AstraZeneca's Nirsevimab Approved in the EU
04 Novembre 2022 - 8:52AM
Dow Jones News
By Anthony O. Goriainoff
AstraZeneca PLC said Friday that nirsevimab, a treatment for the
prevention of respiratory syncytial virus, a
lower-respiratory-tract disease in infants, has been approved in
the European Union.
The Anglo-Swedish pharma major said approval for the treatment,
know commercially as Beyfortus, was based on results from the
Beyfortus clinical development program. This approval follows from
September's recommendation by the Committee for Medicinal Products
for Human Use of the European Medicines Agency, the company
said.
London-listed AstraZeneca said the European Commission was the
first regulatory body to grant Beyfortus an approval.
Beyfortus is being developed jointly by AstraZeneca and Sanofi
and is an investigational long-acting antibody designed for all
infants for protection against RSV disease from birth.
RSV is a common and very contagious seasonal virus, infecting
nearly all children by the age of two, the company said.
Write to Anthony O. Goriainoff at
anthony.orunagoriainoff@dowjones.com
(END) Dow Jones Newswires
November 04, 2022 03:37 ET (07:37 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
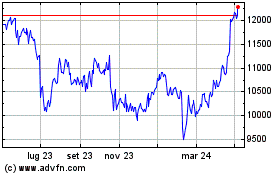
Grafico Azioni Astrazeneca (LSE:AZN)
Storico
Da Mar 2024 a Apr 2024
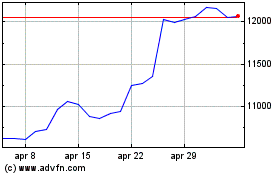
Grafico Azioni Astrazeneca (LSE:AZN)
Storico
Da Apr 2023 a Apr 2024