Saint Herblain (France), February 5,
2025 – Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a
specialty vaccine company, today announced that the Medicines and
Healthcare products Regulatory Agency (MHRA) has granted marketing
authorization in the United Kingdom (UK) for the world’s first and
only chikungunya vaccine, IXCHIQ®. The single-dose vaccine is
indicated for active immunization for the prevention of disease
caused by chikungunya virus (CHIKV) in individuals 18 years of age
and older. The vaccine is manufactured at Valneva’s leading vaccine
production site in Livingston, Scotland.
The approval is based on IXCHIQ®’s final pivotal
Phase 3 data, published in The Lancet, which included more than
4,000 participants and demonstrated that a single dose of the
live-attenuated IXCHIQ® vaccine induces a rapid and strong immune
response. Data has since shown this response can be maintained for
at least three years in both younger and older adults1.
The UK approval marks the fourth regulatory
approval Valneva has received for its single-shot chikungunya
vaccine. The vaccine is currently approved in the United States
(U.S.)2, Europe3 and Canada4 in adults 18 years of age and older.
Valneva expects to receive marketing approval in Brazil in the
first quarter of 2025, which would represent the first approval in
an endemic country. Valneva submitted label extension applications
to the U.S. Food and Drug Administration (FDA)5, the European
Medicines Agency (EMA) and Health Canada6 to potentially extend the
use of IXCHIQ® to adolescents aged 12 to 17 years. The Company now
also plans to submit a label extension application to MHRA.
Juan Carlos Jaramillo, M.D., Chief
Medical Officer of Valneva, commented, “This latest
approval is a further recognition of IXCHIQ®’s strong product
profile and the medical need for a chikungunya vaccine. Travelers
from the UK consistently rank among India’s largest tourist groups
given cultural ties and shared history. With the current outbreak
in India, it is critical to ensure UK travelers have access to this
vaccine, not only for their protection when travelling to India or
other chikungunya endemic countries but also to prevent potential
chikungunya transmission when returning to the UK. Considering the
significant risk chikungunya poses to individuals living in or
traveling to endemic areas, it's crucial to ensure that the vaccine
is accessible to people of all ages and capable of potentially
offering long-term protection from a single shot.”
In 2023, 920,000 UK travellers visited India7,
which recorded the second highest number of chikungunya cases
worldwide over five years, with 370,000 cases reported between
January 2019 and July 20248. The number of cases in India is
rapidly increasing due to the current outbreak in the Indian states
of Maharashtra and Telangana, for which the U.S. Centers for
Disease Control and Prevention (CDC) issued a travel notice after
identifying higher-than-expected numbers of chikungunya cases in
returning travelers9.
Dr. Richard Hatchett, Chief Executive
Officer of the Coalition for Epidemic Preparedness
Innovations (CEPI), supporting late-stage
development and broader access to the vaccine,
commented, “In a warming world, mosquito-borne diseases like
chikungunya are causing more frequent and severe outbreaks around
the world, so it is vital that we keep people safe from this
debilitating illness. Today’s MHRA approval of Valneva’s IXCHIQ®
vaccine is an important step forward in protecting UK citizens
travelling to affected countries – but the fight is not over. Our
work now focusses on expanding access to vaccine doses, at an
affordable price, in those endemic regions. As a major investor in
CEPI, the UK Government is providing vital support to advance this
goal, helping to make the vaccine accessible to those in Low- and
Middle- Income Countries (LMICs) who are most at risk from the
disease, while also protecting their own population.”
Valneva entered a partnership with CEPI in
201910, with support from the European Union (EU) Horizon program,
to support late-stage development of IXCHIQ® and expand access to
the vaccine for at-risk populations in affected countries. Valneva
and CEPI expanded their partnership in the third quarter of 202411,
with support from the EU, through a $41.3 million grant to advance
broader access to the vaccine in LMICs, including outbreak-affected
countries, post-marketing studies and research to support potential
label extensions in children, adolescents and pregnant women.
Within the framework of this partnership,
Valneva recently announced the signing of an exclusive license
agreement with the Serum Institute of India (SII), the world’s
largest manufacturer of vaccines by number of doses12, enabling the
supply of the vaccine in Asia, with a commitment to priority supply
of the chikungunya vaccine at an affordable price to public health
markets in LMICs. This new agreement complements the license
agreement Valneva signed in 2021 with Instituto Butantan in Brazil
for the development, manufacturing and marketing of a local
chikungunya vaccine at an affordable price for distribution in
Latin American countries and selected LMICs affected by the
disease.
About ChikungunyaChikungunya
virus (CHIKV) is a mosquito-borne viral disease spread by the bites
of infected Aedes mosquitoes which causes fever, severe joint and
muscle pain, headache, nausea, fatigue and rash. Joint pain is
often debilitating and can persist for weeks to years13. In 2004,
the disease began to spread quickly, causing large-scale outbreaks
around the world. Since the re-emergence of the virus, CHIKV has
now been identified in over 110 countries in Asia, Africa, Europe
and the Americas14. Between 2013 and 2023, more than 3.7 million
cases were reported in the Americas15 and the economic impact is
considered to be significant. The medical and economic burden is
expected to grow with climate change as the mosquito vectors that
transmit the disease continue to spread geographically. As such,
the World Health Organization (WHO) has highlighted chikungunya as
a major public health problem.16
About Valneva SEWe are a
specialty vaccine company that develops, manufactures, and
commercializes prophylactic vaccines for infectious diseases
addressing unmet medical needs. We take a highly specialized and
targeted approach, applying our deep expertise across multiple
vaccine modalities, focused on providing either first-, best- or
only-in-class vaccine solutions.We have a strong track record,
having advanced multiple vaccines from early R&D to approvals,
and currently market three proprietary travel vaccines, including
the world’s first and only chikungunya vaccine, as well as certain
third-party vaccines.Revenues from our growing commercial business
help fuel the continued advancement of our vaccine pipeline. This
includes the only Lyme disease vaccine candidate in advanced
clinical development, which is partnered with Pfizer, the world’s
most clinically advanced Shigella vaccine candidate, as well as
vaccine candidates against the Zika virus and other global public
health threats. More information is available at
www.valneva.com.
About CEPICEPI was launched in
2017 as an innovative partnership between public, private,
philanthropic and civil organizations. Its mission is to accelerate
the development of vaccines and other biologic countermeasures
against epidemic and pandemic threats so they can be accessible to
all people in need. CEPI has supported the development of more than
50 vaccine candidates or platform technologies against multiple
known high-risk pathogens or a future Disease X. Central to CEPI’s
pandemic-beating five-year plan for 2022-2026 is the ‘100 Days
Mission’ to compress the time taken to develop safe, effective,
globally accessible vaccines against new threats to just 100
days.Learn more at CEPI.net. Follow us on X (@CEPIvaccines),
LinkedIn and Facebook.
About Horizon EuropeHorizon
Europe — #HorizonEU — is the European Union's flagship
Research and Innovation programme, part of the EU-long-term
Multiannual Financial Framework (MFF) with a budget of €95,5
billion to spend over a seven-year period
(2021-2027). Under Horizon Europe, health research will
be supported with the aim to find new ways to keep people healthy,
prevent diseases, develop better diagnostics and more effective
therapies, use personalised medicine approaches to improve
healthcare and wellbeing, and take up innovative health
technologies, such as digital ones.
| Valneva
Investor and Media ContactsLaetitia Bachelot-FontaineVP
Global Communications & European Investor RelationsM +33 (0)6
4516
7099laetitia.bachelot-fontaine@valneva.com |
Joshua Drumm,
Ph.D.VP Global Investor RelationsM +001 917 815
4520joshua.drumm@valneva.com |
CEPI Media Contactpress@cepi.net +44 7387
055214
Forward-Looking Statements
This press release contains certain
forward-looking statements relating to the business of Valneva,
including with respect to the progress, timing, results and
completion of research, development and clinical trials for product
candidates, to regulatory approval of product candidates and review
of existing products. In addition, even if the actual results or
development of Valneva are consistent with the forward-looking
statements contained in this press release, those results or
developments of Valneva may not be sustained in the future. In some
cases, you can identify forward-looking statements by words such as
“could,” “should,” “may,” “expects,” “anticipates,” “believes,”
“intends,” “estimates,” “aims,” “targets,” or similar words. These
forward-looking statements are based largely on the current
expectations of Valneva as of the date of this press release and
are subject to a number of known and unknown risks and
uncertainties and other factors that may cause actual results,
performance or achievements to be materially different from any
future results, performance or achievement expressed or implied by
these forward-looking statements. In particular, the expectations
of Valneva could be affected by, among other things, uncertainties
and delays involved in the development and manufacture of vaccines,
unexpected clinical trial results, unexpected regulatory actions or
delays, competition in general, currency fluctuations, the impact
of the global and European credit crisis, and the ability to obtain
or maintain patent or other proprietary intellectual property
protection. Success in preclinical studies or earlier clinical
trials may not be indicative of results in future clinical trials.
In light of these risks and uncertainties, there can be no
assurance that the forward-looking statements made in this press
release will in fact be realized. Valneva is providing this
information as of the date of this press release and disclaims any
intention or obligation to publicly update or revise any
forward-looking statements, whether as a result of new information,
future events, or otherwise.
1 Valneva Reports Positive Three-Year Antibody Persistence Data
for its Single-Shot Chikungunya Vaccine IXCHIQ® - Valneva2 Valneva
Announces U.S. FDA Approval of World’s First Chikungunya Vaccine,
IXCHIQ® - Valneva3 Valneva Receives Marketing Authorization in
Europe for the World’s First Chikungunya Vaccine, IXCHIQ® -
Valneva4 Valneva Announces Health Canada Approval of the World’s
First Chikungunya Vaccine, IXCHIQ® - Valneva5 Valneva Submits Label
Extension Application for its Chikungunya Vaccine, IXCHIQ®, to the
U.S. FDA - Valneva6 Valneva Submits Label Extension Applications
for its Chikungunya Vaccine, IXCHIQ®, to EMA and Health Canada -
Valneva7 United Kingdom Becomes Third Largest Tourism Source Market
for India - Travel And Tour World8
https://bluedot.global/vaccines-on-the-table-as-chikungunya-outbreak-intensifies-in-india/9
https://wwwnc.cdc.gov/travel/notices/level2/chikungunya-telangana-india10
CEPI awards up to US$23.4 million to Valneva for late-stage
development of a single-dose Chikungunya vaccine | CEPI11 CEPI
Expands Partnership with Valneva with a $41.3 Million Grant to
Support Broader Access to the World’s First Chikungunya Vaccine -
Valneva12 Valneva Successfully Expands Access to Asia for its
Chikungunya Vaccine with Serum Institute of India - Valneva13
https://jvi.asm.org/content/jvi/88/20/11644.full.pdf 14
https://cmr.asm.org/content/31/1/e00104-1615 PAHO/WHO data: Number
of reported cases of chikungunya fever in the Americas (Cumulative
Cases 2018-2023 and Cases per year 2013-2017).
https://www.paho.org/data/index.php/en/mnu-topics/chikv-en/550-chikv-weekly-en.html.
Last accessed 01 Aug 2023.16 Geographical expansion of cases of
dengue and chikungunya beyond the historical areas of transmission
in the Region of the Americas (who.int)
- 2025_02_05_IXCHIQ_MHRA_Approval_PR_EN_Final
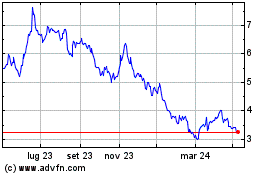
Grafico Azioni Valneva (EU:VLA)
Storico
Da Gen 2025 a Feb 2025
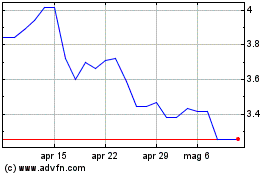
Grafico Azioni Valneva (EU:VLA)
Storico
Da Feb 2024 a Feb 2025