FDA Warns on Safety Issue With Philips CPAP Devices -- Update
29 Novembre 2023 - 12:39AM
Dow Jones News
By Ben Glickman
The U.S. Food and Drug Administration warned of an emerging
safety issue involving a continuous positive airway pressure, or
CPAP, machine made by Philips.
The FDA issued a safety communication about thermal issues with
the Philips Respironics' DreamStation 2 CPAP machines, which are
used to treat forms of sleep apnea, and recommended patients
monitor machines.
The agency said it had received reports of issues such as fire,
smoke, burns and other signs of overheating. The FDA said it is in
discussions with the company about strategies to address the safety
issue.
The FDA said that it noted a "sharp increase" in the number of
medical device reports related to the machines between Aug. 1 and
Nov. 15.
Philips said in a statement that its priority is patient safety
and quality, and that it filed reports with the FDA following a
three-year retrospective review of complaints linked to the
product.
The company said the devices can still be used if the safety
instructions for the product are followed.
The FDA said that it continues to monitor the Philips' handling
of a June 2021 recall involving sleep therapy and respirator
machines, which was related to a foam used in certain products.
The FDA said it does not believe the safety issue is related to
the foam used in the DreamStation 2.
Write to Ben Glickman at ben.glickman@wsj.com
(END) Dow Jones Newswires
November 28, 2023 18:24 ET (23:24 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
Grafico Azioni Koninklijke Philips NV (EU:PHIA)
Storico
Da Giu 2024 a Lug 2024
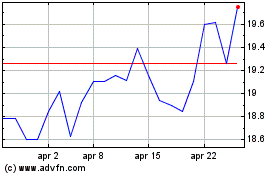
Grafico Azioni Koninklijke Philips NV (EU:PHIA)
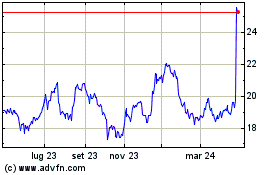
Storico
Da Lug 2023 a Lug 2024