false
0001454789
0001454789
2025-01-13
2025-01-13
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of
the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
January 13, 2025
Astria Therapeutics, Inc.
(Exact name of registrant as specified in
its charter)
| Delaware |
|
001-37467 |
|
26-3687168 |
(State
or other jurisdiction
of incorporation) |
|
(Commission
File
Number) |
|
(IRS
Employer
Identification No.) |
22 Boston Wharf Road
10th Floor |
|
|
|
|
| Boston,
Massachusetts |
|
|
|
02110 |
| (Address
of principal executive offices) |
|
|
|
(Zip
Code) |
Registrant’s telephone number, including
area code: (617) 349-1971
(Former Name or Former Address, if Changed
Since Last Report)
Check the appropriate box below if the Form 8-K
filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions :
| ¨ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered
pursuant to Section 12(b) of the Act:
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange on which
registered |
| Common Stock, $0.001 par
value per share |
|
ATXS |
|
The Nasdaq Stock
Market LLC |
Indicate by check mark
whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of
this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging
growth company ¨
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
| Item 7.01 | Regulation FD Disclosure. |
On January 13, 2025, Astria Therapeutics, Inc. (the “Company”)
issued a press release (the “Press Release”) announcing its planned design of the ALPHA-ORBIT Phase 3 clinical trial of navenibart
in people with hereditary angioedema. On January 13, 2025, the Company also published on its website an updated corporate presentation
(the “Corporate Presentation”). Copies of the Press Release and Corporate Presentation are furnished herewith as Exhibit 99.1
and Exhibit 99.2, respectively, to this Current Report on Form 8-K.
The information furnished under this Item 7.01, including Exhibit 99.1
and Exhibit 99.2, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934,
as amended, or subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended.
The information in this Item 7.01, including Exhibit 99.1 and Exhibit 99.2, shall not be deemed incorporated by reference into
any other filing with the Securities and Exchange Commission made by the Company, whether made before or after the date hereof, regardless
of any general incorporation language in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934,
the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | Astria
Therapeutics, INC. |
| | |
| Date: January 13, 2025 |
By: |
/s/
Ben Harshbarger |
| | |
Ben
Harshbarger |
| | |
Chief Legal Officer |
Exhibit 99.1

Astria Therapeutics Announces Design of ALPHA-ORBIT
Pivotal Phase 3 Trial of Navenibart in HAE
--
Single Pivotal Trial Designed to Demonstrate Efficacy and Safety of Every 3- and Every 6-Month Administration in a 6-Month Treatment Period
--
-- Pioneering Patient-Centric Dosing Flexibility
in HAE with Potential Market-Leading First-Choice Profile --
-- Phase 3 Initiation On-Track, Expected in
Q1 2025 --
-- Strong Financial Position, Funded Through
Expected Top-Line Phase 3 Results --
BOSTON, Mass., January 13, 2025 – Astria Therapeutics, Inc.
(NASDAQ:ATXS), a biopharmaceutical company focused on developing life-changing therapies for allergic and immunologic diseases, today
announced its planned design of the ALPHA-ORBIT Phase 3 clinical trial of navenibart in people with hereditary angioedema (HAE), which
will include both every 3- (Q3M) and every 6-month (Q6M) treatment arms with the primary analysis at 6 months. Global start-up activities
are underway, and ALPHA-ORBIT is expected to initiate in Q1 2025, with top-line results anticipated in early 2027.
“We are thrilled to announce our planned Phase 3 design, which
reflects feedback from regulators and is intended to support global registration for both Q3M and Q6M administration,” said Jill
C. Milne, Ph.D., Chief Executive Officer at Astria. “With navenibart, we are pioneering patient-centric dosing flexibility in HAE
with the goal of maximizing attack rate reduction with a compellingly low burden of treatment. Assuming approval, we believe navenibart
will become the market-leading, first-choice therapy for HAE.”
“Our Phase 3 program was designed in collaboration with the patient
community and physicians, is based on input from global regulatory authorities, and addresses the importance of providing options to patients
for a disease that’s highly variable,” said Christopher Morabito, M.D., Chief Medical Officer at Astria. “Phase 3 preparations
are underway, with trial initiation on-track and expected for this quarter. We are driven by the goal of bringing a potentially life-changing
therapy to patients with HAE.”
ALPHA-ORBIT is designed as a global, randomized, double-blind, placebo-controlled
Phase 3 pivotal clinical trial to evaluate the efficacy and safety of navenibart over a 6-month treatment period in up to 145 patients
with Type 1 or Type 2 HAE. Patients will be randomized to receive one of three navenibart dose arms: 1) an initial 600 mg dose and followed
by 300 mg Q3M, 2) 600 mg Q6M, and 3) 600 mg Q3M, or placebo. The dose arms support the potential to provide patient-centric dosing flexibility
to people with HAE. The primary endpoint is time-normalized monthly HAE attacks at 6 months, and a key secondary endpoint includes the
proportion of participants who are attack-free at 6 months. After 6 months, patients may be eligible to enter a long-term extension trial,
in which all patients will be treated with navenibart (open-label) and which will include an open-label, patient-centric flexible dosing
period. The navenibart Phase 3 program will consist of the ALPHA-ORBIT Phase 3 trial and the long-term extension trial, which are designed
to support registration globally. The Phase 3 program was designed with input from the European Medicines Agency and the Company’s
end of Phase 2 meeting with the U.S. Food and Drug Administration (FDA) held in December 2024.
Planned doses for the Phase 3 ALPHA-ORBIT program were selected based
on positive final top-line results from target enrollment in the Phase 1b/2 ALPHA-STAR trial of navenibart, announced in December 2024,
which showed rapid onset of robust and durable efficacy, favorable safety and tolerability, and pharmacokinetics and pharmacodynamics
consistent with sustained plasma kallikrein inhibition for both Q3M and Q6M administration. Final results included reduction in mean monthly
attack rate of 90-95% and up to a 67% attack-free rate over 6 months. The Company will present these data at an upcoming scientific conference.
Additional details regarding the Company’s planned Phase 3 program
and other business updates are contained in the Company’s Corporate Presentation, which is available on the “Events and Presentations”
page of the “For Investors” section of the Company’s website.
About Astria Therapeutics:
Astria Therapeutics is a biopharmaceutical
company, and our mission is to bring life-changing therapies to patients and families affected by allergic and immunologic diseases. Our
lead program, navenibart (STAR-0215), is a monoclonal antibody inhibitor of plasma kallikrein in clinical development for the treatment
of hereditary angioedema. Our second program, STAR-0310, is a monoclonal antibody OX40 antagonist in preclinical development for the treatment
of atopic dermatitis. Learn more about our company on our website, www.astriatx.com, or follow us on Instagram @AstriaTx and on Facebook
and LinkedIn.
About Navenibart:
Navenibart is a monoclonal antibody inhibitor of plasma kallikrein
in development for the treatment of HAE. Our goal with navenibart is to provide rapid and sustained HAE attack prevention with a validated
mechanism and trusted modality administered every 3 and 6 months. We aim to empower people with HAE to live life without limitations from
their disease.
Forward Looking Statements:
This press release contains forward-looking statements within the meaning
of applicable securities laws and regulations including, but not limited to, statements regarding: the expected design, timing of initiation
and receipt of topline results from the ALPHA-ORBIT trial; the goals and objectives of the ALPHA-ORBIT trial and the long-term extension
trial, including that they would support registration of Q3M and Q6M administration, and potentially accelerate the availability of Q6M
administration; our expectations for the dosing regimens of navenibart and the efficacy data of navenibart in the ALPHA-ORBIT trial; the
potential therapeutic benefits of navenibart as a treatment for HAE; the potential attributes and profile of navenibart as a treatment
for HAE, including our expectation that it will be the market-leading, first choice and a potentially life-changing treatment for patients
with HAE; our overall vision and goals for the navenibart program; expectations about being funded through top-line Phase 3 results; and
our corporate strategy and vision, including our mission to bring life-changing therapies to patients and families affected by allergic
and immunologic diseases. The use of words such as, but not limited to, “anticipate,” “believe,” “continue,”
“could,” “estimate,” “expect,” “goals,” “intend,” “may,” “might,”
“plan,” “potential,” “predict,” “project,” “should,” “target,”
“will,” “would,” or "vision," and similar words and expressions are intended to identify forward-looking
statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on Astria’s
current beliefs, expectations and assumptions regarding the future of its business, future plans and strategies, future financial performance,
results of pre-clinical studies and clinical trials of Astria’s product candidates and other future conditions. Actual results may
differ materially from those indicated by such forward-looking statements as a result of various important factors, including risks and
uncertainties related to: changes in applicable laws or regulations; the possibility that we may be adversely affected by other economic,
business, and/or competitive factors; risks inherent in pharmaceutical research and development, such as: adverse results in our drug
discovery, preclinical and clinical development activities, the risk that the results of preclinical studies may not be replicated in
clinical trials, that the preliminary, initial or interim results from clinical trials may not be indicative of the final results, that
the results of early stage clinical trials, such as the results from the ALPHA-STAR Phase 1b/2 clinical trial, may not be replicated in
later stage clinical trials, such as the ALPHA-ORBIT trial and the open-label extension trial, the risk that we may not be able to enroll
sufficient patients in our clinical trials on a timely basis, and the risk that any of our clinical trials, including ALPHA-ORBIT, may
not commence, continue or be completed on our anticipated timelines, or at all; decisions made by, and feedback received from, the FDA
and other regulatory authorities on our clinical trial design, including for ALPHA-ORBIT, and on our regulatory and clinical trial submissions,
including receipt of FDA minutes from our December 2024 end of Phase 2 meeting, and other feedback from potential clinical trial
sites, including investigational review boards at such sites, and other review bodies with respect to navenibart, STAR-0310, and any other
future development candidates, decisions that we make about the design of clinical trials in response to regulatory feedback, including
the design of the ALPHA-ORBIT trial and the long-term extension trial; our ability to manufacture sufficient quantities of drug substance
and drug product for navenibart, STAR-0310, and any other future product candidates on a cost-effective and timely basis, and to develop
dosages and formulations for navenibart, STAR-0310, and any other future product candidates that are patient-friendly and competitive;
our ability to develop sufficient data to enable the use of planned devices with navenibart, STAR-0310 and any other future product candidates
at commercial launch or otherwise as planned; our ability to develop biomarker and other assays, along with the testing protocols therefor;
our ability to obtain, maintain and enforce intellectual property rights for navenibart, STAR-0310 and any other future product candidates;
our potential dependence on collaboration partners; competition with respect to navenibart, STAR-0310, or any of our other future product
candidates; the risk that survey results, modeling data and market research may not be accurate predictors of the commercial landscape
for HAE, the ability of navenibart to compete in HAE and the anticipated position and attributes of navenibart in HAE based on clinical
data to date, its preclinical profile, pharmacokinetic modeling, market research and other data; risks that any of our clinical trials
of STAR-0310 may not commence, continue or be completed on time, or at all; risks that results of preclinical studies of STAR-0310 will
not be replicated in clinical trials; our ability to manage our cash usage and the possibility of unexpected cash expenditures; our ability
to obtain necessary financing to conduct our planned activities and to manage unplanned cash requirements; the risks and uncertainties
related to our ability to recognize the benefits of any additional acquisitions, licenses or similar transactions; and general economic
and market conditions; as well as the risks and uncertainties discussed in the “Risk Factors” section of our Annual Report
on Form 10-K for the period ended December 31, 2023 and in other filings that we may make with the Securities and Exchange Commission.
New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Astria may not
actually achieve the forecasts or expectations disclosed in our forward-looking statements, and investors and potential investors should
not place undue reliance on Astria’s forward-looking statements.
Neither Astria, nor its affiliates, advisors or representatives, undertake
any obligation to publicly update or revise any forward-looking statement, whether as result of new information, future events or otherwise,
except as required by law. These forward-looking statements should not be relied upon as representing Astria’s views as of any date
subsequent to the date hereof.
###
Astria Contact:
Investor Relations and Media:
Elizabeth Higgins
investors@astriatx.com
Exhibit 99.2

Corporate Presentation January 2025 1

FLS This presentation contains forward - looking statements within the meaning of applicable securities laws and regulations including , but not limited to, statements regarding: the expected design, timing of initiation and receipt of topline results from the ALPHA - ORBIT trial; the goals and objectives of the ALPHA - ORBIT trial and the long - term extension trial, including that they would support registration of Q3M and Q6M administration, and potentially accelerate the availability of Q6M administration; our expectations for the dosing regimens of navenibart and the efficacy data of navenibart in the ALPHA - ORBIT trial; the potential therapeutic benefits of navenibart as a treatment for HAE; the potential attributes and profile of navenibart as a treatment for HAE, including our expectation that it will be the market - leading, first choice and a potentially life - chang ing treatment for patients with HAE; our overall vision and goals for the navenibart program; expectations about being funded through top - line Phase 3 results and our cash runway ; and our corporate strategy and vision, including our mission to bring life - changing therapies to patients and families affecte d by allergic and immunologic diseases. The use of words such as, but not limited to, “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “goals,” “intend,” “ma y,” “might,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would,” or "vision," and similar words and expressions are intended to identify forward - looking statements. Forward - looking statements are neither historical facts nor ass urances of future performance. Instead, they are based on Astria’s current beliefs, expectations and assumptions regarding the future of its business, future plans and strategies, future financial performance, results of pre - clinical studies and clinical trials of Astria’s product candidates and other future conditions. Actual results may differ materially from tho se indicated by such forward - looking statements as a result of various important factors, including risks and uncertainties related to: changes in applicable laws or regulations; the possibility that we may be adversely affected by other economic, business, and/or competitive factors; risks inherent in pharmaceutical research and development, such as: adverse results in our drug discovery, preclinical and clinical development activities, the risk that the results of preclinical studies may not be replicated in clinical trials, that the preliminary, initial or interim results from clinical trials may not be indicative of the final results, that the results of early stage clinical trials, such as the results from the ALPHA - STAR Phase 1b/2 clinical trial, may not be replicated in later stage clinical trials, such as the ALPHA - ORBIT trial and the open - label extension trial, the risk that we may not be able to enroll sufficient patients in our clinical trials on a timely basis, and the risk that any of our clinical trials, including ALPHA - ORBIT, may not commence, continue or be completed on our anticipated timelines, or at all; deci sions made by, and feedback received from, the FDA and other regulatory authorities on our clinical trial design, including for ALPHA - ORBIT, and on our regulatory and clinical trial submissions, including receipt of FDA minutes from our Decem ber 2024 end of Phase 2 meeting, and other feedback from potential clinical trial sites, including investigational review boards at such sites, and other review bodies with respect to navenibart , STAR - 0310, and any other future development candidates, decisions that we make about the design of clinical trials in response to regulatory feedback, including the design of the ALPHA - ORBIT trial and the long - term extension trial; our ability to manufacture sufficient quantities of drug substance and drug product for navenibart , STAR - 0310, and any other future product candidates on a cost - effective and timely basis, and to develop dosages and formulations for navenibart , STAR - 0310, and any other future product candidates that are patient - friendly and competitive; our ability to develop sufficien t data to enable the use of planned devices with navenibart , STAR - 0310 and any other future product candidates at commercial launch or otherwise as planned; our ability to develop biomark er and other assays, along with the testing protocols therefor; our ability to obtain, maintain and enforce intellectual property rights for navenibart , STAR - 0310 and any other future product candidates; our potential dependence on collaboration partners; competition with respec t to navenibart , STAR - 0310, or any of our other future product candidates; the risk that survey results, modeling data and market research may not be accurate predictors of the commercial lan dscape for HAE, the ability of navenibart to compete in HAE and the anticipated position and attributes of navenibart in HAE based on clinical data to date, its preclinical profile, pharmacokinetic modeling, market research and other data; ris ks that any of our clinical trials of STAR - 0310 may not commence, continue or be completed on time, or at all; risks that results of preclinical studies of STAR - 0310 will not be replicated in clinical trials; our ability to manage our cash usage and the possibility of unexpected cash expenditures; our ability to obtain necessary financing to conduct our planned activities and to manage unplanned cash requirements; the risks and uncertainties related to our ability to recognize th e benefits of any additional acquisitions, licenses or similar transactions; and general economic and market conditions; as well as the risks and uncertainties discussed in the “Risk Factors” section of our Annual Report on Form 10 - K for the period ended December 31, 2023 and in other filings that we may make with the Securities and Exchange Commission. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncerta int ies. Astria may not actually achieve the forecasts or expectations disclosed in our forward - looking statements, and investors and potential investors should not place undue reliance on Astria’s forward - looking statements. Neither Astria, nor its affiliates, advisors or representatives, undertake any obligation to publicly update or revise any fo rwa rd - looking statement, whether as result of new information, future events or otherwise, except as required by law. These forward - looking statements should not be relied upon as representing Astria’s views as of any date subsequent to the date hereof. 2
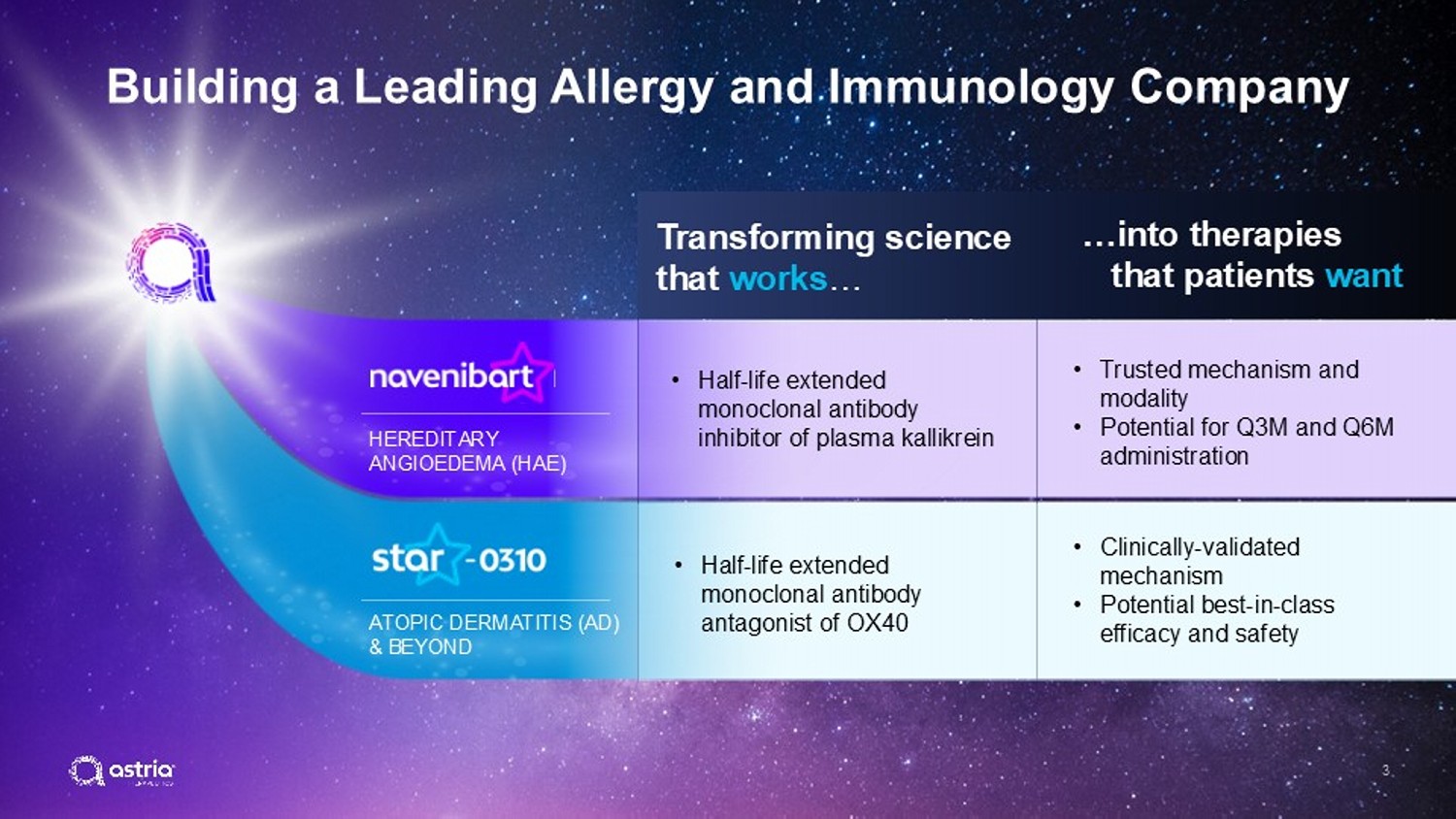
3 ATOPIC DERMATITIS (AD) & BEYOND HEREDITARY ANGIOEDEMA (HAE) Building a Leading Allergy and Immunology Company • Half - life extended monoclonal antibody antagonist of OX40 • Clinically - validated mechanism • Potential best - in - class efficacy and safety • Half - life extended monoclonal antibody inhibitor of plasma kallikrein • Trusted mechanism and modality • Potential for Q3M and Q6M administration Transforming science that works … …into therapies that patients want
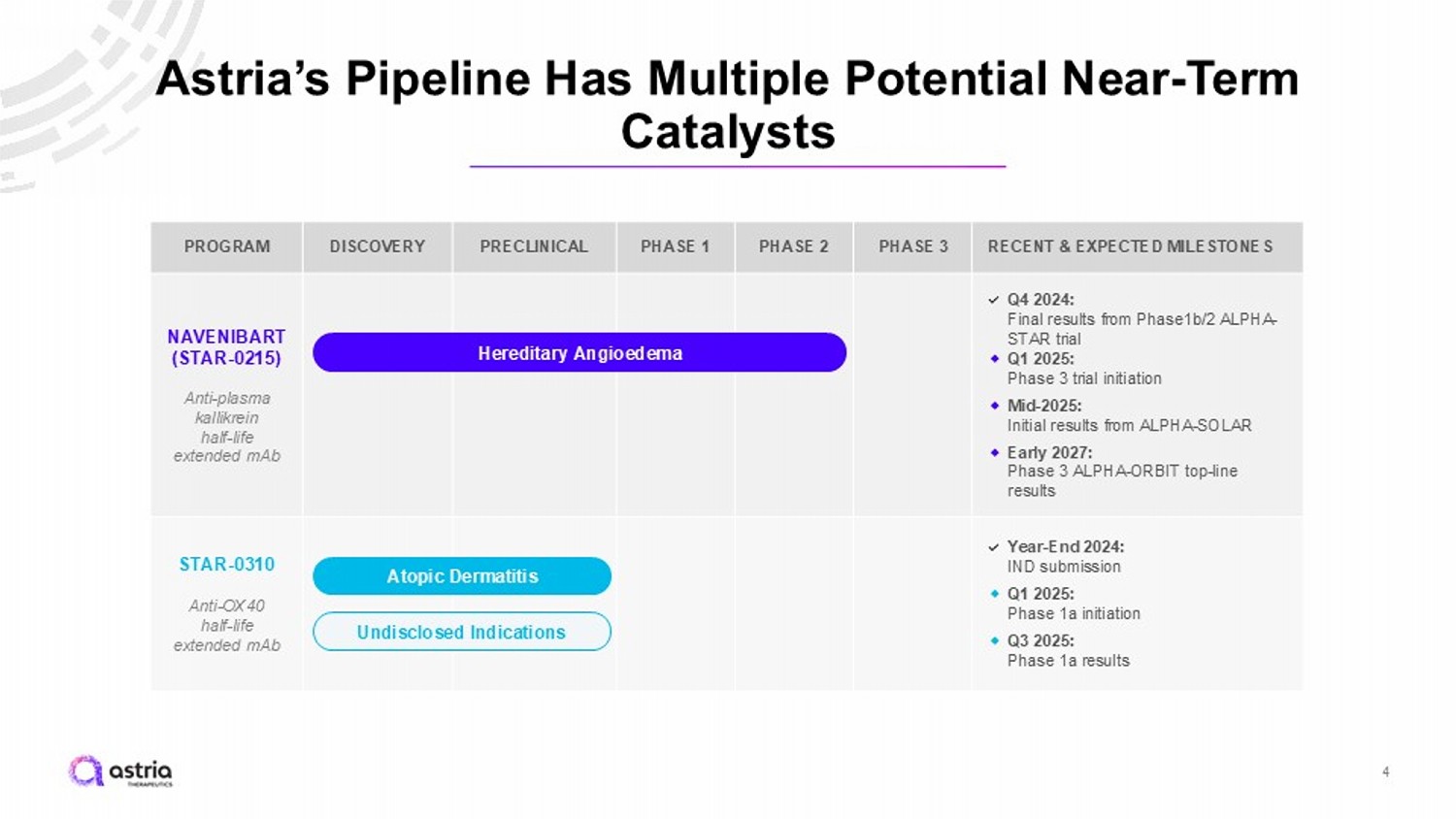
RECENT & EXPECTED MILESTONES PHASE 3 PHASE 2 PHASE 1 PRECLINICAL DISCOVERY PROGRAM Q4 2024: Final results from Phase1b/2 ALPHA - STAR trial ⬥ Q1 2025: Phase 3 trial initiation ⬥ Mid - 2025: Initial results from ALPHA - SOLAR ⬥ Early 2027: Phase 3 ALPHA - ORBIT top - line results NAVENIBART (STAR - 0215) Anti - plasma kallikrein half - life extended mAb Year - End 2024: IND submission ⬥ Q1 2025: Phase 1a initiation ⬥ Q3 2025: Phase 1a results STAR - 0310 Anti - OX40 half - life extended mAb Hereditary Angioedema Atopic Dermatitis Undisclosed Indications Astria’s Pipeline Has Multiple Potential Near - Term Catalysts 4

Developing the Potential Market - Leading HAE Treatment: Navenibart Phase 3 Program • Single 6 - month pivotal Phase 3 trial and long - term extension designed with both Q3M and Q6M administration • Pioneering expected patient - centric dosing flexibility in HAE with potential market - leading first - choice profile • Expected to initiate this quarter, with top - line results expected in early 2027 5 1. As of 12/31/2024
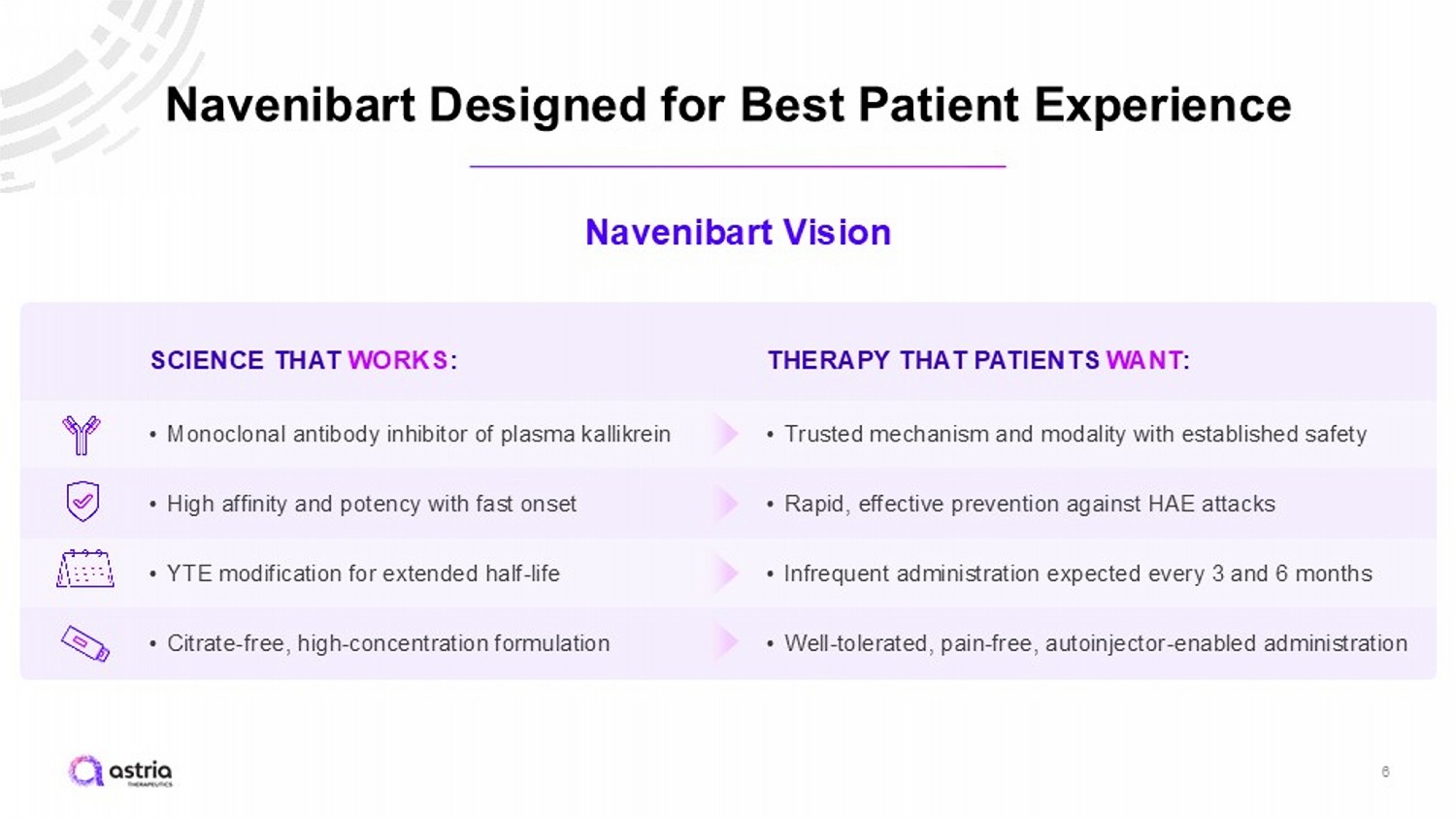
Navenibart Designed for Best Patient Experience 6 Navenibart Vision SCIENCE THAT WORKS : • Monoclonal antibody inhibitor of plasma kallikrein • High affinity and potency with fast onset • YTE modification for extended half - life • Citrate - free, high - concentration formulation THERAPY THAT PATIENTS WANT : • Trusted mechanism and modality with established safety • Rapid, effective prevention against HAE attacks • Infrequent administration expected every 3 and 6 months • Well - tolerated, pain - free, autoinjector - enabled administration
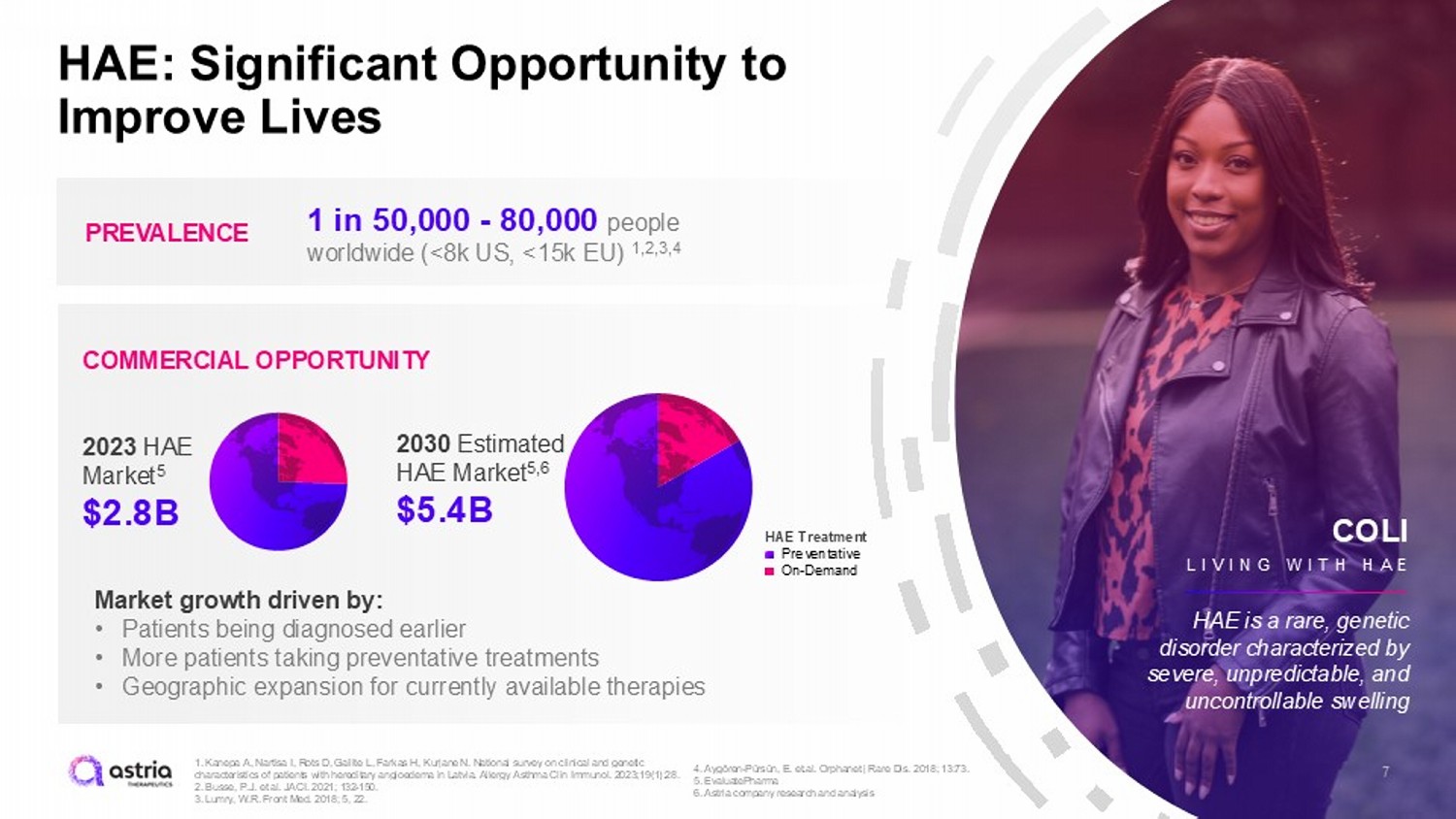
7 HAE: Significant Opportunity to Improve Lives PREVALENCE COMMERCIAL OPPORTUNITY 1 in 50,000 - 80,000 people worldwide (<8k US, <15k EU) 1,2,3,4 Market growth driven by: • Patients being diagnosed earlier • More patients taking preventative treatments • Geographic expansion for currently available therapies COLI LIVING WITH HA E HAE is a rare, genetic disorder characterized by severe, unpredictable, and uncontrollable swelling 2023 HAE Market 5 $2.8B 2030 Estimated HAE Market 5,6 $5.4B HAE Treatment Preventative On - Demand 1. Kanepa A, Nartisa I, Rots D, Gailite L, Farkas H, Kurjane N. National survey on clinical and genetic characteristics of patients with hereditary angioedema in Latvia. Allergy Asthma Clin Immunol. 2023;19(1):28. 2. Busse, P.J. et al. JACI. 2021; 132 - 150. 3. Lumry , W.R. Front Med. 2018; 5, 22. 4. Aygören - Pürsün, E. et.al. Orphanet j Rare Dis. 2018; 13:73. 5. EvaluatePharma 6. Astria company research and analysis

Phase 3 Trial Strategy • Our goal is to revolutionize the way that patients manage their HAE • Phase 3 designed to evaluate both Q3M and Q6M regimens with the goal of providing options for patients that, if approved, would ultimately create flexibility in how patients manage their disease • Planned Phase 3 dose selection determined from cumulative program data • Phase 3 program was designed with input from the EMA and end of Phase 2 meeting with the FDA held in December 2024 8
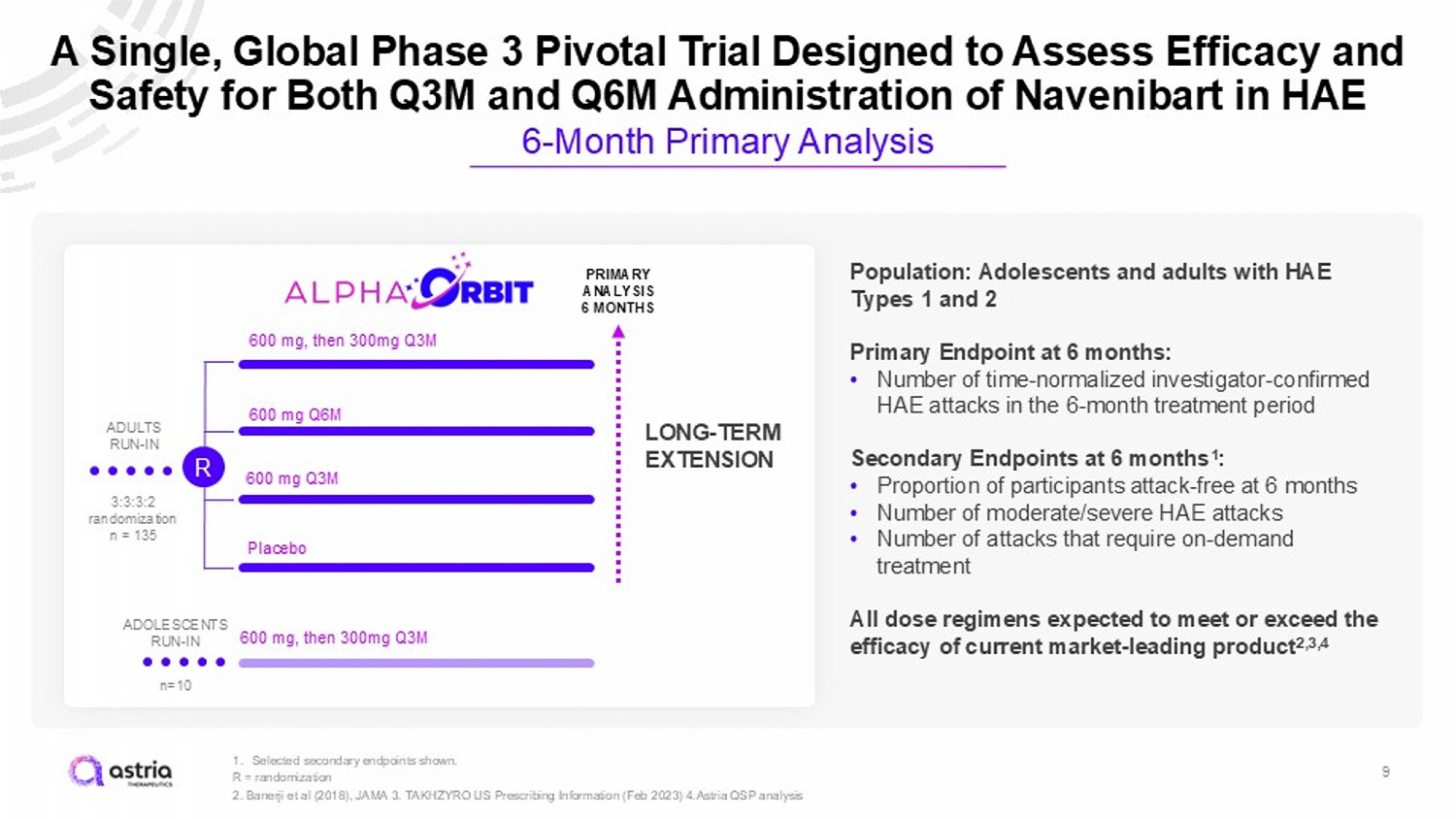
9 1. Selected secondary endpoints shown. R = randomization 2. Banerji et al (2018), JAMA 3. TAKHZYRO US Prescribing Information (Feb 2023) 4.Astria QSP analysis A Single, Global Phase 3 Pivotal Trial Designed to Assess Efficacy and Safety for Both Q3M and Q6M Administration of Navenibart in HAE 6 - Month Primary Analysis 3:3:3:2 randomization n = 135 600 mg Q3M 600 mg, then 300mg Q3M 600 mg Q6M Placebo PRIMARY ANALYSIS 6 MONTHS ADULTS RUN - IN LONG - TERM EXTENSION R Population: Adolescents and adults with HAE Types 1 and 2 Primary Endpoint at 6 months: • Number of time - normalized investigator - confirmed HAE attacks in the 6 - month treatment period Secondary Endpoints at 6 months 1 : • Proportion of participants attack - free at 6 months • Number of moderate/severe HAE attacks • Number of attacks that require on - demand treatment All dose regimens expected to meet or exceed the efficacy of current market - leading product 2,3,4 600 mg, then 300mg Q3M n=10 ADOLESCENTS RUN - IN
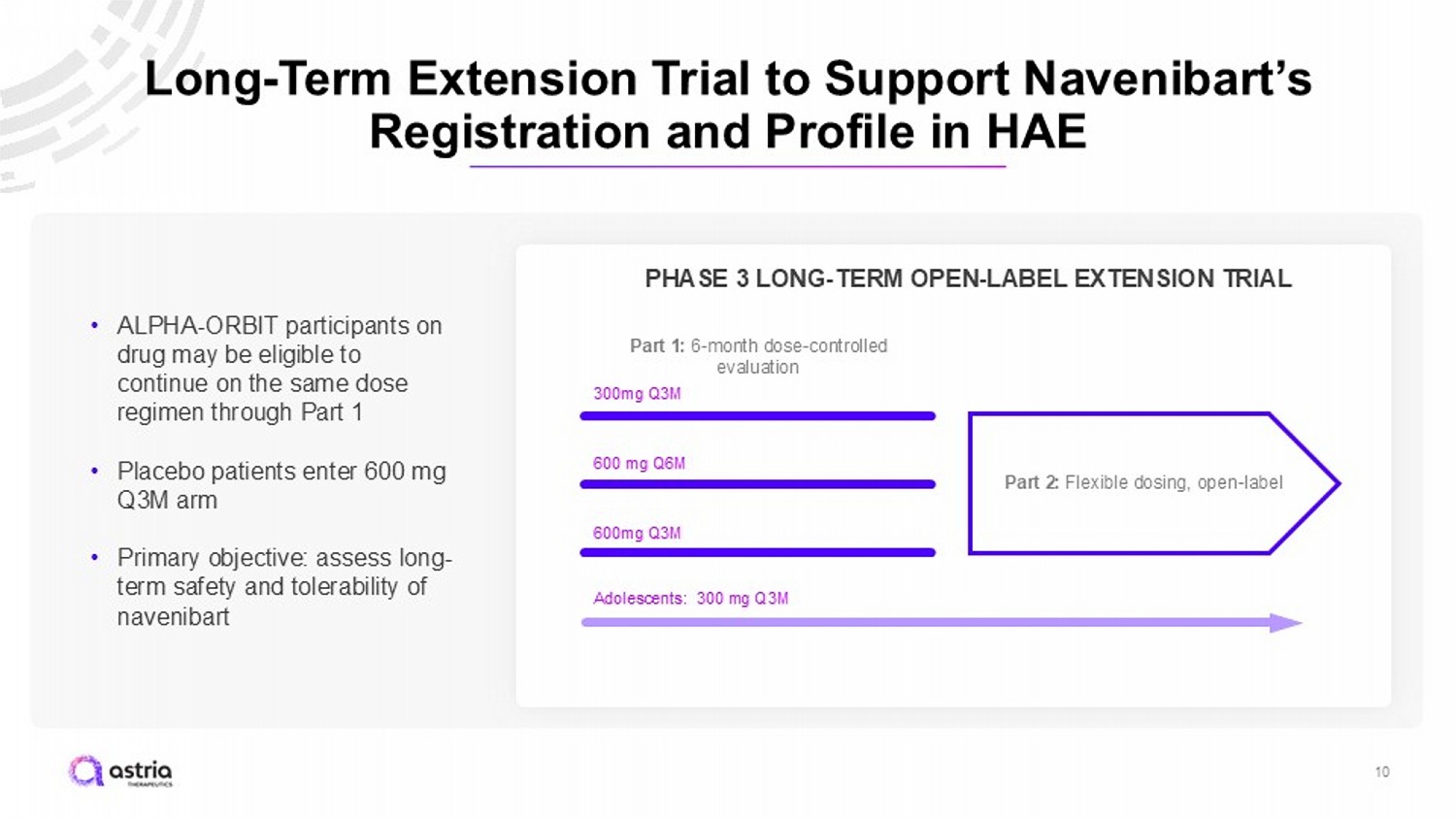
Long - Term Extension Trial to Support Navenibart’s Registration and Profile in HAE 10 600mg Q3M 300mg Q3M 600 mg Q6M PHASE 3 LONG - TERM OPEN - LABEL EXTENSION TRIAL Part 1: 6 - month dose - controlled evaluation Part 2: Flexible dosing, open - label • ALPHA - ORBIT participants on drug may be eligible to continue on the same dose regimen through Part 1 • Placebo patients enter 600 mg Q3M arm • Primary objective: assess long - term safety and tolerability of navenibart Adolescents: 300 mg Q3M
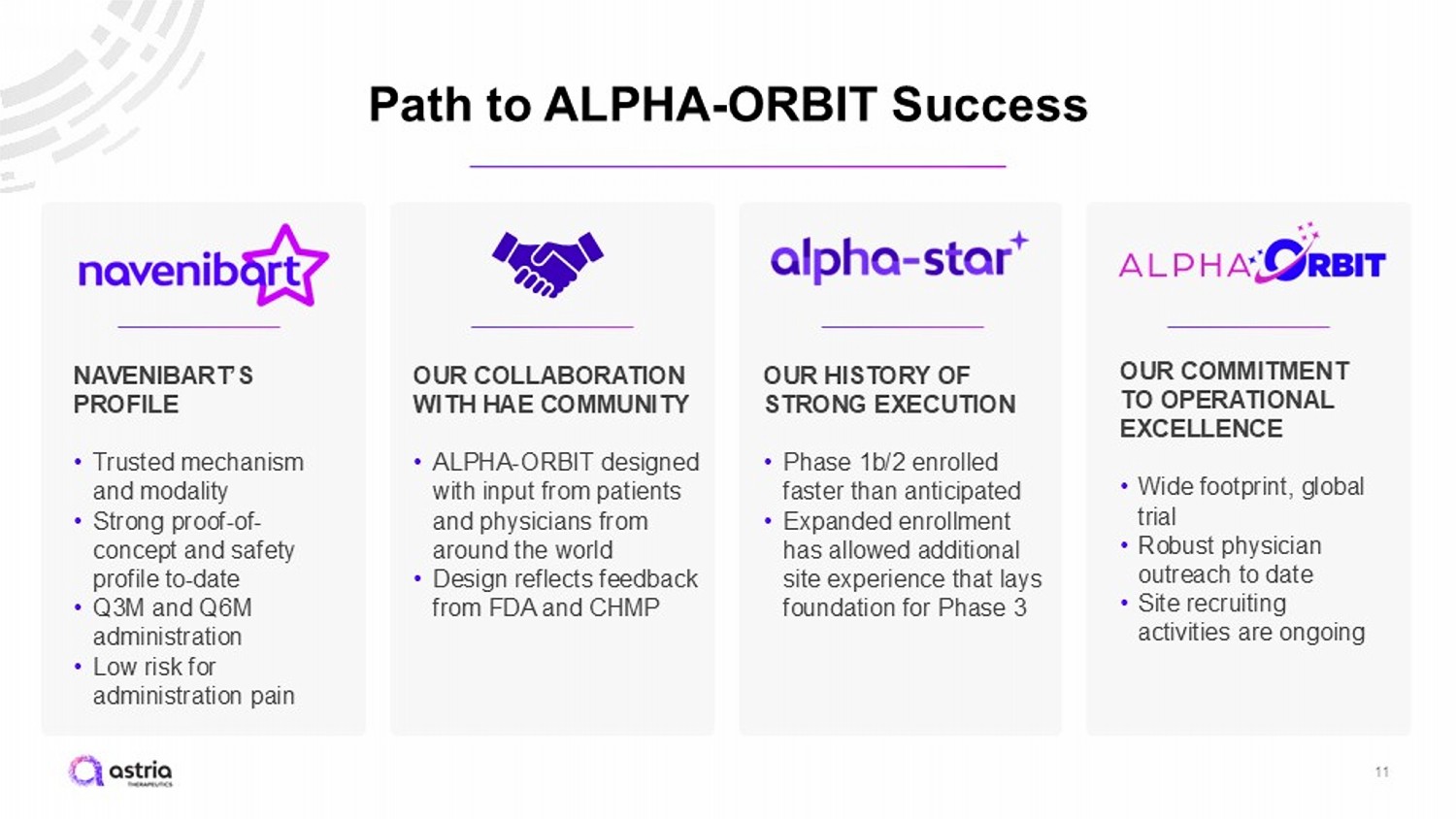
Path to ALPHA - ORBIT Success 11 OUR COMMITMENT TO OPERATIONAL EXCELLENCE • Wide footprint, global trial • Robust physician outreach to date • Site recruiting activities are ongoing OUR HISTORY OF STRONG EXECUTION • Phase 1b/2 enrolled faster than anticipated • Expanded enrollment has allowed additional site experience that lays foundation for Phase 3 OUR COLLABORATION WITH HAE COMMUNITY • ALPHA - ORBIT designed with input from patients and physicians from around the world • Design reflects feedback from FDA and CHMP NAVENIBART’S PROFILE • Trusted mechanism and modality • Strong proof - of - concept and safety profile to - date • Q3M and Q6M administration • Low risk for administration pain
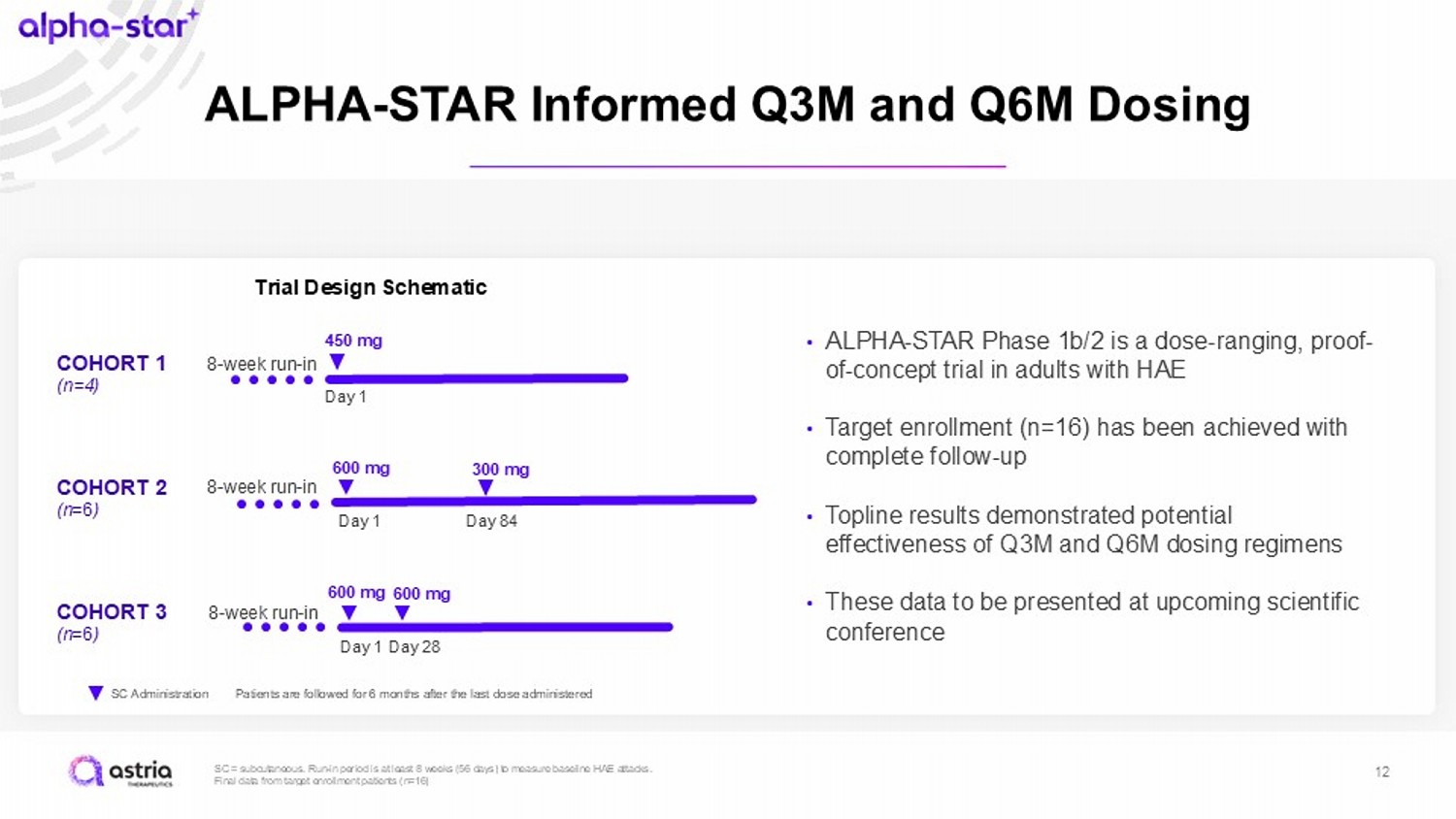
SC = subcutaneous. Run - in period is at least 8 weeks (56 days) to measure baseline HAE attacks. Final data from target enrollment patients (n=16) 12 ALPHA - STAR Informed Q3M and Q6M Dosing SC Administration Trial Design Schematic 8 - week run - in 600 mg 600 mg COHORT 3 (n =6 ) Day 1 Day 28 8 - week run - in 600 mg 300 mg COHORT 2 (n =6 ) Day 1 Day 84 8 - week run - in 450 mg COHORT 1 (n=4) Day 1 Patients are followed for 6 months after the last dose administered • ALPHA - STAR Phase 1b/2 is a dose - ranging, proof - of - concept trial in adults with HAE • Target enrollment (n=16) has been achieved with complete follow - up • Topline results demonstrated potential effectiveness of Q3M and Q6M dosing regimens • These data to be presented at upcoming scientific conference
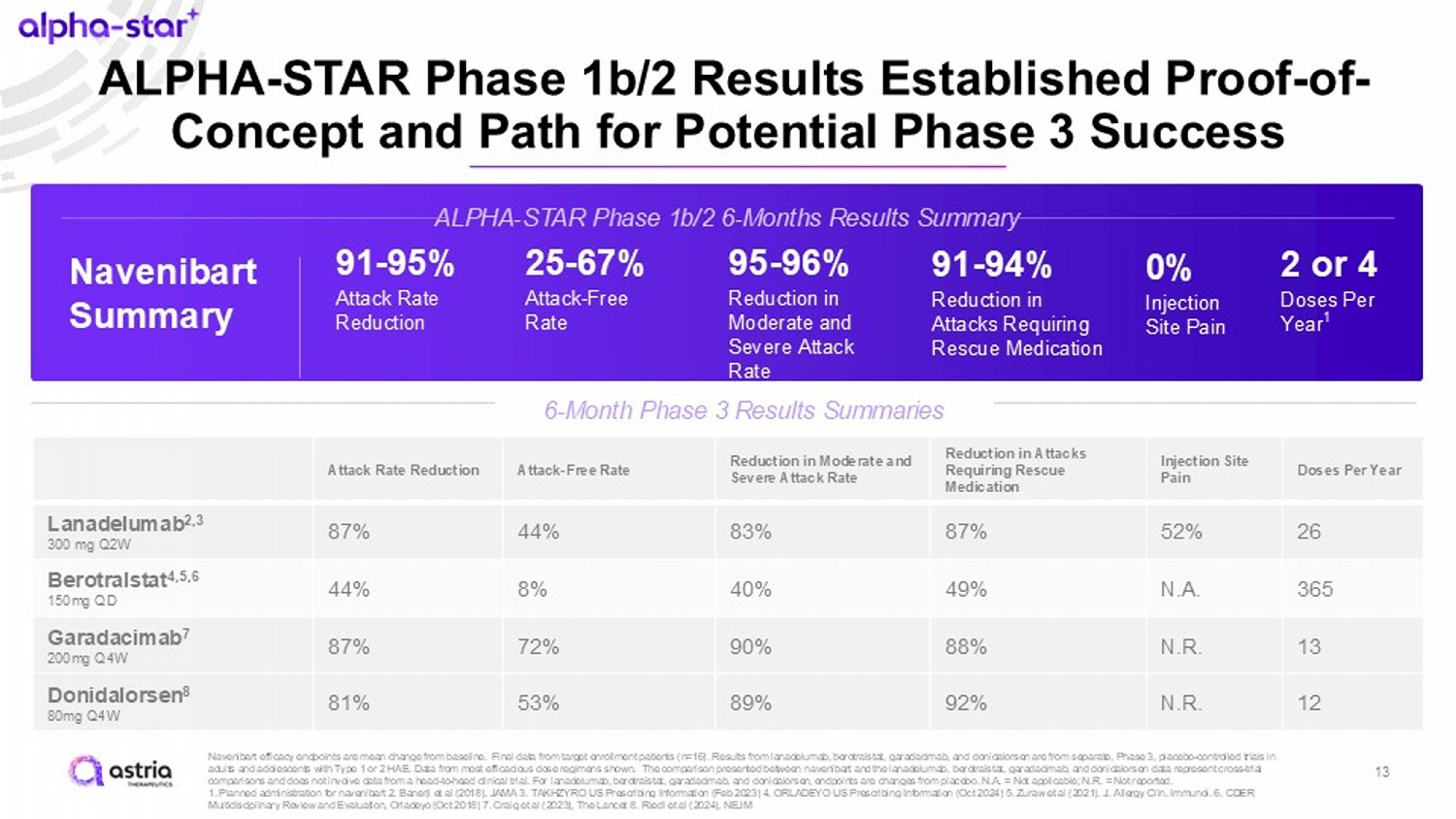
ALPHA - STAR Phase 1b/2 Results Established Proof - of - Concept and Path for Potential Phase 3 Success Navenibart efficacy endpoints are mean change from baseline. Final data from target enrollment patients (n=16). Results from lanadelumab , berotralstat , garadacimab , and donidalorsen are from separate, Phase 3, placebo - controlled trials in adults and adolescents with Type 1 or 2 HAE. Data from most efficacious dose regimens shown. The comparison presented betwee n n avenibart and the lanadelumab , berotralstat , garadacimab , and donidalorsen data represent cross - trial comparisons and does not involve data from a head - to - head clinical trial. For lanadelumab , berotralstat , garadacimab , and donidalorsen , endpoints are changes from placebo. N.A. = Not applicable; N.R. = Not reported. 1. Planned administration for navenibart 2. Banerji et al (2018), JAMA 3. TAKHZYRO US Prescribing Information (Feb 2023) 4. O RLA DEYO US Prescribing Information (Oct 2024) 5. Zuraw et al (2021). J. Allergy Clin. Immunol. 6. CDER Multidisciplinary Review and Evaluation, Orladeyo (Oct 2018) 7. Craig et al (2023), The Lancet 8. Riedl et al (2024), NEJM ALPHA - STAR Phase 1b/2 6 - Months Results Summary 6 - Month Phase 3 Results Summaries Doses Per Year Injection Site Pain Reduction in Attacks Requiring Rescue Medication Reduction in Moderate and Severe Attack Rate Attack - Free Rate Attack Rate Reduction 26 52% 87% 83% 44% 87% Lanadelumab 2,3 300 mg Q2W 365 N.A. 49% 40% 8% 44% Berotralstat 4,5,6 150mg QD 13 N.R. 88% 90% 72% 87% Garadacimab 7 200mg Q4W 12 N.R. 92% 89% 53% 81% Donidalorsen 8 80mg Q4W Navenibart Summary 91 - 95% Attack Rate Reduction 25 - 67% Attack - Free Rate 95 - 96% Reduction in Moderate and Severe Attack Rate 91 - 94% Reduction in Attacks Requiring Rescue Medication 0% Injection Site Pain 2 or 4 Doses Per Year 1 13
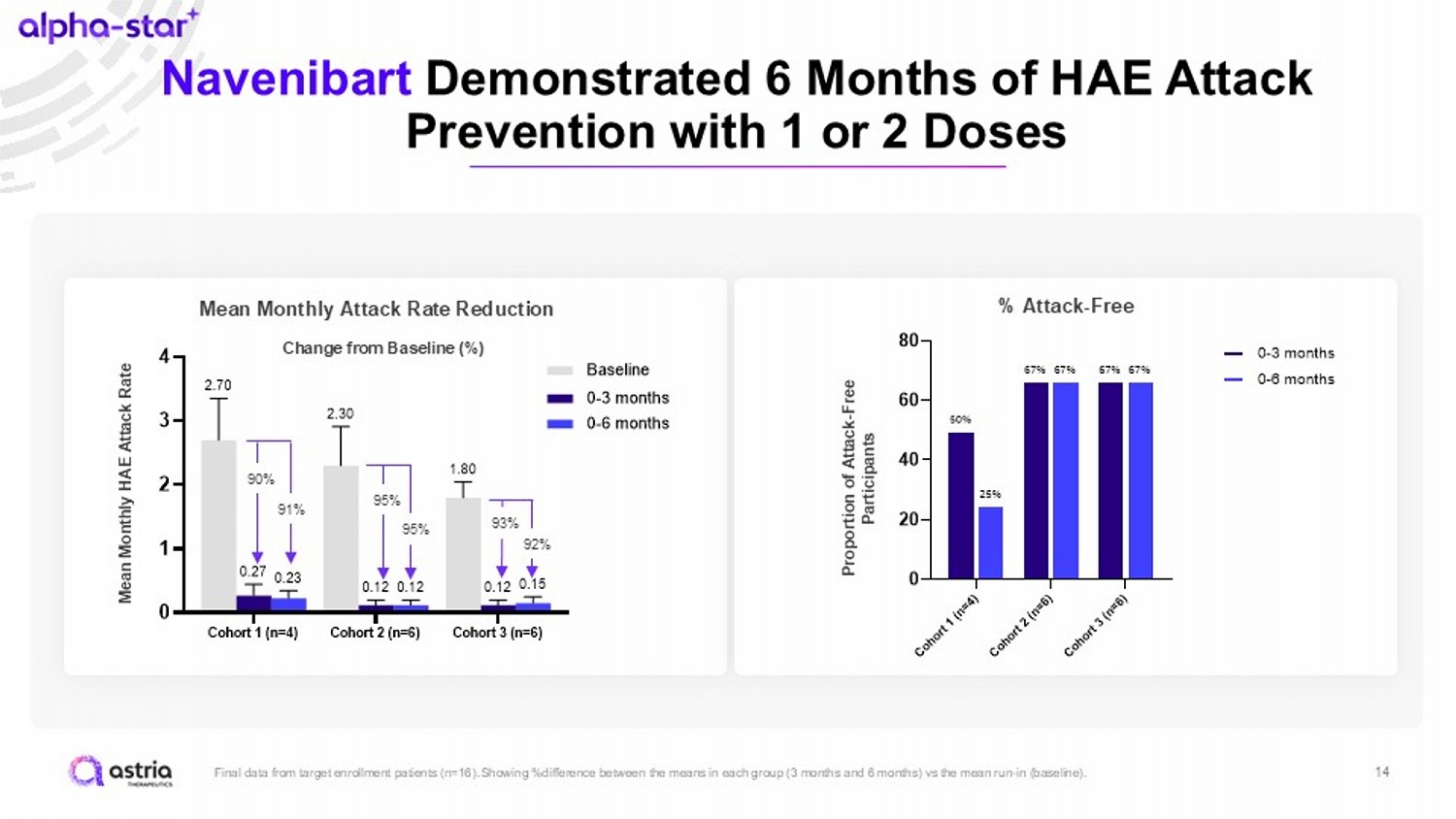
14 Navenibart Demonstrated 6 Months of HAE Attack Prevention with 1 or 2 Doses Final data from target enrollment patients (n=16). Showing %difference between the means in each group (3 months and 6 months) vs the mean run - in (baseline). C o h o r t 1 ( n = 4 ) C o h o r t 2 ( n = 6 ) C o h o r t 3 ( n = 6 ) 0 20 40 60 80 25% 67% 67% 50% 67% 67% 0-3 months 0-6 months Cohort 1 (n=4) Cohort 2 (n=6) Cohort 3 (n=6) 0 1 2 3 4 0.23 0.12 0.15 0.27 0.12 0.12 2.70 2.30 1.80 M e a n m o n t h l y H A E a t t a c k r a t e Baseline 0-3 months 0-6 months 90% 91% 95% 95% 93% 92% Change from Baseline (%) Mean Monthly HAE Attack Rate Mean Monthly Attack Rate Reduction % Attack - Free Proportion of Attack - Free Participants
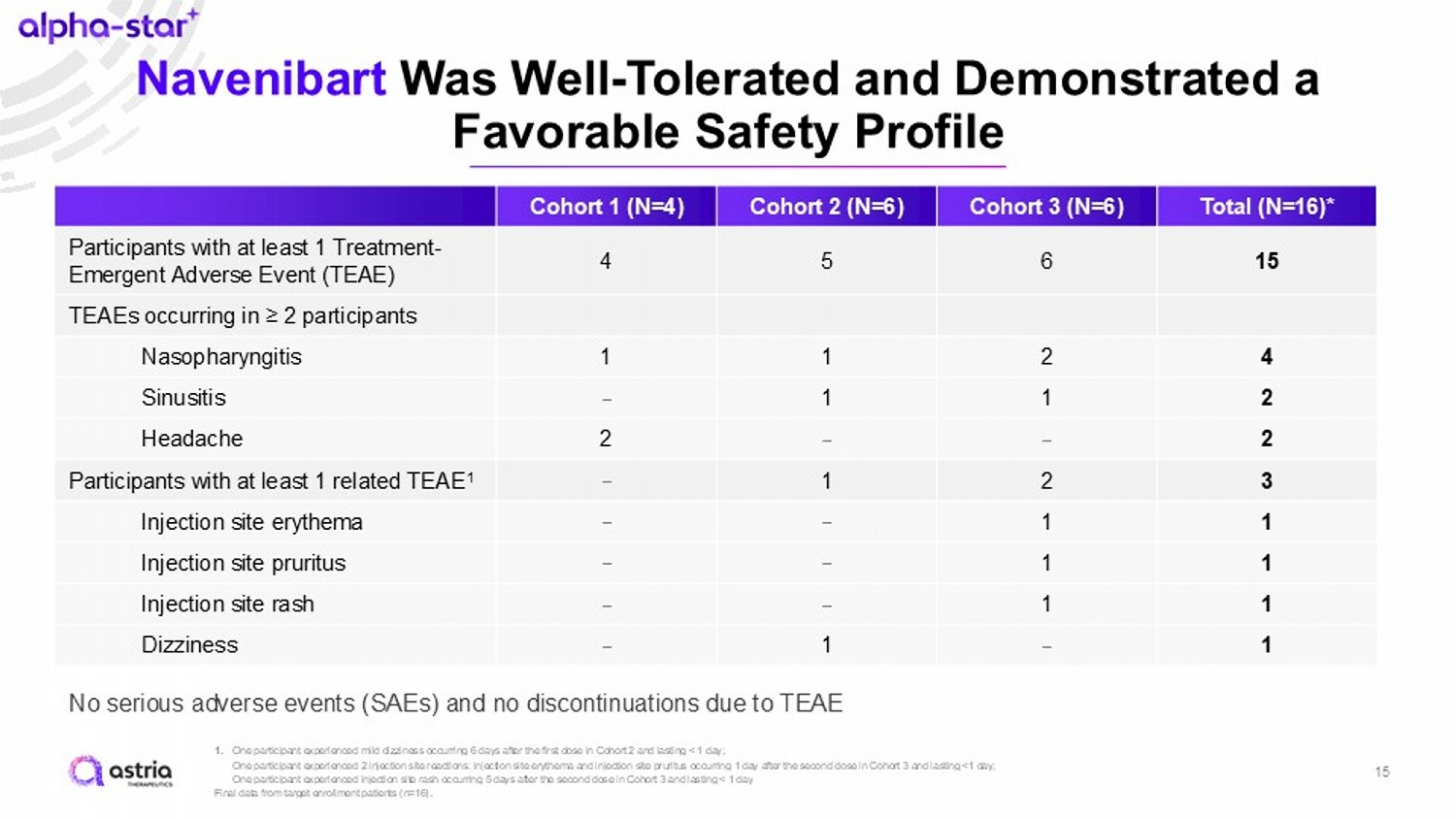
Navenibart Was Well - Tolerated and Demonstrated a Favorable Safety Profile 15 1. One participant experienced mild dizziness occurring 6 days after the first dose in Cohort 2 and lasting < 1 day; One participant experienced 2 injection site reactions: injection site erythema and injection site pruritus occurring 1 day a fte r the second dose in Cohort 3 and lasting <1 day; One participant experienced injection site rash occurring 5 days after the second dose in Cohort 3 and lasting < 1 day Final data from target enrollment patients (n=16). Total (N=16)* Cohort 3 (N=6) Cohort 2 (N=6) Cohort 1 (N=4) 15 6 5 4 Participants with at least 1 Treatment - Emergent Adverse Event (TEAE) TEAEs occurring in ≥ 2 participants 4 2 1 1 Nasopharyngitis 2 1 1 – Sinusitis 2 – – 2 Headache 3 2 1 – Participants with at least 1 related TEAE 1 1 1 – – Injection site erythema 1 1 – – Injection site pruritus 1 1 – – Injection site rash 1 – 1 – Dizziness No serious adverse events (SAEs) and no discontinuations due to TEAE
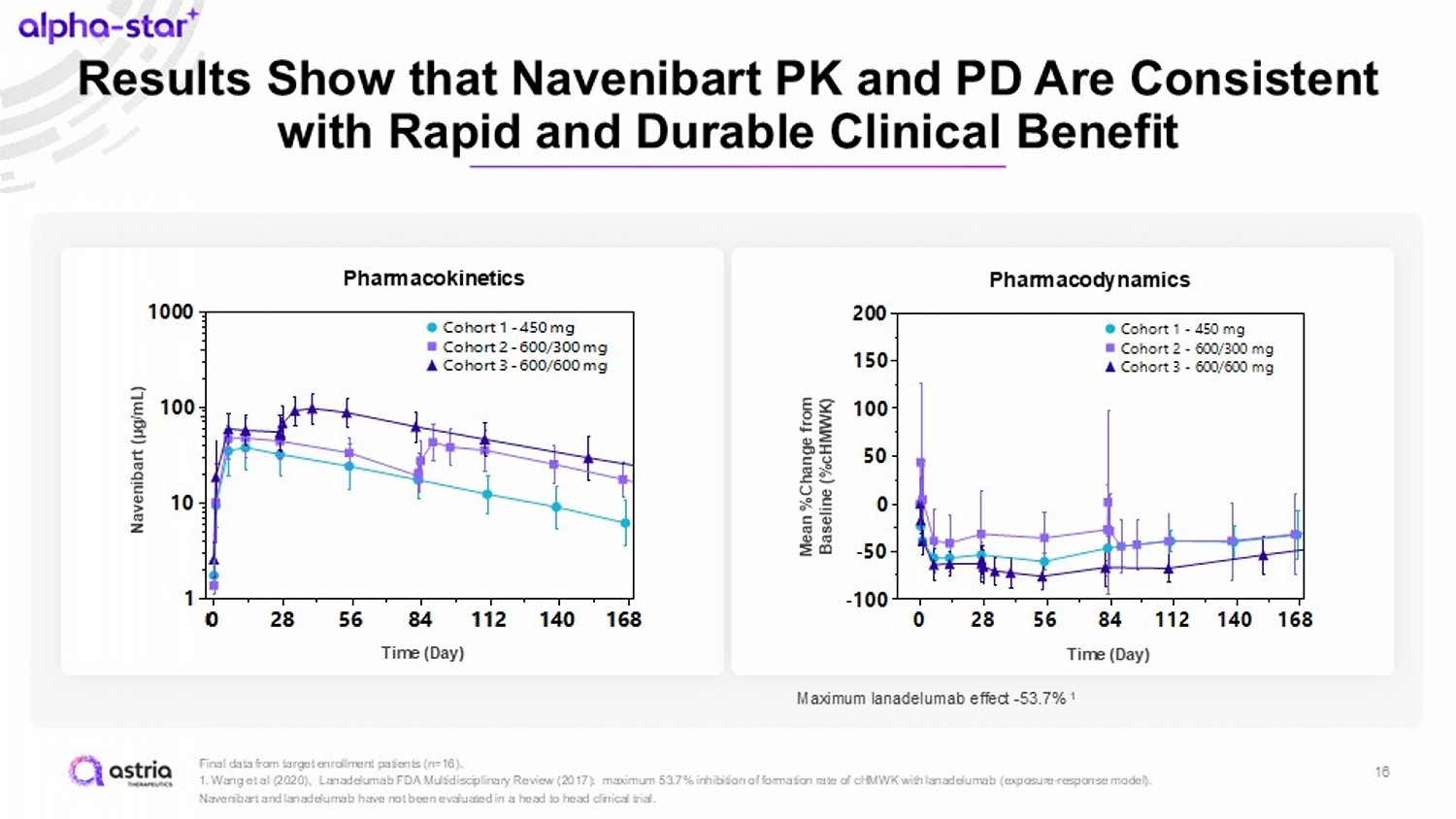
Results Show that Navenibart PK and PD Are Consistent with Rapid and Durable Clinical Benefit 16 Final data from target enrollment patients (n=16). 1 . Wang et al (2020), Lanadelumab FDA Multidisciplinary Review (2017): maximum 53.7% inhibition of formation rate of cHMWK with lanadelumab (exposure - response model). Navenibart and lanadelumab have not been evaluated in a head to head clinical trial. Time (Day) 0 28 56 84 112 140 168 -100 -50 0 50 100 150 200 Cohort 1 - 450 mg Cohort 2 - 600/300 mg Cohort 3 - 600/600 mg Pharmacodynamics Mean %Change from Baseline (% cHMWK ) Time (Day) Maximum lanadelumab effect - 53.7% 1 Time (Day) 0 28 56 84 112 140 168 0 1 2 3 Cohort 1 - 450 mg Cohort 2 - 600/300 mg Cohort 3 - 600/600 mg Pharmacokinetics Time (Day) Time (Day) 0 28 56 84 112 140 168 1 10 100 1000 Cohort 1 - 450 mg Cohort 2 - 600/300 mg Cohort 3 - 600/600 mg Navenibart (µg/mL)
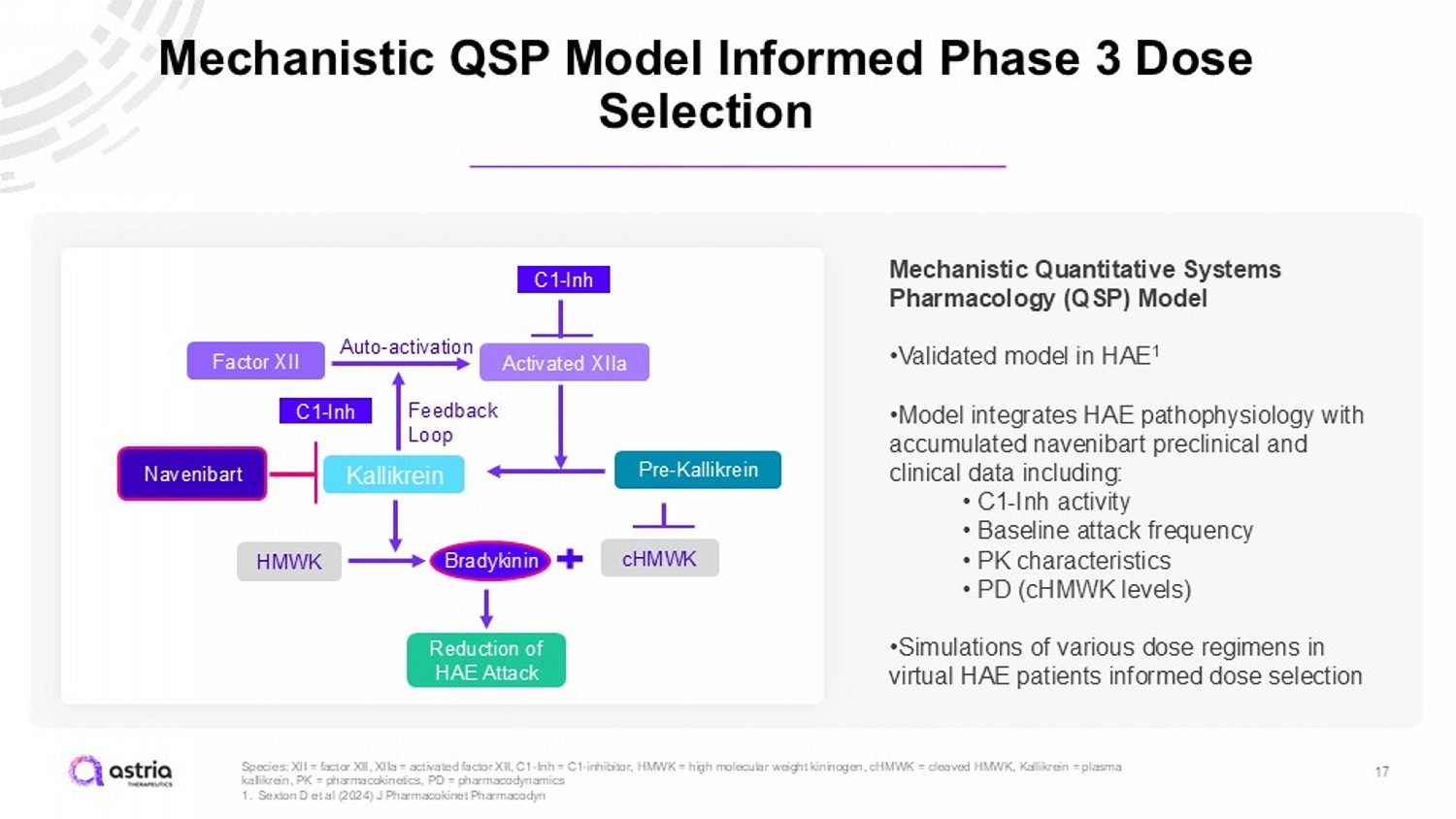
17 Species: XII = factor XII, XIIa = activated factor XII, C1 - Inh = C1 - inhibitor, HMWK = high molecular weight kininogen, cHMWK = cleaved HMWK, Kallikrein = plasma kallikrein, PK = pharmacokinetics, PD = pharmacodynamics 1. Sexton D et al (2024) J Pharmacokinet Pharmacodyn Mechanistic QSP Model Informed Phase 3 Dose Selection C1 - Inh Activated XIIa Factor XII Auto - activation Feedback Loop Pre - Kallikrein Kallikrein Navenibart HMWK cHMWK Bradykinin C1 - Inh Reduction of HAE Attack Mechanistic Quantitative Systems Pharmacology (QSP) Model • Validated model in HAE 1 • Model integrates HAE pathophysiology with accumulated navenibart preclinical and clinical data including: • C1 - Inh activity • Baseline attack frequency • PK characteristics • PD (cHMWK levels) • Simulations of various dose regimens in virtual HAE patients informed dose selection
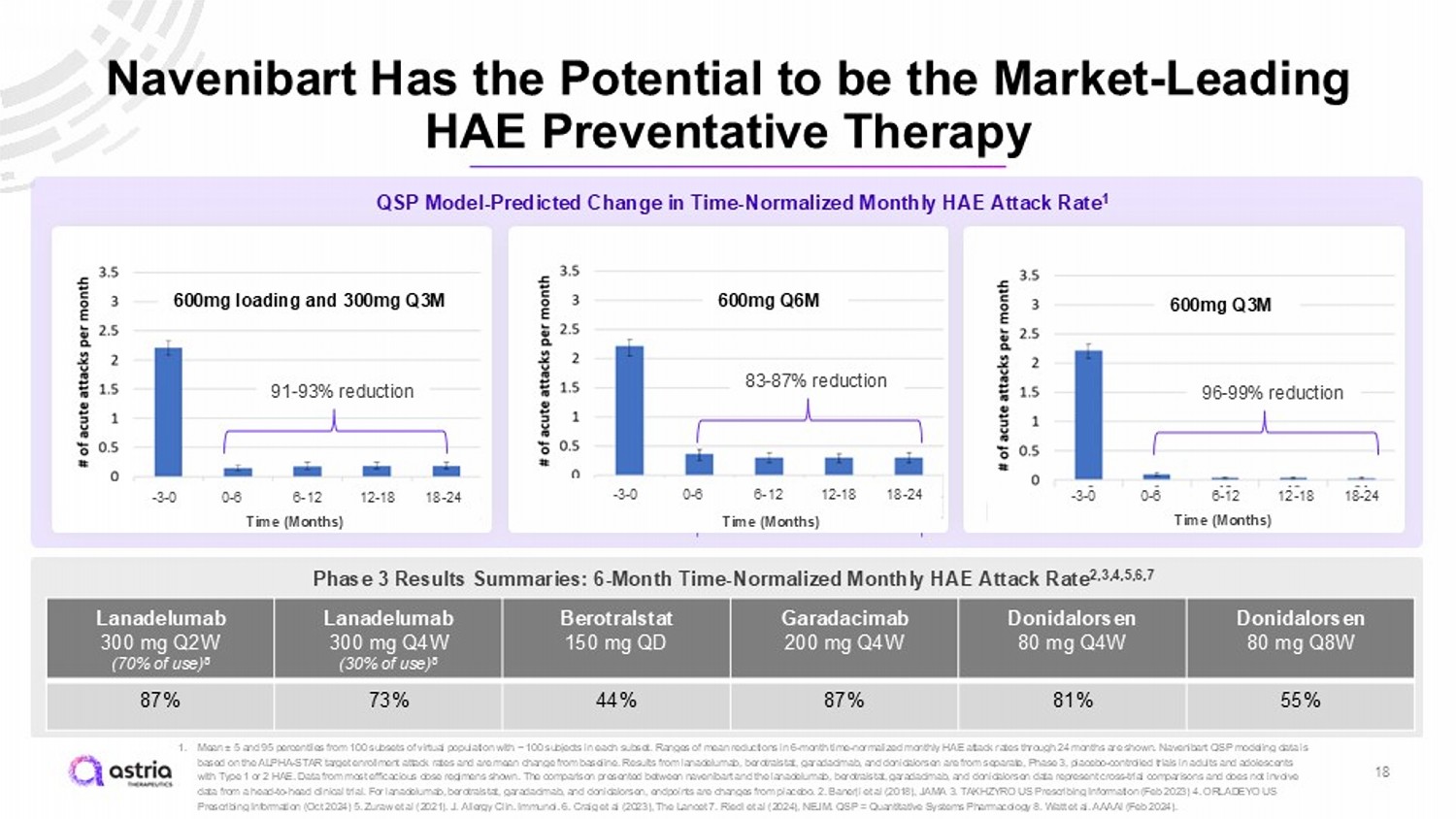
Navenibart Has the Potential to be the Market - Leading HAE Preventative Therapy 1. Mean “ 5 and 95 percentiles from 100 subsets of virtual population with ~ 100 subjects in each subset. Ranges of mean reductions in 6 - month time - normalized monthly HAE attack rates through 24 months are shown. Navenibart QSP modeling data is based on the ALPHA - STAR target enrollment attack rates and are mean change from baseline. Results from lanadelumab , berotralstat , garadacimab , and donidalorsen are from separate, Phase 3, placebo - controlled trials in adults and adolescents with Type 1 or 2 HAE. Data from most efficacious dose regimens shown. The comparison presented between navenibart and the lanadelumab , berotralstat , garadacimab , and donidalorsen data represent cross - trial comparisons and does not involve data from a head - to - head clinical trial. For lanadelumab , berotralstat , garadacimab , and donidalorsen , endpoints are changes from placebo . 2. Banerji et al (2018), JAMA 3. TAKHZYRO US Prescribing Information (Feb 2023) 4. ORLADEYO US Prescribing Information (Oct 2024) 5. Zuraw et al (2021). J. Allergy Clin. Immunol. 6. Craig et al (2023), The Lancet 7. Riedl et al (2024), NEJM. QSP = Quantitative Systems Pharmacology 8. Watt et al. AAAAI (Feb 2024). QSP Model - Predicted Change in Time - Normalized Monthly HAE Attack Rate 1 600mg Q6M 18 600mg Q3M 600mg loading and 300mg Q3M 91 - 93% reduction 96 - 99% reduction 83 - 87% reduction - 3 - 0 0 - 6 6 - 12 12 - 18 18 - 24 - 3 - 0 0 - 6 6 - 12 12 - 18 18 - 24 - 3 - 0 0 - 6 6 - 12 12 - 18 18 - 24 Phase 3 Results Summaries: 6 - Month Time - Normalized Monthly HAE Attack Rate 2,3,4,5,6,7 Donidalorsen 80 mg Q8W Donidalorsen 80 mg Q4W Garadacimab 200 mg Q4W Berotralstat 150 mg QD Lanadelumab 300 mg Q4W (30% of use) 8 Lanadelumab 300 mg Q2W (70% of use) 8 55% 81% 87% 44% 73% 87% 600mg Q6M 83 - 87% reduction - 3 - 0 0 - 6 6 - 12 12 - 18 18 - 24 Time (Months) Time (Months) Time (Months)

Navenibart Dosing Flexibility Has the Potential to Transform the Treatment of HAE 19 1. Roche. YTD September 2024 sales (2024). 2. Takeda Pharmaceutical Co. Takeda Quarterly Financial Report For the Quarter Ended March 31, 2024. (2024). 3. Takeda Pharmaceutical Co. Takeda Quarterly Financial Report For the Quarter Ended June 30, 2024. (2024). 4. Takeda Pharmaceutical Co. Takeda Quarterly Financial Report For the Quarter Ended September 30, 2024. (2024). Patients and physicians increasingly recognize flexible dosing as most appropriate care. For example, VABYSMO ( faricimab - svoa ) revolutionized the wet AMD and DME markets with dosing flexibility ($3.2B USD in sales as of 9/30/2024 1 ) TAKHZYRO ( lanadelumab ), the current HAE market - leading product ($1.0B USD in sales as of 9/30/2024 2,3,4 ) is dosed every 2 weeks with the potential to extend the dosing interval to every 4 weeks Navenibart Phase 3 program is designed to enable dosing flexibility Navenibart has potential to deliver efficacy at or better than TAKHZYRO with the ability for patients and clinicians to decide what works best for them with dosing every 3 or 6 months
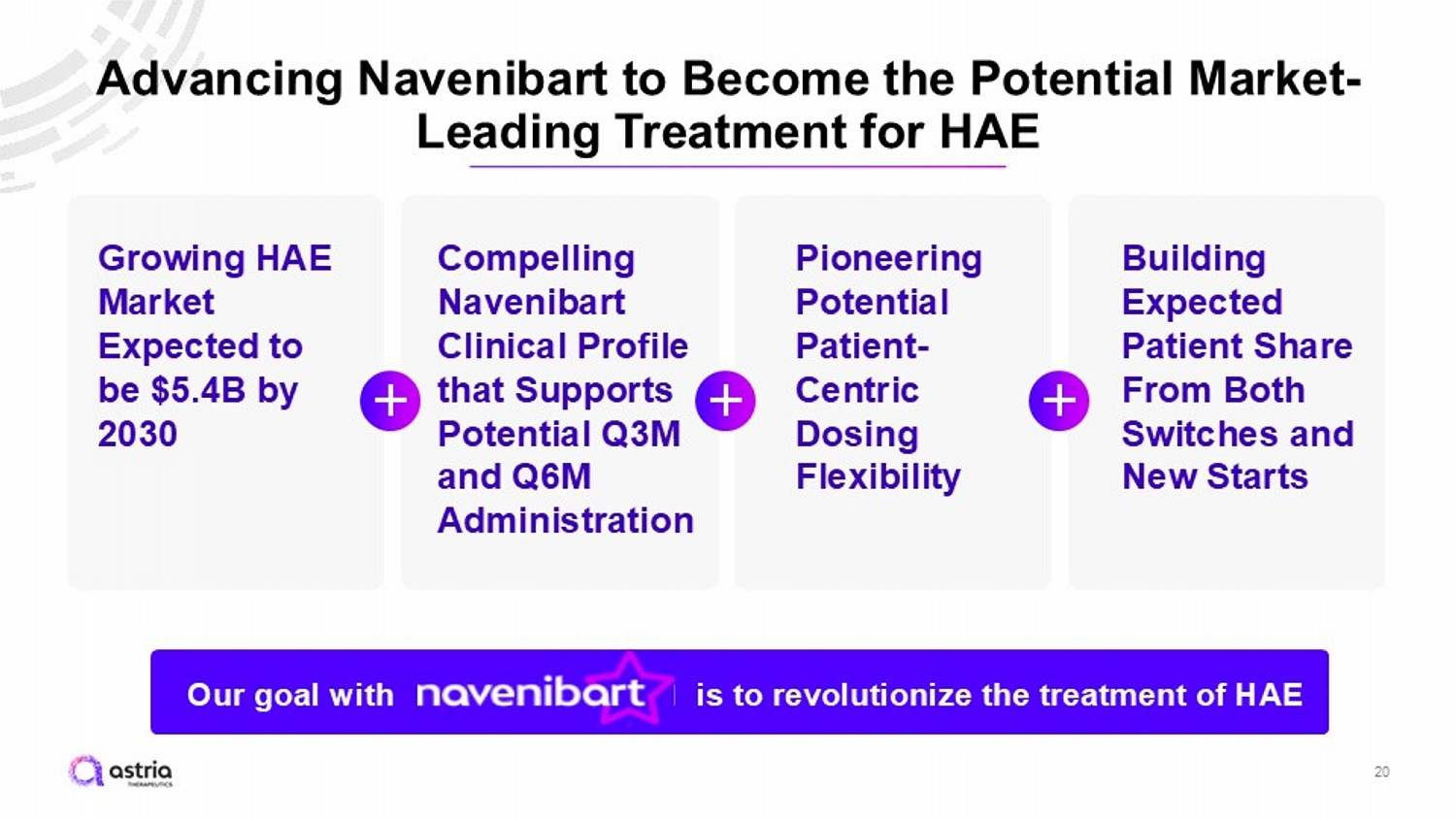
Advancing Navenibart to Become the Potential Market - Leading Treatment for HAE 20 Compelling Navenibart Clinical Profile that Supports Potential Q3M and Q6M Administration Growing HAE Market Expected to be $5.4B by 2030 Pioneering Potential Patient - Centric Dosing Flexibility Building Expected Patient Share From Both Switches and New Starts Our goal with is to revolutionize the treatment of HAE
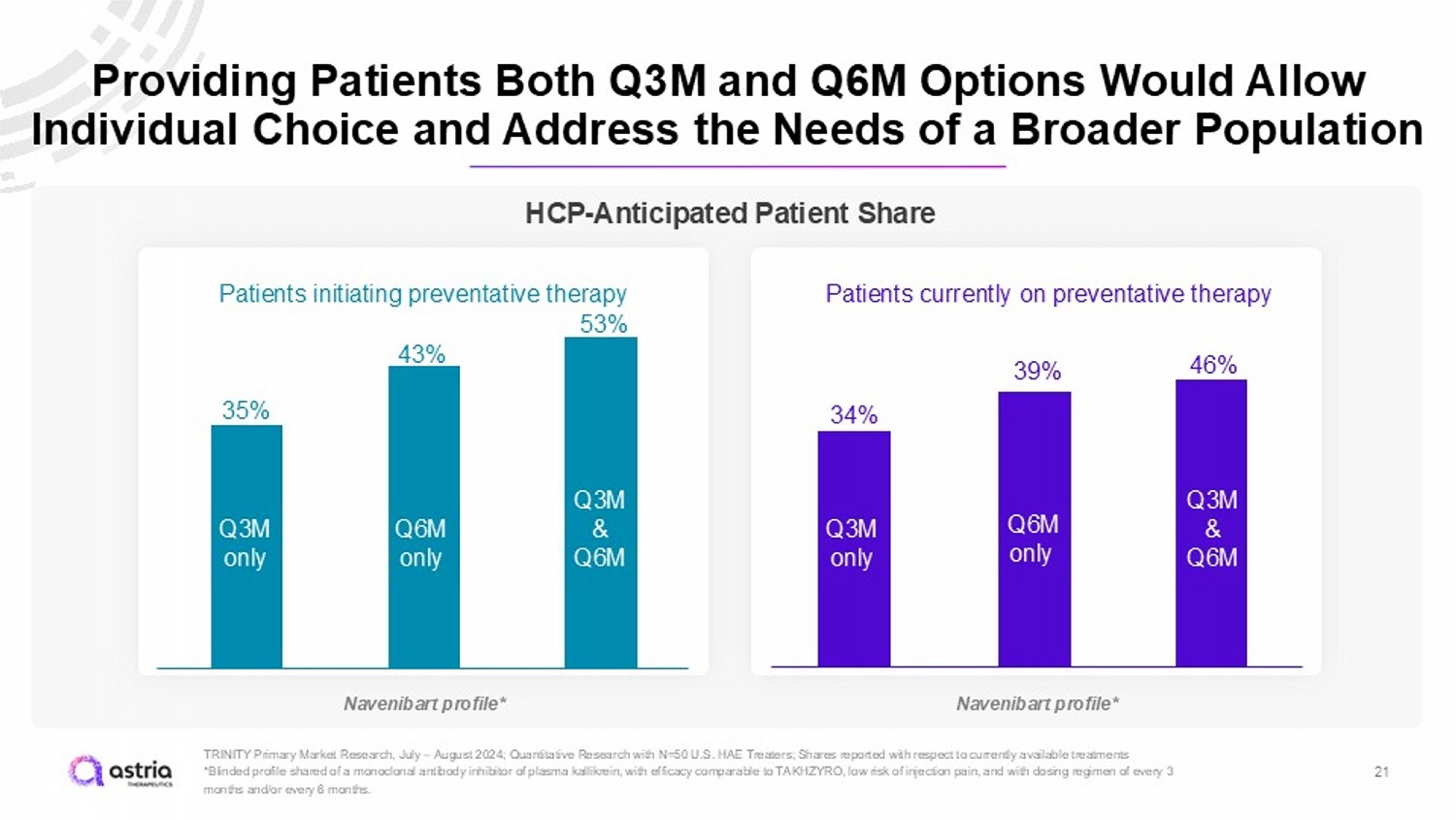
Providing Patients Both Q3M and Q6M Options Would Allow Individual Choice and Address the Needs of a Broader Population 21 TRINITY Primary Market Research, July – August 2024; Quantitative Research with N=50 U.S. HAE Treaters; Shares reported with res pect to currently available treatments *Blinded profile shared of a monoclonal antibody inhibitor of plasma kallikrein, with efficacy comparable to TAKHZYRO, low ri sk of injection pain, and with dosing regimen of every 3 months and/or every 6 months. Patients initiating preventative therapy Patients currently on preventative therapy 35% 43% 34% 39% 53% 46% Q3M only Q6M only Q3M & Q6M Q3M only Q6M only Q3M & Q6M HCP - Anticipated Patient Share Navenibart profile* Navenibart profile*
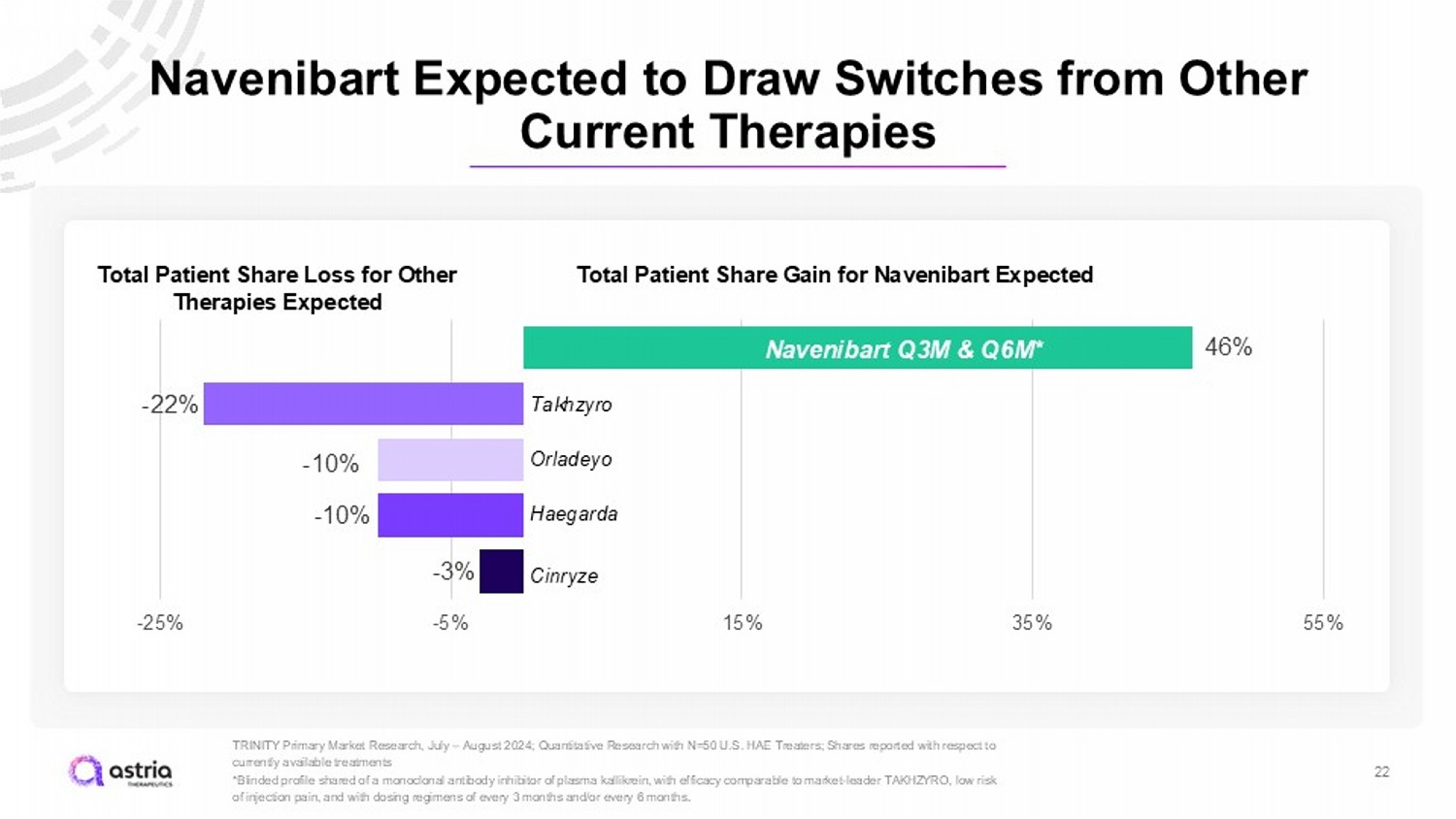
Navenibart Expected to Draw Switches from Other Current Therapies 22 TRINITY Primary Market Research, July – August 2024; Quantitative Research with N=50 U.S. HAE Treaters; Shares reported with res pect to currently available treatments *Blinded profile shared of a monoclonal antibody inhibitor of plasma kallikrein, with efficacy comparable to market - leader TAKHZ YRO, low risk of injection pain, and with dosing regimens of every 3 months and/or every 6 months . 46% - 22% - 10% - 10% - 3% -25% -5% 15% 35% 55% Takhzyro Orladeyo Haegarda Cinryze Total Patient Share Gain for Navenibart Expected 46% N avenibart Q3M & Q6M* Total Patient Share Loss for Other Therapies Expected
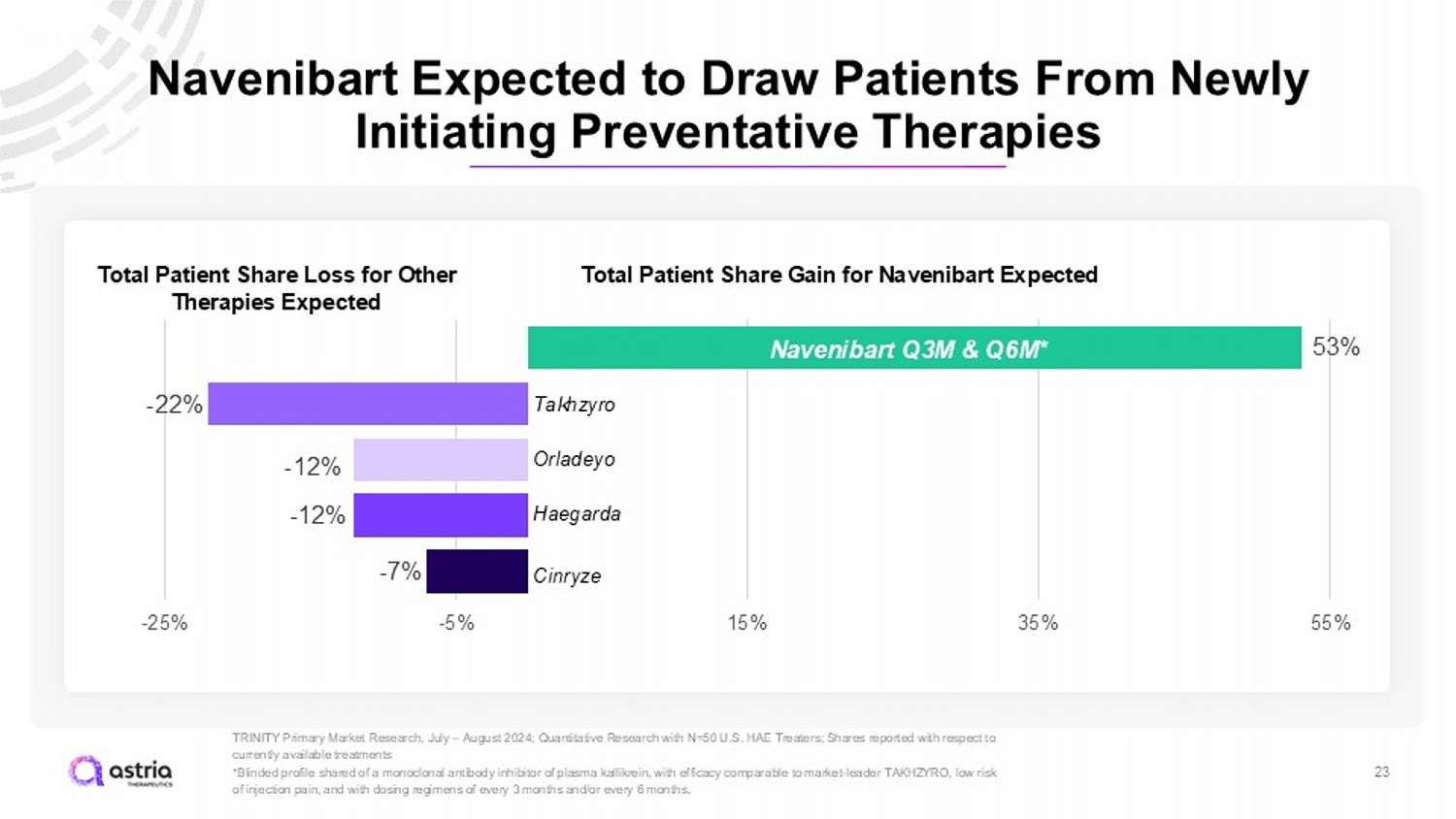
Navenibart Expected to Draw Patients From Newly Initiating Preventative Therapies 23 TRINITY Primary Market Research, July – August 2024; Quantitative Research with N=50 U.S. HAE Treaters; Shares reported with res pect to currently available treatments *Blinded profile shared of a monoclonal antibody inhibitor of plasma kallikrein, with efficacy comparable to market - leader TAKHZ YRO, low risk of injection pain, and with dosing regimens of every 3 months and/or every 6 months . 53% - 22% - 12% - 12% - 7% -25% -5% 15% 35% 55% Takhzyro Orladeyo Haegarda Cinryze 46% N avenibart Q3M & Q6M* Total Patient Share Gain for Navenibart Expected Total Patient Share Loss for Other Therapies Expected

Taking a medication 2 or 4 times a year would mean freedom for me. That is the closest thing to a normal life that I could imagine. I could travel. I could make plans without checking the day of the week.” COLI ”
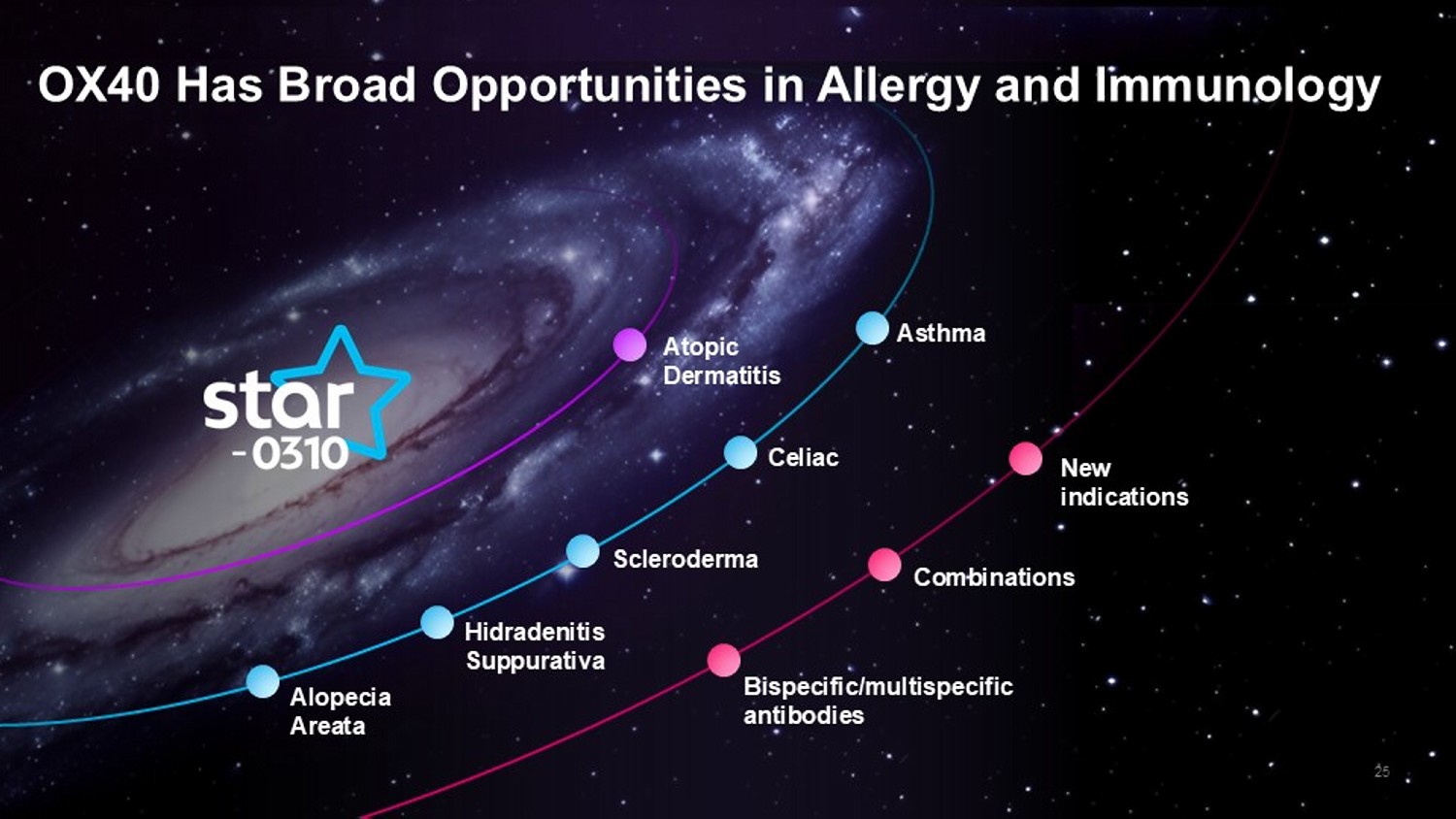
25 OX40 Has Broad Opportunities in Allergy and Immunology Atopic Dermatitis Asthma Celiac Scleroderma Hidradenitis Suppurativa Alopecia Areata New indications Combinations Bispecific/ multispecific antibodies
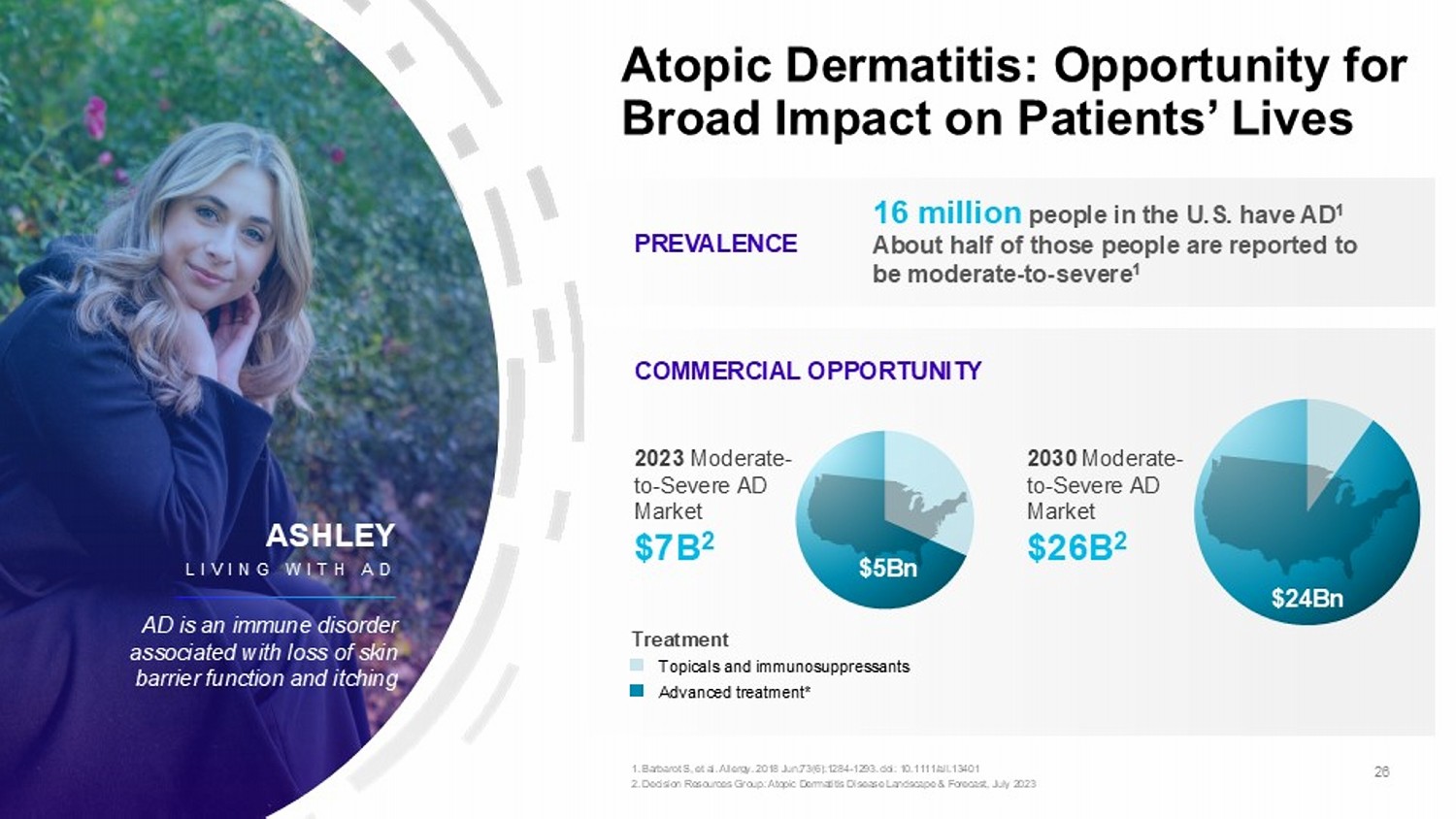
Atopic Dermatitis: Opportunity for Broad Impact on Patients’ Lives PREVALENCE COMMERCIAL OPPORTUNITY 16 million people in the U.S. have AD 1 About half of those people are reported to be moderate - to - severe 1 $5Bn $24Bn Treatment Topicals and immunosuppressants Advanced treatment* 2023 Moderate - to - Severe AD Market $7B 2 2030 Moderate - to - Severe AD Market $26B 2 26 1. Barbarot S, et al. Allergy. 2018 Jun;73(6):1284 - 1293. doi : 10.1111/all.13401 2. Decision Resources Group: Atopic Dermatitis Disease Landscape & Forecast, July 2023 ASHLEY LIVING WITH AD AD is an immune disorder associated with loss of skin barrier function and itching
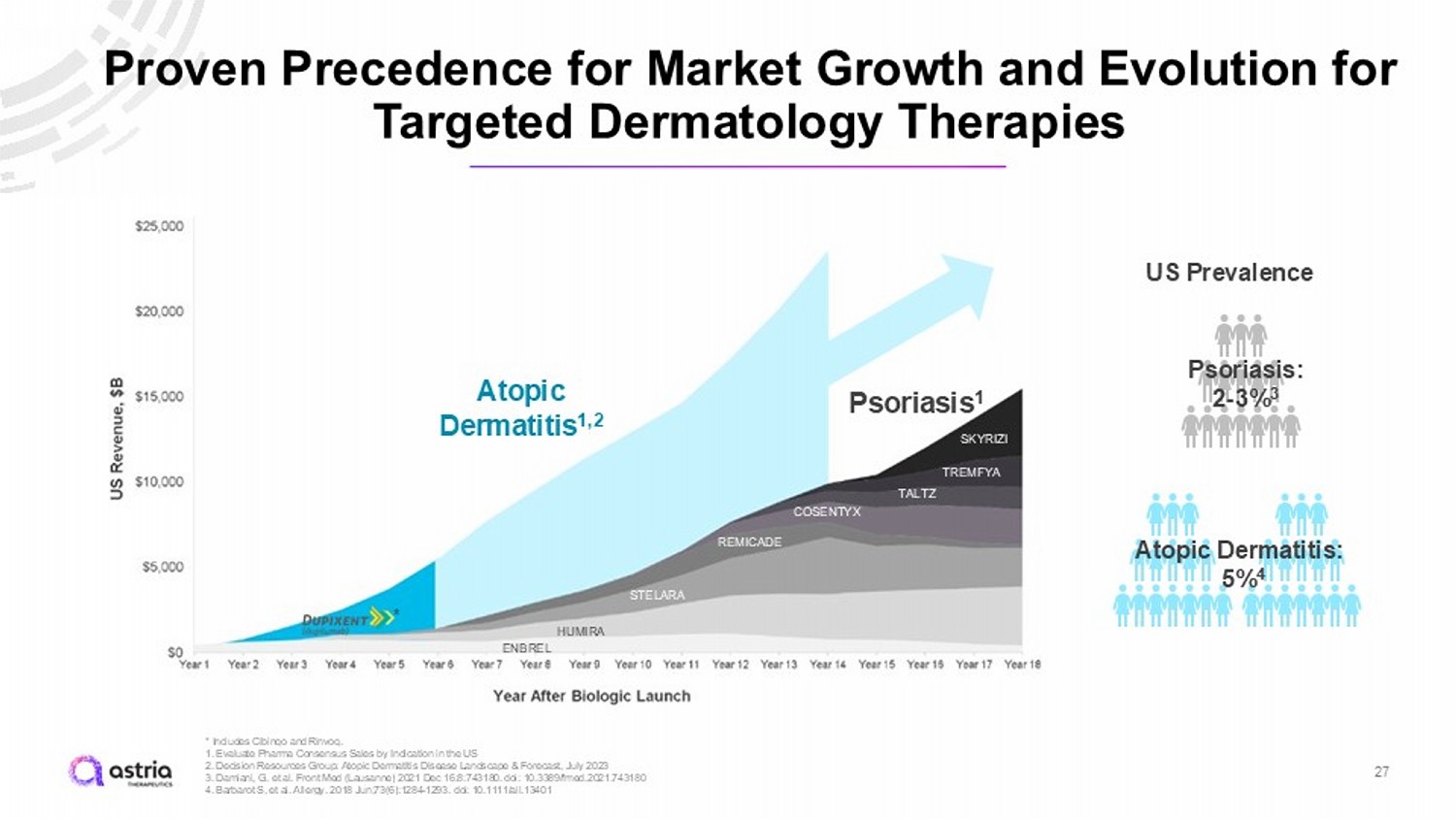
US Prevalence Psoriasis: 2 - 3% 3 Atopic Dermatitis: 5% 4 Proven Precedence for Market Growth and Evolution for Targeted Dermatology Therapies * Includes Cibinqo and Rinvoq . 1. Evaluate Pharma Consensus Sales by Indication in the US 2. Decision Resources Group: Atopic Dermatitis Disease Landscape & Forecast, July 2023 3. Damiani, G. et al. Front Med (Lausanne) 2021 Dec 16:8:743180. doi : 10.3389/fmed.2021.743180 4. Barbarot S, et al. Allergy. 2018 Jun;73(6):1284 - 1293. doi : 10.1111/all.13401 27 Atopic Dermatitis 1,2 Psoriasis 1 ENBREL HUMIRA STELARA REMICADE COSENTYX TALTZ TREMFYA SKYRIZI *
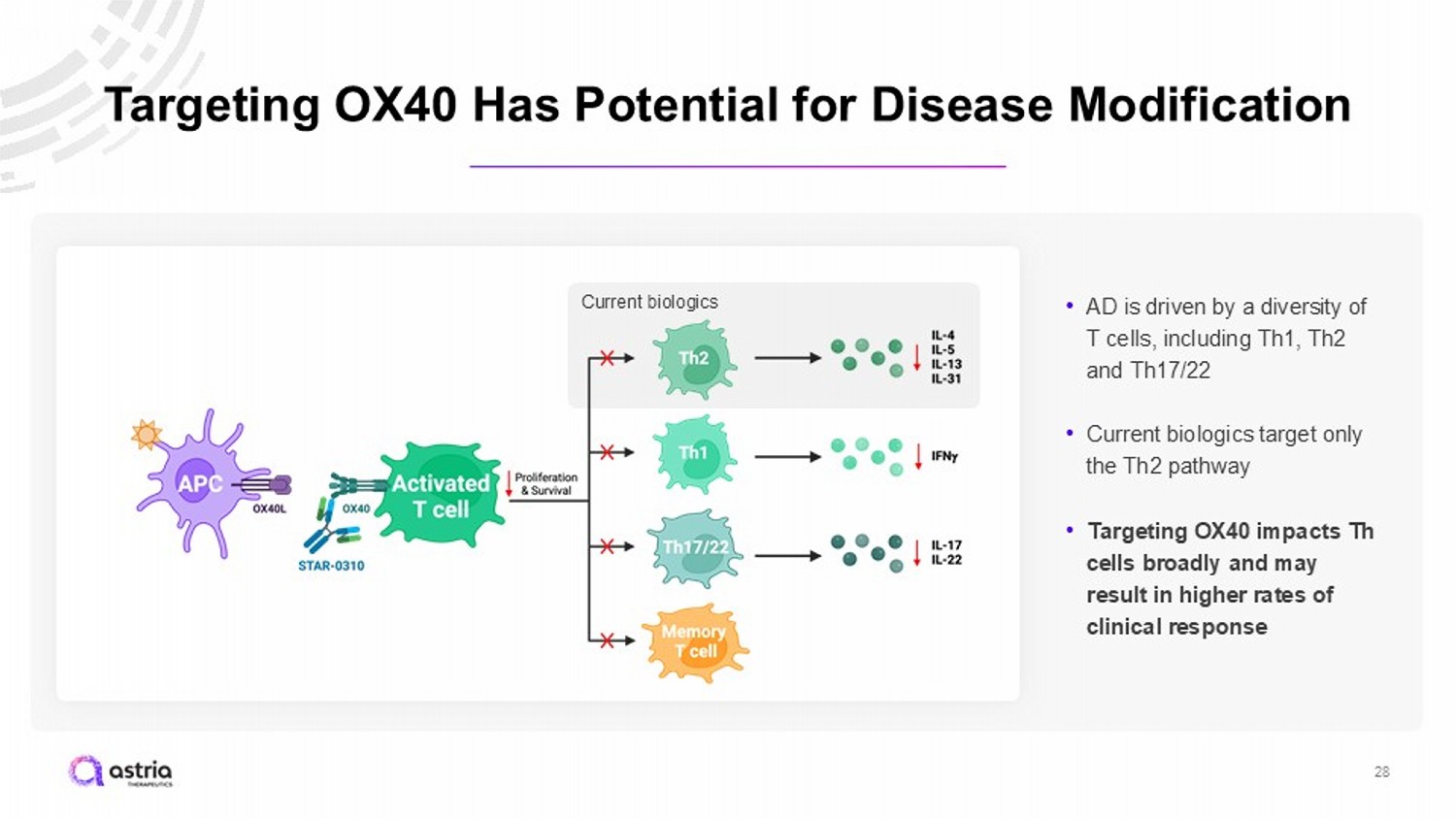
Targeting OX40 Has Potential for Disease Modification 28 • AD is driven by a diversity of T cells, including Th1, Th2 and Th17/22 • Current biologics target only the Th2 pathway • Targeting OX40 impacts Th cells broadly and may result in higher rates of clinical response Current biologics
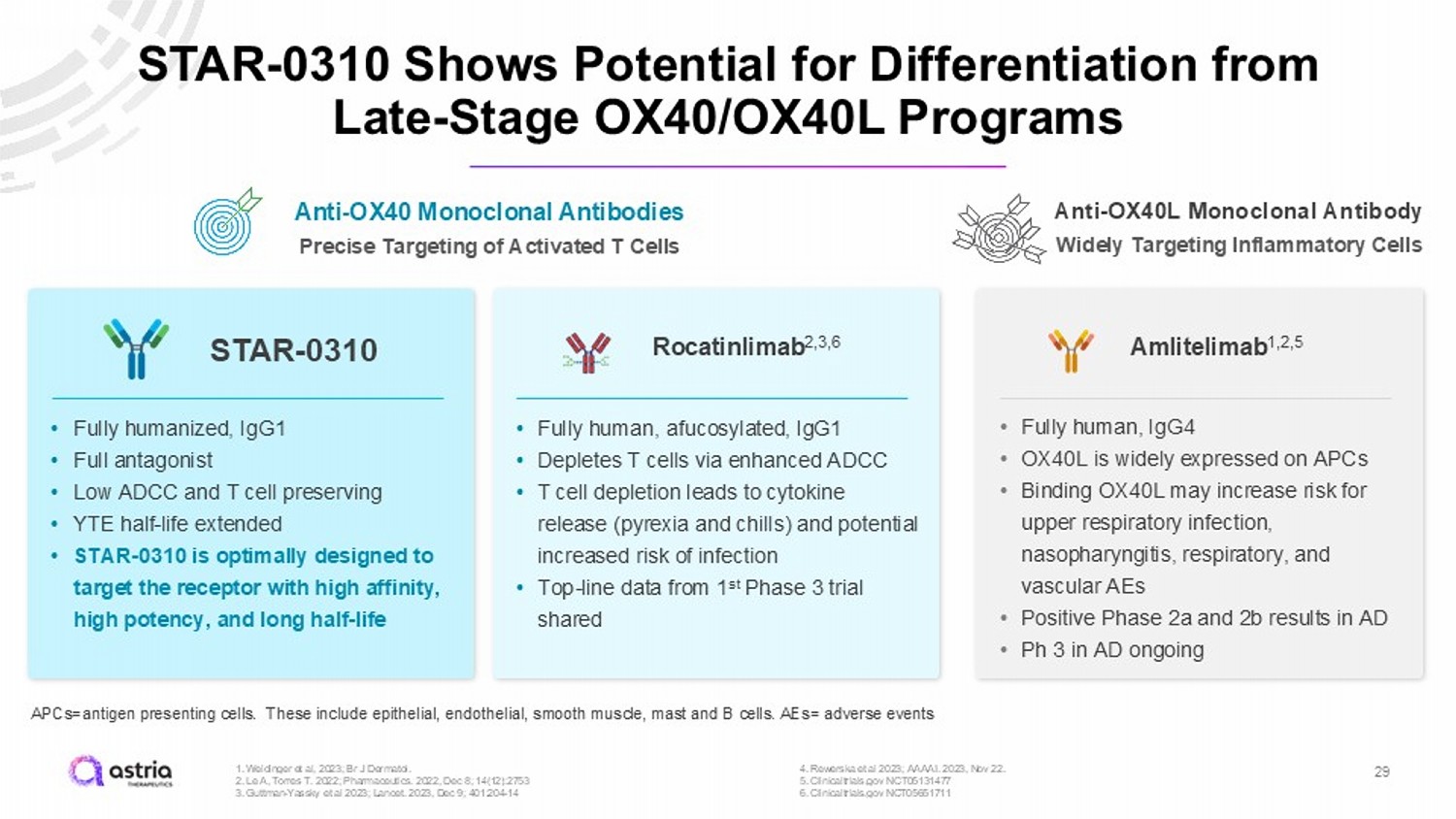
STAR - 0310 Shows Potential for Differentiation from Late - Stage OX40/OX40L Programs 29 Rocatinlimab 2,3,6 Amlitelimab 1,2,5 STAR - 0310 APCs=antigen presenting cells. These include epithelial, endothelial, smooth muscle, mast and B cells. AEs= adverse events 1. Weidinger et al, 2023; Br J Dermatol. 2. Le A, Torres T. 2022; Pharmaceutics. 2022, Dec 8; 14(12):2753 3. Guttman - Yassky et al 2023; Lancet. 2023, Dec 9; 401:204 - 14 4. Rewerska et al 2023; AAAAI. 2023, Nov 22. 5. Clinicaltrials.gov NCT05131477 6. Clinicaltrial s.gov NCT05651711 Anti - OX40 Monoclonal Antibodies Precise Targeting of Activated T Cells Anti - OX40L Monoclonal Antibody Widely Targeting Inflammatory Cells • Fully humanized, IgG1 • Full antagonist • Low ADCC and T cell preserving • YTE half - life extended • STAR - 0310 is optimally designed to target the receptor with high affinity, high potency, and long half - life • Fully human, afucosylated , IgG1 • Depletes T cells via enhanced ADCC • T cell depletion leads to cytokine release (pyrexia and chills) and potential increased risk of infection • Top - line data from 1 st Phase 3 trial shared • Fully human, IgG4 • OX40L is widely expressed on APCs • Binding OX40L may increase risk for upper respiratory infection, nasopharyngitis, respiratory, and vascular AEs • Positive Phase 2a and 2b results in AD • Ph 3 in AD ongoing
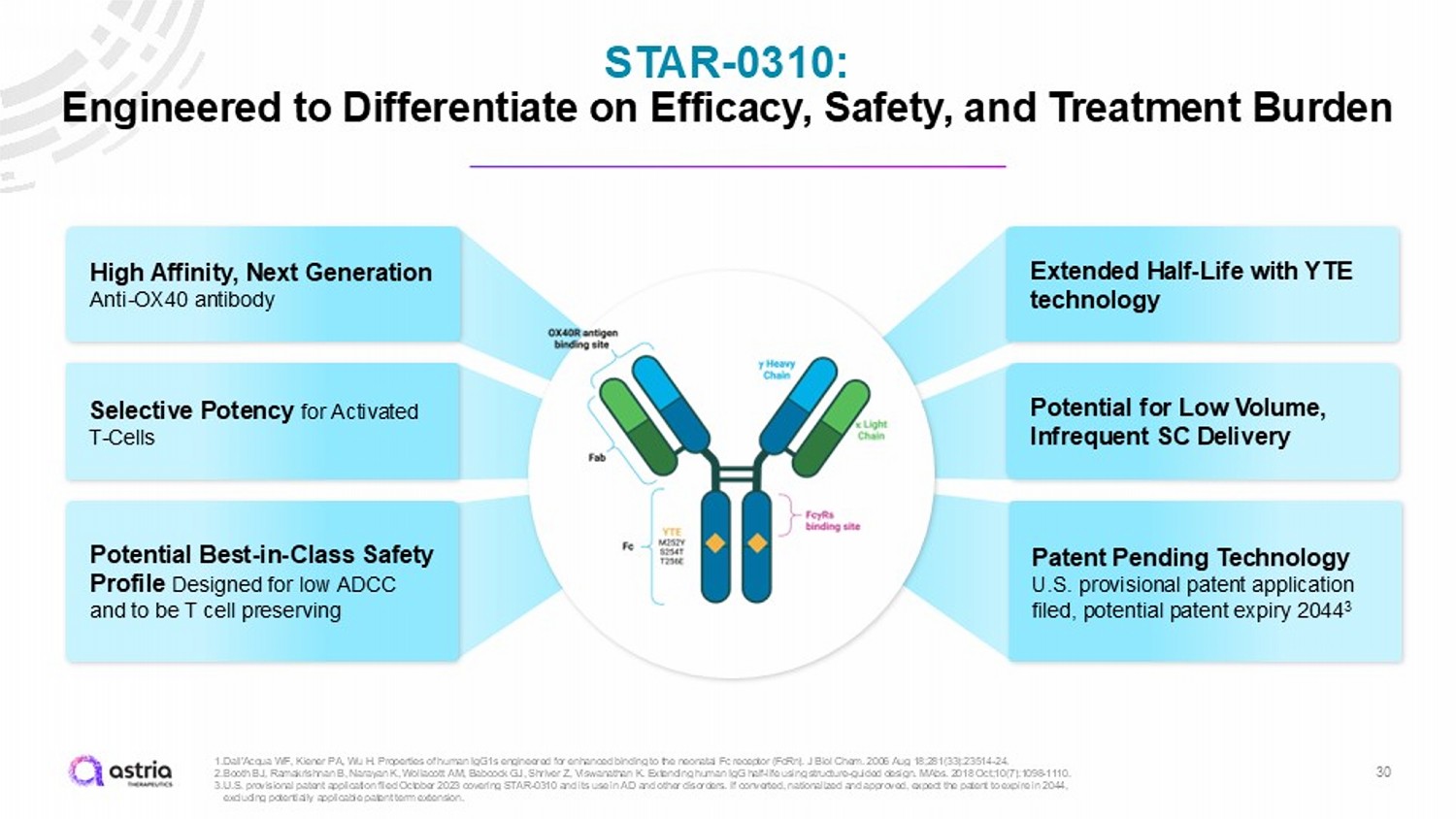
STAR - 0310: Engineered to Differentiate on Efficacy, Safety, and Treatment Burden 30 Extended Half - Life with YTE technology Potential Best - in - Class Safety Profile Designed for low ADCC and to be T cell preserving High Affinity, Next Generation Anti - OX40 antibody Selective Potency for Activated T - Cells Patent Pending Technology U.S. provisional patent application filed, potential patent expiry 2044 3 Potential for Low Volume, Infrequent SC Delivery 1. Dall'Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor ( FcRn ). J Biol Chem. 2006 Aug 18;281(33):23514 - 24. 2. Booth BJ, Ramakrishnan B, Narayan K, Wollacott AM, Babcock GJ, Shriver Z, Viswanathan K. Extending human IgG half - life using structure - guided design. MAbs . 2018 Oct;10(7):1098 - 1110. 3. U.S. provisional patent application filed October 2023 covering STAR - 0310 and its use in AD and other disorders. If converted, n ationalized and approved, expect the patent to expire in 2044, excluding potentially applicable patent term extension.
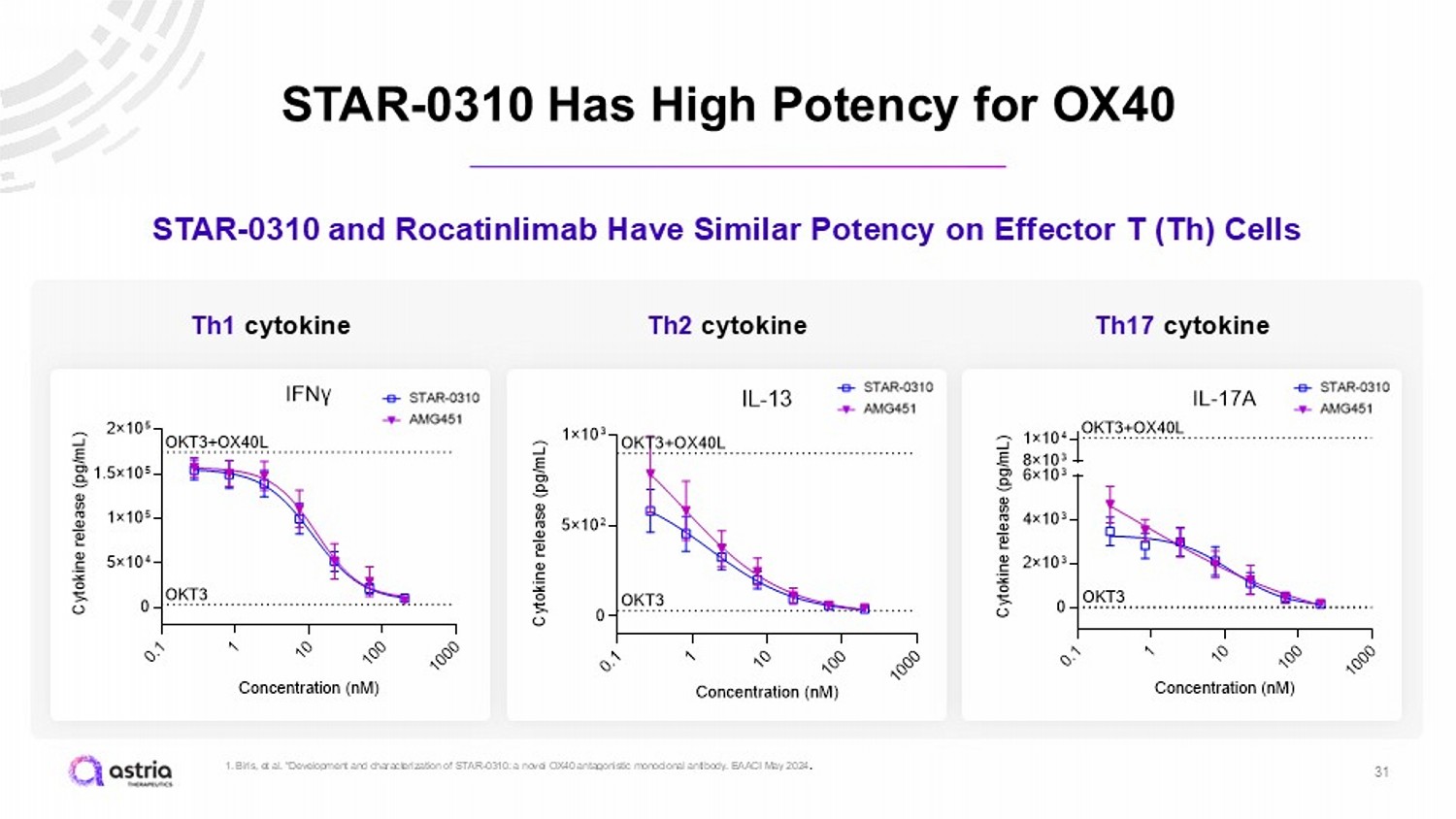
STAR - 0310 Has High Potency for OX40 31 STAR - 0310 and Rocatinlimab Have Similar Potency on Effector T (Th) Cells Th1 cytokine 0 . 1 1 1 0 1 0 0 1 0 0 0 0 5×10 4 1×10 5 1.5×10 5 2×10 5 IFNγ Concentration (nM) C y t o k i n e r e l e a s e ( p g / m L ) OKT3+OX40L OKT3 0 . 1 1 1 0 1 0 0 1 0 0 0 0 5×10 2 1×10 3 IL-13 Concentration (nM) C y t o k i n e r e l e a s e ( p g / m L ) OKT3+OX40L OKT3 0 . 1 1 1 0 1 0 0 1 0 0 0 0 2×10 3 4×10 3 6×10 3 8×10 3 1×10 4 IL-17A Concentration (nM) C y t o k i n e r e l e a s e ( p g / m L ) OKT3 OKT3+OX40L Th2 cytokine Th17 cytokine 1. Biris , et al. “Development and characterization of STAR - 0310: a novel OX40 antagonistic monoclonal antibody. EAACI May 2024 .
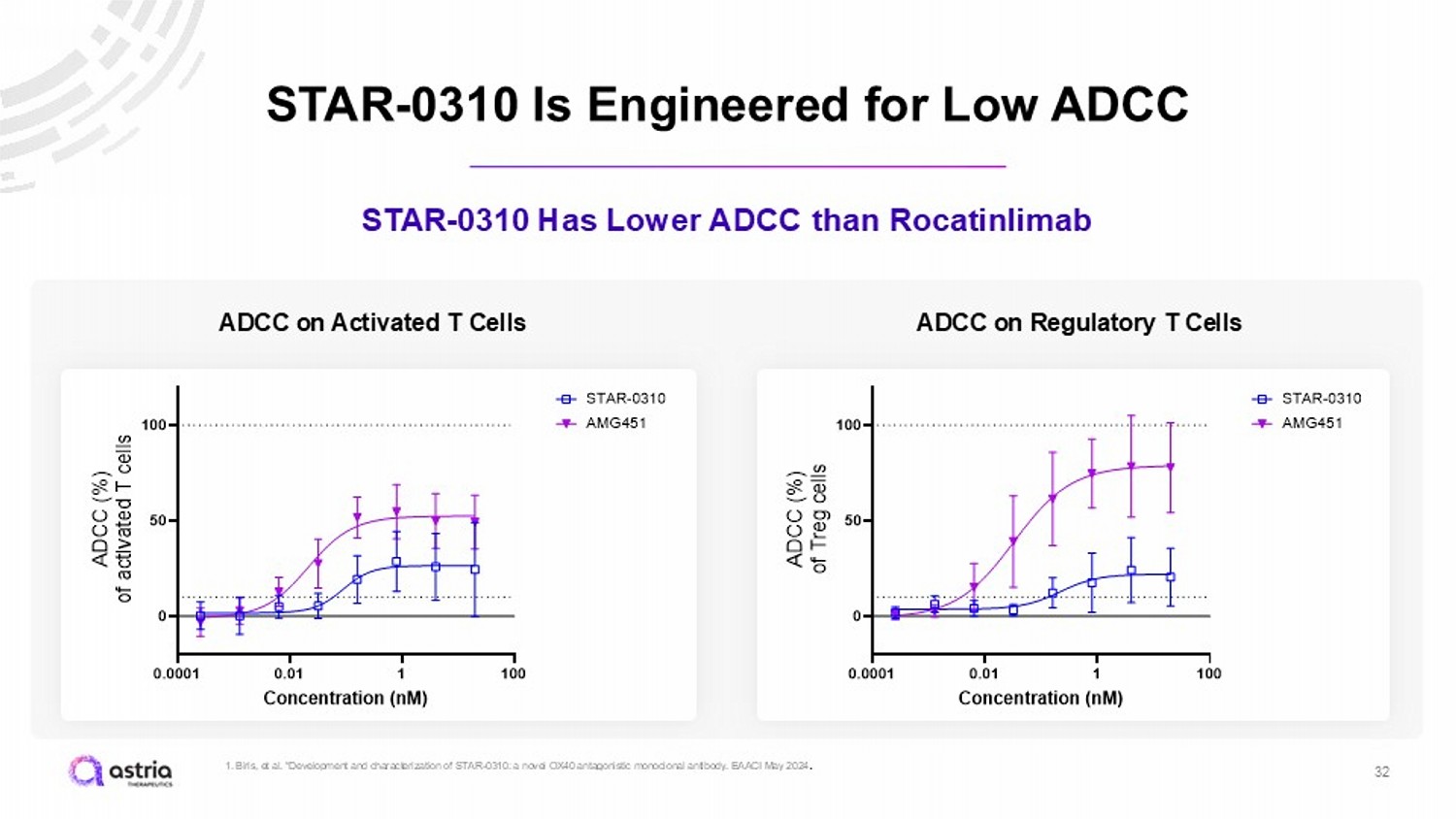
STAR - 0310 Is Engineered for Low ADCC 32 STAR - 0310 Has Lower ADCC than Rocatinlimab 0.0001 0.01 1 100 0 50 100 Concentration (nM) A D C C ( % ) o f a c t i v a t e d T c e l l s STAR-0310 AMG451 ADCC on Activated T Cells 0.0001 0.01 1 100 0 50 100 Concentration (nM) A D C C ( % ) o f T r e g c e l l s STAR-0310 AMG451 ADCC on Regulatory T Cells 1. Biris , et al. “Development and characterization of STAR - 0310: a novel OX40 antagonistic monoclonal antibody. EAACI May 2024 .
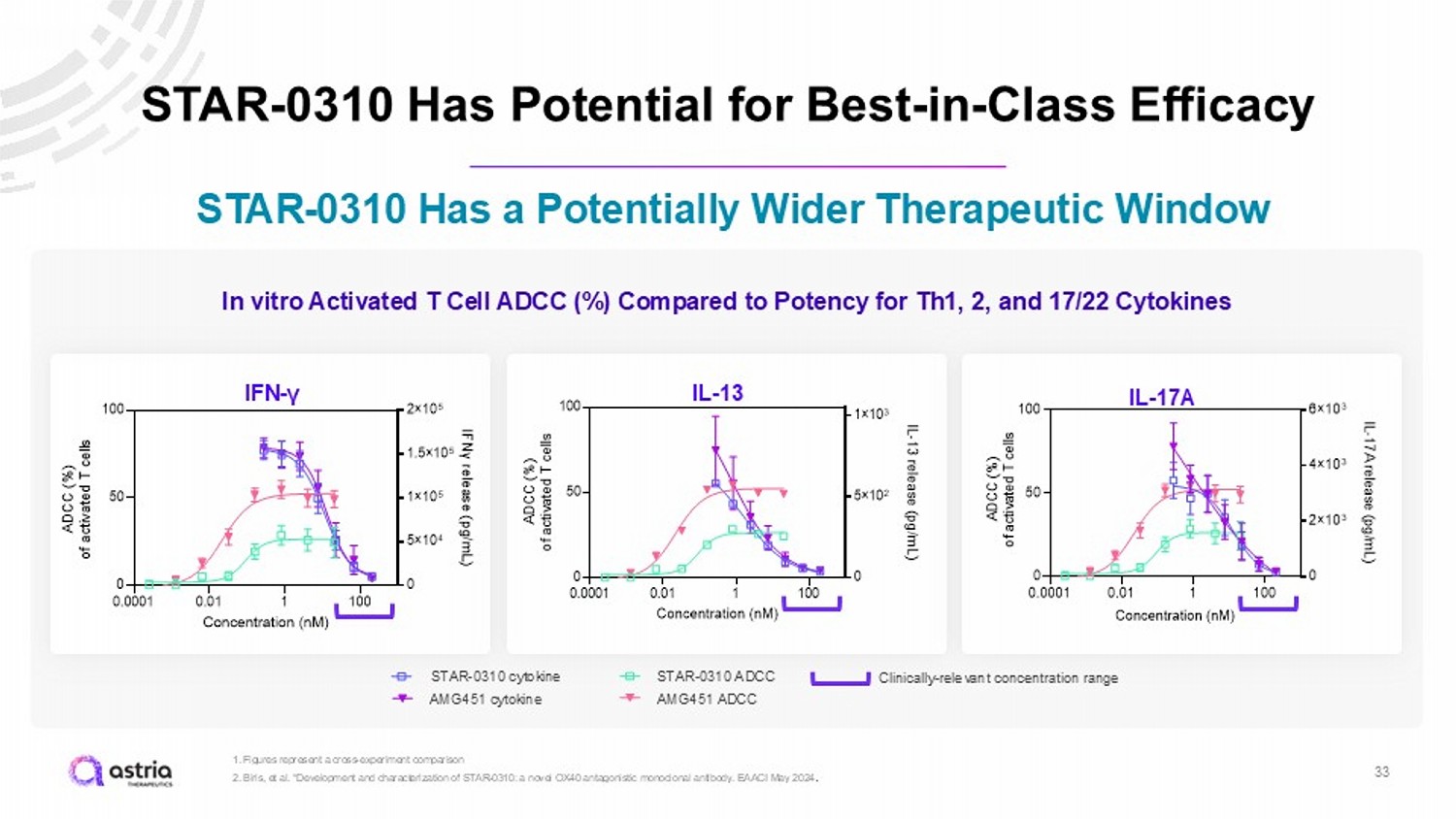
STAR - 0310 Has Potential for Best - in - Class Efficacy 33 1. Figures represent a cross - experiment comparison 2. Biris , et al. “Development and characterization of STAR - 0310: a novel OX40 antagonistic monoclonal antibody. EAACI May 2024 . STAR - 0310 Has a Potentially Wider Therapeutic Window IFN - γ IL - 13 IL - 17A 0.0001 0.01 1 100 0 50 100 0 5×10 4 1×10 5 1.5×10 5 2×10 5 Concentration (nM) A D C C ( % ) o f a c t i v a t e d T c e l l s I F N γ r e l e a s e ( p g / m L ) AMG451-IFNγ STAR-0310 ADCC Tact AMG-451-ADCC Tact STAR-0310 IFNγ 0.0001 0.01 1 100 0 50 100 0 5×10 2 1×10 3 Concentration (nM) A D C C ( % ) o f a c t i v a t e d T c e l l s I L - 1 3 r e l e a s e ( p g / m L ) AMG451-IL13 STAR-0310 ADCC Tact AMG-451-ADCC Tact STAR-0310-IL13 In vitro Activated T Cell ADCC (%) Compared to Potency for Th1, 2, and 17/22 Cytokines STAR - 0310 ADCC STAR - 0310 cytokine AMG451 cytokine AMG451 ADCC Clinically - relevant concentration range
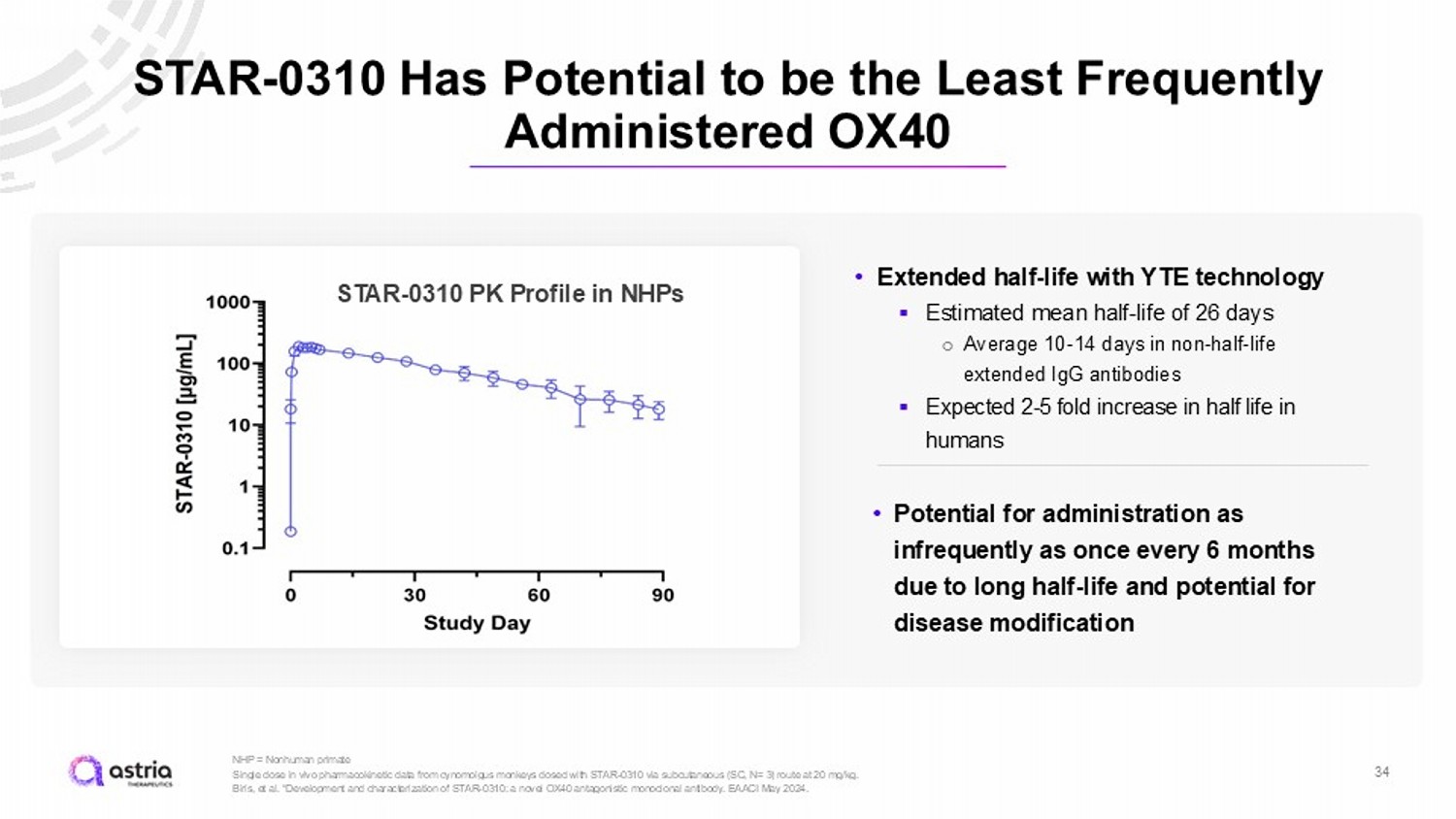
STAR - 0310 Has Potential to be the Least Frequently Administered OX40 34 NHP = Nonhuman primate Single dose in vivo pharmacokinetic data from cynomolgus monkeys dosed with STAR - 0310 via subcutaneous (SC, N= 3) route at 20 mg /kg. Biris, et al. “Development and characterization of STAR - 0310: a novel OX40 antagonistic monoclonal antibody. EAACI May 2024. STAR - 0310 PK Profile in NHPs • Extended half - life with YTE technology ▪ Estimated mean half - life of 26 days ○ Average 10 - 14 days in non - half - life extended IgG antibodies ▪ Expected 2 - 5 fold increase in half life in humans • Potential for administration as infrequently as once every 6 months due to long half - life and potential for disease modification
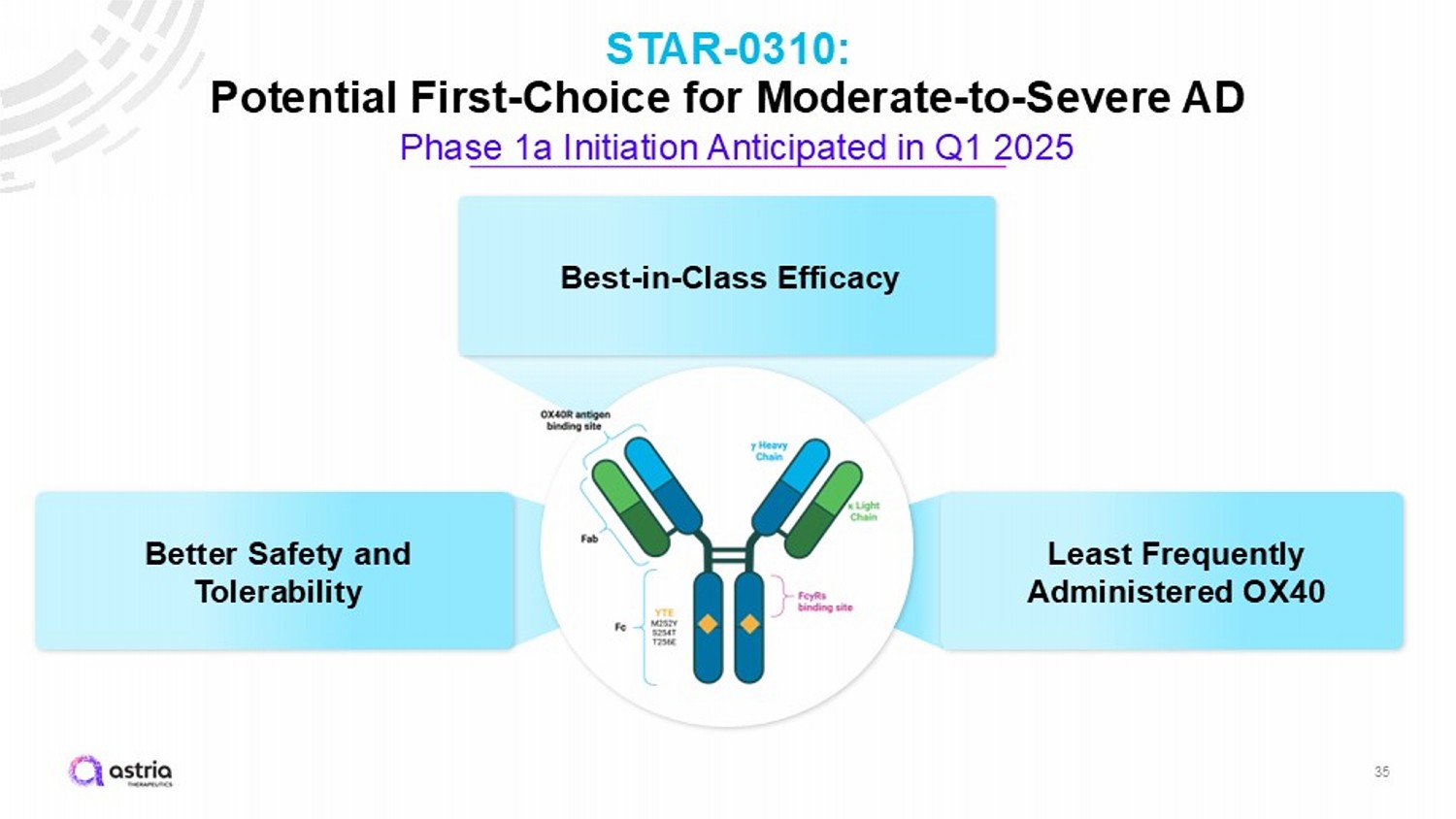
35 STAR - 0310: Potential First - Choice for Moderate - to - Severe AD Phase 1a Initiation Anticipated in Q1 2025 Better Safety and Tolerability Best - in - Class Efficacy Least Frequently Administered OX40
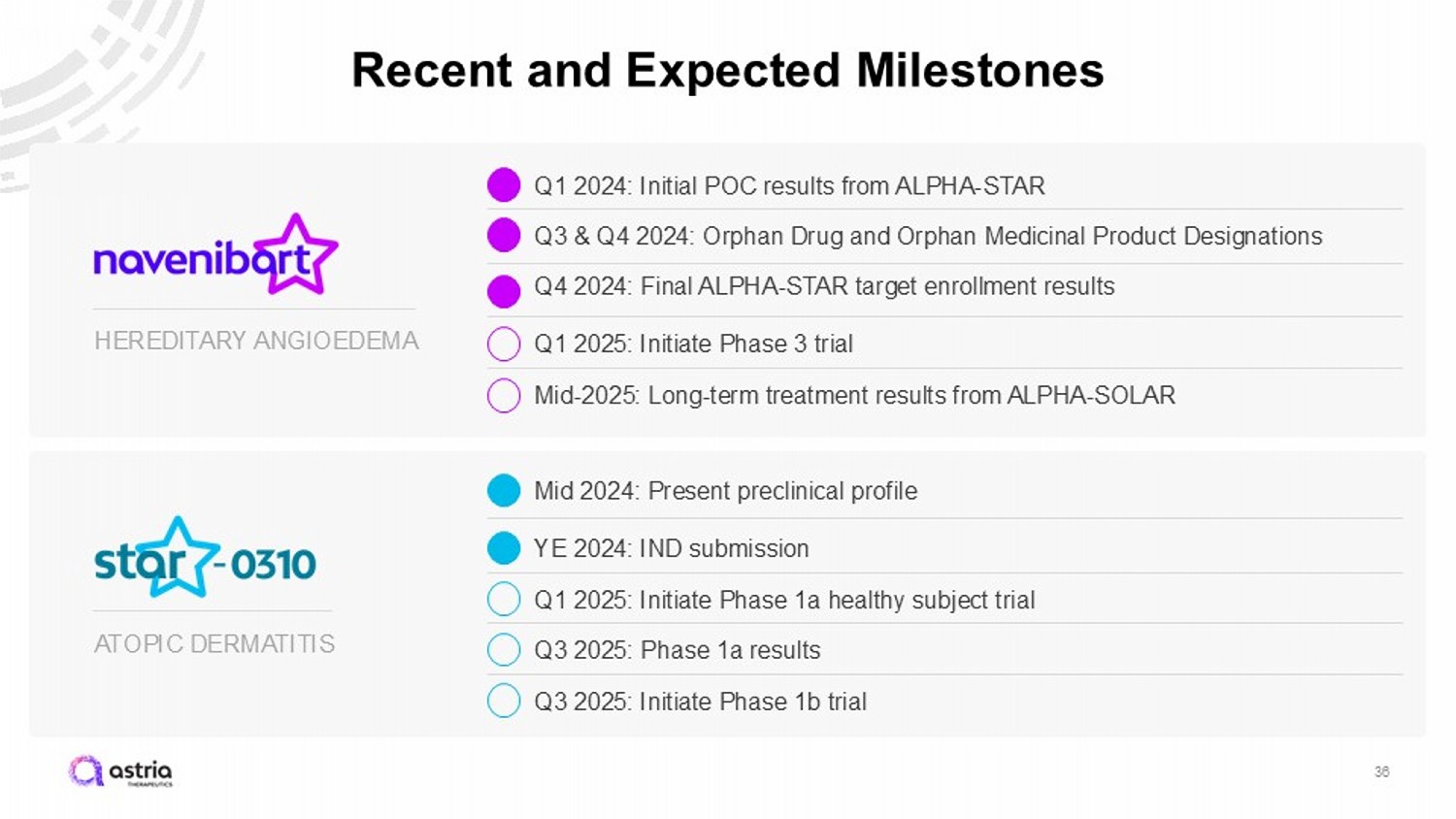
ATOPIC DERMATITIS HEREDITARY ANGIOEDEMA Recent and Expected Milestones 36 Q3 & Q4 2024: Orphan Drug and Orphan Medicinal Product Designations Q1 2025: Initiate Phase 3 trial Mid - 2025: Long - term treatment results from ALPHA - SOLAR YE 2024: IND submission Q1 2025: Initiate Phase 1a healthy subject trial Q3 2025: Phase 1a results Q3 2025: Initiate Phase 1b trial Q4 2024: Final ALPHA - STAR target enrollment results Mid 2024: Present preclinical profile Q1 2024: Initial POC results from ALPHA - STAR
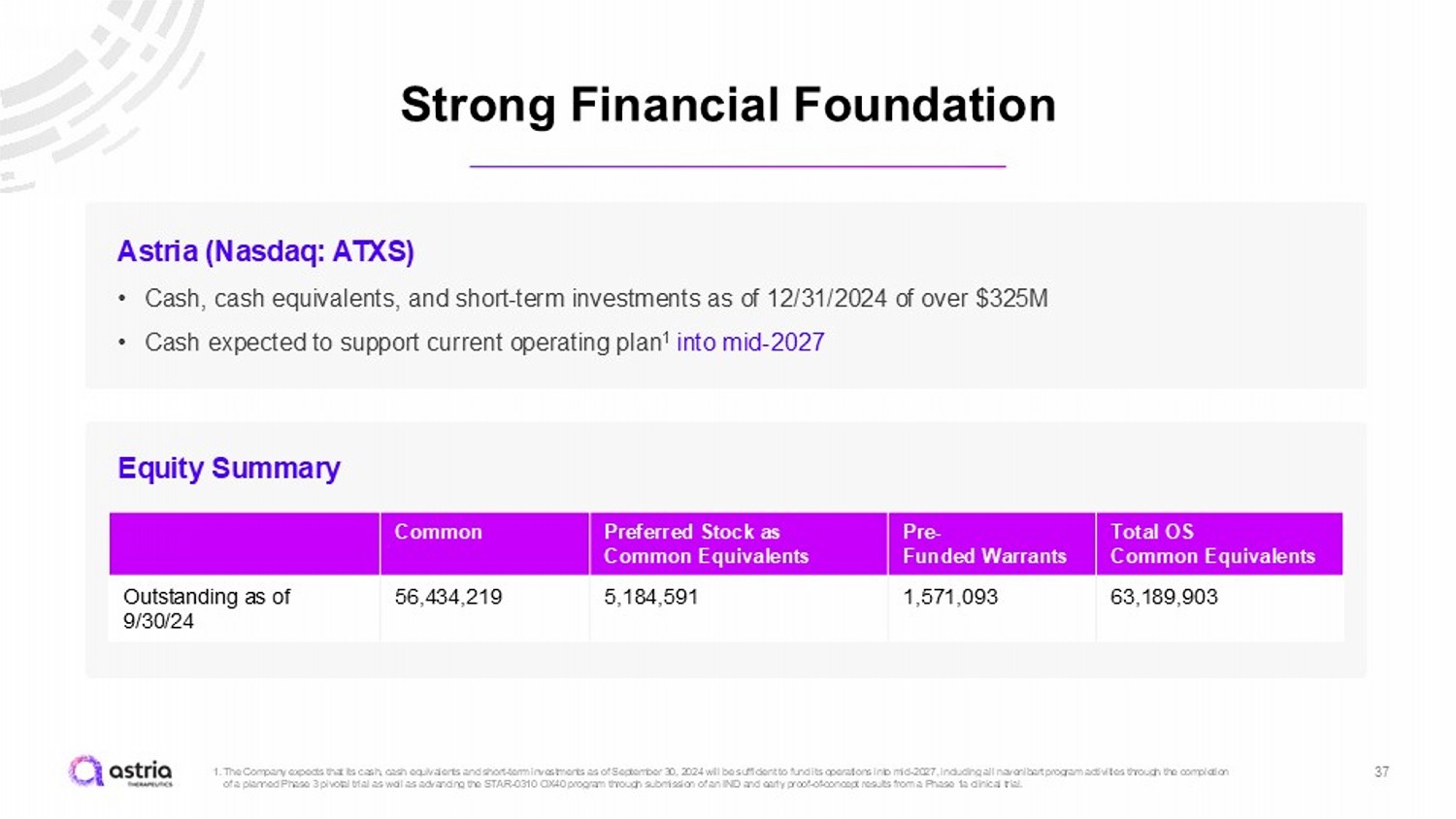
Strong Financial Foundation 37 1. The Company expects that its cash, cash equivalents and short - term investments as of September 30, 2024 will be sufficient to fu nd its operations into mid - 2027, including all navenibart program activities through the completion of a planned Phase 3 pivotal trial as well as advancing the STAR - 0310 OX40 program through submission of an IND and early proof - of - concept results from a Phase 1a clinical trial. Astria (Nasdaq: ATXS) • Cash, cash equivalents, and short - term investments as of 12/31/2024 of over $325M • Cash expected to support current operating plan 1 into mid - 2027 Total OS Common Equivalents Pre - Funded Warrants Preferred Stock as Common Equivalents Common 63,189,903 1,571,093 5,184,591 56,434,219 Outstanding as of 9/30/24 Equity Summary

v3.24.4
Cover
|
Jan. 13, 2025 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jan. 13, 2025
|
| Entity File Number |
001-37467
|
| Entity Registrant Name |
Astria Therapeutics, Inc.
|
| Entity Central Index Key |
0001454789
|
| Entity Tax Identification Number |
26-3687168
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
22 Boston Wharf Road
|
| Entity Address, Address Line Two |
10th Floor
|
| Entity Address, City or Town |
Boston
|
| Entity Address, State or Province |
MA
|
| Entity Address, Postal Zip Code |
02110
|
| City Area Code |
617
|
| Local Phone Number |
349-1971
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, $0.001 par
value per share
|
| Trading Symbol |
ATXS
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Grafico Azioni Astria Therapeutics (NASDAQ:ATXS)
Storico
Da Dic 2024 a Gen 2025

Grafico Azioni Astria Therapeutics (NASDAQ:ATXS)
Storico
Da Gen 2024 a Gen 2025
