Mithra Receives EUR 1.25 Million Milestone Payment from Fuji Pharma under Estelle® Licensing Agreement
03 Agosto 2023 - 7:30AM

Mithra Receives EUR 1.25 Million Milestone Payment from Fuji Pharma
under Estelle® Licensing Agreement
Mithra Receives EUR 1.25
Million Milestone Payment from Fuji Pharma
under
Estelle®
Licensing Agreement
- Relates to completion of the interim
clinical study reports of Phase 3 trials in patients with
dysmenorrhea or endometriosis
- Marks strong progress in 2016
agreement and further boosts Mithra cash position
- Additional EUR 12.5 million milestone
payments scheduled prior to commercialization
- Mithra eligible for revenue from
supply of the manufactured product post-commercialization
Liege,
Belgium,
3 August
2023
–
07:30
CEST – Mithra
(Euronext Brussels: MITRA), a company dedicated to Women’s Health,
today announces it has received a milestone payment of EUR 1.25
million from its partner Fuji Pharma, the Japanese leader in
women’s health, under a license and supply agreement for its
Estetrol (E4) native estrogen-based product Estelle® in Japan and
ASEAN territories.
The payment was triggered by completion of the
interim clinical study reports of Phase 3 trials investigating
Estelle® for the treatment of Japanese patients with dysmenorrhea
or endometriosis. Fuji announced positive top-line results from the
double-blind, placebo-controlled studies in February 2023, showing
the first trial met its primary endpoint by demonstrating a
statistically significant difference for the change in the total
dysmenorrhea score when compared to placebo. The second trial in
patients with endometriosis also met its primary endpoint by
demonstrating a statistically significant difference for the change
in the Visual Analog Scale for the most severe pelvic pain when
compared to placebo.
It is the second milestone payment received by
Mithra under this agreement with Fuji, which was signed in 2016.
The first milestone of EUR 1.25 million was received by Mithra upon
completion of Phase 3 trials in Europe and the United States.
Mithra remains eligible to receive a further EUR 12.5 million in
total milestones prior to commercialization and to receive revenue
from supply of the manufactured product post-commercialization.
David Horn Solomon,
Chief Executive Officer
of Mithra, commented: “We are pleased
with the strong progress made by our partner Fuji Pharma with
Estelle®. This EUR 1.25 million milestone payment marks strong
Phase 3 results, which indicate that this could be an excellent
treatment for patients with dysmenorrhea and endometriosis, and
will further boost Mithra’s balance sheet, as we pursue our aim of
becoming a global leader in women's health. We are looking forward
to continuing our close cooperation with Fuji as we work to bring
Estelle® to patients in Japan and ASEAN territories.”
Estelle®, Mithra’s first E4-based product
composed of 15 mg Estetrol (E4) and 3 mg Drospirenone (DRSP), is
commercialized as a combined oral contraceptive in the US
(NEXTSTELLIS®), Canada (NEXTSTELLIS®), and Europe (DROVELIS® and
LYDISILKA®).
Mithra’s core asset Estetrol (E4) is a native
estrogen produced by the human fetus during pregnancy, passing into
maternal blood at relatively high levels. Because of its unique
mode of action and safety profile, Estetrol could represent a major
breakthrough in various therapeutic fields of women’s health and
beyond.
For more information, please
contact:
|
Mithra Pharmaceuticals
SADavid Horn SolomonChief Executive
Officerinvestorrelations@mithra.com |
Investor &
media relationsChris MaggosCohesion
Bureauchris.maggos@cohesionbureau.com+41 79 367 6254 |
About
Mithra
Mithra Pharmaceuticals SA (Euronext: MITRA) is a
Belgian biopharmaceutical company dedicated to transforming Women’s
Health by offering new choices through innovation, with a
particular focus on contraception and menopause. Mithra’s goal is
to develop products offering better efficacy, safety and
convenience, meeting women’s needs throughout their life span.
Mithra explores the potential of the unique native estrogen
estetrol in a wide range of applications in women health and
beyond. After having successfully launched the first estetrol-based
product in 2021, the contraceptive pill Estelle®, Mithra is now
focusing on its second product Donesta®, the next-generation
hormone therapy. Mithra also offers partners a complete spectrum of
solutions from early drug development, clinical batches and
commercial manufacturing of complex polymeric products (vaginal
ring, implants) and complex liquid injectables and biologicals
(vials, pre-filled syringes or cartridges) at its technological
platform Mithra CDMO. Active in more than 100 countries around the
world, Mithra has an approximate headcount of 230 staff members and
is headquartered in Liège, Belgium. www.mithra.com
NEXTSTELLIS®, LYDISILKA®, ESTELLE® and DONESTA®
are registered trademarks of Mithra Pharmaceuticals or one of its
affiliates.
DROVELIS® is a registered trademark of Richter
Gedeon Nyrt.
Important information
The contents of this announcement include
statements that are, or may be deemed to be, "forward-looking
statements". These forward-looking statements can be identified by
the use of forward-looking terminology, including the words
"believes", "estimates," "anticipates", "expects", "intends",
"may", "will", "plans", "continue", "ongoing", "potential",
"predict", "project", "target", "seek" or "should", and include
statements the Company makes concerning the intended results of its
strategy. By their nature, forward-looking statements involve risks
and uncertainties and readers are cautioned that any such
forward-looking statements are not guarantees of future
performance. The Company's actual results may differ materially
from those predicted by the forward-looking statements. The Company
undertakes no obligation to publicly update or revise
forward-looking statements, except as may be required by law.
|
Subscribe to our mailing list on investors.mithra.com to receive
our press releases by email or follow us on our social media
:Linkedin • Twitter • Facebook |
- 2023-08-03_Mithra Fuji Estelle_FP_Final_FR
Grafico Azioni Mithra Pharmaceuticals (EU:MITRA)
Storico
Da Apr 2024 a Mag 2024
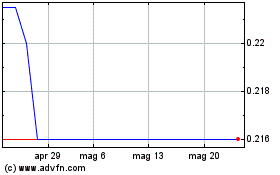
Grafico Azioni Mithra Pharmaceuticals (EU:MITRA)
Storico
Da Mag 2023 a Mag 2024