Immuron Shares Rise 40% After Award for Travelan Study
12 Gennaio 2022 - 4:39PM
Dow Jones News
By Chris Wack
Immuron Ltd. shares were up 40% to $3.66 after the company said
it was awarded 4.8 million Australian dollars ($3.4 million) in
funding in a new research agreement with the U.S Department of
Defense for a study to clinically evaluate a military strength
dosing regimen for Travelan.
The biopharmaceutical company said the U.S. Naval Medical
Research Center received A$1.4 million in funding to support the
Travelan clinical development effort.
The company said the focus of the new agreement is aimed at
testing and confirming the efficacy of a single larger dose regimen
of Travelan in a controlled human infection model clinical study
using the enterotoxigenic Escherichia coli strain H10407.
Immuron said this single larger dosing regime is potentially
more amenable for use in military populations. Up to 60 volunteers
will be enrolled in the clinical study and will be randomly
assigned to receive either a once-daily dose of 1200 mg of Travelan
or placebo.
Results of the proposed clinical study also will inform on
dosing in the pivotal Phase 3 registration trials for Biologics
License Application licensure. A project kickoff meeting for this
award has been scheduled for the end of January with the U.S
government sponsors.
The stock hit its 52-week low of $2.32 on Dec. 28.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
January 12, 2022 10:24 ET (15:24 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
Grafico Azioni Immuron (ASX:IMC)
Storico
Da Gen 2025 a Feb 2025
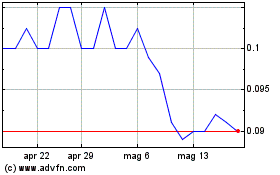
Grafico Azioni Immuron (ASX:IMC)
Storico
Da Feb 2024 a Feb 2025
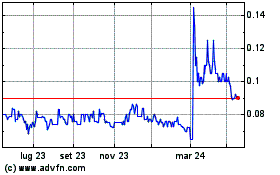
Notizie in Tempo Reale relative a Immuron Limited (Borsa Australiana): 0 articoli recenti
Più Immuron Limited Articoli Notizie