false 0001404644 0001404644 2024-03-04 2024-03-04
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 OR 15(d)
of The Securities Exchange Act of 1934
Date of Report (date of earliest event reported): March 4, 2024
Neurogene Inc.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
001-36327 |
|
98-0542593 |
| (State or other jurisdiction of incorporation or organization) |
|
(Commission File Number) |
|
(I.R.S. Employer
Identification No.) |
|
| 535 W 24th Street, 5th Floor New York, NY 10011 |
| (Address of principal executive offices, including zip code) |
Registrant’s telephone number, including area code: (877) 237-5020
N/A
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, $0.000001 par value |
|
NGNE |
|
The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 |
Regulation FD Disclosure. |
On March 4, 2024, Neurogene Inc. (the “Company”) issued a press release announcing updates to the expansion of its ongoing Phase 1/2 gene therapy clinical trial for NGN-401 for female pediatric patients with Rett syndrome and updates to enable more rapid enrollment in the trial. A copy of the press release is furnished as Exhibit 99.1 to this Current Report on Form 8-K. Also on March 4, 2024, the Company posted an updated corporate presentation on its website. A copy of the corporate presentation is furnished as Exhibit 99.2 to this Current Report on Form 8-K.
The information in Item 7.01 of this Current Report on Form 8-K, including the information in the press release attached as Exhibit 99.1 and the presentation attached as Exhibit 99.2, is furnished pursuant to Item 7.01 of Form 8-K and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section. Furthermore, the information in Item 7.01 of this Current Report on Form 8-K, including the information in the press release attached as Exhibit 99.1 and the presentation attached as Exhibit 99.2, shall not be deemed to be incorporated by reference in the filings of the Company under the Securities Act of 1933, as amended.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
NEUROGENE INC. |
|
|
|
|
| Date: March 4, 2024 |
|
|
|
By: |
|
/s/ Christine Mikail |
|
|
|
|
|
|
Name: Christine Mikail |
|
|
|
|
|
|
Title: President and Chief Financial Officer |
Exhibit 99.1
Neurogene Announces Expansion and Plans for More Rapid Patient Enrollment of Rett
Syndrome Gene Therapy Clinical Trial
Ongoing Phase 1/2 clinical trial for NGN-401 to include additional patients in Cohort 1 and a
dose-escalation cohort
Both previously planned updates are expected to provide a more robust dataset to inform
future registrational trial design
Removal of staggered dosing in Cohort 1 expected to enable the anticipated completion of
Cohort 1 dosing in the second half of 2024
Third patient dosed in Cohort 1
NEW YORK
– March 4, 2024 – Neurogene Inc. (NASDAQ: NGNE) (“Neurogene” or “the Company”), a clinical-stage company founded to bring life-changing genetic medicines to patients and families affected by rare
neurological diseases, today announced the expansion of its ongoing Phase 1/2 gene therapy clinical trial for NGN-401 for female pediatric patients with Rett syndrome and updates to enable more rapid
enrollment in the trial.
“We are excited to share that we have met our first 2024 program milestones, including dosing the third patient in the NGN-401 Phase 1/2 trial for Rett syndrome and expansion of the trial to include more patients in the current dosing cohort and the addition of a high dose cohort,” said Founder and Chief Executive Officer,
Rachel McMinn, Ph.D. “Our clinical development strategy has been to build flexibility and optionality early in the program with two concurrent dose cohorts designed to generate a more complete data package, which we expect will inform future
registration discussions with global health authorities. We expect that expansion of the clinical trial and the removal of staggered dosing in Cohort 1 will enable us to treat more patients in a shorter period of time. Based on this update, we
expect to complete enrollment of Cohort 1 in the second half of 2024.”
Rett Syndrome Program Update
The U.S. Phase 1/2 clinical protocol for NGN-401 has been amended as follows:
| |
• |
|
Cohort 1, which specifies a total dose of
1×1015 total vector genomes delivered via intracerebroventricular (ICV) administration, was expanded from five patients to eight patients. The dosing stagger has been removed from
Cohort 1, enabling the remaining patients to be dosed in parallel. |
| |
• |
|
Cohort 2, which specifies a total dose of
3×1015 total vector genomes delivered via ICV administration, was added and is expected to include a total of eight patients. |
| |
• |
|
The first three patients in Cohort 2 will be dosed in a staggered manner, with first patient dosing expected in
the second quarter of 2024; pending Data and Safety Monitoring Board review of the safety data for the first three patients, the protocol will allow parallel enrollment for the remaining patients in Cohort 2. |
| |
• |
|
In addition, the protocol includes a targeted immunosuppression regimen for Cohort 2, designed as a preventative
measure to aid in avoiding potential adeno-associated virus (AAV)-related immune responses that have been observed with other AAV-based products in this dose range. The immunosuppression regimen includes the
use of rituximab and sirolimus, along with a shortened course of corticosteroids. Cohort 1 immunosuppression remains unchanged with corticosteroids alone. |
A similar protocol amendment was submitted to the UK regulatory authorities. These changes are consistent
with the Company’s guidance issued in January 2024. Importantly, in comprehensive nonclinical studies, the EXACT transgene regulation technology embedded in NGN-401 was shown to mechanistically constrain
MECP2 transgene expression levels, allowing for the potential to dose escalate and enhance biodistribution to the brain, without the commensurate increase in MECP2 transgene expression observed with conventional gene therapy. Both
doses in the updated protocol are below the “no observed adverse effect” level established in rodent and nonhuman primate models.
A third
patient was dosed in the trial early in the first quarter. NGN-401 has been generally well-tolerated and there have been no treatment-emergent or procedure-related serious adverse events, or signs of
overexpression-related toxicity observed in any patient.
Neurogene remains on track to report interim clinical data from Cohort 1 in the fourth quarter
of 2024 and additional data, including from Cohort 2, in the second half of 2025.
About EXACT
Neurogene’s novel and proprietary EXACT gene regulation platform technology is a self-contained transgene regulation platform that can be tuned to deliver
a desired level of transgene expression within a narrow and therapeutically relevant range, with the goal of avoiding transgene-related toxicities associated with conventional gene therapy. EXACT is compatible with viral and non-viral delivery platforms.
About NGN-401
NGN-401 is an investigational AAV9 gene therapy being developed as a one-time
treatment for Rett syndrome. It is the first clinical candidate to deliver the full-length human MECP2 gene under the control of Neurogene’s EXACT technology. The EXACT technology utilized in
NGN-401 is an important advancement in gene therapy for Rett syndrome, specifically because the disorder requires a treatment approach that enables targeted levels of MECP2 transgene
expression without causing overexpression-related toxic effects associated with conventional gene therapy. The robust nonclinical data package for NGN-401 provides evidence of a potentially compelling efficacy
and safety profile in Rett syndrome.
About Neurogene
Neurogene’s mission is to treat devastating neurological diseases to improve the lives of patients and families impacted by these rare diseases. Neurogene
is developing novel approaches and treatments to address the limitations of conventional gene therapy in central nervous system disorders. This includes selecting a delivery approach to maximize distribution to target tissues and by designing
products to maximize potency and purity for an optimized efficacy and safety profile. The Company’s novel and proprietary EXACT transgene regulation platform technology allows for the delivery of therapeutic levels while limiting transgene
toxicity associated with conventional gene therapy. Neurogene has constructed a state-of-the-art gene therapy manufacturing
facility in Houston, Texas. GMP production of NGN-401 was conducted in this facility and will support pivotal clinical development activities. For more information, visit www.neurogene.com.
Neurogene Contacts:
Investor Relations:
Melissa Forst
Argot Partners
Neurogene@argotpartners.com
Media:
David Rosen
Argot Partners
david.rosen@argotpartners.com
Cautionary Note Regarding Forward-Looking Statements
Statements in this press release which are not historical in nature are intended to be, and hereby are identified as, forward-looking statements within the
meaning of the Private Securities Litigation Reform Act of 1995. These statements may discuss goals, intentions and expectations as to future plans, trends, events, results of operations or financial condition, or otherwise, based on current
expectations and beliefs of the management of Neurogene, as well as assumptions made by, and information currently available to, management of Neurogene, including, but not limited to, statements regarding the therapeutic potential and utility,
efficacy and clinical benefits of NGN-401, the safety and tolerability profile of NGN-401, trial designs, clinical development plans and timing for NGN-401, including completion of Cohort 1 dosing, first patient dosing of Cohort 2 and anticipated clinical data results in NGN-401 Phase 1/2 trial for Rett syndrome and
anticipated impact of expansion of Phase 1/2 trial and removal of staggered dosing in Cohort 1. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and
include words such as “may,” “will,” “should,” “would,” “expect,” “anticipate,” “plan,” “likely,” “believe,” “estimate,” “project,”
“intend,” “on track,” and other similar expressions or the negative or plural of these words, or other similar expressions that are predictions or indicate future events or prospects, although not all forward-looking statements
contain these words. Forward looking statements are subject to risks, uncertainties, and assumptions that are difficult to predict with regard to timing, extent, likelihood, and degree of occurrence, which could cause actual result to differ
materially from anticipated results and many of which are outside of Neurogene’s control. Such risks, uncertainties and assumptions include, among other things, risks related the timing and success of enrolling patients in the expanded cohort
of our Phase 1/2 clinical trial of NGN-401 for the treatment of Rett syndrome, the expected timing and results of dosing of patients in our clinical trials, including
NGN-401, the potential that we may not be able to expand our Phase 1/2 clinical trial of NGN-401 for the treatment of Rett syndrome into the UK based on a variety of
factors, including but not limited to any decisions of regulatory authorities, costs of expanding the trial in the UK, the availability of suitable clinical test sites, the ability to enroll patients in the UK or other reasons, the potential for
negative impacts to patients resulting from using a higher dose of NGN-401 in Cohort 2 of the Phase 1/2 clinical trial for the treatment of Rett syndrome, the risk that we may not be able to report our data on
the predicted timeline, our limited operating history; the risk that we may not be able to raise adequate additional capital to finance our operations, complete our clinical trials and commercialize our products, risks related to our ability to
obtain regulatory approval for, and ultimately commercialize, our product candidates, including NGN-401; risks related to the outcome of non-clinical testing and early
clinical trials for our product candidates, including the ability of those trials to satisfy relevant governmental or regulatory requirements; risks related to our limited experience in designing clinical trials and lack of
experience in conducting clinical trials; expectations regarding the market and potential for Neurogene’s current product candidates, including
NGN-401; the substantial competition we face in discovering, developing, or commercializing products, including NGN-401; expectations regarding the potential
tolerability, safety or efficacy for our current product candidates, including NGN-401; our ability to attract, hire, and retain skilled executive officers and employees; our ability to protect our
intellectual property and proprietary technologies; risks related to our reliance on third parties, contract manufacturers, and contract research organizations and legislative, regulatory, political and economic developments and general market
conditions. These and other risks and uncertainties are identified under the heading “Risk Factors” included in our periodic reports that we file with the Securities and Exchange Commission.
Nothing in this communication should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or
that the contemplated results of any such forward-looking statements will be achieved. Forward-looking statements in this communication speak only as of the day they are made and are qualified in their entirety by reference to the cautionary
statements herein. Except as required by applicable law, we do not undertake any obligation to revise or update any forward-looking statement, or to make any other forward-looking statements, whether as a result of new information, future events or
otherwise.
This communication contains hyperlinks to information that is not deemed to be incorporated by reference into this communication.

Exhibit 99.2 Corporate Presentation March 2024

Disclaimer Forward Looking Statements This communication contains
forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may discuss goals, intentions and expectations as to future plans, trends, events, results of operations or financial condition,
or otherwise, based on current expectations and beliefs of the management of Neurogene, as well as assumptions made by, and information currently available to, management of Neurogene, including, but not limited to, statements regarding the
therapeutic potential and utility, efficacy and clinical benefits of NGN-401 and NGN-101; the safety and tolerability profile of NGN-401 and NGN-101, trial designs, clinical development plans and timing for NGN-401 and NGN-101, including completion
of Cohort 1 dosing, first patient dosing of Cohort 2 and anticipated clinical data results in NGN-401 Phase 1/2 trial for Rett syndrome and anticipated clinical data results in NGN-101 Phase 1/2 trial for CLN5 Batten disease; anticipated impact of
expansion of Phase 1/2 trial and removal of staggered dosing in Cohort 1 and anticipated early-stage discovery. Forward- looking statements generally include statements that are predictive in nature and depend upon or refer to future ev ents or
conditions, and include words such as “may,” “will,” “should,” “would,” “expect,” “anticipate,” “plan,” “likely,” “believe,”
“estimate,” “project,” “intend,” and other similar expressions or the negative or plural of these words, or other similar expressions that are predictions or indicate future events or prospects, although not all
forward-looking statements contain these words. Statements that are not historical facts are forward-looking statements. Forward-looking statements in this communication include, but are not limited to, statements regarding the expected expansion
and enrollment of, and timing of data from, Neurogene’s Phase 1/2 clinical trials; statements regarding the potential of, and expectations regarding, Neurogene’s programs, including its EXACT technology, NGN-101, NGN-401 and its research
stage opportunities; statements regarding market opportunities for Neurogene’s product candidates; the expected dosing of additional patients in Neurogene’s Phase 1/2 clinical trial of NGN-401; statements regarding the potential
expansion of Neurogene’s Phase 1/2 clinical trial in Rett syndrome into the United Kingdom, the expansion of Cohort 1 to include additional patients and the expansion of the clinical trial to include Cohort 2 as a dose escalation cohort;
statements regarding future interactions with U.S. or foreign regulatory authorities; and statements regarding Neurogene’s cash runway. Forward-looking statements are based on current beliefs and assumptions that are subject to risks and
uncertainties and are not guarantees of future performance. Actual results could differ materially from those contained in any forward-looking statement as a result of various factors, including, without limitation: Neurogene’s limited
operating history; the significant net losses incurred since inception of Neurogene; the ability to raise additional capital to finance operations; the ability to advance product candidates through non-clinical and clinical development; the ability
to obtain regulatory approval for, and ultimately commercialize, Neurogene’s product candidates; the outcome of non-clinical testing and early clinical trials for Neurogene’s product candidates, including the ability of those trials to
satisfy relevant governmental or regulatory requirements; Neurogene’s limited experience in designing clinical trials and lack of experience in conducting clinical trials; the ability to identify and pivot to other programs, product
candidates, or indications that may be more profitable or successful than Neurogene’s current product candidates; expectations regarding the market and potential for Neurogene’s current product candidates; the substantial competition
Neurogene faces in discovering, developing, or commercializing products; expectations regarding the potential tolerability, safety or efficacy for Neurogene’s current product candidates; the ability to attract, hire, and retain skilled
executive officers and employees; the ability of Neurogene to protect its intellectual property and proprietary technologies; reliance on third parties, contract manufacturers, and contract research organizations; the ability to attract, hire, and
retain skilled executive officers and employees; the ability of Neurogene to protect its intellectual property and proprietary technologies; risks related to Neurogene’s ability to correctly estimate its respective operating expenses,
including its projected cash runway, and any unexpected costs, charges or expenses resulting from the merger with Neoleukin Therapeutics, Inc. (“Neoleukin”); and legislative, regulatory, political and economic developments and general
market conditions. . The foregoing rev iew of important factors that could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and
elsewhere, including the risk factors included in the Company’s most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q filed with the Securities and Exchange Commission (SEC), the registration statement on Form S-4 filed
with the SEC in connection with the merger of Neurogene and Neoleukin, as well as risk factors associated with companies, such as Neurogene, that operate in the biopharma industry. These forward-looking statements involve a number of risks,
uncertainties (some of which are beyond Neurogene’s control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements. Nothing in this
communication should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that the contemplated results of any such forward-looking statements will be achieved. Forward-looking
statements in this communication speak only as of the day they are made and are qualified in their entirety by reference to the cautionary statements herein. Except as required by applicable law, Neurogene undertakes no obligation to revise or
update any forward-looking statement, or to make any other forward-looking statements, whether as a result of new information, future ev ents or otherwise. Industry and Market Data Certain information contained in this Presentation relates to or is
based on studies, publications, surveys and Neurogene’s own internal estimates and research. In this Presentation, Neurogene relies on, and refers to, publicly available information and statistics regarding market participants in the sector in
which Neurogene competes and other industry data. Any comparison of Neurogene to any other entity assumes the reliability of the information available to Neurogene. Neurogene obtained this information and statistics from third-party sources,
including reports by market research firms and company filings. In addition, all of the market data included in this Presentation involve a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of
such assumptions. Finally, while Neurogene believes its internal research is reliable, such research has not been verified by any independent source and Neurogene has not independently verified the information. Trademarks This Presentation may
contain trademarks, serv ice marks, trade names and copyrights of other companies, which are the property of their respective owners. Solely for conv enience, some of the trademarks, serv ice marks, trade names and copyrights referred to in this
Presentation may be listed without the TM, SM © or ® symbols, but Neurogene will assert, to the fullest extent under applicable law, the righ2 ts of the applicable owners, if any, to these trademarks, serv ice marks, trade names and
copyrights.

Neurogene is a Differentiated Clinical-Stage Company Utilizing EXACT
Technology to Treat Complex Neurological Diseases Novel EXACT technology designed to overcome key limitations of conventional gene therapy $ Pipeline addresses attractive market opportunities, including Rett syndrome Internal manufacturing provides
financial and strategic pipeline flexibility 2H:26 cash runway enables operations beyond clinical inflection points Internal manufacturing provides financial and strategic pipeline flexibility EXACT: Expression Attenuation via Construct Tuning
3

Funding for Key Near Term Milestones Obtained in Reverse Merger and
Concurrent Private Financing Completed in 2023 Reverse merger and concurrent financing secured funding to position Transaction Highlights Neurogene to deliver on anticipated near term milestones: • Merger closed on December 18, 2023 Rett
syndrome (NGN-401) Expanded ongoing Phase 1/2 clinical trial to enroll a larger cohort of patients, • Post-merger company trades on Nasdaq including dose-escalation cohort as Neurogene Inc. with ticker “NGNE” • Interim Phase
1/2 clinical data 4Q:24 • Simultaneously closed on ~$95M • Additional Phase 1/2 clinical data from expanded low dose and a high dose concurrent private financing cohort in 2H:25 • 16,887,060 shares of common stock CLN5 Batten
disease (NGN-101) outstanding at closing* • Interim Phase 1/2 clinical data in 2H:24 • Cash balance of approximately $200M at • Engage in FDA discussions regarding a streamlined registrational pathway in closing 2H:24 •
Expected cash runway to fund operations Early-stage discovery into 2H:26 • Advance one early-stage program into the clinic in 2025 *After the closing of merger, private financing, and 1-for-4 reverse stock split. This number includes 4,063,364
Neurogene Pre-Funded Warrants. 4
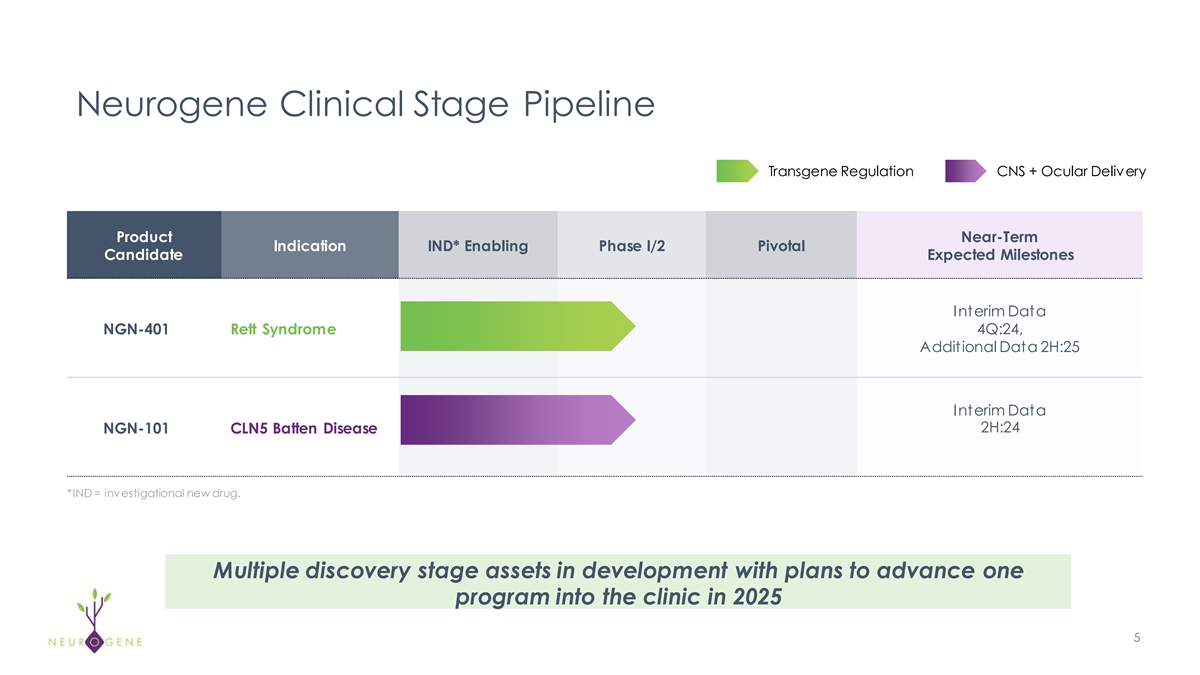
Neurogene Clinical Stage Pipeline Transgene Regulation CNS + Ocular
Delivery Product Near-Term Indication IND* Enabling Phase I/2 Pivotal Candidate Expected Milestones Interim Data NGN-401 Rett Syndrome 4Q:24, Additional Data 2H:25 Interim Data 2H:24 NGN-101 CLN5 Batten Disease *IND = investigational new drug.
Multiple discovery stage assets in development with plans to advance one program into the clinic in 2025 5

Developed to Solve the Limitations of Conventional Gene Therapy in
Complex Neurological Disorders Today’s Gene Therapy Neurogene’s is Limited By: Solutions: Novel, modular EXACT gene regulation technology and other regulatory elements designed to optimize transgene Variable Gene Expression expression to
maximize the therapeutic window Novel and proprietary EXACT gene regulation technology designed to avoid transgene related toxicity associated Safety Limitations with conventional gene therapy Design products to maximize Select ICV delivery approach
potency and purity for to maximize AAV9 distribution Inefficient Gene Delivery potentially optimized to target CNS tissues efficacy/safety profile ICV = intracerebroventricular AAV = adeno-associated virus 6 CNS = central nervous system

Wholly-Owned and Fully Integrated In-House AAV Manufacturing GMP
Manufacturing 42,000 sq ft facility in Houston, with 6,000 sq ft of cleanrooms Analytical Toxicology Batch Development Manufacturing Quality Process Control Development • Flexibility to manufacture AAV product at low cost • Own product
quality and development timelines Quality • Process development expertise supports both HEK293 and Sf9/rBV manufacturing platforms Assurance • Flexibility to rapidly adapt CMC execution to program needs Current research and
clinical-grade manufacturing capabilities are designed for commercial-grade product to avoid potential future comparability challenges 7

Experienced Leadership Team Backed by Top Tier Investors Management Team
Rachel McMinn, Ph.D. Christine Mikail, J.D. Stuart Cobb, Ph.D. Julie Jordan, M.D. Founder and CEO President and CFO CSO CMO Effie Albanis, M.D. Andrew Mulberg, M.D. Arvind Sreedharan Ricardo Jimenez SVP, Early Clinical and SVP, Regulatory Affairs
SVP, Business Operations SVP, Technical Operations Translationall Research Backed by a Syndicate of Thought-Leading Investors AVIDITY PARTNERS Healthcare Investment Fund 8

NGN-401 for Rett Syndrome Leveraging EXACT gene regulation
technology

Rett Syndrome – Devastating Disorder with High Unmet Need
Genetics • X-Linked disorder causing mutations in the gene encoding for methyl-CpG binding protein 2 (MeCP2) • One of the most common genetic causes of developmental and intellectual impairment in females • Unknown incidence in
boys, but typically lethal by ~3 years of age due to no healthy copy of MeCP2 Compelling Market Opportunity • U.S. prevalence - ~6,000-9,000 patients • WW incidence - 1:10,000-1:15,000 live female births High Unmet Need • There are
no approved treatments that address root cause of disease • Significant unmet need remains for new treatment options U.S. prevalence estimate based on published incidence rates; Laurvick CL, et al. J Pediatr 2006;148(3):347–35. WW
incidence estimate based on published incidence rates; Pini G, et al. Orphanet Journal of Rare Diseases (2016) 11:132. 10

Rett Syndrome Treatment Requires Tight Gene Regulation MeCP2 Rett
Syndrome* Duplication Disorder** Balanced Too little Too much treatment goal gene expression drives disease gene expression drives disease ~50% of cells express WT levels of MeCP2 ~100% cells express 2x MeCP2 levels ~50% are MeCP2 deficient •
Rett syndrome (RTT) is a severe neurological disorder caused by mosaic mutations in X-linked MeCP2 gene • Mice modeling RTT recapitulate many neurological phenotypes observed clinically; disease reversibility has been demonstrated in both
immature and mature adult animals NGN-401 is designed to deliver therapeutic levels of MeCP2 to deficient cells while maintaining a non-toxic level in unaffected cells *Represents female Rett syndrome; **Represents male duplication disorder; WT =
wildtype 11 Pini G, et al. Orphanet Journal of Rare Diseases (2016) 11:132.

Acts As a Genetic Thermostat, Limiting Transgene Expression EXACT miRNA
controls transgene levels to targeted range Regulatory elements designed to avoid off- target effects EXACT is expected to enable gene therapy for Rett syndrome and other complex Protein Expressed disorders 12

Designed to Widen Therapeutic Window and Enable Gene Therapy for Rett
Syndrome EXACT Dampens Variable AAV Wider Therapeutic Window Overexpression Transduction Endogenous Well tolerated in animal models Endogenous+NGN-401 Deficient Shows therapeutic effect in gold Deficient +NGN-401 standard model Functional MeCP2
protein levels ~50% of cells express WT levels of MeCP2 ~50% are MeCP2 deficient 13

NGN-401 Demonstrates Efficacy and Safety in Mecp2 Mouse Models ICV
Delivery of NGN-401 Delivers Targeted MeCP2 Levels AAV9 capsid EXACT Full length EXACT Promoter recognition sites miRNA human MECP2 Survival in Male Knockout Survival in Female Het NGN-401 (regulated) 100 100 EXACT1 MECP2 75 75 23 37wks 9
Unregulated 50 50 MECP2 25 25 NGN-401 1e11 vg 0 0 NGN-401 3e11 vg 0 5 10 15 20 25 30 35 40 45 50 0 5 10 15 20 25 Unregulated 1e11 vg Age (weeks) Age (weeks) Unregulated 3e11 vg WT + Vehicle Male or female + Vehicle Het=heterozygous for Mecp2,
mirroring genetic makeup of human females with Rett syndrome 14 Survival (%) Survival (%)

ICV Administration Significantly Better Distribution Than IT-L To Key
Areas of the Nervous System Underlying Rett Syndrome in NHPs Key areas of Rett syndrome pathology ICV M o to r C o rte x Speech F ro n ta l C o rte x Motor H ip p o c a m p u s C e re b e llu m B ra in s te m C e rv ic a l Autonomic T h o ra c ic
Intrathecal L u m b a r 0 1 0 0 0 0 2 0 0 0 0 3 0 0 0 0 4 0 0 0 0 Vector genome copies/µg DNA

NGN-401 Preclinical Data Enabled Pediatric Clinical Approach Delivery
of full-length MECP2 Promising efficacy, favorable safety profile MAXIMIZES THERAPEUTIC POTENTIAL DEMONSTRATED CONTROLLED MeCP2 LEVELS No evidence of off-target or MeCP2 tox Robust MeCP2 levels to key brain areas PROVIDES TRANSLATIONAL FOUNDATION
GENERATED COMPREHENSIVE FOR HUMANS SAFETY PACKAGE U.S. FDA and UK MHRA cleared dosing directly into pediatric patients 16

Cardinal Clinical Features of Rett Syndrome Inability to Impaired Fine
and Autonomic Additional Disease Communicate Gross Motor Skills Dysfunction Manifestations • Severe apnea • Loss of purposeful • Loss of hand function • Seizures episodes • Gait abnormalities hand use & •
Anxiety • Hyperventilation involuntary hand • Ambulation requiring • Scoliosis • Constipation • Muscle contractures movements assistance or • Difficulty swallowing non-ambulatory • Loss of spoken •
Sleep disturbance language GI tube placement common “Relative” stability Developmental delay Normal Spinal fusion surgery common Risk of scoliosis increases Regression of gained skills Significant muscle rigidity/contractures Risk of
seizures developing Hand stereotypies Increased mobility loss Hand function loss Adolescents to adults ~1-4 yrs Birth ~4-10 yrs Pini G, et al. Orphanet Journal of Rare Diseases (2016) 11:132. 17

Phase 1/2 Trial for NGN-401 Designed to Inform Future Pivotal Clinical
Trial Enrollment • Cohort 1 dose of 1E15 vg (total), Cohort 2 dose of 3E15 vg (total) expected to Cohort 2 initiate 2Q24 • Cohorts to enroll concurrently N=8 • Both doses within GLP toxicology safety margin Currently Cohort 1
enrolling • Key assessments at 3, 6, 9 and 12 months, which include caregiver and clinician assessments – RSBQ, CGI-I and CGI-S N=8 Efficacy Assessments of Interest Key Eligibility Criteria • Female, age ≥4 to ≤10 years
with Autonomic Function Objective device to monitor breathing Classic Rett syndrome • Clinical diagnosis & genetic Hand Function Physician assessment of improvement confirmation of pathogenic MeCP2 mutation • Clinical Global
Impression- Communication Physician assessment of improvement Severity (CGI-S)score of 4-6 Gross Motor Function Physician assessment of improvement 18 GLP = Good Laboratory Practice, CGI-I=Clinician Global Impression of Improvement, RSBQ=Rett
syndrome behavior questionnaire (more details on Slide 35)

NGN-401 Study Inclusion Criteria is Driven by Severity of Rett Syndrome
Domains Under CGI-S Modest Limited impairment Eligible for Phase 1/2 clinical trial impairment Clinical CGI-S=1 CGI-S=2 CGI-S=3 CGI-S=4 CGI-S=5 CGI-S=6 CGI-S=7 domains Language/ Normal May have unusual Phrases-sentences. May <5 words No words
Vocalizations No words Communication features (eg have conversations or Babbles Babbles Occasionally screams No vocalizations echolalia, reading echolalia Makes choices 25%- Makes choices Rarely or makes no Screams disability) 50% ≤25% choices
No choices Ambulation No Normal, may have Walks, able to use Walks independently Walks with Stands with support or Cannot sit impairment slight evidence of stairs/run Unable to use stairs assistance independently Doesn’t stand or dystonia/
ataxia/ May ride tricycle or climb or run May walk with support walk dyspraxia Sits independently or with support Hand use Normal, no Normal, may have Bilateral pincer grasp. May Reaches for objects, Reaches Rarely-occasionally None impairment
slight fine motor issue use pen to write but has raking grasp or No grasps reaches out fine motor issues like tremor unilateral pincer No grasp May use utensils/cup Social (eye Normal Occasional eye gaze Appropriate eye contact, Eye contact <20s
Eye contact Eye contact, None contact) avoidance >30s <10s inconsistent 5s Autonomic None Minimal No or minimal breathing Breathing Breathing Breathing dysrhythmia Breathing abnormalities (<5%) warm, dysrhythmia <50% dysrhythmia 50%
50-100% dysrhythmia pink extremities No cynanosis No cynanosis May have cynanosis constantly with Cool UE, Pink LE Cold UE, Pink LE Cool UE or LE, may be cynanosis blue Cold UE and LE, Mottled/blue Seizures None None or controlled None, with or
without meds Monthly-weekly Weekly Weekly-daily Daily Attentiveness Normal Occasional Attentive to conversation, 50-100% 50% <50% 0% inattention follows commands 19

NGN-401 Phase 1/2 Clinical Trial Status Update and Anticipated
Milestones Phase 1/2 Clinical Trial Status q First patient dosed 3Q:23, second patient dosed 4Q:23, third patient dosed 1Q:24 q No treatment-emergent, procedure-related serious adverse events or overexpression toxicity observed to date
2024 Anticipated Key Milestones q Expand ongoing Phase 1/2 clinical trial in 1H:24 to enroll a larger cohort of patients q Initiate dosing of Cohort 2 in 2Q:24 q Complete dosing of Cohort 1 in 2H:24 q Interim Phase 1/2 clinical
data 4Q:24 q Additional Phase 1/2 clinical data from expanded low dose and high dose cohorts in 2H:25 DSMB = Data and Safety Monitoring Board 20

NGN-101 for CLN5 Batten Disease Treating both CNS and vision through
dual route of administration

CLN5 Batten Disease - Fatal, Neurodegenerative Disease With No
Disease-Specific Treatment Options CLN5 Batten disease has no available treatment options Brineura, approved globally for a similar indication, CLN2, has transformed clinical outcomes in Batten disease Simonati A et al, Phenotype and natural history
of variant late infantile ceroid-lipofuscinosis 5. Dev Med Child Neurol. 2017 Aug;59(8):815-821. 22

NGN-101 Dual Delivery Supported by Compelling Preclinical Data Dual
route of administration NGN-101 dosing (ICV+IVT) in CLN5 knockout sheep First clinical gene therapy study targeting both Combination dosing leads to halting of disease progression neurodegeneration and vision loss 30 20 +/- +/- CLN5 Control CLN5
Control -/- -/- CC LN LN 5 5 UntrU en att er deated -/- -/- CLN5 Treated CLN5 High Dose 10 0 NGN-101 product design 0 5 10 15 20 25 Age (months) AAV9 Full length Promoter NGN-101 dosing Human CLN5 capsid IVT = Intravitreal 23 Clinical score
(/24)

Clinical Study Design For NGN-101 Addresses Vision and CNS Currently
enrolling Fully enrolled High dose Fully enrolled Mid dose • Dose selection based on sheep studies Low dose Cohort 3 showing significant treatment effects Cohort 2 • Key assessments every 6 and 12 months Cohort 1 N=3 N=1 N=2 Efficacy
Endpoints/Markers of Interest Key Eligibility Criteria Optical Coherence Preservation of key retinal layers is a leading • Age ≥3 to ≤9 years Tomography (OCT) indicator of vision stability • Genetic diagnosis of CLN5 •
Onset of disease ≤5 years of age Stability in treated eye vs. worsening in untreated Visual Acuity • Score of ≥1 on the Hamburg eye could provide evidence of clinical benefit motor domain at minimum, the equivalent of 20/200 visual
acuity Scale has been used previously to support BMRN’s or better at the time of screening Hamburg Motor Scale ® ERT Brineura for CLN2 disease ERT = Enzyme replacement therapy 24

NGN-101 — Defining a Registration Path FDA meeting focused on
finalizing CMC plans Plan to request FDA meeting in 2H:24 to align on completed 4Q:23 clinical requirements for streamlined registration Potency Assay Complete enrollment of high dose FDA accepted proposed potency cohort in 2024 assay strategy, a
first milestone in determining continuation of the program Continue collection of clinical trial data on vision and motor for analysis Improved Manufacturing Process FDA alignment on proposed comparability strategy for using Ongoing natural history
data Neurogene-made material with collection and analysis substantially improved profile to Phase 1/2 drug product Alignment with FDA on streamlined registration pathway required to move program forward 25

Key Anticipated Milestone Events

Key Upcoming Anticipated Milestones and Pipeline Developments Rett
syndrome (NGN-401) ❑ Expand ongoing Phase 1/2 clinical trial in 1H:24 to enroll a larger cohort of patients ❑ Interim Phase 1/2 clinical data 4Q:24 ❑ Additional Phase 1/2 clinical data from expanded low dose and high dose
cohorts in 2H:25 CLN5 Batten disease (NGN-101) ❑ Interim Phase 1/2 clinical data in 2H:24 ❑ Engage in FDA discussions regarding a streamlined registrational pathway in 2H:24 Early-stage discovery ❑ Advance one program into the
clinic (2025) Approximately $200 million cash on hand at mid-December closing expected to fund operations into 2H:26 27

Why Neurogene? Unlocking multi-billion dollar neurological disease
markets Proprietary capabilities and technology enable addressing complex diseases Strategy focused on efficiency and maximizing probability of success Leadership team with deep operational, technological and clinical experience Leading life
sciences investor syndicate Strong balance sheet and fiscally disciplined approach 28

Appendix

Rett Syndrome Primarily Results from Loss of MECP2 Function in the
Brain, Making the Brain the Key Target Area for Gene Therapy Mecp2 Knockout • Limiting expression of MeCP2 Peripheral Mecp2 Knock Out Mouse to only the brain/spinal cord results in a near normal mouse Peripheral knockout WT • NHP
biodistribution study shows 10-100x greater NHP AAV9 Biodistribution Across Key Brain areas distribution for ICV/ICM compared to IT-L • Delivery of NGN-401 via ICV chosen to maximize MECP2 expression in the brain Ross et al., 2016 Hum. Mol.
Genetics. PMID: 28173151 30 NHP data from ASGCT 2021 Annual meeting May 11-14
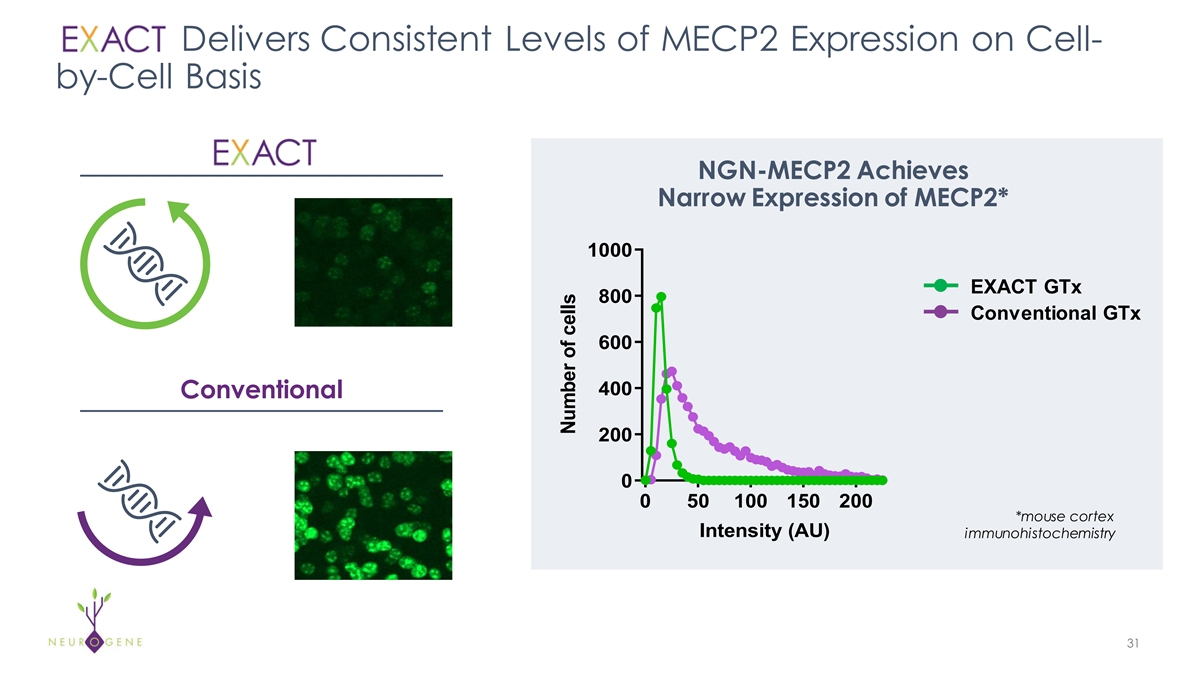
Delivers Consistent Levels of MECP2 Expression on Cell- by-Cell Basis
NGN-MECP2 Achieves Narrow Expression of MECP2* 1000 EXACT GTx 800 Conventional GTx 600 Unregulated Regulated 400 Conventional 200 0 0 50 100 150 200 *mouse cortex Intensity (AU) immunohistochemistry 31 Number of cells

NGN-401 Demonstrates Tight MECP2 Regulation That Translates to
Compelling Outcomes in a Knockout Mouse Model ICV Delivery of NGN-401 Delivers Targeted MECP2 Levels AAV9 capsid EXACT Full length EXACT Promoter recognition sites miRNA human MECP2 Clinical Score* Survival 100 30 25 75 NGN-401 1e11 vg Key domains
improved: 20 NGN-401 3e11 vg 23 37wks 9 50 15• Motor 10• Gait 25 5 • Breathing 0 0 0 5 10 15 20 25 30 0 5 10 15 20 25 30 35 40 45 50 Age (weeks) Age (weeks) *RTT scored 0-5 for six domains: 32 mobility, gait, clasping, breathing,
tremor, body condition Survival (%) RTT score

NGN-401 Via ICV Delivery Well Tolerated in Multiple Studies While
Conventional Unregulated Gene Therapy is Toxic NGN-401 Well Tolerated in NHP studies, NGN-401 Well Tolerated in Female Tight mRNA Levels in NHPs for Mouse Model, Unregulated While Unregulated MeCP2 NGN-401, While Unregulated Has Demonstrates Early
Toxicity MeCP2 Highly Toxic Substantially Greater Variance 100 75 50 150 Regulated* Unregulated 100 25 50 2 0 NGN-401 low dose 0 5 10 15 20 25 2 NGN-401 high dose 5 Age (weeks) 6 3 4 AAV9-RTT251 low dose 5 3 13 1 AAV9-RTT251 high dose 4 2 1 3 0 2 1
0 0 5 10 15 20 25 NCV unaltered Age (weeks) NCV reduced >3m/s WT Vehicle (n=20) 13 Complete loss of NCV response NGN-401 3.7 × 10 vg Het Vehicle (n=13) 14 NGN-401 1.1 × 10 vg NGN-401 1e11 vg (n=11) 13 Unregulated 3.7 × 10 vg
NGN-401 3e11 vg (n=17) 14 Unregulated 1.1 × 10 vg Unregulated 1e11 vg (n=9) Unregulated 3e11 vg (n=10) NOTE: toxicity scoring developed to capture phenotypes associated with MeCP2 overexpression including general condition, tremor, loss of limb
use. *Regulated includes NGN-401 and another EXACT vector; data at 30 days 33 NCV=nerve conduction velocity; NHP = non-human primates Frontal Cortex Motor Cortex Sensory Cortex Hippocampus Striatum Thalamus Midbrain Pons Cerebellum Spinal Cord Liver
Survival (%) Toxicity score Fold change over NGN-401 (copies per ug RNA)

NGN-401 Distribution and Expression Levels in NHPs Support Encouraging
Profile for Human Testing • NGN-401 distributes to key regions underlying RTT pathophysiology in WT non-human primates • Degree of mRNA expression tracks vector genome biodistribution of AAV9 across key brain regions • Aggregate
transgene expression below levels of endogenous MECP2 mRNA (100% of cells), avoiding overexpression concerns Vector Biodistribution with ICV Administration Addresses NGN-401 mRNA Expression Levels Below Key Areas of the Brain Affected in Rett
Syndrome Endogenous Vector biodistribution Motor Sensory motor Seizure 8 Endogenous MECP2 Speech Behavior Autonomic 10 8 10 7 10 7 10 NGN-401 low 6 10 6 10 NGN-401 3.7e13 vg dose NGN-401 high 5 NGN-401 1.1e14 vg 5 10 10 dose 4 4 10 10 3 3 10 10 2 2
10 10 1 1 10 10 34 Motor Cortex Frontal Cortex Sensory Cortex Hippocampus Cerebellum Midbrain Thalamus Pons Cervical Spinal Cord Motor Cortex Frontal Cortex Sensory Cortex Hippocampus Cerebellum Midbrain Thalamus Pons Cervical Spinal Cord vg/μg
DNA copies/μg RNA

GLP Toxicology in NHPs Support Favorable Safety Profile • NGN-401
evaluated in GLP NHP toxicology study with 90-day and 180-day cohorts • No signs or symptoms of MeCP2 overexpression observed • >4x safety margin relative to NGN-401 clinical starting dose in Phase 1/2 • Overall toxicology
profile consistent with typical profile of intra-CSF administered AAV9 product • Slight to minimal non-adverse pathology detected in the dorsal root ganglion (DRG) nerves • Early and transient liver enzyme elevations observed, which
resolved quickly without intervention 35

Explanation of CGI-I and RSBQ 5=Minimally 1=Very much Worse Improved
CGI-I (Clinician Global Impression of Improvement) 6=Much Worse 2=Much Improved 4=No Change 7=Very much 3=Minimally Worsened Improved Domain Total Possible Points (90) RSBQ General mood 16 (Rett Syndrome Behavior Questionnaire) Breathing problems 10
Score Definition Hand behaviors 12 0 not true 1 somewhat or sometimes true Repetitive face movements 8 2 very true Body rocking and expressionless face 12 Nighttime behaviors 6 Fear/anxiety 8 Walking/standing 4 Other 14 36
v3.24.0.1
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Grafico Azioni Neoleukin Therapeutics (NASDAQ:NLTX)
Storico
Da Nov 2024 a Dic 2024

Grafico Azioni Neoleukin Therapeutics (NASDAQ:NLTX)
Storico
Da Dic 2023 a Dic 2024
