Immuron in Travelan Clinical Trial Agreement with U.S.-based Pharmaron
04 Ottobre 2022 - 12:45PM
Dow Jones News
By Chris Wack
Immuron Ltd. said Tuesday that it has executed a master service
agreement with U.S.-based Pharmaron CPC Inc.
The biopharmaceutical company said its clinical trial agreement
will allow for a Phase II trial to evaluate the efficacy of a
single dose regimen of Travelan in a controlled human infection
model using the enterotoxigenic Escherichia coli strain H10407.
Travelan is an orally administered passive immunotherapy that
prophylactically reduces the likelihood of contracting travelers'
diarrhea, a digestive tract disorder that is commonly caused by
pathogenic bacteria and the toxins they produce.
The new planned clinical study will enroll up to 60 volunteers.
Each will be randomly assigned to receive either a once-daily dose
of 1200 mg of Travelan or a placebo. The proposed clinical
development program is being funded in part by a $4.5 million award
from the U.S. Department of Defense.
Immuron is currently on track to submit the Investigational New
Drug application to the U.S. Food and Drug Administration by end of
2022, and will be the sponsor of the clinical study, which is
planned to begin in the first half of 2023, with headline results
from the clinical trial expected in the second half of 2023.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
October 04, 2022 06:30 ET (10:30 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
Grafico Azioni Immuron (ASX:IMC)
Storico
Da Gen 2025 a Feb 2025
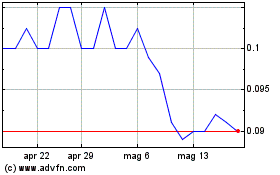
Grafico Azioni Immuron (ASX:IMC)
Storico
Da Feb 2024 a Feb 2025
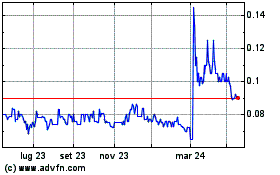
Notizie in Tempo Reale relative a Immuron Limited (Borsa Australiana): 0 articoli recenti
Più Immuron Limited Articoli Notizie