false000176230300017623032025-03-172025-03-17
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): March 17, 2025 |
AVITA Medical, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-39059 |
85-1021707 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
28159 Avenue Stanford Suite 220 |
|
Valencia, California |
|
91355 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: 661 367-9170 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, par value $0.0001 per share |
|
RCEL |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 1.01 Entry into a Material Definitive Agreement.
Contract Manufacturing Agreement
On March 17, 2025, AVITA Medical Americas, LLC (“AVITA Medical”), a wholly-owned subsidiary of AVITA Medical, Inc. (the “Company”), entered into a contract manufacturing agreement (the “Manufacturing Agreement”) with Stedical Scientific, Inc. (“Stedical Scientific”) to manufacture PermeaDerm® Biosynthetic Wound Matrix (“PermeaDerm”) in the United States. PermeaDerm is cleared by the Food and Drug Administration as a transparent biosynthetic wound matrix for use in the treatment of a variety of wound types until healing is achieved.
Under the terms of the Manufacturing Agreement, AVITA Medical will manufacture PermeaDerm in the United States for the purposes of (i) sale in the United States under the terms of the existing Distribution Agreement (defined below) and (ii) sale to Stedical Scientific for sale or distribution outside the United States. The term of the Manufacturing Agreement is ten years.
Amendment Two to the Exclusive Distribution Agreement
On March 17, 2025, AVITA Medical and Stedical Scientific entered into an Amendment Two (the “Amendment”) of their existing exclusive distribution agreement under which AVITA Medical distributes PermeaDerm in the United States (the “Distribution Agreement”).
Under the terms of the Amendment, AVITA Medical's share of revenue from PermeaDerm sales increases from 50% to 60% and Stedical Scientific becomes eligible for certain milestone payments conditioned upon AVITA Medical's achievement of specified sales targets. The Amendment revises the initial term of the Distribution Agreement to ten years from the date of the Amendment.
Item 7.01. Regulation FD.
On March 17, 2025, the Company issued a press release announcing the entry into the Manufacturing Agreement and the Amendment. A copy of the press release is attached to this Current Report on Form 8-K as Exhibit 99.1.
The information under Item 7.01 and Exhibit 99.1 is being furnished and shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and shall not be deemed incorporated by reference into any filing made under the Securities Act of 1933 (except as expressly set forth by specific reference in such filing).
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
AVITA Medical, Inc |
|
|
|
|
Date: |
March 17, 2025 |
By: |
/s/ David O'Toole |
|
|
|
David O'Toole
Chief Financial Officer |
Exhibit 10.1

Contract Manufacturing Agreement
This Contract Manufacturing Agreement, (the "Agreement"), is effective as of March 17, 2025 (the “Effective Date”) and is entered into by and between AVITA Medical Americas, LLC having its principle place of business at 28159 Avenue Stanford, Suite 220 Valencia, CA ("Seller"), and Stedical Scientific, Inc. having its principle place of business at 2888 Loker Avenue East, Suite 319 Carlsbad, CA 92010 ("Buyer", and together with Seller, the "Parties", and each, a "Party").
WHEREAS, Seller is in the business of manufacturing and commercializing medical devices and supplies;
WHEREAS, Seller currently distributes certain Goods (as defined below) in the Territory (as defined below) on behalf of Seller under an existing Distribution Agreement (as defined below);
WHEREAS, Buyer wishes for Seller to now also manufacture such Goods;
WHEREAS, Buyer wishes to purchase Goods (as defined below) from Seller for the purpose of selling such Goods outside the Territory; and
WHEREAS, Seller desires to manufacture the Goods for sale to Buyer for the purposes outlined above and for sale to Seller’s customers under the Distribution Agreement.
NOW, THEREFORE, in consideration of the mutual covenants, terms and conditions set forth herein, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties agree as follows:
1.Definitions. Capitalized terms have either (1) the meanings set out or referred to in this Agreement; or (2) the meanings attributed to them in the Distribution Agreement (as defined below) if not defined in this Agreement.
"Action" means any claim, action, cause of action, demand, lawsuit, arbitration, inquiry, audit, notice of violation, proceeding, litigation, citation, summons, subpoena or investigation of any nature, civil, criminal, administrative, regulatory, or other, whether at law, in equity or otherwise.
"Affiliate" of a Person means any other Person that directly or indirectly, through one or more intermediaries, Controls, is Controlled by, or is under common Control with, such Person.
"Basic Purchase Order Terms" means, collectively, any one or more of the following terms specified by Buyer in a Purchase Order pursuant to Section 7.2: (a) a list of the Goods to be purchased, including SKU; (b) the quantity of each of the Goods ordered; (c) the Requested Delivery Date; (d) the unit Price for each of the Goods to be purchased; (e) the billing address; and (f) the Delivery Location. For the avoidance of doubt, the term "Basic Purchase Order Terms" does not include any general terms or conditions of any Purchase Order.
"Business Day" means any day except Saturday, Sunday, or any other day on which commercial banks located in California are authorized or required by Law to be closed for business.
"Buyer" has the meaning set forth in the preamble to this Agreement.
"Buyer Contracts" means all contracts or agreements to which Buyer is a party or to which any of its material assets are bound.
"Buyer’s Intellectual Property Rights" means all Intellectual Property Rights owned by or licensed to Buyer.
"Control" (and with correlative meanings, the terms "Controlled by" and "under common Control with") means, with respect to any Person, the possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of another Person, whether through the ownership of voting securities, by contract, or otherwise.
"Defective" means not conforming to the Product Warranty.
"Defective Goods" means goods shipped by Seller to Buyer pursuant to this Agreement that are Defective.
"Delivery Location" means the street address within the Territory for delivery of the Goods specified in the applicable Purchase Order.
"Distribution Agreement" means that certain Distribution Agreement entered into between Buyer and Seller on January 10, 2024 as amended from time to time wherein Buyer is designated as “Seller” or “Stedical” and Seller is designated as the “Distributor”.
"Forecast" means, with respect to any calendar quarter, a good faith projection or estimate of Buyer's requirements for Goods during each month during the quarter, which approximates, as nearly as possible, based on information available at the time to Buyer, the quantity of Goods that Buyer may order for each such month.
"Goods" means the goods identified on Schedule A and described in the Specifications.
"Governmental Authority" means any federal, state, local or foreign government or political subdivision thereof, or any agency or instrumentality of such government or political subdivision, or any self-regulated organization or other non-governmental regulatory authority or quasi-governmental authority (to the extent that the rules, regulations or orders of such organization or authority have the force of Law), or any arbitrator, court or tribunal of competent jurisdiction.
"Governmental Order" means any order, writ, judgment, injunction, decree, stipulation, award, or determination entered by or with any Governmental Authority.
"Intellectual Property Rights" means all industrial and other intellectual property rights comprising or relating to: (a) Patents; (b) Trademarks; (c) internet domain names, whether or not Trademarks, registered by any authorized private registrar or Governmental Authority, web addresses, web pages, website, and URLs; (d) works of authorship, expressions, designs, and design registrations, whether or not copyrightable, including copyrights and copyrightable works, data, data files, and databases and other specifications and documentation; (e) Trade Secrets; (f) know-how; and (g) all industrial and other intellectual property rights, and all rights, interests, and protections that are associated with, equivalent or similar to, or required for the exercise of, any of the foregoing, however arising, in each case whether registered or unregistered and including all registrations and applications for, and renewals or extensions of, such rights or forms of protection pursuant to the Laws of any jurisdiction throughout in any part of the world. For clarity and notwithstanding the foregoing, Buyer
and Seller's ownership in and of their separate and pre-existing Intellectual Property Rights shall continue in full force and effect.
"Law" means any statute, law, ordinance, regulation, rule, code, constitution, treaty, common law, Governmental Order, or other requirement or rule of law of any Governmental Authority.
"Legacy Goods" means Goods manufactured in the Territory under any existing agreement entered into by Buyer as of the Effective Date.
"Nonconforming Goods" means any goods received by Buyer from Seller pursuant to a Purchase Order that: (a) do not conform to the SKUs listed in the applicable Purchase Order; (b) do not fully conform to the Specifications; or (c) materially exceed the quantity of Goods ordered by Buyer pursuant to this Agreement or any Purchase Order. Where the context requires, Nonconforming Goods are deemed to be Goods for purposes of this Agreement.
"Patents" means all patents (including all reissues, divisionals, provisionals, continuations and continuations-in-part, re-examinations, renewals, substitutions and extensions thereof), patent applications, and other patent rights and any other Governmental Authority-issued indicia of invention ownership (including inventor's certificates, petty patents, and patent utility models).
"Person" means any individual, partnership, corporation, trust, limited liability entity, unincorporated organization, association, Governmental Authority, or any other entity.
"Personnel" of a Party means any agents, employees, contractors, or subcontractors engaged or appointed by such Party.
"Purchase Order" means Buyer's purchase order issued to Seller hereunder.
“Quality Agreement” means a written document agreed to and signed by both Parties defining each Party’s respective obligations under relevant regulations and quality management standards pertaining to the manufacture of medical devices.
"Representatives" means a Party's Affiliates and each of their respective Personnel, officers, directors, partners, shareholders, attorneys, third-party advisors, successors, and permitted assigns.
"Requested Delivery Date" means the requested delivery date for Goods ordered hereunder that is set forth in a Purchase Order, which must be a Business Day no less than thirty days following delivery of the applicable Purchase Order to Seller.
"Seller" has the meaning set forth in the preamble to this Agreement.
"Seller Distributed Goods" means Goods manufactured by Seller for distribution in the Territory by the Seller.
"Seller's Intellectual Property Rights" means all Intellectual Property Rights owned by or licensed to Seller.
"Seller's Trademarks" means all Trademarks owned by or licensed to Seller.
"Specifications" means the specifications for the manufacturing of Goods established by the Buyer and transmitted to Seller as modified from time to time by mutual agreement of the Parties, and subject to the applicable Quality Agreement(s).
"Taxes" means any and all present and future sales, income, stamp, and other taxes, levies, imposts, duties, deductions, charges, fees or withholdings imposed, levied, withheld, or assessed by any Governmental Authority, together with any interest or penalties imposed thereon.
"Territory" means the US and its territories and possessions.
"Trademarks" means all rights in and to US and foreign trademarks, service marks, trade dress, trade names, brand names, logos, corporate names and domain names, and other similar designations of source, sponsorship, association, or origin, together with the goodwill symbolized by any of the foregoing, in each case whether registered or unregistered and including all registrations and applications for, and renewals or extensions of, such rights and all similar or equivalent rights or forms of protection in any part of the world.
"Trade Secrets" means all inventions, discoveries, trade secrets, business and technical information and know-how, databases, data collections, patent disclosures, and other confidential and proprietary information and all rights therein.
"US" means the United States of America.
2.Exclusive Appointment. Buyer appoints Seller as its exclusive authorized manufacturer of the Goods in the Territory, other than Legacy Goods, during the Term and Seller accepts such appointment.
3.Conduct of the Parties.
3.1Upon receipt of a reasonable request for a specific action, the receiving Party shall reply within five business days stating either (i) the date upon which it will provide the corresponding deliverable, (ii) a counter proposal for achieving the same business goal, or (iii) its intent to not comply with the request.
3.2Should any governmental entity with jurisdiction over the use or sale of the Goods in the Territory request information that is in the other Party’s possession, that Party shall have three business days from the date of receipt of such request to (i) provide the information to the requesting Party; or (ii) propose an alternative due date for the deliverable.
3.3Should either Party experience difficulty in meeting the terms in this Section, that Party shall promptly communicate the difficulty to the other Party’s designated contact.
4.Use of Buyer’s Intellectual Property. To enable the manufacture of Goods under this Agreement as well as their commercialization under the Distribution Agreement or otherwise, Buyer hereby grants to Seller an irrevocable, fully paid-up, and transferrable right to use all of Buyer’s Intellectual Property Rights, exclusive in the Territory, needed for Seller to manufacture, market, and sell the Goods in the Territory for so long as the Agreement is in effect (the “Buyer IP License”). For clarity, the Buyer IP License shall not include a grant of ownership in or to any of Buyer’s Intellectual Property Rights.
5.Regulatory Obligations.
5.1Seller shall modify the Goods only in accordance with the Specifications.
5.2The Parties shall specify their respective regulatory obligations with respect to the performance of this Agreement by entering into a separate Quality Agreement or an amendment to the existing Quality Agreement between the Parties prior to or contemporaneously with the execution of this Agreement and Amendment Two to the Distribution Agreement.
6.Purchase and Sale of Goods.
6.1Purchase and Sale. Subject to the terms and conditions of this Agreement, during the Term, Buyer shall purchase from Seller, and Seller shall manufacture and sell to Buyer, all Goods ordered by Buyer, subject to Section 7.1. Schedule A contains a description of the Goods to be manufactured and sold hereunder. The Parties may, from time to time, amend Schedule A to reflect any agreed revisions to any of the terms described above; provided that no such revisions will modify this Agreement or be binding on the Parties unless such revisions have been fully approved in a signed writing by authorized Representatives of both Parties.
6.2Terms of Agreement Prevail Over Buyer's Purchase Order. Any additional, contrary, or different terms contained in any Purchase Order or other request or communication by Buyer pertaining to the sale of Goods, and any attempt to modify, supersede, supplement or otherwise alter this Agreement, will not modify this Agreement or be binding on the Parties unless such terms have been fully approved in a signed writing by authorized Representatives of both Parties.
7.1Non-binding Forecasts of Buyer's Requirements. Within thirty days following the full execution of this Agreement, Buyer shall provide to Seller a Forecast for the calendar quarter beginning on April 1, 2025. No later than thirty days prior to the first day of each subsequent calendar quarter, Buyer shall deliver to Seller a Forecast for the period beginning with the first day of such calendar quarter. Forecasts are for informational purposes only and do not create any binding obligations on behalf of either Party; provided, however, that Seller shall not be required to manufacture and sell to Buyer any quantity of Goods that is unreasonably disproportionate to any Forecast for the period covered by such Forecast.
7.2Purchase Orders. Buyer shall issue to Seller Purchase Orders (containing applicable Basic Purchase Order Terms that are consistent with the terms of this Agreement), in written form via email or other electronic exchange agreed to by the Parties. By issuing a Purchase Order to Seller, Buyer makes an offer to purchase Goods pursuant to the terms and conditions of this Agreement and the Basic Purchase Order Terms contained in such Purchase Order, and on no other terms. For the avoidance of doubt, any variations made to the terms and conditions of this Agreement by Buyer in any Purchase Order are void and have no effect. Buyer shall be obligated to purchase from Seller quantities of Goods specified in a Purchase Order.
7.3Acceptance, Rejection, and Cancellation of Purchase Orders. Seller accepts a Purchase Order by confirming the order in writing or by delivering the applicable Goods to Buyer, whichever occurs first. Seller may reject a Purchase Order or cancel a previously accepted Purchase Order, which it may do without liability or penalty, and without constituting a waiver of any of Seller's rights or
remedies under this Agreement or any Purchase Order, by providing written notice to Buyer specifying the applicable date of rejection or cancellation:
(a)if any one or more of the events described under Sections 10.3-10.4 has occurred;
(b)pursuant to Seller's rights under the last sentence of Section 9.5.
8.Shipment, Delivery, Acceptance, and Inspection.
8.1Shipment. Unless otherwise expressly agreed by the Parties in writing, Seller shall select the method of shipment of, and the carrier for, the Goods purchased by Byer. Seller may, in its sole discretion, without liability or penalty, make partial shipments of such Goods to Buyer. Each shipment will constitute a separate sale and Buyer shall pay for the Goods shipped, in accordance with the payment terms specified in Section 9.3, whether such shipment is in whole or partial fulfillment of a Purchase Order. All shipments are FCA Seller’s facility.
8.2Packaging and Labeling. Seller shall properly pack and ship Goods and provide Buyer with shipment documentation showing the Purchase Order number, Seller's identification number for the subject Goods, the quantity of pieces in shipment, the number of cartons or containers in shipment, Seller's name, the bill of lading number and the country of origin.
8.3Delivery. Unless otherwise expressly agreed by the Parties in writing, Seller shall deliver the Goods to the Delivery Location, using Seller's standard methods for packaging and shipping such Goods.
8.4Late Delivery. Any time quoted for delivery is an estimate only; provided, however, that Seller shall use commercially reasonable efforts to deliver all Goods on or before the Requested Delivery Date.
8.5Transfer of Title and Risk of Loss.
(a)Title to Goods shipped under any Purchase Order passes to Buyer upon Seller's tender of the Goods to the carrier at Seller’s location.
(b)Risk of loss to Goods shipped under any Purchase Order passes to Buyer upon Seller's tender of the Goods to the carrier at Seller’s location.
8.6Inspection. Buyer shall inspect Goods received under this Agreement within five Business Days receipt of such Goods ("Inspection Period") and either accept or, only if any such Goods are Nonconforming Goods, reject such Goods. Buyer will be deemed to have accepted Goods unless it provides Seller with written Notice of any Nonconforming Goods within five days following the Inspection Period, stating with specificity all defects and nonconformities, and furnishing such other written evidence or other documentation as may be reasonably required by Seller (including the subject Goods, or a representative sample thereof, which Buyer contends are Nonconforming Goods). All defects and nonconformities that are not so specified will be deemed waived by Buyer, such Goods shall be deemed to have been accepted by Buyer, and no attempted revocation of acceptance will be effective. If Buyer timely notifies Seller of any Nonconforming Goods, Seller shall determine, in its reasonable discretion, whether the Goods are Nonconforming Goods. If Seller determines that such Goods are Nonconforming Goods, Seller shall, in its sole discretion, either:
(a)replace such Nonconforming Goods with conforming Goods; or
(b)refund to Buyer such amount paid by Buyer to Seller for such Nonconforming Goods returned by Buyer to Seller.
Buyer shall ship, at Buyer's expense and risk of loss, all Nonconforming Goods to Seller's facility or to such other location as Seller may instruct Buyer in writing. If Seller exercises its option to replace Nonconforming Goods, Seller shall ship to the Delivery Location, at Seller's expense and risk of loss, the replacement Goods.
THE REMEDIES SET FORTH IN THIS SECTION ARE BUYER'S EXCLUSIVE REMEDY FOR THE DELIVERY OF NONCONFORMING GOODS, SUBJECT TO BUYER'S RIGHTS UNDER SECTION 13.5 WITH RESPECT TO ANY SUCH GOODS FOR WHICH BUYER HAS ACCEPTED DELIVERY UNDER THIS SECTION.
8.7Limited Right of Return. Except as provided under Section 8.6, Section 13.5, and Section 13.7, Buyer has no right to return Goods shipped to Buyer pursuant to this Agreement.
9.1Price. Buyer shall purchase the Goods from Seller at 110% of Seller’s cost to manufacture the Goods (“Seller’s Cost”). An estimate of Seller’s Cost is attached as Schedule B. For any Goods not listed in Schedule B as of the Effective Date, Seller shall use commercially reasonable efforts to provide an updated Schedule B covering all Goods under this Agreement. Seller shall promptly provide a revised Schedule B upon any material change to Seller's Cost.
9.2Shipping Charges, Insurance, and Taxes. Buyer shall pay for all shipping charges and insurance costs for all Goods purchased under this Agreement. In addition, all Prices are exclusive of, and Buyer is solely responsible for and shall pay all Taxes, with respect to, or measured by, the manufacture, sale, shipment, use, or Price of the Goods (including interest and penalties thereon).
9.3Payment Terms. Seller shall issue monthly invoices to Buyer for all Goods ordered by Buyer in the previous month. Buyer shall pay to Seller all invoiced amounts within thirty days of Buyer's receipt of such invoice. Buyer shall make all payments in US dollars by check or wire transfer.
9.4Invoice Disputes. Buyer shall notify Seller in writing of any dispute with any invoice (along with substantiating documentation and a reasonably detailed description of the dispute) within five Business Days from Buyer's receipt of such invoice. Buyer will be deemed to have accepted all invoices for which Seller does not receive timely notification of dispute and shall pay all undisputed amounts due under such invoices within the period set forth in Section 9.3. The Parties shall seek to resolve any such disputes expeditiously. Notwithstanding anything to the contrary, Buyer shall continue performing its obligations under this Agreement during any such dispute, including Buyer's obligation to pay all due and undisputed invoice amounts in accordance with the terms of this Agreement.
9.5Late Payments. Except for invoiced payments that Buyer has successfully disputed, Buyer shall pay interest on all late payments (whether during the Term or after the expiration or earlier termination of the Term), calculated daily and compounded monthly, at the lesser of the rate of 1.5% per month or the highest rate permissible under applicable Law. Buyer shall also reimburse Seller for all costs incurred by Seller in collecting any late payments, including attorneys' fees and court costs.
In addition to all other remedies available under this Agreement or at Law (which Seller does not waive by the exercise of any rights under this Agreement), if Buyer fails to pay any undisputed amounts when due under this Agreement, Seller may (a) suspend the delivery of any Goods, (b) reject Buyer's Purchase Orders or cancel accepted Purchase Orders pursuant to the terms of Section 7.3 or (c) terminate this Agreement pursuant to the terms of Sections 10.3-10.4.
9.6No Set-off Right. Buyer shall not, and acknowledges that it will have no right, under this Agreement, any Purchase Order, any other agreement, document or Law to, withhold, offset, recoup or debit any amounts owed (or to become due and owing) to Seller or any of its Affiliates, whether under this Agreement or otherwise, against any other amount owed (or to become due and owing) to it by Seller or Seller's Affiliates, whether relating to Seller's or its Affiliates' breach or non-performance of this Agreement, any Purchase Order, any other agreement between (a) Buyer or any of its Affiliates and (b) Seller or any of its Affiliates, or otherwise.
10.1Initial Term. The term of this Agreement commences on the Effective Date and continues for a period of ten years unless it is earlier terminated pursuant to the terms of this Agreement or applicable Law (the "Initial Term").
10.2Renewal Term. Upon expiration of the Initial Term, this agreement will renew if, and to the same extent as, the Distribution Agreement renews (the “Renewal Term”).
10.3Mutual Termination Rights. Either Party may terminate this Agreement upon prior written notice to the other Party if the other Party is in material breach of this Agreement and either the breach cannot be cured or, if the breach can be cured, it is not cured within forty-five days following the other Party's receipt of notice of such breach. Either Party may also terminate this Agreement if the other Party:
(a)becomes insolvent or is generally unable to pay, or fails to pay, its debts as they become due;
(b)files or has filed against it, a petition for voluntary or involuntary bankruptcy or otherwise becomes subject, voluntarily, or involuntarily, to any proceeding under any domestic or foreign bankruptcy or insolvency law;
(c)seeks reorganization, arrangement, adjustment, winding-up, liquidation, dissolution, composition, or other relief with respect to it or its debts;
(d)makes or seeks to make a general assignment for the benefit of its creditors;
(e)applies for or has a receiver, trustee, custodian, or similar agent appointed by order of any court of competent jurisdiction to take charge of or sell any material portion of its property or business; or
(f)is indicted under any Anti-Bribery Law or conducts itself in such a way that raises a reasonable suspicion that it has violated any Anti-Bribery Law as defined in Section 13.1.
10.4Seller's Right to Terminate. Seller may terminate this Agreement by providing written Notice to Buyer:
(a)if Buyer fails to pay any amount when due under this Agreement ("Payment Failure");
(b)if Buyer sells or attempts to sell Goods, including Legacy Goods, in the Territory;
(c)if Seller terminates the Distribution Agreement due to Buyer’s or Buyer’s Affiliates’ breach or non-performance thereof;
(d)if without obtaining Seller's prior written consent, (i) Buyer sells, leases, or exchanges a material portion of Buyer's assets, (ii) Buyer merges or consolidates with or into another Person, or (iii) a change in Control of Buyer occurs.
Any termination under this Section will be effective on Buyer's receipt of Seller's written Notice of termination or such later date (if any) set forth in such Notice.
10.5Conditions Precedent to Buyer's Right to Terminate. As a condition precedent to Buyer's right to terminate this Agreement pursuant to this Section, within five days following the date of Buyer's termination Notice, Buyer shall pay to Seller all amounts due to Seller for Goods delivered by Seller to Buyer prior to Seller's receipt of the termination Notice and reimburse Seller for all of Seller's out-of-pocket costs and expenses (including raw materials, machinery and equipment purchases) incurred by Seller prior to receipt of Buyer's termination Notice that arise from or relate to this Agreement or any Purchase Order issued by Buyer to Seller prior to Seller's receipt of such notice (each, a "Reimbursement Payment"). Any termination under this Section will be effective on the latest to occur of Seller's receipt of Buyer's written Notice of termination, Seller's receipt of the Reimbursement Payment or such other later date (if any) set forth in such termination Notice (if and to the extent that such later date is approved by Seller in writing).
10.6Effect of Expiration or Termination.
(a)Upon the expiration or earlier termination of this Agreement, all indebtedness of Buyer to Seller under this Agreement, any other agreement or otherwise, of any kind, shall become immediately due and payable to Seller, without further notice to Buyer.
(b)Expiration or termination of the Term will not affect any rights or obligations of the Parties that:
(i)come into effect upon or after termination or expiration of this Agreement; or
(ii)otherwise survive the expiration or earlier termination of this Agreement pursuant to Section 22.4 and were incurred by the Parties prior to such expiration or earlier termination.
(c)Any Notice of termination under this Agreement automatically operates as a cancellation of any deliveries of Goods to Buyer that are scheduled to be made subsequent to the effective date of termination, whether or not any orders for such Goods had been accepted by Seller. With respect to any Goods that are still in transit upon termination of this Agreement,
Seller may require, in its sole discretion, that all sales and deliveries of such Goods be made on either a cash-only or certified-check basis.
(d)In the event of a termination by Seller under 10.4(b), the Buyer IP License shall become perpetual and shall survive the termination of this Agreement.
(e)Upon the expiration or earlier termination of this Agreement, Buyer shall:
(i)return to Seller or destroy all documents and tangible materials (and any copies) containing, reflecting, incorporating, or based on Seller's Confidential Information;
(ii)permanently erase all of Seller's Confidential Information from its computer systems except for copies that are maintained as archive copies on its disaster recovery and/or information technology backup systems; and
(iii)certify in writing to Seller that it has complied with the requirements of this clause within five business days of the expiration or earlier termination of this Agreement.
(f)Subject to Section 10.6(b), the Party terminating this Agreement, or in the case of the expiration of this Agreement, each Party, shall not be liable to the other Party for any damage of any kind (whether direct or indirect) incurred by the other Party by reason of the expiration or earlier termination of this Agreement. Termination of this Agreement will not constitute a waiver of any of either Party's rights, remedies or defenses under this Agreement, at law, in equity or otherwise.
11.Certain Obligations of Buyer.
11.1Certain Prohibited Acts. Notwithstanding anything to the contrary in this Agreement, neither Buyer nor any Buyer Personnel shall:
(a)Sell, distribute, or otherwise attempt to commercialize Goods, including Legacy Goods, within the Territory
(b)make any representations, warranties, guarantees, indemnities, similar claims, or other commitments:
(i)actually, apparently or ostensibly on behalf of Seller, or
(ii)to any customer or other Person with respect to the Goods, which are additional to or inconsistent with any then-existing representations, warranties, guarantees, indemnities, similar claims, or other commitments in this Agreement or any written documentation provided by Seller to Buyer; or
(c)engage in any unfair, competitive, misleading, or deceptive practices respecting Seller, Seller's Trademarks or the Goods, including any product disparagement;
11.2Sales Outside the Territory. When selling or distributing Goods outside the Territory, Buyer shall
(a)assure Goods are shipped, handled, marked, marketed, sold or otherwise commercialized in compliance with all applicable laws and regulations; and
(b)hold Seller harmless from any requirements under non-US Law, regarding the shipping, handling, storage, marketing, sale, or other commercialization of Goods outside the Territory.
11.3Government Contracts. Unless otherwise separately agreed in writing between Seller and Buyer, no provisions required in any contract with any Government Authority or subcontract related thereto shall be a part of this Agreement or imposed upon or binding upon Seller, and this Agreement shall not be deemed an acceptance of any government provisions that may be included or referenced in Buyer's request for quotation, Purchase Order or any other document.
11.4Credit Risk on Resale of the Goods to Customers. Buyer shall be responsible for all credit risks with respect to, and for collecting payment for, all products (including Goods) sold to its customers or other third parties, whether or not Buyer has made full payment to Seller for such products. The inability of Buyer to collect the purchase price for any product shall not affect Buyer's obligation under this Agreement to pay Seller any and all amounts due and payable.
12.Compliance with Laws. Buyer shall at all times comply with all Laws applicable to this Agreement, Buyer's performance of its obligations hereunder, and Buyer's use, commercialization, sale, or distribution of the Goods. Without limiting the generality of the foregoing, Buyer shall (a) at its own expense, maintain all certifications, credentials, licenses, and permits necessary to conduct its business relating to the purchase, use, commercialization, sale, or distribution of the Goods and (b) not engage in any activity or transaction involving the Goods that violates any Law.
13.Representations and Warranties.
13.1Anti-Bribery Representations and Warranties. This Agreement is subject to the Anti-Bribery Representations and Warranties of the Distribution Agreement which are incorporated by reference.
13.2Buyer's Representations and Warranties. Buyer represents and warrants to Seller that:
(a)it is a corporation, duly organized, validly existing, and in good standing under the laws of the State of Delaware;
(b)it is duly qualified to do business and is in good standing in every jurisdiction in which such qualification is required;
(c)it has the full right, corporate power and authority to enter into this Agreement and to perform its obligations hereunder;
(d)the execution of this Agreement by its Representative whose signature is set forth at the end of this Agreement, and the delivery of this Agreement by Buyer, have been duly authorized by all necessary corporate action on the part of Buyer;
(e)the execution, delivery, and performance of this Agreement by Buyer will not violate, conflict with, require consent under or result in any breach or default under (i) any of
Buyer's organizational documents, (ii) any applicable Law or (iii) with or without notice or lapse of time or both, the provisions of any Buyer Contract;
(f)this Agreement has been executed and delivered by Buyer and (assuming due authorization, execution, and delivery by Seller) constitutes the legal, valid, and binding obligation of Buyer, enforceable against Buyer in accordance with its terms;
(g)it is in compliance with all applicable Laws and Buyer Contracts relating to this Agreement, the Goods and the operation of its business;
(h)it has obtained all licenses, authorizations, approvals, consents, or permits required by applicable Laws to conduct its business generally and to perform its obligations under this Agreement;
(i)it is not insolvent and is paying all of its debts as they become due; and
(j)all financial information that it has provided to Seller is true and accurate and fairly represents Buyer's financial condition.
13.3Limited Product Warranty. Subject to the provisions of Sections 13.4 through 13.7, Seller warrants to Buyer (the "Product Warranty") that:
(a)for a period of twelve months from the date of shipment of a Good (the "Warranty Period"), each Good will materially conform to the Specifications and will be free from significant defects in material and workmanship; and
(b)Buyer will receive good and valid title to all Goods, free and clear of all encumbrances and liens of any kind.
13.4Product Warranty Limitations. The Product Warranty does not apply to any Good that:
(a)has been subjected to abuse, misuse, neglect, negligence, accident, improper testing, improper installation, improper storage, improper handling, abnormal physical stress, abnormal environmental conditions or use contrary to any instructions issued by Seller;
(b)has been reconstructed, repaired, or altered by Persons other than Seller or its authorized Representative; or
(c)has been used with any third-party products, hardware, or product that has not been previously approved in writing by Seller.
13.5Buyer's Exclusive Remedy for Defective Goods. Notwithstanding any other provision of this Agreement (except for Section 13.7), this Section contains Buyer's exclusive remedy for Defective Goods. Buyer's remedy under this Section is conditioned upon Buyer's compliance with its obligations below. During the Warranty Period, with respect to any allegedly Defective Goods:
(a)Buyer shall notify Seller, in writing, of any alleged claim or defect within five Business Days from the date Buyer discovers, or upon reasonable inspection should have discovered, such alleged claim or defect (but in any event before the expiration of the applicable Warranty Period);
(b)Buyer shall ship, at its expense and risk of loss, such allegedly Defective Goods to Seller's facility for inspection and testing by Seller;
(c)if Seller's inspection and testing reveal, to Seller's reasonable satisfaction, that such Goods are Defective and any such defect has not been caused or contributed to by any of the factors described under Section 13.4 above, Seller shall in its sole discretion and at its expense, repair or replace such Defective Goods; and
(d)Seller shall ship to Buyer, at Seller's expense, the repaired or replaced Goods to a location designated by Buyer in the Territory.
Buyer has no right to return for repair, replacement, credit, or refund any Good except as set forth in this Section (or if otherwise applicable, Section 8.6 or Section 13.7). In no event shall Buyer reconstruct, repair, alter or replace any Good, in whole or in part, either itself or by or through any third party.
THIS SECTION SETS FORTH SELLER'S ENTIRE LIABILITY FOR ANY BREACH OF, AND BUYER'S SOLE REMEDY RELATING TO, THE LIMITED PRODUCT WARRANTY.
13.6DISCLAIMER OF OTHER REPRESENTATIONS AND WARRANTIES; NON-RELIANCE. EXCEPT FOR THE PRODUCT WARRANTY SET FORTH ABOVE, (A) NEITHER SELLER NOR ANY PERSON ON SELLER'S BEHALF HAS MADE OR MAKES ANY EXPRESS OR IMPLIED REPRESENTATION OR WARRANTY WHATSOEVER, EITHER ORAL OR WRITTEN, INCLUDING ANY WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, TITLE, NON-INFRINGEMENT, PERFORMANCE OF GOODS OR PRODUCTS TO STANDARDS SPECIFIC TO THE COUNTRY OF IMPORT, WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE, ALL OF WHICH ARE EXPRESSLY DISCLAIMED, AND (B) BUYER ACKNOWLEDGES THAT IT HAS NOT RELIED UPON ANY REPRESENTATION OR WARRANTY MADE BY SELLER, OR ANY OTHER PERSON ON SELLER'S BEHALF, EXCEPT AS SPECIFICALLY PROVIDED IN THIS AGREEMENT.
13.7Withdrawal of Goods. If Seller determines that any Goods sold to Buyer may be Defective, or if any Governmental Authority issues a lawful order to Seller that the Good’s must be withdrawn from commerce, at Seller's request, Buyer shall withdraw all similar Goods from sale and, at Seller's option, either return such Goods to Seller or destroy the Goods and provide Seller with written certification of such destruction. Notwithstanding other limitations in this Agreement, if Buyer returns all withdrawn Goods or destroys all withdrawn Goods and provides Seller with written certification of such destruction within thirty days following Seller's withdrawal request, in either case, consistent with Seller's instructions, unless any such defect has not been caused or contributed to by any of the factors described under Section 13.4, Seller shall (a) repair or replace all such returned Goods or (b) replace such destroyed Goods, in either case, pursuant to the terms of this Section. THIS SECTION SETS FORTH SELLER'S ENTIRE LIABILITY FOR, AND BUYER'S SOLE REMEDY RELATING TO, ANY GOODS THAT ARE WITHDRAWN PURSUANT TO THIS SECTION.
14.1Buyer Indemnification. Subject to the terms and conditions of this Agreement, Buyer (as "Indemnifying Party") shall indemnify, defend and hold harmless Seller and its successors and
permitted assigns (collectively, "Indemnified Party") against any and all losses, damages, liabilities, deficiencies, claims, actions, judgments, settlements, interest, awards, penalties, fines, costs, or expenses of whatever kind, including reasonable attorneys' fees, fees and the costs of enforcing any right to indemnification under this Agreement and the cost of pursuing any insurance providers, (collectively, "Losses"), resulting from any third-party Claim alleging:
(a)a material breach or non-fulfillment of any representation, warranty or covenant under this Agreement by Indemnifying Party or Indemnifying Party's Personnel;
(b)any grossly negligent or more culpable act or omission of Indemnifying Party or its Personnel (including any recklessness or willful misconduct) in connection with the performance of this Agreement;
(c)any bodily injury, death of any Person or damage to real or tangible personal property caused by the grossly negligent acts or omissions of Indemnifying Party or its Personnel;
(d)any failure by Indemnifying Party or its Personnel to materially comply with any applicable Laws.
Notwithstanding anything to the contrary in this Agreement, this Section does not apply to any Claim (whether direct or indirect) for which a sole or exclusive remedy is provided for under another section of this Agreement.
14.2Exceptions and Limitations on Indemnification. Notwithstanding anything to the contrary in this Agreement, an Indemnifying Party is not obligated to indemnify or defend (if applicable) an Indemnified Party against any Claim if such Claim or corresponding Losses arise out of or result from the Indemnified Party's or its Personnel's:
(a)gross negligence or more culpable act or omission (including willful misconduct); or
(b)bad faith failure to materially comply with any of its obligations set forth in this Agreement;
14.3EXCLUSIVE REMEDY. THIS SECTION SETS FORTH THE ENTIRE LIABILITY AND OBLIGATION OF EACH INDEMNIFYING PARTY AND THE SOLE AND EXCLUSIVE REMEDY FOR EACH INDEMNIFIED PARTY FOR ANY DAMAGES COVERED BY THIS SECTION.
15.Limitation of Liability.
15.1NO LIABILITY FOR CONSEQUENTIAL OR INDIRECT DAMAGES. EXCEPT FOR OBLIGATIONS TO MAKE PAYMENT UNDER THIS AGREEMENT, LIABILITY FOR INDEMNIFICATION, LIABILITY FOR BREACH OF CONFIDENTIALITY, OR LIABILITY FOR INFRINGEMENT OR MISAPPROPRIATION OF INTELLECTUAL PROPERTY RIGHTS, IN NO EVENT SHALL SELLER OR ITS REPRESENTATIVES BE LIABLE FOR CONSEQUENTIAL, INDIRECT, INCIDENTAL, SPECIAL, EXEMPLARY, OR PUNITIVE DAMAGES ARISING OUT OF OR RELATING TO ANY BREACH OF THIS AGREEMENT, REGARDLESS OF (A) WHETHER SUCH DAMAGES WERE FORESEEABLE, (B) WHETHER OR NOT BUYER WAS
ADVISED OF THE POSSIBILITY OF SUCH DAMAGES AND (C) THE LEGAL OR EQUITABLE THEORY (CONTRACT, TORT OR OTHERWISE) UPON WHICH THE CLAIM IS BASED, AND NOTWITHSTANDING THE FAILURE OF ANY AGREED OR OTHER REMEDY OF ITS ESSENTIAL PURPOSE.
15.2MAXIMUM LIABILITY FOR DAMAGES. IN NO EVENT SHALL SELLER'S AGGREGATE LIABILITY ARISING OUT OF OR RELATED TO THIS AGREEMENT, WHETHER ARISING OUT OF OR RELATED TO BREACH OF CONTRACT, TORT (INCLUDING NEGLIGENCE) OR OTHERWISE, EXCEED 50% OF THE TOTAL OF THE AMOUNTS PAID AND AMOUNTS ACCRUED BUT NOT YET PAID TO SELLER PURSUANT TO THIS AGREEMENT IN THE TWELVE-MONTH PERIOD PRECEDING THE EVENT GIVING RISE TO THE CLAIM.
15.3ASSUMPTION OF RISK. WITHOUT LIMITING THE GENERALITY OF THE FOREGOING, BUYER ASSUMES ALL RISK AND LIABILITY FOR THE RESULTS OBTAINED BY THE USE OF ANY GOODS IN THE PRACTICE OF ANY PROCESS, WHETHER IN TERMS OF OPERATING COSTS, GENERAL EFFECTIVENESS, SUCCESS OR FAILURE, AND REGARDLESS OF ANY ORAL OR WRITTEN STATEMENTS MADE BY SELLER, BY WAY OF TECHNICAL ADVICE OR OTHERWISE, RELATED TO THE USE OF THE GOODS.
16.Intellectual Property Rights.
16.1Ownership. Each Party acknowledges and agrees that:
(a)Each Party’s Intellectual Property Rights are the sole and exclusive property of that Party or its licensors; and
(b)Neither Party shall acquire any ownership interest in the other Party's Intellectual Property Rights under this Agreement.
16.2Prohibited Acts. Neither Party shall:
(a)take any action that interferes with the other Party’s Intellectual Property Rights, including ownership or exercise thereof;
(b)challenge any right, title, or interest of the other Party’s Intellectual Property Rights;
(c)make any claim or take any action adverse to the ownership of the other Party’s Intellectual Property Rights;
(d)register or apply for registrations, anywhere in the world, for the other Party’s Trademarks or any other Trademark that is similar to the other Party’s Trademarks or that incorporates the other Party’s Trademarks;
(e)use any mark, anywhere, that is confusingly similar to the other Party's Trademarks;
(f)engage in any action that tends to disparage, dilute the value of, or reflect negatively on the Goods;
(g)misappropriate any of the other Party’s Trademarks for use as a domain name without prior written consent from the other Party; or
(h)alter, obscure, or remove any of the other Party’s Trademarks or trademark or copyright notices or any other proprietary rights notices placed on the products purchased under this Agreement (including Goods), marketing materials, or other materials provided by the other Party.
17.Confidentiality. This Agreement is subject to the confidentiality provisions of the Distribution Agreement which are herein incorporated by reference.
18.Tooling. All Tooling, other than Tooling listed in Schedule C, used to manufacture the Goods is owned by Seller ("Seller Tooling"). Buyer has no right, title, or interest in or to any of the Seller Tooling.
19.Access and Audit Rights. Seller hereby grants Buyer access to Seller's operations, facilities, books and records, correspondence, writings, drawings, and receipts related to the Goods for the purpose of ensuring Seller's compliance with the terms of this Agreement and any other agreements between Buyer and Seller. Seller shall maintain all pertinent books and records for a period of one year after expiration of the Term. Seller shall also cooperate fully with Buyer with respect to all reasonable requests of Buyer relating to the foregoing access rights; provided that any physical inventory inspection may take place no more frequently than annually. For the avoidance of doubt, these access and audit rights include information necessary to calculate Seller’s Cost.
20.Insurance. This Agreement is subject to the insurance provisions of the Distribution Agreement which are herein incorporated by reference.
21.Contingency & Read Together. This Agreement is contingent upon the execution and delivery of Amendment Two to the Distribution Agreement. In the event that Amendment Two to the Distribution Agreement (attached as Exhibit A) is not signed within two days of the execution of this Agreement, this Agreement shall be null and void and neither Party shall have any rights or obligations under this Agreement. This Agreement shall be read and construed in conjunction with the Distribution Agreement and any amendments thereto.
22.1Further Assurances. Upon a Party's reasonable request, the other Party shall, at its sole cost and expense, execute and deliver all such further documents and instruments, and take all such further acts, necessary to give full effect to this Agreement.
22.2Relationship of the Parties. The relationship between Seller and Buyer is solely that of vendor and vendee, and they are independent contracting parties. Nothing in this Agreement creates any agency, joint venture, partnership, or other form of joint enterprise, employment, or fiduciary relationship between the Parties. Neither Party has any express or implied right or authority to assume or create any obligations on behalf of or in the name of the other Party or to bind the other Party to any contract, agreement, or undertaking with any third party.
22.3Entire Agreement. This Agreement, including and together with the Basic Purchase Order Terms and any related exhibits and schedules, as well as all applicable terms of the Distribution Agreement, constitutes the sole and entire agreement of the Parties with respect to the subject matter
contained herein and therein and supersedes all prior and contemporaneous understandings, agreements, representations, and warranties, both written and oral, with respect to such subject matter.
22.4Survival; Statute of Limitations. Subject to the limitations and other provisions of this Agreement: the representations and warranties of the Parties contained herein and any other provision that, in order to give proper effect to its intent, should survive such expiration or termination, will survive the expiration or earlier termination of this Agreement for a period of twelve months after such expiration or termination. All other provisions of this Agreement will not survive the expiration or earlier termination of this Agreement. Notwithstanding any right under any applicable statute of limitations to bring a claim, no Action based upon or arising in any way out of this Agreement may be brought by either Party after the expiration of the applicable survival or other period set forth in this Section and the Parties waive the right to file any such Action after the expiration of the applicable survival or other period; provided, however, that the foregoing waiver and limitation do not apply to the collection of any amounts due to Seller under this Agreement.
22.5Notices. All notices, requests, consents, claims, demands, waivers, and other communications under this Agreement (each, a "Notice") must be in writing and addressed to the other Party at its address set forth below (or to such other address that the receiving Party may designate from time to time in accordance with this Section). All Notices must be delivered by personal delivery, nationally recognized overnight courier, or certified or registered mail (in each case, return receipt requested, postage prepaid). Notice by facsimile or e-mail (with confirmation of transmission) will satisfy the requirements of this Section Except as otherwise provided in this Agreement, a Notice is effective only (a) on receipt by the receiving Party, and (b) if the Party giving the Notice has complied with the requirements of this Section.
|
|
Notice to Seller: |
2888 Loker Avenue East, Suite 319 Carlsbad, California, 92010 |
|
Attention: Scott Moote, Director, Operation, |
|
[***] |
|
CC: Shuang Miao, Manager, Internal Control, [***] |
|
|
Notice to Buyer: |
28159 Avenue Stanford, Suite 220 Valencia, CA, 91355 |
|
Attention: David Melbye, SVP Operations |
|
[***] |
|
CC: Legal Department legal@avitamedical.com |
22.6Headings. The headings in this Agreement are for reference only and do not affect the interpretation of this Agreement.
22.7Severability. If any term or provision of this Agreement is invalid, illegal, or unenforceable in any jurisdiction, such invalidity, illegality or unenforceability does not affect any other term or
provision of this Agreement or invalidate or render unenforceable such term or provision in any other jurisdiction.
22.8Amendment and Modification. No amendment or termination of this Agreement is effective unless it is in writing and signed by each Party.
(a)No waiver under this Agreement is effective unless it is in writing and signed by the Party waiving its right.
(b)Any waiver authorized on one occasion is effective only in that instance and only for the purpose stated and does not operate as a waiver on any future occasion.
(c)None of the following constitutes a waiver or estoppel of any right, remedy, power, privilege, or condition arising from this Agreement:
(i)any failure or delay in exercising any right, remedy, power or privilege or in enforcing any condition under this Agreement; or
(ii)any act, omission, or course of dealing between the Parties.
22.10Cumulative Remedies. All rights and remedies provided in this Agreement are cumulative and not exclusive, and the exercise by either Party of any right or remedy does not preclude the exercise of any other rights or remedies that may now or subsequently be available at law, in equity, by statute, in any other agreement between the Parties or otherwise. Notwithstanding the previous sentence, the Parties intend that Buyer's rights under Section 8.4, Section 8.6, Section 13.5, and each of the Parties' rights under Section 14 are exclusive remedies for the events specified therein.
22.11Equitable Remedies. Buyer acknowledges and agrees that (a) a breach or threatened breach by such Party of any of its obligations under Sections 10.4(b), 16.2, and 17 would give rise to irreparable harm to Seller for which monetary damages would not be an adequate remedy and (b) in the event of a breach or a threatened breach by Buyer of any such obligations, Seller shall, in addition to any and all other rights and remedies that may be available to Seller at law, at equity or otherwise in respect of such breach, be entitled to equitable relief, including a temporary restraining order, an injunction, specific performance and any other relief that may be available from a court of competent jurisdiction, without any requirement to post a bond or other security, and without any requirement to prove actual damages or that monetary damages will not afford an adequate remedy. Buyer agrees that Buyer will not oppose or otherwise challenge the appropriateness of equitable relief or the entry by a court of competent jurisdiction of an order granting equitable relief, in either case, consistent with the terms of this Section.
22.12Assignment. Buyer may not assign any of its rights or delegate any of its obligations under this Agreement without the prior written consent of Seller. Seller may assign any of its rights or delegate any of its obligations to any Person acquiring all or substantially all of Seller's assets. Any purported assignment or delegation in violation of this Section is null and void. No assignment or delegation relieves the assigning or delegating Party of any of its obligations under this Agreement.
22.13Successors and Assigns. This Agreement is binding on and inures to the benefit of the Parties and their respective permitted successors and permitted assigns.
22.14No Third-Party Beneficiaries. This Agreement benefits solely the parties to this Agreement and their respective permitted successors and permitted assigns, and nothing in this Agreement, express or implied, confers on any other Person any legal or equitable right, benefit or remedy of any nature whatsoever under or by reason of this Agreement.
22.15Dispute Resolution. This Agreement is subject to the dispute resolution provisions of the Distribution Agreement which are herein incorporated by reference.
22.16Governing Law. This Agreement, including all exhibits, schedules, attachments and appendices attached hereto and thereto, and all matters arising out of or relating to this Agreement, are governed by and construed in accordance with, the Laws of the State of Delaware, United States of America, without regard to the conflict of laws provisions thereof. The Parties agree that the United Nations Convention on Contracts for the International Sale of Goods does not apply to this Agreement.
22.17Choice of Forum. Each Party irrevocably and unconditionally agrees that it shall not commence any action, litigation or proceeding of any kind whatsoever against the other Party in any way arising from or relating to this Agreement, including all exhibits, schedules, attachments and appendices attached hereto and thereto, and all contemplated transactions in any forum other than US District Court for the Central District of California or the courts of the State of California sitting in Los Angeles, and any appellate court from any thereof. Each Party irrevocably and unconditionally submits to the exclusive jurisdiction of such courts and agrees to bring any such action, litigation or proceeding only in such courts. Each Party agrees that a final judgment in any such action, litigation, or proceeding is conclusive and may be enforced in other jurisdictions by suit on the judgment or in any other manner provided by Law.
22.18Counterparts. This Agreement may be executed in counterparts, each of which is deemed an original, but all of which together are deemed to be one and the same agreement.
22.19Force Majeure. This Agreement is subject to the force majeure provisions of the Distribution Agreement which are herein incorporated by reference.
22.20No Public Announcements or Trademark Use. Buyer shall not:
(a)make any statement (whether oral or in writing) in any press release, external advertising, marketing, or promotion materials regarding the subject matter of this Agreement, Seller, or its business unless:
(i)it has received the express written consent of Seller, or
(ii)it is required to do so by Law or under the rules of any stock exchange to which it is subject.
(b)use Seller's Trademarks, service marks, trade names, logos, symbols or brand names, in each case, without the prior written consent of Seller.
[SIGNATURE PAGE FOLLOWS]
IN WITNESS WHEREOF, the Parties hereto have executed this Contract Manufacturing Agreement as of the date first set forth above.
|
|
|
AVITA Medical Americas, LLC |
|
By_____________________ Name: Jim Corbett Title: CEO |
|
Stedical Scientific, Inc. |
|
By_____________________ Name: Lin Sun Title: Chairman |
Schedule A
GOODS
|
|
|
|
SKU |
Product |
UOM |
QTY |
C2050500 |
PermeaDerm® C - 5" x 5" |
CT |
10 |
B1051000 |
PermeaDerm® B - 5" x 10" |
CT |
8 |
B2101500 |
PermeaDerm® B - 10" x 15" |
CT |
3 |
B4153000 |
PermeaDerm® B -15" x 30" |
CT |
1 |
G2S00000 |
PermeaDerm® Glove S |
CT |
2 |
G3M00000 |
PermeaDerm® Glove M |
CT |
2 |
G4L10000 |
PermeaDerm® Glove L |
CT |
2 |
G5XL0000 |
PermeaDerm® Glove XL |
CT |
2 |
G6XXL000 |
PermeaDerm® Glove XXL |
CT |
2 |
Exhibit 10.1

SCHEDULE B
Seller’s Cost
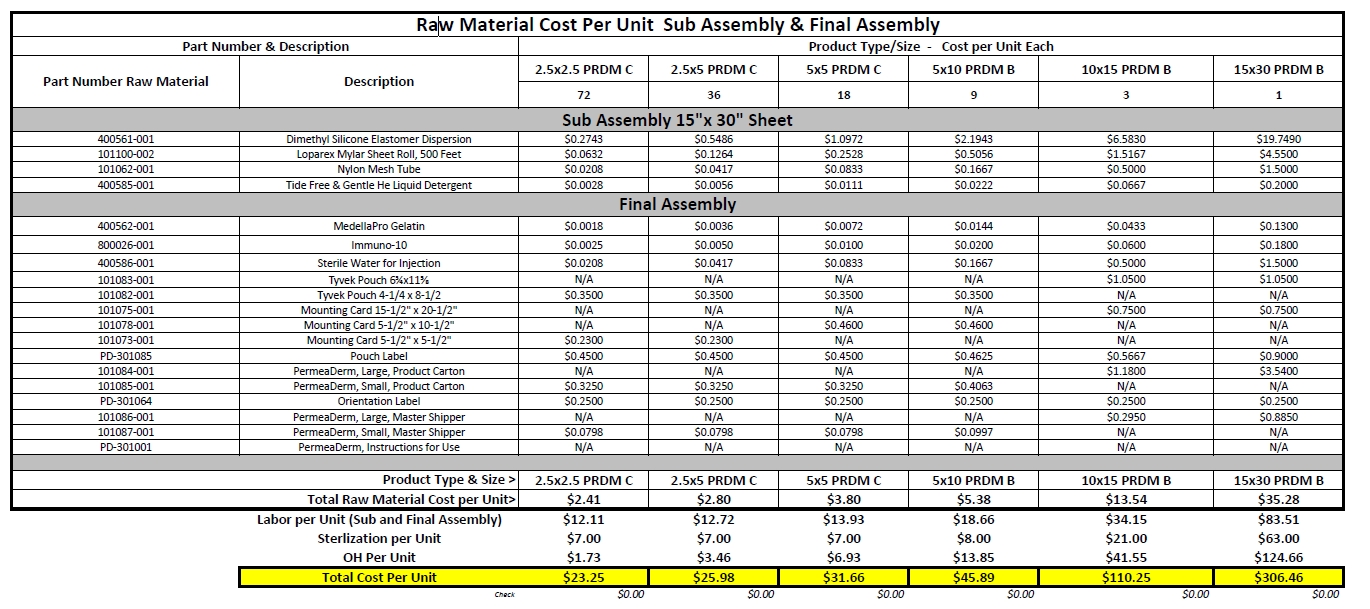
Exhibit 10.1

SCHEDULE C
Buyer-owned Tooling
|
|
Equipment |
Qty |
Large BioDot |
1 |
Size B slitter |
1 |
Size C slitter |
1 |
Tools |
Qty |
Layering Tool |
2 |
Layering Frame |
24 |
Die Cut Tool SM |
1 |
Die Cut Tool M |
1 |
Die Cut Tool L |
1 |
Die Cut Tool XL |
1 |
Die Cut Tool XXL |
1 |
Exhibit 10.2

Amendment Two to the Exclusive Distribution Agreement
This Amendment Two (this “Amendment Two”), is effective as of March 17, 2025 (the “Effective Date”), and amends the Exclusive Distribution Agreement with an Effective Date of January 10, 2024, previously amended on April 30, 2024 (the “Agreement”), by and between AVITA Medical Americas, LLC (“Distributor”) and Stedical Scientific, Inc. (“Seller” or “Stedical”). Distributor and Stedical are referred to herein individually as “Party”, or collectively as “Parties.”
WHEREAS, the Parties now desire to amend the terms of the Agreement to better align with their ongoing needs.
NOW, THEREFORE, in consideration of the mutual covenants contained below, the Parties agree to amend the Agreement as follows:
1.The definition of Stedical Scientific, Inc as “(“Seller”)” is revised to “(“Seller” or “Stedical”)”
2.The first “WHEREAS” clause is deleted in its entirety and replaced with the following:
“WHEREAS, Stedical desires to have the Products (as defined in Schedule A) manufactured, distributed, and sold;
WHEREAS, Distributor intends to manufacture the Products under the terms of a separate Manufacturing Agreement with Stedical (the “Manufacturing Agreement”);”
3.Section 3.1(l) is deleted in its entirety.
4.Section 3.2(h) is deleted in its entirety.
5.Section 4 is deleted in its entirety and replaced with the following:
“4. Revenue Sharing Arrangement; Milestone Payment Opportunities; Growth Targets.
4.1 Revenue Sharing. For all Products sold by Distributor under this Agreement, Distributor shall retain 60% of the revenue from each sale and, in exchange for the Buyer IP License granted in the Manufacturing Agreement (as that term is defined in the Manufacturing Agreement), remit the remaining 40% of revenue to Stedical, less the manufacturing cost and overhead allocation for Seller Distributed Goods (as that term is defined in the Manufacturing Agreement).
4.2 Payment Terms. Distributor shall pay all amounts due to Stedical on a monthly basis. Distributor shall make all payments in USD by wire transfer or automated clearing house. Stedical’s bank wire information is provided in Schedule A.
4.3 Milestone Payment Opportunities. During the Term and any Renewal Term, Stedical will have the opportunity to earn certain performance-based payments (each a “Milestone Payment”) on following terms and conditions:
(a) If Distributor achieves total sales of the Products in the Territory greater than $45,000,000, then Distributor shall pay to Stedical a milestone payment of $1,000,000 (equal to 2% of the first $45,000,000 in gross sales of the Products) within ninety days of the
end of the month in which the achievement is accomplished (the “First Milestone Payment”).
(b) If Distributor achieves total sales of the Products in the Territory greater than $90,000,000, then Distributor shall pay to Stedical a milestone payment of $1,500,000 (equal to 3% of the second $45,000,000 in gross sales of the Products) within ninety days of the end of the month in which the achievement is accomplished (the “Second Milestone Payment”)
(c) If Distributor achieves the First and Second Milestone Payments, then Distributor shall pay to Stedical, on a quarterly basis, an additional Milestone Payment equal to 2.5% of all amounts that are paid to Stedical under Section 4.1 of this Agreement after the payment of the First and Second Milestone Payments.”
4.4 Growth Minimums and Goals. Distributor shall commit to certain annual revenue minimums for sale of Products under this Agreement. For 2025, Distributor shall achieve $6,000,000 in gross sales of Products (the “Initial Growth Minimum”). For every year thereafter, Distributor shall achieve a minimum 20% increase in revenue from sale of the Products (“Annual Growth Minimum(s)”). Additionally, and also for every year thereafter, Distributor and Stedical shall negotiate in good faith to agree upon an annual revenue goal for sale of Products under this Agreement that is reasonably tied to Distributor’s annual revenue growth rate, excluding revenue from Products (each, a “Growth Goal”). Distributor may fail to meet a single Initial Growth Minimum or Annual Growth Minimum (each a “Growth Minimum”) without any effect. In the event that Distributor fails to achieve a Growth Minimum for two subsequent years, Distributor shall be able to cure such failure by making a cash payment equal to the difference between the revenue that would have been remitted to Stedical under Section 4.1 if Distributor had achieved such Growth Minimums and the revenue actually remitted to Stedical over that same time period.
6.Sections 6-7 are each re-titled “RESERVED” and are otherwise deleted in their entirety.
7.Section 9.1 is deleted in its entirety and replaced with the following:
“9.1 Term. The term of this Agreement, as amended, commences on the Effective Date of Amendment Two and terminates on the tenth anniversary of that date, unless terminated earlier under the terms of this Agreement (the "Term"). At least thirty days before the expiration of the Term, the Parties may extend the Term by a mutual written agreement. If Distributor has successfully achieved $90,000,000 in sales in the Territory during the Term, the Agreement shall automatically renew for an additional ten-year period (a “Renewal Term”), unless terminated earlier under the terms of this Agreement.”
8.Sections 9.3 is deleted in its entirety and replaced with the following:
“9.3 Distributor Termination Rights. Distributor may terminate this Agreement by providing written notice to Stedical:
(a) if, after thirty (30) days’ written notice from Distributor and Stedical’s failure to take corrective action, Stedical sells or attempts to sell Products in the Territory;
(b) if Distributor terminates the Manufacturing Agreement due to Stedical’s or Stedical’s Affiliates’ breach or non-performance thereof;”
9. Section 9.4 is re-titled “RESERVED” and otherwise deleted in its entirety.
10.In Section 12.1 the words “the Territory” are deleted and replaced with “any relevant
11.Section 13 is re-titled “RESERVED” and otherwise deleted in its entirety.
12.Section 19 is deleted in its entirety and replaced with the following:
“19. Contingency & Read Together. This Amendment Two is contingent upon the execution and delivery of the Manufacturing Agreement. In the event that the Manufacturing Agreement (attached as Exhibit A) is not signed within two days of the execution of this Amendment Two, this Amendment Two shall be null and void. This Amendment Two shall be read and construed in conjunction with the Agreement, any previous amendments thereto, and the Manufacturing Agreement.
13.Schedule A is deleted in its entirety and replaced with the attached Schedule A.
14.Schedule B is deleted in its entirety and replaced with the attached Schedule B.
15.To the extent any terms of the Agreement conflict with the terms herein, this Amendment Two shall govern. All other terms and conditions of the Agreement will remain unchanged and in full force and effect.
16.This Amendment Two may be executed in any number of counterparts, each of which shall be deemed an original, and all of which shall together constitute one and the same instrument. To the maximum extent permitted by law or any applicable governmental authority, any document may be signed and transmitted by PDF or facsimile with the same validity as if it were an ink-signed document.
IN WITNESS WHEREOF, the Parties have caused this Amendment Two to be executed by their duly authorized officers as of the Effective Date.
|
|
AVITA Medical Americas, LLC |
Stedical Scientific, Inc. |
By: |
By: |
Name: Jim Corbett |
Name: Lin Sun |
Title: CEO |
Title: Chairman |
Date: |
Date: |
Schedule A
Products
·“Products” or “Products” means all models of Stedical’s variable porosity dressing products with the trade name PermeaDerm Biosynthetic Wound Matrix and any new releases, enhancements or modifications thereof as agreed by Distributor and Stedical from time to time.
• [SELLER BANKING INFORMATION]
|
|
Beneficiary Name |
Stedical Scientific, Inc. |
Account Number |
|
Beneficiary Address |
2888 Loker Ave E STE 319, Carlsbad, CA 92010, USA |
SWIFT CODE |
|
|
|
ACH Routing Number |
|
ABA Routing Number |
|
Receiving Bank Name |
Bank of America, N.A. |
Receiving Bank Address |
Bank of America, N.A., 222 Broadway, New York, NY 10038 (U.S. dollars) Bank of America, N.A., 555 California St, San Francisco, CA 94104 (Foreign currency) |
Schedule B
Monthly Reporting Parameters
Distributor shall provide to Stedical, beginning on the tenth business day after the one-month anniversary of the Effective Date, monthly reports that provide the following information from the previous calendar month:
•Summary report of all customer complaints reported in accordance with Section 5;
•Number of Products sold, by Products name;
•Sale price of Products on both a monthly and quarterly basis, by Products name;
•Number of hospitals purchasing;
•Number of new customers;
•Number of patients treated, by indication; (using reasonable commercial efforts to reach
a realistic estimate)
•Market intelligence of competitive activity;
•Emerging training deficits (if any);
•New physician studies involving the Products of which Distributor becomes aware; and
•Any other pertinent information related to the performance of the Agreement.
EXHIBIT A
[MANUFACTURING AGREEMENT]
Exhibit 99.1

AVITA Medical Announces Exclusive Manufacturing and Distribution Agreements with Stedical Scientific
VALENCIA, Calif., March 17, 2025 (GLOBE NEWSWIRE) — AVITA Medical, Inc. (NASDAQ: RCEL, ASX: AVH), a leading therapeutic acute wound care company delivering transformative solutions, today announced it has entered into a new Contract Manufacturing Agreement for PermeaDerm® Biosynthetic Wound Matrix, along with an Amendment to its existing Exclusive Distribution Agreement with Stedical Scientific, Inc. These agreements further strengthen the strategic relationship between the two companies to expand the reach and availability of PermeaDerm.
Under the terms of the new Contract Manufacturing Agreement, effective as of March 17, 2025, AVITA Medical is to manufacture PermeaDerm at its state-of-the-art manufacturing facility in Ventura, California. This agreement ensures the continued availability of this innovative transparent biosynthetic wound matrix while optimizing AVITA Medical’s production capabilities to meet growing market demand. AVITA Medical will utilize its existing infrastructure to support the further commercialization of PermeaDerm in the U.S. while streamlining production, increasing scale, and optimizing manufacturing cost efficiencies to drive greater value.
The Exclusive Distribution Agreement originally executed in January 2024 has been amended to align with the new manufacturing arrangement. This revised agreement modifies revenue-sharing terms, establishes performance-based milestones, and extends the contract term. Under these new terms, AVITA Medical will retain 60% of the average sales price from PermeaDerm sales while remitting 40% to Stedical after deducting manufacturing costs. Prior to the amendment, each party retained 50% of the average sales price from the sale of PermeaDerm.
“These strategic agreements reflect our shared commitment to driving innovation and expanding market access, while ensuring that patients continue to receive first-in-class care,” said Jim Corbett, CEO of AVITA Medical. "We look forward to leveraging our expertise to continue to drive innovation and growth in therapeutic acute wound care."
Lin Sun, Chairman of Stedical Scientific, added, "PermeaDerm has demonstrated great healing results when used for various wounds. We are excited to expand our collaboration with AVITA Medical through this manufacturing agreement. This partnership ensures a robust supply chain for PermeaDerm and strengthens our commitment to delivering cutting-edge solutions to the global market."
PermeaDerm is a biosynthetic matrix that facilitates wound healing while also providing a high level of permeability and biocompatibility. PermeaDerm is cleared by the FDA for use in the treatment of a variety of wound types until healing is achieved. For partial and full-thickness injuries treated with Spray-On Skin™Cells prepared with AVITA Medical’s RECELL® devices, PermeaDerm can be applied to further support healing. Additionally, PermeaDerm is eligible for reimbursement in the U.S. across inpatient and outpatient settings, making it more accessible to healthcare providers and patients.
About AVITA Medical, Inc.
AVITA Medical® is a leading therapeutic acute wound care company delivering transformative solutions. Our technologies are designed to optimize wound healing, effectively accelerating the time to patient recovery. At the forefront of our platform is the RECELL® System, approved by the FDA for the treatment of thermal burn wounds and full-thickness skin defects. RECELL harnesses the healing properties of a patient’s own skin to create Spray-On Skin™ Cells, offering an innovative solution for improved clinical outcomes at the point-of-care. In the U.S., AVITA Medical also holds the exclusive rights to market, sell, and distribute both Cohealyx™, an AVITA Medical-branded collagen-based dermal matrix, and PermeaDerm®, a biosynthetic wound matrix.
In international markets, the RECELL System is approved to promote skin healing in a wide range of applications including burns and full-thickness skin defects. The RECELL System, excluding RECELL GO®, is TGA-registered in Australia, has received CE mark approval in Europe, and has PMDA approval in Japan.
To learn more, visit www.avitamedical.com.
About Stedical Scientific, Inc.
Stedical Scientific, Inc. is an innovative tissue engineering and regenerative medicine company that develops, manufactures and sells cutting-edge products delivered from a proprietary biosynthetic technology platform for the treatment of acute and chronic wounds, burns, as well as for plastic and reconstructive surgery. Stedical Scientific’s mission is to expand its portfolios of disruptive innovations and to serve millions of people around the globe. Stedical Scientific has brought extraordinary clinical experience to patients through its signature product, PermeaDerm, revolutionizing wound care.
Stedical Scientific’s growth strategy, in addition to marketing products in the U.S., is to expand extensively in the international market, while committed to substantially improving patient and clinician experiences through breakthrough technologies and changing the course of human health.
To learn more, visit www.stedical.com.
Forward-Looking Statements
This press release may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements are subject to significant risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements. Forward-looking statements generally may be identified by the use of words such as “anticipate,” “expect,” “intend,” “could,” “would,” “may,” “will,” “believe,” “continue,” “estimate,” “look forward,” “forecast,” “goal,” “target,” “project,” “outlook,” “guidance,” “future,” and similar words or expressions, and the use of future dates. Forward-looking statements include, but are not limited to, statements relating to the timing and realization of regulatory approvals of our products; physician acceptance, endorsement, and use of our products; failure to achieve the anticipated benefits from approval or adoption of our products; the effect of regulatory actions; product liability claims; risks associated with international operations and expansion; and risks of other business effects, including the effects of industry, as well as other economic or political conditions outside of the Company’s control. These statements are made as of the date of this release, and the Company undertakes no obligation to publicly update or revise any of these statements, except as required by law. For additional information and other important factors that
may cause actual results to differ materially from forward-looking statements, please see the “Risk Factors” section of the Company’s latest Annual Report on Form 10-K and other publicly available filings for a discussion of these and other risks and uncertainties.
Investor & Media Contact:
Jessica Ekeberg
Phone +1-661-904-9269
investor@avitamedical.com
media@avitamedical.com
Authorized for release by the Chief Financial Officer of AVITA Medical, Inc.
v3.25.1
Document And Entity Information
|
Mar. 17, 2025 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Mar. 17, 2025
|
| Entity Registrant Name |
AVITA Medical, Inc.
|
| Entity Central Index Key |
0001762303
|
| Entity Emerging Growth Company |
true
|
| Entity File Number |
001-39059
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Tax Identification Number |
85-1021707
|
| Entity Address, Address Line One |
28159 Avenue Stanford
|
| Entity Address, Address Line Two |
Suite 220
|
| Entity Address, City or Town |
Valencia
|
| Entity Address, State or Province |
CA
|
| Entity Address, Postal Zip Code |
91355
|
| City Area Code |
661
|
| Local Phone Number |
367-9170
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Ex Transition Period |
false
|
| Title of 12(b) Security |
Common Stock, par value $0.0001 per share
|
| Trading Symbol |
RCEL
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Grafico Azioni Avita Medical (NASDAQ:RCEL)
Storico
Da Feb 2025 a Mar 2025

Grafico Azioni Avita Medical (NASDAQ:RCEL)
Storico
Da Mar 2024 a Mar 2025
