Philips Respironics alerts customers worldwide of updated instructions and labeling of specific sleep therapy masks that contain magnetic headgear clips due to potential risk of serious injury
06 Settembre 2022 - 10:00PM

Philips Respironics alerts customers worldwide of updated
instructions and labeling of specific sleep therapy masks that
contain magnetic headgear clips due to potential risk of serious
injury
September 6, 2022
These masks may continue to be used according to the updated
instructions and labeling if patients or people in close proximity
to them do not have implanted metallic medical devices or metallic
objects in the body
Pittsburgh, Pennsylvania – Royal Philips’
(NYSE: PHG; AEX: PHIA) subsidiary Philips Respironics is alerting
users of certain CPAP or Bi-Level PAP therapy masks with magnetic
headgear clips or straps that these devices should not be used by
or near patients and their household members, caregivers and bed
partners who have metallic implanted devices or metallic objects
(such as metallic splinters) in the body. The magnetic
headgear clips are used to attach the headgear straps to the masks,
which is a method that is commonly used in the sleep therapy
devices industry.
This is a voluntary notification to users of specific CPAP or
Bi-Level PAP therapy masks containing such magnetic clips to inform
them of the updated instructions and labeling. All users should
read and follow Philips Respironics’ voluntarily updated warning
and added contraindication described below. This represents a new
and industry-leading practice.
Contraindication: Use of the mask is
contraindicated for patients and their household members,
caregivers and bed partners that may be in close vicinity to
patients using the masks, that have implanted devices that may be
affected by magnets, including but not limited to:
- Pacemakers
- Implantable cardioverter defibrillators (ICD)
- Neurostimulators
- Magnetic metallic implants/electrodes/valves placed in upper
limbs, torso, or higher (i.e., neck and head)
- Cerebral spinal fluid (CSF) shunts (e.g., ventriculo peritoneal
(VP) shunt)
- Aneurysm clips
- Embolic coils
- Intracranial aneurysm intravascular flow disruption
devices
- Metallic cranial plates, screws, burr hole covers, and bone
substitute devices
- Metallic splinters in the eye
- Ocular implants (e.g., glaucoma implants, retinal
implants)
- Certain contact lenses with metal
- Implants to restore hearing or balance that have an implanted
magnet (such as cochlear implants, implanted bone conduction
hearing devices, and auditory brainstem implants)
- Magnetic denture attachments
- Metallic gastrointestinal clips
- Metallic stents (e.g., aneurysm, coronary, tracheobronchial,
biliary)
- Implantable ports and pumps (e.g., insulin pumps)
- Hypoglossal nerve stimulators
- Devices labeled as MR (Magnetic Resonance) unsafe
- Magnetic metallic implants not labeled for MR or not evaluated
for safety in a magnetic field
Patients should stop using the affected mask if the
implant/medical device is contraindicated against the mask magnets.
Patients should consult their physician immediately to determine if
another mask can be used for their therapy. In the interim, switch
to a non-magnetic mask if available, for continued therapy.
Patients should properly dispose of the mask that has magnets after
an alternative is obtained.
These masks may continue to be used according to the updated
instructions and labeling if patients or people in close proximity
to them do not have implanted metallic medical devices or metallic
objects in the body.
Warning: Magnets with a magnetic field strength
of 400 mT are used in the mask. With the exception of the devices
identified in the contraindication, ensure that the mask is kept at
least 6 inches (approx. 15 cm) away from any other medical implants
or medical devices that can be impacted by the magnetic fields to
avoid possible effects from localized magnetic fields. This
includes household members, caregivers, and bed partners that may
be in close vicinity to patients that use the masks.
Impacted masks include (see attached image):
- Amara View Full Face Mask
- DreamWisp Nasal Mask
- DreamWear Full Face Mask
- Wisp and Wisp Youth Nasal Mask
- Therapy Mask 3100 NC/SP
More than 17 million masks containing magnetic clips have been
distributed by Philips Respironics to date. As of August 30, 2022,
Philips Respironics has received 14 reports of patients suggesting
that the mask magnets may have impacted their medical devices
including pacemaker interference, pacemaker failure leading to
replacement, need of shunt adjustment, resetting of automatic
implantable cardioverter defibrillator (AICD), seizures,
defibrillator shutting off periodically, arrhythmia, irregular
blood pressure, change in heartbeats, and cognitive issues.
Patients with questions may contact Philips Respironics’
customer service at 1-800-345-6443, (Monday – Friday; 8:30 AM ET to
8:00 PM ET) for more information about non-magnetic mask options.
Patients may also contact their Durable Medical Equipment (DME)
provider, which supplied the masks affected by this notice.
Any adverse events experienced with the use of masks containing
magnetic clips should be reported to the FDA’s MedWatch Program by
phone at 1-800-FDA-1088, by fax at 1-800-FDA-0178, by mail at
MedWatch, HF-2, FDA, 5600 Fishers Lane, Rockville, MD 20852-9787,
or on the MedWatch Web site at www.fda.gov/medwatch.
This voluntary medical device corrective action has been
reported to the regulatory agencies in the countries where these
masks are available.
Media contact:Mario FantePhilips Global Press
OfficeTel.: +1 603 560 9226E-mail: mario.fante@philips.com
About Royal PhilipsRoyal Philips (NYSE: PHG,
AEX: PHIA) is a leading health technology company focused on
improving people's health and enabling better outcomes across the
health continuum from healthy living and prevention, to diagnosis,
treatment and home care. Philips leverages advanced technology and
deep clinical and consumer insights to deliver integrated
solutions. Headquartered in the Netherlands, the company is a
leader in diagnostic imaging, image-guided therapy, patient
monitoring and health informatics, as well as in consumer health
and home care. Philips generated 2021 sales of EUR 17.2 billion and
employs approximately 79,000 employees with sales and services in
more than 100 countries. News about Philips can be found at
www.philips.com/newscenter.
- Overview of impacted masks
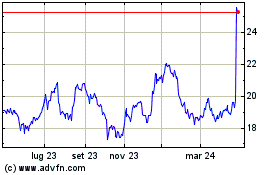
Grafico Azioni Koninklijke Philips NV (EU:PHIA)
Storico
Da Mar 2024 a Apr 2024
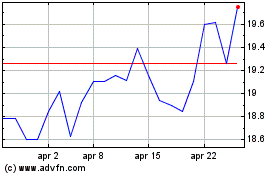
Grafico Azioni Koninklijke Philips NV (EU:PHIA)
Storico
Da Apr 2023 a Apr 2024