false--12-310001498382Kintara Therapeutics, Inc.0001498382dei:FormerAddressMember2024-10-182024-10-1800014983822024-10-182024-10-18
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): October 18, 2024 |
TUHURA BIOSCIENCES, INC.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Nevada |
001-37823 |
99-0360497 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
10500 University Dr., Suite 110 |
Tampa, Florida 33612 |
(Address of Principal Executive Offices, including zip code) |
|
Registrant’s Telephone Number, Including Area Code: (813) 875-6600 |
|
Kintara Therapeutics, Inc. 9920 Pacific Heights Blvd, Suite 150 San Diego, California 92121 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
|
|
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, $0.001 par value per share |
|
HURA |
|
The Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Explanatory Note
On October 18, 2024, the Nevada corporation formerly known as “Kintara Therapeutics, Inc.” completed its previously announced merger transaction in accordance with the terms of the Agreement and Plan of Merger, dated as of April 2, 2024 (the “Merger Agreement”), by and among Kintara Therapeutics, Inc. (“Kintara”), TuHURA Biosciences, Inc. (“TuHURA”), and Kayak Mergeco, Inc., a direct wholly owned subsidiary of Kintara (“Merger Sub” and collectively with Kintara and TuHURA, the “Parties”), pursuant to which Merger Sub merged with and into TuHURA, with TuHURA surviving as a direct wholly owned subsidiary of Kintara and the surviving corporation of the merger (the “Merger”). Additionally, as a result of the Merger, Kintara changed its name (the “Name Change”) from “Kintara Therapeutics, Inc.” to “TuHURA Biosciences, Inc.” Unless the context otherwise requires, “we,” “us,” “our,” and the “Company” refer to TuHURA Biosciences, Inc., a Nevada corporation, and its wholly owned subsidiaries. See Item 2.01 for additional information regarding completion of the Merger.
|
|
Item 1.01. |
Entry into a Material Definitive Agreement. |
Contingent Value Rights Agreement
In connection with the Merger, the Company entered into a Contingent Value Rights Agreement (the “CVR Agreement”) with Equiniti Trust Company, LLC (the “Rights Agent”), pursuant to which the Kintara common stockholders and Kintara common stock warrant holders of record as of immediately prior to the consummation of the Merger and Reverse Stock Split (as defined below) received one contingent value right (“CVR”) for each outstanding share of common stock of Kintara held by such stockholder (or, in the case of warrants, each share of common stock of Kintara for which such warrant is exercisable into). Pursuant to the CVR Agreement, upon the achievement of the Milestone (as defined below), the holders of CVRs are entitled, in aggregate, to receive approximately 1,539,918 shares of common stock of the Company (which gives effect to the Reverse Stock Split) (collectively, the “CVR Shares”).
Each CVR shall entitle the holder thereof to receive its portion of the CVR Shares if the Company (i) enrolls a minimum of ten cutaneous metastatic breast cancer patients in a study to determine whether a dose of REM-001 lower than 1.2 mg/kg elicits a treatment effect similar to that seen in prior studies of REM-001 at the 1.2 mg/kg dose and (ii) such patients enrolled in the study complete eight weeks of follow-up, in each case, on or before December 31, 2025 (the “Milestone”).
The payment date for the CVR Shares will be within 10 business days after the Rights Agent receives the CVR Shares as the payment for achievement of the Milestone. In the event that the Milestone is not achieved, holders of the CVRs will not receive any CVR Shares pursuant to the CVR Agreement. There can be no assurances that any holders of CVRs will receive any CVR Shares with respect thereto. Some CVR Shares that would otherwise be received may be withheld on account of taxes if the Company or the applicable withholding agent determines that tax withholding is required in connection with the delivery of CVR Shares or that there was a failure to withhold adequately in respect of the distribution of the CVRs.
The CVRs are not transferable, except in certain limited circumstances as will be provided in the CVR Agreement, will not be certificated or evidenced by any instrument, and will not be listed for trading on any exchange. The foregoing description of the CVR Agreement contained herein does not purport to be complete and is qualified in its entirety by reference to the full text of the CVR Agreement, which is attached hereto as Exhibit 10.1 and incorporated herein by reference.
Indemnification Agreements
In connection with the Merger, on October 18, 2024, the Company entered into indemnification agreements with each of its new directors and executive officers. Each indemnification agreement provides for indemnification and advancements by the Company of certain expenses and costs relating to claims, suits or proceedings arising from each individual’s service to the Company as an officer or director, as applicable, to the maximum extent permitted by applicable law.
The foregoing description of the indemnification agreements is qualified in its entirety by the full text of the form of indemnification agreement, which is attached hereto as Exhibit 10.2 and incorporated herein by reference.
Waiver
On October 18, 2024, the Parties entered into a Waiver Agreement (the “Waiver Agreement”) pursuant to which the Parties waived the requirements in the Merger Agreement that (i) Kintara deliver a Parent Closing Net Cash amount at Closing in accordance with Annex I of the Merger Agreement and (ii) TuHURA deliver Lock-Up Agreements representing no less than 50% of the outstanding shares of TuHURA common stock on an “as converted” basis. TuHURA delivered at closing, Lock-Up Agreements representing approximately 34% of the “as converted’ shares of TuHURA.
The preceding summary of the Waiver Agreement does not purport to be complete and is qualified in its entirety by reference to the Waiver Agreement, which is filed as Exhibit 2.1 hereto, and which is incorporated herein by reference.
|
|
Item 2.01. |
Completion of Acquisition or Disposition of Assets. |
As previously disclosed, on April 2, 2024, Kintara, Merger Sub and TuHURA entered into the Merger Agreement, pursuant to which Merger Sub merged with and into TuHURA, with TuHURA surviving as a direct wholly owned subsidiary of Kintara and the surviving corporation of the Merger. On October 18, 2024, Kintara, Merger Sub and TuHURA consummated the transactions contemplated by the Merger Agreement. Effective at 12:01 a.m. Eastern Time on October 18, 2024, Kintara effected a 1-for-35 reverse stock split of its common stock (the “Reverse Stock Split”). Effective at 12:03 a.m. Eastern Time on October 18, 2024, the Company completed the Merger, and effective at 12:04 a.m. Eastern Time on October 18, 2024, the Company changed its name to “TuHURA Biosciences, Inc.” (the “Name Change”). Following the completion of the Merger, the business conducted by the Company became primarily the business conducted by TuHURA, which is a clinical stage immune-oncology company developing novel personalized cancer vaccine product candidates designed to overcome primary resistance to immunotherapies like checkpoint inhibitors. Unless noted otherwise, all references to share and per share amounts in this Current Report on Form 8-K reflect the Reverse Stock Split.
Under the terms of the Merger, immediately prior to the effective time of the Merger, shares of TuHURA’s preferred stock were converted into shares of TuHURA’s common stock and all of the convertible notes issued in TuHURA’s private placement (the “TuHURA Note Financing”) were converted into shares of TuHURA common stock pursuant to the terms therein. At the effective time of the Merger, the Company issued an aggregate of approximately 40,441,605 shares of its common stock to TuHURA stockholders, based on an exchange ratio of 0.1789 (after giving effect to the Reverse Stock Split) shares of the Company’s common stock for each share of TuHURA common stock outstanding immediately prior to the Merger. As a result, after giving effect to the issuance, the Company had approximately 42,030,336 shares of the Company’s common stock issued and outstanding. Immediately following the Merger, TuHURA stockholders as of immediately prior to the Merger owned, in aggregate and on a fully-diluted basis, approximately 97.15% of the Company (or 94.55% of the Company after giving effect to the issuance of the CVR Shares assuming the Milestone has been achieved) and Kintara securityholders as of immediately prior to the Merger owned, in aggregate and on a fully-diluted basis, approximately 2.85% of the Company (or 5.45%) of the Company after giving effect to the issuance of the CVR Shares assuming the Milestone has been achieved).
The issuance of the shares of the Company’s common stock to the former stockholders of TuHURA was registered with the SEC on the Company’s Registration Statement on Form S-4 (File No. 333-279368), as amended.
The shares of Kintara’s common stock listed on the Nasdaq Capital Market, previously trading through the close of business on Thursday, October 17, 2024 under the ticker symbol “KTRA,” commenced trading on the Nasdaq Capital Market on a post-Reverse Stock Split adjusted basis and post-Merger basis under the ticker symbol “HURA” on Friday, October 18, 2024. The Company’s common stock is represented by a new CUSIP number, 898920 103.
The foregoing description of the Merger and the Merger Agreement contained herein does not purport to be complete and is qualified in its entirety by reference to the full text of the Merger Agreement, which is attached hereto as Exhibit 2.1 and incorporated herein by reference.
FORM 10 INFORMATION
Cautionary Note Regarding Forward-Looking Statements
This Current Report on Form 8-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”) and 21E of the Exchange Act of 1934, as amended (the “Exchange Act”), including statements regarding the anticipated benefits of the Merger and the financial condition, results of operations, and prospects of the Company. These statements may discuss goals, intentions and expectations as to future plans, trends, events, results of operations or financial condition, or otherwise, based on current expectations and beliefs of the management of the Company, as well as assumptions made by, and information currently available to, the management of the Company. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as “may,” “will,” “should,” “would,” “expect,” “anticipate,” “plan,” “likely,” “believe,” “estimate,” “project,” “intend,” and other similar expressions or the negative or plural of these words, or other similar expressions that are predictions or indicate future events or prospects, although not all forward-looking statements contain these words. Statements that are not historical facts are forward-looking statements. Forward-looking statements in this communication include, but are not limited to, the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in this Current Report on Form 8-K. Forward-looking statements are based on current beliefs and assumptions that are subject to risks and uncertainties and are not guarantees of future performance. Forward-looking statements include, but are not limited to, any statements regarding the strategies, prospects, plans, expectations or objectives of management the Company for future operations, the progress, scope or timing of the development of the Company’s product candidates, the expectations surrounding the potential safety, efficacy, and regulatory and clinical progress of the Company’s product candidates, and anticipated milestones and timing therefor, the benefits that may be derived from any future products or the commercial or market opportunity with respect to any future products of the Company, the ability of the Company to protect its intellectual property rights, the anticipated operations, financial position, ability to raise capital to fund operations, revenues, costs or expenses of the Company, statements regarding future economic conditions or performance, statements of belief and any statement of assumptions underlying any of the foregoing. Forward-looking statements may also include any statements regarding the Merger, including the location and management of the Company, the percentage ownership of the Company, the contingent payments contemplated by the CVRs, the Company’s expected cash and the sufficiency of the Company’s cash, cash equivalents and short-term investments to fund operations, and any statement of assumptions underlying any of the foregoing.
The foregoing review of important factors that could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors included in the “Risk Factors” section of this Current Report on Form 8-K and other documents to be filed by the Company from time to time with the SEC discussions of potential risks, uncertainties, and other important factors in the Company’s subsequent filings with the SEC, and risk factors associated with companies, such as the Company, that operate in the biopharma industry. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond the Company’s control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements. Nothing in this communication should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that the contemplated results of any such forward-looking statements will be achieved. Forward-looking statements in this communication speak only as of the day they are made and are qualified in their entirety by reference to the cautionary statements herein. Except as required by applicable law, the Company undertakes no obligation to revise or update any forward-looking statement, or to make any other forward-looking statements, whether as a result of new information, future events or otherwise.
Business, Facilities and Legal Proceedings
Overview
We are a clinical stage immuno-oncology company developing novel personalized cancer vaccine product candidates designed to overcome primary resistance to immunotherapies like checkpoint inhibitors. We have entered into a Special Protocol Assessment agreement with the FDA for a single Phase 3 randomized placebo and injection controlled trial for IFx-2.0, our lead personalized cancer vaccine product candidate, as adjunctive therapy to pembrolizumab (Keytruda®) in the first line treatment of patients with advanced or metastatic Merkel cell carcinoma who are checkpoint inhibitor naïve utilizing the FDA’s accelerated approval pathway. We are also developing novel
bi-functional antibody drug conjugates, or ADCs, targeting myeloid derived suppressor cells, or MDSCs, to modulate their immunosuppressive effects on the tumor microenvironment to overcome acquired resistance to immunotherapies.
IFx Personalized Cancer Vaccines
We have developed Immune FxTM, or IFx, as a personalized cancer vaccine technology designed to “trick” the body’s immune system to attack tumor cells by making tumor cells look like bacteria and to thereby harness the natural power of innate immunity by leveraging natural mechanisms conserved throughout evolution to recognize threats from foreign pathogens like bacteria or viruses. Our personalized cancer vaccine product candidates are delivered either via intratumoral injection (in the case of the company’s pDNA vaccine product candidate) or tumor targeted via intravenous or autologous whole-cell administration (in the case of our mRNA vaccine product candidate).
Our IFx-2.0 personalized cancer vaccine, the company’s lead product candidate, is comparatively simple to administer and involves only the injection into a patient’s tumor of a relatively small amount of pDNA that is designed to encode for an immunogenic bacterial protein that gets expressed on the surface of the patient’s tumor so that the surface of the tumor looks like a bacterium. By making the surface of a tumor look like a bacterium, it is designed to trigger a patient-specific immune response and use each patient’s tumor itself as the source of distinctive foreign neoantigens to prime and initiate a patient’s innate immune response against the tumor irrespective of whether the tumor escaped immune recognition prior to IFx-2.0 administration. In doing so, IFx-2.0 is designed to harness the power of the patient’s innate immune response, which has evolved over time to be conserved to detect foreign pathogens like bacterial proteins.
We have entered into a Special Protocol Assessment agreement with the FDA for a single Phase 3 randomized placebo and injection controlled trial for IFx-2.0 as adjunctive therapy to pembrolizumab (Keytruda®) in the first line treatment of patients with advanced or metastatic Merkel cell carcinoma, or MCC, who are checkpoint inhibitor-naïve utilizing the FDA’s accelerated approval pathway. We believe that our company has worked with the FDA on a unique trial design such that data from the primary endpoint of overall response rate, or ORR, that is of sufficient magnitude and duration and with a favorable risk/benefit profile could be sufficient to support accelerated approval. Further, we believe that results from a key secondary endpoint of progression-free survival, or PFS, that is adequately powered with statistical assumptions in the statistical analysis plan provided to the FDA, if achieved without a detrimental effect on overall survival, or OS, could be adequate to support conversion to regular approval satisfying the requirement for a confirmatory trial.
As set forth in a January 2024 partial clinical trial hold letter from the FDA regarding the chemistry, manufacturing, and controls (CMC) requirements for our planned Phase 3 trial for IFx-2.0 to be conducted under the Special Protocol Assessment agreement, the FDA is requiring that, prior to initiating the trial, we must provide additional drug substance and drug product information from our contract manufacturers for the trial because the final drug product is intended for a registration-directed trial with potential accelerated approval. In addition, we must qualify and validate a potency assay and qualify the mixing process for IFx-2.0 to be used at the clinical site. We are working with our contract manufacturers to provide the required additional information and, based on correspondence following a type C meeting with the FDA, have planned and are undertaking ongoing in vitro testing, development, and validation adequate intended to address the other requirements to initiate the Phase 3 clinical trial. The company currently believes it may be in position to initiate the Phase 3 study in the first quarter of 2025 if the results of the in-mixing studies and potency assay testing are acceptable to the FDA, but there is no assurance that we will be able to satisfy the requirements set forth in the partial clinical trial hold letter on a timely basis or at all. We anticipate that enrollment for the Phase 3 would take approximately 12 months, with topline data potentially being available 6 to 7 months following the last patient enrolled. If successful, this Phase 3 trial would form the basis of a Biologics License Application, or BLA. A Special Protocol Assessment agreement is a binding written agreement between the U.S. Food and Drug Administration (FDA) and a trial sponsor that indicates the study’s design and analysis are adequate to support an application submission. A Special Protocol Assessment agreement does not increase the likelihood of marketing approval for the product and may not lead to a faster or less costly development, review, or approval process.
We are also developing our IFx-3.0 cancer vaccine product candidate, an mRNA cancer vaccine candidate for intravenous or autologous whole cell administration for blood-related cancers, to expand the utility of our IFx™ technology to tumor types not accessible by intra-tumoral injection. We are designing our mRNA vaccine to be carried by a unique spleen and/or bone marrow compartment targeted lipid nanoparticle (“LNP”) coupled to an
antibody which is intended to recognize and target CD22, a receptor overexpressed on B cell cancers like lymphoma. We believe that our novel LNP-anti CD22 construct may be the first intravenously administered, tumor-targeted mRNA vaccine product candidate in preclinical development. Subject to further testing and development, we believe that systemically targeting a tumor with our mRNA vaccine should induce a more widespread innate immune response given the larger tumor burden associated with blood-related malignancies than with localized injection into small cutaneous or other accessible lesions.
Bi-Functional Antibody Drug Conjugates (ADCs): Delta Receptor Technology
In addition to its cancer vaccine product candidates, we are using proprietary Delta receptor technology to develop small molecule or bifunctional ADCs designed to inhibit the immune suppressing effects of MDSCs on the tumor microenvironment to prevent T cell exhaustion and acquired resistance to checkpoint inhibitors. Our Delta receptor technology was acquired in January 2023 when the company acquired the intellectual property assets of TuHURA Biopharma, Inc.
The tumor microenvironment, or TME, is the tissue surrounding a tumor, including the normal cells, blood vessels, and molecules that surround and feed a tumor cell and shield it from immune attack and eradication. MDSCs are a heterogeneous group of immature myeloid cells that are characterized by the ability to suppress both innate and adaptive immune responses. Myeloid cells are a type of blood cell that originates in the bone marrow and mature into adult blood cells that take on different roles in the bloodstream. MDSCs are generally believed to be responsible for T cell exhaustion (which is the loss of ability of T cells to kill cancer cells) and for acquired resistance to checkpoint inhibitors and cellular therapies like T cell therapies. We are developing small molecule Delta receptor selective inhibitors, to incorporate into its bifunctional ADCs, which we believe represents a paradigm shift from conventional, in development or marketed, ADCs. ADCs are a class of drugs in which a monoclonal antibody is chemically linked to a cancer-fighting substances. Our bifunctional ADCs in development are designed to utilize a small molecule drug to target and inhibit the Delta receptor, reprograming MDSCs’ function and removing their potent immune suppressing effects on the tumor microenvironment while simultaneously localizing an immune effector like a checkpoint inhibitor to where the tumor resides to overcome acquired resistance to immunotherapies and reduce potential indiscriminate toxicity to normal tissues by checkpoint released cytotoxic T cells.
Our Pipeline
We are leveraging our technology platforms to advance several diversified product candidates, including the following:
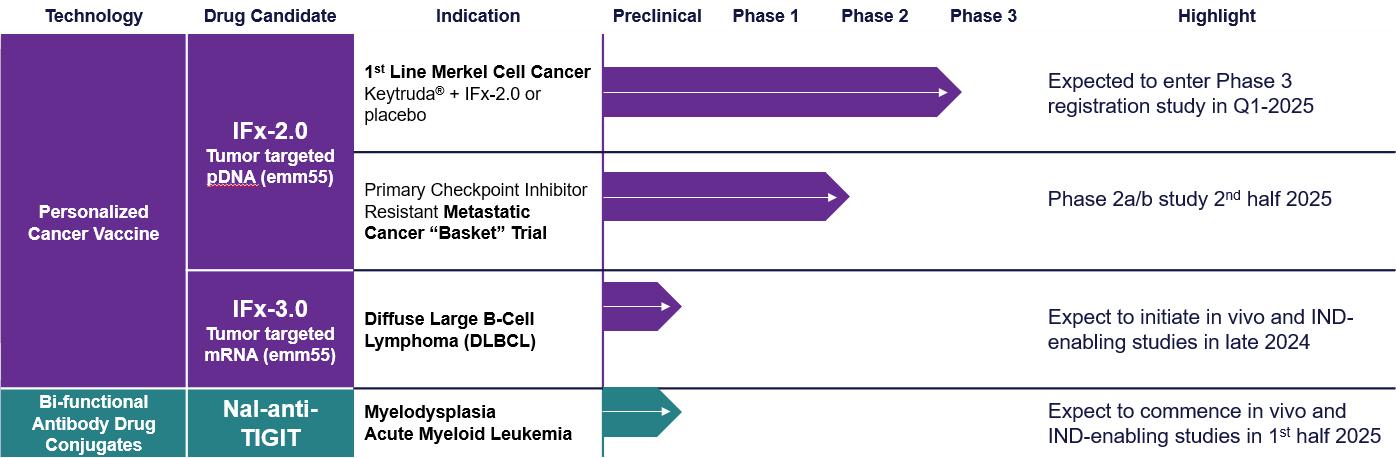
IFx-2.0 Personalized Cancer Vaccine. IFx-2.0 is our lead personalized cancer vaccine product candidate. We received guidance from and worked with the FDA’s Office of Tissues and Advanced Therapies and Oncology Center of Excellence in developing the Phase 3 trial for IFx-2.0. For a description of the planned Phase 3 trial.
IFx-2.0 Phase 1b/2a Basket Trial. We are planning a Phase 1b/2a trial referred to as a “basket” trial, which is a type of clinical trial that tests a new product candidate in patients who have different types of cancer but common biologic reason for resistance to checkpoint inhibitors. The phase 2a stage of the trial will examine the feasibility and safety of Keytruda® and adjunctive IFx-2.0 where IFx-2.0 is administered via interventional radiology into lesions in the liver, retroperitoneal or lungs of patients who have advanced and metastatic Merkel cell carcinoma who are checkpoint inhibitor naïve. The Phase 2b stage of the trial will include patients with checkpoint inhibitor resistant ovarian and triple negative breast cancer. We currently anticipate initiating this study in second half of 2025. If successful, this trial could expand the utility of IFx-2.0 beyond advanced MCC.
IFx-3.0. IFx-3.0 is our mRNA cancer vaccine product candidate for intravenous or autologous whole cell administration. We believe that advancing an mRNA personalized cancer vaccine candidate for systemic or autologous whole cell administration may allow our company to expand the utility of its cancer vaccine technology to blood-related cancers, which are not amenable to intratumoral administration. The first planned application of IFx-3.0, is to target the CD22 receptor, which is over expressed on a number of B cell cancers like aggressive lymphomas. We plan on identifying a lead candidate for IFx-3.0 by mid-2024 and begin initiating in vivo and IND-enabling studies in late 2024.
Nal-TIGIT antibody drug conjugate. We are also developing novel bifunctional ADCs to modulate the tumor microenvironment by reprograming MDSCs’ immune suppressing capabilities through inhibition of Delta receptors on MDSCs. The company has constructed several ADCs using Nal, a small molecule prototype Delta receptor specific inhibitor coupled to anti-PD-1 antibody, and plans on examining other checkpoint inhibitors like an anti-TIGIT antibody. TIGIT is a checkpoint receptor associated with inhibition of NK and T cell cytotoxicity and is associated with disease progression and immune escape observed in pre-leukemia called myelodysplastic syndromes (“MDS”). Since both TIGIT and MDSCs play a central role in myelodysplastic syndromes, the company believes that reprograming MDSC function while inhibiting TIGIT may provide a novel, bifunctional approach in the treatment of MDS. TuHURA is also working on developing and expanding a portfolio of novel Delta specific small molecule inhibitors of MDSC immunosuppressive functions as potential modulators of TME alone or conjugated to an immune effector to construct its bi-functional ADCs. We plan on making a novel Delta specific inhibitor conjugated to an anti-TIGIT antibody which we refer to asTBS-2025 for investigation in preclinical models of MDS.
Continuation of REM-001 Study
Prior to the Merger, Kintara’s lead product candidate was REM-001, a late-stage photodynamic therapy (“PDT”) for the treatment of cutaneous metastatic breast cancer (“CMBC”). PDT is a treatment that uses light sensitive compounds, or photosensitizers, that, when exposed to specific wavelengths of light, act as a catalyst to produce a form of reactive oxygen that induces local tumor cell death. The Company has agreed to continue
Kintara’s REM-001 program (the “REM-OO1 Program”), which is currently enrolled in an NIH-sponsored and funded open label 15-patient study in CMBC. Once 10 patients are enrolled and tracked in this study to determine whether a lower dose of REM-001 has an acceptable safety profile and elicits a treatment effect similar to that seen in prior REM-001 studies, the Company expects to enroll the remaining patients and complete the trial and thereafter evaluate whether the REM-001 technology has potential future value that could be realized by the Company. The Company has agreed to use commercially reasonable efforts to develop REM-001 to achieve the CVR Milestone with the expenditure of up to no greater than $700,000 (in addition to available NIH grants that may be up to $2.0 million).
Our History and Team
We were founded in 1995 by Drs. Patricia and Michael Lawman. The company’s IFx technology was developed in the laboratory of Dr. Michael Lawman at the Walt Disney Memorial Cancer Institute, where Dr. Michael Lawman was formerly a Director of the Institute, and Dr. Patricia Lawman was formerly Division Director of Cancer Molecular Biology at the Institute. Dr. Michael Lawman is a Fellow of the Royal Society of Biology, former Associate Professor at University of South Florida, and former Scientific Research Director of Pediatric Hematology/Oncology at St. Joseph’s Children’s Hospital. Dr. Patricia Lawman also serves as an Adjunct Professor at University of South Florida. Drs. Patricia and Michael Lawman are each inventors on numerous U.S. and foreign patents.
With respect to our bifunctional ADC technology, our Delta receptor peptide antibody and ADC technology was developed in the laboratory of Dr. Mark McLaughlin at the Moffitt Cancer Center and at the West Virginia University Research Corporation. Dr. McLaughlin was previously a Senior Member of the Drug Discovery Department at the Moffitt Cancer Center and is currently Professor of Medicinal Chemistry and Member WVU Cancer Institute, where his research focuses on protein-protein interaction inhibitor design and molecular targeted immunotherapy. The discovery that the Delta receptor is highly expressed on MDSCs was jointly discovered by scientists at Moffitt Cancer Center and TuHURA Biopharma, Inc., a separate company whose intellectual property assets we acquired in January 2023.
Our CEO, Dr. James Bianco, is a 30-year veteran of the biopharmaceutical industry. Dr. Bianco is the principal founder of CTI Biopharma, where he served as its CEO from 1992 to October 2016. Dr. Bianco’s experience spans all aspects of drug development from phase I-IV clinical trials, regulatory approval, and pricing reimbursement to sales and marketing. He has extensive experience in financing, negotiating and execution of pharmaceutical development and commercial license agreements. During his tenure at CTI Biopharma, Dr. Bianco was responsible for strategic portfolio development and identifying, acquiring, licensing, purchasing, or acquiring through international merger and acquisition, five drug candidates, four of which have since been approved by the FDA and with three receiving accelerated or conditional regulatory approval in the U.S. and/or E.U. In 2013, Dr. Bianco led CTI Biopharma in the identification and negotiation of the asset purchase for VONJO® (pacritinib), a novel JAK2 selective tyrosine kinase inhibitor. He also led CTI Biopharma in the negotiation of the development and commercial license agreement with Baxalta. As CEO of CTI Biopharma, Dr. Bianco was also responsible for the PERSIST-2 Phase 3 trial design and conduct, the results of which served as the basis for the 2022 FDA accelerated approval of pacritinib and the subsequent acquisition of CTI Biopharma by SOBI for $1.75 billion
Our Strategy
Our goal is to become a leading immuno-oncology company by developing personalized cancer vaccine candidates designed to harness the power of the innate immune system to overcome primary resistance to immunotherapies, broadening the impact of therapies such as checkpoint inhibitors. With the acquisition of the intellectual property assets of TuHURA Biopharma, Inc. in January 2023, we are also developing novel bifunctional ADCs to modulate the tumor microenvironment by reprograming MDSCs’ immune suppressing capabilities through inhibition of Delta receptors on MDSCs to overcome acquired resistance to immunotherapies.
Our strategy leverages our technologies and novel product candidates to overcome primary and acquired resistance to checkpoint inhibitors, molecularly modified immune therapies and cellular therapies. The key elements of this strategy include:
|
|
|
|
|
• |
|
Shortening the time and cost to product registration. We are working to shorten the time and cost to product registration by focusing on patient populations that qualify for accelerated approval, such as patients with advanced and metastatic MCC in the company’s planned Phase 3 trial for IFx-2.0. We believe this trial could significantly reduce the time and cost to potential approval and the cost associated with precluding the need for a postmarketing confirmatory trial. |
|
|
|
|
|
• |
|
Expanding the application of the IFx-2.0 personalized cancer vaccine. We plan to pursue the potential expansion of IFx-2.0 to other cancers beyond MCC by conducting the planned basket trial described above. We plan on examining IFx-2.0 in patients with any type of advanced cancer where their tumor exhibits primary resistance to and who fail checkpoint inhibitor therapy. If successful, this basket trial is intended to potentially expand the use of IFx-2.0 to many types of cancer for which there are no effective or approved therapies for patients who fail to respond to checkpoint inhibitors or whose cancers are known not to respond to checkpoint inhibitors. |
|
|
|
|
|
• |
|
Leverage the IFx technology platform to develop next generation candidates to expand into hematologic cancer indications. We are also developing IFx-3.0, its mRNA based personalized cancer vaccine candidate, for systemic (intravenous) or autologous whole cell administration targeting the CD22 receptor on malignant B cells as a potential treatment for blood related cancers like aggressive lymphoma, with the intention of expanding the application of IFx technology to blood related cancers not amenable to intratumoral administration. The company believes this would be the first systemically targeted mRNA cancer vaccine product candidate known to be in development. |
|
|
|
|
|
• |
|
Establish a leadership position in developing bi-functional ADCs. Through its January 2023 acquisition of the intellectual property assets of TuHURA Biopharma, Inc., we believe that we may be the first company to identify a novel Delta receptor that controls the regulation of multiple immune suppressive functions of MDSCs, the primary contributor to tumor microenvironment immunosuppression. The company believes that inhibiting MDSC functionality may represent a novel way to overcome acquired resistance to immunotherapies. The company believes that its bifunctional ADCs represent a paradigm shift in this important class of therapeutics and has the potential to position the company to take the lead on advancing these novel bifunctional ADCs to clinical trials. |
|
• |
|
Establish Development and Commercial License Collaborations. Leveraging our CEO’s track record of successfully establishing development and commercial partnerships, we intend to seek and establish partnerships with large pharmaceutical or biotech companies as a source of non-dilutive capital and funding to advance the global development of its product candidates. |
Our business, facilities and legal proceedings are further described in the Proxy Statement/Prospectus filed with the SEC on August 19, 2024 (the “Proxy Statement/Prospectus”) in the section entitled “Information about TuHURA Business” beginning on page 243 and is incorporated herein by reference.
Risk Factors
The information set forth in the section of the Proxy Statement/Prospectus entitled “Risk Factors” beginning on page 27 is incorporated herein by reference.
Financial Information
The information set forth in Item 9.01 of this Current Report on Form 8-K concerning the financial information of TuHURA and Kintara is incorporated herein by reference. The unaudited pro forma condensed combined financial information of TuHURA and Kintara as of and for the six months ended June 30, 2024 and the year ended December 31, 2023 is set forth in Exhibit 99.4 hereto and is incorporated herein by reference.
Management’s Discussion and Analysis of Financial Condition and Results of Operations
Management’s Discussion and Analysis of Financial Condition and Results of Operations of Kintara, for the years ended June 30, 2023 and June 30, 2022, and TuHURA, for the years ended December 31, 2023 and 2022 is set forth in the section of the Proxy Statement/Prospectus entitled “Kintara’s Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “TuHURA Management’s Discussion and Analysis of Financial Condition and Results of Operations” beginning on pages 335 and 298, respectively, and is incorporated herein by reference.
Management’s Discussion and Analysis of Financial Condition and Results of Operations for Kintara for the year end June 30, 2024 is included in Kintara’s annual report on Form 10-K for that was filed with the SEC on October 7, 2024, and is incorporated herein by reference.
Management’s Discussion and Analysis of Financial Condition and Results of Operations for TuHURA for the six months ended June 30, 2024 and 2023 is set forth in Exhibit 99.2 to this Current Report on Form 8-K, and is incorporated herein by reference.
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information regarding beneficial ownership of the Company’s common stock as of immediately following the consummation of the Merger (the “Closing Date”) and reflects the 1-for-35 reverse stock split of the Company’s common stock effected on October 18, 2024.
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Under those rules, beneficial ownership includes any shares as to which the individual or entity has sole or shared voting power or investment power with respect to the securities as well as any shares of common stock that the individual or entity has the right to acquire within 60 days of the Closing Date, such as through the exercise of stock options or other rights. These shares are deemed to be outstanding and beneficially owned by the person holding those options for the purpose of computing the percentage ownership of that person, but they are not treated as outstanding for the purpose of computing the percentage ownership of any other person. Except as noted by footnote, and subject to community property laws where applicable, the Company believes, based on the information provided to them, that the persons and entities named in the table below have sole voting and investment power with respect to all common stock shown as beneficially owned by them.
The table lists applicable percentage ownership based on 42,030,226 shares of common stock outstanding as the Closing Date. The number of shares beneficially owned includes shares of common stock that each person has the right to acquire within 60 days of the Closing Date, including upon the exercise of stock options or warrants and the vesting of restricted stock units.
These stock options, warrants, and restricted stock units shall be deemed to be outstanding for the purpose of computing the percentage of outstanding shares of the Company’s common stock expected to be owned by such person but shall not be deemed to be outstanding for the purpose of computing the percentage of outstanding shares of the combined organization’s common stock expected to be owned by any other person.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Name of Beneficial Owner(1) |
|
|
Common
Stock
Beneficially
Owned |
|
|
% |
|
Directors and Named Executive Officers |
|
|
|
|
|
|
|
|
|
James Bianco(2) |
|
|
|
2,704,791 |
|
|
|
6.4 |
% |
Dan Dearborn(3) |
|
|
|
267,971 |
|
|
|
* |
|
George Ng(4) |
|
|
|
62,891 |
|
|
|
* |
|
Alan List(5) |
|
|
|
27,106 |
|
|
|
* |
|
James Manuso(6) |
|
|
|
27,106 |
|
|
|
* |
|
Robert E. Hoffman(7) |
|
|
|
5,385 |
|
|
|
* |
|
All Officers and Directors as a group (7 Total)(8) |
|
|
|
3,095,360 |
|
|
|
7.2 |
% |
|
|
|
|
|
|
|
|
|
|
Greater than 5% Stockholders |
|
|
|
|
|
|
|
|
|
Vijay Patel(9) |
|
|
|
12,364,430 |
|
|
|
26.7 |
% |
CA Patel F&F Investments, LLC (10) |
|
|
|
2,572,582 |
|
|
|
6.1 |
% |
KP Biotech Group, LLC(11) |
|
|
|
2,572,582 |
|
|
|
6.1 |
% |
Samir Patel(12) |
|
|
|
2,466,377 |
|
|
|
5.8 |
% |
Michael Lawman, M.D. (13) |
|
|
|
2,279,547 |
|
|
|
5.4 |
% |
Patricia Lawman, M.D. (14) |
|
|
|
2,298,884 |
|
|
|
5.4 |
% |
Charles Theofilos, M.D. (15) |
|
|
|
2,506,321 |
|
|
|
5.9 |
% |
|
|
* |
Represents beneficial ownership of less than 1%. |
|
|
(1) |
Except as otherwise indicated, the address of each beneficial owner is c/o TuHURA Biosciences, Inc., 10500 University Center Dr., Suite 110, Tampa, FL 33612. |
|
|
|
(2) Consists of (i) 2,323,307 shares of Company common stock and (ii) 381,594 options to purchase Company common stock held directly by Dr. Bianco exercisable within 60 days after the Closing Date. |
|
|
(3) |
Consists of 267,971 options to purchase Company common stock held directly by Mr. Dearborn exercisable within 60 days after the Closing Date. |
|
|
(4) |
Consists of 62,891 options to purchase Company common stock held directly by Mr. Ng exercisable within 60 days after the Closing Date. |
|
|
(5) |
Consists of 27,106 options to purchase Company common stock held directly by Dr. List exercisable within 60 days after the Closing Date. |
|
|
(6) |
Consists of 27,106 options to purchase Company common stock held directly by Dr. Manuso exercisable within 60 days after the Closing Date. |
|
|
(7) |
Consists of (i) 1,854 shares of Company common stock and (ii) 3,531 shares issuable upon the exercise of vested stock options within 60 days of the Closing Date. |
|
|
(8) |
On December 19, 2023 TuHURA and Dennis Yamashita entered into an employment agreement for Mr. Yamashita’s employment as Chief Science Officer of TuHURA. Mr. Yamashita was an officer as of fiscal year end 2023 and is included in the Directors and Officers Group, but he does not meet the definition of a named executive officer for such period. |
|
|
(9) |
Consists of (i) 7,999,557 shares of Company common stock held by K&V Investment One and (ii) 4,364,873 shares of Company common stock issuable pursuant to currently exercisable warrants that are held by K&V Investment One. Mr. Vijay Patel is the manager of K&V Investment One and may therefore be deemed to have voting and dispositive power over the shares held by such entity. Mr. Patel disclaims beneficial ownership of the shares held by K&V Investment One except to the extent of his pecuniary interest therein. |
|
|
(10) (11) |
Consists of (i) 2,125,332 shares of Company common stock held directly by CA Patel F&F Investments, LLC (“CA Patel”) and (ii) 447,250 of Company common stock issuable pursuant to currently exercisable warrants that are held directly by CA Patel. Under the so-called “rule of three,” if voting and dispositive decisions regarding an entity’s securities are made by a majority comprised of two or more individuals of a three-member (or greater) board, and a voting and dispositive decision requires the approval of a majority of those individuals, none of the individuals is deemed a beneficial owner of the entity’s securities. Based on the foregoing, no individual person exercises voting or dispositive control over any of the securities held by CA Patel. Consists of (i) 2,125,332 shares of Company common stock held directly by KP Biotech Group, LLC (“KP Biotech”) and (ii) 447,250 of Company common stock issuable pursuant to currently exercisable warrants that are held directly by KP Biotech. Under the so-called “rule of three,” if voting and dispositive decisions regarding an entity’s securities are made by a majority comprised of two or more individuals of a three-member (or greater) board, and a voting and dispositive decision requires the approval of a majority of those individuals, none of the individuals is deemed a beneficial owner of the entity’s securities. Based on the foregoing, no individual person exercises voting or dispositive control over any of the securities held by KP Biotech. |
|
|
(12) (13) (14) (15) |
Consists of (i) 1,735,715shares of Company common stock held directly by Pranabio Investments, LLC (“Pranabio”), (ii) 694,882 shares of Company common stock issuable pursuant to currently exercisable warrants that are held by Pranabio, and (iii) 35,780 shares of Company common stock held directly by Garden Street House LLC (“Garden Street”). Mr. Samir Patel is the sole manager and member of Pranabio and Garden Street. Mr. Samir Patel disclaims beneficial ownership of the shares held by Pranabio and Garden Street except to the extent of his pecuniary interest therein. Consists of (i) 1,617,784 shares of Company common stock held directly by Dr. Michael Lawman, (ii) 243,137 shares of Company common stock issuable upon the exercise of vested stock options held directly by Dr. Michael Lawman, and (iii) 447,250 shares of Company common stock held directly by the ML 2018 Irrevocable Trust, U/A/D March 26, 2018 (the “ML Trust”). Dr. Michael Lawman is the trustee of the ML Trust and may therefore be deemed to have voting and dispositive power over the shares held by such entity. Dr. Michael Lawman disclaims beneficial ownership of the shares held by the ML Trust except to the extent of his pecuniary interest therein. Dr. Michael Lawman is the spouse of Dr. Patricia Lawman but maintains sole voting and dispositive power over his shares. Consists of (i) 1,617,784 shares of Company common stock held directly by Dr. Patricia Lawman, (ii) 268,795 shares of Company common stock issuable upon the exercise of vested stock options held directly by Dr. Patricia Lawman, and (iii) 447,250 shares of Company common stock held directly by the PL 2018 Irrevocable Trust, U/A/D March 26, 2018 (the “PL Trust”). Dr. Patricia Lawman is the trustee of the PL Trust and may therefore be deemed to have voting and dispositive power over the shares held by such entity. Dr. Patricia Lawman disclaims beneficial ownership of the shares held by the ML Trust except to the extent of her pecuniary interest therein. Dr. Patricia Lawman is the spouse of Dr. Michael Lawman but maintains sole voting and dispositive power over her shares. Consists of (i) 473,559 shares of Company common stock directly held by the Charles S. Theofilos, MD IRA, an IRA account for Dr. Theofilos’ benefit (the “Theofilos IRA”), (ii) 197,316 shares of Company common stock issuable pursuant to currently exercisable warrants that are held by the Theofilos IRA, (iii) 1,506,586 shares of Company common stock held directly by Charles S. Theofilos, MD and Kathryn N. Theofilos, as tenants by the entireties (the “Charles and Kathryn”), and (iv) 328,860 shares of Company common stock issuable pursuant to currently exercisable warrants that are held by Charles and Kathryn. |
Information about Directors and Executive Officers; Director Compensation and Director Independence; Executive Compensation
The information set forth in Item 5.02 of this Current Report on Form 8-K is incorporated herein by reference.
The information set forth in the section of the Proxy Statement/Prospectus entitled “Management After the Merger” beginning on page 344 is incorporated herein by reference.
A description of the compensation of the named executive officers of TuHURA and the compensation of the executive officers of Kintara before the consummation of the Transactions is set forth in the Proxy Statement/Prospectus in the sections titled “TuHURA’s Executive Compensation” beginning on page 290 of the Proxy Statement/Prospectus and “Kintara’s Executive Compensation” beginning on page 331 of the Proxy Statement/Prospectus, respectively, and that information is incorporated herein by reference.
Certain Relationships and Related Party Transactions and Director Independence
The information set forth in the section of the Proxy Statement/Prospectus entitled “Certain TuHURA Relationships and Related Party Transactions” beginning on page 311 and entitled “Director Independence” beginning on page 347 is incorporated herein by reference.
Market Price of and Dividends on the Registrant’s Common Equity and Related Stockholder Matters
Shares of Kintara’s common stock were historically listed on the Nasdaq Capital Market under the symbol “KTRA.” Shares of the Company’s common stock commenced trading on the Nasdaq Capital Market on a post-Reverse Stock Split adjusted and post-Merger basis on October 18, 2024 under the symbol “HURA.”
As of the closing and following the completion of the Merger and after giving effect to the Reverse Stock Split, the Company had approximately 42,030,226 shares of common stock issued and outstanding held of record by approximately 814 holders. The number of holders of record does not include a substantially greater number of “street name” holders or beneficial holders whose shares of Company common stock are held of record by banks, brokers and other financial institutions.
The information set forth in the section of the Proxy Statement/Prospectus entitled “Market Price and Dividend Information” on page 26 is incorporated herein by reference.
Description of Registrant’s Securities
The information set forth in the section of the Proxy Statement/Prospectus entitled “Description of Kintara’s Securities” beginning on page 351 and in the section entitled “Comparison of Rights of Holders of Kintara Stock and TuHURA Stock” beginning on page 357 is incorporated herein by reference.
Indemnification of Directors and Officers
The information set forth in Item 1.01 of this Current Report on Form 8-K under the heading “Indemnification Agreements” is incorporated herein by reference.
A description of the Company’s indemnification obligations in respect of its directors and officers is included in the Proxy Statement/Prospectus in the section entitled “The Merger Agreement-Indemnification and Insurance for Directors and Officers” beginning on page 162 and is incorporated herein by reference.
Financial Statements and Supplementary Data
The information set forth in Item 9.01 of this Current Report on Form 8-K is incorporated herein by reference.
WHERE YOU CAN FIND MORE INFORMATION
The Company is subject to the informational requirements of the Exchange Act and in accordance therewith, files annual, quarterly and current reports, proxy statements and other information with the SEC electronically, and the
SEC maintains a website that contains the Company’s filings as well as reports, proxy and information statements, and other information issuers file electronically with the SEC at www.sec.gov.
The Company also makes available free of charge on or through its website at https://tuhurabio.com/ its Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after the Company electronically files such material with or otherwise furnishes it to the SEC. The website addresses for the SEC and the Company are inactive textual references and except as specifically incorporated by reference into this Current Report on Form 8-K, information on those websites is not part of this Current Report on Form 8-K.
If you would like to request documents from the Company, please send a request in writing or by telephone to the following address:
TuHURA Biosciences, Inc.
10500 University Center Dr, Suite 110
Tampa, FL 33612
Attn: Dan Dearborn
(813) 875-6600
Email: admin@tuhurabio.com
|
|
Item 2.02. |
Results of Operations and Financial Condition. |
To the extent required by Item 2.02 of Form 8-K, the information incorporated in Items 2.01 and 9.01 of this Current Report on Form 8-K is incorporated by reference herein.
|
|
Item 3.03. |
Material Modification to Rights of Security Holders. |
To the extent required by Item 3.03 of Form 8-K, the information contained in Item 2.01 of this Current Report on Form 8-K is incorporated by reference herein.
As previously disclosed, at a special meeting of Kintara’s stockholders held on October 4, 2024, (the “Special Meeting”), Kintara’s stockholders approved an amendment to Kintara’s Articles of Incorporation, as amended (the “Articles of Incorporation”) to effect the Reverse Stock Split. On October 18, 2024, Kintara amended its Articles of Incorporation to effect the Reverse Stock Split, effective as of 12:01 a.m. Eastern Time on October 18, 2024 (the “Reverse Stock Split Amendment”).
As a result of the Reverse Stock Split, every 35 shares of Kintara’s common stock held by a stockholder immediately prior to the Reverse Stock Split, were combined and reclassified into one share of Kintara’s common stock. No fractional shares were issued in connection with the Reverse Stock Split. Any fractional shares resulting from the Reverse Stock Split were rounded up to the nearest whole number. The par value of Kintara’s common stock remained unchanged at $0.001 per share after the Reverse Stock Split. In addition, the Reverse Stock Split did not affect the authorized number of shares of the Company’s common stock.
On October 18, 2024, the Company also amended its Articles of Incorporation to effect the Name Change, effective as of 12:04 a.m. Eastern Time on October 18, 2024 (the “Name Change Amendment”).
The foregoing descriptions of the certificates of amendment to the Articles of Incorporation of Kintara and the Company are not complete and are subject in their entirety by reference to the Reverse Stock Split Amendment and the Name Change Amendment, copies of which are attached hereto as Exhibit 3.1 and Exhibit 3.2, respectively, and are incorporated herein by reference.
|
|
Item 5.01. |
Changes in Control of Registrant. |
The information set forth in Item 2.01 of this Current Report on Form 8-K regarding the Merger and the information set forth in Item 5.02 of this Current Report on Form 8-K regarding the board of directors and executive officers of the Company following the Merger are incorporated by reference into this Item 5.01.
|
|
Item 5.02. |
Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers. |
Resignation of Directors
In accordance with the Merger Agreement, immediately prior to the Merger, Laura Johnson, Tamara A. Favorito and Robert J. Toth, Jr. resigned from the Kintara’s board of directors and committees of the Kintara’s board of directors on which they respectively served, which resignations were not the result of any disagreements with the Company relating to its operations, policies or practices.
Appointment of Directors
Effective upon the closing of the Merger on October 18, 2024, the size of the board of directors was increased to five members and the board of directors of the Company was reconstituted as follows: (i) Robert E. Hoffman (designated by Kintara), and (ii) James Manuso, M.D., James Bianco, Alan List and George Ng (designated by TuHURA). Under the Nasdaq Listing Rules, a majority of the members of the board of directors must qualify as “independent,” as affirmatively determined by the board of directors. Under the Nasdaq Listing Rules, a director will only qualify as an “independent director” if, in the opinion of that company’s board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. The Company’s board of directors has determined that each of James Manuso, M.D., Alan List and George Ng qualify as “independent directors” as defined by the Nasdaq Listing Rules.
Immediately after the closing of the Merger on October 18, 2024, the board of directors of the Company reconstituted its various standing committees as follows:
Audit Committee
Dr. List, Mr. Ng and Dr. Manuso were appointed to the Audit Committee of the board of directors. Dr. Manuso was appointed chair of the Audit Committee and designated as the “audit committee financial expert.”
Compensation Committee
Dr. Manuso and Dr. List were appointed to the Compensation Committee of the board of directors. Dr. List was appointed chair of the Compensation Committee.
Nominating and Corporate Governance Committee
Mr. Ng and Dr. List were appointed to the Nominating and Corporate Governance Committee of the board of directors. Mr. Ng was appointed chair of the Nominating and Corporate Governance Committee.
Each of the newly appointed non-employee directors’ biographical information is set forth below.
Non-Employee Directors
James S. Manuso, Ph.D., MBA, age 75, has served as a director of TuHURA since November 2022. Dr. Manuso has also served as Chairman and Chief Executive Officer of Talfinium Investments, Inc., an investment entity and financial consultancy, since 2014. Since 2018, Dr. Manuso has served as managing member of Laurelside LLC, a family office, which he founded. Dr. Manuso has served on the board of Ocuphire Pharma, Inc., a public company (NASDAQ:OCUP) developing Nyxol in advanced clinical trials for the treatment of multiple visual disorders, since November 2020. From 2015 until 2018, Dr. Manuso served as President, Chief Executive Officer and Vice Chairman of RespireRx Pharmaceuticals Inc. (OTC QB:RSPI), a Phase 3-ready, clinical-stage respiratory and neurological pharmaceutical company. From July 2011 until October 2013, Dr. Manuso served as Chairman and Chief Executive Officer of Astex Pharmaceuticals, Inc. (Nasdaq:ASTX) and led the sale of Astex Pharmaceuticals, Inc. to Otsuka Pharmaceutical Co., Ltd. (“Otsuka Pharmaceutical”). In 2013, he was a senior mergers and acquisitions advisor to Otsuka Pharmaceuticals’ executive management. Dr. Manuso has served as board chairman and chairman of the audit, governance and nominating, pricing and compensation committees of multiple companies’ boards, including Biotechnology Industry Organization, Novelos Therapeutics, Inc., Merrion Pharmaceuticals Ltd. (MERR:IEX; Dublin, Ireland), Inflazyme Pharmaceuticals, Inc. (IZP-TSE; Vancouver, Canada), Symbiontics, Inc., which he co-founded (sold to BioMarin Pharmaceutical Inc. as ZyStor, Inc.), Montigen Pharmaceuticals, Inc., Quark Pharmaceuticals, Inc., Galenica Pharmaceuticals, Inc., Supratek Pharma, Inc., EuroGen, Ltd. (London, UK), where he
was chairman, and the Greater San Francisco Bay Area Leukemia & Lymphoma Society, where he also served as vice president.
Dr. Manuso holds a B.A. with honors in Economics and Chemistry from New York University, a Ph.D. in Experimental Psychology and Genetics from the New School University, and an Executive M.B.A. from Columbia Business School. Dr. Manuso is the author of numerous chapters, articles and books on topics including health care cost containment and biotechnology company management. The Company believes that Dr. Manuso’s extensive experience in the biopharmaceutical industry in finance, business development and management, and his experience as a member of the boards of directors of multiple pharmaceutical companies, both domestic and foreign, provide him with the qualifications and skills to serve as a director of the Company.
George Ng, age 50, has served as a director of TuHURA since February 2020. Mr. Ng has also served as a director of Calidi Biotherapeutics, Inc. (NYSE American: CLDI) since October 2019 and as its President and Chief Operating Officer since February 1, 2022, as well as a director and Chief Executive Officer of Processa Pharmaceuticals, Inc. (Nasdaq: PCSA) since August 8, 2023. In addition, Mr. Ng is currently a partner at PENG Life Science Ventures since September 2013, a director, co-founder, and chief business officer at IACTA Pharmaceuticals, Inc. since January 2020. Mr. Ng’s experience further includes serving in various executive-level positions for multiple publicly-traded and private global biotechnology and pharmaceutical firms. Mr. Ng previously served as a director of Inflammatory Response Research, Inc. from May 2019 to April 2020, as a director of Invent Medical Corp from July 2019 to January 2020, as a director of ImmuneOncia Therapeutics Inc. from June 2016 to 2019, and as a director of Virttu Biologics Limited from April 2017 to April 2019. Mr. Ng was also the Executive Vice President and Chief Administrative Officer of Sorrento Therapeutics, Inc. (Nasdaq: SRNE) from March 2015 to April 2019, the Co-Founder and President, Business of Scilex Pharmaceuticals Inc. from September 2012 to April 2019, and the Senior Vice President and General Counsel of BioDelivery Sciences International Inc. (Nasdaq: BDSI) from December 2012 to March 2015. Mr. Ng holds a JD degree from the University of Notre Dame School of Law, as well as a B.AS double degree in Biochemistry and Economics from the University of California, Davis. The Company believes Mr. Ng’s experience with the launch and commercialization efforts of multiple pharmaceutical drug products, experience in clinical research procedures, and his executive experience in the biotechnology industry, provide him with the qualifications and skills to serve as a director of the Company.
Alan List, M.D., age 70, has served as a director of TuHURA since November 2022. Dr. List has also served as Chief Medical Officer of Precision BioSciences, Inc. (Nasdaq: DTIL) (“Precision BioSciences”), a clinical stage gene editing company, since April 2021 and, prior to that, had been a strategic clinical advisor to Precision BioSciences and its board since April 2020, providing advice regarding its clinical stage and pre-clinical allogeneic CAR T programs. Prior to joining Precision BioSciences, Dr. List served in various roles at the Moffitt Cancer Center, including as President and Chief Executive Officer from 2012 to December 2019, Executive Vice President, Physician in Chief from 2008 to 2012 and Chief of the Malignant Hematology Division from 2003 to 2008. Prior to joining the Moffitt Cancer Center, Dr. List held academic and clinical appointments at the University of Arizona. Dr. List is internationally recognized for his many contributions in the development of effective treatment strategies for myelodysplastic syndrome (“MDS”) and acute myeloid leukemia. His pioneering work led to the development of Revlimid (lenalidomide), a transformational treatment for patients with MDS and multiple myeloma.
Dr. List is the author of numerous peer-reviewed articles and books. He previously served as the President for the Society of Hematologic Oncology as well as a member of the MDS Foundation Board of Directors. Dr. List is also an active member of the American Society of Clinical Oncology, the American Society of Hematology and the American Association for Cancer Research. He is a Charter Fellow in the National Academy of Inventors, an inductee in the Florida Inventors Hall of Fame. Dr. List received B.S. and M.S. degrees from Bucknell University and earned his M.D. from the University of Pennsylvania. He is board certified in internal medicine, hematology, and medical oncology. The Company believes Dr. List’s extensive clinical development experience together with his experience with biotechnology businesses provide him with the qualifications and skills to serve as a director of the Company.
Robert E. Hoffman, age 58, has served as a director of Kintara since April 2018, as Chairman of Kintara since June 2018, as Chief Executive Officer and President of Kintara since November 2021, and as interim Chief Financial Officer of Kintara since June 1, 2023. He has served as a member of board of directors of ASLAN Pharmaceuticals, Inc. (Nasdaq: ASLN), a publicly-held, clinical-stage immunology focused biopharmaceutical company, since October 2018, and as a member of the board of directors of FibroGenesis, a clinical-stage regenerative medicine company, since April 2021. He has also served as a member of board of directors, on the audit committee, and on the Human Resources and compensation committee of Antibe Therapeutics Inc. (“Antibe”), a publicly-held clinical-stage
biotechnology company, since November 2020, and as Chairman of Antibe’s board of directors from May 2022 to April 2024. Mr. Hoffman served as Senior Vice President and Chief Financial Officer of Heron Therapeutics, Inc., a publicly-held pharmaceutical company, from April 2017 to October 2020. From July 2015 to September 2016, Mr. Hoffman served as Chief Financial Officer of AnaptysBio, Inc., a publicly-held biotechnology company. From June 2012 to July 2015, Mr. Hoffman served as the Senior Vice President, Finance and Chief Financial Officer of Arena Pharmaceuticals, Inc. (“Arena”), a biopharmaceutical company, prior to its acquisition by Pfizer Inc. in March 2022. From August 2011 to June 2012 and previously from December 2005 to March 2011, he served as Arena’s Vice President, Finance and Chief Financial Officer and in a number of various roles of increasing responsibility from 1997 to December 2005. Mr. Hoffman formerly served as a member of the board of directors of Saniona AB, a biopharmaceutical company, from September 2021 to May 2022, and as a member of the board of directors of Kura Oncology, Inc., a cancer research company, from March 2015 to August 2021. He also previously served as a member of the board of directors of CombiMatrix Corporation, a molecular diagnostics company, MabVax Therapeutics Holdings, Inc., a biopharmaceutical company, and Aravive, Inc., a clinical stage biotechnology company. Mr. Hoffman serves as a member of the steering committee of the Association of Bioscience Financial Officers. Mr. Hoffman formerly served as a director and President of the San Diego Chapter of Financial Executives International and was an advisor to the Financial Accounting Standard Board (FASB) for 10 years (2010 to 2020) advising the United States accounting rulemaking organization on emerging issues and new financial guidance. Mr. Hoffman holds a B.B.A. from St. Bonaventure University. The Company believes Mr. Hoffman’s financial and executive business experience qualifies him to serve as a director of the Company.
Departure of Executive Officers
Immediately prior to the Merger, Robert Hoffman resigned from all of his offices with Kintara and all of its subsidiaries, if applicable, including President, Chief Executive Officer, Interim Chief Financial Officer and Principal Executive Officer and Principal Financial and Accounting Officer of Kintara.
Appointment of Executive Officers
On October 18, 2024, the Company’s board of directors appointed James Bianco, M.D. as the Chief Executive Officer, Daniel Dearborn as the Chief Financial Officer and Dennis Yamashita, Ph.D as the Chief Scientific Officer.
There are no family relationships among any of the newly appointed executive officers. None of the newly appointed executive officers has a direct or indirect material interest in any transaction required to be disclosed pursuant to Item 404(a) of Regulation S-K.
The information set forth in the section of the Proxy Statement/Prospectus entitled “TuHURA Executive Compensation” beginning on page 290 through page 294 is incorporated herein by reference.
Each of the newly appointed executive officers’ biographical information is set forth below.
James Bianco, M.D., age 68, has served as TuHURA’s Chief Executive Officer and as a director of TuHURA since July 1, 2021. Dr. Bianco was also the founder, Chief Executive Officer and Chairman of TuHURA Biopharma, Inc., a biotechnology company, from its inception in November 2018 through its dissolution in January 2023, following the transfer of its assets to TuHURA. Dr. Bianco is a 30-year veteran of the biopharmaceutical industry. In 1991, Dr. Bianco founded CTI Biopharma, Inc. (“CTI”) and from 1992 to 2016 was the Chief Executive Officer of CTI. During his tenure at CTI, Dr. Bianco was responsible for strategic portfolio development and identifying, acquiring, licensing, purchasing, or acquiring through international merger and acquisition, five drug candidates, four of which have since been approved by the FDA and with three receiving accelerated or conditional regulatory approval in the U.S. and/or E.U.
Dr. Bianco earned his M.D. from the Mount Sinai Icahn School of Medicine and completed his residency and chief residency at the Mount Sinai Medical Center in New York City. He completed his fellowship in Hematology/Oncology at the University of Washington/Fred Hutchinson Cancer Research Center (FHCRC) where he was appointed Assistant Professor of Medicine, Assistant Member FHCRC and Director of the Bone Marrow Transplant Unit at a “Hutch” affiliate (SVAMC).
The Company believes Dr. Bianco’s experience in building and leading biotechnology businesses as well as his extensive clinical development experience provide him with the qualifications and skills to serve as a director of the Company.
Dan Dearborn, age 57, joined TuHURA in 2018 as its Chief Financial Officer. Mr. Dearborn is a CPA with over 25 years of finance experience exclusively with health care and biotechnology companies. Prior to TuHURA, from 2015 to 2017, Mr. Dearborn was Chief Financial Officer at MYMD Pharmaceuticals, Inc., an emerging biotechnology firm. Mr. Dearborn is an alumnus of Loyola University in Maryland and joined Ernst & Young early in his career. He was with Pharmerica, a long-term care pharmaceutical company, for fifteen years and advanced quickly to a Director role. He then moved to BioDelivery Sciences International as Controller. During his time at BioDelivery Sciences International, the company signed two very large commercial partnership agreements and was listed on Nasdaq. Mr. Dearborn later joined Welldyne, Inc. (“Welldyne”) as its Chief Financial Officer. Welldyne is a pharmacy benefit manager that also had several related health care businesses and employed associates in Florida and Colorado. During his time with Welldyne, the company was sold to Carlyle Group, Inc., one of the largest private equity firms in the world.
Dennis Yamashita, Ph.D., age 59, has served as our Chief Scientific Officer and Head of Discovery Research and Early Development since December 19, 2023. Dr. Yamashita has over 30 years of experience in research and development drug discovery in pharmaceutical and biotechnology companies. Prior to joining TuHURA, he most recently served as the Executive Vice President of Chemistry at Cambrian BioPharma, Inc. from September 2020 until December 2023. From August 2018 to September 2020, he was Vice President of Medicinal Chemistry at Axial Therapeutics, Inc. where he led an immuno-oncology project to improve immune checkpoint inhibitor efficacy. From October 2017 to May 2018, he was the Vice President of Drug Discovery at ORIC Pharmaceuticals Inc. and led projects aimed at overcoming drug resistance of oncology medicines. He began his biotechnology career as the Vice President of Chemistry at Trevena, Inc., which was founded by Nobel laureate Robert Lefkowitz. He was co-inventor of Olinvyk (oliceridine), an FDA-approved Mu opioid G-protein biased ligand for treating post-surgical pain. He began his career at GSK plc where he expanded his expertise in medicinal chemistry over a 20-year period with his last role leading drug discovery projects and research collaborations with premier academic institutions, and he identified four clinical drug candidates to treat cancer and osteoporosis. He has also served as the President and Chairman of the Board of three emerging private companies focused on treating and preventing diseases driven by aging.
Dr. Yamashita holds a B.S. from MIT in Chemistry and a Ph.D. in Organic Chemistry from Yale. His Ph.D. thesis was on the synthesis of calicheamicin, a potent natural product cytotoxic agent that was later incorporated into an antibody drug conjugate called Mylotarg used to treat acute myeloid leukemia. Additionally, he is an active volunteer as a mentor at the MIT Sandbox, an entrepreneurship program for MIT students that aims to move business ideas from concept to societal impacts.
Incentive Plan
At the Special Meeting, Kintara’s stockholders considered and approved the TuHURA Biosciences, Inc. 2024 Equity Incentive Plan (the “2024 Plan”), which became effective at the closing of the Merger and following the Reverse Stock Split. As of the effective time of the Merger, there were 11,000,000 shares of the Company’s common stock available for grant under the 2024 Plan which number does not reflect any options previously granted under the 2017 Plan or granted to certain officers and directors immediately following the closing of the Merger (including as described in Section 5.02 of this Current Report on Form 8-K).
A more complete summary of the terms of the 2024 Plan is set forth in the Proxy Statement/Prospectus under the section titled “Proposal No. 4: Approval of the Kintara Therapeutics, Inc., 2024 Omnibus Equity Incentive Plan” and is incorporated by reference herein. That summary and the foregoing description of the 2024 Plan do not purport to be complete and are qualified in their entirety by reference to the text of the 2024 Plan, a copy of which is attached to this Current Report on Form 8-K as Exhibit 10.4 hereto and are incorporated herein by reference.
|
|
Item 5.03. |
Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year. |
To the extent required by this Item, the information included in Item 2.01 and Item 3.03 of this Current Report on Form 8-K is incorporated herein by reference.
On October 18, 2024, the Company’s common stock commenced trading on the Nasdaq Capital Market under the symbol “HURA.” The change in trading symbol is related solely to the Name Change.
In connection with the closing of the Merger, the Company changed its fiscal year end from June 30 to December 31, the fiscal year end of TuHURA prior to the Merger. Accordingly, the Company will file annual and quarterly reports based on the December 31 fiscal year-end.
|
|
Item 5.06. |
Change in Shell Company Status. |
As a result of the Merger, Kintara ceased to be a shell company (as defined in Rule 12b-2 of the Exchange Act) as of the closing of the Merger. A description of the Merger and the terms of the Merger Agreement are included in the Proxy Statement/Prospectus in the section entitled “Proposal No. 1-The Nasdaq Proposal” beginning on page 189 of the Proxy Statement/Prospectus. Further reference is made to the information contained in Item 2.01 of this Current Report on Form 8-K.
On October 18, 2024, the Company issued a press release announcing, among other things, the closing of the Merger. A copy of the press release is filed as Exhibit 99.1 to this Current Report on Form 8-K and incorporated herein by reference, except that the information contained on the websites referenced in the press releases is not incorporated herein by reference.
|
|
Item 9.01. |
Financial Statements and Exhibits. |
(a) Financial Statements of Businesses Acquired
The audited consolidated financial statements of TuHURA as of and for the years ended December 31, 2023 and 2022 and the related notes thereto are included in the Proxy Statement/Prospectus, and are incorporated herein by reference.
The audited consolidated financial statements of Kintara as of and for the years ended June 30, 2024 and 2023 and the related notes are included in Kintara’s annual report on Form 10-K for the year ended June 30, 2024 that was filed with the SEC on October 7, 2024, and are incorporated herein by reference.
The unaudited condensed interim financial statements of TuHURA as of June 30, 2024 and for the six months ended June 30, 2024 and 2023 are filed herewith as Exhibit 99.3 to this Current Report on Form 8-K and are incorporated herein by reference.
(b) Pro Forma Financial Information
The unaudited pro forma financial information for the year ended December 31, 2023 and the six months ended June 30, 2024 are filed herewith as Exhibit 99.4 to this Current Report on Form 8-K and are incorporated herein by reference.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits:
|
|
|
Exhibit No. |
|
Description |
2.1**†† |
|
Agreement and Plan of Merger, dated as of April 2, 2024, by and among Kintara Therapeutics, Inc., Kayak Mergeco, Inc., and TuHURA Biosciences, Inc. (incorporated by reference to Exhibit 2.1 of Kintara’s Current Report on Form 8-K filed with the SEC on April 3, 2024). |
|
|
|
2.2 |
|
Waiver Agreement to Agreement and Plan of Merger, dated as of September 25, 2024, by and among Kintara Therapeutics, Inc., Kayak Mergeco, Inc., and TuHURA Biosciences, Inc. (incorporated by reference to Exhibit 2.1 of Kintara’s Current Report on Form 8-K filed with the SEC on September 25, 2024) |
|
|
|
2.3* |
|
Waiver Agreement to Agreement and Plan of Merger, dated as of October 18, 2024, by and among Kintara Therapeutics, Inc., Kayak Mergeco, Inc., and TuHURA Biosciences, Inc. |
|
|
|
3.1* |
|
Articles of Amendment to Articles of Incorporation of Kintara Therapeutics, Inc., as filed with the Secretary of State of Nevada, effective October 18, 2024 (Reverse Stock Split) |
|
|
|
3.2* |
|
Articles of Amendment to the Articles of Incorporation of Kintara Therapeutics, Inc., as filed with the Secretary of State of Nevada, effective October 18, 2024 (Name Change Amendment) |
|
|
|
4.1** |
|
Form of TuHURA Biosciences, Inc. (f/k/a Morphogenesis, Inc.) Common Stock Purchase Warrant issued in Series A Preferred Offering (incorporated by reference to Exhibit 4.12 to the Registration Statement on Form S-4 filed on May 13, 2024 (Registration No. 333-279368)) |
|
|
|
4.2** |
|
Form of TuHURA Biosciences, Inc. (f/k/a Morphogenesis, Inc.) Common Stock Purchase Warrant, dated June 1, 2019, issued for advisory services (incorporated by reference to Exhibit 4.13 to the Registration Statement on Form S-4 filed on May 13, 2024 (Registration No. 333-279368)) |
|
|
|
4.3** |
|
Form of TuHURA Biosciences, Inc. (f/k/a Morphogenesis, Inc.) Common Stock Purchase Warrant issued in Series A-1 Preferred Stock Offering (incorporated by reference to Exhibit 4.14 to the Registration Statement on Form S-4 filed on May 13, 2024 (Registration No. 333-279368)) |
|
|
|
4.4** |
|
Form of TuHURA Biosciences, Inc. (f/k/a Morphogenesis, Inc.) Common Stock Purchase Warrant issued in Note Conversion Transaction (incorporated by reference to Exhibit 4.15 to the Registration Statement on Form S-4 filed on May 13, 2024 (Registration No. 333-279368)) |
|
|
|
4.5** |
|
Form of TuHURA Biosciences, Inc. (f/k/a Morphogenesis, Inc.) Common Stock Purchase Warrant issued in Series B Preferred Stock Offering (incorporated by reference to Exhibit 4.16 to the Registration Statement on Form S-4 filed on May 13, 2024 (Registration No. 333-279368)) |
|
|
|
4.6** |
|
Form of TuHURA Biosciences, Inc. Common Stock Warrant issued in TuHURA Note Financing (incorporated by reference to Exhibit 4.17 to the Registration Statement on Form S-4 filed on May 13, 2024 (Registration No. 333-279368)) |
|
|
|
|
|
|
4.7** |
|
Form of TuHURA Biosciences, Inc. Series A Preferred Stock Warrant Amendment Agreement (incorporated by reference to Exhibit 4.19 to the Registration Statement on Form S-4/a filed on August 8, 2024 (Registration No. 333-279368)) |
|
|
|
10.1** |
|
Contingent Value Rights Agreement, dated October 18, 2024, by and between Kintara Therapeutics, Inc. and Equiniti Trust Company, LLC (incorporated by reference to Exhibit 10.4 to the Registration Statement on Form 8-K filed on April 3, 2024 (Registration No. 333-279368)) |
|
|
|
10.2*+ |
|
Form of Indemnification Agreement by and between TuHURA Biosciences, Inc and each of its directors and executive officers. |
|
|
|
10.3** |
|
Form of Lock-up Agreement (incorporated by reference to Exhibit 10.3 of Kintara’s Current Report on Form 8-K filed with the SEC on April 3, 2024) |
|
|
|
10.4*+ |
|
TuHURA Biosciences, Inc. 2024 Equity Incentive Plan. |
|
|
|
10.5**+ |
|
Second Amended and Restated Employment Agreement, dated March 29, 2024, between TuHURA Biosciences, Inc. and Dan Dearborn (incorporated by reference to Exhibit 10.34 to the Registration Statement on Form S-4 filed on May 13, 2024 (Registration No. 333-279368)) |
|
|
|
10.6**+ |
|
Second Amended and Restated Employment Agreement, dated March 29, 2024, between TuHURA Biosciences, Inc. and James Bianco, M.D (incorporated by reference to Exhibit 10.35 to the Registration Statement on Form S-4 filed on May 13, 2024 (Registration No. 333-279368)) |
|
|
|
10.7**+ |
|
Employment Agreement, dated December 19, 2023, between TuHURA Biosciences, Inc. and Dennis Yamashita (incorporated by reference to Exhibit 10.36 to the Registration Statement on Form S-4 filed on May 13, 2024 (Registration No. 333-279368)) |
|
|
|
10.8**† |
|
Exclusive License Agreement, dated March 29, 2019, between Morphogenesis, Inc. and H. Lee Moffitt Cancer Center and Research Institute, Inc., as amended (incorporated by reference to Exhibit 10.37 to the Registration Statement on Form S-4 filed on May 13, 2024 (Registration No. 333-279368)) |
|
|
|
10.9**† |
|
Exclusive License Agreement, dated April 23, 2021, between Morphogenesis, Inc. and H. Lee Moffitt Cancer Center and Research Institute, Inc., as amended (incorporated by reference to Exhibit 10.38 to the Registration Statement on Form S-4 filed on May 13, 2024 (Registration No. 333-279368)) |
|
|
|
10.10**† |
|
Restated and Amended Exclusive License Agreement, effective September 7, 2022, between TuHURA Biopharma, Inc. and West Virginia Research Corporation (incorporated by reference to Exhibit 10.39 to the Registration Statement on Form S-4 filed on May 13, 2024 (Registration No. 333-279368)) |
|
|
|
10.11**† |
|
Asset Purchase Agreement, dated January 26, 2023, between TuHURA Biopharma Inc. and Morphogenesis, Inc. (incorporated by reference to Exhibit 10.40 to the Registration Statement on Form S-4 filed on May 13, 2024 (Registration No. 333-279368)) |
|
|
|
10.12**† |
|
Exclusivity and Right of First Offer Agreement, dated July 3, 2024, between TuHURA Biosciences, Inc. and Kineta, Inc. (incorporated by reference to Exhibit 10.41 to the Registration Statement on Form S-4/A filed on July 19, 2024 (Registration No. 333-279368)) |
|
|
|
21.1* |
|
List of Subsidiaries |
|
|
|
99.1* |
|
Press release issued on October 18, 2024 regarding the closing of the Merger. |
|
|
|
99.2* |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations of TuHURA for the six months ended June 30, 2024 and 2023. |
|
|
† |
Confidential treatment is requested for certain confidential portions of this exhibit pursuant to Rule 24b-2 under the Exchange Act. In accordance with Rule 24b-2, these confidential portions have been omitted from this exhibit and filed separately with the Securities and Exchange Commission. |
|
|
†† |
Schedule has been omitted pursuant to Item 601(a)(5) of Regulation S-K. Kintara hereby undertakes to furnish copies of any of the omitted schedules upon request by the Securities and Exchange Commission. |
* |
Filed herewith |
** + |
Previously filed Indicates a management contract or any compensatory plan, contract or arrangement. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
TUHURA BIOSCIENCES, INC. |
|
|
|
|
Date: |
October 21, 2024 |
By: |
/s/ Dan Dearborn |
|
|
|
Name: Dan Dearborn
Title: Chief Financial Officer |
Exhibit 2.3
WAIVER AGREEMENT
This WAIVER AGREEMENT (this “Waiver”), dated as of October 18, 2024, is entered into by and among Kintara Therapeutics, Inc. (“Kintara”), Kayak Mergeco, Inc., a wholly-owned subsidiary of Kintara incorporated in the State of Delaware (“Merger Sub”), and TuHURA Biosciences, Inc., a Delaware corporation (the “Company”). Capitalized terms used herein but not otherwise defined shall have the meanings ascribed to them in the Merger Agreement (as defined below).
RECITALS
WHEREAS, Kintara, the Company, and Merger Sub (individually referred to as a “Party” and collectively as the “Parties”) have previously entered into that certain Agreement and Plan of Merger, dated April 2, 2024 (as may be amended, modified or supplemented from time to time, the “Merger Agreement”);
WHEREAS, Section 6.21 of the Merger Agreement provides that Kintara will prepare and deliver to the Company prior to the Closing a schedule (the “Net Cash Schedule”) setting forth, in reasonable detail, Kintara’s good faith, estimated calculation of the Parent Closing Net Cash (as defined in the Merger Agreement), including each component thereof (the “Net Cash Calculation” as of the close of business on the last Business Day prior to the Closing Date) prepared and certified by Parent’s principal financial or accounting officer;
WHEREAS, Section 7.3(c) and (e) of the Merger Agreement provides that the obligation of each Party to effect the Merger and otherwise consummate the transactions contemplated by the Merger Agreement at the Closing is subject to, among other things, receipt by the Company from Kintara of the Net Cash Schedule and further, that the Parent Closing Net Cash shall be not less than the amounts set forth in Annex I of the Merger Agreement;
WHEREAS, Section 7.2(f) of the Merger Agreement provides that as an obligation of each Party to effect the Merger and otherwise consummate the transactions contemplated by the Merger Agreement at the Closing is subject to, among other things, stockholders of the Company representing no less than 50% of the outstanding shares of Company Common Stock on an “as-converted” basis as of immediately prior to the Effective Time have executed and delivered to Kintara Lock-Up Agreements (the “Lock-Up Requirement”);WHEREAS, despite the Company’s attempts to obtain Lock-Up Agreements to satisfy the Lock-Up Requirement, the Company has received Lock-Up Agreements representing approximately 60% of the Lock-Up Requirement (the “Lock-Up Shares”);
WHEREAS, notwithstanding the failure to obtain a number of Lock-Up Agreements to satisfy the Lock-Up Requirement, certain stockholders of the Company who are affiliates of the Company immediately prior to the Merger, will be prohibited from transferring their shares they receive in the Merger until a year after the combined company files its Form 10 information with the Securities and Exchange Commission (the “Restricted Stockholders” and the percentage of shares of Company Common Stock to which such Restricted Stockholders own, the “Restricted Shares”);
WHEREAS, in sum, the Restricted Shares and the Lock-Up Shares would satisfy the Lock-Up Requirement had the Restricted Stockholders signed Lock-Up Agreements;
WHEREAS, the Parties desire to waive the requirement (i) that the Parent Closing Net Cash be not less than the amounts set forth in Annex I of the Merger Agreement and (ii) that the Company satisfy the Lock-Up Requirement;
WHEREAS, the conditions precedent to Closing may be waived pursuant to Section 7.1 of the Merger Agreement upon the written waiver by each of Kintara, Merger Sub, and the Company.
NOW THEREFORE, for good and valuable consideration, the receipt and adequacy of which are hereby acknowledged, the Parties agree as follows:
1.Waiver of Parent Closing Net Cash Condition. Each Party hereby waives compliance by Kintara with the Parent Closing Net Cash Requirements set forth in Section 7.3(e) and Annex I of the Merger Agreement as a condition precedent to the Parties’ obligation to effect the Merger.
2.Waiver of Lock-Up Requirement. Each Party hereby waives compliance by the Company to obtain the Lock-Up Requirement as a condition precedent to the Parties’ obligation to effect the Merger.
3.Continuing Effect. Except as expressly set forth herein, all of the terms and conditions of the Merger Agreement shall remain in full force and effect and are hereby ratified and confirmed by the Parties. Without limiting the generality of the foregoing, nothing contained herein shall be deemed a waiver of any other provision of the Merger Agreement or as a waiver of or consent to any further or future action on the part of any Party that would require the waiver or consent of another Party.
4.Counterparts; Choice of Law. This Waiver may be executed in several identical counterparts all of which shall constitute one and the same instrument. This Waiver shall be construed and enforced in accordance with the laws of the State of Delaware, without regard to the principles of conflicts of law thereof.
5.Further Assurances. Each of the Parties hereto shall execute and deliver, at the reasonable request of the other Party hereto, such additional documents, instruments, conveyances and assurances and take such further actions as such other Party may reasonably request to carry out the provisions hereof and give effect to the transactions contemplated by this Waiver.
[signature page follows]
IN WITNESS WHEREOF, the Parties hereto have caused this Waiver to be duly executed as of the day and year written above.
COMPANY:
TUHURA BIOSCIENCES, INC.
By: /s/ James A. Bianco
Name: James A. Bianco, M.D.
Title: Chief Executive Officer
KINTARA:
KINTARA THERAPEUTICS, INC.
By: /s/ Robert Hoffman
Name: Robert Hoffman
Title: Chief Executive Officer
MERGER SUB:
KINTARA MERGECO, INC.
By: /s/ Robert Hoffman
Name: Robert Hoffman
Title: President and Secretary
[Signature Page to Waiver Agreement]
Exhibit 3.1



Exhibit 3.2


Exhibit 10.2
INDEMNITY AGREEMENT
This Indemnity Agreement (this “Agreement”) dated as of ___________ _____, _____, is made by and between TuHURA Biosciences, Inc., a Nevada corporation (the “Company”), and _________________ (“Indemnitee”).
Recitals
A.The Company desires to attract and retain the services of highly qualified individuals as directors, officers, employees and agents.
B. The Company’s Bylaws, as amended (the “Bylaws”), provide that the Company shall indemnify its directors, and empowers the Board of Directors of the Company to cause the Company to indemnify its officers, employees and other agents, as authorized by the Nevada Revised Statutes, as amended (the “Code”), under which the Company is organized and such Bylaws do not prohibit the Company from entering into separate agreements with its directors, officers and other persons to set forth specific indemnification provisions.
C. Indemnitee does not regard the protection currently provided by applicable law, the Bylaws, the Company’s other governing documents, and available insurance as adequate under the present circumstances, and the Company has determined that Indemnitee and other directors, officers, employees and agents of the Company may not be willing to serve or continue to serve in such capacities without additional protection.
D. The Company desires and has requested Indemnitee to serve or continue to serve as a director, officer, employee or agent of the Company, as the case may be, and has proffered this Agreement to Indemnitee as an additional inducement to serve in such capacity.
E. Indemnitee is willing to serve, or to continue to serve, as a director, officer, employee or agent of the Company, as the case may be, if Indemnitee is furnished the indemnity provided for herein by the Company.
Agreement
Now Therefore, in consideration of the mutual covenants and agreements set forth herein, the parties hereto, intending to be legally bound, hereby agree as follows:
1. Definitions.
(a) Agent. For purposes of this Agreement, the term “Agent” of the Company means any person who: (i) is or was a director, officer, employee, agent, or other fiduciary of the Company or a subsidiary of the Company; or (ii) is or was serving at the request or for the convenience of, or representing the interests of, the Company or a subsidiary of the Company, as a director, officer, employee, agent, or other fiduciary of a foreign or domestic corporation, partnership, joint venture, trust or other enterprise.
(b) Change in Control. For purposes of this Agreement, a “Change in Control” shall be deemed to have occurred if (i) any “person” (as such term is used in Sections 13(d) and 14(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), other than a trustee or other fiduciary holding securities under an employee benefit plan of the Company or a corporation owned directly or indirectly by the stockholders of the Company in substantially the same proportions as their ownership of stock of the Company, is or becomes the “beneficial owner” (as defined in Rule 13d-3 under the Exchange Act), directly or indirectly, of securities of the Company representing 20% or more of the total voting power represented by the Company’s then outstanding Voting Securities, (ii) individuals who on the date of this Agreement are members of the Board (the “Incumbent Board”) cease for any reason to constitute at least a majority of the members of the Board (provided, however, that if the appointment or election (or nomination for election) of any new Board member was approved or recommended by a majority vote of the members of the Incumbent Board then still in office, such new member shall be considered as a member of the Incumbent Board), or (iii) the stockholders of the Company approve a merger or consolidation of the Company with any other corporation, other than a merger or consolidation which would result in the Voting Securities of the Company outstanding immediately prior thereto continuing to represent (either by remaining outstanding or by being converted into Voting Securities of the surviving entity) at least 80% of the total voting power represented by the Voting Securities of the Company or such surviving entity outstanding immediately after such merger or
consolidation, or the stockholders of the Company approve a plan of complete liquidation of the Company or an agreement for the sale or disposition by the Company of (in one transaction or a series of transactions) all or substantially all of the Company’s assets.
(c) Expenses. For purposes of this Agreement, the term “Expenses” shall be broadly construed and shall include, without limitation, all direct and indirect costs of any type or nature whatsoever (including, without limitation, all attorneys’, witness, or other professional fees and related disbursements, and other out-of-pocket costs of whatever nature, actually and reasonably incurred by Indemnitee in connection with the investigation, defense or appeal of a proceeding or establishing or enforcing a right to indemnification under this Agreement, the Code or otherwise. The term “Expenses” shall also include reasonable compensation for time spent by Indemnitee for which he or she is not compensated by the Company or any subsidiary or third party: (i) for any period during which Indemnitee is not an Agent, in the employment of, or providing services for compensation to, the Company or any subsidiary; and (ii) if the rate of compensation and estimated time involved is approved by the directors of the Company who are not parties to any action with respect to which Expenses are incurred, for Indemnitee while an Agent of, employed by, or providing services for compensation to, the Company or any subsidiary.
(d) Independent Counsel. For purposes of this Agreement, the term “Independent Counsel” means a law firm, or a partner (or, if applicable, member) of such a law firm, that is experienced in matters of corporation law and neither presently is, nor in the past five (5) years has been, retained to represent: (i) the Company or Indemnitee in any matter material to either such party, or (ii) any other party to the proceeding giving rise to a claim for indemnification hereunder. Notwithstanding the foregoing, the term “Independent Counsel” shall not include any person who, under the applicable standards of professional conduct then prevailing, would have a conflict of interest in representing either the Company or Indemnitee in an action to determine Indemnitee’s rights under this Agreement. The Company will pay the reasonable fees and expenses of the Independent Counsel referred to above and to fully indemnify such counsel against any and all expenses, claims, liabilities and damages arising out of or relating to this Agreement or its engagement pursuant hereto.
(e) Liabilities. For purposes of this Agreement, the term “Liabilities” shall be broadly construed and shall include, without limitation, judgments, damages, deficiencies, liabilities, losses, penalties, excise taxes, fines, assessments and amounts paid in settlement, including any interest and any federal, state, local or foreign taxes imposed as a result of the actual or deemed receipt of any payment under this Agreement.
(f) Proceedings. For purposes of this Agreement, the term “proceeding” shall be broadly construed and shall include, without limitation, any threatened, pending, or completed action, suit, claim, counterclaim, cross claim, arbitration, mediation, alternate dispute resolution mechanism, investigation, inquiry, administrative hearing, or any other actual, threatened or completed proceeding, whether brought in the right of the Company or otherwise and whether of a civil, criminal, administrative or investigative nature, and whether formal or informal in any case, in which Indemnitee was, is or will be involved as a party, potential party, non-party witness, or otherwise by reason of: (i) the fact that Indemnitee is or was a director or officer of the Company; (ii) the fact that any action taken by Indemnitee (or a failure to take action by Indemnitee) or of any action (or failure to act) on Indemnitee’s part while acting as an Agent; or (iii) the fact that Indemnitee is or was serving at the request of the Company as a director, officer, employee or agent of another corporation, partnership, joint venture, trust, employee benefit plan, or other enterprise, and in any such case described above, whether or not serving in any such capacity at the time any liability or Expense is incurred for which indemnification, reimbursement, or advancement of Expenses may be provided under this Agreement. If the Indemnitee believes in good faith that a given situation may lead to or culminate in the institution of a proceeding, this shall be considered a proceeding under this paragraph.
(g) Subsidiary. For purposes of this Agreement, the term “subsidiary” means any corporation, limited liability company, or other entity, of which more than 50% of the outstanding voting securities or equity interests are owned, directly or indirectly, by the Company and one or more of its subsidiaries, and any other corporation, limited liability company, partnership, joint venture, trust, employee benefit plan or other enterprise of which Indemnitee is or was serving at the request of the Company as an Agent.
(h) Voting Securities. For purposes of this Agreement, “Voting Securities” shall mean any securities of the Company that vote generally in the election of directors.
2. Agreement to Serve. Indemnitee will serve, or continue to serve, as the case may be, as an Agent, faithfully and to the best of his or her ability, at the will of such entity designated by the Company and at the request of the Company (or under separate agreement, if such agreement exists), in the capacity Indemnitee currently serves such entity, so long as Indemnitee is duly appointed or elected and qualified in accordance with the applicable provisions of the governance documents of such entity, or until such time as Indemnitee tenders his or her resignation in writing; provided, however, that nothing contained in this Agreement is intended as an employment agreement between Indemnitee and the Company or any of its subsidiaries or to create any right to continued employment of Indemnitee with the Company or any of its subsidiaries in any capacity.
The Company acknowledges that it has entered into this Agreement and assumes the obligations imposed on it hereby, in addition to and separate from its obligations to Indemnitee under the Bylaws, to induce Indemnitee to serve, or continue to serve, as an Agent, and the Company acknowledges that Indemnitee is relying upon this Agreement in serving as an Agent.
3. Indemnification.
(a) Indemnification in Third Party Proceedings. Subject to Section 10 below, the Company shall indemnify Indemnitee to the fullest extent permitted by the Code, as the same may be amended from time to time (but, to the fullest extent of the law, only to the extent that such amendment permits Indemnitee to broader indemnification rights than the Code permitted prior to adoption of such amendment), if Indemnitee is a party to or threatened to be made a party to or otherwise involved in any proceeding, other than a proceeding by or in the right of the Company to procure a judgment in its favor, for any and all Expenses and Liabilities (including all interest, assessments and other charges paid or payable in connection with or in respect of such Expenses and Liabilities) incurred by Indemnitee in connection with the investigation, defense, settlement or appeal of such proceeding, if Indemnitee acted in good faith and in a manner Indemnitee reasonably believed to be in or not opposed to the best interests of the Company and, in the case of a criminal proceeding had no reasonable cause to believe that Indemnitee's conduct was unlawful. The parties hereto intend that this Agreement shall provide to the fullest extent permitted by law for indemnification in excess of that expressly permitted by statute, including, without limitation, any indemnification provided by the Certificate of Incorporation of the Company, the Bylaws, vote of its stockholders or disinterested directors, or applicable law.
(b) Indemnification in Derivative Actions and Direct Actions by the Company. Subject to Section 10 below, the Company shall indemnify Indemnitee to the fullest extent permitted by the Code, as the same may be amended from time to time (but, fullest extent permitted by applicable law, only to the extent that such amendment permits Indemnitee to broader indemnification rights than the Code permitted prior to adoption of such amendment), if Indemnitee is a party to or threatened to be made a party to or otherwise involved in any proceeding by or in the right of the Company to procure a judgment in its favor, against any and all Expenses actually and reasonably incurred by Indemnitee in connection with the investigation, defense, settlement, or appeal of such proceedings, if Indemnitee acted in good faith and in a manner Indemnitee reasonably believed to be in or not opposed to the best interests of the Company. No indemnification for Expenses shall be made under this Section 3(b) in respect of any claim, issue or matter as to which Indemnitee shall have been finally adjudged by a court competent jurisdiction to be liable to the Company, unless and only to the extent that the Nevada District Court or any court in which the proceeding was brought shall determine upon application that, despite the adjudication of liability but in view of all the circumstances of the case, Indemnitee is fairly and reasonably entitled to indemnification.
4. Indemnification of Expenses of Successful Party. Notwithstanding any other provision of this Agreement, in circumstances where indemnification is not available under Section 3(a) or 3(b), as the case may be, to the fullest extent permitted by law and to the extent that Indemnitee is a party to (or a participant in) any proceeding and has been successful on the merits or otherwise in defense of any proceeding or in defense of any claim, issue or matter therein, in whole or part, including the dismissal of any action without prejudice, the Company shall indemnify Indemnitee against all Expenses and Liabilities in connection with the investigation, defense or appeal of such proceeding. If Indemnitee is not wholly successful in such proceeding but is successful, on the merits or otherwise, as to one or more but less than all claims, issues or matters in such proceeding, the Company shall indemnify Indemnitee against all Expenses and Liabilities incurred by Indemnitee or on Indemnitee’s behalf in connection with or related to each successfully resolved claim, issue or matter to the fullest extent permitted by law.
5. Partial Indemnification; Witness Indemnification. If Indemnitee is entitled under any provision of this Agreement to indemnification by the Company for some or a portion of any Expenses and Liabilities incurred by Indemnitee in the investigation, defense, settlement or appeal of a proceeding, but is precluded by applicable law or the specific terms of this Agreement to indemnification for the total amount thereof, the Company shall nevertheless indemnify Indemnitee for the portion thereof to which Indemnitee is entitled. Notwithstanding any other provision of this Agreement, to the fullest extent permitted by applicable law and to the extent that Indemnitee is, by reason of Indemnitee’s acting as an Agent, a witness or otherwise asked to participate in any proceeding to which Indemnitee is not a party, Indemnitee shall be indemnified against all Expenses incurred by Indemnitee or on Indemnitee’s behalf in connection therewith.
6. Advancement of Expenses. To the extent not prohibited by law, the Company shall advance the Expenses incurred by Indemnitee in connection with any proceeding, and such advancement shall be made within twenty (20) days after the receipt by the Company of a statement or statements requesting such advances (which shall include invoices received by Indemnitee in connection with such Expenses but, in the case of invoices in connection with legal services, any references to legal work performed or to expenditures made that would cause Indemnitee to waive any privilege accorded by applicable law shall not be included with the invoice) and upon request of the Company, an undertaking to repay the advancement of Expenses if and to the extent that it is ultimately determined by a court of competent jurisdiction in a final judgment, not subject to appeal, that Indemnitee is not entitled to be indemnified by the Company. Advances shall be unsecured, interest free and without regard to Indemnitee’s ability to repay the Expenses. Advances shall include any and all Expenses incurred by Indemnitee pursuing an action to enforce Indemnitee’s right to indemnification under this Agreement or otherwise and this right of advancement, including expenses incurred preparing and forwarding statements to the Company to support the advances claimed. Indemnitee acknowledges that the execution and delivery of this Agreement shall constitute an undertaking providing that Indemnitee shall, to the fullest extent required by law, repay the advance (without interest) if and to the extent that it is ultimately determined by a court of competent jurisdiction in a final judgment, not subject to appeal, that Indemnitee is not entitled to be indemnified by the Company. The right to advances under this Section shall continue until final disposition of any proceeding, including any appeal therein. This Section 6 shall not apply to any claim made by Indemnitee for which indemnity is excluded pursuant to Section 10(b).
7. Notice and Other Indemnification Procedures.
(a) Notification of Proceeding. Indemnitee will notify the Company in writing promptly upon being served with any summons, citation, subpoena, complaint, indictment, information or other document relating to any proceeding or matter which may be subject to indemnification or advancement of Expenses covered hereunder. The written notification to the Company shall include a description of the nature of the proceeding and the facts underlying the proceeding. The failure of Indemnitee to so notify the Company shall not relieve the Company of any obligation which it may have to Indemnitee under this Agreement or otherwise and any delay in so notifying the Company shall not constitute a waiver by Indemnitee of any rights under this Agreement.
(b) Request for Indemnification Payments. To obtain indemnification under this Agreement, Indemnitee shall submit to the Company a written request, including therein or therewith such documentation and information as is reasonably available to Indemnitee and is reasonably necessary to determine whether and to what extent Indemnitee is entitled to indemnification under the terms of this Agreement, and shall request payment thereof by the Company.
(c) Determination of Right to Indemnification Payments. Upon written request by Indemnitee for indemnification pursuant to the Section 7(b) hereof, a determination with respect to Indemnitee’s entitlement thereto shall be made in the specific case by one of the following four methods, which shall be at the election of the Board of Directors: (1) by a majority vote of the disinterested directors, even though less than a quorum, (2) by a committee of disinterested directors designated by a majority vote of the disinterested directors, even though less than a quorum, (3) if there are no disinterested directors or if the disinterested directors so direct, by Independent Counsel in a written opinion to the Board of Directors, a copy of which shall be delivered to the Indemnitee, or (4) if so directed by the Board of Directors, by the stockholders of the Company; provided, however, that if there has been a Change in Control, then such determination shall be made by Independent Counsel selected by Indemnitee and approved by the Company (which approval shall not be unreasonably withheld). For purposes hereof, disinterested directors are those members of the board of directors of the Company who are not parties to the action, suit or proceeding in respect of
which indemnification is sought by Indemnitee. Indemnification payments requested by Indemnitee under Section 7(b) hereof shall be made by the Company no later than sixty (60) days after receipt of the written request of Indemnitee. Claims for advancement of Expenses shall be made under the provisions of Section 6 herein.
(d) Application for Enforcement. In the event the Company fails to make timely payments as set forth in Sections 6 or 7(b) above, Indemnitee shall have the right to apply to any court of competent jurisdiction for the purpose of enforcing Indemnitee’s right to indemnification or advancement of Expenses pursuant to this Agreement. In such an enforcement hearing or proceeding, the burden of proof shall be on the Company to prove that indemnification or advancement of Expenses to Indemnitee is not required under this Agreement or permitted by applicable law. Any determination by the Company (including its Board of Directors, a committee thereof, Independent Counsel) or stockholders of the Company, that Indemnitee is not entitled to indemnification hereunder, shall not be a defense by the Company to the action nor create any presumption that Indemnitee is not entitled to indemnification or advancement of Expenses hereunder.
(e) Indemnification of Certain Expenses. The Company shall indemnify Indemnitee against all Expenses incurred in connection with any hearing or proceeding under this Section 7 unless the Company prevails in such hearing or proceeding on the merits in all material respects.
8. Assumption of Defense. In the event the Company shall be requested by Indemnitee to pay the Expenses of any proceeding, the Company, if appropriate, shall be entitled to assume the defense of such proceeding, or to participate to the extent permissible in such proceeding, with counsel reasonably acceptable to Indemnitee. Upon assumption of the defense by the Company and the retention of such counsel by the Company, the Company shall not be liable to Indemnitee under this Agreement for any fees of counsel subsequently incurred by Indemnitee with respect to the same proceeding, provided that Indemnitee shall have the right to employ separate counsel in such proceeding at Indemnitee’s sole cost and expense. Notwithstanding the foregoing, if Indemnitee’s counsel delivers a written notice to the Company stating that such counsel has reasonably concluded that there may be a conflict of interest between the Company and Indemnitee in the conduct of any such defense or the Company shall not, in fact, have employed counsel or otherwise actively pursued the defense of such proceeding within a reasonable time, then in any such event the fees and Expenses of Indemnitee’s counsel to defend such proceeding shall be subject to the indemnification and advancement of Expenses provisions of this Agreement.
9. Insurance. To the extent that the Company maintains an insurance policy or policies providing liability insurance for Agents (“D&O Insurance”), Indemnitee shall be covered by such policy or policies in accordance with its or their terms to the maximum extent of the coverage available for any such Agent under such policy or policies. If, at the time of the receipt of a notice of a claim pursuant to the terms hereof, the Company has D&O Insurance in effect or otherwise potentially available, the Company shall give prompt notice of the commencement of such proceeding to the insurers in accordance with the procedures set forth in the respective policies. The Company shall thereafter take all necessary or desirable action to cause such insurers to pay, on behalf of Indemnitee, all amounts payable as a result of such proceeding in accordance with the terms of such policies.
In the event of a change of control of the Company or the Company dissolving or liquidating (including being placed into receivership or entering the federal bankruptcy process and the like), the Company shall maintain in force any and all insurance policies then maintained by the Company in providing insurance in respect of Indemnitee (directors’ and officers’ liability, fiduciary, employment practices or otherwise) for a period of at least six years thereafter (a “Tail Policy”). If such coverage is not placed with the incumbent insurance carriers using the policies that were in place at the time of the change of control or insolvency event, the Tail Policy shall be substantially comparable in scope and amount as the expiring policies, and the insurance carriers for the Tail Policy shall have an AM Best rating that is the same or better than the AM Best ratings of the expiring policies.
10. Exceptions.
(a) Certain Matters. Any provision herein to the contrary notwithstanding, the Company shall not be obligated pursuant to the terms of this Agreement to indemnify Indemnitee on account of any proceeding with respect to: (i) remuneration paid to Indemnitee if it is determined by final judgment or other final adjudication that such remuneration was in violation of law (and, in this respect, both the Company and Indemnitee have been advised that the Securities and Exchange Commission believes that indemnification for liabilities arising under the federal
securities laws is against public policy and is, therefore, unenforceable and that claims for indemnification should be submitted to appropriate courts for adjudication, as indicated in Section 10(d) below); (ii) a final judgment rendered against Indemnitee for an accounting, disgorgement or repayment of profits made from the purchase or sale by Indemnitee of securities of the Company against Indemnitee or in connection with a settlement by or on behalf of Indemnitee to the extent it is acknowledged by Indemnitee and the Company that such amount paid in settlement resulted from Indemnitee's conduct from which Indemnitee received monetary personal profit, pursuant to the provisions of Section 16(b) of the Exchange Act or other provisions of any federal, state or local statute or rules and regulations thereunder; (iii) a final judgment or other final adjudication that Indemnitee’s conduct was in bad faith, knowingly fraudulent or deliberately dishonest or constituted willful misconduct (but only to the extent of such specific determination); or (iv) on account of conduct that is established by a final judgment as constituting a breach of Indemnitee’s duty of loyalty to the Company or resulting in any personal profit or advantage to which Indemnitee is not legally entitled. For purposes of the foregoing sentence, a final judgment or other adjudication may be reached in either the underlying proceeding or action in connection with which indemnification is sought or a separate proceeding or action to establish rights and liabilities under this Agreement.
(b) Claims Initiated by Indemnitee. Any provision herein to the contrary notwithstanding, the Company shall not be obligated to indemnify or advance Expenses to Indemnitee with respect to proceedings or claims initiated or brought by Indemnitee against the Company or its Agents and not by way of defense, except (i) with respect to proceedings brought to establish or enforce a right to indemnification or advancement under this Agreement or under any other agreement, provision in the Bylaws or the Certificate of Incorporation or applicable law, or (ii) with respect to any other proceeding initiated by Indemnitee that is either approved by the Board of Directors or Indemnitee’s participation is required by applicable law. However, indemnification or advancement of Expenses may be provided by the Company in specific cases if the Board of Directors determines it to be appropriate.
(c) Unauthorized Settlements. Any provision herein to the contrary notwithstanding, the Company shall not be obligated pursuant to the terms of this Agreement to indemnify Indemnitee under this Agreement for any amounts paid in settlement of a proceeding effected without the Company’s written consent. Neither the Company nor Indemnitee shall unreasonably withhold consent to any proposed settlement; provided, however, that the Company may in any event decline to consent to (or to otherwise admit or agree to any liability for indemnification hereunder in respect of) any proposed settlement if the Company is also a party in such proceeding and determines in good faith that such settlement is not in the best interests of the Company and its stockholders.
(d) Securities Act Liabilities. Any provision herein to the contrary notwithstanding, the Company shall not be obligated pursuant to the terms of this Agreement to indemnify Indemnitee or otherwise act in violation of any undertaking appearing in and required by the rules and regulations promulgated under the Securities Act of 1933, as amended (the “Securities Act”), or in any registration statement filed with the SEC under the Securities Act. Indemnitee acknowledges that paragraph (h) of Item 512 of Regulation S-K currently generally requires the Company to undertake in connection with any registration statement filed under the Securities Act to submit the issue of the enforceability of Indemnitee’s rights under this Agreement in connection with any liability under the Securities Act on public policy grounds to a court of appropriate jurisdiction and to be governed by any final adjudication of such issue. Indemnitee specifically agrees that any such undertaking shall supersede the provisions of this Agreement and to be bound by any such undertaking.
(e) Prior Payments Any provision herein to the contrary notwithstanding, the Company shall not be obligated pursuant to the terms of this Agreement to indemnify or advance Expenses to Indemnitee under this Agreement for which payment has actually been made to or on behalf of Indemnitee under any insurance policy or other indemnity provision, except with respect to any excess beyond the amount paid under any insurance policy or indemnity policy.
11. Nonexclusivity and Survival of Rights. The provisions for indemnification and advancement of Expenses set forth in this Agreement shall not be deemed exclusive of any other rights which Indemnitee may at any time be entitled under any provision of applicable law, the Company’s Certificate of Incorporation, the Bylaws or other agreements, both as to action in Indemnitee’s official capacity and Indemnitee’s action as an Agent, in any court in which a proceeding is brought, and Indemnitee’s rights hereunder shall continue after Indemnitee has ceased acting as an Agent and shall inure to the benefit of the heirs, executors, administrators and assigns of Indemnitee. The obligations and duties of the Company to Indemnitee under this Agreement shall be binding on the Company and its
successors and assigns until terminated in accordance with its terms. The Company shall require any successor (whether direct or indirect, by purchase, merger, consolidation or otherwise) to all or substantially all of the business or assets of the Company, expressly to assume and agree to perform this Agreement in the same manner and to the same extent that the Company would be required to perform if no such succession had taken place.
No amendment, alteration or repeal of this Agreement or of any provision hereof shall limit or restrict any right of Indemnitee under this Agreement in respect of any action taken or omitted by such Indemnitee in his or her corporate status prior to such amendment, alteration or repeal. To the extent that a change in the Code, whether by statute or judicial decision, permits greater indemnification or advancement of Expenses than would be afforded currently under the Company’s Certificate of Incorporation, the Bylaws and this Agreement, it is the intent of the parties hereto that Indemnitee shall enjoy by this Agreement the greater benefits so afforded by such change. No right or remedy herein conferred is intended to be exclusive of any other right or remedy, and every other right and remedy shall be cumulative and in addition to every other right and remedy given hereunder or now or hereafter existing at law or in equity or otherwise. The assertion or employment of any right or remedy hereunder, or otherwise, by Indemnitee shall not prevent the concurrent assertion or employment of any other right or remedy by Indemnitee.
12. Term. This Agreement shall continue until and terminate upon the later of: (a) five (5) years after the date that Indemnitee shall have ceased to serve as an Agent; or (b) one (1) year after the final termination of any proceeding, including any appeal then pending, in respect to which Indemnitee was granted rights of indemnification or advancement of Expenses hereunder.
No legal action shall be brought and no cause of action shall be asserted by or in the right of the Company against an Indemnitee or an Indemnitee's estate, spouse, heirs, executors or personal or legal representatives after the expiration of five (5) years from the date of accrual of such cause of action, and any claim or cause of action of the Company shall be extinguished and deemed released unless asserted by the timely filing of a legal action within such five-year period; provided, however, that if any shorter period of limitations is otherwise applicable to such cause of action, such shorter period shall govern.
13. Subrogation. In the event of payment under this Agreement, the Company shall be subrogated to the extent of such payment to all of the rights of recovery of Indemnitee, who, at the request and expense of the Company, shall execute all papers required and shall do everything that may be reasonably necessary to secure such rights, including the execution of such documents necessary to enable the Company effectively to bring suit to enforce such rights.
14. Interpretation of Agreement. It is understood that the parties hereto intend this Agreement to be interpreted and enforced so as to provide indemnification and advancement of Expenses to Indemnitee to the fullest extent now or hereafter permitted by law.
15. Severability. If any provision of this Agreement shall be held to be invalid, illegal or unenforceable for any reason whatsoever, (a) the validity, legality and enforceability of the remaining provisions of the Agreement (including without limitation, all portions of any paragraphs of this Agreement containing any such provision held to be invalid, illegal or unenforceable, that are not themselves invalid, illegal or unenforceable) shall not in any way be affected or impaired thereby; and (b) to the fullest extent possible, the provisions of this Agreement (including, without limitation, all portions of any paragraph of this Agreement containing any such provision held to be invalid, illegal or unenforceable, that are not themselves invalid, illegal or unenforceable) shall be construed so as to give effect to the intent manifested by the provision held invalid, illegal or unenforceable and to give effect to Section 14 hereof.
16. Amendment and Waiver. No supplement, modification, amendment, or cancellation of this Agreement shall be binding unless executed in writing by the parties hereto. No waiver of any of the provisions of this Agreement shall be deemed or shall constitute a waiver of any other provision hereof (whether or not similar) nor shall such waiver constitute a continuing waiver.
17. Notice. Except as otherwise provided herein, any notice or demand which, by the provisions hereof, is required or which may be given to or served upon the parties hereto shall be in writing and, if by electronic transmission, shall be deemed to have been validly served, given or delivered when sent, if by overnight delivery, courier or personal delivery, shall be deemed to have been validly served, given or delivered upon actual delivery and,
if mailed, shall be deemed to have been validly served, given or delivered three (3) business days after deposit in the United States mail, as registered or certified mail, with proper postage prepaid and addressed to the party or parties to be notified at the addresses set forth on the signature page of this Agreement (or such other address(es) as a party may designate for itself by like notice). If to the Company, notices and demands shall be delivered to the attention of the Secretary or Chief Financial Officer of the Company.
18. Governing Law. This Agreement shall be governed exclusively by and construed according to the laws of the State of Nevada, as applied to contracts between Nevada residents entered into and to be performed entirely within Nevada.
19. Counterparts. This Agreement may be executed in one or more counterparts, each of which shall for all purposes be deemed to be an original but all of which together shall constitute but one and the same Agreement. Only one such counterpart need be produced to evidence the existence of this Agreement.
20. Headings. The headings of the sections of this Agreement are inserted for convenience only and shall not be deemed to constitute part of this Agreement or to affect the construction hereof.
21. Entire Agreement. Subject to Section 11 hereof, this Agreement constitutes the entire agreement between the parties with respect to the subject matter hereof and supersedes all prior agreements, understandings and negotiations, written and oral, between the parties with respect to the subject matter of this Agreement; provided, however, that this Agreement is a supplement to and in furtherance of the Company’s Certificate of Incorporation, the Bylaws, the Code and any other applicable law, and shall not be deemed a substitute therefor, and does not diminish or abrogate any rights of Indemnitee thereunder.
22. Contribution. To the fullest extent permissible under applicable law, if the indemnification provided for in this Agreement is unavailable to Indemnitee for any reason whatsoever, the Company, in lieu of indemnifying Indemnitee, shall contribute to the amount incurred by Indemnitee, whether for judgments, fines, penalties, excise taxes, amounts paid or to be paid in settlement and/or for Expenses, in connection with any claim relating to an indemnifiable event under this Agreement, in such proportion as is deemed fair and reasonable in light of all of the circumstances of such proceeding in order to reflect (i) the relative benefits received by the Company and Indemnitee as a result of the event(s) and/or transaction(s) giving cause to such proceeding; and/or (ii) the relative fault of the Company and Indemnitee in connection with such event(s) and/or transaction(s).
23. Consent to Jurisdiction. The Company and Indemnitee hereby irrevocably and unconditionally (i) agree that any action or proceeding arising out of or in connection with this Agreement shall be brought only in the Nevada District Court (the “Nevada Court”), and not in any other state or federal court in the United States of America or any court in any other country, (ii) consent to submit to the exclusive jurisdiction of the Nevada Court for purposes of any action or proceeding arising out of or in connection with this Agreement, (iii) agree to appoint, to the extent such party is not otherwise subject to service of process in the State of Nevada, an agent in the State of Nevada as such party's agent for acceptance of legal process in connection with any such action or proceeding against such party with the same legal force and validity as if served upon such party personally within the State of Nevada, (iv) waive any objection to the laying of venue of any such action or proceeding in the Nevada Court, and (v) waive, and agree not to plead or to make, any claim that any such action or proceeding brought in the Nevada Court has been brought in an improper or inconvenient forum.
In Witness Whereof, the parties hereto have entered into this Agreement effective as of the date first above written.
|
|
|
|
TUHURA BIOSCIENCES, INC. |
|
|
|
|
By: |
|
|
Name: |
|
|
Title: |
|
|
|
|
|
INDEMNITEE |
|
|
|
|
|
|
Signature of Indemnitee |
|
|
|
|
|
|
Print or Type Name of Indemnitee |
Exhibit 10.4
OMNIBUS EQUITY INCENTIVE PLAN
TUHURA BIOSCIENCES, INC.
2024 EQUITY INCENTIVE PLAN
1. Purpose; Effective Date; Effect on Prior Plan.
(a) Purpose. The TuHURA Biosciences, Inc. 2024 Equity Incentive Plan (the “Plan”) has two complementary purposes: (i) to attract and retain outstanding individuals to serve as officers, directors, employees, and consultants, and (ii) to increase stockholder value. The Plan will provide participants with incentives to increase stockholder value by offering the opportunity to acquire shares of the Company’s common stock, receive monetary payments based on the value of such common stock, or receive other incentive compensation, on the potentially favorable terms that this Plan provides.
(b) Effective Date; Effect on Prior Plan. The Plan shall become effective at the Effective Time (as defined in the Merger Agreement) (the “Effective Date”), provided that the Company’s stockholders have approved the Plan on or before such date. The Plan will terminate as provided in Section 15. Following the Effective Date, no additional awards will be made under the Company’s 2017 Omnibus Equity Incentive Plan, as amended and restated (the “Prior Plan”), although awards previously granted under the Prior Plan and still outstanding as of the Effective Date will remain outstanding and continue to be subject to all terms and conditions of the Prior Plan.
2. Definitions. Capitalized terms used and not otherwise defined in this Plan or in any Award agreement have the following meanings:
(a) “10% Stockholder” means an individual who owns more than ten percent (10%) of the total combined voting power of all classes of outstanding stock of the Company, its parent or any of its Subsidiaries. In determining stock ownership, the attribution rules of Section 424(d) of the Code shall be applied.
(b) “Administrator” means the Board or the Committee; provided that, to the extent the Board or the Committee has delegated authority and responsibility as an Administrator of the Plan to one or more committees or officers of the Company as permitted by Section 3(b), the term “Administrator” shall also mean such committee(s) and/or officer(s).
(c) “Affiliate” has the meaning ascribed to such term in Rule 12b-2 under the Exchange Act. Notwithstanding the foregoing, for purposes of determining those individuals to whom an Option or a Stock Appreciation Right may be granted, the term “Affiliate” means any entity that, directly or through one or more intermediaries, is controlled by or is under common control with, the Company within the meaning of Code Sections 414(b) or (c); provided that, in applying such provisions, the phrase “at least 20 percent” shall be used in place of “at least 80 percent” each place it appears therein.
(d) “Applicable Exchange” means the national securities exchange or automated trading system on which the Stock is principally traded at the applicable time.
(e) “Award” means a grant of Options, Stock Appreciation Rights, Performance Shares, Performance Units, Stock, Restricted Stock, Restricted Stock Units, a Cash Incentive Award, or any other type of award permitted under this Plan.
(f) “Board” means the Board of Directors of the Company.
(g) “Cash Incentive Award” means the right to receive a cash payment to the extent Performance Goals are achieved (or other requirements are met), as described in Section 10.
(h) “Cause” means, with respect to a Participant, one of the following, which are listed in order of priority:
(i) the meaning given in a Participant’s employment, retention, change of control, severance or similar agreement with the Company or any Affiliate; or if none then
(ii) the meaning given in the Award agreement; or if none then
(iii) the Administrator determines that such Participant has: (A) committed any felony or any crime involving fraud, dishonesty or moral turpitude under the laws of the United States or any state thereof; (B) attempted to commit or participate in a fraud or act of dishonesty against the Company or an Affiliate; (C) intentionally and materially violated any contract or agreement between the Participant and the Company or an Affiliate or of any statutory duty owed to the Company or an Affiliate; (D) used or disclosed the Company’s (or an Affiliate’s) confidential information or trade secrets in an unauthorized manner; or (E) committed gross misconduct.
(i) A “Change of Control” shall be deemed to occur upon the first to occur of the following events:
(i) a Person (other than an Excluded Person) either (A) acquires twenty percent (20%) or more of the combined voting power of the outstanding securities of the Company having the right to vote in elections of directors and such acquisition shall not have been approved within sixty (60) days following such acquisition by a majority of the Continuing Directors then in office, or (B) acquires fifty percent (50%) or more of the combined voting power of the outstanding securities of the Company having a right to vote in elections of directors; or
(ii) Continuing Directors shall for any reason cease to constitute a majority of the Board; or
(iii) the Company disposes of all or substantially all of the business of the Company to a party or parties other than a Subsidiary or other Affiliate of the Company pursuant to a partial or complete liquidation of the Company, sale of the Company’s assets (including stock of a subsidiary of the Company) or otherwise; or
(iv) there is consummated a merger, consolidation or share exchange of the Company with any other corporation or the issuance of voting securities of the Company in connection with a merger, consolidation or share exchange of the Company (or any direct or indirect subsidiary of the Company), other than (A) a merger, consolidation or share exchange which would result in the voting securities of the Company outstanding immediately prior to such merger, consolidation or share exchange continuing to represent (either by remaining outstanding or by being converted into voting securities of the surviving entity or any parent thereof) at least fifty percent (50%) of the combined voting power of the voting securities of the Company or such surviving entity or any parent thereof outstanding immediately after such merger, consolidation or share exchange, or (B) a merger, consolidation or share exchange effected to implement a recapitalization of the Company (or similar transaction) in which no Person (other than an Excluded Person) is or becomes the beneficial owner, directly or indirectly, of securities of the Company (not including in the securities beneficially owned by such Person any securities acquired directly from the Company or its Affiliates after the Effective Date pursuant to express authorization by the Board that refers to this exception) representing twenty percent (20%) or more of either the then outstanding shares of Stock or the Company or the combined voting power of the Company’s then outstanding voting securities.
Notwithstanding the foregoing, (A) no Change of Control shall be deemed to have occurred if there is consummated any transaction or series of integrated transactions immediately following which the record holders of the Stock immediately prior to such transaction or series of transactions continue to own, directly or indirectly, in the same proportions as their ownership in the Company, an entity that owns all or substantially all of the assets or voting securities of the Company immediately following such transaction or series of transactions; and (B) for purposes of an Award (1) that provides for the payment of deferred compensation that is subject to Code Section 409A or (2) with respect to which the Company permits a deferral election, the definition of “Change of Control” shall be deemed amended to conform to the requirements of Code Section 409A to the extent necessary for the Award and deferral election to comply with Code Section 409A.
(j) “Code” means the Internal Revenue Code of 1986, as amended. Any reference to a specific provision of the Code includes any successor provision and the regulations promulgated under such provision.
(k) “Committee” means the Compensation Committee of the Board, any successor committee thereto or such other committee of the Board that is designated by the Board with the same or similar authority. The Committee
shall consist only of Non-Employee Directors (not fewer than two (2)) who meet the definition of “non-employee director” under Rule 16b-3(b)(3) promulgated under the Exchange Act to the extent necessary for the Plan and Awards to comply with Rule 16b-3 promulgated under the Exchange Act.
(l) “Company” means TuHURA Biosciences, Inc. (f/k/a Kintara Therapeutics, Inc.), a Nevada corporation, or any successor thereto.
(m) “Continuing Director” means a member of the Board who either was a member of the Board on the Effective Date or who subsequently became a Director and whose election, or nomination for election, was approved by a vote of at least two-thirds (2/3) of the Continuing Directors then in office.
(n) “Director” means a member of the Board.
(o) “Dividend Equivalent Unit” means the right to receive a payment, in cash or Shares, equal to the cash dividends or other cash distributions paid with respect to a Share.
(p) “Exchange Act” means the Securities Exchange Act of 1934, as amended. Any reference to a specific provision of the Exchange Act includes any successor provision and the regulations and rules promulgated under such provision.
(q) “Excluded Person” means (i) the Company or its subsidiaries, (ii) a trustee or other fiduciary holding securities under any employee benefit plan of the Company or its subsidiaries, (iii) an underwriter temporarily holding securities pursuant to an offering of such securities, or (iv) a corporation owned, directly or indirectly, by the shareholders of the Company in substantially the same proportions as their ownership of stock in the Company
(r) “Fair Market Value” means, as of a given date, the closing sale price of a Share on the Applicable Exchange on such date or, if there shall be no such sale on such date, on the next preceding day on which such a sale shall have occurred; provided that, if so determined by the Administrator, Fair Market Value may instead mean a price that is based on the opening, closing, actual, high or low sale price, or the arithmetic mean of selling prices of, a Share, on the Applicable Exchange on the applicable date, the preceding trading day, the next succeeding trading day, or the arithmetic mean of selling prices on all trading days over a specified averaging period weighted by volume of trading on each trading day in the period that is within 30 days before or 30 days after the applicable date, as determined by the Administrator in its discretion; provided further that, if an arithmetic mean of prices is used to set a grant price or an exercise price for an Option or Stock Appreciation Right, the commitment to grant the applicable Award based on such arithmetic mean must be irrevocable before the beginning of the specified averaging period in accordance with Treasury Regulation §1.409A-1(b)(5)(iv)(A). The method of determining Fair Market Value with respect to an Award shall be determined by the Administrator and may differ depending on whether Fair Market Value is in reference to the grant, exercise, vesting, settlement, or payout of an Award. If the Stock is not traded on an established stock exchange, the Administrator shall determine in good faith the Fair Market Value in whatever manner it considers appropriate, but based on objective criteria; provided that, to the extent required to secure an exemption from Code Section 409A, Fair Market Value shall be determined using a reasonable application of a reasonable valuation method. Notwithstanding the foregoing, in the case of an actual sale of Shares, the actual sale price shall be the Fair Market Value of such Shares.
(s) “Merger Agreement” means the Agreement and Plan of Merger, dated as of April 2, 2024, by and among the Company, Kayak Mergeco, Inc. and TuHURA Biosciences, Inc.
(t) “Non-Employee Director” means a Director who is not also an employee of the Company or its Subsidiaries.
(u) “Option” means the right to purchase Shares at a stated price for a specified period of time.
(v) “Participant” means an individual selected by the Administrator to receive an Award.
(w) “Performance Goals” means any objective or subjective goals the Administrator establishes with respect to an Award. Performance Goals may include, but are not limited to, the performance of the Company or any one or more of its Subsidiaries, Affiliates or its or their business units (or any combination thereof) with respect to the following measures: (a) net earnings or net income; (b) operating earnings or operating income; (c) pretax earnings; (d) earnings per share; (f) share price, including growth measures and total stockholder return; (g) earnings before interest and taxes and related margin; (h) earnings before interest, taxes, depreciation and/or amortization and related margin; (i) sales or revenue growth, whether in general, by type of product, application or service, or by type of customer; (j) gross or operating profit or margins; (k) return measures, including return on assets, capital, investment, equity, sales or revenue; (l) economic value add with or without a capital charge; (m) cash flow, including operating cash flow, free cash flow, cash flow return on equity and cash flow return on investment; (n) productivity ratios; (o) expense targets; (p) market share; (q) financial ratios as provided in credit agreements of the Company and its subsidiaries and interest expense; (r) working capital targets; (s) completion of acquisitions of businesses or companies; (t) completion of divestitures and asset sales; (u) operating metrics; and (v) any combination of any of the foregoing business criteria and associated margins. Performance Goals may also relate to a Participant’s individual performance.
The Administrator reserves the right to adjust Performance Goals, or modify the manner of measuring or evaluating a Performance Goal, for any reason the Administrator determines is appropriate, including but not limited to: (i) by excluding the effects of charges for reorganizing and restructuring; discontinued operations; asset write-downs; gains or losses on the disposition of a business; mergers, acquisitions or dispositions; and extraordinary, unusual and/or non-recurring items of gain or loss; (ii) excluding the costs of litigation, claims, judgments or settlements; (iii) excluding the effects of changes laws or regulations affecting reported results, or changes in tax or accounting principles, regulations or law; and (iv) excluding any accruals of amounts related to payments under the Plan or any other compensation arrangement maintained by the Company or an Affiliate.
The inclusion in an Award agreement of specific adjustments or modifications shall not be deemed to preclude the Administrator from making other adjustments or modifications, in its discretion, as described herein, unless the Award agreement provides that the adjustments or modifications described in such agreement shall be the sole adjustments or modifications.
(x) “Performance Shares” means the right to receive Shares to the extent Performance Goals are achieved (or other requirements are met).
(y) “Performance Unit” means the right to receive a cash payment and/or Shares valued in relation to a unit that has a designated dollar value or the value of which is equal to the Fair Market Value of one or more Shares, to the extent Performance Goals are achieved (or other requirements are met).
(z) “Person” has the meaning given in Section 3(a)(9) of the Exchange Act, as modified and used in Sections 13(d) and 14(d) thereof, or any group of Persons acting in concert that would be considered “persons acting as a group” within the meaning of Treas. Reg. § 1.409A-3(i)(5).
(aa) “Plan” means this TuHURA Biosciences, Inc. 2024 Equity Incentive Plan, as it may be amended from time to time.
(bb) “Restricted Stock” means Shares that are subject to a risk of forfeiture or restrictions on transfer, or both a risk of forfeiture and restrictions on transfer, which may lapse upon the achievement or partial achievement of Performance Goals or upon the completion of a period of service, or both.
(cc) “Restricted Stock Unit” means the right to receive a Share or a cash payment the value of which is equal to the Fair Market Value of one Share.
(dd) “Section 16 Participants” means Participants who are subject to the provisions of Section 16 of the Exchange Act.
(ee) “Share” means a share of Stock.
(ff) “Stock” means the Company’s common stock, par value $0.001 per Share.
(gg) “Stock Appreciation Right” or “SAR” means the right to receive a cash payment, and/or Shares with a Fair Market Value, equal to the appreciation of the Fair Market Value of a Share during a specified period of time measured as the excess of (i) the Fair Market Value of the Shares subject to the SAR at the time of exercise over (ii) the grant price of the SAR, as established on the date of grant.
(hh) “Subsidiary” means any corporation, limited liability company or other limited liability entity in an unbroken chain of entities beginning with the Company if each of the entities (other than the last entities in the chain) owns the stock or equity interest possessing more than fifty percent (50%) of the total combined voting power of all classes of stock or other equity interests in one of the other entities in the chain.
3. Administration.
(a) Administration. In addition to the authority specifically granted to the Administrator in this Plan, the Administrator has full discretionary authority to administer this Plan, including but not limited to the authority to: (i) interpret the provisions of this Plan or any agreement covering an Award; (ii) prescribe, amend and rescind rules and regulations relating to this Plan; (iii) correct any defect, supply any omission, or reconcile any inconsistency in the Plan, any Award or any agreement covering an Award in the manner and to the extent it deems desirable to carry this Plan or such Award into effect; and (iv) make all other determinations necessary or advisable for the administration of this Plan. All Administrator determinations shall be made in the sole discretion of the Administrator and are final and binding on all interested parties.
(b) Delegation to Other Committees or Officers. To the extent applicable law permits, the Board may delegate to another committee of the Board, or the Committee may delegate to a subcommittee of the Committee, or either may delegate to one or more officers of the Company, any or all of their respective authority and responsibility as an Administrator of the Plan; provided that no such delegation is permitted with respect to Stock-based Awards made to Section 16 Participants at the time any such delegated authority or responsibility is exercised unless the delegation is to another committee of the Board consisting entirely of Non-Employee Directors. If the Board or the Committee has made such a delegation, then all references to the Administrator in this Plan include such other committee, subcommittee or one or more officers to the extent of such delegation.
(c) No Liability; Indemnification. No member of the Board or the Committee, and no officer or member of any other committee to whom a delegation under Section 3(b) has been made, will be liable for any act done, or determination made, by the individual in good faith with respect to the Plan or any Award. The Company will indemnify and hold harmless each such individual as to any acts or omissions, or determinations made, in each case done or made in good faith, with respect to this Plan or any Award to the maximum extent that the law and the Company’s By-Laws permit.
4. Eligibility. The Administrator may designate any of the following as a Participant from time to time, to the extent of the Administrator’s authority: any officer or other employee of the Company or its Affiliates; any individual that the Company or an Affiliate has engaged to become an officer or employee; any consultant or advisor who provides services to the Company or its Affiliates; and any Director, including a Non-Employee Director. The Administrator’s designation of, or granting of an Award to, a Participant will not require the Administrator to designate such individual as a Participant or grant an Award to such individual at any future time. The Administrator’s granting of a particular type of Award to a Participant will not require the Administrator to grant any other type of Award to such individual.
5. Types of Awards. Subject to the terms of this Plan, the Administrator may grant any type of Award to any Participant it selects, but only employees of the Company or a Subsidiary may receive grants of incentive stock options within the meaning of Code Section 422. Awards may be granted alone or in addition to, in tandem with, or (subject to the prohibition on repricing set forth in Section 15(e)) in substitution for any other Award (or any other award granted under another plan of the Company or any Affiliate, including the plan of an acquired entity).
6. Shares Reserved under this Plan.
(a) Plan Reserve. Subject to adjustment as provided in Section 17, an aggregate of 11,000,000 Shares are reserved for issuance under this Plan. The aggregate number of Shares reserved for issuance under this Plan shall be increased annually on the first day of each fiscal year of the Company after the Effective Date, commencing on the first day of the Company’s fiscal year 2025 and with a final increase on the first day of the 2034 fiscal year, by a number of Shares equal to the lesser of: (i) 5.0% of the outstanding shares of all classes of the Company’s common stock as of the last day of the immediately preceding fiscal year or (ii) such other number of Shares (which may be zero) as the Board may determine. The Shares reserved for issuance may be either authorized and unissued Shares or Shares reacquired at any time and now or hereafter held as treasury stock. Notwithstanding the foregoing, no more than 11,000,000 Shares may be issued pursuant to Incentive Stock Options.
(b) Depletion and Replenishment of Shares Under this Plan.
(i) The aggregate number of Shares reserved under Section 6(a) shall be depleted on the date of grant of an Award by the maximum number of Shares, if any, with respect to which such Award is granted. Notwithstanding the foregoing, an Award that may be settled solely in cash shall not cause any depletion of the Plan’s Share reserve at the time such Award is granted.
(ii) To the extent (A) an Award lapses, expires, terminates or is cancelled without the issuance of Shares under the Award (whether due currently or on a deferred basis) or is settled in cash, (B) it is determined during or at the conclusion of the term of an Award that all or some portion of the Shares with respect to which the Award was granted will not be issuable on the basis that the conditions for such issuance will not be satisfied, (C) Shares are forfeited under an Award, or (D) Shares are issued under any Award and the Company subsequently reacquires them pursuant to rights reserved upon the issuance of the Shares, then such Shares shall be recredited to the Plan’s reserve and may again be used for new Awards under this Plan, but Shares recredited to the Plan’s reserve pursuant to clause (D) may not be issued pursuant to incentive stock options. Notwithstanding the foregoing, in no event shall the following Shares be recredited to the Plan’s reserve: (I) Shares tendered or withheld in payment of the exercise price of an Option or as a result of the net settlement of an outstanding Stock Appreciation Right, (II) Shares tendered or withheld to satisfy federal, state or local tax withholding obligations, or (III) Shares purchased by the Company (subject to compliance with applicable law) using proceeds from Option exercises.
(c) Non-Employee Director Award Limitation. Subject to adjustment as provided in Section 17, the maximum number of Shares subject to any Award(s) that may be granted during any fiscal year to any individual Non-Employee Director shall not exceed that number of Shares that has a grant date fair value of, when added to any cash compensation received by such Non-Employee Director, $1,000,000 (the “Director Limit”); provided,
however, that in the fiscal year in which a Non-Employee Director first joins the Board or is first designated as Chairman of the Board or Lead Director, the maximum number of Shares subject to Awards granted to the Participant may have a grant date fair value of, when added to any cash compensation received by such Non-Employee Director, up to $2,000,000; provided further that the Non-Employee Director receiving such additional compensation may not participate in the decision to award such compensation.
7. Options.
(a) General. Subject to the terms of this Plan, the Administrator will determine all terms and conditions of each Option, including but not limited to: (i) whether the Option is an “incentive stock option” which meets the requirements of Code Section 422, or a “nonqualified stock option” which does not meet the requirements of Code Section 422; (ii) the grant date, which may not be any day prior to the date that the Administrator approves the grant; (iii) the number of Shares subject to the Option; (iv) the exercise price, which may never be less than the Fair Market Value of the Shares subject to the Option as determined on the date of grant (110% of the Fair Market Value in the case of an incentive stock option granted to a 10% Stockholder); (v) the terms and conditions of vesting and exercise; (vi) the term, except that an Option must terminate no later than ten (10) years after the date of grant (five (5) years in the case of an incentive stock option granted to a 10% Stockholder); and (vii) the manner of payment of the exercise price. Except to the extent otherwise set forth in an Award agreement, a Participant shall have no rights as a holder of Stock as a result of the grant of an Option until the Option is exercised, the exercise price and applicable withholding taxes are paid and the Shares subject to the Option are issued thereunder.
(b) Incentive Stock Options.
(i) The terms of any incentive stock option should comply with the provisions of Code Section 422 except to the extent the Administrator determines otherwise.
(ii) If an Option that is intended to be an incentive stock option fails to meet the requirements thereof, the Option shall automatically be treated as a nonqualified stock option to the extent of such failure.
(iii) If any Participant shall make any disposition of Shares issued pursuant to the exercise of an incentive stock option under the circumstances described in Code Section 421(b) (relating to certain disqualifying dispositions), such Participant shall notify the Company of such disposition within ten (10) days thereof.
(c) Payment of Exercise Price. To the extent previously approved by the Administrator (which approval may be set forth in an Award agreement or in administrative rules), and subject to such procedures as the Administrator may specify, the payment of the exercise price of Options may be made by (i) delivery of cash or other Shares or other securities of the Company (including by attestation) having a then Fair Market Value equal to the purchase price of such Shares, (ii) by delivery (including by fax) to the Company or its designated agent of an executed irrevocable option exercise form together with irrevocable instructions to a broker-dealer to sell or margin a sufficient portion of the Shares and deliver the sale or margin loan proceeds directly to the Company to pay for the exercise price, (iii) by surrendering the right to receive Shares otherwise deliverable to the Participant upon exercise of the Award having a Fair Market Value at the time of exercise equal to the total exercise price, or (iv) by any combination of (i), (ii) and/or (iii).
8. Stock Appreciation Rights. Subject to the terms of this Plan, the Administrator will determine all terms and conditions of each SAR, including but not limited to: (a) the grant date, which may not be any day prior to the date that the Administrator approves the grant; (b) the number of Shares to which the SAR relates; (c) the grant price, which may never be less than the Fair Market Value of the Shares subject to the SAR as determined on the date of grant; (d) the terms and conditions of exercise or maturity, including vesting; (e) the term, provided that an SAR must terminate no later than ten (10) years after the date of grant; and (f) whether the SAR will be settled in cash, Shares or a combination thereof.
9. Performance and Stock Awards.
(a) General. Subject to the terms of this Plan, the Administrator will determine all terms and conditions of each award of Shares, Restricted Stock, Restricted Stock Units, Performance Shares or Performance Units, including but not limited to: (a) the number of Shares or units to which such Award relates; (b) whether, as a condition for the Participant to realize all or a portion of the benefit provided under the Award, one or more Performance Goals must be achieved during such period as the Administrator specifies; (c) the length of the vesting or performance period and, if different, the date on which payment of the benefit provided under the Award will be made; (d) with respect to Performance Units, whether to measure the value of each unit in relation to a designated dollar value or the Fair Market Value of one or more Shares; and (e) with respect to Restricted Stock Units and Performance Units, whether to settle such Awards in cash, in Shares (including Restricted Stock), or in a combination of cash and Shares.
(b) Stockholder Rights. Except to the extent the Administrator provides otherwise and subject to the restrictions set forth in Section 11(a), holders of Restricted Stock and Stock shall have the right to vote the Shares subject to such Awards and the right to receive any dividends declared or paid with respect to such Shares. Except to the extent the Administrator provides otherwise, holders of other types of Awards shall not have any rights as stockholders of the Company with respect to such Awards. A holder of Restricted Stock Units, Performance Shares or Performance Units shall have no rights other than those of a general creditor of the Company; such Awards represent an unfunded and unsecured obligation of the Company, subject to the terms and conditions of this Plan and the applicable Award agreement.
10. Cash Incentive Awards. Subject to the terms of this Plan, the Administrator will determine all terms and conditions of a Cash Incentive Award, including but not limited to the Performance Goals, performance period, the potential amount payable, and the timing of payment.
11. Dividends and Dividend Equivalent Units.
(a) Prohibitions. In no event may dividends or Dividend Equivalent Units be awarded with respect to Options, SARs or any other stock-based award that is not a grant of Stock, Restricted Stock, Restricted Stock Units, Performance Shares or Performance Units. Notwithstanding anything to the contrary in this Plan, and for the avoidance of doubt, this Plan expressly prohibits the payment of dividends or Dividend Equivalent Units on unvested Awards for all equity Award types.
(b) Dividends. If cash dividends are paid while Restricted Stock is unvested, then such dividends will either, at the discretion of the Administrator, be (i) automatically reinvested as additional Shares of Restricted Stock that are subject to the same terms and conditions, including the risk of forfeiture, as the original grant of Restricted Stock, or (ii) paid in cash at the same time and the same extent that the Restricted Stock vests. For clarity, in no event will dividends be distributed to a Participant unless, until and to the same extent as the underlying Shares of Restricted Stock vests.
(c) Dividend Equivalent Units. The Administrator may grant Dividend Equivalent Units only in tandem with Restricted Stock Units, Performance Shares or Performance Units. Dividend Equivalent Units will either, at the discretion of the Administrator, be (i) accumulated and paid, in cash or Shares in the Administrator’s discretion, at the same time and to the same extent that the underlying Award vests or is earned or (ii) reinvested in additional units that are subject to the same terms and conditions (including vesting and forfeiture) as the underlying Award. The Administrator will determine all other terms and conditions of each award of Dividend Equivalent Units. For clarity, in no event will a Participant receive payment with respect to a Dividend Equivalent Unit unless, until and to the same extent as the underlying Award vests and is paid.
12. Other Stock-Based Awards. Subject to the terms of this Plan, the Administrator may grant to a Participant shares of unrestricted Stock as replacement for other compensation to which the Participant is entitled, such as in payment of director fees, in lieu of cash compensation, in exchange for cancellation of a compensation right, or as a bonus.
13. Discretion to Accelerate Vesting. The Administrator may accelerate the vesting of an Award or deem an Award to be earned, in whole or in part, in the event of a Participant’s death, disability (as defined by the Administrator), retirement, or termination without Cause, or as provided in Section 17(c) or upon any other event as determined by the Administrator in its sole and absolute discretion.
14. Transferability. Awards may not be sold, transferred for value, pledged, assigned, or otherwise alienated or hypothecated by a Participant, including to any financial institution, other than by will or the laws of descent and distribution, unless and to the extent the Administrator allows a Participant to: (a) designate in writing a beneficiary to exercise the Award or receive payment under the Award after the Participant’s death; (b) transfer an Award to the former spouse of the Participant as required by a domestic relations order incident to a divorce; or (c) otherwise transfer an Award; provided, however, that (i) in each case the assignee shall not further sell, pledge, transfer, assign or otherwise alienate or hypothecate such Award, and (ii) with respect to clause (c) the Participant may not receive consideration for such a transfer of an Award.
15. Term of Plan; Termination and Amendment; Survival; Repricing and Backdating Prohibited; Foreign Participation; Deferrals.
(a) Term of Plan. Unless the Board earlier terminates this Plan pursuant to Section 15(b), this Plan will terminate on, and no further Awards may be granted under this Plan after, the tenth (10th) anniversary of the latest date on which this Plan, or any amendment thereto or restatement thereof, has been approved by the Company’s stockholders.
(b) Termination and Amendment. The Board or the Administrator may amend, alter, suspend, discontinue or terminate this Plan at any time, subject to the following limitations:
(i) the Board must approve any amendment of this Plan to the extent the Company determines such approval is required by: (A) prior action of the Board, (B) applicable corporate law, or (C) any other applicable law;
(ii) stockholders must approve any amendment of this Plan (which may include an amendment to materially increase the number of Shares specified in Section 6(a), except as permitted by Section 17) to the extent the Company determines such approval is required by: (A) Section 16 of the Exchange Act, (B) the Code, (C) the listing requirements of any principal securities exchange or market on which the Shares are then traded, or (D) any other applicable law; and
(iii) stockholders must approve an amendment that would diminish the protections afforded by Section 15(e).
If the Board or the Administrator takes any action under this Plan that is not, at the time of such action, authorized by this Plan, but that could be authorized by this Plan as amended by the Board or the Administrator, as applicable, the Board or Administrator action will be deemed to constitute an amendment to this Plan to authorize such action to the extent permissible under applicable law and the requirements of any principal securities exchange or any Applicable Exchange.
(c) Amendment, Modification, Cancellation and Disgorgement of Awards.
(i) Except as provided in Section 15(e) and subject to the requirements of this Plan, the Administrator may modify, amend or cancel any Award, or waive any restrictions or conditions applicable to any Award or the exercise of the Award; provided that, except as otherwise provided in the Plan or the Award agreement, any modification or amendment that materially diminishes the rights of the Participant, or the cancellation of an Award, shall be effective only if agreed to by the Participant or any other person(s) as may then have an interest in such Award, but the Administrator need not obtain Participant (or other interested party) consent for the modification, amendment or cancellation of an Award pursuant to the provisions of subsection (ii) or Section 17 or as follows: (A) to the extent the Administrator deems such action necessary to comply with any applicable law or the listing requirements of any Applicable Exchange; (B) to the extent the Administrator deems necessary to preserve favorable accounting or tax treatment of any Award for the Company; or (C) to the extent the Administrator determines that such action does not materially and adversely affect the value of an Award or that such action is in the best interest of the affected Participant (or any other person(s) as may then have an interest in the Award). Notwithstanding the foregoing, unless determined otherwise by the Administrator, any such amendment shall be made in a manner that will enable an Award intended to be exempt from Code Section 409A to continue to be so exempt, or to enable an Award intended to comply with Code Section 409A to continue to so comply.
(ii) Notwithstanding anything to the contrary in an Award agreement, the Administrator shall have full power and authority to terminate or cause the Participant to forfeit the Award, and require the Participant to disgorge to the Company any gains attributable to the Award, if the Participant engages in any action constituting, as determined by the Administrator in its discretion, Cause for termination or a breach of a material Company policy, any Award agreement or any other agreement between the Participant and the Company or an Affiliate concerning noncompetition, nonsolicitation, confidentiality, trade secrets, intellectual property, nondisparagement or similar obligations.
(iii) Any Awards granted pursuant to this Plan, and any Stock issued or cash paid pursuant to an Award, shall be subject to any recoupment or clawback policy that is adopted by the Company, including, but not limited to any clawback pursuant to the listing standards of any national securities exchange or association on which the Company’s securities are listed or as is otherwise required by the Rule 10D-1 under the Exchange Act or other applicable law, or any recoupment or similar requirement otherwise made applicable by law, regulation or listing standards to the Company from time to time. No recovery of compensation under such a clawback policy will be an event giving rise to a right to resign for “good reason” or be deemed a “constructive termination” (or any similar term) as such terms are used in any agreement between any Participant and the Company.
(d) Survival of Authority and Awards. Notwithstanding the foregoing, the authority of the Board and the Administrator under this Section 15 and to otherwise administer the Plan with respect to then-outstanding Awards will extend beyond the date of this Plan’s termination. In addition, termination of this Plan will not affect the rights of Participants with respect to Awards previously granted to them, and all unexpired Awards will continue in force and effect after termination of this Plan except as they may lapse or be terminated by their own terms and conditions.
(e) Repricing and Backdating Prohibited. Notwithstanding anything in this Plan to the contrary, and except for the adjustments provided for in Section 17, neither the Administrator nor any other person may, without stockholder approval (i) amend the terms of outstanding Options or SARs to reduce the exercise or grant price of such outstanding Options or SARs; (ii) cancel outstanding Options or SARs in exchange for Options or SARs with an exercise or grant price that is less than the exercise or grant price of the original Options or SARs; (iii) cancel outstanding Options or SARs with an exercise or grant price above the current Fair Market Value of a Share in exchange for cash or other securities; or (iv) take any other action with respect to an Award that would be treated as a repricing under generally accepted accounting principles. In addition, the Administrator may not make a grant of an Option or SAR with a grant date that is effective prior to the date the Administrator takes action to approve such Award.
(f) Foreign Participation. To assure the viability of Awards granted to Participants employed or residing in foreign countries, the Administrator may provide for such special terms as it may consider necessary or appropriate to accommodate differences in local law, tax policy, accounting or custom. Moreover, the Administrator may approve such supplements to, or amendments, restatements or alternative versions of, this Plan as it determines is necessary or appropriate for such purposes. Any such amendment, restatement or alternative versions that the Administrator approves for purposes of using this Plan in a foreign country will not affect the terms of this Plan for any other country. In addition, all such supplements, amendments, restatements or alternative versions must comply with the provisions of Section 15(b)(ii).
(g) Deferrals. The Administrator may permit or require the deferral of any Award or Award payment into a deferred compensation arrangement, subject to such rules and procedures as it may establish. Any such deferrals shall be made in a manner that complies with Code Section 409A.
16. Taxes.
(a) Withholding. In the event the Company or one of its Affiliates is required to withhold any Federal, state or local taxes or other amounts in respect of any income recognized by a Participant as a result of the grant, vesting, payment or settlement of an Award or disposition of any Shares acquired under an Award, the Company may satisfy such obligation by:
(i) If cash is payable under an Award, deducting (or requiring an Affiliate to deduct) from such cash payment the amount needed to satisfy such obligation;
(ii) If Shares are issuable under an Award, then to the extent previously approved by the Administrator (which approval may be set forth in an Award agreement or in administrative rules), and subject to such procedures as the Administrator may specify, (A) withholding Shares having a Fair Market Value equal to such obligations; or (B) allowing the Participant to elect to (I) have the Company or its Affiliate withhold Shares otherwise issuable under the Award, (II) tender back Shares received in connection with such Award or (III) deliver other previously owned Shares, in each case having a Fair Market Value equal to the amount to be withheld; provided that the amount to be withheld under this clause (ii) may not exceed the total maximum statutory tax withholding obligations associated with the transaction to the extent needed for the Company and its Affiliates to avoid an accounting charge. If an election is provided, the election must be made on or before the date as of which the amount of tax to be withheld is determined and otherwise as the Administrator requires; or
(iii) Deducting (or requiring an Affiliate to deduct) the amount needed to satisfy such obligation from any wages or other payments owed to the Participant, requiring such Participant to pay to the Company or its Affiliate, in cash, promptly on demand, or make other arrangements satisfactory to the Company or its Affiliate regarding the payment to the Company or its Affiliate of the amount needed to satisfy such obligation.
(b) No Guarantee of Tax Treatment. Notwithstanding any provisions of this Plan to the contrary, the Company does not guarantee to any Participant or any other Person with an interest in an Award that (i) any Award intended to be exempt from Code Section 409A shall be so exempt, (ii) any Award intended to comply with Code Section 409A or Code Section 422 shall so comply, or (iii) any Award shall otherwise receive a specific tax
treatment under any other applicable tax law, nor in any such case will the Company or any Affiliate be required to indemnify, defend or hold harmless any individual with respect to the tax consequences of any Award.
17. Adjustment and Change of Control Provisions.
(a) Adjustment of Shares. If (i) the Company shall at any time be involved in a merger or other transaction in which the Shares are changed or exchanged; (ii) the Company shall subdivide or combine the Shares or the Company shall declare a dividend payable in Shares, other securities (other than stock purchase rights issued pursuant to a stockholder rights agreement) or other property; (iii) the Company shall effect a cash dividend the amount of which, on a per Share basis, exceeds ten percent (10%) of the Fair Market Value of a Share at the time the dividend is declared, or the Company shall effect any other dividend or other distribution on the Shares in the form of cash, or a repurchase of Shares, that the Board determines by resolution is special or extraordinary in nature or that is in connection with a transaction that the Company characterizes publicly as a recapitalization or reorganization involving the Shares; or (iv) any other event shall occur, which, in the case of this clause (iv), in the judgment of the Administrator necessitates an adjustment to prevent dilution or enlargement of the benefits or potential benefits intended to be made available under this Plan, then the Administrator shall, in such manner as it may deem equitable to prevent dilution or enlargement of the benefits or potential benefits intended to be made available under this Plan, adjust any or all of: (A) the number and type of shares subject to this Plan (including the number and type of shares described in Section 6(a)) and which may after the event be made the subject of Awards; (B) the number and type of shares subject to outstanding Awards; (C) the grant, purchase, or exercise price with respect to any Award; and (D) the Performance Goals of an Award. In any such case, the Administrator may also (or in lieu of the foregoing) make provision for a cash payment to the holder of an outstanding Award in exchange for the cancellation of all or a portion of the Award (without the consent of the holder of an Award) in an amount determined by the Administrator effective at such time as the Administrator specifies (which may be the time such transaction or event is effective). However, in each case, with respect to Awards of incentive stock options, no such adjustment may be authorized to the extent that such authority would cause this Plan to violate Code Section 422(b). Further, the number of Shares subject to any Award payable or denominated in Shares must always be a whole number. In any event, previously granted Options or SARs are subject to only such adjustments as are necessary to maintain the relative proportionate interest the Options and SARs represented immediately prior to any such event and to preserve, without exceeding, the value of such Options or SARs.
Without limitation, in the event of any reorganization, merger, consolidation, combination or other similar corporate transaction or event, whether or not constituting a Change of Control (other than any such transaction in which the Company is the continuing corporation and in which the outstanding Stock is not being converted into or exchanged for different securities, cash or other property, or any combination thereof), the Administrator may substitute, on an equitable basis as the Administrator determines, for each Share then subject to an Award and the Shares subject to this Plan (if the Plan will continue in effect), the number and kind of shares of stock, other securities, cash or other property to which holders of Stock are or will be entitled in respect of each Share pursuant to the transaction.
Notwithstanding the foregoing, in the case of a stock dividend (other than a stock dividend declared in lieu of an ordinary cash dividend) or subdivision or combination of the Shares (including a reverse stock split), if no action is taken by the Administrator, adjustments contemplated by this subsection that are proportionate shall nevertheless automatically be made as of the date of such stock dividend or subdivision or combination of the Shares.
(b) Issuance or Assumption. Notwithstanding any other provision of this Plan, and without affecting the number of Shares otherwise reserved or available under this Plan, in connection with any merger, consolidation, acquisition of property or stock, or reorganization, the Administrator may authorize the issuance or assumption of awards under this Plan upon such terms and conditions as it may deem appropriate.
(c) Effect of Change of Control.
(i) Upon a Change of Control, except to the extent otherwise provided in an applicable Award agreement, if the successor or surviving corporation (or parent thereof) so agrees, then, without the consent of any Participant (or other person with rights in an Award), some or all outstanding Awards may be assumed, or
replaced with the same type of award with similar terms and conditions, by the successor or surviving corporation (or parent thereof) in the Change of Control transaction, subject to the following requirements:
(A) Each Award which is assumed by the successor or surviving corporation (or parent thereof) shall be appropriately adjusted, immediately after such Change of Control, to apply to the number and class of securities which would have been issuable to the Participant upon the consummation of such Change of Control had the Award been exercised, vested or earned immediately prior to such Change of Control, and such other appropriate adjustments in the terms and conditions of the Award shall be made.
(B) If the securities to which the Awards relate after the Change of Control are not listed and traded on a national securities exchange, then (1) the Participant shall be provided the option, upon exercise or settlement of an Award, to elect to receive, in lieu of the issuance of such securities, cash in an amount equal to the fair value equal of the securities that would have otherwise been issued and (2) for purposes of determining such fair value, no reduction shall be taken to reflect a discount for lack of marketability, minority interest or any similar consideration.
(C) Upon the Participant’s termination of employment within two years following the Change of Control (1) by the successor or surviving corporation without Cause, (2) by reason of death or disability, or (3) by the Participant for “good reason,” as defined in any Award agreement or any employment, retention, change of control, severance or similar agreement between the Participant and the Company or any Affiliate, if any, all of the Participant’s Awards that are in effect as of the date of such termination shall vest in full or be deemed earned in full (assuming target performance goals provided under such Award were met, if applicable) effective on the date of such termination. In the event of any other termination of employment within two years after a Change of Control that is not described herein, the terms of the Award agreement shall apply.
(ii) To the extent the purchaser, successor or surviving entity (or parent thereof) in the Change of Control transaction does not assume the Awards or issue replacement awards as provided in clause (i) (including, for the avoidance of doubt, by reason of a Participant’s termination of employment in connection with the Change of Control), then, except to the extent otherwise provided in an applicable Award agreement or another agreement between the Participant and the Company or an Affiliate, or unless the Administrator otherwise determines:
(A) Each Option or SAR that is then held by a Participant who is employed by or in the service of the Company or an Affiliate shall either (I) become immediately exercisable and remain so for a period of fifteen (15) days prior to the consummation of the Change of Control (with any exercisability being conditioned and effective upon such consummation and any unexercised Options or SARs terminating upon such consummation) or (II) be cancelled (whether or not then vested) on the date of the Change of Control in exchange for a payment in cash or securities having a value (as determined by the Administrator) equal to the excess of the Change of Control Price (as defined below) of the Shares covered by the Option or SAR that is so cancelled over the purchase or grant price of such Shares under the Award; provided, however, that all Options and SARs that have a purchase or grant price that is greater than the Change of Control Price shall be cancelled for no consideration;
(B) Restricted Stock and Restricted Stock Units (that are not Performance Awards) that are not then vested shall vest in full as of immediately prior to the Change of Control and may, in the Administrator’s discretion, be cancelled in exchange for a payment in cash or securities having a value (as determined by the Administrator) equal to the Change of Control Price of the Shares covered by the Award that is so cancelled;
(C) All Performance Shares, Performance Units, and Cash Incentive Awards for which the performance period has expired shall be paid based on actual performance (and assuming all employment or other requirements had been met in full); and all Performance Shares, Performance Units and Cash Incentive Awards for which the performance period has not expired shall be cancelled in exchange for a payment in cash or securities having a value (as determined by the Administrator) equal to the amount that would have been due under such Award(s), valued assuming that the target Performance Goals had been met at the time of such Change of Control;
(D) All Dividend Equivalent Units that are not vested shall vest (to the same extent as the Award granted in tandem with the Dividend Equivalent Unit, if applicable) and be paid; and
(E) All other Awards that are not vested shall vest and if an amount is payable under such vested Award, such amount shall be paid in cash or securities based on the value of the Award.
“Change of Control Price” shall mean the per share price paid or deemed paid in the Change of Control transaction, as determined by the Administrator. For purposes of this clause (ii), if the value of an Award is based on the Fair Market Value of a Share, Fair Market Value shall be deemed to mean the Change of Control Price.
(d) Application of Limits on Payments. Notwithstanding any other provision of this Plan or of any other agreement, contract, or understanding heretofore or hereafter entered into by a Participant with the Company or any Affiliate, except an agreement, contract, or understanding that expressly addresses Section 280G or Section 4999 of the Code (an “Other Agreement”), and notwithstanding any formal or informal plan or other arrangement for the direct or indirect provision of compensation to the Participant (including groups or classes of Participants or beneficiaries of which the Participant is a member), whether or not such compensation is deferred, is in cash, or is in the form of a benefit to or for the Participant (a “Benefit Arrangement”), if the Participant is a “disqualified individual,” as defined in Section 280G(c) of the Code, any Option, Restricted Stock, Restricted Stock Unit, Performance Share or Performance Unit held by that Participant and any right to receive any payment or other benefit under this Plan shall not become exercisable or vested (i) to the extent that such right to exercise, vesting, payment, or benefit, taking into account all other rights, payments, or benefits to or for the Participant under this Plan, all Other Agreements, and all Benefit Arrangements, would cause any payment or benefit to the Participant under this Plan to be considered a “parachute payment” within the meaning of Section 280G(b)(2) of the Code as then in effect (a “Parachute Payment”) and (ii) if, as a result of receiving a Parachute Payment, the aggregate after-tax amounts received by the Participant from the Company under this Plan, all Other Agreements, and all Benefit Arrangements would be less than the maximum after-tax amount that could be received by the Participant without causing any such payment or benefit to be considered a Parachute Payment. In the event that the receipt of any such right to exercise, vesting, payment, or benefit under this Plan, in conjunction with all other rights, payments, or benefits to or for the Participant under any Other Agreement or any Benefit Arrangement would cause the Participant to be considered to have received a Parachute Payment under this Plan that would have the effect of decreasing the after-tax amount received by the Participant as described in clause (ii) of the preceding sentence, then the rights, payments, or benefits under this Plan, any Other Agreements, and any Benefit Arrangements shall be reduced or eliminated in the following manner and order: any such reduction or elimination in rights, payments and benefits shall be applied first against the latest scheduled cash payments; then current cash payments; then any equity or equity derivatives that are included under Code Section 280G at full value rather than accelerated value (with the highest value reduced or eliminated first); then any equity or equity derivatives included under Code Section 280G at an accelerated value (and not at full value) shall be reduced or eliminated with the highest value reduced or eliminated first (as such values are determined under Treasury Regulation 1.280G-1, Q&A 24); finally any other non-cash benefits will be reduced or eliminated in the order of latest scheduled payments to earliest scheduled payments.
18. Effect of Termination on Awards.
(a) Termination for Cause. If a Participant’s employment or service is terminated for Cause, then all Awards and grants of every type, whether or not then vested, shall terminate no later than the Participant’s last day of employment. In addition, if the Participant’s employment or service ends for any reason other than Cause, but the Company later determines that the Participant could have been terminated for Cause if all the facts had been known to the Company, then all Awards and grants of every type, whether or not then vested, shall terminate and be forfeited as soon as the Company makes such determination and the Company may require the Participant to disgorge any profits that the Participant earned from the settlement of any Award between the date of the Participant’s termination of employment and the date of the Company’s determination to the maximum extent permitted by applicable law.
(b) Other Terminations. If a Participant’s employment or service terminates for any reason other than Cause, then the Participant’s Awards will be treated in accordance with the terms of the Participant’s employment, retention, change of control, severance or similar agreement with the Company or any Affiliate that discusses the effect of the Participant’s termination of employment or service on the Participant’s Awards, or to the extent no such
agreement discusses the effect of the applicable termination, then in accordance with the terms of the applicable Award agreement.
19. Miscellaneous.
(a) Other Terms and Conditions. The Administrator may provide in any Award agreement such other provisions (whether or not applicable to the Award granted to any other Participant) as the Administrator determines appropriate to the extent not otherwise prohibited by the terms of the Plan. No provision in an Award agreement shall limit the Administrator’s discretion hereunder unless such provision specifically so provides for such limitation.
(b) Employment and Service. The issuance of an Award shall not confer upon a Participant any right with respect to continued employment or service with the Company or any Affiliate, or the right to continue as a Director. Unless determined otherwise by the Administrator, for purposes of the Plan and all Awards, the following rules shall apply:
(i) a Participant who transfers employment between the Company and its Affiliates, or between Affiliates, will not be considered to have terminated employment;
(ii) a Participant who ceases to be a Non-Employee Director because he or she becomes an employee of the Company or an Affiliate shall not be considered to have ceased service as a Director with respect to any Award until such Participant’s termination of employment with the Company and its Affiliates;
(iii) a Participant who ceases to be employed by the Company or an Affiliate and immediately thereafter becomes a Non-Employee Director, a non-employee director of an Affiliate, or a consultant to the Company or any Affiliate shall not be considered to have terminated employment until such Participant’s service as a director of, or consultant to, the Company and its Affiliates has ceased; and
(iv) a Participant employed by an Affiliate will be considered to have terminated employment when such entity ceases to be an Affiliate.
Notwithstanding the foregoing, for purposes of an Award that is subject to Code Section 409A, if a Participant’s termination of employment or service triggers the payment of compensation under such Award, then the Participant will be deemed to have terminated employment or service upon his or her “separation from service” within the meaning of Code Section 409A. Notwithstanding any other provision in this Plan or an Award to the contrary, if any Participant is a “specified employee” within the meaning of Code Section 409A as of the date of his or her “separation from service” within the meaning of Code Section 409A, then, to the extent required to avoid the imposition of additional taxes under Code Section 409A, any payment made to the Participant on account of such separation from service shall not be made before a date that is six months after the date of the separation from service.
(c) No Fractional Shares. No fractional Shares or other securities may be issued or delivered pursuant to this Plan, and the Administrator may determine whether cash, other securities or other property will be paid or transferred in lieu of any fractional Shares or other securities, or whether such fractional Shares or other securities or any rights to fractional Shares or other securities will be canceled, terminated or otherwise eliminated with or without consideration.
(d) Unfunded Plan; Awards Not Includable for Benefits Purposes. This Plan is unfunded and does not create, and should not be construed to create, a trust or separate fund with respect to this Plan’s benefits. This Plan does not establish any fiduciary relationship between the Company and any Participant or other person. To the extent any person holds any rights by virtue of an Award granted under this Plan, such rights are no greater than the rights of the Company’s general unsecured creditors. Income recognized by a Participant pursuant to an Award shall not be included in the determination of benefits under any employee pension benefit plan (as such term is defined in Section 3(2) of the Employee Retirement Income Security Act of 1974, as amended) or group insurance or other benefit plans applicable to the Participant which are maintained by the Company or any Affiliate, except as may be provided under the terms of such plans or determined by resolution of the Board.
(e) Requirements of Law and Securities Exchange. The granting of Awards and the issuance of Shares in connection with an Award are subject to all applicable laws, rules and regulations and to such approvals by any governmental agencies or national securities exchanges as may be required. Notwithstanding any other provision of this Plan or any Award agreement, the Company has no liability to deliver any Shares under this Plan or make any payment unless such delivery or payment would comply with all applicable laws and the applicable requirements of any securities exchange or similar entity, and unless and until the Participant has taken all actions required by the Company in connection therewith. The Company may impose such restrictions on any Shares issued under the Plan as the Company determines necessary or desirable to comply with all applicable laws, rules and regulations or the requirements of any national securities exchanges.
(f) Code Section 409A. Any Award granted under this Plan shall be provided or made in such manner and at such time as to either make the Award exempt from, or comply with, the provisions of Code Section 409A, to avoid a plan failure described in Code Section 409(a)(1), and the provisions of Code Section 409A are incorporated into this Plan to the extent necessary for any Award that is subject to Code Section 409A to comply therewith.
(g) Governing Law; Waiver of Jury. This Plan, and all agreements under this Plan, will be construed in accordance with and governed by the laws of the State of Nevada, without reference to any conflict of law principles. Any legal action or proceeding with respect to this Plan, any Award or any Award agreement, or for recognition and enforcement of any judgment in respect of this Plan, any Award or any Award agreement, may only be brought and determined in a “bench” trial, and any party to such action or proceeding shall agree to waive its right to a jury trial.
(h) Limitations on Actions. Any legal action or proceeding with respect to this Plan, any Award or any Award agreement, must be brought within one year (365 days) after the day the complaining party first knew or should have known of the events giving rise to the complaint.
(i) Construction. Whenever any words are used herein in the masculine, they shall be construed as though they were used in the feminine in all cases where they would so apply; and wherever any words are used in the singular or plural, they shall be construed as though they were used in the plural or singular, as the case may be, in all cases where they would so apply. Titles of sections are for general information only, and this Plan is not to be construed with reference to such titles. Except to the extent otherwise provided in the applicable Award agreement, in the case of any Award that includes a “series of installment payments” (within the meaning of Section 1.409A-2(b)(2)(iii) of the Treasury Regulations), the Award holder’s right to the series of installment payments shall be treated as a right to a series of separate payments and not as a right to a single payment.
(j) Severability. If any provision of this Plan or any Award agreement or any Award (i) is or becomes or is deemed to be invalid, illegal or unenforceable in any jurisdiction, or as to any person or Award, or (ii) would cause this Plan, any Award agreement or any Award to violate or be disqualified under any law the Administrator deems applicable, then such provision should be construed or deemed amended to conform to applicable laws, or if it cannot be so construed or deemed amended without, in the determination of the Administrator, materially altering the intent of this Plan, Award agreement or Award, then such provision should be stricken as to such jurisdiction, person or Award, and the remainder of this Plan, such Award agreement and such Award will remain in full force and effect.
Exhibit 21.1
List of Subsidiaries
Adgero Biopharmaceuticals Holdings, Inc. (Delaware)
Adgero Biopharmaceuticals, Inc. (Delaware)
TuHURA Biosciences, Inc. (Delaware)
Exhibit 99.1
TuHURA Biosciences Completes Merger Transaction with Kintara Therapeutics
•Combined company will operate as TuHURA Biosciences, Inc. and advance pipeline of novel technologies to overcome resistance to cancer immunotherapy
•Lead program entering single Phase 3 accelerated approval registration trial in first half of 2025 for treatment of 1st line Merkel Cell carcinoma under Special Protocol Assessment (SPA) agreement with FDA
•$31 million fully-funded financing in connection with the merger agreement expected to fund planned operations of the combined company into late 2025
•Combined company to commence trading on The Nasdaq Capital Market under the ticker “HURA” on October 18, 2024
TAMPA, FL, October 17, 2024 – TuHURA Biosciences, Inc. (Nasdaq: HURA) (“TuHURA” or the “Company”), a Phase 3 registration-stage immune-oncology company developing novel technologies to overcome resistance to cancer immunotherapy today announced the completion of its previously announced merger with Kintara Therapeutics, Inc. (now TuHURA Biosciences, Inc.). The combined company will operate under the name “TuHURA Biosciences, Inc.” and will focus on advancing TuHURA’s innate immune response agonists and tumor microenvironment modulators, two technologies that seek to overcome the major obstacles that limit the effectiveness of current immunotherapies in treating cancer. The Company is preparing to initiate a single Phase 3 accelerated approval registration trial in the first half of 2025 for treatment of 1st line Merkel Cell carcinoma under SPA agreement with FDA.
The shares of the Company’s common stock, previously trading under the ticker symbol “KTRA,” will commence trading on a post-reverse split and post-business combination basis, on The Nasdaq Capital Market under the ticker symbol “HURA”, effective October 18, 2024. The Company’s common stock is now represented by a new CUSIP number, 898920103.
As a result of the merger, post-merger Kintara equityholders collectively own approximately 2.85% (or approximately 5.45% after giving effect to the shares issued pursuant to the CVR Agreement if the milestones are achieved) of the common stock of the combined company on a pro forma fully diluted basis. TuHURA equityholders collectively own approximately 97.15% (or approximately 94.55% after giving effect to the shares issued pursuant to the CVR Agreement if
the milestones are achieved) of the common stock of the combined company on a pro forma fully diluted basis.
“This marks a transformational milestone for both companies and is a significant step in the evolution of TuHURA. As we look to the future, which I believe has never been brighter, we are working to solve a significant issue with current cancer immunotherapies,” commented Dr. James Bianco, President and Chief Executive Officer of TuHURA. “Our novel technologies are designed to overcome resistance to cancer immunotherapy, and we are planning to initiate a single Phase 3 accelerated approval registration trial in the first half of 2025 with our lead innate immune response agonist, IFx-2.0. If successful, not only does it provide the ability to target additional oncology indications but also unlocks tremendous value for all stakeholders.”
Advancing Novel Technologies to Overcome Resistance to Cancer Immunotherapy
TuHURA is a Phase 3 registration-stage immuno-oncology company developing novel technologies to overcome primary and acquired resistance -- two major obstacles to cancer immunotherapy’s ability to treat and cure cancer.
•Immune Fx (IFx) Innate Immune Response Agonists: TuHURA’s IFx technology utilizes a proprietary plasmid DNA (“pDNA”) or messenger RNA (“mRNA”) which, when introduced into or targeted to a tumor cell, results in the expression of a highly immunogenic bacterial protein (Emm55) on the surface of the tumor cell. TuHURA’s lead innate immune response agonist candidate, IFx-2.0, is designed to overcome primary resistance to checkpoint inhibitors. TuHURA is preparing to initiate a single Phase 3 accelerated approval registration trial of IFx-2.0 administered as an adjunctive therapy to Keytruda® (pembrolizumab) in first line treatment for advanced or metastatic Merkel Cell Carcinoma under a SPA agreement with the US FDA, in the first half of2025.
•Tumor Microenvironment Modulators: Leveraging its Delta receptor technology, TuHURA is developing bi-specific immune modulating Antibody (ADC) or Peptide (PDC) Drug Conjugates targeting Myeloid Derived Suppressor Cells to inhibit their immune suppressing effects on the tumor microenvironment to prevent T cell exhaustion and acquired resistance to checkpoint inhibitors and cellular therapies.
About the Transaction
Under the terms of the merger agreement, Kayak Mergeco, Inc., Kintara's wholly-owned subsidiary, will merge with and into the private-company TuHURA, with TuHURA surviving the merger and becoming the combined company’s direct, wholly-owned subsidiary effective at 12:03 AM Eastern Time on October 18, 2024.
As previously announced, in connection with the merger and pursuant to the Contingent Value Rights Agreement (the "CVR Agreement"), the Company will issue CVRs to legacy Kintara stockholders (or in the case of warrants to purchase shares of Kintara common stock, each share
of Kintara common stock for which such warrant to purchase shares of Kintara stock is exercisable), entitling such holders to an aggregate of approximately 1,539,918 shares of the combined company’s common stock on a post-split basis, upon the achievement of certain milestones as set forth in the CVR Agreement.
The combined company will be led by James Bianco as President and Chief Executive Officer of TuHURA. In addition to Mr. Bianco, the TuHURA leadership team includes current members of management Dan Dearborn as Chief Financial Officer and Dennis Yamashita as Chief Scientific Officer.
The Board of Directors of TuHURA will be composed of James Bianco, James Manuso, Alan List, George Ng and Robert Hoffman.
Advisors
Lucid Capital Markets, LLC acted as the exclusive financial advisor and Lowenstein Sandler LLP acted as legal counsel to Kintara. H.C. Wainwright & Co. acted as the exclusive financial advisor and Foley & Lardner LLP acted as legal counsel to TuHURA.
About TuHURA Biosciences, Inc.
TuHURA Biosciences, Inc. is a Phase 3 registration-stage immuno-oncology company developing novel technologies to overcome resistance to cancer immunotherapy. TuHURA’s lead innate immune response agonist candidate, IFx-2.0, is designed to overcome primary resistance to checkpoint inhibitors. TuHURA is preparing to initiate a single randomized placebo-controlled Phase 3 registration trial of IFx-2.0 administered as an adjunctive therapy to Keytruda® (pembrolizumab) in first line treatment for advanced or metastatic Merkel Cell Carcinoma.
In addition to its innate immune response agonist candidates, TuHURA is leveraging its Delta receptor technology to develop first-in-class bi-specific ADCs, and PDCs targeting Myeloid Derived Suppressor Cells to inhibit their immune suppressing effects on the tumor microenvironment to prevent T cell exhaustion and acquired resistance to checkpoint inhibitors and cellular therapies.
For more information, please visit tuhurabio.com and connect with TuHURA on Facebook, X, and LinkedIn.
Forward-Looking Statements
This news release contains forward-looking statements that are not historical facts within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and other future conditions. In some cases you can identify these statements by forward-looking words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “could,” “should,” “would,”
“project,” “plan,” “expect,” “goal,” “seek,” “future,” “likely” or the negative or plural of these words or similar expressions. Examples of such forward-looking statements include but are not limited to express or implied statements regarding Kintara’s or TuHURA’s management team’s expectations, hopes, beliefs, intentions or strategies regarding the future including, without limitation, statements regarding: the Proposed Transaction and the expected effects, perceived benefits or opportunities and related timing with respect thereto, expectations regarding clinical trials and research and development programs, in particular with respect to TuHURA’s IFx-Hu2.0 product candidate and its TME modulators development program, and any developments or results in connection therewith; the anticipated timing of the results from those studies and trials; expectations regarding the use of capital resources, including the net proceeds from the fully-funded financing; the time period over which the combined company’s capital resources will be sufficient to fund its anticipated operations; and the expected trading of the combined company’s stock on the Nasdaq Capital Market. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. You are cautioned that such statements are not guarantees of future performance and that actual results or developments may differ materially from those set forth in these forward-looking statements. Factors that could cause actual results to differ materially from these forward-looking statements are described in the “Risk Factors” section of Kintara’s Annual Report on Form 10-K for the fiscal year ended June 30, 2023 filed with the SEC and the proxy statement/prospectus dated August 19, 2024, as supplemented. Additional assumptions, risks and uncertainties are described in detail in our registration statements, reports and other filings with the SEC, which are available on the combined company’s website, and at www.sec.gov.
You are cautioned that such statements are not guarantees of future performance and that our actual results may differ materially from those set forth in the forward-looking statements. The forward-looking statements and other information contained in this news release are made as of the date hereof and Kintara does not undertake any obligation to update publicly or revise any forward-looking statements or information, whether as a result of new information, future events or otherwise, unless so required by applicable securities laws. Nothing herein shall constitute an offer to sell or the solicitation of an offer to buy any securities.
Investor Contact:
Jenene Thomas
JTC Team, LLC
tuhura@jtcir.com
Exhibit 99.2
TUHURA MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of TuHURA’s financial condition and results of operations should be read together with TuHURA’s consolidated financial statements and the related notes appearing elsewhere in this proxy statement/prospectus. This discussion and other parts of this proxy statement/prospectus contain forward-looking statements that involve risks and uncertainties, such as statements regarding TuHURA’s plans, objectives, expectations, intentions and projections. TuHURA’s actual results could differ materially from those described in or implied by these forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in the “Risk Factors” section of this proxy statement/prospectus.
Overview
TuHURA is a clinical stage immuno-oncology company developing novel personalized cancer vaccine product candidates designed to overcome primary resistance to immunotherapies like checkpoint inhibitors. TuHURA has entered into a Special Protocol Assessment agreement with the FDA for a single Phase 3 randomized placebo and injection controlled trial for IFx-2.0, the company’s lead personalized cancer vaccine product candidate, as adjunctive therapy to pembrolizumab (Keytruda®) in the first line treatment of patients with advanced or metastatic Merkel cell carcinoma who are checkpoint inhibitor naïve utilizing the FDA’s accelerated approval pathway. TuHURA is also developing novel bi-functional antibody drug conjugates, or ADCs, targeting myeloid derived suppressor cells, or MDSCs, to modulate their immunosuppressive effects on the tumor microenvironment to overcome acquired resistance to immunotherapies.
TuHURA was incorporated under the laws of the State of Florida on May 11, 1995 and reincorporated in Delaware on April 27, 2023. To date, TuHURA has devoted substantially all of its resources to organizing and staffing TuHURA, business planning, raising capital, identifying and developing product candidates, enhancing its intellectual property portfolio, undertaking research, conducting preclinical studies and clinical trials, and securing manufacturing for its development programs. TuHURA does not have any products approved for sale and has not generated any revenue from product sales. TuHURA has funded its operations primarily through the private placement of common and preferred stock and convertible notes.
TuHURA has incurred significant operating losses since its inception, which are mainly attributed to research and development costs associated with TuHURA’s portfolio and general and administrative expenses. TuHURA’s net loss was $29.3 million for the year ended December 31, 2023 (which includes the expensing of the entire $16.2 million purchase price for the assets of TuHURA Biopharma, Inc., of which $15.0 million was paid in the form of TuHURA Common Stock) and $10.1 million for the six months ended June 30, 2024. As of June 30, 2024, TuHURA had an accumulated deficit of $98.6 million. TuHURA’s operating losses may fluctuate significantly from quarter-to-quarter and year-to-year as a result of several factors, including the timing of its preclinical studies and clinical trials and the expenditures related to other research and development activities. TuHURA expects to continue to incur operating losses. TuHURA anticipates these losses will increase substantially as it advances its product candidates through preclinical and clinical development, develops additional product candidates and seeks regulatory approvals for its product candidates. TuHURA does not expect to generate any revenues from product sales unless and until it successfully completes development and obtains regulatory approval for one or more product candidates. In addition, if TuHURA obtains marketing approval for any product candidate, TuHURA expects to incur pre-commercialization expenses and significant commercialization expenses related to marketing, sales, manufacturing and distribution. TuHURA may also incur expenses in connection with the in-licensing of additional product candidates. Furthermore, upon completion of the Merger, TuHURA expects to incur additional costs associated with operating as a public company, including significant legal, accounting, investor relations, compliance and other expenses that TuHURA did not incur as a private company.
As a result, TuHURA will need substantial additional funding to support its continuing operations and pursue its growth strategy. Until such time as TuHURA can generate significant revenue from sales of its product candidates, if ever, TuHURA expects to finance its cash needs through public or private equity offerings, debt
financings, collaborations and licensing arrangements or other capital sources. However, TuHURA may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms or at all. TuHURA’s failure to raise capital or enter into such other arrangements as and when needed would have a negative impact on its financial condition and could force it to delay, limit, reduce or terminate its product development or future commercialization efforts or grant rights to develop and market its product candidates that it would otherwise prefer to develop and market itself.
Because of the numerous risks and uncertainties associated with pharmaceutical product development, TuHURA is unable to accurately predict the timing or amount of increased expenses or when or if it will be able to achieve or maintain profitability. Even if TuHURA is able to generate product sales, it may not become profitable. If TuHURA fails to become profitable or is unable to sustain profitability on a continuing basis, TuHURA may be unable to continue its operations at planned levels and be forced to reduce or terminate its operations.
As of June 30, 2024, TuHURA had cash and cash equivalents of $12.3 million. See “— Liquidity and Capital Resources” below.
Recent Developments
Proposed Merger
On April 2, 2024, TuHURA entered into the Merger Agreement with Kintara and Merger Sub. Pursuant to the Merger Agreement, among other matters, and subject to the satisfaction or waiver of the conditions set forth in the Merger Agreement, Merger Sub will merge with and into TuHURA, with TuHURA surviving the merger and becoming a direct, wholly-owned subsidiary of Kintara. The Merger is intended to qualify for U.S. federal income tax purposes as a tax-free reorganization under the provisions of Section 368(a) of the Code. The Merger Agreement and the Merger were approved by the members of the board of directors of both TuHURA and Kintara.
Subject to the terms and conditions of the Merger Agreement, at the closing of the Merger, (a) each then-outstanding share of TuHURA Common Stock (other than shares held in treasury and excluding dissenting shares), including shares of TuHURA Common Stock issued upon conversion of TuHURA preferred stock and conversion of all TuHURA convertible promissory notes issued in the TuHURA Note Financing, will be converted into the right to receive a number of shares of Kintara Common Stock (after giving effect to the reverse stock split) equal to the Exchange Ratio per the Merger Agreement and (b) each then-outstanding TuHURA stock option and warrant that has not previously been exercised immediately prior to the Effective Time will be assumed by Kintara.
The Merger is expected to close in the fourth quarter of 2024 and is subject to approval by the stockholders of Kintara and TuHURA as well as other customary closing conditions, including the effectiveness of the registration statement of which this proxy/prospectus forms a part and Nasdaq’s approval of the listing of the shares of Kintara Common Stock to be issued in connection with the Merger. If Kintara is unable to satisfy certain closing conditions or if other mutual closing conditions are not satisfied, TuHURA will not be obligated to complete the Merger. The Merger Agreement contains certain termination rights of each of TuHURA and Kintara. Under certain circumstances, TuHURA may be required to pay Kintara a termination fee of $1 million or reimburse Kintara’s expenses up to a maximum of $0.75 million. Kintara may be required to pay TuHURA a termination fee of $1 million or reimburse TuHURA’s expenses up to a maximum of $0.75 million. If the Merger is completed, the business of TuHURA will continue as the business of the combined company.
Kineta Exclusivity Agreement and July 2024 Private Placement
On July 8, 2024, Kintara and TuHURA issued a press release announcing that TuHURA has entered into an Exclusivity and Right of First Offer Agreement (the “Exclusivity Agreement”) with Kineta, Inc. (“Kineta”) for the potential acquisition of Kineta’s KVA12123 anti-VISTA antibody and related rights and assets associated with and derived from the asset.
KVA12123 is a rationally targeted, anti-VISTA antibody checkpoint inhibitor designed to reverse VISTA immune suppression and remodel the tumor microenvironment (TME) to overcome acquired resistance to immunotherapies.
Pursuant to the Exclusivity Agreement, among other things, Kineta has granted TuHURA an exclusive right to acquire Kineta’s worldwide patents, patent rights, patent applications, product and development program assets,
technical and business information, and other rights and assets associated with and derived from its development program related to KVA12123 during the period commencing as of July 3, 2024 (the “Effective Date”) and continuing through the first to occur of (a) the execution of any Definitive Agreement (as defined therein) with respect to a Potential Transaction (as defined therein) by TuHURA or one or more of its affiliates and (b) 11:59 PM Eastern Time on October 1, 2024, subject to extension as noted in the following sentence (the “Exclusivity Period”). In the event that the parties are engaged in good faith discussions regarding a Potential Transaction on the date on which the Exclusivity Period (or any renewal thereof) is scheduled to expire and TuHURA has not yet closed the transactions contemplated by the Merger Agreement, then on such date, the Exclusivity Period shall automatically renew for an additional ten (10) day period (a “Renewal Period”) (up to a total of two (2) Renewal Periods for an aggregate of twenty (20) days).
Under the terms of the Exclusivity Agreement, TuHURA paid Kineta a fee in the amount of $5,000,000, with $2,500,000 paid at signing and an additional $2,500,000 paid on July 15, 2024. The fee is nonrefundable other than in the case of an uncured breach of the Exclusivity Agreement by Kineta. No later than two (2) business days after a Renewal Period has started (to be confirmed in writing by both parties), TuHURA shall pay an additional $150,000 as an additional Exclusivity Payment, in an amount not to exceed $300,000 for the two (2) available Renewal Periods. The Exclusivity Payment will be credited against the initial cash consideration that may be payable to Kineta pursuant to any Definitive Agreement (if any) between Kineta and TuHURA and/or its affiliates with respect to a Potential Transaction.
In conjunction with the Exclusivity Agreement, TuHURA sold 4,009,623 shares of its common stock in a private offering with a purchase price of $5,000,000 (the “July Private Placement”) to an existing TuHURA shareholder (the “Investor”). In connection with the July Private Placement, the Investor is entitled to a 1.5% royalty on certain sales by TuHURA of products based on KVA12123 as set forth in the Investor’s subscription agreement. Due to no definitive transaction agreement with Kineta for the purchase of KVA12123 and the inherent uncertainties surrounding the regulatory approval of KVA12123 and future monetization, TuHURA has not allocated any of the $5,000,000 purchase price consideration to the royalty agreement.
The accounting treatment for the Exclusivity Agreement and the July Private Placement is preliminary in nature and the final accounting treatment will be determined based on a number of factors, including additional analysis of the transaction and consideration of relevant accounting standards.
Special Protocol Assessment Agreement
On January 25, 2024 TuHURA successfully completed its negotiations with FDA and entered into a Special Protocol Assessment Agreement for a single registration directed, randomized, placebo controlled Phase 3 trial for IFx-Hu2.0 as adjunctive therapy to pembrolizumab (Keytruda®) in first line treatment for patients with advanced or metastatic Merkel Cell carcinoma who are checkpoint inhibitor naive. The trial utilizes a novel design recommended by the FDA which incorporates Overall Response Rate (ORR) as the primary endpoint for accelerated approval. The trial also includes Progression Free Survival (PFS) as a key secondary endpoint which, if achieved, without demonstrating a detriment to Overall Survival, could allow conversion from accelerated approval to full approval satisfying the requirement for a post marketing trial. Before initiating this Phase 3 trial TuHURA is required to complete certain manufacturing activities as noted in a partial clinical hold correspondence from FDA. Based on correspondence following a type C meeting with the FDA, TuHURA has ongoing development and validation of several testing and mixing studies which TuHURA believes will be adequate to address the CMC requirements to initiate the Phase 3 clinical trial. TuHURA believes, it will be in position to initiate the phase 3 study late in the second half of 2024 and anticipates enrollment to take approximately 12 months with topline data 6 to 7 months following the last patient enrolled.
Components of TuHURA’s Results of Operations
Revenue
TuHURA did not generate any revenue and does not expect to generate any revenue from the sale of products in the near future.
Research and Development Expenses
To date, TuHURA’s research and development expenses have related primarily to development of IFx-Hu2.0, manufacturing, clinical studies, and other early pre-clinical activities related to TuHURA’s portfolio. Research and development expenses are recognized as incurred and payments made prior to the receipt of goods or services to be used in research and development are capitalized until the goods or services are received.
Research and development expenses include:
• salaries, payroll taxes, employee benefits
• external research and development expenses incurred under agreements with contract research organizations (“CROs”), and consultants to conduct TuHURA’s clinical studies;
• laboratory supplies;
• costs related to manufacturing product candidates, including fees paid to third-party manufacturers and raw material suppliers;
• stock-based compensation charges for those individuals involved in research and development efforts; and
• facilities, depreciation, and other allocated expenses, which include direct and allocated expenses for rent.
Clinical trial costs are a significant component of research and development expenses and include costs associated with third-party contractors. TuHURA outsources a substantial portion of its clinical trial activities, utilizing external entities such as CROs, independent clinical investigators and other third-party service providers to assist it with the execution of its clinical trials.
TuHURA plans to substantially increase its research and development expenses for the foreseeable future as it continues the development of its product candidates and seeks to discover and develop new product candidates. Due to the inherently unpredictable nature of preclinical and clinical development, TuHURA cannot determine with certainty the timing of the initiation, duration or costs of future clinical trials and preclinical studies of product candidates. Clinical and preclinical development timelines, the probability of success and the amount of development costs can differ materially from expectations. TuHURA anticipates that it will make determinations as to which product candidates and development programs to pursue and how much funding to direct to each product candidate or program on an ongoing basis in response to the results of ongoing and future preclinical studies and clinical trials, regulatory developments and TuHURA’s ongoing assessments as to each product candidate’s commercial potential. In addition, TuHURA cannot forecast which product candidates may be subject to future collaborations, when such arrangements will be secured, if at all, and to what degree such arrangements would affect TuHURA’s development plans and capital requirements.
TuHURA’s future clinical development costs may vary significantly based on factors such as:
• per-patient trial costs;
• the number of trials required for regulatory approval;
• the number of sites included in the trials;
• the countries in which the trials are conducted;
• the length of time required to enroll eligible patients;
• the number of patients that participate in the trials;
• the number of doses that patients receive;
• the drop-out or discontinuation rates of patients;
• potential additional safety monitoring requested by regulatory agencies;
• the phase of development of the product candidate; and
• the efficacy and safety profile of the product candidate.
Acquired In-Process Research and Development (“IPR&D”)
Acquired in-process research and development expenses consist of existing research and development projects at the time of the acquisition. Projects that qualify as IPR&D assets represent those that have not yet reached technological feasibility and have no alternative future use. TuHURA acquisitions of assets have included IPR&D assets that had not yet reached technological feasibility and had no alternative future use, which resulted in a write-off of these IPR&D assets to acquired in-process research and development expenses in our consolidated statement of operations.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and employee-related costs, including stock-based compensation, for personnel in TuHURA’s executive, finance, and other administrative functions. Other significant costs include facility related costs, legal fees relating to intellectual property and corporate matters, professional fees for accounting and consulting services and insurance costs. TuHURA anticipates that its general and administrative expenses will increase in the future to support TuHURA’ continued research and development activities, and, if any product candidates receive marketing approval, commercialization activities. TuHURA also anticipates increased expenses related to audit, legal, regulatory, and tax-related services associated with maintaining compliance with exchange listing and SEC requirements, director and officer insurance premiums and investor relations costs associated with operating as a public company.
Other Income (Expense)
Other income (expense) consists of interest income on our cash and cash equivalents, interest expense on borrowings under our convertible note agreements, and non-cash changes in the fair value of our derivative liability associated with the make-whole premium on our convertible notes. Other income (expense) also included grant income from our NIH-funded research grants completed in May 2023, employee retention tax credit for companies with employees affected during the COVID-19 pandemic, and forgiveness of a paycheck protection program loan in April 2022.
Results of Operations
Comparisons for the Three Months Ended June 30, 2024, and June 30, 2023
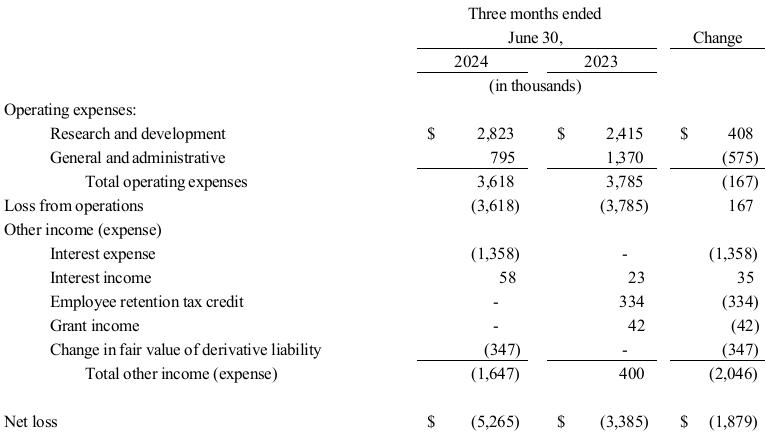
Research and Development Expenses. The following table summarizes TuHURA research and development expenses by program for the periods presented.

Research and development expenses were $2.8 million and $2.4 million for the three months ended June 30, 2024, and 2023, respectively. The increase of $0.4 million related to the following.
•an increase of approximately $0.2 million due to ongoing clinical development of IFx-2.0;
•an increase of $0.2 million due to preclinical research of IFx-3.0 and MDSCs; and
•an increase of less than $0.1 million in facilities, salary and personnel related costs.
General and Administrative Expenses. General and administrative expenses were $0.8 million and $1.4 million for the three months ended June 30, 2024, and 2023, respectively. The decrease of $0.6 million was
primarily due to legal fees associated with the TuHURA Biopharma Inc. asset acquisition and the terminated CohBar merger all that were incurred in the previous year.
Interest Expense. During various dates from December 2023 to June 2024, as part of the TuHURA Note Financing, TuHURA issued convertible notes totaling $21,753,000. The convertible notes included interest at 20% per annum.
Interest Income. For the three months ended June 30, 2024 and 2023, interest income was earned on deposits at two regional banks.
Employee Retention Tax Credit. The IRS provides a refundable tax credit for businesses that had employees and were affected during the COVID-19 pandemic. In October 2022, TuHURA applied for a credit under this program through ADP Totalsource, which manages the TuHURA payroll and benefits. In May 2023, TuHURA received a letter from ADP Totalsource that the credit will be $0.3 million.
Grant Income. Grant income was $0.00 million and less than $0.1 million for the three months ended June 30, 2024 and 2023, respectively. In April 2021, TuHURA received approval from the Department of Health and Human Services for a $0.4 million grant to study cervical cancer and received reimbursements for related expenses associated with the grant in these years. TuHURA received the final payment under this grant in May 2023.
Change in fair value of derivative liability associated with make-whole premium. For the three months ended June 30, 2024, there was a gain of $0.3 million associated with the bifurcated embedded derivative liability related to the make-whole premium on the convertible notes.
Comparisons for the Six Months Ended June 30, 2024, and June 30, 2023
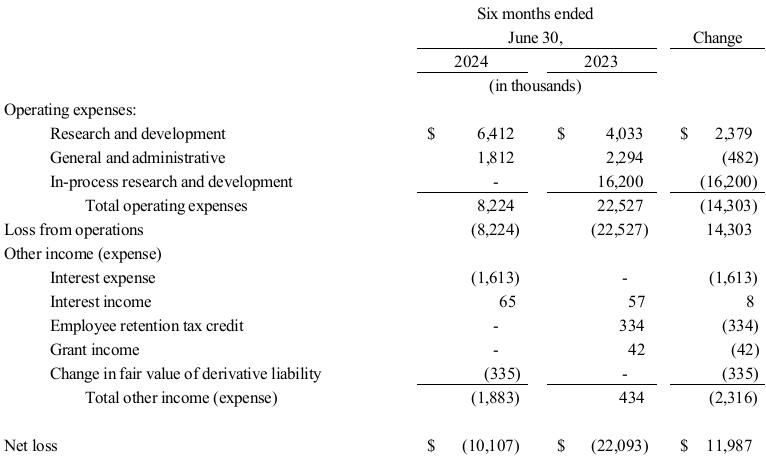
Research and Development Expenses. The following table summarizes TuHURA research and development expenses by program for the periods presented.
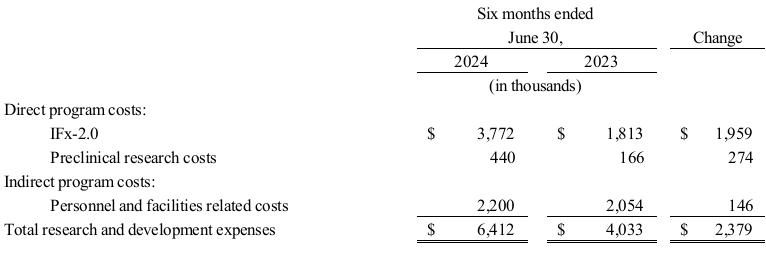
Research and development expenses were $6.4 million and $4.0 million for the six months ended June 30, 2024, and 2023, respectively. The increase of $2.4 million related to the following.
•an increase of approximately $2.0 million due to ongoing clinical development of IFx-2.0;
•an increase of $0.3 million due to preclinical research of IFx-3.0 and MDSCs; and
•an increase of $0.1 million in facilities, salary and personnel related costs.
Acquired in process research and development (“IPR&D”). On January 26, 2023, TuHURA acquired certain assets of TuHURA Biopharma, Inc., for $1.2 million in cash and 22.7 million common shares. The common shares issued to TuHURA have an estimated fair market value of $15.0 million. TuHURA performed the “screen test” and determined that substantially all of the fair value of the gross assets acquired in the TuHURA Biopharma acquisition is concentrated in a single identifiable asset or group of similar identifiable assets. As such, the TuHURA Biopharma acquisition has been accounted for as an asset acquisition. As the underlying asset is in-process research and development, TuHURA immediately expensed the entire $16.2 million purchase price for the six months ended June 30, 2023, in accordance with FASB ASC Topic 730.
General and Administrative Expenses. General and administrative expenses were $1.8 million and $2.3 million for the six months ended June 30, 2024, and 2023, respectively. The decrease of $0.5 million was primarily due to legal fees associated with the TuHURA Biopharma Inc. asset acquisition and proposed merger with CohBar, Inc. which was terminated in accordance with its terms in November 2023.
Employee Retention Tax Credit. The IRS provides a refundable tax credit for businesses that had employees and were affected during the COVID-19 pandemic. In October 2022, TuHURA applied for a credit under this program through ADP Totalsource, which manages the TuHURA payroll and benefits. In May 2023, TuHURA received a letter from ADP Totalsource that the credit will be $0.3 million.
Grant Income. Grant income was $0.0 million and less than $0.1 million for the six months ended June 30, 2024 and 2023, respectively. In April 2021, TuHURA received approval from the Department of Health and Human Services for a $0.4 million grant to study cervical cancer and received reimbursements for related expenses associated with the grant in these years. TuHURA received the final payment under this grant in May 2023.
Interest Expense. In December 2023 to June 2024, as part of the TuHURA Note Financing, TuHURA issued convertible notes totaling $21,753,000. The convertible notes included interest at 20% per annum.
Interest Income. For the six months ended June 30, 2024 and 2023, respectively, interest income was earned on deposits at two regional banks.
Change in fair value of derivative liability associated with make-whole premium. For the six months ended June 30, 2024, there was a gain of $0.3 million associated with the bifurcated embedded derivative liability related to the make-whole premium on the convertible notes.
Liquidity and Capital Resources
TuHURA has incurred net losses and negative cash flows from operations since TuHURA’s inception and anticipates it will continue to incur net losses for the foreseeable future. TuHURA incurred net losses of $10.1 million and $22.1 million for the six months ended June 30, 2024, and 2023, respectively, and used $8.9 million and $6.8 million of cash from TuHURA’s operating activities for the six months ended June 30, 2024, and 2023, respectively. As of June 30, 2024, TuHURA had an accumulated deficit of $98.6 million. The $22.1 million loss for the six months ended June 30, 2023, included the expensing of the entire $16.2 million purchase price for the assets of TuHURA Biopharma, Inc., of which $15 million was paid in the form of TuHURA Common Stock.
As of June 30, 2024, TuHURA had cash and cash equivalents of $12.3 million.
Sources of Liquidity
To date, TuHURA has financed its operations principally through private placements of TuHURA’s common and preferred stock and issuance of convertible notes.
Series A Preferred Stock Financing
In August 2017 through April 2018, TuHURA issued an aggregate of 33,186,952 shares of its Series A Preferred Stock at a purchase price of $0.52 per share for aggregate net proceeds of $15.6 million. There were 15,976,413 common stock warrants associated with these preferred shares.
Series A-1 Preferred Stock Financing
From October 2020 to October 2021, TuHURA issued an aggregate of 14,288,076 shares of its Series A-1 Preferred Stock at a purchase price of $0.66 per share for aggregate consideration of $9,430,000. There were 6,468,026 common stock warrants associated with these preferred shares.
Series B Preferred Stock Financing
From June through August 2022, TuHURA issued Series B preferred shares and received $16.6 million for 25,153,030 Series B shares at a purchase price of $0.66 along with 18,864,773 warrants that are exercisable at a fixed price of $0.66.
Prior Convertible Note Financing
From May 2019 through December 2020, TuHURA issued $4,995,000 aggregate principal amount of convertible notes, which bear interest at the rate of 10% per annum.
On February 24, 2021, a majority of note holders elected to voluntarily convert their notes under the terms of a non-qualified financing in the Note. This forced a conversion of all Notes into preferred shares. The conversion price was set by the same terms offered in the non-qualified financing. As a result, the $4,995,000 Note principal plus $277,000 accrued interest was converted into 7,988,169 Series A-1 preferred shares at $0.66 a share. There were 3,765,851 common stock warrants associated with this conversion.
TuHURA Note Financing
On April 2, 2024, TuHURA completed a private placement under which it offered and sold convertible promissory notes (the “TuHURA Notes”) to approximately 40 accredited investors during the period from December 2023 through April 2, 2024 (the “TuHURA Note Financing”). In the transaction, TuHURA received subscriptions for an aggregate principal amount of $31,253,000 of TuHURA Notes, of which the entire amount was funded as of September 30, 2024.
The TuHURA Notes are general unsecured obligations of TuHURA that have various maturity dates through 2026, and that bear interest at a rate of 20% per annum, simple interest. The TuHURA Notes contain a make-whole provision under which, upon payment or conversion of the TuHURA Notes, the holders of the notes will receive additional interest equal to the amount of interest that would have accrued through the first anniversary of the initial
closing of the TuHURA Note Financing (if the notes are paid or converted prior to such first anniversary), through the 18-month anniversary of the initial closing (if the notes are paid or converted on or after the first anniversary and before the 18-month anniversary), or though the maturity date (if the notes are paid or converted after the 18-month anniversary of the initial closing). The TuHURA Notes provide that, immediately prior to the completion of the Merger, all principal and accrued and unpaid interest and make-whole amounts under the notes will automatically convert into shares of TuHURA Common Stock at a conversion price $0.68 per share of TuHURA Common Stock. In the event that the Merger is not completed, the TuHURA Notes would convert upon an alternative merger transaction or initial public offering that occurs prior to the maturity date of the TuHURA Notes, if any.
In the TuHURA Note Financing, the investors that purchased at least $4.0 million in principal amount of TuHURA Notes, together with their affiliates, were issued warrants to purchase an aggregate of 18,797,794 additional shares of TuHURA Common Stock (the “TuHURA Common Warrants”). The TuHURA Common Warrants have an exercise price of $1.02 per share of TuHURA Common Stock and have an expiration date of 3 years following the respective issue dates of the warrants. The TuHURA Common Warrants are exercisable at any time prior to the expiration date of the warrants, and the warrants are exercisable for cash and, at such time as there is no registration statement covering the resale of the shares issuable upon the exercise of the warrants, on a cashless basis. The TuHURA Common Warrants contain customary adjustments to the exercise price and number of underlying warrant shares by reason of stock splits, stock dividends, reverse stock split, and the like.
In connection with the TuHURA Note Financing, TuHURA will issue an aggregate of 229,040 shares of TuHURA Common Stock to a placement agent for the private placement.
Private Placement of Common Stock
In July 2024, TuHURA sold 4,009,623 shares of its common stock in a private offering with a purchase price of $5,000,000 to an existing TuHURA shareholder.
Cash Flows
The following table sets forth a summary of the net cash flow activity for the six months ended June 30, 2024 and 2023, respectively:
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
March 31, |
|
|
2024 |
|
2023 |
|
|
(in thousands) |
Net cash provided by (used in): |
|
|
|
|
|
|
|
|
Operating activities |
|
$ |
(8,900 |
) |
|
$ |
(6,784 |
) |
Investing activities |
|
|
(37) |
|
|
|
(1,222 |
) |
Financing activities |
|
|
17,583 |
|
|
|
(24) |
|
Net increase (decrease) in cash |
|
$ |
8,646 |
|
|
$ |
(8,030 |
) |
Operating Activities
Net cash used in operating activities was $8.9 million and $6.8 million for the six months ended June 30, 2024 and 2023, respectively. The $2.1 million increase in cash used during the six months ended June 30, 2024 was due to increases in TuHURA’s research and development associated with preclinical, clinical, regulatory, and drug product.
Investing Activities
Net cash used in investing activities was less than $0.1 million and $1.2 million for the six months ended June 30, 2024 and 2023, respectively. On January 26, 2023 TuHURA acquired certain assets of TuHURA Biopharma, Inc. for $1.2 million in cash and 22.7 million common shares. The cash component of the transaction is considered an investing activity. The entire transaction was valued at $16.2 million.
Financing Activities
Net cash provided by financing activities was $17.6 million for the six months ended June 30, 2024, which consisted of net proceeds from convertible notes issued as part of the TuHURA Note Financing and deferred offering costs paid in connection with the proposed merger with Kintara.
Funding Requirements
Upon the closing of the Merger, TuHURA expects to incur additional costs associated with operating as a public company. In addition, TuHURA anticipates that it will need substantial additional funding in connection with its continuing operations. TuHURA believes that its existing cash and cash equivalents, together with the estimated net proceeds from the TuHURA Note Financing, will be sufficient to meet its anticipated cash requirements through the end of 2025.
However, TuHURA’s forecast of the period through which its financial resources will be adequate to support its operations is a forward-looking statement that involves risks and uncertainties, and actual results could vary materially. Management based projections of operating capital requirements on TuHURA’s current operating plan, which includes several assumptions that may prove to be incorrect, and TuHURA may deplete its available capital resources sooner than management expects. TuHURA’s future capital requirements will depend on many factors, including:
• the initiation, progress, timing, costs and results of drug discovery, preclinical studies and clinical trials of IFx-Hu2.0, IFx-Hu3.0 and any other future product candidates;
• the costs associated with hiring additional personnel and consultants as TuHURA’s preclinical and clinical activities increase;
• the outcome, timing and costs of seeking regulatory approvals;
• the cost of manufacturing IFx-Hu2.0 and IFx-Hu3.0 and future product candidates for clinical trials in preparation for marketing approval and in preparation for commercialization;
• the emergence of competing therapies and other adverse market developments;
• the ability to establish and maintain strategic licensing or other arrangements and the financial terms of such agreements; and
• the costs of operating as a public company.
Until such time, if ever, as TuHURA can generate substantial product revenues to support its capital requirements, TuHURA expects to finance its cash needs through a combination of public or private equity offerings, debt financings, collaborations and licensing arrangements or other capital sources. To the extent that TuHURA raises additional capital through the sale of equity or convertible debt securities, the ownership interest of TuHURA’s stockholders will be or could be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of holders of TuHURA’s Common Stock. Debt financing and equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures, or declaring dividends. If TuHURA raises funds through collaborations, or other similar arrangements with third parties, TuHURA may need to relinquish valuable rights to its product candidates, future revenue streams or research programs or may have to grant licenses on terms that may not be favorable to it and/or may reduce the value of TuHURA’s Common Stock. If TuHURA is unable to raise additional funds through equity or debt financings as and when needed, TuHURA may be required to delay, limit, reduce or terminate its product development or future commercialization efforts or grant rights to develop and market its product candidates even if TuHURA would otherwise prefer to develop and market such product candidates themselves.
Critical Accounting Policies and Significant Judgments and Estimates
TuHURA’s management’s discussion and analysis of its financial condition and results of operations is based on its financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these financial statements requires TuHURA to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses and the disclosure of contingent assets and liabilities in TuHURA’s financial statements. On an ongoing basis, TuHURA evaluates its estimates and judgments, including those related to accrued expenses and stock-based compensation. TuHURA bases its estimates on historical experience, known trends and events, and various other factors that TuHURA believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. TuHURA’s actual results may differ from these estimates under different assumptions or conditions. While TuHURA’s significant accounting policies are described in more detail in Note 2 of its financial statements appearing elsewhere in this proxy statement/prospectus, TuHURA believes the following accounting policies and estimates to be most critical to the preparation of its financial statements.
Accrued Research and Development Expenses
As part of the process of preparing TuHURA’s financial statements, TuHURA is required to estimate its accrued expenses as of each balance sheet date. This process involves reviewing open contracts and purchase orders, communicating with TuHURA’s personnel to identify services that have been performed on TuHURA’s behalf and estimating the level of service performed and the associated cost incurred for the service when TuHURA has not yet been invoiced or otherwise notified of the actual cost. TuHURA makes estimates of its accrued expenses as of each balance sheet date based on facts and circumstances known to it at that time. TuHURA periodically confirms the accuracy of its estimates with the service providers and adjusts, if necessary. The significant estimates in TuHURA’s accrued research and development expenses include the costs incurred for services performed by its vendors in connection with research and development activities for which TuHURA has not yet been invoiced.
TuHURA bases its expenses related to research and development activities on its estimates of the services received and efforts expended pursuant to quotes and contracts with vendors that conduct research and development on TuHURA’s behalf. The financial terms of these agreements are subject to negotiation, vary from contract-to-contract and may result in uneven payment flows. There may be instances in which payments made to TuHURA’s vendors will exceed the level of services provided and result in a prepayment of the research and development expense. In accruing service fees, TuHURA estimates the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from TuHURA’s estimate, TuHURA adjusts the accrual or prepaid expense accordingly. Advance payments for goods and services that will be used in future research and development activities are expensed when the activity has been performed or when the goods have been received rather than when the payment is made.
Although TuHURA does not expect its estimates to be materially different from amounts actually incurred, if TuHURA’s estimates of the status and timing of services performed differ from the actual status and timing of services performed, it could result in TuHURA reporting amounts that are too high or too low in any particular period. To date, there have been no material differences between TuHURA’s estimates of such expenses and the amounts actually incurred.
Stock-Based Compensation Expense
Stock-based compensation expense represents the cost of the grant date fair value of equity awards recognized over the requisite service period of the awards (usually the vesting period) on a straight-line basis. TuHURA estimates the fair value of equity awards using the Black-Scholes option pricing model and recognizes forfeitures as they occur. Estimating the fair value of equity awards as of the grant date using valuation models, such as the Black-Scholes option pricing model, is affected by assumptions regarding a number of variables, including the risk-free interest rate, the expected stock price volatility, the expected term of stock options, the expected dividend yield and the fair value of the underlying common stock on the date of grant. Changes in the assumptions can materially affect the fair value and ultimately how much stock-based compensation expense is recognized. These inputs are
subjective and generally require significant analysis and judgment to develop. See Note 2 of TuHURA’s financial statements included elsewhere in this proxy statement/prospectus for information concerning certain of the specific assumptions TuHURA used in applying the Black-Scholes option pricing model to determine the estimated fair value of TuHURA’s stock options granted.
Common stock valuations
TuHURA is required to estimate the fair value of the common stock underlying its equity awards when performing fair value calculations. The fair value of the common stock underlying its equity awards was determined on each grant taking into account input from management and taking into account the pricing offered in TuHURA’s equity raises. All options to purchase shares of TuHURA’s Common Stock are intended to be granted with an exercise price per share no less than the fair value per share of TuHURA’s Common Stock underlying those options on the date of grant, based on the information known to TuHURA on the date of grant. In the absence of a public trading market for TuHURA’s Common Stock, on each grant date TuHURA develops an estimate of the fair value of its common stock in order to determine an exercise price for the option grants. TuHURA’s determinations of the fair value of its common stock were made by considering the prices of preferred stock sold to investors in arm’s length transactions and the rights, preferences and privileges of TuHURA’s preferred stock relative to those of its common stock.
In determining the fair value of TuHURA’s Common Stock underlying stock option grants for the six months ended June 30, 2024 and 2023, TuHURA used the market approach by reference to the closest round of equity financing, preceding the date of valuation and analysis of the trading values of publicly traded companies deemed comparable to TuHURA.
Recently Issued and Adopted Accounting Pronouncements
A description of recently issued and adopted accounting pronouncements that may potentially impact TuHURA’s financial position and results of operations is disclosed in Note 2 to TuHURA’s financial statements appearing elsewhere in this proxy statement/prospectus.
Off-Balance Sheet Arrangements
During the periods presented, TuHURA did not have, nor does it currently have, any off-balance sheet arrangements as defined under SEC rules.
Quantitative and Qualitative Disclosures about Market Risk
TuHURA is exposed to market risks in the ordinary course of its business. These risks primarily include interest rate risks and inflation risks. Periodically, TuHURA maintains deposits in accredited financial institutions in excess of federally insured limits. TuHURA deposits its cash in financial institutions that it believes has high credit quality and have not experienced any losses on such accounts and does not believe it is exposed to any unusual credit risk beyond the normal credit risk associated with commercial banking relationships.
Interest Rate Risk
TuHURA’s cash consists of cash in readily-available checking accounts. TuHURA may also invest in short-term money market fund investments. Such interest-earning instruments carry a degree of interest rate risk; however, historical fluctuations in interest income have not been significant.
Inflation Risk
Inflation generally affects TuHURA by increasing its cost of labor and research and development contract costs. TuHURA does not believe inflation has had a material effect on its results of operations during the periods presented.
Exhibit 99.4
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
The following unaudited pro forma condensed combined financial information presents the combination of the financial information of Kintara and TuHURA adjusted to give effect to the Merger and related transactions. The following unaudited pro forma condensed combined financial information has been prepared in accordance with Article 11 of Regulation S-X. Defined terms included below have the same meaning as terms defined and included elsewhere in Kintara’s proxy statement/prospectus dated August 13, 2024 and filed with the SEC on August 19, 2024.
TuHURA and Kintara have different fiscal year ends. TuHURA’s year end is December 31, and Kintara’s year end is June 30. The following unaudited pro forma condensed combined financial statements have been prepared to present the combination of the historical financial statements of TuHURA and the historical financial statements of Kintara, on a pro forma basis adjusted to give effect to the Merger and related transactions. Following the Merger, the surviving company will have a fiscal year end of December 31. The unaudited pro forma condensed combined financial information includes (all financial information is prepared in accordance with GAAP):
(a) The unaudited pro forma condensed combined balance sheet as of June 30, 2024 combines (i) the unaudited condensed consolidated balance sheet of TuHURA as of June 30, 2024, as derived from its historical financial statements and (ii) the audited consolidated balance sheet of Kintara as of June 30, 2024, as filed on Kintara’s Form 10-K with the SEC on October 7, 2024, on a pro forma basis as if the Merger and related transactions had been consummated on June 30, 2024.
(b) The unaudited pro forma condensed combined statement of operations for the six months ended June 30, 2024 combines (i) the unaudited condensed consolidated statement of operations of TuHURA for the six months ended June 30, 2024, as derived from its historical financial statements and (ii) the unaudited condensed consolidated statement of operations of Kintara for the six months ended June 30, 2024, as calculated by (a) subtracting the unaudited interim condensed consolidated statement of operations of Kintara for the six months ended December 31, 2023, as filed on Kintara’s Form 10-Q with the SEC on February 14, 2024, from (b) the audited consolidated statement of operations of Kintara for the year ended June 30, 2024, as filed on Kintara’s Form 10-K with the SEC on October 7, 2024, on a pro forma basis as if the Merger and related transactions had been consummated on January 1, 2023.
(c) The unaudited pro forma condensed combined statement of operations for the year ended December 31, 2023 combines (i) the audited consolidated statement of operations of TuHURA for the year ended December 31, 2023, as derived from its historical financial statements, and (ii) the unaudited condensed consolidated statement of operations of Kintara for the year ended December 31, 2023 as calculated by (a) adding the unaudited interim condensed consolidated statement of operations of Kintara for the six months ended December 31, 2023, as filed on Kintara’s Form 10-Q with the SEC on February 14, 2024, to (b) the unaudited condensed consolidated statement of operations of Kintara for the six months ended June 30, 2023, as calculated by subtracting the unaudited interim condensed consolidated statement of operations of Kintara for the six months ended December 31, 2022, as filed on Kintara’s Form 10-Q with the SEC on February 14, 2023 from the audited consolidated statement of operations of Kintara for the year ended June 30, 2023, as filed on Kintara’s Form 10-K with the SEC on September 18, 2023, on a pro forma basis as if the Merger and related transactions had been consummated on January 1, 2023.
Such unaudited pro forma financial information has been prepared on a basis consistent with the financial statements of TuHURA, as TuHURA has been determined to be the accounting acquirer. This information should be read together with the financial statements of Kintara and TuHURA and related notes thereto, the sections titled “Kintara Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “TuHURA Management’s Discussion and Analysis of Financial Condition and Results of Operations” and other information included elsewhere in Kintara’s filings with the SEC, including the Merger Agreement and the descriptions of certain terms thereof set forth in the section titled the “Nasdaq Proposal” or “Proposal No. 1” in Kintara’s proxy
statement/prospectus dated August 13, 2024 and filed with the SEC on August 19, 2024 and this Current Report on Form 8-K.
The Merger is accounted for as a reverse recapitalization, in accordance with GAAP. Under this method of accounting, Kintara will be treated as the “acquired” company for financial reporting purposes. Accordingly, the Merger will be treated as the equivalent of TuHURA issuing stock for the net assets of Kintara, accompanied by a recapitalization. The net assets of Kintara will be stated at historical cost, with no goodwill or other intangible assets recorded. There will be no accounting effect or change in the carrying amount of the assets and liabilities as a result of the recapitalization.
TuHURA has been determined to be the accounting acquirer in the Merger for financial reporting purposes based on evaluation of the following facts and circumstances with regard to the combined company immediately after the Closing, including: (i) former TuHURA securityholders own approximately 96.0% of the Kintara Common Stock outstanding immediately following the Effective Time, (ii) TuHURA designated four of the five initial members of the board of directors of the combined company, (iii) TuHURA’s current senior management holds both (two of two) positions in the senior management of the combined company and (iv) TuHURA represents a significant majority of operations of the combined company. Total assets held by TuHURA and Kintara as of June 30, 2024 were $14,093 thousand and $6,202 thousand, respectively, as noted below, and included cash and cash equivalents held by TuHURA of $12,311 thousand and cash and cash equivalents of $4,909 thousand held by Kintara at June 30, 2024. After the Closing, the combined operations will be primarily TuHURA’s operations with the focus mainly on TuHURA’s in-process research and development assets. As a result of TuHURA being treated as the acquiring company for financial reporting purposes, the historical financial statements of TuHURA are the historical consolidated financial statements of the combined company. It is noted that the Kintara chief executive officer is not an assumed employee of the surviving company and as such is not part of the assembled workforce of the surviving company. Kintara is in the process of launching the REM-001 Study (defined below), which is a second-generation PDT photosensitizer agent, and is designed to test the 0.8 mg dose as well as optimize the study design in advance of a Phase 3 trial initiation. In addition, Kintara does not believe that the registrational study of VAL-083 is suitable for further development.
As noted in the CVR Agreement, the combined company is contractually obligated to use commercially reasonable efforts (see Note 1) until December 31, 2025 to achieve the Milestone. TuHURA anticipates the successful enrollment of the ten CMBC patients and that such patients will complete the required follow-up in accordance with the CVR Agreement. The CVR is not contingent on any future outcome of the study, clinical trials, commercialization, or economic benefit to be derived from the REM-001 Study. TuHURA’s management has concluded that it is probable that the Milestone of the Kintara legacy clinical studies pursuant to the CVR Agreement will be achieved and the CVR Shares will be issued. The REM-001 Study is currently in the early stages of the study process with no clinical trials passed or proven efficacy. Once 10 patients are enrolled and tracked in this study to determine whether a lower dose of REM-001 has an acceptable safety profile and elicits a treatment effect similar to that seen in prior REM-001 studies, the combined company expects to enroll the remaining patients and complete the NIH-funded trial and thereafter evaluate whether the REM-001 technology has potential future value that could be realized by the combined company. However, TuHURA currently anticipates no significant value derived from any in-process research and development assets of Kintara as of the Merger. Other than the REM-001 Study, TuHURA does not currently expect a restart or to advance any legacy Kintara technologies acquired. See Note 1 to the Notes to the Unaudited Pro Forma Condensed Financial Statements for the background and impact related to the potential issuance of the CVR Shares.
The unaudited pro forma condensed combined balance sheet as of June 30, 2024 combines the historical balance sheets of TuHURA and Kintara on a pro forma basis as if the Merger and related transactions had been consummated on June 30, 2024. The unaudited pro forma condensed combined statements of operations for the six months ended June 30, 2024 and for the year ended December 31, 2023 give pro forma effect to the Merger and related transactions as if they had occurred on January 1, 2023, the beginning of the earliest period presented. TuHURA and Kintara have
not had any historical operating relationship prior to the Merger. Accordingly, no pro forma adjustments were required to eliminate activities between the companies. These unaudited pro forma condensed combined financial statements are for informational purposes only. They do not purport to indicate the results that would have been obtained had the Merger and related transactions actually been completed on the assumed date or for the periods presented, or which may be realized in the future. The pro forma adjustments are based on the information currently available and the assumptions and estimates underlying the pro forma adjustments are described in the accompanying notes. Actual results may differ materially from the assumptions within the accompanying unaudited pro forma condensed combined financial information.
Description of the Merger Agreement, the TuHURA Note Financing, Exclusivity Agreement and July 2024 Private Placement
Merger Agreement
On April 2, 2024, Kintara, Merger Sub, and TuHURA entered into the Merger Agreement, pursuant to which, among other things, Merger Sub merged with and into TuHURA at the Effective Time, with TuHURA continuing as a wholly owned subsidiary of Kintara, and TuHURA Biosciences, Inc. being the surviving corporation of the Merger. At the closing of the Merger, the corporate name of Kintara was changed to “TuHURA Biosciences, Inc.”
At Effective Time, (i) each outstanding share of TuHURA Common Stock (other than any shares held in treasury and Dissenting Shares (as defined in the Merger Agreement)) was converted into shares of Kintara Common Stock equal to the Exchange Ratio (which is calculated to be 0.1789 following the effect of the 1-35 reverse share split for purposes of these unaudited pro forma condensed combined financial statements), (ii) each outstanding TuHURA Option was assumed and converted into an option to purchase shares of Kintara Common Stock and (iii) each outstanding TuHURA Warrant was assumed and converted into and exchangeable for a warrant of like tenor entitling the holder to purchase shares of Kintara Common Stock.
Immediately after the Merger, on a pro forma basis, pre-Merger TuHURA stockholders owned approximately 96.0% of the combined company, pre-Merger Kintara stockholders owned approximately 4.0% of the combined company (excluding in each such case the effect of out-of-the-money options and warrants of Kintara that remain outstanding after the Merger). The Exchange Ratio was equal to the quotient obtained by dividing (a) TuHURA Merger Shares by (b) TuHURA Outstanding Shares, as those terms are defined and further described in the Merger Agreement, which had the effect and purpose of determining the number of shares to be issued to pre-Merger TuHURA stockholders (or issuable to pre-Merger TuHURA option and warrant holders in respect of such options and warrants) based on the relative valuations and fully-diluted shares of each of Kintara and TuHURA as of immediately prior to the closing of the Merger. For purposes of calculating the Exchange Ratio, (i) shares of Kintara Common Stock underlying Kintara stock options and warrants outstanding as of immediately prior to the closing of the Merger with an exercise price per share of less than or equal to $0.20 (subject to adjustment pursuant to the Merger Agreement) were deemed to be outstanding and (ii) all shares of TuHURA Common Stock underlying outstanding TuHURA preferred stock, TuHURA Options, and TuHURA Warrants were deemed to be outstanding.
Based on the pre-Merger and post-Merger modification of the fair values of the options and warrants there are no material differences identified or noted.
TuHURA Note Financing
On December 1, 2023 (the “Initial Closing”), TuHURA’s board of directors approved the private offering of convertible promissory notes to certain accredited investors in an aggregate principal amount of up to $15 million to be used primarily to fund TuHURA’s clinical development plan and general corporate expenses (the “Convertible Debt”). The convertible promissory notes bore simple interest at a rate of 20% per annum, which was computed on the basis of a 365-day year (each a “Note,” and together, the “Notes”).
On March 29, 2024, TuHURA’s board of directors approved increasing the aggregate principal amount of the Convertible Debt to $35 million as well as that in the event a holder subscribes to purchase Notes in the aggregate principal amount of $4.0 million or more, then such holders would will be granted a TuHURA Warrant to purchase TuHURA Common Stock equal to (i) 50% of the aggregate principal amount of the Note purchased by such holder divided by (ii) $0.68. The threshold for obtaining TuHURA Warrants in connection with the TuHURA Note Financing includes principal amounts issued prior to March 29, 2024, as well as subsequent Note issuances.
The TuHURA Warrants issued in the TuHURA Note Financing may be exercised by such holder at any time within three years of the issuance date. The exercise price for one share of TuHURA Common Stock under such TuHURA Warrant is $1.02, payable in cash.
All outstanding principal and accrued but unpaid interest under the Notes was automatically converted into shares of TuHURA Common Stock immediately prior to the consummation of the Merger.
Kineta Exclusivity Agreement and July 2024 Private Placement
On July 8, 2024, Kintara and TuHURA issued a press release announcing that TuHURA has entered into an Exclusivity and Right of First Offer Agreement (the “Exclusivity Agreement”) with Kineta, Inc. (“Kineta”) for the potential acquisition of Kineta’s KVA12123 anti-VISTA antibody and related rights and assets associated with and derived from the asset.
KVA12123 is a rationally targeted, anti-VISTA antibody checkpoint inhibitor designed to reverse VISTA immune suppression and remodel the tumor microenvironment (TME) to overcome acquired resistance to immunotherapies.
Pursuant to the Exclusivity Agreement, among other things, Kineta has granted TuHURA an exclusive right to acquire Kineta’s worldwide patents, patent rights, patent applications, product and development program assets, technical and business information, and other rights and assets associated with and derived from its development program related to KVA12123 during the period commencing as of July 3, 2024 (the “Effective Date”) and continuing through the first to occur of (a) the execution of any Definitive Agreement (as defined therein) with respect to a Potential Transaction (as defined therein) by TuHURA or one or more of its affiliates and (b) 11:59 PM Eastern Time on October 1, 2024, subject to extension as noted in the following sentence (the “Exclusivity Period”). In the event that the parties are engaged in good faith discussions regarding a Potential Transaction on the date on which the Exclusivity Period (or any renewal thereof) is scheduled to expire and TuHURA has not yet closed the transactions contemplated by the Merger Agreement, then on such date, the Exclusivity Period shall automatically renew for an additional ten (10) day period (a “Renewal Period”) (up to a total of two (2) Renewal Periods for an aggregate of twenty (20) days).
Under the terms of the Exclusivity Agreement, TuHURA paid Kineta a fee in the amount of $5,000,000, with $2,500,000 paid at signing and an additional $2,500,000 paid on July 15, 2024. The fee is nonrefundable other than in the case of an uncured breach of the Exclusivity Agreement by Kineta. No later than two (2) business days after a Renewal Period has started (to be confirmed in writing by both parties), TuHURA shall pay an additional $150,000 as an additional Exclusivity Payment, in an amount not to exceed $300,000 for the two (2) available Renewal Periods. On October 4, 2024, TuHURA paid Kineta $150,000 as an additional Exclusivity Payment for the first Renewal Period. On October 15, 2024, TuHURA paid Kineta $150,000 as an additional Exclusivity Payment for the second Renewal Period. The Exclusivity Payments will be credited against the initial cash consideration that may be payable to Kineta pursuant to any Definitive Agreement (if any) between Kineta and TuHURA and/or its affiliates with respect to a Potential Transaction.
In conjunction with the Exclusivity Agreement, TuHURA sold 4,009,623 shares of its common stock in a private offering with a purchase price of $5,000,000 (the “July Private Placement”) to an existing TuHURA shareholder (the “Investor”). In connection with the July Private Placement, the Investor is entitled to a 1.5% royalty on certain sales by TuHURA of products based on KVA12123 as set forth in the Investor’s subscription agreement. Due to no definitive transaction agreement with Kineta for the purchase of KVA12123 and the inherent uncertainties surrounding the regulatory approval of KVA12123 and future monetization, TuHURA has not allocated any of the $5,000,000 purchase price consideration to the royalty agreement.
The accounting treatment for the Exclusivity Agreement and the July Private Placement is preliminary in nature and the final accounting treatment will be determined based on a number of factors, including additional analysis of the transaction and consideration of relevant accounting standards.
UNAUDITED PRO FORMA CONDENSED COMBINED BALANCE SHEET
AS OF JUNE 30, 2024
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
Kintara (Historical) |
|
TuHURA (Historical) |
|
Additional Financings |
|
Pro Forma Adjustments |
|
Pro Forma Combined |
ASSETS |
|
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ 4,909 |
|
$ 12,311 |
|
$ 9,500 |
A |
$ (5,300) |
C |
$ 22,043 |
|
|
|
|
|
|
4,700 |
B |
(4,077) |
D |
|
Deferred offering costs |
|
— |
|
920 |
|
— |
|
(920) |
D |
— |
Prepaid expenses and other current assets |
|
414 |
|
407 |
|
— |
|
— |
|
821 |
Clinical trial deposit |
|
205 |
|
— |
|
— |
|
— |
|
205 |
Total current assets |
|
5,528 |
|
13,638 |
|
14,200 |
|
(10,297) |
|
23,069 |
Property and equipment, net |
|
674 |
|
149 |
|
— |
|
— |
|
823 |
Right of use lease asset |
|
— |
|
272 |
|
— |
|
— |
|
272 |
Other noncurrent assets |
|
— |
|
34 |
|
— |
|
— |
|
34 |
Total assets |
|
$ 6,202 |
|
$ 14,093 |
|
$ 14,200 |
|
$ (10,297) |
|
$ 24,198 |
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIT) |
|
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
|
Accounts payable and accrued expenses |
|
$ 2,207 |
|
$ 2,600 |
|
$ — |
|
$ (196) |
D |
$ 4,611 |
Derivative liability |
|
— |
|
2,007 |
|
877 |
A |
(2,884) |
G |
— |
Related party payables |
|
52 |
|
— |
|
— |
|
— |
|
52 |
Lease liability, current |
|
— |
|
150 |
|
— |
|
— |
|
150 |
Total current liabilities |
|
2,259 |
|
4,757 |
|
877 |
|
(3,080) |
|
4,813 |
Long-term liabilities |
|
|
|
|
|
|
|
|
|
|
Milestone payment liabilities |
|
186 |
|
— |
|
— |
|
— |
|
186 |
Convertible note payable, net |
|
— |
|
16,169 |
|
6,200 |
A |
(22,369) |
G |
— |
Lease liability, long term |
|
— |
|
125 |
|
— |
|
— |
|
125 |
Total long-term liabilities |
|
186 |
|
16,294 |
|
6,200 |
|
(22,369) |
|
311 |
Total liabilities |
|
$ 2,445 |
|
$ 21,051 |
|
$ 7,077 |
|
$ (25,449) |
|
$ 5,124 |
|
|
|
|
|
|
|
|
|
|
|
Stockholders' Equity (Deficit): |
|
|
|
|
|
|
|
|
|
|
Preferred stock issued and outstanding 279 Series A shares |
|
279 |
|
— |
|
— |
|
(279) |
E |
— |
Preferred stock issued and outstanding 14 Series C shares |
|
9,973 |
|
— |
|
— |
|
(9,973) |
E |
— |
Preferred stock |
|
— |
|
8 |
|
— |
|
(8) |
G |
— |
Common stock |
|
55 |
|
7 |
|
4 |
B |
(55) |
E |
42 |
|
|
|
|
|
|
|
|
31 |
G |
|
Additional paid-in capital |
|
153,305 |
|
91,609 |
|
2,423 |
A |
(973) |
D |
126,742 |
|
|
|
|
|
|
4,696 |
B |
10,307 |
E |
|
|
|
|
|
|
|
|
|
(159,855) |
F |
|
|
|
|
|
|
|
|
|
25,230 |
G |
|
Accumulated deficit |
|
(159,876) |
|
(98,582) |
|
— |
|
(3,828) |
D |
(107,710) |
|
|
|
|
|
|
— |
|
159,876 |
F |
|
|
|
|
|
|
|
|
|
(5,300) |
C |
|
Accumulated other comprehensive income |
|
21 |
|
— |
|
— |
|
(21) |
F |
— |
|
|
|
|
|
|
|
|
|
|
|
Total stockholders' equity (deficit) |
|
3,757 |
|
(6,958) |
|
7,123 |
|
15,152 |
|
19,074 |
Total liabilities and stockholders' equity (deficit) |
|
$ 6,202 |
|
$ 14,093 |
|
$ 14,200 |
|
$ (10,297) |
|
$ 24,198 |
See accompanying notes to the unaudited pro forma condensed combined financial statements.
UNAUDITED PRO FORMA CONDENSED COMBINED STATEMENT OF OPERATIONS
FOR THE SIX MONTHS ENDED JUNE 30, 2024
(in thousands, except share and per share amounts)
|
|
|
|
|
|
|
|
|
Kintara Historical |
|
TuHURA Historical |
|
Pro Forma Adjustments |
|
Pro Forma Combined |
Operating expenses: |
|
|
|
|
|
|
|
Research and development expenses |
693 |
|
6,412 |
|
— |
|
7,105 |
Acquired in-process research and development |
— |
|
— |
|
— |
|
— |
General and administrative expenses |
3,777 |
|
1,812 |
|
— |
|
5,589 |
Total operating expenses |
4,470 |
|
8,224 |
|
— |
|
12,694 |
|
|
|
|
|
|
|
|
Loss from operations |
(4,470) |
|
(8,224) |
|
— |
|
(12,694) |
|
|
|
|
|
|
|
|
Other income (expense): |
|
|
|
|
|
|
|
Interest income (expense), net |
135 |
|
(1,548) |
|
1,613 |
AA |
200 |
Change in fair value of derivative liability associated with make-whole premium |
— |
|
(335) |
|
335 |
BB |
— |
Total other income (loss) |
135 |
|
(1,883) |
|
1,948 |
|
200 |
Net loss |
$ (4,335) |
|
$ (10,107) |
|
$ 1,948 |
|
$ (12,494) |
|
|
|
|
|
|
|
|
Net loss per share: |
|
|
|
|
|
|
|
Net loss |
(4,335) |
|
(10,107) |
|
|
|
|
Series A Preferred cash dividend |
(4) |
|
— |
|
|
|
|
Series C Preferred cash dividend |
— |
|
— |
|
|
|
|
Net loss for the period attributable to common stockholders |
$ (4,339) |
|
$ (10,107) |
|
|
|
|
Basic and fully diluted loss per share |
$ (0.16) |
|
$ (0.13) |
|
|
|
|
Basic and fully diluted weighted average number of shares |
26,352,189 |
|
80,561,229 |
|
|
|
|
Net loss per share - basic and diluted |
|
|
|
|
|
|
$ (0.30) |
Weighted average shares outstanding - basic and diluted(1) |
|
|
|
|
|
|
42,030,363 |
(1) Based on the 1-35 reverse share split effected at the discretion of Kintara’s Board of Directors immediately prior to the consummation of the Merger on October 18, 2024.
UNAUDITED PRO FORMA CONDENSED COMBINED STATEMENT OF OPERATIONS
FOR THE SIX MONTHS ENDED JUNE 30, 2024
(in thousands, except share and per share amounts)
See accompanying notes to the unaudited pro forma condensed combined financial statements.
UNAUDITED PRO FORMA CONDENSED COMBINED STATEMENT OF OPERATIONS
FOR THE YEAR ENDED DECEMBER 31, 2023
(in thousands, except share and per share amounts)
|
|
|
|
|
|
|
|
|
Kintara Historical |
|
TuHURA Historical |
|
Pro Forma Adjustments |
|
Pro Forma Combined |
Operating expenses: |
|
|
|
|
|
|
|
Research and development expenses |
6,051 |
|
9,402 |
|
5,300 |
CC |
20,753 |
Acquired in-process research and development |
— |
|
16,218 |
|
— |
|
16,218 |
General and administrative expenses |
4,581 |
|
4,145 |
|
3,828 |
DD |
12,554 |
Total operating expenses |
10,632 |
|
29,765 |
|
9,128 |
|
49,525 |
|
|
|
|
|
|
|
|
Loss from operations |
(10,632) |
|
(29,765) |
|
(9,128) |
|
(49,525) |
|
|
|
|
|
|
|
|
Other income (expense): |
|
|
|
|
|
|
|
Foreign exchange |
(9) |
|
— |
|
— |
|
(9) |
Employee Retention Tax Credit |
— |
|
334 |
|
— |
|
334 |
Grant income |
— |
|
42 |
|
— |
|
42 |
Interest income, net |
57 |
|
71 |
|
19 |
EE |
147 |
Total other income |
48 |
|
447 |
|
19 |
|
514 |
Net loss |
$ (10,584) |
|
$ (29,318) |
|
$ (9,109) |
|
$ (49,011) |
|
|
|
|
|
|
|
|
Net loss per share: |
|
|
|
|
|
|
|
Net loss |
(10,584) |
|
(29,317) |
|
|
|
|
Series A Preferred cash dividend |
(8) |
|
— |
|
|
|
|
Series C Preferred cash dividend |
(173) |
|
— |
|
|
|
|
Net loss for the period attributable to common stockholders |
$ (10,765) |
|
$ (29,317) |
|
|
|
|
Basic and fully diluted loss per share |
$ (4.56) |
|
$ (0.36) |
|
|
|
|
Basic and fully diluted weighted average number of shares |
2,361,952 |
|
80,561,229 |
|
|
|
|
Net loss per share - basic and diluted |
|
|
|
|
|
|
$ (1.17) |
Weighted average shares outstanding - basic and diluted(1) |
|
|
|
|
|
|
42,030,363 |
(1) Based on the 1-35 reverse share split effected at the discretion of Kintara’s Board of Directors immediately prior to the consummation of the Merger on October 18, 2024.
UNAUDITED PRO FORMA CONDENSED COMBINED STATEMENT OF OPERATIONS
FOR THE YEAR ENDED DECEMBER 31, 2023
(in thousands, except share and per share amounts)
See accompanying notes to the unaudited pro forma condensed combined financial statements.
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
Note 1. Description of Transactions
Merger Transaction
On April 2, 2024, Kintara entered into the Merger Agreement with Merger Sub and TuHURA. Pursuant to the terms of the Merger Agreement, a combination of Kintara and TuHURA was effected through the merger of Merger Sub with and into TuHURA, with TuHURA continuing as a wholly owned subsidiary of Kintara.
At the Effective Time, all shares of TuHURA Common Stock outstanding immediately prior to the Effective Time (after giving effect to the conversion of TuHURA preferred stock and excluding certain excluded and dissenting shares) were converted into and became exchangeable for approximately 54.4 million shares issued in completion of the Merger in the aggregate, under the reverse share split of 1-35 effected by the Board of Directors on October 18, 2024, of the then-issued and outstanding Kintara Common Stock based on an Exchange Ratio calculated as follows:
|
|
|
1-35 reverse share split |
(a) TuHURA's estimated ownership of Merger Shares post-merger on a fully-diluted basis |
54,423,998 |
(b) TuHURA's pre-merger outstanding shares on a fully-diluted basis |
304,226,947 |
Estimated Exchange Ratio: Equal to (a) divided by (b) |
0.1789 |
The Exchange Ratio was calculated by dividing (a) Company Merger Shares by (b) the Company Outstanding Shares (each as defined in the Merger Agreement), which had the effect and purpose of determining the number of shares of Kintara Common Stock to be issued to pre-Merger TuHURA stockholders (or issuable to pre-Merger TuHURA Option and TuHURA Warrant holders in respect of such options and warrants) based on the relative valuations and fully-diluted shares of each of Kintara and TuHURA as of immediately prior to the closing of the Merger. For purposes of calculating the Exchange Ratio, (i) shares of Kintara Common Stock underlying Kintara stock options and warrants outstanding as of immediately prior to the closing of the Merger with an exercise price per share of less than or equal to $0.20 were deemed to be outstanding and (ii) all shares of TuHURA Common Stock underlying outstanding TuHURA preferred stock, TuHURA Options, and TuHURA Warrants were deemed to be outstanding.
Based on the relative valuations, there is no material difference between the fair value and cash value of the options and warrants and as such, they are presented at cash value on the unaudited pro forma condensed combined financial statements.
After taking into account the conversion of the Convertible Debt, immediately after the Merger, pre-Merger TuHURA stockholders owned approximately 96.0% of the combined company and pre-Merger Kintara stockholders owned approximately 4.0% of the combined company. The unaudited pro forma condensed combined financial information were prepared giving effect to the reverse share split that took place immediately prior to the closing of the Merger of the then-issued and outstanding shares of Kintara Common Stock (1-35 reverse share split):
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
|
|
|
|
|
Shares (after 1-35 reverse share split)(1)(2)(3)(4) |
|
Approx. % |
TuHURA existing shareholders |
40,439,947 |
|
96.0 % |
Kintara existing public stockholders |
1,590,416 |
|
4.0 % |
Pro forma Common Stock |
42,030,363 |
|
100.0 % |
|
|
|
|
(1) Includes (i) 13,460,559 shares issued to historical TuHURA common stockholders, (ii) 14,552,460 shares issued to historical TuHURA preferred stockholders and 2,500,315 shares included within the preferred dividends, and (iii) 9,926,612 shares issued to holders of TuHURA convertible notes to be converted upon closing of the Merger. |
(2) Excludes (i) 3,313,383 shares underlying the options issued to TuHURA stockholders, (ii) 7,307,878 shares underlying the warrants issued to TuHURA stockholders and (iii) 3,362,789 shares underlying the warrants issued to TuHURA Note holders. |
(3) Includes (i) 1,590,302 shares issued to historical Kintara common stockholders, and (ii) 114 shares issued to holders of the Kintara restricted stock units that vested upon closing of the Merger. |
(4) Excludes 1,539,918 shares underlying the CVR Agreement. |
TuHURA Note Financing
On the Initial Closing, TuHURA’s board of directors approved the private offering of the Convertible Debt to certain accredited investors in an aggregate principal amount of up to $15 million to be used primarily to fund TuHURA’s clinical development plan and general corporate expenses. The Notes bore simple interest at a rate of 20% per annum, which was computed on the basis of a 365-day year.
On March 29, 2024, TuHURA’s board of directors approved increasing the aggregate principal amount of the Convertible Debt to $35 million as well as that in the event a holder subscribes to purchase Notes in the aggregate principal amount of $4.0 million or more, then such holders would be granted a TuHURA Warrant to purchase TuHURA Common Stock equal to (i) 50% of the aggregate principal amount of the Note purchased by such holder divided by (ii) $0.68. The threshold for obtaining TuHURA Warrants in connection with the TuHURA Note Financing includes principal amounts issued prior to March 29, 2024, as well as subsequent Note issuances.
The TuHURA Warrants issued in the TuHURA Note Financing may be exercised by such holder at any time within three years of the issuance date. The exercise price for one share of TuHURA Common Stock under such TuHURA Warrant is $1.02, payable in cash.
All outstanding principal and accrued but unpaid interest under the Notes was automatically converted into shares of TuHURA Common Stock immediately prior to the consummation of the Merger.
Contingent Value Rights Agreement
Kintara entered into the CVR Agreement with the Rights Agent, on October 18, 2024, pursuant to which holders of record of Kintara Common Stock and Kintara Common Stock warrants, in each case, immediately prior to the effected reverse share split, received one CVR for each outstanding share of Kintara Common Stock held by such stockholder (or, in the case of the warrants, each share of Kintara Common Stock for which such warrant was exercisable. Each CVR entitled the holder thereof to receive its portion of 53,897,125 CVR Shares (or 1,539,918 shares after the effect of the 1-35 reverse share split) if Kintara achieves the Milestone.
The issuance of the CVR Shares is solely based on conducting a study of REM-001 with a certain number of participants and duration and is not contingent on any future outcome of the study, clinical trials, commercialization, or economic benefit to be derived from REM-001. TuHURA is not obligated to develop REM-001 besides using commercially reasonable efforts to achieve the Milestone and commercial reasonable efforts shall not require
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
TuHURA to expend monetary resources in excess of $700,000 after taking into account the amount Kintara reasonably believes it is eligible for and will be reimbursed (or already reimbursed) by $2 million in NIH grants under Federal Award Number 1R44CA281615-01.
TuHURA has determined that any in-process research and development assets of Kintara potentially remaining as of the Merger would not have significant value when compared to the gross assets obtained through the Merger and, other than completing the NIH-funded 15-patient REM-001 Study as described above, TuHURA does not intend to start up development efforts for any of Kintara’s legacy clinical studies following the Merger. However, TuHURA anticipates the successful enrollment of the ten CMBC patients and that such patients will complete the required follow-up. Based on these factors, TuHURA’s management has concluded that it is probable that the Milestone of the Kintara legacy clinical studies pursuant to the CVR Agreement will be achieved and the CVR shares to be issued.
Based on management’s analysis, the CVRs were identified as freestanding financial instruments and determined to be indexed to Kintara’s own stock, as they are to be settled in Kintara Common Stock. Further, the CVR financial instruments are not mandatorily redeemable as the instruments do not require Kintara to redeem them for cash or other assets at a fixed or determinable date, or upon an event that is certain to occur and the CVRs do not represent an unconditional obligation requiring Kintara to redeem the instruments. The CVRs do not represent outstanding shares of Kintara Common Stock, and the CVRs do not obligate Kintara to buy back some or all of its shares. As such, the CVRs are not precluded from being classified within equity. Given the CVRs are initially being recorded within Equity, if the CVR Milestone were to be achieved, the Company would issue additional Common Stock, thereby resulting in a reclass of the CVRs from Additional paid-in capital - CVRs to Common Stock and Additional paid-in capital. As a result, the accounting for the CVR is determined to have zero net effect on total equity within the unaudited pro forma condensed combined balance sheet as of June 30, 2024.
Equity-classified contracts are initially measured at fair value (or allocated value). Subsequent changes in fair value are not recognized if the contracts continue to be classified in equity. Kintara estimated the valuation of the CVR arrangement. Since the Milestone is based on ten participants in the REM-001 study and 8 weeks of follow-up, management determined that the achievement of the Milestone is probable at the time of the filing of this registration statement. The Merger Agreement specifies achievement of the Milestone will result in the issuance of the CVR Shares. Kintara leveraged the fair value level 1 input of the closing price of Kintara’s Common Stock on October 17, 2024, prior to the effect of the 1-35 reverse share split, of $0.2154 multiplied by 53,897,125 shares resulting in an estimated valuation of the CVR Shares of approximately $11,609,441.
Kineta Exclusivity Agreement and July 2024 Private Placement
On July 8, 2024, Kintara and TuHURA issued a press release announcing that TuHURA has entered into the Exclusivity Agreement with Kineta for the potential acquisition of Kineta’s KVA12123 anti-VISTA antibody and related rights and assets associated with and derived from the asset.
KVA12123 is a rationally targeted, anti-VISTA antibody checkpoint inhibitor designed to reverse VISTA immune suppression and remodel the tumor microenvironment (TME) to overcome acquired resistance to immunotherapies.
Pursuant to the Exclusivity Agreement, among other things, Kineta has granted TuHURA an exclusive right to acquire Kineta’s worldwide patents, patent rights, patent applications, product and development program assets, technical and business information, and other rights and assets associated with and derived from its development program related to KVA12123, during the period commencing as of the Effective Date and continuing through the first to occur of (a) the execution of any Definitive Agreement with respect to a Potential Transaction by TuHURA or one or more of its affiliates and (b) Exclusivity Period. In the event that the parties are engaged in good faith discussions regarding a Potential Transaction on the date on which the Exclusivity Period (or any renewal thereof) is scheduled to expire and TuHURA has not yet closed the transactions contemplated by the Merger Agreement then on
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
such date, the Exclusivity Period shall automatically renew for Renewal Period (up to a total of two (2) Renewal Periods for an aggregate of twenty (20) days).
Under the terms of the Exclusivity Agreement, TuHURA paid Kineta a fee in the amount of $5,000,000, with $2,500,000 paid at signing and, subject to certain provisions, an additional $2,500,000 paid on July 15, 2024. The fee is nonrefundable other than in the case of an uncured breach of the Exclusivity Agreement by Kineta. No later than two (2) business days after a Renewal Period has started (to be confirmed in writing by both parties), TuHURA shall pay an additional $150,000 as an additional Exclusivity Payment, in an amount not to exceed $300,000 for the two (2) available Renewal Periods. On October 4, 2024, TuHURA paid Kineta $150,000 as an additional Exclusivity Payment for the first Renewal Period. On October 15, 2024, TuHURA paid Kineta $150,000 as an additional Exclusivity Payment for the second Renewal Period. The Exclusivity Payments will be credited against the initial cash consideration that may be payable to Kineta pursuant to any Definitive Agreement (if any) between Kineta and TuHURA and/or its affiliates with respect to a Potential Transaction.
In conjunction with the Exclusivity Agreement, TuHURA sold 4,009,623 of shares of its common stock with a purchase price of $5,000,000 in the July Private Placement to the Investor. In connection with the July Private Placement, the Investor is entitled to a 1.5% royalty on certain sales by TuHURA of products based on KVA12123 as set forth in the Investor’s subscription agreement. Due to no definitive transaction agreement with Kineta for the purchase of KVA12123 and the inherent uncertainties surrounding the regulatory approval of KVA12123 and future monetization, TuHURA has not allocated any of the $5,000,000 purchase price consideration to the royalty agreement.
On August 19, 2024, TuHURA and Kintara announced in a new press release that Kineta has reopened enrollment in its ongoing VISTA-101 Phase 1/2 clinical trial. Kineta and TuHURA are cooperating on the reinitiation of patient enrollment into this trial during TuHURA’s due diligence period with respect to the KVA12123 assets. 30 of a projected 39 patients have been enrolled in the clinical trial to date, including a monotherapy arm with KVA12123 and a combination arm utilizing KVA12123 together with Merck’s anti-PD1 therapy, KEYTRUDA® (pembrolizumab).
To date, KVA12123 has cleared the fifth of six monotherapy dose levels and two of the four cohorts in combination with Merck's anti-PD1 therapy, KEYTRUDA® (pembrolizumab). Initial results demonstrating partial response and stable disease in the combination cohorts, and durable stable disease observed in monotherapy cohorts, were reported earlier this year at the American Association of Cancer Research (AACR) Annual Meeting 2024. Additionally, the initial results of KVA12123 showed a favorable clinical safety and tolerability profile with no dose limiting toxicities and no evidence of cytokine release syndrome (CRS)-associated cytokines at any dose level.
The accounting treatment for the Exclusivity Agreement and the July Private Placement is preliminary in nature and the final accounting treatment will be determined based on a number of factors, including additional analysis of the transaction and consideration of relevant accounting standards.
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
Note 2. Basis of Presentation
The Merger was accounted as a reverse recapitalization, where the assets and liabilities of Kintara are recorded at their carrying values, with no goodwill or other intangible assets recorded, in accordance with GAAP. Under this method of accounting, Kintara was treated as the “accounting acquiree” and TuHURA as the “accounting acquirer” for financial reporting purposes. The determination of TuHURA as the accounting acquirer was primarily based on the evaluation of the following facts and circumstances:
•The pre-combination equity holders of TuHURA hold the majority of voting rights after the Merger,
•TuHURA holds four of the five board seats after the Merger,
•Executive management of TuHURA comprises the executive management after the Merger, and
•Operations of TuHURA comprise the ongoing operations after the Merger.
Accordingly, for accounting purposes, the Merger was treated as the equivalent of TuHURA issuing shares for the net assets of Kintara, followed by a recapitalization. The net assets of TuHURA are stated at historical cost. Operations prior to the Merger will be those of TuHURA.
The unaudited pro forma condensed combined balance sheet as of June 30, 2024 gives effect to the Merger and related transactions as if they occurred on June 30, 2024. The unaudited pro forma condensed combined statements of operations for the six months ended June 30, 2024 and for the year ended December 31, 2023 give effect to the Merger and related transactions as if they occurred on January 1, 2023. These periods are presented on the basis that TuHURA is the acquirer for accounting purposes.
The pro forma adjustments reflecting the consummation of the Merger and the related transaction are based on certain currently available information and certain assumptions and methodologies that TuHURA management believes are reasonable under the circumstances. The unaudited condensed combined pro forma adjustments, which are described in the accompanying notes, may be revised as additional information becomes available and is evaluated. Therefore, it is likely that the actual adjustments will differ from the pro forma adjustments, and it is possible that the difference may be material. TuHURA management believes that its assumptions and methodologies provide a reasonable basis for presenting all of the significant effects of the Merger and the related transactions based on information available to management at this time and that the pro forma adjustments give appropriate effect to those assumptions and are properly applied in the unaudited pro forma condensed combined financial information.
The unaudited pro forma condensed combined financial information does not give effect to any anticipated synergies, operating efficiencies, tax savings, or cost savings that may be associated with the Merger. The unaudited pro forma condensed combined financial information is not necessarily indicative of what the actual results of operations and financial position would have been had the Merger and related transactions taken place on the dates indicated, nor are they indicative of the future consolidated results of operations or financial position of the post-combination company. They should be read in conjunction with the separate historical unaudited financial statements and notes thereto of Kintara and TuHURA included elsewhere in the filings of Kintara with the SEC.
Immediately prior to the Effective Time, TuHURA preferred stock was converted into TuHURA Common Stock that was subsequently converted into and was exchanged for shares of Kintara Common Stock issued upon completion of the Merger at the Effective Time and in accordance with the Merger Agreement and the Exchange Ratio.
To the extent there are significant changes to the business following completion of the Merger, the assumptions and estimates set forth in the unaudited pro forma condensed combined financial statements could change significantly. Accordingly, the pro forma adjustments are subject to further adjustments as additional information
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
becomes available and as additional analyses are conducted following the completion of the Merger. There can be no assurances that these additional analyses will not result in material changes to the estimates.
The unaudited pro forma condensed combined financial information does not reflect the income tax effects of the pro forma adjustments as any change in the deferred tax balance would be offset by an increase in the valuation allowance given that TuHURA incurred significant losses during the historical periods presented.
The pro forma basic and diluted loss per share amounts presented in the unaudited pro forma condensed combined statements of operations are based upon the number of the combined company’s common shares outstanding following the reverse share split of 1-35 as effected on October 18, 2024, and assuming the Merger occurred on January 1, 2023.
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
Note 3. Accounting Polices
Management will perform a comprehensive review of the two entities’ accounting policies. As a result of the review, management may identify differences between the accounting policies of the two entities which, when conformed, could have a material impact on the financial statements of the post-combination company. Based on its initial analysis, management did not identify any differences that would have a material impact on the unaudited pro forma condensed combined financial information. As a result, the unaudited pro forma condensed combined financial information does not assume any differences in accounting policies.
Note 4. Adjustments to Unaudited Pro Forma Condensed Combined Financial Information
The pro forma adjustments were based on the preliminary information available at the time of the preparation of the unaudited pro forma condensed combined financial information. The unaudited pro forma condensed combined financial information, including the notes thereto, are qualified in their entirety by reference to, and should be read in conjunction with, the separate historical unaudited financial statements of TuHURA and Kintara included elsewhere in the filings of Kintara with the SEC.
The unaudited pro forma condensed combined financial information has been prepared to illustrate the effect of the Merger Agreement and related transactions and has been prepared for informational purposes only.
The following unaudited pro forma condensed combined financial information has been prepared in accordance with Article 11 of Regulation S-X. The unaudited pro forma condensed combined financial information has been prepared to illustrate the effect of the Business Combination and related transactions and has been prepared for informational purposes only. The Company includes additional financing transactions and transaction accounting adjustments in the unaudited pro forma condensed combined financial information as if they had occurred as of June 30, 2024.
The pro forma basic and diluted loss per share amounts presented in the unaudited pro forma condensed combined statements of operations are based upon the number of the combined company’s common shares outstanding following the reverse share split of 1-35 as effected on October 18, 2024, and assuming the Merger occurred on January 1, 2023.
TuHURA Note Financing
On the Initial Closing, TuHURA’s board of directors approved the private offering of the Convertible Debt to certain accredited investors in an aggregate principal amount of up to $15 million to be used primarily to fund TuHURA’s clinical development plan and general corporate expenses. The Notes bore simple interest at a rate of 20% per annum, which was computed on the basis of a 365-day year.
On March 29, 2024, TuHURA’s board of directors approved increasing the aggregate principal amount of the Convertible Debt to $35 million as well as that in the event a holder subscribes to purchase Notes in the aggregate principal amount of $4.0 million or more, then such holders would be granted a TuHURA Warrant to purchase TuHURA Common Stock equal to (i) 50% of the aggregate principal amount of the Note purchased by such holder divided by (ii) $0.68. The threshold for obtaining TuHURA Warrants in connection with the TuHURA Note Financing includes principal amounts issued prior to March 29, 2024, as well as subsequent Note issuances.
The TuHURA Warrants issued in the TuHURA Note Financing may be exercised by such holder at any time within three years of the issuance date. The exercise price for one share of TuHURA Common Stock under such TuHURA Warrant is $1.02, payable in cash.
All outstanding principal and accrued but unpaid interest under the Notes was automatically converted into shares of TuHURA Common Stock immediately prior to the consummation of the Merger.
Kineta Exclusivity Agreement and July 2024 Private Placement
On July 8, 2024, Kintara and TuHURA issued a press release announcing that TuHURA has entered into the Exclusivity Agreement with Kineta for the potential acquisition of Kineta’s KVA12123 anti-VISTA antibody and related rights and assets associated with and derived from the asset.
Pursuant to the Exclusivity Agreement, among other things, Kineta has granted TuHURA an exclusive right to acquire Kineta’s worldwide patents, patent rights, patent applications, product and development program assets,
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
technical and business information, and other rights and assets associated with and derived from its development program related to KVA12123, Kinteta’s VISTA blocking immunotherapy, during the period commencing as of the Effective Date and continuing through the first to occur of (a) the execution of any Definitive Agreement with respect to a Potential Transaction by TuHURA or one or more of its affiliates and (b) Exclusivity Period. In the event that the parties are engaged in good faith discussions regarding a Potential Transaction on the date on which the Exclusivity Period (or any renewal thereof) is scheduled to expire and TuHURA has not yet closed the transactions contemplated by the Merger Agreement then on such date, the Exclusivity Period shall automatically renew for Renewal Period (up to a total of two (2) Renewal Periods for an aggregate of twenty (20) days).
Under the terms of the Exclusivity Agreement, TuHURA paid Kineta a nonrefundable amount of $5,000,000, with $2,500,000 paid at signing and, subject to certain provisions, an additional $2,500,000 paid on July 15, 2024. No later than two (2) business days after a Renewal Period has started (to be confirmed in writing by both parties), TuHURA shall pay an additional $150,000 as an additional Exclusivity Payment, in an amount not to exceed $300,000 for the two (2) available Renewal Periods. On October 4, 2024, TuHURA paid Kineta $150,000 as an additional Exclusivity Payment for the first Renewal Period. On October 15, 2024, TuHURA paid Kineta $150,000 as an additional Exclusivity Payment for the second Renewal Period. The Exclusivity Payments will be credited against the initial cash consideration that may be payable to Kineta pursuant to any Definitive Agreement (if any) between Kineta and TuHURA and/or its affiliates with respect to a Potential Transaction.
In conjunction with the Exclusivity Agreement, TuHURA sold 4,009,623 shares of its common stock with a purchase price of $5,000,000 in the July Private Placement to the Investor. In connection with the July Private Placement, the Investor is entitled to a 1.5% royalty on certain sales by TuHURA of products based on KVA12123 as set forth in the Investor’s subscription agreement. Due to no definitive transaction agreement with Kineta for the purchase of KVA12123 and the inherent uncertainties surrounding the regulatory approval of KVA12123 and future monetization, TuHURA has not allocated any of the $5,000,000 purchase price consideration to the royalty agreement.
On August 19, 2024, TuHURA and Kintara announced in a new press release that Kineta has reopened enrollment in its ongoing VISTA-101 Phase 1/2 clinical trial. Kineta and TuHURA are cooperating on the reinitiation of patient enrollment into this trial during TuHURA’s due diligence period with respect to the KVA12123 assets. 30 of a projected 39 patients have been enrolled in the clinical trial to date, including a monotherapy arm with KVA12123 and a combination arm utilizing KVA12123 together with Merck’s anti-PD1 therapy, KEYTRUDA® (pembrolizumab).
To date, KVA12123 has cleared the fifth of six monotherapy dose levels and two of the four cohorts in combination with Merck's anti-PD1 therapy, KEYTRUDA® (pembrolizumab). Initial results demonstrating partial response and stable disease in the combination cohorts, and durable stable disease observed in monotherapy cohorts, were reported earlier this year at the American Association of Cancer Research (AACR) Annual Meeting 2024. Additionally, the initial results of KVA12123 showed a favorable clinical safety and tolerability profile with no dose limiting toxicities and no evidence of cytokine release syndrome (CRS)-associated cytokines at any dose level.
The accounting treatment for the Exclusivity Agreement and the July 2024 Private Placement is preliminary in nature and the final accounting treatment will be determined based on a number of factors, including additional analysis of the transaction and consideration of relevant accounting standards.
Adjustments related to the Additional Financing Transactions to Unaudited Pro Forma Condensed Combined Balance Sheet
The pro forma adjustments for additional financing transactions represent significant transactions completed by TuHURA and Kintara subsequent to June 30, 2024 as follows:
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
A.Represents anticipated cash proceeds of $9,500,000 in relation to the portion of the TuHURA signed subscription agreements totaling $31,253,000, of which $21,753,000 in aggregate subscriptions were funded as of June 30, 2024. The Company received the remaining cash proceeds of $9,500,000 during the third quarter of 2024; and the recording of the Convertible Debt and embedded features including, the fair value of the derivative liability related to the debt discount on the additional funding of $877,000, the fair value of warrants, attached to the debt, that are equity classified of $2,422,865, and the remaining value that was allocated to the debt liability of $6,200,135, pursuant to the financing transaction. The Convertible Debt will be converted to Merger Shares – see Adjustment G below.
B.Represents the issuance of 4,009,623 shares of TuHURA’s common stock, par value $0.001 per share, in the July 2024 Private Placement to the Investor, for proceeds of $5,000,000, less equity issuance costs of $300,000. The par value of $4,010 is recorded to Common stock and the net amount recorded within additional paid-in capital related to this issuance is $4,695,990.
Transaction Accounting Adjustments to Unaudited Pro Forma Condensed Combined Balance Sheet
The transaction accounting adjustments included in the unaudited pro forma condensed combined balance sheet as of June 30, 2024 are as follows:
C.Reflects the nonrefundable cash payments and associated expense, in connection with TuHURA’s payments of $5,000,000 for the exclusive right to acquire Kineta’s worldwide patents, patent rights, patent applications, product and development program assets, technical and business information, and other rights and assets associated with and derived from its development program related to KVA12123, Kineta’s VISTA blocking immunotherapy. On October 4, 2024, TuHURA paid Kineta $150,000 as an additional Exclusivity Payment for the first Renewal Period. On October 15, 2024, TuHURA paid Kineta $150,000 as an additional Exclusivity Payment for the second Renewal Period. The accounting treatment for the Exclusivity Agreement is preliminary in nature and the final accounting treatment will be determined based on a number of factors, including additional analysis of the transaction and consideration of relevant accounting standards.
D.Reflects (i) payment of total estimated unpaid transaction costs (including $527,248 recorded in TuHURA’s historical accounts payable as of June 30, 2024 and $1,573,000 recorded in Kintara’s historical accounts payable as of June 30, 2024) of $2,100,248, and (ii) payment of one-time special bonus costs upon consummation of the Merger of $327,030, and (iii) the accrual of additional transaction costs that will remain unpaid upon consummation of the Merger of $2,295,571. Approximately $972,928 of the payment was transaction costs incurred in consummating the Merger relate to the equity issuance, and as such are reflected as a reduction against proceeds in additional paid-in capital (net of the $527,248 already recorded as of June 30, 2024). In addition, the recognition of the deferred offering costs upon the closing of the Merger of $920,956 is reflected as a reduction against proceeds in additional paid-in capital. The effect on accumulated deficit amount consists of $2,295,571 in additional transaction expenses incurred between June 30, 2024 and closing and $327,030 for the one-time special bonus payment at the close of the Merger.
E.To record the elimination of Kintara’s historical equity carrying value.
F.To record the elimination of Kintara’s historical accumulated deficit and historical accumulated other comprehensive income.
G.To record the elimination of the historical TuHURA outstanding shares of 226,056,925 (including 71,136,072 common shares, 81,347,397 preferred shares, 13,976,616 preferred dividends to be paid by the
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
issuance of common shares, 4,009,623 shares included within the July 2024 Private Placement, 98,040 shares included within the issuance of common stock to Paulson, 55,489,176 shares upon the conversion of $22,242,770 of Convertible Debt and the elimination of the derivative liability for the conversion option included within the Convertible Debt of $2,884,000, all at par value of $0.0001; and the conversion of these shares at the Exchange Ratio of 0.1789 into 40,439,947 shares to be issued upon completion of the Merger, par value of $0.001 (after the effect of the 1-35 reverse share split) and record the conversion of historical shares of Kintara Common Stock (1,590,302 common shares and 114 shares in restricted stock units vesting) into 1,590,416 shares to be issued upon completion of the Merger, par value of $0.001 (after the effect of the 1-35 reverse share split).
Adjustments to Unaudited Pro Forma Condensed Combined Statements of Operations
The pro forma adjustments included in the unaudited pro forma condensed combined statement of operations for the six months ended June 30, 2024 are as follows:
AA. Reflects the reversal of interest expense incurred on the Convertible Debt for the six months ended June 30, 2024 of $1,612,610.
BB. Reflects the reversal of the Change in fair value of derivative liability associated with make-whole premium that is related to the signed subscription agreements for the six months ended June 30, 2024 of $(335,001).
The pro forma adjustments included in the unaudited pro forma condensed combined statement of operations for the year ended December 31, 2023 are as follows:
CC. Reflects the associated expense in connection with TuHURA’s nonrefundable payments of $5,000,000 for the exclusive right to acquire Kineta’s worldwide patents, patent rights, patent applications, product and development program assets, technical and business information, and other rights and assets associated with and derived from its development program related to KVA12123, Kinteta’s VISTA blocking immunotherapy. On October 4, 2024, TuHURA paid Kineta $150,000 as an additional Exclusivity Payment for the first Renewal Period. On October 15, 2024, TuHURA paid Kineta $150,000 as an additional Exclusivity Payment for the second Renewal Period.
DD. Reflects estimated transaction costs in the amount of $3,827,530 which includes (i) one-time special bonus costs upon consummation of the Merger in the amount of $327,030, (ii) estimated transaction costs incurred by Kintara of $5,073,500, net of (iii) $1,573,000 in transaction costs that have been expensed during the six months ended June 30, 2024, assumed expensed on January 1, 2023.
EE. Reflects the reversal of interest expense incurred on the Convertible Debt for the year ended December 31, 2023 of $18,688.
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
Note 5. Net Loss per Share
Net loss per share was calculated using the historical weighted average shares outstanding, and the issuance of additional shares in connection with the Merger, assuming the shares were outstanding since January 1, 2023. As the Merger is being reflected as if it had occurred at the beginning of the period presented, the calculation of weighted average shares outstanding for basic and diluted net loss per share assumes that the shares issuable relating to the Merger have been outstanding for the entirety of all periods presented.
The unaudited pro forma condensed combined financial information has been prepared to present the Merger Shares for the six months ended June 30, 2024 and for the year ended December 31, 2023 (in thousands, except share and per share amounts):
|
|
|
|
|
For the Six Months Ended June 30, 2024 (1) |
|
For the Year Ended December 31, 2023 (1) |
|
(1-35 reverse share split) |
|
(1-35 reverse share split) |
Numerator: |
|
|
|
Pro forma net loss |
$ (12,494) |
|
$ (49,011) |
Denominator: |
|
|
|
Weighted average shares outstanding - basic and diluted(2) |
42,030,363 |
|
42,030,363 |
Net loss per share: |
|
|
|
Pro forma net loss per share - basic and diluted |
$ (0.30) |
|
$ (1.17) |
Excluded securities: |
|
|
|
TuHURA Warrants(2) |
7,307,878 |
|
7,307,878 |
TuHURA Options(2) |
3,313,383 |
|
3,313,383 |
Kintara CVR Shares(2) |
1,539,918 |
|
1,539,918 |
|
|
|
|
(1) Pro forma net loss per share includes the related pro forma adjustments as referred to within the section “Unaudited Pro Forma Condensed Combined Financial Information.” |
(2) The potentially dilutive outstanding securities were excluded from the computation of pro forma net loss per share, basic and diluted, because their effect would have been anti-dilutive and/or issuance or vesting of such shares is contingent upon the satisfaction of certain conditions which were not satisfied by the end of the periods presented. |
v3.24.3
Document And Entity Information
|
Oct. 18, 2024 |
| Document Information [Line Items] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Oct. 18, 2024
|
| Entity Registrant Name |
Kintara Therapeutics, Inc.
|
| Entity Central Index Key |
0001498382
|
| Entity Emerging Growth Company |
false
|
| Entity File Number |
001-37823
|
| Entity Incorporation, State or Country Code |
NV
|
| Entity Tax Identification Number |
99-0360497
|
| Entity Address, Address Line One |
10500 University Dr.
|
| Entity Address, Address Line Two |
Suite 110
|
| Entity Address, City or Town |
Tampa
|
| Entity Address, State or Province |
FL
|
| Entity Address, Postal Zip Code |
33612
|
| Current Fiscal Year End Date |
--12-31
|
| City Area Code |
813
|
| Local Phone Number |
875-6600
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, $0.001 par value per share
|
| Trading Symbol |
HURA
|
| Security Exchange Name |
NASDAQ
|
| Former Address |
|
| Document Information [Line Items] |
|
| Entity Information, Former Legal or Registered Name |
Kintara Therapeutics, Inc.
|
| Entity Address, Address Line One |
9920 Pacific Heights Blvd
|
| Entity Address, Address Line Two |
Suite 150
|
| Entity Address, City or Town |
San Diego
|
| Entity Address, State or Province |
CA
|
| Entity Address, Postal Zip Code |
92121
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Grafico Azioni Kintara Therapeutics (NASDAQ:KTRA)
Storico
Da Dic 2024 a Gen 2025

Grafico Azioni Kintara Therapeutics (NASDAQ:KTRA)
Storico
Da Gen 2024 a Gen 2025
