Mesoblast Shares Rise 10% After New Info Submitted to FDA for Remestemcel-L
03 Ottobre 2022 - 7:13PM
Dow Jones News
By Chris Wack
Mesoblast Ltd. shares were up 10% to $2.80 after the company
said it submitted substantial new information to the U.S. Food and
Drug Administration on clinical and potency assay items identified
in a Complete Response Letter from FDA.
The company said the CRL was received in September 2020 in
response to the Biologics License Application for remestemcel-L in
the treatment of children with steroid-refractory acute graft
versus host disease.
Mesoblast has maintained an active dialog with the FDA since
receiving the CRL, and the new information submitted to the
Investigational New Drug file for remestemcel-L in the treatment of
children with SR-aGVHD, as guided by FDA, represents a major
milestone in its complete response to the FDA.
Remestemcel-L has been granted Fast Track Designation and BLA
Priority Review from the FDA.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
October 03, 2022 12:58 ET (16:58 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
Grafico Azioni Mesoblast (ASX:MSB)
Storico
Da Apr 2024 a Mag 2024
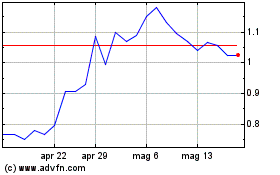
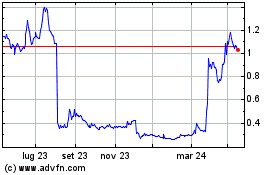
Grafico Azioni Mesoblast (ASX:MSB)
Storico
Da Mag 2023 a Mag 2024