0001293971FALSE00012939712024-01-082024-01-08
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
__________________________
FORM 8-K
__________________________
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 8, 2024
__________________________
bluebird bio, Inc.
(Exact name of Registrant as Specified in Its Charter)
_____________________________________________________________ | | | | | | | | |
| Delaware | 001-35966 | 13-3680878 |
(State or Other Jurisdiction
of Incorporation) | (Commission File Number) | (IRS Employer
Identification No.) |
| | |
455 Grand Union Boulevard, Somerville, MA | | 02145 |
| (Address of Principal Executive Offices) | | (Zip Code) |
(339) 499-9300
(Registrant’s telephone number, including area code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
_____________________________________________________________
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instructions A.2. below): | | | | | |
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act: | | | | | | | | |
| Title of each class | Trading
Symbol(s) | Name of each exchange on which registered |
| Common Stock, $0.01 par value per share | BLUE | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| | | | | |
Item 2.02 | Results of Operations and Financial Condition. |
On January 8, 2024, bluebird bio, Inc. (the “Company”) announced that as of December 31, 2023, the Company’s cash, cash equivalents and marketable securities balance was approximately $275 million, including restricted cash of approximately $53 million. A copy of the press release containing this announcement is furnished as Exhibit 99.1 to this Current Report on Form 8-K (the “Current Report”).
The cash, cash equivalents and marketable securities information above is based on preliminary unaudited information and management estimates for the year ended December 31, 2023, is not a comprehensive statement of the Company’s financial results as of and for the fiscal year ended December 31, 2023, and is subject to completion of the Company’s financial closing procedures. The Company’s independent registered public accounting firm has not conducted an audit or review of, and does not express an opinion or any other form of assurance with respect to, this preliminary estimate.
| | | | | |
Item 7.01 | Regulation FD Disclosure. |
The Company plans to present a corporate update on January 9, 2024 at the 2024 J.P. Morgan Healthcare Conference. A copy of the presentation that will be used is furnished as Exhibit 99.2 to this Current Report.
The information contained in Items 2.02 and 7.01 of this Current Report, including Exhibits 99.1 and 99.2 attached hereto, is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act or the Exchange Act except as expressly set forth by specific reference in such filing.
| | | | | |
Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
| | | | | | | | |
Exhibit
No. | | Description |
| 99.1 | | |
| 99.2 | | |
| 104 | | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
| | |
Forward-Looking Statements
This Current Report contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this Current Report that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation statements regarding the Company’s preliminary unaudited cash position as of December 31, 2023. Statements using words such as “expect”, “anticipate”, “believe”, “may”, “will” and similar terms are also forward-looking statements. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause the Company’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the important factors discussed under the caption “Risk Factors” in the Company’s Annual Report on Form 10-K for the year ended December 31, 2022, as updated by its subsequent Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings with the Securities and Exchange Commission. Except as required by law, the Company undertakes no obligations to make any revisions to the forward-looking statements contained in this Current Report or to update them to reflect events or circumstances occurring after the date of this Current Report, whether as a result of new information, future developments or otherwise.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | | | | | | | | | | |
| | | |
| Date: January 8, 2024 | bluebird bio, Inc.
|
| | | |
| By: | /s/ Joseph Vittiglio |
| Name: | Joseph Vittiglio |
| Title: | Chief Legal & Business Officer and Secretary |
Exhibit 99.1
bluebird bio Provides Update on Commercial Launch Progress, Program Milestones,
and 2024 Financial Outlook
Significant momentum behind LYFGENIA launch with 35 Qualified Treatment Centers accepting patient referrals and payer agreements in place covering approximately 200 million U.S. lives
Anticipate 85 to 105 patient starts (cell collections) across commercial portfolio in 2024 with first patient start for LYFGENIA expected in Q1 2024
Cash, cash equivalents and marketable securities expected to fund current operations into Q1 2025
SOMERVILLE, Mass.—(BUSINESS WIRE)—Jan. 8, 2024—bluebird bio, Inc. (Nasdaq: BLUE) (the Company; bluebird) today announced updates to be presented at the 42nd Annual J.P. Morgan Healthcare conference including commercial launch progress, 2024 program milestones and financial outlook. Andrew Obenshain, chief executive officer, is scheduled to present Tuesday, January 9 at 10:30 a.m. PT/1:30 p.m. ET.
“In 2023 bluebird cemented our status as a gene therapy leader, securing our third FDA approval in under two years and establishing a commercial footprint that will support growth in the coming year and beyond,” said Andrew Obenshain, chief executive officer, bluebird bio. “In 2024 we are leveraging our validated commercial strategy to accelerate the launch of LYFGENIA and drive continued strong uptake for ZYNTEGLO. We are extremely pleased with the indicators of demand from both patients and providers in the weeks following FDA approval of LYFGENIA and are focused on using our real-world experience to support timely and equitable access and deliver a positive treatment experience.”
Highlights from the Company’s update include:
Synergies with ZYNTEGLO commercial network are accelerating LYFGENIA commercial launch in 2024
•Established Qualified Treatment Center (QTC) network in place, with 48 centers activated for ZYNTEGLO for beta-thalassemiai as of January 5, 2024.
•35 of 48 centers ready to receive referrals for LYFGENIA for sickle cell disease as of January 5, 2024.
•All centers are anticipated to be ready to treat with both ZYNTEGLO and LYFGENIA by end of Q1 2024.
Validated access and reimbursement strategy is driving favorable coverage landscape for LYFGENIA and ZYNTEGLO
•bluebird has signed outcomes-based agreements for LYFGENIA with national payer organizations representing dozens of downstream plans and covering approximately 200 million U.S. lives.
•Advanced discussions are ongoing with additional commercial payers and with more than 15 Medicaid agencies representing 80% of individuals with sickle cell disease in the U.S.
•bluebird has designed outcomes-based agreements that are unique to LYFGENIA and offer payers meaningful risk sharing tied to VOE-related hospitalizations, with patients followed for three years. Informed by input from state Medicaid agencies, the Company has designed an
offering specifically for Medicaid that addresses the need for predictability and operational ease that is essential for states grappling with resource constraints.
•Outcomes-based agreements are in place for ZYNTEGLO with both commercial and Medicaid payers, and more than 200 million U.S. lives are covered through contracts or favorable coverage policies. Timely access to ZYNTEGLO for people living with beta-thalassemia continues, with zero ultimate denials across both Medicaid and commercial payers.
•bluebird also continues to engage with Center for Medicare and Medicaid Innovation (CMMI) on its Cell and Gene Therapy Access Model, which is anticipated to be implemented in 2025.
Strong commercial momentum is poised to translate into sustained revenue recognition
•26 patient starts were completed in 2023 across bluebird’s commercial portfolio, including 20 for ZYNTEGLO and 6 for SKYSONA. 2023 patient starts will drive revenue recognition in 2024 as patients complete the gene therapy treatment journey.
•bluebird anticipates the first patient start for LYFGENIA in Q1 2024.
•The Company anticipates 85 to 105 patient starts combined across all three of its FDA approved therapies (LYFGENIA, ZYNTEGLO, SKYSONA) in 2024.
Liquidity and Cash Runway Update
The Company’s preliminary unaudited cash and cash equivalents and marketable securities balance was approximately $275 million, including restricted cash of approximately $53 million, as of December 31, 2023. bluebird expects its cash, cash equivalents, and marketable securities, excluding restricted cash, will be sufficient to meet bluebird’s planned operating expenses and capital expenditure requirements into the first quarter of 2025 as bluebird progresses its launch of LYFGENIA gene therapy for sickle cell disease and continues to scale its launches of ZYNTEGLO and SKYSONA for beta-thalassemia and cerebral adrenoleukodystrophy, respectively.
The Company has taken additional steps to strengthen its financial position by entering into an accounts receivable factoring agreement which will accelerate cash collection related to patient starts across its portfolio of approved therapies.
Presentation at the 2023 J.P. Morgan Healthcare Conference
Andrew Obenshain, chief executive officer, bluebird bio, will present a corporate update on Tuesday, January 9 at 10:30 a.m. PT/1:30 p.m. ET. A live webcast of the presentation will be available on the “Events & Presentations” page within the Investors & Media section of the bluebird bio website at http://investor.bluebirdbio.com. A replay of the webcast will be available on the bluebird bio website for 30 days following the event.
About bluebird bio
bluebird bio is pursuing curative gene therapies to give patients and their families more bluebird days.
Founded in 2010, bluebird has been setting the standard for gene therapy for more than a decade—first as a scientific pioneer and now as a commercial leader. bluebird has an unrivaled track record in bringing the promise of gene therapy out of clinical studies and into the real-world setting, having secured FDA
approvals for three therapies in under two years. Today, we are proving and scaling the commercial model for gene therapy and delivering innovative solutions for access to patients, providers, and payers.
With a dedicated focus on severe genetic diseases, bluebird has the largest and deepest ex-vivo gene therapy data set in the field, with industry-leading programs for sickle cell disease, β-thalassemia and cerebral adrenoleukodystrophy. We custom design each of our therapies to address the underlying cause of disease and have developed in-depth and effective analytical methods to understand the safety of our lentiviral vector technologies and drive the field of gene therapy forward.
bluebird continues to forge new paths as a standalone commercial gene therapy company, combining our real-world experience with a deep commitment to patient communities and a people-centric culture that attracts and grows a diverse flock of dedicated birds.
bluebird bio Cautionary Statement Regarding Forward-Looking Statements
This press release contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. All statements that are not statements of historical facts are, or may be deemed to be, forward-looking statements, such as statements regarding the number of anticipated patient starts across bluebird’s portfolio of therapies, the Company’s anticipated cash runway, the Company’s expectations with respect to the commercialization of LYFGENIA, including without limitation, patient demand for the therapy, bluebird’s ability to establish commercial infrastructure to support timely, equitable access to LYFGENIA, its ability to successfully partner with payers and CMMI, its expectations on timing for activating QTCs, its expectations on the timing and size of its QTC network and the timing of LYFGENIA’s availability at its QTCs. Such forward-looking statements are based on historical performance and current expectations and projections about bluebird’s future goals, plans and objectives and involve inherent risks, assumptions and uncertainties, including internal or external factors that could delay, divert or change any of them in the next several years, that are difficult to predict, may be beyond bluebird’s control and could cause bluebird’s future goals, plans and objectives to differ materially from those expressed in, or implied by, the statements. No forward-looking statement can be guaranteed. Forward-looking statements in this press release should be evaluated together with the many risks and uncertainties that affect bluebird bio’s business, particularly those identified in the risk factors discussion in bluebird bio’s Annual Report on Form 10-K for the year ended December 31, 2022, as updated by its subsequent Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings with the Securities and Exchange Commission. These risks and uncertainties include, but are not limited to: delays and challenges in bluebird’s commercialization and manufacturing of its products; the internal and external costs required for bluebird’s ongoing and planned activities, and the resulting impact on expense and use of cash, has been, and may in the future be, higher than expected which has caused bluebird, and may in the future cause bluebird to use cash more quickly than it expects or change or curtail some of its plans or both; substantial doubt exists regarding bluebird’s ability to continue as a going concern; bluebird’s expectations as to expenses, cash usage and cash needs may prove not to be correct for other reasons such as changes in plans or actual events being different than bluebird’s assumptions; the risk that the efficacy and safety results from bluebird’s prior and ongoing clinical trials will not continue or be seen in the commercial context; the risk that bluebird is not able to activate QTCs on the timeframe that it expects; the risk that the QTCs experience delays in their ability to enroll or treat patients; the risk that bluebird experiences delays in establishing operational readiness across its supply chain following approval to support treatment in the commercial context; the risk that there is not sufficient patient demand or payer reimbursement to
support continued commercialization of LYFGENIA; the risk of insertional oncogenic or other safety events associated with lentiviral vector, drug product, or myeloablation, including the risk of hematologic malignancy; and the risk that bluebird’s products, including LYFGENIA, will not be successfully commercialized. The forward-looking statements included in this document are made only as of the date of this document and except as otherwise required by applicable law, bluebird bio undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events, changed circumstances or otherwise.
Financial Disclosure Advisory
The cash and cash equivalents and marketable securities balance disclosed in this press release is based on preliminary unaudited information and management estimates for the year ended December 31, 2023, is not a comprehensive statement of the Company’s financial results as of and for the fiscal year ended December 31, 2023, and is subject to completion of the Company’s financial closing procedures. The Company’s independent registered public accounting firm has not conducted an audit or review of, and does not express an opinion or any other form of assurance with respect to, this preliminary estimate.
Investors:
Courtney O’Leary, 978-621-7347
coleary@bluebirdbio.com
Media:
Jess Rowlands, 857-299-6103
jess.rowlands@bluebirdbio.com
i Defined as signed MSA

bluebird bio J.P. Morgan Presentation January 2024 NASDAQ: BLUE Exhibit 99.2

CONFIDENTIAL forward-looking statements 2 These slides and the accompanying oral presentation contain forward-looking statements and information. The use of words such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” or “continue,” and other similar expressions are intended to identify forward-looking statements. For example, all statements we make regarding our expectations regarding our programs and therapies, including but not limited to the timing or likelihood of regulatory filings and approvals; our manufacturing and commercialization plans, including without limitation, patient demand for our therapies, our ability to establish commercial infrastructure to support timely, equitable access to our therapies, our ability to successfully partner with payers and CMMI, our expectations on timing for activating QTCs, and our expectations on the timing and size of our QTC network and the timing of our therapies’ availability at our QTCs; addressable market for our therapies; our preliminary unaudited cash position as of December 31, 2023; and our cash runway are forward looking. All forward-looking statements are based on estimates and assumptions by our management that, although we believe to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected. These statements are also subject to a number of material risks and uncertainties that are described in our most recent quarterly report on Form 10-Q, as well as our subsequent filings with the Securities and Exchange Commission. Any forward-looking statement speaks only as of the date on which it was made. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law.

CONFIDENTIAL 3 to give patients and their families more bluebird days

CONFIDENTIAL Only commercial gene therapy company with three FDA approved products 4

CONFIDENTIAL Momentum building with commercial launches; opportunity to deliver significant value for patients and shareholders 5 1.3K – 1.5K Patients potentially eligible for ZYNTEGLO™ for beta-thalassemia 40 Patients potentially eligible for SKYSONA™ for cerebral adrenoleukodystrophy F U TU RE G ROW TH CO M M ER CI AL O PP O RT U N IT Y NUMBER OF PATIENTS 20K Patients potentially eligible for LYFEGNIA™ for sickle cell disease
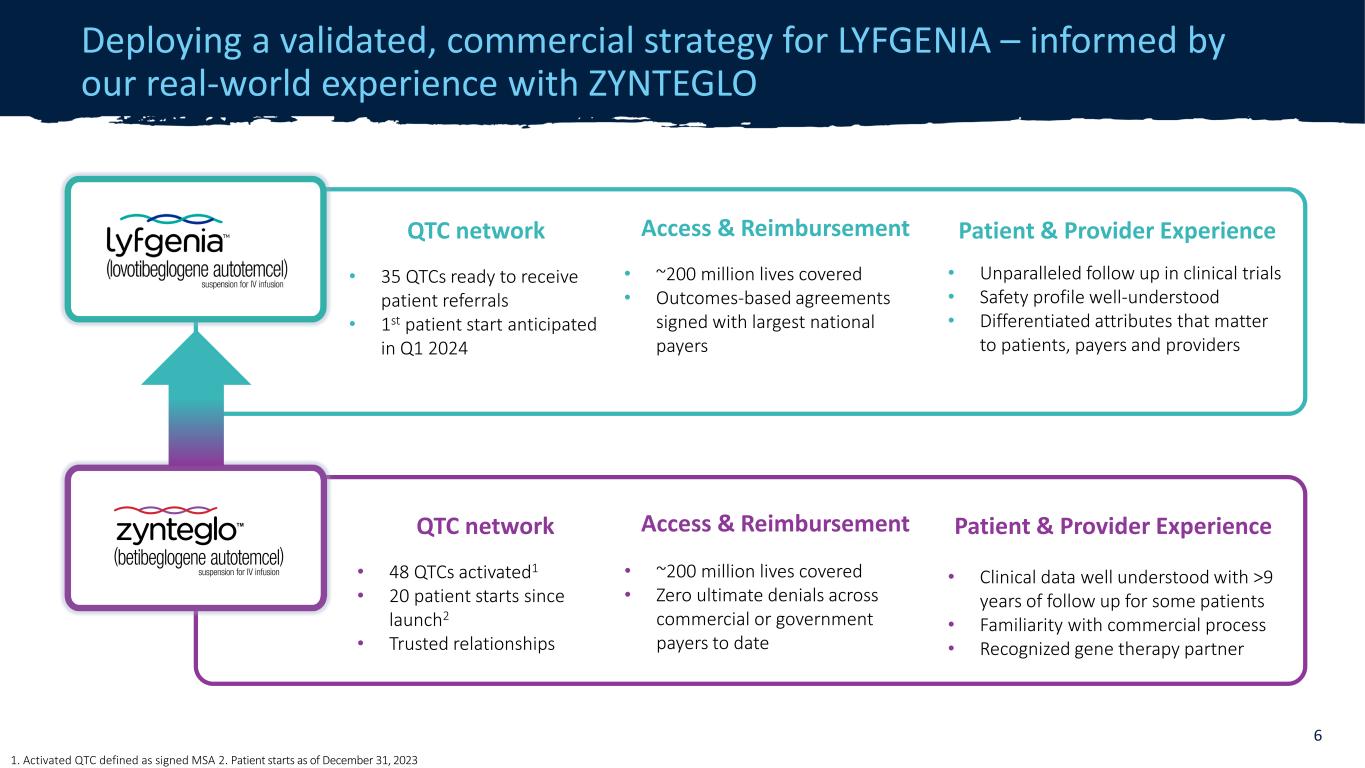
CONFIDENTIAL Access & Reimbursement • ~200 million lives covered • Outcomes-based agreements signed with largest national payers Patient & Provider Experience • Unparalleled follow up in clinical trials • Safety profile well-understood • Differentiated attributes that matter to patients, payers and providers QTC network • 35 QTCs ready to receive patient referrals • 1st patient start anticipated in Q1 2024 Deploying a validated, commercial strategy for LYFGENIA – informed by our real-world experience with ZYNTEGLO 6 QTC network • 48 QTCs activated1 • 20 patient starts since launch2 • Trusted relationships Access & Reimbursement • ~200 million lives covered • Zero ultimate denials across commercial or government payers to date Patient & Provider Experience • Clinical data well understood with >9 years of follow up for some patients • Familiarity with commercial process • Recognized gene therapy partner 1. Activated QTC defined as signed MSA 2. Patient starts as of December 31, 2023

7 QTC Network

CONFIDENTIAL 48 Qualified Treatment Centers (QTCs) activated for ZYNTEGLO and quickly onboarding for LYFGENIA 8 35 QTCs are ready to receive SCD patient referrals now Activated ZYNTEGLO QTC* Shading indicates target SCD market Planned QTC network expansion in 2024 100% of ZYNTEGLO QTCs have initiated the activation process for LYFGENIA Anticipate ZYNTEGLO network will be fully activated for LYFGENIA by end of Q1 2024 *Activated QTC defined as signed MSA

CONFIDENTIAL Optimized QTC network designed to reach individuals living with SCD 9 1. Potential addressable population of 20,000; 2. 70% figure supported by seven years of internal and external market research; 3. Data on file; 4. Komodo claims data STRONG DEMAND FOR GENE THERAPY POISED TO MEET PATIENTS WHERE THEY ARE 95% of SCD patients2 are within 200 miles of a planned QTC3 88% of target SCD patients are actively being treated in the healthcare system4 80% of providers want both LYFEGNIA and its competitor available at their institution3 >70% of SCD patients1 would consider gene therapy if recommended by their doctor2

Access & Reimbursement 10

CONFIDENTIAL Value of ZYNTEGLO is recognized 11 ~200M lives covered under contract or coverage policy ~90% published coverage policies positive for ZYNTEGLO ZERO ultimate denials to date across commercial and government payers Patients with beta-thalassemia are achieving access

CONFIDENTIAL Validated access and reimbursement strategy designed to enable timely, equitable access to LYFGENIA for sickle cell disease 12 Signed outcomes-based agreements representing ~200 million covered lives Advanced discussions with >15 Medicaid agencies representing ~80% of individuals with SCD in the US1 Active engagement with CMMI on innovative payment demonstration (anticipated 2025) Encouraging payer interactions Demonstrated robust and sustained clinical benefit (out more than 5 years) Reflects lifetime impact of reducing or eliminating VOEs • Healthcare utilization • Future earnings • Life opportunities $3.1M price tied to value Meaningful risk sharing Tied to VOE related hospitalizations Patients followed for 3 years Commercial payer and Medicaid options designed to offer predictability and operational ease Outcomes-based agreement offerings 1. Data based on Prevalence rates of SCD per 1,000 Medicaid Beneficiaries in 2012.

13 Treatment Experience
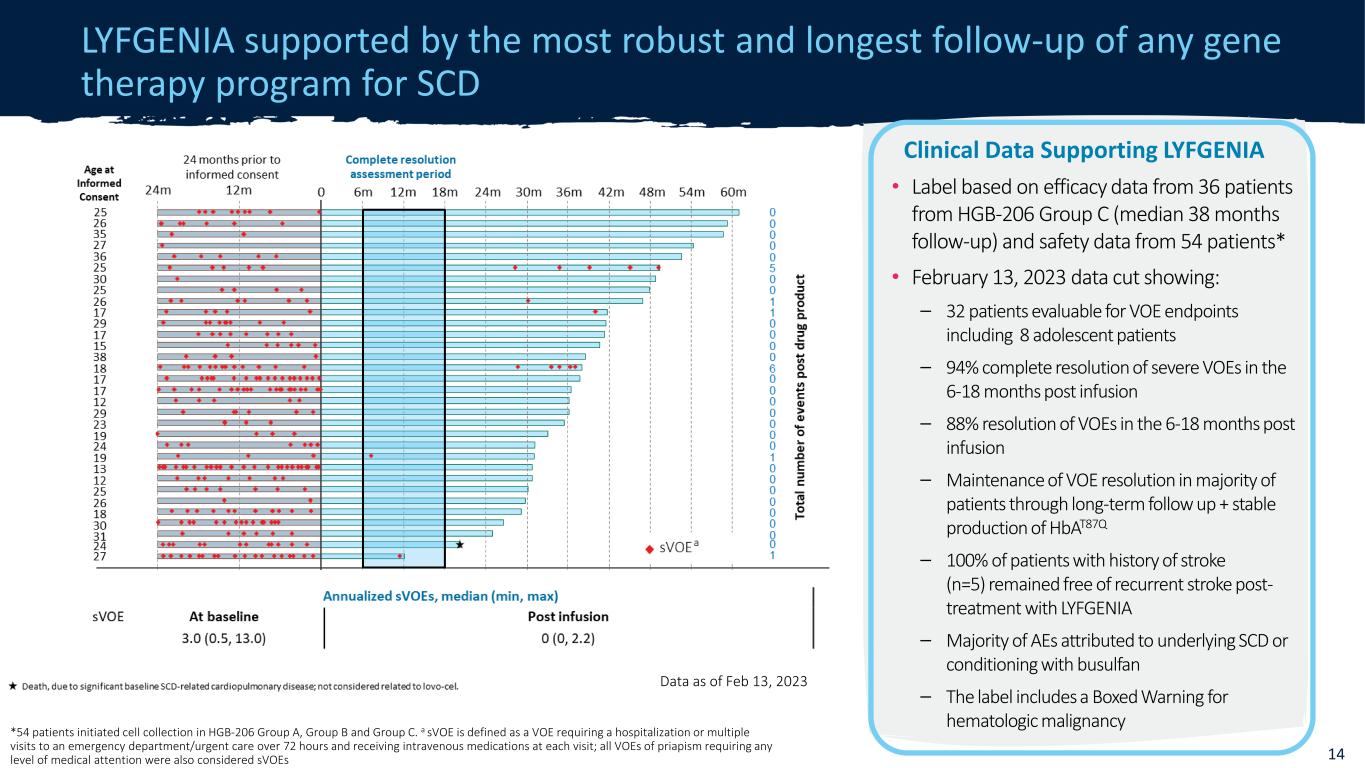
CONFIDENTIAL LYFGENIA supported by the most robust and longest follow-up of any gene therapy program for SCD Data as of Feb 13, 2023 14 *54 patients initiated cell collection in HGB-206 Group A, Group B and Group C. a sVOE is defined as a VOE requiring a hospitalization or multiple visits to an emergency department/urgent care over 72 hours and receiving intravenous medications at each visit; all VOEs of priapism requiring any level of medical attention were also considered sVOEs Clinical Data Supporting LYFGENIA • Label based on efficacy data from 36 patients from HGB-206 Group C (median 38 months follow-up) and safety data from 54 patients* • February 13, 2023 data cut showing: – 32 patients evaluable for VOE endpoints including 8 adolescent patients – 94% complete resolution of severe VOEs in the 6-18 months post infusion – 88% resolution of VOEs in the 6-18 months post infusion – Maintenance of VOE resolution in majority of patients through long-term follow up + stable production of HbAT87Q – 100% of patients with history of stroke (n=5) remained free of recurrent stroke post- treatment with LYFGENIA – Majority of AEs attributed to underlying SCD or conditioning with busulfan – The label includes a Boxed Warning for hematologic malignancy
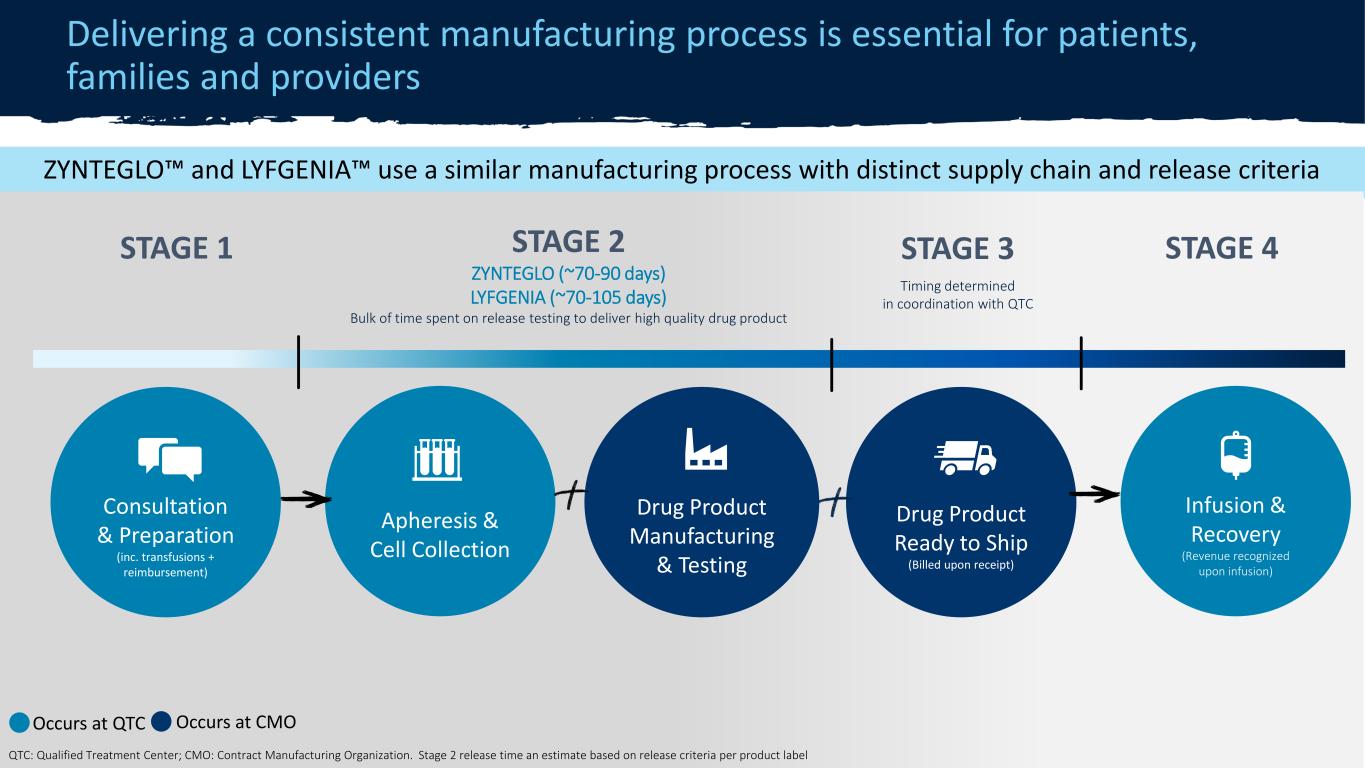
CONFIDENTIAL ZYNTEGLO® manufacturing process Delivering a consistent manufacturing process is essential for patients, families and providers 15 STAGE 2◇ 70-90 days Bulk of time spent on release testing to deliver high quality drug product Apheresis & Cell Collection Drug Product Manufacturing & Testing Drug Product Ready to Ship (QTC billed) Infusion & Recovery (Revenue recognized upon infusion) Consultation & Preparation (inc. hyper transfusions + reimbursement confirmation) STAGE 1* STAGE 3* *Occurs at QTC ◇ Occurs at CMO QTC: Qualified Treatment Center; CMO: Contract Manufacturing Organization In clinical trials, recollections occurred in approximately 20% of cases. In commercial setting, all patients have [completed] the treatment process following recollection. ZYNTEGLO™ and LYFGENIA™ use a similar manufacturing process with distinct supply chain and release criteria STAGE 2 ZYNTEGLO (~70-90 days) LYFGENIA (~70-105 days) Bulk of time spent on release testing to deliver high quality drug product (Billed upon r ceipt) Consultation & Preparation (inc. transfusions + reimbursement) STAGE 1 STAGE 4 Occurs at QTC Occurs at CMO QT lified Treatment C nter; CMO: Contr ct Manufacturi g Organization. Stage 2 release time an estimate based on release criteria per product label STAGE 3 Timing determined in oordination with QTC

CONFIDENTIAL Differentiated attributes of LYFGENIA that matter to patients, payers and providers 16 Underpinned by clinical attributes – including >5 years of follow up, in-depth safety analyses, and data addressing SCD complications, including stroke Cell collections In clinical trials, 85% required <2 cell collections for LYFGENIA1 Drug product delivery Process is designed to take between 70-105 days from cell collection to drug product delivery to the QTC Engraftment time Median time to neutrophil engraftment 20 days, a key step to enabling patient discharge 1. ASH 2023
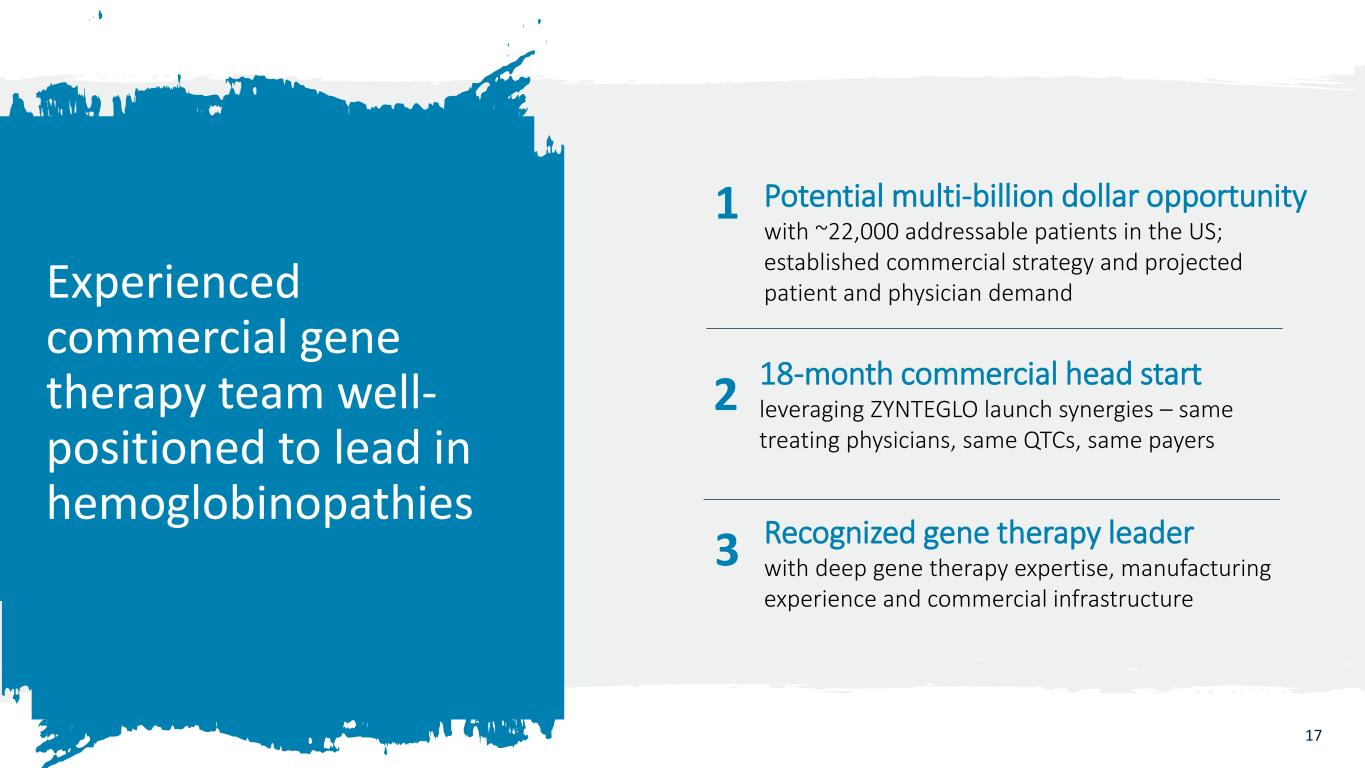
17 2 Potential multi-billion dollar opportunity with ~22,000 addressable patients in the US; established commercial strategy and projected patient and physician demandExperienced commercial gene therapy team well- positioned to lead in hemoglobinopathies 18-month commercial head start leveraging ZYNTEGLO launch synergies – same treating physicians, same QTCs, same payers 1 3 Recognized gene therapy leader with deep gene therapy expertise, manufacturing experience and commercial infrastructure

SKYSONA™ 18

CONFIDENTIALI SKYSONA™ for cerebral adrenoleukodystrophy Commercial • SKYSONA is indicated to slow the progression of neurologic dysfunction in boys 4-17 years of age with early, active cerebral adrenoleukodystrophy (CALD) • 6 patient starts since launch*; 4 QTCs activated; zero ultimate denials across government and commercial payers Clinical • 67 patients treated across all clinical trials • Accelerated approval based on post-hoc analysis of 11 patients; estimated 72% likelihood of major functional disability free survival at 24 months • Five boys treated in clinical trials developed myelodysplastic syndrome; label includes boxed warning for hematologic malignancy** 21Patient starts is defined as a cell collection (apheresis); Activated QTC defined as Qualified Treatment Center with a signed MSA. *Patient starts as of December 31, 2023 **bluebird closely monitors potential and diagnosed cases of hematologic malignancy in patients treated with SKYSONA and additional cases are expected to arise over time. bluebird is communicating regularly with treating physicians and regulatory authorities.

Closing 20

CONFIDENTIAL Established gene therapy leader poised to deliver shareholder value 21 Established Clinical Leadership • 10+ years of gene therapy research • 200+ patients treated • 8 clinical trials Demonstrated Regulatory Success • Established track record for LVV platform • 3 FDA-approved gene therapies Commercial Gene Therapy Leader • Scaled for 3 commercial launches • Synergistic transplant and cell therapy infrastructure • Proven reimbursement Current Financial Position $275M unaudited cash, cash equivalents, restricted cash & marketable securities balance as of December 31, 20231 85-105 patient starts combined across LYFGENIA, ZYNTEGLO and SKYSONA anticipated in 2024 1. Cash balance contains $53m in restricted cash; 2. Cash Runway is calculated using the cash balance / net burn rate (cash from revenue less cash paid for expenses) and is inclusive of cash, cash equivalents and marketable securities and excludes restricted cash; Patient start defined as cell collection Zero debt on balance sheet today Evaluating additional non-dilutive funding to bridge to near term profitability Cash runway into Q1 20252

CONFIDENTIAL bluebird occupies a unique strategic position as a standalone gene therapy company 22 • 10+ years of gene therapy research • 200 patients treated • 8 clinical trials • Established track record for LVV platform • 3 FDA approvals • 3 commercial launches • Transplant and cell therapy infrastructure • Proven reimbursementCl in ic al & p re -c lin ic al c om pa ni es Large cap pharm a Manufacturing experience R&D expertise Commercial infrastructure
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Grafico Azioni bluebird bio (NASDAQ:BLUE)
Storico
Da Mar 2024 a Apr 2024
Grafico Azioni bluebird bio (NASDAQ:BLUE)
Storico
Da Apr 2023 a Apr 2024