AstraZeneca PLC Says Selumetinib Granted FDA Orphan Drug Status
15 Febbraio 2018 - 9:00AM
Dow Jones News
By Oliver Griffin
AstraZeneca PLC (AZN.LN) said Thursday that the U.S. Food and
Drug Administration has granted orphan drug designation for
Selumetinib, a drug used to treat incurable genetic condition
Neurofibromatosis Type 1.
The pharmaceutical company said a U.S. National Cancer
Institute-sponsored phase one and two trial is exploring the use of
Selumetinib to treat NF1 in pediatric patients. Phase two trial
results are expected later in 2018, AstraZeneca said.
The FDA's orphan-drug designation program provides orphan status
to medicines that are intended to treat fewer than 200,000 people
in the U.S. suffering from rare diseases.
AstraZeneca also said that Selumetinib is being investigated in
phase three trials of post-surgery and radiotherapy patients
diagnosed with differentiated thyroid cancer.
Write to Oliver Griffin at oliver.griffin@dowjones.com
(END) Dow Jones Newswires
February 15, 2018 02:45 ET (07:45 GMT)
Copyright (c) 2018 Dow Jones & Company, Inc.
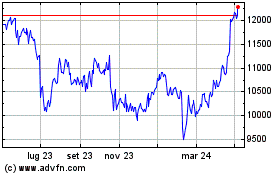
Grafico Azioni Astrazeneca (LSE:AZN)
Storico
Da Mar 2024 a Apr 2024
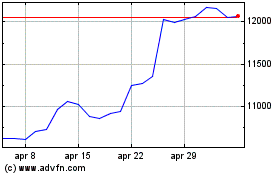
Grafico Azioni Astrazeneca (LSE:AZN)
Storico
Da Apr 2023 a Apr 2024