FALSE000111646300011164632024-01-042024-01-04
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported): January 4, 2024
OraSure Technologies, Inc.
(Exact Name of Registrant as Specified in Charter)
| | | | | | | | |
| Delaware | 001-16537 | 36-4370966 |
(State or Other Jurisdiction of Incorporation) | (Commission File Number) | (I.R.S. Employer Identification No.) |
| | | | | |
220 East First Street Bethlehem, Pennsylvania | 18015-1360 |
| (Address of Principal Executive Offices) | (Zip Code) |
Registrant’s telephone number, including area code: 610-882-1820
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | |
| Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
| Common Stock, $0.000001 par value per share | | OSUR | | The Nasdaq Stock Market LLC |
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the Registrant under any of the following provisions:
o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Indicate by a check mark whether the Registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company o
If an emerging growth company, indicate by check mark if the Registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
ITEM 7.01 Regulation FD Disclosure.
OraSure Technologies, Inc. (the “Company”) hereby furnishes the Investor Presentation that the Company will present to analysts and investors on or after the date hereof, which is attached as Exhibit 99.1 to this Current Report on Form 8-K (“Current Report”), is incorporated herein by reference and will be available on the Company’s website at www.orasure.com.
The information in this Item and attached Exhibit shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934 or otherwise subject to the liabilities of that section, nor shall such information and Exhibit be deemed incorporated by reference in any filing under the Securities Act of 1933, except as shall be expressly set forth by specific reference in such a filing. The fact that the information and Exhibit are being furnished should not be deemed an admission as to the materiality of any information contained therein. The Company undertakes no duty or obligation to publicly update or revise the information contained in this Current Report or attached Exhibit.
ITEM 8.01 Other Events
On January 4, 2024, the Company issued a press release announcing the Company’s investment in KKR Sapphiros, L.P. (“Sapphiros”) and the Company’s entry into a wide-ranging distribution relationship with Sapphiros and certain of its related entities. A copy of the press release is attached as Exhibit 99.2 to this Current Report and is incorporated herein by reference.
Forward Looking Statements
This Current Report contains certain “forward-looking statements” within the meaning of the Federal securities laws, including with respect to products, product development and manufacturing activities, the investment of the Company in, and distribution relationship with Sapphiros and certain of its related entities, revenue growth, cash flow, increasing margins and other matters. Words such as “expects,” “estimates,” “forecasts,” “intends,” “plans,” “projects,” “could,” “may,” “should,” “will” or other similar words and expressions are intended to identify these forward-looking statements. Forward-looking statements are not guarantees of future performance or results. Known and unknown factors that could cause actual performance or results to be materially different from those expressed or implied in these statements include, but are not limited to: Sapphiros’ and its related entities’ ability to seek and obtain regulatory approval for products in development; the Company’s ability to satisfy customer demand; ability to reduce the Company’s spending rate, capitalize on manufacturing efficiencies and drive profitable growth; ability to achieve the anticipated cost savings as a result of the Company’s business restructuring; ability to market and sell products, whether through the Company’s internal, direct sales force or third parties; impact of significant customer concentration in the genomics business; failure of distributors or other customers to meet purchase forecasts, historic purchase levels or minimum purchase requirements for the Company’s products; ability to manufacture or have manufactured products in accordance with applicable specifications, performance standards and quality requirements; ability to obtain, and timing and cost of obtaining, necessary regulatory approvals for new products or new indications or applications for existing products; ability to comply with applicable regulatory requirements; ability to effectively resolve warning letters, audit observations and other findings or comments from the FDA or other regulators; the impact of the novel coronavirus (“COVID-19”) pandemic on the Company’s business, supply chain, labor force, ability to successfully develop new products, validate the expanded use of existing collector products, receive necessary regulatory approvals and authorizations and commercialize such products for COVID-19 testing, and demand for the Company’s COVID-19 testing products; changes in relationships, including disputes or disagreements, with strategic partners such as Sapphiros or other parties and reliance on strategic partners for the performance of critical activities under collaborative arrangements; ability to meet increased demand for the Company’s products; impact of replacing distributors; inventory levels at distributors and other customers; ability of the Company to achieve its financial and strategic objectives and continue to increase its revenues, including the ability to expand international sales and the ability to continue to reduce costs; impact of competitors, competing products and technology changes; reduction or deferral of public funding available to customers; competition from new or better technology or lower cost products; ability to develop, commercialize and market new products; market acceptance of oral fluid or urine testing, collection or other products; market acceptance and uptake of microbiome informatics, microbial genetics technology and related analytics services; changes in market acceptance of products based on product performance or other factors, including changes in testing guidelines, algorithms or other recommendations by the Centers for Disease Control and Prevention or other agencies; ability to fund research and development and other products and operations; ability to obtain and maintain new or existing product distribution channels; reliance on sole supply sources for critical products and components; availability of related products produced by third parties or products required for use of the Company’s products; impact of contracting with the U.S. government; impact of negative economic conditions; ability to maintain sustained profitability; ability to utilize net operating loss carry forwards or other deferred tax assets; volatility of the Company’s stock price; uncertainty relating to patent protection and potential patent infringement claims; uncertainty and costs of litigation relating to patents and other intellectual property; availability of licenses to patents
or other technology; ability to enter into international manufacturing agreements; obstacles to international marketing and manufacturing of products; ability to sell products internationally, including the impact of changes in international funding sources and testing algorithms; adverse movements in foreign currency exchange rates; loss or impairment of sources of capital; ability to attract and retain qualified personnel; exposure to product liability and other types of litigation; changes in international, federal or state laws and regulations; customer consolidations and inventory practices; equipment failures and ability to obtain needed raw materials and components; cybersecurity breaches or other attacks involving the Company’s systems or those of the Company’s third-party contractors and IT service providers; the impact of terrorist attacks, civil unrest, hostilities and war; and general political, business and economic conditions, including inflationary pressures and banking stability. These and other factors that could affect the Company’s results are discussed more fully in the Company’s filings with the Securities and Exchange Commission (the “SEC”), including the Company’s registration statements, Annual Report on Form 10-K for the year ended December 31, 2022, Quarterly Reports on Form 10-Q, and other filings with the SEC. Although forward-looking statements help to provide information about future prospects, readers should keep in mind that forward-looking statements may not be reliable. Readers are cautioned not to place undue reliance on the forward-looking statements. The forward-looking statements are made as of the date of this Current Report and the Company undertakes no duty to update these statements.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| | | | | | | | |
| | |
Exhibit Number | | Description |
|
|
|
| 99.1 | | |
| 99.2 | | |
| 104 |
| Cover Page Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | | | | | | | |
| ORASURE TECHNOLOGIES, INC. |
| |
| Date: January 4, 2024 | By: | /s/ Carrie Eglinton Manner |
| | Carrie Eglinton Manner |
| | President and Chief Executive Officer |

OraSure Technologies, Inc. STRATEGIC PARTNERSHIP & INVESTMENT IN SAPPHIROS JANUARY 4, 2024 Exhibit 99.1

© 2024 OraSure Technologies, Inc. 2 This presentation contains certain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including with respect to products, product development and manufacturing activities, the investment of OraSure Technologies, Inc. (the “OraSure”) in, and distribution relationship with, KKR Sapphiros, L.P. (together with its subsidiaries and related entities “Sapphiros”), revenue growth, cash flow, increasing margins and other matters. Words such as “expects,” “estimates,” “forecasts,” “intends,” “plans,” “projects,” “could,” “may,” “should,” “will” or other similar words and expressions are intended to identify these forward-looking statements. Forward-looking statements are not guarantees of future performance or results. Known and unknown factors that could cause actual performance or results to be materially different from those expressed or implied in these statements include, but are not limited to: Sapphiros’ and its related entities’ ability to seek and obtain regulatory approval for products in development; OraSure’s ability to satisfy customer demand; ability to reduce OraSure’s spending rate, capitalize on manufacturing efficiencies and drive profitable growth; ability to achieve the anticipated cost savings as a result of OraSure’s business restructuring; ability to market and sell products, whether through OraSure’s internal, direct sales force or third parties; impact of significant customer concentration in the genomics business; failure of distributors or other customers to meet purchase forecasts, historic purchase levels or minimum purchase requirements for OraSure’s products; ability to manufacture or have manufactured products in accordance with applicable specifications, performance standards and quality requirements; ability to obtain, and timing and cost of obtaining, necessary regulatory approvals for new products or new indications or applications for existing products; ability to comply with applicable regulatory requirements; ability to effectively resolve warning letters, audit observations and other findings or comments from the FDA or other regulators; the impact of the novel coronavirus (“COVID-19”) pandemic on OraSure’s business, supply chain, labor force, ability to successfully develop new products, validate the expanded use of existing collector products, receive necessary regulatory approvals and authorizations and commercialize such products for COVID-19 testing, and demand for OraSure’s COVID-19 testing products; changes in relationships, including disputes or disagreements, with strategic partners such as Sapphiros or other parties and reliance on strategic partners for the performance of critical activities under collaborative arrangements; ability to meet increased demand for OraSure’s products; impact of replacing distributors; inventory levels at distributors and other customers; ability of OraSure to achieve its financial and strategic objectives and continue to increase its revenues, including the ability to expand international sales and the ability to continue to reduce costs; impact of competitors, competing products and technology changes; reduction or deferral of public funding available to customers; competition from new or better technology or lower cost products; ability to develop, commercialize and market new products; market acceptance of oral fluid or urine testing, collection or other products; market acceptance and uptake of microbiome informatics, microbial genetics technology and related analytics services; changes in market acceptance of products based on product performance or other factors, including changes in testing guidelines, algorithms or other recommendations by the Centers for Disease Control and Prevention or other agencies; ability to fund research and development and other products and operations; ability to obtain and maintain new or existing product distribution channels; reliance on sole supply sources for critical products and components; availability of related products produced by third parties or products required for use of OraSure’s products; impact of contracting with the U.S. government; impact of negative economic conditions; ability to maintain sustained profitability; ability to utilize net operating loss carry forwards or other deferred tax assets; volatility of OraSure’s stock price; uncertainty relating to patent protection and potential patent infringement claims; uncertainty and costs of litigation relating to patents and other intellectual property; availability of licenses to patents or other technology; ability to enter into international manufacturing agreements; obstacles to international marketing and manufacturing of products; ability to sell products internationally, including the impact of changes in international funding sources and testing algorithms; adverse movements in foreign currency exchange rates; loss or impairment of sources of capital; ability to attract and retain qualified personnel; exposure to product liability and other types of litigation; changes in international, federal or state laws and regulations; customer consolidations and inventory practices; equipment failures and ability to obtain needed raw materials and components; cybersecurity breaches or other attacks involving OraSure’s systems or those of OraSure’s third-party contractors and IT service providers; the impact of terrorist attacks, civil unrest, hostilities and war; and general political, business and economic conditions, including inflationary pressures and banking stability. These and other factors that could affect OraSure’s results are discussed more fully in OraSure’s filings with the Securities and Exchange Commission (the “SEC”), including OraSure’s registration statements, Annual Report on Form 10-K for the year ended December 31, 2022, Quarterly Reports on Form 10-Q, and other filings with the SEC. Although forward-looking statements help to provide information about future prospects, readers should keep in mind that forward-looking statements may not be reliable. Readers are cautioned not to place undue reliance on the forward-looking statements. The forward-looking statements are made as of the date of this presentation and OraSure undertakes no duty to update these statements. Forward-Looking Statement

© 2024 OraSure Technologies, Inc. 3 Executive Summary Strategic Alignment • OTI enters strategic relationship with Sapphiros, securing exclusive distribution rights to a select portfolio of key products in development that address opportunities and needs in our current portfolio o Sample management solutions: self-collected, small-volume blood o Diagnostics: visual lateral flow, digital lateral flow, next-generation molecular diagnostics • Opportunity for co-funding and co-development of additional products • OTI investing $30M to lead Sapphiros' Series B funding round Transaction Overview • Strengthens our innovation pipeline and expands portfolio opportunities: o Expands Sample Management Solutions with new sample type - blood o Expands Diagnostics with new tests for infectious diseases, sexually transmitted infections, respiratory conditions & other diseases, as well as next-generation molecular diagnostics • Opens opportunities for new segments & customers with low-cost, highly scalable manufacturing • Leverages our strong customer relationships & commercial capabilities • Amplifies both companies’ opportunity to improve access, quality, & affordability of healthcare Notes: Reference to Sapphiros is intended to include its related companies unless the context requires otherwise. All products are subject to receipt of regulatory approvals in applicable markets.

© 2024 OraSure Technologies, Inc. 4 Strategic Transformation & Financial Impact Partnership with Sapphiros is expected to accelerate profitable growth • Sales of Sapphiros products are expected to add at least 2 percentage points of revenue growth for our Core business beginning in 2025 o Initial product for self-collected blood expected to launch later in 2024 … multiple additional products expected to launch in 2025 and beyond (subject to regulatory approvals) o OTI granted exclusive distribution rights for blood self-collection devices and diagnostic assays • Expected to be accretive to OraSure’s operating profit beginning in 2026 o Attractive margin for OTI due to our ability to leverage our existing infrastructure, capabilities & customer relationships o Modest amount of incremental SG&A by OTI needed in 2024-25 to successfully launch Sapphiros’ products Capital-efficient minority investment … flexibility for additional organic & inorganic innovation

© 2024 OraSure Technologies, Inc. 5 Aligned in Vision & Mission Transforming health through actionable insight: powering the shift that connects people to healthcare wherever they are Improving access, quality, and value of healthcare with innovation in effortless tests, sample management solutions & services Knowledge empowers us to navigate through life’s choices and decisions. With solutions that provide real-time, definitive results; at Sapphiros we work together to help global communities act on these insights with confidence Knowing Now Moves Us™

© 2024 OraSure Technologies, Inc. 6 Move to Point-of-Care & Home Testing Growth of Precision Medicine Healthcare Consumerism OraSure’s Imperatives Shared Focus on the Future of Healthcare

© 2024 OraSure Technologies, Inc. 7 Analytes (ex. proteins) Sample Types (ex. blood) Partnership with Sapphiros expected to expand our offerings with new sample type (blood) New & expanded offerings – sample types, analytes, applications & clearances Leading tools & chemistries to collect, stabilize, transport & store Comprehensive sample types / analytes / applications … preferred partner across market segments & geographies OTI Molecular Sample Management Solutions Sample collection & stabilization innovation drives access & discovery Applications (ex. liquid biopsy) OTI Portfolio - Sample Management Solutions Goal Clearances (ex. US FDA)

© 2024 OraSure Technologies, Inc. 8 OTI Portfolio – Diagnostics Broad array of infectious disease – respiratory & sexual health – assays, ex. COVID-19, HIV, HCV Substance abuse testing OTI Diagnostics Actionable POC & self-testing increases affordability & access to care Clinical lab molecular Increasing performance Increasing cost & challenge to access “Molecular- like” LFDs Lateral flow diagnostics (LFDs) (visual & digital) POC molecular Partnership with Sapphiros accelerates our innovation pipeline with new tests & platforms New offerings – more tests & next-gen platformsGoal Current Future
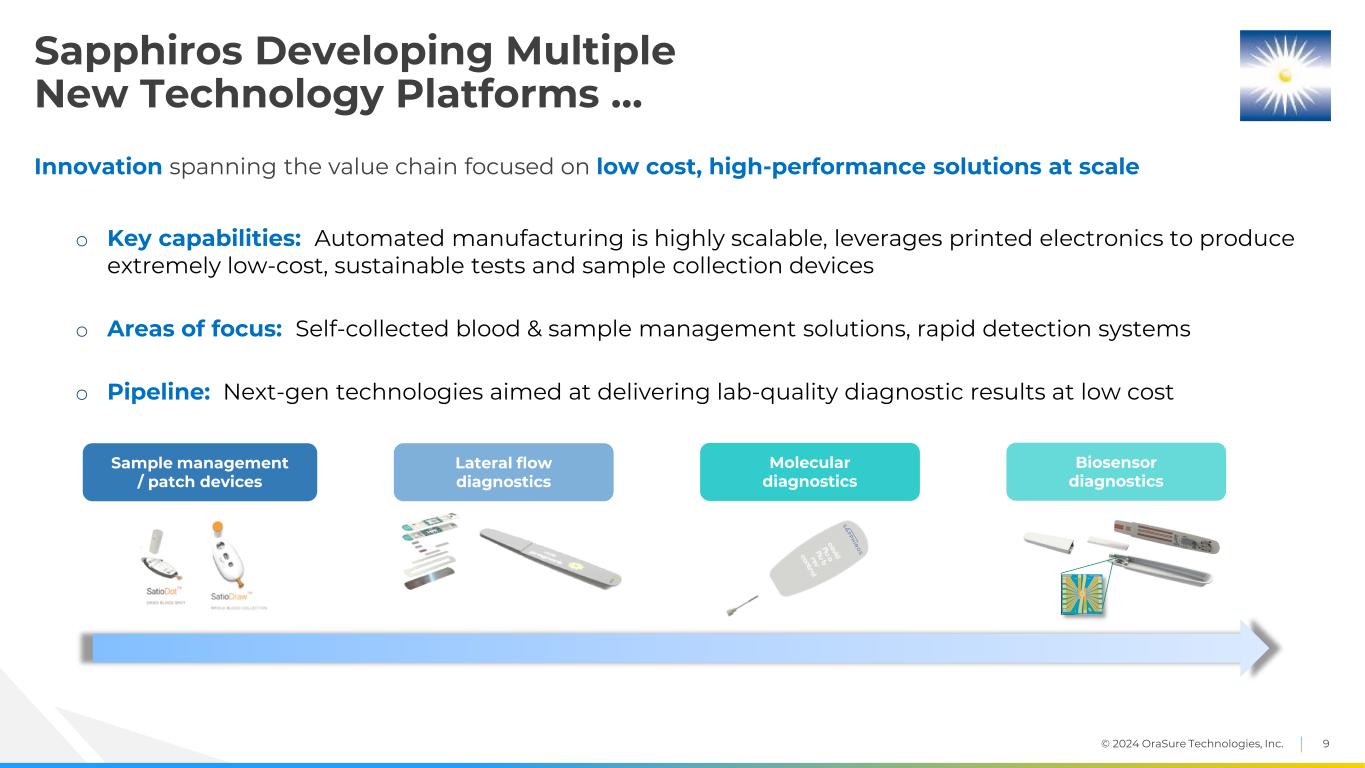
© 2024 OraSure Technologies, Inc. 9 Sapphiros Developing Multiple New Technology Platforms … Innovation spanning the value chain focused on low cost, high-performance solutions at scale o Key capabilities: Automated manufacturing is highly scalable, leverages printed electronics to produce extremely low-cost, sustainable tests and sample collection devices o Areas of focus: Self-collected blood & sample management solutions, rapid detection systems o Pipeline: Next-gen technologies aimed at delivering lab-quality diagnostic results at low cost Lateral flow diagnostics Sample management / patch devices Molecular diagnostics Biosensor diagnostics
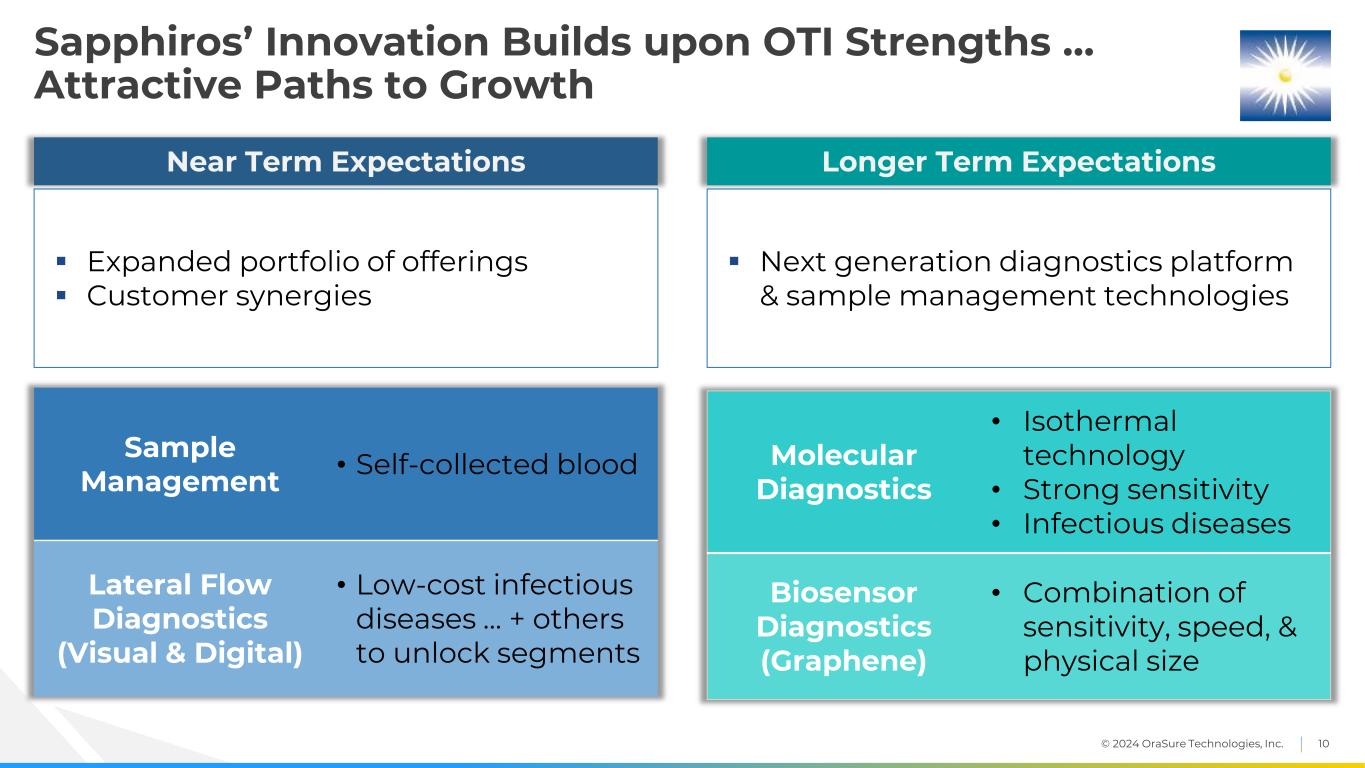
© 2024 OraSure Technologies, Inc. 10 Sapphiros’ Innovation Builds upon OTI Strengths … Attractive Paths to Growth Sample Management • Self-collected blood Lateral Flow Diagnostics (Visual & Digital) • Low-cost infectious diseases … + others to unlock segments Expanded portfolio of offerings Customer synergies Near Term Expectations Next generation diagnostics platform & sample management technologies Longer Term Expectations Molecular Diagnostics • Isothermal technology • Strong sensitivity • Infectious diseases Biosensor Diagnostics (Graphene) • Combination of sensitivity, speed, & physical size

© 2024 OraSure Technologies, Inc. 11 Public Health Clinical Settings Self-Testing OraSure’s ImperativesLeverages OTI’s Existing Commercial Strengths & Expands Opportunities in New & Existing Segments Expands OTI’s portfolio of high-performance sample management solutions and diagnostic tests Sapphiros’ product roadmap aligns with OTI’s capabilities in the areas of infectious disease, sexual health, and respiratory diseases Sapphiros’ next-generation manufacturing capabilities complement OTI’s operational strengths to increase access to affordable innovation in growth markets

© 2024 OraSure Technologies, Inc. 12 Partnership with Sapphiros Supports Key Elements for Long-Term Success Key Elements Partnership Opportunities Product portfolio & pipeline • Comprehensive portfolio increases diversity & breadth of solutions spanning sample management & diagnostic testing • Low-cost, high performance, scalable manufacturing broadens access in new & existing market segments Platforms for the future • Provides access to future next-generation diagnostics, including molecular & molecular-like technologies • Co-funding & co-development opportunities for additional products Commercial fit • Technologies complement our core business & enable segment expansion • Leverages our strengths, including in distribution & customer relationships Financials • Expect meaningful contribution to long-term growth & profitability • Minority ownership stake - participates in Sapphiros’ success • Flexibility for further organic & inorganic investments Culture • Purpose-driven focus to improve global health access, quality, affordability • Highly motivated teams with track records of success
EXHIBIT 99.2
| | | | | |
Investor Contact: Jason Plagman VP, Investor Relations investorinfo@orasure.com | Media Contact: Amy Koch Director, Corporate Communications media@orasure.com |
OraSure Technologies, Inc. Secures Strategic Distribution Rights and Invests in Sapphiros,
a Next-Generation Consumer Diagnostics Company
The relationship expands OraSure’s innovation pipeline with access to a broad portfolio of key Sapphiros products in development.
Distribution of Sapphiros’ products is expected to accelerate OraSure’s core revenue growth rate
beginning in 2025.
BETHLEHEM, Pa., January 4, 2024 (Globe Newswire) – OraSure Technologies, Inc. (NASDAQ: OSUR), a leader in point-of-care and home diagnostic tests, specimen collection devices, and microbiome laboratory and analytical services, today announced it is leading the Series B financing and has entered wide-ranging strategic distribution agreements with Sapphiros, a privately held consumer diagnostics portfolio company based in Boston, and certain of its related entities. Sapphiros was conceived in 2020 by experienced healthcare executive Namal Nawana and launched along with leading global investment firm KKR in 2021. The company has since developed innovative capabilities including novel sample collection, next-generation detection systems, computational biology, and printed electronics to help consumers access diagnostic results.
Through this strategic relationship, OraSure expects to be able to offer a more comprehensive range of low-cost diagnostic tests and sample management solutions to its customers globally. OraSure has secured exclusive distribution rights to key products in Sapphiros’ development pipeline that align with and enhance OraSure’s existing areas of expertise, including self-collected blood samples and diagnostic tests for sexually transmitted infections, respiratory conditions, and other diseases.
“Our partnership and investment in Sapphiros significantly expands and accelerates our product and innovation pipeline. It also advances our vision to improve the access, quality, and affordability of healthcare, including addressing unmet needs in the growing consumer diagnostic market,” said Carrie Eglinton Manner, President and CEO of OraSure. “We are excited to collaborate with the Sapphiros team to fully unlock the value and benefits of this relationship.”
This partnership connects Sapphiros’ innovation and robust product pipeline with OraSure’s strength in commercial distribution to serve new and existing market segments. It also creates opportunities for further collaboration in co-developing future products and advancing Sapphiros’ novel platforms for next-generation lateral flow devices, break-through molecular technologies, and advanced biosensors. Initial product distribution is expected to begin in 2024, with multiple additional products expected to launch in 2025 and beyond, subject to regulatory approvals. Distribution of Sapphiros’ products is expected to accelerate revenue growth in OraSure’s core business beginning in 2025.
"Our relationship with OraSure is a significant milestone for Sapphiros, and we are excited to partner with an organization that shares our focus on expanding access to diagnostic insights. We believe OraSure’s existing commercial infrastructure will allow us to quickly and efficiently scale following regulatory approvals to the populations that need them the most," said Mark Gladwell, President and CEO of Sapphiros. "We are looking forward to an innovation-filled future that will have a real impact on global health."
Conference Call Information
OraSure will host a conference call to discuss its strategic relationship with Sapphiros at 9 a.m. ET on Thursday, Jan. 4, 2024. A webcast of the conference call will be available on the investor relations page of OraSure’s website at https://orasure.gcs-web.com/events-and-presentations. Please click on the webcast link and follow the prompts for registration and access at least 10 minutes prior to the call. The webcast will be archived on OraSure’s website shortly after the call has ended and will be available for approximately 90 days.
To participate in the live conference call, please follow the link below to pre-register. After registering, you will be provided with access details via email.
https://register.vevent.com/register/BIeb0ab4e711c74d0f90b2c435f5a4266f
About OraSure Technologies, Inc.
OraSure Technologies (the “Company”) transforms health through actionable insight and powers the shift that connects people to healthcare wherever they are. The Company improves access, quality, and value of healthcare with innovation in effortless tests, sample management solutions, and services. OraSure, together with its wholly-owned subsidiaries, DNA Genotek, Diversigen, and Novosanis, provides its customers with end-to-end solutions that encompass diagnostics, tools, and services. The OraSure family of companies is a leader in the development, manufacture, and distribution of rapid diagnostic tests, sample collection and stabilization devices, and molecular services solutions designed to discover and detect critical medical conditions. OraSure’s portfolio of products is sold globally to clinical laboratories, hospitals, physician’s offices, clinics, public health and community-based organizations, research institutions, government agencies, pharmaceutical companies, commercial entities, and direct to consumers. For more information on OraSure Technologies, please visit www.orasure.com
About Sapphiros
Sapphiros, backed by KKR and Neoenta, is a privately held consumer diagnostics company. Sapphiros’ portfolio of capabilities and technologies includes novel sample collection, next-generation diagnostics, computational biology, and printed electronics, which help consumers access important diagnostic results globally. Knowing Now Moves Us™
Forward Looking Statements
This press release contains certain forward-looking statements, including with respect to products, product development and manufacturing activities, our investment in, and distribution relationship with, Sapphiros and its related entities, revenue growth, cost savings, cash flow, increasing margins and other matters. Forward-looking statements are not guarantees of future performance or results. Known and unknown factors that could cause actual performance or results to be materially different from those expressed or implied in these statements include, but are not limited to: Sapphiros’ and its related entities’ ability to seek and obtain regulatory approval for products in development; our ability to satisfy customer demand; ability to reduce our spending rate, capitalize on manufacturing efficiencies and drive profitable growth; ability to achieve the anticipated cost savings as a result of our business restructuring; ability to market and sell products, whether through our internal, direct sales force or third parties; impact of significant customer concentration in the genomics business; failure of distributors or other customers to meet purchase forecasts, historic purchase levels or minimum purchase requirements for products; ability to manufacture or have manufactured products in accordance with applicable specifications, performance standards and quality requirements; ability to obtain, and timing and cost of obtaining, necessary regulatory approvals for new products or new indications or applications for existing products; ability to comply with applicable regulatory requirements; ability to effectively resolve warning letters, audit observations and other findings or comments from the FDA or other regulators; the impact of the novel coronavirus (“COVID-19”) pandemic on the Company's business, supply chain, labor force, ability to successfully develop new products, validate the expanded use of existing collector products, receive necessary regulatory approvals and authorizations and commercialize such products for COVID-19 testing, and demand for our COVID-19 testing products; changes in relationships, including disputes or disagreements, with strategic partners such as Sapphiros or other parties and reliance on
strategic partners for the performance of critical activities under collaborative arrangements; ability to meet increased demand for the Company’s products; impact of replacing distributors; inventory levels at distributors and other customers; ability of the Company to achieve its financial and strategic objectives and continue to increase its revenues, including the ability to expand international sales and the ability to continue to reduce costs; impact of competitors, competing products and technology changes; reduction or deferral of public funding available to customers; competition from new or better technology or lower cost products; ability to develop, commercialize and market new products; market acceptance of oral fluid or urine testing, collection or other products; market acceptance and uptake of microbiome informatics, microbial genetics technology and related analytics services; changes in market acceptance of products based on product performance or other factors, including changes in testing guidelines, algorithms or other recommendations by the Centers for Disease Control and Prevention or other agencies; ability to fund research and development and other products and operations; ability to obtain and maintain new or existing product distribution channels; reliance on sole supply sources for critical products and components; availability of related products produced by third parties or products required for use of our products; impact of contracting with the U.S. government; impact of negative economic conditions; ability to maintain sustained profitability; ability to utilize net operating loss carry forwards or other deferred tax assets; volatility of the Company’s stock price; uncertainty relating to patent protection and potential patent infringement claims; uncertainty and costs of litigation relating to patents and other intellectual property; availability of licenses to patents or other technology; ability to enter into international manufacturing agreements; obstacles to international marketing and manufacturing of products; ability to sell products internationally, including the impact of changes in international funding sources and testing algorithms; adverse movements in foreign currency exchange rates; loss or impairment of sources of capital; ability to attract and retain qualified personnel; exposure to product liability and other types of litigation; changes in international, federal or state laws and regulations; customer consolidations and inventory practices; equipment failures and ability to obtain needed raw materials and components; cybersecurity breaches or other attacks involving our systems or those of our third-party contractors and IT service providers; the impact of terrorist attacks, civil unrest, hostilities and war; and general political, business and economic conditions, including inflationary pressures and banking stability. These and other factors that could affect our results are discussed more fully in our SEC filings, including our registration statements, Annual Report on Form 10-K for the year ended December 31, 2022, Quarterly Reports on Form 10-Q, and other filings with the SEC. Although forward-looking statements help to provide information about future prospects, readers should keep in mind that forward-looking statements may not be reliable. Readers are cautioned not to place undue reliance on the forward-looking statements. The forward-looking statements are made as of the date of this press release and OraSure Technologies undertakes no duty to update these statements.
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Grafico Azioni OraSure Technologies (NASDAQ:OSUR)
Storico
Da Nov 2024 a Dic 2024

Grafico Azioni OraSure Technologies (NASDAQ:OSUR)
Storico
Da Dic 2023 a Dic 2024
