AstraZeneca to Discontinue Epanova Trial Following Disappointing Data
13 Gennaio 2020 - 8:37AM
Dow Jones News
By Adriano Marchese
AstraZeneca PLC (AZN.LN) said Monday that it will discontinue
the trial for the drug Epanova following a recommendation from an
independent data-monitoring committee.
The company said it has decided to close the phase three trial
for the drug due to its low likelihood of demonstrating a benefit
to patients with mixed dyslipidaemia who are at risk of
cardiovascular disease.
"We are disappointed by these results, but we remain committed
to addressing the needs of patients in the cardiovascular space
where we have an extensive pipeline," Executive Vice President of
BioPharmaceuticals R&D Mene Pangalos said.
Write to Adriano Marchese at adriano.marchese@wsj.com
(END) Dow Jones Newswires
January 13, 2020 02:22 ET (07:22 GMT)
Copyright (c) 2020 Dow Jones & Company, Inc.
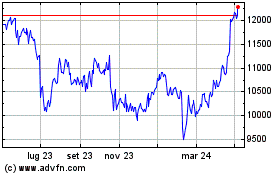
Grafico Azioni Astrazeneca (LSE:AZN)
Storico
Da Mar 2024 a Apr 2024
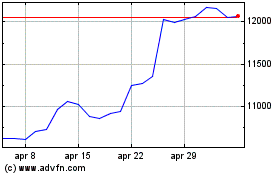
Grafico Azioni Astrazeneca (LSE:AZN)
Storico
Da Apr 2023 a Apr 2024