Form 8-K - Current report
21 Maggio 2024 - 11:00PM
Edgar (US Regulatory)
NONE0001792581false00017925812024-05-212024-05-21
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): May 21, 2024
KIROMIC BIOPHARMA, INC.
(Exact name of registrant as specified in its charter)
| | | | |
Delaware | | 001-39619 | | 46-4762913 |
(State or other jurisdiction of incorporation) | | (Commission File Number) | | (IRS Employer Identification No.) |
7707 Fannin, Suite 140
Houston, TX, 77054
(Address of principal executive offices) (Zip Code)
Registrant's telephone number, including area code (832) 968-4888
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| | | | |
Title of Each Class | | Trading Symbol(s) | | Name of Each Exchange on Which Registered |
Common Stock, $0.001 par value | | KRBP | | The OTC QB Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01Regulation FD Disclosure
Kiromic BioPharma, Inc. (the “Company”) intends to conduct meetings with third parties in which its corporate slide presentation will be presented. A copy of the presentation materials is attached as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
The information in this Item 7.01 and the document attached as Exhibit 99.1 is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities and Exchange Act of 1934, as amended (the “Exchange Act”), nor otherwise subject to the liabilities of that section, nor incorporated by reference in any filing under the Securities Act of 1933 or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing.
Item 9.01.Financial Statements and Exhibits
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | |
| Kiromic BioPharma, Inc. |
| |
Date: May 21, 2024 | By: | /s/ Pietro Bersani |
| | Pietro Bersani |
| | Chief Executive Officer |
Exhibit 99.1

| Revolutionizing CAR T-Cell Therapy
BioPharma
OTCQB: KRBP
Kiromic.com
May 2024 |

| PAGE
|
Forward-Looking Statements 2
This presentation contains forward-looking statements that involve substantial risks and uncertainties. Kiromic makes
such forward-looking statements pursuant to the safe harbor provisions of the United States Private Securities Litigation
Reform Act, Section 21E of the Securities Exchange Act of 1934, as amended, and other federal securities laws. All
statements other than statements of historical facts are forward-looking statements. In some cases, you can identify
forward-looking statements by terms such as: “will,” “potential,” “could,” “can,” “believe,” “intends,” “continue,” “plans,”
“expects,” “anticipates,” “estimates,” “may,” or the negative of these terms or other comparable terminology. These
forward-looking statements include, but are not limited to, statements regarding: Kiromic’s current and anticipated IND
applications including statements regarding the scope of and timing for submission of an IND application; the Deltacel
product platform; the sponsored research agreement and the data that will be generated as a result of such collaboration;
the timing for submitting and activating Kiromic’s IND applications; the benefits of utilizing non-genetically engineered
Gamma Delta T cells as our first in-human study; Kiromic’s ability to achieve its objectives; and the timing for the
initiation and successful completion of Kiromic’s clinical trials of its product candidates. These forward-looking
statements involve known and unknown risks, uncertainties and other factors that may cause actual results, levels of
activity, performance, or achievements to be materially different from the information expressed or implied expressed or
implied by these forward-looking statements. These risks and uncertainties include, but are not limited to, the risks and
uncertainties discussed in our Annual Report on Form 10-K for the year ended December 31, 2022, and as detailed from
time to time in our SEC filings. You should not rely upon forward-looking statements as predictions of future events.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot
guarantee that the future results, levels of activity, performance, or events and circumstances reflected in the forward-looking statements will be achieved or occur. Moreover, neither we nor any other person assumes responsibility for the
accuracy and completeness of the forward-looking statements. Such forward-looking statements relate only to events as
of the date of this press release. We undertake no obligation to update any forward-looking statements except to the
extent required by law. |

| PAGE
| 3
Gamma Delta T-cell (GDT) Therapy:
Mechanism of Action (MOA), Product Pipeline, cGMP Manufacturing
Current Status and Path Forward
The Kiromic Difference and Market Opportunity
Diamond AI (Artificial Intelligence)
Contents |
| PAGE
| 4
1American Cancer Society, Cancer Facts & Figures,
2022..https://www.cancer.org/research/cancer-facts-statistics.html.
1 3
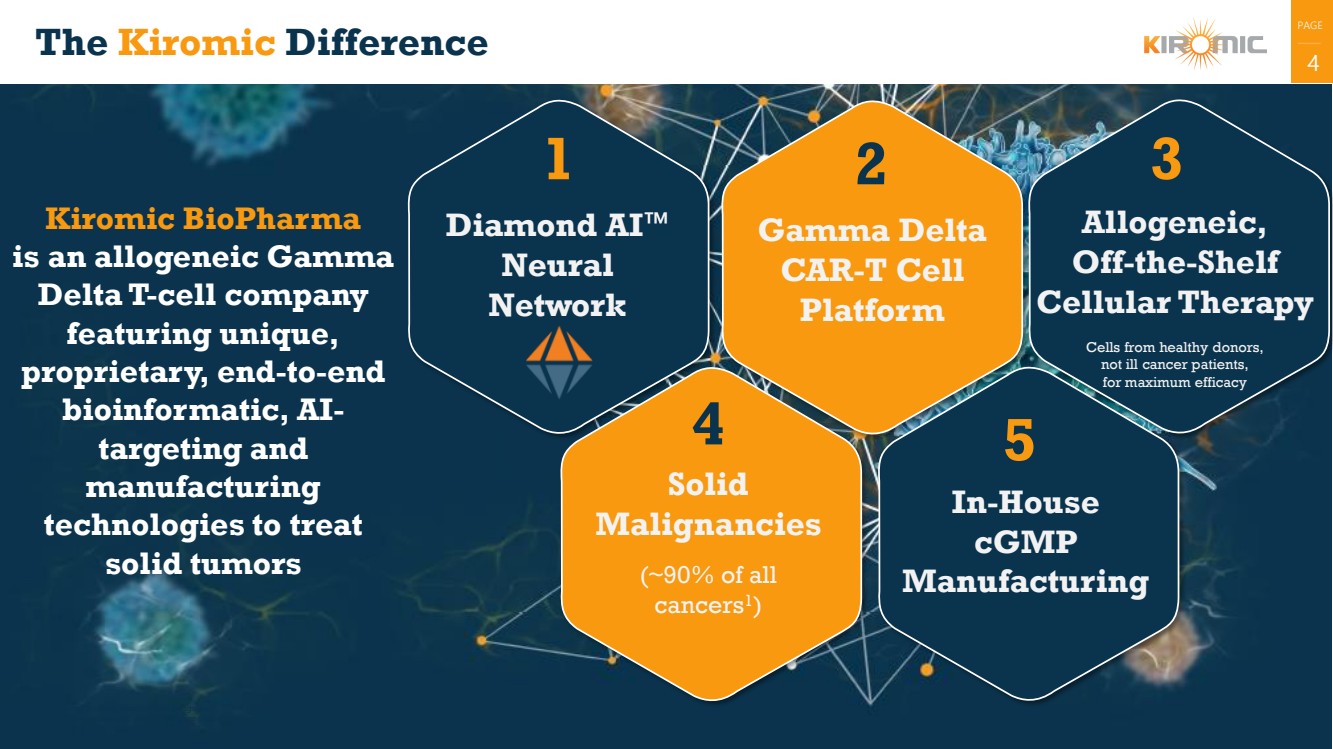
The Kiromic Difference
Gamma Delta
CAR-T Cell
Platform
In-House
cGMP
Manufacturing
Diamond AI
Neural
Network
4
5
Solid
Malignancies
(~90% of all
cancers1
)
Allogeneic,
Off-the-Shelf
Cellular Therapy
Cells from healthy donors,
not ill cancer patients,
for maximum efficacy
Kiromic BioPharma
is an allogeneic Gamma
Delta T-cell company
featuring unique,
proprietary, end-to-end
bioinformatic, AI-targeting and
manufacturing
technologies to treat
solid tumors
2
4 |
| PAGE
|
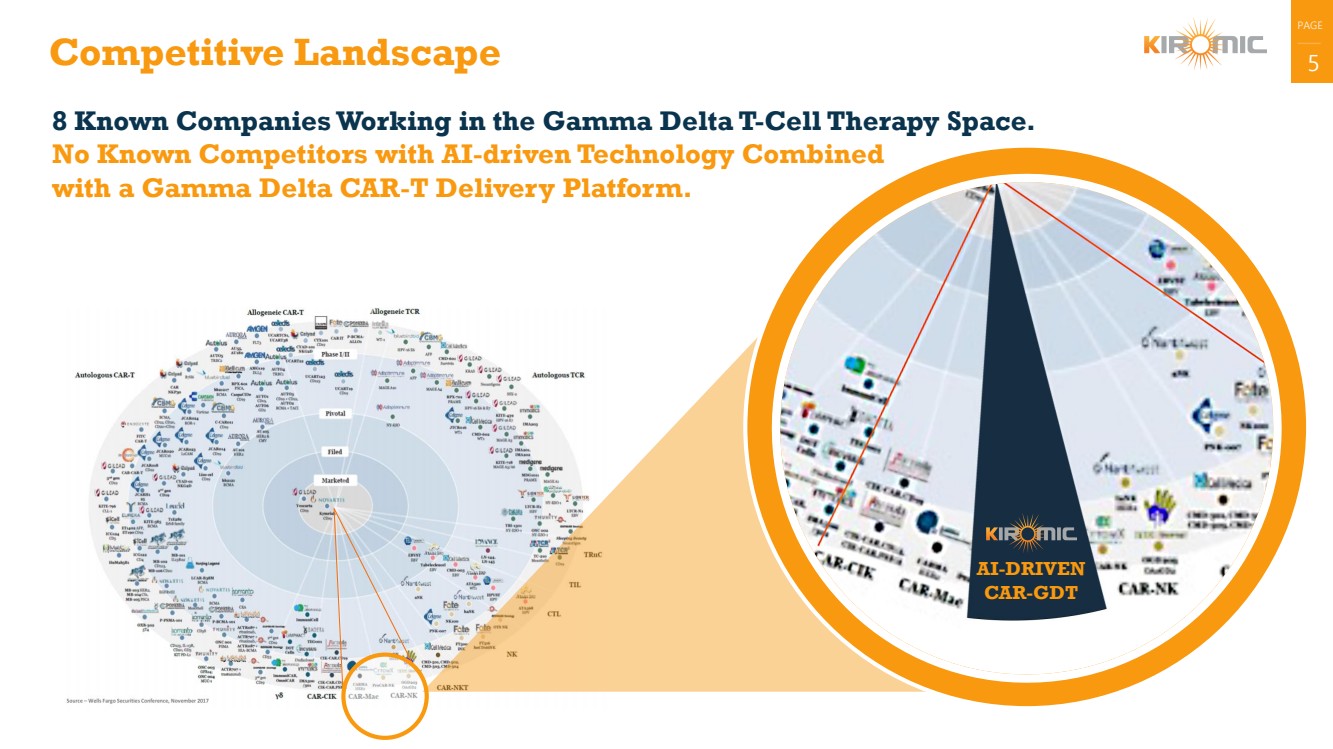
Competitive Landscape 5
AI-DRIVEN
CAR-GDT
8 Known Companies Working in the Gamma Delta T-Cell Therapy Space.
No Known Competitors with AI-driven Technology Combined
with a Gamma Delta CAR-T Delivery Platform. |
| PAGE
| 6
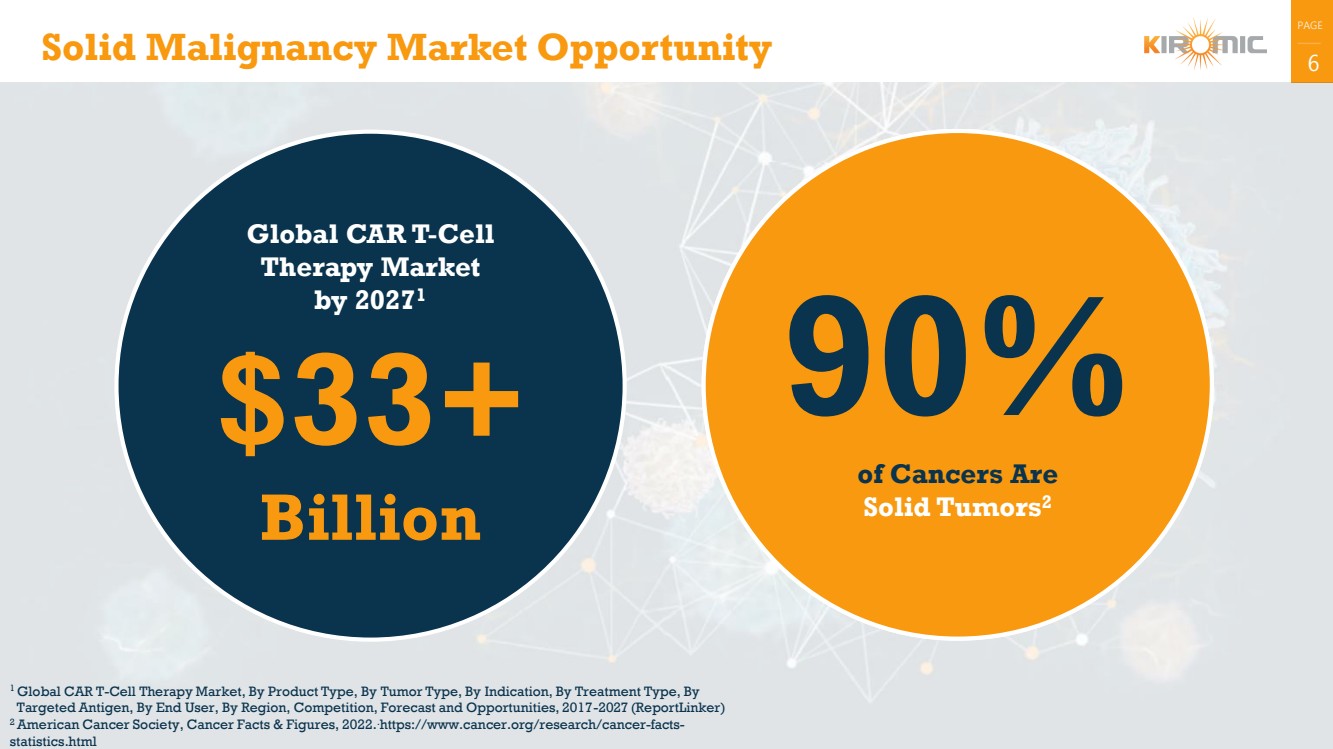
Global CAR T-Cell
Therapy Market
by 20271
$33+
Billion
1 Global CAR T-Cell Therapy Market, By Product Type, By Tumor Type, By Indication, By Treatment Type, By
Targeted Antigen, By End User, By Region, Competition, Forecast and Opportunities, 2017-2027 (ReportLinker)
2 American Cancer Society, Cancer Facts & Figures, 2022..https://www.cancer.org/research/cancer-facts-statistics.html
Solid Malignancy Market Opportunity
90%
of Cancers Are
Solid Tumors2 |
| PAGE
|
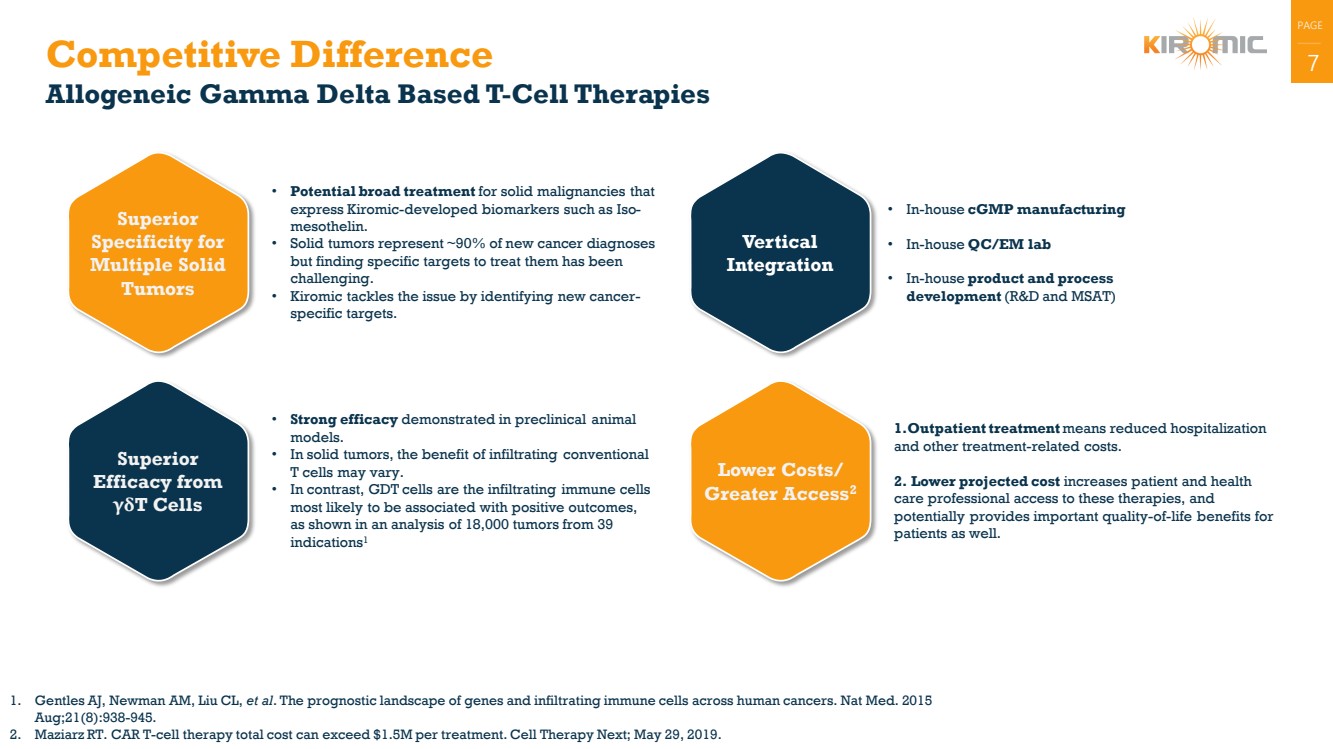
Competitive Difference 7
Allogeneic Gamma Delta Based T-Cell Therapies
Superior
Specificity for
Multiple Solid
Tumors
• Potential broad treatment for solid malignancies that
express Kiromic-developed biomarkers such as Iso-mesothelin.
• Solid tumors represent ~90% of new cancer diagnoses
but finding specific targets to treat them has been
challenging.
• Kiromic tackles the issue by identifying new cancer-specific targets.
Superior
Efficacy from
γδT Cells
• Strong efficacy demonstrated in preclinical animal
models.
• In solid tumors, the benefit of infiltrating conventional
T cells may vary.
• In contrast, GDT cells are the infiltrating immune cells
most likely to be associated with positive outcomes,
as shown in an analysis of 18,000 tumors from 39
indications1
Vertical
Integration
• In-house cGMP manufacturing
• In-house QC/EM lab
• In-house product and process
development (R&D and MSAT)
Lower Costs/
Greater Access2
1.Outpatient treatment means reduced hospitalization
and other treatment-related costs.
2. Lower projected cost increases patient and health
care professional access to these therapies, and
potentially provides important quality-of-life benefits for
patients as well.
1. Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015
Aug;21(8):938-945.
2. Maziarz RT. CAR T-cell therapy total cost can exceed $1.5M per treatment. Cell Therapy Next; May 29, 2019. |

| PAGE
| 8
Gamma Delta T-cell (GDT) Therapy:
Mechanism of Action (MOA), Product Pipeline, cGMP Manufacturing
Current Status and Path Forward
The Kiromic Difference and Market Opportunity
Diamond AI (Artificial Intelligence)
Contents |
| PAGE
|
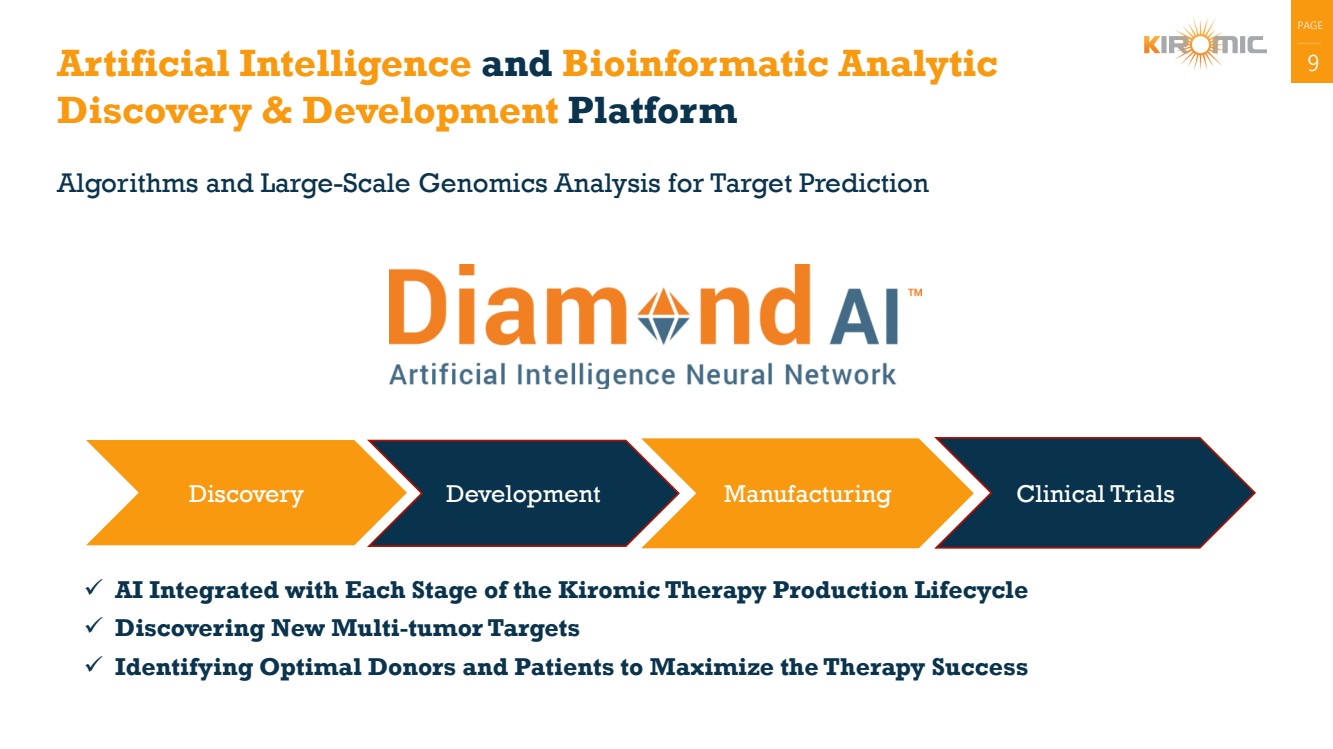
Artificial Intelligence and Bioinformatic Analytic 9
Discovery & Development Platform
Algorithms and Large-Scale Genomics Analysis for Target Prediction
Discovery Development Manufacturing Clinical Trials
✓ AI Integrated with Each Stage of the Kiromic Therapy Production Lifecycle
✓ Discovering New Multi-tumor Targets
✓ Identifying Optimal Donors and Patients to Maximize the Therapy Success |
| PAGE
|
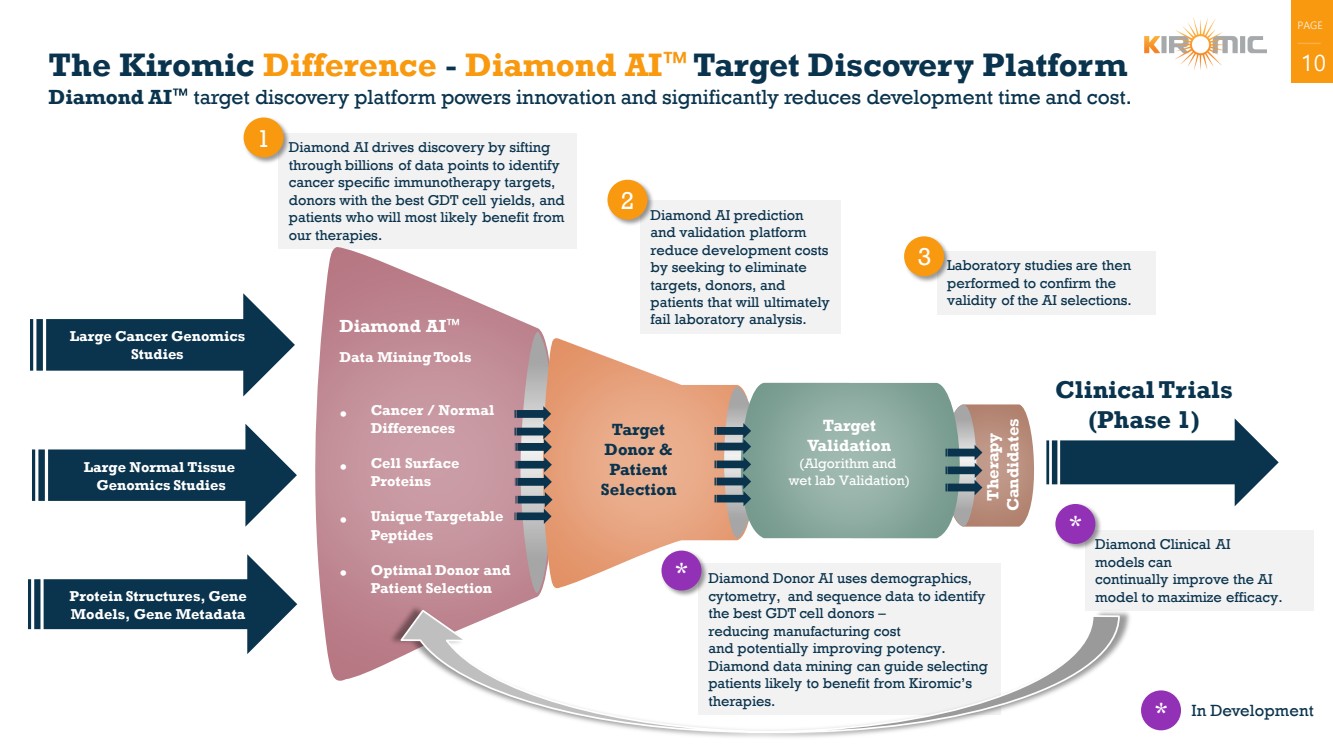
The Kiromic Difference - Diamond AI Target Discovery Platform 10
Diamond AI target discovery platform powers innovation and significantly reduces development time and cost.
Clinical Trials
(Phase 1)
Diamond AI drives discovery by sifting
through billions of data points to identify
cancer specific immunotherapy targets,
donors with the best GDT cell yields, and
patients who will most likely benefit from
our therapies.
1
Diamond AI prediction
and validation platform
reduce development costs
by seeking to eliminate
targets, donors, and
patients that will ultimately
fail laboratory analysis.
2
Laboratory studies are then
performed to confirm the
validity of the AI selections.
3
Protein Structures, Gene
Models, Gene Metadata
Large Cancer Genomics
Studies
Large Normal Tissue
Genomics Studies
Diamond Clinical AI
models can
continually improve the AI
model to maximize efficacy.
*
Diamond Donor AI uses demographics,
cytometry, and sequence data to identify
the best GDT cell donors –
reducing manufacturing cost
and potentially improving potency.
Diamond data mining can guide selecting
patients likely to benefit from Kiromic’s
therapies.
*
* In Development
Therapy
Candidates
Diamond AI
Data Mining Tools
•
Cancer / Normal
Differences
•
Cell Surface
Proteins
•
Unique Targetable
Peptides
•
Optimal Donor and
Patient Selection
Target
Donor &
Patient
Selection
Target
Validation
(Algorithm and
wet lab Validation) |

| PAGE
| 11
Gamma Delta T-cell (GDT) Therapy:
Mechanism of Action (MOA), Product Pipeline, cGMP Manufacturing
Current Status and Path Forward
The Kiromic Difference and Market Opportunity
Diamond AI (Artificial Intelligence)
Contents |
| PAGE
|
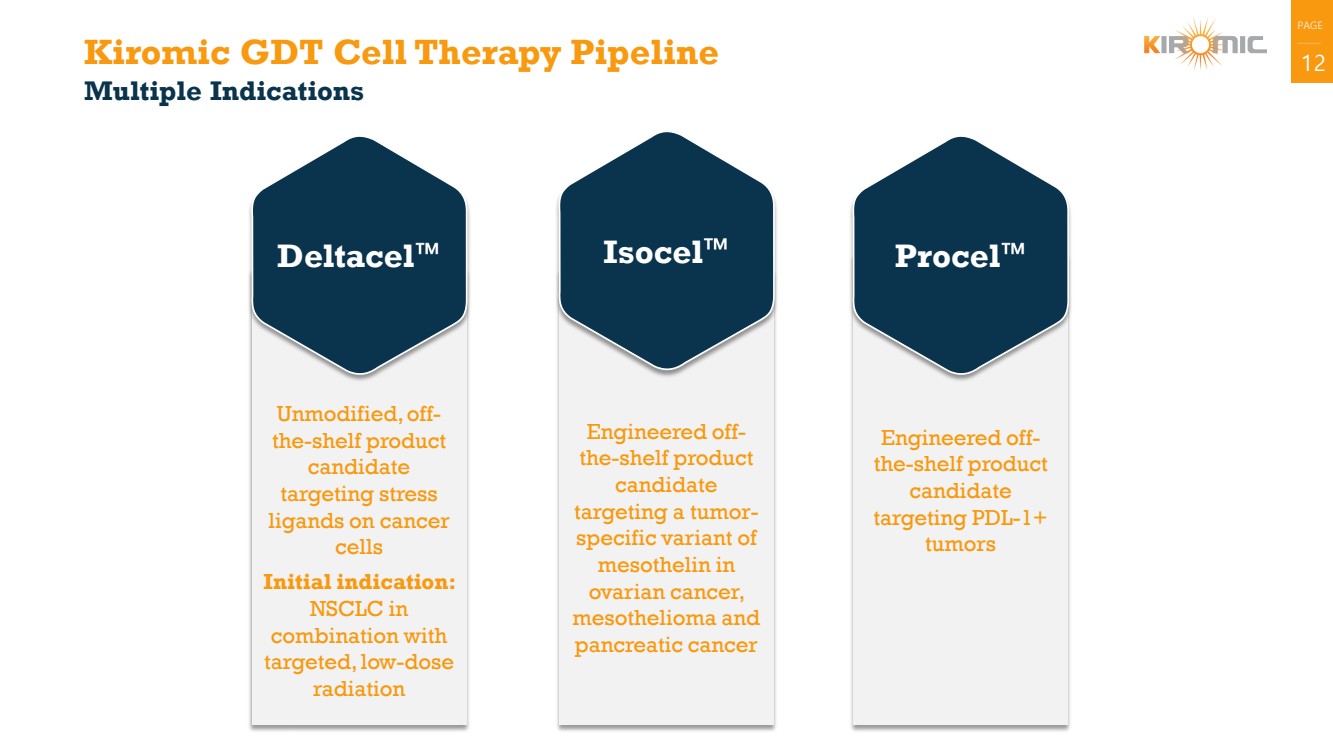
Kiromic GDT Cell Therapy Pipeline 12
Multiple Indications
Unmodified, off-the-shelf product
candidate
targeting stress
ligands on cancer
cells
Initial indication:
NSCLC in
combination with
targeted, low-dose
radiation
Deltacel
Engineered off-the-shelf product
candidate
targeting PDL-1+
tumors
Procel
Engineered off-the-shelf product
candidate
targeting a tumor-specific variant of
mesothelin in
ovarian cancer,
mesothelioma and
pancreatic cancer
Isocel |
| PAGE
| 13
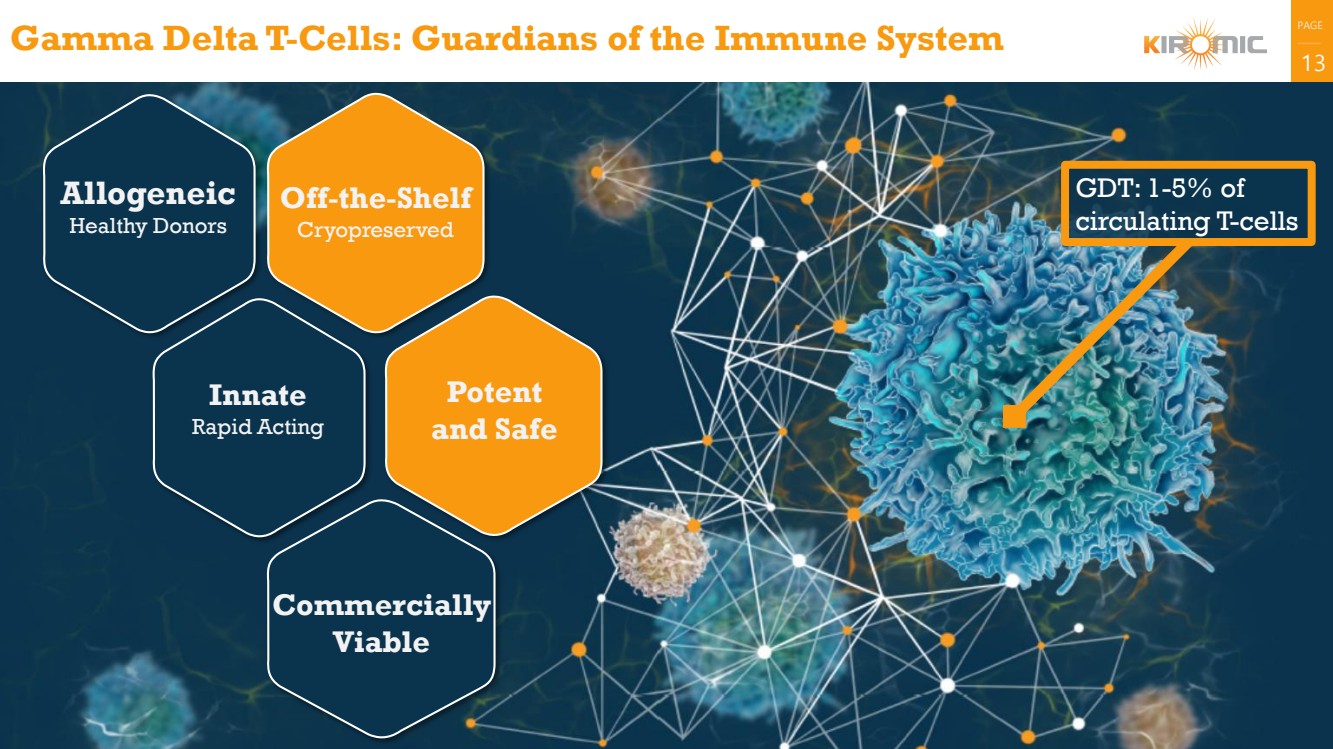
Gamma Delta T-Cells: Guardians of the Immune System
Off-the-Shelf
Cryopreserved
Allogeneic
Healthy Donors
Innate
Rapid Acting
Potent
and Safe
Commercially
Viable
GDT: 1-5% of
circulating T-cells |
| PAGE
| 14
Deltacel: Non-Viral Gamma Delta T-Cell Development
Kiromic
Proprietary
In-house GDT
Cell Isolation and
Expansion
Bridge Between
the Innate and
Adaptive
Immune
Response
Rapid Response
to Attack
Cancer Cells
Decreased
Toxicity Risk
Profile
Virus Free
Expansion and
Production
GDT: 1-5% of
circulating T-cells |
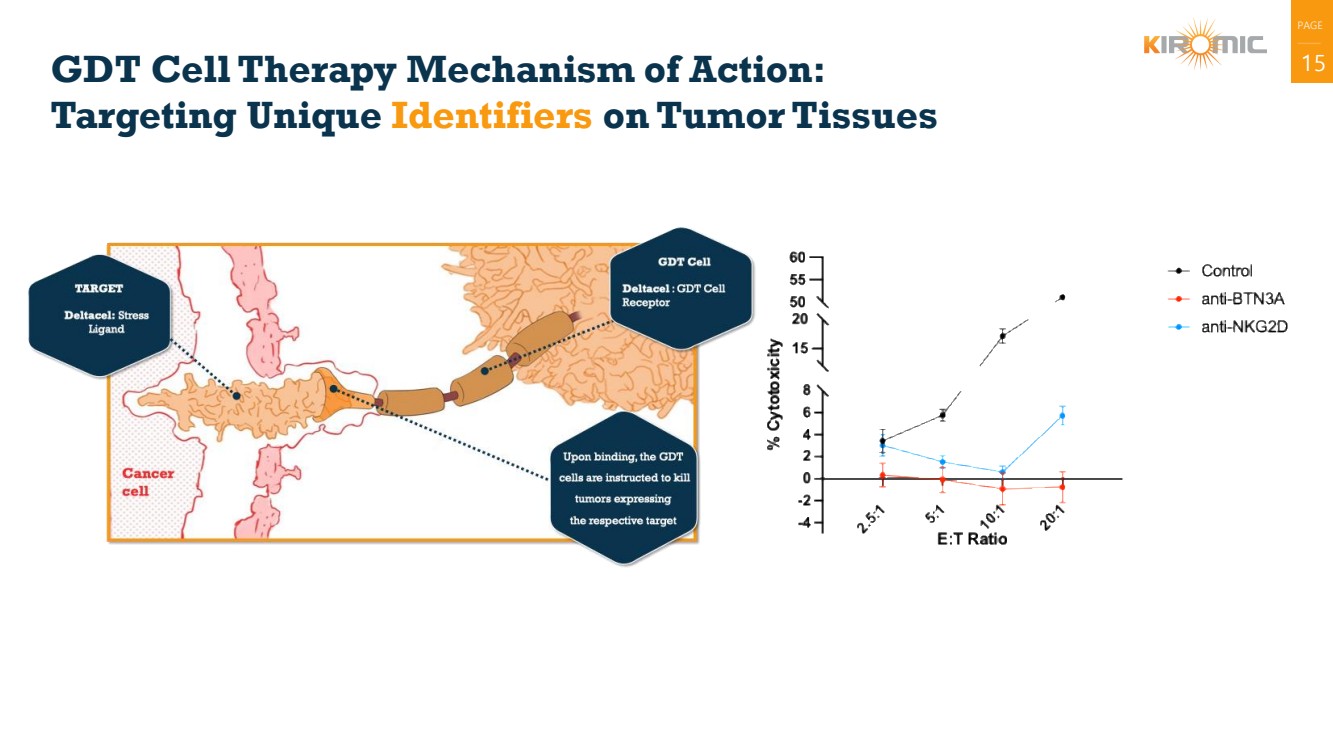
| PAGE
|
GDT Cell Therapy Mechanism of Action: 15
Targeting Unique Identifiers on Tumor Tissues |
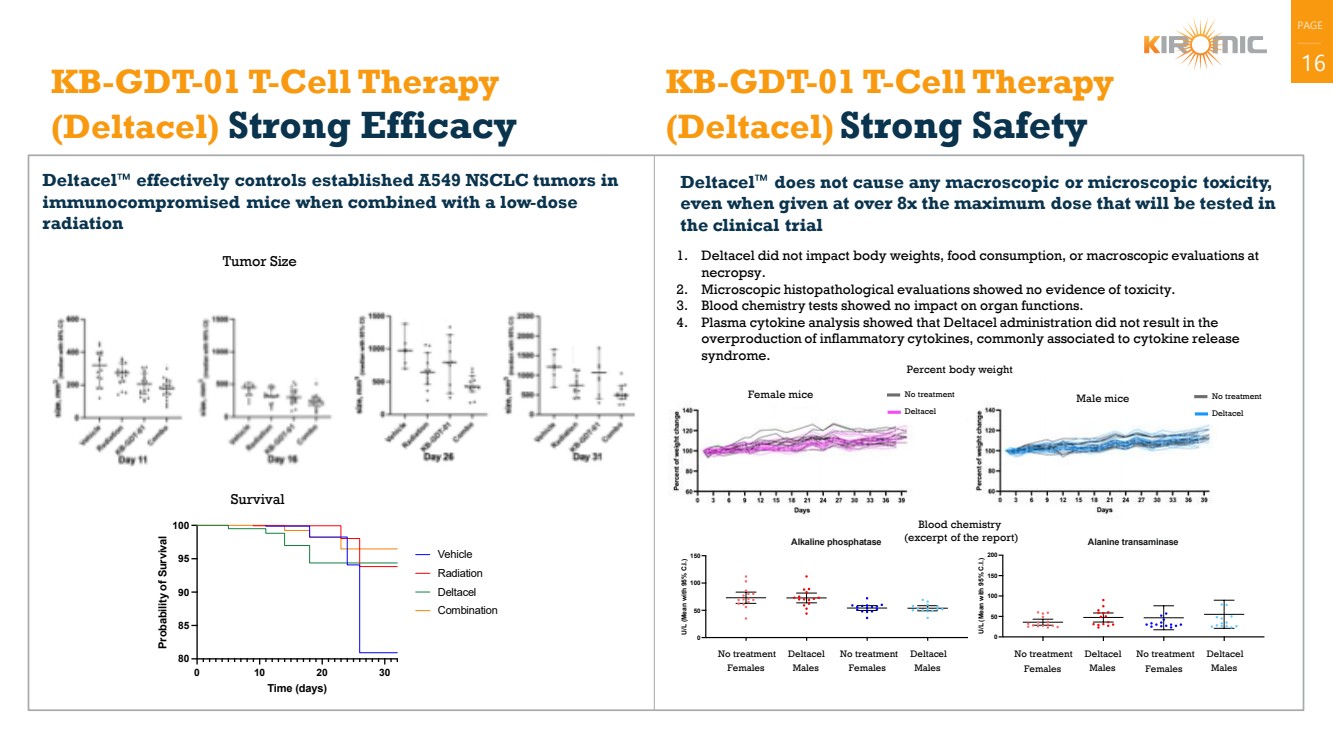
| PAGE
| 16 KB-GDT-01 T-Cell Therapy
(Deltacel) Strong Efficacy
Deltacel effectively controls established A549 NSCLC tumors in
immunocompromised mice when combined with a low-dose
radiation
Deltacel does not cause any macroscopic or microscopic toxicity,
even when given at over 8x the maximum dose that will be tested in
the clinical trial
KB-GDT-01 T-Cell Therapy
(Deltacel) Strong Safety
1. Deltacel did not impact body weights, food consumption, or macroscopic evaluations at
necropsy.
2. Microscopic histopathological evaluations showed no evidence of toxicity.
3. Blood chemistry tests showed no impact on organ functions.
4. Plasma cytokine analysis showed that Deltacel administration did not result in the
overproduction of inflammatory cytokines, commonly associated to cytokine release
syndrome.
0 10 20 30
80
85
90
95
100
Time (days)
Probability of Survival
Vehicle
Radiation
Deltacel
Combination
No treatment
Deltacel
No treatment
Deltacel
Female mice
Tumor Size
Survival
Females Tumor, CS5
Females Tumor gdT
Males Tumor CS5
Males Tumor gdT
0.0
0.2
0.4
0.6
mg/dL (Mean with 95% C.I.)
Total bilirubin
Females Tumor, CS5
Females Tumor gdT
Males Tumor CS5
Males Tumor gdT
0
50
100
150
200
U L/ (Mean with 95% C.I.)
Alanine transaminase
Females Tumor, CS5
Females Tumor gdT
Males Tumor CS5
Males Tumor gdT
0
50
100
150
U/L (Mean with 95% C.I.)
Alkaline phosphatase
Females Tumor, CS5
Females Tumor gdT
Males Tumor CS5
Males Tumor gdT
0
200
400
600
U/L (Mean with 95% C.I.)
Aspartate aminotransferase
Females Tumor, CS5
Females Tumor gdT
Males Tumor CS5
Males Tumor gdT
0.0
0.2
0.4
0.6
mg/dL (Mean with 95% C.I.)
Total bilirubin
Females Tumor, CS5
Females Tumor gdT
Males Tumor CS5
Males Tumor gdT
0
50
100
150
200
U/L (Mean with 95% C.I.)
Alanine transaminase
Females Tumor, CS5
Females Tumor gdT
Males Tumor CS5
Males Tumor gdT
0
50
100
150
U/L (Mean with 95% C.I.)
Alkaline phosphatase
Females Tumor, CS5
Females Tumor gdT
Males Tumor CS5
Males Tumor gdT
0
200
400
600
U/L (Mean with 95% C.I.)
Aspartate aminotransferase No treatment Deltacel No treatment Deltacel No treatment Deltacel No treatment Deltacel
Females Males Females Males Females Males Females Males
Blood chemistry
(excerpt of the report)
Percent body weight
Male mice |
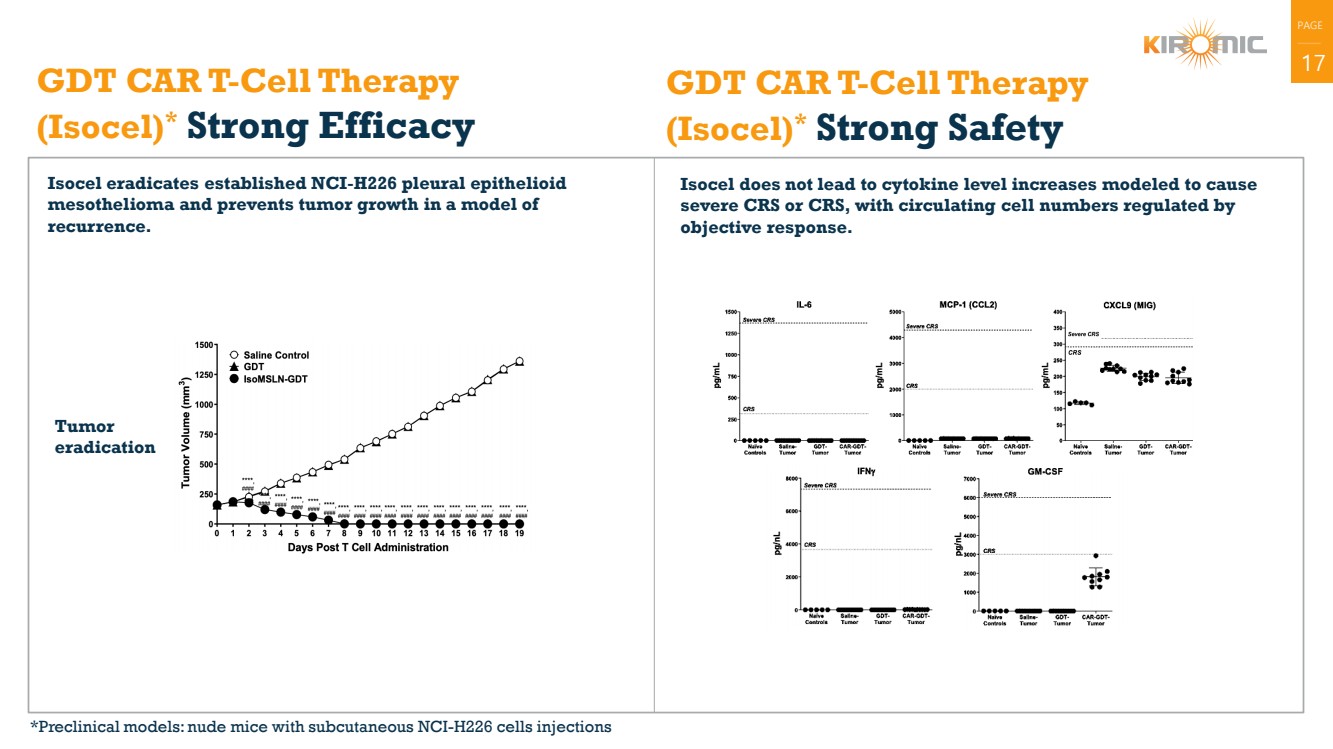
| PAGE
| 17 GDT CAR T-Cell Therapy
(Isocel)* Strong Efficacy
Isocel eradicates established NCI-H226 pleural epithelioid
mesothelioma and prevents tumor growth in a model of
recurrence.
Isocel does not lead to cytokine level increases modeled to cause
severe CRS or CRS, with circulating cell numbers regulated by
objective response.
GDT CAR T-Cell Therapy
(Isocel)* Strong Safety
Tumor
eradication
*Preclinical models: nude mice with subcutaneous NCI-H226 cells injections |
| PAGE
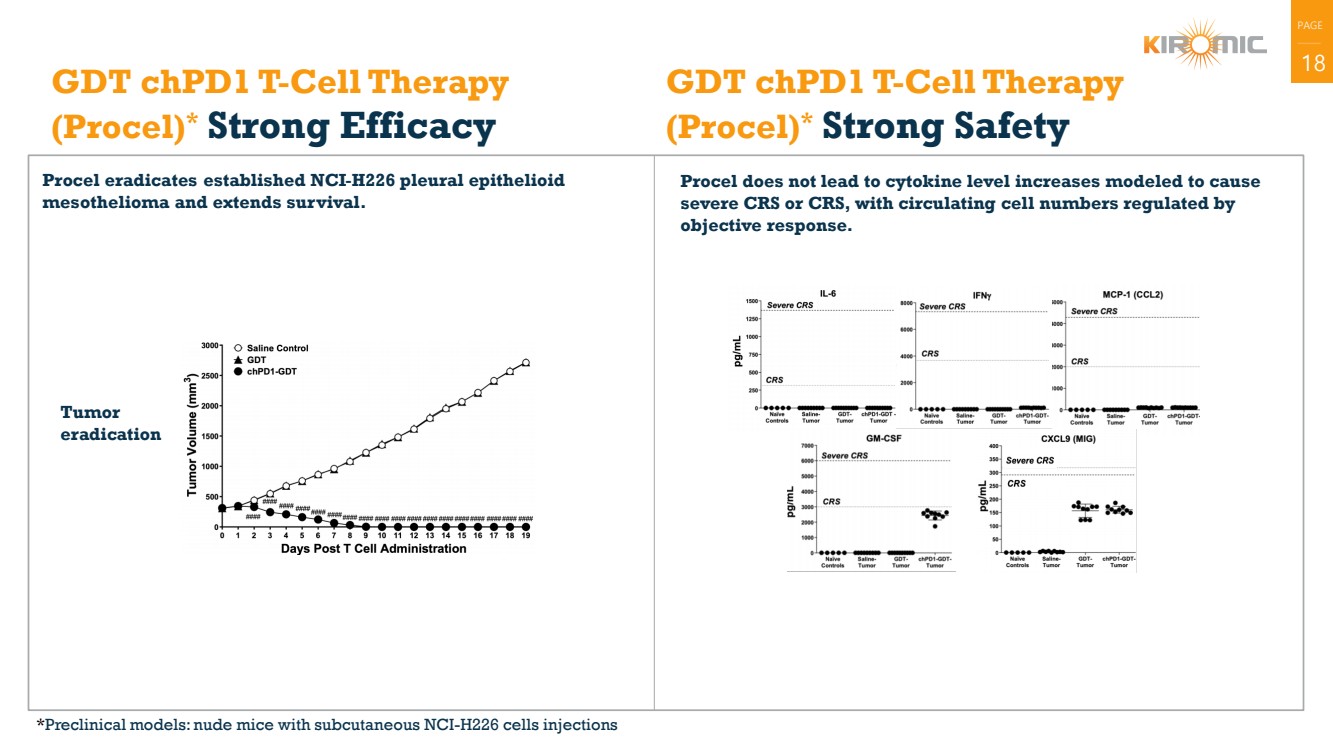
| 18 GDT chPD1 T-Cell Therapy
(Procel)* Strong Efficacy
Procel eradicates established NCI-H226 pleural epithelioid
mesothelioma and extends survival.
Procel does not lead to cytokine level increases modeled to cause
severe CRS or CRS, with circulating cell numbers regulated by
objective response.
GDT chPD1 T-Cell Therapy
(Procel)* Strong Safety
Tumor
eradication
*Preclinical models: nude mice with subcutaneous NCI-H226 cells injections |
| PAGE
|
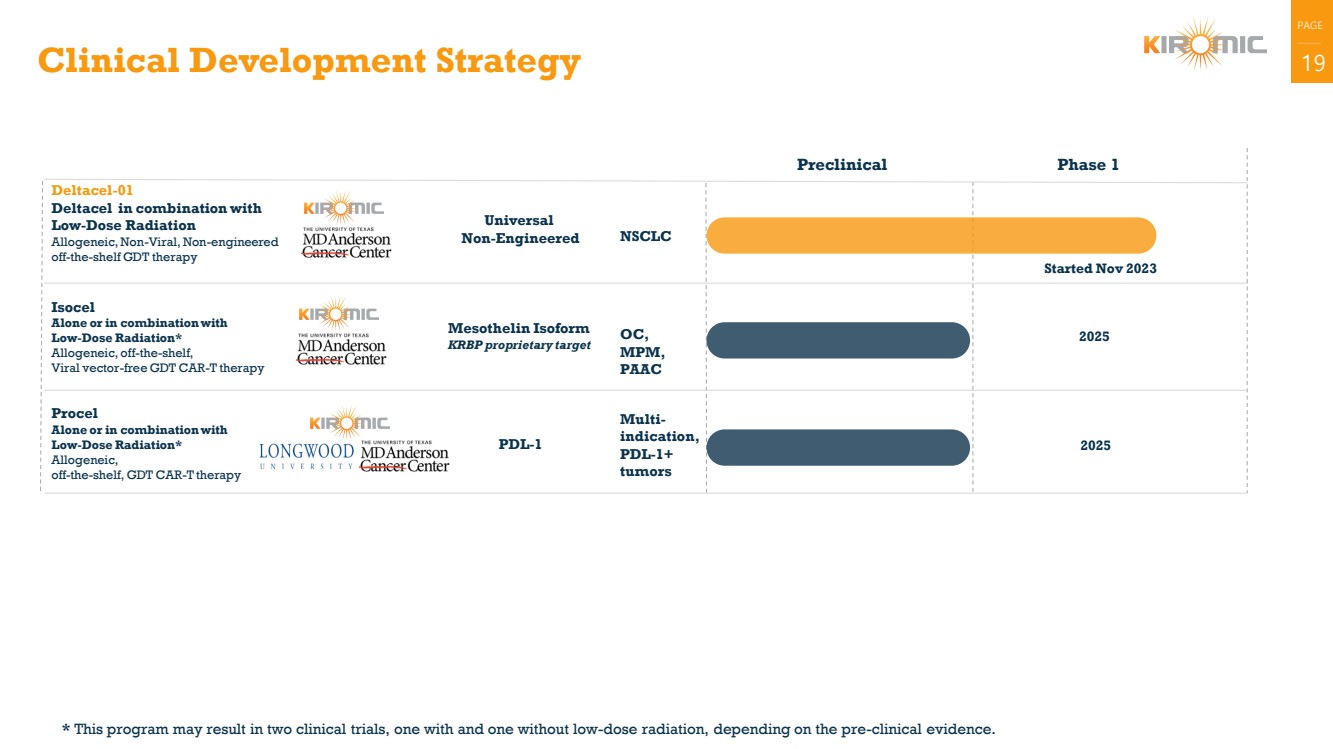
Clinical Development Strategy 19
Procel
Alone or in combination with
Low-Dose Radiation*
Allogeneic,
off-the-shelf, GDT CAR-T therapy
Deltacel-01
Deltacel in combination with
Low-Dose Radiation
Allogeneic, Non-Viral, Non-engineered
off-the-shelf GDT therapy
Started Nov 2023
Universal
Non-Engineered
Isocel
Alone or in combination with
Low-Dose Radiation*
Allogeneic, off-the-shelf,
Viral vector-free GDT CAR-T therapy
Mesothelin Isoform 2025 KRBP proprietary target
Preclinical Phase 1
PDL-1 2025
NSCLC
OC,
MPM,
PAAC
Multi-indication,
PDL-1+
tumors
* This program may result in two clinical trials, one with and one without low-dose radiation, depending on the pre-clinical evidence. |
| PAGE
| 20
In-House cGMP Manufacturing Creates De-Risked Value
Dedicated
Product
Development
Suite
34,000 sq ft
Facility
Operations
12,000 sq ft
R&D Lab &
Manufacturing
Facility
Dedicated
cGLP
Microbiology
and QC Lab
Clinical-Grade,
cGMP-Compliant
Cell Therapy
Manufacturing |

| PAGE
| 21
Gamma Delta T-cell (GDT) Therapy:
Mechanism of Action (MOA), Product Pipeline, cGMP Manufacturing
Current Status and Path Forward
The Kiromic Difference and Market Opportunity
Diamond AI (Artificial Intelligence)
Contents |
| PAGE
|
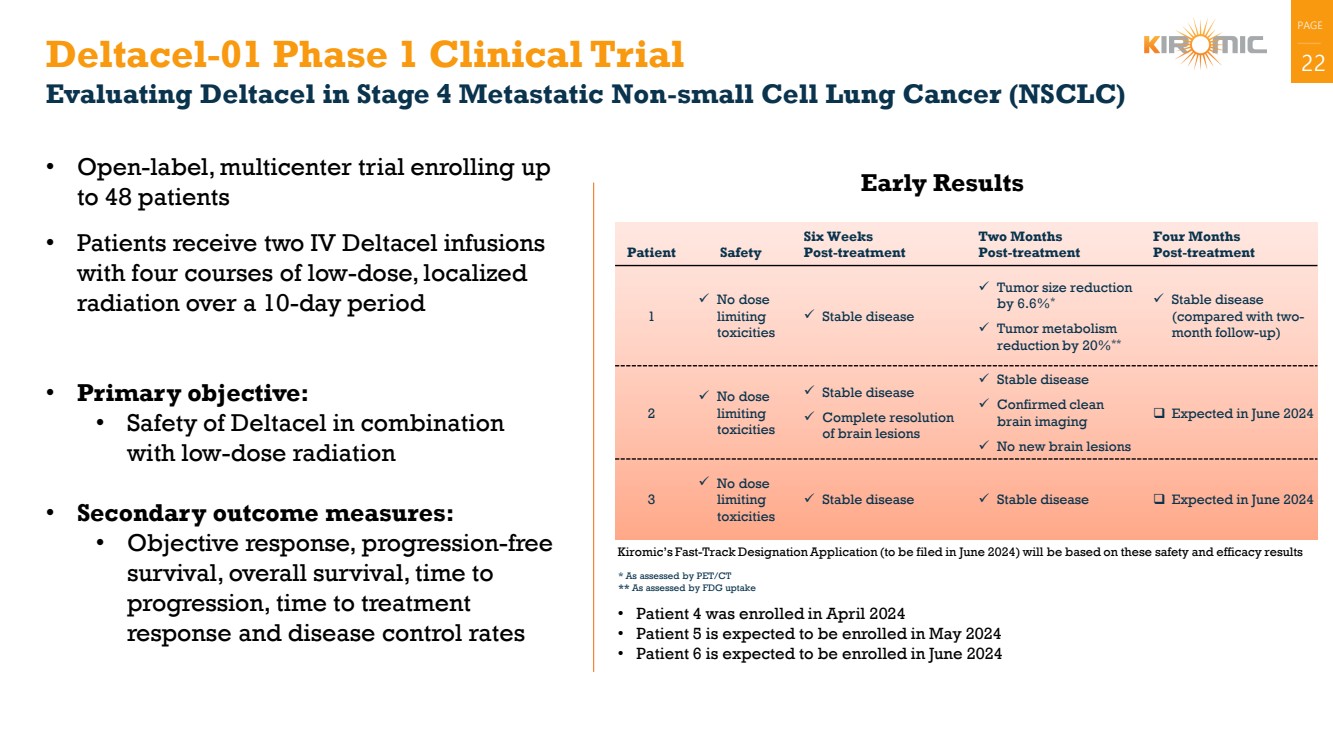
Deltacel-01 Phase 1 Clinical Trial 22
Evaluating Deltacel in Stage 4 Metastatic Non-small Cell Lung Cancer (NSCLC)
• Open-label, multicenter trial enrolling up
to 48 patients
• Patients receive two IV Deltacel infusions
with four courses of low-dose, localized
radiation over a 10-day period
• Primary objective:
• Safety of Deltacel in combination
with low-dose radiation
• Secondary outcome measures:
• Objective response, progression-free
survival, overall survival, time to
progression, time to treatment
response and disease control rates
Early Results
Patient Safety
Six Weeks
Post-treatment
Two Months
Post-treatment
Four Months
Post-treatment
1
✓ No dose
limiting
toxicities
✓ Stable disease
✓ Tumor size reduction
by 6.6%*
✓ Tumor metabolism
reduction by 20%**
✓ Stable disease
(compared with two-month follow-up)
2
✓ No dose
limiting
toxicities
✓ Stable disease
✓ Complete resolution
of brain lesions
✓ Stable disease
✓ Confirmed clean
brain imaging
✓ No new brain lesions
❑ Expected in June 2024
3
✓ No dose
limiting
toxicities
✓ Stable disease ✓ Stable disease ❑ Expected in June 2024
* As assessed by PET/CT
** As assessed by FDG uptake
Kiromic’s Fast-Track Designation Application (to be filed in June 2024) will be based on these safety and efficacy results
• Patient 4 was enrolled in April 2024
• Patient 5 is expected to be enrolled in May 2024
• Patient 6 is expected to be enrolled in June 2024 |
| PAGE
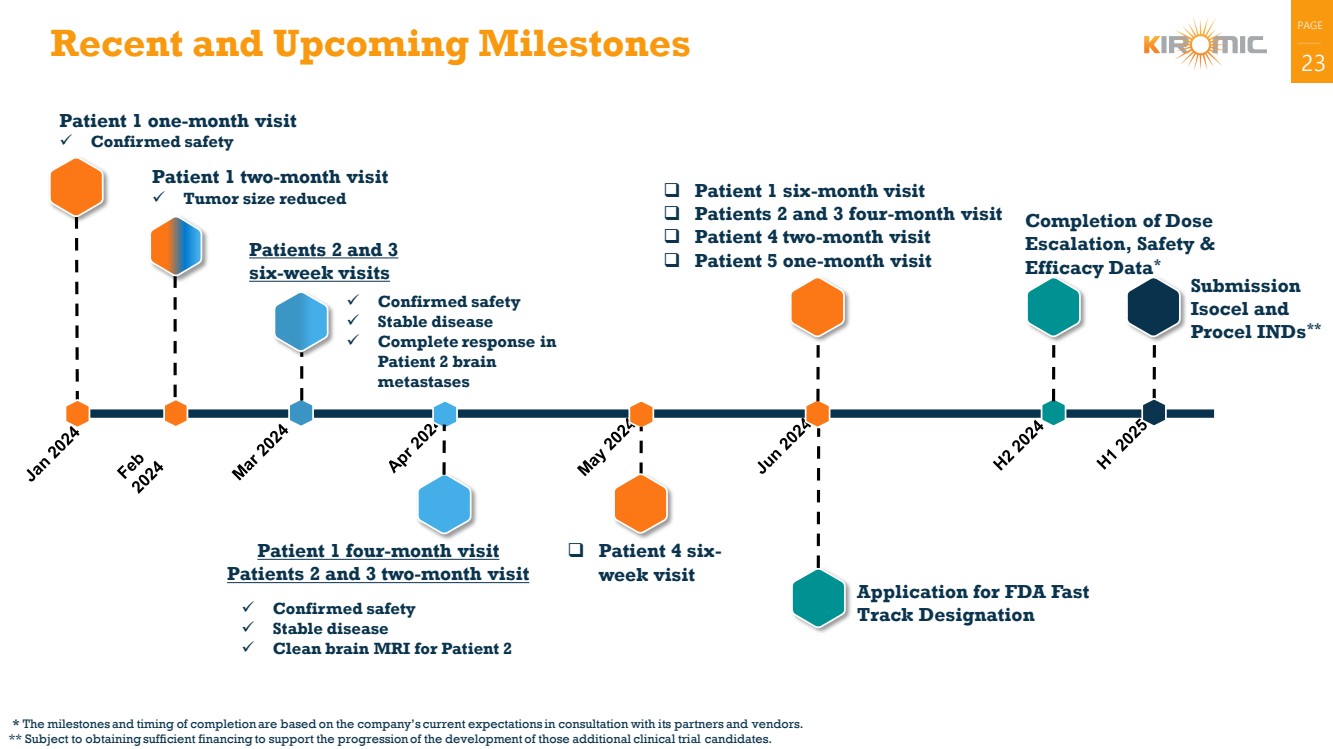
| 23 Recent and Upcoming Milestones
Patient 1 one-month visit
✓ Confirmed safety
Submission
Isocel and
Procel INDs**
Patient 1 two-month visit
✓ Tumor size reduced
Completion of Dose
Escalation, Safety &
Efficacy Data*
✓ Confirmed safety
✓ Stable disease
✓ Complete response in
Patient 2 brain
metastases
Patients 2 and 3
six-week visits
* The milestones and timing of completion are based on the company’s current expectations in consultation with its partners and vendors.
** Subject to obtaining sufficient financing to support the progression of the development of those additional clinical trial candidates.
✓ Confirmed safety
✓ Stable disease
✓ Clean brain MRI for Patient 2
Patient 1 four-month visit
Patients 2 and 3 two-month visit
Application for FDA Fast
Track Designation
❑ Patient 1 six-month visit
❑ Patients 2 and 3 four-month visit
❑ Patient 4 two-month visit
❑ Patient 5 one-month visit
❑ Patient 4 six-week visit |

| PAGE
| 24
Brian
Hungerford
CPA,CGMA
Leadership Team
Scott
Dahlbeck
M.D., Pharm.D.
COSO
Texas Tech Univ
Health Science
Center
University of TX
Health Science
Center Houston
College
of Pharmacy
CEO
Pietro
Bersani
CPA, CGMA
CSO/INTERIM
COO
Leonardo
Mirandola
Ph.D.
CFO
CellMark
CellMark |

| PAGE
| 25 Board of Directors
Michael
Nagel
Chairperson
Pam
Misajon
Director
Pietro
Bersani
CPA, CGMA
Independent
Director
Independent
Director
Michael
Catlin |
| PAGE
|
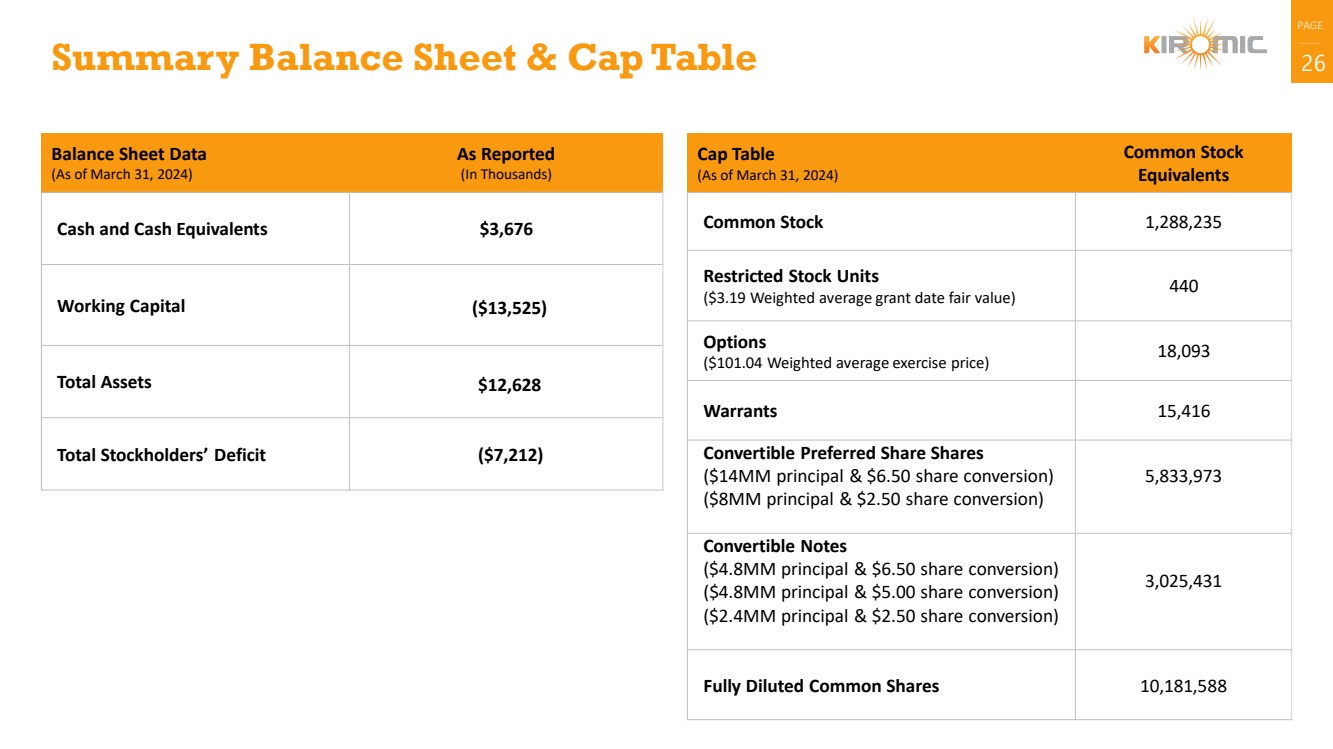
Summary Balance Sheet & Cap Table 26
Balance Sheet Data
(As of March 31, 2024)
As Reported
(In Thousands)
Cash and Cash Equivalents $3,676
Working Capital ($13,525)
Total Assets $12,628
Total Stockholders’ Deficit ($7,212)
Cap Table
(As of March 31, 2024)
Common Stock
Equivalents
Common Stock 1,288,235
Restricted Stock Units
($3.19 Weighted average grant date fair value) 440
Options
($101.04 Weighted average exercise price) 18,093
Warrants 15,416
Convertible Preferred Share Shares
($14MM principal & $6.50 share conversion)
($8MM principal & $2.50 share conversion)
5,833,973
Convertible Notes
($4.8MM principal & $6.50 share conversion)
($4.8MM principal & $5.00 share conversion)
($2.4MM principal & $2.50 share conversion)
3,025,431
Fully Diluted Common Shares 10,181,588 |
| PAGE
| 27
1American Cancer Society, Cancer Facts & Figures,
2022..https://www.cancer.org/research/cancer-facts-statistics.html.
1 2 3
Value Proposition Summary
Gamma Delta
CAR-T Cell
Therapy
Platform
In-House
cGMP
Manufacturing
Diamond AI
Neural
Network
4 5
Solid
Malignancies
(~90% of all
cancers1)
Allogeneic,
Off-the-Shelf
Cellular Therapy
Cells from healthy donors,
not ill cancer patients,
for maximum efficacy |

| Revolutionizing CAR T-Cell Therapy
BioPharma
OTCQB: KRBP
Kiromic.com
May 2024 |
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Grafico Azioni Kiromic BioPharma (NASDAQ:KRBP)
Storico
Da Dic 2024 a Gen 2025

Grafico Azioni Kiromic BioPharma (NASDAQ:KRBP)
Storico
Da Gen 2024 a Gen 2025
