Pleco Therapeutics receives FDA Orphan Drug Designation
16 Gennaio 2024 - 5:30PM

The U.S. Food and Drug Administration (FDA) has granted Orphan Drug
Designation to Pleco Therapeutics BV’s lead compound, PTX-252 for
the treatment of Acute Myeloid Leukaemia (AML). PTX-252 is a novel
molecular entity developed to increase the sensitivity of cancer
cells to chemotherapy.
The FDA’s designation acknowledges the potential
of PTX-252 in addressing AML, a rare blood cancer with a poor
prognosis. Orphan Drug Designation is granted to medicines that
treat or prevent life-threatening or chronically debilitating rare
disease, and with either no currently approved method of prevention
or treatment or with significant clinical benefit when compared
with existing treatments.
Orphan Drug Designation qualifies companies for
incentives, including tax credits for clinical trials, exemption of
user fees, and seven years of market exclusivity after
approval.
"We are very excited to receive this designation
for PTX-252 from the FDA," states Ivo Timmermans, Chief Executive
Officer of Pleco Therapeutics. "This milestone underscores our
commitment to innovative therapies for rare diseases and it brings
hope to AML patients who have limited treatment options. Our team
is dedicated to advancing this therapy through clinical development
as swiftly as possible."
In the development of the lead compound PTX-252,
Pleco has collaborated with Belgian company Hyloris Pharmaceuticals
SA (Euronext Brussels: HYL). Stijn Van Rompay, Chief Executive
Officer of Hyloris, comments: "Securing orphan drug designation for
PTX-252 stands as a testament to our unwavering commitment to
advancing the frontiers of scientific discovery within the
repurposing space."
For more information, please
contact:
Julie Powell, Commercial: M: +44 (0) 7774 827205
E: julie.powell@plecotherapeutics.com
Helen Pope, Media: M: +44 (0) 7879 818247 E:
helen@helenpopepr.co.uk
About Acute Myeloid Leukaemia
(AML)
AML is a heterogenous haematological malignancy
that originates from immature white blood cells (blasts) in the
bone marrow. AML generally spreads quickly to the bloodstream and
can then spread to other parts of the body including lymph nodes,
spleen, central nervous system, and testicles. AML is an orphan
disease, and it is the most common type of acute leukaemia in
adults. The median age of newly diagnosed AML patients is around 67
years. Worldwide the incidence of AML is estimated to be 350,000
cases per year (4.7 cases per 100,000 population, Globocan). In the
US, there were an estimated 20,050 new cases of AML in 2022 and
11,540 deaths. It can arise de novo or secondary to the progression
of other diseases or following treatment with cytotoxic agents.
About Pleco
Therapeutics
Pleco Therapeutics is a clinical stage specialty
biopharmaceutical company that aims to improve the survival rate of
cancer patients through its novel Plecoid™ therapies that increase
the effectiveness of current cancer treatments. A private company,
Pleco is headquartered in Nijmegen, the Netherlands, with a U.S.
subsidiary, Pleco Therapeutics USA Inc, based in Newark, New
York.
For more information about Pleco Therapeutics
and its work in rare diseases, including AML, visit
www.plecotherapeutics.comFollow us on LinkedIn:
https://www.linkedin.com/company/pleco-therapeutics-bv/
About Hyloris Pharmaceuticals
SA
Hyloris is a specialty biopharma company focused
on innovating, reinventing, and optimizing existing medications to
address important healthcare needs and deliver relevant
improvements for patients, healthcare professionals and payors.
Hyloris has built a broad, patented portfolio of 17 reformulated
and repurposed value-added medicines that have the potential to
offer significant advantages over available alternatives. Hyloris
is based in Liège, Belgium.
For more information, visit www.hyloris.com
Follow us on LinkedIn:
https://www.linkedin.com/company/hyloris-pharmaceuticals/
A photo accompanying this announcement is
available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/21824f03-e3b6-4adb-b65d-90899c54ce73
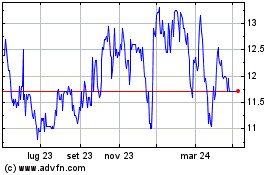
Grafico Azioni Hyloris Pharmaceuticals (EU:HYL)
Storico
Da Nov 2024 a Dic 2024
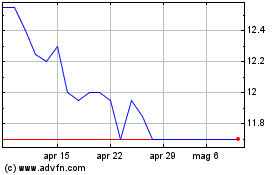
Grafico Azioni Hyloris Pharmaceuticals (EU:HYL)
Storico
Da Dic 2023 a Dic 2024