false 0001865494 0001865494 2024-01-08 2024-01-08
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(D)
OF THE SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported): January 8, 2024
IO BIOTECH, INC.
(Exact name of Registrant as Specified in its Charter)
|
|
|
|
|
| Delaware |
|
001-41008 |
|
87-0909276 |
| (State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
Ole Maaløes Vej 3
DK-2200 Copenhagen N
Denmark
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area code: +45 7070 2980
N/A
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, par value $0.001 per share |
|
IOBT |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01. |
Regulation FD Disclosure. |
Beginning on January 8, 2024, IO Biotech, Inc., a Delaware corporation (the “Company”) will participate in the 42nd Annual J.P. Morgan Healthcare Conference (the “Conference”). The Company has updated the corporate presentation that it intends to use at the Conference and in meetings with investors, a copy of which was posted to the Company’s website on January 8, 2024 (the “Investor Deck”). The Investor Deck includes, among other things, confirmation of its projected cash runway through the fourth quarter of 2025.
A copy of the Investor Deck is attached hereto as Exhibit 99.1 and is incorporated by reference herein.
The information contained in this Current Report on Form 8-K, including Exhibit 99.1, is being furnished and shall not be deemed to be “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
IO BIOTECH, INC. |
|
|
|
|
| Date: January 8, 2024 |
|
|
|
By: |
|
/s/ Mai-Britt Zocca, Ph.D. |
|
|
|
|
Name: |
|
Mai-Britt Zocca, Ph.D. |
|
|
|
|
Title: |
|
Chief Executive Officer |

Break Boundaries. Ignite Change.
Nasdaq: IOBT Corporate Presentation January 2024 Exhibit 99.1

DISCLAIMER | Forward Looking
Statements Certain information contained in this presentation includes “forward-looking statements”, within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as
amended, related to our business plan, clinical trials and regulatory submissions. We may, in some cases, use terms such as “may,” “should,” “would,” “expects,” “plans,”
“anticipates,” “could,” “intends,” “target,” “projects,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or
“continue” or other words that convey uncertainty of the future events or outcomes to identify these forward-looking statements. Our forward-looking statements are based on current beliefs and expectations of our management team that
involve risks, potential changes in circumstances, assumptions, and uncertainties. Any or all of the forward-looking statements may turn out to be wrong or be affected by inaccurate assumptions we might make or by known or unknown risks and
uncertainties. These forward-looking statements are subject to risks and uncertainties including risks related to the execution of our business plan, success and timing of our clinical trials or other studies and the other risks set forth in our
filings with the U.S. Securities and Exchange Commission. For all these reasons, actual results and developments could be materially different from those expressed in or implied by our forward-looking statements. You are cautioned not to place
undue reliance on these forward-looking statements, which are made only as of the date of this presentation. We undertake no obligation to publicly update such forward-looking statements to reflect subsequent events or circumstances.
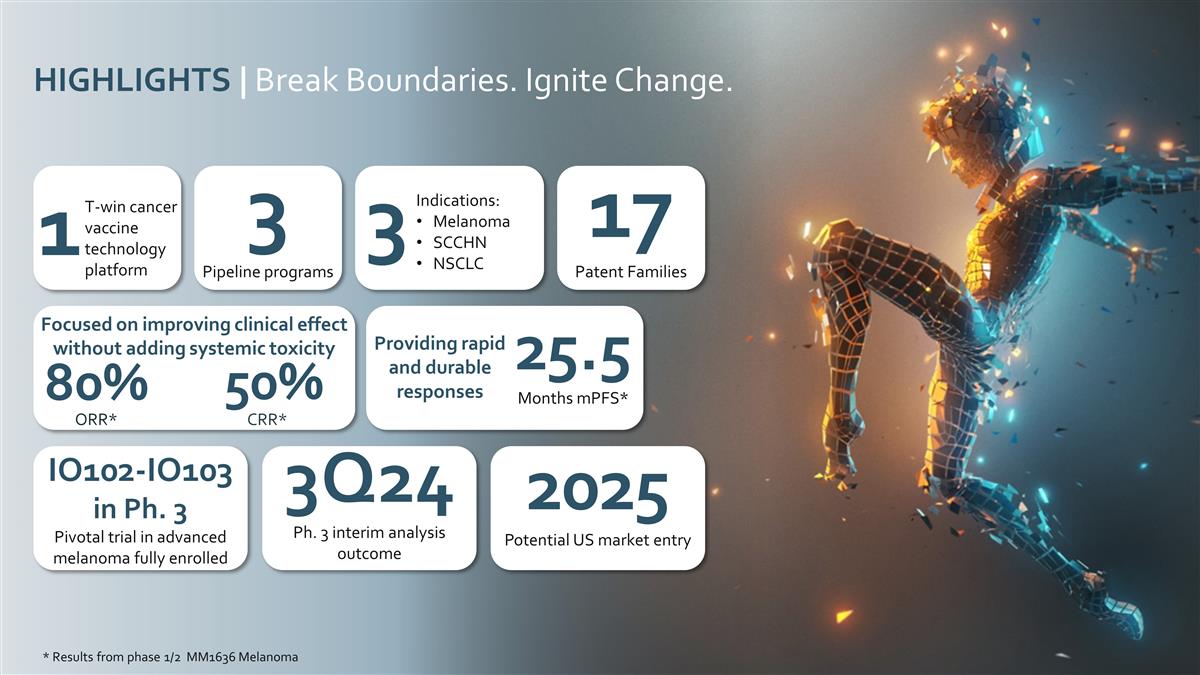
Indications: Melanoma SCCHN NSCLC 3
T-win cancer vaccine technology platform 1 3 Pipeline programs 17 Patent Families Focused on improving clinical effect without adding systemic toxicity 50% 80% * Results from phase 1/2 MM1636 Melanoma 25.5 Months mPFS* Providing rapid and durable
responses HIGHLIGHTS | Break Boundaries. Ignite Change. 3Q24 Ph. 3 interim analysis outcome IO102-IO103 in Ph. 3 Pivotal trial in advanced melanoma fully enrolled 2025 Potential US market entry ORR* CRR*
PATIENT AND MARKET PERSPECTIVE 1 OUR
UNIQUE VALUE PROPOSITION 2 OUR PIPELINE AND THE SCIENCE BEHIND IT 3 GROWTH STRATEGY AND OUTLOOK 4 THE IO BIOTECH TEAM 5 CONTENT
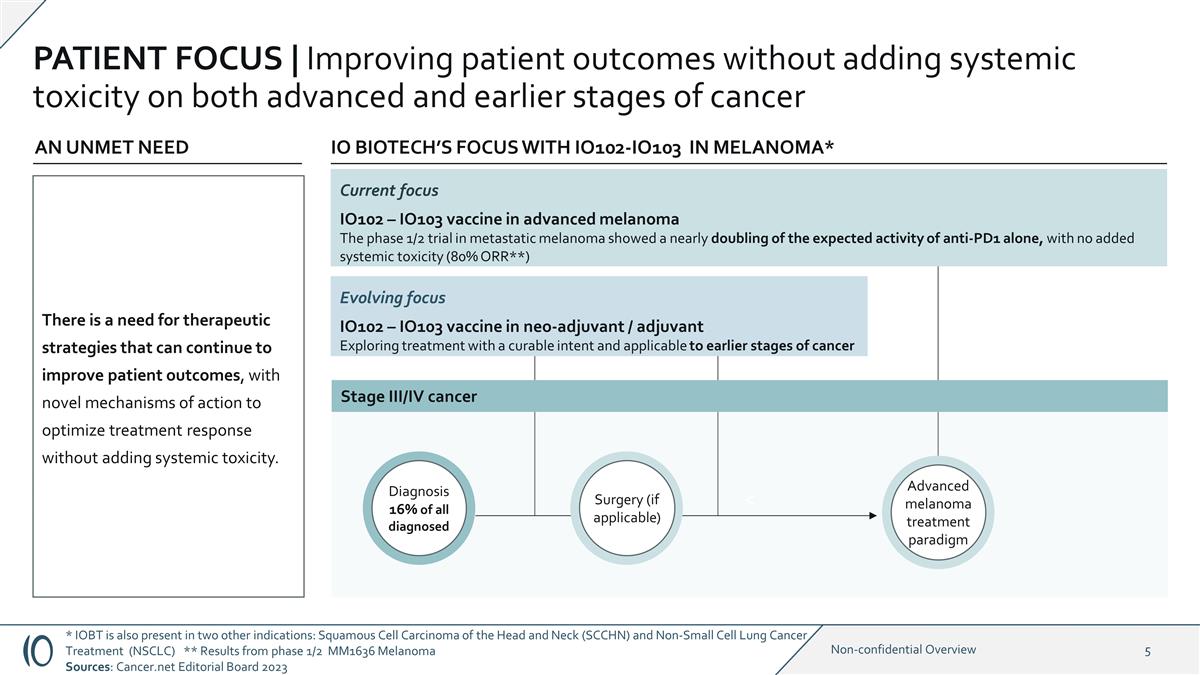
PATIENT FOCUS | Improving patient
outcomes without adding systemic toxicity on both advanced and earlier stages of cancer * IOBT is also present in two other indications: Squamous Cell Carcinoma of the Head and Neck (SCCHN) and Non-Small Cell Lung Cancer Treatment (NSCLC) ** Results
from phase 1/2 MM1636 Melanoma Sources: Cancer.net Editorial Board 2023 Evolving focus IO102 – IO103 vaccine in neo-adjuvant / adjuvant Exploring treatment with a curable intent and applicable to earlier stages of cancer IO BIOTECH’S
FOCUS WITH IO102-IO103 IN MELANOMA* Current focus IO102 – IO103 vaccine in advanced melanoma The phase 1/2 trial in metastatic melanoma showed a nearly doubling of the expected activity of anti-PD1 alone, with no added systemic toxicity
(80% ORR**) AN UNMET NEED < Diagnosis 16% of all diagnosed Advanced melanoma treatment paradigm Surgery (if applicable) Stage III/IV cancer There is a need for therapeutic strategies that can continue to improve patient outcomes, with novel
mechanisms of action to optimize treatment response without adding systemic toxicity.
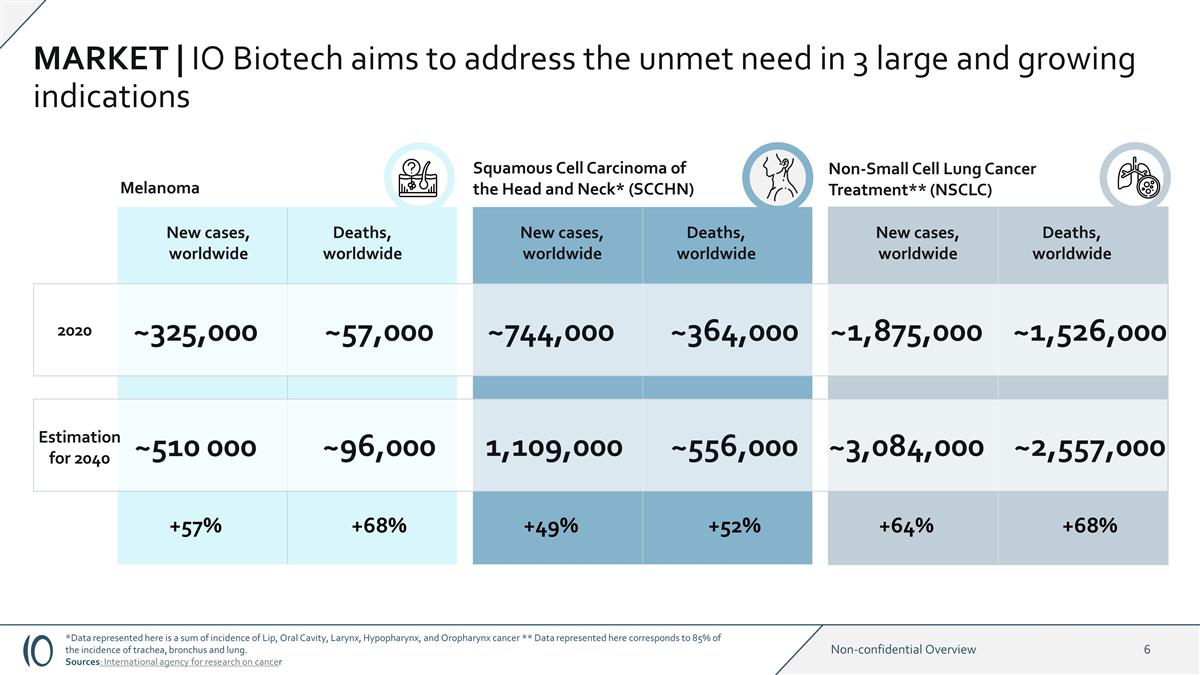
MARKET | IO Biotech aims to address
the unmet need in 3 large and growing indications Squamous Cell Carcinoma of the Head and Neck* (SCCHN) Non-Small Cell Lung Cancer Treatment** (NSCLC) Melanoma New cases, worldwide Deaths, worldwide New cases, worldwide Deaths, worldwide New cases,
worldwide Deaths, worldwide 2020 Estimation for 2040 ~510 000 ~325,000 ~57,000 ~96,000 ~744,000 1,109,000 ~364,000 ~1,875,000 ~3,084,000 ~1,526,000 ~556,000 ~2,557,000 +57% +68% +49% +52% +64% +68% *Data represented here is a sum of incidence
of Lip, Oral Cavity, Larynx, Hypopharynx, and Oropharynx cancer ** Data represented here corresponds to 85% of the incidence of trachea, bronchus and lung. Sources: International agency for research on cancer
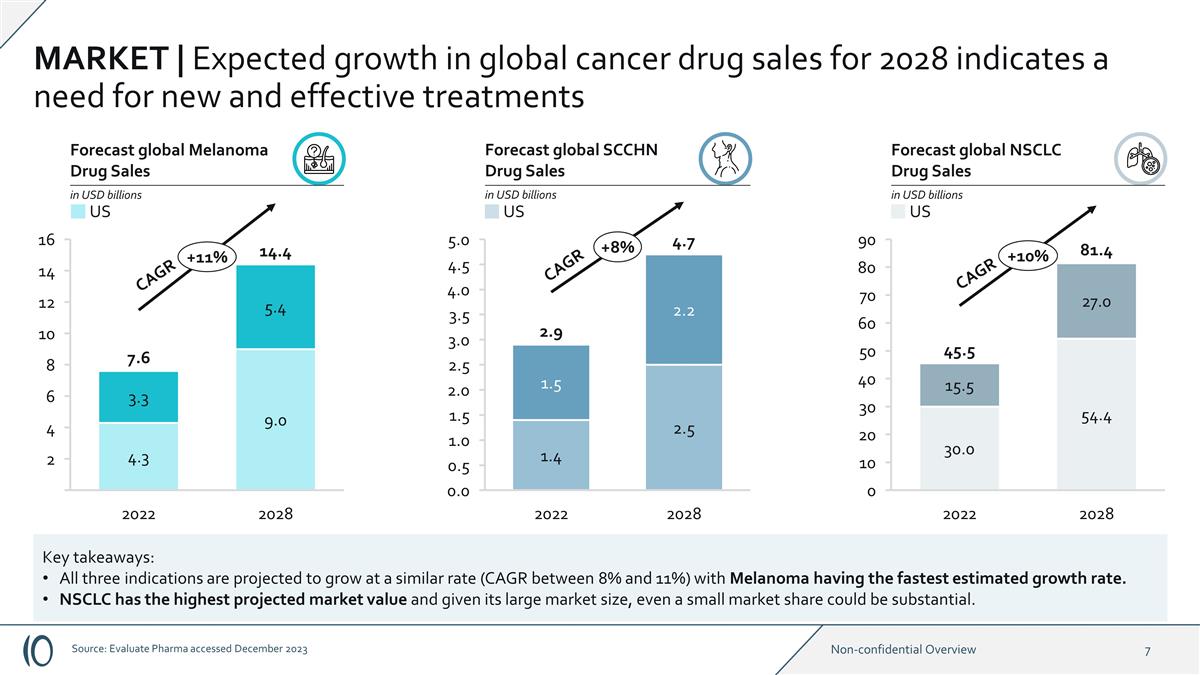
2028 2022 16 14 12 10 8 6 4 2 +11%
2028 2022 2028 2022 Forecast global Melanoma Drug Sales in USD billions Forecast global SCCHN Drug Sales in USD billions Forecast global NSCLC Drug Sales in USD billions 0.0 4.0 5.0 4.5 3.5 3.0 2.5 2.0 1.5 1.0 0.5 +8% 0 90 80 70 60 50 40 30 4.3 9.0
5.4 3.3 20 10 +10% CAGR 30.0 54.4 27.0 15.5 14.4 7.6 2.9 4.7 CAGR 45.5 81.4 CAGR MARKET | Expected growth in global cancer drug sales for 2028 indicates a need for new and effective treatments Key takeaways: All three indications are projected to
grow at a similar rate (CAGR between 8% and 11%) with Melanoma having the fastest estimated growth rate. NSCLC has the highest projected market value and given its large market size, even a small market share could be substantial. US US US Source:
Evaluate Pharma accessed December 2023
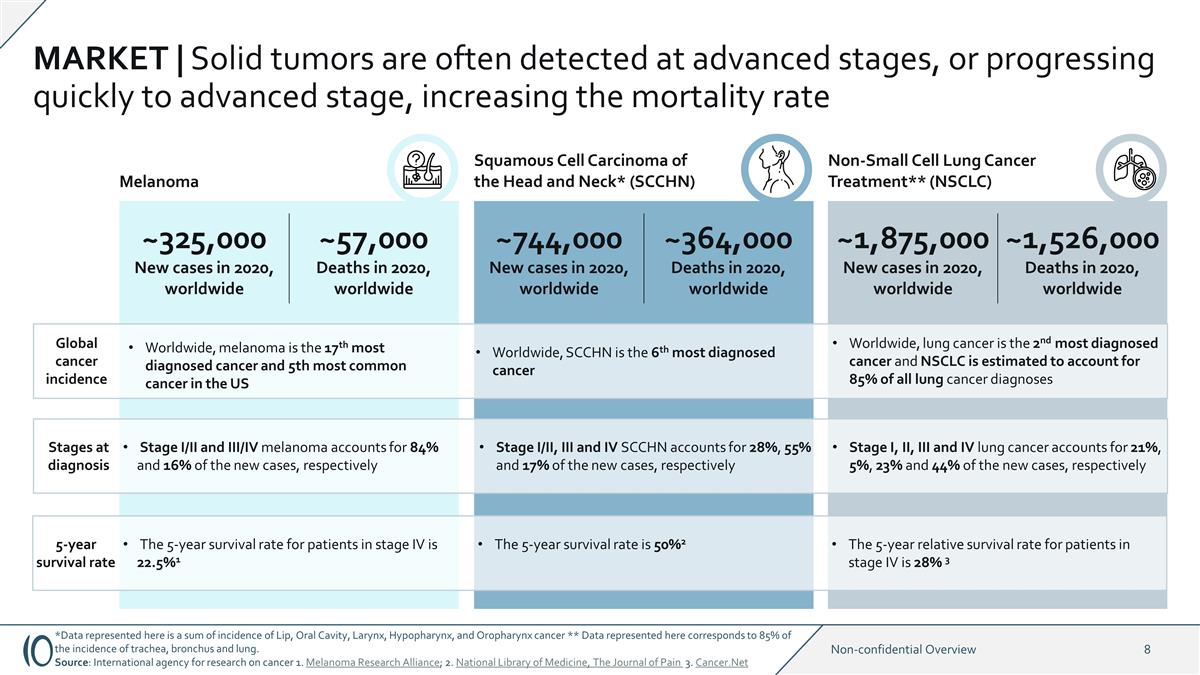
MARKET | Solid tumors are often
detected at advanced stages, or progressing quickly to advanced stage, increasing the mortality rate ~325,000 New cases in 2020, worldwide ~57,000 Deaths in 2020, worldwide ~744,000 New cases in 2020, worldwide ~364,000 Deaths in 2020, worldwide
Melanoma Non-Small Cell Lung Cancer Treatment** (NSCLC) Squamous Cell Carcinoma of the Head and Neck* (SCCHN) ~1,875,000 New cases in 2020, worldwide ~1,526,000 Deaths in 2020, worldwide Global cancer incidence Worldwide, lung cancer is the 2nd most
diagnosed cancer and NSCLC is estimated to account for 85% of all lung cancer diagnoses 5-year survival rate The 5-year survival rate for patients in stage IV is 22.5%1 The 5-year survival rate is 50%2 The 5-year relative survival rate for patients
in stage IV is 28% 3 Stages at diagnosis Stage I/II and III/IV melanoma accounts for 84% and 16% of the new cases, respectively Stage I/II, III and IV SCCHN accounts for 28%, 55% and 17% of the new cases, respectively Stage I, II, III and IV lung
cancer accounts for 21%, 5%, 23% and 44% of the new cases, respectively Worldwide, SCCHN is the 6th most diagnosed cancer Worldwide, melanoma is the 17th most diagnosed cancer and 5th most common cancer in the US *Data represented here is a sum of
incidence of Lip, Oral Cavity, Larynx, Hypopharynx, and Oropharynx cancer ** Data represented here corresponds to 85% of the incidence of trachea, bronchus and lung. Source: International agency for research on cancer 1. Melanoma Research Alliance;
2. National Library of Medicine, The Journal of Pain 3. Cancer.Net
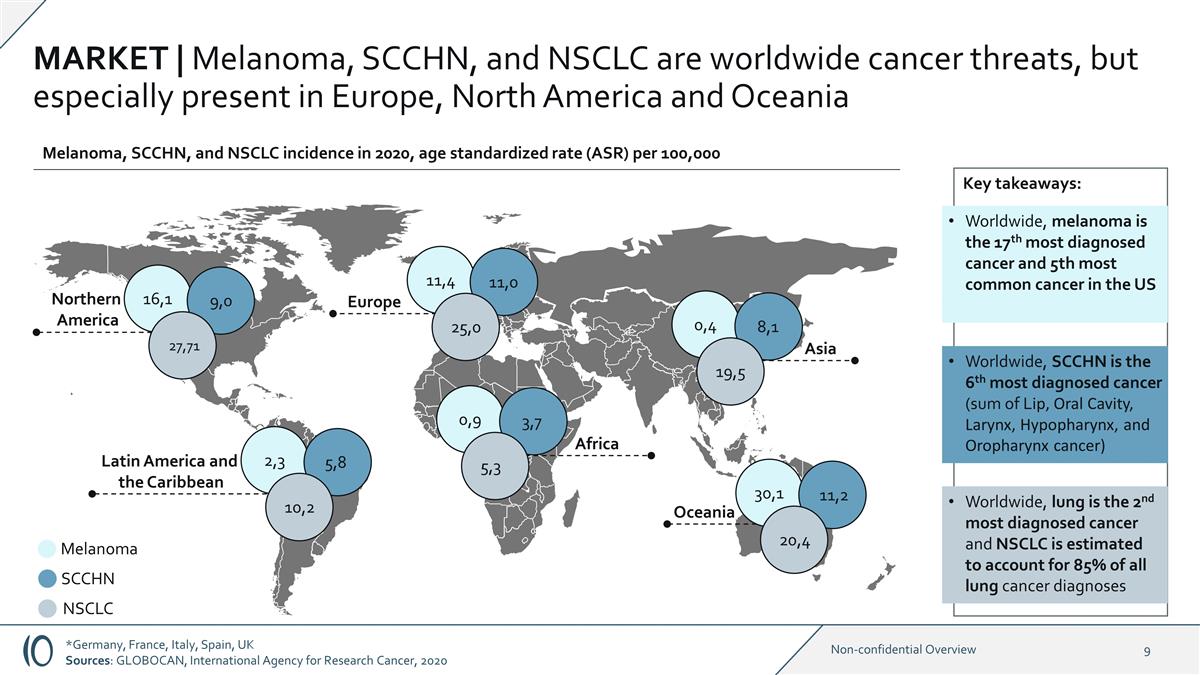
MARKET | Melanoma, SCCHN, and NSCLC
are worldwide cancer threats, but especially present in Europe, North America and Oceania 16,1 Northern America Oceania Europe 9,0 Melanoma SCCHN NSCLC 27,71 11,4 11,0 25,0 30,1 11,2 20,4 Asia 0,4 8,1 19,5 Melanoma, SCCHN, and NSCLC incidence in
2020, age standardized rate (ASR) per 100,000 Key takeaways: Worldwide, melanoma is the 17th most diagnosed cancer and 5th most common cancer in the US Worldwide, SCCHN is the 6th most diagnosed cancer (sum of Lip, Oral Cavity, Larynx, Hypopharynx,
and Oropharynx cancer) Worldwide, lung is the 2nd most diagnosed cancer and NSCLC is estimated to account for 85% of all lung cancer diagnoses Latin America and the Caribbean 2,3 5,8 10,2 Africa 0,9 3,7 5,3 *Germany, France, Italy, Spain, UK
Sources: GLOBOCAN, International Agency for Research Cancer, 2020
PATIENT AND MARKET PERSPECTIVE 1
OUR UNIQUE VALUE PROPOSITION 2 OUR PIPELINE AND THE SCIENCE BEHIND IT 3 GROWTH STRATEGY AND OUTLOOK 4 THE IO BIOTECH TEAM 5 CONTENT
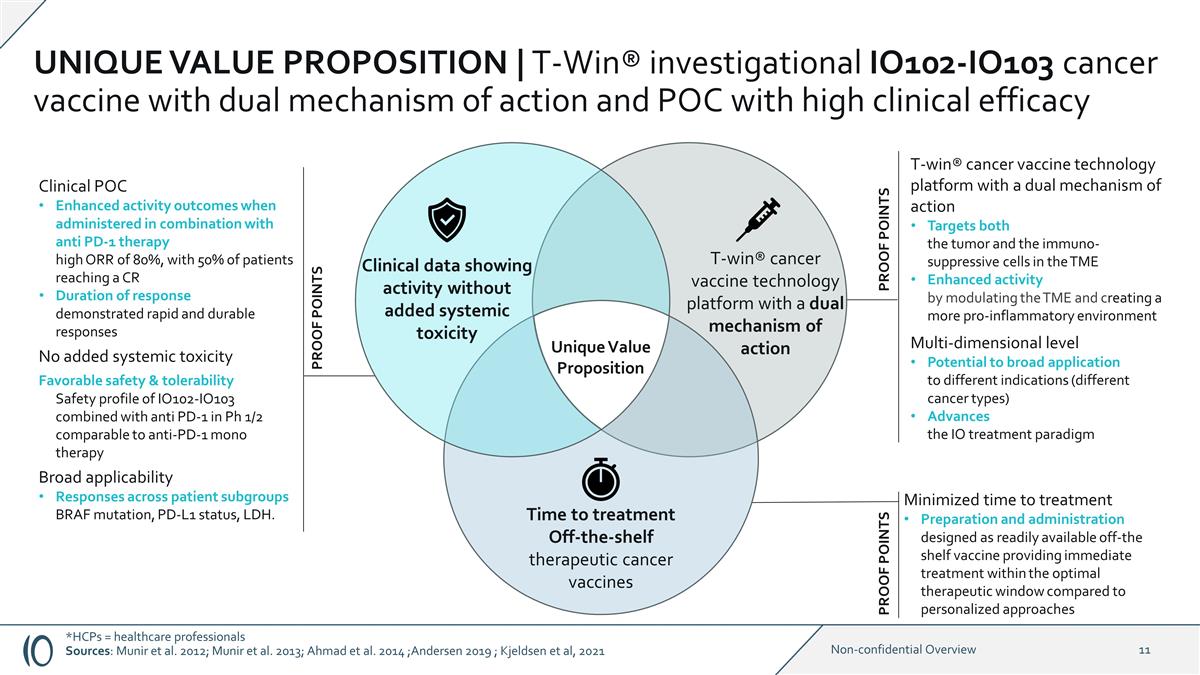
Clinical POC Enhanced activity
outcomes when administered in combination with anti PD-1 therapy high ORR of 80%, with 50% of patients reaching a CR Duration of response demonstrated rapid and durable responses No added systemic toxicity Favorable safety & tolerability
Safety profile of IO102-IO103 combined with anti PD-1 in Ph 1/2 comparable to anti-PD-1 mono therapy Broad applicability Responses across patient subgroups BRAF mutation, PD-L1 status, LDH. Minimized time to treatment Preparation and
administration designed as readily available off-the shelf vaccine providing immediate treatment within the optimal therapeutic window compared to personalized approaches T-win® cancer vaccine technology platform with a dual mechanism of action
Targets both the tumor and the immuno- suppressive cells in the TME Enhanced activity by modulating the TME and creating a more pro-inflammatory environment Multi-dimensional level Potential to broad application to different indications (different
cancer types) Advances the IO treatment paradigm Clinical data showing activity without added systemic toxicity T-win® cancer vaccine technology platform with a dual mechanism of action Time to treatment Off-the-shelf therapeutic cancer
vaccines Unique Value Proposition PROOF POINTS PROOF POINTS PROOF POINTS *HCPs = healthcare professionals Sources: Munir et al. 2012; Munir et al. 2013; Ahmad et al. 2014 ;Andersen 2019 ; Kjeldsen et al, 2021 UNIQUE VALUE PROPOSITION |
T-Win® investigational IO102-IO103 cancer vaccine with dual mechanism of action and POC with high clinical efficacy
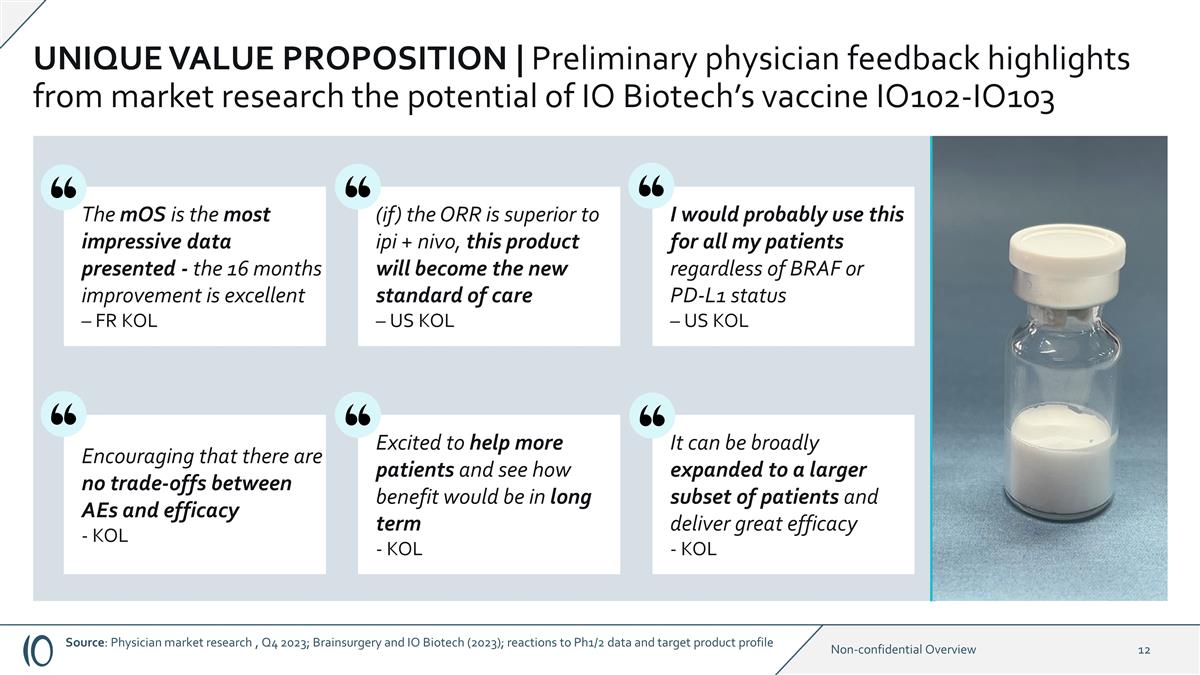
UNIQUE VALUE PROPOSITION |
Preliminary physician feedback highlights from market research the potential of IO Biotech’s vaccine IO102-IO103 The mOS is the most impressive data presented - the 16 months improvement is excellent – FR KOL (if) the ORR is superior to
ipi + nivo, this product will become the new standard of care – US KOL I would probably use this for all my patients regardless of BRAF or PD-L1 status – US KOL Excited to help more patients and see how benefit would be in long term -
KOL Encouraging that there are no trade-offs between AEs and efficacy - KOL It can be broadly expanded to a larger subset of patients and deliver great efficacy - KOL Source: Physician market research , Q4 2023; Brainsurgery and IO Biotech (2023);
reactions to Ph1/2 data and target product profile
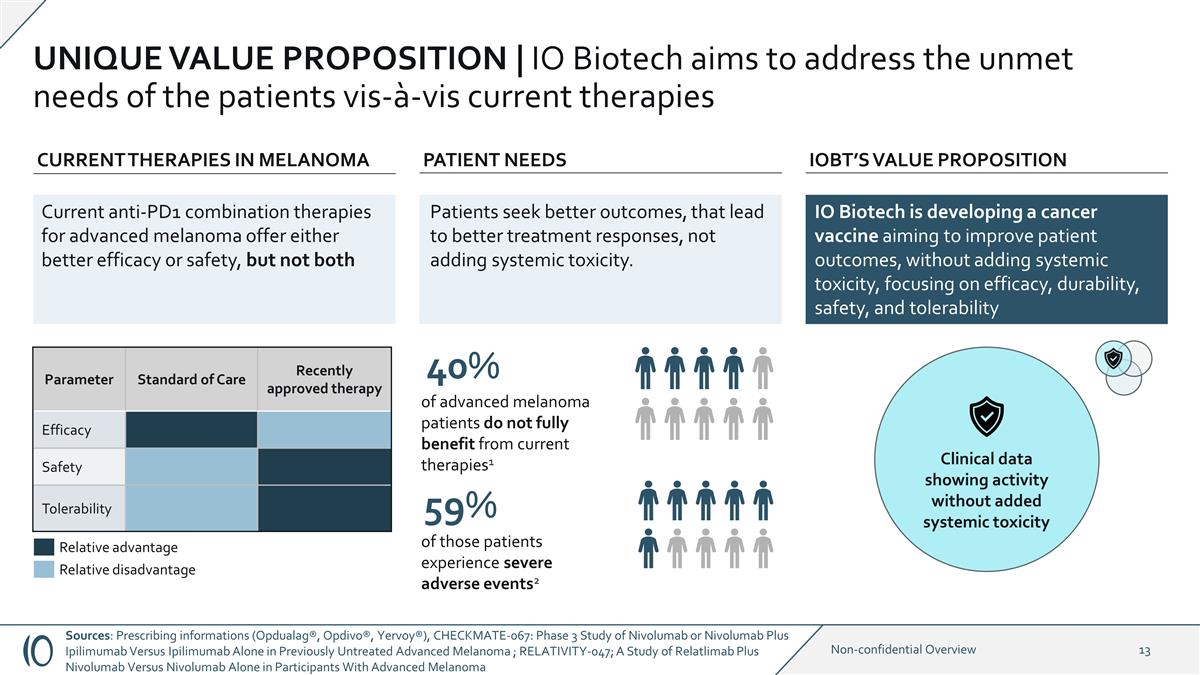
IO Biotech is developing a cancer
vaccine aiming to improve patient outcomes, without adding systemic toxicity, focusing on efficacy, durability, safety, and tolerability UNIQUE VALUE PROPOSITION | IO Biotech aims to address the unmet needs of the patients vis-à-vis current
therapies Parameter Standard of Care Recently approved therapy Efficacy Safety Tolerability CURRENT THERAPIES IN MELANOMA IOBT’S VALUE PROPOSITION Initials: Initials: 59% of those patients experience severe adverse events2 40% of advanced
melanoma patients do not fully benefit from current therapies1 Clinical data showing activity without added systemic toxicity Current anti-PD1 combination therapies for advanced melanoma offer either better efficacy or safety, but not both PATIENT
NEEDS 1/10 2/10 3/10 5/10 6/10 7/10 8/10 9/10 10/10 1/10 2/10 3/10 5/10 7/10 8/10 9/10 10/10 Relative disadvantage Relative advantage Patients seek better outcomes, that lead to better treatment responses, not adding systemic toxicity. Sources:
Prescribing informations (Opdualag®, Opdivo®, Yervoy®), CHECKMATE-067: Phase 3 Study of Nivolumab or Nivolumab Plus Ipilimumab Versus Ipilimumab Alone in Previously Untreated Advanced Melanoma ; RELATIVITY-047; A Study of Relatlimab
Plus Nivolumab Versus Nivolumab Alone in Participants With Advanced Melanoma
PATIENT AND MARKET PERSPECTIVE 1
OUR UNIQUE VALUE PROPOSITION 2 OUR PIPELINE AND THE SCIENCE BEHIND IT 3 GROWTH STRATEGY AND OUTLOOK 4 THE IO BIOTECH TEAM 5 CONTENT
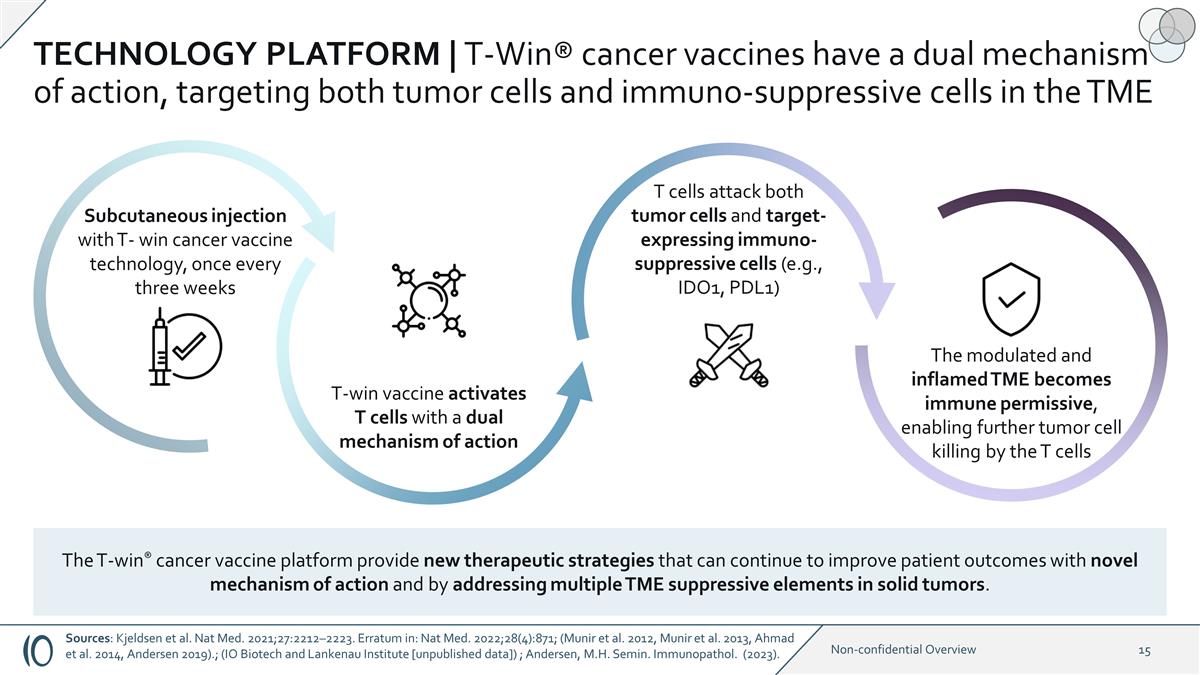
TECHNOLOGY PLATFORM | T-Win®
cancer vaccines have a dual mechanism of action, targeting both tumor cells and immuno-suppressive cells in the TME T cells attack both tumor cells and target-expressing immuno-suppressive cells (e.g., IDO1, PDL1) The modulated and inflamed TME
becomes immune permissive, enabling further tumor cell killing by the T cells T-win vaccine activates T cells with a dual mechanism of action Subcutaneous injection with T- win cancer vaccine technology, once every three weeks The T-win® cancer
vaccine platform provide new therapeutic strategies that can continue to improve patient outcomes with novel mechanism of action and by addressing multiple TME suppressive elements in solid tumors. Sources: Kjeldsen et al. Nat
Med. 2021;27:2212–2223. Erratum in: Nat Med. 2022;28(4):871; (Munir et al. 2012, Munir et al. 2013, Ahmad et al. 2014, Andersen 2019).; (IO Biotech and Lankenau Institute [unpublished data]) ; Andersen, M.H.
Semin. Immunopathol. (2023).
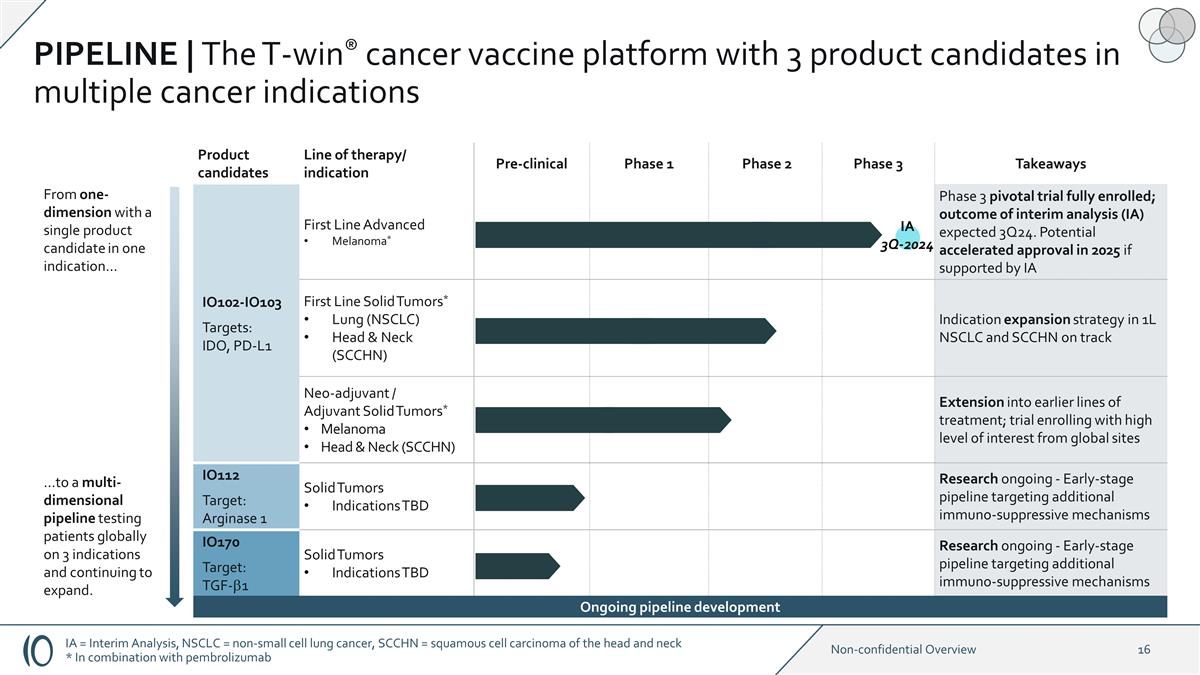
IA 3Q-2024 Product candidates Line
of therapy/ indication Pre-clinical Phase 1 Phase 2 Phase 3 Takeaways IO102-IO103 Targets: IDO, PD-L1 First Line Advanced Melanoma* Phase 3 pivotal trial fully enrolled; outcome of interim analysis (IA) expected 3Q24. Potential
accelerated approval in 2025 if supported by IA IO102–IO103 First Line Solid Tumors* Lung (NSCLC) Head & Neck (SCCHN) Indication expansion strategy in 1L NSCLC and SCCHN on track Neo-adjuvant / Adjuvant Solid Tumors* Melanoma Head &
Neck (SCCHN) Extension into earlier lines of treatment; trial enrolling with high level of interest from global sites IO112 Target: Arginase 1 Solid Tumors Indications TBD Research ongoing - Early-stage pipeline targeting additional
immuno-suppressive mechanisms IO170 Target: TGF-b1 Solid Tumors Indications TBD Research ongoing - Early-stage pipeline targeting additional immuno-suppressive mechanisms From one-dimension with a single product candidate in one indication…
…to a multi-dimensional pipeline testing patients globally on 3 indications and continuing to expand. Ongoing pipeline development PIPELINE | The T-win® cancer vaccine platform with 3 product candidates in multiple cancer indications IA =
Interim Analysis, NSCLC = non-small cell lung cancer, SCCHN = squamous cell carcinoma of the head and neck * In combination with pembrolizumab
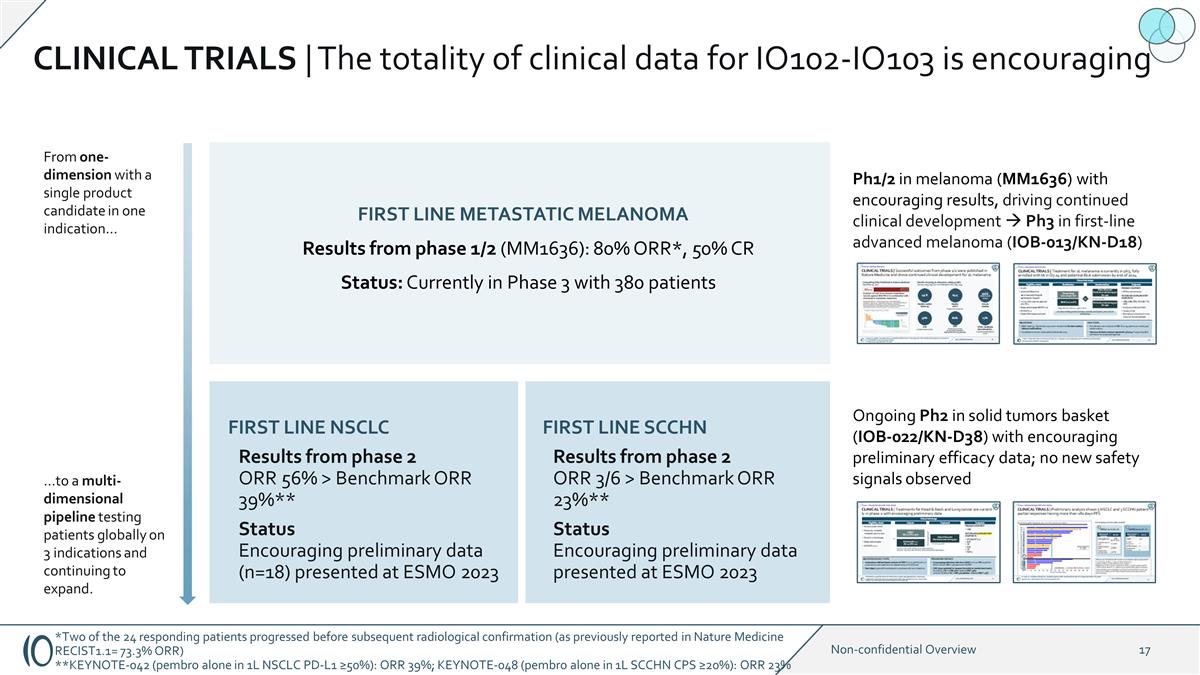
CLINICAL TRIALS | The totality of
clinical data for IO102-IO103 is encouraging Ph1/2 in melanoma (MM1636) with encouraging results, driving continued clinical development à Ph3 in first-line advanced melanoma (IOB-013/KN-D18) Ongoing Ph2 in solid tumors basket (IOB-022/KN-D38)
with encouraging preliminary efficacy data; no new safety signals observed FIRST LINE METASTATIC MELANOMA Results from phase 1/2 (MM1636): 80% ORR*, 50% CR Status: Currently in Phase 3 with 380 patients FIRST LINE NSCLC Results from phase 2 ORR 56%
> Benchmark ORR 39%** Status Encouraging preliminary data (n=18) presented at ESMO 2023 FIRST LINE SCCHN Results from phase 2 ORR 3/6 > Benchmark ORR 23%** Status Encouraging preliminary data presented at ESMO 2023 From one-dimension with a
single product candidate in one indication… …to a multi-dimensional pipeline testing patients globally on 3 indications and continuing to expand. *Two of the 24 responding patients progressed before subsequent radiological
confirmation (as previously reported in Nature Medicine RECIST1.1= 73.3% ORR) **KEYNOTE-042 (pembro alone in 1L NSCLC PD-L1 ≥50%): ORR 39%; KEYNOTE-048 (pembro alone in 1L SCCHN CPS ≥20%): ORR 23%
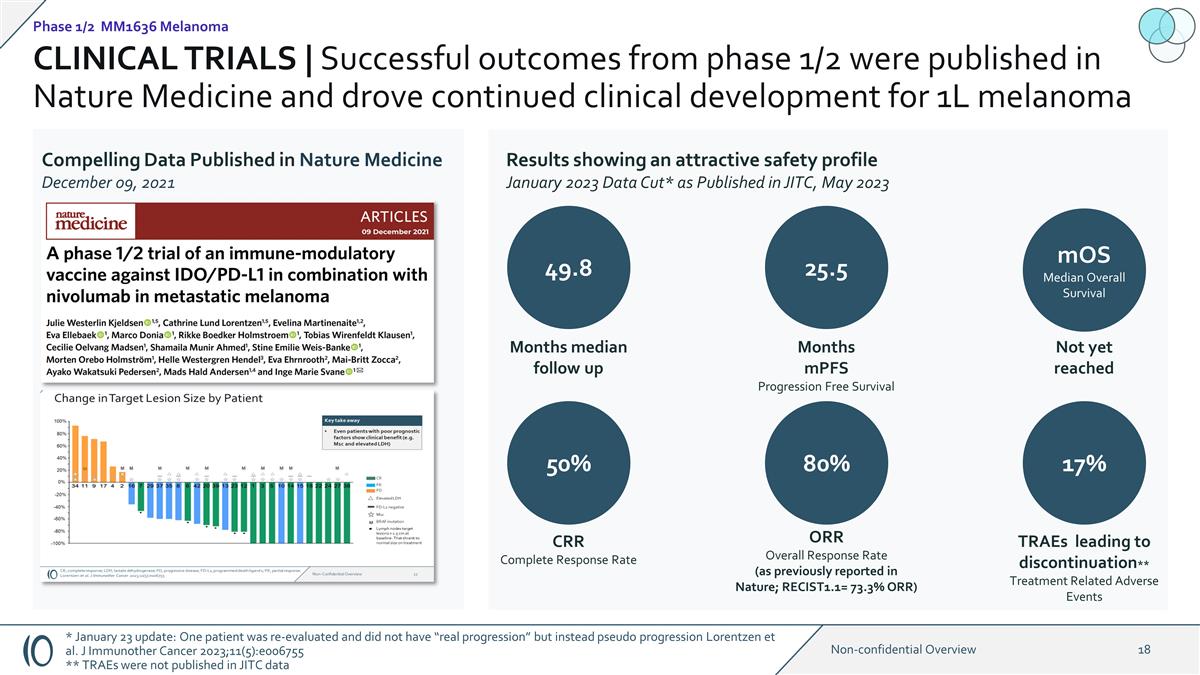
CLINICAL TRIALS | Successful
outcomes from phase 1/2 were published in Nature Medicine and drove continued clinical development for 1L melanoma Compelling Data Published in Nature Medicine December 09, 2021 Results showing an attractive safety profile January 2023 Data Cut* as
Published in JITC, May 2023 49.8 Months median follow up 25.5 mOS Median Overall Survival Not yet reached 50% 80% 17% CRR Complete Response Rate ORR Overall Response Rate (as previously reported in Nature; RECIST1.1= 73.3% ORR) TRAEs leading to
discontinuation** Treatment Related Adverse Events Months mPFS Progression Free Survival Phase 1/2 MM1636 Melanoma * January 23 update: One patient was re-evaluated and did not have “real progression” but instead pseudo progression
Lorentzen et al. J Immunother Cancer 2023;11(5):e006755 ** TRAEs were not published in JITC data
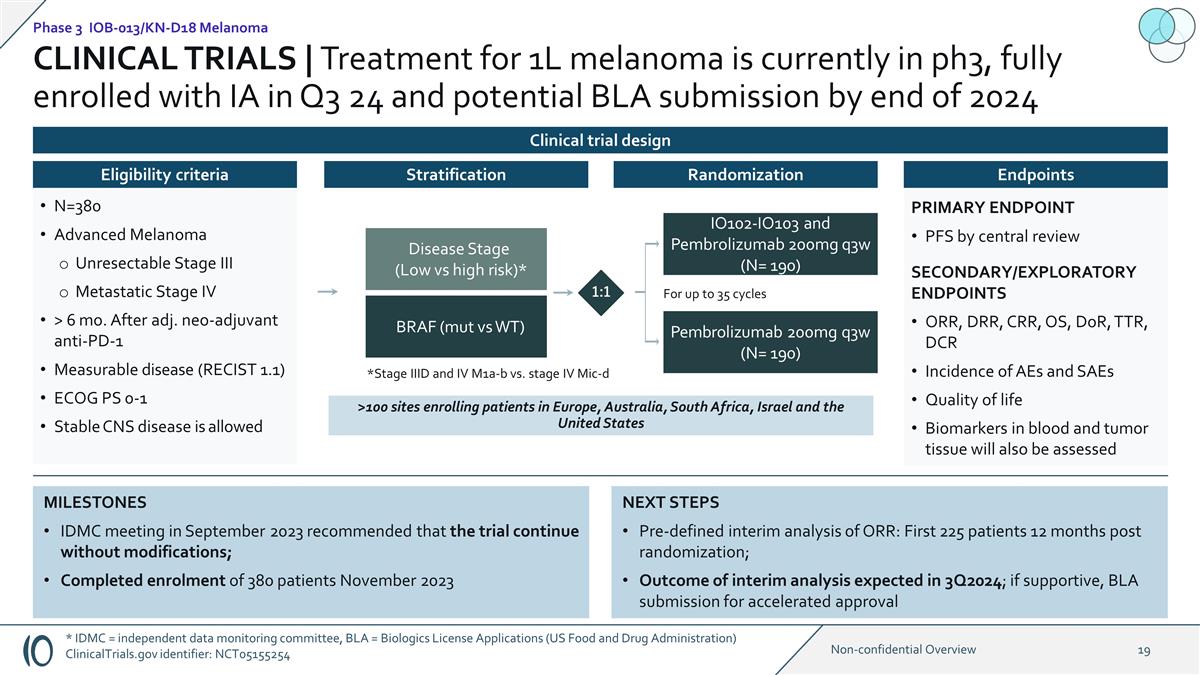
NEXT STEPS Pre-defined interim
analysis of ORR: First 225 patients 12 months post randomization; Outcome of interim analysis expected in 3Q2024; if supportive, BLA submission for accelerated approval MILESTONES IDMC meeting in September 2023 recommended that the trial continue
without modifications; Completed enrolment of 380 patients November 2023 >100 sites enrolling patients in Europe, Australia, South Africa, Israel and the United States PRIMARY ENDPOINT PFS by central review SECONDARY/EXPLORATORY ENDPOINTS ORR,
DRR, CRR, OS, DoR, TTR, DCR Incidence of AEs and SAEs Quality of life Biomarkers in blood and tumor tissue will also be assessed Disease Stage (Low vs high risk)* BRAF (mut vs WT) IO102-IO103 and Pembrolizumab 200mg q3w (N= 190) Pembrolizumab
200mg q3w (N= 190) For up to 35 cycles 1:1 *Stage IIID and IV M1a-b vs. stage IV Mic-d Stratification Endpoints Randomization N=380 Advanced Melanoma Unresectable Stage III Metastatic Stage IV > 6 mo. After adj. neo-adjuvant anti-PD-1 Measurable
disease (RECIST 1.1) ECOG PS 0-1 Stable CNS disease is allowed Eligibility criteria CLINICAL TRIALS | Treatment for 1L melanoma is currently in ph3, fully enrolled with IA in Q3 24 and potential BLA submission by end of 2024 Phase 3 IOB-013/KN-D18
Melanoma Clinical trial design * IDMC = independent data monitoring committee, BLA = Biologics License Applications (US Food and Drug Administration) ClinicalTrials.gov identifier: NCT05155254
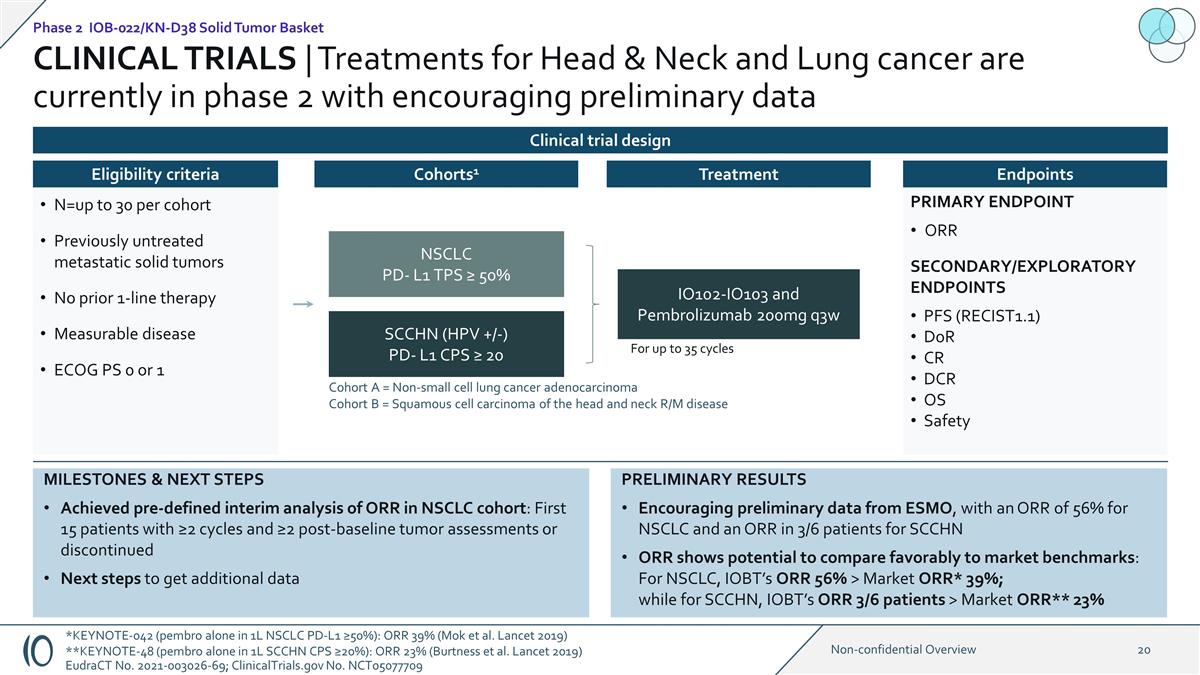
PRIMARY ENDPOINT ORR
SECONDARY/EXPLORATORY ENDPOINTS PFS (RECIST1.1) DoR CR DCR OS Safety CLINICAL TRIALS | Treatments for Head & Neck and Lung cancer are currently in phase 2 with encouraging preliminary data 1:1 IO102-IO103 and Pembrolizumab 200mg q3w NSCLC PD- L1
TPS ≥ 50% SCCHN (HPV +/-) PD- L1 CPS ≥ 20 Cohort A = Non-small cell lung cancer adenocarcinoma Cohort B = Squamous cell carcinoma of the head and neck R/M disease For up to 35 cycles N=up to 30 per cohort Previously untreated metastatic
solid tumors No prior 1-line therapy Measurable disease ECOG PS 0 or 1 Eligibility criteria Cohorts1 Treatment Endpoints PRELIMINARY RESULTS Encouraging preliminary data from ESMO, with an ORR of 56% for NSCLC and an ORR in 3/6 patients for SCCHN
ORR shows potential to compare favorably to market benchmarks: For NSCLC, IOBT’s ORR 56% > Market ORR* 39%; while for SCCHN, IOBT’s ORR 3/6 patients > Market ORR** 23% MILESTONES & NEXT STEPS Achieved pre-defined interim
analysis of ORR in NSCLC cohort: First 15 patients with ≥2 cycles and ≥2 post-baseline tumor assessments or discontinued Next steps to get additional data Phase 2 IOB-022/KN-D38 Solid Tumor Basket Clinical trial design
*KEYNOTE-042 (pembro alone in 1L NSCLC PD-L1 ≥50%): ORR 39% (Mok et al. Lancet 2019) **KEYNOTE-48 (pembro alone in 1L SCCHN CPS ≥20%): ORR 23% (Burtness et al. Lancet 2019) EudraCT No. 2021-003026-69; ClinicalTrials.gov No. NCT05077709
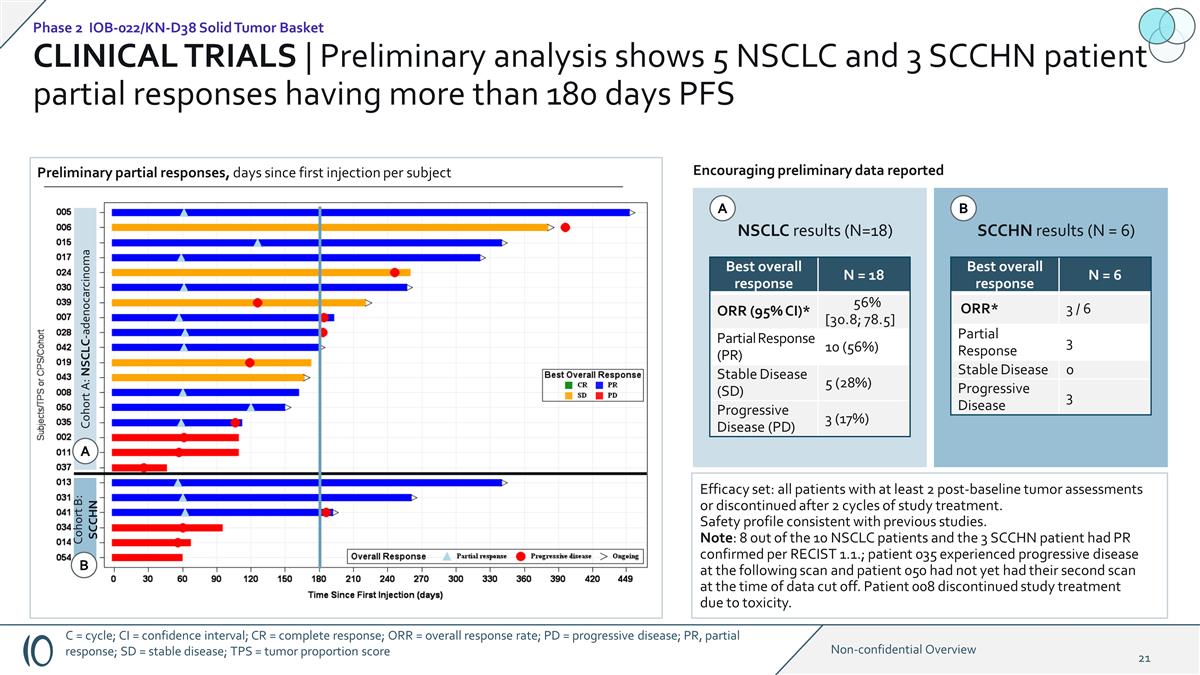
CLINICAL TRIALS | Preliminary
analysis shows 5 NSCLC and 3 SCCHN patient partial responses having more than 180 days PFS Cohort A: NSCLC-adenocarcinoma Cohort B: SCCHN NSCLC results (N=18) SCCHN results (N = 6) Encouraging preliminary data reported Best overall response N = 18
ORR (95% CI)* 56% [30.8; 78.5] Partial Response (PR) 10 (56%) Stable Disease (SD) 5 (28%) Progressive Disease (PD) 3 (17%) Efficacy set: all patients with at least 2 post-baseline tumor assessments or discontinued after 2 cycles of study treatment.
Safety profile consistent with previous studies. Note: 8 out of the 10 NSCLC patients and the 3 SCCHN patient had PR confirmed per RECIST 1.1.; patient 035 experienced progressive disease at the following scan and patient 050 had not yet had their
second scan at the time of data cut off. Patient 008 discontinued study treatment due to toxicity. Best overall response N = 6 ORR* 3 / 6 Partial Response 3 Stable Disease 0 Progressive Disease 3 Preliminary partial responses, days since first
injection per subject A A B B Phase 2 IOB-022/KN-D38 Solid Tumor Basket C = cycle; CI = confidence interval; CR = complete response; ORR = overall response rate; PD = progressive disease; PR, partial response; SD = stable disease; TPS = tumor
proportion score
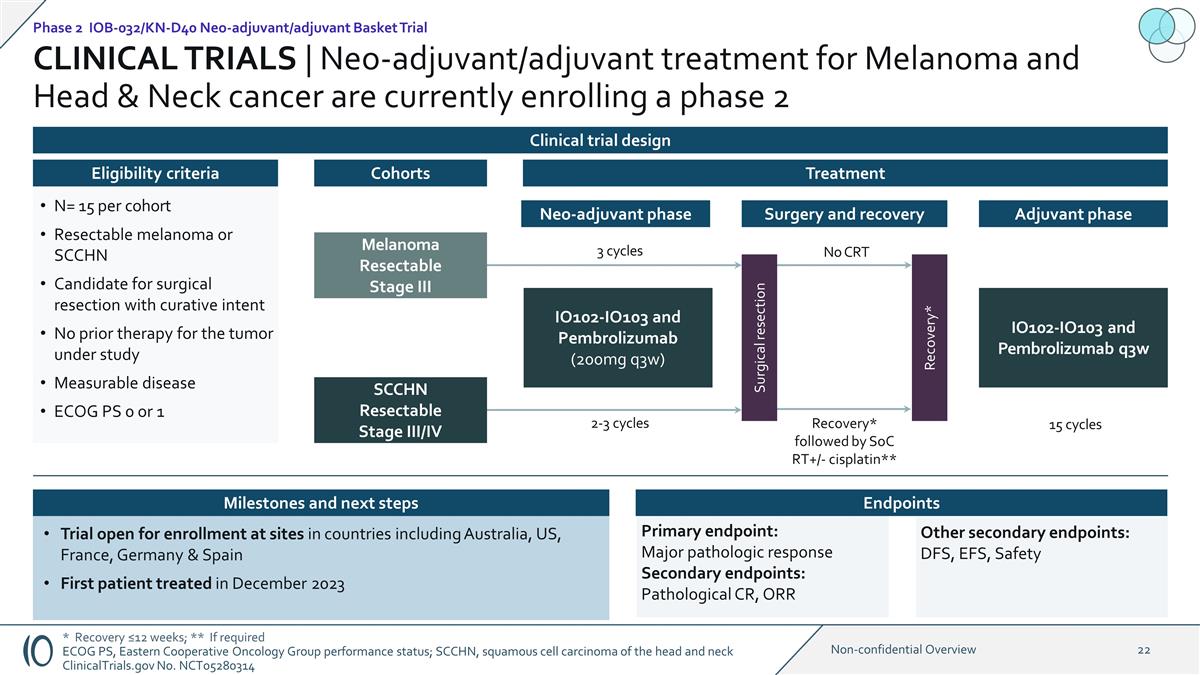
CLINICAL TRIALS |
Neo-adjuvant/adjuvant treatment for Melanoma and Head & Neck cancer are currently enrolling a phase 2 1:1 IO102-IO103 and Pembrolizumab (200mg q3w) Melanoma Resectable Stage III SCCHN Resectable Stage III/IV N= 15 per cohort Resectable melanoma
or SCCHN Candidate for surgical resection with curative intent No prior therapy for the tumor under study Measurable disease ECOG PS 0 or 1 Eligibility criteria Cohorts Treatment Trial open for enrollment at sites in countries including
Australia, US, France, Germany & Spain First patient treated in December 2023 Phase 2 IOB-032/KN-D40 Neo-adjuvant/adjuvant Basket Trial Clinical trial design Neo-adjuvant phase 2-3 cycles Surgical resection Surgery and recovery
Recovery* IO102-IO103 and Pembrolizumab q3w 15 cycles Adjuvant phase 3 cycles * Recovery ≤12 weeks; ** If required ECOG PS, Eastern Cooperative Oncology Group performance status; SCCHN, squamous cell carcinoma of the head and neck
ClinicalTrials.gov No. NCT05280314 Recovery* followed by SoC RT+/- cisplatin** No CRT Primary endpoint: Major pathologic response Secondary endpoints: Pathological CR, ORR Other secondary endpoints: DFS, EFS, Safety Milestones and next steps
Endpoints
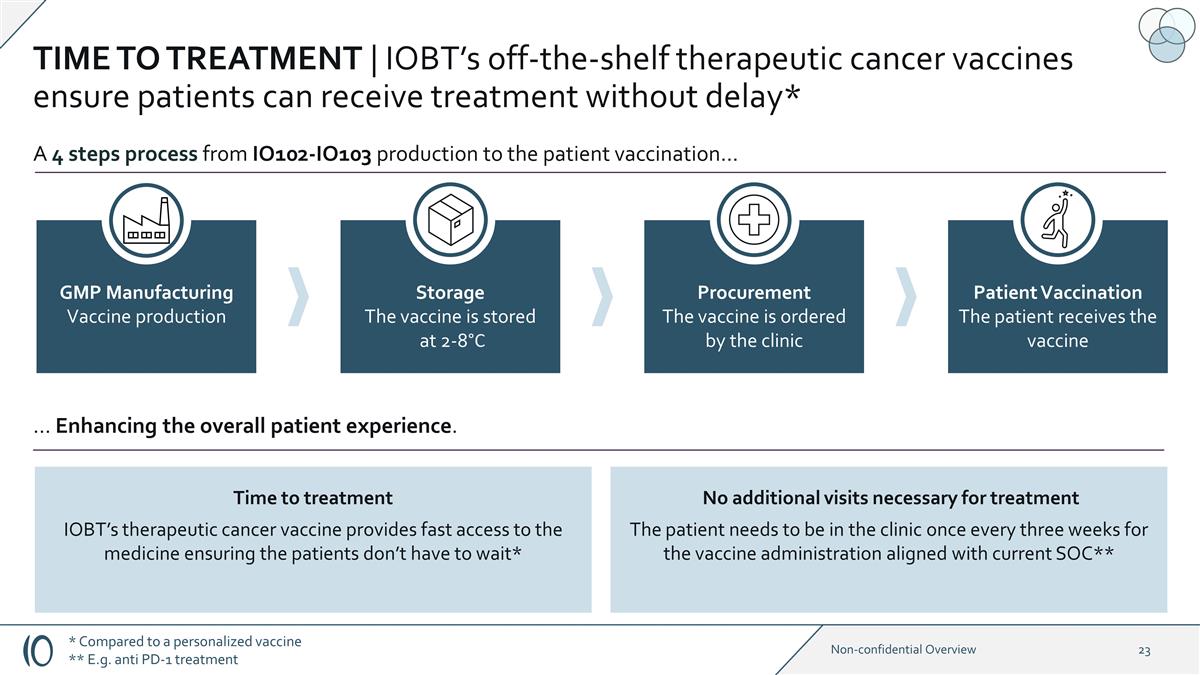
TIME TO TREATMENT | IOBT’s
off-the-shelf therapeutic cancer vaccines ensure patients can receive treatment without delay* GMP Manufacturing Vaccine production Storage The vaccine is stored at 2-8°C Procurement The vaccine is ordered by the clinic Patient Vaccination The
patient receives the vaccine A 4 steps process from IO102-IO103 production to the patient vaccination… Time to treatment IOBT’s therapeutic cancer vaccine provides fast access to the medicine ensuring the patients don’t have to
wait* … Enhancing the overall patient experience. No additional visits necessary for treatment The patient needs to be in the clinic once every three weeks for the vaccine administration aligned with current SOC** * Compared to a personalized
vaccine ** E.g. anti PD-1 treatment
PATIENT AND MARKET PERSPECTIVE 1
OUR UNIQUE VALUE PROPOSITION 2 OUR PIPELINE AND THE SCIENCE BEHIND IT 3 GROWTH STRATEGY AND OUTLOOK 4 THE IO BIOTECH TEAM 5 CONTENT
IOB-022 – basket trial data
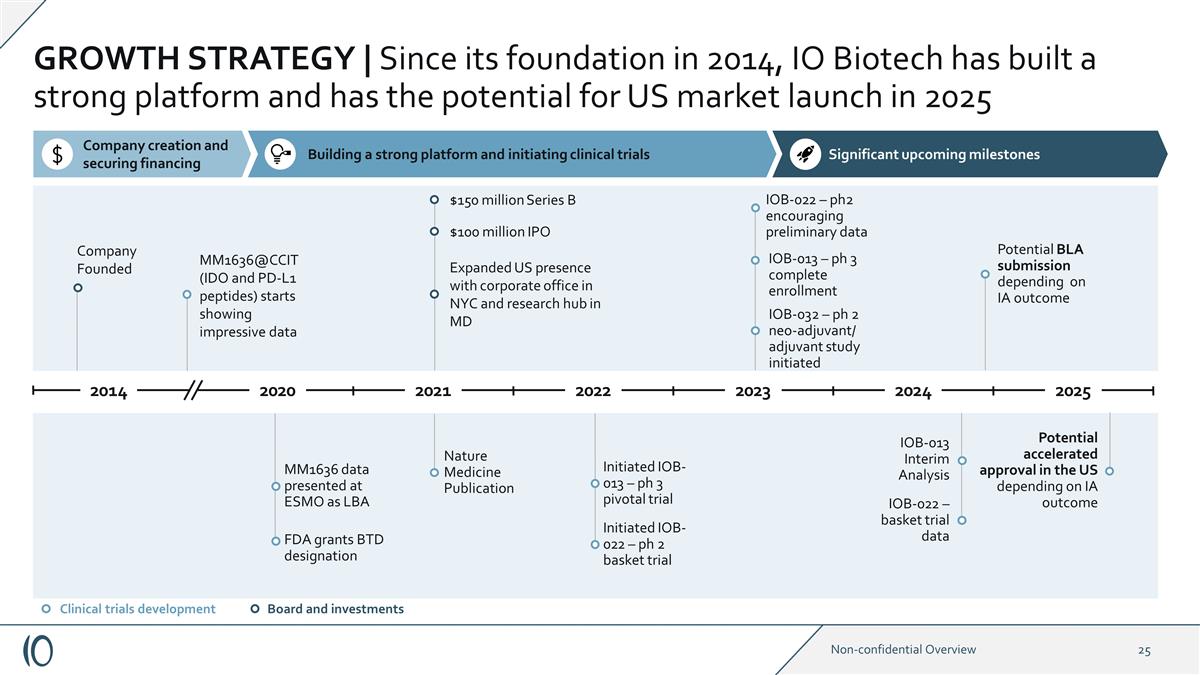
GROWTH STRATEGY | Since its foundation in 2014, IO Biotech has built a strong platform and has the potential for US market launch in 2025 Company Founded MM1636@CCIT (IDO and PD-L1 peptides) starts showing impressive data FDA grants BTD designation
MM1636 data presented at ESMO as LBA Nature Medicine Publication $150 million Series B $100 million IPO Expanded US presence with corporate office in NYC and research hub in MD Initiated IOB-022 – ph 2 basket trial Initiated IOB-013 – ph
3 pivotal trial IOB-022 – ph2 encouraging preliminary data Board and investments Clinical trials development IOB-013 Interim Analysis IOB-013 – ph 3 complete enrollment Potential BLA submission depending on IA outcome Potential
accelerated approval in the US depending on IA outcome Significant upcoming milestones Building a strong platform and initiating clinical trials Company creation and securing financing 2021 2020 2014 2022 2023 2024 2025 IOB-032 – ph 2
neo-adjuvant/ adjuvant study initiated
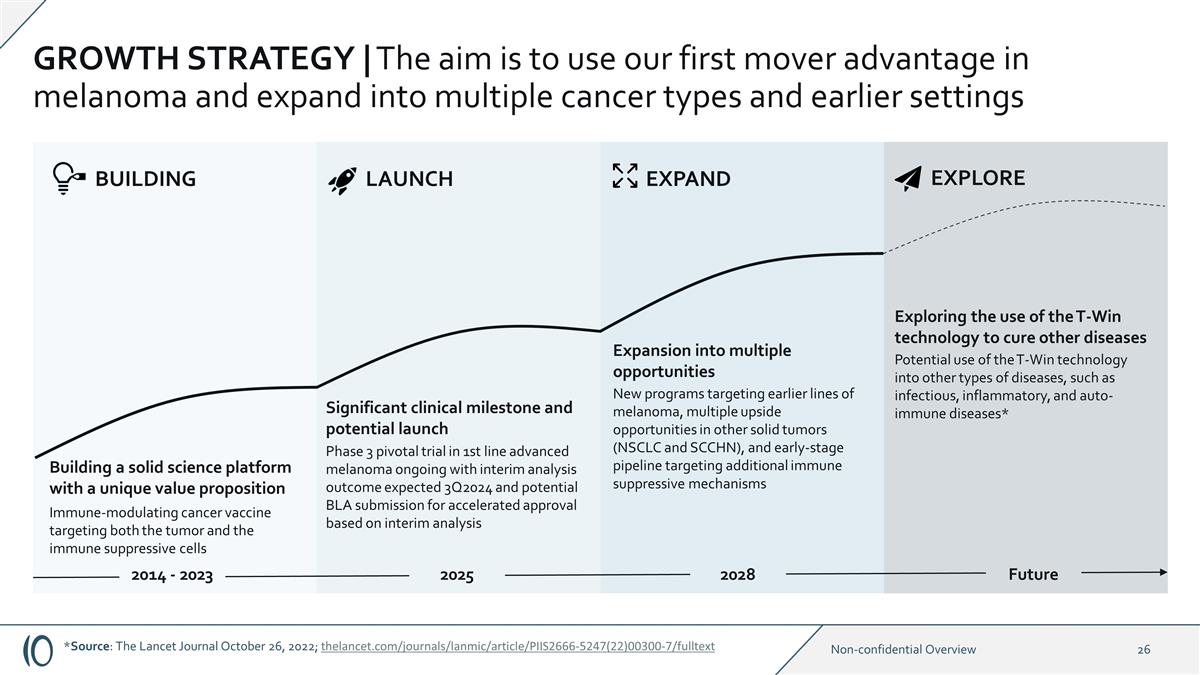
GROWTH STRATEGY | The aim is to use
our first mover advantage in melanoma and expand into multiple cancer types and earlier settings Building a solid science platform with a unique value proposition Immune-modulating cancer vaccine targeting both the tumor and the immune suppressive
cells Significant clinical milestone and potential launch Phase 3 pivotal trial in 1st line advanced melanoma ongoing with interim analysis outcome expected 3Q2024 and potential BLA submission for accelerated approval based on interim analysis New
programs targeting earlier lines of melanoma, multiple upside opportunities in other solid tumors (NSCLC and SCCHN), and early-stage pipeline targeting additional immune suppressive mechanisms Expansion into multiple opportunities 2025 2028 EXPAND
BUILDING LAUNCH 2014 - 2023 Potential use of the T-Win technology into other types of diseases, such as infectious, inflammatory, and auto-immune diseases* Exploring the use of the T-Win technology to cure other diseases Future EXPLORE *Source: The
Lancet Journal October 26, 2022; thelancet.com/journals/lanmic/article/PIIS2666-5247(22)00300-7/fulltext
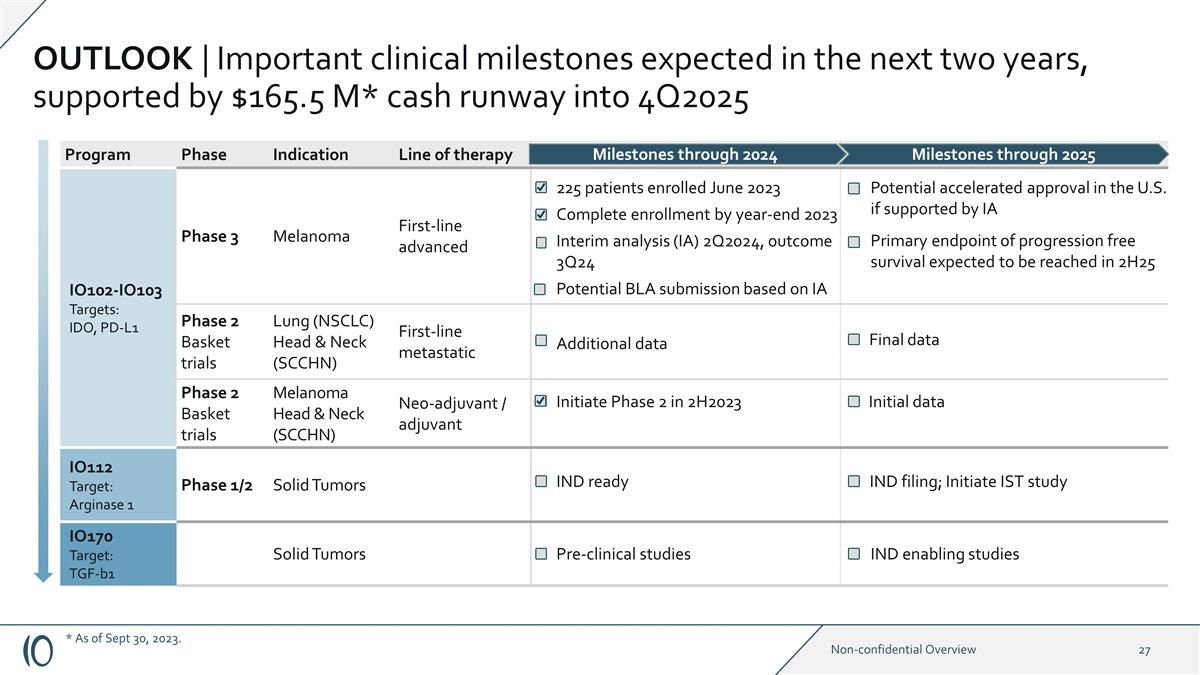
OUTLOOK | Important clinical
milestones expected in the next two years, supported by $165.5 M* cash runway into 4Q2025 Program Phase Indication Line of therapy IO102-IO103 Targets: IDO, PD-L1 Phase 3 Melanoma First-line advanced IO102–IO103 Phase 2 Basket
trials Lung (NSCLC) Head & Neck (SCCHN) First-line metastatic Phase 2 Basket trials Melanoma Head & Neck (SCCHN) Neo-adjuvant / adjuvant IO112 Target: Arginase 1 Phase 1/2 Solid Tumors IO170 Target: TGF-b1 Solid Tumors Milestones through
2024 Milestones through 2025 225 patients enrolled June 2023 Complete enrollment by year-end 2023 Interim analysis (IA) 2Q2024, outcome 3Q24 Potential accelerated approval in the U.S. if supported by IA Primary endpoint of progression free survival
expected to be reached in 2H25 Additional data Final data Initiate Phase 2 in 2H2023 Initial data IND ready IND filing; Initiate IST study Pre-clinical studies IND enabling studies Potential BLA submission based on IA * As of Sept 30, 2023.
PATIENT AND MARKET PERSPECTIVE 1
OUR UNIQUE VALUE PROPOSITION 2 OUR PIPELINE AND THE SCIENCE BEHIND IT 3 GROWTH STRATEGY AND OUTLOOK 4 THE IO BIOTECH TEAM 5 CONTENT

THE TEAM | We have a strong
management team with large biopharma and biotech experience Mai-Britt Zocca, Ph.D. Chief Executive Officer Qasim Ahmad, M.D. Chief Medical Officer Amy Sullivan, M.B.A. Chief Financial Officer Eric Faulkner, M.B.A. Chief Technical Officer Devin Smith
General Counsel Dan Mannix, Ph.D. SVP Regulatory

Board of Directors THE TEAM | Our
management team is supported by the Board of Directors and the Scientific Advisory Board Peter Hirth, Ph.D. Chairman Kathleen Sereda Glaub, M.B.A. Member Christian Elling, Ph.D. Member – Lundbeckfonden Heidi Hunter Member Jack B. Nielsen, M.Sc
Member – Vivo Capital David V. Smith, M.B.A. Member Mai-Britt Zocca, Ph.D. President and CEO Scientific Advisory Board Inge Marie Svane, M.D., Ph.D Founder, Clinical Advisor Alexander Eggermont, M.D., Ph.D. Sr. Clinical Advisor Kapil Dhingra,
M.D. Strategic R&D Advisor Mads Hald Andersen, M.D., Ph.D. Founder, Scientific Advisor Hellen Collins, M.D. Member
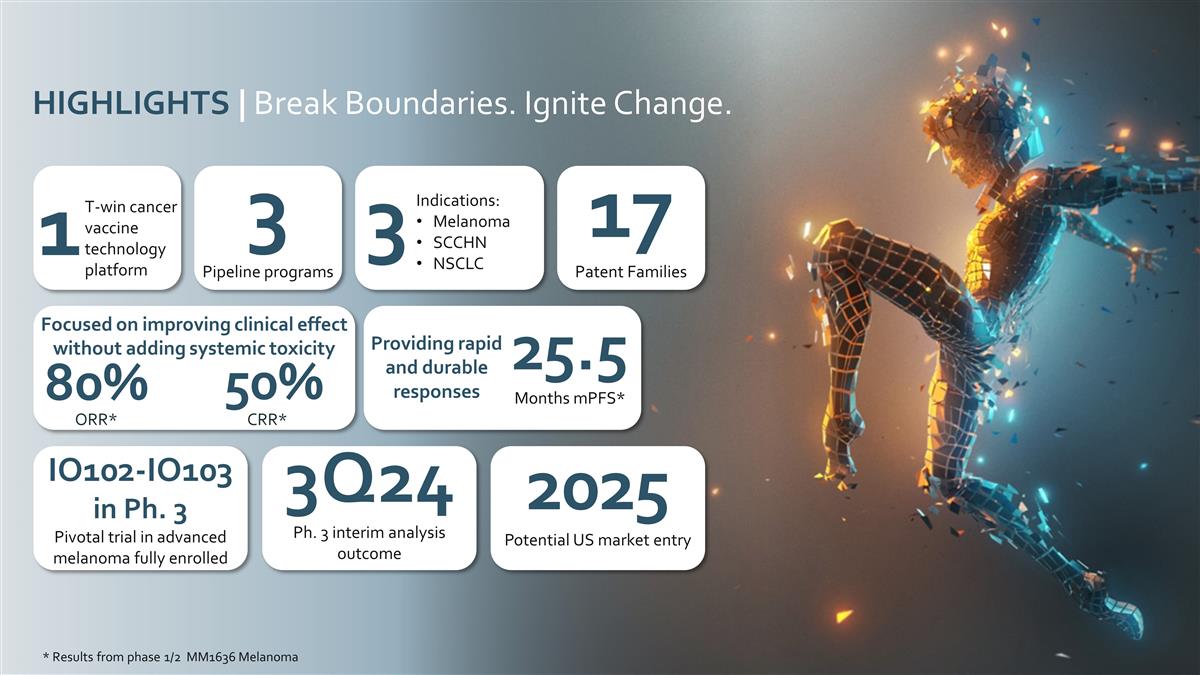
Indications: Melanoma SCCHN NSCLC 3
T-win cancer vaccine technology platform 1 3 Pipeline programs 17 Patent Families Focused on improving clinical effect without adding systemic toxicity 50% 80% * Results from phase 1/2 MM1636 Melanoma 25.5 Months mPFS* Providing rapid and durable
responses HIGHLIGHTS | Break Boundaries. Ignite Change. 3Q24 Ph. 3 interim analysis outcome IO102-IO103 in Ph. 3 Pivotal trial in advanced melanoma fully enrolled 2025 Potential US market entry ORR* CRR*
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionISO 3166-1 alpha-2 country code.
| Name: |
dei_EntityAddressCountry |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:countryCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Grafico Azioni IO Biotech (NASDAQ:IOBT)
Storico
Da Nov 2024 a Dic 2024

Grafico Azioni IO Biotech (NASDAQ:IOBT)
Storico
Da Dic 2023 a Dic 2024
