technological investments, which could harm its ability to attract customers
and have a material and adverse effect on its business, results of operations, financial condition and future prospects. In addition,
GEDi Cube may not be able to effectively implement new technology-driven products and services as quickly as its competitors or be successful
in marketing these products and services to potential customers. If GEDi Cube is unable to successfully and timely innovate, GEDi Cube
could experience reputational damage and decreased demand for its AI platform and other products and technologies and its growth, business,
results of operations, financial condition and future prospects could be materially and adversely affected.
GEDi Cube’s
AI platform and other related products may become obsolete due to fast growing technological innovations or the entry of competitors
with more financial and brand power.
AI
is a fast growing industry and GEDi Cube must successfully adapt and manage
technological advancements in AI and AI-related markets, as well as effectively compete with the emergence of additional competitors
in the AI industry in order to maintain and grow GEDi Cube’s AI business and products. Thus, the success of GEDi Cube’s AI
platform, other products and business depends in large part on its ability to keep pace with rapid technological changes in the development
and implementation of AI products. For example, the development of groundbreaking technological innovations in AI, or innovations that
would render AI obsolete, would harm GEDi Cube’s business and make its platform or other products less durable. Further, the entry
of competitors into the AI market that have more financial and brand power could cause GEDi Cube’s share of the market to be significantly
reduced thereby negatively affecting its business, operating results and financial condition.
Risks Related
to the Combined Company
The combined company does not expect
to pay dividends in the foreseeable future. Any return on investment may be limited to the value of its Common Stock.
The current expectation
is that the combined company will retain its future earnings to fund the development and growth of its business and, as such, that
it will not pay cash dividends on the Common Stock in the foreseeable future. The payment of dividends on the Common Stock will
depend on earnings, financial condition and other business and economic factors affecting it at such time as the combined company’s
board of directors may consider relevant. As a result, capital appreciation, if any, of the shares of the combined company’s
Common Stock will be your sole source of gain, if any, for the foreseeable future.
The financial and operational projections
that GEDi Cube or the combined company has made or may make from time to time are subject to inherent risks.
The projections of GEDi
Cube provided herein or that, following the Closing, the management of the combined company may provide from time to time (including,
but not limited to, those relating to potential clinical and regulatory timelines, production and supply matters, commercial launch
dates, potential sales amounts and other financial or operational matters) reflect numerous assumptions made by GEDi Cube’s
or the combined company’s management, as applicable, including assumptions with respect to its specific as well as general
business, regulatory, economic, market and financial conditions and other matters, all of which are difficult to predict and many
of which are beyond GEDi Cube’s or the combined company’s control. Accordingly, there is a risk that the assumptions
made in preparing the projections, or the projections themselves, will prove inaccurate. There may be differences between actual
and projected results, and actual results may be materially different from those contained in the projections.
If securities or industry analysts do
not publish research or publish inaccurate or unfavorable research about the combined company’s business, the stock price
and trading volume of the combined company’s Common Stock could decline.
The trading market for the Common Stock of the combined company following the Closing will depend in part
on the research and reports that securities or industry analysts publish about the combined company and its business. Securities
and industry analysts do not currently, and may never, publish research on the combined company. If no or only very few securities
analysts commence coverage, or if industry analysts cease coverage, of the combined company, the trading price for the combined
company’s Common Stock would be negatively affected. If one or more of the analysts who cover the combined company downgrade
the Common Stock or publish inaccurate or unfavorable research about the combined company’s business, the Common Stock price
would likely decline. If one or more of these analysts cease coverage of the combined company or fail to publish reports on the
combined company regularly, demand for the Common Stock could decrease, which might cause the price and trading volume of the Common
Stock to decline.
The combined
company’s quarterly results of operations may fluctuate significantly or may fall below the expectations of investors or
securities analysts, each of which may cause the combined company’s stock price to fluctuate or decline.
Following
the Closing, it is expected that the combined company’s results of operations will be subject to quarterly fluctuations.
The combined company’s net loss and other operating results will be affected by numerous factors, including:
| |
● |
the combined company’s ability to successfully develop, market and sell its products; |
| |
● |
the prices at which the combined company is able to sell its products; |
| |
● |
the impact of competitive developments or the combined company’s response thereto; |
| |
● |
disruptions in the combined company’s business due to manufacturing, supply, security breaches, outages, or other issues; |
| |
● |
the cost of performing next-generation sequencing and other general information and communications technology costs, such as cloud services; |
| |
● |
the extent to which the combined company’s products are deemed eligible or ineligible for coverage or reimbursement from third-party payors; |
| |
● |
changes in coverage or reimbursement or in reimbursement-related laws directly affecting the combined company’s business; |
| |
● |
regulatory developments affecting the combined company’s products or competing products; |
| |
● |
timing of expenditures in connection with the combined company’s additional clinical validation or any future clinical studies; and |
| |
● |
non-routine cash and non-cash expenses and write-offs, whether associated with acquisitions, restructuring activities, litigation, investigations, or otherwise. |
If,
following the Closing, the combined company’s quarterly results of operations fall below the expectations of investors or
securities analysts, the value of the Common Stock could decline substantially. Furthermore, any quarterly fluctuations in the
combined company’s results of operations may, in turn, cause the value of the Common Stock to fluctuate substantially. Accordingly,
quarterly comparisons of the combined company’s financial results are not necessarily meaningful and should not be relied
upon as an indication of its future performance.
Acquisitions
or other strategic transactions may increase the combined company’s capital requirements, dilute its stockholders, cause
it to incur debt or assume contingent liabilities, and subject it to other risks.
Following
the Closing, the combined company may engage in various additional acquisitions and strategic partnerships in the future, including
licensing or acquiring complementary intellectual property rights, technologies, or businesses. Any acquisition or strategic partnership
may entail numerous risks, including:
| |
● |
increased operating expenses and cash requirements; |
| |
● |
the assumption of indebtedness or contingent liabilities; |
| |
● |
the issuance of the combined company’s equity securities that would result in dilution to its stockholders; |
| |
● |
assimilation of operations, intellectual property and products of an acquired company; |
| |
● |
difficulties associated with integrating new personnel; |
| |
● |
retention of key employees, the loss of key personnel and uncertainties in the combined company’s ability to maintain key business relationships; |
| |
● |
the diversion of the its management’s attention from its existing product programs and initiatives in pursuing such an acquisition or strategic partnership; |
| |
● |
risks and uncertainties associated with the other party to such a transaction, including the prospects of that party and their existing products or product candidates and regulatory approvals, and the validity and enforceability of their intellectual property; |
| |
● |
inability to consummate acquisitions on which the combined company spends a significant amount of time and resources; |
| |
● |
possible write-offs or impairment charges relating to acquired businesses; and |
| |
● |
the combined company’s inability to generate revenue from acquired intellectual property, technology, or tests sufficient to meet its objectives or offset the associated transaction costs. |
In
addition, as GEDi Cube and Renovaro integrate and the combined company’s strategy evolves, the combined company may opt to
discontinue, deprioritize, or dispose of assets, technologies, or acquired businesses.
The combined
company’s business activities are subject to the Foreign Corrupt Practices Act (the “FCPA”) and similar anti-bribery and anti-corruption laws.
Following
the Closing, the combined company’s business activities will be subject to the FCPA and similar anti-bribery or anti-corruption laws,
regulations, or rules of other countries in which the combined company will operate, including the U.K. Bribery Act. The FCPA generally
prohibits offering, promising, giving, or authorizing others to give anything of value, either directly or indirectly, to a non-U.S. government official
in order to influence official action, or otherwise obtain or retain business. The FCPA also requires public companies to make
and keep books and records that accurately and fairly reflect the transactions of the corporation and to devise and maintain an
adequate system of internal accounting controls. GEDi Cube’s and Renovaro’s respective businesses are, and the combined
company’s business will be, heavily regulated and, therefore, do and will involve significant interaction with public officials,
including officials of foreign governments. Additionally, in many other countries, the healthcare providers who
administer diagnostic tests are employed by their government, and the purchasers of diagnostics tests are government entities.
Therefore, the combined company’s dealings with these providers and purchasers will be subject to regulation under the FCPA.
The SEC and United States Department of Justice (the “DOJ”)
have increased their FCPA enforcement activities with respect to biotechnology and pharmaceutical companies. There is no certainty
that all of the combined company’s employees, agents, contractors, or collaborators, or those of its affiliates, will comply
with all applicable laws and regulations, particularly given the high level of complexity of these laws. Violations of these laws
and regulations could result in fines, criminal sanctions against the combined company and its officers and employees, the closing
down of the combined company’s facility, requirements to obtain export licenses, cessation of business activities in sanctioned
countries, implementation of compliance programs, and prohibitions on the conduct of the combined company’s business. Any
such violations could include prohibitions on the combined company’s ability to offer its products in one or more countries
and could materially damage the combined company’s reputation, brand, international expansion efforts, ability to attract
and retain employees, business, prospects, operating results and financial condition.
FORWARD-LOOKING STATEMENTS
This proxy statement contains “forward-looking statements” (including
within the meaning of Section 21E of the U.S. Securities Exchange Act of 1934, as amended (the “Exchange Act”), and Section
27A of the Securities Act), relating to Renovaro, GEDi Cube, the Transaction and the other proposed transactions contemplated by the Stock
Purchase Agreement. These forward-looking statements include express or implied statements relating to Renovaro’s or GEDi Cube’s
expectations, hopes, beliefs, intentions or strategies regarding the future, including the future business and results of operations of
the combined company. These statements may discuss goals, intentions and expectations as to future plans, trends, events, results of operations
or financial condition, or otherwise, based on current beliefs of the management of Renovaro or GEDi Cube, as well as assumptions made
by, and information currently available to, management. In addition, any statements that refer to projections, forecasts or other characterizations
of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words “anticipate,”
“believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,”
“intend,” “may,” “might,” “plan,” “possible,” “potential,” “approximate,”
“predict,” “project,” “should,” “will,” “would” and similar expressions may
identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. These forward-looking
statements are based on current expectations and beliefs concerning future developments and their potential effects. There can be no assurance
that future developments affecting Renovaro, GEDi Cube, the combined company after the Transaction, or the proposed Transaction will be
those that have been anticipated. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond
Renovaro’s and GEDi Cube’s control) or other assumptions that may cause actual results or performance to be materially different
from those expressed or implied by these forward-looking statements. Factors that might cause these differences include, but are not limited
to, those described in this proxy statement under the heading “Risk Factors,” and the following factors:
| ● | the risk that the conditions to the Closing are not satisfied, including the failure to obtain stockholder approval of the
Share Issuance Proposal and the Authorized Share Proposal as described in this proxy statement; |
| ● | uncertainties as to the timing of the consummation of the Transaction and the ability of each of Renovaro and GEDi Cube to
consummate the Transaction; |
| ● | the combined company may not be able to obtain the equity or debt financing necessary to support the research, development,
licensing, manufacture, and marketing of product candidates, products and platforms, and its anticipated level of operations; |
| ● | uncertainties regarding the impact that any delay in the Closing, or other future liabilities or expenses, would have on the
anticipated cash resources of Renovaro or the combined company’s cash resources; |
| ● | the occurrence of any event, change or other circumstance or condition that could give rise to the termination of the Stock
Purchase Agreement; |
| ● | the effect of the announcement or pendency of the Transaction on Renovaro’s or GEDi Cube’s business relationships,
operating results and business generally; |
| ● | the initiation or outcome of any legal proceedings that may be instituted against Renovaro, GEDi Cube or any of their respective
directors or officers related to the Stock Purchase Agreement or the transactions contemplated thereby; |
| ● | competitive responses to the Transaction; |
| ● | unexpected costs, charges or expenses resulting from the Transaction; |
| ● | the combined company’s ability to continue as a going concern and ability to raise additional capital, if needed; |
| ● | the combined company’s dependence on the services of experts, including third parties to research and develop product
candidates in cooperation with the combined company’s employees and officers; |
| ● | the difficulty or impossibility of predicting future clinical trial results and regulatory outcomes of the combined company’s
product candidates based upon pre-clinical or earlier clinical trial performance; |
| ● | the application of heightened regulatory and commercial scrutiny to the combined company’s gene, cell, and immunotherapy
products given their novel nature and concomitant potential for actual or perceived safety issues; |
| ● | the combined company’s ability to compete in a rapidly developing field, and the potential impact to its financial condition,
product marketability, and operational capacities of a competitor receiving regulatory approval before the combined company, or
a competitor developing a more advanced or efficacious therapy than the combined company’s product; |
| ● | potential delays or total failures of third parties, such as universities, non-profits, and clinical research centers, to perform
obligations on which the combined company’s product research and development rely; |
| ● | the impact on the combined company’s competitive position, business operations and financial condition of implementation
of amended healthcare laws and regulations related to healthcare pricing and reimbursement; |
| ● | the dependence of certain of the combined company’s pipelines on intellectual property licensed from licensors, and the
severe adverse impact to its business operations of a disruption of one of its licensing relationships; |
| ● | the potential monetary costs of defending the combined company’s intellectual property rights in a dispute, and the possibility
that an intellectual property dispute will not be settled in our favor; |
| ● | the possibility that the combined company’s patents and patent applications, even if unchallenged, will not sufficiently
protect or provide exclusive use of the combined company’s intellectual property, which could jeopardize its ability to commercialize
its products and dissuade companies from subsequently collaborating with the combined company; |
| ● | the negative impact to the combined company’s competitive position and the value of its technology of its failure to
protect trade secrets through the use of non-disclosure and confidentiality agreements, or the unavailability of adequate recourse
for breach of such agreements; |
| ● | the fluctuation and volatility of the market price of the Common Stock due to its limited public market, and the possibility
that these issues will compound and strain the Renovaro stockholders’ ability to resell their Common Stock; |
| ● | the combined company’s significant dependence on sophisticated management with highly technical expertise to oversee
business operations, and its ability to attract and retain qualified personnel to sustain growth; |
| ● | the combined company’s ability to adapt to future growth by training an expanding employee base and shifting away from
reliance on third-party contractors; |
| ● | the risk of liability arising from claims of environmental damage, personal injury, and property damages in connection with
the combined company’s research and development activities, including those that involve the use of hazardous materials; |
| ● | the possibility that enforcement actions to suspend or severely restrict the combined company’s business operations will
be brought against the combined company for its failure to comply with laws or regulations and the potential costs of defending
against such actions; |
| ● | the combined company’s reliance on adequate maintenance of the security and integrity of its information technology systems
to effectively operate its business; and |
| ● | other risks and uncertainties detailed from time to time in Renovaro’s SEC filings. |
The foregoing review of important factors that
could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with
statements that are included herein and elsewhere in this proxy statement. Many of the important factors that will determine these
results are beyond Renovaro’s and GEDi Cube’s ability to control or predict. Should one or more of these risks or uncertainties
materialize, or should any of Renovaro’s assumptions prove incorrect, actual results of Renovaro, GEDi Cube or the combined
company could differ materially from the forward-looking statements. There may be additional risks that Renovaro considers immaterial
or that are unknown. It is not possible to predict or identify all such risks. Forward-looking statements only speak as of the
date they are made, and neither Renovaro nor GEDi Cube undertake any obligation to (and expressly disclaims any such obligation
to) update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except
as may be required under applicable laws.
For a discussion of the factors that may
cause Renovaro’s, GEDi Cube’s or the combined company’s actual results, performance or achievements to
differ materially from any future results, performance or achievements expressed or implied in such forward-looking
statements, or for a discussion of risks associated with the ability of Renovaro and GEDi Cube to complete the Transaction
and the effect of the Transaction on the business of Renovaro, GEDi Cube and the combined company, please see the section
titled “Risk Factors” beginning on page 22 of this proxy statement. Additional factors that could cause
actual results to differ materially from those expressed in the forward-looking statements are also discussed in reports
filed with the SEC by Renovaro.
THE SPECIAL MEETING OF RENOVARO STOCKHOLDERS
General
The special meeting of the Renovaro stockholders, or
the Special Meeting, will be held on Thursday, January 25, 2024, at 12:00 p.m., Eastern Standard Time, at K&L Gates LLP, 200 S Biscayne
Blvd., Suite 3900, Miami, FL 33131, unless postponed or adjourned to a later date.
This proxy statement, together with copies of the Renovaro
2023 Form 10-K and the Renovaro First Quarter 2024 Form 10-Q, is expected to be first mailed to stockholders of Renovaro on or about January
4, 2024.
Record Date and Shares Outstanding
Stockholders of record at the close of business on December 29, 2023, the Record Date,
are entitled to notice of the Special Meeting. At the Record Date, 67,224,089 shares of Common Stock and 561,010 shares of Series A Preferred
Stock were outstanding and entitled to vote at the Special Meeting.
Voting Rights
Only holders of Common
Stock and Series A Preferred Stock at the close of business on the Record Date are entitled to notice of, and to vote at, the Special
Meeting. Each share of Common Stock is entitled to one vote on all matters to be voted on at the Special Meeting, and each share
of Series A Preferred Stock is entitled to ten (10) votes on all matters to be voted on at the Special Meeting. The presence
in person or by proxy of at least one-third (1/3) of the issued and outstanding shares of Common Stock and
Series A Preferred Stock entitled to vote at the Special Meeting constitutes a quorum.
Broker Non-Votes
If you do not give instructions to your broker or other nominee on how to vote, your nominee may vote your shares
on matters that are considered to be “routine” under applicable stock exchange rules, but will not be permitted to
vote your shares with respect to “non-routine” items. We believe that, under the applicable stock exchange rules, the
Share Issuance Proposal, the Authorized Share Proposal, the Repricing Proposal, the 2023 Plan Amendment Proposal and the Adjournment
Proposal are non-routine matters. A “broker non-vote” occurs when a broker, bank or other nominee that is a holder
of record holding shares for a beneficial owner properly executes and returns a proxy without voting on a particular proposal because
such holder of record does not have discretionary voting power for that particular item. Broker non-votes will not be counted as
votes “FOR” or “AGAINST” any proposal to be considered at the Special Meeting but will be counted in determining
whether there is a quorum for the Special Meeting. We strongly encourage you to submit your voting instructions and exercise your
right to vote as a stockholder.
Purposes of the Special Meeting
The purposes of the Special Meeting are:
| ● | Share Issuance Proposal: To approve, for purposes of Nasdaq Listing Rule 5635, the issuance
of shares of Common Stock pursuant to the Stock Purchase Agreement, which approval is necessary to consummate the transactions
contemplated by the Stock Purchase Agreement; |
| ● | Authorized Share Proposal: To approve an amendment to the Certificate of Amendment to increase the total
number of authorized shares of capital stock of Renovaro from one hundred ten million (110,000,000) to three hundred sixty million (360,000,000)
and to increase the total number of authorized shares of Common Stock from one hundred million (100,000,000) to three hundred fifty million
(350,000,000), which approval is necessary to consummate the transactions contemplated by the Stock Purchase Agreement; |
| ● | Repricing Proposal: To approve a decrease in the exercise price of the outstanding stock
options held by Renovaro’s employees and consultants granted pursuant to the Enochian BioSciences Inc. 2019 Equity Incentive
Plan, or the 2019 Plan, and the Renovaro Biosciences Inc. 2023 Equity Incentive Plan, or the 2023 Plan, with current exercise prices
above the closing price of the Common Stock as reported on Nasdaq on the date of the Closing to such reported closing price, as
required by Nasdaq Listing Rule 5635 and the terms of the Plans; |
| ● | 2023 Plan Amendment Proposal: To approve an amendment to the 2023 Plan to increase the number of shares
of Common Stock available for awards under the 2023 Plan by five million (5,000,000) shares; and |
| ● | Adjournment Proposal: To adjourn the Special Meeting, if necessary, for the purpose of soliciting
additional proxies to approve any one or more of the matters identified above for consideration at the Special Meeting. |
If Renovaro is to
complete the Transaction with GEDi Cube, stockholders must approve Proposals 1 and 2. The approval of Proposals 3, 4
and/or 5 is not a condition to the completion of the Transaction.
As of the date of this proxy statement, the Renovaro Board does
not know of any business to be presented at the Special Meeting other than as set forth in the notice accompanying this proxy statement.
If any other matters should properly come before the Special Meeting, it is intended that the shares represented by proxies will
be voted with respect to such matters in accordance with the judgment of the persons voting the proxies.
Recommendation of the Renovaro Board
After careful consideration, the Renovaro Board has unanimously approved the Stock Purchase Agreement, the Transaction
and each of the proposals referred to above, and has determined that they are advisable, fair and in the best interests of Renovaro’s
stockholders. Accordingly, the Renovaro Board unanimously recommends that Renovaro’s stockholders vote “FOR”
the Share Issuance Proposal, “FOR” the Authorized Share Proposal, “FOR” the Repricing Proposal, “FOR”
the 2023 Plan Amendment Proposal and “FOR” the Adjournment Proposal.
Voting and Revocation of Proxies
The proxy accompanying this proxy statement is
solicited on behalf of the Renovaro Board for use at the Special Meeting and any adjournments or postponements of the Special Meeting.
If you are a stockholder of record of Renovaro
as of the Record Date, you may vote:
| ● | over the Internet at www.AALvote.com/RENBSM; |
| ● | by telephone at 1-866-804-9616; |
| ● | if you elected to receive printed proxy materials by mail, by signing and returning the proxy card provided; and |
| ● | by attending the Special Meeting and voting in person. |
Whether or not you plan to attend the Special Meeting, Renovaro urges you to vote
by proxy to ensure your vote is counted. You may still attend the Special Meeting and vote in person if you have already voted by proxy.
If you vote by proxy, your vote must be received by 11:59 p.m., Eastern Standard Time, on January 24, 2024 to be counted.
If your shares are held in the name of a bank,
broker, trustee or other nominee holder of record (i.e., in “street name”), you may vote by following the voting
instructions provided to you by your bank or broker.
All properly executed proxies that are not revoked will be voted at the Special Meeting and at any adjournment
or postponement of the Special Meeting in accordance with the voting instructions contained in the proxy. If a holder of Common
Stock or Series A Preferred Stock executes and returns a proxy or otherwise votes without marking voting selections, the shares
represented by that proxy will be voted “FOR” the Share Issuance Proposal, the Authorized Share Proposal, the Repricing
Proposal, the 2023 Plan Amendment Proposal and the Adjournment Proposal.
A stockholder of record as of the Record Date
who has submitted a proxy may change or revoke the proxy. You may change your vote or revoke your proxy at any time prior to the
taking of the vote at the Special Meeting as follows:
| ● | By submitting a written notice of revocation to our Secretary at our principal executive office, Attention: Luisa Puche, Chief
Financial Officer and Corporate Secretary; |
| ● | By timely delivering a properly executed, later-dated proxy or submitting a proxy with new voting instructions using the telephone
or Internet voting system; or |
| ● | By voting during the Special Meeting. |
For all methods of voting, the last vote cast will supersede all
previous votes. Attendance at the Special Meeting alone will not have the effect of revoking a proxy. The method by which you vote
by proxy will in no way limit your right to vote at the Special Meeting if you decide to attend the Special Meeting.
If your shares are held in the name of a broker
or other nominee, you may change or revoke your vote by following the specific directions provided to you by your broker or other
nominee.
Required Vote
The presence at the Special Meeting, in person or by proxy, of the holders
of one-third (1/3) of the issued and outstanding shares of Common Stock and Series A Preferred Stock entitled to
vote at the Special Meeting will constitute a quorum. At the close of business on the Record Date, Renovaro had 67,224,089 shares of Common
Stock outstanding and entitled to vote at the Special Meeting and 561,010 shares of Series A Preferred Stock outstanding and entitled
to vote at the Special Meeting. Abstentions and broker non-votes will be counted for purposes of determining whether a quorum is present
at the Special Meeting.
Each of Proposals 1 and 2 must be approved by
the affirmative vote of a majority of the votes cast in person or by proxy at the Special Meeting, as required by Nasdaq Listing
Rule 5635.
Each of Proposal 3 and Proposal 4 must be approved by the affirmative vote of a majority of the votes cast in person
or by proxy at the Special Meeting, as required by the General Corporation Law of the State of Delaware.
Proposal 5 must be approved by the
affirmative vote of a majority of the votes cast in person or by proxy at the Special Meeting, as required by the
By-laws.
For these proposals, broker non-votes, if any,
and a properly marked “ABSTAIN” with respect to any such matter will not be voted for or against a proposal and will
not be counted as a vote cast for or against a proposal for purposes of determining the number of votes cast with respect to such
proposals.
Appraisal Rights
Renovaro’s stockholders are not entitled
to appraisal rights in connection with the Transaction.
Solicitation of Proxies
In addition to solicitation
by mail, the directors, officers, employees and agents of Renovaro may solicit proxies from Renovaro stockholders by telephone,
letter, email or in person without compensation. Renovaro will reimburse banks, brokerage firms
and other custodians, nominees, and fiduciaries for their reasonable out-of-pocket expenses for sending proxy materials to stockholders
and obtaining their votes.
THE TRANSACTION
This section and the section titled “The Stock Purchase Agreement” beginning on page 64 of this proxy
statement describe the material aspects of the Transaction, including the Stock Purchase Agreement. While Renovaro believes that
this description covers the material terms of the Transaction and the Stock Purchase Agreement, it may not contain all of the information
that is important to you. You should carefully read this entire proxy statement, including the Stock Purchase Agreement, which
is attached as Annex A-I to this proxy statement, including the First Amendment to Stock Purchase Agreement attached as Annex A-II
to this proxy statement, and the other documents to which Renovaro has referred or which have been incorporated by reference herein.
For a more detailed description of where you can find those other documents, please see the section titled “Where You Can
Find More Information” beginning on page 178 of this proxy statement.
Background of the Transaction
The following chronology summarizes the key
meetings and events that led to the signing of the Stock Purchase Agreement. The following chronology does not purport to catalogue
every conversation among the Renovaro Board, the Transaction Committee (as defined below), members of Renovaro management or Renovaro’s
representatives and other parties.
During the second quarter of 2023, Renovaro’s management began actively
exploring options to strengthen Renovaro’s ability to advance its existing product pipeline and enhance stockholder value. These
options included licensing or acquiring rights to other product candidates, or acquisitions of, or mergers with, other companies with
other products, product candidates or technologies. Management had regular conversations with various Renovaro Board members, including
Rene Sindlev and Gregg Alton, about market competition and opportunities. In April 2023, Nina Storms, an advisor to GEDi Cube, spoke with
René Sindlev, Chairman of Renovaro, regarding a potential business relationship. On May 17, 2023, Mr. Sindlev met with Ms. Storms
regarding GEDi Cube and the possible benefits of a transaction. Mr. Sindlev conducted multiple calls and in-person meetings with Ms. Storms
in the weeks that followed, including a meeting with Frank Van Asch, the inventor of the GEDi Cube technology.
On June 8, 2023, Renovaro announced it had received FDA comments on pre-Investigational
New Drug Application (“IND”) for pancreatic cancer therapy, RENB-DC11: Genetically modified Allogeneic Dendritic Cell Therapeutic
Vaccine as Potential Product for Long-term Remission of Solid Tumors (“RENB-DC11”). Renovaro considered the comments to be
thorough and highly informative setting a clear pathway for IND. Renovaro developed extensive pre-clinical and clinical plans to achieve
IND and conduct Phase 1 and 2 clinical trials. Ongoing pre-clinical studies in animal models conducted through Dr. Anahid Jewett at UCLA
continued to demonstrate promising results. Renovaro projected IND submission and commencement of human trials for the second half of
2024, with the potential for approval for human use by the end of 2025 or early 2026.
On June 9, 2023, the Renovaro Board held a special
meeting at which members of Renovaro management and representatives of K&L Gates LLP (“K&L”), Renovaro’s outside
legal counsel, were present. At this meeting, the Renovaro management discussed the strategic, financial and operational challenges of
operating Renovaro’s business in the then-current environment, including the macro-economic, industry and market conditions negatively
impacting pre-clinical-stage cell and gene therapy companies such as Renovaro. The Renovaro Board discussed the need for significant
capital investment to complete Renovaro’s pre-clinical and clinical trials of RENB-DC11 and its other therapeutic platforms in
development, support the filing of an IND application for RENB-DC11, fund its manufacturing network, fund its innovative research and
development efforts, hire the appropriate personnel necessary to execute on those priorities, retain critical talent, and operate generally
as a public company, as well as the risks and uncertainties associated with conducting pre-clinical and clinical trials for RENB-DC11,
pursuing related regulatory approvals and, if successful, launching and commercializing RENB-DC11. The Renovaro Board also discussed
the risks and uncertainties associated with Renovaro’s cash requirements, which had severely negatively impacted Renovaro’s
stock price and its ability to raise sufficient capital through equity financings. The Renovaro Board further discussed Renovaro’s
current cash position, its near-term priorities and related cash requirements, and the lack of attractive financing alternatives given
then-current market conditions. The Renovaro Board further discussed changing the Company’s name to Renovaro, a variant of the
Latin verb “to renew”.
Throughout June, Ms. Storms and Mr. Sindlev continued to have discussions
regarding a possible transaction. On June 19, 2023, Ms. Storms sent an email to Mr. Sindlev with information regarding GEDi Cube.
On June 20, 2023, Mr. Sindlev forwarded the information
provided by Ms. Storms concerning GEDi Cube to Dr. Mark Dybul, the Chief Executive Officer of Renovaro, and other representatives
of Renovaro.
Additional, preliminary and high-level exploratory communications followed between representatives of Renovaro,
including Dr. Dybul, Mr. Sindlev, Ms. Luisa Puche, Renovaro’s Chief Financial Officer and Francois Binette, Renovaro’s
Chief Operating Officer, and representatives of GEDi Cube, including Ms. Storms, GEDi Cube representatives including incoming Chief
Executive Officer, and Rhys Tombling, GEDi Cube’s Finance Director throughout late June 2023 and early July 2023.
During late June 2023 and early July 2023, Dr.
Dybul had discussions with the senior management of a biotech company (“Party A”) with, among other assets, advanced
pre-clinical potential mRNA vaccines for two infectious diseases with sizeable potential commercial markets. Party A was in the
midst of a sale process and was looking to sell, license or otherwise partner these products to Renovaro. Because of the market
size and complementarity to Renovaro’s pipeline, on July 6, 2023, management began working on strategies to secure funding of
Renovaro’s acquisition of Party A and discussions were held with potential investor relations consultants regarding such
strategies.
During early July 2023, there were many discussions
among Renovaro management, Mr. Sindlev, Gregg Alton, a member of the Renovaro Board and K&L regarding Party A and the ability
to finance the acquisition of the specified pipelines.
On July 10, 2023, Dr. Dybul wrote to representatives
of GEDi Cube to request certain additional information regarding GEDi Cube. Ms. Storms provided a response to that request via
email on July 10, 2023, which included discussions of the preparation of a confidentiality agreement and a preliminary term sheet
for a possible business combination.
On July 15, 2023, Mr. Sindlev had a further communication
with Ms. Storms regarding the terms of the potential business combination.
On July 16, 2023, members of Renovaro’s
management had communications with the K&L team to discuss matters related to the structure of the potential business combination
and due diligence of GEDi Cube.
Renovaro and GEDi Cube entered into a mutual
confidentiality disclosure agreement, effective as of July 17, 2023 (the “NDA”), to enable the parties to engage in
confidential discussions in respect of a potential negotiated business arrangement between the parties. The NDA did not contain
standstill obligations.
On July 20, 2023, Renovaro provided a draft of a non-binding letter of intent to representatives of GEDi Cube
(the “LOI”). The LOI proposed to combine Renovaro and GEDi Cube in an all-stock transaction. Among other terms,
the LOI offered consideration of Renovaro Common Stock for each outstanding ordinary share of GEDi Cube such that GEDi Cube shareholders
immediately prior to consummation of the proposed transaction would own approximately 50% of the combined company immediately following
closing of the proposed transaction. The LOI also proposed a 120-day exclusivity period and that GEDi Cube would receive board
representation on the combined company’s board of directors.
On July 20, 2023, Dr, Dybul, Ms. Storms and Mr.
Tombling had a call to discuss the LOI in which they requested the clarification that the 50% ownership was on a fully-diluted
basis.
On July 21, 2023 Renovaro management sent the
Renovaro Board the draft letter of intent, including the request for the 50% ownership on a fully diluted basis, and a presentation
prepared by GEDi Cube about the company and called a meeting of the Renovaro Board on July 25, 2023 to discuss.
On July 25, 2023, the Renovaro Board had a telephonic board meeting at which
members of Renovaro management and representatives of K&L were present. Dr. Dybul updated the Renovaro Board on the potential strategic
opportunities for Renovaro. Dr. Dybul informed the Renovaro Board that Renovaro had been deemed ineligible for a grant Renovaro had been
pursuing because of its limited cash reserves and other issues. Dr. Dybul advised the Renovaro Board on the discussions with Party A and
that management had not yet been able to identify financing to proceed with the acquisition of Party A’s pipelines. Dr. Dybul also
advised the Renovaro Board on the potential opportunity with GEDi Cube, including GEDi Cube’s technology, senior management, possible
synergies, strategic rationale and considerations in evaluating a potential business combination transaction. The Renovaro Board and management
also discussed the terms of the LOI. A representative of K&L provided the Renovaro Board with an overview of the terms of the LOI.
Following discussion, the Renovaro Board directed management to continue negotiations on the July LOI with GEDi Cube on the terms
previously reviewed by and discussed with the Renovaro Board at the meeting. In addition, the Renovaro Board approved the change in the
Company’s name to Renovaro Biosciences Inc.
On July 26, 2023, there was a joint meeting of the senior scientific consultants
and management of Renovaro and GEDi Cube to explore areas of synergy. The Renovaro team was impressed with the depth of experience and
knowledge of the GEDi Cube team and identified clear areas to mutually reinforce each of Renovaro’s platforms as well as areas for
collaboration to develop novel approaches across the companies. There were additional post-meeting interactions between senior members
of each team to discuss further.
On July 28, 2023, the Renovaro Board had a telephonic meeting at which Dr.
Dybul informed of the additional diligence conversations that had taken place with GEDi Cube management and that GEDi Cube had expressed
an interest in executing the LOI previously presented to the Renovaro Board. The Renovaro Board authorized Dr. Dybul to execute the LOI.
On August 1st, Dr. Dybul and Karen Brink, a director
of GEDi Cube, executed the LOI.
Between August 1, 2023 and August 6, 2023, representatives
of Renovaro and GEDi Cube, along with input from a third-party publication relations firm, discussed the contents of the public announcement
of the entry into the LOI.
On August 8, 2023 and August 9, 2023, Dr. Dybul,
Mr. Sindlev, representatives from K&L, Ms. Storms, Mr. Rhodes and Mr. Tombling met in person in Cannes, France where the parties
discussed the status of the parties’ confirmatory diligence, negotiation of the definitive agreement, and the expected communications
regarding announcement of the execution of the LOI and the proposed transaction as well as the short-term working capital needs
of GEDi Cube and the possibility of Renovaro lending funds to GEDi Cube to meet those needs. On August 9, 2023, Renovaro issued
a press release announcing the entry into the LOI.
On August 10, 2023, Dr. Dybul circulated to the
Renovaro Board a written consent for the approval of a loan of up to $1.1 million to GEDi Cube for its short-term working capital
needs. On August 10th, the Renovaro Board approved a loan of up to $1.1 million to GEDi Cube. On August 11th, Renovaro loaned GEDi
Cube $550,000, as evidenced by a six-month promissory note, and on August 18th, Renovaro loaned GEDi Cube $500,000, evidenced by
a second six-month promissory note.
Between August 11, 2023 and August 20, 2023, Dr. Dybul, Mr. Sindlev and representatives
from K&L had various discussions on the confirmatory due diligence process and the expected timeline for the execution of a definitive
agreement. On August 22, 2023, the Renovaro Board held a telephonic board meeting at which they discussed the Transaction and formed a
transaction committee of Mr. Alton, Jayne McNicol, a Renovaro Board member, and Mr. Sindlev (the “Transaction Committee”).
On August 29, 2023, K&L sent Greenberg Traurig,
P.A. (“Greenberg”), counsel to GEDi Cube, an initial draft of a definitive agreement. Among other things, the draft
Stock Purchase Agreement provided for (i) representations and warranties and interim operating covenants customary for transactions
of this type, (ii) largely reciprocal non-solicitation restrictions prohibiting either party from soliciting or engaging in discussions
with competing bidders, and (iii) a termination right, exercisable by either party, if the Transaction had not closed by February
28, 2024. K&L also provided a draft of the Stock Purchase Agreement to the Transaction Committee and received comments from
the Transaction Committee thereon.
During the period from August 30, 2023 through
September 28, 2023, representatives and advisors of Renovaro and GEDi Cube reviewed due diligence materials provided by the
other party and engaged in business, financial and legal due diligence discussions with representatives and advisors of the other
party and submitted a number of requests for supplemental due diligence information. The mutual diligence was focused on assessing
potential synergies and risks for the combined company, legal and compliance matters, intellectual property matters and accounting
matters.
On September 10, 2023, Renovaro management received a due diligence report
it had independently contracted for from an AI expert on the GEDi Cube technology (the “Due Diligence Report”).
On September 11, 2023, Greenberg sent a revised
draft of the Stock Purchase Agreement to K&L, which, among other things, (i) provided for reciprocal representations and warranties,
interim operating covenants, closing conditions and indemnification obligations of Renovaro, and (ii) added an expense reimbursement
obligation of Renovaro for the cost of the completion of the GEDi Cube audit of its 2022 financial statements.
From September 11, 2023, through September 28,
2023, representatives of K&L, with input from the Renovaro Transaction Committee and Renovaro management, and GEDi Cube’s
representatives and representatives of Greenberg exchanged drafts and participated in discussions regarding the terms of the definitive
agreement, the registration rights agreement and related documents.
On September 27, 2023, the Renovaro Board held a meeting at which members
of Renovaro management and representatives of K&L were present. Representatives from K&L reviewed with members of the Renovaro
Board the status of due diligence and the status of the negotiations on the definitive agreement, including the probability that less
than 100% of the shareholders of GEDi Cube would sign the definitive agreement, but that the Stock Purchase Agreement would contain a
condition to closing that 100% of the stockholders sign joinders prior to closing. The Renovaro Board asked questions and provided feedback
and direction to Renovaro management on these matters. Dr. Dybul provided a summary to the Renovaro Board of the information contained
in a valuation report which GEDi Cube obtained on the valuation of itself as well as a summary of the conclusions of the Due Diligence
Report. The Renovaro Board asked questions of Dr. Dybul regarding these diligence matters. Representatives of K&L then discussed,
in response to questions from the Renovaro Board, certain financial terms of the Stock Purchase Agreement (including the 50%/50% split
of the ownership of the post-closing combined entity and the possible change in the Renovaro stock price between signing of the LOI and
closing). After discussion, the Renovaro Board directed management to continue negotiation of the Stock Purchase Agreement and that the
Renovaro Board would reconvene to consider the revised definitive agreement.
On September 28, 2023, the Renovaro Board held
a meeting at which members of Renovaro management and representatives of K&L were present. Representatives from K&L reviewed
with members of the Renovaro Board the status of the negotiations on the definitive agreement. After discussion and consideration
of the definitive agreement, the members of the Renovaro Board present at the meeting unanimously approved the terms of the Stock
Purchase Agreement and the other transactions contemplated by the Stock Purchase Agreement and directed Renovaro management to
execute the Stock Purchase Agreement.
In the evening of September 28, 2023, Renovaro, GEDi
Cube and GEDi Cube shareholders holding 77.5% of the outstanding GEDi Cube Shares executed the Stock Purchase Agreement.
On September 29, 2023, Renovaro and GEDi Cube issued
a joint press release announcing the parties’ entry into the Stock Purchase Agreement.
On November 17, 2023, Renovaro received an
executed joinder by a GEDi Cube shareholder who owns 16.7% of the outstanding GEDi Cube Shares.
On December 20, 2023, Renovaro, GEDi Cube and the shareholders party to the
Stock Purchase Agreement executed a First Amendment to Stock Purchase Agreement, which is attached as Annex A-II to this proxy statement,
pursuant to which the parties agreed that the 1 million shares of Common Stock issued to Avram Miller pursuant to the Miller Consulting
Agreement would not be counted for the calculation of the Closing Consideration as well as to correct the definition of “Shareholder
Approval”.
Reasons for the Transaction
At a meeting held on September 28, 2023, among
other things, the Renovaro Board unanimously (i) determined that the Transaction and the other transactions contemplated by the
Stock Purchase Agreement are fair to, advisable and in the best interests of Renovaro and its stockholders, (ii) approved and
declared advisable the Stock Purchase Agreement and the transactions contemplated thereby, including the issuance of shares of
Common Stock to the Sellers, and (iii) determined to recommend, upon the terms and subject to the conditions set forth in the
Stock Purchase Agreement, that the stockholders approve the Share Issuance Proposal and the Authorized Share Proposal, each of
which is required to consummate the Transaction.
In reaching its decision to approve the Stock
Purchase Agreement and the transactions contemplated thereby, including the Transaction, the Renovaro Board considered a number
of factors that it viewed as supporting this decision, including:
| ● | the financial condition and prospects of Renovaro and the risks associated with continuing to operate Renovaro
on a standalone basis, particularly in light of the then-current environment, including the macro-economic, industry and market
conditions negatively impacting pre-clinical-stage cell and gene therapy companies such as Renovaro; |
| ● | the Renovaro Board has undertaken a comprehensive and thorough process of reviewing and analyzing potential
strategic acquisitions as well as reaching out to partnering candidates for a variety of strategic transactions to identify the
opportunity that would, in the Renovaro Board’s opinion, create the most value for Renovaro’s stockholders; |
| ● | the results of the due diligence review of GEDi Cube’s business and operations by Renovaro’s management; |
| ● | the volatility of the market price of the Common Stock; |
| ● | the consequences of current market conditions, the depressed stock price of Renovaro, continuing net operating losses and
the likelihood that the resulting consequences for Renovaro, on a standalone basis, would not change for the benefit of the Renovaro
stockholders in the foreseeable future; |
| |
● |
the highly complementary product candidates and technologies of Renovaro and GEDi Cube and the expectation that, following the Closing, the combined company would be in a better position to accelerate product development and to pursue the new therapeutic approaches for cancer and other diseases; |
| |
|
|
| |
● |
the expectation that GEDi Cube’s AI platform could aid Renovaro in its planned clinical trial for RENB-DC11; |
| ● | the Renovaro Board believes that, as a result of arm’s length negotiations with GEDi Cube, Renovaro and its representatives
negotiated the most favorable value and form for the consideration to be paid by Renovaro in the Transaction to which GEDi Cube
and the Sellers were willing to agree, and that the terms of the Stock Purchase Agreement include the most favorable terms to Renovaro
in the aggregate to which GEDi Cube and the Sellers were willing to agree; |
| ● | the Renovaro Board believes that the Transaction would provide the existing Renovaro stockholders opportunities as a result
of the Transaction to participate in the prospective growth of the combined company given Renovaro’s potential to commercialize
its pipeline and GEDi Cube’s potential to enhance its AI capabilities; |
| ● | the likelihood that the combined company would possess sufficient financial resources to allow the management team to focus
on plans for developing and commercializing Renovaro’s product candidates and the potential achievement of important clinical
milestones in the near future; |
| ● | the possibility that the combined company would be able to raise capital in the future from a broader array of sources as a
result of the combination of Renovaro’s public company structure with Renovaro and GEDi Cube’s combined businesses; |
| ● | the fact that the combined company will continue to be led by an experienced industry chief executive officer and a team many
of whom have extensive expertise in cancer treatment, research and development, business, and healthcare regulation, and a board
of directors with representation from the current Renovaro and GEDi Cube boards; and |
| ● | the expectation that the Transaction would result in meaningful synergies by combining key assets, personnel,
capabilities and intellectual property, as well as ongoing access to world-leading scientific and clinical collaborators, which
is expected to deliver long-term value for stockholders of the combined company. |
The Renovaro Board also reviewed the terms and
conditions of the Stock Purchase Agreement and concluded that such terms and conditions were reasonable under the circumstances:
| |
● |
the estimated number of shares of Common Stock to be issued in the Transaction, the resulting ownership of Renovaro by the Sellers and the impact of changes in the number of outstanding shares of Common Stock between the date of the Stock Purchase Agreement and the Closing and the fact that the number of shares of Common Stock to be issued would not be adjusted based on the market price of Renovaro’s Common Stock; |
| |
● |
the number and nature of the conditions of
Renovaro’s and GEDi Cube’s respective obligations to complete the Transaction and the likelihood that the
Transaction will be completed on a timely basis, as more fully described below under “The Stock Purchase
Agreement—Conditions to Completion of the Transaction” beginning on page 71 of this proxy
statement; |
| |
● |
the condition to Renovaro’s obligation to consummate the Transaction that all holders of GEDi Cube Shares immediately prior to the Closing have joined the Stock Purchase Agreement as Sellers, so that, following the Closing, Renovaro shall be the sole holder of GEDi Cube Shares; |
| |
● |
the composition of the Renovaro Board following closing with respect to the representatives to be appointed by each party and the continued leadership of Renovaro under its current executive management team; |
| |
|
|
| |
● |
the belief that the terms of the Stock Purchase Agreement, including the parties’ representations, warranties, covenants and the conditions to their respective obligations, are reasonable under the circumstances; |
| |
● |
the length of the survival of, and reasonableness of the limitations for, the post-closing indemnification by the Sellers to Renovaro for breaches of representations, warranties, covenants and agreements in the Stock Purchase Agreement, claims by current or former holders of GEDi Cube’s securities and pre-closing taxes; |
| |
● |
the respective rights of, and limitations on, Renovaro and GEDi Cube under the Stock Purchase Agreement to consider and engage in discussions regarding unsolicited acquisition proposals under certain circumstances, as more fully described below under “The Stock Purchase Agreement—Exclusivity” beginning on page 70 of this proxy statement; |
| |
● |
the fact that the Stock Purchase Agreement contains restrictions on GEDi Cube’s conduct of business prior to the completion of the Transaction; and |
| |
|
|
| |
● |
the fact that the Exchange Consideration was the result of extensive arm’s-length negotiations between the parties. |
In the course of its deliberations and in addition
to the analyses and recommendation of the Renovaro Board, the Renovaro Board also considered a variety of risks and other countervailing
factors related to entering into the Transaction, including:
| |
● |
the prohibition on Renovaro to solicit alternative acquisition proposals during the pendency of the Transaction, subject to certain exceptions; |
| |
● |
the substantial expenses to be incurred by Renovaro in connection with the Transaction; |
| |
● |
the possibility of disruptive stockholder litigation following announcement of the Transaction; |
| |
● |
the obligation of Renovaro to obtain stockholder approval of the Share Issuance Proposal and the Authorized Share Proposal to consummate the Transaction; |
| |
● |
the fact that Renovaro’s stockholders would experience dilution by virtue of the issuance of Common Stock as Exchange Consideration in the Transaction; |
| |
|
|
| |
● |
the risk that, because the Exchange Consideration will not be adjusted based on the market price of Renovaro’s Common Stock, the then-current trading price of the Common Stock to be issued to GEDi Cube shareholders upon the consummation of the Transaction could be significantly higher than the trading price prevailing at the time the Stock Purchase Agreement was executed; |
| |
|
|
| |
● |
the risk that GEDi Cube’s financial performance may not meet Renovaro’s expectations; |
| |
|
|
| |
● |
the risk that the technologies of the combined company may not be as complementary as expected and that perceived technology advantages cannot be realized; |
| |
● |
the risk to Renovaro’s business, operations and financial results in the event that the Transaction is not consummated; |
| |
|
|
| |
● |
the risk of diverting Renovaro management’s focus and resources from other strategic opportunities and from operational matters, and potential disruption of Renovaro management associated with the Transaction and integrating the two companies; |
| ● | the technical, regulatory and other risks and uncertainties associated with the integration of Renovaro’s and GEDi Cube’s
business and the continued development of their product candidates and technologies; |
| |
● |
the risk that the combined company will not be successful in commercializing Renovaro’s product candidates or GEDi Cube’s platform or future products and that the combined company will not be able to secure funding for such commercialization on commercially reasonable terms or at all, and the risk that the revenues from the combined company’s future products and technology will be less than expected; |
| ● | the risks, challenges and costs inherent in combining the operations of the two companies and the expenses to be incurred in
connection with the Transaction; |
| ● | the strategic direction of the combined company following the completion of the Transaction, which will be determined by a
board of directors initially comprised of a majority of the directors designated by Renovaro; and |
| ● | the various other risks associated with the combined company and the Transaction, including those described in the sections
entitled “Risk Factors” and “Forward-Looking Statements” in this proxy statement. |
After evaluating
the proposed Transaction and taking into account all of the factors previously discussed and considered by the Renovaro Board,
the Renovaro Board unanimously approved the Transaction and authorized management to negotiate and enter into definitive agreements
on terms consistent in material respects with the terms presented to the Renovaro Board. In making its determination, the Renovaro
Board considered the range of percentages of the combined company that would be held by Renovaro stockholders, the existing business
and future business prospects of GEDi Cube, the overall structure of the Transaction, the terms of the Stock Purchase Agreement
and the factors and considerations described above.
The foregoing information and factors considered by the Renovaro Board are not intended to be exhaustive but are
believed to include the material factors considered by the Renovaro Board in its decision to approve the Stock Purchase Agreement
and the transactions contemplated thereby. The Renovaro Board viewed its recommendation to approve the Transaction as being based
upon its business judgment in light of Renovaro’s financial position and the totality of the information presented and considered,
and the overall effect of the Transaction on the stockholders of Renovaro compared to other alternatives. In view of the wide variety
of factors considered in connection with its evaluation of the Transaction and the complexity of these matters, the Renovaro Board
did not find it useful, and did not attempt, to quantify, rank or otherwise assign relative weights to these factors. In considering
the factors described above, individual members of the Renovaro Board may have given different weight to different factors. The
Renovaro Board conducted an overall consideration of the factors described above, including discussions with, and questioning of,
Renovaro’s management and Renovaro’s legal advisors, and considered the factors overall to be favorable to, and to
support, its determination.
Interests of the Renovaro
Directors and Executive Officers in the Transaction
In considering the recommendation of the Renovaro
Board with respect to the Share Issuance Proposal and the other matters to be acted upon by the Renovaro stockholders at the Special
Meeting, the Renovaro stockholders should be aware that certain members of the Renovaro Board, and certain executive officers of
Renovaro, have interests in the Transaction that may be different from, or in addition to, the interests of the Renovaro stockholders.
Members of the Renovaro Board were aware of and considered these interests, among other matters, in evaluating and negotiating
the Stock Purchase Agreement and the Transaction, and in recommending to Renovaro’s stockholders to vote in favor of the
proposals outlined herein.
These interests relate to or arise from, among
other things:
| ● | the current executive officers of Renovaro, including Mark R. Dybul,
M.D., Luisa Puche and François Binette, PhD, are expected to continue
as executive officers of the combined company after the consummation of the Transaction and will be eligible to receive cash and
equity compensation as officers of the combined company; |
| ● | certain of Renovaro’s non-employee directors are expected to remain directors of
the combined company immediately following the consummation of the Transaction and will continue to be eligible to receive cash
and equity compensation as non-employee directors; |
| ● | the Stock Purchase Agreement provides that, at or prior to the Closing, Renovaro will
enter into with each new director of the Renovaro Board a customary director indemnification agreement, in form and substance acceptable
to such director to be effective upon the later of the Closing or the date of the director’s appointment; |
| ● | the Stock Purchase Agreement provides that, for a period of six years following the
Effective Time, Renovaro and GEDi Cube shall cause the Certificate of Incorporation and the By-laws of Renovaro and the
organizational documents of GEDi Cube to contain provisions no less favorable with respect to exculpation, indemnification and
advancement of expenses to any current or former directors and officers of Renovaro and GEDi Cube and each person who served as a
director, officer, member, trustee or fiduciary of another corporation, partnership, joint venture, trust, pension or other employee
benefit plan or enterprise at the request of Renovaro or GEDi Cube than as set forth in such Certificate of Incorporation, By-laws
or organizational documents, as applicable, to the extent permitted by applicable law; and |
| ● | the Stock Purchase Agreement requires Renovaro to amend its directors’ and officers’
insurance policies in order to provide for the new directors of the Renovaro Board and any new officers of Renovaro appointed after
the date of the Stock Purchase Agreement with at least as favorable terms and scope of coverage as those of such policy covering
Renovaro’s existing officers and directors. |
See “Proposal 3: Approval of the Repricing of Outstanding
Stock Options Granted under the Enochian BioSciences Inc. 2019 Equity Incentive Plan and the Renovaro Biosciences Inc. 2023 Equity
Incentive Plan—Eligible Option Holders” for additional information regarding the Eligible Options (as defined below)
held by Renovaro’s executive officers that may be subject to the Option Repricing.
Ownership Interests
As of December 29, 2023, Renovaro’s current directors
and executive officers held, in the aggregate, approximately 21.00% of the outstanding shares of Renovaro’s capital stock and 20.18%
of the outstanding shares of Common Stock (in each case, which excludes any shares of Common Stock issuable upon exercise or settlement
of outstanding stock options or restricted stock units under the Plans and outstanding warrants held by such individuals).
Executive Officers Following the
Closing
The current executive officers of Renovaro are
expected to continue as executive officers of the combined company following the Closing, which includes the following individuals:
| Name |
Position with the Combined Company |
Current Position |
| Mark R. Dybul, M.D. |
Chief Executive Officer |
Chief Executive Officer |
| Luisa Puche |
Chief Financial Officer & Corporate Secretary |
Chief Financial Officer & Corporate Secretary |
| François Binette, PhD |
Chief Operating Officer & Executive Vice President for Research & Development |
Chief Operating Officer & Executive Vice President for Research & Development |
Director Positions Following the
Closing
Mark R. Dybul, M.D.,
currently the Chief Executive Officer of Renovaro and a member of the Renovaro Board, will continue as the Chief Executive Officer
and a director of the combined company after the Closing. In addition, pursuant to the Stock Purchase Agreement, the board of directors
of the combined company effective as of the Closing will include, of the nine total directors serving thereon, four persons
designated by Renovaro (at least three of whom must qualify as an “independent director” under Nasdaq Listing Rules).
Accordingly, it is expected that certain of Renovaro’s current directors will continue to serve on the board of directors
of the combined company following the Closing.
Indemnification and Insurance
Under the terms of the Stock Purchase Agreement,
at or prior to the Closing, Renovaro must provide to each director appointed to service on the board of directors of the combined
company following the Closing a customary director indemnification agreement, in form and substance reasonably acceptable to such
director, to be effective upon the later of the Closing or the date of such director’s appointment.
For a discussion of the indemnification and insurance
provisions related to the Renovaro directors and officers under the Stock Purchase Agreement, please see the section titled “The
Stock Purchase Agreement—Indemnification” beginning on page 73 below.
Federal Securities Law
Consequences
The issuance
of Common Stock in the Transaction to Renovaro stockholders will be effected by means of a private placement, which is exempt from
registration under the Securities Act, in reliance on Section 4(a)(2) of the Securities Act and Rule 506 of Regulation D or Regulation
S promulgated thereunder, and such shares will be “restricted securities.” The shares issued in connection with the
Transaction will not be registered under the Securities Act upon issuance and will not be freely transferable. Holders of such
shares may not sell their respective shares unless the shares are registered under the Securities Act or an exemption is available
under the Securities Act. Upon the consummation of the Transaction, Renovaro will enter into a registration rights agreement with
each Seller granting to each such Seller certain customary registration rights with respect to such Seller’s shares of Common
Stock acquired in the Transaction, as described in further detail under “Agreements Related to the Transaction—Registration
Rights Agreement” beginning on page 76 of this proxy statement.
Material U.S. Federal
Income Tax Consequences of the Transaction
Regardless
of whether the Transaction will qualify for U.S. federal income tax purposes as a reorganization within the meaning of Section
368(a) of the Code, the Transaction will not result in any taxable gain or loss for U.S. federal income tax purposes to Renovaro,
GEDi Cube or any Renovaro stockholder in his, her or its capacity as a Renovaro stockholder.
The foregoing
discussion is for general information purposes only and is not intended to be a complete analysis or description of all potential
U.S. federal income tax consequences of the Transaction. In addition, the discussion does not address tax consequences that may
vary with, or are contingent on, your individual circumstances. Moreover, the discussion does not address any non-income tax or
any non-U.S., state or local tax consequences of the Transaction. Accordingly, you are strongly encouraged to consult with your
own tax advisor as to the tax consequences of the Transaction in your particular circumstances, including the applicability and
effect of the alternative minimum tax and any state, local or non-U.S. and other tax laws and of changes in those laws.
Regulatory Approvals
Renovaro must comply with applicable federal
and state securities laws and the rules and regulations of Nasdaq in connection with the issuance of shares of the Common Stock
in the Transaction and the filing of this proxy statement with the SEC. Renovaro does not intend to seek any regulatory approval
from antitrust authorities to consummate the Transaction.
Anticipated Accounting Treatment
Renovaro will apply the acquisition method of
accounting for business combinations in accordance with U.S. GAAP to the Transaction. Under the acquisition method of accounting,
Renovaro will record the acquisition based on the fair value of the consideration given, which includes the market value of its
shares of Common Stock issued in connection with the Transaction (based on the closing price of the Common Stock as reported on
Nasdaq on the effective date of the Closing). Renovaro will allocate the purchase price to the identifiable assets acquired and
liabilities assumed based on their respective fair values at the date of the Closing. Any excess of the value of consideration
paid over the aggregate fair value of those net assets will be recorded as goodwill.
Appraisal Rights
Under the General Corporation Law of the State
of Delaware, Renovaro stockholders are not entitled to appraisal rights in connection with the Transaction.
THE STOCK PURCHASE AGREEMENT
The following is a summary of selected provisions of the Stock Purchase Agreement.
While Renovaro believes that this description covers the material terms of the Stock Purchase Agreement, it may not contain all of the
information that is important to you. The Stock Purchase Agreement and the First Amendment to Stock Purchase Agreement have been attached
as Annexes A-I and A-II to this proxy statement to provide you with information regarding the terms of the Stock Purchase Agreement.
They are not intended to provide any other factual information about Renovaro, GEDi Cube or the Sellers. The following description does
not purport to be complete and is qualified in its entirety by reference to the Stock Purchase Agreement, including the First Amendment
to Stock Purchase Agreement. You should refer to the full text of the Stock Purchase Agreement, including the First Amendment to Stock
Purchase Agreement, for details of the Transaction and the terms and conditions thereof.
The Stock Purchase Agreement contains representations
and warranties that Renovaro, on the one hand, and GEDi Cube and the Sellers, on the other hand, have made to one another as of
specific dates. These representations and warranties have been made for the benefit of the other parties to the Stock Purchase
Agreement and may be intended not as statements of fact but rather as a way of allocating certain risks to one of the parties if
those statements prove to be incorrect. In addition, the assertions embodied in the representations and warranties are qualified
by information in confidential disclosure schedules exchanged by the parties in connection with signing the Stock Purchase Agreement.
While Renovaro does not believe that these disclosure schedules contain information required to be publicly disclosed under applicable
securities laws, other than information that has already been so disclosed, the disclosure schedules do contain information that
modifies, qualifies and creates exceptions to the representations and warranties set forth in the Stock Purchase Agreement. Accordingly,
you should not rely on the representations and warranties as current characterizations of factual information about Renovaro, GEDi
Cube or the Sellers, because they were made as of specific dates, may be intended merely as a risk allocation mechanism between
Renovaro, on the one hand, and GEDi Cube and the Sellers, on the other hand, and are modified by the disclosure schedules.
The Transaction and Effective Time of the Transaction
The Stock Purchase
Agreement provides that, at the Effective Time, the Sellers will sell and transfer to Renovaro, and Renovaro will purchase and
acquire from the Sellers, all of the GEDi Cube Shares owned by the Sellers that are issued and outstanding as of immediately prior
to the Effective Time in exchange for the Exchange Consideration, as described below. The Closing will occur as soon as practicable
but no later than three (3) business days after the satisfaction or waiver of the last to be fulfilled of the closing conditions
set forth in the Stock Purchase Agreement, or at such other date and place as Renovaro, GEDi Cube and the Sellers may agree to
in writing. The Transaction will be deemed effective as of the closing of business on the Closing Date (which is referred to herein
as the “Effective Time”). Renovaro expects that the Closing will take place during the first half of 2024,
subject to satisfying the closing conditions set forth in the Stock Purchase Agreement, including the approval by Renovaro’s
stockholders of the Share Issuance Proposal and the Authorized Share Proposal. Because the Closing is subject to such conditions,
Renovaro cannot predict if or exactly when the Closing will occur.
Exchange Consideration
If the Transaction
is consummated, in exchange for the sale and transfer of the issued and outstanding GEDi Cube Shares to Renovaro, the Sellers shall
become entitled to receive from Renovaro (i) an aggregate number of shares of Common Stock equal to a specified percentage
of the shares of Common Stock issued and outstanding as of the Effective Time (minus (a) 1 million shares of Common Stock issued
to a consultant assisting with the Transaction and (b) 1 million shares of Common Stock issued to Avram Miller prior to the Closing
pursuant to the Miller Consulting Agreement), which percentage shall be a ratio of the aggregate number of GEDi Cube Shares owned
by the Sellers divided by the aggregate number of GEDi Cube Shares issued and outstanding, in each case, as of the Effective Time
(which is referred to herein as the “Closing Consideration”), and (ii) earn-out shares of Common Stock to be issued
pro rata to the Sellers upon the exercise or conversion of any of Renovaro’s derivative securities (subject to certain exceptions)
which are outstanding at the Effective Time (which is referred to herein as the “Earnout Shares” and, together with
the Closing Consideration, the “Exchange Consideration”). The aggregate number of Earnout Shares to be issued to the
Sellers will be equal to a percentage of the shares of Common Stock issued upon exercise or conversion of Renovaro’s derivative
securities, which percentage shall be a ratio of the aggregate number of GEDi Cube Shares owned by the Sellers divided by the aggregate
number of GEDi Cube Shares issued and outstanding, in each case, as of the Effective Time. Each
Seller will receive its pro rata portion of the Exchange Consideration based on such Seller’s pro rata ownership of the GEDi
Cube Shares transferred to Renovaro.
Immediately following the Closing, the Sellers are expected to own approximately 49%
of the issued and outstanding shares of Common Stock (before giving effect to the issuance of the Earnout Shares).
Fractional Shares
No fractional shares of Common Stock will be
issued in connection with the Transaction, and no cash will be paid for fractional shares. Any fractional shares of Common Stock
that a Seller would otherwise be entitled to receive will be rounded down to the nearest whole share.
Renovaro Stock Approval; Increase in Authorized Shares
The Stock Purchase Agreement provides that it
is a condition to the parties’ obligation to consummate the Transaction that the Renovaro stockholders have approved, at
the Special Meeting, the Share Issuance Proposal and the Authorized Share Proposal, as described in this proxy statement. Upon
approval by the Renovaro stockholders of the amendment to the Certificate of Incorporation effecting the increase in the number
of authorized shares of Common Stock, Renovaro will file such amendment to its Certificate of Incorporation with the Secretary
of State of the State of Delaware to be effective upon the Effective Time. Pursuant to the terms of the Stock Purchase Agreement,
Renovaro will also amend its Certificate of Incorporation, effective as of the Effective Time, to change its name to “Renovaro
AI” and to provide for the changes to the size and structure of the post-Closing board of directors of Renovaro as contemplated
by the Stock Purchase Agreement (discussed in further detail below under “—Board of Directors and Officers of the
Combined Company”).
Surrender of GEDi Cube Shares
At the Closing, each Seller will deliver to
Renovaro an executed share transfer form in respect of the GEDi Cube Shares to be transferred to Renovaro and the certificate(s)
representing such shares. In the event that any such certificate is lost, stolen or destroyed, in lieu of delivery thereof to
Renovaro, the Seller may instead deliver to Renovaro a statutory declaration of lost certificate and an indemnity of loss in form
and substance reasonably acceptable to Renovaro and GEDi Cube, which may, in the reasonable discretion of Renovaro, include a
requirement that such Seller deliver a bond in such sum as Renovaro may reasonably direct as indemnity against a claim that may be
made against Renovaro or GEDi Cube with respect to the GEDi Cube Shares represented by the lost, stolen or destroyed
certificate.
Board of Directors and Officers of the Combined Company
The Stock Purchase Agreement obligates the parties
to take all necessary action so that, effective as of the Closing, the combined company’s board of directors will consist
of nine (9) individuals, including the Chief Executive Officer of Renovaro, four (4) additional persons designated by Renovaro
(at least three (3) of whom must qualify as an “independent director” under Nasdaq Listing Rules) and four (4) additional
persons designated by GEDi Cube (at least two (2) of whom must qualify as an “independent director” under Nasdaq Listing
Rules); provided that the combined company’s board of directors as so composed must meet all diversity and other requirements
under applicable law and Nasdaq Listing Rules. The board of directors of GEDi Cube immediately after the Closing shall be composed
of the same individuals as the combined company’s board of directors as of the Closing.
The Stock Purchase Agreement does not address
the composition of the combined company’s executive officers following the Closing. Renovaro expects that the current executive
officers of Renovaro will continue, immediately following the Closing, as the executive officers of the combined company, including
Mark R. Dybul, M.D. as Chief Executive Officer, Luisa Puche as Chief Financial Officer and Corporate Secretary and François
Binette as Chief Operating Officer and Executive Vice President for Research & Development.
Representations and Warranties
The Stock Purchase Agreement contains generally
similar representations and warranties of Renovaro with respect to Renovaro and of GEDi Cube and the Sellers with respect to GEDi
Cube regarding:
| ● | corporate organization, standing and power; |
| ● | capitalization and related matters; |
| ● | no conflict, required consents, notices and approvals of any governmental entity or third party required to complete the Transaction,
except as contemplated by the Stock Purchase Agreement; |
| ● | availability, accuracy and compliance with generally accepted accounting principles of financial statements and financial records; |
| ● | no pending legal proceedings; |
| ● | absence of undisclosed liabilities; |
| ● | compliance with applicable law and validity of permits; |
| ● | ethical practices in Renovaro’s or GEDi Cube’s operations, as applicable; |
| ● | no owned real property and validity and compliance with leases for leased real property; |
| ● | intellectual property rights and, with respect to GEDi Cube only, ownership and use of AI technologies by GEDi Cube; |
| ● | privacy and information security; |
| ● | no investment bank, broker, finder, or other intermediary retained, except as disclosed; |
| ● | absence of certain changes; |
| ● | completeness of representations; |
| ● | independent investigation by Renovaro or GEDi Cube, as applicable; and |
| ● | information supplied by Renovaro or GEDi Cube, as applicable. |
In addition, the Stock Purchase Agreement contains
further representations and warranties of Renovaro as to, among other things:
| ● | valid issuance of the Exchange Consideration; |
| ● | filing and material accuracy of SEC filings and related matters; |
| ● | listing of Common Stock on Nasdaq and compliance with Nasdaq rules; |
| ● | effectiveness, availability and coverage of directors’ and officers’ insurance policies; and |
| ● | compliance with Regulation S in connection with the Transaction. |
In addition, each Seller represents and warrants
as to the following matters with respect to such Seller:
| ● | organization and standing of any Seller that is not an individual; |
| ● | authority of such Seller; |
| ● | such Seller’s ownership of GEDi Cube Shares; |
| ● | no pending legal proceedings; |
| ● | status as an “accredited investor” or that such Seller is not a “U.S. Person”; and |
| ● | investment intent with respect to shares of Common Stock acquired in the Transaction and other related matters. |
The representations and warranties have been
made solely for the benefit of the parties in connection with the Stock Purchase Agreement and are not intended to be relied upon
by any other person, including the stockholders of Renovaro. In addition, the representations and warranties are qualified by specific
disclosures made to the other parties in connection with the Stock Purchase Agreement and should not be relied upon by any other
person as current characterizations of factual information about Renovaro, GEDi Cube or the Sellers. Moreover, many of the representations
and warranties are subject to materiality and knowledge qualifications contained in the Stock Purchase Agreement and are made only
as of the date of the Stock Purchase Agreement or such other date as is specified in the Stock Purchase Agreement.
Covenants; Conduct of Business Pending the Transaction
Under the Stock Purchase Agreement, each of Renovaro
and GEDi Cube has agreed, during the period from the date of the Stock Purchase Agreement and continuing until the earlier of the
termination of the Stock Purchase Agreement or the Closing (the “Interim Period”), except as set forth in the Stock
Purchase Agreement or unless the other party consents in advance in writing, to carry on its business in the ordinary course consistent
with past practice, continue to observe its obligations to comply with applicable legal and regulatory requirements, and use commercially
reasonable efforts to, subject to certain exceptions:
| ● | preserve intact its present business organization and assets; |
| ● | keep available the services of its officers, consultants and employees; |
| ● | maintain goodwill and relationships with manufacturers, customers, suppliers, contractors, distributors, and other business
relationships; and |
| ● | maintain its books, records and financials in accordance with generally accepted accounting principles. |
In addition, each of Renovaro and GEDi Cube has
agreed, during the Interim Period, except as set forth in the Stock Purchase Agreement or unless the other party consents in advance
in writing, not to take any of the following actions, among others:
| ● | increase any compensation or benefits of any current or former directors, officers, employees or consultants, as applicable,
establish or amend any benefit plans, terminate any employee other than for cause and grant any severance or termination pay to
any employee; |
| ● | assign, transfer, dispose of, or license ownership of any intellectual property rights or other assets; |
| ● | abandon or permit to lapse any of its intellectual property rights; |
| ● | disclose any of its trade secrets to any third party, other than pursuant to confidentiality agreements; |
| ● | declare, set aside or pay any distribution with respect to any equity interests of such party or purchase, redeem or acquire
outstanding equity interests of such party or other ownership interests in such party or otherwise change the capitalization of
such party; |
| ● | amend its organizational documents or comparable charter or governance documents; |
| ● | incur, create, assume, prepay or otherwise become liable for indebtedness, make a loan or advance to or investment in any third
party, or guarantee or endorse a third party’s indebtedness, liability or organization; |
| ● | commence a lawsuit or settle, compromise or otherwise terminate any litigation, claim, investigation or settlement negotiation; |
| ● | make any payment to any third party in excess of $100,000; |
| ● | extend an employment offer for compensation exceeding $300,000; |
| ● | enter into any joint venture, partnership, limited liability company, or operating agreement with any third party; |
| ● | enter into any contract that would require disclosure as a “Company Material Contract” or a “Buyer Material
Contract” (as each is defined the Stock Purchase Agreement), as applicable, under the Stock Purchase Agreement, or breach,
modify, amend, or terminate any Company Material Contract or Buyer Material Contract, as applicable, or waive, release, or assign
any rights or claims under any such contracts; |
| ● | voluntarily incur any liability or obligation in excess of $300,000 individually or $600,000 in the aggregate other than pursuant
to existing contractual terms; |
| ● | adopt a plan of complete or partial liquidation, dissolution, merger, consolidation, restructuring, recapitalization or other
reorganization; |
| ● | acquire or dispose of capital stock of any third party or merge or consolidate with any third party; |
| ● | establish any subsidiary or enter into any new line of business; |
| ● | fail to use commercially reasonable efforts to keep in force insurance policies providing insurance coverage with respect to
its assets, operations and activities in such amount and scope of coverage substantially similar to that which is currently in
effect; |
| ● | sell, lease, license, transfer, exchange or swap, mortgage or otherwise pledge or encumber, or otherwise dispose of any material
portion of its properties, assets or rights; or |
| ● | knowingly take any action that would or is reasonably likely to make any representation or warranty of such party contained
in the Stock Purchase Agreement inaccurate, result in any of the closing conditions not being satisfied or otherwise impair the
ability of such party to consummate the Transaction. |
In addition, GEDi Cube has further agreed, during
the Interim Period, except as set forth in the Stock Purchase Agreement or unless Renovaro consents in advance in writing, not
to issue, grant, sell, dispose of or propose to do any of the foregoing with respect to any equity interests, other securities or
rights to acquire or sell any equity interests or other securities of GEDi Cube or engage in any hedging transactions with respect
to such securities.
In addition, each Seller has agreed that, during
the Interim Period, such Seller will not sell, transfer or dispose of any GEDi Cube Shares owned by such Seller unless the transferee
thereof becomes a party to the Stock Purchase Agreement as a Seller and executes and delivers each document required to be delivered
by such transferee as a Seller under the Stock Purchase Agreement.
Renovaro and GEDi Cube also agreed to (i) confer
with the other party (and, in the case of GEDi Cube, the Sellers) and its representatives about operations and integration matters
to the extent permitted by law, (ii) notify the other party in writing promptly after becoming aware of any event that would cause
or constitute a material breach of any of the representations and warranties of such party and use commercially reasonable efforts
to remedy such breach; (iii) promptly notify the other party if it fails to comply with or satisfy any of its covenants in the
Stock Purchase Agreement in any material respect; (iv) promptly notify the other party of any change in the normal course of any
business, operations or financial condition of such party or its respective assets or properties or any emergency related to the
same; and (v) afford the other party (and, in the case of GEDi Cube, the Sellers) and its representatives reasonable access during
normal business hours during the Interim Period to all information concerning the business, properties and personnel of such party,
as may be requested by the other party to complete the Transaction and prepare for post-closing transition of GEDi Cube’s
business.
Additional Agreements
Each of Renovaro and GEDi Cube has agreed to
use its commercially reasonable efforts to (i) apply for or otherwise seek, or obtain the consents (if any) of, or provide notice
to, a third party or governmental entity required to be obtained or provided in connection with the Transaction (including, with
respect to Renovaro, the approval by the Renovaro stockholders of the Share Issuance Proposal and the Authorized Share Proposal);
(ii) make any filings required with respect to the consummation of the Transaction; (iii) effect the Transaction and fulfill or
cause to be fulfilled the conditions to Closing under the Stock Purchase Agreement; and (iv) defend against any proceedings challenging
the Stock Purchase Agreement and the consummation of the Transaction, seeking to have any preliminary injunction, temporary restraining
order, stay or other legal restraint or prohibition entered or imposed by any court or other governmental entity that is not yet
final and nonappealable, vacated or reversed.
GEDi Cube and the Sellers further agreed that:
| ● | each of them shall not, while in possession of material nonpublic information, purchase or sell any securities of Renovaro
(other than to engage in the Transaction), communicate such information to any third party, take any other action with respect
to Renovaro in violation of the restrictions imposed by U.S. federal securities laws and other applicable foreign and domestic
laws on a person possessing material nonpublic information about a publicly traded company, or cause or encourage any third party
to do any of the foregoing; |
| ● | GEDi Cube shall, immediately prior to the Closing, issue to a consultant assisting the parties on the Transaction a number
of GEDi Cube Shares that would result in such consultant receiving 1 million shares of Common Stock as a part of the Closing Consideration
to be issued by Renovaro at Closing and use commercially reasonable efforts to cause such consultant to become a party to the Stock
Purchase Agreement as a Seller; |
| ● | GEDi Cube shall, pursuant to its contractual obligation, immediately prior to the Closing, issue to an employee a number of
GEDi Cube Shares required pursuant to such contractual obligation and use commercially reasonable efforts to cause such employee
to become a party to the Stock Purchase Agreement as a Seller; and |
| ● | GEDi Cube and the Sellers will use commercially reasonable efforts to cause all holders of GEDi Cube Shares to become parties
to the Stock Purchase Agreement as a Seller. |
Exclusivity
Each of Renovaro, on the one hand, and GEDi Cube
and the Sellers, on the other hand, agreed to not, and to cause their respective representatives to not, subject to certain exceptions
under the Stock Purchase Agreement, during the Interim Period:
| ● | solicit, assist, initiate or facilitate the making, submission or announcement of, or intentionally encourage, any Acquisition
Proposal; |
| ● | furnish any non-public information regarding such party or its affiliates or their respective businesses, operations, assets,
liabilities, financial condition, prospects or employees to any person or group of persons (other than the other parties to the
Stock Purchase Agreement or their respective representatives) in connection with or in response to an Acquisition Proposal, other
than as required by applicable law; |
| ● | engage or participate in discussions or negotiations with any person or group of persons with respect to, or that could reasonably
be expected to lead to, an Acquisition Proposal; |
| ● | approve, endorse or recommend, or publicly propose to approve, endorse or recommend, any Acquisition Proposal; |
| ● | negotiate or enter into any letter of intent, agreement in principle, acquisition agreement or other similar agreement related
to any Acquisition Proposal; or |
| ● | release any third person from, or waive any provision of, any confidentiality agreement to which such party is a party. |
Each of Renovaro, on the one hand, and GEDi Cube
and the Sellers, on the other hand, agreed to notify the other parties or party as promptly as practicable (but in any event within
three (3) business days) in writing of the receipt of (i) any bona fide inquiries, proposals or offers, requests for information
or requests for discussions or negotiations regarding or constituting any Acquisition Proposal or any bona fide inquiries, proposals
or offers, requests for information or requests for discussions or negotiations that could be expected to result in an Acquisition
Proposal and (ii) any request for non-public information relating to Renovaro or GEDi Cube, as applicable, or its affiliates in
connection with any Acquisition Proposal.
For purposes of the Stock Purchase Agreement,
an “Acquisition Proposal” means any inquiry, proposal or offer or any indication of interest in making an offer or
proposal from any person or group at any time relating to any of the following, as applicable:
| ● | with respect to GEDi Cube, the Sellers and their respective affiliates, a transaction (other than the Transaction) concerning
the sale of (i) all or any material part of the business or assets of GEDi Cube and its direct and indirect subsidiaries (other
than in the ordinary course of business consistent with past practice) or (ii) any of the shares or other equity interests or profits
of GEDi Cube and its direct and indirect subsidiaries, in any case, whether such transaction takes the form of a sale of shares
or other equity interests, assets, merger, consolidation, issuance of debt securities, management contract, joint venture or partnership,
or otherwise; and |
| ● | with respect to Renovaro and its affiliates, a transaction (other than the Transaction) concerning a merger,
consolidation or other business combination involving Renovaro, the issuance of equity interests in Renovaro resulting in a change
of control of Renovaro, or the sale of all or a substantial portion of the assets of Renovaro or its subsidiaries. |
Renovaro Special Meeting and Proxy Statement
Renovaro is obligated under the Stock Purchase
Agreement to use its reasonable best efforts to obtain approval of its stockholders at the Special Meeting, and to take all action
necessary or advisable to secure the required vote or consent of its stockholders to approve the Share Issuance Proposal and the
Authorized Share Proposal. For such purpose, Renovaro was required to prepare and file with the SEC a notice of meeting and this
proxy statement relating to the Special Meeting. If the Authorized Share Proposal is approved, Renovaro is further obligated to
amend its Certificate of Incorporation promptly after the Special Meeting and prior to the Closing to reflect the amendment so
approved by its stockholders.
Indemnification and Insurance of Directors and Officers
The Stock Purchase Agreement provides that all
rights to exculpation, indemnification and advancement of expenses existing in favor of any current or former directors and officers
of Renovaro or GEDi Cube and each person who served as a director, officer, member, trustee or fiduciary of another corporation,
partnership, joint venture, trust, pension or other employee benefit plan or enterprise at the request of Renovaro or GEDi Cube
(collectively, the “D&O Indemnified Persons”) as provided in their respective organizational documents or under
any indemnification, employment or similar agreements between any such D&O Indemnified Person and Renovaro or GEDi Cube, as
applicable, in each case as in effect on the date of the Stock Purchase Agreement, shall survive the Closing and continue in full
force and effect in accordance with their terms to the extent permitted by applicable law. Each of Renovaro and GEDi Cube agree
to cause their respective organizational documents to contain, for a period of six (6) years following the Closing, provisions
no less favorable with respect to exculpation and indemnification of and advancement of expenses to D&O Indemnified Persons
than are set forth in their respective organizational documents as of the date of the Stock Purchase Agreement to the extent permitted
by applicable law. The Stock Purchase Agreement further provides that Renovaro will amend its directors’ and officers’
insurance policy in form reasonably satisfactory to GEDi Cube to provide to the members of the combined company’s board of
directors and any new officers of Renovaro appointed after the date of the Stock Purchase Agreement with at least as favorable
terms and scope of coverage as its directors’ and officers’ insurance policy in existence as of the date of the Stock
Purchase Agreement.
Conditions to Completion of the Transaction
The obligation of Renovaro, on the one hand,
and the Sellers and GEDi Cube, on the other hand, to consummate the Transaction is subject to certain customary conditions, unless
otherwise waived, including the following:
| ● | approval of Proposals 1 and 2 by the Renovaro stockholders; |
| ● | receipt of the consents required to be obtained in connection with the Transaction, except for those consents as Renovaro and
GEDi Cube have agreed not to obtain or except whether the failure to obtain such consent would not have a material adverse effect
on the business of Renovaro or GEDi Cube, as applicable, or the ability of Renovaro, or the Sellers or GEDi Cube, as applicable,
to consummate the Transaction or perform their respective obligations under the Stock Purchase Agreement; |
| ● | no governmental entity of a competent jurisdiction having enacted, issued, promulgated, enforced, or entered any order in effect
as of the Closing that has the effect of making the Transaction illegal or otherwise prohibiting consummation of the Transaction
(which illegality or prohibition have a material adverse effect on the business of Renovaro or GEDi Cube, as applicable, or the
ability of Renovaro, or the Sellers or GEDi Cube, as applicable, to consummate the Transaction or perform their respective obligations
under the Stock Purchase Agreement); |
| ● | Renovaro having received evidence reasonably satisfactory to Renovaro, the Seller’s Representative and GEDi Cube that
immediately after the Closing and after giving effect to the issuance of the Exchange Shares and the Transaction, the shares of
Common Stock will remain listed on Nasdaq; |
| ● | accuracy of the respective representations and warranties of Renovaro, on the one hand, and GEDi Cube and the Sellers, on the
other hand, as of the Closing, except that, in the case of representations and warranties other than those deemed to be fundamental
representations, except where such inaccuracy would not have a material adverse effect on the business of Renovaro or GEDi Cube,
as applicable, or the ability of Renovaro, or the Sellers or GEDi Cube, as applicable, to consummate the Transaction or perform
their respective obligations under the Stock Purchase Agreement; |
| ● | compliance in all material respects by Renovaro, on the one hand, and the Sellers and GEDi Cube, on the other hand, with their
respective covenants and obligations in the Stock Purchase Agreement; |
| ● | no material adverse effect occurring with respect to the business of Renovaro or GEDi Cube, as applicable, or the ability of
Renovaro, or the Sellers or GEDi Cube, as applicable, to consummate the Transaction or perform their respective obligations under
the Stock Purchase Agreement; and |
| ● | Renovaro, on the one hand, and the Sellers and GEDi Cube, on the other hand, executing and delivering all documents and instruments
required to be delivered by such party or parties at the Closing under the Stock Purchase Agreement. |
In addition, the obligation of Renovaro to consummate
the Transaction is subject to the following additional conditions, unless waived by Renovaro:
| ● | each Seller, as mutually agreed upon by Renovaro and GEDi Cube, executing and delivering a non-competition agreement to Renovaro; |
| ● | no legal proceeding pending or being threatened that (i) challenges or seeks to retain or prohibit the consummation of the
Transaction, or seeks to obtain any material damages or any award of attorney fees in connection with the Transaction or (ii) seeks
to prohibit or impose any material limitation on Renovaro’s ownership or operation of all or any portion of GEDi Cube’s
business or to compel Renovaro to dispose of or hold separate all or any material portion of the assets of GEDi Cube as a result
of the Transaction; |
| ● | termination of certain designated contracts of GEDi Cube; |
| ● | all of the holders of GEDi Cube Shares immediately prior to the Closing having delivered a joinder to become a party to the
Stock Purchase Agreement as a Seller; |
| ● | receipt by Renovaro of the consolidated financial statements of GEDi Cube required to be included in this proxy statement in
the form prepared in accordance with the applicable filing requirements with the SEC in respect to the Transaction; and |
| ● | repayment to GEDi Cube of all distributions by GEDi Cube to any affiliate since August 1, 2023. |
In addition, the obligation of GEDi Cube and
the Sellers to consummate the Transaction is subject to the following additional conditions, unless waived by GEDi Cube:
| ● | the combined company’s board of directors being composed of those individuals designated by the Stock Purchase Agreement
and otherwise specified by Renovaro or GEDi Cube, where required, as of the Effective Time; and |
| ● | amendment of Renovaro’s organizational documents to reflect the amendment proposed by the Authorized Share Proposal. |
Indemnification
The Stock Purchase Agreement provides that each
Seller will indemnify Renovaro and its respective officers, directors, agents, consultants, advisors, representatives and equity
holders (each, a “Buyer Indemnified Person” and, collectively, “Buyer Indemnified Persons”) from all documented
and out-of-pocket costs, expenses, losses or damages incurred by Buyer Indemnified Persons arising out of, relating to or resulting
from:
| ● | any inaccuracy in or breach of any representation or warranty of GEDi Cube or such Seller contained in the Stock Purchase Agreement; |
| ● | any breach or non-fulfillment of any covenant, agreement or obligation of GEDi Cube or such Seller under the Stock Purchase
Agreement (other than as a direct result of the failure of Renovaro to perform its obligations under the Stock Purchase Agreement); |
| ● | any claims by any then current or former holder or alleged then current or former holder of any GEDi Cube Shares, arising out
of, resulting from or in connection the Transaction or the Stock Purchase Agreement or such person’s status or alleged status
as a holder of GEDi Cube Shares at any time at or prior to the Closing, whether for breach of fiduciary duty or otherwise or any
person to the effect that such Person is entitled to any GEDi Cube Shares or any payment in connection with the Transaction by
virtue of such GEDi Cube Shares; and |
Each Seller shall only be liable to Buyer Indemnified
Persons for indemnification with respect to breaches of representations and warranties or claims under the third matter identified
above with respect to claims by third parties in proportion to such Seller’s pro rata portion such losses based on such Seller’s
pro rata ownership of the GEDi Cube Shares to be transferred to Renovaro. No Seller shall have any liability to a Buyer Indemnified
Person with respect to a breach of representation, warranty or covenant under the Stock Purchase Agreement to the extent that Renovaro
knew of such breach as of the Closing.
The Stock Purchase Agreement provides that Renovaro
will indemnify each Seller and its respective officers, directors, agents, consultants, advisors, representatives and equity holders,
and successors and assigns (each, a “Seller Indemnified Person” and, collectively, “Seller Indemnified Persons”)
from all documented and out-of-pocket costs, expenses, losses or damages incurred by Seller Indemnified Persons arising out of,
relating to or resulting from:
| ● | any inaccuracy in or breach of any representation or warranty of Renovaro contained in the Stock Purchase Agreement; |
| ● | any breach or non-fulfillment of any covenant, agreement or obligation of Renovaro under the Stock Purchase Agreement (other
than as a direct result of the failure of GEDi Cube or any Seller to perform its obligations under the Stock Purchase Agreement); |
| ● | any claims by any then current or former holder or alleged then current or former holder of any equity interests of Renovaro
or securities convertible or exchangeable into equity interests of Renovaro, arising out of, resulting from or in connection the
Transaction or the Stock Purchase Agreement or any person to the effect that such person is entitled to any equity interests of
Renovaro or securities convertible or exchangeable into equity interests of Renovaro or any payment in connection with the Transaction
by virtue of such equity interests or convertible or exchangeable securities; |
| ● | any action, claim or proceeding arising out of or relating to Serhat Gumrukcu, William Anderson Wittekind, their affiliates,
or their relationship with Renovaro as current or former stockholders; or |
| ● | any legal proceeding relating to any inaccuracy, breach, claim or expense of the type referred to above. |
Renovaro shall have no liability to a Seller
Indemnified Person with respect to a breach of representation, warranty or covenant under the Stock Purchase Agreement to the extent
that the Sellers knew of such breach as of the Closing.
The Stock Purchase Agreement provides for customary
survival periods and limitations on the parties’ respective indemnification obligations, including the following (subject,
in all cases, to fraud by the indemnifying party and the other exceptions described below):
| ● | the representations and warranties of each party deemed to be fundamental representations
under the Stock Purchase Agreement will survive for six (6) years following the Closing; |
| ● | the representations and warranties of each party pertaining to tax matters shall survive until ninety (90) days after the expiration
of the applicable statute of limitations; |
| ● | all other representations and warranties of such party shall survive until the date that is twenty-four (24) months after the
Closing; |
| ● | the Sellers and Renovaro are not liable for indemnification for breaches of representations and
warranties until the individual amount of each claim equals or exceeds $250,000, except for indemnification for any representations
and warranties deemed to be fundamental representations under the Stock Purchase Agreement; |
| ● | except for indemnification for any representations and warranties deemed to be fundamental
representations under the Stock Purchase Agreement, the maximum amount of losses which may be recovered
from the Sellers, on the one hand, or Renovaro, on the other hand, under such parties’ or party’s indemnification obligations
in the Stock Purchase Agreement shall be limited to (i) with respect to breaches of representations and warranties by Renovaro
(except with respect to taxes), an amount equal to fifteen percent (15%) or the Exchange Consideration received by the Sellers
at Closing, and with respect to breaches of representations and warranties by GEDi Cube or the Sellers (except with respect to
taxes), an amount equal to such Seller’s pro rata share of fifteen percent (15%) or the Exchange Consideration received by
the Sellers at Closing; and (ii) with respect to breaches of representations and warranties by Renovaro pertaining to taxes, an
amount equal to fifty percent (50%) or the Exchange Consideration received by the Sellers at Closing, and with respect to breaches
of representations and warranties by GEDi Cube or the Sellers pertaining to taxes, an amount equal to such Seller’s pro rata
share of fifty percent (50%) or the Exchange Consideration received by the Sellers at Closing; |
| ● | no Seller shall have any liability for indemnification under the Stock Purchase Agreement in excess of such Seller’s
pro rata share of the Exchange Consideration received at the Closing; and |
| ● | Renovaro shall have no liability for indemnification under the Stock Purchase Agreement in excess of an amount equal to the
Exchange Consideration received at the Closing. |
Termination
The Stock Purchase Agreement may be terminated
by Renovaro or by GEDi Cube or the Sellers’ Representative upon written notice by such party under certain circumstances,
including, among others:
| ● | By mutual written consent of Renovaro and GEDi Cube; |
| ● | By Renovaro or the Sellers’ Representative if any of the conditions to the Closing under the Stock Purchase Agreement
have not been satisfied or waived by February 28, 2024, so long as no breach or violation by the terminating party or any of its
representations, warranties, covenants or obligations under the Stock Purchase Agreement was the cause of, or otherwise resulted
in, the failure of the Closing to occur before such date; |
| ● | By Renovaro or GEDi Cube if a governmental entity of competent jurisdiction has issued an order or taken any other action that
permanently restricts, restrains, enjoins or otherwise prohibits the Transaction and such order or other action has become final
and non-appealable, so long as no failure of the terminating party or any of its affiliates to comply with any provision of the
Stock Purchase Agreement has been a substantial cause of, or substantially resulted in, such action by such governmental entity; |
| ● | By Renovaro or GEDi Cube, as applicable, upon a breach of any representation, warranty, covenant or agreement on the part of
GEDi Cube or any Seller, or on the part of Renovaro, respectively, set forth in the Stock Purchase Agreement, or if any representation
or warranty of such other parties or party shall have become inaccurate, in either case, such that the closing conditions concerning
accuracy of such other parties’ or party’s representations and warranties and compliance with covenants would not be
satisfied as of the later of the date of the Stock Purchase Agreement or the time of such breach or inaccuracy, if such breach
or inaccuracy is incapable of being cured or is not cured within the earlier of twenty (20) days after prior written notice of
such breach or inaccuracy provided by the terminating party or February 28, 2024; |
| ● | By Renovaro upon a material adverse effect occurring with respect to the business of GEDi Cube or the ability of the Sellers
or GEDi Cube to consummate the Transaction or perform their respective obligations under the Stock Purchase Agreement; |
| ● | By Renovaro or GEDi Cube, if the Special Meeting is held and has concluded, and the Renovaro stockholders have voted on Proposals
1 and 2 and any of such proposals have not been approved or adopted at the Special Meeting; |
| ● | By Renovaro, if there has been a material adverse change, in Renovaro’s reasonable determination, to GEDi Cub’s
AI technologies, intellectual property rights or capital structure, except as contemplated by the Stock Purchase Agreement on or
prior to the date that is five (5) business days following the date of the delivery of GEDi Cube’s disclosure schedules to
the Stock Purchase Agreement; provided that Renovaro is not in material uncured breach of the Stock Purchase Agreement; or |
| ● | By the Sellers’ Representative, if there has been a material adverse change, in the Sellers’ Representative’s
reasonable determination, to Renovaro’s intellectual property rights or capital structure, except as contemplated by the
Stock Purchase Agreement on or prior to the date that is five (5) business days following the date of the delivery of Renovaro’s
disclosure schedules to the Stock Purchase Agreement; provided that none of GEDi Cube or the Sellers are in material uncured breach
of the Stock Purchase Agreement. |
In the event of termination, the Stock Purchase
Agreement (other than certain provisions as set forth in the Stock Purchase Agreement) will become void and of no effect with no
liability or obligation on the part of any party to the Stock Purchase Agreement or any of its officers or directors, except that
no party will be relieved of any liability or damages arising from fraud or willful breach by such party of its representations,
warranties or covenants in the Stock Purchase Agreement.
Fees and Expenses
Generally, each party will pay its own fees and
expenses incurred in connection with the Stock Purchase Agreement, whether or not the Transaction is completed. However, the Stock
Purchase Agreement provides that Renovaro will pay for all costs of GEDi Cube in connection with the preparation and audit of the
consolidated financial statements of GEDi Cube included in this proxy statement.
AGREEMENTS RELATED TO THE TRANSACTION
Registration Rights Agreement
The Stock Purchase
Agreement contemplates that, at the Closing, Renovaro and each of the Sellers will enter into a Registration Rights Agreement,
pursuant to which, among other matters, Renovaro will agree to undertake certain shelf registration obligations in accordance with
the Securities Act and each Seller will be granted customary demand and piggyback registration rights with respect to its
shares of Common Stock acquired in the Transaction. The Registration Rights Agreement also provides that Renovaro will pay certain
expenses related to such registrations and will indemnify the Sellers against certain liabilities arising from such registrations.
Under the Registration Rights Agreement, Renovaro
further agrees to take such action as the Sellers may reasonably request, and to the extent required from time to time, to (i)
enable such Sellers to sell their shares of Common Stock acquired in the Transaction without registration under the Securities
Act within the limitation of the exemptions provided by Rule 144 under the Securities Act and, (ii) within two (2) business days
of receipt of written notice from any Seller wishing to transfer or sell any of such Seller’s shares without registration
under the Securities Act within the limitation of the exemption provided by Rule 144, provide, execute or deliver, as applicable,
all notifications, certifications, legal opinions, instruction letters, and any other documents or instruments reasonably requested
by such Seller or Renovaro’s transfer agent for such Seller to be able to sell or transfer such shares within the limitation
of the exemption provided by Rule 144.
Promissory Notes
On August 11, 2023, and August 18, 2023, Renovaro
entered into two Promissory Notes (the “Notes”) in the amounts $550,000 and $500,000, respectively, to lend a total
of $1.05 million to GEDi Cube to further develop GEDi Cube’s intellectual property and technology, which will
become part of the combined company following the Closing. Pursuant to the Notes, GEDi Cube promised to pay Renovaro the outstanding
principal and related accrued interest at a rate of 6% per annum on the applicable maturity date, February 11 and February 18,
2024, respectively. The outstanding aggregate balance of the Notes at September 30, 2023, was $1,057,875.
MATTERS BEING SUBMITTED TO A VOTE OF RENOVARO’S
STOCKHOLDERS
PROPOSAL 1:
Approval of the Issuance of Common Stock in the Transaction Nasdaq Listing Rule 5635
At the Special Meeting, Renovaro stockholders will be asked to approve the
issuance of shares of Common Stock as the Exchange Consideration to the Sellers pursuant to the Stock Purchase Agreement. Based on the
number of shares of Common Stock outstanding as of December 29, 2023 and the terms of the Stock Purchase Agreement governing the determination
of the Closing Consideration, Renovaro estimates that, immediately following the Closing, the Sellers will own approximately 49% of the
issued and outstanding shares of Common Stock (before giving effect to the issuance of the Earnout Shares). This percentage is subject
to certain assumptions and may change prior to the Closing, including as a result of future changes in the number of shares of Common
Stock outstanding prior to the Closing, the securityholders of GEDi Cube who become a party to the Stock Purchase Agreement and the Effective
Time of the Transaction, see “The Stock Purchase Agreement—Exchange Consideration.” As a result, the stockholders
of Renovaro and the securityholders of GEDi Cube immediately before the Effective Time of the Transaction could own a greater, or lesser,
percentage amount of the outstanding shares of the combined company immediately after the Effective Time of the Transaction.
The terms of, reasons for, and other aspects
of the Stock Purchase Agreement, the Transaction, and the issuance of the Exchange Consideration to the Sellers pursuant to the
Stock Purchase Agreement, are described in detail in other sections of this proxy statement.
Reasons for Stockholder Approval
The Common Stock is listed on Nasdaq, and, as
such, Renovaro is subject to the applicable Nasdaq Listing Rules, including Nasdaq Listing Rule 5635. In order to comply with the
Nasdaq Listing Rules and to satisfy conditions under the Stock Purchase Agreement, Renovaro is seeking stockholder approval of
this Proposal 1. Nasdaq Listing Rule 5635 requires stockholder approval in connection with the acquisition of the stock or assets
of another company if, due to the present or potential issuance of common stock, the common stock of the issuer has or will have
upon issuance voting power equal to or in excess of 20% of the voting power outstanding before the issuance of stock or securities
convertible into or exercisable for common stock of the issuer.
Renovaro is seeking stockholder approval of the
Share Issuance Proposal in order to satisfy the requirements of Nasdaq Listing Rule 5635 with respect to the issuance of the Common
Stock in the Transaction in excess of the 20% of the voting power outstanding before the issuance.
The Stock Purchase Agreement requires Renovaro
to submit this Proposal 1 to its stockholders at the Special Meeting. Approval of this Proposal 1 will constitute approval pursuant
to the Nasdaq Listing Rules.
Dilution
If this Proposal 1 is approved, existing Renovaro stockholders will suffer significant dilution in ownership
interests and voting rights as a result of the issuance of shares of Common Stock in the Transaction. In the case of the Transaction,
the number of shares of Common Stock to be issued will be based on the number of issued and outstanding shares of Common Stock
as of the Effective Time and the number of securityholders of GEDi Cube who join as Sellers under the Stock Purchase Agreement.
This number would not give effect to any other future issuances of Common Stock. The sale into the public market of these shares
could also materially and adversely affect the market price of the Common Stock.
Vote Required; Recommendation of the Renovaro Board
The affirmative vote
of a majority of the votes cast in person or by proxy at the Special Meeting is required for approval of Proposal 1. A failure
to submit a proxy card or vote at the Special Meeting, or an abstention for Proposal 1 will have no effect on the outcome of Proposal
1.
THE
RENOVARO BOARD UNANIMOUSLY RECOMMENDS THAT RENOVARO’S STOCKHOLDERS VOTE “FOR” PROPOSAL 1 TO APPROVE THE ISSUANCE OF
THE COMMON STOCK PURSUANT TO THE STOCK PURCHASE AGREEMENT UNDER THE NASDAQ LISTING RULES. THE APPROVAL OF EACH OF PROPOSALS 1 AND 2 IS
REQUIRED TO CONSUMMATE THE TRANSACTION.
PROPOSAL 2:
Approval of Amendment to Renovaro’s Certificate of Incorporation to Increase the Number of Authorized Common Shares of Renovaro
On December 21, 2023, the
Renovaro Board approved and declared advisable, subject to stockholder approval, an amendment to the Certificate of Incorporation to
increase the total number of authorized shares of capital stock of Renovaro from one hundred ten million (110,000,000) to three hundred
sixty million (360,000,000) and to increase the total number of authorized shares of Common Stock from one hundred million (100,000,000)
to three hundred fifty million (350,000,000). The Certificate of Incorporation currently authorizes one hundred ten million (110,000,000)
shares of capital stock of Renovaro, including one hundred million (100,000,000) shares of Common Stock and ten million (10,000,000)
shares of preferred stock, par value $0.0001 per share (“Preferred Stock”). As of December 29, 2023, out of the authorized
shares of Common Stock, 9,696,664 remained available for future issuance and 90,303,336 shares were issued or issuable, as follows:
| ● | 67,224,089 shares of Common Stock are issued and outstanding; |
| ● | 5,130,110 shares of Common Stock are issuable upon the exercise of outstanding stock options pursuant to the Plans; |
| ● | 5,913,616 shares of Common Stock are reserved for future issuance under the 2023 Plan; |
| ● | 5,827,407 shares of Common Stock are issuable upon the exercise of outstanding warrants; |
| ● | 5,610,100 shares of Common Stock are reserved for the future conversion of Series A Preferred Stock; and |
| ● | 598,014 shares of Common Stock are issuable for the future conversion of the Convertible Notes and the additional convertible promissory notes issued by Renovaro since
September 30, 2023, in each case, as described below. |
For
a description of the Convertible Notes, see Note 7 to Renovaro’s unaudited condensed consolidated financial statements for
the quarter ended September 30, 2023, which are included in the First Quarter 2024 Form 10-Q.
As described in greater detail in the Share Issuance Proposal, Renovaro will
be required to reserve approximately 82,389,720 shares of Common Stock for issuance as Exchange Consideration in connection with the Transaction
pursuant to the terms of the Stock Purchase Agreement. As a result, even if Renovaro’s stockholders approve the Share Issuance Proposal,
Renovaro will not have sufficient authorized shares of Common Stock for issuance of the Exchange Consideration, and the Transaction may
not be completed unless this Proposal 2 is approved. In addition, approval of this Proposal 2 by the Renovaro stockholders is required
to consummate the Transaction, unless waived by GEDi Cube. The proposed amendment to the Certificate of Incorporation would not increase
or otherwise affect Renovaro’s authorized Preferred Stock. As of September 30, 2023, there were 561,010 shares of Series A Preferred
Stock issued and outstanding.
The Common Stock is
all of a single class, with equal voting, distribution, liquidation and other rights. The additional Common Stock to be authorized
by adoption of the proposed amendment to the Certificate of Incorporation would have rights identical to Renovaro’s currently
outstanding Common Stock. A copy of the amendment to the Certificate of Incorporation is attached as Annex B to this proxy statement.
If Renovaro’s stockholders approve this Proposal 2, subject to the discretion of the Renovaro Board, Renovaro intends to
file the amendment to the Certificate of Incorporation with the Secretary of State of the State of Delaware immediately prior to
the Effective Time of the Transaction.
Purpose
After consideration and
consultation with its senior management and its financial and legal advisors, the Renovaro Board determined that the Stock Purchase
Agreement and the Transaction are advisable and in the best interests of Renovaro and its stockholders. The Renovaro Board
considered various reasons to reach its determination, as discussed elsewhere in this proxy statement. The amendment to the
Certificate of Incorporation as described in this Proposal 2 is necessary for Renovaro to have sufficient authorized shares of
Common Stock to issue the Exchange Consideration to the Sellers under the Stock Purchase Agreement and stockholder approval of this
Proposal 2 is a condition to the Sellers’ and GEDi Cube’s obligation to consummate the Transaction. At this time,
Renovaro does not have any plans, commitments, arrangements, understandings or agreements regarding the issuance of Common Stock
following the increase of its authorized shares, except as described in this Proposal 2.
Possible Effects of the Amendment
If the amendment to
the Certificate of Incorporation to effect the increase in number of authorized shares of Common Stock is adopted and filed with
the Secretary of State of the State of Delaware, the additional authorized shares of Common Stock would be available for issuance
at the discretion of the Renovaro Board and without further stockholder approval, except as may be required by law or the rules
of Nasdaq on which the Common Stock is listed. The additional authorized shares of Common Stock would have the same rights and
privileges as the shares of Common Stock currently issued and outstanding. Holders of Common Stock have no preemptive rights. The
issuance of additional shares of Common Stock is likely to, among other things, have a dilutive effect on earnings per share and
on stockholders’ equity and voting rights. Furthermore, future sales of substantial amounts of Common Stock, or the perception
that these sales might occur, could adversely affect the prevailing market price of the Common Stock or limit Renovaro’s
ability to raise additional capital.
Vote Required; Recommendation of the Renovaro Board
The affirmative vote
of a majority of the votes cast in person or by proxy at the Special Meeting is required for approval of Proposal 2. A failure
to submit a proxy card or vote at the Special Meeting, or an abstention for Proposal 2 will have no effect on the outcome of Proposal
2.
THE RENOVARO BOARD UNANIMOUSLY RECOMMENDS
A VOTE “FOR” THE APPROVAL OF THE AMENDMENT TO THE CERTIFICATE OF INCORPORATION OF RENOVARO TO INCREASE THE NUMBER OF
AUTHORIZED SHARES OF COMMON STOCK. THE APPROVAL OF EACH OF PROPOSALS 1 AND 2 IS REQUIRED
TO CONSUMMATE THE TRANSACTION.
PROPOSAL 3:
Approval of the Repricing of Outstanding Stock Options Granted under the Enochian BioSciences Inc. 2019 Equity Incentive Plan and
the Renovaro Biosciences Inc. 2023 Equity Incentive Plan
Renovaro
is seeking stockholder approval of a decrease in the exercise price (the
“Option Repricing”) of outstanding stock options held by employees and consultants of Renovaro (“Eligible Participants”)
covering up to an aggregate of 3,931,430 shares of Common Stock granted under the Plans, which currently have exercise prices above the
closing price of the Common Stock as reported on Nasdaq (the “Reported Price”) on the date of the Closing (the “Repricing
Date”). To qualify for the Option Repricing,
Eligible Participants must hold as of the Repricing
Date options that: (i) were issued pursuant to the 2019 Plan or 2023 Plan, (ii) have a per share exercise price that is in excess of the
Reported Price, and (iii) are outstanding and unexercised (the “Eligible Options”). Options held by Eligible Participants
that have a per share exercise price that is in excess of the Reported Price will not be considered Eligible Options and will not be impacted
by the Option Repricing in any manner.
On December 21, 2023, the Renovaro
Board approved, upon the recommendation of the Compensation Committee of Renovaro, subject to the approval of the stockholders at the
Special Meeting, the repricing of the Eligible Options, as of the Repricing Date to the Reported Price (the “Repriced Exercise Price”).
In approving the Option Repricing, the Renovaro Board considered the impact of the current exercise prices of the stock options on the
incentives provided to Renovaro’s employees and consultants, the lack of retention value provided by the current stock options,
and the impact of such options on the capital structure of Renovaro. On December 21, 2023, the date on which the Renovaro Board approved
the Option Repricing, the closing price of the Common Stock on Nasdaq was $3.34 per share.
There will be no other changes to the stock options outstanding under the Plans. The Renovaro Board has determined
that the Option Repricing is in the best interest of Renovaro and its stockholders.
Specifics of the Option Repricing
Under
the Option Repricing, on the Repricing Date, the Eligible Options held by Eligible Participants as of the Repricing Date will automatically
be repriced to the Repriced Exercise Price without any action on the part of the Eligible Participants. If any holder of an Eligible
Option ceases to be an employee or consultant, as applicable, with Renovaro before the Repricing
Date, the holder’s Eligible Options will not be repriced on the Repricing Date. The following
table provides information, as of December 29, 2023, regarding the Eligible Options eligible for the Option Repricing:
| Exercise Price of Eligible | |
Number of | |
Weighted | |
Weighted Average Remaining Term of |
| Options | |
Shares | |
Average | |
Eligible Option (Years) |
| Position | |
From: | |
To: | |
Underlying | |
Price of | |
|
| | |
| |
| |
Eligible | |
Eligible | |
|
| | |
| |
| |
Options | |
Options | |
|
| Named Executive Officers | |
$ | 2.15 | | |
$ | 8.58 | | |
| 3,557,790 | | |
$ | 4.98 | | |
| 7.35 | |
| Executive Officers | |
$ | 2.15 | | |
$ | 8.58 | | |
| 166,500 | | |
$ | 5.08 | | |
| 8.30 | |
| Non- Executive Employees and Consultant | |
$ | 1.12 | | |
$ | 8.05 | | |
| 207,141 | | |
$ | 2.60 | | |
| 8.76 | |
Option Repricing
The
Renovaro Board has determined that adverse changes in the market price of the Common Stock since the Eligible Options were granted
could materially interfere with Renovaro’s efforts to retain the service of employees and consultants.
Therefore, the Renovaro Board recommends the Option Repricing to encourage an increasing alignment of employees’ and consultants’
interests with those of Renovaro’s stockholders and increased stake of Renovaro’s employees and consultants in the
long-term performance and success of Renovaro. When the market price for the Common Stock is significantly below the applicable
exercise price of a stock option (often referred to as “underwater” or “out-of-the-money” option), for
example, the Renovaro Board believes that the option holder is not likely to exercise that stock option and will not have the desired
incentive that the stock option was intended to provide.
Alternatives Considered
The Renovaro Board considered
several alternatives in arriving at this Proposal 3, namely:
| ● | Allowing outstanding stock options to remain “underwater” or “out-of-the-money.”
The Renovaro Board is concerned that if Renovaro does not improve the Eligible Option holders’ prospects of receiving long-term
value from their stock options, Renovaro will undermine their long-term commitment to Renovaro, which in turn could limit Renovaro’s
ability to successfully implement its business plan and the business plan of the combined company if the Transaction is consummated.
Renovaro will also forgo an opportunity to better align their interests with those of Renovaro’s stockholders. |
| ● | Issuing additional stock options or other types of equity awards. However, this alternative would
result in increasing Renovaro’s overhang of outstanding equity awards and lowering its shares available for future grant
under the 2023 Plan, and Renovaro believes that adjusting already outstanding stock options would better serve the interests of
Renovaro’s stockholders. |
| ● | Exchanging options on a basis of less than one for one as a means of offsetting the increase in
value resulting from repricing options. Any exchange proposal would have required compliance with tender offer rules and resulted
in added costs, complexities and burdens on Renovaro’s resources in addition to the costs already being incurred in connection
with the Transaction. |
Eligible Option Holders
The
Option Repricing, if approved, would
benefit 62% of Renovaro’s employees, including Renovaro’s executive officers and one consultant. The following
table lists, for each of our named executive officers, all of our current executive officers as a group, and all of our employees (other
than our executive officers) as a group, the following information as of December 29, 2023: (i) the number of shares of Common Stock
subject to Eligible Options and (ii) the per share weighted average exercise prices of the Eligible Options. Each holder of an Eligible
Option must continue to be an employee or consultant of Renovaro, as applicable, through the Repricing Date in order to participate in
the Option Repricing, and any such holder that terminates service with Renovaro before the Repricing Date will not have the holder’s
Eligible Options repriced under the Option Repricing.
| Name of Individual or Group | |
Number of Shares Subject to Eligible Options | |
Weighted Average Exercise Price Eligible Option |
| Mark Dybul, Chief Executive Officer | |
| 3,112,790 | | |
$ | 5.04 | |
| Luisa Puche, Chief Financial Officer | |
| 275,000 | | |
$ | 4.49 | |
| Francois Binette, Chief Operating Officer | |
| 170,000 | | |
$ | 4.63 | |
| Executive Group | |
| 166,500 | | |
$ | 5.08 | |
| Non-Executive Employee Group and Consultant | |
| 207,141 | | |
$ | 2.60 | |
Accounting Treatment of the Option Repricing
Under Financial Accounting
Standards Codification Topic 718, Renovaro will recognize any incremental compensation cost of the Eligible Options subject to
the Option Repricing. Renovaro believes that the incremental compensation cost will be measured on the Repricing Date, if any,
of the fair value of the repriced Eligible Options immediately following the Option Repricing over the fair value of the Eligible
Options immediately prior to the Option Repricing.
Certain U.S. Federal Income Tax Consequences
Under U.S. federal tax laws,
the repricing of an Eligible Option is treated as a new option granted as of the Repricing Date, including with respect to the
incentive stock option rules. The rules concerning the federal income tax consequences with respect to options granted pursuant
to the Plans are quite technical. Moreover, the applicable statutory provisions are subject to change, as are their interpretations
and applications, which may vary in individual circumstances. Therefore, the following is designed to provide a general understanding
of the U.S. federal income tax consequences with respect to such grants. In addition, the following discussion does not set forth
any gift, estate, social security or state or local tax consequences that may be applicable and is limited to the U.S. federal
income tax consequences to individuals who are citizens or residents of the United States, other than those individuals who are
taxed on a residence basis in a non-U.S. country.
Incentive Stock Options
No taxable income is reportable
when an incentive stock option is granted or exercised, although the exercise may subject the optionee to the alternative minimum
tax or may affect the determination of the optionee’s alternative minimum tax (unless the shares are sold or otherwise disposed
of in the same year). If the optionee exercises the option and then later sells or otherwise disposes of the shares acquired more
than two years after the grant date and more than one year after the exercise date, the difference between the sale price and the
exercise price will be taxed as capital gain or loss. If the optionee exercises the option and then later sells or otherwise disposes
of the shares before the end of the two- or one-year holding periods described above, he or she generally will have ordinary income
at the time of the sale equal to the fair market value of the shares on the exercise date (or the sale price, if less) minus the
exercise price of the option. For purposes of the alternative minimum tax, the difference between the option exercise price and
the fair market value of the shares on the exercise date is treated as an adjustment item in computing the optionee’s alternative
minimum taxable income in the year of exercise. In addition, special alternative minimum tax rules may apply to certain subsequent
disqualifying dispositions of the shares or provide certain basis adjustments or tax credits for alternative minimum tax purposes.
Nonqualified Stock Options
No taxable income is reportable
when a nonstatutory stock option with a per share exercise price at least equal to the fair market value of a share of the underlying
stock on the date of grant is granted to an optionee. Upon exercise, the optionee will recognize ordinary income in an amount equal
to the excess of the fair market value (on the exercise date) of the shares purchased over the exercise price of the exercised
shares subject to the option. Any taxable income recognized in connection with an option exercise by an employee is subject to
tax withholding by Renovaro. Any additional gain or loss recognized upon any later disposition of the shares would be capital gain
or loss to the optionee.
Tax Effect for Renovaro
Renovaro generally will
be entitled to a tax deduction in connection with the repriced Eligible Options in an amount equal to the ordinary income realized
by the holder at the time the holder recognizes such income (for example, the exercise of a nonstatutory stock option). Special
rules limit the deductibility of compensation paid to Renovaro’s Chief Executive Officer and other “covered employees”
within the meaning of Code Section 162(m). Under Code Section 162(m), the annual compensation paid to any of these specified employees
will be deductible only to the extent that it does not exceed $1,000,000.
Vote Required; Recommendation of the Renovaro Board
The affirmative vote
of a majority of the votes cast in person or by proxy at the Special Meeting is required for approval of Proposal 3. A failure
to submit a proxy card or vote at the Special Meeting, or an abstention for Proposal 3 will have no effect on the outcome of Proposal
3.
THE
RENOVARO BOARD UNANIMOUSLY RECOMMENDS A VOTE “FOR” APPROVAL OF THE Repricing of Outstanding
Stock Options Granted under the Enochian BioSciences Inc. 2019 Equity Incentive Plan and the Renovaro Biosciences Inc. 2023 Equity Incentive
Plan.
PROPOSAL 4:
Approval of Amendment to the Renovaro Biosciences Inc. 2023 Equity Incentive Plan to Authorize 5,000,000 Additional Shares
At the 2023 annual meeting
of stockholders of Renovaro held on July 21, 2023 (the “2023 Annual Meeting”), the stockholders approved the 2023 Plan,
including approval of a reserve of 4,000,000 shares of Common Stock available for the grant of awards under the 2023 Plan. Following
the consummation of the Transaction, it is expected that the officers, employees and certain consultants and advisors of GEDi Cube
will be eligible to receive awards under the 2023 Plan. With the expansion of the potential recipients under the 2023 Plan, Renovaro
is requesting that its stockholders approve an amendment to increase the reserve of shares of Common Stock available for the grant
of awards under the 2023 Plan to enable the Renovaro Board to continue to make grants to eligible officers, employees, consultants,
advisors and non-employee directors of the combined company and its affiliates following the consummation of the Transaction. If
Renovaro’s stockholders approve the amendment proposed under this Proposal 4 at the Special Meeting, it will become effective
on the date of the Special Meeting. The effectiveness of this amendment is not subject to approval of any other proposals at the
Special Meeting or consummation of the Transaction.
On December 21, 2023, upon the recommendation of the Compensation Committee
of the Renovaro Board, the Renovaro Board unanimously adopted, subject to approval by Renovaro’s stockholders, an amendment to the
2023 Plan authorizing five million (5,000,000) additional shares of Common Stock for delivery in connection with awards under the 2023
Plan, increasing the total number of shares of Common Stock available for the grant of awards under the 2023 Plan to nine million (9,000,000)
(subject to adjustment under the terms of the 2023 Plan), plus the number of shares of Common Stock available for the grant of awards
under the 2019 Plan as of July 21, 2023. A copy of the 2023 Plan, as proposed to be amended, is attached as Annex C to this proxy statement.
With 2,205,489 shares of Common Stock remaining available for the grant of awards under the 2019 Plan as of July 21, 2023 (the date of
the 2023 Annual Meeting at which the 2023 Plan was approved) and 291,873 shares of Common Stock already subject to outstanding awards,
or having already been issued in connection with awards, under the 2023 Plan as of December 29, 2023, the total number of shares of Common
Stock available for the grant of awards under the 2023 Plan would be 10,913,616 shares of Common Stock if the amendment proposed under
this Proposal 4 is approved at the Special Meeting. No additional changes to the 2023 Plan are proposed for consideration by the Renovaro
stockholders at the Special Meeting.
The purposes of the 2023
Plan are to enhance Renovaro’s ability to attract and retain highly qualified officers, non-employee directors, key employees
and consultants, and to motivate those service providers to serve Renovaro and to expend maximum effort to improve Renovaro’s
business results and earnings, by providing to those service providers an opportunity to acquire or increase a direct proprietary
interest in Renovaro’s operations and future success of Renovaro. Renovaro believes that, following the consummation of the
Transaction, the increase in the number of shares of Common Stock available for future grants to eligible individuals under the
2023 Plan is vital to the combined company’s ability to continue offering competitive equity-based compensation to further
the purposes of the 2023 Plan.
The
following is a summary of the material terms of the 2023 Plan. The only
change proposed under the 2023 Plan Amendment Proposal is to increase the number of shares available for the grant of awards under the
2023 Plan by five million (5,000,000) shares. The summary is not intended to be a complete description of the 2023 Plan and is qualified
in its entirety by the actual text of the 2023 Plan, as proposed to be amended, which is attached as Annex C to this proxy statement.
Description of the 2023 Plan
Eligibility
and Participation. Awards may be granted under the 2023 Plan to officers, employees, consultants, advisors and non-employee directors
of Renovaro and its affiliates. Incentive stock options may be granted only to employees of Renovaro or its subsidiaries. As of
December 29, 2023, approximately 24 individuals
were eligible to receive awards under the 2023 Plan, including three named executive officers, ten other employees, nine non-employee
directors and two non-employee scientific
advisors.
Plan Administration. The
Renovaro Board has the power and authority to administer the 2023 Plan
as is consistent with the Certificate of Incorporation and the By-laws, and applicable law. Pursuant to the 2023 Plan, the Renovaro Board
has the authority to delegate such power and authority to administer the 2023 Plan to a committee of two or more non-employee directors.
Type of Awards. The following
types of awards are available for grant under the 2023 Plan: stock options, stock appreciation rights (“SARs”), restricted
shares, restricted stock units (“RSUs”), other stock-based awards and cash awards.
Number of Authorized Shares. Under
the 2023 Plan, as proposed to be amended, and subject to adjustment as
provided by the terms of the 2023 Plan, the maximum number of shares of Common Stock with respect to which awards may be granted under
the 2023 Plan is 9,000,000 shares, plus the number of shares of Common Stock available for the grant of awards under the 2019 Plan as
of July 21, 2023. Of this maximum amount, as of December 29, 2023, approximately 291,873 shares of Common Stock are already subject to
outstanding awards, or have already been issued in connection with awards, under the 2023 Plan. All such shares may be granted as Incentive
Stock Options (“ISOs”). In addition, any shares subject to outstanding awards under the 2019 Plan or the 2023 Plan that subsequently
expire, terminate, or are surrendered or forfeited for any reason without issuance of shares will automatically become available for issuance
under the 2023 Plan. The shares of Common Stock issuable under the 2023 Plan will consist of authorized and unissued shares, treasury
shares, or shares purchased on the open market or otherwise.
Share Counting
Each share granted in connection with an award
is counted as one share against the number of shares available.
Share Recycling. If any award is
surrendered, terminates, expires or lapses for any reason prior to the issuance of shares, or if shares are issued under the 2023
Plan and thereafter are forfeited to Renovaro, the shares subject to such awards and the forfeited shares will again be available
for grant under the 2023 Plan. In addition, any issued shares that are repurchased by Renovaro at no more than cost will again
be available for grant under the 2023 Plan.
The following items will not count against
the aggregate number of shares of Common Stock available for grant under the 2023 Plan: (i) any award that is settled in cash rather
than by issuance of shares of Common Stock, or (ii) awards granted in assumption of or in substitution for awards previously granted.
Shares tendered or withheld to pay the exercise price for an option or tax withholding for any type of award will continue to count
against the aggregate number of shares of Common Stock available for grant under the 2023 Plan. In addition, the total number of
shares covering stock-settled SARs or net-settled options will be counted against the pool of available shares, not just the net
shares issued upon exercise.
Stand-Alone, Additional, Tandem, and
Substitute Awards
The Renovaro Board may grant awards either
alone, in addition to, in tandem with, or in substitution or exchange for (i) any other award, (ii) any award granted under another
plan or (iii) any other right to receive payment from Renovaro.
Stock Options
Option Price. The Renovaro Board may
grant either ISOs, which must comply with Section 422 of the Code, or nonstatutory stock options (“NSOs”) (together
“options”). Options confer on the grantee a right to purchase a specified number of shares of Common Stock at a specified
price (the “option price”) subject to the terms and conditions of the award agreement. The option price per share will
be at least 100% of the fair market value of a share of Common Stock on the grant date. In the case of a grant of an option intended
to qualify as an ISO to a grantee that owns more than 10% of the total combined voting power of Renovaro (a “10% Stockholder”),
the option price will not be less than 110% of the fair market value of a share of Common Stock on the grant date.
Vesting and Exercise. The Renovaro Board
will determine the terms and conditions under which an option will become exercisable. No option may be exercised for a fraction
of a share. The Renovaro Board will establish the terms, conditions, and method of exercise. An option may be exercised by delivery
of a notice of exercise to Renovaro, setting forth the number of shares with respect to which the option is to be exercised, accompanied
by full payment for the shares.
Special Limitations on ISOs. An option
will constitute an ISO only (i) if the grantee is an employee of Renovaro or a subsidiary of Renovaro, (ii) to the extent the option
is specifically designated as an ISO in the related award agreement, and (iii) to the extent that the aggregate fair market value
(determined at the time the option is granted) of the shares of Common Stock with respect to which all ISOs held by the grantee
become exercisable for the first time during any calendar year (under the 2023 Plan, the 2019 Plan and all other plans of the grantee’s
employer and its affiliates) does not exceed $100,000.
Expiration. Options will expire no more
than 10 years from its grant date, or in the case of an ISO held by a 10% Stockholder, not more than five years from its grant
date.
Stock Appreciation Rights
SAR Exercise Price. A SAR will confer on the grantee a right to receive, upon exercise, a payment
of the excess of (i) the fair market value of one share of Common Stock on the date of exercise over (ii) the SAR exercise price, which
will be determined by the Renovaro Board (the “SAR exercise price”). The SAR exercise price will be fixed at no less than
the fair market value of a share of Common Stock on the grant date and will be specified in the award agreement. SARs granted in tandem
with an outstanding option following the grant date of such option will have a SAR exercise price that is equal to the option price, except
that the SAR’s exercise price may not be less than the fair market value of a share of Common Stock on the grant date of the SAR.
Vesting and Exercise. The Renovaro Board
will determine the terms and conditions under which a SAR will become exercisable.
Expiration. SARs will expire no more
than 10 years from their grant date.
Restricted Shares and RSUs
Restricted Shares. At the time
a grant of restricted shares is made, the Renovaro Board may establish an applicable “restricted period” during which
all or any portion of the shares will be forfeited if the grantee terminates service during the period and prescribes other restrictions,
including the satisfaction of corporate or individual performance objectives, which will be specified in the award agreement. Unless
the Renovaro Board otherwise provides in an award agreement, holders of restricted shares will have the right to vote and the right
to receive any dividends declared or paid with respect to the stock subject to the awards. All distributions, if any, received
by a grantee with respect to restricted stock as a result of any stock split, stock dividend, combination of shares, or other similar
transaction will be subject to the restrictions applicable to the original grant.
RSUs. An RSU is a bookkeeping entry
representing the right to receive cash or shares of Common Stock in the future. At the time a grant of RSUs is made, the Renovaro
Board will establish the events upon which the RSUs will become vested and payable, including either service-based vesting conditions
or performance-based vesting conditions based on the satisfaction of corporate or individual performance objectives, which will
be specified in the award agreement. RSUs will not confer stockholder rights to grantees. A grantee of RSUs shall have no rights
other than those of a general creditor of Renovaro.
Neither restricted shares nor RSUs can be sold,
transferred, assigned, pledged, or otherwise encumbered or disposed of during the restricted period, prior to vesting and settlement,
or before satisfaction of any other applicable restrictions.
Other Stock-Based Awards
The Renovaro Board may, in its discretion,
grant other stock-based awards, valued in whole or in part by reference to, or otherwise based upon, Common Stock. The terms of
other stock-based awards will be set forth in the applicable award agreements.
Performance Awards
The Renovaro Board may condition the grant,
exercise, vesting, or settlement of any award on performance conditions as it may specify. The Renovaro Board may select corporate
or individual performance objectives as it deems appropriate.
Effect of Certain Transactions
Adjustments for Changes in Capitalization. If
changes in Renovaro’s Common Stock occur by reason of any recapitalization, reclassification, stock split, reverse split,
combination of shares, exchange of shares, stock dividend or other distribution payable in stock, or other increase or decrease
without receipt of consideration by Renovaro, or if there occurs any spin-off, split-up, extraordinary cash dividend or other distribution
of assets by Renovaro, the number and kind of securities for which grants of awards may be made or settled under the 2023 Plan
will be equitably adjusted by Renovaro. In addition, if there occurs any spin-off, split-up, extraordinary cash dividend or other
distribution of assets by Renovaro, the number and kind of securities subject to any outstanding awards and the exercise price
of any outstanding options or SARs will be equitably adjusted by Renovaro.
Adjustments for Certain Transactions.
Except as otherwise provided in an award agreement, in the event of a recapitalization, reorganization, merger, consolidation,
combination, exchange, consolidation, sale of all or substantially all of Renovaro’s assets, or the acquisition of assets
or stock of another entity by Renovaro, or other corporate transaction involving Renovaro (a “transaction”), the 2023
Plan and the awards issued pursuant to the 2023 Plan will continue in effect in accordance with their respective terms, except
that following a transaction either (i) each outstanding award will be treated as provided for in the agreement entered into in
connection with the transaction or (ii) if not so provided in the agreement, each grantee will be entitled to receive in respect
of each share of our Common Stock subject to any outstanding awards, upon exercise or payment or transfer in respect of any award,
the same number and kind of stock, securities, cash, property or other consideration that each holder of a share of Common Stock
was entitled to receive in the transaction in respect of a share of Common Stock, except that, unless otherwise determined by the
Renovaro Board, such stock, securities, cash, property or other consideration will remain subject to all of the terms and conditions
(including performance criteria) which were applicable to the awards prior to the transaction. The treatment of outstanding options
and SARs in connection with a transaction in which the consideration paid or distributed to our stockholders is not entirely shares
of Common Stock of the acquiring or resulting corporation may include the cancellation of outstanding options and SARs upon consummation
of the transaction as long as, at the election of the Renovaro Board, (i) the holders of affected options and SARs have been given
a period of at least 15 days prior to the date of the consummation of the transaction to exercise the options or SARs (to the extent
otherwise exercisable) or (ii) the holders of the affected options and SARs are paid (in cash or cash equivalents) in respect of
each share covered by the option or SAR being canceled an amount equal to the excess, if any, of the per share price paid or distributed
to Renovaro’s stockholders in the transaction (the value of any non-cash consideration to be determined by the Renovaro Board
in its sole discretion) over the option or SAR exercise price, as applicable.
Change in Control. Under the 2023 Plan,
the Renovaro Board will determine how outstanding shares will be treated in the event of a change in control. The Renovaro Board
may provide that:
| |
(i) |
Upon a grantee’s separation from service prior to, upon, or immediately following a change in control for any reason other than cause, the award will immediately accelerate; |
| |
|
|
| |
(ii) |
In the event of a change in control, awards will (a) be assumed, (b) substituted for an equivalent award of the replacing entity’s stock, or (c) continue to be an obligation of Renovaro; or |
| |
|
|
| |
(iii) |
In the event of a change in control, each or any award shall be cancelled in exchange for a payment with respect to each vested share in cash, stock of a company that is a party to the change in control, or other property. |
Term of 2023 Plan. Unless terminated earlier by the Renovaro Board, the authority to make grants
under the 2023 Plan will terminate on July 21, 2033.
Amendment and Termination. The
Renovaro Board may, at any time and from time to time, amend, suspend, or terminate the 2023 Plan as to any shares of Common Stock
as to which awards have not been made. An amendment will be contingent on approval of Renovaro’s stockholders to the extent
stated by the Renovaro Board, required by applicable law or required by applicable stock exchange listing requirements. No awards
will be made after termination of the 2023 Plan. No amendment, suspension, or termination of the 2023 Plan will, without the consent
of the grantee, materially impair rights or obligations under any award theretofore awarded under the 2023 Plan.
No Repricing. Stockholder approval
will be required if any modification to an award would result in repricing of the award.
Clawback Policy. All awards received
or outstanding under the 2023 Plan shall be subject to clawback, cancellation, recoupment, rescission, payback, reduction, or other
similar action in accordance with any Company clawback or similar policy or any applicable law related to such actions. In addition,
a grantee may be required to repay Renovaro previously paid compensation, whether provided pursuant to the 2023 Plan or an award
agreement in accordance with Renovaro’s clawback policy. A grantee’s acceptance of an award constitutes the grantee’s
acknowledgement of and consent to Renovaro’s clawback or similar policy or any applicable law related to such actions and
the grantee’s agreement that Renovaro may take any actions that may be necessary to effectuate any such policy or applicable
law, without further consideration or action.
Separation from Service for Cause.
Renovaro may annul an award if the grantee is terminated from Renovaro’s employment for cause.
Deferral Arrangement. The Renovaro
Board may permit or require the deferral of any award payment into a deferred compensation arrangement, subject to rules and procedures
as Renovaro may establish and in accordance with Code Section 409A.
Transferability. Awards are not
transferable other than by will or the laws of descent, except that in certain instances transfers may be made to or for the benefit
of designated family members of the grantee for no value.
New Plan Benefits. The participants
to be granted awards and the amount and nature of awards to be granted to a particular participant under the 2023 Plan are within
the discretion of the Renovaro Board and, therefore, cannot be determined at this time.
Aggregate Past Grants. The following
table provides information, as of June 30, 2023, regarding the number of shares of Common Stock that may be issued under Renovaro’s
2014 Equity Incentive Plan (the “2014 Plan”) and the 2019 Plan:
| Plan Category | |
Number of securities to be issued upon exercise of outstanding options, warrants and rights | |
Weighted-average exercise price of outstanding options, warrants and rights | |
Number of securities remaining available for future issuance under equity compensation plans |
| Equity compensation plans approved by security holders | |
| 4,751,211 | | |
| 4. 62 | | |
| 2,292,515 | (1) |
| Equity compensation plans not approved by security holders | |
| — | | |
| — | | |
| — | |
| Total | |
| 4,751,211 | | |
| 4. 62 | | |
| 2,292,515 | (1) |
(1) On February 6, 2014, the Renovaro Board
adopted the 2014 Plan, and Renovaro had reserved 1,206,000 shares of Common Stock for issuance in accordance with the terms of
the 2014 Plan. On October 30, 2019, the Renovaro Board approved, and on October 31, 2019, Renovaro’s stockholders adopted
the 2019 Plan, which became effective on December 12, 2019 (the “Plan Effective Date”) and replaced the 2014 Plan.
The 2019 Plan included a reserve of (i) 6,000,000 new shares of Common Stock, (ii) the number of shares of Common Stock available
under the 2014 Plan for the grant of awards as of the Plan Effective Date, and (iii) shares of Common Stock underlying outstanding
awards granted under the 2014 Plan that, after the Plan Effective Date, expire or are terminated, surrendered, or forfeited for
any reason without the issuance of shares. The remaining shares of Common Stock available for grant related to the 2014 Plan was
655,769. As of the Plan Effective Date, this amount, along with the new 6,000,000 shares of Common Stock, totaled 6,655,769 shares
of Common Stock available for grant immediately after the Plan Effective Date. After June 30, 2023, the Renovaro Board and Renovaro’s
stockholders approved the 2023 Plan.
Federal Tax Consequences
The following is a brief summary of the U.S.
federal income tax consequences of the 2023 Plan generally applicable to Renovaro and to grantees in the 2023 Plan who are subject
to U.S. federal taxes. The summary is based on the Code, applicable Treasury Regulations, and administrative and judicial interpretations
thereof, each as in effect on the date of this proxy statement, and is, therefore, subject to future changes in the law, possibly
with retroactive effect.
THE FOLLOWING INFORMATION IS ONLY A SUMMARY
OF THE TAX CONSEQUENCES OF THE AWARDS AND IS NOT INTENDED TO COVER ALL TAX CONSEQUENCES NOR IS IT INTENDED TO BE USED BY ANY TAXPAYER
TO AVOID PENALTIES WHICH MAY BE IMPOSED. WE ENCOURAGE GRANTEES TO CONSULT WITH THEIR OWN TAX ADVISORS WITH RESPECT TO THE TAX CONSEQUENCES
INHERENT IN THE OWNERSHIP, EXERCISE, VESTING OR SETTLEMENT OF THEIR AWARDS, AND THE OWNERSHIP AND DISPOSITION OF ANY UNDERLYING
SECURITIES. TAX CONSEQUENCES FOR ANY PARTICULAR INDIVIDUAL OR UNDER STATE OR NON-U.S. TAX LAWS MAY BE DIFFERENT.
Nonqualified Stock Options. A
grantee generally will not recognize taxable income upon the grant or vesting of a nonqualified stock option with an exercise price
that is at least equal to the fair market value of Common Stock on the date of grant and that does not otherwise provide for the
deferral of compensation. Upon the exercise of a nonqualified stock option, a grantee generally will recognize compensation taxable
as ordinary income in an amount equal to the difference between the fair market value of the shares underlying the stock option
on the date of exercise and the exercise price of the stock option. When a grantee sells the shares, the grantee will have short-term
or long-term capital gain or loss, as the case may be, equal to the difference between the amount the grantee received from the
sale and the tax basis of the shares sold. The tax basis of the shares generally will be equal to the greater of the fair market
value of the shares on the exercise date or the exercise price of the stock option.
Incentive Stock Options. A grantee
generally will not recognize taxable income upon the grant of an incentive stock option. If a grantee exercises an incentive stock
option during employment or within three months after employment ends (12 months in the case of permanent and total disability),
the grantee will not recognize taxable income at the time of exercise for regular U.S. federal income tax purposes (although the
grantee generally will have taxable income for alternative minimum tax purposes at that time as if the stock option were a nonqualified
stock option). If a grantee sells or otherwise disposes of the shares acquired upon exercise of an incentive stock option after
the later of (i) one year from the date the grantee exercised the option and (ii) two years from the grant date of the stock option,
the grantee generally will recognize long-term capital gain or loss equal to the difference between the amount the grantee received
in the disposition and the exercise price of the stock option. If a grantee sells or otherwise disposes of shares acquired upon
exercise of an incentive stock option before these holding period requirements are satisfied, the disposition will constitute a
“disqualifying disposition,” and the grantee generally will recognize taxable ordinary income in the year of disposition
equal to the excess of the fair market value of the shares on the date of exercise over the exercise price of the stock option
(or, if less, the excess of the amount realized on the disposition of the shares over the exercise price of the stock option).
The balance of the grantee’s gain on a disqualifying disposition, if any, will be taxed as short-term or long-term capital
gain, as the case may be.
With respect to both nonqualified stock options
and incentive stock options, special rules apply if a grantee uses shares of Common Stock already held by the grantee to pay the
exercise price or if the shares received upon exercise of the stock option are subject to a substantial risk of forfeiture by the
grantee.
Stock Appreciation Rights. A
grantee generally will not recognize taxable income upon the grant or vesting of a SAR with a grant price at least equal to the
fair market value of our Common Stock on the date of grant and that does not otherwise provide for the deferral of compensation.
Upon the exercise of a SAR, a grantee generally will recognize compensation taxable as ordinary income in an amount equal to the
difference between the fair market value of the shares underlying the SAR on the date of exercise and the grant price of the SAR.
Restricted Share Awards, RSUs, and Performance
Awards. A grantee generally will not have taxable income upon the grant of restricted shares, restricted stock units or
performance awards. Instead, the grantee will recognize ordinary income at the time of vesting or payout equal to the fair market
value (on the vesting or payout date) of the shares or cash received minus any amount paid. For restricted shares only, a grantee
may instead elect to be taxed at the time of grant.
Other Stock-Based Awards. The
U.S. federal income tax consequences of other stock or cash-based awards will depend on the specific terms of each award.
Tax Consequences to Renovaro.
In the foregoing cases, Renovaro generally will be entitled to a deduction at the same time, and in the same amount, as a grantee
recognizes ordinary income, subject to certain limitations imposed under the Code.
Section 409A. Renovaro intends
that awards granted under the 2023 Plan comply with, or otherwise be exempt from, Code Section 409A, but make no representation
or warranty to that effect.
Tax Withholding. Renovaro is
authorized to deduct or withhold from any award granted or payment due under the 2023 Plan, or require a grantee to remit, the
amount of any withholding taxes due in respect of the award or payment and to take such other action as may be necessary to satisfy
all obligations for the payment of applicable withholding taxes. Renovaro is not required to issue any shares of Common Stock or
otherwise settle an award under the 2023 Plan until all tax withholding obligations are satisfied.
Vote Required; Recommendation of the Renovaro Board
The affirmative vote
of a majority of the votes cast virtually or by proxy at the virtual Special Meeting is required for approval of Proposal 4. A
failure to submit a proxy card or vote at the Special Meeting, or an abstention for Proposal 4 will have no effect on the outcome
of Proposal 4.
THE RENOVARO BOARD UNANIMOUSLY RECOMMENDS
A VOTE “FOR” APPROVAL OF THE proposed amendment to the 2023 plan to Increase the number of shares of Common Stock available
for awards BY FIVE MILLION (5,000,000) SHARES.
PROPOSAL 5:
Approval of Possible Adjournment of the Special Meeting, if Necessary, to Solicit Additional Proxies
General
The Renovaro Board believes that if there are insufficient votes to constitute a quorum, or if Renovaro fails
to receive a sufficient number of votes to approve Proposals 1, 2, 3 and/or 4, it is in the best interests of the stockholders
to enable the Renovaro Board to continue to seek to obtain a sufficient number of additional affirmative votes to approve such
proposals, and as a result our proxy holders may move to adjourn the Special Meeting, for a period of not more than sixty (60)
days, for the purpose of soliciting additional proxies to approve such proposals. Renovaro currently does not intend to propose
adjournment at the Special Meeting if there are sufficient votes to approve Proposals 1, 2, 3 and 4. If a quorum is present, the
affirmative vote of a majority of votes cast in person or in person on the proposal (not counting “abstentions” or
“broker non-votes” as votes cast) at the Special Meeting is required to approve the adjournment of the Special Meeting
for the purpose of soliciting additional proxies to approve Proposals 1, 2, 3 and/or 4.
In this Proposal 5, Renovaro is asking its stockholders to authorize the holder of any proxy solicited by the
Renovaro Board to vote in favor of adjourning the Special Meeting to another time and place, if necessary, to solicit additional
proxies in the event there are not sufficient votes to approve Proposals 1, 2, 3 and/or 4. If the Renovaro stockholders approve
this Proposal 5, Renovaro could adjourn the Special Meeting and any adjourned or postponed session of the Special Meeting and use
the additional time to solicit additional proxies, including the solicitation of proxies from the Renovaro stockholders that have
previously voted. Among other things, approval of this Proposal 5 could mean that, even if Renovaro had received proxies representing
a sufficient number of votes to defeat Proposal 1, 2, 3 or 4, we could adjourn the Special Meeting without a vote on such proposal
to solicit additional proxies and votes in favor of such proposal.
If it is necessary to adjourn the Special Meeting,
except as may be required by applicable law or SEC regulations, no notice of the adjourned meeting is required to be given to Renovaro’s
stockholders, other than an announcement at the Special Meeting of the date, time and place to which the Special Meeting is adjourned,
and the means of remote communication, if any, by which stockholders may participate in the meeting, so long as the meeting is
adjourned for 30 days or less and no new record date is fixed for the adjourned meeting. At the adjourned meeting, Renovaro
may transact any business which might have been transacted at the original meeting.
Vote Required; Recommendation of the Renovaro Board
The affirmative vote of a majority of the votes cast in person or by proxy at the Special Meeting is required
for approval of Proposal 5. A failure to submit a proxy card or vote at the Special Meeting, or an abstention for Proposal 5 will
have no effect on the outcome of Proposal 5.
THE RENOVARO BOARD UNANIMOUSLY RECOMMENDS A VOTE “FOR” APPROVAL
OF THE PROPOSAL TO ADJOURN THE SPECIAL MEETING, IF NECESSARY, TO SOLICIT ADDITIONAL PROXIES IF THERE ARE NOT SUFFICIENT VOTES IN
FAVOR OF PROPOSALS 1, 2, 3 and/or 4.
RENOVARO’S BUSINESS
In addition to the information
set forth below, please refer to the Renovaro 2023 Form 10-K, the Renovaro First Quarter 2024 Form 10-Q, and the other documents
filed with the SEC and incorporated by reference into this proxy statement for additional information regarding Renovaro’s
business.
Unless otherwise indicated
or the context otherwise requires, all references in this section to “we,” “us,” “our,” or
“Renovaro” are to Renovaro Biosciences Inc., a Delaware corporation together with its wholly owned subsidiaries, Renovaro
Biopharma, Inc., a Delaware corporation (“Renovaro Biopharma”), Renovaro BioSciences Denmark ApS, a Danish limited
company, organized under the Danish Act on Limited Companies of the Kingdom of Denmark, and Renovaro Technologies, Inc., a Nevada
corporation (“Renovaro Technologies”).
Our Business
We
are a biotechnology company committed to developing advanced allogeneic cell and gene therapies to promote stronger immune system
responses potentially for long-term or life-long cancer remission in some of the deadliest cancers, and potentially to treat or
cure serious infectious diseases such as Human Immunodeficiency Virus (HIV) and Hepatitis B virus (HBV) infection.
Our
Product Development strategy is anchored in the use of “non-self” or allogeneic cells that enhance the immune response
that we seek to elicit.
Over the past several years,
Renovaro has evolved from a company with a single product candidate as a potential cure for HIV, adding two additional pipeline
candidates for HIV, a pipeline for HBV, and with a significant expansion into cancer immune therapies to address high unmet needs
from difficult-to-treat solid tumors.
The oncology platform is
now at the forefront of our development activities, beginning with pancreatic cancer.
Many operational aspects
of our platforms can be quickly adapted to multiple disease states from a single therapeutic approach, potentially streamlining
and accelerating development and regulatory process, as well as manufacturing operations. Moreover, because our product candidates
do not require specialized delivery devices and surgical procedures, our potentially groundbreaking interventions could have worldwide
applicability.
Renovaro responds quickly
to new data and perceived development opportunities and risk assessments. Based on the maturation of our pipelines, Renovaro makes
business decisions to prioritize the programs that could move more rapidly through development and commercial processes.
Therapeutic Areas
of focus
Solid
Tumors
Cancer is caused by an uncontrolled
proliferation of abnormal cells. The immune system plays a key role in identifying and destroying those abnormal cells. In the
past 10 years, there has been significant investment in research and development to retrain the immune system in people with cancer
to restore the effectiveness of that immune response – so called “immune-therapy”. Immune-therapies have made
substantial advances to treat various types of blood cell-derived cancers. However, solid tumors still represent a key challenge
for long-term remission or cure. Certain solid tumors have evolved mechanisms that can either hide from normal immune control and
immune-therapies or release certain signaling factors that can block attempts by the immune system to recognize and destroy cancer
cells.
Renovaro is developing innovative,
proprietary approaches that involve gene- and/or cell-therapy to promote cancer fighting cells that are designed to potentially
overcome those mechanisms and enhance the ability of a person living with a solid tumor to more easily recognize cancer cells and
mount a much more robust and effective immune reaction to destroy them.
Pancreatic cancer remains
a very deadly disease with only 5-10 percent of patients surviving 5 years. Initial preclinical in vitro and proof
of concept in vivo studies of our immune-therapeutic approach for pancreatic cancer have demonstrated promising
results.
The flexibility provided
by the platform technology for quick adaptations during research and development and manufacturing processes could accelerate the
development of potential products for other solid tumors beyond pancreatic cancer. For example, triple-negative breast cancer,
glioblastoma and renal cell carcinoma are all solid tumors with poor survival rates and limited treatment options. The platform
might also allow for non-specific immune enhancement that could have impact against a broad array of solid tumors. As currently
conceived, our approach could potentially allow for outpatient therapy without significant impairment of the patient’s immune
system, as many current approaches require.
Because many types of solid
tumors do not have adequate therapies, are difficult to treat and have relatively low survival rates, there is a large market potential.
Infectious Diseases
Infectious diseases such
as HIV and HBV cause disease and death in hundreds of millions of people every year. In a similar way to solid tumors, those viruses
escape from natural immune response by hiding inside of human cells, creating sanctuaries or reservoirs of infection that can evade
the immune system. Advances in anti-viral drugs have made a huge impact on the ability to extend and improve the quality of life
for many, but are often associated with side effects and can be very expensive.
Through its advanced cell-
and gene- therapy platforms, Renovaro is developing unique tools potentially to enhance a person’s ability to both recognize
and fight infections. Because those mechanisms aim to eliminate the cells that serve as a reservoir for viral replication, our
therapy could potentially either cure or at least provide long term remission to people living with chronic infections.
HIV
HIV
attacks the human immune system, specifically killing off CD4+ T-cells, a central part of a person’s ability to control other
infections and certain cancers. Left untreated, over time, the number of CD4+ T-cells drops to such low levels that people die
from those infections and cancers.
Thanks
to scientific advances, there are over 30 antiretroviral drugs, or ARVs, approved by the FDA to treat HIV that allow many
people to live almost as long as people without HIV. But, as mentioned, the drugs are expensive, require taking pills every
day, and can have significant side effects over time.
According
to the World Health Organization, more than 30 million people are living with HIV. In addition, as many as 1 million people, including
people in high-income countries, continue to die each year from HIV due to the ability of the virus to evolve to evade the effects
of the drugs, in particular in people who have been taking various drugs for many years. To date, there are no treatments that
can eliminate the reservoir of immune cells that are infected with HIV from the body. Consequently, treatment for HIV is life-long.
HBV
Despite the availability
of an effective vaccine and treatment that can control infection if it is taken daily for life, HBV is the world’s most common
serious liver infection. According to the Hepatitis B Foundation, two billion people have been infected with HBV, approximately
300 million have chronic HBV infection, and nearly one million people die every year around the world. HBV remains the leading
cause of liver cancer and the second leading cause of cancer deaths in the world.
While vaccines are increasingly
required for children, many adults have not been vaccinated. Life-long treatment access can be limited and also can be difficult
for certain people to follow due to its side effects.
Current efforts to develop
novel treatments or cure largely focus on approaches to deplete the pool of the covalently closed circular DNA (cccDNA), a type
of HBV DNA that is the root cause of HBV chronicity. Renovaro is exploring the development of an innovative gene therapy approach
to co-opt HBV polymerase, a key factor that the virus needs to reproduce itself and to induce the death of liver cells infected
with the virus.
Therapeutic Platforms
Renovaro’s general
approach with gene- and/or cell-therapy is to enhance the immune system to allow a person to better fight diseases. Our vision
is for a world free from toxic chemotherapy and healthy longevity for those with cancer and other diseases. Renovaro is leveraging
general principles and advances in the knowledge of the immune response to engineer cells with enhanced attributes to promote the
recognition and elimination of diseased cells.
Advanced Allogeneic Cell
Therapy
The strategic benefit of
cell therapy platforms is to potentially allow for manufacture of large, “off-the-shelf” banks of therapeutic cells
that could be accessed on demand by health care professionals to potentially decrease the time between diagnosis and treatment.
In addition, because we
focus on cells from donors, the strategy could potentially enhance the ability of the therapeutic candidates to induce a more robust
response once injected into patients. The human immune system is designed to recognize and distinguish “self” from
“non-self” and destroy “otherness” such as bacteria, viruses, and damaged or diseased cells such as cancer
cells. Alloreactivity (reacting against another person’s cells) is the most powerful response the immune system generates.
Several of our technologies take advantage of the alloreactivity to hyper stimulate a person’s immune response to better
attack a chronic infection (e.g., HIV) or solid tumor.
In certain treatments (e.g.,
HIV and cancer), cells taken from healthy donors are sometimes genetically modified to introduce signaling molecules that are designed
to enhance the ability of specific immune cells to recognize diseased cells, and to help recruit other cells that will destroy
cancer or virus infected cells.
Renovaro believes that the
combination of off-the-shelf allogeneic cells, combined with genetic modifications designed to enhance immune signaling, could
potentially generate therapeutic candidates that have unique attributes that will increase the likelihood of success.
Cell Therapy enabling
technology
In addition to the platform
described above, Renovaro has an innovative gene therapy approach to enhance the selection and engraftment (uptake) of cells carrying
therapeutic attributes. Enhanced uptake or engraftment could play a critical role in some cases to increase the likelihood of therapeutic
benefit. This technology was initially developed for autologous cell therapy from a person living with HIV, and genetically modifying
those cells so they cannot be infected with most variants of HIV, plus a gene modification to enhance uptake. We have sublicensed
under a profit-sharing agreement our technology to potentially increase engraftment for potential use in CAR-T therapy as a potential
cure for HIV.
HBV Gene Therapy
Renovaro is exploring various
approaches for gene therapy design elements to potentially eliminate virus-infected cells with an innovative molecular mechanism
that co-opts the virus’ machinery to induce the death of infected cells rather than reproducing and causing more infection
and exacerbate disease.
Our Product Candidates

Oncology:
RENB-DC11: Genetically
modified Allogeneic Dendritic Cell Therapeutic Vaccine as Potential Product for Long-term Remission of Solid Tumors – Starting
with Pancreatic Cancer
Allogeneic Cell Therapy
Platform – Advanced Pre-Clinical
Based
on learning from peer-reviewed publications of Phase I/IIa trials, we have designed an innovative therapeutic vaccination platform
that could potentially be used to induce life-long remission from some of the deadliest solid tumors. The survival rate in pancreatic
cancer is currently only 5 to 10 percent at 5 years.
Initial
preclinical in vitro and proof of concept in vivo studies have been compelling. The platform
might also allow for non-specific immune enhancement that could have impact against a broad array of solid tumors. We initially
plan to target pancreatic cancer. Other potential targets for later development could include triple-negative breast cancer, liver
or mesothelioma. As with HIV, our approach would potentially allow for outpatient therapy without wiping out or significantly impairing
the patient’s immune system, as many current approaches require.
Renovaro has initiated a collaboration with Dr. Anahid Jewett, a leading cancer
immunology researcher recently identified by Stanford University as being among the top 3% of researchers in the world, from UCLA to study
further the in vitro and in vivo effectiveness of the approach in pancreatic cancer. Dr. Jewett created
an innovative pancreatic cancer mouse model that comprises the human immune system repertoire in combination with implanted human cancer
cells. Reproducible results from four independently conducted studies show promising substantial tumor size reduction, significant infiltration
of the tumor sack by effector immune cells, indicating ongoing killing of cancer cells, markers of significant immune activation and,
in a recent study with our most refined technology, no metastases. Multiple experiments in different humanized mouse models are consistently
showing with only one cycle of therapy – in humans five to ten are likely - what Dr. Jewett calls “the Holy Grail of cancer
research”:
| 1. | 80-90% substantial tumor size reduction (volume and weight) |
| 2. | Remnant of tumor sack significantly infiltrated with effector immune cells indicating ongoing killing
of cancer. |
| 3. | Significant correlation with expected immune response important to fight cancer detected in blood,
and |
Recently, a leading research and
product development company conducted a study of the effects of the therapeutic vaccine in more advanced pancreatic cancer. In the treated
group, there were no metastases, and there was a significant reduction in tumor size – perhaps even elimination – despite
using approximately half as many gene-modified immune cells and using a general tumor lysate rather than matching it to the specific cancer
compared with previous studies. In addition, Dr. Jewett conducted a study in liver cancer with promising results, including no metastases
in mice treated with the vaccine compared to metastases in all the animals that received no treatment.
Finally, we have finalized the
design of the clinical protocol and have identified the principal investigator from the Department of Oncology at UCLA to conduct the
first in-human studies, which is projected to be at the end of 2024. Because our Senior Vice President for Clinical Operations has 35
years of experience at Pfizer, including creating and leading teams that managed human trials of cancer cell and gene therapies, we will
retain complete control of the study with subcontractors who have already been identified rather than outsourcing to a clinical research
organization, which we expect will support the combined Phase 1 and 2 trial’s quality and speed and reduce the estimated cost by
50% or greater.
The confirming reproducibility and robustness of the therapeutic response in
an aggressive form of human pancreatic cancer in several models is promising. We received FDA input from pre-IND interactions, which helped
solidify our IND-enabling plan as well as our investigational plan. We are now fully committed to process development/improvements and
IND-enabling activities. We believe that we can complete IND-enabling activities in the second half of 2024, which, if successful, would
enable the start of clinical trials in humans during the second half of 2024. The investigational plan discussed with the FDA includes
phase 1 safety testing broadly in all solid tumor types, followed by a phase 2a focusing on a few solid tumor types that are difficult
to treat and have poor life expectancy, for example triple negative breast, second-line liver, head and neck cancers. Phase 2b would expand
cohorts in cancers with the strongest response in phase 2a.
RENB-DC-12--XX: Genetically
modified Allogeneic Dendritic Cell Therapeutic Vaccine as Potential Product for Long-term Remission of Additional Indications
The technology is a platform
that could potentially be adapted to other solid tumors first line and/or salvage therapy, by itself or, potentially, in combination
with other cancer treatments. Additional cancer vaccine designs are being evaluated strategically to balance risk and opportunity
to advance therapeutic development quickly in cancer indications with few treatment options.
Infectious Diseases:
HIV:
RENB-HV 12: HIV Therapeutic Vaccines for Potential
Long-term Remission/Cure
Allogeneic Cell Therapy
Platform - Advanced Pre-Clinical Stage; Non-Human Primate Studies Ongoing.
In
persons living with HIV who are controlling the spread of virus with anti-retroviral (ARV) treatment, boosting the immune system
in a different way than the virus already has through infection, could allow for control of HIV after stopping ARVs.
Renovaro
is developing RENB-HV12 that utilizes a novel cellular and immunotherapy approach that could potentially provide therapeutic vaccines
for HIV. A non-human primate study of the therapeutic vaccine in primates at the Fred Hutchinson Cancer Research Center is ongoing.
Animals began receiving the first injections of the potential therapeutic vaccine in August, 2022. Preliminary results assessment
may potentially be available in the second half of 2023. A Pre-IND request could be submitted in the second half of 2024, with
IND submission and the beginning of Phase I clinical trials by mid- to end-2025.
RENB-HV-01: Autologous Transplant with Genetically
Modified Cells
FDA INTERACT Meeting Held February 2020 -
Advanced Pre-Clinical Stage
We have pioneered a novel
enabling technology (ALDH gene modification) that we believe will allow sufficient engraftment of the CCR5 gene-modified Hematopoietic
Stem Cell (HSC) to eliminate the need for Antiretroviral Treatment (ART.)
Management conducted a successful
FDA INTERACT Meeting in alignment with Renovaro’s experimental plan. Although in vitro and in vivo studies
have demonstrated promising results, further development of RENB-HV01 at this time was deemed costly and a long-term undertaking.
While Renovaro plans to return to full development of the approach when resources are available, it has become less attractive
and been deprioritized for business reasons, while pipelines that could move more quickly have been prioritized (e.g., RENB-DC11).
Therefore, a business decision was made to sub-license the ALDH gene modification.
RENB-HV01 was sub-licensed
to Caring Cross with a profit share arrangement. Caring Cross is developing a CAR-T approach that they believe, when combined with
Renovaro ALDH gene modification, could enhance engraftment of their CAR-T cell therapy and enhance their likelihood of success.
RENB-HV-21: Immunotherapy with Allogeneic
NK/GDT Cells
Allogeneic Cell Therapy Platform -Pre-IND
conducted - Advanced Pre-Clinical with Human Data through a Collaboration
We are also exploring RENB-HV21,
an innovative treatment for HIV with allogeneic Natural Killer (NK) and Gamma Delta T-Cells (GDT). It is believed that the GDT
cells, a small subset of immune cells that can be infected with HIV, could both be infected by, and be a key factor in controlling,
the virus. The initial scientific findings were presented during the American Society of Gene & Cell Therapy (ASCGT) Annual
Meeting in 2021. Renovaro has an exclusive license to use the underlying patent to develop RENB-HV21 for potential treatment or
cure of HIV. A successful investigator-initiated Pre-IND was completed in October 2021. However, due to a shift in priorities to
the Oncology pipeline, Renovaro does not plan to pursue the IND and potential clinical trial in the medium- to long-term.
HBV:
RENB-HB-01: Potential
Cure for HBV
HBV Gene Therapy - Pre-Clinical
RENB-HB01 is in an early
pre-clinical phase as we explore various approaches for gene therapy design elements. If those explorations are successful, it
is possible we could begin the regulatory process at the earliest in the second half of 2024. However, our highest priority is
currently the oncology platform, beginning with pancreatic cancer and other solid tumors with poor life expectancy.
Collaborations
We have established strategic partnerships with leading scientists and research
centers, such as the University of California, Los Angeles, Fred Hutchinson Cancer Research Center, and Caring Cross for some of our programs.
We will continue to pursue partnerships and collaborations when appropriate with selected philanthropic, pharmaceutical and biotechnology
companies to fund internal research and development activities, and to assist in product development and commercialization. We are applying
our technology platform to several potential commercial applications in which our products provide us and our strategic partners and collaborators
with potential technical, competitive and economic advantages.
Our Intellectual Property
Patents and licenses are key to our business. Our strategy is to file for patent
applications to protect technology, inventions and improvements to inventions that we consider important for the development of our business.
We rely on a combination of patent, copyright, trademark and trade secret laws, as well as continuing technological innovations, proprietary
knowledge and various third-party agreements, including, without limitation, confidentiality agreements, materials transfer agreements,
research agreements and licensing agreements to establish and protect our proprietary rights. We aim to take advantage of all of the intellectual
property rights that are available to us and seek the protection of those rights so that we can fully exploit our innovations.
We also protect our proprietary information by requiring our employees, consultants,
contractors and other advisors to execute nondisclosure and assignment of invention agreements upon commencement of their respective employment
or engagement. Our patent filings are discussed briefly below.
Internally Developed Intellectual Property
Protocol for generating dendritic cells (2005 DK, 2008 PCT)
This patent family is directed
to the generation of dendritic cells based on a blood sample by culturing monocytes at reduced temperatures. Dendritic cells exposed
to tumor antigens followed by treatment with T(h) 1-polarizing differentiation signals have paved the way for the development of
dendritic cell-based cancer vaccines. Issued claims are directed to a method of generating immature dendritic cells under certain
temperature settings, which by further activation has been shown to give a high yield of homogeneous and fully matured dendritic
cells. The patent expiration date is December 2026 subject to any applicable patent term extension, patent term adjustment, or
supplementary protection certificates that may be available in a country or jurisdiction. This patent has been issued in the USA,
Canada, China, Eurasia, Russia, Europe, Israel, Mexico, Malaysia and New Zealand. This patent is owned by Renovaro and was not
licensed from third parties.
Assigned Intellectual Property
On August 16, 2022, the USPTO issued U.S. Patent No. 11,413,338 B2, “Methods
and Compositions Using Recombinant Dendritic Cells for Cancer Therapy,” pertaining to methods and compositions for treating cancer
by eliciting an immune response by administering dendritic cells expressing heterologous proteins. This patent protects RENB-DC-11: Genetically
modified Allogeneic Dendritic Cells as Potential Product for Long-term Remission of Solid Tumors – Starting with Pancreatic Cancer and
potential future products RENB-DC-12-XX: Genetically modified Allogeneic Dendritic Cells as Potential Product for
Long-term Remission of Additional Indications for twenty years. This patent is owned by Renovaro through assignment as of July
15, 2019.
On June 17, 2020, a patent application was filed entitled “Allogeneic
T-Cell-Based HIV Vaccine to Induce Cellular and Humoral Immunity,” US 2021/0030795 A1, for the composition and method of use concepts
for HV-12. This patent application is owned by Renovaro through assignment as of September 28, 2021.
In-Licensed Technology
On February 16, 2018, Renovaro
Biopharma, Renovaro’s wholly owned subsidiary, entered into a License Agreement (the “HIV License Agreement”)
with Weird Science, LLC (“Weird Science”). The License Agreement contains, among other things, the following terms:
(a) a perpetual, fully paid-up, royalty-free, sublicensable, and exclusive (including to the exclusion of Weird Science) worldwide
license from Weird Science to Renovaro Biopharma to use Weird Science’s intellectual property and technology for the prevention,
treatment, and/or amelioration of and/or therapy for HIV in humans, and research and development exclusively relating to HIV in
humans (the “Field”) worldwide; (b) a nonexclusive, royalty-free, sublicensable license from Renovaro Biopharma to
Weird Science to use the Renovaro technology to commercialize products outside of the Field worldwide; (c) a nonexclusive, royalty-free
license from Renovaro Biopharma to Weird Science to use the results of a study with syngeneic and humanized mice models outside
the Field and, at Weird Science’s own expense, to prosecute patents relating to the results of the study, which Weird Science
will own, and (d) a perpetual, fully paid-up, royalty-free, sublicensable, and sole and exclusive (including to the exclusion of
Weird Science) worldwide license from Weird Science to Renovaro Biopharma (which will be part of the license described in (a) above)
to use patent applications and patents related to the study results disclosed in (d) above solely in the Field, and to make, have
made, use, sell, offer to sell and import inventions claimed in such patent applications and patents solely in the Field. Our current
product candidates covered by this license include RENB-HV-01: Autologous Transplant with Genetically Modified Cells.
On January 31, 2020, Renovaro
entered into a Statement of Work and License Agreement (the “HBV License Agreement”) by and among Renovaro, G Tech
Bio, LLC (“G-Tech”), and Seraph Research Institute (“SRI”) (formerly G Health Research Foundation), and,
a not for profit entity organized under the laws of California doing business as Seraph Research Institute (“SRI”),
whereby Renovaro acquired a perpetual, sublicensable, exclusive license (the “HBV License”) for a treatment under development
(aimed to treat HBV infections in accordance with its agreement in principle with G-Tech and SRI announced by Renovaro on November
25, 2019. The HBV License Agreement states that in consideration for the HBV License, Renovaro would provide cash funding for research
costs and equipment and certain other in-kind funding related to the licensed treatment over a 24-month period. Renovaro paid an
upfront payment of $1.2 million on February 6, 2020. Our current product candidate under this license is RENB-HB-01 HBV Gene Therapy
(see Note 9 to the consolidated financial statements included in the Renovaro 2023 Form 10-K and to the condensed consolidated
financial statements included in the Renovaro First Quarter 2024 Form 10-Q).
On August 25, 2021, Renovaro
entered into an ALC Patent License and Research Funding Agreement in the HIV Field (the “ALC License Agreement”) with
SRI whereby Renovaro was granted an exclusive, worldwide, perpetual, fully paid-up, royalty-free license (the “ALC License”),
with the right to sublicense, the proprietary technology subject to a U.S. patent application, to make, use, offer to sell, sell
or import products for use solely for the prevention, treatment, amelioration of or therapy exclusively for HIV in humans, and
research and development exclusively relating to HIV in humans; provided the licensor retained the right to conduct HIV research
in the HIV Field. Pursuant to the ALC License Agreement, Renovaro granted a non-exclusive license back to licensor, under any patents
or other intellectual property owned or controlled by Renovaro, to the extent arising from the ALC License, to make, use, offer
to sell, sell or import products for use in the diagnosis, prevention, treatment, amelioration or therapy of any (i) HIV comorbidities
and (ii) any other diseases or conditions outside the HIV Field. Renovaro made an initial payment to SRI of $600,000 and agreed
to fund future HIV research, as mutually agreed to by the parties. Our current product candidate under this license is RENB-HV-21:
HIV Natural Killer and Gamma Delta T Cell Treatment or Cure (see Note 9 to the consolidated financial statements included in the
Renovaro 2023 Form 10-K and to the condensed consolidated financial statements included in the Renovaro First Quarter 2024 Form
10-Q).
Trade Secrets and Proprietary Know-How
In addition to intellectual
property protected by patents and copyrights, we have trade secrets and proprietary know-how relating to our products, production
processes and future strategies.
Competition
The biotechnology and pharmaceutical
industries, including in the field of gene therapy, are characterized by rapidly advancing technologies, intense competition and a strong
emphasis on intellectual property. While we believe that our technology platforms, strong intellectual property portfolio and scientific
expertise in the gene therapy field provide us with competitive advantages, we face potential competition from many different sources,
including larger and better-funded pharmaceutical and biotechnology companies, new market entrants and new technologies.
We are aware of several
companies focused on other methods for editing genes and regulating their expression, and a limited number of commercial and academic
groups pursuing the development of gene regulation and genome editing technology. The field of applied gene regulation and genome editing
is highly competitive, and we expect competition to persist and intensify in the future from several different sources, including pharmaceutical
and biotechnology companies, academic and research institutions and government agencies.
Accordingly, our competitors may
succeed in obtaining patent protection, receiving FDA approval or commercializing competitive products before us. If we commence commercial
product sales, we may be competing against companies with greater marketing and manufacturing capabilities, areas in which we have limited
or no experience. In addition, any product candidate that we successfully develop may compete with existing products that have long histories
of safe and effective use.
The competitive landscape
that we are facing is as follows:
Gene therapy companies
developing gene-based products in clinical trials. uniQure N.V.’s product for lipoprotein lipase deficiency and GlaxoSmithKline
plc, or GSK’s, product for severe combined immunodeficiency due to adenosine deaminase deficiency are approved in Europe.
No other gene therapy products have yet been approved. Our competitors in this category may include, but not be limited to, Sangamo
Therapeutics, Inc., uniQure N.V., bluebird bio, Inc., Regenxbio Inc., Shire, Pfizer Pharmaceutical, and GSK.
Cell therapy companies
developing cell-based products. Our competitors in this category may include Novartis AG, Adaptimmune Therapeutics PLC,
Atara Biotherapeutics, Inc., bluebird bio, Inc., Cellectis S.A., Juno Therapeutics, Inc., Kite Pharma, and Iovance Biotechnologies,
Inc.
For RENB-DC-11, the competitive
landscape is more complex.
Immunotherapy is an active
area of research and a number of immune-related products have been identified in recent years that are alleged to modulate the
immune system. Many of these products utilize dendritic cells, a form of immune cell that presents cancer target peptides to T
cells and that can in turn result in T cell activation. More recently, bi-specific antibodies and checkpoint inhibitors (for instance
PD-1/PD-L1 antibodies) have been identified as having utility in the treatment of cancer. Bi-specific antibodies commonly target
both the cancer peptide and the T cell receptors (“TCR”), thus bringing both cancer cells and T cells into close proximity
to maximize the chance of TCR binding and hence an immune response to the cancer cells. Checkpoint inhibitors on the other hand
work by targeting receptors that inhibit T cell effectiveness and proliferation and essentially activate T cells. Other immunotherapies
that are being actively investigated include antibody-drug complexes, TCR-mimic antibodies, oncolytic viruses and cancer vaccines.
A variety of cell-based autologous and allogeneic approaches are also being researched and developed.
CAR-T in solid tumors
In addition to hematological
malignancies, a growing number of pharmaceutical, biotechnology, and academic institutions are researching and developing autologous
and allogeneic chimeric antigen receptor T cell (“CAR-T”) therapies in the solid tumor setting. These CAR-T cell therapies
are at a variety of stages of preclinical and clinical development, as well as directed towards a broad target spectrum. Four CAR-T
therapies have been approved for treatment of leukemia.
TCR T cells
Competitors are developing
TCR T cells (including affinity engineered T cells) that are directed towards a multitude of targets. Juno Therapeutics has developed
an engineered TCR therapeutic candidate where the end TCR is purported to have enhanced affinity through stem-cell selection.
Other cell-based approaches
In addition to all the adoptive
cell therapy approaches above, our competitors are also investigating the potential of Gamma Delta T cell, Chimeric Antigen Receptor
- Natural Killer (CAR-NK) cell, Natural Killer (NK) cell, (NKT) cell and Cytotoxic T-cells (CTLs) either in a preclinical or clinical
setting (both hematologic malignancies and solid tumors). In addition, Bristol Myers Squibb’s Abraxane is used for pancreatic
cancer.
For RENB-HV-12, we are aware of
a few biotech companies developing an HIV vaccine such as Geovax, Biosantech SA, and FIT Biotech, among a few others.
For RENB-HV-01,
we are aware of two companies developing a gene therapy for HIV/AIDS: Sangamo and American Gene Technology.
For RENB-HB-01, there
is an approved vaccine to prevent HBV infection. In addition, several approved combination antivirals can suppress replication,
but do not cure HBV. Several companies are pursuing cures, mostly targeting the depletion of ccc-DNA.
Manufacturing
Our intent is to rely on contract manufacturing organizations (CMOs) and contract
development and manufacturing organizations (CDMOs), to help develop processes and manufacture our product candidates in accordance with
FDA and EMA mandated regulations, also known as current good manufacturing practices, (“cGMPs”). We employ a technical operations
staff in the areas of process development, analytical development, quality control, quality assurance, project management and manufacturing,
which will facilitate appropriate oversight of our CMOs, support of our regulatory filings and execution of clinical trials.
Government Regulation
FDA Review and Approval
Government authorities in
the United States, at the federal, state, and local levels, and in other countries extensively regulate, among other things, the
research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion,
advertising, distribution, post-approval monitoring and reporting, marketing and export and import of products such as those we
are developing. Any products we develop will require regulatory review and allowance to proceed prior to conducting clinical trials
and additional regulatory approvals prior to commercialization. In the United States, the FDA regulates drugs under the Federal
Food, Drug and Cosmetic Act (FDCA) and the Public Health Service Act (PHSA) and their implementing regulations govern, among other
things, biopharmaceutical testing, manufacturing, safety, efficacy, labeling, storage, recordkeeping, advertising, and other promotional
practices.
Obtaining FDA approval is
a costly and time-consuming process. Generally, FDA approval requires that preclinical studies be conducted in the laboratory and
in animal model systems to gain preliminary information on efficacy and to identify any major safety concerns. The results of these
studies are then submitted as a part of an IND, which the FDA must review and allow before human clinical trials can start. The
IND includes a detailed description of the proposed clinical investigations. An independent Institutional Review Board (“IRB”)
must also review and approve the clinical protocol and each clinical site.
A company must submit an
IND for each investigational medical product and specific indication(s) and must conduct clinical studies to demonstrate the safety
and efficacy of the product necessary to obtain FDA approval. The FDA receives reports on the progress of each phase of clinical
testing and may require the modification, suspension or termination of clinical trials if an unwarranted risk is presented to
participants including patients.
Obtaining FDA approval prior
to marketing a biopharmaceutical product in the United States typically requires several phases of clinical trials to demonstrate
the safety and efficacy of the product candidate. Clinical trials are the means by which experimental treatments are tested in
humans and are conducted following preclinical testing. Clinical trials may be conducted within the United States or in foreign
countries. If clinical trials are conducted in foreign countries, the products under development as well as the trials are subject
to regulations of the FDA and/or its regulatory counterparts in the other countries. Upon successful completion of clinical trials,
approval to market the treatment for a particular patient population may be requested from the FDA in the United States and/or
its counterparts in other countries.
Clinical trials for therapeutic products are normally conducted in three phases.
Phase 1 clinical trials are typically conducted with a small number of subjects/patients to evaluate the safety, determine a safe dosage
range, identify side effects and, if possible, gain early evidence of effectiveness. Phase 2 clinical trials are conducted with a larger
group of patients to evaluate the effectiveness of an investigational product for a defined patient population and to determine common
short-term side effects and risks associated with the drug. Phase 3 clinical trials involve large scale, multi-center, comparative trials
that are conducted to evaluate the overall benefit-risk relationship of the investigational product and to provide an adequate basis for
product labeling. In some special cases where the efficacy testing of a product may present a special challenge to testing in humans,
such as in the case of a vaccine to protect healthy humans from a life-threatening disease that is not a naturally occurring threat, effectiveness
testing may be required in animals. For certain advanced therapies that meet eligibility criteria for expedited program designations,
clinical development may be accelerated.
Clinical trials involve
the administration of the treatment/drug product candidate to healthy volunteers or patients under the supervision of qualified
investigators who generally are physicians not employed by, or under, the control of the trial sponsor. Clinical trials are conducted
under written study protocols detailing, among other things, the objectives of the clinical trial, dosing procedures, subject selection
and exclusion criteria and the parameters to be used to monitor subject safety, including stopping rules that assure a clinical
trial will be stopped if certain adverse events should occur. Each protocol and any amendments to the protocol must be submitted
to the FDA as part of the IND. Clinical trials must be conducted and monitored in accordance with the FDA’s regulations comprising
the Good Clinical Practice (“GCP”) requirements, and any additional requirements for the protection of human research
subjects and their health information including the requirement that all research subjects provide informed consent.
Further, each clinical trial
must be reviewed and approved by an IRB at or servicing each institution at which the clinical trial will be conducted. An IRB
is charged with protecting the welfare and rights of trial participants and considers items such as whether the risks to individuals
participating in the clinical trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves
the form and content of the informed consent that must be signed by each clinical trial subject, or their legal representative,
reviews and approves the study protocol, and must monitor the clinical trial until completed. Clinical trials involving recombinant
DNA also must be reviewed by an institutional biosafety committee, or IBC, a local institutional committee that reviews and oversees
basic and clinical research that utilizes recombinant DNA at that institution. The IBC assesses the safety of the research and
identifies any potential risk to public health or the environment.
After completion of clinical
trials of a new product, FDA marketing approval must be obtained. If the product is regulated as a biologic, a Biologics License
Application, or BLA, is required. If the product is classified as a new drug, a New Drug Application, or NDA is required. The NDA
or BLA must include results of product development activities, preclinical studies and clinical trials in addition to detailed
chemistry, manufacturing and control information.
Applications submitted to
the FDA are subject to an unpredictable and potentially prolonged approval process. Despite good-faith communication and collaboration
between the applicant and the FDA during the development process, the FDA may ultimately decide, upon final review of the data,
that the application does not satisfy its criteria for approval or requires additional product development or further preclinical
or clinical studies. Even if FDA regulatory approval(s) are obtained, a marketed product is subject to continual review, and later
discovery of previously unknown problems or failure to comply with the applicable regulatory requirements may result in restrictions
on the marketing of a product or withdrawal of the product from the market as well as possible civil or criminal sanctions.
Before marketing approval can be secured for a product, the facility in which
the product is manufactured must be inspected by the FDA and must comply with the FDA’s cGMP regulations. In addition, after marketing
approval is secured, the manufacturing facility must be inspected periodically for cGMP compliance by FDA inspectors, and, if the facility
is located in California, by inspectors from the Food and Drug Branch of the California Department of Health Services. As of the date of this proxy statement, we have made substantial progress
toward demonstrating cGMP compliance and Chemistry, Manufacturing and Controls Management (CMC) required for FDA regulatory approval.
Sponsors of clinical trials
are required to register, and report results for, all controlled, clinical investigations, other than Phase 1 investigations, of
a product subject to FDA regulation. Trial registration may require public disclosure of certain confidential commercial development
data.
The process of obtaining
regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations
require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at
any time during the product development process, approval process or after approval, may subject an applicant to administrative
or judicial sanctions. FDA sanctions could include, among other actions, refusal to approve pending applications, withdrawal of
an approval, a clinical hold, warning letters, product recalls or withdrawals from the market, product seizures, total or partial
suspension of production or distribution injunctions, fines, refusals of government contracts, restitution, disgorgement or civil
or criminal penalties. Any agency or judicial enforcement action could have a material adverse effect on our business, financial
condition, results of operations and cash flows.
The FDA offers several programs
to expedite the development of products that treat serious or life-threatening illnesses and that provide meaningful therapeutic
benefits to patients over existing treatments.
RMAT designation:
A drug is eligible
for designation as an RMAT if: the drug is a regenerative medicine therapy, which is defined as a cell therapy, therapeutic tissue
engineering product, human cell and tissue product or any combination product using such therapies or products, except for those
regulated solely under certain other sections; the drug is intended to treat, modify, reverse or cure a serious or life-threatening
disease or condition; and preliminary clinical evidence indicates that the drug has the potential to address unmet medical needs
for such disease or condition. Some of our current and future products may be eligible for RMAT designation.
Orphan designation:
Under the Orphan
Drug Act, the FDA may grant orphan designation to a product intended to treat a rare disease or condition, which is generally a
disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the
United States and for which there is no reasonable expectation that the cost of developing and making a product available for this
type of disease or condition will be recovered from sales of the product. Orphan designation must be requested before submitting
an NDA or BLA. Orphan designation does not convey any advantage in or shorten the duration of the regulatory review and approval
process. If a product that has orphan designation subsequently receives the first FDA approval for such product for the disease
or condition for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA
may not approve any other applications to market a product containing the same active moiety for the same use or indication for
seven years,
except in limited circumstances, such as a showing of clinical superiority to the product with orphan exclusivity.
A product is clinically superior if it is safer, more effective or makes a major contribution to patient care. Any claims of clinical
superiority could require a head-to-head clinical trial between such drugs. Competitors may receive approval of different products
for the indication for which the orphan product has exclusivity or obtain approval for the same product but for a different indication
for which the orphan product has exclusivity. If a product designated as an orphan product receives marketing approval for an indication
broader than what is designated, it may not be entitled to orphan product exclusivity.
Other Healthcare
Laws and Compliance Regulations
Although we currently do not have any products on the market, we may also be subject
to additional healthcare regulation and enforcement by the federal government and by authorities in the states and foreign jurisdictions
in which we conduct our business. In the United States, among other things, the research, manufacturing, distribution, sale and promotion
of pharmaceutical and biological products are potentially subject to regulation and enforcement by various federal, state and local authorities
in addition to the FDA, including the CMS, other divisions of the United States Department of Health and Human Services (e.g., the Office
of Inspector General), the Drug Enforcement Administration, the Consumer Product Safety Commission, the Federal Trade Commission, the
Occupational Safety and Health Administration, the Environmental Protection Agency, state Attorneys General and other state and local
government agencies. Our current and future business activities, including for example, sales, marketing, and scientific/educational grant
programs, must comply with health care regulatory laws, as applicable, including, without limitation:
| ● | the federal anti-kickback statute, which is a criminal statute that makes it a felony for individuals
or entities to knowingly and willfully offer or pay, or to solicit or receive, direct or indirect remuneration, in order to induce
the purchase, order, lease, or recommending of items or services, or the referral of patients for services, that are reimbursed
under a federal health care program, including Medicare and Medicaid; |
| ● | the federal False Claims Act, which prohibits, among other things, individuals and entities from
knowingly submitting, or causing to be submitted, false or fraudulent claims for payment of government funds, with penalties that
include three times the government’s damages plus civil penalties for each false claim; in addition, the False Claims Act
permits a person with knowledge of fraud, referred to as a qui tam plaintiff, to file a lawsuit on behalf of the government against
the person or business that committed the fraud, and, if the action is successful, the qui tam plaintiff is rewarded with a percentage
of the recovery; |
| ● | federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program
or making false statements relating to healthcare matters; |
| ● | the Health Insurance Portability and Accountability Act of 1996, or HIPAA, which governs the conduct
of certain electronic healthcare transactions and protects the security and privacy of protected health information; |
| ● | the federal Physician Payments Sunshine Act, which requires certain manufacturers of drugs, devices,
biologics and medical suppliers to report annually to CMS information related to payments and other transfers of value to physicians,
other healthcare professionals and teaching hospitals, and ownership and investment interests held by physicians and other healthcare
professionals and their immediate family members; and |
| ● | state and foreign law equivalents of each of the above federal laws, such as state anti-kickback
and false claims laws which may impose stricter requirements than federal law and may apply to items or services reimbursed by
any payor (including commercial insurers and cash-paying patients); state laws that require pharmaceutical companies to comply
with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the
federal government or otherwise restrict payments that may be made to healthcare professionals and other potential referral sources;
state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians
and other healthcare professionals or marketing expenditures; and state laws governing the privacy and security of health information
in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating
compliance efforts. |
If our operations are found
to be in violation of any of such laws or any other governmental laws or regulations that apply, they may be subject to penalties,
including, without limitation, civil and criminal penalties, damages, fines, disgorgement, the curtailment or restructuring of
operations, exclusion from participation in federal and state healthcare programs, additional program integrity obligations, individual
imprisonment, injunctions, recall or seizure of products, total or partial suspension of production, denial or withdrawal of product
approvals, refusal to permit us to enter into supply contracts, including government contracts, contractual damages, reputational
harm, administrative burdens, diminished profits, and future earnings, any of which could have a material adverse effect on our
business, financial condition, result of operations and cash flows. These additional healthcare regulations could affect our current
and future arrangements with healthcare professionals, principal investigators, consultants, customers and third-party payors.
Moreover, the introduction
of legislation, implementation of new regulations, or enforcement of existing regulations that have a negative impact on the commercial
prospects for the types of products we are developing could negatively impact our share price and our ability to raise capital.
Coverage
and Reimbursement
Significant uncertainty
exists as to the coverage and reimbursement status of any product candidate that receives regulatory approval. In the United States
and markets in other countries, sales of our product candidates, if approved, will depend, in part, on the extent to which third-party
payors provide coverage and establish adequate reimbursement levels.
In the United States, third-party
payors include federal and state healthcare programs, government authorities, private managed care providers, private health insurers
and other organizations. Third-party payors are increasingly challenging the price, examining the medical necessity and reviewing
the cost-effectiveness of medical drug products and medical services, in addition to questioning their safety and efficacy. Such
payors may limit coverage to specific drug products on an approved list, also known as a formulary, which might not include all
the FDA-approved drugs for a particular indication. Third-party payor coverage may be more limited than the purposes for which
the product is approved by the FDA or foreign regulatory authorities. Further, one payor’s determination to provide coverage
for a drug product does not assure that other payors will also provide coverage for the drug product.
Moreover, the process for
determining whether a third-party payor will provide coverage for a drug product may be separate from the process for setting the
price of a drug product or for establishing the reimbursement rate that such a payor will pay for the drug product. A payor’s
decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved or that the
product will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and
distribution. There may be significant delays in obtaining reimbursement for approved products, and reimbursement rates may fluctuate
over time or vary according to the use of the product or clinical setting in which a product is used. Net prices for products may
be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation
of laws that presently restrict imports of products from countries where they may be sold at lower prices than in the United States.
Further, third-party payers
are increasingly challenging the price of medical products and services, and there is increasing pressure on biotechnology companies
to reduce healthcare costs. If purchasers or users of our products are not able to obtain adequate reimbursement for the cost of
using our products, they may forgo or reduce their use. Significant uncertainty exists as to the reimbursement status of newly
approved healthcare products, and whether adequate third-party coverage will be available. Our inability to promptly obtain coverage
and profitable payment rates from both government funded and private payors for future products we develop could have a material
adverse effect on our operating results, our ability to raise capital needed to commercialize potential products, and our overall
financial condition.
Healthcare
Reform
In March 2010, former President
Obama signed into law The Patient Protection and Affordable Care Act and the Health Care and Education Affordability Reconciliation
Act of 2010 (collectively, the “Affordable Care Act”), which substantially changed the way healthcare is financed by
both governmental and private insurers in the United States and significantly affected the pharmaceutical industry. The Affordable
Care Act contains a number of provisions, including those governing enrollments in federal healthcare programs, reimbursement adjustments
and fraud and abuse changes. Additionally, the Affordable Care Act increases the minimum level of Medicaid rebates payable by manufacturers
of brand name drugs; requires collection of rebates for drugs paid by Medicaid managed care organizations; requires manufacturers
to participate in a coverage gap discount program, under which they must agree to offer point-of-sale discounts off negotiated
prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s
outpatient drugs to be covered under Medicare Part D; and imposes a non-deductible annual fee on pharmaceutical manufacturers
or importers who sell “branded prescription drugs” to specified federal government programs.
Since its enactment, there
have been judicial and Congressional challenges to certain aspects of the Affordable Care Act, and we expect there will be additional
challenges and amendments to the Affordable Care Act in the future. Other legislative changes have been proposed and adopted since
the Affordable Care Act was enacted, including aggregate reductions of Medicare payments to providers and reduced payments to several
types of Medicare providers. Moreover, there has recently been heightened governmental scrutiny over the manner in which manufacturers
set prices for their marketed products, which has resulted in several Congressional inquiries and proposed bills designed to, among
other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs,
and reform government program reimbursement methodologies for drug products. Individual states in the United States have also become
increasingly active in implementing regulations designed to control pharmaceutical product pricing, including price or patient
reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency
measures, and, in some cases, proposing to encourage importation from other countries and bulk purchasing. We cannot predict
what healthcare reform initiatives may be adopted in the future.
We also are subject to various federal, state and local laws, regulations,
and recommendations relating to safe working conditions, laboratory and manufacturing practices, the experimental use of animals and the
use and disposal of hazardous or potentially hazardous substances, including radioactive compounds and infectious disease agents, used
in connection with our research. The extent of government regulation that might result from any future legislation or administrative action
cannot be accurately predicted.
Foreign
Corrupt Practices Act
Our business activities may be subject to the FCPA and similar anti-bribery
or anti-corruption laws, regulations, or rules of other countries in which we operate. The FCPA generally prohibits offering, promising,
giving, or authorizing others to give anything of value, either directly or indirectly, to a non-U.S. government official in order to
influence official action, or otherwise obtain or retain business. The FCPA also requires public companies to make and keep books and
records that accurately and fairly reflect the transactions of the corporation and to devise and maintain an adequate system of internal
accounting controls. Our business is heavily regulated and therefore involves significant interaction with public officials, including
officials of non-U.S. governments. Additionally, in many other countries, the health care providers who prescribe pharmaceuticals are
employed by their government, and the purchasers of pharmaceuticals are government entities; therefore, our dealings with these prescribers
and purchasers are subject to regulation under the FCPA. There is no certainty that all of our employees, agents, suppliers, manufacturers,
contractors, or collaborators, or those of our affiliates, will comply with all applicable laws and regulations, particularly given the
high level of complexity of these laws. Violations of these laws and regulations could result in fines, criminal sanctions against us,
our officers, or our employees, the closing down of facilities, including those of our suppliers and manufacturers, requirements to obtain
export licenses, cessation of business activities in sanctioned countries, implementation of compliance programs, and prohibitions on
the conduct of our business. Any such violations could include prohibitions on our ability to offer our products in one or more countries
as well as difficulties in manufacturing or continuing to develop our products, and could materially damage our reputation, our brand,
our international expansion efforts, our ability to attract and retain employees, and our business, prospects, operating results, and
financial condition.
Employees
As of November 30, 2023,
we had 13 full-time employees. In July 2022, Renovaro began to streamline the organization to focus around two of its therapies
(oncology and HIV therapeutic vaccine). Renovaro has tailored its workforce to focus on these therapies. We believe that we have
good relations with our employees.
Corporate Information
On September 28, 2023, Renovaro
entered into the Stock Purchase Agreement with GEDi Cube. Upon the terms and subject to the conditions set forth in the Stock Purchase
Agreement, Renovaro will acquire 100% of the GEDi Cube Shares from the Sellers and GEDi Cube will become a wholly-owned subsidiary
of Renovaro. On September 28, 2023, the Renovaro Board and the board of managers of GEDi Cube unanimously approved the Stock Purchase
Agreement. The completion of the Transaction is subject to the satisfaction or waiver of customary closing conditions. The Stock
Purchase Agreement contains certain termination rights for both Renovaro and GEDi Cube described herein.
There can be no assurance
that the Transaction will be fully realized or may take longer to realize than expected; the possibility that stockholders of Renovaro
may not approve the issuance of new shares of Common Stock in the proposed Transaction; the risk that a condition to closing of
the proposed Transaction may not be satisfied, that either party may terminate the Transaction Agreement or that the Closing of
the proposed Transaction might be delayed or not occur at all.
We trade on Nasdaq under
the ticker “RENB.”
Our website is http://www.renovarobio.com.
We make available free of charge, on or through our website, our annual, quarterly, and current reports and any amendments to those
reports filed or furnished pursuant to Section 13(a) of the Exchange Act as soon as reasonably practicable after we electronically
file such material with, or furnish it to, the SEC. Information contained in our website is not part of, nor incorporated by reference
into, this proxy statement.
Legal Proceedings
For a description of material legal proceedings
to which Renovaro is a party, please refer to the section titled “Item 3. Legal Proceedings” set forth in the Renovaro
2023 Form 10-K and the section titled “Item 1. Legal Proceedings” set forth in the Renovaro First Quarter 2024 Form
10-Q, each of which is incorporated by reference herein.
Property
For a description of Renovaro’s property,
please refer to the section titled “Item 2. Properties” set forth in the Renovaro 2023 Form 10-K, which is incorporated
by reference herein.
GEDI CUBE’S BUSINESS
Overview
GEDi Cube is an AI-driven
healthcare technology company focusing on the earliest possible detection of cancer and its recurrence. GEDi Cube has developed
a proprietary AI platform that analyzes genetics using Explainable AI (as defined below) to provide earlier and more accurate cancer
diagnosis. This platform uses a multi-omics approach to search for individual biomarkers that are present even in asymptomatic
patients. This approach is combined with differential molecular capabilities that are designed to identify, differentiate and pinpoint
the exact source. GEDi Cube’s process also involves the mining of biomarker panels, which are integrated into a machine learning
library referred to as “GEDiCube” to further enhance diagnosis.
GEDi Cube also aims to utilize
its proprietary AI platform in the development of commercial products to support clinical, research and pharmaceutical organizations
that are trying to improve patient care through precision diagnosis, prediction of success of therapy, new drug discovery, treatment
protocols or clinical trials. Specifically, GEDi Cube specializes in developing products and services aimed at (i) early cancer
characterization, (ii) personalized treatment selection, (iii) prediction and tracking response to therapies, (iv) recurrence detection
and efficacy monitoring, and (v) ultimately, drug discovery.
GEDi Cube was initially
incorporated as Grace Systems B.V. (“Grace Systems”) in 2013 under the laws of the Netherlands to develop unique data
mining algorithms to enable banking, finance and government entities to extract business insights from data. Grace Systems began
applying its algorithms to biological data in 2018 to uncover cancer-associated patterns. Beginning in 2018, Grace Systems pivoted
its platform to focus only on healthcare. On August 22, 2023, GEDi Cube Intl. Ltd. (formerly known as GEDi Health Group Ltd.),
initially incorporated in June 2023, acquired 100% of the shares of Grace Systems. Since this acquisition, GEDi Cube has focused
on developing its AI technology for early cancer detection and its recurrence and has developed a pipeline of in silico validated
biomarker panels for 13 types of cancer.
GEDi Cube has now focused
on commercialization of its AI technology. GEDi Cube believes that it has developed a unique approach to the early detection and
diagnosis of cancer and its recurrence and, in time, other rare diseases through the systematic analysis of data using AI technologies,
data mining procedures and algorithms for health technology.
GEDi Cube’s technology
has also been applied to complex heterogeneous cancer data and has been shown to find patterns associated with cancer in public
and private data resources. With the help of GEDi Cube’s algorithms, discovered patterns may be translated into biomarkers
that can be used in a clinical setting to target various aspects of cancer diagnosis and treatment.
Cancer is a complex set
of diseases. The abnormalities that cause cancer and drive its progression can often be observed at multiple molecular layers,
including genome, transcriptome, proteome, and even microbiome. The growing prominence of large multi-omics datasets resulting
from research studies give GEDi Cube the opportunity to integrate wide-spread molecular alterations underlying cancer and gain
actionable knowledge in a precision medicine framework. However, the heterogeneous nature of cancer makes data highly divergent
from patient to patient, which makes it difficult to extract meaningful patterns that can be utilized in a clinical setting. GEDi
Cube hopes to address the need for more powerful tools to extract information from big data and augment the ability of clinicians
to identify cancer quickly and accurately.
GEDi Cube’s Strategy
GEDi Cube’s product
development focuses on four core areas:
| ● | Early Detection. Multi-cancer early detection (“MCED”) blood tests are advanced diagnostic tools that analyze
cell-derived molecules present in the bloodstream. These tests specifically look for abnormal genetic, epigenetic or proteomic
patterns of these cell-derived molecules, which can indicate the presence of cancer cells. By examining the molecules shed from
various cells, including cancer cells, MCED tests aim to detect cancer at an early stage. This approach holds promise for improving
cancer detection and potentially saving lives.
|
| ● | Recurrence of cancer. A recurrence refers to the return of cancer after a period of remission.
A cancer recurrence happens because, in spite of the efforts to kill the cancer, some cells may remain, which grow and eventually
cause symptoms. In rare instances, a patient may develop a new cancer that’s completely unrelated to the originally diagnosed
cancer, which is referred to as a second primary cancer. An early warning system could help to identify a recurrence as early as
possible, thereby helping to accelerate any the treatment and diagnosis. The different types of recurrence include: |
| o | Local recurrence, meaning that the cancer has returned in the same place it first started; |
| o | Regional recurrence, meaning that the cancer has returned to the lymph nodes near the place
it first started; and |
| o | Distant recurrence, meaning the cancer has returned in another part of the body. |
| ● | Rare Disease. In the United States, the government defines a disease as rare if it impacts
fewer than 200,000 individuals. The European Union defines a rare disease as a condition which affects less than 1 in 2,000 people.
According to the National Institutes of Health, it is currently estimated that there are 7,000 known rare diseases, with
new conditions continually being identified as research advances. Certain cancers are classified as rare disease. While rare diseases
may have an identified genetic origin, they can also be caused by disordered immunity, infections, allergies, deterioration of
body tissues and organs or disruption to development while in the womb. Although rare diseases are individually rare they can be
collectively common, with 1 in 17 people in the United Kingdom being affected by a rare disease at some point in their lifetime
according to the Genetic Alliance U.K. One of GEDi Cube’s goals is to support the diagnostic process of finding a rare disease,
which can sometimes take years. |
| ● | Clinical trials. Clinical trials involve a type of research that studies new tests and treatments
and evaluates their effects on human health outcomes. People volunteer to take part in clinical trials to test medical interventions
including drugs, cells and other biological products, surgical procedures, radiological procedures, devices, behavioral treatments
and preventive care. Clinical trials are carefully designed, reviewed and completed, and need to be approved before they can start. |
In response to these four
core areas, the key components of GEDi Cube’s product development are to build a software and hardware platform that:
| ● | Uses data science to develop novel insights into the characterization of diseases such as cancer.
GEDi Cube intends to apply its proprietary technology to biological data from multiple sources to enable the typification (or classification)
of disease entities and sub-entities to provide insights about the nature and behavior of diseases to payers, providers, pharmaceutical
companies and patients. |
| ● | Enables more accurate diagnosis and earlier detection of cancer and other diseases with the goal
of maximizing outcomes and minimizing the costs of treatment. GEDi Cube intends to develop a system to understand the smallest
fragments of cancer in the blood of the patient. Presently, GEDi Cube is developing a product to analyze results from liquid biopsy
run through an Oxford Nanopore Sequencer. The GEDi Cube product will subsequently identify, train and validate explainable biomarkers,
panels and models on different molecular layers. Multiple models will be individually trained for optimal stratification through
the entire health journey, ensuring the right accuracy for a therapeutic decision in every stage. GEDi Cube will integrate different
modalities and molecular data sources into a differential diagnostic report. Diagnostics and prognosis will be explainable with
quality control reports and biomarker insights for different disciplines ensuring maximum trust and insight in medical decision
making. |
| ● | Assists in clinical trials with patient cohort selection and response tracking, to be used by companies
like Renovaro on their patient cohort selection for their clinical trials, by looking at which patients are reacting positively,
negatively and have no reaction. This data becomes more important through the progression of the different phases of drug development,
as more and more patients are added. GEDi Cube will provide multi-omic data analysis, looking for specific changes in the patients
that might indicate a change in their molecular make-up. This can then be used for the next phase of a clinical trial to look for
the specific molecular data that has showed a positive reaction in the previous phase. It also provides insights for more effective
response tracking, which GEDi Cube believes is important to the providers of care as well as the development and evaluation of
new pharmaceuticals and immune therapies in clinical trials. Patient response to treatment can be used to focus the target audience
for drugs in development and in subsequent clinical practice. As GEDi Cube collects more data, longitudinal about treatment and
response, it will have the ability to train prognostic models to give an insight in disease progression and treatment response,
both critical for enrolling in clinical trials and eventually every treatment. Because GEDi Cube consists of many independently
trained and validated models, it will have the ability to assist in virtually every therapeutic decision, for different subtypes
and groups (stratifications). The multi-omics and multi-modal pipelines could allow the use of multiple combinations of tissue
samples and diagnostic platforms. The detailed diagnostic reports will allow and support insights for multiple disciplines such
as cancer biology, genomics and pathology to look at underpinning biomarkers, pathways and clinical annotations. |
| ● | Provides insight into patients who have had cancer previously. These insights will provide for
more effective recurrence monitoring, which GEDi Cube believes is important to the providers of care and patients during follow-up
monitoring of remissions. GEDi Cube anticipates that payers want to detect and re-treat recurrences at the earliest possible stage
to maximize patients’ outcomes in terms of time and cost and that, similarly, patients with a recurrence are keen to re-engage
with effective treatment at the earliest opportunity. A key aspect of this will be taking blood from the patients, sequencing this
blood and running it through the GEDi Cube platform which will identify if the patient has any indication of the recurrence of
the same or a new cancer. For recurrence monitoring, GEDi Cube will focus on a highly sensitive combination of lab and information
technology. Lab protocols, sequence post processing and machine learning are all designed, trained and validated to get the best
signal with the highest sensitivity to catch early signals of recurrence. This will be done on a regular basis allowing surveillance
analysis over time. |
| ● | Create value through advancing more sophisticated typification of diseases in an effort to address
some of the pressing problems faced by modern healthcare, including healthcare costs, an aging population and developments in medical
technology that produce a stream of increasingly sophisticated treatments requiring more precise targeting. |
One additional key focus
of GEDi Cube is its multi-modal data analysis. Multi-modal data encompasses the whole aspect of data from a patient perspective,
whether genomics, imaging, phenotypic or even wearable data, which can be cross-analyzed to produce data that could not be previously
produced. GEDi Cube intends to use multi-modal data to bring new insights to the clinical and research teams trying to understand
what to do next with the patient.
GEDi Cube’s Technology and Techniques
GEDi Cube is dedicated to the development of
early cancer detection blood tests and expects to develop partnerships with third-party laboratories across the United Kingdom,
the Netherlands, elsewhere in Europe and the United States and others, as needed. In particular, GEDi Cube is focused on developing
tests and test kits that would analyze samples derived from non-invasive liquid biopsy samples and intends to engage third-party
laboratories to perform these tests for end-users.
For this purpose, GEDi Cube has developed an
AI platform that aims to leverage expertise in both biological and computational sciences and to go beyond traditional tumor signals
by detecting the body’s early warning signs of cancer. GEDi Cube’s goal is to provide accurate and reliable tests that
can aid in the early diagnosis and treatment of cancer. GEDi Cube’s AI technology is created to detect a wide range of biological
signs to enhance the accuracy and sensitivity of early cancer detection and, thereby, enable earlier intervention and potentially
improved patient outcomes.
GEDi Cube’s AI technology
aims to address three critical facets of medical needs within the domain of cancer diagnosis (as illustrated in below):
| ● | type-specific cancer detection; |
| ● | pan-cancer detection; and |

Moreover, the versatility of GEDi Cube’s
AI technology extends to encompass the realm of rare cancers, including cases such as cancer of unknown primary.
Leveraging DNA methylation
data, GEDi Cube has identified and validated biomarker panels tailored for the detection of a wide range of cancers, including
bladder, breast, colon, prostate, thyroid, head and neck, liver, kidney and lung cancer.
The foundational architecture
of GEDi Cube’s AI technology is engineered to facilitate comprehensive pan-cancer analysis through its extensive record of
informative biomarkers discovered across a diverse array of cancer types. This comprehensive repository empowers GEDi Cube’s
AI technology to swiftly cross-reference biomarkers and explore molecular commonalities and distinctions that span multiple tumor
categories.
For example, the capabilities
of GEDi Cube’s AI technology have unearthed biomarkers capable of pinpointing a specific subgroup of thyroid cancer patients
characterized by a distinct genomic alteration, the neurotrophic tyrosine receptor kinase (“NTRK”) gene fusion. Identification
of these NTRK-positive patients provides an actionable therapeutic target.
Uses of GEDi Cube’s AI Technology
GEDi Cube has developed its AI platform to support:
| ● | AI-assisted patient diagnostics; |
| ● | multi-omic data analysis; |
| ● | genome-wide or targeted analysis; |
| ● | different technology platforms (sequence or array); |
| ● | tracking of each sample; |
| ● | AI-guided biomarker discovery for single or multiple cancer types; and |
| ● | logs of analysis steps and outcomes (data preparation, discovery, validation). |
GEDi Cube’s AI platform
is an enterprise software platform that is distinguished from its competitors’ technology by its core attributes encompassing
AI-guided analysis and meticulous record-keeping of data handling procedures within audit trails, logs, and data discoveries. GEDi
Cube designed this technology to support and validate every phase of the process, from the initial handling of raw data to the
creation of essential biomarker panels. GEDi Cube’s AI platform also facilitates the integration of data originating from
diverse sources, including public databases and collaborative partnership data.
Illustrated below is the
three-phase workflow behind GEDi Cube’s AI platform for biomarker discovery using DNA methylation data. This workflow commences
with the identification of pertinent single- and multi-omic data best suited to address the specific inquiries of GEDi Cube’s
clients, and the subsequent stages involve the meticulous pre-processing and loading of this data into the platform. This process
culminates in the availability of a dashboard offering the client insights into the data’s characteristics, such as data
quality, the technology employed, and associated metadata.

| ● | Phase I of the workflow behind GEDi Cube’s AI platform primarily centers on the pivotal process
of biomarker discovery. This intricate procedure unfolds through the application of data mining algorithms and statistical methodologies
integrated into the AI platform. The paramount objective of Phase I is to reduce the plethora of genomic features displaying variations
across samples, which is accomplished by systematically eliminating extraneous or inconsequential features while preserving those
features that exhibit the greatest potential for accurately detecting cancer. |
| ● | Phase II of the workflow builds upon the foundation of selected biomarkers by focusing on understanding
the dynamic interplay among these chosen biomarkers, culminating in the creation of composite panels. The goal of Phase II is to
pinpoint biomarker combinations that not only demonstrate robustness in detecting cancer but also maintain their efficacy across
diverse contexts. GEDi Cube believes that its AI algorithms are adept at uncovering multiple combinations across a spectrum of
panels, which is supported by GEDi Cube’s AI-guided panel mining, a proprietary combinatorial optimization technique used
by GEDi Cube’s AI technology. This approach, coupled with the capacity to explore numerous panels, significantly enhances
the likelihood of discovering panels that align with specific metric criteria, such as sensitivity, specificity, precision, and
recall and allows for tailoring criteria to align with clients’ unique needs, such as the number of biomarkers included per
panel or the inclusion of biomarkers associated with the expression of specific genes. The performance of the top-tier panels is
further fine-tuned through the application of machine learning models. Subsequently, the efficacy of these biomarker panels in
detecting cancer is validated through independent data sets. |
| ● | Phase III of the workflow involves GEDi Cube’s collaboration with its clinical partners to
validate the performance of the biomarker panels. Through this collaboration, GEDi Cube is able to confirm the utility and accuracy
of its biomarker panels in real-world clinical contexts. |
AI-Assisted Diagnostics
The process of biomarker
discovery facilitated by GEDi Cube’s AI technology has yielded a set of data that enables scrutiny of the genomic distinctions
and commonalities inherent in diverse cancer types. This data set can support the diagnosis of cancers when their type or origin
remains unidentified.
In addition to this role
in biomarker discovery and the development of diagnostic tests, GEDi Cube’s AI technology also integrates AI-guided molecular
profiling of patient samples and furnishes diagnostic patient reports. These diagnostic reports reflect the outcomes of molecular
profiling, coupled with interpretations provided by GEDi Cube’s team, to facilitate the process of cancer diagnostics by
a qualified healthcare provider, who can consider these reports in the context of a patient’s medical history, clinical signs,
and symptoms, among other factors.
Quality Control Process
GEDi Cube undertakes post-processing
of data generated from sequence and arrays in an effort to ensure accurate and meaningful results. These post-processing steps
for omic data include:
| 1. | Quality Control: Quality control is performed to assess the overall data quality and to
identify any technical issues or anomalies. |
| 2. | Normalization: arrays can introduce various sources of technical variation, such as batch
effects, intensity variations, and probe-specific biases. |
| 3. | Quality Filtering: After genotype calling, additional quality filtering may be performed
to remove low-quality SNPs based on criteria like call rates, minor allele frequency, Hardy-Weinberg equilibrium p-values, and
linkage disequilibrium. |
Other post-processing steps
may include genotype calling, population stratification and association analysis. Specific post-processing steps may vary depending
on the type of array used, the study design, and the analytical goals.
Planning for Commercialization
Commercial Relationships
Existing Partnerships
NVIDIA
Corporation (“NVIDIA”) pioneered accelerated computing to tackle challenges no one else can solve. NVIDIA’s work
in AI and the industrial metaverse is aimed at transforming the world’s largest industries and profoundly impacting society.
GEDi is a formal Inception Partner of Nvidia, having signed a contract with them. This partnership gives GEDi access to their resources
for infrastructure and scaling, and specific advice and guidance from their Inception Team.
Partnerships in Development
To enhance multi-omic and
multi-modal capacity, and to work to validate those capabilities with human samples including liquid-biopisy-based tests/test kits,
GEDiCube is actively pursuing relationships with leading academic cancer centers, pathology and imagery centers in Europe, the
USA and the Middle East. In certain cases, scopes of work are in process.
Resources
GEDi Cube intends to hire
additional staff to increase the speed and velocity of its organization, including the development of the AI platform and the opportunities
to deploy the AI platform for research perspective and ultimately for clinical practice and into clinical trials.
In addition, GEDi Cube intends
to build out its infrastructure by leasing space for storage, networking and hosting facilities as well as a space to facilitate
our build of a supercomputer cluster.
Market Segments
GEDi Cube’s intended
customers will be hospitals, clinics, insurance companies, pharmaceutical companies, biotech companies, research centers, physicians
and individual patients.
GEDi Cube aims to utilize
its AI technology to commercialize products and test kits for healthcare providers, hospitals, clinics and doctors that will expedite
diagnosis and the selection of appropriate treatment for various types of cancer. GEDi Cube intends to differentiate its products
based on the following factors:
| ● | Proprietary and unique panel mining algorithms to create multiple biomarker stratifications per cancer; |
| ● | Explainable AI, offering traceability between the prediction and the exact biomarkers, panels and genes; |
| ● | Differential diagnosis, inclusion and exclusion of cancer types based on facts; and |
| ● | Precision diagnosis, with a high accuracy percentage with machine-learning tuning. |
The multi-omic design of
GEDi Cube’s AI platform enables the use of different molecular layers, such as epigenomics, transcriptomics, and metabolomics,
together with genomics and clinical data.
Panel Mining
The unique panel mining
technique in GEDi Cube’s technology repeatedly investigates genes to identify relevant biomarkers. The proprietary technique
in GEDi Cube’s technology not only searches for individual biomarkers, but also integrates validated panels for different
cancer types into the “GEDiCube” machine learning library. This process enables precision diagnosis, by including one
cancer and excluding others on the basis of statistically, scientifically and clinically validated machine-learning panels.
Panel mining is designed
to combine biomarkers into panels in such a way that the final panel meets:
| ● | performance metric criteria; |
| ● | technical criteria, such as a minimum or maximum amount of biomarkers for the selected assay; |
| ● | biological criteria, non-annotated genes inclusion; and |
| ● | stratification criteria. |
Explainable AI
The term “Explainable
AI” refers to the ability of an AI system or model to provide human-understandable explanations for its decision-making process
or predictions. This feature aims to bridge the gap between the “black box” nature of many AI algorithms and the need
for transparency, interpretability, and accountability in AI applications.
In traditional machine learning
approaches, such as deep neural networks, the internal workings of the model can be complex and difficult to interpret. This lack
of interpretability poses challenges in critical domains where decisions have significant implications, such as healthcare.
GEDi Cube believes that
Explainable AI is crucial for ensuring transparency, fairness, and accountability in AI systems. GEDi Cube’s AI platform
includes Explainable AI by design. All data points, calculations and results are traceable, and all calculations are verifiable
and reproducible with the same result.
Disease prognosis is one
of the diagnostic capabilities of the Explainable AI feature of GEDi Cube’s technology. Disease prognosis gives more insight
for a specific patient that empowers healthcare providers, patients, and their families to make well-informed decisions about treatment,
care, and future planning, thereby enhancing patient-centered care, optimizing resource utilization, and contributing to improved
patient outcomes and quality of life.
Differential Diagnosis
GEDi Cube’s AI platform
offers differential diagnosis by design due to its approach with a multitude of models for different diseases and the ability to
include and exclude diseases.
Diseases like cancer are
very homogenous, meaning that markers like TP53 or BRCA are expressed with multiple cancers. To address this homogeneity, differential
diagnosis distinguishes between two or more conditions or diseases that share similar signs, symptoms or characteristics. The
goal of differential diagnosis is to consider and evaluate all possible diagnoses for the patient’s symptoms to determine
the most likely cause. Differential diagnosis therefore aims to identify the underlying condition accurately and guide appropriate
treatment and management strategies.
Differential diagnosis is important for several
reasons:
| 1. | Accurate Diagnosis: Differential diagnosis helps healthcare professionals arrive at the
correct diagnosis by systematically considering all possible explanations for the patient’s symptoms. This ensures that the
appropriate treatment and interventions are provided, leading to better patient outcomes. |
| 2. | Avoiding Misdiagnosis: Many medical conditions have similar or overlapping symptoms, and
misdiagnosis can have serious consequences. Differential diagnosis helps to avoid misdiagnosing one condition as another, preventing
unnecessary treatments, delays in appropriate care or potential harm to the patient. |
| 3. | Tailored Treatment: Different conditions require different treatments. Identifying the correct
diagnosis through differential diagnosis allows healthcare professionals to develop a targeted treatment plan based on the specific
condition, improving the chances of successful management and recovery. |
| 4. | Avoiding Overtreatment or Undertreatment: Some conditions may require aggressive interventions,
while others may resolve with minimal treatment or simply require symptomatic management. Differential diagnosis helps prevent
overtreating or undertreating patients by ensuring that interventions are appropriate for the specific condition. |
| 5. | Identifying Underlying Causes: In some cases, multiple conditions may present with similar
symptoms, but the underlying causes may be distinct. Differential diagnosis helps identify the root cause of the symptoms, which
is crucial for implementing effective long-term management strategies and preventing complications. |
In summary, differential
diagnosis is a critical process in healthcare that supports accurate identification of the underlying condition, tailored treatment
plans, and improved patient outcomes.
Lung Cancer Validation
GEDi Cube has received both
technical and bio-scientific independent validation of cohorts for lung cancer, which was carried out in June 2022 by the Antwerp
University Hospital in Antwerp, Belgium (the “UZA”). The goal of this study was to validate the performance (specifically
the sensitivity and specificity) of predicted lung-cancer diagnostic biomarkers identified by GEDi Cube’s AI platform on
a newly generated, independent dataset provided by the UZA. The average sensitivity of the machine learning models tested on the
samples was 95%, illustrating the capacity for the biomarkers to identify previously unseen cancer in concordance with the data
provided by UZA.
Precision Diagnostics
Precision diagnostics and
personalized care is about focusing on what is required for a specific patient, what are their individual needs, how can we understand
more about them to be more precise when delivering care and identifying the right clinical pathway to move them through treatment.
GEDi Cube technology will bring a high level of detail with multi-omic data, allowing individual genes to be identified and different
possible cancers under suspicion. Due to the structure of the data produced, GEDi Cube can bring it into any format of user interface,
documentation, or database. We will provide the flexibility to respond to the different requirements for delivering data to the
right person at the right place at the right time. We will comply to user interface guidance and usability regulations (WC3C) to
ensure that they meet the correct standards to enable the data to be displayed, read and understood. Personalized care is what
we all in the healthcare system are striving for, we are one component that can make a huge difference.
Future Development
In driving towards future
commercialization, GEDi Cube intends to undertake or continue the following activities to enable the development of its AI platform,
bolster the credibility of this platform and open up revenue opportunities:
| ● | Increase in-kind contribution projects with hospitals, research centers and pharmaceutical companies,
focusing on rare disease, cancer diagnosis and other opportunities for clinical trials; |
| ● | Work to develop long-term strategic partnerships with clinical organizations, research centers
and pharmaceutical companies to advance existing research in multi-modal analysis and open up potential revenue streams; |
| ● | Develop GEDi Cube’s multi-modal platform architecture and first prototypes, with the goal
of providing integrated multi-modal solutions that would be sold as a fully deployed and fully supported solution, subject to compliance
with regulatory standards for hosting and support and for production of algorithms being deployed in a clinical environment; |
| ● | Build GEDi Cube’s service and support models for its AI platform; |
| ● | Build a “supercomputer” that will be utilized for processing genomic data, the training
of algorithms and the development of GEDi Cube’s solutions; |
| ● | Continue business development across key territories in the Europe, Middle East, and Africa (“EMEA”)
region, starting in the United Kingdom, the Netherlands and Germany, under the management of in-country managers supported by centralized
teams in Amsterdam and London and progress to rolling out in the US leveraging the Renovaro US based staff; |
| ● | Deploy a sequencing lab in the EMEA region that will allow GEDi Cube to control the sample preparation
and medical device, thereby enabling faster commercialization analysis and quality control; and |
| ● | Expand its team to include biomedical scientists, data scientists, machine-learning engineers,
specialized medical doctors (oncologist, geneticist), high-performance-computer engineers and software engineers. |
Sales and Marketing
Given its
current stage of development, GEDi Cube has initiated the process of establishing a commercial organization and infrastructure.
In the first quarter of 2024, we anticipate welcoming a Chief Commercial Officer with medical expertise, who will recruit country
managers and collaborate with strategic partners across various European locations. Additionally, we plan to onboard product managers
to bolster all sales and marketing endeavors. The groundwork has already been laid with a high-quality branding and communications
organization, and we will unveil this in the upcoming months.
Our first
products for early cancer detection and recurrence monitoring, along with companion diagnostics tests under research use only restrictions
will initially be marketed to hospitals, clinics, and doctors, and we intend to launch image campaigns directed at these professional
organizations with the aim of raising awareness among patients of the potential of our early detection and monitoring process.
Emphasizing the benefits of our AI platform, we intend to highlight its capacity to detect cancers through our comprehensive multi-omics
approach.
Furthermore,
we are preparing a project to delineate cohort stratification for Renovaro’s future pancreatic cancer clinical trials. We
also intend to market certain of our products to biopharmaceutical companies, such as customized biomarker panels and then leverage
those strategic relationships to develop other unique products.
It’s
important to note that the rollout and implementation of our initiatives in different countries are contingent on securing additional
funding, which we expect will materialize as needed.
Competition
Once GEDi Cube achieves
commercialization, the products offered from its AI platform will compete in the testing and diagnostic products industry, which
is a competitive arena. GEDi Cube has competitors both in the Europe and abroad, including Grail, Inc., Exact Sciences Corporation
(Thrive Earlier Detection Corp.), Freenome, Inc. and Haystack Oncology, that have stated that they are developing tests designed
to detect cancer. GEDi Cube’s competitors have, or may have, substantially greater financial, technical and other resources,
such as larger research and development staff and well-established marketing and sales forces, and they may operate in jurisdictions
where lower standards of evidence are required to bring products to market. Several factors may influence GEDi Cube’s ability
to compete in this industry, including those discussed under “GEDi Cube’s business and results of operations will suffer
if it fails to compete effectively” under the heading “Risk Factors” beginning on page 22 in this proxy
statement. However, GEDi Cube intends to differentiate its products within the market using the factors discussed under “Planning
of Commercialization” above.
Intellectual Property
Proprietary Biomarker Discovery
GEDi Cube’s AI technology
contains a proprietary method of interactive and real-time biomarker discovery across omics and diseases. Biomarker discovery is
the process of identifying and validating molecules or characteristics, known as biomarkers, that are associated with a particular
disease, physiological condition, or biological process. Biomarkers can be various types of molecules, such as proteins, nucleic
acids, metabolites, or even physical characteristics. Biomarkers can then be stored for client intellectual property protection.
Searches for biomarkers or panels can be performed sequentially and in parallel, whereby in-silico cross-performance analysis can
be performed.
Employees
As of December 20, 2023, GEDi Cube has 11 full-time employees and four independent contractors, including two
full-time. From time to time, GEDi Cube may engage consultants or independent contractors in certain research and development activities.
None of GEDi Cube’s employees is represented by a labor union or covered by a collective bargaining agreement. GEDi Cube
believes that its relations with its employees are good.
RENOVARO’S MANAGEMENT’S DISCUSSION
AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
For Renovaro’s Management’s
Discussion and Analysis of Financial Condition and Results of Operations for the fiscal year ended June 30, 2023, and the quarterly
period ended September 30, 2023, please refer respectively to the section titled “Item 7. Management’s Discussion and
Analysis of Financial Condition and Results of Operations” set forth in the Renovaro 2023 Form 10-K, and the section titled
“Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations” set forth in
the Renovaro First Quarter 2024 Form 10-Q, each of which is incorporated by reference herein. Such sections should be read together
with the consolidated financial statements of Renovaro and accompanying notes appearing in the Renovaro 2023 Form 10-K and the
condensed consolidated financial statements of Renovaro and accompanying notes appearing in the Renovaro First Quarter 2024 Form
10-Q.
QUANTITATIVE AND QUALITATIVE DISCLOSURES
ABOUT MARKET RISK
Renovaro
Renovaro is a “smaller reporting
company” as defined by Rule 12b-2 of the Exchange Act, and is not required to provide this information.
GEDi Cube
GEDi Cube is not currently subject to the
reporting requirements of either Section 13(a) or 15(d) of the Exchange Act. If GEDi Cube was subject to such reporting requirements,
GEDi would likely qualify as a “smaller reporting company” as defined by Rule 12b-2 of the Exchange Act and, as such,
would not be required to provide this information.
GEDI CUBE’S MANAGEMENT’S DISCUSSION
AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following Management’s Discussion and Analysis of Financial
Condition and Results of Operations should be read together with GEDI Cube’s consolidated financial statements and the notes
related thereto appearing at the end of this proxy statement. As a result of many factors, including those factors set forth in
the section entitled “Risk Factors—Risks Related to GEDi Cube,” GEDi Cube’s actual results could differ
materially from the results described in or implied by the forward-looking statements contained in the following discussion and
analysis. You should carefully read the section entitled “Risk Factors—Risks Related to GEDi Cube” to gain an
understanding of the important factors that could cause actual results to differ materially from the forward- looking statements
contained in the following discussion and analysis. Please also see the section entitled “Forward-Looking Statements.”
Unless otherwise indicated or the context otherwise requires,
references in this Management’s Discussion and Analysis of Financial Condition and Results of Operations to “GEDi Cube”
refer to Grace Systems B.V. and its subsidiary prior to the closing of the acquisition of Grace Systems B.V. by GEDi Cube on August
23, 2023 (the “Share Acquisition Closing”) described in Note 3 to the unaudited consolidated financial statements of
GEDi Cube for the period ended September 30, 2023, included in this proxy statement and refer to GEDi Cube Intl Ltd. and its subsidiaries
following the Share Acquisition Closing.
Overview
GEDi Cube is an AI-driven healthcare technology company focusing
on the earliest possible detection of cancer and its recurrence. GEDi Cube specializes in data science and data mining and plans
to be disruptive in the industry with a broad range of cutting edge software, services, methods and models to tackle big data challenges.
GEDi Cube provides services in the field of software development and maintenance, data mining and data science and focuses on the
high-end of advanced and predictive analytics, which contributes to its ability to combine big data stores, execute scientific
algorithms and visualize analytics to business users. GEDi Cube aims to provide and accelerate precision, personalized medicine
for longevity powered by mutually reinforcing AI and biotechnology platforms for early diagnosis, better targeted treatments and
drug discovery.
GEDi Cube’s approach is distinguished by the fusion of proprietary
AI technology with diverse biological data sources, including genomics, proteomics, transcriptomics and epigenomics. GEDi Cube
uses a multi-omics approach to search for individual biomarkers that are present even in asymptomatic patients. Moreover, GEDi
Cube incorporates critical imaging data and the potential integration of pertinent patient health records encompassing medical
history, medication history and comorbidities.
Central to GEDi Cube’s mission is its multidisciplinary team
and its proprietary AI platform referred to as “GEDiCube”. GEDi Cube believes that it has developed a unique approach
to the early detection and diagnosis of cancer and other rare diseases through the systematic analysis of data using AI technologies,
data mining procedures and algorithms for health technology.
GEDi Cube’s operations to date have
been limited to business planning, raising capital, developing GEDi Cube’s AI technology, identifying potential products
and other research and development. To date, GEDi Cube has financed operations primarily through private placements, debt and funds
received from license and collaboration agreements. As of September 30, 2023, GEDI had cash and cash equivalents totaling
€339,985.
GEDi Cube does not expect to generate revenue
unless and until it successfully completes development of, obtains marketing approval for, and commercializes, its products, either
alone or in collaboration with third parties. GEDi Cube expects these activities may take
several years and its success in these efforts is subject to significant uncertainty. Accordingly, GEDi Cube expects it will need
to raise additional capital prior to the commercialization of, and any future regulatory approval required for, its platform and
any of its future products. Until such time, if ever, that GEDi Cube generates significant revenue, GEDi Cube’s viability
will depend on its ability to raise additional capital to finance its operations. GEDi Cube’s failure to raise additional
capital as needed will have a negative impact on its financial condition and its ability to continue to pursue its business strategies,
including commercialization of its AI platform and future products.
On
November 22, 2023, GEDi Cube entered into a loan agreement (the “DMZ Facility”) with DMZ Invest I APS (“DMZ”)
pursuant to which DMZ agreed to loan GEDi Cube up to £500,000 (€572,775 as of November 22, 2023), which will be used
to fund GEDi Cube’s operations through the Closing. GEDi Cube believes that, based on its current operating plan, its cash
and cash equivalents as of September 30, 2023 and its anticipated funding from the DMZ Facility will enable GEDi Cube to fund its
operating expenses and capital expenditure requirements into February 2024. Therefore, there is substantial doubt about GEDi Cube’s
ability to continue as a going concern as GEDi Cube does not believe that its cash, cash equivalents and investments will be sufficient
to fund operations for at least the next twelve months.
Acquisition of Grace Systems
In June 2023, Grace Systems B.V. (“Grace Systems”) and GEDi
Cube (formerly GEDi Health Group Ltd.) entered into a Binding Head of Terms for the acquisition by GEDi Cube of 100% of the outstanding
shares of Grace Systems. On August 23, 2023, Grace Systems and GEDi Cube entered into a Share Purchase Agreement (the “Share Purchase
Agreement”) with the shareholders of Grace Systems, pursuant to which GEDi Cube acquired 100% of the outstanding shares of Grace
Systems from its existing shareholders in exchange for ordinary shares of GEDi Cube, representing a 49% ownership interest in GEDi Cube,
and an additional cash investment in Grace Systems by GEDi Cube of €1 million. See Note 3 to the unaudited consolidated financial
statements included in this proxy statement for a description of this transaction.
Stock Purchase Agreement
On September 28, 2023, GEDi
Cube entered into a Stock Purchase Agreement, dated September 28, 2023 (the “Stock Purchase Agreement”), with Renovaro,
the shareholders of GEDi Cube party thereto (together with the other shareholders of GEDi Cube who deliver to Renovaro a joinder
to the Stock Purchase Agreement, the “Sellers” and each, a “Seller”), and Yalla Yalla Ltd., a private limited
liability company registered and incorporated under the laws of Malta, in its capacity as the representative of the Sellers (the
“Sellers’ Representative”), pursuant to which Renovaro will acquire all of the issued and outstanding shares
and other equity interests of GEDi Cube from the Sellers after which GEDi Cube will become a wholly-owned subsidiary of Renovaro.
Key Components of GEDi Cube’s
Results of Operations
Revenues
Historical revenues consisted of revenues
recognized under GEDi Cube’s strategic collaboration agreements for consulting services provided to third parties to assist
with their research and development activities. GEDi Cube’s strategic collaboration agreements generally outline overall
development plans and include payments GEDi Cube receives for services provided. For these activities and payments, GEDi Cube utilizes
judgment to assess the nature of the performance obligations to determine whether the performance obligations are satisfied over
time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue.
GEDi Cube has not recognized any royalty revenue or revenue related to the commercialisation of its AI technology to date. GEDi
Cube does not expect to generate any revenue from any products that it develops unless and until GEDi Cube commercializes its products
or enters into other collaborative agreements with third parties.
Operating Expenses
Operating expenses consist primarily of
personnel costs, including wages and benefits, related to GEDi Cube’s executive, sales and marketing personnel, as well as
management fees, marketing fees and professional fees, including legal and accounting fees.
GEDi Cube expects its general and administrative
expenses to increase in the near term, both in absolute dollars and as a percentage of revenue, largely driven by hiring additional
personnel to support the growth of its business. Following the Closing, there will also be significant additional expenses to GEDi
Cube associated with operating as a public company as part of the combined company. Such increases may include increased insurance
premiums, investor relations expenses, legal and accounting fees associated with the expansion of GEDi Cube’s business and
corporate governance, financial reporting expenses and expenses related to the Sarbanes-Oxley Act and other regulatory compliance
obligations.
Total Other Income (Expense)
Interest Expense
Interest expense
consists primarily of interest and amortization of debt related to the Notes entered into with Renovaro under which GEDi Cube borrowed
an aggregate of €1 million in August 2023. The Notes’ applicable interest rate is
6% per annum. GEDi Cube has made no payments of principal or interest on the Notes.
Net Loss
Net loss for
the year ended December 31, 2022 was €623,982 and net income for the year ended December 31, 2021 was €131,416, representing
a decrease in net income of €755,398. The decrease in net income was primarily due to the
decrease of revenues of €1,041,660, which was primarily attributable to GEDi Cube’s strategic focus pivoting from providing
consulting services to the development of its AI platform for early cancer detection and its recurrence. This decrease in revenues
was partially offset by the €138,480 decrease in operating expenses.
Results of Operations
Comparison of Fiscal Years Ended
December 31, 2022 and 2021
The following table
sets forth certain statements of operations data for the periods indicated:
| | |
Year Ended
December 31, |
| | |
2022 | |
2021 | |
Change |
| Revenue | |
€ | — | | |
€ | 1,041,660 | | |
€ | (1,041,660 | ) |
| Cost of Revenue | |
| — | | |
| (147,782 | ) | |
| 147,782 | |
| Gross (Loss) Profit | |
€ | — | | |
€ | 893,878 | | |
| (893,878 | ) |
| Operating expenses: | |
| | | |
| | | |
| | |
| Selling, general and administrative | |
| 135,398 | | |
| 166,287 | | |
| (30,889 | ) |
| Depreciation expense | |
| 14,591 | | |
| 12,270 | | |
| 2,321 | |
| Management fees | |
| 378,000 | | |
| 372,275 | | |
| 5,725 | |
| Advisory Fees | |
| 13,455 | | |
| 4,481 | | |
| 8,974 | |
| Salaries and wages | |
| 82,538 | | |
| 207,149 | | |
| (124,611 | ) |
| Total operating expenses | |
€ | 623,982 | | |
€ | 762,462 | | |
| (138,480 | ) |
| | |
| | | |
| | | |
| | |
| Net Profit (Loss) | |
€ | (623,982 | ) | |
€ | 131,416 | | |
| (755,398 | ) |
Revenues
Revenues for the years ended December 31, 2022 and 2021 were €Nil
and €1,041,660, respectively, representing a decrease in revenue of €1,041,660 or 100%. The revenues earned in 2021 related
to the consulting services provided to a third-party healthcare technology company. In 2021, GEDi Cube shifted its focus from providing
consulting services to the development of its AI technology for early cancer detection and its recurrence, and, therefore, no revenue
was generated in 2022. GEDi Cube is in the development stage of its lifecycle and has not yet commenced operations. GEDi Cube has
never generated revenues relating to the utilisation of its AI technology. GEDi Cube anticipates that it will continue to incur
primarily net losses for the foreseeable future.
Operating Expenses
GEDi Cube’s operating expenses for the years ended December 31,
2022 and 2021 were €623,982 and €762,462, respectively, representing a decrease of €138,480 or 18%. The largest contributors
to the decrease in operating expenses for the year ended December 31, 2022, were the decrease in the selling, general and administrative
expenses and the decrease in salaries and wage expenses of €30,889 and €124,611, respectively. After GEDi Cube pivoted its
business model towards developing its AI technology, the personnel team was streamlined to focus on development, which allowed GEDi Cube
to save money on its sales and marketing personnel. Housing costs were also reduced by €73,593 from 2021 to 2022 due to GEDi Cube
adapting to a work-from-home model during the coronavirus (“COVID-19”) pandemic. These decreases were partially offset by
GEDi Cube increasing its advisory costs in 2022 versus 2021 by €8,974, which related primarily to advisory costs associated with
the clinical aspect of the development of the AI platform, and increasing management fees in 2022 versus 2021 by €5,725 related
to an agreed inflationary increase.
Comparison of Nine Months Ended September
30, 2023 and 2022
The following table
sets forth certain statements of operations data for the periods indicated:
| | |
Successor & Predecessor
Nine Months Ended
September 30, |
| | |
2023 | |
2022 | |
Change |
| | |
| |
| |
|
| Operating expenses: | |
| | | |
| | | |
| | |
| Selling, general and administrative | |
| 266,103 | | |
| 100,077 | | |
| 166,026 | |
| Depreciation expense | |
| 2,130 | | |
| 10,913 | | |
| (8,783 | ) |
| Management fees | |
| 283,816 | | |
| 290,104 | | |
| (6,288 | ) |
| In process research and development expense | |
| 464,335 | | |
| — | | |
| 464,335 | |
| Advisory Fees | |
| 139,909 | | |
| 1,975 | | |
| 137,935 | |
| Salaries and wages | |
| 267,119 | | |
| 65,359 | | |
| 201,760 | |
| Total operating expenses | |
| 1,423,412 | | |
| 468,427 | | |
| 954,985 | |
| Loss from operations | |
| (1,423,412 | ) | |
| (468,427 | ) | |
| 954,985 | |
| Other income (expense): | |
| | | |
| | | |
| | |
| Gain from deconsolidation | |
| 176,266 | | |
| — | | |
| (176,266 | ) |
| Interest Expense | |
| (7,346 | ) | |
| — | | |
| 7,346 | |
| Gain on exchange | |
| (27,468 | ) | |
| — | | |
| 27,468 | |
| Net Loss | |
€ | (1,281,960 | ) | |
€ | (468,427 | ) | |
€ | 813,533 | |
Operating Expenses
GEDi Cube’s operating expenses for the nine months ended September 30,
2023 and 2022 were €1,423,412 and €468,427, respectively, representing an increase of €954,985. The largest contributors
to the increase in operating expenses for the nine months ended September 30, 2023, were the increase in the selling, general and administrative
expenses and the increase in salaries and wage expenses of €166,026 and €201,760,
respectively. Since the acquisition of Grace Systems in August 2023, GEDi Cube has increased its headcount significantly
from a focus on research and development personnel to include technical developers and medical professionals. The increase in salaries
and wage expenses for the first nine months of 2023 partly related to the loss of subsidy income recorded during the nine months
ended September 30, 2022 as the result of a one-time Emergency Bridging Measure for Job Opportunities (NOW) wage subsidy implemented
by the Dutch government as emergency funding for all companies as a result of the COVID-19 outbreak where qualifying expenses are
reimbursed by the authorities.
GEDi Cube’s advisory fees for the
nine months ended September 30, 2023 and 2022 were €139,909 and €1,975 respectively, representing an increase of €137,935.
This increase was driven by the increase in legal and advisory fees related to the Transaction with Renovaro. There were no such
costs in the nine months ended September 30, 2022 where advisory fees related to accountancy fees.
In process research and development
expense
GEDi Cube’s in process research and
development expense for the nine months ended September 30, 2023 and 2022 was €464,335 and €Nil, respectively, representing
an increase of €464,335. GEDi Cube’s acquisition of Grace Systems encompasses the acquisition of the research-and-development
project for Grace Systems’ AI platform. This project, known as In-Process Research and Development (“IPR&D”),
includes not only the current research but also historical expertise, protocols, designs and procedures required to facilitate
the future testing phase of the AI technology.
Total Other Income (Expense)
Gain from deconsolidation
The gain from deconsolidation for the nine
months ended September 30, 2023 and 2022 was €176,266 and €Nil, respectively, representing an increase of €176,266.
GEDi Cube recorded a gain of €176,266 on the deconsolidation of Grace Datascience B.V. in 2023 with no similar disposal for
the nine months ended September 30, 2022. See Note 12 to the unaudited consolidated financial statements of GEDi Cube included
in this proxy statement for further details regarding the deconsolidation of Grace Datascience B.V.
Interest Expenses
The interest expense for the nine months ended September 30, 2023 and 2022
was €7,346 and €Nil, respectively, representing an increase of €7,346. Interest expense consists primarily of interest
and amortization of debt related to the Notes entered into with Renovaro under which GEDi Cube borrowed an aggregate of €1 million
in August 2023. The Notes’ applicable interest rate is 6% per annum. GEDi Cube has made no payments of principal or interest on
the Notes. There was no such similar expense for the nine months ended September 30, 2022.
Loss on Exchange
The loss on exchange for the nine months ended September 30, 2023 and 2022
was €27,468 and €Nil, respectively, representing an increase of €27,468. Loss on exchange consists primarily of exchange
rate differences between GEDi Cube’s functional currency of euros and the U.S. dollar currency on the borrowings under the Notes.
There was no such similar expense for the nine months ended September 30, 2022.
Liquidity and Capital Resources
Sources of Liquidity
Since inception, GEDi Cube has not generated any revenue from product
sales or its current AI platform and has a history of primarily net losses and negative cash flows from its operations. GEDi Cube
expects to continue to incur significant expenses and net losses for the foreseeable future as it advances the clinical development
of its AI platform. GEDi Cube expects that its research and development and general and administrative costs will continue to increase
significantly, including in connection with conducting clinical validation and technological development costs for its AI platform
and future products to support commercialization and providing general and administrative support for its operations,
including
the costs associated with operating as a public company as a part of the combined company following the Closing. As a result, GEDi
Cube will need additional capital to fund its operations. GEDi Cube’s failure to raise
additional capital as needed will have a negative impact on its financial condition and its ability to continue to pursue its business
strategies, including commercialization of its AI platform and future products. See the section entitled “Risk
Factors—Risks Related to GEDi Cube” for additional risks associated with GEDi Cube’s substantial capital
requirements.
As of September 30, 2023, GEDi Cube had cash and cash equivalents
of €339,985. Since the Share Acquisition Closing, GEDi Cube has funded its operations primarily through working capital and
the net proceeds of €1 million under the Notes entered into with Renovaro in August 2023.
GEDi
Cube expects to have access to €572,775 of funding through the
DMZ Facility, which will be used to fund operations through the Closing. GEDi Cube believes that, based on its current operating
plan, its cash and cash equivalents as of September 30, 2023 and its anticipated funding from the DMZ Facility will enable GEDi
Cube to fund its operating expenses and capital expenditure requirements into February 2024. Therefore, there is substantial doubt
about GEDi Cube’s ability to continue as a going concern as GEDi Cube does not believe that its cash, cash equivalents and
investments will be sufficient to fund operations for at least the next twelve months.
Future Capital Requirements
Management expects GEDi Cube’s expenses to increase in connection
with its ongoing activities as described in greater detail below. GEDi Cube is subject to the risks incident in the development
of new biotechnological products, and it may encounter unforeseen expenses, difficulties, complications, delays, and other unknown
factors that may harm GEDi Cube’s business. See the section entitled “Risk Factor—Risks Related to GEDi Cube”
for risks related to the development of GEDi Cube’s products.
In order to complete the development of GEDi Cube’s AI platform
and to build the sales, marketing and distribution infrastructure that management believes will be necessary to commercialize its
platform and future products, GEDi Cube will require substantial additional capital. Accordingly, until such time that GEDI Cube
can generate a sufficient amount of revenue from product sales or other sources, if ever, management expects to seek to raise any
necessary additional capital through loans or other capital sources, which could include income from collaborations, partnerships
or other marketing, distribution, licensing or other strategic arrangements with third parties, or from grants. If GEDi Cube raises
capital through collaborations, partnerships and other similar arrangements with third parties, it may be required to grant rights
to develop and market technological products that GEDi Cube would otherwise prefer to develop and market itself. GEDi Cube may
be unable to raise additional capital from these sources on favorable terms, or at all. GEDi Cube’s ability to raise additional
capital may be adversely impacted by potential worsening global economic conditions and the recent disruptions to, and volatility
in, the credit and financial markets in the European Union and worldwide. The failure to obtain sufficient capital on acceptable
terms when needed could have a material adverse effect on GEDi Cube’s business, results of operations or financial condition,
including by requiring GEDi Cube to delay, reduce or curtail its research, technological or product development or future commercialization
efforts. GEDi Cube may also be required to license the rights to its AI technology at an earlier stage of development or on less
favorable terms than GEDi Cube would otherwise choose. Management cannot provide assurance that GEDi Cube will ever generate positive
cash flow from its operating activities.
Cash Flows
The following table summarizes GEDi Cube’s cash flows for
the periods indicated:
| |
|
Successor Nine Months Ended September 30, |
|
Predecessor Year Ended
December 31, |
| |
|
2023 |
|
2022 |
|
2022 |
|
2021 |
| Net cash used in operating activities |
|
€ |
(622,252 |
) |
|
€ |
(246,843 |
) |
|
€ |
(246,157 |
) |
|
€ |
154,376 |
|
| Net cash used in investing activities |
|
|
(1,982 |
) |
|
|
(1,057 |
) |
|
|
(1,057 |
) |
|
|
(42,867 |
) |
| Net cash received from financing activities |
|
|
964,218 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Net increase (decrease) in cash and cash equivalents |
|
€ |
339,985 |
|
|
€ |
(247,900 |
) |
|
€ |
(247,214 |
) |
|
€ |
111,508 |
|
Cash Flows from Operating Activities
For the nine months ended September 30, 2023, GEDi Cube used €622,252
of net cash in operating activities. Net cash used in operating activities reflected GEDi Cube’s net loss of €1,152,388, which
primarily resulted from the lack of revenue during the first nine months of 2023 and increased operating costs during the third quarter
of 2023 associated with investments to increase GEDi Cube’s operating capacity. The primary use of cash was to fund GEDi Cube’s
operations related to the development of its AI technology.
For the nine months ended September 30, 2022, GEDi Cube used €246,843
of net cash in operating activities. Net cash used in operating activities reflected GEDi Cube’s net loss of €468,427,
which primarily resulted from the lack of revenue in 2022. This lack of revenue was partially offset via cost reductions as GEDi
Cube pivoted its focus from providing consulting services to the development of its AI technology for early cancer detection and
its recurrence, primarily related to the reduction in GEDi Cube’s headcount and housing costs. The primary use of cash was
to fund GEDi Cube’s operations related to the development of its AI technology.
For the year ended December 31, 2022, GEDi Cube used €246,157
of net cash in operating activities. Net cash used in operating activities reflected GEDi Cube’s net loss of €623,982,
which primarily resulted from the lack of revenue in 2022. This lack of revenue was partially offset via cost reductions as GEDi
Cube began reducing its headcount and related housing costs as it pivoted to focus on the development of its AI technology. The
primary use of cash was to fund GEDi Cube’s operations related to the development of its AI technology.
For the year ended December 31, 2021,
net cash provided by operating activities of €154,376 reflected GEDi Cube’s net profit
of €131,416, which primarily resulted from €1.04 million of consulting revenue earned in 2021. This revenue was offset
primarily by salaries and wages of employees who were engaged to support GEDi Cube’s consulting activities. The primary use
of cash was to fund GEDi Cube’s revenue-generating consulting operations until GEDi Cube shifted its focus to the development
of its AI technology.
Cash Flows from Investing Activities
For the nine months ended September 30,
2023, net cash flows used in investing activities consisted of net proceeds from the purchase and sale of property and equipment
of €1,982, which related primarily to laptops and information technology peripherals for employees.
For the nine months ended September 30,
2022, net cash flows used in investing activities consisted of purchases of property and equipment of €1,057, which
related primarily to laptops and information technology peripherals for employees.
For the year ended December 31, 2022,
net cash flows used in investing activities consisted of purchases of property and equipment of €1,057, which related primarily
to laptops and information technology peripherals for employees.
For the year ended December 31, 2021,
net cash flows used in investing activities consisted of purchases of property and equipment of €42,867 which related primarily
to laptops and information technology peripherals for employees and office furnishings.
Cash Flows from Financing Activities
For the nine months ended September 30,
2023, net cash flows provided by financing activities consisted of proceeds of €964,218 from the borrowings from Renovaro
under the Notes entered into in August 2023. See Note 13 to the unaudited consolidated financial statements of GEDi Cube included
in this proxy statement for further information regarding the Notes.
For the nine months ended September 30,
2022, net cash flows used or provided by financing activities was €Nil.
For the year ended December 31, 2022,
net cash flows used or provided by financing activities was €Nil.
For the year ended December 31, 2021,
net cash flows used or provided by financing activities was €Nil.
Lease Obligations
GEDi Cube leases space under a 30-month operating lease agreement for administrative
offices in Amsterdam, The Netherlands, which has a minimum cancellation notice period of three months which management has determined
will likely continue and expire in February 2026. GEDi Cube also leases an additional space under a 23.4-month operating lease agreement
for an apartment in Amsterdam, The Netherlands, which has a minimum cancellation notice period of one month. As at September 30, 2023,
future minimum lease payments required under the two operating leases total €615,959.
Off-Balance Sheet Arrangements
As of December 31, 2022, and 2021, GEDi Cube had no off-balance
sheet arrangements. GEDi Cube is not aware of any material transactions which are not disclosed in its consolidated financial statements.
Significant Accounting Policies and Critical Accounting Estimates
Basis of Presentation and Principles
of Consolidation
The accompanying consolidated financial
statements have been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”)
as defined by the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”).
The consolidated financial statements include the accounts of Gedi Cube Intl Ltd and its subsidiaries.
All material inter-company balances and transactions have been eliminated.
In the opinion of GEDi Cube, all adjustments (consisting of normal recurring
accruals) considered necessary for a fair presentation have been included. Interim results for the period
from August 24, 2023 to September 30, 2023 are not necessarily indicative of the results for the full year ending December 31,
2023. These interim unaudited consolidated financial statements should be read in conjunction with the Grace
Systems B.V. audited consolidated financial statements and notes for the years ended December 31,
2022 and 2021.
In June 2023 Grace Systems B.V. ("Grace"), ("Predecessor")
and Gedi Cube Intl Ltd ("GEDiCube Int"), ("Successor") entered into a Binding
Head of Terms for the acquisition of 100% of the share capital in Grace. On August 23, 2023,
Grace and Gedi Cube Int executed a Share Purchase Agreement ("Agreement") for the acquisition
of 100% of the share capital in Grace, involving a €1 million investment into Grace by GEDiCube Int. In exchange, existing
shareholders of Grace were allocated 49% ownership in GEDiCube Int through a share-exchange transaction, ensuring their continued
involvement in the development and commercialization of Grace's underlying Intellectual Property ("IP") and Trade Secrets.
The unaudited consolidated financial statements of Successor
and Predecessor are not comparable due to the acquisition described above. Gedi Cube Int. was incorporated in June 2023 with no
operational history and has no prior comparative period financial statements presented. Therefore, the reporting period has been
separated by a black line in the consolidated financial statements with the Predecessor representing the pre-Closing Date period
(January 1, 2023 through August 23, 2023) and the Successor representing the post-Closing
Date period (August 24, 2023 through September 30, 2023).
GEDi Cube noted that the Predecessor includes financial information related to the Grace Systems, while
the Successor includes financial information related to the newly formed company after the asset acquisition.
Cash
As of September 30, 2023 and December 31,
2022 GEDi Cube's cash balances were €339,985 and
€1,583, respectively. Cash is recorded at cost, which approximates fair value. As of September 30,
2023 and December 31, 2022, cash consisted of checking and savings deposits.
Property and Equipment
Property, plant and equipment is stated
at cost, less accumulated depreciation. Maintenance and repairs are charged to expense when incurred. Additions and improvements
that extend the economic useful life of the asset are capitalized and depreciated over the remaining useful lives of the assets.
The cost and accumulated depreciation of assets sold or retired are removed from the respective accounts, and any resulting gain
or loss is reflected in current earnings. Depreciation is recognized using the straight-line method in amounts considered to be
sufficient to allocate the cost of the assets to operations over the estimated useful lives of the assets, all of which are 5
years.
Revenue Recognition
Revenue is recognized when promised goods or services are transferred to customers in an amount that reflects the
consideration to which GEDi Cube expects to be entitled in exchange for those goods or services
by following a five-step process: (1) identify the contract with a customer, (2) identify the performance obligations in the contract,
(3) determine the transaction price, (4) allocate the transaction price, and (5) recognize revenue when or as GEDi
Cube satisfies a performance obligation. There was no revenue recognized during the 12 months
ending December 31, 2022 and the nine months ending September 30, 2023.
Contract Acquisition Costs
Contract acquisition costs primarily consist
of commissions that GEDi Cube incurs to obtain a contract with a customer. These costs are incremental and recoverable. However,
as the amortization period of such amounts would not exceed one year, commission costs, as permitted by the practical expedient,
are expensed as incurred.
Contract Assets and Liabilities
Contract assets consist of work performed by
GEDi Cube in which revenue has been recognized but an invoice has not been generated. Contract liabilities consist of deferred
revenue which represents milestones achieved, invoices issued, or consideration received prior to transferring control of goods
or services to the customer.
Business Combinations
GEDi Cube accounts for business combinations under Financial Accounting Standards Board (“FASB”) Accounting Standards
Codification (“ASC”) 805 “Business Combinations” using the acquisition method of accounting, and accordingly,
the assets and liabilities of the acquired business are recorded at their fair values at the date of acquisition. All acquisition
costs are expensed as incurred. Upon acquisition, the accounts and results of operations are included as of and subsequent to
the acquisition date.
In
analyzing the business combination for proper accounting treatment, management utilized ASC 805 & ASU 2017-01. ASU 2017-01
created a new framework that companies must use to evaluate whether an integrated set of assets and activities is a business and
should be accounted for as a business acquisition. FASB defines a business as a set of activities and assets that include an input
and a substantive process; however, the fair value of the set is not usually concentrated in single or multiple assets. This set
of inputs and processes usually provides goods and services to customers and return to stakeholders (ASC 805-10-55-3A and ASC
805-10-55-4). The new guidance requires an initial screening test to determine if substantially all of the fair value of the assets
acquired is concentrated in one asset or a group of similar assets.
ASC
805 also provides a screen test to assess whether substantially all of the fair value of the gross assets acquired is concentrated
in a single identifiable asset or group of similar identifiable assets and allows organizations to assess whether acquired activities
or assets are considered a business. The screen test reduces the number of transactions that might otherwise be considered a business
combination by providing a method for aggregating similar assets into one acquired asset group. This aggregation provides that
substantially all of the assets in the transaction be part of this asset group to qualify under the practical screen.
The
acquisition of Grace Systems by Gedi Cube Int LTD was treated as an asset acquisition.
Leases
In
accordance with ASC Topic 842, GEDi Cube determined the initial classification
and measurement of its right-of-use assets and lease liabilities at the lease commencement date and thereafter. The lease terms
include any renewal options and termination options that GEDi Cube is reasonably assured to exercise,
if applicable. The present value of lease payments is determined by using the implicit interest rate in the lease, if that rate
is readily determinable; otherwise, GEDi Cube develops an incremental borrowing rate based on
the information available at the commencement date in determining the present value of the future payments. Rent expense for operating
leases is recognized on a straight-line basis, unless the operating lease right of use assets have been impaired, over the reasonably
assured lease term based on the total lease payments and is included in operating expenses in the consolidated statements of operations.
For operating leases that reflect impairment, GEDi Cube will recognize the amortization of the
operating lease right-of-use assets on a straight-line basis over the remaining lease term with rent expense still included in
selling, general and administrative expenses in the unaudited consolidated statements of operations.
GEDi Cube has elected the practical expedient to not separate lease and non-lease components.
GEDi Cube’s non-lease components are primarily related to property maintenance, insurance,
and taxes, which vary based on future outcomes, and thus are recognized in selling, general and administrative
expenses when incurred.
UNAUDITED PRO FORMA COMBINED
FINANCIAL STATEMENTS
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
The following unaudited pro forma condensed combined financial information
is presented to illustrate the estimated effects of the proposed Transaction.
The unaudited pro forma condensed combined balance sheet information as
of September 30, 2023, is based upon and derived from the unaudited historical financial information of Renovaro and GEDi Cube and gives
effect to the Transaction as if such acquisition had occurred on July 1, 2023.
The unaudited pro forma condensed combined statements of operations for the twelve months ended June 30, 2023,
combines the historical audited consolidated statement of operations of Renovaro for the twelve months ended June 30, 2023, with
GEDi Cube’s unaudited financial results for the twelve months ended June 30, 2023, and give effect to the Transaction as
if it occurred on July 1, 2022. Renovaro and GEDi Cube have different fiscal years. Renovaro’s fiscal year ends on June 30,
whereas GEDi Cube’s ends on December 31. GEDi Cube’s unaudited financial results for the twelve months ended June 30,
2023, have been derived from (i) its unaudited statement of operations for the six months ended June 30, 2023, and (ii) its audited
statement of operations for the year ended December 31, 2022 removing its results of operations for the six months ended June 30,
2022 derived from its unaudited statement of operations for the six months ended June 30, 2022. In addition, the unaudited pro
forma condensed combined statements of operations for the year ended June 30, 2023, are also based upon and derived from the historical
financial information of Grace Systems BV or Grace (Predecessor), which was acquired by GEDi Cube (Successor) on August 24, 2023,
and give effect to the Grace acquisition as if it occurred on July 1, 2022.
The unaudited pro forma condensed combined statements of operations for
the three months ended September 30, 2023, combines the historical unaudited consolidated statement of operations of Renovaro for the
three months ended September 30, 2023, with GEDi Cube’s unaudited financial results for the three months ended September 30, 2023.
The
unaudited pro forma condensed combined financial information has been derived from and should be read in conjunction with:
| ● | Renovaro’s
audited consolidated financial statements and accompanying notes as of and for the year ended
June 30, 2023, as contained in its Annual Report on Form 10-K filed on October 2, 2023, with
the SEC. |
| ● | Renovaro’s
unaudited condensed consolidated financial statements and accompanying notes as of and for
the three-month period ended September 30, 2023, as contained in its Quarterly Report on
Form 10-Q filed on November 14, 2023, with the SEC. |
| ● | GEDi
Cube’s Predecessor’s audited consolidated financial statements and accompanying
notes as of and for the years ended December 31, 2021, and 2022, and the Predecessor’s
unaudited consolidated financial statements and the accompanying notes for the year ended
June 30, 2023, included elsewhere in this proxy statement/prospectus; |
| ● | GEDi
Cube’s unaudited condensed consolidated financial statements and accompanying notes
as of and for the three months ended September 30, 2023, included elsewhere in this
proxy statement/prospectus; |
Additional information about the basis of presentation of this information
is provided in Note 1 hereto.
The unaudited pro forma condensed combined financial information is provided for illustrative purposes only and
is not necessarily indicative of the operating results or financial position that would have occurred if the Transaction had been
completed as of the dates set forth above, nor is it indicative of the future results or financial position of the combined company.
In connection with the pro forma financial information, Renovaro allocated the purchase price using its best estimates of fair
value (using a 10-day volume-weighted average price preceding December 10, 2023). The closing price will be used on the date of acquisition. Accordingly,
the pro forma Transaction price adjustments are preliminary and subject to further adjustments as
additional information becomes available and as additional analyses are performed. The unaudited pro forma condensed combined financial
information also does not give effect to the potential impact of current financial conditions, any anticipated synergies, operating efficiencies,
or cost savings that may result from the Transaction or any integration costs. The unaudited pro forma condensed combined statements of
operations do not reflect certain amounts resulting from the Transaction that were determined to be of a non-recurring nature.
The unaudited pro forma condensed combined financial information was prepared
in accordance with Article 11 of Regulation S-X. Renovaro has accounted for the Transaction using the acquisition method of accounting,
in accordance with FASB Accounting Standards Codification, or ASC, Topic 805 “Business Combinations”, or ASC 805. Renovaro
preliminarily determined that the Transaction constitutes a business combination.
After the closing of the Transaction, Renovaro will complete the valuations
necessary to finalize the required purchase price allocation based upon the fair market values as of the actual closing date of the Transaction,
at which time the final allocation of the purchase price will be determined. The pro forma financial information contained in this joint
proxy statement/prospectus is also based upon certain assumptions with respect to the number of shares of Renovaro common stock to be
issued to GEDi Cube shareholders at the closing of the Transaction. Differences between preliminary estimates in the pro forma financial
information presented herein and the final acquisition accounting will occur and could have a material impact on the pro forma financial
information. In this regard, differences between those preliminary estimates and the number of shares of Renovaro common stock ultimately
issued to GEDi Cube shareholders at closing of the Transaction would result in changes to various components of the unaudited pro forma
condensed combined balance sheet, including intangible assets, common stock and additional paid-in capital, and various components of
the unaudited pro forma condensed combined statements of operations, including net loss per share and weighted-average shares used in
computing net loss per share.
The unaudited pro forma condensed combined financial information has been
prepared by Renovaro in accordance with SEC Regulation S-X Article 11 and is not necessarily indicative of the condensed combined financial
position or results of operations that would have been realized had the Transaction been completed as of the dates indicated above, nor
is it meant to be indicative of any anticipated combined financial position or future results of operations that Renovaro will experience
after the Transaction is completed. In addition, the accompanying unaudited pro forma condensed combined statements of operations do not
include any pro forma adjustments to reflect expected cost savings or restructuring actions which may be achievable or the impact of any
non-recurring expenses and one-time transaction-related costs that may be incurred as a result of the Transaction.
The Transaction and Effective Time of the Transaction
The Stock Purchase Agreement provides that, at the Effective Time, the
Sellers will sell and transfer to Renovaro, and Renovaro will purchase and acquire from the Sellers all of the GEDi Cube Shares owned
by the Sellers that are issued and outstanding as of immediately prior to the Effective Time in exchange for the Exchange Consideration,
as described below. The Closing will occur as soon as practicable but no later than three (3) business days after the satisfaction or
waiver of the last to be fulfilled of the closing conditions set forth in the Stock Purchase Agreement or at such other date and place
as Renovaro, GEDi Cube, and the Sellers may agree to in writing. The Transaction will be deemed effective as of the closing of business
on the Closing Date (which is referred to herein as the “Effective Time”). Renovaro expects that the Closing will take place
during the first half of 2024, subject to satisfying the closing conditions set forth in the Stock Purchase Agreement, including the approval
by Renovaro’s stockholders of the Share Issuance Proposal and the Authorized Share Proposal. Because the Closing is subject to such
conditions, Renovaro cannot predict if or exactly when the Closing will occur.
Exchange Consideration
If the Transaction is consummated, in exchange for the sale and transfer
of the issued and outstanding GEDi Cube Shares to Renovaro, the Sellers shall become entitled to receive from Renovaro (i) an aggregate
number of shares of Common Stock equal to a specified percentage of the shares of Common Stock issued and outstanding as of the Effective
Time (minus (a) 1 million shares of Common Stock issued to a consultant assisting with the Transaction and (b) 1 million shares of Common
Stock issued to Avram Miller prior to the Closing pursuant to the Miller Consulting Agreement), which percentage shall be a ratio of the
aggregate number of GEDi Cube Shares owned by the Sellers divided by the aggregate number of GEDi Cube Shares issued and outstanding,
in each case, as of the Effective Time (which is referred to herein as the “Closing Consideration”), and (ii) earn-out shares
of Common Stock to be issued pro rata to the Sellers upon the exercise or conversion of any of Renovaro’s derivative securities
(subject to certain exceptions) which are outstanding at the Effective Time (which is referred to herein as the “Earnout Shares”
and, together with the Closing Consideration, the “Exchange Consideration”). The aggregate number of Earnout Shares to be
issued to the Sellers will be equal to a percentage of the shares of Common Stock issued upon exercise or conversion of Renovaro’s
derivative securities, which percentage shall be a ratio of the aggregate number of GEDi Cube Shares owned by the Sellers divided by the
aggregate number of GEDi Cube Shares issued and outstanding, in each case, as of the Effective Time. Each Seller will receive its
pro rata portion of the Exchange Consideration based on such Seller’s pro rata ownership of the GEDi Cube Shares transferred to
Renovaro.
| Table 1 – Earnout Share Breakdown: | |
Amount |
| Estimated Conversion of 561,010 Preferred Shares at a 10:1 ratio | |
| 5,610,100 | |
| Estimated outstanding warrants at the closing date | |
| 5,827,407 | |
| Estimated Stock options outstanding at closing date | |
| 5,130,110 | |
| Estimated Common Stock issuance related to Convertible Notes | |
| 598,014 | |
| Estimated Earnout Shares related to outstanding Renovaro Derivatives | |
| 17,165,631 | |
| Table 2 – Estimated Exchange Consideration | |
Amount |
| GEDi Cube Common Stock outstanding as of September 30, 2023 | |
| 2,157 | |
| Estimated total GEDi Cube Common Stock | |
| 2,157 | |
| Exchange ratio | |
| 38,174 | |
| Estimated Closing Consideration | |
| 65,224,089 | |
| Estimated Earnout Shares (see Table 1 above) | |
| 17,165,631 | |
| Estimated Renovaro Common Stock to be issued | |
| 82,389,720 | |
| Estimated closing price (10-day average VWAP[1]) | |
$ | 3.34 | |
| Estimated Exchange Consideration | |
$ | 275,181,665 | |
[1]
The estimated closing price was calculated using a 10-day Volume Weighted Average Price (VWAP) for Renovaro that preceded December 10,
2023. On the acquisition date, the closing price of the Renovaro shares.
Accounting for the Transaction
The Transaction is expected to be accounted for as a business combination using the acquisition method with Renovaro
as the accounting acquirer in accordance with ASC 805. Under this method of accounting, the Transaction consideration will be allocated
to GEDi Cube’s assets acquired and liabilities assumed based on their estimated fair values at the date of completion of
the Transaction, which is expected to close in the first half of 2024. The process of valuing the net assets of GEDi Cube immediately
prior to the Transaction, as well as evaluating accounting policies for conformity, is preliminary.
In addition, the acquisition method of accounting requires the acquirer
to recognize the consideration transferred at fair value. Because this is an all-stock transaction, the fair value of the Transaction
consideration fluctuates with changes in the market price of Renovaro. That consideration is fixed on the date of completion of the Transaction.
Any differences between the estimated fair value of the Transaction consideration and the estimated fair value of the assets acquired
and liabilities assumed will be recorded as goodwill. Alternatively, any excess of the estimated fair value of such assets and liabilities
over the Transaction consideration would be recorded as bargain purchase gain. Accordingly, the Transaction consideration allocation and
related adjustments reflected in this unaudited pro forma condensed combined financial information are preliminary and subject to revision
based on a final determination of fair value. Refer to Note 1, “Basis of Presentation” for more information.
As a result of the foregoing, the unaudited pro forma condensed combined
financial information is based on the preliminary information available and management’s preliminary valuation of the fair value
of tangible and intangible assets acquired and liabilities assumed and the preliminary value of the consideration transferred. The actual
accounting may vary based on final analyses of the valuation of assets acquired and liabilities assumed, particularly in regard to indefinite-lived
intangible assets, which could be material. Renovaro will finalize the accounting for the Transaction as soon as practicable within the
measurement period in accordance with ASC 805 but in no event later than one year from the closing of the Transaction.
UNAUDITED PRO FORMA CONDENSED COMBINED BALANCE
SHEET
As of September 30, 2023
| | |
Renovaro Biosciences Inc. | |
GEDi Cube Intl Ltd | |
| |
| |
|
| | |
September 30, | |
September 30, | |
| |
| |
|
| | |
2023 | |
2023 | |
Adjustments | |
Notes | |
Pro-Forma Combined |
| | |
(Unaudited) | |
(Unaudited) | |
(Unaudited) | |
| |
(Unaudited) |
| ASSETS | |
| | | |
| | | |
| | | |
| | | |
| | |
| CURRENT ASSETS: | |
| | | |
| | | |
| | | |
| | | |
| | |
| Cash | |
$ | 523,474 | | |
$ | 359,840 | | |
| | | |
| | | |
| 883,314 | |
| Notes receivable | |
| 1,057,875 | | |
| — | | |
| (1,057,875 | ) | |
| [A] | | |
| — | |
| Prepaids and other assets | |
| 324,966 | | |
| 131,585 | | |
| | | |
| | | |
| 456,551 | |
| Total Current Assets | |
| 1,906,315 | | |
| 491,425 | | |
| (1,057,875 | ) | |
| | | |
| 1,339,865 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Property and equipment, net | |
| 482,510 | | |
| 15,355 | | |
| — | | |
| | | |
| 497,865 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| OTHER ASSETS: | |
| | | |
| | | |
| | | |
| | | |
| | |
| Definite life intangible assets, net | |
| 37,641 | | |
| — | | |
| | | |
| | | |
| 37,641 | |
| Indefinite life intangible assets | |
| 42,611,000 | | |
| — | | |
| 218,010,768 | | |
| [B] | | |
| 317,795,928 | |
| | |
| | | |
| | | |
| 57,174,160 | | |
| [B] | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Goodwill | |
| 11,640,000 | | |
| — | | |
| | | |
| | | |
| 11,640,000 | |
| Deposits and other assets | |
| 21,742 | | |
| — | | |
| | | |
| | | |
| 21,742 | |
| Operating lease right-of-use assets | |
| 863,598 | | |
| 651,931 | | |
| | | |
| | | |
| 1,515,529 | |
| Total Other Assets | |
| 55,173,981 | | |
| 651,931 | | |
| 275,184,928 | | |
| | | |
| 331,010,840 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| TOTAL ASSETS | |
$ | 57,562,806 | | |
$ | 1,158,711 | | |
| 274,127,053 | | |
| | | |
| 332,848,570 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| LIABILITIES | |
| | | |
| | | |
| | | |
| | | |
| | |
| CURRENT LIABILITIES: | |
| | | |
| | | |
| | | |
| | | |
| | |
| Accounts payable – trade | |
$ | 5,421,126 | | |
$ | 97,967 | | |
| | | |
| | | |
| 5,519,093 | |
| Accrued expenses | |
| 797,778 | | |
| 571,124 | | |
| | | |
| | | |
| 1,368,902 | |
| Other current liabilities | |
| — | | |
| — | | |
| — | | |
| | | |
| — | |
| Current portion of operating lease liabilities | |
| 214,061 | | |
| 262,586 | | |
| | | |
| | | |
| 476,647 | |
| Notes payable, net | |
| 2,909,987 | | |
| 1,057,376 | | |
| (1,057,376 | ) | |
| [A] | | |
| 2,909,987 | |
| Convertible notes payable | |
| 752,741 | | |
| — | | |
| | | |
| | | |
| 752,741.0 | |
| Total Current Liabilities | |
| 10,095,693 | | |
| 1,989,053 | | |
| (1,057,376 | ) | |
| | | |
| 11,027,370 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| NON-CURRENT LIABILITIES: | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Contingent Consideration | |
| — | | |
| — | | |
| 57,174,160 | | |
| [B] | | |
| 57,174,160 | |
| Operating lease liabilities, net of current portion | |
| 720,801 | | |
| 389,345 | | |
| | | |
| | | |
| 1,110,146 | |
| Total Non-Current Liabilities | |
| 720,801 | | |
| 389,345 | | |
| 57,174,160 | | |
| | | |
| 58,284,306 | |
| Total Liabilities | |
| 10,816,494 | | |
| 2,378,398 | | |
| 56,116,784 | | |
| | | |
| 69,311,676 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Commitments and Contingencies | |
| — | | |
| — | | |
| — | | |
| | | |
| — | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| STOCKHOLDERS’ EQUITY: | |
| | | |
| | | |
| | | |
| | | |
| | |
| Preferred stock | |
| 56 | | |
| — | | |
| — | | |
| | | |
| 56 | |
| Common Stock | |
| 6,571 | | |
| 2,634 | | |
| 19,251 | | |
| [B][D] | | |
| 28,456 | |
| Additional paid-in capital | |
| 300,008,449 | | |
| (2,634 | ) | |
| 219,093,536 | | |
| [B][D] | | |
| 519,099,351 | |
| Accumulated deficit | |
| (253,204,281 | ) | |
| (1,219,687 | ) | |
| (1,102,019 | ) | |
| [B][C][D] | | |
| (252,525,987 | ) |
| Accumulated other comprehensive loss | |
| (64,483 | ) | |
| — | | |
| (499 | ) | |
| [A] | | |
| (64,982 | ) |
| Total Stockholders’ Equity | |
| 46,746,312 | | |
| (1,219,687 | ) | |
| 218,010,269 | | |
| | | |
| 263,536,894 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | |
$ | 57,562,806 | | |
$ | 1,158,711 | | |
| 274,127,053 | | |
| | | |
| 332,848,570 | |
See notes to unaudited pro forma condensed combined
financial statements
UNAUDITED
PRO FORMA CONDENSED COMBINED STATEMENT OF OPERATIONS
For
the Year Ended June 30, 2023
| | |
(Audited) | |
(Unaudited) | |
(Unaudited) | |
| |
(Unaudited) |
| | |
Renovaro Biosciences Inc. | |
GEDi Cube Intl Ltd | |
Adjustments | |
Notes | |
Combined |
| Operating Expenses | |
| | | |
| | | |
| | | |
| | | |
| | |
| General and administrative | |
$ | 15,318,198 | | |
$ | 616,092 | | |
$ | 2,364,562 | | |
| [C][D] | | |
$ | 18,298,852 | |
| Research and development | |
| 4,165,197 | | |
| — | | |
| | | |
| | | |
| 4,165,197 | |
| Indefinite life intangible assets impairment charge | |
| 18,960,000 | | |
| — | | |
| | | |
| | | |
| 18,960,000 | |
| Depreciation and amortization | |
| 113,496 | | |
| 10,231 | | |
| | | |
| | | |
| 123,727 | |
| Total Operating Expenses | |
| 38,556,891 | | |
| 626,323 | | |
| 2,364,562 | | |
| | | |
| 41,547,776 | |
| LOSS FROM OPERATIONS | |
| (38,556,891 | ) | |
| (626,323 | ) | |
| (2,364,562 | ) | |
| | | |
| (41,547,776 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Other Income (Expenses) | |
| | | |
| | | |
| | | |
| | | |
| | |
| Loss on extinguishment of contingent consideration liability | |
| (419,182 | ) | |
| — | | |
| | | |
| | | |
| (419,182 | ) |
| Change in fair value of contingent consideration | |
| — | | |
| — | | |
| | | |
| | | |
| — | |
| Interest expense | |
| (580,344 | ) | |
| — | | |
| | | |
| | | |
| (580,344 | ) |
| Gain (loss) on foreign currency transactions | |
| (1,019 | ) | |
| — | | |
| | | |
| | | |
| (1,019 | ) |
| Interest income and other income (expense) | |
| (126,620 | ) | |
| 184,636 | | |
| | | |
| | | |
| 58,016 | |
| Total Other Income (Expenses) | |
| (1,127,165 | ) | |
| 184,636 | | |
| — | | |
| | | |
| (942,529 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Loss Before Income Taxes | |
| (39,684,056 | ) | |
| (441,687 | ) | |
| (2,364,562 | ) | |
| | | |
| (42,490,305 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Income Tax (Expense) Benefit | |
| — | | |
| — | | |
| — | | |
| | | |
| — | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| NET LOSS | |
$ | (39,684,056 | ) | |
$ | (441,687 | ) | |
$ | (2,364,562 | ) | |
| | | |
$ | (42,490,305 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| BASIC AND DILUTED NET LOSS PER COMMON SHARE | |
$ | (0.71 | ) | |
$ | (3,681 | ) | |
| | | |
| | | |
$ | (0.35 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| WEIGHTED AVERAGE NUMBER OF SHARES OF COMMON STOCK OUTSTANDING - BASIC AND DILUTED | |
| 56,265,362 | | |
| 120 | | |
| | | |
| | | |
| 121,310,755 | |
See notes to unaudited pro forma condensed combined
financial statements
UNAUDITED
PRO FORMA CONDENSED COMBINED STATEMENT OF OPERATIONS AND COMPREHENSIVE LOSS
For
the Three Months Ended September 30, 2023
| | |
(Unaudited) | |
(Unaudited) | |
(Unaudited) | |
| |
(Unaudited) |
| | |
Renovaro Biosciences Inc. | |
GEDi Cube Intl Ltd | |
Adjustments | |
Notes | |
Combined |
| Operating Expenses | |
| | | |
| | | |
| | | |
| | | |
| | |
| General and administrative | |
$ | 8,290,210 | | |
$ | 710,564 | | |
$ | 2,717,144 | | |
| [C][D] | | |
$ | 11,717,918 | |
| Research and development | |
| 566,644 | | |
| 505,391 | | |
| — | | |
| | | |
| 1,072,035 | |
| Depreciation and amortization | |
| 27,260 | | |
| 432 | | |
| — | | |
| | | |
| 27,692 | |
| Total Operating Expenses | |
| 8,884,114 | | |
| 1,216,387 | | |
| 2,717,144 | | |
| | | |
| 12,817,6455 | |
| LOSS FROM OPERATIONS | |
| (8,884,114 | ) | |
| (1,216,387 | ) | |
| 2,717,144 | | |
| | | |
| (12,817,645 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Other Income (Expenses) | |
| | | |
| | | |
| | | |
| | | |
| | |
| Loss on extinguishment of debt | |
| (120,018 | ) | |
| — | | |
| — | | |
| | | |
| (120,018 | ) |
| Loss on extinguishment of contingent consideration liability | |
| — | | |
| — | | |
| — | | |
| | | |
| — | |
| Interest expense | |
| (179,271 | ) | |
| (7,996 | ) | |
| 7,875 | | |
| [A] | | |
| (179,392 | ) |
| Interest income and other income (expense) | |
| 8,375 | | |
| (29,897 | ) | |
| (7,875 | ) | |
| [A] | | |
| (29,397 | ) |
| Total Other Income (Expenses) | |
| (290,914 | ) | |
| (37,893 | ) | |
| — | | |
| | | |
| (328,807 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Loss Before Income Taxes | |
| (9,175,028 | ) | |
| (1,254,280 | ) | |
| (2,717,144 | ) | |
| | | |
| (13,146,452 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Income Tax (Expense) Benefit | |
| — | | |
| — | | |
| — | | |
| | | |
| — | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| NET LOSS | |
$ | (9,175,028 | ) | |
$ | (1,254,280 | ) | |
$ | (2,717,144 | ) | |
| | | |
$ | (13,146,452 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| BASIC AND DILUTED NET LOSS PER COMMON SHARE | |
$ | (0.14 | ) | |
$ | (581.49 | ) | |
| | | |
| | | |
$ | (0.10 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| WEIGHTED AVERAGE NUMBER OF SHARES OF COMMON STOCK OUTSTANDING - BASIC AND DILUTED | |
| 64,480,753 | | |
| 2,157 | | |
| | | |
| | | |
| 129,704,842 | |
See notes to unaudited pro forma condensed combined
financial statements
The historical consolidated financial information of Renovaro has been
adjusted in the accompanying unaudited pro forma condensed combined financial information to give effect to pro forma events that are
(i) directly attributable to the Transaction, (ii) factually supportable, and (iii) with respect to the unaudited pro forma condensed
combined statements of operations and comprehensive loss, are expected to have a continuing impact on the results of operations.
The unaudited pro forma condensed combined financial statements were prepared
in accordance with the regulations of the SEC and are intended to show how the Transaction might have affected the historical financial
statements. The unaudited pro forma condensed combined financial information set forth herein is based upon the unaudited historical consolidated
financial statements of Renovaro and GEDi Cube. The unaudited pro forma condensed combined financial statements is presented as if the
Transaction had been completed on September 30, 2023, with respect to the unaudited pro forma condensed combined balance sheet as of September
30, 2023, and as of July 1, 2022, with respect to the unaudited pro forma condensed combined statements of operations for the twelve months
ended June 30, 2023 and the three months ended September 30, 2023 for the Transaction.
Renovaro’s combined financial information has
been prepared in accordance with Generally Accepted Accounting Principles in the United States, or GAAP, as issued by the Financial Accounting
Standards Board, or FASB. GEDi Cube’s financial information has been historically prepared in accordance with GAAP.
The unaudited pro forma condensed combined financial information is presented
for informational purposes only and is not necessarily indicative of the combined financial position or results of operations had the
Transaction occurred as of the dates indicated, nor is it meant to be indicative of any anticipated combined financial position or future
results of operations that the combined company will experience after the completion of the Transaction. To the extent there are significant
changes to the combined company’s business following the completion of the Transaction, the assumptions and estimates set forth
in the unaudited pro forma condensed combined financial statements could change significantly.
Renovaro determined the Transaction constitutes a business combination
and is accounting for the Transaction using the acquisition method.
The unaudited pro forma adjustments included in the unaudited pro forma
condensed combined financial statements are based on preliminary estimates that may change significantly as additional information is
obtained. are as follows:
| A. | The elimination of intercompany Notes and related interest income and expense between Renovaro GEDi Cube. |
| B. | To record the 100% acquisition of GEDi Cube through the issuance of 65,224,089 shares of common stock at the estimated $3.34 per
share price and establish contingent consideration for potential Earnout Shares of 17,165,631 common shares (see Table 1 for
details) at the estimated share price of $3.34, and to eliminate the net stockholders’ equity accounts of GEDi Cube prior to
the acquisition. |
| |
C. |
To eliminate management fees paid by GEDi Cube that are not being carried forward to the combined company. |
| |
|
|
| |
D. |
To record one-time consulting fees of $2.760,000 related to the Transaction that was paid in shares at 10/23/2023 closing price of $2.76. |
INDEX TO CONSOLIDATED FINANCIAL
STATEMENTS
Grace Systems BV
Unaudited Consolidated Interim Financial
Statements
| Unaudited
Consolidated Balance Sheets as of September 30, 2023 (Successor) and December 31, 2022 (audited) (Predecessor) |
137 |
| Unaudited Consolidated Statements of Operations for the Period from August 24, 2023
to September 30, 2023 (Successor), the Period from January 1, 2023 to August 23, 2023 (Predecessor), and Nine Months Ended September
30, 2022 (Predecessor) |
138 |
| Unaudited Consolidated Statements of Changes in Stockholders' Equity (Deficit) for
the Period from August 24, 2023 to September 30, 2023 (Successor), the Period from January 1, 2023 to August 23, 2023 (Predecessor),
and the Nine Months Ended September 30, 2022 (Predecessor) |
139 |
| Unaudited Consolidated Statements of Cash Flows for the Period from August 24, 2023
to September 30, 2023 (Successor), the Period from January 1, 2023 to August 23, 2023 (Predecessor), and the Nine Months Ended
September 30, 2022 (Predecessor) |
140 |
| Notes to the Unaudited Consolidated Financial Statements |
141 |
| |
|
| Audited Consolidated Financial Statements |
|
Gedi Cube Intl Ltd
Unaudited Consolidated Balance Sheets (In Euros)
| |
|
Successor |
|
Predecessor |
| |
|
September 30, 2023 |
|
December 31, 2022 |
| |
|
(unaudited) |
|
|
| Assets |
|
|
|
|
|
|
|
|
| Current assets: |
|
|
|
|
|
|
|
|
| Cash |
|
€ |
339,985 |
|
|
€ |
1,583 |
|
| VAT receivable |
|
|
54,193 |
|
|
|
2,189 |
|
| Deposits |
|
|
45,348 |
|
|
|
— |
|
| Prepaid expenses |
|
|
24,784 |
|
|
|
— |
|
| Total current assets |
|
|
464,310 |
|
|
|
3,772 |
|
| Operating lease right-of-use assets |
|
|
615,959 |
|
|
|
— |
|
| Property, plant, and equipment, net |
|
|
14,508 |
|
|
|
37,552 |
|
| Total assets |
|
€ |
1,094,777 |
|
|
€ |
41,324 |
|
| |
|
|
|
|
|
|
|
|
| Liabilities and Stockholders’ Deficit |
|
|
|
|
|
|
|
|
| Current liabilities: |
|
|
|
|
|
|
|
|
| Accounts payable |
|
|
92,561 |
|
|
|
77,340 |
|
| Accrued expenses |
|
|
494,795 |
|
|
|
284,038 |
|
| Due to related parties |
|
|
44,818 |
|
|
|
14,608 |
|
| Current portion of operating lease liabilities |
|
|
248,097 |
|
|
|
— |
|
| Notes payable |
|
|
999,032 |
|
|
|
— |
|
| Total current liabilities |
|
|
1,879,303 |
|
|
|
375,986 |
|
| Operating lease liabilities, net of current portion |
|
|
367,862 |
|
|
|
— |
|
| Total liabilities |
|
€ |
2,247,165 |
|
|
€ |
375,986 |
|
| |
|
|
|
|
|
|
|
|
| Stockholders’ Deficit: |
|
|
|
|
|
|
|
|
| Predecessor Common shares, €0.01 par value; 120 shares authorized; 120 shares issued and outstanding as of December 31, 2022 |
|
|
|
|
|
|
|
|
| Successor Common shares, £1.00 par value; 2,157 shares authorized; 2,157 shares issued and outstanding as of September 30, 2023 |
|
|
2,489 |
|
|
|
1 |
|
| Additional paid-in capital |
|
|
(2,489 |
) |
|
|
100 |
|
| Accumulated Deficit |
|
|
(1,152,388 |
) |
|
|
(334,763 |
) |
| Total stockholders’ deficit |
|
|
(1,152,388 |
) |
|
|
(334,662 |
) |
| Total liabilities and stockholders’ deficit |
|
€ |
1,094,777 |
|
|
€ |
41,324 |
|
The
accompanying notes are an integral part of these unaudited consolidated financial statements.
Gedi
Cube Intl Ltd
Unaudited Consolidated Statements of Operations (In Euros)
| | |
Successor | |
Predecessor |
| | |
Period from August 24, 2023 to September 30, 2023 | |
Period from January 1, 2023 to August 23, 2023 | |
Nine months ended September 30, 2022 |
| | |
| |
| |
|
| Revenue | |
€ | — | | |
€ | — | | |
€ | — | |
| Cost of Revenue | |
| — | | |
| — | | |
| — | |
| Gross Loss | |
| — | | |
| — | | |
| — | |
| | |
| | | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | |
| Selling, general and administrative | |
| 242,910 | | |
| 23,193 | | |
| 100,077 | |
| Depreciation | |
| 397 | | |
| 1,733 | | |
| 10,913 | |
| Management fees | |
| 39,375 | | |
| 244,441 | | |
| 290,104 | |
| In process research and development expense | |
| 464,335 | | |
| — | | |
| — | |
| Advisory Fees | |
| 133,888 | | |
| 6,021 | | |
| 1,975 | |
| Salaries and wages | |
| 236,669 | | |
| 30,450 | | |
| 65,359 | |
| Total operating expenses | |
| 1,117,574 | | |
| 305,838 | | |
| 468,427 | |
| | |
| | | |
| | | |
| | |
| Loss from operations | |
| (1,117,574 | ) | |
| (305,838 | ) | |
| (468,427 | ) |
| | |
| | | |
| | | |
| | |
| Other (expense) income | |
| | | |
| | | |
| | |
| Gain from deconsolidation | |
| — | | |
| 176,266 | | |
| — | |
| Interest expense | |
| (7,346 | ) | |
| — | | |
| — | |
| Loss on exchange | |
| (27,468 | ) | |
| — | | |
| — | |
| Total other (expense) income | |
| (34,814 | ) | |
| 176,266 | | |
| — | |
| | |
| | | |
| | | |
| | |
| Loss before income taxes | |
| (1,152,388 | ) | |
| (129,572 | ) | |
| (468,427 | ) |
| | |
| | | |
| | | |
| | |
| Provision for income taxes | |
| — | | |
| — | | |
| — | |
| | |
| | | |
| | | |
| | |
| Loss from operations | |
| (1,152,388 | ) | |
| (129,572 | ) | |
| (468,427 | ) |
| | |
| | | |
| | | |
| | |
| Net loss | |
€ | (1,152,388 | ) | |
€ | (129,572 | ) | |
€ | (468,427 | ) |
| | |
| | | |
| | | |
| | |
| Basic and diluted loss per share | |
€ | (534 | ) | |
€ | (1,080 | ) | |
€ | (3,904 | ) |
| | |
| | | |
| | | |
| | |
| Weighted Average number of shares of common stock outstanding - Basic and diluted | |
| 2,157 | | |
| 120 | | |
| 120 | |
The
accompanying notes are an integral part of these unaudited consolidated financial statements.
Gedi Cube Intl Ltd
Unaudited Consolidated Statements of Changes in Stockholders' Deficit (In Euros)
| Predecessor |
| | |
Shares | |
Amount | |
Additional Paid-In Capital | |
Accumulated Deficit | |
Total Stockholders’ Deficit |
| Balance at January 1, 2022 | |
| 120 | | |
€ | 1 | | |
€ | 100 | | |
€ | 289,219 | | |
€ | 289,320 | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| (623,982 | ) | |
| (623,982 | ) |
| Balance at December 31, 2022 | |
| 120 | | |
€ | 1 | | |
€ | 100 | | |
€ | (334,763 | ) | |
€ | (334,662 | ) |
| Net loss | |
| — | | |
| — | | |
| — | | |
| (129,572 | ) | |
| (129,572 | ) |
| Balance at August 23, 2023 | |
| 120 | | |
€ | 1 | | |
€ | 100 | | |
€ | (464,335 | ) | |
€ | (464,234 | ) |
| Successor |
| | |
Shares | |
Amount | |
Additional Paid-In Capital | |
Accumulated Deficit | |
Total Stockholders’ Equity |
| Balance at August 24, 2023 | |
| 1,100 | | |
€ | 1,269 | | |
€ | (1,269 | ) | |
€ | — | | |
€ | — | |
| Net Loss | |
| — | | |
| — | | |
| — | | |
| (1,152,388 | ) | |
| (1,152,388 | ) |
| Shares issued as part of acquisition | |
| 1,057 | | |
| 1,220 | | |
| (1,220 | ) | |
| — | | |
| — | |
| Balance at September 30, 2023 | |
| 2,157 | | |
€ | 2,489 | | |
€ | (2,489 | ) | |
€ | (1,152,388 | ) | |
€ | (1,152,388 | ) |
The
accompanying notes are an integral part of these unaudited consolidated financial statements.
Gedi
Cube Intl Ltd
Unaudited Consolidated Statements of Cash Flows (In Euros)
| | |
Successor | |
Predecessor |
| | |
Period from August 24, 2023 to September 30, 2023 | |
Period January 1 2023 to August 23, 2023 | |
Nine months ended September 30, 2022 |
| Cash flows from operating activities: | |
| | | |
| | | |
| | |
| Net loss | |
€ | (1,152,388 | ) | |
€ | (129,572 | ) | |
€ | (468,427 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | |
| | | |
| | | |
| | |
| Depreciation | |
| 397 | | |
| 1,733 | | |
| 10,913 | |
| Amortization of ROU Asset | |
| 17,575 | | |
| | | |
| | |
| Effect of exchange rates on cash | |
| 27,468 | | |
| — | | |
| — | |
| Gain from deconsolidation | |
| — | | |
| (176,266 | ) | |
| — | |
| Write off of in process research & development intangible asset | |
| 464,335 | | |
| — | | |
| — | |
| Changes in assets/liabilities: | |
| | | |
| | | |
| | |
| Prepaid expenses | |
| (24,784 | ) | |
| — | | |
| (2,279 | ) |
| Deposits | |
| (45,348 | ) | |
| — | | |
| — | |
| Accounts payable | |
| 71,662 | | |
| 24,305 | | |
| 43,942 | |
| Operating lease liabilities | |
| (17,575 | ) | |
| — | | |
| — | |
| Accrued Liabilities | |
| 100,449 | | |
| 183,243 | | |
| 177,948 | |
| Due to related parties | |
| (14,065 | ) | |
| 99,728 | | |
| 12,000 | |
| VAT receivable | |
| (49,978 | ) | |
| (4,721 | ) | |
| (13,531 | ) |
| VAT payable | |
| — | | |
| — | | |
| (7,409 | ) |
| Net cash used in operating activities | |
| (622,252 | ) | |
| (1,550 | ) | |
| (246,842 | ) |
| | |
| | | |
| | | |
| | |
| Cash flows from investing activities: | |
| | | |
| | | |
| | |
| Property, plant, and equipment, net | |
| (1,982 | ) | |
| — | | |
| (1,057 | ) |
| Net cash used in investing activities | |
| (1,982 | ) | |
| — | | |
| (1,057 | ) |
| | |
| | | |
| | | |
| | |
| Cash flows from financing activities: | |
| | | |
| | | |
| | |
| Proceeds from loan payable | |
| 964,218 | | |
| — | | |
| — | |
| Net cash provided by financing activities | |
| 964,218 | | |
| — | | |
| — | |
| | |
| | | |
| | | |
| | |
| Cash: | |
| | | |
| | | |
| | |
| Net change during the period | |
| 339,985 | | |
| (1,550 | ) | |
| (247,900 | ) |
| Balance, beginning of period | |
| — | | |
| 1,583 | | |
| 248,797 | |
| Balance, end of period | |
€ | 339,985 | | |
€ | 33 | | |
€ | 897 | |
| | |
| | | |
| | | |
| | |
| Supplemental disclosure of cash flow information: | |
| | | |
| | | |
| | |
| Interest paid in cash | |
| 2,038 | | |
| — | | |
| — | |
| Income taxes | |
| — | | |
| — | | |
| — | |
| | |
| | | |
| | | |
| | |
| Supplemental disclosure of non-cash activity: | |
| | | |
| | | |
| | |
| Non-cash amounts of lease liabilities arising from obtaining right-of-use assets | |
| 633,534 | | |
| — | | |
| — | |
The
accompanying notes are an integral part of these unaudited consolidated financial statements.
Gedi Cube Intl Ltd
Notes to the Unaudited Consolidated Financial Statements
Note 1. Organization
Gedi Cube Intl Ltd, formed June 14, 2023, and its
subsidiaries (Grace Systems B.V. and GEDi Cube B.V.) (collectively, the ”Company”), headquartered in the United Kingdom,
specialize in data science and data mining and plan to be disruptive in the industry with a broad range of cutting edge software, services,
methods, and models to tackle big data challenges. The Company provides services in the field of software development and maintenance,
data mining, and data science. The Company focuses on the high-end of advanced and predictive analytics which contributes to our ability
to combine big data stores, execute scientific algorithms and visualize analytics to business users. The Company aims to provide and accelerate
precision, personalized medicine for longevity powered by mutually reinforcing AI and biotechnology platforms for early diagnosis, better
targeted treatments and drug discovery. As of the report date, the Company is in the development stage of its lifecycle and has not yet
commenced operations.
Note 2. Summary of Significant Accounting Policies
Basis of Presentation and Principles of Consolidation
The accompanying consolidated financial statements
have been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”) as defined
by the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”). The consolidated
financial statements include the accounts of Gedi Cube Intl Ltd and its subsidiaries. All material inter-company balances and transactions
have been eliminated.
In the opinion of Gedi Cube Intl Ltd , all adjustments
(consisting of normal recurring accruals) considered necessary for a fair presentation have been included. Interim results for the period
from August 24, 2023 to September 30, 2023 are not necessarily indicative of the results for the full year ending December 31, 2023.
These interim unaudited consolidated financial statements should be read in conjunction with the Grace Systems B.V. audited consolidated
financial statements and notes for the years ended December 31, 2022 and 2021.
In June 2023 Grace Systems B.V. (“Grace”), (“Predecessor”)
and Gedi Cube Intl Ltd (“GEDiCube Int”), (“Successor”) entered into a Binding Head of Terms for the acquisition
of 100% of the share capital in Grace. On August 23, 2023, Grace and GEDiCube Int executed a Share Purchase Agreement (“Agreement”)
for the acquisition of 100% of the share capital in Grace, involving a €1 million investment into the Company by GEDiCube Int. In
exchange, existing shareholders of Grace were allocated 49% ownership in GEDiCube Int through a share-exchange transaction, ensuring their
continued involvement in the development and commercialization of Grace’s underlying Intellectual Property (“IP”) and
Trade Secrets.
The unaudited consolidated financial statements of
Successor and Predecessor are not comparable due to the acquisition described above. Gedi Cube Int. was incorporated in June 2023 with
no operational history and has no prior comparative period financial statements presented. Therefore, the reporting period has been separated
by a black line in the consolidated financial statements with the Predecessor representing the pre-Closing Date period (January 1, 2023
through August 23, 2023) and the Successor representing the post-Closing Date period (August 24, 2023 through September 30,
2023). The Company noted that the Predecessor includes financial information related to the Grace Systems (as defined in Note 12), while
the Successor includes financial information related to the newly formed company after the asset acquisition.
Business Combinations
The Company accounts for business combinations under
Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 805 “Business Combinations”
using the acquisition method of accounting, and accordingly, the assets and liabilities of the acquired business are recorded at their
fair values at the date of acquisition. All acquisition costs are expensed as incurred. Upon acquisition, the accounts and results of
operations are included as of and subsequent to the acquisition date.
Gedi
Cube Intl Ltd
Notes to the Unaudited Consolidated Financial Statements
In analyzing the business combination for proper accounting
treatment, management utilized ASC 805 & ASU 2017-01. ASU 2017-01 created a new framework that companies must use to evaluate whether
an integrated set of assets and activities is a business and should be accounted for as a business acquisition. FASB defines a business
as a set of activities and assets that include an input and a substantive process; however, the fair value of the set is not usually concentrated
in single or multiple assets. This set of inputs and processes usually provides goods and services to customers and return to stakeholders
(ASC 805-10-55-3A and ASC 805-10-55-4). The new guidance requires an initial screening test to determine if substantially all of the fair
value of the assets acquired is concentrated in one asset or a group of similar assets.
ASC 805 also provides a screen test to assess whether
substantially all of the fair value of the gross assets acquired is concentrated in a single identifiable asset or group of similar identifiable
assets and allows organizations to assess whether acquired activities or assets are considered a business. The screen test reduces the
number of transactions that might otherwise be considered a business combination by providing a method for aggregating similar assets
into one acquired asset group. This aggregation provides that substantially all of the assets in the transaction be part of this asset
group to qualify under the practical screen.
As discussed in Note 3, the acquisition of Grace Systems
by Gedi Cube Int LTD was treated as an asset acquisition.
Proposed Transaction
On September 29, 2023 the Company and Renovaro Biosciences, Inc. (“Renovaro”)
executed a definitive agreement to combine (the “Proposed Transaction”). The Proposed Transaction is being structured as a
stock-for-stock acquisition and is subject to Renovaro shareholder approval which is still pending as of the date of issuance of these
financial statements.
Going Concern
As of September 30, 2023 (Successor), the Company
had cash of approximately €339,985. For the year ended September 30, 2023 (Successor), the Company used €(622,252) in cash
from operating activities and had an accumulated deficit of €1,152,388. These factors raise substantial doubt regarding the Company’s
ability to continue as a going concern for a period of one year from the issuance of these financials. The continuation of the Company
as a going concern is dependent upon the continued financial support from its stockholders and debt holders. Specifically, continuation
is contingent on the Company’s ability to obtain necessary equity or debt financing to continue operations, and ultimately the Company’s
ability to generate profit from sales and positive operating cash flows, which is not assured. These accompanying audited consolidated
financial statements have been prepared assuming that the Company will continue as a going concern and do not include any adjustments
that might result from the outcome of this uncertainty.
Use of Estimates
The preparation of the consolidated financial statements,
in conformity with U.S. GAAP, requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities
and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue
and expenses during the reporting period. Management evaluates its estimates and assumptions on an ongoing basis using historical experience
and other factors, including the current economic environment, and makes adjustments when facts and circumstances dictate. These estimates
are based on information available as of the date of the financial statements; therefore, actual results could differ from those estimates.
The most significant estimates and judgments include those related to the asset acquisition, valuation allowance for deferred taxes and
the discount rates used in determining right of use assets and related lease liabilities.
Cash and Cash Equivalents
As of September 30, 2023 and December 31,
2022 the Company’s cash balances were €339,985 and €1,583, respectively. Cash is recorded at cost, which approximates
fair value. As of September 30, 2023 and December 31, 2022, cash consisted of checking and savings deposits.
Property, Plant and Equipment
Property, plant and equipment is stated at cost, less
accumulated depreciation. Maintenance and repairs are charged to expense when incurred. Additions and improvements that extend the economic
useful life of the asset are capitalized and depreciated over the remaining useful lives of the assets. The cost and accumulated depreciation
of assets sold or retired are removed from the respective accounts, and any resulting gain or loss is reflected in current earnings. Depreciation
is recognized using the straight-line method in amounts considered to be sufficient to allocate the cost of the assets to operations over
the estimated useful lives of the assets, all of which are 5 years.
Gedi Cube Intl Ltd
Notes to the Unaudited Consolidated Financial Statements
Impairment of Long Lived Assets
Long-lived assets, such as property, plant and equipment,
are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable.
Circumstances which could trigger a review include, but are not limited to: significant decreases in the market price of the asset; significant
adverse changes in the business climate or legal factors; current period cash flow or operating losses combined with a history of losses
or a forecast of continuing losses associated with the use of the asset; and current expectations that the asset will more likely than
not be sold or disposed of significantly before the end of its estimated useful life.
Recoverability of assets to be held and used is measured
by a comparison of the carrying amount of an asset to estimated undiscounted future cash flows expected to be generated by the asset.
If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, an impairment charge is recognized for the amount
by which the carrying amount of the asset exceeds the fair value of the asset. Assets to be disposed of would be separately presented
in the balance sheet and reported at the lower of the carrying amount or fair value less costs to sell and would no longer be depreciated.
The depreciable basis of assets that are impaired and continue in use are their respective fair values.
Revenue Recognition
Revenue is recognized when promised goods or services
are transferred to customers in an amount that reflects the consideration to which the Company expects to be entitled in exchange for
those goods or services by following a five-step process: (1) identify the contract with a customer, (2) identify the performance obligations
in the contract, (3) determine the transaction price, (4) allocate the transaction price, and (5) recognize revenue when or as the Company
satisfies a performance obligation. There was no revenue recognized during the 12 months ending December 31, 2022 and the nine months
ending September 30, 2023.
Value Added Tax
The company collects value added tax (“VAT”)
on sales (“VAT payable”) and offsets it with deducted VAT from eligible purchases (“VAT receivable”). The standard
rate is 21%, with reduced rates applicable to specific goods and services. Amounts are reported quarterly to the tax authorities in April,
July, October, and January directly via an online platform or through the use of certain software. In the case of a net payable VAT, the
company pays directly to the tax authorities within the month, and if the net VAT is receivable, the company receives a letter from the
tax office with a refund date and details, usually within 5 weeks. Periodic VAT returns offer a comprehensive breakdown of both output
and input taxes. Submitting a VAT return is mandatory for all companies registered with the Chamber of Commerce in the Netherlands (“KVK”)
and the United Kingdom.
Leases
In accordance with ASC Topic 842, the Company determined
the initial classification and measurement of its right-of-use assets and lease liabilities at the lease commencement date and thereafter.
The lease terms include any renewal options and termination options that the Company is reasonably assured to exercise, if applicable.
The present value of lease payments is determined by using the implicit interest rate in the lease, if that rate is readily determinable;
otherwise, the Company develops an incremental borrowing rate based on the information available at the commencement date in determining
the present value of the future payments. Rent expense for operating leases is recognized on a straight-line basis, unless the operating
lease right of use assets have been impaired, over the reasonably assured lease term based on the total lease payments and is included
in operating expenses in the consolidated statements of operations. For operating leases that reflect impairment, the Company will recognize
the amortization of the operating lease right-of-use assets on a straight-line basis over the remaining lease term with rent expense still
included in selling, general and administrative expenses in the unaudited consolidated statements of operations. The Company has elected
the practical expedient to not separate lease and non-lease components. The Company’s non-lease components are primarily related
to property maintenance, insurance, and taxes, which vary based on future outcomes, and thus are recognized in selling, general and administrative
expenses when incurred.
Gedi Cube Intl Ltd
Notes to the Unaudited Consolidated Financial Statements
Functional Currency
The local currency, Euros, is the functional currency
for all of the Company’s activity. Gains and losses from foreign currency transactions are included in net loss for the period.
Income Taxes
Income taxes provide for the tax effects of transactions
reported in the consolidated financial statements and consist of primarily deferred tax assets and liabilities. Deferred tax assets and
liabilities are recognized for differences between the basis of assets and liabilities for financial statement and income tax purposes.
The differences relate primarily to net operating losses. The future realization of deferred tax assets is subject to the existence of
sufficient future taxable income. Currently, management believes that it is not more likely than not that the Company will realize the
benefits of any tax loss carryforwards.
Due to the historical net loss position of the Company,
and the lack of sufficient taxable income to realize the deferred tax asset, the Company recorded a full valuation allowance €201,616
and €128,760 as of September 30, 2023 and December 31, 2022.
Segment Information
The Company has one operating segment with one planned
business activity as described in Note 1 The Company’s chief operating decision maker is its Chief Executive Officer, who manages
operations and reviews financial information on a consolidated basis for the purpose of assessing performance and allocating resources.
Net Loss Per Share Calculation
Basic earnings (loss) per common share (“EPS”)
is computed by dividing net income (loss) available to common stockholders by the weighted-average number of common shares outstanding
for the period. Diluted earnings (loss) per share is computed by dividing net income (loss) by the weighted average shares outstanding,
assuming all dilutive potential common shares were issued. Dilutive loss per share excludes all potential common shares if their effect
is anti-dilutive. No potentially dilutive debt or equity instruments were issued or outstanding during the period from August 24, 2023
to September 30, 2023 (Successor), for the nine months ended September 30, 2022, and the period from January 1, 2023 to August 23, 2023.
Recent Accounting Pronouncements
Accounting Pronouncements Recently Adopted
In February 2016, the FASB issued ASU 2016-02, Leases
(Topic 842) (“ASU 2016-02”), which supersedes the current accounting for leases and while retaining two distinct types of
leases, finance and operating, (i) requires lessees to record a right of use asset and a related liability for the rights and obligations
associated with a lease, regardless of lease classification, and recognize lease expense in a manner similar to current accounting, (ii)
eliminates most real estate specific lease provisions, and (iii) aligns many of the underlying lessor model principles with those in the
new revenue standard. Leases with a term of 12 months or less will be accounted for similar to existing guidance for operating leases.
The new standard is effective for fiscal years beginning after December 15, 2021, with early adoption permitted, based on the FASB effective
date deferral per ASU 2020-05. A modified retrospective transition approach is required, applying the new standard to all leases existing
at the date of initial application. An entity may choose to use either (1) its effective date or (2) the beginning of the earliest comparative
period presented in the financial statements as its date of initial application. If an entity chooses the second option, the transition
requirements for existing leases also apply to leases entered into between the date of initial application and the effective date. The
company utilized the effective date and adopted the provisions of this standard on January 1, 2021.
Gedi Cube Intl Ltd
Notes to the Unaudited Consolidated Financial Statements
Note 3. Share Purchase Agreement- Asset Acquisition
On August 24, 2023, Grace Systems B.V. (“Seller”)
and Gedi Cube Int Ltd (“Buyer”) executed a Share Purchase Agreement (the “Agreement”). Existing shareholders of
Grace Systems exchanged their shares for a 49% interest of the Buyer. Additionally, Gedi Cube Int Ltd made an investment in the Company
totaling €1,000,000.
The Buyers acquisition of Grace Systems B.V encompasses
the transfer of a vital research and development project focused on an AI platform designed for early cancer detection. This project,
known as In-Process Research and Development (“IPR&D”), includes not only the current research but also historical expertise,
protocols, designs, and procedures required to facilitate Gedi’s subsequent testing phase, ultimately bringing the project to a
revenue-generating stage.
In evaluating the nature of this transaction and the
relevant accounting guidance, management evaluated the guidance in paragraphs ASC 805-10-55-5A through 55-5C. As such, management concluded
that that the IPR&D AI project qualifies as an identifiable intangible asset within the context of a business combination and represents
over 90% of the fair value of the assets acquired, therefore accounting for the acquisition as an asset acquisition. The Company allocated
substantially all of the value to the IPR&D asset of date of acquisition noting that the amount was expensed as incurred as it was
determined that there is no alternative future use which represents the amount of the assumed net liabilities of the Company of €464,335.
Note 4. Prepaid Expenses
Prepaid expenses consisted of the following:
| | |
Successor | |
Predecessor |
| | |
September 30,
2023 | |
December 31,
2022 |
| Prepaid audit expenses | |
€ | 13,778 | | |
€ | — | |
| Prepaid legal expenses | |
| 7,031 | | |
| — | |
| Prepaid rent expense | |
| 3,975 | | |
| — | |
| Total prepaid expenses | |
€ | 24,784 | | |
€ | — | |
Gedi Cube Intl Ltd
Notes to the Unaudited Consolidated Financial Statements
Note 5. Property, Plant, and Equipment, net
Property, plant, and equipment consisted of the following:
| | |
Successor | |
Predecessor |
| | |
September 30,
2023 | |
December 31,
2022 |
| Computer equipment and software | |
€ | 4,809 | | |
€ | 5,866 | |
| Computer hardware | |
| 10,096 | | |
| 50,665 | |
| Machines and installations | |
| — | | |
| 21,196 | |
| | |
| 14,905 | | |
| 77,727 | |
| Less: Accumulated depreciation | |
| (397 | ) | |
| (40,175 | ) |
| Property, plant, and equipment, net | |
€ | 14,508 | | |
€ | 37,552 | |
The Company uses the straight line method to calculate
depreciation expense. Depreciation expense totaled €397 and €14,591 for the period from August 24, 2023 to September 30, 2023
(Successor) and for the year ended December 31, 2022 (Predecessor), respectively. On April 24, 2023, Grace Datascience B.V., a subsidiary
of Gedi Cube Intl Ltd, entered into bankruptcy proceedings and was deconsolidated from the group financials. The balance of Property,
Plant, and Equipment, net on the Grace Datascience B.V. balance sheet immediately prior to the bankruptcy was €22,894. Refer to Note
12 for additional information related to the bankruptcy.
Gedi Cube Intl Ltd
Notes to the Unaudited Consolidated Financial Statements
Note 6. Accrued Expenses
Accrued expenses consisted of the following:
| | |
Successor | |
Predecessor |
| | |
September 30,
2023 | |
December 31,
2022 |
| Accrued payroll | |
€ | 48,369 | | |
€ | 32,038 | |
| Accrued management fees | |
| 401,625 | | |
| 252,000 | |
| Other accrued expenses | |
| 44,801 | | |
| — | |
| Total accrued expenses | |
€ | 494,795 | | |
€ | 284,038 | |
The other accrued expenses seen in the table above
relate to accruals for normal operating expenses. On April 24, 2023, Grace Datascience B.V., a subsidiary of Gedi Cube Intl Ltd, entered
into bankruptcy proceedings and was deconsolidated from the group financials. The balance of Accrued Expense on the Grace Datascience
B.V. balance sheet immediately prior to the bankruptcy was €65,656 and the total amount was related to accrued payroll. Refer to
Note 12 for additional information related to the bankruptcy.
Gedi Cube Intl Ltd
Notes to the Unaudited Consolidated Financial Statements
Note 7. Commitments and Contingencies
Operating Lease
The Company leases an office facility in Amsterdam,
Netherlands, under a 30 month operating lease agreement commencing on September 1, 2023 with a maturity date of February 28, 2026. In
determining lease asset values, the Company considers fixed and variable payment terms, prepayments, incentives, and options to extend,
terminate or purchase.
The Company leases an apartment in Amsterdam, Netherlands,
under a 23.4 month operating lease agreement commencing on September 19, 2023 with a maturity date of August 31, 2025. In determining
lease asset values, the Company considers fixed and variable payment terms, prepayments, incentives, and options to extend, terminate
or purchase.
Right-of-use asset and lease liability for operating
leases were recorded in the combined and consolidated balance sheets as follows:
| | |
Successor | |
Predecessor |
| | |
Period from August 24, 2023 to September 30, 2023 | |
December 31, 2022 |
| Operating leases: | |
| | | |
| | |
| Operating lease right-of-use asset, net | |
€ | 615,959 | | |
€ | — | |
| | |
| | | |
| | |
| Lease liability, current | |
| 248,097 | | |
| — | |
| Lease liability, long term | |
| 367,862 | | |
| — | |
| Total operating lease liability | |
€ | 615,959 | | |
€ | — | |
The weighted average remaining lease term for the
operating lease is 2.35 years and the incremental borrowing rate is 6.75% as of September 30, 2023.
As of September 30, 2023, future minimum lease payments
required under operating leases are as follows:
| | |
Successor | |
Predecessor |
| | |
Period from August 24, 2023 to September 30, 2023 | |
December 31, 2022 |
| | 2023 | | |
€ | 68,925 | | |
€ | — | |
| | 2024 | | |
| 281,400 | | |
| — | |
| | 2025 | | |
| 272,511 | | |
| — | |
| | 2026 | | |
| 40,314 | | |
| — | |
| | Total minimum lease payments | | |
| 663,150 | | |
| — | |
| | Less: effects of discounting | | |
| (47,191 | ) | |
| — | |
| | Present value of future minimum lease payments | | |
€ | 615,959 | | |
€ | — | |
Gedi Cube Intl Ltd
Notes to the Unaudited Consolidated Financial Statements
Note 8. Stockholder’ Equity (Deficit)
Common Stock - Predecessor
The Predecessor was formed as a partnership and incorporated
on June 3, 2013. Upon the Predecessor’s formation, the Company issued capital in the amount of 120 regular common stock shares with
a par value of €0.01. Each common share affords the holder general voting rights as defined in the Shareholders Agreement and summarized
below.
Common Stock - Successor
On August 24, 2023, Grace Systems B.V. and Gedi Cube
Int Ltd executed a Share Purchase Agreement. Existing shareholders of Grace Systems exchanged their shares for a 49% interest of Gedi
Cube Int Ltd where the remaining controlling interest was purchased by Gedi Cube in exchange for consideration.
Common stock of the Successor and Predecessor are
outlined below:
| | |
Successor | |
Predecessor |
| | |
September 30, 2023 | |
December 31, 2022 |
| | Common Stock shares outstanding as of August 24, 2023 and January 1, 2022, respectively | | |
| 1,100 | | |
| 120 | |
| | Shares issued as part of acquisition | | |
| 1,057 | | |
| — | |
| | Common Stock shares outstanding as of September 30, 2023 and December 31, 2022, respectively | | |
| 2,157 | | |
| 120 | |
Note 9. Income Taxes
During the nine months ended September 30, 2023, the
Company did not record an income tax provision or benefit due to net operating losses. The Company recorded a full valuation allowance
against its deferred tax assets due to the lack of taxable income needed to realize the deferred tax assets. The Company has used a discrete
effective tax rate method to calculate taxes for the period from August 24, 2023 to September 30, 2023 (Successor), for the nine months
ended September 31, 2022, and the period from January 1, 2023 to August 23, 2023. The Company believes that, at this time, the use of
the discrete method is more appropriate than the estimated annual effective tax rate method as the estimated annual effective tax rate
method is not reliable due to a high degree of uncertainty in estimating annual pre-tax earnings.
Gedi Cube Intl Ltd
Notes to the Unaudited Consolidated Financial Statements
Note 10. Risks and Uncertainties
The Company prepares consolidated financial statements
in conformity with U.S. GAAP. It is possible that future requirements could change the current application of U.S. GAAP, resulting in
a material adverse impact on the Company’s balance sheets or results of operations. In addition, the future effective tax rates
could be unfavorably affected by changes in tax laws or the interpretation of tax laws or by changes in the valuation of our deferred
tax assets and liabilities. The Company regularly assess the implementation of applicable accounting principles and the adequacy of provision
for income taxes. The final determination of any tax authority, upon examination of the Company’s tax returns, could have an adverse
effect on the Company’s operating results and balance sheets.
The Company is in the development stage and faces
all of the risks and uncertainties associated with a new and unproven business. The Company’s future is based on an unproven business
plan with no historical facts to support projections and assumptions. The Company’s operations are subject to all of the risks inherent
in the establishment of a new business enterprise. The likelihood of the Company’s success must be considered in light of the problems,
expenses, difficulties, complications and delays frequently encountered in connection with the formation of a pre-revenue business. The
Company’s lack of a significant and relevant operating history makes it difficult to manage operations and predict future operating
results.
Note 11. Related Parties
Related party balances consisted of the following:
| | |
Successor | |
Predecessor |
| | |
September 30, 2023 | |
December 31, 2022 |
| Imbott Group BV | |
€ | — | | |
€ | 500 | |
| RC Grace Bio Omics / formerly Linkin Data | |
| (182 | ) | |
| 13,608 | |
| SEPA Beheer BV | |
| — | | |
| 500 | |
| van Kalken | |
| 45,000 | | |
| — | |
| | |
€ | 44,818 | | |
€ | 14,608 | |
Related parties include Imbott Group BV, SEPA Beheer
BV and C.K. van Kalken Beheer B.V., all of which are stockholders of the Successor, Gedi Cube Int Ltd and former stockholders of the Predecessor,
Grace Systems B.V. and Grace Bio Omics B.V., is an affiliated entity by way of mutual owners. The nature of the transactions making up
this balance relates to short term loans. The loan related party balances were non-interest bearing.
Note 12. Deconsolidation
Grace Datascience B.V. Bankruptcy
In April 2023, the Grace Systems B.V. entered into
a bankruptcy agreement with the Dutch courts for its wholly owned subsidiary, Grace Datascience B.V. As of the bankruptcy date, Grace
Systems is no longer authorized to manage and dispose of Grace Data’s assets where these rights have been transferred to the curator
appointed by the Dutch courts to oversee the liquidation of these assets and satisfaction of outstanding liabilities. Based on the guidance
prescribed in ASC 810 - Consolidation, Grace Systems elected to deconsolidate Grace Data as of the effective date of bankruptcy which
is determined to be April 24, 2023. Grace Systems operations from January 1, 2023 to April 23, 2023 include the operations of Grace Datascience
B.V..
The following tables reflect the balance sheet immediately
prior to submission of the subsidiary into bankruptcy in addition to the activity of the subsidiary for the period controlled by Grace
Systems.
| Grace Datascience B.V. |
| Balance Sheet |
| | |
April 23, 2023 |
| | |
|
| Cash | |
€ | 38 | |
| Property, plant and equipment, net | |
| 22,894 | |
| Tax Assets | |
| 2,694 | |
| Total Assets | |
| 25,626 | |
| | |
| | |
| Accounts payable | |
| 80,746 | |
| Intercompany | |
| 55,491 | |
| Accrued payroll | |
| 65,656 | |
| Total Liabilities | |
| 201,892 | |
| | |
| | |
| Common Stock | |
| 100 | |
| Current Year Accumulated Deficit | |
| (41,526 | ) |
| Accumulated Deficit | |
| (134,840 | ) |
| Total Stockholders Deficit | |
| (176,266 | ) |
| Total Liabilities and Stockholders Deficit | |
€ | 25,626 | |
| Grace Datascience B.V. |
| Statement of Operations |
| | |
|
| | |
Period January 1 2023 to April 23, 2023 |
| | |
|
| Revenue | |
€ | — | |
| Cost of Revenue | |
| — | |
| Gross Profit | |
| — | |
| | |
| | |
| Advisory Fees | |
| 6,021 | |
| Salaries and wages | |
| 30,450 | |
| Selling, general and administrative | |
| 3,197 | |
| Depreciation Expense | |
| 1,858 | |
| Total Operating Expense | |
| 41,526 | |
| | |
| | |
| Net Loss | |
€ | (41,526 | ) |
Gedi Cube Intl Ltd
Notes to the Unaudited Consolidated Financial Statements
Note 13. Notes Payable
During August 2023, the Company entered into two Promissory Notes (“Notes”).
The first note was for $550,000, which translates to €515,601. The second note was for $500,000, which translates to €476,085.
The Company received a total of $1,050,000, which translates to €991,686, from Renovaro to further develop the Company’s IP
and technology. Pursuant to the Notes, the Company promised to pay Renovaro the outstanding principal and related accrued interest at
a rate of 6% per annum on the maturity date of February 11 and February 18 of 2024, respectively. For the period from August 24, 2023
to September 30, 2023, the Company accrued interest of €7,346 and is included in interest expense within other expense (income) on
the Statements of Operations. The total amount of the Notes and the interest owed to Renovaro is €999,032.
Note 14. Subsidies
The Dutch government has previously implemented the
Emergency Bridging Measure for Job Opportunities (NOW) wage subsidy as emergency funding for all companies as a result of the COVID-19
outbreak where qualifying expenses are reimbursed by the authorities. During the nine months ended September 30, 2022, the Company
received and was rewarded €45,333 which was recorded as a reduction to salaries and wages in the Statement of Operations.
Gedi Cube Intl Ltd
Notes to the Unaudited Consolidated Financial Statements
Note 15. Subsequent Events
On November 22, 2023, the Company entered into a loan
agreement (the “DMZ Facility”) with DMZ Invest I APS (“DMZ”) pursuant to which DMZ agreed to loan the Company
up to £500,000 (€572,775 as of November 22, 2023), which will be used to fund the Company’s operations through the Closing.
The DMZ Facility has a repayment date occurring the first business day after the first anniversary of the draw down of the loan. The first
draw down of the loan occurred on November 27, 2023, resulting in a repayment date of November 28, 2024. The Company will pay interest
on the loan at the rate of 10% per annum. Interest is accrued quarterly in arrears on the last business day of March, June, September
and December and is payable on the repayment date.
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of Grace Systems BV:
Opinion on the Financial Statements
We have audited the accompanying consolidated balance
sheets of Grace Systems BV (“the Company”) as of December 31, 2022 and 2021, the related consolidated statements of operations,
stockholders’ equity (deficit), and cash flows for each of the years in the two-year period ended December 31, 2022 and the related
notes (collectively referred to as the “financial statements”). In our opinion, the financial statements referred to above
present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the results of its
operations and its cash flows for each of the years in the two-year period ended December 31, 2022, in conformity with accounting principles
generally accepted in the United States of America.
Explanatory Paragraph Regarding Going Concern
The accompanying financial statements have been prepared
assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has negative
cash flows from operations and an accumulated deficit which raises substantial doubt about its ability to continue as a going concern.
Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments
that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility
of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our
audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”)
and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable
rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards
of the PCAOB and in accordance with auditing standards generally accepted in the United States of America. Those standards require that
we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement,
whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over
financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but
not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly,
we express no such opinion.
Our audit included performing procedures to assess
the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond
to those risks. Such procedures included examining on a test basis, evidence regarding the amounts and disclosures in the financial statements.
Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating
the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the
current-period audit of the financial statements that were communicated or required to be communicated to the audit committee and that
(1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective,
or complex judgments. We determined that there are no critical audit matters.
/Sadler Gibb & Associates, LLC
We have served as the Company’s auditor since 2023.
Draper, UT December 4, 2023
Grace
Systems BV
Consolidated
Balance Sheets (in Euros)
| | |
December 31, |
| | |
2022 | |
2021 |
| Assets | |
| | | |
| | |
| Current assets: | |
| | | |
| | |
| Cash | |
€ | 1,583 | | |
€ | 248,797 | |
| VAT receivable | |
| 2,189 | | |
| — | |
| Total current assets | |
| 3,772 | | |
| 248,797 | |
| Property, plant and equipment, net | |
| 37,552 | | |
| 51,086 | |
| Total assets | |
€ | 41,324 | | |
| 299,883 | |
| | |
| | | |
| | |
| Liabilities and Stockholders’ Equity (Deficit) | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Accounts payable | |
| 77,340 | | |
| 375 | |
| Accrued payroll | |
| 284,038 | | |
| 2,672 | |
| Due to related parties | |
| 14,608 | | |
| 108 | |
| VAT payable | |
| — | | |
| 7,408 | |
| Total current liabilities | |
| 375,986 | | |
| 10,563 | |
| Total liabilities | |
€ | 375,986 | | |
€ | 10,563 | |
| | |
| | | |
| | |
| Stockholders’ (Deficit) Equity: | |
| | | |
| | |
| Common Stock | |
| | | |
| | |
| Common stock, €0.01 par value; 120 shares authorized and 120 shares issued as of December 31, 2022, and 2021 | |
| 1 | | |
| 1 | |
| Additional paid-in capital | |
| 100 | | |
| 100 | |
| (Accumulated Deficit) Retained Earnings | |
| (334,763 | ) | |
| 289,219 | |
| Total stockholders’ (deficit) equity | |
| (334,662 | ) | |
| 289,320 | |
| Total liabilities and stockholders’ (deficit) equity | |
€ | 41,324 | | |
€ | 299,883 | |
The
accompanying notes are an integral part of these audited consolidated financial statements.
Grace
Systems BV
Consolidated
Statements of Operations
| | |
Year Ended December 31, |
| | |
2022 | |
2021 |
| | |
| |
|
| Revenue | |
€ | — | | |
€ | 1,041,660 | |
| Cost of Revenue | |
| — | | |
| 147,782 | |
| Gross Profit | |
| — | | |
| 893,878 | |
| | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | |
| | |
| | | |
| | |
| Selling, general and administrative | |
| 135,398 | | |
| 166,287 | |
| Depreciation expense | |
| 14,591 | | |
| 12,270 | |
| Management fees | |
| 378,000 | | |
| 372,275 | |
| Advisory Fees | |
| 13,455 | | |
| 4,481 | |
| Salaries and wages | |
| 82,538 | | |
| 207,149 | |
| Total operating expenses | |
| 623,982 | | |
| 762,462 | |
| | |
| | | |
| | |
| (Loss) income from operations | |
| (623,982 | ) | |
| 131,416 | |
| | |
| | | |
| | |
| | |
| | | |
| | |
| (Loss) income before income taxes | |
| (623,982 | ) | |
| 131,416 | |
| | |
| | | |
| | |
| Provision for income taxes | |
| — | | |
| — | |
| | |
| | | |
| | |
| Net (loss) income | |
€ | (623,982 | ) | |
€ | 131,416 | |
| | |
| | | |
| | |
| Basic and diluted (loss) earnings per share | |
| (5,200 | ) | |
| 1,095 | |
| | |
| | | |
| | |
| Weighted average number of shares of common stock outstanding - Basic and diluted | |
| 120 | | |
| 120 | |
The
accompanying notes are an integral part of these audited consolidated financial statements.
Grace
Systems BV
Consolidated
Statements of Changes in Stockholders' Equity (Deficit)
| | |
Common Stock | |
| |
| |
|
| | |
Shares | |
Amount | |
Additional Paid-In Capital | |
(Accumulated Deficit) Retained Earnings | |
Total Stockholders’ Equity (Deficit) |
| Balance at January 1, 2021 | |
| 120 | | |
€ | 1 | | |
€ | 100 | | |
€ | 157,803 | | |
€ | 157,904 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net income | |
| | | |
| | | |
| | | |
| 131,416 | | |
€ | 131,416 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance at December 31, 2021 | |
| 120 | | |
€ | 1 | | |
€ | 100 | | |
€ | 289,219 | | |
€ | 289,320 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss | |
| | | |
| | | |
| | | |
| (623,982 | ) | |
€ | (623,982 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance at December 31, 2022 | |
| 120 | | |
€ | 1 | | |
€ | 100 | | |
€ | (334,763 | ) | |
€ | (334,662 | ) |
The
accompanying notes are an integral part of these audited consolidated financial statements.
Grace
Systems BV
Consolidated
Statements of Cash Flows
| | |
Year Ended December 31, |
| | |
2022 | |
2021 |
| Cash flows from operating activities: | |
| | | |
| | |
| Net (loss) income | |
€ | (623,982 | ) | |
€ | 131,416 | |
| Adjustments to reconcile net loss to net cash (used in) provided by operating activities: | |
| | | |
| | |
| Depreciation | |
| 14,591 | | |
| 12,270 | |
| Changes in assets/liabilities: | |
| | | |
| | |
| Accounts payable | |
| 76,965 | | |
| (47,407 | ) |
| Accrued payroll | |
| 281,366 | | |
| 1,507 | |
| Due to related parties | |
| 14,500 | | |
| 68,082 | |
| VAT receivable | |
| (2,189 | ) | |
| — | |
| VAT payable | |
| (7,408 | ) | |
| (11,492 | ) |
| Net cash (used in) provided by operating activities | |
| (246,157 | ) | |
| 154,376 | |
| | |
| | | |
| | |
| Cash flows from investing activities: | |
| | | |
| | |
| Purchases of property and equipment | |
| (1,057 | ) | |
| (42,867 | ) |
| Net cash used in investing activities | |
| (1,057 | ) | |
| (42,867 | ) |
| | |
| | | |
| | |
| Cash flows from financing activities: | |
| | | |
| | |
| Net cash provided by financing activities | |
| — | | |
| — | |
| | |
| | | |
| | |
| Cash: | |
| | | |
| | |
| Net change during the year | |
| (247,214 | ) | |
| 111,508 | |
| Balance, beginning of year | |
| 248,797 | | |
| 137,288 | |
| Balance, end of year | |
€ | 1,583 | | |
€ | 248,796 | |
| | |
| | | |
| | |
| Supplemental Disclosures of Cash Flow Information | |
| | | |
| | |
| Cash paid during the period for | |
| | | |
| | |
| Interest | |
€ | — | | |
€ | — | |
| Income Taxes | |
€ | — | | |
€ | — | |
The
accompanying notes are an integral part of these audited consolidated financial statements.
Grace
Systems BV
Notes
to the Consolidated Financial Statements
Note 1. Organization
Grace Systems BV
and its subsidiary (Grace Datascience BV) (collectively, the ”Company”), headquartered
in the Netherlands, specialize in data science and data mining and plan to be disruptive in the
industry with a broad range of cutting edge software, services, methods, and models to tackle big data challenges. Grace
Systems BV focuses on the high-end of advanced and predictive analytics which contributes to our ability to combine big
data stores, execute scientific algorithms and visualize analytics to business users. As of the report date, the Company is in
the development stage of its lifecycle and has not yet commenced operations.
Note 2. Summary of Significant Accounting
Policies
Basis of Presentation and Principles
of Consolidation
The accompanying consolidated financial statements
have been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”) as
defined by the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”).
The consolidated financial statements include the accounts of Grace Systems BV and its subsidiaries.
All intercompany accounts and transactions have been eliminated in consolidation.
Going Concern
As of December 31,
2022, the Company had cash of approximately €1,583. For the year ended December 31,
2022, the Company used €246,157 in cash for operating activities and had an accumulated
deficit of €334,763. These factors raise substantial doubt regarding the Company’s
ability to continue as a going concern for one year from the issuance of these financials.
The continuation of the Company as a going
concern is dependent upon the continued financial support from its members and debt holders. Specifically, continuation is contingent
on the Company’s ability to obtain necessary equity or debt financing to continue operations, and ultimately the Company’s
ability to generate profit from sales and positive operating cash flows, which is not assured.
The Company’s plans include dissolving
the entity, Grace Datascience BV, as well as obtaining associated debt and equity financing. If the Company is unsuccessful in
completing these planned transactions, it may be required to reduce its spending rate to align with expected revenue levels and
cash reserves, although there can be no guarantee that it will be successful in doing so. Accordingly, the Company may be required
to raise additional cash through debt or equity transactions. It may not be able to secure financing in a timely manner or on favorable
terms, if at all.
These accompanying audited consolidated financial
statements have been prepared assuming that the Company will continue as a going concern and do not include any adjustments that
might result from the outcome of this uncertainty.
Use of Estimates
The preparation of the consolidated financial
statements, in conformity with U.S. GAAP, requires management to make estimates and assumptions that affect the reported amounts
of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements
and the reported amounts of revenue and expenses during the reporting period. Management evaluates its estimates and assumptions
on an ongoing basis using historical experience and other factors, including the current economic environment, and makes adjustments
when facts and circumstances dictate. These estimates are based on information available as of the date of the financial statements;
therefore, actual results could differ from those estimates.
Cash and Cash Equivalents
As of December 31, 2022 and 2021
the Company’s cash balances were €1,583 and €248,797,
respectively. Cash is recorded at cost, which approximates fair value. As of December 31, 2022 and 2021,
cash consisted of checking and savings deposits.
Grace
Systems BV
Notes
to the Consolidated Financial Statements
Property and Equipment
Property and equipment are stated at cost,
less accumulated depreciation. Maintenance and repairs are charged to expense when incurred. Additions and improvements that extend
the economic useful life of the asset are capitalized and depreciated over the remaining useful lives of the assets. The cost and
accumulated depreciation of assets sold or retired are removed from the respective accounts, and any resulting gain or loss is
reflected in current earnings. Depreciation is recognized using the straight-line method in amounts considered to be sufficient
to allocate the cost of the assets to operations over the estimated useful lives of the assets, all of which are 5 years.
Impairment of Long Lived Assets
Long-lived assets, such as property and equipment,
definite and indefinite life intangible assets are reviewed for impairment whenever events or changes in circumstances indicate
that the carrying amount of an asset may not be recoverable. Circumstances which could trigger a review include, but are not limited
to: significant decreases in the market price of the asset; significant adverse changes in the business climate or legal factors;
current period cash flow or operating losses combined with a history of losses or a forecast of continuing losses associated with
the use of the asset; and current expectations that the asset will more likely than not be sold or disposed of significantly before
the end of its estimated useful life.
Recoverability of assets to be held and used
is measured by a comparison of the carrying amount of an asset to estimated undiscounted future cash flows expected to be generated
by the asset. If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, an impairment charge is
recognized for the amount by which the carrying amount of the asset exceeds the fair value of the asset. Assets to be disposed
of would be separately presented in the balance sheet and reported at the lower of the carrying amount or fair value less costs
to sell and would no longer be depreciated. The depreciable basis of assets that are impaired and continue in use are their respective
fair values.
Revenue Recognition
Revenue is recognized when promised goods or
services are transferred to customers in an amount that reflects the consideration to which the Company expects to be entitled
in exchange for those goods or services by following a five-step process: (1) identify the contract with a customer, (2) identify
the performance obligations in the contract, (3) determine the transaction price, (4) allocate the transaction price, and (5) recognize
revenue when or as the Company satisfies a performance obligation. The revenue recognized in 2021 does not relate to the Company’s
planned core operations and is not recurring.
Value Added Tax
The company collects value added tax (“VAT”)
on sales (VAT payable) and offsets it with deducted VAT from eligible purchases (VAT receivable). The standard rate is 21%, with
reduced rates applicable to specific goods and services. Amounts are reported quarterly to the tax authorities in April, July,
October, and January directly via an online platform or through the use of certain software. In the case of a net payable VAT,
the company pays directly to the tax authorities within the month, and if the net VAT is receivable, the company receives a letter
from the tax office with a refund date and details, usually within 5 weeks. Periodic VAT returns offer a comprehensive breakdown
of both output and input taxes. Submitting a VAT return is mandatory for all companies registered with the Chamber of Commerce
in the Netherlands (KVK).
Leases
The Company had one active lease in 2022 for
an office space that commenced in June 2022 and was terminated in November 2022. The Company recognizes rent expense for the periods
in which rent payments were made. As of December 31, 2022, the Company has no active leases. The Company paid €24,071
and €10,454 during the years ended December 31, 2022 and 2021 respectively.
Functional Currency
The local currency, Euros, is the functional
currency for all of the Company’s activity. Gains and losses from foreign currency transactions are included in net income
for the period.
Grace
Systems BV
Notes
to the Consolidated Financial Statements
Income Taxes
Income taxes provide for the tax effects of
transactions reported in the consolidated financial statements and consist of deferred tax assets and liabilities. Deferred tax
assets and liabilities are recognized for differences between the basis of assets and liabilities for financial statement and income
tax purposes. The differences relate primarily to net operating losses. The future realization of deferred tax assets is subject
to the existence of sufficient future taxable income. Currently, management believes that it is not more likely than not that the
Company will realize the benefits of any tax loss carryforwards.
Due to the historical net loss position of
the Company, and the lack of sufficient taxable income to realize the deferred tax asset, the Company recorded a full valuation
allowance of €128,760 and €1,207 as of December 31,
2022 and 2021, respectively.
Segment Information
The Company has one operating segment with
one planned business activity as described in Note 1. The Company’s chief operating decision maker is its Chief Executive
Officer, who manages operations and reviews financial information on a consolidated basis for the purpose of assessing performance
and allocating resources.
Net Loss Per Share Calculation
Basic earnings (loss) per common share (“EPS”)
is computed by dividing net income (loss) available to common stockholders by the weighted-average number of common shares outstanding
for the period. Diluted earnings (loss) per share is computed by dividing net income (loss) by the weighted average shares outstanding,
assuming all dilutive potential common shares were issued. Dilutive loss per share excludes all potential common shares if their
effect is anti-dilutive. No potentially dilutive debt or equity instruments were issued or outstanding for the years ended December 31,
2022 and December 31, 2021.
Recent Accounting Pronouncements
Accounting Pronouncements Recently Adopted
In February 2016, the FASB issued ASU 2016-02,
Leases (Topic 842) (“ASU 2016-02”), which supersedes the current accounting for leases and while retaining two distinct
types of leases, finance and operating, (i) requires lessees to record a right of use asset and a related liability for the rights
and obligations associated with a lease, regardless of lease classification, and recognize lease expense in a manner similar to
current accounting, (ii) eliminates most real estate specific lease provisions, and (iii) aligns many of the underlying lessor
model principles with those in the new revenue standard. Leases with a term of 12 months or less will be accounted for similar
to existing guidance for operating leases. The new standard is effective for fiscal years beginning after December 15, 2021, with
early adoption permitted, based on the FASB effective date deferral per ASU 2020-05. A modified retrospective transition approach
is required, applying the new standard to all leases existing at the date of initial application. An entity may choose to use either
(1) its effective date or (2) the beginning of the earliest comparative period presented in the financial statements as its date
of initial application. If an entity chooses the second option, the transition requirements for existing leases also apply to leases
entered into between the date of initial application and the effective date. The Company adopted the provisions of this standard
on January 1, 2021 noting the Company does not have any leases that qualify under this standard.
Note 3. Property, Plant and Equipment,
net
Property, plant and equipment consisted of
the following:
| | |
December 31, |
| | |
2022 | |
2021 |
| Computer equipment and software | |
€ | 5,866 | | |
€ | 4,809 | |
| Computer hardware | |
| 50,665 | | |
| 50,665 | |
| Machines and installations | |
| 21,196 | | |
| 21,196 | |
| | |
| 77,727 | | |
| 76,670 | |
| Less: Accumulated depreciation | |
| (40,175 | ) | |
| (25,584 | ) |
| Property, plant and equipment, net | |
€ | 37,552 | | |
€ | 51,086 | |
Grace
Systems BV
Notes
to the Consolidated Financial Statements
The Company uses the straight
line method to calculate depreciation expense. Depreciation expense totaled €14,591and
€12,270 for the years ended December 31, 2022 and 2021.
Note 4. Stockholder’ Equity
Common Stock
The Company was formed as a partnership and
incorporated on June 3, 2013. Upon Company formation, the Company issued capital in the amount of 120 regular common stock shares
with a par value of €0.01. Each common share affords the holder general voting rights as defined in the Shareholders Agreement
in addition to providing for the right for dividends to be declared, to which none have been declared to date.
Note 5. Income Taxes
The Company’s net deferred tax assets
are as follows:
| | |
December 31, |
| | |
2022 | |
2021 |
| Deferred tax assets: | |
| | | |
| | |
| Net operating loss carryforwards | |
€ | 128,760 | | |
€ | 1,207 | |
| Total deferred tax assets | |
| 128,760 | | |
| 1,207 | |
| Valuation allowance | |
| (128,760 | ) | |
| (1,207 | ) |
| Deferred tax assets, net of valuation allowance | |
€ | — | | |
€ | — | |
The income tax provisions for the year ended December 31, 2022 and
2021 consists of the following:
| | |
December 31, |
| | |
2022 | |
2021 |
| Foreign | |
€ | — | | |
€ | — | |
| Current | |
| — | | |
| — | |
| Deferred | |
| (127,553 | ) | |
| (1,207 | ) |
| Change in valuation allowance | |
| 127,553 | | |
| 1,207 | |
| Income tax provision | |
€ | — | | |
€ | — | |
As of December 31, 2022, and December 31, 2021,
the Company had available an operating loss carry forward of approximately $499,070 and $4,677, respectively, that may be carried
forward indefinitely for years beginning after January 2022. The net operating loss incurred for the period ending December 31,
2021 was fully utilized on an amended return for the year 2020.
In assessing the realization of deferred tax
assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be
realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the
periods in which temporary differences representing future deductible amounts become deductible. Management considers the scheduled
reversal of deferred tax assets, projected future taxable income and tax planning strategies in making this assessment. After consideration
of all the information available, management believes that significant uncertainty exists with respect to future realization of
the deferred tax assets and has therefore established a full valuation allowance. As of December 31, 2022 and 2021, the valuation
allowance was $128,760 and $1,207, respectively.
A reconciliation of the statutory income tax
rate to the Company’s effective tax rate at December 31, 2022 and 2021 is as follows:
Grace
Systems BV
Notes
to the Consolidated Financial Statements
| | |
December 31, |
| | |
2022 | |
2021 |
| Statutory income tax rate | |
| 25.8 | % | |
| 25.8 | % |
| Non-deductible costs | |
| -3.94 | % | |
| -24.03 | % |
| Previous tax year adjustment | |
| -0.20 | % | |
| 0.00 | % |
| Small-scale investment deduction | |
| 0.00 | % | |
| -2.90 | % |
| Change in valuation allowance | |
| -21.66 | % | |
| 1.13 | % |
| Income tax provision | |
| 0.00 | % | |
| 0.00 | % |
The Company’s effective tax rates for
the periods presented differ from the expected (statutory) rates primarily due to changes in non-deductible costs and the recording
of full valuation allowances on deferred tax assets. The Company files income tax returns in the Netherlands, which remains open
and subject to examination.
Note 6. Risks and Uncertainties
The Company prepares consolidated financial
statements in conformity with U.S. GAAP. It is possible that future requirements could change the current application of US GAAP,
resulting in a material adverse impact on the Company’s balance sheets or results of operations. In addition, the future
effective tax rates could be unfavorably affected by changes in tax laws or the interpretation of tax laws or by changes in the
valuation of our deferred tax assets and liabilities. The Company regularly assess the implementation of applicable accounting
principles and the adequacy of provision for income taxes. The final determination of any tax authority, upon examination of the
Company’s tax returns, could have an adverse effect on the Company’s operating results and balance sheets.
The Company is in the development stage and
faces all of the risks and uncertainties associated with a new and unproven business. The Company’s future is based on an
unproven business plan with no historical facts to support projections and assumptions. The Company’s operations are subject
to all of the risks inherent in the establishment of a new business enterprise. The likelihood of the Company’s success must
be considered in light of the problems, expenses, difficulties, complications and delays frequently encountered in connection with
the formation of a pre-revenue business. The Company’s lack of a significant and relevant operating history makes it difficult
to manage operations and predict future operating results.
Note 7. Related Parties
Related party transactions consisted of the
following:
| | |
December 31, |
| | |
2022 | |
2021 |
| Imbott Group BV | |
€ | 500 | | |
€ | — | |
| RC Grace Bio Omics / formerly Linkin Data | |
| 13,608 | | |
| 108 | |
| SEPA Beheer BV | |
| 500 | | |
| — | |
| Total due to related parties | |
€ | 14,608 | | |
€ | 108 | |
Grace
Systems BV
Notes
to the Consolidated Financial Statements
Related
parties include Imbott Group BV and SEPA Beheer BV all of which are stockholders of Grace Systems B.V. RC Grace Bio Omics,
formerly Linkin Data, is an affiliated entity by way of mutual owners. The nature of the related party transactions related to
short term loans. There is no interest on these loans which have maturity dates less than a year.
Note 8. Subsequent
Events
Grace Datascience B.V. Bankruptcy
In April 2023, the Company entered into a bankruptcy
agreement with the Dutch courts for its wholly owned subsidiary, Grace Datascience B.V.. As of the bankruptcy date, the Company
is no longer authorized to manage and dispose of the Company’s assets where these rights have been to the Curator appointed
by the Dutch courts to oversee the liquidation of these assets and satisfaction of outstanding liabilities. As of the report date,
the Grace Datascience B.V. bankruptcy is still ongoing. The Company will no longer consolidate this entity as of the bankruptcy
date and will record a gain on the deconsolidation of this entity.
Gedi Health Group LTD Share Purchase Agreement
In August 2023, The Company and its shareholders,
(the “Seller”), entered into a share purchase agreement with Gedi Health Group LTD (the “Buyer”). The capital
contribution made by the buyer of €1,000,000 included shareholders of the Company and are for shares in Gedi Health.
Renovaro Transaction
On September 29, 2023 the Company and Renovaro
executed a definitive agreement to merge (the “Proposed Transaction”). The Proposed Transaction is being structured
as a stock-for-stock acquisition and is subject to shareholder approval which is still pending as of the date of issuance of these
financial statements.
PRINCIPAL STOCKHOLDERS OF
RENOVARO
The
following table sets forth information regarding the beneficial ownership
of shares of Common Stock as of the Record Date by:
| |
● |
each person known by Renovaro to be the beneficial owner of more than 5% of outstanding shares of Common Stock; |
| |
|
|
| |
● |
each of Renovaro’s named executive officers for the 2023 fiscal year; |
| |
|
|
| |
● |
each of Renovaro’s directors; and |
| |
|
|
| |
● |
all of Renovaro’s current executive officers and directors as a group. |
Beneficial
ownership of the Common Stock is determined in accordance with the rules
of the SEC and includes any shares of Common Stock over which a person exercises sole or shared voting or dispositive power, or of which
a person has a right to acquire ownership at any time within sixty (60) days. Except as otherwise indicated, Renovaro believes that the
persons named in this table have sole voting and dispositive power with respect to all shares of Common Stock held by them. Applicable
percentage ownership in the following table is based on 67,224,089 shares of Common Stock outstanding as of the Record Date, plus any
securities that the individuals included in this table have the right to acquire within sixty (60) days of the Record Date.
To
Renovaro’s knowledge, except as indicated in the footnotes to this table and pursuant to applicable community property laws,
the persons named in the table have sole voting and dispositive power with respect to all shares of Common Stock beneficially owned
by them. Unless indicated otherwise, the address for the beneficial holders is c/o Renovaro Biosciences Inc., 2080 Century Park
East, Suite 906, Los Angeles, CA, 90067 U.S.A.
| |
|
Renovaro Biosciences Inc. |
| Name of Beneficial Owner |
|
Number of Shares |
|
% Ownership |
| |
|
|
|
|
| Directors/Officers: |
|
|
|
|
|
|
|
|
| Renè Sindlev, Chairman of the Board (1) |
|
|
14,945,971 |
|
|
|
21.43 |
% |
| Mark R. Dybul, M.D., Chief Executive Officer (2) |
|
|
2,279,271 |
|
|
|
3.28 |
% |
| Luisa Puche, Chief Financial Officer & Corporate Secretary (3) |
|
|
217,933 |
|
|
|
* |
% |
| François Binette, PhD, Chief Operating Officer and Executive Vice President for Research & Development (4) |
|
|
143,334 |
|
|
|
* |
% |
| Carol Brosgart, Director (5) |
|
|
107,759 |
|
|
|
* |
% |
| Gregg Alton, Director (6) |
|
|
107,759 |
|
|
|
* |
% |
| James Sapirstein, Director (7) |
|
|
85,895 |
|
|
|
* |
% |
| Jayne McNicol, Director (8) |
|
|
43,390 |
|
|
|
* |
% |
| Henrik Grønfeldt-Sørensen, Director (9) |
|
|
136,889 |
|
|
|
* |
% |
| Leni Boeren, Director (10) |
|
|
— |
|
|
|
* |
% |
| Ruud Hendriks, Director (10) |
|
|
— |
|
|
|
* |
% |
| Avram Miller, Director (11) |
|
|
1,000,000 |
|
|
|
1.49 |
% |
| Directors/Officers Total (12 persons): |
|
|
19,068,201 |
|
|
|
26.22 |
% |
| 5% Shareholders who are not Directors or Officers: |
|
|
|
|
|
|
|
|
| RS Bio ApS |
|
|
14,866,223 |
|
|
|
21.34 |
% |
| Serhat Gümrükcü (12) |
|
|
12,438,431 |
|
|
|
18.50 |
% |
| Anderson Wittekind (13) |
|
|
6,517,945 |
|
|
|
9.70 |
% |
| Paseco ApS (14) |
|
|
11,157,444 |
|
|
|
15.09 |
% |
| 5% Shareholders who are not Directors or Officers Total: |
|
|
44,980,043 |
|
|
|
58.91 |
% |
| * |
Indicates less than 1%. |
(1) Includes 12,432,338 shares of Common Stock, 70,126 of Series A Convertible Preferred
Stock that are presently convertible into 701,260 shares of Common Stock, and warrants exercisable into 1,732,625 shares of Common Stock
owned of record by RS Bio ApS, a Danish entity, and options to purchase 79,748 shares of Common Stock exercisable within sixty (60) days
of the Record Date, owned of record by Mr. Sindlev. Mr. Sindlev, the Chairman of the Renovaro Board, holds the sole voting and dispositive
power of the shares owned by RS Bio ApS.
(2) Includes 66,481 shares of Common Stock and options
to purchase 2,212,790 shares of Common Stock exercisable within sixty (60) days of the Record Date.
(3) Includes 16,266 shares of Common Stock and
options to purchase 201,667 shares of Common Stock exercisable within sixty (60) days of the Record Date.
(4) Includes options to purchase 143,334 shares of
Common Stock exercisable within sixty (60) days of the Record Date.
(5) Represents options to purchase 107,759 shares
of Common Stock exercisable within sixty (60) days of the Record Date.
(6) Represents options to purchase 107,759 shares
of Common Stock exercisable within sixty (60) days of the Record Date.
(7) Represents options to purchase 85,895 shares of
Common Stock exercisable within sixty (60) days of the Record Date.
(8) Represents options to purchase 43,390 shares of
Common Stock exercisable within sixty (60) days of the Record Date.
(9) Includes 50,000 shares of Common Stock and options to purchase 86,889 shares of
Common Stock exercisable within sixty (60) days of the Record Date. Mr. Grønfeldt-Sørensen, a director of Renovaro, holds
the sole voting and dispositive power of the shares of Common Stock owned by Greenfield Holding ApS. Excludes 12,432,338 shares of Common
Stock, 70,126 of Series A Convertible Preferred Stock that are presently convertible into 701,260 shares of Common Stock, and warrants
exercisable into 1,732,625 shares of Common Stock owned of record by RS Bio ApS, a Danish entity, of which Mr. Grønfeldt-Sørensen
is an officer, but Mr. Grønfeldt-Sørensen exercises no voting or dispositive power with respect to such shares.
(10) Each of Ms. Boeren and Mr. Hendriks was appointed
to the Renovaro Board effective as of October 4, 2023, to fill a vacancy.
(11) Includes 1,000,000 shares of restricted stock
issued to Mr. Miller under the Advisory Agreement, dated October 10, 2023, by and between Renovaro and Mr. Miller, under which 166,667
of such shares will vest in 2024, 444,444 of such shares will vest in 2025, and 388,889 of such shares will vest in 2026, subject to Mr.
Miller’s continued service through each applicable vesting date. Mr. Miller was appointed to the Renovaro Board effective as of
October 11, 2023, to fill a vacancy.
(12) All such shares are owned by Gumrukcu (as defined
below).
(13) Based on the Schedule 13D/A filed on
November 3, 2023, by William Anderson Wittekind (“Wittekind”), which reported shared voting and dispositive power
of 12,526,552 shares and sole voting and dispositive power of 6,429,824 shares. Consists of (i) 3,615,757 shares owned by Wittekind;
(ii) 1,313,499 shares owned by Weird Science LLC (“Weird Science”); (iii) 633,921 shares owned by the William Anderson
Wittekind 2020 Annuity Trust, a grantor retained annuity trust of which Wittekind is the sole trustee (the “Wittekind 2020
Annuity Trust”); (iv) 450,568 shares owned by the Dybul 2020 Angel Annuity Trust, a grantor retained annuity trust of which
Wittekind is the sole trustee (the “Dybul 2020 Annuity Trust”); (v) 50,000 shares owned by the Ty Mabry 2021 Annuity
Trust, a grantor retained annuity trust of which Wittekind is sole trustee (the “Mabry 2021 Annuity Trust”); (vi)
366,079 shares owned by the William Anderson Wittekind 2021 Annuity Trust, a grantor retained annuity trust of which Wittekind
is the sole trustee (the “Wittekind 2021 Annuity Trust”, and, together with the Wittekind 2020 Annuity Trust, the
Dybul 2020 Annuity Trust and the Mabry 2021 Annuity Trust, the “Trusts”); and (vii) 88,121 shares owned by Wittekind
and Serhat Gumrukcu, Wittekind’s spouse (“Gumrukcu”), Wittekind’s spouse, as joint tenants with a right
of survivorship. In addition, Wittekind may be deemed a beneficial owner of 12,438,431 shares owned by Gumrukcu (as reported in
the table above), of which Wittekind shares voting power and dispositive power through a power of attorney dated June 24, 2022.
In his capacity as the sole manager of Weird Science, Wittekind has sole voting and dispositive power over the shares owned by
Weird Science. In his capacity as the sole trustee of the Trusts, Wittekind has sole voting and dispositive power over the shares
owned by the Trusts.
(14) Includes (i) 4,462,292 shares of
Common Stock, (ii) 343,619 shares of Series A Preferred Stock convertible into 3,436,190 shares of Common Stock, and (iii)
warrants exercisable into 3,258,965 shares of Common Stock.
COMPENSATION OF DIRECTORS AND EXECUTIVE
OFFICERS OF RENOVARO
Renovaro’s named executive officers
for the year ended June 30, 2023, which consisted of its principal executive officer and its two next most highly compensated executive
officers, are:
| ● | Mark R. Dybul, M.D., Renovaro’s Chief Executive Officer; |
| ● | François Binette, PhD, Renovaro’s Chief Operating Officer and Executive Vice President for Research & Development;
and |
| ● | Luisa Puche, Renovaro’s Chief Financial Officer and Corporate Secretary. |
Summary Compensation Table
The following table sets
forth certain information with respect to compensation for the years ended June 30, 2023 and June 30, 2022, respectively earned
by or paid to the named executive officers of Renovaro. During such periods, no compensation, other than salary, cash bonuses,
stock awards and option awards, was earned by any named executive officer of Renovaro.
| Name and Principal Position | |
Year | |
Salary ($) | |
Bonus | |
Stock
Awards ($) | |
Option Awards
($)(1) | |
Non-equity
incentive
plan
compensation ($) | |
Other
Compensation
($) | |
Total
($) |
| Mark R. Dybul, M.D. (2) | |
| 2023 | | |
$ | 664,583 | | |
$ | 100,000 | | |
$ | — | | |
$ | 640,850 | | |
$ | — | | |
$ | — | | |
$ | 1,405,433 | |
| Chief Executive Officer | |
| 2022 | | |
$ | 850,000 | | |
$ | 100,000 | | |
$ | — | | |
$ | 9,801,000 | | |
$ | — | | |
$ | — | | |
$ | 10,751,000 | |
| François Binette, PhD (3) | |
| 2023 | | |
$ | 389,375 | | |
$ | 115,000 | | |
$ | — | | |
$ | 377,195 | | |
$ | — | | |
$ | — | | |
$ | 881,570 | |
| Chief Operating Officer & EVP for Research & Development | |
| 2022 | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | |
| Luisa Puche | |
| 2023 | | |
$ | 325,000 | | |
$ | 185,000 | | |
$ | — | | |
$ | 130,000 | | |
$ | — | | |
$ | — | | |
$ | 640,000 | |
| Chief Financial Officer & Corporate Secretary | |
| 2022 | | |
$ | 293,750 | | |
$ | 110,000 | | |
$ | 9,812 | | |
$ | 375,780 | | |
$ | — | | |
$ | — | | |
$ | 789,342 | |
(1) The
amounts shown do not reflect compensation actually received by the executive officer. Instead, the amounts shown are the
total grant date valuations of stock option grants awarded during the year as determined pursuant to ASC Topic 718. The
valuations are expensed for financial reporting purposes over the vesting period of the grant. The performance-based options
awarded to Mr. Dybul in the fiscal year ended June 30, 2022, have been completely forfeited as of June 30, 2023.
(2) Effective January 1, 2023, Mr. Dybul took
a reduction in base salary from $850,000 to $550,000.
(3) Mr. Binette was appointed as Chief Operating
Officer of Renovaro, effective November 1, 2022.
Narrative to Summary Compensation Table
The Compensation Committee
of the Renovaro Board (the “Renovaro Compensation Committee”) reviews compensation annually for all of Renovaro’s
executive officers. Compensation awarded to Renovaro’s named executive officers in 2023 and 2022 generally consisted of base
salary, cash bonuses, stock awards and option awards.
The Renovaro Compensation
Committee considered compensation for comparable positions in the market, the historical compensation levels of the executives,
individual performance as compared to its expectations and objectives, the desire to motivate employees to achieve short- and long-term
results that are in the best interests of the Renovaro stockholders, and a long-term commitment to Renovaro. Renovaro does not
target a specific competitive position or a specific mix of compensation among elements of compensation.
Employment Agreements and Potential Payments upon Termination
or Change in Control Provisions
Mark R. Dybul, M.D.
Since January 7, 2019, when Dr. Dybul became Renovaro’s principal executive officer by virtue of his appointment as Executive
Vice-Chair of the Renovaro Board, Dr. Dybul received compensation as Executive Vice Chair of the Renovaro Board under his Amended
and Restated Director’s Agreement, as amended on May 1, 2019 (the “Director Agreement”), which called for cash
compensation of $430,000 per annum, and the grant of options to purchase 300,000 shares of Common Stock, which was granted on November
21, 2018. The Director Agreement did not provide for any payments or other benefits upon a change in control. Dr. Dybul was given
a one-time grant of options to purchase 450,000 shares of Common Stock at a strike price of $8.00 per share on June 11, 2020.
On October 30, 2019, the
Renovaro Compensation Committee approved and presented to the Renovaro Board an employment agreement whereby Dr. Dybul would serve
as Renovaro’s Chief Executive Officer (the “Employment Agreement”) which was recommended by the Renovaro Board
for approval by the Renovaro stockholders. On October 31, 2019, the Renovaro stockholders approved the Employment Agreement via
written consent. Effective July 1, 2021, Dr. Dybul and Renovaro entered into the Employment Agreement in connection with
his appointment to Chief Executive Officer. The Employment Agreement was subsequently amended on December 12, 2022, effective January
1, 2023. The following is a summary of the material terms of the Employment Agreement, as amended.
Term. Dr.
Dybul will serve as Chief Executive Officer of Renovaro for a term of three (3) years with automatic yearly renewal terms thereafter
unless terminated at least 90 days before the expiry of the term.
Duties. Dr.
Dybul will perform duties consistent with the position of Chief Executive Officer, as directed by and reporting to the Renovaro
Board, where he shall remain a director but without further compensation for Board service. Dr. Dybul will devote a substantial
majority of his business time and attention to the performance of his duties with Renovaro, but he will be able to hold positions
with charitable organizations approved by the Renovaro Board, and serve on boards of up to five (5) non-competitive entities, with
prior approval by the Renovaro Board required for publicly traded companies.
Place of Employment
and Expenses. Dr. Dybul shall work out of Renovaro’s headquarters in Los Angeles, commuting as needed. Dr. Dybul
shall be reimbursed for reasonable expenses for accommodations in Los Angeles and a company car.
Cash Compensation.
Dr. Dybul shall be entitled to a base salary of $550,000 per year. Dr. Dybul shall be eligible for a bonus of up to $800,000
per year at the sole discretion of the Renovaro Compensation Committee and in accordance with any short-term incentive plan adopted
by Renovaro.
Benefits. Dr.
Dybul shall receive benefits provided to similarly situated employees of Renovaro and five (5) weeks of vacation per year.
Termination. The
Employment Agreement may be terminated by Renovaro for “Cause” or by Dr. Dybul without “Good Reason” (each
as defined therein), in which case Dr. Dybul will only receive accrued compensation and benefits. In the event Renovaro terminates
the Employment Agreement without Cause or Dr. Dybul terminates the Employment Agreement with Good Reason, Dr. Dybul will receive
his base salary for one (1) year and vesting of one (1) year’s worth of unvested options.
Change in Control.
Upon a change in control, the option grant described below shall immediately vest, and Dr. Dybul shall have the right to
terminate the Employment Agreement for Good Reason.
Restrictive Covenants.
Dr. Dybul shall be subject to restrictive covenants set forth in that certain Confidential and Proprietary Information
Agreement attached to the Employment Agreement, which are independent of the obligations set forth in the Employment Agreement.
The restrictive covenants include non-compete, non-solicitation and non-disparagement obligations for one (1) year, provided that
Renovaro shall continue to pay his base salary for such one (1) year period.
Description of the
Option Grant. Upon appointment to Chief Executive Officer of Renovaro, Dr. Dybul was awarded an option to purchase 3,000,000
shares of Common Stock at an exercise price equivalent to the closing price per share quoted on Nasdaq on the trading day prior
to the grant date. The option has a ten-year term, subject to continued employment, and the option vests with respect to 2,000,000
shares ratably on July 1, 2022, July 1, 2023 and July 1, 2024. As granted, the option vested with respect to one-third of the
remaining 1,000,000 shares at the end of each of the three (3) years beginning with the year ending June 30, 2022, based upon
the achievement by Renovaro of certain benchmarks. However, of these 1,000,000 shares underlying the option, 333,333 shares were
forfeited as of June 30, 2022, and the remaining balance of 666,667 shares was forfeited as of June 30, 2023.
François Binette,
PhD. Pursuant to his offer letter from Renovaro, dated February 22, 2022, Mr. Binette was hired as Renovaro’s Executive
Vice President for Research & Development starting April 2022 with an annual base salary of $375,000, and is eligible for a
discretionary cash bonus, with a target of 40% of his base salary. Mr. Binette also received a grant of options to purchase 65,000
shares of Common Stock, vesting on the first anniversary of the date of hire. On October 18, 2022, Mr. Binette was appointed as
Chief Operating Officer of Renovaro, effective November 1, 2022, and pursuant to an amendment to his offer letter, received an
increase in base salary to $420,000 and 40,000 options, vesting in equal increments over three (3) years.
Luisa Puche.
Pursuant to her offer letter from Renovaro, dated December 28, 2018 (the “Puche Offer Letter”), Ms. Puche received
an annual base salary of $200,000, and is eligible for a discretionary cash bonus, with a target of 40% of her base salary. Ms.
Puche also received a grant of options to purchase 60,000 shares of Common Stock and 15,000 restricted stock units, each vesting
in equal increments over three (3) years. The Puche Offer Letter provides for at-will employment; provided however, that upon termination
of Ms. Puche’s employment by Renovaro without “Cause” (as defined in the Puche Offer Letter), or for a termination
of employment by Ms. Puche for Good Reason (as defined in the Puche Offer Letter), she will receive six (6) months’ salary
and COBRA eligibility. Additionally, if the termination without Cause or for Good Reason occurs within twelve (12) months of a
change in control, Ms. Puche will also be entitled to a pro-rata bonus and immediate vesting of any unvested options or restricted
stock units. Ms. Puche had a base salary of $300,000 for the 2023 fiscal year. Effective October 18, 2022, Ms. Puche received an
increase in base salary to $350,000 following the completion of the 2023 fiscal year and 80,000 options, vesting in equal increments
over three (3) years.
Outstanding Equity Awards at Fiscal Year-End
The following table provides
information concerning outstanding equity awards held by Renovaro’s named executive officers as of June 30, 2023.
| Option Awards | |
Stock Awards |
| Name | |
Number of
Securities
Underlying
Unexercised
Options (#)
Exercisable | |
Number of
Securities
Underlying
Unexercised
Options (#)
Unexercisable | |
Option
Exercise
Price
($) | |
Option
Expiration
Date | |
Number of
Shares
or Shares of
Stock That
Have
Not
Vested
(#) | |
Market
Value of
Shares or
Shares of
Stock
That
Have
Not
Vested
($) |
| Mark R. Dybul, M.D., | |
| 7,563 | | |
| — | | |
$ | 8.00 | | |
02/27/2028 | |
| — | | |
| — | |
| Chief Executive Officer(1) | |
| 5,226 | | |
| — | | |
$ | 5.74 | | |
09/18/2028 | |
| — | | |
| — | |
| | |
| 300,000 | | |
| — | | |
$ | 6.50 | | |
11/21/2028 | |
| — | | |
| — | |
| | |
| 450,000 | | |
| — | | |
$ | 8.00 | | |
06/11/2030 | |
| — | | |
| — | |
| | |
| 666,666 | | |
| 1,333,334 | | |
$ | 4.57 | | |
07/19/2031 | |
| — | | |
| — | |
| | |
| — | | |
| 350,000 | | |
$ | 2.60 | | |
08/25/2032 | |
| — | | |
| — | |
| François Binette, PhD, | |
| 65,000 | | |
| — | | |
$ | 8.40 | | |
02/22/2032 | |
| — | | |
| — | |
| Chief Operating Officer | |
| 39,000 | | |
| 26,000 | | |
$ | 2.38 | | |
07/22/2032 | |
| — | | |
| — | |
| & EVP for Research & Development | |
| — | | |
| 40,000 | | |
$ | 2.15 | | |
10/18/2032 | |
| — | | |
| — | |
| Luisa Puche, Chief Financial Officer | |
| 60,000 | | |
| — | | |
$ | 6.15 | | |
06/06/2029 | |
| — | | |
| — | |
| & Corporate Secretary | |
| 20,000 | | |
| 40,000 | | |
$ | 8.58 | | |
10/26/2031 | |
| — | | |
| — | |
| | |
| 75,000 | | |
| — | | |
$ | 2.38 | | |
07/22/2032 | |
| — | | |
| — | |
| | |
| — | | |
| 80,000 | | |
$ | 2.15 | | |
10/18/2032 | |
| — | | |
| — | |
(1) The performance-based options awarded to
Mr. Dybul in the fiscal year ended June 30, 2022, have been completely forfeited as of June 30, 2023.
Renovaro Board Compensation
The table below sets forth
the compensation earned by Renovaro’s non-employee directors for services during the fiscal year ended June 30, 2023:
| | |
Fees Earned or Paid in | |
| |
Option | |
All Other | |
|
| | |
Cash | |
Stock Awards | |
Awards | |
Compensation | |
Total |
| Name | |
($) | |
($) | |
($)(1) | |
($) | |
($) |
| | |
| |
| |
| |
| |
|
| René Sindlev | |
$ | 100,000 | | |
$ | — | | |
$ | 53,707 | | |
$ | — | | |
$ | 153,707 | |
| James Sapirstein | |
| 77,500 | | |
| — | | |
| 56,121 | | |
| — | | |
| 133,621 | |
| Carol Brosgart | |
| 69,000 | | |
| — | | |
| 55,706 | | |
| — | | |
| 124,706 | |
| Gregg Alton | |
| 77,500 | | |
| — | | |
| 55,706 | | |
| — | | |
| 133,206 | |
| Henrik Grønfeldt- Sørensen | |
| 60,000 | | |
| — | | |
| 55,091 | | |
| — | | |
| 115,091 | |
| Jayne McNicol | |
| 75,000 | | |
| — | | |
| 56,250 | | |
| — | | |
| 131,250 | |
| Total | |
$ | 459,000 | | |
$ | — | | |
$ | 332,581 | | |
$ | — | | |
$ | 791,581 | |
| (1) |
The amounts shown are not intended to reflect the value actually received by the non-employee directors. Instead, the amounts shown are the total fair value of option awards granted in the fiscal year ended June 30, 2023 for financial statement reporting purposes, as determined pursuant to Financial Accounting Standards Board Accounting Standards Codification Topic 718, or ASC Topic 718. These values are amortized as equity compensation expense over the vesting period of the grants. |
Narrative to Director’s Compensation Table
Renovaro’s director
compensation program reflects competitive practices for a Nasdaq-listed company. The resulting compensation package for Renovaro’s
non-employee directors and for committee service (for non-employee members) for the fiscal year ended June 30, 2023, is set forth
in the table below. In addition, Renovaro’s directors are awarded annual options to purchase Common Stock valued at $75,000.
| Compensation Element | |
Value |
| Retainer-Board Chair | |
$ | 100,000 | |
| Retainer-Board Members | |
$ | 60,000 | |
| Audit Committee Chair Fee | |
$ | 15,000 | |
| Compensation Committee Chair Fee | |
$ | 10,000 | |
| Nominating Committee Chair Fee | |
$ | 10,000 | |
| Audit Committee Member Fee | |
$ | 7,500 | |
| Compensation Committee Member Fee | |
$ | 5,000 | |
| Nominating Committee Member Fee | |
$ | 4,000 | |
Pay Versus Performance
The following table and supporting narrative contain information
regarding “compensation actually paid” to our named executive officers and the relationship to company performance.
Pay Versus Performance Table
| Year |
Summary Compensation Table Total for PEO ($) (1) |
Compensation Actually Paid to PEO ($) (1) |
Average Summary Compensation Table Total for Non-PEO Named Executive Officers ($) (2) |
Average Compensation Actually Paid to Non-PEO Named Executive Officers ($) (2) |
Value of $100 fixed investment |
Net Income ($mm) |
| Total Shareholder Return ($) |
| 2023 |
$1,405,433 |
$(1,079,918) |
$760,785 |
$547,183 |
$11 |
$(40) |
| 2022 |
$10,751,000 |
$4,176,667 |
$789,342 |
$503,762 |
$39 |
$(113) |
| (1) | Reflects compensation for our Chief Executive Officer, Dr.
Mark Dybul, who served as our Principal Executive Officer (PEO) in FY2022 and FY2023. |
| (2) | Reflects compensation for Luisa Puche in FY2022 and Luisa
Puche and Francois Binette in FY2023, as shown in the Summary Compensation Table for each respective year. |
To calculate “compensation actually paid”
for our PEO and other NEOs the following adjustments were made to Summary Compensation Table total pay.
| Adjustments |
PEO - Dybul |
Other NEO Average |
| 2023 |
2022 |
2023 |
2022 |
| Summary Compensation Table Total |
$1,405,433 |
$10,751,000 |
$760,785 |
$789,342 |
| Deduction for amount reported in “Stock Awards and Option Awards” column of the Summary Compensation Table |
$(640,850) |
$(9,801,000) |
$(253,598) |
$(385,592) |
| Addition of fair value at fiscal year (FY) end, of equity awards granted during the FY that remained unvested |
$115,500 |
$3,226,667 |
$25,160 |
$66,000 |
| Addition of fair value at vesting date, of equity awards granted during the FY that vested during the FY |
$0 |
$0 |
$47,310 |
$9,812 |
| Addition of change in fair value at FY end versus prior FY end for awards granted in prior FY that remained unvested |
$(1,280,001) |
$0 |
$(17,800) |
$0 |
| Addition of change in fair value at vesting date versus prior FY end for awards granted in prior FY that vested during the FY |
$126,667 |
$0 |
$(14,675) |
$24,200 |
| Reduction of fair value of awards granted during prior FY that were forfeited during applicable FY, determined as of prior FY end |
$(806,667) |
$0 |
$0 |
$0 |
| Compensation Actually Paid |
$(1,079,918) |
$4,176,667 |
$547,183 |
$503,762 |
The equity awards included above comprise of stock options granted
from 2021 through 2022. Measurement date equity fair values are calculated with assumptions derived on a basis consistent with
those used for grant date fair value purposes. Stock options are valued using a Black-Scholes Model. Performance stock options
are valued using a Monte Carlo simulation.
Compensation Actually Paid Versus Company
Performance
The following charts provide a clear, visual
description of the relationships between “compensation actually paid” to our PEOs and the average “compensation
actually paid” for our non-PEO NEOs to aspects of our financial performance.
FUTURE STOCKHOLDER PROPOSALS
To have a proposal intended
to be presented at Renovaro’s 2024 annual meeting of stockholders be considered for inclusion in the proxy statement and
form of proxy relating to that meeting, a Renovaro stockholder must deliver written notice of such proposal in writing to the Corporate
Secretary at Renovaro’s corporate headquarters no later than January 17, 2024 or, if the date of Renovaro’s 2024 annual
meeting of stockholders changes by more than 30 calendar days from the date of its 2023 annual meeting of stockholders (July 21,
2023), such notice must be delivered no later than a reasonable period of time before Renovaro begins to print and send its proxy
materials for the 2024 annual meeting of stockholders. Such proposal must also comply with the requirements as to form and substance
established by the SEC for such a proposal to be included in the proxy statement.
Other stockholder proposals
may be raised at Renovaro’s 2024 annual meeting of stockholders (but not considered for inclusion in the proxy statement
and form of proxy relating to that meeting) if notice of such proposal is delivered to the Corporate Secretary at Renovaro’s
corporate headquarters no later than April 1, 2024 or, if the date of Renovaro’s 2024 annual meeting of stockholders changes
by more than 30 calendar days from the date of its 2023 annual meeting of stockholders (July 21, 2023), such notice must be delivered
no later than a reasonable period of time before Renovaro begins to print and send its proxy materials for the 2024 annual meeting
of stockholders.
In addition to satisfying
the foregoing requirements, to comply with the universal proxy rules under the Exchange Act, stockholders who intend to solicit
proxies in support of director nominees other than our director nominees for Renovaro’s 2024 annual meeting of stockholders
must provide notice that sets forth the information required by Rule 14a-19 under the Exchange Act no later than May
22, 2024 or, if the date of Renovaro’s 2024 annual meeting of stockholders changes by more than 30 calendar days from the
date of its 2023 annual meeting of stockholders (July 21, 2023), such notice must be provided by the later of 60 calendar days
prior to the date of the 2024 annual meeting of stockholders or the 10th calendar day following the day on which public announcement
of the date of the 2024 annual meeting of stockholders is first made by Renovaro.
Renovaro reserves the right
to reject, rule out of order or take other appropriate action with respect to any proposal that does not comply with these and
other applicable requirements.
NO DISSENTERS’ RIGHTS
No dissenters’ or appraisal rights are available to Renovaro’s stockholders on the Record Date under
the General Corporation Law of the State of Delaware, the Certificate of Incorporation or the By-laws in connection with Proposals
1, 2, 3, 4 or 5.
OTHER MATTERS
Transaction of Other Business
Renovaro knows of no other
matters to be submitted to the Renovaro stockholders at the Special Meeting. If any other matters properly come before the Renovaro
stockholders at the Special Meeting, the persons named in the enclosed form of proxy will vote the shares they represent in their
discretion.
Available Information
Renovaro will furnish without
charge to each person whose proxy is being solicited, upon request of any such person, a copy of the Renovaro 2023 Form 10-K, as
filed with the SEC, including the financial statements and schedules thereto, but not the exhibits. In addition, such report is
available, free of charge, through Renovaro’s website, http://www.renovarobio.com, by clicking on Financials and then
SEC Filings. A request for a copy of such report should be directed to Renovaro Biosciences Inc., 2080 Century Park East, Suite
906, Los Angeles, CA, Attention: Chief Financial Officer. A copy of any exhibit to the Renovaro 2023 Form 10-K, as amended, will
be forwarded following receipt of a written request to us.
SPECIAL MEETING PROXY MATERIALS RESULTS
Copies of this proxy statement,
the Renovaro 2023 Form 10-K and the Renovaro First Quarter 2024 Form 10-Q may be found on Renovaro’s website at http://www.renovarobio.com
and at http://www.viewproxy.com/Renovaro/2024. Renovaro intends to publish final results from the Special Meeting in a Current
Report on Form 8-K, which will be filed with the SEC within four (4) business days from the Special Meeting, or as amended thereafter.
You may obtain a copy of this and other reports free of charge at or the SEC at (800) 732-0330 or http://www.sec.gov.
DELIVERY OF DOCUMENTS TO STOCKHOLDERS SHARING
AN ADDRESS
Only one proxy statement
is being delivered to two or more Renovaro stockholders who share an address, unless Renovaro has received contrary instruction
from one or more of such stockholders. Renovaro will promptly deliver, upon written or oral request, a separate copy of the proxy
statement to a stockholder at a shared address to which a single copy of the document was delivered. If you would like to request
additional copies of the proxy statement, or if in the future you would like to receive multiple copies of information or proxy
statements, or annual reports, or, if you are currently receiving multiple copies of these documents and would, in the future,
like to receive only a single copy, please so instruct Renovaro by writing to us at Renovaro Biosciences Inc., 2080 Century Park
East, Suite 906, Los Angeles, CA, Attention: Chief Financial Officer.
WHERE YOU CAN FIND MORE INFORMATION
Accompanying
this proxy statement are copies of the Renovaro 2023 Form 10-K and the Renovaro First Quarter 2024 Form 10-Q. Such reports include
Renovaro’s audited consolidated financial statements for the fiscal years ended June 30, 2023 and June 30, 2022
and Renovaro’s unaudited condensed consolidated financial statements for the quarter ended September 30, 2023 and certain
other financial information, which are incorporated by reference herein. See “Information Incorporated by Reference”
for further information.
Renovaro is subject
to the information requirements of the Exchange Act. Accordingly, Renovaro files annual, quarterly and current reports, proxy statements
as may be required and other information with the SEC. You can review Renovaro’s filings by accessing the website maintained
by the SEC at http://www.sec.gov. The site contains reports, proxy and information statements and other information regarding issuers
that file electronically with the SEC.
In addition to the foregoing,
Renovaro maintains a website at http://www.renovarobio.com. Information contained in this website is not part of, nor incorporated
by reference into, this proxy statement. Renovaro makes available free of charge, on or through its website, the annual, quarterly,
and current reports and any amendments to those reports filed or furnished by Renovaro pursuant to Section 13(a) of the Exchange
Act as soon as reasonably practicable after it electronically files such material with, or furnish it to, the SEC.
INFORMATION INCORPORATED BY REFERENCE
This proxy statement
incorporates by reference the Renovaro 2023 Form 10-K and the Renovaro First Quarter 2024 Form 10-Q, which Renovaro has previously
filed with the SEC (other than, in each case, documents or information deemed to have been furnished and not filed in accordance
with SEC rules) and copies of which have been delivered with this proxy statement. They contain important information about Renovaro.
Those documents are
considered to be a part of this proxy statement, effective as of the date they are filed. In the event of conflicting information
in those documents, the information in the latest filed document should be considered correct.
Renovaro has supplied
all information contained in this proxy statement relating to Renovaro, and GEDi Cube has supplied all information contained in
this proxy statement relating to GEDi Cube.
If you would like
to request documents from Renovaro or GEDi Cube, please send a request in writing or by telephone to either Renovaro or GEDi Cube
at the following addresses:
Renovaro Biosciences
Inc.
Investor Relations
2080 Century Park
East, Suite 906
Los Angeles, CA 90067
Telephone: (305) 918-1980
Email:
ir@renovarobio.com
GEDi Cube Intl Ltd.
71-75 Shelton Street
Covent Garden
London
WC2H 9JQ
E-mail: info@gedicube.com
Annex
A-I
Stock Purchase Agreement
Renovaro
Biosciences Inc., as Buyer,
GEDi
Cube Intl Ltd., as Company
The
Sellers SIGNATORIES Hereto
and
Yalla
Yalla Ltd., in its capacity as Sellers’ Representative
Stock
Purchase AGREEMENT
Dated as of September 28, 2023
| |
TABLE OF CONTENTS |
|
| |
|
Page |
| |
|
|
| ARTICLE I DEFINITIONS |
2 |
| |
|
|
| 1.1 |
Certain Defined Terms |
2 |
| |
|
|
| 1.2 |
Definitions |
14 |
| |
|
|
| ARTICLE II PURCHASE AND SALE; CLOSING |
17 |
| |
|
|
| 2.1 |
Purchase and Sale of Shares |
17 |
| |
|
|
| 2.2 |
Exchange Consideration |
17 |
| |
|
|
| 2.2.1 |
Earnout; Earnout Stock |
17 |
| |
|
|
| 2.3 |
Amended Buyer Organizational Documents |
18 |
| |
|
|
| 2.4 |
Surrender of Purchased Shares |
19 |
| |
|
|
| 2.5 |
Termination of Certain Agreements |
19 |
| |
|
|
| 2.6 |
Taking of Necessary Action |
19 |
| |
|
|
| 2.7 |
Closing |
19 |
| |
|
|
| ARTICLE III REPRESENTATIONS AND WARRANTIES |
19 |
| |
|
|
| 3.1 |
Representations and Warranties Relating to Target Companies |
19 |
| |
|
|
| 3.1.1 |
Organization, Standing, and Power |
20 |
| |
|
|
| 3.1.2 |
Capital Structure |
20 |
| |
|
|
| 3.1.3 |
Authority |
22 |
| |
|
|
| 3.1.4 |
No Conflicts; Consents, Notices and Approvals |
22 |
| |
|
|
| 3.1.5 |
Financial Statements |
22 |
| |
|
|
| 3.1.6 |
Litigation |
23 |
| |
|
|
| 3.1.7 |
Absence of Undisclosed Liabilities |
24 |
| |
|
|
| 3.1.8 |
No Violations |
24 |
| |
|
|
| 3.1.9 |
Compliance with Healthcare Laws |
24 |
| |
|
|
| 3.1.10 |
Ethical Practices |
24 |
| |
TABLE OF CONTENTS |
|
| |
(continued) |
|
| |
|
Page |
| 3.1.11 |
Employment Matters |
25 |
| |
|
|
| 3.1.12 |
Employee Benefit Plans |
26 |
| |
|
|
| 3.1.13 |
Real Property; Leases |
27 |
| |
|
|
| 3.1.14 |
Environmental |
27 |
| |
|
|
| 3.1.15 |
Company Material Contracts |
27 |
| |
|
|
| 3.1.16 |
Taxes. |
29 |
| |
|
|
| 3.1.17 |
Intellectual Property Rights |
30 |
| |
|
|
| 3.1.18 |
Artificial Intelligence |
31 |
| |
|
|
| 3.1.19 |
Privacy and Information Security. |
32 |
| |
|
|
| 3.1.20 |
Brokers and Other Fees |
33 |
| |
|
|
| 3.1.21 |
Insurance |
33 |
| |
|
|
| 3.1.22 |
Affiliate Transactions |
34 |
| |
|
|
| 3.1.23 |
Absence of Certain Changes |
34 |
| |
|
|
| 3.1.24 |
Disclosure |
34 |
| |
|
|
| 3.1.25 |
Independent Investigation |
34 |
| |
|
|
| 3.1.26 |
Information Supplied |
35 |
| |
|
|
| 3.2 |
Representations and Warranties of Buyer |
35 |
| |
|
|
| 3.2.1 |
Organization; Standing and Power |
35 |
| |
|
|
| 3.2.2 |
Capitalization |
36 |
| |
|
|
| 3.2.3 |
Authority |
38 |
| |
|
|
| 3.2.4 |
Valid Issuance of Shares |
39 |
| |
|
|
| 3.2.5 |
No Conflicts; Consents, Notices and Approvals |
39 |
| |
|
|
| 3.2.6 |
SEC Documents; Financial Statements |
39 |
| |
TABLE OF CONTENTS |
|
| |
(continued) |
|
| |
|
Page |
| 3.2.7 |
Litigation |
40 |
| |
|
|
| 3.2.8 |
Absence of Undisclosed Liabilities |
40 |
| |
|
|
| 3.2.9 |
No Violations |
40 |
| |
|
|
| 3.2.10 |
Compliance with Healthcare Laws |
41 |
| |
|
|
| 3.2.11 |
Ethical Practices |
42 |
| |
|
|
| 3.2.12 |
Employment Matters |
42 |
| |
|
|
| 3.2.13 |
Employee Benefit Plans |
44 |
| |
|
|
| 3.2.14 |
Real Property; Lease |
44 |
| |
|
|
| 3.2.15 |
Environmental |
45 |
| |
|
|
| 3.2.16 |
Buyer Material Contracts |
45 |
| |
|
|
| 3.2.17 |
Tax Returns and Payments |
47 |
| |
|
|
| 3.2.18 |
Intellectual Property Rights |
48 |
| |
|
|
| 3.2.19 |
Privacy and Information Security. |
49 |
| |
|
|
| 3.2.20 |
Brokers and Other Fees |
49 |
| |
|
|
| 3.2.21 |
Insurance |
50 |
| |
|
|
| 3.2.22 |
D&O Policy |
50 |
| |
|
|
| 3.2.23 |
Affiliate Transactions |
50 |
| |
|
|
| 3.2.24 |
Absence of Certain Changes |
50 |
| |
|
|
| 3.2.25 |
Compliance with Regulation S |
51 |
| |
|
|
| 3.2.26 |
Disclosure |
51 |
| |
|
|
| 3.2.27 |
Independent Investigation |
51 |
| |
|
|
| 3.2.28 |
Liens on Company and Company Shares |
51 |
| |
|
|
| 3.2.29 |
Information Supplied |
51 |
| |
TABLE OF CONTENTS |
|
| |
(continued) |
|
| |
|
Page |
| 3.3 |
Representations and Warranties with respect to Sellers |
52 |
| |
|
|
| 3.3.1 |
Organization, Standing, and Power |
52 |
| |
|
|
| 3.3.2 |
Authority |
52 |
| |
|
|
| 3.3.3 |
Investment Representations |
53 |
| |
|
|
| ARTICLE IV COVENANTS OF COMPANY AND SELLER |
54 |
| |
|
|
| 4.1 |
Conduct of Business |
54 |
| |
|
|
| 4.1.1 |
Ordinary Course |
54 |
| |
|
|
| 4.1.2 |
Seller Transfers |
56 |
| |
|
|
| 4.1.3 |
Exclusivity; Acquisition Proposals |
57 |
| |
|
|
| 4.1.4 |
Company Financial Statements |
58 |
| |
|
|
| 4.1.5 |
No Trading |
59 |
| |
|
|
| 4.2 |
Notification; Access to Information |
59 |
| |
|
|
| 4.2.1 |
Cooperation |
59 |
| |
|
|
| 4.2.2 |
Access to Information |
59 |
| |
|
|
| 4.3 |
Consents and Notices |
60 |
| |
|
|
| 4.4 |
Commercially Reasonable Efforts |
60 |
| |
|
|
| 4.5 |
Proxy Statement |
60 |
| |
|
|
| 4.6 |
Issuance of Company Oridinary Shares to Consultant and Employee |
60 |
| |
|
|
| ARTICLE V COVENANTS OF BUYER |
60 |
| |
|
|
| 5.1 |
Conduct of Business |
60 |
| |
|
|
| 5.1.1 |
Ordinary Course |
60 |
| |
|
|
| 5.1.2 |
Exclusivity; Acquisition Proposals |
63 |
| |
|
|
| 5.1.3 |
Buyer Public Filings |
63 |
| |
TABLE OF CONTENTS |
|
| |
(continued) |
|
| |
|
Page |
| 5.2 |
Notification; Access to Information |
64 |
| |
|
|
| 5.2.1 |
Cooperation |
64 |
| |
|
|
| 5.2.2 |
Access to Information |
64 |
| |
|
|
| 5.3 |
Consents and Notices |
64 |
| |
|
|
| 5.4 |
Commercially Reasonable Efforts |
65 |
| |
|
|
| 5.5 |
Proxy Statements; Buyer Stockholder Meeting |
65 |
| |
|
|
| 5.5.1 |
Proxy Statements |
65 |
| |
|
|
| 5.5.2 |
Buyer Stockholder Meeting |
65 |
| |
|
|
| 5.5.3 |
Stockholder Approval of Amendment |
66 |
| |
|
|
| 5.6 |
Fairness Opinion |
66 |
| |
|
|
| 5.7 |
D&O Policy |
66 |
| |
|
|
| ARTICLE VI ADDITIONAL AGREEMENTS |
66 |
| |
|
|
| 6.1 |
Confidentiality Agreement |
66 |
| |
|
|
| 6.2 |
Expenses |
66 |
| |
|
|
| 6.3 |
Further Assurances |
67 |
| |
|
|
| 6.4 |
Public Announcements |
67 |
| |
|
|
| 6.4.1 |
Prior Written Consent |
67 |
| |
|
|
| 6.4.2 |
Press Releases and Filings |
67 |
| |
|
|
| 6.5 |
Post-Closing Board of Directors |
68 |
| |
|
|
| 6.6 |
Indemnification of Company’s Board and Officers |
68 |
| |
|
|
| 6.7 |
Post-Closing Tax Matters |
68 |
| |
|
|
| 6.7.1 |
Returns Filed |
68 |
| |
|
|
| 6.7.2 |
Tax Cooperation |
69 |
| |
TABLE OF CONTENTS |
|
| |
(continued) |
|
| |
|
Page |
| 6.7.3 |
No Amendments |
69 |
| |
|
|
| 6.7.4 |
Transfer Taxes |
69 |
| |
|
|
| 6.8 |
Efforts to Consummate; Regulatory Matters and Approvals |
69 |
| |
|
|
| 6.8.1 |
Defense of Proceedings |
69 |
| |
|
|
| 6.9 |
Securities Laws Compliance |
69 |
| |
|
|
| 6.10 |
Delivery of Disclosure Schedules |
69 |
| |
|
|
| 6.11 |
Additional Sellers |
70 |
| |
|
|
| ARTICLE VII CONDITIONS PRECEDENT |
70 |
| |
|
|
| 7.1 |
Conditions to Each Party’s Obligation to Effect the Transactions |
70 |
| |
|
|
| 7.1.1 |
Buyer Stockholder Approval |
70 |
| |
|
|
| 7.1.2 |
Consents |
70 |
| |
|
|
| 7.1.3 |
No Order |
70 |
| |
|
|
| 7.1.4 |
Nasdaq Listing |
70 |
| |
|
|
| 7.2 |
Conditions to Obligations of Buyer |
71 |
| |
|
|
| 7.2.1 |
Representations and Warranties of Company and Sellers |
71 |
| |
|
|
| 7.2.2 |
Agreements and Covenants |
71 |
| |
|
|
| 7.2.3 |
No Company Material Adverse Effect |
72 |
| |
|
|
| 7.2.4 |
Return of All Distributions |
72 |
| |
|
|
| 7.2.5 |
Certain Transaction Documents |
72 |
| |
|
|
| 7.2.6 |
Seller’s and Company’s Closing Deliverables |
72 |
| |
|
|
| 7.2.7 |
Legal Action |
73 |
| |
|
|
| 7.2.8 |
Financial Statements |
73 |
| |
|
|
| 7.2.9 |
Fairness Opinion |
73 |
| |
TABLE OF CONTENTS |
|
| |
(continued) |
|
| |
|
Page |
| 7.2.10 |
Termination of Certain Agreements |
73 |
| |
|
|
| 7.3 |
Conditions to Obligation of Company and Sellers |
73 |
| |
|
|
| 7.3.1 |
Representations and Warranties of Buyer |
73 |
| |
|
|
| 7.3.2 |
Agreements and Covenants |
74 |
| |
|
|
| 7.3.3 |
No Buyer Material Adverse Effect |
74 |
| |
|
|
| 7.3.4 |
Certain Transaction Documents |
74 |
| |
|
|
| 7.3.5 |
Buyer Organizational Documents |
74 |
| |
|
|
| 7.3.6 |
Appointment to the Board |
74 |
| |
|
|
| 7.3.7 |
Buyer Closing Deliverables |
74 |
| |
|
|
| 7.4 |
Frustrations of Conditions |
75 |
| |
|
|
| ARTICLE VIII INDEMNIFICATION |
75 |
| |
|
|
| 8.1 |
Indemnification by Sellers |
75 |
| |
|
|
| 8.2 |
Indemnification by Buyer |
76 |
| |
|
|
| 8.3 |
Third Party Claims |
77 |
| |
|
|
| 8.4 |
Tax Contests |
78 |
| |
|
|
| 8.5 |
Survival |
79 |
| |
|
|
| 8.6 |
Limitations |
79 |
| |
|
|
| 8.6.1 |
Threshold Amounts |
80 |
| |
|
|
| 8.6.2 |
Caps |
80 |
| |
|
|
| 8.7 |
Exclusive Remedy |
81 |
| |
|
|
| 8.8 |
Value of Shares |
82 |
| |
|
|
| 8.9 |
Losses Net of Insurance Coverage |
82 |
| |
|
|
| 8.10 |
No Duplicative Recovery |
82 |
| |
TABLE OF CONTENTS |
|
| |
(continued) |
|
| |
|
Page |
| 8.11 |
Straddle Period |
83 |
| |
|
|
| 8.12 |
Tax Treatment of Indemnification Payments |
83 |
| |
|
|
| ARTICLE IX CLAIMS |
83 |
| |
|
|
| 9.1 |
Notice of Claims and Expenses |
83 |
| |
|
|
| 9.2 |
Resolution of Claims |
83 |
| |
|
|
| 9.3 |
Arbitration |
84 |
| |
|
|
| ARTICLE X TERMINATION, AMENDMENT, AND WAIVER |
84 |
| |
|
|
| 10.1 |
Termination |
84 |
| |
|
|
| 10.1.1 |
Mutual Written Consent |
85 |
| |
|
|
| 10.1.2 |
Written Notice by Buyer or Sellers’ Representatives |
85 |
| |
|
|
| 10.1.3 |
Written Notice of Order |
85 |
| |
|
|
| 10.1.4 |
Written Notice of Breach by Buyer |
85 |
| |
|
|
| 10.1.5 |
Written Notice of Breach by Company or Seller |
85 |
| |
|
|
| 10.1.6 |
Written Notice of Company Material Adverse Effect |
86 |
| |
|
|
| 10.1.7 |
Written Notice of Buyer Stockholder Meeting |
86 |
| |
|
|
| 10.1.8 |
Written Notice of Unsatisfactory Buyer Due Diligence |
86 |
| |
|
|
| 10.1.9 |
Written Notice of Unsatisfactory Seller Due Diligence |
86 |
| |
|
|
| 10.2 |
Effect of Termination |
86 |
| |
|
|
| ARTICLE XI GENERAL PROVISIONS |
86 |
| |
|
|
| 11.1 |
Notices |
86 |
| |
|
|
| 11.2 |
Disclosure Schedule |
88 |
| |
|
|
| 11.3 |
Supplements to Disclosure Schedule |
88 |
| |
|
|
| 11.4 |
Interpretation |
88 |
| |
TABLE OF CONTENTS |
|
| |
(continued) |
|
| |
|
Page |
| 11.5 |
Counterparts |
89 |
| |
|
|
| 11.6 |
Entire Understanding; No Third Party Beneficiaries |
89 |
| |
|
|
| 11.7 |
Governing Law |
90 |
| |
|
|
| 11.8 |
Amendment; Waiver |
90 |
| |
|
|
| 11.9 |
Successors and Assigns |
90 |
| |
|
|
| 11.10 |
Specific Performance |
90 |
| |
|
|
| 11.11 |
Severability |
90 |
| |
|
|
| 11.12 |
Submission to Jurisdiction |
91 |
| |
|
|
| 11.13 |
Waiver of Jury Trial |
91 |
| |
|
|
| 11.14 |
Sellers’ Representative |
91 |
| |
|
|
| 11.14.1 |
Appointment of Seller’s Representative |
91 |
| |
|
|
| 11.14.2 |
Reliance by the Sellers’ Representative |
92 |
| |
|
|
| 11.14.3 |
Expenses of the Sellers’ Representative |
93 |
| |
|
|
| 11.14.4 |
Indemnification of the Sellers’ Representative |
93 |
| |
|
|
| 11.14.5 |
Resignation of the Sellers’ Representative |
93 |
| |
|
|
| 11.15 |
Legal Representation |
93 |
STOCK PURCHASE AGREEMENT
This stock
purchase AGREEMENT (this “Agreement”), dated September 28, 2023 (the “Effective Date”),
is by and among Renovaro Biosciences Inc., a Delaware corporation (“Buyer”), GEDi Cube Intl Ltd., a private
company formed under the laws of England and Wales (“Company”), each of the
shareholders of the Company that are named on signature pages hereto that have executed and delivered a copy of this
Agreement as of the date hereof (collectively the “Signing Sellers”), each of the other shareholders of the
Company that after the Effective Date execute and deliver to the Buyer, the Company and the Seller Representative (as defined below)
a joinder agreement in substantially the form attached as Exhibit A hereto (each a “Seller Joinder”)
to become a party to this Agreement which Seller Joinder is accepted in writing and executed and delivered by Buyer, the Company
and the Seller Representative (collectively, the “Joining Sellers” and, together with the Signing Sellers, each
a “Seller” and collectively, the “Sellers”), and Yalla Yalla Ltd., a private limited liability
company registered and incorporated under the laws of Malta with company registration number C 103531 (“Yalla”),
in its capacity as representative to the Sellers (the “Sellers’ Representative”). Buyer, Company, Sellers
and the Sellers’ Representative are each referred to as a “Party” or collectively as the “Parties.”
WHEREAS, the Signing Sellers
own the issued shares and other equity interests in or of Company set forth opposite such Signing Seller’s name in Exhibit
B hereto (the “Company Shares”);
WHEREAS, Company holds the
legal and beneficial title to all issued and outstanding shares, with a nominal value of EUR 0.01 (one euro cent) each, in the
capital of Grace Systems B.V., a private limited liability company (een besloten vennootschap met beperkte aansprakelijkheid)
organized and existing under the laws of the Netherlands, having its corporate seat (statutaire zetel) in Amsterdam, the
Netherlands, with address at Park Oosterspaarn 48, 2036MB Haarlem, the Netherlands, registered with the Trade Register of the Dutch
Chamber of Commerce under number 58062203 (“Grace”), which such shares constitute the only issued and outstanding
equity interests of Grace;
WHEREAS, Company holds the
legal and beneficial title to all issued and outstanding shares, with a nominal value of EUR 0.01 (one euro cent) each, in the
capital of Gedi Cube B.V., a private limited liability company (een besloten vennootschap met beperkte aansprakelijkheid)
organized and existing under the laws of the Netherlands, having its corporate seat (statutaire zetel) in Amsterdam, the
Netherlands, with address at Park Oosterspaarn 48, 2036MB Haarlem, the Netherlands, registered with the Trade Register of the Dutch
Chamber of Commerce under number 91275237, “GC Sub”),
which such shares constitute the only issued and outstanding equity interests of GC Sub;
Whereas,
Grace and GC Sub are engaged in the business of artificial intelligence machine learning technology with validation in humans for
early diagnosis of lung cancers, as well as silico detection for additional cancers;
WHEREAS, Buyer is a biotechnology
company committed to developing advanced cell and gene therapies to promote stronger immune system responses potentially for long-term
or life-long cancer remission in some of the deadliest cancers, and potentially to treat or cure serious infectious diseases such
as Human Immunodeficiency Virus and potentially Hepatitis B virus infection;
Whereas,
the Signing Sellers, and upon their execution and delivery of this Agreement, the Joining Sellers, desire to sell to Buyer, and
Buyer desires to purchase from the Signing Sellers, and upon their execution and delivery of this Agreement, from the Joining Sellers,
all of the issued and outstanding shares and any other equity interests in or of the Company
in exchange for newly issued shares of Buyer Common Stock (the “Share Exchange”), on the terms and conditions
set forth in this Agreement.
NOW,
Therefore, in consideration of the premises and the mutual representations, warranties, covenants, and agreements in this
Agreement and for other good and valuable consideration, the receipt and sufficiency of which are hereby
acknowledged, the Parties intending to be legally bound, agree as follows:
Article
I
DEFINITIONS
1.1 Certain Defined
Terms. The terms below are defined as follows:
“Acquisition Proposal”
means any inquiry, proposal or offer, or any indication of interest in making an offer or proposal, from any Person or group at
any time relating to an Alternative Transaction.
“AI”
collectively means the AI Inputs, AI Outputs and AI Technology;
“AI Inputs”
means any and all of the following generated by or derived from AI Technology which are, in whole or in part, used or relied upon,
or licensed, sold, otherwise provided or accessed, by or to Company or its subsidiaries, including but not limited to, data, writings,
works of authorship, graphics, pictures, recordings, any electronic or other information, text or numerals, audio or visual content,
or materials of any nature or description;
“AI Outputs”
means any and all services, products, data, writings, works of authorship, graphics, pictures, recordings, any electronic or other
information, text or numerals, audio or visual content, or materials of any nature or description generated or derived by or on
behalf of Company or its subsidiaries, from any AI Technology or AI Inputs, where such outputs are licensed, sold, provided to,
or otherwise made available or accessible by Company or its subsidiaries to any third party;
“AI Technology”
means any and all training, self-improving, or machine learning software, algorithms, hardware or other artificial intelligence
tools or aids of any kind.
“Accounting Principles”
means, (i) with respect to Buyer and the other Renovaro Group members, US GAAP, (ii) with respect to Company, UK GAAP, and
(iii) with respect to the Company’s subsidiaries, Dutch GAAP.
“Applicable Percentage”
means an amount, expressed as a percentage and calculated as of the Closing Date immediately prior to Closing, equal to the ratio
of (a) the aggregate number of Company Shares held by all Sellers, divided by (b) the aggregate Company Ordinary Shares
issued and outstanding.
“as converted basis”
means, as of any date of determination, all issued and outstanding shares of Buyer Common Stock and all Buyer Common Stock issuable
upon the conversion of any outstanding Convertible Securities of Buyer as of such date, whether or not such Buyer Convertible Security
is at the time convertible.
“Assets”
of any Person means all assets and properties of every kind, nature, character and description, that are owned, leased or licensed
by such Person.
“Buyer Common Stock”
means the common stock of Buyer, par value $0.0001 per share.
“Buyer IP Rights”
shall mean Buyer Patent Rights, Buyer Know-How Rights, Trade Secrets and any other Intellectual Property in or to the Buyer Technology.
“Buyer Know-How
Rights” shall mean all Know-How Rights in and to Buyer Technology.
“Buyer Material
Adverse Effect” means any event, circumstance, development, occurrence, fact, condition, effect, or change (each, an
“Effect”) that, individually or in the aggregate, is or would reasonably be expected to be materially adverse
to: (a) the business, results of operations, condition (financial or otherwise), or assets of Buyer and the Renovaro Group, taken
as a whole; or (b) the ability of Buyer to timely perform its obligations under this Agreement or consummate the Transactions contemplated
hereby on a timely basis; provided, however, that a Buyer Material Adverse Effect shall not be deemed to include any Effect
(alone or in combination) arising out of, relating to, or resulting from: (i) changes generally affecting the economy, financial
or securities markets, or political conditions; (ii) the execution and delivery, or consummation of the Transactions contemplated
by this Agreement (it being understood and agreed that this clause shall not apply with respect to any representation or warranty
that is intended to address the consequences of the execution and delivery or consummation of this Agreement); (iii) any changes
in applicable Law or Accounting Principles or other applicable accounting standards; (iv) acts of war or terrorism or the escalation
thereof; (v) natural disasters, epidemics, pandemics, or disease outbreaks (including the COVID-19 virus), public health emergencies
(as declared by the World Health Organization or the Health and Human Services Secretary of the United States), or other force
majeure events; (vi) general conditions in the industry in which Buyer operates; (vii) any failure, in and of itself, by Buyer
to meet any internal or published projections, forecasts, estimates,
or predictions in respect of revenues, earnings, or other
financial or operating metrics for any period (it being understood that any Effect underlying such failure may be deemed to constitute,
or be taken into account in determining whether there has been or would reasonably be expected to become, a Buyer Material Adverse
Effect, to the extent permitted by this definition and not otherwise excepted by another clause of this proviso); (viii) any change,
in and of itself, in the market price or trading volume of Buyer’s securities (it being understood that any Effect underlying
such change may be deemed to constitute, or be taken into account in determining whether there has been or would reasonably be
expected to become, a Buyer Material Adverse Effect, to the extent permitted by this definition and not otherwise excepted by another
clause of this proviso); or (ix) actions taken as required or specifically permitted by the Agreement or actions or omissions taken
with Company’s consent; provided further, however, that any Effect referred to in clauses (i), (iii), (iv),
(v), or (vi) immediately above shall be taken into account in determining whether a Buyer Material Adverse Effect has occurred
or would reasonably be expected to occur if it has a disproportionate effect on Buyer or the Renovaro Group, taken as a whole,
compared to other participants in the industries in which Buyer conducts its businesses.
“Buyer Patent Rights”
shall mean any and all current and future Patent Rights that claim or Cover Company Technology related to Buyer’s business,
including, but not limited to the Patent Rights set forth on Section 3.2.18(a) of the Buyer Disclosure Schedule.
“Buyer Special
Representations” means the representations and warranties set forth in Section 3.2.17 and Section 3.2.18.
“Buyer Technology”
shall mean all Technology that relates to Buyer’s business that is (i) owned by Renovaro Group or licensed to a Renovaro
Group member immediately prior to the Effective Date or (ii) conceived, created, generated, made, derived, developed, reduced to
practice or acquired by or on behalf of Renovaro Group, solely or jointly with any other Person.
“Code”
means the U.S. Internal Revenue Code of 1986, as amended.
“Company IP Rights”
shall mean Company Patent Rights, Company Know-How Rights, Trade Secrets and any other Intellectual Property in or to the Company
Technology.
“Company Know-How
Rights” shall mean all Know-How Rights in and to Company Technology.
“Company Loan Proceed
Shares” means any Renovaro Group Securities issued prior to Closing, the proceeds of which are used to finance the operations
of the Company; provided; however; that in the event any such loan made by Buyer to the Company is prepaid by the
Company prior to the Closing, such Renovaro Group Securities underlying the proceeds of such prepaid loan shall be reduced from
the Company Loan Proceed Shares.
“Company Material
Adverse Effect” means any Effect that, individually or in the aggregate, is or would reasonably be expected to be materially
adverse to: (a) the business, results of operations, condition (financial or otherwise), or assets of Target Companies, taken as
a whole; or (b) the ability of Company or Sellers to timely perform their obligations under this Agreement or consummate the transactions
contemplated hereby on a timely basis; provided, however, that a Company Material Adverse Effect shall not be deemed to
include any Effect (alone or in combination) arising out of, relating to, or resulting from: (i) changes generally affecting the
economy, financial or securities markets, or political conditions; (ii) the execution and delivery, or consummation of the transactions
contemplated by this Agreement (it being understood and agreed that this clause shall not apply with respect to any representation
or warranty that is intended to address the consequences of the execution and delivery or consummation of this Agreement); (iii) any
changes in applicable Law or Accounting Principles or other applicable accounting standards (iv) acts of war or terrorism or the
escalation thereof; (v) natural disasters, epidemics, pandemics, or disease outbreaks (including the COVID-19 virus), public health
emergencies (as declared by the World Health Organization or the Health and Human Services Secretary of the United States), or
other force majeure events; (vi) general conditions in the industry in which any Target Company operates; (vii) any failure, in
and of itself, by any Target Company to meet any internal or published projections, forecasts, estimates, or predictions in respect
of revenues, earnings, or other financial or operating metrics for any period (it being understood that any Effect underlying such
failure may be deemed to constitute, or be taken into account in determining whether there has been or would reasonably be expected
to become, a Company Material Adverse Effect, to the extent permitted by this definition and not otherwise excepted by another
clause of this proviso); or (viii) actions taken as required or specifically permitted by the Agreement or actions or omissions
taken with Buyer’s consent; provided further, however, that any Effect referred to in clauses (i), (iii), (iv),
(v), or (vi) immediately above shall be taken into account in determining whether a Company Material Adverse Effect has occurred
or would reasonably be expected to occur if it has a disproportionate effect on Target Companies, taken as a whole, compared to
other participants in the industries in which any Target Company conducts its business.
“Company Ordinary
Shares” means the 2,157 ordinary shares of £1.00 each in the capital of Company.
“Company Patent
Rights” shall mean any and all current and future Patent Rights that claim or Cover Company Technology related to Target
Companies’ businesses.
“Company Securities”
means, collectively, the Company Ordinary Shares and any Convertible Securities of the Company.
“Company Special
Representations” means the representations and warranties set forth in Section 3.1.16.
“Company Technology”
shall mean all Technology that is (i) owned by any Target Company or licensed to any Target Company (including by any Seller) immediately
prior to the Effective Date or (ii) conceived, created, generated, made, derived, developed, reduced to practice or acquired by
or on behalf of any Target Company, solely or jointly with any other Person.
“Confidential Information”
means any and all confidential or proprietary Information, whether communicated in writing or orally or by any other method, which
is provided by or on behalf of one Party to the other Party in connection with this Agreement or the Transactions, and which is
designated in writing as confidential at the time of such communication or which should, given the facts and circumstances surrounding
the communication reasonably be understood as confidential by the receiving Party, excluding any information that (i) was in the
public domain at the time of disclosure, (ii) is published or otherwise comes into the public domain after its disclosure through
no violation of this Agreement, (iii) is disclosed to the recipient by a third party not under an obligation of confidence, or
(iv) is already known by the recipient at the time of its disclosure as evidenced by written documentation of the recipient existing
prior to such disclosure.
“Consent”
means any consent, approval, waiver, authorization or Permit of, or notice to or declaration or filing with any Governmental Entity
or any other Person.
“Contract”
means any legally binding written or oral contract, lease, sublease, license, sublicense, loan agreement, master services agreement,
mortgage, security agreement, indemnity agreement, surety agreement, trust indenture or other agreement or instrument.
“Control”,
“Controls”, or “Controlled by” means, with respect to any Person, the power to direct or
cause the direction of the management or policies of a Person, whether through the ability to exercise voting power, by contract
or otherwise.
“Convertible Securities”
means, with respect to a Person, collectively, any options, warrants, convertible notes, convertible preferred stock (including,
in the case of Buyer, Preferred Stock) or other rights to subscribe for or purchase any capital stock of such Person or securities
convertible into or exchangeable for, or that otherwise confer on the holder any right to acquire any equity interest of such Person.
For the avoidance of doubt, Convertible Securities shall include any securities, rights and/or profits interests, issued by any
Affiliate, plan, holding company, or other entity which, directly or indirectly, holds equity interests in such Person, and which
can cause the revaluation, valuation, issuance, profits or payment compensation in connection with, or conversion, exercise or
exchange of, any equity interests of such Person.
“Cover”
means, (a) with respect to Know-How Rights, that such Know-How Rights were used in making, having made, using, selling, offering
to sell, importing, having sold, exporting or making improvements to the product, and (b) with respect to a Patent Right, a Valid
Patent Claim would (absent a license thereunder or ownership thereof) be infringed by making, having made, using, selling, offering
to sell, importing, having sold, exporting or making improvements to the product, in each case, including research and development.
Grammatical variations of the word “Cover”, including “Covered”, shall have correlative meanings.
“Dutch GAAP”
means, the provisions of Title 9 of Book 2 of the Dutch Civil Code (Burgerlijk wetboek) and the Regulations for annual reporting
(Richtlijnen voor de Jaarverslaggeving).
“equity interest”
means (i) with respect to a corporation, any and all classes or series of shares of capital stock, (ii) with respect to a partnership,
limited liability company, trust or similar Person, any and all classes or series of units, interests or other partnership/limited
liability company interests and (iii) with respect to any other entity, any other security representing ownership interest, equity
or participation in such entity.
“Environmental
Laws” means any Law, required permit, license or other authorization relating to: (i) releases or threatened releases
of Hazardous Materials or materials containing Hazardous Materials; (ii) the presence, manufacture, refining, production, generation,
handling, transport, use, treatment, recycling, storage, importing, labeling, testing, disposal, cleanup or control of Hazardous
Materials or materials containing Hazardous Materials; (iii) pollution or protection of the environment or natural resources; or
(iv) public health and safety or, as it relates to the handling of or exposure to Hazardous Materials, worker/occupational health
and safety.
“Environmental
Professional” means an individual licensed by a Governmental Entity to act on behalf of such Governmental Entity to oversee
environmental site investigation and remediation.
“Exchange Act”
means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
“Fraud”
means common law fraud under Delaware Law, including the elements of scienter and reliance, with respect to a representation or
warranty (i) that was untrue, (ii) which the Person making the representation knew to be untrue at the time such representation
was made, (iii) with the intent to deceive and for the purpose of inducing the recipient to act upon it.
“Governmental Entity”
means any federal, state, local or foreign government or political subdivision thereof, or any agency, commission, governmental
authority, or instrumentality of such government or political subdivision, or court, or any self-regulated organization or other
non-governmental regulatory authority or quasi-governmental authority.
“Hazardous Material”
means: (i) any substance, material or waste which is regulated by, or for which liability or standards of conduct may be imposed
under, any Environmental Law, including any substance, material or waste which is defined as a “hazardous substance,”
“hazardous material,” “hazardous waste,” “solid waste,” “pollutant,” “contaminant,”
“toxic substance,” “toxic waste” or other similar term or phrase under any applicable Environmental Law;
(ii) petroleum and petroleum products, including crude oil and any fractions thereof; (iii) natural gas, natural gas liquids, synthetic
gas, and any mixtures thereof; (iv) polychlorinated biphenyls, asbestos and asbestos-containing materials, urea formaldehyde, toxic
mold, and radon; and (v) per- or polyfluoroalkyl substances.
“Healthcare Law”
means any Law relating to the regulation of the healthcare or clinical laboratory industry or to the payment for services rendered
by healthcare providers, including, without limitation: Title XVIII of the SSA (Medicare); Title XIX of the SSA (Medicaid); Title
10, Chapter 55 of the U.S.C. (TRICARE); the Federal Anti-Kickback Law (42 U.S.C. § 1320a-7b); the Stark Law (42 U.S.C. §
1395nn); the Federal False Claims Act (31 U.S.C. §§ 3729, et seq.); the Federal Civil Monetary Penalties Law (42 U.S.C.
§ 1320a-7a); the Federal Program Fraud Civil Remedies Act (31 U.S.C. § 3801 et seq.); the Federal Health Care Fraud law
(18 U.S.C. § 1347); the criminal false claims statutes (e.g., 18 U.S.C. §§ 287 and 1001); the Medicare Secondary
Payor Statute (32 U.S.C. § 1395y(b)); the Federal Food, Drug and Cosmetic Act (21 U.S.C. § 321 et seq.) and all regulations
promulgated thereunder; the Health Insurance Portability and Accountability Act of 1996 as amended by the Health Information Technology
for Economic and Clinical Health Act of 2009, and their implementing regulations and any state health care privacy, security or
confidentiality Laws; the Patient Protection and Affordable Care Act (Pub. L 111-148) as amended by the Health Care and Education
Reconciliation Act of 2010 (Pub. L. 111-152) and the regulations adopted thereunder; Laws pertaining to licensure, certification,
registration or operation requirements of healthcare facilities, services or equipment, including, but not limited to, the Clinical
Laboratory Improvement Amendments, as amended; CMS manual guidance, and applicable Medicare administrative contractor guidance
pertaining to coding, coverage, reimbursement, claims submission, billing and collections; state certificate of need or similar
Laws governing the establishment of healthcare facilities or service or the making of healthcare capital expenditures; state Laws
relating to fee-splitting, patient brokering or the corporate practice of medicine; and any state physician self-referral prohibition
or state anti-kickback Laws.
“In-License”
means all licenses and other agreements under which (i) any Target Company or (ii) any member of the Renovaro Group has been granted
a license to any Intellectual Property (other than for “shrink wrapped,” “click-through,” or other form
license-based “off-the-shelf” third party Intellectual Property that is otherwise commercially available on standard,
nondiscriminatory terms, for an annual or one-time license fee of no more than $50,000).
“Indebtedness”
of any Person means, without duplication, (i) all indebtedness of such Person for borrowed money (including the outstanding
principal and accrued but unpaid interest), (ii) all obligations for the deferred purchase price of property or services (other
than trade payables incurred in the ordinary course of business), (iii) any other indebtedness of such Person that is evidenced
by a note, bond, debenture, credit agreement or similar instrument, (iv) all obligations of such Person under leases that
should be classified as finance leases in accordance with its Accounting Principles, (v) all obligations of such Person for
the reimbursement of any obligor on any line or letter of credit, banker’s acceptance, guarantee or similar credit transaction,
in each case, that has been drawn or claimed against, (vi) all obligations of such Person in respect of acceptances issued
or created, (vii) all interest rate and currency swaps, caps, collars and similar agreements or hedging devices under which
payments are obligated to be made by such Person, whether periodically or upon the happening of a contingency, (viii) all
obligations secured by a Lien on any property of such Person, (ix) any premiums, prepayment fees or other penalties, fees,
costs or expenses associated with payment of any Indebtedness of such Person, and (x) all obligations described in clauses
(i) through (ix) above of any other Person which is directly or indirectly guaranteed by such Person or which such Person
has agreed (contingently or otherwise) to purchase or otherwise acquire or in respect of which it has otherwise assured a creditor
against loss.
“Information”
shall mean any and all data, information, materials and know-how (whether patentable or not), including, (a) ideas, discoveries,
inventions, improvements, Technology or trade secrets, (b) pharmaceutical, chemical and biological materials, products, components,
or compositions, (c) methods, procedures, formulas, processes, tests, assays, techniques, regulatory requirements, business plans,
marketing plans, and competitive strategies, (d) biological, chemical, pharmacological, toxicological, pharmaceutical, physical
and analytical, clinical, safety, manufacturing, process and quality control data and information related thereto, (e) technical
and non-technical data and other information related to the foregoing, and (f) drawings, plans, designs, diagrams, sketches, specifications,
invention disclosures, patent applications or other documents containing or relating to such data, information, materials or know-how.
“Infringe”
means any infringement as determined by applicable law, including, without limitation, direct infringement, contributory infringement
or any inducement to infringe.
“Intellectual Property”
means any and all of the following and all rights in, relating to, arising out of, or associated with (whether provisional or non-provisional)
intellectual property and proprietary rights, whether protected, created, or arising under any Law or jurisdiction throughout the
world (including all applications or rights to apply for any of the following, and all registrations, renewals, extensions, future
equivalents, and restorations, now or hereafter in force and effect), including: all United States, international, and foreign:
(1) issued patents and patent applications, and all substitutions, reissues, divisions, reexaminations, provisionals, continuations,
continuations-in-part, revalidations, extensions, supplementary protection certificates, results of inter parties, post grant or
covered business method patent reviews and derivation proceedings, and equivalent or similar rights anywhere in the world in inventions,
discoveries, and designs, including invention disclosures or restorations of any of the foregoing, and
other Governmental Entity-issued indicia of invention ownership (including certificates of invention, petty patents, and patent
utility models) (“Patents”); (2) all Trade Secrets; (3) trademarks, service
marks, brands, certification marks, logos, trade dress, trade names, and other similar indicia of source or origin, together with
the goodwill connected with the use of and symbolized by, and all registrations, applications for registration, and renewals of,
any of the foregoing; (4) copyrights and works of authorship, whether or not copyrightable, and all registrations, applications
for registration, and renewals of any of the foregoing and all other rights corresponding thereto, (including moral rights),
throughout the world; (“Copyrights”); (5) mask works, and all registrations, applications
for registration, and renewals thereof; (6) industrial designs, and all Patents, registrations, applications for registration,
and renewals thereof, whether or not Copyrights; (7) computer programs, operating systems, applications, firmware, and other code,
including all source code, object code, application programming interfaces, data files, databases, protocols, specifications, and
other documentation thereof; (8) rights of publicity, privacy and personality; (9) all
rights in World Wide Web addresses, URLs, domain names, social media accounts and handles, other similar designations, and contract
rights therein and (10) all other intellectual or industrial property, proprietary rights, and
any similar, corresponding, or equivalent rights to any of the foregoing in items (1) through (10) above, anywhere in the world.
“Intellectual Property
Rights” means, collectively, Patent Rights, Know-How Rights, and any other Intellectual Property.
“IP Assignment”
means all agreements under which any Person has sold, conveyed, transferred, assigned or delivered any Intellectual Property to
(i) any Target Company or (ii) the Renovaro Group, as applicable.
“Know-How Rights”
means all Trade Secrets and other know-how rights (whether at law, in equity or otherwise) including, but not limited to, rights
in Confidential Information.
“Knowledge”
means, with respect to (i) Company, the actual knowledge of the executive officers or directors of any Target Company, including,
for the avoidance of a doubt, Frank Van Asch, after reasonable inquiry, or (ii) any other Party, (A) if an entity, the
actual knowledge of its directors and executive officers, after reasonable inquiry, or (B) if a natural person, the actual
knowledge of such Party after reasonable inquiry.
“Law”
means any statute, law, ordinance, regulation, rule, code, order, constitution, treaty, common law, judgment, decree, other requirement
or rule of law of any Governmental Entity.
“Liabilities”
means any and all liabilities, Indebtedness, Proceedings or obligations of any nature (whether absolute, accrued, contingent
or otherwise, whether known or unknown, whether direct or indirect, whether matured or unmatured, whether due or to become due
and whether or not required to be recorded or reflected on a balance sheet under Accounting Principles or other applicable accounting
standards), including Tax liabilities due or to become due.
“Lien”
means any mortgage, pledge, security interest, attachment, right of first refusal, option, proxy, voting trust, encumbrance, lien
or charge of any kind (including any conditional sale or other title retention agreement or lease in the nature thereof), restriction
(whether on voting, sale, transfer, disposition or otherwise), any subordination arrangement in favor of another Person, or any
filing or agreement to file a financing statement as debtor under any Law.
“Nasdaq”
shall mean The Nasdaq Stock Market.
“Order”
means any order, decree, ruling, judgment, injunction, writ, determination, binding decision, verdict, judicial award or other
action that is or has been made, entered, rendered, or otherwise put into effect by or under the authority of any Governmental
Entity.
“Organizational
Documents” means, with respect to any Person that is an entity, its certificate of incorporation or formation, bylaws,
operating agreement, memorandum and articles of association or similar organizational documents, in each case, as amended.
“Patent Rights”
shall mean (i) Patents (which for the purposes of this Agreement shall be deemed to include certificates of invention and applications
for certificates of invention); (ii) any protection certificates, results of inter parties, post-grant, or covered business method
patent reviews and derivation proceedings, and the like of any such patents and patent applications; and (iii) any and all foreign
equivalents of the foregoing throughout the world.
“Permits”
means all permits, licenses, franchises, approvals, consents, permissions, clearances, exceptions, authorizations, registrations,
qualifications certificates, variances and similar rights obtained, or required to be obtained, from Governmental Entities.
“Person”
means any individual, corporation, limited or general partnership, limited liability company, limited liability partnership, trust,
association, estate, joint venture, een besloten vennootschap met beperkte aansprakelijkheid, Governmental Entity, or other
entity or group (which term will include a “group” as such term is defined in Section 13(d)(3) of the Exchange Act).
“Personal Information”
means all information that is defined as “personal information,” “personal data,” “personally identifiable
information,” “non-public information,” “protected health information,” or any similar term under
applicable Privacy Laws, including as applicable, names, addresses, telephone numbers, email addresses, financial information,
biometric information, personal health information, government-issued numbers, Internet Protocol addresses, device identifiers,
or other persistent identifiers, and other such information that identifies, can be used to identify or is otherwise associated
with an individual natural person or device.
“Pre-Closing Straddle
Period” means the portion of any Straddle Period ending on the Closing Date.
“Pre-Closing Tax
Period” means any taxable period ending on or before the Closing Date.
“Pre-Closing Taxes”
means (a) all Taxes imposed upon a Target Company (or for which such Target Company is liable) with respect to any Pre-Closing
Tax Period; (b) with respect to any Straddle Period, all Taxes imposed on any Target Company (or for which such Target Company
is liable) which are allocable, pursuant to Section 8.11, to a Pre-Closing Straddle Period; (c) all Taxes of any member
of an affiliated, consolidated, combined, or unitary group of which any Target Company (or any predecessor of any Target Company)
is or was a member on or prior to the Closing Date, including pursuant to Treasury Regulation Section 1.1502-6 or any analogous
or similar Law; (d) all Taxes of any Person imposed on any Target Company (or for which such Target Company is liable) as a transferee
or successor, by contract or pursuant to any Law, which Taxes relate to an event, transaction, or agreement occurring or entered
into before the Closing.
“Privacy Laws”
means all applicable Laws, Orders, guidance issued by any Governmental Authority concerning the privacy, security, or Processing
of Personal Information and contractual and fiduciary obligations related to data privacy, biometric data, data protection, data
security or marketing.
“Proceeding”
means any claim, action, cause of action, demand, lawsuit, arbitration, inquiry, audit, notice of violation, proceeding, litigation,
citation, summons, subpoena or investigation of any nature, civil, criminal, administrative, regulatory or otherwise, whether at
law or in equity.
“Product”
shall mean any product or process that is Covered by, (i) in the case of Company, Company IP Rights, or that practices Company
Technology, or (ii) in the case of Buyer, Buyer IP Rights, or that practices Buyer Technology.
“Pro Rata Share”
means an amount, expressed as a percentage and calculated as of the Closing Date immediately prior to Closing, equal to the ratio
of (a) the aggregate number of Company Shares held by such Seller, divided by (b) the aggregate number of Company Shares
held by all Sellers.
“Regulatory Authority”
shall mean any country, federal, regional, supranational, state or local regulatory agency, department, bureau or other governmental
or regulatory authority having the administrative authority to regulate the development or marketing of pharmaceutical products
in any country or other jurisdiction, including, to the extent applicable, the United States Food and Drug Administration.
“Renovaro Denmark”
means Renovaro Biosciences Denmark ApS, a Danish limited company.
“Renovaro Group”
means, collectively, Buyer, Renovaro Denmark, Renovaro Biopharma, Inc., a Delaware corporation, and Renovaro Technologies, Inc.,
a Nevada corporation.
“Representatives”
means, as to any Person, such Person’s Affiliates and the respective managers, directors, officers, employees, independent
contractors, consultants, advisors (including financial advisors, counsel and accountants), agents and other legal representatives
of such Person or its Affiliates.
“SEC”
means the U.S. Securities and Exchange Commission.
“Securities Act”
means the Securities Act of 1933, as amended and the rules and regulations promulgated thereunder.
“Stockholder Approval”
means the approval of Buyer’s stockholders: (i) to amend its certificate of incorporation to increase the
number of authorized shares of Buyer Common Stock to allow for the issuance of the Exchange Consideration, (ii) as contemplated
by Nasdaq Rule 5635(a) with respect to the issuance of Buyer Common Stock in excess of the limitations imposed by such rule pursuant
to this Agreement and (iii) as contemplated by Nasdaq Rule 5635(c) with respect to the repricing of outstanding stock options.
“Straddle Period”
means, with respect to the Target Companies, any Tax period that begins before the Closing Date and ends after the Closing Date.
“Target Company”
means each of Company and its direct and indirect subsidiaries.
“Taxes”
(each, a “Tax”) means any and all taxes, fees, levies, duties, tariffs, imposts, and other charges of any kind
(together with any and all interest, penalties, additions to tax and additional amounts imposed with respect thereto) imposed by
any Governmental Entity, including taxes or other charges on or with respect to income, franchises, windfall or other profits,
gross receipts, property, sales, use, capital stock, abandoned and unclaimed property, escheat, payroll, employment, social security,
workers’ compensation, unemployment compensation, or net worth; taxes or other charges in the nature of excise, withholding,
ad valorem, stamp, transfer, value added, or gains taxes; license, registration and documentation fees; and customs’ duties,
tariffs, and similar charges, in each case together with any interest, additions or penalties with respect thereto and any interest
in respect of such additions or penalties.
“Tax Returns”
means any return, declaration, report, election, claim for refund or information return or other statement or form relating to
Taxes, including any schedule or attachment thereto or any amendment thereof.
“Technology”
means all discoveries, inventions (whether or not protectable under patent Laws), designs, developments, works of authorship, data,
information, methods of manufacture or use, know-how, procedures, protocols, techniques, results of experimentation and testing,
and other technology.
“Tax Contests”
any demand, claim, or notice of commencement of a claim, proposed adjustment, assessment, examination or other administrative or
court proceeding with respect to Taxes of the Target Companies for which Buyer may be liable.
“Trade Secrets”
means all trade secrets, know-how and confidential or proprietary ideas and information, including such rights in inventions (whether
or not), discoveries, improvements, Technology, and customer and supplier lists, business and technical information, proprietary
information, processes, formulae, databases and data compilations and collections, tools, methods, protocols, results, technical
data, methodologies, practices, techniques, and other confidential and proprietary information and rights therein.
“Transaction Documents”
means each agreement, instrument or document attached hereto as an exhibit, and the other agreements, certificates and instruments
to be executed or delivered by any of the Parties hereto in connection with or pursuant to this Agreement, including the Seller
Registration Rights Agreement, and the non-competition agreements to be entered into between certain Sellers and the Company.
“Treasury Regulations”
means the US Department of Treasury regulations promulgated under the Code.
“UK GAAP”
means United Kingdom generally accepted accounting principles, as consistently applied.
“US GAAP”
means United States generally accepted accounting principles, as consistently applied.
“Valid Patent Claim”
shall mean a claim of an issued and unexpired patent included within (i) Company Patent Rights, with respect to Company, or (ii)
Buyer Patent Rights, with respect to Buyer, which claim has not been revoked or held unenforceable or invalid by a decision of
a court or other governmental agency of competent jurisdiction (which decision is not appealable or has not been appealed within
the time allowed for appeal), and which claim has not been disclaimed, denied or admitted to be invalid or unenforceable through
reissue, re-examination or disclaimer or otherwise.
“VWAP”
means the volume-weighted average price per share, rounded to the nearest four decimal points, of Buyer Common Stock on the Nasdaq
(as reported on Bloomberg L.P. under the function “VWAP”), for the relevant period.
1.2 Definitions.
The following terms have the meanings set forth in the Sections set forth below:
| Definition |
Location |
| |
|
| “Additional SEC Documents” |
5.1.3(a) |
| “Affiliate” |
11.4 |
| “Agreement” |
Preamble |
| “Alternative Transaction” |
4.1.3 |
| “Amended Buyer Organizational Documents” |
2.3 |
| “Approved Indemnification Claim” |
9.2 |
| “Authorizations” |
3.2.10(a) |
| “beneficial ownership” |
11.4 |
| “beneficially owned” |
11.4 |
| “Benefit Plan” |
3.2.13 |
| “business day” |
11.4 |
| “Buyer” |
Preamble |
| “Buyer Disclosure Schedule” |
3.2 |
| “Buyer Employees” |
5.1.1(a) |
| “Buyer Indemnified Person” |
8.1 |
| “Buyer Lease” |
3.2.14 |
| “Buyer Material Contracts” |
3.2.16(a) |
| “Buyer Post-Closing Representation” |
11.15 |
| “Buyer Stock” |
3.2.2 |
| “Buyer Stockholder Meeting” |
5.5.1 |
| “Claim” |
9.1 |
| “Closing” |
2.7 |
| “Closing Date” |
2.7 |
| “Closing Derivative Securities” |
2.2.1(a) |
| “Closing Filing” |
6.4.2 |
| “Closing Press Release” |
6.4.2 |
| “Company” |
Preamble |
| “Company Certificates” |
2.4 |
| “Company Disclosure Schedule” |
3.1 |
| “Company Financials” |
3.1.5 |
| “Company Lease” |
3.1.13 |
| “Company Material Contracts” |
3.1.15(a) |
| “Company Shares” |
Recitals |
| “Confidentiality Agreement” |
6.1 |
| “Copyrights” |
1.1 |
| “Directors” |
6.5 |
| “Dispute Period” |
9.2 |
| “D&O Indemnified Persons” |
6.6 |
| “D&O Policy” |
3.2.22 |
| “Earnout Stock” |
2.2.1(b) |
| “Effect” |
1.1 |
| “Effective Date” |
Preamble |
| “Employees” |
4.1.1(a) |
| “Exchange Consideration” |
2.2 |
| “Exchange Shares” |
2.2 |
| “Federal Securities Laws” |
4.1.4(c) |
| “hereof,” “herein,” “hereto,”
and “hereunder” |
11.4 |
| “include,” “includes,” and
“including” |
11.4 |
| “Indemnification Dispute Notice” |
9.2 |
| “Indemnified Persons” |
8.2 |
| “Indemnifying Seller” |
8.1 |
| “Latest Balance Sheet Date” |
3.1.5 |
| “Losses” |
8.1 |
| “made available” or “provided to” |
11.4 |
| “Majority Sellers” |
11.14.5 |
| “Notice of Claim” |
9.1 |
| “Notice of Meeting and Preliminary Proxy Statement” |
5.5.1 |
| “OFAC” |
3.1.10(c) |
| “Outside Date” |
10.1.2 |
| “Party” and “Parties” |
Preamble |
| “Patents” |
1.1 |
| “PCAOB” |
4.1.4(a) |
| “Post-Closing Board of Directors” |
6.5 |
| “Preferred Stock” |
3.2.2(a) |
| “Pro Rata Share” |
2.2 |
| “Proxy Statements” |
5.5.1 |
| “Record Date” |
5.5.2 |
| “Record Stockholders” |
5.5.1 |
| “Renovaro Group Securities” |
2.2.1(a) |
| “Rules” |
9.3 |
| “SEC Documents” |
3.2.6 |
| “Security Breach” |
3.1.11(b) |
| “Seller” and “Sellers” |
Preamble |
| “Seller Indemnified Persons” |
8.2 |
| “Seller Registration Rights Agreement” |
6.12 |
| “Sellers’ Representative” |
Preamble |
| “Share Exchange” |
Recitals |
| “Signing Filing” |
6.4.2 |
| “Signing Press Release” |
6.4.2 |
| “subsidiary” and “subsidiaries” |
11.4 |
| “Target Company Benefit Plan” |
3.1.12 |
| “Third Party Claim” |
8.3 |
| “Threshold Amount” |
8.6.1(a) |
| “Transfer Taxes” |
6.7.4 |
| “Trigger Date” |
2.2.1(b) |
| “Transactions” |
3.1.4 |
| “Violation” |
3.1.4 |
| “Union” |
3.1.11(b) |
| “Waiving Parties” |
11.15 |
| “Yalla” |
Preamble |
| “2022 Company Financials” |
4.1.4(a) |
| “2023 Equity Incentive Plan” |
3.2.2(f) |
| |
|
| “2023 Interim Company Financials” |
4.1.4(a) |
Article
II
Purchase and Sale; Closing
2.1 Purchase and Sale
of Shares. At the Closing and subject to and upon the terms
and conditions of this Agreement, Sellers shall sell, transfer, convey, assign and deliver to Buyer, and Buyer shall purchase,
acquire and accept from Sellers, all of the Company Shares, free and clear of all Liens (other than potential restrictions on
resale under applicable securities Laws).
2.2 Exchange Consideration.
At the Closing, subject to and upon the terms and conditions of this Agreement, as consideration
for the Company Shares, Sellers collectively shall be entitled to have issued to them by Buyer, in the aggregate, (i) a number
of shares of Buyer Common Stock equal to (the “Exchange Shares”) the Applicable Percentage of the total
number of issued and outstanding shares of Buyer Common Stock as of the Closing Date (minus 1,000,000 shares of Buyer Common Stock
representing shares issued by Buyer to a consultant assisting the Parties on the Transaction (the “Consultant”))
and (ii) the right to receive from Buyer shares of Buyer Common Stock which may become issuable as Earnout Stock in accordance
with Section 2.2.1 below, if any (together with the Exchange Shares, the “Exchange Consideration”).
Each Seller shall receive its pro rata portion of the Exchange Consideration based on such Seller’s Pro Rata Share. Notwithstanding
anything to the contrary contained herein, no fraction of a share of Buyer Common Stock will be issued by Buyer by virtue of this
Agreement or the Transactions contemplated hereby, and each Seller who would otherwise be entitled to a fraction of a share of
Buyer Common Stock (after aggregating all fractional shares of Buyer Common Stock that would otherwise be received by such Seller)
shall instead have the number of shares of Buyer Common Stock issued to such Seller rounded down in the aggregate to the nearest
whole share of Buyer Common Stock.
2.2.1 Earnout; Earnout
Stock.
(a) Attached hereto
as Exhibit 2.2.1 is a complete list of all outstanding shares of Buyer Common Stock, Renovaro Group equity interests, Convertible
Securities of Renovaro Group, and any other subscriptions, calls, Contracts, commitments, understandings, restrictions, arrangements,
rights or phantom equity, including any rights plan, and any right of conversion or exchange under any outstanding security, instrument
or other agreement, obligating any Renovaro Group entity to issue, deliver, or sell, or cause to be issued, delivered or sold,
equity interests of any Renovaro Group entity or obligating any Renovaro Group entity to grant, extend, or enter into any such
agreement or commitment (collectively, “Renovaro Group Securities”). Buyer represents and warrants that there
are no Renovaro Group Securities, or other securities or other instruments outstanding that are convertible into or exercisable
for Buyer Common Stock or Renovaro Group equity interests, and there are no other obligations to issue Buyer Common Stock, Renovaro
Group equity interests or securities convertible into or exercisable for Buyer Common Stock or Renovaro Group equity interests,
and there will not be any such obligations at Closing except as listed on the certified list of Closing Derivative Securities as
defined below. On the Closing Date, Buyer shall deliver to the Sellers’ Representative an updated certified list of all shares
of Buyer Common Stock and Buyer Convertible Securities, including the name of the holder, the exercise price, and the term (the
“Closing Derivative Securities”).
(b) Upon the issuance
of any shares of Buyer Common Stock upon the exercise or conversion of any Closing Derivative Securities (other than any Company
Loan Proceed Shares) (each, a “Trigger Date”), Buyer shall issue to Sellers, excluding Consultant to the extent
Consultant becomes a Joining Seller (without the payment of any further consideration by Sellers) in the aggregate a number of
shares of Buyer Common Stock equal to the Applicable Percentage of such shares of Buyer Common Stock issued upon the exercise or
conversion of such Closing Derivative Securities on such Trigger Date (“Earnout Stock”), to be distributed proportionately
to such Sellers so that each such Seller receives his, her or its Pro Rata Share of such Earnout Stock
(calculated without taking into account Consultant’s Company Shares).
(c) The right of each
Seller to receive Earnout Stock pursuant to this Section 2.2.1 may only be assigned by operation of law, shall not be evidenced
by negotiable certificates of any kind and shall not be readily marketable.
(d) Delivery of Earnout
Stock shall be made on or before (i) the 10th full trading day following the Trigger Date if the Sellers’ Representative
gives Buyer notice that the Sellers wish to receive certificates representing such shares, or (ii) the 5th trading day following
the Trigger Date if the Sellers’ Representative gives Buyer notice that such shares are to be delivered via the Deposit Withdrawal
at Custodian (DWAC) process of the Depository Trust Company.
2.3 Amended Buyer Organizational
Documents. Effective upon the Closing, Buyer shall amend and restate its Organizational Documents to be
in a form to be mutually agreed upon by Buyer and Company, each acting reasonably (together, the “Amended Buyer Organizational
Documents”), which shall, among other matters, amend the Buyer’s Organizational Documents to (i) increase the number
of authorized shares of Buyer Common Stock to allow for the issuance of the Exchange Consideration, (ii) provide that the name of Buyer
shall be changed to “Renovaro AI”, or such other name as mutually agreed to by Buyer and Company and (iii) provide for
the size and structure of the Post-Closing Buyer Board in accordance with Section 6.5.
2.4 Surrender of Purchased
Shares. At the Closing, each Seller will deliver to Buyer,
a duly executed share transfer form in respect of its Company Ordinary Shares that are Company Shares, and the certificate(s)
representing Company Ordinary Shares (“Company Certificates”) for such Company Shares. In the event that any
Company Certificate shall have been lost, stolen or destroyed, in lieu of delivery of a Company Certificate to Buyer, Seller may
instead deliver to Buyer a statutory declaration of lost certificate and indemnity of loss in form and substance reasonably acceptable
to Buyer and Company; which at the reasonable discretion of Buyer may include a requirement that the owner of such lost, stolen
or destroyed Company Certificate deliver a bond in such sum as it may reasonably direct as indemnity against any claim that may
be made against Buyer or Company with respect to Company Shares represented by Company Certificates alleged to have been lost,
stolen or destroyed.
2.5 Termination of Certain
Agreements. Company and Sellers hereby agree that, effective
at the Closing, any agreement containing pre-emptive and special rights conferred to shareholders/stockholders, or shareholders’/stockholders’,
voting or similar agreement among Company and any of Sellers or among Sellers with respect to Company’s capital stock shall
automatically, and without any further action by any of the Parties, terminate in full and become null and void and of no further
force and effect. Further, each Seller and Company hereby waive any obligations of the parties under any of the foregoing agreements
or Company’s Organizational Documents with respect to the transactions contemplated by this Agreement and the Transaction
Documents, and any failure of the parties to comply with the terms thereof in connection with the Transactions and the Transaction
Documents.
2.6 Taking of Necessary
Action. If, at any time after the Closing, any further
action is necessary or desirable to carry out the purposes of this Agreement and to vest Buyer with full right, title and interest
in, to and under, and/or possession of, all assets, property, rights, privileges, powers and franchises of the Target Companies,
the officers and directors of Buyer are fully authorized in the name and on behalf of Company or any of its subsidiaries, to take
all lawful action necessary or desirable to accomplish such purpose or acts, so long as such action is not inconsistent with this
Agreement.
2.7 Closing.
The closing of the transaction contemplated by this Agreement (the “Closing”)
will take place as soon as practicable but no later than three (3) business days after satisfaction or waiver of the last to be
fulfilled of the conditions in Article VII (the date of such Closing, the “Closing Date”), at the offices
of K&L Gates LLP at 200 South Biscayne Boulevard, Suite 3900, Miami, Florida 33131, or remotely via electronic exchange of
signature counterparts, unless another date or place is agreed to in writing by the Parties. The Closing will be deemed effective
as of the closing of business on the Closing Date.
Article
III
REPRESENTATIONS AND WARRANTIES
3.1 Representations
and Warranties Relating to Target Companies. Except as
provided in a correspondingly numbered disclosure schedule delivered by Company and Sellers to Buyer pursuant to Section 6.10
(the “Company Disclosure Schedule”), Company and each Seller, severally and not jointly, represent to Buyer
as follows:
3.1.1 Organization,
Standing, and Power. Company is a corporation duly incorporated,
validly existing and in good standing under the Laws of England and Wales, and has all requisite corporate power and authority
to own, lease and operate its properties and to carry on its business as now being conducted. Each subsidiary of Company is a
private limited liability company (een besloten vennootschap met beperkte aansprakelijkheid) duly incorporated and validly
existing under the Laws of its jurisdiction of organization and has all requisite corporate power and authority to own, lease
and operate its properties and to carry on its business as now being conducted. Each Target Company is duly qualified or licensed
and in good standing in the jurisdiction in which it is incorporated or registered and in each other jurisdiction where it does
business or operates, to the extent applicable under the Laws of such jurisdictions and to the extent that the character of the
property owned, or leased or operated by it or the nature of the business conducted by it makes such qualification or licensing
necessary, except where the failure to be so qualified has not and would not have a Company Material Adverse Effect. Section
3.1.1 of the Company Disclosure Schedule lists all jurisdictions in which any Target Company is qualified to conduct business
and all names other than its legal name under which any Target Company does business. Company has provided to Buyer accurate and
complete copies of its Organizational Documents and the Organizational Documents of each of its subsidiaries, each as amended
to date and as currently in effect. No Target Company is in violation of any provision of its Organizational Documents in any
material respect.
3.1.2 Capital Structure.
(a) The Company Ordinary
Shares comprise all of the issued shares of the Company. No Company Ordinary Shares are held in treasury. All of the issued Company
Ordinary Shares have been duly authorized and validly issued, are fully paid except as set forth on Disclosure Schedule 3.1.2,
and have not been issued in violation of any purchase option, right of first refusal, preemptive right, subscription right or any
similar right under any provision of the laws of England and Wales, any other applicable Law, the Company Organizational Documents
or any contract to which Company is a party or by which it or its securities are bound. The legal (registered) and beneficial owners
of all of the issued and outstanding Company Ordinary Shares are set forth on Section 3.1.2 of the Company Disclosure Schedule,
all of which Company Shares are owned by the Persons set forth therein free and clear of any Liens, other than those imposed under
applicable securities Laws. No other class of shares or equity interests of Company is authorized or will be outstanding at Closing.
(b) There are no Convertible
Securities of the Company, or preemptive rights or rights of first refusal or first offer, nor are there any Contracts, commitments,
arrangements or restrictions to which Company or, to the Knowledge of Company, any of its stockholders is a party or bound relating
to any equity interests of Company, whether or not outstanding. There are no outstanding or authorized equity appreciation, phantom
equity or similar rights with respect to Company. There are no voting trusts, proxies, shareholder/stockholder agreements or any
other agreements or understandings with respect to the voting of Company’s equity interests. Except as set forth in the Company
Organizational Documents, there are no outstanding contractual obligations of Company to repurchase, redeem or otherwise acquire
any equity interests or securities of Company, nor has Company granted any registration rights to any Person with respect to Company’s
equity interests. All of Company Securities have been granted, offered, sold and issued in compliance with all applicable securities
Laws. As a result of the consummation of the Transactions contemplated by this Agreement, no equity interests of Company are issuable
and no rights in connection with any interests, warrants, rights, options or other securities of Company accelerate or otherwise
become triggered (whether as to vesting, exercisability, convertibility or otherwise).
(c) Except as disclosed
in the Company Financials, since its inception, Company has not declared or paid any distribution or dividend in respect of its
equity interests and has not repurchased, redeemed or otherwise acquired any equity interests of Company, and the board of directors
of Company has not authorized any of the foregoing.
(d) Section 3.1.2(d)
of the Company Disclosure Schedule sets forth the name of each subsidiary of Company, and with respect to each subsidiary (a) its
jurisdiction of organization, (b) its authorized shares or other equity interests (if applicable), (c) the number of issued and
outstanding shares or other equity interests and the record holders and beneficial owners thereof and (d) its Tax election to be
treated as a corporate or a disregarded entity under applicable non-U.S. Tax laws, if any. All of the outstanding equity interests
of each subsidiary of Company are duly authorized and validly issued, fully paid and non-assessable (if applicable), and were offered,
sold and delivered in compliance with all applicable securities Laws, and owned by one or more of Company or its subsidiaries free
and clear of all Liens, other than those imposed under applicable securities Laws. There are no Contracts to which Company or any
of its Affiliates is a party or bound with respect to the voting (including voting trusts or proxies) of the equity interests of
any subsidiary of Company other than the Organizational Documents of any such subsidiary. There are no outstanding or authorized
options, warrants, rights, agreements, subscriptions, convertible securities or commitments to which any subsidiary of Company
is a party or which are binding upon any subsidiary of Company providing for the issuance or redemption of any equity interests
of any subsidiary of Company. There are no outstanding equity appreciation, phantom equity, profit participation or similar rights
granted by any subsidiary of Company. Except as set forth in Section 3.1.2(d) of the Company Disclosure Schedules, no subsidiary
of Company has any limitation, whether by Contract, Order or applicable Law, on its ability to make any distributions or dividends
to its equity holders or repay any debt owed to another Target Company. Except for the equity interests of the subsidiaries of
the Company listed on Section 3.1.2(d) of the Company Disclosure Schedule, Company does not own or have any rights to acquire,
directly or indirectly, any equity interests of, or otherwise Control, any Person. None of Company or its subsidiaries is a participant
in any joint venture, partnership or similar arrangement. There are no outstanding contractual obligations of Company or its subsidiaries
to provide funds to, or make any investment (in the form of a loan, capital contribution or otherwise) in, any other Person.
3.1.3 Authority.
Company has all requisite corporate power and authority to execute and deliver this Agreement
and each Transaction Document to which it is a party, to perform Company’s obligations hereunder and thereunder and to consummate
the Transactions contemplated hereby and thereby. The execution and delivery of this Agreement and each Transaction Document to
which Company is a party and the consummation of the Transactions contemplated hereby and thereby, (a) have been duly and validly
authorized by Company’s board of directors in accordance with the Company’s Organizational Documents, any applicable
Law or any Contract to which Company or any of its shareholders/stockholders is a party or by which it or its securities are bound
and (b) no other corporate proceedings on the part of Company are necessary to authorize the execution and delivery of this Agreement
and each Transaction Document to which it is a party or to consummate the Transactions contemplated hereby and thereby. This Agreement
has been, and each Transaction Document to which Company is or is required to be a party shall be when delivered, duly and validly
executed and delivered by Company and assuming the due authorization, execution and delivery of this Agreement and any such Transaction
Document by the other parties hereto and thereto, constitutes, or when delivered shall constitute, the legal, valid and binding
obligation of Company, enforceable against Company in accordance with its terms, except to the extent that enforceability may
be limited by the effect of (a) any applicable bankruptcy, insolvency, reorganization, moratorium, or other similar laws affecting
the enforcement of creditors rights generally, and (b) general equitable principles, regardless of whether such enforceability
is considered in a proceeding at law or in equity.
3.1.4 No Conflicts;
Consents, Notices and Approvals. The execution, delivery
and performance of this Agreement and the other Transaction Documents, and the consummation of the Transactions contemplated hereby
and thereby (the “Transactions”), do not and will not, conflict with or result in any violation of, or default
(with or without notice or lapse of time, or both) under, or give rise to a right of termination, cancellation, or acceleration
of any obligation or to loss of a material benefit under, or the creation of a Lien on assets (any such conflict, violation, default,
right, loss, or creation, a “Violation”), and no Target Company has received written notice that it would be,
with the passage of time, in Violation under: (a) any provision of their respective Organizational Documents; (b) any loan or
credit agreement, note, bond, mortgage, indenture, or other agreement or instrument including, without limitation each Company
Material Contract; or (c) any Law applicable to any Target Company or their properties or assets. Except as set forth in Section
3.1.4 of the Company Disclosure Schedule, no Consent of or with any Governmental Entity on the part of any Target Company
is required to be obtained or made in connection with the execution, delivery or performance of this Agreement or any Transaction
Documents or the consummation by Company of the Transactions contemplated hereby or thereby other than (a) such filings as
are expressly contemplated by this Agreement or (b) where the failure to obtain or make such Consents or to make such filings
or notifications, would not, individually or in the aggregate, have a Company Material Adverse Effect.
3.1.5 Financial Statements.
(a) As used herein,
the term “Company Financials” means (i) when delivered in accordance with Section 4.1.4 of
this Agreement, the 2022 Company Financials, and (ii) the 2023 Interim Company Financials. When delivered to Buyer pursuant
to Section 4.1.4, the Company Financials (i) shall accurately reflect the books and records of the Target Companies
as of the times and for the periods referred to therein, (ii) were prepared in accordance with US GAAP, consistently applied
throughout and among the periods involved, (iii) comply with all applicable accounting requirements under the Securities Act
and the rules and regulations of the SEC thereunder, and (iv) fairly present in all material respects the financial position
of the Target Companies as of the respective dates thereof. No Target Company has ever been subject to the reporting requirements
of Sections 13(a) and 15(d) of the Exchange Act.
(b) Each
Target Company maintains accurate books and records reflecting its assets and Liabilities and maintains proper and adequate internal
accounting controls that provide reasonable assurance that (i) such Target Company does not maintain any off-the-book accounts
and that such Target Company’s assets are used only in accordance with such Target Company’s management directives,
(ii) transactions are executed with management’s authorization, (iii) transactions are recorded as necessary to
permit preparation of the financial statements of such Target Company and to maintain accountability for such Target Company’s
assets, (iv) access to such Target Company’s assets is permitted only in accordance with management’s authorization,
(v) the reporting of such Target Company’s assets is compared with existing assets at regular intervals and verified
for actual amounts, and (vi) accounts, notes and other receivables and inventory are recorded accurately, and proper and adequate
procedures are implemented to effect the collection of accounts, notes and other receivables on a current and timely basis. All
of the financial books and records of the Target Companies are complete and accurate in all material respects and have been maintained
in the ordinary course consistent with past practice and in accordance with applicable Laws. No Target Company has been subject
to or involved in any material fraud that involves management or other employees who have a significant role in the internal controls
over financial reporting of any Target Company. In the past three (3) years, no Target Company or its Representatives has
received any written complaint, allegation, assertion or claim regarding the accounting or auditing practices, procedures, methodologies
or methods of any Target Company or its internal accounting controls, including any material written complaint, allegation, assertion
or claim that any Target Company has engaged in questionable accounting or auditing practices.
(c) The Target Companies
do not have any Indebtedness other than the Indebtedness owed to Buyer.
3.1.6 Litigation.
Except as set forth in Section 3.1.6 of the Company Disclosure Schedule, there:
(a) are no Proceedings of any nature currently pending or, to Company’s Knowledge, threatened (and no such Proceeding has
been brought or, to Company’s Knowledge, threatened in the past three (3) years); or (b) is no Order now pending
or outstanding or that was rendered by a Governmental Entity, in either case of (a) or (b) by or against any Target
Company, its current or former directors, officers or equity holders (provided, that any litigation involving the directors, officers
or equity holders of a Target Company must be related to the Target Company’s business, equity securities or assets), its
business, equity securities or assets. In the past five (5) years, none of the current or former officers or directors of
any Target Company have been charged with, indicted for, arrested for, or convicted of any felony or any crime involving Fraud.
3.1.7 Absence of Undisclosed
Liabilities. No Target Company is subject to any Liabilities
or obligations which would be required to be reflected on or reserved against in the Company Financials under applicable Law or
Accounting Principles, except for those that are either (i) adequately reflected or reserved on or provided for in the consolidated
balance sheet of Company and its subsidiaries as of the Latest Balance Sheet Date contained in Company Financials or (ii) not
material and that were incurred after the Latest Balance Sheet Date in the ordinary course of business consistent with past practice
(other than Liabilities for breach of any Contract or violation of any Law).
3.1.8 No Violations.
(a) No Target Company
is or has been in material conflict or material non-compliance with, or in material default or violation of, nor has any Target
Company received, since January 1, 2020, any written or, to the Knowledge of Company, oral notice of any material conflict
or non-compliance with, or material default or violation of, any applicable Laws by which it or any of its properties, assets,
employees, business or operations are or were bound or affected.
(b) All Permits required
for the Target Companies to conduct their respective businesses have been obtained by them and are valid and in full force and
effect. All fees and charges with respect to such Permits as of the date hereof have been paid in full. Section 3.1.8(b)
of the Company Disclosure Schedule lists all current Permits issued to Target Company, including the names of the Permits and their
respective dates of issuance and expiration. No loss or expiration of any such Permit is pending or, to the Knowledge of the Target
Companies, threatened, other than expiration in accordance with the terms thereof.
3.1.9 Compliance with
Healthcare Laws.
(a) The Target Companies
are and have been since January 1, 2020, in compliance in all material respects with all applicable Healthcare Laws.
(b) To the extent that
any Target Company is participating in any research or clinical trials project, such research or clinical trials project is performed
in compliance in all material respects with applicable Healthcare Laws.
3.1.10 Ethical Practices.
(a) No Target Company,
nor, to the Target Company’s Knowledge, any of their respective Representatives acting on their behalf has (i) used any funds
for unlawful contributions, gifts, entertainment or other unlawful expenses relating to political activity, (ii) made any unlawful
payment to foreign or domestic government officials or employees, to foreign or domestic political parties or campaigns or violated
any provision of the U.S. Foreign Corrupt Practices Act of 1977, as amended, or any other local or foreign anti-corruption or bribery
Law or (iii) made any other unlawful payment. No Target Company, nor, to the Knowledge of the Target Companies, any of their respective
Representatives acting on their behalf has directly or indirectly, given or agreed to give any unlawful gift or similar benefit
in any material amount to any customer, supplier, governmental employee or other Person who is or may be in a position to help
or hinder any Target Company or assist any Target Company in connection with any actual or proposed transaction.
(b) The operations of
each Target Company are and have been conducted in compliance with anti-money laundering statutes in all applicable jurisdictions,
the rules and regulations thereunder and any related or similar rules, regulations or guidelines, issued, administered or
enforced by any Governmental Entity, and no Proceeding involving a Target Company with respect to any of the foregoing is pending
or, to the Knowledge of Company, threatened.
(c) No Target Company
or any of their respective directors or officers, or, to the Knowledge of Company, any other Representative acting on behalf of
a Target Company is currently identified on the specially designated nationals or other blocked person list or otherwise currently
subject to any U.S. sanctions administered by the Office of Foreign Assets Control of the U.S. Treasury Department (“OFAC”),
and no Target Company has in the last five (5) fiscal years, directly or indirectly, used any funds, or loaned, contributed
or otherwise made available such funds to any subsidiary, joint venture partner or other Person, in connection with any sales or
operations in Cuba, Iran, Syria, Sudan, Myanmar, Russia or any other country sanctioned by OFAC or for the purpose of financing
the activities of any Person currently subject to, or otherwise in violation of, any U.S. sanctions administered by OFAC.
3.1.11 Employment Matters.
(a) Section 3.1.11(a)
of the Company Disclosure Schedule contains a list of all persons who are employees of any Target Company as of the date hereof,
including any employee who is on a leave of absence of any nature, paid or unpaid, authorized or unauthorized, and sets forth for
each such individual the following: (i) name; (ii) title or position (including whether full-time or part-time); (iii) hire or
retention date; (iv) current annual base compensation rate or contract fee; and (v) commission, bonus or other incentive-based
compensation. As of the date hereof, all compensation, including wages, commissions, bonuses, fees and other compensation, payable
to all employees, independent contractors or consultants of any Target Company for services performed on or prior to the date hereof
have been paid in full and there are no outstanding agreements, understandings or commitments of any Target Company with respect
to any compensation, commissions, bonuses or fees.
(b) No Target Company
is, nor has been for the past three (3) years, a party to, bound by, or negotiating any collective bargaining agreement or other
Contract with a union, works council or labor organization (collectively, “Union”), and there is not, and has
not been for the past three (3) years, any Union representing or purporting to represent any employee of a Target Company, and
no Union or group of employees is seeking or has sought to organize employees for the purpose of collective bargaining. There has
never been, nor has there been any threat of, any strike, slowdown, work stoppage, lockout, concerted refusal to work overtime
or other similar labor disruption or dispute affecting a Target Company or any of their employees. No Target Company has a duty
to bargain with any Union.
(c) Each Target Company
(i) is and has been in compliance in all material respects with all applicable Laws respecting employment and employment practices,
terms and conditions of employment, health and safety and wages and hours, and other Laws relating to discrimination, disability,
labor relations, hours of work, payment of wages and overtime wages, pay equity, immigration, workers compensation, working conditions,
employee scheduling, occupational safety and health, family and medical leave, and employee terminations, and has not received
written or, to the Knowledge of Company, oral notice that there is any pending Proceeding involving unfair labor practices against
a Target Company, (ii) is not liable for any material past due arrears of wages or any material penalty for failure to comply
with any of the foregoing, and (iii) is not liable for any material payment to any Governmental Entity with respect to unemployment
compensation benefits, social security or other benefits or obligations for employees, independent contractors or consultants (other
than routine payments to be made in the ordinary course of business and consistent with past practice). There are no Proceedings
pending or, to the Knowledge of Company, threatened against a Target Company brought by or on behalf of any applicant for employment,
any current or former employee, any Person alleging to be a current or former employee, or any Governmental Entity, relating to
any such Law or regulation, or alleging breach of any express or implied contract of employment, wrongful termination of employment,
or alleging any other discriminatory, wrongful or tortious conduct in connection with the employment relationship.
(d) Section 3.1.11(d)
of the Company Disclosure Schedule contains a list of all independent contractors (including consultants) currently engaged
by any Target Company, along with the position, the entity engaging such Person, date of retention, date of expiration and rate
of remuneration, most recent increase (or decrease) in remuneration and amount thereof, for each such Person. Except as set forth
on Section 3.1.11(d) of the Company Disclosure Schedule, all of such independent contractors are a party to a written Contract
with a Target Company. Except as set forth on Section 3.1.11(d) of the Company Disclosure Schedule, each such independent
contractor has entered into customary covenants regarding confidentiality, non-solicitation and assignment of inventions and copyrights
in such Person’s agreement with a Target Company, a copy of which has been provided to Buyer by Company.
3.1.12 Employee Benefit
Plans. Section 3.1.12 of the Company Disclosure
Schedule, contains a true and complete list of each pension, benefit, retirement, compensation, employment, consulting, profit-sharing,
deferred compensation, incentive, bonus, performance award, phantom equity, stock or stock-based, change in control, retention,
severance, vacation,
paid time off (PTO), medical, vision, dental, disability,
welfare, fringe benefit and other similar agreement, plan, policy, program or arrangement (and any amendments thereto), in each
case whether or not reduced to writing and whether funded or unfunded, which is or has been maintained, sponsored, contributed
to, or required to be contributed to by any Target Company for the benefit of any current or former employee, officer, director,
retiree, independent contractor or consultant of any Target Company or any spouse or dependent of such individual, or under which
any Target Company has or may have any Liability, or with respect to which Buyer or any of its Affiliates would reasonably be expected
to have any Liability, contingent or otherwise (as listed on Section 3.1.12 of the Company Disclosure Schedule, each, a
“Target Company Benefit Plan”). Each Target Company Benefit Plan is and has been operated at all times
in compliance with all applicable Laws in all material respects.
3.1.13 Real Property;
Leases. No Target Company owns, or has ever owned, real
property. Section 3.1.13 of the Company Disclosure Schedule sets forth each lease and sublease in effect on the date of
this Agreement under which any Target Company leases or subleases (a) real property (as either a tenant or subtenant), or (b)
personal property (in case of either clause (a) or (b), a “Company Lease”). No default by any Target Company,
or to Company’s Knowledge, by lessor or sublessor, exists under any Company Lease, and all payments of rent, operating expenses,
and other sums due under each Company Lease are current as of the date hereof. No Company Lease is terminable because of the execution
of this Agreement or the consummation of the Transactions. Each Company Lease is in full force and effect in accordance with its
respective terms. No consent is required from any party under any Company Lease in connection with the completion of the Transactions,
and no Target Company has received written notice that a party to any Company Lease intends to cancel, terminate, or refuse to
renew any Company Lease or to exercise any option or other right under such Company Lease, except where the failure to receive
such consent, or where such cancellation, termination, or refusal, would not result, or reasonably be expected to result, individually
or in the aggregate, in a Company Material Adverse Effect.
3.1.14 Environmental.
(a) Each Target Company
has complied in all material respects with all applicable Environmental Laws in effect on or before the date hereof in respect
of any real property leased or subleased by any Target Company.
(b) No Proceeding is
pending and, to Company’s Knowledge, no Proceeding has been threatened by any Governmental Entity against any Target Company
concerning any Environmental Law with respect to any real property leased or subleased at any point in time by any Target Company.
3.1.15 Company Material
Contracts.
(a) Section 3.1.15(a)
of the Company Disclosure Schedule lists all of the following Contracts to which a Target Company is a party (the “Company
Material Contracts”):
(i) any Contract with
an annual payment obligation by the Buyer of more than $300,000 (A) relating to the performance of services by, any employee, consultant,
or other Person, (B) in accordance with which a Target Company is or may become obligated to make any severance, termination, incentive,
or similar payment to any current or former officer, director, or employee, or (C) in accordance with which a Target Company may
be required to provide, or accelerate the vesting of, any payments, benefits, or equity rights upon the occurrence of any of the
Transactions;
(ii) any Contract imposing
any restriction on the right or ability of any Target Company, or that, after consummation of the Transactions, would impose a
restriction on the right or ability of Buyer, to compete in any line of business or in any geographic region with any other Person
or to transact business or deal in any other manner with any other Person;
(iii) any Contract that
provides for indemnification of any officer, director, employee, agent of any Target Company, except for Contracts containing standard
indemnification provisions entered into in the ordinary course of business;
(iv) any Contract with
a third party in accordance with which any Target Company (A) has paid or received $300,000 or more annually;
(v) any Contract or
agreement of partnership or joint venture, limited liability company or operating agreement that would give rise to an obligation
on the part of any Target Company to form a joint venture or to acquire securities of, or any other equity interest in, a third
party;
(vi) any Company Lease;
(vii) any Contract providing
for the development of any Technology or other Intellectual Property, independently or jointly, by or for any Target Company (other
than agreements with employees and independent contractors of any Target Company);
(viii) any settlement,
conciliation or similar agreement under which any Target Company or any counterparty to any such agreement has any outstanding
obligations in excess of $200,000;
(ix) any Contract relating
to the distribution, reselling, whole selling, advertising, marketing or sales of any Target Company’s services or products,
if any;
(x) any Contract that
grants any right of first refusal or right of first offer or similar right or that limits or purports to limit the ability of any
Target Company to own, operate, sell, transfer, pledge or otherwise dispose of any material amount of its assets and properties
or their respective businesses;
(xi) any Contract entered
into since the Latest Balance Sheet Date providing for the acquisition or sale, directly or indirectly, by merger or otherwise
of all or substantially all of the assets of another Person, the equity interests of another Person, or a business or division
of another Person;
(xii) any Contract between
any Target Company, on the one hand, and an Affiliate, on the other hand that provides for consideration in excess of $120,000;
(xiii) any Contract
with any Governmental Entity;
(xiv) any Contract with
a vendor to which total amounts payable exceed $300,000 annually;
(xv) except for trade
indebtedness incurred in the ordinary course of business and except as disclosed in the Company Financials, any instrument evidencing
or related in any way to indebtedness for borrowed money (either owed by or to any Target Company) by way of direct loan, sale
of debt securities, purchase money obligation, conditional sale, guarantee, or otherwise; and
(xvi) Any In-Licenses
or IP Assignments.
(b) Each Company Material
Contract is in full force and effect and is a valid and binding obligation of the applicable Target Company, and, to Company’s
Knowledge, against any other party to such Company Material Contract, except to the extent that enforceability may be limited by
the effect of (a) any applicable bankruptcy, insolvency, reorganization, moratorium, or other similar laws affecting the enforcement
of creditors rights generally, and (b) general equitable principles, regardless of whether enforceability is considered in a Proceeding
at law or in equity. No Target Company, nor, to Company’s Knowledge, any other party to the Company Material Contract, is
in material breach of or default under, any Company Material Contract. None of the parties to the Company Material Contracts has
expressed intent in writing to cancel, terminate, or refuse to renew any Company Material Contract. Company has made available
to Buyer complete copies of all Company Material Contracts.
3.1.16 Taxes.
(a) Each Target Company
has or will have timely filed, or caused to be timely filed, all income and other material Tax Returns required to be filed by
it (taking into account all available extensions), which Tax Returns are true, accurate, correct and complete in all material respects,
and has paid, collected or withheld and remitted, or caused to be paid, collected or withheld and remitted, all Taxes required
to be paid, collected or withheld and remitted (whether or not shown on any Tax Return), other than such Taxes for which adequate
reserves in the Company Financials have been established and which are not yet due and payable.
(b) There is no Proceeding
currently pending or, to the Knowledge of Company, threatened against any Target Company by a Governmental Entity in a jurisdiction
where the Target Company does not file Tax Returns that it is or may be subject to taxation by that jurisdiction.
(c) No Target Company
is being audited by any Tax authority or has been notified in writing or, to the Knowledge of Company, orally by any Tax authority
that any such audit is contemplated or pending. There are no claims, suits, assessments or, to the Knowledge of Company, audits,
examinations, investigations or other Proceedings, pending against any Target Company in respect of any Tax, and no Target Company
has been notified in writing of any proposed Tax claims or assessments against it (other than, in each case, claims or assessments
for which adequate reserves in the Company Financials have been established).
(d) There are no Liens
with respect to any Taxes upon any Target Company’s assets, other than Liens with respect to Taxes not yet due and payable
or Taxes being contested in good faith and for which adequate reserves in the Company Financials when delivered to Buyer have been
established.
(e) No Target Company
has any outstanding waivers or extensions of any applicable statute of limitations to assess any amount of Taxes. There are no
outstanding requests by a Target Company for any extension of time within which to file any Tax Return or within which to pay any
Taxes shown to be due on any Tax Return.
(f) No Target
Company has made any change in accounting method (except as required by a change in Law) or received a ruling from, or signed an
agreement with, any taxing authority that would reasonably be expected to have a material impact on its Taxes following the Closing.
(g) No
Target Company has any Liability for the Taxes of another Person (other than another Target Company) that are not adequately reflected
in the Company Financials (i) under any applicable Tax Law, (ii) as a transferee or successor, or (iii) by Contract
or indemnity (excluding commercial agreements entered into in the ordinary course of business the primary purpose of which is not
the sharing of Taxes). No Target Company is a party to or bound by any Tax indemnity agreement, Tax sharing agreement or Tax allocation
agreement or similar agreement (excluding commercial agreements entered into in the ordinary course of business the primary purpose
of which is not the sharing of Taxes) with respect to Taxes (including advance pricing agreements, closing agreements or other
agreements relating to Taxes with any Governmental Entity) that will be binding on any Target Company with respect to any period
following the Closing Date.
(h) No Target Company
has requested, or is it the subject of or bound by any private letter ruling, technical advice memorandum, closing agreement or
similar ruling, memorandum or agreement with any Governmental Entity with respect to any Taxes, nor is any such request outstanding.
3.1.17 Intellectual
Property Rights.
(a) Section 3.1.17(a)
of the Company Disclosure Schedule attached hereto contains a true and complete list of (i) the Company Patent Rights existing
on the Effective Date that are registered and applied-for with a Governmental Entity and owned by a Target Company and (ii) In-Licenses
relating to the Company Technology as of the Effective Date, excluding any licenses or sublicenses entered with any Target Company’s
suppliers or customers. The Company Patent Rights listed in Section 3.1.17(a) of the Company Disclosure Schedule include
all of the Patent Rights owned by the Target Companies and licenses relating to the Company Technology as of the Effective Date
that relate to the business and its operations.
(b) The Target Companies
are the owner or exclusive licensee of all Company Patent Rights listed in Section 3.1.17(b) of the Company Disclosure Schedule
and, to Company’s Knowledge as of the Effective Date, and Company has no knowledge that the Company Patent Rights are not
unpatentable or unenforceable.
(c) Company (i) is,
as of the Effective Date, the sole and exclusive owner or licensee of all right, title and interest in and to Company IP Rights
and as of the Effective Date free and clear of any Liens; (ii) has not granted to any third party any license or other right with
respect to Company IP Rights that conflicts with or limits in any way the licenses and rights granted to Company in or by any license;
and (iii) to the Company’s Knowledge has not disclosed to any third party any confidential Trade Secrets or Know-how listed
in Section 3.1.17(c) of the Company Disclosure Schedule.
(d) The manufacture,
use, sale, offer for sale or import of any Company Technology does not, to Company’s Knowledge as of the Effective Date,
Infringe any Patent Rights, Trade Secret, or any other Intellectual Property or proprietary right of any third party, and no Target
Company has received written, oral or other notice from any third party claiming that the manufacture, use, sale, testing, offer
for sale or import of any Product to the extent practicing Company Technology Infringes any Patent or other Intellectual Property
rights of any third party, nor to Company’s Knowledge as of the Effective Date is there any reasonable basis for such a claim.
(e) There are no claims,
judgments or settlements against or owed by Company or Sellers (or any of their Affiliates) with respect to Company IP Rights,
and neither Company nor any Seller is a party to any legal action, suit or proceeding relating to Company IP Rights, nor has Company
received any written, oral or other communication from any third party, including, without limitation, any Regulatory Authority
or other Governmental Entity, threatening such action, suit or proceeding or any other claim or proceeding alleging the unpatentability
or unenforceability of any Company Patent Rights; in each case, as of the Effective Date.
3.1.18 Artificial Intelligence.
(a) The Parties acknowledge
that Section 3.1.18(a) of the Company Disclosure Schedule sets forth all (a) AI Technologies, the owners or licensors thereof,
and the Contracts pursuant to which the Target Companies have a license to use or access such AI Technologies; (b) AI Inputs, the
owners or licensors thereof, and the Contracts pursuant to which the Target Companies have a license to use or access such AI Inputs;
and (c) AI Outputs, the recipients thereof, and the Contracts pursuant to which each such recipient have a license to use or access
such AI Outputs, and the means by which the AI Outputs have been provided to such recipient, including whether by remote access,
software as a service or on premises.
(b) The Target Companies
are not, and will not, be subject to any obligations that conflict with any terms in any contained herein which govern any Target
Company’s use or ownership of AI Inputs or AI Technology or its AI Outputs;
(c) The Target Companies
own or possess all rights and licenses required under applicable Law to use the AI Technology and AI Inputs and to generate the
AI Outputs, all as currently used and generated in its business;
(d) The Target Companies’
current use of the AI, does not Infringe any Intellectual Property rights or rights of likeness or publicity, and Target Companies
have acquired proper Consent from individuals where such Consent is necessary or required by applicable Privacy Laws;
(e) The Target Companies’
use of AI complies with all Laws and regulations (including those pertaining to Privacy Laws) as applicable to the Target Companies;
(f) Target Companies
use of AI and generation of AI Outputs conform to prevalent industry standards and practices;
(g) The consummation
of the contemplated Transactions will not adversely affect Target Companies rights in or use of any AI, and Target Companies will
be able to use the AI in a materially similar manner as prior to the Effective Date and Closing Date as it has prior to the Agreement.
(h) There have been
no errors, defects, failures or interruptions in the AI Technology or AI Inputs that have had a material effect on the , or to
Company’s Knowledge, in any Personal Information that may have been used to train the AI, or in the performance of its intended
purpose, and the AI has materially performed in accordance with its specifications and intended purpose.
(i) Target Companies
maintain commercially reasonable security measures, including access controls for the AI. There has been no unauthorized use of
or access to AI and, to the Company’s Knowledge, no AI has been used in violation of any applicable Laws.
3.1.19 Privacy and
Information Security.
(a) Target Companies
are in compliance with all Privacy Laws in the Processing of Personal Information (including employee information) applicable to
the Target Companies’ business;
(b) Target Companies
have not experienced any unauthorized access to: (i) any Personal Information Processed by the Targeted Companies; (ii) any databases,
computers, servers, storage media (e.g.) backup tapes, network devices or other devices or systems that collect or Process Personal
Information owned or maintained by the Target Companies (each, a “Security Breach”), where such Security Breach
resulted in the provision of notice to any Person as required by applicable Privacy Laws;
(c) Target Companies
are not subject to any contractual requirements or other legal obligations that, following the Closing of this Agreement, would
prohibit the Buyer from Processing any Personal Information in the manner in which the Target Companies Processed such Personal
Information;
(d) Target Companies
maintain commercially reasonable security measures, controls, technologies, policies and safeguards designed to protect Personal
Information and any proprietary information in possession of the Target Companies.
(e) Target Companies
have established one or more incident response and disaster recovery and business continuity plans to address any actual or threatened
Security Breach or other security incident or data breach.
3.1.20 Brokers and Other
Fees. Neither Target Companies, nor their stockholders,
officers, directors, or employees has employed any investment banker, broker, finder, or other intermediary that has been retained
by, or is authorized to act on behalf of, any Target Company that would be entitled to any fee or commission from any of the Target
Companies, or the holders of Company Shares in connection with or upon consummation of the Transactions.
3.1.21 Insurance.
(a) Section 3.1.21(a)
of the Company Disclosure Schedule lists all insurance policies (by policy number, insurer, coverage period, coverage amount, annual
premium and type of policy) held by any Target Company relating to a Target Company or a Target Company’s business, properties,
assets, directors, officers and employees, copies of which have been provided to Buyer. All premiums due and payable under all
such insurance policies have been paid and the Target Companies are otherwise in material compliance with the terms of such insurance
policies. Each such insurance policy (i) is legal, valid, binding,
enforceable and in full force and effect and (ii) will
continue to be legal, valid, binding, enforceable, and in full force and effect on identical terms following the Closing. No Target
Company has any self-insurance or co-insurance programs. In the past three (3) years, no Target Company has received any written
notice from, or on behalf of, any insurance carrier relating to or involving any adverse change or any change other than in the
ordinary course of business, in the conditions of insurance, any refusal to issue an insurance policy or non-renewal of a policy.
(b) The Target Companies
maintain and shall continue to maintain commercially reasonable insurance coverage for claims or losses pertaining to the AI. The
AI has not been subject to any claims, suits, demands, rulings, judgements, threats, fines, penalties, or a cease and desist letter
asserted against, or brought by, any Target Company pertaining to intellectual property or any other rights, violation or breach
of any applicable Law.
3.1.22 Affiliate Transactions.
Except for (a) relationships with any Target Company as an officer, director, employee
or consultant (and compensation by a Target Company in consideration of such services which is included in the Company Financials),
(b) relationships with Target Companies as holders of equity interests and (c) as disclosed in Section 3.1.22 of the Company
Disclosure Schedule, none of the officers, directors, consultants or equity interest holders, or to Company’s or Seller’s
Knowledge, any member of any of their immediate families, is presently a party to, or was a party to since inception of such Target
Company, any transaction with a Target Company, including any Contract, agreement, or other arrangement (x) providing for the
furnishing of services to or by, (y) providing for lease or sublease of real or personal property to or from, or (z) otherwise
requiring payments to or from, any such Person or any corporation, partnership, trust, or other entity in which any such Person
has or had a 5% or more interest (as a member, partner, beneficiary, or otherwise) or is or was an officer, director, employee,
consultant, vendor or customer of any Target Company.
3.1.23 Absence of Certain
Changes. Except as set forth in Section 3.1.23 of
the Company Disclosure Schedule, since the Latest Balance Sheet Date, each Target Company has (a) conducted its business
only in the ordinary course of business consistent with past practice, (b) not been subject to a Company Material Adverse
Effect and (c) has not taken any action or committed or agreed to take any action that would be prohibited by Section 4.1.1
of this Agreement if such action were taken on or after the date hereof without the consent of Buyer.
3.1.24 Disclosure.
No representation or warranty made by any Target Company in this Agreement or the other
Transaction Documents contains any untrue statement of a material fact, or omits to state a material fact necessary to make the
statements or facts contained herein or therein, in light of the circumstances under which they were made, not misleading. Company
and Sellers have provided to Buyer or its counsel each document listed in the Company Disclosure Schedule.
3.1.25 Independent Investigation.
Company has conducted its own independent investigation, review and analysis of the business,
results of operations, prospects, condition (financial or otherwise) or assets of Buyer, and acknowledges that it has been provided
adequate
access to the personnel,
properties, assets, premises, books and records, and other documents and data of Buyer for such purpose. Company acknowledges and
agrees that: (a) in making its decision to enter into this Agreement and to consummate the Transactions contemplated hereby, it
has relied solely upon its own investigation and the express representations and warranties of Buyer set forth in this Agreement
(including the related portions of the Buyer Disclosure Schedule and the SEC Documents), and in any certificate delivered to Company
pursuant hereto; and (b) neither Buyer nor any of its Representatives have made any representation or warranty as to Buyer or this
Agreement, except as expressly set forth in this Agreement (including the related portions of the Buyer Disclosure Schedule and
the SEC Documents), or in any certificate delivered to Company pursuant hereto.
3.1.26 Information Supplied.
None of the information supplied or to be supplied by Company expressly for inclusion
or incorporation by reference, and ultimately included or incorporated by reference: (a) in any current report on Form 8-K,
and any exhibits thereto or any other report, form, registration or other filing made with any Governmental Entity or stock exchange
with respect to the Transactions contemplated by this Agreement; (b) the Proxy Statements; or (c) in the mailings or other distributions
to Buyer’s stockholders and/or prospective investors with respect to the consummation of the Transactions contemplated by
this Agreement, or in any amendment to any of the documents identified in (a) through (c), will, when filed, made available, mailed
or distributed, as the case may be, contain any untrue statement of a material fact or omit to state any material fact required
to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they are
made, not misleading; provided, however, that neither Company, nor any Seller, make any representation with respect
to any forward-looking statements. None of the information supplied or to be supplied by Company expressly for inclusion or incorporation
by reference in any of the Signing Press Release, the Signing Filing, the Closing Press Release and the Closing Filing will, when
filed or distributed, as applicable, contain any untrue statement of a material fact or omit to state any material fact required
to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they are
made, not misleading. Notwithstanding the foregoing, Company makes no representation, warranty or covenant with respect to any
information supplied by or on behalf of Buyer or its Affiliates.
3.2 Representations
and Warranties of Buyer. Except: (a) as disclosed in SEC
Documents and to the extent that is reasonably apparent on the face of such disclosure to be applicable to the representation
and warranty set forth herein (other than any disclosures contained or referenced therein under the captions “Risk Factors,”
“Forward-Looking Statements,” “Quantitative and Qualitative Disclosures About Market Risk,” and any other
disclosures contained or referenced therein of information, factors, or risks that are predictive, cautionary, or forward-looking
in nature) (it being acknowledged that nothing disclosed in such an SEC Documents will be deemed to modify or qualify the Buyer
Fundamental Representations); or (b) as provided in a correspondingly numbered disclosure schedule delivered by Buyer to Company
pursuant to Section 6.10 (the “Buyer Disclosure Schedule”), Buyer, represents to Company as follows:
3.2.1 Organization;
Standing and Power.
(a) Buyer is a corporation
duly incorporated, validly existing, and in good standing under the laws of the State of Delaware. Each other member of the Renovaro
Group is a corporation or other entity duly formed, validly existing and in good standing under the Laws of its jurisdiction of
organization. Each member of the Renovaro Group has all requisite corporate power and authority to own, lease, hold its Assets,
and operate its properties and to carry on its business as now being conducted. Each member of the Renovaro Group is duly qualified
or licensed and in good standing in the jurisdiction in which it is incorporated or registered and in each other jurisdiction where
it does business or operates to the extent that the character of the property owned, leased, or operated by it or the nature of
the business conducted by it makes such qualification or licensing necessary, except where the failure to be so qualified has not
and would not have a Buyer Material Adverse Effect. Section 3.2.1(a) of the Buyer Disclosure Schedule lists all jurisdictions
in which any member of the Renovaro Group is qualified to conduct business and all names other than its legal name under which
any member of the Renovaro Group does business.
(b) Buyer has provided
to Sellers and Company correct and complete copies of Buyer’s Organizational Documents, and minute books and the comparable
governing instruments and minutes, as amended as of the date of this Agreement. The minute books of Buyer contains correct and
materially complete records of all material proceedings and actions taken at all meetings of, or effected by written consent of
the stockholders and its board of directors, and the stock records of Buyer contain correct and complete records of all original
issuances, transfers, repurchases, and cancellations of Buyer Common Stock. Except for Buyer’s ownership of other Renovaro
Group members, neither Buyer nor other Renovaro Group members own or Control, directly or indirectly, shares of capital stock of
any other corporation, or any interest in any partnership, joint venture, or other non-corporate business entity or enterprise.
No member of the Renovaro Group is in violation of any provision of its Organizational Documents in any material respect.
3.2.2 Capitalization.
(a) The authorized capital
stock of Buyer consists of (i) 100,000,000 shares of Buyer Common Stock, with a par value of $0.0001 per share, of which 65,698,144
shares are issued and outstanding as of the date of this Agreement, and (ii) 10,000,000 shares of Buyer’s preferred stock,
par value $0.0001 per share, of which 1,000,000 shares have been classified as Series A Convertible Stock (the “Preferred
Stock”, and together with the Buyer Common Stock, the “Buyer Stock”), of which 561,010 shares are
issued and outstanding as of the date of this Agreement. All of the outstanding Buyer Stock has been duly authorized, validly issued,
fully paid, nonassessable, have not been issued in violation of any purchase option, right of first refusal, preemptive right,
subscription right or any similar right under any provision of applicable Law, the Buyer Organizational Documents or any contract
to which Buyer is a party or by which it or its securities are bound, and are not subject to any preemptive rights or similar rights
or Liens under applicable law, Buyer’s Organizational Documents, or any agreement to which Buyer is a party or by which Buyer
may be bound. Section 3.2.2(a) of the Buyer Disclosure Schedule sets forth a list of all instruments that describe the rights
of the holders of Buyer Stock. True and complete copies of all instruments describing the rights of all holders of Buyer Stock
have been provided to or made available to Company.
(b) Buyer owns all of
the equity interest in Renovaro Denmark, organized under the Danish Act on Limited Companies of the Kingdom of Denmark, and all
of the share capital of the Renovaro Biopharma, Inc., a Delaware corporation, organized under the laws of Delaware, and Renovaro
Technologies, Inc., a Nevada corporation, organized under the laws of Nevada, in each case free and clear of any Liens. Other than
as reflected in the previous sentence, Buyer has no other direct or indirect subsidiary, Buyer does not own or Control, directly
or indirectly, an interest in any Person, Buyer does not have any rights to acquire, directly or indirectly, any equity interests
of any Person, and Buyer is not a participant in any joint venture, partnership, or limited liability company. There are no outstanding
contractual obligations of Buyer or its subsidiaries to provide funds to, or make any investment (in the form of a loan, capital
contribution or otherwise) in, any other Person.
(c) Except as disclosed
in the Buyer’s financial statements included in the SEC Documents, since June 30, 2021, Buyer has not declared or paid any
distribution or dividend in respect of its equity interests and has not repurchased, redeemed or otherwise acquired any equity
interests of Buyer, and the board of directors of Company has not authorized any of the foregoing.
(d) All of the outstanding
equity securities of each subsidiary of Buyer are duly authorized and validly issued, fully paid and non-assessable, and were offered,
sold and delivered in compliance with all applicable securities Laws. There are no Contracts to which Buyer or any of its Affiliates
is a party or bound with respect to the voting (including voting trusts or proxies) of the equity interests of any subsidiary of
Buyer other than the Organizational Documents of any such subsidiary. There are no outstanding or authorized options, warrants,
rights, agreements, subscriptions, convertible securities or commitments to which any subsidiary of Buyer is a party or which are
binding upon any subsidiary of Buyer providing for the issuance or redemption of any equity interests of any subsidiary of Buyer.
There are no outstanding equity appreciation, phantom equity, profit participation or similar rights granted by any subsidiary
of Buyer. No subsidiary of Buyer has any limitation, whether by Contract, Order or applicable Law, on its ability to make any distributions
or dividends to its equity holders or repay any debt owed to another member of the Renovaro Group.
(e) Other than Buyer,
the Renovaro Group members do not (i) own, directly or indirectly, any outstanding securities or other equity interests in, any
other Person or (ii) hold the right to acquire any security or interest in any other person.
(f) Buyer has reserved
4,000,000 shares of Buyer Common Stock for issuance pursuant to its 2019 Equity Incentive Plan duly adopted by Buyer’s Board
of Directors and approved by Buyer’s stockholders (the “2019 Equity Incentive Plan”).
(g) Except as set forth
on Section 3.2.2(g) of the Buyer Disclosure Schedule, there are no Convertible Securities of Buyer, or preemptive rights
or rights of first refusal or first offer, nor are there any Contracts, commitments, orally or in writing, arrangements or restrictions
to which Buyer is a party or bound relating to any equity interests of Buyer, whether or not outstanding. To Buyer’s Knowledge,
there are no voting trusts, proxies, shareholder/stockholder agreements or any other agreements or understandings with respect
to the voting of Buyer’s equity interests. Except as set forth in the Buyer Organizational Documents, the SEC Documents or
Section 3.2.2(g) of the Buyer Disclosure Schedule, there are no outstanding contractual obligations of Company to repurchase,
redeem or otherwise acquire any equity interests or securities of Buyer, nor has Buyer granted any registration rights to any Person
with respect to Buyer’s equity interests. All of Buyer’s securities (including Buyer Stock) have been granted, offered,
sold and issued in compliance with all applicable securities Laws. As a result of the consummation of the Transactions contemplated
by this Agreement, no equity interests of Buyer are issuable and no rights in connection with any interests, warrants, rights,
options or other securities of Buyer accelerate or otherwise become triggered (whether as to vesting, exercisability, convertibility
or otherwise). There are no accrued and unpaid dividends with respect to any outstanding shares of Buyer Common Stock. There are
no accrued and unpaid dividends with respect to any outstanding Buyer Stock. To Buyer’s Knowledge, the Buyer Stock is free
and clear of any Liens.
(h) All outstanding
Buyer Stock are duly authorized, validly issued, fully paid, and not subject to any preemptive rights or similar rights or Liens
under applicable Law, Buyer’s Organizational Documents, or any agreement to which Buyer is a party or by which Buyer may
be bound.
(i) Except as set forth
on Section 3.2.2(i) of the Buyer Disclosure Schedule, Buyer does not have outstanding:
(i) any debt, the holders
of which (A) have the right to vote (or are convertible or exercisable into securities having the right to vote) with Buyer stockholders
on any matter, or (B) are or will become entitled to receive any payment as a result of the execution of this Agreement or the
completion of the Transactions; or
(ii) any restricted
stock units, stock appreciation rights, stock performance awards, dividend equivalents, or other stock-based or equity-linked securities
of a similar nature.
3.2.3 Authority.
Buyer has all requisite corporate power and authority to execute and deliver this Agreement
and each Transaction Document. The execution, delivery and performance by Buyer of this Agreement and the Transaction Documents
have been duly and validly authorized by all requisite corporate action on the part of Buyer and upon receipt of Stockholder Approval,
no other proceedings on the part of Buyer are necessary for Buyer to perform its obligations under this Agreement and the Transaction
Documents and to consummate the Transactions contemplated hereby and thereby. This Agreement has been, and upon execution and
delivery thereof, the other Transaction Documents will be, duly and validly executed and
delivered by Buyer, and (assuming due authorization, execution and delivery thereof
by the other parties hereto and thereto) this Agreement constitutes, and upon execution and delivery thereof, the other Transaction
Documents will constitute, the legal, valid and binding obligations of Buyer, enforceable against Buyer in accordance with their
respective terms, except to the extent that enforceability may be limited by the effect of (a) any applicable bankruptcy, insolvency,
reorganization, moratorium, or other similar laws affecting the enforcement of creditors rights generally, and (b) general equitable
principles, regardless of whether such enforceability is considered in a proceeding at law or in equity.
3.2.4 Valid Issuance
of Shares. The Exchange Consideration being issued in accordance
with this Agreement when issued, sold, and delivered in accordance with the terms and for the consideration set forth in this
Agreement, will be validly issued, fully paid, and nonassessable, and free and clear of any Liens (other than those imposed by
applicable securities Laws and the Amended Buyer Organizational Documents). Assuming the accuracy of the representations and warranties
of Sellers herein and subject to any filings described in Section 3.2.5 of this Agreement, the Exchange Consideration
being issued in accordance with this Agreement will be issued in compliance with all Federal Securities Laws and state securities
Laws.
3.2.5 No Conflicts;
Consents, Notices and Approvals. The execution and delivery
of this Agreement and the other Transaction Documents do not, and after receipt of Stockholder Approval, the consummation of the
Transactions will not cause a Violation, and Buyer has not received written notice that it would be, with the passage of time,
in Violation under: (a) any provision of Buyer’s Organizational Documents (b) any loan or credit agreement, note, bond,
mortgage, indenture, or other agreement or instrument including, without limitation each Buyer Material Contract; or (c) Law applicable
to any Renovaro Group member or its respective properties or assets. Except as set forth in Section 3.2.5 of the Buyer
Disclosure Schedule and for the Stockholder Approval, no Consent of or with any (y) third party or (z) Governmental Entity is
required by or with respect to Buyer in connection with the execution, delivery or performance of this Agreement, the Transaction
Documents or the consummation by Buyer of the Transactions other than any filings under any applicable state securities laws.
3.2.6 SEC Documents;
Financial StatementsIn the two (2) years preceding the
date hereof, Buyer has filed all registration statements, prospectuses, reports, schedules, forms, statements and other documents
(including exhibits and schedules thereto and all other information incorporated by reference) required to be filed or furnished
by Buyer with or to the SEC, including pursuant to Section 13(a) or 15(d) of the Exchange Act, together with any amendments, restatements
or supplements thereto (collectively referred to herein as the “SEC Documents”). As of their respective dates,
the SEC Documents complied in all material respects with the applicable requirements of the Securities Act and the Exchange Act,
and the rules and regulations promulgated thereunder. None of the SEC Documents, when filed, contained any untrue statement of
a material fact or omitted to state a material fact required to be stated therein or necessary in order to make the statements
therein, in the light of the circumstances under which they were made, not misleading. Each of the consolidated financial statements
of Buyer (including, in each case, any notes thereto) included in the SEC Documents comply in all material respects with applicable
accounting requirements and the rules and regulations of the
SEC with respect thereto as in effect at the time of filing. Such consolidated financial statements have been prepared in accordance
with US GAAP during the periods involved (except as may be otherwise specified in such financial statements or the notes thereto,
or, in the case of unaudited financial statements may not contain all footnotes required by US GAAP), and fairly present in all
material respects the financial position of Buyer and its consolidated subsidiaries as of and for the respective dates thereof
and the results of operations and cash flows for the respective periods then ended, subject, in the case of unaudited statements,
to normal, immaterial, year-end audit adjustments. Except as set forth in Section 3.2.6 of the Buyer Disclosure Schedule,
Buyer has received no notices or correspondence from the SEC for the two (2) years preceding the date hereof.
(b) As
of the date of this Agreement, except as set forth on Section 3.2.6(b)
of the Buyer Disclosure Schedule, (A) the Buyer Common Stock is registered pursuant to Section 12(b) of the Exchange Act and
is listed for trading on the Nasdaq Capital Market under the symbol “RENB”, (B) Buyer has not received any written
deficiency notice from Nasdaq relating to the continued listing requirements of such Buyer Common Stock, (C) there are no
Proceedings pending or, to the Knowledge of Buyer, threatened against Buyer by Nasdaq, the SEC or the Financial Industry Regulatory
Authority with respect to any intention by such entity to suspend, prohibit or terminate the quoting of such Buyer Common Stock
on the Nasdaq Capital Markets, and (D) Buyer is in compliance with all of the applicable corporate governance rules of
the Nasdaq. None of Buyer or any of its Affiliates has taken any action in an attempt to terminate the registration of the Buyer
Common Stock under the Exchange Act.
3.2.7 Litigation.
Except as disclosed in SEC Documents or on Section 3.2.7 of the Buyer Disclosure
Schedule, there is no (a) Proceeding of any nature currently outstanding or, to the Buyer’s Knowledge, threatened (and
no such Proceeding has been brought or, to the Buyer’s Knowledge, threatened since June 30, 2021); or (b) Order now
pending or outstanding or that was rendered by a Governmental Entity, in either case of (a) or (b) by or against any
member of the Renovaro Group, any of its current or former directors, officers or equity holders, except for any Proceeding or
Order involving any such director, officer or equity interest holder of a member of the Renovaro Group that is not related to
a Renovaro Group member’s business, equity interests or assets. In the past five (5) years, none of the current or
former officers or directors of any member of the Renovaro Group have been charged with, indicted for, arrested for, or convicted
of any felony or any crime involving Fraud.
3.2.8 Absence of Undisclosed
Liabilities. Except as disclosed in SEC Documents, no Renovaro
Group member is subject to any Liabilities or obligations which would be required to be reflected or reserved against in Buyers
consolidated financial statements prepared in accordance with US GAAP, except for those that are either (i) adequately reflected
or reserved on or provided for in the financial statements included in the SEC Documents or (ii) not material and that were incurred
after the date of the most recent financial statements included in the SEC Documents in the ordinary course of business consistent
with past practice (other than Liabilities for breach of any Contract or violation of any Law).
3.2.9 No Violations.
(a) No Renovaro Group
member is or has not been in material conflict or material non-compliance with, or in material default or violation of, nor has
any Renovaro Group member received, since June 30, 2021, any written or, to the Knowledge of Buyer, oral notice of any material
conflict or non-compliance with, or material default or violation of, any applicable Laws by which it or any of its properties,
assets, employees, business or operations are or were bound or affected.
(b) All Permits required
for the Renovaro Group members to conduct their respective business have been obtained by them and are valid and in full force
and effect. All fees and charges with respect to such Permits as of the date hereof have been paid in full. Section 3.2.9(b)
of the Buyer Disclosure Schedule lists all current Permits issued to the Renovaro Group members, including the names of the Permits
and their respective dates of issuance and expiration. No loss or expiration of any such Permit is pending or, to the Knowledge
of the Buyer, threatened, other than expiration in accordance with the terms thereof.
3.2.10 Compliance with
Healthcare Laws.
(a) The Renovaro Group
members are and have been since June 30, 2021, in compliance in all material respects with all applicable Healthcare Laws.
The Renovaro Group members (i) have not received any material written notice of adverse finding, warning letter, untitled
letter or other correspondence or notice from the U.S. Food and Drug Administration or any other Governmental Entity alleging or
asserting material noncompliance with any Laws or any licenses, certificates, approvals, clearances, authorizations, permits and
supplements or amendments thereto required by any such Laws (“Authorizations”); (ii) possess all
material Authorizations and such material Authorizations are valid and in full force and effect and, to Buyer’s knowledge,
are not in violation of any term of any such material Authorizations; (iii) have not received written notice of any Proceeding
or other action from any Governmental Entity or third party alleging that any product, operation or activity is in violation of
any Laws or Authorizations and Buyer has no Knowledge that any such Governmental Entity or third party is considering any such
Proceeding; (iv) have not received written notice that any Governmental Entity has taken, is taking or intends to take action to
limit, suspend, modify or revoke any Authorizations and Buyer has no Knowledge that any such Governmental Entity is considering
such action; and (v) have filed, obtained, maintained or submitted all material reports, documents, forms, notices, applications,
records, claims, submissions and supplements or amendments as required by any Laws or material Authorizations and that all such
reports, documents, forms, notices, applications, records, claims, submissions and supplements or amendments were complete and
correct in all material respects on the date filed (or were corrected or supplemented by a subsequent submission). During the two
year period immediately preceding the date hereof, to Buyer’s Knowledge, the studies, tests and preclinical studies conducted
by or on behalf of the Renovaro Group were and, if still pending, are, in all material respects, being conducted in accordance
with experimental protocols, procedures and controls pursuant to accepted professional scientific standards and all applicable
Laws, including, without limitation, the United States Federal Food, Drug, and Cosmetic Act or any other federal, state, local
or foreign Governmental.
Entity or quasi-governmental body exercising comparable authority. The descriptions of the results of such
studies and tests contained in the SEC Documents are accurate and complete in all material respects and fairly present the data
derived from such studies and tests in all material respects; and to Buyer’s Knowledge there are no other studies whose results
are materially inconsistent with or otherwise materially call into question the results described or referred to in the SEC Documents.
Buyer uses commercially reasonable efforts to review, from time to time, the progress and results of the studies, tests and preclinical
studies that are described in the SEC Documents and, based upon (i) the information provided the Renovaro Group members by the
third parties conducting such studies, tests, and preclinical studies and Buyer’s review of such information, and (ii) Buyer’s
Knowledge, Buyer believes that the descriptions of the results of such studies, tests, and preclinical studies are accurate and
complete in all material respects.
3.2.11 Ethical Practices.
(a) No Renovaro Group
member, nor, to the Knowledge of Buyer, any of their respective Representatives acting on their behalf, has (i) used any funds
for unlawful contributions, gifts, entertainment or other unlawful expenses relating to political activity, (ii) made any
unlawful payment to foreign or domestic government officials or employees, to foreign or domestic political parties or campaigns
or violated any provision of the U.S. Foreign Corrupt Practices Act of 1977, as amended or any other local or foreign anti-corruption
or bribery Law, or (iii) made any other unlawful payment. No Renovaro Group member, nor, to the Knowledge of Buyer, any of
their respective Representatives acting on their behalf has, directly or indirectly, given or agreed to give any unlawful gift
or similar benefit in any material amount to any customer, supplier, governmental employee or other Person who is or may be in
a position to help or hinder a Renovaro Group member or assist any Renovaro Group member in connection with any actual or proposed
transaction.
(b) The operations of
the Renovaro Group are and have been conducted at all times in compliance with money laundering statutes in all applicable jurisdictions,
the rules and regulations thereunder and any related or similar rules, regulations or guidelines, issued, administered or
enforced by any Governmental Entity, and no Proceeding involving the Renovaro Group with respect to any of the foregoing is pending
or, to the Knowledge of Buyer, threatened.
(c) No Renovaro Group
member, nor any of its directors or officers, or, to the Knowledge of Buyer, any other Representative acting on behalf of Buyer
is currently identified on the specially designated nationals or other blocked person list or otherwise currently subject to any
U.S. sanctions administered by OFAC, and Buyer has not in the last five (5) fiscal years, directly or indirectly, used any funds,
or loaned, contributed or otherwise made available such funds to any subsidiary, joint venture partner or other Person, in connection
with any sales or operations in Cuba, Iran, Syria, Sudan, Myanmar, Russia or any other country sanctioned by OFAC or for
the purpose of financing the activities of any Person currently subject to, or otherwise in violation of, any U.S. sanctions administered
by OFAC.
3.2.12 Employment Matters.
(a) Section 3.2.12(a)
of the Buyer Disclosure Schedule contains a list of all persons who are employees of any Renovaro Group member as of the date hereof,
including any employee who is on a leave of absence of any nature, paid or unpaid, authorized or unauthorized, and sets forth for
each such individual the following: (i) name; (ii) title or position (including whether full-time or part-time); (iii) hire or
retention date; (iv) current annual base compensation rate or contract fee; and (v) commission, bonus or other incentive-based
compensation. As of the date hereof, all compensation, including wages, commissions, bonuses, fees and other compensation, payable
to all employees, independent contractors or consultants of the Renovaro Group for services performed on or prior to the date hereof
have been paid in full and there are no outstanding agreements, understandings or commitments of the Renovaro Group with respect
to any compensation, commissions, bonuses or fees.
(b) No Renovaro Group
member is, nor has been for the past five (5) years, a party to, bound by, or negotiating any collective bargaining agreement or
other Contract with a Union, and there is not, and has not been for the past five (5) years, any Union representing or purporting
to represent any employee of the Renovaro Group, and no Union or group of employees is seeking or has sought to organize employees
for the purpose of collective bargaining. There has never been, nor has there been any threat of, any strike, slowdown, work stoppage,
lockout, concerted refusal to work overtime or other similar labor disruption or dispute affecting a Renovaro Group member or any
of its employees. No Renovaro Group member has a duty to bargain with any Union.
(c) Each Renovaro Group
member (i) is and has been in compliance in all material respects with all applicable Laws pertaining to employment and employment
practices to the extent they relate to employees, volunteers, interns, consultants and independent contractors of such Renovaro
Group member, including all Laws relating to labor relations, equal employment opportunities, fair employment practices, employment
discrimination, harassment, retaliation, reasonable accommodation, disability rights or benefits, immigration, wages, hours, overtime
compensation, child labor, hiring, promotion and termination of employees, working conditions, meal and break periods, privacy,
health and safety, workers’ compensation, leaves of absence, paid sick leave and unemployment insurance. All individuals
characterized and treated by the Renovaro Group as independent contractors or consultants are properly treated as independent contractors
under all applicable Laws (ii) is not liable for any material past due arrears of wages or any material penalty for failure
to comply with any of the foregoing, and (iii) is not liable for any material payment to any Governmental Entity with respect
to unemployment compensation benefits, social security or other benefits or obligations for employees, independent contractors
or consultants (other than routine payments to be made in the ordinary course of business and consistent with past practice). All
employees of the Renovaro Group classified as exempt under the Fair Labor Standards Act and state and local wage and hour laws
are properly classified in all material respects. Each Renovaro Group member is in compliance with and has complied with all immigration
laws, including Form I-9 requirements and any applicable mandatory “E-Verify” obligations. There are no Proceedings
against any Renovaro Group member pending, or to Buyer’s Knowledge, threatened to be brought or filed, by or with any Governmental
Entity or arbitrator in
connection with the employment of any current or former applicant, employee, consultant, volunteer, intern
or independent contractor of the Renovaro Group, including, without limitation, any charge, investigation or claim relating to
unfair labor practices, equal employment opportunities, fair employment practices, employment discrimination, harassment, retaliation,
reasonable accommodation, disability rights or benefits, immigration, wages, hours, overtime compensation, employee classification,
child labor, hiring, promotion and termination of employees, working conditions, meal and break periods, privacy, health and safety,
workers’ compensation, leaves of absence, paid sick leave, unemployment insurance or any other employment related matter
arising under applicable Laws.
(d) Section 3.2.12(d)
of the Buyer Disclosure Schedule contains a list of all independent contractors (including consultants) currently engaged by any
Renovaro Group member, along with the position, the entity engaging such Person, date of retention and rate of remuneration, most
recent increase (or decrease) in remuneration and amount thereof, for each such Person. Except as set forth on Section 3.2.12(d)
of the Buyer Disclosure Schedule, all of such independent contractors are a party to a written Contract with a Renovaro Group member.
Except as set forth on Section 3.2.12(d) of the Company Disclosure Schedule, each such independent contractor has entered
into customary covenants regarding confidentiality, non-solicitation and assignment of inventions and copyrights in such Person’s
agreement with a Renovaro Group member, a copy of which has been provided to Company by Buyer.
3.2.13 Employee Benefit
Plans. Section 3.2.13 of the Buyer Disclosure Schedule,
contains a true and complete list of each pension, benefit, retirement, compensation, employment, consulting, profit-sharing,
deferred compensation, incentive, bonus, performance award, phantom equity, stock or stock-based, change in control, retention,
severance, vacation, paid time off (PTO), medical, vision, dental, disability, welfare, fringe benefit and other similar agreement,
plan, policy, program or arrangement (and any amendments thereto), in each case whether or not reduced to writing and whether
funded or unfunded, including each “employee benefit plan” within the meaning of Section 3(3) of ERISA, whether or
not tax-qualified and whether or not subject to ERISA (each a “Benefit Plan”), which is or has been maintained,
sponsored, contributed to, or required to be contributed to by Buyer or any of its subsidiaries for the benefit of any current
or former employee, officer, director, retiree, independent contractor or consultant of Buyer, or any of its subsidiaries, or
any spouse or dependent of such individual, or under which Buyer, any of its subsidiaries, or any of its ERISA Affiliates has
or may have any Liability. Each Benefit Plan is and has been operated at all times in compliance with all applicable Laws
in all material respects.
3.2.14 Real Property;
Lease. Neither Buyer nor any Renovaro Group member own,
or have ever owned, real property. Section 3.2.14 of the Buyer Disclosure Schedule sets forth each lease and sublease in
effect on the date of this Agreement under which any Renovaro Group member leases or subleases (a) real property (as either a
tenant or subtenant), or (b) personal property (in case of either clause (a) or (b), a “Buyer Lease”). No default
by any Renovaro Group member, or to Buyer’s Knowledge, by any lessor, or sublessor, exists under any Buyer Lease, and all
payments of rent, operating expenses, and other sums due under each Buyer Lease are current as of the date hereof. No Buyer Lease
is terminable because of the execution of this Agreement or
the consummation
of the Transactions. Each Buyer Lease is in full force and effect in accordance with its respective terms. No consent is required
from any party under any Buyer Lease in connection with the completion of the Transaction. Buyer has not received notice that a
party to any Buyer Lease intends to cancel, terminate, or refuse to renew any Buyer Lease or to exercise any option or other right
under such Buyer Lease, except where the failure to receive such consent, or where such cancellation, termination, or refusal,
would not result, or reasonably be expected to result, individually or in the aggregate, in a Buyer Material Adverse Effect.
3.2.15 Environmental.
(a) Each Renovaro Group
member has complied in all material respects with all Environmental Law in effect on or before the date hereof in respect of any
real property leased or subleased by any Renovaro Group member.
(b) No Proceeding is
pending and, to Buyer’s Knowledge, no Proceeding has been threatened by any Governmental Entity against any Renovaro Group
member concerning any Environmental Law in respect of any real property leased or subleased at any point in time by any Renovaro
Group member.
3.2.16 Buyer Material
Contracts.
(a) Section 3.2.16(a)
of the Buyer Disclosure Schedule lists all of the following Contracts to which Buyer or which a Renovaro Group member is a party
(the “Buyer Material Contracts”):
(i) any Contract with
an annual payment obligation by the Buyer of more than $300,000 (A) relating to the performance of services by, any employee, consultant,
or other Person, (B) in accordance with which Buyer or any Renovaro Group member is or may become obligated to make any severance,
termination, incentive, or similar payment to any current or former officer, director, or employee, or (C) in accordance with which
Buyer or a Renovaro Group member may be required to provide, or accelerate the vesting of, any payments, benefits, or equity rights
upon the occurrence of any of the Transactions;
(ii) any Contract imposing
any restriction on the right or ability of Buyer or any Renovaro Group member, or that, after consummation of the Transactions,
would impose a restriction on the right or ability of Buyer or any Renovaro Group member, to compete in any line of business or
in any geographic region with any other Person or to transact business or deal in any other manner with any other Person;
(iii) any Contract that
provides for indemnification of any officer, director, employee, agent of Buyer or any Renovaro Group member;
(iv) any Contract with
a third party in accordance with which Buyer (A) has paid or received $300,000 or more annually;
(v) any Contract or
agreement of partnership or joint venture, limited liability company or operating agreement that would give rise to an obligation
on the part of Buyer or any Renovaro Group member to form a joint venture or to acquire securities of, or any other equity interest
in, a third party;
(vi) any Buyer Lease;
(vii) any Contract providing
for the development of any Technology or other Intellectual Property, independently or jointly, by or for Buyer or any Renovaro
Group member (other than agreements with employees and independent contractors of Buyer or any Renovaro Group member);
(viii) any settlement,
conciliation or similar agreement under which Buyer, any Renovaro Group member or any counterparty to any such agreement has any
outstanding obligations in excess of $200,000;
(ix) any Contract relating
to the distribution, reselling, whole selling, advertising, marketing or sales of Buyer’s or any Renovaro Group member’s
services or products, if any;
(x) any Contract that
grants any right of first refusal or right of first offer or similar right or that limits or purports to limit the ability of Buyer
or any Renovaro Group member to own, operate, sell, transfer, pledge or otherwise dispose of any material amount of its assets
and properties or their respective businesses;
(xi) any Contract entered
into since the date of Buyer’s most recent financial statements included in the SEC Documents providing for the acquisition
or sale, directly or indirectly, by merger or otherwise of all or substantially all of the assets of another Person, the equity
interests of another Person, or a business or division of another Person;
(xii) any Contract between
Buyer or any Renovaro Group member, on the one hand, and an Affiliate, on the other hand that provides for consideration in excess
of $120,000;
(xiii) any Contract
with any Governmental Entity;
(xiv) any Contract with
a vendor to which total amounts payable exceed $300,000;
(xv) except for trade
indebtedness incurred in the ordinary course of business and except as disclosed in the financial statements included in the SEC
Documents, any instrument evidencing or related in any way to indebtedness for borrowed money (either owed by or to Buyer or any
Renovaro Group member) by way of direct loan, sale of debt securities, purchase money obligation, conditional sale, guarantee,
or otherwise; and
(xvi) Any In-Licenses
or IP Assignments.
(b) Each Buyer Material
Contract is in full force and effect and is a valid and binding obligation of Buyer or such Renovaro Group member, and, to Buyer’s
Knowledge, against any other party to such Buyer Material Contract, except to the extent that enforceability may be limited by
the effect of (a) any applicable bankruptcy, insolvency, reorganization, moratorium, or other similar laws affecting the enforcement
of creditors rights generally, and (b) general equitable principles, regardless of whether enforceability is considered in a Proceeding
at law or in equity. Neither Buyer, any Renovaro Group member, nor, to Buyer’s Knowledge, any other party to any Buyer Material
Contract, is in material breach of or default under, any Buyer Material Contract. None of the parties to the Buyer Material Contracts
has expressed intent in writing to cancel, terminate, or refuse to renew any Buyer Material Contract. Buyer has made available
to Company complete copies of all Buyer Material Contracts.
3.2.17 Tax Returns and
Payments.
(a) Buyer has or will
have filed, or caused to be filed, all income and other material Tax Returns required to be filed by it or any Renovaro Group member
(taking into account all available extensions), which Tax Returns are true, accurate, correct and complete in all material respects,
and has paid, collected or withheld, or caused to be paid, collected or withheld, all Taxes required to be paid, collected or withheld,
other than such Taxes for which adequate reserves in the Company Financials have been established.
(b) There is no Proceeding
currently pending or, to the Knowledge of Buyer, threatened against a member of the Renovaro Group by a Governmental Entity in
a jurisdiction where a Renovaro Group member does not file Tax Returns that it is or may be subject to taxation by that jurisdiction.
(c) No member of the
Renovaro Group is being audited by any Tax authority or has been notified in writing by any Tax authority that any such audit is
contemplated or pending. There are no claims, suits, assessments or, to the Knowledge of Buyer, audits, examinations, investigations
or other Proceedings, pending against a member of the Renovaro Group in respect of any Tax, and no member of the Renovaro Group
has been notified in writing of any proposed Tax claims or assessments against it (other than, in each case, claims or assessments
for which adequate reserves in the financial statements included in the SEC Documents have been established).
(d) There are no Liens
with respect to any Taxes upon any Renovaro Group member’s assets, other than Liens with respect to Taxes not yet due and
payable or Taxes being contested in good faith and for which adequate reserves in Buyer’s financial statements included in
the SEC Documents have been established.
(e) No Renovaro Group
member has any outstanding waivers or extensions of any applicable statute of limitations to assess any amount of Taxes. There
are no outstanding requests by any Renovaro Group member for any extension of time within which to file any Tax Return or within
which to pay any Taxes shown to be due on any Tax Return.
(f) No Renovaro Group
member has made any change in accounting method (except as required by a change in Law) or received a ruling from, or signed an
agreement with, any taxing authority that would reasonably be expected to have a material impact on its Taxes following the Closing.
(g) No Renovaro Group
member has any Liability for the Taxes of another Person (other than another member of the Renovaro Group) that are not adequately
reflected in the financial statements included in the SEC Documents (i) under any applicable Tax Law, (ii) as a transferee
or successor, or (iii) by Contract or indemnity (excluding commercial agreements entered into in the ordinary course of business
the primary purpose of which is not the sharing of Taxes). No member of the Renovaro Group is a party to or bound by any Tax indemnity
agreement, Tax sharing agreement or Tax allocation agreement or similar agreement (excluding commercial agreements entered into
in the ordinary course of business the primary purpose of which is not the sharing of Taxes) with respect to Taxes (including advance
pricing agreements, closing agreements or other agreements relating to Taxes with any Governmental Entity) that will be binding
on any member of the Renovaro Group with respect to any period following the Closing Date.
(h) No Renovaro Group
member has requested, or is the subject of or bound by any private letter ruling, technical advice memorandum, closing agreement
or similar ruling, memorandum or agreement with any Governmental Entity with respect to any Taxes, nor is any such request outstanding.
3.2.18 Intellectual
Property Rights.
(a) Section 3.2.18(a)
of the Buyer Disclosure Schedule attached hereto contains a true and complete list of (i) the Buyer Patent Rights existing on the
Effective Date that are registered and applied-for with a Governmental Entity and owned by a Renovaro Group member and (ii) In-Licenses
relating to the Buyer Technology as of the Effective Date, excluding any licenses or sublicenses entered with any Renovaro Group
member’s suppliers or customers. The Buyer Patent Rights listed in Section 3.2.18(a) of the Buyer Disclosure Schedule
include all of the Patent Rights owned by the Renovaro Group members and licenses relating to the Buyer Technology as of the Effective
Date that relate to the Renovaro Group’s business and its operations.
(b) Buyer is the owner
or exclusive licensee of all Buyer Patent Rights listed in Section 3.2.18(b) of the Buyer Disclosure Schedule and, to Buyer’s
Knowledge as of the Effective Date, and Buyer has no Knowledge that the Buyer Patent Rights are not unpatentable or unenforceable.
(c) Buyer (i) is, as
of the Effective Date, the sole and exclusive owner or licensee of all right, title and interest in and to Buyer IP Rights and
as of the Effective Date free and clear of any Liens; (ii) has not granted to any third party any license or other right with respect
to Buyer IP Rights that conflicts with or limits in any way the licenses and rights granted to Buyer in or by any license; and
(iii) to the Buyer’s Knowledge has not disclosed to any third party any confidential Trade Secrets or Know-how listed in
Section 3.1.17(b) of the Buyer Disclosure Schedule.
(d) The manufacture,
use, sale, offer for sale or import of any Buyer Technology does not, to Buyer’s Knowledge as of the Effective Date, Infringe
any Patent, Trade Secret, or any other Intellectual Property or proprietary right of any third party, and no Renovaro Group member
has received written, oral or other notice from any third party claiming that the manufacture, use, sale, testing, offer for sale
or import of any Product to the extent practicing Buyer Technology Infringes any Patent or other Intellectual Property rights of
any third party, nor to Buyer’s Knowledge as of the Effective Date is there any reasonable basis for such a claim.
(e) There are no claims,
judgments or settlements against or owed by Buyer (or any of their Affiliates) with respect to Buyer IP Rights, and Buyer is not
a party to any legal action, suit or proceeding relating to Buyer IP Rights, nor has Company received any written, oral or other
communication from any third party, including, without limitation, any Regulatory Authority or other Governmental Entity, threatening
such action, suit or proceeding or any other claim or proceeding alleging the unpatentability or unenforceability of any Buyer
Patent Rights; in each case, as of the Effective Date.
3.2.19 Privacy and
Information Security.
(a) The Renovaro Group
members are in compliance with all Privacy Laws in the Processing of Personal Information (including employee information) applicable
to the its business;
(b) The Renovaro Group
members have not experienced any Security Breach, where such Security Breach resulted in the provision of notice to any Person
as required by applicable Privacy Laws;
(c) The Renovaro Group
members maintain commercially reasonable security measures, controls, technologies, policies and safeguards designed to protect
Personal Information and any proprietary information in possession of the Renovaro Group members.
(d) The Renovaro Group
members have established one or more incident response and disaster recovery and business continuity plans to address any actual
or threatened Security Breach or other security incident or data breach.
3.2.20 Brokers and Other
Fees. Neither Buyer, nor any of its officers, directors,
or employees has employed any investment banker, broker, finder, or other intermediary that has been retained by, or is authorized
to act on behalf of, Buyer that would be entitled to any fee or commission from any of the Renovaro Group members in connection
with or upon consummation of the Transactions.
3.2.21 Insurance.
Section 3.2.21 of the Buyer Disclosure Schedule lists all insurance policies (by
policy number, insurer, coverage period, coverage amount, annual premium and type of policy) held by the Renovaro Group members
relating to the Renovaro Group or its business, properties, assets, directors, officers and employees, copies of which have been
provided to Company. All premiums due and payable under all such insurance policies have been timely paid and the Renovaro Group
members are otherwise in material compliance with the terms of such insurance policies. Each such insurance policy (i) is
legal, valid, binding, enforceable and in full force and effect and (ii) will continue to be legal, valid, binding, enforceable,
and in full force and effect on identical terms following the Closing. No the Renovaro Group member has any self-insurance or
co-insurance programs. Since June 30, 2021, no the Renovaro Group member has received any written notice from, or on behalf of,
any insurance carrier relating to or involving any adverse change or any change other than in the ordinary course of business,
in the conditions of insurance, any refusal to issue an insurance policy or non-renewal of a policy.
3.2.22 D&O Policy.
Buyer has previously provided to Company (through counsel) a copy of its current directors
and officers insurance policy (“D&O Policy”). Neither Buyer nor any of its subsidiaries is in default with
respect to its obligations under any such insurance policy. Such policy is in full force and effect, all premiums due and payable
thereon have been paid, and no notice of cancellation or termination has been received with respect to such policy and, to the
Knowledge of Buyer, no cancellation or termination of such policy is pending or threatened by the current insurer. All of Buyer’s
officers and directors (including those who shall assume such positions in accordance with this Agreement and/or the other Transaction
Documents) shall be covered by the D&O Policy, as amended prior to Closing pursuant to Section 5.7 hereof.
3.2.23 Affiliate Transactions.
Except for (a) relationships with any Renovaro Group member as an officer, director,
employee or consultant (and compensation by a Renovaro Group member in consideration of such services), (b) relationships with
Buyer as an equity interest holder and (c) except as set forth in Section 3.2.24 of the Buyer Disclosure Schedule or as
disclosed in the SEC Documents, none of the officers, directors, consultants or equity interest holders, or to Buyer’s Knowledge,
any member of any of their immediate families, is presently a party to, or was a party to since June 30, 2021, any transaction
with Buyer or Renovaro Group, including any Contract, agreement, or other arrangement (x) providing for the furnishing of services
to or by, (y) providing for lease or sublease of real or personal property to or from, or (z) otherwise requiring payments to
or from, any such Person or any corporation, partnership, trust, or other entity in which any such Person has or had a 5% or more
interest (as a member, partner, beneficiary, or otherwise) or is or was an officer, director, employee, Buyer vendor.
3.2.24 Absence of Certain
Changes. Except as set forth in Section 3.2.24 of the Buyer Disclosure Schedule, since
the date of the most recent financial statements included in the SEC Document, each Renovaro Group member has (a) conducted
its business only in the ordinary course of business consistent with past practice,
(b) not been subject to a Buyer Material Adverse Effect and (c) has not taken any action
or committed or agreed to take any action that would be prohibited by Section 5.1.1 if such action were taken on or
after the date hereof without the consent of Company.
3.2.25 Compliance with
Regulation S. Neither Buyer, nor any of its Affiliates
nor any person acting on its or their behalf has engaged or will engage in any form of general solicitation, general advertising
or “directed selling efforts” (as defined in Rule 902 of Regulation S promulgated under the Securities Act) in connection
with the offering of the Exchange Consideration. Buyer has complied and will comply with the offering restrictions requirement
of Regulation S promulgated under the Securities Act.
3.2.26 Disclosure.
No representation or warranty made by Buyer in this Agreement or the other Transaction Documents contains any untrue statement
of a material fact, or omits to state a material fact necessary to make the statements or facts contained herein or therein, in
light of the circumstances under which they were made, not misleading. Buyer has made available to Company or its counsel each
document listed in the Buyer Disclosure Schedule.
3.2.27 Independent Investigation.
Buyer has conducted its own independent investigation, review and analysis of the business,
results of operations, prospects, condition (financial or otherwise) or assets of the Target Companies, and acknowledges that
it has been provided adequate access to the personnel, properties, assets, premises, books and records, and other documents and
data of the Target Companies for such purpose. Buyer acknowledges and agrees that: (a) in making its decision to enter into
this Agreement and to consummate the Transactions contemplated hereby, it has relied solely upon its own investigation and the
express representations and warranties of Company and Sellers set forth in this Agreement (including the related portions of the
Company Disclosure Schedules) and in any certificate delivered to Buyer pursuant hereto, and the information provided by or on
behalf of Company or Sellers for any registration statement; and (b) none of Company, Sellers nor any of their respective
Representatives have made any representation or warranty as to the Target Companies, Sellers or this Agreement, except as expressly
set forth in this Agreement (including the related portions of the Company Disclosure Schedules) or in any certificate delivered
to Buyer pursuant hereto, or with respect to the information provided by or on behalf of Company for any registration statement.
3.2.28 Liens on
Company and Company Shares. The purchase of the Company Shares by Buyer and the consummation
of the Transactions contemplated hereby shall not result in the creation of any Lien on any assets of the Target Companies or on
any Company Shares under the terms of any loan or credit agreement, note, bond, mortgage, indenture, or other agreement or instrument
including, without limitation each Buyer Material Contract, to which any Renovaro Group member is a party, or any Order or decree
by any Governmental Authority imposed on any Renovaro Group member.
3.2.29 Information SuppliedNone
of the information supplied by Buyer expressly for inclusion or incorporation by reference, and ultimately included or incorporated
by reference in any of the Proxy Statements, Signing Press Release,
Signing Filing, Closing Press Release and Closing Filing will,
when filed, made available, mailed or distributed, as the case may be, contain any untrue statement of a material fact or omit
to state any material fact required to be stated therein or necessary in order to make the statements therein, in light of the
circumstances under which they are made, not misleading; provided, however, that Buyer makes no representation with
respect to any forward-looking statements supplied by or on behalf of Buyer for inclusion in, or relating to information to be
included in the Proxy Statements, Signing Press Release, Signing Filing, Closing Press Release or Closing Filing. Notwithstanding
the foregoing, Buyer makes no representation, warranty or covenant with respect to any information supplied by or on behalf of
the Company, Sellers or their Affiliates.
3.3 Representations
and Warranties with respect to Sellers. Each Seller represents
to Buyer, with respect to such Seller and not with respect to any Seller, as follows:
3.3.1 Organization,
Standing, and Power. Such Seller, if not an individual
person, is an entity duly organized, validly existing and in good standing under the Laws of the jurisdiction of its formation
and has all requisite power and authority to own, lease and operate its properties and to carry on its business as now being conducted.
3.3.2 Authority.
(a) Seller has all requisite
power and authority to execute and deliver this Agreement and to consummate the Transactions. The execution and delivery by Seller
of this Agreement and any other Transaction Document to which said Seller is a party and the performance of any Seller’s
obligations under this Agreement and the other Transaction Documents to which such Seller is a party have been duly and validly
authorized by all necessary action on the part of said Seller and no other action on the part of Seller, or its members or manager,
are necessary to authorize this Agreement or to consummate the Transactions. This Agreement has been duly executed and delivered
by Seller and (assuming due authorization, execution and delivery by Buyer and Company) constitutes a valid and binding obligation
of said Seller enforceable in accordance with its terms, except to the extent that enforceability may be limited by the effect
of (a) any applicable bankruptcy, insolvency, reorganization, moratorium, or other similar laws affecting the enforcement of creditors
rights generally, and (b) general equitable principles, regardless of whether enforceability is considered in a Proceeding at law
or in equity.
(b) The execution and
delivery of this Agreement and compliance with the provisions of this Agreement do not and will not (i) conflict with, or result
in any material violation or breach of, or default (with or without notice) under, any provision of the Organizational Documents
of such Seller, (ii) violate any Law or Order to which such Seller is subject, or (iii) violate any agreement or instrument to
which said Seller is a party or by which said Sellers assets are bound, except, in the case of items (ii) and (iii) above, for
any violation that individually or in the aggregate, would not materially and adversely affect the ability of such Seller to consummate
the Transactions contemplated hereby, and discharge its obligations under, this Agreement and the Transaction Documents to which
such Seller is a party.
(c) Such Seller owns
good, valid and marketable title to the Company Shares set forth opposite such Seller’s name on Exhibit B, free and
clear of any and all Liens, other than those imposed under applicable securities Laws. There are no proxies, voting rights, shareholders’/stockholders’
agreements or other agreements or understandings, to which such Seller is a party or by which such Seller is bound, with respect
to the voting or transfer of any of such Seller’s Company Shares other than this Agreement. Upon delivery of such Seller’s
Company Shares to Buyer on the Closing Date in accordance with this Agreement, the entire legal and beneficial interest in such
Company Shares and good, valid and marketable title to such Company Shares, free and clear of all Liens (other than those imposed
under applicable securities Laws or those incurred by Buyer), will pass to Buyer.
(d) There is no Proceeding
pending or, to the Knowledge of such Seller, threatened, nor any Order is outstanding, against or involving such Seller, whether
at law or in equity, before or by any Governmental Entity, which would reasonably be expected to materially and adversely affect
the ability of such Seller to consummate the Transactions contemplated by, and discharge its obligations under, this Agreement
and the Transaction Documents to which such Seller is a party.
3.3.3 Investment Representations.
Such Seller, as identified on the signature pages hereto: (a) either is (i) an “accredited
investor” as such term is defined in Rule 501(a) of Regulation D promulgated under the Securities Act, or (ii) not
a “U.S. person” as such term is defined in Rule 902 of Regulation S promulgated under the Securities Act, is not acquiring
the Exchange Shares for the account or benefit of any U.S. person, is not, at the time of the execution of this Agreement, and
will not be at the time of Closing, in the United States and is not a “distributor” (as defined in Regulation S);
(b) is acquiring its portion of the Exchange Shares for itself for investment purposes only, and not with a view towards any resale
or distribution of such Exchange Shares; (c) has been advised and understands that the Exchange Shares (i) are being issued in
reliance upon one or more exemptions from the registration requirements of the Securities Act and any applicable state securities
Laws and (ii) have not been and shall not be registered under the Securities Act or any applicable state securities Laws and,
therefore, must be held indefinitely and cannot be resold unless such Exchange Shares are registered under the Securities Act
and all applicable state securities Laws, unless exemptions from registration are available; (d) is aware that an investment in
Buyer is a speculative investment and is subject to the risk of complete loss; and (e) acknowledges that except as set forth in
the Seller Registration Rights Agreement, Buyer is under no obligation hereunder to register the Exchange Shares under the Securities
Act. Such Seller does not have any Contract with any Person to sell, transfer, or grant participations to such Person, or to any
third Person, with respect to the Exchange Shares. By reason of such Seller’s business or financial experience, or by reason
of the business or financial experience of such Seller’s “purchaser representatives” (as that term is defined
in Rule 501(i) under the Securities Act), such Seller is capable of evaluating the risks and merits of an investment in
Buyer and of protecting its interests in connection with this investment. Such Seller has carefully read and understands all materials
provided by or on behalf of Buyer or its Representatives to such Seller or such Seller’s Representatives pertaining to an
investment in Buyer and has consulted, as such Seller has deemed advisable, with its own attorneys, accountants or investment
advisors with respect to the investment contemplated hereby and its suitability for such Seller.
Such Seller acknowledges that the Exchange Shares are subject to dilution for events not under
the control of such Seller. Such Seller has completed its independent inquiry and has relied fully upon the advice of its own legal
counsel, accountant, financial and other Representatives in determining the legal, tax, financial and other consequences of this
Agreement and the Transactions contemplated hereby and the suitability of this Agreement and the Transactions contemplated hereby
for such Seller and its particular circumstances, and, except as set forth herein, has not relied upon any representations or advice
by Buyer or its Representatives. Such Seller acknowledges and agrees that, except as set forth in Section 3.2 (including
the related portions of the Buyer Disclosure Schedules and the SEC Documents), no representations or warranties have been made
by Buyer or any of its Representatives, and that such Seller has not been guaranteed or represented to by any Person, (i) any specific
amount or the event of the distribution of any cash, property or other interest in Buyer or (ii) the profitability or value of
the Exchange Shares in any manner whatsoever. Such Seller: (A) has been represented by independent counsel (or has had the opportunity
to consult with independent counsel and has declined to do so); (B) has had the full right and opportunity to consult with such
Seller’s attorneys and other advisors and has availed itself of this right and opportunity; (C) has carefully read and fully
understands this Agreement in its entirety and has had it fully explained to it or him by such counsel; (D) is fully aware of the
contents hereof and the meaning, intent and legal effect thereof; and (E) is competent to execute this Agreement and has executed
this Agreement free from coercion, duress or undue influence.
Article
IV
COVENANTS OF COMPANY AND SELLER
During the period from the
date of this Agreement and continuing until the earlier of the termination of this Agreement pursuant to Article X or the
Closing, Company agrees (except as expressly contemplated by this Agreement or as otherwise consented to in advance in writing
by Buyer, which consent shall not be unreasonably delayed, conditioned or withheld) as follows:
4.1 Conduct of Business.
4.1.1 Ordinary Course.
Company shall, and shall cause each of the other Target Companies to, carry on its business
in the ordinary course consistent with past practice, will continue to observe its obligations to comply with the requirements
of all applicable Laws and regulations, and will use commercially reasonable efforts to (i) preserve intact its present business
organization and its assets, (ii) keep available the services of its officers, consultants, and employees (other than termination
of such services for cause), (iii) maintain and preserve the goodwill and relationships with manufacturers, customers, suppliers,
contractors, distributors, and others having business relationships with it and (iv) maintain its books, records and financials
in accordance with applicable Accounting Principles. Company will promptly notify Buyer of any event or occurrence not in the
ordinary course of business of any Target Company that would result, or could reasonably be expected to result, individually or
in the aggregate, in a Company Material Adverse Effect. Without limiting the above, prior to the earlier of the termination of
this Agreement pursuant to Article X or the Closing Date, the Company shall not, and shall cause its
subsidiaries to not (except as (i) expressly contemplated by this Agreement, (ii)
as required by applicable Law, or (iii) as otherwise consented to in advance in writing by Buyer, which consent shall not be unreasonably
delayed, conditioned or withheld):
(a) except as required
under the terms of any agreement existing as of the date of this Agreement as disclosed in the Company Disclosure Schedule, (i)
increase the compensation or benefits of any of the current or former directors, officers, employees or consultants of any Target
Company (collectively, “Employees”), (ii) establish or amend any Benefit Plan, (iii) terminate the employment
of any Employee (other than due to terminations for cause), or (iv) grant any severance or termination pay to any Employee, other
than in such Target Company’s ordinary course of business and consistent with past practices;
(b) assign, transfer,
dispose of, or license ownership of Company IP Rights or any other assets;
(c) authorize for issuance,
issue, grant, sell, dispose of or propose to issue, grant, sell or dispose of any of its equity interests, Convertible Securities
or any commitments, subscriptions or rights of any kind to acquire or sell any of its equity interests, Convertible Securities
or other securities, including any securities convertible into or exchangeable for any of its shares or other equity interests
or securities of any class and any other equity-based awards, or engage in any hedging transaction with a third Person with respect
to such securities;
(d) abandon or permit
to lapse any Company IP Rights;
(e) disclose any of
Company’s Trade Secrets to any third party, other than pursuant to confidentiality agreements;
(f) declare, set aside,
or pay any distribution with respect to any Company Shares, or repurchase, redeem, or acquire any outstanding Company Shares or
other ownership interests in Company, or otherwise change the capitalization of any Target Company in any manner from the way it
existed on the date of this Agreement;
(g) amend Company’s
Organizational Documents or comparable charter or governance documents of any Target Company;
(h) incur,
create, assume, prepay or otherwise become liable for any Indebtedness (directly, contingently or otherwise), make a loan or advance
to or investment in any third party (other than advancement of expenses to employees in the ordinary course of business), or guarantee
or endorse any Indebtedness, Liability or obligation of any Person;
(i) commence a lawsuit,
or settle, compromise, or otherwise terminate any litigation, claim, investigation, or other settlement negotiation;
(j) make any payments
to any third parties in excess of $100,000;
(k) extend an offer
of employment to a candidate whose annual compensation shall be in excess of $300,000;
(l) enter into any joint
venture, partnership, limited liability company, or operating agreement with any Person;
(m) enter into any Company
Material Contract or breach, modify, amend, or terminate any Company Material Contracts (including insurance policies covering
Company’s properties or assets), or waive, release, or assign any rights or claims under any Company Material Contracts,
except as expressly required or permitted by this Agreement;
(n) voluntarily
incur any Liability or obligation (whether absolute, accrued, contingent or otherwise) in excess of $300,000 individually or $600,000
in the aggregate other than pursuant to the terms of a Company Material Contract;
(o) adopt a plan of
complete or partial liquidation, dissolution, merger, consolidation, restructuring, recapitalization, or other reorganization;
(p) acquire or dispose
of capital stock of any third party or merge or consolidate with any third party;
(q) establish any subsidiary
or enter into any new line of business;
(r) fail to use commercially
reasonable efforts to keep in force insurance policies or replacement or revised policies providing insurance coverage with respect
to its assets, operations and activities in such amount and scope of coverage substantially similar to that which is currently
in effect;
(s) sell,
lease, license, transfer, exchange or swap, mortgage or otherwise pledge or encumber (including securitizations), or otherwise
dispose of any material portion of its properties, assets or rights;
(t) knowingly take any
action that would or is reasonably likely to (i) make any representation or warranty of Company contained in this Agreement inaccurate,
(ii) result in any of the conditions in Article VII not being satisfied, or (iii) impair the ability of Company to consummate
the Transactions in accordance with the terms of this Agreement; or
(u) authorize, commit,
or agree to take any of the foregoing actions.
4.1.2 Seller Transfers. Without
limiting Section 4.1.1, except as set forth in Section 4.1.2 of the Company Disclosure Schedule, prior to Closing
or termination of this Agreement, without the prior written consent of Buyer, which consent shall not be unreasonably withheld,
delayed, or conditioned (i) Company shall not issue any Company Securities, and (ii) no Seller shall sell, transfer or dispose
of any Company Securities owned by such Seller, in either case of clauses (i) and (ii), unless the recipient or transferee of
such Company Securities (A) becomes a Joining Seller hereunder by executing and delivering to Buyer and Company a Seller
Joinder, which
Seller Joinder is accepted in writing and executed and delivered by Buyer, Company and Seller Representative, and (B) executes
and delivers to Buyer and Company any Transaction Documents which such transferee would have been required to be a party or bound
if such transferee were a Signing Seller on the date of this Agreement or to which the transferring Seller is otherwise bound.
Notwithstanding anything to the contrary in this Agreement, the Parties shall make any appropriate adjustments to Exhibit B
and each Seller’s Pro Rata Share and Earnout Stock to account for any such new Seller.
4.1.3 Exclusivity; Acquisition
Proposals.
(a) For purposes of
this Agreement, an “Alternative Transaction” means (A) with respect to Company, Sellers and their respective
Affiliates, a transaction (other than the Transactions contemplated by this Agreement) concerning the sale of (x) all or any material
part of the business or assets of the Target Companies (other than in the ordinary course of business consistent with past practice)
or (y) any of the shares or other equity interests or profits of the Target Companies, in any case, whether such transaction takes
the form of a sale of shares or other equity interests, assets, merger, consolidation, issuance of debt securities, management
Contract, joint venture or partnership, or otherwise and (B) with respect to Buyer and its Affiliates, a transaction (other than
the Transactions contemplated by this Agreement) concerning a merger, consolidation or other business combination involving Buyer,
the issuance of equity interests in Buyer resulting in a change of Control of Buyer, or the sale of all or a substantial portion
of the assets of the Renovaro Group.
(b) Prior to the earlier
of the termination of this Agreement pursuant to Article X or the Closing, in order to induce Buyer to continue to commit
to expend management time and financial resources in furtherance of the Transactions contemplated hereby, the Target Companies
and Sellers shall not, and shall cause their Representatives to not, without the prior written consent of Buyer, directly or indirectly,
(i) solicit, assist, initiate or facilitate the making, submission or announcement of, or intentionally encourage, any Acquisition
Proposal, (ii) furnish any non-public information regarding the Target Companies or their Affiliates or their respective businesses,
operations, assets, Liabilities, financial condition, prospects or employees to any Person or group of Persons (other than a Party
to this Agreement or their respective Representatives) in connection with or in response to an Acquisition Proposal, other than
as required by applicable Law, (iii) engage or participate in discussions or negotiations with any Person or group of Persons
with respect to, or that could reasonably be expected to lead to, an Acquisition Proposal, (iv) approve, endorse or recommend,
or publicly propose to approve, endorse or recommend, any Acquisition Proposal, (v) negotiate or enter into any letter of intent,
agreement in principle, acquisition agreement or other similar agreement related to any Acquisition Proposal, or (vi) release any
third Person from, or waive any provision of, any confidentiality agreement to which such Party is a party.
(c) Company and any
Seller shall notify Buyer as promptly as practicable (and in any event within three (3) business days) in writing of the receipt
by such Party or any of its Representatives of (i) any bona fide inquiries, proposals or offers,
requests for information or requests
for discussions or negotiations regarding or constituting any Acquisition Proposal or any bona fide inquiries, proposals or offers,
requests for information or requests for discussions or negotiations that could be expected to result in an Acquisition Proposal,
and (ii) any request for non-public information relating to the Target Companies or their Affiliates in connection with any Acquisition
Proposal, specifying in each case, the material terms and conditions thereof (including a copy thereof if in writing or a written
summary thereof if oral) and the identity of the party making such inquiry, proposal, offer or request for information. Each Party
shall keep the others promptly informed of the status of any such inquiries, proposals, offers or requests for information. Prior
to Closing, each Target Company shall, and shall cause its Representatives to, immediately cease and cause to be terminated any
solicitations, discussions or negotiations with any Person with respect to any Acquisition Proposal and shall, and shall direct
its Representatives to, cease and terminate any such solicitations, discussions or negotiations.
4.1.4 Company Financial Statements.
(a) Company shall
deliver to Buyer as promptly as reasonably practicable (but in any event no later than October 30, 2023) following the date of
this Agreement, (i) the audited consolidated financial statements of the Target Companies (including any related notes thereto)
for the years ended December 31, 2022 and 2021, consisting of the audited consolidated balance sheets of the Target Companies as
of December 31, 2022 and 2021, and the related audited consolidated income statements, changes in shareholder/stockholder
equity and statements of cash flows for the fiscal years then ended, audited by a Public Company Accounting Oversight Board (“PCAOB”)
qualified auditor in accordance with US GAAP and PCAOB standards (the “2022 Company Financials”), and (ii) interim
unaudited consolidated financial statements of the Target Companies for the six months ended June 30, 2023 and 2022, consisting
of the interim unaudited consolidated balance sheet of the Target Companies as of June 30, 2023 and 2022, prepared in accordance
with US GAAP and PCAOB standards, subject to year-end audit adjustments and excluding footnotes (the “2023 Interim Company
Financials”). Company shall cause (A) the 2022 Company Financials and the 2023 Interim Company Financials to be prepared
in accordance with US GAAP applied on a consistent basis throughout the periods indicated (except as may be specifically indicated
in the notes thereto), (B) the 2022 Company Financials to be audited in accordance with the standards of the PCAOB and to
contain a report of Company’s auditor and (C) the 2022 Company Financials and the 2023 Interim Company Financials to
comply in all material respects with the applicable accounting requirements and with the rules and regulations of the SEC,
the Exchange Act and the Securities Act in effect as of date of delivery (including Regulation S-X or Regulation S-K, as applicable).
(b) In the event the
Closing does not occur prior to November 15, 2023, the Company shall deliver to Buyer as promptly as reasonably practicable (but
in any event no later than November 15, 2023) interim unaudited consolidated financial statements of the Target Companies for the
nine months ended September 30, 2023 and 2022, consisting of the interim unaudited consolidated balance sheet of the Target Companies
as of September 30, 2023 and 2022, prepared in accordance with US GAAP and PCAOB standards, subject to year-end audit adjustments
and excluding footnotes (the “September Company Financials”).
The Parties agree the term “2023 Interim
Company Financials” shall be modified to include the September Company Financials if their delivery is required prior to
Closing. As used herein, the defined term “Latest Balance Sheet Date” shall mean the date of the balance sheet
contained in the 2023 Interim Company Financials, as modified by the previous sentence.
(c) Prior to Closing,
and beginning with the month ended October 31, 2023, within thirty (30) calendar days following the end of each calendar month
and within thirty (45) calendar days following the end of each three-month quarterly period, Company shall deliver to Buyer an
interim unaudited consolidated income statement and an interim unaudited consolidated balance sheet of the Target Companies for
the period from the Latest Balance Sheet Date through the end of such calendar month or quarterly period and the applicable comparative
period in the preceding fiscal year, in each case accompanied by a certificate of the Chief Financial Officer of Company to the
effect that all such financial statements fairly present the consolidated financial position and results of operations of the Target
Companies as of the date or for the periods indicated, in accordance with US GAAP, subject to year-end audit adjustments and excluding
footnotes. From the date hereof through the Closing Date, Company will also promptly deliver to Buyer copies of any audited consolidated
financial statements of the Target Companies that the Target Companies’ certified public accountants may issue.
4.1.5 No Trading.
Each of Company and Sellers acknowledges and agrees that it is aware, and that their respective Affiliates are aware (and each
of their respective Representatives is aware or, upon receipt of any material nonpublic information of Buyer, will be advised)
of the restrictions imposed by U.S. federal securities laws and the rules and regulations of the SEC and Nasdaq (or other
applicable national exchange) promulgated thereunder or otherwise (the “Federal Securities Laws”) and other applicable
foreign and domestic Laws on a Person possessing material nonpublic information about a publicly traded company. Each of Company
and Sellers hereby agrees that, while it is in possession of such material nonpublic information, it shall not purchase or sell
any securities of Buyer (other than to engage in the Transactions contemplated by this Agreement), communicate such information
to any third party, take any other action with respect to Buyer in violation of such Laws, or cause or encourage any third party
to do any of the foregoing.
4.2 Notification; Access to
Information.
4.2.1 Cooperation.
From the date of this Agreement to the earlier of the Closing Date or the termination of this Agreement in accordance with Article
X, Company will (i) confer with Buyer and its Representatives, at such times as they may request, during normal business hours and
upon reasonable advance notice, about operational and integration matters to the extent permitted by Law, (ii) in the event of, and promptly
after becoming aware of, the occurrence of or the pending or threatened occurrence of any event that would cause or constitute a material
breach of any of the representations and warranties in Section 3.1, give detailed written notice to Buyer and use commercially
reasonable efforts to prevent or promptly remedy such breach or inaccuracy, (iii) promptly notify Buyer if it fails to comply with or
satisfy any covenant, condition or agreement
to be complied with or satisfied by it or its
Affiliates or any Seller hereunder in any material respect; and (iv) promptly notify Buyer of any change in the normal course of
any business, operations, or financial condition of the Target Companies or their respective Assets or properties, or any emergency
related to the same.
4.2.2 Access to Information.
Company will, subject to applicable Law, afford Buyer and its Representatives reasonable access during normal business hours during the
period before the Closing to all information concerning the business, properties, and personnel of the Target Companies, as Buyer may
reasonably request that is necessary to complete the Transactions and prepare for an orderly transition of operations after the Closing;
provided that such access does not disrupt the normal operations of the Target Companies and does not contravene applicable Law.
4.3 Consents and Notices.
Company will use its commercially reasonable efforts, and each Seller will cooperate with Buyer and Company, to promptly (a) apply for
or otherwise seek, and use commercially reasonable efforts to obtain, all consents included in Section 3.1.4 of the Company Disclosure
Schedule (if any), (b) provide all notices included in Section 3.1.4 of the Company Disclosure Schedule (if any), and (c) make
all filings required with respect to any Target Company for the consummation of the Transactions (if any).
4.4 Commercially Reasonable
Efforts. Each Target Company will use commercially reasonable efforts to effect the Transactions applicable
to such Party, including the satisfaction of the respective conditions set forth in Article VII, applicable to Company, and including
to execute and deliver such other instruments and do and perform such other acts and things as may be reasonably necessary for effecting
completely the consummation of the Transactions applicable to Company (including making all filings (if any), giving all notices (if
any) required to be made and given by Company in connection with the Transactions and using commercially reasonable efforts to obtain
each consent (if any) required to be obtained (pursuant to any applicable Law, Contract or otherwise) by such Party in connection with
the Transactions).
4.5 Proxy Statement.
Company and Sellers shall, and shall cause the Target Companies to, furnish all information concerning the Target Companies and Sellers
as Buyer may reasonably request in connection with the preparation of the Proxy Statements, including, without limitation, any information
in response to comments received from the SEC, if applicable.
4.6 Issuance of
Company Ordinary Shares to Consultant. Immediately prior to Closing, Company shall (i) issue
to Consultant that number of Company Ordinary Shares that would result in Consultant receiving 1,000,000 Exchange Shares at Closing
pursuant to Section 2.2 and (ii) use commercially reasonable efforts to cause the Consultant to become a Joining Seller.
Article
V
COVENANTS OF BUYER
During the period from the
date of this Agreement and continuing until the earlier of the termination of this Agreement pursuant to Article X or the
Closing, Buyer agrees (except as expressly contemplated by this Agreement or as otherwise consented to in advance in writing by
Company, which consent shall not be unreasonably delayed, conditioned or withheld) as follows:
5.1 Conduct of Business.
5.1.1 Ordinary Course.
Buyer shall, and shall cause each of the other Renovaro Group members to, carry on its business in the ordinary course consistent with
past practice, will continue to observe its obligations to comply with the requirements of all applicable Laws and regulations, and will
use commercially reasonable efforts to (i) preserve intact its present business organization and its assets, (ii) keep available the
services of its officers, consultants, and employees (other than termination of such services for cause), (iii) maintain and preserve
the goodwill and relationships with manufacturers, customers, suppliers, contractors, distributors, and others having business relationships
with it and (iv) maintain its books, records and financials in accordance with US GAAP. Buyer will promptly notify Sellers and Company
of any event or occurrence not in the ordinary course of business of any Renovaro Group member that would result, or could reasonably
be expected to result, individually or in the aggregate, in a Buyer Material Adverse Effect. Without limiting the above, prior to the
earlier of the termination of this Agreement pursuant to Article X or the Closing Date, Buyer shall not, and shall cause its subsidiaries
to not (except as (i) expressly contemplated by this Agreement, (ii) as required by applicable Law, or (iii) as otherwise consented to
in advance in writing by Company and Sellers, which consent shall not be unreasonably delayed, conditioned or withheld):
(a) except as required
under the terms of any agreement existing as of the date of this Agreement as disclosed in the Buyer Disclosure Schedule, (i) increase
the compensation or benefits of any of the current or former directors, officers, or employees of any Renovaro Group member (collectively,
“Buyer Employees”), (ii) establish or amend any Benefit Plan, (iii) terminate the employment of any Buyer Employee
(other than due to terminations for cause) or (iv) grant any severance or termination pay to any Buyer Employee, other than in
Buyer’s ordinary course of business and consistent with past practices;
(b) assign, transfer,
dispose of, or license ownership of Buyer IP Rights or any other assets;
(c) abandon or permit
to lapse any Buyer IP Rights;
(d) disclose any Buyer’s
Trade Secrets to any third party, other than pursuant to confidentiality agreements;
(e) declare, set aside,
or pay any distribution with respect to any Buyer Common Stock, or repurchase, redeem, or acquire any outstanding Buyer Common
Stock or other ownership interests in Buyer, or otherwise change the capitalization of Buyer in any manner from the way it existed
on the date of this Agreement;
(f) amend the Organizational
Documents of Buyer;
(g) incur,
create, assume, prepay or otherwise become liable for any Indebtedness (directly, contingently or otherwise), make a loan or advance
to or investment in any third party (other than advancement of expenses to employees in the ordinary course of business), or guarantee
or endorse any Indebtedness, Liability or obligation of any Person;
(h) commence a lawsuit,
or settle, compromise, or otherwise terminate any litigation, claim, investigation, or other settlement negotiation;
(i) make any payments
to any third parties in excess of $100,000 outside of the ordinary course of business;
(j) extend an offer
of employment to a candidate whose annual compensation shall be in excess of $300,000;
(k) enter into any joint
venture, partnership, limited liability company, or operating agreement with any Person;
(l) enter into any Buyer
Material Contract or breach, modify, amend, or terminate any Buyer Material Contracts (including insurance policies covering Buyer’s
properties or assets), or waive, release, or assign any rights or claims under any Buyer Material Contracts, except as expressly
required or permitted by this Agreement;
(m) voluntarily
incur any Liability or obligation (whether absolute, accrued, contingent or otherwise) in excess of $300,000 individually or $600,000
in the aggregate other than pursuant to the terms of a Company Material Contract;
(n) adopt a plan of
complete or partial liquidation, dissolution, merger, consolidation, restructuring, recapitalization, or other reorganization;
(o) acquire or dispose
of capital stock of any third party or merge or consolidate with any third party;
(p) establish any subsidiary
or enter into any new line of business;
(q) fail to use commercially
reasonable efforts to keep in force insurance policies or replacement or revised policies providing insurance coverage with respect
to its assets, operations and activities in such amount and scope of coverage substantially similar to that which is currently
in effect;
(r) sell,
lease, license, transfer, exchange or swap, mortgage or otherwise pledge or encumber (including securitizations), or otherwise
dispose of any material portion of its properties, assets or rights;
(s) knowingly take any
action that would or is reasonably likely to (i) make any representation or warranty of Buyer contained in this Agreement inaccurate,
(ii) result in any of the conditions in Article VII not being satisfied, or (iii) impair the ability of Buyer to consummate
the Transactions in accordance with the terms of this Agreement; or
(t) authorize, commit,
or agree to take any of the foregoing actions.
5.1.2 Exclusivity; Acquisition
Proposals.
(a) Prior to the earlier
of the termination of this Agreement pursuant to Article X or the Closing, in order to induce Company and Sellers to continue
to commit to expend management time and financial resources in furtherance of the Transactions contemplated hereby, Buyer shall
not, and shall cause its Representatives to not, without the prior written consent of Company, directly or indirectly, (i) solicit,
assist, initiate or facilitate the making, submission or announcement of, or intentionally encourage, any Acquisition Proposal,
(ii) furnish any non-public information regarding Buyer or its Affiliates or their respective businesses, operations, assets, Liabilities,
financial condition, prospects or employees to any Person or group of Persons (other than a Party to this Agreement or their respective
Representatives) in connection with or in response to an Acquisition Proposal, other than as required by applicable Law, (iii)
engage or participate in discussions or negotiations with any Person or group of Persons with respect to, or that could reasonably
be expected to lead to, an Acquisition Proposal, (iv) approve, endorse or recommend, or publicly propose to approve, endorse or
recommend, any Acquisition Proposal, (v) negotiate or enter into any letter of intent, agreement in principle, acquisition
agreement or other similar agreement related to any Acquisition Proposal, or (vi) release any third Person from, or waive any provision
of, any confidentiality agreement to which such Party is a party.
(b) Buyer shall notify
Company and the Sellers’ Representative as promptly as practicable (and in any event three (3) business days) in writing
of the receipt by Buyer or any of its Representatives of (i) any bona fide inquiries, proposals or offers, requests for information
or requests for discussions or negotiations regarding or constituting any Acquisition Proposal or any bona fide inquiries, proposals
or offers, requests for information or requests for discussions or negotiations that could be expected to result in an Acquisition
Proposal, and (ii) any request for non-public information relating to Buyer or its Affiliates in connection with any Acquisition
Proposal, specifying in each case, the material terms and conditions thereof (including a copy thereof if in writing or a written
summary thereof if oral) and the identity of the party making such inquiry, proposal, offer or request for information. Each Party
shall keep the others promptly informed of the status of any such inquiries, proposals, offers or requests for information. Prior
to Closing, Buyer shall, and shall cause its Representatives to, immediately cease and cause to be terminated any solicitations,
discussions or negotiations with any Person with respect to any Acquisition Proposal and shall, and shall direct its Representatives
to, cease and terminate any such solicitations, discussions or negotiations.
5.1.3 Buyer Public Filings
(a) From the date of
this Agreement to the earlier of the Closing Date or the termination of this Agreement in accordance with Article X, Buyer
will keep current and timely file all of the forms, reports, schedules, statements and other documents required to be filed by
Buyer with the SEC, including all necessary amendments and supplements thereto, and otherwise comply in all material respects with
applicable securities Laws (the “Additional SEC Documents”). All such Additional SEC Documents (including any
financial statements or schedules included therein) shall be prepared in all material respects in accordance with either the requirements
of the Securities Act, the Exchange Act and the Sarbanes-Oxley Act, as the case may be, and the rules and regulations promulgated
thereunder. As used in this Section 5.1.3, the term “file” shall be broadly construed to include any manner
in which a document or information is furnished, supplied or otherwise made available to the SEC or Nasdaq. Any Additional SEC
Documents which discuss or refer to this Agreement or the Transactions shall be subject to the prior review and approval of Company
(not to be unreasonably withheld, delayed or conditioned).
(b) From the date of
this Agreement to the earlier of the Closing Date or the termination of this Agreement, Buyer shall promptly notify the Sellers’
Representative of the filing of any Additional SEC Documents.
(c) From the date of
this Agreement to the earlier of the Closing Date or the termination of this Agreement in accordance with Article X, Buyer
shall use its best efforts to maintain the listing of the Buyer Common Stock on the Nasdaq.
5.2 Notification; Access to
Information.
5.2.1 Cooperation.
From the date of this Agreement to the earlier of the Closing Date or the termination of this Agreement in accordance with Article
X, Buyer will (i) confer with Company, Sellers, and their Representatives, at such times as they may request, during normal business
hours and upon reasonable advance notice, about operations and integration matters to the extent permitted by Law, (ii) in the event
of, and promptly after becoming aware of, the occurrence of or the pending or threatened occurrence of any event that would cause or
constitute a material breach of any of the representations and warranties in Section 3.2, Buyer will give detailed written notice
to Company and will use commercially reasonable efforts to prevent or promptly remedy such breach or inaccuracy; (iii) promptly notify
Company if it fails to comply with or satisfy any covenant, condition or agreement to be complied with or satisfied by it or its Affiliates
hereunder in any material respect; and (iv) promptly notify the Sellers’ Representative of any change in the normal course of any
business, operations, or financial condition of the Renovaro Group or their respective Assets or properties, or any emergency related
to the same.
5.2.2 Access to Information.
Buyer will, subject to applicable Law, afford Company and Sellers and their respective Representatives reasonable access during normal
business hours during the period before the Closing to all information concerning the business, properties, and personnel of Buyer, as
Company and any Seller may reasonably request that is necessary to complete the Transactions and prepare for an orderly transition of
operations after the Closing; provided that such access does not disrupt the normal operations of the Renovaro Group and does
not contravene applicable Law.
5.3 Consents and Notices.
Buyer will use its commercially reasonable efforts, and will cooperate with Company and Seller, to promptly (a) apply for or otherwise
seek, and use commercially reasonable efforts to obtain, all consents included in Section 3.2.5 of the Buyer Disclosure Schedule,
including Stockholder Approval, (b) provide all notices included in Section 3.2.5 of the Buyer Disclosure Schedule (if any), and
(c) make all filings required with respect to Buyer for the consummation of the Transactions (if any).
5.4 Commercially Reasonable
Efforts. Buyer will use commercially reasonable efforts to effect the Transactions and to fulfill and
cause to be fulfilled the conditions to Closing under this Agreement, including the satisfaction of the respective conditions set forth
in Article VII, applicable to Buyer, and including to execute and deliver such other instruments and do and perform such other
acts and things as may be reasonably necessary for effecting completely the consummation of the Transactions applicable to Buyer (including
making all filings (if any), giving all notices (if any) required to be made and given by Buyer in connection with the Transactions and
using commercially reasonable efforts to obtain each consent (if any) required to be obtained (pursuant to any applicable Law, Contract
or otherwise) by such Party in connection with the Transactions).
5.5 Proxy Statements; Buyer
Stockholder Meeting.
5.5.1 Proxy Statements
As promptly as practicable after the date of this Agreement and in any event no later than fifteen (15)
days from the delivery of the 2022 Company Financials and the 2023 Company Interim Financials, Buyer shall prepare and file with the
SEC a notice of meeting and preliminary proxy statement (“Notice of Meeting and Preliminary Proxy Statement”) relating
to a meeting of Buyer’s stockholders (the “Buyer Stockholder Meeting”) to be held for the purpose of voting
on the matters requiring Stockholder Approval. As promptly as practicable after filing such Notice of Meeting and Preliminary Proxy Statement,
but in any event subject to the rules and regulations of the SEC, Buyer shall prepare and file with the SEC, and mail to its stockholders
of record as of the close of business on the Record Date (the “Record Stockholders”), a notice of meeting and definitive
proxy statement relating to the Buyer Stockholder Meeting (the “Notice of Meeting and Definitive Proxy Statement”).
The Notice of Meeting and Preliminary Proxy Statement and Notice of Meeting and Definitive Proxy Statement are hereinafter referred to
as the “Proxy Statements.”
5.5.2 Buyer Stockholder
Meeting The Buyer Stockholder Meeting shall be called for a date which, after taking into consideration
the provisions of the Buyer Organizational Documents, the Delaware General Corporation Law,
the rules and regulations of the SEC and the NASDAQ Listing Rules and the recommendations
of any proxy solicitor engaged by Buyer with respect to the Buyer Stockholder Meeting and the Transactions, is as prompt as practicable
after the Notice of Meeting and Definitive Proxy Statement is filed with the SEC and mailed to the Record Stockholders. Buyer shall
consult with Company in fixing the record date for the Buyer Stockholder Meeting (the “Record Date”) and the
date of the Buyer Stockholder Meeting, give notice to Company of the Buyer Stockholder Meeting and allow Company’s Representatives
to attend the Buyer Stockholder Meeting. Buyer shall use its reasonable best efforts to obtain the approval of the holders of Buyer
Common Stock at Buyer Stockholder Meeting, including by soliciting from its shareholders proxies as promptly as possible in favor
of the matters to be approved by the Stockholder Approval, and shall take all other action necessary or advisable to secure the
required vote or consent of its shareholders therefor. Buyer shall provide the Company with updates with respect to the tabulated
vote counts received by Buyer; provided, however, that Buyer shall not be permitted to postpone the Buyer Stockholder Meeting
more than the earlier of (i) ten (10) business days prior to the Outside Date and (ii) ten (10) calendar days from the date of
the first Buyer Stockholder Meeting without the prior written consent of Company (not to be unreasonably withheld, delayed or conditioned),
and (c) the right to review and comment on all communication sent to holders of Buyer Common Stock and/or proxy solicitation firms.
5.5.3 Stockholder Approval
of Amendment If the Buyer’s stockholders approve the matters to be approved by the Stockholder
Approval at the Buyer Stockholder Meeting, then promptly after the Buyer Stockholder Meeting and prior to the Closing, Buyer shall amend
the Buyer’s Organizational Documents in accordance with the amendments contemplated by the Proxy Statements as described in Section
2.3.
5.6 Fairness Opinion.
Buyer will use its commercially reasonable efforts to cause an investment bank to issue to Buyer an opinion to the effect that, as of
the Closing Date, the consideration to be paid to the Sellers pursuant to the terms of this Agreement is fair, from a financial point
of view, to Buyer.
5.7 D&O Policy.
Buyer shall amend the D&O Policy in form reasonably satisfactory to Company in order to provide the members of the Post-Closing Buyer
Board and any new officers of Buyer appointed after the date hereof with at least as favorable terms and scope of coverage as those of
the D&O Policy covering Buyer’s officers and directors on the date hereof.
Article
VI
ADDITIONAL AGREEMENTS
Buyer and Company each agree
to take the following actions after the execution of this Agreement:
6.1 Confidentiality Agreement.
Company and Buyer agree that the Confidential Disclosure Agreement dated July 17, 2023, by and between Gedi Health Group, Ltd. and Buyer
(“Confidentiality Agreement”) will continue in full force and effect and will be applicable to all “Confidential
Information” (as such term is defined in the Confidentiality Agreement) exchanged in connection with this Agreement and the Transactions.
6.2 Expenses.
All costs and expenses incurred in connection with this Agreement and the Transactions will be paid by the Party incurring such cost
or expense; provided, however, that Buyer shall pay for all costs and expenses of the Company in connection with the preparation
and audit of the 2022 Company Financials, including engaging an accounting firm to assist the Company in the preparation of the 2022
Company Financials and the 2023 Interim Company Financials. For the avoidance of doubt, all expenses in connection with the Signing Press
Release, the Signing Filing, the Closing Press Release, the Closing Filing, the Buyer Stockholder Meeting and all Buyer related disclosure
included in the Proxy Statements shall be borne by Buyer.
6.3 Further Assurances.
Prior to Closing, Buyer, Company, and Sellers, as applicable, shall sign and deliver any documents and instruments and take any further
action that is reasonably necessary or desirable to effect the Closing and to carry out the purposes of this Agreement.
6.4 Public Announcements.
6.4.1 Prior Written Consent
The Parties agree that prior to Closing, no public release, filing or announcement concerning this
Agreement or the Transaction Documents or the Transactions contemplated hereby or thereby, including the existence or status thereof,
shall be issued by any Party or any of their respective Affiliates without the prior written consent of Buyer and Company (which consent
shall not be unreasonably withheld, conditioned or delayed), except as such release or announcement may be required by applicable Law
or the rules or regulations of Nasdaq, in which case the applicable Party shall promptly notify Buyer and Company (as applicable)
of such obligations and shall use its commercially reasonable efforts to allow Buyer and Company (as applicable) reasonable time to review
and comment on, and arrange for any required filing with respect to, such release or announcement in advance of such issuance.
6.4.2 Press Releases and Filings
Buyer and Company shall mutually agree upon and, as promptly as practicable after the execution of
this Agreement (but in any event within four (4) business days thereafter), issue a press release announcing the execution of this Agreement
(the “Signing Press Release”). Promptly after the issuance of the Signing Press Release, Buyer shall file a current
report on Form 8-K (the “Signing Filing”) with the Signing Press Release and a description of this Agreement
and the Transaction Documents as required by Federal Securities Laws, which Company shall review, comment upon and approve (which approval
shall not be unreasonably withheld, conditioned or delayed) prior to filing (with Company reviewing, commenting upon and approving such
Signing Filing in any event no later than the third (3rd) business day after the execution of this Agreement, provided that Company receive
a substantially complete draft of the Signing Press Release and Signing Filing no more than one (1) business day after the date of this
Agreement). Buyer, Company and the Sellers’ Representative shall mutually agree upon and, as promptly as practicable after the
Closing (but in any event within four (4) business days thereafter), issue a press release announcing the consummation of the Transactions
contemplated by this Agreement (the “Closing Press Release”). Promptly after the issuance of the
Closing Press Release, Buyer shall file a current report on Form 8-K (the “Closing Filing”) with
the Closing Press Release and a description of the Closing as required by Federal Securities Laws which the Sellers’ Representative
shall review, comment upon and approve (which approval shall not be unreasonably withheld, conditioned or delayed) prior to filing.
In connection with the preparation of the Signing Press Release, the Signing Filing, the Closing Filing, the Closing Press Release,
or any other report, statement, filing notice or application made by or on behalf of a Party to any Governmental Entity or other
third party in connection with the Transactions contemplated hereby, each Party shall, upon request by any other Party, furnish
the Parties with all information concerning themselves, their respective directors, officers and equity holders, and such other
matters as may be reasonably necessary or advisable in connection with the Transactions contemplated hereby, or any other report,
statement, filing, notice or application made by or on behalf of a Party to any third party and/or any Governmental Entity in connection
with the Transactions contemplated hereby.
6.5 Post-Closing Board of Directors.
The Parties shall take all necessary action, including causing certain directors of Buyer to resign, so that effective as of the Closing,
Buyer’s board of directors (the “Post-Closing Buyer Board”) will consist of nine (9) individuals (such persons,
the “Directors”): (i) one (1) of which shall be the Chief Executive Officer of Buyer, (ii) the four (4) persons that
are designated by Buyer prior to the Closing, at least three (3) of whom shall qualify as an “independent director” under
NASDAQ Listing Rule 5605(a), and (iii) the four (4) persons that are designated by Company prior to the Closing, at least two (2) of
whom shall be required to qualify as an “independent director” under NASDAQ Listing Rule 5605(a); provided that the
Post-Closing Buyer Board will meet all diversity and other requirements under applicable Law and the NASDAQ Listing Rules. The board
of directors of Company immediately after the Closing shall be the same as the Post-Closing Buyer Board. At or prior to the Closing,
Buyer will provide each Director with a customary director indemnification agreement, in form and substance reasonably acceptable to
such Director, to be effective upon the Closing (or if later, such Director’s appointment).
6.6 Indemnification of Company’s
Board and Officers The Parties agree that all rights to exculpation, indemnification and advancement
of expenses existing in favor of the current or former directors and officers of Buyer or Company and each Person who served as a director,
officer, member, trustee or fiduciary of another corporation, partnership, joint venture, trust, pension or other employee benefit plan
or enterprise at the request of Buyer or Company (the “D&O Indemnified Persons”) as provided in its Organizational
Documents or under any indemnification, employment or other similar agreements between any D&O Indemnified Person and Buyer or Company,
in each case as in effect on the date of this Agreement, shall survive the Closing and continue in full force and effect in accordance
with their respective terms to the extent permitted by applicable Law. For a period of six (6) years after the Closing, Buyer and Company
shall cause the Organizational Documents of Buyer and Company to contain provisions no less favorable with respect to exculpation and
indemnification of and advancement of expenses to D&O Indemnified Persons than are set forth as of the date of this Agreement in
the Organizational Documents of, as applicable, Buyer or Company to the extent permitted by applicable Law. The provisions of this Section 6.6
shall survive the Closing and are intended to be for the benefit of, and shall be enforceable by, each of the D&O Indemnified
Persons and their respective heirs and representatives.
6.7 Post-Closing Tax
Matters.
6.7.1 Returns Filed.
Following the Closing, Buyer will prepare and timely file, or cause to be prepared and timely filed, any Tax Return with respect
to a Pre-Closing Tax Period with a due date (including any applicable extensions) after the Closing Date (including any Tax Returns
with respect to a Straddle Period). Such Tax Returns will be prepared in a manner consistent with past practice, except to the
extent otherwise required under applicable law. Buyer will provide all such Tax Returns that are income Tax Returns to the Sellers’
Representative for review at least fifteen (15) days prior to the due date for such Tax Returns (including any applicable extensions),
and Buyer will reasonably and in good faith consider any comments made by the Sellers’ Representative before the due date
for such Tax Returns with respect to such Tax Returns that are consistent with the standard set forth in the preceding sentence.
6.7.2 Tax Cooperation.
Each of Buyer and each Seller will cooperate fully, as and to the extent reasonably requested by any of the others, in connection
with the filing of Tax Returns and any Tax Contest with respect to Pre-Closing Tax Periods.
6.7.3 No Amendments.
Following the Closing Date, Buyer will not amend or cause to be amended any Tax Return, make or change any Tax election, agree
to the extension or waiver of the statute of limitations period or take any other action that has the effect of extending the
period of assessment or collection, initiate discussions or examinations with any Governmental Entity, or make any voluntary disclosures,
in each case with respect to Taxes of Company that relates to a Pre-Closing Tax Period, and in each case except (i) as required
by applicable law as mutually agreed by Buyer and Sellers, (ii) with the consent of the Sellers’ Representative, which consent
will not be unreasonably withheld, delayed, or conditioned, or (iii) if the Buyer, in its sole and absolute discretion, makes
or causes to be made any Section 338 Election.
6.7.4 Transfer Taxes.
All Taxes and fees (including any penalties and interest) incurred in connection with the Transactions shall be borne and paid
by the Party to which it accrues under applicable Law.
6.7.5 Election Under
Section 338 of the Code. Each Party hereto acknowledges and agrees that the Buyer, in its sole
and absolute discretion, may (but is not obligated to) make or cause to be made an election under Section 338(g) of the Code (and
any comparable provision of applicable U.S. state or local Tax Law) with respect to the purchase of the equity interests of one
or more of the Target Companies and if the Buyer so makes, or causes to be made, such an election, each party hereto shall cooperate
with each other and take all actions necessary and appropriate (including filing such additional forms, returns, elections schedules
and other documents as may be required) to effect and preserve a timely election, in accordance with Section 338(a) of the Code
and the applicable Treasury Regulations promulgated thereunder (each such election, a “Section 338 Election”).
6.8 Efforts to Consummate;
Regulatory Matters and Approvals.
6.8.1 Defense of Proceedings.
Except as otherwise provided in this Agreement, each of the Parties agrees to use its commercially reasonable efforts to defend
against any Proceedings challenging this Agreement or the consummation of the Transactions, seeking to have any preliminary injunction,
temporary restraining order, stay or other legal restraint or prohibition entered or imposed by any court or other Governmental
Entity that is not yet final and nonappealable vacated or reversed, and executing any additional instruments reasonably requested
by another Party (without cost or expense to the executing Party) necessary to carry out the Transactions and to fully carry out
the purposes of this Agreement.
6.9 Securities Laws
Compliance. Buyer covenants to take all actions, make all filings and transmit all documents
required under all Federal Securities Laws and state securities laws and regulations with respect to the Transactions contemplated
by this Agreement, the Transaction Documents and all related agreements.
6.10 Delivery of Disclosure
Schedules. Buyer shall deliver the Buyer Disclosure Schedules and Exhibit 2.2.1 to Sellers
and Company, and Sellers and Company shall deliver the Company Disclosure Schedules to Buyer, within ten (10) Business Days from
the date of this Agreement.
6.11 Additional Sellers.
Company and the Signing Sellers shall use commercially reasonable efforts to cause all other holders of Company Ordinary Shares
to become Parties to this Agreement as a “Joining Seller” by executing and delivering to Buyer and Company (i) a Seller
Joinder to this Agreement, which joinder is accepted in writing and executed and delivered by Buyer and Company, and (ii) any
Transaction Documents which such transferee would have been required to be a party or bound if such transferee were a Seller on
the date of this Agreement. Notwithstanding anything to the contrary in this Agreement, the Parties shall make any appropriate
adjustments to Exhibit B and each Seller’s Pro Rata Share and Earnout Stock to account for any such new Seller.
6.12 Seller Registration
Rights Agreement. Sellers and Buyer shall enter into a Registration Rights Agreement, in the
form attached as Exhibit C hereto (the “Seller Registration Rights Agreement”), which Seller Registration
Rights Agreement shall become effective as of the Closing.
6.13 Issuance of Company
Ordinary Shares to Employee. Immediately prior to Closing, pursuant to its contractual obligation,
Company shall (i) issue to an employee that number of Company Ordinary Shares required to be issued pursuant to such contractual
obligation, and (ii) use commercially reasonable efforts to cause such employee to become a Joining Seller. Upon the exchange
of Company Shares for Exchange Shares pursuant to Section 2.2, Buyer shall cause any reverse vesting, forfeiture provision
or any other restrictions applicable to such Company Ordinary Shares issued to such employee prior to Closing to apply to such
Exchange Shares received by such employee.
Article
VII
CONDITIONS PRECEDENT
7.1 Conditions to Each
Party’s Obligation to Effect the Transactions. The respective obligations of each Party
to consummate the Transactions are subject to the satisfaction, or to the extent permitted by applicable law, the written waiver
at or before Closing, of each of the following conditions:
7.1.1 Buyer
Stockholder Approval. Buyer shall have received Stockholder Approval at the Buyer
Stockholder Meeting in accordance with the Proxy Statements.
7.1.2 Consents.
Each Consent listed on Section 3.1.4 of the Company Disclosure Schedule (if any) and Section 3.2.5 of the Buyer
Disclosure Schedule will have been obtained or provided, other than such Consents (a) as Buyer and Company agree Company will
not seek to obtain, or (b) the failure of which to obtain would not result, or reasonably be expected to result, individually
or in the aggregate, in a Company Material Adverse Effect, Buyer Material Adverse Effect, or as a result of the Transactions,
a Buyer Material Adverse Effect or Company Material Adverse Effect.
7.1.3 No Order.
No Governmental Entity of competent jurisdiction will have enacted, issued, promulgated, enforced, or entered any statute, rule,
regulation, executive order, decree, injunction, or other Order (whether temporary, preliminary, or permanent) that (a) is in
effect and (b) has the effect of making the Transactions illegal or otherwise prohibiting consummation of the Transactions (which
illegality or prohibition would have a Company Material Adverse Effect or Buyer Material Adverse Effect if the Transactions were
consummated).
7.1.4 Nasdaq Listing.
Buyer shall have received evidence reasonably satisfactory to Buyer, the Sellers’ Representative and Company that immediately
after the Closing and after giving effect to the issuance of the Exchange Shares and any of the other Transactions contemplated
by this Agreement, the shares of Buyer Common Stock shall remain listed on Nasdaq.
7.2 Conditions to Obligations
of Buyer. In addition to the conditions specified in Section 7.1, the obligations
of Buyer to consummate the Share Exchange and the other Transactions contemplated by this Agreement are subject to the satisfaction
or written waiver (by Buyer) of the following conditions:
7.2.1 Representations
and Warranties of Company and Sellers.
(a) The representations
and warranties of Company and Sellers contained in Section 3.1.1, Section 3.1.2, Section 3.1.3, Section 3.1.4(a), Section 3.1.17,
Section 3.1.18 and Section 3.1.20 (the “Company Fundamental Representations”) shall be true and correct in all
respects as of the Closing Date with the same force and effect as if made on the Closing Date (except to the extent that any such
representation and warranty speaks as of an earlier date, in which case such representation and warranty shall be true and correct
in all respects as of such date). All other representations and warranties of Company and Sellers in this Agreement shall be true
and correct in all respects as of the Closing Date with the same force and effect as if made on the Closing Date (except to the
extent that any such representation and warranty speaks as of an earlier date, in which case such representation and warranty shall
be true and correct in all respects as of such date), except where the failure to be true and correct (without regard to any materiality
or Company Material Adverse Effect qualifiers set forth therein) would not, individually or in the aggregate, reasonably be expected
to have a Company Material Adverse Effect.
(b) The representations
and warranties of Sellers contained in Section 3.3.1, Section 3.3.2(a), Section 3.3.2(b), and Section 3.3.2(c) (the “Seller
Fundamental Representations”) shall be true and correct in all respects as of the Closing Date with the same force and
effect as if made on the Closing Date (except to the extent that any such representation and warranty speaks as of an earlier date,
in which case such representation and warranty shall be true and correct in all respects as of such date). All other representations
and warranties of Sellers in this Agreement shall be true and correct in all respects as of the Closing Date with the same force
and effect as if made on the Closing Date (except to the extent that any such representation and warranty speaks as of an earlier
date, in which case such representation and warranty shall be true and correct in all respects as of such date), except where the
failure to be true and correct (without regard to any materiality or Company Material Adverse Effect qualifiers set forth therein)
would not, individually or in the aggregate, reasonably be expected to have a Company Material Adverse Effect.
7.2.2 Agreements and
Covenants. Company and Sellers shall have performed in all material respects all of their respective
obligations and complied in all material respects with all of their respective agreements and covenants under this Agreement to
be performed or complied with by them on or prior to the Closing Date.
7.2.3 No Company Material
Adverse Effect. No Company Material Adverse Effect shall have occurred with respect to the Target
Companies since the date of this Agreement which is continuing and uncured.
7.2.4 Return of All
Distributions. All distributions made by any Target Company to any Affiliate since August 1,
2023, shall have been repaid to the Target Company.
7.2.5 Certain Transaction
Documents. Each (i) Seller shall have duly executed and delivered to Buyer the Seller Registration
Rights Agreement, and (ii) Seller that Buyer and Company mutually agree upon, in good faith, that is to be a party to a non-competition
agreement shall have duly executed and delivered to Buyer a non-competition agreement.
7.2.6 Seller’s
and Company’s Closing Deliverables(a) Officer’s Certificate. Buyer will
have received from Company a certificate, dated the Closing Date, signed by an executive officer of Company certifying as to the
Company’s satisfaction of the conditions specified in Sections 7.2.1(a), 7.2.2, 7.2.3 and 7.2.4.
(b) Sellers’
Certificates. Buyer will have received a certificate from each Seller, dated the Closing Date, signed by such Seller, certifying
as to such Seller’s satisfaction of the conditions specified in Sections 7.2.1(b), and 7.2.2.
(c) Secretary’s
Certificate. Company shall have delivered to Buyer a certificate from its secretary or other executive officer certifying as
to the validity and effectiveness of, and attaching, (A) copies of Company’s Organizational Documents as in effect as of
the Closing Date (immediately prior to the Closing), (B) the requisite resolutions of Company’s board of directors authorizing
and approving the execution, delivery and performance of this Agreement and each of the Transaction Documents to which Company
is a party or by which it is bound, and the consummation of the Transactions contemplated hereby and thereby, and (C) the
incumbency of officers of Company authorized to execute this Agreement or any Transaction Document to which Company is or is required
to be a party or otherwise bound.
(d) Good Standing.
Company shall have delivered to Buyer good standing certificates (or similar documents applicable for such jurisdictions, in the
case of the Netherlands, such similar document being an excerpt of the Trade Register of the Dutch Chamber of Commerce) for each
Target Company certified as of a date no earlier than thirty (30) days prior to the Closing Date from the proper Governmental Entity
of the Target Company’s jurisdiction of organization, in each case to the extent that good standing certificates or similar
documents are generally available in such jurisdictions.
(e) Share Certificates
and Transfer Instruments. Buyer shall have received from each Seller share certificates representing the Company Shares (or
duly executed affidavits of lost share certificates in form and substance reasonably acceptable to Buyer and consistent with the
Laws of England and Wales), if applicable, together with executed instruments of transfer in respect of Company Shares in favor
of Buyer and in form reasonably acceptable for transfer on the books of Company.
7.2.7 Legal Action.
Since the date of this Agreement, there will not be pending or threatened any Proceeding (a) challenging or seeking to restrain
or prohibit the consummation of the Transactions, or seeking to obtain any material damages, or any award of attorney fees in
connection with the Transactions; or (b) seeking to prohibit or impose any material limitations on Buyer’s ownership or
operation of all or any portion of any Target Company’s respective businesses, or to compel Buyer to dispose of or hold
separate all or any material portion of the assets of Target Company as a result of the Transactions.
7.2.8 Financial Statements.
Company shall have delivered to Buyer the 2022 Company Financials and 2023 Interim Company Financials in the form required for
Buyer’s independent public accounting firm to prepare financial statements in accordance with Buyer’s filing requirements
with the SEC in respect to the Transactions.
7.2.9 Fairness Opinion.
The board of directors of Buyer shall have received an opinion from an investment bank to the effect that, as of the Closing Date,
the consideration to be paid to the Sellers pursuant to the terms of this Agreement is fair, from a financial point of view, to
Buyer.
7.2.10 Termination of
Certain Agreements. All agreements listed in Section 7.2.10 of the Company Disclosure
Schedule (if any) will have been terminated by the parties thereto, if any.
7.2.11 Joinder to this
Agreements. All holders of Company Ordinary Shares immediately prior to the Closing shall have
become Parties to this Agreement as a “Signing Seller” or a “Joining Seller” by executing and delivering
to Buyer and Company a Seller Joinder to this Agreement so that immediately following the Closing, Buyer shall be the sole holder
of Company Ordinary Shares.
7.3 Conditions to Obligation
of Company and Sellers. In addition to the conditions specified in Section 7.1,
the obligations of Company and Sellers to consummate the Share Exchange and the other Transactions contemplated by this Agreement
are subject to the satisfaction or written waiver (by Company) of the following conditions:
7.3.1 Representations
and Warranties of Buyer. The representations and warranties of Buyer contained in Section 3.2.1,
Section 3.2.2, Section 3.2.3, Section 3.2.4, Section 3.2.5(a) and Section 3.2.20 (the “Buyer Fundamental Representations”)
shall be true and correct in all respects as of the Closing Date with the same force and effect as if made on the Closing Date,
except to the extent that any such representation and warranty speaks as of an earlier date, in which case such representation
and warranty shall be true and correct in all respects as of such date. All other representations and warranties of Buyer in this
Agreement shall be true and correct in all respects as of the Closing Date with the same force and effect as if made on the Closing
Date (except to the extent that any such representation and warranty speaks as of an earlier date, in which case such representation
and warranty shall be true and correct in all respects as of such date), except where the failure to be true and correct (without
regard to any materiality or Buyer Material Adverse Effect qualifiers set forth therein) would not, individually or in the aggregate,
reasonably be expected to have a Buyer Material Adverse Effect.
7.3.2 Agreements and
Covenants. Buyer shall have performed, in all material respects, all of Buyer’s obligations
and complied, in all material respects, with all of Buyer’s agreements and covenants under this Agreement to be performed
or complied with by it on or prior to the Closing Date.
7.3.3 No Buyer
Material Adverse Effect. No Buyer Material Adverse Effect shall have occurred with respect
to Buyer since the date of this Agreement which is continuing and uncured.
7.3.4 Certain Transaction
Documents. Buyer shall have duly executed and delivered
to the Sellers’ Representative: (i) the Seller Registration Rights Agreement, and (ii) each non-competition agreement.
7.3.5 Buyer Organizational
Documents. Buyer shall have amended its Organizational
Documents to reflect the Stockholder Approval as described in Section 2.3.
7.3.6 Appointment to
the Board. The members of the Post-Closing Buyer Board shall have been elected or appointed
as of the Closing consistent with the requirements of Section 6.5.
7.3.7 Buyer Closing
Deliverables.
Officer’s Certificate.
Company will have received from Buyer a certificate, dated the Closing Date, signed by an executive officer of Buyer certifying
as to the satisfaction of the conditions specified in Sections 7.3.1, 7.3.2 and 7.3.3.
Secretary’s Certificate.
Buyer shall have delivered to Company a certificate from its secretary or other executive officer certifying as to, and attaching,
(A) copies of the Buyer’s Organizational Documents as in effect as of the Closing Date, (B) the resolutions of Buyer’s
board of directors authorizing and approving the execution, delivery and performance of this Agreement and each of the Transaction
Documents to which it is a party or by which it is bound, and the consummation of the transactions contemplated hereby and thereby,
(C) evidence that the Stockholder Approval has been obtained and (D) the incumbency of officers authorized to execute this Agreement
or any Transaction Document to which Buyer is or is required to be a party or otherwise bound.
Good Standing.
Buyer shall have delivered to Company a good standing certificate (or similar documents applicable for such jurisdictions) for
Buyer certified as of a date no earlier than thirty (30) days prior to the Closing Date from the proper Governmental Entity of
Buyer’s jurisdiction of organization.
(d) Exchange Shares.
Buyer shall have delivered to each Seller, such Seller’s Exchange Shares, in book entry form, in the name of such Seller,
and promptly thereafter on the Closing Date a copy of the records of Securities Transfer Corporation, transfer agent for Buyer,
showing such Seller as the owner of such Exchange Shares.
7.4 Frustrations of
Conditions. Notwithstanding anything contained herein to
the contrary, no Party may rely on the failure of any condition set forth in this Article VII to be satisfied if such failure
was caused by the failure of such Party or its Affiliates (or with respect to Company, any Target Company or any Seller) failure
to comply with or perform any of its covenants or obligations set forth in this Agreement.
Article
VIII
INDEMNIFICATION
8.1 Indemnification
by Sellers. Subject to the limitations in this Article
VIII, if the Closing of the Transactions occurs, from and after Closing, each Seller (the “Indemnifying Seller”)
will defend, indemnify, and hold Buyer and its respective officers, directors, agents, consultants, advisors, Representatives
and equity holders (each of the foregoing being referred to individually as a “Buyer Indemnified Person” and
collectively as “Buyer Indemnified Persons”) harmless from and reimburse Buyer (on behalf of Buyer Indemnified
Persons), without duplication, for all documented and out-of-pocket costs or expenses (including without limitation, reasonable
attorney’s fees), judgments, levies, losses, damages, fines, Liens, Taxes and penalties, except for any punitive, consequential,
indirect or exemplary damages (except, in each case, to the extent reasonably foreseeable) or losses or damages on account of
loss of future opportunities (collectively, “Losses”), incurred by Buyer Indemnified Persons arising out of,
relating to or resulting from any of the following:
(a) any inaccuracy in
or breach of any representation or warranty of Company or Sellers contained in Section 3.1 of this Agreement (as modified
by the Company Disclosure Schedule);
(b) any breach or non-fulfillment
of any covenant, agreement, or obligation to be performed by such Seller under this Agreement (other than as a direct result of
the failure of Buyer to perform its agreements under this Agreement);
(c) any inaccuracy in
or breach of any representation or warranty of such Seller contained in Section 3.3 of this Agreement (as modified by the
Company Disclosure Schedule);
(d) any breach or non-fulfillment
of any covenant, agreement, or obligation to be performed on or prior to Closing by the Company under this Agreement (other than
as a direct result of the failure of Buyer to perform its agreements under this Agreement);
(e) any claims by (A)
any then current or former holder or alleged then-current or former holder of any Company Securities, arising out of, resulting
from or in connection with (I) the Transactions or this Agreement, or (II) such Persons status or alleged status as a holder of
Company Securities at any time at or prior to the Closing, whether for breach of fiduciary duty or otherwise or (B) any Person
to the effect that such Person is entitled to any Company Securities or any payment in connection with the Transactions by virtue
of such Company Securities; and
(f) any Pre-Closing
Taxes;
provided, that (i) and in the case of
Sections 8.1(a), (b), (c) or (d) above, without giving effect to any “materiality” limitations
or references to “Company Material Adverse Effect” in determining Losses (but not in determining whether any breaches
of representations and warranties have occurred); and (ii) each Seller shall only be liable to Buyer Indemnified Persons for indemnification
under Sections 8.1(a), (d) and (e) above in proportion to such Seller’s Pro Rata Share of such Losses.
Notwithstanding the foregoing, in no event shall any Indemnifying Seller have any liability to a Buyer Indemnified Person with
respect to a breach of representation, warranty or covenant under this Agreement to the extent that Buyer knew of such breach as
of the Closing Date.
8.2 Indemnification
by Buyer. Subject to the limitations in this Article
VIII, if the Closing of the Transactions occurs, from and after Closing, Buyer will defend, indemnify, and hold each Seller
and its respective officers, directors, agents, consultants, advisors, Representatives, equity holders, and successors and assigns
(each of the foregoing being referred to individually as a “Seller Indemnified Person” and collectively as
“Seller Indemnified Persons”, and together with Buyer Indemnified Persons, “Indemnified Persons”)
harmless from and reimburse Seller Indemnified Persons, without duplication, for all Losses incurred by Sellers Indemnified Persons
arising out of, relating to or resulting from any of the following:
(a) any inaccuracy in
or breach of any representation or warranty of Buyer contained in Section 3.2 of this Agreement (as modified by the Buyer
Disclosure Schedule);
(b) any breach or non-fulfillment
of any covenant, agreement, or obligation to be performed by Buyer or the Renovaro Group members under this Agreement (other than
as a direct result of the failure of Company or Seller to perform its agreements under this Agreement);
(c) any breach or non-fulfillment
of any covenant, agreement, or obligation to be performed following the Closing by the Company under this Agreement (other than
as a direct result of the failure of Seller to perform its agreements under this Agreement);
(d) any claims by (A)
any then current or former holder of any Buyer equity interests or Convertible Security, arising out of, resulting from or in connection
with the Transactions or this Agreement or (B) any Person to the effect that such Person is entitled to any Buyer equity interests
or Convertible Securities or any payment in connection with the Transactions by virtue of such Buyer equity interests or Convertible
Securities;
(e) any action, claim
or Proceeding arising out of or relating to Serhat Gumrukcu, William Anderson Wittekind, their Affiliates, or their relationship
with Buyer or the other Renovaro Group members as current or former stockholders;
(f) any legal Proceeding
relating to any inaccuracy, breach, claim or expense of the type referred to in the preceding clauses (a) through (e) (including,
without limitation, any legal Proceeding commenced by a Seller for the purpose of enforcing its rights under this Article
VIII if Seller is the prevailing party in any such legal Proceeding);
provided, that in the case of Sections
8.2(a), (b) or (c) above, without giving effect to any “materiality” limitations or references to
“Buyer Material Adverse Effect” in determining Losses (but not in determining whether any breaches of representations
and warranties have occurred). Notwithstanding the foregoing, in no event shall Buyer have any liability to a Seller Indemnified
Person with respect to a breach of representation, warranty or covenant under this Agreement to the extent that Sellers knew of
such breach as of the Closing Date.
8.3 Third Party Claims.
(a) Whenever an Indemnified
Person receives a written notice that a claim or demand has been asserted or threatened by any Person who is not a Party to this
Agreement for which such Indemnified Person may seek indemnification under Article VIII of this Agreement (other than claims
or demands covered by Section 8.4) (a “Third Party Claim”), the Indemnified Person shall notify the Indemnifying
Party of such Third Party Claim and of the related facts within the Indemnified Person’s knowledge within a reasonable time
after receiving such written notice; provided that no delay on the part of the Indemnified Person in notifying the Indemnifying
Party will relieve the Indemnifying Party from any obligation under this Article VIII, except and only to the extent such
delay actually prejudices the Indemnifying Party.
(b) The Indemnifying
Party shall have the right to participate in the defense of any Third Party Claim that is the subject of a notice given by or on
behalf of any Indemnified Person pursuant to this Section 8.3. In addition, the Indemnifying Party will have the right to
defend the Indemnified Person against the Third Party Claim with counsel of its choice reasonably satisfactory to the Indemnified
Person so long as (i) the Indemnifying Party gives written notice to the Indemnified Person within fifteen (15) business days after
the Indemnified Person has given notice of the Third Party Claim under to this Section 8.3 stating that it has elected to
assume the defense of such Third Party Claim, (ii) the Indemnifying Party provides the Indemnified Person with evidence reasonably
acceptable to the Indemnified Person that the Indemnifying Party will have adequate financial resources to defend against the Third
Party Claim, (iii) the Third Party Claim involves only money damages and does not seek an injunction or other equitable relief
against the Indemnified Person, (iv) the Indemnified Person has not been advised by counsel that an actual or probable conflict
exists between the Indemnified Person and the Indemnifying Party in connection with the defense of the Third Party Claim, (v) the
Third Party Claim does not relate to or otherwise arise in connection with any criminal or regulatory enforcement Proceeding and
(vi) the Indemnifying Party conducts the defense of the Third Party Claim actively and diligently at its expense. In the event
that the Indemnifying Party assumes the defense of any Third Party Claim, subject to Section 8.3(c), it shall have the right
to take such action as it deems necessary to avoid, dispute, defend, appeal or make counterclaims pertaining to any such Third
Party Claim in the name and on behalf of the Indemnified Person. The Indemnified Person may retain separate co-counsel at its sole
cost and expense and participate in the defense of the Third Party Claim; provided that the Indemnifying Party will pay
the fees and expenses of separate counsel retained by the Indemnified Person that are incurred prior to the Indemnifying Party’s
assumption of control of the defense of the Third Party Claim.
(c) Notwithstanding
any other provision of this Agreement, the Indemnifying Party will not consent to the entry of any judgment or enter into any compromise
or settlement with respect to the Third Party Claim without the prior written consent of the Indemnified Person unless such judgment,
compromise or settlement (i) provides for the payment by the Indemnifying Party of money as sole relief for the claimant, (ii)
results in the full and general release of all Indemnified Persons from all liabilities arising or relating to, or in connection
with, the Third Party Claim from the Person with which such settlement is made and (iii) involves no finding or admission of any
violation of Laws or the rights of any Persons.
(d) If the Indemnifying
Party elects not to compromise or defend such Third Party Claim, fails to promptly notify the Indemnified Person in writing of
its election to defend as provided in this Agreement, or otherwise at any time fails to conduct the defense of such Third Party
Claim actively and diligently, the Indemnified Person may defend the Third Party Claim and may consent to the entry of any judgment
or enter into any compromise or settlement with respect to, the Third Party Claim in any manner it may deem appropriate. The Indemnifying
Party shall remain responsible for any Losses of the Indemnified Person the Indemnifying Party would be responsible for pursuant
to this Article VIII in the event that (i) the Indemnifying Party does not elect to defend such Third Party Claim pursuant
to Section 8.3(b) or (ii) the Indemnifying Party and the Indemnified Person are parties to or subject to such Third Party
Claim and conflicts of interest exist between the Indemnified Person and the Indemnifying Party.
8.4 Tax Contests.
Notwithstanding anything to the contrary in this Agreement (including Section 8.3), following the Closing Date, Buyer will
have the right, in Buyer’s sole and absolute discretion, to conduct and control, through counsel of Buyer’s choosing,
the defense of any Tax Contest; provided, however, that to the extent that any Tax Contest could reasonably give rise to
an indemnification claim by Buyer under Article VIII Buyer will (i) provide notice of such Tax Contest to the Sellers’
Representative within thirty (30) days after receiving written notice of the commencement of such Tax Contest from the relevant
Governmental Entity (provided that any failure by Buyer to provide such notice to the Sellers’ Representative within such
period will not relieve Sellers of any obligation or liability to Buyer, except and only to the extent such delay actually prejudices
the Sellers), (ii) provide to the Sellers’ Representative all information reasonably requested by the Sellers’ Representative
regarding such Tax Contest, (iii) permit Sellers to evaluate and comment on such Tax Contest at Sellers’ sole expense, and
(iv) reasonably and in good faith consider any such comments of the Sellers’ Representative. Buyer may settle, adjust, or
compromise any such Tax Contest, in Buyer’s sole and absolute discretion, without the consent of Sellers. In the event that
Sellers consent in writing to any settlement, adjustment, or compromise of any Tax Contest, Sellers will not have any power or
authority to object under any provision of Article VIII to the amount of any claim by Buyer for indemnification under Article
VIII with respect to such settlement, adjustment, or compromise.
8.5 Survival.
All representations, warranties, covenants, and agreements set forth in this Agreement
will survive the Closing, subject to the following:
(a) (i) The Company
Fundamental Representations and the Seller Fundamental Representations shall survive the Closing until the date that is six (6)
years from the Closing Date; (ii) the representations and warranties of the Sellers contained in Section 3.1.16 (Tax Matters)
shall survive until 90 days after the expiration of the applicable statute of limitations; and (iii) all other representations
and warranties of the Company and Sellers contained in Section 3.1 and Section 3.3 shall survive the Closing until
the date that is twenty four (24) months from the Closing Date.
(b) (i) The Buyer Fundamental
Representations shall survive the Closing until the date that is six (6) years from the Closing Date; (ii) the representations
and warranties of the Buyer contained in Section 3.2.17 (Tax Returns and Payments) shall survive until 90 days after the
expiration of the applicable statute of limitations; and (iii) all other representations and warranties of Buyer contained in Section
3.2 shall survive the Closing until the date that is twenty four (24) months from the Closing Date.
(c) The covenants and
other agreements of the Parties set forth herein (other than the covenants which by their terms are to be performed prior to the
Closing) shall survive the Closing until the date that is five (5) years from the Closing Date or for the period explicitly specified
therein.
(d) Notwithstanding
anything in this Section 8.5 to the contrary, in the event a Notice of Claim is asserted by the Buyer Indemnified Persons
or the Seller Indemnified Persons related to or arising out of an inaccuracy or breach in any representation, warranty, covenant
or claim during the time periods provided for in Sections 8.5(a), (b), and (c), such representation, warranty,
covenant or claim will continue to survive until such matter has been resolved by settlement, litigation (including all appeals
related thereto), arbitration or otherwise. For purposes of clarity, only a Notice of Claim needs to be given during the applicable
time period, without a requirement to commence Proceedings.
(e) Notwithstanding
anything in this Section 8.5 to the contrary, in the event a claim is asserted by a Buyer Indemnified Person or Seller Indemnified
Person related to or arising out of an inaccuracy or breach in any representation or warranty that results from or constitutes
Fraud by Sellers or Buyer, respectively, such representation or warranty will survive without any time limitation.
8.6 Limitations.
Indemnified Persons will be entitled to indemnification under Article VIII only
if the following conditions included in this Section 8.6 are met.
8.6.1 Threshold Amounts.
(a) Sellers
shall not be liable for any Losses pursuant to Section 8.1 (a)
or Section 8.1 (c) which, individually
considered, are lower than an amount equal to $250,000 (the “Threshold Amount”). Any Losses pursuant to Section
8.1 (a) or Section
8.1 (c) not exceeding the Threshold Amount shall
be considered non-indemnifiable Losses under this Agreement; provided, however, that a series of claims or multiple claims
for Losses arising out of the same or substantially the same set of facts or circumstances shall be deemed to have arisen from
a single event subject to indemnification for purposes of determining the foregoing Threshold Amount. Notwithstanding anything
contained in this Agreement to the contrary, the Threshold Amount limitation shall not apply to (i) claims for Losses made by the
Buyer Indemnified Persons pursuant to any of the Company Fundamental Representations or Seller Fundamental Representations, (ii)
claims for Losses made by the Buyer Indemnified Persons pursuant to Section 8.1
(b), Section 8.1 (d)
and Section 8.1 (e), and (iii)
claims for Losses arising out of Fraud.
(b) Buyer
shall not be liable for any Losses pursuant to Section 8.2 (a)
which, individually considered, are lower than the Threshold Amount. Any Losses pursuant to Section 8.2
(a) not exceeding the Threshold Amount shall be considered non-indemnifiable Losses under
this Agreement; provided, however, that a series of claims or multiple claims for Losses arising out of the same or substantially
the same set of facts or circumstances shall be deemed to have arisen from a single event subject to indemnification for purposes
of determining the foregoing Threshold Amount. Notwithstanding anything contained in this Agreement to the contrary, the Threshold
Amount limitation shall not apply to (i) claims for Losses made by the Seller Indemnified Persons pursuant to any of the Buyer
Fundamental Representations, (ii) claims for Losses made by the Seller Indemnified Persons pursuant to Section 8.2
(b), Section 8.2 (c),
Section 8.2 (d), Section
8.2 (e) and Section 8.2
(e), and (iii) claims for Losses arising out of Fraud.
8.6.2 Caps.
(a) The Indemnifying
Sellers shall always have the right to satisfy any claim for indemnification hereunder with shares of Buyer Common Stock issued
to it hereunder, and other than in respect of Fraud, shall never be required to make a cash payment to satisfy the indemnification
claims, unless the Indemnifying Seller so elects. Notwithstanding anything herein to the contrary, other than in respect of Fraud:
(i) the
maximum aggregate amount of indemnifiable Losses which may be recovered from any Seller for indemnification by the Buyer Indemnified
Persons pursuant Section 8.1(a) and Section 8.1(c) shall be an amount equal to such Seller’s Pro Rata Share
of fifteen percent (15%) of the Exchange Consideration received at Closing, calculated as of the Closing Date in accordance with
Section 8.8 (the “General Cap”); provided, however, that the General Cap will not apply to claims
for Losses made by the Buyer Indemnified Persons for any breaches of, or inaccuracies in, any of the Company Fundamental Representations,
Company Special Representations or the Seller Fundamental Representations;
(ii) the
maximum aggregate amount of indemnifiable Losses which may be recovered from any Seller for indemnification by the Buyer Indemnified
Persons pursuant any breach or inaccuracy in any of the Company Special Representations shall be an amount equal to such Seller’s
Pro Rata Share of fifty percent (50%) of the Exchange Consideration received at Closing, calculated as of the Closing Date in accordance
with Section 8.8 (the “Special Cap”); provided, however, that Sellers’ liability for Losses
under Section 8.1(a) and Section 8.1(c) (excluding for any breaches of, or inaccuracies in, any of the Company Fundamental
Representations or the Seller Fundamental Representations) collectively, shall not exceed the Special Cap in the aggregate; and
(iii) no
Seller shall have any Liability under Section 8.1 in excess of a maximum aggregate amount equal to such Seller’s Pro
Rata Share of the Exchange Consideration received at Closing, calculated as of the Closing Date in accordance with Section 8.8.
(b) Notwithstanding
anything herein to the contrary, other than in respect of Fraud:
(i) the
maximum aggregate amount of indemnifiable Losses which may be recovered from Buyer for indemnification by the Seller Indemnified
Persons pursuant Section 8.2(a) shall be an amount equal to General Cap; provided, however, that the General Cap
will not apply to claims for Losses made by the Seller Indemnified Persons for any breaches of, or inaccuracies in, any of the
Buyer Special Representations or Buyer Fundamental Representations;
(ii) the
maximum aggregate amount of indemnifiable Losses which may be recovered from Buyer for indemnification by the Seller Indemnified
Persons pursuant any breach or inaccuracy in any of the Buyer Special Representations shall be an amount equal to the Special Cap;
provided, however, that Buyer’s liability for Losses under Section 8.2(a) (excluding for any breaches of, or inaccuracies
in, any of the Buyer Fundamental Representations) shall not exceed the Special Cap in the aggregate; and
(iii) Buyer
shall not have any Liability under Section 8.2 in excess of a maximum aggregate amount equal to the Exchange Consideration
received at Closing, calculated as of the Closing Date in accordance with Section 8.8.
(c) Notwithstanding
anything herein to the contrary, no limitations on indemnification set forth in this Article VIII, including with respect to time
limits, the Threshold Amount, the General Cap and the Special Cap shall apply to any claims for Losses arising out of Fraud.
8.7 Exclusive Remedy.
Subject to Section 11.10, with the exception of claims based upon Fraud,
the Parties acknowledge and agree that their sole and exclusive remedy with respect to claims for any breach of any representation,
warranty, covenant or agreement set forth herein or otherwise relating to the subject matter of this Agreement from and after
the Closing Date shall be pursuant to the indemnification provisions set forth in this Article VIII. In furtherance of
the foregoing, subject to Section 11.10, each Party hereby waives, to the fullest extent permitted under Law, any
and all rights, claims and causes of action for any breach of any representation, warranty, covenant or agreement set forth herein
that it may have against the other Parties hereto arising under or based upon any Law, except pursuant to the indemnification
provisions set forth in this Article VIII. Notwithstanding the foregoing, nothing in this Section 8.7 or elsewhere
in this Agreement will affect an Indemnified Person’s right to equitable remedies to the extent available or to seek any
remedy on account of Fraud.
8.8 Value of Shares.
If a Buyer Indemnified Person makes a claim directly against the Indemnifying Seller
for indemnifiable Losses under this Article VIII, the Indemnifying Seller may elect, at the Indemnifying Sellers sole and
absolute discretion, to satisfy such claim with cash or Buyer Common Stock beneficially owned by the Indemnifying Seller. To the
extent any claim for indemnifiable Losses under this Article VIII is to be satisfied by the return and cancellation of
any Buyer Common Stock paid to the Indemnifying Seller, the per share value of any such Buyer Common Stock at the time of satisfaction,
release and cancellation shall be an amount in U.S. dollars, equal to the Buyer Common Stock’s VWAP for the period of thirty
(30) consecutive trading days ending on the trading day immediately prior to such date of payment. The Buyer Indemnified Persons’
sole recourse against the Indemnifying Seller in respect of any finally resolved indemnification obligations of the Indemnifying
Sellers shall be to seek return and cancellation of a portion of the Buyer Common Stock then held by the Indemnifying Seller,
pursuant to the pricing terms and subject to the limitations of this Article VIII. Notwithstanding the immediately preceding
sentence to the contrary, if an Indemnifying Seller, at the time of payment of a claim for which it is liable hereunder, does
not hold a sufficient number of shares to satisfy the claim because it has previously sold shares of Buyer Common Stock acquired
hereunder, then such Indemnifying Seller may be liable hereunder in cash for an amount up to the lesser of (i) the net proceeds
actually received by him for the sale of its shares of Buyer Common Stock acquired hereunder and (ii) the amounts remaining under
the General Cap or Special Cap to the extent applicable to such indemnifiable claim.
8.9 Losses Net of Insurance
Coverage. In calculating amounts payable to an Indemnified
Person, the amount of any indemnified Losses shall be computed net of (i) payments actually recovered by such Person under any
insurance policy, with respect to such Losses, less any increase in the corresponding premium, and (ii) any prior recovery by
such Person from any third party with respect to such Losses.
8.10 No Duplicative
Recovery. Where substantially the same events or circumstances
qualify under one or more single or multiple claims or under one or more provisions of this Agreement, the Indemnified Person
shall not be entitled to double or duplicative recovery of Losses arising out of such events or circumstances, or to calculate
its Losses by duplicating or double counting its Losses arising out of such events or circumstances. For the avoidance of doubt,
if the Indemnified Person is entitled to bring the claim under more than one provision of this Agreement, such Indemnified Person
may choose at its sole and absolute discretion the provision or provisions under which it seeks indemnification.
8.11 Straddle Period.
In the case of a Straddle Period: (a) the amount of any property or similar ad valorem
Taxes of any Target Company for a Straddle Period that relate to the portion of such Straddle Period through the end of the Closing
Date will be deemed to be the total amount of such Taxes for the Straddle Period multiplied by a fraction, (i) the numerator of
which is the number of days in the Straddle Period up to and including the Closing Date, and (ii) the denominator of which is
the total number of days in such Straddle Period, and (b) the amount of any Taxes based on or measured by income, gains, or receipts
of any Target Company for the portion of such Straddle Period through the end of the Closing Date will be determined based on
an interim closing of the books as of the close of business on the Closing Date.
8.12 Tax Treatment of
Indemnification Payments. All indemnification payments
made under this Agreement shall be treated by the parties as an adjustment to the Exchange Consideration for Tax purposes unless
otherwise required by applicable law.
Article
IX
CLAIMS
9.1 Notice of Claims
and Expenses. Promptly after the receipt by Buyer (on behalf of a Buyer Indemnified Person)
or the Sellers’ Representative (on behalf of a Seller Indemnified Person) of notice or discovery of any Proceeding or other
Loss that such Party believes gives rise to a claim for indemnification (a “Claim”) under Article VIII,
such Party will give the other Party (which will be Buyer or the Sellers’ Representative, as the case may be) (the “Indemnifying
Party”) written notice of such Claim (a “Notice of Claim”). Each Notice of Claim will include the
following: (i) a statement that such Party believes that an Indemnified Person is entitled to indemnification under Article
VIII, (ii) the actual Losses being claimed, and (iii) a summary of known, relevant facts with respect to such Claim; provided
that no defect in the information contained in such Notice of Claim will relieve the Indemnifying Party from any obligation
under Article VIII, except to the extent such failure to include information actually prejudices such Indemnifying Party.
9.2 Resolution of Claims.
Following timely provided notice of a Claim under this Agreement in accordance with Section
9.1 (other than a Third Party Claim which is governed by Section 8.3), the Indemnifying Party will have thirty (30)
days from the date notice was provided of such Claim (the “Dispute Period”) to make such investigation of the
Claim as the Indemnifying Party deems necessary or advisable. For purposes of such investigation, the Indemnified Person will
make available to the Indemnifying Party all the information reasonably related to such Claim relied upon by, or in the possession
or control of, the Indemnified Person to substantiate such Claim. If the Indemnifying Party disagrees with the validity or amount
of all or a portion of such Claim made by the Indemnified Person, the Indemnifying Party will provide to the Indemnified Person
written notice thereof (the “Indemnification Dispute Notice”) prior to the expiration of the Dispute Period.
If the Indemnifying Party provides notice that it does not have a dispute with respect to such Claim for indemnification, then
such Claim will be deemed approved and consented to by the Indemnifying Party (such Claim being referred to herein as an “Approved
Indemnification Claim”). The Indemnifying Party will pay the amount of the Approved Indemnification Claim by wire transfer
of immediately available funds (or, in the case of an Indemnifying Seller, by delivery of its shares of Buyer Common Stock to
the extent applicable under Article VIII) within five (5) business days after such Claim is determined to be an Approved
Indemnification Claim. If no Indemnification Dispute Notice is timely provided to the Indemnified Person within the Dispute Period
or if an Indemnification Dispute Notice is provided to the Indemnified Person within the Dispute Period and the Indemnifying Party
and the Indemnified Person do not agree to the validity and/or amount of such disputed Claim, the Indemnifying Party and the Indemnified
Person shall negotiate in good faith for a period of at least sixty (60) days to resolve the dispute. If the Indemnifying Party
and the Indemnified Person are unable to come to an agreement regarding such disputed Claim during such sixty (60) day period,
such dispute shall be resolved in accordance with Section 9.3.
9.3 Arbitration.
If there is a dispute with respect to a Claim and no settlement agreement is reached
despite negotiations between the Parties in accordance with Section 9.2, then either Party may, by written notice to the
other, submit such dispute to binding arbitration to the American Arbitration Association in Wilmington, Delaware, which will
administer the arbitration in accordance with the American Arbitration Association Commercial Arbitration Rules in effect on the
date of the execution of this Agreement (the “Rules”). Arbitration in accordance with this Section 9.3
will be conducted by a sole arbitrator mutually selected by the Parties; provided that if the Parties fail to mutually
select an arbitrator within fifteen (15) business days after such dispute is submitted to the American Arbitration Association,
then Buyer or Seller will follow the arbitrator selection procedures in accordance with the Rules. The arbitrators’ authority
will be confined to determining (a) whether the Indemnified Person is entitled to recover any indemnifiable Losses and (b) the
prevailing party and any award of fees or costs (or both). The final decision of the arbitrator will include the dollar value
of the award to the Indemnified Person, if any, and will be furnished to both parties in writing. The prevailing party, as determined
by the arbitrator, will be entitled to an award of reasonable attorneys’ fees and costs, and responsibility for all costs
of arbitration paid or payable by the prevailing party, as determined by the arbitrator, will be allocated among the parties in
the discretion of the arbitrator and specified in the arbitrators award. The arbitrator will not have the power to alter, amend,
or otherwise affect the terms of these arbitration provisions or any other provision of this Agreement or any other documents
that are executed in connection with this Agreement. The final decision of the arbitrator will constitute the conclusive determination
of the issues in question, binding upon both parties. Judgment upon any award rendered by the arbitrator may be entered in any
court having jurisdiction.
Article
X
TERMINATION, AMENDMENT, AND WAIVER
10.1 Termination.
This Agreement may be terminated and the Transactions abandoned at any time before the
Closing:
10.1.1 Mutual Written
Consent. By mutual written consent of Buyer and Company
(on behalf of itself and Sellers);
10.1.2 Written Notice
by Buyer or Sellers’ Representatives. By written
notice by Buyer or the Sellers’ Representative if any of the conditions to the Closing set forth in Article VII have
not been satisfied or waived by February 28, 2024 (the “Outside Date”); provided, however, the
right to terminate this Agreement under this Section 10.1.2 shall not be available to a Party if the breach or violation
by such Party or its Affiliates (or with respect to Company, any Seller) of any representation, warranty, covenant or obligation
under this Agreement was the cause of, or resulted in, the failure of the Closing to occur on or before the Outside Date;
10.1.3 Written
Notice of Order. By written notice by either Buyer or Company
if a Governmental Entity of competent jurisdiction shall have issued an Order or taken any other action permanently restraining,
enjoining or otherwise prohibiting the Transactions contemplated by this Agreement, and such Order or other action has become
final and non-appealable; provided, however, that the right to terminate this Agreement pursuant to this Section
10.1.3 shall not be available to a Party if the failure by such Party or its Affiliates (or with respect to Company,
any Seller) to comply with any provision of this Agreement has been a substantial cause of, or substantially resulted in, such
action by such Governmental Entity;
10.1.4 Written Notice
of Breach by Buyer. By written notice by Company to Buyer,
if (i) there has been a breach by Buyer of any of its representations, warranties, covenants or agreements contained in this Agreement,
or if any representation or warranty of Buyer shall have become untrue or inaccurate, in any case, which would result in a failure
of a condition set forth in Section 7.3.1 or Section 7.3.2 to be satisfied (treating the Closing Date
for such purposes as the date of this Agreement or, if later, the date of such breach), and (ii) the breach or inaccuracy is incapable
of being cured or is not cured within the earlier of (A) twenty (20) days after written notice of such breach or inaccuracy is
provided to Buyer or (B) the Outside Date; provided, that Company shall not have the right to terminate this Agreement
pursuant to this Section 10.1.4 if at such time Company or any Seller is in material uncured breach of this Agreement;
10.1.5 Written Notice
of Breach by Company or Seller. By written notice by Buyer
to Company, if (i) there has been a breach by Company or any Seller of any of its representations, warranties, covenants or agreements
contained in this Agreement, or if any representation or warranty of such Parties shall have become untrue or inaccurate, in any
case, which would result in a failure of a condition set forth in Section 7.2.1 or Section 7.2.2 to
be satisfied (treating the Closing Date for such purposes as the date of this Agreement or, if later, the date of such breach),
and (ii) the breach or inaccuracy is incapable of being cured or is not cured within the earlier of (A) twenty (20) days after
written notice of such breach or inaccuracy is provided to Company or (B) the Outside Date; provided, that Buyer shall
not have the right to terminate this Agreement pursuant to this Section 10.1.5 if at such time Buyer is in material
uncured breach of this Agreement;
10.1.6 Written Notice
of Company Material Adverse Effect. By written notice by
Buyer to Company, if there shall have been a Company Material Adverse Effect on the Target Companies taken as a whole following
the date of this Agreement which is uncured and continuing;
10.1.7 Written Notice
of Buyer Stockholder Meeting. By written notice by either
Buyer or Company to the other, if the Buyer Stockholder Meeting is held (including any adjournment or postponement thereof) and
has concluded, Buyer’s stockholders have duly voted, and the Stockholder Approval was not obtained;
10.1.8 Written Notice
of Unsatisfactory Buyer Due Diligence. By written notice
by Buyer to the Sellers’ Representative if Buyer reasonably determines that there has been a material adverse change to
the Target Companies’ AI Technologies, Company IP Rights or capital structure,
except as contemplated by this Agreement on or prior to the date that is five (5) Business Days following the date of
the delivery of the Company Disclosure Schedules to Buyer pursuant to Section 6.10; provided, that Buyer shall not
have the right to terminate this Agreement pursuant to this Section 10.1.8 if at such time Buyer is in material uncured
breach of this Agreement; or
10.1.9 Written Notice
of Unsatisfactory Seller Due Diligence. By written notice
by the Sellers’ Representative to Buyer if the Sellers reasonably determined that there has been a material adverse change
to the Buyer IP Rights or capital structure, except as contemplated by this Agreement on or prior to the date that is five (5)
Business Days following the date of the delivery of the Buyer Disclosure Schedules to the Sellers pursuant to Section 6.10;
provided, that Sellers shall not have the right to terminate this Agreement pursuant to this Section 10.1.9 if
at such time Company or any Seller is in material uncured breach of this Agreement.
10.2 Effect of Termination.
In the event of termination of this Agreement by either Company or Buyer, this Agreement
will become void and have no effect, and there will be no liability or obligation on the part of Buyer, Company, any Seller, or,
as applicable, their respective officers or directors, except that (a) the provisions of Sections 6.1 (Confidentiality
Agreement), 6.4 (Public Announcements), 10.2 (Effect of Termination), Article XI (General Provisions), and the Confidentiality
Agreement will survive any termination, and (b) no Party will be relieved of any liability or damages arising from Fraud or the
willful breach by such Party of any of its representations, warranties, or covenants included in this Agreement. Without limiting
and subject to the foregoing, and except as provided in this Section 10.2 and subject to the right to seek injunctions,
specific performance or other equitable relief in accordance with Section 11.10, the Parties’ sole right prior
to the Closing with respect to any breach of any representation, warranty, covenant or other agreement contained in this Agreement
by another Party or with respect to the Transactions contemplated by this Agreement shall be the right, if applicable, to terminate
this Agreement pursuant to Section 10.1.
Article
XI
GENERAL PROVISIONS
11.1 Notices.
All notices, requests, demands, or other communications required or permitted to be given under this Agreement will be in writing
and deemed given and received upon (a) personal delivery, (b) one business day after being sent, if sent by reputable, nationally
recognized overnight courier service, (c) three (3) business days after being mailed, if sent by registered or certified mail,
pre-paid and return receipt requested, in each case to the applicable Party at the following addresses (or at such other address
for a Party as shall be specified by like notice), or (d) affirmative confirmation of a receipt of an email:
| (i) |
if to Buyer, or to Company after Closing: |
Renovaro Biosciences Inc.
Attention: Luisa Puche, Chief Financial Officer
9480 NE 2nd Avenue, #73
Miami, FL 33138
Email: lpuche@renovarobio.com |
| |
With a copy (which will not constitute notice) to: |
K&L
Gates LLP
200 South
Biscayne Boulevard
Suite
3900
Miami,
Florida 33131-2399
Attention:
Clayton E. Parker, Esq.
Facsimile
No.: 305-358-7095
Email: clayton.parker@klgates.com
|
| (ii) |
if to Company (before Closing), to: |
GEDi Cube
Intl Ltd
71-75
Shelton Street
Covent
Garden
London
WC2H 9JQ
Attn.:
Karen Brink, Statutory Director
E-mail: karen@gedicube.com
|
| |
With a copy (which will not constitute notice) to: |
Greenberg Traurig, P.A.
333 S.E. 2nd Avenue
Miami, FL 33131
Attention: John D. Owens, III,
Esq.
Email: owensjohn@gtlaw.com
|
| (iii) |
if to Sellers’ Representative to: |
Yalla Yalla Ltd.
Q2, Level 7, Quad Central,
Triq L-Esporaturi
Central Business District,
Birkirkara, CBD 1040, Malta
Attention: Karen Brink
E-mail: kbri@mac.com
|
11.2 Disclosure Schedule.
Certain information set forth in the schedules to the Buyer Disclosure Schedule and the
Company Disclosure Schedule is included solely for informational purposes and may not be required to be disclosed pursuant to
this Agreement. The disclosure of any information shall not be deemed to constitute an acknowledgment that such information is
required to be disclosed in connection with or expand the representations and warranties made by Buyer, Company, or Seller, as
applicable,
in this Agreement or that such information
is material, nor shall such information be deemed to establish a standard of materiality, nor shall it be deemed an admission of
any liability of, or concession as to any defense available to, Buyer, Company, or any Seller, as applicable. Unless this Agreement
specifically provides otherwise, neither the specification of any item or matter in any representation or warranty contained in
this Agreement nor the inclusion of any specific item in any of the schedules to the Buyer Disclosure Schedule or the Company Disclosure
Schedule is intended to imply that such item or matter, or other items or matters, are or are not in the ordinary course of business,
and no Party may use the fact of the setting forth or the inclusion of any such item or matter in any dispute or controversy between
the Parties as to whether any obligation, item or matter not described herein or included in any of the schedules is or is not
in the ordinary course of business for purposes of this Agreement. The section number headings in the schedules to the Buyer Disclosure
Schedule and the Company Disclosure Schedule correspond to the section numbers in this Agreement and any information disclosed
in any section of the schedules shall be deemed to be disclosed and incorporated into any other section of the schedules (and,
for the avoidance of doubt, for purposes of any other representations and warranties in this Agreement) where the relevance of
such disclosure is reasonably apparent on its face. The Parties do not assume any responsibility to any third person other than
to the extent provided in the Agreement for the form or accuracy of any information herein. In disclosing information in the schedules
included in the Buyer Disclosure Schedule and the Company Disclosure Schedule, Buyer, Company, and Sellers have not and do not
waive any attorney-client privilege associated with such information or any protection afforded by the work-product doctrine with
respect to any of the matters disclosed or discussed herein.
11.3 Supplements to
Disclosure Schedule. From time to time before the Closing,
Buyer will promptly supplement or amend the Buyer Disclosure Schedule, and Company and each Seller will promptly supplement or
amend the Company Disclosure Schedule, with respect to any matter, condition, or occurrence arising, which if existing or occurring
at the date of this Agreement that would have been required to be included in such respective Company Disclosure Schedule or Buyer
Disclosure Schedule. No supplement or amendment to the Buyer Disclosure Schedule or Seller Disclosure Schedule, or filing of any
Additional SEC Document, will (a) cure any breach of any representation or warranty made in this Agreement for the purpose of
determining satisfaction of the closing conditions in Article VIII or Article VII, respectively, or (b) be taken
into account for purposes of determining indemnification obligations in Article VIII. No amendment or supplement to such
respective Company Disclosure Schedule or Buyer Disclosure Schedule will be permitted without the written consent of Company or
Buyer, respectively.
11.4 Interpretation.
As used in this Agreement, the term (a) “subsidiary” or “subsidiaries”
means with respect to any Person, any entity or entities of which such Person directly or indirectly owns an amount of the voting
securities or other voting ownership interests sufficient to elect a majority of its board of directors or other governing body
(or, if there are no such interests, 50% or more of the equity interests); (b) “Affiliate” has the meaning
included in Rule 12b-2 promulgated under the Exchange Act; (c) “business day” means any day other than a Saturday,
Sunday, or a day on which banking institutions in New York,
New York, the Netherlands or the United Kingdom are permitted or obligated by law to
be closed for regular banking business; (d) “beneficially owned” or “beneficial ownership”
shall be determined in accordance with Rule 13d-3 promulgated under the Exchange Act; (e) the words “include,”
“includes,” and “including” when used in this Agreement will be treated in each case as followed
by the words “without limitation”; and (f) The respective Parties to this Agreement and their attorneys
have negotiated this Agreement and any ambiguity or uncertainty in the language of this Agreement will not be presumptively construed
for or against a Party as drafter. The table of contents and headings in this Agreement are for reference purposes only and will
not affect in any way the meaning or interpretation of this Agreement. A reference to a section, schedule, or an exhibit means
a section in, or schedule or exhibit to, this Agreement unless otherwise explicitly provided. A reference in this Agreement to
$ or dollars is to U.S. dollars. The definitions of terms herein shall apply equally to the singular and plural forms of the terms
defined. The words “hereof,” “herein,” “hereby,” “hereto,”
and “hereunder” and words of similar import when used in this Agreement shall refer to this Agreement as a whole
and not to any particular provision of this Agreement. References to “this Agreement” shall include the Company Disclosure
Schedule and the Buyer Disclosure Schedule. References to “made available” or “provided to”
(or words of similar import) when referring to any document or information being made available by Company to Buyer shall mean
posted to the electronic data room established in respect to the Transactions at least two business days prior to the date of this
Agreement.
The Parties have participated
jointly in negotiating and drafting this Agreement. In the event that an ambiguity or a question of intent or interpretation arises,
this Agreement shall be construed as if drafted jointly by the parties, and no presumption or burden of proof shall arise favoring
or disfavoring any Party by virtue of the authorship of any provision of this Agreement.
11.5 Counterparts.
This Agreement may be executed (a) in one or more partially or fully executed counterparts,
each of which will be deemed an original and will bind the signatory, but all of which together will constitute the same instrument,
and (b) by facsimile or electronic mail. The execution and delivery of a signature page in the form annexed to this Agreement
by any Party who will have been furnished the final form of this Agreement will constitute the execution and delivery of this
Agreement by such Party.
11.6 Entire Understanding;
No Third Party Beneficiaries. This Agreement, the Confidentiality
Agreement, and the Transaction Documents constitute the entire agreement among the Parties with respect to the subject matter
of this Agreement and supersede all prior agreements and understandings, both written and oral, among the Parties with respect
to the subject matter of this Agreement. This Agreement (a) is not intended to confer upon any other Person any rights or remedies
under this Agreement (except as otherwise expressly provided in this Agreement); and (b) will not be assigned by operation of
law or otherwise except as otherwise specifically provided.
11.7 Governing Law.
This Agreement shall be governed by, construed and enforced in accordance with the Laws
of the State of Delaware without regard to the conflict of laws principles thereof.
11.8 Amendment; Waiver.
Except as may otherwise be provided in this Agreement and by applicable law, any provision,
exhibit, or schedule of this Agreement may be amended or modified by the Parties before the Closing Date, if and only if such
amendment or modification is in writing and signed on behalf of each of the Parties to this Agreement. This Agreement may not
be amended, modified or supplemented after the Closing Date except by written agreements of the Parties. No waiver by any Party
of any of the provisions of this Agreement will be effective unless explicitly set forth in writing and signed by the Party so
waiving. Except as otherwise set forth in this Agreement, no failure to exercise, or delay in exercising, any rights, remedy,
power or privilege arising from this Agreement will operate or be construed as a waiver nor will any single or partial exercise
of any right, remedy, power or privilege arising from this Agreement preclude any other or further exercise of this Agreement
or the exercise of any other right, remedy, power, or privilege.
11.9 Successors and
Assigns. This Agreement shall not be assigned by operation
of Law or otherwise without the prior written consent of Buyer and Company (and after the Closing, Buyer and Sellers), and any
assignment without such consent shall be null and void; provided that no such assignment shall relieve the assigning Party
of its obligations hereunder. Subject to the preceding sentence, this Agreement will be binding upon, inure to the benefit of,
and be enforceable by, the Parties and their respective permitted successors and assigns.
11.10 Specific Performance.
Except as otherwise provided herein, any and all remedies herein expressly conferred
upon a Party hereto shall be deemed cumulative with and not exclusive of any other remedy conferred hereby, or by law or equity
upon such Party, and the exercise by a Party hereto of any one remedy shall not preclude the exercise of any other remedy and
nothing herein shall be deemed a waiver by any Party hereto of any right to specific performance or injunctive relief. Except
as otherwise provided herein and specifically subject to Section 8.7, the Parties acknowledge and agree that any breach
of the terms of this Agreement would give rise to irreparable harm for which money damages would not be an adequate remedy. Except
as otherwise provided herein, the Parties agree that, in addition to any other remedies, each will be entitled to an injunction
to prevent breaches of this Agreement and to enforce the terms of this Agreement by a decree of specific performance without the
necessity of proving the inadequacy of money damages as a remedy or posting a bond.
11.11 Severability.
If any term or other provision of this Agreement is invalid, illegal, or incapable of
being enforced by any rule of law, or public policy, all other conditions and provisions of this Agreement will nevertheless remain
in full force and effect so long as the economic or legal substance of the Transactions are not affected in any manner materially
adverse to any Party. Upon such determination that any term or other provision is invalid, illegal, or incapable of being enforced,
the Parties will negotiate in good faith to modify this Agreement to effect the original intent of the Parties as closely as possible
in a mutually acceptable manner in order that the Transactions be consummated as originally contemplated to the greatest extent
possible.
11.12 Submission to
Jurisdiction. Subject to the provisions of Section 9.3,
for the purpose of any action arising out of or relating to this Agreement brought by any Party against another Party arising
out of or relating to this Agreement or any of the Transactions (a) each of the Parties irrevocably and unconditionally consents
and submits to the exclusive jurisdiction and venue of the Court of Chancery of the State of Delaware or, if (and only if) the
Court of Chancery of the State of Delaware declines to accept jurisdiction over a particular matter, the Superior Court of the
State of Delaware (Complex Commercial Division) or, if (and only if) the Superior Court of the State of Delaware (Complex Commercial
Division) declines to accept jurisdiction over a particular matter, any federal court sitting in the State of Delaware, and any
appellate courts therefrom, (b) irrevocably waives any objection that it may now or hereafter have to the venue of any such action,
dispute or controversy in any such court or that such legal Proceeding was brought in an inconvenient court and agrees not to
plead or claim the same, and (c) agrees that it shall not bring any legal Proceeding relating to this Agreement or the Transactions
in any court other than the aforesaid courts, and (d) each of the Parties irrevocably consents to service of process by first
class certified mail, return receipt requested, postage prepaid, to the address at which such Party is to receive notice in accordance
with Section 11.1; provided, however, that the foregoing shall not limit the right of a Party to effect service
of process on the other Party by any other legally available method.
11.13 Waiver of Jury
Trial. EACH OF THE PARTIES TO THIS AGREEMENT ACKNOWLEDGES
AND AGREES THAT ANY CONTROVERSY ARISING UNDER THIS AGREEMENT IS LIKELY TO INVOLVE COMPLICATED AND DIFFICULT ISSUES. AS A RESULT
EACH PARTY TO THIS AGREEMENT IRREVOCABLY AND UNCONDITIONALLY WAIVES ANY RIGHT THAT SUCH PARTY MAY HAVE TO A TRIAL BY JURY IN RESPECT
TO LITIGATION ARISING OUT OF THIS AGREEMENT OR THE TRANSACTIONS. EACH PARTY TO THIS AGREEMENT UNDERSTANDS AND HAS CONSIDERED THE
IMPLICATIONS OF THIS WAIVER AND MAKES THIS WAIVER VOLUNTARILY.
11.14 Sellers’
Representative.
11.14.1 Appointment
of Seller’s Representative. Each Seller does hereby
irrevocably appoint the Sellers’ Representative as its true and lawful attorney-in-fact and agent, with full power of substitution
or re-substitution, to act on behalf of such Seller with respect to this Agreement and the Transaction Documents in accordance
with the terms and provisions of this Agreement, and to take any and all actions and make any decisions required or permitted
to be taken by the Sellers’ Representative pursuant to this Agreement or the Transaction Documents, including the power:
(a) to
give and receive notices and communications;
(b) agree
to, negotiate, enter into settlements and compromises of, and comply with orders or otherwise handle any other matters described
in Section 6.7;
(c) execute
and deliver all documents necessary or desirable to carry out the intent of this Agreement and the Transaction Documents;
(d) make
all elections or decisions contemplated by this Agreement and the Transaction Documents;
(e) engage,
employ or appoint any agents or representatives (including attorneys, accountants and consultants) to assist the Sellers’
Representative in complying with his duties and obligations;
(f) to
receive funds, make payments of funds and give receipts for funds;
(g) to
receive funds for the payment of expenses of Sellers and apply such funds in payment for such expenses; and
(h) take
all actions necessary or appropriate in the good faith judgment of the Sellers’ Representative for the accomplishment of
the foregoing.
Notwithstanding the above,
in case any of the aforementioned actions need to be executed locally in the jurisdictions involved, the Sellers are obliged to
grant the necessary documents, instruments and/or powers of attorney in accordance with the formalities and requirements established
by the correspondent legislation. The appointment of the Sellers’ Representative to act on behalf of Sellers shall be deemed
coupled with an interest and shall be irrevocable, and Buyer, Company and any other Person may conclusively and absolutely rely,
without inquiry, upon any action of the Sellers’ Representative in all matters referred to herein. All notices required to
be made or delivered by Buyer to Sellers shall be made to the Sellers’ Representative for the benefit of Sellers and shall
discharge in full all notice requirements of Buyer to Sellers with respect thereto. The Sellers’ Representative shall act
for Sellers on all of the matters set forth in this Agreement in the manner the Sellers’ Representative believes to be in
the best interest of Sellers and consistent with the obligations of Sellers under this Agreement, but the Sellers’ Representative
shall not be responsible to Sellers for any Losses which Sellers may suffer by the performance of the Sellers’ Representative’s
duties under this Agreement, other than Losses arising from Fraud in the performance of such duties under this Agreement. The Sellers’
Representative shall not have any duties or responsibilities except those expressly set forth in this Agreement, and no implied
covenants, functions, responsibilities, duties, obligations or liabilities shall be read into this Agreement or shall otherwise
exist against the Sellers’ Representative.
11.14.2 Reliance by
the Sellers’ Representative. The Sellers’ Representative
shall be entitled to rely, and shall be fully protected in relying, upon any statements furnished to him by Sellers or Buyer or
any other evidence reasonably deemed by the Sellers’ Representative to be reliable, and the Sellers’ Representative
shall be entitled to act on the advice of counsel selected by him.
11.14.3 Expenses of
the Sellers’ Representative. The Sellers’ Representative
shall be entitled to retain counsel and to incur such expenses (including court costs and reasonable attorneys’ fees and
expenses) as the Sellers’ Representative deems to be necessary or appropriate in connection with his performance of his
obligations under this Agreement. All fees and expenses incurred by the Sellers’ Representative in performing his duties
shall be borne by the Sellers.
11.14.4 Indemnification
of the Sellers’ Representative. Each Seller hereby
agrees to indemnify the Sellers’ Representative (in its capacity as such) against, and to hold the Sellers’ Representative
(in its capacity as such) harmless from any and all liabilities of whatever kind which may at any time be imposed upon, incurred
by or asserted against the Sellers’ Representative in such capacity in any way relating to or arising out of the Sellers’
Representative’s action or failure to take action pursuant to this Agreement or in connection herewith or therewith in such
capacity; provided, that Sellers shall not be liable for the payment of any portion of such liabilities to the extent resulting
from the Fraud of the Sellers’ Representative. Sellers hereby authorize the Sellers’ Representative to apply proceeds
otherwise distributable to Sellers pursuant to this Agreement to satisfy any of Sellers’ obligations under this Section
11.14.4.
11.14.5 Resignation
of the Sellers’ Representative. The Sellers’
Representative may resign at any time, and may be removed for any reason or no reason by the vote or written consent of a majority
in interest of the Sellers according to each Seller’s Pro Rata Share (the “Majority Sellers”); provided,
however, in no event shall the Sellers’ Representative resign or be removed without the Majority Sellers having first
appointed a new Sellers’ Representative who shall assume such duties immediately upon the resignation or removal of the
Sellers’ Representative. In the event of the death, incapacity, resignation or removal of the Sellers’ Representative,
a new Sellers’ Representative shall be appointed by the vote or written consent of the Majority Sellers. Notice of such
vote or a copy of the written consent appointing such new Sellers’ Representative shall be sent to Buyer, such appointment
to be effective upon the later of the date indicated in such consent or the date such notice is received by Buyer; provided,
that until such notice is received, Buyer, shall be entitled to rely on the decisions and actions of the prior Sellers’
Representative as described in Section 11.14.1.
11.15 Legal Representation
Each Party, on its own behalf and on behalf of its directors, managers, officers, owners, employees
and Affiliates and each of their successors and assigns (all such parties, the “Waiving Parties”), hereby agrees
that. K&L Gates LLP (or any successor thereto) may represent Buyer or any direct or indirect director, manager, officer, owner,
employee or Affiliate thereof, in connection with any dispute, claim, Proceeding or Liability arising out of or relating to this
Agreement, any Transaction Document or the transactions contemplated hereby or thereby (any such representation, the “Buyer
Post-Closing Representation”) notwithstanding its representation (or any continued representation) of Buyer in connection
with the transactions contemplated by this Agreement, and each Party on behalf of itself and the applicable Waiving Parties hereby
consents thereto and irrevocably waives (and will not assert) any conflict of interest or any objection arising therefrom or relating
thereto, even though the interests of the Buyer Post-Closing Representation may be directly adverse to the applicable Waiving
Parties.
[remainder of page intentionally blank]
IN WITNESS WHEREOF, the Parties hereto have
signed or caused their respective duly authorized officers to sign this Agreement, all as of the date first written above.
| |
BUYER: |
| |
|
| |
RENOVARO BIOSCIENCES INC. |
| |
|
| |
By: |
/s/ Mark Dybul_ |
| |
Name: |
Mark Dybul, M.D. |
| |
Title: |
Chief Executive Officer |
| |
|
| |
COMPANY: |
| |
|
| |
GEDI CUBE INTL LTD. |
| |
|
| |
By: |
/s/ Frank Van Asch |
| |
Name: |
Frank Van Asch |
| |
Title: |
Director |
| |
|
| |
SIGNING SELLERS: |
| |
|
| |
YALLA YALLA LTD. |
| |
|
| |
By: |
/s/ Karen Brink |
| |
Name: |
Karen Brink |
| |
Title: |
Authorized Signature |
| |
|
| |
SEPA BEHEER BV |
| |
|
| |
By: |
/s/ F.Y. Van Asch |
| |
Name: |
F.Y. Van Asch |
| |
Title: |
Director |
| |
|
| |
CK VA KALKEN BEHEER BV |
| |
|
| |
By: |
/s/ CK Van Kalken |
| |
Name: |
CK Van Kalken |
| |
Title: |
Director |
| |
|
|
| |
SELLERS’ REPRESENTATIVE: |
| |
|
| |
YALLA YALLA LTD. |
| |
|
|
| |
By: |
/s/ Karen Brink |
| |
Name: |
Karen Brink |
| |
Title: |
Authorized Signature |
ANNEX A-II
FIRST AMENDMENT TO Stock
Purchase Agreement
This First Amendment (this
“Amendment”) to the Stock Purchase Agreement, dated as of September 28, 2023 (the “Agreement”),
by and among Renovaro Biosciences Inc., a Delaware corporation (“Buyer”), Gedi Cube Intl Ltd., a private limited
company incorporated under the laws of England and Wales (“Company”), each of the shareholders of the Company
signatory thereto (collectively, the “Sellers”) and Yalla Yalla Ltd., a private limited liability company registered
and incorporated under the laws of Malta with company registration number C 103531, in its capacity as representative to the Sellers,
is dated December 20, 2023. Capitalized terms used herein, which are not otherwise defined herein, shall have the meanings assigned
to such terms in the Agreement.
WHEREAS, the undersigned
desire to amend the Agreement pursuant to the terms of and subject to the conditions set forth in this Amendment; and
Whereas,
pursuant to Section 11.8 of the Agreement, the Agreement may be amended or modified by the Parties before the Closing Date,
if the amendment or modification is in writing and signed on behalf of each of the Parties to the Agreement.
NOW, THEREFORE, in
consideration of the premises set forth above and other good and valuable consideration, the receipt and sufficiency of which are
hereby acknowledged, the Parties agree as follows:
1. Recitals. The foregoing
recitals are hereby incorporated herein to the same extent as if hereinafter fully set forth in herein.
2. Amendments for Common Stock
Issued to Avram Miller. Section 2.2 of the Agreement is hereby deleted in its entirety and replaced with the following:
“Exchange Consideration.
At the Closing, subject to and upon the terms and conditions of this Agreement, as consideration for the Company Shares, Sellers
collectively shall be entitled to have issued to them by Buyer, in the aggregate, (i) a number of shares of Buyer Common Stock
equal to (the “Exchange Shares”) the Applicable Percentage of the total number of issued and outstanding
shares of Buyer Common Stock as of the Closing Date (minus (a) 1,000,000 shares of Buyer Common Stock representing shares issued
by Buyer to a consultant assisting the Parties on the Transaction (the “Consultant”) and (b) 1,000,000 shares
of Buyer Common Stock representing shares issued to Avram Miller pursuant to his Advisory Agreement with the Company dated October
11, 2023) and (ii) the right to receive from Buyer shares of Buyer Common Stock which may become issuable as Earnout Stock in accordance
with Section 2.2.1 below, if any (together with the Exchange Shares, the “Exchange Consideration”).
Each Seller shall receive its pro rata portion of the Exchange Consideration based on such Seller’s Pro Rata Share. Notwithstanding
anything to the contrary contained herein,
no fraction of a share of Buyer Common Stock will be issued by Buyer by virtue of this
Agreement or the Transactions contemplated hereby, and each Seller who would otherwise be entitled to a fraction of a share of
Buyer Common Stock (after aggregating all fractional shares of Buyer Common Stock that would otherwise be received by such Seller)
shall instead have the number of shares of Buyer Common Stock issued to such Seller rounded down in the aggregate to the nearest
whole share of Buyer Common Stock.”
3. Amendment to the
definition of “Stockholder Approval.” The definition of “Stockholder Approval” in Section 1.1 of the
Agreement is hereby deleted in its entirety and replaced with the following:
“‘Stockholder
Approval’ means the approval of Buyer’s stockholders: (i) to amend its certificate of incorporation to
increase the number of authorized shares of Buyer Common Stock to allow for the issuance of the Exchange Consideration and
(ii) as contemplated by Nasdaq Rule 5635(a) with respect to the issuance of Buyer Common Stock in excess of the limitations
imposed by such rule pursuant to this Agreement.”
4. No Other Amendment.
Except as specifically amended by the terms herein agreed to by the Parties, the Agreement, and all other provisions thereof, shall
be unchanged and shall remain in full force and effect.
5. Miscellaneous.
The provisions of Sections 11.1, 11.3 to 11.13, and 11.15 of the Agreement are hereby incorporated
into this Amendment as if fully set forth herein and each reference to “Agreement” therein shall be deemed a reference
to this Amendment.
[Remainder of Page Intentionally Left Blank;
Signature Pages Follow]
IN WITNESS WHEREOF, the
undersigned, intending to be legally bound hereby, have duly executed this Amendment as of the date first above written.
| Buyer: |
|
| |
|
| RENOVARO BIOSCIENCES INC. |
|
| |
|
| By: |
/s/ Mark
Dybul, M.D. |
|
| Name: |
Mark Dybul, M.D. |
|
| Title: |
Chief Executive Officer |
|
| Company: |
|
| |
|
| Gedi Cube Intl LTD. |
|
| |
|
| By: |
/s/ Karen
Brink |
|
| Name: |
Karen Brink |
|
| Title: |
Director |
|
| Sellers’ Representative |
|
| |
|
| Yalla Yalla LTD. |
|
| |
|
| By: |
/s/ Matthijs
van Kranenburg |
|
| Name: |
Matthijs van Kranenburg |
|
| Title: |
Authorized Signatory |
|
| Sellers: |
|
| |
|
| Yalla Yalla LTD. |
|
| |
|
| By: |
/s/ Matthijs
van Kranenburg |
|
| Name: |
Matthijs van Kranenburg |
|
| Title: |
Authorized Signatory |
|
| SEPA BEHEER BV |
|
| |
|
| By: |
/s/ Frank van
Asch |
|
| Name: |
Frank van Asch |
|
| Title: |
Director |
|
| CK VA KALKEN BEHEER BV |
|
| |
|
| By: |
/s/ CK Van Kalken |
|
| Name: |
CK Van Kalken |
|
| Title: |
Director |
|
| DMZ INVEST i APS |
|
| |
|
| By: |
/s/ Flemming
Segerlund |
|
| Name: |
Flemming Segerlund |
|
| Title: |
CEO |
|
Annex
B
Proposed Certificate of Amendment to Renovaro’s Certificate of Incorporation Regarding Increase of Authorized Common Stock
CERTIFICATE OF AMENDMENT
OF
CERTIFICATE OF INCORPORATION
OF
RENOVARO BIOSCIENCES INC.
Renovaro Biosciences Inc., a corporation duly organized
and existing under the General Corporation Law of the State of Delaware (the “Corporation”), pursuant to the General
Corporation Law of the State of Delaware (the “DGCL”), does hereby certify that:
1.
This Certificate of Amendment to the Certificate of Incorporation of the Corporation, as amended
to date (the “Certificate of Incorporation”), was duly adopted by the Board of Directors of the Corporation in accordance
with the provisions of Sections 242 and 228 of the DGCL.
2.
This Certificate of Amendment hereby amends the Certificate of Incorporation by deleting the first
paragraph of Article 5 in its entirety and replacing it with the following:
“The total number of shares of capital stock which the Corporation shall have
the authority to issue is three hundred sixty million (360,000,000). These shares shall be divided into two classes with three hundred
fifty million (350,000,000) shares designated as common stock at $.0001 par value (the “Common Stock”) and ten million (10,000,000)
shares designated as preferred stock at $.0001 par value (the “Preferred Stock”).”
IN WITNESS WHEREOF,
the Corporation has caused this Certificate of Amendment to be executed by its duly authorized officer on this _______day of _________, 2024.
| |
RENOVARO BIOSCIENCES INC. |
| |
|
|
| |
By: |
|
| |
Name: |
Mark R. Dybul, M.D. |
| |
Title: |
Chief Executive Officer |
Annex
C
Proposed Renovaro BioSciences Inc. 2023 Equity Incentive Plan, As Amended
RENOVARO
BIOSCIENCES INC.
2023
EQUITY INCENTIVE PLAN
(as proposed to be amended)
[NOTE: Strike-out text herein indicates
deletions, and underlined and bolded text herein indicates additions]
Renovaro BioSciences Inc. (the
“Company”) sets forth herein the terms and conditions of its 2023 Equity Incentive Plan (the “Plan”),
as follows:
The
Plan is intended to enhance the ability of the Company and its Affiliates to attract and retain highly-qualified employees, Consultants
and Non-Employee Directors, and to motivate such employees, Consultants, and Non-Employee Directors to serve the Company and its Affiliates
and to expend maximum effort to improve the business results and earnings of the Company, by providing to such persons an opportunity
to acquire or increase a direct proprietary interest in the operations and future success of the Company. To this end, the Plan provides
for the grant of stock options, stock appreciation rights, restricted stock, RSUs, other stock-based awards, and cash awards. Any of
these awards may, but need not, be made as performance incentives to reward attainment of performance goals in accordance with the terms
and conditions hereof. Upon becoming effective, the Plan replaces, and no further awards may be made under, the Prior Plans. Stock options
granted under the Plan may be non-qualified stock options or incentive options, as provided herein.
For
purposes of interpreting the Plan and related documents (including Award Agreements), the following definitions shall apply:
“Acquiror”
shall have the meaning set forth in Section 15.3(ii).
“Affiliate”
means any company or other trade or business that “controls,” is “controlled by,” or is “under common control
with,” the Company within the meaning of Rule 405 of Regulation C under the Securities Act, including, without limitation, any
Subsidiary.
“Award”
means a grant, under the Plan, of an Option, SAR, Restricted Shares, RSUs, Other Stock-based Award or cash award.
“Award
Agreement” means a written agreement (including an agreement transmitted electronically) between the Company and a Grantee,
or notice from the Company or an Affiliate to a Grantee that evidences and sets out the terms and conditions of an Award.
“Beneficial
Owner” shall have the meaning assigned to such term in Rule 13d-3 and Rule 13d-5 under the Exchange Act, except that in calculating
the beneficial ownership of any particular Person, such Person shall be deemed to have beneficial ownership of all securities that such
Person has the right to acquire by conversion or exercise of other securities, whether such right is currently exercisable or is exercisable
only after the passage of time. The terms “Beneficially Owns” and “Beneficially Owned” have corresponding meanings.
“Board”
means the Board of Directors of the Company.
“Cause”
shall be defined as that term is defined in the Grantee’s offer letter or other applicable employment agreement; or, if there is
no such definition, “Cause” means, as determined by the Company and unless otherwise provided in an applicable Award
Agreement: (i) the Grantee’s willful failure to perform the Grantee’s duties and responsibilities; (ii) the Grantee’s
commission of any act of fraud, embezzlement, dishonesty or willful misconduct; (iii) unauthorized use or disclosure by the Grantee of
any proprietary information of the Company or any Affiliate; or (iv) Grantee’s willful breach of any of the Grantee’s obligations
under any agreement with the Company or any Affiliate. The Committee, in its absolute discretion, shall determine the effect of all matters
and questions relating to the existence of Cause.
“Change
in Control” shall mean, in the case of a particular Award, unless the applicable Award Agreement states otherwise or contains
a different definition of “Change in Control,” the occurrence of any of the following events:
(i) An
acquisition (whether directly from the Company or otherwise) of any voting securities of the Company (the “Voting Securities”)
by any “Person” (as the term person is used for purposes of Section 13(d) or 14(d) of the Securities and Exchange Act of
1934, as amended (the “Exchange Act”)), immediately after which such Person has “Beneficial Ownership”
(within the meaning of Rule 13d-3 promulgated under the Exchange Act) of more than fifty percent (50%) of the combined voting power of
the Company’s then outstanding Voting Securities.
(ii) Consummation
of any definitive agreement, the consummation of which would cause to occur:
(A) A
merger, consolidation or reorganization involving the Company, where either or both of the events described in clause (i) above would
be the result;
(B) A
liquidation or dissolution of or appointment of a receiver, rehabilitator, conservator or similar person for, or the filing by a third
party of an involuntary bankruptcy against, the Company; or
(C) An
agreement for the sale or other disposition of all or substantially all of the assets of the Company to any Person (other than a transfer
to an Affiliate).
Solely
to the extent required by Section 409A, an event described above shall not constitute a Change in Control for purposes of the payment
(but not vesting) terms and conditions of any Award that is determined to be subject to Section 409A unless such event also constitutes
a change in ownership or effective control of the Company or a change in the ownership of a substantial portion of the Company’s
assets within the meaning of Section 409A (a “409A Change in Control Event”); provided, however, that if an event
described in clause (ii) above would be a 409A Change in Control Event upon consummation of the event described therein rather than upon
approval by the Board, then the consummation of such event rather than approval by the Board shall constitute a Change in Control.
“Code”
means the Internal Revenue Code of 1986, as it may be amended from time to time. Any reference to a section of the Code shall be deemed
to include a reference to any valid and binding governmental regulations, court decisions and other regulatory and judicial authority
issued or promulgated thereunder.
“Committee”
means a committee of members of the Board appointed by the Board to administer the Plan in accordance with Section 3. The Board
shall cause the Committee to satisfy the applicable requirements of any stock exchange on which the Common Stock may then be listed.
“Company”
shall have the meaning set forth in the preamble.
“Common
Stock” means the common stock of the Company, par value $0.0001 per share.
“Consultant”
means any person, other than an employee or Non-Employee Director, engaged by the Company or any Affiliate to render bona fide services
to such entity, including as an advisor, and who qualifies as a consultant or advisor under Rule 701 of the Securities Act (during any
period in which the Company is not a public company subject to the reporting requirements of the Exchange Act) or Form S-8 (during any
period in which the Company is a public company subject to the reporting requirements of the Exchange Act).
“Corporate
Transaction” means a recapitalization, reorganization, merger, consolidation, combination, exchange, consolidation, sale of
all or substantially all of the Company’s assets, or the acquisition of assets or stock of another entity by the Company, or other
corporate transaction involving the Company or any of its Affiliates.
“Disability”
means “permanent and total disability” as set forth in Code Section 22(e)(3).
“Effective
Date” means July 21, 2023, the date the Plan was approved by the Company’s stockholders.
“Exchange
Act” means the Securities Exchange Act of 1934, as not in effect or as hereafter amended.
“Fair
Market Value” of a Share as of a particular date shall mean (i) if the Common Stock (A) is listed on a national securities
exchange or (B) is not listed on a national securities exchange, but is quoted by the OTC Markets Group, Inc. (www.otcmarkets.com) or
any successor or alternative recognized over-the-counter market or another inter-dealer quotation system, on a last sale basis, the closing
or last price of the Common Stock reported on such national securities exchange or other inter-dealer quotation system for the applicable
date, or if the applicable date is not a trading day, the trading day immediately preceding the applicable date; or (ii) if the Common
Stock is not listed on a national securities exchange or quoted in an inter-dealer quotation system on a last sale basis, the amount
determined by the Committee to be the fair market value of the Common Stock in good faith in its sole discretion.
“Family
Member” means a person who is a spouse, former spouse, child, stepchild, grandchild, parent, stepparent, grandparent, niece,
nephew, mother-in-law, father-in-law, son-in-law, daughter-in-law, brother, sister, brother-in-law, or sister-in-law, including adoptive
relationships, of the applicable individual, any person sharing the applicable individual’s household (other than a tenant or employee),
a trust in which any one or more of these persons have more than 50% of the beneficial interest, a foundation in which any one or more
of these persons (or the applicable individual) control the management of assets, and any other entity in which one or more of these
persons (or the applicable individual) own more than 50% of the voting interests.
“GAAP”
means U.S. Generally Accepted Accounting Principles.
“Grant
Date” means, as determined by the Board, the latest to occur of (i) the date as of which the Board approves an Award,
(ii) the date on which the recipient of an Award first becomes eligible to receive an Award under Section 6, or (iii) such
other date as may be specified by the Board in the Award Agreement.
“Grantee”
means a person who receives or holds an Award under the Plan.
“Incentive
Stock Option” means an Option that is an “incentive stock option” within the meaning of Code Section 422.
“Issued
Shares” means, collectively, all outstanding Shares issued pursuant to Awards (including without limitation, outstanding Restricted
Shares prior to or after vesting and shares issued in connection with the exercise of an Option or SAR or the settlement of an RSU).
“Non-Employee
Director” means a member of the Board who is not an employee.
“Nonstatutory
Stock Option” means an Option that is not an Incentive Stock Option.
“Option”
means an option to purchase one or more Shares under the Plan, including an Incentive Stock Option and a Nonstatutory Stock Option.
“Option
Price” means the exercise price for each Share subject to an Option.
“Other
Stock-based Award” means Awards consisting of Share units, or other Awards, valued in whole or in part by reference to, or
otherwise based on, Common Stock, other than Options, SARs, Restricted Shares, and RSUs.
“Plan”
shall have the meaning set forth in the preamble.
“Prior
Plans” means the Enochian BioSciences, Inc. 2019 Equity Incentive Plan and the Dandrit Biotech USA, Inc. 2014 Stock Incentive
Plan.
“Purchase
Price” means the purchase price for each Share under a grant of Restricted Shares.
“Restricted
Period” shall have the meaning set forth in Section 10.1.
“Restricted
Shares” means restricted Shares awarded to a Grantee under Section 10.
“RSU”
means a bookkeeping entry representing the equivalent of Shares, awarded to a Grantee under Section 10.
“SAR”
means a right granted to a Grantee under Section 9.
“SAR
Exercise Price” means the per Share exercise price of a SAR granted under Section 9.
“SEC”
means the United States Securities and Exchange Commission.
“Section 409A”
means Code Section 409A.
“Securities
Act” means the Securities Act of 1933, as now in effect or as hereafter amended.
“Separation
from Service” means the termination of the applicable Grantee’s employment with, and performance of services for, the
Company and each Affiliate. Unless otherwise determined by the Company, if a Grantee’s employment or service with the Company or
an Affiliate terminates but the Grantee continues to provide services to the Company or an Affiliate in a non-employee director capacity
or as an employee, officer, or consultant, as applicable, such change in status shall not be deemed a Separation from Service. Approved
temporary absences from employment because of illness, vacation, or leave of absence and transfers among the Company and its Affiliates
shall not be considered Separations from Service. Notwithstanding the foregoing, with respect to any Award that constitutes nonqualified
deferred compensation under Section 409A, “Separation from Service” shall mean a “separation from service” as
defined under Section 409A.
“Service
Provider” means an employee, officer, Non-Employee Director, or Consultant of the Company or an Affiliate.
“Share”
means one share of Common Stock.
“Stockholder”
means a stockholder of the Company.
“Subsidiary”
means any corporation, partnership, joint venture, affiliate, or other entity in which the Company owns more than 50% of the voting stock
or voting ownership interest, as applicable, or any other business entity designated by the Board as a Subsidiary for purposes of the
Plan.
“Substitute
Award” means any Award granted in assumption of or in substitution for an award of a company or business acquired by the Company
or an Affiliate or with which the Company or an Affiliate combines.
“Ten
Percent Stockholder” means an individual who owns more than 10% of the total combined voting power of all classes of outstanding
stock of the Company, its parent or any of its Subsidiaries. In determining stock ownership, the attribution rules of Code Section 424(d)
shall be applied.
“Termination
Date” means the date that is ten years after the Effective Date, unless the Plan is earlier terminated by the Board under Section 5.2.
| 3. | ADMINISTRATION
OF THE PLAN |
3.1.
General
The
Board shall have such powers and authorities related to the administration of the Plan as are consistent with the Company’s articles
of incorporation, bylaws and applicable law, and as further described in Section 3.3. To the extent permitted by applicable law,
the Board shall have the power and authority to delegate its powers and responsibilities hereunder to the Committee, which shall have
full authority to act in accordance with its charter (as in effect from time to time), and with respect to the authority of the Board
to act hereunder. All references to the Board shall be deemed to include a reference to the Committee, to the extent such power or responsibilities
of the Board have been delegated. The Committee shall administer the Plan; provided that the Board shall retain the right to exercise
the authority of the Committee to the extent consistent with applicable law and the applicable requirements of any securities exchange
on which the Common Stock may then be listed.
3.2. Committee
Composition
Except
as otherwise determined by the Board, the Committee shall consist solely of two or more Non-Employee Directors. The Board shall have
discretion to determine whether or not it intends to comply with the exemption requirements of SEC Rule 16b-3. However, if the Board
intends to satisfy such exemption requirements, with respect to any insider subject to Section 16 of the Exchange Act, the Committee
shall be a compensation committee of the Board that at all times consists solely of two or more Non-Employee Directors. To the extent
permitted by applicable law, the Board or the Committee may delegate its authority to grant Awards to any individual or committee of
individuals who are not Non-Employee Directors with respect to Awards that do not involve insiders within the meaning of SEC Rule 16.
To the extent that the Board delegates its authority to make Awards as provided by this Section 3.1, all references in the
Plan to the Board’s authority to make Awards and determinations with respect thereto shall be deemed to include the Board’s
delegate. Any such delegate shall serve at the pleasure of, and may be removed at any time by the Board. Nothing herein shall create
an inference that an Award is not validly granted under the Plan in the event Awards are granted under the Plan by a compensation committee
of the Board that does not at all times consist solely of two or more Non-Employee Directors.
3.3. Authority
of Board
Except
as specifically provided in Section 14 or as otherwise may be required by applicable law, regulatory requirement, or the
articles of incorporation or the bylaws of the Company, the Board shall have full power and authority to take all actions and to make
all determinations required or provided for under the Plan, any Award or any Award Agreement, and shall have full power and authority
to take all such other actions and make all such other determinations, including determinations of fact, not inconsistent with the specific
terms and conditions of the Plan that the Board deems to be necessary or appropriate to the administration of the Plan. The interpretation
and construction by the Board of the Plan, any Award, or any Award Agreement shall be final, binding, and conclusive. Without limitation,
the Board shall have full and final authority, subject to the other terms and conditions of the Plan, to:
| (i) | construe
and interpret the Plan and apply its provisions; |
| (iii) | determine
the type or types of Awards to be made to a Grantee and the applicable Grant Date; |
| (iv) | determine
the number of Shares to be subject to an Award; |
| (v) | establish
the terms and conditions of each Award (including, but not limited to, the Option Price of
any Option, the nature and duration of any restriction or condition (or provision for lapse
thereof) relating to the vesting, exercise, transfer, or forfeiture of an Award or the Shares
subject thereto, and any terms or conditions that may be necessary to qualify Options as
Incentive Stock Options); |
| (vi) | prescribe
the form of each Award Agreement; |
| (vii) | amend,
modify, or supplement the terms and conditions of any outstanding Award, including the authority,
in order to effectuate the purposes of the Plan, to modify Awards to foreign nationals or
individuals who are employed outside the United States to recognize differences in local
law, tax policy, or custom; |
| (viii) | promulgate,
amend and rescind rules and regulations relating to the administration of the Plan; |
| (ix) | to
authorize any person to execute, on behalf of the Company, any instrument required to carry
out the purposes of the Plan; and |
| (x) | to
modify the Option Price or SAR Exercise Price of any outstanding Option or SAR, provided
that if the modification effects a repricing, shareholder approval shall be required before
the repricing is effective. |
| 3.4. | Separation
from Service for Cause; Clawbacks |
| 3.4.1. | Separation
from Service for Cause |
The
Company may annul an Award if the Grantee incurs a Separation from Service for Cause.
All
awards, amounts, or benefits received or outstanding under the Plan shall be subject to clawback, cancellation, recoupment, rescission,
payback, reduction, or other similar action in accordance with any Company clawback or similar policy (“Clawback Policy”)
or any applicable law related to such actions. In addition, a Grantee may be required to repay to the Company previously paid compensation,
whether provided pursuant to the Plan or an Award Agreement in accordance with the Clawback Policy. A Grantee’s acceptance of an
Award shall be deemed to constitute the Grantee’s acknowledgement of and consent to the Company’s application, implementation,
and enforcement of any applicable Company clawback or similar policy that may apply to the Grantee, whether adopted before or after the
Effective Date and whether before or after the Grant Date of an Award, and any applicable law relating to clawback, cancellation, recoupment,
rescission, payback, or reduction of compensation, and the Grantee’s agreement that the Company may take any actions that may be
necessary to effectuate any such policy or applicable law, without further consideration or action.
The
Board may permit or require the deferral of any Award payment into a deferred compensation arrangement, subject to such rules and procedures
as it may establish and in accordance with Section 409A, which may include terms and conditions for the payment or crediting of
interest or dividend equivalents, including converting such credits into deferred units.
No
member of the Board or the Committee shall be liable for any action or determination made in good faith with respect to the Plan, any
Award, or Award Agreement.
Notwithstanding
any other term or condition of the Plan to the contrary, the Company may elect to satisfy any requirement under the Plan for the delivery
of stock certificates through the use of book-entry.
| 4. | shares
SUBJECT TO THE PLAN |
| 4.1. | Authorized
Number of Shares |
Subject
to adjustment under Section 15, the total number of Shares authorized to be awarded under the Plan shall not exceed the sum
of (i) 4,000,000 9,000,000 and (ii) the number of Shares available for the grant of awards as of the Effective
Date under the Prior Plans. In addition, Shares underlying any outstanding award granted under the Prior Plans that, after the Effective
Date, expires, or is terminated, surrendered, or forfeited for any reason without issuance of Shares shall be available for the grant
of new Awards. As provided in Section 1, no new awards shall be granted under the Prior Plans after the Effective Date. Shares
issued under the Plan shall consist in whole or in part of authorized but unissued Shares, treasury Shares, or Shares purchased on the
open market or otherwise, all as determined by the Company from time to time. All of the Shares available under this 4.1 shall be available
for issuance under Incentive Stock Options.
Each
Share granted in connection with an Award shall be counted as one Share against the limit in Section 4.1, subject to this
Section 4.2.
| 4.2.2. | Cash-Settled
Awards |
Any
Award settled in cash shall not be counted as Shares for any purpose under the Plan.
| 4.2.3. | Expired
or Terminated Awards |
If
any Award under the Plan expires, or is terminated, surrendered, or forfeited, in whole or in part, the unissued Shares covered by such
Award shall again be available for the grant of Awards.
| 4.2.4. | Repurchased,
Surrendered, or Forfeited Awards |
If
Issued Shares are repurchased by, or are surrendered or forfeited to the Company at no more than cost, such Shares shall again be available
for the grant of Awards.
| 4.2.5. | Payment
of Option Price or Tax Withholding in Shares |
Notwithstanding
anything to the contrary contained herein: Shares subject to an Award under the Plan shall not again be made available for issuance or
delivery under the Plan if such Shares are (i) Shares tendered in payment of an Option, (ii) Shares delivered or withheld by the Company
to satisfy any tax withholding obligation, (iii) Shares covered by a Share-settled SAR or other Shares that were not issued upon the
settlement of the SAR.
In
the case of any Substitute Award, such Substitute Award shall not be counted against the number of Shares reserved under the Plan.
| 5. | EFFECTIVE
DATE, DURATION, AND AMENDMENTS |
The
Plan shall be effective as of the Effective Date, provided that it has been approved by the Stockholders. The Plan shall terminate automatically
on the ten-year anniversary of the Effective Date and may be terminated on any earlier date as provided in Section 5.2.
| 5.2. | Amendment
and Termination of the Plan |
The
Board may, at any time and from time to time, amend, suspend, or terminate the Plan as to any Awards that have not been made. An amendment
shall be contingent on approval of the Stockholders to the extent stated by the Board, required by applicable law, or required by applicable
securities exchange listing requirements. No Awards may be granted after the Termination Date. The applicable terms and conditions of
the Plan, and any terms and conditions applicable to Awards granted before the Termination Date shall survive the termination of the
Plan and continue to apply to such Awards. No amendment, suspension, or termination of the Plan shall, without the consent of the Grantee,
materially impair rights or obligations under any Award theretofore awarded.
| 6. | AWARD
ELIGIBILITY AND LIMITATIONS |
Awards
may be made to any Service Provider, as the Board may determine and designate from time to time, in its discretion, subject to Section
8.7 in the case of an Incentive Stock Option. The Board may grant an Award to a person who is reasonably expected to become a Service
Provider provided that such grant is contingent upon such person becoming a Service Provider.
Service
Providers may receive more than one Award, subject to such restrictions as are provided herein.
| 6.3. | Stand-Alone,
Additional, Tandem, and Substitute Awards |
The
Board may grant Awards either alone or in addition to, in tandem with, or in substitution or exchange for, any other Award or any award
granted under another plan of the Company, any Affiliate, or any business entity to be acquired by the Company or an Affiliate, or any
other right of a Grantee to receive payment from the Company or any Affiliate. Such additional, tandem, substitute or exchange Awards
may be granted at any time. If an Award is granted in substitution or exchange for another Award, the Board shall have the right to require
the surrender of such other Award in consideration for the grant of the new Award. Subject to Section 3.3(ix), and the requirements
of applicable law, the Board shall have the right, in its discretion, to make Awards in substitution or exchange for any other award
under another plan of the Company, any Affiliate, or any business entity to be acquired by the Company or an Affiliate. In addition,
Awards may be granted in lieu of cash compensation, including in lieu of cash amounts payable under other plans of the Company or any
Affiliate, in which the value of Shares subject to the Award is equivalent in value to the cash compensation (for example, RSUs or Restricted
Shares).
Each
Award shall be evidenced by an Award Agreement, in such form or forms as the Board shall from time to time determine. Without limiting
the foregoing, an Award Agreement may be provided in the form of a notice that provides that acceptance of the Award constitutes acceptance
of all terms and conditions of the Plan and the notice. Award Agreements granted from time to time or at the same time need not contain
similar terms and conditions but shall be consistent with the terms and conditions of the Plan. Each Award Agreement evidencing an Award
of Options shall specify whether such Options are intended to be Nonstatutory Stock Options or Incentive Stock Options, and in the absence
of such specification such options shall be deemed Nonstatutory Stock Options.
| 8. | TERMS
AND CONDITIONS OF OPTIONS |
The
Option Price of each Option shall be fixed by the Board and stated in the related Award Agreement. Each Option shall be separately designated
in the Award Agreement as either an Incentive Stock Option or Nonqualified Option. The Option Price of each Option (except those that
constitute Substitute Awards) shall be at least the Fair Market Value of a Share on the Grant Date; provided, however,
that in the event that a Grantee is a Ten Percent Stockholder as of the Grant Date, the Option Price of an Option granted to such Grantee
that is intended to be an Incentive Stock Option shall be not less than 110% of the Fair Market Value of a Share on the Grant Date. In
no case shall the Option Price of any Option be less than the par value of a Share.
Subject
to Section 8.3, each Option shall become exercisable at such times and under such terms and conditions (including, without
limitation, performance requirements) as may be determined by the Board and stated in the Award Agreement. No Option may be exercised
for a fraction of a Share. The Board may, but shall not be required to, provide for an acceleration of vesting and exercisability in
the terms of any Award Agreement upon the occurrence of a specified event.
8.3.1 General
Each
Option shall terminate, and all rights to purchase Shares thereunder shall cease, upon the expiration of the Option term determined by
the Board and stated in the Award Agreement not to exceed ten years from the Grant Date, or under such circumstances and on any date
before ten years from the Grant Date as may be set forth in the Plan or as may be fixed by the Board and stated in the related Award
Agreement; provided, however, that in the event that the Grantee is a Ten Percent Stockholder, an Option granted to such
Grantee that is intended to be an Incentive Stock Option at the Grant Date shall not be exercisable after the expiration of five years
from its Grant Date.
8.3.2 Separation
from Service
Unless
otherwise provided in an Award Agreement or in an employment agreement the terms of which have been approved by the Board, in the event
a Grantee has a Separation from Service (other than upon the Grantee’s death or Disability), the Grantee may exercise any Option
(to the extent that the Grantee was entitled to exercise such Option as of the date of Separation from Service) but only within such
period of time ending on the earlier of (i) the date three months following the Grantee’s Separation from Service or (ii) the expiration
of the term of the Option as set forth in the Award Agreement; provided that, if the Separation from Service is by the Company
for Cause or if the Grantee’s Separation from Service is due to resignation, all outstanding Options (whether or not vested) shall
immediately terminate and cease to be exercisable. If, after termination, the Grantee does not exercise the Option within the time specified
in the Award Agreement, the Option shall terminate.
8.3.3 Extension
of Termination Date
A
Grantee’s Award Agreement may also provide that if the exercise of the Option following the Grantee’s Separation from Service
for any reason would be prohibited at any time because the issuance of Shares would violate the registration requirements under the Securities
Act or any other state or federal securities law or the rules of any securities exchange or interdealer quotation system, then the Option
shall terminate on the earlier of (i) the expiration of the term of the Option in accordance with Section 8.3.1 or (ii) the expiration
of a period after the Grantee’s Separation from Service that is three months after the end of the period during which the exercise
of the Option would be in violation of such registration or other securities law requirements.
8.3.4 Disability
of Grantee
Unless
otherwise provided in an Award Agreement, in the event of a Grantee’s Separation from Service as a result of the Grantee’s
Disability, the Grantee may exercise any Option (to the extent that the Grantee was entitled to exercise such Option as of the date of
termination), but only within such period of time ending on the earlier of (i) the date 12 months following such termination or (ii)
the expiration of the term of the Option as set forth in the Award Agreement. If, after termination, the Grantee does not exercise the
Option within the time specified herein or in the Award Agreement, the Option shall terminate.
8.3.5 Death
of Grantee
Unless
otherwise provided in an Award Agreement, in the event of a Grantee’s Separation from Service as a result of the Grantee’s
death, then the Option may be exercised (to the extent the Grantee was entitled to exercise such Option as of the date of death) by the
Grantee’s estate, by a person who acquired the right to exercise the Option by bequest or inheritance or by a person designated
to exercise the Option upon the Grantee’s death, but only within the period ending on the earlier of (i) the date 12 months following
the date of death or (ii) the expiration of the term of such Option as set forth in the Award Agreement. If, after the Grantee’s
death, the Option is not exercised within the time specified herein or in the Award Agreement, the Option shall terminate.
| 8.4. | Limitations
on Exercise of Option |
Notwithstanding
any other term or condition of the Plan, in no event may any Option be exercised, in whole or in part, (i) prior to the date the Plan
is approved by the Stockholders as provided herein or (ii) after the occurrence of an event that results in termination of the Option.
An
Option that is exercisable may be exercised by the Grantee’s delivery of a notice of exercise to the Company, setting forth the
number of Shares with respect to which the Option is to be exercised, accompanied by full payment for the Shares. To be effective, notice
of exercise must be made in accordance with procedures established by the Company from time to time.
| 8.6. | Rights
of Holders of Options |
Unless
otherwise stated in the related Award Agreement, an individual holding or exercising an Option shall have none of the rights of a Stockholder
(for example, the right to receive cash or dividend payments or distributions attributable to the subject Shares or to direct the voting
of the subject Shares) until the Shares covered thereby are fully paid and issued to the Grantee. Except as provided in Section 15
or the related Award Agreement, no adjustment shall be made for dividends, distributions, or other rights for which the record date
is before the date of such issuance.
| 8.7. | Limitations
on Incentive Stock Options |
An
Option shall constitute an Incentive Stock Option only (i) if the Grantee of the Option is an employee of the Company or any Subsidiary;
(ii) to the extent specifically provided in the related Award Agreement; and (iii) to the extent that the aggregate Fair Market
Value (determined at the time the Option is granted) of the Shares with respect to which all Incentive Stock Options held by such Grantee
become exercisable for the first time during any calendar year (under the Plan and all other plans of the Grantee’s employer and
its Affiliates) does not exceed $100,000. This limitation shall be applied by taking Options into account in the order in which they
were granted. No Option shall be treated as an Incentive Stock Option unless the Plan has been approved by the Stockholders in a manner
intended to comply with the stockholder approval requirements of Code Section 422; provided that any Option intended
to be an Incentive Stock Option shall not fail to be effective solely on account of a failure to obtain such approval, but rather such
Option shall be treated as a Nonstatutory Stock Option unless and until such approval is obtained.
| 9. | TERMS
AND CONDITIONS OF STOCK APPRECIATION RIGHTS (SARs) |
A
SAR shall confer on the Grantee a right to receive, upon exercise thereof, the excess of (i) the Fair Market Value of one Share
on the date of exercise over (ii) the SAR Exercise Price. The Award Agreement for a SAR (except those that constitute Substitute
Awards) shall specify the SAR Exercise Price, which shall be fixed on the Grant Date as not less than the Fair Market Value of a Share
on that date. SARs may be granted alone or in conjunction with all or part of an Option or at any subsequent time during the term of
such Option or in conjunction with all or part of any other Award. A SAR granted in tandem with an outstanding Option after the Grant
Date of such Option shall have a SAR Exercise Price that is equal to the Option Price; provided, however, that the SAR
Exercise Price may not be less than the Fair Market Value of a Share on the Grant Date of the SAR to the extent required by Section 409A.
The
Board shall determine at the Grant Date, or thereafter, the time or times at which and the circumstances under which a SAR may be exercised
in whole or in part (including based on achievement of performance goals and/or future service requirements), the time or times at which
SARs shall cease to be or become exercisable after Separation from Service or upon other terms or conditions, the method of exercise,
whether or not a SAR shall be in tandem or in combination with any other Award, and any other terms and conditions of any SAR.
The
term of a SAR granted under the Plan shall be determined by the Board, in its sole discretion, and stated in the related Award Agreement;
provided, however, that such term shall not exceed ten years.
| 9.4. | Payment
of SAR Amount |
Upon
exercise of a SAR, a Grantee shall be entitled to receive payment from the Company (in cash or Shares) in an amount determined by multiplying:
| (i) | the
difference between the Fair Market Value of a Share on the date of exercise over the SAR
Exercise Price; by |
| (ii) | the
number of Shares with respect to which the SAR is exercised. |
| 10. | TERMS
AND CONDITIONS OF RESTRICTED SHARES AND RSUs |
At
the time of grant, the Board may, in its sole discretion, establish a period of time (a “Restricted Period”) and any
additional restrictions including the satisfaction of corporate or individual performance objectives applicable to an Award of Restricted
Shares or RSUs. Each Award of Restricted Shares or RSUs may be subject to a different Restricted Period and additional restrictions.
Neither Restricted Shares nor RSUs may be sold, transferred, assigned, pledged, or otherwise encumbered or disposed of during the Restricted
Period or prior to the satisfaction of any other applicable restrictions.
| 10.2. | Restricted
Share Certificates |
The
Company shall issue, in the name of each Grantee to whom Restricted Shares have been granted, stock certificates or other evidence of
ownership representing the total number of Restricted Shares granted to the Grantee, as soon as reasonably practicable after the Grant
Date. The Board may provide in an Award Agreement that either (i) the Secretary of the Company shall hold such certificates for the Grantee’s
benefit until such time as any Restricted Shares are forfeited to the Company or the restrictions lapse, or (ii) such certificates shall
be delivered to the Grantee; provided, however, that such certificates shall bear a legend or legends that comply with the applicable
securities laws and regulations and make appropriate reference to the restrictions imposed under the Plan and the Award Agreement.
| 10.3. | Rights
of Holders of Restricted Shares |
Unless
the Board otherwise provides in an Award Agreement and subject to Section 17.10, holders of Restricted Shares shall have
rights as Stockholders, including voting and dividend rights.
| 10.4. | Rights
of Holders of RSUs |
| 10.4.1. | Settlement
of RSUs |
RSUs
may be settled in cash or Shares, as determined by the Board and set forth in the Award Agreement. The Award Agreement shall also set
forth whether the RSUs shall be settled (i) within the time period specified for “short term deferrals” under Section 409A
or (ii) otherwise within the requirements of Section 409A, in which case the Award Agreement shall specify upon which events
such RSUs shall be settled.
| 10.4.2. | Voting
and Dividend Rights |
Unless
otherwise stated in the applicable Award Agreement and subject to Section 17.10, holders of RSUs shall not have rights as
Stockholders, including no voting or dividend or dividend equivalents rights.
A
holder of RSUs shall have no rights other than those of a general creditor of the Company. RSUs represent an unfunded and unsecured obligation
of the Company, subject to the applicable Award Agreement.
| 10.5. | Purchase
of Restricted Shares |
The
Grantee shall be required, to the extent required by applicable law, to purchase Restricted Shares from the Company at a Purchase Price
equal to the greater of (i) the aggregate par value of the Shares represented by such Restricted Shares or (ii) the Purchase
Price, if any, specified in the related Award Agreement. If specified in the Award Agreement, the Purchase Price may be deemed paid by
services already rendered. The Purchase Price shall be payable in a form described in Section 11 or, if permitted by the
Board, in consideration for past services rendered.
| 10.6. | Delivery
of Stock Certificates |
Upon
the expiration or termination of any Restricted Period and the satisfaction of any other terms and conditions prescribed by the Board,
the restrictions applicable to Restricted Shares or RSUs settled in Shares shall lapse, and, unless otherwise provided in the Award Agreement,
a stock certificate for such Shares shall be delivered, free of all such restrictions, to the Grantee or the Grantee’s beneficiary
or estate, as the case may be.
| 11. | FORM
OF PAYMENT FOR OPTIONS AND RESTRICTED SHARES |
Payment
of the Option Price for an Option or the Purchase Price for Restricted Shares shall be made in cash or in cash equivalents acceptable
to the Company, except as provided in this Section 11. Notwithstanding any provision of this Section 11, during any
period for which the Common Stock is publicly traded (i.e., the Common Stock is listed on any established stock exchange or a national
market system) an exercise by a Non-Employee Director or officer that involves or may involve a direct or indirect extension of credit
or arrangement of an extension of credit by the Company, directly or indirectly, in violation of Section 402(a) of the Sarbanes-Oxley
Act of 2002 shall be prohibited with respect to any Award under this Plan.
To
the extent the Award Agreement so provides, payment of the Option Price for Shares purchased pursuant to the exercise of an Option or
the Purchase Price for Restricted Shares may be made all or in part through the tender to, or withholding by, the Company of Shares that
shall be valued, for purposes of determining the extent to which the Option Price or Purchase Price for Restricted Shares has been paid
thereby, at their Fair Market Value on the date of exercise or surrender. Notwithstanding the foregoing, in the case of an Incentive
Stock Option, the right to make payment in the form of already owned Shares may be authorized only at the time of grant.
With
respect to an Option only (and not with respect to Restricted Shares), to the extent permitted by law and to the extent the Award Agreement
so provides, payment of the Option Price may be made all or in part by delivery (on a form acceptable to the Company) of an irrevocable
direction to a licensed securities broker acceptable to the Company to sell Shares and to deliver all or part of the sales proceeds to
the Company in payment of the Option Price and any withholding taxes described in Section 17.3.
| 11.4. | Other
Forms of Payment |
To
the extent the Award Agreement so provides, payment of the Option Price or the Purchase Price for Restricted Shares may be made in any
other form that is consistent with applicable laws, regulations, and rules, including the Company’s withholding of Shares otherwise
due to the exercising Grantee.
| 12. | TERMS
AND CONDITIONS OF PERFORMANCE AWARDS |
The
right of a Grantee to exercise or receive a grant or settlement of any Award, and the timing thereof, may be subject to such performance
conditions as may be specified by the Board. The Board may use such business criteria and other measures of performance as it may deem
appropriate in establishing any performance conditions, and may exercise its discretion to reduce the amounts payable under any Award
subject to performance conditions.
| 13. | other
sTOCK-based awards |
| 13.1. | Grant
of Other Stock-based Awards |
Other
Stock-based Awards may be granted either alone or in addition to or in conjunction with other Awards. Other Stock-based Awards may be
granted in lieu of other cash or other compensation to which a Service Provider is entitled from the Company or may be used in the settlement
of amounts payable in Shares under any other compensation plan or arrangement of the Company. Subject to the terms and conditions of
the Plan, the Board shall have the sole and complete authority to determine the persons to whom and the time or times at which such Awards
may be made, the number of Shares to be granted under such Awards, and all other terms and conditions of such Awards. Unless the Board
determines otherwise, any such Award shall be confirmed by an Award Agreement, which shall contain such terms and conditions as the Board
determines to be necessary or appropriate to carry out the intent of the Plan with respect to such Award.
| 13.2. | Terms of Other
Stock-based Awards |
Any
Shares subject to Awards made under this Section 12 may not be sold, assigned, transferred, pledged, or otherwise encumbered
before the date on which the Shares are issued, or, if later, the date on which any applicable restriction, performance, or deferral
period lapses.
The
Company shall not be required to sell or issue any Shares under any Award if the sale or issuance of such Shares would constitute a violation
by the Grantee, any other individual, or the Company of any law or regulation of any governmental authority, including any federal or
state securities laws or regulations. If at any time the Company determines that the listing, registration, or qualification of any Shares
subject to an Award upon any securities exchange or under any governmental regulatory body is necessary or desirable as a term or condition
of, or in connection with, the issuance or purchase of Shares hereunder, no Shares may be issued or sold to the Grantee or any other
individual exercising an Option unless such listing, registration, qualification, consent or approval shall have been effected or obtained
free of any terms and conditions not acceptable to the Company, and any delay caused thereby shall in no way affect the date of termination
of the Award. Specifically, in connection with the Securities Act, upon the exercise of any Option or the delivery of any Shares underlying
an Award, unless a registration statement under such Act is in effect with respect to the Shares covered by such Award, the Company shall
not be required to sell or issue such Shares unless the Board has received evidence satisfactory to it that the Grantee or any other
individual exercising an Option may acquire such Shares under an exemption from registration under the Securities Act. Any determination
in this connection by the Board shall be final, binding, and conclusive. The Company may, but shall not be obligated to, register any
securities covered hereby under the Securities Act. The Company shall not be obligated to take any affirmative action in order to cause
the exercise of an Option or the issuance of Shares under the Plan to comply with any law or regulation of any governmental authority.
As to any jurisdiction that expressly imposes the requirement that an Option shall not be exercisable until the Shares covered by such
Option are registered or are exempt from registration, the exercise of such Option (under circumstances in which the laws of such jurisdiction
apply) shall be deemed conditioned upon the effectiveness of such registration or the availability of such an exemption. The Board may
require the Grantee to sign such additional documentation, make such representations, and furnish such information as the Board may consider
appropriate in connection with the grant of Awards or issuance or delivery of Shares in compliance with applicable laws.
The
Plan is intended to comply with Section 25102(o) of the California Corporations Code, to the extent applicable. In that regard, to the
extent required by Section 25102(o), (1) the terms of any Options or SARs, to the extent vested and exercisable upon a Grantee’s
Separation from Service, shall include any minimum exercise periods following Separation from Service specified by Section 25102(o) and
(2) any repurchase right of the Company with respect to Issued Shares shall include a minimum 90-day notice requirement. Any Plan term
that is inconsistent with Section 25102(o) shall, without further act or amendment by the Company or the Board, be reformed to comply
with the requirements of Section 25102(o).
During
any time when the Company has a class of equity security registered under Section 12 of the Exchange Act, it is the intent of the Company
that Awards and the exercise of Options granted to officers and directors hereunder shall qualify for the exemption provided by Rule
16b-3 under the Exchange Act. To the extent that any provision of the Plan or action by the Board or Committee does not comply with the
requirements of Rule 16b-3, it shall be deemed inoperative to the extent permitted by law and deemed advisable by the Board, and shall
not affect the validity of the Plan. In the event that Rule 16b-3 is revised or replaced, the Board may exercise its discretion to modify
this Plan in any respect necessary to satisfy the requirements of, or to take advantage of any features of, the revised exemption or
its replacement.
| 14.4 | Non-Exempt Employees. |
No
Option granted to a Grantee who is a non-exempt employee for purposes of the Fair Labor Standards Act of 1938, as amended, shall be first
exercisable for any shares of Common Stock until at least six months following the date of grant of the Option. Notwithstanding the foregoing,
consistent with the provisions of the Worker Economic Opportunity Act, in the event of the Grantee’s death or Disability, upon
a Change in Control in which the vesting of such Options accelerates, or upon the Grantee’s retirement (as such term may be defined
in the Grantee’s Award Agreement or in another applicable agreement or in accordance with the Company’s then current employment
policies and guidelines) any such vested Options may be exercised earlier than six months following the date of grant. The foregoing
provision is intended to operate so that any income derived by a non-exempt employee in connection with the exercise or vesting of an
Option shall be exempt from the Grantee’s regular rate of pay.
| 15. | EFFECT
OF CHANGES IN CAPITALIZATION |
| 15.1. | Changes
in Common Stock |
If
(i) the number of outstanding Shares is increased or decreased or the Shares are changed into or exchanged for a different number or
kind of shares or other securities of the Company on account of any recapitalization, reclassification, stock split, reverse split, combination
of shares, exchange of shares, stock dividend or other distribution payable in capital stock, or other increase or decrease in such Shares
effected without receipt of consideration by the Company occurring after the Effective Date or (ii) there occurs any spin-off, split-up,
extraordinary cash dividend, or other distribution of assets by the Company, (A) the number and kinds of shares for which grants of Awards
may be made (including the per-Grantee maximums set forth in Section 4), (B) the number and kinds of shares for which outstanding
Awards may be exercised or settled, and (C) the performance goals relating to outstanding Awards, shall be equitably adjusted by the
Company; provided that any such adjustment shall comply with Section 409A. In addition, in the event of any such increase
or decease in the number of outstanding shares or other transaction described in clause (ii) above, the number and kind of shares for
which Awards are outstanding and the Option Price per share of outstanding Options and SAR Exercise Price per share of outstanding SARs
shall be equitably adjusted; provided that any such adjustment shall comply with Section 409A.
| 15.2. | Effect
of Certain Transactions |
Except
as otherwise provided in an Award Agreement, in the event of a Corporate Transaction, the Plan and the Awards shall continue in effect
in accordance with their respective terms, except that after a Corporate Transaction either (i) each outstanding Award shall be treated
as provided for in the agreement entered into in connection with the Corporate Transaction or (ii) if not so provided in such agreement,
each Grantee shall be entitled to receive in respect of each Share subject to any outstanding Awards, upon exercise or payment or transfer
in respect of any Award, the same number and kind of stock, securities, cash, property, or other consideration that each Stockholder
was entitled to receive in the Corporate Transaction in respect of one Share; provided, however, that, unless otherwise
determined by the Board, such stock, securities, cash, property or other consideration shall remain subject to all of the terms and conditions
(including performance criteria) that were applicable to the Awards before such Corporate Transaction. Without limiting the generality
of the foregoing, the treatment of outstanding Options and SARs under this Section 15.2 in connection with a Corporate Transaction
in which the consideration paid or distributed to the Stockholders is not entirely shares of common stock of the acquiring or resulting
corporation may include the cancellation of outstanding Options and SARs upon consummation of the Corporate Transaction as long as, at
the election of the Board, (A) the holders of affected Options and SARs have been given a period of at least 15 days before the date
of the consummation of the Corporate Transaction to exercise the Options or SARs (to the extent otherwise exercisable) or (B) the holders
of the affected Options and SARs are paid (in cash or cash equivalents) in respect of each Share covered by the Option or SAR being canceled
an amount equal to the excess, if any, of the per Share price paid or distributed to Stockholders in the Corporate Transaction (the value
of any noncash consideration to be determined by the Board) over the Option Price or SAR Exercise Price, as applicable. For avoidance
of doubt, (i) the cancellation of Options and SARs under clause (B) of the preceding sentence may be effected notwithstanding any other
term or condition of the Plan or any Award Agreement and (ii) if the amount determined under clause (B) of the preceding sentence is
zero or less, the affected Option or SAR may be cancelled without any payment therefore. The treatment of any Award as provided in this
Section 15.2 shall be conclusively presumed to be appropriate for purposes of Section 15.1.
Subject
to the requirements and limitations of Section 409A, if applicable, the Board may provide for any one or more of the following in connection
with a Change in Control, which such actions need not be the same for all Grantees:
| (i) | Accelerated
Vesting. Unless otherwise provided in any Award Agreement, upon a Grantee’s Separation
from Service immediately prior to, upon, or following a Change in Control for any reason
other than Cause, the exercisability, vesting and/or settlement of an Award shall immediately
accelerate. |
| (ii) | Assumption,
Continuation or Substitution. In the event of a Change in Control, the surviving, continuing,
successor, or purchasing corporation or other business entity or parent thereof, as the case
may be (the “Acquiror”), may, without the consent of any Grantee, either
assume or continue the Company’s rights and obligations under each or any Award or
portion thereof outstanding immediately prior to the Change in Control or substitute for
each or any such outstanding Award or portion thereof a substantially equivalent award with
respect to the Acquiror’s stock, as applicable. For purposes of this Section 15.3,
if so determined by the Board, in its discretion, an Award denominated in Shares shall be
deemed assumed if, following the Change in Control, the Award confers the right to receive,
subject to the terms and conditions of the Plan and the applicable Award Agreement, for each
Share subject to the Award immediately prior to the Change in Control, the consideration
(whether stock, cash, other securities or property or a combination thereof) to which a holder
of a Share on the effective date of the Change in Control was entitled; provided, however,
that if such consideration is not solely common stock of the Acquiror, the Board may, with
the consent of the Acquiror, provide for the consideration to be received upon the exercise
or settlement of the Award, for each Share subject to the Award, to consist solely of common
stock of the Acquiror equal in Fair Market Value to the per share consideration received
by holders of Shares pursuant to the Change in Control. If any portion of such consideration
may be received by holders of Shares pursuant to the Change in Control on a contingent or
delayed basis, the Board may, in its sole discretion, determine such Fair Market Value per
share as of the time of the Change in Control on the basis of the Board’s good faith
estimate of the present value of the probable future payment of such consideration. Any Award
or portion thereof which is neither assumed or continued by the Acquiror in connection with
the Change in Control nor exercised or settled as of the time of consummation of the Change
in Control shall terminate and cease to be outstanding effective as of the time of consummation
of the Change in Control. |
| (iii) | Cash-Out
of Awards. The Board may, in its discretion and without the consent of any Grantee, determine
that, upon the occurrence of a Change in Control, each or any Award or a portion thereof
outstanding immediately prior to the Change in Control and not previously exercised or settled
shall be canceled in exchange for a payment with respect to each vested Share (and each unvested
Share, if so determined by the Board) subject to such canceled Award in (i) cash, (ii) stock
of the Company or of a corporation or other business entity a party to the Change in Control,
or (iii) other property which, in any such case, shall be in an amount having a Fair Market
Value equal to the Fair Market Value of the consideration to be paid per Share in the Change
in Control, reduced by the exercise or purchase price per share, if any, under such Award.
If any portion of such consideration may be received by holders of Shares pursuant to the
Change in Control on a contingent or delayed basis, the Board may, in its sole discretion,
determine such Fair Market Value per share as of the time of the Change in Control on the
basis of the Board’s good faith estimate of the present value of the probable future
payment of such consideration. In the event such determination is made by the Board, the
amount of such payment (reduced by applicable withholding taxes, if any) shall be paid to
Grantees in respect of the vested portions of their canceled Awards as soon as practicable
following the date of the Change in Control and in respect of the unvested portions of their
canceled Awards in accordance with the vesting schedules applicable to such Awards. The Board
may, in its discretion, without payment of any consideration to the Grantee, cancel any outstanding
Award to the extent not vested or exercised immediately prior to the Change in Control and
not otherwise assumed or continued by the Acquiror in accordance with Section 15.3(ii)
above. |
Adjustments
under this Section 15 related to Shares or other securities of the Company shall be made by the Board, whose determination
in that respect shall be final, binding and conclusive. No fractional Shares or other securities shall be issued under any such adjustment,
and any fractions resulting from any such adjustment shall be eliminated in each case by rounding downward to the nearest whole Share.
| 16. | No
Limitations on Company |
The
grant of Awards shall not affect or limit in any way the right or power of the Company to make adjustments, reclassifications, reorganizations,
or changes of its capital or business structure or to merge, consolidate, dissolve, or liquidate, or to sell or transfer all or any part
of its business or assets.
| 17. | TERMS
APPLICABLE GENERALLY TO AWARDS |
| 17.1. | Disclaimer
of Rights |
No
term or condition of the Plan or any Award Agreement shall be construed to confer upon any individual the right to remain in the employ
or service of the Company or any Affiliate, or to interfere in any way with any contractual or other right or authority of the Company
or any Affiliate either to increase or decrease the compensation or other payments to any individual at any time, or to terminate any
employment or other relationship between any individual and the Company. In addition, notwithstanding any other term or condition of
the Plan, unless otherwise stated in the applicable Award Agreement, no Award shall be affected by any change of duties or position of
the Grantee, so long as such Grantee continues to be a Service Provider. The obligation of the Company to pay any benefits under the
Plan shall be interpreted as a contractual obligation to pay only those amounts described herein, in the manner and under the terms and
conditions prescribed herein. The Plan shall in no way be interpreted to require the Company to transfer any amounts to a third party
trustee or otherwise hold any amounts in trust or escrow for payment to any Grantee or beneficiary under the Plan.
| 17.2. | Nonexclusivity
of the Plan |
Neither
the adoption of the Plan nor the submission of the Plan to the Stockholders for approval shall be construed as creating any limitations
upon the right and authority of the Board to adopt such other incentive compensation arrangements (which arrangements may be applicable
either generally to a class or classes of individuals or specifically to a particular individual or particular individuals), including,
without limitation, the granting of Options as the Board determines desirable.
The
Company or an Affiliate, as the case may be, shall have the right to deduct from payments of any kind otherwise due to a Grantee any
federal, state, or local taxes of any kind required by law to be withheld (i) with respect to the vesting of or other lapse of restrictions
applicable to an Award, (ii) upon the issuance of any Shares upon the exercise of an Option or SAR, or (iii) otherwise due in connection
with an Award. At the time of such vesting, lapse, or exercise, the Grantee shall pay to the Company or the Affiliate, as the case may
be, any amount that the Company or the Affiliate may reasonably determine to be necessary to satisfy such withholding obligation. The
Company or the Affiliate, as the case may be, may require or permit the Grantee to satisfy such obligations, in whole or in part, (A)
by causing the Company or the Affiliate to withhold up to the maximum required number of Shares otherwise issuable to the Grantee as
may be necessary to satisfy such withholding obligation or (B) by delivering to the Company or the Affiliate Shares already owned by
the Grantee. The Shares so delivered or withheld shall have an aggregate Fair Market Value equal to such withholding obligations. The
Fair Market Value of the Shares used to satisfy such withholding obligation shall be determined by the Company or the Affiliate as of
the date that the amount of tax to be withheld is to be determined. To the extent applicable, a Grantee may satisfy any withholding obligation
only with Shares that are not subject to any repurchase, forfeiture, unfulfilled vesting, or other similar requirements.
| 17.4. | Other
Terms and Conditions; Employment Agreements |
Each
Award Agreement may contain such other terms and conditions not inconsistent with the Plan as may be determined by the Board, in its
sole discretion. In the event of any conflict between the terms and conditions of an employment agreement and the Plan, the terms and
conditions of the employment agreement shall govern.
If
any term or condition of the Plan or any Award Agreement is determined to be illegal or unenforceable by any court of law in any jurisdiction,
the remaining terms and conditions hereof and thereof shall be severable and enforceable, and all terms and conditions shall remain enforceable
in any other jurisdiction.
The
Plan and all Award Agreements shall be construed in accordance with and governed by the laws of the State of Delaware without regard
to the principles of conflicts of law that could cause the application of the laws of any jurisdiction other than the State of Delaware.
For purposes of resolving any dispute that arises under the Plan, each Grantee, by virtue of receiving an Award, shall be deemed to have
submitted to and consented to the exclusive jurisdiction of the State of Florida and to have agreed that any related litigation shall
be conducted solely in the courts of Miami-Dade County or the federal courts for the U.S. for the Southern District of Florida, where
the Plan is made and to be performed, and no other courts. The Plan is not intended to be subject to the Employee Retirement Income Security
Act of 1974.
The
Plan is intended to comply with Section 409A to the extent subject thereto, and, accordingly, to the maximum extent permitted, the
Plan shall be interpreted and administered to be in compliance therewith. Any payments described in the Plan that are due within the
“short-term deferral period” as defined in Section 409A shall not be treated as deferred compensation unless applicable
laws require otherwise. For purposes of Section 409A, each installment payment under the Plan shall be treated as a separate payment.
Notwithstanding any other term or condition of the Plan, to the extent required to avoid accelerated taxation or tax penalties under
Section 409A, amounts that would otherwise be payable and benefits that would otherwise be provided under the Plan during the six-month
period immediately after the Grantee’s Separation from Service shall instead be paid on the first payroll date after the six-month
anniversary of the Grantee’s Separation from Service (or the Grantee’s death, if earlier). Notwithstanding the foregoing,
neither the Company nor the Board shall have any obligation to take any action to prevent the assessment of any additional tax or penalty
on any Grantee under Section 409A and neither the Company nor the Board shall have any liability to any Grantee for such tax or
penalty.
| 17.8. | Separation
from Service |
The
Board shall determine the effect of a Separation from Service upon Awards, and such effect shall be set forth in the appropriate Award
Agreement. Without limiting the foregoing, the Board may provide in the Award Agreements at the time of grant, or any time thereafter
with the consent of the Grantee, the actions that may be taken upon the occurrence of a Separation from Service, including accelerated
vesting or termination, depending upon the circumstances surrounding the Separation from Service.
| 17.9. | Transferability
of Awards and Issued Shares |
| 17.9.1. | Transfers
in General |
Except
as provided in Section 17.9.2, no Award shall be assignable or transferable by the Grantee to whom it is granted, other than
by will or the laws of descent and distribution, and, during the lifetime of the Grantee, only the Grantee personally (or the Grantee’s
personal representative) may exercise rights under the Plan.
If
authorized in the applicable Award Agreement, a Grantee may transfer, not for value, all or part of an Award (other than Incentive Stock
Options) to any Family Member. For the purpose of this Section 17.9.2, a “not for value” transfer is a transfer
that is (i) a gift, (ii) a transfer under a domestic relations order in settlement of marital property rights; or (iii) a
transfer to an entity in which more than 50% of the voting interests are owned by Family Members (or the Grantee) in exchange for an
interest in that entity. After a transfer under this Section 17.9.2, any such Award shall continue to be subject to the same
terms and conditions as were applicable immediately before transfer. Subsequent transfers of transferred Awards are prohibited except
to Family Members of the original Grantee in accordance with this Section 17.9.2 or by will or the laws of descent and distribution.
| 17.10. | Dividend
Equivalent Rights |
If
specified in the Award Agreement, the recipient of an Award may be entitled to receive dividend equivalent rights with respect to the
Shares or other securities covered by an Award. The terms and conditions of a dividend equivalent right may be set forth in the Award
Agreement. Dividend equivalents credited to a Grantee may be paid in cash or deemed to be reinvested in additional Shares or other securities
of the Company at a price per unit equal to the Fair Market Value of a Share on the date that such dividend was paid to Stockholders.
Notwithstanding the foregoing, dividends or dividend equivalents shall not be paid on any Award or portion thereof that is unvested or
on any Award that is subject to the achievement of performance criteria before the Award has become earned and payable.
A
Grantee’s acceptance of an Award shall be deemed to constitute the Grantee’s acknowledgement of and consent to the collection
and processing of personal data relating to the Grantee so that the Company can meet its obligations and exercise its rights under the
Plan and generally administer and manage the Plan. This data shall include data about participation in the Plan and Shares offered or
received, purchased, or sold under the Plan and other appropriate financial and other data (such as the date on which the Awards were
granted) about the Grantee and the Grantee’s participation in the Plan.
| 17.12. | Disqualifying
Dispositions |
Any
Grantee who shall make a “disposition” (as defined in Section 424 of the Code) of all or any portion of Shares acquired upon
exercise of an Incentive Stock Option within two years from the Grant Date of such Incentive Stock Option or within one year after the
issuance of the Shares acquired upon exercise of such Incentive Stock Option shall be required to immediately advise the Company in writing
as to the occurrence of the sale and the price realized upon the sale of such shares of Common Stock.
In
the Plan, unless otherwise stated, the following uses apply:
| |
(i) |
references to a statute or
law refer to the statute or law and any amendments and any successor statutes or laws, and to all valid and binding governmental regulations,
court decisions, and other regulatory and judicial authority issued or rendered thereunder, as amended, or their successors, as in effect
at the relevant time; |
| |
|
|
| |
(ii) |
in computing periods from a
specified date to a later specified date, the words “from” and “commencing on” (and the like) mean “from
and including,” and the words “to,” “until” and “ending on” (and the like) mean “to and
including”; |
| |
|
|
| |
(iii) |
indications of time of day
shall be based upon the time applicable to the location of the principal headquarters of the Company; |
| |
|
|
| |
(iv) |
the words “include,”
“includes” and “including” (and the like) mean “include, without limitation,” “includes, without
limitation” and “including, without limitation” (and the like), respectively; |
| |
|
|
| |
(v) |
all references to articles
and sections are to articles and sections in the Plan; |
| |
|
|
| |
(vi) |
all words used shall be construed
to be of such gender or number as the circumstances and context require; |
| |
|
|
| |
(vii) |
the captions and headings of
articles and sections have been inserted solely for convenience of reference and shall not be considered a part of the Plan, nor shall
any of them affect the meaning or interpretation of the Plan; |
| |
|
|
| |
(viii) |
any reference to an agreement,
plan, policy, form, document or set of documents, and the rights and obligations of the parties under any such agreement, plan, policy,
form, document or set of documents, shall mean such agreement, plan, policy, form, document or set of documents as amended from time
to time, and any and all modifications, extensions, renewals, substitutions or replacements thereof; and |
| |
|
|
| |
(ix) |
all accounting terms not specifically
defined shall be construed in accordance with GAAP. |

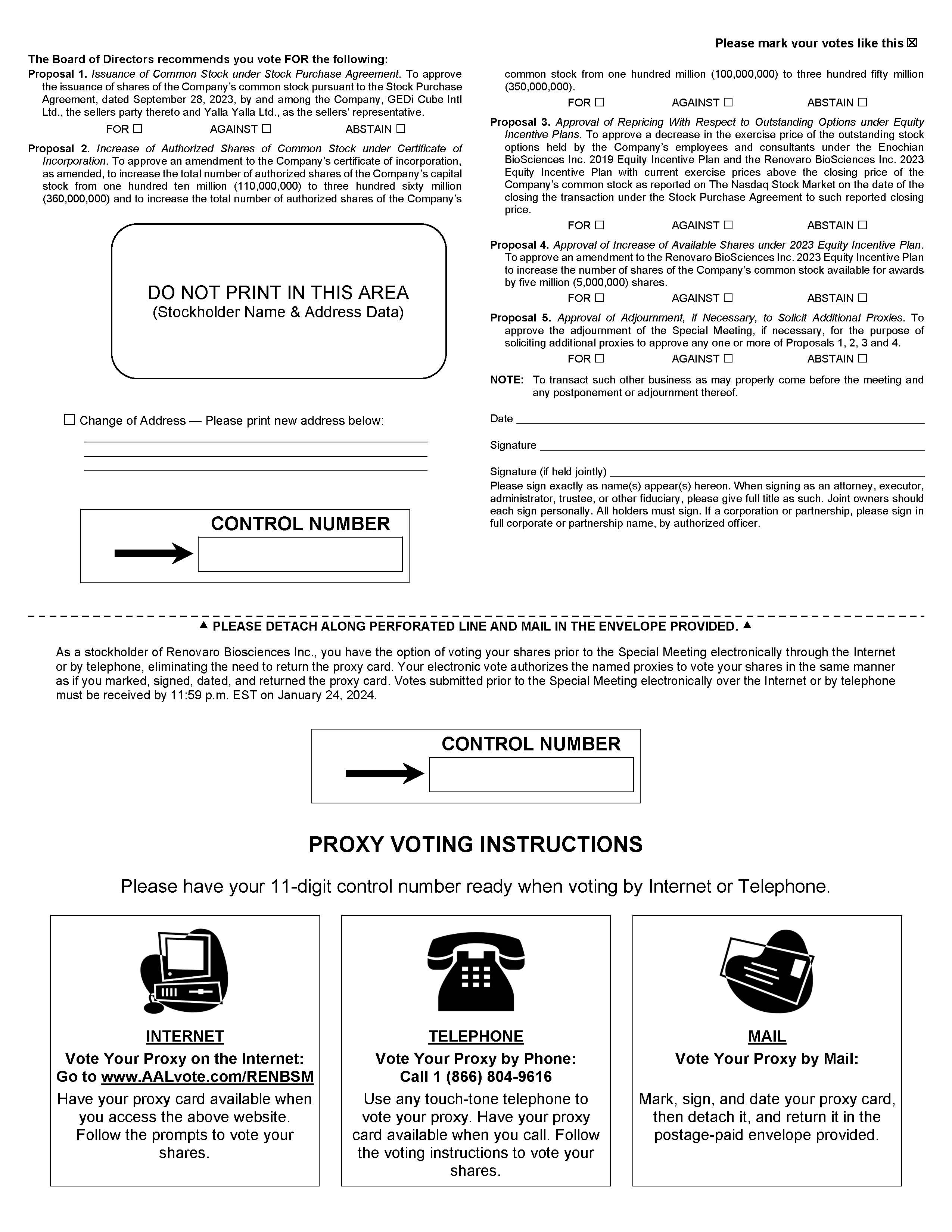
Grafico Azioni Enochian Biosciences (NASDAQ:ENOB)
Storico
Da Nov 2024 a Dic 2024

Grafico Azioni Enochian Biosciences (NASDAQ:ENOB)
Storico
Da Dic 2023 a Dic 2024
