Sanofi, Regeneron Say Dupixent Gets FDA Approval to Treat Prurigo Nodularis Skin Disease
29 Settembre 2022 - 1:05AM
Dow Jones News
By Stephen Nakrosis
Sanofi and Regeneron Pharmaceuticals Inc. said Wednesday the
Food and Drug Administration approved Dupixent to treat patients
with prurigo nodularis.
Dupixent, or dupilumab, is the first and only medicine
specifically indicated to treat prurigo nodularis, a skin disease
that can cause painful lesions, in the U.S. The companies said
there are about 75,000 adults in the U.S. living with prurigo
nodularis.
According to the companies, "Dupixent significantly reduced itch
and skin lesions compared to placebo in direct-to-Phase 3 program
consisting of two pivotal trials."
Dupixent has also been given regulatory approvals in one or more
countries to treat certain patients with atopic dermatitis, asthma
and various other ailments. More than 500,000 patients have been
treated with Dupixent globally, according to the companies.
Write to Stephen Nakrosis at stephen.nakrosis@wsj.com
(END) Dow Jones Newswires
September 28, 2022 18:50 ET (22:50 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
Grafico Azioni Sanofi (EU:SAN)
Storico
Da Giu 2024 a Lug 2024
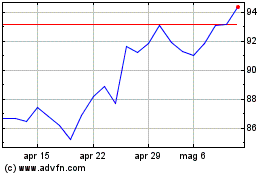
Grafico Azioni Sanofi (EU:SAN)
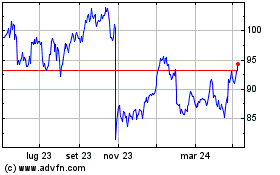
Storico
Da Lug 2023 a Lug 2024