Mithra Announces New Positive Preclinical Data from CSF-1R Inhibitor Program
15 Giugno 2023 - 7:30AM

Mithra Announces New Positive Preclinical Data from CSF-1R
Inhibitor Program
Mithra Announces New
Positive Preclinical Data from CSF-1R
Inhibitor Program
- CSF-1R inhibitors show promising
anti-tumor activity
- Lead compound shows synergy with PD1
cancer immune checkpoint inhibitor
- Preclinical development conducted in
partnership with BCI Pharma
Liege,
Belgium,
15 June
2023
–
7:30
CEST – Mithra
(Euronext Brussels: MITRA), a company dedicated to Women’s Health,
today announces new positive data from preclinical studies on
inhibitors of CSF-1R in development, in collaboration with BCI
Pharma, for treatment of endometriosis, oncology and inflammatory
disorders.
Colony-stimulating factor 1 receptor (CSF-1R) is
a cell-surface tyrosine kinase receptor and a key regulator of
macrophage biology and homeostasis. Tumor-associated macrophages
are key components of the tumor microenvironment and have emerged
as a promising avenue for discovery of novel cancer
immunotherapies. CSF-1R kinase inhibition is therefore a promising
therapeutic strategy for cancer treatment.
Graham Dixon, Chief Scientific Officer
of Mithra, commented: “We are encouraged that the lead
CSF-1R inhibitor demonstrated efficacy as a single agent in 3
different preclinical cancer models and that the data suggest it
may be synergistic when used in combination with PD-1 inhibitors.
This provides a strong rationale supporting further development of
CSF-1R inhibitors in combination with immune checkpoint inhibitors.
We look forward to further evaluating and advancing these promising
small molecule therapeutic candidates.”
A first study, conducted in a widely used
preclinical immune-oncology model, demonstrated that the lead
compound, as well as several backups, showed anti-tumor activity as
a single agent. The well tolerated compounds demonstrated
pronounced and consistent modulation of the tumor microenvironment,
including an increase of T cells, which mediate anti-tumor immune
responses, and infiltration of natural killer (NK) cells,
containing enzymes that can kill tumor cells, into the tumors. Data
showed a decrease in tumor-infiltrating macrophages, a type of
white blood cell that plays an important role in the human immune
system, and repolarization of the tumor-supportive M2 macrophage to
the tumor-suppressive M1 macrophage. These results are consistent
with anti-tumor activity, and tumor growth inhibition of up to
59%.
The efficacy of the lead compound was further
assessed as a single agent or in combination with anti PD1
therapeutic antibody in additional preclinical studies using three
different cancer models: MC38 (colorectal), 4T1 (orthotopic triple
negative breast) and EMT6 (triple negative breast).
The significant anti-tumor activity of the lead
compound used as a single agent was confirmed and was further
enhanced with twice daily administration, compared to once daily.
Additivity or synergy on tumor growth inhibition was observed when
the lead compound was used in combination with anti-PD1 therapy;
the triple negative breast cancer model showed that 50% of the
animals achieved a complete tumor regression after the combination
treatment. Further data will be presented in a peer-reviewed
setting.
The research was conducted under a partnership
with BCI Pharma, first announced in November 2021. Mithra’s primary
focus is on endometriosis and cancers affecting women, including
orphan indications such as triple negative breast cancer (TNBC).
Under the terms of the agreement, Mithra has an option to acquire
from BCI all rights, title and interest in the series of CSF-1R
inhibitors, and part of the BCI rights, title and interest in and
to the results of the research and related intellectual
property.
For more information, please
contact:
|
Mithra Pharmaceuticals
SADavid Horn SolomonChief Executive
Officerinvestorrelations@mithra.com |
Investor &
media relationsChris MaggosCohesion
Bureauchris.maggos@cohesionbureau.com+41 79 367 6254 |
About
Mithra
Mithra Pharmaceuticals SA (Euronext: MITRA) is a
Belgian biopharmaceutical company dedicated to transforming Women’s
Health by offering new choices through innovation, with a
particular focus on contraception and menopause. Mithra’s goal is
to develop products offering better efficacy, safety and
convenience, meeting women’s needs throughout their life span.
Mithra explores the potential of the unique native estrogen
estetrol in a wide range of applications in women health and
beyond. After having successfully launched the first estetrol-based
product in 2021, the contraceptive pill Estelle®, Mithra is now
focusing on its second product Donesta®, the next-generation
hormone therapy. Mithra also offers partners a complete spectrum of
solutions from early drug development, clinical batches and
commercial manufacturing of complex polymeric products (vaginal
ring, implants) and complex liquid injectables and biologicals
(vials, pre-filled syringes or cartridges) at its technological
platform Mithra CDMO. Active in more than 100 countries around the
world, is headquartered in Liège, Belgium. www.mithra.com
ESTELLE®, and DONESTA® are registered trademarks
of Mithra Pharmaceuticals or one of its affiliates.
Important information
The contents of this announcement include
statements that are, or may be deemed to be, "forward-looking
statements". These forward-looking statements can be identified by
the use of forward-looking terminology, including the words
"believes", "estimates," "anticipates", "expects", "intends",
"may", "will", "plans", "continue", "ongoing", "potential",
"predict", "project", "target", "seek" or "should", and include
statements the Company makes concerning the intended results of its
strategy. By their nature, forward-looking statements involve risks
and uncertainties and readers are cautioned that any such
forward-looking statements are not guarantees of future
performance. The Company's actual results may differ materially
from those predicted by the forward-looking statements. The Company
undertakes no obligation to publicly update or revise
forward-looking statements, except as may be required by law.
|
Subscribe to our mailing list on investors.mithra.com to receive
our press releases by email or follow us on our social media
:Linkedin • Twitter • Facebook |
- 2023-06-14_Mithra CSF-1R Preclin Data_Final_FR
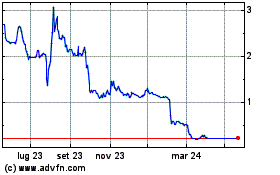
Grafico Azioni Mithra Pharmaceuticals (EU:MITRA)
Storico
Da Apr 2024 a Mag 2024
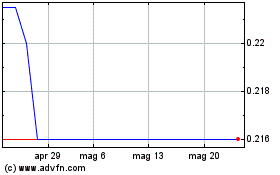
Grafico Azioni Mithra Pharmaceuticals (EU:MITRA)
Storico
Da Mag 2023 a Mag 2024