ABVC BioPharma and its Subsidiary Receive $460M From AiBtl BioPharma as the First Milestone Payment of Global Licensing Fees
03 Gennaio 2024 - 1:24PM

via NewMediaWire – ABVC BioPharma,
Inc. (NASDAQ: ABVC) and its subsidiary BioLite, Inc.
("Company"), a clinical-stage biopharmaceutical company developing
therapeutic solutions in ophthalmology, CNS (central nervous
systems), and oncology/hematology, announced today that the Company
and its subsidiary received an aggregate of 46 million shares from
AiBtl BioPharma Inc. ("AiBtl"), as its first milestone payment
under a global licensing agreement. The agreement between the
Company and AiBtl placed a value of $460 ($10 per share) on such
payment. AiBtl is a private company, and the share value is based
on the terms of the agreement between the parties and the valuation
report done by an independent third party.
The Company entered into a definitive global licensing agreement
with AiBtl on November 12, 2023, for the Company's CNS drugs with
the indications of MDD (Major Depressive Disorder) and ADHD
(Attention Deficit Hyperactivity Disorder). According to the terms
of the agreement, AiBtl delivered 46M shares to the Company as the
first milestone payment. The Company expects AiBtl to achieve the
further milestones under the agreement, upon which the Company may
receive the remaining licensing fees of up to $7M cash and 5%
royalties of net sales, up to
$200M. "I
am pleased with the progress of ABVC and AiBtl's collaborative
work, which reflects ABVC's strength to grow and increase cash
flow," said Uttam Patil, Ph.D., Chief Executive Officer of
ABVC. He continued, "After this licensing payment, AiBtl becomes a
subsidiary of ABVC, which holds 57% of the consolidated shares of
AiBtl; accordingly, AiBtl is now also considered a related
party.“We believe this milestone payment marks the beginning of
ABVC's revenue generation through licensing deals and opens new
avenues that could potentially increase ABVC's revenue. AiBtl will
help ABVC conduct international business development for the MDD
and ADHD markets and bridge the partnership with international
pharmaceutical companies."
"Completing out-licensing for the licensed products is our
priority to strengthen our collaboration with ABVC," said AiBtl's
Chief Executive Officer Russman Jaimes. He further commented,
"AiBtl is a US company registered in Delaware. We are working on
progressive joint ventures in Asia to expand the healthcare
business and are poised to strengthen it. With ABVC's strong
pipeline of products, we believe AiBtl has a strong future."
Management believes the Company's pipeline of products has
excellent market potential. As per the Future Market Insights
report, the MDD market was valued at $11.51 billion in 2022 and is
expected to reach $14.96 billion by 2032 with a CAGR of 2.8% over
the forecast period[1]. According to the Polaris market
research report, the global ADHD treatment market was valued at
$15.23 billion in 2022 and is expected to grow at a CAGR of 7.3%
over the forecast period between 2023-2032.[2] Straits
Research reports that the global botanical drug market size was
valued at $163 million in 2021 and is expected to be valued at $3.2
billion; the market is expected to grow at a CAGR of 39% during the
forecast period (2022–2030).[3]
About ABVC BioPharma & Its IndustryABVC BioPharma is
a clinical-stage biopharmaceutical company with an active pipeline
of six drugs and one medical device (ABV-1701/Vitargus®) under
development. For its drug products, the Company utilizes
in-licensed technology from its network of world-renowned research
institutions to conduct proof-of-concept trials through Phase II of
clinical development. The Company's network of research
institutions includes Stanford University, University of California
at San Francisco, and Cedars-Sinai Medical Center. For Vitargus®,
the Company intends to conduct global clinical trials for PMA
(pre-Market Approval).
Forward-Looking StatementsThis press release contains
"forward-looking statements." Such statements may be preceded by
the words "intends," "may," "will," "plans," "expects,"
"anticipates," "projects," "predicts," "estimates," "aims,"
"believes," "hopes," "potential," or similar words. Forward-looking
statements are not guarantees of future performance, are based on
certain assumptions, and are subject to various known and unknown
risks and uncertainties, many of which are beyond the Company's
control, and cannot be predicted or quantified, and, consequently,
actual results may differ materially from those expressed or
implied by such forward-looking statements. None of the outcomes
expressed herein are guaranteed. Such risks and uncertainties
include, without limitation, risks and uncertainties associated
with (i) our inability to manufacture our product candidates on a
commercial scale on our own, or in collaboration with third
parties; (ii) difficulties in obtaining financing on commercially
reasonable terms; (iii) changes in the size and nature of our
competition; (iv) loss of one or more key executives or scientists;
and (v) difficulties in securing regulatory approval to proceed to
the next level of the clinical trials or to market our product
candidates. More detailed information about the Company and the
risk factors that may affect the realization of forward-looking
statements is set forth in the Company's filings with the
Securities and Exchange Commission (SEC), including the Company's
Annual Report on Form 10-K and its Quarterly Reports on Form 10-Q.
Investors are urged to read these documents free of charge on the
SEC's website at http://www.sec.gov. The Company assumes no
obligation to publicly update or revise its forward-looking
statements as a result of new information, future events or
otherwise.
This press release does not constitute an offer to sell, or the
solicitation of an offer to buy any of the Company's securities,
nor shall such securities be offered or sold in the United States
absent registration or an applicable exemption from registration,
nor shall there be any offer, solicitation or sale of any of the
Company's securities in any state or jurisdiction in which such
offer, solicitation or sale would be unlawful prior to registration
or qualification under the securities laws of such state or
jurisdiction.
Contact:Leeds
ChowEmail: leedschow@ambrivis.com
[1] https://www.futuremarketinsights.com/reports/major-depressive-disorder-treatment-market#:~:text=The%20major%20depressive%20disorder%20(MDD,US%24%2011.51%20billion%20in%202022[2] https://www.polarismarketresearch.com/industry-analysis/attention-deficit-hyperactivity-disorder-market[3] https://straitsresearch.com/report/botanical-drugs-market
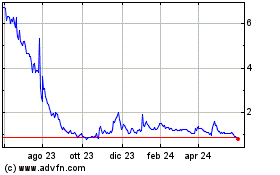
Grafico Azioni ABVC BioPharma (NASDAQ:ABVC)
Storico
Da Dic 2024 a Gen 2025
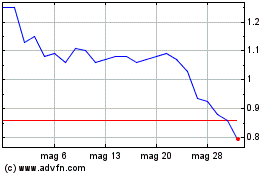
Grafico Azioni ABVC BioPharma (NASDAQ:ABVC)
Storico
Da Gen 2024 a Gen 2025