false
--09-30
2024
Q1
0001314052
0001314052
2023-10-01
2023-12-31
0001314052
2024-02-07
0001314052
2023-12-31
0001314052
2023-09-30
0001314052
2022-10-01
2022-12-31
0001314052
us-gaap:CommonStockMember
2023-09-30
0001314052
us-gaap:AdditionalPaidInCapitalMember
2023-09-30
0001314052
us-gaap:RetainedEarningsMember
2023-09-30
0001314052
us-gaap:CommonStockMember
2022-09-30
0001314052
us-gaap:AdditionalPaidInCapitalMember
2022-09-30
0001314052
us-gaap:RetainedEarningsMember
2022-09-30
0001314052
2022-09-30
0001314052
us-gaap:CommonStockMember
2023-10-01
2023-12-31
0001314052
us-gaap:AdditionalPaidInCapitalMember
2023-10-01
2023-12-31
0001314052
us-gaap:RetainedEarningsMember
2023-10-01
2023-12-31
0001314052
us-gaap:CommonStockMember
2022-10-01
2022-12-31
0001314052
us-gaap:AdditionalPaidInCapitalMember
2022-10-01
2022-12-31
0001314052
us-gaap:RetainedEarningsMember
2022-10-01
2022-12-31
0001314052
us-gaap:CommonStockMember
2023-12-31
0001314052
us-gaap:AdditionalPaidInCapitalMember
2023-12-31
0001314052
us-gaap:RetainedEarningsMember
2023-12-31
0001314052
us-gaap:CommonStockMember
2022-12-31
0001314052
us-gaap:AdditionalPaidInCapitalMember
2022-12-31
0001314052
us-gaap:RetainedEarningsMember
2022-12-31
0001314052
2022-12-31
0001314052
avxl:MichaelJFoxMember
2023-10-01
2023-12-31
0001314052
avxl:AnavexMember
2022-10-01
2023-09-30
0001314052
avxl:AnavexMember
2020-10-01
2021-09-30
0001314052
currency:AUD
2023-10-01
2023-12-31
0001314052
currency:AUD
2022-10-01
2022-12-31
0001314052
currency:AUD
2023-12-31
0001314052
currency:AUD
2023-09-30
0001314052
avxl:EquityOfferingSalesAgreementMember
avxl:CantorFitzgeraldAndCoMember
2023-10-01
2023-12-31
0001314052
avxl:EquityOfferingSalesAgreementMember
2023-10-01
2023-12-31
0001314052
avxl:EquityOfferingSalesAgreementMember
2022-10-01
2022-12-31
0001314052
avxl:PurchaseAgreement2023Member
avxl:LincolnParkCapitalFundLLCMember
2023-02-02
2023-02-03
0001314052
avxl:PurchaseAgreement1Member
avxl:LincolnParkCapitalFundLLCMember
2023-02-02
2023-02-03
0001314052
avxl:ThirdPartyMember
2023-12-31
0001314052
avxl:PurchaseAgreement1Member
avxl:LincolnParkCapitalFundLLCMember
2023-10-01
2023-12-31
0001314052
avxl:PurchaseAgreement1Member
avxl:LincolnParkCapitalFundLLCMember
2022-10-01
2022-12-31
0001314052
avxl:StockOptionPlan2015Member
2023-12-31
0001314052
avxl:StockOptionPlan2019Member
2023-10-01
2023-12-31
0001314052
avxl:StockOptionPlan2022Member
2021-10-01
2022-09-30
0001314052
avxl:StockOptionPlan2022Member
2023-10-01
2023-12-31
0001314052
avxl:StockOptionPlan2022Member
2023-12-31
0001314052
2022-10-01
2023-09-30
0001314052
avxl:PurchaseWarrantsMember
2023-12-31
0001314052
avxl:PurchaseWarrants1Member
2023-12-31
0001314052
us-gaap:StockOptionMember
2022-09-30
0001314052
us-gaap:StockOptionMember
2022-10-01
2023-09-30
0001314052
us-gaap:StockOptionMember
2023-09-30
0001314052
us-gaap:StockOptionMember
2023-10-01
2023-12-31
0001314052
us-gaap:StockOptionMember
2023-12-31
0001314052
us-gaap:StockOptionMember
avxl:OptionPrice1Member
2023-10-01
2023-12-31
0001314052
us-gaap:StockOptionMember
avxl:OptionPrice1Member
2023-12-31
0001314052
us-gaap:StockOptionMember
avxl:OptionPrice2Member
2023-10-01
2023-12-31
0001314052
us-gaap:StockOptionMember
avxl:OptionPrice2Member
2023-12-31
0001314052
us-gaap:StockOptionMember
avxl:OptionPrice3Member
2023-10-01
2023-12-31
0001314052
us-gaap:StockOptionMember
avxl:OptionPrice3Member
2023-12-31
0001314052
us-gaap:StockOptionMember
avxl:OptionPrice4Member
2023-10-01
2023-12-31
0001314052
us-gaap:StockOptionMember
avxl:OptionPrice4Member
2023-12-31
0001314052
us-gaap:StockOptionMember
avxl:OptionPrice5Member
2023-10-01
2023-12-31
0001314052
us-gaap:StockOptionMember
avxl:OptionPrice5Member
2023-12-31
0001314052
us-gaap:GeneralAndAdministrativeExpenseMember
2023-10-01
2023-12-31
0001314052
us-gaap:GeneralAndAdministrativeExpenseMember
2022-10-01
2022-12-31
0001314052
us-gaap:ResearchAndDevelopmentExpenseMember
2023-10-01
2023-12-31
0001314052
us-gaap:ResearchAndDevelopmentExpenseMember
2022-10-01
2022-12-31
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
xbrli:pure
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
10-Q
(Mark One)
☒
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended: December
31, 2023
☐ TRANSITION
REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _____to _____
Commission File Number: 001-37606
ANAVEX LIFE SCIENCES CORP.
(Exact name
of registrant as specified in its charter)
| Nevada |
98-0608404 |
| (State or other jurisdiction of |
(IRS Employer |
| incorporation or organization) |
Identification No.) |
630 5th Avenue, 20th Floor, New York,
NY USA 10111
(Address of principal
executive offices) (Zip Code)
1-844-689-3939
(Registrant’s
telephone number, including area code)
Securities Registered Pursuant to Section 12(b)
of the Act:
| Title of Each Class |
|
Trading Symbol |
|
Name of Each Exchange on Which Registered |
| Common Stock Par Value $0.001 |
|
AVXL |
|
NASDAQ Stock Market LLC |
Indicate by check mark whether the registrant
(1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding
12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such
filing requirements for the past 90 days.
☒ Yes ☐
No
Indicate by check mark whether the registrant
has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405
of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
☒
Yes ☐ No
Indicate by check mark whether the registrant
is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth
company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting
company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer |
☒ |
|
Accelerated filer |
☐ |
| Non-accelerated filer |
☐ |
|
Smaller reporting company |
☐ |
| |
|
|
Emerging growth company |
☐ |
If an emerging growth company, indicate by check
mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting
standards provided pursuant to Section 13(a) of the Exchange Act
☐
Indicate by check mark whether the registrant is
a shell company (as defined in Rule 12b-2 of the Exchange Act).
☐
Yes ☒ No
Indicate the number of shares outstanding
of each of the issuer’s classes of Common Stock, as of the latest practicable date: 82,112,511 shares of Common Stock
outstanding as of February 7, 2024.
PART
I – FINANCIAL INFORMATION
ITEM
1. FINANCIAL STATEMENTS
Anavex
Life Sciences Corp.
Condensed Consolidated Interim Financial Statements
December
31, 2023
(Unaudited)
| Anavex
Life Sciences Corp. |
| Condensed
Consolidated Interim Balance Sheets |
| (in thousands,
except share and per share amounts) |
| | |
| |
|
|
| | |
| | | |
| | |
| | |
December
31, | |
September
30, |
| | |
2023 | |
2023 |
| | |
(Unaudited) | |
|
| Assets | |
| | | |
| | |
| Current | |
| | | |
| | |
| Cash
and cash equivalents | |
$ | 143,765 | | |
$ | 151,024 | |
| Incentive
and tax receivables | |
| 3,549 | | |
| 2,709 | |
| Prepaid
expenses and other current assets | |
| 756 | | |
| 653 | |
| Total
Assets | |
$ | 148,070 | | |
$ | 154,386 | |
| | |
| | | |
| | |
| Liabilities
and Stockholders' Equity | |
| | | |
| | |
| Current
Liabilities | |
| | | |
| | |
| Accounts
payable | |
$ | 4,292 | | |
$ | 4,322 | |
| Accrued
liabilities - Note 4 | |
| 7,286 | | |
| 7,295 | |
| Deferred
grant income - Note 3 | |
| 917 | | |
| 917 | |
| Total
Liabilities | |
| 12,495 | | |
| 12,534 | |
| | |
| | | |
| | |
| Commitments
and Contingencies - Note 6 | |
| — | | |
| — | |
| | |
| | | |
| | |
| Capital
stock | |
| | | |
| | |
| Authorized: | |
| | | |
| | |
| 10,000,000
preferred stock, par value $0.001 per share | |
| — | | |
| — | |
| 200,000,000 common stock, par value $0.001 per share | |
| | | |
| | |
| Issued
and outstanding: | |
| | | |
| | |
| 82,086,511
common shares (September 30, 2023 - 82,066,511) | |
| 82 | | |
| 82 | |
| Additional
paid-in capital | |
| 437,184 | | |
| 434,839 | |
| Accumulated
deficit | |
| (301,691 | ) | |
| (293,069 | ) |
| Total
Stockholders' Equity | |
| 135,575 | | |
| 141,852 | |
| Total
Liabilities and Stockholders' Equity | |
$ | 148,070 | | |
$ | 154,386 | |
See
Accompanying Notes to Condensed Consolidated Interim Financial Statements
| Anavex
Life Sciences Corp. |
| Condensed
Consolidated Interim Statements of Operations and Comprehensive Loss |
| (in thousands,
except share and per share amounts) |
| (Unaudited) |
| | |
| |
|
| | |
| | | |
| | |
| | |
Three
months ended December 31, |
| | |
2023 | |
2022 |
| Operating
expenses | |
| | | |
| | |
| General
and administrative | |
$ | 2,609 | | |
$ | 3,317 | |
| Research
and development | |
| 8,684 | | |
| 12,067 | |
| | |
| | | |
| | |
| Total
operating expenses | |
| 11,293 | | |
| 15,384 | |
| Operating
loss | |
| (11,293 | ) | |
| (15,384 | ) |
| | |
| | | |
| | |
| Other
income | |
| | | |
| | |
| Grant
income | |
| — | | |
| 25 | |
| Research
and development incentive income | |
| 592 | | |
| 733 | |
| Interest
income, net | |
| 2,008 | | |
| 1,268 | |
| Foreign
exchange gain | |
| 156 | | |
| 366 | |
| | |
| | | |
| | |
| Total
other income, net | |
| 2,756 | | |
| 2,392 | |
| | |
| | | |
| | |
| Income
tax recovery (expense), current | |
| (85 | ) | |
| 20 | |
| | |
| | | |
| | |
| Net
loss and comprehensive loss | |
$ | (8,622 | ) | |
$ | (12,972 | ) |
| | |
| | | |
| | |
| Net Loss
per share | |
| | | |
| | |
| Basic
and diluted | |
$ | (0.11 | ) | |
$ | (0.17 | ) |
| | |
| | | |
| | |
| Weighted
average number of shares outstanding | |
| | | |
| | |
| Basic
and diluted | |
| 82,077,815 | | |
| 77,977,112 | |
See
Accompanying Notes to Condensed Consolidated Interim Financial Statements
Anavex Life Sciences Corp.
Condensed Consolidated Interim Statements of Changes
in Stockholders' Equity
For the three months ended December 31, 2023 and
2022
(in thousands, except share and per share amounts)
(Unaudited)
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
Common
Stock | |
Additional
Paid-in | |
Accumulated | |
|
| | |
Shares | |
Par
Value | |
Capital | |
Deficit | |
Total |
| | |
| |
| |
| |
| |
|
| Balance,
October 1, 2023 | |
| 82,066,511 | | |
$ | 82 | | |
$ | 434,839 | | |
$ | (293,069 | ) | |
$ | 141,852 | |
| Shares
issued pursuant to exercise of stock options | |
| 20,000 | | |
| — | | |
| 59 | | |
| — | | |
| 59 | |
| Share based
compensation | |
| — | | |
| — | | |
| 2,286 | | |
| — | | |
| 2,286 | |
| Net
loss | |
| — | | |
| — | | |
| — | | |
| (8,622 | ) | |
| (8,622 | ) |
| Balance,
December 31, 2023 | |
| 82,086,511 | | |
| 82 | | |
| 437,184 | | |
| (301,691 | ) | |
| 135,575 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance,
October 1, 2022 | |
| 77,942,815 | | |
$ | 78 | | |
$ | 387,977 | | |
$ | (245,564 | ) | |
| 142,491 | |
| Shares
issued pursuant to exercise of stock options | |
| 89,320 | | |
| — | | |
| 258 | | |
| — | | |
| 258 | |
| Share based
compensation | |
| — | | |
| — | | |
| 5,347 | | |
| — | | |
| 5,347 | |
| Net
loss | |
| — | | |
| — | | |
| — | | |
| (12,972 | ) | |
| (12,972 | ) |
| Balance,
December 31, 2022 | |
| 78,032,135 | | |
| 78 | | |
| 393,582 | | |
| (258,536 | ) | |
| 135,124 | |
See
Accompanying Notes to Condensed Consolidated Interim Financial Statements
| Anavex Life Sciences Corp. |
| Condensed Consolidated Interim Statements of Cash Flows |
| (in thousands, except share and per share amounts) |
| (Unaudited) |
| | |
| |
|
| | |
| | | |
| | |
| | |
Three months ended December 31, |
| | |
2023 | |
2022 |
| | |
| |
|
| Cash Flows used in Operating Activities | |
| | | |
| | |
| Net loss | |
$ | (8,622 | ) | |
$ | (12,972 | ) |
| Adjustments to reconcile net loss to net cash used in operations: | |
| | | |
| | |
| Share based compensation | |
| 2,286 | | |
| 5,347 | |
| Changes in working capital balances related to operations: | |
| | | |
| | |
| Incentive and tax receivables | |
| (840 | ) | |
| (902 | ) |
| Prepaid expenses and deposits | |
| (103 | ) | |
| (301 | ) |
| Accounts payable | |
| (30 | ) | |
| 1,463 | |
| Accrued liabilities | |
| (9 | ) | |
| 1,098 | |
| Deferred grant income | |
| — | | |
| 473 | |
| Net cash used in operating activities | |
| (7,318 | ) | |
| (5,794 | ) |
| | |
| | | |
| | |
| Cash Flows provided by Financing Activities | |
| | | |
| | |
| Proceeds from exercise of stock options | |
| 59 | | |
| 258 | |
| Net cash provided by financing activities | |
| 59 | | |
| 258 | |
| | |
| | | |
| | |
| Decrease in cash and cash equivalents during the period | |
| (7,259 | ) | |
| (5,536 | ) |
| Cash and cash equivalents, beginning of period | |
| 151,024 | | |
| 149,158 | |
| Cash and cash equivalents, end of period | |
$ | 143,765 | | |
$ | 143,622 | |
| | |
| | | |
| | |
| Supplemental Cash Flow Information | |
| | | |
| | |
| Cash paid for state and local minimum income taxes | |
$ | 47 | | |
$ | 50 | |
See
Accompanying Notes to Condensed Consolidated Interim Financial Statements
Anavex
Life Sciences Corp.
Notes to the Condensed Consolidated Interim Financial Statements
December 31, 2023
(Unaudited)
Note 1 Business Description
Business
Anavex Life Sciences Corp. (“Anavex” or
the “Company”) is a clinical stage biopharmaceutical company engaged in the development of differentiated therapeutics by
applying precision medicine to central nervous system (“CNS”) diseases with high unmet need. Anavex analyzes genomic data
from clinical trials to identify biomarkers, which are used in the analysis of its clinical trials for the treatment of neurodegenerative
and neurodevelopmental diseases.
The Company’s lead compound ANAVEX®2-73
is being developed to treat Alzheimer’s disease, Parkinson’s disease and potentially other central nervous system diseases,
including rare diseases, such as Rett syndrome, a rare severe neurological monogenic disorder caused by mutations in the X-linked gene,
methyl-CpG-binding protein 2 (“MECP2”).
Note 2 Basis of Presentation
Basis of Presentation
These accompanying unaudited condensed consolidated interim financial statements
have been prepared pursuant to the rules and regulations of the Securities and Exchange Commission (“SEC”) and accounting
principles generally accepted in the United States of America (“U.S. GAAP”) for interim reporting. Accordingly, certain information
and note disclosures normally included in the annual financial statements in accordance with U.S. GAAP have been condensed or omitted
pursuant to such rules and regulations. In the opinion of management, the disclosures are adequate to make the information presented not
misleading.
These accompanying unaudited condensed consolidated interim financial statements
reflect all adjustments, consisting of normal recurring adjustments, which in the opinion of management are necessary for fair presentation
of the information contained herein. The consolidated balance sheet as of September 30, 2023 was derived from the audited annual financial
statements but does not include all disclosures required by U.S. GAAP. The accompanying unaudited condensed consolidated interim financial
statements should be read in conjunction with the audited consolidated financial statements and notes thereto included in the Company’s
annual report on Form 10-K for the year ended September 30, 2023 filed with the SEC on November 27, 2023. The Company follows the same
accounting policies in the preparation of interim reports.
Operating results for the three months ended December
31, 2023 are not necessarily indicative of the results that may be expected for the year ending September 30, 2024.
Liquidity
All of the Company’s potential drug compounds
are in the clinical development stage and the Company cannot be certain that its research and development efforts will be successful or,
if successful, that its potential drug compounds will ever be approved for sales to pharmaceutical companies or generate commercial revenues.
To date, we have not generated any revenues from our operations. The Company expects the business to continue to experience negative cash
flows from operations for the foreseeable future and cannot predict when, if ever, our business might become profitable.
Anavex
Life Sciences Corp.
Notes to the Condensed Consolidated Interim Financial Statements
December 31, 2023
(Unaudited)
Management believes that the current working capital position will be sufficient to
meet the Company’s working capital requirements beyond the next 12 months after the date that these condensed consolidated interim
financial statements are issued. The process of drug development can be costly, and the timing and outcomes of clinical trials are uncertain. The
assumptions upon which the Company has based its estimates are routinely evaluated and may be subject to change. The actual amount
of the Company’s expenditures will vary depending upon a number of factors including but not limited to the design, timing and duration
of future clinical trials, the progress of the Company’s research and development programs and the level of financial resources
available. The Company has the ability to adjust its operating plan spending levels based on the timing of future clinical trials.
Other than our rights related to the 2023
Purchase Agreement (as defined below in Note 5), there can be no assurance that additional financing will be available to us when
needed or, if available, that it can be obtained on commercially reasonable terms. If the Company is not able to obtain the
additional financing on a timely basis, if and when it is needed, it will be forced to delay or scale down some or all of its
research and development activities.
Use of Estimates
The preparation of financial statements in accordance
with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the
date of the financial statements and the reported amounts of revenue and expenses in the reporting period. The Company regularly evaluates
estimates and assumptions related to accounting for research and development costs, incentive income receivable, valuation and recoverability
of deferred tax assets, share based compensation, and loss contingencies. The Company bases its estimates and assumptions on current facts,
historical experience, and various other factors that it believes to be reasonable under the circumstances, the results of which form
the basis for making judgments about the carrying values of assets and liabilities and the accrual of costs and expenses that are not
readily apparent from other sources. The actual results experienced by the Company may differ materially and adversely from the Company’s
estimates. To the extent there are material differences between the estimates and the actual results, future results of operations will
be affected.
Principles of Consolidation
These consolidated financial statements include the
accounts of Anavex Life Sciences Corp. and its wholly-owned subsidiaries, Anavex Australia Pty Limited (“Anavex Australia”),
a company incorporated under the laws of Australia, Anavex Germany GmbH, a company incorporated under the laws of Germany, and Anavex
Canada Ltd., a company incorporated under the laws of the Province of Ontario, Canada. All inter-company transactions and balances have
been eliminated.
Fair Value Measurements
The fair value hierarchy under GAAP is based on three
levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value which
are the following:
Anavex
Life Sciences Corp.
Notes to the Condensed Consolidated Interim Financial Statements
December 31, 2023
(Unaudited)
Level 1 - quoted prices (unadjusted) in active
markets for identical assets or liabilities;
Level 2 - observable inputs other than Level
1, quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets and
liabilities in markets that are not active, and model-derived prices whose inputs are observable or whose significant value
drivers are observable; and
Level
3 - assets and liabilities whose significant value drivers are unobservable by little or no market activity and that are significant
to the fair value of the assets or liabilities.
At December 31, 2023 and September 30, 2023, the Company
did not have any Level 3 assets or liabilities.
Basic and Diluted Loss per Share
Basic income/(loss) per common share is computed by
dividing net income/(loss) available to common stockholders by the weighted average number of common shares outstanding during the period.
Diluted income/(loss) per common share is computed by dividing net income/(loss) available to common stockholders by the sum of (1) the
weighted-average number of common shares outstanding during the period, (2) the dilutive effect of the assumed exercise of options and
warrants using the treasury stock method and (3) the dilutive effect of other potentially dilutive securities. For purposes of the diluted
net loss per share calculation, options and warrants are potentially dilutive securities and are excluded from the calculation of diluted
net loss per share because their effect would be anti-dilutive.
As of December 31, 2023 loss per share excludes 14,269,363
(December 31, 2022: 13,525,296) potentially dilutive common shares related to outstanding options and warrants, as their effect was anti-dilutive.
Recently Adopted Accounting
Pronouncements
In November 2023, the Financial
Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) No. 2023-07, “Segment Reporting: Improvements to Reportable
Segment Disclosures.” This guidance requires disclosure of incremental segment information on an annual and interim basis. This
amendment is effective for our fiscal year ending September 30, 2025 and our interim periods within the fiscal year ending September 30,
2026. The Company is currently assessing the impact of this guidance on its disclosures.
In December 2023, the FASB
issued ASU No. 2023-09, “Income Taxes: Improvements to Income Tax Disclosures.” This guidance requires consistent categories
and greater disaggregation of information in the rate reconciliation and disclosures of income taxes paid by jurisdiction. This amendment
is effective for our fiscal year ending September 30, 2026. The Company is currently assessing the impact of this guidance on its disclosures.
Note 3 Other Income
Grant
Income
As
of December 31, 2023, the Company had received a $1.0 million research grant awarded by the Michael J. Fox Foundation for Parkinson’s
Research. The grant will be used to fund a clinical trial of the Company’s lead compound, ANAVEX®2-73 related
to Parkinson’s disease. Of the total, $0.5 million was received during the year ended September 30, 2023 and $0.5 million
was received during the year ended September 30, 2021.
Anavex
Life Sciences Corp.
Notes to the Condensed Consolidated Interim Financial Statements
December 31, 2023
(Unaudited)
The
grant income has been deferred when received and is being amortized to other income as the related research and development expenditures
are incurred. During the three months ended December 31, 2023, the Company recognized $0 (three months ended December 31, 2022:
$25,000) of this grant on its statements of operations as grant income. At December 31, 2023, an amount of $0.9 million (September
30, 2023: $0.9 million) of this grant is recorded as deferred grant income, representing the amount of this grant which has not
yet been recognized to other income. The Company will recognize this income on its statement of operations as the relating expenditures
are incurred to offset the income.
Research
and development incentive income
Research and development
incentive income represents the income earned by Anavex Australia of the Australia R&D credit. This cash incentive is received by
Anavex Australia, upon filing of a claim in connection with Anavex Australia’s annual income tax return.
During the three months ended
December 31, 2023 the Company recorded research and development incentive income of $0.6 million (AUD 0.9 million) (2022: $0.7 million
(AUD 1.1 million)) in respect of the Australia R&D credit for eligible research and development expenses incurred during the period.
This amount is included within Other income on the consolidated statements of operations.
At December 31, 2023, Incentive
and tax receivables includes $3.3 million (AUD 4.9 million) (September 30, 2023: $2.5 million (AUD 3.9 million)) relating to Australia
R&D credits earned during the year that are expected to be reimbursed upon filing of the Company’s annual claim under this program.
The Australia R&D credit
program is a self-assess program whereby the Company must assess its eligibility each year to determine (i) if the entity is eligible
(ii) if the specific R&D activities are eligible and (iii) if the individual R&D expenditures have nexus to such R&D activities.
The Company evaluates its eligibility under the tax incentive program as of each balance sheet date based on the most current and relevant
data available. Anavex Australia is able to continue to claim the R&D tax incentive for as long as it remains eligible and continues
to incur eligible research and development expenditures.
Although the Company believes
that it has complied with all the relevant conditions of eligibility under the program for all periods claimed, the Australian Tax Office
(ATO) has the right to review the Company’s qualifying programs and related expenditures for a period of four years. If such a review
were to occur, the ATO may have different interpretations of certain eligibility requirements. If the ATO disagreed with the Company’s
assessments and any related subsequent appeals, it could require adjustment to and repayment of current or previous years’ claims
already received. Additionally, if the Company was unable to demonstrate a reasonably arguable position taken on such claims, the ATO
could also assess penalties and interest on such adjustment.
Currently, the Company’s
tax incentive claims from 2019 to 2022 are open to potential review by the ATO. Additionally, the period open for review is indefinite
if the ATO suspects fraud. The Company has not provided any allowance for any such potential adjustments, should they occur in the future.
Anavex
Life Sciences Corp.
Notes to the Condensed Consolidated Interim Financial Statements
December 31, 2023
(Unaudited)
Note 4 Accrued Liabilities
The principal components of accrued liabilities consist of (in thousands):
| Schedule of accrued liabilities | |
| | | |
| | |
| | |
December 31 | |
September 30 |
| | |
2023 | |
2023 |
| Accrued clinical site and patient visits costs | |
$ | 2,058 | | |
$ | 2,006 | |
| Accrued compensation and benefits | |
| 1,785 | | |
| 1,360 | |
| Fixed contract accruals | |
| 364 | | |
| 38 | |
| Milestone based contract accruals | |
| 989 | | |
| 1,267 | |
| All other accrued liabilities | |
| 2,090 | | |
| 2,624 | |
| Total accrued liabilities | |
$ | 7,286 | | |
$ | 7,295 | |
Note 5 Equity Offerings
Common Stock
Common shares are voting and are entitled to dividends
as declared at the discretion of the Board of Directors (the “Board”).
Preferred Stock
The Company’s Board has the authority to
issue preferred stock in one or more series and to fix the rights, preferences, privileges, restrictions and the number of shares constituting
any series or the designation of the series.
Sales Agreement
The Company entered into a Controlled Equity Offering
Sales Agreement on July 6, 2018, which was amended and restated on May 1, 2020 (the “Sales Agreement”) with Cantor Fitzgerald &
Co. and SVB Leerink LLC (together the “Sales Agents”), pursuant to which the Company may offer and sell shares of common stock
(“Shares”) registered under an effective registration statement from time to time through the Sales Agents (the “Offering”).
Upon delivery of a placement notice based on the Company’s
instructions and subject to the terms and conditions of the Sales Agreement, the Sales Agents may sell the Shares by methods deemed to
be an “at the market offering” offering, in negotiated transactions at market prices prevailing at the time of sale or at
prices related to such prevailing market prices, or by any other method permitted by law, including negotiated transactions, subject to
the prior written consent of the Company. The Company is not obligated to make any sales of Shares under the Sales Agreement. The Company
or Sales Agents may suspend or terminate the offering of Shares upon notice to the other party, subject to certain conditions. The Sales
Agents will act as agent on a commercially reasonable efforts basis consistent with their normal trading and sales practices and applicable
state and federal law, rules and regulations and the rules of Nasdaq.
Anavex
Life Sciences Corp.
Notes to the Condensed Consolidated Interim Financial Statements
December 31, 2023
(Unaudited)
The Company has agreed to pay the Sales Agents commissions
for their services of up to 3.0% of the gross proceeds from the sale of the Shares pursuant to the Sales Agreement. The Company also agreed
to provide the Sales Agents with customary indemnification and contribution rights. During the three months ended December 31, 2023 and
2022, no shares were sold pursuant to the Offering. At December 31, 2023, an amount of $142.4 million (September 30, 2023: $142.4 million)
was registered pursuant to an effective registration statement. The Company currently is unable to sell shares of common stock under the
Sales Agreement.
2023 Purchase Agreement
On February 3, 2023, the Company entered into a $150.0
million purchase agreement (the “2023 Purchase Agreement”) with Lincoln Park Capital Fund, LLC (“Lincoln Park”),
pursuant to which the Company has the right to sell and issue to Lincoln Park, and Lincoln Park is obligated to purchase, up to $150.0
150,000,000
million in value of its shares of common stock from time to time over a three-year period until February 3, 2026.
In consideration for entering into the 2023 Purchase
Agreement, the Company issued to Lincoln Park 75,000 shares of common stock as a commitment fee (the “initial commitment shares”)
and agreed to issue up to an additional 75,000 shares pro rata, when and if, Lincoln Park purchased, at the Company’s discretion,
the $150.0 million aggregate commitment. The Company determined the fair value of the initial commitment shares was $0.8 million with
reference to the closing price of the Company’s shares on the Purchase Agreement date. In addition, the Company incurred third party
expenses of $0.1 million in connection with entering into the Purchase Agreement. These amounts were expensed to other financing expense
on the statements of operations during the year ended September 30, 2023.
During the three months ended December 31, 2023 and
2022, the Company did not issue any shares of common stock under the 2023 Purchase Agreement.
At December 31, 2023, an amount of $122.1 million
remained available under the 2023 Purchase Agreement.
Note 6 Commitments and Contingencies
Leases
The Company leases office space under
an operating lease with an initial term of 12 months or less. Under the terms of the office lease, the Company is required to pay its
proportionate share of operating costs.
During the three months ended December
31, 2023 and 2022, operating lease costs were as follows (in thousands):
| Schedule of operating lease costs | |
| |
|
| | |
2023 | |
2022 |
| Operating lease costs | |
$ | 30 | | |
$ | 30 | |
Anavex
Life Sciences Corp.
Notes to the Condensed Consolidated Interim Financial Statements
December 31, 2023
(Unaudited)
Employee 401(k) Benefit Plan
The Company has
a defined-contribution savings plan under Section 401(k) of the Internal Revenue Code. The plan covers all United States based employees.
United States based employees eligible to participate in the plan may contribute up to the current statutory limits under the Internal
Revenue Service regulations. The 401(k) plan permits the Company to make additional matching contributions on behalf of contributing employees.
During the three months ended December 31, 2023
and 2022, the Company made matching contributions under the 401(k) plan as
follows (in thousands):
| Schedule of contribution plan | |
| |
|
| | |
2023 | |
2022 |
| Contributions to 401(k) plan | |
$ | 73 | | |
$ | 44 | |
Litigation
The Company is subject
to claims and legal proceedings that arise in the ordinary course of business. Such matters are inherently uncertain, and there can be
no guarantee that the outcome of any such matter will be decided favorably to the Company or that the resolution of any such matter will
not have a material adverse effect upon the Company’s consolidated financial statements. The Company does not believe that any of
such pending claims and legal proceedings will have a material adverse effect on its consolidated financial statements.
Share Purchase Warrants
At December 31, 2023 and September 30, 2023, the Company
had 160,000 warrants outstanding at a weighted average exercise price of $3.72 as follows:
| Schedule of share purchase warrants outstanding | |
| |
|
| Number | |
Exercise Price | |
Expiry Date |
| | 150,000 | | |
$ | 3.17 | | |
May 6, 2024 |
| | 10,000 | | |
$ | 12.00 | | |
April 21, 2026 |
| | 160,000 | | |
| | | |
|
Stock–based Compensation
Plan
2015 Stock Option Plan
On September 18, 2015, the Company’s Board approved
a 2015 Omnibus Incentive Plan (the “2015 Plan”), which provided for the grant of stock options and restricted stock awards
to directors, officers, employees and consultants of the Company.
Anavex
Life Sciences Corp.
Notes to the Condensed Consolidated Interim Financial Statements
December 31, 2023
(Unaudited)
The maximum number of our common shares reserved for
issue under the plan was 6,050,553 shares, subject to adjustment in the event of a change of the Company’s capitalization.
2019 Stock Option Plan
On January 15, 2019, the Board approved the 2019 Omnibus
Incentive Plan (the “2019 Plan”), which provides for the grant of stock options and restricted stock awards to directors,
officers, employees, consultants and advisors of the Company.
The maximum number of our common shares reserved for
issue under the plan was 6,000,000 shares, subject to adjustment in the event of a change of the Company’s capitalization.
During the year ended September 30, 2022, 406,453
options previously available under the 2019 Plan and the 2015 Plan became available under the 2022 Plan (as defined below).
2022 Stock Option Plan
On March 25, 2022, the Board approved the 2022 Omnibus
Incentive Plan (the “2022 Plan”). The 2022 Plan was approved by stockholders on May 24, 2022. Under the terms of the 2022
Plan, 10,000,000 additional shares of Common Stock will be available for issuance under the plan, in addition to the shares available
under the 2019 Plan and the 2015 Plan. Any awards outstanding under a previous stock option plan will remain subject to and be paid under
such plan, and any shares subject to outstanding awards under a previous plan that subsequently cease to be subject to such awards (other
than by reason of settlement of the awards in shares) will automatically become available for issuance under the 2022 Plan.
The 2022 Plan provides that it may be administered
by the Board, or the Board may delegate such responsibility to a committee. The exercise price will be determined by the Board at the
time of grant shall be at least equal to the fair market value on such date. If the grantee is a 10% stockholder on the grant date, then
the exercise price shall not be less than 110% of fair market value of the Company’s shares of common stock on the grant date. Stock
options may be granted under the 2022 Plan for an exercise period of up to ten years from the date of grant of the option or such lesser
periods as may be determined by the Board, subject to earlier termination in accordance with the terms of the 2022 Plan. At December 31,
2023, 3,892,000 options had been issued under the 2022 Plan and 6,643,952 options were available for issue under the 2022 Plan.
Anavex
Life Sciences Corp.
Notes to the Condensed Consolidated Interim Financial Statements
December 31, 2023
(Unaudited)
The following summarizes information about stock option
activity during the year ended September 30, 2023 and three months ended December 31, 2023:
| Schedule of outstanding stock purchase options | | |
| | | |
| | | |
| | | |
| | |
| | |
Number of Options | |
Weighted Average Exercise Price
($) | |
Weighted Average Grant Date Fair Value
($) | |
Aggregate intrinsic value
($) |
| Outstanding, September 30, 2022 | | |
| 13,169,616 | | |
| 6.61 | | |
| 4.96 | | |
| 62,267,309 | |
| Granted | | |
| 1,959,000 | | |
| 9.30 | | |
| 6.60 | | |
| — | |
| Exercised | | |
| (759,753 | ) | |
| 2.34 | | |
| 0.95 | | |
| 4,629,026 | |
| Forfeited | | |
| (257,083 | ) | |
| 12.00 | | |
| 6.74 | | |
| — | |
| Outstanding, September 30, 2023 | | |
| 14,111,780 | | |
| 7.12 | | |
| 5.27 | | |
| 22,290,069 | |
| Granted | | |
| 155,000 | | |
| 7.40 | | |
| 4.88 | | |
| — | |
| Exercised | | |
| (20,000 | ) | |
| 2.96 | | |
| 2.23 | | |
| 58,600 | |
| Forfeited | | |
| (137,417 | ) | |
| 12.19 | | |
| 6.78 | | |
| — | |
| Outstanding, December 31, 2023 | | |
| 14,109,363 | | |
| 7.08 | | |
| | | |
| 47,674,002 | |
| Exercisable, December 31, 2023 | | |
| 9,663,446 | | |
| 5.26 | | |
| | | |
| 45,388,166 | |
The following summarizes information about stock options
at December 31, 2023 by a range of exercise prices:
| | Schedule of summarized information about stock options | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Range of exercises prices | |
Number of outstanding | |
Weighted average remaining contractual life | |
Weighted average | |
Number of vested | |
Weighted average |
| From | |
To | |
options | |
(in years) | |
exercise price | |
options | |
exercise price |
| $ | 0.92 | | |
$ | 3.00 | | |
| 3,260,309 | | |
| 4.47 | | |
$ | 2.38 | | |
| 3,260,309 | | |
$ | 2.38 | |
| $ | 3.01 | | |
$ | 5.00 | | |
| 2,017,500 | | |
| 4.07 | | |
$ | 3.28 | | |
| 2,017,500 | | |
$ | 3.28 | |
| $ | 5.01 | | |
$ | 9.00 | | |
| 5,375,054 | | |
| 6.00 | | |
$ | 6.91 | | |
| 3,329,970 | | |
$ | 6.11 | |
| $ | 9.01 | | |
$ | 13.00 | | |
| 1,909,000 | | |
| 8.10 | | |
$ | 10.53 | | |
| 496,083 | | |
$ | 11.36 | |
| $ | 13.01 | | |
$ | 25.00 | | |
| 1,547,500 | | |
| 7.23 | | |
$ | 18.24 | | |
| 559,584 | | |
$ | 18.64 | |
| | | | |
| | | |
| 14,109,363 | | |
| 5.79 | | |
$ | 7.08 | | |
| 9,663,446 | | |
$ | 5.26 | |
Anavex
Life Sciences Corp.
Notes to the Condensed Consolidated Interim Financial Statements
December 31, 2023
(Unaudited)
The weighted average grant date fair value of options
vested at December 31, 2023 was $4.00 (September 30, 2023: $3.94). At December 31, 2023, the weighted average contractual life of options
outstanding was 5.8 years (September 30, 2023: 6.0 years) and for options exercisable was 4.5 years (September 30, 2023: 4.75 years).
The aggregate intrinsic value is calculated as the
difference between the exercise price of the underlying awards and the quoted market price of the Company’s stock for the options
that were in-the-money at December 31, 2023.
During the three months ended December 31, 2023,
the Company recognized stock-based compensation expense of $2.3
million (2022: $5.3
million) in connection with the issuance and vesting of stock options and warrants in exchange for services. These amounts have
been included in general and administrative expenses and research and development expenses on the Company’s condensed
consolidated interim statement of operations as follows (in thousands):
| Schedule of general and administrative expenses and research and development expenses | |
| | | |
| | |
| | |
December 31 |
| | |
2023 | |
2022 |
| General and administrative | |
$ | 926 | | |
$ | 1,743 | |
| Research and development | |
| 1,360 | | |
| 3,604 | |
| Total stock-based compensation | |
$ | 2,286 | | |
$ | 5,347 | |
An amount of approximately $27.7 million in stock-based
compensation is expected to be recorded over the remaining term of such options through fiscal 2026.
The fair value of each option award
granted during the three months ended December 31, 2023 and 2022 is estimated on the date of grant using the Black Scholes option pricing
model based on the following weighted average assumptions:
| Schedule of weighted average
assumptions | |
| | | |
| | |
| | |
2023 | |
2022 |
| Risk-free interest rate | |
| 4.47 | % | |
| 4.07 | % |
| Expected life of options (years) | |
| 5.29 | | |
| 4.98 | |
| Annualized volatility | |
| 80.61 | % | |
| 84.01 | % |
| Dividend rate | |
| 0.00 | % | |
| 0.00 | % |
The fair value of stock compensation charges recognized
during the three months ended December 31, 2023 and 2022 was determined with reference to the quoted market price of the Company’s
shares on the grant date.
ITEM 2. MANAGEMENT’S DISCUSSION AND
ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
Forward-Looking Statements
This Quarterly Report on Form 10-Q includes
forward-looking statements. All statements other than statements of historical facts contained in this Quarterly Report on Form
10-Q, including statements regarding our anticipated future clinical and regulatory milestone events, future financial position,
business strategy and plans and objectives of management for future operations, are forward-looking statements. The words “believe,”
“may,” “estimate,” “continue,” “anticipate,” “intend,” “expect”
“should,” “forecast,” “potential,” “predict”, “could,” “would,”
“will,” “suggest,” “plan” and similar expressions, as they relate to us, are intended to identify
forward-looking statements. Such forward-looking statements include, without limitation, statements regarding:
| |
● |
volatility in our stock price and in the markets in general; |
| |
● |
our ability to successfully conduct preclinical studies and clinical trials for our product candidates; |
| |
● |
our ability to raise additional capital on favorable terms and the impact of such activities on our stockholders and stock price; |
| |
● |
our ability to generate any revenue or to continue as a going concern; |
| |
● |
our ability to execute our research and development plan on time and on budget; |
| |
● |
our products candidates’ ability to demonstrate efficacy or an acceptable safety profile; |
| |
● |
our ability to obtain the support of qualified scientific collaborators; |
| |
● |
our ability, whether alone or with commercial partners, to successfully commercialize any of our product candidates that may be approved for sale; |
| |
● |
our ability to identify and obtain additional product candidates; |
| |
● |
our reliance on third parties in non-clinical studies and clinical trials; |
| |
● |
our ability to defend against product liability claims; |
| |
● |
our ability to safeguard against security breaches; |
| |
● |
our ability to obtain and maintain sufficient intellectual property protection for our product candidates; |
| |
● |
our ability to comply with our intellectual property licensing agreements; |
| |
● |
our ability to defend against claims of intellectual property infringement; |
| |
● |
our ability to comply with the maintenance requirements of the government patent agencies; |
| |
● |
our ability to protect our intellectual property rights throughout the world; |
| |
● |
competition; |
| |
● |
the anticipated start dates, durations and completion dates of our ongoing and future clinical trials; |
| |
● |
the anticipated designs of our future clinical trials; |
| |
● |
our ability to attract and retain qualified employees; |
| |
● |
the impact of Fast Track designation on receipt of actual FDA approval; |
| |
● |
our anticipated future regulatory submissions and our ability to receive regulatory approvals to develop and market our product candidates, including any orphan drug or Fast Track designations; and |
| |
● |
our anticipated future cash position and ability to obtain funding for our operations. |
We have based these forward-looking statements
largely on our current expectations and projections about future events, including the responses we expect from regulatory authorities
and financial trends that we believe may affect our financial condition, results of operations, business strategy, preclinical
studies and clinical trials, and financial needs. These forward-looking statements are subject to a number of risks, uncertainties
and assumptions including without limitation the risks described in “Risk Factors” in Part I, Item 1A of our Annual
Report on Form 10-K filed with the Securities and Exchange Commission on November 27, 2023. These risks are not exhaustive. Other
sections of this Quarterly Report on Form 10-Q include additional factors which could adversely impact our business and financial
performance. Moreover, we operate in a very competitive and rapidly changing environment. New risk factors emerge from time to
time and it is not possible for our management to predict all risk factors, nor can we assess the impact of all factors on our
business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those
contained in any forward-looking statements. You should not rely upon forward-looking statements as predictions of future events.
We cannot assure you that the events and circumstances reflected in the forward-looking statements will be achieved or occur and
actual results could differ materially from those projected in the forward-looking statements. Except as required by applicable
laws including the securities laws of the United States, we assume no obligation to update or supplement forward-looking statements.
As used in this Quarterly Report on Form 10-Q,
the terms “we,” “us,” “our,” “Company”, and “Anavex” mean Anavex Life
Sciences Corp., unless the context clearly indicates otherwise.
Our Current Business
Anavex Life Sciences Corp. is a clinical stage
biopharmaceutical company engaged in the development of differentiated therapeutics by applying precision medicine to central nervous
system (“CNS”) diseases with high unmet need. We analyze genomic data from clinical trials to identify biomarkers,
which we use in the analysis of our clinical trials.
Our lead product candidate, ANAVEX®2-73
(blarcamesine), is being developed to treat Alzheimer’s disease, Parkinson’s disease and potentially other central
nervous system diseases, including rare diseases, such as Rett syndrome, a rare severe neurological monogenic disorder caused by
mutations in the X-linked gene, methyl-CpG-binding protein 2 (“MECP2”).
We currently have two core programs and two
seed programs. Our core programs are at various stages of clinical and preclinical development, in neurodegenerative and neurodevelopmental
diseases.
The following table summarizes key information about our programs:
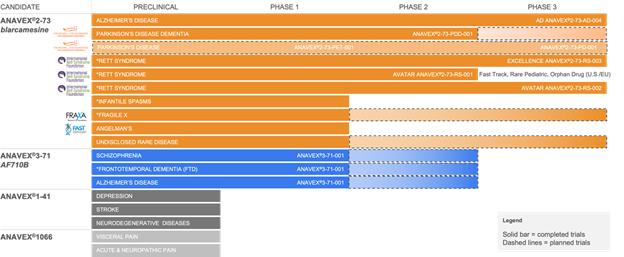
* = Orphan Drug Designation by the FDA
Anavex has a portfolio of compounds varying
in sigma-1 receptor (SIGMAR1) binding activities. The SIGMAR1 gene encodes the SIGMAR1 protein, which is an intracellular chaperone
protein with important roles in cellular communication. SIGMAR1 is also involved in transcriptional regulation at the nuclear envelope
and restores homeostasis and stimulates recovery of cell function when activated. In order to validate the ability of our compounds
to activate quantitatively the SIGMAR1, we performed, in collaboration with Stanford University, a quantitative Positron Emission
Tomography (PET) imaging scan in mice, which demonstrated a dose-dependent ANAVEX®2-73 (blarcamesine) target engagement
or receptor occupancy with SIGMAR1 in the brain.
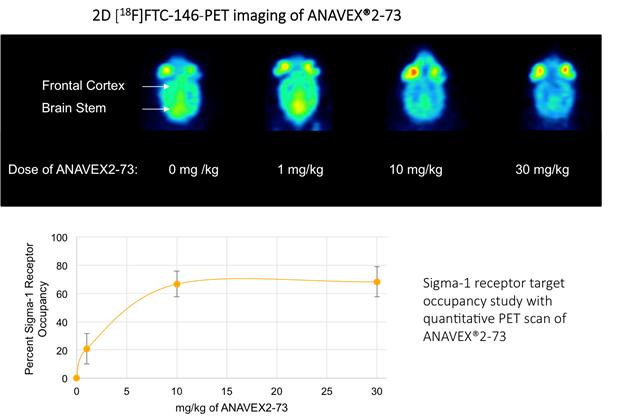
Source:
Reyes S et al., Sci Rep. 2021 Aug 25; 11(1):17150
Cellular Homeostasis
Many diseases are possibly directly caused
by chronic homeostatic imbalances or cellular stress of brain cells. In pediatric diseases, such as Rett syndrome or infantile
spasms, the chronic cellular stress is possibly caused by the presence of a constant genetic mutation. In neurodegenerative diseases,
such as Alzheimer’s and Parkinson’s diseases, chronic cellular stress is possibly caused by age-correlated buildup
of cellular insult and hence chronic cellular stress. Specifically, defects in homeostasis of protein or ribonucleic acid (“RNA”)
lead to the death of neurons and dysfunction of the nervous system. The spreading of protein aggregates resulting in a proteinopathy,
a characteristic found in Alzheimer’s and Parkinson’s diseases that results from disorders of protein synthesis, trafficking,
folding, processing or degradation in cells. The clearance of macromolecules in the brain is particularly susceptible to imbalances
that result in aggregation and degeneration in nerve cells. For example, Alzheimer’s disease pathology is characterized by
the presence of amyloid plaques, and neurofibrillary tangles, which are aggregates of hyperphosphorylated Tau protein that are
a marker of other diseases known as tauopathies as well as inflammation of microglia. With the SIGMAR1 activation through SIGMAR1
agonists like ANAVEX®2-73 (blarcamesine), our approach is to restore cellular balance (i.e. homeostasis). Therapies
that correct defects in cellular homeostasis might have the potential to halt or delay neurodevelopmental and neurodegenerative
disease progression.
ANAVEX®2-73 (blarcamesine) specific
Biomarkers
As part of some of our clinical trials, we
have incorporated a genomic analysis to better understand potential populations for whom our clinical programs might benefit. In
our clinical trials, a full genomic analysis of Alzheimer’s disease patients treated with ANAVEX®2-73 (blarcamesine)
has helped us identify actionable genetic variants. A significant impact of the genomic biomarkers SIGMAR1, the direct target of
ANAVEX®2-73 (blarcamesine) and COMT, a gene involved in memory function, on the drug response level was identified,
leading to an early ANAVEX®2-73 (blarcamesine) specific biomarker hypothesis. We believe that excluding patients
with SIGMAR1 identified biomarker variant (approximately 10%-20% of the population) in prospective studies would identify approximately
80%-90% patients that would display clinically significant improved functional and cognitive scores. The consistency between the
identified DNA and RNA data related to ANAVEX®2-73 (blarcamesine), which are considered independent of Alzheimer’s
disease pathology, as well as multiple endpoints and time-points, provides support for the potential precision medicine clinical
development of ANAVEX®2-73 (blarcamesine) by using genetic biomarkers identified within the trial population itself
to either confirm the mechanism of action of ANAVEX®2-73 (blarcamesine) or target patients who are most likely to
respond to ANAVEX®2-73 (blarcamesine) treatment. We may in the future utilize such an approach in Alzheimer’s
disease as well as indications like Parkinson’s disease dementia or Rett syndrome in which ANAVEX®2-73 (blarcamesine)
is currently being studied.
Clinical Trials Overview
Alzheimer’s Disease
In November 2016, we completed a Phase 2a clinical
trial, consisting of Part A and Part B, which lasted a total of 57 weeks, for ANAVEX®2-73 in mild-to-moderate Alzheimer’s
patients. This open-label randomized trial in Australia met both primary and secondary endpoints and was designed to assess the
safety and exploratory efficacy of ANAVEX®2-73 in 32 patients. ANAVEX®2-73 targets sigma-1 and muscarinic
receptors, which have been shown in preclinical studies to reduce stress levels in the brain believed to restore cellular homeostasis
and to reverse the pathological hallmarks observed in Alzheimer’s disease. In October 2017, we presented positive pharmacokinetic
(“PK”) and pharmacodynamic (“PD”) data from the Phase 2a clinical trial, which established a concentration-effect
relationship between ANAVEX®2-73 and trial measurements. These measures obtained from all patients who participated
in the entire 57 weeks include exploratory cognitive and functional scores as well as biomarker signals of brain activity. Additionally,
the clinical trial appeared to show that ANAVEX®2-73 activity was enhanced by its active metabolite (ANAVEX19-144),
which also targets the SIGMAR1 receptor and has a half-life approximately twice as long as the parent molecule.
Two consecutive trial extensions for the Phase
2a trial have allowed participants who completed the 52-week Part B of the trial to continue taking ANAVEX®2-73,
providing an opportunity to gather extended safety data for a cumulative time period of five years. In August 2020, patients completing
these Phase 2a trial extensions were granted continued access to treatment with ANAVEX®2-73 through the Australian
Government Department of Health – Therapeutic Goods Administration’s compassionate use Special Access Scheme.
A larger Phase 2b/3 double-blind, placebo-controlled
trial of ANAVEX®2-73 in Alzheimer’s disease commenced in August 2018. The Phase 2b/3 trial enrolled 509 patients,
which were treated with a convenient once-daily oral formulation of ANAVEX®2-73 for 48 weeks, randomized 1:1:1 to
two different ANAVEX®2-73 doses or placebo. The trial took place at 52 sites across North America, Europe and Australia.
Primary and secondary endpoints to assess safety and both cognitive and functional efficacy, were measured through the Alzheimer’s
Disease Assessment Scale – Cognitive Subscale test (“ADAS-Cog”), Alzheimer’s Disease Cooperative Study
– Activities of Daily Living (“ADCS-ADL”) and Clinical Dementia Rating – Sum of Boxes for cognition and
function (“CDR-SB”). In addition to the primary endpoints, the ANAVEX®2-73 Phase 2b/3 trial design incorporated
pre-specified statistical analyses related to potential genomic precision medicine biomarkers previously identified in the ANAVEX®2-73
Phase 2a clinical trial. The trial was completed in mid-2022 and, in December 2022, the Company presented positive topline results
from the Phase 2b/3 clinical trial.
ANAVEX®2-73 met the co-primary
endpoints ADAS-Cog and ADCS-ADL and key secondary endpoint CDR-SB. ANAVEX®2-73 treatment slowed decline of cognition
and function in patients with early Alzheimer’s disease over 48 weeks. Patients treated with ANAVEX®2-73 had
1.84 times higher odds, or likelihood, to improve cognitively compared to placebo, with a ADAS-Cog score threshold change of -0.5
points or better [Odds Ratio = 1.84 (p = 0.015)]. At clinically significant levels of improvement in function (ADCS-ADL score threshold
change of +3.5 points or better), patients treated with ANAVEX®2-73 had 2.67 times higher odds, or likelihood, to
improve function compared to placebo [Odds Ratio = 2.67 (p = 0.0255)]. Additionally, treatment with
ANAVEX®2-73 reduced cognitive decline at end of treatment, measured with the ADAS-Cog, as compared to placebo, by
45%, representing a treatment difference in mean score change of -1.85 points (p=0.033). Compared to placebo, ANAVEX®2-73
reduced clinical decline of cognition and function by 27% with mean score difference of -0.42 points (p=0.040) as measured by the
CDR-SB. ANAVEX®2-73 was generally safe and well tolerated. All statistical analyses were
performed by outside consultancy companies.
In September 2023, we provided additional data
demonstrating that the clinical effect was complemented by two independent biomarkers. A significant reduction in pathological
amyloid beta levels in plasma, as well as a significant slowing in the rate of pathological brain atrophy on Magnetic Resonance
Imaging (MRI) scans. Validated biomarkers of amyloid beta pathology, plasma Aβ42/40 ratio increased significantly (P = 0.048),
demonstrating strong anti-amyloid effects of ANAVEX®2-73 in Alzheimer’s disease patients, while MRI revealed
significant reduction in brain volume loss, including whole brain (P = 0.0005), comparing treatment to placebo.
Furthermore, all pre-specified clinical endpoints
were further analyzed using a mixed model for repeated measures (MMRM). Under the multiplicity control rule, a trial is successful
in meeting the co-primary endpoints if the significance of each endpoint is P < 0.05, or if the significance of only one co-primary
endpoint is P < 0.025. If only one primary endpoint is significant at an α level of 0.025, then the secondary endpoint
will be evaluated at the same level of 0.025. The trial was successful, since the differences in the least-squares mean (LSM) change
from baseline to 48 weeks between the ANAVEX®2-73 and placebo groups were −1.783 [95% CI, −3.314 to
−0.251]; (P = 0.0226) for ADAS-Cog13, and −0.456 [95% CI, −0.831 to −0.080]; (P = 0.0175) for CDR-SB in
patients with early Alzheimer’s disease.
In the respective safety population, common
treatment-emergent adverse events included dizziness, which was transient and mostly mild to moderate in severity, and occurred
in 120 participants (35.8%) during titration and in 76 participants (25.2%) during maintenance with ANAVEX®2-73 and 10 (6.0%)
during titration and 9 (5.6%) during maintenance with placebo.
A subsequent long-term open label extension
study of ANAVEX®2-73, entitled the ATTENTION-AD trial was initiated for patients who have completed the 48-week
Phase 2b/3 placebo-controlled trial referenced above. This trial extension for an additional 96 weeks is currently ongoing, and
provides an opportunity to evaluate longer term safety and efficacy of ANAVEX®2-73 in persons with Alzheimer’s
disease.
Rett Syndrome
In February 2016, we presented positive preclinical
data for ANAVEX®2-73 in Rett syndrome, a rare neurodevelopmental disease. The data demonstrated dose related and
significant improvements in an array of behavioral and gait paradigms in a mouse model with an MECP2-null mutation that causes
neurological symptoms that mimic Rett syndrome. The study was funded by the International Rett Syndrome Foundation (“Rettsyndrome.org”).
In January 2017, we were awarded a financial grant from Rettsyndrome.org of a minimum of $0.6 million to cover some of the costs
of a multicenter Phase 2 clinical trial of ANAVEX®2-73 for the treatment of Rett syndrome. This award was received
in quarterly instalments which commenced during fiscal 2018.
In March 2019, we commenced the first Phase
2 clinical trial in a planned Rett syndrome program of ANAVEX®2-73 for the treatment of Rett syndrome. The clinical
trials are being conducted in a range of patient age demographics and geographic regions, utilizing an oral liquid once-daily formulation
of ANAVEX®2-73.
The first Phase 2 trial, (ANAVEX®2-73-RS-001),
which took place in the United States, was completed in December 2020. This trial was a randomized double-blind, placebo-controlled
safety, tolerability, PK and efficacy trial of oral liquid ANAVEX®2-73 formulation in 25 adult female patients with
Rett syndrome over a 7-week treatment period including ANAVEX®2-73-specific genomic precision medicine biomarkers.
The primary endpoint of the trial was safety. The dosing of 5 mg ANAVEX®2-73 was well-tolerated and demonstrated
dose-proportional PK. All secondary efficacy endpoints of the trial showed statistically significant and clinically meaningful
response in the Rett Syndrome Behaviour Questionnaire (“RSBQ”) response, when compared to placebo, in the intent to
treat (“ITT”) cohort (all participants, p = 0.011). 66.7% of ANAVEX®2-73 treated subjects showed a statistically
significant improvement in RSBQ response as compared to 10% of the subjects on placebo in the ITT cohort (all participants, p =
0.011). ANAVEX®2-73 treatment resulted in a sustained improvement in Clinical Global Impression Improvement (CGI-I)
response throughout the 7-week clinical trial, when compared to placebo in the ITT cohort (all participants, p = 0.014). Consistent
with previous ANAVEX®2-73 clinical trials, patients carrying the common form of the SIGMAR1 gene treated with ANAVEX®2-73
experienced stronger improvements in the prespecified efficacy endpoints.
The
second, international trial of ANAVEX®2-73 for the treatment of Rett syndrome, called the AVATAR trial, commenced
in June 2019. This trial took place in Australia and the United Kingdom using a higher dose than the U.S. based Phase 2
trial for Rett syndrome. The trial was a Phase 3 randomized, double-blind, placebo-controlled trial to evaluate the safety and
efficacy of ANAVEX®2-73 in 33 adult patients over a 7-week treatment period including ANAVEX®2-73
specific precision medicine biomarkers. Based upon the input from the successful U.S. Phase 2 Rett syndrome trial (ANAVEX®2-73-RS-001),
we updated the endpoints for the AVATAR trial (ANAVEX®2-73-RS-002) to appropriately assess the clinically meaningful
outcome following International Conference on Harmonization (ICH) guidelines. These updates were approved by the respective regulatory
authorities in the U.K. and in Australia, respectively, where the AVATAR trial was conducted.
The data from the AVATAR trial was released
in February 2022. The clinical trial met all primary and secondary efficacy and safety endpoints, with consistent improvements
in primary efficacy endpoint, RSBQ response (p = 0.037), and secondary efficacy endpoints, Anxiety, Depression, and Mood Scale
(ADAMS) (p = 0.010) and CGI-I (p = 0.037) response. Efficacy endpoints demonstrated statistically significant and clinically meaningful
reductions in Rett syndrome symptoms. Convenient once daily oral liquid doses of up to 30 mg of ANAVEX®2-73 were
also well tolerated with good medication compliance. All patients who participated in the trial were eligible to receive ANAVEX®2-73
under a voluntary open label extension protocol and subsequent Compassionate Use Program.
The very first trial of ANAVEX®2-73
in pediatric Rett syndrome patients, the EXCELLENCE trial, completed enrollment in February 2023. This randomized, double-blind,
placebo-controlled Phase 2/3 trial in pediatric patients with Rett syndrome included trial sites in Canada, Australia, and the
United Kingdom. 92 pediatric patients with Rett syndrome between the ages of 5 through 17 years were treated daily with up to 30
mg ANAVEX®2-73. Participants were randomized 2:1 (ANAVEX®2-73:placebo) for 12 weeks, followed by
a week 16 safety visit and topline results from this trial were announced in early January 2024.
After 12 weeks, the study showed improvement
on the key co-primary endpoint RSBQ, which is a detailed 45-item questionnaire for assessing multiple Rett syndrome characteristics
by the patients’ caregivers. The other co-primary endpoint, the CGI-I, which represents a less granular assessment by the
site investigators using a seven-point scoring (one=“very much improved” to seven=“very much worse”), was
not met.
In an ad-hoc analysis, using the predefined
mixed-effect model for repeated measure (MMRM) method, after 12 weeks of treatment, ANAVEX®2-73-treated patients
improved LS Mean (SE) -12.93 (2.150) points on their RSBQ total score compared to LS Mean (SE) -8.32 (2.537) points in placebo-treated
patients. The LS Mean difference (SE) of -4.61 (2.439) points between treated and placebo groups did not reach statistical significance
(n=77; p=0.063). ANAVEX®2-73-treated patients demonstrated a rapid onset of action with improvements at 4 weeks
after treatment with a RSBQ total score LS Mean (SE) -10.32 (2.086) points in the drug-treated group compared to a LS Mean (SE)
-5.67 (2.413) points in placebo-treated patients. The LS Mean difference of -4.65 (2.233) points between treated and placebo groups
was statistically significant (n=77; p=0.041).
The key secondary endpoint, the ADAMS, trended
favorably. In the same analysis, scores for all RSBQ and ADAMS subscales improved over the course of the study. Collectively, the
RSBQ and ADAMS demonstrated improvements in multiple areas, impacting positively in particular repetitive movements, nighttime
disruptive behaviors and social avoidance.
A preliminary review of the safety results
indicates there were no new safety signals in the EXCELLENCE study, reinforcing the favorable and manageable safety profile observed
with ANAVEX®2-73 to date.
All patients who participated in the trial
were eligible to receive ANAVEX®2-73 under a voluntary open label extension protocol.
A high enrollment rate in the OLE of over 91%
and the high level of requests for the Compassionate Use Program (93%) provide solid numerical evidence for the reported positive
Real World Evidence (RWE) from patients with Rett syndrome under Compassionate Use Authorization. Families whose children were
previously on drug or placebo in the placebo-controlled trial commented favorably on the improvement of their child’s daily
life due to ANAVEX®2-73 treatment in the Compassionate Use Program.
Parkinson’s Disease
In September 2016, we presented positive preclinical
data for ANAVEX®2-73 in an animal model of Parkinson’s disease, which demonstrated significant improvements
on behavioral, histopathological, and neuroinflammatory endpoints. The study was funded by the Michael J. Fox Foundation. Additional
data announced in October 2017 indicated that ANAVEX®2-73 induced robust neurorestoration in experimental Parkinsonism.
We believe the encouraging results we have gathered in this preclinical model, coupled with the favorable profile of this product
candidate in the Alzheimer’s disease trial, support the notion that ANAVEX®2-73 has the potential to treat
Parkinson’s disease dementia.
In October 2020, we completed a double-blind,
randomized, placebo-controlled proof-of-concept Phase 2 trial with ANAVEX®2-73 in Parkinson’s disease dementia
in Spain and Australia, to study the effect of the compound on both the cognitive and motor impairment of Parkinson’s disease.
The Phase 2 trial enrolled approximately 132 patients for 14 weeks, randomized 1:1:1 to two different ANAVEX®2-73
doses, 30 mg and 50 mg, or placebo. The ANAVEX®2-73 Phase 2 Parkinson’s disease dementia trial design incorporated
genomic precision medicine biomarkers identified in the ANAVEX®2-73 Phase 2a Alzheimer’s disease trial.
The trial demonstrated that ANAVEX®2-73
was safe and well tolerated in oral doses up to 50 mg once daily. The results showed clinically meaningful, dose-dependent, and
statistically significant improvements in the Cognitive Drug Research (“CDR”) computerized assessment system analysis.
Treatment with ANAVEX®2-73 also resulted in clinically meaningful improvements as measured by the global composite
score of Parkinson’s disease symptom severity, MDS-Unified Parkinson’s Disease Rating Scale (“MDS-UPDRS”)
total score on top of standard of care including dopaminergic therapy, levodopa and other anti-PD medications after 14 weeks of
treatment, suggesting ANAVEX®2-73’s potential capability of slowing and reversing symptoms that progress in
Parkinson’s disease. In addition, the trial confirmed the precision medicine approach of targeting SIGMAR1 as a genetic biomarker
in response to ANAVEX®2-73 may result in improved clinical outcomes.
A 48-week Open Label Extension (“OLE”)
ANAVEX2-73-PDD-EP-001 Phase 2 trial was offered to participants after completion of the double-blind placebo-controlled ANAVEX2-73-PDD-001
Phase 2 trial discussed above. The OLE trial assessed safety, tolerability and efficacy, measuring among others, MDS-Unified Parkinson’s
Disease Rating Scale Parts I, II, III, REM Sleep Behavior Disorder Screening Questionnaire (RBDSQ), Clinical Global Impression
– Improvement (CGI-I), as well as cognitive efficacy endpoint Montreal Cognitive Assessment (MoCA) over a 48-week period.
In March 2023, we reported the preliminary
ANAVEX2-73-PDD-EP-001 OLE trial data, which demonstrated longitudinal beneficial effects of ANAVEX®2-73 on the pre-specified
primary and secondary objectives. Preliminary analysis reveals that ANAVEX®2-73 was found to be generally safe and
well tolerated; and safety findings in this trial were consistent with the known safety profile of ANAVEX®2-73.
In respect to efficacy, across all efficacy endpoints, patients performed better while on ANAVEX®2-73. While all
patients were on drug holiday due to COVID-19 between the DB EOT and the OLE Baseline, the respective efficacy endpoints, including
the MDS-UPDRS Part II + III and CGI-I, measured at the end of trial of the double-blind study (DB EOT) and the OLE Baseline, were
worsening, as expected in a progressive disease like Parkinson’s. However, when patients resumed daily oral ANAVEX®2-73
treatment, a consistent improvement was observed during the extension phase from OLE Baseline through OLE Week 24, and OLE Week
48, respectively. These results are consistent with the pattern observed for all efficacy measures in the extension phase. The
two endpoints, MDS-UPDRS Part II + III and CGI-I measured in this study are the planned primary and key secondary endpoints in
our forthcoming pivotal 6-month Parkinson’s disease study.
In January 2021, we were awarded a research
grant of $1.0 million from The Michael J. Fox Foundation for Parkinson’s Research to develop ANAVEX®2-73
for the treatment of Parkinson’s disease. The award will explore utilization of PET imaging biomarkers to enable measurement
of target engagement and pathway activation of the SIGMAR1 with clinically relevant doses including in people with Parkinson’s
disease.
Schizophrenia, Frontotemporal Dementia and
Alzheimer’s disease
In July 2020, we commenced the First-in-Human
Phase 1 clinical trial of ANAVEX®3-71. ANAVEX®3-71 was previously granted orphan drug designation
for the treatment of Frontotemporal Dementia (“FTD”) by the FDA. ANAVEX®3-71 is an orally administered
small molecule targeting sigma-1 and M1 muscarinic receptors that is designed to be beneficial for neurodegenerative diseases.
In preclinical studies, ANAVEX®3-71 demonstrated disease-modifying activity
against the major hallmarks of Alzheimer’s disease in transgenic (3xTg-AD) mice, including cognitive deficits, amyloid and
tau pathologies, as well as beneficial effects on mitochondrial dysfunction and neuroinflammation.
The Phase
1 clinical trial was a prospective double-blind, randomized, placebo-controlled trial in Australia. A total of 36 healthy male
and female subjects were included. Single escalating doses of ANAVEX®3-71 were administered in order to evaluate
the safety, tolerability, and PK of ANAVEX®3-71 and the effects of food and gender on its PK in healthy volunteers.
The trial
met its primary and secondary endpoints of safety, with no serious adverse events (“SAEs”) or dose-limiting toxicities
observed. ANAVEX®3-71 was well tolerated in all cohorts receiving ANAVEX®3-71 in single doses ranging
from 5 mg to 200 mg daily with no SAEs and no significant lab abnormalities in any subject. In the trial, ANAVEX®3-71
exhibited linear PK. Its pharmacokinetics was also dose proportional for doses up to 160 mg. Gender had no effect on the PK of
the drug and food had no effect on the bioavailability of ANAVEX®3-71. The trial also met the secondary objective
of characterizing the effect of ANAVEX®3-71 on electrocardiogram (“ECG”) parameters. There were no clinically
significant ECG parameters throughout the trial. Participant QTcF measures were normal across all dose groups with no difference
between ANAVEX®3-71 and placebo.
In October
2023 a peer-reviewed publication in the journal Neurobiology of Aging, titled “Early treatment with an M1 and sigma-1 receptor
agonist prevents cognitive decline in a transgenic rat model displaying Alzheimer-like amyloid pathology”, featured the orally
available small molecule ANAVEX®3-71 (AF710B). The preclinical study described the potential disease-modifying properties
of ANAVEX®3-71 on Alzheimer’s disease pathology as a possible drug candidate for a potential once daily oral
preventive strategy for Alzheimer’s disease.
In January
2024, in another peer-reviewed publication in the journal Clinical Pharmacology in Drug Development, entitled, ‘Population-Based
Characterization of the Pharmacokinetics and Food Effect of ANAVEX3-71, a Novel Sigma-1 Receptor and Allosteric M1 Muscarinic Receptor
Agonist in Development for Treatment of Frontotemporal Dementia, Schizophrenia, and Alzheimer Disease’, reported the
Population-based characterization of the PK and food effect of ANAVEX®3-71 as part of the single ascending dose
study in healthy participants with the primary objective of assessing dose proportionality of ANAVEX®3-71, and to
characterize the effect of food on the PK of ANAVEX®3-71. The results from this PK evaluation demonstrated that
ANAVEX®3-71, at single ascending doses of 5 to 200 mg, is linear, dose proportional, and time invariant. Food had
no effect on the PK of ANAVEX®3-71. This data also expands the safety objectives met in this first-in-human study
of ANAVEX®3-71, further supporting its drug development program.
Based
on these results, and ANAVEX®3-71’s pre-clinical profile, we intend to advance ANAVEX®3-71
into a biomarker-driven clinical development dementia program for the treatment of schizophrenia, FTD and Alzheimer’s disease,
evaluating longitudinal effect of treatment with ANAVEX®3-71.
The next
study with ANAVEX®3-71 is the U.S. FDA cleared placebo-controlled Phase 2 ANAVEX®3-71-SZ-001 study, will consist of two
parts to explore multiple ascending doses in individuals with schizophrenia followed by a 28-day treatment period in a larger
cohort. The study will utilize standard clinical outcome measures for schizophrenia including the Positive and Negative Symptoms
Scale (PANSS) and novel electrophysiological biomarkers identified by the ERP Biomarker Qualification Consortium for use in schizophrenia
clinical trials.
Our Pipeline
Our research and development pipeline includes
ANAVEX®2-73 currently in three different clinical trial indications, and several other compounds in different stages
of clinical and pre-clinical development.
Our proprietary SIGMACEPTOR™ Discovery
Platform produced small molecule drug candidates with unique modes of action, based on our understanding of sigma receptors. Sigma
receptors may be targets for therapeutics to combat many human diseases, both of neurodegenerative nature, including Alzheimer’s
disease, as well as of neurodevelopmental nature, like Rett syndrome. When bound by the appropriate ligands, sigma receptors influence
the functioning of multiple biochemical signals that are involved in the pathogenesis (origin or development) of disease. Multiple
viruses including SARS-CoV-2 (COVID-19) induce cellular stress by intrinsic mitochondrial apoptosis and other related cellular
processes, in order to ensure survival and replication. Hence, it is possible that SIGMAR1 could play a role in modulating the
cellular response to viral infection and ameliorate pathogenesis.
Compounds that have been subjects of our research
include the following:
ANAVEX®2-73 (blarcamesine)
We believe ANAVEX®2-73 may offer
a disease-modifying approach in neurodegenerative and neurodevelopmental diseases by activation of SIGMAR1. ANAVEX®2-73
is being developed in an oral liquid once-daily formulation for rare diseases such as Rett syndrome as well as an oral once-daily
capsule formulation for diseases such as Alzheimer’s disease.
In Rett syndrome, administration of ANAVEX®2-73
in liquid form resulted in both significant and dose-related improvements in an array of behavioral paradigms in the MECP2 HET
Rett syndrome disease model. In addition, in a further experiment sponsored by Rettsyndrome.org, ANAVEX®2-73 was
evaluated in automatic visual response and respiration tests in 7-month-old mice, an age at which advanced pathology is evident.
Vehicle-treated MECP2 mice demonstrated fewer automatic visual responses than wild-type mice. Treatment with ANAVEX®2-73
for four weeks significantly increased the automatic visual response in the MECP2 Rett syndrome disease mice. Additionally, chronic
oral dosing daily for 6.5 weeks of ANAVEX®2-73 starting at ~5.5 weeks of age was conducted in the MECP2 HET Rett
syndrome disease mouse model assessed the different aspects of muscular coordination, balance, motor learning and muscular strengths,
some of the core deficits observed in Rett syndrome. Administration of ANAVEX®2-73 resulted in both significant
and dose related improvements in an array of these behavioral paradigms in the MECP2 HET Rett syndrome disease model.
In May 2016 and June 2016, the FDA granted
Orphan Drug Designation to ANAVEX®2-73 for the treatment of Rett syndrome and infantile spasms, respectively. In
November 2019, the FDA granted to ANAVEX®2-73 the Rare Pediatric Disease (RPD) designation for the treatment of
Rett syndrome. The RPD designation is intended to encourage the development of treatments for rare pediatric diseases.
Further, in February 2020, the FDA granted
Fast Track designation for the ANAVEX®2-73 clinical development program for the treatment of Rett syndrome. The
FDA Fast Track program is designed to facilitate and expedite the development and review of new drugs to address unmet medical
needs in the treatment of serious and life-threatening conditions.
For Parkinson’s disease, data demonstrates
significant improvements and restoration of function in a disease modifying animal model of Parkinson’s disease. Significant
improvements were seen on all measures tested: behavioral, histopathological, and neuroinflammatory endpoints. In October 2020,
we completed a double-blind, randomized, placebo-controlled proof-of-concept Phase 2 trial with ANAVEX®2-73 in Parkinson’s
disease dementia, to study the effect of the compound on both the cognitive and motor impairment of Parkinson’s disease.
The Phase 2 trial enrolled approximately 132 patients for 14 weeks, randomized 1:1:1 to two different ANAVEX®2-73
doses, 30mg and 50mg, or placebo. The ANAVEX®2-73 Phase 2 Parkinson’s disease dementia trial design incorporated
genomic precision medicine biomarkers identified in the ANAVEX®2-73 Phase 2a Alzheimer’s disease trial.
The trial demonstrated that ANAVEX®2-73
was safe and well tolerated in oral doses up to 50mg once daily. The results showed clinically meaningful, dose-dependent, and
statistically significant improvements in the CDR computerized assessment system analysis. We anticipate conducting further clinical
trials of ANAVEX®2-73 in Parkinson’s disease dementia after submitting the results of the trial to the FDA
to obtain regulatory guidance.
In Alzheimer’s disease animal models,
ANAVEX®2-73 has shown pharmacological, histological and behavioral evidence as a potential neuroprotective, anti-amnesic,
anti-convulsive and anti-depressive therapeutic agent, due to its potent affinity to SIGMAR1 and moderate affinities to M1-4 type
muscarinic receptors. In addition, ANAVEX®2-73 has shown a potential dual mechanism which may impact amyloid, tau
pathology and inflammation. In a transgenic Alzheimer’s disease animal model Tg2576, ANAVEX®2-73 induced a
statistically significant neuroprotective effect against the development of oxidative stress in the mouse brain, as well as significantly
increased the expression of functional and synaptic plasticity markers that is apparently amyloid-beta independent. It also statistically
alleviated the learning and memory deficits developed over time in the animals, regardless of sex, both in terms of spatial working
memory and long-term spatial reference memory.
Based on the results of pre-clinical testing,
we initiated and completed a Phase 1 single ascending dose (SAD) clinical trial of ANAVEX®2-73. In this Phase 1
SAD trial, the maximum tolerated single dose was defined per protocol as 55-60 mg. This dose is above the equivalent dose shown
to have positive effects in mouse models of Alzheimer’s disease. There were no significant changes in laboratory or ECG parameters.
ANAVEX®2-73 was well tolerated below the 55-60 mg dose with only mild adverse events in some subjects. Observed
adverse events at doses above the maximum tolerated single dose included headache and dizziness, which were moderate in severity
and reversible. These side effects are often seen with drugs that target CNS conditions, including Alzheimer’s disease.
In November 2016, we completed a Phase 2a clinical
trial for ANAVEX®2-73, for the treatment of Alzheimer’s disease. The open-label randomized trial was designed
to assess the safety and exploratory efficacy of ANAVEX®2-73 in 32 patients with mild-to-moderate Alzheimer’s
disease. The Phase 2a trial met both primary and secondary objectives of the trial.
In July 2018, we presented
the results of a genomic DNA and RNA evaluation of the participants in the Phase 2a clinical trial. More than 33,000 genes were
analyzed using unbiased, data driven, machine learning, artificial intelligence (AI) system for analyzing DNA and RNA data in patients
treated with ANAVEX®2-73. The analysis identified genetic variants that impacted response to ANAVEX®2-73,
among them variants related to the SIGMAR1, the target for ANAVEX®2-73. Results showed that trial participants with
the common SIGMAR1 wild type gene variant, which is estimated to be about 80% of the population worldwide, demonstrated improved
cognitive (MMSE) and functional (ADCS-ADL) scores. The results from this evaluation supported the continued evaluation of genomic
information in subsequent clinical trials, since these signatures can now be applied to neurological indications tested in future
clinical trials with ANAVEX®2-73 including Alzheimer’s disease, Parkinson’s disease dementia and Rett
syndrome.
ANAVEX®2-73
data met prerequisite information in order to progress into a Phase 2b/3 placebo-controlled trial. On July 2, 2018, the Human Research
Ethics Committee in Australia approved the initiation of our Phase 2b/3, double-blind, randomized, placebo-controlled 48-week safety
and efficacy trial of ANAVEX®2-73 for the treatment of early Alzheimer’s disease. Clinical trial sites in
Canada, the United Kingdom, the Netherlands and Germany were also added. This Phase 2b/3 trial design incorporates inclusion of
genomic precision medicine biomarkers identified in the ANAVEX®2-73 Phase 2a trial.
We believe preclinical data from our studies
also supports further research into the use of ANAVEX®2-73 as a potential platform drug for other neurodegenerative
diseases beyond Alzheimer’s disease, Parkinson’s disease or Rett syndrome, more specifically, epilepsy, infantile spasms,
Fragile X syndrome, Angelman syndrome, multiple sclerosis, and, more recently, tuberous sclerosis complex (TSC). ANAVEX®2-73
demonstrated significant improvements in all of these indications in the respective preclinical animal models.
In a preclinical study sponsored by the Foundation
for Angelman Syndrome, ANAVEX®2-73 was assessed in a mouse model for the development of audiogenic seizures. The
results indicated that ANAVEX®2-73 administration significantly reduced audiogenic-induced seizures in mice. In
a study sponsored by FRAXA Research Foundation regarding Fragile X syndrome, data demonstrated that ANAVEX®2-73
restored hippocampal brain-derived neurotrophic factor (BDNF) expression to normal levels. BDNF under-expression has been observed
in many neurodevelopmental and neurodegenerative pathologies. BDNF signaling promotes maturation of both excitatory and inhibitory
synapses. ANAVEX®2-73 normalization of BDNF expression could be a contributing factor for the positive preclinical
data observed in both neurodevelopmental and neurodegenerative disorders like Angelman and Fragile X syndromes.
In addition, preclinical data to-date also
indicates that ANAVEX®2-73 has the potential to demonstrate protective effects of mitochondrial enzyme complexes
during pathological conditions, which, if impaired, may play a role in the pathogenesis of neurodegenerative and neurodevelopmental
diseases.
In addition, preclinical data on ANAVEX®2-73
related to multiple sclerosis indicates that ANAVEX®2-73 may promote remyelination in multiple sclerosis disease.
Further, our data also demonstrates that ANAVEX®2-73 has the potential to provide protection for oligodendrocytes
(“OL’s”) and oligodendrocyte precursor cells (“OPC’s”), as well as central nervous system neurons
in addition to helping repair by increasing OPC proliferation and maturation in tissue culture.
In March 2018, we presented preclinical data
of ANAVEX®2-73 in a genetic mouse model of tuberous sclerosis complex (“TSC”). TSC is a rare genetic
disorder characterized by the growth of numerous benign tumors in many parts of the body with a high incidence of seizures. The
preclinical data demonstrated that treatment with ANAVEX®2-73 significantly increased survival and reduced seizures
in those mice.
ANAVEX®3-71
ANAVEX®3-71 is a clinical drug
candidate with a novel mechanism of action via SIGMAR1 activation and M1 muscarinic allosteric modulation, which has been shown
to enhance neuroprotection and cognition in Alzheimer’s disease models. ANAVEX®3-71 is a CNS-penetrable potential
disease modifying treatment for cognitive impairments. We believe it is effective in very small doses against the major Alzheimer’s
hallmarks in transgenic (3xTg-AD) mice, including cognitive deficits, amyloid and tau pathologies, and also has beneficial effects
on inflammation and mitochondrial dysfunctions. ANAVEX®3-71 indicates extensive therapeutic advantages in Alzheimer’s
and other protein-aggregation-related diseases given its ability to enhance neuroprotection and cognition via SIGMAR1 activation
and M1 muscarinic allosteric modulation.
A preclinical study examined the response of
ANAVEX®3-71 in aged transgenic animal models and showed a significant reduction in the rate of cognitive deficit,
amyloid beta pathology and inflammation with the administration of ANAVEX®3-71. In April 2016, the FDA granted Orphan
Drug Designation to ANAVEX®3-71 for the treatment of FTD.
During pathological conditions ANAVEX®3-71
demonstrated the formation of new synapses between neurons (synaptogenesis) without causing an abnormal increase in the number
of astrocytes. In neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease, synaptogenesis is believed
to be impaired. Additional preclinical data presented also indicates that in addition to reducing oxidative stress, ANAVEX®3-71
has the potential to demonstrate protective effects of mitochondrial enzyme complexes during pathological conditions, which, if
impaired, are believed to play a role in the pathogenesis of neurodegenerative and neurodevelopmental diseases.
In July 2020, we commenced the first Phase
1 clinical trial of ANAVEX®3-71. The trial took place in Australia and was a double-blind, randomized, placebo-controlled,
Phase 1 trial to evaluate safety and tolerability, and PK of oral escalating doses of ANAVEX®3-71
including effects of food and gender in healthy volunteers. The trial met its primary and
secondary endpoints of safety, respectively, with no serious adverse events (SAEs) or dose-limiting toxicities observed, as
more fully described above under Clinical Trials Overview – Schizophrenia, Frontotemporal Dementia and Alzheimer’s
disease.
Based
on these results, and ANAVEX®3-71 pre-clinical profile, the Company intends to advance ANAVEX®3-71
into a biomarker-driven clinical development dementia program for the treatment of schizophrenia, FTD and Alzheimer’s disease,
evaluating longitudinal effect of treatment with ANAVEX®3-71. We believe the results of this clinical trial and
preclinical study could serve as a basis for advancing into respective registration trials in the U.S.
ANAVEX®1-41
ANAVEX®1-41 is a sigma-1 agonist.
Pre-clinical tests revealed significant neuroprotective benefits (i.e., protects nerve cells from degeneration or death) through
the modulation of endoplasmic reticulum, mitochondrial and oxidative stress, which damages and impairs cell viability. In addition,
in animal models, ANAVEX®1-41 prevented the expression of caspase-3, an enzyme that plays a key role in apoptosis
(programmed cell death) and loss of cells in the hippocampus, the part of the brain that regulates learning, emotion and memory.
These activities involve both muscarinic and SIGMAR1 systems through a novel mechanism of action.
Preclinical data presented also indicates that
ANAVEX®1-41 has the potential to demonstrate protective effects of mitochondrial enzyme complexes during pathological
conditions, which, if impaired, are believed to play a role in the pathogenesis of neurodegenerative and neurodevelopmental diseases.
ANAVEX®1066
ANAVEX®1066, a mixed sigma-1/sigma-2
ligand, is designed for the potential treatment of neuropathic and visceral pain. ANAVEX®1066 was tested in two
preclinical models of neuropathic and visceral pain that have been extensively validated in rats. In the chronic constriction injury
model of neuropathic pain, a single oral administration of ANAVEX®1066 dose-dependently restored the nociceptive
threshold in the affected paw to normal levels while leaving the contralateral healthy paw unchanged. Efficacy was rapid and remained
significant for two hours. In a model of visceral pain, chronic colonic hypersensitivity was induced by injection of an inflammatory
agent directly into the colon and a single oral administration of ANAVEX®1066 returned the nociceptive threshold
to control levels in a dose-dependent manner. Companion studies in rats demonstrated the lack of any effects on normal gastrointestinal
transit with ANAVEX®1066 and a favorable safety profile in a battery of behavioral measures.
ANAVEX®1037
ANAVEX®1037 is designed for
the treatment of prostate and pancreatic cancer. It is a low molecular weight, synthetic compound exhibiting high affinity for
SIGMAR1 at nanomolar levels and moderate affinity for sigma-2 receptors and sodium channels at micromolar levels. In advanced pre-clinical
studies, this compound revealed antitumor potential. It has also been shown to selectively kill human cancer cells without affecting
normal/healthy cells and also to significantly suppress tumor growth in immune-deficient mice models. Scientific publications highlight
the possibility that these ligands may stop tumor growth and induce selective cell death in various tumor cell lines. Sigma receptors
are highly expressed in different tumor cell types. Binding by appropriate sigma-1 and/or sigma-2 ligands can induce selective
apoptosis. In addition, through tumor cell membrane reorganization and interactions with ion channels, we believe our drug candidates
may play an important role in inhibiting the processes of metastasis (spreading of cancer cells from the original site to other
parts of the body), angiogenesis (the formation of new blood vessels) and tumor cell proliferation.
ANAVEX®1037 is currently in
the pre-clinical and clinical testing stages of development, and there is no guarantee that the activity demonstrated in pre-clinical
models will be shown in human testing.
We continue to identify and initiate discussions
with potential strategic and commercial partners to most effectively advance our programs and increase stockholder value. Further,
we may acquire or develop new intellectual property and assign, license, or otherwise transfer our intellectual property to further
our goals.
Our Target Indications
We are developing compounds with potential
application to two broad categories and several specific indications, including:
Central Nervous System Diseases
| |
● |
Alzheimer’s disease – In 2023, an estimated 6.7 million Americans aged 65 and older suffered from Alzheimer’s disease. The Alzheimer’s Association® estimates that the annual number of new cases of Alzheimer’s and other dementias is projected to double by 2050. Medications on the market today treat only the symptoms of Alzheimer’s disease and do not have the ability to stop its onset or its progression. We believe that there is an urgent and unmet need for both a disease modifying cure for Alzheimer’s disease as well as for better symptomatic treatments. |
| |
● |
Parkinson’s disease – Parkinson’s disease is a progressive disease of the nervous system marked by tremors, muscular rigidity, and slow, imprecise movement. It is associated with degeneration of the basal ganglia of the brain and a deficiency of the neurotransmitter dopamine. Parkinson’s disease currently is estimated to afflict more than 10 million people worldwide, typically middle-aged and elderly people. The Parkinson’s disease market is expected to reach $11.5 billion by 2029, according to GlobalData. |
| |
● |
Rett syndrome – Rett syndrome is a rare
X-linked genetic neurological and developmental disorder that affects the way the brain develops, including protein transcription,
which is altered and as a result leads to severe disruptions in neuronal homeostasis. It is considered a rare, progressive neurodevelopmental
disorder and is caused by a single mutation in the MECP2 gene. Because males have a different chromosome combination from females,
boys who have the genetic MECP2 mutation are affected in devastating ways. Most of them die before birth or in early infancy. For
females who survive infancy, Rett syndrome leads to severe impairments, affecting nearly every aspect of the child’s life;
severe mental retardation, their ability to speak, walk and eat, sleeping problems, seizures and even the ability to breathe easily.
Rett syndrome affects approximately 1 in every 10,000-15,000 females.
|
| |
● |
Schizophrenia - Schizophrenia is a persistent and often disabling mental illness impacting how a person thinks, feels, and behaves, and affects nearly 24 million people worldwide, including 2.8 million people in the U.S., according to the World Health Organization. It is characterized by three symptom domains: positive symptoms (hallucinations and delusions), negative symptoms (difficulty enjoying life and withdrawal from others), and cognitive impairment (deficits in memory, concentration, and decision-making). In part due to limitations with current treatments, people living with schizophrenia often struggle to maintain employment, live independently, and manage relationships. While current treatments can be effective in managing select symptoms, approximately 30% of people do not respond to therapy, with an additional 50% experiencing only a partial improvement in symptoms or unacceptable side effects, according to the World Health Organization. |
| |
|
|
| · | Fragile X – Fragile X syndrome (FXS) is the most prevalent genetic form of intellectual disability
and autism spectrum disorder, primarily affecting boys. As with most neurodevelopmental disorders, FXS is considered a condition
of synaptic development and function. The disease has a range of clinical presentations depending on the specific genetic changes
associated with an “expansion” of the FMR1 gene. The disease is characterized by deficits in long-term potentiation
and homeostatic plasticity. FXS has been detected in all populations and ethnic groups. Researchers do not know the exact number
for how many Americans could have full mutation FXS. Studies estimate that the disease affects approximately 1:4,000 males and
1:6,000-8,000 females. Worldwide, more than 1,400,000 people could be affected by FXS. |
| |
|
|
| |
● |
Depression – Depression is a major cause of morbidity worldwide according to the World Health Organization. The global antidepressant drug market is projected to reach $21 Billion by 2030 according to Allied Market Research. Pharmaceutical treatment for depression has been historically dominated by blockbuster brands. However, the dominance of the leading brands is waning, largely due to an increase in the number of approvals for antidepressant drugs. |
| |
● |
Epilepsy – Epilepsy is a common chronic neurological disorder characterized by recurrent unprovoked seizures. These seizures are transient signs and/or symptoms of abnormal, excessive or synchronous neuronal activity in the brain. According to the Centers for Disease Control and Prevention, in 2015 epilepsy affected 3.4 million Americans. Today, epilepsy is often controlled, but not cured, with medication that is categorized as older traditional anti-epileptic drugs and second generation anti-epileptic drugs. Because epilepsy afflicts sufferers in different ways, there is a need for drugs used in combination with both traditional anti-epileptic drugs and second generation anti-epileptic drugs. |
| |
● |
Neuropathic Pain – We define neuralgia, or neuropathic pain, as pain that is not related to activation of pain receptor cells in any part of the body. Neuralgia is more difficult to treat than some other types of pain because it does not respond well to normal pain medications. Special medications have become more specific to neuralgia and typically fall under the category of membrane stabilizing drugs or antidepressants. |
Cancer
| |
● |
Malignant Melanoma – Predominantly a skin cancer, malignant melanoma can also occur in melanocytes found in the bowel and the eye. Malignant melanoma accounts for a large majority of skin cancer deaths. The treatment includes surgical removal of the tumor, adjuvant treatment, chemo and immunotherapy, or radiation therapy. According to iHealthcareAnalyst, Inc. the worldwide malignant melanoma market is expected to grow to $7.5 billion by 2029. |
| |
● |
Prostate Cancer – Specific to men, prostate cancer is a form of cancer that develops in the prostate, a gland in the male reproductive system. Cancer cells may metastasize from the prostate to other parts of the body, particularly the bones and lymph nodes. Drug therapeutics for prostate cancer are expected to increase to nearly $10.1 billion by the end of 2030 according to Market Research Future. |
| |
● |
Pancreatic Cancer – Pancreatic cancer is a malignant neoplasm of the pancreas. In the United States, approximately 62,000 new cases of pancreatic cancer will be diagnosed this year and approximately 50,000 patients will die as a result of their cancer, according to the American Cancer Society. Sales predictions by Market Data Forecast predict that the market for the global pharmaceutical treatment of pancreatic cancer will increase to $3.7 billion by 2027. |
Patents, Trademarks and Intellectual
Property
We hold ownership or exclusive rights to twenty-four
(24) U.S. patents, twenty-three U.S. patent applications, and various PCT or ex-U.S. patent applications relating to our drug candidates,
methods associated therewith, and to our research programs.
We own one issued U.S. patent entitled “ANAVEX®2-73
and certain anticholinesterase inhibitors composition and method for neuroprotection” claims a composition of matter of ANAVEX®2-73
directed to a novel and synergistic neuroprotective compound combined with donepezil and other cholinesterase inhibitors. This
patent is expected to expire in June 2034, absent any patent term extension for regulatory delays. We own one issued U.S. patent
entitled “A2-73 crystalline polymorph compositions of matter and methods of use thereof”. It claims crystals of A2-73
freebase or its fumarate salt, dosage forms and pharmaceutical formulations. This patent is expected to expire in July 2039, absent
any patent term extension for regulatory delays. We own four issued U.S. patents each with claims directed to crystalline forms
of ANAVEX®2-73. The first of these four patents claims crystalline forms of ANAVEX®2-73, dosage forms
and compositions containing crystalline ANAVEX®2-73, and methods of treatment for Alzheimer’s disease using
them. This patent is expected to expire in July 2036, absent any patent term extension for regulatory delays. The second of these
four patents claims pharmaceutical compositions containing a crystalline form of ANAVEX®2-73, and methods of treatment
for Alzheimer’s disease using the compositions. This patent is expected to expire in June 2036, absent any patent term extension
for regulatory delays. The third of these four patents claims pharmaceutical compositions containing a crystalline form of ANAVEX®2-73,
and methods of treatment for Alzheimer’s disease using the compositions. This patent is expected to expire in June 2036,
absent any patent term extension for regulatory delays. The fourth of these four patents claims method of making certain crystalline
forms ANAVEX®2-73. This patent is expected to expire in October 2036, absent any patent term extension for regulatory
delays. We also own three issued U.S. patents for seizure treatment. The first of these three patents claims methods and dosage
forms for treating seizures, the dosage forms containing a low-dose anti-epilepsy drug combined with either: (i) ANAVEX®2-73
and its active metabolite ANAVEX®19-144; or (ii) ANAVEX®19-144. The second of these three patents
further claims a combination seizure treatment involving administration of an anti-epilepsy drug combined with (i) ANAVEX®19-144,
or (ii) ANAVEX 19-144® and ANAVEX 2-73®. The third of these three patents claims a dosage form for
seizure reduction, comprising (i) ANAVEX®19-144, (ii) ANAVEX®2-73, or (iii) a combination of ANAVEX®19-144
and ANAVEX®2-73; and optionally further comprising a low-dose anti-epilepsy drug. All three patents are expected
to expire in October 2035, absent any patent term extension for regulatory delays. We also own four issued U.S. patents with claims
directed to treating neurodevelopmental disorders. These patents claim methods for treating a neurodevelopmental disorder, multiple
sclerosis, their related biochemical and functional abnormalities, or loss-of-function associated with a neurodevelopmental disorder,
by administering ANAVEX®2-73, ANAVEX®19-144, and/or ANAVEX®1-41 (another sigma receptor
ligand similar to ANAVEX®2-73), or compositions thereof. All four patents are expected to expire in January 2037,
absent any patent term extension for regulatory delays. In addition, we own one issued U.S. Patent with claims directed to methods
of treating melanoma with a compound related to ANAVEX®2-73. This patent is expected to expire in February 2030,
absent any patent term extension for regulatory delays. We also own an issued U.S. patent that claims crystalline forms of ANAVEX®19-144,
dosage forms and compositions containing the crystalline forms of ANAVEX®19-144, and methods of treatment for Alzheimer’s
disease. This patent is expected to expire in July 2036, absent any patent term extension for regulatory delays. Further, we own
one issued U.S. Patent with claims directed to methods of treating cardiac dysfunction with ANAVEX®2-73. This patent
is expected to expire in July 2038, absent any patent term extension for regulatory delays. Additionally, we own two issued U.S.
Patent for the treatment of insomnia, anxiety, or agitation. The first of the two patents claims methods of treating insomnia or
anxiety with ANAVEX®2-73, ANAVEX®19-144, and/or ANAVEX®1-41. This patent is expected
to expire in September 2038. The second of the two patents claims a dosage form comprising any of, or any combination of ANAVEX®2-73,
ANAVEX®19-144, and/or ANAVEX®1-41. This patent is expected to expire in July 2038, absent any patent
term extension for regulatory delays. Further, we own one issued U.S. Patent with claims directed to a method of treating systolic
hypertension using ANAVEX®2-73. This patent is expected to expire in July 2039, absent any patent term extension
for regulatory delays. Additionally, we own one issued U.S. Patent with claims directed to pharmaceutical dosage forms of (-) enantiomer
of ANAVEX®2-73. This patent is expected to expire in July 2036, absent any patent term extension for regulatory
delays.
We also own two issued U.S. patents related
to ANAVEX®1066. The first of these two patents claims methods for treating or preventing pain using (+) ANAVEX®1066
isomer. The second patent claims methods for treating or preventing pain using (-) ANAVEX®1066 isomer. Both patents
are expected to expire in November 2036, absent any patent term extension for regulatory delays.
For ANAVEX®2-73, ANAVEX®19-144,
ANAVEX®1-41, and ANAVEX®1066, we also have granted or pending applications in Australia, Canada,
China, Europe, Japan, and Hong Kong, which are expected to expire after 2035.
With regard to ANAVEX®3-71,
we own exclusive rights to two issued U.S. patents with claims respectively directed to the ANAVEX®3-71 compound
and methods of treating various diseases including Alzheimer’s with the same. These patents are expected to expire in April
2030, and January 2030, respectively, absent any patent term extension for regulatory delays. We also own exclusive rights to related
patents or applications that are granted or pending in Australia, Canada, China, Europe, Japan, Korea, New Zealand, Russia, and
South Africa, which are expected to expire in January 2030.
We also own other patent applications directed
to enantiomers, crystals, formulations, uses, and patient selection methods that may provide additional protection for one or more
of our product candidates.
We regard patents and other intellectual property
rights as corporate assets. Accordingly, we attempt to optimize the value of intellectual property in developing our business strategy
including the selective development, protection, and exploitation of our intellectual property rights. In addition to filings made
with intellectual property authorities, we protect our intellectual property and confidential information by means of carefully
considered processes of communication and the sharing of information, and by the use of confidentiality and non-disclosure agreements
and provisions for the same in contractor’s agreements. While no agreement offers absolute protection, such agreements provide
some form of recourse in the event of disclosure, or anticipated disclosure.
Our
intellectual property position, like that of many biomedical companies, is uncertain and involves complex legal and technical questions
for which important legal principles are unresolved. For more information regarding challenges to our existing or future patents,
see “Risk Factors” in Part I, Item 1A of our Annual Report on Form 10-K filed with the Securities and Exchange Commission
on November 27, 2023.
Financial Overview
The following discussion should be read in conjunction with our condensed consolidated
interim financial statements and related notes thereto contained elsewhere in this report. Past operating results are not necessarily
indicative of results that may occur in future periods. The discussion contains forward-looking statements, which involve a number of
risks and uncertainties. See “Forward Looking Statements” included elsewhere in this report.
We are in the development stage and have not
earned any revenue since our inception in 2004. We do not anticipate earning any revenues until we can establish an alliance with
other companies to develop, co-develop, license, acquire or market our products.
Our operating costs consist primarily of research
and development activities including the cost of clinical trials and clinical supplies as well as clinical drug manufacturing and
formulation. Research and development expenses also include personnel related costs such as salaries and wages, and third-party
contract research organization (CRO) expenses in support of these clinical trials. Personnel costs include salaries and wages,
benefits, and non-cash stock-based compensation charges associated with options and other equity awards granted to employees and
consultants who are directly engaged in support of our research and development activities.
General and administrative expenses consist
of personnel costs, expenses for outside professional services and expenses associated with operating as a public company. Personnel
costs consist of salaries and wages, benefits and stock-based compensation for general and administrative personnel. Outside professional
services and public company expenses include expenses related to compliance and reporting, additional insurance expenses, audit
and SOX compliance, expenses associated with patent research, applications and filings, investor and stockholder relations activities
and other administrative expenses and professional services.
Comparison of the three months ended December
31, 2023 and 2022
Operating Expenses
Total operating expenses for the quarter ended
December 31, 2023 were $11.3 million, compared to $15.4 million for the comparable quarter ended December 31, 2022.
Our research and development expenses for the
three months ended December 31, 2023 were $8.7 million, as compared to $12.1 million for the three months ended December 31, 2022.
The decrease in research and development expenses is primarily related to the following;
| (i) | a decrease in stock-based compensation expense of $2.2 million as a result of the vesting of previously
awards options and the extended timeline of milestone based vesting awards; |
| (ii) | a decrease of approximately $0.8 million in expenditures over the comparable
period relating to our Rett syndrome program as a result of the completion of the EXCELLENCE trial and the respective open label extension
of the AVATAR
trial |
| (iii) | a decrease of approximately $1.2 million in expenditures over the comparable period relating to
our Alzheimer’s program as a result of the completion of the Phase 2b/3 clinical trial in the comparable period. |
The following table summarizes our research
and development expenses for the three months ended December 31, 2023 and 2022 (in thousands):
| | |
2023 | |
2022 |
| Cost of external service providers | |
$ | 4,565 | | |
$ | 6,054 | |
| Personnel costs | |
| 2,748 | | |
| 2,381 | |
| Stock-based compensation | |
| 1,360 | | |
| 3,604 | |
| Other common costs | |
| 11 | | |
| 28 | |
| Total research and development costs | |
$ | 8,684 | | |
$ | 12,067 | |
During the three months ended December 31,
2023 and 2022, external service providers cost by product candidate was as follows (in thousands):
| | |
2023 | |
2022 |
| ANAVEX®2-73 | |
$ | 3,811 | | |
$ | 5,240 | |
| ANAVEX®3-71 | |
| 597 | | |
| 712 | |
| All other product candidates | |
| 5 | | |
| — | |
| Other external service provider costs | |
| 152 | | |
| 102 | |
| Total external service provider costs | |
$ | 4,565 | | |
$ | 6,054 | |
General and administrative expenses were $2.6
million for the three months ended December 31, 2023, as compared to $3.3 million for the same quarter of fiscal 2022. The primary
reason for the decrease in general and administrative expenses was a reduction in stock-based compensation charges of $0.8 million
as a result of the vesting of previously awards stock options and the extended timeline of milestone based vesting awards.
We expect to see our research and development
expenditures increase from current levels as we advance our clinical programs, including ongoing extension trials of our Alzheimer’s
and Rett syndrome programs, planned initiation of an ANAVEX®3-71 trial in Schizophrenia, planned advancement of
ANAVEX®2-73 for Parkinson’s disease, planned initiation of an ANAVEX®2-73 for Fragile X clinical
trial, and as we continue to add additional staffing to manage and support these clinical initiatives.
Other income (net)
The net amount of other income for the three
months ended December 31, 2023 was $2.8 million as compared to $2.4 million for the comparable three months ended December 31,
2022. The increase in other income for the quarter is primarily related to an increase in return on excess cash invested in cash
equivalents due to a marketwide increase in interest rates.
Net loss
Net loss for the three months ended December
31, 2023, was $8.6 million, or $0.11 per share, as compared to $13.0 million, or $0.17 per share in the comparative quarter of
fiscal 2023. The decrease in net loss for the quarter is primarily related to a decrease in research and development expenditures
and an increase in other income, as discussed above.
Liquidity and Capital Resources
Working Capital (in thousands)
| | |
December 31, 2023 | |
September 30, 2023 |
| Current Assets | |
$ | 148,070 | | |
$ | 154,386 | |
| Current Liabilities | |
| 12,495 | | |
| 12,534 | |
| Working Capital | |
$ | 135,575 | | |
$ | 141,852 | |
At December 31, 2023, we had net current assets
of $135.6 million, a decrease of $6.3 million from September 30, 2023. The decrease in net current assets primarily relates to
a decrease in cash and cash equivalents of $7.3 million due to cash utilized in operations, which were funded using cash and cash
equivalents on hand.
During the first three months of fiscal 2023,
we utilized cash and cash equivalents of $7.3 million to fund our operations, compared to $5.8 million during the same period of
fiscal 2022. Our cash position was $143.8 million at December 31, 2023 compared to $151.0 million at our fiscal year ended September
30, 2023.
We intend
to continue to use our capital resources to advance our clinical trials for ANAVEX®2-73 and ANAVEX®3-71,
and to perform the work necessary to prepare for future development of our pipeline compounds.
Cash Flows
The following
table summarizes cash flows during the three months ended December 31, 2023 and 2022 (in thousands):
| | |
2023 | |
2022 |
| Net cash flows used in operating activities | |
$ | (7,318 | ) | |
$ | (5,794 | ) |
| Net cash flows from financing activities | |
| 59 | | |
| 258 | |
| Decrease in cash and cash equivalents | |
$ | (7,259 | ) | |
$ | (5,536 | ) |
Cash flow used in operating activities
Net cash used in operating activities for the
three months ended December 31, 2023 was $7.3 million, compared to $5.8 million during the comparable period ended December 31,
2022. The principal reason for this increase in net cash used in operating activities in the current period is due to a net increase
in accounts payable and accrued liabilities in the comparable quarter.
Cash flow provided by financing activities
Cash provided by financing activities for the
three months ended December 31, 2023 was $0.06 million, from the exercise of stock options during the period. Cash provided
by financing activities for the three months ended December 31, 2022 was $0.3 million, also attributable to the exercise
of stock options during that period. The Company did not sell any shares under the 2023 Purchase Agreement or Sales Agreement,
both described below, during either of the periods.
Other Financings
2023 Purchase Agreement
On February 3, 2023, the Company entered into
a $150,000,000 purchase agreement (the “2023 Purchase Agreement”) with Lincoln Park Capital Fund, LLC (“Lincoln
Park”), pursuant to which the Company has the right to sell and issue to Lincoln Park, and Lincoln Park is obligated to purchase,
up to $150.0 million in value of its shares of Common Stock from time to time over a three-year period until February 3, 2026.
On any business day and subject to certain
customary conditions, the Company may direct Lincoln Park to purchase up to 200,000 shares of Common Stock (such purchases, “Regular
Purchases”). The amount of a Regular Purchase may increase under certain circumstances based on the market price of the Common
Stock; provided, however, that Lincoln Park’s committed obligation under any Regular Purchase shall not exceed $4.0 million.
The purchase price of shares of Common Stock will be based on the then prevailing market prices of such shares at the time of sales
as described in the Purchase Agreement. There are no limits on the price per share that Lincoln Park may pay to purchase Common
Stock under the Purchase Agreement. In addition, if the Company has directed Lincoln Park to purchase the full amount of Common
Stock available as a Regular Purchase on a given day, it may direct Lincoln Park to purchase additional amounts as “accelerated
purchases” and “additional accelerated purchases,” each as set forth in the Purchase Agreement.
The Purchase Agreement limits the Company’s
sale of shares of Common Stock to Lincoln Park to 15,606,426 shares of Common Stock, representing 19.99% of the shares of the Common
Stock outstanding on the date of the Purchase Agreement unless (i) stockholder approval is obtained to issue more than such amount
or (ii) the average price of all applicable sales of Common Stock to Lincoln Park under the Purchase Agreement equals or exceeds
the lower of (A) the closing price of the Common Stock on the Nasdaq Capital Market immediately preceding the Execution Date or
(B) the average of the closing price of the Common Stock on the Nasdaq Capital Market for the five Business Days immediately preceding
the Execution Date.
The Purchase Agreement also prohibits the Company
from directing Lincoln Park to purchase any shares of Common Stock if those shares, when aggregated with all other shares of Common
Stock then beneficially owned by Lincoln Park and its affiliates, would result in Lincoln Park and its affiliates having beneficial
ownership, at any single point in time, of more than 4.99% of the then total outstanding shares of Common Stock, as calculated
pursuant to Section 13(d) of the Securities Exchange Act of 1934, as amended, and Rule 13d-3 thereunder.
In consideration for entering into the 2023
Purchase Agreement, the Company issued to Lincoln Park 75,000 shares of Common Stock as a commitment fee (the “initial commitment
shares”) during the year ended September 30, 2023 and agreed to issue up to 75,000 shares pro rata (collectively with the
initial commitment shares, the “commitment shares”), when and if, Lincoln Park purchased, at the Company’s discretion,
the $150.0 million aggregate commitment.
During the three months ended December 31,
2023 and 2022, the Company did not issue any shares of Common Stock to Lincoln Park under the 2023 Purchase Agreement.
As of December 31, 2023, an amount of
$122.1 million in shares of our Common Stock remains available for purchase by Lincoln Park under the 2023 Purchase Agreement.
Controlled Equity Offering Sales Agreement
On May 1, 2020, we entered into an Amended
and Restated Sales Agreement (the “Sales Agreement”) with Cantor Fitzgerald & Co. and SVB Leerink LLC (the “Sales
Agents”), pursuant to which we may offer and sell shares of common stock registered under an effective registration statement
from time to time through the Sales Agents (the “At-the-Market Offering”).
Upon delivery of a placement notice based on
our instructions and subject to the terms and conditions of the Sales Agreement, the Sales Agents may sell shares of common stock
by methods deemed to be an “at the market offering”, in negotiated transactions at market prices prevailing at the
time of sale or at prices related to such prevailing market prices, or by any other method permitted by law, including negotiated
transactions, subject to our prior written consent. We are not obligated to make any sales of shares under the Sales Agreement.
We or the Sales Agents may suspend or terminate the At-the-Market Offering upon notice to the other party, subject to certain conditions.
The Sales Agents will act as agents on a commercially reasonable efforts basis consistent with their normal trading and sales practices,
applicable state and federal law, and rules and regulations and the rules of Nasdaq.
We have agreed to pay the Sales Agents’
commissions for their services of 3.0% of the gross proceeds from the sale of shares of Common Stock pursuant to the Sales Agreement.
We have also agreed to provide the Sales Agents with customary indemnification and contribution rights.
No shares were sold during the three months
ended December 31, 2023 and 2022 under the Sales Agreement. The Company currently is unable to sell shares of common stock under
the Sales Agreement.
Off-Balance Sheet
Arrangements
We have no off-balance
sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in
financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are
material to our stockholders.
CRITICAL ACCOUNTING POLICIES
We prepare our condensed consolidated interim financial statements in accordance
with accounting principles generally accepted in the United States of America and make estimates and assumptions that affect our reported
amounts of assets, liabilities, revenue and expenses, and the related disclosures of contingent liabilities. We base our estimates on
historical experience and other assumptions that we believe are reasonable in the circumstances. Actual results may differ from these
estimates.
There have been no
significant changes in the critical accounting policies and estimates described in our Annual Report on Form 10-K for the year
ended September 30, 2023, as filed with the SEC on November 27, 2023.
RECENT ACCOUNTING
PRONOUNCEMENTS
Please refer to Note 2 “Recent Accounting Pronouncements” in notes
to our Condensed Consolidated Interim Financial Statements included in this Form 10-Q.
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET
RISKS.
There have been no
material changes in our exposure to market risk from that disclosed in Item 7A of our Annual Report on Form 10-K for the year ended
September 30, 2023.
ITEM 4. CONTROLS AND PROCEDURES
Disclosure Controls
and Procedures
We maintain disclosure
controls and procedures that are designed to provide reasonable assurance that material information required to be disclosed in
our periodic reports filed under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified
in the SEC’s rules and forms and to provide reasonable assurance that such information is accumulated and communicated to
our management, our chief executive officer and our principal financial officer, to allow timely decisions regarding required disclosure.
We carried out an
evaluation, under the supervision and with the participation of our management, including our principal executive officer and principal
financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rule
13a-15(e) under the Exchange Act, as of the end of the period covered by this Quarterly Report on Form 10-Q. Based on this evaluation,
our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective
as of December 31, 2023.
Changes in Internal
Control over Financial Reporting
During the quarter
ended December 31, 2023, there were no changes to our internal control over financial reporting identified in management’s
evaluation pursuant to Rules 13a 15(d) or 15d 15 (d) of the Exchange Act that materially affected, or are reasonably likely to
materially affect, our internal controls over financial reporting.
PART II – OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
We
know of no material pending legal or governmental proceedings, other than ordinary routine litigation incidental to our business,
to which our Company or our subsidiaries are a party or of which any of their property is subject. There are no proceedings in
which any of our directors, officers or affiliates, or any registered or beneficial stockholder holding more than 5% of our shares,
or any associate of such persons, is an adverse party or has a material interest adverse to our or our subsidiaries’ interest.
ITEM 1A. RISK FACTORS
There have been no material changes to the
risk factors discussed in “Risk Factors” in Part I, Item 1A of our Annual Report on Form 10-K for the fiscal year ended
September 30, 2023, filed with the SEC on November 27, 2023.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
During the period covered by this Quarterly
Report on Form 10-Q, we have not sold any equity securities that were not registered under the Securities Act of 1933 that were
not previously reported in a Current Report on Form 8-K.
ITEM 3. DEFAULTS UPON SENIOR SECURITIES
None.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
ITEM 5. OTHER INFORMATION
None
ITEM 6. EXHIBITS
* Filed herewith.
** Furnished herewith.
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly
authorized.
ANAVEX LIFE SCIENCES CORP.
| /s/Christopher Missling, PhD |
|
| |
|
| Christopher Missling, PhD |
|
| Chief Executive Officer |
|
| (Principal Executive Officer) |
|
| Date: February 7, 2024 |
|
| /s/Sandra Boenisch |
|
| |
|
| Sandra Boenisch, CPA, CGA |
|
| Principal Financial Officer |
|
| (Principal Financial and Accounting Officer) |
|
| Date: February 7, 2024 |
|
42
Exhibit 31.1
CERTIFICATION
I, Christopher Missling, certify that:
1. I have reviewed this Quarterly Report on
Form 10-Q for the three months ended December 31, 2023 of Anavex Life Sciences Corp. (the “registrant”);
2. Based on my knowledge, this report does
not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in
light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements,
and other financial information included in this report, fairly present in all material respects the financial condition, results
of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant’s other certifying
officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules
13a–15(e) and 15d–15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a–15(f)
and 15d–15(f)) for the registrant and have:
(a) designed such disclosure controls
and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material
information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities,
particularly during the period in which this report is being prepared;
(b) designed such internal control
over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide
reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external
purposes in accordance with generally accepted accounting principles;
(c) evaluated the effectiveness
of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness
of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d) disclosed in this report any
change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent
fiscal quarter that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control
over financial reporting; and
5. The registrant’s other certifying
officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s
auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
(a) all significant deficiencies
and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to
adversely affect the registrant’s ability to record, process, summarize and report financial information; and
(b) any fraud, whether or not material,
that involves management or other employees who have a significant role in the registrant’s internal control over financial
reporting.
| Date: February 7, 2024 |
|
| |
|
| /s/Christopher Missling, PhD |
|
| Christopher Missling, PhD |
|
| Chief Executive Officer, President and Secretary |
|
| (Principal Executive Officer) |
|
Exhibit
31.2
CERTIFICATION
I, Sandra Boenisch, certify that:
1. I have reviewed this Quarterly Report on
Form 10-Q for the three months ended December 31, 2023 of Anavex Life Sciences Corp. (the “registrant”);
2. Based on my knowledge, this report does
not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in
light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements,
and other financial information included in this report, fairly present in all material respects the financial condition, results
of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant’s other certifying
officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act
Rules 13a–15(e) and 15d–15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a–15(f)
and 15d–15(f)) for the registrant and have:
(a) designed such disclosure controls
and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material
information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities,
particularly during the period in which this report is being prepared;
(b) designed such internal control
over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide
reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external
purposes in accordance with generally accepted accounting principles;
(c) evaluated the effectiveness
of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness
of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d) disclosed in this report any
change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent
fiscal quarter that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control
over financial reporting; and
5. The registrant’s other certifying
officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s
auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
(a) all significant deficiencies
and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to
adversely affect the registrant’s ability to record, process, summarize and report financial information; and
(b) any fraud, whether or not material,
that involves management or other employees who have a significant role in the registrant’s internal control over financial
reporting.
| Date: February 7, 2024 |
|
| |
|
| /s/Sandra Boenisch |
|
| Sandra Boenisch, CPA, CGA |
|
| Principal Financial Officer, Treasurer |
|
| (Principal Financial and Accounting Officer) |
|
Exhibit 32.1
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Quarterly Report of
Anavex Life Sciences Corp. (the “Company”) on Form 10-Q for the three months ended December 31, 2023 as filed with
the Securities and Exchange Commission on the date hereof (the “Report”), the undersigned, in the capacities and on
the date indicated below, hereby certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley
Act of 2002, that to the best of our knowledge:
(1) the Report fully complies with
the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2) the information contained in
the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
| Date: February 7, 2024 |
/s/Christopher Missling,
PhD |
| |
Christopher Missling, PhD |
| |
Chief Executive Officer, President, Secretary |
| |
(Principal Executive Officer) |
| |
/s/Sandra Boenisch |
| |
Sandra Boenisch, CPA, CGA |
| |
Principal Financial Officer, Treasurer |
| |
(Principal Financial and Accounting Officer) |
The foregoing certification is being furnished
solely to accompany the Report pursuant to 18 U.S.C. § 1350, and is not being filed for purposes of Section 18 of the Securities
Exchange Act of 1934, as amended, and is not to be incorporated by reference into any filing of the Company, whether made before
or after the date hereof, regardless of any general incorporation language in such filing. A signed original of this written statement
required by Section 906, or other document authenticating, acknowledging, or otherwise adopting the signature that appears in typed
form within the electronic version of this written statement required by Section 906, has been provided to the Company and will
be retained by the Company and furnished to the Securities and Exchange Commission or its staff upon request.
v3.24.0.1
Cover - shares
|
3 Months Ended |
|
Dec. 31, 2023 |
Feb. 07, 2024 |
| Cover [Abstract] |
|
|
| Document Type |
10-Q
|
|
| Amendment Flag |
false
|
|
| Document Quarterly Report |
true
|
|
| Document Transition Report |
false
|
|
| Document Period End Date |
Dec. 31, 2023
|
|
| Document Fiscal Period Focus |
Q1
|
|
| Document Fiscal Year Focus |
2024
|
|
| Current Fiscal Year End Date |
--09-30
|
|
| Entity File Number |
001-37606
|
|
| Entity Registrant Name |
ANAVEX LIFE SCIENCES CORP.
|
|
| Entity Central Index Key |
0001314052
|
|
| Entity Tax Identification Number |
98-0608404
|
|
| Entity Incorporation, State or Country Code |
NV
|
|
| Entity Address, Address Line One |
630 5th Avenue
|
|
| Entity Address, Address Line Two |
20th Floor
|
|
| Entity Address, City or Town |
New York
|
|
| Entity Address, State or Province |
NY
|
|
| Entity Address, Country |
US
|
|
| Entity Address, Postal Zip Code |
10111
|
|
| City Area Code |
844
|
|
| Local Phone Number |
689-3939
|
|
| Title of 12(b) Security |
Common Stock Par Value $0.001
|
|
| Trading Symbol |
AVXL
|
|
| Security Exchange Name |
NASDAQ
|
|
| Entity Current Reporting Status |
Yes
|
|
| Entity Interactive Data Current |
Yes
|
|
| Entity Filer Category |
Large Accelerated Filer
|
|
| Entity Small Business |
false
|
|
| Entity Emerging Growth Company |
false
|
|
| Entity Shell Company |
false
|
|
| Entity Common Stock, Shares Outstanding |
|
82,112,511
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY.
| Name: |
dei_DocumentFiscalPeriodFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fiscalPeriodItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006.
| Name: |
dei_DocumentFiscalYearFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as an quarterly report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-Q
-Number 240
-Section 308
-Subsection a
| Name: |
dei_DocumentQuarterlyReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as a transition report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Forms 10-K, 10-Q, 20-F
-Number 240
-Section 13
-Subsection a-1
| Name: |
dei_DocumentTransitionReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionISO 3166-1 alpha-2 country code.
| Name: |
dei_EntityAddressCountry |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:countryCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument.
| Name: |
dei_EntityCommonStockSharesOutstanding |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionIndicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure.
| Name: |
dei_EntityCurrentReportingStatus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-T
-Number 232
-Section 405
| Name: |
dei_EntityInteractiveDataCurrent |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityShellCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Condensed Consolidated Interim Balance Sheets - USD ($)
$ in Thousands |
Dec. 31, 2023 |
Sep. 30, 2023 |
| Current |
|
|
| Cash and cash equivalents |
$ 143,765
|
$ 151,024
|
| Incentive and tax receivables |
3,549
|
2,709
|
| Prepaid expenses and other current assets |
756
|
653
|
| Total Assets |
148,070
|
154,386
|
| Current Liabilities |
|
|
| Accounts payable |
4,292
|
4,322
|
| Accrued liabilities - Note 4 |
7,286
|
7,295
|
| Deferred grant income - Note 3 |
917
|
917
|
| Total Liabilities |
12,495
|
12,534
|
| Commitments and Contingencies - Note 6 |
|
|
| Capital stock Authorized:10,000,000 preferred stock, par value $0.001 per share |
|
|
| Capital stock Authorized:200,000,000 common stock, par value $0.001 per share 82,086,511 common shares (September 30, 2023 - 82,066,511) |
82
|
82
|
| Additional paid-in capital |
437,184
|
434,839
|
| Accumulated deficit |
(301,691)
|
(293,069)
|
| Total Stockholders' Equity |
135,575
|
141,852
|
| Total Liabilities and Stockholders' Equity |
$ 148,070
|
$ 154,386
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable, pertaining to costs that are statutory in nature, are incurred on contractual obligations, or accumulate over time and for which invoices have not yet been received or will not be rendered. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionValue received from shareholders in common stock-related transactions that are in excess of par value or stated value and amounts received from other stock-related transactions. Includes only common stock transactions (excludes preferred stock transactions). May be called contributed capital, capital in excess of par, capital surplus, or paid-in capital. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapitalCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are recognized. Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 26: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the caption on the face of the balance sheet to indicate that the entity has entered into (1) purchase or supply arrangements that will require expending a portion of its resources to meet the terms thereof, and (2) is exposed to potential losses or, less frequently, gains, arising from (a) possible claims against a company's resources due to future performance under contract terms, and (b) possible losses or likely gains from uncertainties that will ultimately be resolved when one or more future events that are deemed likely to occur do occur or fail to occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(15))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 210
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03.17)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.25)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommitmentsAndContingencies |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred income excluding obligation to transfer product and service to customer for which consideration has been received or is receivable, classified as current. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section 25
-Paragraph 2
-SubTopic 10
-Topic 470
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481174/470-10-25-2
| Name: |
us-gaap_DeferredIncomeCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all liabilities that are recognized. Liabilities are probable future sacrifices of economic benefits arising from present obligations of an entity to transfer assets or provide services to other entities in the future. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 22: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19-26)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable preferred shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(21))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.0.1
Condensed Consolidated Interim Balance Sheets (Parenthetical) - $ / shares
|
Dec. 31, 2023 |
Sep. 30, 2023 |
| Statement of Financial Position [Abstract] |
|
|
| Preferred stock, shares authorized |
10,000,000
|
10,000,000
|
| Preferred stock, par value |
$ 0.001
|
$ 0.001
|
| Common stock, shares authorized |
200,000,000
|
200,000,000
|
| Common stock, par value |
$ 0.001
|
$ 0.001
|
| Common stock, shares issued |
82,086,511
|
82,066,511
|
| Common stock, shares outstanding |
82,086,511
|
82,066,511
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StatementOfFinancialPositionAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Condensed Consolidated Interim Statements of Operations and Comprehensive Loss (Unaudited) - USD ($)
$ in Thousands |
3 Months Ended |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Operating expenses |
|
|
| General and administrative |
$ 2,609
|
$ 3,317
|
| Research and development |
8,684
|
12,067
|
| Total operating expenses |
11,293
|
15,384
|
| Operating loss |
(11,293)
|
(15,384)
|
| Other income |
|
|
| Grant income |
|
25
|
| Research and development incentive income |
592
|
733
|
| Interest income, net |
2,008
|
1,268
|
| Foreign exchange gain |
156
|
366
|
| Total other income, net |
2,756
|
2,392
|
| Net loss before provision for income taxes |
(8,537)
|
(12,992)
|
| Income tax recovery (expense), current |
(85)
|
20
|
| Net loss and comprehensive loss |
$ (8,622)
|
$ (12,972)
|
| X |
- References
+ Details
| Name: |
avxl_NonOperatingIncomeFromGrants |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_ResearchAndDevelopmentIncentiveIncome |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, before tax, of realized and unrealized gain (loss) from foreign currency transaction. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 35
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482014/830-20-35-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481956/830-20-45-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481926/830-20-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481839/830-10-45-17
| Name: |
us-gaap_ForeignCurrencyTransactionGainLossBeforeTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total of expenses of managing and administering the affairs of an entity, including affiliates of the reporting entity, which are not directly or indirectly associated with the manufacture, sale or creation of a product or product line. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_GeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe net amount of operating interest income (expense). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04.10)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_InterestIncomeExpenseNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of income or expense from ancillary business-related activities (that is to say, excluding major activities considered part of the normal operations of the business). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.7)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_NonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NonoperatingIncomeExpenseAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGenerally recurring costs associated with normal operations except for the portion of these expenses which can be clearly related to production and included in cost of sales or services. Includes selling, general and administrative expense.
| Name: |
us-gaap_OperatingExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate costs incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process whether intended for sale or the entity's use, during the reporting period charged to research and development projects, including the costs of developing computer software up to the point in time of achieving technological feasibility, and costs allocated in accounting for a business combination to in-process projects deemed to have no alternative future use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482916/730-10-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482517/912-730-25-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 985
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481283/985-20-50-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.24.0.1
Condensed Consolidated Interim Statements of Operations and Comprehensive Loss (Unaudited) (Parenthetical) - $ / shares
|
3 Months Ended |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Income Statement [Abstract] |
|
|
| Net Loss per share, Basic |
$ (0.11)
|
$ (0.17)
|
| Net Loss per share, Diluted |
$ (0.11)
|
$ (0.17)
|
| Weighted average number of shares outstanding, Basic |
82,077,815
|
77,977,112
|
| Weighted average number of shares outstanding, Diluted |
82,077,815
|
77,977,112
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncomeStatementAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Condensed Consolidated Interim Statements of Changes in Stockholders' Equity (Unaudited) - USD ($)
$ in Thousands |
Common Stock [Member] |
Additional Paid-in Capital [Member] |
Retained Earnings [Member] |
Total |
| Beginning balance, value at Sep. 30, 2022 |
$ 78
|
$ 387,977
|
$ (245,564)
|
$ 142,491
|
| Beginning balance, shares at Sep. 30, 2022 |
77,942,815
|
|
|
|
| Shares issued pursuant to exercise of stock options |
|
258
|
|
258
|
| Shares issued upon exercise of stock options, shares |
89,320
|
|
|
|
| Share based compensation |
|
5,347
|
|
5,347
|
| Net loss |
|
|
(12,972)
|
(12,972)
|
| Ending balance, value at Dec. 31, 2022 |
$ 78
|
393,582
|
(258,536)
|
135,124
|
| Ending balance, shares at Dec. 31, 2022 |
78,032,135
|
|
|
|
| Beginning balance, value at Sep. 30, 2023 |
$ 82
|
434,839
|
(293,069)
|
141,852
|
| Beginning balance, shares at Sep. 30, 2023 |
82,066,511
|
|
|
|
| Shares issued pursuant to exercise of stock options |
|
59
|
|
59
|
| Shares issued pursuant to exercise of stock options, shares |
20,000
|
|
|
|
| Share based compensation |
|
2,286
|
|
2,286
|
| Net loss |
|
|
(8,622)
|
(8,622)
|
| Ending balance, value at Dec. 31, 2023 |
$ 82
|
$ 437,184
|
$ (301,691)
|
$ 135,575
|
| Ending balance, shares at Dec. 31, 2023 |
82,086,511
|
|
|
|
| X |
- References
+ Details
| Name: |
avxl_SharesIssuedPursuantToExerciseOfStockOptions |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_SharesIssuedPursuantToExerciseOfStockOptionsShares |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_SharesIssuedUponExerciseOfStockOptionsShares |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase to additional paid-in capital (APIC) for recognition of cost for award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481089/718-20-55-13
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481089/718-20-55-12
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalSharebasedCompensationRequisiteServicePeriodRecognitionValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued which are neither cancelled nor held in the treasury.
| Name: |
us-gaap_SharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.0.1
Condensed Consolidated Interim Statements of Cash Flows (Unaudited) - USD ($)
$ in Thousands |
3 Months Ended |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Cash Flows used in Operating Activities |
|
|
| Net loss |
$ (8,622)
|
$ (12,972)
|
| Adjustments to reconcile net loss to net cash used in operations: |
|
|
| Share based compensation |
2,286
|
5,347
|
| Changes in working capital balances related to operations: |
|
|
| Incentive and tax receivables |
(840)
|
(902)
|
| Prepaid expenses and deposits |
(103)
|
(301)
|
| Accounts payable |
(30)
|
1,463
|
| Accrued liabilities |
(9)
|
1,098
|
| Deferred grant income |
|
473
|
| Net cash used in operating activities |
(7,318)
|
(5,794)
|
| Cash Flows provided by Financing Activities |
|
|
| Proceeds from exercise of stock options |
59
|
258
|
| Net cash provided by financing activities |
59
|
258
|
| Decrease in cash and cash equivalents during the period |
(7,259)
|
(5,536)
|
| Cash and cash equivalents, beginning of period |
151,024
|
149,158
|
| Cash and cash equivalents, end of period |
143,765
|
143,622
|
| Supplemental Cash Flow Information |
|
|
| Cash paid for state and local minimum income taxes |
$ 47
|
$ 50
|
| X |
- References
+ Details
| Name: |
avxl_IncreaseDecreaseInDeferredGrantIncome |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsNoncashItemsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; excluding effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481877/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseExcludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate amount of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate amount of expenses incurred but not yet paid. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccruedLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the amount of outstanding money paid in advance for goods or services that bring economic benefits for future periods. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesContinuingOperationsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesContinuingOperationsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow from exercise of option under share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2A
-Subparagraph (a)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2A
| Name: |
us-gaap_ProceedsFromStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.24.0.1
Business Description
|
3 Months Ended |
Dec. 31, 2023 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Business Description |
Note 1 Business Description
Business
Anavex Life Sciences Corp. (“Anavex” or
the “Company”) is a clinical stage biopharmaceutical company engaged in the development of differentiated therapeutics by
applying precision medicine to central nervous system (“CNS”) diseases with high unmet need. Anavex analyzes genomic data
from clinical trials to identify biomarkers, which are used in the analysis of its clinical trials for the treatment of neurodegenerative
and neurodevelopmental diseases.
The Company’s lead compound ANAVEX®2-73
is being developed to treat Alzheimer’s disease, Parkinson’s disease and potentially other central nervous system diseases,
including rare diseases, such as Rett syndrome, a rare severe neurological monogenic disorder caused by mutations in the X-linked gene,
methyl-CpG-binding protein 2 (“MECP2”).
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for organization, consolidation and basis of presentation of financial statements disclosure. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480424/946-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480424/946-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//810/tableOfContent
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//205/tableOfContent
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Basis of Presentation
|
3 Months Ended |
Dec. 31, 2023 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Basis of Presentation |
Note 2 Basis of Presentation
Basis of Presentation
These accompanying unaudited condensed consolidated interim financial statements
have been prepared pursuant to the rules and regulations of the Securities and Exchange Commission (“SEC”) and accounting
principles generally accepted in the United States of America (“U.S. GAAP”) for interim reporting. Accordingly, certain information
and note disclosures normally included in the annual financial statements in accordance with U.S. GAAP have been condensed or omitted
pursuant to such rules and regulations. In the opinion of management, the disclosures are adequate to make the information presented not
misleading.
These accompanying unaudited condensed consolidated interim financial statements
reflect all adjustments, consisting of normal recurring adjustments, which in the opinion of management are necessary for fair presentation
of the information contained herein. The consolidated balance sheet as of September 30, 2023 was derived from the audited annual financial
statements but does not include all disclosures required by U.S. GAAP. The accompanying unaudited condensed consolidated interim financial
statements should be read in conjunction with the audited consolidated financial statements and notes thereto included in the Company’s
annual report on Form 10-K for the year ended September 30, 2023 filed with the SEC on November 27, 2023. The Company follows the same
accounting policies in the preparation of interim reports.
Operating results for the three months ended December
31, 2023 are not necessarily indicative of the results that may be expected for the year ending September 30, 2024.
Liquidity
All of the Company’s potential drug compounds
are in the clinical development stage and the Company cannot be certain that its research and development efforts will be successful or,
if successful, that its potential drug compounds will ever be approved for sales to pharmaceutical companies or generate commercial revenues.
To date, we have not generated any revenues from our operations. The Company expects the business to continue to experience negative cash
flows from operations for the foreseeable future and cannot predict when, if ever, our business might become profitable.
Management believes that the current working capital position will be sufficient to
meet the Company’s working capital requirements beyond the next 12 months after the date that these condensed consolidated interim
financial statements are issued. The process of drug development can be costly, and the timing and outcomes of clinical trials are uncertain. The
assumptions upon which the Company has based its estimates are routinely evaluated and may be subject to change. The actual amount
of the Company’s expenditures will vary depending upon a number of factors including but not limited to the design, timing and duration
of future clinical trials, the progress of the Company’s research and development programs and the level of financial resources
available. The Company has the ability to adjust its operating plan spending levels based on the timing of future clinical trials.
Other than our rights related to the 2023
Purchase Agreement (as defined below in Note 5), there can be no assurance that additional financing will be available to us when
needed or, if available, that it can be obtained on commercially reasonable terms. If the Company is not able to obtain the
additional financing on a timely basis, if and when it is needed, it will be forced to delay or scale down some or all of its
research and development activities.
Use of Estimates
The preparation of financial statements in accordance
with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the
date of the financial statements and the reported amounts of revenue and expenses in the reporting period. The Company regularly evaluates
estimates and assumptions related to accounting for research and development costs, incentive income receivable, valuation and recoverability
of deferred tax assets, share based compensation, and loss contingencies. The Company bases its estimates and assumptions on current facts,
historical experience, and various other factors that it believes to be reasonable under the circumstances, the results of which form
the basis for making judgments about the carrying values of assets and liabilities and the accrual of costs and expenses that are not
readily apparent from other sources. The actual results experienced by the Company may differ materially and adversely from the Company’s
estimates. To the extent there are material differences between the estimates and the actual results, future results of operations will
be affected.
Principles of Consolidation
These consolidated financial statements include the
accounts of Anavex Life Sciences Corp. and its wholly-owned subsidiaries, Anavex Australia Pty Limited (“Anavex Australia”),
a company incorporated under the laws of Australia, Anavex Germany GmbH, a company incorporated under the laws of Germany, and Anavex
Canada Ltd., a company incorporated under the laws of the Province of Ontario, Canada. All inter-company transactions and balances have
been eliminated.
Fair Value Measurements
The fair value hierarchy under GAAP is based on three
levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value which
are the following:
Level 1 - quoted prices (unadjusted) in active
markets for identical assets or liabilities;
Level 2 - observable inputs other than Level
1, quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets and
liabilities in markets that are not active, and model-derived prices whose inputs are observable or whose significant value
drivers are observable; and
Level
3 - assets and liabilities whose significant value drivers are unobservable by little or no market activity and that are significant
to the fair value of the assets or liabilities.
At December 31, 2023 and September 30, 2023, the Company
did not have any Level 3 assets or liabilities.
Basic and Diluted Loss per Share
Basic income/(loss) per common share is computed by
dividing net income/(loss) available to common stockholders by the weighted average number of common shares outstanding during the period.
Diluted income/(loss) per common share is computed by dividing net income/(loss) available to common stockholders by the sum of (1) the
weighted-average number of common shares outstanding during the period, (2) the dilutive effect of the assumed exercise of options and
warrants using the treasury stock method and (3) the dilutive effect of other potentially dilutive securities. For purposes of the diluted
net loss per share calculation, options and warrants are potentially dilutive securities and are excluded from the calculation of diluted
net loss per share because their effect would be anti-dilutive.
As of December 31, 2023 loss per share excludes 14,269,363
(December 31, 2022: 13,525,296) potentially dilutive common shares related to outstanding options and warrants, as their effect was anti-dilutive.
Recently Adopted Accounting
Pronouncements
In November 2023, the Financial
Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) No. 2023-07, “Segment Reporting: Improvements to Reportable
Segment Disclosures.” This guidance requires disclosure of incremental segment information on an annual and interim basis. This
amendment is effective for our fiscal year ending September 30, 2025 and our interim periods within the fiscal year ending September 30,
2026. The Company is currently assessing the impact of this guidance on its disclosures.
In December 2023, the FASB
issued ASU No. 2023-09, “Income Taxes: Improvements to Income Tax Disclosures.” This guidance requires consistent categories
and greater disaggregation of information in the rate reconciliation and disclosures of income taxes paid by jurisdiction. This amendment
is effective for our fiscal year ending September 30, 2026. The Company is currently assessing the impact of this guidance on its disclosures.
|
| X |
- DefinitionThe entire disclosure for the basis of accounting, or basis of presentation, used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
| Name: |
us-gaap_BasisOfAccounting |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Other Income
|
3 Months Ended |
Dec. 31, 2023 |
|---|
| Other Income |
|
| Other Income |
Note 3 Other Income
Grant
Income
As
of December 31, 2023, the Company had received a $1.0 million research grant awarded by the Michael J. Fox Foundation for Parkinson’s
Research. The grant will be used to fund a clinical trial of the Company’s lead compound, ANAVEX®2-73 related
to Parkinson’s disease. Of the total, $0.5 million was received during the year ended September 30, 2023 and $0.5 million
was received during the year ended September 30, 2021.
The
grant income has been deferred when received and is being amortized to other income as the related research and development expenditures
are incurred. During the three months ended December 31, 2023, the Company recognized $0 (three months ended December 31, 2022:
$25,000) of this grant on its statements of operations as grant income. At December 31, 2023, an amount of $0.9 million (September
30, 2023: $0.9 million) of this grant is recorded as deferred grant income, representing the amount of this grant which has not
yet been recognized to other income. The Company will recognize this income on its statement of operations as the relating expenditures
are incurred to offset the income.
Research
and development incentive income
Research and development
incentive income represents the income earned by Anavex Australia of the Australia R&D credit. This cash incentive is received by
Anavex Australia, upon filing of a claim in connection with Anavex Australia’s annual income tax return.
During the three months ended
December 31, 2023 the Company recorded research and development incentive income of $0.6 million (AUD 0.9 million) (2022: $0.7 million
(AUD 1.1 million)) in respect of the Australia R&D credit for eligible research and development expenses incurred during the period.
This amount is included within Other income on the consolidated statements of operations.
At December 31, 2023, Incentive
and tax receivables includes $3.3 million (AUD 4.9 million) (September 30, 2023: $2.5 million (AUD 3.9 million)) relating to Australia
R&D credits earned during the year that are expected to be reimbursed upon filing of the Company’s annual claim under this program.
The Australia R&D credit
program is a self-assess program whereby the Company must assess its eligibility each year to determine (i) if the entity is eligible
(ii) if the specific R&D activities are eligible and (iii) if the individual R&D expenditures have nexus to such R&D activities.
The Company evaluates its eligibility under the tax incentive program as of each balance sheet date based on the most current and relevant
data available. Anavex Australia is able to continue to claim the R&D tax incentive for as long as it remains eligible and continues
to incur eligible research and development expenditures.
Although the Company believes
that it has complied with all the relevant conditions of eligibility under the program for all periods claimed, the Australian Tax Office
(ATO) has the right to review the Company’s qualifying programs and related expenditures for a period of four years. If such a review
were to occur, the ATO may have different interpretations of certain eligibility requirements. If the ATO disagreed with the Company’s
assessments and any related subsequent appeals, it could require adjustment to and repayment of current or previous years’ claims
already received. Additionally, if the Company was unable to demonstrate a reasonably arguable position taken on such claims, the ATO
could also assess penalties and interest on such adjustment.
Currently, the Company’s
tax incentive claims from 2019 to 2022 are open to potential review by the ATO. Additionally, the period open for review is indefinite
if the ATO suspects fraud. The Company has not provided any allowance for any such potential adjustments, should they occur in the future.
|
| X |
- References
+ Details
| Name: |
avxl_DisclosureOtherIncomeAbstract |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_OtherIncomeDisclosureTextBlock |
| Namespace Prefix: |
avxl_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Accrued Liabilities
|
3 Months Ended |
Dec. 31, 2023 |
|---|
| Payables and Accruals [Abstract] |
|
| Accrued Liabilities |
Note 4 Accrued Liabilities
The principal components of accrued liabilities consist of (in thousands):
| Schedule of accrued liabilities | |
| | | |
| | |
| | |
December 31 | |
September 30 |
| | |
2023 | |
2023 |
| Accrued clinical site and patient visits costs | |
$ | 2,058 | | |
$ | 2,006 | |
| Accrued compensation and benefits | |
| 1,785 | | |
| 1,360 | |
| Fixed contract accruals | |
| 364 | | |
| 38 | |
| Milestone based contract accruals | |
| 989 | | |
| 1,267 | |
| All other accrued liabilities | |
| 2,090 | | |
| 2,624 | |
| Total accrued liabilities | |
$ | 7,286 | | |
$ | 7,295 | |
|
| X |
- DefinitionThe entire disclosure for accounts payable, accrued expenses, and other liabilities that are classified as current at the end of the reporting period.
| Name: |
us-gaap_AccountsPayableAccruedLiabilitiesAndOtherLiabilitiesDisclosureCurrentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_PayablesAndAccrualsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Equity Offerings
|
3 Months Ended |
Dec. 31, 2023 |
|---|
| Equity [Abstract] |
|
| Equity Offerings |
Note 5 Equity Offerings
Common Stock
Common shares are voting and are entitled to dividends
as declared at the discretion of the Board of Directors (the “Board”).
Preferred Stock
The Company’s Board has the authority to
issue preferred stock in one or more series and to fix the rights, preferences, privileges, restrictions and the number of shares constituting
any series or the designation of the series.
Sales Agreement
The Company entered into a Controlled Equity Offering
Sales Agreement on July 6, 2018, which was amended and restated on May 1, 2020 (the “Sales Agreement”) with Cantor Fitzgerald &
Co. and SVB Leerink LLC (together the “Sales Agents”), pursuant to which the Company may offer and sell shares of common stock
(“Shares”) registered under an effective registration statement from time to time through the Sales Agents (the “Offering”).
Upon delivery of a placement notice based on the Company’s
instructions and subject to the terms and conditions of the Sales Agreement, the Sales Agents may sell the Shares by methods deemed to
be an “at the market offering” offering, in negotiated transactions at market prices prevailing at the time of sale or at
prices related to such prevailing market prices, or by any other method permitted by law, including negotiated transactions, subject to
the prior written consent of the Company. The Company is not obligated to make any sales of Shares under the Sales Agreement. The Company
or Sales Agents may suspend or terminate the offering of Shares upon notice to the other party, subject to certain conditions. The Sales
Agents will act as agent on a commercially reasonable efforts basis consistent with their normal trading and sales practices and applicable
state and federal law, rules and regulations and the rules of Nasdaq.
The Company has agreed to pay the Sales Agents commissions
for their services of up to 3.0% of the gross proceeds from the sale of the Shares pursuant to the Sales Agreement. The Company also agreed
to provide the Sales Agents with customary indemnification and contribution rights. During the three months ended December 31, 2023 and
2022, no shares were sold pursuant to the Offering. At December 31, 2023, an amount of $142.4 million (September 30, 2023: $142.4 million)
was registered pursuant to an effective registration statement. The Company currently is unable to sell shares of common stock under the
Sales Agreement.
2023 Purchase Agreement
On February 3, 2023, the Company entered into a $150.0
million purchase agreement (the “2023 Purchase Agreement”) with Lincoln Park Capital Fund, LLC (“Lincoln Park”),
pursuant to which the Company has the right to sell and issue to Lincoln Park, and Lincoln Park is obligated to purchase, up to $150.0
150,000,000
million in value of its shares of common stock from time to time over a three-year period until February 3, 2026.
In consideration for entering into the 2023 Purchase
Agreement, the Company issued to Lincoln Park 75,000 shares of common stock as a commitment fee (the “initial commitment shares”)
and agreed to issue up to an additional 75,000 shares pro rata, when and if, Lincoln Park purchased, at the Company’s discretion,
the $150.0 million aggregate commitment. The Company determined the fair value of the initial commitment shares was $0.8 million with
reference to the closing price of the Company’s shares on the Purchase Agreement date. In addition, the Company incurred third party
expenses of $0.1 million in connection with entering into the Purchase Agreement. These amounts were expensed to other financing expense
on the statements of operations during the year ended September 30, 2023.
During the three months ended December 31, 2023 and
2022, the Company did not issue any shares of common stock under the 2023 Purchase Agreement.
At December 31, 2023, an amount of $122.1 million
remained available under the 2023 Purchase Agreement.
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480237/815-40-50-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(e)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//505/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 16
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-16
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
| Name: |
us-gaap_StockholdersEquityNoteDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Commitments and Contingencies
|
3 Months Ended |
Dec. 31, 2023 |
|---|
| Commitments and Contingencies Disclosure [Abstract] |
|
| Commitments and Contingencies |
Note 6 Commitments and Contingencies
Leases
The Company leases office space under
an operating lease with an initial term of 12 months or less. Under the terms of the office lease, the Company is required to pay its
proportionate share of operating costs.
During the three months ended December
31, 2023 and 2022, operating lease costs were as follows (in thousands):
| Schedule of operating lease costs | |
| |
|
| | |
2023 | |
2022 |
| Operating lease costs | |
$ | 30 | | |
$ | 30 | |
Employee 401(k) Benefit Plan
The Company has
a defined-contribution savings plan under Section 401(k) of the Internal Revenue Code. The plan covers all United States based employees.
United States based employees eligible to participate in the plan may contribute up to the current statutory limits under the Internal
Revenue Service regulations. The 401(k) plan permits the Company to make additional matching contributions on behalf of contributing employees.
During the three months ended December 31, 2023
and 2022, the Company made matching contributions under the 401(k) plan as
follows (in thousands):
| Schedule of contribution plan | |
| |
|
| | |
2023 | |
2022 |
| Contributions to 401(k) plan | |
$ | 73 | | |
$ | 44 | |
Litigation
The Company is subject
to claims and legal proceedings that arise in the ordinary course of business. Such matters are inherently uncertain, and there can be
no guarantee that the outcome of any such matter will be decided favorably to the Company or that the resolution of any such matter will
not have a material adverse effect upon the Company’s consolidated financial statements. The Company does not believe that any of
such pending claims and legal proceedings will have a material adverse effect on its consolidated financial statements.
Share Purchase Warrants
At December 31, 2023 and September 30, 2023, the Company
had 160,000 warrants outstanding at a weighted average exercise price of $3.72 as follows:
| Schedule of share purchase warrants outstanding | |
| |
|
| Number | |
Exercise Price | |
Expiry Date |
| | 150,000 | | |
$ | 3.17 | | |
May 6, 2024 |
| | 10,000 | | |
$ | 12.00 | | |
April 21, 2026 |
| | 160,000 | | |
| | | |
|
Stock–based Compensation
Plan
2015 Stock Option Plan
On September 18, 2015, the Company’s Board approved
a 2015 Omnibus Incentive Plan (the “2015 Plan”), which provided for the grant of stock options and restricted stock awards
to directors, officers, employees and consultants of the Company.
The maximum number of our common shares reserved for
issue under the plan was 6,050,553 shares, subject to adjustment in the event of a change of the Company’s capitalization.
2019 Stock Option Plan
On January 15, 2019, the Board approved the 2019 Omnibus
Incentive Plan (the “2019 Plan”), which provides for the grant of stock options and restricted stock awards to directors,
officers, employees, consultants and advisors of the Company.
The maximum number of our common shares reserved for
issue under the plan was 6,000,000 shares, subject to adjustment in the event of a change of the Company’s capitalization.
During the year ended September 30, 2022, 406,453
options previously available under the 2019 Plan and the 2015 Plan became available under the 2022 Plan (as defined below).
2022 Stock Option Plan
On March 25, 2022, the Board approved the 2022 Omnibus
Incentive Plan (the “2022 Plan”). The 2022 Plan was approved by stockholders on May 24, 2022. Under the terms of the 2022
Plan, 10,000,000 additional shares of Common Stock will be available for issuance under the plan, in addition to the shares available
under the 2019 Plan and the 2015 Plan. Any awards outstanding under a previous stock option plan will remain subject to and be paid under
such plan, and any shares subject to outstanding awards under a previous plan that subsequently cease to be subject to such awards (other
than by reason of settlement of the awards in shares) will automatically become available for issuance under the 2022 Plan.
The 2022 Plan provides that it may be administered
by the Board, or the Board may delegate such responsibility to a committee. The exercise price will be determined by the Board at the
time of grant shall be at least equal to the fair market value on such date. If the grantee is a 10% stockholder on the grant date, then
the exercise price shall not be less than 110% of fair market value of the Company’s shares of common stock on the grant date. Stock
options may be granted under the 2022 Plan for an exercise period of up to ten years from the date of grant of the option or such lesser
periods as may be determined by the Board, subject to earlier termination in accordance with the terms of the 2022 Plan. At December 31,
2023, 3,892,000 options had been issued under the 2022 Plan and 6,643,952 options were available for issue under the 2022 Plan.
The following summarizes information about stock option
activity during the year ended September 30, 2023 and three months ended December 31, 2023:
| Schedule of outstanding stock purchase options | | |
| | | |
| | | |
| | | |
| | |
| | |
Number of Options | |
Weighted Average Exercise Price
($) | |
Weighted Average Grant Date Fair Value
($) | |
Aggregate intrinsic value
($) |
| Outstanding, September 30, 2022 | | |
| 13,169,616 | | |
| 6.61 | | |
| 4.96 | | |
| 62,267,309 | |
| Granted | | |
| 1,959,000 | | |
| 9.30 | | |
| 6.60 | | |
| — | |
| Exercised | | |
| (759,753 | ) | |
| 2.34 | | |
| 0.95 | | |
| 4,629,026 | |
| Forfeited | | |
| (257,083 | ) | |
| 12.00 | | |
| 6.74 | | |
| — | |
| Outstanding, September 30, 2023 | | |
| 14,111,780 | | |
| 7.12 | | |
| 5.27 | | |
| 22,290,069 | |
| Granted | | |
| 155,000 | | |
| 7.40 | | |
| 4.88 | | |
| — | |
| Exercised | | |
| (20,000 | ) | |
| 2.96 | | |
| 2.23 | | |
| 58,600 | |
| Forfeited | | |
| (137,417 | ) | |
| 12.19 | | |
| 6.78 | | |
| — | |
| Outstanding, December 31, 2023 | | |
| 14,109,363 | | |
| 7.08 | | |
| | | |
| 47,674,002 | |
| Exercisable, December 31, 2023 | | |
| 9,663,446 | | |
| 5.26 | | |
| | | |
| 45,388,166 | |
The following summarizes information about stock options
at December 31, 2023 by a range of exercise prices:
| | Schedule of summarized information about stock options | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Range of exercises prices | |
Number of outstanding | |
Weighted average remaining contractual life | |
Weighted average | |
Number of vested | |
Weighted average |
| From | |
To | |
options | |
(in years) | |
exercise price | |
options | |
exercise price |
| $ | 0.92 | | |
$ | 3.00 | | |
| 3,260,309 | | |
| 4.47 | | |
$ | 2.38 | | |
| 3,260,309 | | |
$ | 2.38 | |
| $ | 3.01 | | |
$ | 5.00 | | |
| 2,017,500 | | |
| 4.07 | | |
$ | 3.28 | | |
| 2,017,500 | | |
$ | 3.28 | |
| $ | 5.01 | | |
$ | 9.00 | | |
| 5,375,054 | | |
| 6.00 | | |
$ | 6.91 | | |
| 3,329,970 | | |
$ | 6.11 | |
| $ | 9.01 | | |
$ | 13.00 | | |
| 1,909,000 | | |
| 8.10 | | |
$ | 10.53 | | |
| 496,083 | | |
$ | 11.36 | |
| $ | 13.01 | | |
$ | 25.00 | | |
| 1,547,500 | | |
| 7.23 | | |
$ | 18.24 | | |
| 559,584 | | |
$ | 18.64 | |
| | | | |
| | | |
| 14,109,363 | | |
| 5.79 | | |
$ | 7.08 | | |
| 9,663,446 | | |
$ | 5.26 | |
The weighted average grant date fair value of options
vested at December 31, 2023 was $4.00 (September 30, 2023: $3.94). At December 31, 2023, the weighted average contractual life of options
outstanding was 5.8 years (September 30, 2023: 6.0 years) and for options exercisable was 4.5 years (September 30, 2023: 4.75 years).
The aggregate intrinsic value is calculated as the
difference between the exercise price of the underlying awards and the quoted market price of the Company’s stock for the options
that were in-the-money at December 31, 2023.
During the three months ended December 31, 2023,
the Company recognized stock-based compensation expense of $2.3
million (2022: $5.3
million) in connection with the issuance and vesting of stock options and warrants in exchange for services. These amounts have
been included in general and administrative expenses and research and development expenses on the Company’s condensed
consolidated interim statement of operations as follows (in thousands):
| Schedule of general and administrative expenses and research and development expenses | |
| | | |
| | |
| | |
December 31 |
| | |
2023 | |
2022 |
| General and administrative | |
$ | 926 | | |
$ | 1,743 | |
| Research and development | |
| 1,360 | | |
| 3,604 | |
| Total stock-based compensation | |
$ | 2,286 | | |
$ | 5,347 | |
An amount of approximately $27.7 million in stock-based
compensation is expected to be recorded over the remaining term of such options through fiscal 2026.
The fair value of each option award
granted during the three months ended December 31, 2023 and 2022 is estimated on the date of grant using the Black Scholes option pricing
model based on the following weighted average assumptions:
| Schedule of weighted average
assumptions | |
| | | |
| | |
| | |
2023 | |
2022 |
| Risk-free interest rate | |
| 4.47 | % | |
| 4.07 | % |
| Expected life of options (years) | |
| 5.29 | | |
| 4.98 | |
| Annualized volatility | |
| 80.61 | % | |
| 84.01 | % |
| Dividend rate | |
| 0.00 | % | |
| 0.00 | % |
The fair value of stock compensation charges recognized
during the three months ended December 31, 2023 and 2022 was determined with reference to the quoted market price of the Company’s
shares on the grant date.
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for commitments and contingencies. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//450/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 954
-SubTopic 440
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480327/954-440-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 440
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//440/tableOfContent
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Basis of Presentation (Policies)
|
3 Months Ended |
Dec. 31, 2023 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Basis of Presentation |
Basis of Presentation
These accompanying unaudited condensed consolidated interim financial statements
have been prepared pursuant to the rules and regulations of the Securities and Exchange Commission (“SEC”) and accounting
principles generally accepted in the United States of America (“U.S. GAAP”) for interim reporting. Accordingly, certain information
and note disclosures normally included in the annual financial statements in accordance with U.S. GAAP have been condensed or omitted
pursuant to such rules and regulations. In the opinion of management, the disclosures are adequate to make the information presented not
misleading.
These accompanying unaudited condensed consolidated interim financial statements
reflect all adjustments, consisting of normal recurring adjustments, which in the opinion of management are necessary for fair presentation
of the information contained herein. The consolidated balance sheet as of September 30, 2023 was derived from the audited annual financial
statements but does not include all disclosures required by U.S. GAAP. The accompanying unaudited condensed consolidated interim financial
statements should be read in conjunction with the audited consolidated financial statements and notes thereto included in the Company’s
annual report on Form 10-K for the year ended September 30, 2023 filed with the SEC on November 27, 2023. The Company follows the same
accounting policies in the preparation of interim reports.
Operating results for the three months ended December
31, 2023 are not necessarily indicative of the results that may be expected for the year ending September 30, 2024.
|
| Liquidity |
Liquidity
All of the Company’s potential drug compounds
are in the clinical development stage and the Company cannot be certain that its research and development efforts will be successful or,
if successful, that its potential drug compounds will ever be approved for sales to pharmaceutical companies or generate commercial revenues.
To date, we have not generated any revenues from our operations. The Company expects the business to continue to experience negative cash
flows from operations for the foreseeable future and cannot predict when, if ever, our business might become profitable.
Management believes that the current working capital position will be sufficient to
meet the Company’s working capital requirements beyond the next 12 months after the date that these condensed consolidated interim
financial statements are issued. The process of drug development can be costly, and the timing and outcomes of clinical trials are uncertain. The
assumptions upon which the Company has based its estimates are routinely evaluated and may be subject to change. The actual amount
of the Company’s expenditures will vary depending upon a number of factors including but not limited to the design, timing and duration
of future clinical trials, the progress of the Company’s research and development programs and the level of financial resources
available. The Company has the ability to adjust its operating plan spending levels based on the timing of future clinical trials.
Other than our rights related to the 2023
Purchase Agreement (as defined below in Note 5), there can be no assurance that additional financing will be available to us when
needed or, if available, that it can be obtained on commercially reasonable terms. If the Company is not able to obtain the
additional financing on a timely basis, if and when it is needed, it will be forced to delay or scale down some or all of its
research and development activities.
|
| Use of Estimates |
Use of Estimates
The preparation of financial statements in accordance
with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the
date of the financial statements and the reported amounts of revenue and expenses in the reporting period. The Company regularly evaluates
estimates and assumptions related to accounting for research and development costs, incentive income receivable, valuation and recoverability
of deferred tax assets, share based compensation, and loss contingencies. The Company bases its estimates and assumptions on current facts,
historical experience, and various other factors that it believes to be reasonable under the circumstances, the results of which form
the basis for making judgments about the carrying values of assets and liabilities and the accrual of costs and expenses that are not
readily apparent from other sources. The actual results experienced by the Company may differ materially and adversely from the Company’s
estimates. To the extent there are material differences between the estimates and the actual results, future results of operations will
be affected.
|
| Principles of Consolidation |
Principles of Consolidation
These consolidated financial statements include the
accounts of Anavex Life Sciences Corp. and its wholly-owned subsidiaries, Anavex Australia Pty Limited (“Anavex Australia”),
a company incorporated under the laws of Australia, Anavex Germany GmbH, a company incorporated under the laws of Germany, and Anavex
Canada Ltd., a company incorporated under the laws of the Province of Ontario, Canada. All inter-company transactions and balances have
been eliminated.
|
| Fair Value Measurements |
Fair Value Measurements
The fair value hierarchy under GAAP is based on three
levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value which
are the following:
Level 1 - quoted prices (unadjusted) in active
markets for identical assets or liabilities;
Level 2 - observable inputs other than Level
1, quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets and
liabilities in markets that are not active, and model-derived prices whose inputs are observable or whose significant value
drivers are observable; and
Level
3 - assets and liabilities whose significant value drivers are unobservable by little or no market activity and that are significant
to the fair value of the assets or liabilities.
At December 31, 2023 and September 30, 2023, the Company
did not have any Level 3 assets or liabilities.
|
| Basic and Diluted Loss per Share |
Basic and Diluted Loss per Share
Basic income/(loss) per common share is computed by
dividing net income/(loss) available to common stockholders by the weighted average number of common shares outstanding during the period.
Diluted income/(loss) per common share is computed by dividing net income/(loss) available to common stockholders by the sum of (1) the
weighted-average number of common shares outstanding during the period, (2) the dilutive effect of the assumed exercise of options and
warrants using the treasury stock method and (3) the dilutive effect of other potentially dilutive securities. For purposes of the diluted
net loss per share calculation, options and warrants are potentially dilutive securities and are excluded from the calculation of diluted
net loss per share because their effect would be anti-dilutive.
As of December 31, 2023 loss per share excludes 14,269,363
(December 31, 2022: 13,525,296) potentially dilutive common shares related to outstanding options and warrants, as their effect was anti-dilutive.
|
| Recently Adopted Accounting Pronouncements |
Recently Adopted Accounting
Pronouncements
In November 2023, the Financial
Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) No. 2023-07, “Segment Reporting: Improvements to Reportable
Segment Disclosures.” This guidance requires disclosure of incremental segment information on an annual and interim basis. This
amendment is effective for our fiscal year ending September 30, 2025 and our interim periods within the fiscal year ending September 30,
2026. The Company is currently assessing the impact of this guidance on its disclosures.
In December 2023, the FASB
issued ASU No. 2023-09, “Income Taxes: Improvements to Income Tax Disclosures.” This guidance requires consistent categories
and greater disaggregation of information in the rate reconciliation and disclosures of income taxes paid by jurisdiction. This amendment
is effective for our fiscal year ending September 30, 2026. The Company is currently assessing the impact of this guidance on its disclosures.
|
| X |
- References
+ Details
| Name: |
avxl_LliquidityPolicyTextBlock |
| Namespace Prefix: |
avxl_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for basis of accounting, or basis of presentation, used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS).
| Name: |
us-gaap_BasisOfAccountingPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy regarding (1) the principles it follows in consolidating or combining the separate financial statements, including the principles followed in determining the inclusion or exclusion of subsidiaries or other entities in the consolidated or combined financial statements and (2) its treatment of interests (for example, common stock, a partnership interest or other means of exerting influence) in other entities, for example consolidation or use of the equity or cost methods of accounting. The accounting policy may also address the accounting treatment for intercompany accounts and transactions, noncontrolling interest, and the income statement treatment in consolidation for issuances of stock by a subsidiary. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1
| Name: |
us-gaap_ConsolidationPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for computing basic and diluted earnings or loss per share for each class of common stock and participating security. Addresses all significant policy factors, including any antidilutive items that have been excluded from the computation and takes into account stock dividends, splits and reverse splits that occur after the balance sheet date of the latest reporting period but before the issuance of the financial statements. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-2
| Name: |
us-gaap_EarningsPerSharePolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for fair value measurements of financial and non-financial assets, liabilities and instruments classified in shareholders' equity. Disclosures include, but are not limited to, how an entity that manages a group of financial assets and liabilities on the basis of its net exposure measures the fair value of those assets and liabilities.
| Name: |
us-gaap_FairValueMeasurementPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact.
| Name: |
us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Accrued Liabilities (Tables)
|
3 Months Ended |
Dec. 31, 2023 |
|---|
| Payables and Accruals [Abstract] |
|
| Schedule of accrued liabilities |
| Schedule of accrued liabilities | |
| | | |
| | |
| | |
December 31 | |
September 30 |
| | |
2023 | |
2023 |
| Accrued clinical site and patient visits costs | |
$ | 2,058 | | |
$ | 2,006 | |
| Accrued compensation and benefits | |
| 1,785 | | |
| 1,360 | |
| Fixed contract accruals | |
| 364 | | |
| 38 | |
| Milestone based contract accruals | |
| 989 | | |
| 1,267 | |
| All other accrued liabilities | |
| 2,090 | | |
| 2,624 | |
| Total accrued liabilities | |
$ | 7,286 | | |
$ | 7,295 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_PayablesAndAccrualsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the components of accrued liabilities.
| Name: |
us-gaap_ScheduleOfAccruedLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Commitments and Contingencies (Tables)
|
3 Months Ended |
Dec. 31, 2023 |
|---|
| Commitments and Contingencies Disclosure [Abstract] |
|
| Schedule of operating lease costs |
| Schedule of operating lease costs | |
| |
|
| | |
2023 | |
2022 |
| Operating lease costs | |
$ | 30 | | |
$ | 30 | |
|
| Schedule of contribution plan |
| Schedule of contribution plan | |
| |
|
| | |
2023 | |
2022 |
| Contributions to 401(k) plan | |
$ | 73 | | |
$ | 44 | |
|
| Schedule of share purchase warrants outstanding |
| Schedule of share purchase warrants outstanding | |
| |
|
| Number | |
Exercise Price | |
Expiry Date |
| | 150,000 | | |
$ | 3.17 | | |
May 6, 2024 |
| | 10,000 | | |
$ | 12.00 | | |
April 21, 2026 |
| | 160,000 | | |
| | | |
|
|
| Schedule of outstanding stock purchase options |
| Schedule of outstanding stock purchase options | | |
| | | |
| | | |
| | | |
| | |
| | |
Number of Options | |
Weighted Average Exercise Price
($) | |
Weighted Average Grant Date Fair Value
($) | |
Aggregate intrinsic value
($) |
| Outstanding, September 30, 2022 | | |
| 13,169,616 | | |
| 6.61 | | |
| 4.96 | | |
| 62,267,309 | |
| Granted | | |
| 1,959,000 | | |
| 9.30 | | |
| 6.60 | | |
| — | |
| Exercised | | |
| (759,753 | ) | |
| 2.34 | | |
| 0.95 | | |
| 4,629,026 | |
| Forfeited | | |
| (257,083 | ) | |
| 12.00 | | |
| 6.74 | | |
| — | |
| Outstanding, September 30, 2023 | | |
| 14,111,780 | | |
| 7.12 | | |
| 5.27 | | |
| 22,290,069 | |
| Granted | | |
| 155,000 | | |
| 7.40 | | |
| 4.88 | | |
| — | |
| Exercised | | |
| (20,000 | ) | |
| 2.96 | | |
| 2.23 | | |
| 58,600 | |
| Forfeited | | |
| (137,417 | ) | |
| 12.19 | | |
| 6.78 | | |
| — | |
| Outstanding, December 31, 2023 | | |
| 14,109,363 | | |
| 7.08 | | |
| | | |
| 47,674,002 | |
| Exercisable, December 31, 2023 | | |
| 9,663,446 | | |
| 5.26 | | |
| | | |
| 45,388,166 | |
|
| Schedule of summarized information about stock options |
| | Schedule of summarized information about stock options | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Range of exercises prices | |
Number of outstanding | |
Weighted average remaining contractual life | |
Weighted average | |
Number of vested | |
Weighted average |
| From | |
To | |
options | |
(in years) | |
exercise price | |
options | |
exercise price |
| $ | 0.92 | | |
$ | 3.00 | | |
| 3,260,309 | | |
| 4.47 | | |
$ | 2.38 | | |
| 3,260,309 | | |
$ | 2.38 | |
| $ | 3.01 | | |
$ | 5.00 | | |
| 2,017,500 | | |
| 4.07 | | |
$ | 3.28 | | |
| 2,017,500 | | |
$ | 3.28 | |
| $ | 5.01 | | |
$ | 9.00 | | |
| 5,375,054 | | |
| 6.00 | | |
$ | 6.91 | | |
| 3,329,970 | | |
$ | 6.11 | |
| $ | 9.01 | | |
$ | 13.00 | | |
| 1,909,000 | | |
| 8.10 | | |
$ | 10.53 | | |
| 496,083 | | |
$ | 11.36 | |
| $ | 13.01 | | |
$ | 25.00 | | |
| 1,547,500 | | |
| 7.23 | | |
$ | 18.24 | | |
| 559,584 | | |
$ | 18.64 | |
| | | | |
| | | |
| 14,109,363 | | |
| 5.79 | | |
$ | 7.08 | | |
| 9,663,446 | | |
$ | 5.26 | |
|
| Schedule of general and administrative expenses and research and development expenses |
| Schedule of general and administrative expenses and research and development expenses | |
| | | |
| | |
| | |
December 31 |
| | |
2023 | |
2022 |
| General and administrative | |
$ | 926 | | |
$ | 1,743 | |
| Research and development | |
| 1,360 | | |
| 3,604 | |
| Total stock-based compensation | |
$ | 2,286 | | |
$ | 5,347 | |
|
| Schedule of weighted average assumptions |
| Schedule of weighted average
assumptions | |
| | | |
| | |
| | |
2023 | |
2022 |
| Risk-free interest rate | |
| 4.47 | % | |
| 4.07 | % |
| Expected life of options (years) | |
| 5.29 | | |
| 4.98 | |
| Annualized volatility | |
| 80.61 | % | |
| 84.01 | % |
| Dividend rate | |
| 0.00 | % | |
| 0.00 | % |
|
| X |
- References
+ Details
| Name: |
avxl_ScheduleOfSharePurchaseWarrantsOutstandingTableTextBlock |
| Namespace Prefix: |
avxl_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of defined contribution pension plans or defined contribution other postretirement plans, separately for pension plans and other postretirement benefit plans. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 715
-SubTopic 70
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480794/715-70-50-1
| Name: |
us-gaap_DefinedContributionPlanDisclosuresTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of lessee's lease cost. Includes, but is not limited to, interest expense for finance lease, amortization of right-of-use asset for finance lease, operating lease cost, short-term lease cost, variable lease cost and sublease income. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_LeaseCostTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of allocation of amount expensed and capitalized for award under share-based payment arrangement to statement of income or comprehensive income and statement of financial position. Includes, but is not limited to, corresponding line item in financial statement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (h)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfEmployeeServiceShareBasedCompensationAllocationOfRecognizedPeriodCostsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure for stock option plans. Includes, but is not limited to, outstanding awards at beginning and end of year, grants, exercises, forfeitures, and weighted-average grant date fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the significant assumptions used during the year to estimate the fair value of stock options, including, but not limited to: (a) expected term of share options and similar instruments, (b) expected volatility of the entity's shares, (c) expected dividends, (d) risk-free rate(s), and (e) discount for post-vesting restrictions. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (f)(2)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedPaymentAwardStockOptionsValuationAssumptionsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the change in stock options.
| Name: |
us-gaap_ScheduleOfStockOptionsRollForwardTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
| X |
- DefinitionSecurities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) or earnings per unit (EPU) in the future that were not included in the computation of diluted EPS or EPU because to do so would increase EPS or EPU amounts or decrease loss per share or unit amounts for the period presented. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Other Income (Details Narrative) - USD ($)
|
3 Months Ended |
12 Months Ended |
Dec. 31, 2023 |
Dec. 31, 2022 |
Sep. 30, 2023 |
Sep. 30, 2021 |
| Research and development incentive income |
$ 600,000
|
$ 700,000
|
|
|
| Grant income |
0
|
25,000
|
|
|
| Grant income, amount |
900,000
|
|
$ 900,000
|
|
| Incentive and tax receivables |
3,300,000
|
|
2,500,000
|
|
| Australia, Dollars |
|
|
|
|
| Research and development incentive income |
900,000
|
$ 1,100,000
|
|
|
| Incentive and tax receivables |
4,900,000
|
|
3,900,000
|
|
| Anavex [Member] |
|
|
|
|
| Research and development incentive income |
|
|
$ 500,000
|
$ 500,000
|
| Michael J Fox [Member] |
|
|
|
|
| Research and development incentive income |
$ 1,000,000
|
|
|
|
| X |
- References
+ Details
| Name: |
avxl_DeferredGrantIncome |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
avxl_IncentiveAndTaxReceivables |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
avxl_NonOperatingIncomeFromGrant |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_CurrencyAxis=currency_AUD |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=avxl_AnavexMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
Accrued liabilities (Details) - USD ($)
$ in Thousands |
Dec. 31, 2023 |
Sep. 30, 2023 |
| Payables and Accruals [Abstract] |
|
|
| Accrued clinical site and patient visits costs |
$ 2,058
|
$ 2,006
|
| Accrued compensation and benefits |
1,785
|
1,360
|
| Fixed contract accruals |
364
|
38
|
| Milestone based contract accruals |
989
|
1,267
|
| All other accrued liabilities |
2,090
|
2,624
|
| Total accrued liabilities |
$ 7,286
|
$ 7,295
|
| X |
- References
+ Details
| Name: |
avxl_AccruedClinicalSiteAndPatientVisitsCosts |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
avxl_FixedContractAccruals |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
avxl_MilestoneBasedContractAccruals |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable for incentive compensation awarded to employees and directors or earned by them based on the terms of one or more relevant arrangements. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedBonusesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable, pertaining to costs that are statutory in nature, are incurred on contractual obligations, or accumulate over time and for which invoices have not yet been received or will not be rendered. Examples include taxes, interest, rent and utilities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 210
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03.15(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_AccruedLiabilitiesCurrentAndNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of expenses incurred but not yet paid classified as other, due within one year or the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_PayablesAndAccrualsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Equity Offerings (Details Narrative) - USD ($)
|
|
3 Months Ended |
|
Feb. 03, 2023 |
Dec. 31, 2023 |
Dec. 31, 2022 |
Sep. 30, 2023 |
| Collaborative Arrangement and Arrangement Other than Collaborative [Line Items] |
|
|
|
|
| Sales of agreement amount |
|
$ 142,400,000
|
|
$ 142,400,000
|
| Fair value of the initial commitment |
|
800,000
|
|
|
| Third Party [Member] |
|
|
|
|
| Collaborative Arrangement and Arrangement Other than Collaborative [Line Items] |
|
|
|
|
| Incurred expenses |
|
$ 100,000
|
|
|
| Equity Offering Sales Agreement [Member] |
|
|
|
|
| Collaborative Arrangement and Arrangement Other than Collaborative [Line Items] |
|
|
|
|
| Number of common stock sold |
|
0
|
0
|
|
| Equity Offering Sales Agreement [Member] | Cantor Fitzgerald And Co [Member] |
|
|
|
|
| Collaborative Arrangement and Arrangement Other than Collaborative [Line Items] |
|
|
|
|
| Percentage of gross proceeds from sales |
|
3.00%
|
|
|
| Purchase Agreement 2023 [Member] | Lincoln Park Capital Fund L L C [Member] |
|
|
|
|
| Collaborative Arrangement and Arrangement Other than Collaborative [Line Items] |
|
|
|
|
| Value Of Shares Obligated To Purchase |
$ 150,000,000
|
|
|
|
| Purchase Agreement 1 [Member] | Lincoln Park Capital Fund L L C [Member] |
|
|
|
|
| Collaborative Arrangement and Arrangement Other than Collaborative [Line Items] |
|
|
|
|
| Share issued for offering, shares |
75,000
|
0
|
0
|
|
| Pro rata basic number of shares obligated to purchase |
75,000
|
|
|
|
| Proceeds from Issuance or sale of equity |
$ 150,000,000
|
|
|
|
| Amount of shares remain available |
|
$ 122,100,000
|
|
|
| X |
- References
+ Details
| Name: |
avxl_FairValueOfInitialCommitment |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
avxl_IncurredExpenses |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
avxl_NumberOfSharesObligatedToPurchaseProrataBasic |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_PercentageOfGrossProceedsFromSales |
| Namespace Prefix: |
avxl_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_SalesOfAgreementAmount |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
avxl_StockIssuedDuringPeriodValueNewIssues1 |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_ValueOfSharesObligatedToPurchase |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 808
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479402/808-10-50-1
| Name: |
us-gaap_CollaborativeArrangementsAndNoncollaborativeArrangementTransactionsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the issuance of common stock, preferred stock, treasury stock, stock options, and other types of equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ProceedsFromIssuanceOrSaleOfEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares issued or sold by the subsidiary or equity method investee per stock transaction.
| Name: |
us-gaap_SaleOfStockNumberOfSharesIssuedInTransaction |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_HealthCareOrganizationRevenueSourcesAxis=avxl_ThirdPartyMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=avxl_EquityOfferingSalesAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=avxl_CantorFitzgeraldAndCoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=avxl_PurchaseAgreement2023Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=avxl_LincolnParkCapitalFundLLCMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=avxl_PurchaseAgreement1Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of single lease cost, calculated by allocation of remaining cost of lease over remaining lease term. Includes, but is not limited to, single lease cost, after impairment of right-of-use asset, calculated by amortization of remaining right-of-use asset and accretion of lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.24.0.1
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow for pension benefit. Includes, but is not limited to, employer contribution to fund plan asset and payment to retiree. Excludes other postretirement benefit. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (g)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_PensionContributions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.24.0.1
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of fully vested and expected to vest options outstanding that can be converted into shares under option plan. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted-average exercise price, at which grantee can acquire shares reserved for issuance, for fully vested and expected to vest options outstanding. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionExpiration date of outstanding warrant and right embodying unconditional obligation requiring redemption by transferring asset at specified or determinable date or upon event certain to occur, in YYYY-MM-DD format. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_WarrantsAndRightsOutstandingMaturityDate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=avxl_PurchaseWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=avxl_PurchaseWarrants1Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
Commitments and Contingencies (Details 3) - Equity Option [Member] - USD ($)
|
3 Months Ended |
12 Months Ended |
Dec. 31, 2023 |
Sep. 30, 2023 |
| Offsetting Assets [Line Items] |
|
|
| Number of Options Outstanding at Beginning |
14,111,780
|
13,169,616
|
| Weighted Average Exercise Price Outstanding at Beginning |
$ 7.12
|
$ 6.61
|
| Weighted Average Grant Date Fair Value at Beginning |
$ 5.27
|
$ 4.96
|
| Aggregate Intrinsic Value Outstanding at Beginning |
$ 22,290,069
|
$ 62,267,309
|
| Number of Options, Granted |
155,000
|
1,959,000
|
| Weighted Average Exercise Price, Granted |
$ 7.40
|
$ 9.30
|
| Weighted Average Grant Date Fair Value, Granted |
$ 4.88
|
$ 6.60
|
| Number of Options, Exercised |
(20,000)
|
(759,753)
|
| Weighted Average Exercise Price, Exercised |
$ 2.96
|
$ 2.34
|
| Weighted Average Grant Date Fair Value, Exercised |
$ 2.23
|
$ 0.95
|
| Aggregate Intrinsic Value, Exercised |
$ 58,600
|
$ 4,629,026
|
| Number of Options, Forfeited |
(137,417)
|
(257,083)
|
| Weighted Average Exercise Price, Forfeited |
$ 12.19
|
$ 12.00
|
| Weighted Average Grant Date Fair Value, Forfeited |
$ 6.78
|
$ 6.74
|
| Number of Options Outstanding at Ending |
14,109,363
|
14,111,780
|
| Weighted Average Exercise Price Outstanding at Ending |
$ 7.08
|
$ 7.12
|
| Aggregate Intrinsic Value Outstanding at Ending |
$ 47,674,002
|
$ 22,290,069
|
| Number of Options, Exercisable |
9,663,446
|
|
| Weighted Average Exercise Price, Exercisable |
$ 5.26
|
|
| Aggregate Intrinsic Value, Exercisable |
$ 45,388,166
|
|
| X |
- References
+ Details
| Name: |
avxl_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExercisesInPeriodWeightedAverageExercisesPrice |
| Namespace Prefix: |
avxl_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsForfeituresInPeriodWeightedAverageExercisesPrice |
| Namespace Prefix: |
avxl_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_OffsettingAssetsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPer share or unit weighted-average fair value of nonvested award under share-based payment arrangement. Excludes share and unit options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of shares into which fully or partially vested stock options outstanding as of the balance sheet date can be currently converted under the option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe weighted-average price as of the balance sheet date at which grantees can acquire the shares reserved for issuance on vested portions of options outstanding and currently exercisable under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated difference between fair value of underlying shares on dates of exercise and exercise price on options exercised (or share units converted) into shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisesInPeriodTotalIntrinsicValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares under options that were cancelled during the reporting period as a result of occurrence of a terminating event specified in contractual agreements pertaining to the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average grant-date fair value of options granted during the reporting period as calculated by applying the disclosed option pricing methodology. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount by which the current fair value of the underlying stock exceeds the exercise price of options outstanding. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingIntrinsicValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which option holders acquired shares when converting their stock options into shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExercisesInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which grantees could have acquired the underlying shares with respect to stock options that were terminated. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsForfeituresInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of difference between fair value of the underlying shares reserved for issuance and exercise price of vested portions of options outstanding and currently exercisable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableIntrinsicValue1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of share options (or share units) exercised during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=us-gaap_StockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
Commitments and Contingencies (Details 4) - $ / shares
|
3 Months Ended |
12 Months Ended |
|
Dec. 31, 2023 |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Offsetting Assets [Line Items] |
|
|
|
| Weighted average remaining contractual life (in years) |
5 years 9 months 18 days
|
6 years
|
|
| Equity Option [Member] |
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
| Number of outstanding options |
14,109,363
|
14,111,780
|
13,169,616
|
| Weighted average remaining contractual life (in years) |
5 years 9 months 14 days
|
|
|
| Weighted average exercise price |
$ 7.08
|
$ 7.12
|
$ 6.61
|
| Number of vested options |
9,663,446
|
|
|
| Weighted average exercise price options vested |
$ 5.26
|
|
|
| Equity Option [Member] | Option Price 1 [Member] |
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
| Range of exercise prices, lower range limit |
0.92
|
|
|
| Range of exercise prices, upper range limit |
$ 3.00
|
|
|
| Number of outstanding options |
3,260,309
|
|
|
| Weighted average remaining contractual life (in years) |
4 years 5 months 19 days
|
|
|
| Weighted average exercise price |
$ 2.38
|
|
|
| Number of vested options |
3,260,309
|
|
|
| Weighted average exercise price options vested |
$ 2.38
|
|
|
| Equity Option [Member] | Option Price 2 [Member] |
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
| Range of exercise prices, lower range limit |
3.01
|
|
|
| Range of exercise prices, upper range limit |
$ 5.00
|
|
|
| Number of outstanding options |
2,017,500
|
|
|
| Weighted average remaining contractual life (in years) |
4 years 25 days
|
|
|
| Weighted average exercise price |
$ 3.28
|
|
|
| Number of vested options |
2,017,500
|
|
|
| Weighted average exercise price options vested |
$ 3.28
|
|
|
| Equity Option [Member] | Option Price 3 [Member] |
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
| Range of exercise prices, lower range limit |
5.01
|
|
|
| Range of exercise prices, upper range limit |
$ 9.00
|
|
|
| Number of outstanding options |
5,375,054
|
|
|
| Weighted average remaining contractual life (in years) |
6 years
|
|
|
| Weighted average exercise price |
$ 6.91
|
|
|
| Number of vested options |
3,329,970
|
|
|
| Weighted average exercise price options vested |
$ 6.11
|
|
|
| Equity Option [Member] | Option Price 4 [Member] |
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
| Range of exercise prices, lower range limit |
9.01
|
|
|
| Range of exercise prices, upper range limit |
$ 13.00
|
|
|
| Number of outstanding options |
1,909,000
|
|
|
| Weighted average remaining contractual life (in years) |
8 years 1 month 6 days
|
|
|
| Weighted average exercise price |
$ 10.53
|
|
|
| Number of vested options |
496,083
|
|
|
| Weighted average exercise price options vested |
$ 11.36
|
|
|
| Equity Option [Member] | Option Price 5 [Member] |
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
| Range of exercise prices, lower range limit |
13.01
|
|
|
| Range of exercise prices, upper range limit |
$ 25.00
|
|
|
| Number of outstanding options |
1,547,500
|
|
|
| Weighted average remaining contractual life (in years) |
7 years 2 months 23 days
|
|
|
| Weighted average exercise price |
$ 18.24
|
|
|
| Number of vested options |
559,584
|
|
|
| Weighted average exercise price options vested |
$ 18.64
|
|
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_OffsettingAssetsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted-average exercise price, at which grantee can acquire shares reserved for issuance, for fully vested and expected to vest exercisable or convertible options. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestExercisableWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe floor of a customized range of exercise prices for purposes of disclosing shares potentially issuable under outstanding stock option awards on all stock option plans and other required information pertaining to awards in the customized range. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansExercisePriceRangeLowerRangeLimit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe ceiling of a customized range of exercise prices for purposes of disclosing shares potentially issuable under outstanding stock option awards on all stock option plans and other required information pertaining to awards in the customized range. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansExercisePriceRangeUpperRangeLimit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average remaining contractual term for option awards outstanding, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (e)(1)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsOutstandingWeightedAverageRemainingContractualTerm2 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of options vested.
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsVestedNumberOfShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=us-gaap_StockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=avxl_OptionPrice1Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=avxl_OptionPrice2Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=avxl_OptionPrice3Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=avxl_OptionPrice4Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=avxl_OptionPrice5Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 460
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482425/460-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 450
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483076/450-20-50-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483076/450-20-50-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 450
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483076/450-20-50-4
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483076/450-20-50-9
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 450
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483076/450-20-50-9
| Name: |
us-gaap_LossContingenciesLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_GeneralAndAdministrativeExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_ResearchAndDevelopmentExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated measure of the percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe risk-free interest rate assumption that is used in valuing an option on its own shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Commitments and Contingencies (Details Narrative) - USD ($)
$ / shares in Units, $ in Millions |
3 Months Ended |
12 Months Ended |
Dec. 31, 2023 |
Dec. 31, 2022 |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
| Warrants outstanding |
160,000
|
|
160,000
|
|
| Warrants outstanding weighted average exercise |
$ 3.72
|
|
$ 3.72
|
|
| Option issued |
3,892,000
|
|
|
|
| Weighted average grant date fair value of options vested |
$ 4.00
|
|
$ 3.94
|
|
| Weighted average contractual life of options outstanding |
5 years 9 months 18 days
|
|
6 years
|
|
| Weighted average contractual life of options exercisable |
4 years 6 months
|
|
4 years 9 months
|
|
| Share based compensation expense |
$ 2.3
|
$ 5.3
|
|
|
| Remaining stock based compensation |
$ 27.7
|
|
|
|
| Stock Option Plan 2015 [Member] |
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
| Common shares reserved for future issuance |
6,050,553
|
|
|
|
| Stock Option Plan 2019 [Member] |
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
| Additional shares of common stock available for issuance |
6,000,000
|
|
|
|
| Stock Option Plan 2022 [Member] |
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
| Additional shares of common stock available for issuance |
10,000,000
|
|
|
|
| Option granted |
|
|
|
406,453
|
| Option available issuance |
6,643,952
|
|
|
|
| X |
- References
+ Details
| Name: |
avxl_OptionIssued |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_WarrantsOutstandingWeightedAverageExercise |
| Namespace Prefix: |
avxl_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of expense for award under share-based payment arrangement. Excludes amount capitalized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.F)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_AllocatedShareBasedCompensationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of warrants or rights outstanding.
| Name: |
us-gaap_ClassOfWarrantOrRightOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate number of common shares reserved for future issuance. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.29)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockCapitalSharesReservedForFutureIssuance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cost to be recognized for option under share-based payment arrangement. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedStockOptions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe weighted average fair value as of grant date pertaining to an equity-based award plan other than a stock (or unit) option plan for which the grantee gained the right during the reporting period, by satisfying service and performance requirements, to receive or retain shares or units, other instruments, or cash in accordance with the terms of the arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of additional shares authorized for issuance under share-based payment arrangement.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfAdditionalSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe difference between the maximum number of shares (or other type of equity) authorized for issuance under the plan (including the effects of amendments and adjustments), and the sum of: 1) the number of shares (or other type of equity) already issued upon exercise of options or other equity-based awards under the plan; and 2) shares (or other type of equity) reserved for issuance on granting of outstanding awards, net of cancellations and forfeitures, if applicable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAvailableForGrant |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNet number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average remaining contractual term for option awards outstanding, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (e)(1)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsOutstandingWeightedAverageRemainingContractualTerm2 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average remaining contractual term of exercisable stock options, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (e)(2)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationSharesAuthorizedUnderStockOptionPlansExercisePriceRangeExercisableOptionsWeightedAverageRemainingContractualTerm2 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=avxl_StockOptionPlan2015Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=avxl_StockOptionPlan2019Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=avxl_StockOptionPlan2022Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Grafico Azioni Anavex Life Sciences (NASDAQ:AVXL)
Storico
Da Ott 2024 a Nov 2024
Grafico Azioni Anavex Life Sciences (NASDAQ:AVXL)
Storico
Da Nov 2023 a Nov 2024