false 0001744659 0001744659 2025-01-27 2025-01-27
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 27, 2025
Akero Therapeutics, Inc.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
001-38944 |
|
81-5266573 |
| (State or other jurisdiction |
|
(Commission |
|
(I.R.S. Employer |
| of incorporation) |
|
File Number) |
|
Identification No.) |
|
|
|
|
|
| 601 Gateway Boulevard, Suite 350 |
|
|
| South San Francisco, CA |
|
94080 |
| (Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code (650) 487-6488
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, par value $0.0001 per share |
|
AKRO |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 2.02 |
Results of Operation and Financial Condition. |
On January 27, 2025, Akero Therapeutics, Inc. (the “Company”) provided an update regarding the amount of cash, cash equivalents and marketable securities it had on hand as of December 31, 2024. Although the Company has not finalized its financial results for such period, the Company currently anticipates that its cash, cash equivalents and marketable securities were approximately $800 million as of December 31, 2024. This information is preliminary and unaudited and does not present all information necessary for an understanding of the Company’s financial condition as of December 31, 2024, and is subject to change upon completion of the Company’s financial statement closing procedures and the audit of the Company’s consolidated financial statements.
The information furnished under this Item 2.02 shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or incorporated by reference in any filing under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act, except as expressly set forth by specific reference in such filing.
| Item 7.01. |
Regulation FD Disclosure. |
On January 27, 2025, the Company issued a press release titled “Akero Therapeutics Reports Preliminary Topline Results Showing Statistically Significant Reversal of Compensated Cirrhosis (F4) Due to MASH—by Both Completer and ITT Analyses—at Week 96 in Phase 2b SYMMETRY Study.” A copy of the press release is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The Company from time to time presents and/or distributes to the investment community slide presentations to provide updates and summaries of its business. Copies of its SYMMETRY Study slide presentation and its corporate presentation are being furnished herewith as Exhibit 99.2 and Exhibit 99.3, respectively, to this Current Report on Form 8-K. The information under this Item 7.01, including Exhibit 99.1, Exhibit 99.2 and Exhibit 99.3 hereto, is being furnished herewith and shall not be deemed “filed” for the purposes of Section 18 of the Exchange Act, or otherwise subject to the liabilities of that section, nor shall such information be deemed incorporated by reference into any filing under the Securities Act or the Exchange Act, except as expressly set forth by specific reference in such filing.
On January 27, 2025, the Company released preliminary topline week 96 results from SYMMETRY, a Phase 2b study evaluating the efficacy and safety of its lead product candidate efruxifermin (“EFX”) in patients with biopsy-confirmed compensated cirrhosis (F4), Child-Pugh Class A, due to metabolic dysfunction-associated steatohepatitis (“MASH”). Among patients with baseline and week 96 biopsies (n=134), 39% of patients treated with 50mg EFX (n=46) (p=0.009) experienced reversal of cirrhosis with no worsening of MASH, compared to 15% for placebo (n=47). In the Intent to Treat (ITT) population (n=181), with all missing week 96 biopsies treated as failures, 29% of patients in the 50mg EFX group (n=63) (p=0.031) experienced reversal of cirrhosis with no worsening of MASH, compared to approximately 12% in the placebo group (n=61).
With more than a doubling of effect size from weeks 36 to 96 in the 50mg group (from 10% to 24%), the SYMMETRY study underscores the benefit of longer EFX treatment for patients with compensated cirrhosis (F4).
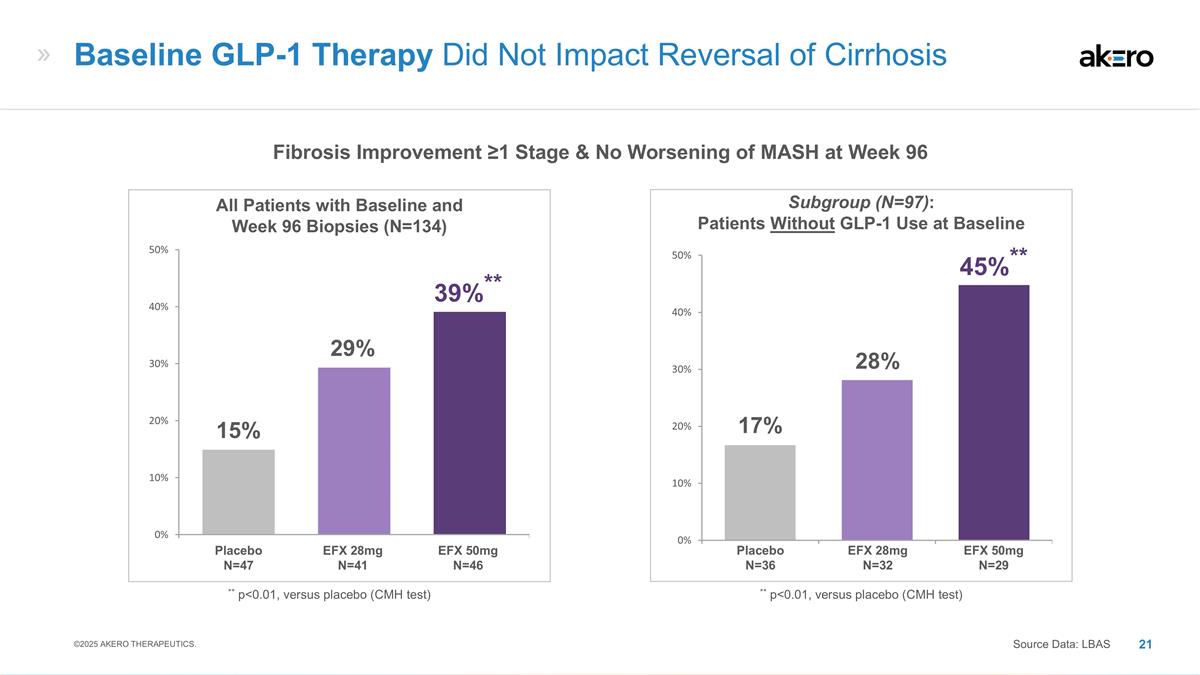
In a subgroup of patients with baseline and week 96 biopsies who were not taking GLP-1 at baseline (n=97), 45% in the 50mg EFX group experienced reversal of cirrhosis with no worsening of MASH (n=29) (p=0.009) compared to 17% for placebo (n=36), suggesting that the observed reversal of cirrhosis was not attributable to GLP-1 therapy.
Summary of Week 96 Reversal of Cirrhosis Endpoint
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Primary Analysis (N=134)1 |
|
|
ITT Analysis (N=181)2 |
|
| Histology Endpoint3 (Proportion of Patients) |
|
Placebo
(N=47) |
|
|
28mg
(N=41) |
|
|
50mg
(N=46) |
|
|
Placebo
(N=61) |
|
|
28mg
(N=57) |
|
|
50mg
(N=63) |
|
| ≥1 stage fibrosis improvement without worsening MASH (%) |
|
|
15 |
|
|
|
29 |
|
|
|
39 |
** |
|
|
12 |
|
|
|
21 |
|
|
|
29 |
* |
| 1 |
All patients with baseline and week 96 biopsies |
| 2 |
The 47 randomized and dosed patients who had missing biopsies at week 96 are treated as failures in the ITT analysis (without imputation) |
| 3 |
Biopsies scored independently by two pathologists; third available to adjudicate (which was not required) |
| * |
p<0.05, ** p<0.01, versus placebo (Cochran-Mantel-Haenszel test (“CMH”)) |
Summary of Week 96 Changes in Key Noninvasive Measures of Liver Fibrosis and Injury
|
|
|
|
|
|
|
|
|
|
|
|
|
| Measure (LS Mean Change From Baseline to Week 96) |
|
Placebo
(n=49) |
|
|
28mg
(n=40-41) |
|
|
50mg
(n=47) |
|
| ELF Score |
|
|
+0.22 |
|
|
|
-0.34 |
*** |
|
|
-0.53 |
*** |
| Liver Stiffness (%) (FibroScan) |
|
|
-8 |
|
|
|
-18 |
|
|
|
-24 |
* |
| ALT (U/L) |
|
|
-6.8 |
|
|
|
-10.5 |
|
|
|
-11.1 |
|
| AST (U/L) |
|
|
-1.6 |
|
|
|
-8.1 |
|
|
|
-11.2 |
** |
| * |
p<0.05, *** p<0.001, versus placebo (MMRM) |
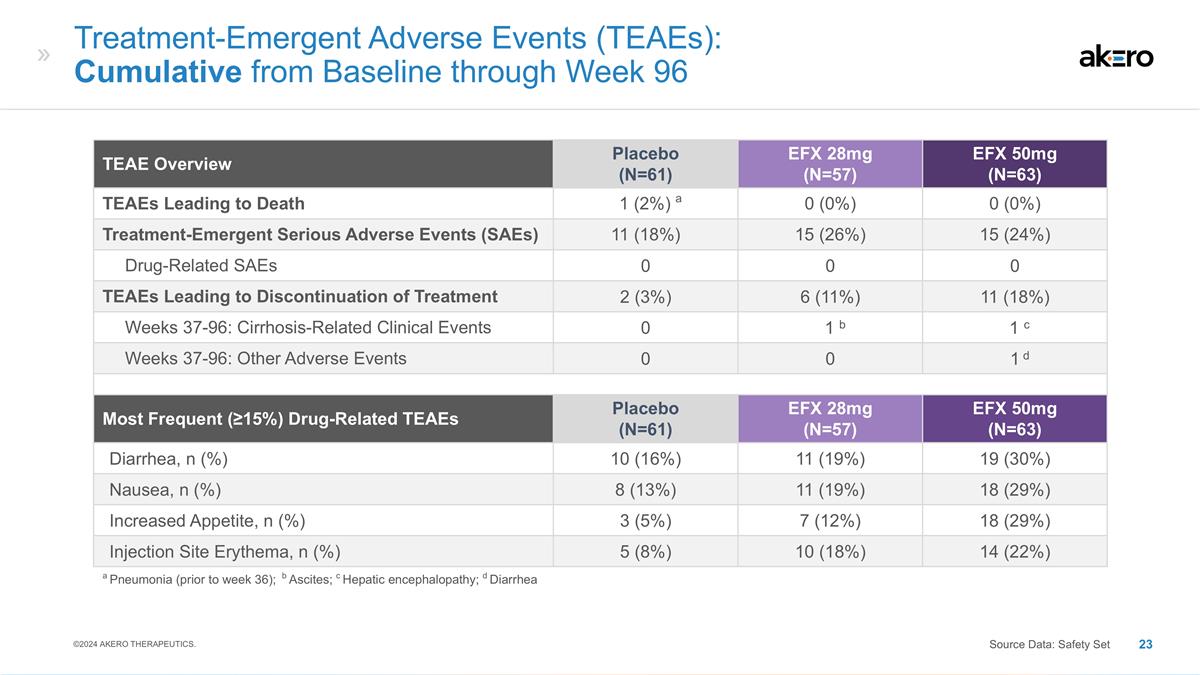
EFX was reported to be generally well-tolerated. There were no deaths on EFX, but one death in the placebo arm due to pneumonia. None of the Serious Adverse Events were determined to be related to study drug. Across both EFX groups, the most frequent adverse events (“AEs”) were grade 1 or 2, gastrointestinal in origin (diarrhea, nausea, and increased appetite) and transient in nature.
Forward-Looking Statements
This Current Report on Form 8-K and certain materials furnished or filed herewith contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, implied and express statements regarding: the Company’s business plans and objectives, including future plans or expectations for EFX and ongoing clinical studies, including the anticipated or potential therapeutic effects of EFX, as well as the dosing, safety and tolerability of EFX; and the expected cash, cash equivalents and marketable securities as of December 31, 2024.
Any forward-looking statements are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. Risks that contribute to the uncertain nature of the forward-looking statements include: the success, cost, and timing of the Company’s product candidate development activities and planned clinical trials; the Company’s ability to execute on its strategy; positive results from any of its clinical studies may not necessarily be predictive of the results of future or ongoing clinical studies; regulatory developments in the United States and foreign countries; the Company’s ability to fund operations. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause the Company’s actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in the Company’s annual report on Form 10-K filed, with the United States Securities and Exchange Commission (“SEC”) and quarterly reports on Form 10-Q filed with the SEC, as well as discussions of potential risks, uncertainties, and other important factors in the Company’s other filings with the SEC. All forward-looking statements contained in this presentation speak only as of the date on which they were made. The Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.
| Item 9.01. |
Financial Statements and Exhibits. |
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Exchange Act, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
| Date: January 27, 2025 |
|
AKERO THERAPEUTICS, INC. |
|
|
|
|
|
|
|
|
By: |
|
/s/ Andrew Cheng |
|
|
|
|
|
|
Andrew Cheng, M.D., Ph.D. |
|
|
|
|
|
|
President and Chief Executive Officer |
Exhibit 99.1
Akero Therapeutics Reports Preliminary Topline Results Showing Statistically Significant Reversal of Compensated Cirrhosis (F4) Due to
MASH—by Both Completer and ITT Analyses—at Week 96 in Phase 2b SYMMETRY Study
Among patients with baseline and week 96
biopsies, 39% of the 50mg EFX group (p=0.009) demonstrated ≥1 stage improvement in fibrosis with no worsening of MASH, representing a 24% effect size over placebo at 15%
By ITT analysis, with all missing week 96 biopsies treated as failures, 29% of the 50mg EFX group (p=0.031) demonstrated
≥1 stage improvement in fibrosis with no worsening of MASH, representing a 17% effect size over placebo at 12%
Investor webcast at 8:00 am ET Monday, January 27, 2025
SOUTH SAN FRANCISCO, Calif., January 27, 2025 — Akero Therapeutics, Inc. (Nasdaq: AKRO), a clinical-stage company developing transformational
treatments for patients with serious metabolic disease marked by high unmet medical need, today released preliminary topline week 96 results from SYMMETRY, a Phase 2b study evaluating the efficacy and safety of its lead product candidate
efruxifermin (EFX) in patients with biopsy-confirmed compensated cirrhosis (F4), Child-Pugh Class A, due to metabolic dysfunction-associated steatohepatitis (MASH). Among patients with baseline and week 96 biopsies (n=134), 39% of patients
treated with 50mg EFX (n=46) (p=0.009) experienced reversal of cirrhosis with no worsening of MASH, compared to 15% for placebo (n=47). In the Intent to Treat (ITT) population (n=181), with all missing week 96 biopsies treated as failures, 29% of
patients in the 50mg EFX group (n=63) (p=0.031) experienced reversal of cirrhosis with no worsening of MASH, compared to approximately 12% in the placebo group (n=61).
With more than a doubling of effect size from weeks 36 to 96 in the 50mg group (from 10% to 24%), the SYMMETRY study underscores the benefit of longer EFX
treatment for patients with compensated cirrhosis (F4).
In a subgroup of patients with baseline and week 96 biopsies who were not taking GLP-1 at baseline (n=97), 45% in the 50mg EFX group experienced reversal of cirrhosis with no worsening of MASH (n=29) (p=0.009) compared to 17% for placebo (n=36), suggesting that the observed reversal of cirrhosis
was not attributable to GLP-1 therapy.
“Until today, we’ve not had the prospect of an effective
treatment for compensated cirrhosis due to MASH, which is associated with high rates of short-term morbidity and mortality,” said Mazen Nourredin, M.D., Professor of Medicine and Transplant Hepatologist at Houston Methodist Hospital, and
principal investigator for the SYMMETRY study. “Now we have reason to be optimistic about the future potential of EFX as a much-needed treatment for cirrhosis, if approved. I’m so happy for my patients and patients all around the
world.”
Summary of Week 96 Reversal of Cirrhosis Endpoint
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Primary Analysis (N=134)1 |
|
|
ITT Analysis (N=181)2 |
|
| Histology
Endpoint3 (Proportion of Patients) |
|
Placebo
(N=47) |
|
|
28mg
(N=41) |
|
|
50mg
(N=46) |
|
|
Placebo
(N=61) |
|
|
28mg
(N=57) |
|
|
50mg
(N=63) |
|
| ≥1 stage fibrosis improvement without worsening MASH (%) |
|
|
15 |
|
|
|
29 |
|
|
|
39 |
** |
|
|
12 |
|
|
|
21 |
|
|
|
29 |
* |
| 1 |
All patients with baseline and week 96 biopsies |
| 2 |
The 47 randomized and dosed patients who had missing biopsies at week 96 are treated as failures in the ITT
analysis (without imputation) |
| 3 |
Biopsies scored independently by two pathologists; third available to adjudicate (which was not required)
|
| * |
p<0.05, ** p<0.01, versus placebo
(Cochran-Mantel-Haenszel test (CMH)) |
“We believe today’s first-ever public report of reversal of cirrhosis due to MASH,
whether by completer or ITT analysis, sets EFX apart from other approved or investigational treatments in the MASH landscape as a compound with transformational potential,” said Andrew Cheng, M.D., Ph.D., president and chief executive officer
of Akero. “We look forward to continuing evaluation of 50mg EFX in our ongoing Phase 3 SYNCHRONY Outcomes study in patients with compensated cirrhosis due to MASH.”
The reversal of cirrhosis, as quantified by a consensus of two histopathologists, is supported by improvements in noninvasive measures of liver fibrosis and
injury.
Summary of Week 96 Changes in Key Noninvasive Measures of Liver Fibrosis and Injury
|
|
|
|
|
|
|
|
|
|
|
|
|
| Measure (LS Mean Change From Baseline to Week 96) |
|
Placebo
(n=49) |
|
|
28mg
(n=40-41) |
|
|
50mg
(n=47) |
|
| ELF Score |
|
|
+0.22 |
|
|
|
-0.34 |
*** |
|
|
-0.53 |
*** |
| Liver Stiffness (%) (FibroScan) |
|
|
-8 |
|
|
|
-18 |
|
|
|
-24 |
* |
| ALT (U/L) |
|
|
-6.8 |
|
|
|
-10.5 |
|
|
|
-11.1 |
|
| AST (U/L) |
|
|
-1.6 |
|
|
|
-8.1 |
|
|
|
-11.2 |
** |
| * |
p<0.05, *** p<0.001, versus placebo (MMRM)
|
EFX was reported to be generally well-tolerated. There were no deaths on EFX, but one death in the placebo arm due to pneumonia. None
of the Serious Adverse Events were determined to be related to study drug. Across both EFX groups, the most frequent adverse events (AEs) were grade 1 or 2, gastrointestinal in origin (diarrhea, nausea, and increased appetite) and transient in
nature.
Conference Call / Webcast Details
Akero
will host a conference call and webcast with slide presentation at 8:00 a.m. ET today. The live webcast will be available on the Events & Presentations page of Akero’s website, with the recording and presentation available
immediately following the event.
About Cirrhosis Due to MASH
Cirrhosis due to MASH (metabolic dysfunction-associated steatohepatitis) is a life-threatening disease with high risk of liver failure, cancer and eventually
death. By 2030, an estimated 3 million Americans are projected to have MASH cirrhosis, which is the fastest growing cause of liver transplants and liver cancer in the United States and Europe.
About the SYMMETRY Study
The Phase 2b SYMMETRY study is a multicenter, randomized, double-blind, placebo-controlled, dose-ranging trial in adult patients with biopsy-confirmed
compensated cirrhosis (F4, Child-Pugh A) due to MASH. The study enrolled a total of 182 patients, randomized to receive once-weekly subcutaneous dosing of 28mg or 50mg EFX, or placebo for 36 weeks, 181 of whom received at least one study dose. The
primary efficacy endpoint for the study was the proportion of patients who achieve at least one-stage fibrosis improvement without worsening of MASH at week 36. Week 96 secondary measures included ≥1
stage fibrosis improvement and no worsening of MASH, MASH resolution, change from baseline in liver enzymes, noninvasive markers of liver fibrosis, glycemic control, and lipoproteins, as well as safety and tolerability measures.
About EFX
Efruxifermin (EFX), Akero’s lead product
candidate for MASH, is currently being evaluated in three ongoing Phase 3 studies. In multiple Phase 2 studies, EFX has been observed to reverse fibrosis (including compensated cirrhosis), resolve MASH, reduce
non-invasive markers of fibrosis and liver injury, and improve insulin sensitivity and lipoprotein profile. This holistic profile offers the potential to address the complex, multi-system disease state of all
stages of MASH, including improvements in lipoprotein risk factors linked to cardiovascular disease – the leading cause of death among MASH patients. Engineered to mimic the biological activity profile of native FGF21, EFX is designed to
offer convenient once-weekly dosing and has been generally well-tolerated in clinical trials to date.
About Akero Therapeutics
Akero Therapeutics is a clinical-stage company developing transformational treatments for patients with serious metabolic diseases marked by high unmet
medical need, including metabolic dysfunction-associated steatohepatitis (MASH). Akero’s lead product candidate, EFX, is currently being evaluated in three ongoing Phase 3 clinical studies: SYNCHRONY Histology in patients with pre-cirrhotic MASH (F2-F3 fibrosis), SYNCHRONY Outcomes in patients with compensated cirrhosis due to MASH, and SYNCHRONY Real-World in patients with MASH or
MASLD (Metabolic Dysfunction Associated Steatotic Liver Disease). The Phase 3 SYNCHRONY program builds on the results of two Phase 2b clinical trials, the HARMONY study in patients with pre-cirrhotic MASH and
the SYMMETRY study in patients with compensated cirrhosis due to MASH. Akero is headquartered in South San Francisco. Visit us at akerotx.com and follow us on LinkedIn and X for more information.
Forward Looking Statements
Statements contained in this
press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Because such statements are subject to risks and uncertainties,
actual results may differ materially from those expressed or implied by such forward-looking statements, including, but not limited to, statements regarding Akero’s business plans and objectives, including future plans or expectations for EFX
and ongoing clinical studies, the therapeutic effects of EFX, as well as the dosing, safety and tolerability of EFX; and the future potential of EFX following the preliminary topline week 96 results of Akero’s Phase 2b SYMMETRY study, which are
subject to audit and verification procedures and additional data that could result in material changes in the final data. Any forward-looking statements in this press release are based on management’s current expectations of future events and
are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. Risks that contribute to the uncertain nature of the
forward-
looking statements include:; the success, cost, and timing of Akero’s product candidate development activities and planned clinical trials; Akero’s ability to execute on its strategy;
positive results from a clinical study may not necessarily be predictive of the results of future or ongoing clinical studies; regulatory developments in the United States and foreign countries; Akero’s ability to fund operations; as well as
those risks and uncertainties set forth more fully under the caption “Risk Factors” in Akero’s most recent Annual Report on Form 10-K and Quarterly Report on Form
10-Q, as filed with the Securities and Exchange Commission (SEC) as well as discussions of potential risks, uncertainties and other important factors in Akero’s other filings and reports with the SEC. All
forward-looking statements contained in this press release speak only as of the date on which they were made. Akero undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which
they were made.
Investor Contact:
Christina
Tartaglia
Precision AQ
212.362.1200
christina.tartaglia@precisionaq.com
Media Contact:
Peg Rusconi
Deerfield Group
617.910.6217
Peg.rusconi@deerfieldgroup.com
Topline Preliminary Week 96 Results
From Phase 2b SYMMETRY Study in Patients with Compensated Cirrhosis (F4) Due to MASH January 27, 2025 Restoring balance. Renewing Life. Exhibit 99.2

Safe Harbor and Legal Disclaimers This
presentation may contain “forward-looking statements” of Akero Therapeutics, Inc. (“we,” “us,” “our,” “Akero” or the “Company”) within the meaning of the Private Securities
Litigation Reform Act of 1995 relating to our business, operations, and financial conditions, including but not limited to current express or implied beliefs, expectations, and assumptions regarding: the future of our business; future plans and
strategies, including our expectations around the therapeutic potential and clinical benefits of Efruxifermin (“EFX”); our development plans for EFX, including our belief in the unique potential of EFX as a foundational MASH therapy; our
preclinical and clinical results, including the week 96 results from our Phase 2b SYMMETRY study, which are preliminary, topline results that are subject to audit and verification procedures and additional data that could result in material changes
in the final data; the SYNCHRONY Phase 3 program, including the SYNCHRONY Histology, SYNCHRONY Real-World, and SYNCHRONY Outcomes studies, their respective trial designs; and risks related to the competitive landscape. Words such as, but not limited
to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should,” and “could,” and
similar expressions or words, identify forward-looking statements. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Except as required by law, we assume no obligation to update
these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. For a discussion of these
and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in our most recent
annual report on Form 10-K and quarterly report on Form 10-Q filed with the Securities and Exchange Commission (the “SEC”), as well as discussions of potential risks, uncertainties, and other important factors in our other subsequent
filings with the SEC. All information in this presentation is as of the date hereof, and we undertake no duty to update this information unless required by law. Certain information contained in this presentation relates to or is based on studies,
publications, surveys, and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not
independently verified, and makes no representation as to the adequacy, fairness, accuracy, or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of
assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source.
©2025 AKERO THERAPEUTICS.
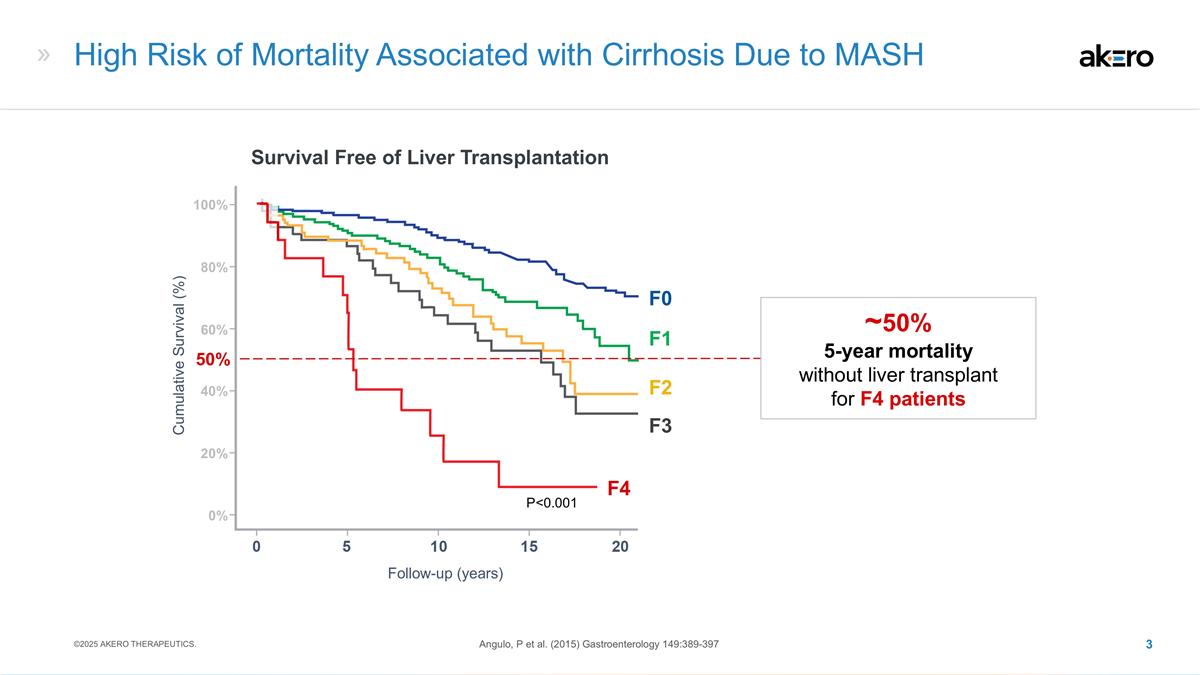
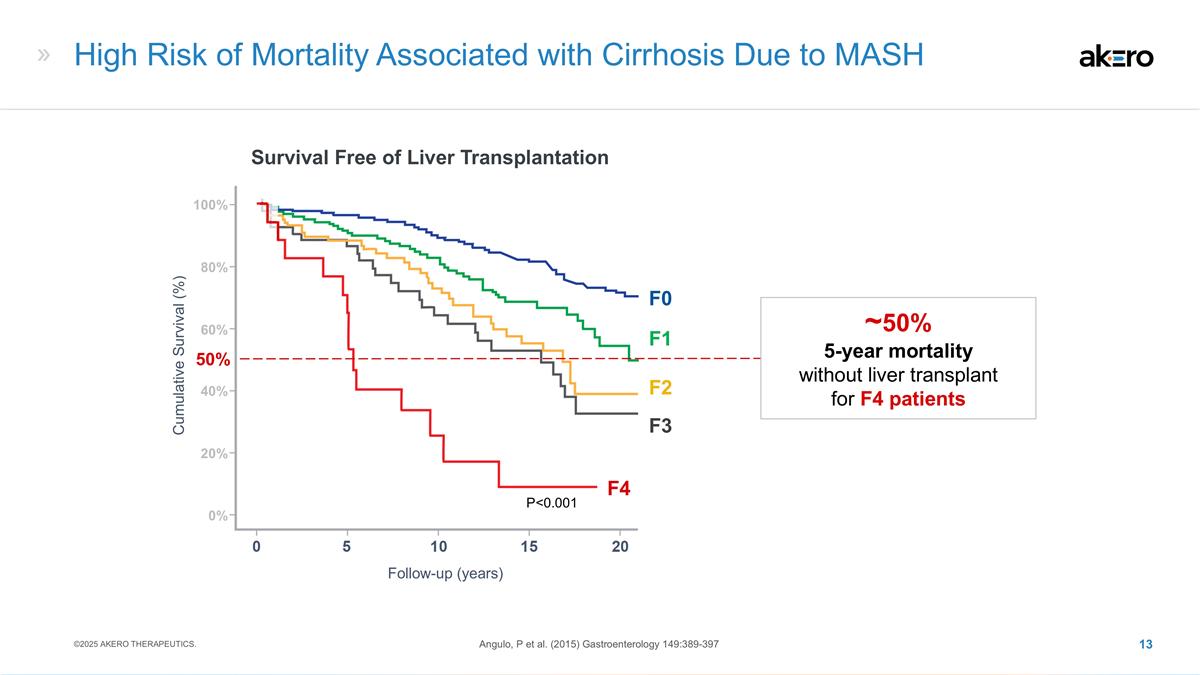
High Risk of Mortality Associated with
Cirrhosis Due to MASH Survival Free of Liver Transplantation Follow-up (years) Cumulative Survival (%) Angulo, P et al. (2015) Gastroenterology 149:389-397 P<0.001 F4 50% ~50% 5-year mortality without liver transplant for F4 patients 40% 20% 0%
100% 80% 60% ©2025 AKERO THERAPEUTICS. F3 F2 F1 F0
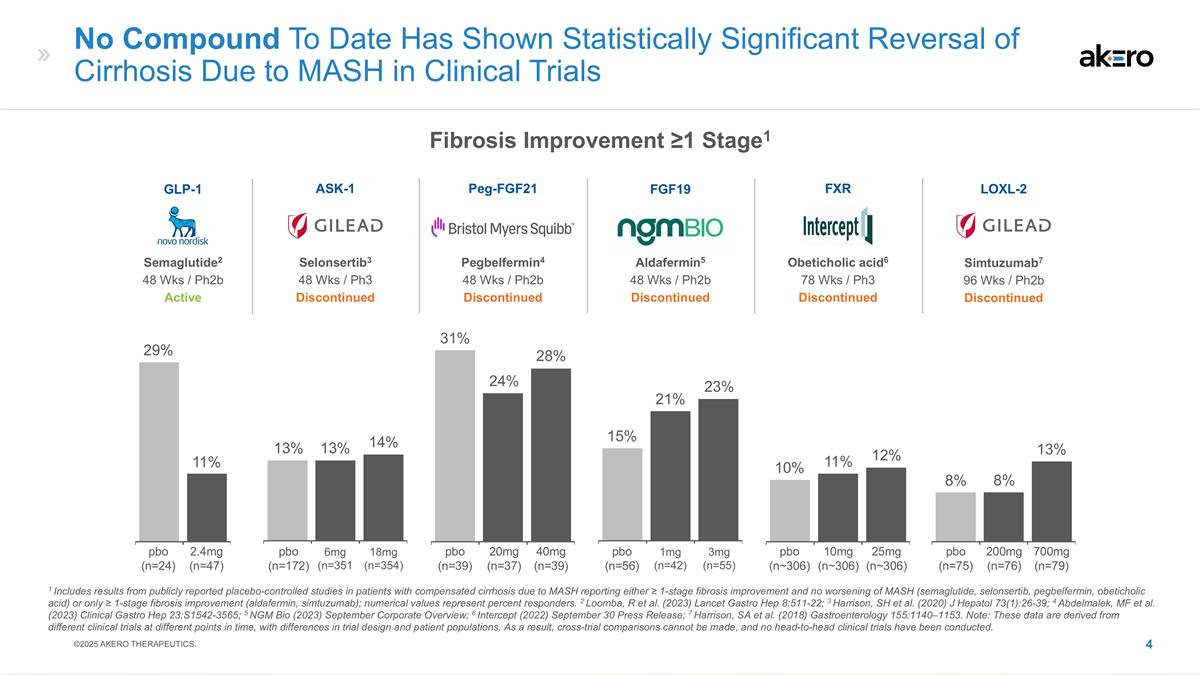
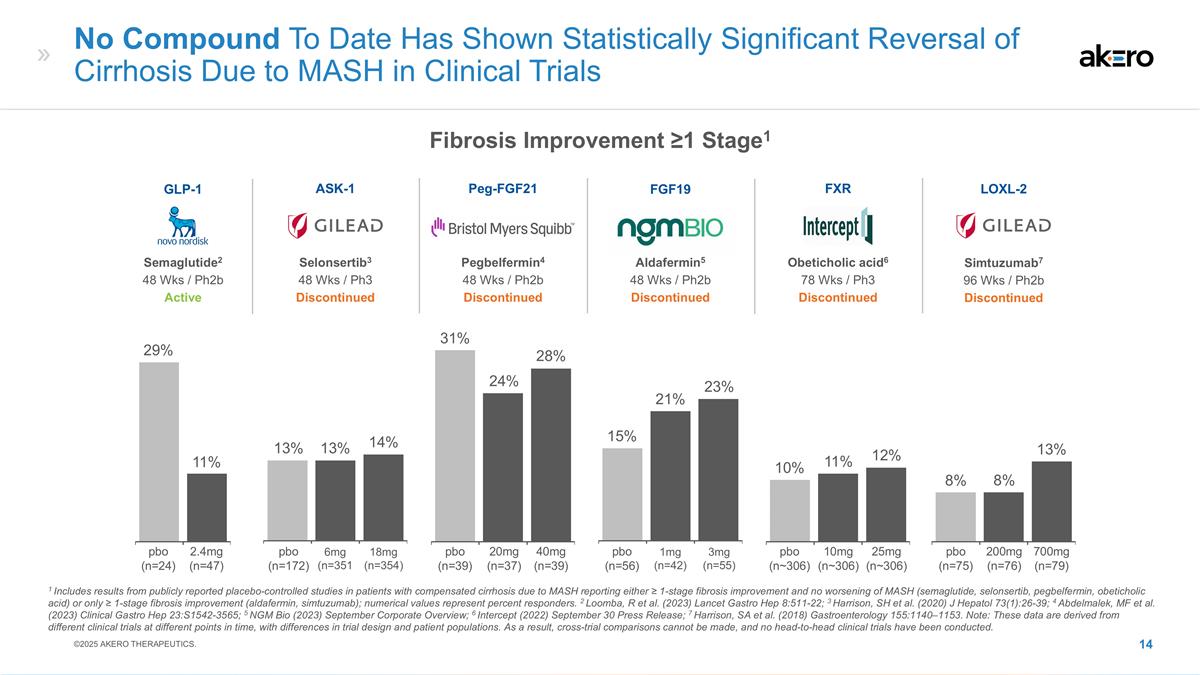
1mg (n=42) 3mg (n=55) 21% 15% No
Compound To Date Has Shown Statistically Significant Reversal of Cirrhosis Due to MASH in Clinical Trials 6mg (n=351 18mg (n=354) 20mg (n=37) 40mg (n=39) 14% 13% 31% 24% 25mg (n~306) 10mg (n~306) 10% 12% 2.4mg (n=47) 11% 200mg (n=76) 700mg (n=79) 8%
8% Aldafermin5 48 Wks / Ph2b Discontinued FGF19 Selonsertib3 48 Wks / Ph3 Discontinued ASK-1 Peg-FGF21 Pegbelfermin4 48 Wks / Ph2b Discontinued FXR Obeticholic acid6 78 Wks / Ph3 Discontinued Semaglutide2 48 Wks / Ph2b Active GLP-1 Simtuzumab7 96
Wks / Ph2b Discontinued LOXL-2 1 Includes results from publicly reported placebo-controlled studies in patients with compensated cirrhosis due to MASH reporting either ≥ 1-stage fibrosis improvement and no worsening of MASH (semaglutide,
selonsertib, pegbelfermin, obeticholic acid) or only ≥ 1-stage fibrosis improvement (aldafermin, simtuzumab); numerical values represent percent responders. 2 Loomba, R et al. (2023) Lancet Gastro Hep 8:511-22; 3 Harrison, SH et al. (2020) J
Hepatol 73(1):26-39; 4 Abdelmalek, MF et al. (2023) Clinical Gastro Hep 23:S1542-3565; 5 NGM Bio (2023) September Corporate Overview; 6 Intercept (2022) September 30 Press Release; 7 Harrison, SA et al. (2018) Gastroenterology 155:1140–1153.
Note: These data are derived from different clinical trials at different points in time, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been
conducted. 29% 13% 28% 23% 11% 13% pbo (n=24) pbo (n=172) pbo (n=39) pbo (n=56) pbo (n~306) pbo (n=75) ©2025 AKERO THERAPEUTICS. Fibrosis Improvement ≥1 Stage1
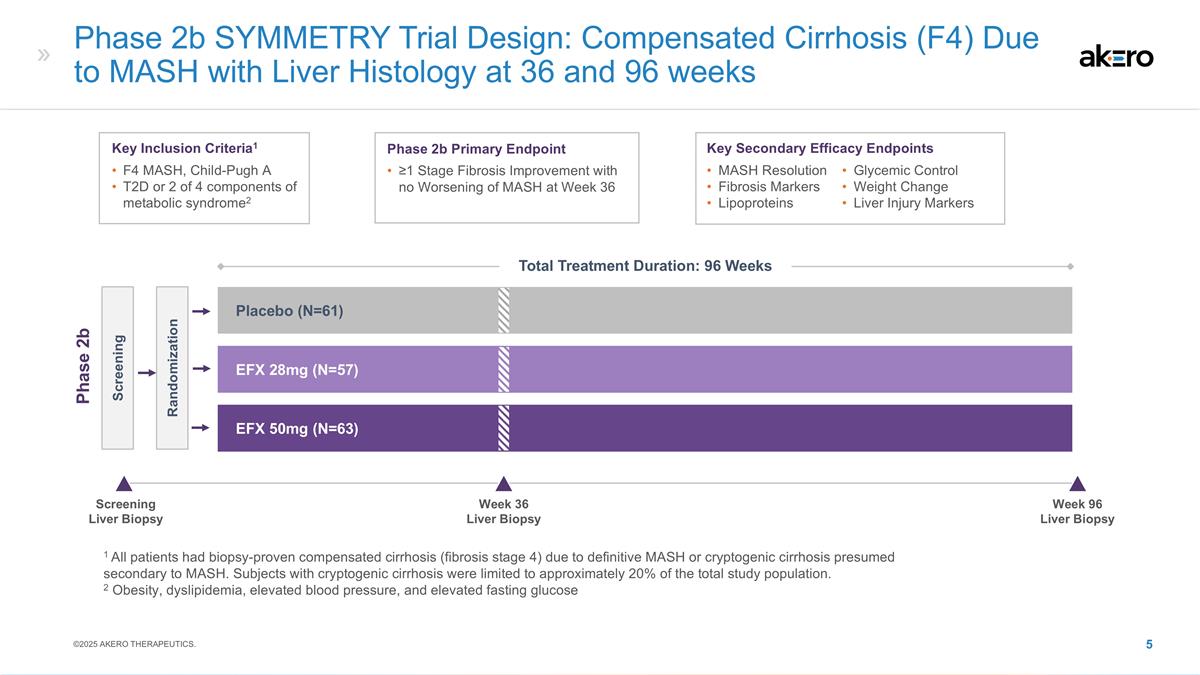
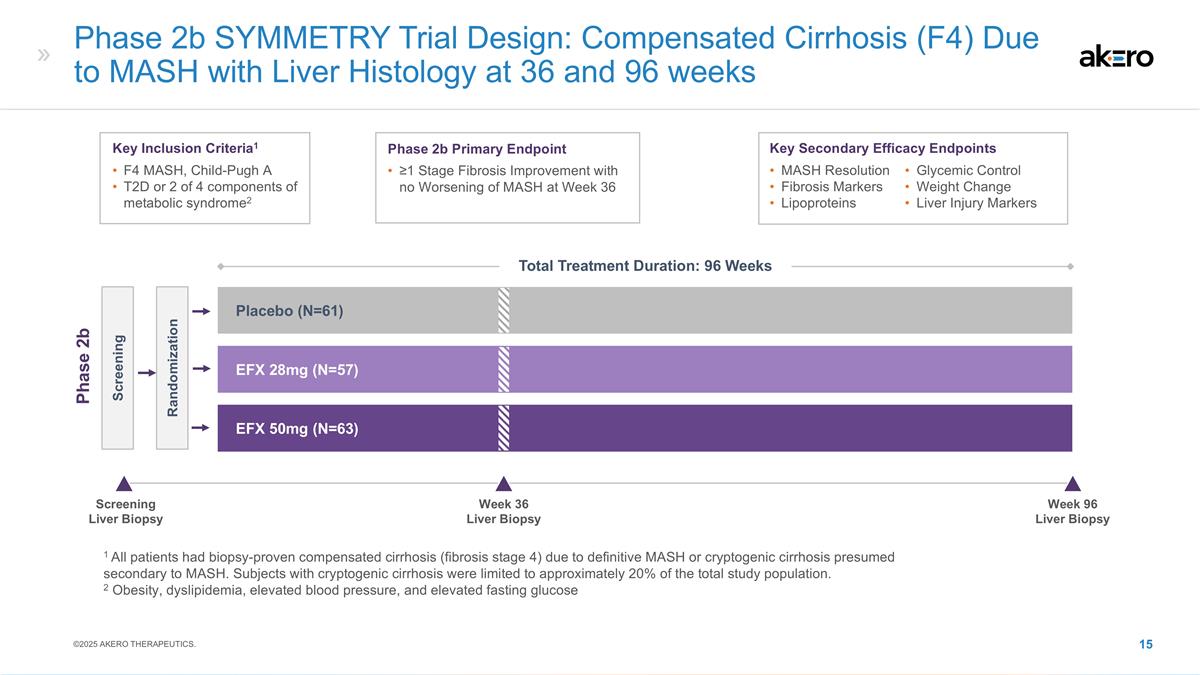
Phase 2b SYMMETRY Trial Design:
Compensated Cirrhosis (F4) Due to MASH with Liver Histology at 36 and 96 weeks EFX 28mg (N=57) Placebo (N=61) EFX 50mg (N=63) Screening Randomization Phase 2b Key Inclusion Criteria1 F4 MASH, Child-Pugh A T2D or 2 of 4 components of metabolic
syndrome2 Phase 2b Primary Endpoint ≥1 Stage Fibrosis Improvement with no Worsening of MASH at Week 36 Key Secondary Efficacy Endpoints MASH Resolution Fibrosis Markers Lipoproteins Glycemic Control Weight Change Liver Injury Markers 1 All
patients had biopsy-proven compensated cirrhosis (fibrosis stage 4) due to definitive MASH or cryptogenic cirrhosis presumed secondary to MASH. Subjects with cryptogenic cirrhosis were limited to approximately 20% of the total study population. 2
Obesity, dyslipidemia, elevated blood pressure, and elevated fasting glucose Screening Liver Biopsy Week 36 Liver Biopsy Week 96 Liver Biopsy ©2025 AKERO THERAPEUTICS. Total Treatment Duration: 96 Weeks
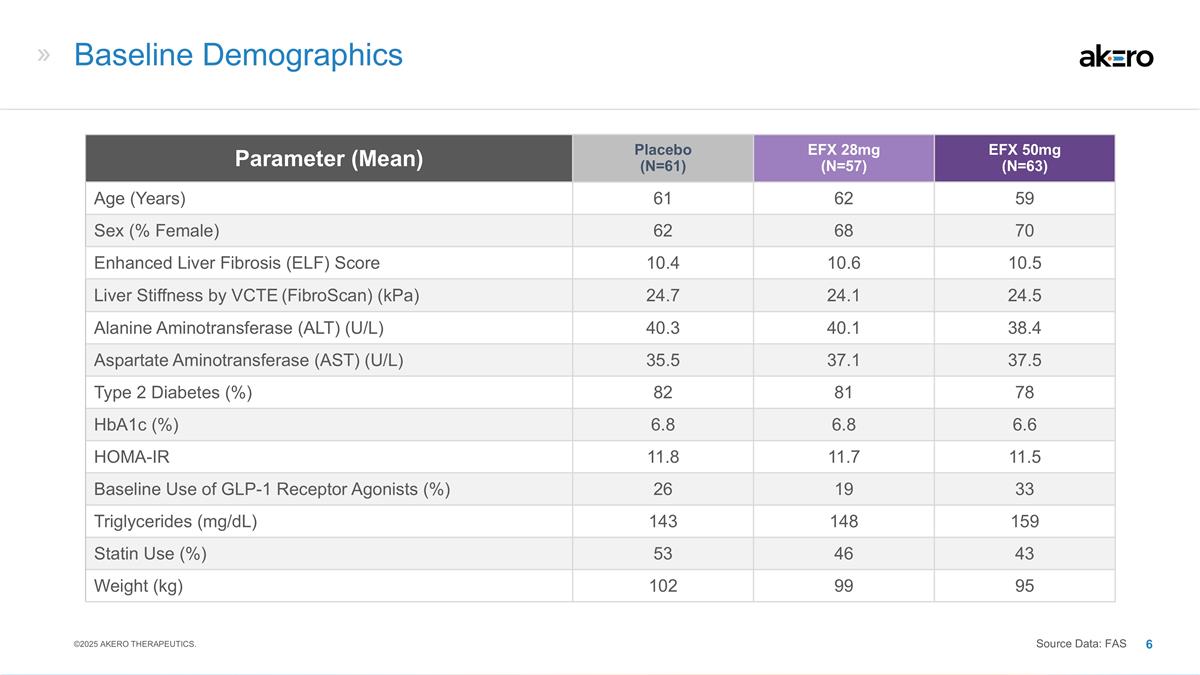
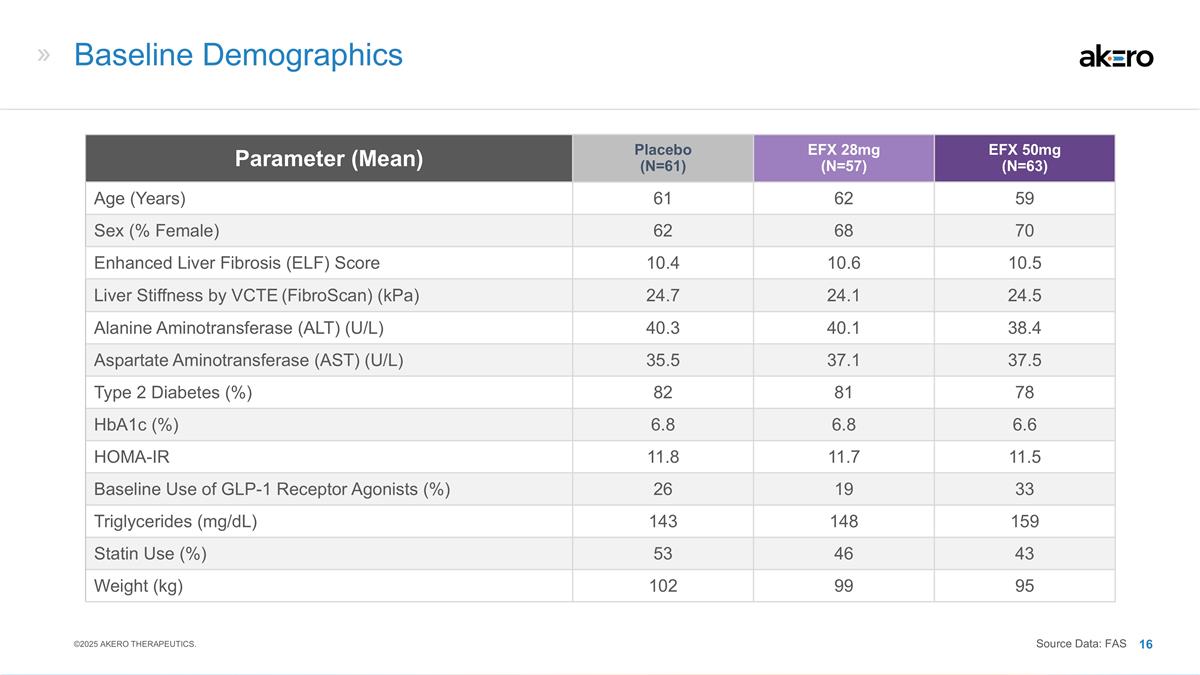
Baseline Demographics Parameter (Mean)
Placebo (N=61) EFX 28mg (N=57) EFX 50mg (N=63) Age (Years) 61 62 59 Sex (% Female) 62 68 70 Enhanced Liver Fibrosis (ELF) Score 10.4 10.6 10.5 Liver Stiffness by VCTE (FibroScan) (kPa) 24.7 24.1 24.5 Alanine Aminotransferase (ALT) (U/L) 40.3 40.1
38.4 Aspartate Aminotransferase (AST) (U/L) 35.5 37.1 37.5 Type 2 Diabetes (%) 82 81 78 HbA1c (%) 6.8 6.8 6.6 HOMA-IR 11.8 11.7 11.5 Baseline Use of GLP-1 Receptor Agonists (%) 26 19 33 Triglycerides (mg/dL) 143 148 159 Statin Use (%) 53 46 43
Weight (kg) 102 99 95 ©2025 AKERO THERAPEUTICS. Source Data: FAS
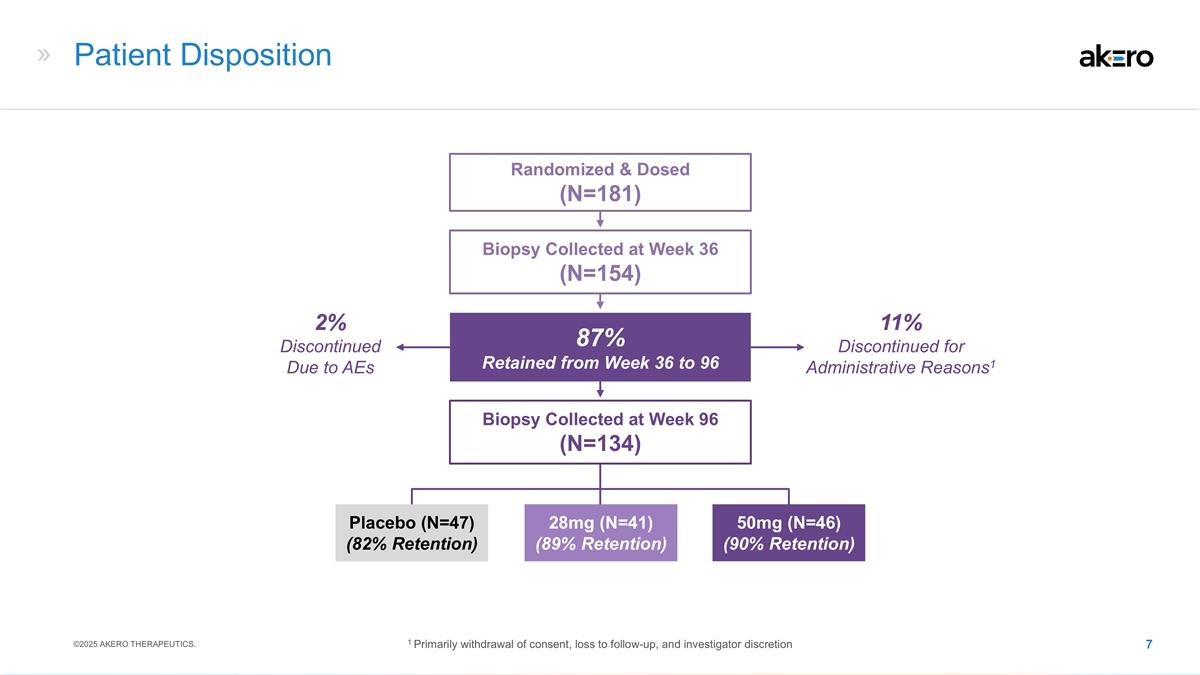
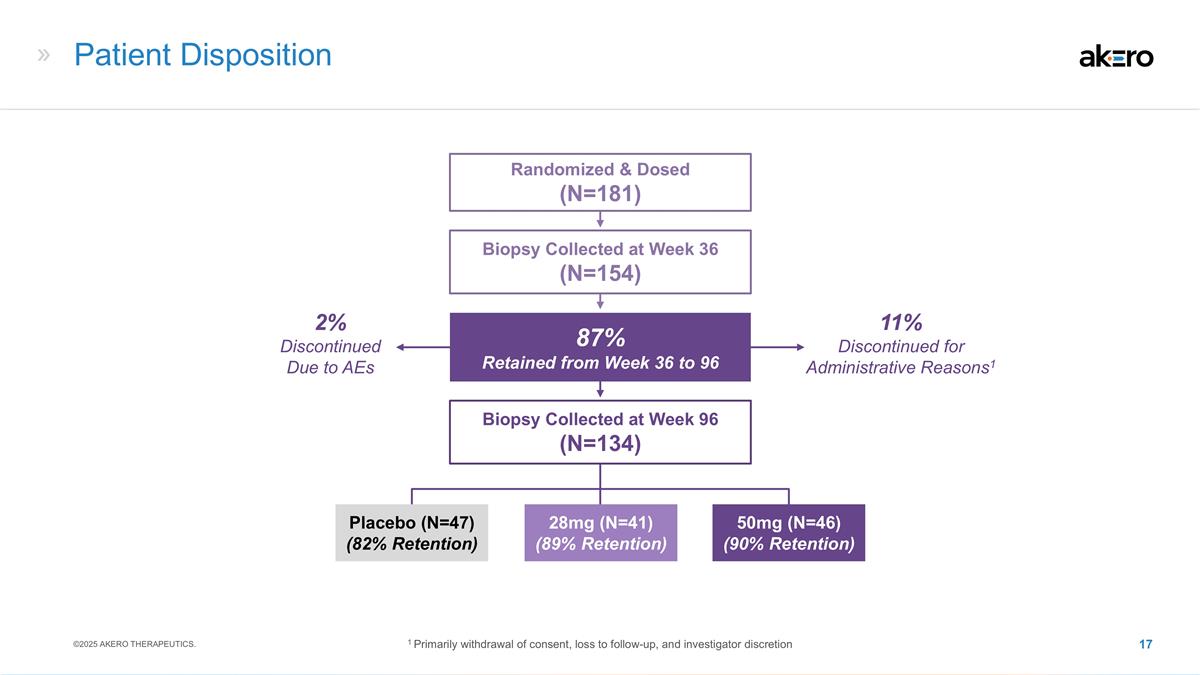
11% Discontinued for Administrative
Reasons1 Patient Disposition 28mg (N=41) (89% Retention) 50mg (N=46) (90% Retention) Placebo (N=47) (82% Retention) Biopsy Collected at Week 36 (N=154) Randomized & Dosed (N=181) Biopsy Collected at Week 96 (N=134) 87% Retained from Week 36 to
96 2% Discontinued Due to AEs ©2025 AKERO THERAPEUTICS. 1 Primarily withdrawal of consent, loss to follow-up, and investigator discretion
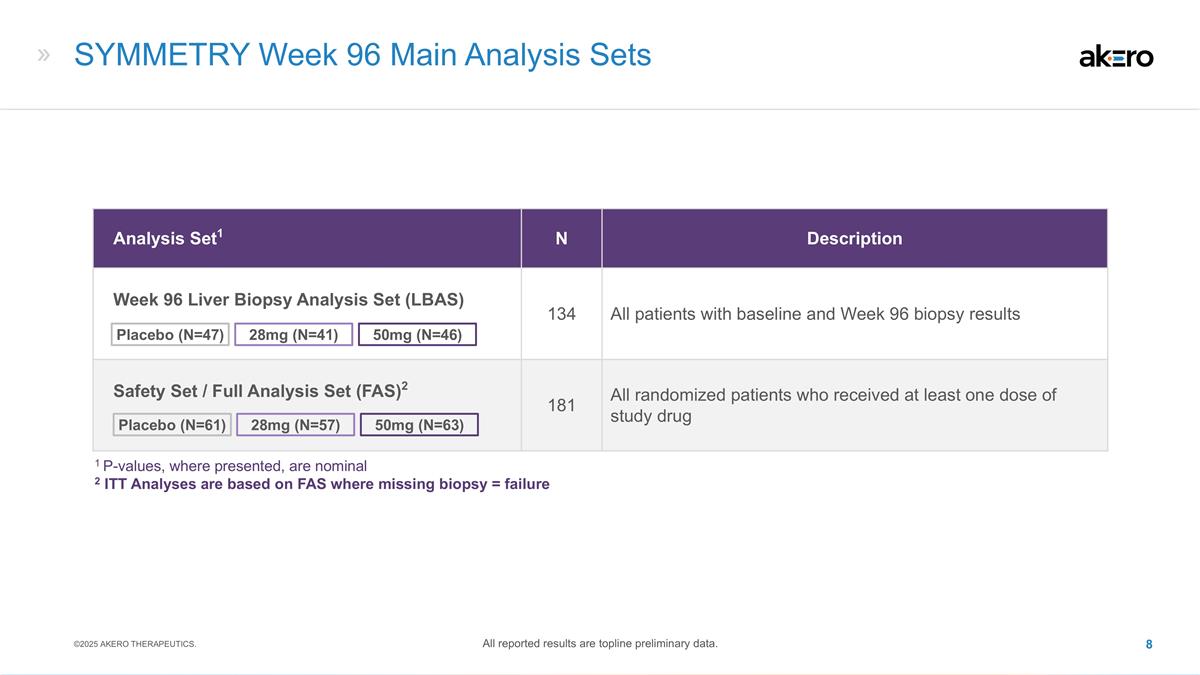
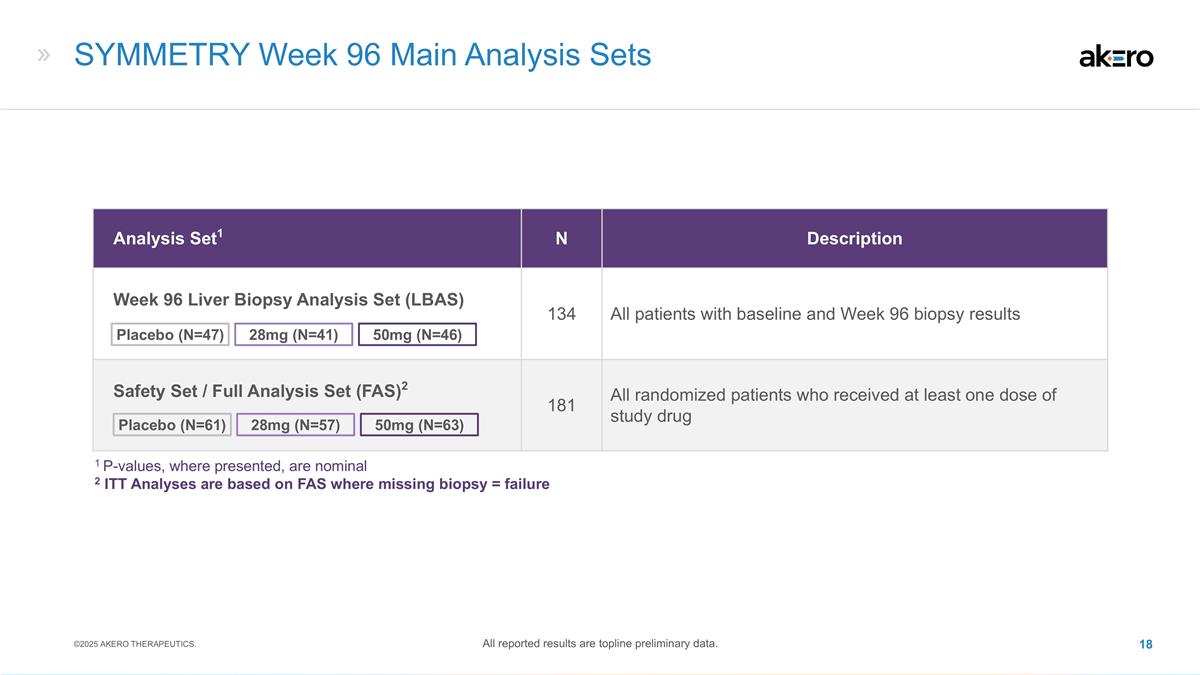
SYMMETRY Week 96 Main Analysis Sets
Analysis Set1 N Description Week 96 Liver Biopsy Analysis Set (LBAS) 134 All patients with baseline and Week 96 biopsy results Safety Set / Full Analysis Set (FAS)2 181 All randomized patients who received at least one dose of study drug 28mg (N=41)
50mg (N=46) Placebo (N=47) 50mg (N=63) 28mg (N=57) Placebo (N=61) 1 P-values, where presented, are nominal 2 ITT Analyses are based on FAS where missing biopsy = failure ©2025 AKERO THERAPEUTICS. All reported results are topline preliminary
data.
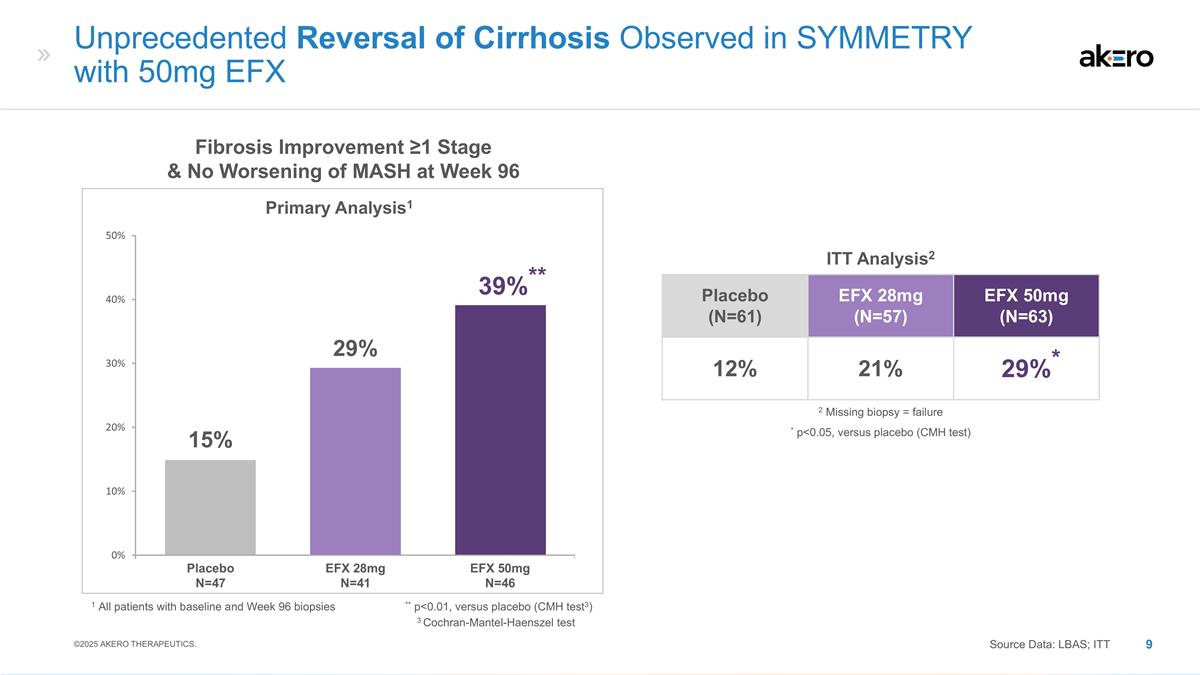
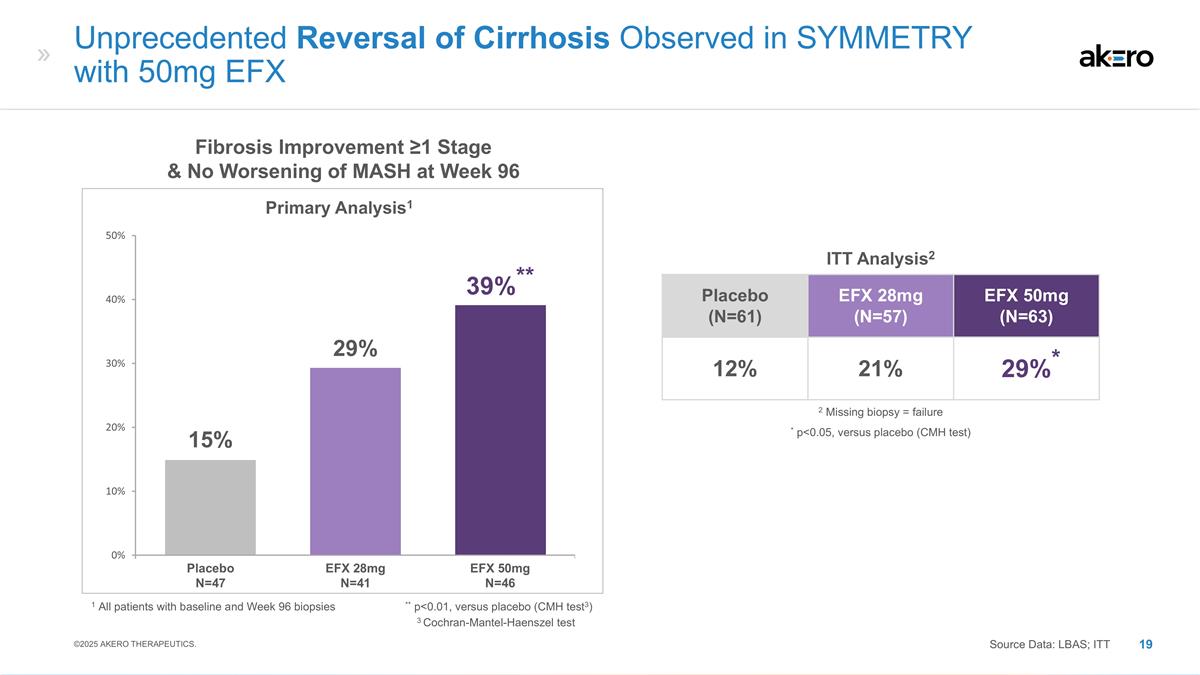
Placebo (N=61) EFX 28mg (N=57) EFX
50mg (N=63) 12% 21% *29%* EFX 28mg N=41 EFX 50mg N=46 Placebo N=47 Fibrosis Improvement ≥1 Stage & No Worsening of MASH at Week 96 Primary Analysis1 1 All patients with baseline and Week 96 biopsies ** p<0.01, versus placebo (CMH test3)
** 39%** 29% 15% Unprecedented Reversal of Cirrhosis Observed in SYMMETRY with 50mg EFX ITT Analysis2 * p<0.05, versus placebo (CMH test) 2 Missing biopsy = failure Source Data: LBAS; ITT ©2025 AKERO THERAPEUTICS. 3 Cochran-Mantel-Haenszel
test
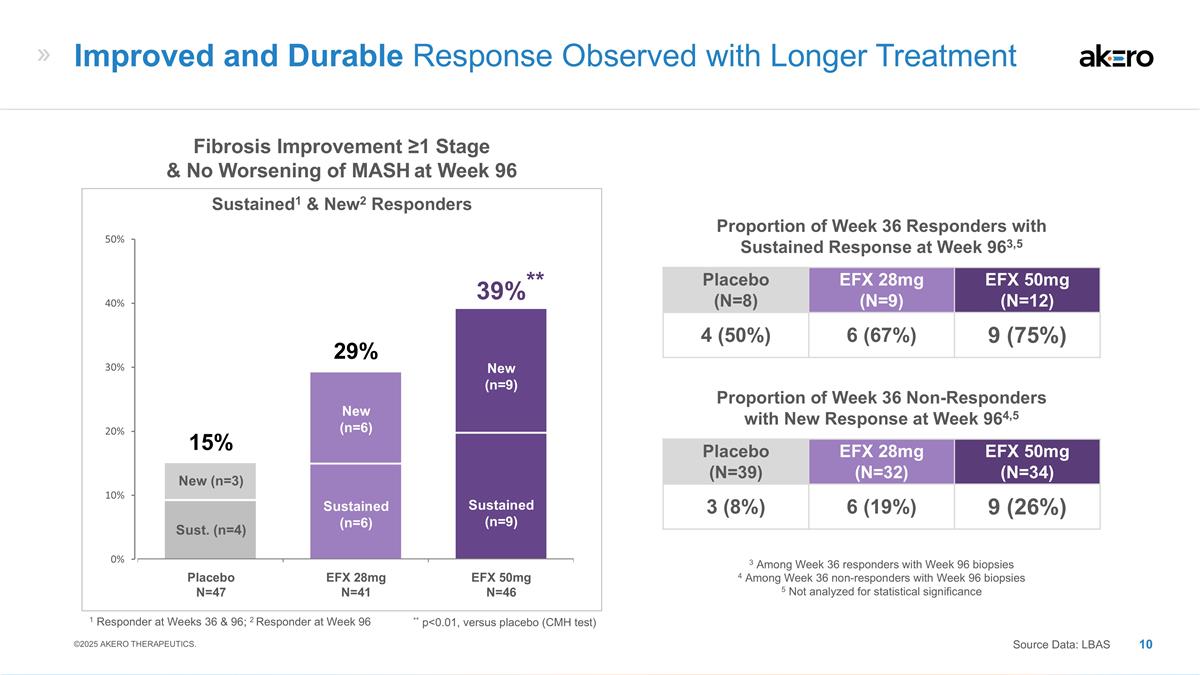
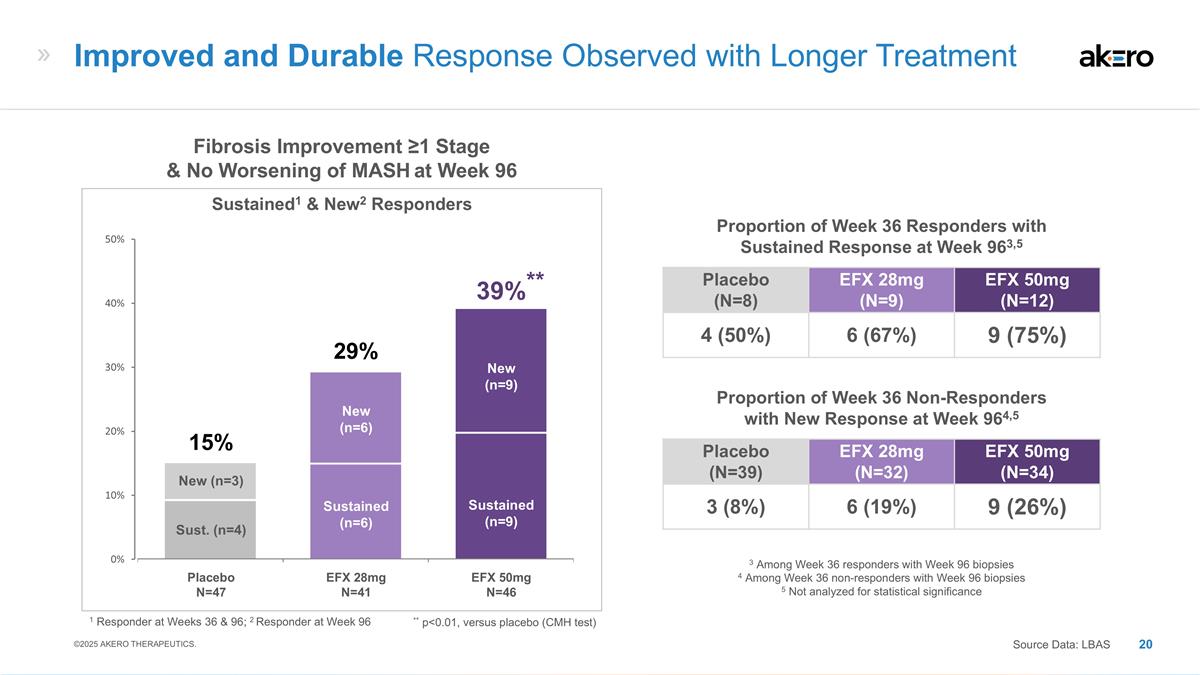
Sustained (n=6) New (n=6) Sustained
(n=9) New (n=9) Sust. (n=4) New (n=3) **39%** EFX 28mg N=41 EFX 50mg N=46 Placebo N=47 29% 15% Fibrosis Improvement ≥1 Stage & No Worsening of MASH at Week 96 Sustained1 & New2 Responders Placebo (N=8) EFX 28mg (N=9) EFX 50mg (N=12) 4
(50%) 6 (67%) 9 (75%) Proportion of Week 36 Responders with Sustained Response at Week 963,5 Placebo (N=39) EFX 28mg (N=32) EFX 50mg (N=34) 3 (8%) 6 (19%) 9 (26%) Proportion of Week 36 Non-Responders with New Response at Week 964,5 3 Among Week 36
responders with Week 96 biopsies 4 Among Week 36 non-responders with Week 96 biopsies 5 Not analyzed for statistical significance Improved and Durable Response Observed with Longer Treatment Source Data: LBAS ©2025 AKERO THERAPEUTICS. 1
Responder at Weeks 36 & 96; 2 Responder at Week 96 ** p<0.01, versus placebo (CMH test)
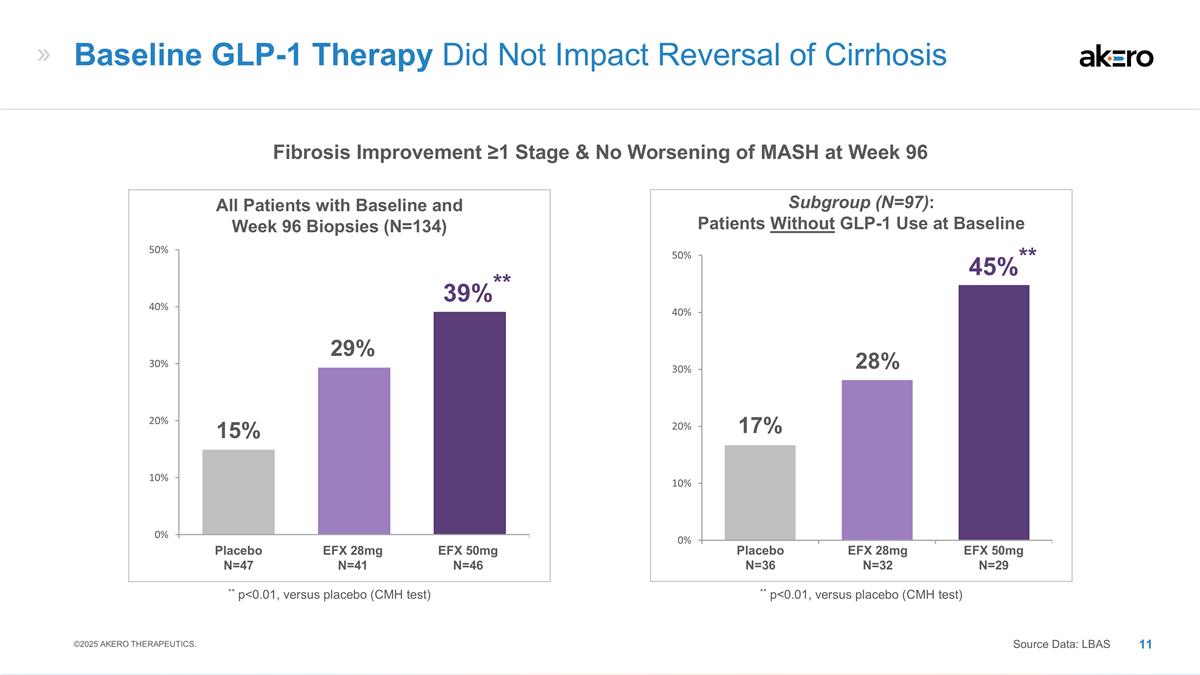
EFX 28mg N=32 EFX 50mg N=29 Placebo
N=36 **45%** 28% 17% Subgroup (N=97): Patients Without GLP-1 Use at Baseline EFX 28mg N=41 EFX 50mg N=46 Placebo N=47 ** p<0.01, versus placebo (CMH test) **39%** 29% 15% All Patients with Baseline and Week 96 Biopsies (N=134) Baseline GLP-1
Therapy Did Not Impact Reversal of Cirrhosis Source Data: LBAS ** p<0.01, versus placebo (CMH test) Fibrosis Improvement ≥1 Stage & No Worsening of MASH at Week 96 ©2025 AKERO THERAPEUTICS.
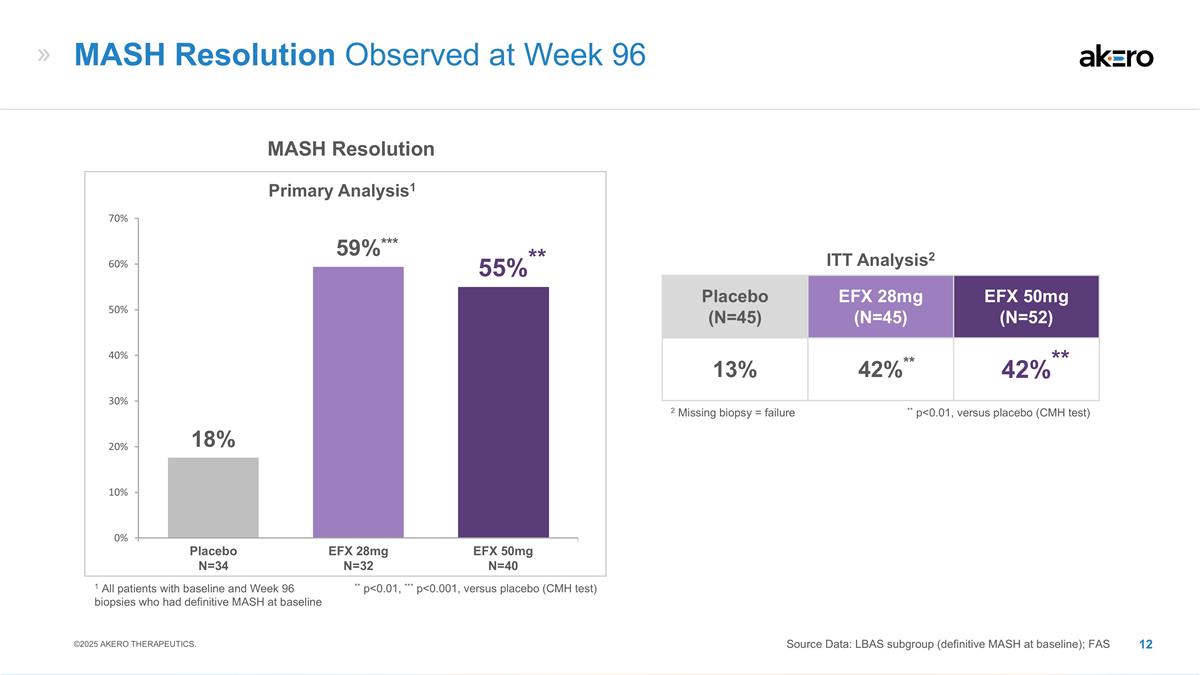
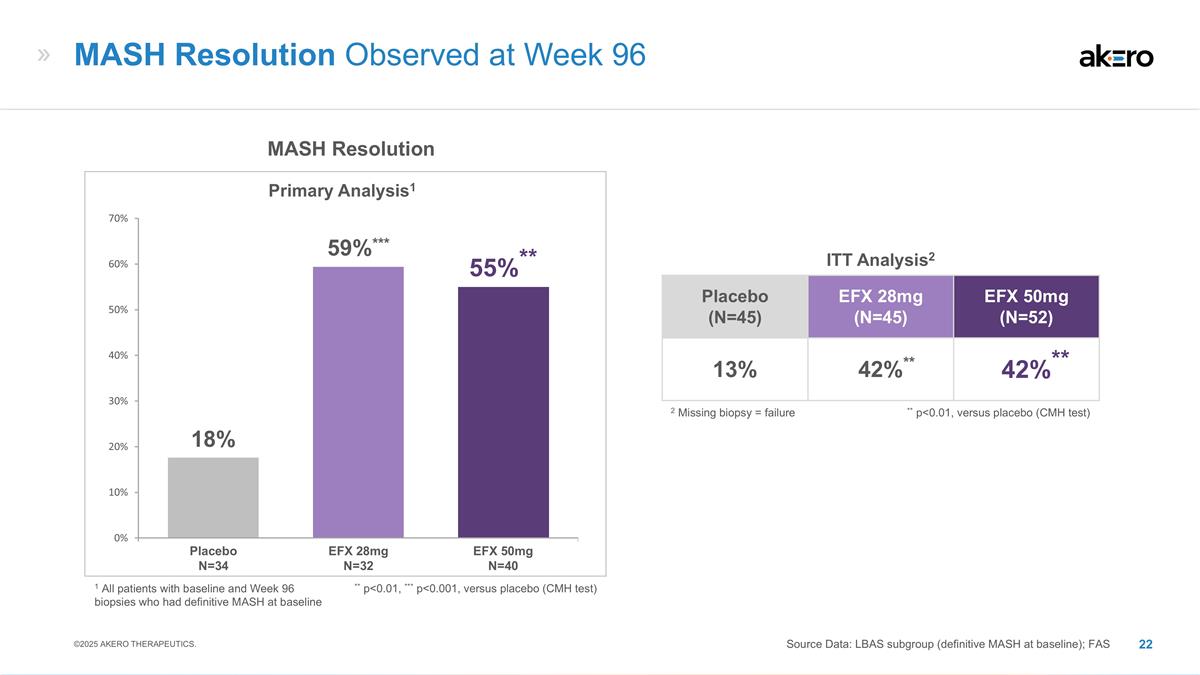
EFX 28mg N=32 EFX 50mg N=40 Placebo
N=34 MASH Resolution Primary Analysis1 ** p<0.01, *** p<0.001, versus placebo (CMH test) **55%** ***59%*** 18% MASH Resolution Observed at Week 96 Source Data: LBAS subgroup (definitive MASH at baseline); FAS ©2025 AKERO THERAPEUTICS.
Placebo (N=45) EFX 28mg (N=45) EFX 50mg (N=52) 13% **42%** **42%** ITT Analysis2 ** p<0.01, versus placebo (CMH test) 2 Missing biopsy = failure 1 All patients with baseline and Week 96 biopsies who had definitive MASH at baseline
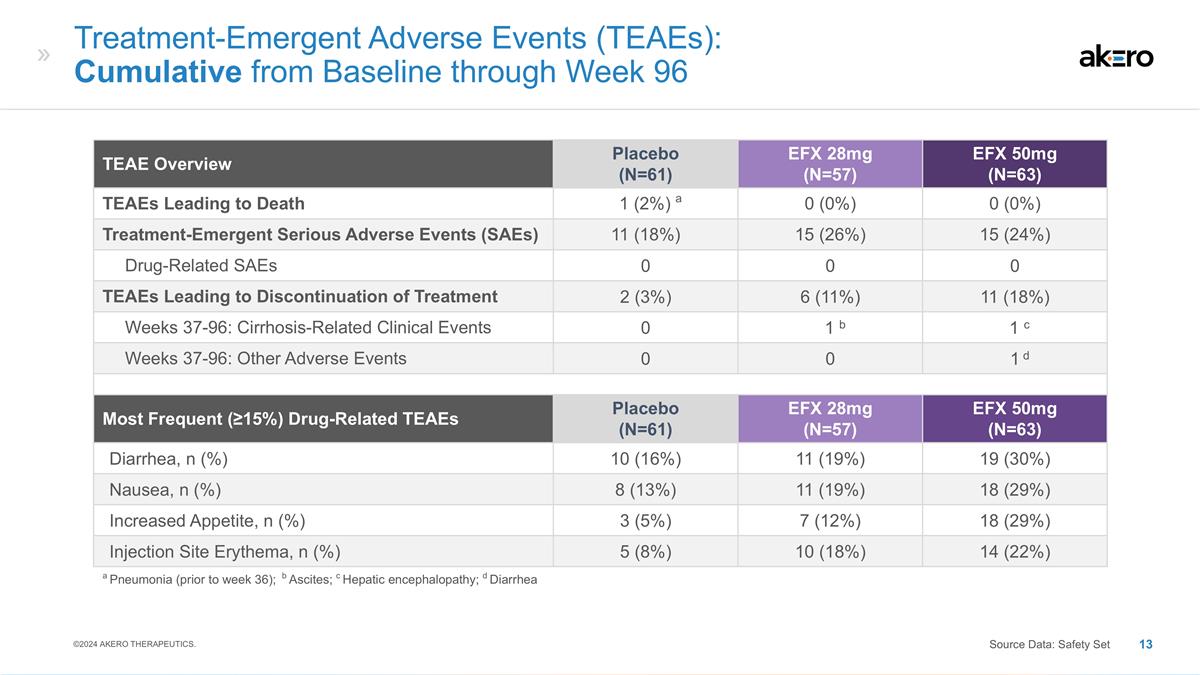
Treatment-Emergent Adverse Events
(TEAEs): Cumulative from Baseline through Week 96 TEAE Overview Placebo (N=61) EFX 28mg (N=57) EFX 50mg (N=63) TEAEs Leading to Death a 1 (2%) a 0 (0%) 0 (0%) Treatment-Emergent Serious Adverse Events (SAEs) 11 (18%) 15 (26%) 15 (24%) Drug-Related
SAEs 0 0 0 TEAEs Leading to Discontinuation of Treatment 2 (3%) 6 (11%) 11 (18%) Weeks 37-96: Cirrhosis-Related Clinical Events 0 b 1 b c 1 c Weeks 37-96: Other Adverse Events 0 0 d 1 d Most Frequent (≥15%) Drug-Related TEAEs Placebo (N=61)
EFX 28mg (N=57) EFX 50mg (N=63) Diarrhea, n (%) 10 (16%) 11 (19%) 19 (30%) Nausea, n (%) 8 (13%) 11 (19%) 18 (29%) Increased Appetite, n (%) 3 (5%) 7 (12%) 18 (29%) Injection Site Erythema, n (%) 5 (8%) 10 (18%) 14 (22%) a Pneumonia (prior to week
36); b Ascites; c Hepatic encephalopathy; d Diarrhea ©2024 AKERO THERAPEUTICS. Source Data: Safety Set

Additional Safety Observations for
50mg EFX (Phase 3 Dose) Versus Placebo at Week 96 Bone Mineral Density 43% of 50mg EFX and 41% of placebo patients had osteopenia1 at baseline, but only 3% of 50mg EFX and 7% of placebo patients were treated with bisphosphonates Placebo-adjusted,
significant relative reductions in bone mineral density (5%) for spine and hip were observed for 50mg EFX, or about 2-3% per year For context, treatment with a diabetic dose of GLP-1 was recently reported to be associated with about a 2% reduction
in bone mineral density over one year.2 Equal number of fractures (4 each) observed for 50mg EFX and placebo Markers of Hemostasis and Liver Function Markers of hemostasis and liver function, including bilirubin, MELD score, INR, platelet count,
albumin and Child-Pugh score, remained stable or trended toward improvement for 50mg EFX Electrocardiograms (ECGs), Vital Signs & Body Weight No statistically significant changes in ECGs, heart rate, or blood pressure were observed for 50mg EFX
versus placebo Weight reduction of ~2% was observed for 50mg EFX and placebo ©2025 AKERO THERAPEUTICS. 1 T-score < -1.0 by DXA 2 M. S. Hansen et al., eClinicalMedicine. 72, 102624 (2024)
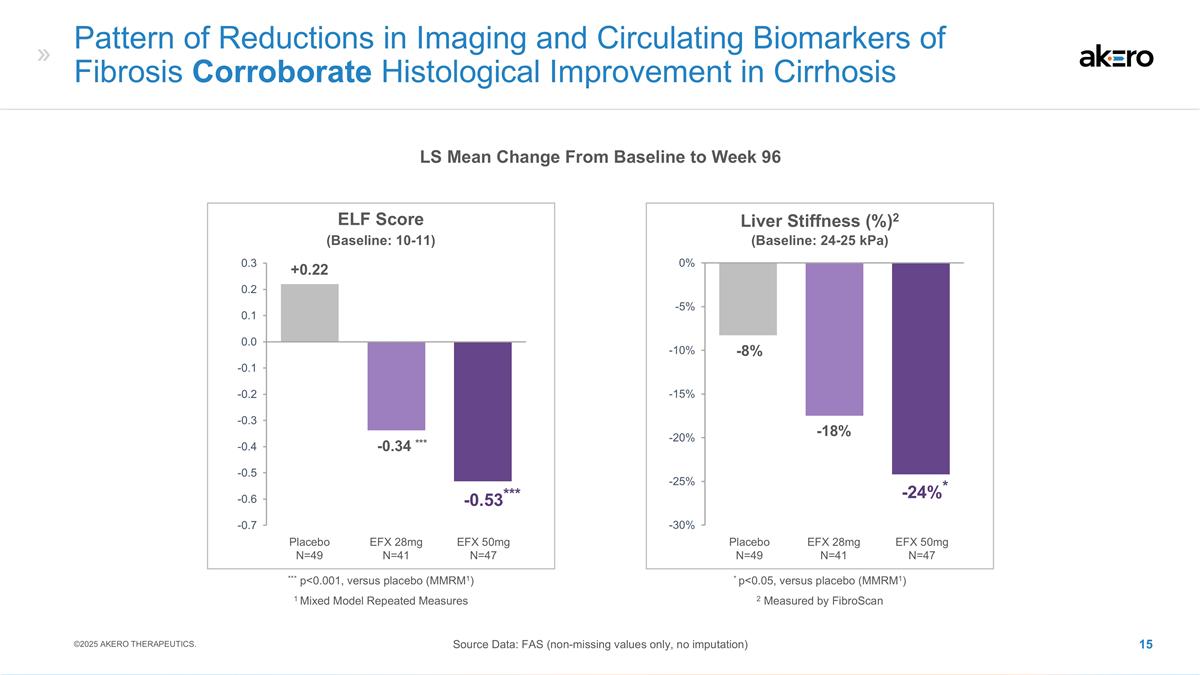
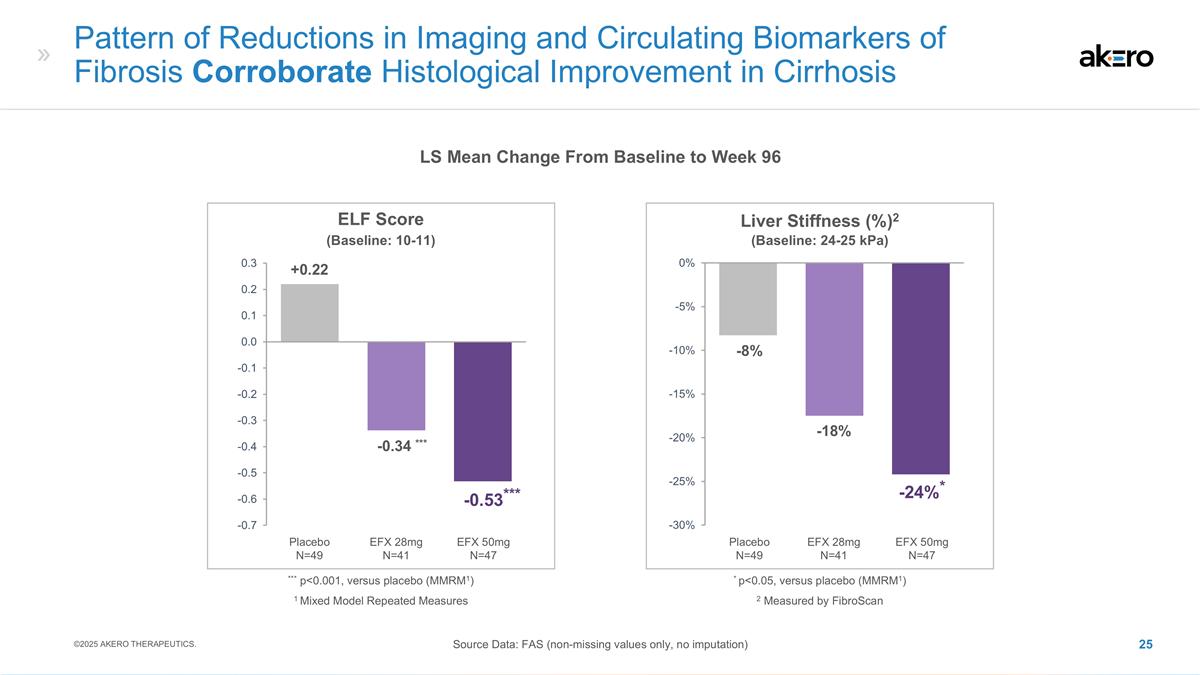
Pattern of Reductions in Imaging
and Circulating Biomarkers of Fibrosis Corroborate Histological Improvement in Cirrhosis LS Mean Change From Baseline to Week 96 Source Data: FAS (non-missing values only, no imputation) ©2025 AKERO THERAPEUTICS. Liver Stiffness (%)2 -18% -8%
EFX 50mg N=47 Placebo N=49 EFX 28mg N=41 *-24%* 2 Measured by FibroScan * p<0.05, versus placebo (MMRM1) (Baseline: 24-25 kPa) ELF Score EFX 50mg N=47 Placebo N=49 EFX 28mg N=41 ***-0.34 *** +0.22 ***-0.53*** *** p<0.001, versus placebo
(MMRM1) (Baseline: 10-11) 1 Mixed Model Repeated Measures
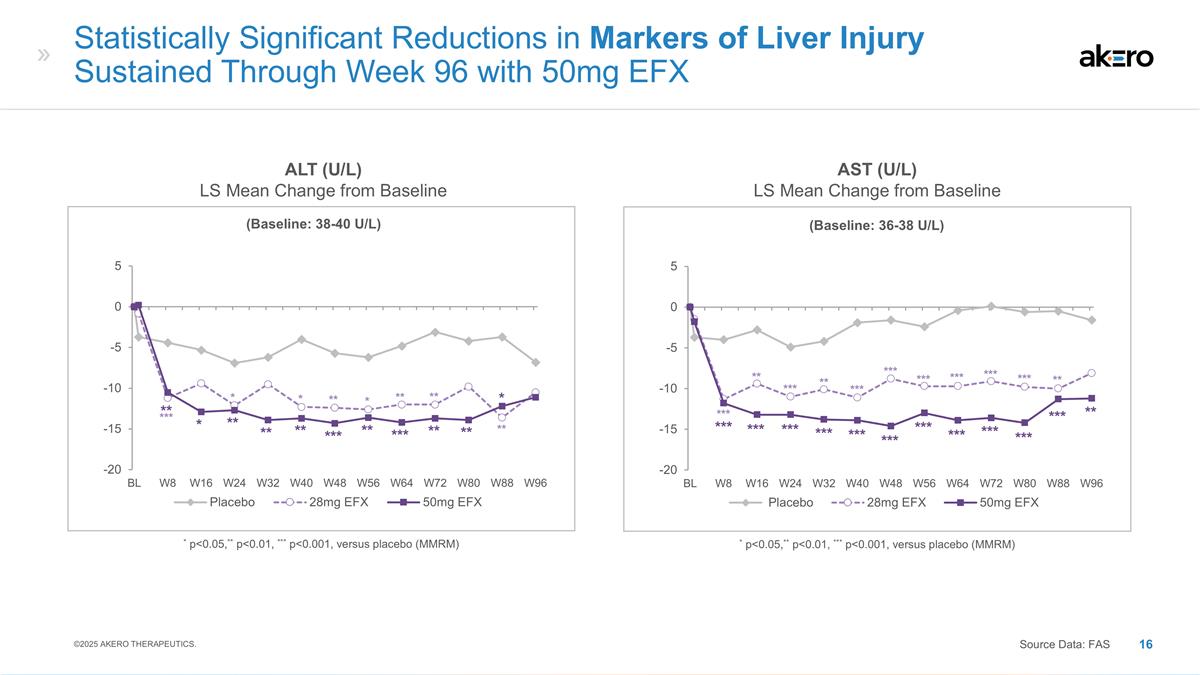
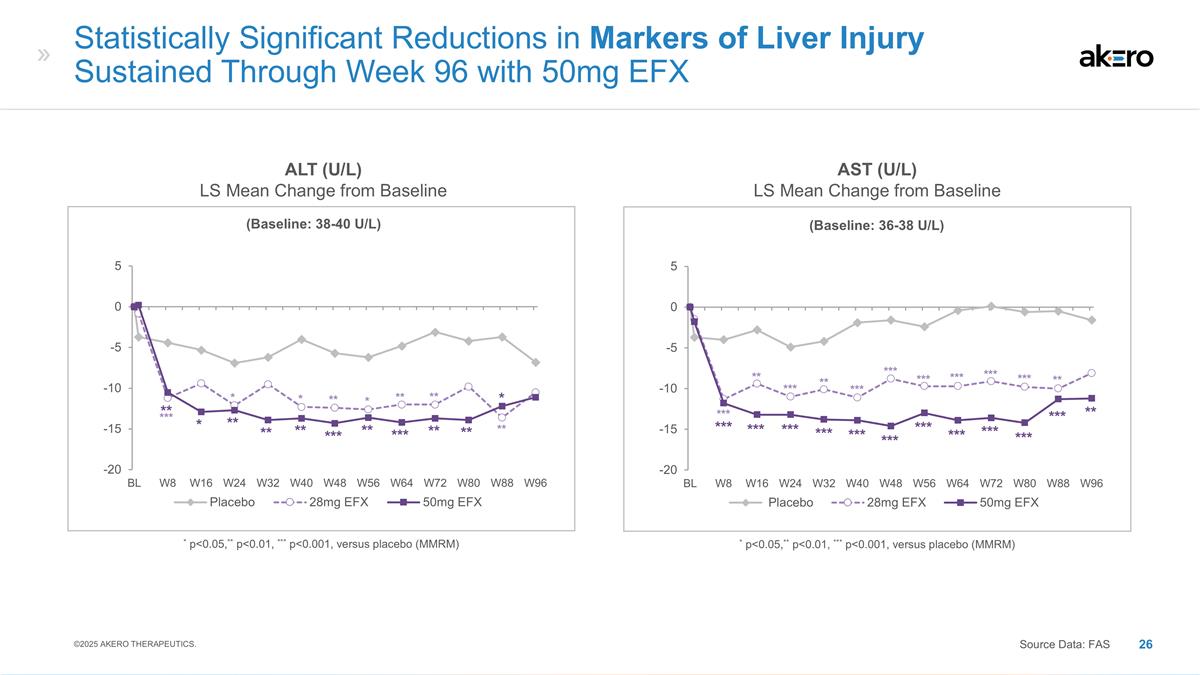
(Baseline: 38-40 U/L) *
p<0.05,** p<0.01, *** p<0.001, versus placebo (MMRM) Statistically Significant Reductions in Markers of Liver Injury Sustained Through Week 96 with 50mg EFX ALT (U/L) LS Mean Change from Baseline AST (U/L) LS Mean Change from Baseline *** *
* ** * ** ** ** ** * ** ** ** *** ** *** ** ** * ©2025 AKERO THERAPEUTICS. Source Data: FAS (Baseline: 36-38 U/L) * p<0.05,** p<0.01, *** p<0.001, versus placebo (MMRM) *** *** *** *** *** *** *** *** *** *** *** ** ** ** *** *** ***
*** *** ** *** *** ***
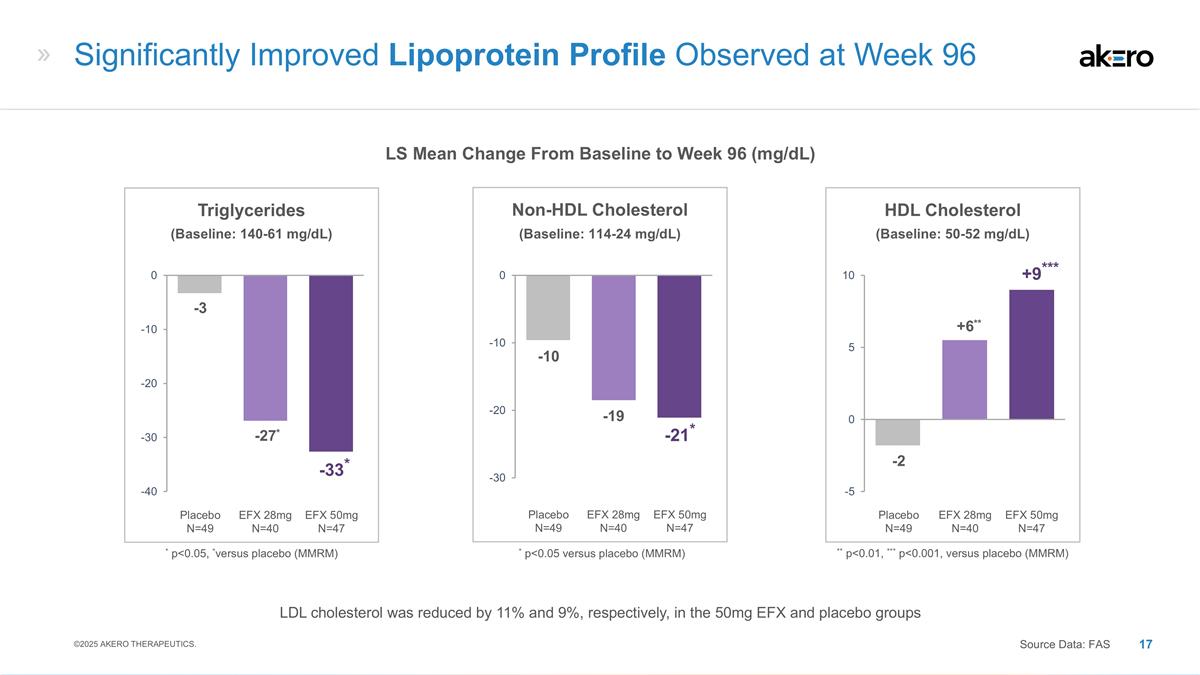
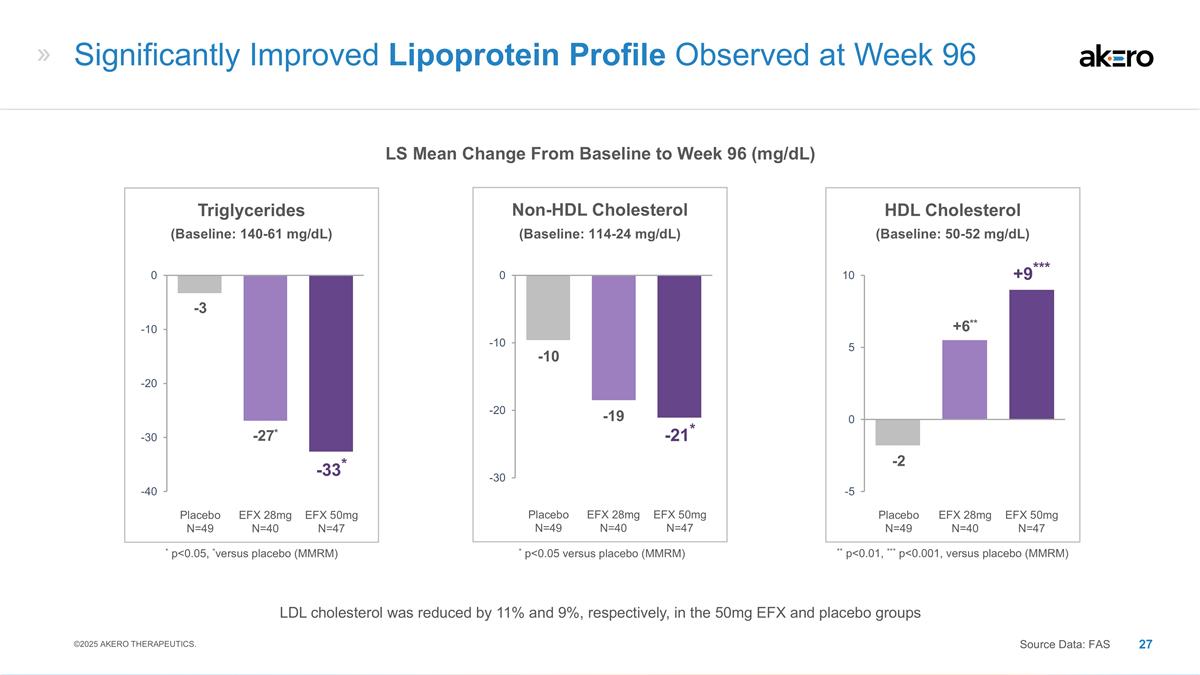
EFX 50mg N=47 Placebo N=49 EFX 28mg
N=40 HDL Cholesterol **+6** -2 ***+9*** ** p<0.01, *** p<0.001, versus placebo (MMRM) EFX 50mg N=47 Placebo N=49 EFX 28mg N=40 Non-HDL Cholesterol -19 -10 -21* EFX 50mg N=47 Placebo N=49 EFX 28mg N=40 Triglycerides *-27* -3 *-33* * p<0.05,
*versus placebo (MMRM) Significantly Improved Lipoprotein Profile Observed at Week 96 LS Mean Change From Baseline to Week 96 (mg/dL) ©2025 AKERO THERAPEUTICS. Source Data: FAS * p<0.05 versus placebo (MMRM) (Baseline: 114-24 mg/dL)
(Baseline: 50-52 mg/dL) (Baseline: 140-61 mg/dL) LDL cholesterol was reduced by 11% and 9%, respectively, in the 50mg EFX and placebo groups
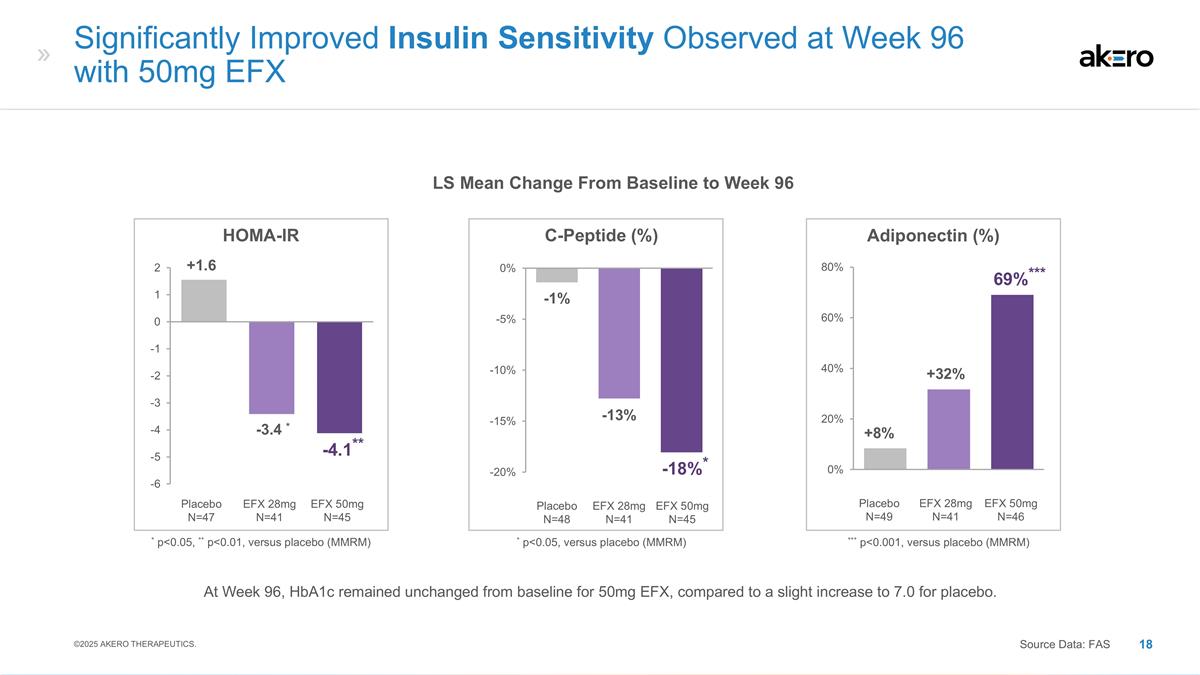
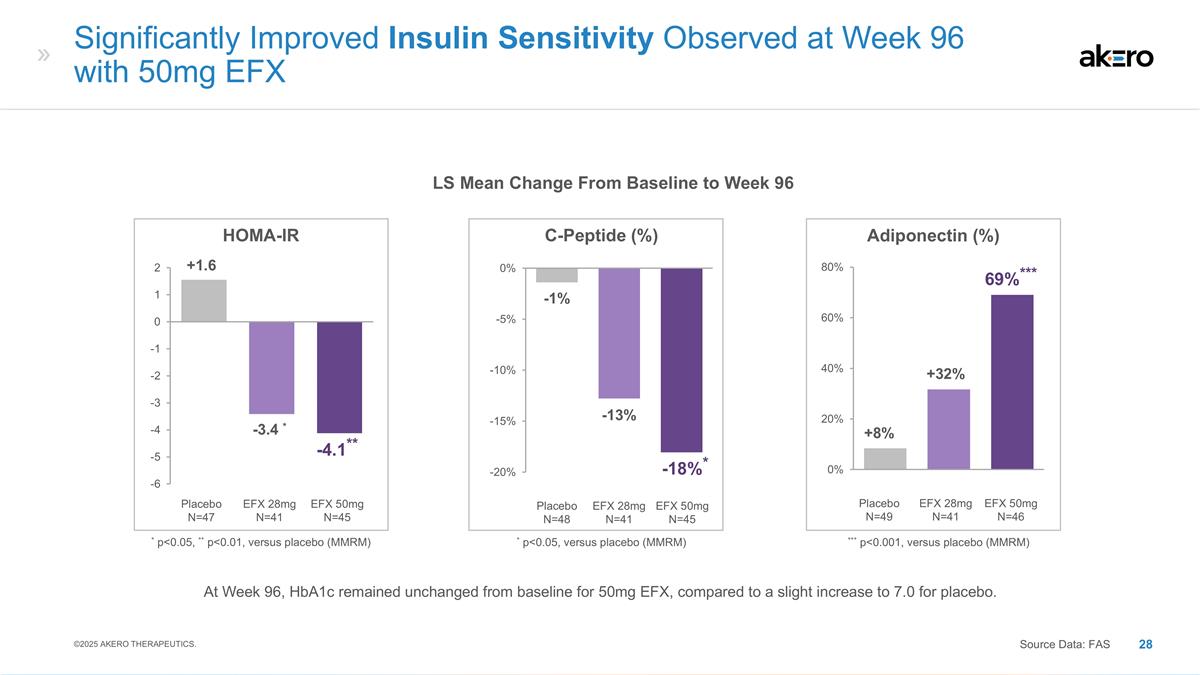
EFX 50mg N=45 Placebo N=48 EFX 28mg
N=41 C-Peptide (%) * p<0.05, versus placebo (MMRM) -13% -1% *-18%* EFX 50mg N=46 Placebo N=49 EFX 28mg N=41 Adiponectin (%) +32% +8% ***69%*** *** p<0.001, versus placebo (MMRM) Significantly Improved Insulin Sensitivity Observed at Week 96
with 50mg EFX Source Data: FAS LS Mean Change From Baseline to Week 96 EFX 50mg N=45 Placebo N=47 EFX 28mg N=41 HOMA-IR * -3.4 * +1.6 **-4.1** * p<0.05, ** p<0.01, versus placebo (MMRM) ©2025 AKERO THERAPEUTICS. At Week 96, HbA1c remained
unchanged from baseline for 50mg EFX, compared to a slight increase to 7.0 for placebo.
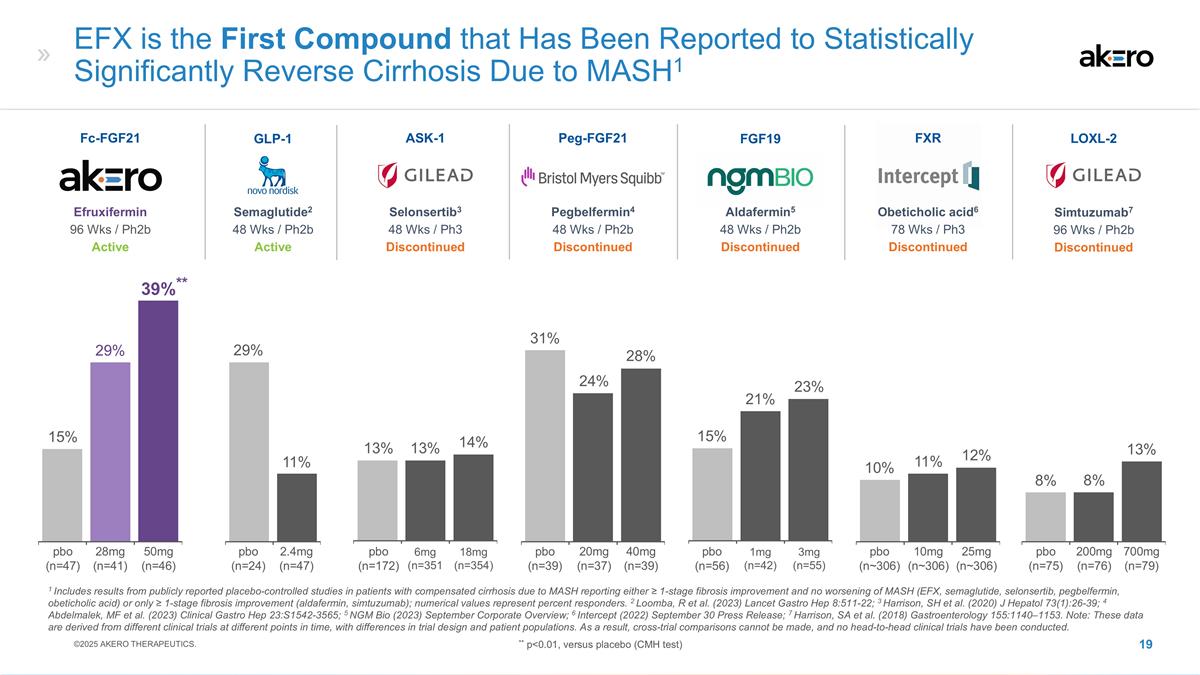
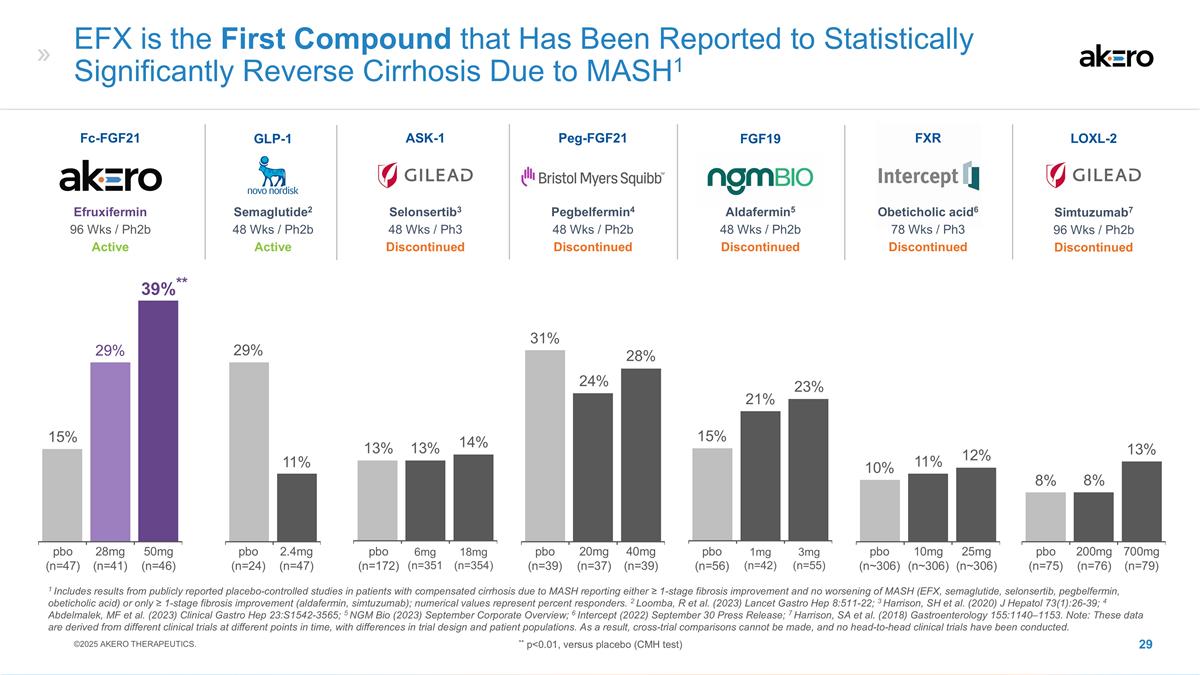
Aldafermin5 48 Wks / Ph2b
Discontinued FGF19 1mg (n=42) 3mg (n=55) 21% 15% EFX is the First Compound that Has Been Reported to Statistically Significantly Reverse Cirrhosis Due to MASH1 Selonsertib3 48 Wks / Ph3 Discontinued ASK-1 Peg-FGF21 Pegbelfermin4 48 Wks / Ph2b
Discontinued 6mg (n=351 18mg (n=354) 20mg (n=37) 40mg (n=39) 14% 13% 31% 24% FXR Obeticholic acid6 78 Wks / Ph3 Discontinued 25mg (n~306) 10mg (n~306) 10% 12% Semaglutide2 48 Wks / Ph2b Active GLP-1 2.4mg (n=47) 11% Simtuzumab7 96 Wks / Ph2b
Discontinued LOXL-2 200mg (n=76) 700mg (n=79) 8% 8% 1 Includes results from publicly reported placebo-controlled studies in patients with compensated cirrhosis due to MASH reporting either ≥ 1-stage fibrosis improvement and no worsening of
MASH (EFX, semaglutide, selonsertib, pegbelfermin, obeticholic acid) or only ≥ 1-stage fibrosis improvement (aldafermin, simtuzumab); numerical values represent percent responders. 2 Loomba, R et al. (2023) Lancet Gastro Hep 8:511-22; 3
Harrison, SH et al. (2020) J Hepatol 73(1):26-39; 4 Abdelmalek, MF et al. (2023) Clinical Gastro Hep 23:S1542-3565; 5 NGM Bio (2023) September Corporate Overview; 6 Intercept (2022) September 30 Press Release; 7 Harrison, SA et al. (2018)
Gastroenterology 155:1140–1153. Note: These data are derived from different clinical trials at different points in time, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no
head-to-head clinical trials have been conducted. 29% 13% 28% 23% 11% 13% Efruxifermin 96 Wks / Ph2b Active Fc-FGF21 50mg (n=46) 28mg (n=41) **39%** 29% 15% pbo (n=47) pbo (n=24) pbo (n=172) pbo (n=39) pbo (n=56) pbo (n~306) pbo (n=75) ©2025
AKERO THERAPEUTICS. ** p<0.01, versus placebo (CMH test)
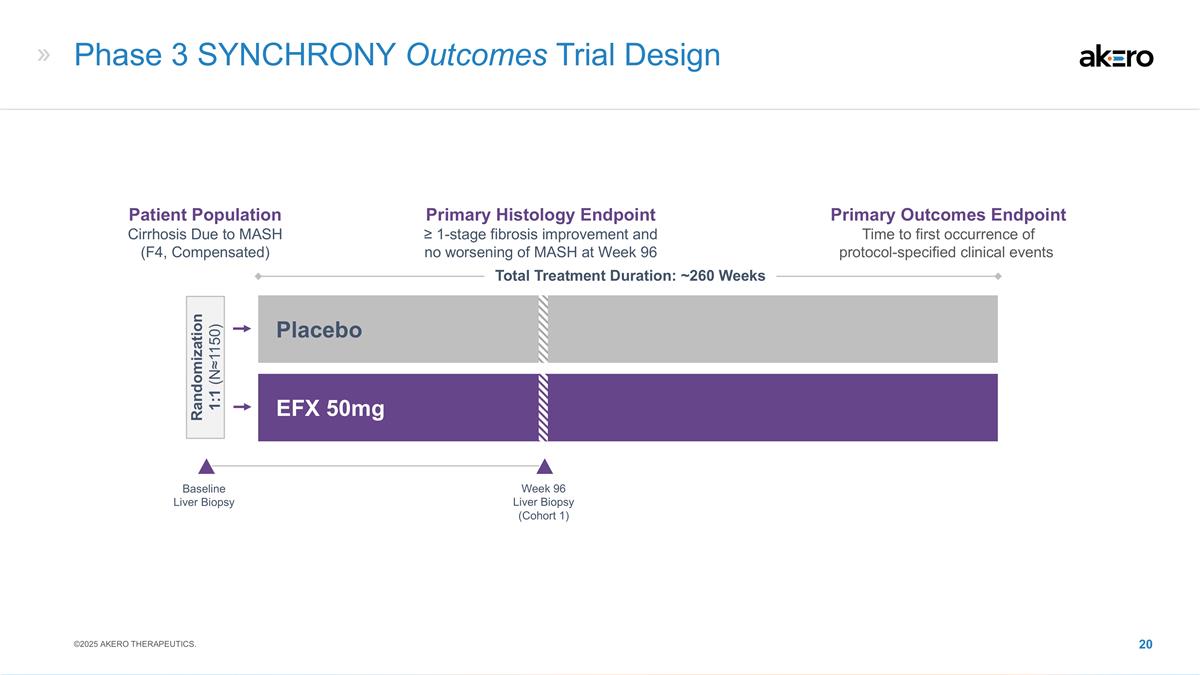
Phase 3 SYNCHRONY Outcomes Trial
Design Baseline Liver Biopsy EFX 50mg Placebo Total Treatment Duration: ~260 Weeks Week 96 Liver Biopsy (Cohort 1) Patient Population Cirrhosis Due to MASH (F4, Compensated) Primary Histology Endpoint ≥ 1-stage fibrosis improvement and no
worsening of MASH at Week 96 Primary Outcomes Endpoint Time to first occurrence of protocol-specified clinical events Randomization 1:1 (N≈1150) ©2025 AKERO THERAPEUTICS.
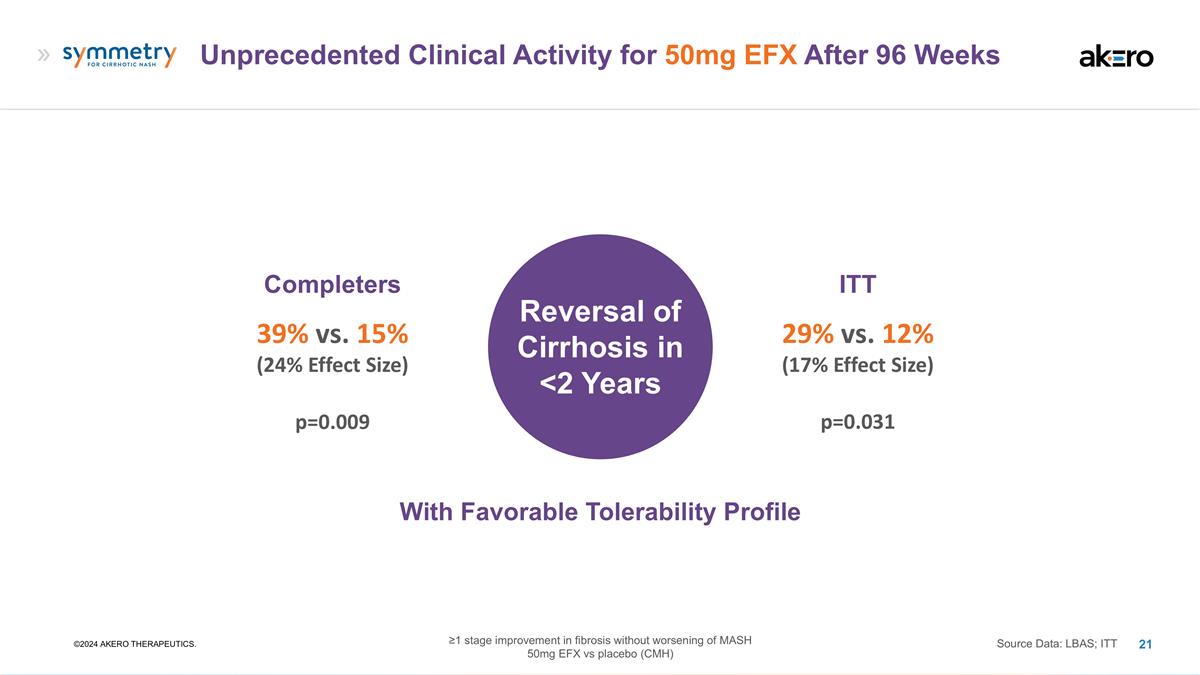
Unprecedented Clinical Activity for
50mg EFX After 96 Weeks ©2024 AKERO THERAPEUTICS. Completers 39% vs. 15% (24% Effect Size) Source Data: LBAS; ITT ITT 29% vs. 12% (17% Effect Size) Reversal of Cirrhosis in <2 Years p=0.009 p=0.031 With Favorable Tolerability Profile
≥1 stage improvement in fibrosis without worsening of MASH 50mg EFX vs placebo (CMH)

AKERO THERAPEUTICS 601 Gateway
Boulevard Suite 350 South San Francisco, CA 94080 Nasdaq: AKRO

Corporate Presentation Restoring
Balance. Renewing Life. January 2025 Exhibit 99.3

Safe Harbor and Legal Disclaimers This
presentation may contain “forward-looking statements” of Akero Therapeutics, Inc. (“we,” “us,” “our,” “Akero” or the “Company”) within the meaning of the Private Securities
Litigation Reform Act of 1995 relating to our business, operations, and financial conditions, including but not limited to current express or implied beliefs, expectations and assumptions regarding: the future of our business; future plans and
strategies, including our expectations around the therapeutic potential and clinical benefits of Efruxifermin (“EFX”), including in combination with GLP-1 receptor agonist therapies; our development plans for EFX, including our belief in
the unique potential of EFX as a foundational metabolic dysfunction-associated steatohepatitis (“MASH”) therapy; our preclinical and clinical results, including our safety/tolerability, laboratory measures and histology data from our
Phase 2b HARMONY study, Phase 2b SYMMETRY study, the Cohort D expansion of our Phase 2b SYMMETRY study, and the week 96 results of the SYMMETRY study, which are preliminary topline results that are subject to audit and verification procedures and
additional data that could result in material changes in the final data; the potential benefits resulting from the PRIME, Breakthrough Therapy and Fast Track designations of EFX; the SYNCHRONY Phase 3 program, including the SYNCHRONY Histology,
SYNCHRONY Real-World, and SYNCHRONY Outcomes studies, their respective trial designs and expected timing to report results for the respective primary endpoints; the availability of a new drug product formulation to support Phase 3 clinical trials;
risks related to the competitive landscape; the timing and potential benefits of our regulatory interactions; and our use of capital, expenses and other future financial results, including the expected cash, cash equivalents and short-term
marketable securities as of December 31, 2024, and cash runway. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,”
“plan,” “would,” “should” and “could,” and similar expressions or words, identify forward-looking statements. New risks and uncertainties may emerge from time to time, and it is not possible to predict
all risks and uncertainties. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward-looking
statements, even if new information becomes available in the future. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the
forward-looking statements, see the section entitled “Risk Factors” in our most recent annual report on Form 10-K and quarterly report on Form 10-Q filed with the Securities and Exchange Commission (the “SEC”), as well as
discussions of potential risks, uncertainties, and other important factors in our other subsequent filings with the SEC. All information in this presentation is as of the date hereof, and we undertake no duty to update this information unless
required by law. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the
Company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from
third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe
our own internal research is reliable, such research has not been verified by any independent source. ©2025 AKERO THERAPEUTICS.
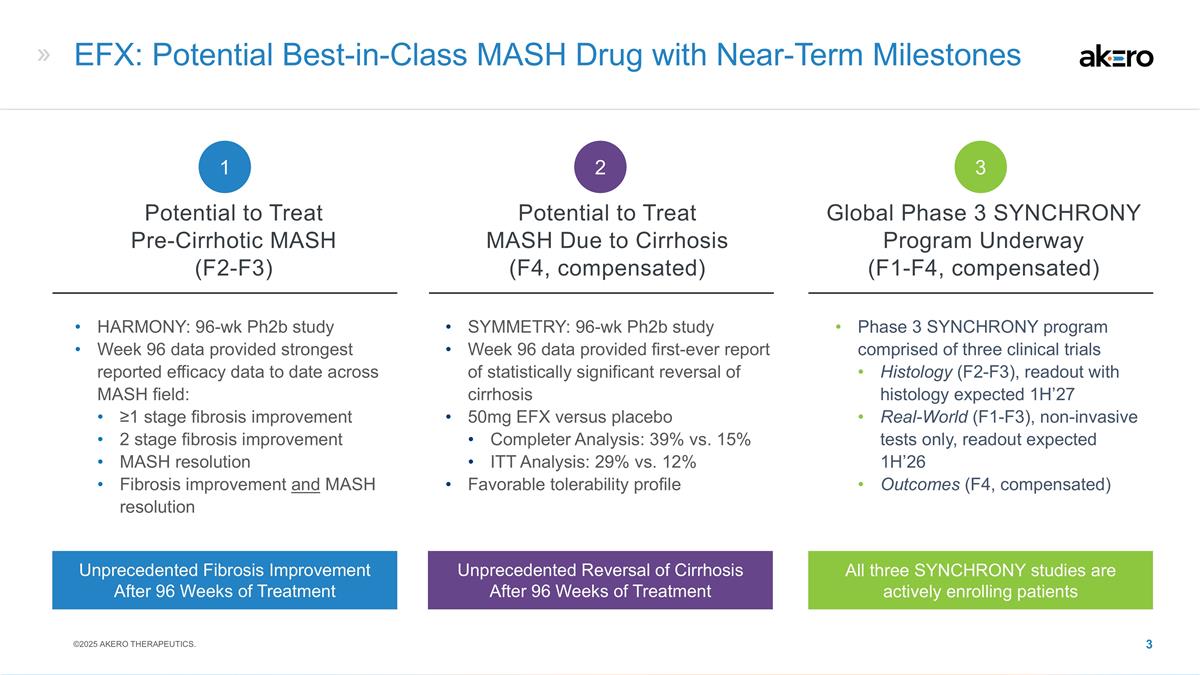
HARMONY: 96-wk Ph2b study Week 96 data
provided strongest reported efficacy data to date across MASH field: ≥1 stage fibrosis improvement 2 stage fibrosis improvement MASH resolution Fibrosis improvement and MASH resolution SYMMETRY: 96-wk Ph2b study Week 96 data provided
first-ever report of statistically significant reversal of cirrhosis 50mg EFX versus placebo Completer Analysis: 39% vs. 15% ITT Analysis: 29% vs. 12% Favorable tolerability profile EFX: Potential Best-in-Class MASH Drug with Near-Term Milestones
Phase 3 SYNCHRONY program comprised of three clinical trials Histology (F2-F3), readout with histology expected 1H’27 Real-World (F1-F3), non-invasive tests only, readout expected 1H’26 Outcomes (F4, compensated) Potential to Treat
Pre-Cirrhotic MASH (F2-F3) Potential to Treat MASH Due to Cirrhosis (F4, compensated) Global Phase 3 SYNCHRONY Program Underway (F1-F4, compensated) 1 2 3 Unprecedented Reversal of Cirrhosis After 96 Weeks of Treatment All three SYNCHRONY studies
are actively enrolling patients Unprecedented Fibrosis Improvement After 96 Weeks of Treatment ©2025 AKERO THERAPEUTICS.
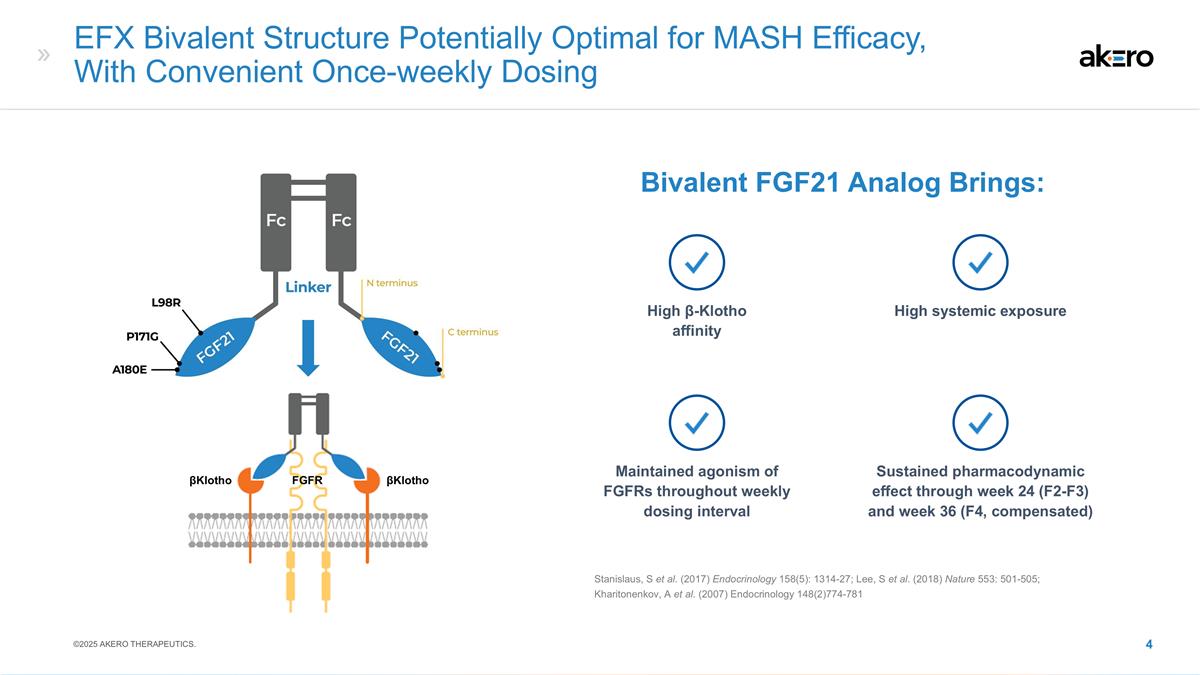
EFX Bivalent Structure Potentially
Optimal for MASH Efficacy, With Convenient Once-weekly Dosing Bivalent FGF21 Analog Brings: Stanislaus, S et al. (2017) Endocrinology 158(5): 1314-27; Lee, S et al. (2018) Nature 553: 501-505; Kharitonenkov, A et al. (2007) Endocrinology
148(2)774-781 High β-Klotho affinity High systemic exposure Maintained agonism of FGFRs throughout weekly dosing interval Sustained pharmacodynamic effect through week 24 (F2-F3) and week 36 (F4, compensated) βKlotho FGFR βKlotho
©2025 AKERO THERAPEUTICS.
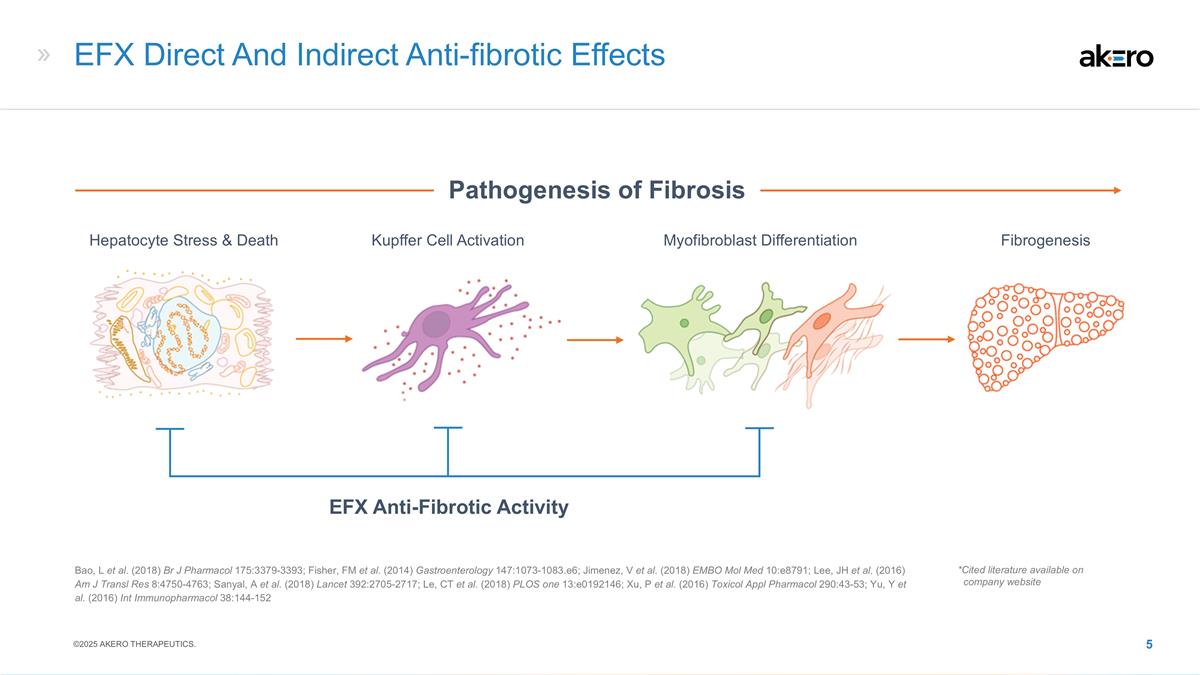
EFX Direct And Indirect Anti-fibrotic
Effects EFX Anti-Fibrotic Activity Hepatocyte Stress & Death Myofibroblast Differentiation Kupffer Cell Activation Fibrogenesis Pathogenesis of Fibrosis Bao, L et al. (2018) Br J Pharmacol 175:3379-3393; Fisher, FM et al. (2014) Gastroenterology
147:1073-1083.e6; Jimenez, V et al. (2018) EMBO Mol Med 10:e8791; Lee, JH et al. (2016) Am J Transl Res 8:4750-4763; Sanyal, A et al. (2018) Lancet 392:2705-2717; Le, CT et al. (2018) PLOS one 13:e0192146; Xu, P et al. (2016) Toxicol Appl Pharmacol
290:43-53; Yu, Y et al. (2016) Int Immunopharmacol 38:144-152 *Cited literature available on company website ©2025 AKERO THERAPEUTICS.
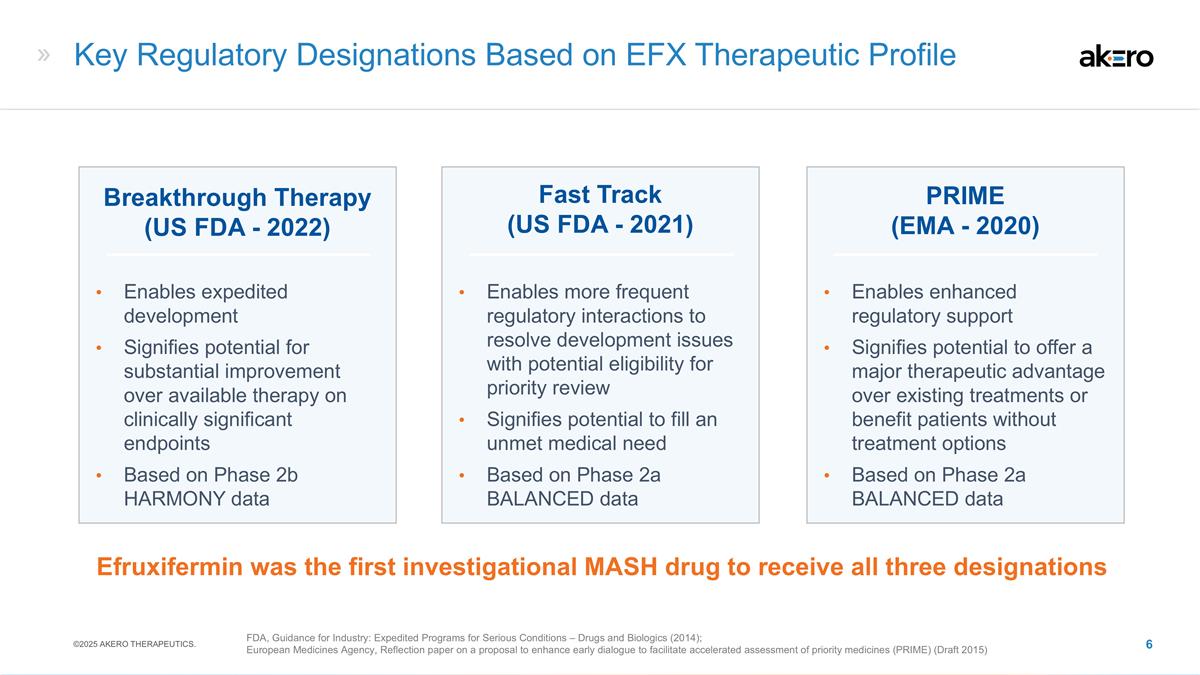
Key Regulatory Designations Based on
EFX Therapeutic Profile Fast Track (US FDA - 2021) Breakthrough Therapy (US FDA - 2022) Enables expedited development Signifies potential for substantial improvement over available therapy on clinically significant endpoints Based on Phase 2b
HARMONY data PRIME (EMA - 2020) Enables more frequent regulatory interactions to resolve development issues with potential eligibility for priority review Signifies potential to fill an unmet medical need Based on Phase 2a BALANCED data Enables
enhanced regulatory support Signifies potential to offer a major therapeutic advantage over existing treatments or benefit patients without treatment options Based on Phase 2a BALANCED data Efruxifermin was the first investigational MASH drug to
receive all three designations FDA, Guidance for Industry: Expedited Programs for Serious Conditions – Drugs and Biologics (2014); European Medicines Agency, Reflection paper on a proposal to enhance early dialogue to facilitate accelerated
assessment of priority medicines (PRIME) (Draft 2015) ©2025 AKERO THERAPEUTICS.
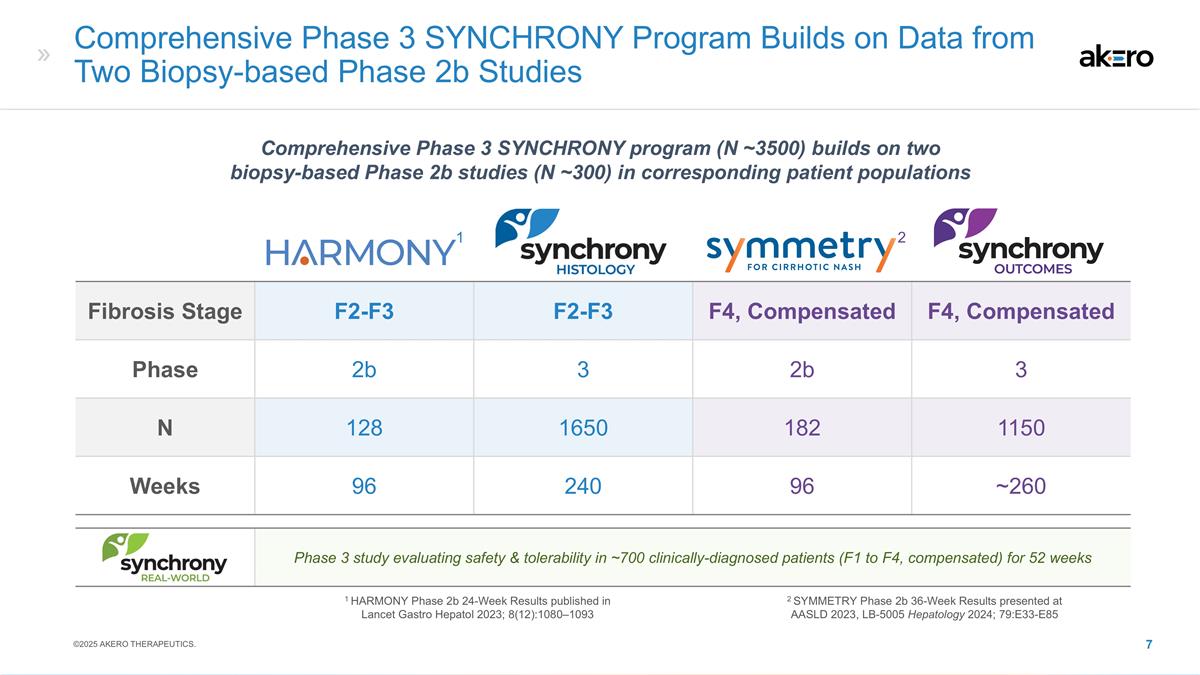
Fibrosis Stage F2-F3 F2-F3 F4,
Compensated F4, Compensated Phase 2b 3 2b 3 N 128 1650 182 1150 Weeks 96 240 96 ~260 Phase 3 study evaluating safety & tolerability in ~700 clinically-diagnosed patients (F1 to F4, compensated) for 52 weeks Comprehensive Phase 3 SYNCHRONY
Program Builds on Data from Two Biopsy-based Phase 2b Studies 1 HARMONY Phase 2b 24-Week Results published in Lancet Gastro Hepatol 2023; 8(12):1080–1093 Comprehensive Phase 3 SYNCHRONY program (N ~3500) builds on two biopsy-based Phase 2b
studies (N ~300) in corresponding patient populations 1 2 2 SYMMETRY Phase 2b 36-Week Results presented at AASLD 2023, LB-5005 Hepatology 2024; 79:E33-E85 ©2025 AKERO THERAPEUTICS.
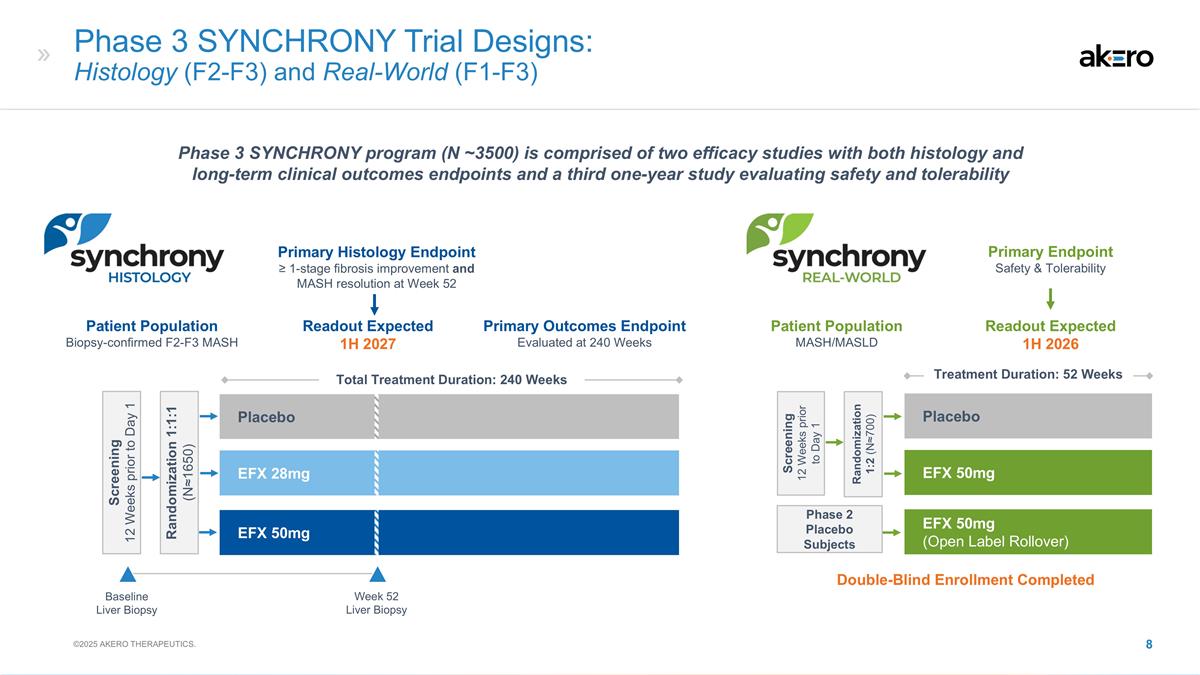
Phase 3 SYNCHRONY Trial Designs:
Histology (F2-F3) and Real-World (F1-F3) Baseline Liver Biopsy Screening 12 Weeks prior to Day 1 Randomization 1:1:1 (N≈1650) EFX 28mg Placebo Total Treatment Duration: 240 Weeks EFX 50mg EFX 50mg Placebo EFX 50mg (Open Label Rollover) Primary
Endpoint Safety & Tolerability Screening 12 Weeks prior to Day 1 Randomization 1:2 (N≈700) Week 52 Liver Biopsy Phase 2 Placebo Subjects Treatment Duration: 52 Weeks Patient Population Biopsy-confirmed F2-F3 MASH Patient Population
MASH/MASLD Primary Histology Endpoint ≥ 1-stage fibrosis improvement and MASH resolution at Week 52 Primary Outcomes Endpoint Evaluated at 240 Weeks Readout Expected 1H 2027 Readout Expected 1H 2026 Phase 3 SYNCHRONY program (N ~3500) is
comprised of two efficacy studies with both histology and long-term clinical outcomes endpoints and a third one-year study evaluating safety and tolerability ©2025 AKERO THERAPEUTICS. Double-Blind Enrollment Completed
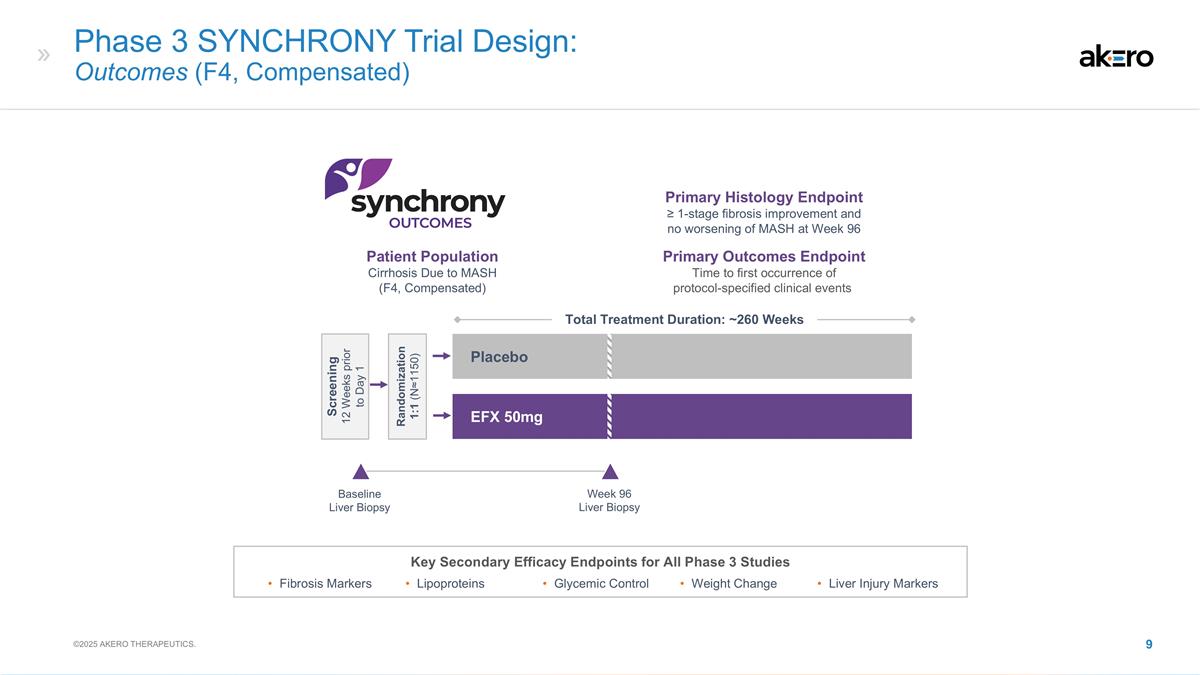
Phase 3 SYNCHRONY Trial Design:
Outcomes (F4, Compensated) Key Secondary Efficacy Endpoints for All Phase 3 Studies Fibrosis Markers Lipoproteins Glycemic Control Weight Change Liver Injury Markers Baseline Liver Biopsy EFX 50mg Placebo Total Treatment Duration: ~260 Weeks Week 96
Liver Biopsy Patient Population Cirrhosis Due to MASH (F4, Compensated) Primary Histology Endpoint ≥ 1-stage fibrosis improvement and no worsening of MASH at Week 96 Primary Outcomes Endpoint Time to first occurrence of protocol-specified
clinical events Screening 12 Weeks prior to Day 1 Randomization 1:1 (N≈1150) ©2025 AKERO THERAPEUTICS.
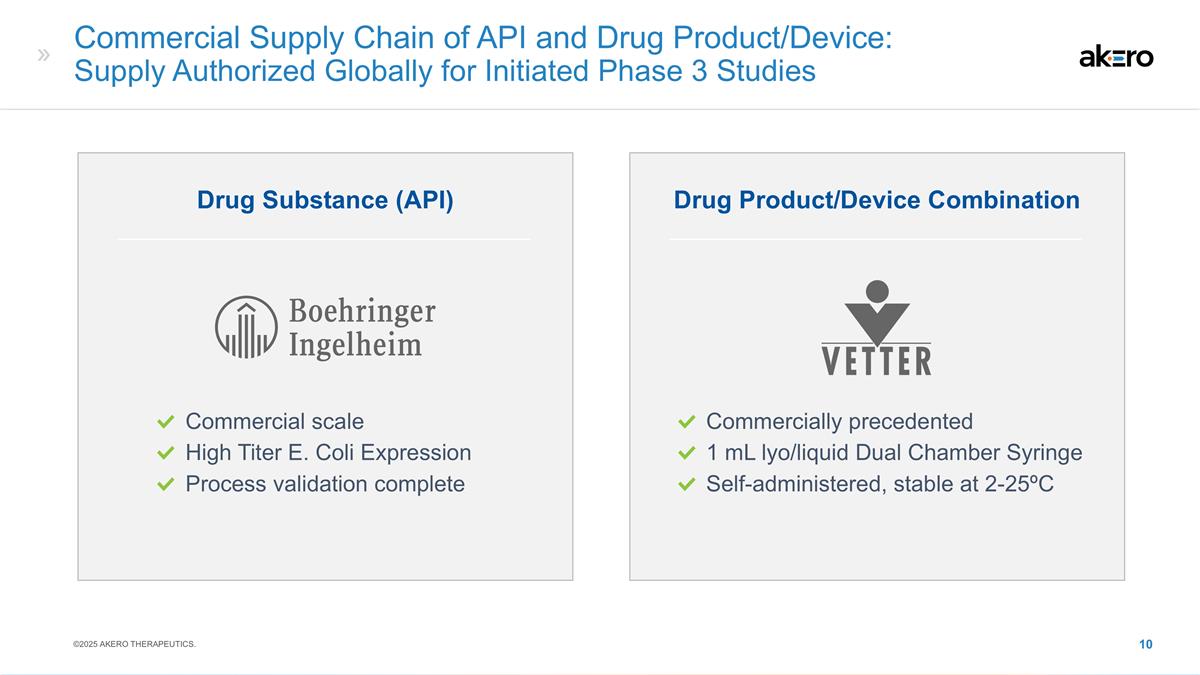
Commercial Supply Chain of API and
Drug Product/Device: Supply Authorized Globally for Initiated Phase 3 Studies Drug Product/Device Combination Drug Substance (API) Commercial scale High Titer E. Coli Expression Process validation complete Commercially precedented 1 mL lyo/liquid
Dual Chamber Syringe Self-administered, stable at 2-25ºC ©2025 AKERO THERAPEUTICS.
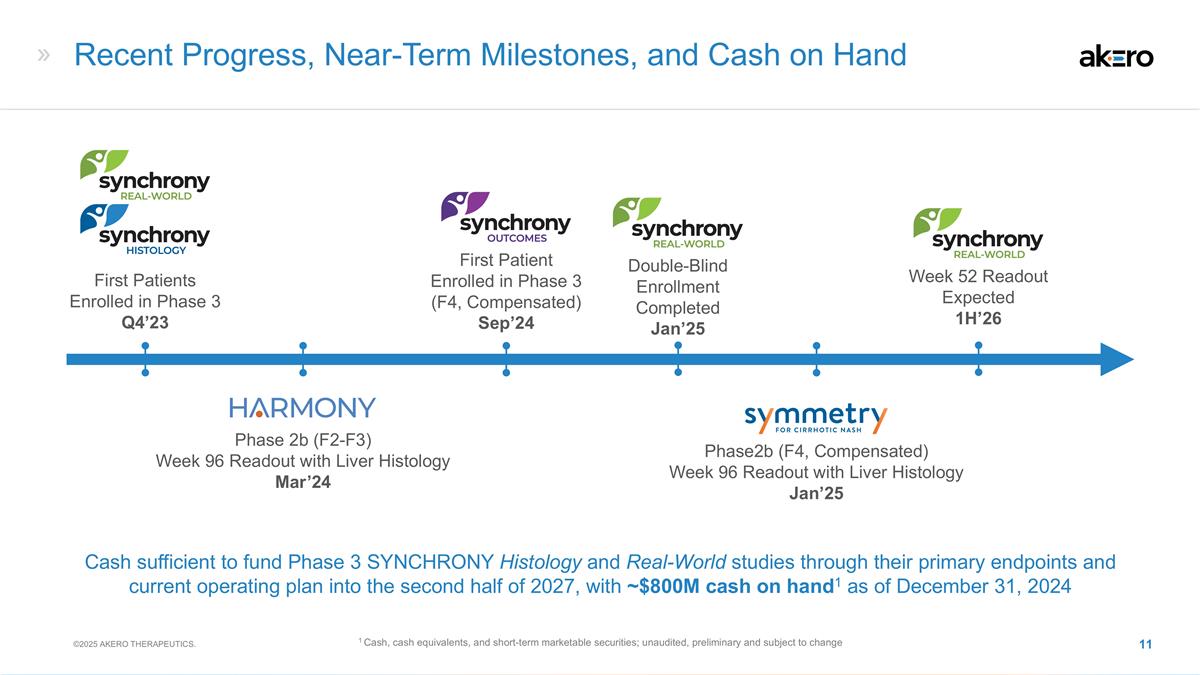
Recent Progress, Near-Term
Milestones, and Cash on Hand First Patients Enrolled in Phase 3 Q4’23 Phase 2b (F2-F3) Week 96 Readout with Liver Histology Mar’24 Phase2b (F4, Compensated) Week 96 Readout with Liver Histology Jan’25 First Patient Enrolled in
Phase 3 (F4, Compensated) Sep’24 Cash sufficient to fund Phase 3 SYNCHRONY Histology and Real-World studies through their primary endpoints and current operating plan into the second half of 2027, with ~$800M cash on hand1 as of December 31,
2024 1 Cash, cash equivalents, and short-term marketable securities; unaudited, preliminary and subject to change ©2025 AKERO THERAPEUTICS. Double-Blind Enrollment Completed Jan’25 Week 52 Readout Expected 1H’26

EFX: Treatment of Compensated
Cirrhosis (F4) Due to MASH Statistically Significant Reversal of Cirrhosis after 96 Weeks ©2025 AKERO THERAPEUTICS.
High Risk of Mortality Associated
with Cirrhosis Due to MASH Survival Free of Liver Transplantation Follow-up (years) Cumulative Survival (%) Angulo, P et al. (2015) Gastroenterology 149:389-397 P<0.001 F4 50% ~50% 5-year mortality without liver transplant for F4 patients 40% 20%
0% 100% 80% 60% ©2025 AKERO THERAPEUTICS. F3 F2 F1 F0
1mg (n=42) 3mg (n=55) 21% 15% No
Compound To Date Has Shown Statistically Significant Reversal of Cirrhosis Due to MASH in Clinical Trials 6mg (n=351 18mg (n=354) 20mg (n=37) 40mg (n=39) 14% 13% 31% 24% 25mg (n~306) 10mg (n~306) 10% 12% 2.4mg (n=47) 11% 200mg (n=76) 700mg (n=79) 8%
8% Aldafermin5 48 Wks / Ph2b Discontinued FGF19 Selonsertib3 48 Wks / Ph3 Discontinued ASK-1 Peg-FGF21 Pegbelfermin4 48 Wks / Ph2b Discontinued FXR Obeticholic acid6 78 Wks / Ph3 Discontinued Semaglutide2 48 Wks / Ph2b Active GLP-1 Simtuzumab7 96
Wks / Ph2b Discontinued LOXL-2 1 Includes results from publicly reported placebo-controlled studies in patients with compensated cirrhosis due to MASH reporting either ≥ 1-stage fibrosis improvement and no worsening of MASH (semaglutide,
selonsertib, pegbelfermin, obeticholic acid) or only ≥ 1-stage fibrosis improvement (aldafermin, simtuzumab); numerical values represent percent responders. 2 Loomba, R et al. (2023) Lancet Gastro Hep 8:511-22; 3 Harrison, SH et al. (2020) J
Hepatol 73(1):26-39; 4 Abdelmalek, MF et al. (2023) Clinical Gastro Hep 23:S1542-3565; 5 NGM Bio (2023) September Corporate Overview; 6 Intercept (2022) September 30 Press Release; 7 Harrison, SA et al. (2018) Gastroenterology 155:1140–1153.
Note: These data are derived from different clinical trials at different points in time, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been
conducted. 29% 13% 28% 23% 11% 13% pbo (n=24) pbo (n=172) pbo (n=39) pbo (n=56) pbo (n~306) pbo (n=75) ©2025 AKERO THERAPEUTICS. Fibrosis Improvement ≥1 Stage1
Phase 2b SYMMETRY Trial Design:
Compensated Cirrhosis (F4) Due to MASH with Liver Histology at 36 and 96 weeks EFX 28mg (N=57) Placebo (N=61) EFX 50mg (N=63) Screening Randomization Phase 2b Key Inclusion Criteria1 F4 MASH, Child-Pugh A T2D or 2 of 4 components of metabolic
syndrome2 Phase 2b Primary Endpoint ≥1 Stage Fibrosis Improvement with no Worsening of MASH at Week 36 Key Secondary Efficacy Endpoints MASH Resolution Fibrosis Markers Lipoproteins Glycemic Control Weight Change Liver Injury Markers 1 All
patients had biopsy-proven compensated cirrhosis (fibrosis stage 4) due to definitive MASH or cryptogenic cirrhosis presumed secondary to MASH. Subjects with cryptogenic cirrhosis were limited to approximately 20% of the total study population. 2
Obesity, dyslipidemia, elevated blood pressure, and elevated fasting glucose Screening Liver Biopsy Week 36 Liver Biopsy Week 96 Liver Biopsy ©2025 AKERO THERAPEUTICS. Total Treatment Duration: 96 Weeks
Baseline Demographics Parameter
(Mean) Placebo (N=61) EFX 28mg (N=57) EFX 50mg (N=63) Age (Years) 61 62 59 Sex (% Female) 62 68 70 Enhanced Liver Fibrosis (ELF) Score 10.4 10.6 10.5 Liver Stiffness by VCTE (FibroScan) (kPa) 24.7 24.1 24.5 Alanine Aminotransferase (ALT) (U/L) 40.3
40.1 38.4 Aspartate Aminotransferase (AST) (U/L) 35.5 37.1 37.5 Type 2 Diabetes (%) 82 81 78 HbA1c (%) 6.8 6.8 6.6 HOMA-IR 11.8 11.7 11.5 Baseline Use of GLP-1 Receptor Agonists (%) 26 19 33 Triglycerides (mg/dL) 143 148 159 Statin Use (%) 53 46 43
Weight (kg) 102 99 95 ©2025 AKERO THERAPEUTICS. Source Data: FAS
11% Discontinued for Administrative
Reasons1 Patient Disposition 28mg (N=41) (89% Retention) 50mg (N=46) (90% Retention) Placebo (N=47) (82% Retention) Biopsy Collected at Week 36 (N=154) Randomized & Dosed (N=181) Biopsy Collected at Week 96 (N=134) 87% Retained from Week 36 to
96 2% Discontinued Due to AEs ©2025 AKERO THERAPEUTICS. 1 Primarily withdrawal of consent, loss to follow-up, and investigator discretion
SYMMETRY Week 96 Main Analysis Sets
Analysis Set1 N Description Week 96 Liver Biopsy Analysis Set (LBAS) 134 All patients with baseline and Week 96 biopsy results Safety Set / Full Analysis Set (FAS)2 181 All randomized patients who received at least one dose of study drug 28mg (N=41)
50mg (N=46) Placebo (N=47) 50mg (N=63) 28mg (N=57) Placebo (N=61) 1 P-values, where presented, are nominal 2 ITT Analyses are based on FAS where missing biopsy = failure ©2025 AKERO THERAPEUTICS. All reported results are topline preliminary
data.
Placebo (N=61) EFX 28mg (N=57) EFX
50mg (N=63) 12% 21% *29%* EFX 28mg N=41 EFX 50mg N=46 Placebo N=47 Fibrosis Improvement ≥1 Stage & No Worsening of MASH at Week 96 Primary Analysis1 1 All patients with baseline and Week 96 biopsies ** p<0.01, versus placebo (CMH test3)
39%** 29% 15% Unprecedented Reversal of Cirrhosis Observed in SYMMETRY with 50mg EFX ITT Analysis2 * p<0.05, versus placebo (CMH test) 2 Missing biopsy = failure Source Data: LBAS; ITT ©2025 AKERO THERAPEUTICS. 3 Cochran-Mantel-Haenszel
test
Sustained (n=6) New (n=6) Sustained
(n=9) New (n=9) Sust. (n=4) New (n=3) **39%** EFX 28mg N=41 EFX 50mg N=46 Placebo N=47 29% 15% Fibrosis Improvement ≥1 Stage & No Worsening of MASH at Week 96 Sustained1 & New2 Responders Placebo (N=8) EFX 28mg (N=9) EFX 50mg (N=12) 4
(50%) 6 (67%) 9 (75%) Proportion of Week 36 Responders with Sustained Response at Week 963,5 Placebo (N=39) EFX 28mg (N=32) EFX 50mg (N=34) 3 (8%) 6 (19%) 9 (26%) Proportion of Week 36 Non-Responders with New Response at Week 964,5 3 Among Week 36
responders with Week 96 biopsies 4 Among Week 36 non-responders with Week 96 biopsies 5 Not analyzed for statistical significance Improved and Durable Response Observed with Longer Treatment Source Data: LBAS ©2025 AKERO THERAPEUTICS. 1
Responder at Weeks 36 & 96; 2 Responder at Week 96 ** p<0.01, versus placebo (CMH test)
EFX 28mg N=32 EFX 50mg N=29 Placebo
N=36 45%** 28% 17% Subgroup (N=97): Patients Without GLP-1 Use at Baseline EFX 28mg N=41 EFX 50mg N=46 Placebo N=47 ** p<0.01, versus placebo (CMH test) 39%** 29% 15% All Patients with Baseline and Week 96 Biopsies (N=134) Baseline GLP-1 Therapy
Did Not Impact Reversal of Cirrhosis Source Data: LBAS ** p<0.01, versus placebo (CMH test) Fibrosis Improvement ≥1 Stage & No Worsening of MASH at Week 96 ©2025 AKERO THERAPEUTICS.
EFX 28mg N=32 EFX 50mg N=40 Placebo
N=34 MASH Resolution Primary Analysis1 ** p<0.01, *** p<0.001, versus placebo (CMH test) 55%** 59%*** 18% MASH Resolution Observed at Week 96 Source Data: LBAS subgroup (definitive MASH at baseline); FAS ©2025 AKERO THERAPEUTICS. Placebo
(N=45) EFX 28mg (N=45) EFX 50mg (N=52) 13% **42%** **42%** ITT Analysis2 ** p<0.01, versus placebo (CMH test) 2 Missing biopsy = failure 1 All patients with baseline and Week 96 biopsies who had definitive MASH at baseline
Treatment-Emergent Adverse Events
(TEAEs): Cumulative from Baseline through Week 96 TEAE Overview Placebo (N=61) EFX 28mg (N=57) EFX 50mg (N=63) TEAEs Leading to Death a 1 (2%) a 0 (0%) 0 (0%) Treatment-Emergent Serious Adverse Events (SAEs) 11 (18%) 15 (26%) 15 (24%) Drug-Related
SAEs 0 0 0 TEAEs Leading to Discontinuation of Treatment 2 (3%) 6 (11%) 11 (18%) Weeks 37-96: Cirrhosis-Related Clinical Events 0 b 1 b c 1 c Weeks 37-96: Other Adverse Events 0 0 d 1 d Most Frequent (≥15%) Drug-Related TEAEs Placebo (N=61)
EFX 28mg (N=57) EFX 50mg (N=63) Diarrhea, n (%) 10 (16%) 11 (19%) 19 (30%) Nausea, n (%) 8 (13%) 11 (19%) 18 (29%) Increased Appetite, n (%) 3 (5%) 7 (12%) 18 (29%) Injection Site Erythema, n (%) 5 (8%) 10 (18%) 14 (22%) a Pneumonia (prior to week
36); b Ascites; c Hepatic encephalopathy; d Diarrhea ©2024 AKERO THERAPEUTICS. Source Data: Safety Set

Additional Safety Observations for
50mg EFX (Phase 3 Dose) Versus Placebo at Week 96 Bone Mineral Density 43% of 50mg EFX and 41% of placebo patients had osteopenia1 at baseline, but only 3% of 50mg EFX and 7% of placebo patients were treated with bisphosphonates Placebo-adjusted,
significant relative reductions in bone mineral density (5%) for spine and hip were observed for 50mg EFX, or about 2-3% per year For context, treatment with a diabetic dose of GLP-1 was recently reported to be associated with about a 2% reduction
in bone mineral density over one year.2 Equal number of fractures (4 each) observed for 50mg EFX and placebo Markers of Hemostasis and Liver Function Markers of hemostasis and liver function, including bilirubin, MELD score, INR, platelet count,
albumin and Child-Pugh score, remained stable or trended toward improvement for 50mg EFX Electrocardiograms (ECGs), Vital Signs & Body Weight No statistically significant changes in ECGs, heart rate, or blood pressure were observed for 50mg EFX
versus placebo Weight reduction of ~2% was observed for 50mg EFX and placebo ©2025 AKERO THERAPEUTICS. 1 T-score < -1.0 by DXA 2 M. S. Hansen et al., eClinicalMedicine. 72, 102624 (2024)
Pattern of Reductions in Imaging
and Circulating Biomarkers of Fibrosis Corroborate Histological Improvement in Cirrhosis LS Mean Change From Baseline to Week 96 Source Data: FAS (non-missing values only, no imputation) ©2025 AKERO THERAPEUTICS. Liver Stiffness (%)2 -18% -8%
EFX 50mg N=47 Placebo N=49 EFX 28mg N=41 -24%* 2 Measured by FibroScan * p<0.05, versus placebo (MMRM1) (Baseline: 24-25 kPa) ELF Score EFX 50mg N=47 Placebo N=49 EFX 28mg N=41 ***-0.34 *** +0.22 ***-0.53*** *** p<0.001, versus placebo (MMRM1)
(Baseline: 10-11) 1 Mixed Model Repeated Measures
(Baseline: 38-40 U/L) *
p<0.05,** p<0.01, *** p<0.001, versus placebo (MMRM) Statistically Significant Reductions in Markers of Liver Injury Sustained Through Week 96 with 50mg EFX ALT (U/L) LS Mean Change from Baseline AST (U/L) LS Mean Change from Baseline *** *
* ** * ** ** ** ** * ** ** ** *** ** *** ** ** * ©2025 AKERO THERAPEUTICS. Source Data: FAS (Baseline: 36-38 U/L) * p<0.05,** p<0.01, *** p<0.001, versus placebo (MMRM) *** *** *** *** *** *** *** *** *** *** *** ** ** ** *** *** ***
*** *** ** *** *** ***
EFX 50mg N=47 Placebo N=49 EFX 28mg
N=40 HDL Cholesterol +6** -2 +9*** ** p<0.01, *** p<0.001, versus placebo (MMRM) EFX 50mg N=47 Placebo N=49 EFX 28mg N=40 Non-HDL Cholesterol -19 -10 -21* EFX 50mg N=47 Placebo N=49 EFX 28mg N=40 Triglycerides -27* -3 -33* * p<0.05, *versus
placebo (MMRM) Significantly Improved Lipoprotein Profile Observed at Week 96 LS Mean Change From Baseline to Week 96 (mg/dL) ©2025 AKERO THERAPEUTICS. Source Data: FAS * p<0.05 versus placebo (MMRM) (Baseline: 114-24 mg/dL) (Baseline: 50-52
mg/dL) (Baseline: 140-61 mg/dL) LDL cholesterol was reduced by 11% and 9%, respectively, in the 50mg EFX and placebo groups
EFX 50mg N=45 Placebo N=48 EFX 28mg
N=41 C-Peptide (%) * p<0.05, versus placebo (MMRM) -13% -1% *-18%* EFX 50mg N=46 Placebo N=49 EFX 28mg N=41 Adiponectin (%) +32% +8% 69%*** *** p<0.001, versus placebo (MMRM) Significantly Improved Insulin Sensitivity Observed at Week 96 with
50mg EFX Source Data: FAS LS Mean Change From Baseline to Week 96 EFX 50mg N=45 Placebo N=47 EFX 28mg N=41 HOMA-IR -3.4 * +1.6 -4.1** * p<0.05, ** p<0.01, versus placebo (MMRM) ©2025 AKERO THERAPEUTICS. At Week 96, HbA1c remained
unchanged from baseline for 50mg EFX, compared to a slight increase to 7.0 for placebo.
Aldafermin5 48 Wks / Ph2b
Discontinued FGF19 1mg (n=42) 3mg (n=55) 21% 15% EFX is the First Compound that Has Been Reported to Statistically Significantly Reverse Cirrhosis Due to MASH1 Selonsertib3 48 Wks / Ph3 Discontinued ASK-1 Peg-FGF21 Pegbelfermin4 48 Wks / Ph2b
Discontinued 6mg (n=351 18mg (n=354) 20mg (n=37) 40mg (n=39) 14% 13% 31% 24% FXR Obeticholic acid6 78 Wks / Ph3 Discontinued 25mg (n~306) 10mg (n~306) 10% 12% Semaglutide2 48 Wks / Ph2b Active GLP-1 2.4mg (n=47) 11% Simtuzumab7 96 Wks / Ph2b
Discontinued LOXL-2 200mg (n=76) 700mg (n=79) 8% 8% 1 Includes results from publicly reported placebo-controlled studies in patients with compensated cirrhosis due to MASH reporting either ≥ 1-stage fibrosis improvement and no worsening of
MASH (EFX, semaglutide, selonsertib, pegbelfermin, obeticholic acid) or only ≥ 1-stage fibrosis improvement (aldafermin, simtuzumab); numerical values represent percent responders. 2 Loomba, R et al. (2023) Lancet Gastro Hep 8:511-22; 3
Harrison, SH et al. (2020) J Hepatol 73(1):26-39; 4 Abdelmalek, MF et al. (2023) Clinical Gastro Hep 23:S1542-3565; 5 NGM Bio (2023) September Corporate Overview; 6 Intercept (2022) September 30 Press Release; 7 Harrison, SA et al. (2018)
Gastroenterology 155:1140–1153. Note: These data are derived from different clinical trials at different points in time, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no
head-to-head clinical trials have been conducted. 29% 13% 28% 23% 11% 13% Efruxifermin 96 Wks / Ph2b Active Fc-FGF21 50mg (n=46) 28mg (n=41) **39%** 29% 15% pbo (n=47) pbo (n=24) pbo (n=172) pbo (n=39) pbo (n=56) pbo (n~306) pbo (n=75) ©2025
AKERO THERAPEUTICS. ** p<0.01, versus placebo (CMH test)
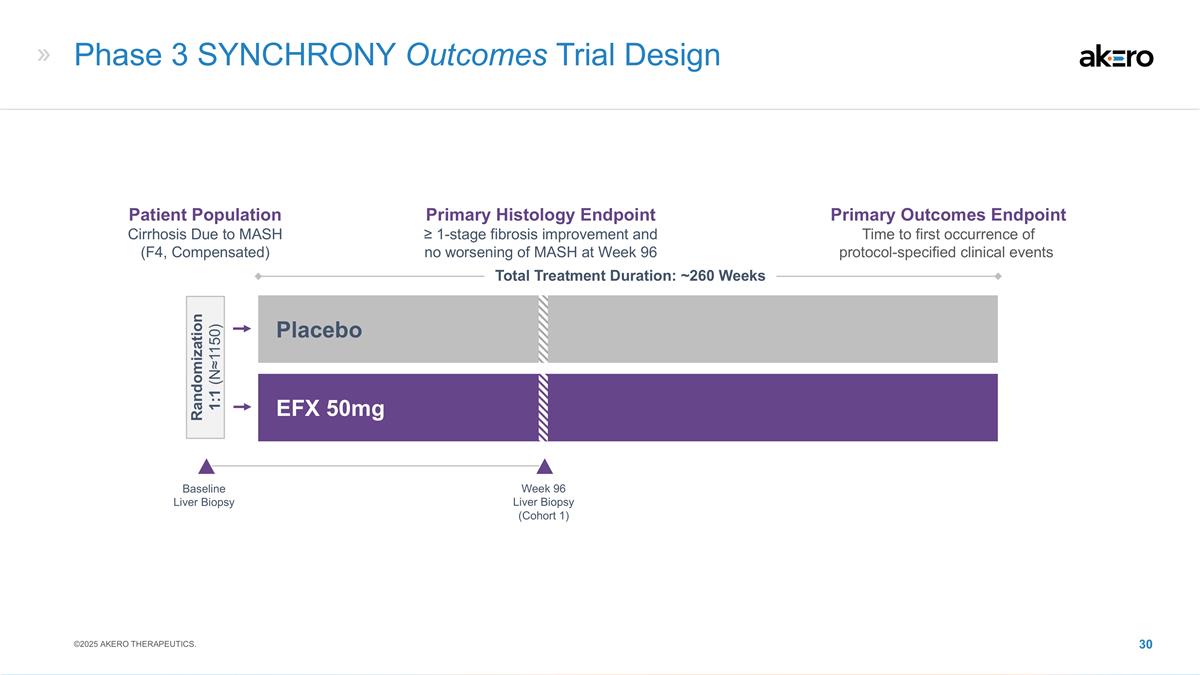
Phase 3 SYNCHRONY Outcomes Trial
Design Baseline Liver Biopsy EFX 50mg Placebo Total Treatment Duration: ~260 Weeks Week 96 Liver Biopsy (Cohort 1) Patient Population Cirrhosis Due to MASH (F4, Compensated) Primary Histology Endpoint ≥ 1-stage fibrosis improvement and no
worsening of MASH at Week 96 Primary Outcomes Endpoint Time to first occurrence of protocol-specified clinical events Randomization 1:1 (N≈1150) ©2025 AKERO THERAPEUTICS.
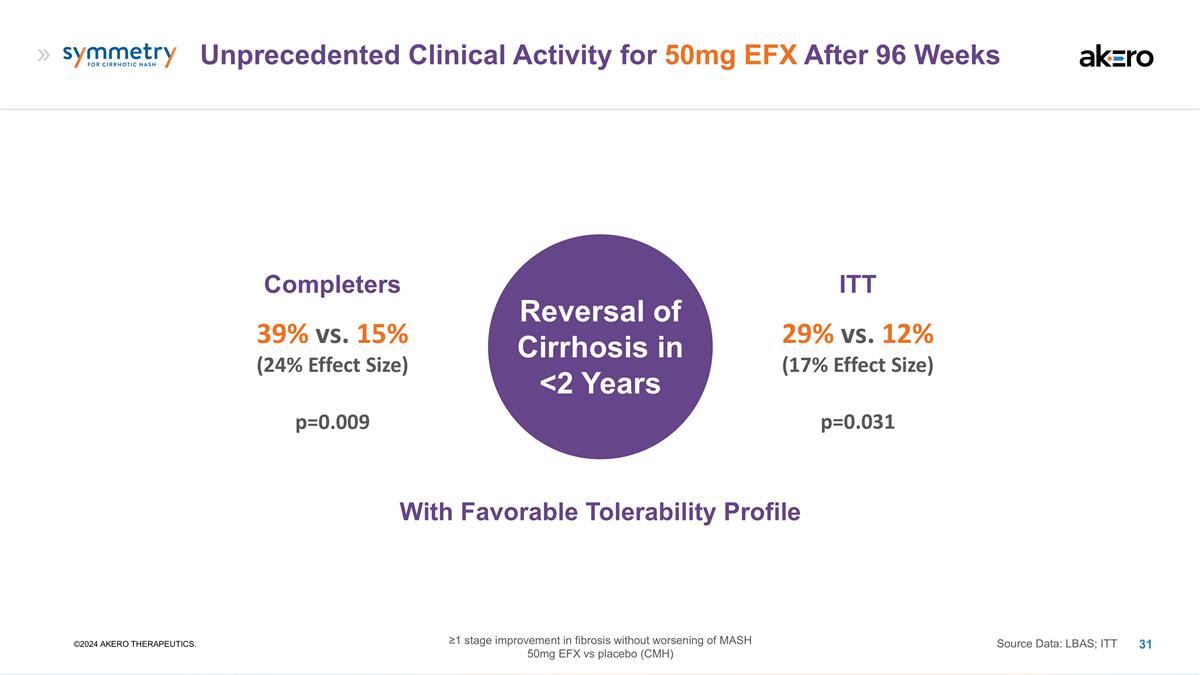
Unprecedented Clinical Activity for
50mg EFX After 96 Weeks ©2024 AKERO THERAPEUTICS. Completers 39% vs. 15% (24% Effect Size) Source Data: LBAS; ITT ITT 29% vs. 12% (17% Effect Size) Reversal of Cirrhosis in <2 Years p=0.009 p=0.031 With Favorable Tolerability Profile
≥1 stage improvement in fibrosis without worsening of MASH 50mg EFX vs placebo (CMH)
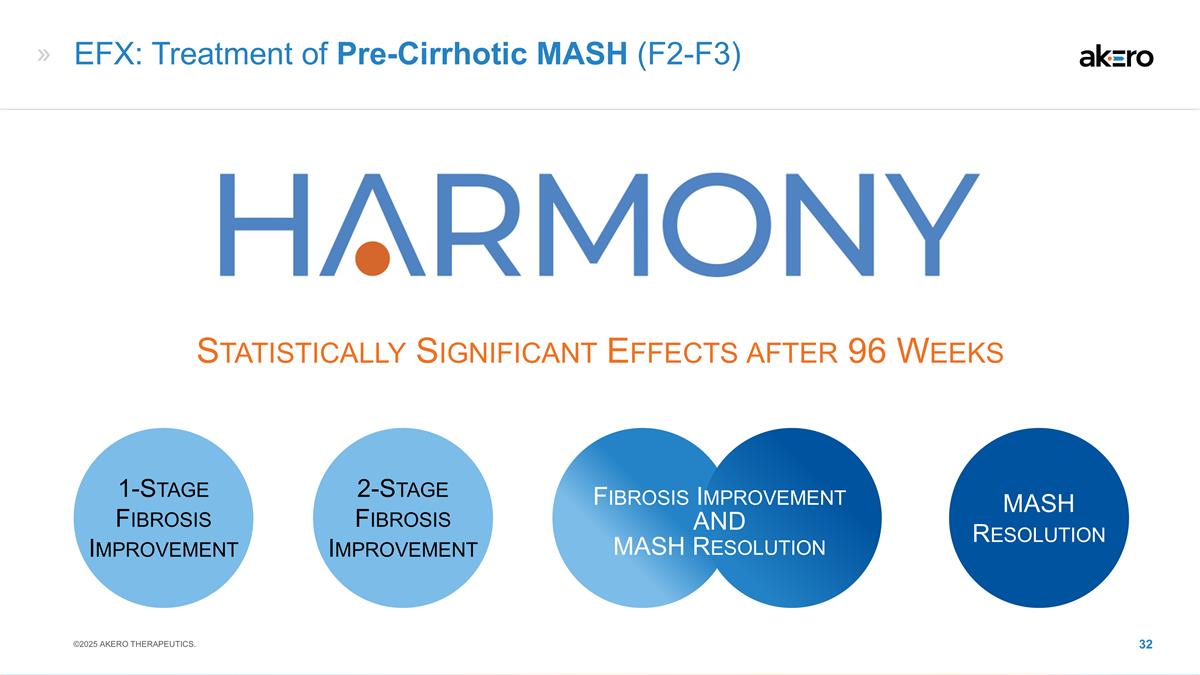
EFX: Treatment of Pre-Cirrhotic
MASH (F2-F3) Statistically Significant Effects after 96 Weeks 1-Stage Fibrosis Improvement MASH Resolution Fibrosis Improvement AND MASH Resolution 2-Stage Fibrosis Improvement ©2025 AKERO THERAPEUTICS.
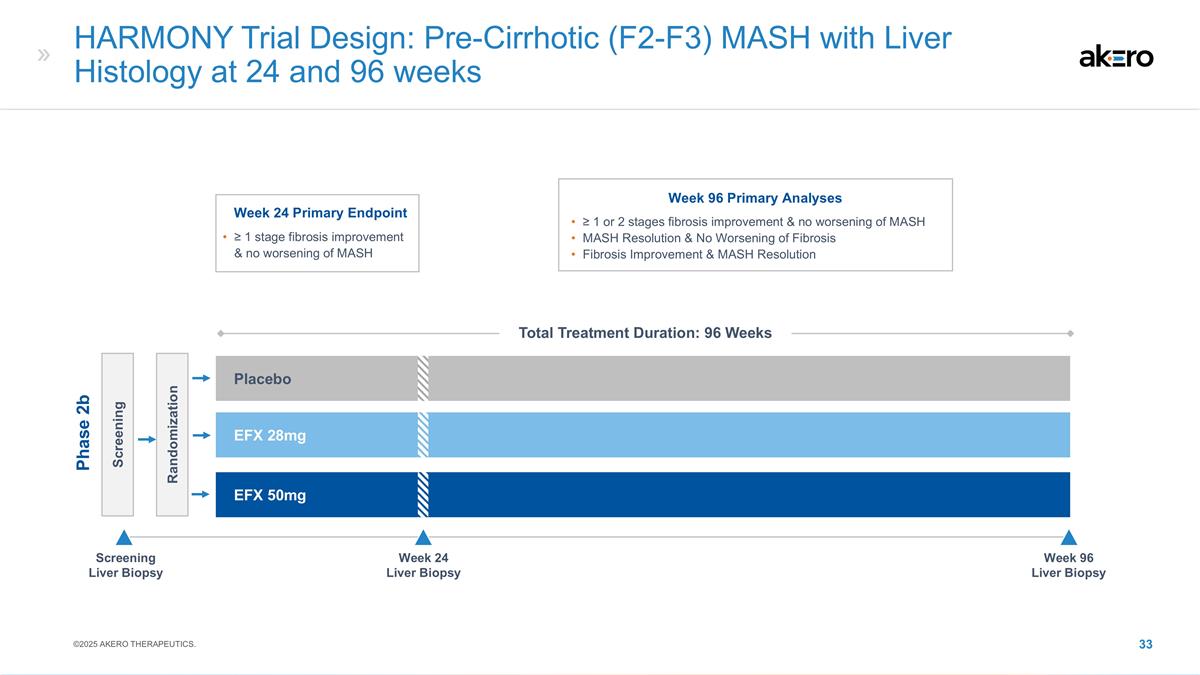
HARMONY Trial Design: Pre-Cirrhotic
(F2-F3) MASH with Liver Histology at 24 and 96 weeks Screening Randomization EFX 28mg Placebo EFX 50mg Phase 2b Screening Liver Biopsy Week 24 Liver Biopsy Week 96 Liver Biopsy Week 96 Primary Analyses ≥ 1 or 2 stages fibrosis improvement
& no worsening of MASH MASH Resolution & No Worsening of Fibrosis Fibrosis Improvement & MASH Resolution Week 24 Primary Endpoint ≥ 1 stage fibrosis improvement & no worsening of MASH ©2025 AKERO THERAPEUTICS. Total
Treatment Duration: 96 Weeks
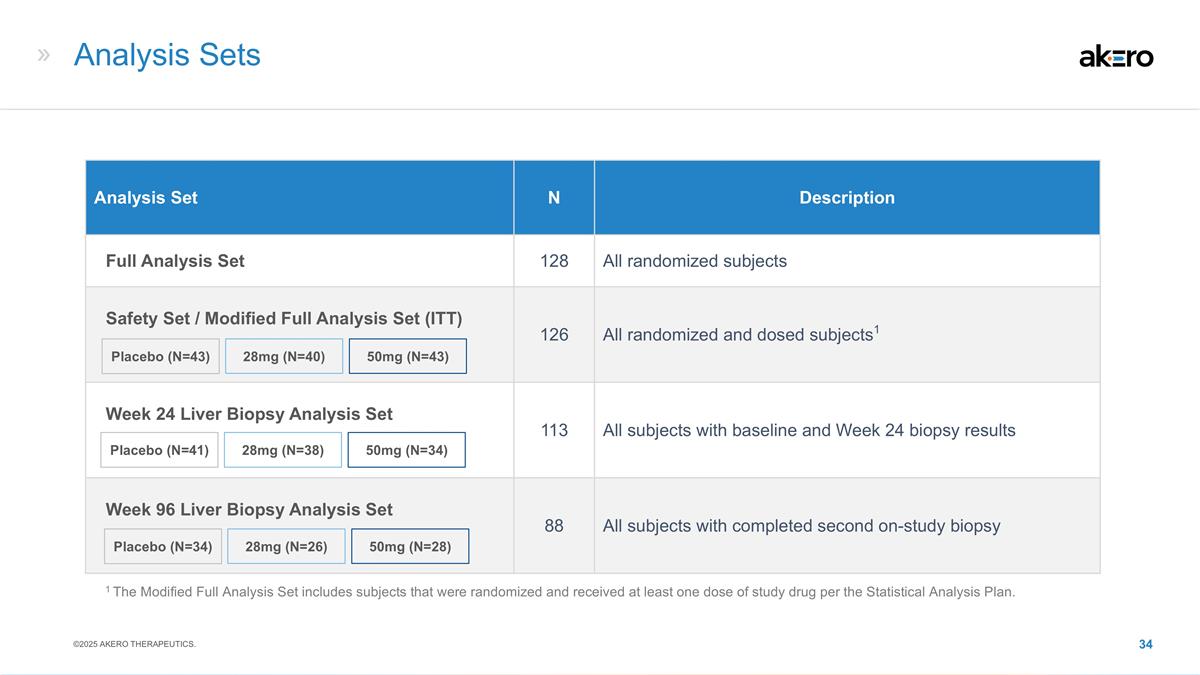
Analysis Sets Analysis Set N
Description Full Analysis Set 128 All randomized subjects Safety Set / Modified Full Analysis Set (ITT) 126 All randomized and dosed subjects1 Week 24 Liver Biopsy Analysis Set 113 All subjects with baseline and Week 24 biopsy results Week 96 Liver
Biopsy Analysis Set 88 All subjects with completed second on-study biopsy 28mg (N=26) 50mg (N=28) Placebo (N=34) 28mg (N=40) 50mg (N=43) Placebo (N=43) 28mg (N=38) 50mg (N=34) Placebo (N=41) 1 The Modified Full Analysis Set includes subjects that
were randomized and received at least one dose of study drug per the Statistical Analysis Plan. ©2025 AKERO THERAPEUTICS.
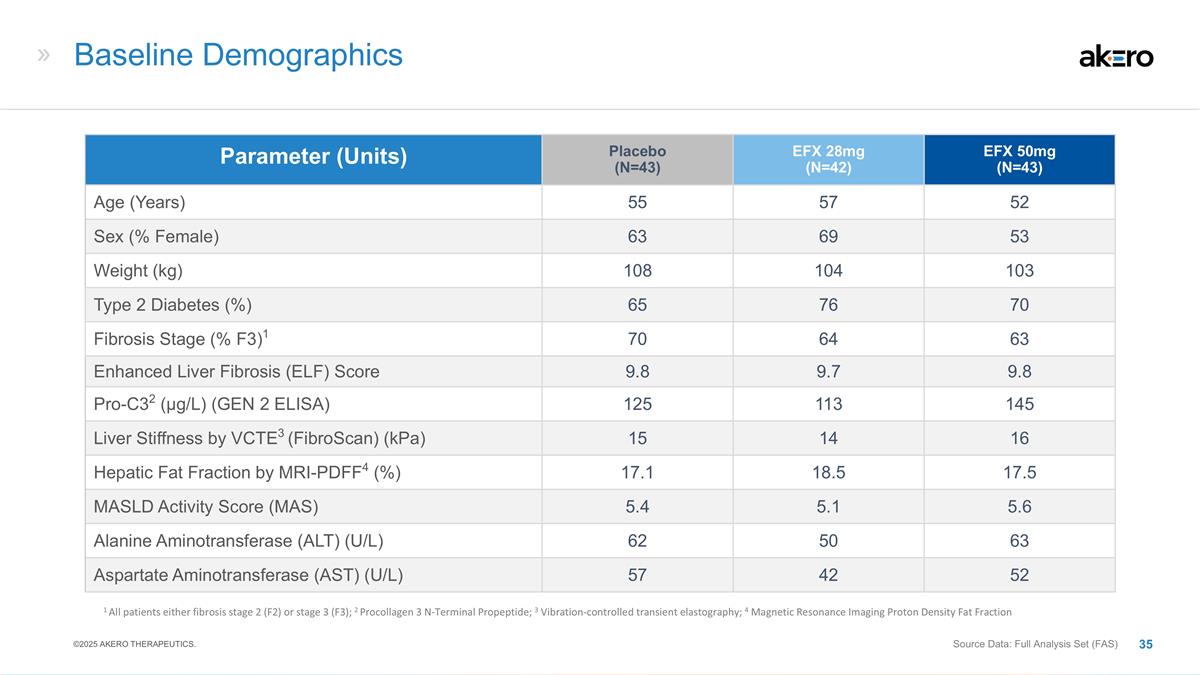
Baseline Demographics Parameter
(Units) Placebo (N=43) EFX 28mg (N=42) EFX 50mg (N=43) Age (Years) 55 57 52 Sex (% Female) 63 69 53 Weight (kg) 108 104 103 Type 2 Diabetes (%) 65 76 70 Fibrosis Stage (% F3)1 70 64 63 Enhanced Liver Fibrosis (ELF) Score 9.8 9.7 9.8 Pro-C32
(μg/L) (GEN 2 ELISA) 125 113 145 Liver Stiffness by VCTE3 (FibroScan) (kPa) 15 14 16 Hepatic Fat Fraction by MRI-PDFF4 (%) 17.1 18.5 17.5 MASLD Activity Score (MAS) 5.4 5.1 5.6 Alanine Aminotransferase (ALT) (U/L) 62 50 63 Aspartate
Aminotransferase (AST) (U/L) 57 42 52 1 All patients either fibrosis stage 2 (F2) or stage 3 (F3); 2 Procollagen 3 N-Terminal Propeptide; 3 Vibration-controlled transient elastography; 4 Magnetic Resonance Imaging Proton Density Fat Fraction Source
Data: Full Analysis Set (FAS) ©2025 AKERO THERAPEUTICS.
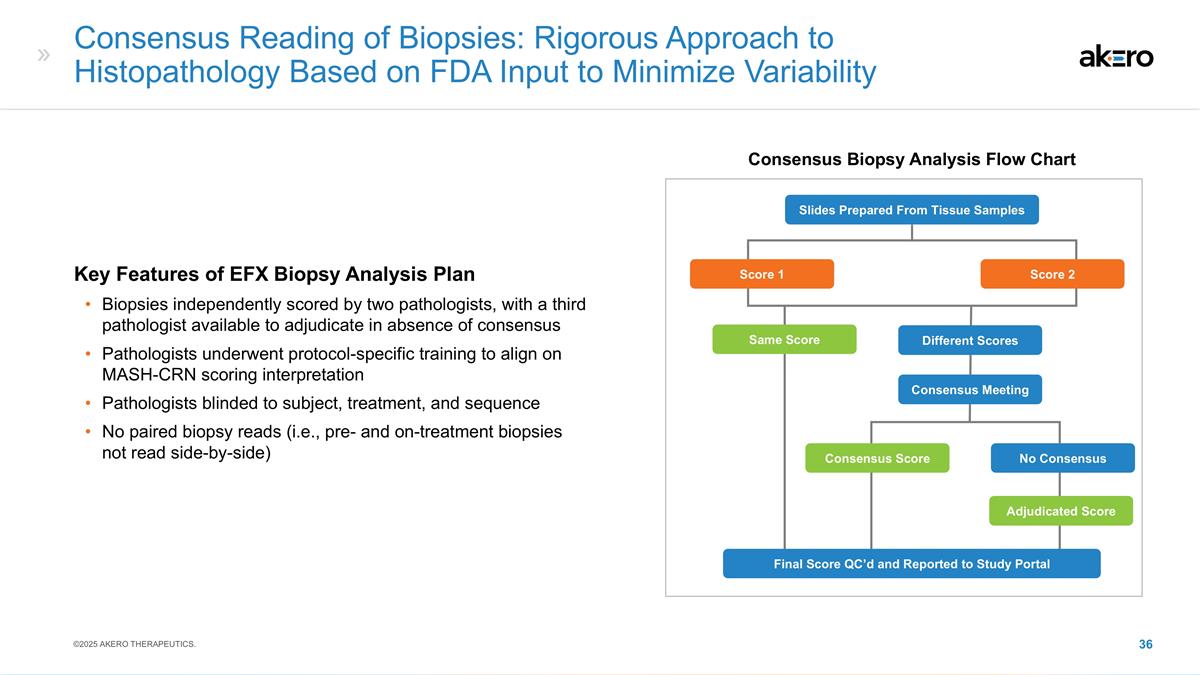
Consensus Reading of Biopsies:
Rigorous Approach to Histopathology Based on FDA Input to Minimize Variability Different Scores Slides Prepared From Tissue Samples Score 1 Score 2 Same Score Consensus Score Adjudicated Score No Consensus Consensus Meeting Final Score QC’d
and Reported to Study Portal Key Features of EFX Biopsy Analysis Plan Biopsies independently scored by two pathologists, with a third pathologist available to adjudicate in absence of consensus Pathologists underwent protocol-specific training to
align on MASH-CRN scoring interpretation Pathologists blinded to subject, treatment, and sequence No paired biopsy reads (i.e., pre- and on-treatment biopsies not read side-by-side) Consensus Biopsy Analysis Flow Chart ©2025 AKERO THERAPEUTICS.
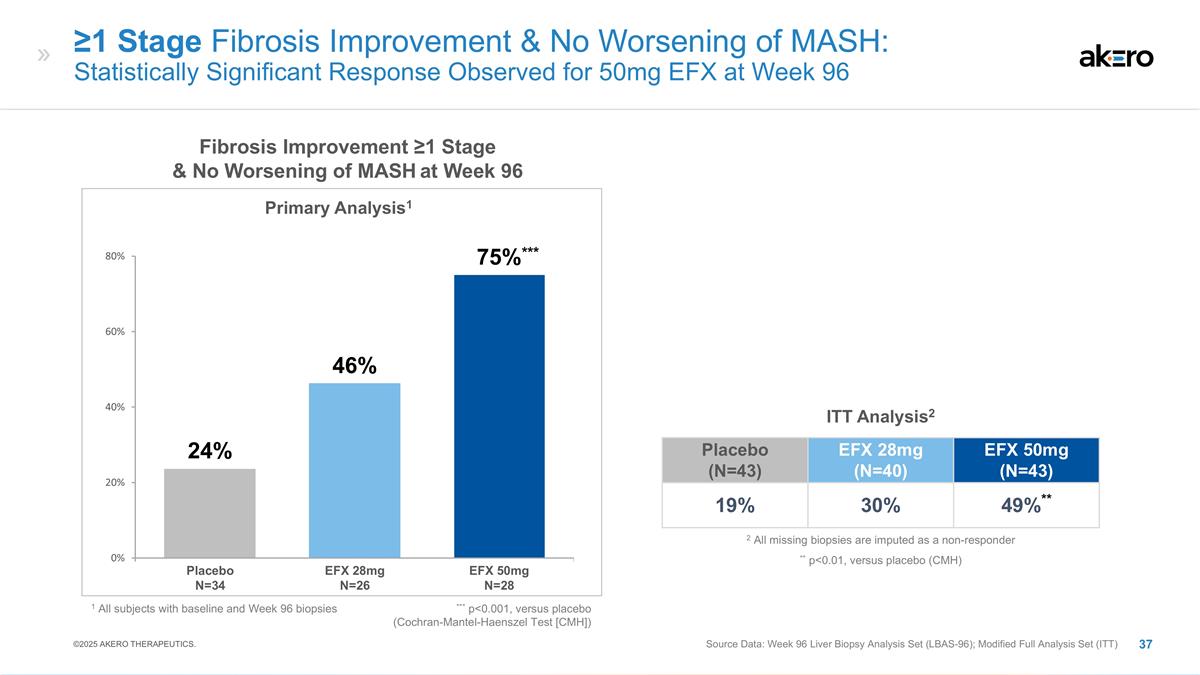
***75%*** ≥1 Stage Fibrosis
Improvement & No Worsening of MASH: Statistically Significant Response Observed for 50mg EFX at Week 96 Source Data: Week 96 Liver Biopsy Analysis Set (LBAS-96); Modified Full Analysis Set (ITT) EFX 28mg N=26 EFX 50mg N=28 Placebo N=34 Fibrosis
Improvement ≥1 Stage & No Worsening of MASH at Week 96 Placebo (N=43) EFX 28mg (N=40) EFX 50mg (N=43) 19% 30% 49%** ITT Analysis2 Primary Analysis1 ** p<0.01, versus placebo (CMH) 2 All missing biopsies are imputed as a non-responder 1
All subjects with baseline and Week 96 biopsies 46% 24% *** p<0.001, versus placebo (Cochran-Mantel-Haenszel Test [CMH]) ©2025 AKERO THERAPEUTICS.
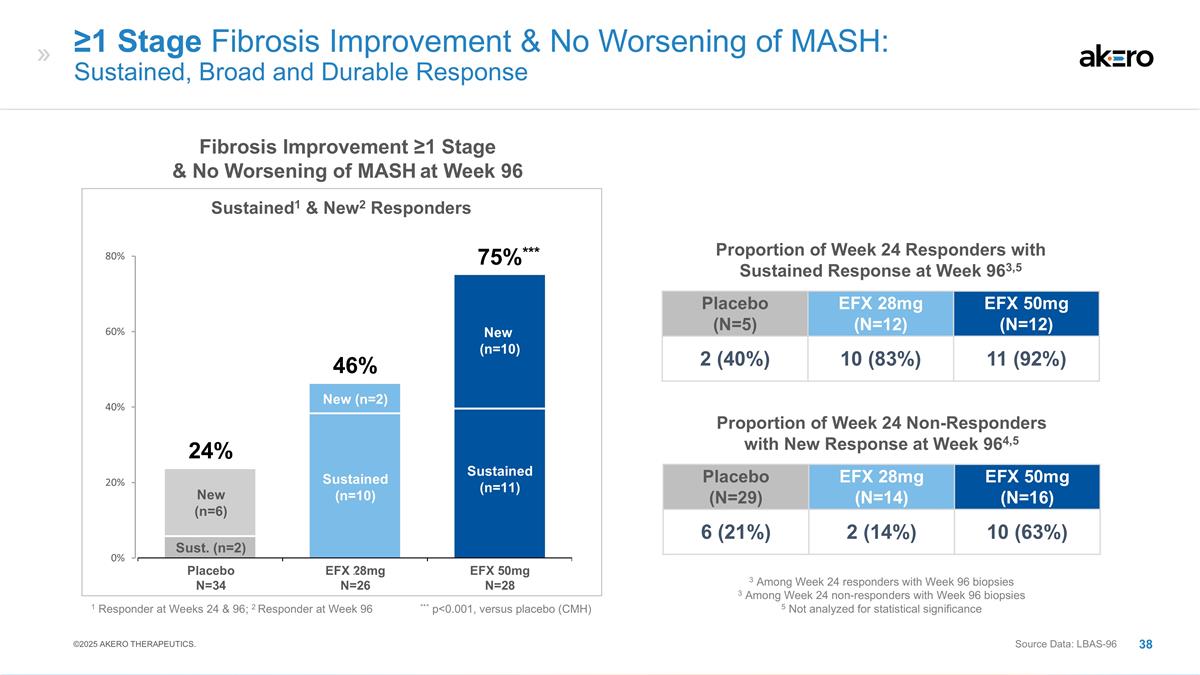
Placebo (N=29) EFX 28mg (N=14) EFX
50mg (N=16) 6 (21%) 2 (14%) 10 (63%) Proportion of Week 24 Non-Responders with New Response at Week 964,5 3 Among Week 24 responders with Week 96 biopsies 3 Among Week 24 non-responders with Week 96 biopsies 5 Not analyzed for statistical
significance ≥1 Stage Fibrosis Improvement & No Worsening of MASH: Sustained, Broad and Durable Response Sustained (n=10) New (n=2) Sustained (n=11) New (n=10) Sust. (n=2) New (n=6) ***75%*** EFX 28mg N=26 EFX 50mg N=28 Placebo N=34 46%
24% 1 Responder at Weeks 24 & 96; 2 Responder at Week 96 *** p<0.001, versus placebo (CMH) Placebo (N=5) EFX 28mg (N=12) EFX 50mg (N=12) 2 (40%) 10 (83%) 11 (92%) Proportion of Week 24 Responders with Sustained Response at Week 963,5 Fibrosis
Improvement ≥1 Stage & No Worsening of MASH at Week 96 Sustained1 & New2 Responders Source Data: LBAS-96 ©2025 AKERO THERAPEUTICS.
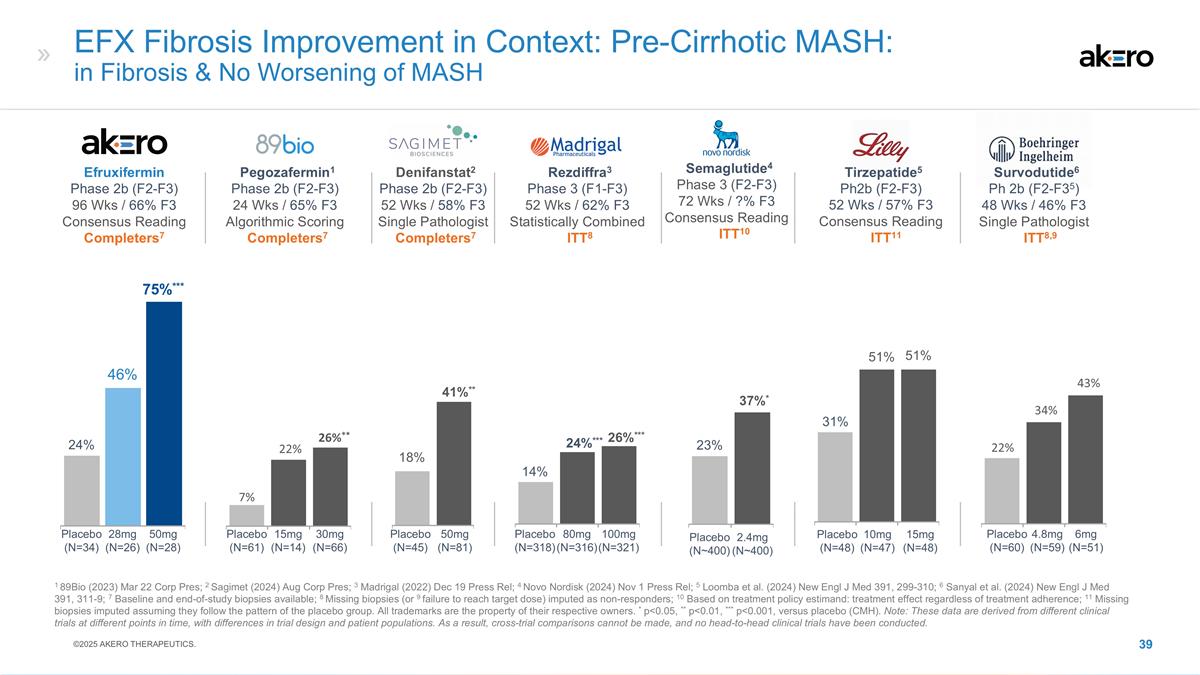
EFX Fibrosis Improvement in
Context: Pre-Cirrhotic MASH: in Fibrosis & No Worsening of MASH 1 89Bio (2023) Mar 22 Corp Pres; 2 Sagimet (2024) Aug Corp Pres; 3 Madrigal (2022) Dec 19 Press Rel; 4 Novo Nordisk (2024) Nov 1 Press Rel; 5 Loomba et al. (2024) New Engl J Med
391, 299-310; 6 Sanyal et al. (2024) New Engl J Med 391, 311-9; 7 Baseline and end-of-study biopsies available; 8 Missing biopsies (or 9 failure to reach target dose) imputed as non-responders; 10 Based on treatment policy estimand: treatment effect
regardless of treatment adherence; 11 Missing biopsies imputed assuming they follow the pattern of the placebo group. All trademarks are the property of their respective owners. * p<0.05, ** p<0.01, *** p<0.001, versus placebo (CMH). Note:
These data are derived from different clinical trials at different points in time, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been
conducted. 2Denifanstat2 Phase 2b (F2-F3) 52 Wks / 58% F3 Single Pathologist 7Completers7 18% **41%** Placebo (N=45) 50mg (N=81) Efruxifermin Phase 2b (F2-F3) 96 Wks / 66% F3 Consensus Reading Completers7 75%*** 46% 24% 28mg (N=26) 50mg (N=28)
Placebo (N=34) 3Rezdiffra3 Phase 3 (F1-F3) 52 Wks / 62% F3 Statistically Combined 8ITT8 Placebo (N=318) 80mg (N=316) 100mg (N=321) ***26%*** ***%*** 14% 1Pegozafermin1 Phase 2b (F2-F3) 24 Wks / 65% F3 Algorithmic Scoring 7Completers7 **26%** Placebo
(N=61) 15mg (N=14) 30mg (N=66) *22% 7% 5Tirzepatide5 Ph2b (F2-F3) 52 Wks / 57% F3 Consensus Reading 11ITT11 Placebo (N=48) 10mg (N=47) 15mg (N=48) 31% 51% 51% 6Survodutide6 Ph 2b (F2-F35) 48 Wks / 46% F3 Single Pathologist 8,9ITT8,9 **43% *34% 22%
Placebo (N=60) 4.8mg (N=59) 6mg (N=51) Placebo (N~400) 4Semaglutide4 Phase 3 (F2-F3) 72 Wks / ?% F3 Consensus Reading 510ITT10 23% *37%* 2.4mg (N~400) ©2025 AKERO THERAPEUTICS.
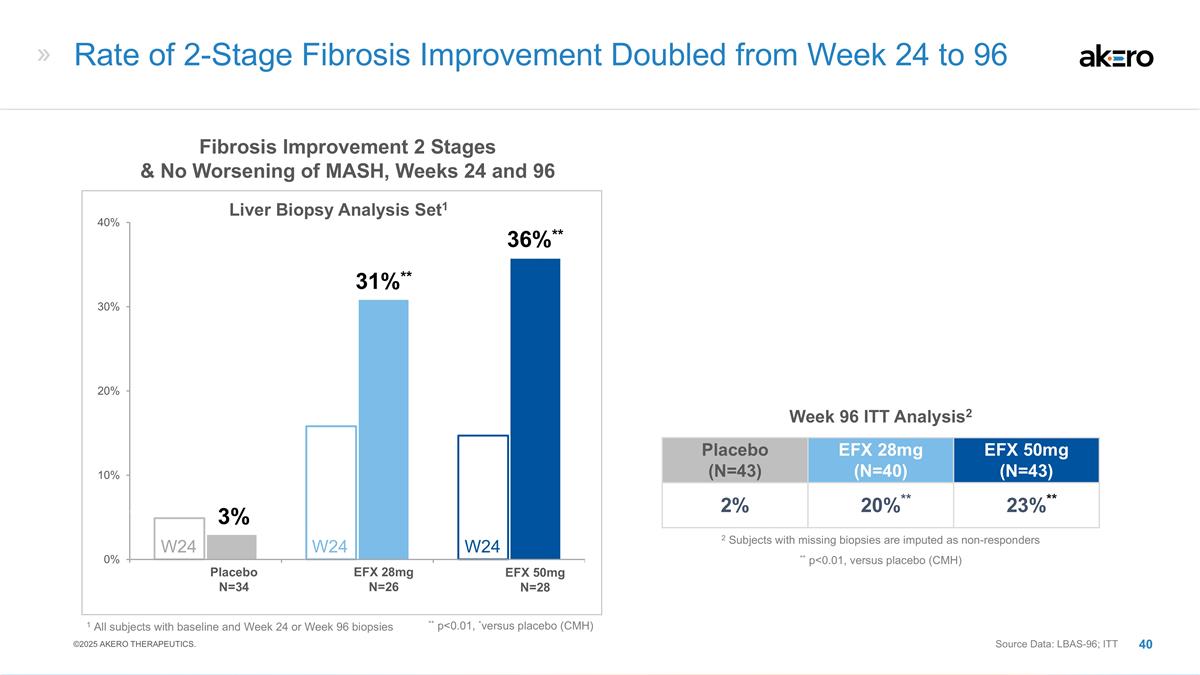
Fibrosis Improvement 2 Stages &
No Worsening of MASH, Weeks 24 and 96 1 All subjects with baseline and Week 24 or Week 96 biopsies ** p<0.01, *versus placebo (CMH) Placebo N=34 EFX 28mg N=26 EFX 50mg N=28 3% 31%** 36%** W24 W24 W24 Rate of 2-Stage Fibrosis Improvement Doubled
from Week 24 to 96 Placebo (N=43) EFX 28mg (N=40) EFX 50mg (N=43) 2% **20%** **23%** Week 96 ITT Analysis2 ** p<0.01, versus placebo (CMH) 2 Subjects with missing biopsies are imputed as non-responders Source Data: LBAS-96; ITT Liver Biopsy
Analysis Set1 ©2025 AKERO THERAPEUTICS.
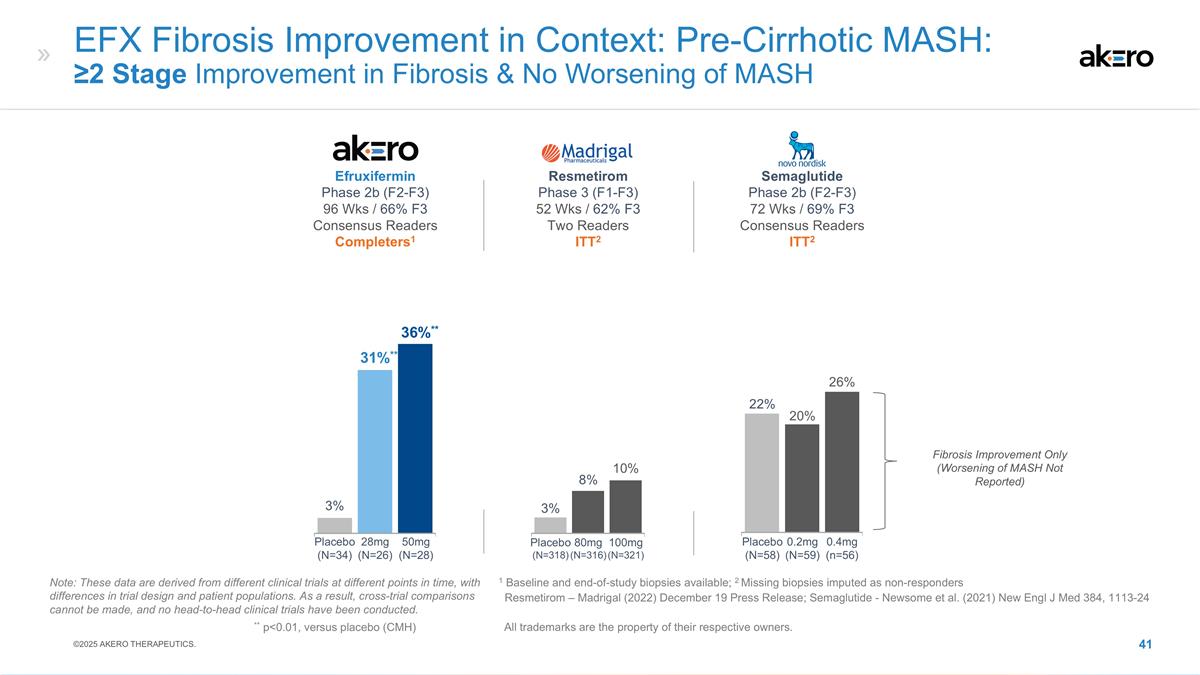
Efruxifermin Phase 2b (F2-F3) 96
Wks / 66% F3 Consensus Readers Completers1 **36%** **31%** 3% 28mg (N=26) 50mg (N=28) Placebo (N=34) EFX Fibrosis Improvement in Context: Pre-Cirrhotic MASH: ≥2 Stage Improvement in Fibrosis & No Worsening of MASH Semaglutide Phase 2b
(F2-F3) 72 Wks / 69% F3 Consensus Readers ITT2 Resmetirom Phase 3 (F1-F3) 52 Wks / 62% F3 Two Readers ITT2 Placebo (N=318) 80mg (N=316) 100mg (N=321) 10% 8% 3% Placebo (N=58) 22% 26% 0.2mg (N=59) 0.4mg (n=56) 20% Fibrosis Improvement Only (Worsening
of MASH Not Reported) 1 Baseline and end-of-study biopsies available; 2 Missing biopsies imputed as non-responders Resmetirom – Madrigal (2022) December 19 Press Release; Semaglutide - Newsome et al. (2021) New Engl J Med 384,
1113-24 Note: These data are derived from different clinical trials at different points in time, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have
been conducted. ** p<0.01, versus placebo (CMH) All trademarks are the property of their respective owners. ©2025 AKERO THERAPEUTICS.
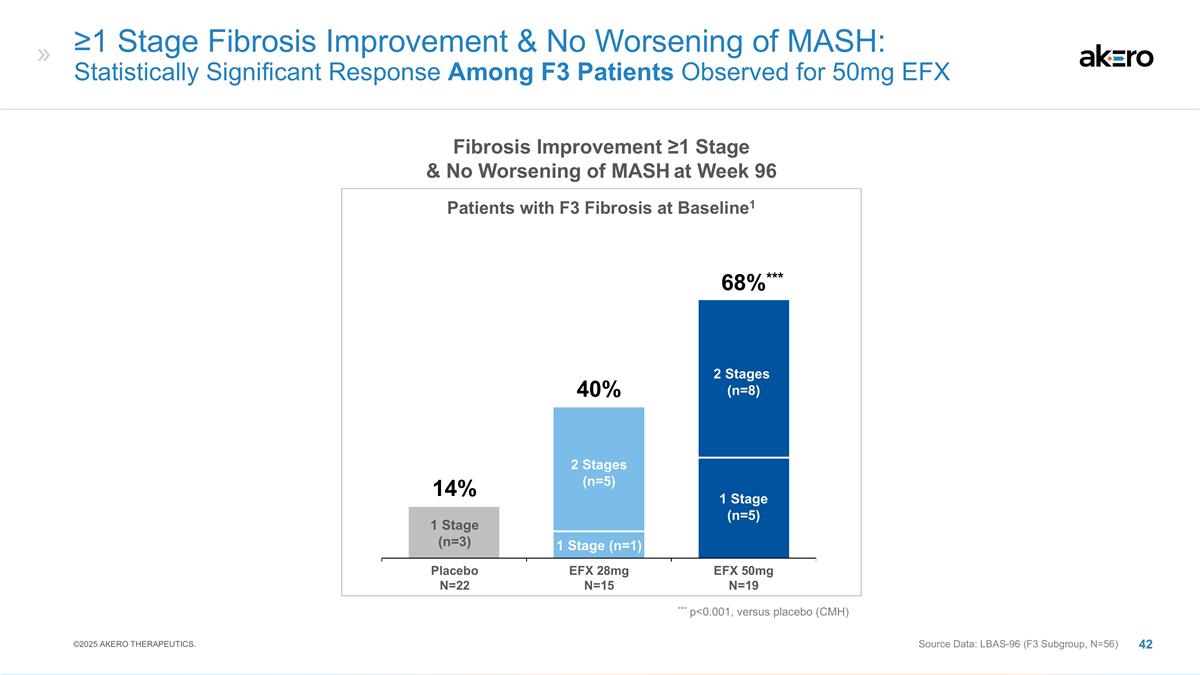
≥1 Stage Fibrosis Improvement
& No Worsening of MASH: Statistically Significant Response Among F3 Patients Observed for 50mg EFX 2 Stages (n=5) 1 Stage (n=1) 1 Stage (n=5) 2 Stages (n=8) 1 Stage (n=3) ***68%*** EFX 28mg N=15 EFX 50mg N=19 Placebo N=22 40% 14% *** p<0.001,
versus placebo (CMH) Fibrosis Improvement ≥1 Stage & No Worsening of MASH at Week 96 Patients with F3 Fibrosis at Baseline1 Source Data: LBAS-96 (F3 Subgroup, N=56) ©2025 AKERO THERAPEUTICS.
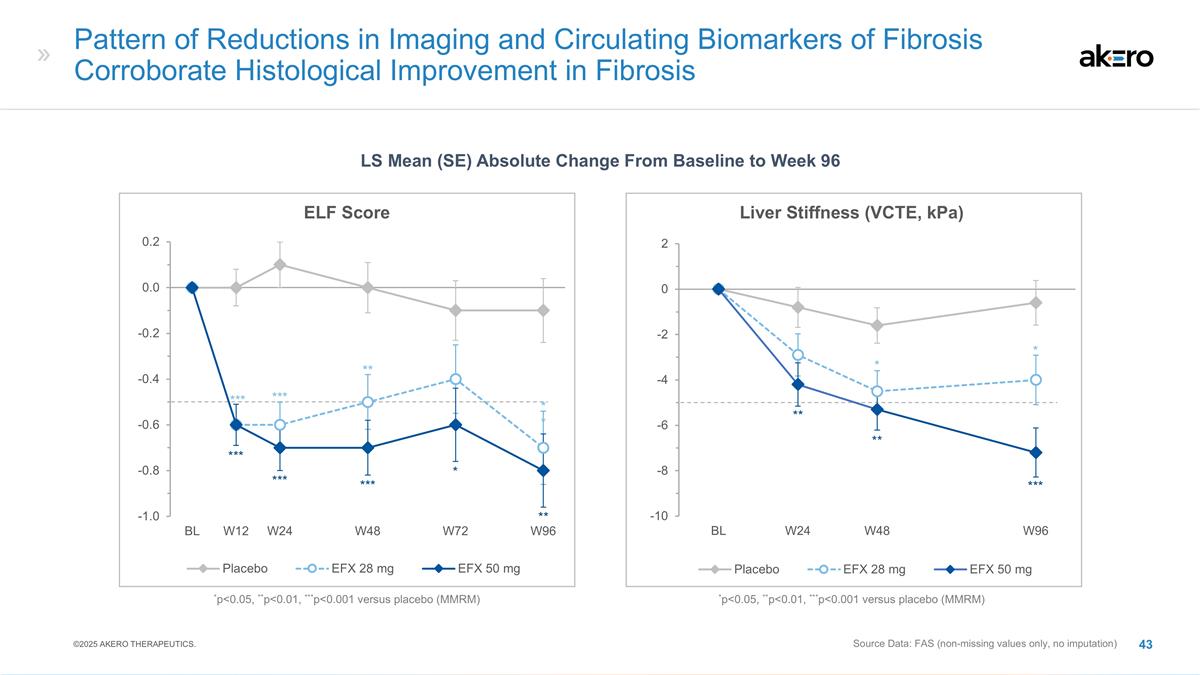
Pattern of Reductions in Imaging
and Circulating Biomarkers of Fibrosis Corroborate Histological Improvement in Fibrosis Source Data: FAS (non-missing values only, no imputation) LS Mean (SE) Absolute Change From Baseline to Week 96 Liver Stiffness (VCTE, kPa) ** ** * * ***
*p<0.05, **p<0.01, ***p<0.001 versus placebo (MMRM) ELF Score *** *** * ** *** *** *** ** *p<0.05, **p<0.01, ***p<0.001 versus placebo (MMRM) ** ©2025 AKERO THERAPEUTICS.
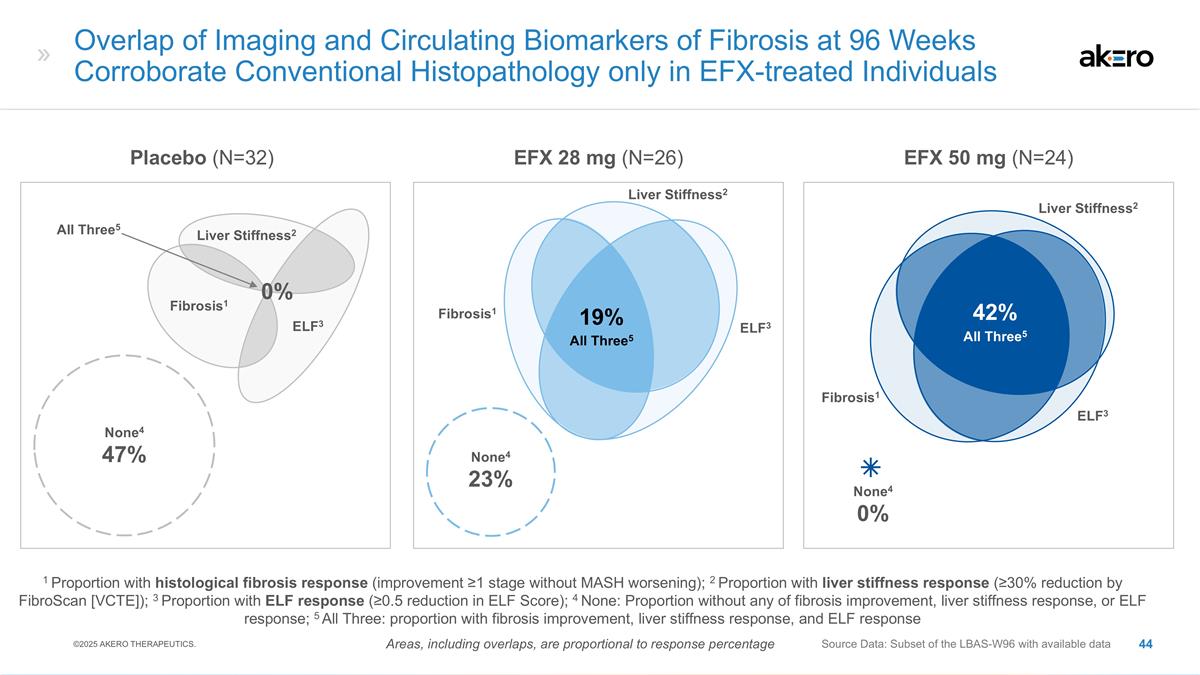
None4 0% Liver Stiffness2 Fibrosis1
ELF3 Liver Stiffness2 Fibrosis1 ELF3 EFX 28 mg (N=26) EFX 50 mg (N=24) Source Data: Subset of the LBAS-W96 with available data Overlap of Imaging and Circulating Biomarkers of Fibrosis at 96 Weeks Corroborate Conventional Histopathology only in
EFX-treated Individuals 1 Proportion with histological fibrosis response (improvement ≥1 stage without MASH worsening); 2 Proportion with liver stiffness response (≥30% reduction by FibroScan [VCTE]); 3 Proportion with ELF response
(≥0.5 reduction in ELF Score); 4 None: Proportion without any of fibrosis improvement, liver stiffness response, or ELF response; 5 All Three: proportion with fibrosis improvement, liver stiffness response, and ELF response Placebo (N=32)
None4 47% None4 23% Liver Stiffness2 Fibrosis1 ELF3 19% All Three5 All Three5 0% Areas, including overlaps, are proportional to response percentage 42% All Three5 ©2025 AKERO THERAPEUTICS.
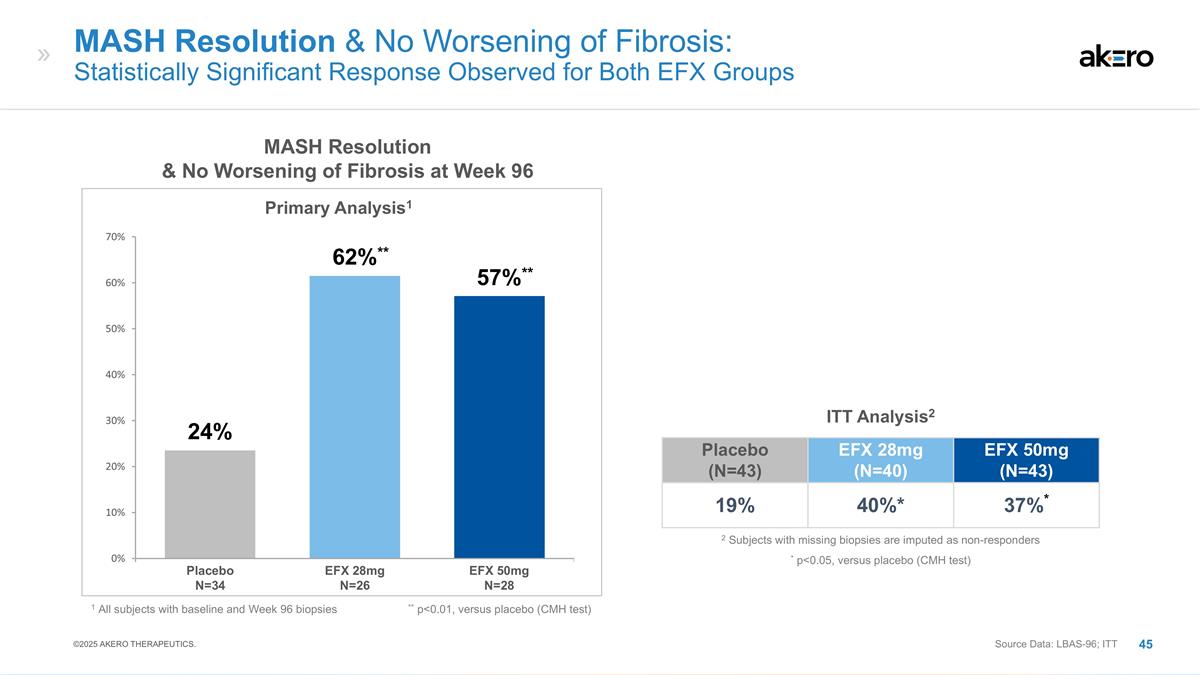
MASH Resolution & No Worsening
of Fibrosis: Statistically Significant Response Observed for Both EFX Groups EFX 28mg N=26 EFX 50mg N=28 Placebo N=34 MASH Resolution & No Worsening of Fibrosis at Week 96 Primary Analysis1 1 All subjects with baseline and Week 96 biopsies **
p<0.01, versus placebo (CMH test) **57%** **62%** 24% Placebo (N=43) EFX 28mg (N=40) EFX 50mg (N=43) 19% 40%* 37%* ITT Analysis2 * p<0.05, versus placebo (CMH test) 2 Subjects with missing biopsies are imputed as non-responders Source Data:
LBAS-96; ITT ©2025 AKERO THERAPEUTICS.
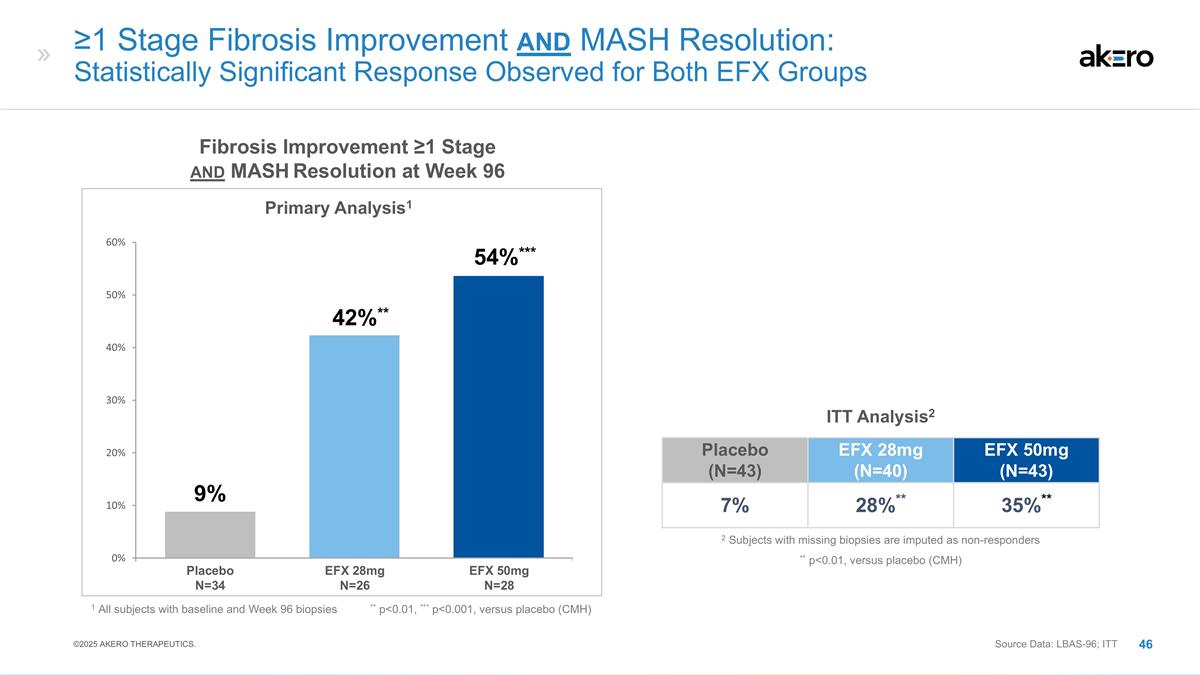
≥1 Stage Fibrosis Improvement
and MASH Resolution: Statistically Significant Response Observed for Both EFX Groups Placebo (N=43) EFX 28mg (N=40) EFX 50mg (N=43) 7% 28%** 35%** ITT Analysis2 ** p<0.01, versus placebo (CMH) 2 Subjects with missing biopsies are imputed as
non-responders **54%*** EFX 28mg N=26 EFX 50mg N=28 Placebo N=34 Fibrosis Improvement ≥1 Stage and MASH Resolution at Week 96 Primary Analysis1 1 All subjects with baseline and Week 96 biopsies **42%** 9% ** p<0.01, *** p<0.001, versus
placebo (CMH) Source Data: LBAS-96; ITT ©2025 AKERO THERAPEUTICS.
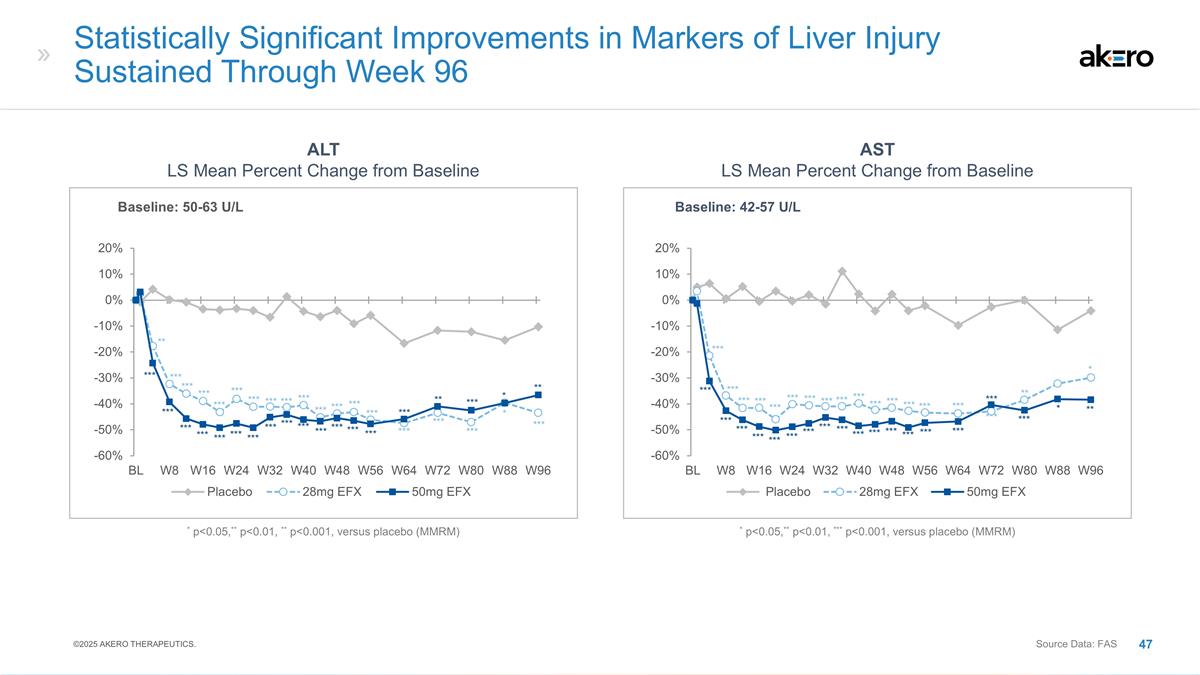
Statistically Significant
Improvements in Markers of Liver Injury Sustained Through Week 96 Source Data: FAS ALT LS Mean Percent Change from Baseline AST LS Mean Percent Change from Baseline Baseline: 50-63 U/L Baseline: 42-57 U/L *** *** *** *** *** *** *** *** *** *** ***
*** *** *** *** *** *** ** ** *** *** *** *** *** *** *** *** *** *** *** *** *** *** * *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** *** * ** * *** *** *** *** ** *** * ** *
p<0.05,** p<0.01, *** p<0.001, versus placebo (MMRM) * p<0.05,** p<0.01, ** p<0.001, versus placebo (MMRM) ©2025 AKERO THERAPEUTICS.
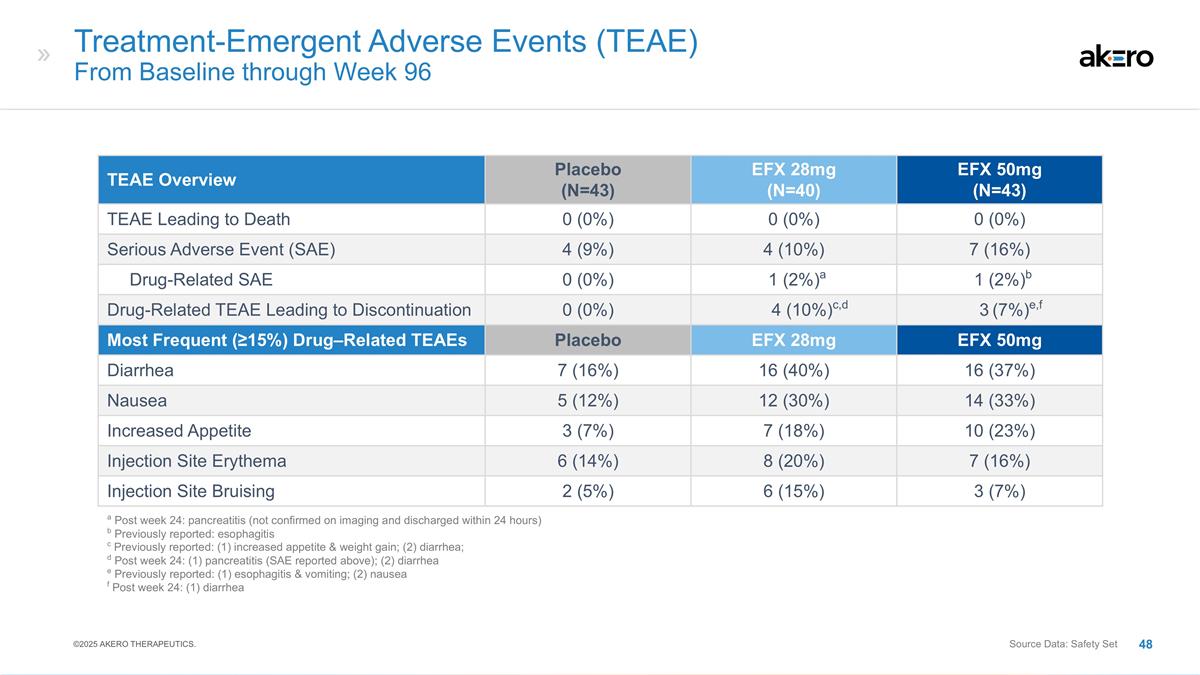
Treatment-Emergent Adverse Events
(TEAE) From Baseline through Week 96 TEAE Overview Placebo (N=43) EFX 28mg (N=40) EFX 50mg (N=43) TEAE Leading to Death 0 (0%) 0 (0%) 0 (0%) Serious Adverse Event (SAE) 4 (9%) 4 (10%) 7 (16%) Drug-Related SAE 0 (0%) a1 (2%)a b1 (2%)b Drug-Related
TEAE Leading to Discontinuation 0 (0%) c,d,e,f4 (10%)c,d g,h,i3 (7%)e,f Most Frequent (≥15%) Drug–Related TEAEs Placebo EFX 28mg EFX 50mg Diarrhea 7 (16%) 16 (40%) 16 (37%) Nausea 5 (12%) 12 (30%) 14 (33%) Increased Appetite 3 (7%) 7
(18%) 10 (23%) Injection Site Erythema 6 (14%) 8 (20%) 7 (16%) Injection Site Bruising 2 (5%) 6 (15%) 3 (7%) c Previously reported: (1) increased appetite & weight gain; (2) diarrhea; d Post week 24: (1) pancreatitis (SAE reported above); (2)
diarrhea e Previously reported: (1) esophagitis & vomiting; (2) nausea f Post week 24: (1) diarrhea Source Data: Safety Set a Post week 24: pancreatitis (not confirmed on imaging and discharged within 24 hours) b Previously reported: esophagitis
©2025 AKERO THERAPEUTICS.

Safety Overview Blood Pressure No
statistical difference versus placebo in systolic & diastolic BP at week 96 Markers of Liver Function and Hemostasis Remained stable, including platelets, bilirubin, INR1, MELD2 and CP3 score Progression to Cirrhosis Balanced across dose groups
Bone Mineral Density At week 48, no significant changes versus placebo for lumbar spine and femoral neck regions At week 96, significant reductions versus placebo for lumbar spine (3-4%, both EFX groups) and femoral neck regions (< 3%, 50mg EFX
only) One vertebral fracture (L1) observed in placebo group; no vertebral fractures observed in EFX groups 1 Prothrombin International Normalized Ratio; 2 Model for End-Stage Liver Disease; 3 Child-Pugh ©2025 AKERO THERAPEUTICS.
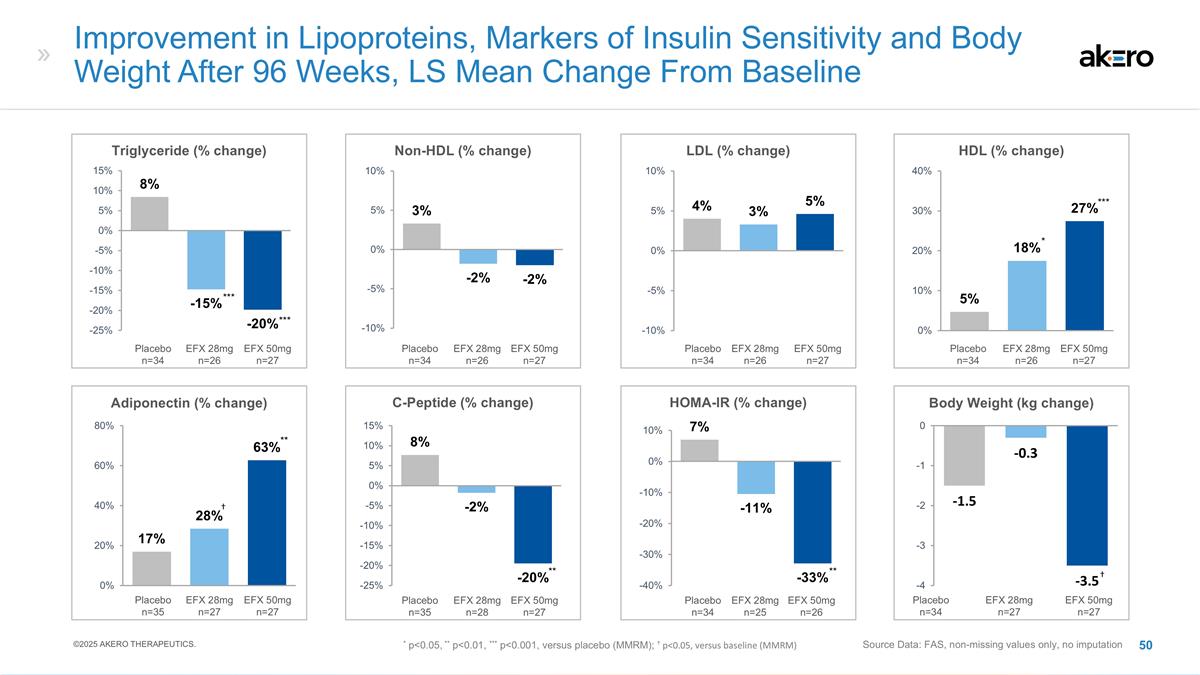
Improvement in Lipoproteins,
Markers of Insulin Sensitivity and Body Weight After 96 Weeks, LS Mean Change From Baseline Source Data: FAS, non-missing values only, no imputation Non-HDL (% change) LDL (% change) Triglyceride (% change) HDL (% change) C-Peptide (% change)
HOMA-IR (% change) Adiponectin (% change) Body Weight (kg change) EFX 28mg n=26 Placebo n=34 EFX 50mg n=27 EFX 28mg n=26 Placebo n=34 EFX 50mg n=27 EFX 28mg n=26 Placebo n=34 EFX 50mg n=27 EFX 28mg n=26 Placebo n=34 EFX 50mg n=27 EFX 28mg n=25
Placebo n=34 EFX 50mg n=26 EFX 28mg n=28 Placebo n=35 EFX 50mg n=27 EFX 28mg n=27 Placebo n=35 EFX 50mg n=27 EFX 28mg n=27 Placebo n=34 EFX 50mg n=27 * p<0.05, ** p<0.01, *** p<0.001, versus placebo (MMRM); † p<0.05, versus
baseline (MMRM) *** *** *** * ** † ** ** † ©2025 AKERO THERAPEUTICS.
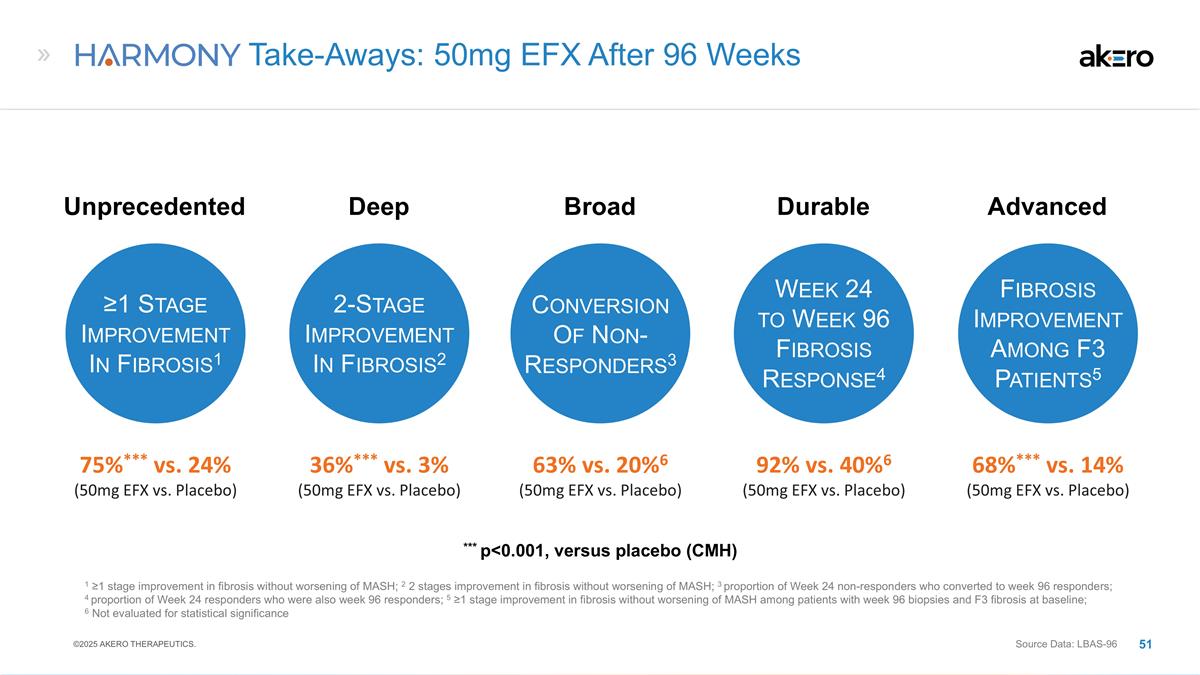
Fibrosis Improvement Among F3
Patients5 Advanced 68%*** vs. 14% (50mg EFX vs. Placebo) Take-Aways: 50mg EFX After 96 Weeks Unprecedented 75%*** vs. 24% (50mg EFX vs. Placebo) ≥1 Stage Improvement In Fibrosis1 Deep 36%*** vs. 3% (50mg EFX vs. Placebo) 2-Stage Improvement In
Fibrosis2 Week 24 to Week 96 Fibrosis Response4 Durable 92% vs. 40%6 (50mg EFX vs. Placebo) 1 ≥1 stage improvement in fibrosis without worsening of MASH; 2 2 stages improvement in fibrosis without worsening of MASH; 3 proportion of Week 24
non-responders who converted to week 96 responders; 4 proportion of Week 24 responders who were also week 96 responders; 5 ≥1 stage improvement in fibrosis without worsening of MASH among patients with week 96 biopsies and F3 fibrosis at
baseline; 6 Not evaluated for statistical significance *** p<0.001, versus placebo (CMH) Conversion Of Non-Responders3 Broad 63% vs. 20%6 (50mg EFX vs. Placebo) Source Data: LBAS-96 ©2025 AKERO THERAPEUTICS.

Backup Slides
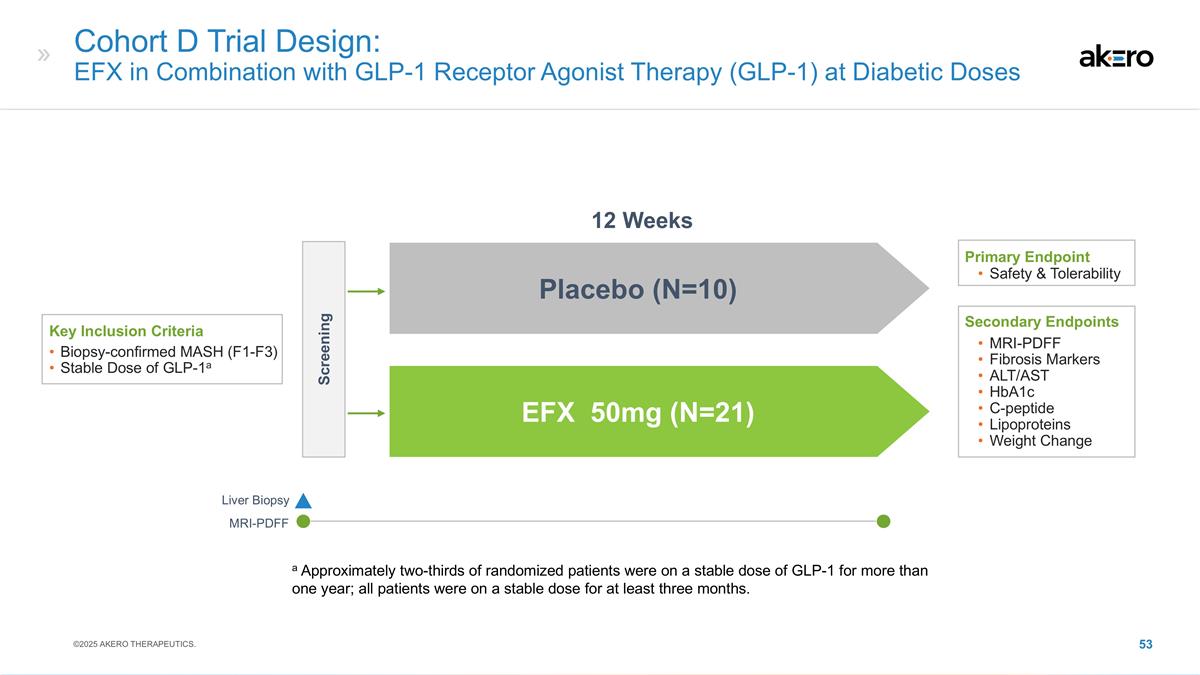
Cohort D Trial Design: EFX in
Combination with GLP-1 Receptor Agonist Therapy (GLP-1) at Diabetic Doses Screening 12 Weeks EFX 50mg (N=21) Placebo (N=10) Key Inclusion Criteria Biopsy-confirmed MASH (F1-F3) Stable Dose of GLP-1a Primary Endpoint Safety & Tolerability
Secondary Endpoints MRI-PDFF Fibrosis Markers ALT/AST HbA1c C-peptide Lipoproteins Weight Change MRI-PDFF Liver Biopsy a Approximately two-thirds of randomized patients were on a stable dose of GLP-1 for more than one year; all patients were on a
stable dose for at least three months. ©2025 AKERO THERAPEUTICS.
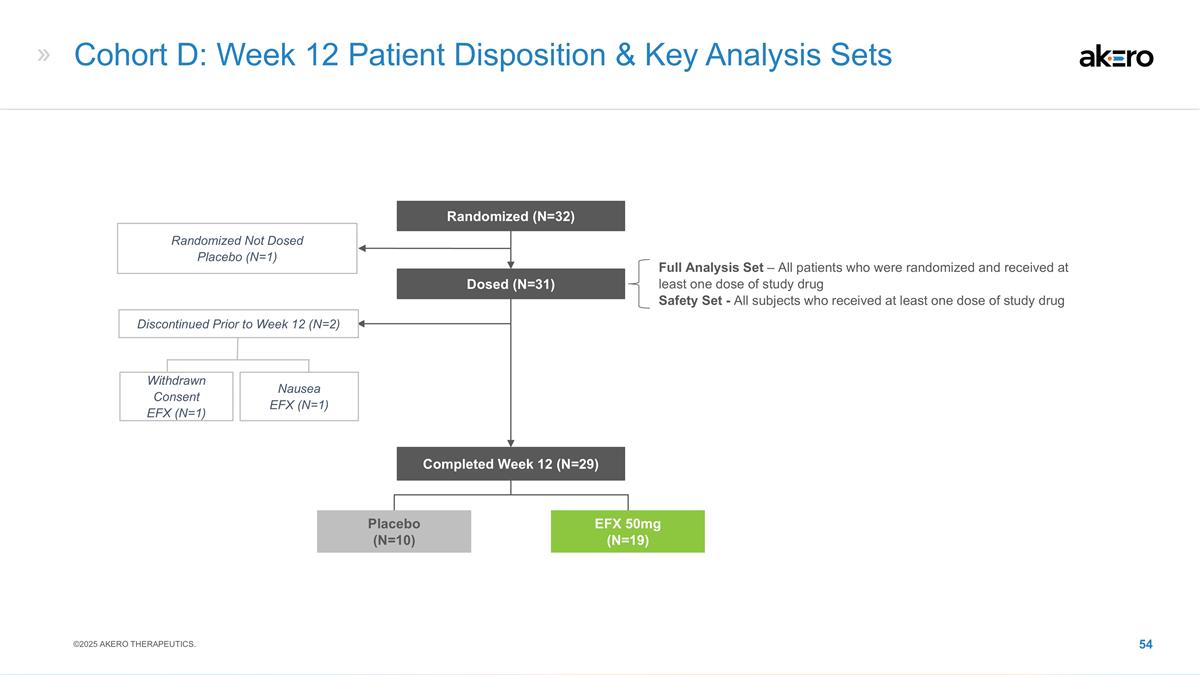
Cohort D: Week 12 Patient
Disposition & Key Analysis Sets Randomized (N=32) Completed Week 12 (N=29) Randomized Not Dosed Placebo (N=1) Dosed (N=31) Full Analysis Set – All patients who were randomized and received at least one dose of study drug Safety Set - All
subjects who received at least one dose of study drug EFX 50mg (N=19) Placebo (N=10) Discontinued Prior to Week 12 (N=2) Nausea EFX (N=1) Withdrawn Consent EFX (N=1) ©2025 AKERO THERAPEUTICS.
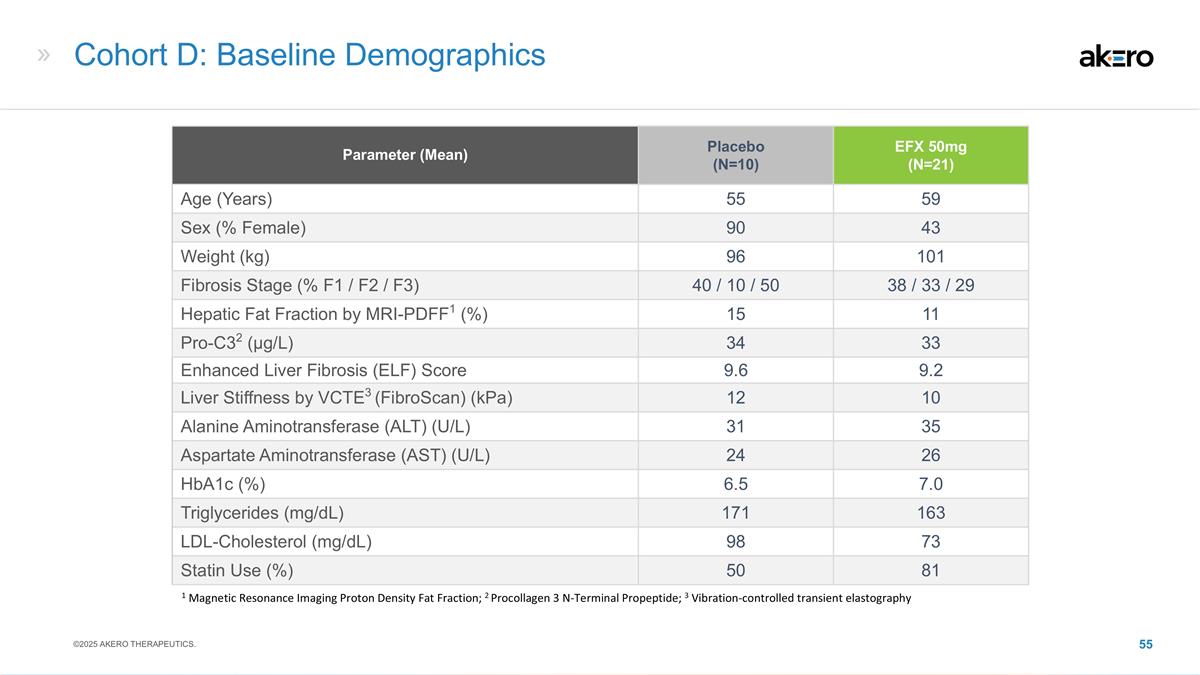
Cohort D: Baseline Demographics
Parameter (Mean) Placebo (N=10) EFX 50mg (N=21) Age (Years) 55 59 Sex (% Female) 90 43 Weight (kg) 96 101 Fibrosis Stage (% F1 / F2 / F3) 40 / 10 / 50 38 / 33 / 29 Hepatic Fat Fraction by MRI-PDFF1 (%) 15 11 Pro-C32 (μg/L) 34 33 Enhanced Liver
Fibrosis (ELF) Score 9.6 9.2 Liver Stiffness by VCTE3 (FibroScan) (kPa) 12 10 Alanine Aminotransferase (ALT) (U/L) 31 35 Aspartate Aminotransferase (AST) (U/L) 24 26 HbA1c (%) 6.5 7.0 Triglycerides (mg/dL) 171 163 LDL-Cholesterol (mg/dL) 98 73
Statin Use (%) 50 81 1 Magnetic Resonance Imaging Proton Density Fat Fraction; 2 Procollagen 3 N-Terminal Propeptide; 3 Vibration-controlled transient elastography ©2025 AKERO THERAPEUTICS.
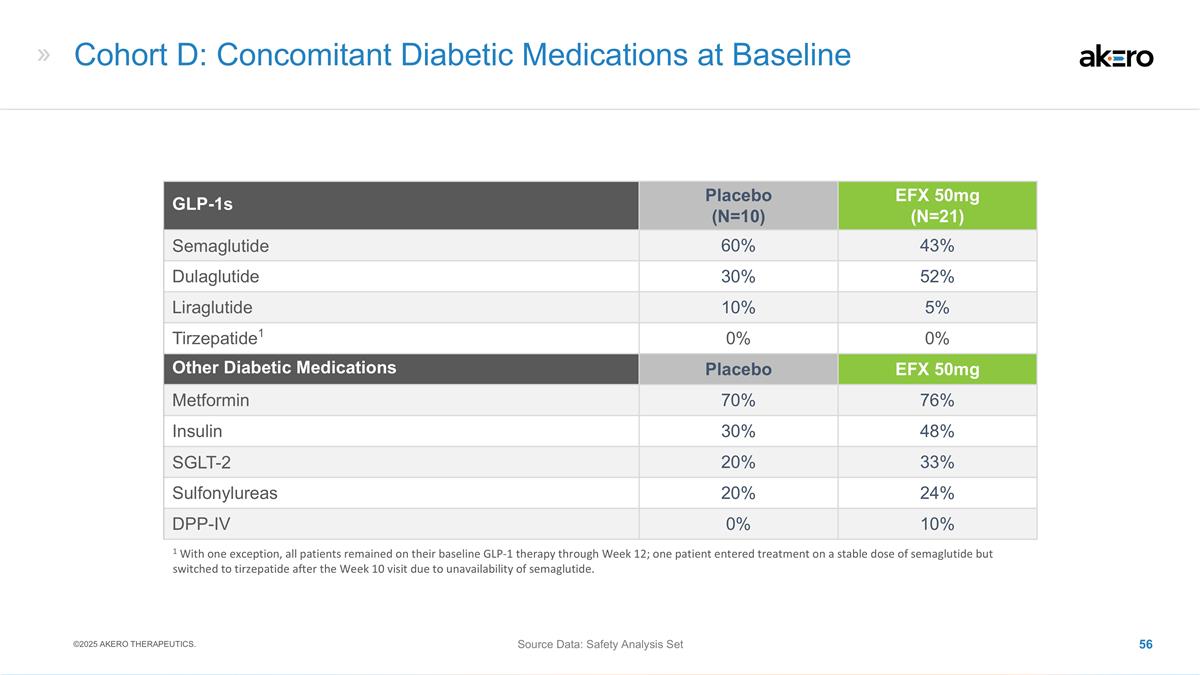
Cohort D: Concomitant Diabetic
Medications at Baseline GLP-1s Placebo (N=10) EFX 50mg (N=21) Semaglutide 60% 43% Dulaglutide 30% 52% Liraglutide 10% 5% Tirzepatide1 0% 0% Other Diabetic Medications Placebo EFX 50mg Metformin 70% 76% Insulin 30% 48% SGLT-2 20% 33% Sulfonylureas
20% 24% DPP-IV 0% 10% Source Data: Safety Analysis Set 1 With one exception, all patients remained on their baseline GLP-1 therapy through Week 12; one patient entered treatment on a stable dose of semaglutide but switched to tirzepatide after the
Week 10 visit due to unavailability of semaglutide. ©2025 AKERO THERAPEUTICS.
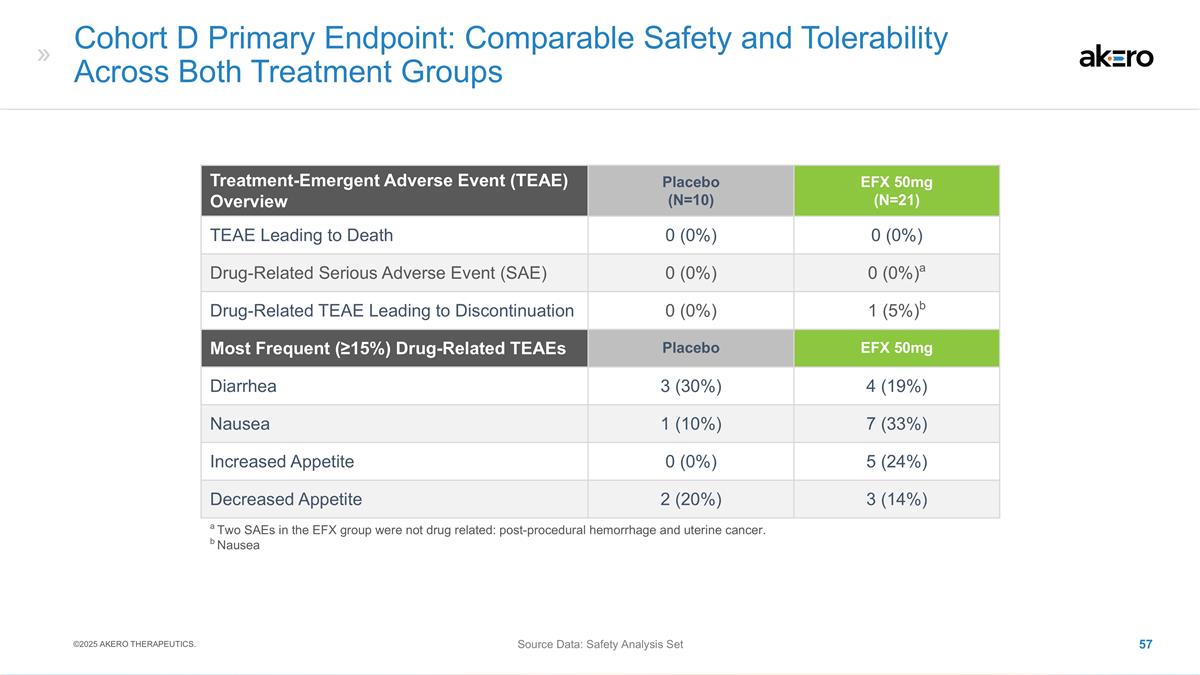
Cohort D Primary Endpoint:
Comparable Safety and Tolerability Across Both Treatment Groups Source Data: Safety Analysis Set Treatment-Emergent Adverse Event (TEAE) Overview Placebo (N=10) EFX 50mg (N=21) TEAE Leading to Death 0 (0%) 0 (0%) Drug-Related Serious Adverse Event
(SAE) 0 (0%) 0 (0%)a Drug-Related TEAE Leading to Discontinuation 0 (0%) 1 (5%)b Most Frequent (≥15%) Drug-Related TEAEs Placebo EFX 50mg Diarrhea 3 (30%) 4 (19%) Nausea 1 (10%) 7 (33%) Increased Appetite 0 (0%) 5 (24%) Decreased Appetite 2
(20%) 3 (14%) a Two SAEs in the EFX group were not drug related: post-procedural hemorrhage and uterine cancer. b Nausea ©2025 AKERO THERAPEUTICS.
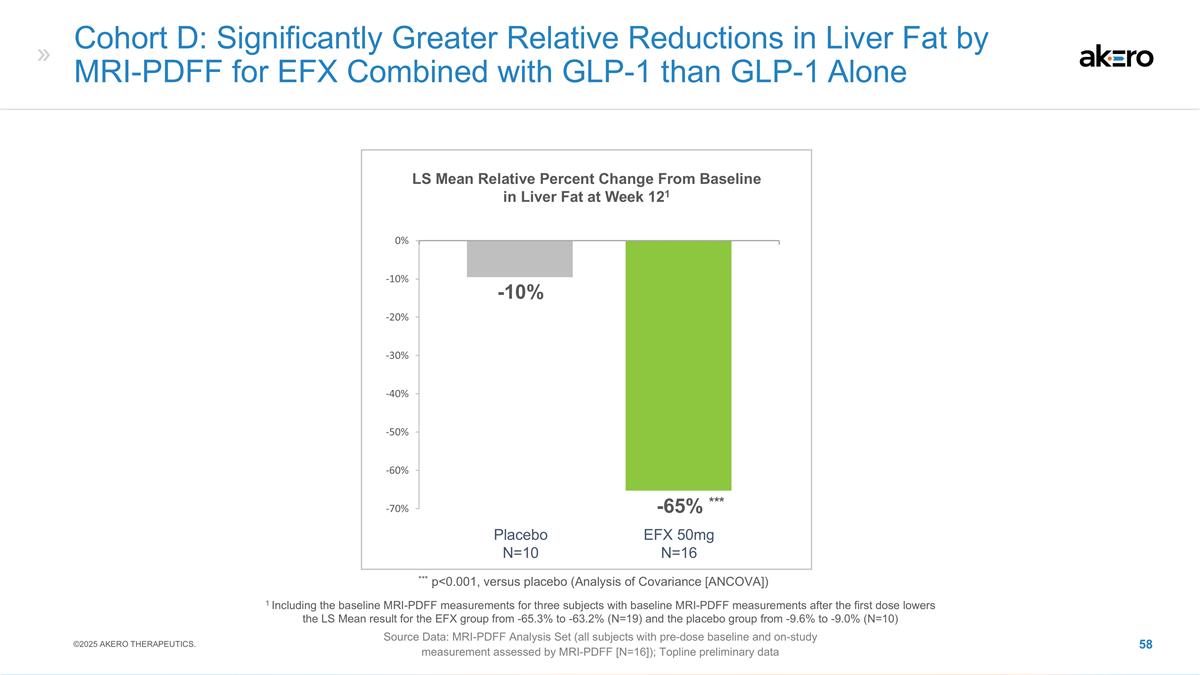
Cohort D: Significantly Greater
Relative Reductions in Liver Fat by MRI-PDFF for EFX Combined with GLP-1 than GLP-1 Alone -10% LS Mean Relative Percent Change From Baseline in Liver Fat at Week 121 *** p<0.001, versus placebo (Analysis of Covariance [ANCOVA]) *** -65% ***
Source Data: MRI-PDFF Analysis Set (all subjects with pre-dose baseline and on-study measurement assessed by MRI-PDFF [N=16]); Topline preliminary data EFX 50mg Placebo N=16 N=10 1 Including the baseline MRI-PDFF measurements for three subjects with
baseline MRI-PDFF measurements after the first dose lowers the LS Mean result for the EFX group from -65.3% to -63.2% (N=19) and the placebo group from -9.6% to -9.0% (N=10) ©2025 AKERO THERAPEUTICS.
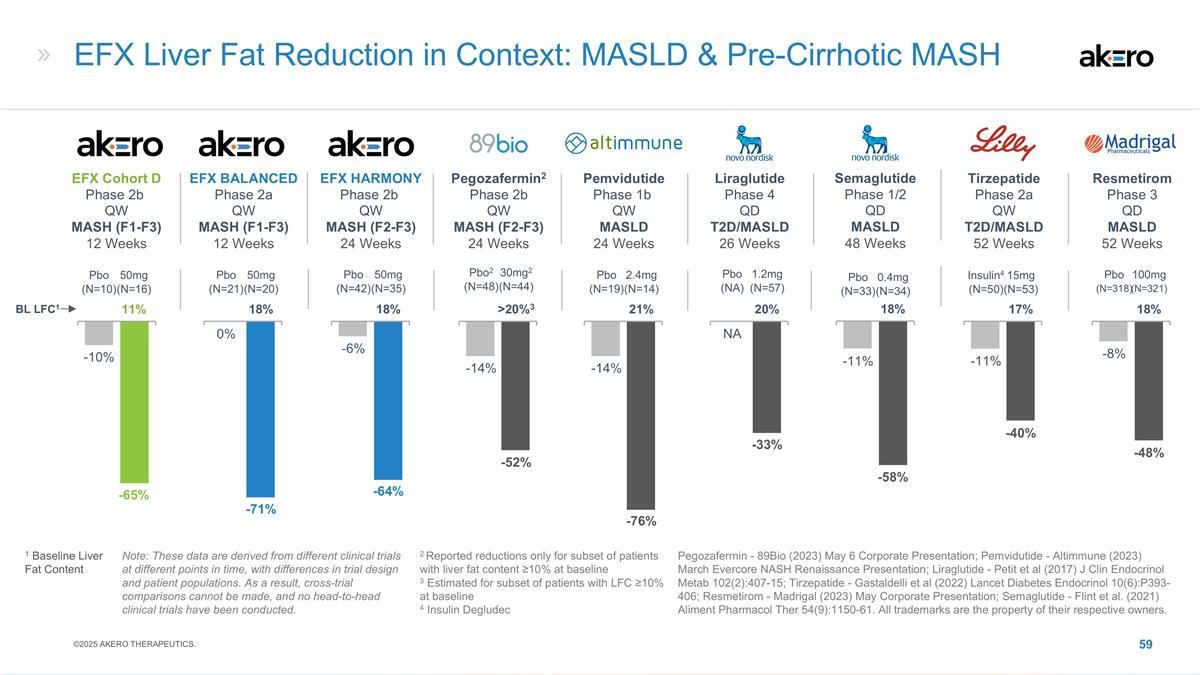
Madrigal Resmetirom Phase 3 QD
MASLD 52 Weeks (N=318) (N=321) 100mg Pbo -48% -8% 18% Semaglutide Phase 1/2 QD MASLD 48 Weeks (N=33) (N=34) 0.4mg Pbo -58% -11% 18% Inventiva Pemvidutide Phase 1b QW MASLD 24 Weeks (N=19) (N=14) 2.4mg Pbo -76% -14% 21% Akero EFX HARMONY Phase 2b QW
MASH (F2-F3) 24 Weeks (N=42) (N=35) 50mg Pbo -64% -6% 18% Novo Nordisk Liraglutide Phase 4 QD T2D/MASLD 26 Weeks (NA) (N=57) 1.2mg Pbo -33% NA 20% EFX Liver Fat Reduction in Context: MASLD & Pre-Cirrhotic MASH Pegozafermin - 89Bio (2023) May 6
Corporate Presentation; Pemvidutide - Altimmune (2023) March Evercore NASH Renaissance Presentation; Liraglutide - Petit et al (2017) J Clin Endocrinol Metab 102(2):407-15; Tirzepatide - Gastaldelli et al (2022) Lancet Diabetes Endocrinol
10(6):P393-406; Resmetirom - Madrigal (2023) May Corporate Presentation; Semaglutide - Flint et al. (2021) Aliment Pharmacol Ther 54(9):1150-61. All trademarks are the property of their respective owners. Note: These data are derived from
different clinical trials at different points in time, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been conducted. Novo Nordisk Tirzepatide
Phase 2a QW T2D/MASLD 52 Weeks (N=50) (N=53) 15mg Insulin4 -40% -11% 17% Madrigal Pegozafermin2 Phase 2b QW MASH (F2-F3) 24 Weeks (N=48) (N=44) 30mg2 Pbo2 -52% -14% >20%3 Akero EFX Cohort D Phase 2b QW MASH (F1-F3) 12 Weeks (N=10) (N=16) 50mg Pbo
-65% -10% 11% Akero EFX BALANCED Phase 2a QW MASH (F1-F3) 12 Weeks (N=21) (N=20) 50mg Pbo -71% 0% 18% BL LFC1 1 Baseline Liver Fat Content 2 Reported reductions only for subset of patients with liver fat content ≥10% at baseline 3 Estimated
for subset of patients with LFC ≥10% at baseline 4 Insulin Degludec ©2025 AKERO THERAPEUTICS.
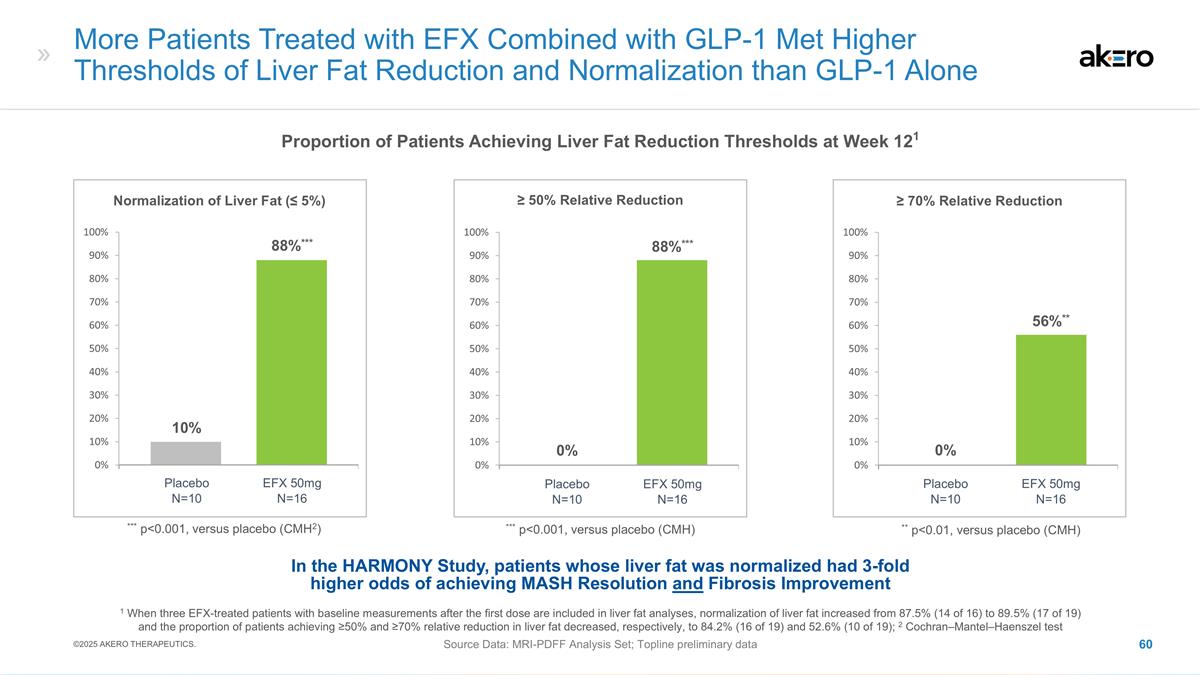
*** p<0.001, versus placebo
(CMH) ≥ 50% Relative Reduction EFX 50mg Placebo N=16 N=10 ≥ 70% Relative Reduction 0% 88%*** EFX 50mg Placebo N=16 N=10 0% 56%** ** p<0.01, versus placebo (CMH) Normalization of Liver Fat (≤ 5%) EFX 50mg Placebo N=16 N=10 10%
88%*** *** p<0.001, versus placebo (CMH2) More Patients Treated with EFX Combined with GLP-1 Met Higher Thresholds of Liver Fat Reduction and Normalization than GLP-1 Alone Source Data: MRI-PDFF Analysis Set; Topline preliminary data Proportion
of Patients Achieving Liver Fat Reduction Thresholds at Week 121 In the HARMONY Study, patients whose liver fat was normalized had 3-fold higher odds of achieving MASH Resolution and Fibrosis Improvement 1 When three EFX-treated patients with
baseline measurements after the first dose are included in liver fat analyses, normalization of liver fat increased from 87.5% (14 of 16) to 89.5% (17 of 19) and the proportion of patients achieving ≥50% and ≥70% relative reduction in
liver fat decreased, respectively, to 84.2% (16 of 19) and 52.6% (10 of 19); 2 Cochran–Mantel–Haenszel test ©2025 AKERO THERAPEUTICS.
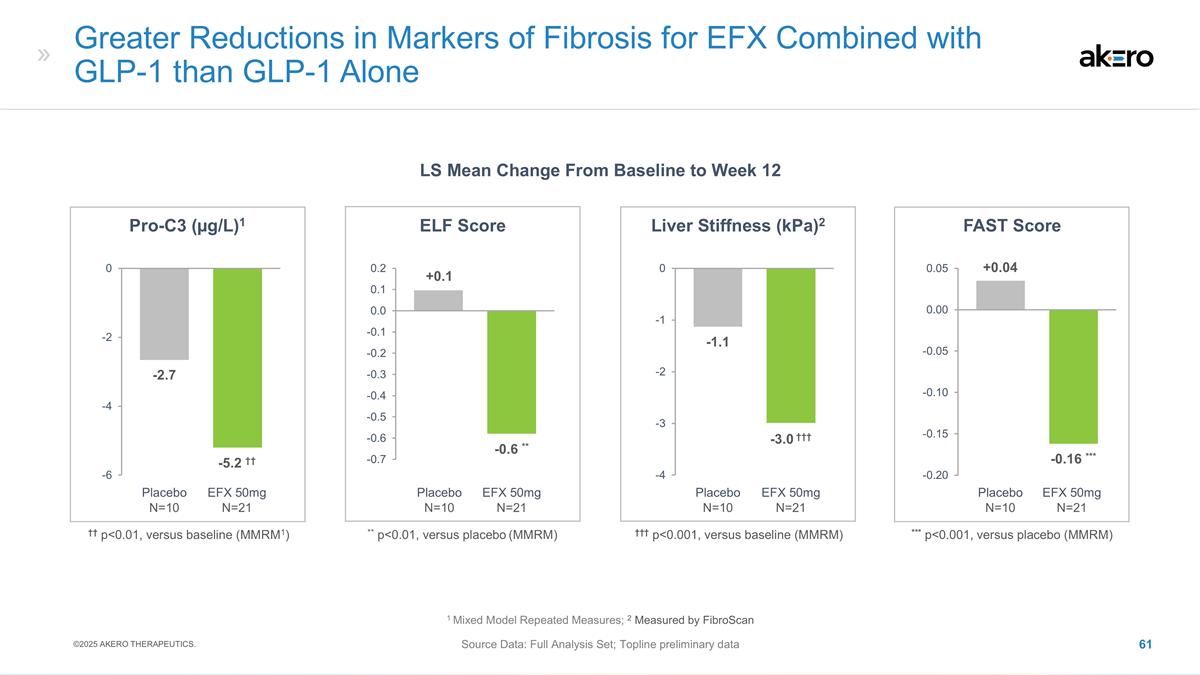
Greater Reductions in Markers of
Fibrosis for EFX Combined with GLP-1 than GLP-1 Alone Source Data: Full Analysis Set; Topline preliminary data ELF Score -0.6 ** +0.1 EFX 50mg Placebo N=21 N=10 ** p<0.01, versus placebo (MMRM) -5.2 †† -2.7 Pro-C3 (µg/L)1 EFX
50mg Placebo N=21 N=10 †† p<0.01, versus baseline (MMRM1) -3.0 ††† -1.1 Liver Stiffness (kPa)2 EFX 50mg Placebo N=21 N=10 ††† p<0.001, versus baseline (MMRM) -0.16 *** +0.04 FAST Score EFX 50mg
Placebo N=21 N=10 *** p<0.001, versus placebo (MMRM) LS Mean Change From Baseline to Week 12 1 Mixed Model Repeated Measures; 2 Measured by FibroScan ©2025 AKERO THERAPEUTICS.
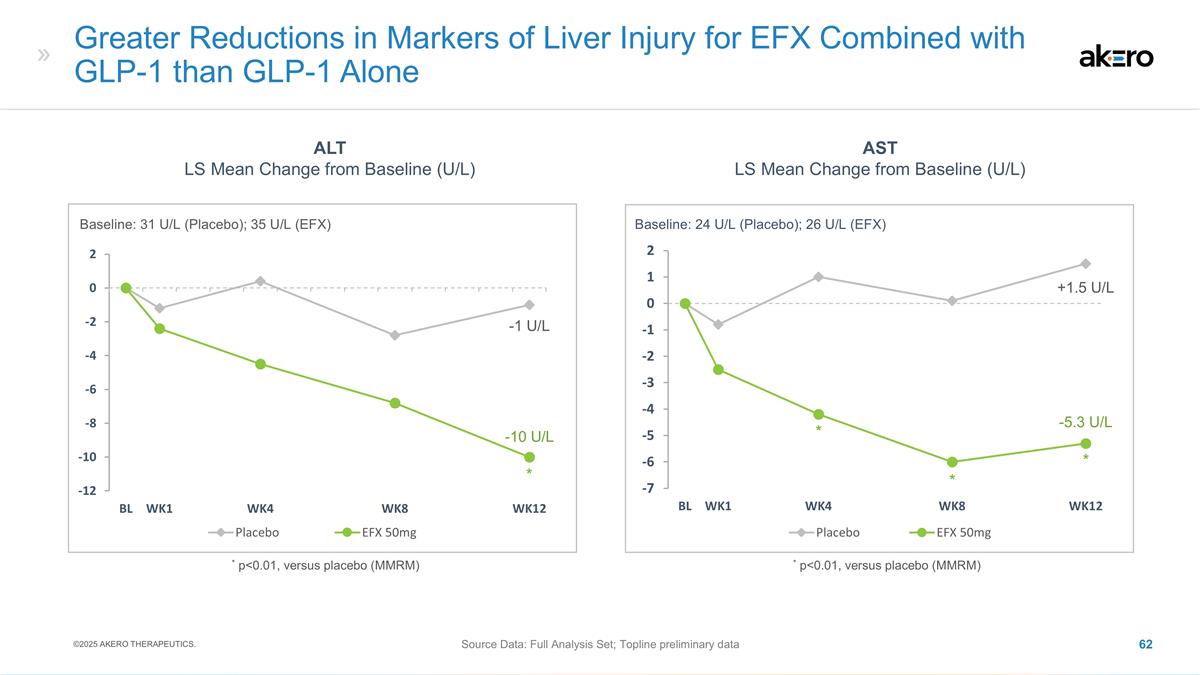
Greater Reductions in Markers of
Liver Injury for EFX Combined with GLP-1 than GLP-1 Alone * p<0.01, versus placebo (MMRM) * p<0.01, versus placebo (MMRM) * * ALT LS Mean Change from Baseline (U/L) * * AST LS Mean Change from Baseline (U/L) Baseline: 31 U/L (Placebo); 35 U/L
(EFX) Baseline: 24 U/L (Placebo); 26 U/L (EFX) -10 U/L -5.3 U/L Source Data: Full Analysis Set; Topline preliminary data -1 U/L +1.5 U/L ©2025 AKERO THERAPEUTICS.
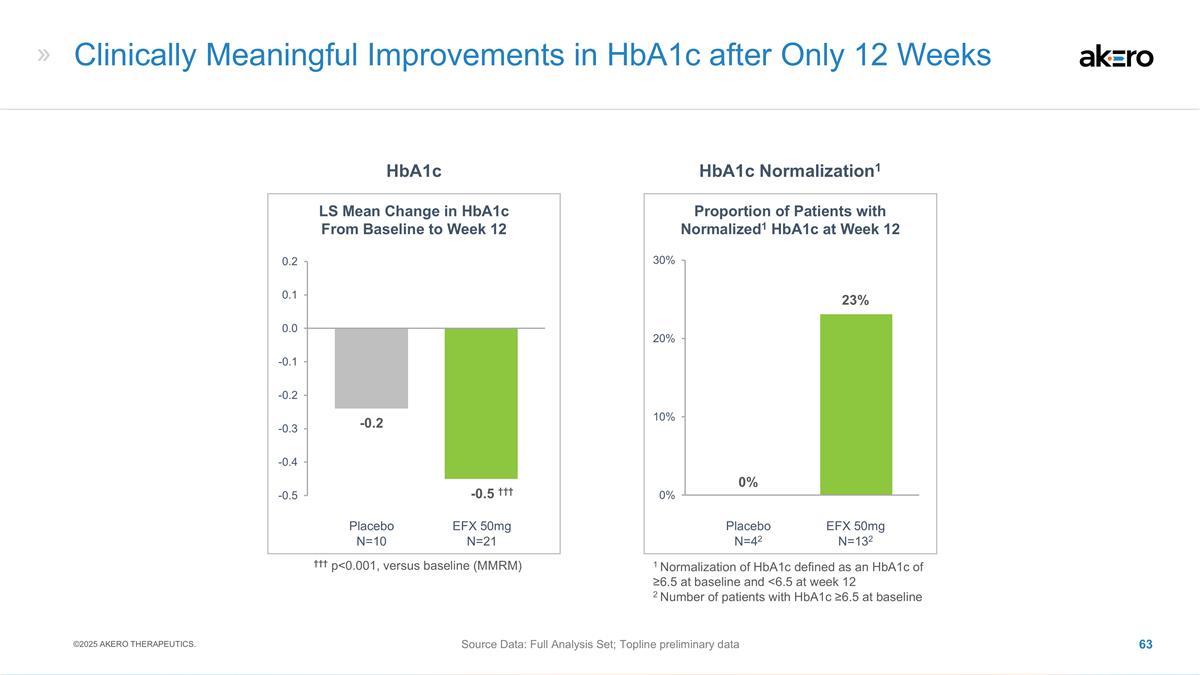
Clinically Meaningful Improvements
in HbA1c after Only 12 Weeks Source Data: Full Analysis Set; Topline preliminary data 23% 0% -0.2 LS Mean Change in HbA1c From Baseline to Week 12 Proportion of Patients with Normalized1 HbA1c at Week 12 EFX 50mg Placebo N=21 N=10 EFX 50mg Placebo
N=132 N=42 1 Normalization of HbA1c defined as an HbA1c of ≥6.5 at baseline and <6.5 at week 12 2 Number of patients with HbA1c ≥6.5 at baseline ††† p<0.001, versus baseline (MMRM) ††† -0.5
††† HbA1c HbA1c Normalization1 ©2025 AKERO THERAPEUTICS.
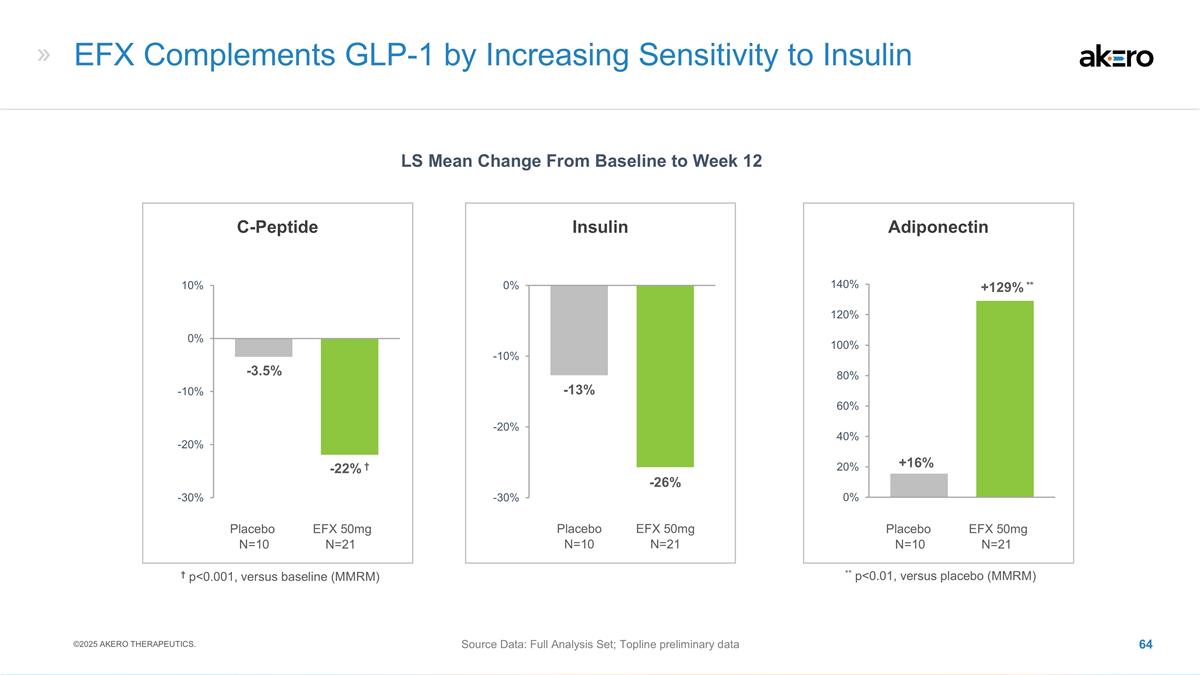
C-Peptide -3.5% EFX 50mg Placebo
N=21 N=10 -22% † † p<0.001, versus baseline (MMRM) EFX Complements GLP-1 by Increasing Sensitivity to Insulin Source Data: Full Analysis Set; Topline preliminary data +16% Adiponectin LS Mean Change From Baseline to Week 12 EFX 50mg
Placebo N=21 N=10 +129% ** +129% ** p<0.01, versus placebo (MMRM) Insulin EFX 50mg Placebo N=21 N=10 -13% -26% ©2025 AKERO THERAPEUTICS.
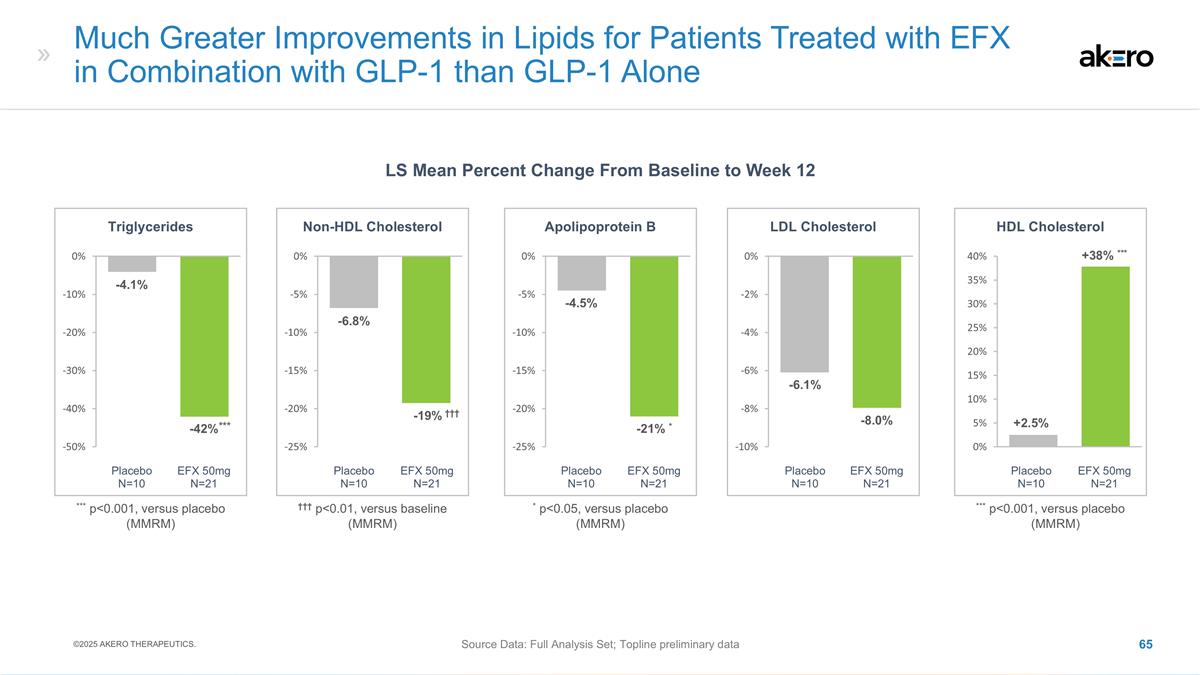
*** p<0.001, versus placebo
(MMRM) *** p<0.001, versus placebo Much Greater Improvements in Lipids for Patients Treated with EFX in Combination with GLP-1 than GLP-1 Alone LS Mean Percent Change From Baseline to Week 12 Source Data: Full Analysis Set; Topline preliminary
data ††† p<0.01, versus baseline HDL Cholesterol LDL Cholesterol Non-HDL Cholesterol Triglycerides EFX 50mg Placebo N=21 N=10 EFX 50mg Placebo N=21 N=10 EFX 50mg Placebo N=21 N=10 EFX 50mg Placebo N=21 N=10 EFX 50mg Placebo N=21
N=10 (MMRM) Apolipoprotein B * p<0.05, versus placebo (MMRM) (MMRM) -4.1% ***-42%*** -42% -6.8% † †† -19% ††† -6.1% -8.0% -4.5% -21% * +2.5% +38% *** ©2025 AKERO THERAPEUTICS.
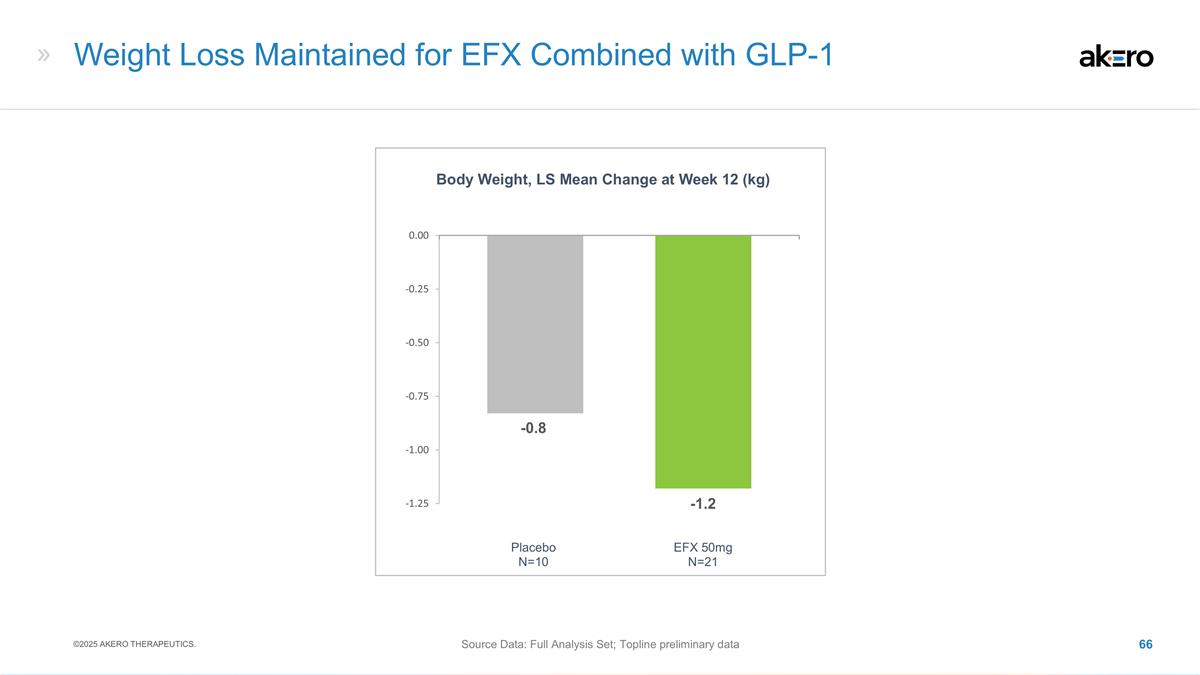
Weight Loss Maintained for EFX
Combined with GLP-1 Source Data: Full Analysis Set; Topline preliminary data -0.8 Body Weight, LS Mean Change at Week 12 (kg) -1.2 EFX 50mg Placebo N=21 N=10 ©2025 AKERO THERAPEUTICS.
Normalized Liver Fat Complementing
GLP-1 Potential for EFX on Top of GLP-1 to be More Effective than GLP-1 Alone Improved Lipoprotein Profile Improved Fibrosis Markers Improved Glycemic Control Weight Loss Maintained Cohort D Adds to a Growing Body of Evidence for EFX’s
Potential as a Cornerstone MASH Treatment Key Take-Aways EFX and GLP-1 have complementary mechanisms of action. Addition of EFX to GLP-1 in patients with MASH and type 2 diabetes was well tolerated, without additive GI side effects. EFX with GLP-1
showed multiple benefits over GLP-1 alone: reduced markers of liver steatosis, injury and fibrosis with improved glycemic control, dyslipidemia and weight loss maintained. The Cohort D EFX profile was comparable to that seen in the previous BALANCED
and HARMONY studies with EFX. After 12 Weeks in Cohort D ©2025 AKERO THERAPEUTICS.
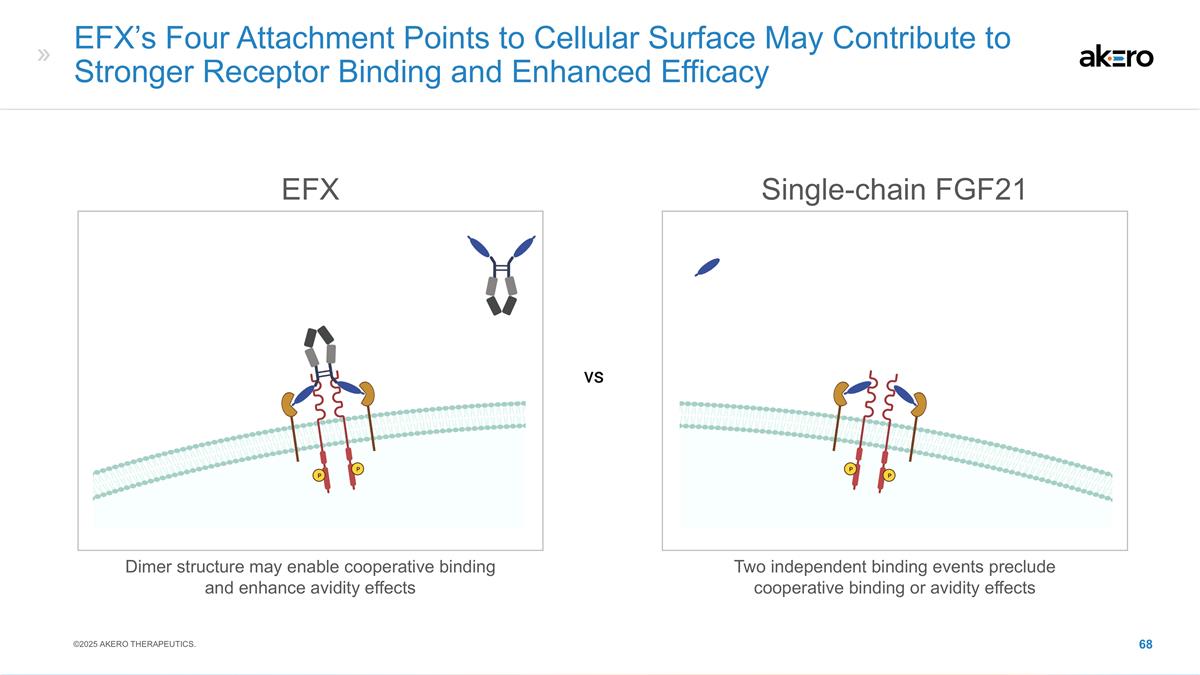
EFX’s Four Attachment Points
to Cellular Surface May Contribute to Stronger Receptor Binding and Enhanced Efficacy Dimer structure may enable cooperative binding and enhance avidity effects EFX Single-chain FGF21 Two independent binding events preclude cooperative binding or
avidity effects ©2025 AKERO THERAPEUTICS.
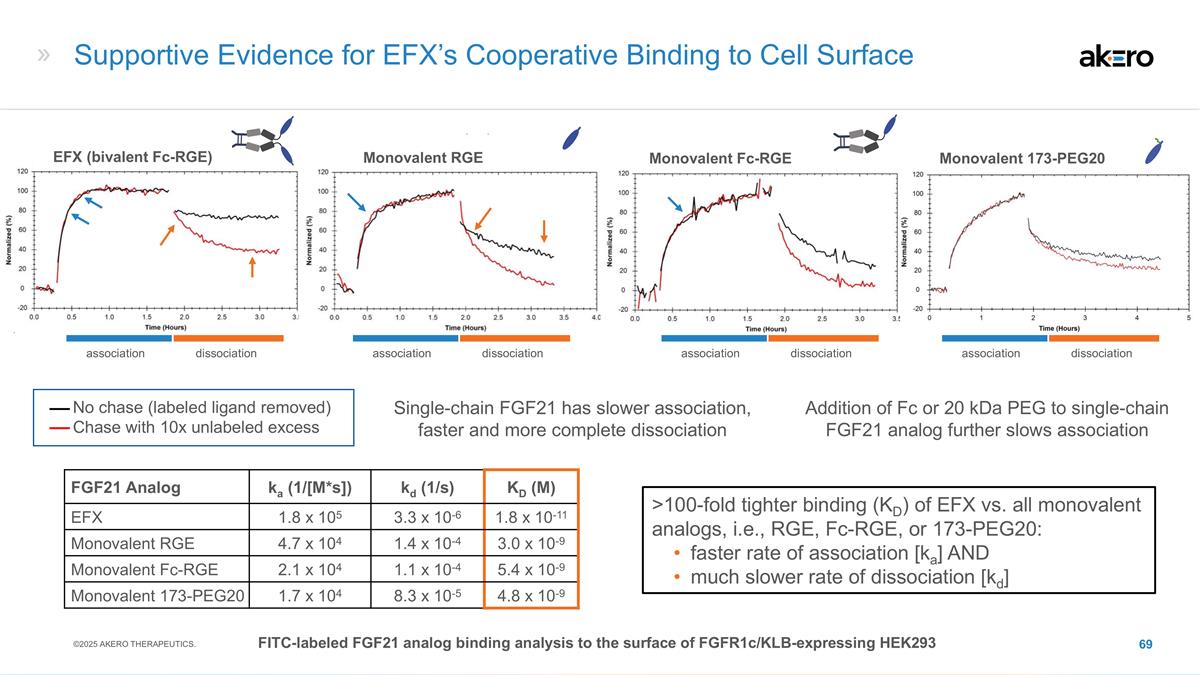
Supportive Evidence for EFX’s
Cooperative Binding to Cell Surface Single-chain FGF21 has slower association, faster and more complete dissociation Addition of Fc or 20 kDa PEG to single-chain FGF21 analog further slows association FGF21 Analog ka (1/[M*s]) kd (1/s) KD (M) EFX
1.8 x 105 3.3 x 10-6 1.8 x 10-11 Monovalent RGE 4.7 x 104 1.4 x 10-4 3.0 x 10-9 Monovalent Fc-RGE 2.1 x 104 1.1 x 10-4 5.4 x 10-9 Monovalent 173-PEG20 1.7 x 104 8.3 x 10-5 4.8 x 10-9 association dissociation Monovalent RGE No chase (labeled ligand
removed) Chase with 10x unlabeled excess FITC-labeled FGF21 analog binding analysis to the surface of FGFR1c/KLB-expressing HEK293 EFX (bivalent Fc-RGE) >100-fold tighter binding (KD) of EFX vs. all monovalent analogs, i.e., RGE, Fc-RGE, or
173-PEG20: faster rate of association [ka] AND much slower rate of dissociation [kd] association dissociation association dissociation association dissociation Monovalent Fc-RGE Monovalent 173-PEG20 ©2025 AKERO THERAPEUTICS.
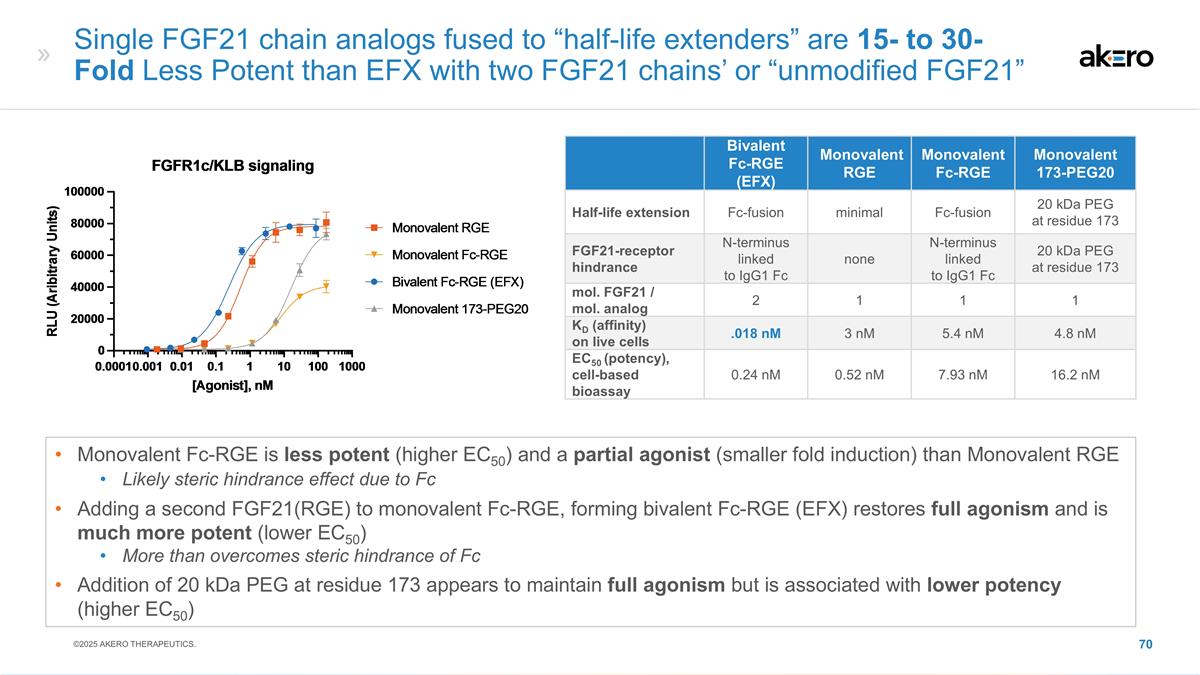
Single FGF21 chain analogs fused to
“half-life extenders” are 15- to 30-Fold Less Potent than EFX with two FGF21 chains’ or “unmodified FGF21” Monovalent Fc-RGE is less potent (higher EC50) and a partial agonist (smaller fold induction) than Monovalent
RGE Likely steric hindrance effect due to Fc Adding a second FGF21(RGE) to monovalent Fc-RGE, forming bivalent Fc-RGE (EFX) restores full agonism and is much more potent (lower EC50) More than overcomes steric hindrance of Fc Addition of 20 kDa PEG
at residue 173 appears to maintain full agonism but is associated with lower potency (higher EC50) Bivalent Fc-RGE (EFX) Monovalent RGE Monovalent Fc-RGE Monovalent 173-PEG20 Half-life extension Fc-fusion minimal Fc-fusion 20 kDa PEG at
residue 173 FGF21-receptor hindrance N-terminus linked to IgG1 Fc none N-terminus linked to IgG1 Fc 20 kDa PEG at residue 173 mol. FGF21 / mol. analog 2 1 1 1 KD (affinity) on live cells .018 nM 3 nM 5.4 nM 4.8 nM EC50 (potency), cell-based bioassay
0.24 nM 0.52 nM 7.93 nM 16.2 nM ©2025 AKERO THERAPEUTICS.

AKERO THERAPEUTICS 601 Gateway
Boulevard Suite 350 South San Francisco, CA 94080
v3.24.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Grafico Azioni Akero Therapeutics (NASDAQ:AKRO)
Storico
Da Gen 2025 a Feb 2025

Grafico Azioni Akero Therapeutics (NASDAQ:AKRO)
Storico
Da Feb 2024 a Feb 2025
