false000180886500018088652024-01-102024-01-10
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): January 10, 2024 |
iTeos Therapeutics, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-39401 |
84-3365066 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
321 Arsenal Street |
|
Watertown , Massachusetts |
|
02472 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: 339 217 0161 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, $0.001 par value per share |
|
ITOS |
|
The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On January 10, 2024, Michel Detheux, Ph.D., President and Chief Executive Officer of iTeos Therapeutics, Inc., will present at the 42nd Annual J.P. Morgan Healthcare Conference (the "Conference"). The slides that will be presented by Dr. Detheux at the Conference are furnished with this report as Exhibit 99.1, which is incorporated herein by reference.
The information in this Item 7.01 is furnished pursuant to Item 7.01 and shall not be deemed “filed” for the purposes of Section 18 of the Securities and Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section. It may only be incorporated by reference in another filing under the Exchange Act or the Securities Act of 1933, as amended, if such subsequent filing specifically references the information furnished pursuant to Item 7.01 of this report.
Item 9.01 Financial Statements and Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
|
iTeos Therapeutics, Inc. |
|
|
|
|
Date: |
January 10, 2024 |
By: |
/s/ Michel Detheux |
|
|
|
Michel Detheux, Ph.D.
President and Chief Executive Officer |

Cancer Immunotherapies�by design™ Nasdaq: ITOS January 2024

Forward-Looking Statements This presentation contains forward-looking statements. Any statements that are not solely statements of historical fact are forward-looking statements. Words such as “believe,” “anticipate,” “plan,” “expect,” “will,” “may,” “intend,” “prepare,” “look,” “potential,” “possible” and similar expressions are intended to identify forward-looking statements. These forward-looking statements include statements relating to the potential benefits of our product candidates and combinations, including the potential of belrestotug to be the highest quality TIGIT in the field, the potential of EOS-984’s mechanism to have profound effects as a monotherapy or in combinations, and the potential of inupadenant to enhance chemotherapy therapeutic response; the expectation that 2024 will be a defining year for iTeos; our clinical and data generation plans for 2024, including initiating a TIGIT Phase 3 registrational study, having clinical data from GALAXIES Lung-201 and TIG-006 HNSCC, having clinical data from the dose escalation portion of A2A-005 in late 2024, presenting preclinical mechanism of action data from EOS-984 in the second quarter of 2024, and having topline data from the Phase 1 dose escalation trial in advanced malignancies in late 2024; our goal to gain commercial approval for belrestotug in 1L NSCLC and branch into earlier lines and potentially to a variety of IO amenable tumors; the potential of our biomarker for TIGIT in identifying indications to target and subpopulations; our market opportunities and number of patients potentially eligible for our product candidates; the potential benefits of our collaboration with GSK and the expectation that 2024 will be a year of significant momentum for this collaboration; and our expected cash runway through 2026, which contemplates the launch of multiple TIGIT Phase 3 trials. These forward-looking statements involve risks and uncertainties, many of which are beyond iTeos’ control. Actual results could materially differ from those stated or implied by these forward-looking statements as a result of such risks and uncertainties. Known risk factors include the following: success in preclinical testing and early clinical trials does not ensure that later clinical trials will be successful, and early results from a clinical trial do not necessarily predict final results; the data for our product candidates may not be sufficient to support regulatory approval; iTeos may encounter unanticipated costs or may expend cash more rapidly or more slowly than currently anticipated due to challenges and uncertainties inherent in product research and development and biologics manufacturing; the expected benefits and opportunities related to the agreement between iTeos and GSK may not be realized or may take longer to realize than expected due to a variety of reasons, including any inability of the parties to perform their commitments and obligations under the agreement, challenges and uncertainties inherent in product research and development and manufacturing limitations; iTeos may not be able to execute on its business plans, including meeting its expected or planned regulatory milestones and timelines, research and clinical development plans, and bringing its product candidates to market, for various reasons, some of which may be outside of iTeos’ control, including possible limitations of company financial and other resources, manufacturing limitations that may not be anticipated or resolved for in a timely manner, negative developments in the field of immuno-oncology, such as adverse events or disappointing results, including in connection with competitor therapies, and regulatory, court or agency decisions such as decisions by the United States Patent and Trademark Office with respect to patents that cover our product candidates; and those risks identified under the heading “Risk Factors” in iTeos’ Quarterly Report on Form 10-Q for the nine months ended September 30, 2023 filed with the Securities and Exchange Commission (SEC) as well as other SEC filings made by the Company which you are encouraged to review. Statements regarding the Company’s cash runway do not indicate when the Company may access the capital markets. Any of the foregoing risks could materially and adversely affect iTeos’ business, results of operations and the trading price of iTeos’ common stock. We caution investors not to place considerable reliance on the forward-looking statements contained in this press release. iTeos does not undertake any obligation to publicly update its forward-looking statements based on events or circumstances after the date hereof. 2

2024 A Defining Year for iTeos Promising TIGIT:PD-1 Doublet 1 Two Data Readouts Anticipated in 2024 Unlocking Adenosine Pathway 2 Two Data Readouts Anticipated in 2024 Funded Through 2026 3 $645M in cash as of 3Q23
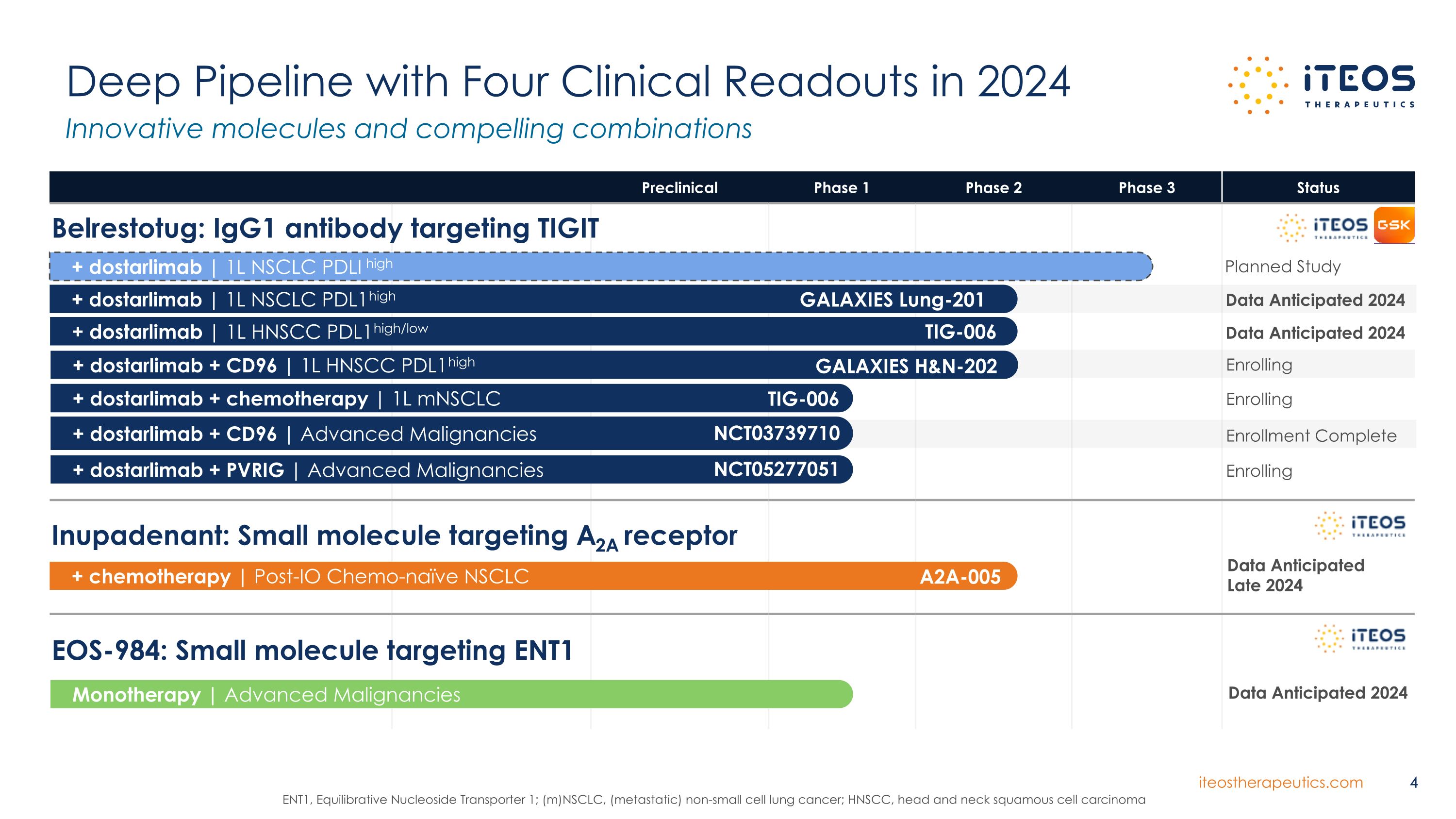
Preclinical Phase 1 Phase 2 Phase 3 Status Deep Pipeline with Four Clinical Readouts in 2024 Innovative molecules and compelling combinations 4 ENT1, Equilibrative Nucleoside Transporter 1; (m)NSCLC, (metastatic) non-small cell lung cancer; HNSCC, head and neck squamous cell carcinoma Inupadenant: Small molecule targeting A2A receptor + chemotherapy | Post-IO Chemo-naïve NSCLC A2A-005 Data Anticipated Late 2024 Monotherapy | Advanced Malignancies EOS-984: Small molecule targeting ENT1 Data Anticipated 2024 + dostarlimab | 1L NSCLC PDL1high + dostarlimab | 1L HNSCC PDL1high/low + dostarlimab | 1L NSCLC PDLI high Belrestotug: IgG1 antibody targeting TIGIT GALAXIES Lung-201 TIG-006 + dostarlimab + chemotherapy | 1L mNSCLC TIG-006 + dostarlimab + CD96 | Advanced Malignancies NCT03739710 + dostarlimab + PVRIG | Advanced Malignancies NCT05277051 + dostarlimab + CD96 | 1L HNSCC PDL1high GALAXIES H&N-202 Data Anticipated 2024 Data Anticipated 2024 Enrolling Enrolling Enrollment Complete Enrolling Planned Study

Belrestotug�EOS-448 / GSK4428859A iTeos and GSK are uniquely positioned to leverage the TIGIT/CD226 axis
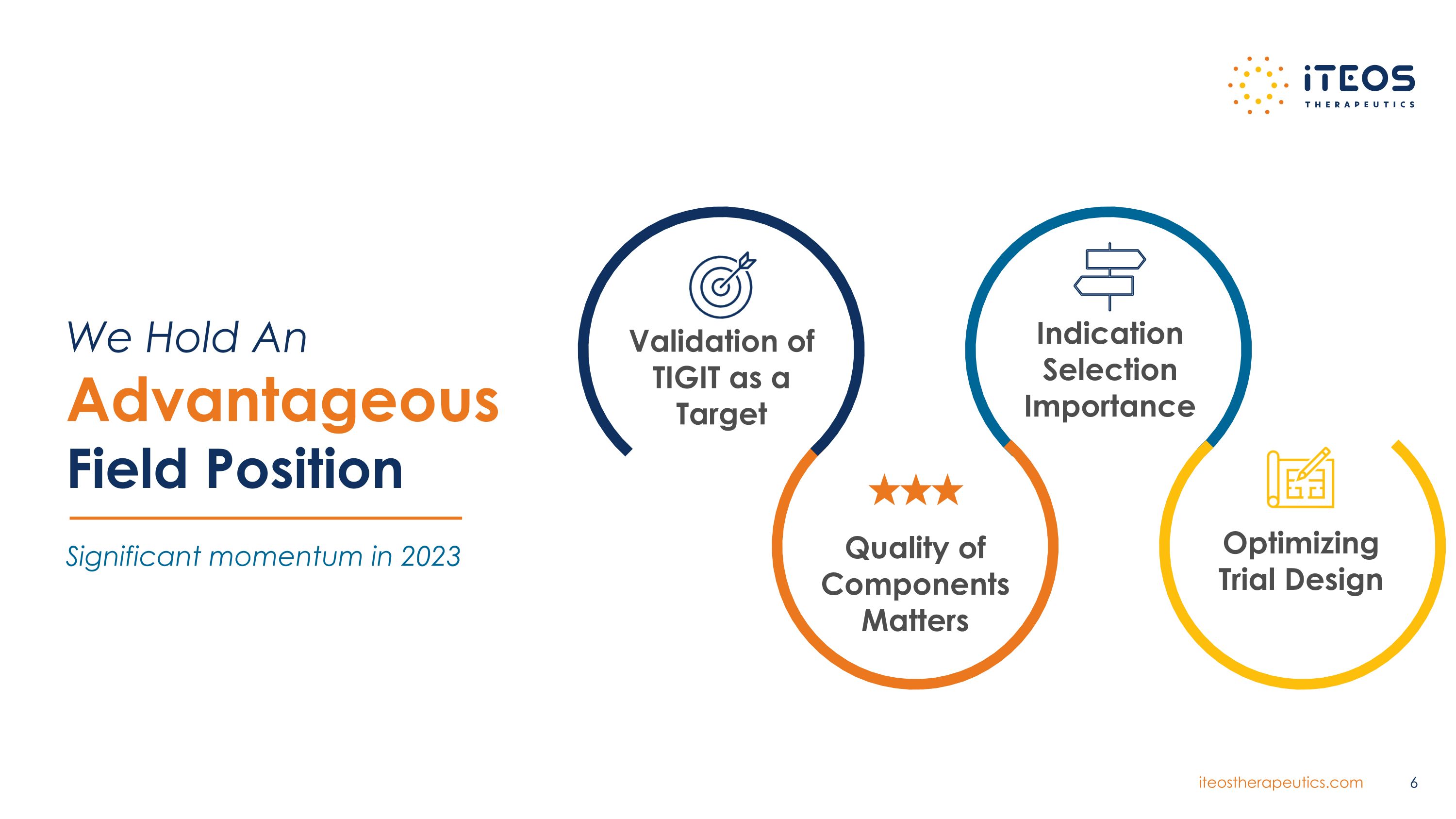
We Hold An Advantageous Field Position Significant momentum in 2023 Validation of TIGIT as a Target Quality of Components Matters Indication Selection Importance Optimizing Trial Design
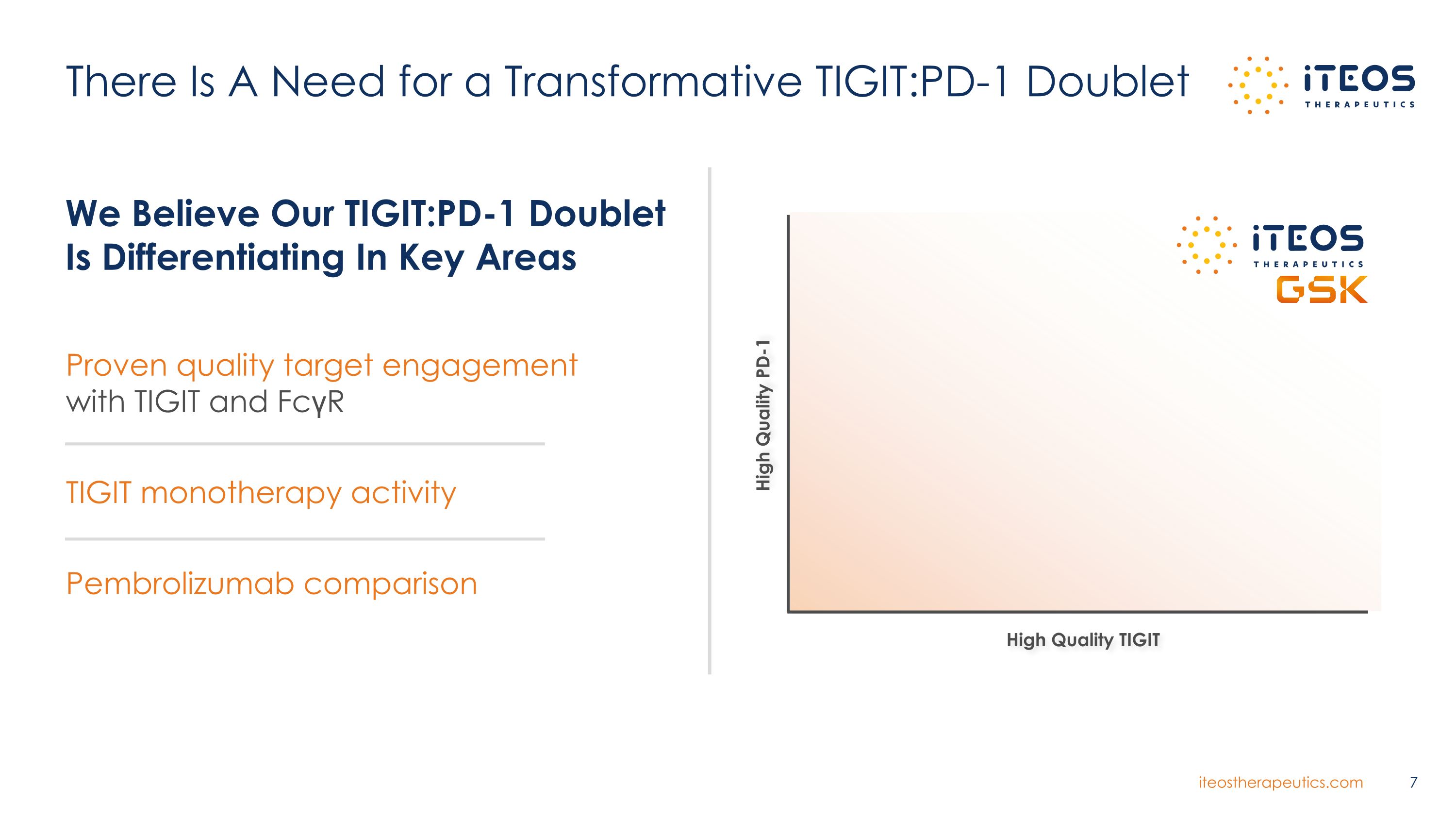
Proven quality target engagement with TIGIT and FcγR TIGIT monotherapy activity Pembrolizumab comparison There Is A Need for a Transformative TIGIT:PD-1 Doublet High Quality PD-1 High Quality TIGIT 7 We Believe Our TIGIT:PD-1 Doublet Is Differentiating In Key Areas
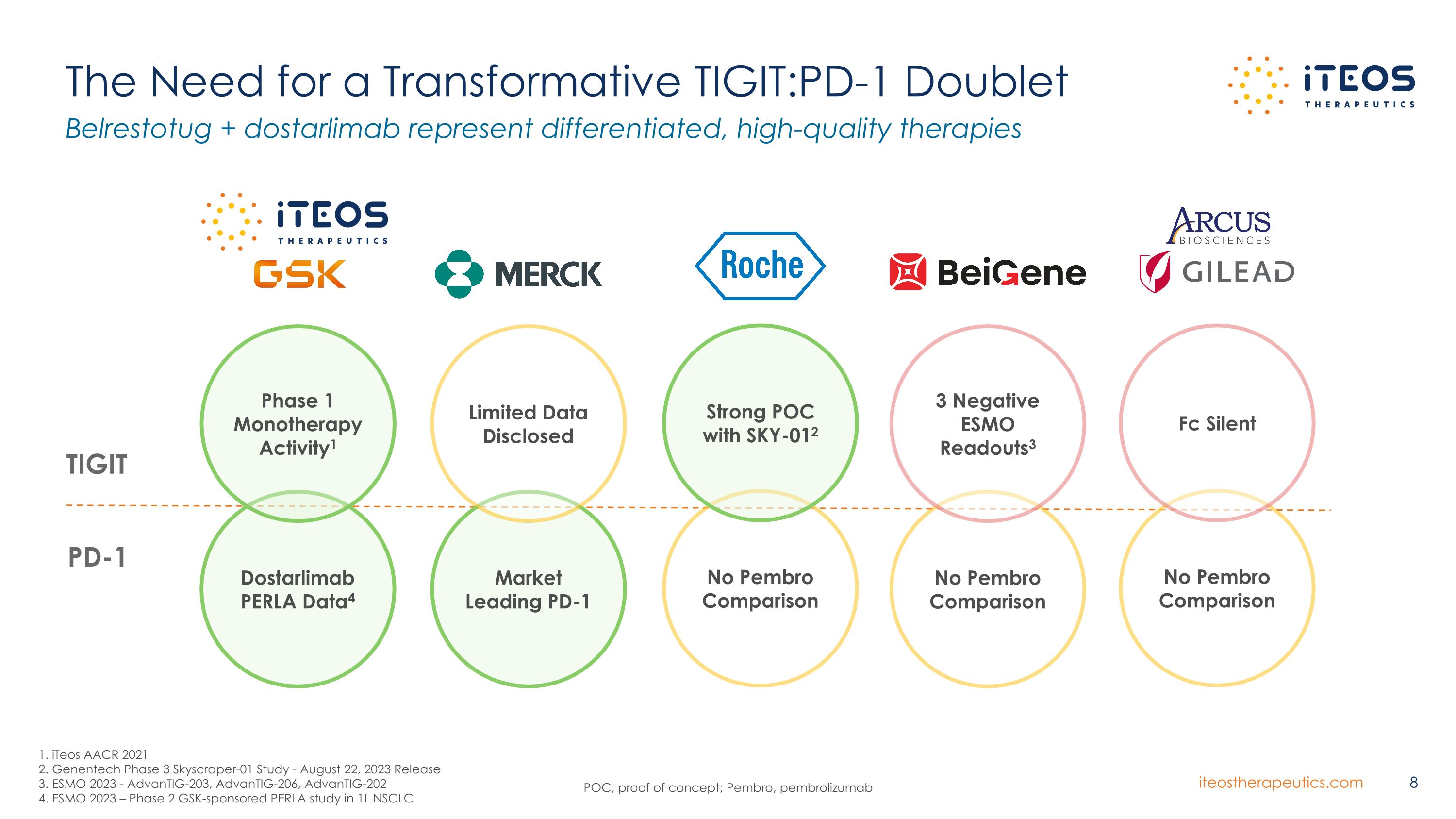
POC, proof of concept; Pembro, pembrolizumab The Need for a Transformative TIGIT:PD-1 Doublet Belrestotug + dostarlimab represent differentiated, high-quality therapies TIGIT PD-1 Dostarlimab PERLA Data4 Market Leading PD-1 Phase 1 Monotherapy Activity1 Limited Data Disclosed No Pembro Comparison Strong POC with SKY-012 No Pembro Comparison Fc Silent No Pembro Comparison 3 Negative ESMO Readouts3 1. iTeos AACR 2021 2. Genentech Phase 3 Skyscraper-01 Study - August 22, 2023 Release 3. ESMO 2023 - AdvanTIG-203, AdvanTIG-206, AdvanTIG-202 4. ESMO 2023 – Phase 2 GSK-sponsored PERLA study in 1L NSCLC
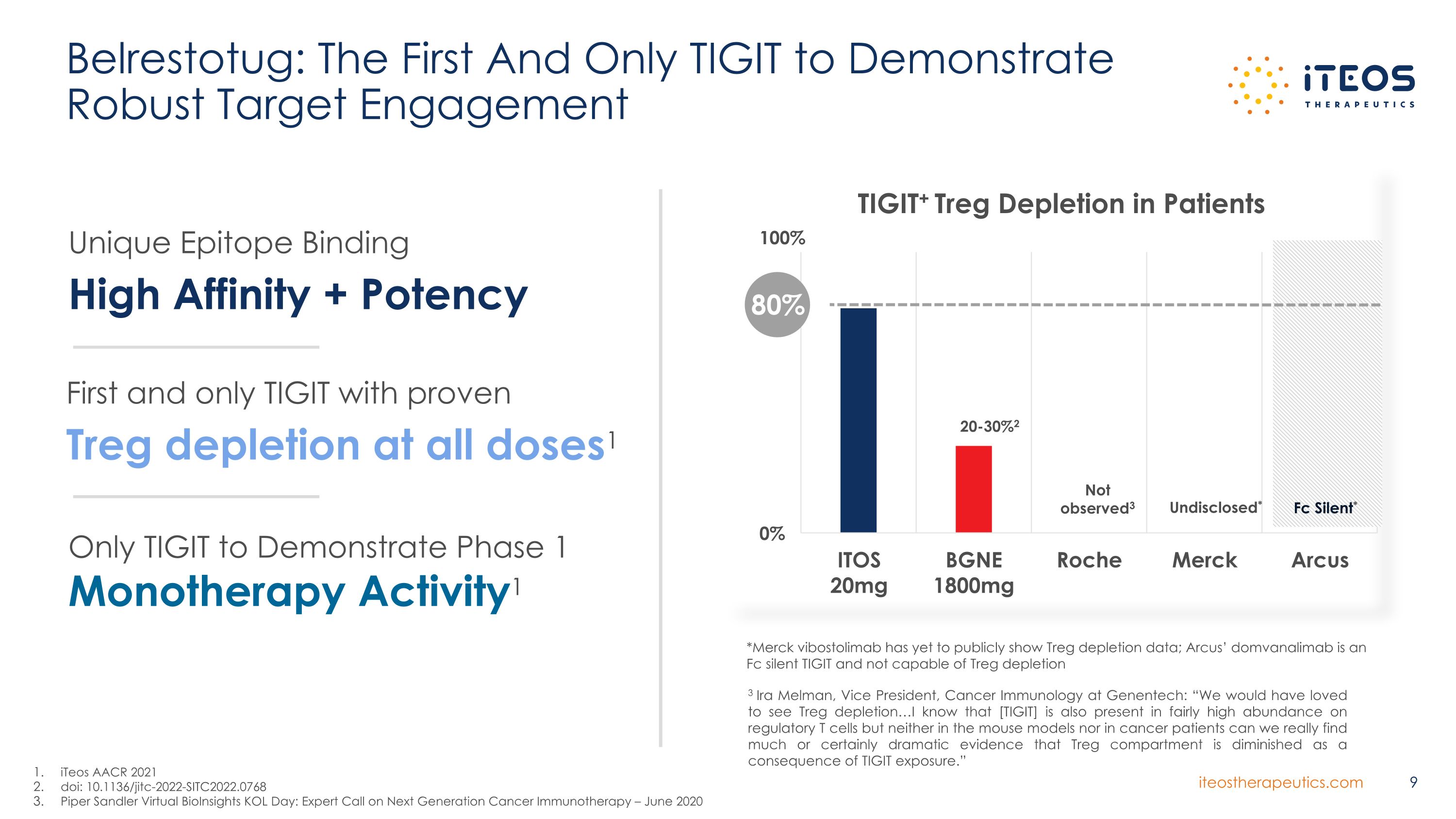
Belrestotug: The First And Only TIGIT to Demonstrate Robust Target Engagement iTeos AACR 2021 doi: 10.1136/jitc-2022-SITC2022.0768 Piper Sandler Virtual BioInsights KOL Day: Expert Call on Next Generation Cancer Immunotherapy – June 2020 *Merck vibostolimab has yet to publicly show Treg depletion data; Arcus’ domvanalimab is an Fc silent TIGIT and not capable of Treg depletion Fc Silent* Undisclosed* 100% 3 Ira Melman, Vice President, Cancer Immunology at Genentech: “We would have loved to see Treg depletion…I know that [TIGIT] is also present in fairly high abundance on regulatory T cells but neither in the mouse models nor in cancer patients can we really find much or certainly dramatic evidence that Treg compartment is diminished as a consequence of TIGIT exposure.” 20-30%2 Not observed3 Unique Epitope Binding High Affinity + Potency First and only TIGIT with proven Treg depletion at all doses1 Only TIGIT to Demonstrate Phase 1 Monotherapy Activity1 80%
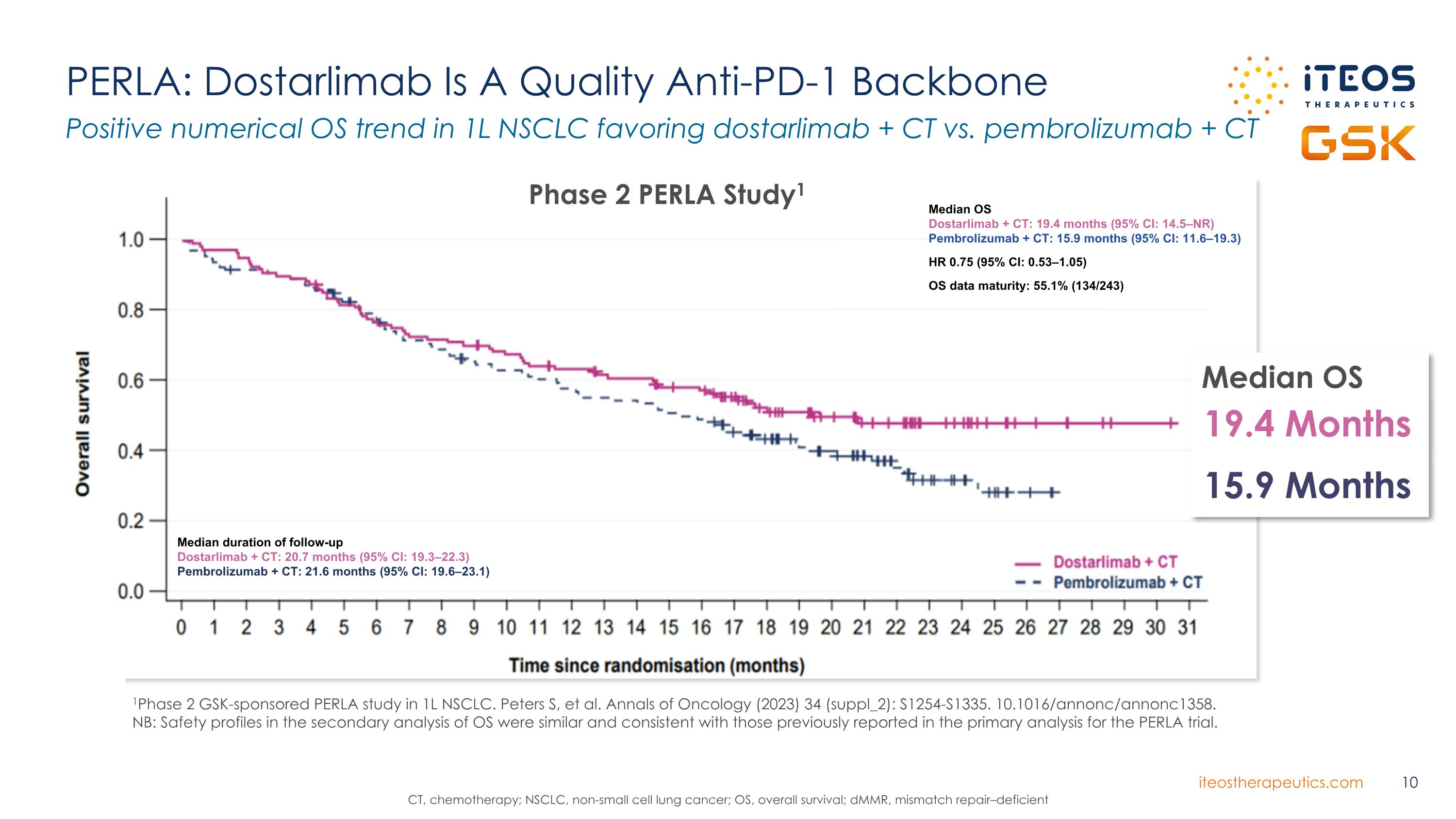
PERLA: Dostarlimab Is A Quality Anti-PD-1 Backbone Positive numerical OS trend in 1L NSCLC favoring dostarlimab + CT vs. pembrolizumab + CT CT, chemotherapy; NSCLC, non-small cell lung cancer; OS, overall survival; dMMR, mismatch repair–deficient Dostarlimab + CT Pembrolizumab + CT Phase 2 PERLA Study1 Median OS Dostarlimab + CT: 19.4 months (95% CI: 14.5–NR) Pembrolizumab + CT: 15.9 months (95% CI: 11.6–19.3) HR 0.75 (95% CI: 0.53–1.05) OS data maturity: 55.1% (134/243) Median duration of follow-up Dostarlimab + CT: 20.7 months (95% CI: 19.3–22.3) Pembrolizumab + CT: 21.6 months (95% CI: 19.6–23.1) Median OS 19.4 Months 15.9 Months 1Phase 2 GSK-sponsored PERLA study in 1L NSCLC. Peters S, et al. Annals of Oncology (2023) 34 (suppl_2): S1254-S1335. 10.1016/annonc/annonc1358. NB: Safety profiles in the secondary analysis of OS were similar and consistent with those previously reported in the primary analysis for the PERLA trial.
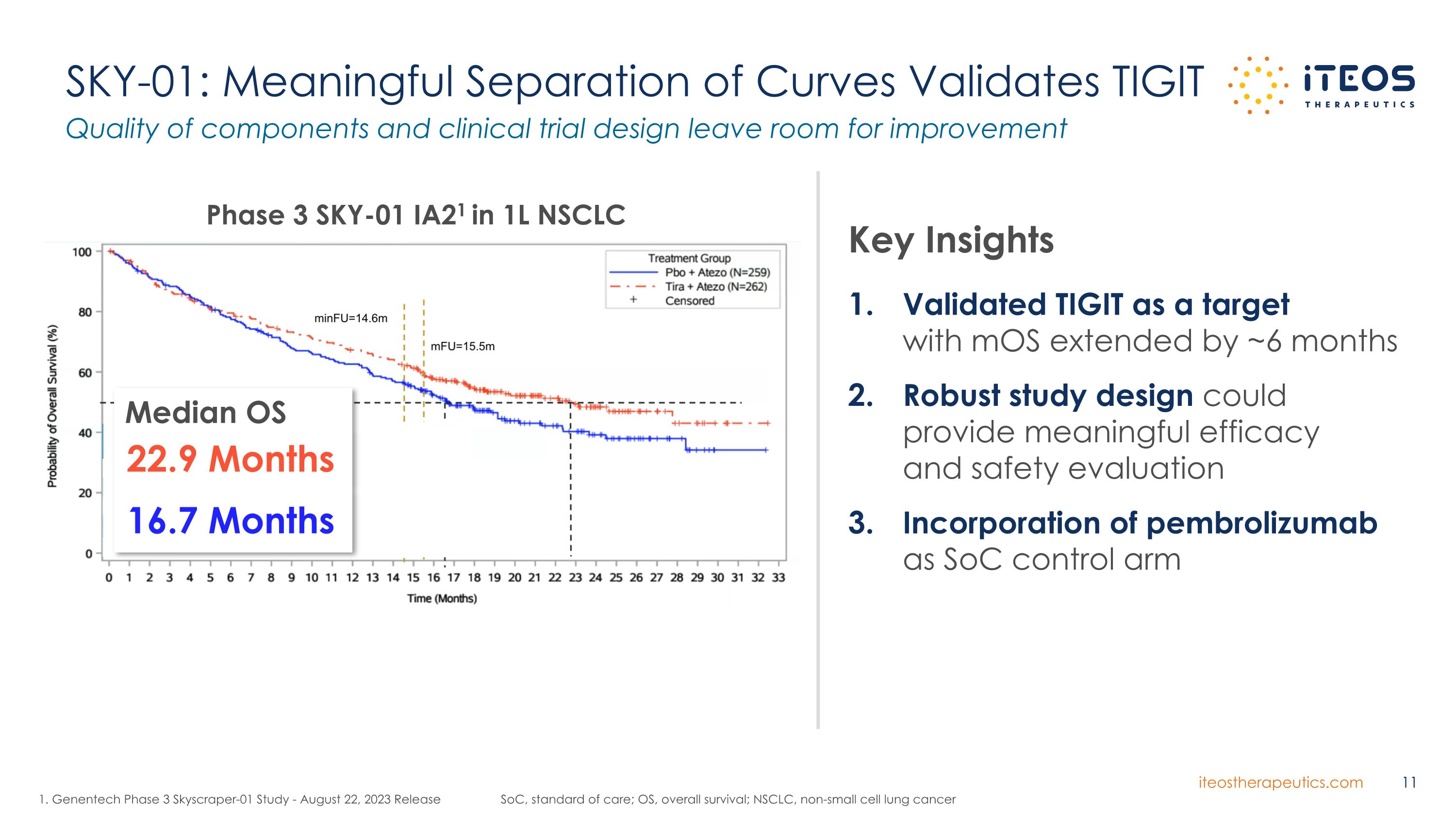
SKY-01: Meaningful Separation of Curves Validates TIGIT SoC, standard of care; OS, overall survival; NSCLC, non-small cell lung cancer Quality of components and clinical trial design leave room for improvement Validated TIGIT as a target with mOS extended by ~6 months Robust study design could provide meaningful efficacy and safety evaluation Incorporation of pembrolizumab as SoC control arm Key Insights 1. Genentech Phase 3 Skyscraper-01 Study - August 22, 2023 Release Phase 3 SKY-01 IA21 in 1L NSCLC Median OS 22.9 Months 16.7 Months
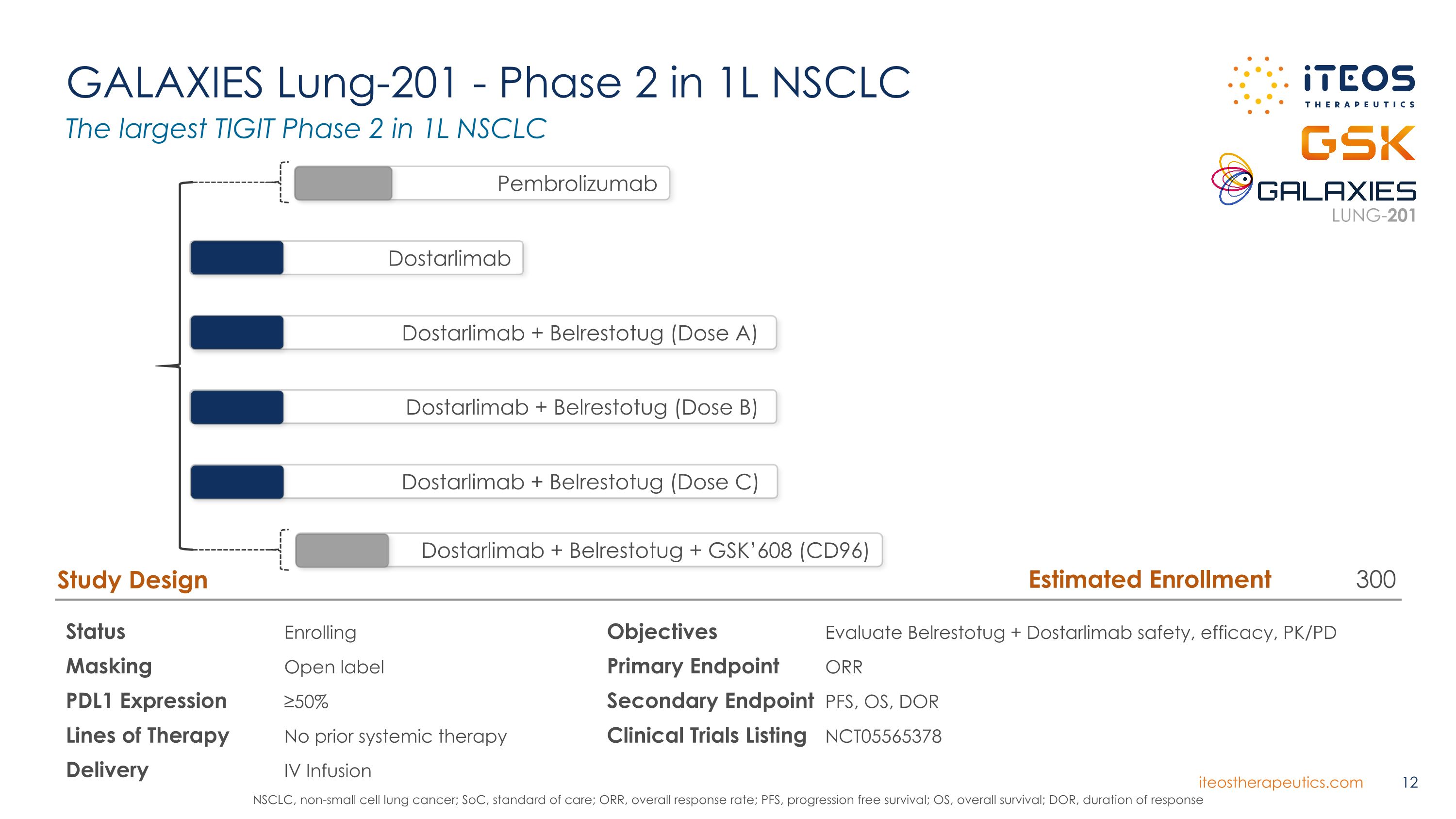
GALAXIES Lung-201 - Phase 2 in 1L NSCLC Status Enrolling Masking Open label PDL1 Expression ≥50% Lines of Therapy No prior systemic therapy Delivery IV Infusion Study Design Objectives Evaluate Belrestotug + Dostarlimab safety, efficacy, PK/PD Primary Endpoint ORR Secondary Endpoint PFS, OS, DOR Clinical Trials Listing NCT05565378 Dostarlimab + Belrestotug + GSK’608 (CD96) Pembrolizumab Dostarlimab Dostarlimab + Belrestotug (Dose A) Dostarlimab + Belrestotug (Dose B) Dostarlimab + Belrestotug (Dose C) The largest TIGIT Phase 2 in 1L NSCLC NSCLC, non-small cell lung cancer; SoC, standard of care; ORR, overall response rate; PFS, progression free survival; OS, overall survival; DOR, duration of response LUNG-201 Estimated Enrollment 300
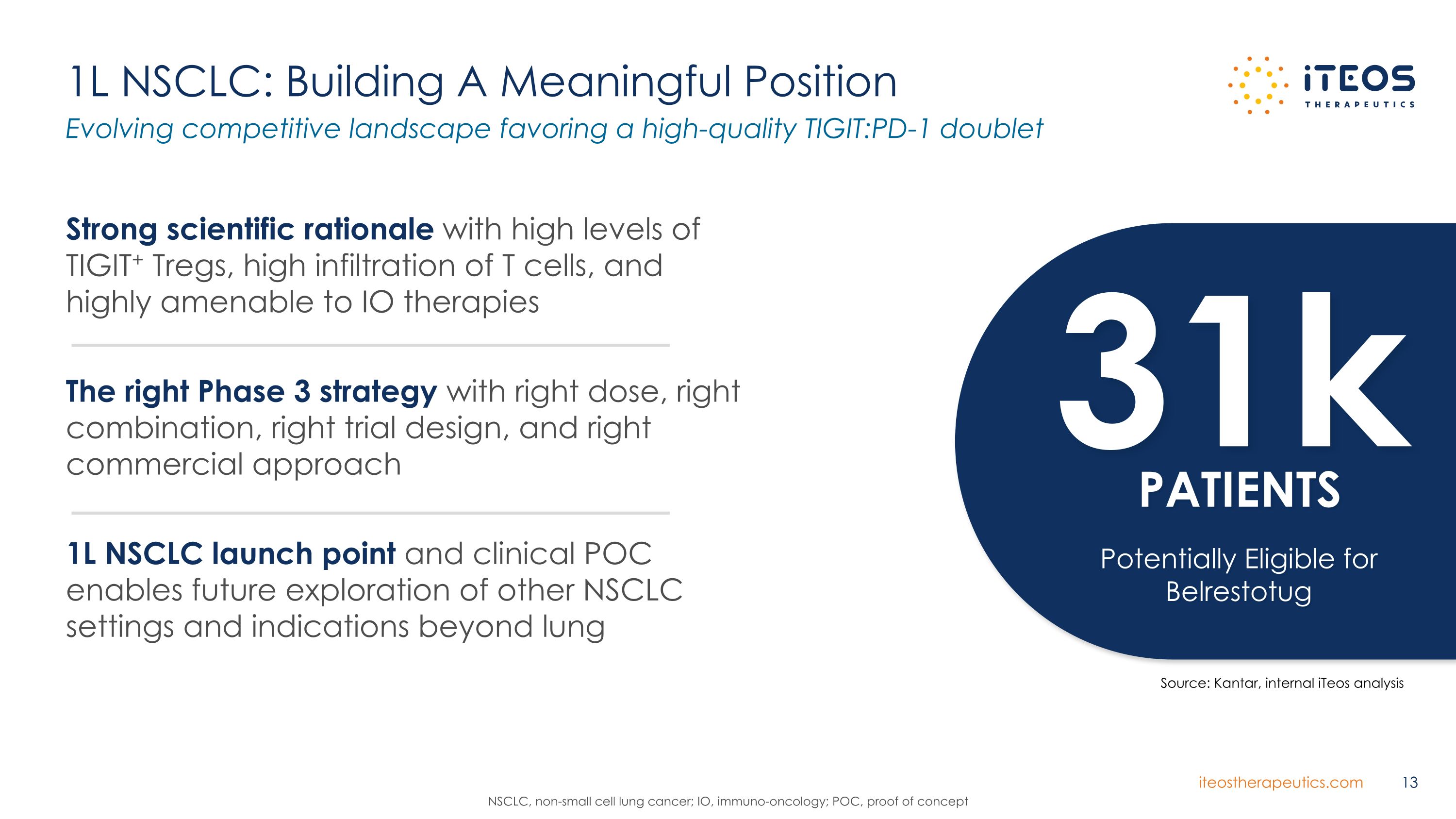
1L NSCLC: Building A Meaningful Position Evolving competitive landscape favoring a high-quality TIGIT:PD-1 doublet NSCLC, non-small cell lung cancer; IO, immuno-oncology; POC, proof of concept 31k PATIENTS Potentially Eligible for Belrestotug Strong scientific rationale with high levels of TIGIT+ Tregs, high infiltration of T cells, and highly amenable to IO therapies The right Phase 3 strategy with right dose, right combination, right trial design, and right commercial approach 1L NSCLC launch point and clinical POC enables future exploration of other NSCLC settings and indications beyond lung Source: Kantar, internal iTeos analysis
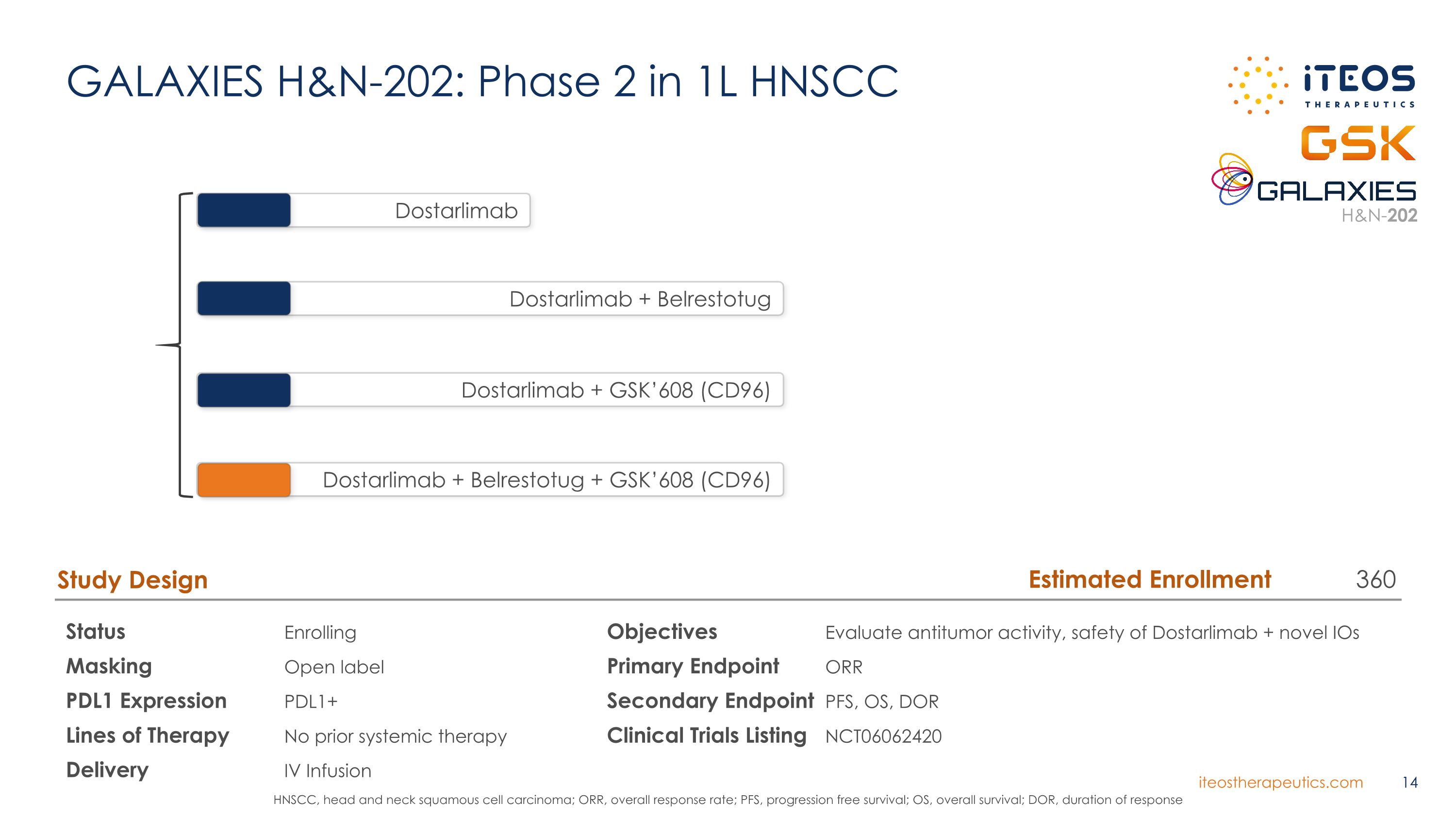
GALAXIES H&N-202: Phase 2 in 1L HNSCC Status Enrolling Masking Open label PDL1 Expression PDL1+ Lines of Therapy No prior systemic therapy Delivery IV Infusion Study Design Objectives Evaluate antitumor activity, safety of Dostarlimab + novel IOs Primary Endpoint ORR Secondary Endpoint PFS, OS, DOR Clinical Trials Listing NCT06062420 Dostarlimab Dostarlimab + Belrestotug Dostarlimab + GSK’608 (CD96) Dostarlimab + Belrestotug + GSK’608 (CD96) HNSCC, head and neck squamous cell carcinoma; ORR, overall response rate; PFS, progression free survival; OS, overall survival; DOR, duration of response Estimated Enrollment 360 H&N-202

TIG-006 – Phase 2 in 1L HNSCC PDL1High/Low Status Enrolling Masking Open label PDL1 Expression PDL1+ Lines of Therapy No prior systemic therapy Delivery IV Infusion Study Design Objectives Evaluate Belrestotug + Dostarlimab in two CPS populations Primary Endpoint ORR Secondary Endpoint PFS, OS, DOR Clinical Trials Listing NCT05060432 CPS High ≥ 20 Dostarlimab + Belrestotug (Dose B) CPS Low < 20 Dostarlimab + Belrestotug (Dose B) Estimated Enrollment 80

1L HNSCC: Potential First-to-Market Opportunity Under-served population with strong biological rationale seeking advances HNSCC, head and neck squamous cell carcinoma; mOS, median overall survival 17k PATIENTS Potentially Eligible for Belrestotug Source: Kantar, internal iTeos analysis Strong scientific rationale with high levels of TIGIT+ Tregs, high infiltration of T cells and the indication being amenable to PD-1 therapy Significant market opportunity due to no ongoing Phase 3 studies, potential to be first-to-market, and the opportunity to expand to the locally advanced setting
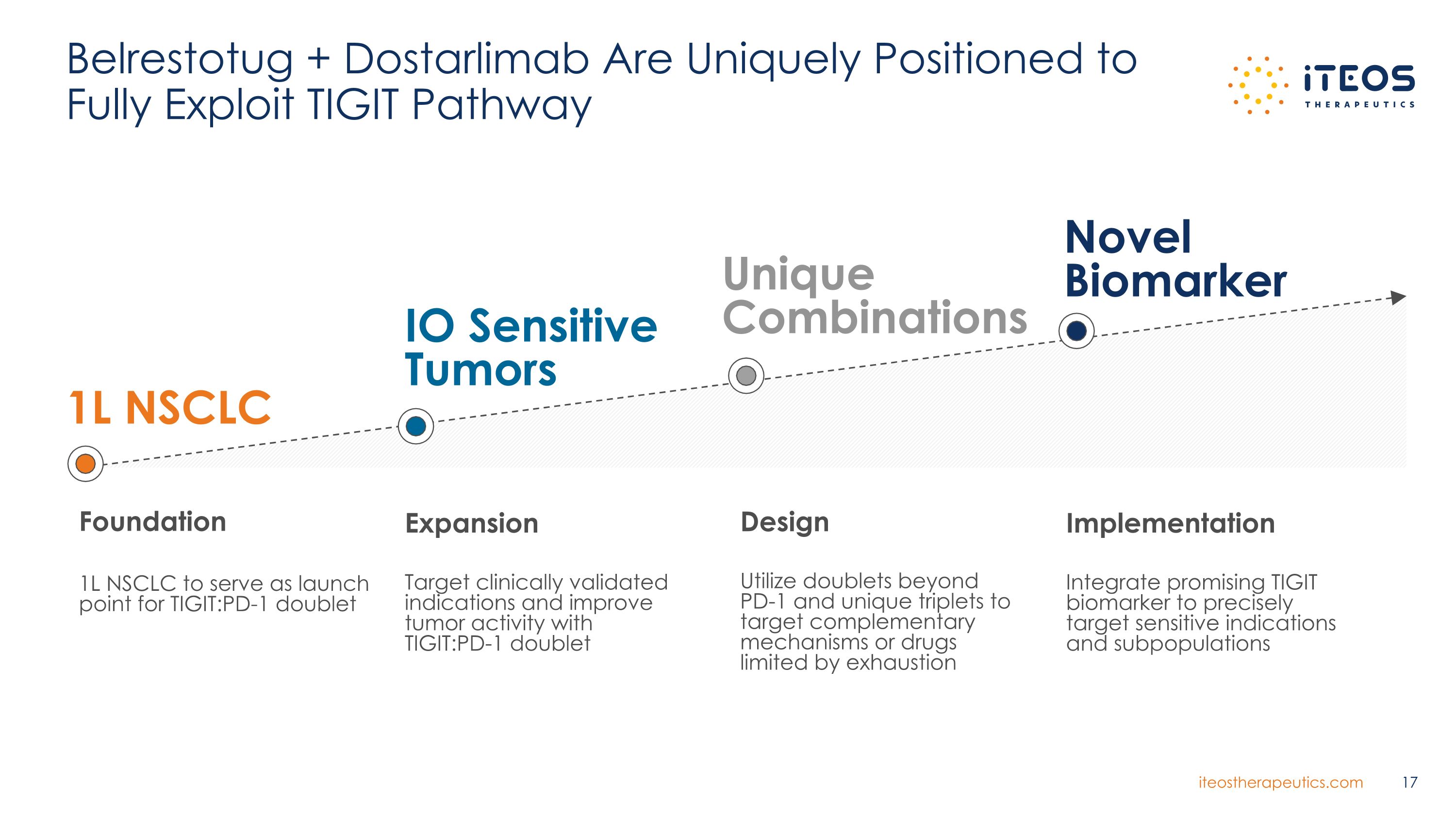
Belrestotug + Dostarlimab Are Uniquely Positioned to Fully Exploit TIGIT Pathway 1L NSCLC Foundation 1L NSCLC to serve as launch point for TIGIT:PD-1 doublet IO Sensitive Tumors Unique Combinations Expansion Target clinically validated indications and improve tumor activity with TIGIT:PD-1 doublet Design Utilize doublets beyond PD-1 and unique triplets to target complementary mechanisms or drugs limited by exhaustion Implementation Integrate promising TIGIT biomarker to precisely target sensitive indications and subpopulations Novel Biomarker
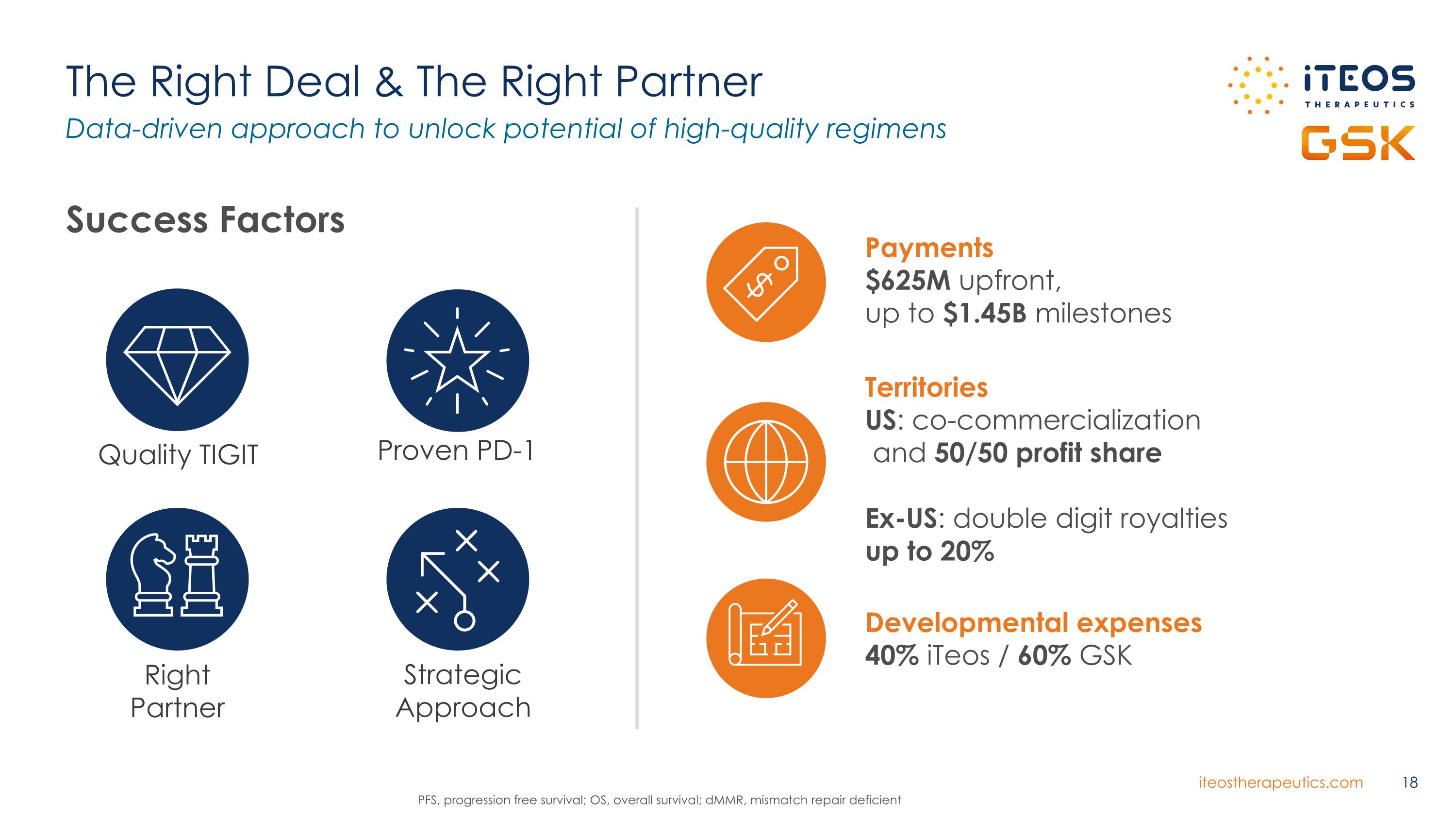
The Right Deal & The Right Partner Data-driven approach to unlock potential of high-quality regimens Proven PD-1 Right Partner Quality TIGIT PFS, progression free survival; OS, overall survival; dMMR, mismatch repair deficient Success Factors Strategic �Approach Payments $625M upfront, �up to $1.45B milestones Developmental expenses 40% iTeos / 60% GSK Territories US: co-commercialization � and 50/50 profit share Ex-US: double digit royalties �up to 20%

Adenosine Pathway Unlocking one of the most promising targets responsible for immunosuppression
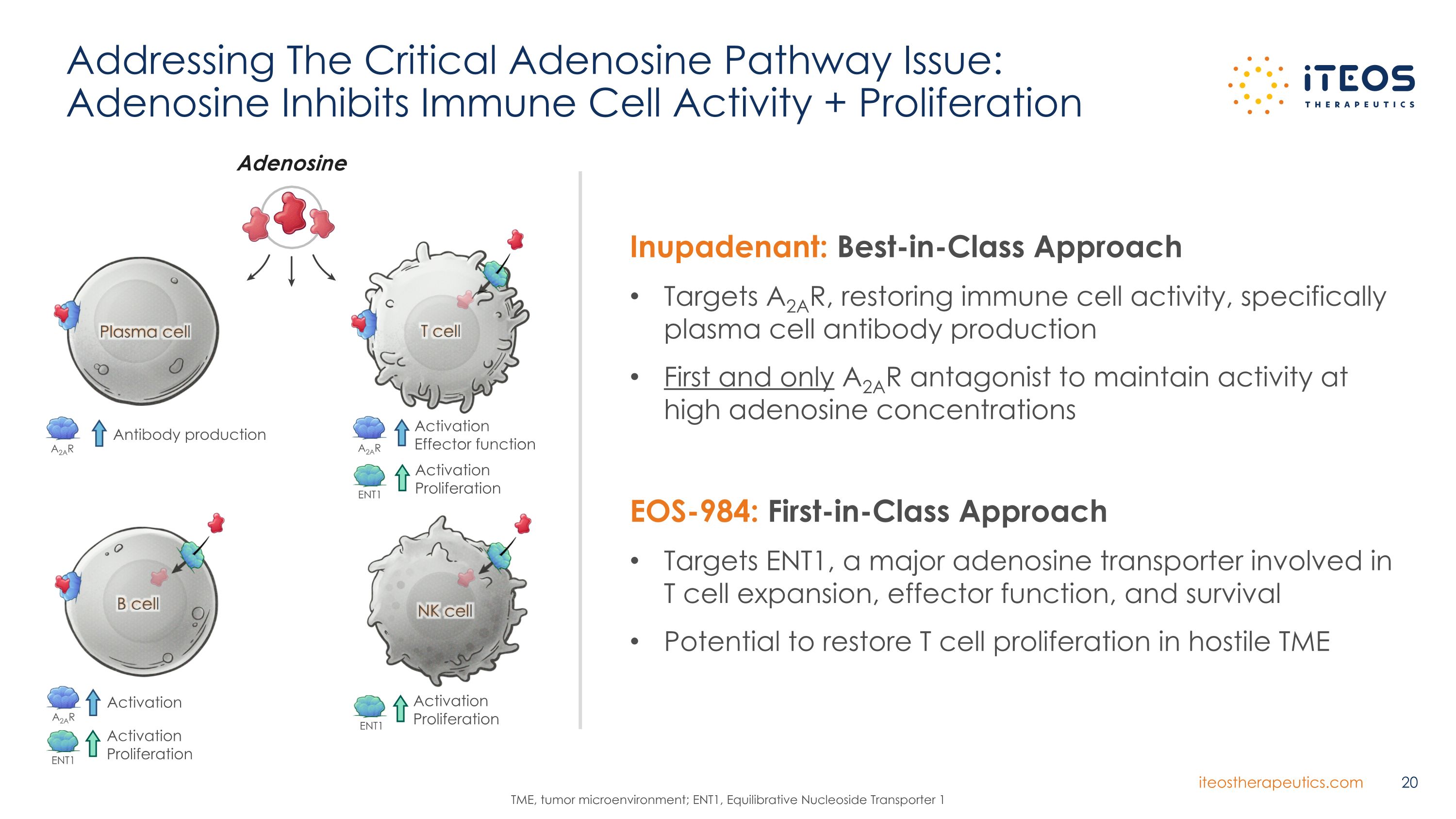
Addressing The Critical Adenosine Pathway Issue: �Adenosine Inhibits Immune Cell Activity + Proliferation TME, tumor microenvironment; ENT1, Equilibrative Nucleoside Transporter 1 Inupadenant: Best-in-Class Approach Targets A2AR, restoring immune cell activity, specifically plasma cell antibody production First and only A2AR antagonist to maintain activity at high adenosine concentrations EOS-984: First-in-Class Approach Targets ENT1, a major adenosine transporter involved in T cell expansion, effector function, and survival Potential to restore T cell proliferation in hostile TME Plasma cell Antibody production A2AR T cell Activation Effector function A2AR Activation Proliferation ENT1 B cell ENT1 Activation Proliferation Activation A2AR NK cell Activation Proliferation ENT1 Adenosine
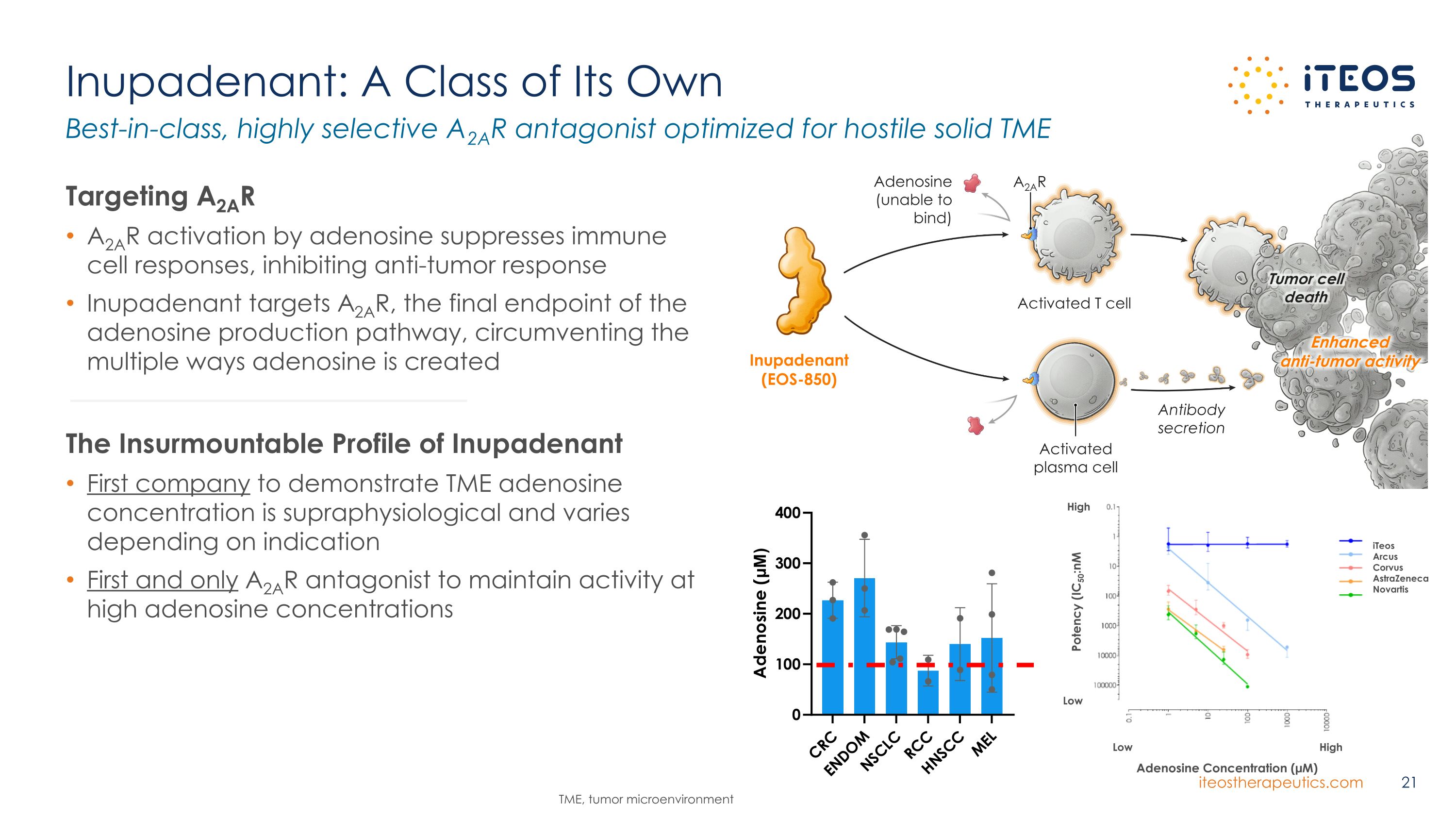
Low High Adenosine Concentration (µM) Potency (IC50:nM Low High iTeos Arcus Corvus AstraZeneca Novartis Inupadenant: A Class of Its Own Targeting A2AR A2AR activation by adenosine suppresses immune cell responses, inhibiting anti-tumor response Inupadenant targets A2AR, the final endpoint of the adenosine production pathway, circumventing the multiple ways adenosine is created The Insurmountable Profile of Inupadenant First company to demonstrate TME adenosine concentration is supraphysiological and varies depending on indication First and only A2AR antagonist to maintain activity at high adenosine concentrations Best-in-class, highly selective A2AR antagonist optimized for hostile solid TME TME, tumor microenvironment A2AR Tumor cell death Activated T cell Activated plasma cell Antibody secretion Enhanced anti-tumor activity Adenosine (unable to bind) Inupadenant (EOS-850)
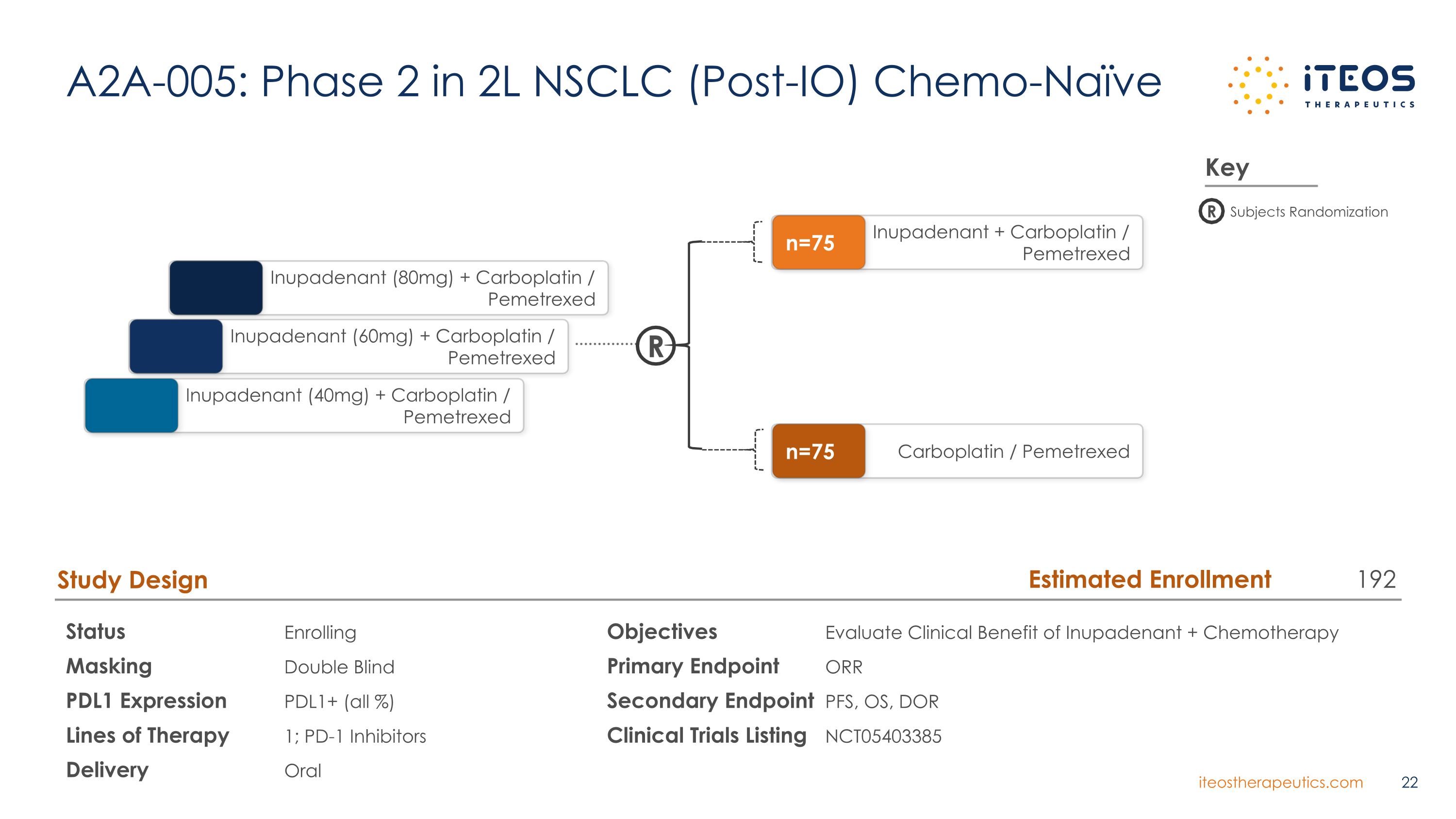
A2A-005: Phase 2 in 2L NSCLC (Post-IO) Chemo-Naïve Status Enrolling Masking Double Blind PDL1 Expression PDL1+ (all %) Lines of Therapy 1; PD-1 Inhibitors Delivery Oral Study Design Objectives Evaluate Clinical Benefit of Inupadenant + Chemotherapy Primary Endpoint ORR Secondary Endpoint PFS, OS, DOR Clinical Trials Listing NCT05403385 R Inupadenant (60mg) + Carboplatin / Pemetrexed Inupadenant (40mg) + Carboplatin / Pemetrexed Inupadenant (80mg) + Carboplatin / Pemetrexed Inupadenant + Carboplatin / Pemetrexed n=75 Carboplatin / Pemetrexed n=75 Key R Subjects Randomization Estimated Enrollment 192
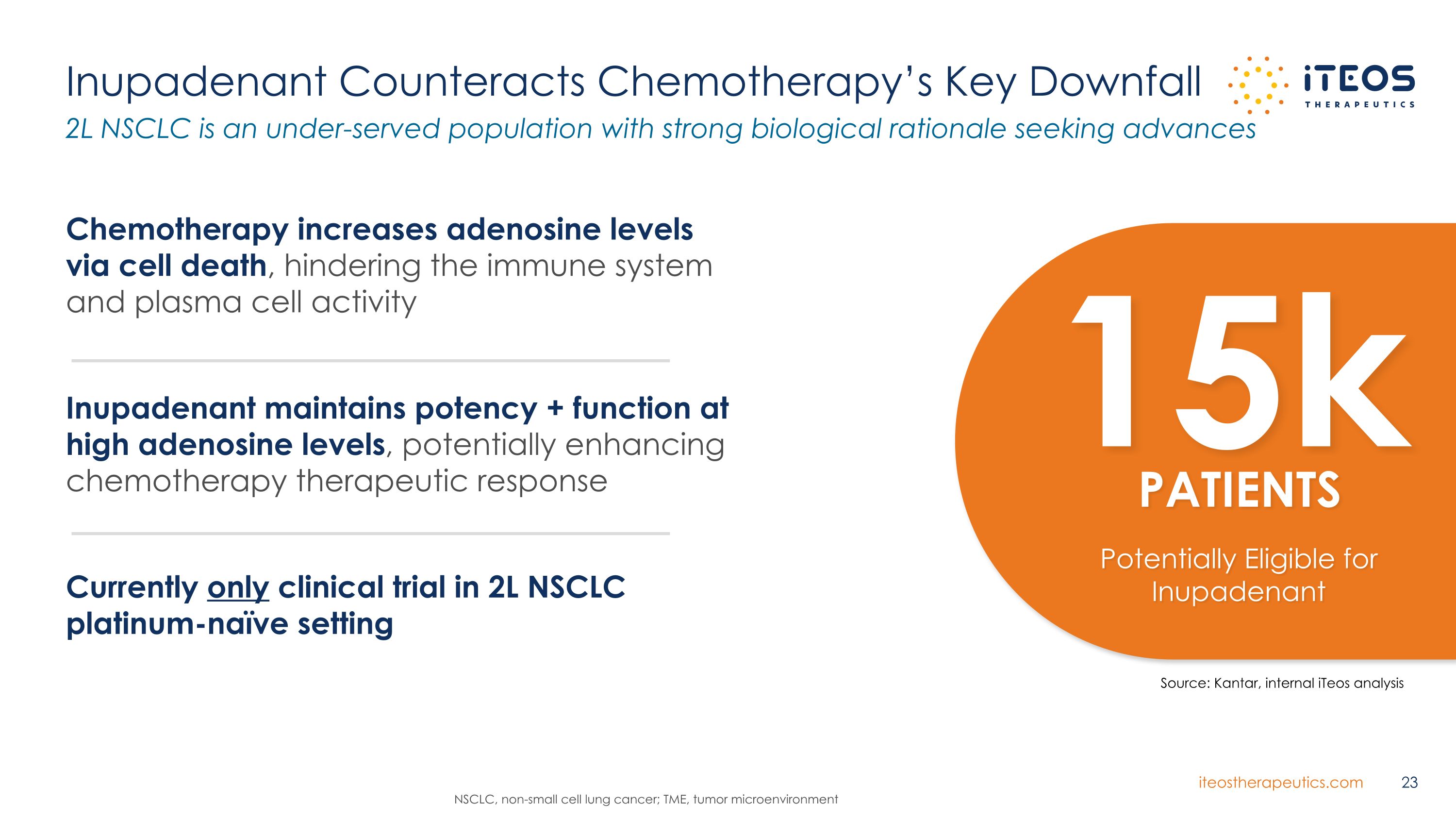
Inupadenant Counteracts Chemotherapy’s Key Downfall Source: Kantar, internal iTeos analysis Chemotherapy increases adenosine levels via cell death, hindering the immune system and plasma cell activity Inupadenant maintains potency + function at high adenosine levels, potentially enhancing chemotherapy therapeutic response Currently only clinical trial in 2L NSCLC platinum-naïve setting 2L NSCLC is an under-served population with strong biological rationale seeking advances NSCLC, non-small cell lung cancer; TME, tumor microenvironment 15k PATIENTS Potentially Eligible for Inupadenant
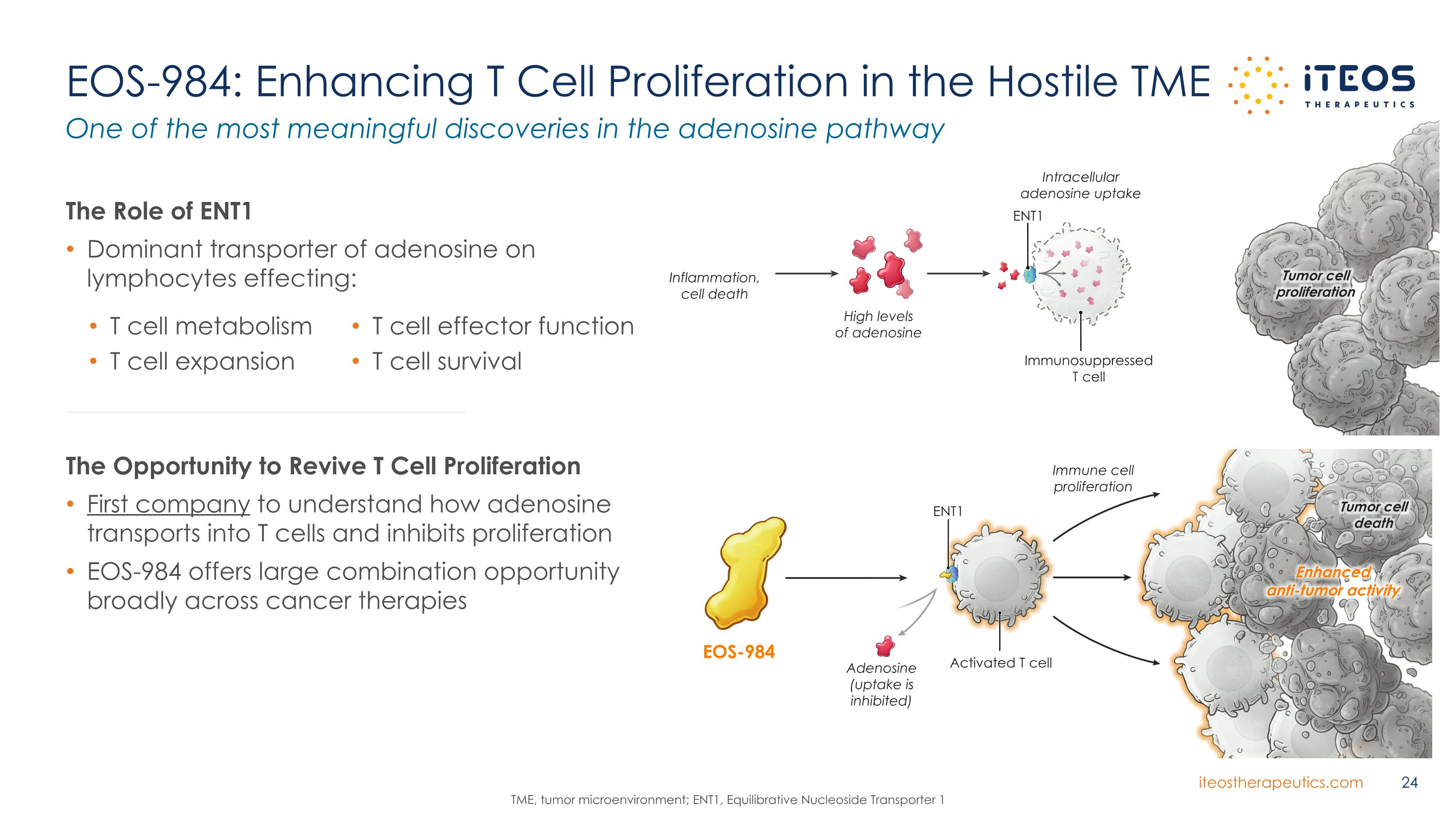
EOS-984: Enhancing T Cell Proliferation in the Hostile TME The Opportunity to Revive T Cell Proliferation First company to understand how adenosine transports into T cells and inhibits proliferation EOS-984 offers large combination opportunity broadly across cancer therapies One of the most meaningful discoveries in the adenosine pathway TME, tumor microenvironment; ENT1, Equilibrative Nucleoside Transporter 1 T cell effector function T cell survival The Role of ENT1 Dominant transporter of adenosine on lymphocytes effecting: T cell metabolism T cell expansion ENT1 Inflammation, cell death Tumor cell proliferation High levels of adenosine Immunosuppressed T cell Intracellular adenosine uptake ENT1 Tumor cell death Adenosine (uptake is inhibited) Activated T cell Immune cell proliferation Enhanced anti-tumor activity EOS-984
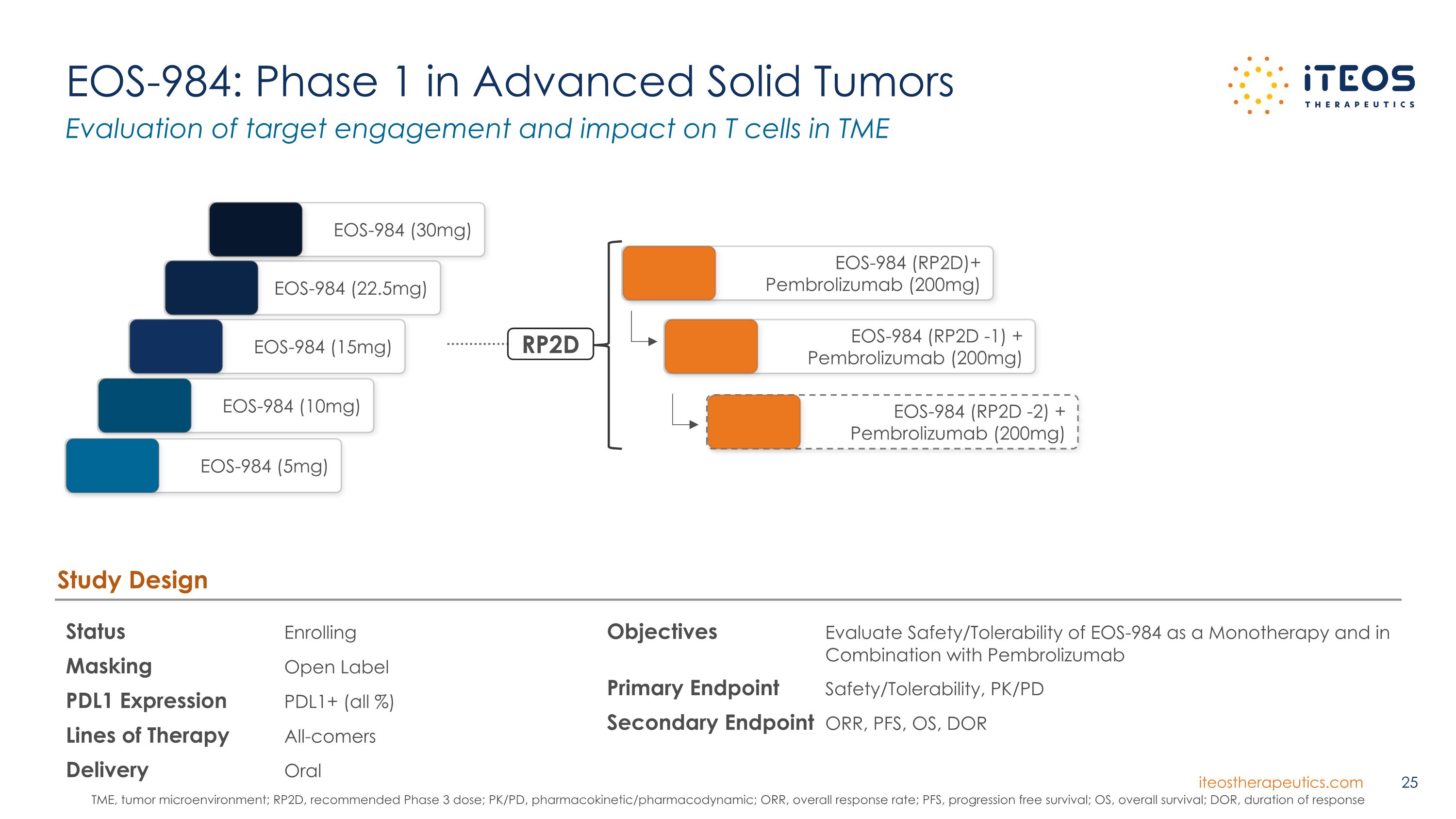
EOS-984: Phase 1 in Advanced Solid Tumors Status Enrolling Masking Open Label PDL1 Expression PDL1+ (all %) Lines of Therapy All-comers Delivery Oral Study Design Objectives Evaluate Safety/Tolerability of EOS-984 as a Monotherapy and in Combination with Pembrolizumab Primary Endpoint Safety/Tolerability, PK/PD Secondary Endpoint ORR, PFS, OS, DOR EOS-984 (15mg) EOS-984 (10mg) EOS-984 (22.5mg) EOS-984 (RP2D -1) + Pembrolizumab (200mg) EOS-984 (5mg) EOS-984 (30mg) RP2D EOS-984 (RP2D)+ Pembrolizumab (200mg) EOS-984 (RP2D -2) + Pembrolizumab (200mg) Evaluation of target engagement and impact on T cells in TME TME, tumor microenvironment; RP2D, recommended Phase 3 dose; PK/PD, pharmacokinetic/pharmacodynamic; ORR, overall response rate; PFS, progression free survival; OS, overall survival; DOR, duration of response
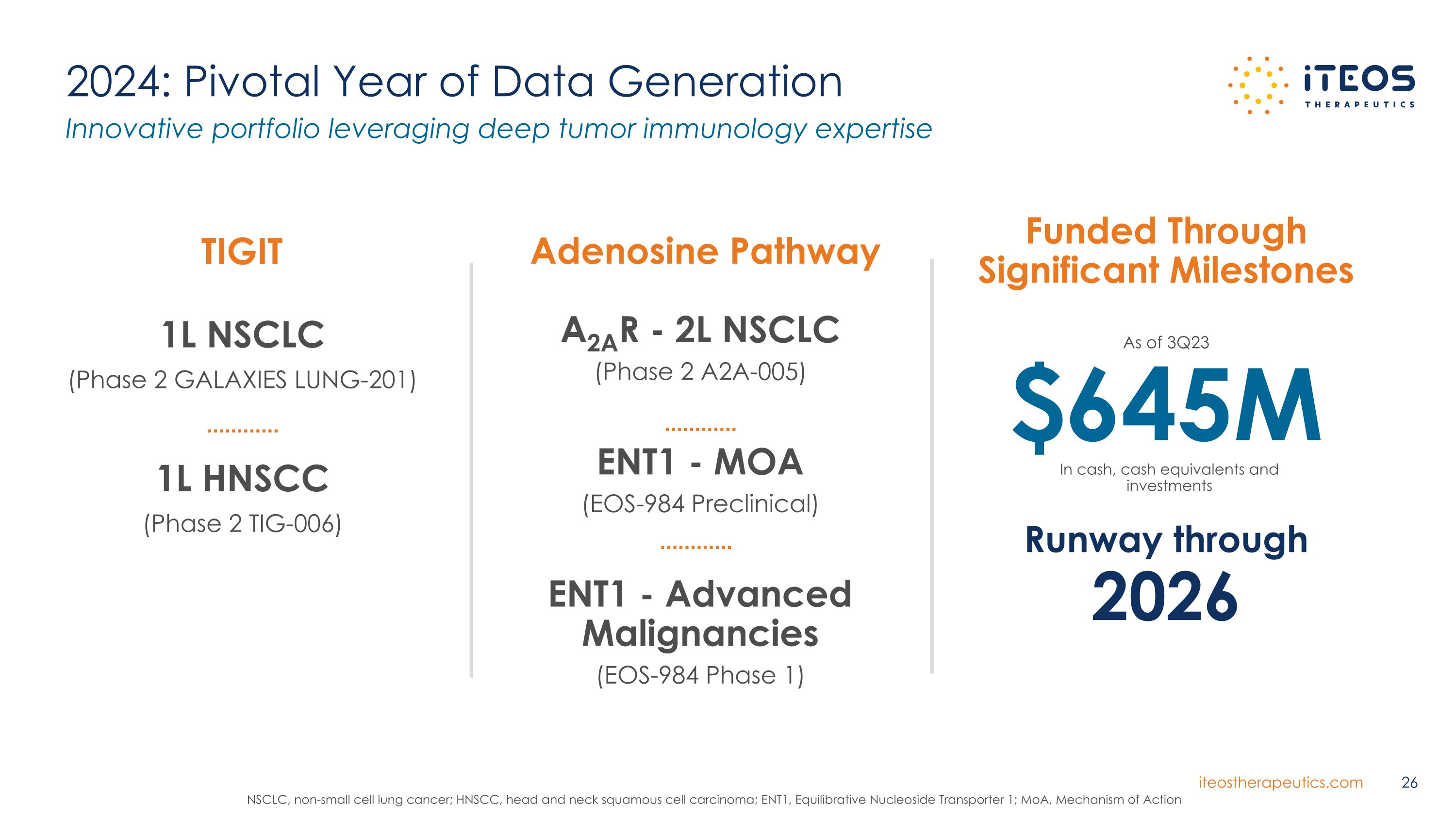
2024: Pivotal Year of Data Generation Innovative portfolio leveraging deep tumor immunology expertise TIGIT $645M As of 3Q23 Runway through 2026 In cash, cash equivalents and investments Adenosine Pathway 1L NSCLC (Phase 2 GALAXIES LUNG-201) 1L HNSCC (Phase 2 TIG-006) A2AR - 2L NSCLC (Phase 2 A2A-005) ENT1 - MOA (EOS-984 Preclinical) ENT1 - Advanced Malignancies (EOS-984 Phase 1) Funded Through Significant Milestones NSCLC, non-small cell lung cancer; HNSCC, head and neck squamous cell carcinoma; ENT1, Equilibrative Nucleoside Transporter 1; MoA, Mechanism of Action

Cancer Immunotherapies�by design™ Nasdaq: ITOS January 2024
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Grafico Azioni iTeos Therapeutics (NASDAQ:ITOS)
Storico
Da Nov 2024 a Dic 2024

Grafico Azioni iTeos Therapeutics (NASDAQ:ITOS)
Storico
Da Dic 2023 a Dic 2024
