Pfizer Gets European Approval for Vyndaqel in ATTR-CM
18 Febbraio 2020 - 8:09PM
Dow Jones News
By Colin Kellaher
Pfizer Inc. on Tuesday said the European Commission approved
Vyndaqel for the treatment of wild-type or hereditary transthyretin
amyloidosis in adults with cardiomyopathy, or ATTR-CM.
The New York drug maker said Vyndaqel is the first and only
treatment approved in the European Union for the rare,
life-threatening disease characterized by the buildup of abnormal
deposits of misfolded protein called amyloid in the heart.
Pfizer said prior treatment options for patients with ATTR-CM
were restricted to symptom management and, in rare cases,
transplant.
The European Medicines Agency's Committee for Medicinal Products
for Human Use in December recommended approval Vyndaqel for
ATTR-CM.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
February 18, 2020 13:54 ET (18:54 GMT)
Copyright (c) 2020 Dow Jones & Company, Inc.
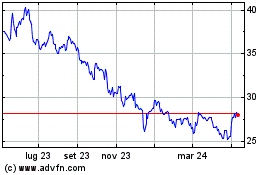
Grafico Azioni Pfizer (NYSE:PFE)
Storico
Da Mar 2024 a Apr 2024
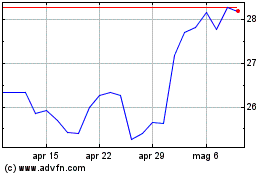
Grafico Azioni Pfizer (NYSE:PFE)
Storico
Da Apr 2023 a Apr 2024