Smith+Nephew takes centre court sponsoring players competing at Wimbledon; global Sports Medicine technology leader helping athletes get back in the game
26 Giugno 2024 - 9:00AM
UK Regulatory
Smith+Nephew takes centre court sponsoring players competing at
Wimbledon; global Sports Medicine technology leader helping
athletes get back in the game
Smith+Nephew (LSE:SN, NYSE:SNN), the global medical technology
company, today announces it will sponsor tennis players
participating in The Championships, Wimbledon 2024 to spotlight its
advanced Sports Medicine portfolio alongside some of the greatest
athletes in the world. Smith+Nephew endeavours to help people live
their ‘Life Unlimited’ by aiding in their recovery from injury and
get them back to doing what they love.

Click to play video - Smith+Nephew Proud
Player Sponsor at The Championships, Wimbledon
2024
Tennis is an elegant sport but can be rough on the body –
especially to a player’s joints. Two common injuries1,2
that tennis players suffer are rotator cuff tears in the shoulder
and meniscus tears in the knee. Smith+Nephew offers advanced
solutions for both of these injuries that are supported by clinical
evidence and time-tested results.
Smith+Nephew’s comprehensive Advanced Healing Solutions
portfolio is redefining biological healing in rotator cuff repair.
Backed by 10 years of clinical evidence and more than 100,000
procedures completed globally since its introduction in 2014, the
REGENETEN Bioinductive Implant has had a transformative impact,
offering a better solution for more than 1 million3
patients having surgery for a rotator cuff tear each year. The
collagen-based implant supports the body’s natural healing response
to facilitate the formation of new tendon-like tissue to
biologically augment the existing tendon and change the course of
rotator cuff tear progression.4-10
Smith+Nephew also has an established track record of success in
knee repair including a 30-year legacy of saving the meniscus
rather than removing it. One of the most frequently published
meniscal repair devices on the market, the FAST-FIX◊
Family of meniscal repair solutions has helped surgeons repair
hundreds of thousands11 of meniscal tears over the past
30 years due to its ease of use,12 high fixation
strength,*13-16 and strong, reliable
repairs.17-19 With an 88% mean success rate of
all-inside repairs,20 the FAST-FIX Family has truly
helped patients return to a Life Unlimited.
“Both Smith+Nephew and the players participating in Wimbledon
share a common thread as leaders in their respective fields and
being among the best at what they do,” said Christie Van Geffen,
Senior Vice President of Global Marketing for Sports Medicine at
Smith+Nephew. “It’s an honour to sponsor players competing in this
year’s Championship and we look forward to helping athletes all
over the world get back to performing at the highest level.”
If you would like to find out more about Smith+Nephew’s leading
Sports Medicine technology portfolio – including the REGENETEN
Bioinductive Implant and FAST-FIX Family of meniscal repair
solutions – please visit www.smith-nephew.com.
- ends –
Media Enquiries
Dave Snyder +1
(978) 749-1440
Smith+Nephew
david.snyder@smith-nephew.com
*As demonstrated in biomechanical testing
References
- Dines, JS et al. Tennis Injuries: Epidemiology,
Pathophysiology, and Treatment. J Am Acad Orth Surg.
2015;23(3):181-189
- Majewski M et al.Epidemiology of athletic knee injuries: A
10-year study. The Knee. 2006;13(3):184-188
- iData Research. Rotator cuff repair and reconstruction market
size, share and trends analysis (2023). Available at:
https://idataresearch.com/product/rotator-cuff-repair-reconstruction-market-size-share-and-trends-analysis-global-2023-2029-medsuite-includes-grafts-allografts-xenograft-synthetic-and-1-more/#.
Accessed December 19, 2023.
- Ruiz Ibán MA, Navlet MG, Marco SM, et al. Augmentation of a
transosseous equivalent repair in posterosuperior non-acute rotator
cuff tears with a bioinductive collagen implant decreases the
re-tear rate at one year. A randomised controlled trial.
Arthroscopy. Published online 12/27/2023.
- Bokor DJ, Sonnabend D, Deady L, et al. Evidence of healing of
partial-thickness rotator cuff tears following arthroscopic
augmentation with a collagen implant: a 2-year MRI follow-up.
Muscles, Ligaments Tendons J. 2016;6(1):16-25.
- Schlegel TF, Abrams JS, Bushnell BD, Brock JL, Ho CP.
Radiologic and clinical evaluation of a bioabsorbable collagen
implant to treat partial-thickness tears: a prospective multicenter
study. J Shoulder Elbow Surg. 2018 27(2):242-251.
- Van Kampen C, Arnoczky S, Parks P, et al. Tissue-engineered
augmentation of a rotator cuff tendon using a reconstituted
collagen scaffold: a histological evaluation in sheep. Muscles
Ligaments Tendons J. 2013;3(3):229-235.
- Arnoczky SP, Bishai SK, Schofield B, et al. Histologic
Evaluation of Biopsy Specimens Obtained After Rotator Cuff
Repair Augmented With a Highly Porous Collagen Implant.
Arthroscopy. 2017;33(2):278-283
- Bokor DJ, Sonnabend DH, Deady L, et al. Healing of
partial-thickness rotator cuff tears following arthroscopic
augmentation with a highly porous collagen implant: a 5-year
clinical and MRI follow-up. Muscles, Ligaments Tendons J.
2019;9(3):338-347.
- McElvany MD, McGoldrick E, Gee AO, Neradilek MB, Matsen FA,
3rd. Rotator cuff repair: published evidence on factors
associated with repair integrity and clinical outcome. Am J Sports
Med. 2015;43(2):491-500.
- Internal Smith+Nephew sales data from 2023 FY STGP RM
- Smith+Nephew 2015.Ver/Val, Protocol/Report, FAST-FIX 360
Delivery System. Internal Report. 15000994 Rev. E.
- Smith+Nephew 2004. Suture performance in standard arthroscopic
knots - Effects of material and design. Internal Report.
1061539 Rev. A.
- Smith+Nephew 2007. Internal Test Request - ULTRA FAST-FIX.
Internal Report. ITR-3457.
- Smith+Nephew 2010.The biomechanical performance of the FAST-FIX
360 meniscal repair system. Internal Report. DOF 10600596.
- Smith+Nephew 2007.Evaluation of the mechanical properties of
the ULTRA FAST-FIX meniscal repair system in a bovine meniscus
model. Internal Report. DOF 10600342
- Uzun E, Misir A, Kizkapan TB, et al. Evaluation of Midterm
Clinical and Radiographic Outcomes of Arthroscopically
Repaired Vertical Longitudinal and Bucket-Handle Lateral Meniscal
Tears. Orthop J Sports Med. 2019;7(5):1 - 8.
- Shearman A, Foster A, Wilson A, Risebury M, Yasen S. Excellent
medium-term survival of an all-inside tensionable knotted
suture device justifies repair of most meniscal tears encountered
during reconstructive knee ligament surgery. Knee Surg Sports
Traumatol Arthrosc. 2020:1-8.
- Laurendon L, Neri T, Farizon F, Philippot R. Prognostic factors
for all-inside meniscal repair. A 87-case series. OTSR.
2017;103(7):1017 - 1020.
- Johnson, D., Souter, P., Sedgwick, M.. Clinical Performance of
an All-inside Meniscal Repair Device: A Systematic Literature
Review with Meta-analysis. ISAKOS. 2023.
About Smith+Nephew
Smith+Nephew is a portfolio medical technology business focused
on the repair, regeneration and replacement of soft and hard
tissue. We exist to restore people’s bodies and their self-belief
by using technology to take the limits off living. We call this
purpose ‘Life Unlimited’. Our 18,000 employees deliver this mission
every day, making a difference to patients’ lives through the
excellence of our product portfolio, and the invention and
application of new technologies across our three global
business units of Orthopaedics, Sports Medicine & ENT and
Advanced Wound Management.
Founded in Hull, UK, in 1856, we now operate in more than 100
countries, and generated annual sales of $5.5 billion in 2023.
Smith+Nephew is a constituent of the FTSE100 (LSE:SN, NYSE:SNN).
The terms ‘Group’ and ‘Smith+Nephew’ are used to refer to Smith
& Nephew plc and its consolidated subsidiaries, unless the
context requires otherwise.
For more information about Smith+Nephew, please visit
www.smith-nephew.com and follow us on X, LinkedIn, Instagram
or Facebook.
Forward-looking Statements
This document may contain forward-looking statements that
may or may not prove accurate. For example, statements regarding
expected revenue growth and trading profit margins, market trends
and our product pipeline are forward-looking statements. Phrases
such as "aim", "plan", "intend", "anticipate", "well-placed",
"believe", "estimate", "expect", "target", "consider" and similar
expressions are generally intended to identify forward-looking
statements. Forward-looking statements involve known and unknown
risks, uncertainties and other important factors that could cause
actual results to differ materially from what is expressed or
implied by the statements. For Smith+Nephew, these factors include:
conflicts in Europe and the Middle East, economic and financial
conditions in the markets we serve, especially those affecting
healthcare providers, payers and customers; price levels for
established and innovative medical devices; developments in medical
technology; regulatory approvals, reimbursement decisions or other
government actions; product defects or recalls or other problems
with quality management systems or failure to comply with related
regulations; litigation relating to patent or other claims; legal
and financial compliance risks and related investigative, remedial
or enforcement actions; disruption to our supply chain or
operations or those of our suppliers; competition for qualified
personnel; strategic actions, including acquisitions and disposals,
our success in performing due diligence, valuing and integrating
acquired businesses; disruption that may result from transactions
or other changes we make in our business plans or organisation to
adapt to market developments; relationships with healthcare
professionals; reliance on information technology and
cybersecurity; disruptions due to natural disasters, weather and
climate change related events; changes in customer and other
stakeholder sustainability expectations; changes in taxation
regulations; effects of foreign exchange volatility; and numerous
other matters that affect us or our markets, including those of a
political, economic, business, competitive or reputational nature.
Please refer to the documents that Smith+Nephew has filed with the
U.S. Securities and Exchange Commission under the U.S. Securities
Exchange Act of 1934, as amended, including Smith+Nephew's most
recent annual report on Form 20-F, which is available on the SEC’s
website at www. sec.gov, for a discussion of certain of these
factors. Any forward-looking statement is based on information
available to Smith+Nephew as of the date of the statement. All
written or oral forward-looking statements attributable to
Smith+Nephew are qualified by this caution. Smith+Nephew does not
undertake any obligation to update or revise any forward-looking
statement to reflect any change in circumstances or in
Smith+Nephew's expectations.
◊ Trademark of Smith+Nephew. Certain
marks registered in US Patent and Trademark Office.


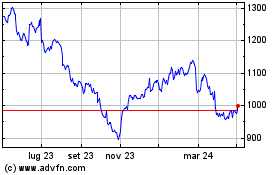
Grafico Azioni Smith & Nephew (LSE:SN.)
Storico
Da Dic 2024 a Gen 2025
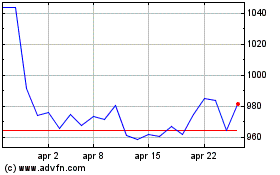
Grafico Azioni Smith & Nephew (LSE:SN.)
Storico
Da Gen 2024 a Gen 2025