Mesoblast Receives FDA Approval For Bone Marrow Transplant Trial
07 Luglio 2011 - 4:19AM
Dow Jones News
Stem cell medicine developer Mesoblast Ltd. (MSB.AU) said
Thursday it has received approval from U.S. authorities to begin an
advanced trial of a treatment that could boost the number of bone
marrow transplants for patients who cannot find a matched
donor.
The company said in a statement the Food and Drug Administration
had cleared its Phase Three trial for bone marrow regeneration in
patients with blood cancers.
"We hope that this particular product will make bone marrow
transplantation a more widely used and safer option for critically
ill patients who undergo chemotherapy to potentially cure blood
cancer," Chief Executive Silviu Itescu said in the statement.
Mesoblast's shares rose after the announcement, and at 0124 GMT
were up 3.4% at A$8.75, outperforming the broader benchmark
S&P/ASX 200, which was down 0.4%.
The Melbourne-based company uses adult stem cells to develop
regenerative therapies.
Mesoblast aims to produce a product that can be used in bone
marrow transplants where a perfectly matched donor cannot be
found.
It said the therapy could expand the number of unrelated donor
transplants by three to four fold.
Itescu said the bone marrow product could be the company's first
revenue generating biologic therapy in the U.S. and Europe.
-By Gavin Lower, Dow Jones Newswires; 61-3-9292-2095; gavin.lower@dowjones.com
Grafico Azioni Mesoblast (ASX:MSB)
Storico
Da Apr 2024 a Mag 2024
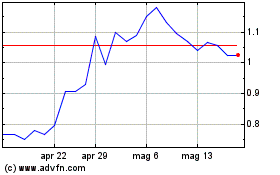
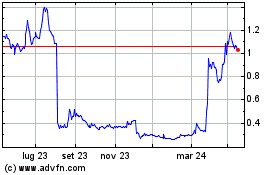
Grafico Azioni Mesoblast (ASX:MSB)
Storico
Da Mag 2023 a Mag 2024