false 0001217234 0001217234 2024-10-15 2024-10-15
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported): October 15, 2024
CAREDX, INC.
(Exact Name of Registrant as Specified in its Charter)
|
|
|
|
|
| Delaware |
|
001-36536 |
|
94-3316839 |
| (State or Other Jurisdiction of Incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
8000 Marina Boulevard, 4th Floor
Brisbane, California 94005
(Address of Principal Executive Offices) (Zip Code)
(415) 287-2300
Registrant’s telephone number, including area code
N/A
(Former Name, or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Exchange Act:
|
|
|
|
|
| (Title of each class) |
|
(Trading
Symbol) |
|
(Name of exchange on which registered) |
| Common Stock, $0.001 Par Value |
|
CDNA |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 2.02 |
Results of Operations and Financial Condition. |
On October 15, 2024, CareDx, Inc. (the “Company”) issued a press release announcing its preliminary financial results for the quarter ended September 30, 2024. A copy of the press release is attached hereto as Exhibit 99.1 and is incorporated herein by reference.
The information in this Item 2.02, including the press release attached hereto as Exhibit 99.1, is intended to be furnished under Item 2.02 and Item 9.01 of Form 8-K and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that Section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing.
| Item 7.01 |
Regulation FD Disclosure. |
The Company is furnishing a corporate presentation, attached as Exhibit 99.2 to this Current Report on Form 8-K (the “Corporate Presentation”), which the Company intends to post on the Company’s website. The Corporate Presentation is current as of October 15, 2024, and the Company disclaims any obligation to update this material in the future.
The information in this Item 7.01, including the Corporate Presentation attached hereto as Exhibit 99.2, shall not be deemed “filed” for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities of that Section, nor shall it be deemed incorporated by reference in any filing under the Securities Act or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits.
- 2 -
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
| Date: October 15, 2024 |
|
|
|
CAREDX, INC. |
|
|
|
|
|
|
|
|
By: |
|
/s/ John W. Hanna |
|
|
|
|
|
|
John W. Hanna |
|
|
|
|
|
|
President and Chief Executive Officer |
- 3 -
Exhibit 99.1
CareDx Reports Preliminary Financial Results for Third Quarter 2024
Third Quarter 2024 Financial Results to be Reported on November 4, 2024
BRISBANE, Calif., October 15, 2024 — CareDx, Inc. (Nasdaq: CDNA)- The Transplant Company™
— a leading precision medicine company focused on the discovery, development, and commercialization of clinically differentiated, high-value healthcare solutions for transplant patients and caregivers —today reported preliminary financial
results for the third quarter ended September 30, 2024.
Third Quarter 2024 Highlights
| |
• |
|
Third quarter revenue is expected to be in the range of $82 million to $83 million, an increase of
approximately 23% year-over-year. |
| |
• |
|
Grew Testing Services volume to approximately 44,600, an increase of approximately 16% year-over-year.
|
| |
• |
|
Testing services revenue is expected to be in the range of $60 million to $61 million, an increase of
approximately 26% year-over-year. This includes approximately $1.2 million in revenue for tests performed in prior quarters. |
| |
• |
|
Patient and Digital Solutions revenue is expected to be approximately $11.9 million, and Products is
expected to be approximately $10.2 million, up 20% and 7% year-over-year, respectively. |
| |
• |
|
Ended the quarter with cash, cash equivalents, and marketable securities of approximately $240 million with
no debt. |
The preliminary financial information presented in this press release is based on CareDx’s current expectations and may
be adjusted as a result of, among other things, the completion of the quarterly review procedures of CareDx’s third quarter 2024 financial statements.
“We are pleased to report another quarter of growth across all our lines of business. Our performance continues to be strong as we head into the fourth
quarter,” said John W. Hanna, CareDx President and CEO.
Upcoming Earnings Event
CareDx plans to report its third quarter 2024 financial results for the period ending September 30, 2024, on November 4, 2024, after market close.
Company management will host a corresponding conference call beginning at 1:30 p.m. Pacific Time / 4:30 p.m. Eastern Time
Individuals interested in listening to the conference call may do so by dialing
1-800-343-4849 for domestic callers or 1-203-518-9783 for international callers. Please reference Conference ID: CareDx. To listen to a live webcast, please visit the investor relations section of CareDx’s website at:
investors.caredxinc.com.
About CareDx – The Transplant Company
CareDx, Inc., headquartered in Brisbane, California, is a leading precision medicine solutions company focused on the discovery, development, and
commercialization of clinically differentiated, high-value healthcare solutions for transplant patients and caregivers. CareDx offers testing services, products, and digital healthcare solutions along the pre-
and post-transplant patient journey and is the leading provider of genomics-based information for transplant patients. For more information, please visit: www.CareDx.com.
Forward Looking Statements
This press release includes
forward-looking statements, including expectations regarding CareDx’s third quarter 2024 revenue, and cash, cash equivalents, and marketable securities as of September 30, 2024, its ability to advance transplant patient care, its prospects
in 2024, and its anticipation to report third quarter 2024 financial results during its earnings call on November 4, 2024. These forward-looking statements are based upon information that is currently available to CareDx and its current
expectations, speak only as of the date hereof, and are subject to numerous risks and uncertainties that could cause actual results to differ materially from those projected, including the completion of quarterly review procedures off CareDx’s
third quarter 2024 financial statements, and its current expectations, and general economic and market factors; and other risks discussed in CareDx’s filings with the SEC, including the Annual Report on Form
10-K for the fiscal year ended December 31, 2023 filed by CareDx with the SEC on February 28, 2024, the Quarterly Report on Form 10-Q for the fiscal quarter
ended March 31, 2024 filed by CareDx with the SEC on May 9, 2024 and the Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2024 filed by CareDx with the SEC on July 31,
2024, and other reports that CareDx has filed with the SEC. Any of these may cause CareDx’s actual results, performance, or achievements to differ materially and adversely from those anticipated or implied by CareDx’s forward-looking
statements. CareDx expressly disclaims any obligation, except as required by law, or undertaking to update or revise any such forward-looking statements.
CareDx, Inc.
Media Relations
Anna Czene
818-731-2203
aczene@caredx.com
Investor Relations
Greg Chodaczek
investor@caredx.com

Exhibit 99.2 2024 INVESTOR DAY O C T O B E R 1 5 , 2 0 2 4

SAFE HARBOR STATEMENT These slides, and the accompanying oral
presentation, contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. All statements other than statements of historical fact
contained in this presentation, including statements regarding the future financial position of CareDx®, Inc. (together with its subsidiaries, “CareDx” or the “Company”), including financial targets and expectations,
business strategy, and plans and objectives for future operations, are forward-looking statements. The words believe, may, will, potentially, estimate, continue, anticipate, intend, could, should, would, project, plan, target, contemplate, predict,
expect, and the negative and plural forms of these words and similar expressions are intended to identify that CareDx has based these forward-looking statements on its own estimates and assumptions and its current expectations and projections about
future events. These forward-looking statements are subject to a number of risks, uncertainties, and assumptions, including those contained in the “Risk Factors” section of the Company’s most recent Annual Report on Form 10-K for
the fiscal year ended December 31, 2023, filed with the U.S. Securities and Exchange Commission (the SEC ) on February 28, 2024, the Quarterly Report on Form 10- Q for the fiscal quarter ended March 31, 2024, filed with the SEC on May 9, 2024 and
the Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2024, filed with the SEC on July 31, 2024. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this presentation
are inherently uncertain and may not occur, and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Accordingly, you should not rely upon forward-looking statements as predictions
of future events. CareDx undertakes no obligation to update publicly or revise any forward-looking statements for any reason after the date of this presentation or to conform these statements to actual results or to changes in CareDx’s
expectations. These slides and the accompanying oral presentation contain certain non-GAAP financial measures, which are provided to assist in an understanding of the business and performance of CareDx. These measures should always be considered
only as a supplement to, and not as superior to, financial measures prepared in accordance with GAAP. Further information regarding our non-GAAP financial measures can be found in our filings with the SEC. Certain data in this presentation was
obtained from various external sources, and neither the Company nor its affiliates, advisers or representatives has verified such data with independent sources. Accordingly, neither the Company nor any of its affiliates, advisers or representatives
makes any representations as to the accuracy or completeness of that data or undertakes any obligation to update such data after the date of this presentation. Such data involves risks and uncertainties and is subject to change based on various
factors. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the products or services of the Company. 2

Establishing CareDx as the Most Innovative Company in Diagnostics John
Hanna President and CEO

TODAY’S AGENDA John Hanna Jessica Meng President & CEO Chief
Commercial Officer 8:00-8:25 Establishing CareDx as the Most Innovative Company in Diagnostics John Hanna 8:25-8:45 Unlocking Building Blocks of Growth Jessica Meng 8:45-9:00 Securing Coverage & Adoption Through Evidence Robert Woodward
9:00-9:15 Driving Operational Leverage Keith Kennedy 9:15- 9:25 Charting a Clear Path to Profitable Growth Abhishek Jain Robert Woodward, PhD Keith Kennedy Chief Scientific Officer Chief Operating Officer 9:25-9:45 Expanding Our Footprint Beyond $8B
TAM Marica Grskovic 9:45-10:00 Activating Our Strategy John Hanna Abhishek Jain Marica Grskovic, PhD Chief Financial Officer Chief Strategy Officer 4
OBSERVATIONS OVER FIRST 100 DAYS TEAM MARKET PRODUCTS 5

T E A M TEAM PASSIONATE John Hanna Jessica Meng Abhishek Jain Keith
Kennedy ABOUT INNOVATION Chief Commercial Officer Chief Financial Officer President & CEO Chief Operating Officer & PATIENT CARE 650 180 Marica Grskovic, PhD Robert Woodward, PhD Jing Huang, PhD CareDx Employees Globally Field Employees
Supporting Chief Strategy Officer Chief Scientific Officer Chief Data and AI Officer Headquartered in South San Customers and Patients Francisco & Stockholm 110 80 Scientists & Researchers Software Programmers Stacey Follon GS Jha Jeff
Novack, JD Kashif Rathore Building the Next Innovations Building and Enhancing Our Head of Human Resources Head of Software Engineering Chief Information Officer General Counsel in Transplant Digital Solutions 6
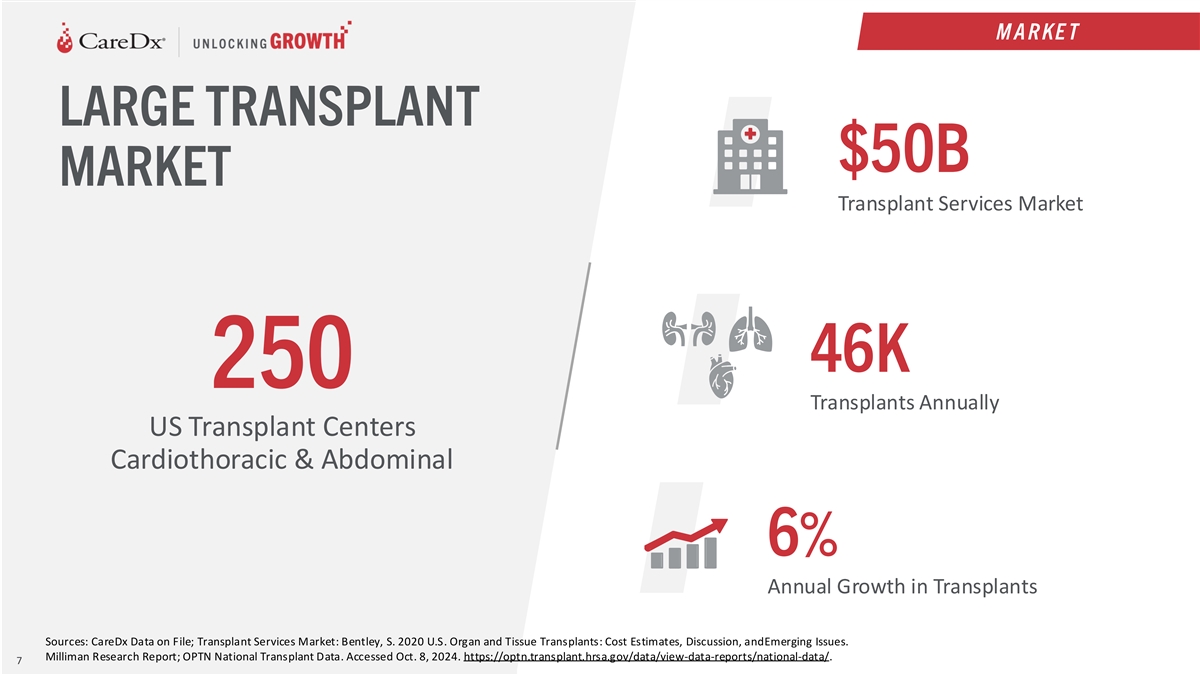
M A R KE T LARGE TRANSPLANT $50B MARKET Transplant Services Market 46K
250 Transplants Annually US Transplant Centers Cardiothoracic & Abdominal 6% Annual Growth in Transplants Sources: CareDx Data on File; Transplant Services Market: Bentley, S. 2020 U.S. Organ and Tissue Transplants: Cost Estimates, Discussion,
and Emerging Issues. Milliman Research Report; OPTN National Transplant Data. Accessed Oct. 8, 2024. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/. 7
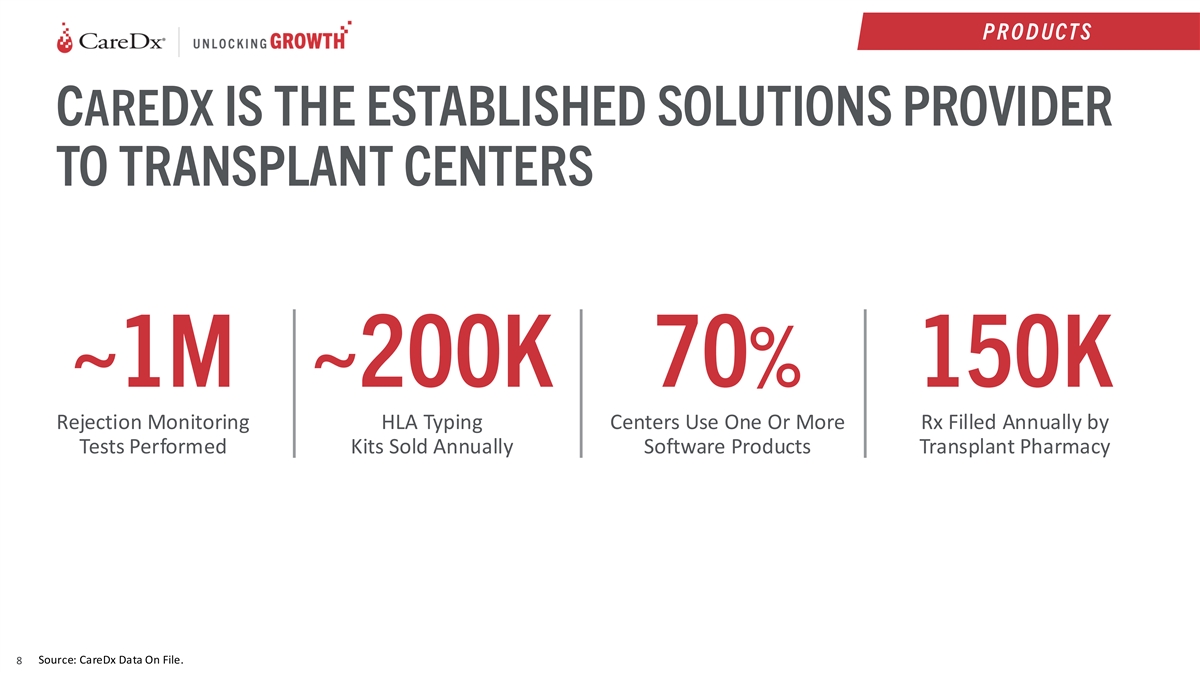
P R O D UCT S CAREDX IS THE ESTABLISHED SOLUTIONS PROVIDER TO TRANSPLANT
CENTERS ~1M ~200K 70% 150K Rejection Monitoring HLA Typing Centers Use One Or More Rx Filled Annually by Tests Performed Kits Sold Annually Software Products Transplant Pharmacy Source: CareDx Data On File. 8
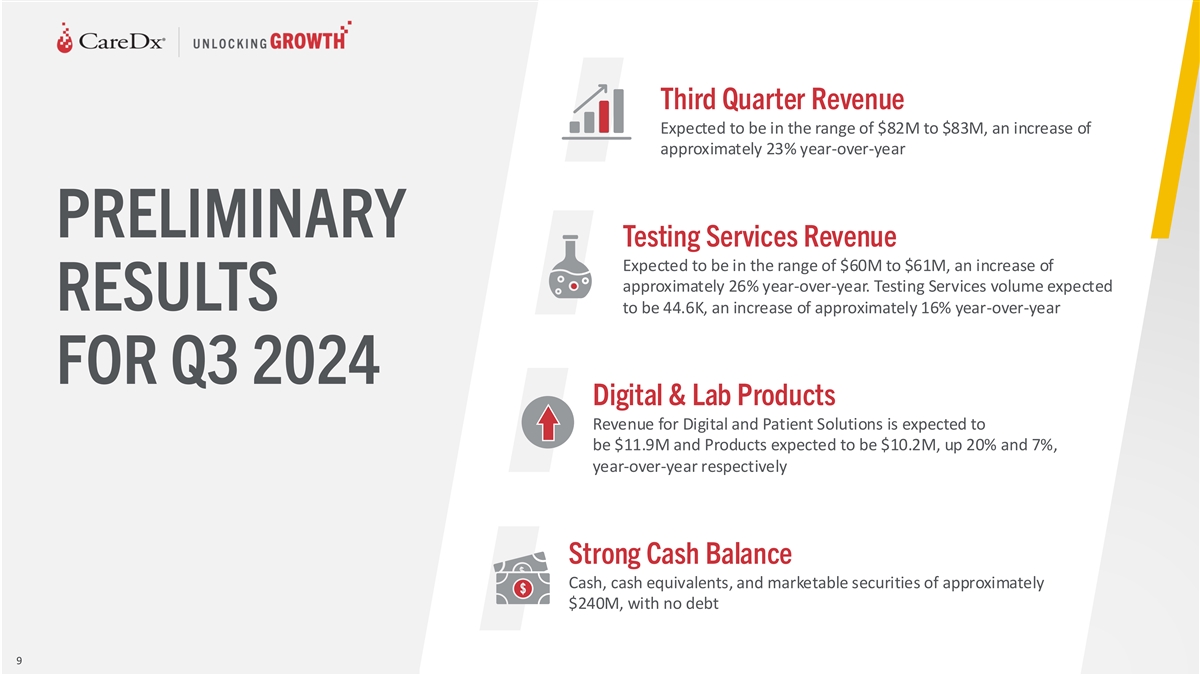
Third Quarter Revenue Expected to be in the range of $82M to $83M, an
increase of approximately 23% year-over-year PRELIMINARY Testing Services Revenue Expected to be in the range of $60M to $61M, an increase of approximately 26% year-over-year. Testing Services volume expected RESULTS to be 44.6K, an increase of
approximately 16% year-over-year FOR Q3 2024 Digital & Lab Products Revenue for Digital and Patient Solutions is expected to be $11.9M and Products expected to be $10.2M, up 20% and 7%, year-over-year respectively Strong Cash Balance Cash, cash
equivalents, and marketable securities of approximately $240M, with no debt 9
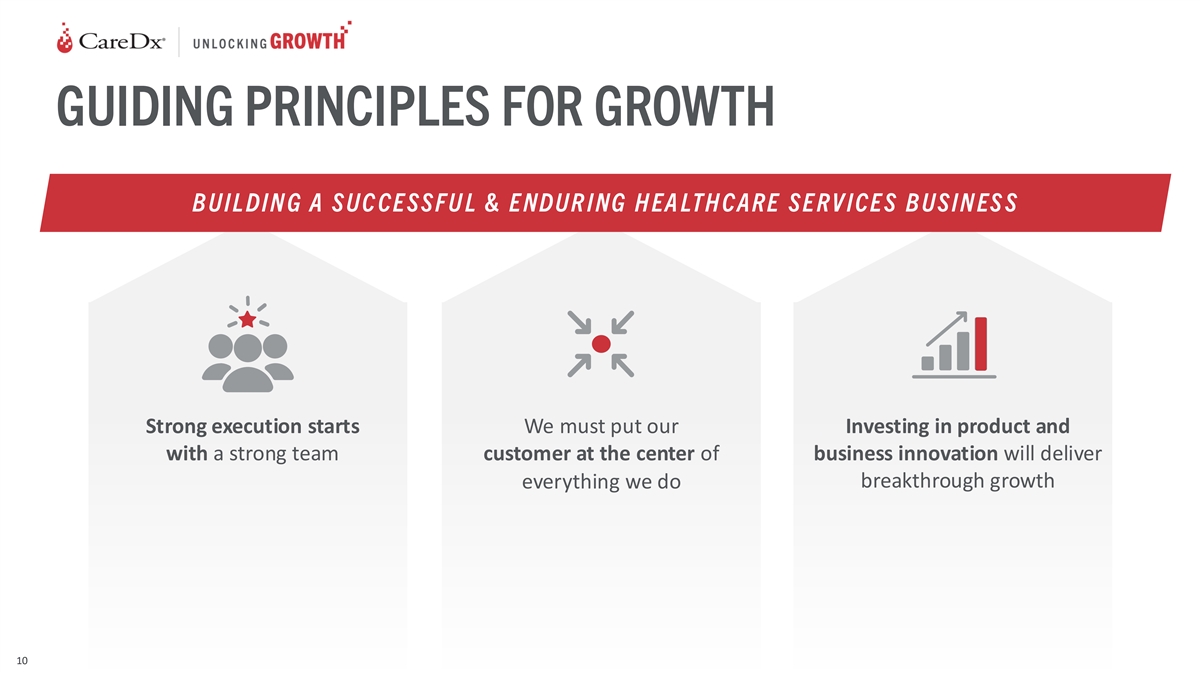
GUIDING PRINCIPLES FOR GROWTH B UIL DIN G A SUC CE SSFU L & E NDUR
ING H EA LTH CARE SE RV ICE S B USIN ES S Strong execution starts We must put our Investing in product and with a strong team customer at the center of business innovation will deliver breakthrough growth everything we do 10
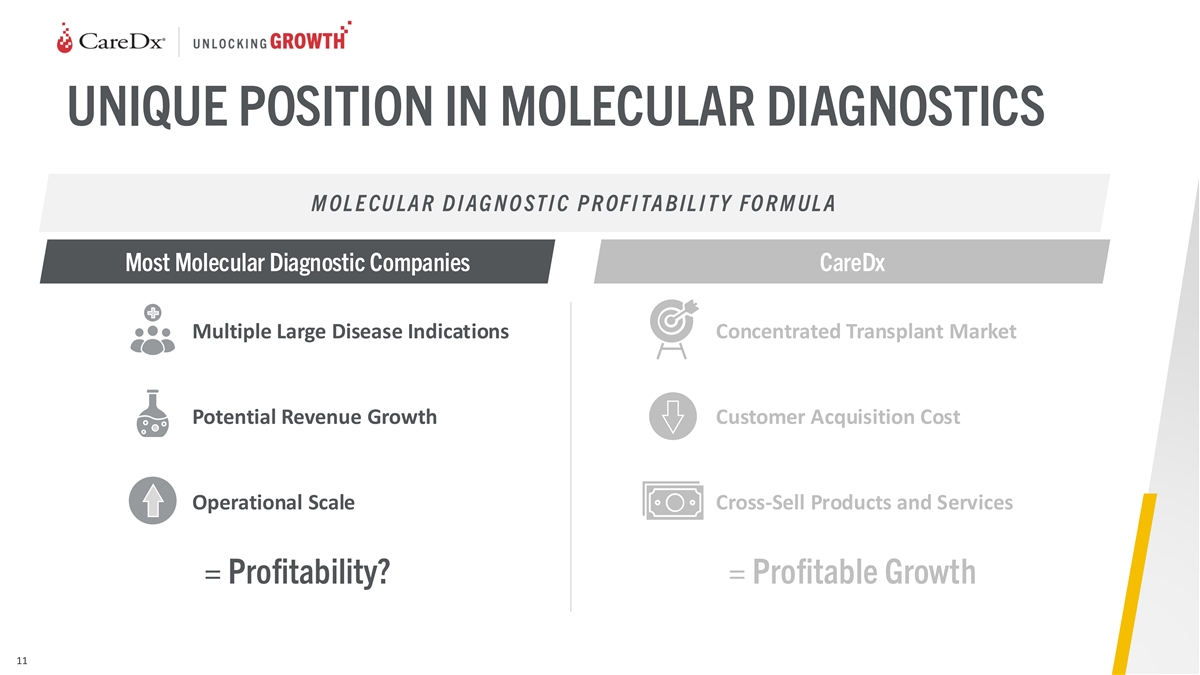
UNIQUE POSITION IN MOLECULAR DIAGNOSTICS M OL E CU LA R D I AG N O ST I
C P R OF I TA B IL I T Y FO R M UL A Most Molecular Diagnostic Companies CareDx Multiple Large Disease Indications Concentrated Transplant Market Potential Revenue Growth Customer Acquisition Cost Operational Scale Cross-Sell Products and Services =
Profitability? = Profitable Growth 11
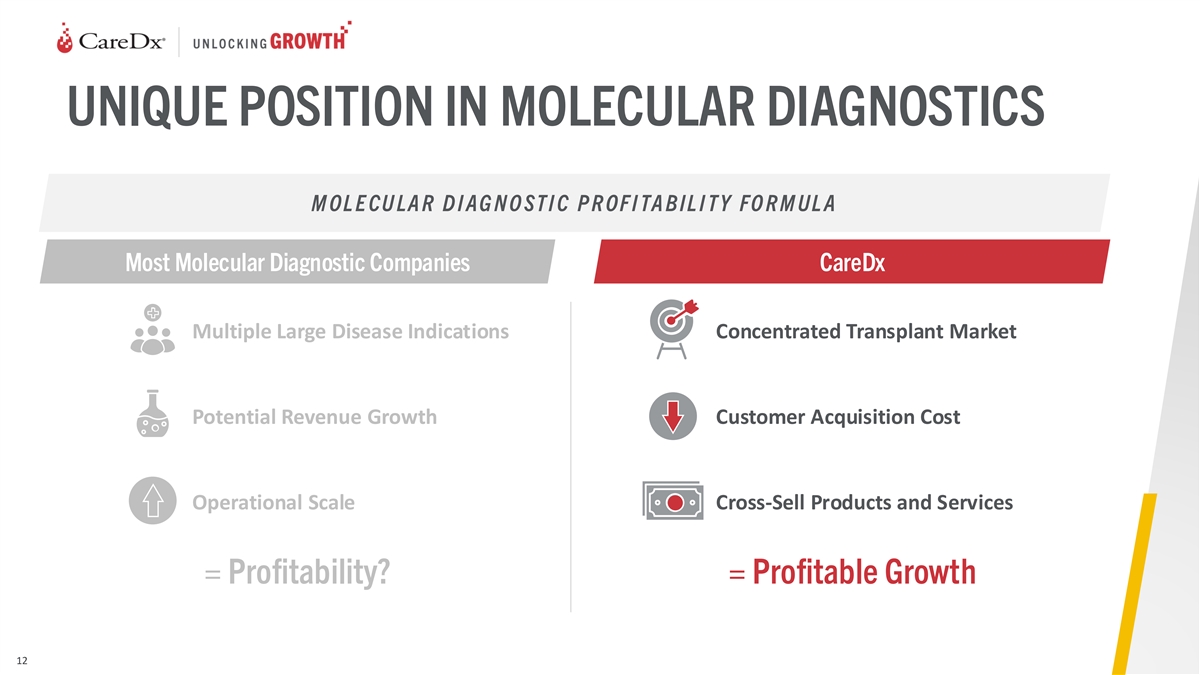
UNIQUE POSITION IN MOLECULAR DIAGNOSTICS M OL E CU LA R D I AG N O ST I
C P R OF I TA B IL I T Y FO R M UL A Most Molecular Diagnostic Companies CareDx Multiple Large Disease Indications Concentrated Transplant Market Potential Revenue Growth Customer Acquisition Cost Operational Scale Cross-Sell Products and Services =
Profitability? = Profitable Growth 12

CAREDX STRATEGIC PRIORITIES PROFITABLE GROWTH 1 OPERATIONAL EXCELLENCE
2 DEFINE TRANSPLANT+ 3 ELEVATE PERFORMANCE CULTURE 4 13

Unlocking Building Blocks of Growth Jessica Meng Chief Commercial
Officer

UNLOCKING 46K 400K 100K+ Transplants Organ Transplant Transplant Wait
List OUR PATIENT in 2023 Patients in the U.S. IMPACT 15 Sources: OPTN National Transplant Data. Accessed Oct. 8, 2024. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/.; Pullen BLC. Am J Transplant. 2023;23(1):1-2.
doi:10.1016/j.ajt.2022.12.003
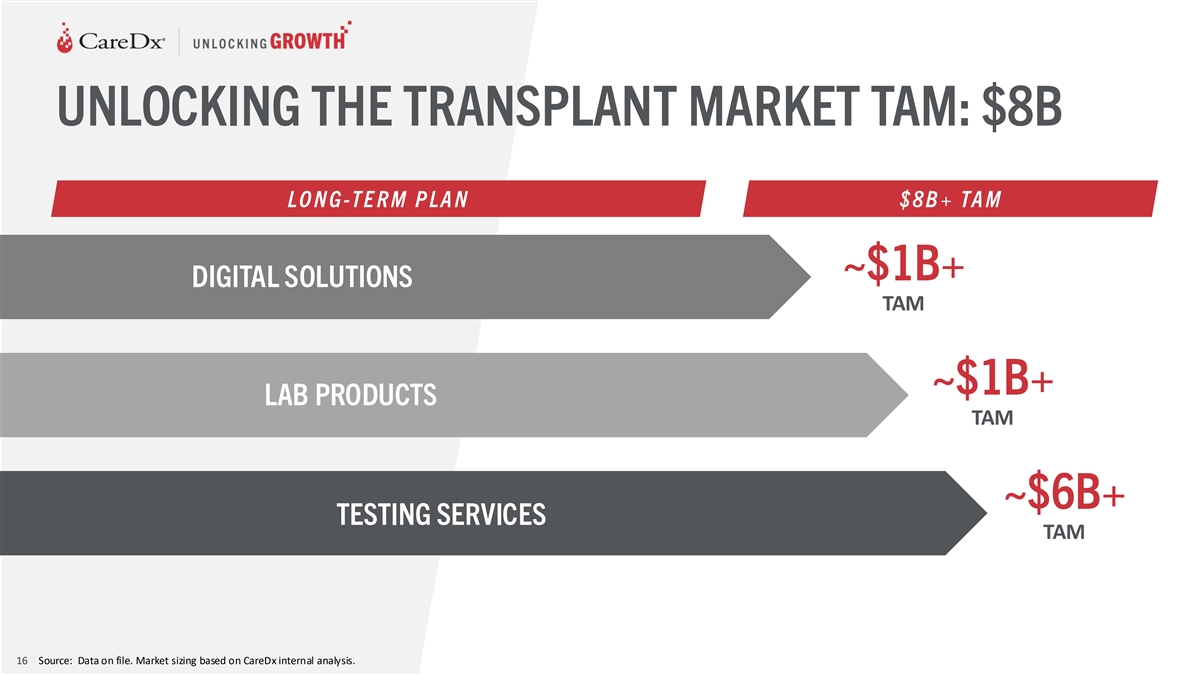
UNLOCKING THE TRANSPLANT MARKET TAM: $8B LO N G -T E R M P LA N $ 8 B +
TA M ~$1B+ DIGITAL SOLUTIONS TAM ~$1B+ LAB PRODUCTS TAM ~$6B+ TESTING SERVICES TAM 16 Source: Data on file. Market sizing based on CareDx internal analysis.
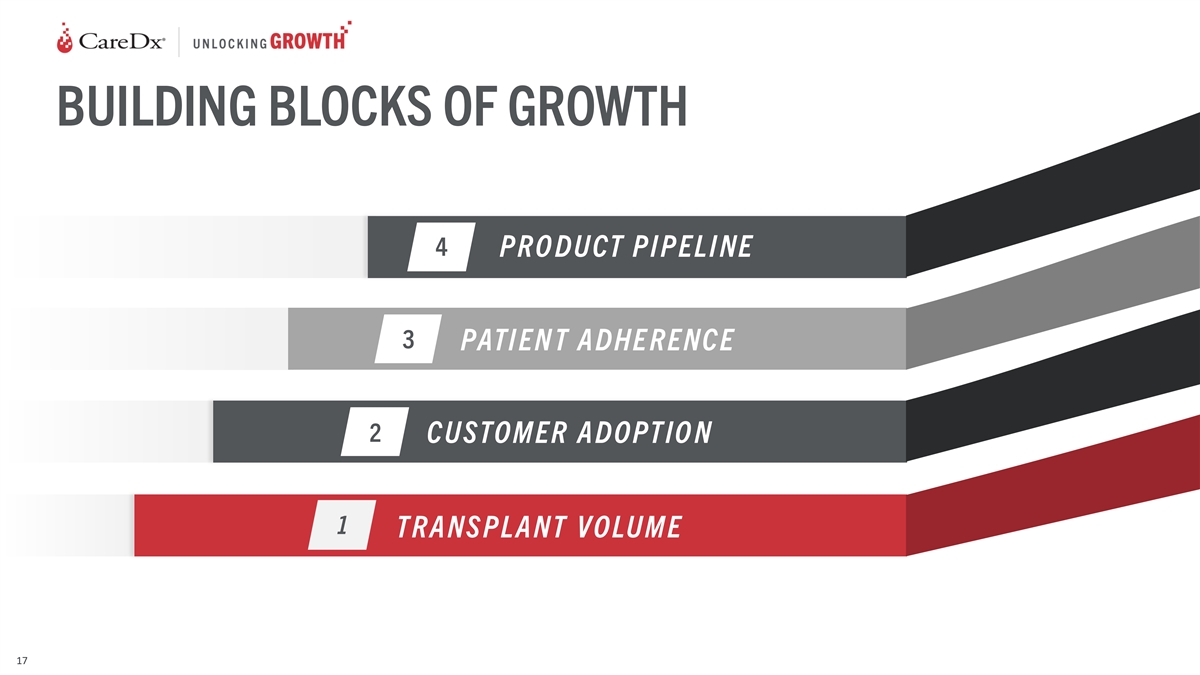
BUILDING BLOCKS OF GROWTH 4 PRO DUCT PI PELI NE 3 PATI EN T ADHE RENCE
2 CUSTOMER ADOPTIO N 1 TRANS PLANT VOLUME 17

T R A N S P L AN T V O LU ME 1 FORCES DRIVING TRANSPLANT VOLUME GROWTH
Growing government efforts Technological advances are working to expand transplantation access are improving organ viability IOTA Model Increasing Organ Transplant Access OPTN Organ Procurement and Transplantation Network 18 18
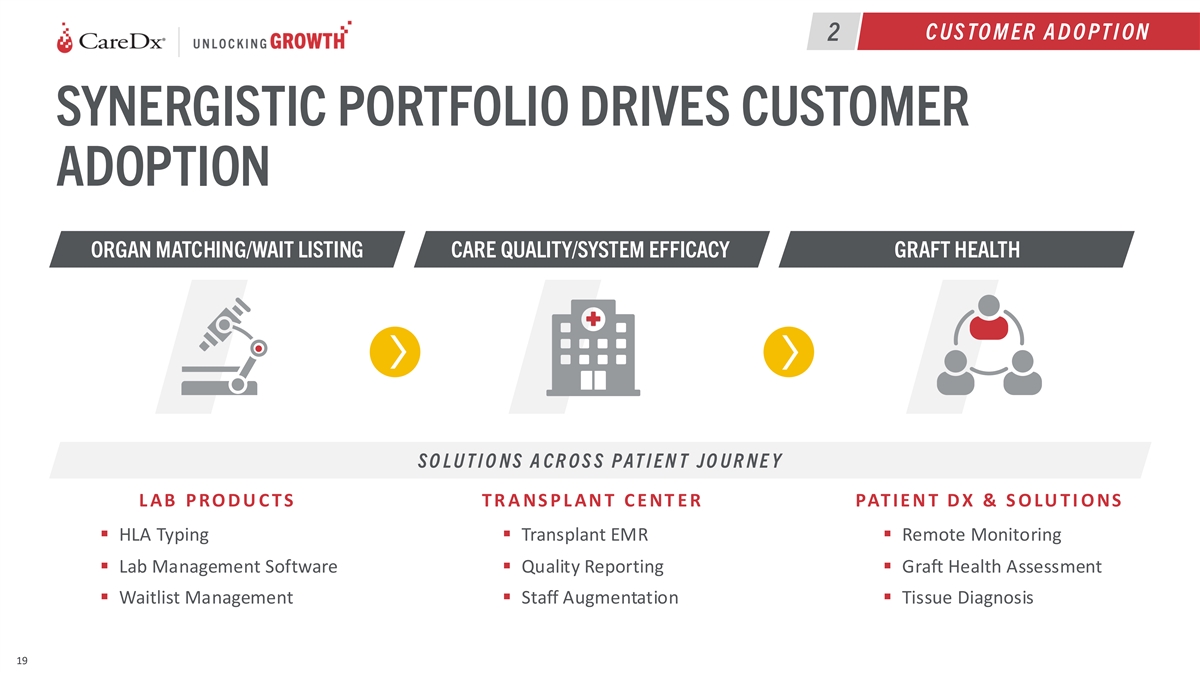
C US TO M ER A D O PT I ON 2 SYNERGISTIC PORTFOLIO DRIVES CUSTOMER
ADOPTION ORGAN MATCHING/WAIT LISTING CARE QUALITY/SYSTEM EFFICACY GRAFT HEALTH S O L U T I O N S A C R O S S P AT I E N T JO U R N E Y L AB P RO D U C T S T R A NS P L AN T C E NT E R PAT IE N T D X & S O LU T I O NS ▪ HLA Typing▪
Transplant EMR▪ Remote Monitoring ▪ Lab Management Software▪ Quality Reporting▪ Graft Health Assessment ▪ Waitlist Management ▪ Staff Augmentation▪ Tissue Diagnosis 19
C US TO M ER A D O PT I ON 2 CUSTOMER ADOPTION EXAMPLE TESTING SERVICES
LAB PRODUCTS DIGITAL SOLUTIONS E M R IN T E G R AT I O N 20
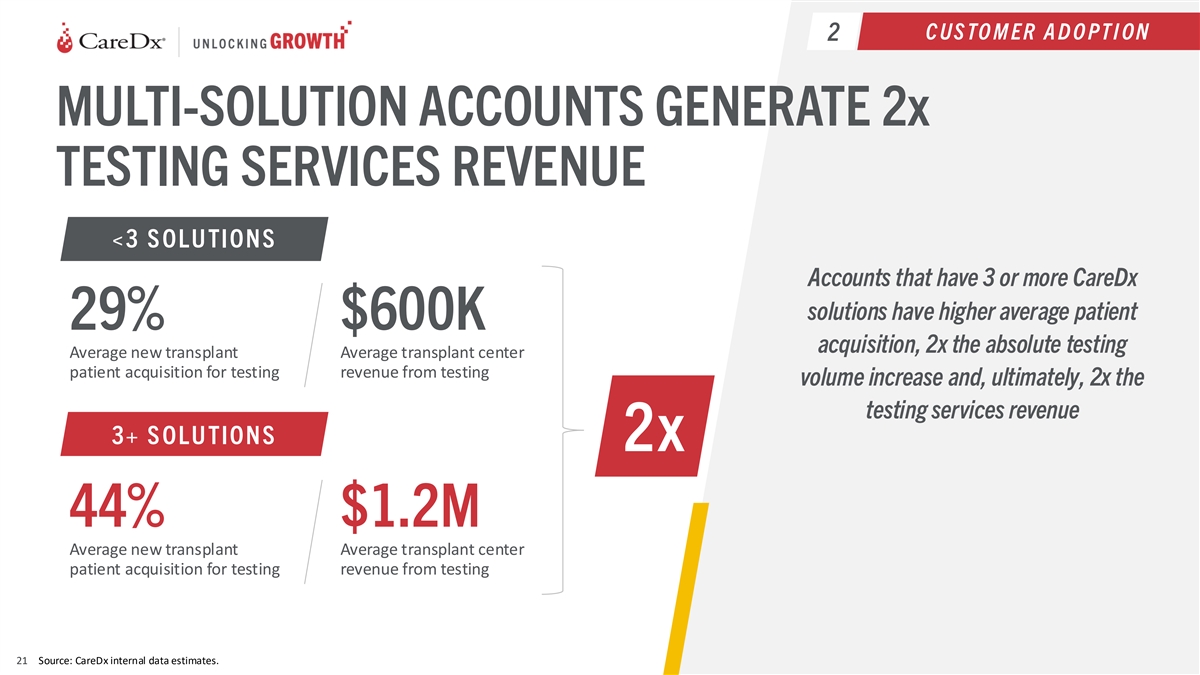
C US TO M ER A D O PT I ON 2 MULTI-SOLUTION ACCOUNTS GENERATE 2x
TESTING SERVICES REVENUE <3 S OLU TION S Accounts that have 3 or more CareDx solutions have higher average patient 29% $600K acquisition, 2x the absolute testing Average new transplant Average transplant center patient acquisition for testing
revenue from testing volume increase and, ultimately, 2x the testing services revenue 3+ S OLU TION S 2x 44% $1.2M Average new transplant Average transplant center patient acquisition for testing revenue from testing 21 Source: CareDx internal data
estimates.
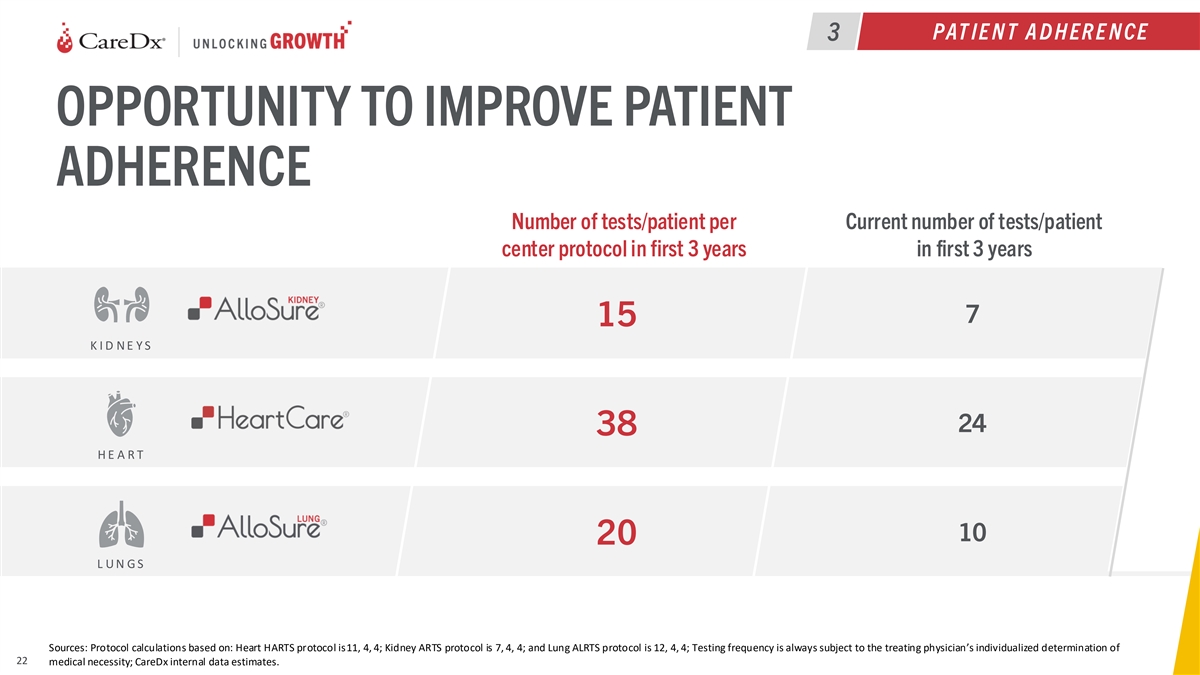
PAT IE N T A D H ER E N CE 3 OPPORTUNITY TO IMPROVE PATIENT ADHERENCE
Number of tests/patient per Current number of tests/patient center protocol in first 3 years in first 3 years 7 15 K I D N E Y S 24 38 H E A R T 10 20 L U N G S Sources: Protocol calculations based on: Heart HARTS protocol is11, 4, 4; Kidney ARTS
protocol is 7, 4, 4; and Lung ALRTS protocol is 12, 4, 4; Testing frequency is always subject to the treating physician’s individualized determination of 22 medical necessity; CareDx internal data estimates.
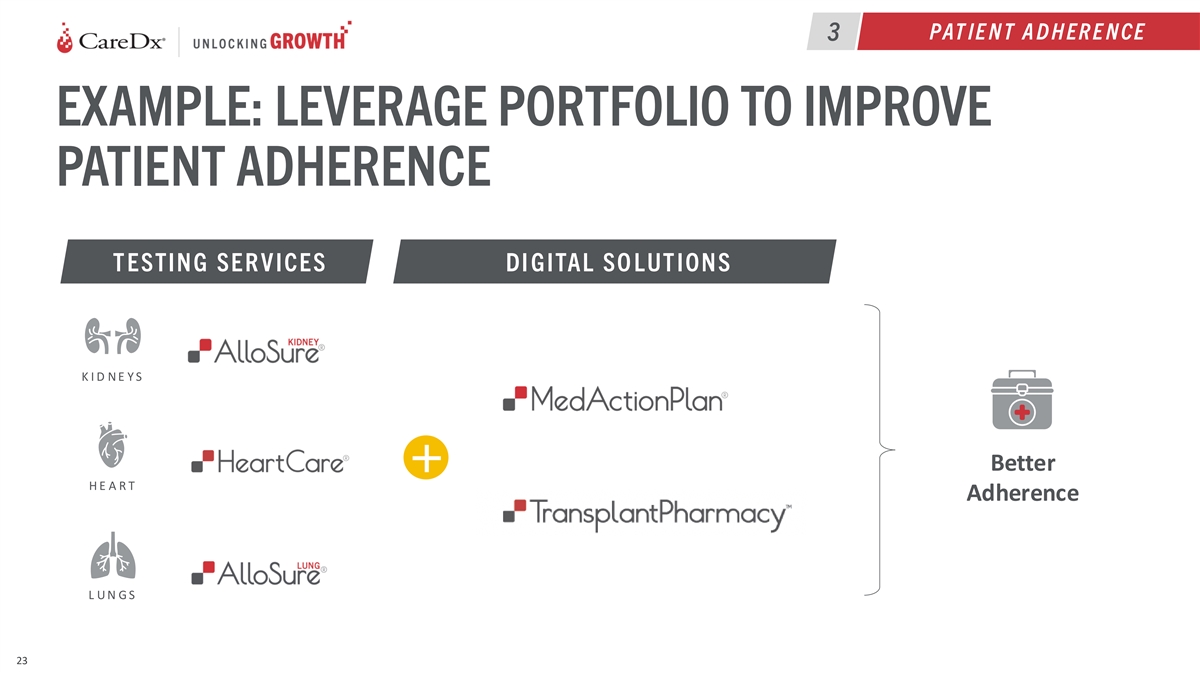
PAT IE N T A D H ER E N CE 3 EXAMPLE: LEVERAGE PORTFOLIO TO IMPROVE
PATIENT ADHERENCE T ESTIN G SER V ICES DI GITAL SO LUT ION S K I D N E Y S Better + H E A R T Adherence L U N G S 23
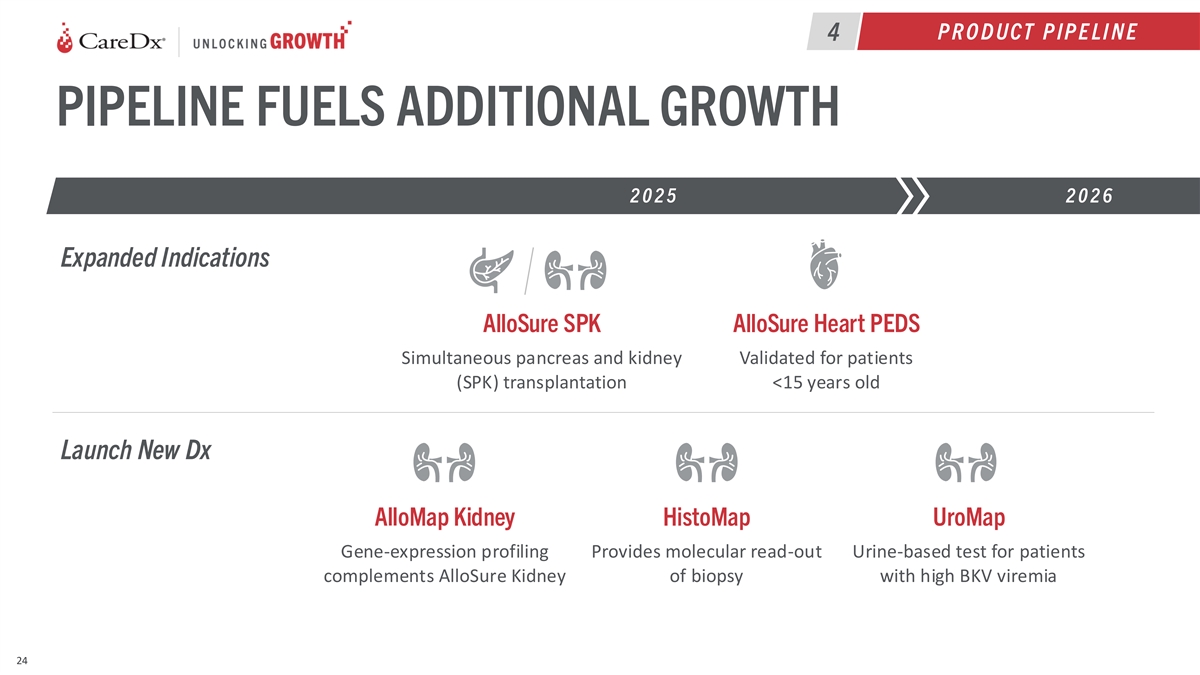
P R O D UCT P IP E LI N E 4 PIPELINE FUELS ADDITIONAL GROWTH 2025 2026
Expanded Indications AlloSure SPK AlloSure Heart PEDS Simultaneous pancreas and kidney Validated for patients (SPK) transplantation <15 years old Launch New Dx AlloMap Kidney HistoMap UroMap Gene-expression profiling Provides molecular read-out
Urine-based test for patients complements AlloSure Kidney of biopsy with high BKV viremia 24
BUILDING BLOCKS OF GROWTH 4 PRO DUCT PI PELI NE 3 PATI EN T ADH EREN CE
2 CUSTOMER ADOPTIO N 1 TRANS PLANT VOLUME 25

Securing Coverage and Adoption Through Evidence Robert Woodward, PhD
Chief Scientific Officer
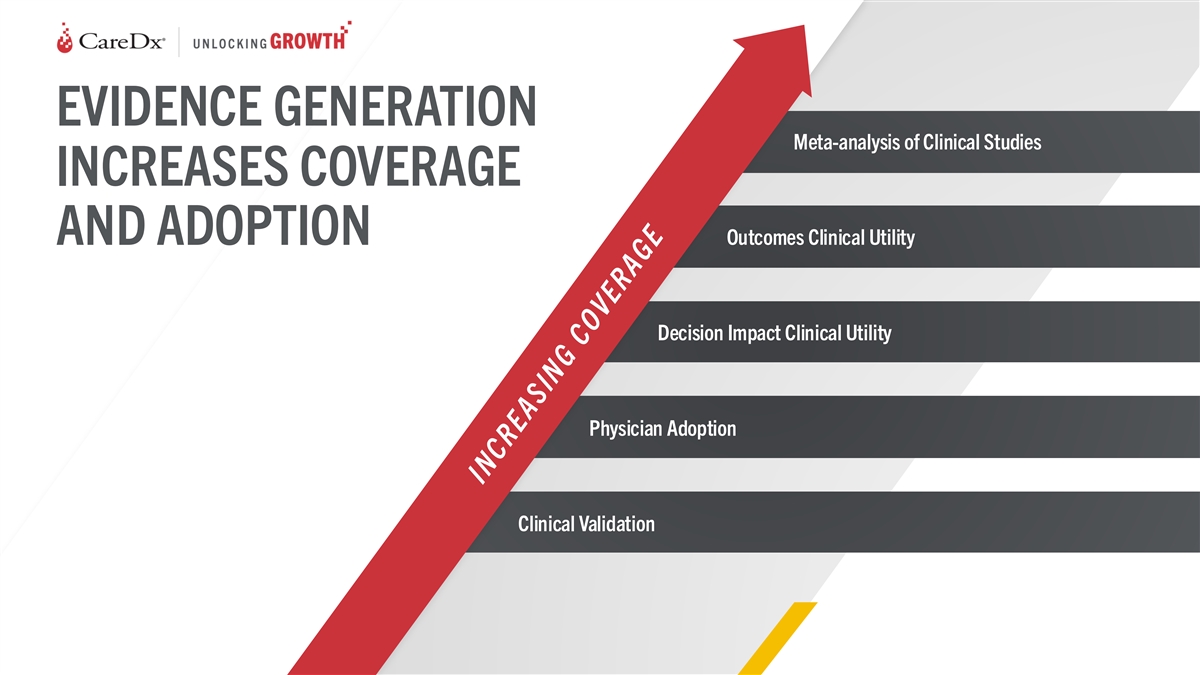
EVIDENCE GENERATION Meta-analysis of Clinical Studies INCREASES
COVERAGE AND ADOPTION Outcomes Clinical Utility Decision Impact Clinical Utility Physician Adoption Clinical Validation 27
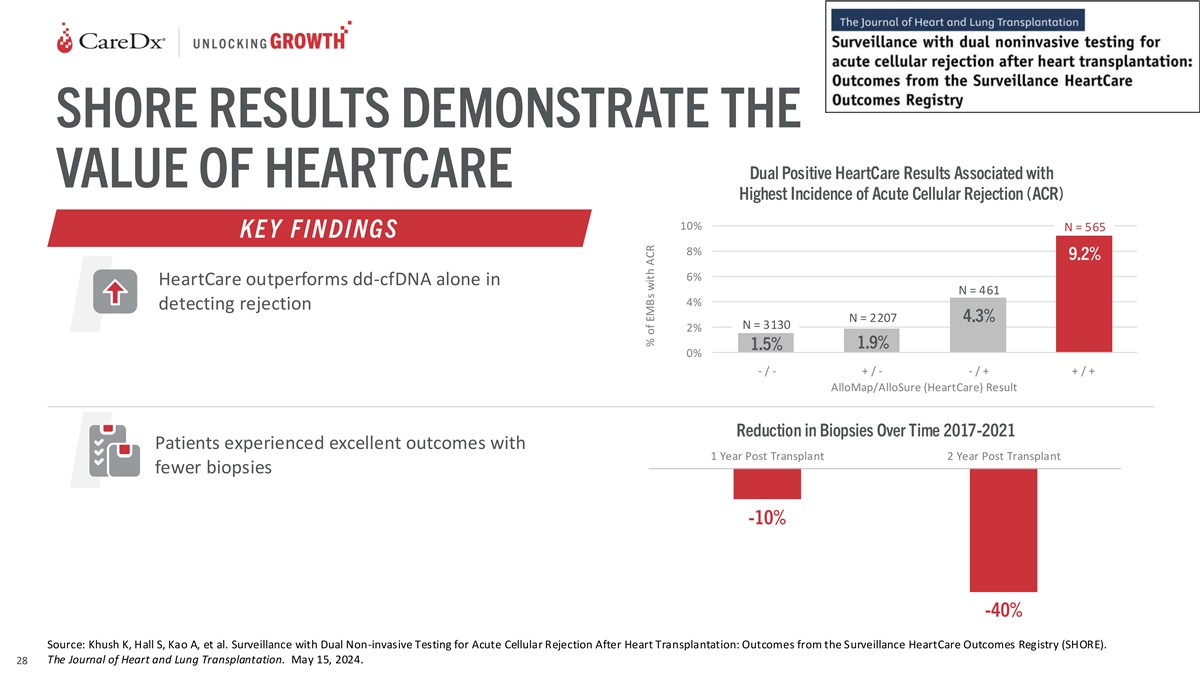
SHORE RESULTS DEMONSTRATE THE Dual Positive HeartCare Results
Associated with VALUE OF HEARTCARE Highest Incidence of Acute Cellular Rejection (ACR) 10% N = 565 KE Y FIN DINGS 8% 9.2% 6% HeartCare outperforms dd-cfDNA alone in N = 461 4% detecting rejection N = 2207 4.3% N = 3130 2% 1.9% 1.5% 0% - / - + / - -
/ + + / + `- / - `+ / - ` - /+ `+ / + AlloMap/AlloSure (HeartCare) Result Reduction in Biopsies Over Time 2017-2021 Patients experienced excellent outcomes with 1 Year Post Transplant 2 Year Post Transplant fewer biopsies -10% -40% Source: Khush K,
Hall S, Kao A, et al. Surveillance with Dual Non-invasive Testing for Acute Cellular Rejection After Heart Transplantation: Outcomes from the Surveillance HeartCare Outcomes Registry (SHORE). The Journal of Heart and Lung Transplantation. May 15,
2024. 28 % of EMBs with ACR
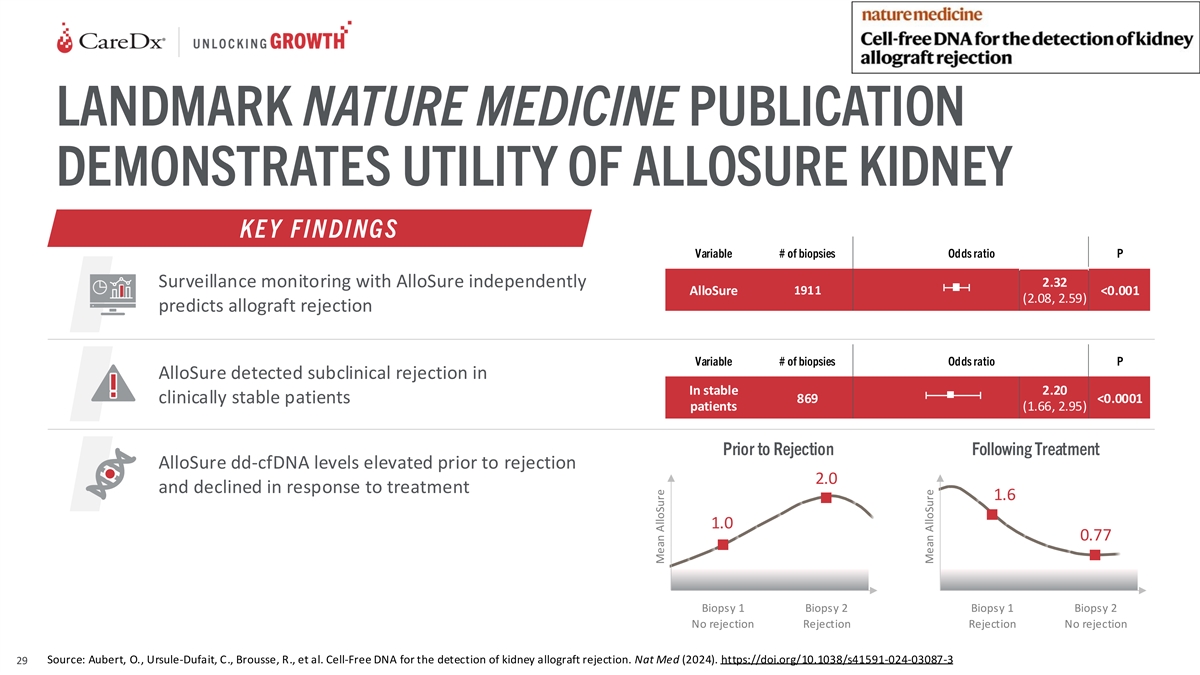
LANDMARK NATURE MEDICINE PUBLICATION DEMONSTRATES UTILITY OF ALLOSURE
KIDNEY KE Y FIN DINGS Variable # of biopsies Odds ratio P Surveillance monitoring with AlloSure independently 2.32 AlloSure 1911 <0.001 (2.08, 2.59) predicts allograft rejection Variable # of biopsies Odds ratio P AlloSure detected subclinical
rejection in In stable 2.20 clinically stable patients 869 <0.0001 patients (1.66, 2.95) Prior to Rejection Following Treatment AlloSure dd-cfDNA levels elevated prior to rejection 2.0 and declined in response to treatment 1.6 1.0 0.77 Biopsy 1
Biopsy 2 Biopsy 1 Biopsy 2 No rejection Rejection Rejection No rejection Source: Aubert, O., Ursule-Dufait, C., Brousse, R., et al. Cell-Free DNA for the detection of kidney allograft rejection. Nat Med (2024).
https://doi.org/10.1038/s41591-024-03087-3 29 Mean AlloSure Mean AlloSure
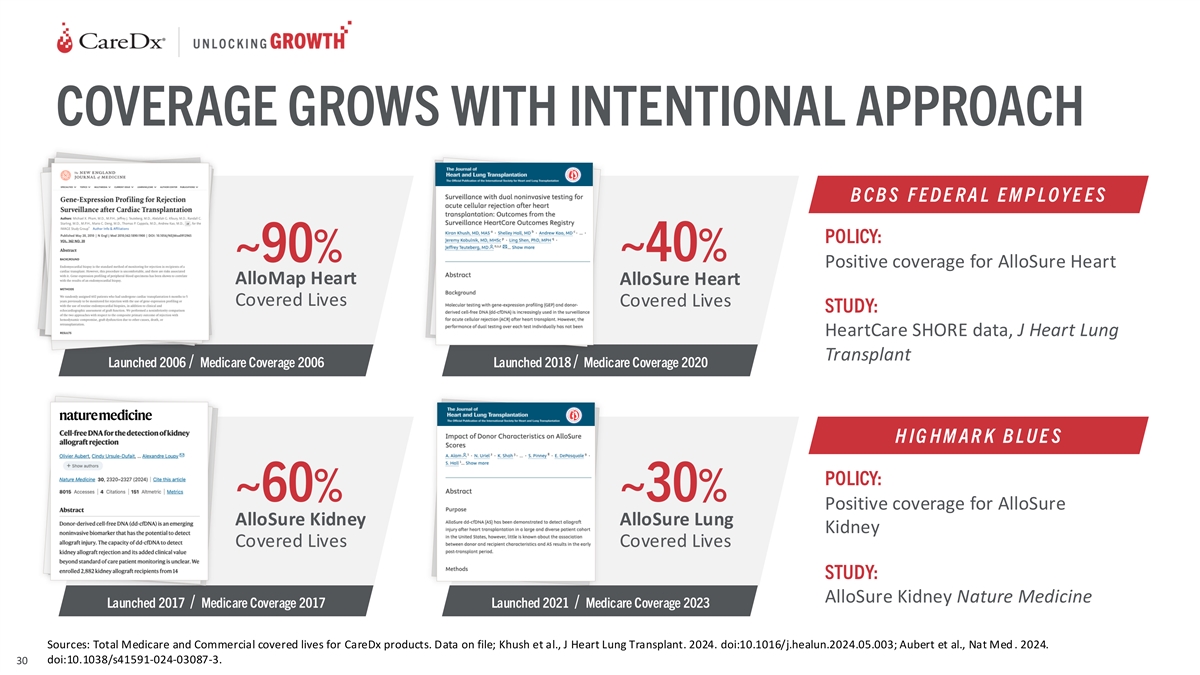
COVERAGE GROWS WITH INTENTIONAL APPROACH B C B S F E D E R A L E M P L
O Y E E S POLICY: ~40% ~90% Positive coverage for AlloSure Heart AlloMap Heart AlloSure Heart Covered Lives Covered Lives STUDY: HeartCare SHORE data, J Heart Lung Transplant Launched 2006 / Medicare Coverage 2006 Launched 2018 / Medicare Coverage
2020 H IG H M A R K B L U E S POLICY: ~60% ~30% Positive coverage for AlloSure AlloSure Kidney AlloSure Lung Kidney Covered Lives Covered Lives STUDY: AlloSure Kidney Nature Medicine Launched 2017 / Medicare Coverage 2017 Launched 2021 / Medicare
Coverage 2023 Sources: Total Medicare and Commercial covered lives for CareDx products. Data on file; Khush et al., J Heart Lung Transplant. 2024. doi:10.1016/j.healun.2024.05.003; Aubert et al., Nat Med . 2024. doi:10.1038/s41591-024-03087-3.
30
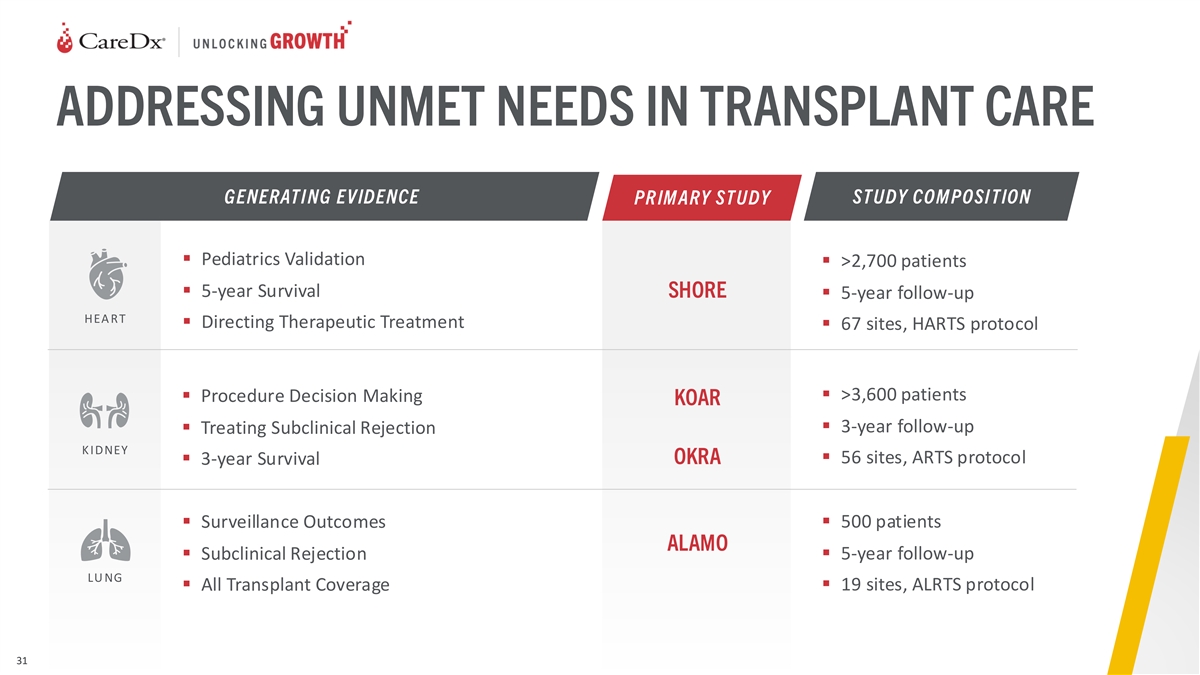
ADDRESSING UNMET NEEDS IN TRANSPLANT CARE GENERATING EVIDENCE STUDY COM
POSITION PR IM ARY STUDY ▪ Pediatrics Validation ▪ >2,700 patients ▪ 5-year Survival SHORE ▪ 5-year follow-up HEA RT ▪ Directing Therapeutic Treatment ▪ 67 sites, HARTS protocol ▪ >3,600 patients
▪ Procedure Decision Making KOAR ▪ 3-year follow-up ▪ Treating Subclinical Rejection K IDNEY OKRA▪ 56 sites, ARTS protocol ▪ 3-year Survival ▪ Surveillance Outcomes▪ 500 patients ALAMO ▪ Subclinical
Rejection▪ 5-year follow-up LU NG ▪ All Transplant Coverage▪ 19 sites, ALRTS protocol 31

Driving Operational Leverage Keith Kennedy Chief Operating
Officer
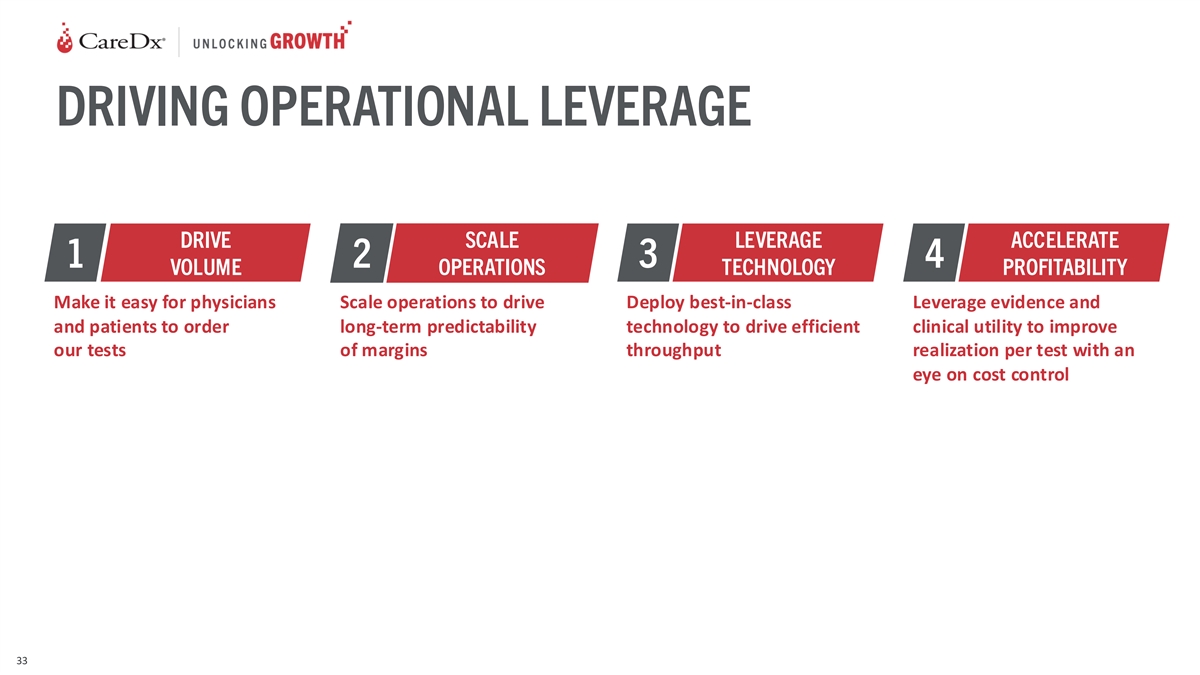
DRIVING OPERATIONAL LEVERAGE DRIVE SCALE LEVERAGE ACCELERATE 1 2 3 4
VOLUME OPERATIONS TECHNOLOGY PROFITABILITY Make it easy for physicians Scale operations to drive Deploy best-in-class Leverage evidence and and patients to order long-term predictability technology to drive efficient clinical utility to improve our
tests of margins throughput realization per test with an eye on cost control 33
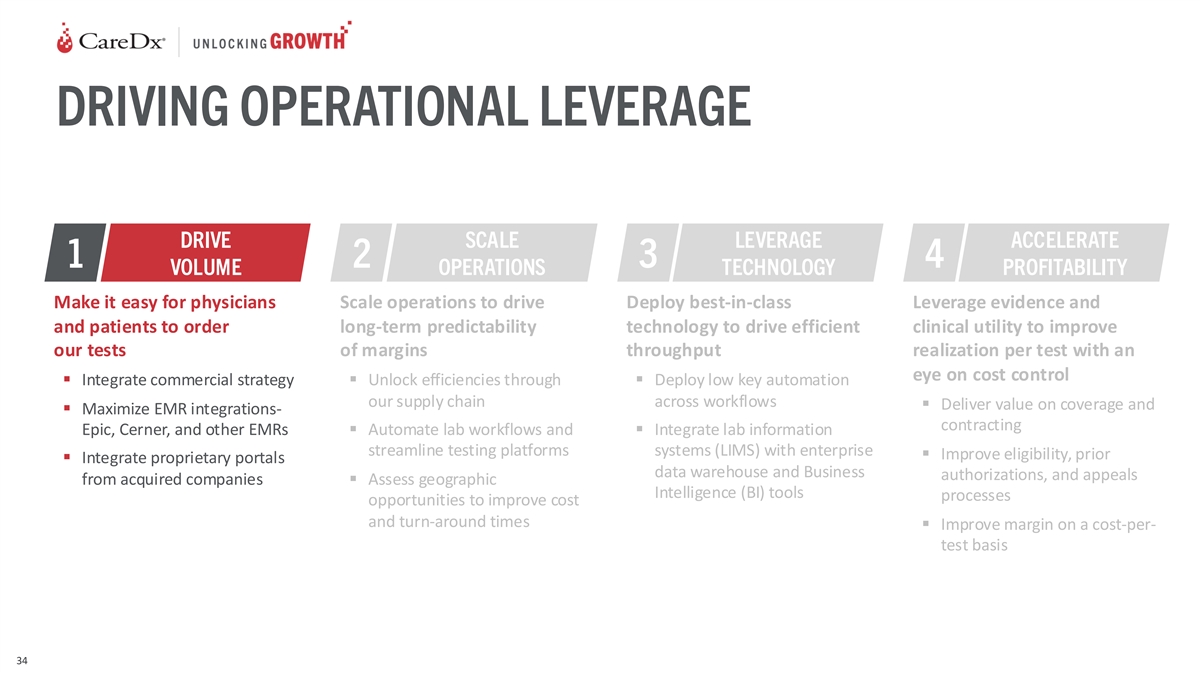
DRIVING OPERATIONAL LEVERAGE DRIVE SCALE LEVERAGE ACCELERATE 1 2 3 4
VOLUME OPERATIONS TECHNOLOGY PROFITABILITY Make it easy for physicians Scale operations to drive Deploy best-in-class Leverage evidence and and patients to order long-term predictability technology to drive efficient clinical utility to improve our
tests of margins throughput realization per test with an eye on cost control ▪ Integrate commercial strategy▪ Unlock efficiencies through ▪ Deploy low key automation our supply chain across workflows ▪ Deliver value on
coverage and ▪ Maximize EMR integrations- contracting Epic, Cerner, and other EMRs▪ Automate lab workflows and ▪ Integrate lab information streamline testing platforms systems (LIMS) with enterprise ▪ Improve eligibility,
prior ▪ Integrate proprietary portals data warehouse and Business authorizations, and appeals from acquired companies▪ Assess geographic Intelligence (BI) tools processes opportunities to improve cost and turn-around times ▪
Improve margin on a cost-per- test basis 34
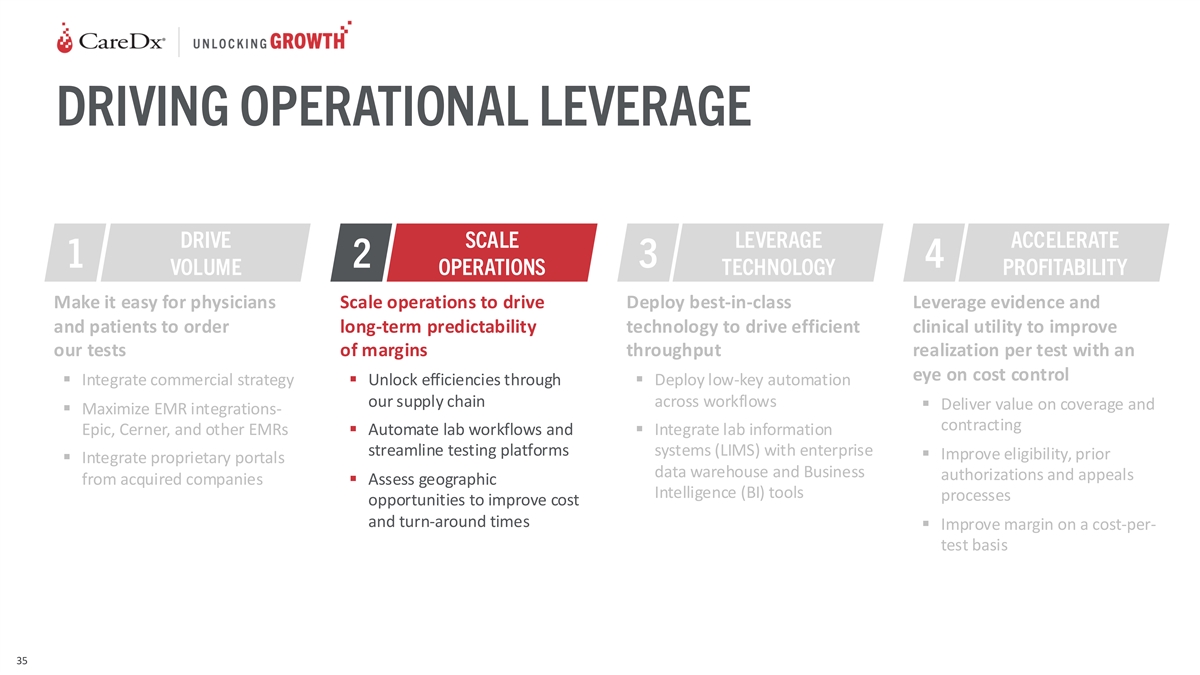
DRIVING OPERATIONAL LEVERAGE DRIVE SCALE LEVERAGE ACCELERATE 1 2 3 4
VOLUME OPERATIONS TECHNOLOGY PROFITABILITY Make it easy for physicians Scale operations to drive Deploy best-in-class Leverage evidence and and patients to order long-term predictability technology to drive efficient clinical utility to improve our
tests of margins throughput realization per test with an eye on cost control ▪ Integrate commercial strategy▪ Unlock efficiencies through ▪ Deploy low-key automation our supply chain across workflows ▪ Deliver value on
coverage and ▪ Maximize EMR integrations- contracting Epic, Cerner, and other EMRs▪ Automate lab workflows and ▪ Integrate lab information streamline testing platforms systems (LIMS) with enterprise ▪ Improve eligibility,
prior ▪ Integrate proprietary portals data warehouse and Business authorizations and appeals from acquired companies▪ Assess geographic Intelligence (BI) tools processes opportunities to improve cost and turn-around times ▪ Improve
margin on a cost-per- test basis 35
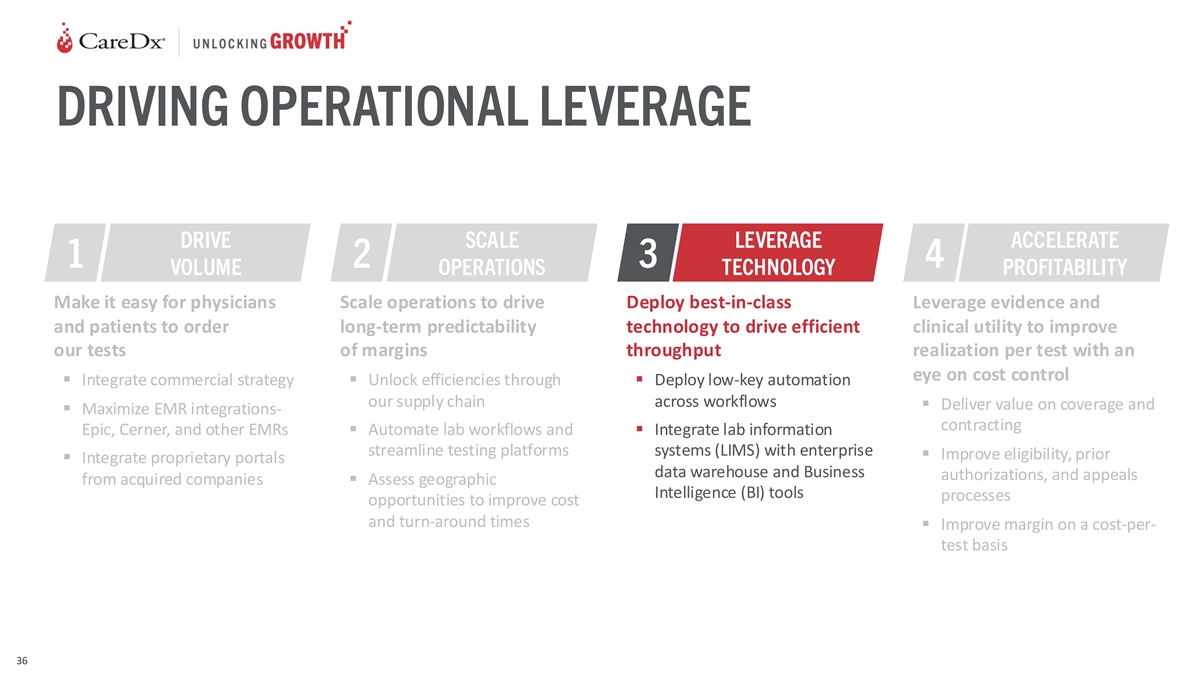
DRIVING OPERATIONAL LEVERAGE DRIVE SCALE LEVERAGE ACCELERATE 1 2 3 4
VOLUME OPERATIONS TECHNOLOGY PROFITABILITY Make it easy for physicians Scale operations to drive Deploy best-in-class Leverage evidence and and patients to order long-term predictability technology to drive efficient clinical utility to improve our
tests of margins throughput realization per test with an eye on cost control ▪ Integrate commercial strategy▪ Unlock efficiencies through ▪ Deploy low-key automation our supply chain across workflows ▪ Deliver value on
coverage and ▪ Maximize EMR integrations- contracting Epic, Cerner, and other EMRs▪ Automate lab workflows and ▪ Integrate lab information streamline testing platforms systems (LIMS) with enterprise ▪ Improve eligibility,
prior ▪ Integrate proprietary portals data warehouse and Business authorizations, and appeals from acquired companies▪ Assess geographic Intelligence (BI) tools processes opportunities to improve cost and turn-around times ▪
Improve margin on a cost-per- test basis 36
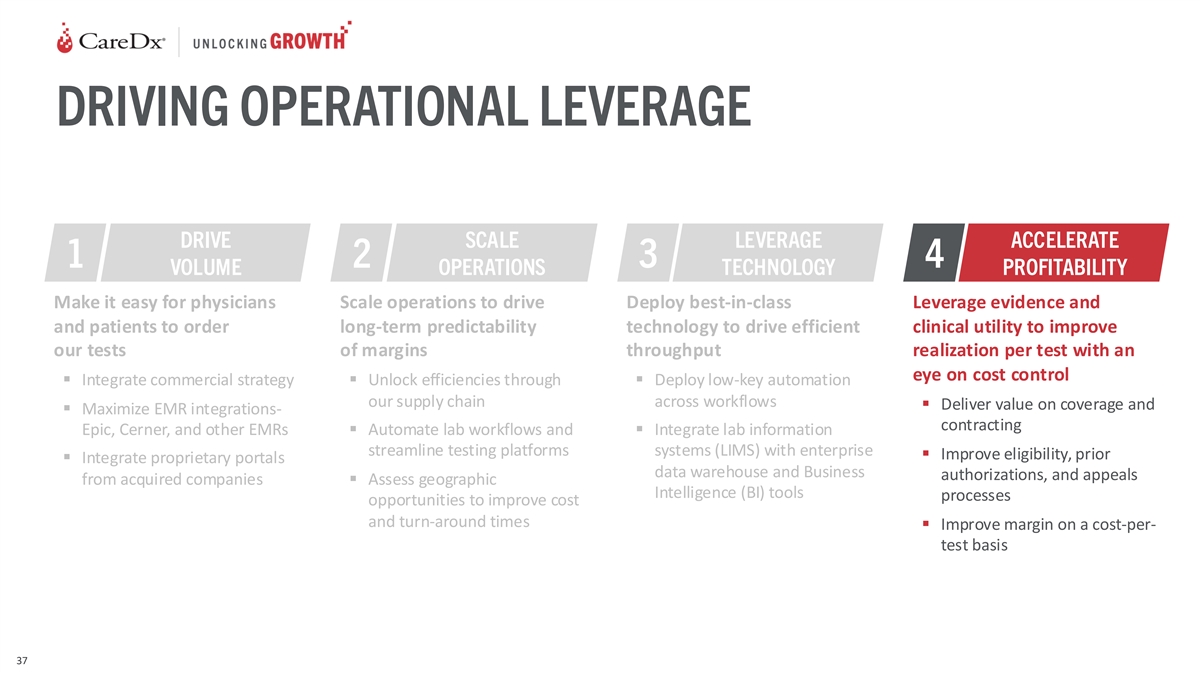
DRIVING OPERATIONAL LEVERAGE DRIVE SCALE LEVERAGE ACCELERATE 1 2 3 4
VOLUME OPERATIONS TECHNOLOGY PROFITABILITY Make it easy for physicians Scale operations to drive Deploy best-in-class Leverage evidence and and patients to order long-term predictability technology to drive efficient clinical utility to improve our
tests of margins throughput realization per test with an eye on cost control ▪ Integrate commercial strategy▪ Unlock efficiencies through ▪ Deploy low-key automation our supply chain across workflows ▪ Deliver value on
coverage and ▪ Maximize EMR integrations- contracting Epic, Cerner, and other EMRs▪ Automate lab workflows and ▪ Integrate lab information streamline testing platforms systems (LIMS) with enterprise ▪ Improve eligibility,
prior ▪ Integrate proprietary portals data warehouse and Business authorizations, and appeals from acquired companies▪ Assess geographic Intelligence (BI) tools processes opportunities to improve cost and turn-around times ▪
Improve margin on a cost-per- test basis 37
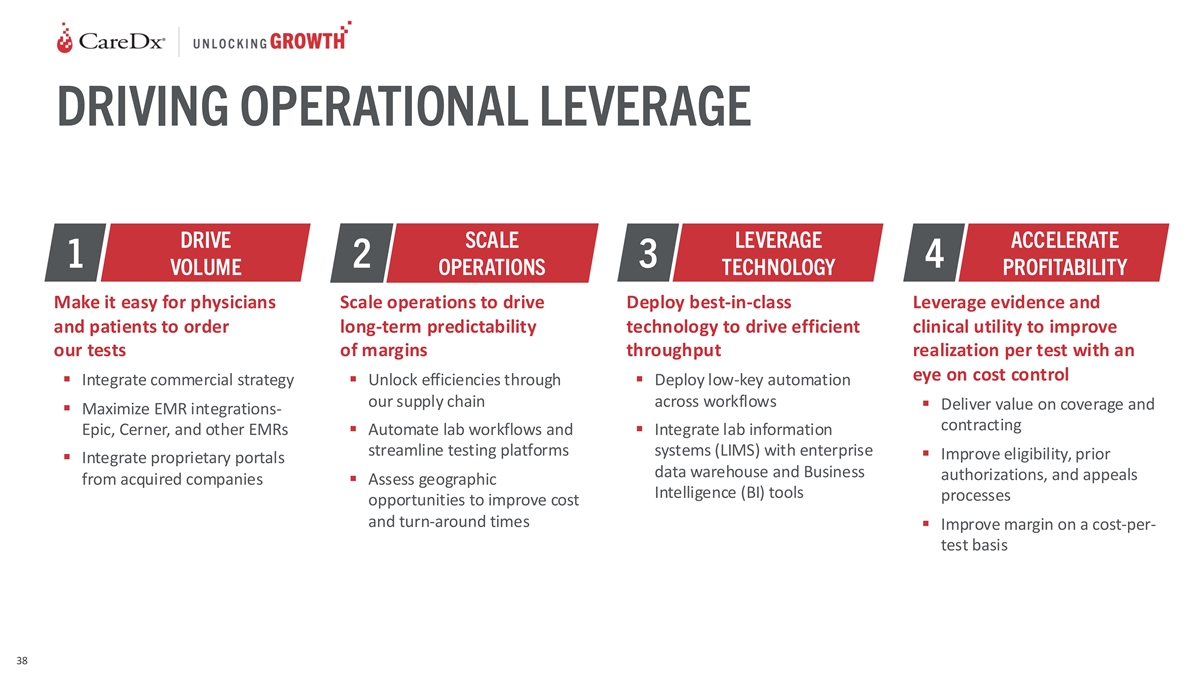
DRIVING OPERATIONAL LEVERAGE DRIVE SCALE LEVERAGE ACCELERATE 1 2 3 4
VOLUME OPERATIONS TECHNOLOGY PROFITABILITY Make it easy for physicians Scale operations to drive Deploy best-in-class Leverage evidence and and patients to order long-term predictability technology to drive efficient clinical utility to improve our
tests of margins throughput realization per test with an eye on cost control ▪ Integrate commercial strategy▪ Unlock efficiencies through ▪ Deploy low-key automation our supply chain across workflows ▪ Deliver value on
coverage and ▪ Maximize EMR integrations- contracting Epic, Cerner, and other EMRs▪ Automate lab workflows and ▪ Integrate lab information streamline testing platforms systems (LIMS) with enterprise ▪ Improve eligibility,
prior ▪ Integrate proprietary portals data warehouse and Business authorizations, and appeals from acquired companies▪ Assess geographic Intelligence (BI) tools processes opportunities to improve cost and turn-around times ▪
Improve margin on a cost-per- test basis 38
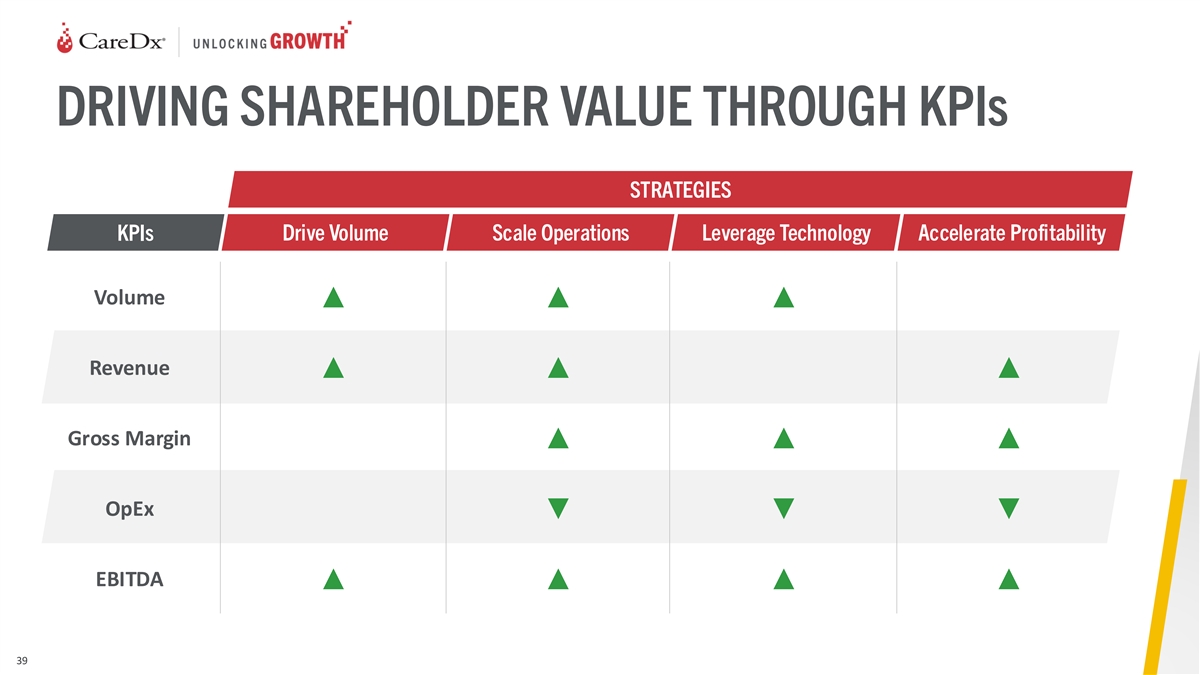
DRIVING SHAREHOLDER VALUE THROUGH KPIs STRATEGIES KPIs Drive Volume
Scale Operations Leverage Technology Accelerate Profitability Volume ▲▲▲ Revenue ▲▲▲ Gross Margin ▲▲▲ OpEx ▼▼▼ EBITDA ▲▲▲▲ 39

Best-in-Class Scalable operations IMPACT Industry Leading OVER THE NEXT
Operating metrics 24 MONTHS Driving Shareholder value 40

Charting a Clear Path to Profitable Growth Abhishek Jain Chief
Financial Officer

CHARTING A PATH TO PROFITABLE GROWTH AND STRATEGIC CAPITAL ALLOCATION
PROFITABLE GROWTH PROFITS AND CASH CAPITAL ALLOCATION ▪ Compounded ▪ Adjusted EBITDA▪ M&A Annual Growth ▪ Cash Generation ▪ Invest in Core Rate (CAGR) Business ▪ Gross Margin ▪ Share Buyback
42

DRIVING PROFITABLE GROWTH 15% >70% 3-Year Revenue 2027 Gross Margin
CAGR Target Target Compounded annual growth rate based on 2024 revenue guidance at the mid-point. 43
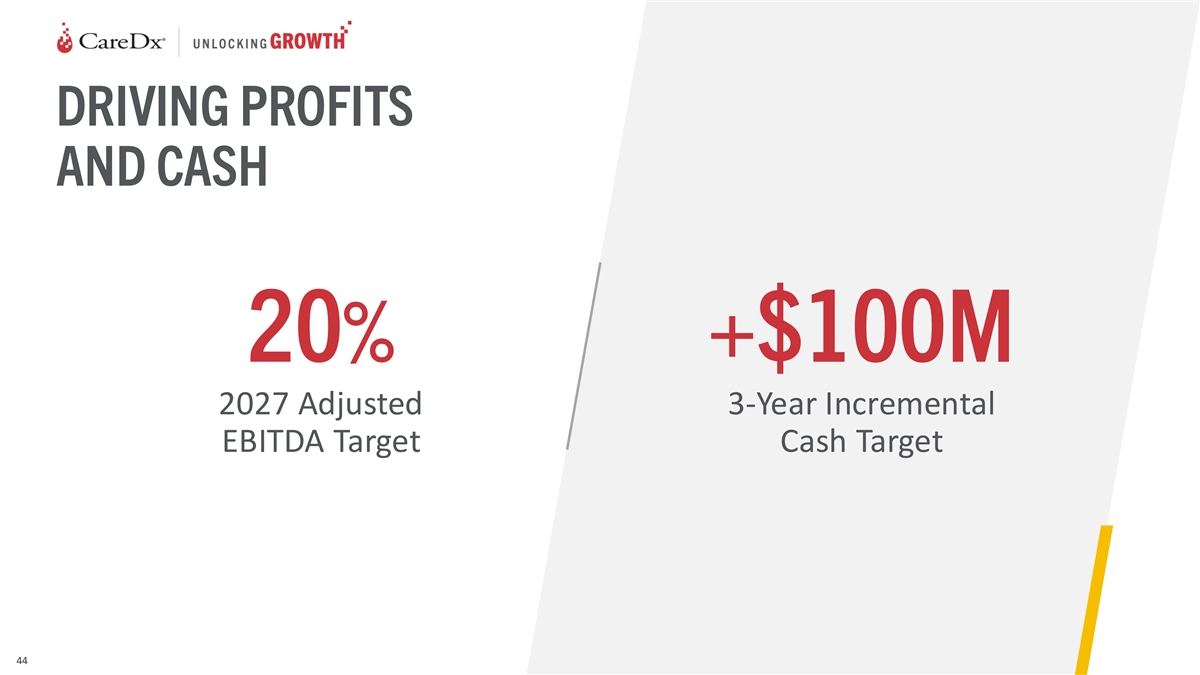
DRIVING PROFITS AND CASH 20% +$100M 2027 Adjusted 3-Year Incremental
EBITDA Target Cash Target 44
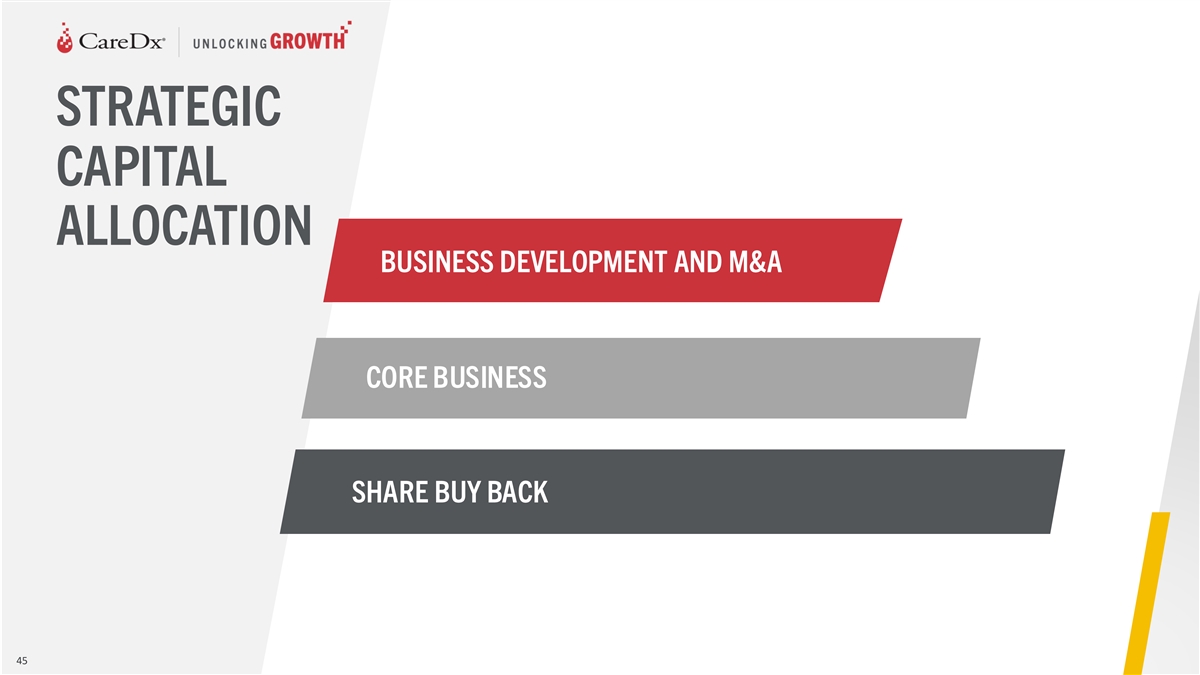
STRATEGIC CAPITAL ALLOCATION BUSINESS DEVELOPMENT AND M&A CORE
BUSINESS SHARE BUY BACK 45

Expanding Our Footprint Beyond $8B TAM Marica Grskovic, PhD Chief
Strategy Officer
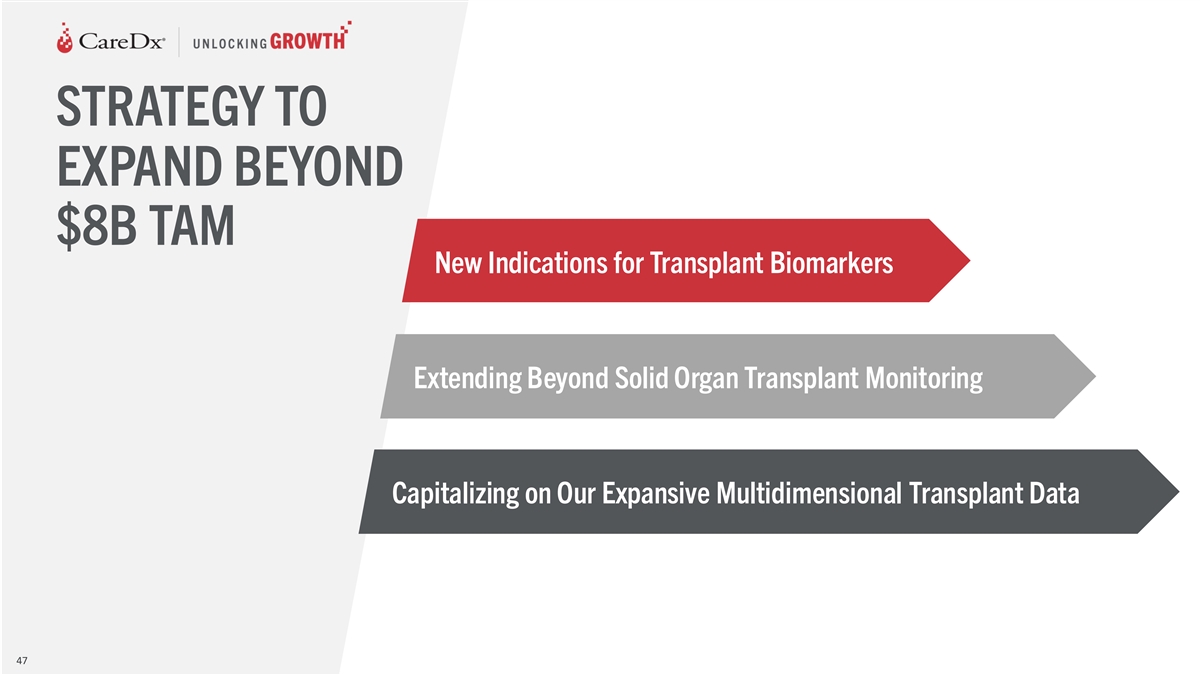
STRATEGY TO EXPAND BEYOND $8B TAM New Indications for Transplant
Biomarkers Extending Beyond Solid Organ Transplant Monitoring Capitalizing on Our Expansive Multidimensional Transplant Data 47
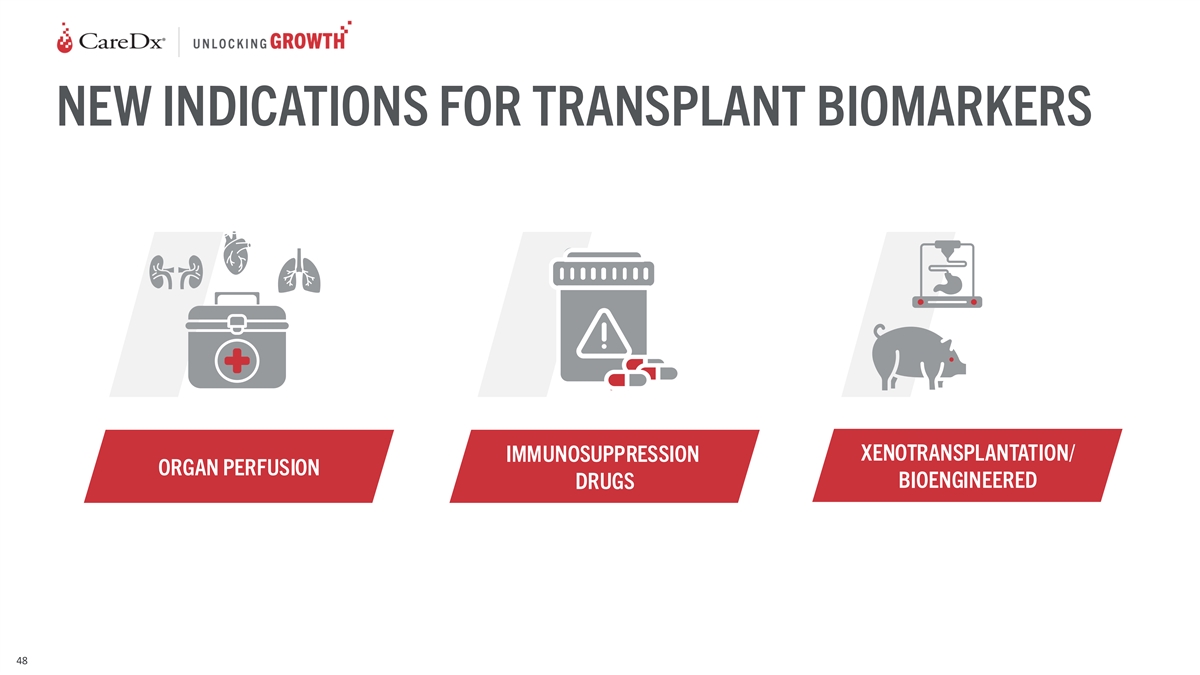
NEW INDICATIONS FOR TRANSPLANT BIOMARKERS XENOTRANSPLANTATION/
IMMUNOSUPPRESSION ORGAN PERFUSION BIOENGINEERED DRUGS 48
EXTENDING BEYOND SOLID ORGAN TRANSPLANT MONITORING â Blood Solid
Autoimmune Cancer Cancer Disorders 49
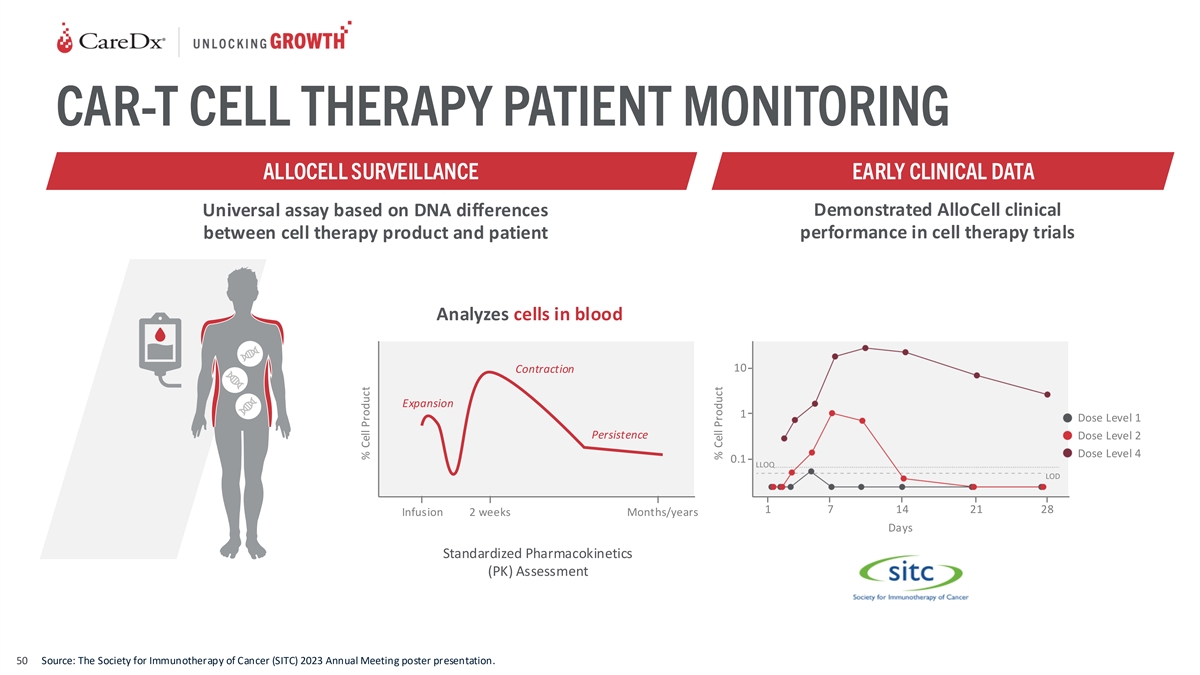
CAR-T CELL THERAPY PATIENT MONITORING ALLOCELL SURVEILLANCE EARLY
CLINICAL DATA Universal assay based on DNA differences Demonstrated AlloCell clinical between cell therapy product and patient performance in cell therapy trials Analyzes cells in blood 10 Contraction Expansion 1 Dose Level 1 Persistence Dose Level
2 Dose Level 4 0.1 LLOQ LOD 1 7 14 21 28 Infusion 2 weeks Months/years Days Standardized Pharmacokinetics (PK) Assessment 50 Source: The Society for Immunotherapy of Cancer (SITC) 2023 Annual Meeting poster presentation. % Cell Product % Cell
Product
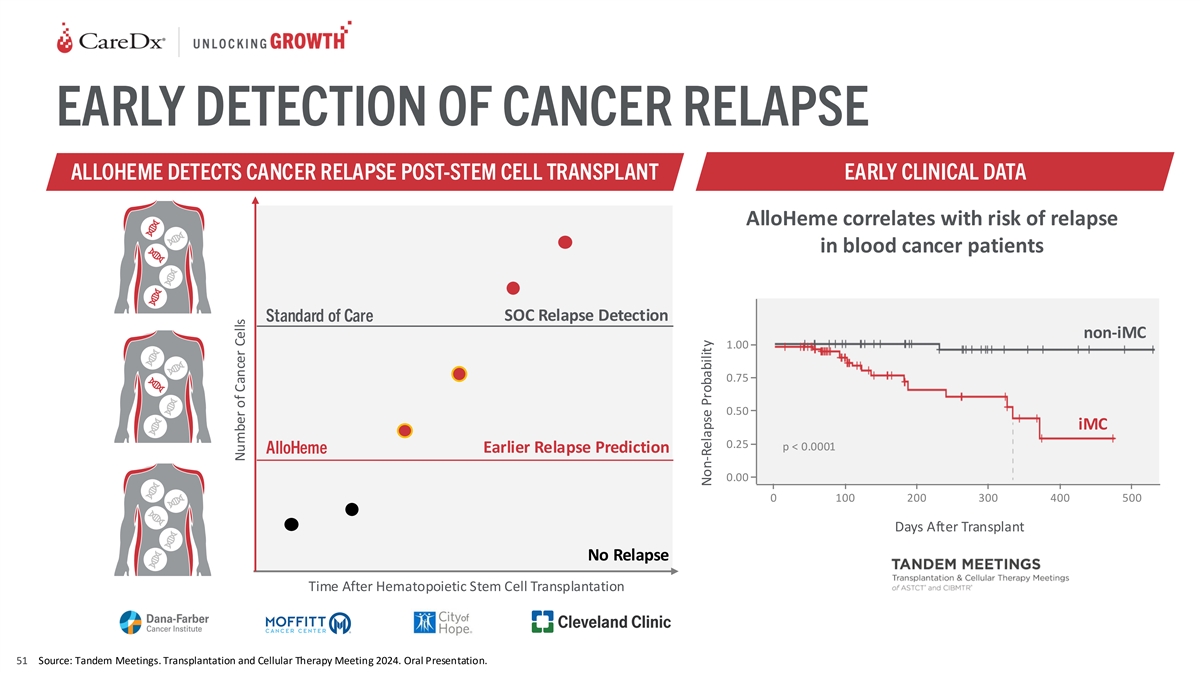
EARLY DETECTION OF CANCER RELAPSE ALLOHEME DETECTS CANCER RELAPSE
POST-STEM CELL TRANSPLANT EARLY CLINICAL DATA AlloHeme correlates with risk of relapse in blood cancer patients SOC Relapse Detection Standard of Care non-iMC 1.00 0.75 0.50 iMC 0.25 p < 0.0001 Earlier Relapse Prediction AlloHeme 0.00 0 100 200
300 400 500 Days After Transplant No Relapse Time After Hematopoietic Stem Cell Transplantation 51 Source: Tandem Meetings. Transplantation and Cellular Therapy Meeting 2024. Oral Presentation. Number of Cancer Cells Non-Relapse
Probability
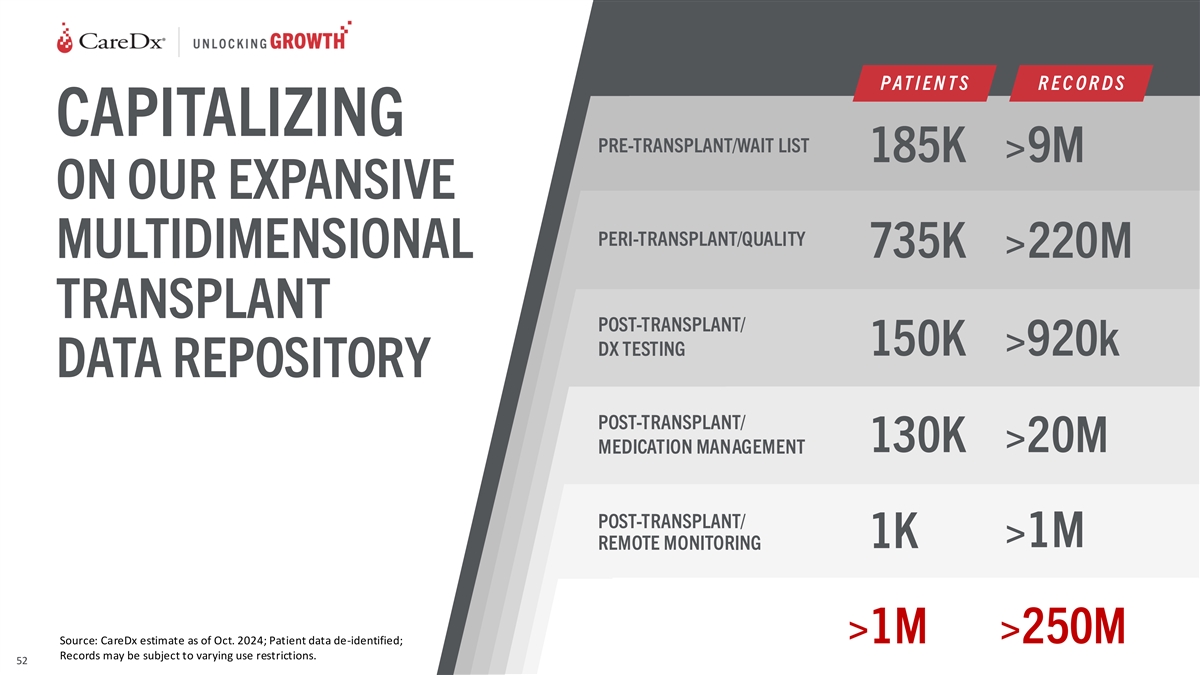
P AT I E N T S R E C O R D S CAPITALIZING PRE-TRANSPLANT/WAIT LIST 185K
>9M ON OUR EXPANSIVE PERI-TRANSPLANT/QUALITY 735K >220M MULTIDIMENSIONAL TRANSPLANT POST-TRANSPLANT/ DX TESTING 150K >920k DATA REPOSITORY POST-TRANSPLANT/ 130K >20M MEDICATION MANAGEMENT POST-TRANSPLANT/ >1M 1K REMOTE MONITORING
>1M >250M Source: CareDx estimate as of Oct. 2024; Patient data de-identified; Records may be subject to varying use restrictions. 52
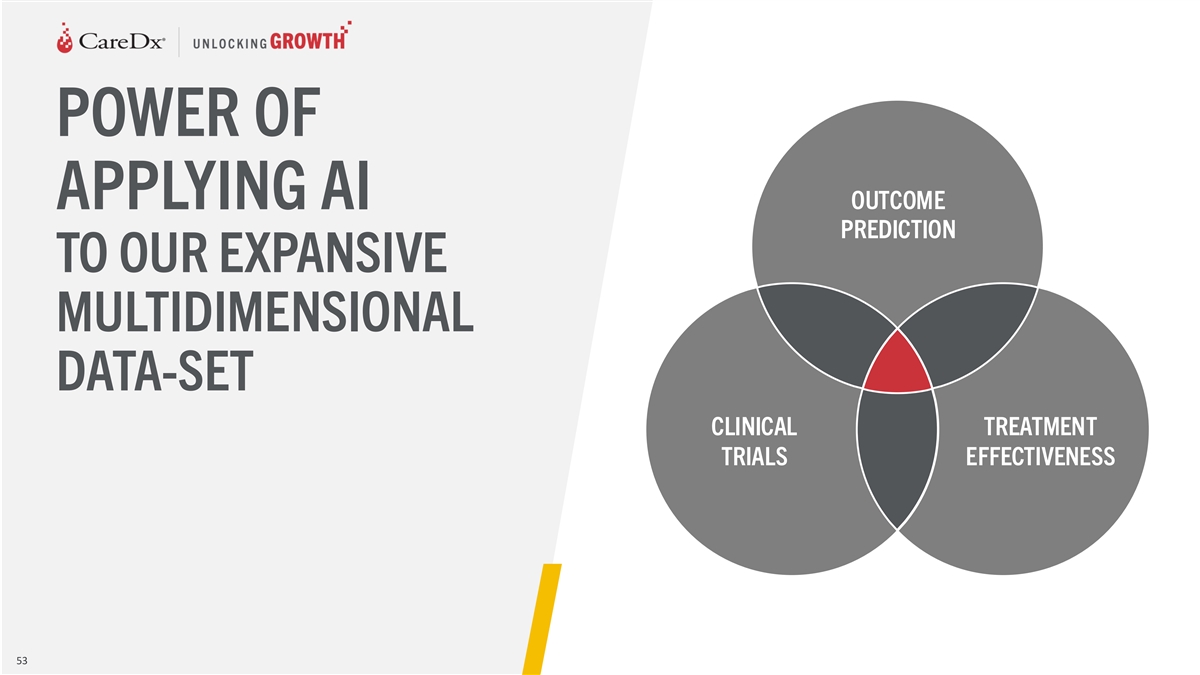
POWER OF OUTCOME APPLYING AI PREDICTION TO OUR EXPANSIVE
MULTIDIMENSIONAL DATA-SET CLINICAL TREATMENT TRIALS EFFECTIVENESS 53
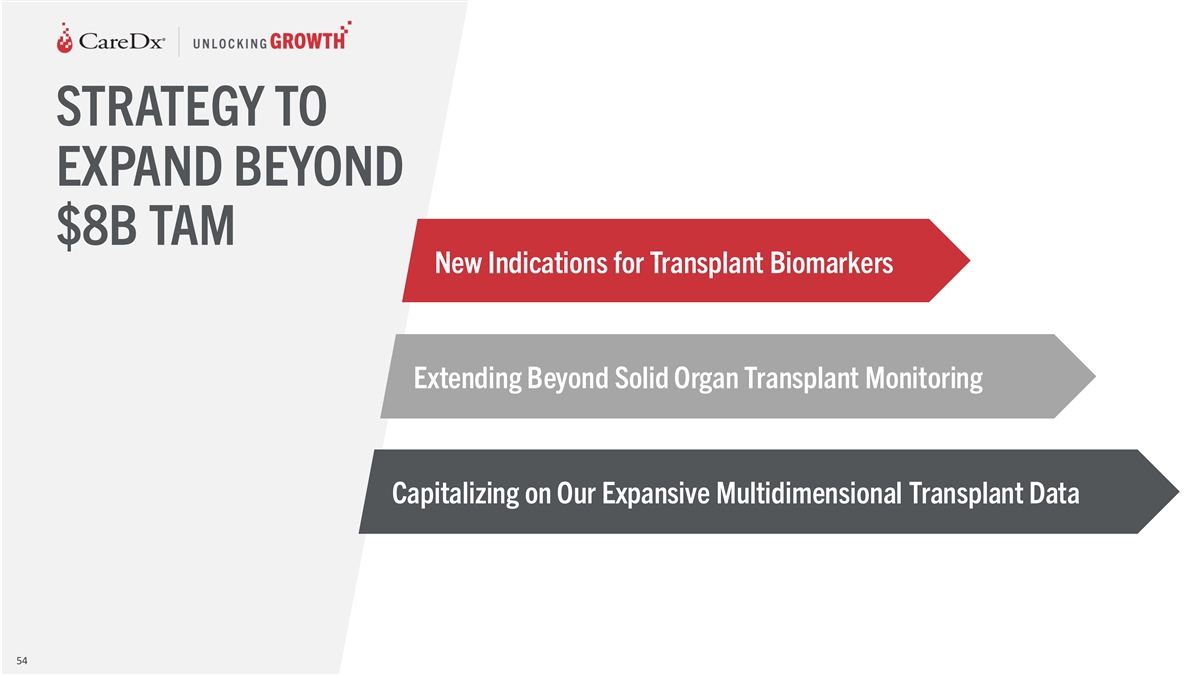
STRATEGY TO EXPAND BEYOND $8B TAM New Indications for Transplant
Biomarkers Extending Beyond Solid Organ Transplant Monitoring Capitalizing on Our Expansive Multidimensional Transplant Data 54

Activating Our Strategy John Hanna President and CEO

CAREDX STRATEGIC PRIORITIES PROFITABLE GROWTH 1 OPERATIONAL EXCELLENCE
2 DEFINE TRANSPLANT+ 3 ELEVATE PERFORMANCE CULTURE 4 56
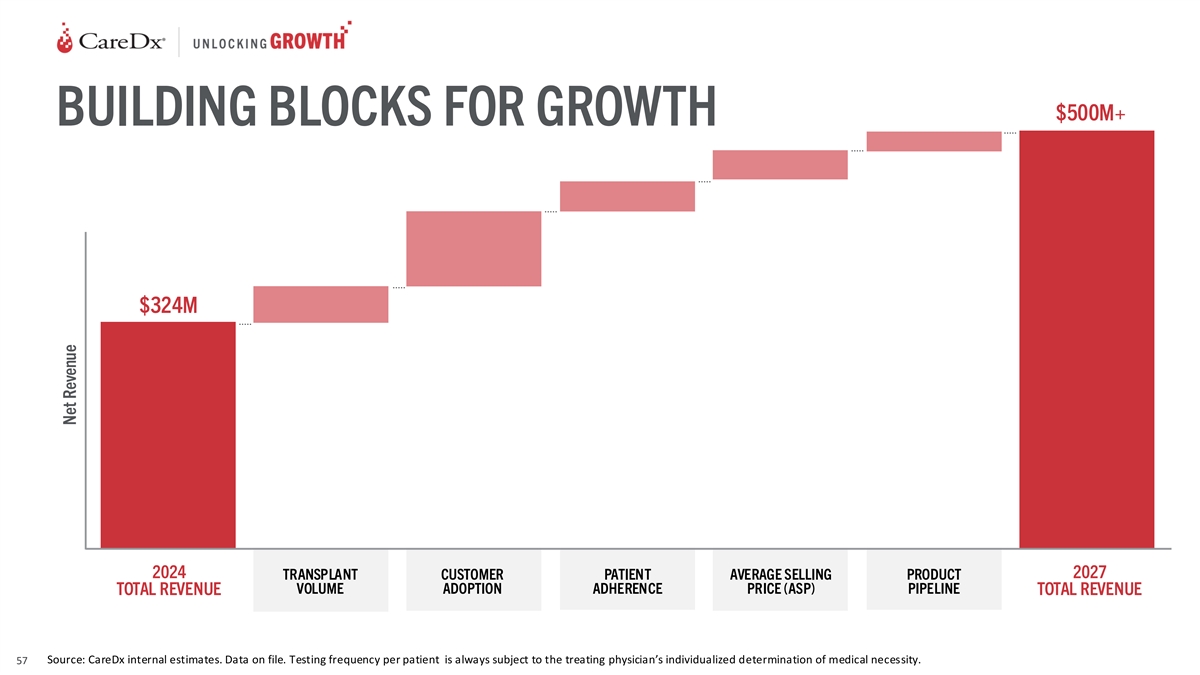
$500M+ BUILDING BLOCKS FOR GROWTH $324M 2024 2027 TRANSPLANT CUSTOMER
PATIENT AVERAGE SELLING PRODUCT VOLUME ADOPTION ADHERENCE PRICE (ASP) PIPELINE TOTAL REVENUE TOTAL REVENUE Source: CareDx internal estimates. Data on file. Testing frequency per patient is always subject to the treating physician’s
individualized determination of medical necessity. 57 Net Revenue
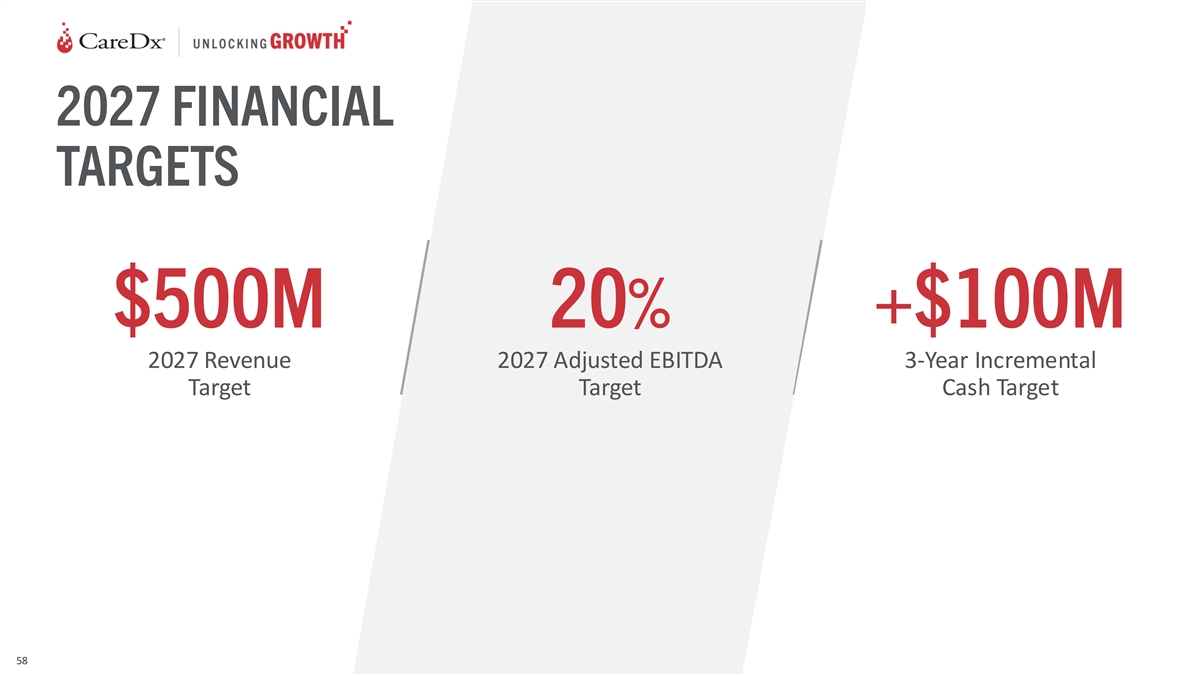
2027 FINANCIAL TARGETS $500M 20% +$100M 2027 Revenue 2027 Adjusted
EBITDA 3-Year Incremental Target Target Cash Target 58

ACTIVATING OUR STRATEGY PUT IN PLACE THE RIGHT TEAM IDENTIFIED THE
MARKET OPPORTUNITIES LAUNCHING INNOVATIVE SOLUTIONS TO RESOLVE UNMET MEDICAL NEEDS 59

THE MOST INNOVATIVE COMPANY IN DIAGNOSTICS 60
v3.24.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Grafico Azioni CareDx (NASDAQ:CDNA)
Storico
Da Dic 2024 a Gen 2025

Grafico Azioni CareDx (NASDAQ:CDNA)
Storico
Da Gen 2024 a Gen 2025
