Filed
pursuant to Rule 424(b)(5)
Registration
No. 333-272516
Prospectus
Supplement
(To
Prospectus dated June 14, 2023)

Up
to $4,000,000
Ordinary
Shares
We
have entered into an At Market Issuance Sales Agreement (the “Sales Agreement”) with B. Riley Securities, Inc. (the “Agent”
or “B. Riley”), relating to our ordinary shares, no par value per share, offered by this prospectus supplement and the accompanying
prospectus. In accordance with the terms of the Sales Agreement, we may offer and sell our ordinary shares having an aggregate offering
price of up to $4.0 million from time to time through or to B. Riley, acting as sales agent or principal.
Our ordinary shares are listed
on The Nasdaq Capital Market under the symbol “OKYO”. On December 19, 2023, the last reported price of our ordinary shares
on The Nasdaq Capital Market was $2.01 per share.
Sales of our ordinary shares,
if any, under this prospectus supplement may be made in sales deemed to be “at the market offerings” as defined in Rule 415(a)(4)
promulgated under the Securities Act of 1933, as amended (the “Securities Act”). B. Riley is not required to sell any specific
number or dollar amount of securities, but will act as a sales agent using commercially reasonable efforts consistent with its normal
trading and sales practices, on mutually agreed terms between B. Riley and us. There is no arrangement for funds to be received in any
escrow, trust or similar arrangement.
B. Riley will be entitled to compensation
at a fixed commission rate of up to 3.0% of the gross sales price per ordinary share sold under the Sales Agreement. In connection with
the sale of our ordinary shares on our behalf, B. Riley will be deemed to be an “underwriter” within the meaning of the Securities
Act and the compensation of B. Riley will be deemed to be underwriting commissions or discounts. We have also agreed to provide indemnification
and contribution to B. Riley with respect to certain liabilities, including liabilities under the Securities Act or the Securities Exchange
Act of 1934, as amended (the “Exchange Act”).
As of the date of this
prospectus supplement, the aggregate market value of our outstanding ordinary shares held by non-affiliates, or our public float,
was $43,941,615 based on 21,861,500 outstanding ordinary shares held by non-affiliates and a per share price of $2.01, the closing
price of our ordinary shares on December 19, 2023, which is the highest closing price of our ordinary shares on The Nasdaq Capital
Market within the prior 60 days. During the 12 calendar month period that ends on, and
includes, the date of this prospectus supplement (excluding this offering), we have offered and sold our ordinary shares at an
aggregate sales price of $10,606,101 pursuant to General Instruction I.B.6. of Form F-3 and, accordingly, may offer and sell
additional ordinary shares having an aggregate offering price of up to $4 million from time to time through B. Riley in accordance
with the terms of the Sales Agreement.
We are an “emerging growth
company,” as defined by the Jumpstart Our Business Startups Act of 2012, or JOBS Act, and as such, have elected to comply with certain
reduced public company reporting requirements for this prospectus supplement and future filings.
Investing in our securities
involves a high degree of risk. Please carefully consider the risks discussed in this prospectus supplement under “Risk Factors”
on page S-6 in this prospectus supplement, page 7 in the accompanying prospectus and in the documents incorporated by reference in this
prospectus supplement for a discussion of the factors you should carefully consider before deciding to purchase our securities.
Neither the U.S. Securities
and Exchange Commission, any U.S. state securities commission, nor any other foreign securities commission has approved or disapproved
of these securities or determined if this prospectus supplement or the accompanying prospectus is truthful or complete. Any representation
to the contrary is a criminal offense.
B. Riley Securities
The
date of this prospectus supplement is December 20, 2023.
Table
of Contents
Prospectus
Supplement
Prospectus
ABOUT
THIS PROSPECTUS SUPPLEMENT
This
prospectus supplement and the accompanying prospectus are part of a “shelf” registration statement on Form F-3 (File No. 333-272516) that
we originally filed with the U.S. Securities and Exchange Commission (the “SEC”), on June 8, 2023 and which became effective
on June 14, 2023.
This
document is in two parts. The first part is the prospectus supplement, including the documents incorporated by reference, which describes
the specific terms of this offering. The second part, the accompanying prospectus, including the documents incorporated by reference,
provides more general information. Generally, when we refer to this prospectus supplement, we are referring to both parts of this document
combined. We urge you to carefully read this prospectus supplement and the accompanying prospectus, and the documents incorporated by
reference herein and therein, before buying any of the securities being offered under this prospectus supplement. This prospectus supplement
may add, update or change information contained in the accompanying prospectus. To the extent that any statement that we make in this
prospectus supplement is inconsistent with statements made in the accompanying prospectus or any documents incorporated by reference
therein filed prior to the date of this prospectus supplement, the statements made in this prospectus supplement will be deemed to modify
or supersede those made in the accompanying prospectus and such documents incorporated by reference therein.
You
should rely only on the information contained or incorporated herein by reference in this prospectus supplement and contained or incorporated
therein by reference in the accompanying prospectus. We have not authorized anyone to provide you with different or additional information.
If anyone provides you with different, additional or inconsistent information, you should not rely on it.
We
are offering to sell the securities only in jurisdictions where such offers and sales are permitted. The distribution of this prospectus
supplement and the accompanying prospectus and the offering of the securities in certain jurisdictions or to certain persons within such
jurisdictions may be restricted by law. Persons outside the United States who come into possession of this prospectus supplement and
the accompanying prospectus must inform themselves about and observe any restrictions relating to the offering of the securities and
the distribution of this prospectus supplement and the accompanying prospectus outside the United States. This prospectus supplement
and the accompanying prospectus do not constitute, and may not be used in connection with, an offer to sell, or a solicitation of an
offer to buy, any securities offered by this prospectus supplement and the accompanying prospectus by any person in any jurisdiction
in which it is unlawful for such person to make such an offer or solicitation.
You
should assume that the information in this prospectus supplement and the accompanying prospectus is accurate only as of the date on the
front of the applicable document and that any information we have incorporated by reference is accurate only as of the date of the document
incorporated by reference, regardless of the time of delivery of this prospectus supplement or the accompanying prospectus, or any sale
of a security. Our business, financial condition, results of operations and prospects may have changed since those dates. You should
read this prospectus supplement, the accompanying prospectus and the documents incorporated by reference in this prospectus supplement
and the accompanying prospectus when making your investment decision. You should also read and consider the information in the documents
we have referred you to in the sections of this prospectus supplement and the accompanying prospectus titled “Where You Can Find
More Information” and “Incorporation of Documents by Reference.”
The
representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated
by reference herein or in the accompanying prospectus were made solely for the benefit of the parties to such agreement, including, in
some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation,
warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly,
such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
This
prospectus supplement, the accompanying prospectus, and the information incorporated herein and therein by reference includes trademarks,
service marks and trade names owned by us or other companies. All trademarks, service marks and trade names included or incorporated
by reference into this prospectus supplement or the accompanying prospectus are the property of their respective owners.
Unless
otherwise indicated or the context otherwise requires, all references in this registration statement to the terms “OKYO,”
“OKYO Pharma Limited,” the “Company,” “we,” “us” and “our” refer to OKYO
Pharma Limited and our wholly owned subsidiary OKYO Pharma US Inc.
PROSPECTUS
SUPPLEMENT SUMMARY
This
summary highlights selected information about us and this offering and does not contain all of the information that you should consider
in making your investment decision. You should carefully read this entire prospectus supplement and the accompanying prospectus, including
the information set forth under the sections of this prospectus supplement entitled “Risk Factors,” “Cautionary Statement
Regarding Forward-Looking Statements,” and similar headings in the other documents that are incorporated by reference into this
prospectus supplement and the accompanying prospectus. If you invest in our securities, you are assuming a high degree of risk.
Overview
We
are a clinical-stage biopharmaceutical company developing next-generation therapeutics to improve the lives of patients suffering from
inflammatory eye diseases and ocular pain. Our research program is focused on a novel G Protein-Coupled Receptor, or GPCR, which we believe
plays a key role in the pathology of these inflammatory eye diseases of high unmet medical need. Our therapeutic approach is focused
on targeting inflammatory and pain modulation pathways that drive these conditions. We are presently developing OK-101, our lead clinical
product candidate, for the treatment of dry eye disease (“DED”). We also plan to evaluate its potential in benefiting patients
with ocular neuropathic pain, uveitis and allergic conjunctivitis. We have also been evaluating OK-201, a bovine adrenal medulla, or
BAM, lipidated-peptide preclinical analogue candidate that is currently in developmental stage.
OK-101
OK-101,
our lead clinical-stage product candidate, is focused on keratoconjunctivitis sicca, commonly referred to as DED, which is a multifactorial
disease caused by an underlying inflammation resulting in the lack of lubrication and moisture in the surface of the eye. DED is one
of the most common ophthalmic conditions encountered in clinical practice. Symptoms of DED include constant discomfort and irritation
accompanied by inflammation of the ocular surface, visual impairment and potential damage to the ocular surface. There are presently
approximately 20 million people suffering from DED in the U.S. alone (Farrand et al. AJO 2017; 182:90), with the disease affecting approximately
up to 34% of the population aged 50+ (Dana et al. AJO 2019; 202:47), and with women representing approximately two-thirds of those affected
(Matossian et al. J Womens Health (Larchmt) 2019; 28:502–514). Prevalence of DED is anticipated to increase substantially in the
next 10-20 years due to aging populations in the U.S., Europe, Japan and China and use of contact lenses in the younger population. We
believe this increase in prevalence of DED represents a major expanding economic burden to public healthcare. According to Market Research
Report, Dry Eye Disease, December 2020, the global DED market in 2019 was approximately $5.22 billion, with the market size expected
to reach $6.54 billion by 2027. In addition, DED causes approximately $3.8 billion annually in healthcare costs and represents a major
economic burden to public healthcare, accounting for more than $50 billion to the U.S. economy annually.
At
present, there are 5 prescription drugs available to treat DED: 1) Restasis (0.05% cyclosporine), 2) Cequa (0.09% cyclosporine), 3) Xiidra
(5% lifitegrast), 4) Tyrvaya (0.03 mg varenicline), and 5) Eysuvis (0.25% loteprednol – a corticosteroid for short term use only).
However, DED continues to be a major unmet medical need due to the large number of patients not well served by the treatments available
to them through the medical community.
The
development of new drugs to treat DED has been particularly challenging due to the heterogeneous nature of the patient population suffering
from DED, and due to the difficulties in demonstrating an improvement in both signs and symptoms of the disease in well-controlled clinical
trials. The evidence from over 40 years of scientific literature, however, suggests inflammation as the most common underlying element
of DED. Consequently, development of new therapeutic agents that target inflammatory pathways is looking to be an attractive approach
in improving symptoms in DED patients. Moreover, large number of dry eye patients suffer from ocular neuropathic pain, making their condition
more resistant to topical anti-inflammatory therapy, and a drug capable of targeting both of these aspects of DED would be a significant
addition to the ocular-care practitioner’s arsenal for the treatment of DED.
The
chemerin receptor (CMKLR1 or ChemR23) is a chemokine like GCPR expressed on select populations of cells including inflammatory mediators,
epithelial and endothelial cells as well as neurons and glial cells in the dorsal root ganglion, spinal cord, and retina. Activation
of CMKLR1 by chemerin has been shown to resolve the inflammation and pain in animal models of asthma and pain, respectively. We have
been pioneering the development of OK-101, a lipidated-chemerin analogue, which is an agonist of CMKLR1, in treating DED and other ocular
inflammatory conditions. OK-101 was first identified in a program developed by OTT using membrane-tethered ligand technology.
To
expand our understanding of the structure-activity relationships of the lipidated-chemerin analogues, such as OK-101, as agonists of
the chemerin receptor, we synthesized a small library of analogues of OK-101. We screened these analogues in a cell-line based receptor
binding assay to characterize the agonist potency of these lipidated-chemerin analogues. This work has also been coupled to an evaluation
of a subset of these analogues’ potential in treating DED by using a variety of preclinical studies and dry eye animal model studies.
After evaluating a number of our analogues in a mouse model of acute DED by looking at their ability to reduce corneal permeability,
a measure of dry-eye effectiveness, as well as the analogues’ impact on immune response, we determined that OK-101 was in fact
the most potent analogue in reducing corneal permeability and down-regulating immune response. In addition, in a separate set of animal
model experiments, OK-101 was shown to exhibit potent ocular pain-reducing activity in a ciliary nerve ligation mouse model of corneal
neuropathic pain. Following these studies, we evaluated the ocular tolerance of OK-101 via repeated ocular instillation in rabbits
followed by clinical ophthalmic observations. Rabbit ocular tolerance tests on OK-101 showed no adverse signs such as inflammation, chemosis
or hyperemia and no signs of local irritation. With potential anti-inflammatory and neuropathic pain reducing characteristics, we are
developing OK-101 for the treatment of DED.
Based
on the results from the DED animal model, the neuropathic corneal pain model as well as the rabbit ocular tolerance studies, we moved
forward over the past 18 months with plans to file an IND on OK-101 to treat DED to enable us to begin clinical trials soon thereafter.
During the fourth quarter of 2021 we successfully manufactured a 200-gram batch of OK-101 drug substance needed for initiating the IND-enabling
studies that were begun during the first quarter of 2022. In support of this work, we also had previously signed an agreement on April
13, 2021, with Ora, Inc., or Ora, a major clinical research organization, or CRO, specializing in ophthalmic drug development.
On
February 15, 2022, we announced the successful completion of the pre-IND meeting facilitated by Ora with the FDA regarding development
plans for OK-101 to treat DED. Both nonclinical and clinical development milestones were covered in the pre-IND meeting, with the FDA
agreeing that our first human trial would be a Phase 2 safety and efficacy trial in DED patients. The FDA also provided guidance on the
planned protocol for this trial in DED patients, concurring with one particular option OKYO has considered for the protocol which is
to designate co-primary efficacy endpoints covering both a sign and a symptom of DED in the clinical trial. Notably, the final decision
we recently took to, in fact, designate these two primary efficacy endpoints in the clinical protocol of the ongoing phase 2 trial is
significant as should this phase 2 trial then meet these prespecified endpoints, the trial should considerably affect the timeline to
an NDA filing with the FDA for OK-101 to treat DED.
On
May 2, 2023, we announced that the first patient had been screened for our Phase 2, multi-center, randomized, double-blinded, placebo-controlled
trial of OK-101. Because the drug is designed to be administered topically, we were able to skip the standard Phase 1 studies typically
expected with orally delivered or injectable drug candidates in non-life-threatening conditions and we opened the first trial with OK-101
as a Phase 2 clinical trial in DED patients (See OKYO Pipeline below). This trial is planned to be conducted in approximately 200 to
240 DED patients. The study is being designed in conjunction with and is being managed and monitored by Ora, well known for its leadership
of ophthalmic clinical trial activities. The Phase 2 trial is expected to be completed in 6-8 months from enrollment of the first patient.
OK-201
MAS-Related
G Protein-Coupled Receptors, or MRGPRs, mainly expressed in the sensory neurons, are involved in the perception of pain, thus making
them a promising analgesic target. Activation of MRGPR by Bovine Adrenal Medulla, or BAM, peptide inhibits pain perception by modulating
Ca2+ influx. OK-201, a BAM peptide analogue, licensed from TMC on May 1, 2018, is a potent agonist of human MRGPR and a promising candidate
for the treatment of neuropathic and inflammatory pain.
On
August 6, 2019, we signed a collaborative agreement with TMC and Pedram Hamrah, MD, Professor of Ophthalmology at Tufts University School
of Medicine, Boston, MA as Principal Investigator to evaluate OK-201 and other proprietary lead compounds to suppress corneal neuropathic
pain using a mouse ocular pain model recently developed in Dr. Hamrah’s laboratory. Our goal was to further develop this lipidated
peptide, as well as explore additional analogues, for their potential use in treating ocular pain, and for potentially treating long-term
chronic pain.
On
April 28, 2021, we announced positive results of OK-201, a non-opioid analgesic drug candidate delivered topically in Dr. Hamrah’s
mouse neuropathic corneal pain model, as a potential drug to treat acute and chronic ocular pain. Importantly, OK-201 demonstrated a
reduced corneal pain response equivalent to that of gabapentin, a commonly used oral drug for neuropathic pain. These observations demonstrated
preclinical ‘proof-of-concept’ for the topical administration of OK-201 as a potential non-opioid analgesic for ocular pain.
Current treatments for corneal pain are limited to short term non-steroidal anti-inflammatory drugs, or NSAIDs, steroids, and oral gabapentin
and opioids in severe cases.
Although
the results with OK-201 were encouraging, due to subsequent success obtained with OK-101 (see section above on OK-101) in follow-on animal
model studies utilizing the same mouse corneal neuropathic pain model as for OK-201, we have decided to maintain this drug candidate
at the exploratory level while we focus our primary energy on the OK-101 program to treat DED, based on OK-101’s combination of
anti-inflammatory and corneal pain-reducing activities in animal models of these conditions.
Corporate
Information
We
were originally incorporated in the British Virgin Islands as a British Virgin Islands Business Company on July 4, 2007 under the BVI
Business Companies Act 2004 with company number 1415559 under the name Jellon Enterprises, Inc. Our legal and commercial name was changed
to Minor Metals & Mining, Inc. on October 24, 2007, to Emerging Metals Limited on November 28, 2007, to West African Minerals Corporation
on December 9, 2011, and to OKYO Pharma Corporation on January 10, 2018. On March 9, 2018, shareholders approved the cancellation of
our AIM listing and migration to Guernsey. On July 3, 2018, following the approval of the Guernsey Companies Registry, we were registered
under the Guernsey Companies Law under the name OKYO Pharma Limited, as a Guernsey company with limited liability, an indefinite life
and company number 65220. We are domiciled in Guernsey. On July 17, 2018, our Ordinary Shares were admitted to listing on the standard
segment of the Official List of the FCA and admitted to trading on the Main Market of the London Stock Exchange. On May 22, 2023, we
delisted our ordinary shares from the standard segment of Official List of the FCA, and trading ceased on the Main Market of the London
Stock Exchange. We are no longer subject to the Takeover Code.
Our
registered office is located at Martello Court, Admiral Park, St. Peter Port, Guernsey GY1 3HB and our telephone number is +44 (0) 20
7495 2379. Our website address is www.okyopharma.com. The reference to our website is an inactive textual reference only and the information
contained in, or that can be accessed through, our website is not a part of this prospectus supplement or the accompanying prospectus.
Our agent for service of process in the United States is OKYO Pharma US, Inc.
THE
OFFERING
| Ordinary
shares offered by us in this offering |
|
Ordinary
shares having an aggregate offering price of up to $4.0 million. |
| |
|
|
| Ordinary
shares outstanding immediately after this offering (1) |
|
Up
to 35,226,100 ordinary shares, assuming the sale of up to 1,990,050 ordinary shares hereunder at a price of $2.01 per share,
the closing price per share on The Nasdaq Capital Market on December 19, 2023. The actual number of shares issued may vary depending
on the price at which shares may be sold from time to time during this offering. |
| |
|
| Use
of proceeds |
|
We
currently expect to use the net proceeds, if any, for clinical development of our product candidates, general corporate purposes
and working capital. See “Use of Proceeds” on page S-8.
|
| Form
of offering |
|
“At
the market offering” that may be made from time to time through or to B. Riley as sales
agent or principal. See “Plan of Distribution” on page S-10 of this prospectus
supplement.
|
| |
|
| Risk
factors |
|
See
“Risk Factors” beginning on page S-6 of this prospectus supplement, page 7 of the accompanying prospectus and
all other information included in, or incorporated by reference into, this prospectus supplement and the accompanying prospectus
for a discussion of certain factors you should carefully consider before deciding to invest in our securities. |
| |
|
| Nasdaq
Capital Market symbol for Ordinary shares |
|
Our
ordinary shares are listed on the Nasdaq Capital Market under the symbol “OKYO.” |
(1)
The number of ordinary shares to be outstanding after this offering is based on 33,236,050 ordinary shares outstanding as of December
14, 2023, and excludes as of such date:
| |
● |
2,146,451 ordinary shares issuable upon the exercise of share options at
a weighted average exercise price of $3.44 per ordinary share of which 618,354 ordinary shares are currently exercisable and 1,528,097
are exercisable between July 27, 2023 and November 23, 2033; and |
| |
● |
538,461
ordinary shares that currently may be issued upon the exercise of warrants to purchase ordinary shares at a weighted average exercise
price of $3.68 per ordinary share. |
RISK
FACTORS
An
investment in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you should consider
carefully the risks described below and discussed under the section captioned “Risk Factors” contained in our Annual Report
on Form 20-F for the year ended March 31, 2023, as amended, which is incorporated by reference in this prospectus supplement
and the accompanying prospectus in its entirety, and any amendment or update thereto reflected in subsequent filings with the SEC, together
with other information in this prospectus supplement, the accompanying prospectus, the information and documents incorporated herein
and therein by reference, as updated by our subsequent filings under the Exchange Act and in any free writing prospectus that we have
authorized for use in connection with this offering. If any of these risks actually occurs, our business, financial condition, results
of operations or cash flow could be seriously harmed. This could cause the trading price of our ordinary shares to decline, resulting
in a loss of all or part of your investment.
Risks
Related to this Offering and our Nasdaq Listing
Management
will have broad discretion as to the use of the proceeds from this offering, and we may not use the proceeds effectively.
Our
management will have broad discretion in the application of the net proceeds we receive in this offering, including for any of the purposes
described in the section entitled “Use of Proceeds,” and you will not have the opportunity as part of your investment decision
to assess whether our management is using the net proceeds appropriately. Because of the number and variability of factors that will
determine our use of our net proceeds from this offering their ultimate use may vary substantially from their currently intended use.
You
may experience immediate and substantial dilution as a result of this offering.
The
offering price per share in this offering may exceed the net tangible book value per share of our ordinary shares outstanding prior to
this offering. Assuming that an aggregate of 1,990,050 ordinary shares are sold during the term of the Sales Agreement at an assumed
public offering price of $2.01 per share, the last reported sale price of our ordinary shares on The Nasdaq Capital Market on December
19, 2023, for aggregate gross proceeds of $4,000,000, after deducting commissions and estimated aggregate offering expenses payable by
us, you will experience immediate dilution of $1.16 per share, representing the difference between our pro forma as adjusted net tangible
book value per share as of March 31, 2023 after giving effect to this offering and the assumed offering price. The exercise of outstanding
stock options and warrants may result in further dilution of your investment. See the section entitled “Dilution” below for
a more detailed illustration of the dilution you would incur if you participate in this offering.
If
we raise additional capital in the future, your ownership in us could be diluted.
In
order to raise additional capital, we may at any time, including during this offering, offer additional ordinary shares or other securities
convertible into or exchangeable for our ordinary shares at prices that may not be the same as the price per share in this offering.
We may sell shares or other securities in any other offering at a price per share that is less than the price per share paid by investors
in this offering, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders,
including investors who purchase ordinary shares in this offering. The price per share at which we sell additional ordinary shares or
securities convertible into ordinary shares in future transactions may be higher or lower than the price per share in this offering.
Until
such time, if ever, as we can generate substantial revenue from our operations, we anticipate financing our cash needs through a combination
of equity offerings, debt financings and license agreements. To the extent that we raise additional capital through the further sale
of equity securities or convertible debt securities, your ownership interest will be diluted.
We
have never declared or paid any cash dividends on our ordinary shares and, accordingly, shareholders must rely on stock appreciation
for any return on their investment.
We
have never declared or paid any cash dividends on our ordinary shares, and we do not intend to pay any cash dividends on our ordinary
shares. Rather, we currently intend to retain all available funds and any future earnings, if any, to fund the development and expansion
of our business and for general corporate purposes, and we do not anticipate paying any cash dividends in the foreseeable future. Consequently,
investors must rely on sales of their ordinary shares after price appreciation, which may never occur, as the primary way to realize
any gains on their investment.
The
actual number of ordinary shares we will issue under the Sales Agreement, at any one time or in total, is uncertain.
Subject
to certain limitations in the Sales Agreement and compliance with applicable law, we have the discretion to deliver a placement notice
to B. Riley at any time throughout the term of the Sales Agreement. The number of shares that are sold by B. Riley after delivering a
placement notice will fluctuate based on the market price of our ordinary shares during the sales period and limits we set with B. Riley.
Because the price per share of each share sold will fluctuate based on the market price of our ordinary shares during the sales period,
it is not possible at this stage to predict the number of shares that will be ultimately issued.
The
ordinary shares offered hereby will be sold in “at the market offerings,” and investors who buy shares at different times
will likely pay different prices.
Investors
who purchase shares in this offering at different times will likely pay different prices, and so may experience different outcomes in
their investment results. We will have discretion, subject to market demand, to vary the timing, prices and numbers of shares sold, and
there is no minimum or maximum sales price. Investors may experience a decline in the value of their shares as a result of share sales
made at prices lower than the prices they paid.
Our
ordinary shares could be delisted from The Nasdaq Capital Market.
We
may be unable to maintain the listing of our ordinary shares on The Nasdaq Capital Market. In the event that our ordinary shares were
to be delisted from The Nasdaq Capital Market, we expect that they would be traded on the OTCQB or OTCQX, which are unorganized, inter-dealer, over-the-counter markets
which provide significantly less liquidity than the Nasdaq or other national securities exchanges. In the event that our ordinary shares
were to be delisted from The Nasdaq Capital Market, it may have a material adverse effect on the trading and price of our ordinary shares.
If,
for any reason, Nasdaq should delist our ordinary shares from trading on its exchange and we are unable to obtain listing on another
national securities exchange or take action to restore our compliance with the Nasdaq continued listing requirements, a material adverse
effect on our shareholders may occur due to a reduction in some or all of the following: the market price of our ordinary shares; the
liquidity of our ordinary shares; our ability to obtain financing for the continuation of our operations; the number of market makers
in our ordinary shares; and the number of institutional and general investors that will consider investing in our ordinary shares.
In
the event that our ordinary shares were to be delisted from The Nasdaq Capital Market, they may be considered a “penny stock.”
Securities broker-dealers participating in sales of our ordinary shares would then be subject to the “penny stock” regulations
set forth in Rules 15g-2 through 15g-9 promulgated under the Exchange Act. Generally, brokers may be less willing
to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for investors to
dispose of our ordinary shares and cause a decline in the market value of our stock.
CAUTIONARY
STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus supplement, the accompanying prospectus, the documents incorporated by reference herein and therein, and any free writing
prospectus that we have authorized for use in connection with this offering, contain forward-looking statements that involve substantial
risks and uncertainties. The forward-looking statements are contained principally in the sections of this prospectus supplement titled
“About this Prospectus Supplement,” “Risk Factors,” and “Prospectus Supplement Summary.” All statements,
other than statements of historical facts, contained in this prospectus supplement, including statements regarding our future results
of operations and financial position, business strategy, prospective products, product approvals, research and development costs, timing
and likelihood of success, plans and objectives of management for future operations, and future results of current and anticipated products,
are forward-looking statements. These statements relate to future events or to our future financial performance and involve known and
unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different
from any future results, performance or achievements expressed or implied by the forward-looking statements. The words “anticipate,”
“assume,” “believe,” “contemplate,” “continue,” “could,” “estimate,”
“expect,” “goal,” “intend,” “may,” “might,” “objective,” “plan,”
“potential,” “predict,” “project,” “positioned,” “seek,” “should,”
“target,” “will,” “would,” or the negative of these terms or other similar expressions are intended
to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking
statements are based on current expectations, estimates, forecasts and projections about our business and the industry in which we operate
and management’s beliefs and assumptions, are not guarantees of future performance or development and involve known and unknown
risks, uncertainties and other factors.
Actual
results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we
make. As a result, any or all of our forward-looking statements in this prospectus supplement may turn out to be inaccurate. We have
included important factors in the cautionary statements included in this prospectus supplement, particularly in the section of this prospectus
supplement titled “Risk Factors,” that we believe could cause actual results or events to differ materially from the forward-looking
statements that we make. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements,
and you should not place undue reliance on our forward-looking statements. Moreover, we operate in a highly competitive and rapidly changing
environment in which new risks often emerge. It is not possible for our management to predict all risks, nor can we assess the impact
of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially
from those contained in any forward-looking statements we may make. Our forward-looking statements do not reflect the potential impact
of any future acquisitions, mergers, dispositions, joint ventures or investments we may make.
You
should read this prospectus supplement, the accompanying prospectus, and the documents that we reference in this prospectus supplement
and have filed as exhibits to the registration statement of which this prospectus supplement is a part completely and with the understanding
that our actual future results may be materially different from what we expect. The forward-looking statements contained in this prospectus
supplement are made as of the date of this prospectus supplement, and we do not assume any obligation to update any forward-looking statements
except as required by applicable law and regulation.
USE
OF PROCEEDS
We
may issue and sell our ordinary shares having aggregate gross sales proceeds of up to $4.0 million from time to time. The amount of proceeds
from this offering will depend upon the number of ordinary shares sold and the market price at which they are sold. There can be no assurance
that we will be able to sell any shares under or fully utilize the Sales Agreement with B. Riley as a source of financing.
We
currently intend to use the net proceeds to us from this offering for clinical development of our product candidates, general corporate
purposes and working capital. This expected use of our net proceeds from this offering represents our intentions based upon our current
plans and business conditions, which could change in the future as our plans and business conditions evolve. The amounts and timing of
our actual expenditures may vary significantly depending on numerous factors, including any unforeseen cash needs. Accordingly, we will
have broad discretion over the uses of the net proceeds from this offering and investors will be relying on the judgment of our management
regarding the application of the net proceeds from this offering. The timing and amount of our actual expenditures will be based on many
factors, including cash flows from operations and the anticipated growth of our business.
Investors
are further cautioned that the proceeds from this offering are expected to be sufficient to enable us to continue operations for only
a short period of time. We expect that we will have to raise such additional funds through the sale of additional equity or equity backed
securities. Any future equity or equity linked financing that we may need may not be able available on terms favorable to us or at all.
Pending
any use, as described above, we plan to deposit the net proceeds in money market accounts with our primary bank or otherwise invest the
net proceeds in high-quality, short-term, interest-bearing securities.
DILUTION
If
you invest in our securities in this offering, your ownership interest will be diluted to the extent of the difference between the offering
price per ordinary share in this offering and the as adjusted net tangible book value per ordinary share immediately after this offering.
The net tangible book value of our ordinary shares as of March 31, 2023 was approximately $(2,053,237), or approximately $(0.08) per
ordinary share based upon 25,519,882 ordinary shares outstanding at that time. Net tangible book value per share is equal to our total
tangible assets, less our total liabilities, divided by the total number of ordinary shares outstanding as of March 31, 2023.
Net
tangible book value dilution per ordinary share to investors participating in this offering represents the difference between the effective
offering price per ordinary share paid by purchasers of securities in this offering and the pro forma as adjusted net tangible book value
per ordinary share immediately after this offering. After giving effect to the issuance of (i) the issuance of 2,666,670 ordinary shares
in September 2023 at an offering price of $1.50 per share, (ii) the issuance of 1,092,600 ordinary shares in October 2023 at an offering
price of $1.50 per share, (iii) the issuance of 2,766,667 ordinary shares to certain creditors in October 2023, (iv) the issuance of
400,000 ordinary shares in December 2023 at an offering price of $1.50 per share and (v) the issuance of 144,800 ordinary shares in December
2023 to certain creditors in December 2023, our pro forma net tangible book value as of March 31, 2023 would have been approximately
$8.5 million, or $0.26 per ordinary share. After giving effect to the sale of our ordinary shares in the aggregate amount of $4.0 million
in this offering at an assumed public offering price of $2.01 per share (which was the last reported sale price of our ordinary shares
on the Nasdaq Capital Market on December 19, 2023), and after deducting estimated offering expenses and after deducting estimated sales
agent commissions payable by us, our pro forma as adjusted net tangible book value as of March 31, 2023 would have been approximately
$12.5 million, or $0.35 per ordinary share. This represents an immediate increase in net tangible book value of $0.09 per ordinary share
to existing stockholders and immediate dilution of $1.16 per ordinary share to investors purchasing our securities in this offering at
the offering price. The following table illustrates this dilution on a per ordinary share basis:
| Assumed public offering price per
share | |
| | | |
$ | 2.01 | |
| Pro forma net tangible book
value per share as of March 31, 2023 | |
$ | 0.26 | | |
| | |
| Increase
in net tangible per share attributable to this offering | |
| 0.09 | | |
| | |
| Pro forma as adjusted
net tangible book value per share as of March 31, 2023 | |
| | | |
| 0.35 | |
| Dilution
in net tangible book value per share to investors participating in this offering | |
| | | |
$ | 1.16 | |
The discussion of
dilution, and the table quantifying it, assumes no exercise of any outstanding options or warrants or other potentially dilutive securities.
The exercise of potentially dilutive securities having an exercise price less than the offering price would increase the dilutive effect
to new investors.
In particular, the table
above excludes the following securities as of March 31, 2023:
| |
● |
1,956,451
ordinary shares issuable upon the exercise of share options at a weighted average exercise price of $3.63 per ordinary share of which
618,354 ordinary shares are currently exercisable and 1,338,097 are exercisable between July 27, 2023 and July 26, 2033; and |
| |
|
|
| |
● |
538,461
ordinary shares that currently may be issued upon the exercise of warrants to purchase ordinary shares at a weighted average exercise
price of $3.68 per ordinary share. |
To
the extent that any outstanding stock options or warrants are exercised, new options are issued under our equity incentive plans and
subsequently exercised or we issue additional ordinary shares in the future, there will be further dilution to new investors participating
in this offering.
PLAN
OF DISTRIBUTION
We
have entered into an At Market Issuance Sales Agreement (the “Sales Agreement”) with B. Riley (the “Agent”).
Pursuant to this prospectus supplement, we may offer and sell our ordinary shares having an aggregate gross sales price of up to $4.0
million from time to time through or to the Agent acting as sales agent or principal.
The
Agent may sell the ordinary share by any method permitted by law deemed to be an “at the market offering” as defined in Rule
415(a)(4) under the Securities Act.
Each
time that we wish to sell ordinary shares under the Sales Agreement, we will provide the Agent with a placement notice describing the
number of ordinary shares to be sold, the time period during which sales are requested to be made, any limitation on the number of ordinary
shares that may be sold in any one day and any minimum price below which sales may not be made.
Upon
receipt of a placement notice from us, and subject to the terms and conditions of the Sales Agreement, the Agent has agreed to use its
commercially reasonable efforts consistent with its normal trading and sales practices to sell such ordinary shares up to the amount
specified on such terms. Unless otherwise specified, the settlement between us and the Agent will occur on the second trading day following
the date on which the sale was made. The obligation of the Agent under the Sales Agreement to sell ordinary shares pursuant to a placement
notice is subject to a number of conditions. There is no arrangement for funds to be received in an escrow, trust or similar arrangement.
We
will pay the Agent a commission of up to 3% of the gross proceeds of the sales price of all ordinary shares sold through the Agent as
sales agent under the Sales Agreement. Because there is no minimum offering amount required as a condition to closing this offering,
the actual total public offering amount, commissions and proceeds to us, if any, are not determinable at this time.
We
estimate that the total expenses for the offering, excluding compensation payable to the Agent under the terms of the Sales Agreement,
will be approximately US$50,000. We have also agreed to reimburse B. Riley for its reasonable out-of-pocket expenses, including
attorney’s fees, in an amount not to exceed an aggregate of $75,000 incurred in connection with entering into the transactions
contemplated by the Sales Agreement and up to $5,000 per calendar quarter, for ongoing diligence arising from the transactions contemplated
by the Sales Agreement.
In
connection with the sale of ordinary shares contemplated in this prospectus supplement, the Agent will be deemed to be an “underwriter”
within the meaning of the Securities Act, and the compensation paid to the Agent will be deemed to be underwriting commissions or discounts.
We have agreed to provide indemnification and contribution to B. Riley against certain civil liabilities, including liabilities under
the Securities Act.
The
offering of ordinary shares pursuant to the Sales Agreement will terminate on the earlier of (1) the sale of all of the ordinary shares
subject to the Sales Agreement or (2) termination of the Sales Agreement by us or the Agent.
This
summary of the material provisions of the Sales Agreement does not purport to be a complete statement of its terms and conditions. A
copy of the Sales Agreement has been or will be filed with the SEC as an exhibit to registration statement of which this prospectus supplement
is a part.
The
Agent does not have any relationship with us other than its role as a sales agent for our current “at the market offerings”
of ordinary shares. The Agent and its affiliates may in the future provide various investment banking and other financial services for
us, for which services they may in the future receive customary fees.
LEGAL
MATTERS
Certain
legal matters with respect to English law and Guernsey law in connection with the validity of our ordinary shares registered hereby
will be passed upon for us by Orrick, Herrington & Sutcliffe (UK) LLP, United Kingdom and Carey Olsen (Guernsey) LLP, Bailiwick
of Guernsey, respectively. Certain matters of U.S. federal law will be passed upon for us by Sheppard, Mullin, Richter & Hampton
LLP, New York, New York. B. Riley Securities, Inc. is being represented in this offering by Duane Morris LLP, New York, New
York.
EXPERTS
The
consolidated financial statements of OKYO Pharma Limited as of March 31, 2023 and for the year then ended, included in our Form 20-F
annual report for the year ended March 31, 2023, as amended, and incorporated by reference in this prospectus supplement have
been audited by PKF Littlejohn LLP, an independent registered public accounting firm, given on the authority of said firm as experts
in auditing and accounting. The audit report contains an explanatory paragraph regarding our ability to continue as a going concern.
The
consolidated financial statements of OKYO Pharma Limited as of March 31, 2022, and 2021 and for each of the years then ended, included
in our Form 20-F annual report for the year ended March 31, 2023 and incorporated by reference in this prospectus
supplement have been audited by Mazars LLP, an independent registered public accounting firm, given on the authority of said firm
as experts in auditing and accounting. The audit report contains an explanatory paragraph regarding our ability to continue as a going
concern. The registered business address of Mazars LLP is 30 Old Bailey, London EC4M 7AU, United Kingdom.
INCORPORATION
OF DOCUMENTS BY REFERENCE
This
prospectus supplement is part of the registration statement, but the registration statement includes and incorporates by reference additional
information and exhibits. The SEC permits us to “incorporate by reference” the information contained in documents we file
with the SEC, which means that we can disclose important information to you by referring you to those documents rather than by including
them in this prospectus supplement and the accompanying base prospectus. Information that is incorporated by reference is considered
to be part of this prospectus supplement and the accompanying base prospectus and you should read it with the same care that you read
this prospectus supplement and the accompanying base prospectus. Information that we file later with the SEC will automatically update
and supersede the information that is either contained, or incorporated by reference, in this prospectus supplement and the accompanying
base prospectus, and will be considered to be a part of this prospectus supplement and the accompanying base prospectus from the date
those documents are filed.
We
incorporate by reference the documents listed below, all filings filed by us pursuant to the Exchange Act after the date of the registration
statement of which this prospectus supplement and the and the accompanying base prospectus form a part, and any future filings we make
with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act prior to the time that all securities covered by this prospectus
supplement have been sold; provided, however, that we are not incorporating any information furnished under either Item 2.02 or Item
7.01 of any Current Report on Form 6-K and exhibits furnished on such form that relate to such items:
| ● |
|
Our Annual Report on Form 20-F for the fiscal year ended March 31, 2023 filed with the SEC on August 15, 2023, as amended on August 25, 2023; |
| |
|
|
| ● |
|
our Reports on Form 6-K
furnished to the SEC on May
18, 2022, May
19, 2022, May
20, 2022, June
9, 2022, August
16, 2022, August
19, 2022, August
30, 2022, September
7, 2022, September
8, 2022, October
20, 2022, October
24, 2022, November
10, 2022, November
21, 2022, November
25, 2022, December
1, 2022, December
6, 2022, December
21, 2022, December
22, 2022, December
30, 2022,
January 3, 2023, January
4, 2023, February
14, 2023, February
21, 2023, February
23, 2023, February
28, 2023, March
13, 2023, March
14, 2023, March
15, 2023 (2), March
16, 2023, March
24, 2023, March
29, 2023, March
30, 2023, April
4, 2023, April
5, 2023, April
25, 2023, May
2, 2023, May
3, 2023 (3), May
11, 2023 (2), May
15, 2023, May
19, 2023, May
22, 2023, June
6, 2023, July
27, 2023, July
28, 2023, July
31, 2023, August
15, 2023, August
29, 2023, August
30, 2023, September
8, 2023, September
13, 2023, September
14, 2023, September
15, 2023, October
5, 2023, October
10, 2023, October
31, 2023, November
1 2023, November
29, 2023, December
4, 2023 and December
18, 2023; and |
| |
|
|
| ● |
|
the description of our
ordinary shares contained in our Registration Statement on Form 8-A filed with the SEC on May 10, 2022, as amended by Form 8-A/A filed with the SEC on June 2, 2023, including any amendments or reports filed for the purpose of updating such description. |
We
are also incorporating by reference all subsequent Annual Reports on Form 20-F that we file with the SEC and certain reports on Form
6-K that we furnish to the SEC after the date of this prospectus supplement (if they state that they are incorporated by reference into
this prospectus supplement) prior to the termination of this offering. In all cases, you should rely on the later information over different
information included in this prospectus supplement.
Unless
expressly incorporated by reference, nothing in this prospectus supplement shall be deemed to incorporate by reference information
furnished to, but not filed with, the SEC. Copies of all documents incorporated by reference in this prospectus supplement, other than
exhibits to those documents unless such exhibits are specifically incorporated by reference in this prospectus supplement, will
be provided at no cost to each person, including any beneficial owner, who receives a copy of this prospectus supplement on the
written or oral request of that person made to:
OKYO
Pharma Limited
Martello
Court
Admiral
Park
St.
Peter Port
Guernsey
GY1 3HB
+44
(0)20 7495 2379
You
may also access these documents on our website, www.okyopharma.com. The information contained on, or that can be accessed through,
our website is not a part of this prospectus supplement. We have included our website address in this prospectus supplement solely as
an inactive textual reference.
You
should rely only on information contained in, or incorporated by reference into, this prospectus supplement. We have not authorized anyone
to provide you with information different from that contained in this prospectus supplement or incorporated by reference in this prospectus
supplement. We are not making offers to sell the securities in any jurisdiction in which such an offer or solicitation is not authorized
or in which the person making such offer or solicitation is not qualified to do so or to anyone to whom it is unlawful to make such offer
or solicitation.
WHERE YOU CAN FIND MORE INFORMATION
This
prospectus supplement constitutes a part of the registration statement on Form F-3 that we have filed with the SEC under the Securities
Act. As permitted by the SEC’s rules, this prospectus supplement and any accompanying prospectus, which forms a part of the registration
statement, do not contain all of the information that is included in the registration statement. You will find additional information
about us in the registration statement. Any statement made in this prospectus supplement or any accompanying prospectus concerning legal
documents are not necessarily complete and you should read the documents that are filed as exhibits to the registration statement or
otherwise filed with the SEC for a more complete understanding of the document or matter.
We
are subject to the informational requirements of the Exchange Act. Our Annual Report on Form 20-F for the year ending March 31, 2023
has been filed with the SEC. The company has also filed periodic reports with the SEC on Form 6-K. You may inspect and copy reports and
other information filed with the SEC at the Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Information on the operation
of the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains an Internet website
that contains reports and other information about issuers, like us, that file electronically with the SEC. The address of that website
is www.sec.gov.
As
a foreign private issuer, we are exempt under the Exchange Act from, among other things, the rules prescribing the furnishing and
content of proxy statements, and our executive officers, directors and principal shareholders are exempt from the reporting and
short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we will not be required under the
Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose
securities are registered under the Exchange Act.
PROSPECTUS

$100,000,000
Ordinary
Shares
Warrants
Units
We
may offer, issue and sell from time to time up to $100,000,000, or its equivalent in any other currency, currency units, or composite
currency or currencies, of our ordinary shares, warrants to purchase ordinary shares, and a combination of such securities, separately
or as units, in one or more offerings. This prospectus provides a general description of offerings of these securities that we may undertake.
We
refer to our ordinary shares, warrants, and units collectively as “securities” in this prospectus.
Each
time we sell our securities pursuant to this prospectus, we will provide the specific terms of such offering in a supplement to this
prospectus. The prospectus supplement may also add, update, or change information contained in this prospectus. You should read this
prospectus, the accompanying prospectus supplement, together with the additional information described under the heading “Where
You Can Find More Information,” before you make your investment decision.
We
may, from time to time, offer to sell the securities, through public or private transactions, directly or through underwriters, agents
or dealers, on or off The Nasdaq Capital Market, at prevailing market prices or at privately negotiated prices. If any underwriters,
agents or dealers are involved in the sale of any of these securities, the applicable prospectus supplement will set forth the names
of the underwriter, agent or dealer and any applicable fees, commissions or discounts.
Our
ordinary shares are listed on The Nasdaq Capital Market under the symbol “OKYO”. On June 7, 2023, the last reported price
of our ordinary shares on The Nasdaq Capital Market was $1.45 per share.
We
are an “emerging growth company,” as defined by the Jumpstart Our Business Startups Act of 2012, or JOBS Act, and as such,
have elected to comply with certain reduced public company reporting requirements for this prospectus and future filings.
Investing
in our securities involves a high degree of risk. Please carefully consider the risks discussed in this prospectus under “Risk
Factors” in this prospectus, in any accompanying prospectus supplement and in the documents incorporated by reference in this prospectus
for a discussion of the factors you should carefully consider before deciding to purchase our securities.
Neither
the U.S. Securities and Exchange Commission, any U.S. state securities commission, nor any other foreign securities commission has approved
or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a
criminal offense.
The
date of this prospectus is June 14, 2023.
Table
of Contents
ABOUT
THIS PROSPECTUS
This
prospectus is part of a registration statement that we filed with the U.S. Securities and Exchange Commission, or SEC, using a “shelf”
registration process. Under this shelf registration process, we may sell our securities described in this prospectus in one or more offerings
up to a total dollar amount of $100,000,000. Each time we offer our securities, we will provide you with a supplement to this prospectus
that will describe the specific amounts, prices and terms of the securities we offer. The prospectus supplement may also add, update
or change information contained in this prospectus. This prospectus, together with applicable prospectus supplements and the documents
incorporated by reference in this prospectus and any prospectus supplements, includes all material information relating to an offering
of our securities. Please read carefully both this prospectus and any prospectus supplement together with additional information described
below under “Where You Can Find More Information” and “Incorporation of Certain Information by Reference.”
You
should rely only on the information contained in or incorporated by reference in this prospectus and any applicable prospectus supplement.
We have not authorized anyone to provide you with different or additional information. If anyone provides you with different or inconsistent
information, you should not rely on it. The information contained in this prospectus is accurate only as of the date of this prospectus,
regardless of the time of delivery of this prospectus or any sale of securities described in this prospectus. This prospectus is not
an offer to sell our securities and it is not soliciting an offer to buy our securities in any jurisdiction where the offer or sale is
not permitted. You should assume that the information appearing in this prospectus or any prospectus supplement, as well as information
we have previously filed with the SEC and incorporated by reference, is accurate as of the date on the front of those documents only.
Our business, financial condition, results of operations and prospects may have changed since those dates. This prospectus may not be
used to consummate a sale of our securities unless it is accompanied by a prospectus supplement.
Throughout
this prospectus, unless otherwise designated, the terms “OKYO,” “OKYO Pharma Limited,” “the company,”
“we,” “us” and “our” refer to OKYO Pharma Limited and its wholly-owned subsidiary, OKYO Pharma US,
Inc. References to “ordinary shares”, “warrants” and “share capital” refer to the ordinary shares,
warrants and share capital, respectively, of OKYO Pharma Limited.
Certain
figures included in this prospectus have been subject to rounding adjustments. Accordingly, figures shown as totals in certain tables
may not be an arithmetic aggregation of the figures that precede them.
We
have not authorized anyone to provide you with information that is different from that contained in this prospectus, any amendment or
supplement to this prospectus, or in any free writing prospectus we may authorize to be delivered or made available to you. We take no
responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus
is not an offer to sell securities, and it is not soliciting an offer to buy securities, in any jurisdiction where the offer or sale
is not permitted. The information contained in this prospectus is accurate only as of the date on the front of this prospectus, regardless
of the time of delivery of this prospectus or any sale of the securities. For investors outside of the United States: We have not taken
any action to permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose
is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to
this offering and the distribution of this prospectus.
The
industry in which we operate is subject to a high degree of uncertainty and risk due to a variety of factors, including those described
in the section titled “Risk Factors.” These and other factors could cause results to differ materially from those expressed
in the estimates made by the independent parties and by us.
We
qualify as an “emerging growth company,” as defined in the JOBS Act. An emerging growth company may take advantage of specified
reduced reporting and regulatory requirements in contrast to those otherwise applicable generally to public companies. These provisions
include, but are not limited to, an exemption from the auditor attestation requirement in the assessment of our internal control over
financial reporting pursuant to Section 404 the Sarbanes-Oxley Act of 2002, as amended.
We
may take advantage of these reduced reporting and other regulatory requirements until such time that we are no longer an emerging growth
company. We will remain an “emerging growth company” until the earliest of (i) the last day of the fiscal year in which we
have total annual gross revenues of $1.07 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the
date of our initial public offering; (iii) the date on which we have issued more than $1 billion in non-convertible debt during the previous
three years; or (iv) the date on which we are deemed to be a “large accelerated filer” as defined in Rule 12b-2 of the Securities
Exchange Act of 1934, as amended, or the Exchange Act. In addition, the JOBS Act provides that an emerging growth company may delay adopting
new or revised accounting standards until those standards apply to private companies.
We
are a “foreign private issuer” as defined in Rule 3b-4 under the Exchange Act. As a result, our proxy solicitations are not
subject to the disclosure and procedural requirements of Regulation 14A under the Exchange Act and transactions in our equity securities
by our officers and directors are exempt from Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act
to file periodic reports and financial statements as frequently or as promptly as U.S. companies whose securities are registered under
the Exchange Act.
CAUTIONARY
STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus contains forward-looking statements that involve substantial risks and uncertainties. The forward-looking statements are contained
principally in the sections of this prospectus titled “About this Prospectus,” “Risk Factors,” and “Prospectus
Summary.” All statements, other than statements of historical facts, contained in this prospectus, including statements regarding
our future results of operations and financial position, business strategy, prospective products, product approvals, research and development
costs, timing and likelihood of success, plans and objectives of management for future operations, and future results of current and
anticipated products, are forward-looking statements. These statements relate to future events or to our future financial performance
and involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements
to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements.
The words “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,”
“estimate,” “expect,” “goal,” “intend,” “may,” “might,” “objective,”
“plan,” “potential,” “predict,” “project,” “positioned,” “seek,”
“should,” “target,” “will,” “would,” or the negative of these terms or other similar
expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying
words. These forward-looking statements are based on current expectations, estimates, forecasts and projections about our business and
the industry in which we operate and management’s beliefs and assumptions, are not guarantees of future performance or development
and involve known and unknown risks, uncertainties and other factors.
Actual
results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we
make. As a result, any or all of our forward-looking statements in this prospectus may turn out to be inaccurate. We have included important
factors in the cautionary statements included in this prospectus, particularly in the section of this prospectus titled “Risk Factors,”
that we believe could cause actual results or events to differ materially from the forward-looking statements that we make. We may not
actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance
on our forward-looking statements. Moreover, we operate in a highly competitive and rapidly changing environment in which new risks often
emerge. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the
extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking
statements we may make. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions,
joint ventures or investments we may make.
You
should read this prospectus and the documents that we reference in this prospectus and have filed as exhibits to the registration statement
of which this prospectus is a part completely and with the understanding that our actual future results may be materially different from
what we expect. The forward-looking statements contained in this prospectus are made as of the date of this prospectus, and we do not
assume any obligation to update any forward-looking statements except as required by applicable law and regulation.
PROSPECTUS
SUMMARY
You
should read the following summary together with the more detailed information about us, the securities that may be sold from time to
time, and our financial statements and the notes thereto, all of which appear elsewhere in this prospectus or in the documents incorporated
by reference in this prospectus.
Overview
We
are a clinical-stage biopharmaceutical company developing next-generation therapeutics to improve the lives of patients suffering from
inflammatory eye diseases and ocular pain. Our research program is focused on a novel G Protein-Coupled Receptor, or GPCR, which we believe
plays a key role in the pathology of these inflammatory eye diseases of high unmet medical need. Our therapeutic approach is focused
on targeting inflammatory and pain modulation pathways that drive these conditions. We are presently developing OK-101, our lead preclinical
product candidate, for the treatment of dry eye disease (“DED”). We also plan to evaluate its potential in benefiting patients
with ocular neuropathic pain, uveitis and allergic conjunctivitis. We have also been evaluating OK-201, a bovine adrenal medulla, or
BAM, lipidated-peptide preclinical analogue candidate that is currently in developmental stage.
On
February 21, 2018, we announced that we successfully obtained (via assignment from Panetta Partners Ltd., a related party) a license
from On Target Therapeutics LLC, or OTT, to patents owned or controlled by OTT and a sub-license from OTT to certain patents licensed
by OTT from Tufts Medical Center Inc., or TMC, to support our ophthalmic disease drug programs. These licenses gave us the right to exploit
the intellectual property, or IP estate which is directed to compositions-of-matter and methodologies for treating ocular inflammation,
DED, with chemerin or lipid-linked chemerin analogues. We also have a license from TMC to a separate IP estate for treating symptoms
of ocular neuropathic pain and uveitis associated pain. On August 6, 2019, we signed a collaborative agreement with TMC on a research
program focused on ocular neuropathic pain.
On
January 7, 2021, we announced the appointment of Mr. Gabriele Cerrone as Non-Executive Chairman and Director, and Gary S. Jacob, Ph.D.
as Chief Executive Officer and Director. The addition of these two individuals was a significant step for us, highlighting a careful
realignment of the strategic focus of our research and development program, with the aim of facilitating advancement of both of our preclinical
programs. We believed this realignment would allow us to file investigational new drug, or IND, applications on our drug candidates with
the U.S. Food and Drug Administration, or FDA, in the shortest time possible.
OK-101
OK-101,
our lead clinical-stage product candidate, is focused on keratoconjunctivitis sicca, commonly referred to as DED, which is a multifactorial
disease caused by an underlying inflammation resulting in the lack of lubrication and moisture in the surface of the eye. DED is one
of the most common ophthalmic conditions encountered in clinical practice. Symptoms of DED include constant discomfort and irritation
accompanied by inflammation of the ocular surface, visual impairment and potential damage to the ocular surface. There are presently
approximately 20 million people suffering from DED in the U.S. alone (Farrand et al. AJO 2017; 182:90), with the disease affecting approximately
up to 34% of the population aged 50+ (Dana et al. AJO 2019; 202:47), and with women representing approximately two-thirds of those affected
(Matossian et al. J Womens Health (Larchmt) 2019; 28:502–514). Prevalence of DED is anticipated to increase substantially in the
next 10-20 years due to aging populations in the U.S., Europe, Japan and China and use of contact lenses in the younger population. We
believe this increase in prevalence of DED represents a major expanding economic burden to public healthcare. According to Market Research
Report, Dry Eye Disease, December 2020, the global DED market in 2019 was approximately $5.22 billion, with the market size expected
to reach $6.54 billion by 2027. In addition, DED causes approximately $3.8 billion annually in healthcare costs and represents a major
economic burden to public healthcare, accounting for more than $50 billion to the U.S. economy annually.
At
present, there are 5 prescription drugs available to treat DED: 1) Restasis (0.05% cyclosporine), 2) Cequa (0.09% cyclosporine), 3) Xiidra
(5% lifitegrast), 4) Tyrvaya (0.03 mg varenicline), and 5) Eysuvis (0.25% loteprednol – a corticosteroid for short term use only).
However, DED continues to be a major unmet medical need due to the large number of patients not well served by the treatments available
to them through the medical community.
The
development of new drugs to treat DED has been particularly challenging due to the heterogeneous nature of the patient population suffering
from DED, and due to the difficulties in demonstrating an improvement in both signs and symptoms of the disease in well-controlled clinical
trials. The evidence from over 40 years of scientific literature, however, suggests inflammation as the most common underlying element
of DED. Consequently, development of new therapeutic agents that target inflammatory pathways is looking to be an attractive approach
in improving symptoms in DED patients. Moreover, large number of dry eye patients suffer from ocular neuropathic pain, making their condition
more resistant to topical anti-inflammatory therapy, and a drug capable of targeting both of these aspects of DED would be a significant
addition to the ocular-care practitioner’s arsenal for the treatment of DED.
The
chemerin receptor (CMKLR1 or ChemR23) is a chemokine like GCPR expressed on select populations of cells including inflammatory mediators,
epithelial and endothelial cells as well as neurons and glial cells in the dorsal root ganglion, spinal cord, and retina. Activation
of CMKLR1 by chemerin has been shown to resolve the inflammation and pain in animal models of asthma and pain, respectively. We have
been pioneering the development of OK-101, a lipidated-chemerin analogue, which is an agonist of CMKLR1, in treating DED and other ocular
inflammatory conditions. OK-101 was first identified in a program developed by OTT using membrane-tethered ligand technology.
To
expand our understanding of the structure-activity relationships of the lipidated-chemerin analogues, such as OK-101, as agonists of
the chemerin receptor, we synthesized a small library of analogues of OK-101. We screened these analogues in a cell-line based receptor
binding assay to characterize the agonist potency of these lipidated-chemerin analogues. This work has also been coupled to an evaluation
of a subset of these analogues’ potential in treating DED by using a variety of preclinical studies and dry eye animal model studies.
After evaluating a number of our analogues in a mouse model of acute DED by looking at their ability to reduce corneal permeability,
a measure of dry-eye effectiveness, as well as the analogues’ impact on immune response, we determined that OK-101 was in fact
the most potent analogue in reducing corneal permeability and down-regulating immune response. In addition, in a separate set of animal
model experiments, OK-101 was shown to exhibit potent ocular pain-reducing activity in a ciliary nerve ligation mouse model of corneal
neuropathic pain. Following these studies, we evaluated the ocular tolerance of OK-101 via repeated ocular instillation in rabbits
followed by clinical ophthalmic observations. Rabbit ocular tolerance tests on OK-101 showed no adverse signs such as inflammation, chemosis
or hyperemia and no signs of local irritation. With potential anti-inflammatory and neuropathic pain reducing characteristics, we are
developing OK-101 for the treatment of DED.
Based
on the results from the DED animal model, the neuropathic corneal pain model as well as the rabbit ocular tolerance studies, we moved
forward over the past 18 months with plans to file an IND on OK-101 to treat DED to enable us to begin clinical trials soon thereafter.
During the fourth quarter of 2021 we successfully manufactured a 200-gram batch of OK-101 drug substance needed for initiating the IND-enabling
studies that were begun during the first quarter of 2022. In support of this work, we also had previously signed an agreement on April
13, 2021, with Ora, Inc., or Ora, a major clinical research organization, or CRO, specializing in ophthalmic drug development.
We
recently completed the final stages of a concerted effort to complete all IND enabling activities and filed with FDA the IND on OK-101
to treat DED on November 18, 2022. On December 22, 2022 we announced that we received clearance of the IND application from the FDA to
enable us to initiate a Phase 2, first-in-human, clinical study of OK-101 for the treatment of DED.
On
February 15, 2022, we announced the successful completion of the pre-IND meeting facilitated by Ora with the FDA regarding development
plans for OK-101 to treat DED. Both nonclinical and clinical development milestones were covered in the pre-IND meeting, with the FDA
agreeing that our first human trial would be a Phase 2 safety and efficacy trial in DED patients. The FDA also provided guidance on the
planned protocol for this trial in DED patients, concurring with one particular option OKYO has considered for the protocol which is
to designate co-primary efficacy endpoints covering both a sign and a symptom of DED in the clinical trial. Notably, the final decision
we recently took to, in fact, designate these two primary efficacy endpoints in the clinical protocol of the ongoing phase 2 trial is
significant as should this phase 2 trial then meet these prespecified endpoints, the trial should considerably affect the timeline to
an NDA filing with the FDA for OK-101 to treat DED.
On
May 2, 2023, we announced that the first patient has been screened for our Phase 2, multi-center, randomized, double-blinded, placebo-controlled
trial of OK-101. Because the drug is designed to be administered topically, we were able to skip the standard Phase 1 studies typically
expected with orally delivered or injectable drug candidates in non-life-threatening conditions and we opened the first trial with OK-101
as a Phase 2 clinical trial in DED patients (See OKYO Pipeline below). This trial is planned to be conducted in approximately 200 to
240 DED patients. The study is being designed in conjunction with and is being managed and monitored by Ora, well known for its leadership
of ophthalmic clinical trial activities. The Phase 2 trial is expected to be completed in 6-8 months from enrollment of the first patient.
OKYO
Pipeline
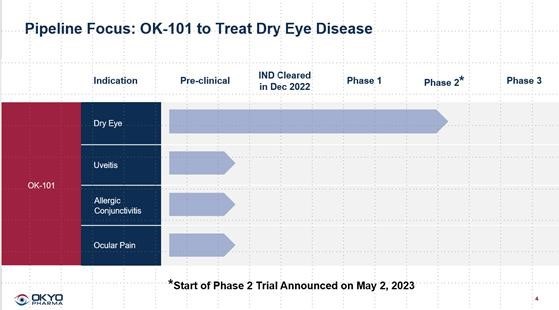
Risks
Associated with Our Business
Our
business is subject to a number of risks of which you should be aware before making an investment decision. You should carefully consider
all of the information set forth in this prospectus and, in particular, should evaluate the specific factors set forth in the section
titled “Risk Factors” before deciding whether to invest in our ordinary shares. These important risks include, but are not
limited to, the following:
●
We have only recently committed to our new business and our product candidates are in the early stages of development and it may be some
years until we generate revenue, if at all.
●
Our product candidates have not been evaluated in clinical trials and results in the clinic may not be reproduced in human trials.
●
There is a high degree of failure for product candidates as they progress through clinical trials and clinical trial data may be interpreted
in varying ways which may delay, limit or prevent future regulatory approvals.
●
The development of pharmaceutical products carries significant risk of failure in early and late stage development programs.
●
We anticipate that we will continue to incur significant losses for the foreseeable future.
●
We will need to spend extensively on further research activities and there can be no guarantee that we will have access to sufficient
funds to fully realize our research and development plan or to commercialize any products derived from research activities.
●
Even if we successfully develop a product which shows efficacy in human subjects there remain high barriers to commercial success.
●
We face significant competition from pharmaceutical companies. We have competitors internationally, including major multinational pharmaceutical
companies, universities and research institutions. In respect of OK-101 as an indication for the treatment of DED, there are a number
of established companies engaged in the development and marketing of preparations addressing the DED market. In addition, there are a
wide range of products addressing the DED market currently approved and marketed by a number of large and small pharmaceutical companies.
●The
expiration of certain intellectual property rights or an inability to obtain, maintain or enforce adequate intellectual property rights
for products that are marketed or in development may result in additional competition from other third-party products. Third parties
may have blocking intellectual property rights which could prevent the sale of products by us or require that compensation be paid to
such third parties.
●
Our product candidates could infringe patents and other intellectual property rights of third parties.
●
COVID-19 has adversely affected our business, and any new pandemic, epidemic or outbreak of an infectious disease may further adversely
affect our business.
●
The relationship of the UK with the EU could impact our ability to operate efficiently in certain jurisdictions or in certain markets.
●
Even if we complete the necessary clinical trials, we cannot predict when, or if, we will obtain regulatory approval to commercialize
our product candidates and the approval may be for a more narrow indication than we seek.
●
If our competitors are able to obtain orphan drug exclusivity for products that constitute the same drug and treat the same indications
as our product candidates, we may not be able to have competing products approved by applicable regulatory authorities for a significant
period of time. In addition, even if we obtain orphan drug exclusivity for any of our products, such exclusivity may not protect us from
competition.
●
Even if we obtain regulatory approval for a product candidate, our product candidates will remain subject to regulatory oversight.
●
Even if we obtain and maintain approval for our product candidates in a major pharmaceutical market such as the United States, we may
never obtain approval for our product candidates in other major markets.
●
We may seek a conditional marketing authorization in the United Kingdom and EU for some or all of our current product candidates, but
we may not be able to obtain or maintain such designation.
●
Healthcare legislative reform measures may have a negative impact on our business and results of operations.
●
We are subject to governmental regulation and other legal obligations related to privacy, data protection and data security. Our actual
or perceived failure to comply with such obligations could harm our business.
●
We do not know whether an active, liquid and orderly trading market will develop for our ordinary share’s or what the market price
of our ordinary shares will be. As a result, it may be difficult for ordinary shareholders to sell their ordinary share’s.
●
Holders of our ordinary shares may experience substantial dilution upon the exercise of outstanding options and warrants.
●
The rights of our shareholders may differ from the rights typically offered to shareholders of a U.S. corporation.
●
If we are a passive foreign investment company, there could be adverse U.S. federal income tax consequences to U.S. holders.
Corporate
Information
We
were originally incorporated in the British Virgin Islands as a British Virgin Islands Business Company on July 4, 2007 under the BVI
Business Companies Act 2004 with company number 1415559 under the name Jellon Enterprises, Inc. Our legal and commercial name was changed
to Minor Metals & Mining, Inc. on October 24, 2007, to Emerging Metals Limited on November 28, 2007, to West African Minerals Corporation
on December 9, 2011, and to OKYO Pharma Corporation on January 10, 2018. On March 9, 2018, shareholders approved the cancellation of
our AIM listing and migration to Guernsey. On July 3, 2018, following the approval of the Guernsey Companies Registry, we were registered
under the Guernsey Companies Law under the name OKYO Pharma Limited, as a Guernsey company with limited liability, an indefinite life
and company number 65220. We are domiciled in Guernsey. On July 17, 2018, our Ordinary Shares were admitted to listing on the standard
segment of the Official List of the FCA and admitted to trading on the Main Market of the London Stock Exchange. We are subject to the
Takeover Code.
Our
registered office is located at Martello Court, Admiral Park, St. Peter Port, Guernsey GY1 3HB and our telephone number is +44 (0) 20
7495 2379. Our website address is www.okyopharma.com. The reference to our website is an inactive textual reference only and the information
contained in, or that can be accessed through, our website is not a part of this registration statement. Our agent for service of process
in the United States is OKYO Pharma US, Inc.
“OKYO,”
the OKYO logo and other trademarks or service marks of OKYO Pharma Limited appearing in this prospectus are the property of OKYO or our
subsidiary. This prospectus contains additional trade names, trademarks and service marks of others, which are the property of their
respective owners. Solely for convenience, trademarks and trade names referred to in this prospectus may appear without the ® or
TM symbols.
Implications
of Being an Emerging Growth Company
We
are an EGC as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. As such, we may take advantage of certain
exemptions from various reporting requirements that are applicable to other publicly traded entities that are not EGCs. These exemptions
include:
| |
● |
the
option to present only two years of audited financial statements and related discussion in the section titled “Management’s
Discussion and Analysis of Financial Condition and Results of Operations” in this prospectus; |
| |
|
|
| |
● |
not
being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or Sarbanes-Oxley
Act; |
| |
|
|
| |
● |
not
being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory
audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial
statements (i.e., an auditor discussion and analysis); |
| |
|
|
| |
● |
not
being required to submit certain executive compensation matters to stockholder advisory votes, such as “say-on-pay,”
“say-on-frequency,” and “say-on-golden parachutes;” and |
| |
|
|
| |
● |
not
being required to disclose certain executive compensation related items such as the correlation between executive compensation and
performance and comparisons of the chief executive officer’s compensation to median employee compensation. |
Section
107 of the JOBS Act also provides that an EGC can take advantage of the extended transition period provided in Section 13(a) of the Exchange
Act, for complying with new or revised accounting standards. As a result, an EGC can delay the adoption of certain accounting standards
until those standards would otherwise apply to private companies.
We
will remain an EGC until the earliest of: (1) the last day of the first fiscal year in which our annual gross revenues exceed $1.235
billion; (2) the last day of our fiscal year following the fifth anniversary of the completion of our initial public offering; (3) the
date that we become a “large accelerated filer” as defined in Rule 12b-2 under the Exchange Act, which would occur on the
last day of any fiscal year that the aggregate worldwide market value of our common equity held by non-affiliates exceeds $700 million
as of the last business day of our most recently completed second fiscal quarter; or (4) the date on which we have issued more than $1.0
billion in non-convertible debt securities during any three-year period.
Implications
of Being a Foreign Private Issuer
We
currently report under the Exchange Act as a non-U.S. company with FPI status. Even after we no longer qualify as an EGC, as long as
we qualify as an FPI under the Exchange Act we will be exempt from certain provisions of the Exchange Act that are applicable to U.S.
domestic public companies, including:
| |
● |
the
sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered
under the Exchange Act; |
| |
|
|
| |
● |
the
sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability
for insiders who profit from trades made in a short period of time; and |
| |
|
|
| |
● |
the
rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q containing unaudited financial and
other specific information, and current reports on Form 8-K upon the occurrence of specified significant events. |
FPIs
are also exempt from certain more stringent executive compensation disclosure rules. Thus, even if we no longer qualify as an EGC, but
remain an FPI, we will continue to be exempt from the more stringent compensation disclosures required of companies that are neither
an EGC nor an FPI.
RISK
FACTORS
Investing
in our securities involves significant risk. The prospectus supplement applicable to each offering of our securities will contain a discussion
of the risks applicable to an investment in our company. Prior to making a decision about investing in our securities, you should carefully
consider the specific factors discussed under the heading “Risk Factors” in the applicable prospectus supplement, together
with all of the other information contained or incorporated by reference in the prospectus supplement or appearing or incorporated by
reference in this prospectus. You should also consider the risks, uncertainties and assumptions discussed under the heading “Risk
Factors” included in our most recent Annual Report on Form 20-F and any subsequent Annual Reports on Form 20-F we file after the
date of this prospectus, and all other information contained in or incorporated by reference into this prospectus or the registration
statement of which this prospectus forms a part, as updated by our subsequent filings under the Exchange Act and the risk factors and
other information contained in any applicable prospectus supplement before acquiring any of our securities. Additional risks and uncertainties
not presently known to us or that we currently deem immaterial may also affect our operations. The occurrence of any of these risks might
cause you to lose all or part of your investment in the offered securities.
CAPITALIZATION
A
prospectus supplement or report on Form 6-K incorporated by reference into the registration statement of which this prospectus forms
a part will include information on our consolidated capitalization.
USE
OF PROCEEDS
Except
as otherwise provided in the applicable prospectus supplement, we intend to use the net proceeds from the sale of the securities offered
by this prospectus for general corporate purposes, which may include working capital, capital expenditures, research and development
expenditures, regulatory affairs expenditures, clinical trial expenditures, acquisitions of new technologies and investments, and the
repayment, refinancing, redemption or repurchase of indebtedness or capital stock.
The
intended application of proceeds from the sale of any particular offering of securities using this prospectus will be described in the
accompanying prospectus supplement relating to such offering. The precise amount and timing of the application of these proceeds will
depend on our funding requirements and the availability and costs of other funds.
DESCRIPTION
OF SHARE CAPITAL AND MEMORANDUM OF ASSOCIATION
We
were originally incorporated in the British Virgin Islands as a British Virgin Islands Business Company on 4 July 2007 under the BVI
Business Companies Act 2004 with company number 1415559 under the name Jellon Enterprises, Inc. Our legal and commercial name was changed
to Minor Metals & Mining, Inc. on October 24, 2007, to Emerging Metals Limited on November 28, 2007, to West African Minerals Corporation
on December 9, 2011, and to OKYO Pharma Corporation on January 10, 2018. On March 9, 2018, shareholders approved the cancellation of
the Company’s AIM listing and migration to Guernsey. On July 3, 2018, following the approval of the Guernsey Companies Registry,
the Company was registered under the Guernsey Companies Law under the name OKYO Pharma Limited, as a Guernsey company with limited liability,
an indefinite life and company number 65220. The Company is domiciled in Guernsey.
Our
registered office is located at Martello Court, Admiral Park, St. Peter Port, Guernsey, GY1 3HB and our telephone number is +44 (0) 20
7495 2379. Our website address is www.okyopharma.com. The reference to our website is an inactive textual reference only and the information
contained in, or that can be accessed through, our website is not a part of this registration statement.
Current
authorized share capital
Not
applicable.
Current
issued share capital
As
of May 26, 2023, our issued share capital was 25,519,774 ordinary shares, no par value. Each issued ordinary share is fully paid.
Options
As
of May 26, 2023, there were vested options to purchase 550,856 ordinary shares outstanding with a weighted average exercise price of
$4.41 per ordinary share. The remaining options to purchase 76,164,333 ordinary shares vest between January 31, 2023 and March 14, 2027.
Warrants
As
of May 26, 2023, there were warrants to purchase 538,461 ordinary shares outstanding as follows:
| No.
outstanding |
|
|
Exercise
Price |
|
|
Exercise
Price |
|
|
Final
Exercise Date |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 538,461 |
|
|
$ |
3.610 |
|
|
£ |
2.925 |
|
|
|
07/17/2023 |
|
Warrants
to purchase 538,491 ordinary shares are exercisable based on the achievement of milestones, with a final exercise date of July 17, 2023.
Information
about the Ordinary Shares
In
accordance with our Articles, the following summarizes the rights of holders of our ordinary shares:
| |
● |
each
holder of our ordinary shares is entitled to one vote per ordinary share on all matters to be voted on by shareholders generally; |
| |
|
|
| |
● |
the
holders of the ordinary shares shall be entitled to receive notice of, attend, speak and vote at our general meetings; and |
| |
|
|
| |
● |
holders
of our ordinary shares are entitled to receive such dividends as are recommended by our directors and declared by our shareholders. |
Articles
We
are incorporated in Guernsey as a non-cellular company limited by shares under company number 65220. We are governed by our Articles
and the Guernsey Companies Law.
Our
Articles were adopted by a special resolution of our shareholders at our annual general meeting held on September 25, 2020. The summary
below is not a complete copy of the terms of the Articles.
The
Articles contain no specific restrictions on our purpose and therefore our purpose is unrestricted.
The
Articles contain, among other things, provisions to the following effect:
Share
Capital
Our
share capital currently consists of ordinary shares. Subject to the Guernsey Companies Law and to any rights attached to existing shares,
we may issue shares with such rights or restrictions as may be determined by the board. In addition, shares which are to be redeemed,
or are liable to be redeemed at our option or the holder of such shares may be issued with the board determining the terms and conditions
of such redemption.
All
of our issued and outstanding ordinary shares are fully paid. Holders of ordinary shares do not have conversion or redemption rights.
There are no provisions in our Articles discriminating against a shareholder as a result of such shareholder’s ownership of a particular
number of shares.
Preferred
Shares
Our
board of directors may provide for other classes of shares, including series of preferred shares. If any preferred shares are issued,
the rights, preferences and privileges of our ordinary shares will be subject to, and may be adversely affected by, the rights of holders
of such preferred shares.
Voting
Subject
to any rights or restrictions attached to any shares, on a show of hands every shareholder who is present in person or by proxy at a
general meeting shall have one vote. On a poll, every shareholder present in person or by proxy at a general meeting shall have one vote
for every ordinary share held by such shareholder. A proxy need not be a shareholder of ours.
A
shareholder shall not be entitled, in respect of any shares held by such shareholder, to vote (either personally or by proxy) at any
general meeting of ours unless all amounts payable by such shareholder in respect of that share in our capital have been paid or credited
as having been paid, or where such shareholder is in default of the provisions in the Articles requiring disclosure of ownership of shares
and we have served a direction notice on such shareholder advising such shareholder that such shares may not be voted.
Variation
of Rights
All
or any of the rights, privileges or conditions attached to any class of shares in issue may only be varied with the consent in writing
of the holders of 75% in value of the issued shares of that class (excluding treasury shares) or with the sanction of a special resolution
passed at a separate general meeting of the holders of the shares of that class. A quorum for the separate class meeting is two persons
(in person or by proxy) holding one-third of the voting rights of the shares of that class or group.
Alteration
of capital
We
may by ordinary resolution:
(a)
consolidate and divide all or any of our share capital into shares of a larger amount than our existing shares;
(b)
sub-divide all or any of our shares into shares of a smaller amount than is fixed by our Articles or by ordinary resolution;
(c)
cancel any shares which, at the date of passing the resolution have not been taken up or agreed to be taken up;
(d)
convert the whole, or any particular class, of our shares into redeemable shares;
(e)
redesignate the whole, or any particular class, of our shares into shares of another class;
(f)
covert all or any of our shares into shares of a nominal amount of a different currency, at the exchange rate; and
(g)
where our shares were expressed in a particular currency, denominate or redenominate it.
Dividends
(a)
Subject to the Guernsey Companies Law, our directors may authorize dividends and distributions to be paid to Shareholders. If any share
is issued on terms providing that it shall rank for dividend or distribution as from a particular date, such share shall rank for dividend
or distribution accordingly.
(b)
Our directors may direct that any dividend or distribution shall be satisfied wholly or partly by the distribution of assets, and in
particular of paid-up shares, debentures, or other securities of any other company.
(c)
No dividend or distribution payable shall bear interest against us.
(d)
A transfer of shares shall not pass the right to any dividend or distribution declared thereon before the registration of the transfer.
(e)
All dividends or distribution unclaimed for a period of one year from the date on which such dividend or distribution was declared may
be invested or otherwise made use of by our directors for the benefit of until claimed.
(f)
All dividends or distribution unclaimed for a period of six years from the date on which such dividend or distribution was declared shall,
if our directors so resolve, be forfeited and shall revert to us.
(g)
Subject to the Guernsey Companies Law or in the terms of issue of any share in our capital, for the purposes of making any distribution
or paying any dividend, our directors may determine that those persons who are entered on the register of members at the close of business
on a day determined by our directors shall be the persons who are entitled to receive such dividends or distributions.
(h)
Payments of dividends or distributions may be made by electronic transfer in such manner as agreed between the member and us or by cheque
or warrant.
Transfer
of Ordinary Shares
A
shareholder may transfer all or any of their shares (i) in the case of certificated shares by transfer in writing in any usual or common
form or in any other form acceptable to our directors; and (ii) in the case of uncertificated shares, in the manner provided for in the
rules and procedures of the operator of the “relevant system” (i.e., the CREST System) and in accordance with and subject
to the CREST Regulations.
The
instrument of transfer of a certified share shall be signed by or on behalf of the transferor and, if the share is not fully paid, by
or on behalf of the transferee.
Our
board of directors may, in its absolute discretion and without assigning any reason, decline to register any transfer of certificated
share or uncertified shares unless it is:
| |
(a) |
in
respect of a share which is fully paid up; |
| |
|
|
| |
(b) |
in
respect of a share in which we have no lien; |
| |
|
|
| |
(c) |
in
respect of only one class of share; |
| |
|
|
| |
(d) |
in
favor of a single transferee or not more than four joint transferees; and |
| |
|
|
| |
(e) |
in
relation to a certificated share, delivered for registration to our registered office (or such other place as our board of directors
may from time to time determine) accompanied by the relevant share certificate(s) and such other evidence as our board of directors
may reasonably require, to show the right of the transferor to make the transfer. |
Our
board of directors shall not refuse to register any transfer or renunciation of partly paid shares which are listed on the Main Market
of the London Stock Exchange on the grounds that they are partly paid shares in circumstances where that refusal would prevent dealings
in any such shares from taking place on an open and proper basis.
Disclosure
of Ownership
Our
directors may by notice in writing require a shareholder to disclose to us the identity of any person other than such shareholder who
has, or has had, at any time during the three years immediately preceding the date on which the notice is issued, any interest (whether
direct or indirect) in the shares held by such shareholder.
If
a shareholder, or any other person appearing to be interested in shares held by that shareholder, has been issued with such a notice
and has failed in relation to any shares, or the Default Interests, to give the Company the information thereby required within the prescribed
period from the service of the notice, our directors may in their discretion serve a direction notice on such shareholder which may direct
that:
(a)
the shareholder shall not be entitled in respect of the Default Interests to be present or to vote (either in person or by proxy) at
any general meeting or at any separate meeting of the holders of any class of shares or on any poll or to exercise any other right conferred
by membership in relation to any such meeting or poll; and
(b)
where the Default Interests represent at least 0.25%. of the number of shares in issue of the class concerned:
(i)
any dividend, distribution or other money payable in respect of the shares shall be withheld by us, which shall not have any obligation
to pay interest on it; and
(ii)
no transfer of the Default Interests held by us shall be registered unless: (I) the shareholder is not themself in default as regards
supplying the information requested; and (II) the shareholder proves to the satisfaction of our directors that no person in default as
regards supplying such information is interested in any of the shares the subject of the transfer.
Requirement
to disclose interests
Each
shareholder shall be under an obligation to comply with the disclosure and notification requirements set out in Chapter 5 of the DTRs.
If the Company determines that a shareholder, or the Defaulting Member, has not complied with the provisions of Chapter 5 of the DTRs
with respect to some or all of such shares held by such Shareholder, or the Default Shares, we shall have the right by delivery of notice
to the Defaulting Member, or a Default Notice, to:
(a)
suspend the right of such Defaulting Member to vote on the Default Shares in person or by proxy at any meeting of ours; and/or
(b)
(i) withhold, without any obligation to pay interest thereon, any dividend or other amount payable with respect to the Default Shares,
(ii) render ineffective any election to receive our shares instead of cash in respect of any dividend or part thereof, and/or (iii) prohibit
the transfer of any of our shares held by the Defaulting Member except with the consent of ours.
Pre-emptive
Rights
Neither
the laws of Guernsey nor the Articles provide shareholders with pre-emptive rights when new shares are issued by the Company.
Board
of Directors
Unless
otherwise determined by the company by ordinary resolution, the number of directors (other than any alternate directors) shall not be
less than one, but there shall be no maximum number of directors.
The
business and affairs of the Company shall be managed by, or under the direction or supervision of the our directors who may pay all expenses
incurred in promoting and registering the Company, and may exercise all such powers necessary for managing, and for directing and supervising
the management of, the our business and affairs as are not, by the Guernsey Companies Law or by the Articles, required to be exercised
by us in a general meeting, subject to the Articles, to the provisions of the Guernsey Companies Law and to such regulations as may be
prescribed by us by special resolution provided that such regulations are not inconsistent with the Articles or the provisions of the
Guernsey Companies Law.
Subject
to the Articles, our directors may meet together for the dispatch of business, adjourn and otherwise regulate their meetings as they
think fit. The quorum necessary for the transaction of the business is two unless otherwise resolved by our directors. A meeting of our
directors at which a quorum is present shall be competent to exercise all powers and discretions for the time being exercisable by our
directors.
A
director who is in any way, directly or indirectly, interested in a proposed transaction or arrangement with us, or in a transaction
or arrangement that has been entered into by us, must declare the nature and extent of such director’s interest to our directors.
The declaration must be made at a meeting of our board of directors, or by written notice, or by general notice, in accordance with the
Guernsey Companies Law and the Articles.
Our
directors shall have power at any time and from time to time to appoint any person to be a director, either to fill a casual vacancy
or as an addition to our existing directors.
Subject
to the provisions of the Guernsey Companies Law and provided the director has disclosed their interest to our other directors, such director
notwithstanding their office may:
(a)
be a party to, or otherwise interested in, any transaction or arrangement with us, or in which we are otherwise interested;
(b)
act by themself or through their firm in a professional capacity for us be entitled to remuneration as if they were not a director;
(c)
be a director or officer of, or employed by, or a party to any transaction or arrangement with, a shareholder of or otherwise directly
or indirectly interested in, any corporate entity promoted by us, or with which we have entered into any transaction with or are interested
in; and
(d)
not by reason of his office, be accountable to us for any benefit which such director derives from any such office or employment or from
any such transaction or arrangement or from any interest in any such body corporate and no such transaction or arrangement shall be liable
to be avoided on the ground of any such interest or benefit.
A
director shall be counted in the quorum at any meeting in relation to any resolution in respect of which such director has declared an
interest and may vote thereon.
A
person must not be appointed as a director unless such person has consented in writing and submitted their declaration that they are
not ineligible to act as a director under the Guernsey Companies Law. A director need not be a shareholder but shall be entitled to receive
notice of and attend all of our general meetings.
No
person shall, unless recommended by our directors, be eligible for election to the office of director at any general meeting unless not
less than three nor more than 21 days before the date appointed for the meeting there shall have been left at our registered office a
notice in writing signed by a shareholder, their intention to propose such a person for election (this must be accompanied by that persons
willingness to be elected and their signed declaration).
Our
directors shall be paid such remuneration (by way of fee) for their services as may be determined by our directors in their absolute
discretion. Our directors shall also be entitled to be repaid all travelling, hotel and other expenses of travelling to and from board
meetings, committee meetings, general meetings, or otherwise incurred while engaged on our business.
Subject
to the provisions of the Guernsey Companies Law, every director shall have the power to purchase and maintain insurance for or for the
benefit of any persons who are or were at any time our directors, officers or employees (including any other company which is its holding
company or in which we have any direct or indirect interest in) against any liability incurred by such persons in respect of any act
or omission in the actual or purported execution and /or discharge of their duties or exercise or purported exercise of their powers
in relation to or in connection with their duties, powers or offices in relation to us or any other such company or subsidiary.
Any
director may at any time by writing appoint any person to be their alternate director and may in like manner at any time terminate such
appointment.
The
office of director shall, ipso facto, be vacated if such director:
(a)
resigns his office by writing under his hand and it is deposited at our registered office and we may agree to accept this at a later
date than specified;
(b)
shall have absented themselves from meetings of the directors for six months in succession and all our other directors have resolved
that such director should vacate their office;
(c)
becomes bankrupt, suspends payment or compounds with such director’s creditors, or is adjudged insolvent or has his affairs declared
en désastre;
(d)
dies;
(e)
becomes ineligible to act as a director under the Guernsey Companies Law;
(f)
is removed by resolution of our directors in writing signed by all of our other directors (being not less than two in number); or
(g)
if we shall by ordinary resolution declare that such person shall cease to be a director.
Indemnity
Our
directors (including any alternate director), secretary and other officer or employee for the time being shall be indemnified out of
the our assets to the fullest extent permitted by the Guernsey Companies Law from and against all actions, costs, charges, losses, damages
and expenses in respect of which they may lawfully be indemnified which they or any of them shall or may incur or sustain by reason of
any contract entered into or any act done, concurred in, or omitted, in or about the execution of their duty or supposed duty or in relation
thereto.
Limitations
on the Rights to Own Our Securities
We
are not aware of any limitations on the rights to own our securities, including rights of non-resident or foreign shareholders to hold
or exercise voting rights on our securities, imposed by foreign law or by our Articles.
General
Meetings
An
annual general meeting of ours shall be held in each calendar year (provided that no more than fifteen months may elapse between one
annual general meeting and the next) at such time and place as may be determined by our directors.
Our
directors may convene a general meeting whenever they think fit. General meetings shall also be convened by the directors within 21 days
of a requisition by our shareholders as provided for by the Guernsey Companies Law.
Unless
special notice is required in accordance with the Guernsey Companies Law, not less than fourteen days’ notice in respect of all
general meeting shall be given to all Shareholders (other than those who, under the provisions of the Articles or otherwise, are not
entitled to receive notices from the Company).
Every
notice shall specify the place, the date and the time of the meeting and the general nature of the business of the meeting. Any general
meeting may be held in Guernsey, or elsewhere, as our directors may from time to time determine. There is no age limit at which a director
is required to retire.
For
the purpose of determining which persons are entitled to attend and vote at any general meeting and how many votes such persons may cast,
the Company may specify in the relevant notice of general meeting a time, not more than forty eight hours (excluding any days which are
not business days) before the time fixed for the meeting, by which a person must be entered on the register of members in order to have
the right to attend and vote at the meeting.
No
business shall be transacted unless the requisite quorum is present when the meeting proceeds to business. Two shareholders present in
person or by proxy and entitled to vote shall be a quorum, save where we only have one shareholder.
If
within half an hour from the time appointed for the general meeting a quorum is not present, if convened on the requisition of the shareholders
the meeting shall be dissolved. In any other case the meeting shall be adjourned to the same day in the next week at the same time and
place and no notice of such adjournment need be given. At any such adjourned meeting, those shareholders present in person or by proxy
shall be a quorum. If no shareholders are present at the adjourned meeting, the meeting shall be dissolved.
Every
question submitted to a general meeting shall be determined in the first instance by a show of hands of the shareholders present in person
or by proxy or by attorney and entitled to vote, but a poll may be demanded by no fewer than five shareholders having the right to vote
on the resolution, or one or more of the shareholders present in person or by proxy representing at least 10%. of the total voting rights
of all of the shareholders having the right to vote on the resolution.
Corporate
representatives
Any
corporation which is a shareholder may by resolution of its directors or other governing body authorize such person as it thinks fit
to act as its representative at any meeting of ours or of any class of shareholders, and the person so authorized shall be entitled to
exercise the same powers on behalf of the corporation which he represents as that corporation could exercise if it were an individual
shareholder.
Borrowing
Powers
Subject
to the Articles and the Guernsey Companies Law, our board of directors may exercise all of the powers of the company to:
| |
(a) |
borrow
money; |
| |
|
|
| |
(b) |
indemnify
and guarantee; |
| |
|
|
| |
(c) |
mortgage
or charge; |
| |
|
|
| |
(d) |
create
and issue debentures and other securities; and |
| |
|
|
| |
(e) |
give
security either outright or as collateral security for any debt, liability or obligation of the company or of any third party. |
Uncertificated
Shares
Subject
to the Guernsey Companies Law, our board of directors may permit title to shares of any class to be issued or held otherwise than by
a certificate and to be transferred by means of a “relevant system” (i.e., the CREST System) without a certificate.
Our
board of directors may take such steps as it sees fit in relation to the evidencing of and transfer of title to uncertificated shares,
any records relating to the holding of uncertificated shares and the conversion of uncertificated shares to certificated shares, or vice-versa.
Our
board of directors may by notice to the holder of an uncertificated share, require that share to be converted into certificated form.
Our
board of directors may take such other action that the board considers appropriate to achieve the sale, transfer, disposal, forfeiture,
re-allotment or surrender of an uncertified share or otherwise to enforce a lien in respect of it.
Winding
up
Subject
to any preferred, deferred or other special rights, or subject to such conditions or restrictions to which any shares in our capital
may be issued, on a winding-up or other return of capital, the holders of our ordinary shares are entitled to share in any surplus assets
pro rata to their holdings of such ordinary shares. A liquidator may, with the sanction of a special resolution of ours and any
other sanction required by the Guernsey Companies Law, divide amongst our shareholders in specie or in kind the whole or any part of
our assets (whether or not the assets shall consist of property of one kind or shall consist of property of different kinds), those assets
to be set at such value as such liquidator deems fair. A liquidator may also vest the whole or any part of our assets in trustees on
trusts for the benefit of the shareholders as the liquidator shall think fit.
Where
the Company is proposed to be or is in the course of being wound up and the whole or part of its business or property is proposed to
be transferred or sold to another company the liquidator may, with the sanction of an ordinary resolution, receive in compensation for
the transfer or sale, shares, policies or other like interests in such other company for distribution among our shareholders or may enter
into any other arrangement whereby our shareholders may, in lieu of receiving cash, shares, policies or other like interests, or in addition
thereto, participate in the profits of or receive any other benefits from such company.
Issue
of shares and share rights
Our
directors may exercise the power of the company for an unlimited duration to issue an unlimited number of shares or grant rights to subscribe
for, or to convert any security into shares.
We
may issue shares which: (i) are redeemable shares; (ii) confer preferential rights to distribution of capital or income; (iii) do not
entitle the holder to voting rights; and (iv) entitle the holder to restricted voting rights. Our directors may issue shares which have
a nominal or par value, no par value, in any number they see fit and in fractions of a share. Subject to “Variation of Rights”
above, we may convert all or any classes of our shares into redeemable shares.
Our
directors may make arrangements on the issue of shares to distinguish between shareholders as to the amounts and the times of payments
of calls on their shares and issue shares that provide for the payment of dividends and distributions in differing proportions.
Acquisition
of own shares
Subject
to the provisions of the Guernsey Companies Law and the rights of holders of any class of shares, we may purchase our own shares, including
redeemable shares.
Liens,
Calls on Shares and Forfeiture
In
respect of any shares we issue that are not fully paid, we will have a first and paramount lien on every share (not being a fully paid
share) for all moneys payable at a fixed time or called in respect of such share. Our board of directors may make calls upon shareholders
for any amounts unpaid in respect of their shares, subject to the terms of allotment (whether in respect of nominal value or premium).
If
a call remains unpaid after it has become due and payable, then, following notice by our board of directors requiring payment of the
unpaid amount together with any accrued interest and expenses incurred, such share may be forfeited by a resolution of our board of directors.
A
shareholder whose shares have been forfeited will cease to be a shareholder in respect of such share, but will, notwithstanding the forfeiture,
remain liable to us for all moneys which at the date of forfeiture were presently payable together with interest. A forfeited share may
be sold, re-allotted or otherwise disposed of as our board of directors sees fit.
Provisions
that Would Delay, Defer or Prevent a Change of Control
There
are no provisions in our Articles that would have the effect of delaying, deferring or preventing a change in control of us and that
would operate only with respect to a merger, acquisition or corporate restructuring involving us or any of our subsidiaries.
Other
Relevant Laws and Regulations
Mandatory
Bid
The
U.K. City Code on Takeovers and Mergers, or Takeover Code, ceased to apply to the Company on May 22, 2023 when the London Main Market
listing was terminated.
Shareholder
rights under Guernsey Law
The
following is a summary of the rights of shareholders under the Guernsey Companies Law and other applicable laws in Guernsey. Prospective
shareholders are advised that this is not a complete statement of the rights of Shareholders under applicable law in Guernsey or under
the Articles.
(a)
Company alterations
Under
the Guernsey Companies Law, it is possible for a Guernsey company to merge with another Guernsey company or an overseas company with
the approval by a special resolution of members, provided that there is a short form amalgamation process for amalgamations between a
company and its wholly-owned subsidiary or between two or more wholly- owned subsidiaries of the same company which does not require
a special resolution of the members of each company.
Under
the Guernsey Companies Law, a compromise or arrangement is permitted between the company and its creditors or shareholders, or any class
thereof, whether for the purpose of facilitating the company’s reconstruction or its merger with another company, or otherwise.
An application must be made to court which court will then order a meeting of the company’s creditors or shareholders. It is necessary
for 75%. in value of the creditors or 75% of the voting rights of the shareholders, or class thereof, as the case may be, to agree to
the compromise or arrangement and if such compromise or arrangement is sanctioned by the court, it will be binding on the creditors or
shareholders, or class thereof, as appropriate.
The
Guernsey Companies Law also requires the approval of the shareholders by special resolution for the removal of a company from the Guernsey
Register of Companies for the purpose of becoming registered as a company under the law of a district, territory or place outside Guernsey.
Under
the Guernsey Companies Law, amendments to a company’s articles of incorporation so permitted may be authorized by way of a special
resolution of the company’s shareholders (provided that certain provisions within a company’s articles of incorporation can
be embedded with a higher voting threshold required for change).
(b)
Rights of dissent and appraisal
The
Guernsey Companies Law contains rights of dissent (the granting of which is discretionary on the part of the court), which are applicable
where the company resolves to:
(i)
amalgamate with another corporation (other than vertical or horizontal short form amalgamations);
(ii)
transfer of its registration into another jurisdiction; or
(iii)
carry out a takeover transaction.
(c)
Shareholder derivative actions
The
laws of Guernsey permit derivative actions to be brought by a shareholder, or such person as the court directs who, in the discretion
of the court, is a proper person to make an application to court to bring a derivative action. Under the laws of Guernsey, the complainant
must obtain permission of the court to commence a derivative action.
(d)
Sale of undertaking
The
Companies Law does not contain provisions in relation to shareholder authority for the sale of a company’s undertaking and, accordingly,
the sale, lease or exchange of all or substantially all the property of the company will be governed by the articles of incorporation
of a company.
(e)
Unfair prejudice
A
member of a company may apply to the court on the ground that the affairs of the company are conducted in a manner that is unfairly prejudicial
to the interests of members generally or of some part of its members (including at least themselves), or an actual or proposed act or
omission of the company is or would be so prejudicial.
If
the court is satisfied that an application is well founded it may make such orders as it sees fit, which may include without limitation:
(a) requiring the company to refrain from doing or continuing to do an act, or require it to do any act which the applicant has complained
it has omitted to do; or (b) providing for the purchase of shares of any member of the company by other members of the company or by
the company itself (and the reduction of the company’s capital accordingly).
Differences
in Corporate Law
As
a non-cellular company limited by shares incorporated in Guernsey, we are governed by the Guernsey Companies Law. The applicable provisions
of the Guernsey Companies Law differ from laws applicable to U.S. corporations and their shareholders. Set forth below is a summary of
certain differences between the provisions of the Guernsey Companies Law applicable to us and the Delaware General Corporation Law relating
to shareholders’ rights and protections. This summary is not intended to be a complete discussion of the respective rights and
it is qualified in its entirety by reference to the laws of Guernsey and Delaware law.
| |
|
Guernsey
|
|
Delaware |
| Shareholder
Meetings |
|
● |
Unless
a company’s memorandum or articles of incorporation state otherwise, the directors are required to call a general meeting once
the company receives requests to do so from shareholders who hold more than 10% of the capital of the company that carries the right
of voting at general meetings (excluding any capital held as treasury shares). |
|
● |
Shareholders
generally do not have the right to call meetings of shareholders unless that right is granted in the certificate of incorporation
or bylaws. |
| |
|
|
|
|
● |
May
be held at such time or place as designated in the certificate of incorporation or the bylaws, or if not so designated, as determined
by the board of directors |
| |
|
|
|
|
|
|
| |
|
● |
Unless
the shareholders pass a resolution exempting the company from holding an annual general meeting, a company must hold a general meeting
of its members within a period of 18 months beginning on the date on which it was incorporated and thereafter at least once every
calendar year (with no more than 15 months elapsing between one annual general meeting and the next). |
|
●
● |
May
be held inside or outside Delaware
Notice: |
| |
|
|
|
|
|
|
| |
|
● |
Subject
to the articles of incorporation, a meeting may be held at any place in Guernsey or elsewhere. |
|
—
Whenever shareholders are required to take any action at a meeting, a written notice of the meeting shall be given which shall state
the place, if any, date and hour of the meeting, and the means of remote communication, if any. |
| |
|
|
|
|
|
|
| |
|
● |
Notice: |
|
|
|
| |
|
|
|
|
|
|
| |
|
—
A meeting must be called by at least 10 days’ notice or such longer period as provided by the articles of incorporation. |
|
|
|
| |
|
|
|
|
|
| |
|
—
A meeting may be called by shorter notice if all shareholders entitled to attend and vote so agree. |
|
|
|
| |
|
|
|
|
|
| |
|
—
The notice shall specify the date, time and place of the meeting, the information of any resolutions to be passed at the meeting
and such other information as is required by the articles of incorporation. |
|
|
|
| |
|
|
|
|
|
| Shareholders’
Voting Rights |
|
● |
Unless
the memorandum or articles of incorporation provide otherwise, directors are appointed by ordinary resolution of the shareholders. |
|
● |
With
limited exceptions, and unless the certificate of incorporation provides otherwise, shareholders may act by written consent to elect
directors. |
| |
|
|
|
|
|
|
| |
|
● |
Any
shareholder may appoint another person or persons to be their proxy to exercise all or any of their rights to attend, speak and vote
at a meeting. |
|
● |
Each
stockholder entitled to vote may authorize another person or persons to act for such shareholder by proxy. |
| |
|
|
|
|
|
|
| |
|
● |
Subject
to the articles of incorporation, the quorum shall be two shareholders holding 5% of the total voting rights of the company between
them. |
|
● |
The
certificate of incorporation or bylaws may specify the number to constitute a quorum, but in no event shall a quorum consist of less
than one-third of shares entitled to vote at a meeting. In the absence of such specifications, a majority of shares entitled to vote,
present in person or represented by proxy, shall constitute a quorum. |
| |
|
|
|
|
|
|
| |
|
● |
Subject
to certain limited exceptions, a provision of the articles of incorporation is void to the extent that it would have the effect of
excluding or making ineffective a demand for a poll at general meeting. |
|
● |
The
certificate of incorporation may provide for cumulative voting. |
| |
|
Guernsey |
|
Delaware |
| Directors |
|
● |
Subject
to the articles of incorporation, the board of directors must consist of at least one director and is not subject to a maximum number
of directors. |
|
● |
The
board of directors must consist of at least one director and is not subject to a maximum number of directors. |
| |
|
|
|
|
|
|
| |
|
● |
Subject
to the articles of incorporation, the board of directors may determine the remuneration or other benefits given to a director. |
|
● |
The
number of directors shall be fixed by the bylaws, unless the certificate of incorporation fixes such number, in which case a change
in the number shall be made only by amendment of the certificate of incorporation. |
| |
|
|
|
|
|
|
| |
|
● |
A
person will cease to be a director if such person: |
|
● |
A
classified board is permitted. |
| |
|
|
|
|
|
|
| |
|
—
provides written notice of their resignation to the company; |
|
● |
The
board of directors has the authority to fix the compensation of directors, unless otherwise restricted by the certificate of incorporation
or bylaws. |
| |
|
|
|
|
|
|
| |
|
—
is removed in accordance with the memorandum and articles of incorporation; |
|
● |
Removal |
| |
|
|
|
|
|
|
| |
|
—
becomes ineligible to be a director under the laws of Guernsey; |
|
|
|
| |
|
|
|
|
|
|
| |
|
—
dies; or |
|
–
Any or all of the directors may be removed, with or without cause, by the holders of a majority of the shares entitled to vote unless
the certificate of incorporation provides otherwise. |
| |
|
|
|
|
|
|
| |
|
—
otherwise vacates office in accordance with the memorandum and articles of incorporation. |
|
–
In the case of a classified board, shareholders may affect removal only for cause. |
| |
|
|
|
|
| Interested
Shareholders’ Transactions |
|
● |
The
Guernsey Companies Law does not contain any specific prohibition on interested shareholder transactions. |
|
● |
The
Delaware General Corporation Law contains a business combination statute applicable to corporations whereby, unless the corporation
has specifically elected not to be governed by such statute, it is prohibited from engaging in certain business combinations with
an “interested shareholder” for three years following the date that such shareholder becomes an interested shareholder.
An interested shareholder generally is a person or a group that owns at least 15% of the corporation’s outstanding voting stock. |
| |
|
|
|
|
|
|
| Interested
Director Transactions |
|
● |
A
director must, immediately after becoming aware of the fact that such director is interested in a transaction or proposed transaction
with the company, disclose to the board the nature and extent of such director’s interest. |
|
● |
Interested
director transactions are permissible and may not be legally voided if: |
| |
|
|
|
|
|
|
| |
|
|
Subject
to the memorandum and articles of incorporation, a director who is interested in a transaction may vote, attend board meetings, sign
documents and do any other thing in such director’s capacity as a director in relation to a transaction in which such director
is interested as if such director was not interested in the transaction provided that such director has made the necessary declarations. |
|
—
the material facts of the director’s interest are disclosed and a majority of the disinterested directors approve the transaction;
|
| |
|
|
|
|
|
|
| |
|
A
transaction in which a director is interested is voidable by the company at any time within 3 months of the date after which the
transaction is disclosed to the board unless: |
|
—
the material facts of the director’s interest are disclosed and a majority of the shareholders entitled to vote approve the
transaction; or |
| |
|
|
|
|
|
|
| |
|
—
the director’s interest was disclosed at the time the transaction was entered into or a disclosure was not required (for example,
if the transaction is entered into in the ordinary course of business and on usual terms and conditions); |
|
—
the transaction is determined to have been fair to the corporation at the time it is authorized, approved or ratified by the board
of directors, a committee thereof or the shareholders. |
| |
|
|
|
|
|
|
| |
|
—
the transaction is ratified by the shareholders; or |
|
|
|
| |
|
|
|
|
|
|
| |
|
—
the company received fair value for the transaction. |
|
|
|
| |
|
Guernsey |
|
Delaware |
| Dividends |
|
● |
A
company may pay a dividend if the board of directors is satisfied on reasonable grounds that the company will, immediately after
payment of the dividend, satisfy the statutory solvency test contained in the Guernsey Companies Law as well as any other requirement
of the memorandum or articles of incorporation. |
|
● |
The
board of directors may declare and pay dividends, subject to any restrictions contained in the certificate of incorporation, upon
the shares of the corporation’s capital stock either: out of its surplus or, in case there is no surplus, out of its net profits
for the fiscal year in which the dividend is declared or the preceding fiscal year. |
| |
|
|
|
|
|
|
| |
|
● |
A
dividend may be of such amount, be paid at such time and be paid to such members as the board of directors thinks fit; provided
that the directors must not authorize a dividend in respect of some but not all of the shares in a class or that is of a greater
value per share in respect of some shares of a class than in respect of other shares of that class. |
|
|
|
| |
|
|
|
|
|
|
| |
|
● |
Subject
to the articles of incorporation, there is no requirement for dividends to be paid out of a particular account or source. |
|
|
|
| |
|
|
|
|
|
|
| Variation
of Rights of Class of Shares |
|
● |
A
company may only vary the rights of a class of shareholders in accordance with the provisions of the articles of incorporation or,
in the absence of such provisions, with the consent in writing from the holders of at least 75% in value of the issued shares of
that class or by means of a special resolution passed by at least 75% in value of the issued shares of that class at a separate meeting
of shareholders of that class. |
|
● |
A
corporation may vary the rights of a class of shares with the approval of a majority of the outstanding shares of such class, unless
the certificate of incorporation provides otherwise. |
| |
|
|
|
|
|
|
| Mergers
and Similar Arrangements |
|
Subject
to the articles of incorporation, a merger, consolidation, sale, lease or transfer of all or substantially all of the assets of a
company may be negotiated and approved by the board of directors. Depending on the structure of such a transaction, a separate shareholder
approval may be required. |
|
● |
Under
the Delaware General Corporation Law, with certain exceptions, a merger, consolidation, sale, lease or transfer of all or substantially
all of the assets of a corporation must be approved by the board of directors and a majority of the outstanding shares entitled to
vote thereon.
|
| |
|
If,
within a period of four months after the date of an offer being made in respect of a transfer of shares, the offer is approved or
accepted by the shareholders comprising not less than 90% in value of the shares affected, the offeree may, within two months immediately
after the last day on which the offer can be approved or accepted, give notice to any dissenting shareholders of its desire to acquire
the remaining shares. On the expiration of one month from the date of the notice to acquire, the offeror will be entitled to acquire
the shares of the dissenting shareholder(s) by sending them a copy of the notice to acquire and by paying or transferring to them
the consideration that such shareholder(s) are entitled to in respect of those shares, at which point the offeror shall be registered
as the holder of those shares. |
|
● |
The
Delaware General Corporation Law also provides that a parent corporation may, by resolution of its board of directors, merge with
any subsidiary of which it owns at least 90% of each class of capital stock without a vote by the shareholders of such subsidiary. |
| |
|
|
|
|
|
|
| Appraisal
Rights |
|
● |
The
Guernsey Companies Law does not specifically provide for any appraisal rights of shareholders. The Guernsey Companies Law does, however,
give the courts of Guernsey broad authority in respect of orders made pursuant to successful unfair prejudice claims under the Guernsey
Companies Law. |
|
● |
A
shareholder of a corporation participating in certain major transactions may, under certain circumstances, be entitled to appraisal
rights under which the shareholder may receive cash in the amount of the fair value of the shares held by such shareholder in lieu
of the transaction consideration. |
| |
|
Guernsey |
|
Delaware |
| Shareholder
Suits |
|
● |
A
shareholder may commence or continue a claim as a representative of those with the same interests in the claim. Unless the court
directs otherwise, any judgment in which a party is acting as a representative will be binding on all persons represented. |
|
● |
Class
actions and derivative actions generally are available to shareholders for, among other things, breach of fiduciary duty, corporate
waste, and actions not taken in accordance with applicable law. In such actions, the court has discretion to permit the winning party
to recover attorneys’ fees incurred in connection with such action. |
| |
|
|
|
|
|
|
| |
|
● |
Derivative
actions are also available to shareholders in respect of a cause of action arising from an actual or proposed act or omission involving:
negligence, default, breach of duty and/or breach of trust by a director of the company. |
|
|
|
| |
|
|
|
|
|
|
| |
|
● |
Costs
are awarded by the court at its discretion. The normal order is for the winning party to recover its costs incurred in connection
the action. |
|
|
|
| |
|
|
|
|
|
|
| Limitations
on Directors’ Liability and Indemnification of Directors and Officers |
|
● |
A
company may include in its articles of incorporation provisions limiting the liability of its directors (and officers or other persons);
however, any provision that purports to exempt a director from any liability in connection with any negligence, default, breach of
duty or breach of trust in relation to the company is void. |
|
● |
A
corporation may include in its certificate of incorporation provisions limiting the personal liability of its directors to the corporation
or its shareholders for monetary damages for certain breaches of fiduciary duty. However, such provisions may not limit liability
for any breach of the duty of loyalty, acts or omissions not in good faith or that involve intentional misconduct or a knowing violation
of law, the authorization of unlawful dividends, stock purchases, or redemptions, or any transaction from which a director derived
an improper personal benefit. |
| |
|
|
|
|
|
|
| |
|
● |
Any
provision by which a company directly or indirectly provides an indemnity for a director of the company, or any associated company,
against any liability in connection with any negligence, default, breach of duty or breach of trust is void, except that: |
|
● |
A
corporation may indemnify a director or officer of the corporation against expenses, judgments, fines and amounts paid in settlement
actually and reasonably incurred in defense of any action, suit or proceeding by reason of such person’s position if (i) the
person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests of the corporation
and (ii) with respect to any criminal action or proceeding, the person had no reasonable cause to believe the conduct was unlawful. |
| |
|
|
|
|
|
|
| |
|
—
a company is not prevented from purchasing and maintaining for a director of the company, or any associated company, insurance against
any such liability; and |
|
|
|
| |
|
|
|
|
|
|
| |
|
—
such restriction does not apply to a qualifying third-party indemnity provision, which is a provision for indemnity against liability
incurred by a director to a person other than the company or an associated company that does not provide any indemnity against a
prescribed list of liabilities, including certain fines and penalties and liabilities incurred in defending certain proceedings. |
|
|
|
| |
|
Guernsey |
|
Delaware |
| Directors’
Fiduciary Duties |
|
● |
The
duties of directors in Guernsey are generally owed to the company and its shareholders as a whole rather than to any other person
or particular shareholders (subject to certain exceptions) and arise from customary laws, statutory laws and contractual obligations. |
|
● |
Directors
of a Delaware corporation have a fiduciary duty to the corporation and its shareholders. This duty has two components: the duty of
care and the duty of loyalty. |
| |
|
|
|
|
|
|
| |
|
● |
Customary
law duties of directors include: |
|
—
The duty of care requires that a director act in good faith, with the care that an ordinarily prudent person would exercise under
similar circumstances. Under this duty, a director must inform themselves of, and disclose to shareholders, all material information
reasonably available regarding a significant transaction. |
| |
|
|
|
|
|
|
| |
|
—
a duty to act in good faith, in the best interests of the company, and not for any collateral purpose; |
|
—
The duty of loyalty requires that a director act in a manner he or she reasonably believes to be in the best interests of the corporation.
He or she must not use their corporate position for personal gain or advantage. This duty prohibits self-dealing by a director and
mandates that the best interest of the corporation and its shareholders take precedence over any interest possessed by a director,
officer or controlling shareholder and not shared by the shareholders generally. |
| |
|
|
|
|
|
|
| |
|
—
a duty to exercise powers for a proper purpose. Even if a director is acting in good faith and in the best interests of the company,
such director must nevertheless use their powers for the proper purpose for which they were conferred; |
|
|
|
| |
|
|
|
|
|
|
| |
|
—
a duty to avoid and mitigate conflicts of interest; and |
|
|
|
| |
|
|
|
|
|
|
| |
|
—
a duty to account for profits. As a fiduciary, a director may not take a personal profit from opportunities arising from such director’s
office, even if the director is acting honestly and in the best interests of the company. Any such profit must be paid to the company.
A director’s entitlement to remuneration and payment of expenses will be governed by the company’s articles of incorporation. |
|
● |
In
general, actions of a director are presumed to have been made on an informed basis, in good faith and in the honest belief that the
action taken was in the best interests of the corporation. However, this presumption may be rebutted by evidence of a breach of one
of the fiduciary duties. Should such evidence be presented concerning a transaction by a director, such director must prove the procedural
fairness of the transaction, and that the transaction was of fair value to the corporation. |
| |
|
|
|
|
|
|
| |
|
● |
Statutory
duties of directors include: |
|
|
|
| |
|
|
|
|
|
|
| |
|
—
a general duty to manage the business and affairs of the company; and |
|
|
|
| |
|
|
|
|
|
|
| |
|
—
the directors are responsible for considering a solvency test in various circumstances, including in authorizing distributions by
the company to its shareholders. |
|
|
|
| |
|
Guernsey |
|
Delaware |
| Inspection
of Books and Records |
|
● |
The
register and index of members, register of directors, register of secretaries and copies of all resolutions of shareholders passed
other than at general meetings and minutes of the proceedings of general meetings, in each case, in the last six years, must be open
for the inspection by any shareholder of the company without charge during ordinary business hours. They must also be open to inspection
by any other person upon payment of such fee as may be prescribed by the Guernsey Committee for Economic Development or such lesser
fee as the company may request. |
|
● |
All
shareholders have the right, upon written demand, to inspect or obtain copies of the corporation’s shares ledger and its other
books and records for any purpose reasonably related to such person’s interest as a shareholder. |
| |
|
|
|
|
|
|
| |
|
● |
When
a company receives a request to inspect its records, the company must comply with that request or apply to the Guernsey courts for
a direction not to comply. |
|
|
|
| |
|
|
|
|
|
|
| Amendments
of Governing Documents |
|
● |
Subject
to certain exceptions, such as the alteration of the statement of the company’s name, a company may only make or alter a provision
of its memorandum of incorporation in accordance with the terms of the memorandum of incorporation or by unanimous resolution of
all of its shareholders. |
|
|
Amendments
to the certificate of incorporation require the affirmative vote of the holders of a majority of the outstanding shares entitled
to vote thereon, unless the certificate of incorporation provides otherwise. Bylaws may be amended with the approval of a majority
of the outstanding shares entitled to vote and may, if provided in the certificate of incorporation, also be amended by the board
of directors. |
| |
|
|
|
|
|
|
| |
|
● |
A
company may alter its articles of incorporation by means of a special resolution passed by at least 75% of the shareholders. |
|
|
|
| |
|
|
|
|
|
|
| Dissolution
and Winding Up |
|
● |
A
company may be dissolved by means of a compulsory or voluntary winding up or a compulsory or voluntary striking off. |
|
|
Unless
the board of directors approves the proposal to dissolve, dissolution must be approved by all of the shareholders. Only if the dissolution
is initiated by the board of directors may it be approved by a simple majority of the corporation’s outstanding shares. |
| |
|
|
|
|
|
|
| |
|
● |
An
application for voluntary winding up requires a special resolution of the members passed by a majority of at least 75%. |
|
|
|
| |
|
|
|
|
|
|
| |
|
● |
An
application for the voluntary striking off of a company must be made by the board of directors and be accompanied by a declaration
of compliance confirming that all requirements of Guernsey law with respect to the striking off have been complied with. |
|
|
|
Other
Guernsey Law Considerations
Registered
Shares
We
are required by the Guernsey Companies Law to keep a register of our shareholders. Under the laws of Guernsey, the ordinary shares are
deemed to be issued when the name of the shareholder is entered in our share register. The share register therefore is prima facie evidence
of the identity of our shareholders, and the shares that they hold. The share register generally provides limited, or no, information
regarding the ultimate beneficial owners of our ordinary shares. Our share register is maintained by our registrar, Computershare Investor
Services (Guernsey) Limited.
We
will perform all procedures necessary to update the share register to reflect any ordinary shares being sold in any potential offering,
including updating the share register with the number of ordinary shares to be issued to the depositary upon the closing of any such
offering in the future. We also are required by the Guernsey Companies Law to register a transfer of shares (or give the transferee notice
of and reasons for refusal as the transferee may reasonably request) as soon as practicable and in any event within two months of receiving
notice of the transfer.
We,
any of our shareholders, or any other affected person may apply to the court for rectification of the share register if:
| |
● |
the
name of any person, without sufficient cause, is entered in or omitted from our register of members; or |
| |
|
|
| |
● |
default
is made or unnecessary delay takes place in entering on the register the fact of any person having ceased to be a member or on which
we have a lien, provided that such delay does not prevent dealings in the shares taking place on an open and proper basis. |
A
shareholder may transfer all or any of his shares (i) in the case of certificated shares by transfer in writing in any usual or common
form or in any other form acceptable to the Directors; and (ii) in the case of uncertificated shares, in the manner provided for in the
rules and procedures of the operator of the relevant system and in accordance with and subject to the CREST Regulations.
The
instrument of transfer of a certified share shall be signed by or on behalf of the transferor and, if the share is not fully paid, by
or on behalf of the transferee.
The
Board may, in its absolute discretion and without assigning any reason, decline to register any transfer of certificated share or uncertified
shares unless it is:
| |
(a) |
in
respect of a share which is fully paid up; |
| |
|
|
| |
(b) |
in
respect of a share in which the Company has no lien; |
| |
|
|
| |
(c) |
in
respect of only one class of share; |
| |
|
|
| |
(d) |
in
favor of a single transferee or not more than four joint transferees; and |
| |
|
|
| |
(e) |
in
relation to a certificated share, delivered for registration to the registered office of the Company (or such other place as the
Board may from time to time determine) accompanied by the relevant share certificate(s) and such other evidence as the Board may
reasonably require to prove the right of the transferor to make the transfer. |
The
Board shall not refuse to register any transfer or renunciation of partly paid shares which are listed on the Main Market of the London
Stock Exchange on the grounds that they are partly paid shares in circumstances where that refusal would prevent dealings in any such
shares from taking place on an open and proper basis
Distributions
and Dividends
(a)
Subject to the Guernsey Companies Law, our directors may authorize dividends and distributions to be paid to shareholders. If any share
is issued on terms providing that it shall rank for dividend or distribution as from a particular date, such share shall rank for dividend
or distribution accordingly.
(b)
Our directors may direct that any dividend or distribution shall be satisfied wholly or partly by the distribution of assets, and in
particular of paid-up shares, debentures, or other securities of any other company.
(c)
No dividend or distribution payable shall bear interest against us.
(d)
A transfer of shares shall not pass the right to any dividend or distribution declared thereon before the registration of the transfer.
(e)
Unless otherwise directed, any dividend or distribution may be paid by way of electronic transfer in such manner as agreed between the
shareholder and us or by cheque or warrant sent through the post to the registered address of such shareholder entitled thereto, or in
the case of joint holders to that one whose name stands first on our register of members in respect of the joint holding.
(f)
All dividends or distribution unclaimed for a period of one year from the date on which such dividend or distribution was declared may
be invested or otherwise made use of by our directors for our benefit until claimed.
(g)
All dividends or distribution unclaimed for a period of six years from the date on which such dividend or distribution was declared shall,
if our directors so resolve, be forfeited and shall revert to us.
(h)
Subject to the Guernsey Companies Law or in the terms of issue of any share in our capital, for the purposes of making any distribution
or paying any dividend, our directors may determine that those persons who are entered on the register of members at the close of business
on a day determined by our directors shall be the persons who are entitled to receive such dividends or distributions.
Limitation
on Owning Securities
Our
Articles do not restrict in any way the ownership or voting of our shares by non-residents.
Purchase
of Own Shares
Our
Articles, a summary of which is provided above, do not prohibit us from purchasing our own shares.
Our
Articles do not have conditions governing changes to our capital which are more stringent that those required by law.
Shareholder
Rights
Certain
rights granted under the Guernsey Companies Law, including the right to requisition a general meeting or require a resolution to be put
to shareholders at the annual general meeting, are only available to our members. For Guernsey law purposes, our members are the persons
who are registered as the owners of the legal title to the shares and whose names are recorded in our register of members. In the case
of shares held in a settlement system operated by the Depository Trust Company, or DTC, the registered member will be DTC’s nominee,
Cede & Co. If a person who holds their ordinary share’s in DTC wishes to exercise certain of the rights granted under the Guernsey
Companies Law, they may be required to first take steps to withdraw their ordinary share’s from the settlement system operated
by DTC and become the registered holder of the shares in our register of members. A withdrawal of shares from DTC may have tax implications,
for additional information on the potential tax implications of withdrawing your shares from the settlement system operated by DTC, see
“Material Tax Considerations—Guernsey Taxation.”
Exchange
Controls and Other Limitations Affecting Security Holders
Under
Guernsey law, there are currently no restrictions on the export or import of capital, including foreign exchange controls or restrictions
that affect the remittance of dividends, interest or other payments to nonresidents holders of our ordinary shares.
Enforcement
of Civil Liabilities
U.S.
laws do not necessarily extend either to us or our officers or directors. We are incorporated under the laws of Guernsey. Some of our
directors and officers reside outside of the United States. Substantially all of the assets of both us and our directors and officers
are located outside the United States. As a result, it may not be possible for investors to effect service of process on either us or
our officers and directors within the United States, or to enforce against these persons or us, either inside or outside the United States,
a judgment obtained in a U.S. court predicated upon the civil liability provisions of the federal securities or other laws of the United
States or any State in the United States.
We
have appointed OKYO Pharma US, Inc., as our agent to receive service of process with respect to any action brought against us in the
United States under the federal securities laws of the United States or of the laws of any state of the United States.
A
judgment of a U.S. court is not directly enforceable in Guernsey, but constitutes a cause of action which may be enforced by Guernsey
courts provided that:
| |
● |
the
applicable U.S. courts had jurisdiction over the case, as recognized under Guernsey law; |
| |
|
|
| |
● |
the
judgment is given on the merits and is final, conclusive and non-appealable; |
| |
|
|
| |
● |
the
judgment relates to the payment of a sum of money, not being taxes, fines or similar governmental penalties; |
| |
|
|
| |
● |
the
defendant is not immune under the principles of public international law; |
| |
|
|
| |
● |
the
same matters at issue in the case were not previously the subject of a judgment or disposition in a separate court; |
| |
|
|
| |
● |
the
judgment was not obtained by fraud; and |
| |
|
|
| |
● |
the
recognition and enforcement of the judgment is not contrary to public policy in Guernsey. |
Guernsey
courts award compensation for the loss or damage actually sustained by the plaintiff. Although punitive damages are generally unknown
to the Guernsey legal system, there is no prohibition on them either by statute or customary law. Whether a particular judgment may be
deemed contrary to Guernsey public policy depends on the facts of each case, though judgments found to be exorbitant, unconscionable,
or excessive will generally be deemed as contrary to public policy. Moreover, certain defendants may qualify for protection under Protection
of Trading Interests Act 1980, an act of the UK extended to Guernsey by the Protection of Trading Interests Act 1980 (Guernsey) Order,
1983. This Act provides that a qualifying defendant is not liable for multiple damages, in excess of that required for actual compensation.
A “qualifying defendant” for these purposes is a citizen of the UK and its Colonies (as defined in the Act), a corporation
or other limited liability entity organized under the laws of the UK, Guernsey or other territory for whose international relations the
UK is responsible or a person conducting business in Guernsey.
Guernsey
courts cannot enter into the merits of the foreign judgment and cannot act as a court of appeal or review over the foreign courts. It
is doubtful that an original action based on U.S. federal or state securities laws could be brought before Guernsey courts. In addition,
a plaintiff who is not resident in Guernsey may be required to provide a security bond in advance to cover the potential of the expected
costs of any case initiated in Guernsey. In addition, Clarivate has been further advised by our legal counsel in Guernsey that it is
uncertain as to whether the courts of Guernsey would entertain original actions or enforce judgments from U.S. courts against us or our
officers and directors which originated from actions alleging civil liability under U.S. federal or state securities laws.
DESCRIPTION
OF WARRANTS
We
may issue and offer warrants under the material terms and conditions described in this prospectus and any accompanying prospectus supplement.
The accompanying prospectus supplement may add, update or change the terms and conditions of the warrants as described in this prospectus.
We
may issue warrants to purchase our ordinary shares. Warrants may be issued independently or together with any securities and may be attached
to or separate from those securities. The warrants may be issued under warrant or subscription agreements to be entered into between
us and a bank or trust company, as warrant agent, all of which will be described in the prospectus supplement relating to the warrants
we are offering. The warrant agent will act solely as our agent in connection with the warrants and will not have any obligation or relationship
of agency or trust for or with any holders or beneficial owners of warrants.
The
particular terms of the warrants, the warrant or subscription agreements relating to the warrants and the warrant certificates representing
the warrants will be described in the applicable prospectus supplement, including, as applicable:
| |
● |
the
title of such warrants; |
| |
|
|
| |
● |
the
aggregate number of such warrants; |
| |
|
|
| |
● |
the
price or prices at which such warrants will be issued and exercised; |
| |
● |
the
currency or currencies in which the price of such warrants will be payable; |
| |
|
|
| |
● |
the
date on which the right to exercise such warrants shall commence and the date on which such right shall expire; |
| |
|
|
| |
● |
if
applicable, the minimum or maximum amount of such warrants which may be exercised at any one time; |
| |
|
|
| |
● |
if
applicable, the designation and terms of the securities with which such warrants are issued and the number of such warrants issued
with each such security; |
| |
|
|
| |
● |
if
applicable, the date on and after which such warrants and the related securities will be separately transferable; |
| |
|
|
| |
● |
if
applicable, any provisions for cashless exercise of the warrants; |
| |
|
|
| |
● |
if
applicable, any exercise limitations with respect to the ownership limitations by the holder exercising the warrant; |
| |
|
|
| |
● |
information
with respect to book-entry procedures, if any; |
| |
|
|
| |
● |
any
material U.K. and United States federal income tax consequences; |
| |
|
|
| |
● |
the
anti-dilution provisions of the warrants, if any; and |
| |
|
|
| |
● |
any
other terms of such warrants, including terms, procedures and limitations relating to the exchange and exercise of such warrants. |
Holders
of warrants will not be entitled, solely by virtue of being holders, to vote, to consent, to receive dividends, to receive notice as
shareholders with respect to any meeting of shareholders for the election of directors or any other matters, or to exercise any rights
whatsoever as a holder of the equity securities purchasable upon exercise of the warrants.
The
description in the applicable prospectus supplement of any warrants we offer will not necessarily be complete and will be qualified in
its entirety by reference to the applicable warrant agreement and warrant certificate, which will be filed with the SEC if we offer warrants.
For more information on how you can obtain copies of the applicable warrant agreement if we offer warrants, see “Where You Can
Find More Information” and “Incorporation of Certain Information by Reference.” We urge you to read any applicable
prospectus supplement and the applicable warrant agreement and form of warrant certificate in their entirety.
DESCRIPTION
OF UNITS
We
may issue units comprised of one or more of the other securities described in this prospectus in any combination. Each unit will be issued
so that the holder of the unit is also the holder of each security included in the unit. Thus, the holder of a unit will have the rights
and obligations of a holder of each included security. The unit agreement under which a unit is issued may provide that the securities
included in the unit may not be held or transferred separately, at any time or at any time before a specified date.
The
applicable prospectus supplement will describe:
| |
● |
the
designation and terms of the units and of the securities comprising the units, including whether and under what circumstances those
securities may be held or transferred separately; |
| |
|
|
| |
● |
any
unit agreement under which the units will be issued; |
| |
|
|
| |
● |
any
provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units; and
|
| |
|
|
| |
● |
whether
the units will be issued in fully registered or global form. |
The
applicable prospectus supplement will describe the terms of any units. The preceding description and any description of units in the
applicable prospectus supplement does not purport to be complete and is subject to and is qualified in its entirety by reference to the
unit agreement and, if applicable, collateral arrangements and depositary arrangements relating to such units. For more information on
how you can obtain copies of the applicable unit agreement if we offer units, see “Where You Can Find More Information” and
“Incorporation of Certain Information by Reference.” We urge you to read the applicable unit agreement and any applicable
prospectus supplement in their entirety.
PLAN
OF DISTRIBUTION
The
securities being offered by this prospectus may be sold:
| |
● |
through
agents; |
| |
|
|
| |
● |
to
or through one or more underwriters on a firm commitment or agency basis; |
| |
|
|
| |
● |
through
put or call option transactions relating to the securities; |
| |
|
|
| |
● |
to
or through dealers, who may act as agents or principals, including a block trade (which may involve crosses) in which a broker or
dealer so engaged will attempt to sell as agent but may position and resell a portion of the block as principal to facilitate the
transaction; |
| |
|
|
| |
● |
through
privately negotiated transactions; |
| |
|
|
| |
● |
purchases
by a broker or dealer as principal and resale by such broker or dealer for its own account pursuant to this prospectus; |
| |
|
|
| |
● |
directly
to purchasers, including our affiliates, through a specific bidding or auction process, on a negotiated basis or otherwise; |
| |
|
|
| |
● |
exchange
distributions and/or secondary distributions; |
| |
|
|
| |
● |
ordinary
brokerage transactions and transactions in which the broker solicits purchasers; |
| |
● |
in
“at-the-market” offerings, within the meaning of Rule 415(a)(4) of the Securities Act to or through a market maker or
into an existing trading market, on an exchange or otherwise; |
| |
|
|
| |
● |
transactions
not involving market makers or established trading markets, including direct sales or privately negotiated transactions; |
| |
|
|
| |
● |
transactions
in options, swaps or other derivatives that may or may not be listed on an exchange; |
| |
|
|
| |
● |
through
any other method permitted pursuant to applicable law; or |
| |
|
|
| |
● |
through
a combination of any such methods of sale. |
At
any time a particular offer of the securities covered by this prospectus is made, a revised prospectus or prospectus supplement, if required,
will be distributed which will set forth the aggregate amount of securities covered by this prospectus being offered and the terms of
the offering, including the name or names of any underwriters, dealers, brokers or agents, any discounts, commissions, concessions and
other items constituting compensation from us and any discounts, commissions or concessions allowed or re-allowed or paid to dealers.
Such prospectus supplement, and, if necessary, a post-effective amendment to the registration statement of which this prospectus is a
part, will be filed with the SEC to reflect the disclosure of additional information with respect to the distribution of the securities
covered by this prospectus. In order to comply with the securities laws of certain states, if applicable, the securities sold under this
prospectus may only be sold through registered or licensed broker-dealers. In addition, in some states the securities may not be sold
unless they have been registered or qualified for sale in the applicable state or an exemption from registration or qualification requirements
is available and is complied with.
The
distribution of securities may be effected from time to time in one or more transactions, including block transactions and transactions
on The Nasdaq Capital Market or any other organized market where the securities may be traded. The securities may be sold at a fixed
price or prices, which may be changed, or at market prices prevailing at the time of sale, at prices relating to the prevailing market
prices or at negotiated prices. The consideration may be cash or another form negotiated by the parties. Agents, underwriters or broker-dealers
may be paid compensation for offering and selling the securities. That compensation may be in the form of discounts, concessions or commissions
to be received from us or from the purchasers of the securities. Any dealers and agents participating in the distribution of the securities
may be deemed to be underwriters, and compensation received by them on resale of the securities may be deemed to be underwriting discounts.
If any such dealers or agents were deemed to be underwriters, they may be subject to statutory liabilities under the Securities Act.
Agents
may from time to time solicit offers to purchase the securities. If required, we will name in the applicable prospectus supplement any
agent involved in the offer or sale of the securities and set forth any compensation payable to the agent. Unless otherwise indicated
in the prospectus supplement, any agent will be acting on a best efforts basis for the period of its appointment. Any agent selling the
securities covered by this prospectus may be deemed to be an underwriter, as that term is defined in the Securities Act, of the securities.
To
the extent that we make sales to or through one or more underwriters or agents in at-the-market offerings, we will do so pursuant to
the terms of a distribution agreement between us and the underwriters or agents. If we engage in at-the-market sales pursuant to a distribution
agreement, we will sell any of our listed securities to or through one or more underwriters or agents, which may act on an agency basis
or on a principal basis. During the term of any such agreement, we may sell any of our listed securities on a daily basis in exchange
transactions or otherwise as we agree with the underwriters or agents. The distribution agreement will provide that any of our listed
securities which are sold will be sold at prices related to the then prevailing market prices for our listed securities. Therefore, exact
figures regarding proceeds that will be raised or commissions to be paid cannot be determined at this time and will be described in a
prospectus supplement. Pursuant to the terms of the distribution agreement, we also may agree to sell, and the relevant underwriters
or agents may agree to solicit offers to purchase, blocks of our listed securities. The terms of each such distribution agreement
will be set forth in more detail in a prospectus supplement to this prospectus.
If
underwriters are used in a sale, securities will be acquired by the underwriters for their own account and may be resold from time to
time in one or more transactions, including negotiated transactions, at a fixed public offering price or at varying prices determined
at the time of sale, or under delayed delivery contracts or other contractual commitments. Securities may be offered to the public either
through underwriting syndicates represented by one or more managing underwriters or directly by one or more firms acting as underwriters.
If an underwriter or underwriters are used in the sale of securities, an underwriting agreement will be executed with the underwriter
or underwriters, as well as any other underwriter or underwriters, with respect to a particular underwritten offering of securities,
and will set forth the terms of the transactions, including compensation of the underwriters and dealers and the public offering price,
if applicable. The prospectus and prospectus supplement will be used by the underwriters to resell the securities.
If
a dealer is used in the sale of the securities, we or an underwriter will sell the securities to the dealer, as principal. The dealer
may then resell the securities to the public at varying prices to be determined by the dealer at the time of resale. To the extent required,
we will set forth in the prospectus supplement the name of the dealer and the terms of the transactions.
We
may directly solicit offers to purchase the securities and may make sales of securities directly to institutional investors or others.
These persons may be deemed to be underwriters within the meaning of the Securities Act with respect to any resale of the securities.
To the extent required, the prospectus supplement will describe the terms of any such sales, including the terms of any bidding or auction
process, if used.
Agents,
underwriters and dealers may be entitled under agreements which may be entered into with us to indemnification by us against specified
liabilities, including liabilities incurred under the Securities Act, or to contribution by us to payments they may be required to make
in respect of such liabilities. If required, the prospectus supplement will describe the terms and conditions of the indemnification
or contribution. Some of the agents, underwriters or dealers, or their affiliates may be customers of, engage in transactions with or
perform services for us or our subsidiaries.
Any
person participating in the distribution of securities registered under the registration statement that includes this prospectus will
be subject to applicable provisions of the Exchange Act and the applicable SEC rules and regulations, including, among others, Regulation
M, which may limit the timing of purchases and sales of any of our securities by that person. Furthermore, Regulation M may restrict
the ability of any person engaged in the distribution of our securities to engage in market-making activities with respect to our securities.
These restrictions may affect the marketability of our securities and the ability of any person or entity to engage in market-making
activities with respect to our securities.
Certain
persons participating in an offering may engage in over-allotment, stabilizing transactions, short-covering transactions, penalty bids
and other transactions that stabilize, maintain or otherwise affect the price of the offered securities. These activities may maintain
the price of the offered securities at levels above those that might otherwise prevail in the open market, including by entering stabilizing
bids, effecting syndicate covering transactions or imposing penalty bids, each of which is described below:
| |
● |
a
stabilizing bid means the placing of any bid, or the effecting of any purchase, for the purpose of pegging, fixing or maintaining
the price of a security. |
| |
|
|
| |
● |
a
syndicate covering transaction means the placing of any bid on behalf of the underwriting syndicate or the effecting of any purchase
to reduce a short position created in connection with the offering. |
| |
|
|
| |
● |
a
penalty bid means an arrangement that permits the managing underwriter to reclaim a selling concession from a syndicate member in
connection with the offering when offered securities originally sold by the syndicate member are purchased in syndicate covering
transactions. |
These
transactions may be effected on an exchange or automated quotation system, if the securities are listed on that exchange or admitted
for trading on that automated quotation system, or in the over-the-counter market or otherwise.
If
so indicated in the applicable prospectus supplement, we will authorize agents, underwriters or dealers to solicit offers from certain
types of institutions to purchase offered securities from us at the public offering price set forth in such prospectus supplement pursuant
to delayed delivery contracts providing for payment and delivery on a specified date in the future. Such contracts will be subject only
to those conditions set forth in the prospectus supplement and the prospectus supplement will set forth the commission payable for solicitation
of such contracts.
In
addition, the securities may be issued upon conversion of or in exchange for debt securities or other securities.
Any
underwriters to whom offered securities are sold for public offering and sale may make a market in such offered securities, but such
underwriters will not be obligated to do so and may discontinue any market making at any time without notice. The offered securities
may or may not be listed on a national securities exchange. No assurance can be given that there will be a market for the offered securities.
Any
securities that qualify for sale pursuant to Rule 144 or Regulation S under the Securities Act, may be sold under Rule 144 or Regulation
S rather than pursuant to this prospectus.
In
connection with offerings made through underwriters or agents, we may enter into agreements with such underwriters or agents pursuant
to which we receive our outstanding securities in consideration for the securities being offered to the public for cash. In connection
with these arrangements, the underwriters or agents may also sell securities covered by this prospectus to hedge their positions in these
outstanding securities, including in short sale transactions. If so, the underwriters or agents may use the securities received from
us under these arrangements to close out any related open borrowings of securities.
We
may enter into derivative transactions with third parties or sell securities not covered by this prospectus to third parties in privately
negotiated transactions. If the applicable prospectus supplement indicates, in connection with those derivatives, such third parties
(or affiliates of such third parties) may sell securities covered by this prospectus and the applicable prospectus supplement, including
in short sale transactions. If so, such third parties (or affiliates of such third parties) may use securities pledged by us or borrowed
from us or others to settle those sales or to close out any related open borrowings of shares, and may use securities received from us
in settlement of those derivatives to close out any related open borrowings of shares. The third parties (or affiliates of such third
parties) in such sale transactions will be underwriters and will be identified in the applicable prospectus supplement (or a post-effective
amendment).
We
may loan or pledge securities to a financial institution or other third party that in turn may sell the securities using this prospectus.
Such financial institution or third party may transfer its short position to investors in our securities or in connection with a simultaneous
offering of other securities offered by this prospectus or in connection with a simultaneous offering of other securities offered by
this prospectus.
TAXATION
The
material U.S. federal income tax consequences relating to the purchase, ownership and disposition of any of the securities offered by
this prospectus will be set forth in the prospectus supplement offering those securities.
EXPENSES
The
following is a statement of expenses in connection with the distribution of the securities registered. All amounts shown are estimates
except the SEC registration fee and FINRA fee. The estimates do not include expenses related to offerings of particular securities. Each
prospectus supplement describing an offering of securities will reflect the estimated expenses related to the offering of securities
under that prospectus supplement.
| U.S.
Securities and Exchange Commission registration fee |
|
$ |
11,020 |
|
| FINRA
fee |
|
|
15,500 |
|
| Legal
fees and expenses |
|
|
25,000 |
|
| Accounting
fees and expenses |
|
|
15,000 |
|
| Other
miscellaneous fees and expenses |
|
|
3,480 |
|
| Total |
|
$ |
70,000 |
|
LEGAL
MATTERS
Certain
legal matters with respect to English law and Guernsey law in connection with the validity of our ordinary shares registered hereby will
be passed upon for us by Orrick, Herrington & Sutcliffe (UK) LLP, United Kingdom and Carey Olsen (Guernsey) LLP, Bailiwick of Guernsey,
respectively. Certain matters of U.S. federal law will be passed upon for us by Sheppard, Mullin, Richter & Hampton LLP, New York,
New York.
EXPERTS
The
consolidated financial statements of OKYO Pharma Limited as of March 31, 2022, and 2021 and for each of the years then ended, included
in our Form 20-F annual report for the year ended March 31, 2022 and incorporated by reference in this prospectus have been audited by
Mazars LLP, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
The audit report contains an explanatory paragraph regarding our ability to continue as a going concern. The registered business address
of Mazars LLP is 30 Old Bailey, London EC4M 7AU, United Kingdom.
SERVICE
OF PROCESS AND ENFORCEMENT OF LIABILITIES
We
are incorporated and currently existing under the laws of Guernsey. In addition, certain of our directors and officers reside outside
the United States, and most of the assets of our non-U.S. subsidiaries are located outside the United States. As a result, it may not
be possible for investors to effect service of process within the United States upon OKYO or those persons or to enforce against OKYO
or them, either inside or outside the United States, judgments obtained in U.S. courts, or to enforce in U.S. courts, judgments obtained
against them in courts in jurisdictions outside the U.S., in any action predicated upon civil liability provisions of the federal securities
laws of the United States. Both in original actions and in actions for the enforcement of judgments of U.S. courts, there is doubt as
to whether civil liabilities predicated solely upon the U.S. federal securities laws are enforceable in Guernsey. See “Description
of Share Capital and Memorandum and Articles of Incorporation—Enforcement of Civil Liabilities.”
INCORPORATION
OF CERTAIN INFORMATION BY REFERENCE
The
SEC allows us to “incorporate by reference” information into this prospectus, which means that we can disclose important
information to you by referring you to other documents which we have filed or will file with the SEC. The information incorporated by
reference is considered a part of this prospectus and should be read carefully. Certain information in this prospectus supersedes information
incorporated by reference that we filed with the SEC prior to the date of this prospectus. Certain information that we file later with
the SEC will automatically update and supersede the information in this prospectus. Any statement so modified or superseded shall not
be deemed, except as so modified or superseded, to constitute a part of this prospectus.
We
incorporate by reference into this prospectus and the registration statement of which it is a part the following documents, including
any amendments to such filings:
| ● |
|
our
Annual Report on Form 20-F for the fiscal year ended March 31, 2022; |
| |
|
|
| ● |
|
our
Reports on Form 6-K furnished to the SEC on May 18, 2022, May 19, 2022, May 20, 2022, June 9, 2022, August 16, 2022, August 19, 2022,
August 30, 2022, September 7, 2022, September 8, 2022, October 20, 2022, October 24, 2022, November 10, 2022, November 21, 2022,
November 25, 2022, December 1, 2022, December 6, 2022, December 21, 2022, December 22, 2022, December 30, 2022, January 3, 2023,
January 4, 2023, February 14, 2023, February 21, 2023, February 23, 2023, February 28, 2023, March 13, 2023, March 14, 2023, March 15, 2023 (2), March 16, 2023, March 24, 2023, March 29, 2023, March 30, 2023, April 4, 2023, April 5, 2023, April 25, 2023, May 2, 2023, May 3, 2023 (3), May 11, 2023 (2), May 15, 2023, May 19, 2023, May 22, 2023, and June 6, 2023; and |
| |
|
|
| ● |
|
the
description of our common shares contained in our Registration Statement on Form 8-A filed with the SEC on May 10, 2022, as amended
by Form 8-A/A filed with the SEC on June 2, 2023, including any amendments or reports filed for the purpose of updating such description. |
We
are also incorporating by reference all subsequent Annual Reports on Form 20-F that we file with the SEC and certain reports on Form
6-K that we furnish to the SEC after the date of this prospectus (if they state that they are incorporated by reference into this prospectus)
prior to the termination of this offering. In all cases, you should rely on the later information over different information included
in this prospectus or any accompanying prospectus supplement.
Unless
expressly incorporated by reference, nothing in this prospectus shall be deemed to incorporate by reference information furnished to,
but not filed with, the SEC. Copies of all documents incorporated by reference in this prospectus, other than exhibits to those documents
unless such exhibits are specifically incorporated by reference in this prospectus, will be provided at no cost to each person, including
any beneficial owner, who receives a copy of this prospectus on the written or oral request of that person made to:
OKYO
Pharma Limited
Martello
Court
Admiral
Park
St.
Peter Port
Guernsey
GY1 3HB
+44
(0)20 7495 2379
You
may also access these documents on our website, www.okyopharma.com. The information contained on, or that can be accessed through,
our website is not a part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
You
should rely only on information contained in, or incorporated by reference into, this prospectus. We have not authorized anyone to provide
you with information different from that contained in this prospectus or incorporated by reference in this prospectus. We are not making
offers to sell the securities in any jurisdiction in which such an offer or solicitation is not authorized or in which the person making
such offer or solicitation is not qualified to do so or to anyone to whom it is unlawful to make such offer or solicitation.
WHERE
YOU CAN FIND MORE INFORMATION
We
have filed with the SEC a registration statement (including amendments and exhibits to the registration statement) on Form F-3 under
the Securities Act. This prospectus, which is part of the registration statement, does not contain all of the information set forth in
the registration statement and the exhibits and schedules to the registration statement. For further information, we refer you to the
registration statement and the exhibits and schedules filed as part of the registration statement. If a document has been filed as an
exhibit to the registration statement, we refer you to the copy of the document that has been filed. Each statement in this prospectus
relating to a document filed as an exhibit is qualified in all respects by the filed exhibit.
We
are subject to the informational requirements of the Exchange Act. Our Annual Report on Form 20-F for the year ending March 31, 2022
has been filed with the SEC. The company has also filed periodic reports with the SEC on Form 6-K. You may inspect and copy reports and
other information filed with the SEC at the Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Information on the operation
of the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains an Internet website
that contains reports and other information about issuers, like us, that file electronically with the SEC. The address of that website
is www.sec.gov.
As
a foreign private issuer, we are exempt under the Exchange Act from, among other things, the rules prescribing the furnishing and content
of proxy statements, and our executive officers, directors and principal shareholders are exempt from the reporting and short-swing profit
recovery provisions contained in Section 16 of the Exchange Act. In addition, we will not be required under the Exchange Act to file
periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered
under the Exchange Act.

$4,000,000
Ordinary Shares
PROSPECTUS SUPPLEMENT
B. Riley Securities
December 20, 2023
Grafico Azioni OKYO Pharma (NASDAQ:OKYO)
Storico
Da Ott 2024 a Nov 2024

Grafico Azioni OKYO Pharma (NASDAQ:OKYO)
Storico
Da Nov 2023 a Nov 2024
