false 0001759425 0001759425 2024-01-08 2024-01-08
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 OR 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 8, 2024
Mirum Pharmaceuticals, Inc.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
001-38981 |
|
83-1281555 |
| (State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(I.R.S. Employer Identification No.) |
|
|
|
| 950 Tower Lane, Suite 1050 Foster City, California |
|
94404 |
| (Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (650) 667-4085
N/A
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| |
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common stock, par value $0.0001 per share |
|
MIRM |
|
Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 2.02 |
Results of Operations and Financial Condition. |
On January 8, 2024, Mirum Pharmaceuticals, Inc. (the “Company”) issued a press release announcing, among other things, the Company’s preliminary unaudited revenues and net product sales for the fiscal year ended December 31, 2023 and preliminary unaudited net product sales of LIVMARLI® (maralixibat) oral solution. The full text of the press release is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
| Item 7.01 |
Regulation FD Disclosure. |
On January 8, 2024, in connection with its participation in the J.P. Morgan Healthcare Conference, the Company posted a corporate slide presentation in the “Investors” portion of its website at www.mirumpharma.com. A copy of the presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.2.
The information in this Current Report on Form 8-K, including Exhibits 99.1 and 99.2, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that Section, nor shall it be deemed to be incorporated by reference into any filing of the Company under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
Mirum Pharmaceuticals, Inc. |
|
|
|
|
| Date: January 8, 2024 |
|
|
|
By: |
|
/s/ Christopher Peetz |
|
|
|
|
|
|
Christopher Peetz |
|
|
|
|
|
|
President and Chief Executive Officer |
Exhibit 99.1

Mirum Pharmaceuticals Announces Preliminary Unaudited 2023 Net Revenue and Provides Corporate Updates
| - |
$186-188 million in total revenue and $178-180 million net product sales expected for 2023, preliminary and unaudited |
| - |
LIVMARLI net products sales of $141-143 million expected for
2023, representing approximately 89% year-over-year growth, preliminary and unaudited |
FOSTER CITY, Calif. –
January 8, 2023 - Mirum Pharmaceuticals, Inc. (Nasdaq: MIRM) today provided its preliminary and unaudited estimates for full-year 2023 revenue and net product sales, corporate updates, and full-year 2024 outlook.
“2023 was a transformative year for Mirum as we cemented our position as a leader in rare disease and dramatically advanced our operating and financial
scale. We expanded our reach and impact through strong global adoption of LIVMARLI and the addition of CHOLBAM and CHENODAL,” said Chris Peetz, president and chief executive officer. “In the year ahead, we are excited about the growth
prospects of all three commercial medicines, the potential label expansion opportunities for LIVMARLI and CHENODAL and key volixibat analyses in primary sclerosing cholangitis and primary biliary cholangitis.”
2023 Highlights
| |
• |
|
Established a leading pediatric hepatology franchise with three commercial medicines. |
| |
• |
|
2023 estimated LIVMARLI net product sales of $141-143 million
representing approximately 89% growth over 2022 LIVMARLI net product sales . |
| |
• |
|
Total expected net product sales of $69-71 million in Q4 2023
including $41-43 million in LIVMARLI net sales and approximately $28 million in net sales from CHOLBAM and CHENODAL. |
| |
• |
|
Acquired two therapies commercially available in the U.S.: CHOLBAM and CHENODAL |
| |
• |
|
Announced positive Phase 3 data from the RESTORE study evaluating CHENODAL in cerebrotendinous
xanthomatosis (CTX) patients |
| |
• |
|
Expanded LIVMARLI’s US label in Alagille syndrome (ALGS) to include infants 3 months of age and
older. |
| |
• |
|
Grew international business to 18 countries with reimbursed access |
| |
• |
|
Company fully financed with strong balance sheet and financial performance |
| |
• |
|
Announced the appointment of Eric Bjerkholt as Chief Financial Officer |
The foregoing amounts relating to 2023 financial data are unaudited and preliminary and are subject to completion of financial closing procedures. Additional
information and disclosure would be required for a more complete understanding of the company’s financial position and results of operations as of December 31, 2023.

2024 Expectations and Milestones
| |
• |
|
Expect continued revenue growth across all three commercial medicines |
| |
• |
|
U.S. Food and Drug Administration (FDA) Prescription Drug User Free Act (PDUFA) date for LIVMARLI in progressive
familial intrahepatic cholestasis (PFIC) is March 13, 2024. |
| |
• |
|
New Drug Application (NDA) submission for CHENODAL in CTX planned in first half 2024 |
| |
• |
|
Volixibat VISTAS study in primary sclerosing cholangitis blinded interim analysis expected first half 2024
|
| |
• |
|
Volixibat VANTAGE study for primary biliary cholangitis interim analysis expected in first half 2024
|
Mirum will present at the 42nd annual J.P. Morgan Healthcare Conference in San
Francisco on Wednesday, January 10, 2024 at 10:30 a.m. PT. The presentation and question and answer session will be webcast live and can be accessed by visiting the Investors and Media section of Mirum’s corporate website. The
replay of the webcast will be available for 30 days.
About LIVMARLI® (maralixibat) oral
solution
LIVMARLI® (maralixibat) oral solution is an orally administered, once-daily, ileal
bile acid transporter (IBAT) inhibitor and the only approved medication by the U.S. Food and Drug Administration for the treatment of cholestatic pruritus in patients with Alagille syndrome (ALGS) three months of age and older. LIVMARLI is also
approved by the European Commission for the treatment of cholestatic pruritus in patients with ALGS two months and older. For more information for U.S. residents, please visit LIVMARLI.com.
Mirum has also submitted LIVMARLI for approval in the U.S. in cholestatic pruritus in PFIC patients three months of age and older, and in Europe, in PFIC for
patients two months of age and older.
LIVMARLI is currently being evaluated in late-stage clinical studies in other rare cholestatic liver diseases.
LIVMARLI has received Breakthrough Therapy designation for ALGS and PFIC type 2 and orphan designation for ALGS and PFIC. To learn more about ongoing clinical trials with LIVMARLI, please visit Mirum’s clinical trials section on the
company’s website.
IMPORTANT SAFETY INFORMATION
LIVMARLI can cause side effects, including:
Changes in
liver tests. Changes in certain liver tests are common in patients with Alagille syndrome and can worsen during treatment with LIVMARLI. These changes may be a sign of liver injury and can be serious. Your healthcare provider should do blood
tests before starting and during treatment to check your liver function. Tell your healthcare provider right away if you get any signs or symptoms of liver problems, including nausea or vomiting, skin or the white part of the eye turns yellow, dark
or brown urine, pain on the right side of the stomach (abdomen) or loss of appetite.
Stomach and intestinal (gastrointestinal) problems. LIVMARLI
can cause stomach and intestinal problems, including diarrhea, stomach pain, and vomiting during treatment. Tell your healthcare provider right away if you have any of these symptoms more often or more severely than normal for you.

A condition called Fat Soluble Vitamin (FSV) Deficiency caused by low levels of certain vitamins
(vitamin A, D, E, and K) stored in body fat. FSV deficiency is common in patients with Alagille syndrome but may worsen during treatment. Your healthcare provider should do blood tests before starting and during treatment.
Other common side effects reported during treatment were bone fractures and gastrointestinal bleeding.
US Prescribing Information
EU SmPC
About Volixibat
Volixibat is an oral, minimally absorbed
agent designed to selectively inhibit the apical sodium dependent bile acid transporter (ASBT). Volixibat may offer a novel approach in the treatment of adult cholestatic diseases by blocking the recycling of bile acids, through inhibition of ASBT,
thereby reducing bile acids systemically and in the liver. Phase 1 and Phase 2 studies of volixibat demonstrated on-target fecal bile acid excretion, a pharmacodynamic marker of ASBT inhibition, in addition to
decreases in LDL cholesterol and increases in 7αC4 which are markers of bile acid synthesis. Volixibat has been evaluated in more than 400 individuals across multiple clinical trials. The most common adverse events reported were mild to
moderate gastrointestinal events observed in the volixibat groups.
Volixibat is currently being evaluated in Phase 2b studies for primary sclerosing
cholangitis (VISTAS study), and primary biliary cholangitis (VANTAGE study).
About CHOLBAM® (cholic acid) capsules
The FDA approved CHOLBAM® (cholic acid) capsules in March 2015, the first FDA-approved treatment for pediatric and adult patients with bile acid synthesis disorders due to single
enzyme defects, and for adjunctive treatment of patients with peroxisome biogenesis disorder-Zellweger spectrum disorder. The effectiveness of CHOLBAM® has been demonstrated in clinical trials
for bile acid synthesis disorders and the adjunctive treatment of peroxisomal disorders.
CHOLBAM® (cholic acid) Indication
CHOLBAM is a bile acid indicated for
| |
• |
|
Treatment of bile acid synthesis disorders due to single enzyme defects. |
| |
• |
|
Adjunctive treatment of peroxisomal disorders, including Zellweger spectrum disorders, in patients who exhibit
manifestations of liver disease, steatorrhea, or complications from decreased fat-soluble vitamin absorption. |
LIMITATIONS OF USE
The safety and effectiveness of
CHOLBAM on extrahepatic manifestations of bile acid synthesis disorders due to single enzyme defects or peroxisomal disorders, including Zellweger spectrum disorders, have not been established.

IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS – Exacerbation of liver impairment
| |
• |
|
Monitor liver function and discontinue CHOLBAM in patients who develop worsening of liver function while on
treatment. |
| |
• |
|
Concurrent elevations of serum gamma glutamyltransferase (GGT) and alanine aminotransferase (ALT) may indicate
CHOLBAM overdose. |
| |
• |
|
Discontinue treatment with CHOLBAM at any time if there are clinical or laboratory indicators of worsening liver
function or cholestasis. |
ADVERSE REACTIONS
| |
• |
|
The most common adverse reactions (≥1%) are diarrhea, reflux esophagitis, malaise, jaundice, skin lesion,
nausea, abdominal pain, intestinal polyp, urinary tract infection, and peripheral neuropathy. |
Please see full Prescribing
Information for additional Important Safety Information.
About
CHENODAL® (chenodiol) tablets
CHENODAL® is a synthetic oral form of chenodeoxycholic acid (CDCA), a naturally occurring primary bile
acid synthesized from cholesterol in the liver. The FDA approved CHENODAL for the treatment of people with radiolucent stones in the gallbladder. In 2010, CHENODAL was granted orphan drug designation for the treatment of cerebrotendinous
xanthomatosis (CTX), a rare autosomal recessive lipid storage disease.
While CHENODAL® is not
currently approved for CTX, it received a medical necessity determination in the U.S. by the FDA and has been used as the standard of care for more than three decades. Efforts are being made to obtain FDA approval of CHENODAL for the treatment of
CTX and a Phase 3 clinical trial for this indication was initiated in January 2020.
About Mirum Pharmaceuticals, Inc.
Mirum Pharmaceuticals, Inc. is a biopharmaceutical company dedicated to transforming the treatment of rare diseases affecting children and adults. Mirum has
three approved medications: LIVMARLI® (maralixibat) oral solution, CHOLBAM® (cholic acid)
capsules, and CHENODAL® (chenodiol) tablets.
LIVMARLI, an IBAT inhibitor,
is approved for the treatment of cholestatic pruritus in patients with Alagille syndrome in the U.S. (three months and older), in Europe (two months and older), and in Canada. Mirum has also submitted LIVMARLI for approval in the U.S. in cholestatic
pruritus in PFIC patients three months of age and older and in Europe in PFIC for patients two months of age and older. CHOLBAM is FDA-approved for the treatment of bile acid synthesis disorders due to single
enzyme defects and adjunctive treatment of peroxisomal disorders in patients who show signs or symptoms or liver disease. CHENODAL has received medical necessity recognition by the FDA to treat patients with cerebrotendinous xanthomatosis (CTX).
Mirum’s late-stage pipeline includes two investigational treatments for high-need rare diseases. Volixibat, also an IBAT inhibitor, is being
evaluated in two potentially registrational studies including the Phase 2b VISTAS study for primary sclerosing cholangitis and Phase 2b VANTAGE study for primary biliary cholangitis. CHENODAL, has been evaluated in
a Phase 3 clinical study, RESTORE, to treat patients with CTX.

To learn more about Mirum, visit mirumpharma.com and follow Mirum
on Facebook, LinkedIn, Instagram and Twitter.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of
the Private Securities Litigation Reform Act of 1995. Such forward-looking statements include statements regarding, among other things: continued commercial success and growth prospects for LIVMARLI, CHENODAL and CHOLBAM, including growth in
LIVMARLI year-over-year net product sales, planned LIVMARLI launches in additional international markets and label expansion into PFIC, the timing and results of LIVMARLI’s PDUFA date, CHENODAL’s potential full indication in CTX,
becoming a global leader in rare disease, the results, conduct and progress of Mirum’s ongoing and planned studies for its product candidates and the regulatory approval path for its product candidates globally. Because such statements are
subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “forward,” “planned,” “poised,” “positioned,”
“potential”, “will,” “prospects,” “opportunities” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon Mirum’s current expectations
and involve assumptions that may never materialize or may prove to be incorrect. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without
limitation, risks and uncertainties associated with Mirum’s business in general, the impact of geopolitical and macroeconomic events, and the other risks described in Mirum’s filings with the Securities and Exchange
Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made and are based on management’s assumptions and estimates as of such date. Mirum undertakes no obligation to update such
statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
Contacts
Investor Contacts:
Andrew McKibben
ir@mirumpharma.com
Sam Martin
Argot Partners
ir@mirumpharma.com
Media Contact:
Erin Murphy
media@mirumpharma.com

January 2024 Mirum Pharmaceuticals:
Transforming Lives in Rare Disease Exhibit 99.2

Forward-Looking Statements This
presentation contains "forward-looking" statements that are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include all statements other than statements of
historical fact contained in this presentation, including information concerning our business strategy, objectives and opportunities, including as a result of our acquisition of CHOLBAM and CHENODAL and the anticipated benefits resulting from the
same. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors that may cause our actual results, performance or achievements to differ materially and adversely from those anticipated or implied
by our forward-looking statements, including, but not limited to: the results, conduct and progress of, as well as our plans and expectations for, commercializing LIVMARLI, CHOLBAM and CHENODAL in the United States and rest of world; the costs of
our commercialization plans and development programs, and the financial impact or revenues from any commercialization we undertake; estimates of the number of patients impacted by the diseases or related diseases that we seek to treat and who are
appropriate for treatment with our commercial products; the potential clinical benefits of LIVMARLI, CHOLBAM and CHENODAL and any other product candidates; the potential benefits of our business model and expected growth; our ability to
obtain necessary regulatory approvals for our product candidates and, if and when approved, market acceptance of our products; our dependence on third-party clinical research organizations, manufacturers, suppliers and distributors; the design,
implementation, timelines and outcomes of our clinical trials; the impact of competitive products and therapies; our ability to obtain necessary additional capital; our ability to attract and retain key employees; our ability to manage the growth
and complexity of our organization; our ability to maintain, protect and enhance our intellectual property; and our ability to continue to stay in compliance with applicable laws and regulations. You should refer to the section entitled “Risk
Factors” set forth in our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and other filings we make with the Securities and Exchange Commission (SEC) from time to time (available at http://www.sec.gov) for a discussion of important
factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Neither we nor any other person
assumes responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation to update any forward-looking statements after the date of this presentation except as may be required by law. This presentation
also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. These data involve a number of assumptions and limitations, and you are cautioned not to
give undue weight to such estimates. Projections, assumptions and estimates of the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. The trademarks included herein are the
property of the owners thereof and are used for reference purposes only. We use our website (www.mirumpharma.com), LinkedIn page (www.linkedin.com/company/mirum-pharmaceuticals), and Twitter account (https://twitter.com/mirumpharma) as channels
of distribution of information about our company, product candidates, planned announcements, attendance at upcoming conferences and other matters. Such information may be deemed material information and we may use these channels to comply with our
disclosure obligations under Regulation FD. Therefore, investors should monitor our website, LinkedIn page, and Twitter account in addition to following our SEC filings, press releases, public conference calls and webcasts.

Established leader in the development
and commercialization of life changing rare disease medicines Standard of Care Medicines Late-Stage Indications Countries with Reimbursed Access 3 4 18
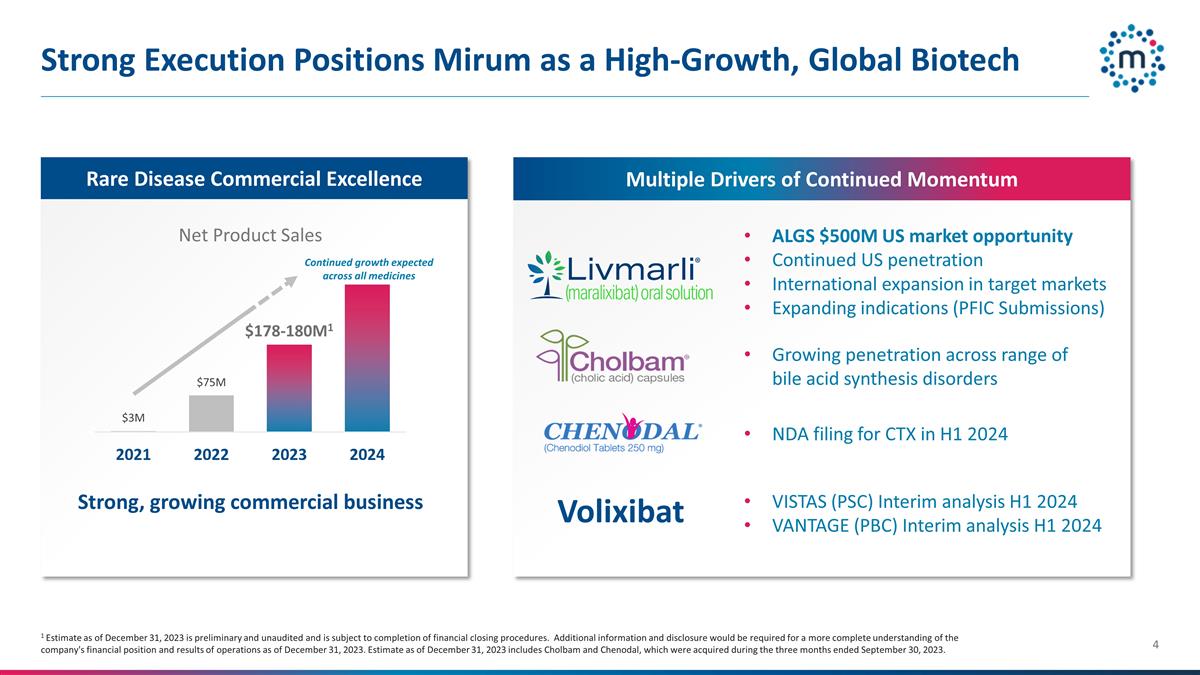
Strong Execution Positions Mirum as a
High-Growth, Global Biotech ALGS $500M US market opportunity Continued US penetration International expansion in target markets Expanding indications (PFIC Submissions) Rare Disease Commercial Excellence Net Product Sales Growing penetration
across range of bile acid synthesis disorders NDA filing for CTX in H1 2024 Multiple Drivers of Continued Momentum Strong, growing commercial business VISTAS (PSC) Interim analysis H1 2024 VANTAGE (PBC) Interim analysis H1 2024 Volixibat Continued
growth expected across all medicines 1 Estimate as of December 31, 2023 is preliminary and unaudited and is subject to completion of financial closing procedures. Additional information and disclosure would be required for a more complete
understanding of the company's financial position and results of operations as of December 31, 2023. Estimate as of December 31, 2023 includes Cholbam and Chenodal, which were acquired during the three months ended September 30, 2023.
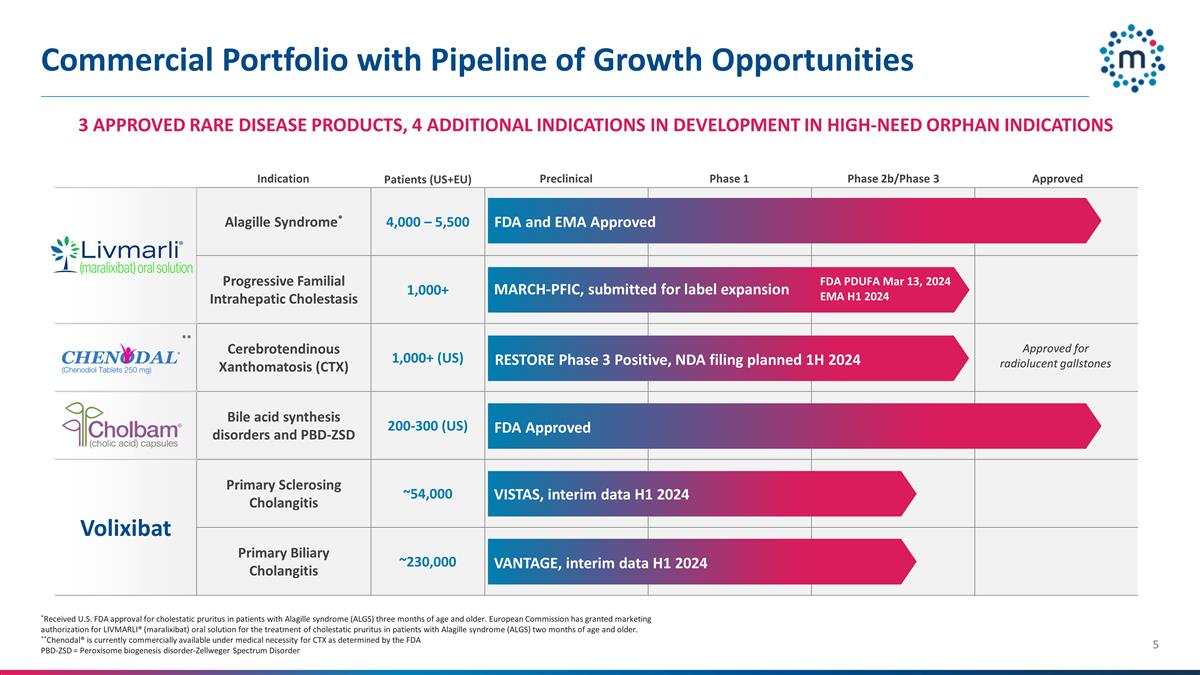
Commercial Portfolio with Pipeline of
Growth Opportunities *Received U.S. FDA approval for cholestatic pruritus in patients with Alagille syndrome (ALGS) three months of age and older. European Commission has granted marketing authorization for LIVMARLI® (maralixibat) oral solution
for the treatment of cholestatic pruritus in patients with Alagille syndrome (ALGS) two months of age and older. **Chenodal® is currently commercially available under medical necessity for CTX as determined by the FDA PBD-ZSD = Peroxisome
biogenesis disorder-Zellweger Spectrum Disorder Alagille Syndrome* 4,000 – 5,500 LIVMARLI (maralixibat) Oral Solution Progressive Familial Intrahepatic Cholestasis 1,000+ Cerebrotendinous Xanthomatosis (CTX) 1,000+ (US) Bile acid synthesis
disorders and PBD-ZSD 200-300 (US) Volixibat Primary Sclerosing Cholangitis ~54,000 Primary Biliary Cholangitis ~230,000 VISTAS, interim data H1 2024 VANTAGE, interim data H1 2024 RESTORE Phase 3 Positive, NDA filing planned 1H 2024 Phase 1
Approved Preclinical Phase 2b/Phase 3 MARCH-PFIC, submitted for label expansion FDA PDUFA Mar 13, 2024 EMA H1 2024 3 APPROVED RARE DISEASE PRODUCTS, 4 ADDITIONAL INDICATIONS IN DEVELOPMENT IN HIGH-NEED ORPHAN INDICATIONS FDA and EMA Approved FDA
Approved Indication Patients (US+EU) ** Approved for radiolucent gallstones

5 Clinical Programs 2024 Strategic
Goals to Build Value INDUSTRY LEADING TEAM DELIVERING REMARKABLE TREATMENTS TO PATIENTS WORLDWIDE Drive continued growth across all three commercial medicines Expand labels for LIVMARLI (PFIC) & CHENODAL (CTX) Leverage IBAT Inhibition Expertise
in Adult Cholestatic Liver Disease Expand Pipeline, Leveraging Rare Disease & Commercial Expertise $306M* Cash Balance, Well Positioned to Execute on Our Strategy * Cash, cash equivalents and investments as of September 30, 2023.

Commercial Overview
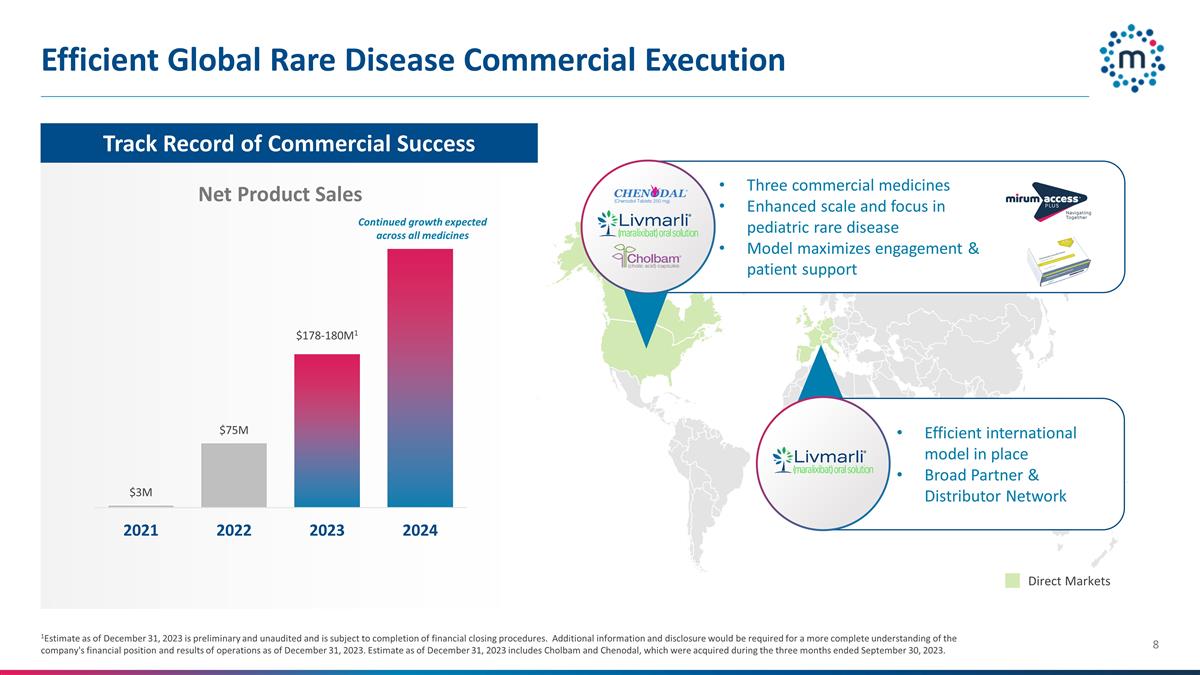
Efficient Global Rare Disease
Commercial Execution 1Estimate as of December 31, 2023 is preliminary and unaudited and is subject to completion of financial closing procedures. Additional information and disclosure would be required for a more complete understanding of the
company's financial position and results of operations as of December 31, 2023. Estimate as of December 31, 2023 includes Cholbam and Chenodal, which were acquired during the three months ended September 30, 2023. Direct Markets Net Product Sales
Three commercial medicines Enhanced scale and focus in pediatric rare disease Model maximizes engagement & patient support Track Record of Commercial Success Efficient international model in place Broad Partner & Distributor Network
Continued growth expected across all medicines

Long-term data set showing
significant effects on pruritus and event-free survival LIVMARLI® (maralixibat) Oral Solution in ALGS
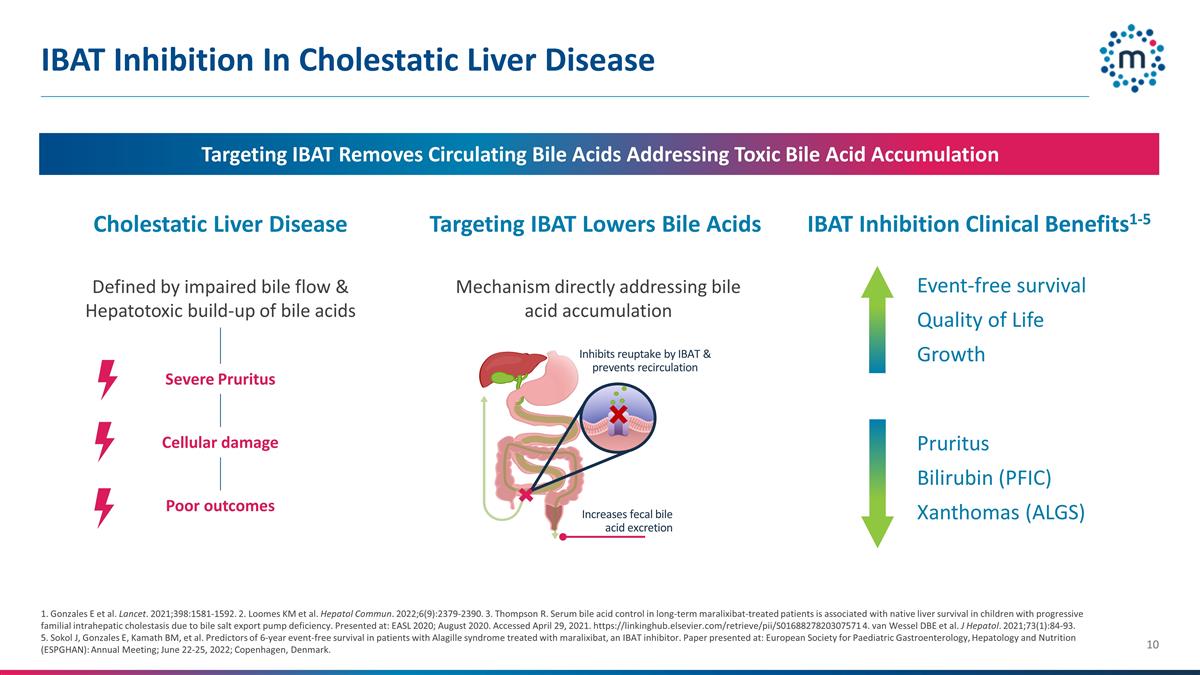
IBAT Inhibition In Cholestatic
Liver Disease Targeting IBAT Removes Circulating Bile Acids Addressing Toxic Bile Acid Accumulation Pruritus Bilirubin (PFIC) Xanthomas (ALGS) Event-free survival Quality of Life Growth 1. Gonzales E et al. Lancet. 2021;398:1581-1592. 2. Loomes KM
et al. Hepatol Commun. 2022;6(9):2379-2390. 3. Thompson R. Serum bile acid control in long-term maralixibat-treated patients is associated with native liver survival in children with progressive familial intrahepatic cholestasis due to bile salt
export pump deficiency. Presented at: EASL 2020; August 2020. Accessed April 29, 2021. https://linkinghub.elsevier.com/retrieve/pii/S0168827820307571 4. van Wessel DBE et al. J Hepatol. 2021;73(1):84-93. 5. Sokol J, Gonzales E, Kamath BM, et al.
Predictors of 6-year event-free survival in patients with Alagille syndrome treated with maralixibat, an IBAT inhibitor. Paper presented at: European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN): Annual Meeting; June
22-25, 2022; Copenhagen, Denmark. Inhibits reuptake by IBAT & prevents recirculation Increases fecal bile acid excretion Cholestatic Liver Disease Defined by impaired bile flow & Hepatotoxic build-up of bile acids Targeting IBAT Lowers Bile
Acids Mechanism directly addressing bile acid accumulation IBAT Inhibition Clinical Benefits1-5 Severe Pruritus Cellular damage Poor outcomes
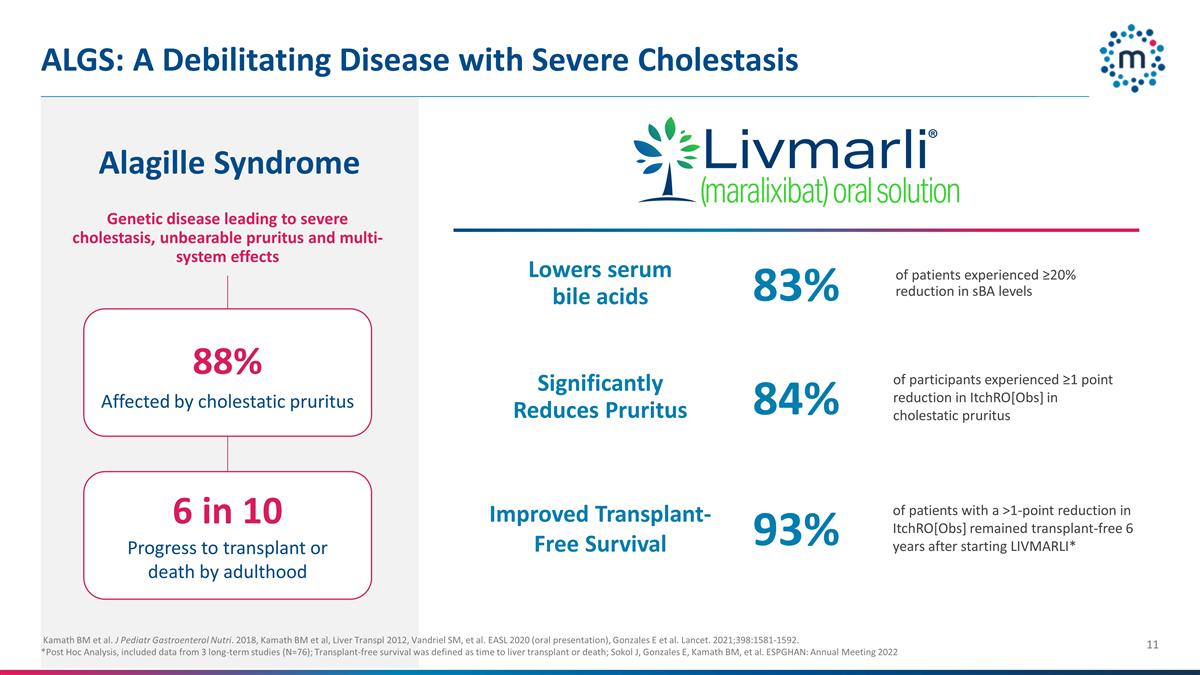
ALGS: A Debilitating Disease with
Severe Cholestasis Kamath BM et al. J Pediatr Gastroenterol Nutri. 2018, Kamath BM et al, Liver Transpl 2012, Vandriel SM, et al. EASL 2020 (oral presentation), Gonzales E et al. Lancet. 2021;398:1581-1592. *Post Hoc Analysis, included data
from 3 long-term studies (N=76); Transplant-free survival was defined as time to liver transplant or death; Sokol J, Gonzales E, Kamath BM, et al. ESPGHAN: Annual Meeting 2022 Genetic disease leading to severe cholestasis, unbearable pruritus and
multi-system effects 88% Affected by cholestatic pruritus 6 in 10 Progress to transplant or death by adulthood Improved Transplant-Free Survival Lowers serum bile acids Alagille Syndrome Significantly Reduces Pruritus 84% 83% of patients with a
>1-point reduction in ItchRO[Obs] remained transplant-free 6 years after starting LIVMARLI* of patients experienced ≥20% reduction in sBA levels of participants experienced ≥1 point reduction in ItchRO[Obs] in cholestatic pruritus 93%
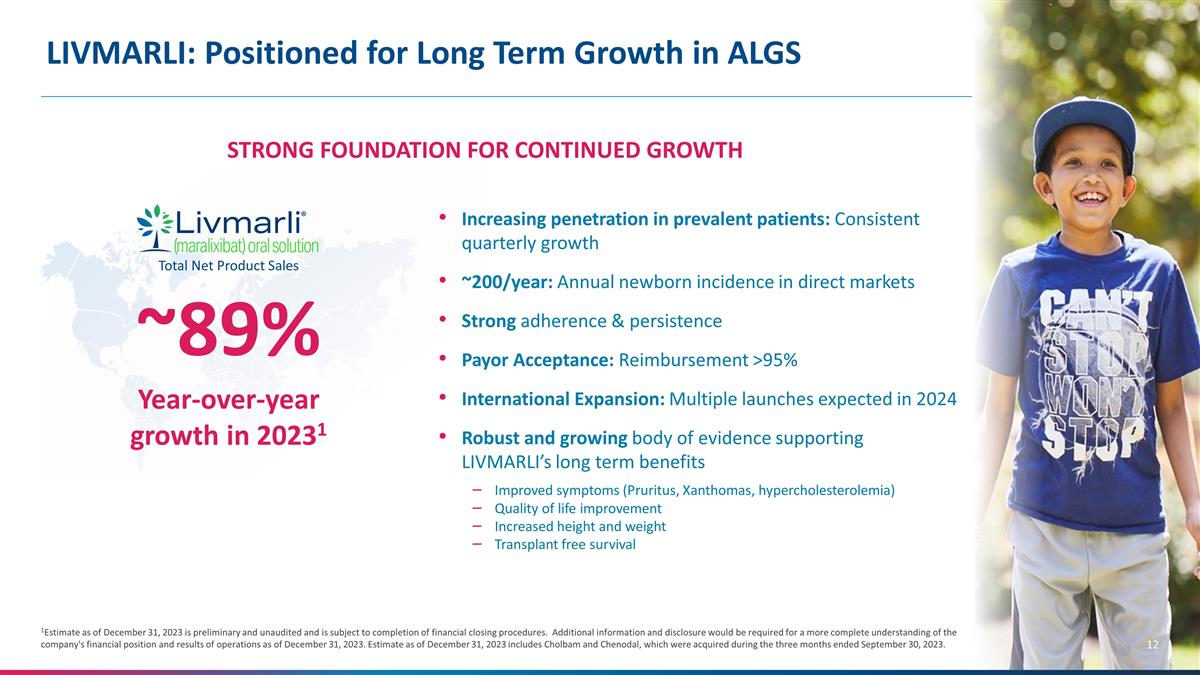
LIVMARLI: Positioned for Long Term
Growth in ALGS 1Estimate as of December 31, 2023 is preliminary and unaudited and is subject to completion of financial closing procedures. Additional information and disclosure would be required for a more complete understanding of the company's
financial position and results of operations as of December 31, 2023. Estimate as of December 31, 2023 includes Cholbam and Chenodal, which were acquired during the three months ended September 30, 2023. Increasing penetration in prevalent patients:
Consistent quarterly growth ~200/year: Annual newborn incidence in direct markets Strong adherence & persistence Payor Acceptance: Reimbursement >95% International Expansion: Multiple launches expected in 2024 Robust and growing body of
evidence supporting LIVMARLI’s long term benefits Improved symptoms (Pruritus, Xanthomas, hypercholesterolemia) Quality of life improvement Increased height and weight Transplant free survival STRONG FOUNDATION FOR CONTINUED GROWTH Total Net
Product Sales ~89% Year-over-year growth in 20231

LIVMARLI Well-Characterized Safety
Profile Safety Data of LIVMARLI in ALGS Includes 5 Years of Follow-up from 3 Randomized Studies Most common adverse events were diarrhea, abdominal pain (41.6 and 38.6 events per 100 person-years respectively) GI adverse reactions were generally
mild or moderate severity and self-limiting 6% of patients experienced dose reductions or interruptions due to diarrhea, abdominal pain, or vomiting

CHENODAL & CHOLBAM Bile Acid
Portfolio
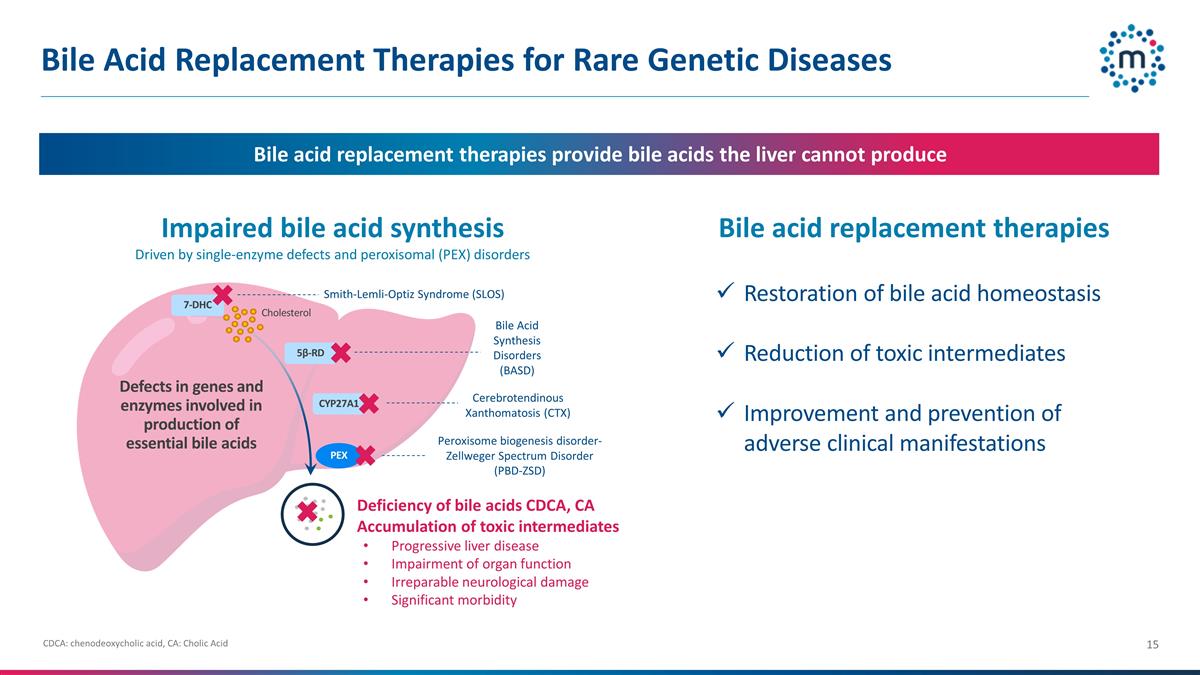
Bile Acid Replacement Therapies for
Rare Genetic Diseases Impaired bile acid synthesis Driven by single-enzyme defects and peroxisomal (PEX) disorders Bile acid replacement therapies provide bile acids the liver cannot produce Peroxisome biogenesis disorder-Zellweger Spectrum Disorder
(PBD-ZSD) Smith-Lemli-Optiz Syndrome (SLOS) Deficiency of bile acids CDCA, CA Accumulation of toxic intermediates Progressive liver disease Impairment of organ function Irreparable neurological damage Significant morbidity 7-DHC 5β-RD CYP27A1
Cholesterol Defects in genes and enzymes involved in production of essential bile acids PEX Bile acid replacement therapies Restoration of bile acid homeostasis Reduction of toxic intermediates Improvement and prevention of adverse clinical
manifestations Bile Acid Synthesis Disorders (BASD) Cerebrotendinous Xanthomatosis (CTX) CDCA: chenodeoxycholic acid, CA: Cholic Acid
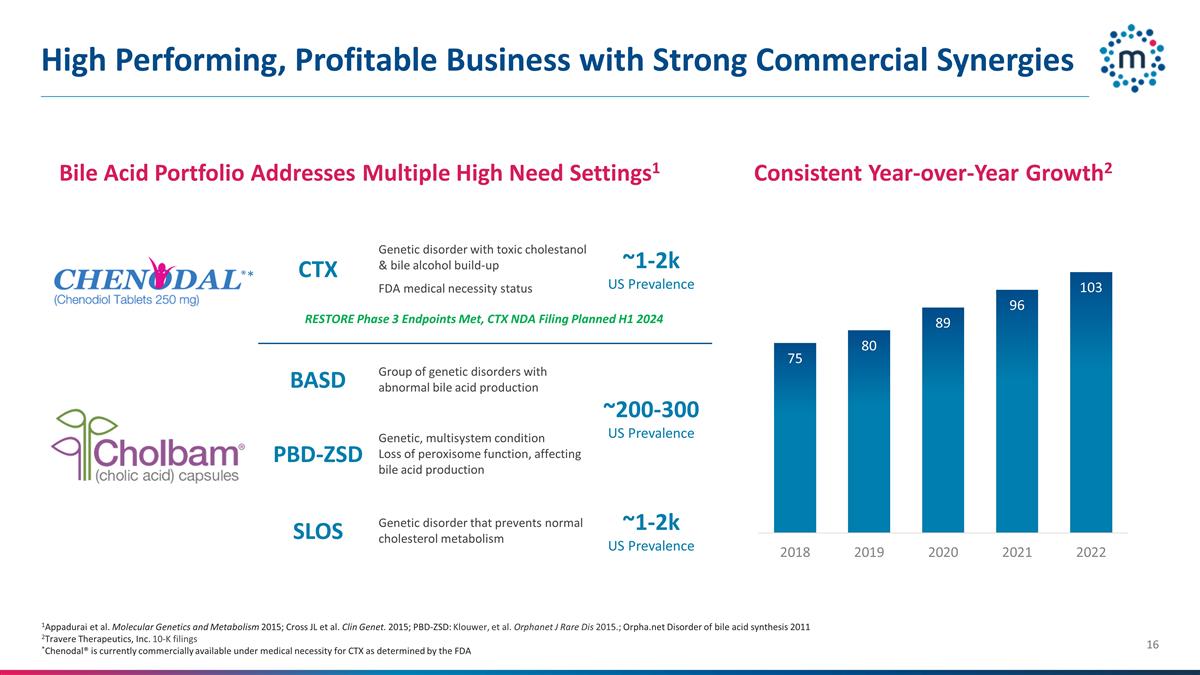
High Performing, Profitable
Business with Strong Commercial Synergies CTX Genetic disorder with toxic cholestanol & bile alcohol build-up FDA medical necessity status ~1-2k US Prevalence BASD Group of genetic disorders with abnormal bile acid production ~200-300 US
Prevalence PBD-ZSD Genetic, multisystem condition Loss of peroxisome function, affecting bile acid production ~1k SLOS Genetic disorder that prevents normal cholesterol metabolism ~1-2k US Prevalence Bile Acid Portfolio Addresses Multiple High Need
Settings1 1Appadurai et al. Molecular Genetics and Metabolism 2015; Cross JL et al. Clin Genet. 2015; PBD-ZSD: Klouwer, et al. Orphanet J Rare Dis 2015.; Orpha.net Disorder of bile acid synthesis 2011 2Travere Therapeutics, Inc. 10-K
filings *Chenodal® is currently commercially available under medical necessity for CTX as determined by the FDA Consistent Year-over-Year Growth2 RESTORE Phase 3 Endpoints Met, CTX NDA Filing Planned H1 2024 *

Significant expansion opportunities
within pipeline indications Pipeline

Phase 3 MARCH-PFIC Study: Highly
statistically significant effects in All-PFIC LIVMARLI (maralixibat) in PFIC
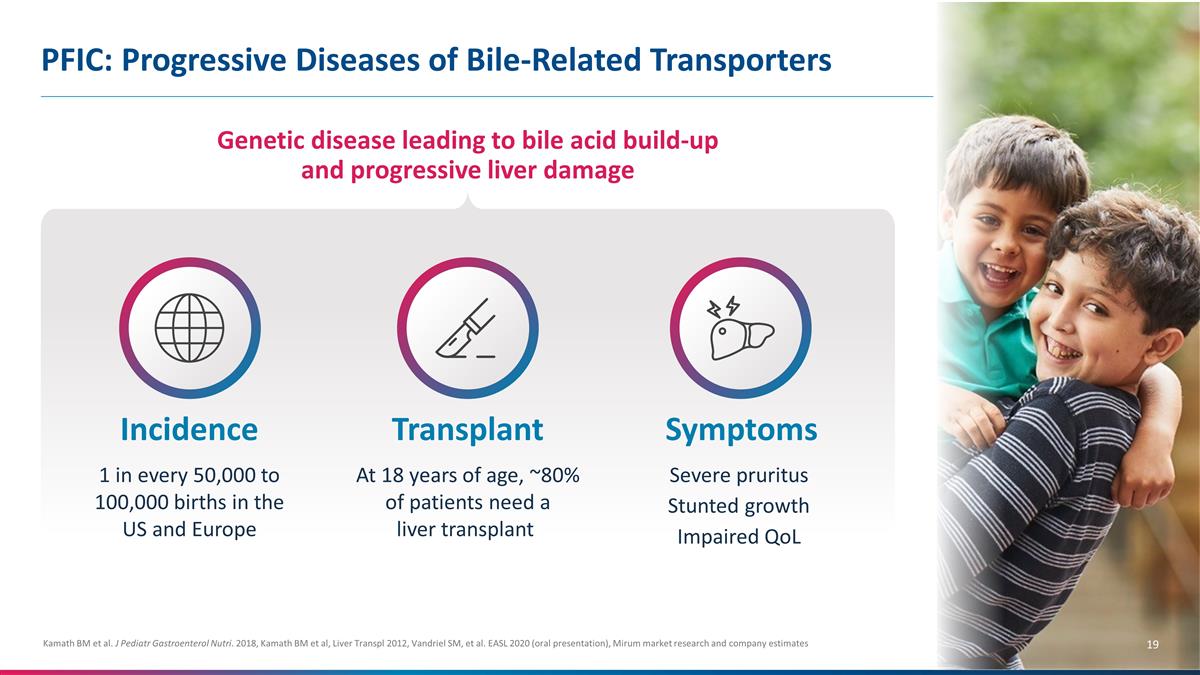
PFIC: Progressive Diseases of
Bile-Related Transporters Kamath BM et al. J Pediatr Gastroenterol Nutri. 2018, Kamath BM et al, Liver Transpl 2012, Vandriel SM, et al. EASL 2020 (oral presentation), Mirum market research and company estimates Genetic disease leading to bile
acid build-up and progressive liver damage Incidence 1 in every 50,000 to 100,000 births in the US and Europe Transplant At 18 years of age, ~80% of patients need a liver transplant Symptoms Severe pruritus Stunted growth Impaired
QoL
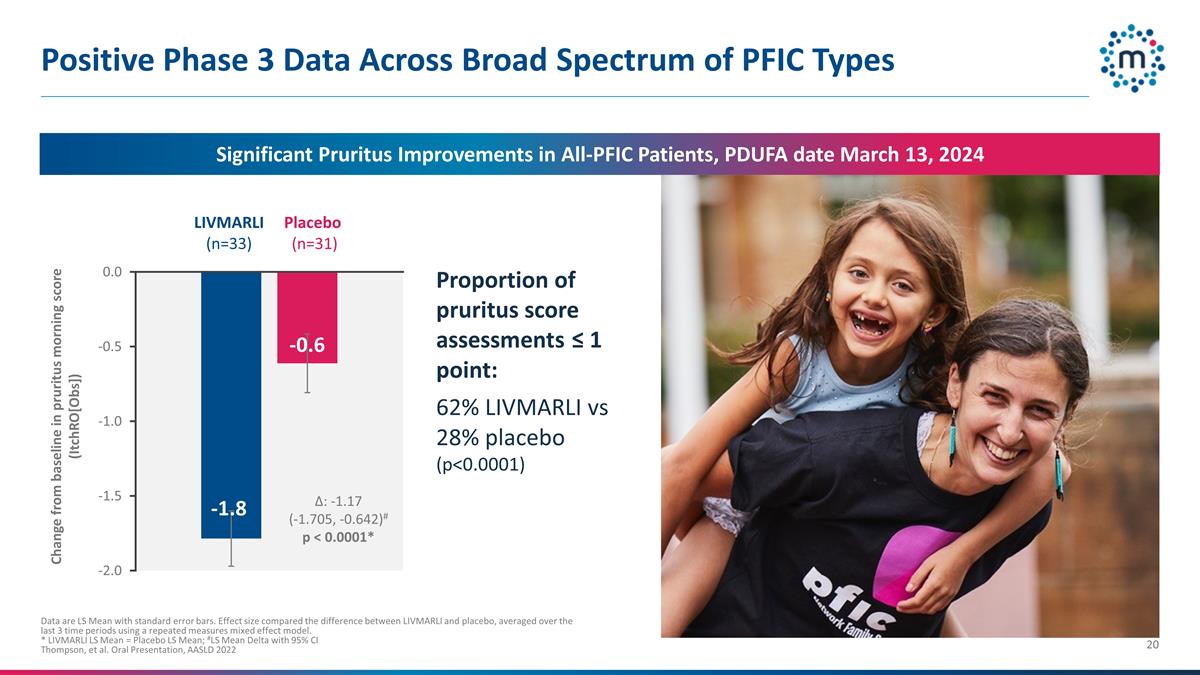
Positive Phase 3 Data Across Broad
Spectrum of PFIC Types Data are LS Mean with standard error bars. Effect size compared the difference between LIVMARLI and placebo, averaged over the last 3 time periods using a repeated measures mixed effect model. * LIVMARLI LS Mean = Placebo LS
Mean; #LS Mean Delta with 95% CI Thompson, et al. Oral Presentation, AASLD 2022 LIVMARLI (n=33) Placebo (n=31) Change from baseline in pruritus morning score (ItchRO[Obs]) Δ: -1.17 (-1.705, -0.642)# p < 0.0001* Proportion of pruritus score
assessments ≤ 1 point: 62% LIVMARLI vs 28% placebo (p<0.0001) Significant Pruritus Improvements in All-PFIC Patients, PDUFA date March 13, 2024
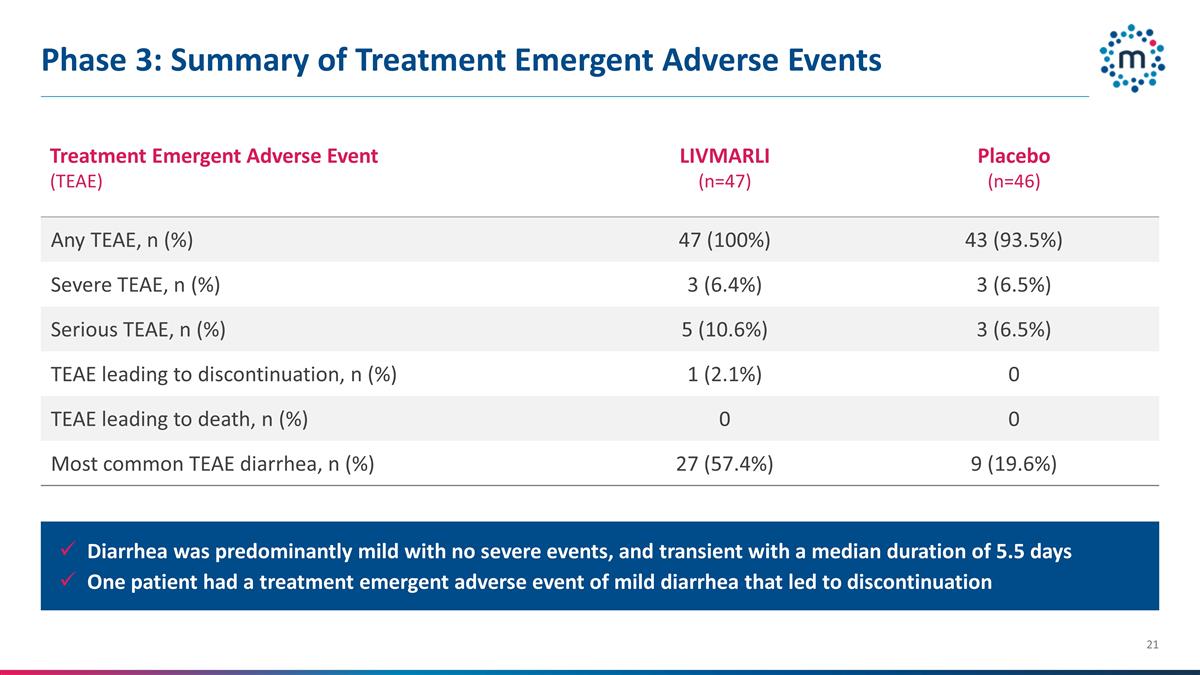
Phase 3: Summary of Treatment
Emergent Adverse Events Treatment Emergent Adverse Event (TEAE) LIVMARLI (n=47) Placebo (n=46) Any TEAE, n (%) 47 (100%) 43 (93.5%) Severe TEAE, n (%) 3 (6.4%) 3 (6.5%) Serious TEAE, n (%) 5 (10.6%) 3 (6.5%) TEAE leading to discontinuation, n (%) 1
(2.1%) 0 TEAE leading to death, n (%) 0 0 Most common TEAE diarrhea, n (%) 27 (57.4%) 9 (19.6%) Diarrhea was predominantly mild with no severe events, and transient with a median duration of 5.5 days One patient had a treatment emergent adverse
event of mild diarrhea that led to discontinuation

IBAT Inhibitor for Cholestasis in
Adults Volixibat
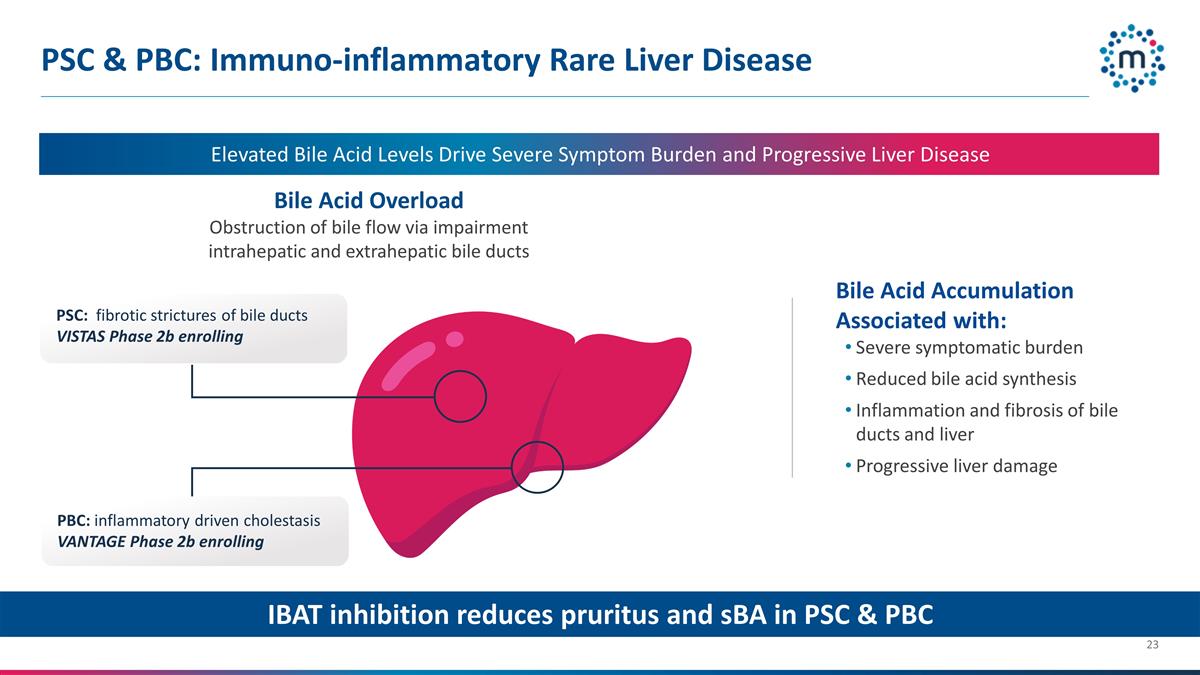
PSC & PBC: Immuno-inflammatory
Rare Liver Disease Bile Acid Overload Obstruction of bile flow via impairment intrahepatic and extrahepatic bile ducts Elevated Bile Acid Levels Drive Severe Symptom Burden and Progressive Liver Disease Bile Acid Accumulation Associated with: Severe
symptomatic burden Reduced bile acid synthesis Inflammation and fibrosis of bile ducts and liver Progressive liver damage PSC: fibrotic strictures of bile ducts VISTAS Phase 2b enrolling PBC: inflammatory driven cholestasis VANTAGE Phase 2b
enrolling IBAT inhibition reduces pruritus and sBA in PSC & PBC

Primary Sclerosing
Cholangitis
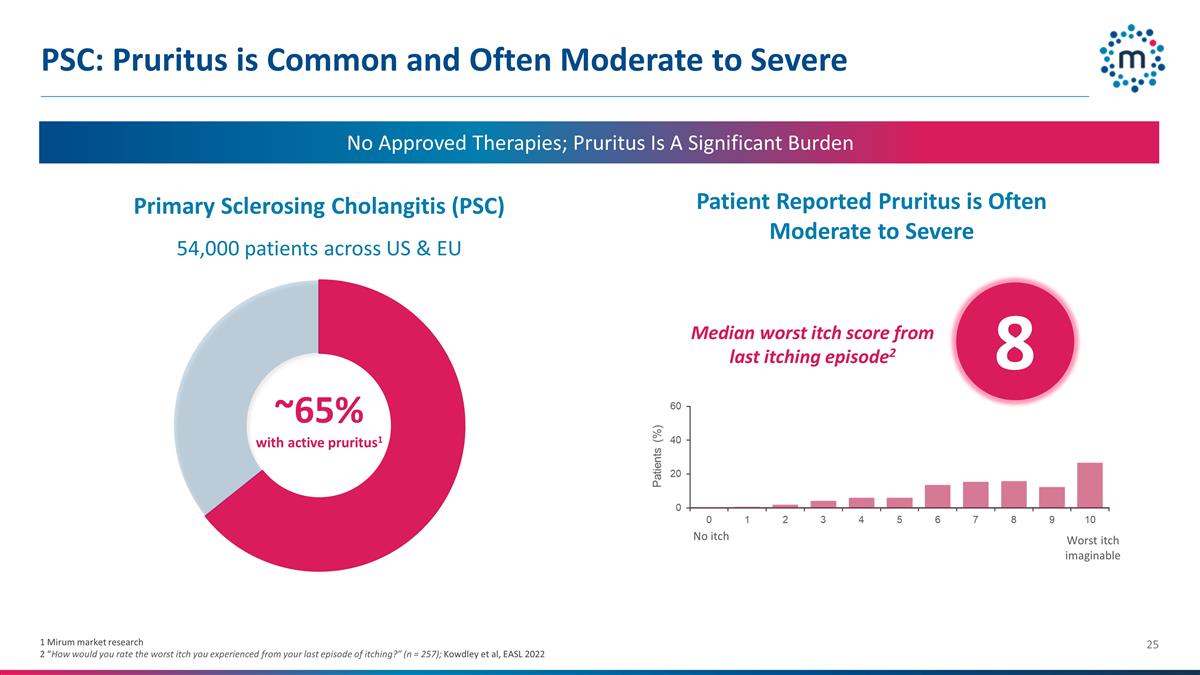
PSC: Pruritus is Common and Often
Moderate to Severe 54,000 patients across US & EU ~65% with active pruritus1 1 Mirum market research 2 “How would you rate the worst itch you experienced from your last episode of itching?” (n = 257); Kowdley et al, EASL 2022 No
Approved Therapies; Pruritus Is A Significant Burden Primary Sclerosing Cholangitis (PSC) Patient Reported Pruritus is Often Moderate to Severe No itch Worst itch imaginable 8 Median worst itch score from last itching episode2
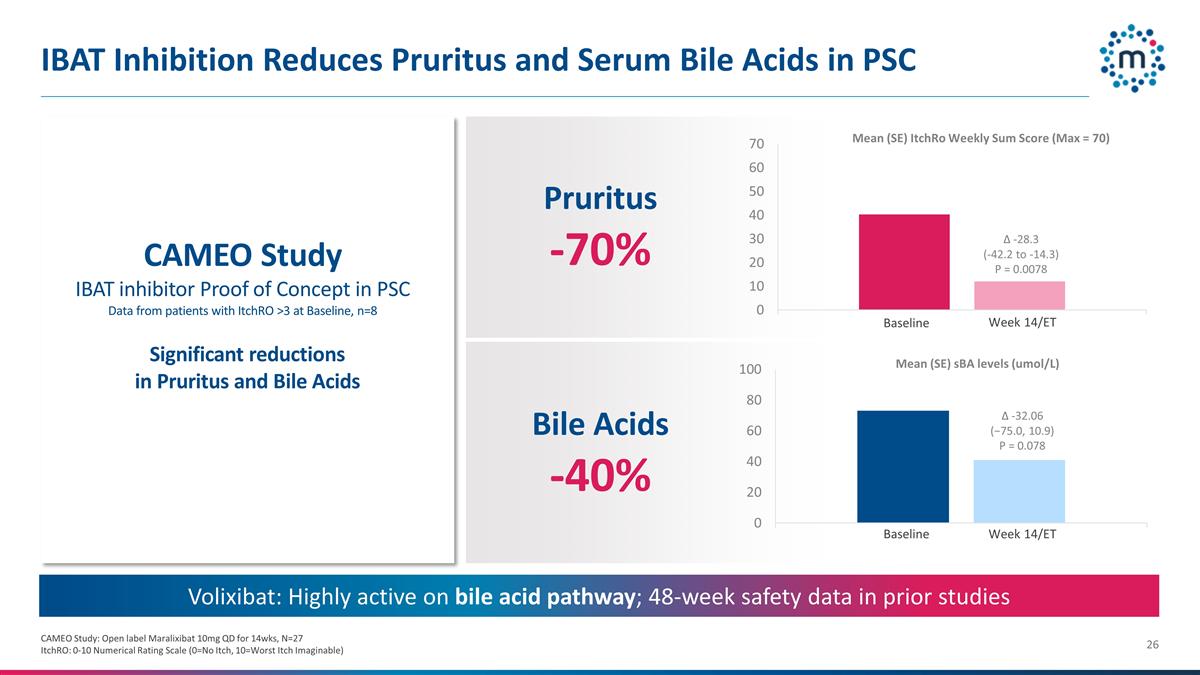
IBAT Inhibition Reduces Pruritus
and Serum Bile Acids in PSC CAMEO Study: Open label Maralixibat 10mg QD for 14wks, N=27 ItchRO: 0-10 Numerical Rating Scale (0=No Itch, 10=Worst Itch Imaginable) Volixibat: Highly active on bile acid pathway; 48-week safety data in prior studies
CAMEO Study IBAT inhibitor Proof of Concept in PSC Data from patients with ItchRO >3 at Baseline, n=8 Baseline Week 14/ET Pruritus -70% Mean (SE) ItchRo Weekly Sum Score (Max = 70) Bile Acids -40% Mean (SE) sBA levels (umol/L) Baseline Week 14/ET
Δ -28.3 (-42.2 to -14.3) P = 0.0078 Δ -32.06 (−75.0, 10.9) P = 0.078 Significant reductions in Pruritus and Bile Acids
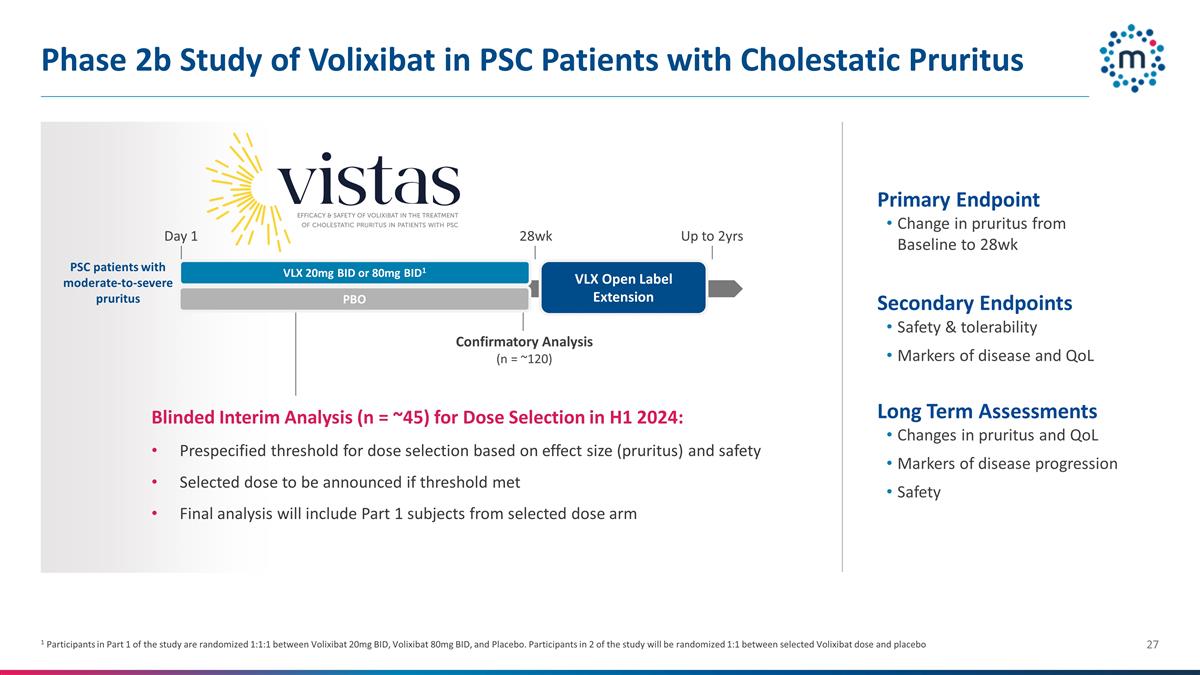
Phase 2b Study of Volixibat in PSC
Patients with Cholestatic Pruritus Primary Endpoint Change in pruritus from Baseline to 28wk Secondary Endpoints Safety & tolerability Markers of disease and QoL Long Term Assessments Changes in pruritus and QoL Markers of disease progression
Safety Up to 2yrs Blinded Interim Analysis (n = ~45) for Dose Selection in H1 2024: Prespecified threshold for dose selection based on effect size (pruritus) and safety Selected dose to be announced if threshold met Final analysis will include Part
1 subjects from selected dose arm Confirmatory Analysis (n = ~120) VLX 20mg BID or 80mg BID1 VLX Open Label Extension 28wk Day 1 1 Participants in Part 1 of the study are randomized 1:1:1 between Volixibat 20mg BID, Volixibat 80mg BID, and Placebo.
Participants in 2 of the study will be randomized 1:1 between selected Volixibat dose and placebo PBO PSC patients with moderate-to-severe pruritus

Primary Biliary
Cholangitis
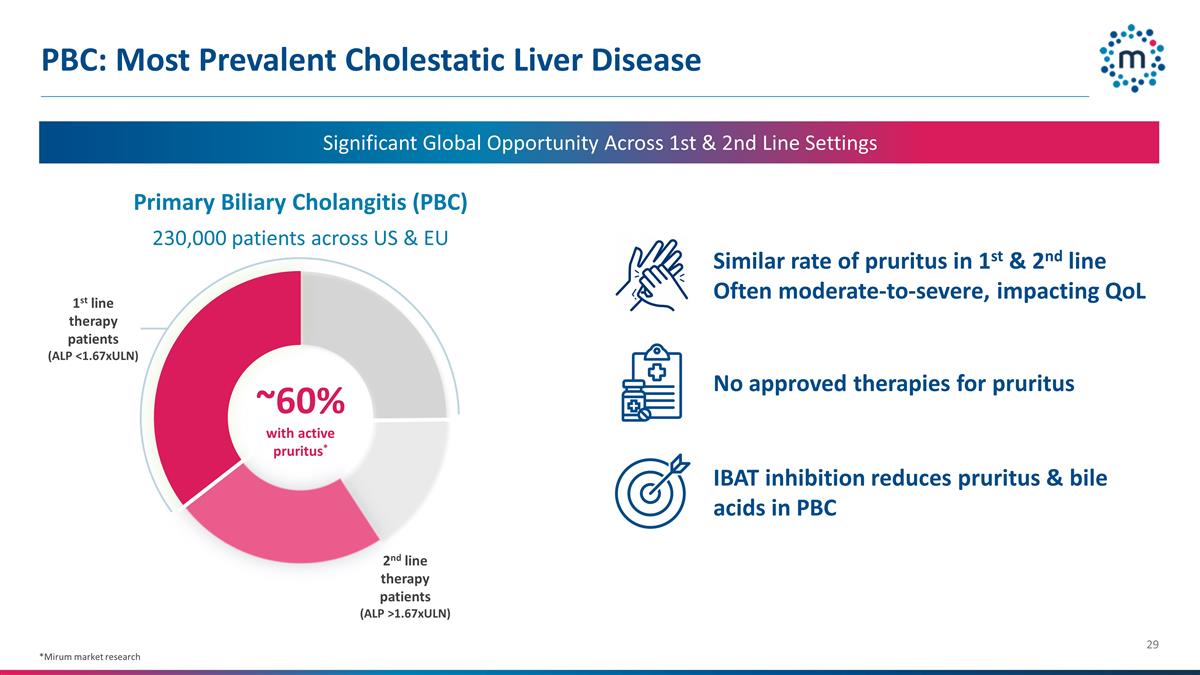
PBC: Most Prevalent Cholestatic
Liver Disease 230,000 patients across US & EU *Mirum market research Significant Global Opportunity Across 1st & 2nd Line Settings 1st line therapy patients (ALP <1.67xULN) 2nd line therapy patients (ALP >1.67xULN) ~60% with active
pruritus* Primary Biliary Cholangitis (PBC) Similar rate of pruritus in 1st & 2nd line Often moderate-to-severe, impacting QoL No approved therapies for pruritus IBAT inhibition reduces pruritus & bile acids in PBC
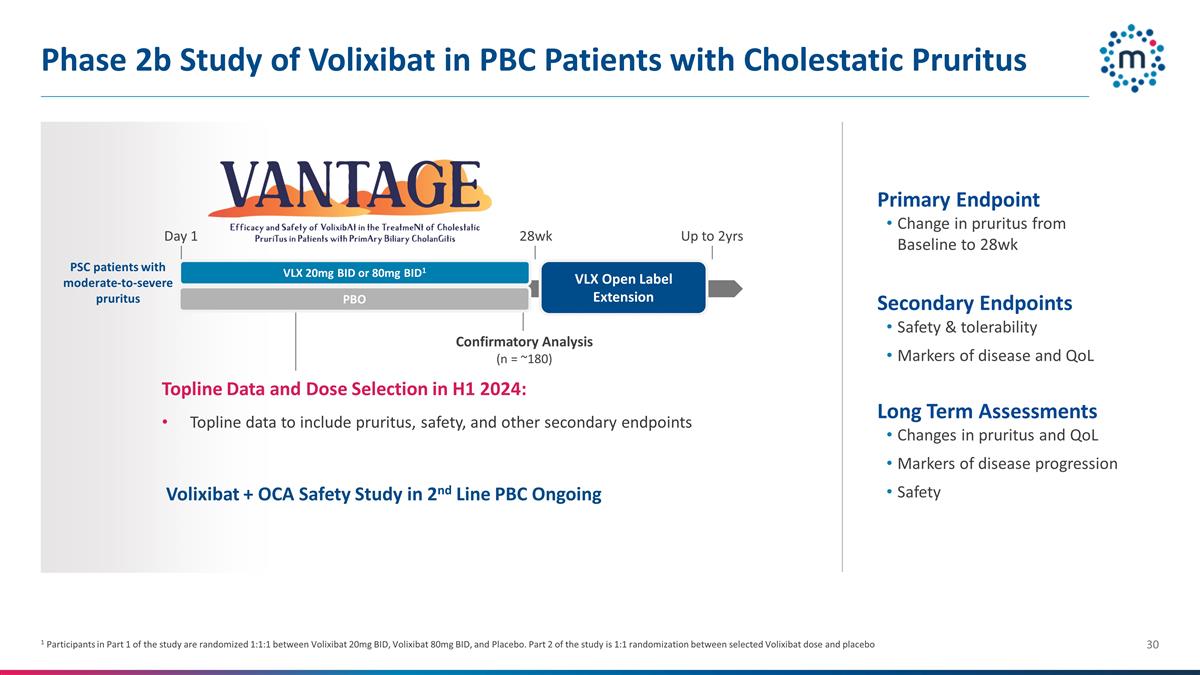
Phase 2b Study of Volixibat in PBC
Patients with Cholestatic Pruritus Primary Endpoint Change in pruritus from Baseline to 28wk Secondary Endpoints Safety & tolerability Markers of disease and QoL Long Term Assessments Changes in pruritus and QoL Markers of disease progression
Safety Up to 2yrs Topline Data and Dose Selection in H1 2024: Topline data to include pruritus, safety, and other secondary endpoints Confirmatory Analysis (n = ~180) VLX Open Label Extension 28wk Day 1 1 Participants in Part 1 of the study are
randomized 1:1:1 between Volixibat 20mg BID, Volixibat 80mg BID, and Placebo. Part 2 of the study is 1:1 randomization between selected Volixibat dose and placebo Volixibat + OCA Safety Study in 2nd Line PBC Ongoing VLX 20mg BID or 80mg BID1 PBO PSC
patients with moderate-to-severe pruritus

Dedicated to transforming the
treatment of rare diseases Mirum
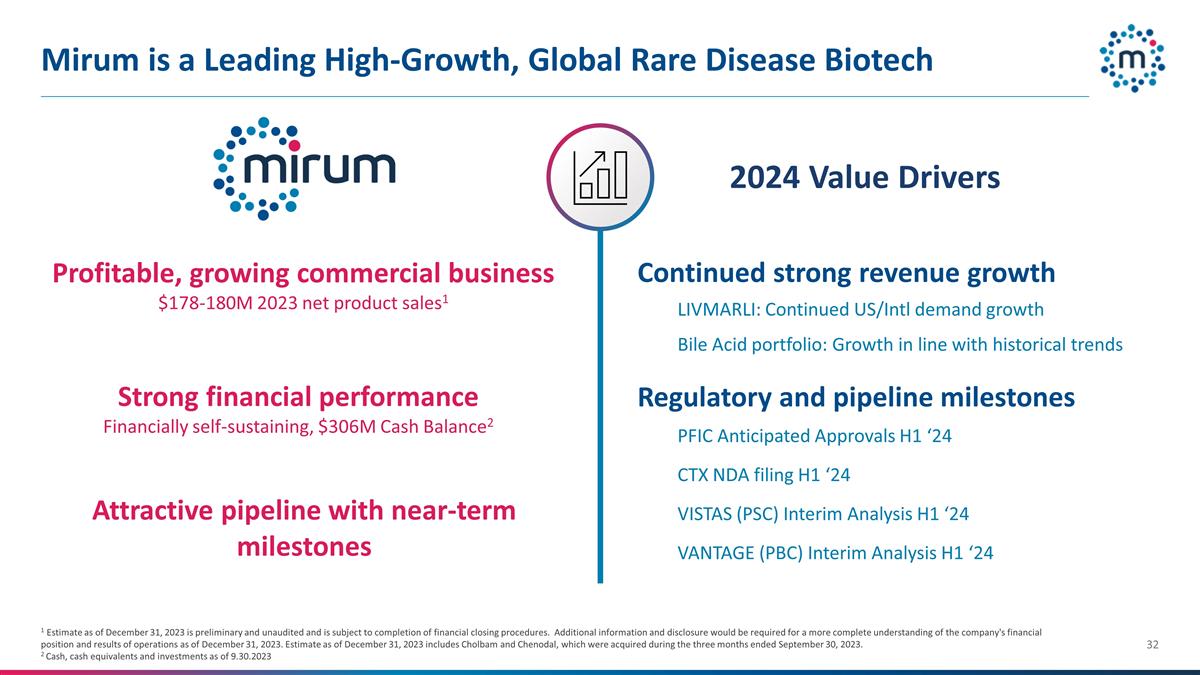
Mirum is a Leading High-Growth,
Global Rare Disease Biotech 1 Estimate as of December 31, 2023 is preliminary and unaudited and is subject to completion of financial closing procedures. Additional information and disclosure would be required for a more complete understanding of
the company's financial position and results of operations as of December 31, 2023. Estimate as of December 31, 2023 includes Cholbam and Chenodal, which were acquired during the three months ended September 30, 2023. 2 Cash, cash equivalents and
investments as of 9.30.2023 Profitable, growing commercial business $178-180M 2023 net product sales1 Strong financial performance Financially self-sustaining, $306M Cash Balance2 Attractive pipeline with near-term milestones Continued strong
revenue growth Bile Acid portfolio: Growth in line with historical trends LIVMARLI: Continued US/Intl demand growth PFIC Anticipated Approvals H1 ‘24 CTX NDA filing H1 ‘24 VISTAS (PSC) Interim Analysis H1 ‘24 VANTAGE (PBC) Interim
Analysis H1 ‘24 2024 Value Drivers Regulatory and pipeline milestones

Thank You

Supplemental Materials

LIVMARLI (maralixibat) oral
solution Important Safety Information IMPORTANT SAFETY INFORMATION LIVMARLI can cause serious side effects, including: Changes in liver tests: Changes in certain liver tests are common in patients with Alagille syndrome but may worsen during
treatment with LIVMARLI. These changes may be a sign of liver injury and can be serious. Your health care provider should do blood tests before starting and during treatment to check your liver function. Stomach and intestinal
(gastrointestinal) problems: LIVMARLI can cause stomach and intestinal problems, including diarrhea, stomach pain, and vomiting during treatment. Fat Soluble Vitamin Deficiency: This vitamin deficiency is common in patients with Alagille syndrome
but may worsen during treatment.
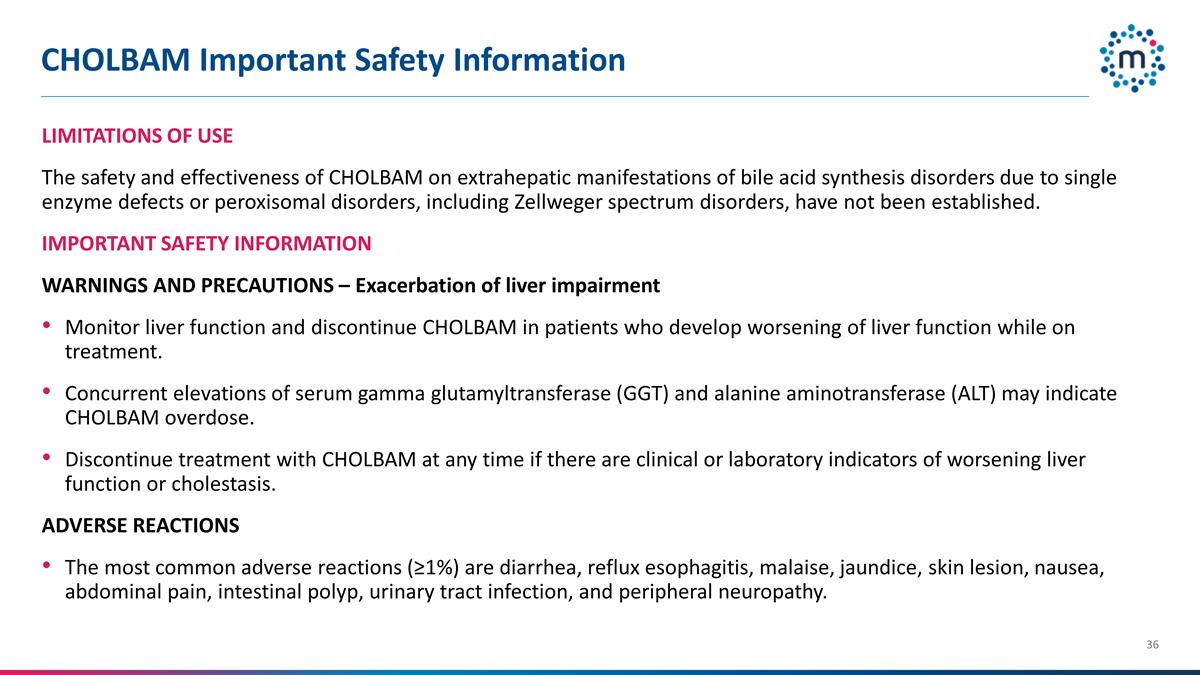
CHOLBAM Important Safety
Information LIMITATIONS OF USE The safety and effectiveness of CHOLBAM on extrahepatic manifestations of bile acid synthesis disorders due to single enzyme defects or peroxisomal disorders, including Zellweger spectrum disorders, have not been
established. IMPORTANT SAFETY INFORMATION WARNINGS AND PRECAUTIONS – Exacerbation of liver impairment Monitor liver function and discontinue CHOLBAM in patients who develop worsening of liver function
while on treatment. Concurrent elevations of serum gamma glutamyltransferase (GGT) and alanine aminotransferase (ALT) may indicate CHOLBAM overdose. Discontinue treatment with CHOLBAM at any time if there are clinical or
laboratory indicators of worsening liver function or cholestasis. ADVERSE REACTIONS The most common adverse reactions (≥1%) are diarrhea, reflux esophagitis, malaise, jaundice, skin lesion, nausea, abdominal pain,
intestinal polyp, urinary tract infection, and peripheral neuropathy.
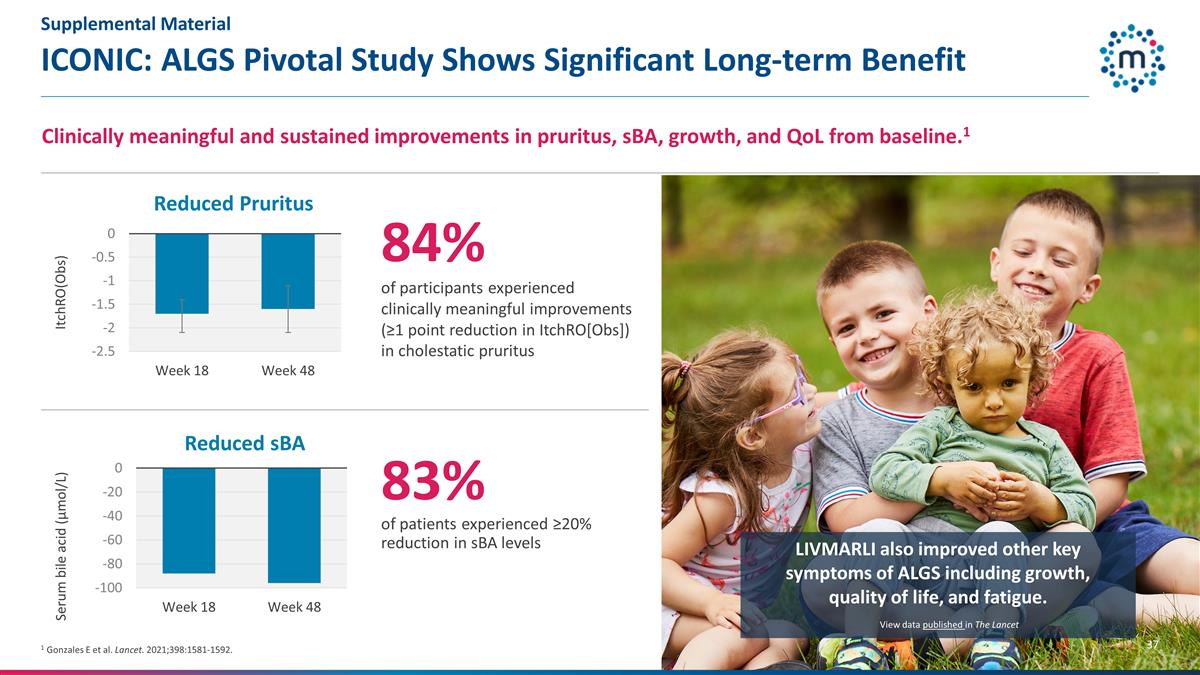
ICONIC: ALGS Pivotal Study Shows
Significant Long-term Benefit 1 Gonzales E et al. Lancet. 2021;398:1581-1592. 84% of participants experienced clinically meaningful improvements (≥1 point reduction in ItchRO[Obs]) in cholestatic pruritus LIVMARLI also improved other key
symptoms of ALGS including growth, quality of life, and fatigue. Clinically meaningful and sustained improvements in pruritus, sBA, growth, and QoL from baseline.1 83% of patients experienced ≥20% reduction in sBA levels View data published in
The Lancet Supplemental Material
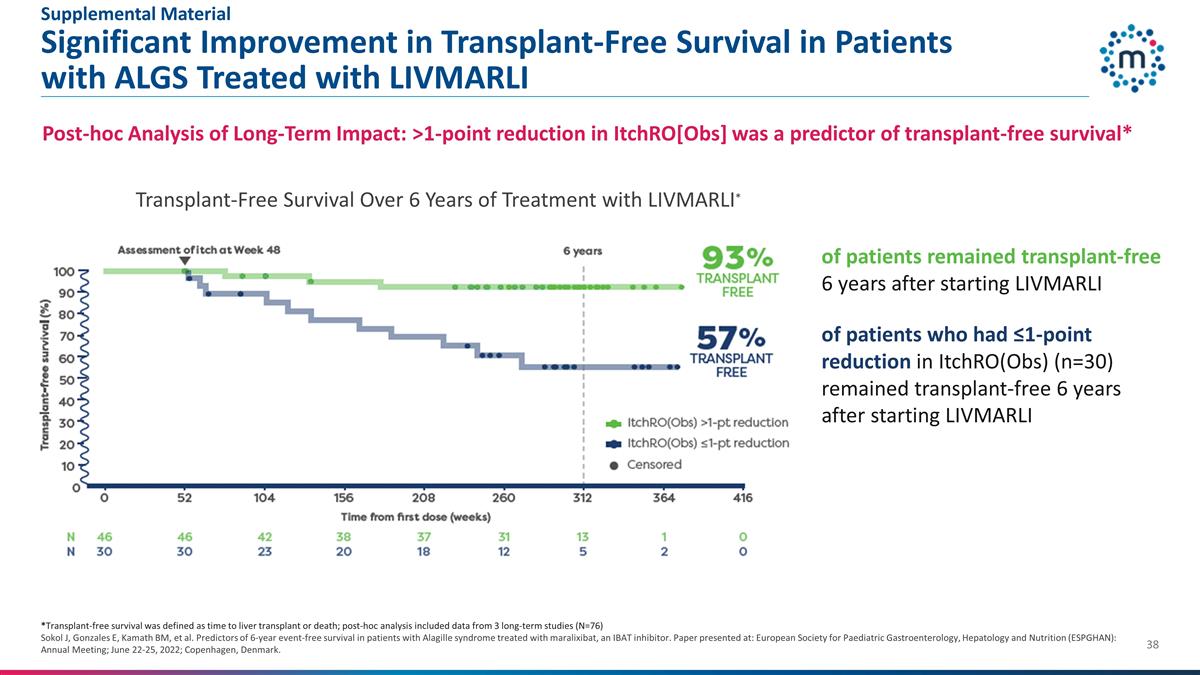
Significant Improvement in
Transplant-Free Survival in Patients with ALGS Treated with LIVMARLI *Transplant-free survival was defined as time to liver transplant or death; post-hoc analysis included data from 3 long-term studies (N=76) Sokol J, Gonzales E, Kamath BM, et al.
Predictors of 6-year event-free survival in patients with Alagille syndrome treated with maralixibat, an IBAT inhibitor. Paper presented at: European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN): Annual Meeting; June
22-25, 2022; Copenhagen, Denmark. Transplant-Free Survival Over 6 Years of Treatment with LIVMARLI* Post-hoc Analysis of Long-Term Impact: >1-point reduction in ItchRO[Obs] was a predictor of transplant-free survival* of patients remained
transplant-free 6 years after starting LIVMARLI of patients who had ≤1-point reduction in ItchRO(Obs) (n=30) remained transplant-free 6 years after starting LIVMARLI Supplemental Material
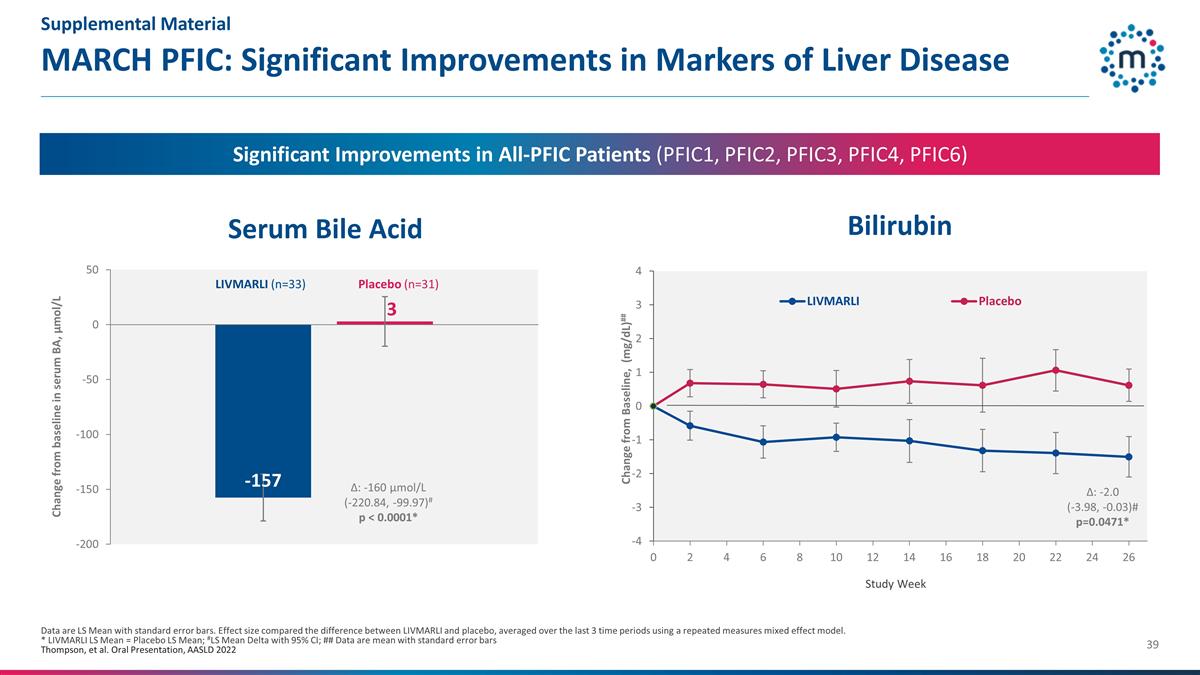
MARCH PFIC: Significant
Improvements in Markers of Liver Disease Data are LS Mean with standard error bars. Effect size compared the difference between LIVMARLI and placebo, averaged over the last 3 time periods using a repeated measures mixed effect model. * LIVMARLI LS
Mean = Placebo LS Mean; #LS Mean Delta with 95% CI; ## Data are mean with standard error bars Thompson, et al. Oral Presentation, AASLD 2022 3 Change from baseline in serum BA, µmol/L LIVMARLI (n=33) Placebo (n=31) Change from Baseline,
(mg/dL)## Δ: -160 µmol/L (-220.84, -99.97)# p < 0.0001* Bilirubin Serum Bile Acid Significant Improvements in All-PFIC Patients (PFIC1, PFIC2, PFIC3, PFIC4, PFIC6) Δ: -2.0 (-3.98, -0.03)# p=0.0471* Supplemental Material
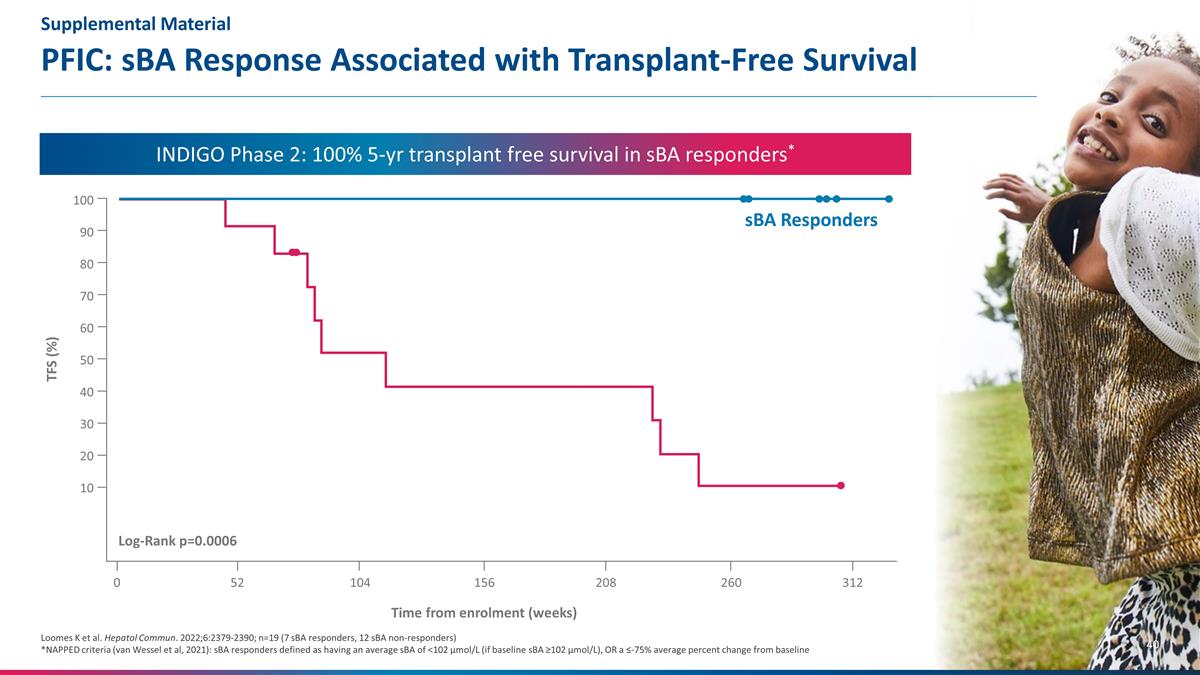
PFIC: sBA Response Associated with
Transplant-Free Survival Loomes K et al. Hepatol Commun. 2022;6:2379-2390; n=19 (7 sBA responders, 12 sBA non-responders) *NAPPED criteria (van Wessel et al, 2021): sBA responders defined as having an average sBA of <102 μmol/L (if baseline
sBA ≥102 μmol/L), OR a ≤-75% average percent change from baseline Time from enrolment (weeks) TFS (%) 100 60 40 20 80 10 30 50 70 90 0 52 208 260 312 104 156 Log-Rank p=0.0006 sBA Responders INDIGO Phase 2: 100% 5-yr transplant free
survival in sBA responders* Supplemental Material
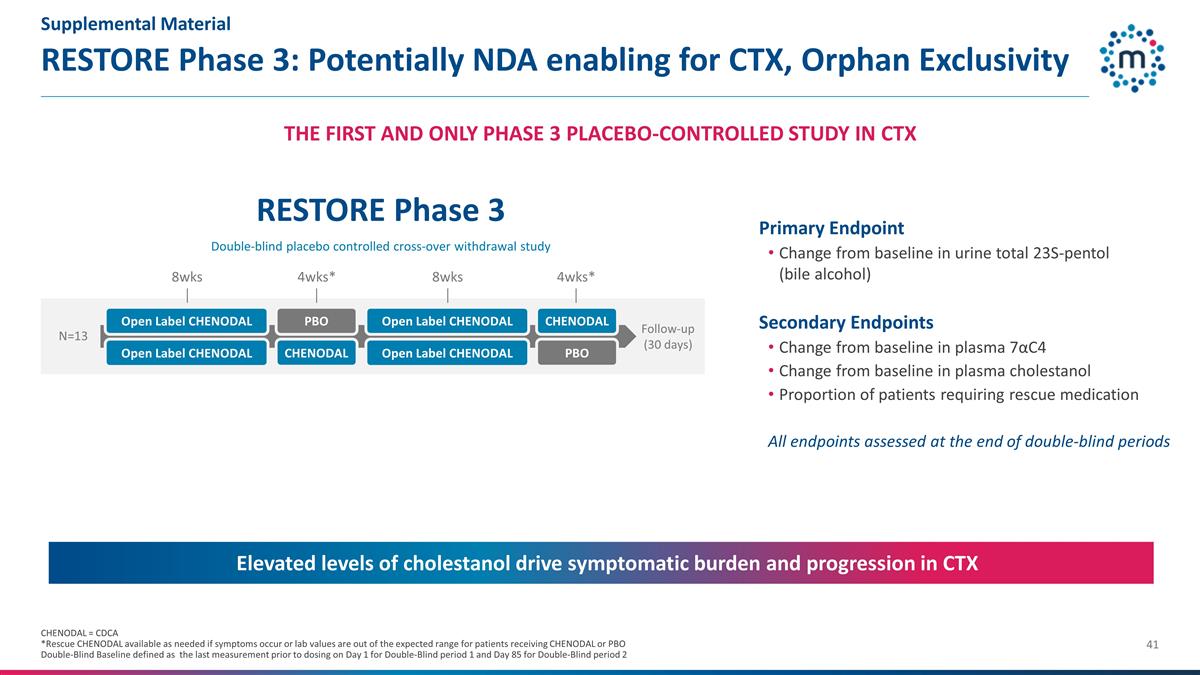
RESTORE Phase 3: Potentially NDA
enabling for CTX, Orphan Exclusivity RESTORE Phase 3 Double-blind placebo controlled cross-over withdrawal study Primary Endpoint Change from baseline in urine total 23S-pentol (bile alcohol) Secondary Endpoints Change from baseline in plasma
7αC4 Change from baseline in plasma cholestanol Proportion of patients requiring rescue medication All endpoints assessed at the end of double-blind periods Follow-up (30 days) 8wks 4wks* PBO 4wks* Open Label CHENODAL 8wks N=13
CHENODAL CHENODAL PBO Open Label CHENODAL Open Label CHENODAL Open Label CHENODAL Elevated levels of cholestanol drive symptomatic burden and progression in CTX CHENODAL = CDCA *Rescue CHENODAL available as needed if symptoms occur or lab values are
out of the expected range for patients receiving CHENODAL or PBO Double-Blind Baseline defined as the last measurement prior to dosing on Day 1 for Double-Blind period 1 and Day 85 for Double-Blind period 2 THE FIRST AND ONLY PHASE 3
PLACEBO-CONTROLLED STUDY IN CTX Supplemental Material
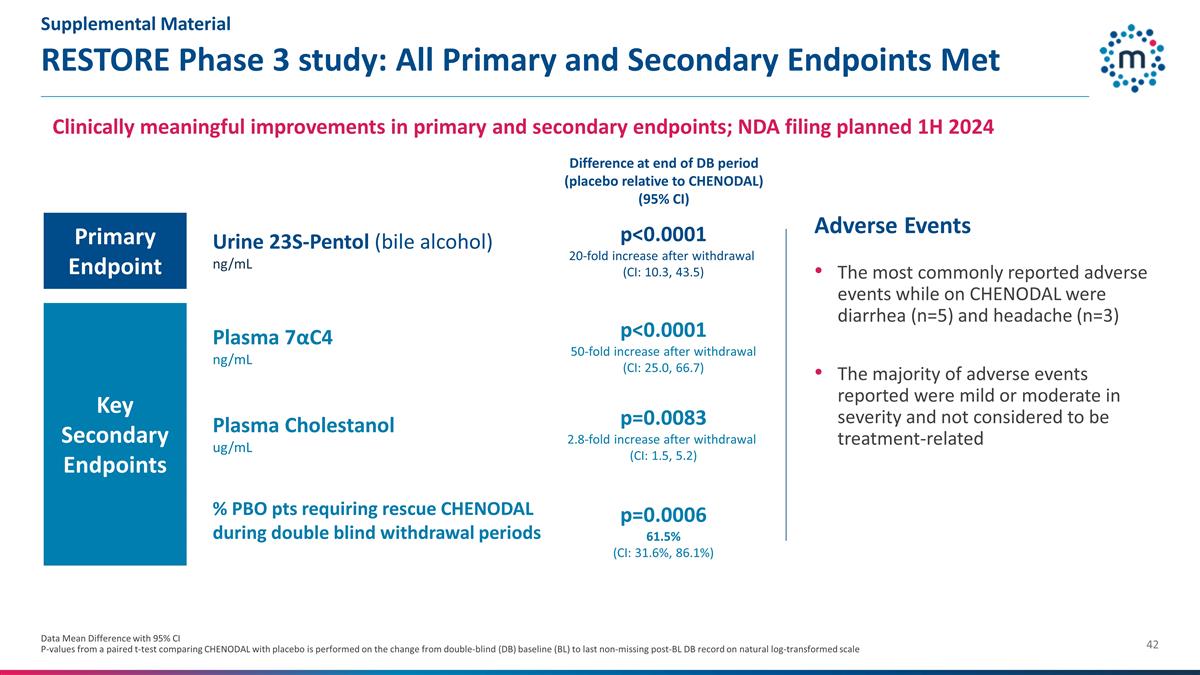
RESTORE Phase 3 study: All Primary
and Secondary Endpoints Met Data Mean Difference with 95% CI P-values from a paired t-test comparing CHENODAL with placebo is performed on the change from double-blind (DB) baseline (BL) to last non-missing post-BL DB record on natural
log-transformed scale Adverse Events The most commonly reported adverse events while on CHENODAL were diarrhea (n=5) and headache (n=3) The majority of adverse events reported were mild or moderate in severity and not considered to be
treatment-related Urine 23S-Pentol (bile alcohol) ng/mL p<0.0001 20-fold increase after withdrawal (CI: 10.3, 43.5) Plasma 7αC4 ng/mL p<0.0001 50-fold increase after withdrawal (CI: 25.0, 66.7) Plasma Cholestanol ug/mL
p=0.0083 2.8-fold increase after withdrawal (CI: 1.5, 5.2) % PBO pts requiring rescue CHENODAL during double blind withdrawal periods p=0.0006 61.5% (CI: 31.6%, 86.1%) Clinically meaningful improvements in primary and secondary
endpoints; NDA filing planned 1H 2024 Difference at end of DB period (placebo relative to CHENODAL) (95% CI) Primary Endpoint Key Secondary Endpoints Supplemental Material
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Grafico Azioni Mirum Pharmaceuticals (NASDAQ:MIRM)
Storico
Da Apr 2024 a Mag 2024

Grafico Azioni Mirum Pharmaceuticals (NASDAQ:MIRM)
Storico
Da Mag 2023 a Mag 2024
